Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
106 results about "Rifaximin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat diarrhea caused by the common bacteria known as E.
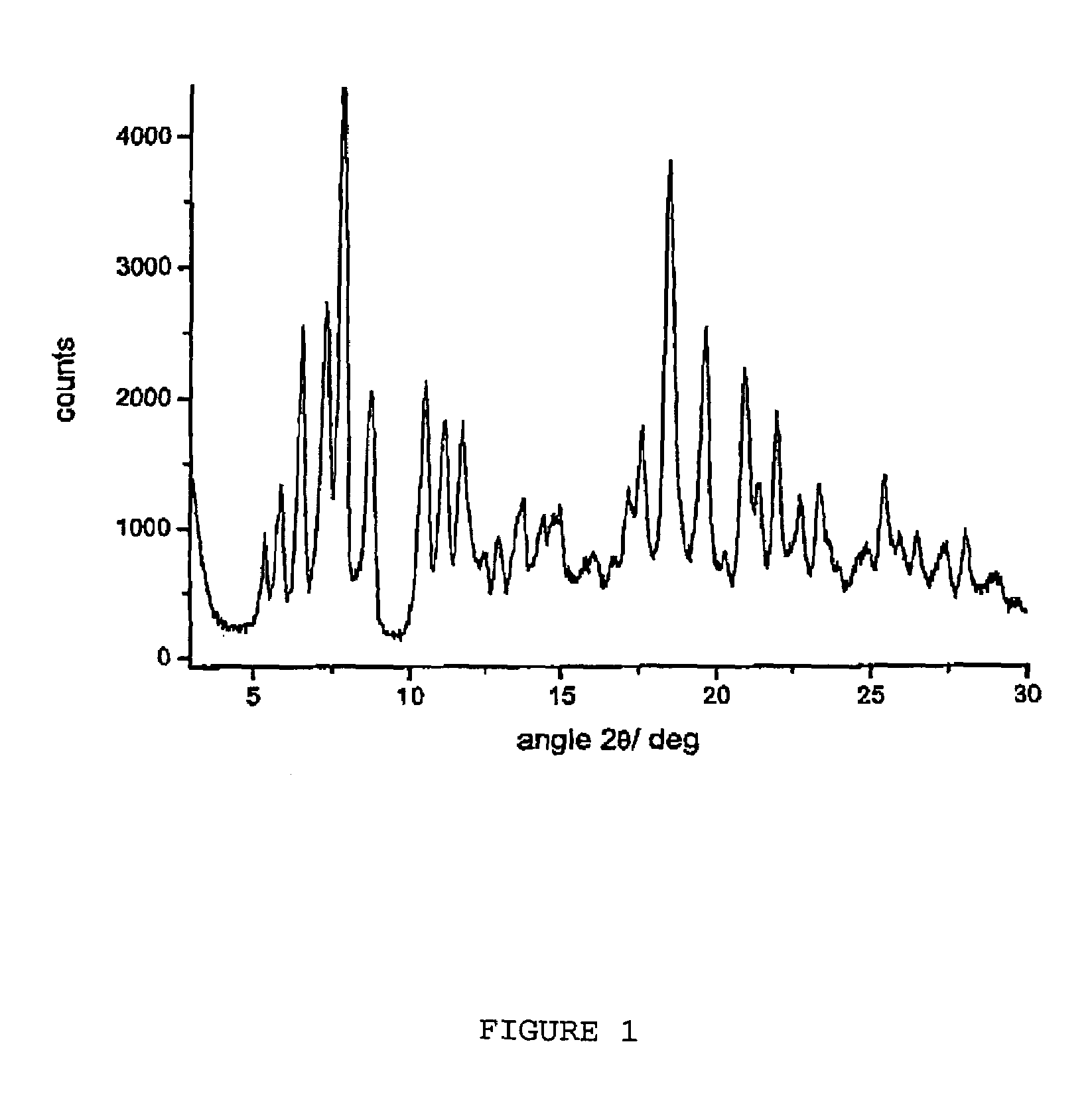
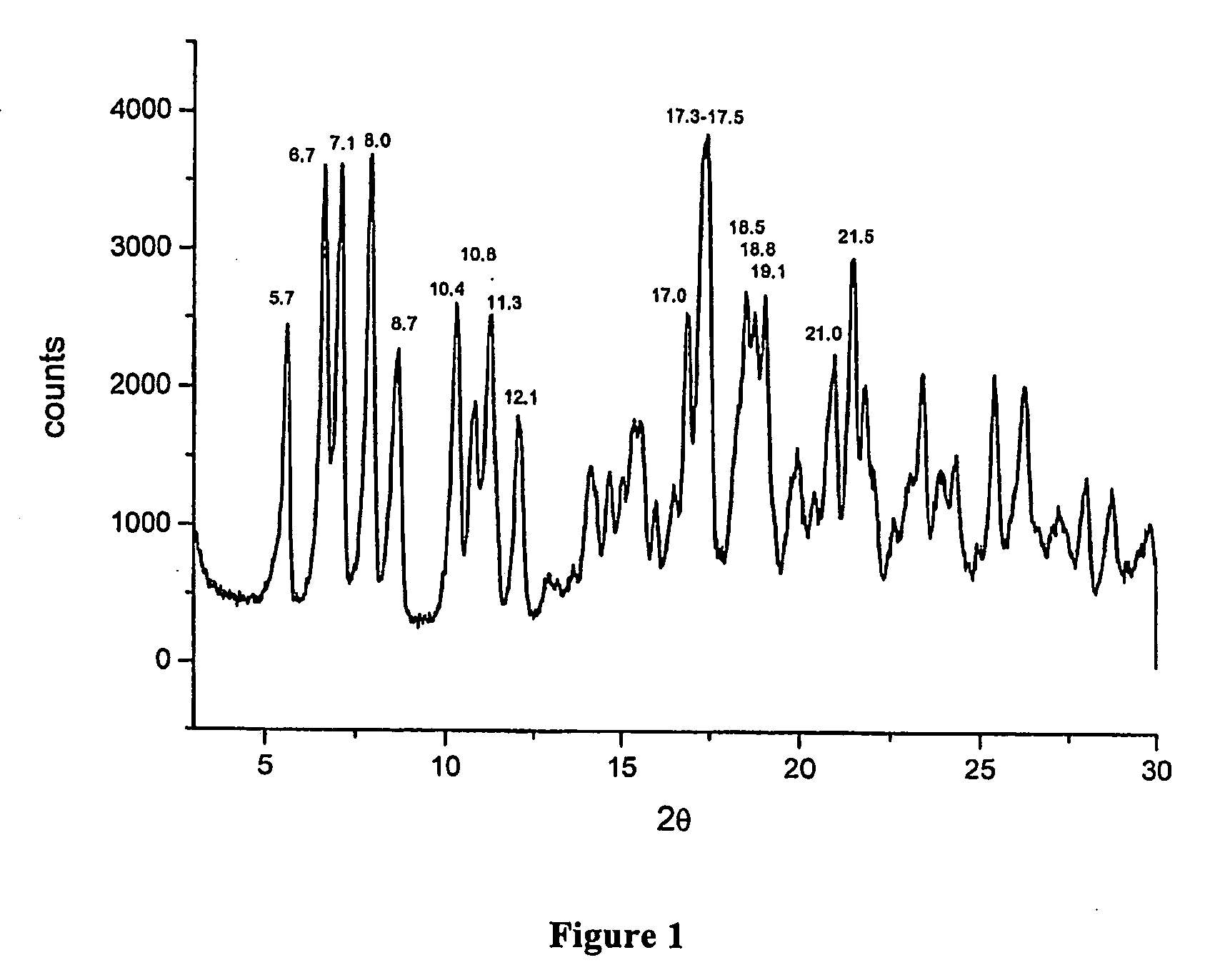
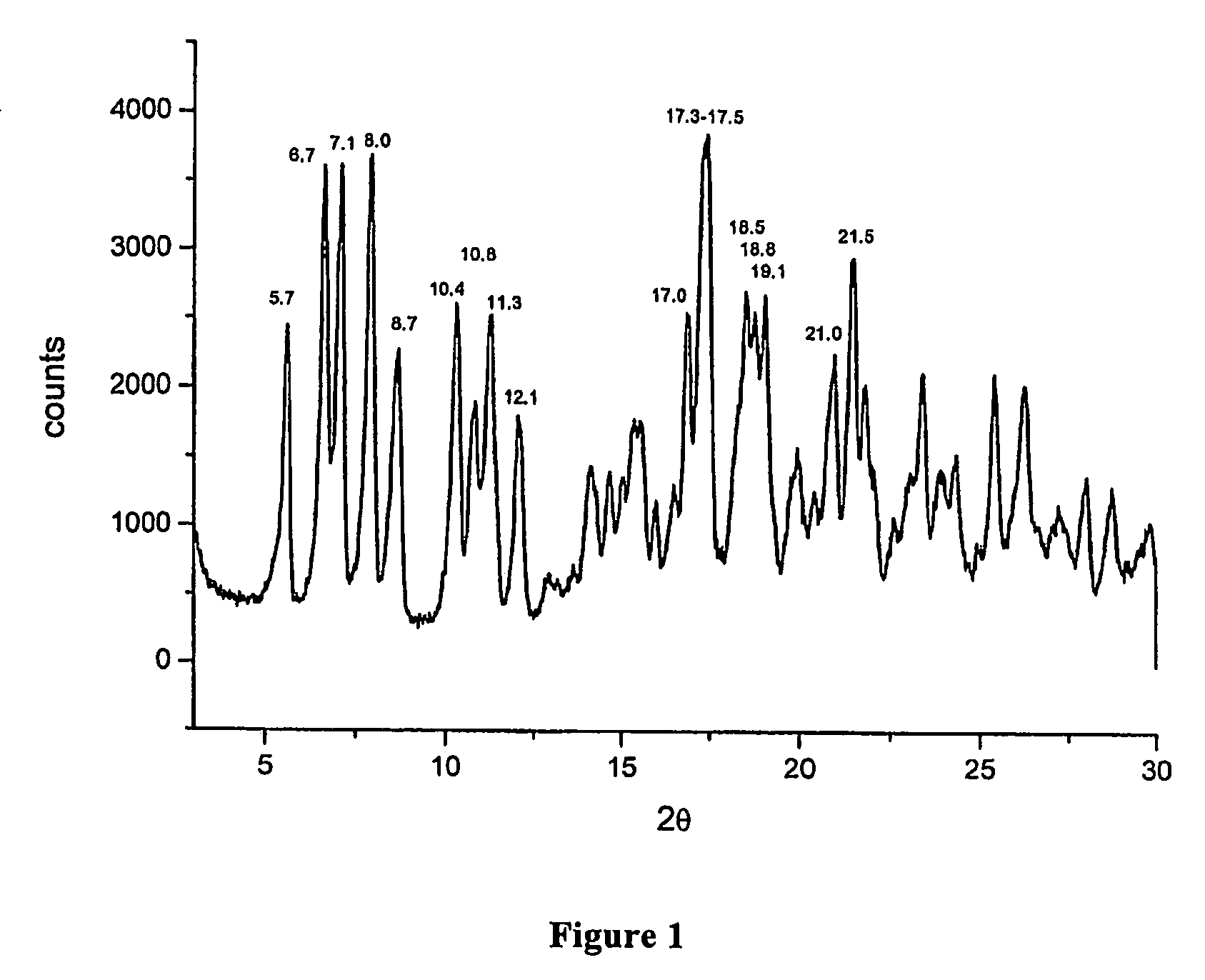
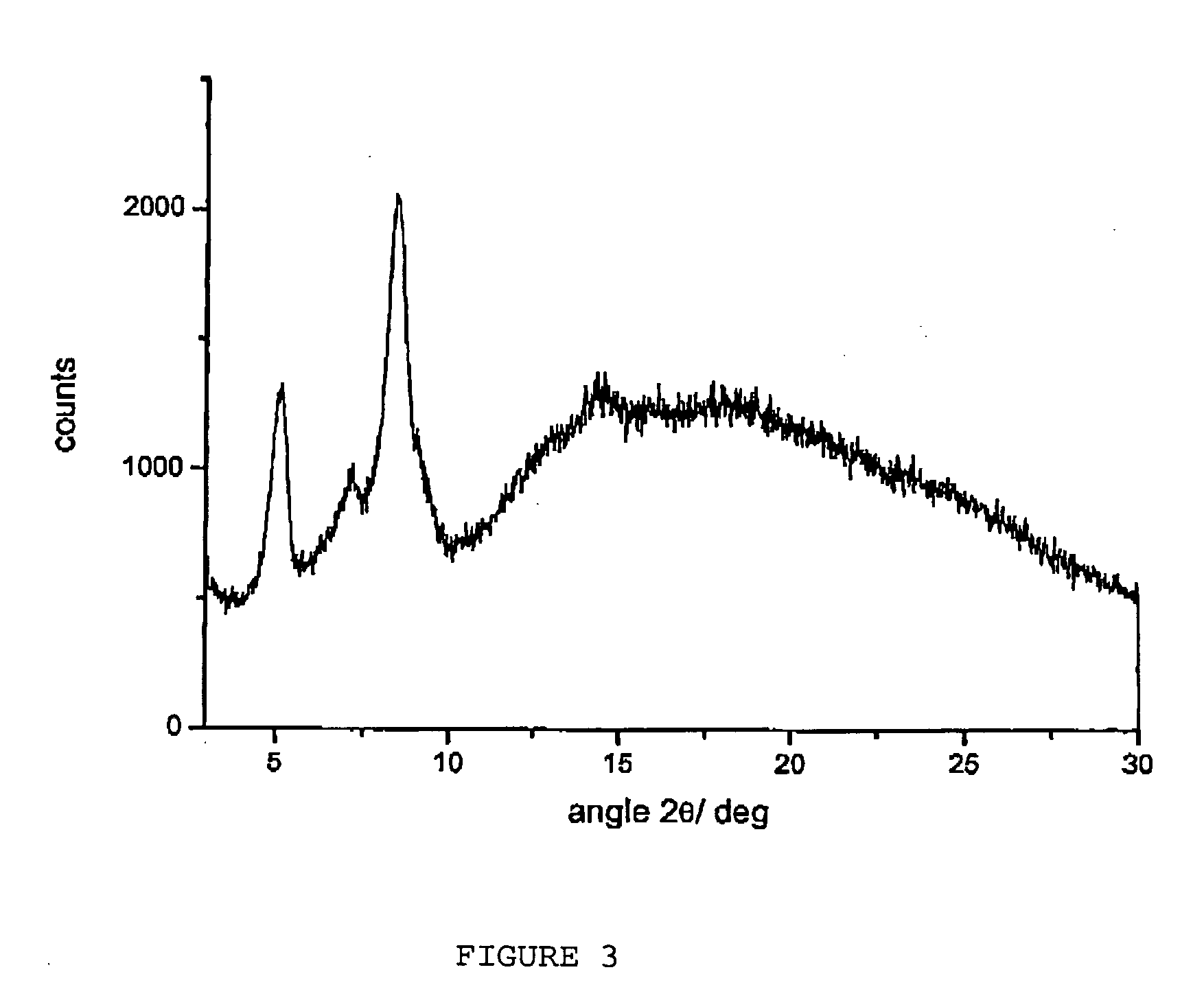
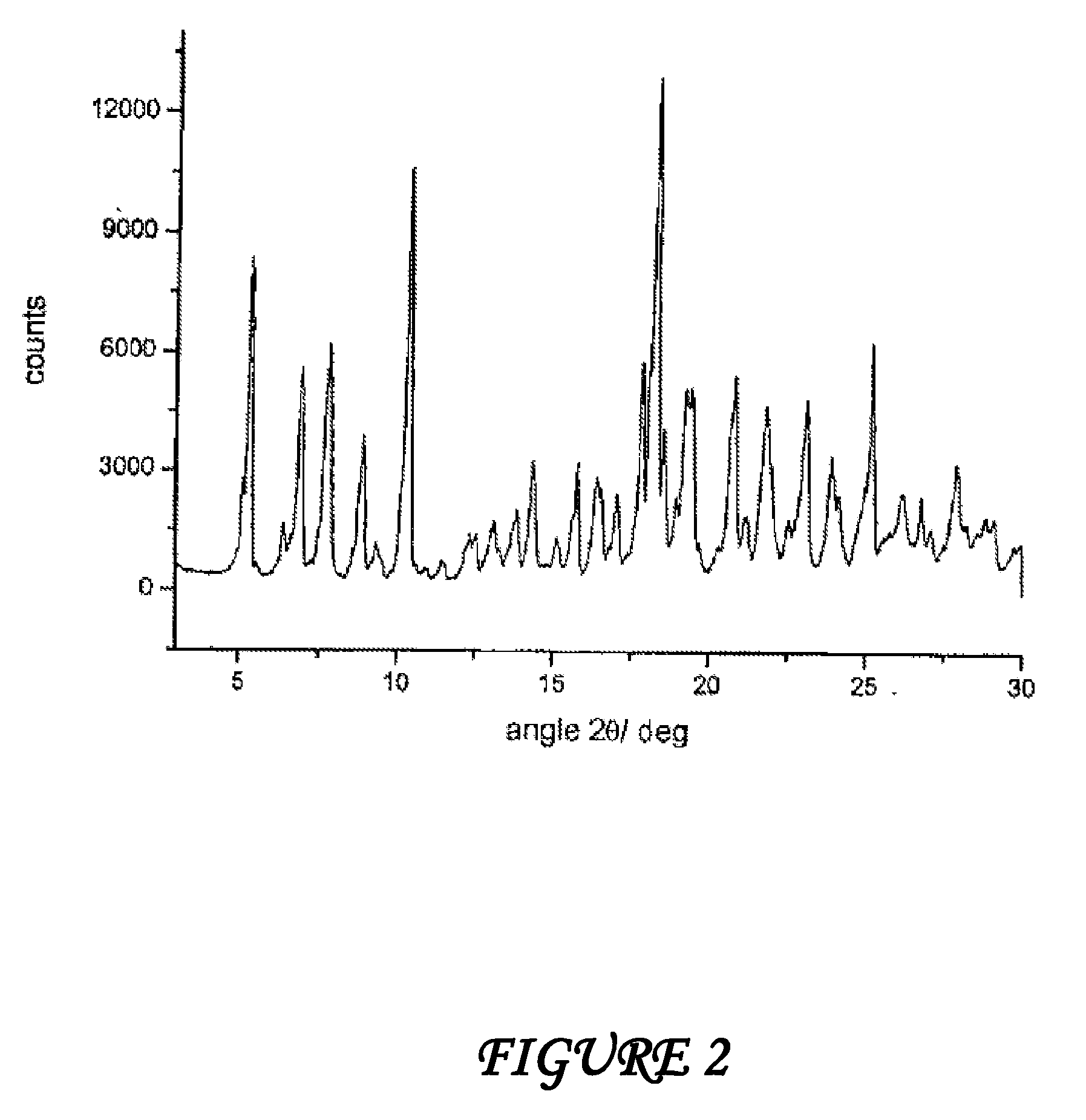
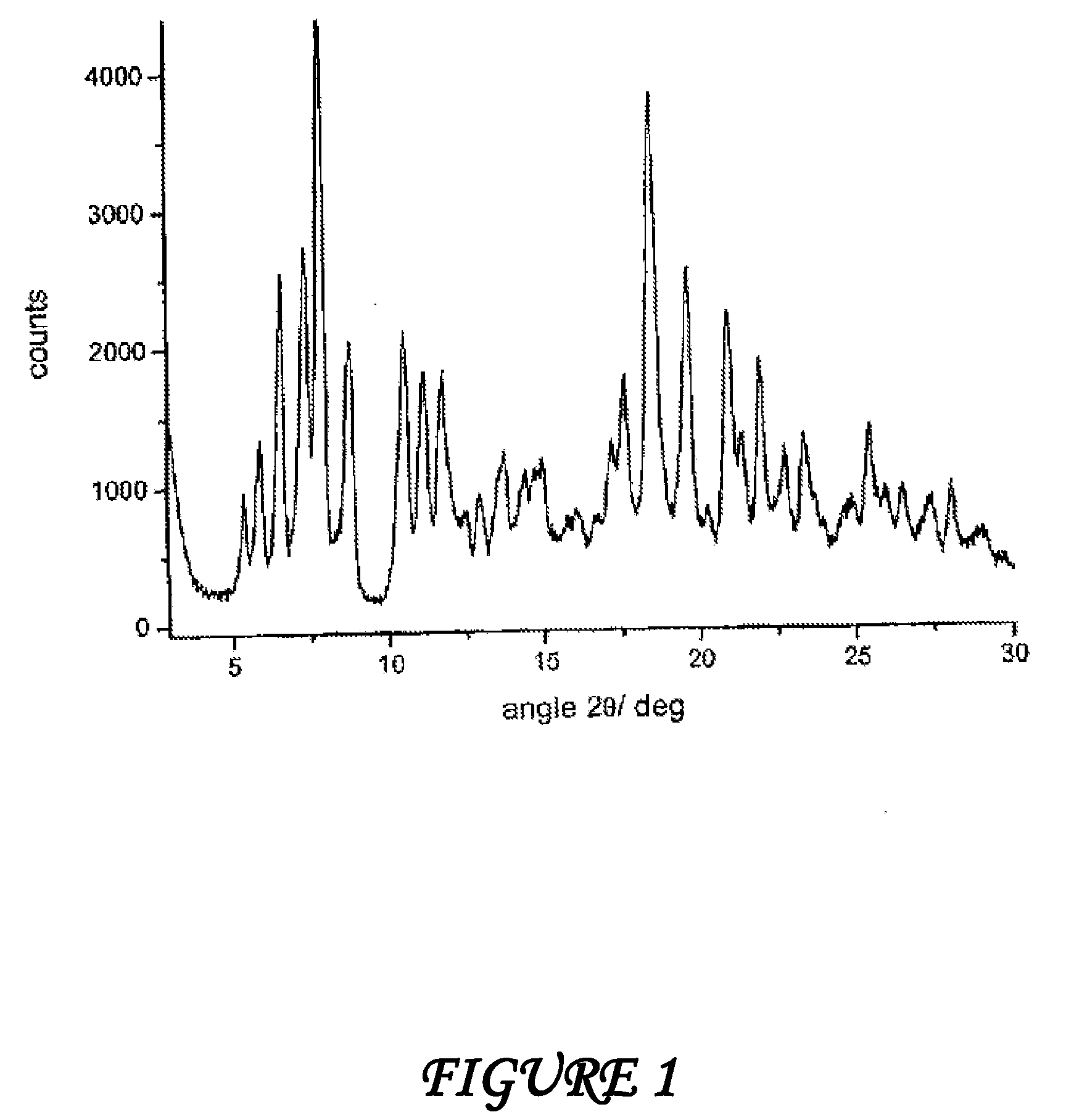
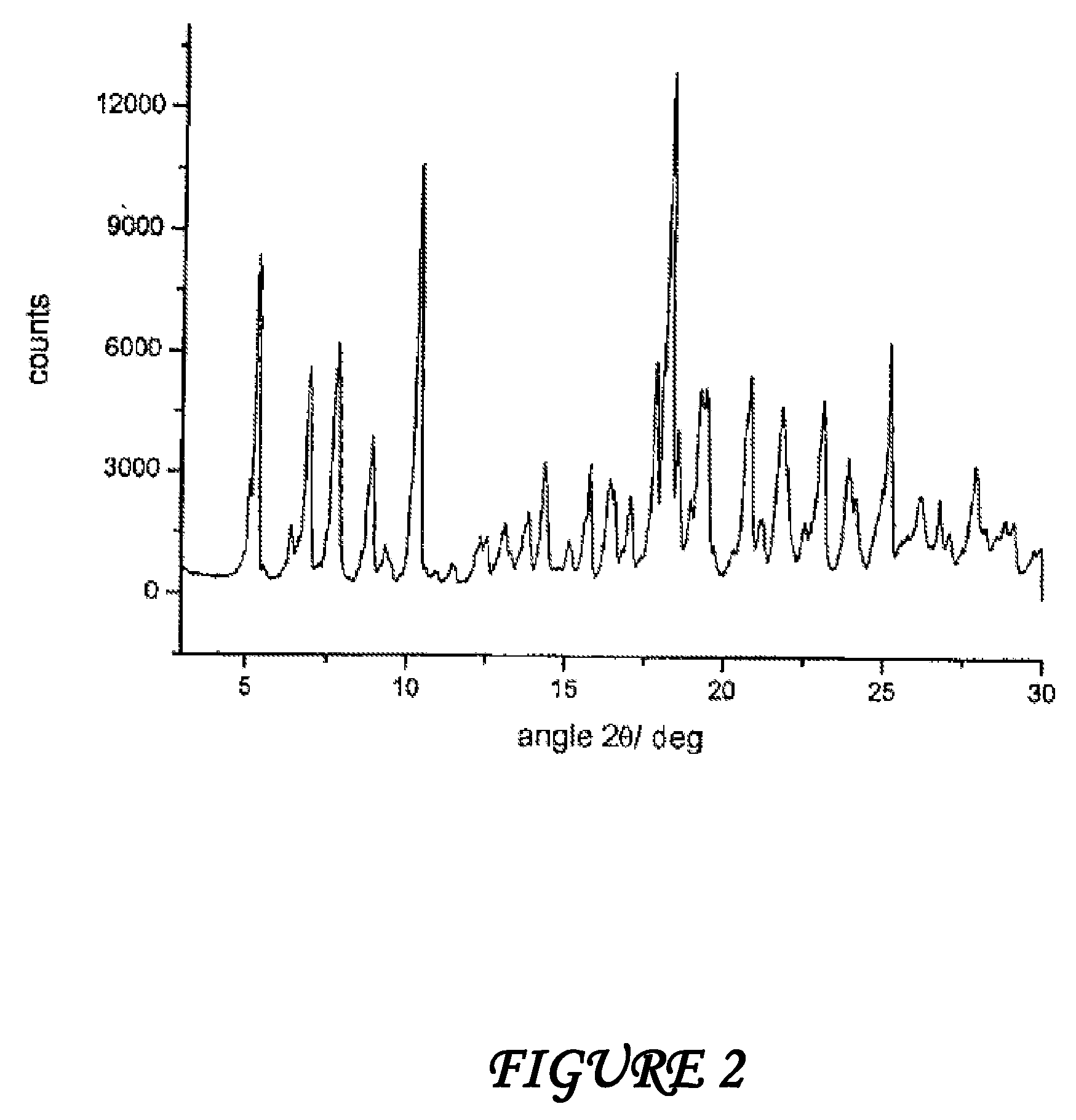
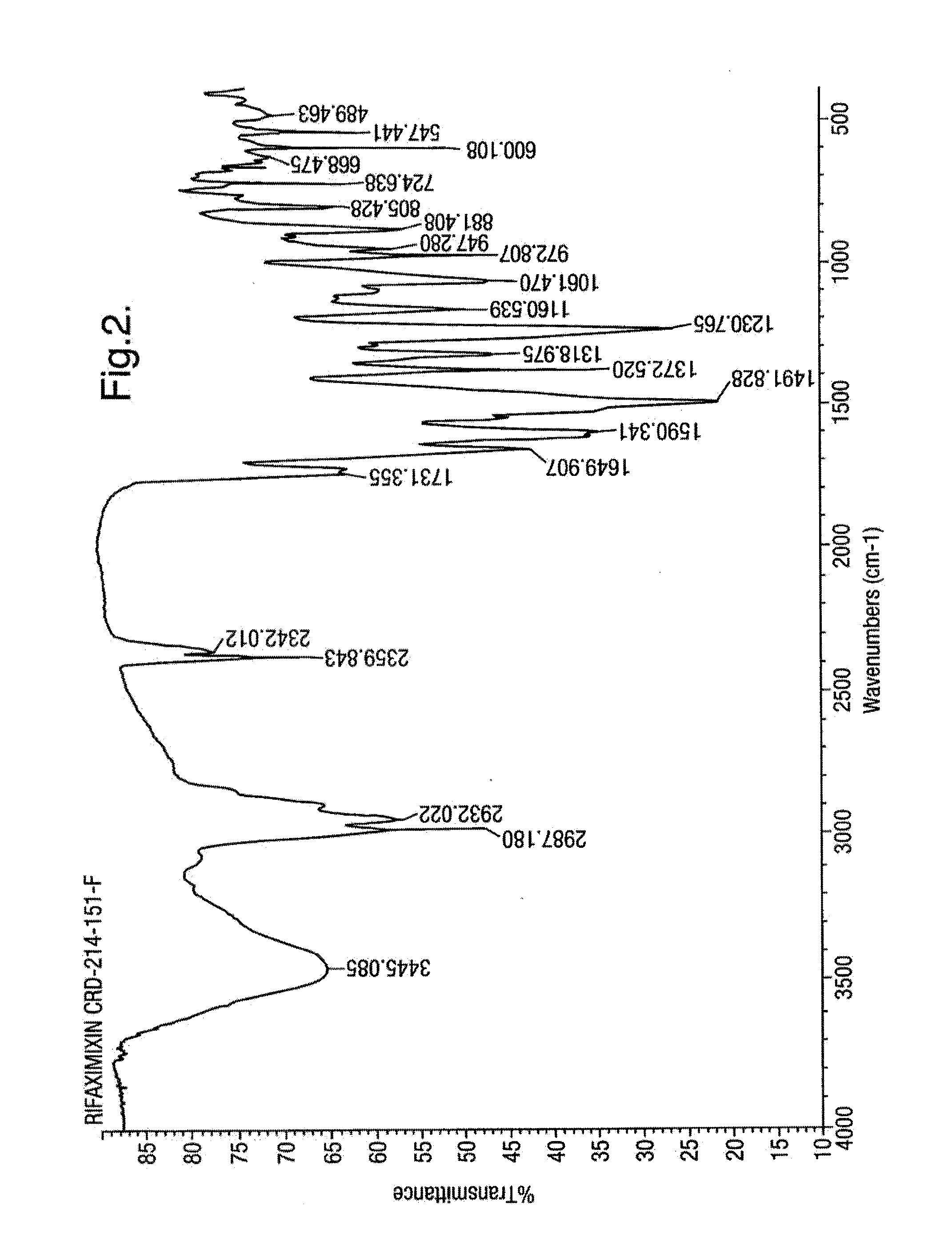
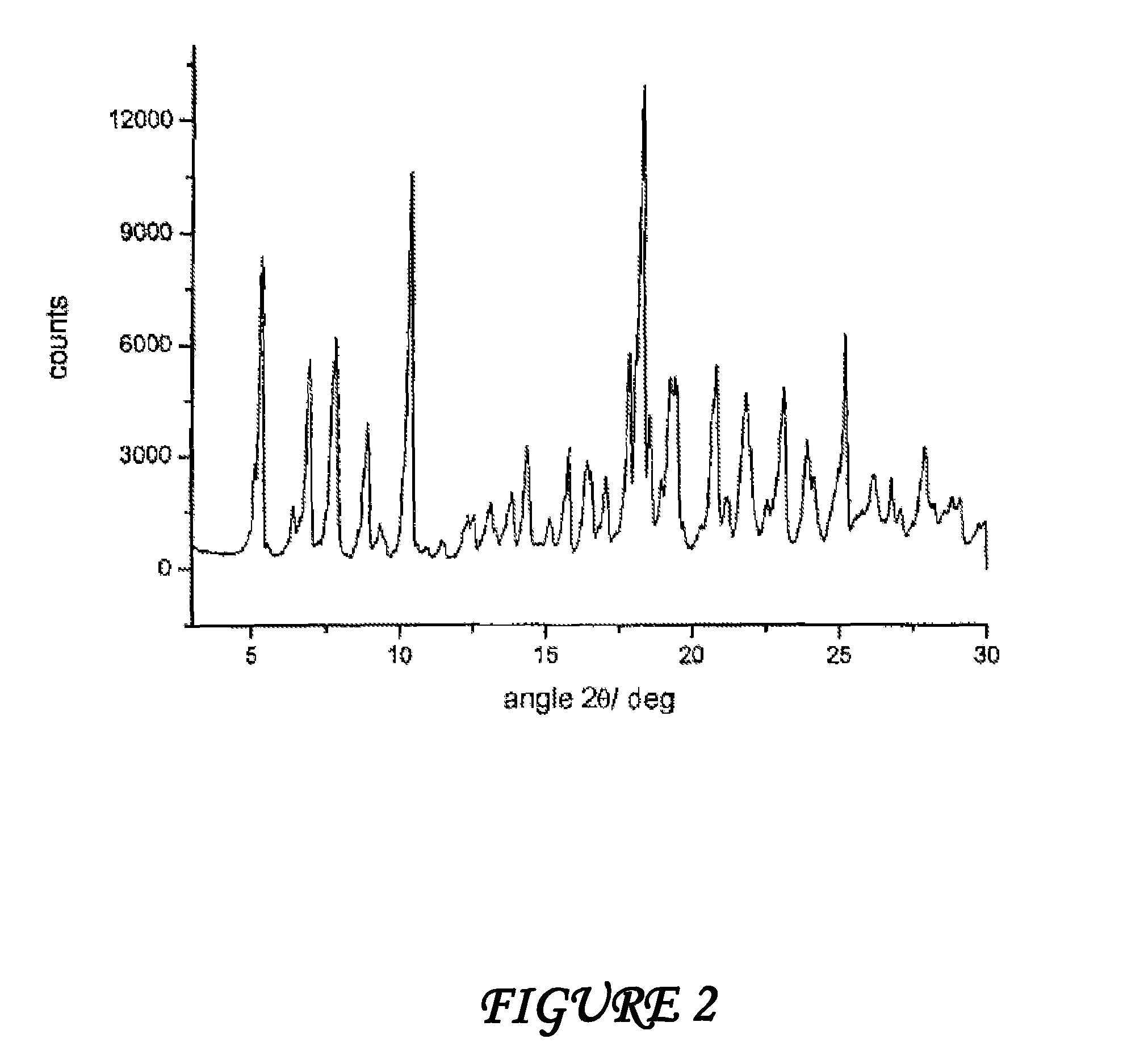
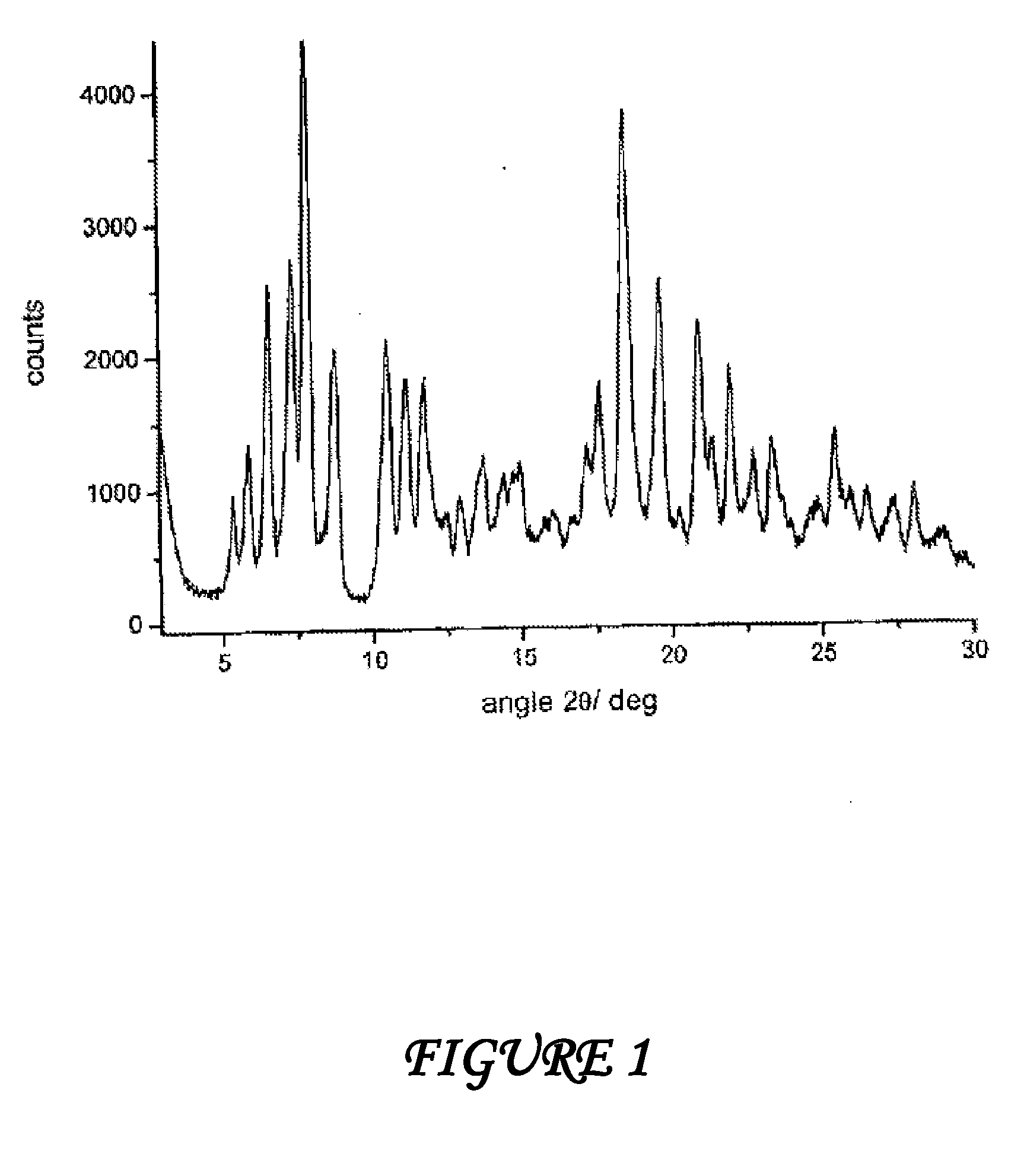
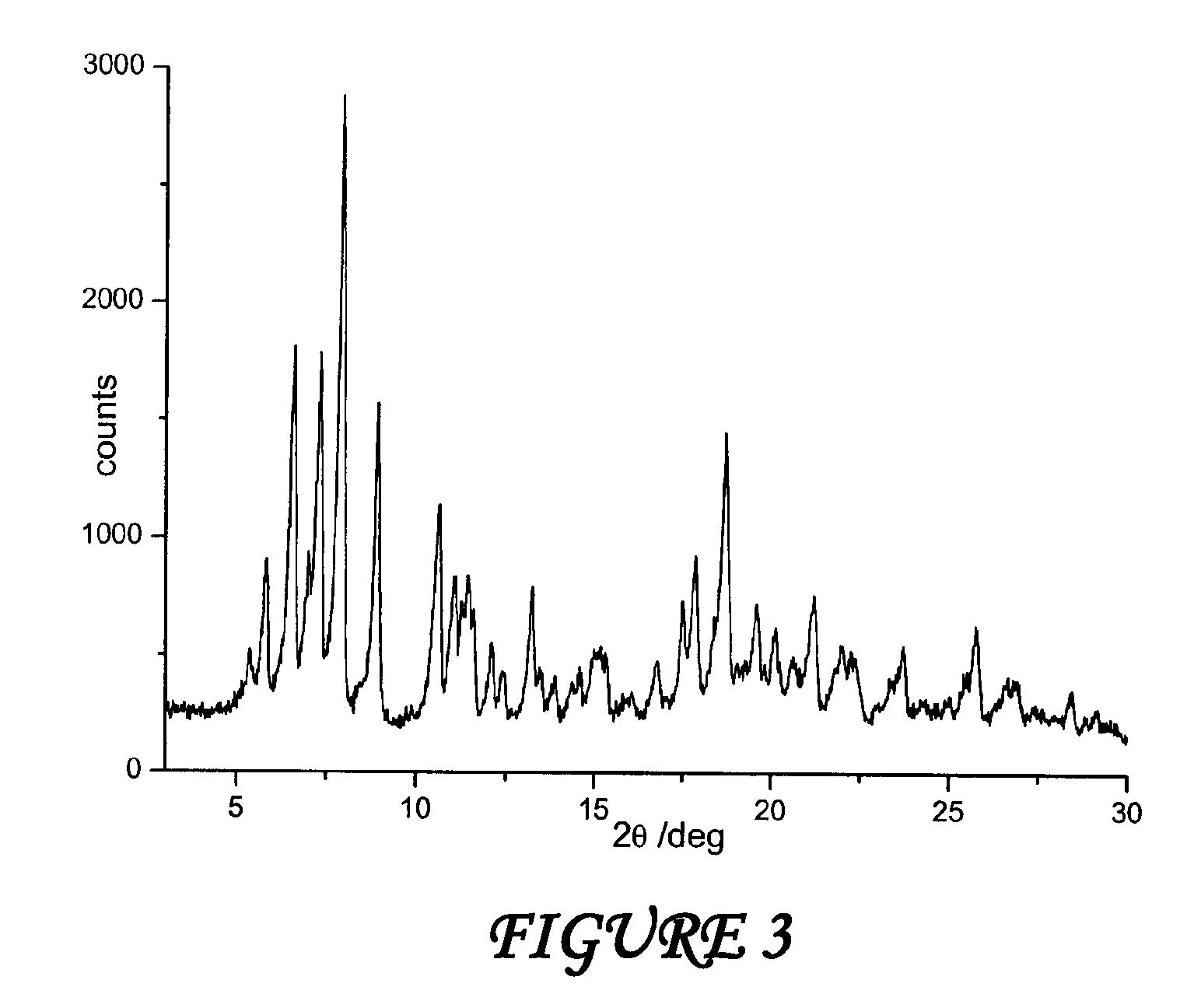
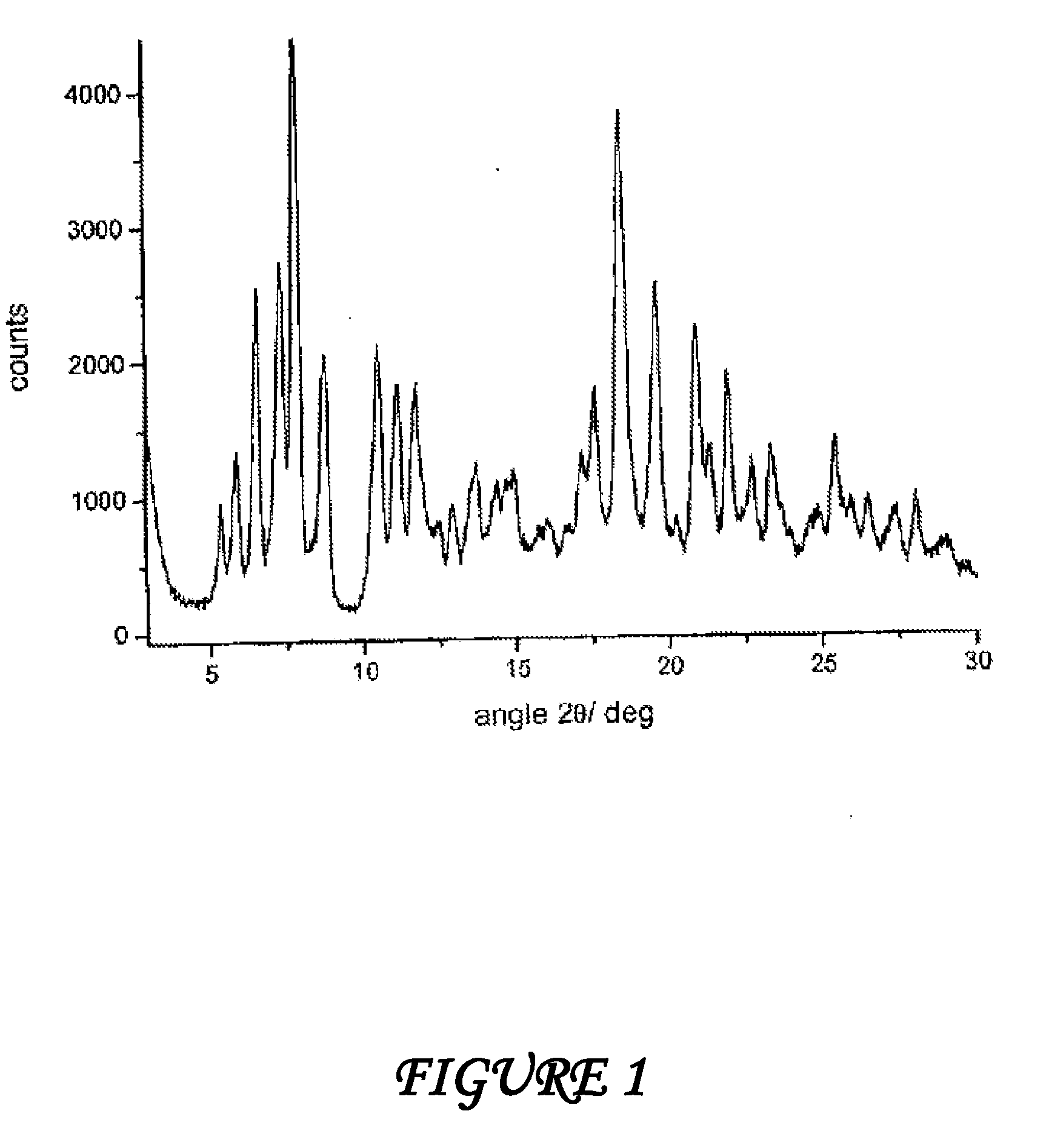
Polymorphous forms of rifaximin, processes for their production and use thereof in medicinal preparations
Crystalline polymorphous forms of the rifaximin (INN) antibiotic named rifaximin α and rifaximin β, and a poorly crystalline form named rifaximin γ have been discovered. These forms are useful in the production of medicinal preparations for oral and topical use and can be obtained by means of a crystallization process carried out by hot-dissolving the raw rifaximin in ethyl alcohol and by causing the crystallization of the product by the addition of water at a determinate temperature and for a determinate period of time. The crystallization is followed by drying carried out under controlled conditions until a specific water content is reached in the end product.
Owner:ALFASIGMA SPA
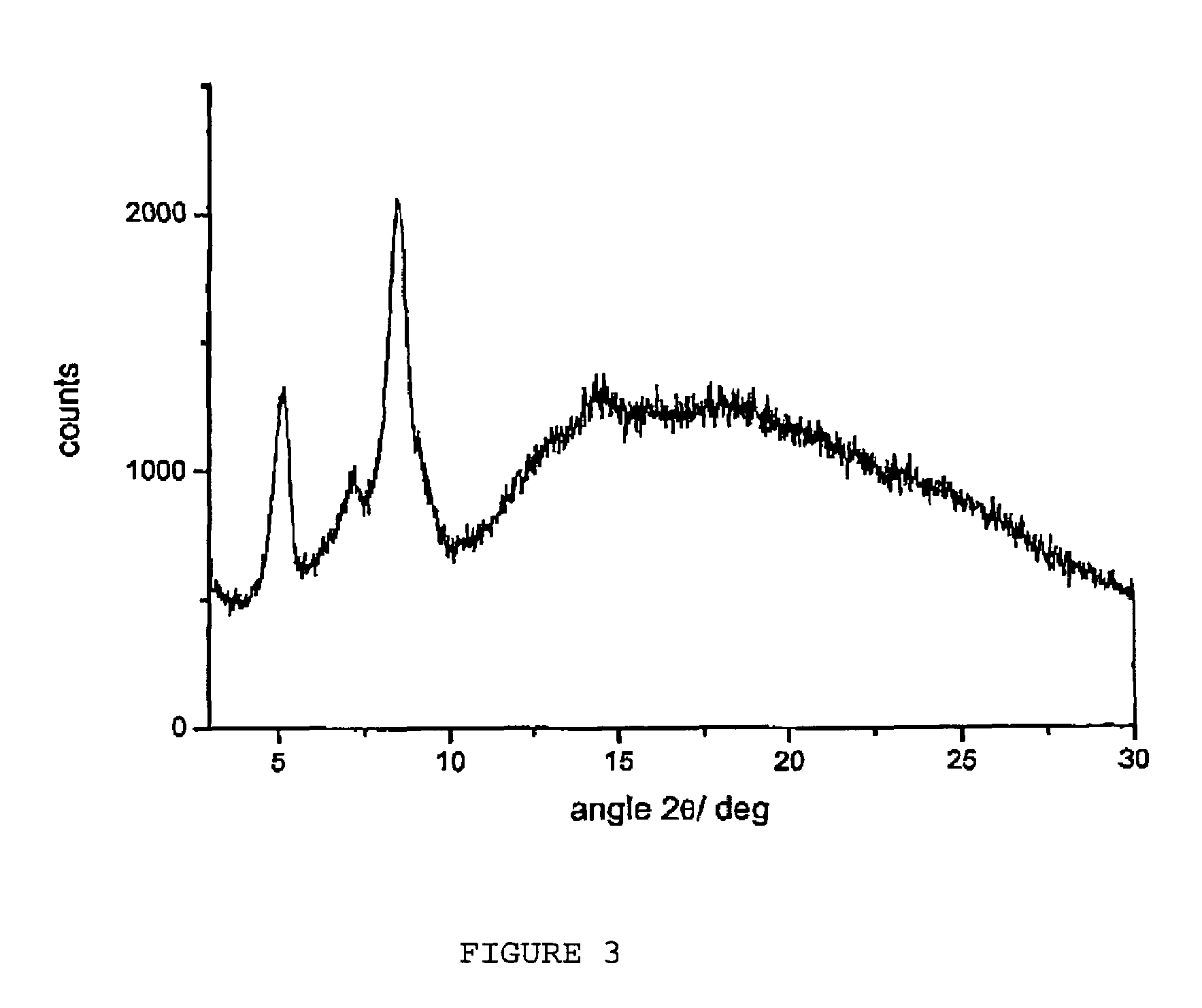
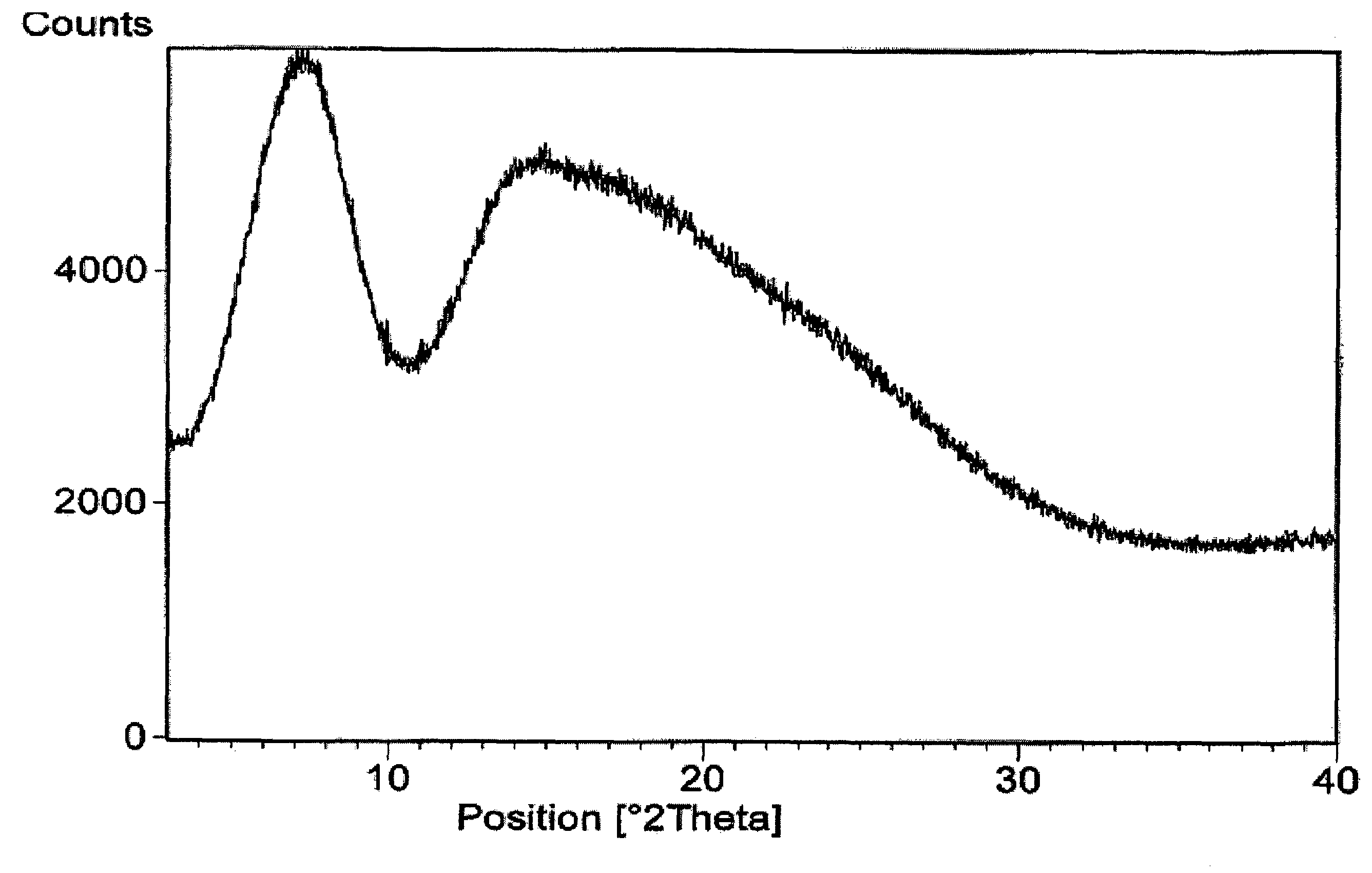
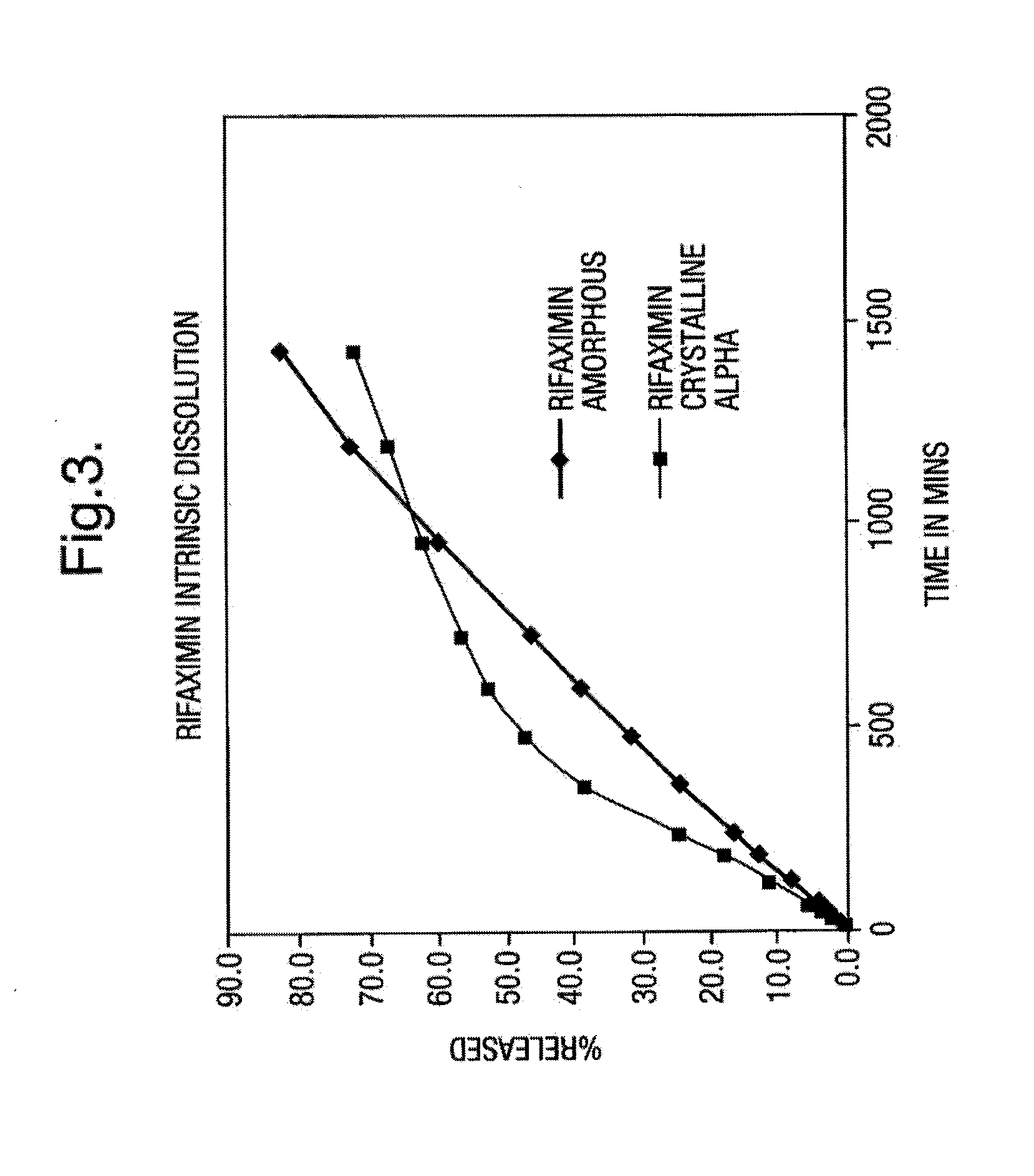
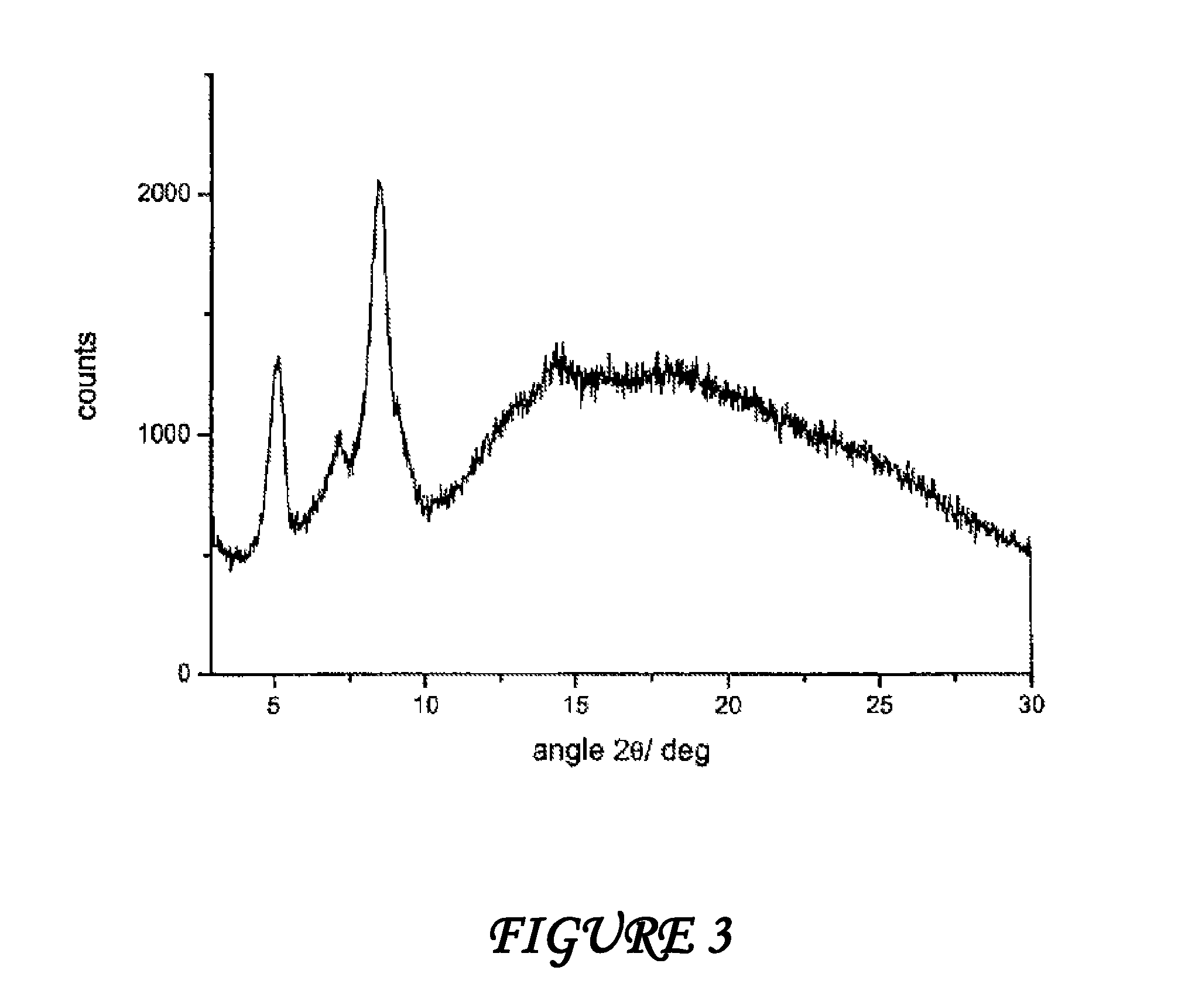
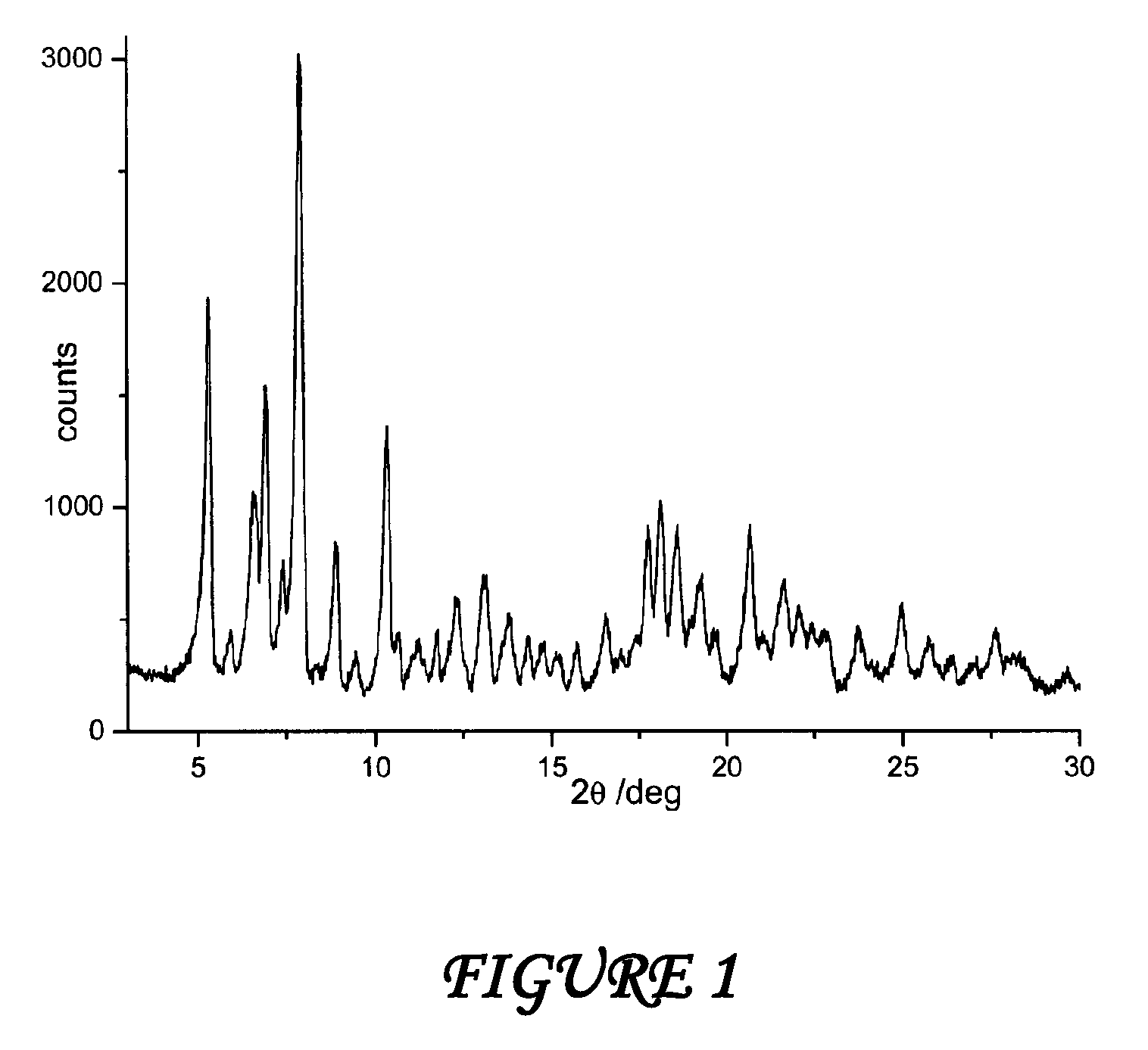
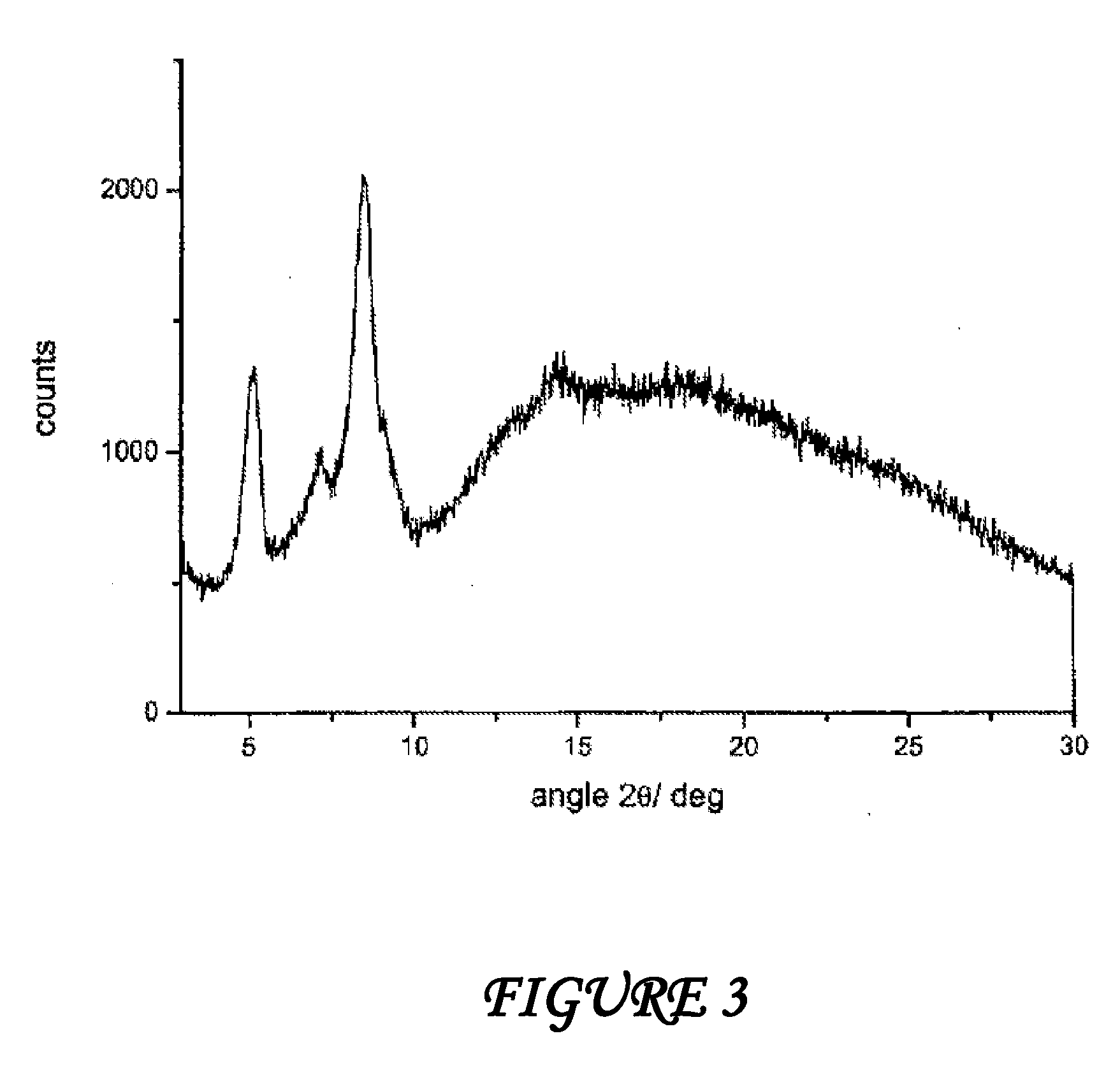
Amorphous form of rifaximin and processes for its preparation
A stable amorphous form of rifaximin is disclosed. This form is chemically and polymorphically stable on storage and can be prepared by dissolving rifaximin in a solvent to form a solution, which is precipitated by adding an anti-solvent and isolating of the precipitated amorphous rifaximin as an end product.
Owner:SALIX PHARMA INC
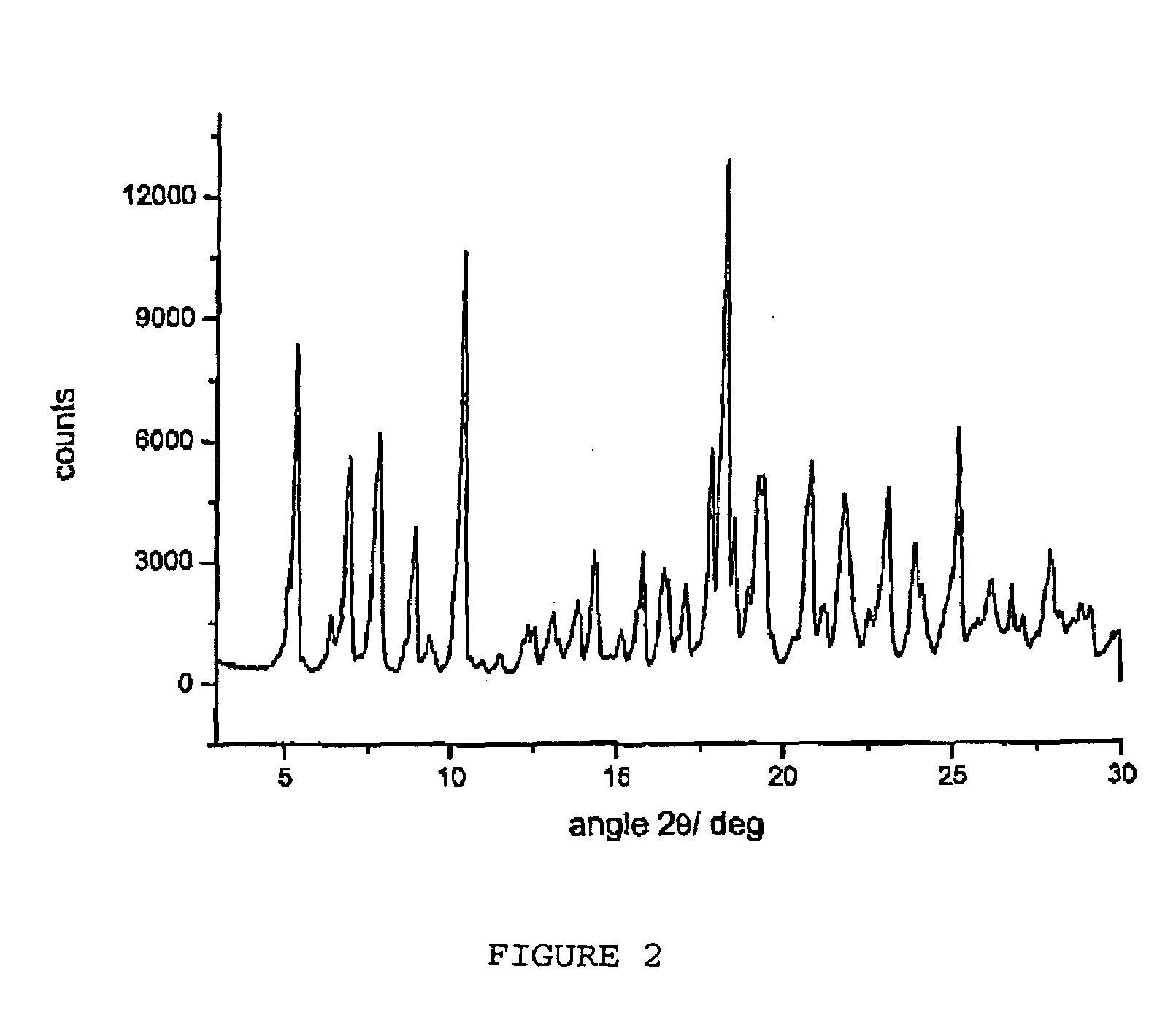
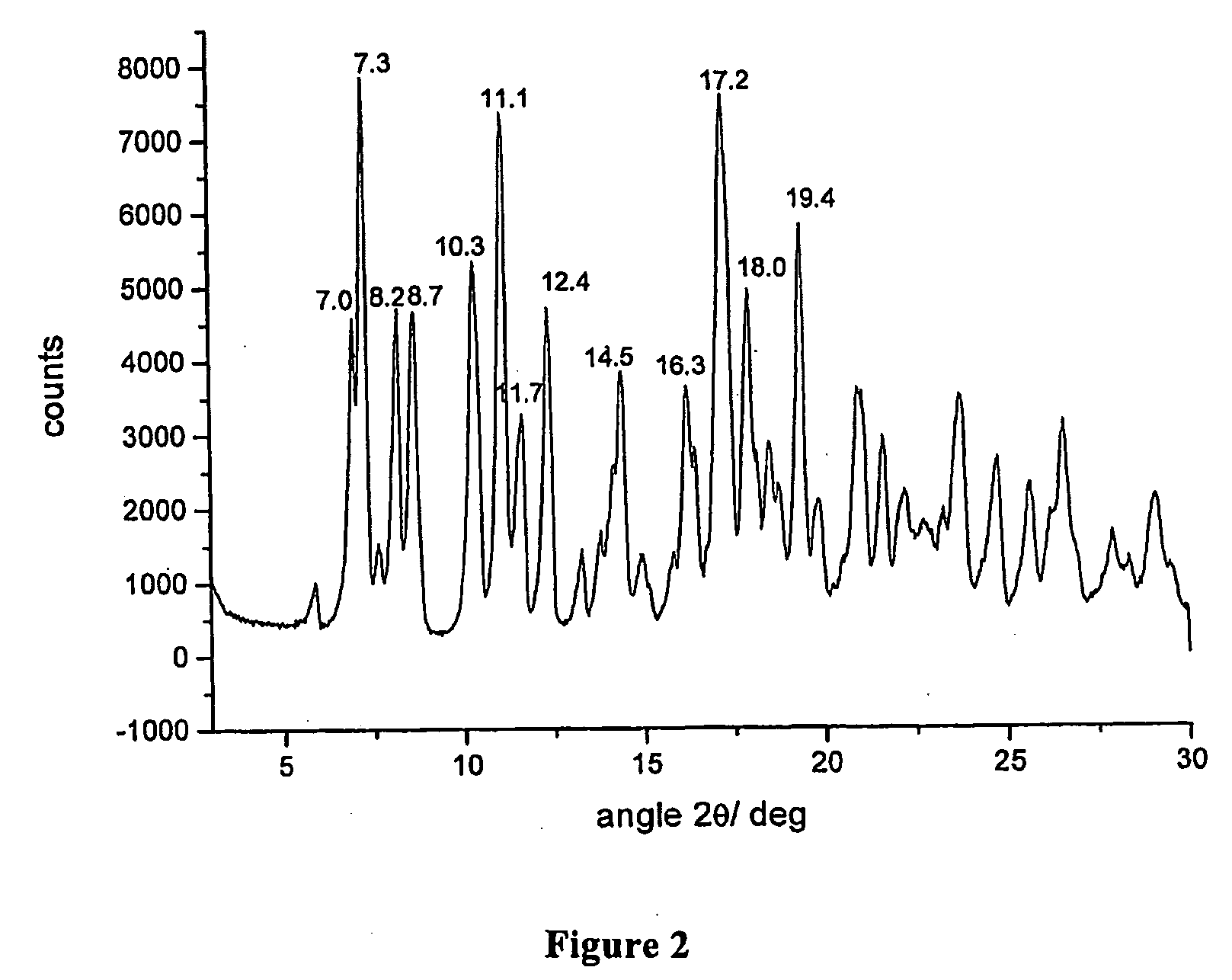
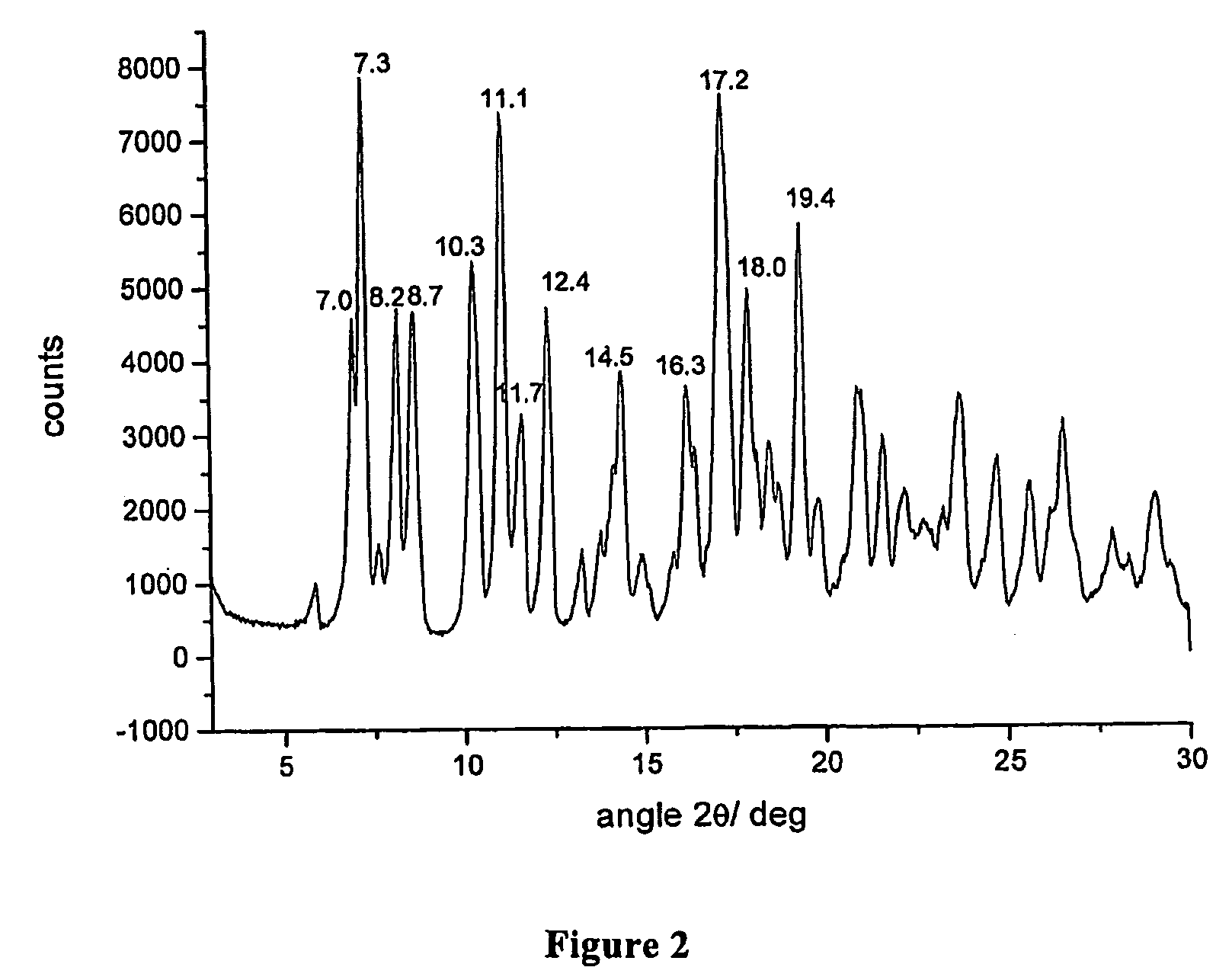
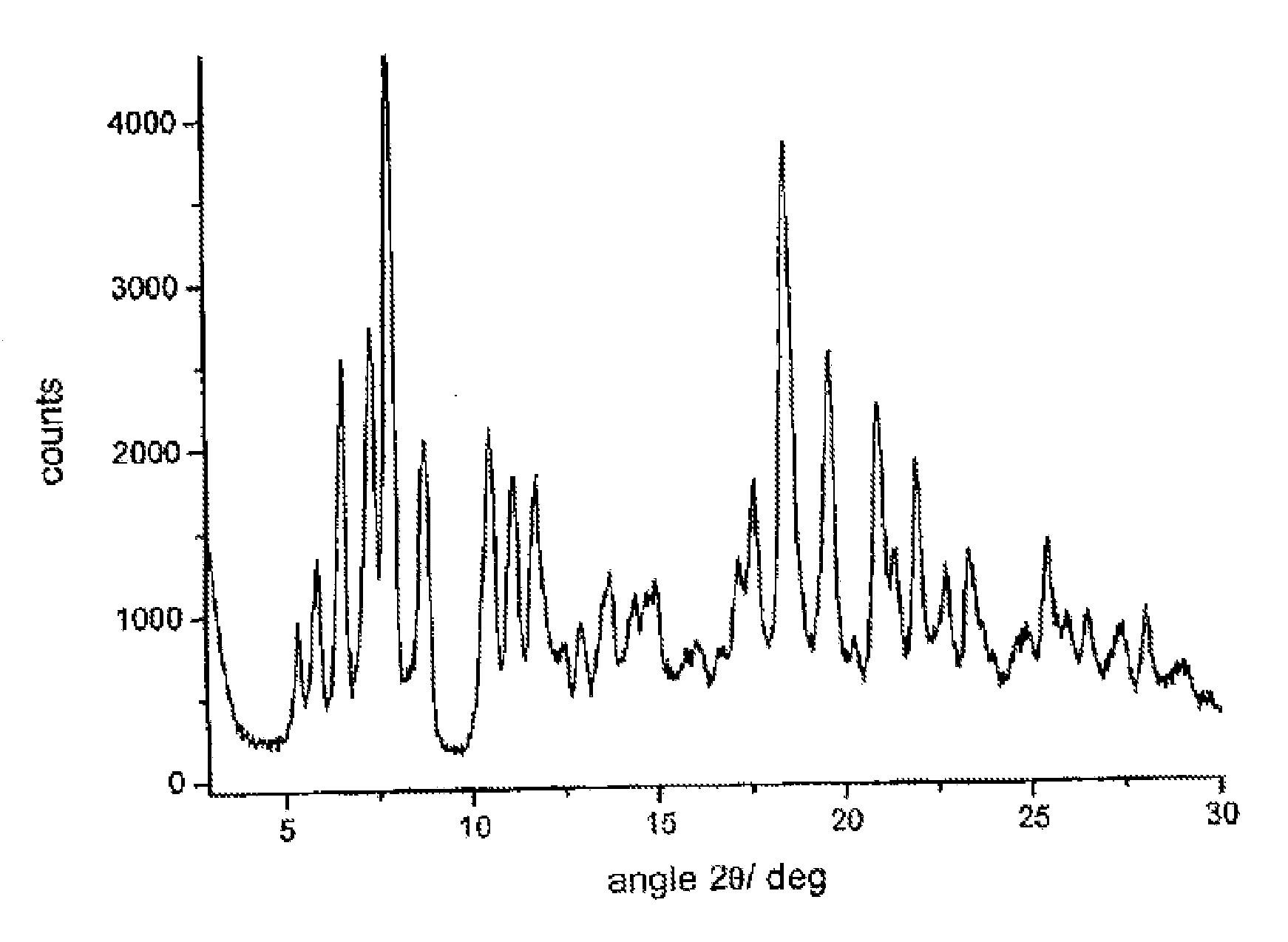
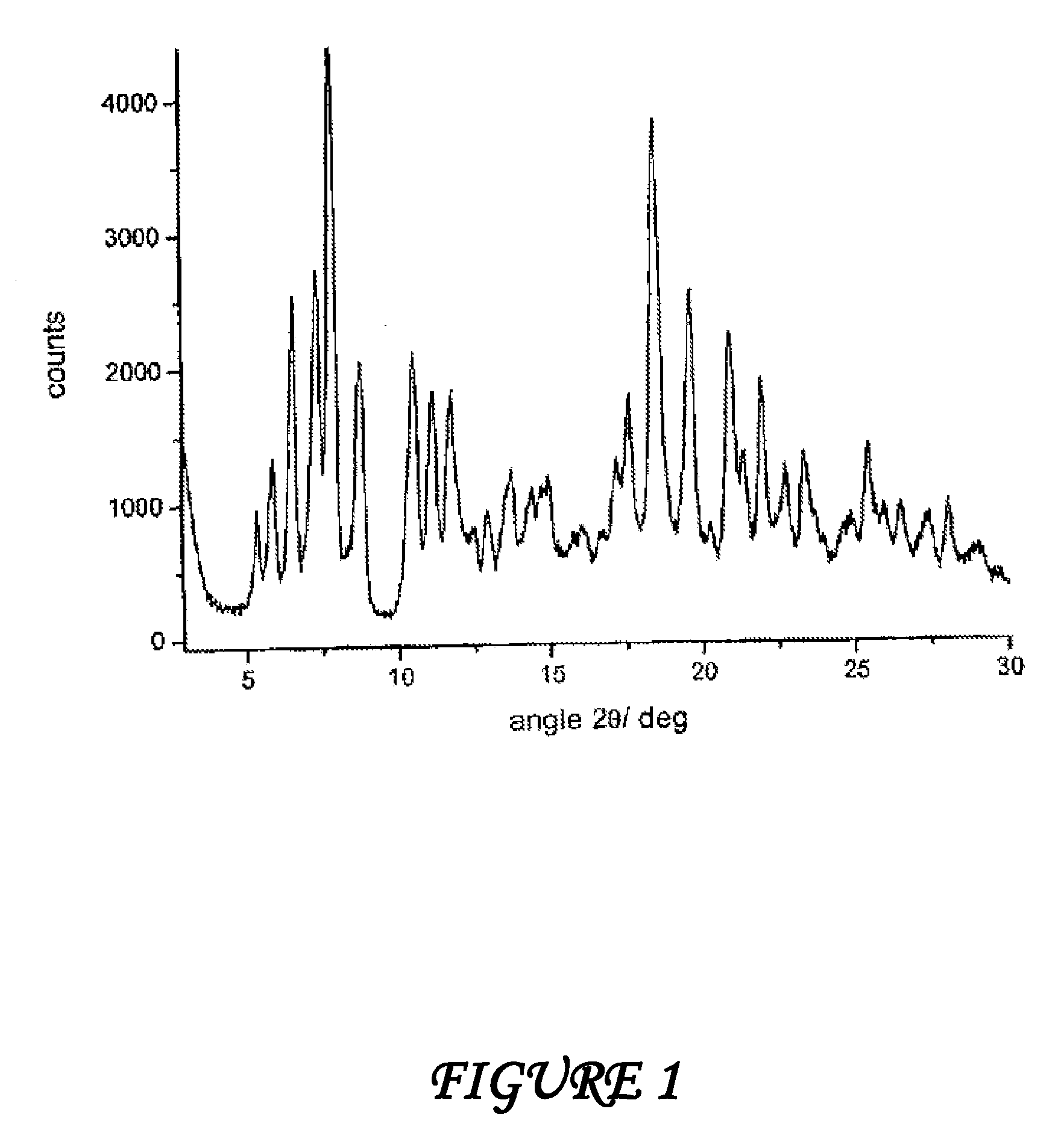
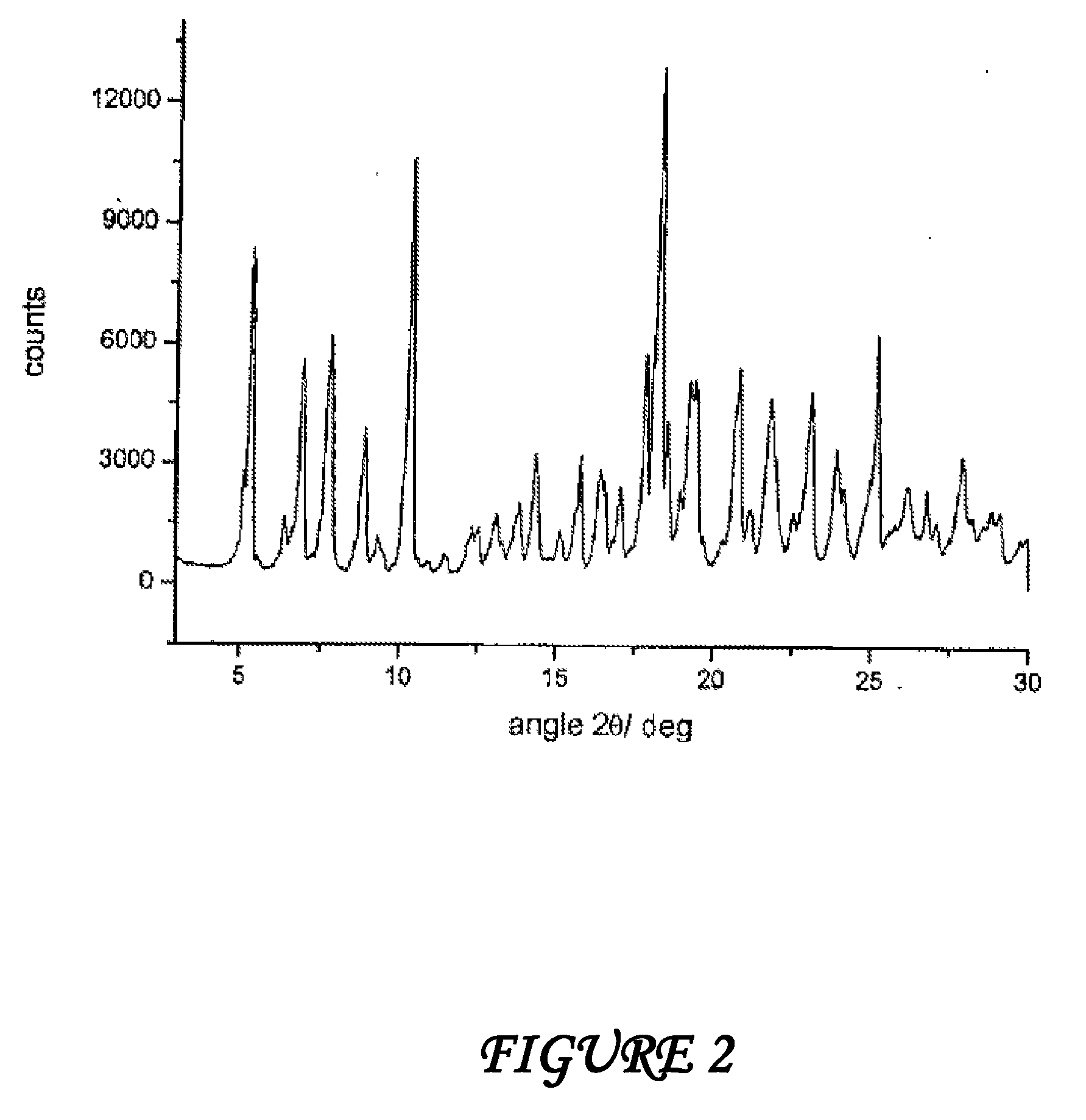
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Crystalline polymorphous forms of the rifaximin (INN) antibiotic named rifaximin δ and rifaximin ε useful in the production of medicinal preparations containing rifaximin for oral and topical use and obtained by means of a crystallization process carried out by hot-dissolving the raw rifaximin in ethyl alcohol and by causing the crystallization of the product by addition of water at a determinate temperature and for a determinate period of time, followed by a drying carried out under controlled conditions until reaching a settled water content in the end product, are the object of the invention.
Owner:ALFASIGMA SPA
Amorphous form of rifaximin and processes for its preparation
A stable amorphous form of rifaximin is disclosed. This form is chemically and polymorphically stable on storage and can be prepared by dissolving rifaximin in a solvent to form a solution, which is precipitated by adding an anti-solvent and isolating of the precipitated amorphous rifaximin as an end product.
Owner:SALIX PHARMA INC
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Crystalline polymorphous forms of the rifaximin (INN) antibiotic named rifaximin δ and rifaximin ε useful in the production of medicinal preparations containing rifaximin for oral and topical use and obtained by means of a crystallization process carried out by hot-dissolving the raw rifaximin in ethyl alcohol and by causing the crystallization of the product by addition of water at a determinate temperature and for a determinate period of time, followed by a drying carried out under controlled conditions until reaching a settled water content in the end product, are the object of the invention.
Owner:ALFASIGMA SPA
Polymorphous forms of rifaximin, processes for their production and use thereof in medicinal preparations
Crystalline polymorphous forms of the rifaximin (INN) antibiotic named rifaximin α and rifaximin β, and a poorly crystalline form named rifaximin γ have been discovered. These forms are useful in the production of medicinal preparations for oral and topical use and can be obtained by means of a crystallization process carried out by hot-dissolving the raw rifaximin in ethyl alcohol and by causing the crystallization of the product by the addition of water at a determinate temperature and for a determinate period of time. The crystallization is followed by drying carried out under controlled conditions until a specific water content is reached in the end product.
Owner:ALFASIGMA SPA
Polymorphic forms alpha, beta and gamma of rifaximin
ActiveUS20080262220A1Influence pharmaco-toxicologic propertyEfficient productionBiocideOrganic chemistry methodsAlcoholDrugs preparations
Crystalline polymorphous forms of rifaximin (INN) antibiotic named rifaximin α and rifaximin β, and a poorly crystalline form named rifaximin γ, useful in the production of medicinal preparations containing rifaximin for oral and topical use and obtained by means of a crystallization carried out by hot-dissolving the raw rifaximin in ethyl alcohol and by causing the crystallization of the product by addition of water at a determinate temperature and for a determinate period of time, followed by a drying carried out under controlled conditions until reaching a settled water content in the end product, are the object of the invention.
Owner:ALFASIGMA SPA
Use of rifaximin for the prevention of aspiration pneumonia and/or sepsis
InactiveUS20060210592A1Organic active ingredientsDispersion deliveryAspiration pneumoniaEndocrinology
An oral preparation consisting of a non-systemic antibiotic and a proton pump blocker used for prevention of aspiration pneumonia and sepsis; and a method of prevention of aspiration pneumonia and / or sepsis by orally administering to a subject in need of such treatment a composition containing a therapeutically effective amount of rifaximin.
Owner:KODSI ROBERT E
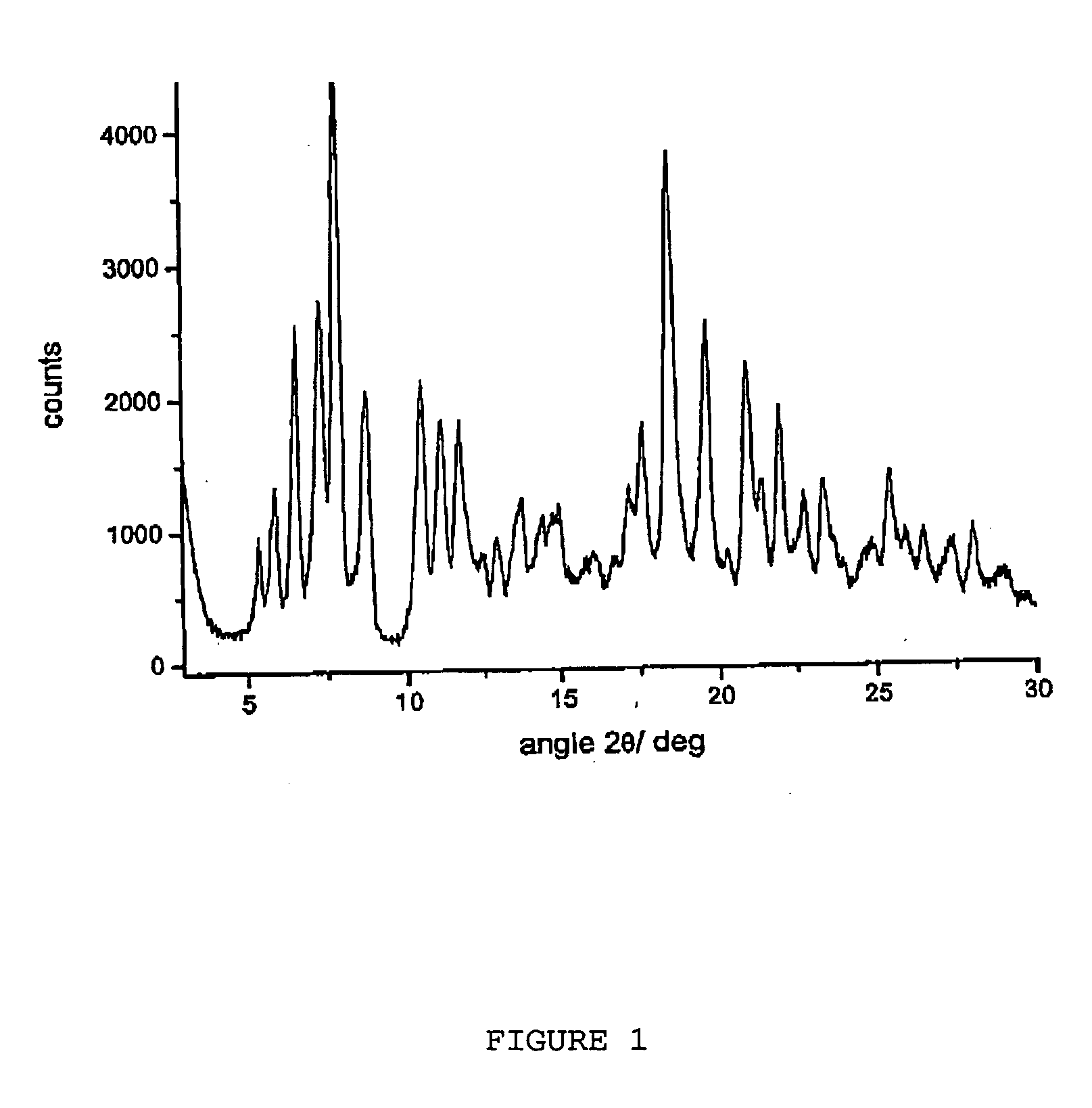
Processes for the production of polymorphic forms of rifaximin
ActiveUS20080262232A1Influence pharmaco-toxicologic propertyEfficient productionOrganic chemistryAlcoholMedicinal chemistry
Crystalline polymorphous forms of rifaximin (INN) antibiotic named rifaximin α and rifaximin β, and a poorly crystalline form named rifaximin γ, useful in the production of medicinal preparations containing rifaximin for oral and topical use and obtained by means of a crystallization carried out by hot-dissolving the raw rifaximin in ethyl alcohol and by causing the crystallization of the product by addition of water at a determinate temperature and for a determinate period of time, followed by a drying carried out under controlled conditions until reaching a settled water content in the end product, are the object of the invention.
Owner:ALFASIGMA SPA
Gastroresistant Pharmaceutical Formulations Containing Rifaximin
ActiveUS20090011020A1Increase intakeWide modulationAntibacterial agentsBiocideCrohn's diseaseMedicine
The object of the invention consists of pharmaceutical formulations containing rifaximin in the shape of microgranules made gastroresistant by an insoluble polymer at pH values between 1.5 and 4.0 and soluble at pH values between 5.0 and 7.5, by their preparation and by their use in the manufacture of medicinal preparations useful in the treatment of inflammatory bowel diseases (IBD) and mainly Crohn's disease.
Owner:ALFASIGMA SPA
Rifaximin compositions and method of use
InactiveUS20080262024A1Influence pharmaco-toxicologic propertyEfficient productionBiocideOrganic chemistryAntibiotic YTopical uses
Owner:ALFA WASSERNANN SPA
Processes for the production of polymorphic forms of rifaximin
ActiveUS7923553B2Influence pharmaco-toxicologic propertyEfficient productionOrganic chemistryAlcoholDrugs preparations
Crystalline polymorphous forms of rifaximin (INN) antibiotic named rifaximin α and rifaximin β, and a poorly crystalline form named rifaximin γ, useful in the production of medicinal preparations containing rifaximin for oral and topical use and obtained by means of a crystallization carried out by hot-dissolving the raw rifaximin in ethyl alcohol and by causing the crystallization of the product by addition of water at a determinate temperature and for a determinate period of time, followed by a drying carried out under controlled conditions until reaching a settled water content in the end product, are the object of the invention.
Owner:ALFASIGMA SPA
Use of polyols to obtain stable polymorphous forms of rifaximin
Polyols stabilize polymorphous form of rifaximin, in particular the β form. When polyols having at least two hydroxy groups are added to rifaximin powder, polymorph β is stable and remains stable in time independently from the environment humidity. In this invention a method to prepare formulations constituted by pure and stable polymorphous forms able to give a pharmaceutical product is described.
Owner:ALFASIGMA SPA
Pharmaceutical Compositions Comprising Rifaximin, Processes For Their Preparation And Their Use In The Treatment Of Vaginal Infections
ActiveUS20130028971A1Effective treatmentReduce riskAntibacterial agentsBiocideDiseaseBacterial vaginosis
The invention relates generally to pharmaceutical compositions comprising rifaximin effective at treating vaginal infections, and in particular bacterial vaginosis. The pharmaceutical compositions comprising rifaximin granules are characterized in that they release rifaximin in the vagina in a controlled way. The present invention also relates to processes for preparation of the rifaximin pharmaceutical compositions and their use in the treatment of vaginal infections. Effective dosages and courses of treatment useful and effective at recovering from the disease and preventing any possible relapse are also provided.
Owner:ALFASIGMA SPA
Rifaximin compositions and method of use
InactiveUS20110086871A1Influence pharmaco-toxicologic propertyEfficient productionBiocideOrganic chemistryAntibiotic YTopical uses
Owner:ALFA WASSERNANN SPA
Use of polyols to obtain stable polymorphous forms of rifaximin
Polyols stabilize polymorphous form of rifaximin, in particular the β form. When polyols having at least two hydroxy groups are added to rifaximin powder, polymorph β is stable and remains stable in time independently from the environment humidity. In this invention a method to prepare formulations constituted by pure and stable polymorphous forms able to give a pharmaceutical product is described.
Owner:ALFASIGMA SPA
Rifaximin vaginal suppository for livestock and preparation method for same
ActiveCN102512358AImprove antibacterial propertiesBroad spectrum antibacterialAntibacterial agentsOrganic active ingredientsWater bathsVaginal Suppository
The invention belongs to the field of veterinary medicine, and particularly relates to a rifaximin vaginal suppository for livestock and a preparation method for the same. The suppository contains the following components: 8.5-25.5 parts by weight of rifaximin solid dispersion, 30-88 parts by weight of matrix, 6.0-12.5 parts by weight of water, 0.01-1.0 parts by weight of surfactant, 0.01-10.0 parts by weight of synergist and 0.01-5.0 parts by weight of preservative, wherein the rifaximin solid dispersion is obtained by melting 7.3-21.9 parts by weight of solid dispersion carrier in a water bath at 70-80 DEG C, and then adding the used 2-16 parts by weight of absolute ethyl alcohol to dissolve 1.2-3.6 parts by weight of rifaximin to obtain rifaximin solution, uniformly stirring, steaming away the absolute ethyl alcohol in the water bath at 70-80 DEG C, and then rapidly cooling and solidifying. The suppository disclosed by the invention can ensure the uniform dispersion of main medicine and overcome the problems of low heat resistance and long melting time limit of glycerin gelatine matrix.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
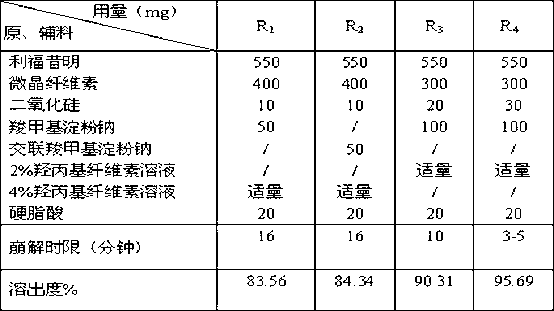
Rifaximin medicine composition and preparation method thereof
InactiveCN103340856ARapid dissolutionEvenly dispersedOrganic active ingredientsNervous disorderMedicineHepatic encephalopathy
The invention discloses a rifaximin medicine composition and a preparation method of the rifaximin medicine composition. The rifaximin medicine composition comprises the following ingredients in percentages by mass: 40-60% of rifaximin, 10-50% of microcrystalline cellulose, 1-30% of sodium carboxymethyl starch or sodium carboxymethyl starch, 0.5-6% of silicon dioxide, 0.5-6% of stearic acid and a little amount of aqueous solution of hydroxy propyl cellulose with the mass concentration of 0.5-6%. The medicine composition can be made into tablets and capsules, each dose comprises 550mg of rifaximin respectively, and a coating solution for the preparation is an aqueous solution of opadry with the mass concentration of 5-30%. The invention also provides the preparation method of the rifaximin medicine composition. According to the rifaximin medicine composition and the preparation method of the rifaximin medicine composition disclosed by the invention, the preparation of the medicine composition can be rapidly disintegrated to fully take medicine efficacy, so as to improve the health of a patient suffering from liver cirrhosis recurrent hepatic encephalopathy.
Owner:WORLDCO INT
Gastroresistant Pharmaceutical Formulations Containing Rifaximin
InactiveUS20100330129A1Modulate the dose strengthIncrease intakeAntibacterial agentsBiocideCrohn's diseaseMedicine
The object of the invention consists of pharmaceutical formulations containing rifaximin in the shape of microgranules made gastroresistant by an insoluble polymer at pH values between 1.5 and 4.0 and soluble at pH values between 5.0 and 7.5, by their preparation and by their use in the manufacture of medicinal preparations useful in the treatment of inflammatory bowel diseases (IBD) and mainly Crohn's disease.
Owner:ALFA WASSERNANN SPA
Rifaximin suspension containing montmorillonite and preparation method thereof
ActiveCN102218078ASmall particle sizeImprove bioavailabilityAntibacterial agentsOrganic active ingredientsMortality rateMontmorillonite
The invention discloses rifaximin suspension containing montmorillonite, which belongs to the field of veterinary preparations. The rifaximin suspension comprises the following components by weight percent: 5-15% of rifaximin, 7.5-22.5% of the montmorillonite, 0.5-2% of suspending agent, 0.2-1% of surface active agent, 0.2-1% of deflocculating agent and the balance of water. The invention further discloses a preparation method of the rifaximin suspension, which comprises the following steps: adding the suspending agent, the surface active agent and the deflocculating agent into the water according to proportion, uniformly mixing and performing water bathing for 1-3 hours at the temperature of 65 DEG C; further adding the rifaximin and the montmorillonite into a stirrer for stirring; and adding the water to volume, homogenizing and sterilizing. The rifaximin in the rifaximin suspension is small in particle size, the release amount and the release speed of active ingredients are greatly improved, and the bioavailability of the rifaximin is improved; secondly, the mortality rate of livestock and poultry with diseases caused by diarrhea can be reduced; in addition, the rifaximin suspension is good in stability and suitable for long-term preservation.
Owner:江苏基宇生物医药科技有限公司
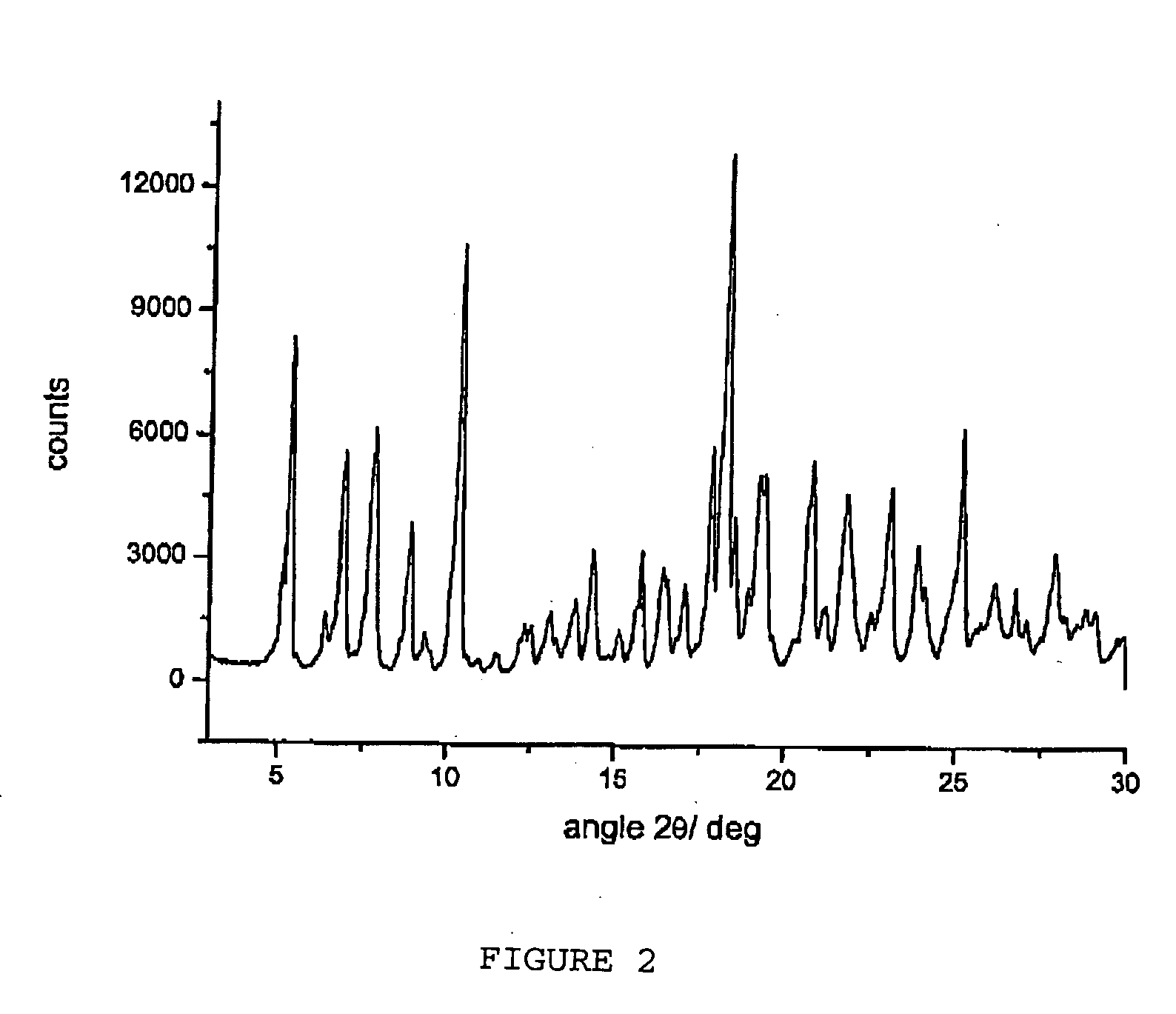
Polymorphic forms alpha, beta, and gamma of rifaximin
ActiveUS20090234114A1Influence pharmaco-toxicologic propertyEfficient productionOrganic chemistry methodsAlcoholDrugs preparations
Crystalline polymorphous forms of rifaximin (INN) antibiotic named rifaximin α and rifaximin β, and a poorly crystalline form named rifaximin γ, useful in the production of medicinal preparations containing rifaximin for oral and topical use and obtained by means of a crystallization carried out by hot-dissolving the raw rifaximin in ethyl alcohol and by causing the crystallization of the product by addition of water at a determinate temperature and for a determinate period of time, followed by a drying carried out under controlled conditions until reaching a settled water content in the end product, are the object of the invention.
Owner:ALFASIGMA SPA
Compound rifaximin dry suspension for preventing and treating endometritis of livestock and preparation method for same
ActiveCN102512417ALess resistant bacteriaAntibacterial and anti-inflammatoryAntibacterial agentsPowder deliverySodium new houttuyfonateVeterinary Drugs
The invention belongs to the field of veterinary medicine, and particularly relates to a compound rifaximin dry suspension for preventing and treating the endometritis of livestock and a preparation method for the same. The rifaximin and sodium new houttuyfonate dry suspension is obtained by using rifaximin and sodium new houttuyfonate as active ingredients, and preparing with pharmaceutically acceptable auxiliary materials. The preparation method comprises the following steps of: performing superfine crushing treatment on raw materials at first; uniformly mixing the treated rifaximin and sodium new houttuyfonate with right amount of filler, suspending aid, surfactant, lubricant, adsorbent and pH buffer according to an equivalent incremental mixing method; subpackaging and sterilizing for1 hour by flowing steam at 100 DEG C. Rifaximin is a novel rifamycin board-spectrum semisynthetic antibiotic medicine, with the advantages of board-spectrum antibacterium, antitoxin and the like, andcapable of preventing and treating the endometritis of dairy cow well; and sodium new houttuyfonate is antibacterial, anti-inflammatory and capable of enhancing immunity as well as resisting bacteriaand diminishing inflammation by cooperating with rifaximin, and has great effect of preventing and treating the endometritis of dairy cow.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Rifaximin suspension mix granule formulation
InactiveCN1485034AEasy to swallowFull effectAntibacterial agentsOrganic active ingredientsCelluloseAluminium chloride
A dried rifaximin suspensoid pelletized granule, which is prepared by mixing rifaximin as the active component and medicinal auxiliary materials at the weight ratio of 1í†5í½35. The medicinal auxiliary materials mainly comprise the suspending agent and the flocculating agent at the weight ratio of 1í†0.05í½2. The suspending agent comprises two or three of microcrystal cellulose, methylcellulose, sodium methylol cellulose, hydroxypropyl methylcellulose, sodium alginate, gum arabic and pectine. The flocculating agent is one of citrate, tartrate, phosphate and aluminum chloride. Other auxiliary materials contain cane sugar, starch, food sweetening agent, preservative and flavoring and so on.
Owner:天津合益达生物医学技术有限公司
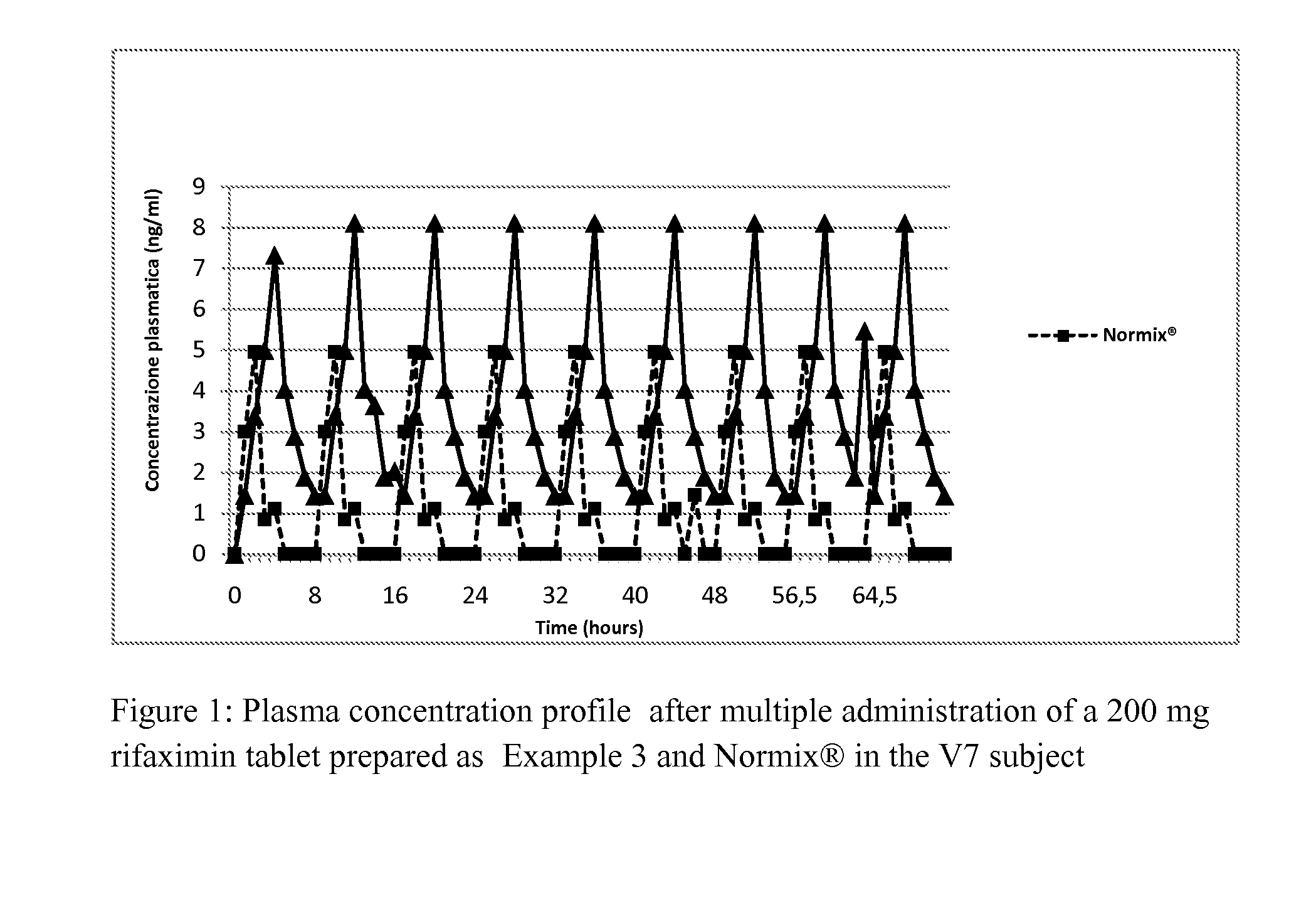
Rifaximin powder, process for preparing the same and controlled release compositions containing said rifaximin useful for obtaining a long-lasting effect
ActiveUS20130004576A1Improve treatment efficiencyConstant levelAntibacterial agentsPowder deliveryControl releaseOral medication
The present invention describes rifaximin powder and to a process for preparing the same. The invention relates also to a pharmaceutical composition in solid form comprising said rifaximin, pharmaceutically acceptable excipients and optionally other ingredients. The compositions according to the invention are suitable for oral administration and are characterized by producing a controlled release of rifaximin, whereby a long-lasting effect is obtained in a patient.
Owner:ALFASIGMA SPA
New use of rifamycin-nitroimidazole coupling molecule
InactiveCN106822119AHigh antibacterial activityLow frequency of drug resistanceAntibacterial agentsOrganic active ingredientsNitroimidazoleAnaerobic bacteria
The invention discloses application of a rifamycin-nitroimidazole coupling molecule represented in a formula I in inhibition of anaerobic or facultative anaerobic ammonia producing floras in gastrointestinal tracts. The rifamycin-nitroimidazole coupling molecule represented in the formula I is similar to an antibacterial spectrum of rifaximin and has relatively strong antibacterial activity to common ammonia producing floras in the gastrointestinal tracts; and meanwhile, the rifamycin-quinolizidone dual-target molecule has the property of low spontaneous drug resistance frequency and has very good application prospects in prevention and treatment of infection of hepatic encephalopathy and relevant anaerobic bacteria. The formula is as shown in the specification.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
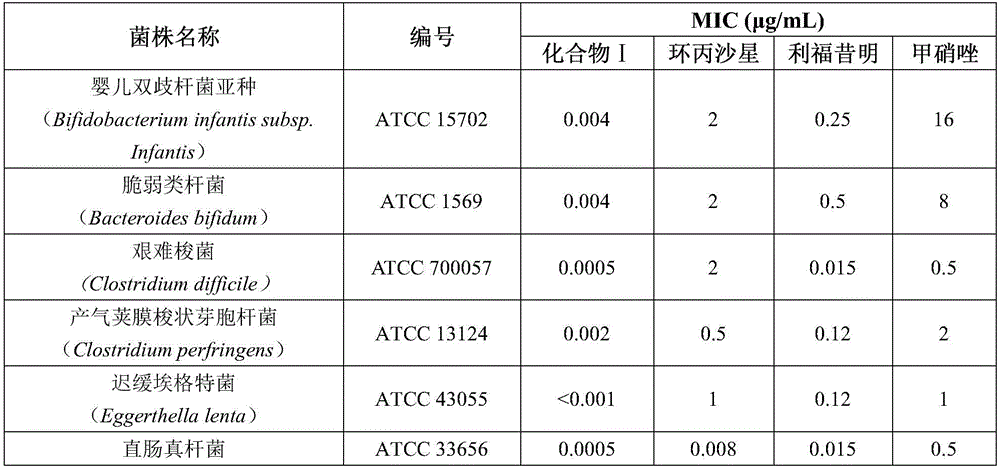
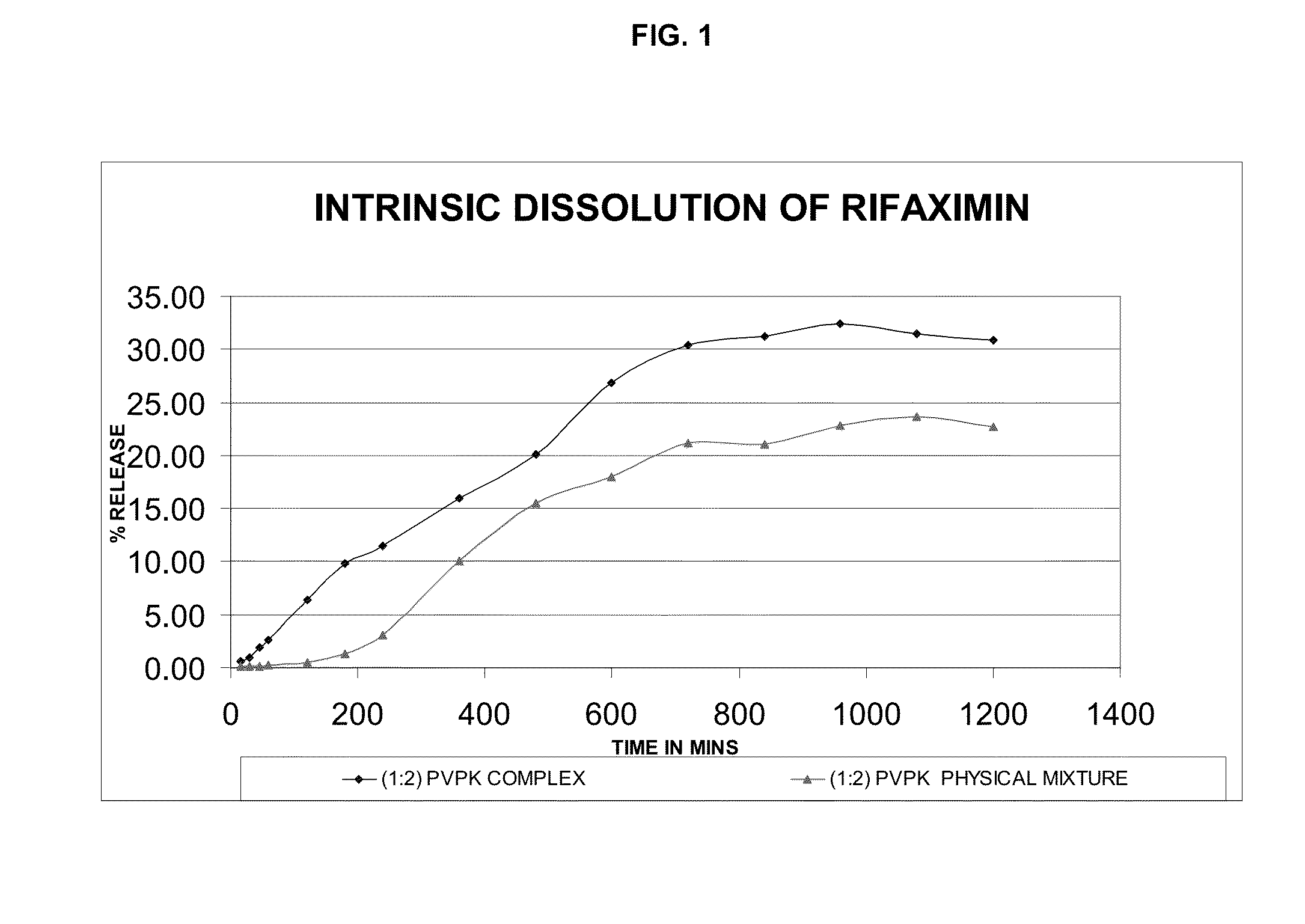
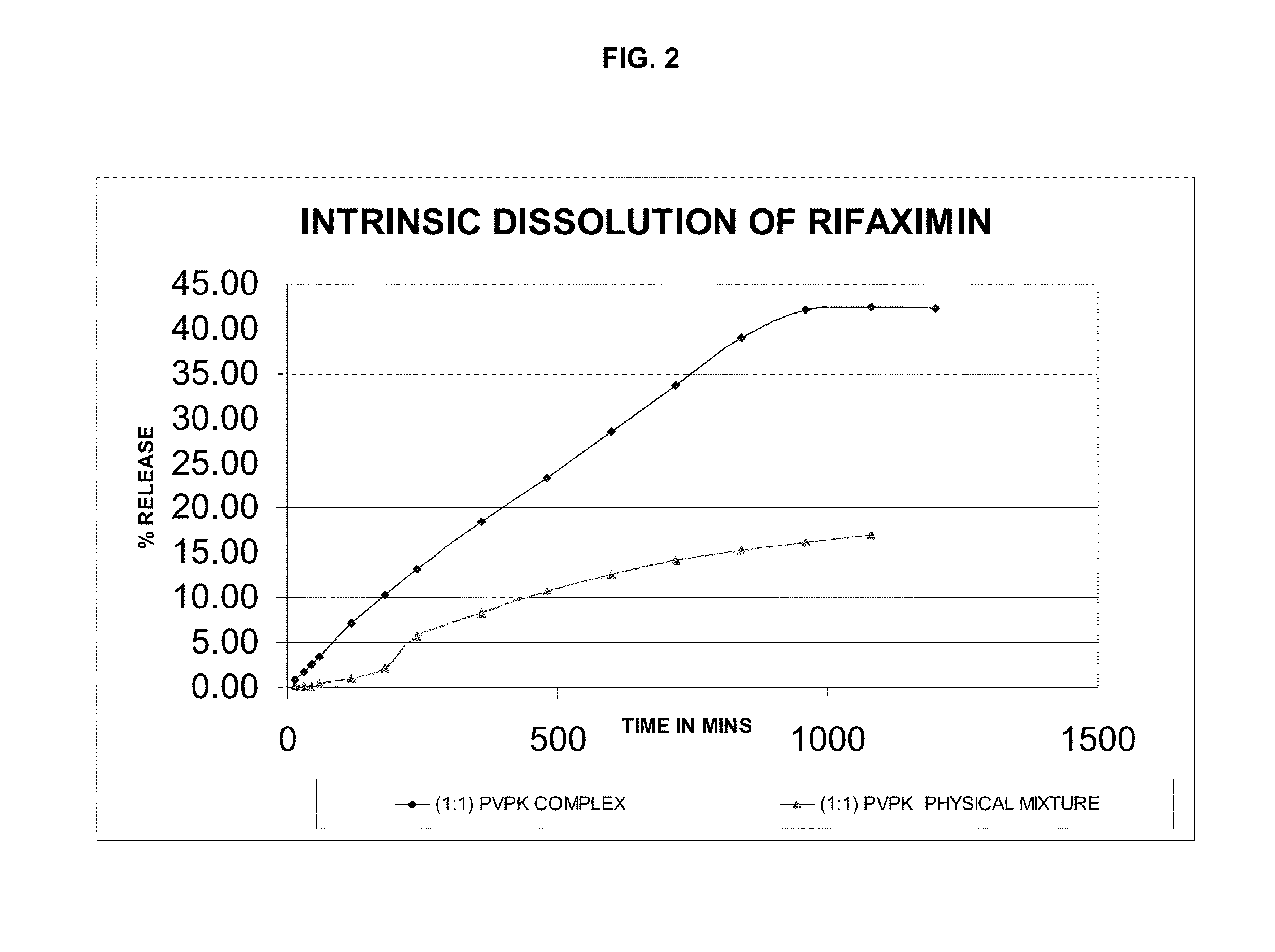
Rifaximin complexes
ActiveUS8916193B2Improve solubility and stabilityImprove stabilityAntibacterial agentsPowder deliveryCyclodextrinPolyvinyl pyrrolidone
There is provided a complex comprising rifaximin and a complexing agent, wherein the complexing agent is a polyvinyl pyrrolidone (PVP) or a cyclodextrin. There is also provided a process for preparing the complex, a pharmaceutical composition including the complex, and therapeutic uses of the complex.
Owner:CIPLA LTD
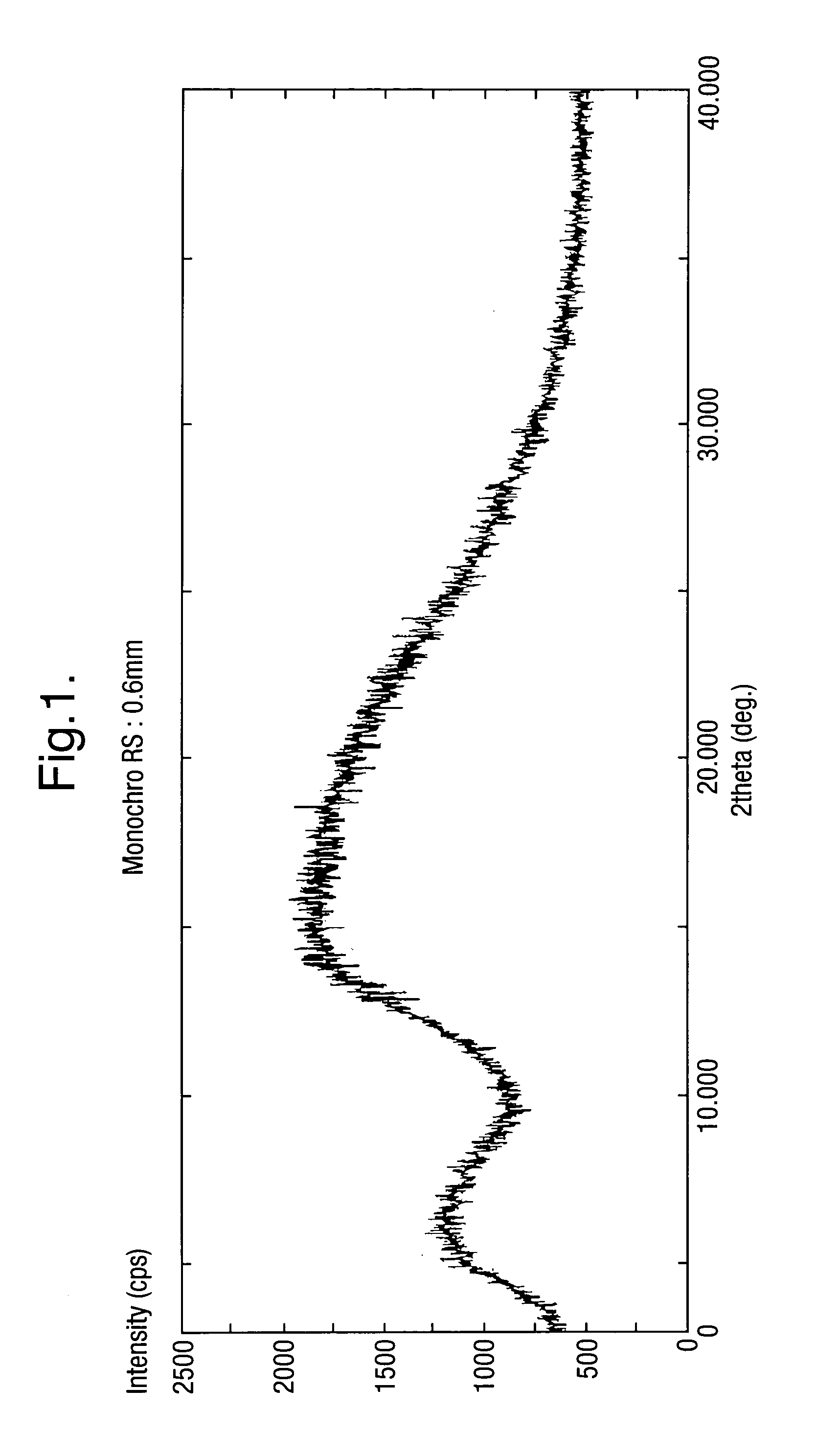
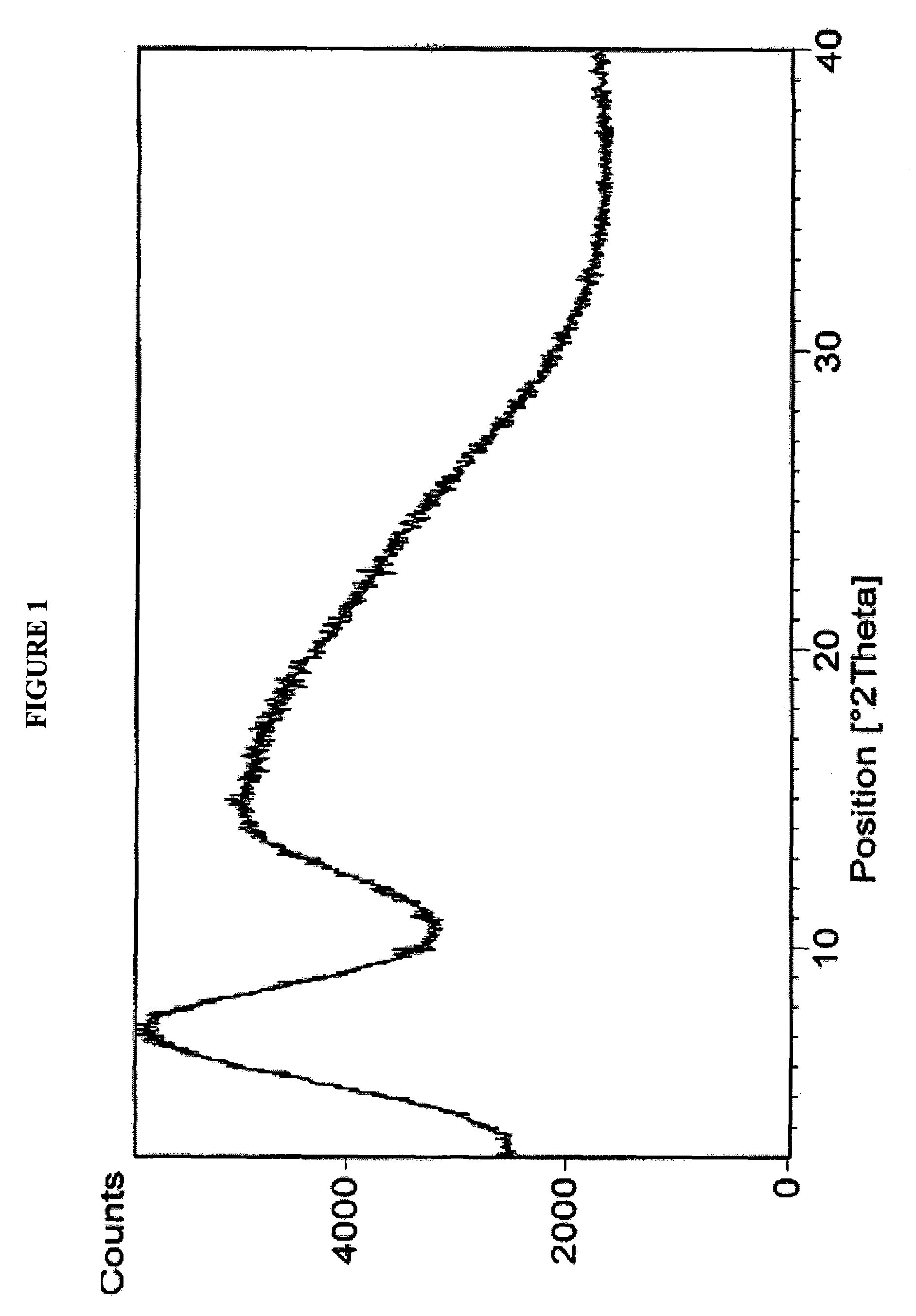
Method for the production of amorphous rifaximin
ActiveUS20120289532A1Large solubilityImprove drug bioavailabilityAntibacterial agentsBiocideHigh energyMedicine
The present invention relates to a new amorphous form of rifaximin and to methods for the preparation thereof by means of high energy milling or Spray drying. The present invention further relates to a new amorphous form for use as medicament and to the pharmaceutical compositions composing it.
Owner:UNILAB S A S DI LAVAGNA SILVIO MASSIMO & C
Process for preparing rifaximin
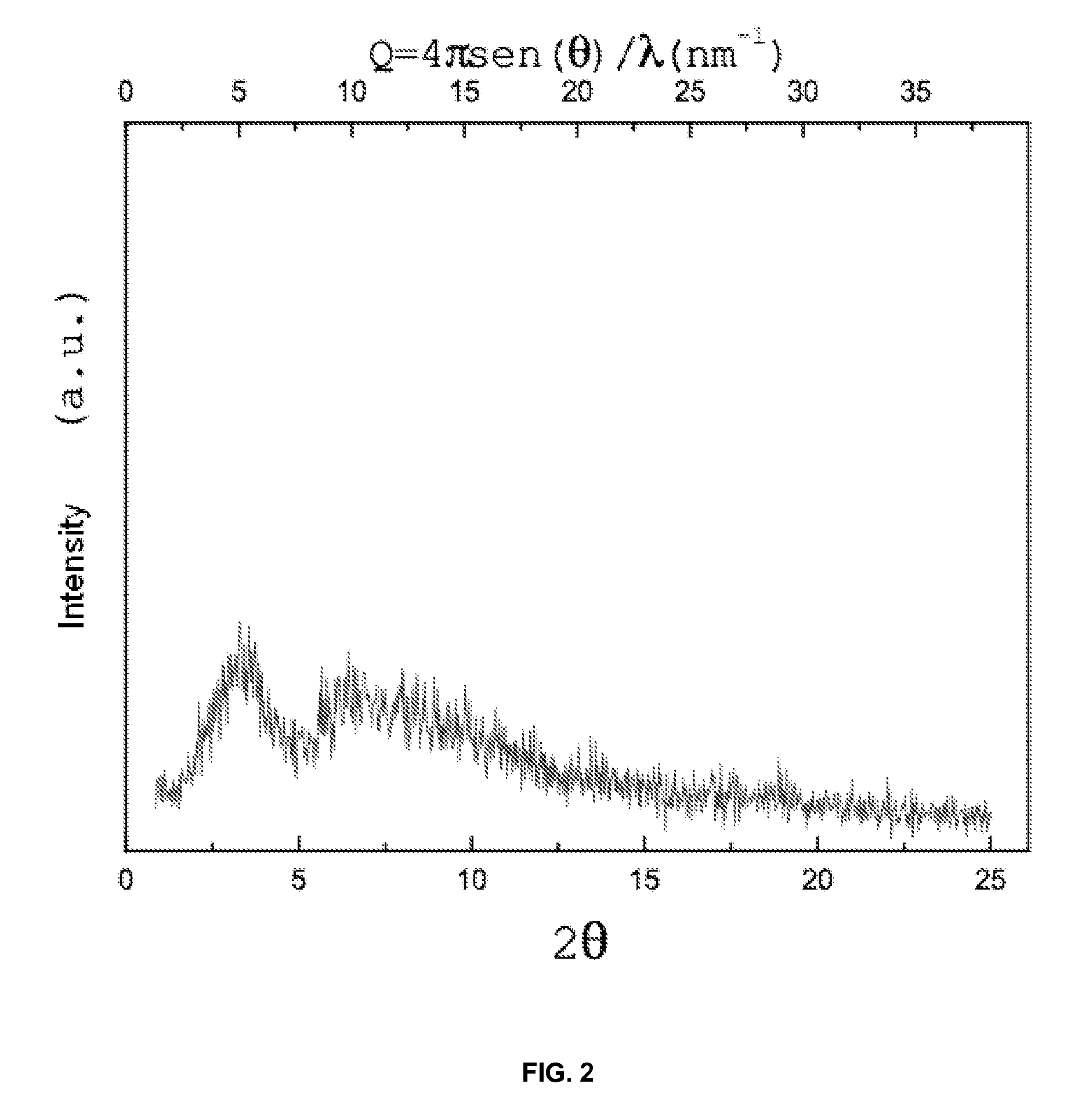
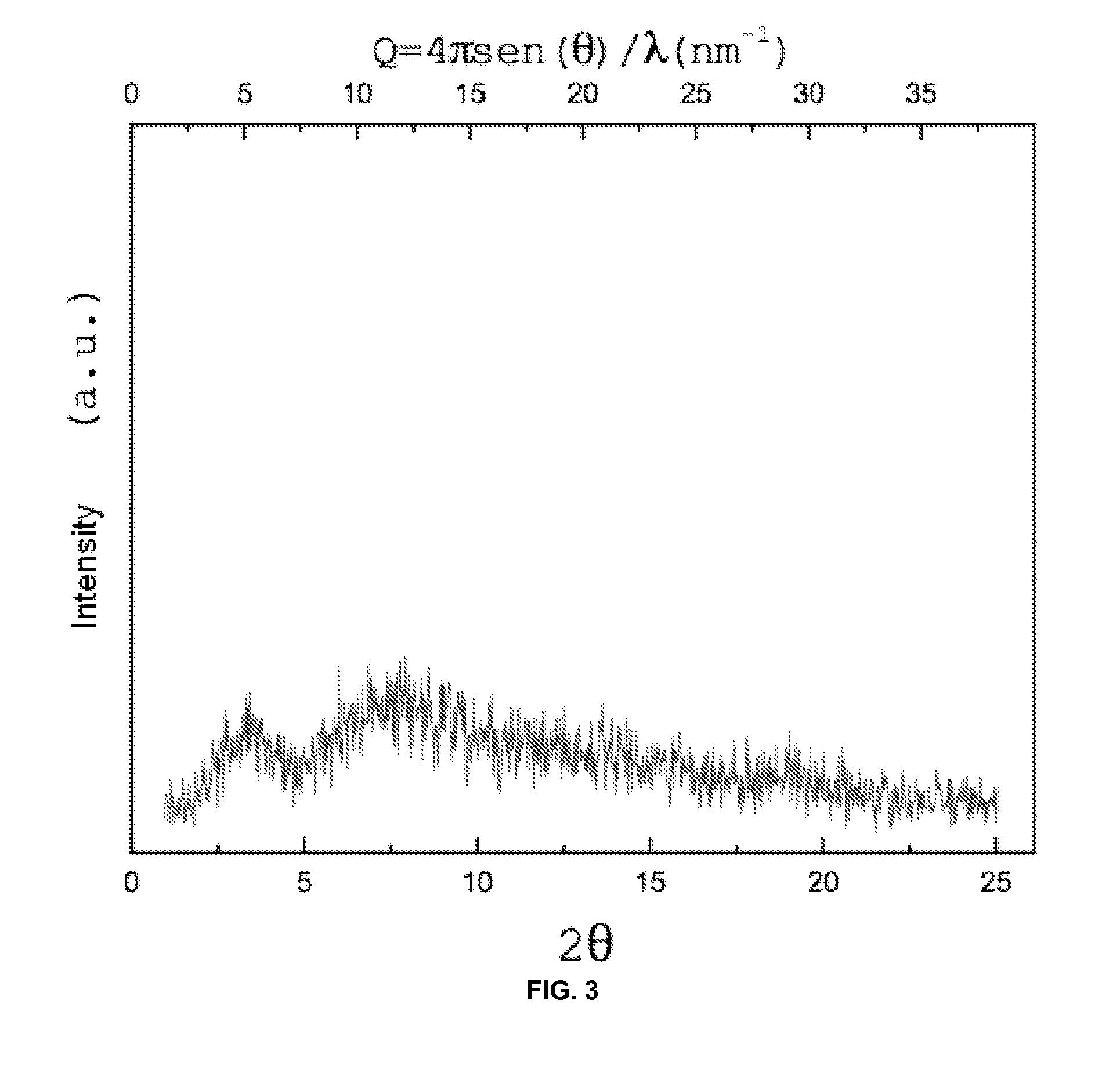
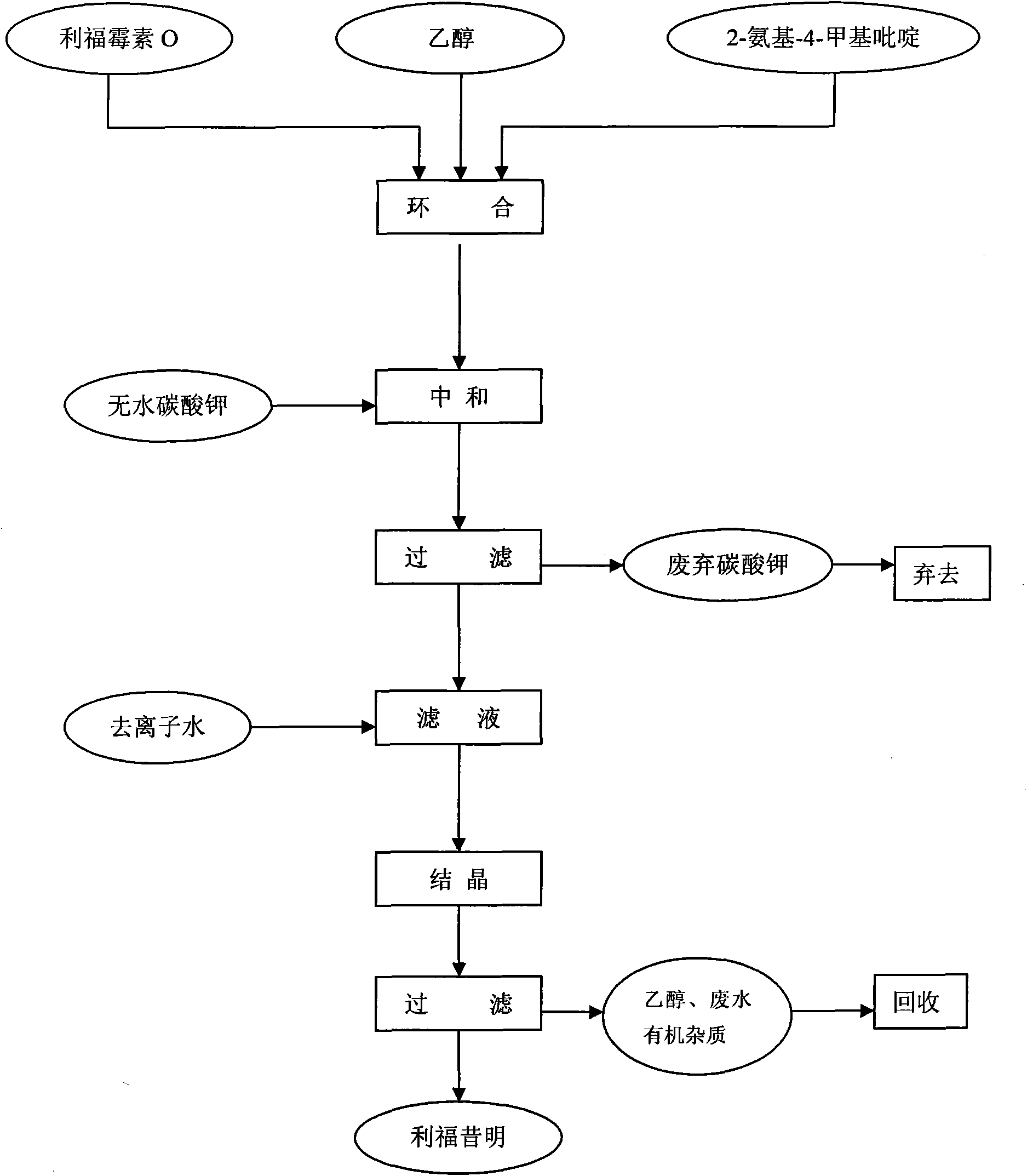
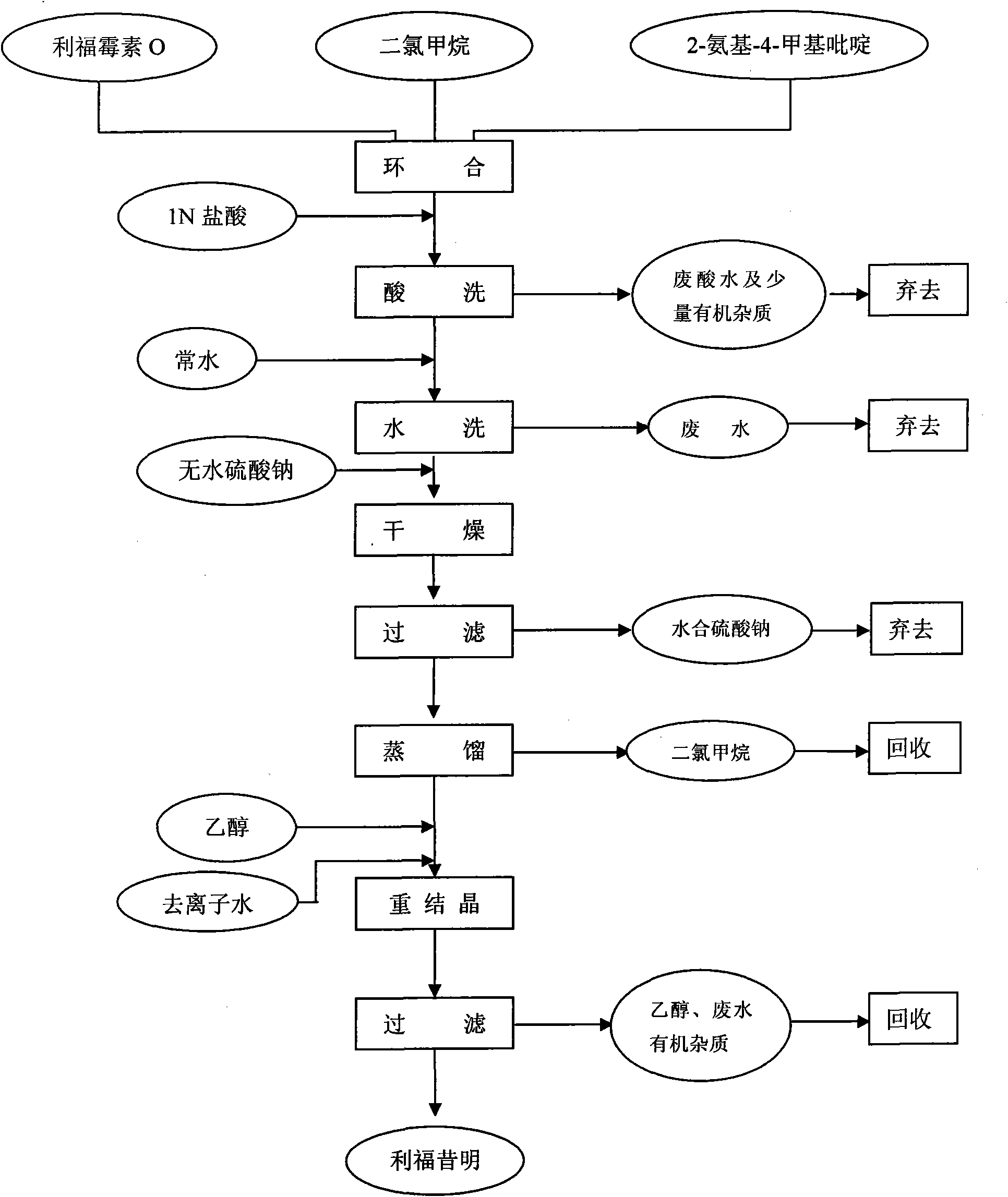
ActiveCN101585843AHigh yieldReduce consumptionOrganic chemistryAntiinfectivesReaction temperatureSolvent
The invention discloses a process for preparing rifaximin, which comprises the following steps: fortimicin O is reacted with excessive 2-amino-4-picolyl for 20-24 hours at 35-45 DEG C by using ethanol as a reaction solvent; after the reaction, anhydrous potassium carbonate is added, wherein the mole number of the added anhydrous potassium carbonate is 0.9-1.1 times of that of the fortimicin O; the obtained mixture is stirred and filtered, and deionized water is added to obtained filter liquor for crystallizing; obtained filter mass is filtered; and after the filter mass is dried, a finished rifaximin product is obtained. The invention has the advantages of short time of the preparation process, simple and convenient post treatment, environmental protection, low consumption, high yield and low cost.
Owner:重庆赛诺生物药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com