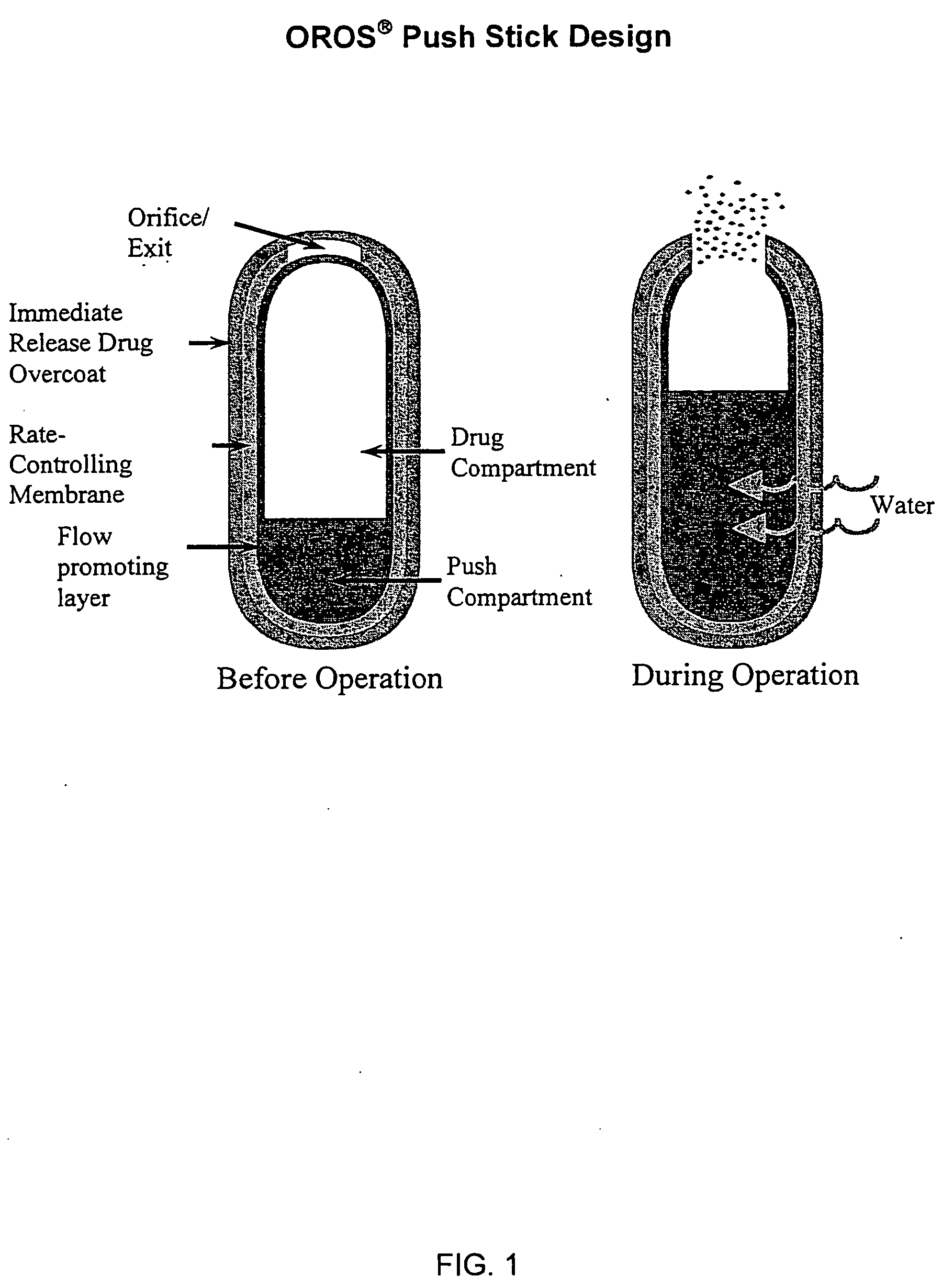
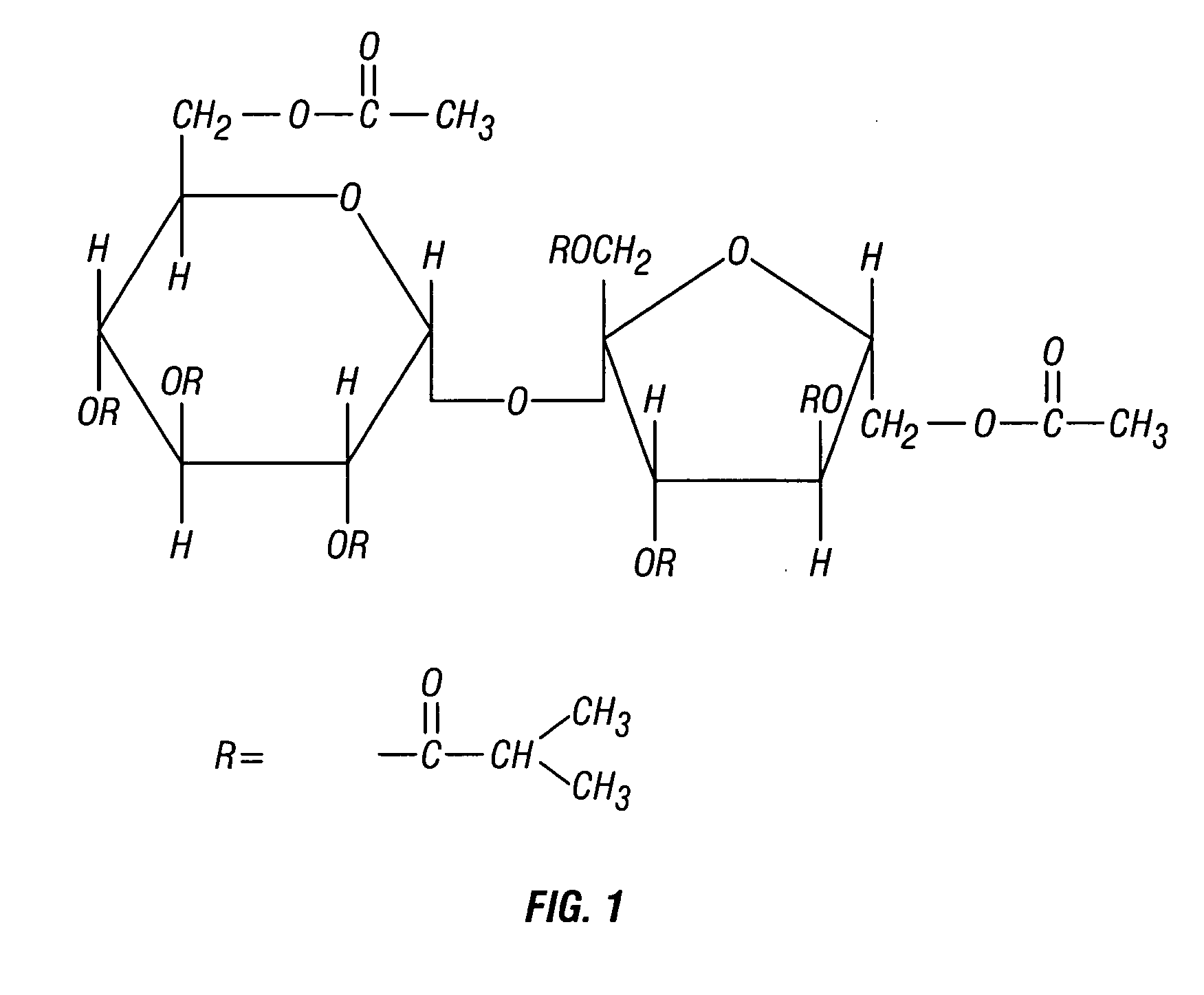
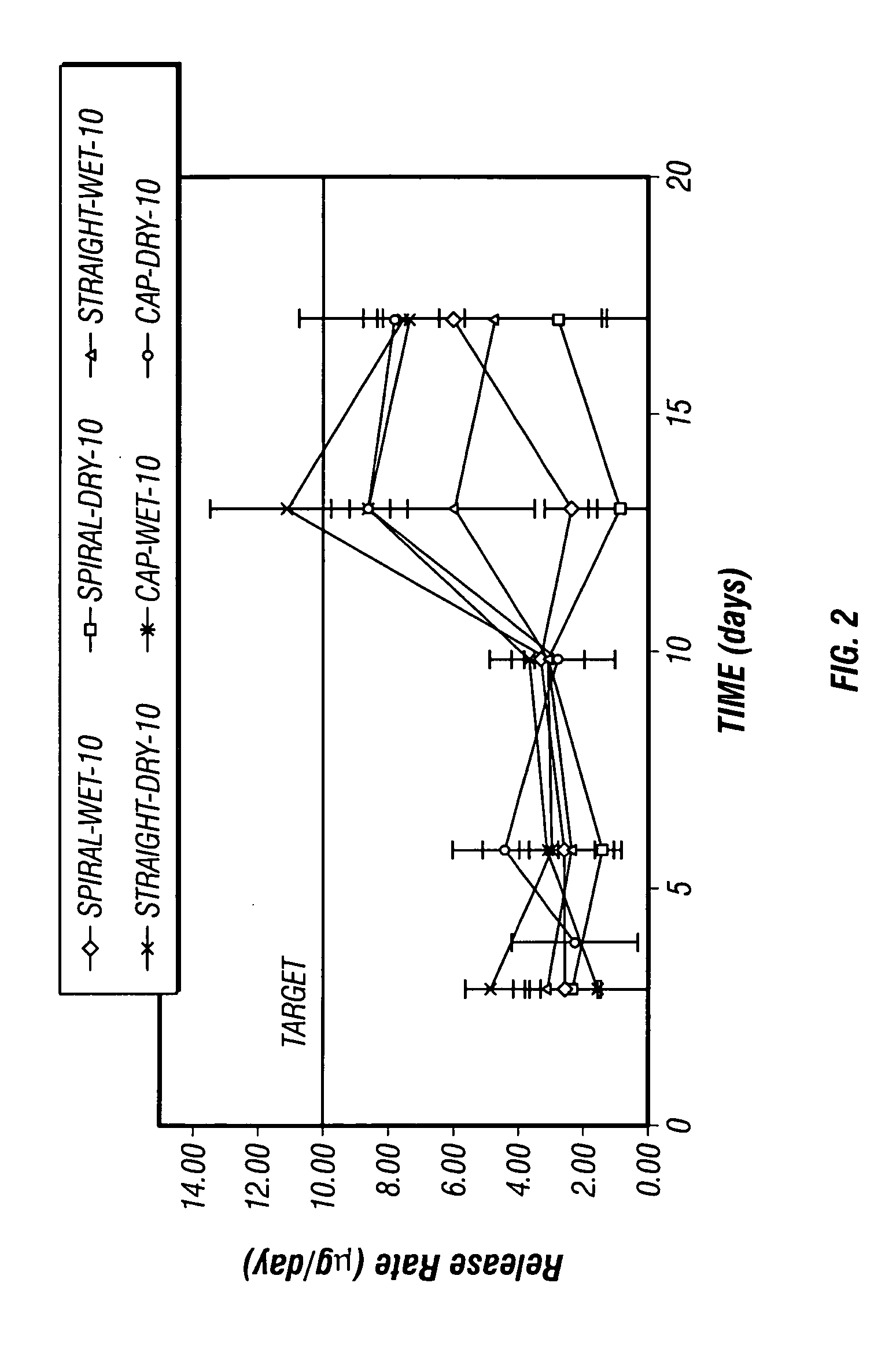
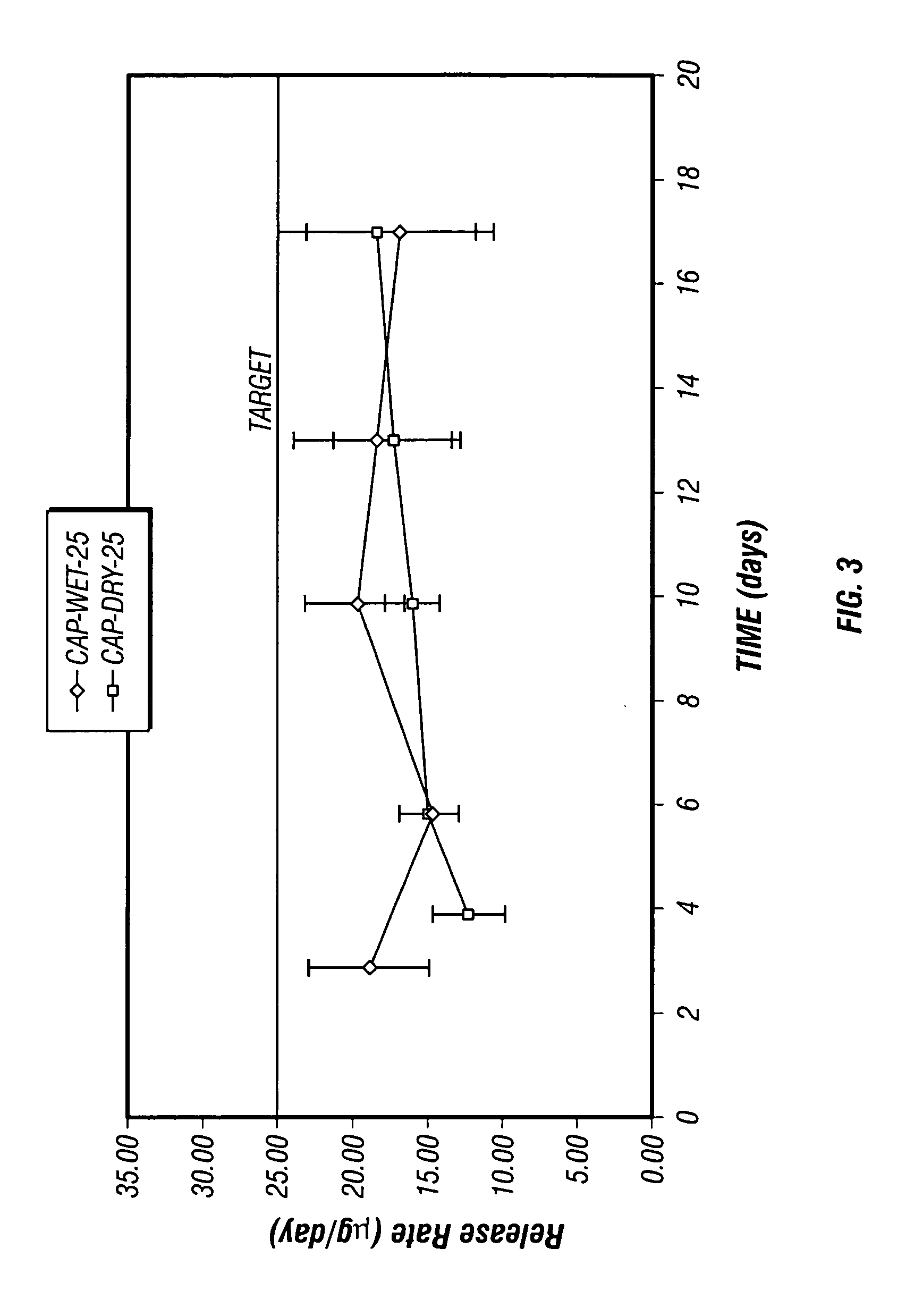
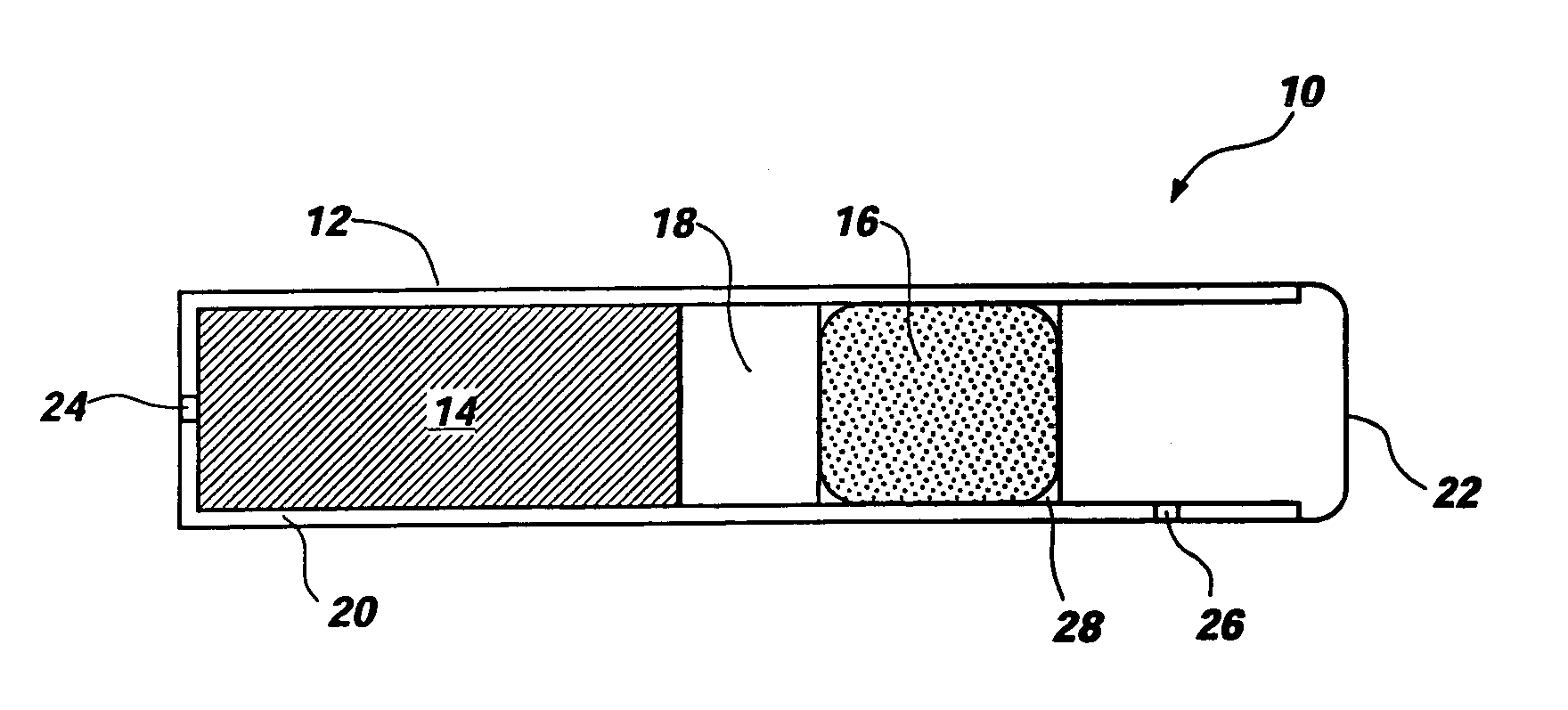
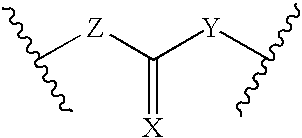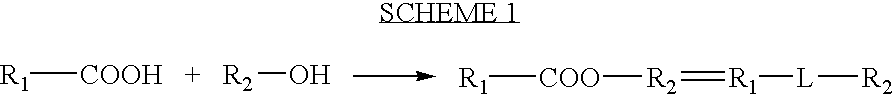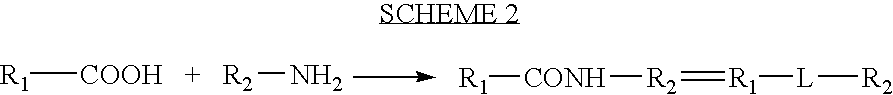Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1770results about "Osmotic delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
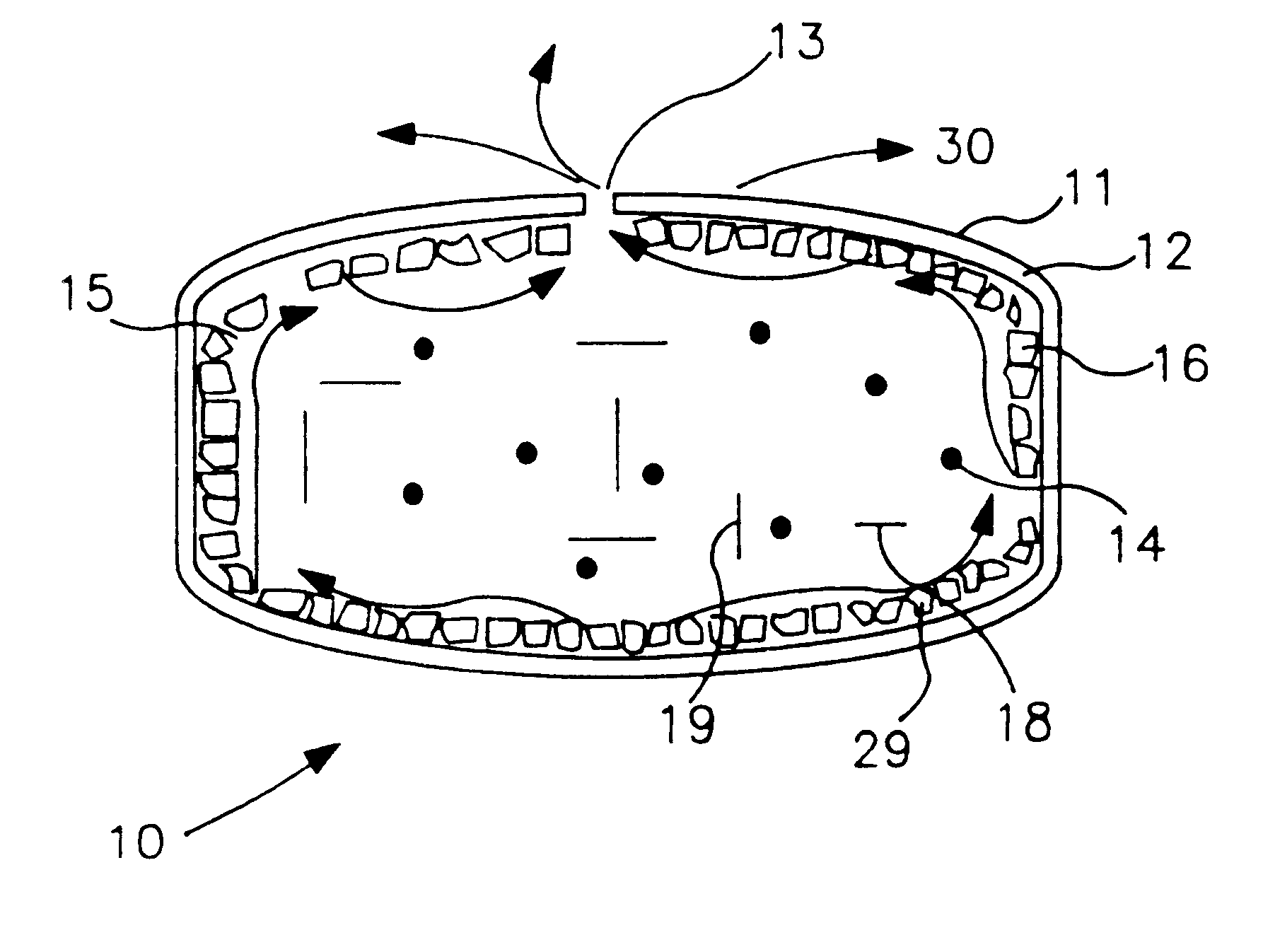
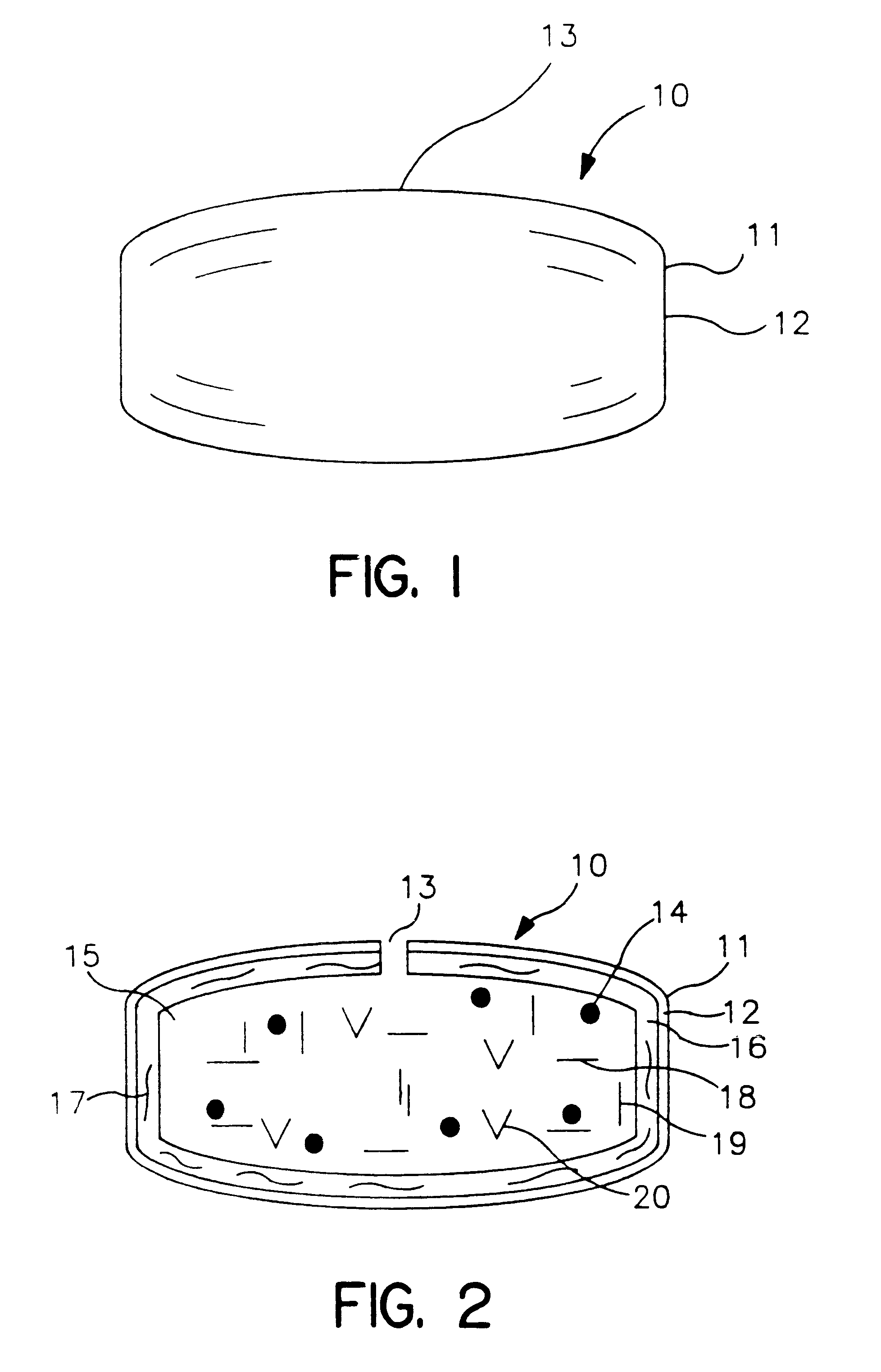
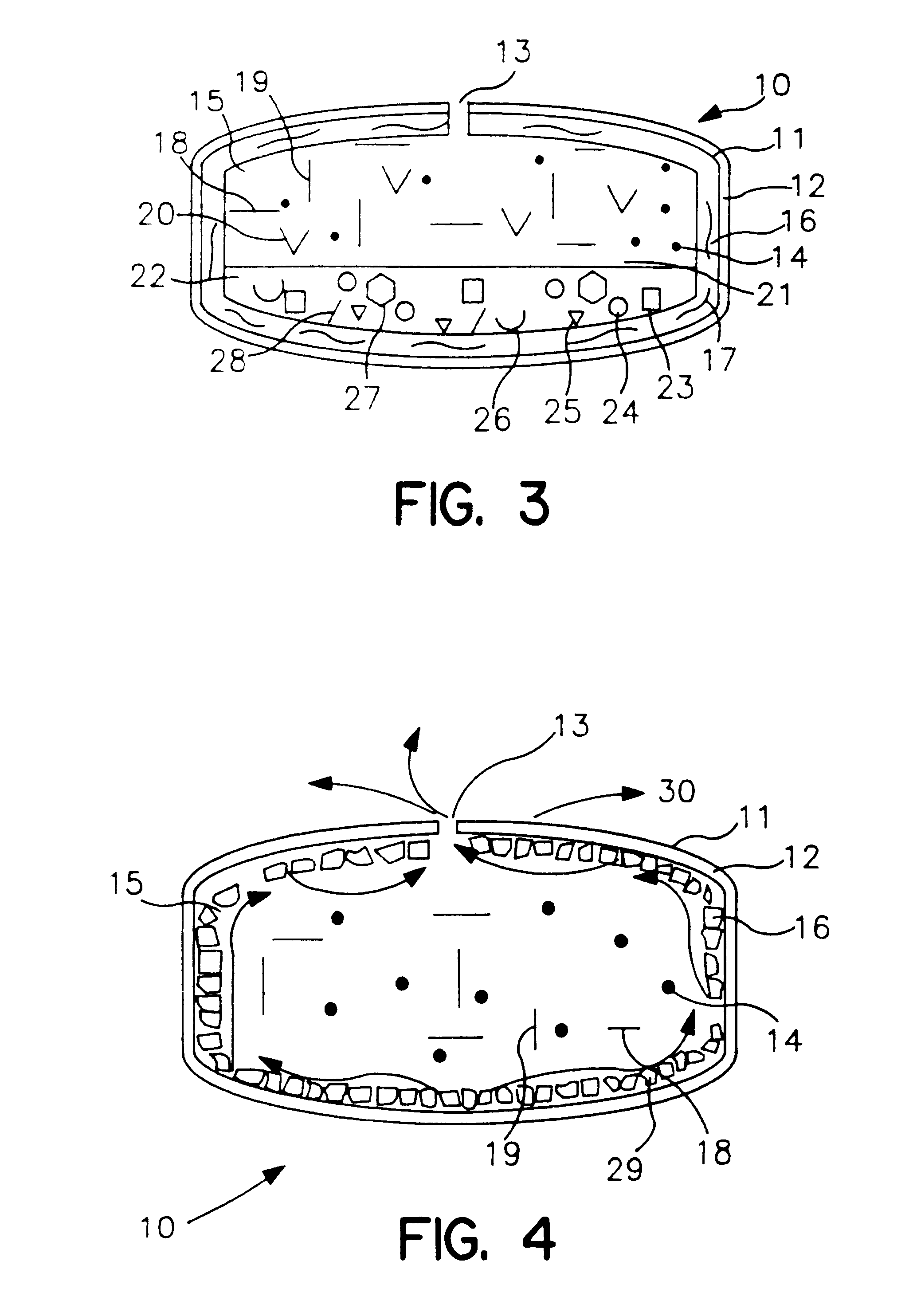
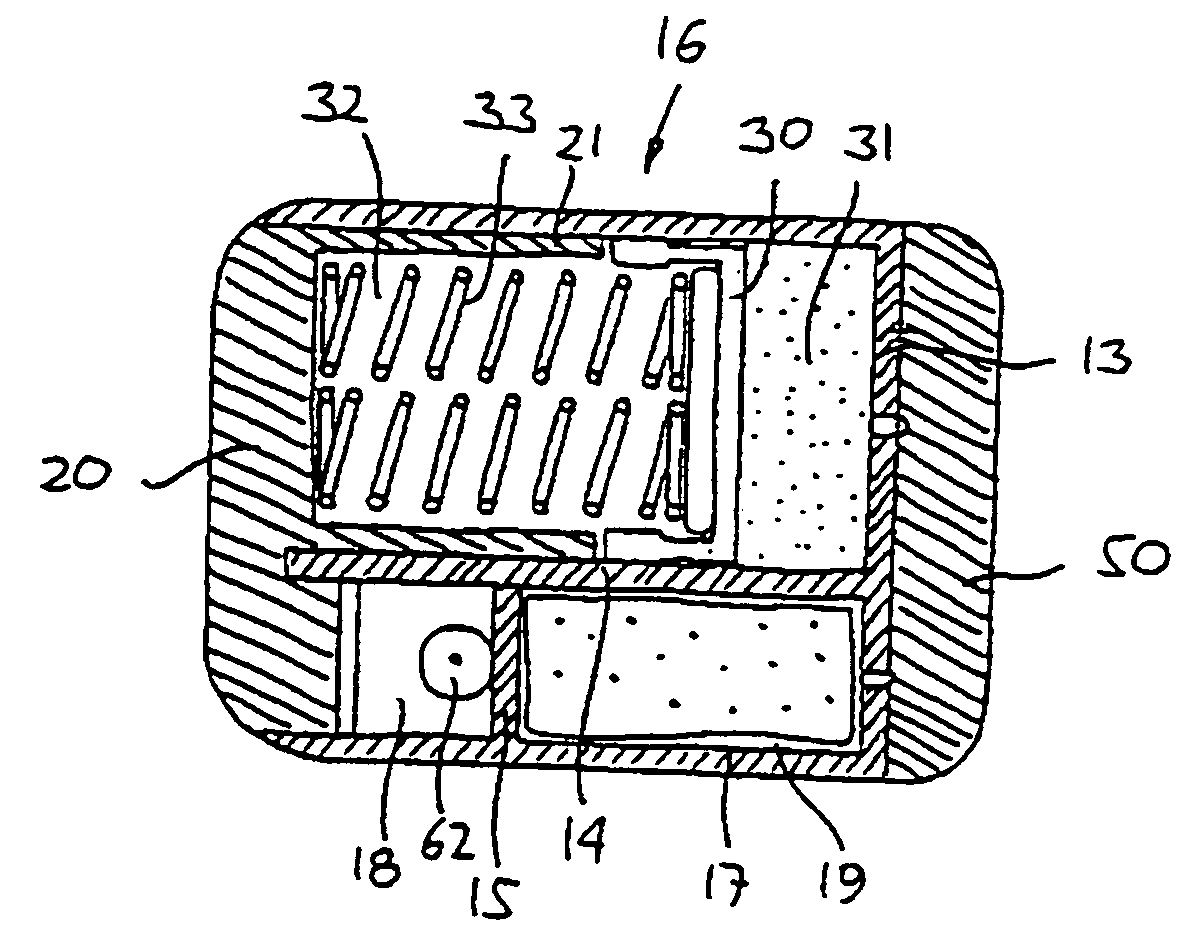
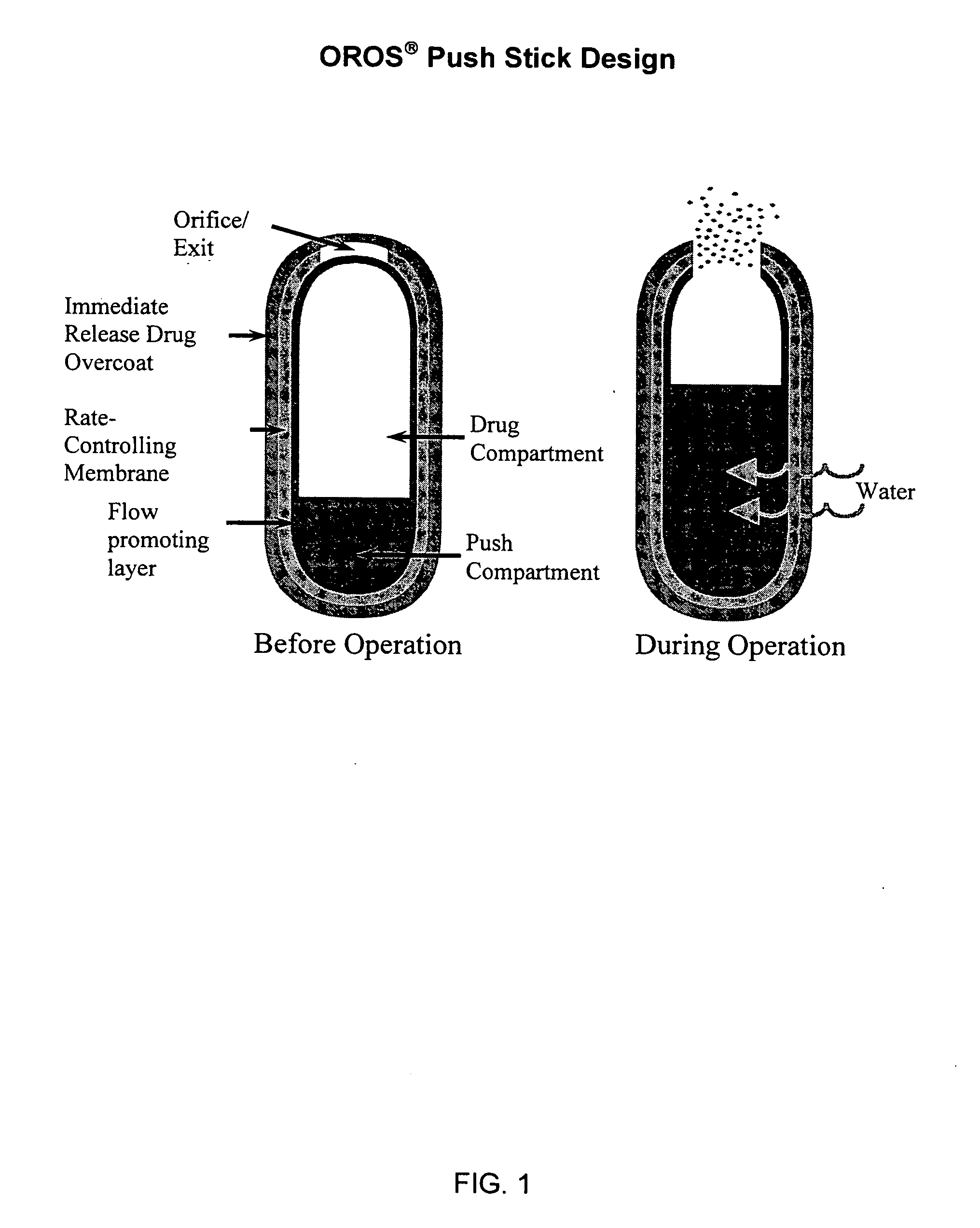
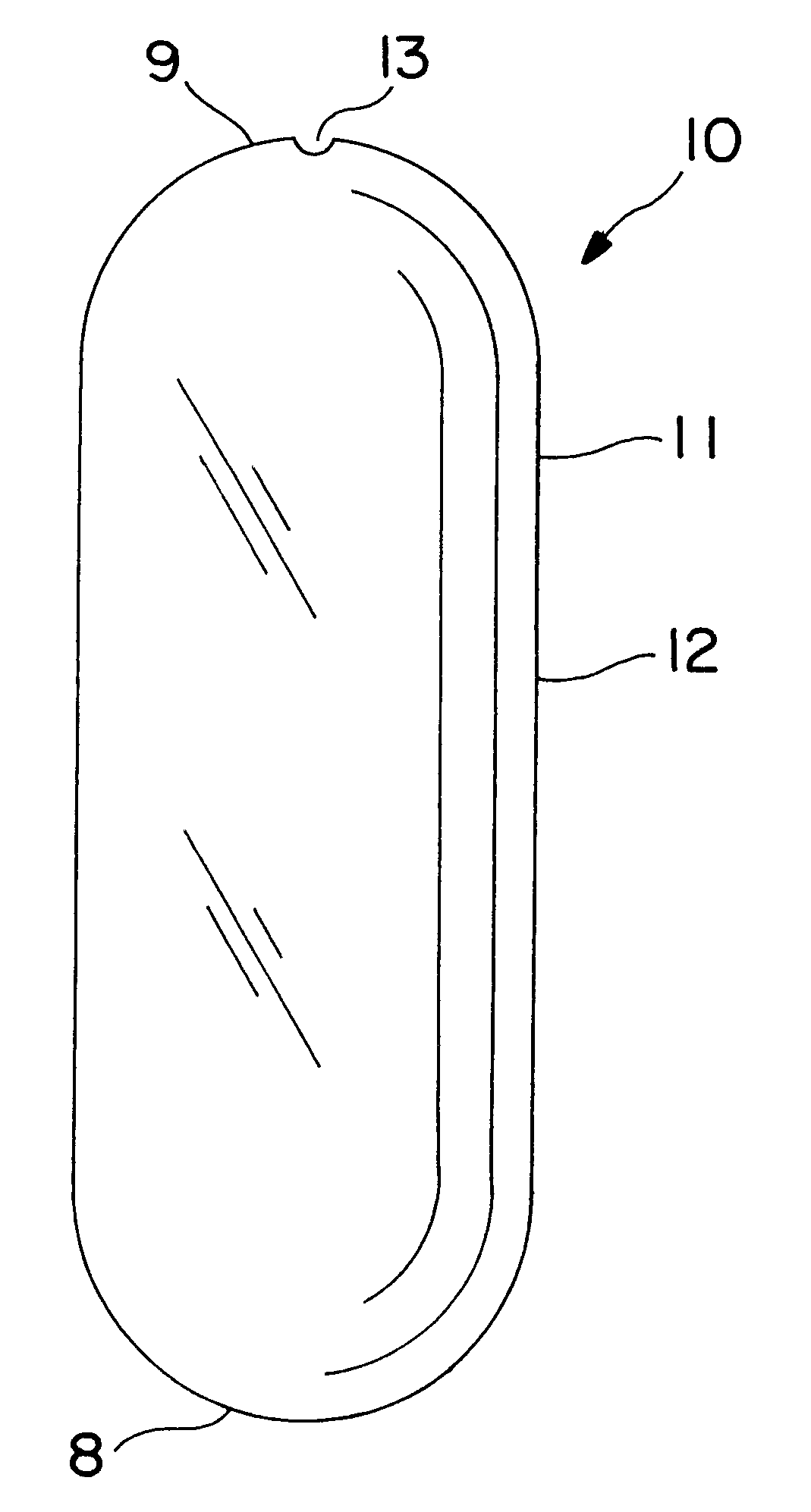
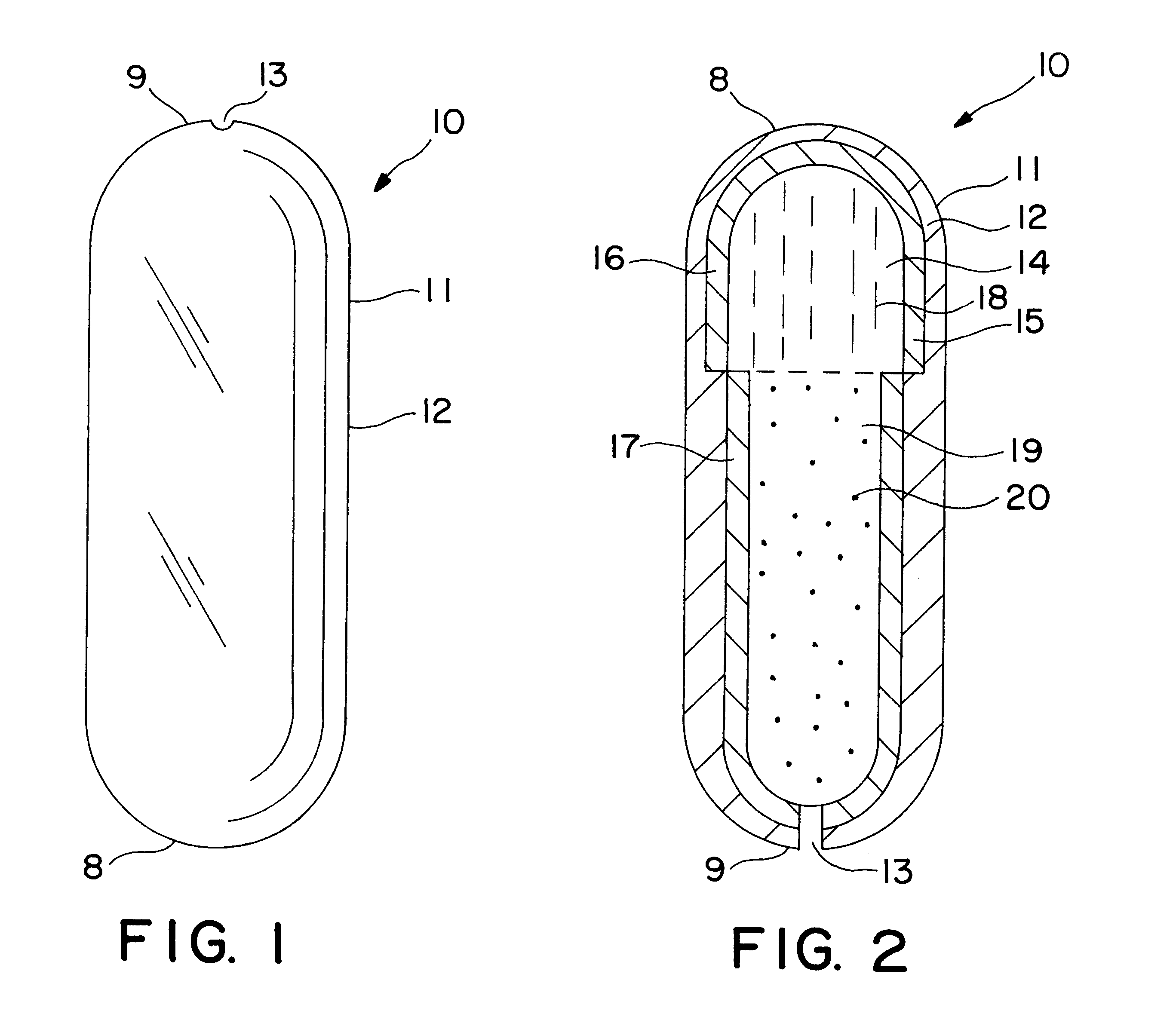
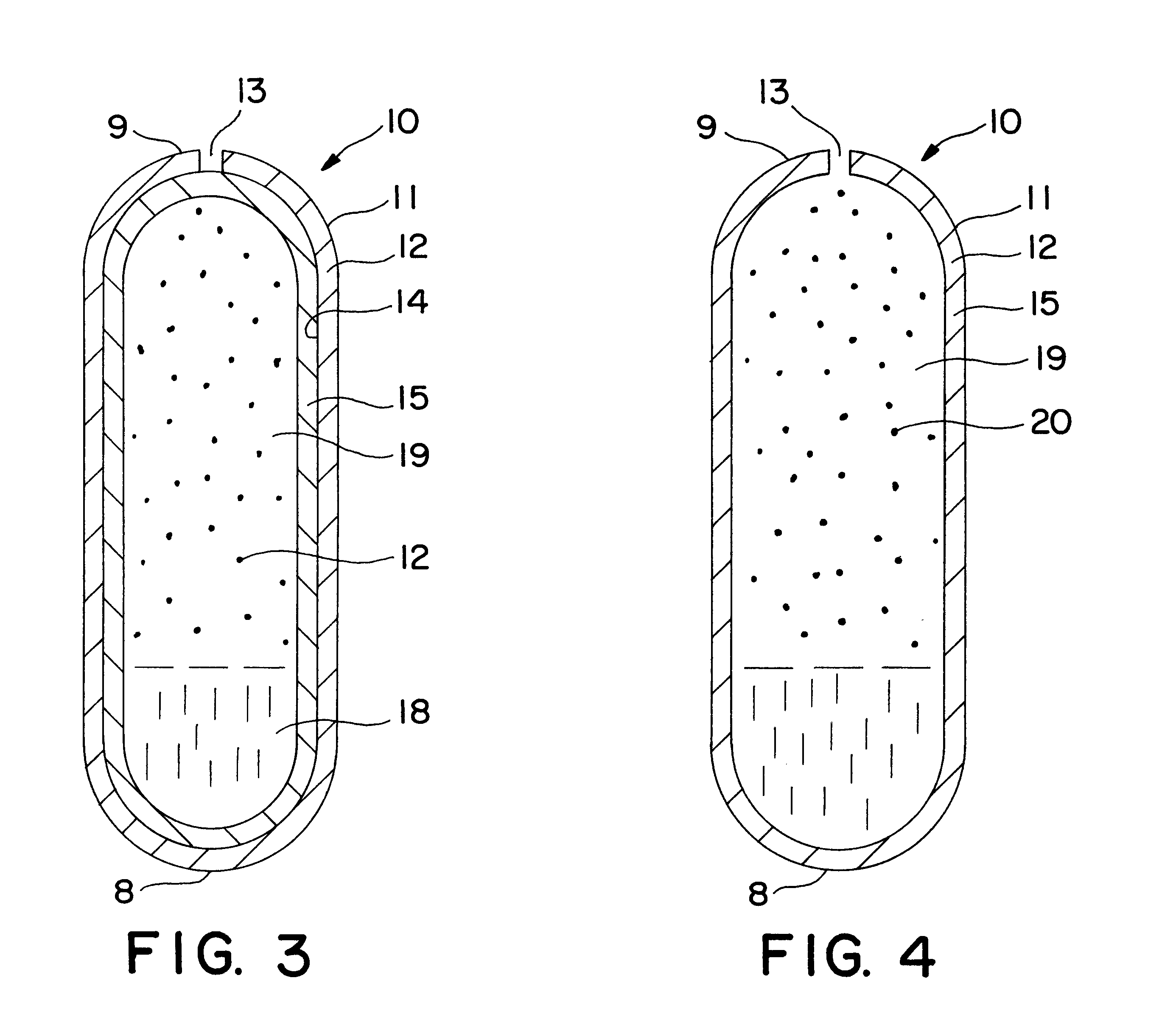
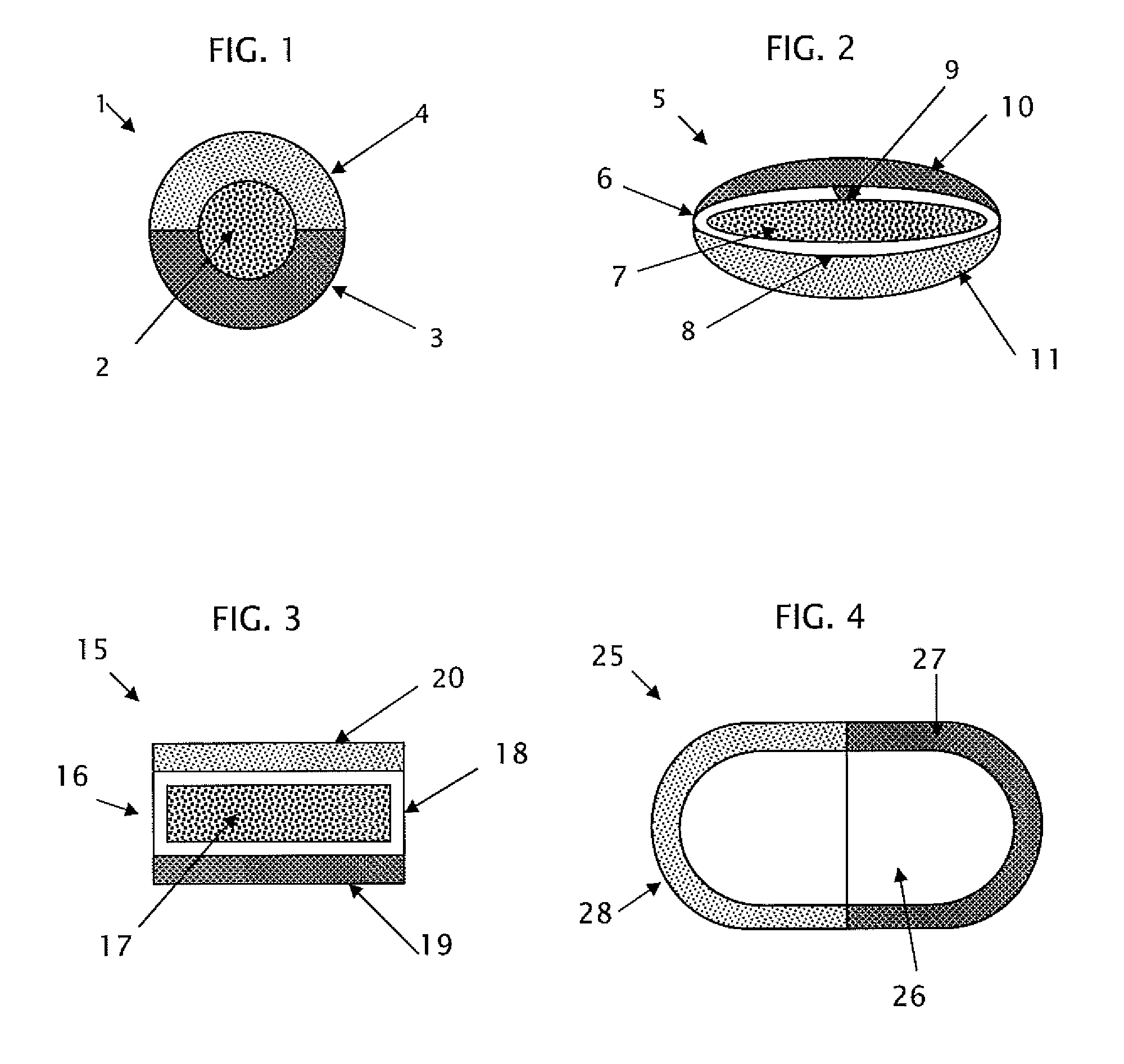
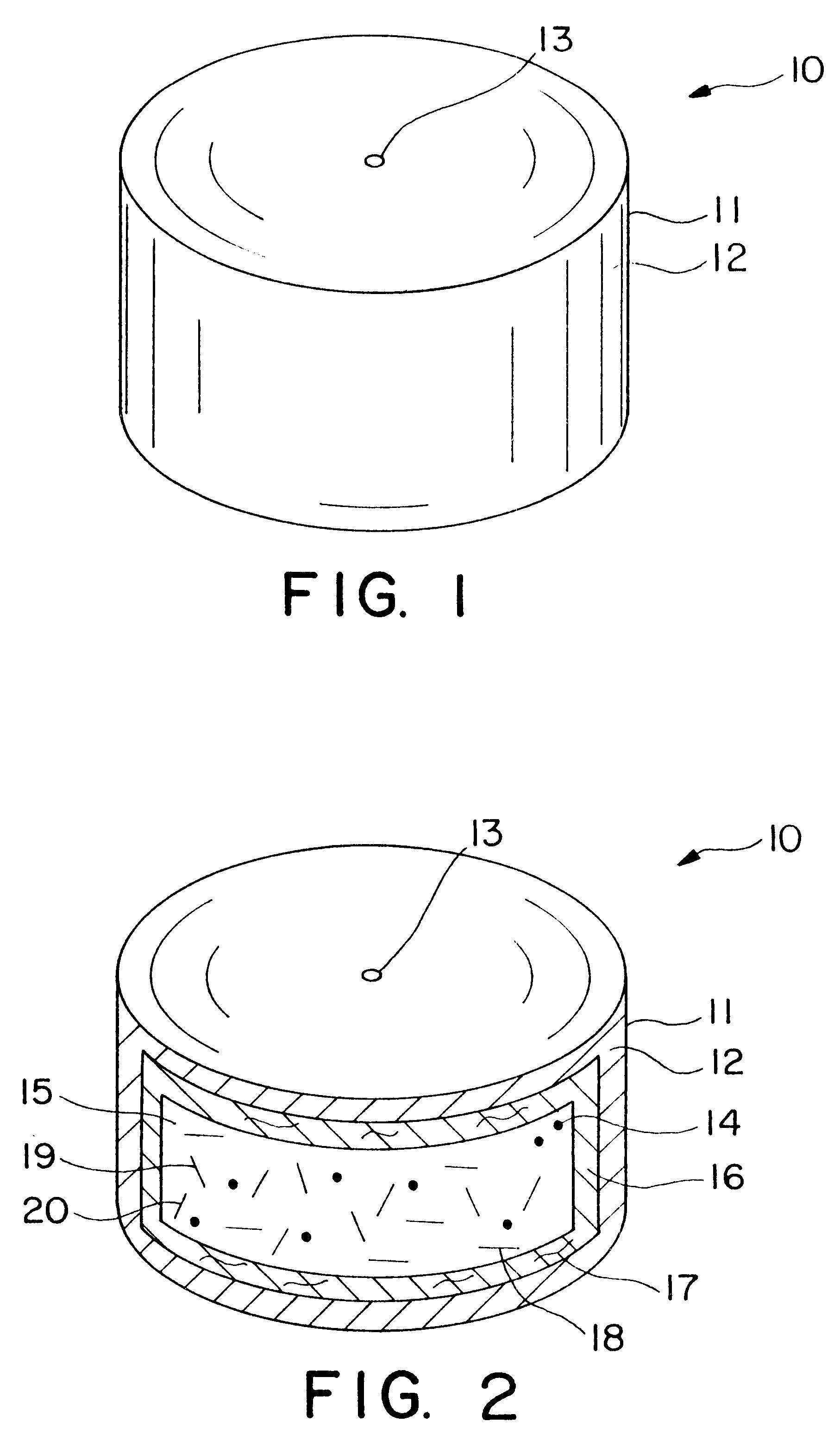
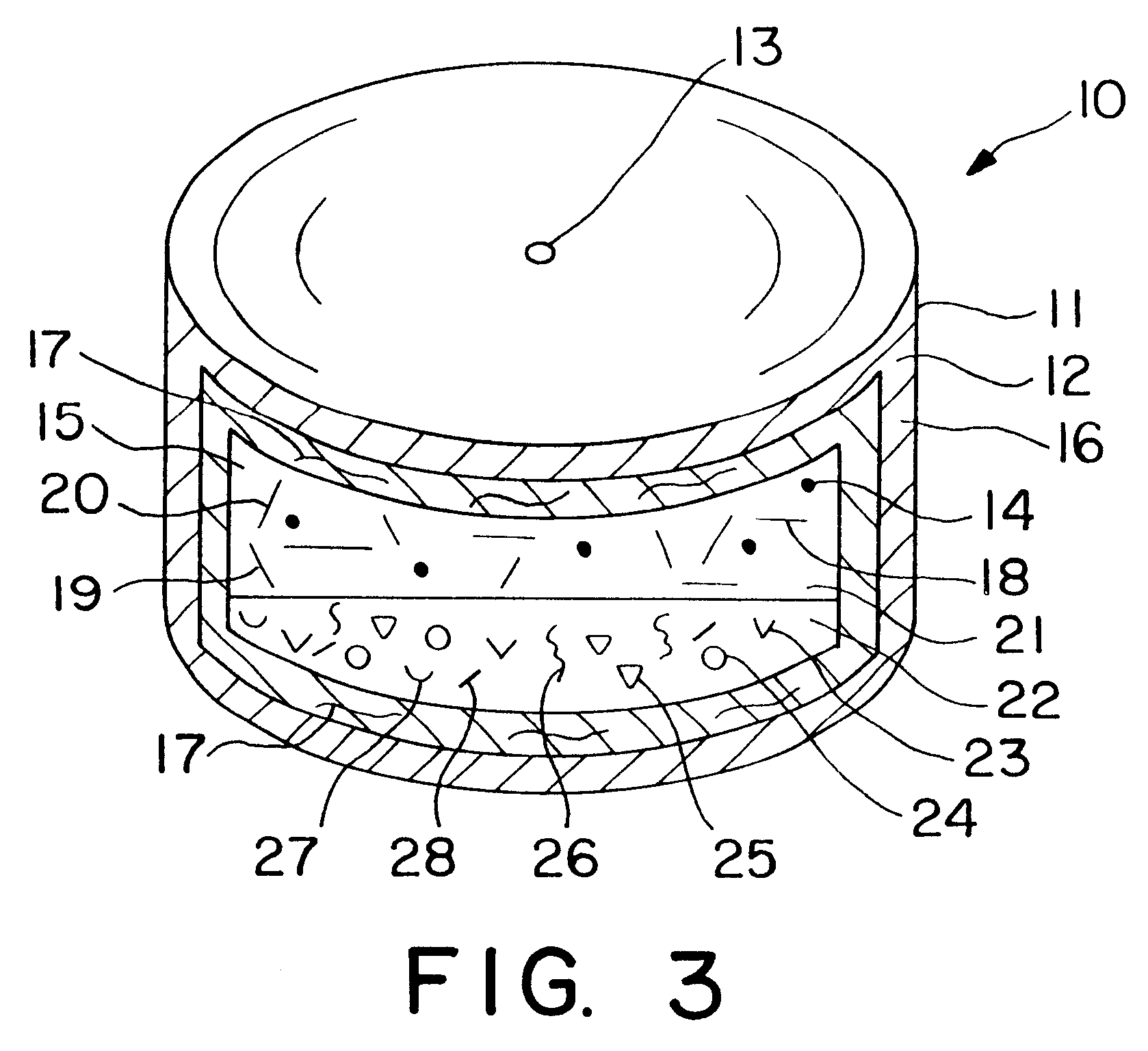
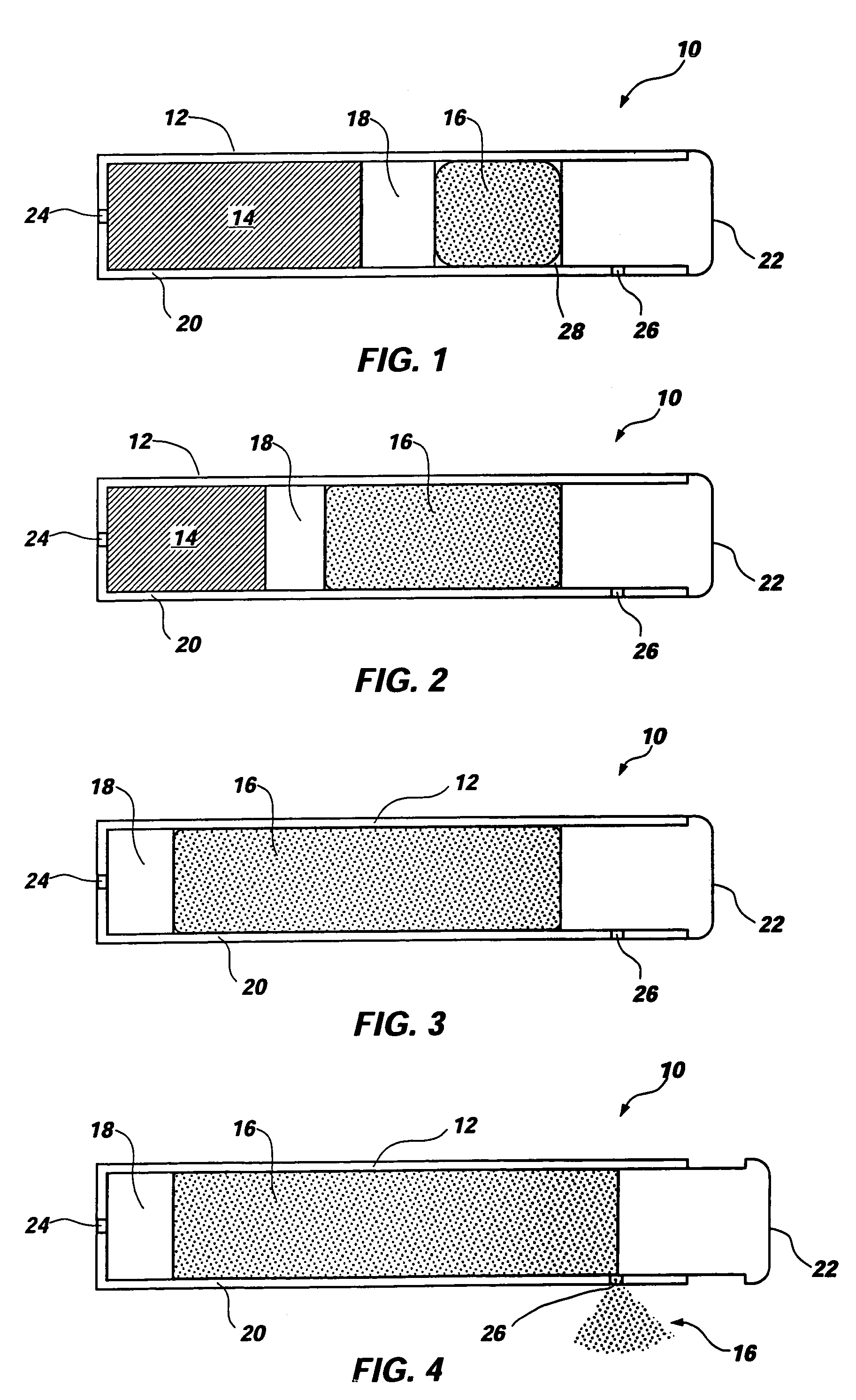
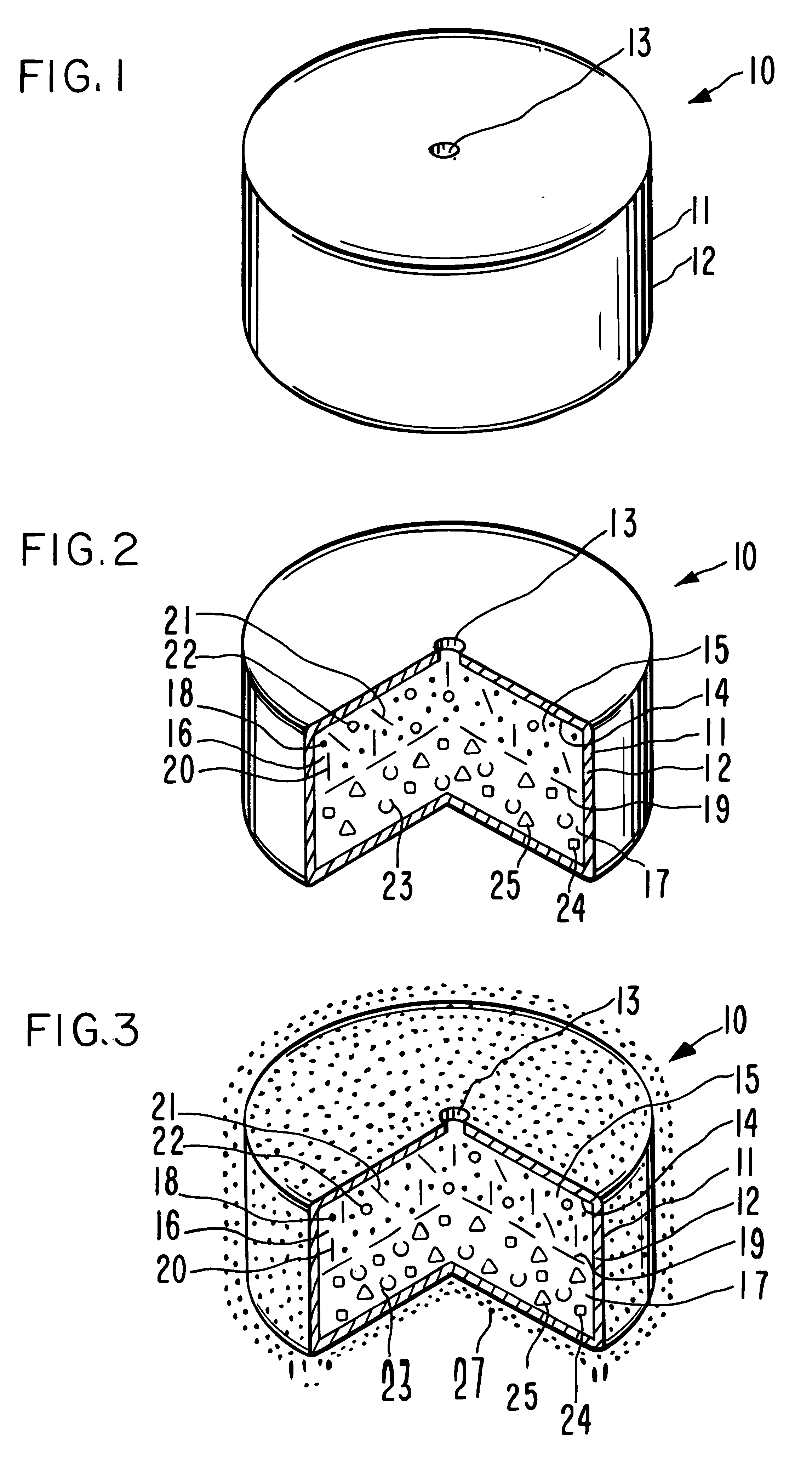
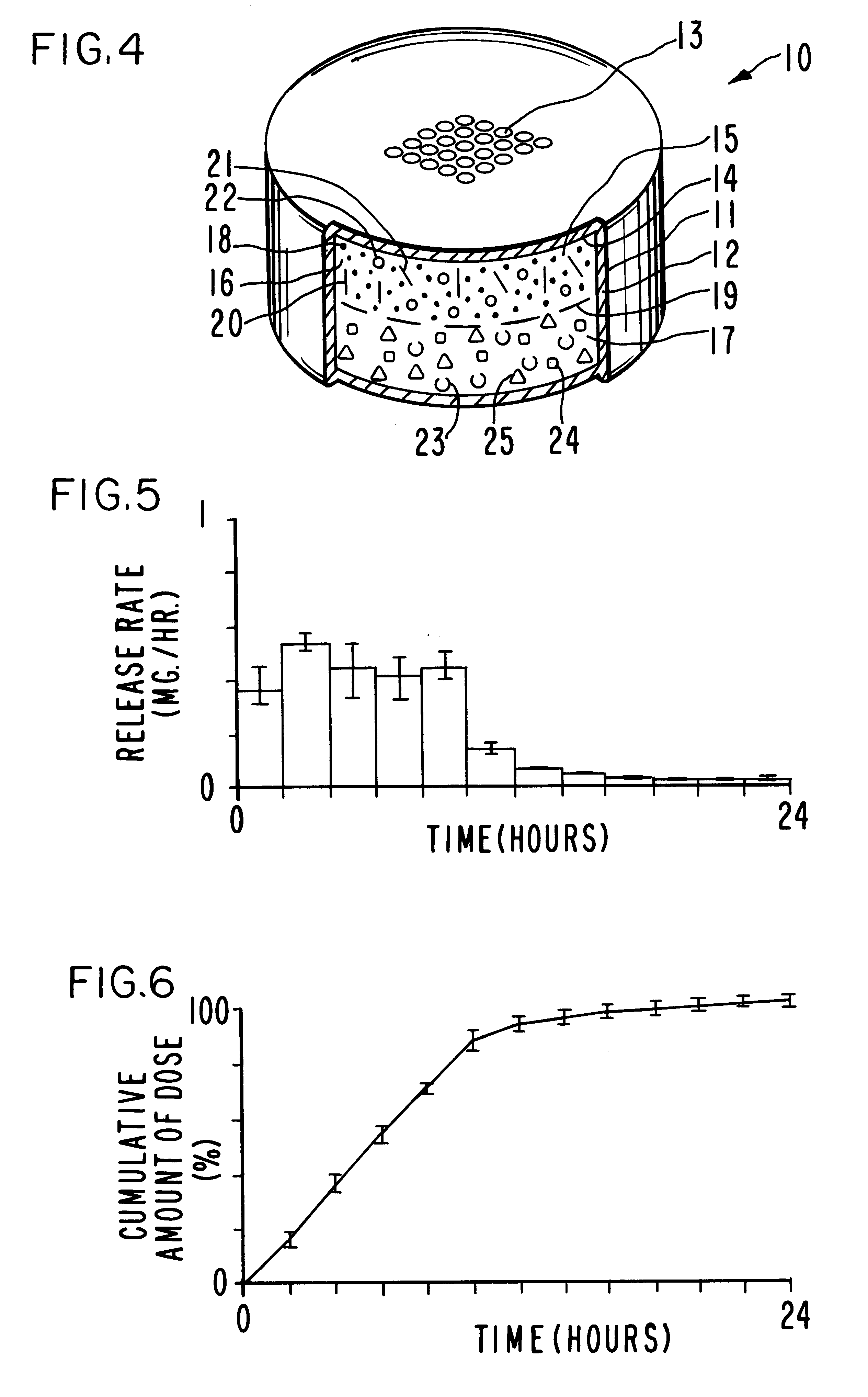
Extended release dosage form
InactiveUS6245357B1Free to changeOrganic active ingredientsNervous disorderExtended Release Dosage Form
A dosage form comprising a composition comprising a drug surrounded by an interior and an exterior wall with an exit for administering the drug to a patient; and a method of using the dosage form are disclosed for an indicated therapy.
Owner:ALZA CORP
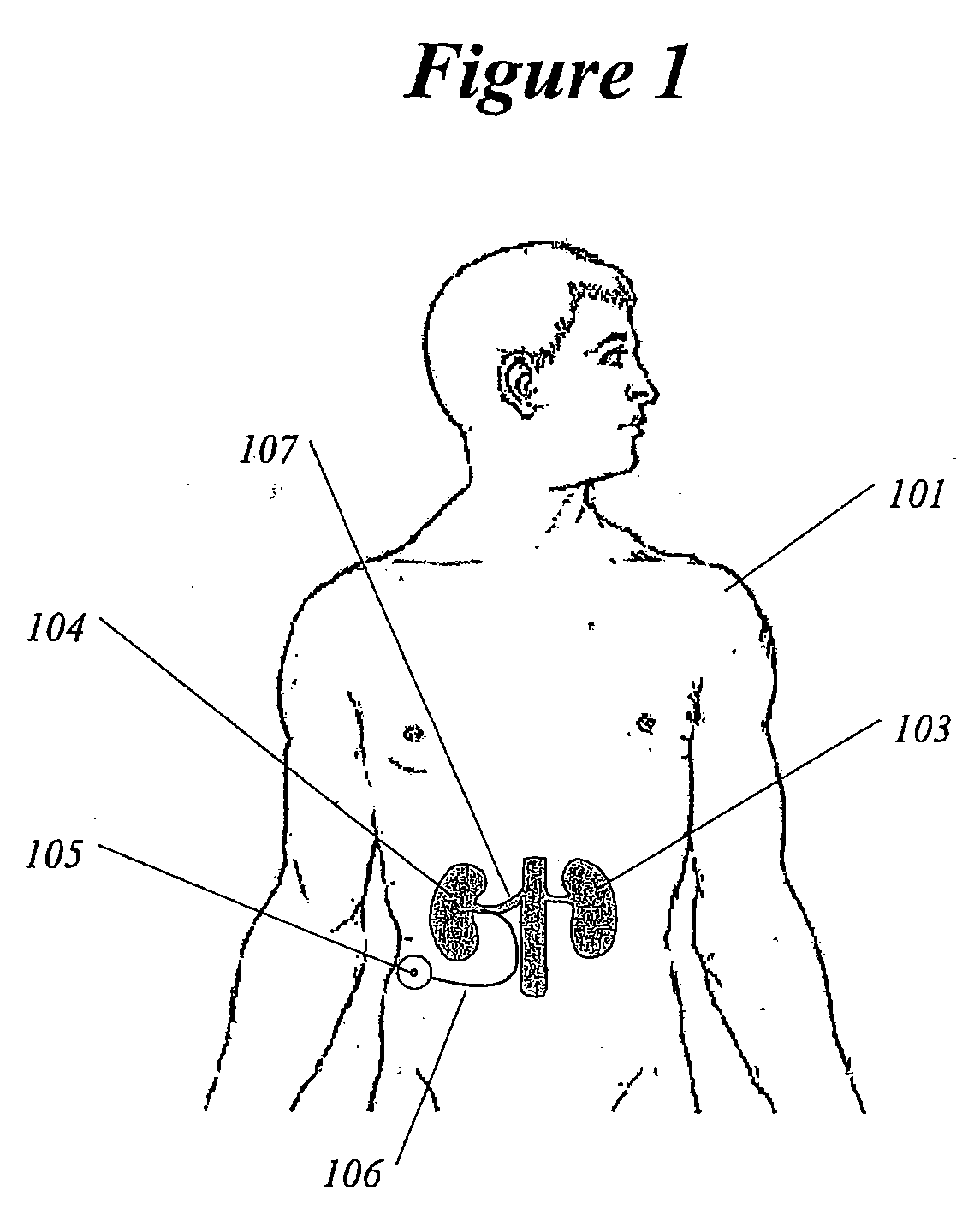
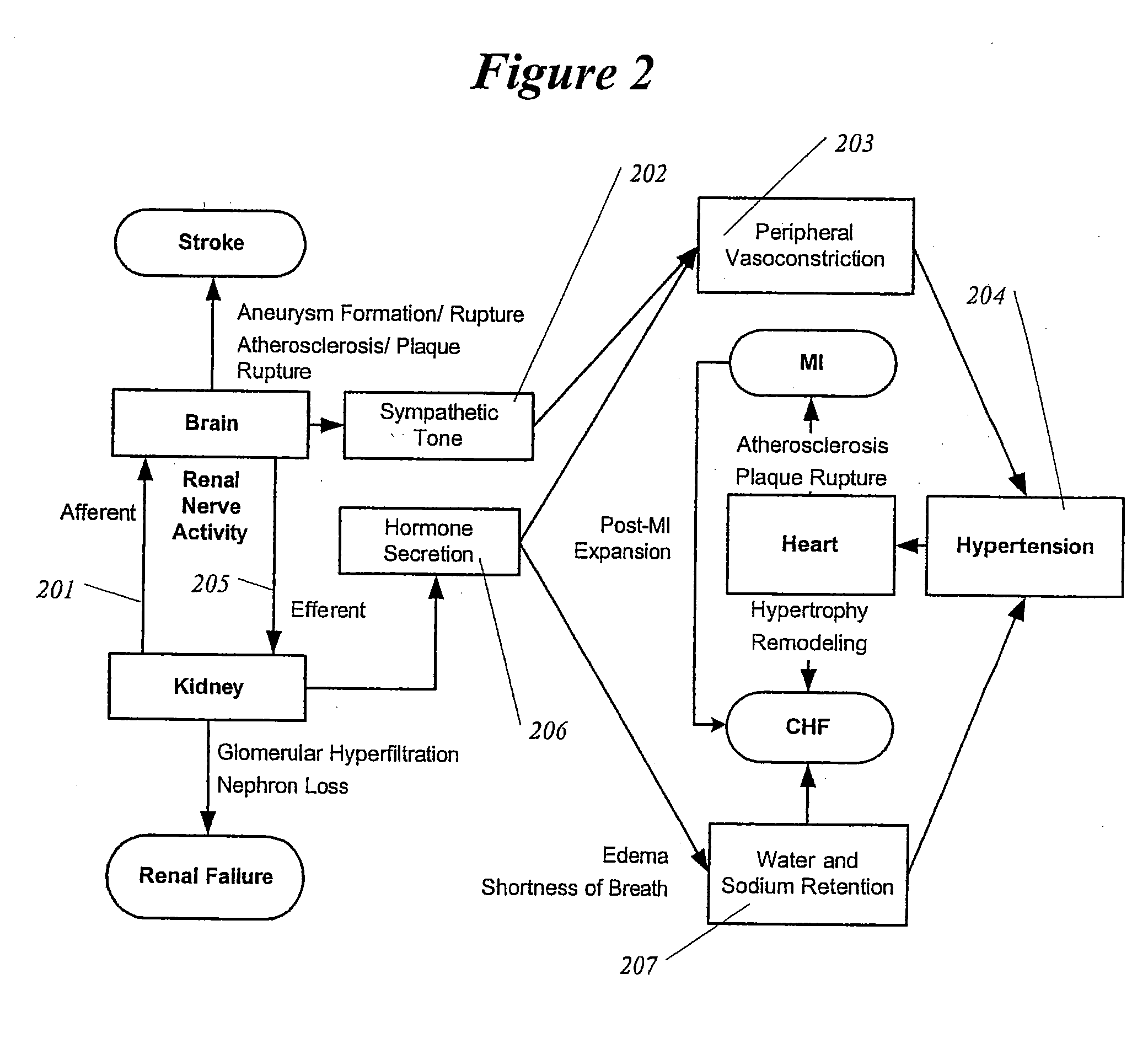
Methods and devices for renal nerve blocking
InactiveUS20080213331A1Shorten the progressResolution of overloadSpinal electrodesMedical devicesDiseaseRenal nerve
A method and apparatus for treatment of cardiac and renal diseases associated with the elevated sympathetic renal nerve activity by implanting a device to block the renal nerve signals to and from the kidney. The device can be a drug pump or a drug eluding implant for targeted delivery of a nerve-blocking agent to the periarterial space of the renal artery.
Owner:ARDIAN
Once-a-day, oral, controlled-release, oxycodone dosage forms
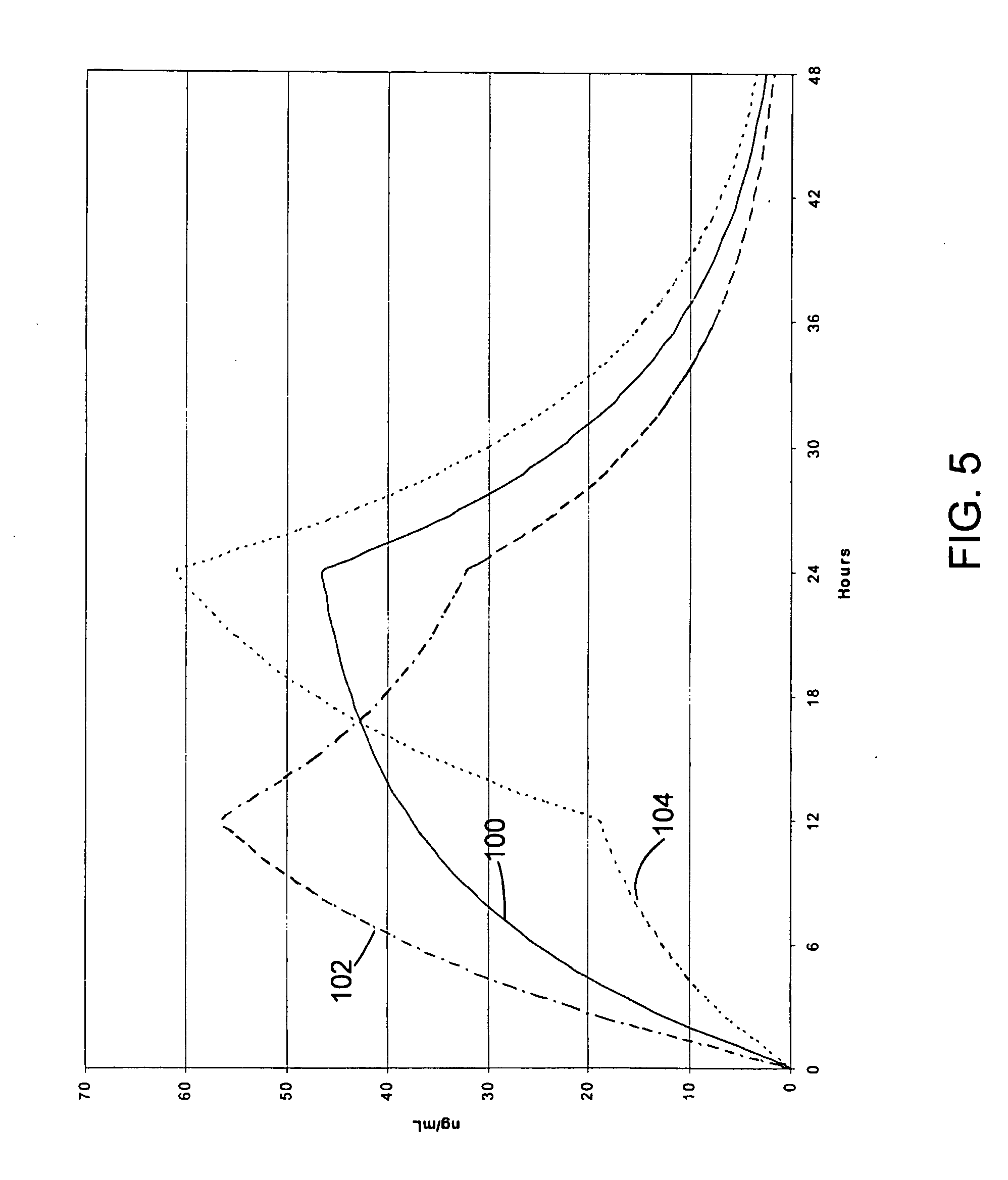
Oxycodone formulations are provided which produce substantially flat in vivo steady state plasma profiles. Tolerance levels associated with such profiles and tolerance levels associated with biphasic profiles are shown not to be statistically different. The substantially flat in vivo steady state plasma profiles are produced by dosage forms having substantially zero order in vitro release profiles. Such release profiles produce low single dose in vivo Cmax levels which can reduce the probability of adverse side effects.
Owner:ALZA CORP
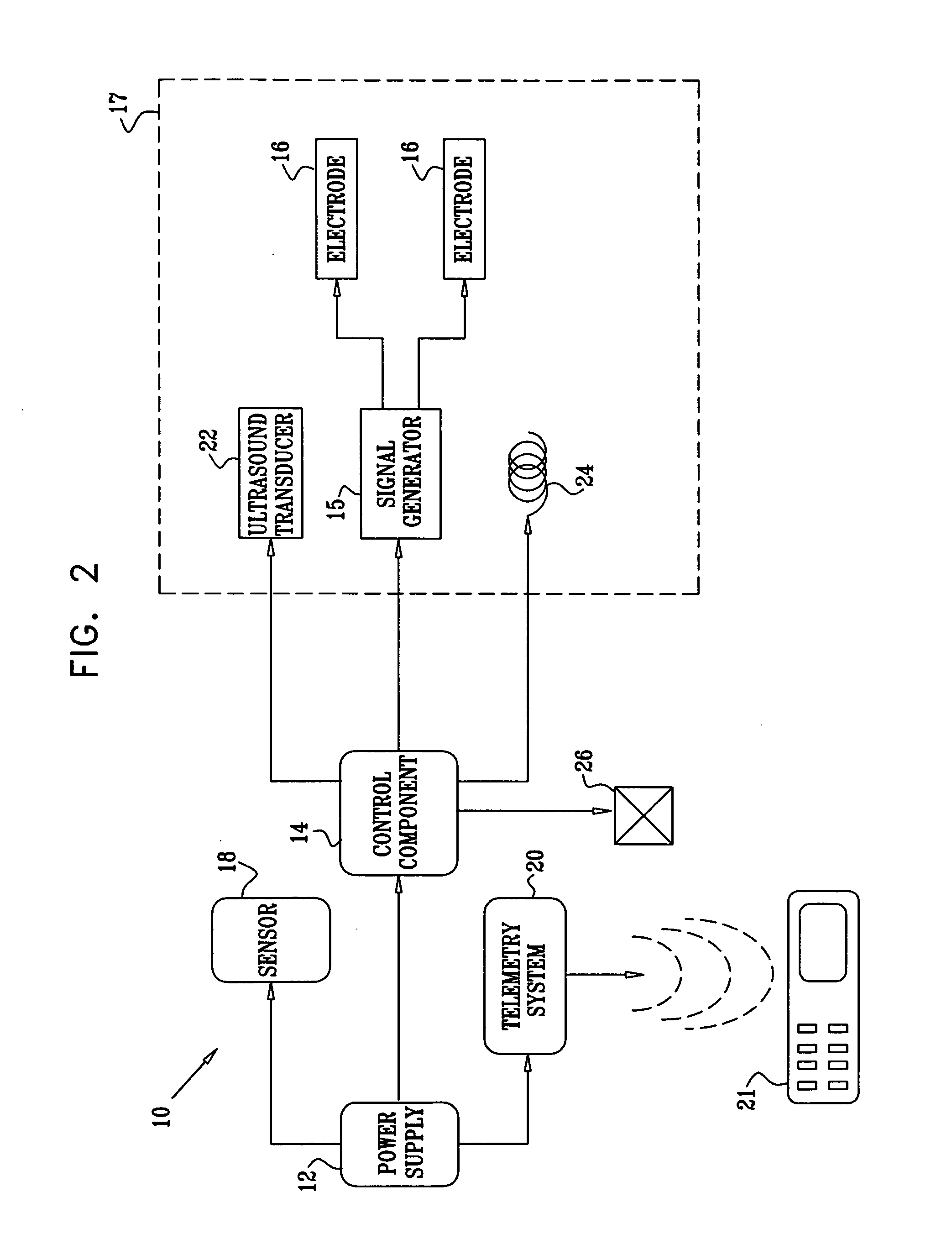
Active drug delivery in the gastrointestinal tract
InactiveUS20050058701A1Easy accessPromote absorptionInternal electrodesBody temperature measurementMedicineDrug administration
Apparatus for drug administration is provided, including an ingestible capsule, which includes a drug, stored by the capsule, and an environmentally-sensitive mechanism, adapted to change a state thereof responsively to a disposition of the capsule within a gastrointestinal (GI) tract of a subject. The capsule further includes first and second electrodes, and a control component, adapted to facilitate passage of the drug, in response to a change of state of the environmentally-sensitive mechanism, through an epithelial layer of the GI tract by driving the first and second electrodes to apply a series of pulses at a current of less than about 5 mA, at a frequency of between about 12 Hz and about 24 Hz, and with a pulse duration of between about 0.5 milliseconds and about 3 milliseconds.
Owner:E PILL PHARMA
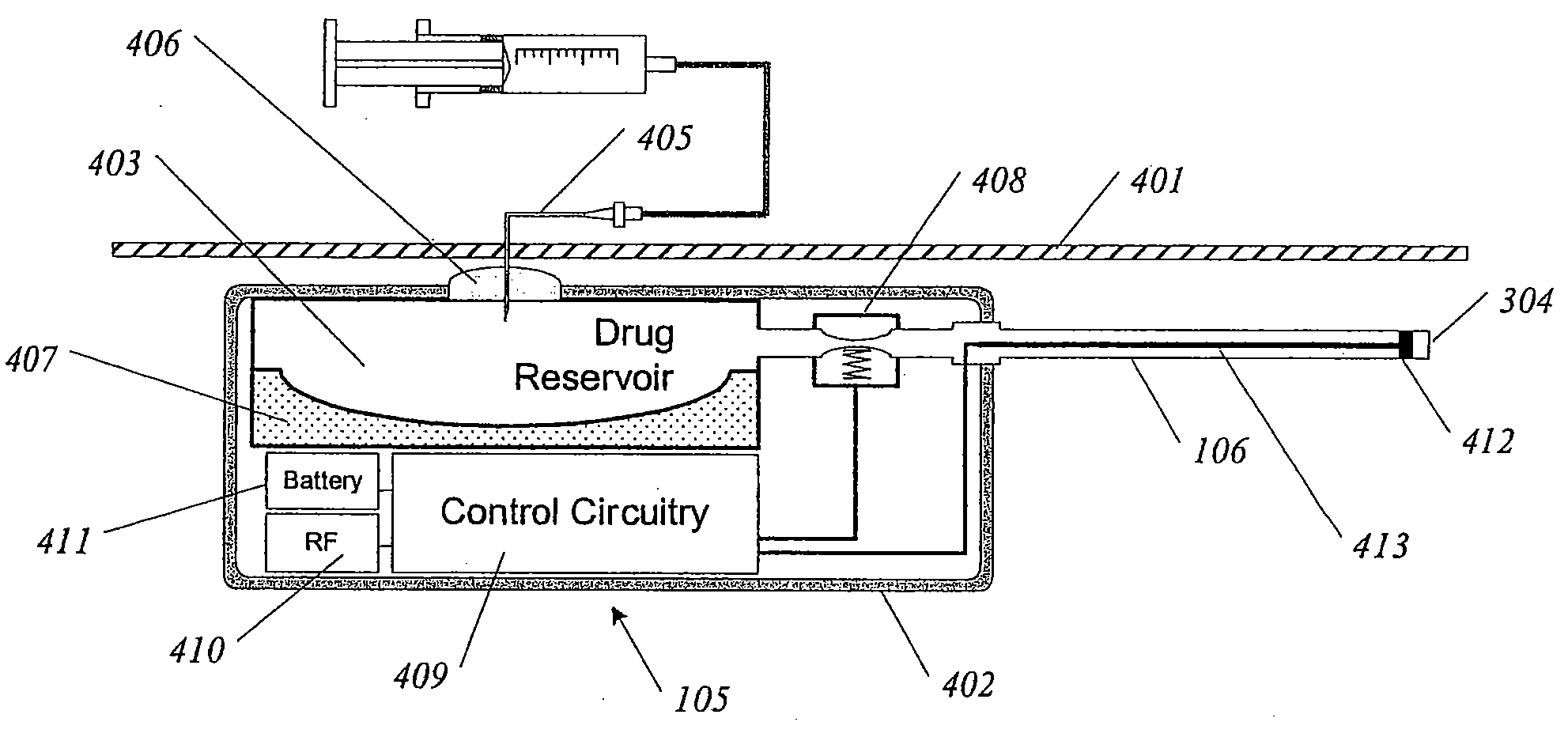
Delivery device
InactiveUS20060069382A1Safe and easy identificationImprove securityInfusion syringesMedical devicesDrug reservoirEngineering
This invention relates to a delivery device of the bleeding hole type, where a primary drive fluid, e.g. silicon oil, is used to expel a secondary fluid, e.g. a drug, contained in a reservoir. To provide a desired drug flow rate, the primary fluid is forced from a first reservoir through a flow restrictor into a second reservoir displacing a portion of the drug reservoir, thereby expelling the drug from its reservoir. The idea is to provide a drive fluid outlet, i.e. a flow restrictor inlet, which protrudes into the first reservoir. By this arrangement the amount of particles and air-bubbles entering the narrow flow restrictor will be reduced. The reduction is achieved because particles and air-bubbles will normally concentrate in the top or bottom of the reservoir, whereas the protrusion will primarily connect to the centre of the first reservoir.
Owner:NOVO NORDISK AS
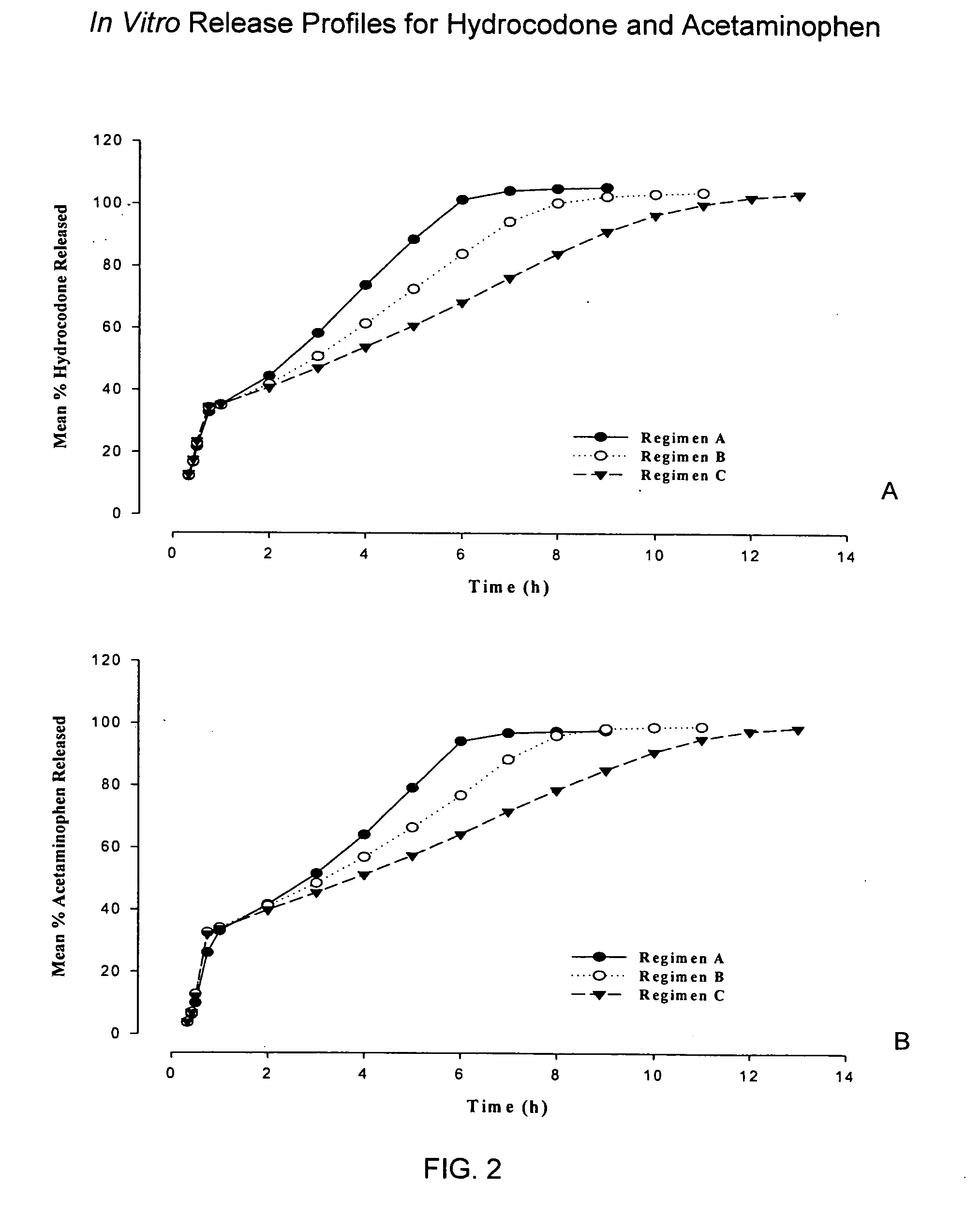
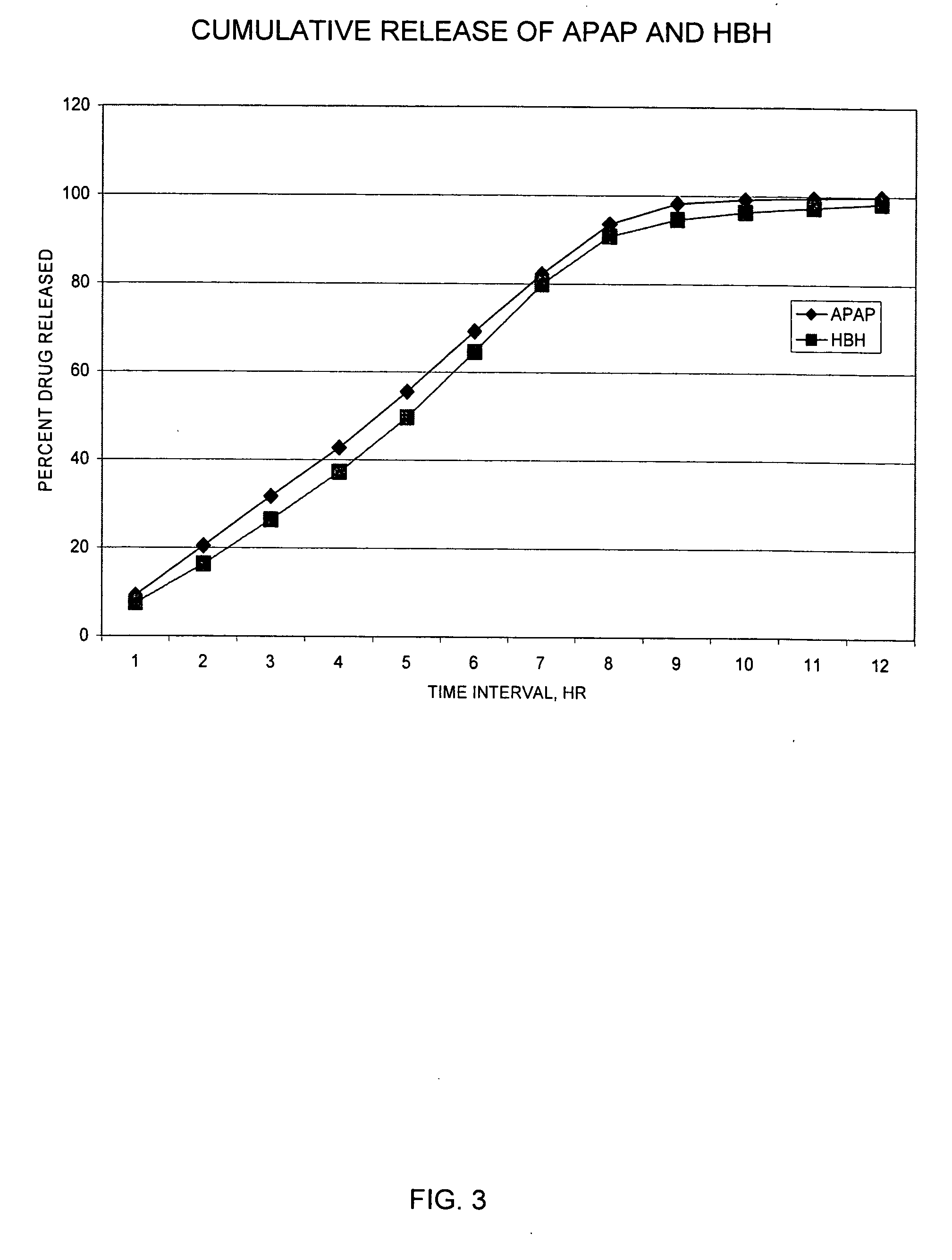
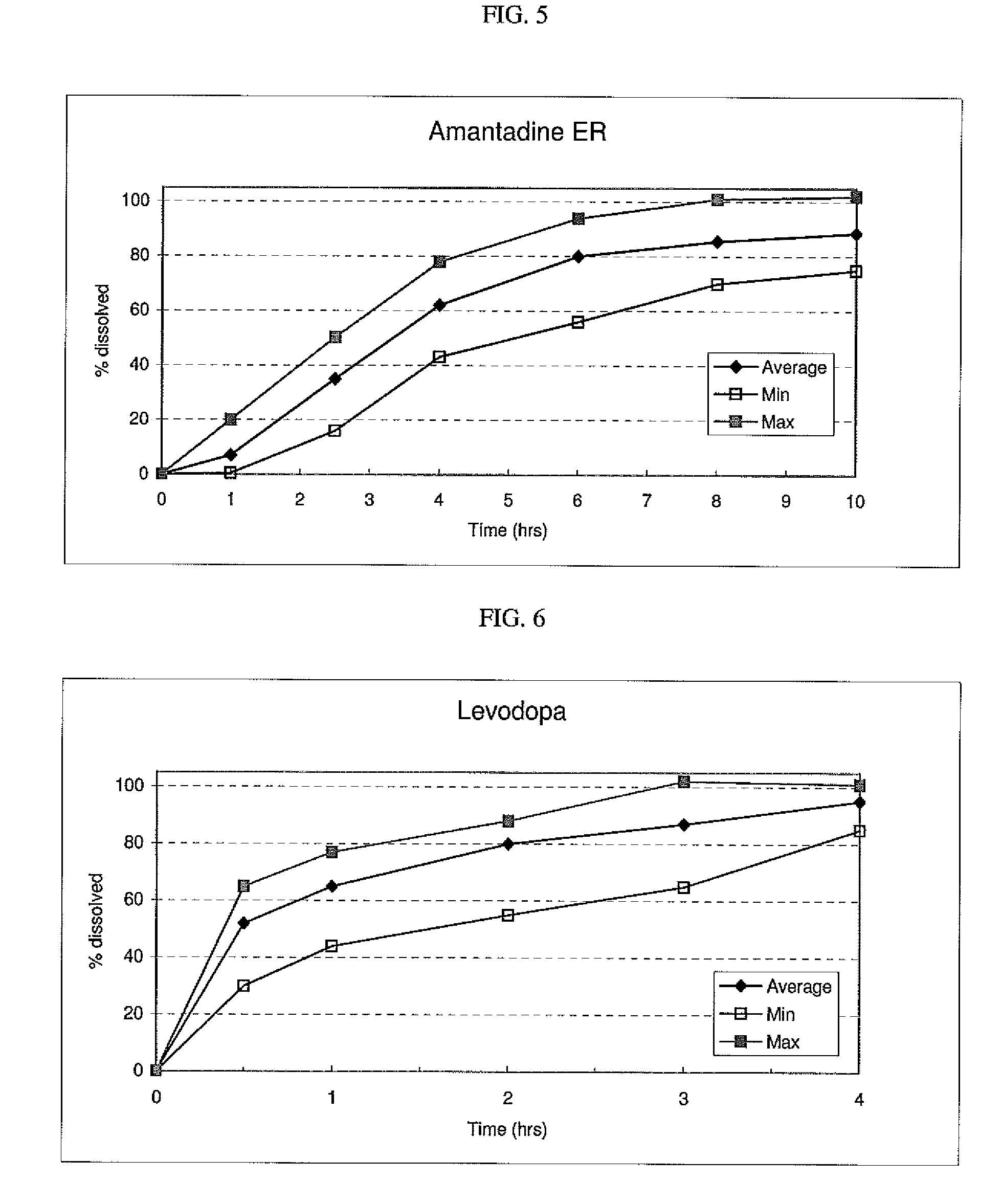
Controlled release formulations of opioid and nonopioid analgesics
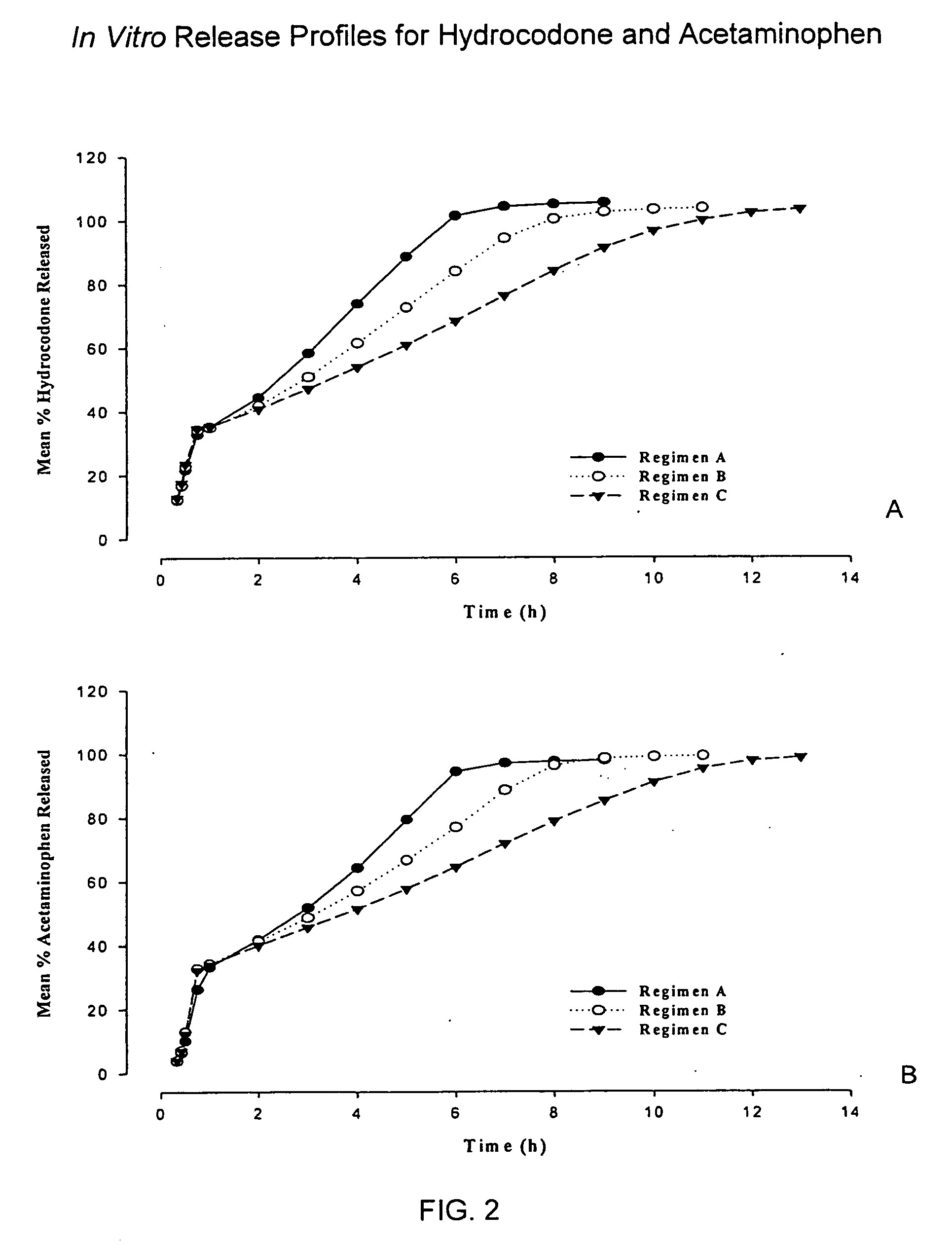
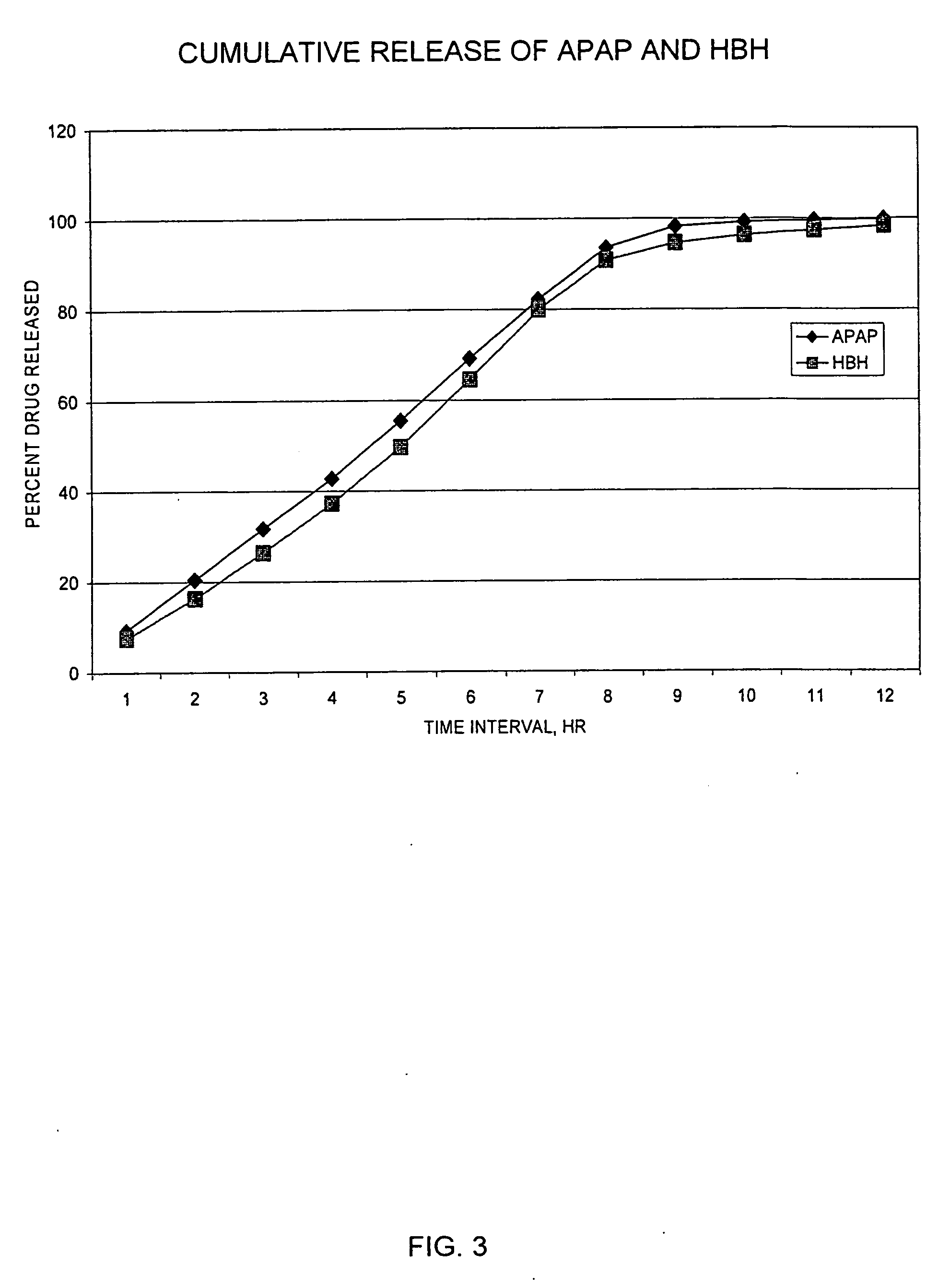
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Dosage form comprising liquid formulation
InactiveUS6174547B1Improve oral bioavailabilityImprove bioavailabilityCapsule deliveryEmulsion deliveryPharmaceutical formulationDosage form
Owner:ENCINAL PHARMA INVESTMENTS
Abuse-safeguarded dosage form
A pharmaceutical dosage form that is safeguarded against abuse containing at least one active substance that is susceptible to abuse and at least two of the following constituents (a) through (d): (a) at least one substance that irritates the nasal and / or pharyngeal region; (b) at least one viscosity increasing agent that together with a required minimum quantity of an aqueous liquid forms a gel in an extract obtained from the dosage form, which gel can still be discerned after being introduced into an additional quantity of aqueous liquid; (c) at least one antagonist for the at least one active substance that is susceptible to abuse; and (d) at least one emetic.
Owner:GRUNENTHAL GMBH
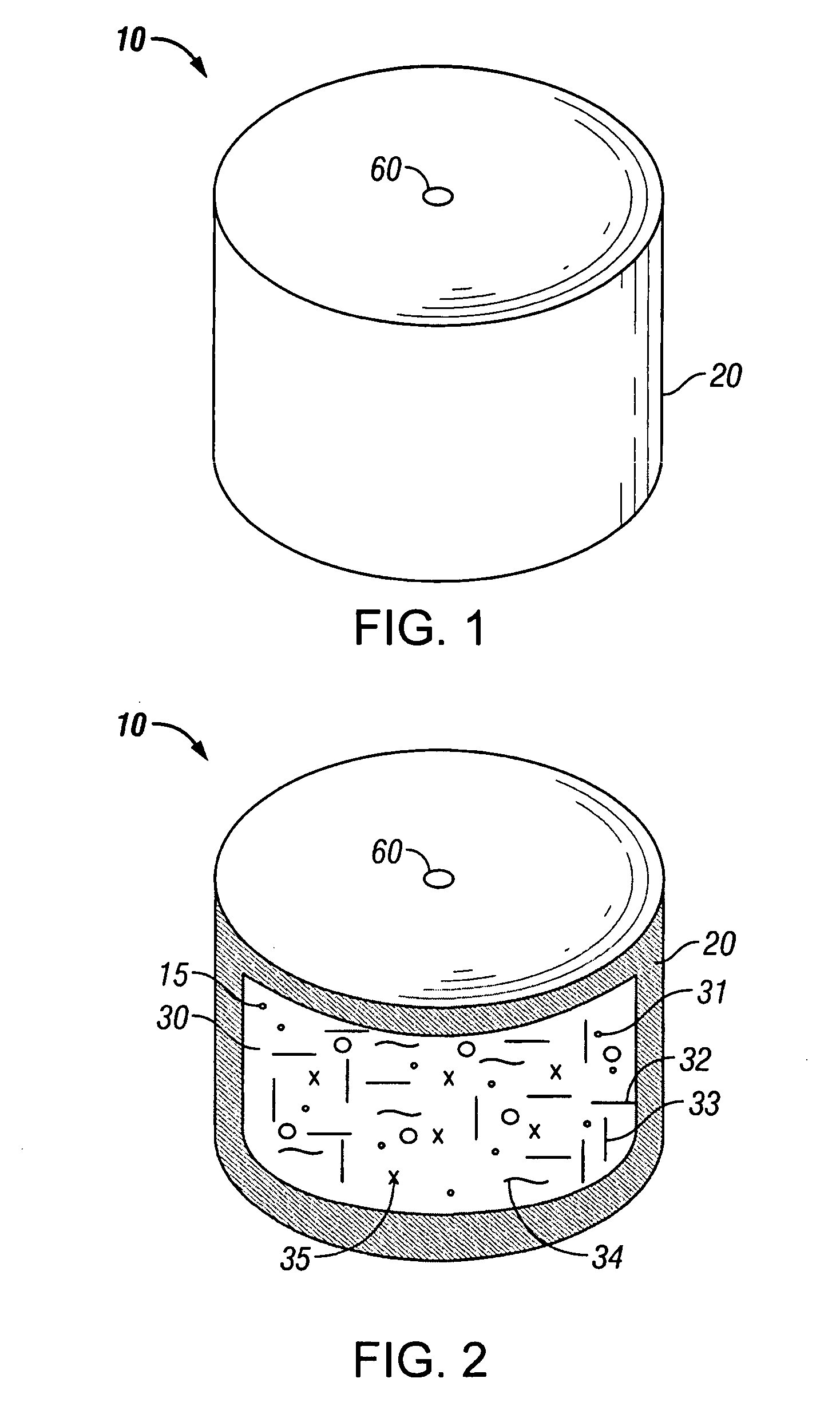
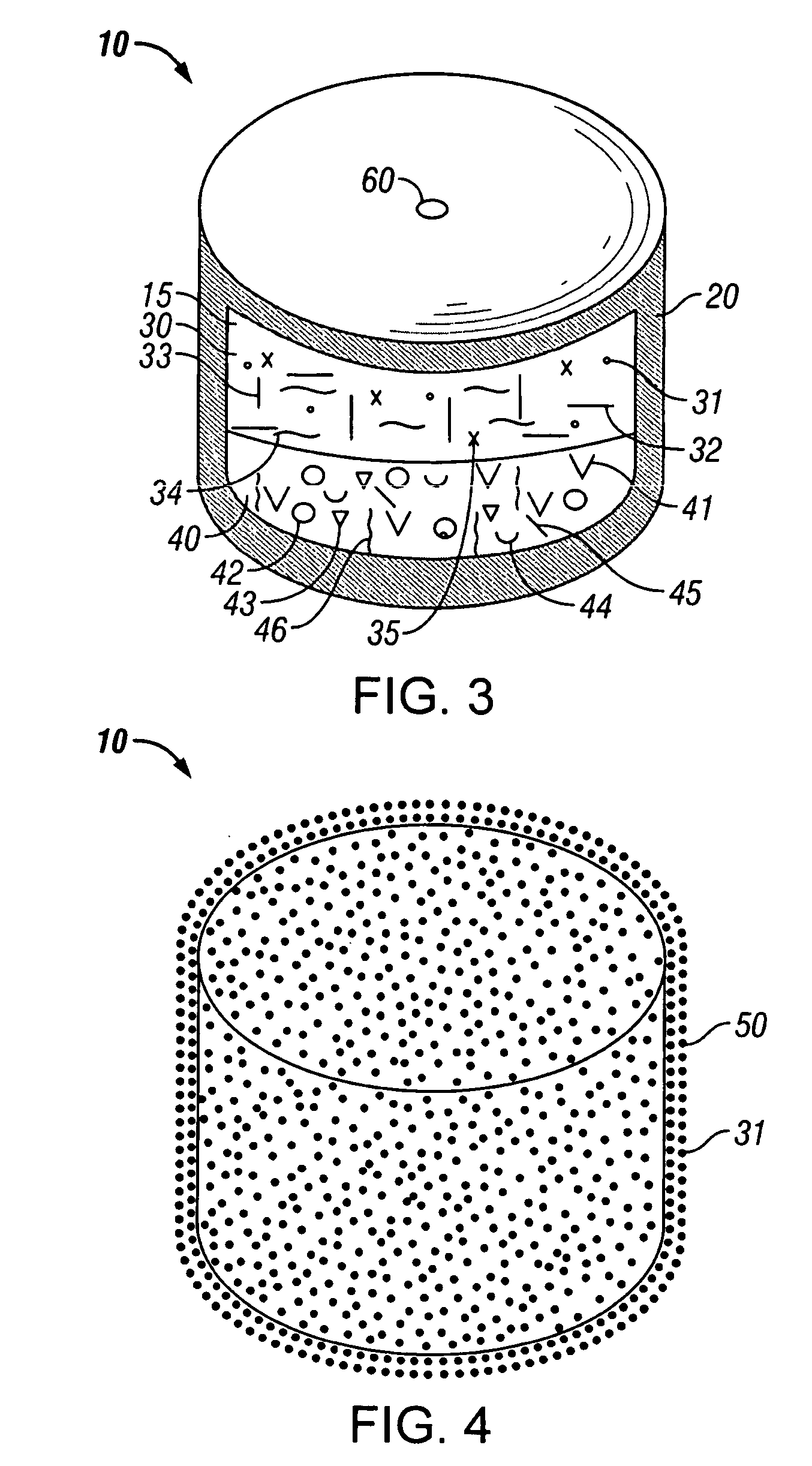
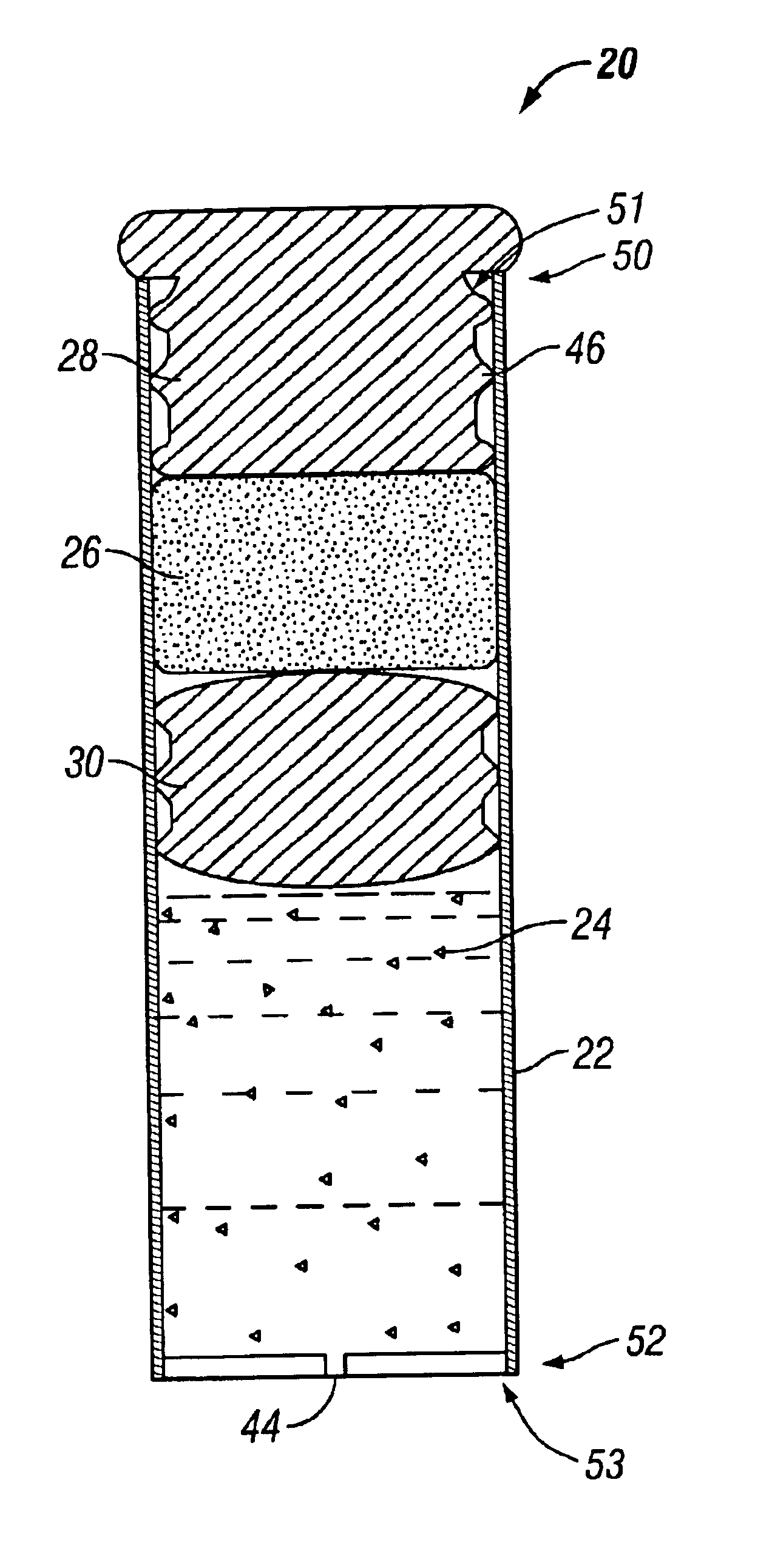
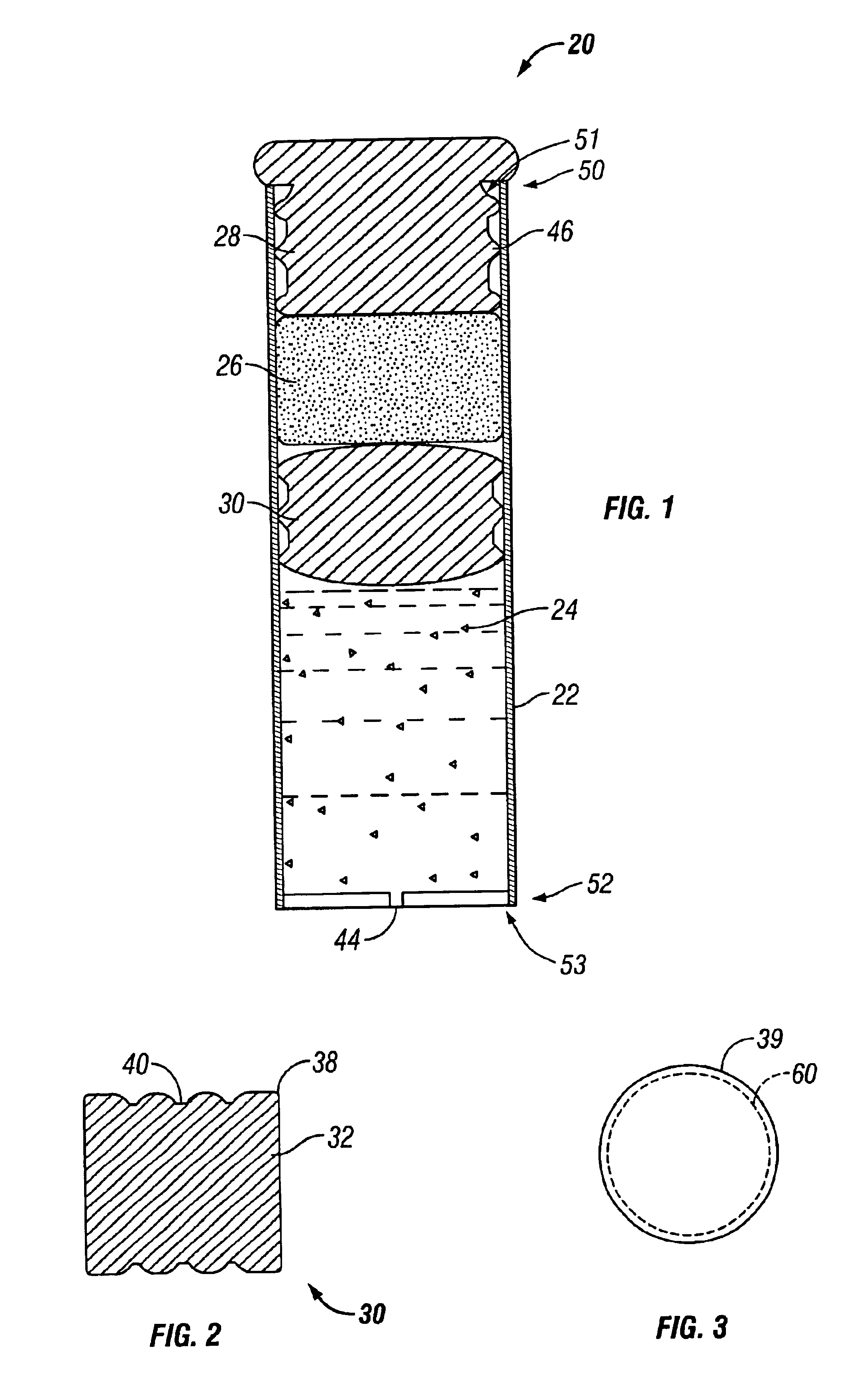
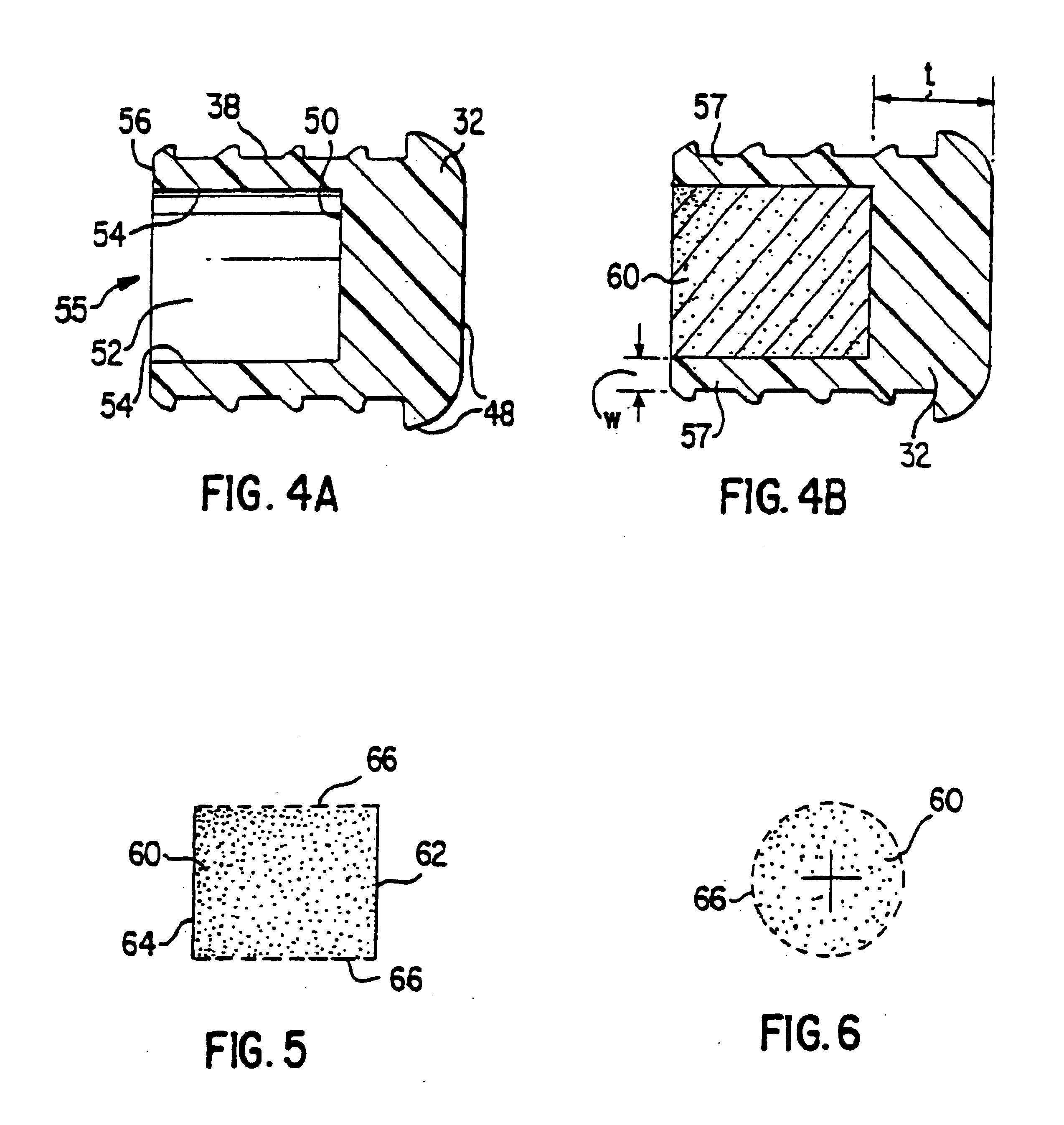
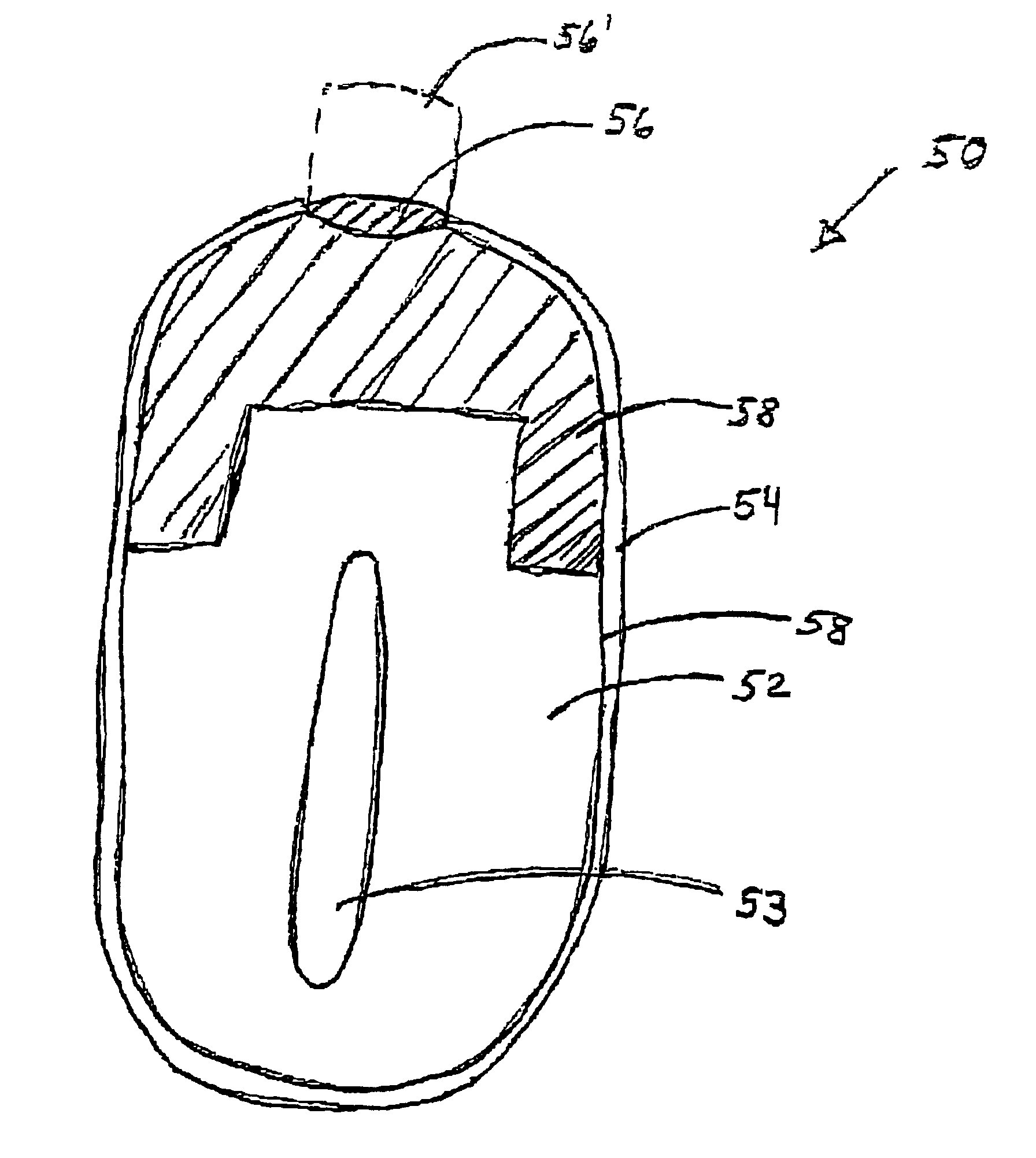
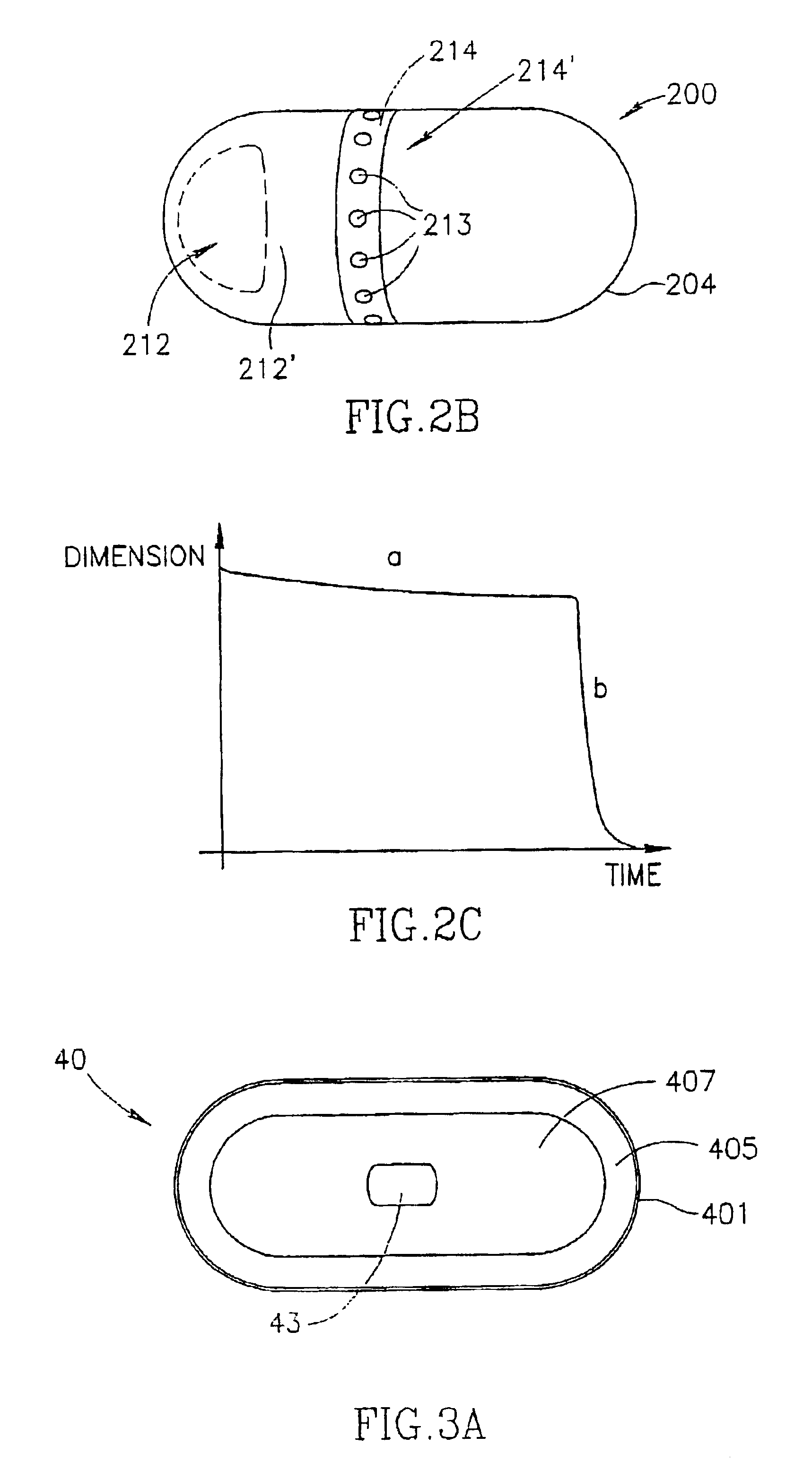
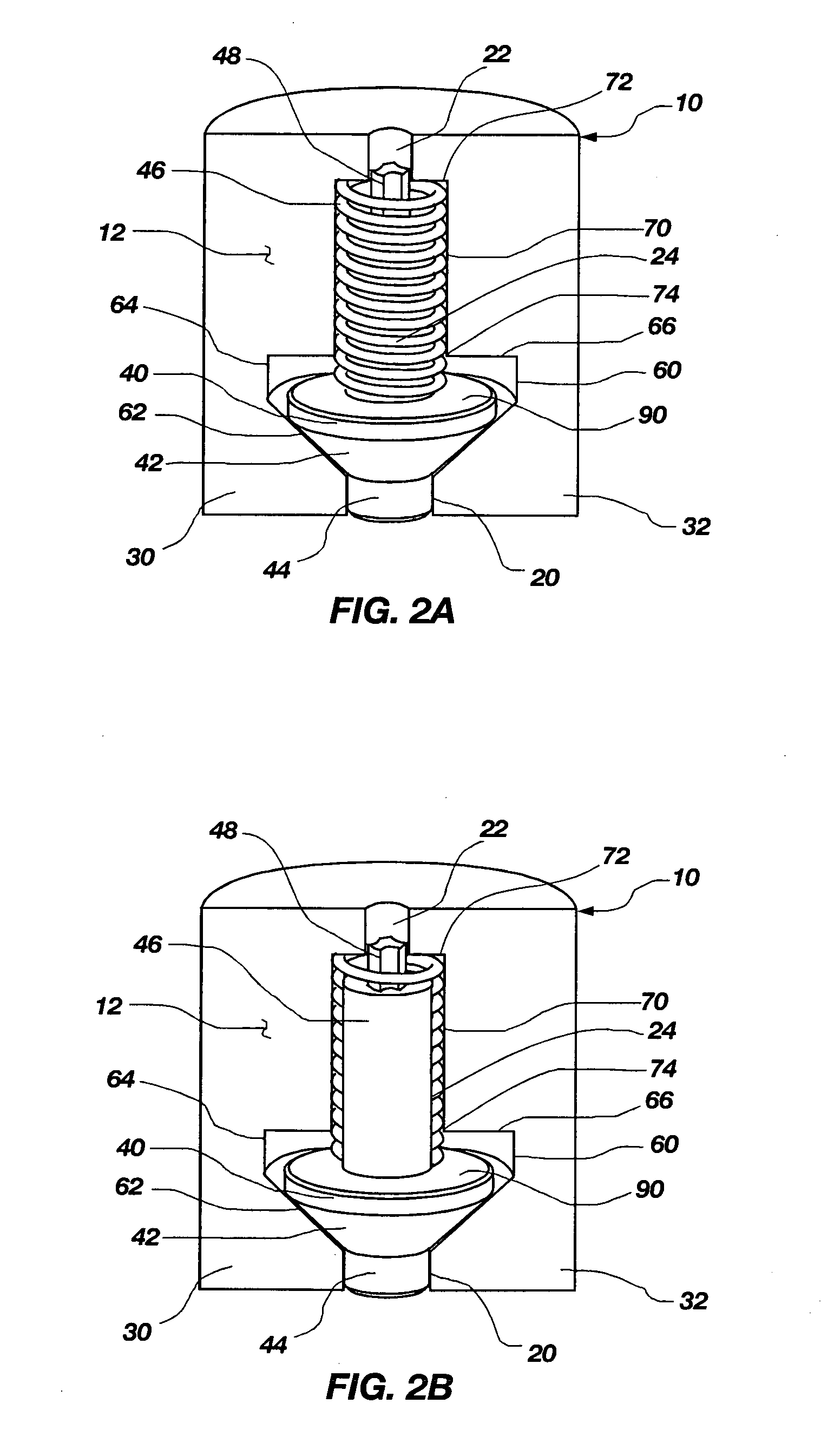
Minimally compliant, volume efficient piston for osmotic drug delivery systems
ActiveUS6939556B2Improve space efficiencyIncrease in sizeMedical devicesPressure infusionDiameter ratioOsmotic pump
An osmotic pump having a minimally compliant, volume-efficient piston positioned within a capsule is provided. The capsule has an interior surface, a beneficial agent, and an osmotic agent. The piston is movable with respect to an interior surface of the capsule, and defines a movable seal with the interior surface of the capsule. The movable seal separates the osmotic agent from the beneficial agent. The piston has a length-to-total-diameter ratio of about 1.1:1 and a core-diameter-to-total-diameter ratio of about 0.9:1. The piston enables greater beneficial agent and / or osmotic agent payload without increasing the size of the capsule. The osmotic agent imbibes liquid from a surrounding environment through a semipermeable body to cause the piston to move and, in turn, cause delivery of the beneficial agent from the capsule.
Owner:INTARCIA THERAPEUTICS INC
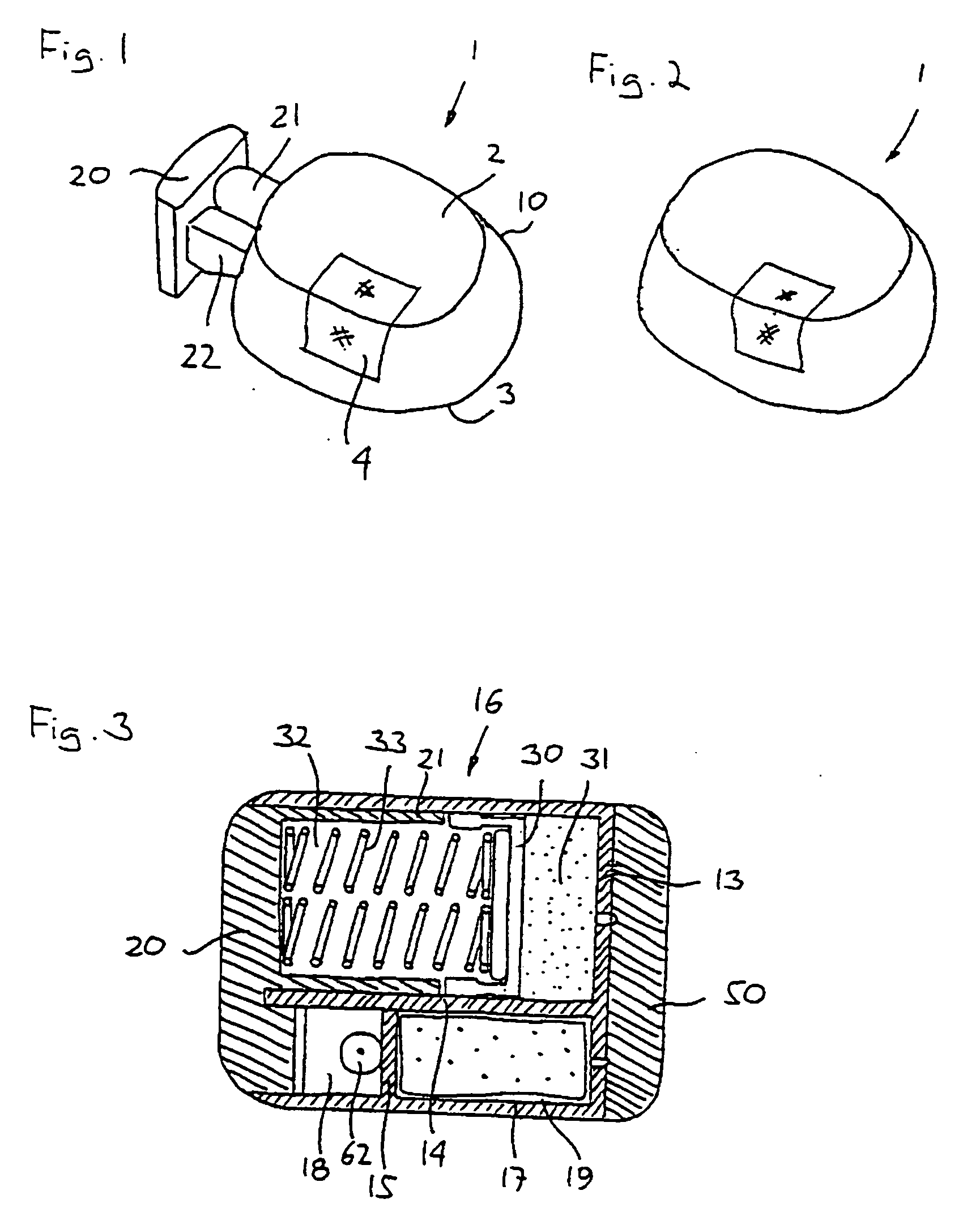
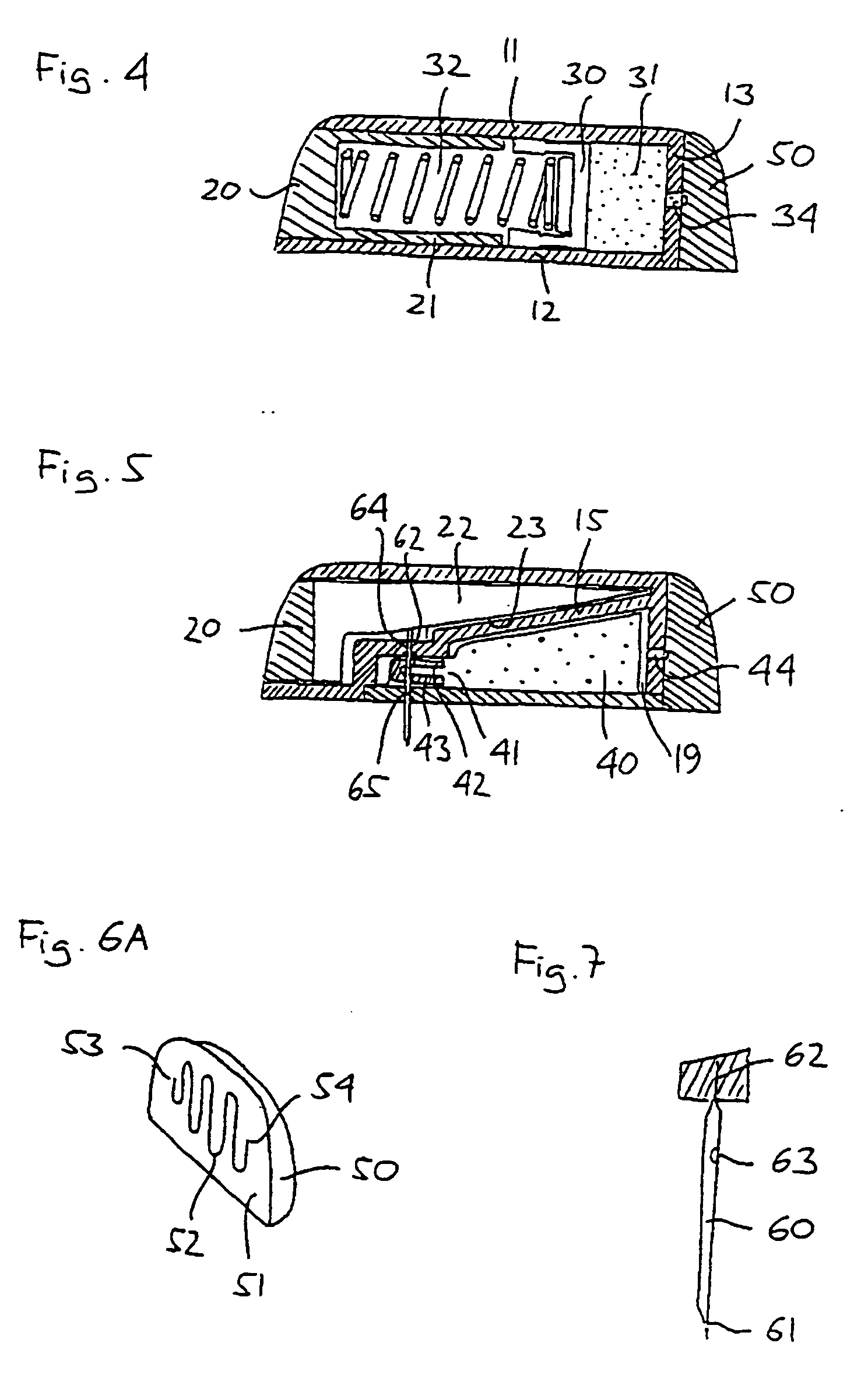
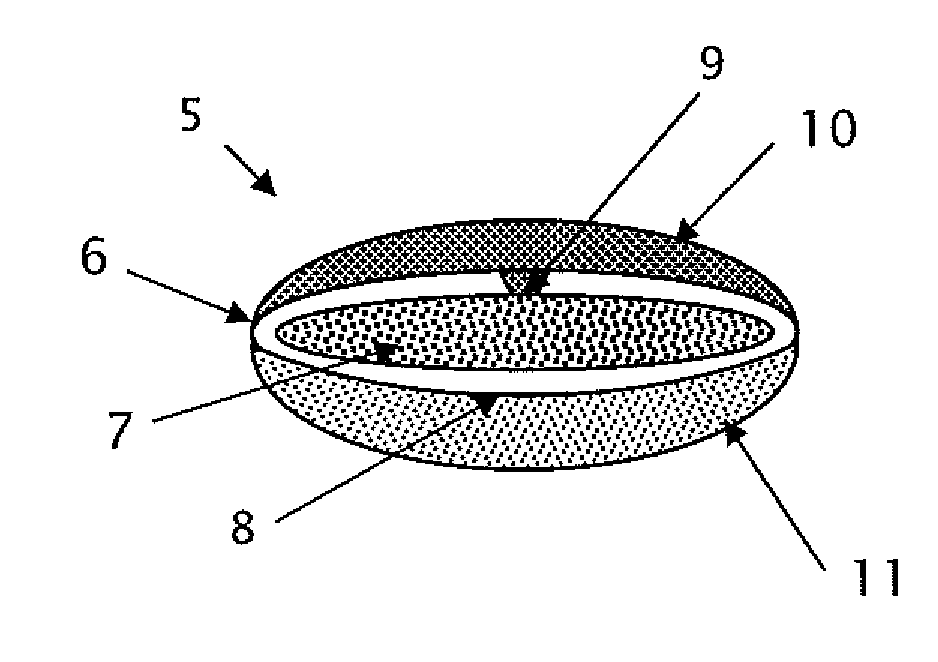
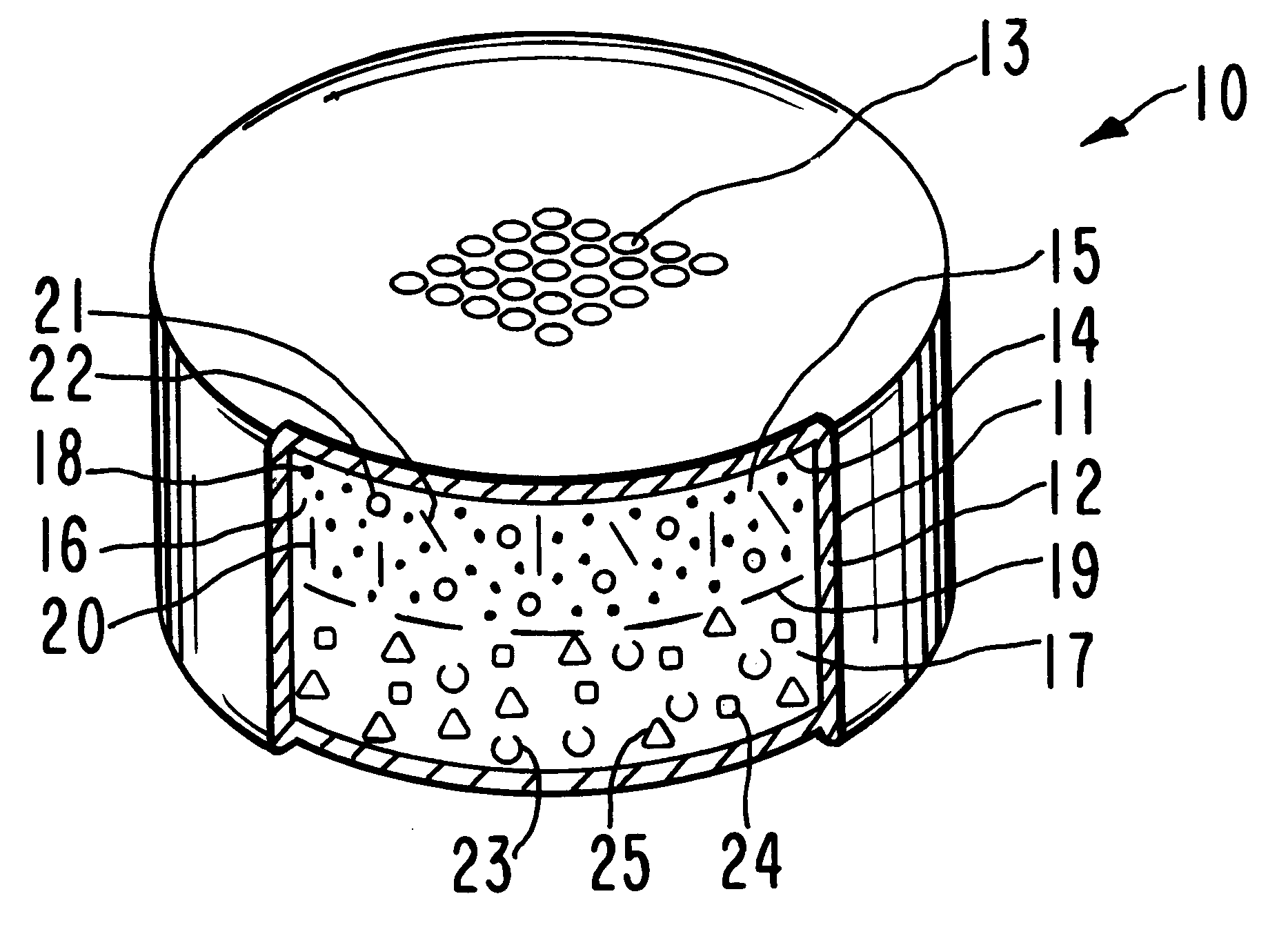
Triple Combination Release Multi-Layered Tablet
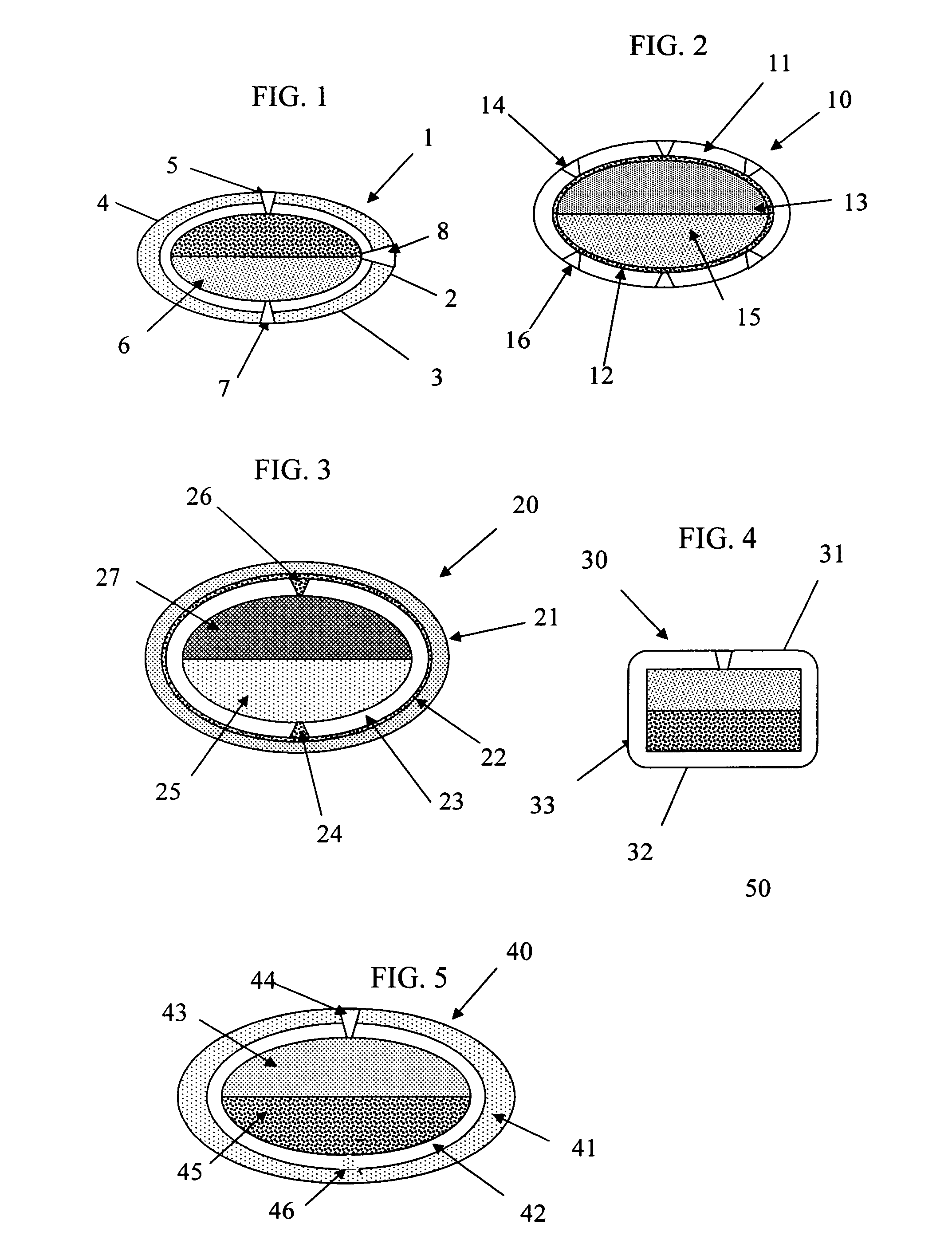
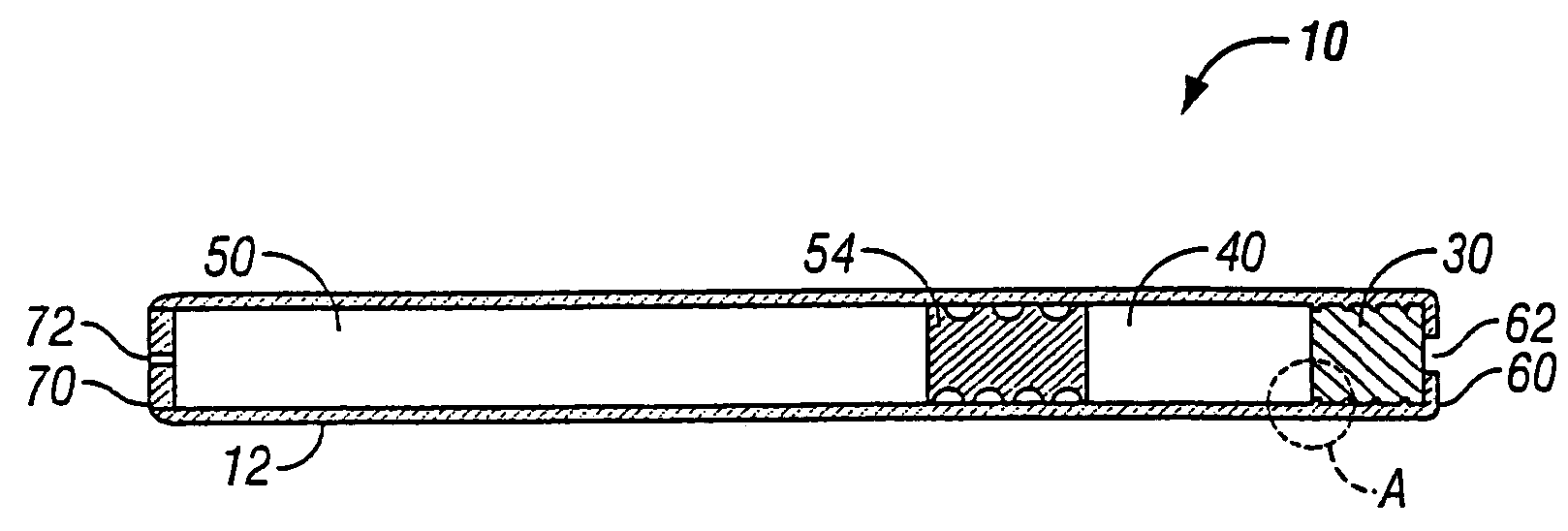
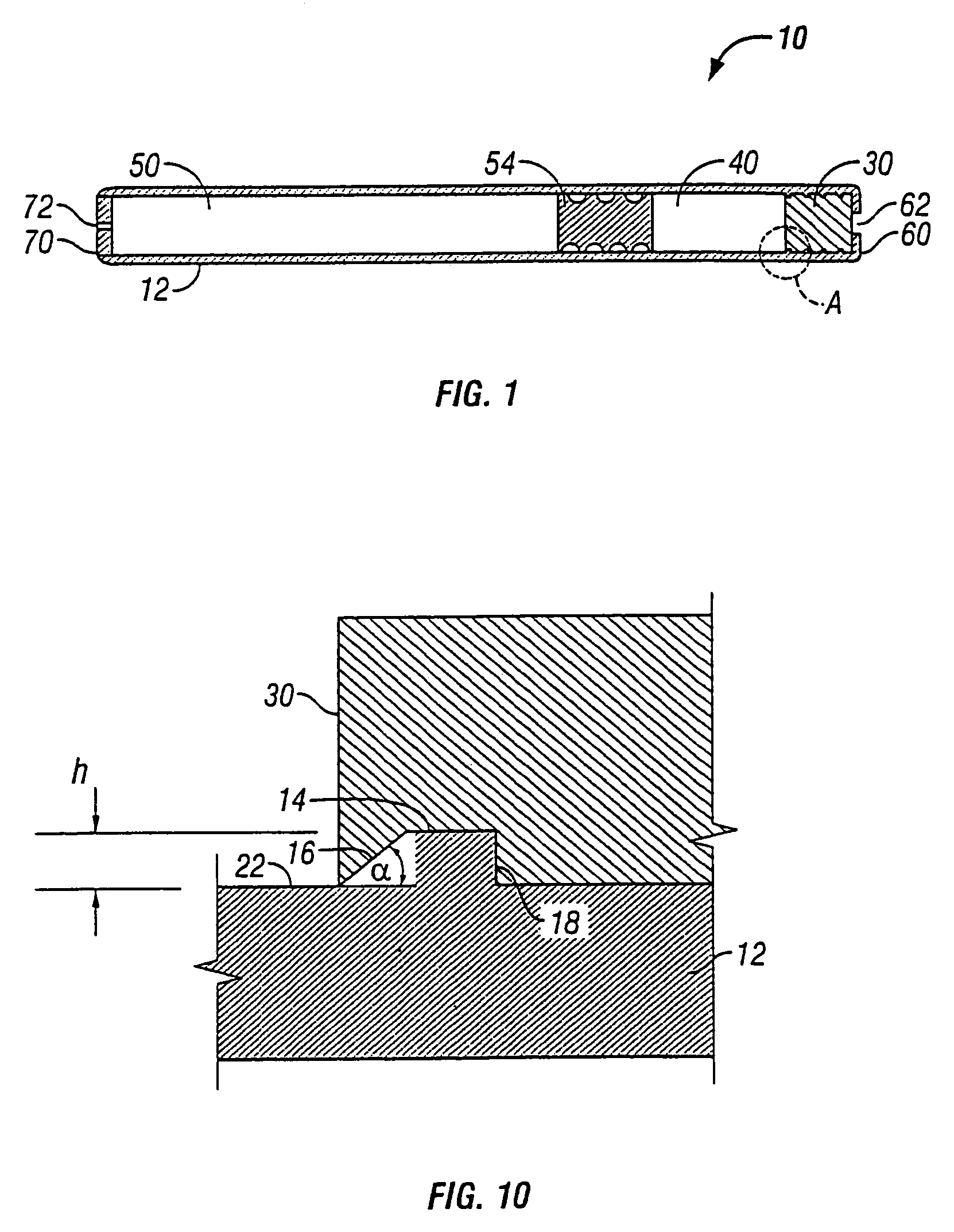
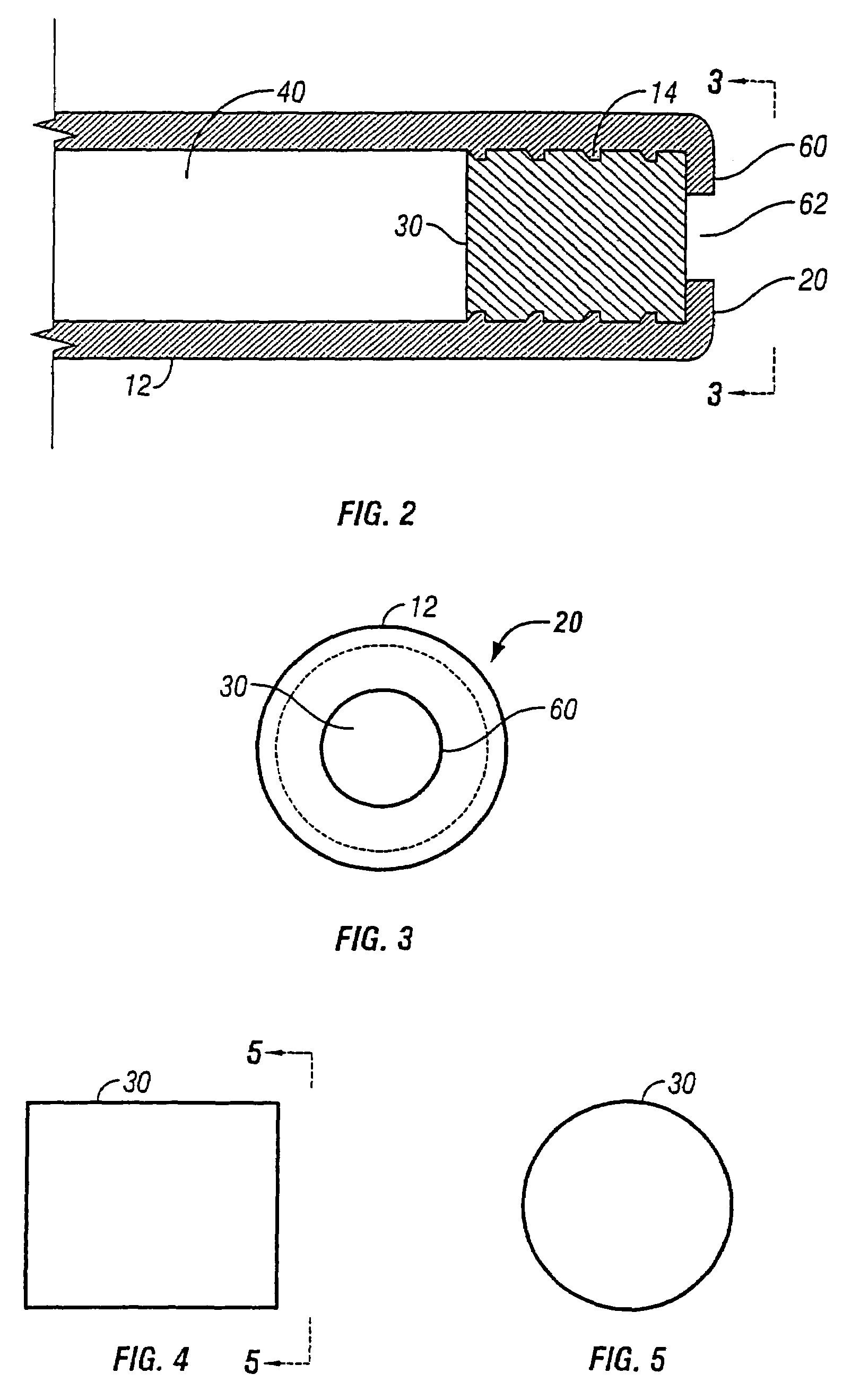
This invention pertains to a multi-layered tablet for a triple combination release of active agents to an environment of use. More particularly, the invention pertains to a multi-layered tablet (1) comprising two external drug-containing layers (2 and 3) in stacked arrangement with respect to and on opposite sides of an oral dosage form (4) that provides a triple combination release of at least one active agent. In one embodiment of the invention the dosage form is an osmotic device. In another embodiment of the invention the dosage form is a gastro-resistant coated core. In yet another embodiment of the invention the dosage form is a matrix tablet. In a different embodiment the dosage form is a hard capsule.
Owner:ACELLA HLDG LLC +1
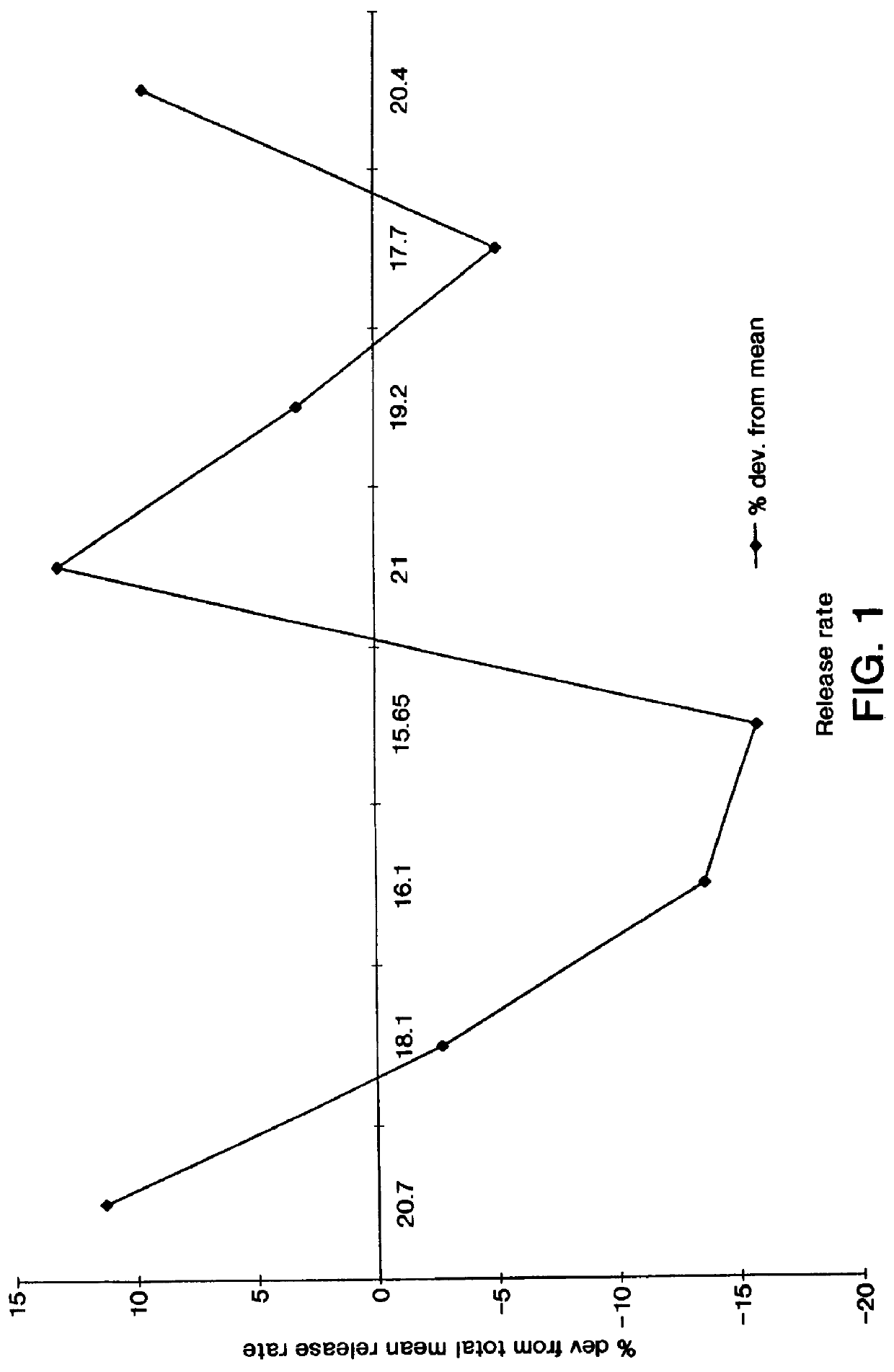
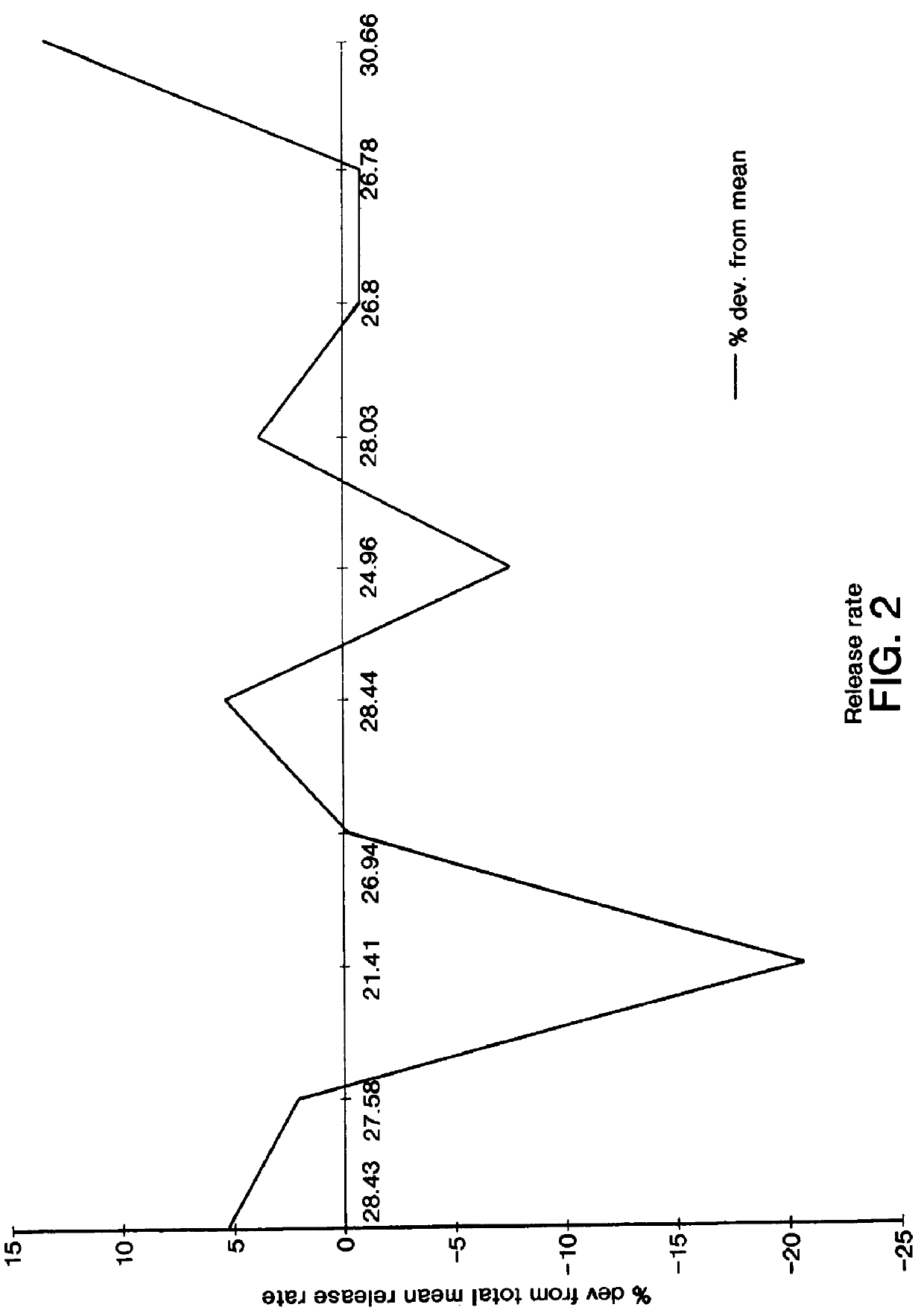
Osmotic delivery system, osmotic delivery system semipermeable body assembly, and method for controlling delivery rate of beneficial agents from osmotic delivery systems
InactiveUS6923800B2Reduce needHigh liquid uptakePill deliveryOsmotic deliveryPermeationBiomedical engineering
Osmotic delivery system semipermeable body assemblies that control the delivery rate of a beneficial agent from an osmotic delivery system incorporating one of the semipermeable body assemblies are provided. A semipermeable body assembly or plug includes a semipermeable body which is positionable in an opening of an osmotic delivery system. The semipermeable body has a hollow interior portion having a size selected to obtain a predetermined liquid permeation rate through the semipermeable body. Because the beneficial agent in the osmotic delivery system is delivered at substantially the same rate, the osmotic agent imbibes liquid which has permeated through the plug from a surrounding environment, and the liquid permeation rate through the plug controls the delivery rate of the beneficial agent from the osmotic delivery system. The liquid permeation rate through a semipermeable body may be varied to control the delivery rate of beneficial agent from an osmotic delivery system by changing the thickness of the semipermeable body or by changing an amount of surface area of the semipermeable body that is exposed to liquid when the osmotic delivery system is located in a liquid environment of use.
Owner:INTARCIA THERAPEUTICS INC
Dosage form having first and second coats
A dosage form comprising a composition comprising a drug surrounded by a first coat and a second coat with an exit for administering the drug to a patient; and a method of using the dosage form are disclosed for an indicated therapy.
Owner:ALZA CORP
Dosage form, process of making and using same
The invention disclosed pertains to a dosage form comprising an agent formulation comprising drug and pharmaceutical carrier of cooperating particle size and means for dispensing the agent formulation from the dosage form.
Owner:ALZA CORP
Device and method for examining a body lumen
InactiveUS7083578B2Simple and safe processTesting motility in the GI tractGeneral/multifunctional contrast agentsPerson identificationIn vivoBiomedical engineering
An in vivo examining device and method are described. The in vivo examining device has two operational phases; an initial phase in which the device is of initial dimensions and a final phase in which the device is of final dimensions. In the initial phase the device can pass freely through a normally configured body lumen whereas it may not be able to pass freely through an abnormally configured lumen. In the final phase the device can pass freely through a body lumen even if it is abnormally configured.
Owner:GIVEN IMAGING LTD
Pharmaceutical co-crystal compositions
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
Processes for forming a drug delivery device
A drug delivery device can, in whole or in part, be formed by co-extruding a drug core and an outer tube. The outer tube may be permeable, semi-permeable, or impermeable to the drug. The drug core may include a polymer matrix which does not significantly affect the release rate of the drug. The outer tube, the polymer matrix of the drug core, or both may be bioerodible. The co-extruded product can be segmented into drug delivery devices. The devices may be left uncoated so that their respective ends are open, or the devices may be coated with, for example, a layer that is permeable to the drug, semi-permeable to the drug, or bioerodible.
Owner:CONTROL DELIVERY SYST
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Osmotic delivery device having a two-way valve and a dynamically self-adjusting flow channel
ActiveUS7014636B2Prevent backdiffusionAvoid communicationOsmotic deliveryBandagesHigh pressureVariable geometry
An implantable osmotically driven delivery system having a dynamic, two-way valve and a self-adjusting, variable geometry fluid flow channel. As pressure within the agent delivery system increases, the fluid channel narrows, thereby restricting flow. At exceptionally high pressures, the valve can be designed to close altogether at the orifice or delivery end, or it can provide a minimal leak path so that a maximum fluid flow is never exceeded. At zero or very low pressures, the valve will close completely at the beneficial agent reservoir end, isolating the beneficial agent formulation from external fluid infiltration and thereby eliminating diffusion of external fluid into the beneficial agent formulation.
Owner:INTARCIA THERAPEUTICS INC
Conversion of liquid filled gelatin capsules into controlled release systems by multiple coatings
InactiveUS6929803B2Pretreated surfacesMacromolecular non-active ingredientsControl releaseActive agent
A dosage form comprising a gelatin capsule formed with a composite wall and containing a liquid, active agent formulation where the wall comprises a barrier layer formed over the external surface of the gelatin capsule, an expandable layer formed over the barrier layer and a semipermeable layer formed over the expandable layer is described. The dosage forms and methods provide for the conversion of standard gelatin, liquid formulation capsules into controlled, release dosage forms that permit the controlled release of the active agent into the environment of use over time.
Owner:ENCINAL PHARMA INVESTMENTS
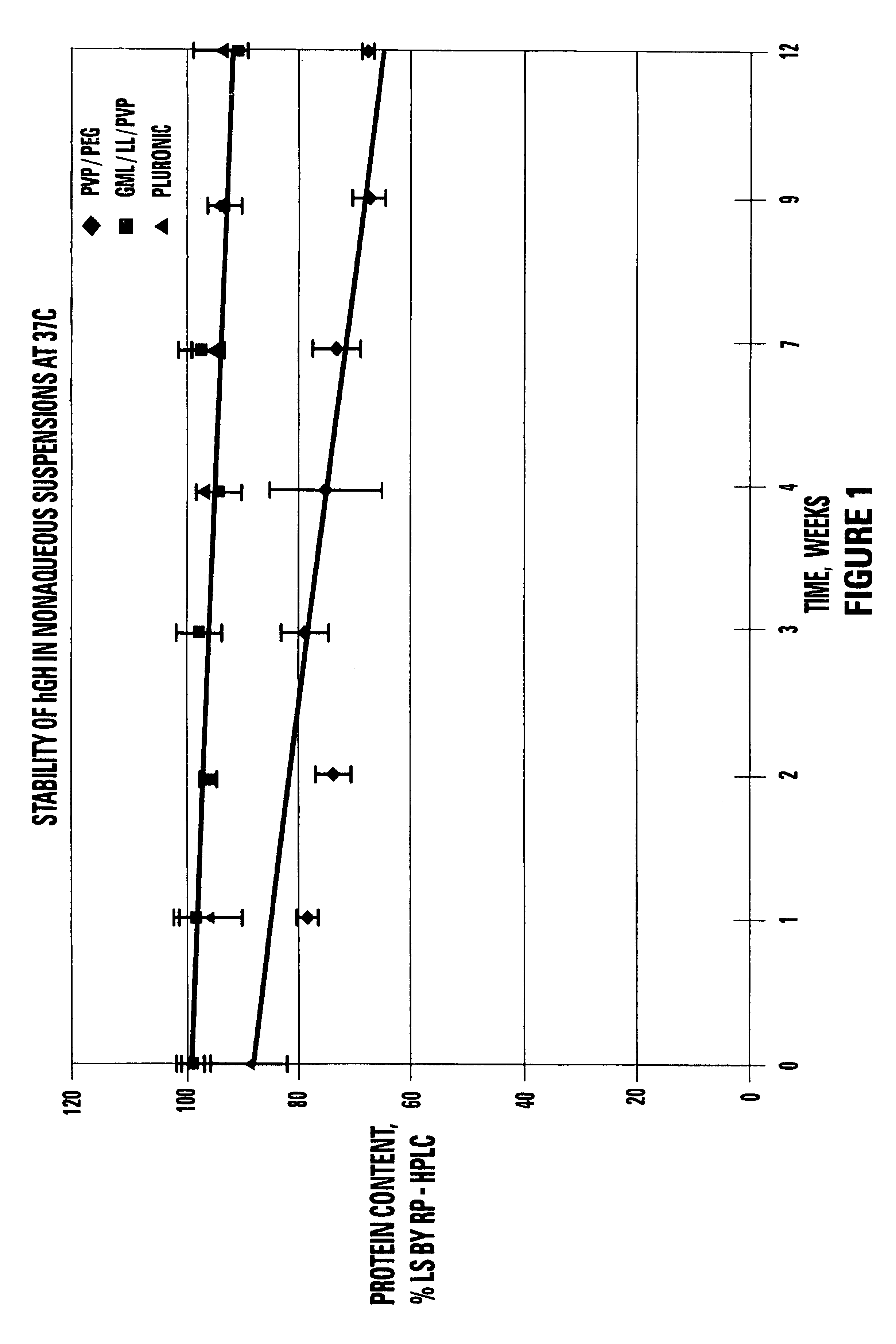
Non-aqueous single phase vehicles and formulations utilizing such vehicles
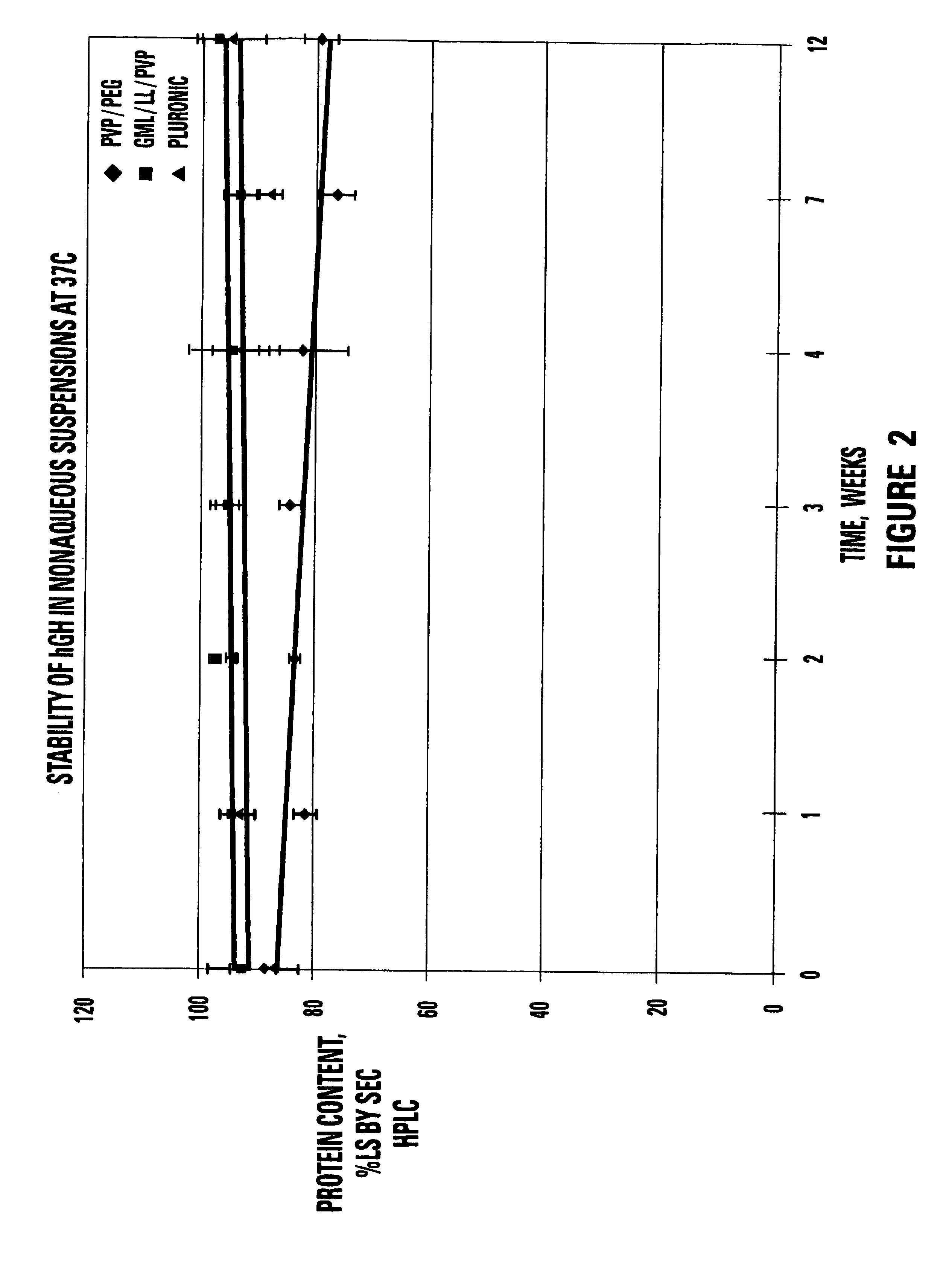
InactiveUS20050008661A1Increase stickinessReduce occlusionPowder deliveryPeptide/protein ingredientsEngineeringSolvent
The present invention includes materials and methods for providing vehicles useful for providing drug formulations that address the potential drawbacks of known nonaqueous formulations. In particular, the present invention includes nonaqueous vehicles that are formed using a combination of polymer and solvent that results in a vehicle that is miscible in water. The nonaqueous vehicles facilitate the formulation of drug formulations that are stable over time, even when stored at, or exposed to, elevated temperatures. Moreover, the miscible vehicles of the present invention allow the preparation of drug formulations that work to reduce the occurrence of partial or complete occlusions of the delivery conduits included in delivery devices used to administer the drug formulations.
Owner:DURECT CORP
Dual controlled release dosage form
A dosage form that provides a controlled release of at least two different active agents is provided. Particular embodiments include a dosage form that provides therapeutically effective levels of a first active agent and a second active agent in a mammal for an extended period of time following oral administration. An osmotic device containing a bi-layered core is provided. The osmotic device provides a dual controlled release of both drugs from the core. The layers of the core are in stacked, substantially concentric or substantially eccentric arrangement.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Stable non-aqueous single phase viscous vehicles and formulations utilizing such vehicle
InactiveUS7258869B1Zero order release rateReduce flow rateBiocidePeptide/protein ingredientsMedicineShear rate
This invention relates to stable non-aqueous single phase viscous vehicles and to formulations utilizing such vehicles. The formulations comprise at least one beneficial agent uniformly suspended in the vehicle. The formulation is capable of being stored at temperatures ranging from cold to body temperature for long periods of time. The formulations are capable of being uniformly delivered from drug delivery systems at an exit shear rate of between about 1 to 1×10−7 reciprocal second.
Owner:INTARCIA THERAPEUTICS INC
Osmotic implant with membrane and membrane retention means
InactiveUS7163688B2Prevent ejectionReduces expulsionMedical devicesCapsule deliverySystems designMedicine
An osmotic delivery system for controlled delivery of a beneficial agent includes an implantable capsule containing a beneficial agent and an osmotic engine that swells on contact with water, thereby causing the release of the beneficial agent over time. The osmotic delivery system has a membrane material that allows a controlled amount of fluid to enter from an exterior of the capsule, while preventing the compositions within the capsule from passing out of the capsule. The osmotic delivery system is designed to meet at least the operating pressures of 1000 psi. The membrane material is cast, calendered or extruded followed by machining (i.e., die-cutting, stamping or otherwise cutting to shape) to provide a uniform nonribbed membrane material. The capsule also includes a membrane material-retaining means that is positioned at a fluid uptake end to retain the membrane material within the capsule, even under periods of high pressure.
Owner:INTARCIA THERAPEUTICS INC
Stable, non-aqueous, single-phase gels and formulations thereof for delivery from an implantable device
The present invention provides a suspension vehicle and suspension formulations deliverable from an implantable delivery device. In particular, the suspension vehicle of the present invention allows the formulation of beneficial agent suspensions that are stable over time at ambient and physiological temperatures. In addition, the beneficial agent suspensions formed using the suspension vehicle of the present invention allow controlled delivery of beneficial agent from an implanted delivery device over sustained periods of time, even when such delivery occurs at low-flow rates, through a small-diameter delivery channel. Also included in the present invention are implantable delivery devices.
Owner:INTARCIA THERAPEUTICS INC
Osmotic pump with means for dissipating internal pressure
ActiveUS7207982B2Simple designReducing and minimizing likelihoodMedical devicesPressure infusionInternal pressurePhysical separation
The present invention includes an osmotic pump that includes a means for venting an osmotic composition included in the pump before the internal pressure of the pump has the opportunity to build to such an extent that the pump is structurally compromised, such as when one or more components of the pump are physically separated. The means for venting osmotic material included in an osmotic pump according to the present invention includes a vent that allows the material included in the osmotic composition of the pump to dissipate into an environment of operation at a rate that results in dissipation of the pressure created within the osmotic pump and a reduced potential for subject discomfort or irritation.
Owner:INTARCIA THERAPEUTICS INC
Pharmaceutical formulations of potassium ATP channel openers and uses thereof
InactiveUS20060051418A1Enhanced drug releaseReduces food uptakeBiocideOrganic active ingredientsPotassium channel openerPotassium
Provided are immediate or prolonged administration of certain potassium ATP (KATP) channel openers to a subject to achieve novel pharmacodynamic, pharmacokinetic, therapeutic, physiological, metabolic and compositional outcomes in the treatment of diseases or conditions involving KATP channels. Also provided are pharmaceutical formulations, methods of administration and dosing of KATP channel openers that achieve these outcomes and reduce the incidence of adverse effects in treated individuals. Further provided are method of co-administering KATP channel openers with other drugs to treat diseases of humans and animals.
Owner:ESSENTIALIS INC
Antiparkinson dosage form
InactiveUS6217905B1Exclude influenceAdministration limitationPill deliveryOsmotic deliveryDiseaseAntiparkinsonian drugs
A dosage form is disclosed comprising an anti-Parkinson's disease drug for administering to a patient in need of anti-Parkinson's disease therapy.
Owner:ENCINAL PHARMA INVESTMENTS
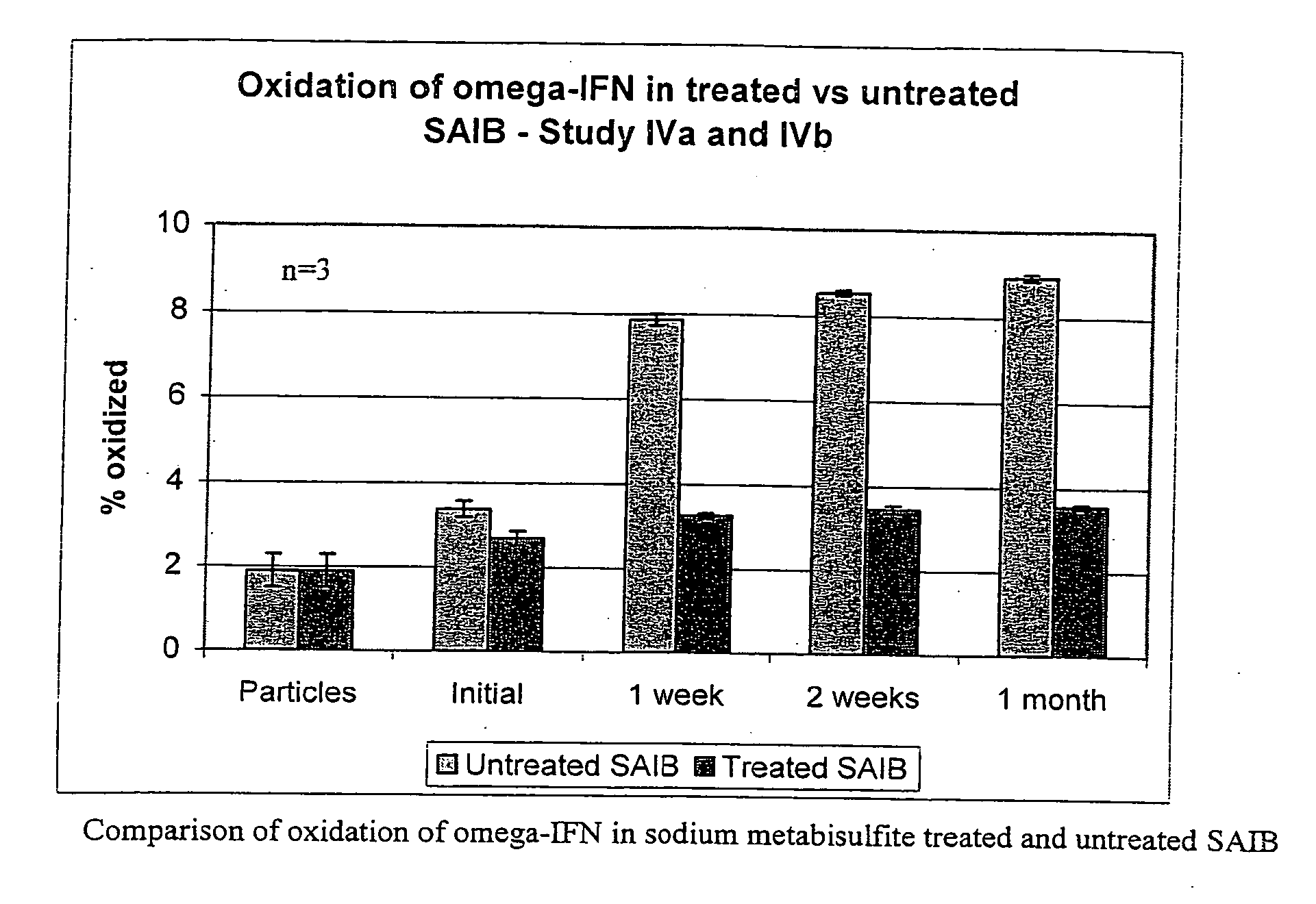
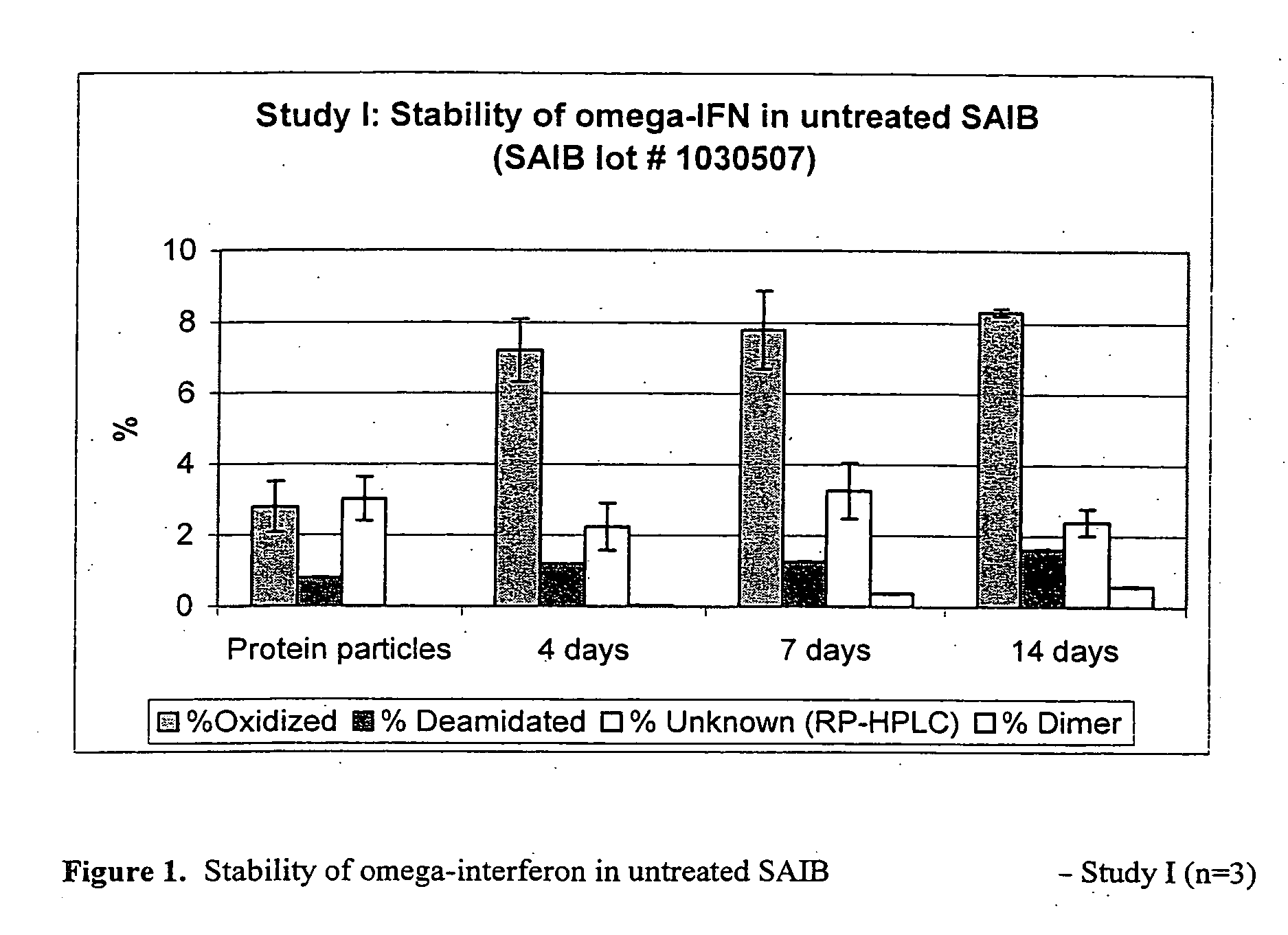
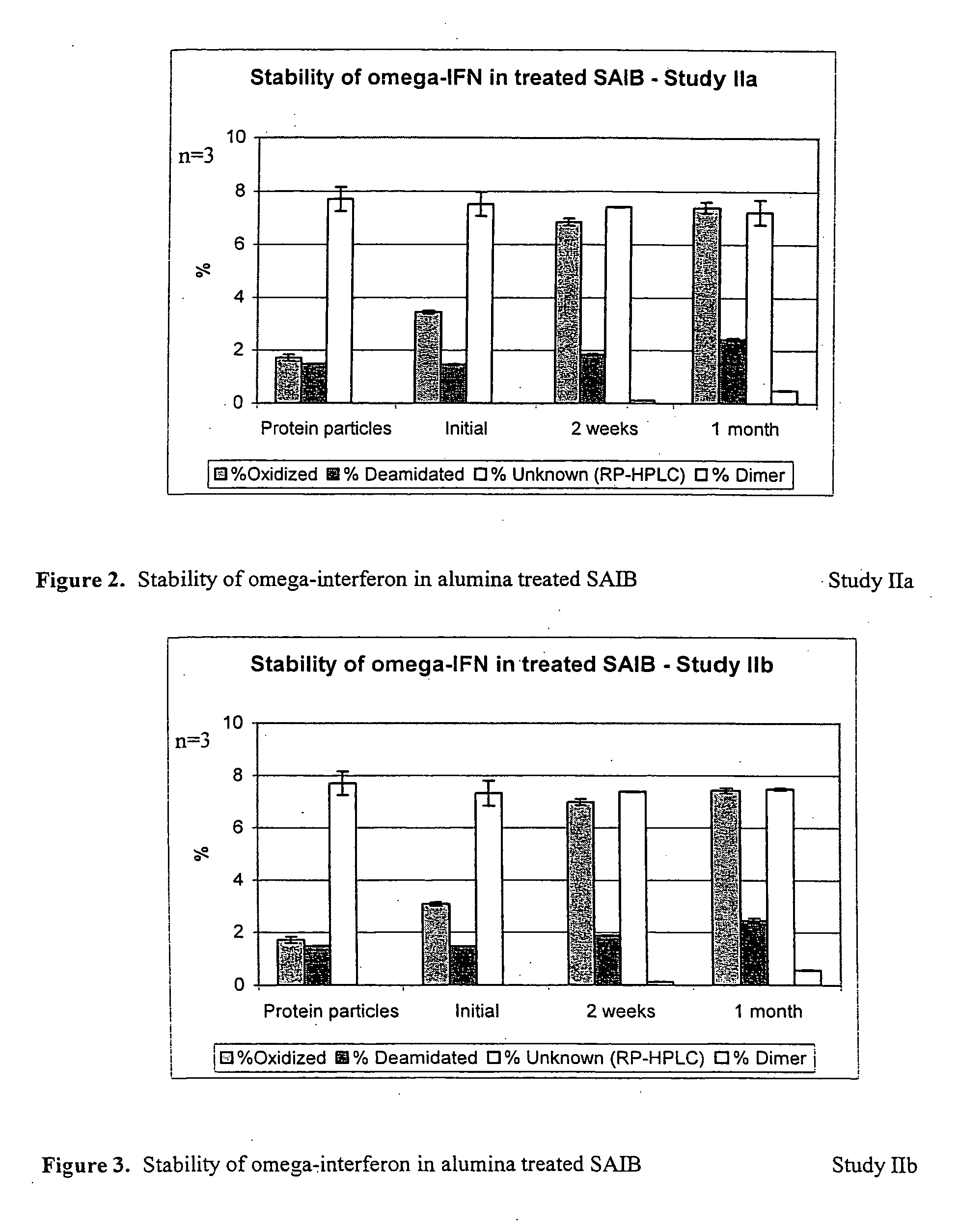
Peroxide removal from drug delivery vehicle
InactiveUS20070027105A1Improve drug stabilityReduced level of peroxideOrganic active ingredientsBiocideMedicineSucrose acetate isobutyrate
Owner:DURECT CORP
Method and apparatus treating tissue adjacent a bodily conduit with thermocompression and drugs
InactiveUS7833220B2Guaranteed functionAvoid heatElectrotherapyDrug compositionsSufficient timeHeat sensitive
Owner:MEDIFOCUS
Capillary moderator for osmotic delivery system
InactiveUS20050175701A1Prevent backflowPressure infusionPill deliveryBiomedical engineeringDelivery system
The present invention relates to apparatus and methods for preventing backflow into a beneficial agent dispensing osmotic delivery system.
Owner:INTARCIA THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com