New use of rifamycin-nitroimidazole coupling molecule
A technology of nitroimidazolium and rifamycin, which is applied in the field of medicinal chemistry, can solve the problem of no documented antibacterial activity, and achieve the effects of low frequency of drug resistance, strong antibacterial activity, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
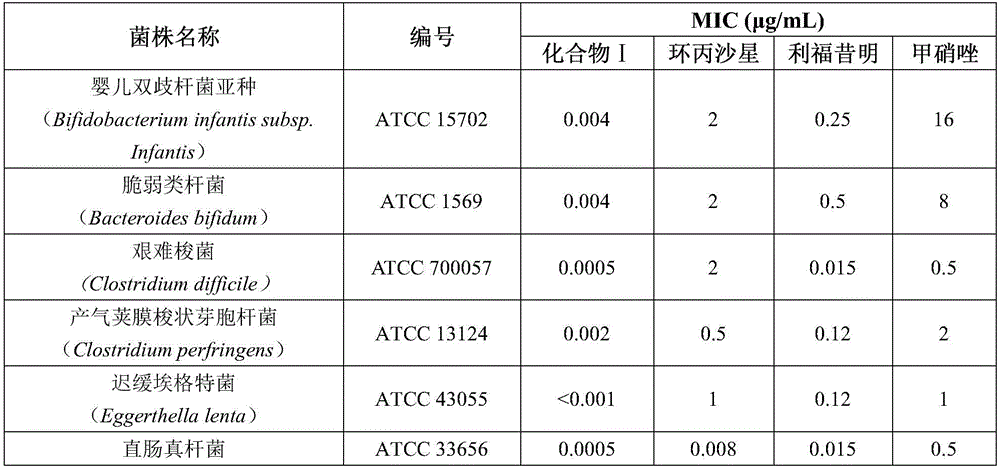
[0019] This example provides the application of the rifamycin-nitroimidazole coupling molecule represented by formula I in inhibiting anaerobic or facultative anaerobic ammonia-producing bacteria in the gastrointestinal tract;
[0020]
[0021] Wherein, the anaerobic or facultative anaerobic ammonia-producing bacteria group in the gastrointestinal tract includes Bifidobacterium infantis subspecies, Bacteroides fragilis, Clostridium perfringens, Eggerthia tarda, Fusobacterium necrosis, A combination of one or more of Eubacterium rectum, Clostridium difficile, Helicobacter pylori, Lactobacillus salivarius and Peptostreptococcus prausinii.
[0022] In this example, compound I rifamycin-nitroimidazole conjugated molecules were tested for drug susceptibility to pathogenic bacteria related to hepatic encephalopathy, and the pathogenic bacteria included the above-mentioned anaerobic ammonia-producing bacteria group. Tests were performed under anaerobic conditions using an agar dil...
Embodiment 2
[0040] This example provides the prescription and preparation method of a fast-release oral preparation of the rifamycin-nitroimidazole conjugate molecule represented by formula I.
[0041]
[0042] Weigh the rifamycin-nitroimidazole coupling molecule and auxiliary materials represented by the formula I according to the above-mentioned prescription amount. Dissolve povidone K30 (PVP K30) and sodium dodecyl sulfate (SDS) in purified water, stir for 1 hour, and use it as an adhesive for later use; couple rifamycin-nitroimidazole shown in formula I Molecule, mannitol and carboxymethyl starch sodium (DST) are passed through a 30-mesh sieve, added to the granulator, pre-mixed, the impeller stirring speed is 700rpm, and the time is about 15 minutes. Then use a peristaltic pump to add an appropriate amount of purified water and binder to the mixture of the granulator at a fixed speed (145-165g / min). The stirring speed of the impeller of the granulator is 400rpm, and the time is ab...
Embodiment 3
[0045] This example provides a method for preparing an injection of the rifamycin-nitroimidazole conjugate molecule represented by formula I.
[0046]
[0047] Add mannitol, acetaldehyde sodium sulfoxylate, and Tween-80 to an appropriate amount of water for injection under nitrogen protection, add the rifamycin-nitroimidazole coupling molecule shown in formula I, and stir at a medium speed for 10-15 minutes. Wet the rifamycin-nitroimidazole coupling molecule shown in formula I, and slowly add 1N NaOH dropwise, which takes about 175 minutes (fast at the beginning and slow at the end), to the rifamycin-nitroimidazole shown in formula I The coupling molecules are completely dissolved, filtered through two microporous membranes of 0.45+0.22 μm, the filtrate is filled into 10mL glass bottles, each bottle contains 3.5mL, the glass bottles are transferred to a lyophilizer for lyophilization, and the formula is obtained after capping The freeze-dried powder injection of the rifamyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com