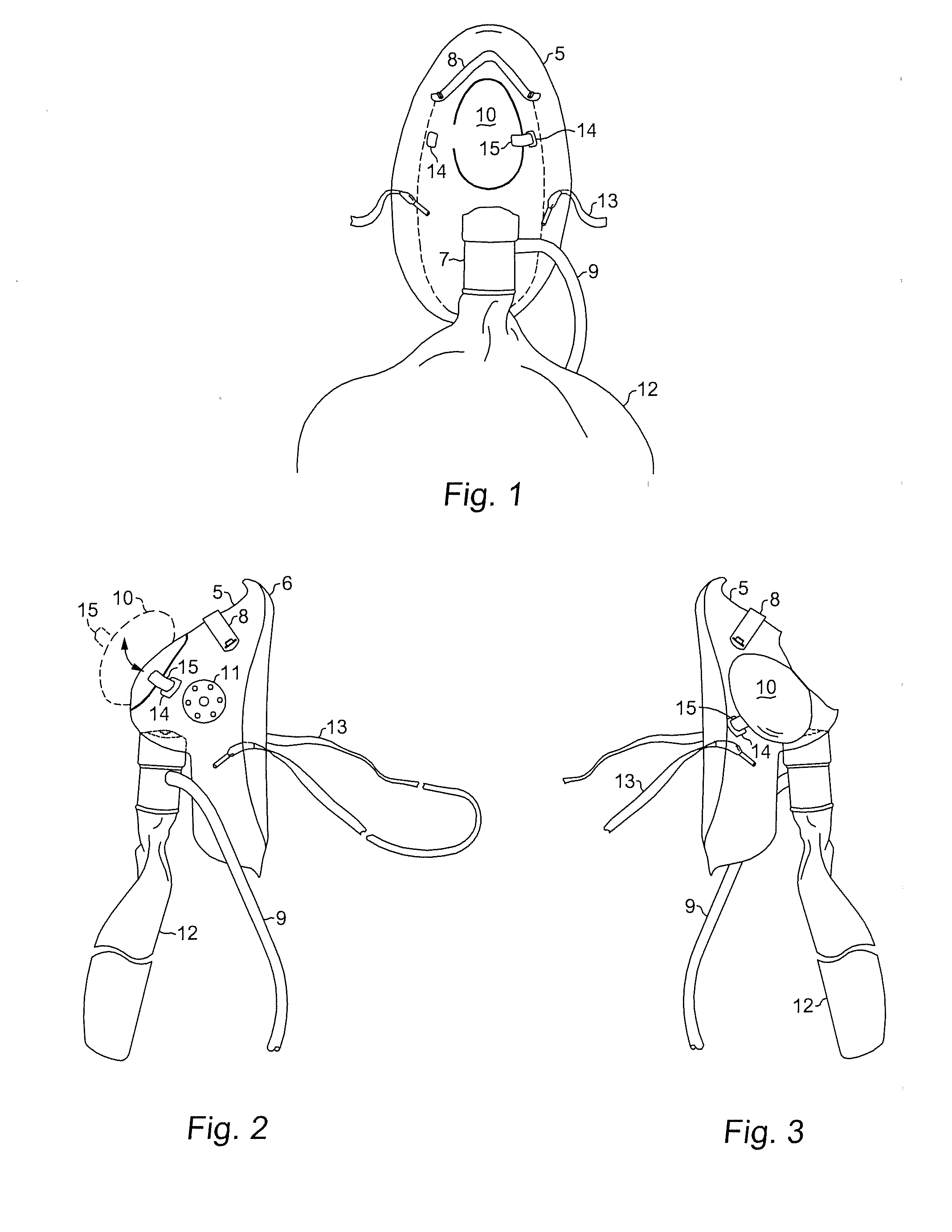Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
242 results about "Upper gastrointestinal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
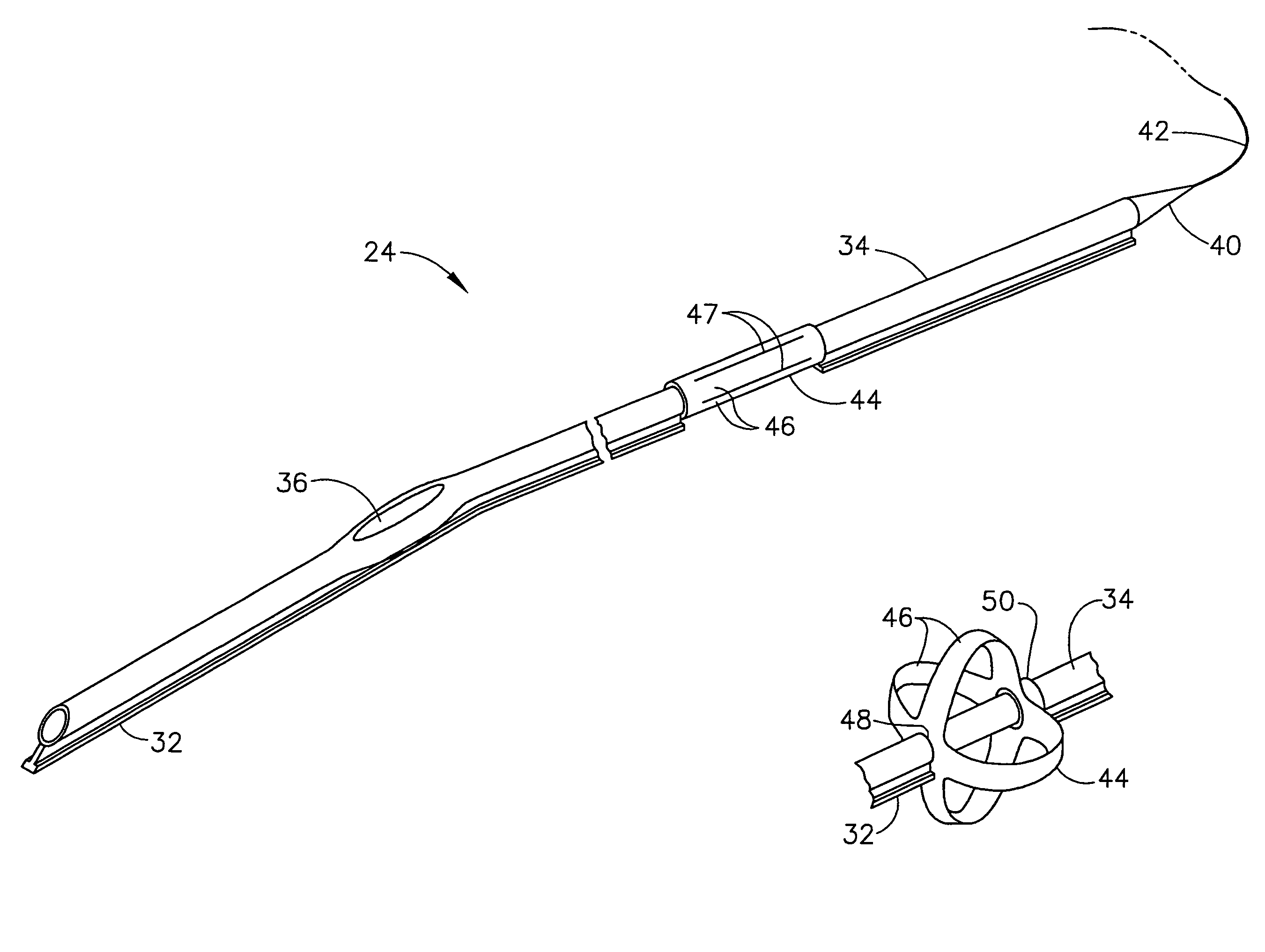
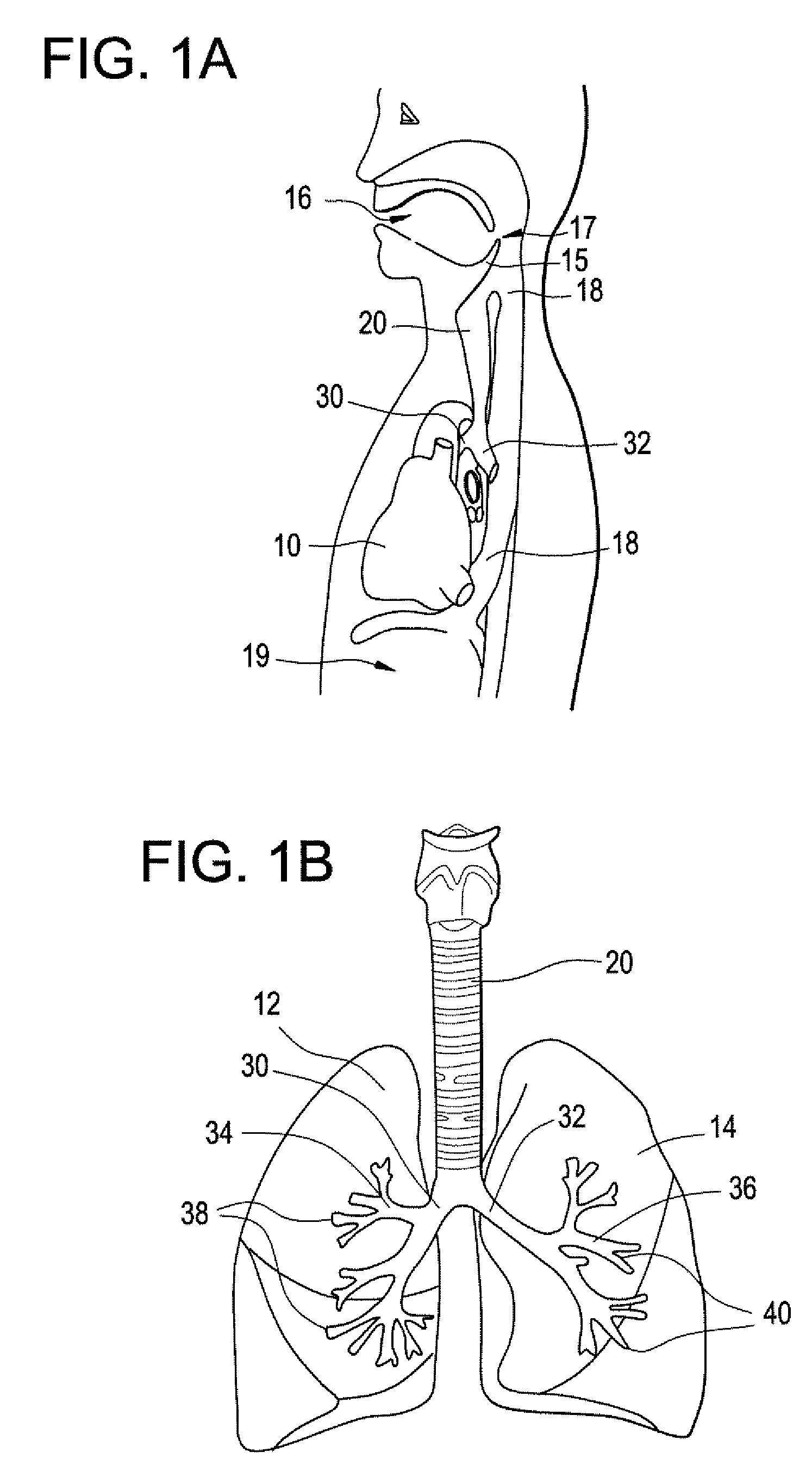
Intubation device for enteral feeding
An intubation device is provided for use with a guide apparatus having a track that is adapted to be associated with an endoscope such that bending of the track is substantially decoupled from bending of the endoscope. The intubation device includes an elongated, flexible tube and a mating member attached to the tube and adapted to slidingly engage the track external of the endoscope. The intubation device further includes a tissue bolster disposed on the proximal portion of the tube and changeable between a collapsed and an expanded configuration. The tube is positionable inside the upper gastrointestinal tract of a patient such that the proximal end of the tube is externalized through the gastric and abdominal walls of the patient, and wherein the tissue bolster is securable against the inner gastric wall when the tissue bolster is in the expanded configuration.
Owner:ETHICON ENDO SURGERY INC
Dosage forms of bisphosphonates
ActiveUS20050260262A1Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyUpper gastrointestinal
Oral dosage forms of a bisphosphonate comprised of a safe and effective amount of a pharmaceutical composition comprising a bisphosphonate, a chelating agent, and, means for effecting delayed release of the bisphosphonate and the chelating agent in the lower gastrointestinal tract provide delivery of the pharmaceutical composition to the lower gastrointestinal tract of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between bisphosphonates and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of the bisphosphonate and the chelating agent to the lower GI tract, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
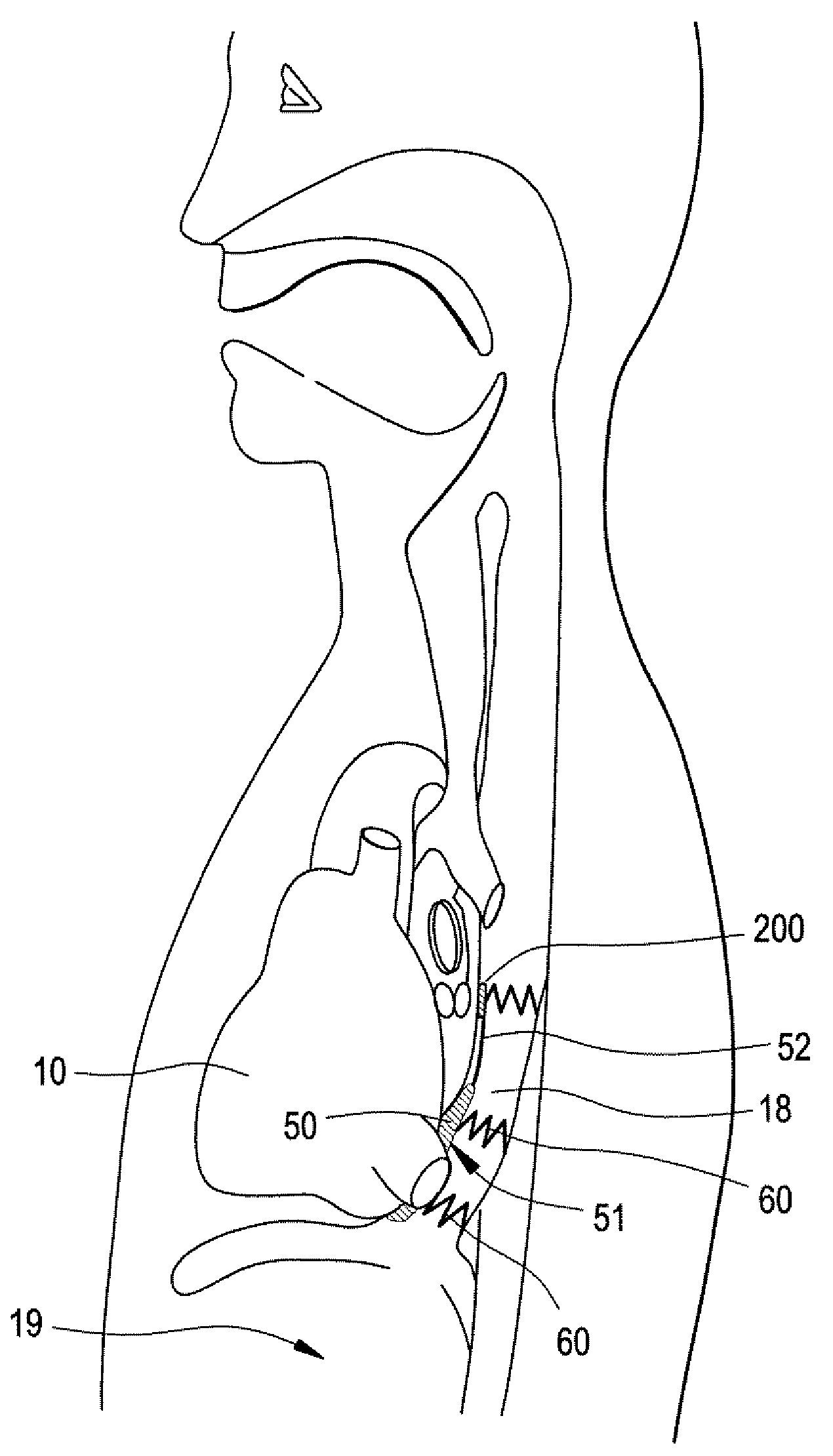
Implantable Devices and Methods for Stimulation of Cardiac or Other Tissues
An implantable stimulation system is provided for stimulation of the heart, phrenic nerve, gastric system, or other tissue structures accessible via a patient's upper gastrointestinal system or airway. The stimulation system includes an implantable controller housing including a pulse generator; at least one electrical lead attachable to the pulse generator; and at least one electrode carried by the electrical lead that is positionable and fixable within the upper gastrointestinal tract or airway. The controller housing may be adaptable for subcutaneous implantation, or within the upper gastrointestinal tract or airway, wherein the controller housing is proportioned to substantially permit fluid and solid flow through the upper gastrointestinal tract or airway about the controller housing. The pulse generator may be operable to deliver one or more electrical pulses effective in cardiac pacing, cardiac defibrillation, cardioversion, cardiac resynchronization therapy, diaphragm pacing, phrenic nerve stimulation, gastric electrical stimulation, or a combination thereof.
Owner:E PACING
Pulsatile gastric retentive dosage forms
Dosage forms for delayed and pulsed release of therapeutic agents into the stomach are described. The dosage forms are gastric retentive dosage forms that achieve release of the therapeutic agent into the stomach and upper gastrointestinal tract subsequent to administration of the dosage form. The dosage forms find particular use in administration of acid-labile active agents such as proton pump inhibitors, and in treating gastric acid secretion such as gastro-esophageal reflux disease (GERD) and nocturnal acid breakthrough (NAB).
Owner:DEPOMED SYST INC
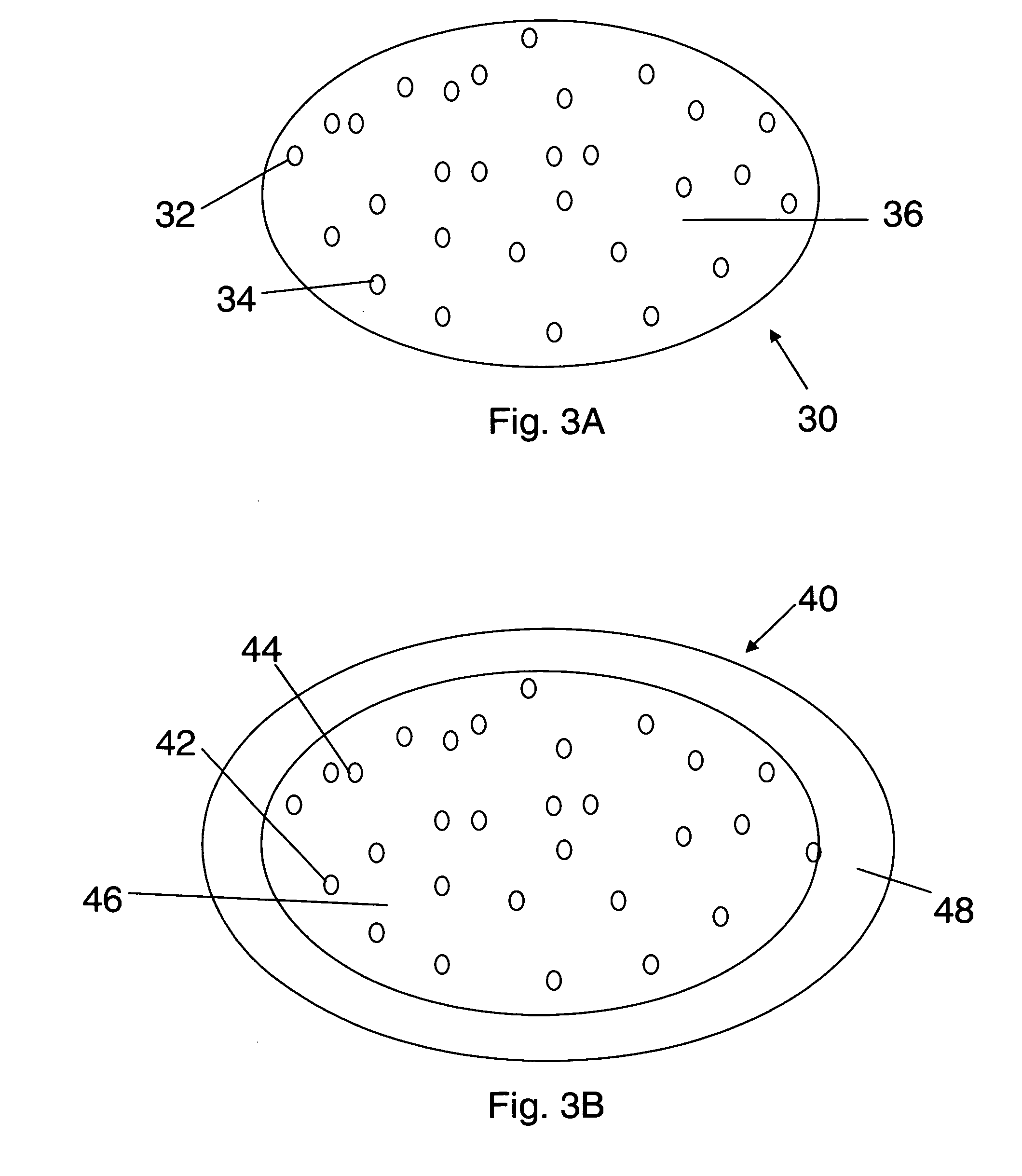
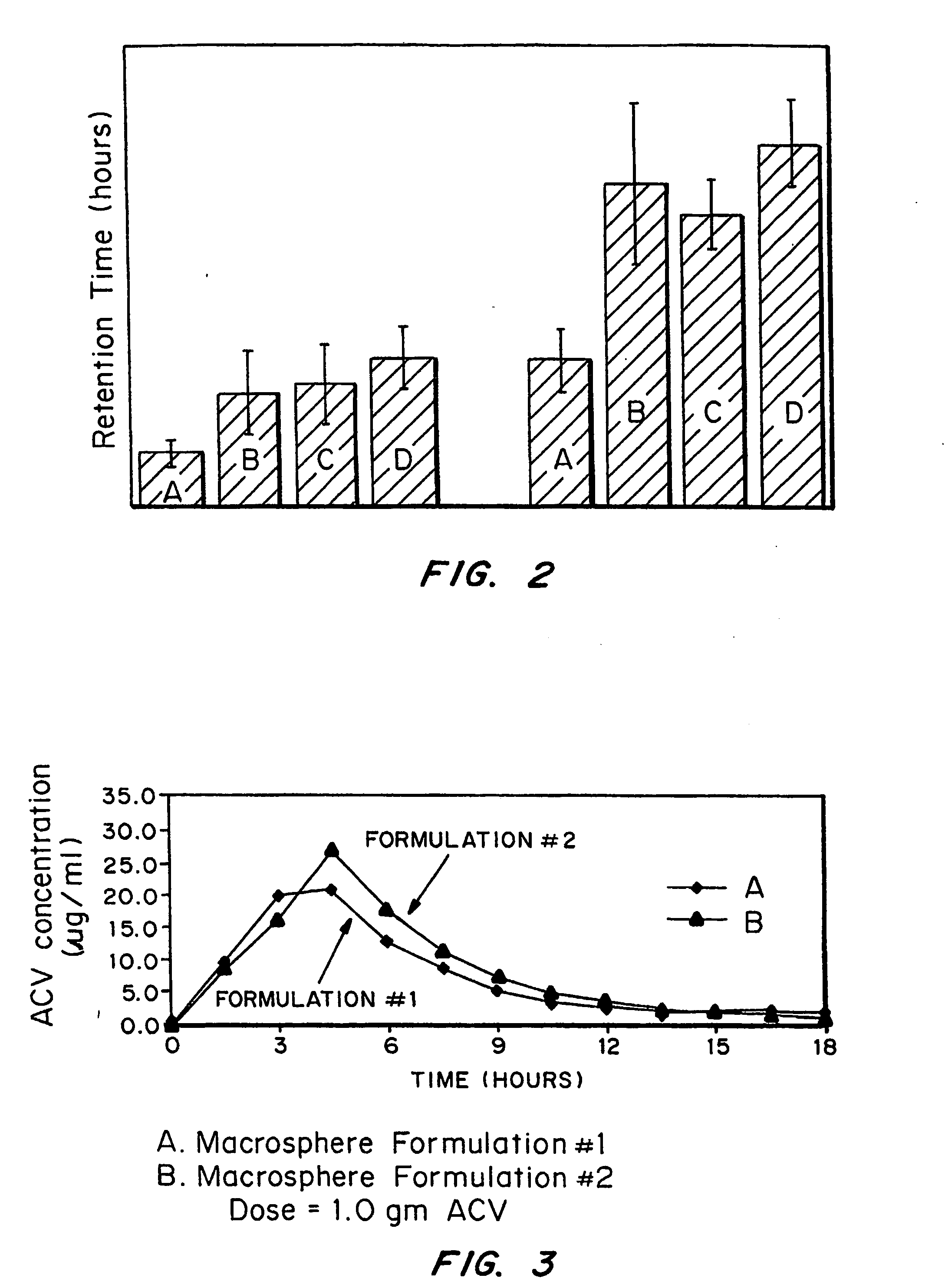
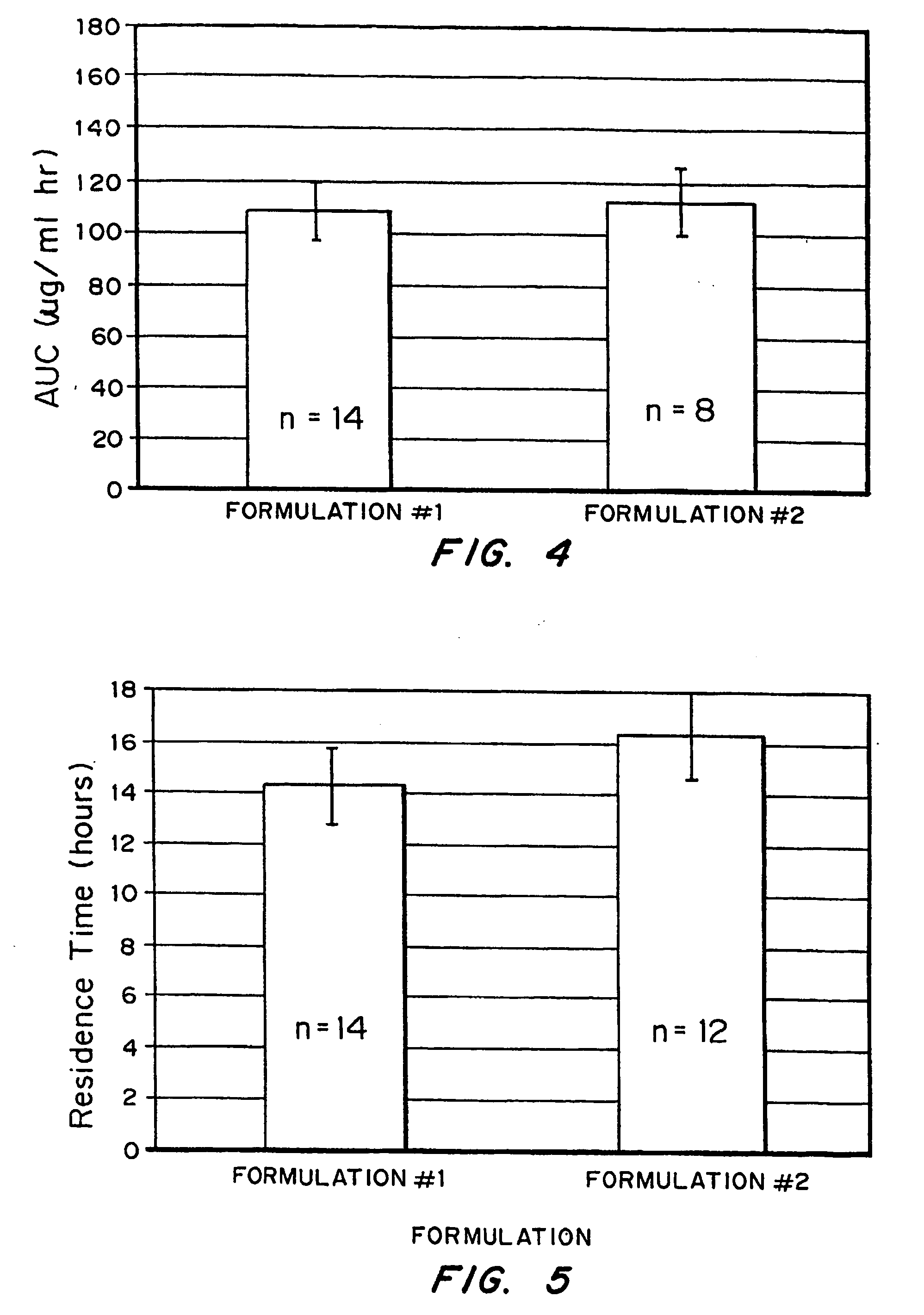
Bioadhesive drug delivery system with enhanced gastric retention
InactiveUS20050064027A1Prolonged gastric retention timeHigh retention rateBiocideCosmetic preparationsWhole bodyRetention time
Bioadhesive macrosphere delivery systems (“BDDS”) having prolonged gastric retention time due to bioadhesion rather than physical density or size are described. In general, the macrospheres have diameters that are greater than 200 microns, more preferably greater than 500 microns. The bioadhesive macrospheres are released in the stomach where they reside in close proximity to the gastric mucosa for a prolonged period of time. Increased residence of BDDS in the upper GI can lead to increased systemic absorption of drug in the preferred site of systemic absorption, namely the upper GI tract (upper to mid-jejunum). The BDDS may be engineered either as a capsule with drug delivery controlled by a diffusion-limited membrane or degradable shell, or as a solid matrix system with drug delivery controlled by a combination of diffusion and polymer degradation kinetics.
Owner:SPHERICS
Cooling agents, pharmaceutical compositions having cooling agents and processes for making and using same
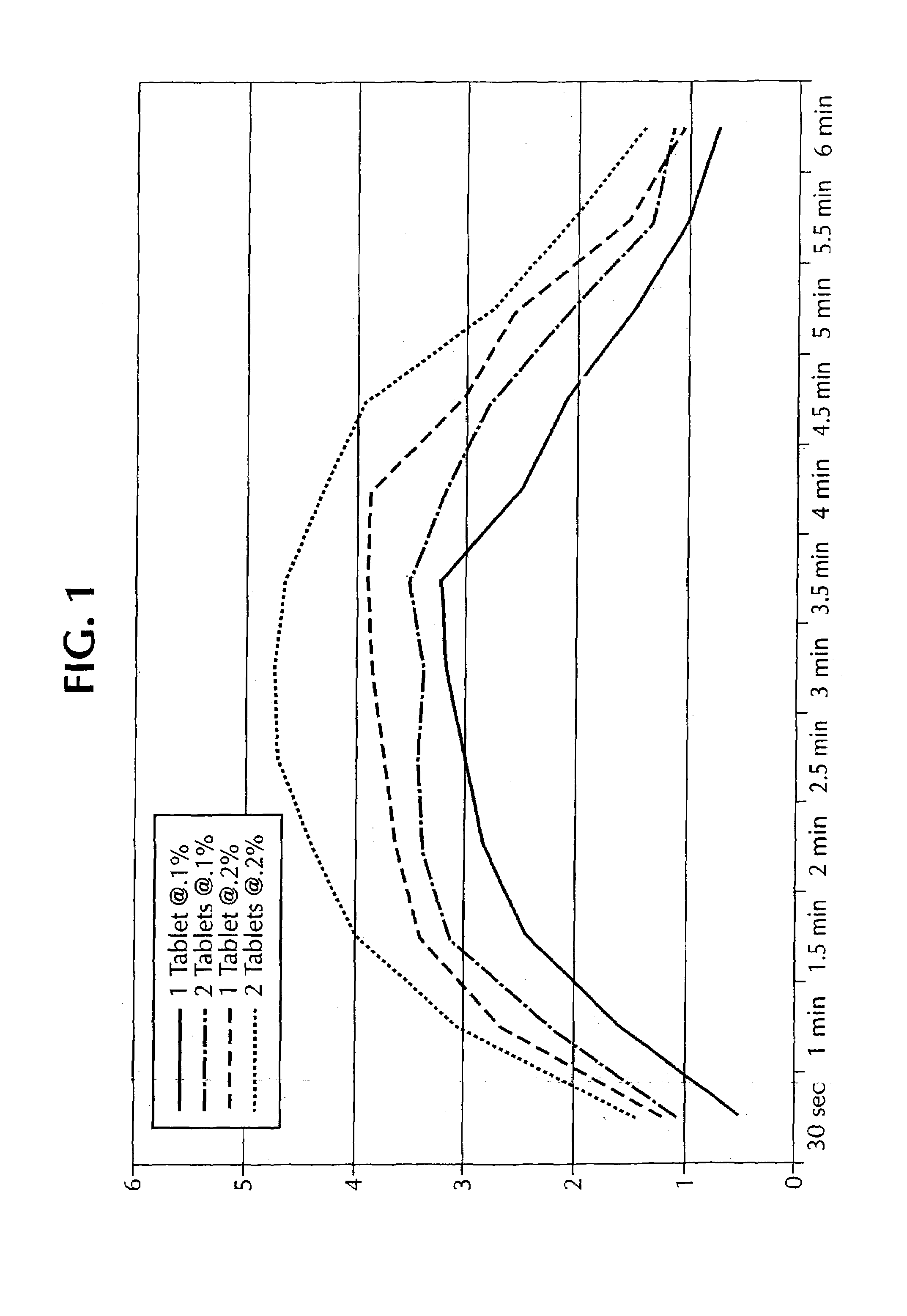
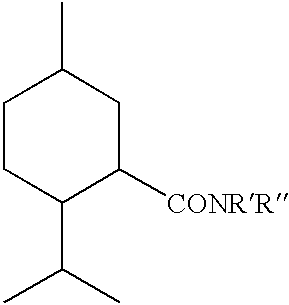
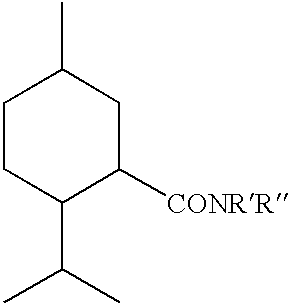
The invention pertains to cooling agents comprising N-substituted p-menthane-3-carboxamides, menthyl acetate and solubilizer, and methods for making the cooling agents. The invention also concerns pharmaceutical compositions comprising the cooling agents, including tablets, suspensions and liquid solutions having active pharmaceutical agents for treating upper gastrointestinal tract distress, and methods for treating upper gastrointestinal tract distress in humans.
Owner:FIRMENICH SA
Compound feed for teaching piglets to eat foods other than breast milk
InactiveCN102178115AHealthy micro-ecological balance environmentAvoid damageFood processingAnimal feeding stuffDiseaseSucrose
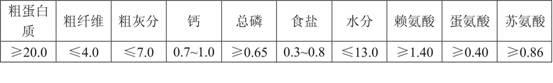
The invention provides compound feed for teaching piglets to eat foods other than breast milk, which is used for weaning piglets and teaching the piglets to eat foods other than breast milk, and comprises the following components in part by weight: 212.4 parts of corn starch, 154.0 parts of bulked corn, 100.0 parts of 46 percent of bulked bean pulp, 40 parts of fish meal, 100 parts of wheat flour, 5 parts of calcium powder, 21 parts of calcium hydrophosphate, 2 parts of table salt, 20 parts of soybean oil, 5 parts of lysine, 2 parts of threonine, 1.5 parts of choline chloride, 10 parts of premix, 0.2 part of complex enzyme, 0.3 part of pig Duowei, 0.2 part of sweetener, 0.3 part of flavouring agent, 3 parts of baking soda, 20 parts of cane sugar, 30 parts of glucose, 50 parts of egg powder, 70 parts of soy protein concentrate, 50 parts of fermented bean pulp, 6 parts of acidizer, 100 parts of whey powder, 0.5 part of mould removing agent, 0.3 part of antioxidant and 0.3 part of mildewpreventive. In the invention, the problems of incomplete immune functions, low disease resistance, inadequate digestive ferment, incomplete upper gastrointestinal development, low digestion and absorption ability, susceptibility to diarrhea and the like of piglets.
Owner:AGRI SCI & TECH INST CO LTD OF CHENGDU WEST HOPE GRP
Rosiglitazone formulations
Rosiglitazone is a drug used to treat type 2 diabetes. Methods for the formation of amorphous rosiglitazone and formulations comprising the amorphous rosiglitazone are described. Other formulations include pulsed-release formulations and formulations for retention in the stomach and upper gastrointestinal tract. Controlled-release dosage form include those wherein the maximum plasma concentration of rosiglitazone occurs greater than one hour after administration to a human and / or wherein less than 75 percent by weight of the rosiglitazone is released at 1 hour after immersion in simulated gastric fluid.
Owner:ACTAVIS GRP PTC EHF
Cooling agents, pharmaceutical compositions having cooling agents and processes for making and using same
The invention pertains to cooling agents comprising N-substituted p-menthane-3-carboxamides, menthyl acetate and solubilizer, and methods for making the cooling agents. The invention also concerns pharmaceutical compositions comprising the cooling agents, including tablets, suspensions and liquid solutions having active pharmaceutical agents for treating upper gastrointestinal tract distress, and methods for treating upper gastrointestinal tract distress in humans.
Owner:FIRMENICH SA
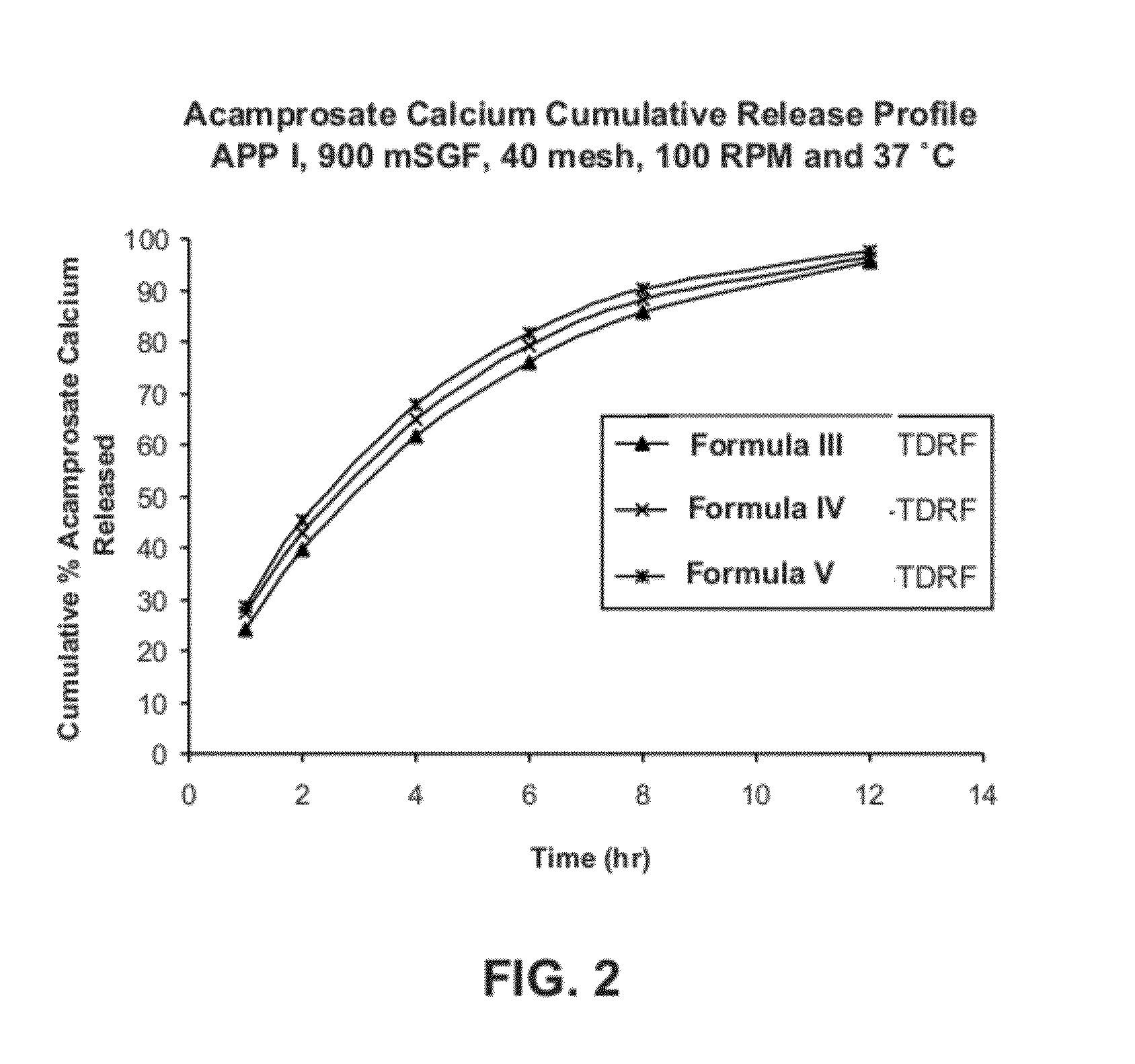
Gastric retentive dosage forms for extended release of acamprosate into the upper gastrointestinal tract
Gastric retentive dosage forms for sustained release of acamprosate are described which may allow once- or twice-daily dosing for both acute and long-term treatment of a disorder including alcohol dependence, tinnitus, sleep apnea, Parkinson's disease, levodopa-induced dyskinesias in Parkinson's disease, Alzheimer's disease, Huntington's disease, Amyotrophic lateral sclerosis, Cortical spreading depression, migraine, schizophrenia, anxiety, tardive dyskinesia, spasticity, multiple sclerosis, various types pain, or binge eating. Methods of treatment using the dosage forms and methods of making the dosage forms are also described.
Owner:DEPOMED SYST INC
Dosage form for delivery of multiple drug forms
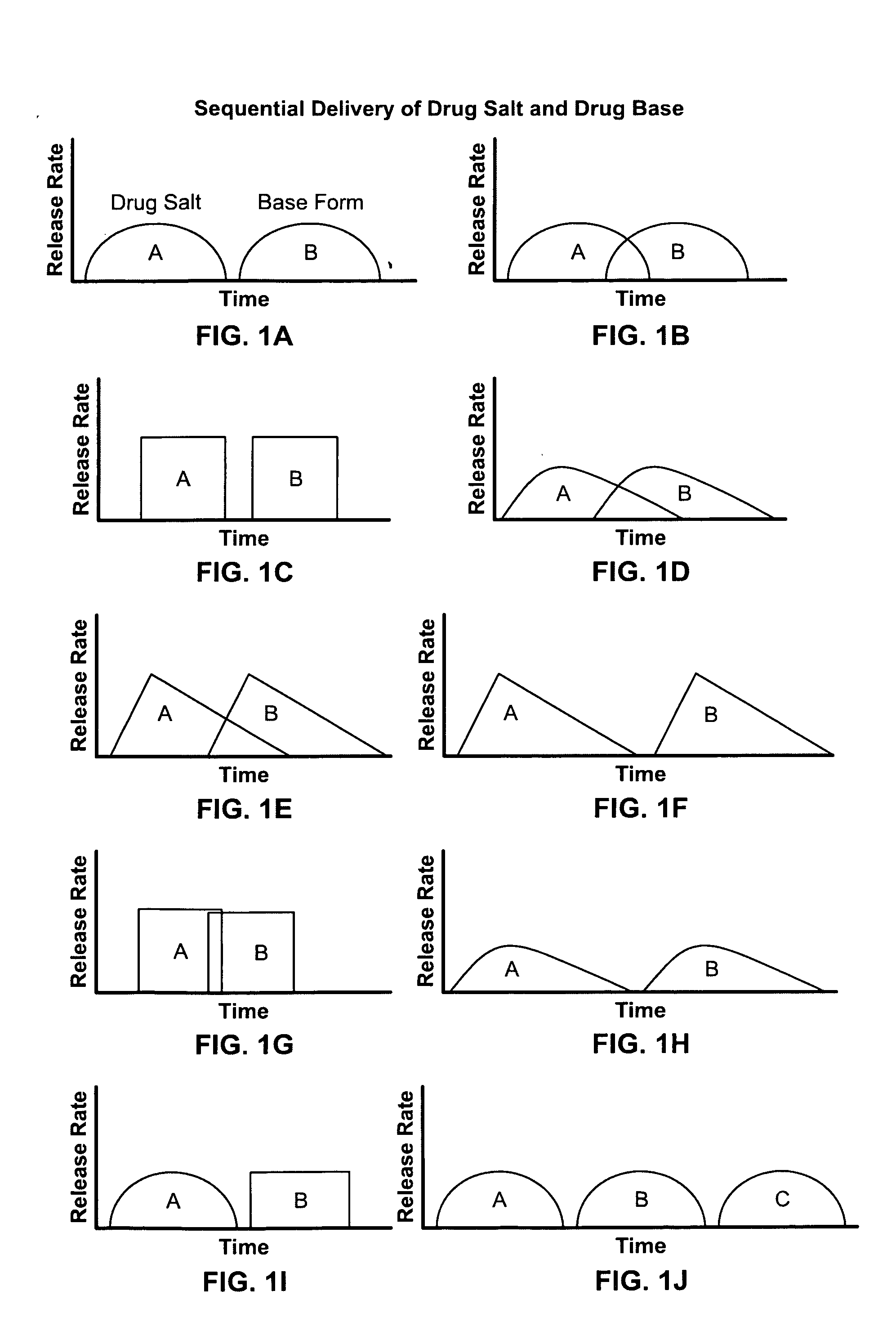
Disclosed are controlled release dosage forms and related methods including: (a) a micronized or liquid base form of a drug; (b) either a pharmaceutically acceptable salt form of the drug or starting materials that are capable of reacting to form a pharmaceutically acceptable salt form of the drug; (c) an upper gastrointestinal system pharmaceutically acceptable salt form releasing structure; and (d) a colonic system base form releasing structure.
Owner:ALZA CORP
Rosiglitazone and metformin formulations
InactiveUS20050163842A1Powder deliveryOrganic active ingredientsUpper gastrointestinalRosiglitazone metformin
Rosiglitazone and metformin are drugs used to treat type 2 diabetes. Formulations comprising amorphous rosiglitazone and metformin are described. Other formulations include formulations for retention in the stomach and upper gastrointestinal tract. Controlled-release dosage forms in which the release of the rosiglitazone, the metformin, or both are controlled are described.
Owner:ACTAVIS GRP PTC EHF
Powder injection for treating peptic ulcers and preparation method thereof
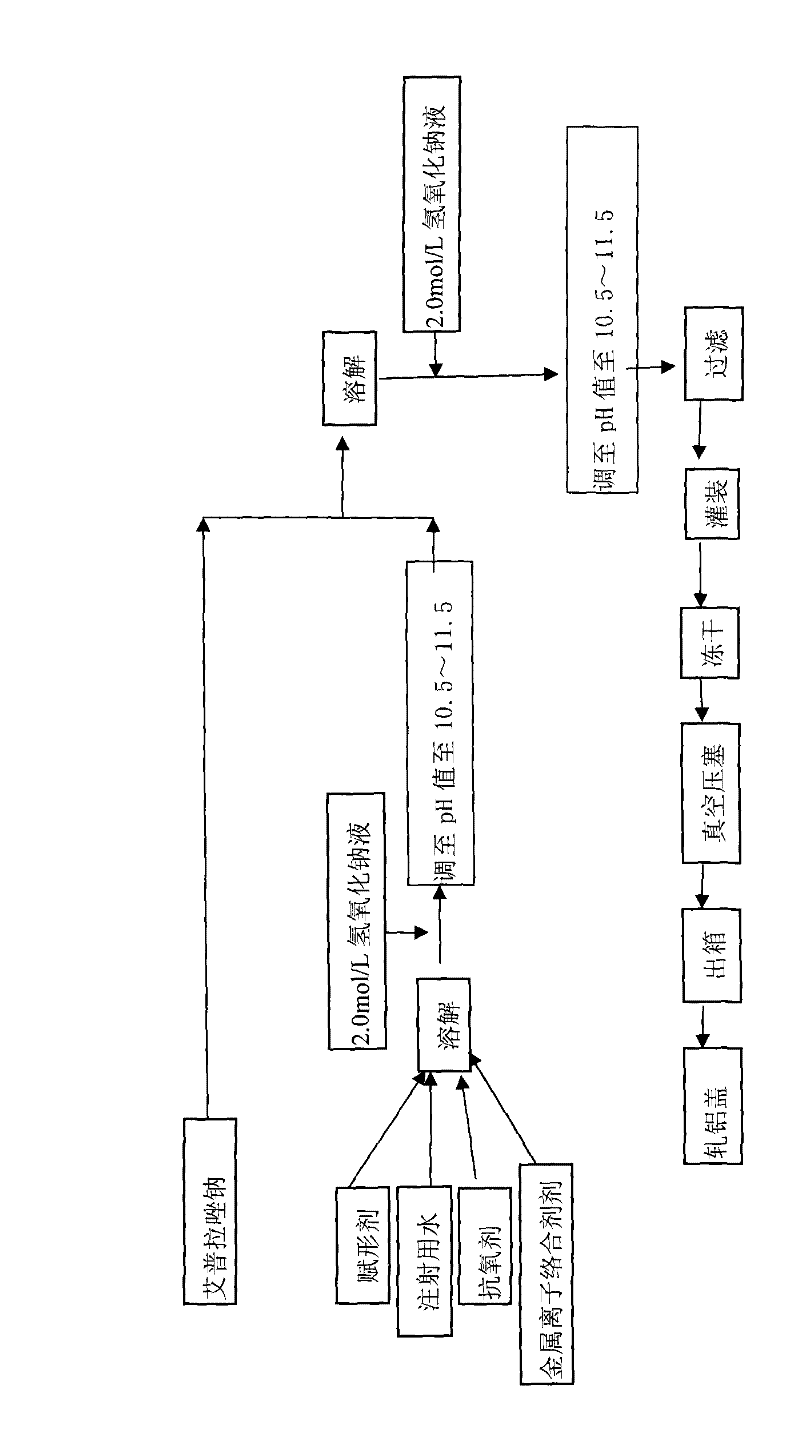
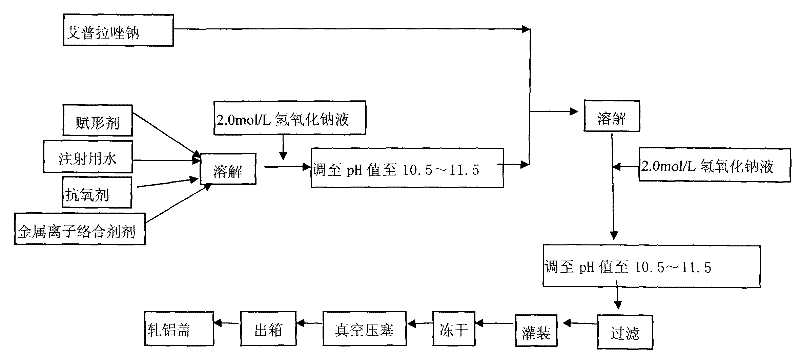
The invention provides an injection for treating a peptic ulcers and a preparation method thereof. The powder injection provided by the invention comprises the active ingredients of Ilaprazole sodium and excipient, wherein the ratio of the both in parts by weight is (1:1)-(1:30), and the preferable ratio is (1:10)-(1:18). The preferable prescription of the powder injection provided by the invention comprises 1 part of Ilaprazole sodium, 1-30 parts of excipient, 0-10 parts of antioxidant and / or 0-0.3 part of metal ion complexing agent; and a right amount of inorganic base is added to regulate the pH value to 9.0-12.0. The Ilaprazole sodium freeze-dried powder injection provided by the invention has stable quality, and is suitable for treating peptic ulcer bleeding and stress ulcers and preventing upper gastrointestinal bleeding caused by serious diseases.
Owner:LIVZON PHARM GRP INC
Dosage forms of risedronate
ActiveUS20060110452A1Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyImmediate release
Oral dosage forms of a risedronate comprised of a safe and effective amount of a pharmaceutical composition comprising risedronate, a chelating agent, and, means for effecting delayed release of the risedronate and the chelating agent in the small intestine provide immediate release of the pharmaceutical composition to the small intestine of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between risedronate and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of risedronate and the chelating agent to the small intestine, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
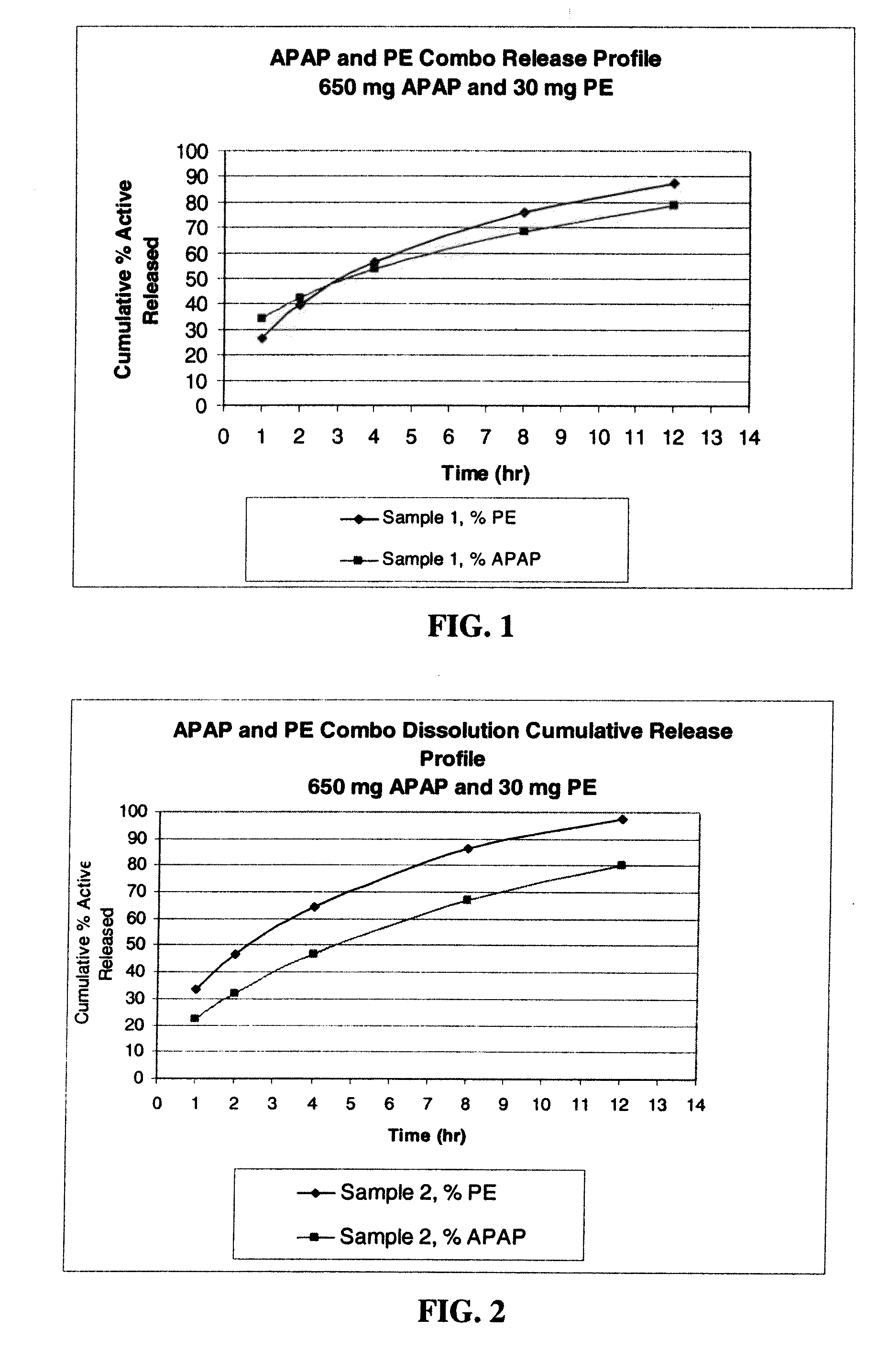
Gastric Retentive Extended-Release Dosage Forms Comprising Combinations of a Non-Opioid Analgesic and an Opioid Analgesic
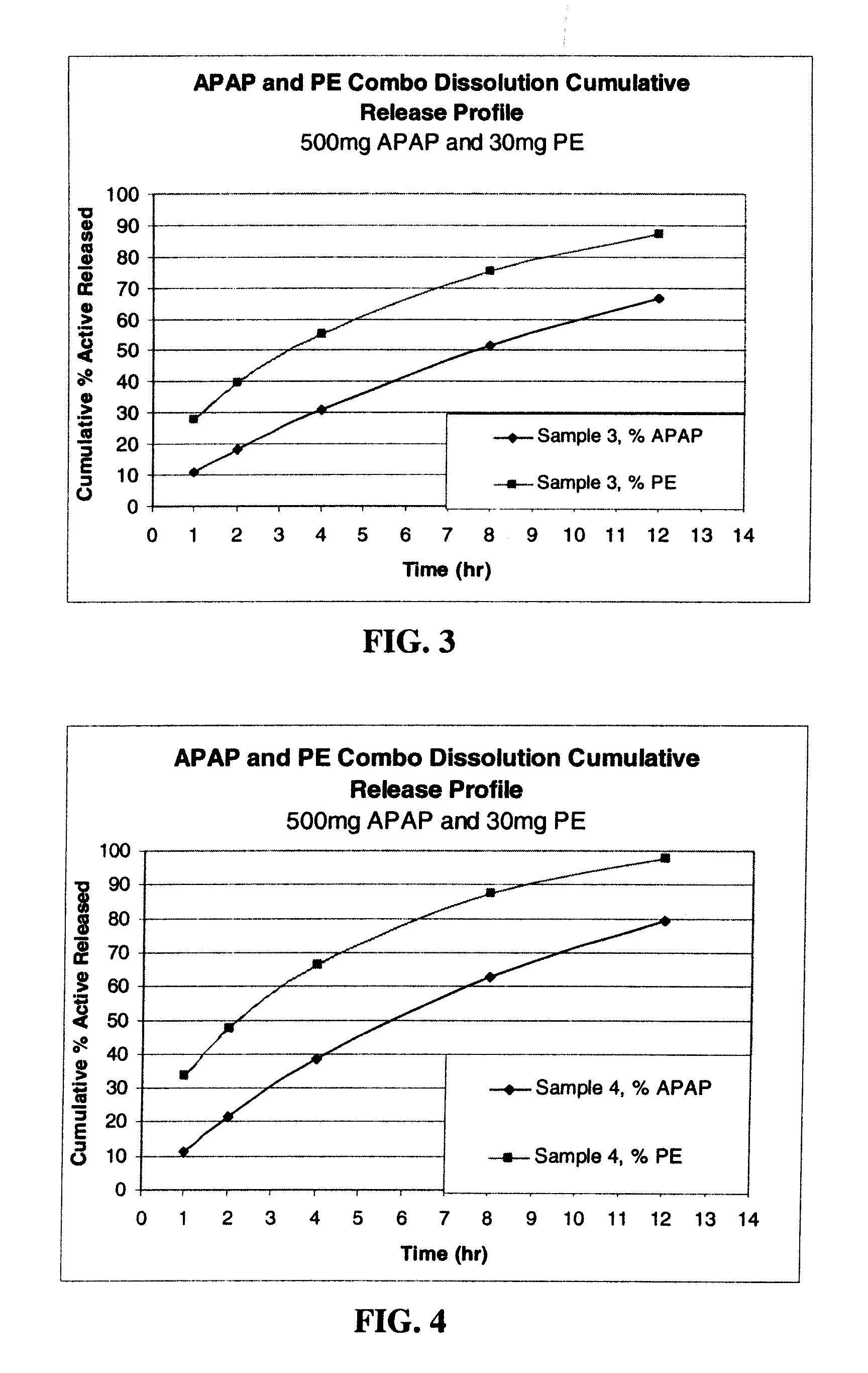
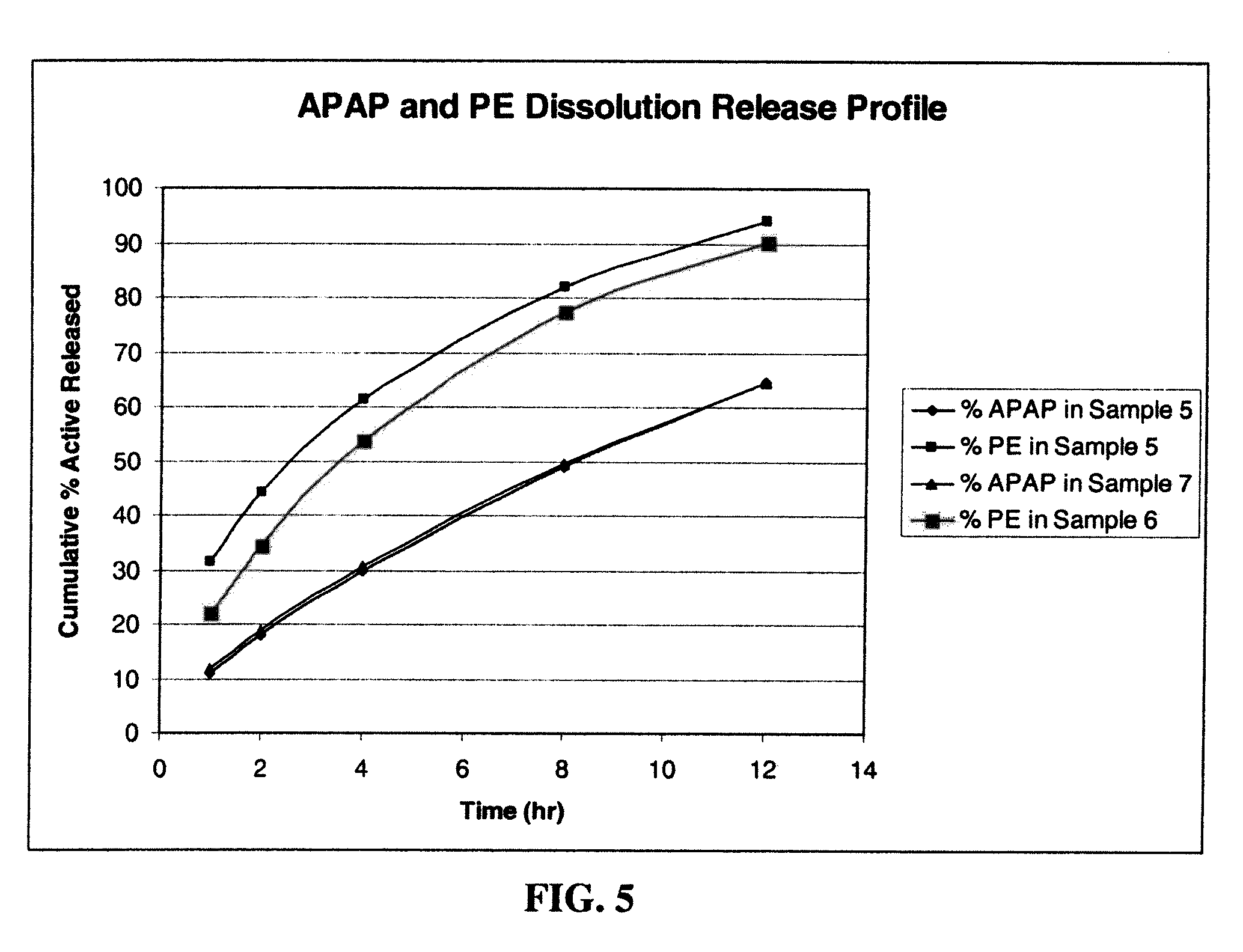
Compositions and methods for the treatment of pain in a mammal are described. More specifically, a dosage form designed for release of acetaminophen and an opioid is described, wherein the dosage form provides delivery of the drugs to the upper gastrointestinal tract (“GI”) of a mammal for an extended period of time.
Owner:DEPOMED SYST INC
Oxygen mask adaptable for upper gastrointestinal (UGI) endoscopy procedures providing enhanced oxygen concentration to maintain patient oxygenation
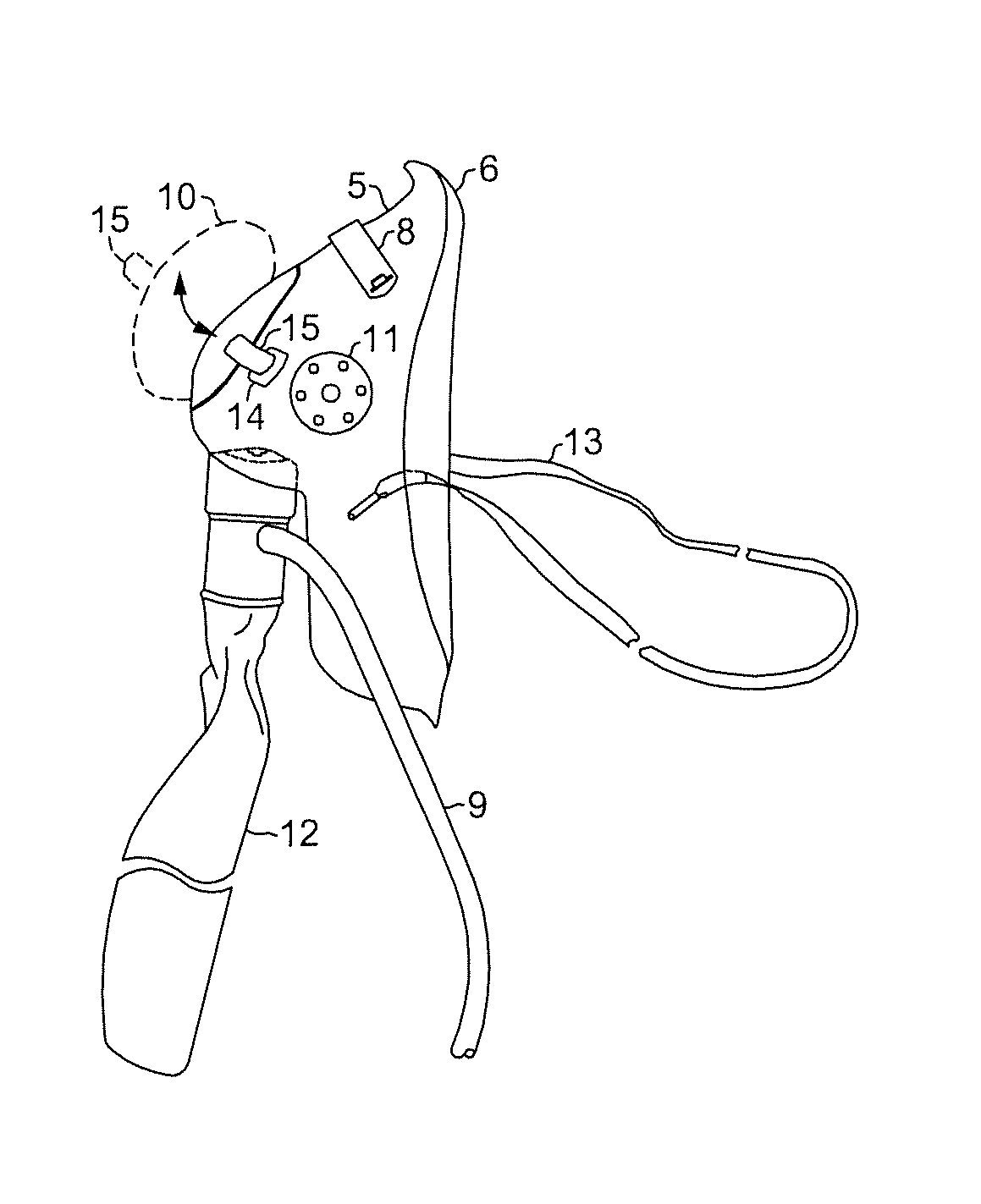
InactiveUS20140243600A1Increase oxygen concentrationMaintain oxygenationGastroscopesOesophagoscopesUpper gastrointestinalOxygen mask
An oxygen mask adaptable for upper gastrointestinal (UGI) endoscopy procedures providing enhanced oxygen concentration to maintain patient oxygenation. An oxygen mask in accordance with the present invention provides an aperture of sufficient dimensions to enable substantially unimpeded placement and manipulation of an endoscope during an endoscopic procedure while an overlying closure is in an open position and yet may also be closed to cover the aperture when the procedure is not being performed.
Owner:EISENBERGER MICHELLE
Gastric retentive oral dosage form with restricted drug release in the lower gastrointestinal tract
InactiveUS20110301129A1Minimizes variabilityIncreasing and decreasing drug loadingAntibacterial agentsBiocideDrug release rateErosion rate
Owner:DEPOMED SYST INC
Ph sensitive matrix formulation
InactiveUS20100081672A1Quick releaseImprove bioavailabilityBiocideDigestive systemUpper gastrointestinalDrugs exposure
The present invention provides formulations of therapeutic agents that benefit from a prolonged time of controlled release in the stomach and upper gastrointestinal (GI) tract, and from an enhanced drug exposure to the upper GI tract. The formulations of the invention comprise a therapeutic agent and one or more pH sensitive polymers that are designed for accelerated hydration, expansion, disintegration and dissolution at the higher pH of the upper GI tract, thereby, ensuring that any therapeutic agent that has not been released in the stomach is released in the upper GI tract, thus maximizing absorption of therapeutic agent that has a window of absorption located at the upper GI tract.
Owner:MERCK SHARP & DOHME CORP
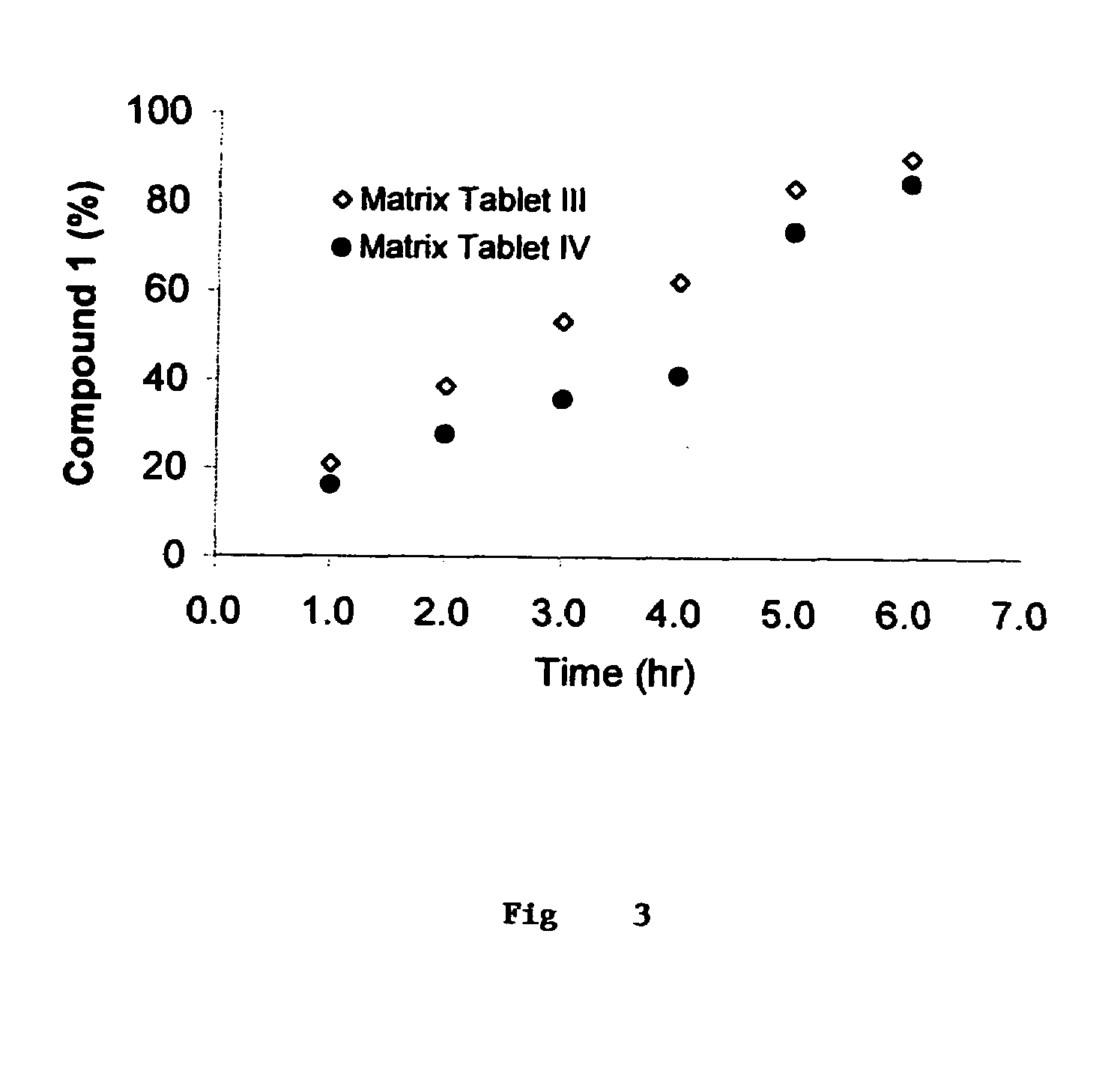
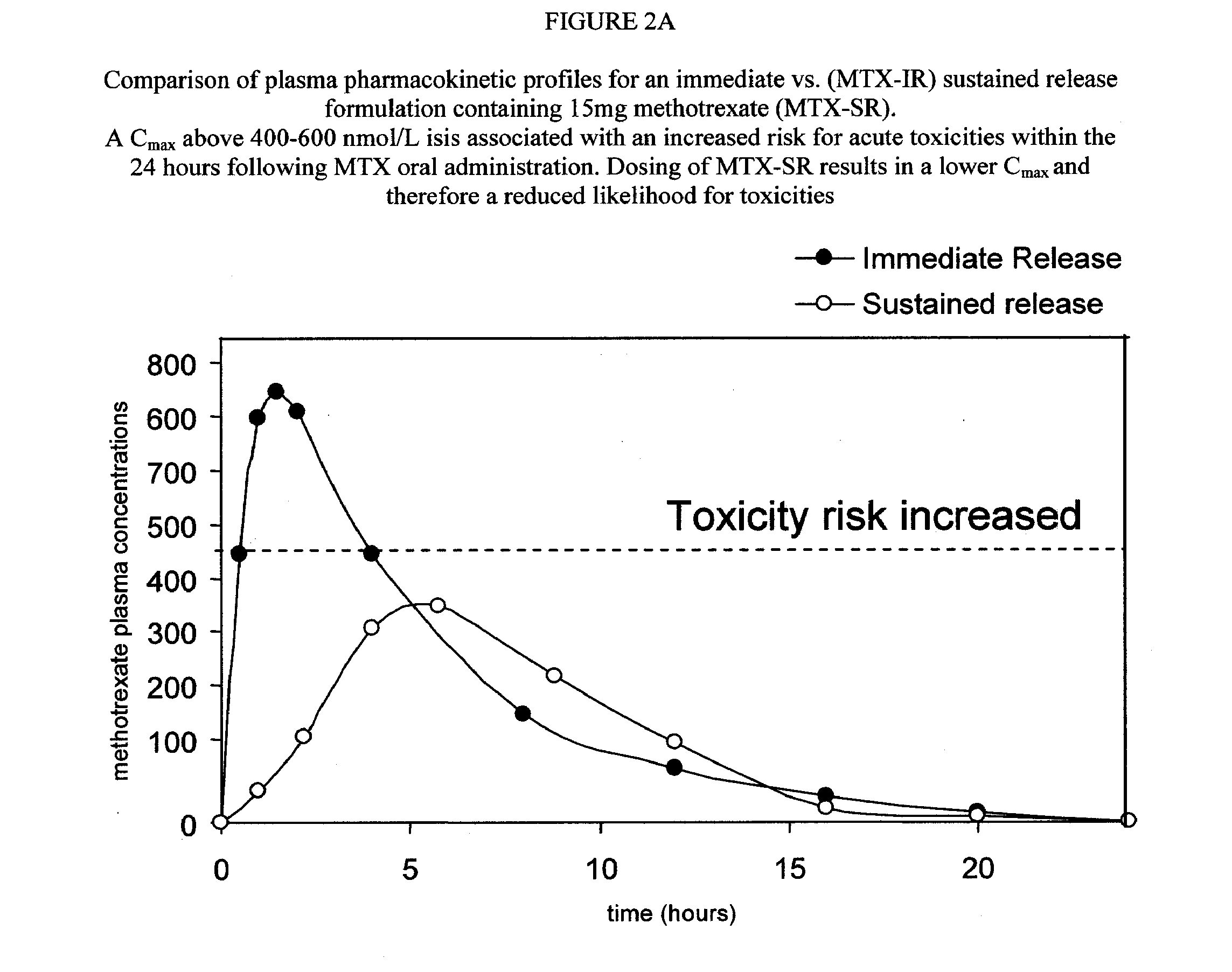
Sustained release methotrexate formulations and methods of use thereof
InactiveUS20080268045A1Reduce morbidityGood curative effectBiocideSkeletal disorderDiseaseOral medication
Described herein are methods of treating a disease by treatment with oral sustained release methotrexate alone or in combination with folates. In some embodiments, these approaches improve the pharmacotherapeutic performance of methotrexate therapy.Described herein are novel pharmaceutical compositions for oral administration. Also described herein are novel pharmaceutical compositions for the controlled, sustained delivery of one or more drugs to the stomach or upper gastrointestinal tract. Further described are novel pharmaceutical compositions with increased gastrointestinal residence time. More particularly, novel pharmaceutical compositions which can simultaneously, float in gastric fluid, adhere to the mucosal surfaces of the gastrointestinal tract, swell to a size which delays passage through the pylorus, are described herein. In some embodiments, the pharmaceutical compositions comprise methotrexate. In some embodiments, the pharmaceutical compositions comprise methotrexate and a folate compound. Also described herein are methods for treating or preventing diseases, by administration of the pharmaceutical compositions described herein.
Owner:CYPRESS BIOSCI
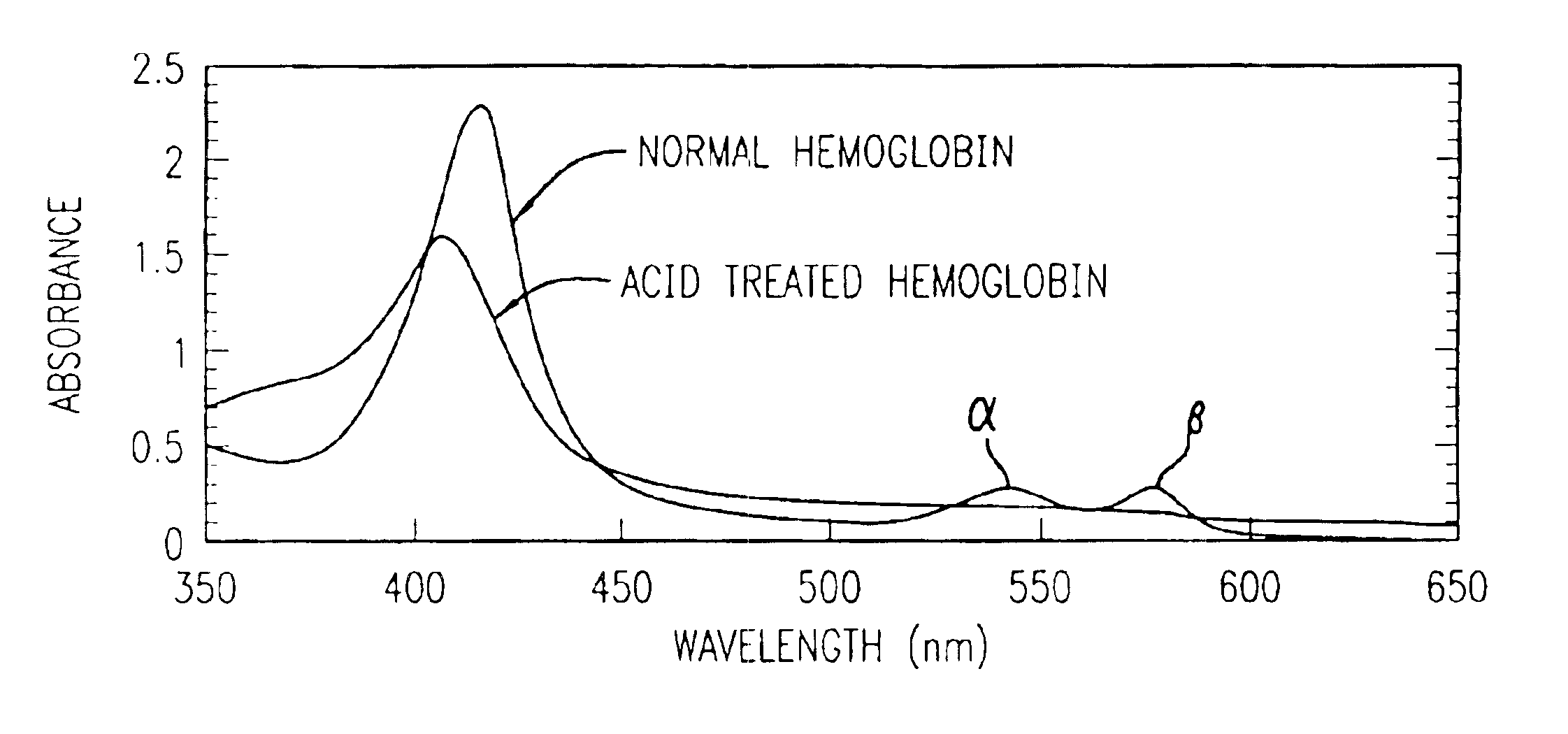
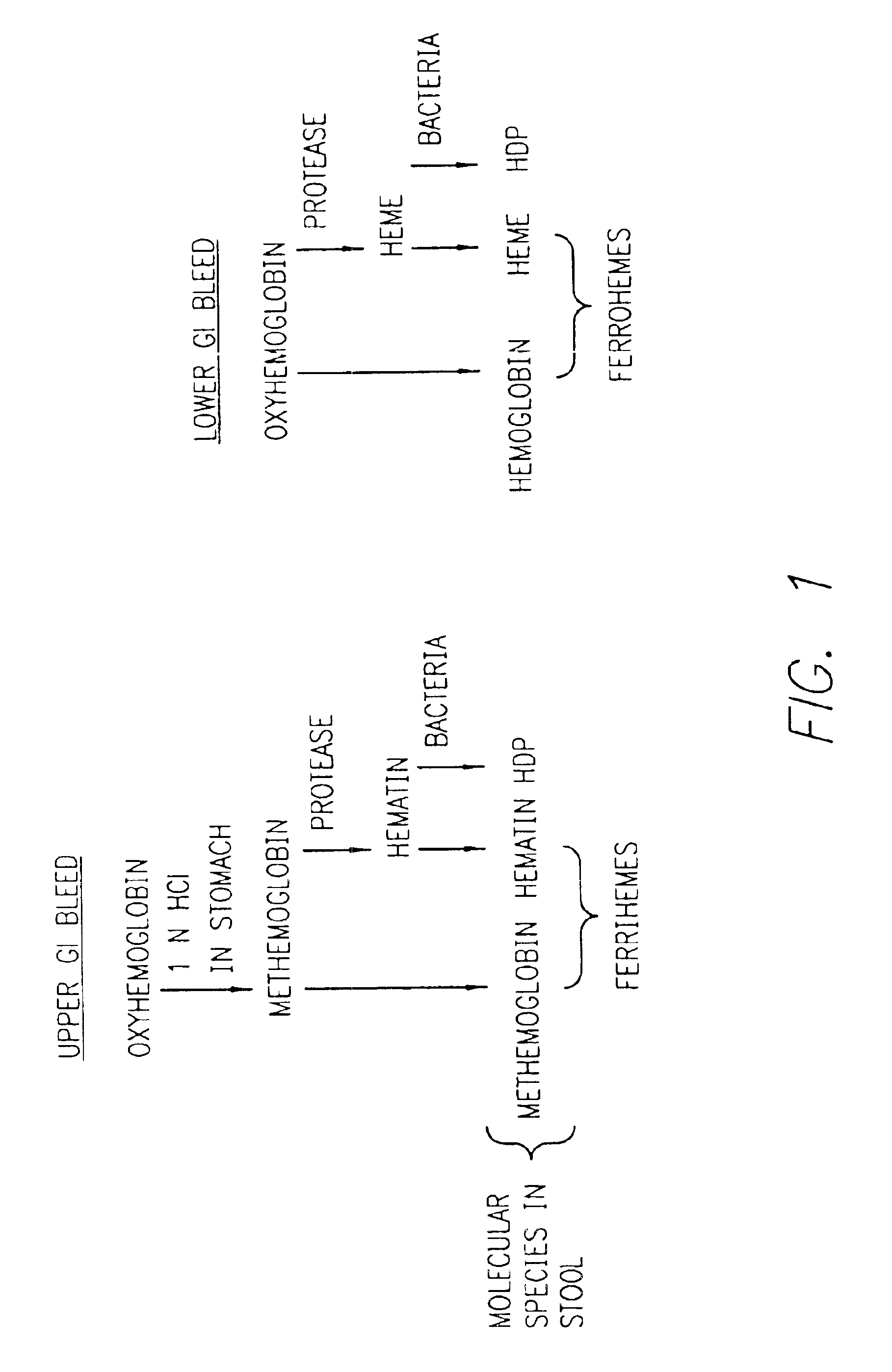
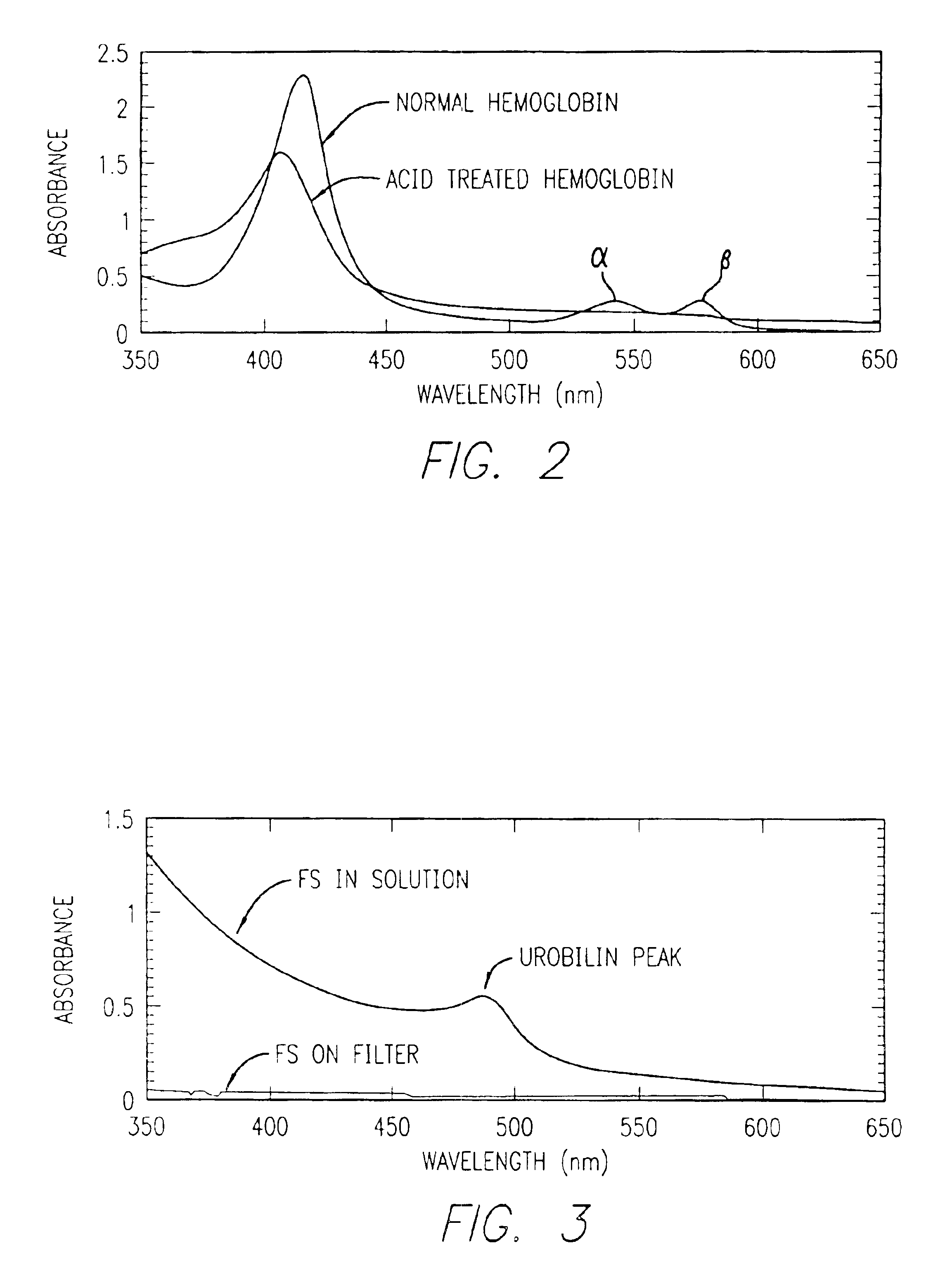
Method for determining location of gastrointestinal bleeding
InactiveUS6844195B2Rapid and economicalPerformance requirementAnalysis using chemical indicatorsPreparing sample for investigationFecesLower Gastrointestinal Tract
A method for determining if blood in a stool sample originated from the upper or lower gastrointestinal tract. This includes a method for purifying and concentrating hemoglobin and its products from a stool sample to allow a simple and sensitive spectrophotometric analysis. A rapid, noninvasive determination of whether the blood originated from an upper gastrointestinal or lower gastrointestinal site is made on the basis of changes in the absorption spectra of hemoglobin that occur when hemoglobin is exposed to a highly acidic environment.
Owner:WESTERN RES
Gastroretentive gel formulations
InactiveUS20170319698A1Easy to swallowAcceptable to patientAntibacterial agentsAntipyreticGastric emptyingUpper gastrointestinal
The present invention provides a gastric retentive gel composition comprising: (a) a hydrophobic or amphiphilic liquid gelled with an organogelator; (b) an active agent; and (c) a hard wax or wax-like additive, for controlled delivery of the active agent to or through the upper gastrointestinal tract, in particular to or through the stomach. The gel composition forms a stable, floating and coherent raft in the gastric environment, and is not directly expelled from the stomach as a result of gastric emptying. The active agent is released from the composition in a controlled manner for absorption or local action.
Owner:JAGOTEC AG
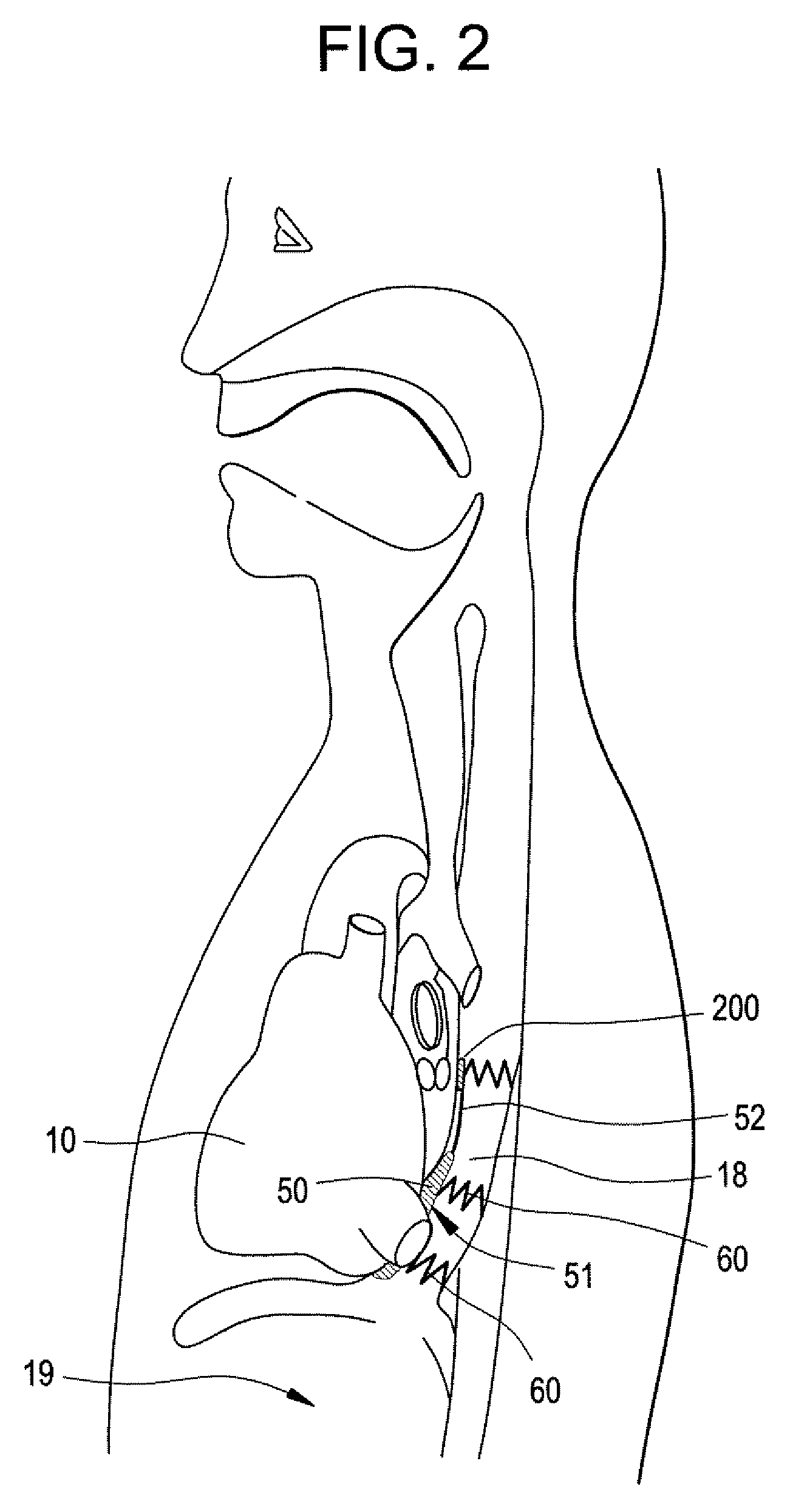
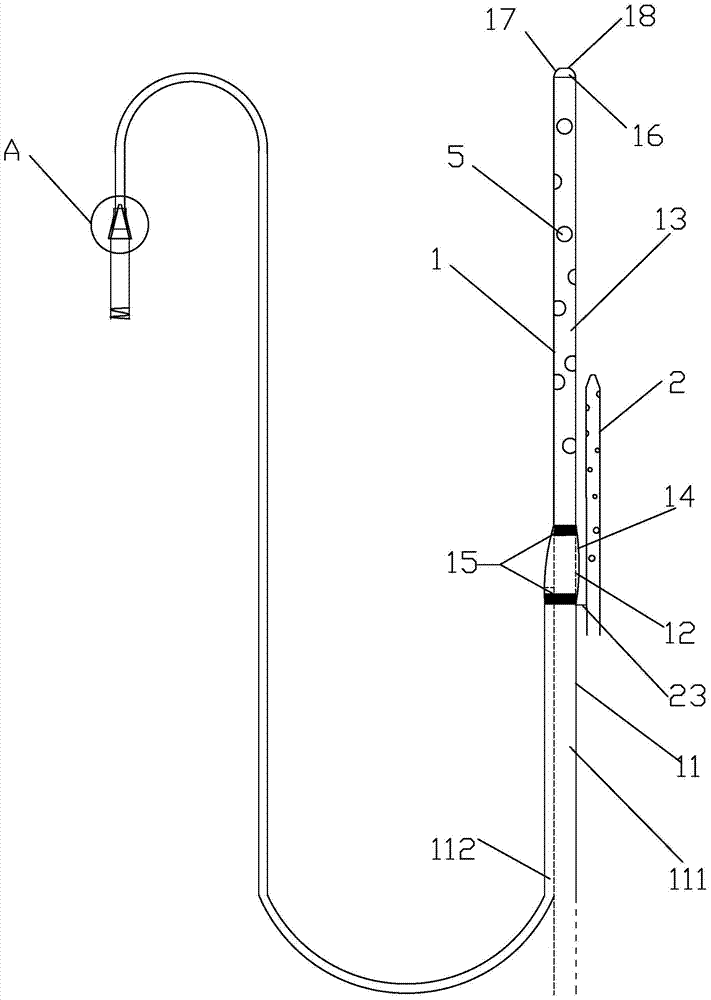
Integrated bile and pancreatic juice drainage pipe
The invention discloses an integrated bile and pancreatic juice drainage pipe which comprises a water sac type bile outer drainage pipe and a pancreatic juice inner drainage pipe. The water sac type bile outer drainage pipe comprises a drainage pipe section I, a drainage pipe section II and a drainage pipe section III which are communicated in sequence. The outer wall of the drainage pipe section II is sleeved with a balloon thin film. The pancreatic juice inner drainage pipe comprises a drainage pipe body, a pipe cap and a drainage pipe tail portion. The end, far away from the drainage pipe section II, of the drainage pipe section III is matched with the drainage pipe tail portion. The integrated bile and pancreatic juice drainage pipe has strong tension force, can have the function of avoiding disengagement of the water sac type bile outer drainage pipe, is suitable for bile ducts of various diameters, has no damage to bile duct mucosa, duodenal papillae and upper gastrointestinal tract mucosa, and cannot cause obstruction of biliary tracts while avoiding displacement of the water sac type bile outer drainage pipe. The pancreatic juice inner drainage pipe is connected with the water sac type bile outer drainage pipe through a line, and the function of avoiding displacement or disengagement of the pancreatic juice inner drainage pipe is achieved.
Owner:DALIAN UNIV
Methods of treating eosinophilic esophagitis
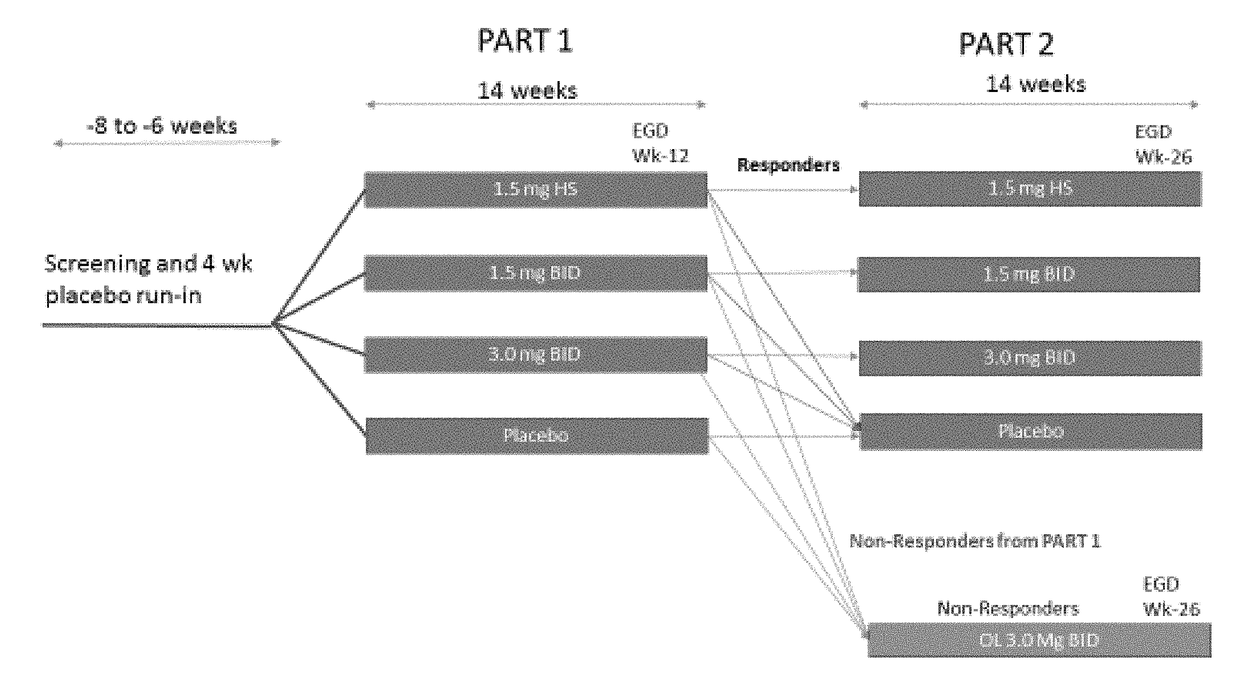
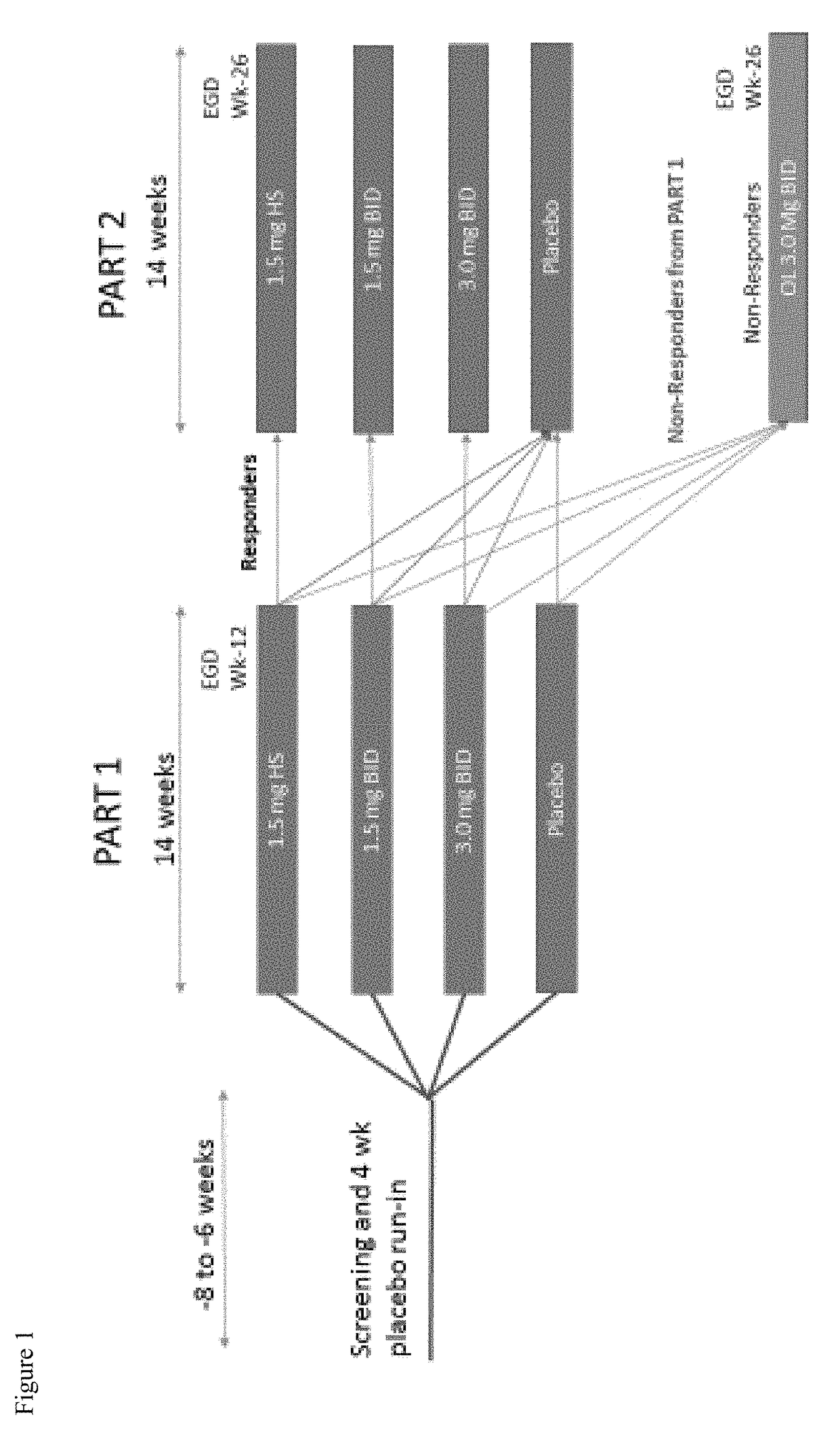
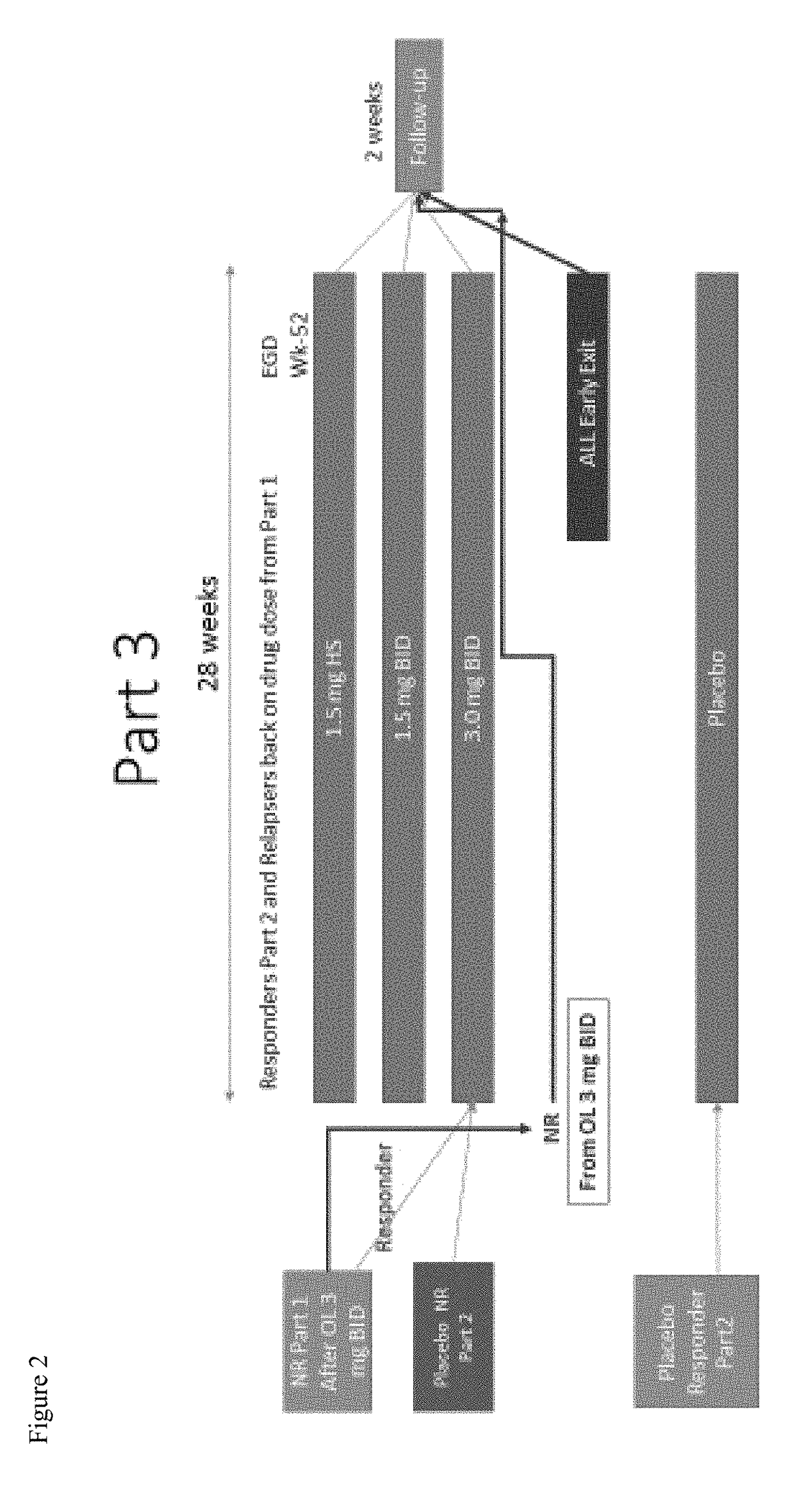
ActiveUS20180133145A1Raise countNo worseningOrganic active ingredientsAntipyreticGastrointestinal inflammationUpper gastrointestinal
The present disclosure provides methods of treating inflammation of the upper gastrointestinal tract, especially the esophagus, by administering an oral corticosteroid. In some cases, the methods include treating eosinophilic esophagitis (EoE) by administering an oral corticosteroid in an induction phase and a maintenance phase to improve peak eosinophilic counts and symptoms. In embodiments, the methods include treating EoE by administering the oral corticosteroid at nighttime and / or while the patient is lying down.
Owner:ELLODI PHARM LP
Traditional Chinese medicine enteric oral liquor using leech extractive as active component, and its preparation method
InactiveCN1911250AAnthropod material medical ingredientsPill deliveryMedicinal herbsUpper gastrointestinal
An orally taken enteric Chinese medicine in the form of tablet, capsule, dripping pill, or soft capsule for treating unstable angina pectoris, acute myocardial infarction, cerebral haemorrhage, etc and preventing deep phlebothrombosis is prepared from leech. Its preparing process is also disclosed.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Y-type bile pancreatic juice drainage tube with water bag
The invention discloses a Y-type bile pancreatic juice drainage tube with a water bag. The Y-type bile pancreatic juice drainage tube comprises a water bag type bile outer drainage tube and a pancreatic juice inner drainage tube. The water bag type bile outer drainage tube comprises a drainage tube section I, a drainage tube section II and a drainage tube section III. The drainage tube section II is sleeved with a balloon film, and the pancreatic juice inner drainage tube comprises a drainage tube body and a tube cap I located at the upper end of the drainage tube body. The side wall of the drainage tube body is connected with the side wall of the drainage tube section I through a connecting part. The Y-type bile pancreatic juice drainage tube has the effects of preventing the water bag type bile outer drainage tube from disengaging and preventing the pancreatic juice inner drainage tube from shifting or disengaging, is suitable for bile ducts of various diameters and has no injury to the bile duct mucous membrane, the duodenal papilla and the upper digestive duct mucosa in the placing-in and taking-out processes, bile duct obstruction will not be caused while the water bag type bile outer drainage tube is prevented form shifting, and while the water bag type bile outer drainage tube is taken out, the pancreatic juice inner drainage tube can be taken out.
Owner:DALIAN UNIV
Carbazochrome Sodium Sulfonate infusion and its preparation method
InactiveCN1557302AGood effectEasy to operateOrganic active ingredientsPharmaceutical delivery mechanismDiseaseUpper gastrointestinal
The present invention belongs to the field of medicine technology. The Carbazochrome liquid contains Carbazochrome 0.002-0.5 wt%, antioxidant 0-10 wt%, osmotic pressure regulator 0.8-75 wt%, and injection water 20-99.1 wt%. The preparation process of the Carbazochrome liquid includes dissolving osmotic pressure regulator with partial injection water in a compounding tank; adding injection level active carbon through heating and stirring for adsorption for 30 min; filtering and decarbonizing with titanium rod and adding rest injection water to obtain the solution I; dissolving Carbazochrome and antioxidant in solution I, adding injection level active carbon for adsorption for 30 min, filtering and decarbonizing with titanium rod, regulating pH value, fine filtering, detecting, packing in bottle, disinfecting and other steps. The medicine is used in intravenous transfusion for treating various hemorrhage diseases.
Owner:肖广常 +1
Composition and method for treatment of NASH
ActiveUS9314444B2Increase productionReduce contentPeptide/protein ingredientsCapsule deliveryUpper gastrointestinalL-Glutamin
The present invention relates to a method of treating NASH or NAFLD by delivery of an effective amount of a composition comprising L-glutamine or butyric acid formulated for release in the colon by bypassing the upper digestive tract and stomach.
Owner:BIOKIER
Liquid enteral nutritional composition suitable for tube feeding, minimizing lower and upper tract digestive conditions
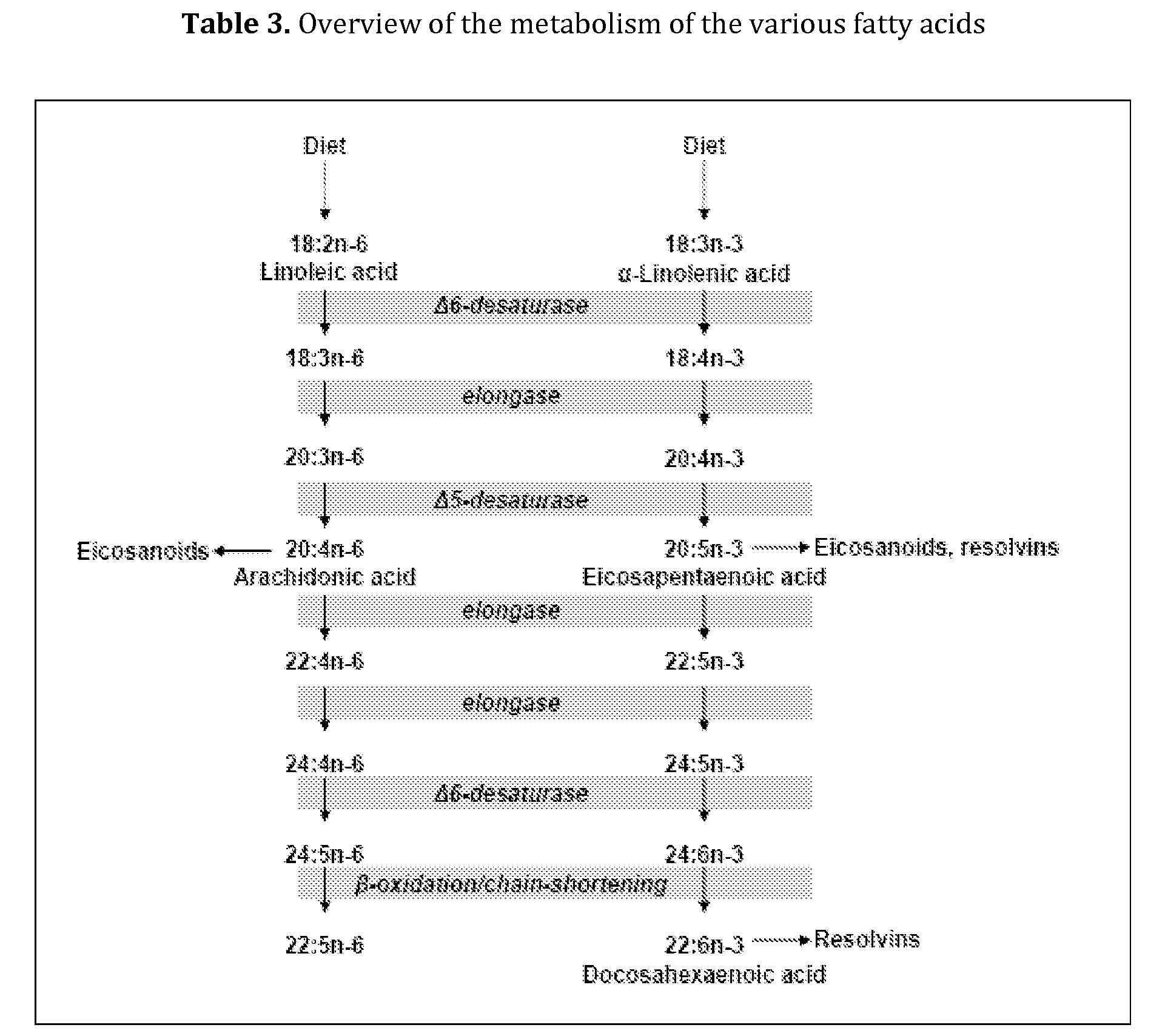
ActiveUS8618047B2Reduction of serum inflammatory markersReduce ratePeptide/protein ingredientsMetabolism disorderDocosahexaenoic acidUpper tract
The invention is directed to liquid enteral nutritional compositions comprising a protein fraction comprising more than 25 weight % and up to 80 weight % of a vegetable protein comprising at least a source of pea protein, and a fat fraction comprising (a) 8 to 15 weight % of linoleic acid; (b) 3.0 to 6.0 weight % of a combination of alpha-linolenic acid, docosahexaenoic acid and eicosapentaenoic acid, wherein the amount of ALA is >2.5 weight % and the combined amount of DHA and EPA is ≦2.5 weight %; (c) 10 to 20 weight % of at least one medium-chain fatty acid; and (d) 35 to 79 weight % of at least one mono-unsaturated fatty acid. The compositions provide for a healthy and balanced diet, which is well-tolerated and minimises clinical complications that are frequently associated with the administration of enteral nutrition in patients using tube feeding, especially with respect to a reduced gastric emptying.
Owner:NV NUTRICIA
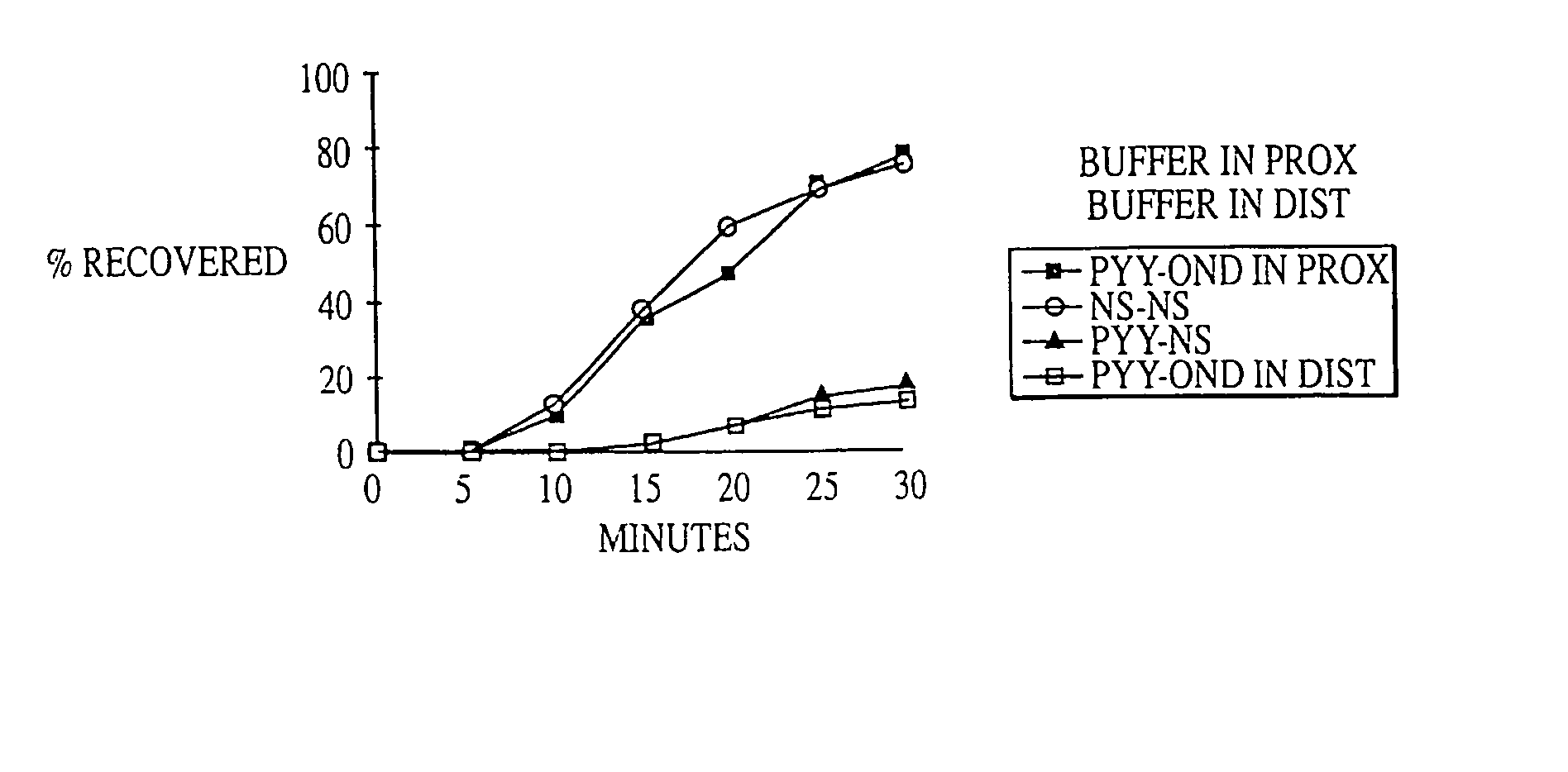
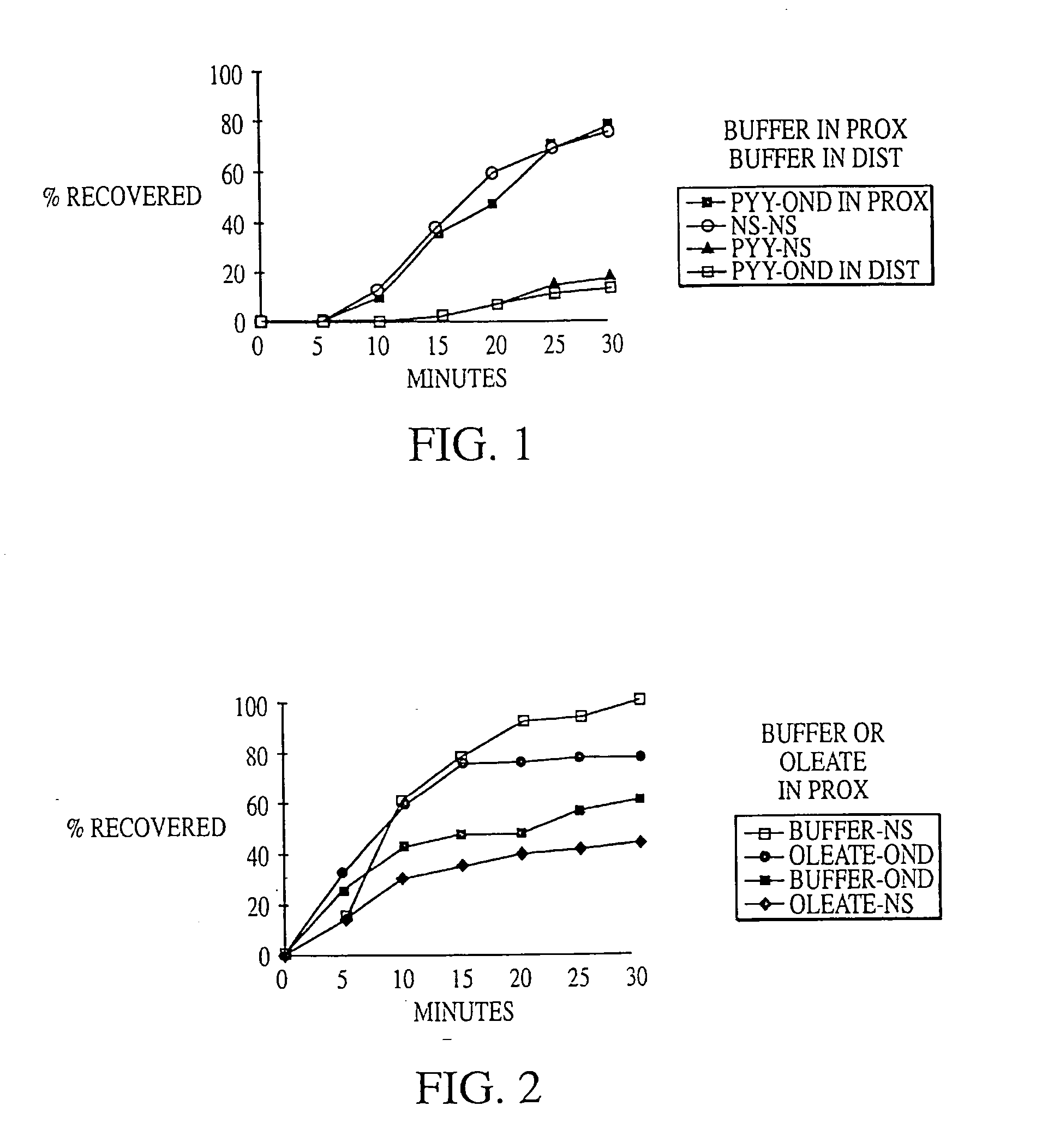
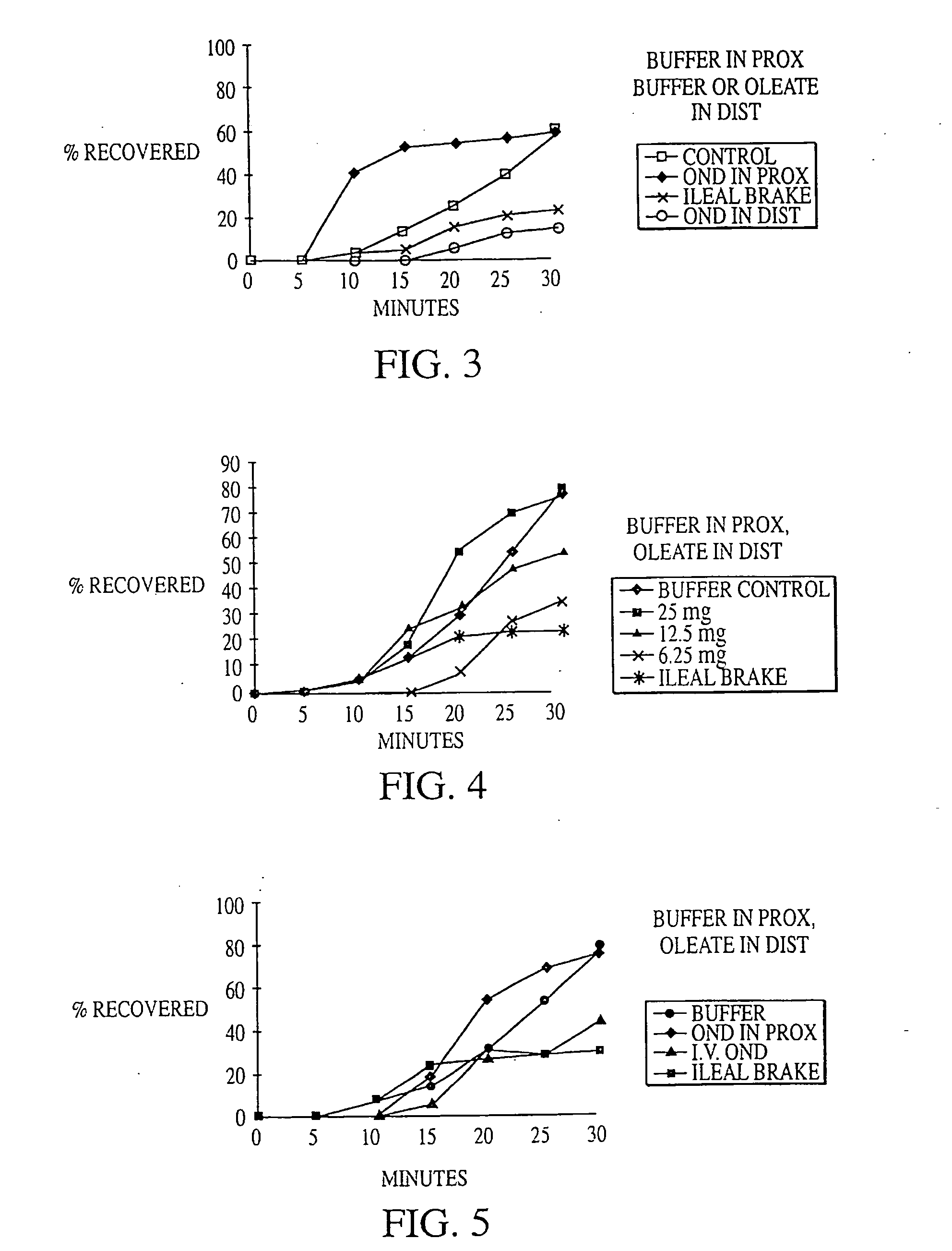
Methods for manipulating upper gastrointestinal transit, blood flow, and satiety, and for treating visceral hyperalgesia
InactiveUS20050014693A1Reduced sensationImprove bioavailabilityCompounds screening/testingAntibacterial agentsAdministered substanceMammal
Disclosed is a method of manipulating the rate of upper gastrointestinal transit of a substance in a mammal. Also disclosed are methods of manipulating satiety and post-prandial visceral blood flow. A method of treating visceral pain or visceral hypersensitivity in a human subject is also described. A method for prolonging the residence time of an orally or enterally administered substance by promoting its dissolution, bioavailability and / or absorption in the small intestine is also described. These methods are related to a method of transmitting to and replicating at a second location in the central nervous system a serotonergic neural signal originating at a first location in the proximal or distal gut of a mammal and / or a method of transmitting to and replicating at a second location in the upper gastrointestinal tract a serotonergic neural signal originating at a first location in the proximal or distal gut.
Owner:CEDARS SINAI MEDICAL CENT
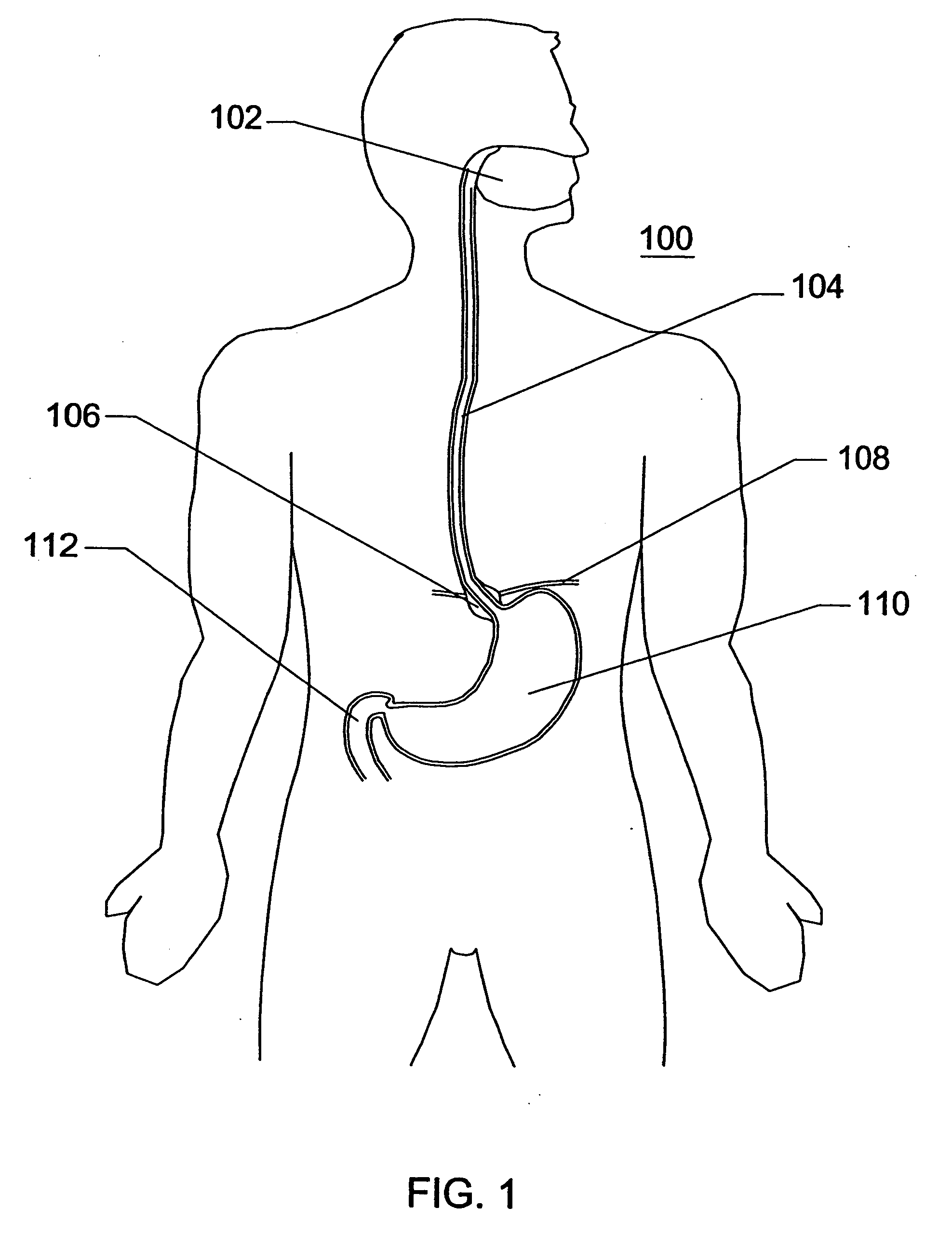
Expandable sheath for percutaneous upper gastrointestinal tract access
InactiveUS20060259061A1Reduce manufacturing costStable or stiff platform for esophageal sphincter repairMulti-lumen catheterSurgeryAccess routeStomach walls
Disclosed is an expandable percutaneous sheath, for introduction into the body while in a first, low cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the upper gastrointestinal tract and has utility in the performance of procedures in the esophagus and stomach. The access route is through the anterior abdominal wall to the stomach. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement through the abdominal wall and into the stomach. The distal end of the sheath is subsequently expanded using a radial dilatation device. In an exemplary application, the sheath is utilized to provide access for a diagnostic or therapeutic procedure such as diagnosis and repair of gastro esophageal reflux disease. The sheath further can be secured within the gastrointestinal system and be used to draw the stomach wall against the abdominal wall.
Owner:ONSET MEDICAL CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com