Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
263results about How to "Improve drug stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
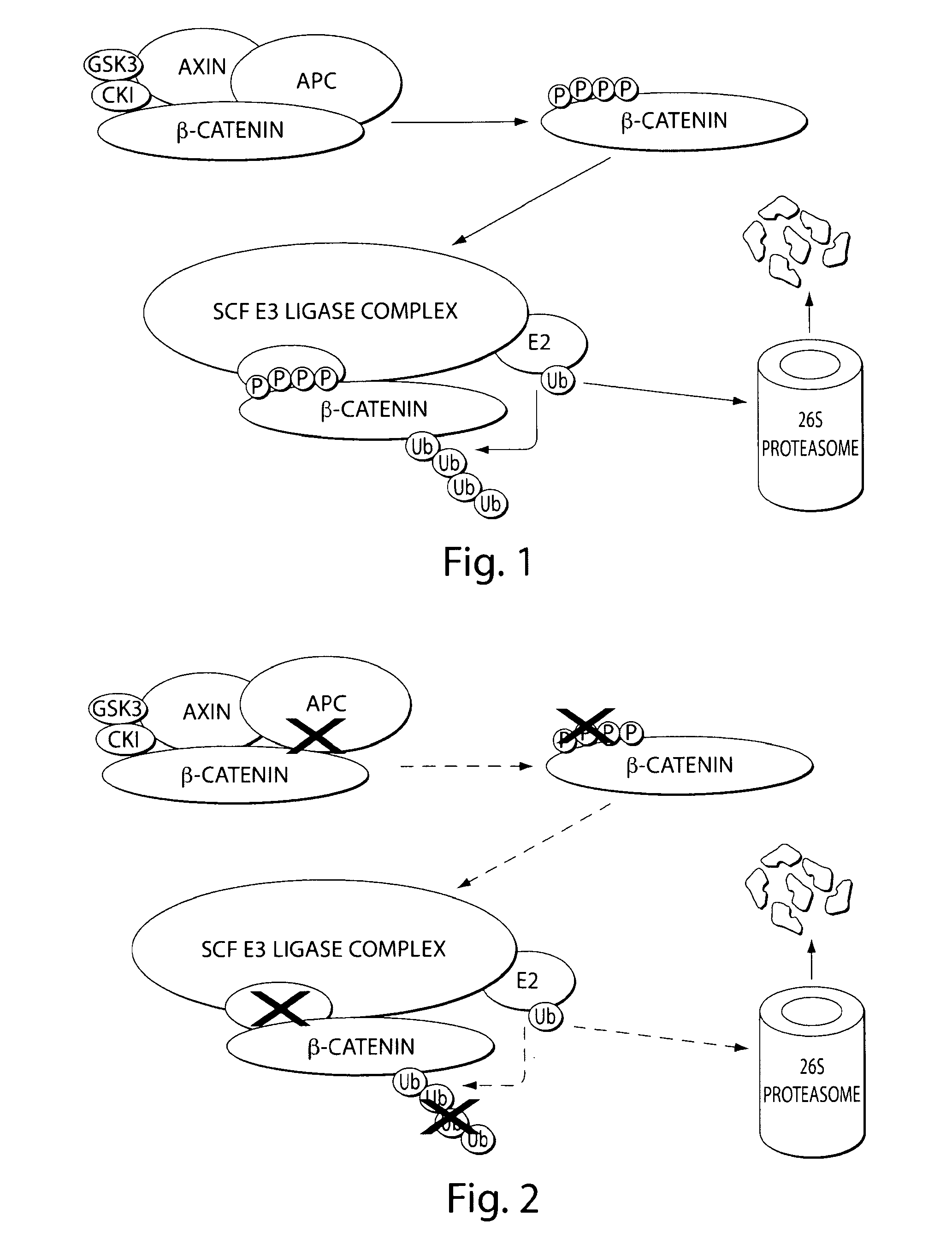
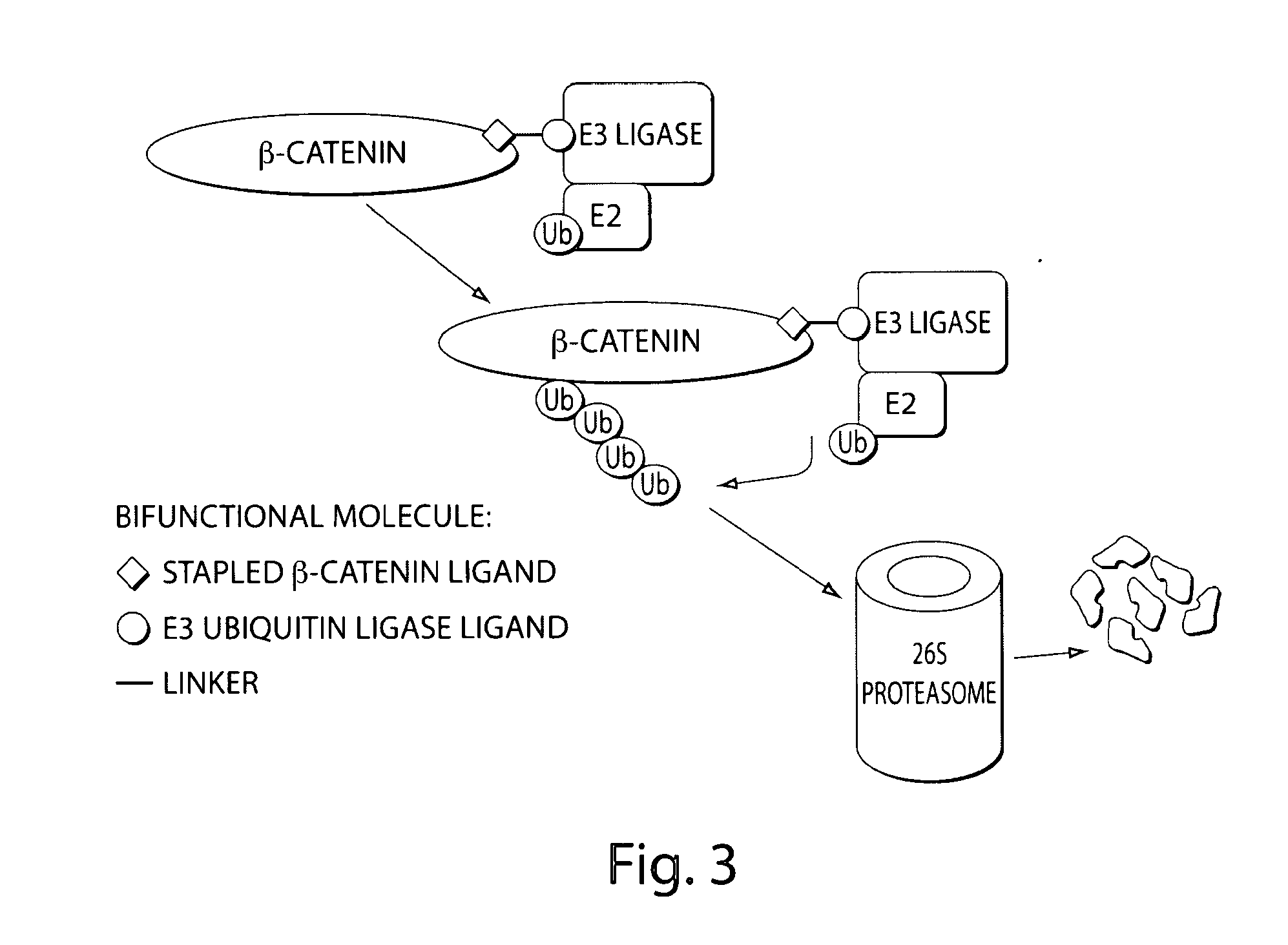
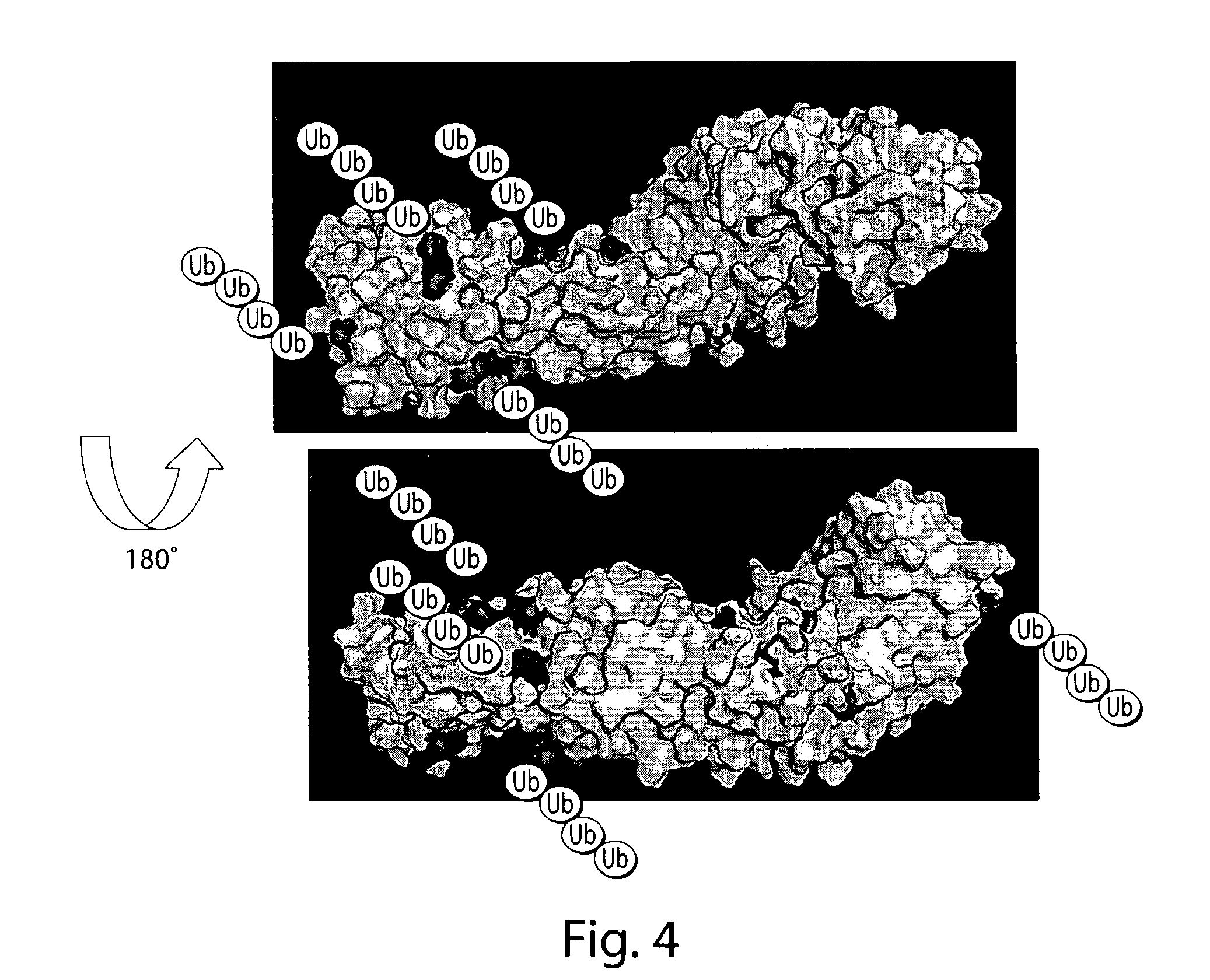
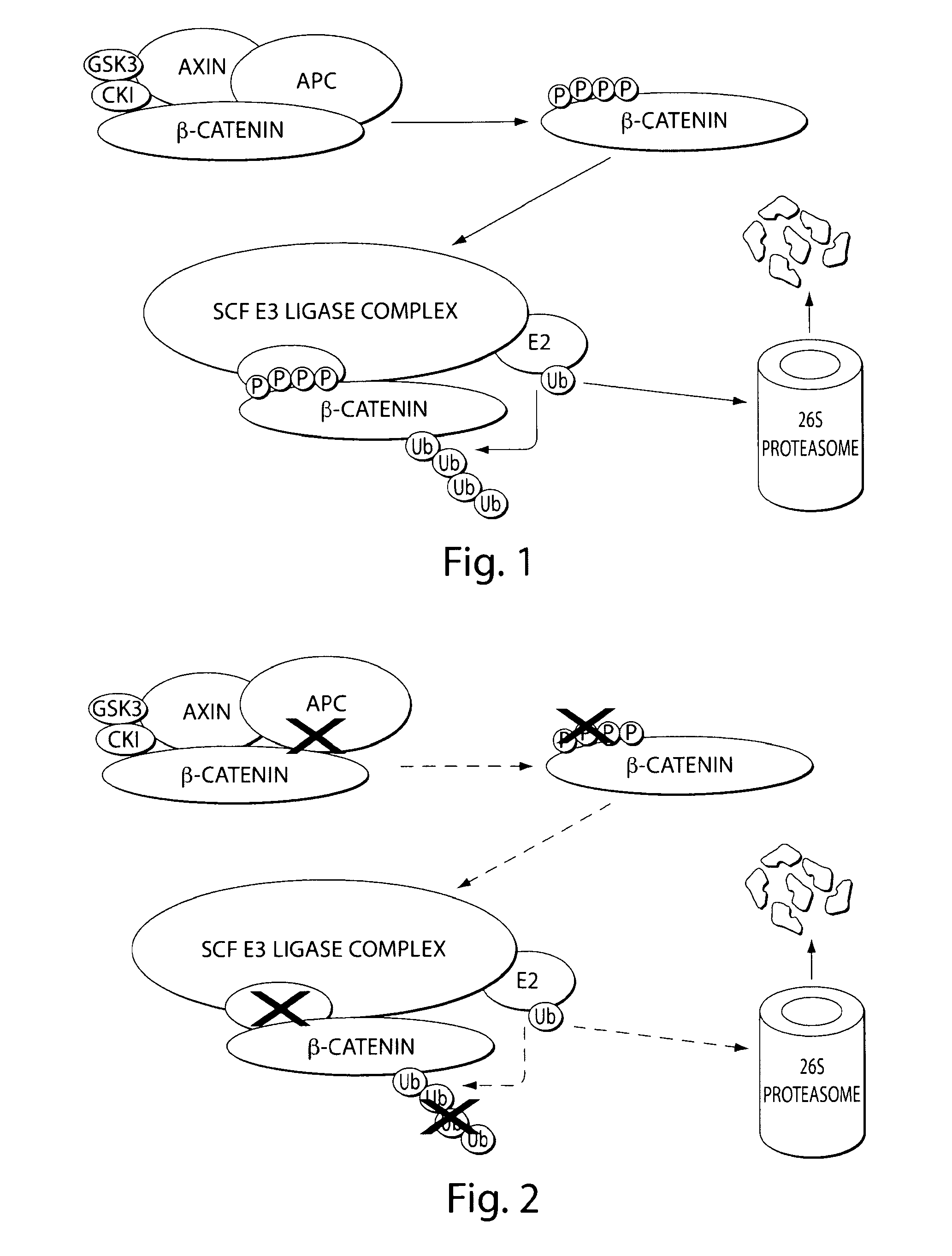
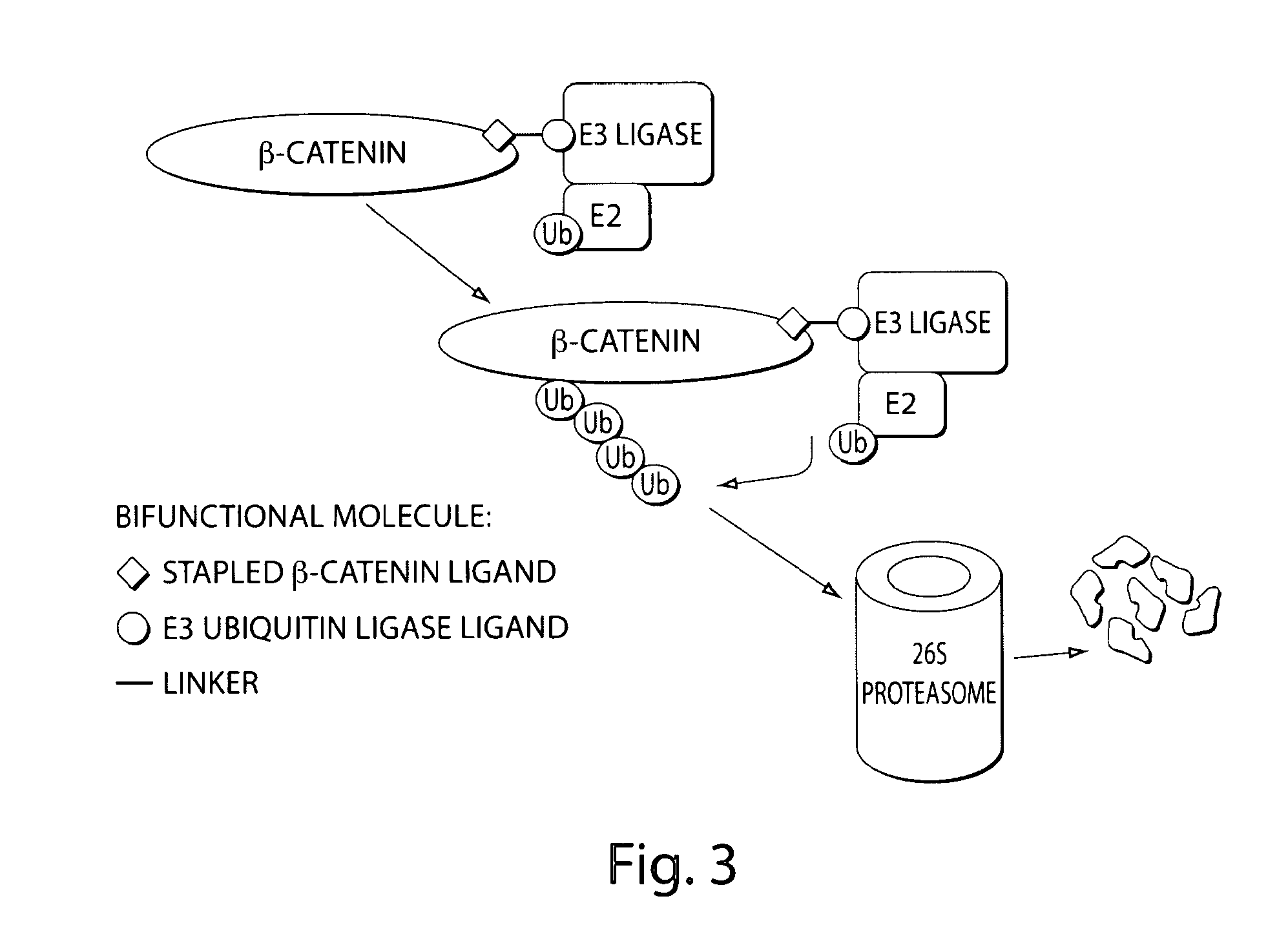
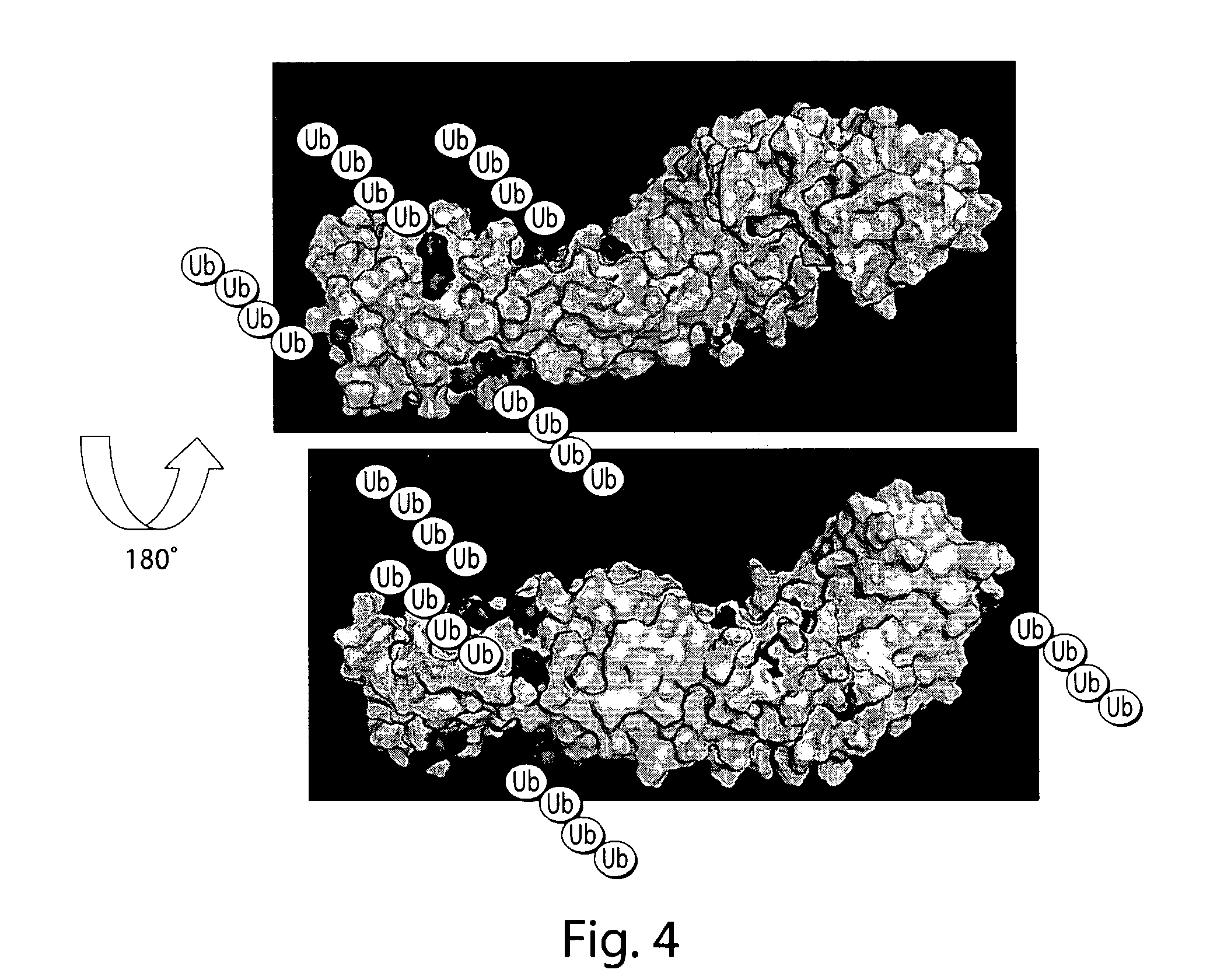
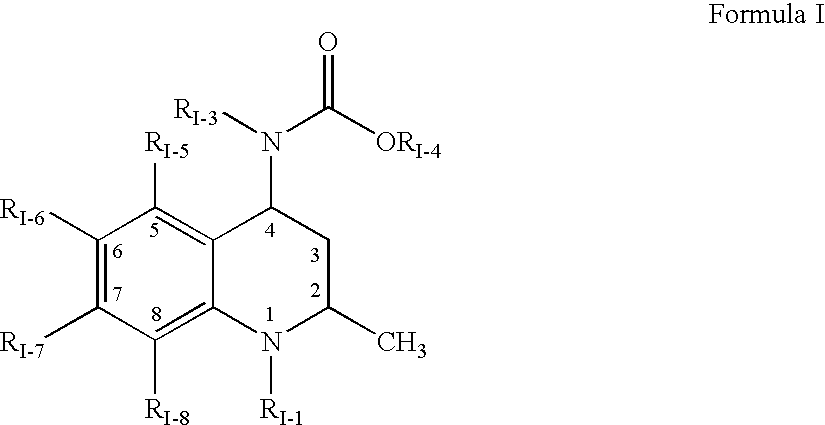
Bifunctional stapled polypeptides and uses thereof
ActiveUS20120270800A1Increase productionAvoid unfoldingPeptide/protein ingredientsAntibody mimetics/scaffoldsPeptideBifunctional
The invention relates to bifunctional stapled or stiched peptides comprising a targeting domain, a linker moiety, and an effector domain, that can be used to tether, or to bring into close proximity, at least two cellular entities (e.g., proteins). Certain aspects relate to bifunctional stapled or stiched peptides that bind to an effector biomolecule through the effector domain and bind to a target biomolecule through the targeting domain. Polypeptides and / or polypeptide complexes that are tethered by the bifunctional stapled or stiched peptides of the invention, where the effector polypeptide bound to the effector domain of the bifunctional stapled or stiched peptide modifies or alters the target polypeptide bound to the targeting domain of the bifunctional peptide. Uses of the inventive bifunctional stapled or stiched peptides including methods for treatment of disease (e.g., cancer, inflammatory diseases) are also provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
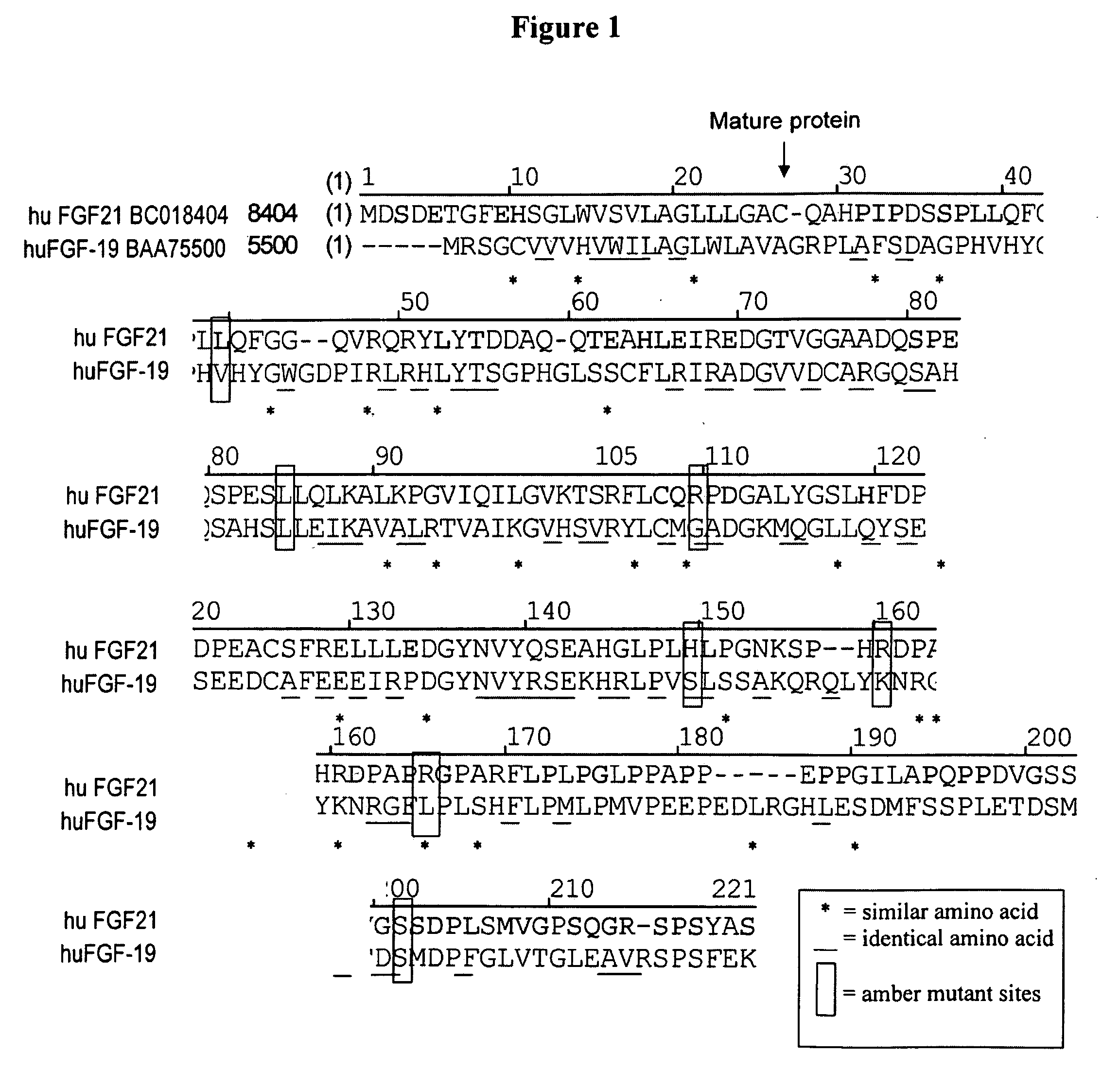
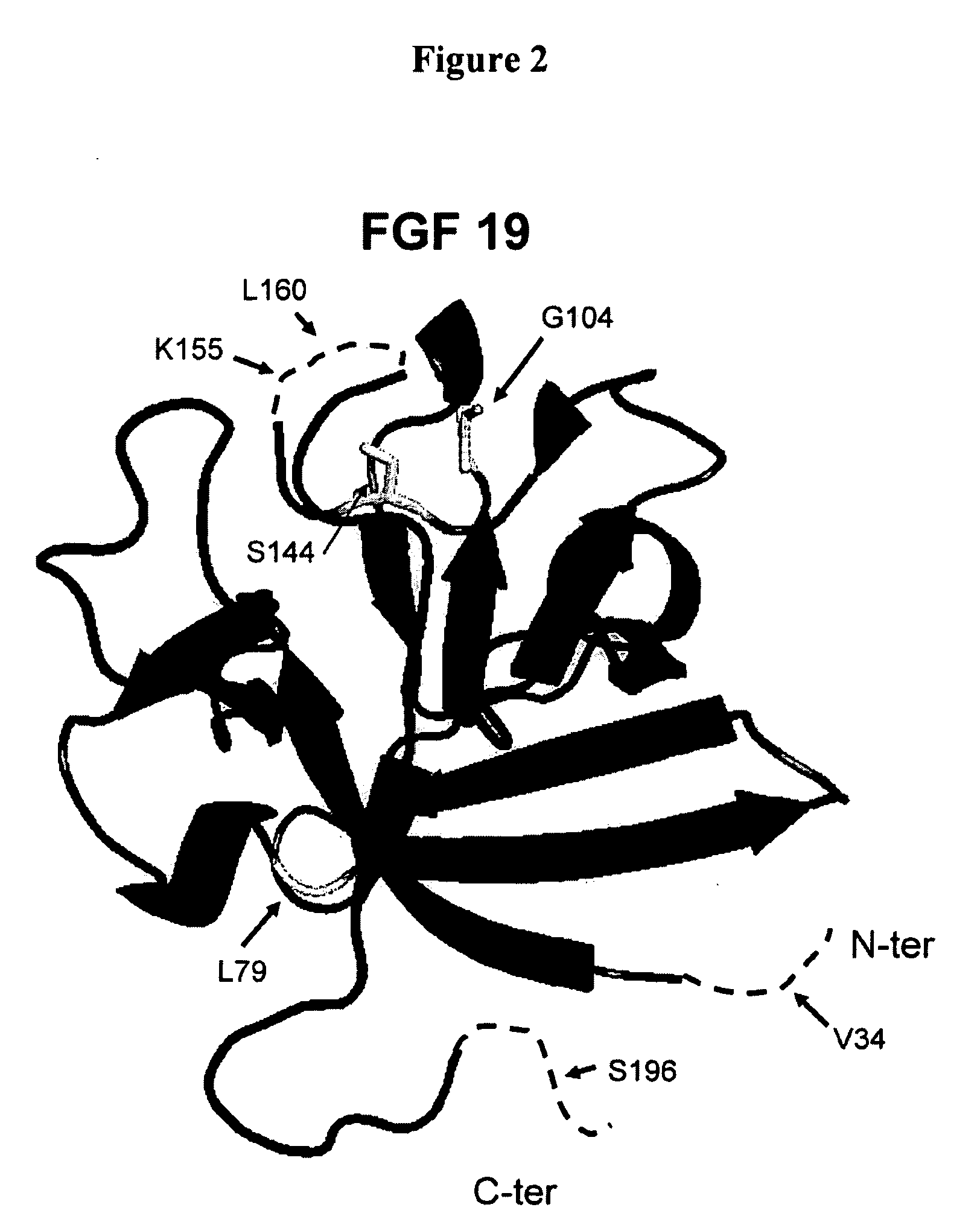
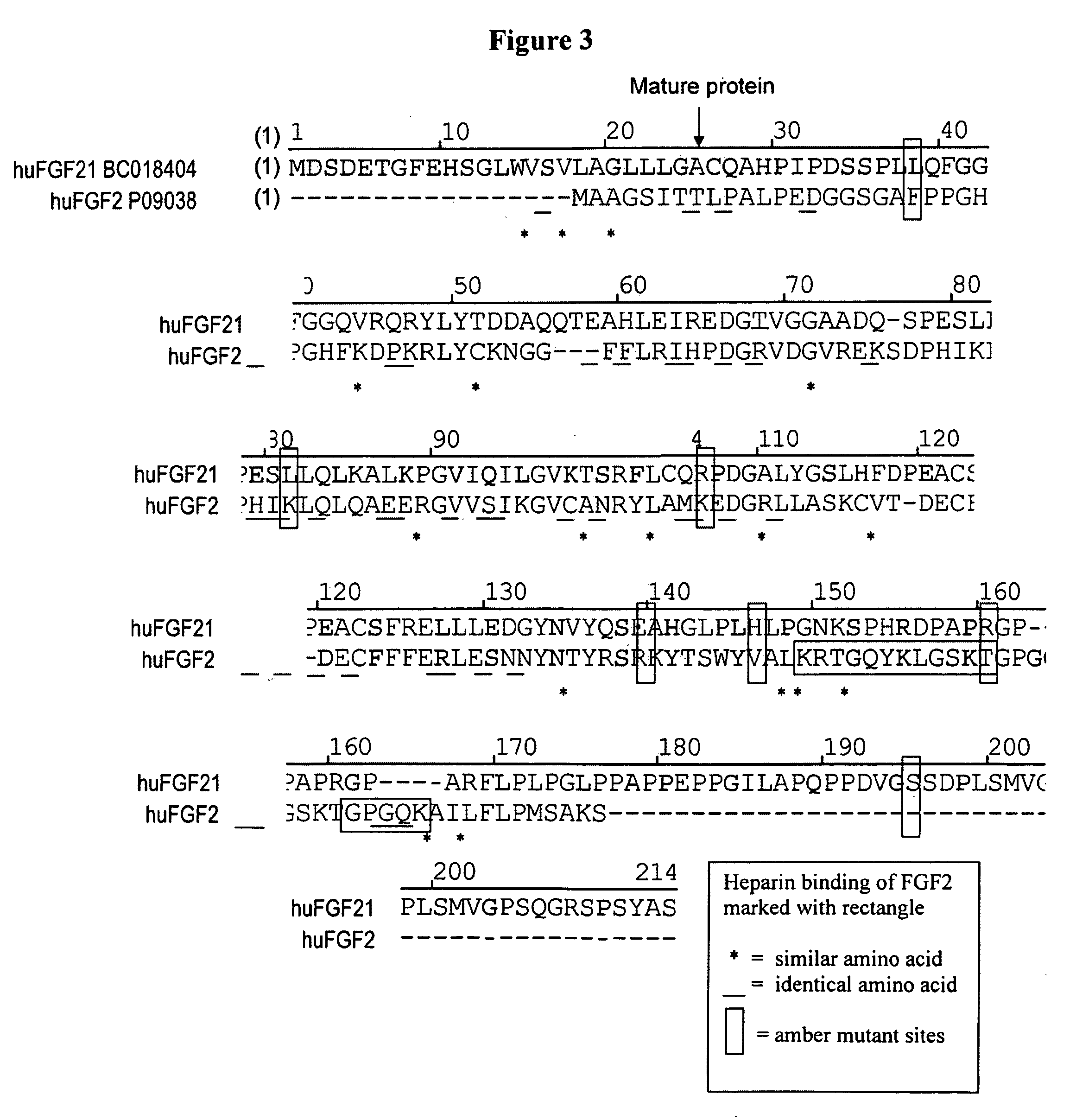
Modified FGF-21 Polypeptides and Their Uses
ActiveUS20080255045A1Increase in glucose uptakeFaster and efficient glucose utilizationAntibacterial agentsFungiChemistry
Owner:AMBRX
Liquid composition of biodegradable block copolymer for drug delivery system and process for the preparation thereof
InactiveUS6916788B2Rule out the possibilityAvoid hydrolysisAntibacterial agentsOrganic active ingredientsPolyethylene glycolWater soluble
The present invention relates to a liquid polymeric composition capable of forming a physiologically active substance-containing implant when it is injected into a living body and a method of preparation thereof. The composition comprises a water-soluble biocompatible liquid polyethylene glycol derivative, a biodegradable block copolymer which is insoluble in water but soluble in the water-soluble biocompatible liquid polyethylene glycol derivative and a physiologically active substance.
Owner:SAMYANG BIOPHARMLS CORP
Muteins of fibroblast growth factor 21
ActiveUS7622445B2Reduce sensitivityReduced O-glycosylationPeptide/protein ingredientsMetabolism disorderMutated proteinNucleic acid sequence
The present invention relates to novel muteins of human fibroblast growth factor-21 with reduced susceptibility for proteolytic degradation when expressed in yeast. Both protein and the respective encoding nucleic acid species are disclosed. The invention also embodies vectors and host cells for the propagation of said nucleic acid sequences and the production of said muteins. Also disclosed are methods for treating type 2 diabetes, obesity, or metabolic syndrome.
Owner:ELI LILLY & CO
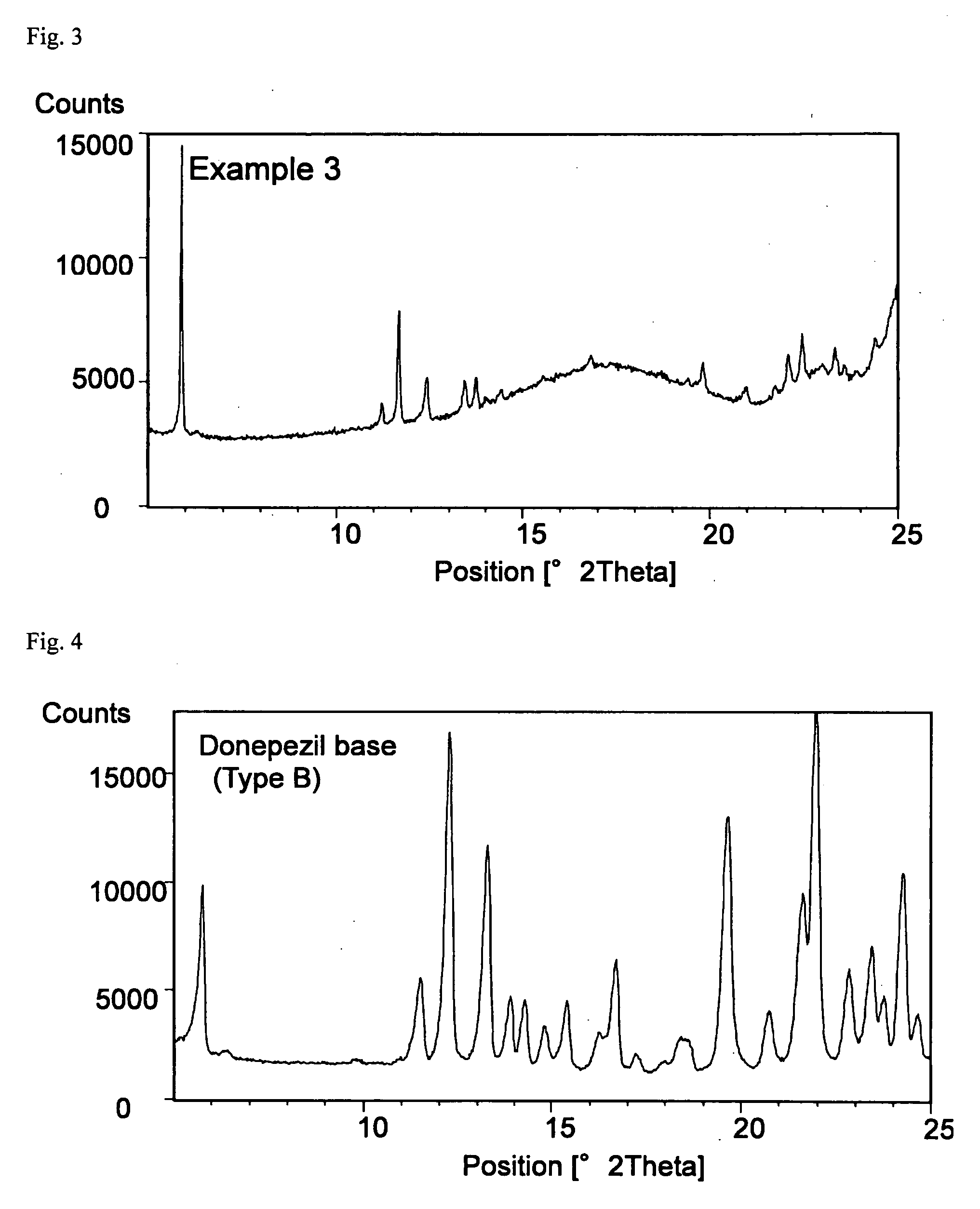
Transdermally absorbable Donepezil Preparation
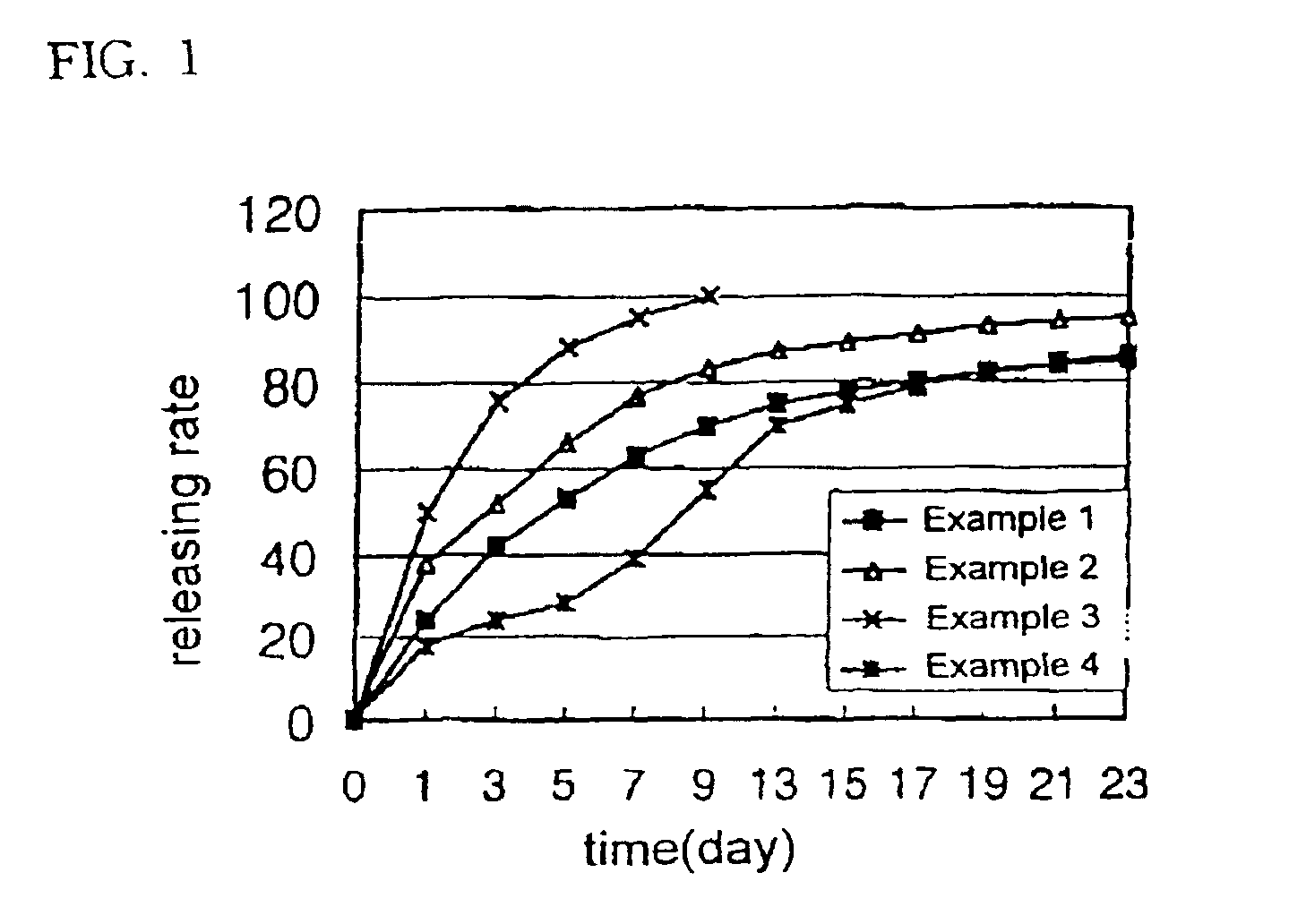
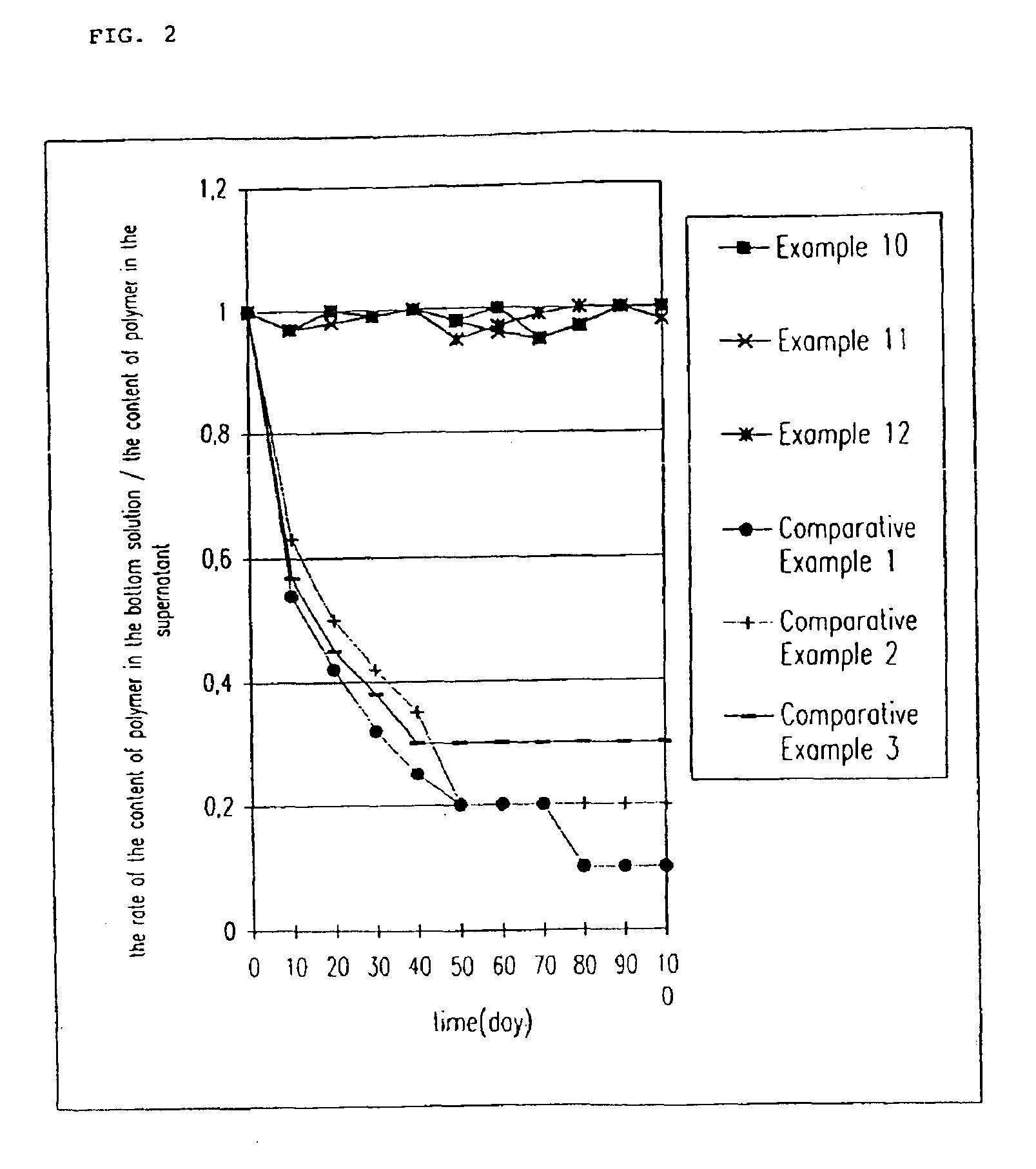
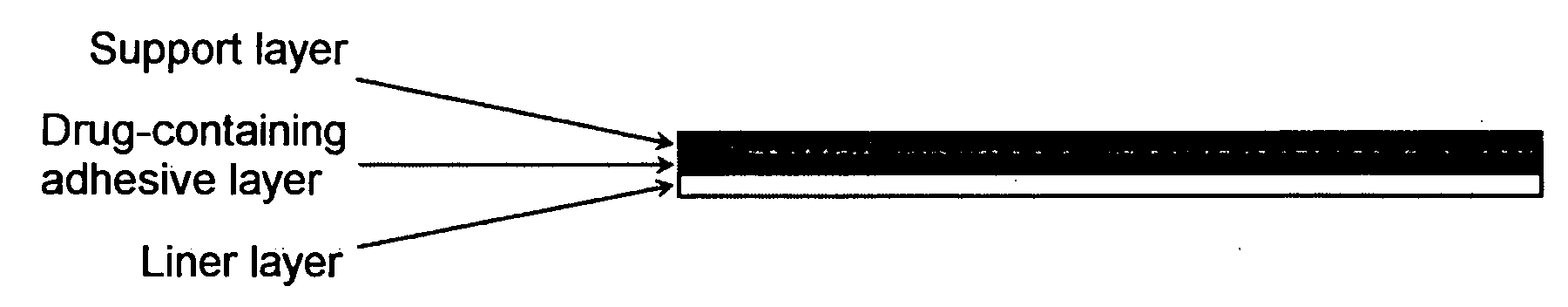
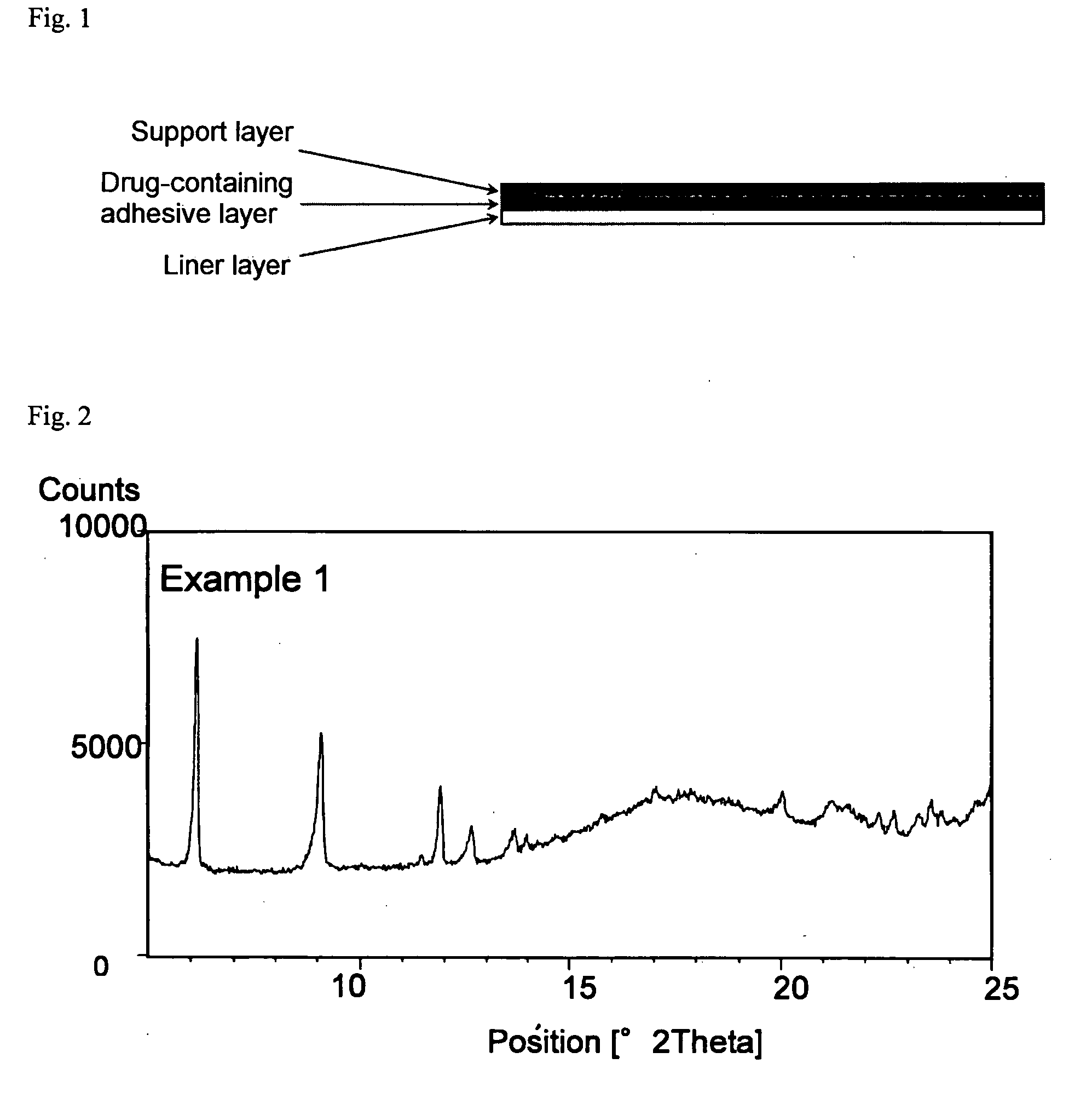
InactiveUS20090175929A1Improve drug stabilityLess localized stimulationBiocideNervous disorderChemistryUnexpected therapeutic effect
Disclosed is a donepezil-containing transdermally absorbable preparation which develops reduced adverse side effects and shows a satisfactory level of therapeutic effect. The preparation comprises an adhesive and a donepezil component (containing crystalline donepezil having type-B crystal polymorphism) and / or a salt thereof, wherein the donepezil component or the salt thereof is contained in an amount of 9 to 50% by mass relative to the total weight of the adhesive. The preparation (particularly, one having a non-aqueous adhesive layer) shows an excellent penetration of donepezil and / or a salt thereof into the skin, retains good stability of the active ingredient therein, and is remarkably reduced in local stimulation and adverse side effects.
Owner:HISAMITSU PHARM CO INC
Peptide boronic acid inhibitors
InactiveUS20060084592A1Low variabilityImprove bioavailabilityPeptide/protein ingredientsPeptide sourcesArylSulfur
A pharmaceutically acceptable base addition salt of an organoboronic acid of formula (XXX): wherein: P is hydrogen or an amino-group protecting moiety; R is hydrogen or alkyl; A is 0, 1 or 2; R1, R2 and R3 are independently hydrogen, alkyl, cycloalkyl, aryl or —CH2—R5; R5, in each instance, is one of aryl, aralkyl, alkaryl, cycloalkyl, heterocyclyl, heteroaryl, or —W—R6, where W is a chalcogen and R6 is alkyl; and where the ring portion of any of said aryl, aralkyl, alkaryl, cycloalkyl, heterocyclyl, or heteroaryl in R1, R2, R3 or R5 can be optionally substituted.
Owner:TRIGEN
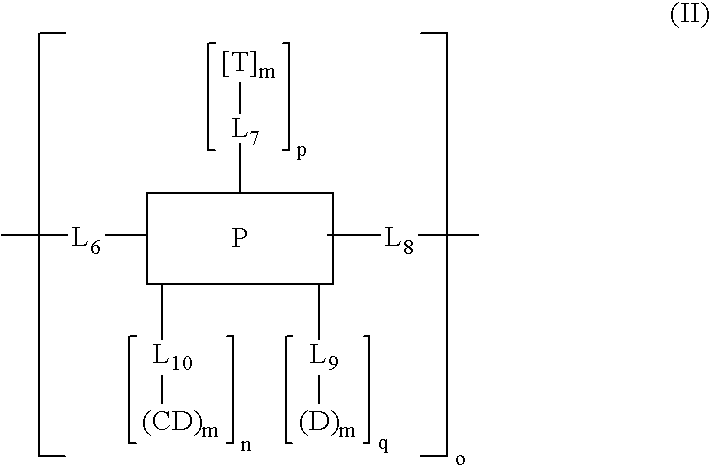
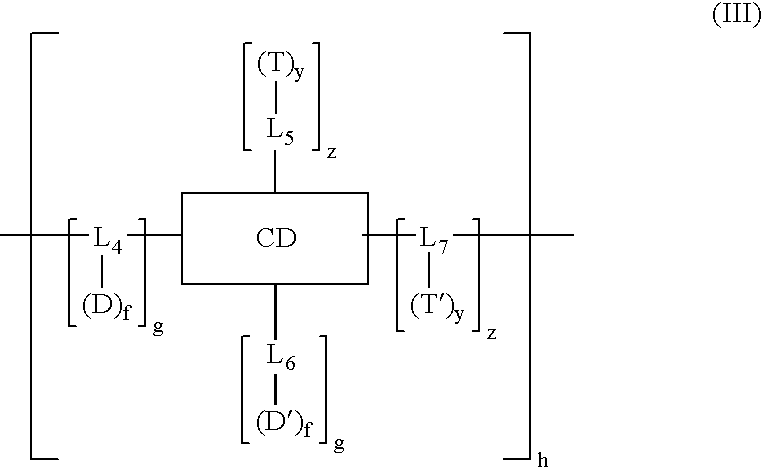
Cyclodextrin-based polymers for therapeutics delivery
InactiveUS20060210527A1Improve drug stabilityImprove solubilityNanomedicinePharmaceutical non-active ingredientsPresent methodCyclodextrin
The present invention relates to novel compositions of therapeutic polymeric compounds designed as carriers for small molecule therapeutics delivery and pharmaceutical compositions thereof. In some embodiments, the small molecule therapeutic is attached to the polymer by a photocleavable linker. The polymeric compounds may also employ targeting agents. By selecting from a variety of linker groups and targeting ligands the polymers present methods for controlled delivery of the therapeutic agents. On reaching a targeted site in the body of a patient, the linker can then be cleaved by the shining of ultraviolet, visible, or infrared wavelength light onto the site. The methods provide reduced toxicity and local delivery of therapeutics. The invention also relates to methods of treating subjects with the therapeutic compositions described herein. The invention further relates to methods for conducting a pharmaceutical business comprising manufacturing, licensing, or distributing kits containing or relating to the polymeric compounds described herein.
Owner:CERULEAN PHARMA
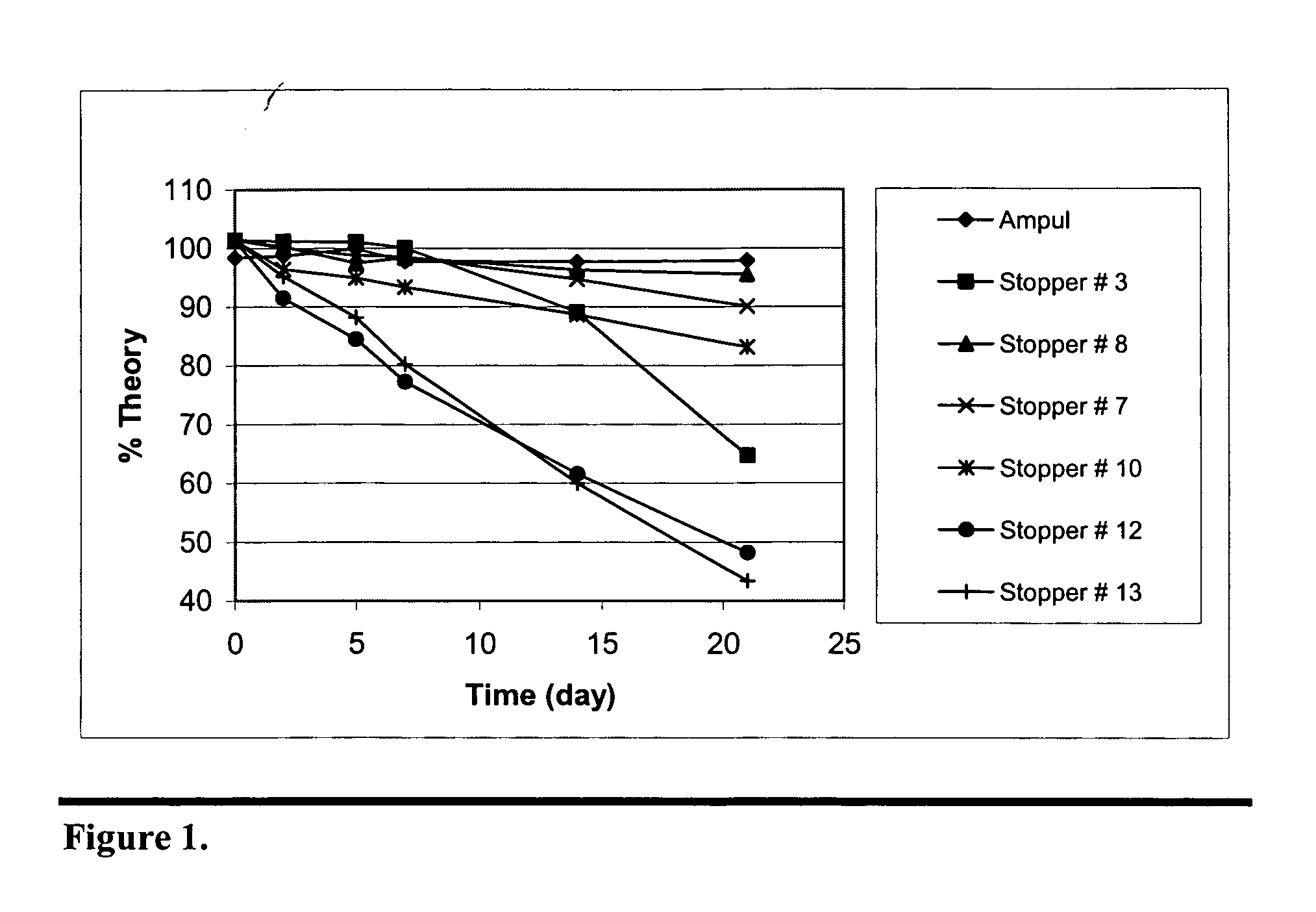
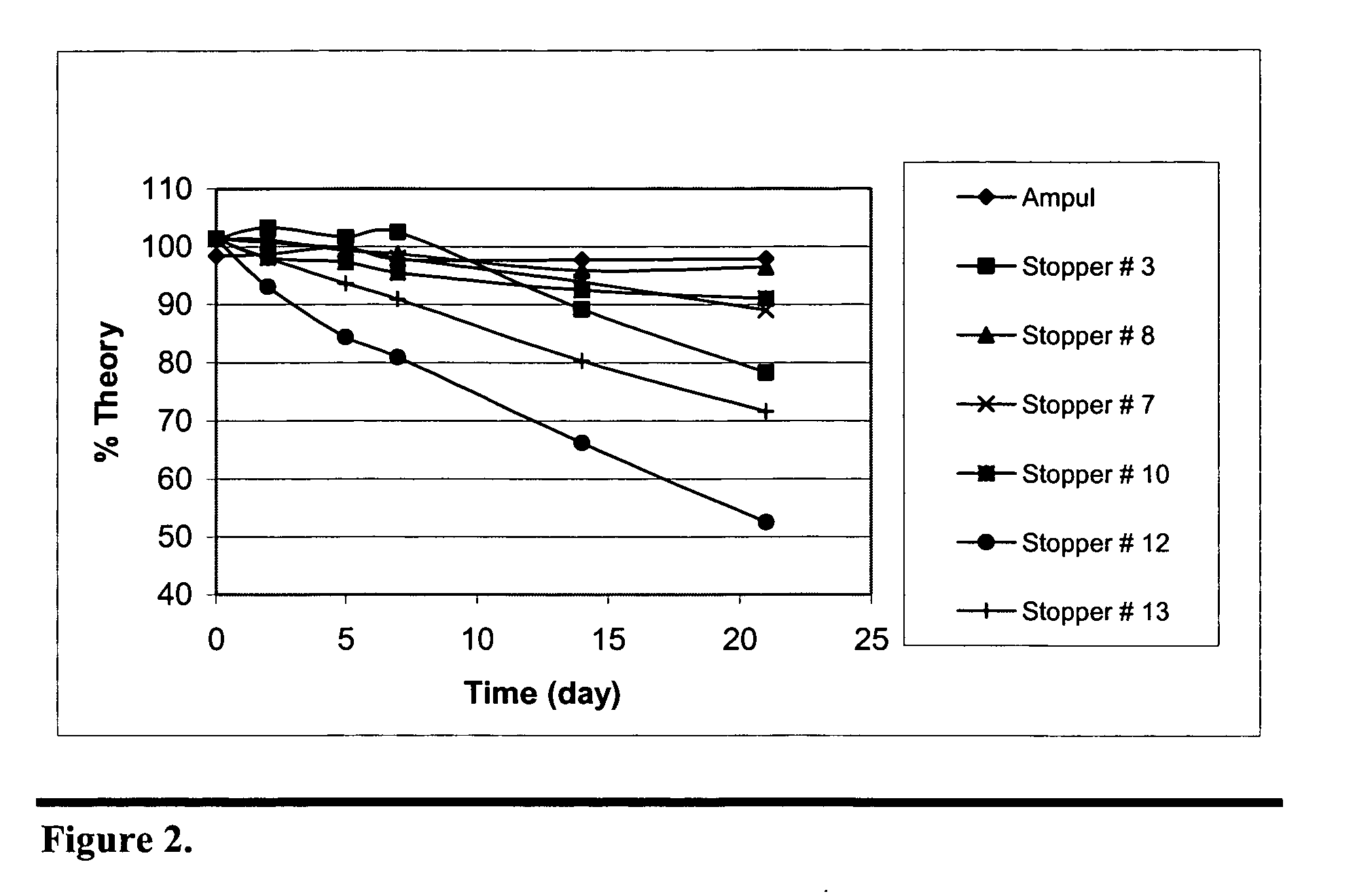
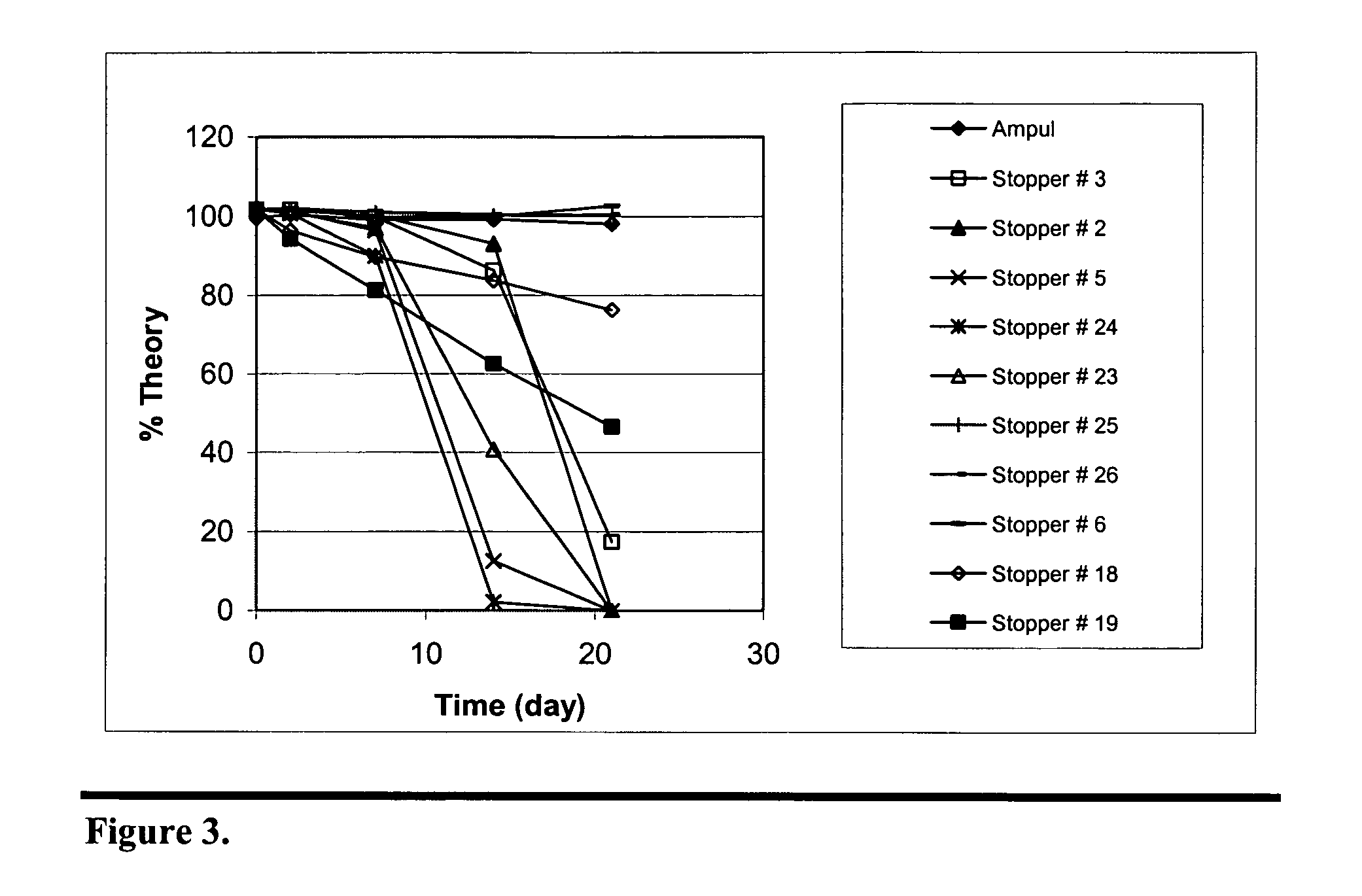
Stabilization of paricalcitol using chlorobutyl or chlorinated butyl stoppers
InactiveUS20070166187A1Extended shelf lifeImprove drug stabilityPharmaceutical containersPharmaceutical delivery mechanismParicalcitolEnvironmental chemistry
This invention relates to a method of enhancing the stability of paricalcitol solution in a container by using a chlorobutyl or chlorinated butyl stopper in the container.
Owner:ABBOTT LAB INC
Methods and compositions for reducing body fat and adipocytes
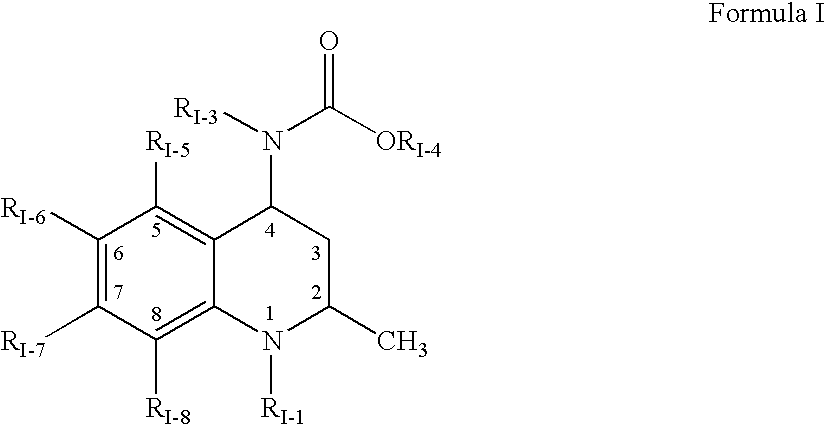
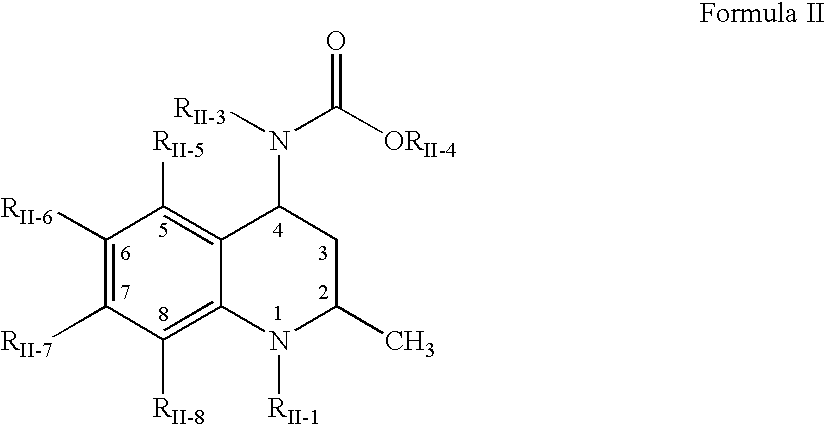
ActiveUS8426471B1Improve propertiesEnhance pharmaceuticallyCosmetic preparationsBiocideReducing bodiesProdrug
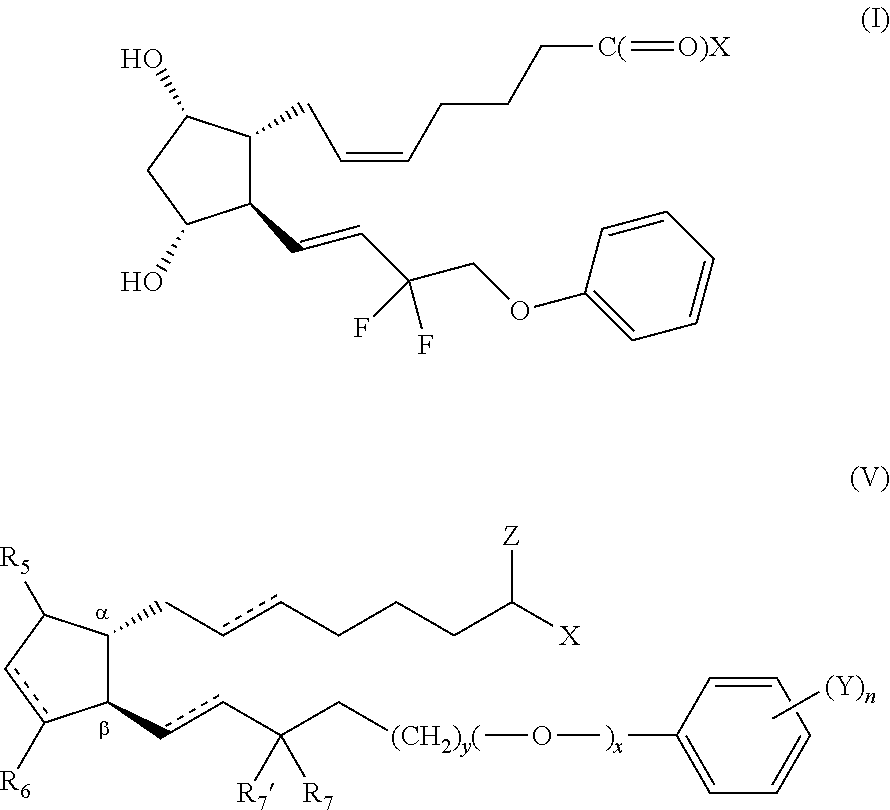
Provided are methods of reducing body fat in a subject, comprising locally (e.g., topically) administering one or more compounds of the Formula (I):or a pharmaceutically acceptable salt, hydrate, solvate, stereoisomer, polymorph, tautomer, isotopically enriched derivative, or prodrug thereof, wherein X is —OR1, —SR2, or —NR3R4, and R1, R2, R3, and R4 are as defined herein.
Owner:TOPOKINE THERAPEUTICS
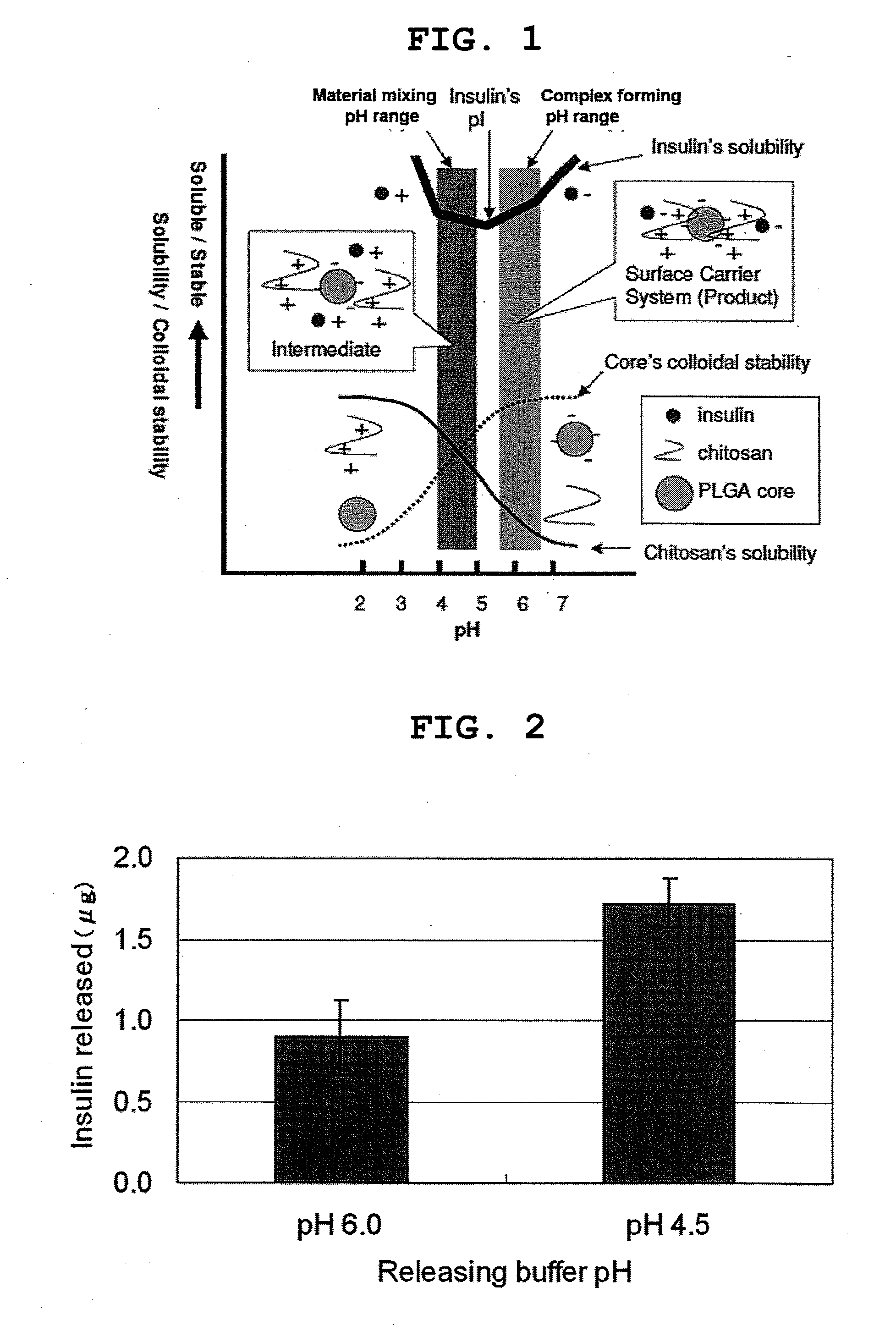
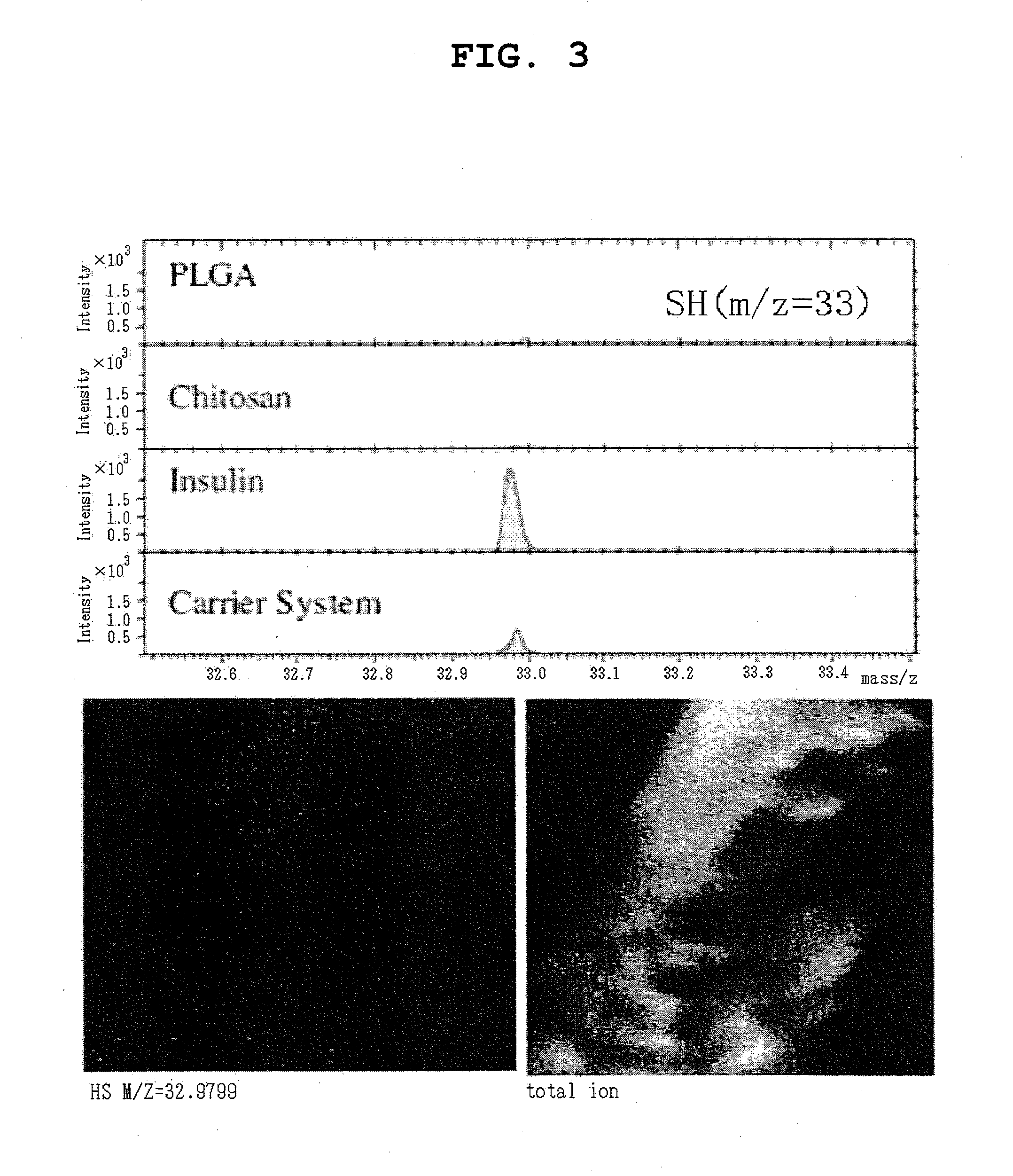
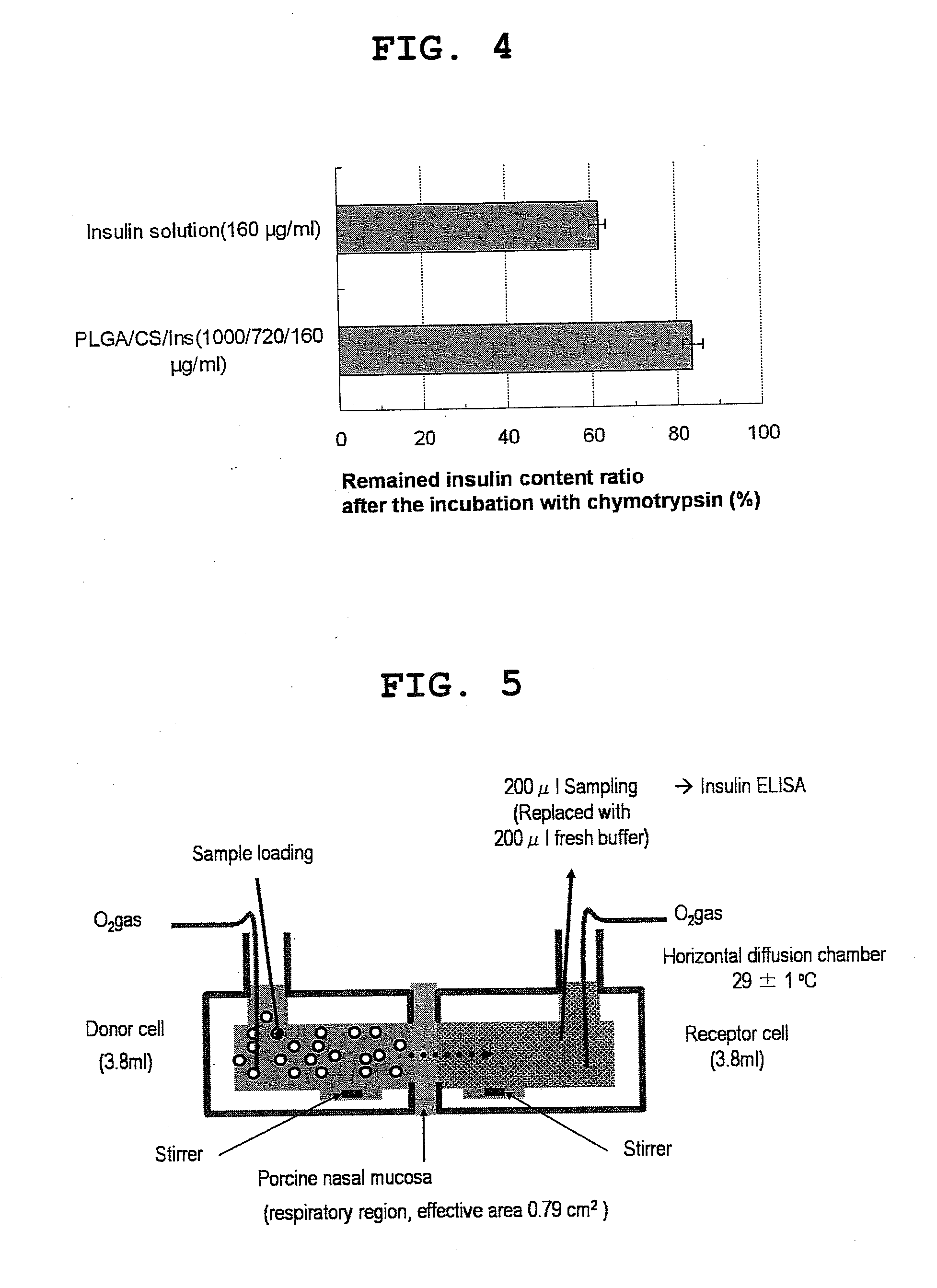
Pharmaceutical composition containing surface-coated microparticles
InactiveUS20110189299A1Increase capacityImprove efficiencyPowder deliveryOrganic active ingredientsMedicineMicroparticle
The invention provides a pharmaceutical composition that can be used for efficient administration of low-molecular weight drugs and polymeric compounds such as peptides and proteins by methods other than injection, as well as a method for producing the composition. The pharmaceutical composition is for transmucosal administration and comprises (a) a drug having a positive or negative charge at a predetermined pH, (b) a pharmaceutically acceptable small particle and (c) a pharmaceutically acceptable surface-coating polymer capable of being electrically charged at the pH, wherein the surface of the small particle is coated by the surface-coating polymer, the drug is immobilized on the surface of the small particle via the surface-coating polymer, and a complex is formed by a noncovalent interaction between the small particle and the surface-coating polymer and a concurrent electrostatic interaction between the surface-coating polymer and the drug.
Owner:NITTO DENKO CORP
Bifunctional stapled polypeptides and uses thereof
ActiveUS9163330B2Increase productionAvoid unfoldingPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseBifunctional
The invention relates to bifunctional stapled or stitched peptides comprising a targeting domain, a linker moiety, and an effector domain, that can be used to tether, or to bring into close proximity, at least two cellular entities (e.g., proteins). Certain aspects relate to bifunctional stapled or stitched peptides that bind to an effector biomolecule through the effector domain and bind to a target biomolecule through the targeting domain. Polypeptides and / or polypeptide complexes that are tethered by the bifunctional stapled or stitched peptides of the invention, where the effector polypeptide bound to the effector domain of the bifunctional stapled or stitched peptide modifies or alters the target polypeptide bound to the targeting domain of the bifunctional peptide. Uses of the inventive bifunctional stapled or stitched peptides including methods for treatment of disease (e.g., cancer, inflammatory diseases) are also provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
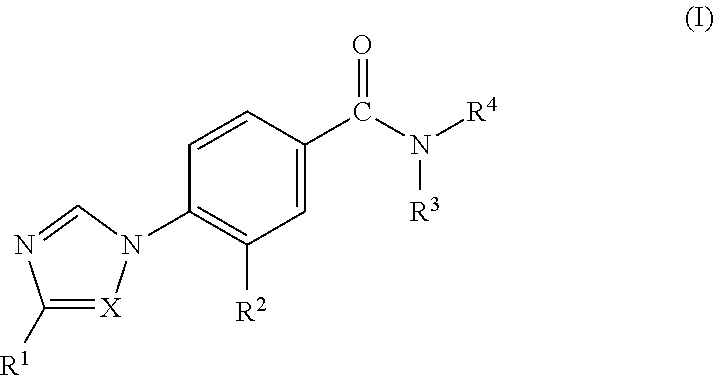
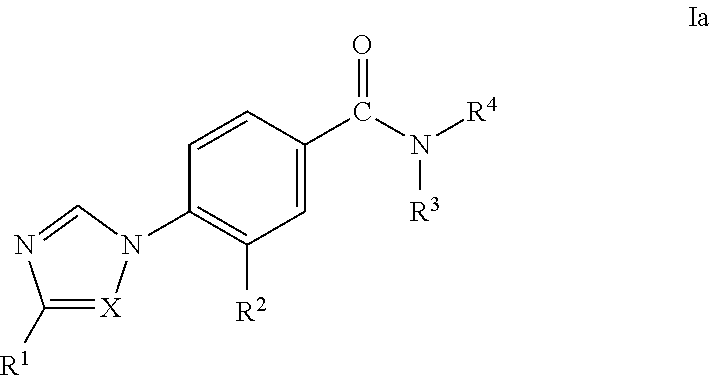
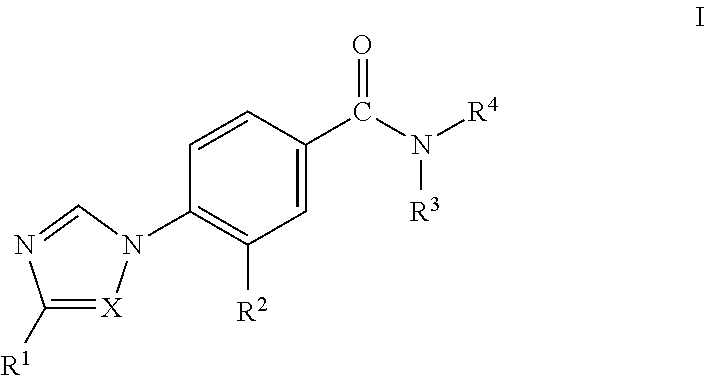
Novel 4-(Substituted Amino)-7H-Pyrrolo[2,3-d] Pyrimidines As LRRK2 Inhibitors
ActiveUS20140005183A1Desirable solubility in waterImprove drug stabilityBiocideNervous disorderLeprosyMedicine
The present invention provides novel 4,5-disubstituted-7H-pyrrolo[2,3-d]pyrimidine derivatives of Formula I, and the pharmaceutically acceptable salts thereofwherein R1, R2, R3, R4 and R5 are as defined in the specification. The invention is also directed to pharmaceutical compositions comprising the compounds of formula I and to use of the compounds in the treatment of diseases associated with LRRK2, such as neurodegenerative diseases including Parkinson's disease or Alzheimer's disease, cancer, Crohn's disease or leprosy.
Owner:PFIZER INC
Pharmaceutical Compositions of Adsorbates of Amorphous Drug
ActiveUS20080292707A1Improve concentrationImprove drug stabilityAntibacterial agentsPowder deliverySolubilityHigh surface area
Pharmaceutical compositions comprise a low-solubility drug adsorbed onto a high surface area substrate to form an adsorbate. The compositions in some embodiments include a concentration-enhancing polymer.
Owner:BEND RES
Use of proton sequestering agents in drug formulations
InactiveUS20050013867A1Extended shelf lifeImprove drug stabilityPowder deliveryOrganic active ingredientsCombinatorial chemistrySpray dried
Methods are provided for preparing spray-dried, drug-containing particles comprising the steps of: (a) selecting drug, an aqueous solution, and a proton-sequestering agent; (b) adding the drug and the proton-sequestering agent to the solution to form a feed solution; and (c) spray drying the feed solution to form the spray-dried, drug-containing particles, wherein at least a portion of the proton-sequestering agent remains mixed with the drug in the spray-dried, drug containing particles, particles and pharmaceutical formulations comprising the prepared particles as well as methods of use are also provided.
Owner:NOVARTIS FARMA
Methods and compositions for reducing body fat and adipocytes
ActiveUS20130178525A1Improve propertiesEnhance pharmaceuticallyBiocideCosmetic preparationsReducing bodiesProdrug
Owner:TOPOKINE THERAPEUTICS
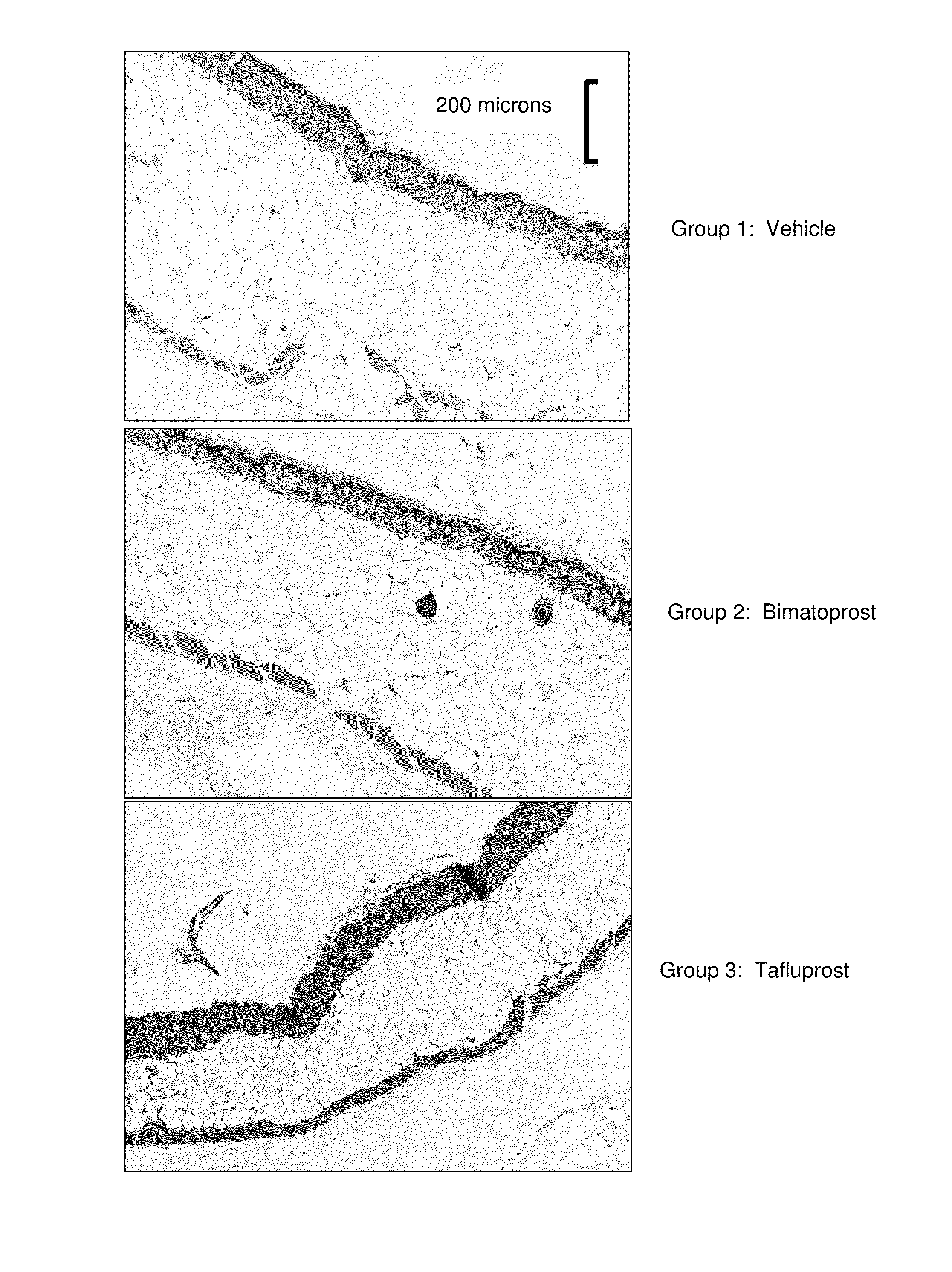
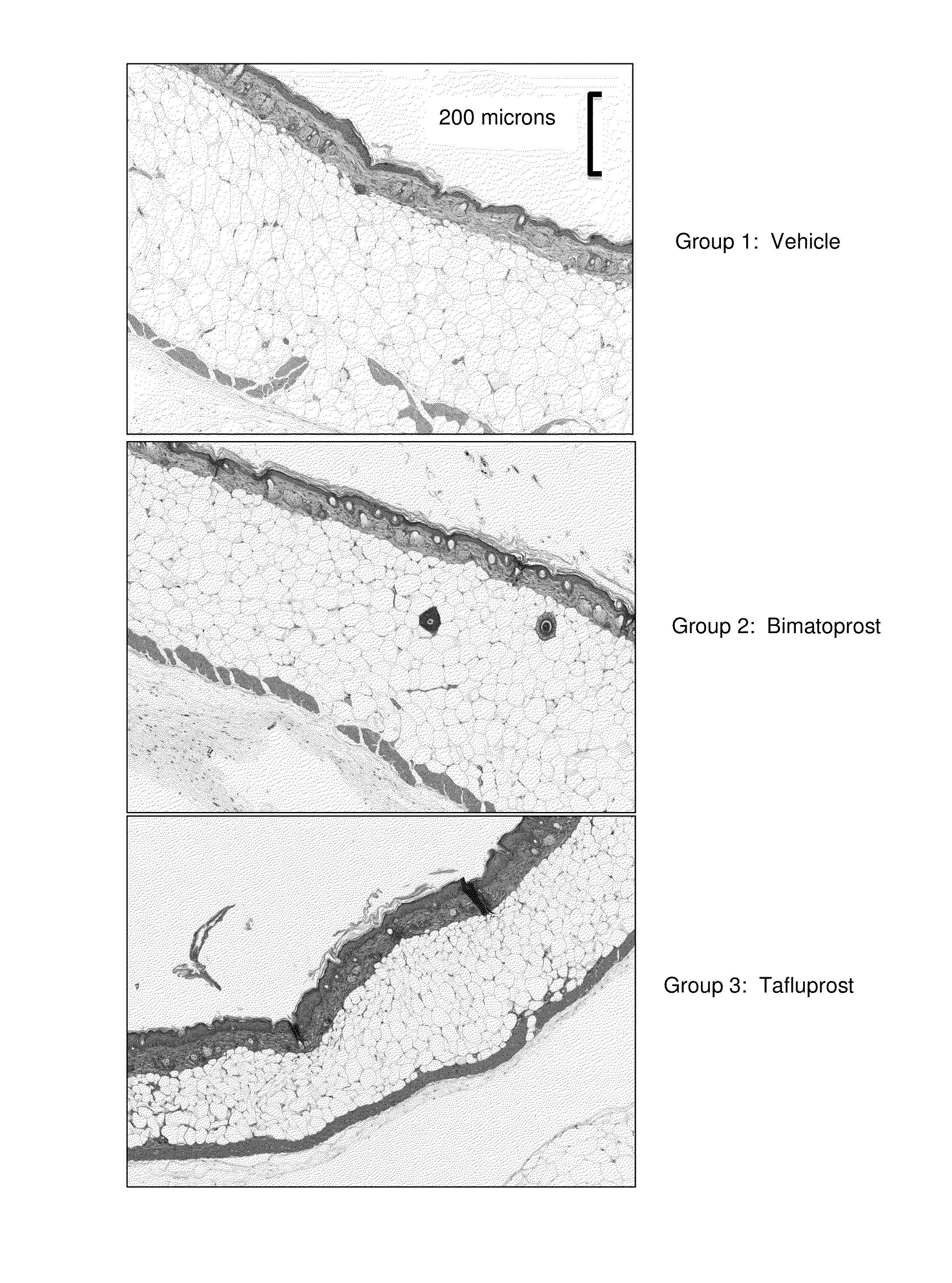
Therapeutic drug compositions and implants for delivery of same
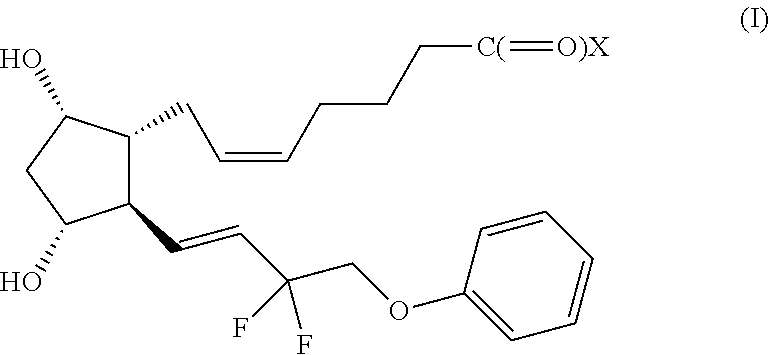
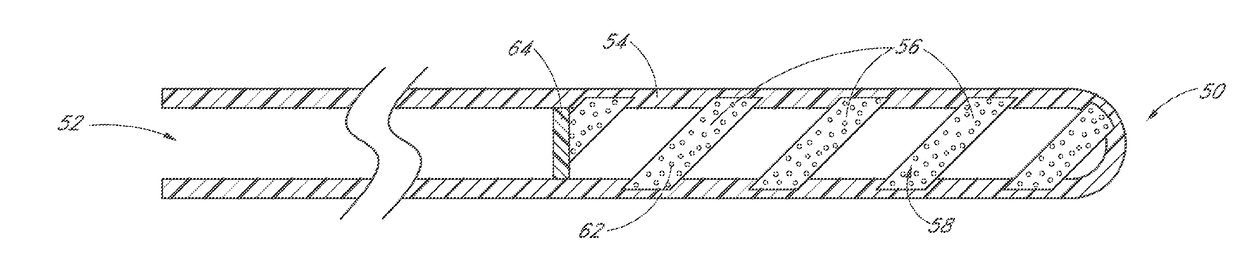
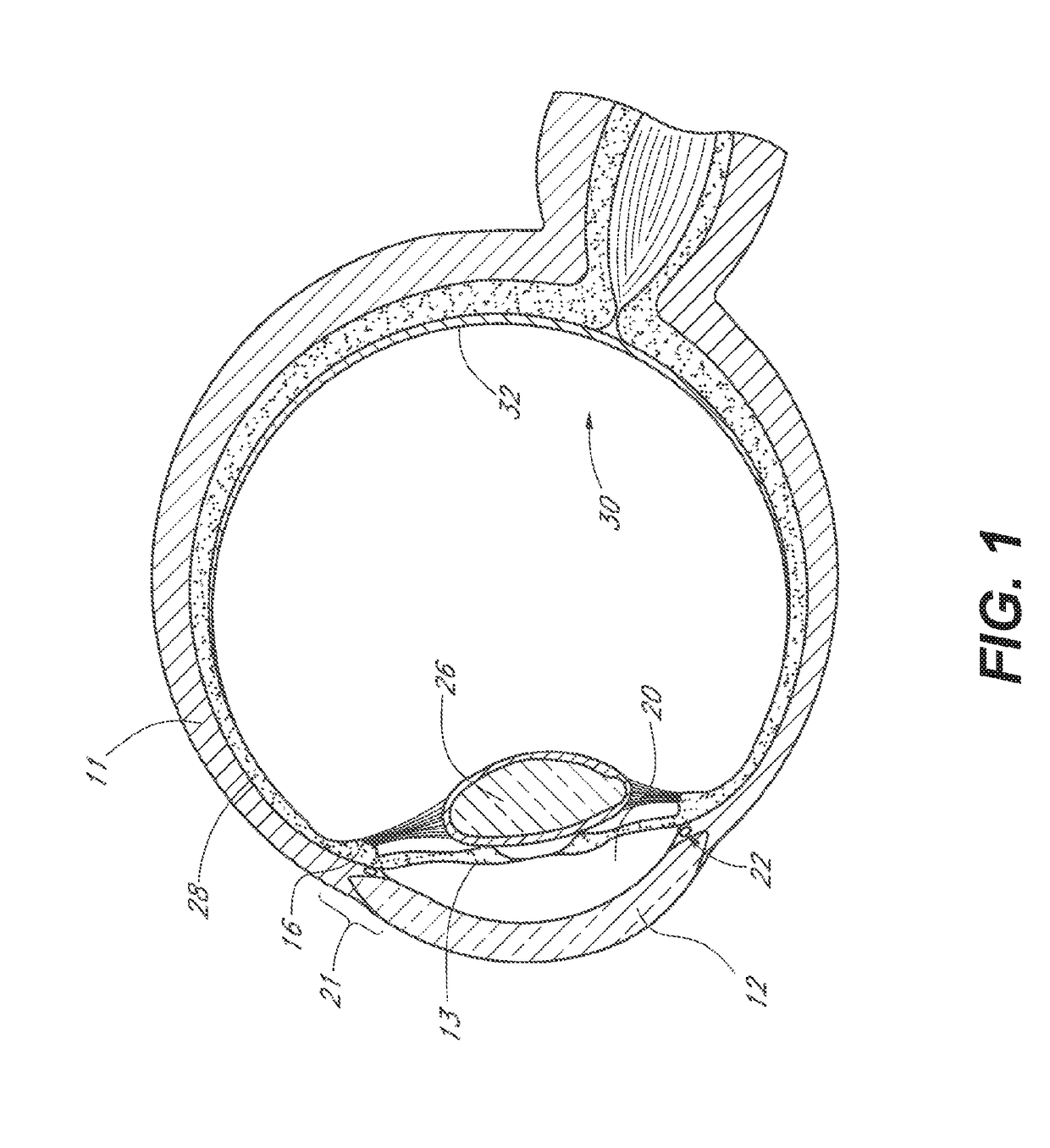
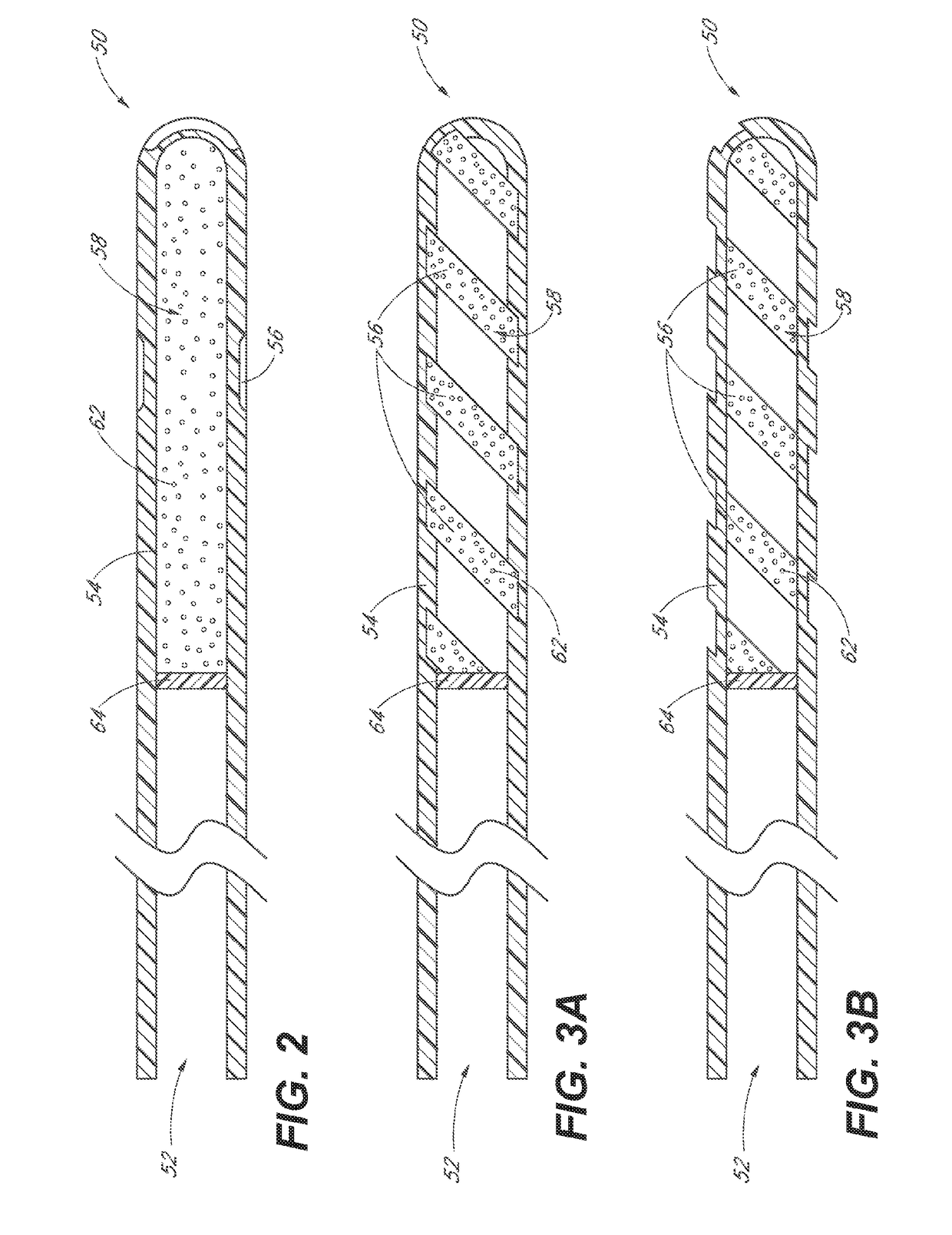
InactiveUS20180280194A1Improve drug stabilityImprove stabilityOrganic active ingredientsEye treatmentTherapeutic effectExtended time
Disclosed herein are drug delivery devices and methods for the treatment of ocular disorders requiring targeted and controlled administration of a drug to an interior portion of the eye for reduction or prevention of symptoms of the disorder. In several embodiments, the devices are configured to release a pro-drug form of a drug into a target tissue site, wherein the pro-drug is converted to an active drug that yields a therapeutic effect. The use of the device and pro-drug form advantageously, in several embodiments, provide a stable drug composition that can yield a therapeutic effect over an extended time period.
Owner:GLAUKOS CORP
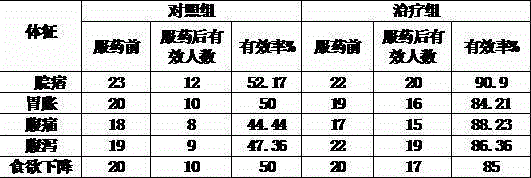
Yueju Baohe dispersible tablets and preparing method thereof
InactiveCN106728634AWith industrial productionImprove solubilityDigestive systemPill deliveryMaterials preparationTraditional medicine
The invention discloses Yueju Baohe dispersible tablets and a preparing method thereof. The Yueju Baohe dispersible tablets are prepared from, by weight, 10-50% of traditional Chinese medicine extract, 10-80% of filler, 5-50% of internally added disintegrant, 5-15% of externally added disintegrant, 0.1-10% of corrigent, 0.1-20% of wetting agent and 0.05-20% of lubricant. The traditional Chinese medicine extract comprises, by weight, 60-120 parts of ginger-processed fructus gardeniae, 60-120 parts of medicated leaven stir-fried with bran, 60-120 parts of vinegar-processed rhizoma cyperi, 60-120 parts of rhizoma chuanxiong, 60-120 parts of rhizoma atractylodis, 60-120 parts of radix aucklandiae and 60-120 parts of semen arecae. The preparing method comprises the steps of material preparation, extraction, tablet preparation and packaging. The Yueju Baohe dispersible tablets can soothe liver and dispel melancholy, whet the appetite and promote digestion, and are used for treating symptoms like dyspepsia, qi depression and stasis, poor appetite and thoracico-abdominal swelling pain.
Owner:HARBIN SHENGJI PHARMA
Novel Phenyl Imidazoles and Phenyl Triazoles As Gamma-Secretase Modulators
InactiveUS20120053165A1Ease of detectabilityEasy to prepareBiocideSenses disorderGamma secretaseTriazole
Owner:PFIZER INC
Negatively charged amphiphilic block copolymer as drug carrier
InactiveUS6890560B2Increase blood concentrationImprove drug stabilityPowder deliveryMaterial nanotechnologyBlood concentrationDrug carrier
The present invention provides an anionic group-containing amphiphilic block copolymer that is biocompatible and biodegradable and, when used as a drug carrier for a cationic drug, provides several advantages such as increased blood concentration and improved stability of the drug.
Owner:SAMYANG BIOPHARMLS CORP
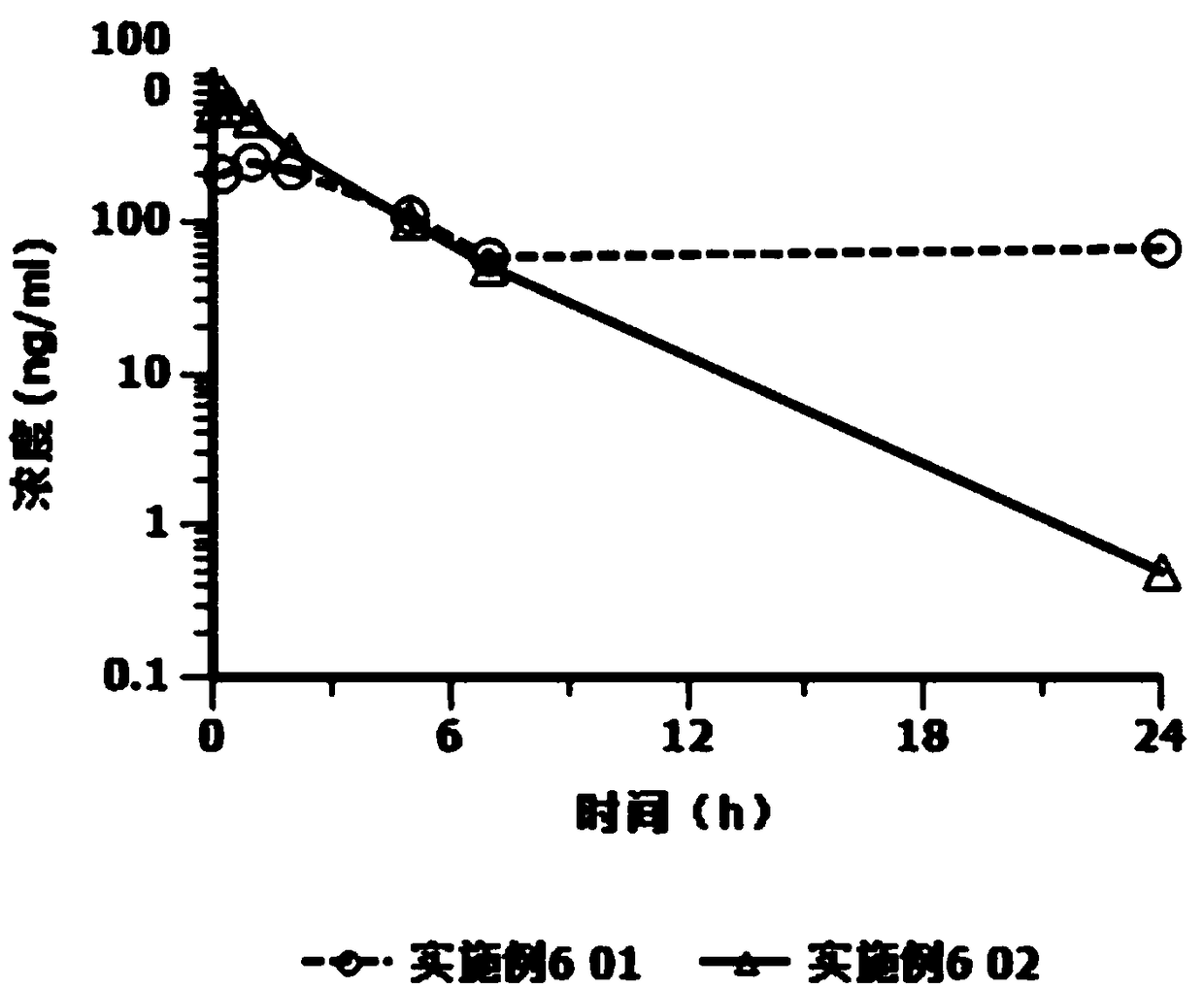
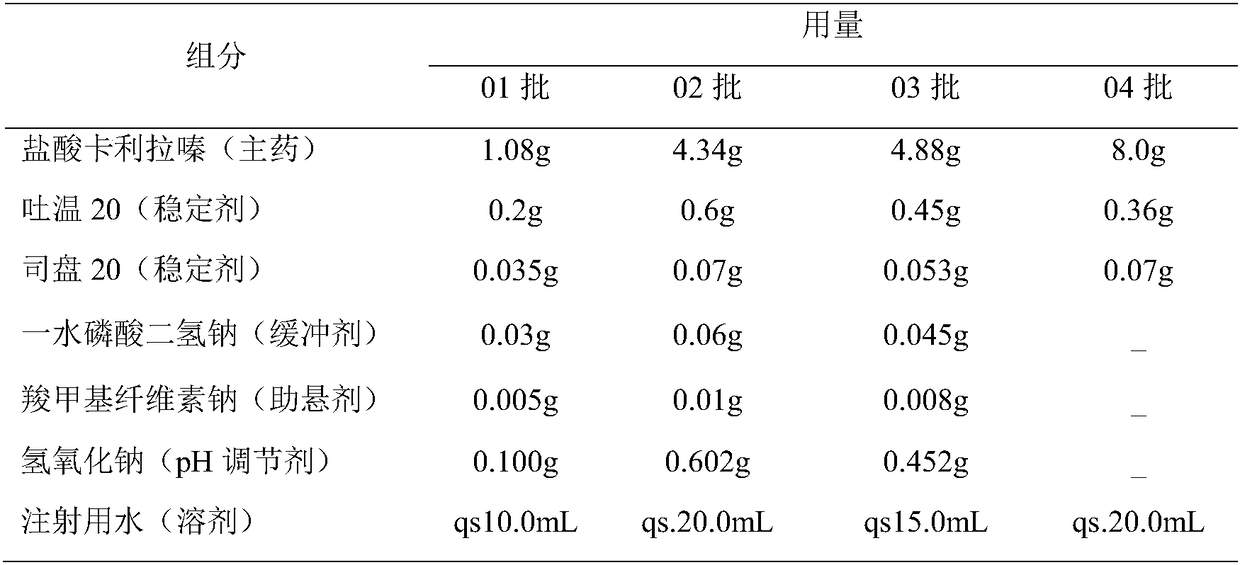
Cariprazine hydrochloride injection preparation, and preparation method and use thereof
ActiveCN108261394AImprove drug stabilitySmall batch-to-batch varianceOrganic active ingredientsPowder deliveryHigh concentrationInjection volume
The invention provides a cariprazine hydrochloride injection preparation, and a preparation method and a use thereof. The injection preparation is an aqueous suspension when used, cariprazine hydrochloride has a high concentration and a good stability, a high dosage can be obtained within a limited injection volume, the dosage can be flexibly adjusted according to the long-acting administration time, the particle size distribution and the injection dosage are controlled to achieve the long-acting effect, the cariprazine hydrochloride is continuously released in at least one week after the preparation is injected, and the preparation is administrated every one week or more to increase the compliance of patients. The invention also provides the preparation method of the cariprazine hydrochloride injection preparation. The injection preparation prepared by the method has the advantages of good stability and high safety, and the method is simple, is easy to implement, and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Heterocyclic substituted hexahydropyrano[3,4-d][1,3]thiazin-2-amine compounds
InactiveUS8962616B2Desirable solubility in waterImprove drug stabilitySenses disorderNervous disorderTautomer
Owner:PFIZER INC
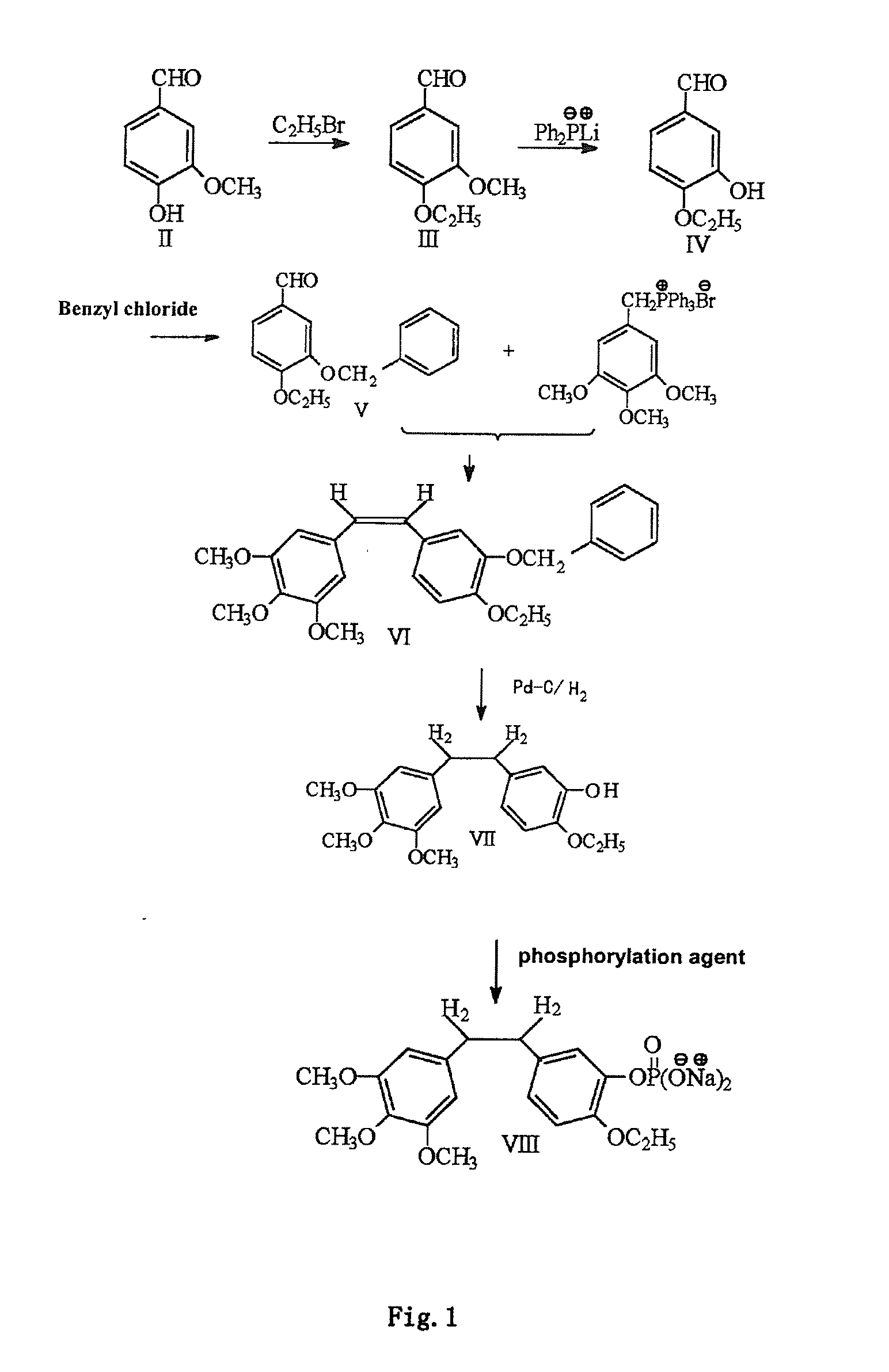
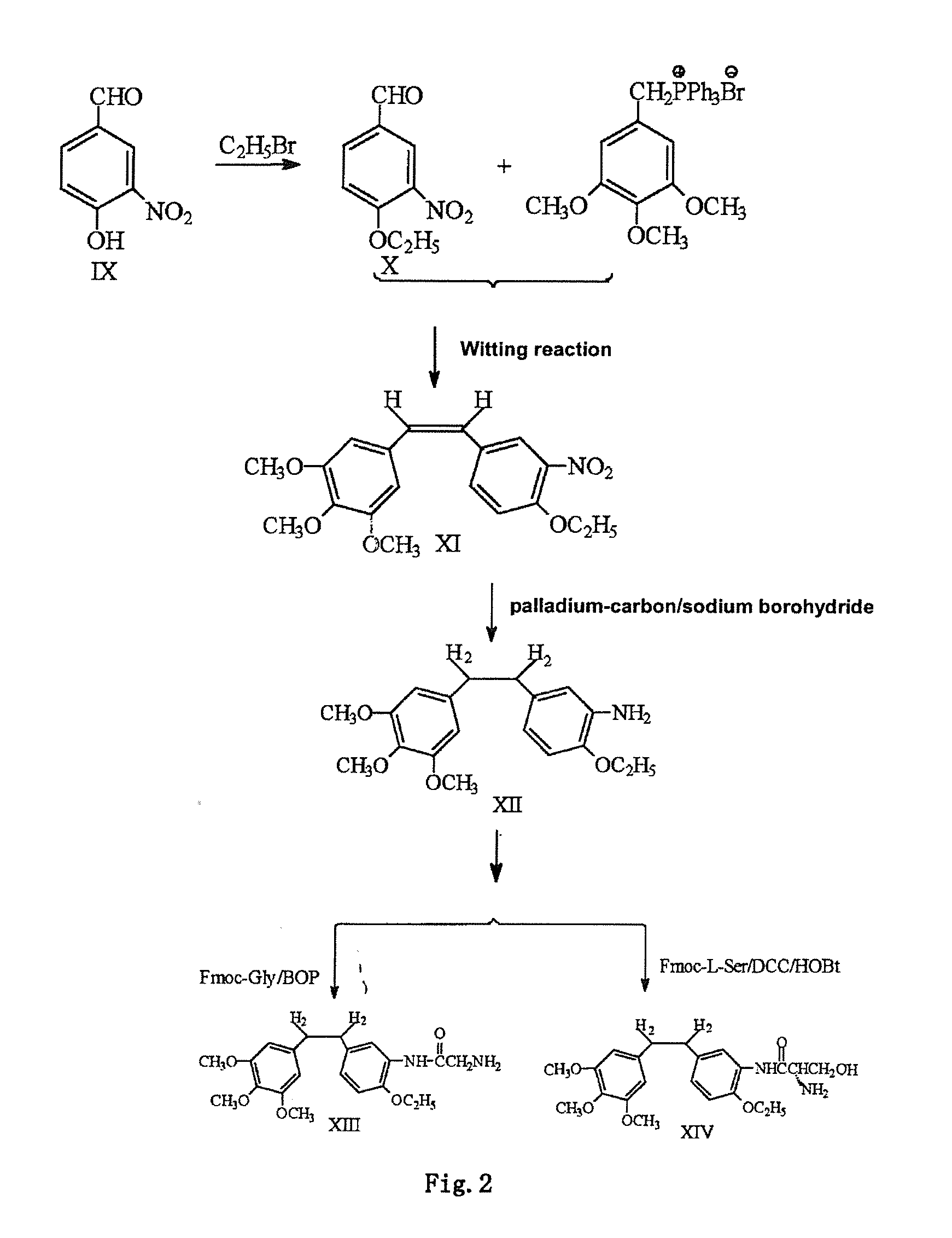
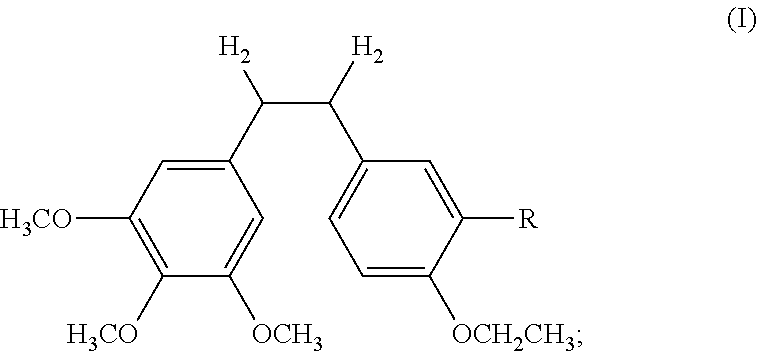
Ethoxy Diphenyl Ethane Derivatives, Preparation Processes and Uses Thereof
ActiveUS20120046492A1Improve drug stabilityLow toxicityOrganic compound preparationGroup 5/15 element organic compoundsAmino acid side chainVascular endothelium
The invention discloses an ethoxydiphenylethane derivative and a synthetic method and uses thereof 4′ position of phenylethane B aromatic ring is chemically modified by ethoxy and hydroxy at position 3′ thereof is simultaneously modified to water soluble prodrug such as phosphate, and similarly, amino acid side chain is introduced to amino at position 3′ to form amino acid amide water soluble prodrug having the structure shown as formula (I)the ethoxydiphenylethane derivative and the prodrug thereof include strong tubulin aggregation inhibiting ability and obvious target damage effect for tumor vessels, selectively cause dysfunction and structural damage of tumor vessels and induce apoptosis of vascular endothelial cells in order to play the role of killing tumor cells or inhibiting tumor metastasis in case that the tumor cells are free from the support of nutrition and oxygen.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD +1
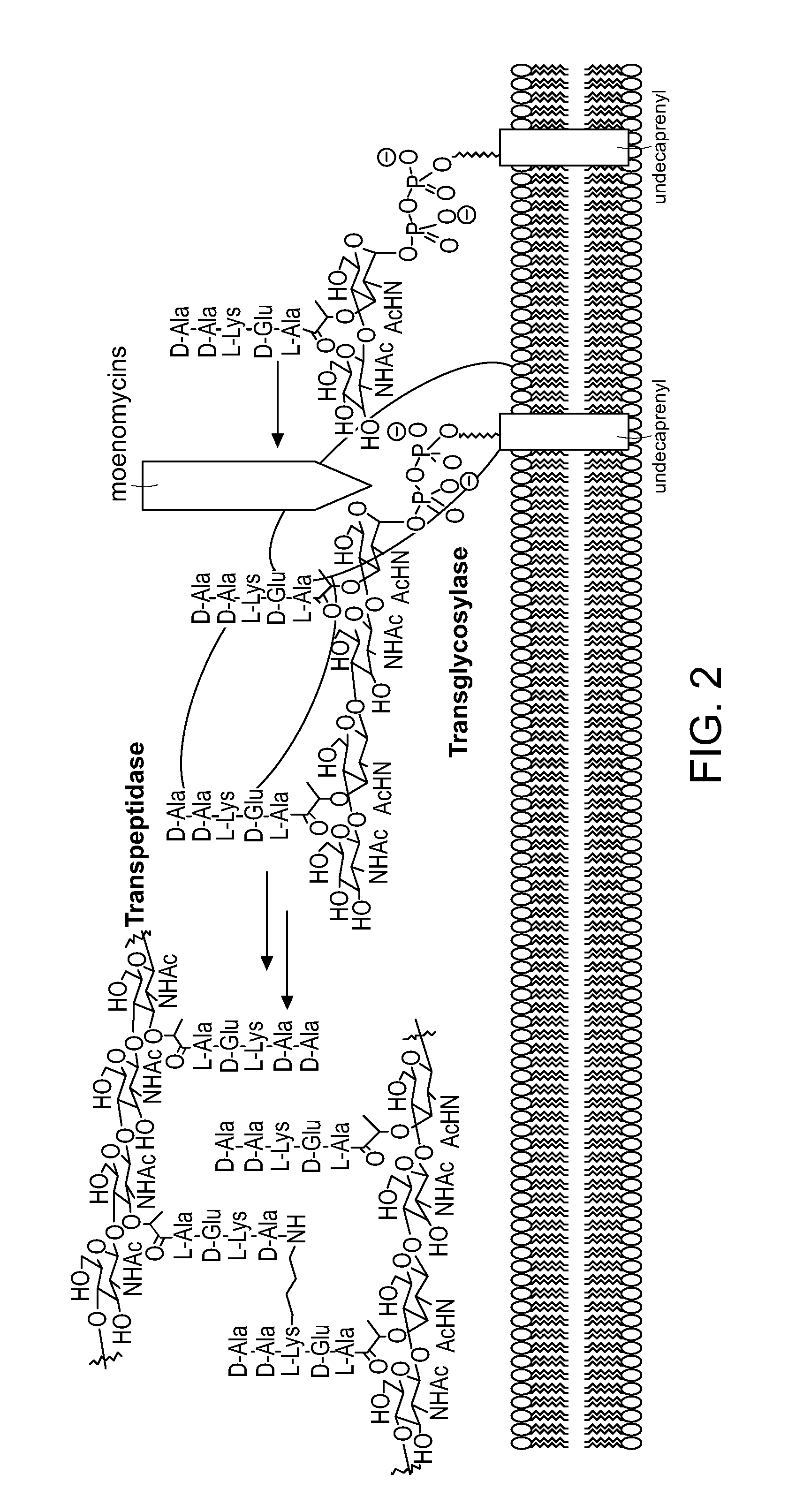
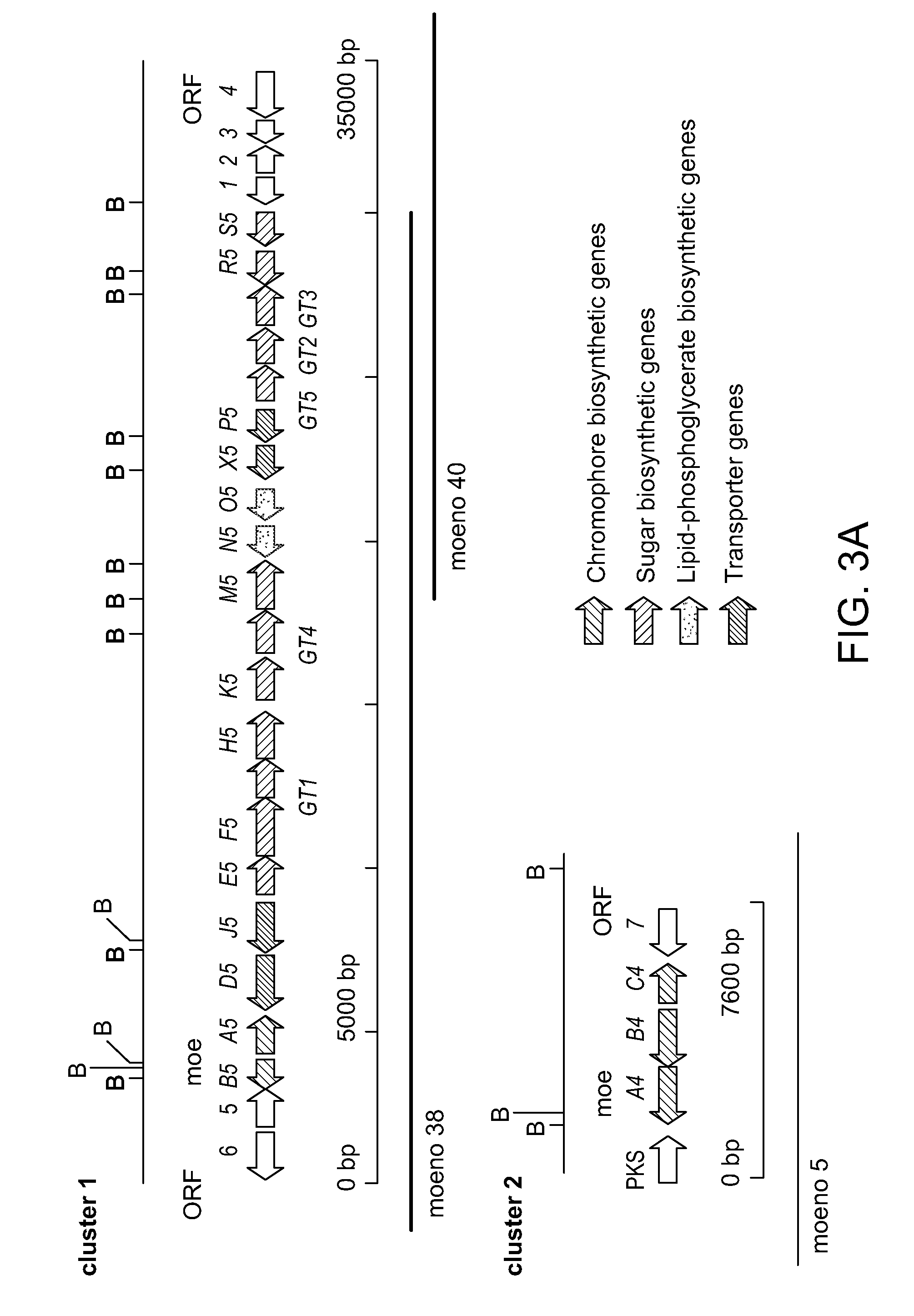
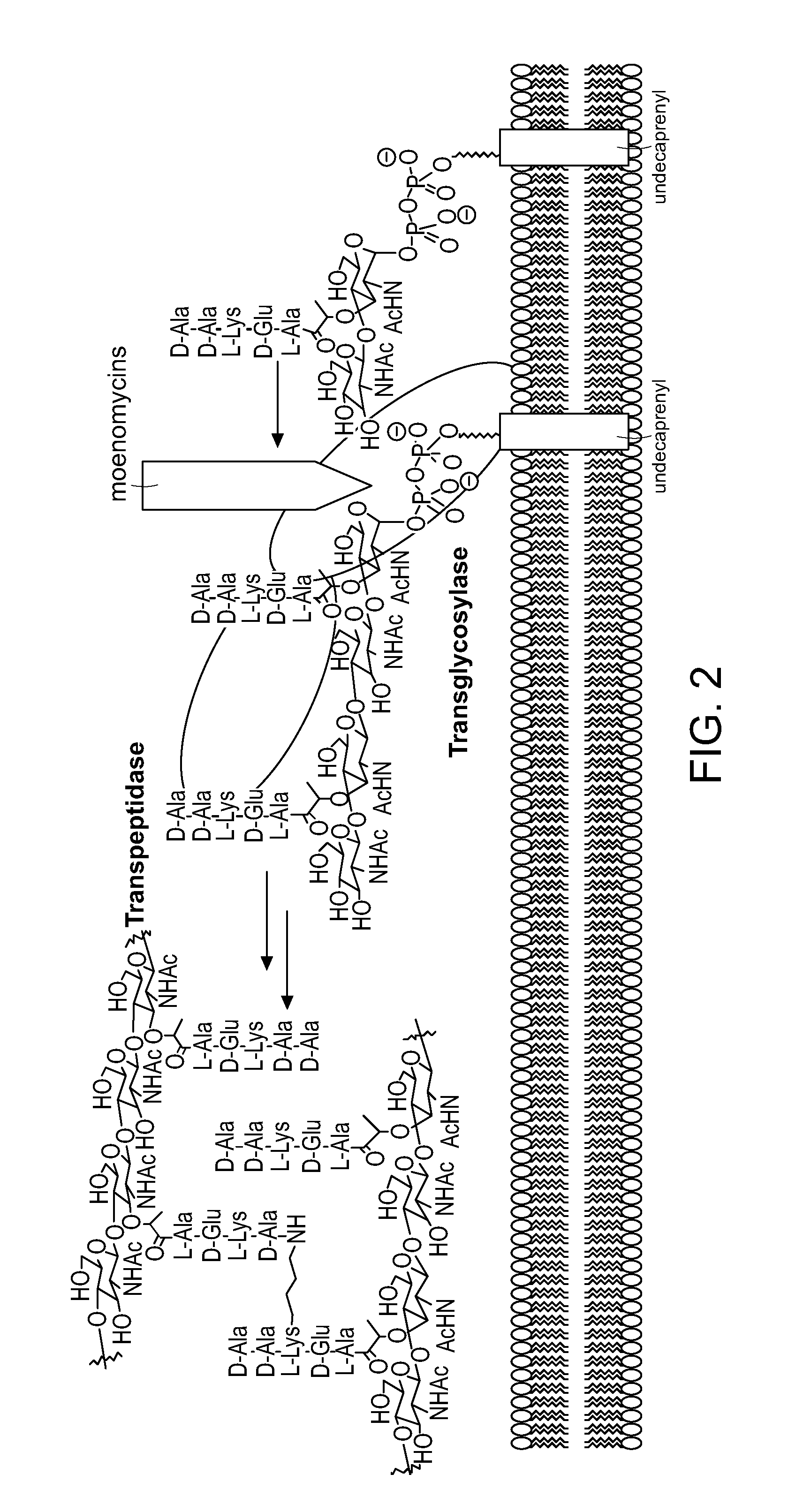
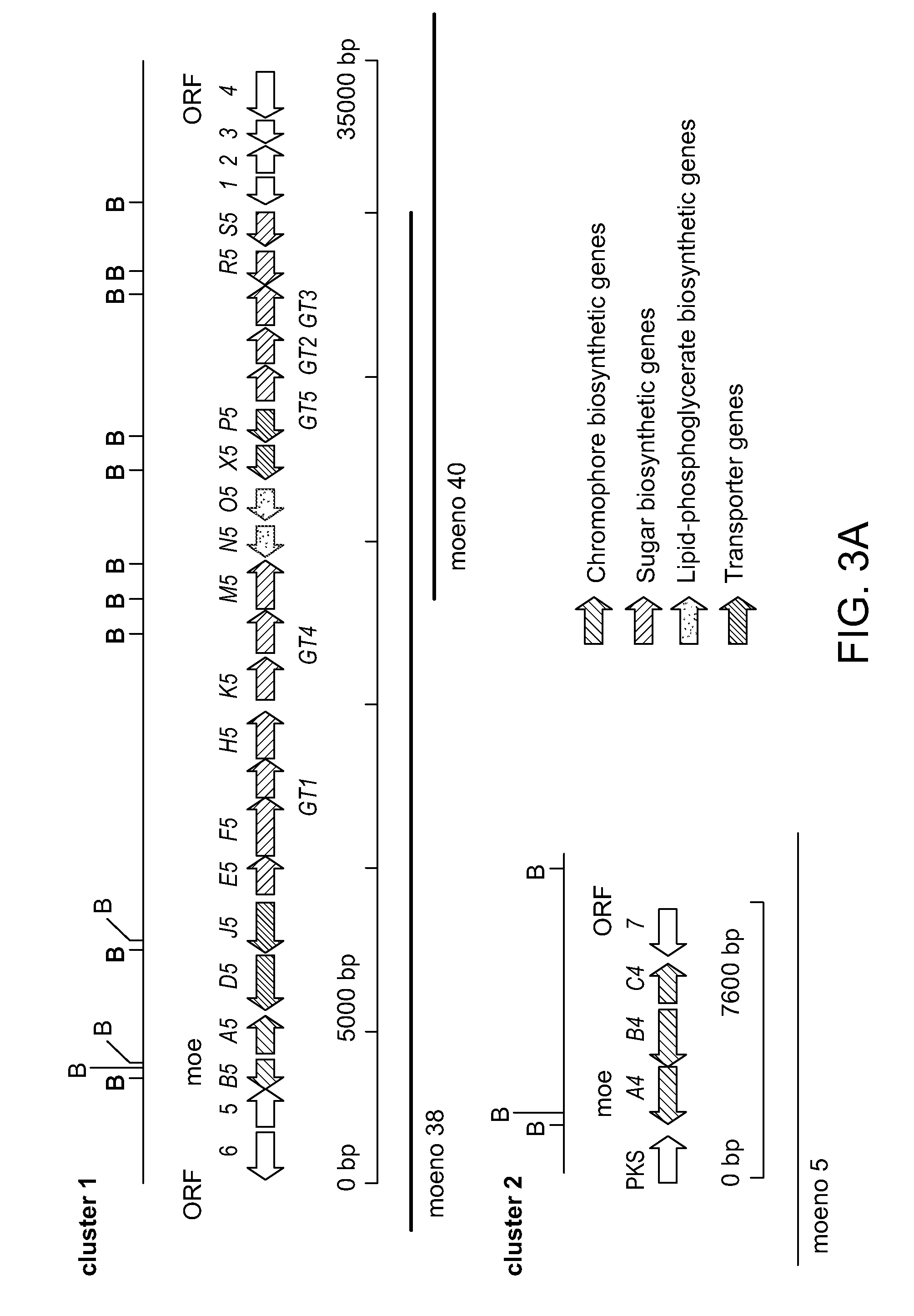
Moenomycin analogs, methods of synthesis, and uses thereof
The present invention provides novel moenomycin analogs as well as pharmaceutical compositions thereof, methods of synthesis, and methods of use in treating an infection by administering an inventive compound to a subject in need thereof. The moenomycin analogs may be prepared synthetically, biosynthetically, or semi-synthetically. The analogs are particularly useful in treating or preventing infections caused by Gram-positive organisms. Certain inventive compounds may have a broader spectrum of coverage, which includes Gram-negative organisms.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Pharmaceutical Compositions of Adsorbates of Amorphous Drug
ActiveUS20070148236A1Improve concentrationImprove drug stabilityAntibacterial agentsPowder deliverySolubilityHigh surface area
Pharmaceutical compositions comprise a low-solubility drug adsorbed onto a high surface area substrate to form an adsorbate. The compositions in some embodiments include a concentration-enhancing polymer.
Owner:BEND RES
Cell-free preparation of carbapenems
ActiveUS9469861B2Improve propertiesEnhance pharmaceuticallyAntibacterial agentsBiocideCell freePharmaceutical drug
Owner:GREENLIGHT BIOSCIENCES INC
Preparation method of polymeric micelles composition containing a poorly water-soluble drug
InactiveUS20110251269A1Reduce processing timeImprove stabilityOrganic active ingredientsBiocideWater solubleOrganic solvent
Provided is a method for preparing a drug-containing polymeric micelle composition, which includes: dissolving a drug and an amphiphilic block copolymer into an organic solvent; and adding an aqueous solution to the resultant mixture in the organic solvent to form polymeric micelles, wherein the method requires no separate operation to remove the organic solvent prior to the formation of micelles. The method for preparing a drug-containing polymeric micelle composition is simple, reduces the processing time, and is amenable to mass production.
Owner:SAMYANG BIOPHARMLS CORP
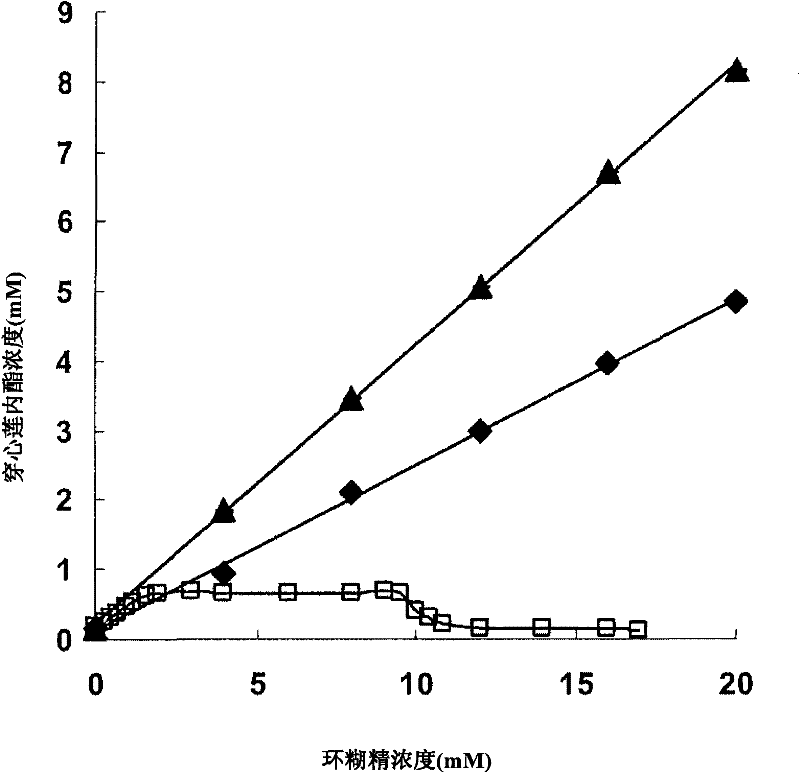
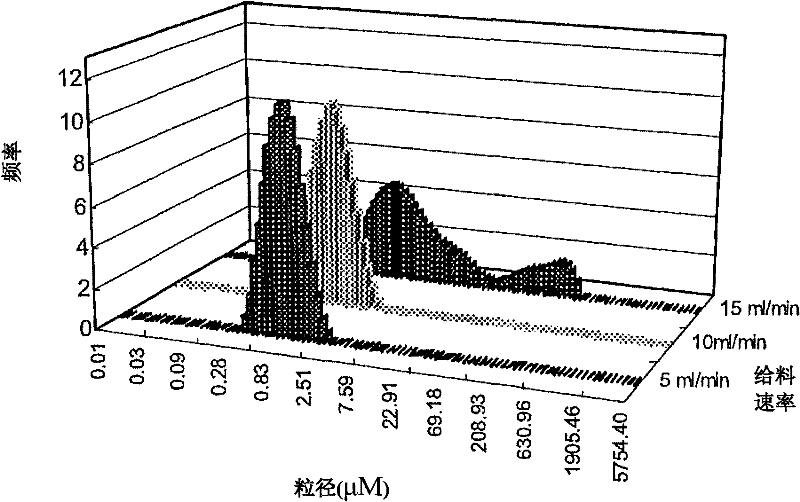
Andrographolide cyclodextrin inclusion compound, preparation method and application thereof
InactiveCN102343096AImprove solubilityImprove lipophilicityAntibacterial agentsOrganic active ingredientsSolubilityMicrometer
The present invention provides an andrographolide cyclodextrin inclusion compound. The basic components of the andrographolide cyclodextrin inclusion compound comprise: a) andrographolide, and b) pharmaceutically-acceptable cyclodextrin. The inclusion compound is prepared through carrying out spray drying for the CD and the andrographolide, wherein the entrance temperature is 100-200 DEG C, the material feeding speed is less than or equal to 20 ml / min, a molar ratio of the CD to the andrographolide is more than or equal to 1:1. The present invention further provides a preparation method for the inclusion compound, and an application of the inclusion compound. The inclusion compound provided by the present invention has excellent solubility, excellent drug stability and excellent bioavailability. With the method provided by the present invention, the inclusion compound of the andrographolide and the cyclodextrin or the inclusion compound of other plant compounds and the cyclodextrin can be prepared, wherein the inclusion compound has the particle size from micrometer to submicron, such that the bioavailability and the stability of the andrographolide are increased, a new delivery system for the diterpene plant compound is provided.
Owner:CITY UNIVERSITY OF HONG KONG
Moenomycin analogs, methods of synthesis, and uses thereof
InactiveUS20110136759A1Improve propertyEnhanced water solubilityAntibacterial agentsBiocideGram-positive bacteriumChemical compound
The present invention provides novel moenomycin analogs as well as pharmaceutical compositions thereof, methods of synthesis, and methods of use in treating an infection by administering an inventive compound to a subject in need thereof. The moenomycin analogs may be prepared synthetically, biosynthetically, or semi-synthetically. The analogs are particularly useful in treating or preventing infections caused by Gram-positive organisms. Certain inventive compounds may have a broader spectrum of coverage, which includes Gram-negative organisms.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Self-microemulsifying calcium alginate gel pellets for loading drugs and preparation method thereof
InactiveCN103655482AImprove drug stabilityEasy to adjustKetone active ingredientsGranular deliveryIonDrug
The invention discloses self-microemulsifying calcium alginate gel pellets and a preparation method thereof. The preparation method comprises the following steps: uniformly mixing an oil phase, a surfactant and a co-surfactant, adding an indissolvable drug until the indissolvable drug is completely dissolved; uniformly mixing the prepared liquid drug-loading self-microemulsifying preparation and a sodium alginate solution, performing complex crosslinking between the sodium alginate and calcium ions in calcium chloride to prepare the self-microemulsifying calcium alginate gel pellets for loading drugs by an ion gelatinizing method. According to the method, sustained-release and controlled release of a medicine can be controlled by changing the concentration of sodium alginate, the concentration of calcium chloride and other conditions. By virtue of the method, a solid self-microemulsifying preparation can be prepared from the liquid self-microemulsifying preparation, so that various defects of a liquid self-microemulsifying preparation can be effectively avoided. The preparation method is simple in process, low in production cost and easy for industrial production.
Owner:CHONGQING MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
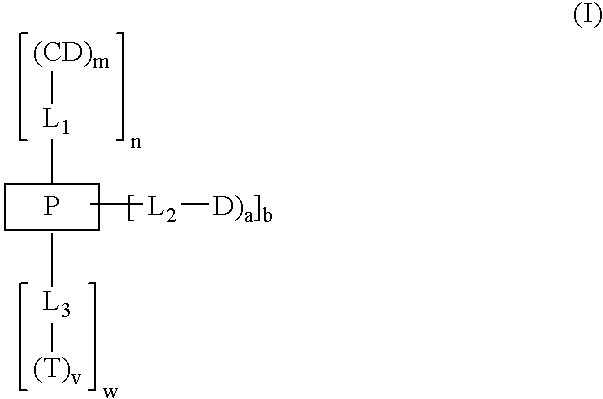
![Novel 4-(Substituted Amino)-7H-Pyrrolo[2,3-d] Pyrimidines As LRRK2 Inhibitors Novel 4-(Substituted Amino)-7H-Pyrrolo[2,3-d] Pyrimidines As LRRK2 Inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/65221143-e5cc-4f03-a8db-2a000ff43859/US20140005183A1-20140102-C00001.png)
![Novel 4-(Substituted Amino)-7H-Pyrrolo[2,3-d] Pyrimidines As LRRK2 Inhibitors Novel 4-(Substituted Amino)-7H-Pyrrolo[2,3-d] Pyrimidines As LRRK2 Inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/65221143-e5cc-4f03-a8db-2a000ff43859/US20140005183A1-20140102-C00002.png)
![Novel 4-(Substituted Amino)-7H-Pyrrolo[2,3-d] Pyrimidines As LRRK2 Inhibitors Novel 4-(Substituted Amino)-7H-Pyrrolo[2,3-d] Pyrimidines As LRRK2 Inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/65221143-e5cc-4f03-a8db-2a000ff43859/US20140005183A1-20140102-C00003.png)
![Heterocyclic Substituted Hexahydropyrano[3,4-d][1,3]Thiazin-2-Amine Compounds Heterocyclic Substituted Hexahydropyrano[3,4-d][1,3]Thiazin-2-Amine Compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f09bfe5-356d-4f07-9392-666b0977fd0b/US20130296308A1-20131107-C00001.png)
![Heterocyclic Substituted Hexahydropyrano[3,4-d][1,3]Thiazin-2-Amine Compounds Heterocyclic Substituted Hexahydropyrano[3,4-d][1,3]Thiazin-2-Amine Compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f09bfe5-356d-4f07-9392-666b0977fd0b/US20130296308A1-20131107-C00002.png)
![Heterocyclic Substituted Hexahydropyrano[3,4-d][1,3]Thiazin-2-Amine Compounds Heterocyclic Substituted Hexahydropyrano[3,4-d][1,3]Thiazin-2-Amine Compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f09bfe5-356d-4f07-9392-666b0977fd0b/US20130296308A1-20131107-C00003.png)
![Heterocyclic substituted hexahydropyrano[3,4-d][1,3]thiazin-2-amine compounds Heterocyclic substituted hexahydropyrano[3,4-d][1,3]thiazin-2-amine compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9cfc7678-803d-4d4c-bb6f-61a844a3945c/US08962616-20150224-C00001.PNG)
![Heterocyclic substituted hexahydropyrano[3,4-d][1,3]thiazin-2-amine compounds Heterocyclic substituted hexahydropyrano[3,4-d][1,3]thiazin-2-amine compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9cfc7678-803d-4d4c-bb6f-61a844a3945c/US08962616-20150224-C00002.PNG)
![Heterocyclic substituted hexahydropyrano[3,4-d][1,3]thiazin-2-amine compounds Heterocyclic substituted hexahydropyrano[3,4-d][1,3]thiazin-2-amine compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9cfc7678-803d-4d4c-bb6f-61a844a3945c/US08962616-20150224-C00003.PNG)