Pulsatile gastric retentive dosage forms
a gastric retentive and pulsatile technology, applied in the field of gastric retentive dosage forms, can solve the problems of affecting the release of the drug from the dosage form, hindering the absorption of the drug once released, and additional obstacles in delivering an effective dose to the patien
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Method of Treating GERD and Preventing or Reducing NAB
[0172]A study was conducted to demonstrate the limited colonic absorption of omeprazole. Nine healthy subjects were entered into the study with an intention to complete treatment of at least six subjects. The study was a four-way crossover study with the following doses administered: (i) simulated control release (SCR): 20 mg omeprazole divided into 17 doses, administered at 30 minute intervals (8 hr of delivery), in the fed state; (ii) 20 mg omeprazole in the fed state; (iii) 20 mg omeprazole in the fasted state; and (iv) 20 mg omeprazole delivered to the ascending colon via the ENTERION™ capsule (radio controlled capsule to control release of drug; the position in the GI tract is determined by scintigraphy). A period of at least four days for washout was allowed between dosing.
[0173]Seven subjects completed the study. Table 1 lists the mean±SD of the pharmacokinetic parameters determined from the blood plasma drug concentration...
example 2
Method of Treating GERD and / or NAB
[0176]A randomized, open-label, two-period crossover study in GERD patients between 18 and 65 years of age, inclusive, with nocturnal reflux after receiving PPIs for at least 3 months, was conducted to demonstrate the efficacy of a two-pulse dosing regimen for treating GERD and / or NAB. Sixteen patients with a history of GERD, all of whom experienced recurrent nighttime reflux for at least three months while taking proton pump inhibitors, were enrolled. The study was an open label crossover study in which 14 of the 16 patients participated in each of two treatment arms separated by a washout period. In one treatment arm, the patients received 40 mg of omeprazole 30 minutes before dinner, for six days. In the other treatment arm, the patients received 20 mg of omeprazole at dinner followed by an additional 20 mg of omeprazole four hours later, for six days. Ambulatory 24-hour gastric pH was recorded and blood samples taken for PK analysis on days 6-7....
example 3
Shell and Core Tablet
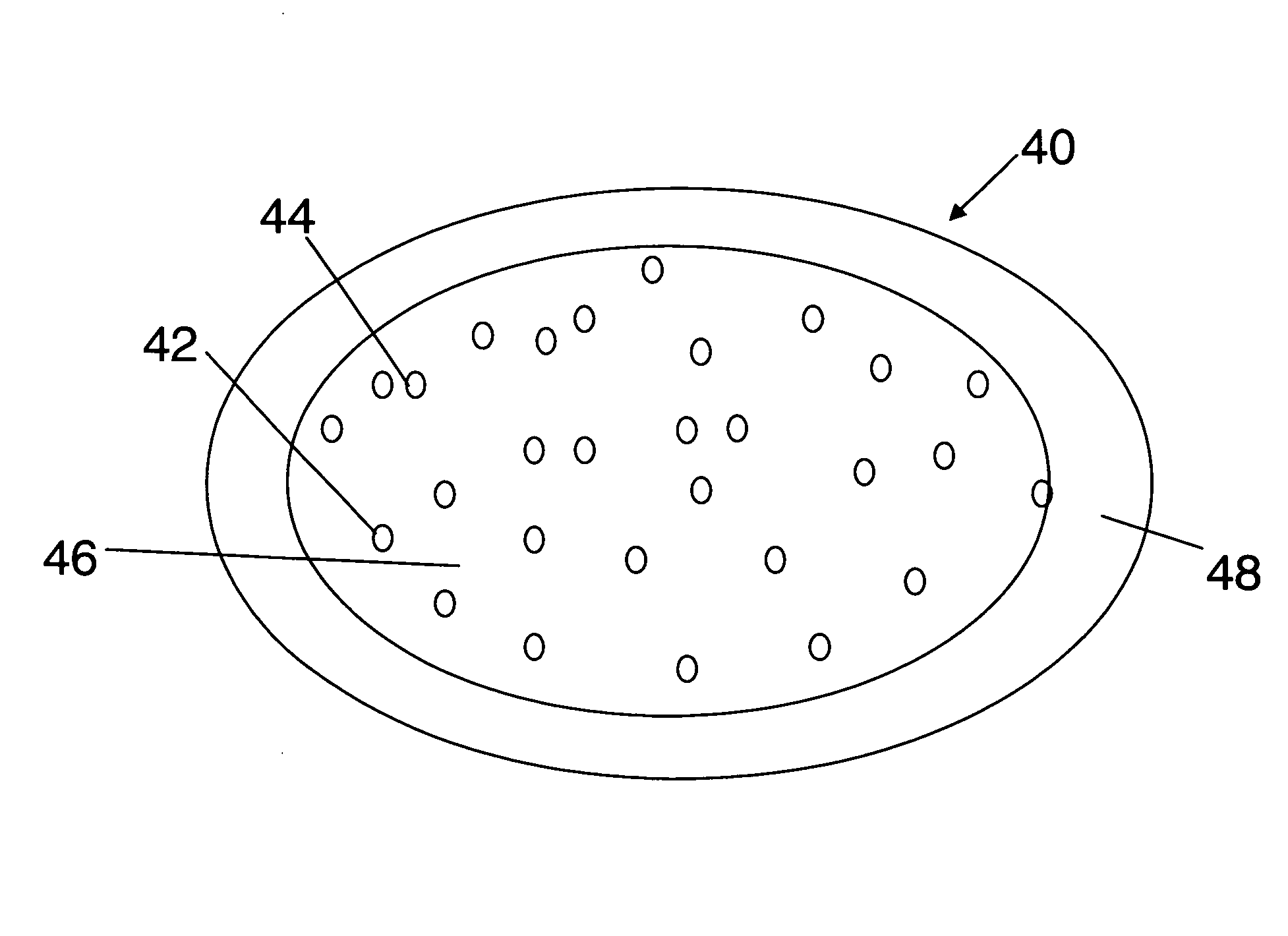
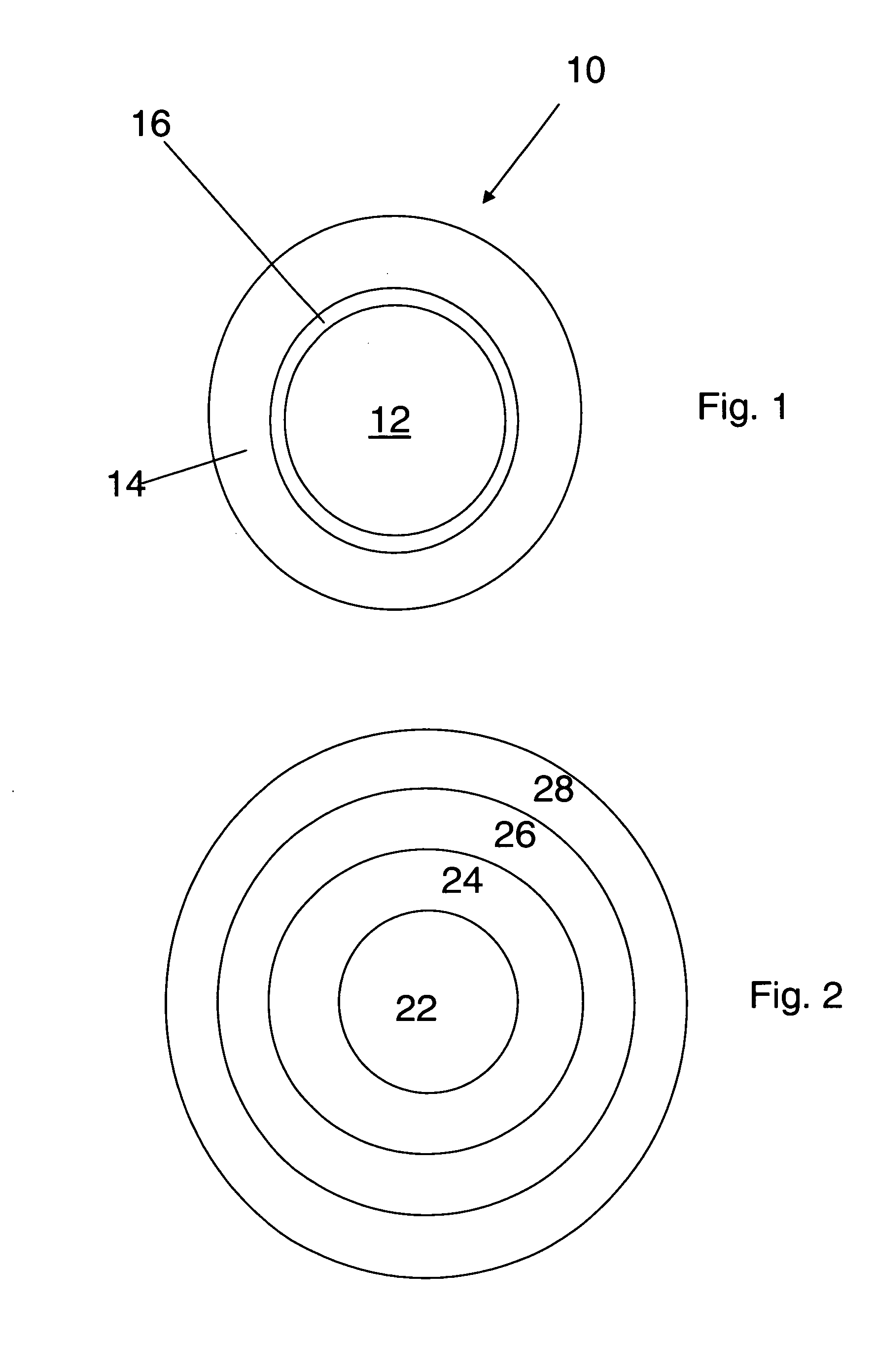
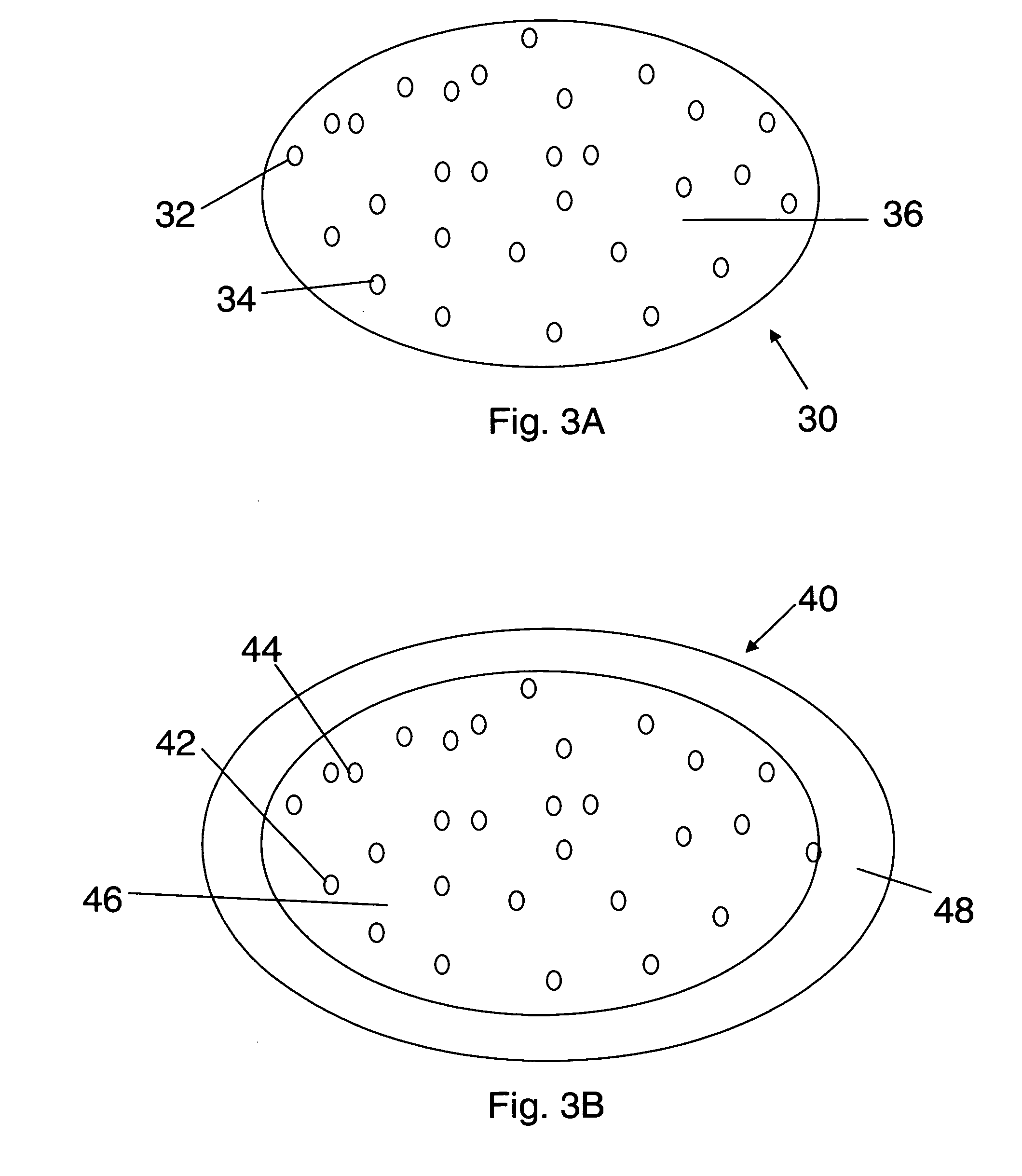
[0183]In one embodiment, a dosage form that provides a delayed pulse of drug release created by a core tablet or pellet containing the drug that is surrounded by a coating or shell such that the dosage form releases the drug in a pulse (optionally, the drug is an acid-protected PPI; the acid-protected PPI can be an enteric or delayed release coated particle, bead or pellet or alternatively a particle bead or pellet containing base) after a delay (relative to the time of ingestion) is provided. This dosage form can be referred to as a “press coated” tablet or a “shell and core” tablet. This example describes a dosage form comprising a drug-containing core surrounded by an erodible, swellable, layer designed to promote gastric retention and retard the release of a drug for a pre-selected period of time, between about 1 and 12 hours. If the drug in the dosage form is omeprazole or another acid labile drug, then the drug-containing particle can be protected from the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| bead outer diameter | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com