Self-microemulsifying calcium alginate gel pellets for loading drugs and preparation method thereof
A technology of calcium alginate gel pellets and self-microemulsification, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of inability to achieve effective drug loading and solid adsorption To avoid problems such as large amount of dosage, to achieve the effect of increasing drug stability, low production cost, and convenient adjustment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] Preparation of puerarin self-microemulsifying gel pellets
[0019] The composition of the self-microemulsifying formulation is as follows:
[0020] Caprylic capric acid glyceride (ODO): 0.175g
[0021] Polyoxyethylene hydrogenated castor oil (Cremophor RH40): 0.550g
[0022] Diethylene glycol monoethyl ether (Transcutol P): 0.275g
[0023] Puerarin: 50.0mg
[0024] Mix oil phase ODO, surfactant Cremophor RH40 and co-surfactant Transcutol P, add puerarin raw material drug, stir at 37°C until the drug is completely dissolved, and obtain a clear and transparent puerarin self-microemulsification preparation; take 0.3g puerarin Add the self-microemulsifying preparation into 3.0% sodium alginate solution, mix well and add 3.2M CaCl dropwise 2 In the solution, after gelation reaction for 1 hour, filter, rinse with distilled water three times, and dry at 50°C to obtain puerarin self-microemulsified calcium alginate gel pellets.
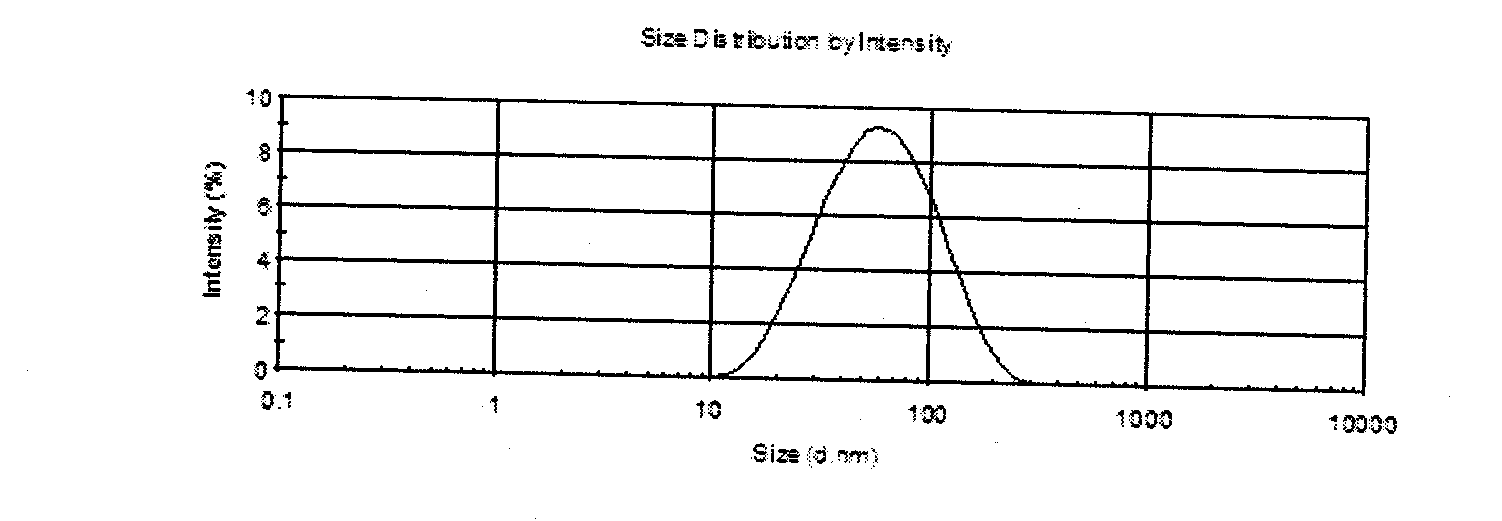
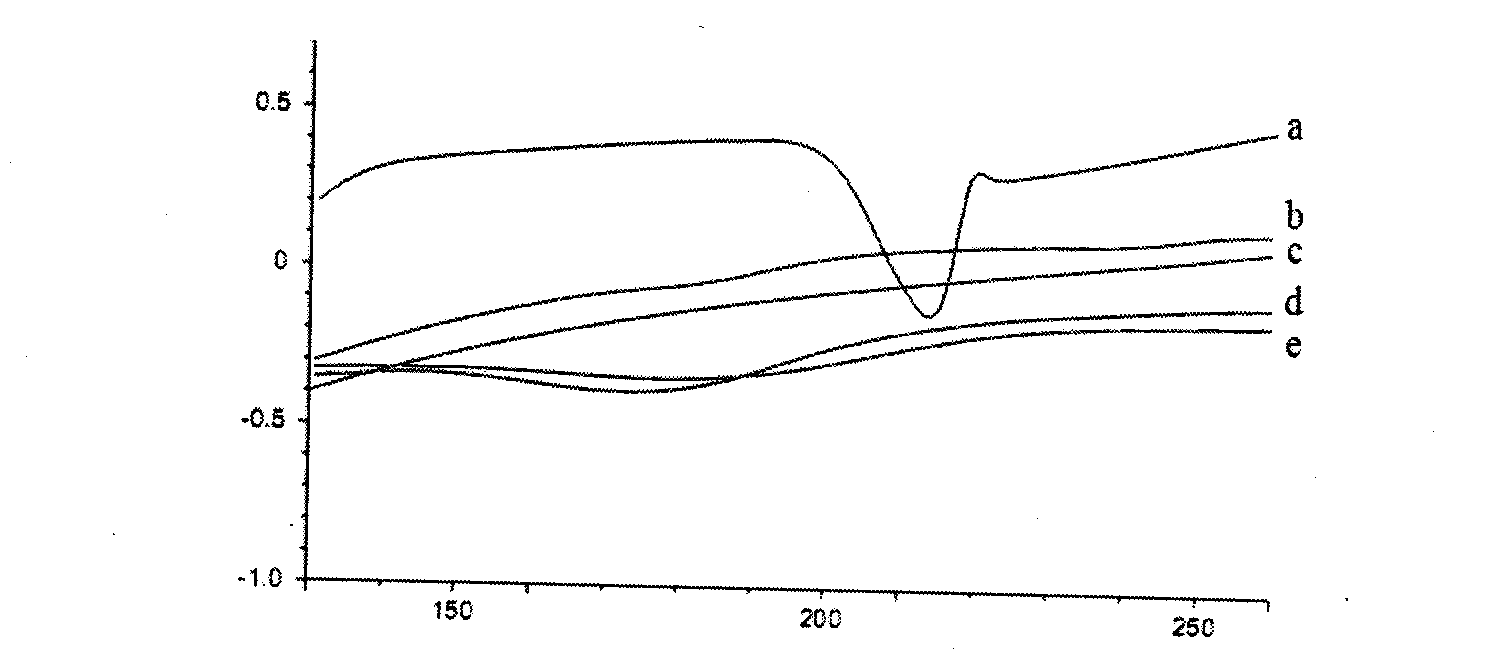
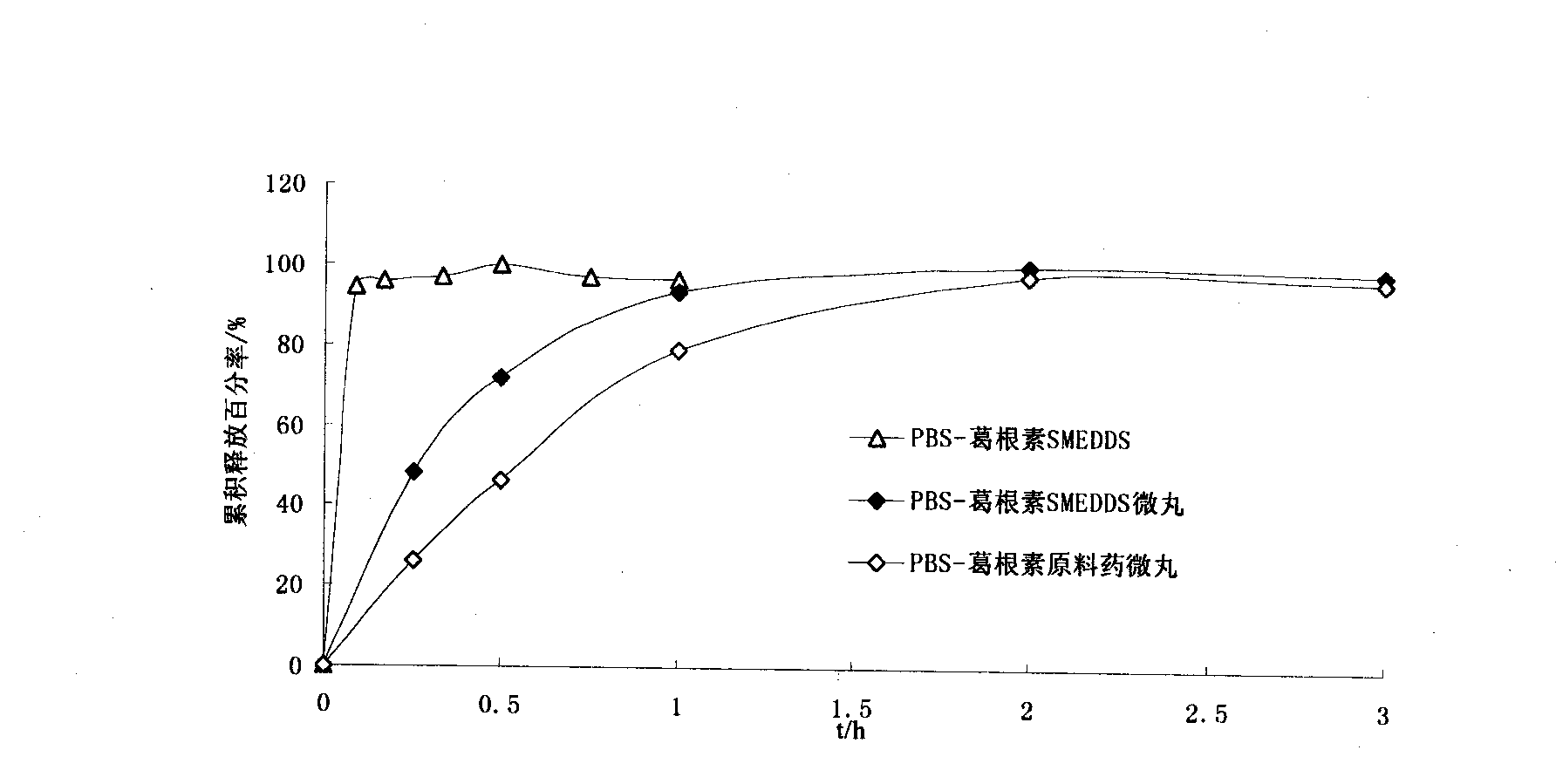
[0025] The prepared puerarin self-microemul...
example 2
[0029] Preparation of baicalein self-microemulsifying gel pellets
[0030] The composition of the self-microemulsifying formulation is as follows:
[0031] Caprylic capric acid glyceride (ODO): 0.2500g
[0032] Polyoxyethylene hydrogenated castor oil (Cremophor RH40): 0.5357g
[0033] Diethylene glycol monoethyl ether (Transcutol P): 0.2143g
[0034] Baicalein: 30.0mg
[0035] Mix oil phase ODO, surfactant Cremophor RH40 and co-surfactant Transcutol P, add baicalein raw material drug, stir at 37°C until the drug is completely dissolved, and obtain a clear and transparent baicalein self-microemulsification preparation; take baicalein self-emulsification Add 0.3g of the preparation to 1ml of 3% sodium alginate solution and mix well; add the mixed solution dropwise to 100ml of CaCl with a concentration of 0.4M 2 In the solution, after 60 minutes of cross-linking reaction, filter and wash with distilled water, and dry at 50°C to obtain baicalein self-microemulsified calcium al...
example 3
[0039] Preparation of naftopidil self-microemulsifying gel pellets
[0040] The composition of the self-microemulsifying formulation is as follows:
[0041] Caprylic capric acid glyceride (ODO): 0.25g
[0042] Polyoxyethylene hydrogenated castor oil (Cremophor RH40): 0.50g
[0043] Diethylene glycol monoethyl ether (Transcutol P): 0.25g
[0044] Naftopidil: 20.0mg
[0045] After mixing the oil phase ODO, surfactant Cremophor RH40 and co-surfactant Transcutol P, add naftopidil crude drug, and stir at 37°C until the drug is completely dissolved to obtain a clear and transparent naftopidil self-microemulsion preparation; Add 0.3g of naftopidil self-emulsifying preparation into 1ml of 3% sodium alginate solution, and mix well; add the mixed solution dropwise to 100ml of CaCl with a concentration of 0.6M 2 In the solution, after 60 minutes of cross-linking reaction, filter and wash with distilled water, and dry at 50° C. to obtain naftopidil self-microemulsified calcium alginat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com