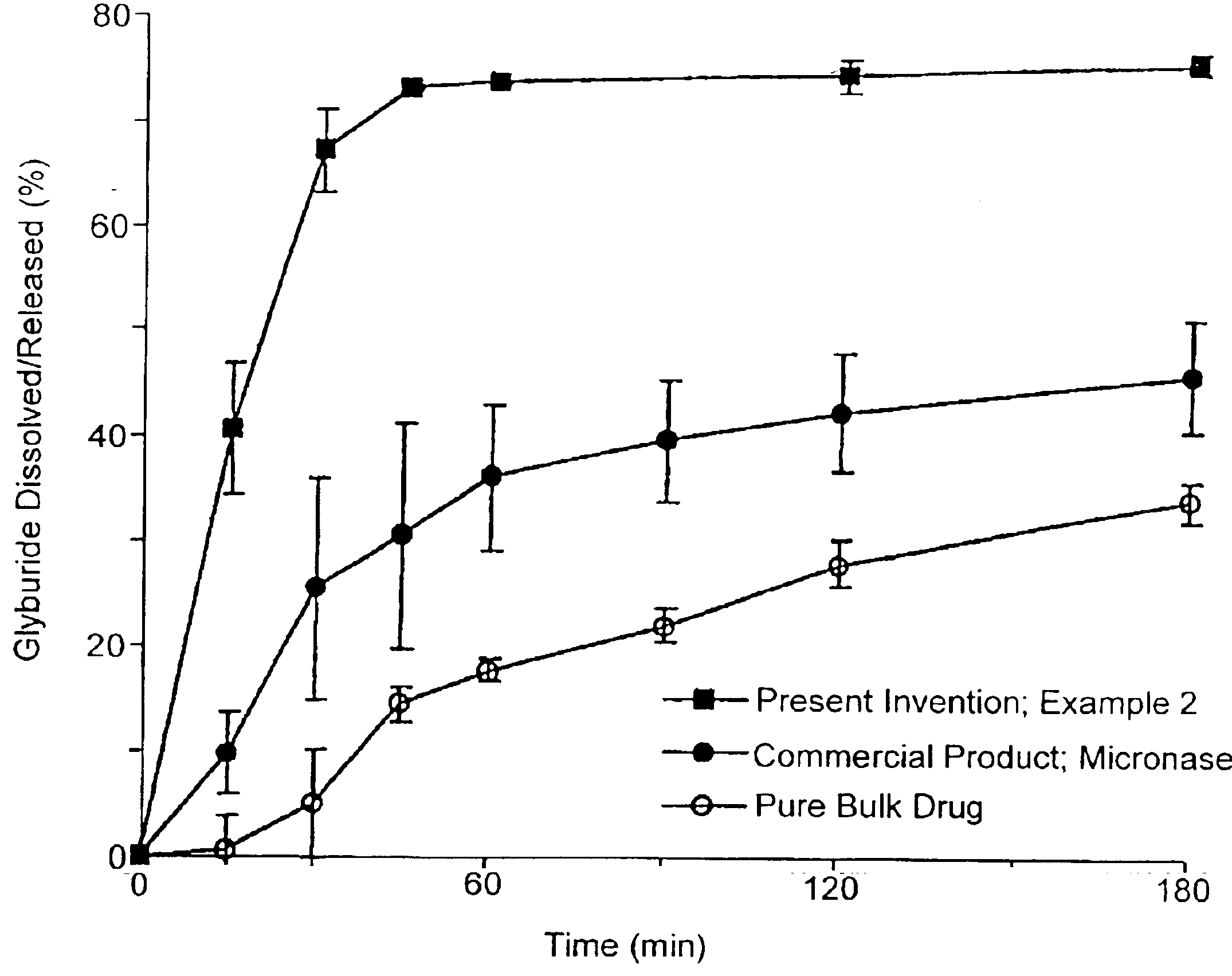
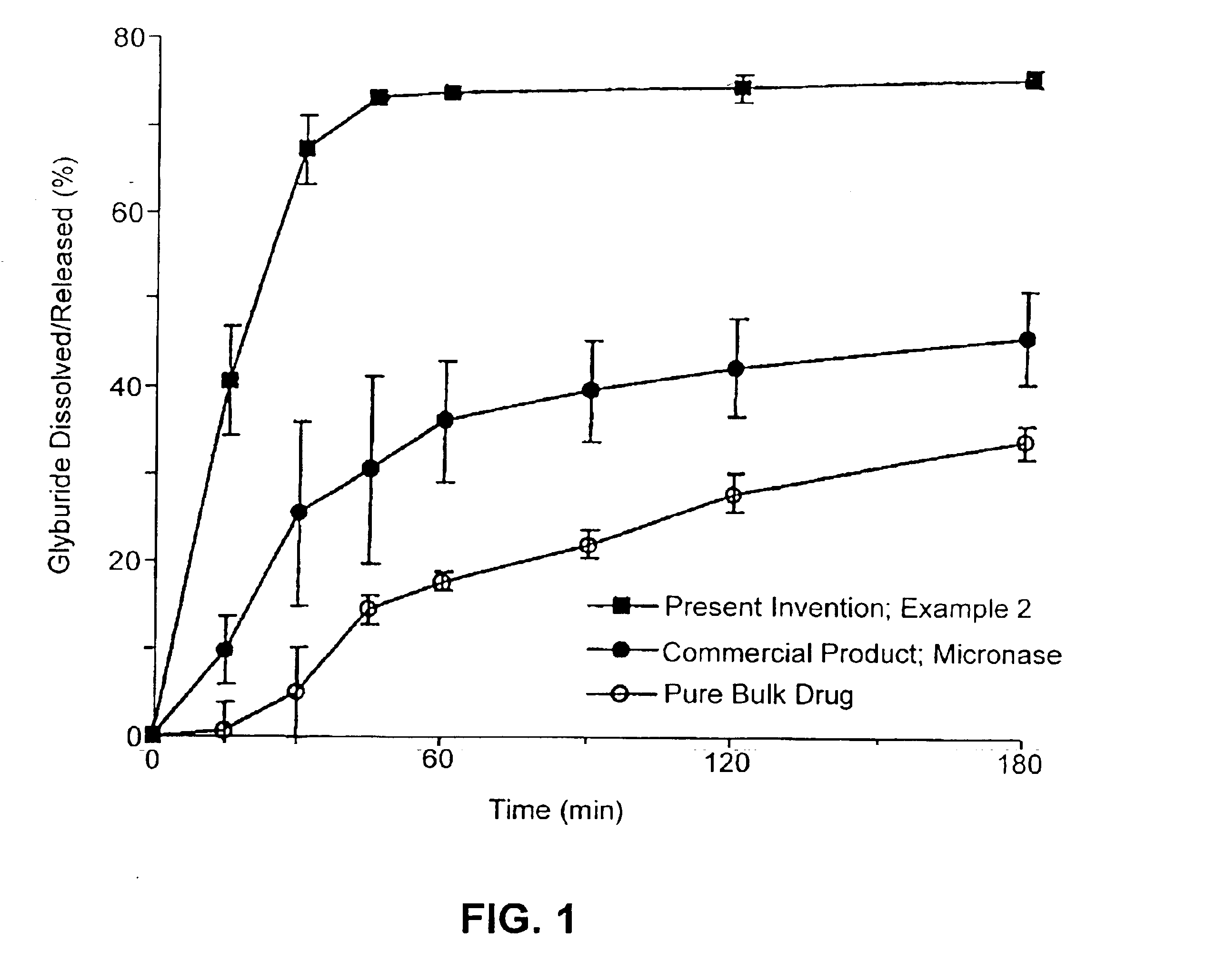
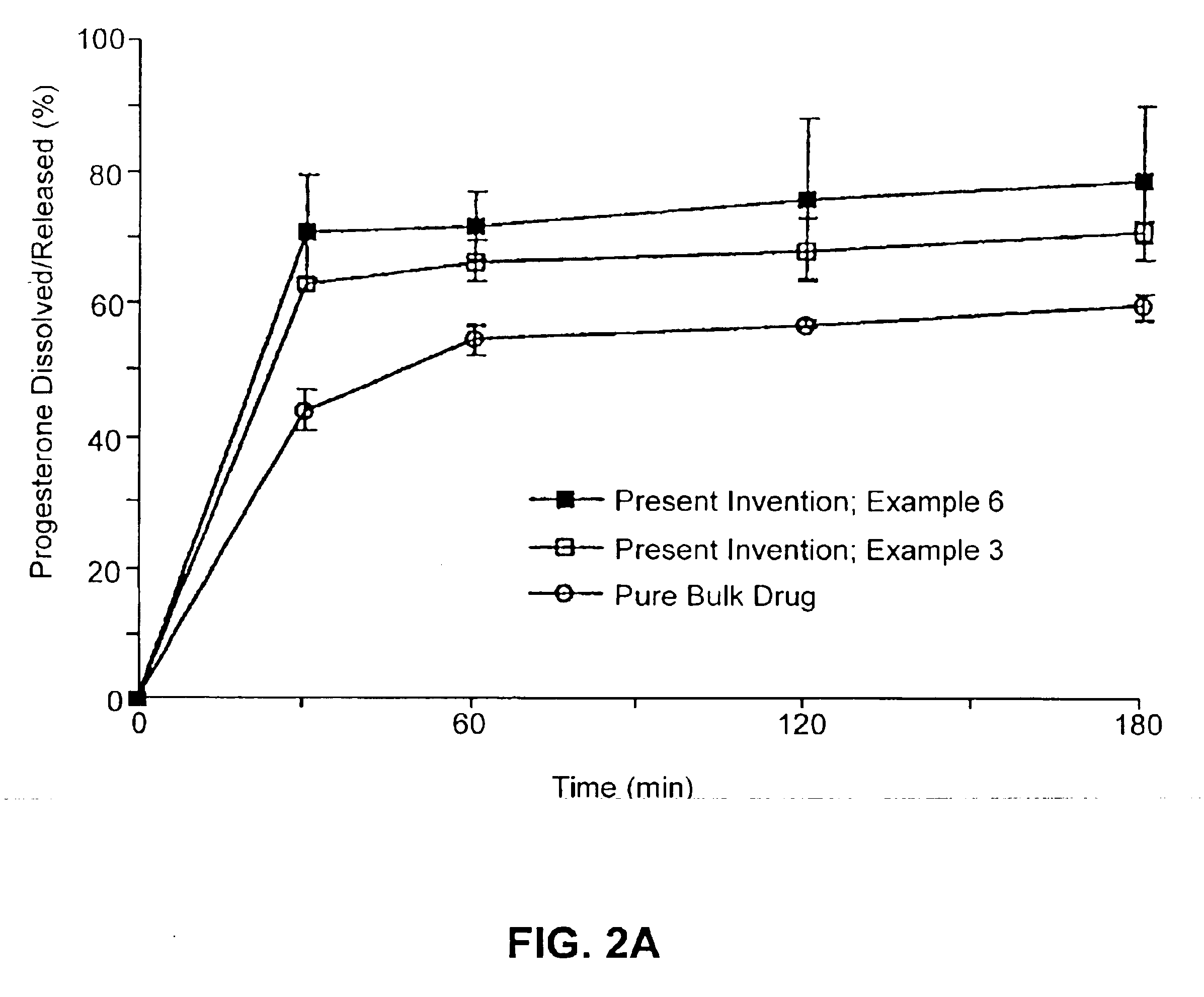
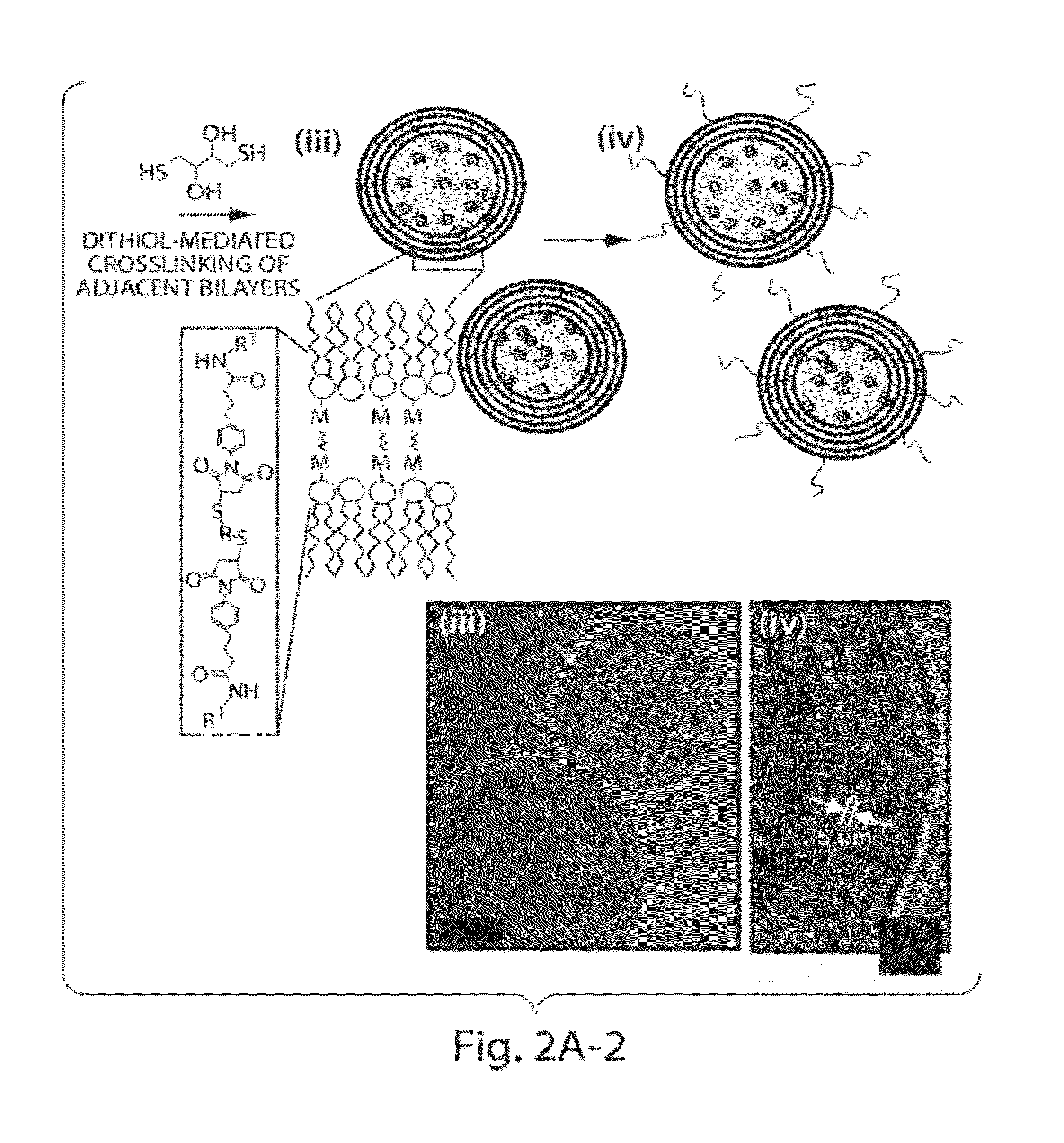
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
9489results about "Oil/fats/waxes non-active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
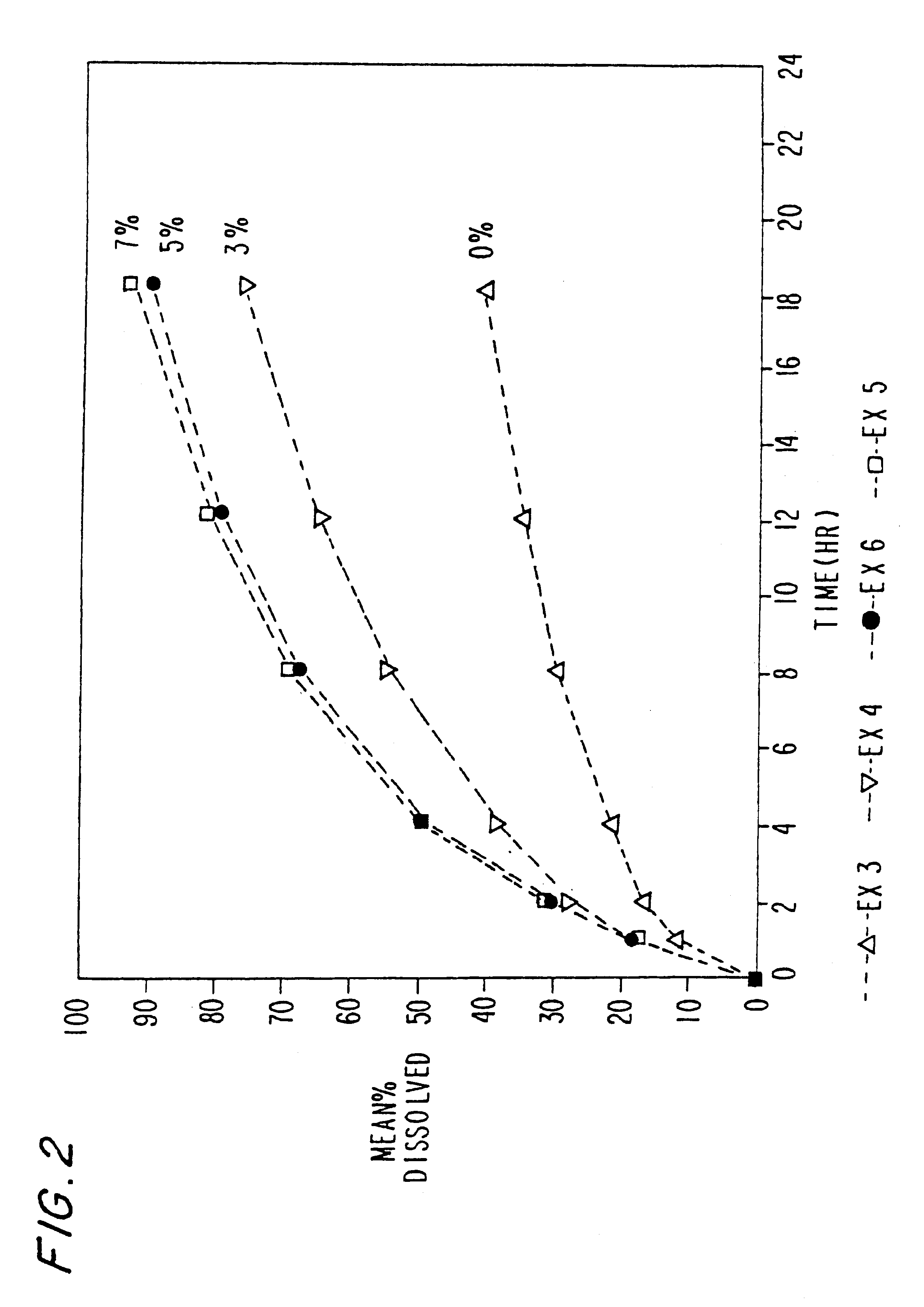
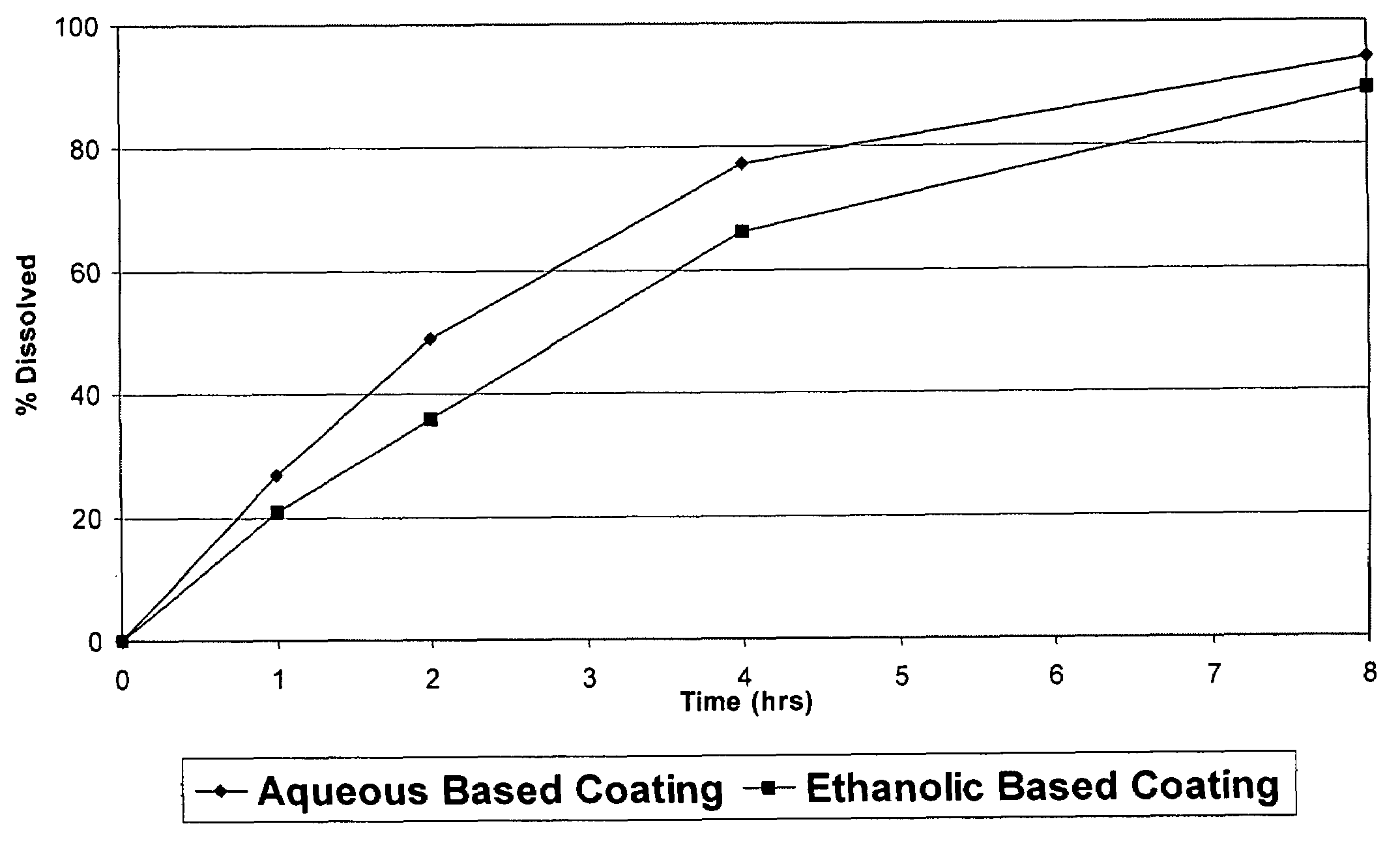
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Emulsion compositions
Owner:UK RES & INNOVATION LTD
Melt-extruded orally administrable opioid formulations
InactiveUS6261599B1Sustained effectSlow and control releaseBiocideOrganic active ingredientsMelt extrusionDosage form
Bioavailable sustained release oral opioid analgesic dosage forms, comprising a plurality of multiparticulates produced via melt extrusion techniques disclosed.
Owner:PURDUE PHARMA LP
Apolipoprotein A-I agonists and their use to treat dyslipidemic disorders
The present invention provides peptides and peptide analogues that mimic the structural and pharmacological properties of human ApoA-I. The peptides and peptide analogues are useful to treat a variety of disorders associated with dyslipidemia.
Owner:DASSEUX JEAN LOUIS +5
Pharmaceutical and cosmetic carrier or composition for topical application
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
Transdermal delivery system
The present invention relates to the discovery of a transdermal delivery system that can deliver high molecular weight pharmaceuticals and cosmetic agents to skin cells. A novel transdermal delivery system with therapeutic and cosmetic application and methods of use of the foregoing is disclosed.
Owner:ORIX +1
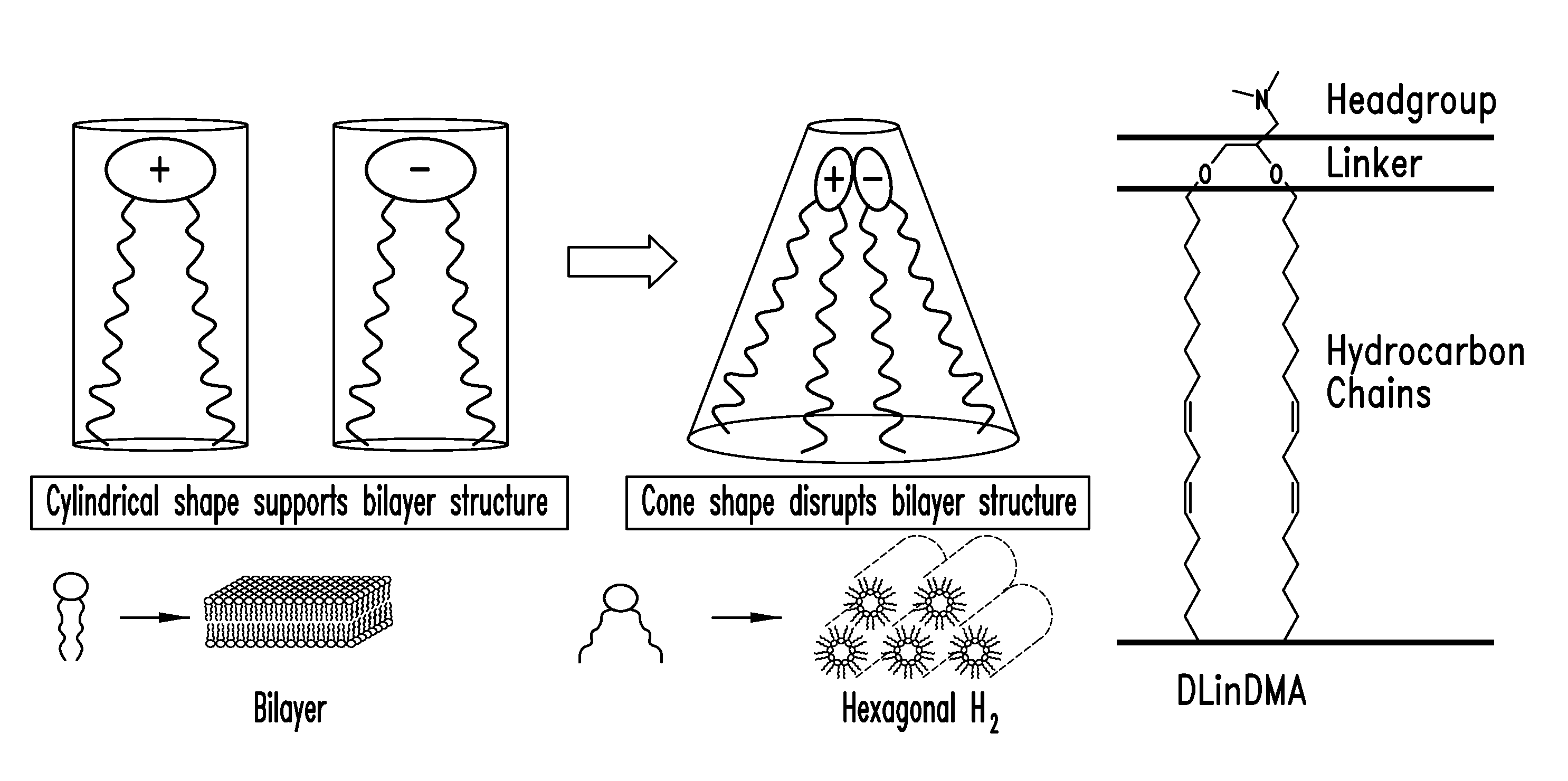
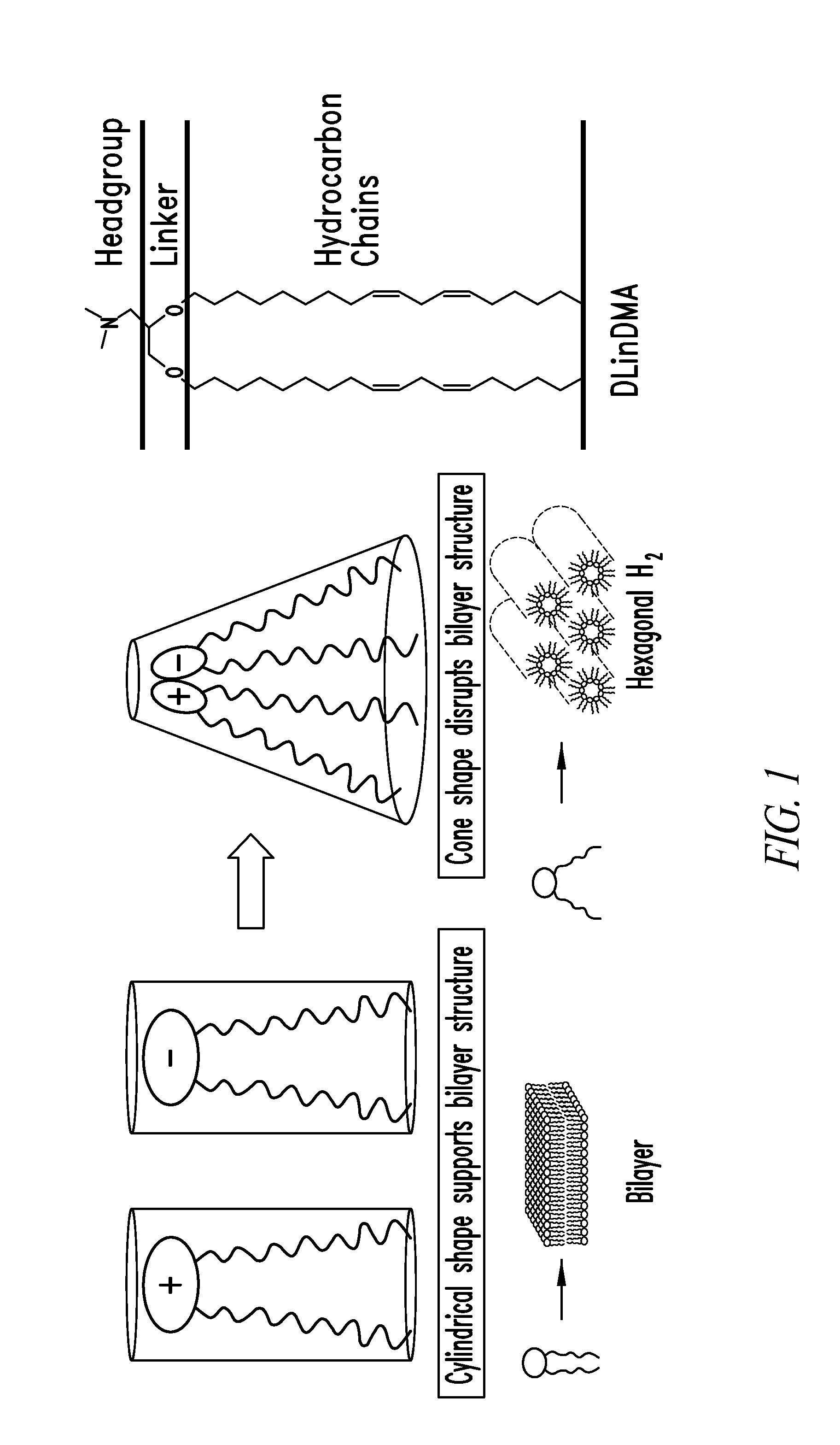
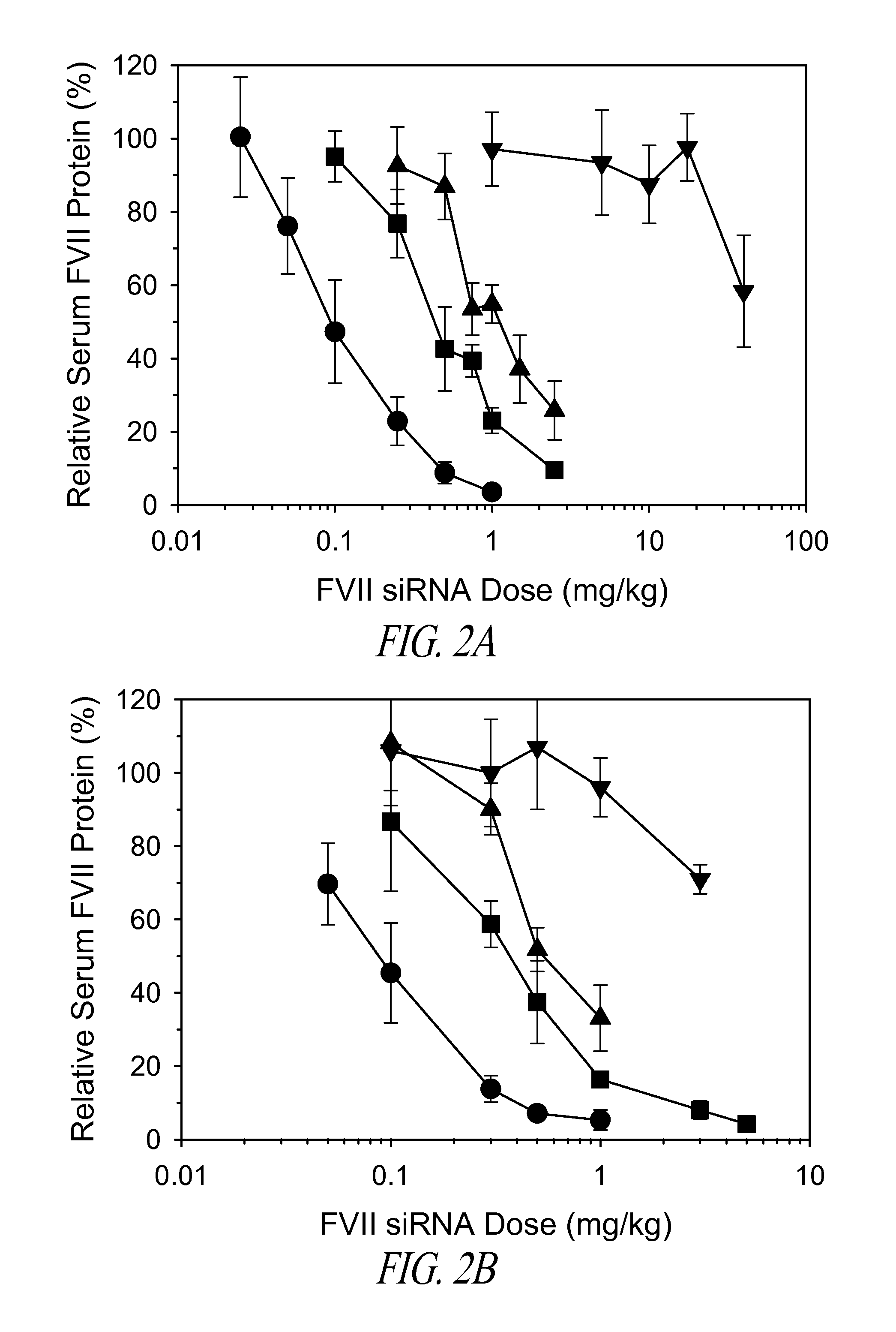
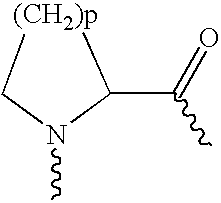
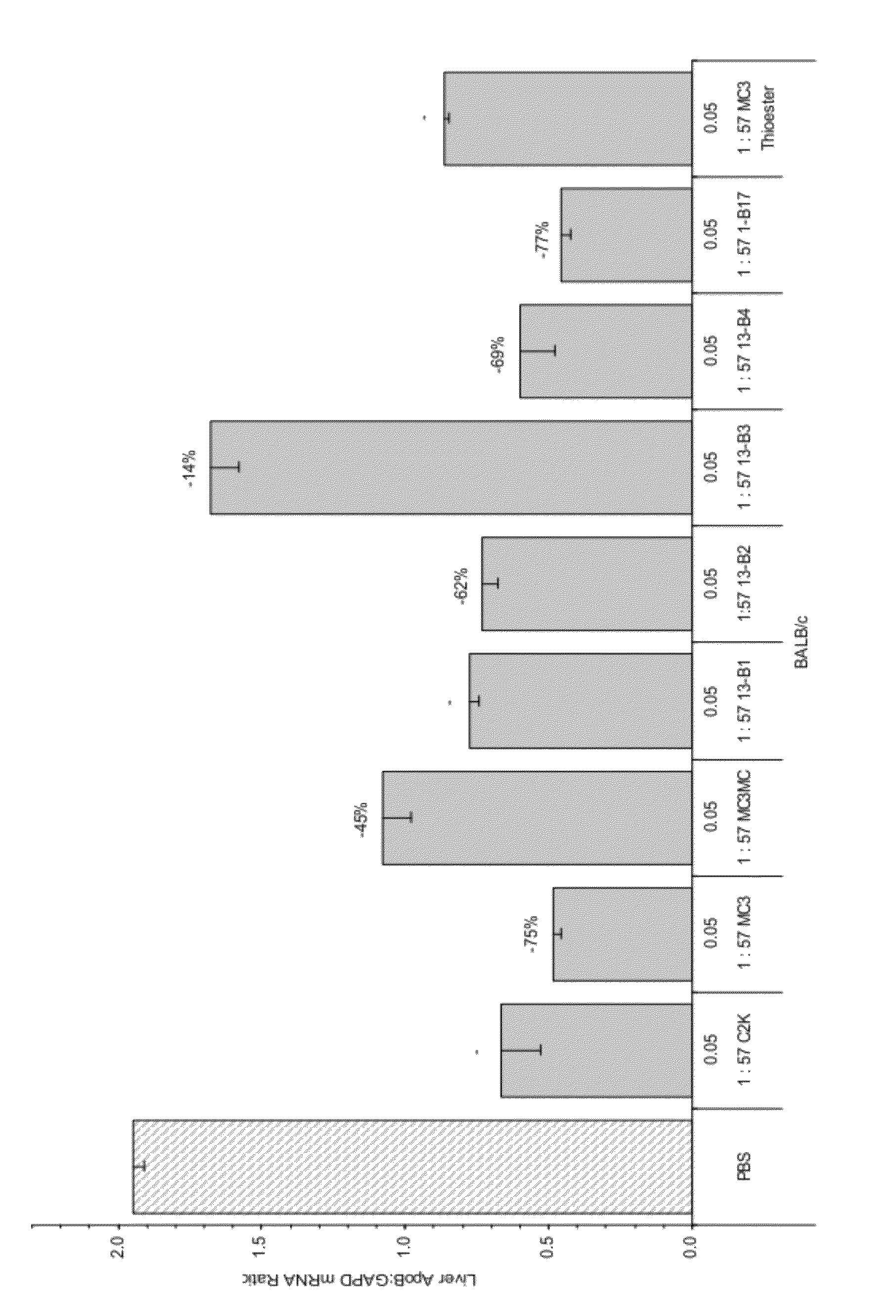
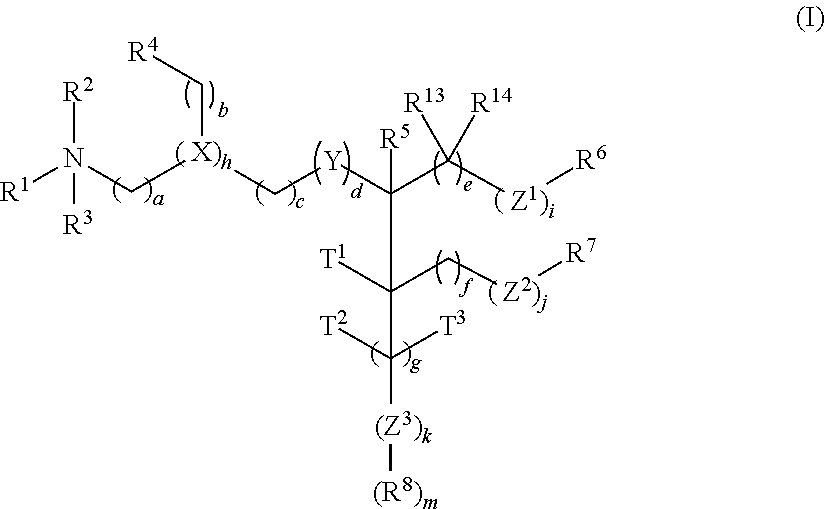
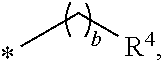
Amino lipids and methods for the delivery of nucleic acids
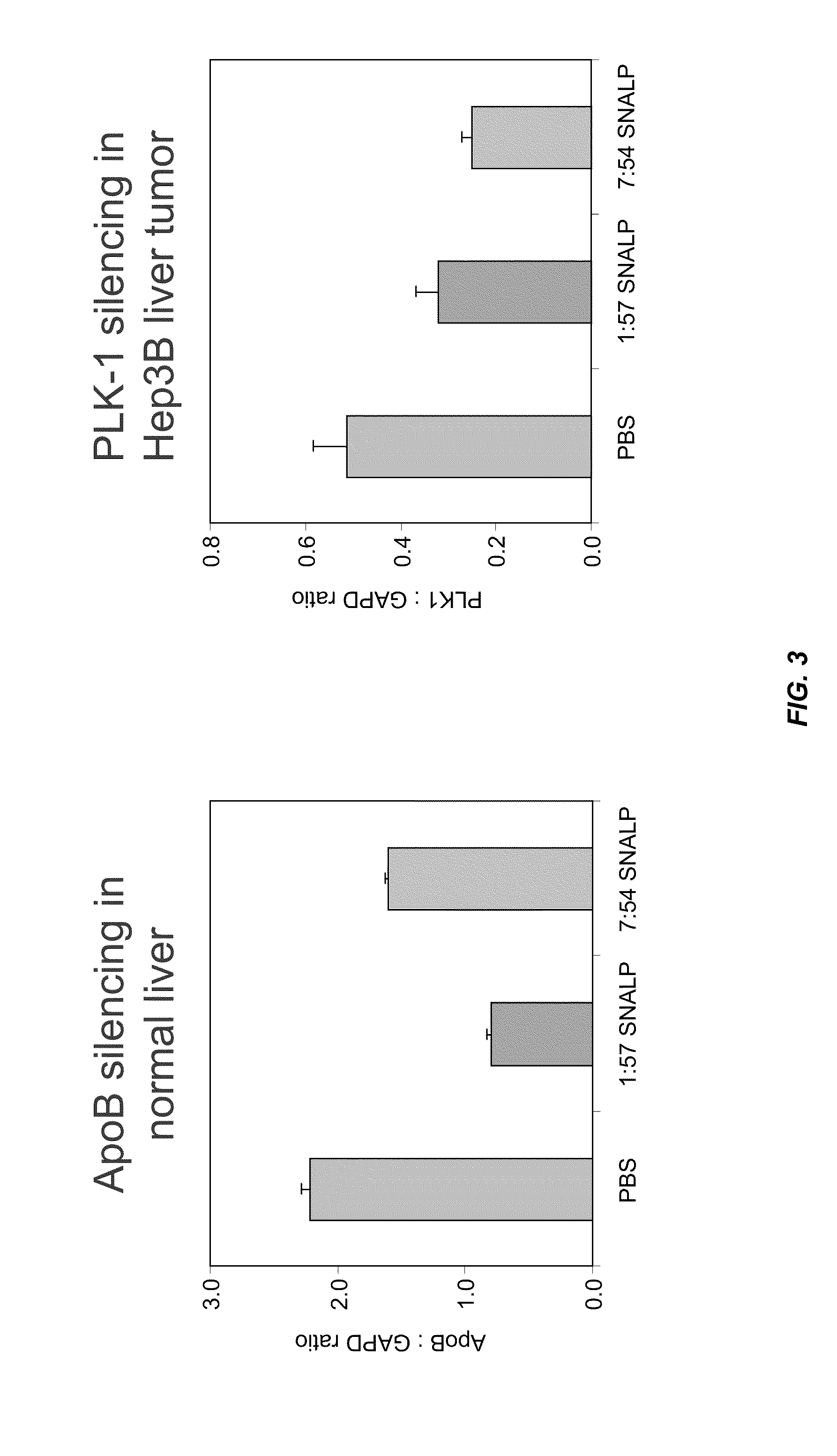
The present invention provides superior compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target proteins at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION +1
Lipid formulations for nucleic acid delivery
ActiveUS8283333B2Improve effectivenessHigh activityOrganic active ingredientsSugar derivativesLipid particleActive agent
The present invention provides novel, serum-stable lipid particles comprising one or more active agents or therapeutic agents, methods of making the lipid particles, and methods of delivering and / or administering the lipid particles. More particularly, the present invention provides serum-stable nucleic acid-lipid particles (SNALP) comprising a nucleic acid (e.g., one or more interfering RNA molecules), methods of making the SNALP, and methods of delivering and / or administering the SNALP (e.g., for the treatment of cancer). In particular embodiments, the present invention provides tumor-directed lipid particles that preferentially target solid tumors. The tumor-directed formulations of the present invention are capable of preferentially delivering a payload such as a nucleic acid to cells of solid tumors compared to non-cancerous cells.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
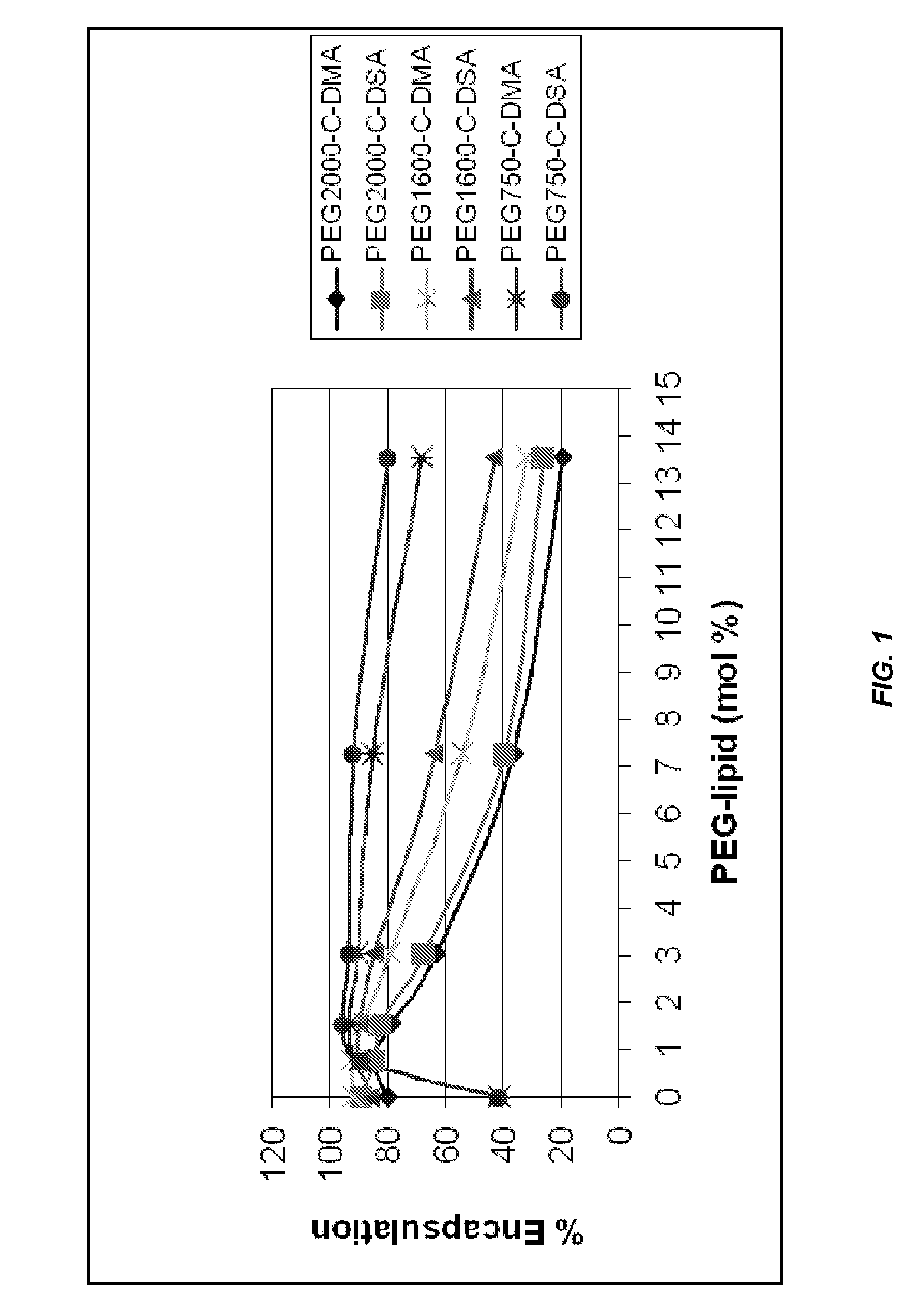
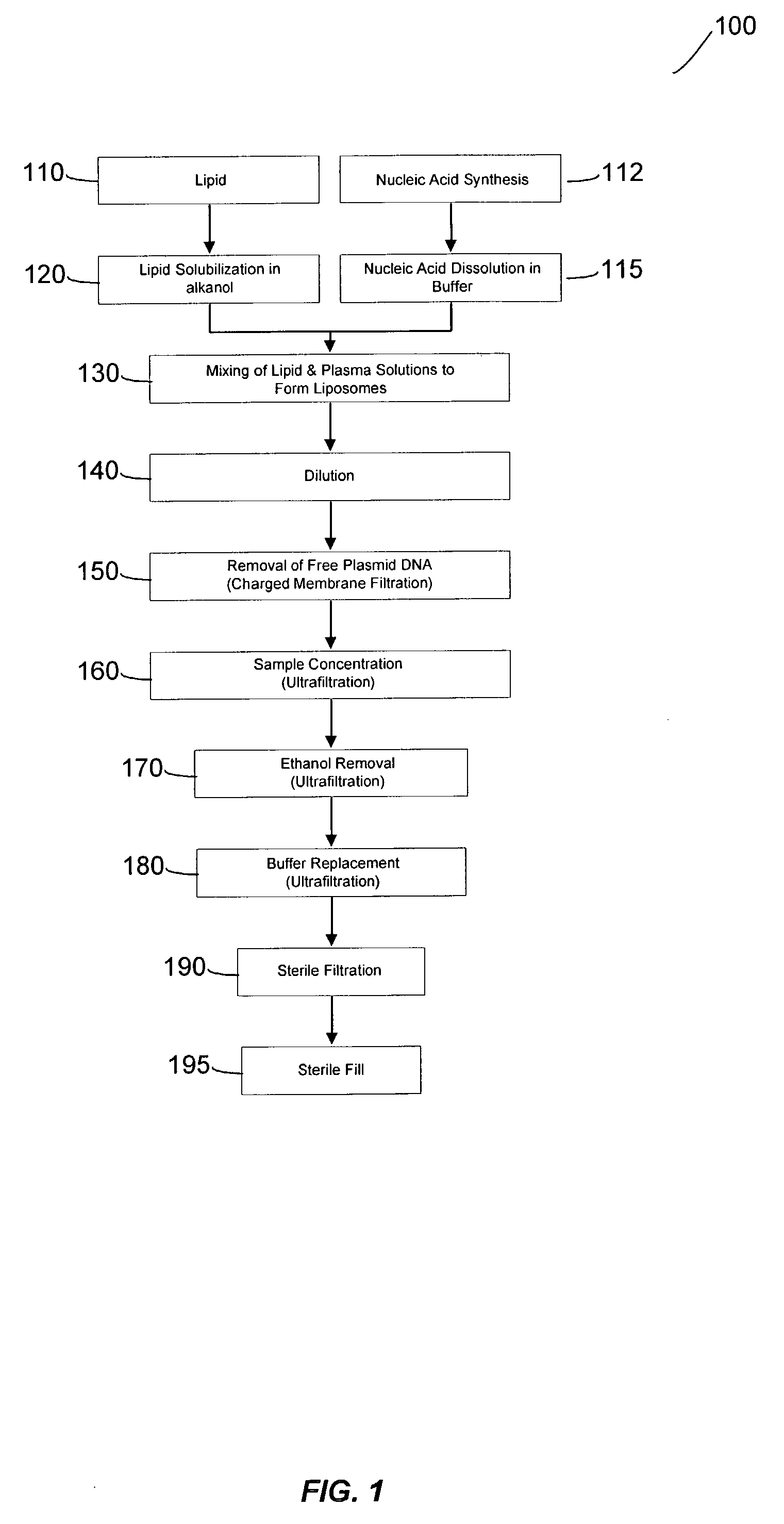
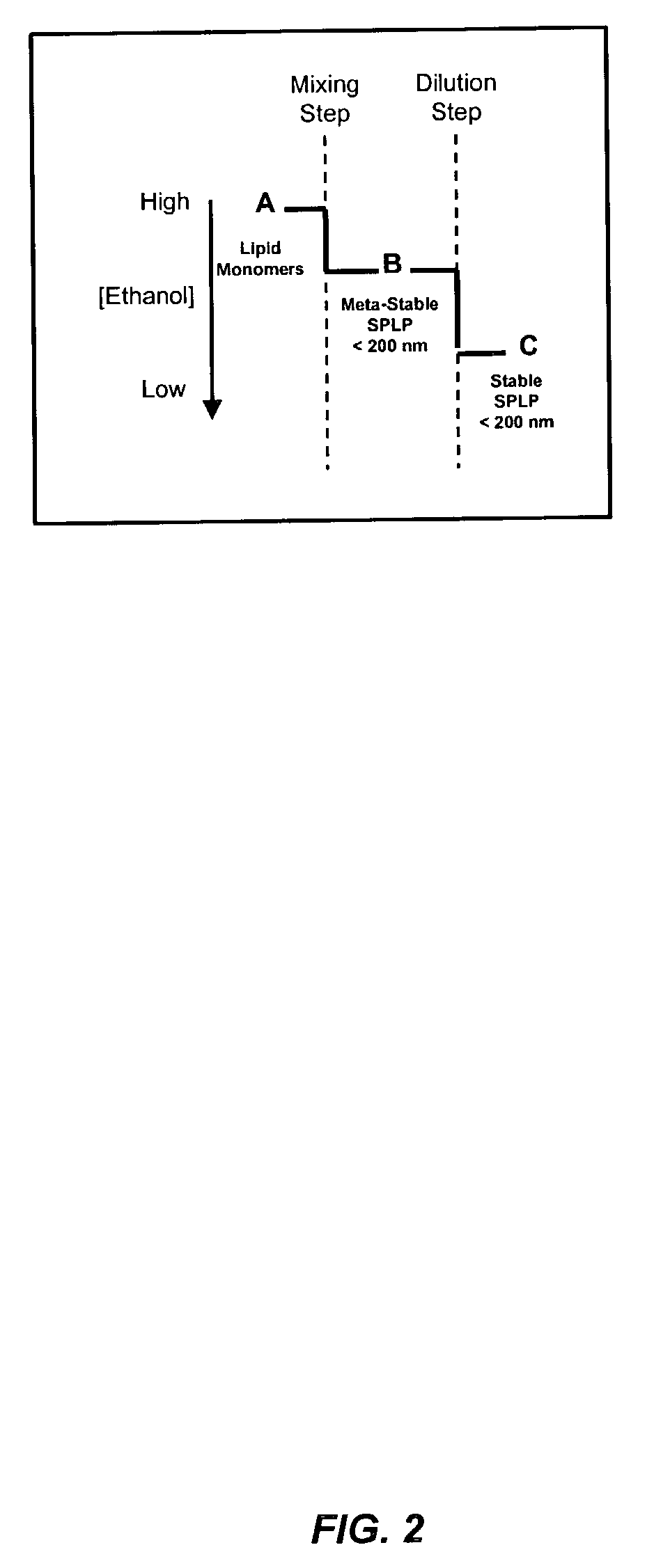
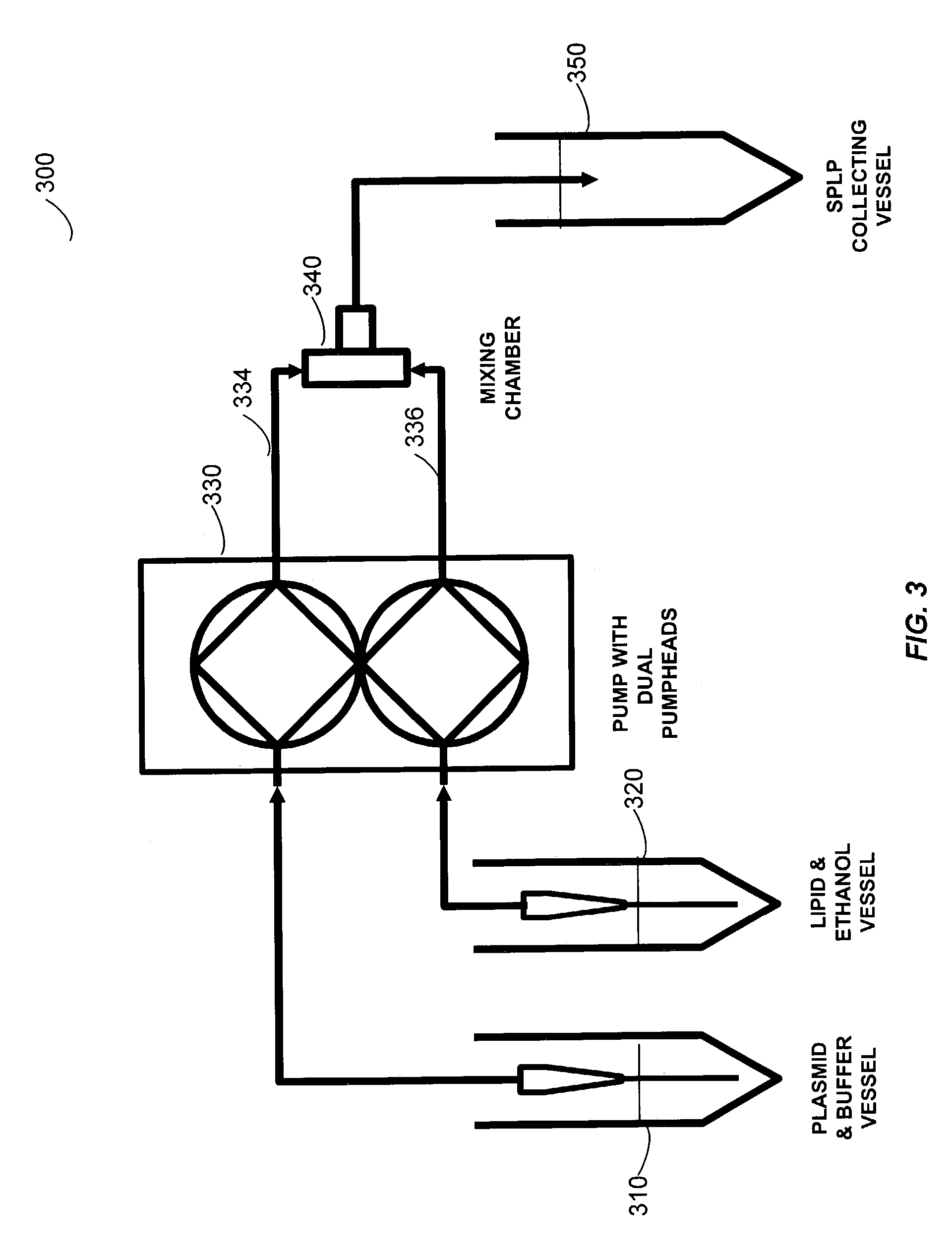
Liposomal apparatus and manufacturing methods
ActiveUS7901708B2High encapsulation efficiencyBiocideSugar derivativesLipid formationOrganic solvent
The present invention provides apparatus and processes for producing liposomes. By providing a buffer solution in a first reservoir, and a lipid solution in a second reservoir, continuously diluting the lipid solution with the buffer solution in a mixing chamber produces a liposome. The lipid solution preferably comprises an organic solvent, such as a lower alkanol.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
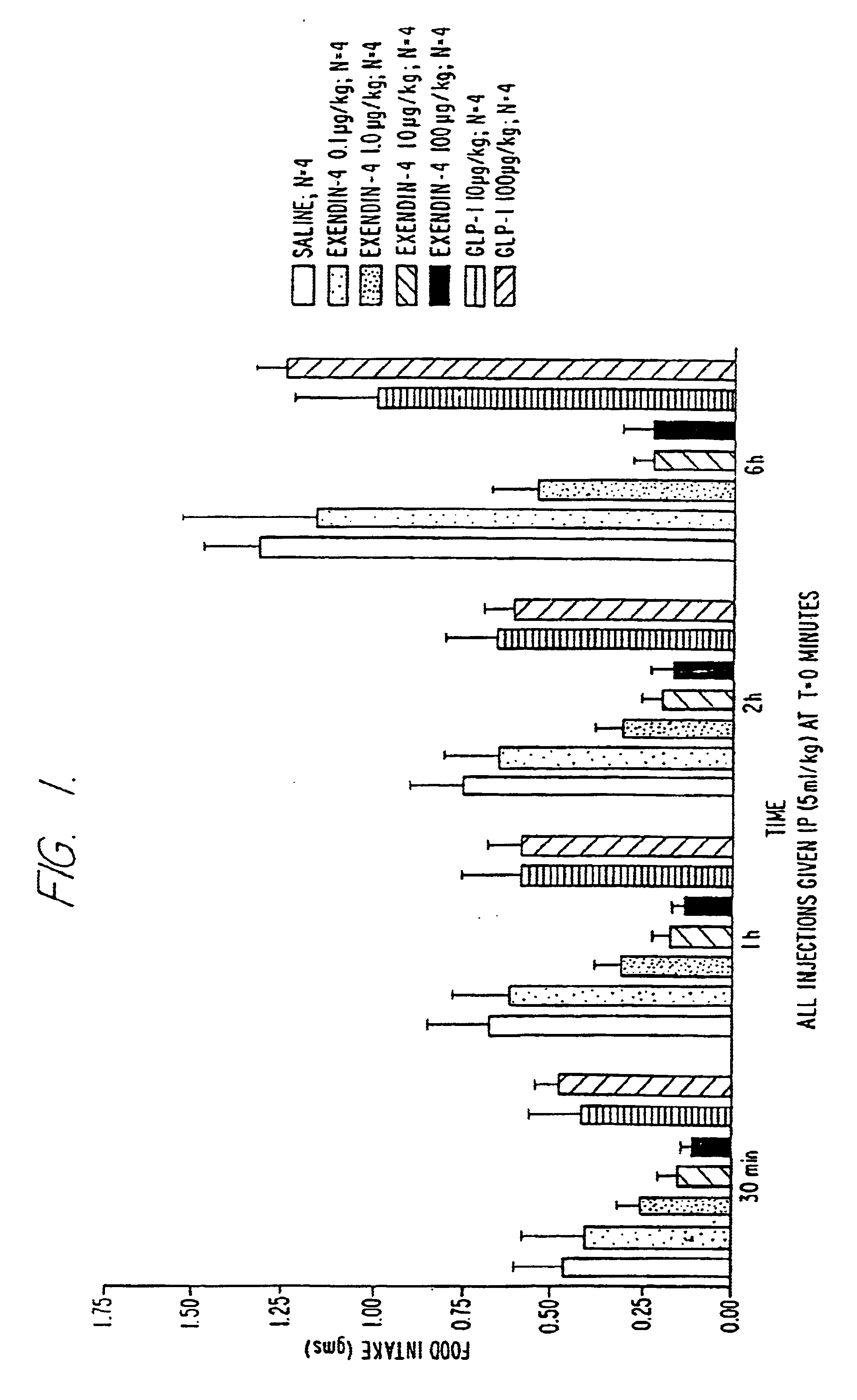
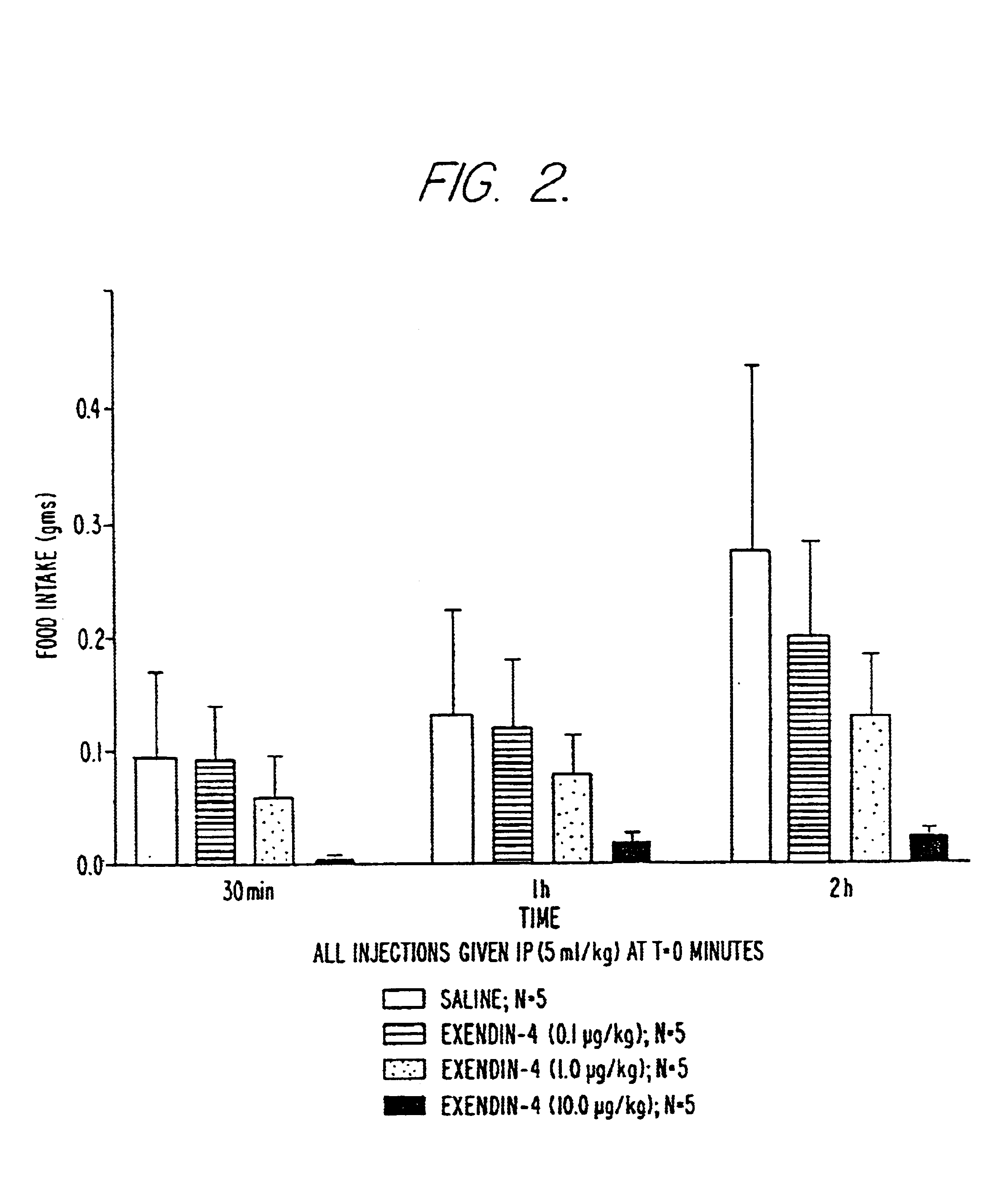
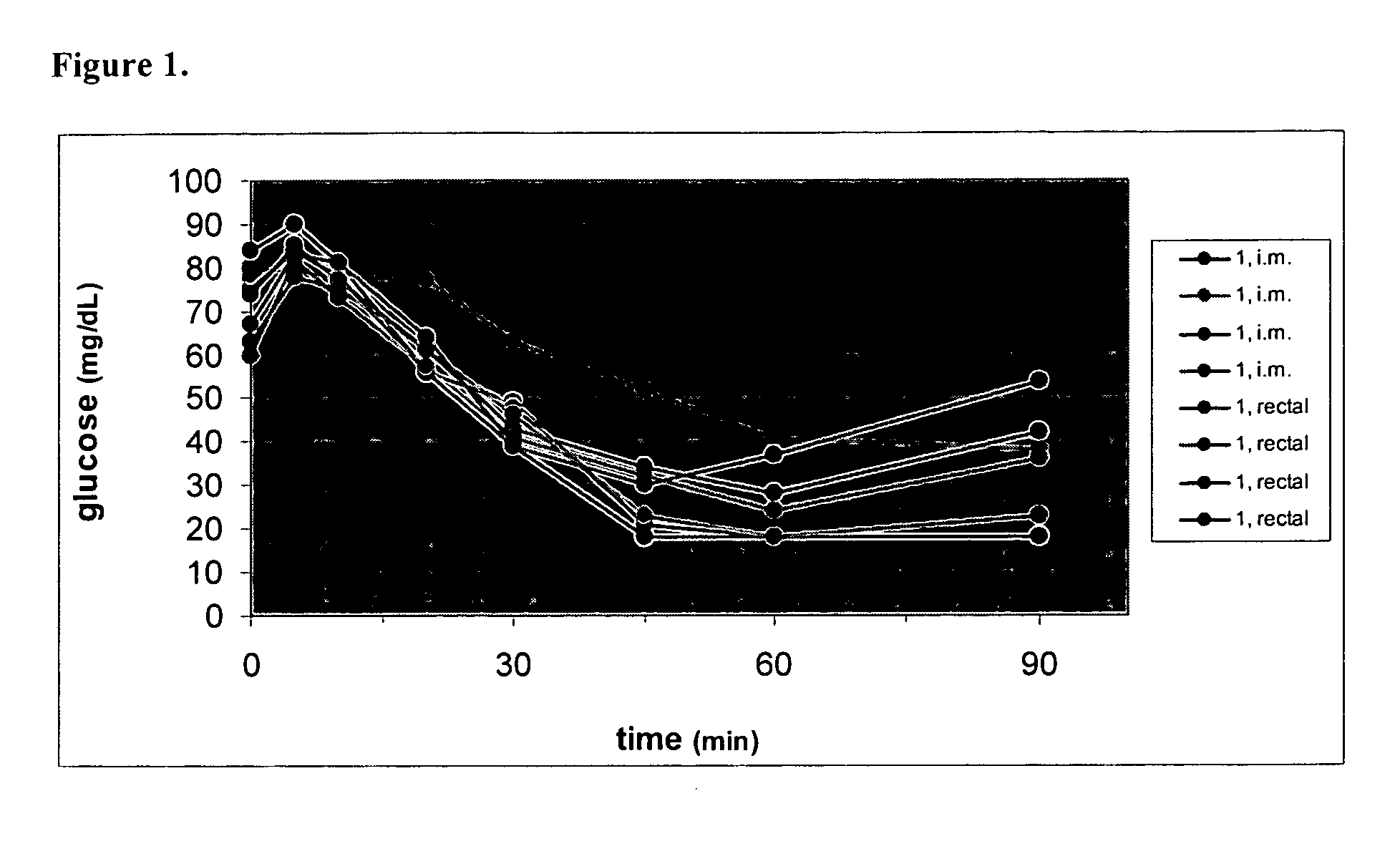
Use of exendins for the reduction of food intake
InactiveUS6956026B2Reduce appetiteReduce cardiac riskPeptide/protein ingredientsPharmaceutical delivery mechanismFeeding disabilityCvd risk
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an exendin or an exendin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. The methods are also useful for lowering the plasma glucose level, lowering the plasma lipid level, reducing the cardiac risk, reducing the appetite, and reducing the weight of subjects. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:ASTRAZENECA PHARMA LP
Thin film with non-self-aggregating uniform heterogeneity and drug delivery systems made therefrom
The invention relates to the film products and methods of their preparation that demonstrate a non-self-aggregating uniform heterogeneity. Desirably the films disintegrate in water and may be formed by a controlled drying process, or other process that maintains the required uniformity of the film.
Owner:AQUESTIVE THERAPEUTICS INC
Abuse-resistant pharmaceutical compositions
An abuse-resistant controlled release pharmaceutical composition comprising a pharmaceutically effective amount of discrete particles of an active capable of abuse, wherein surfaces of said particles are wetted with a water insoluble coating material, and preferably wherein said composition comprises a matrix, in which said particles are distributed, and which renders the abuse-capable compound within the matrix difficult to separate from the matrix; and a method for the preparation of a controlled release pharmaceutical composition having a reduced potential for abuse, comprising applying a pressure force to a mixture comprising a water insoluble material, and particles of a pharmaceutically active compound capable of inducing in a subject a reaction that is physiologically or psychologically detrimental if administered in an immediate release dosage form, thereby resulting in surface coated particles, and incorporating said surface coated particles into a pharmaceutical composition
Owner:ELAN PHRMA INT LTD
Vaccine formulations
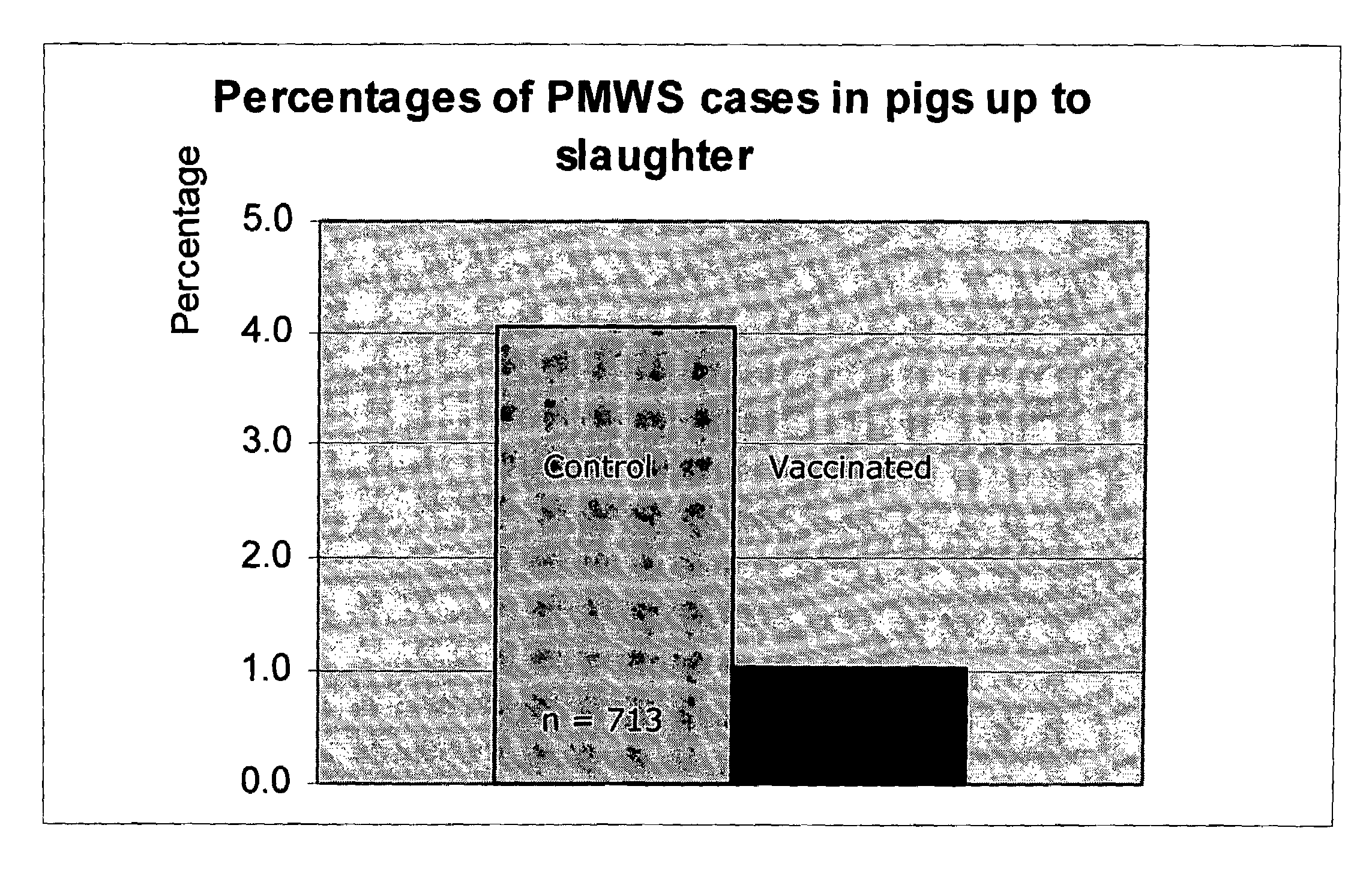
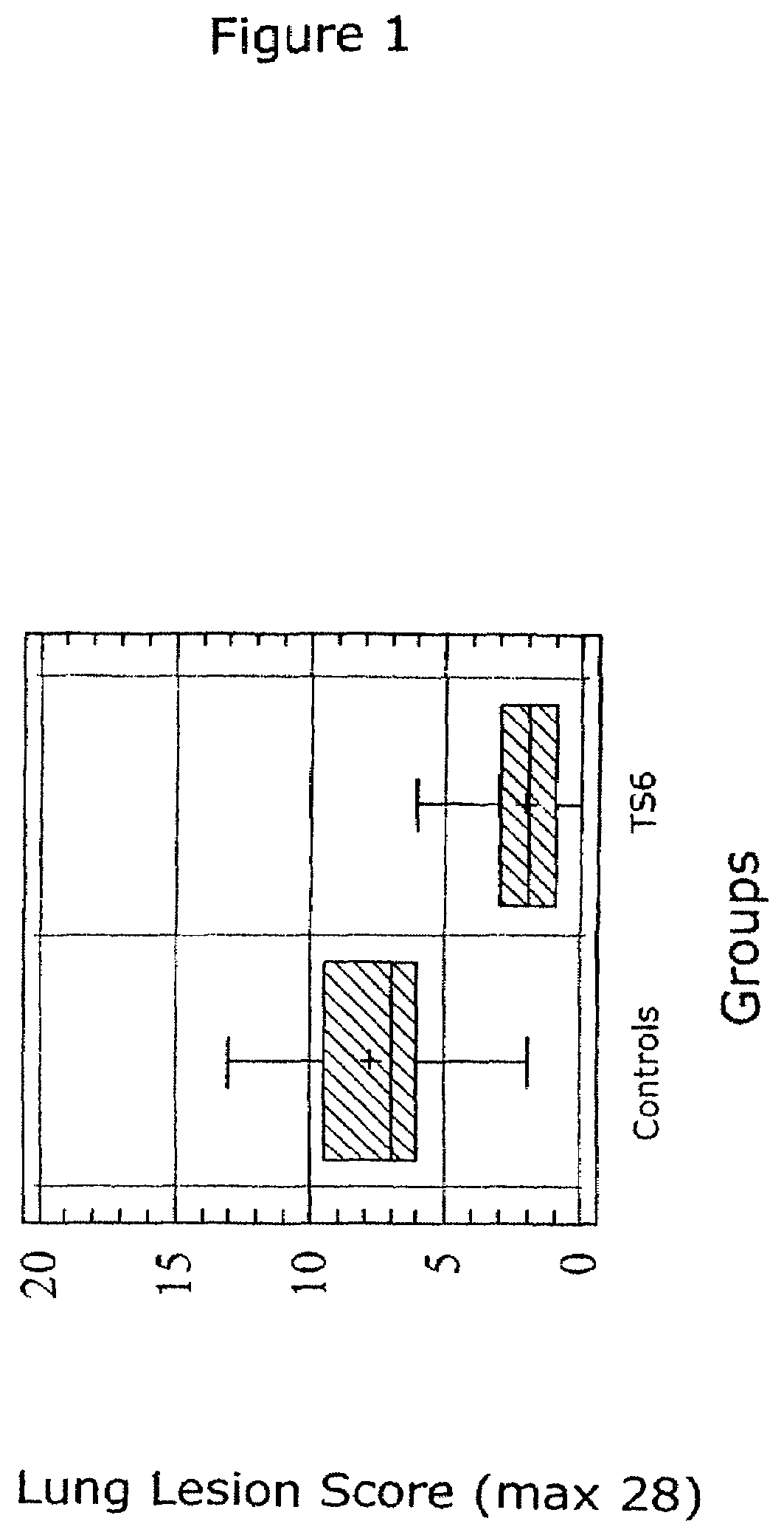
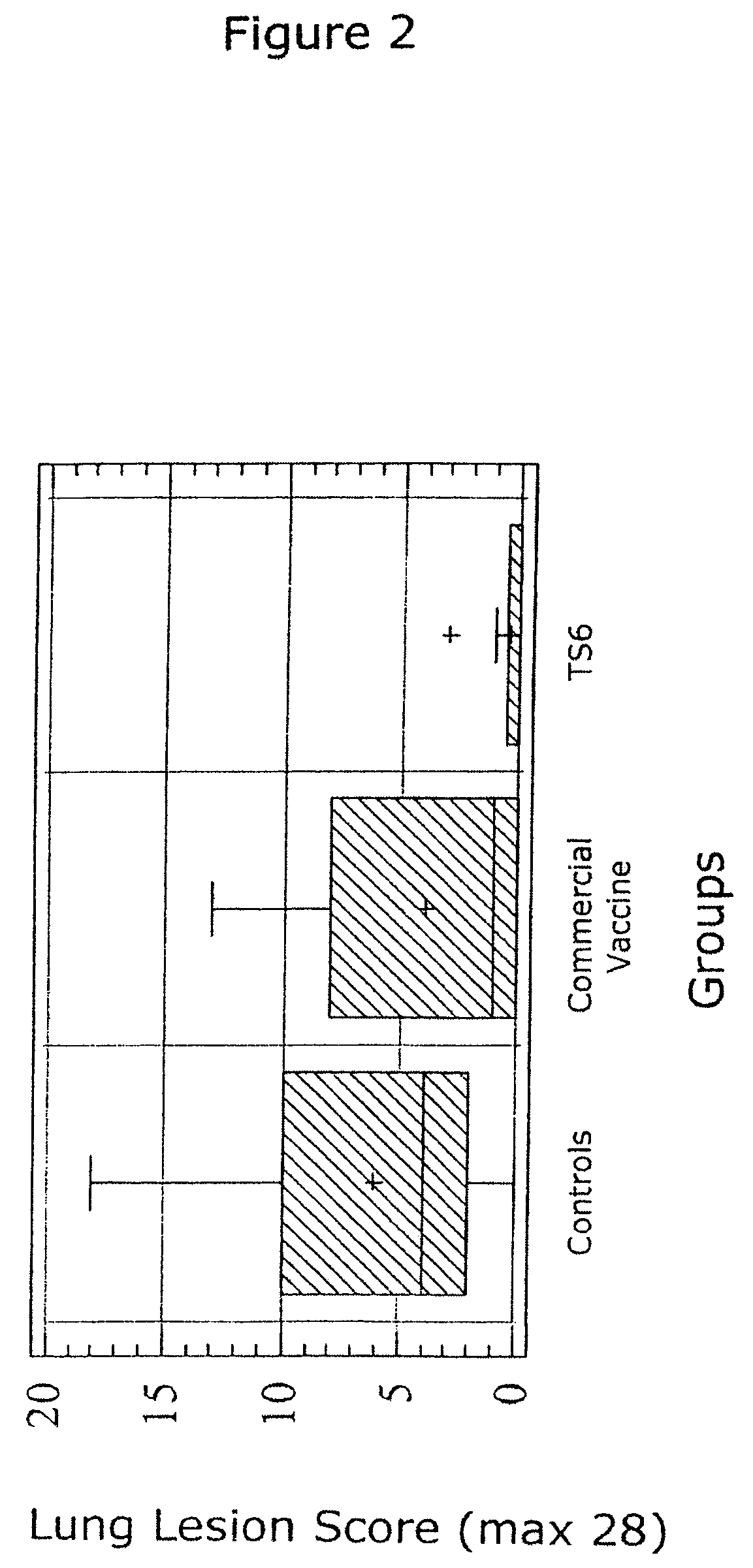
ActiveUS7371395B2Improve stabilityStable and safe and easily administrableSsRNA viruses negative-senseAntibacterial agentsPlasmidBacilli
Owner:MERIAL INC
Pharmaceutical compositions for buccal and pulmonary application
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and at least three different micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal region of the mouth.
Owner:GENEREX PHARMA INC +1
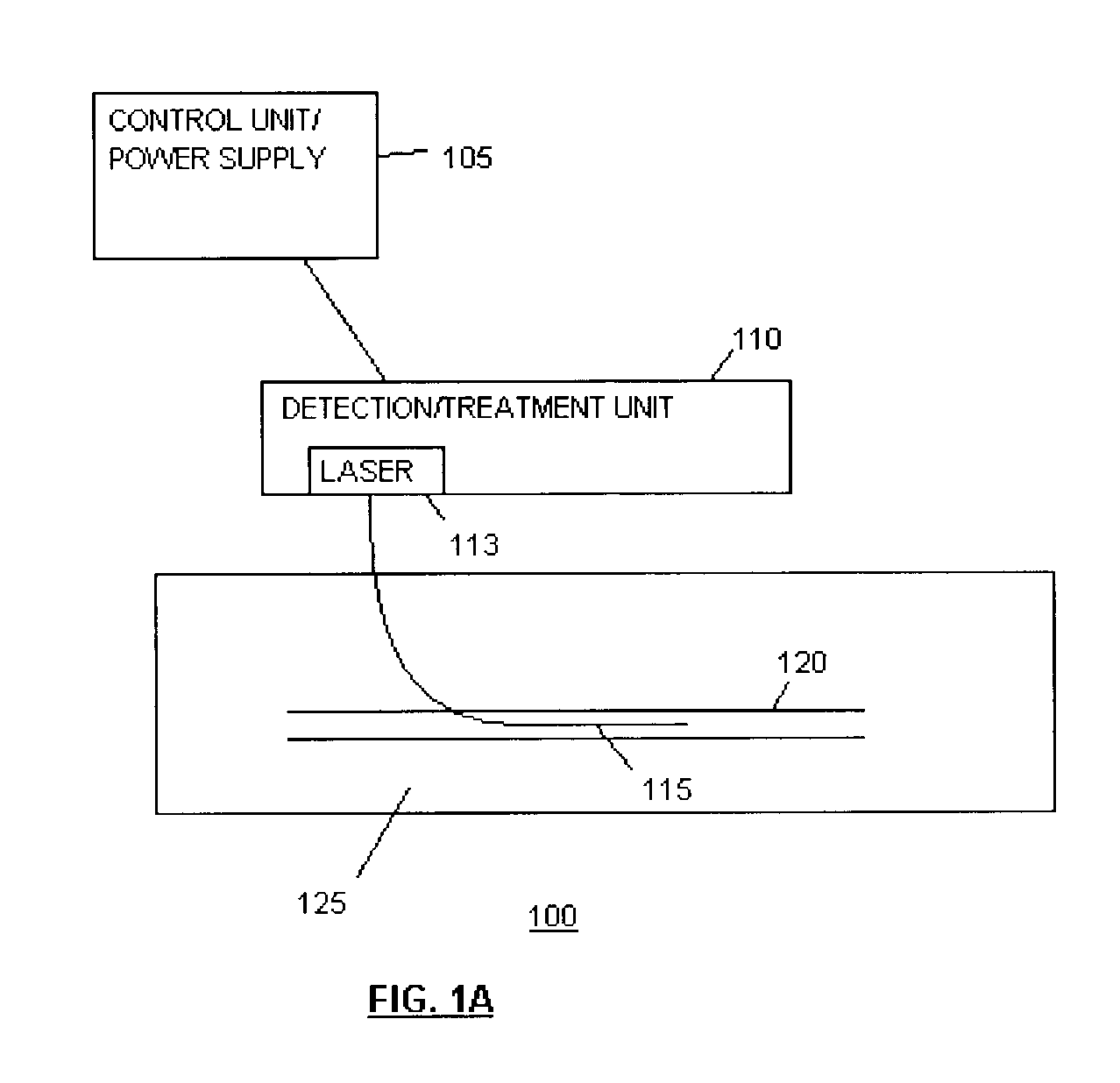
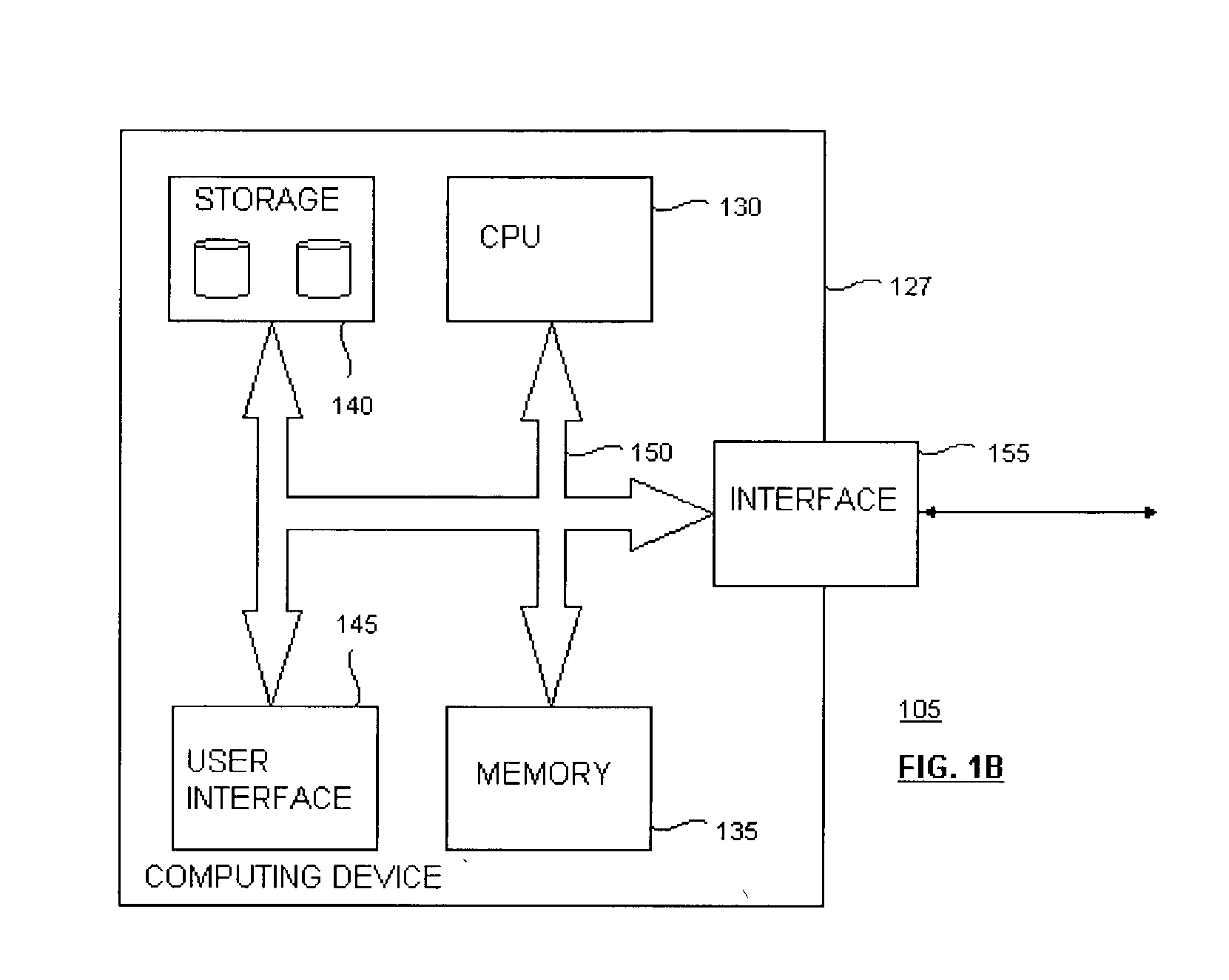
Methods and devices for detection and therapy of atheromatous plaque
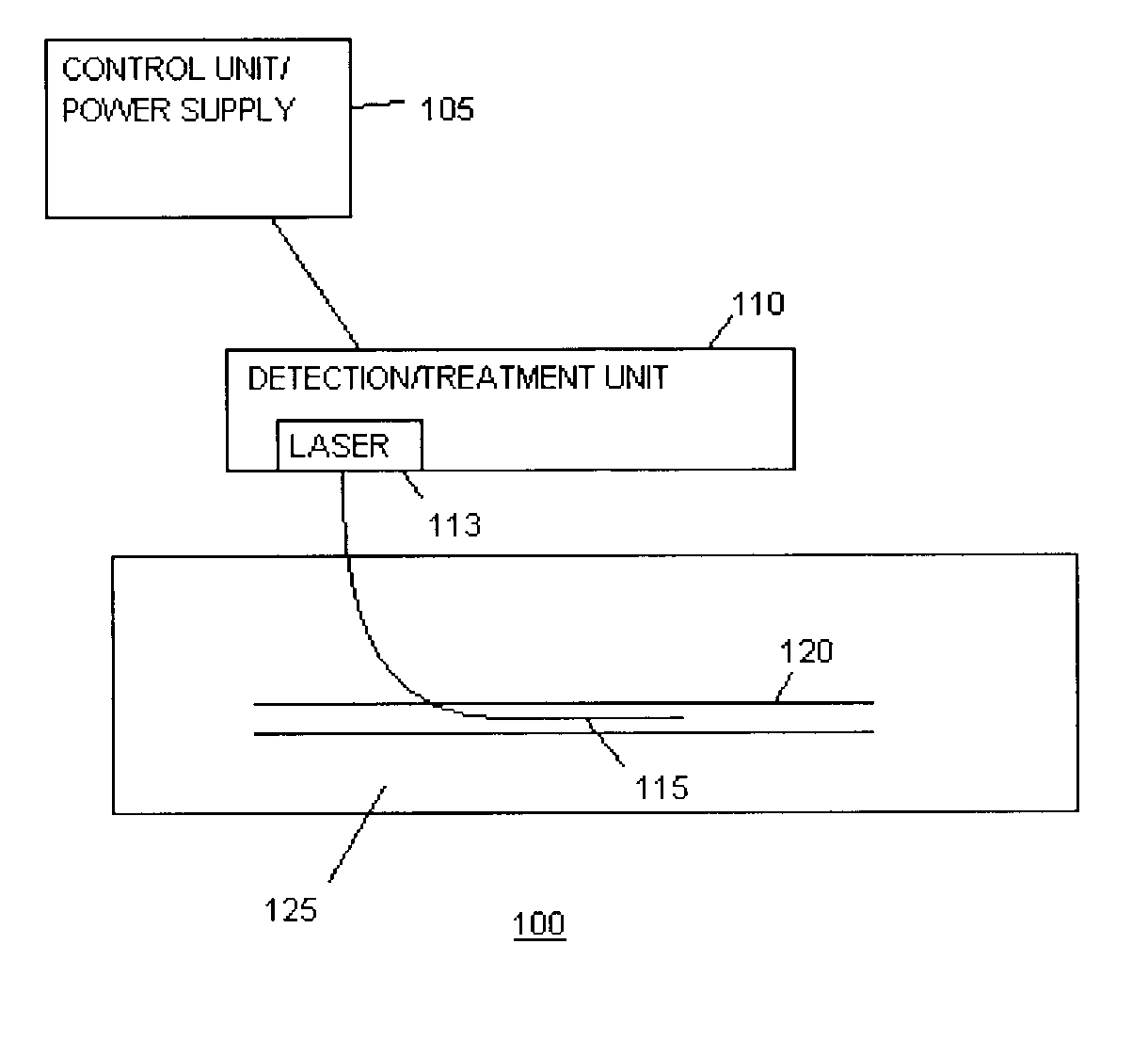
InactiveUS20030082105A1Easy to detectHigh sensitivityUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsVulnerable plaqueFluorescence
The present invention relates to devices for detection and therapy of active atheromatous plaque and / or thin-capped fibro-atheroma ("vulnerable plaque"), using selectively targeted fluorescent, radiolabeled, or fluorescent and radiolabeled compositions. The present invention further relates to methods and devices for detection and theraphy of active atheromatous plaques and / or vulnerable plaques, using selectively targeted beta-emitting compositions, optionally comprising fluorescent compositions.
Owner:THE GENERAL HOSPITAL CORP
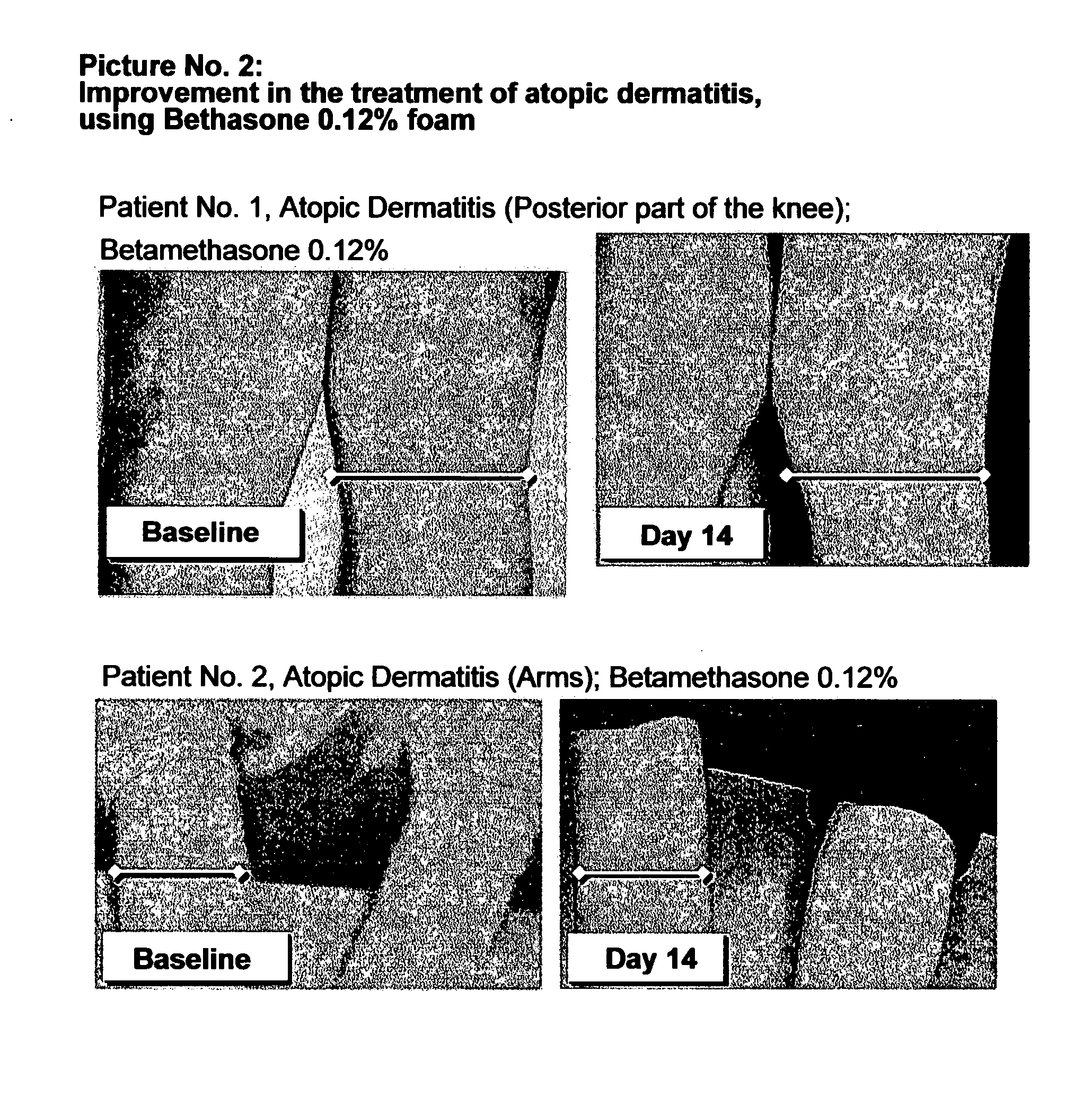
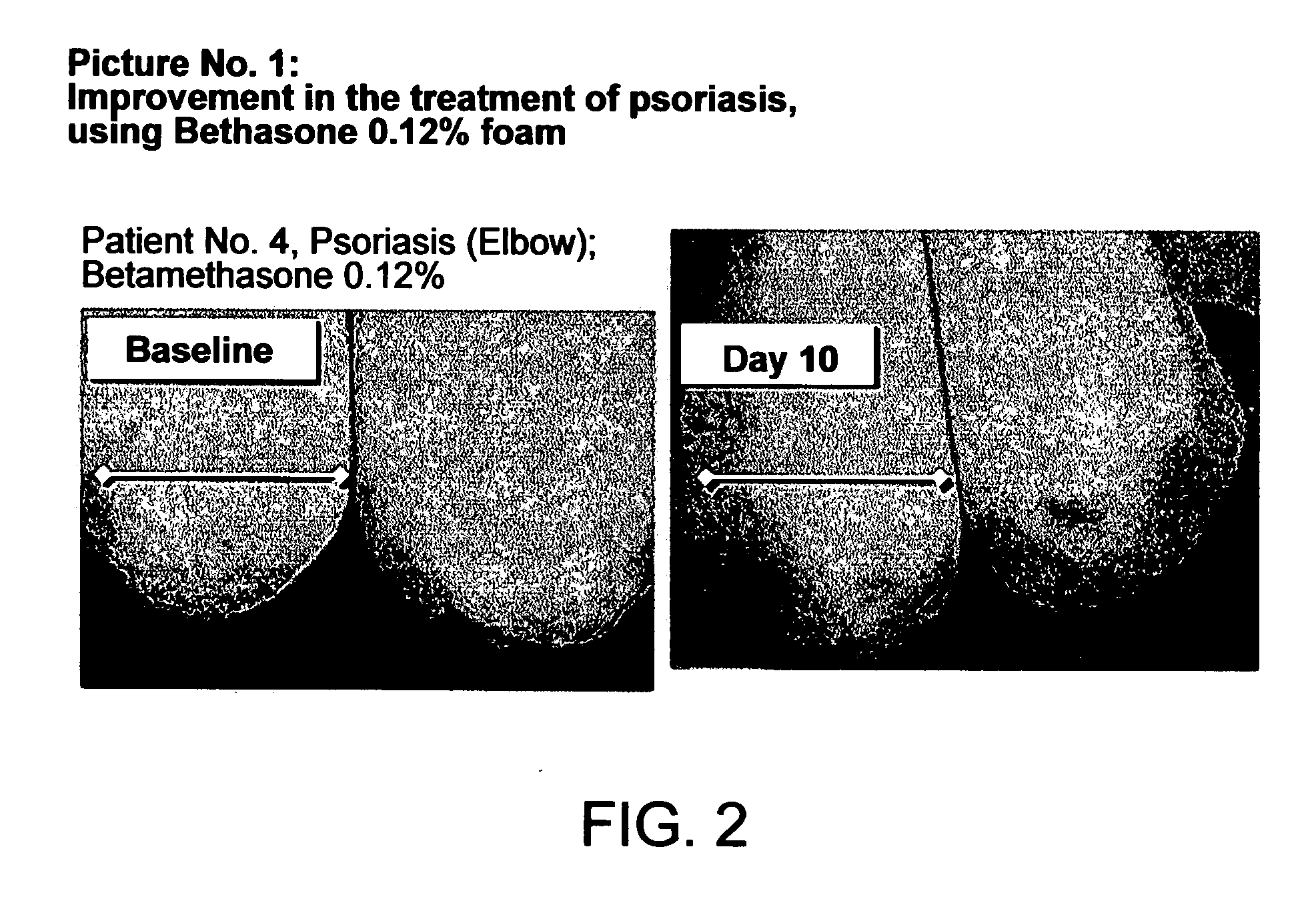
Cosmetic and pharmaceutical foam
InactiveUS20080031907A1Efficient ConcentrationReduce sensitivityAntibacterial agentsBiocideAlcohol freeVegetable oil
The invention relates to uses of an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a hydrophobic solvent, a foam adjuvant agent, a surface-active agent and a water gelling agent as a flame retardant or flame resistant foam. The hydrophobic solvent is preferably mineral oil; medium chain triglycerides; isopropyl myristearate or octyl dodecanol, silicone oil or vegetable oil or mixtures thereof. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, also making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil-soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Trialkyl cationic lipids and methods of use thereof
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
Antimicrobial compositions and methods
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including, in particular, an antimicrobial lipid component, such as a fatty acid ester, fatty ether, or alkoxide derivative thereof. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
Topical compositions and methods for treating pain
InactiveUS6638981B2Avoid painComposition is stableBiocideNervous disorderNR1 NMDA receptorPreventing pain
Topical compositions and methods for treating pain. The invention provides oil-in-water emulsions comprising an antidepressant; an NMDA-receptor antagonists; a lipophilic component; water; and a surfactant. The compositions induce a local-anesthetic effect when topically administered to intact skin thereby treating or preventing pain, for example, neuropathic pain.
Owner:EPICEPT CORP
Lipid vesicle compositions and methods of use
ActiveUS20120177724A1Increase load capacityImprove the level ofPeptide/protein ingredientsMicroencapsulation basedAntigenVesicle/vacuole
The invention provides delivery systems comprised of stabilized multilamellar vesicles, as well as compositions, methods of synthesis, and methods of use thereof. The stabilized multilamellar vesicles comprise terminal-cysteine-bearing antigens or cysteine-modified antigens, at their surface and / or internally.
Owner:MASSACHUSETTS INST OF TECH +1
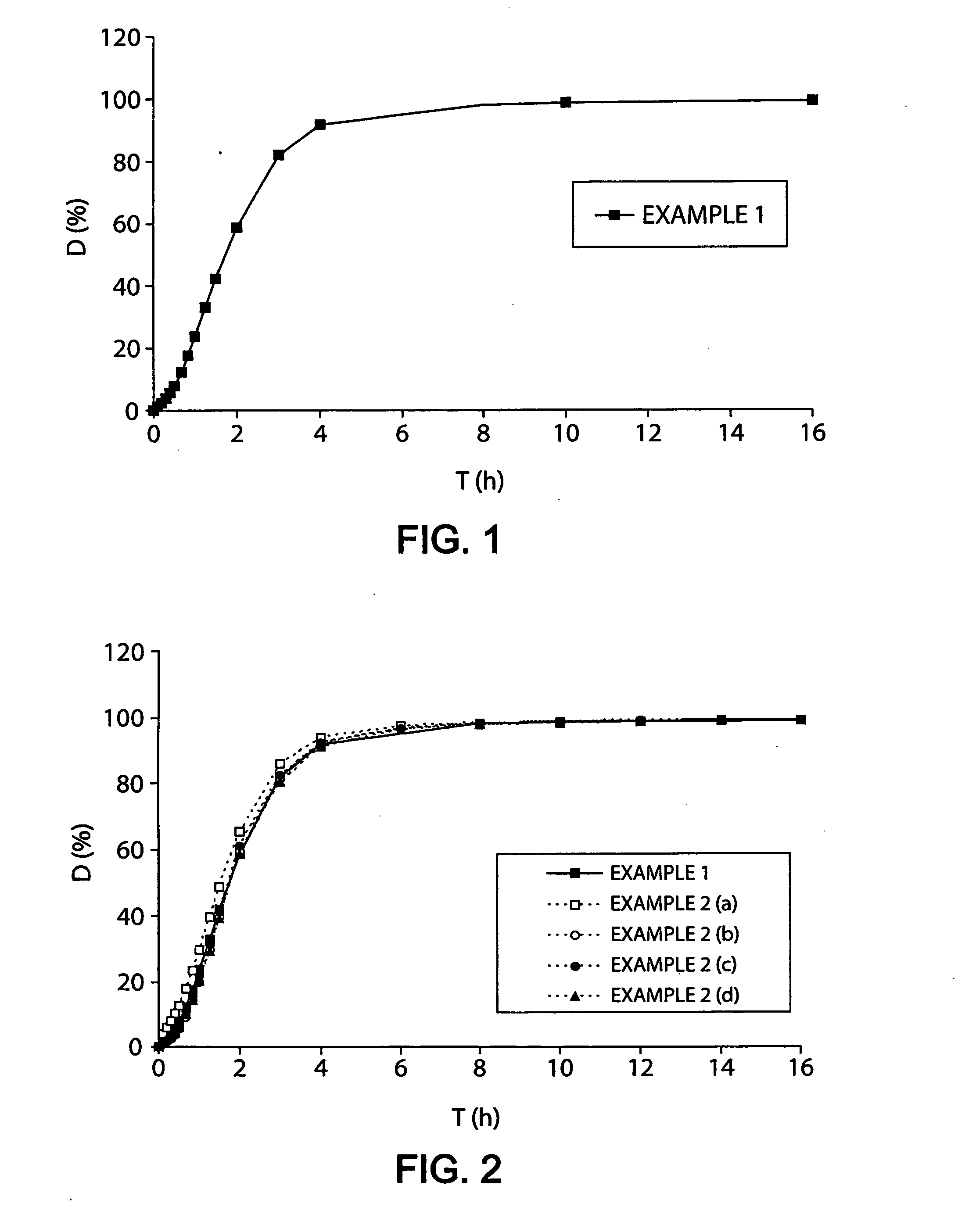
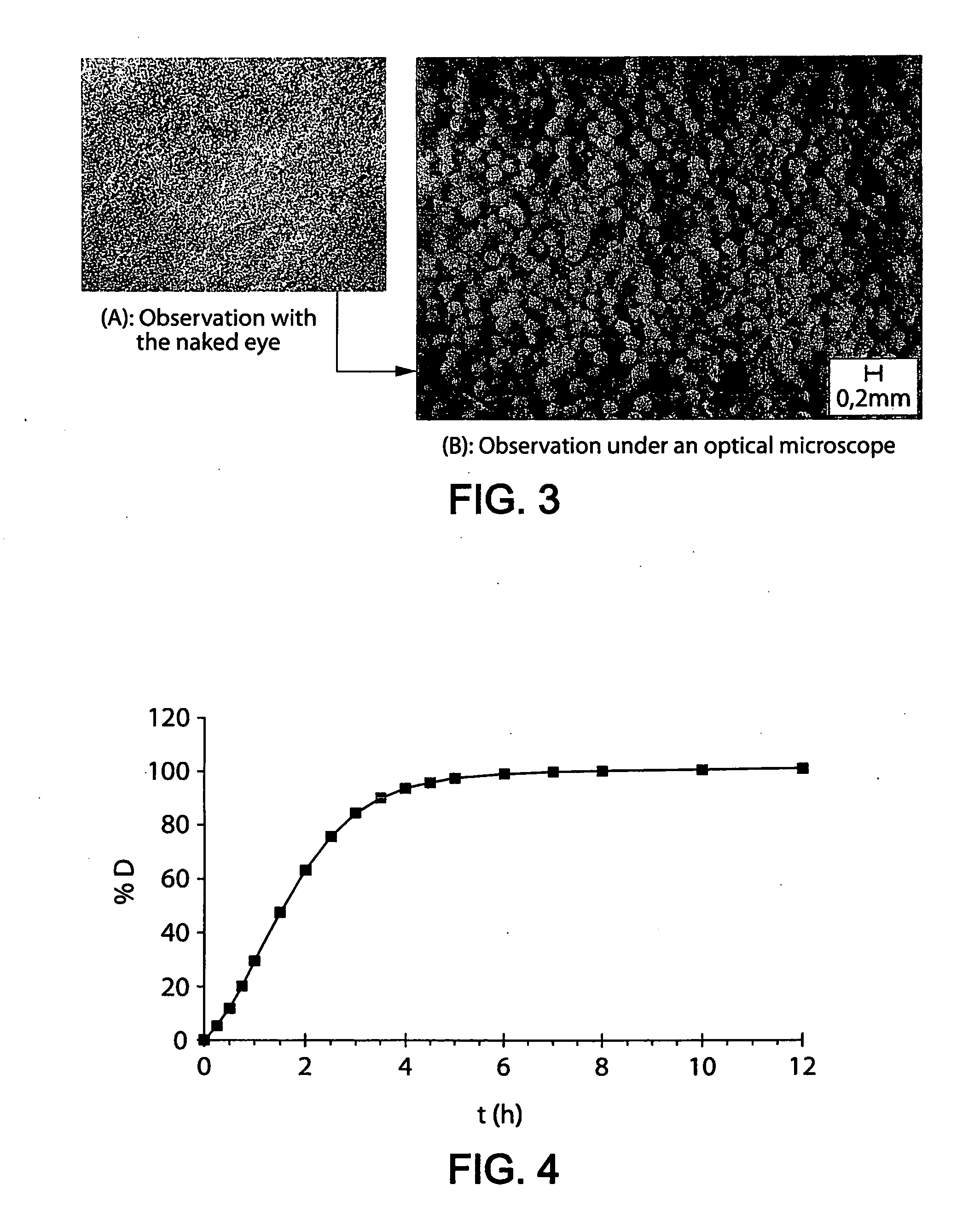
Controlled release preparation
InactiveCN101987081AImprove stabilityRelease impact mitigationInorganic non-active ingredientsSuppositories deliveryParticulatesChemical reaction
The invention discloses a controlled release preparation with improved performance. The controlled release preparation comprises a core containing medicament and a controlled release film covering the outside of the core and being almost insoluble in water as well as stomach and intestines digestive juice. The controlled release film comprises particulate matters of a water soluble medicinal additive, the water-soluble medicinal additive is covered by a polymer film which can be soluble in the stomach and / or intestines digestive juice but almost insoluble in water, the polymer and the medicinal additive can not produce chemical reaction or can produce chemical reaction but do not produce water-insoluble non-gaseous products and the pharmaceutically unacceptable products, and the amount of the polymer is no more 700% of that of the medicinal additive. The invention also discloses a preparation method of the controlled release preparation. The controlled release preparation has the advantages of improved medicament release reproducibility, reduced medicament release lag time, accelerated medicament release and improved bioavailability, can realize located controlled release, delayed controlled release and interval type or pulse type controlled release of the medicament in the gastrointestinal tract, and the like.
Owner:钟术光
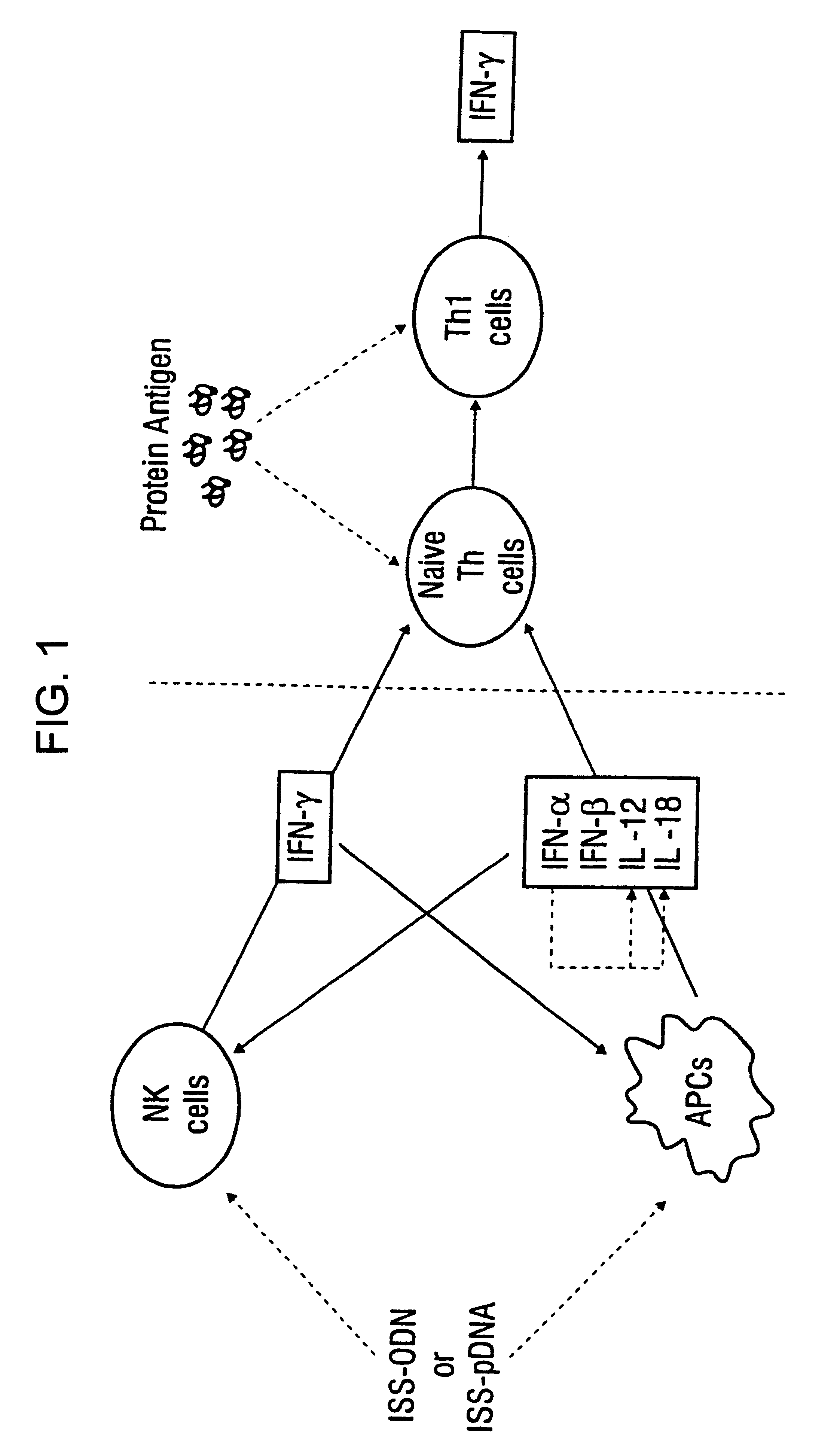
Immunization-free methods for treating antigen-stimulated inflammation in a mammalian host and shifting the host's antigen immune responsiveness to a Th1 phenotype
InactiveUS6498148B1Treatment and prevention of inflammationSuppresses antigen-stimulated granulocyte infiltrationOrganic active ingredientsSenses disorderAntigen stimulationTherapeutic intent
The invention relates to methods for preventing or reducing antigen-stimulated, granulocyte-mediated inflammation in tissue of an antigen-sensitized mammal host by delivering an immunostimulatory oligonucleotide to the host. In addition, methods for using the immunostimulatory oligonucleotides to boost a mammal host's immune responsiveness to a sensitizing antigen (without immunization of the host by the antigen) and shifting the host's immune responsiveness to a Th1 phenotype to achieve various therapeutic ends are provided. Kits for practicing the methods of the invention are also provided.
Owner:RGT UNIV OF CALIFORNIA
Abuse resistant drug formulation
A pharmaceutical composition may include a coated particulate which may include at least one active pharmaceutical ingredient, particularly one susceptible to abuse by an individual. The coated particles may include a fat / wax and have improved controlled release and / or crush resistance. Method of making these coated particulate and dosage forms therewith are also described.
Owner:CIMA LABS
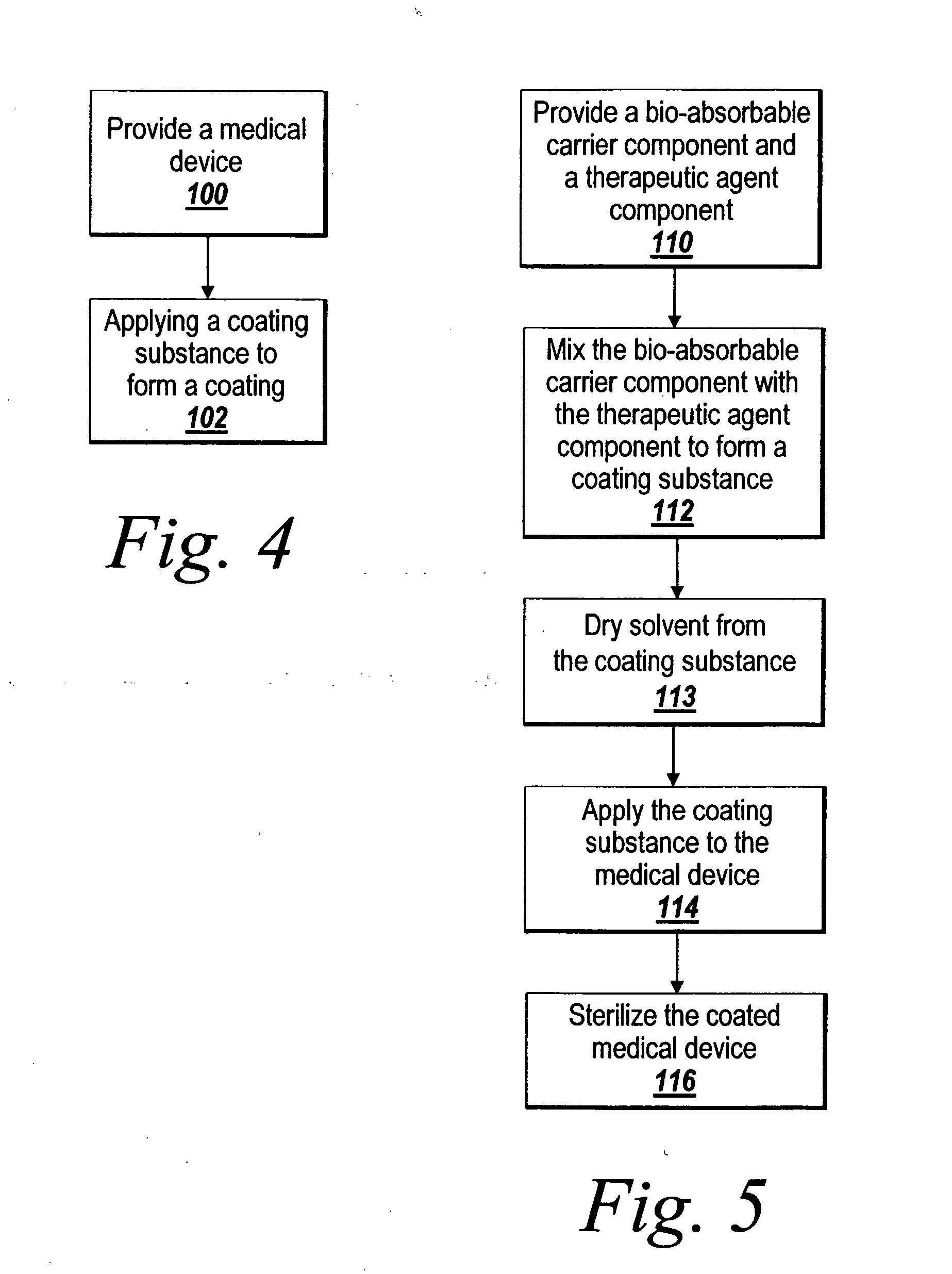
Pre-dried drug delivery coating for use with a stent
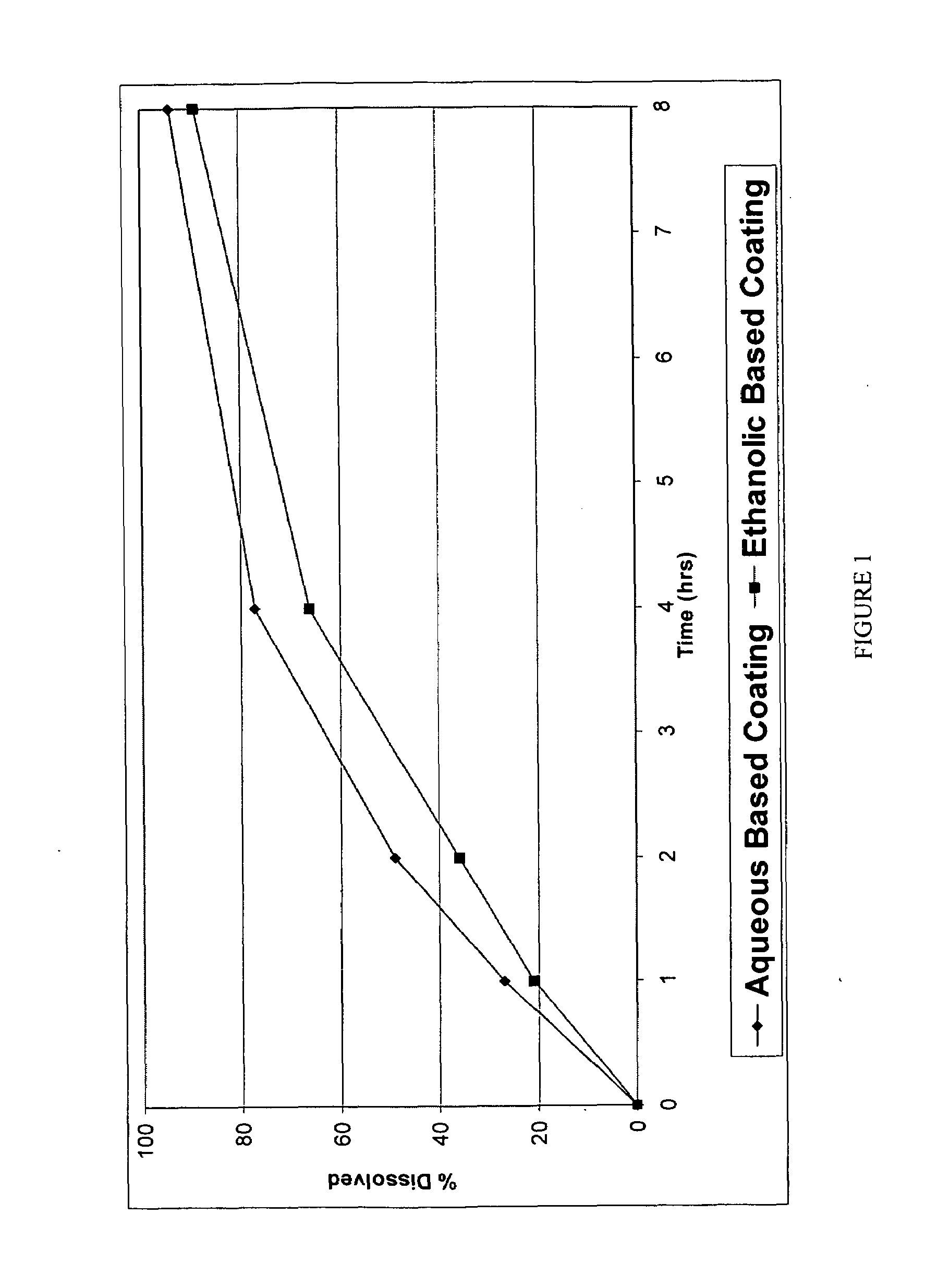
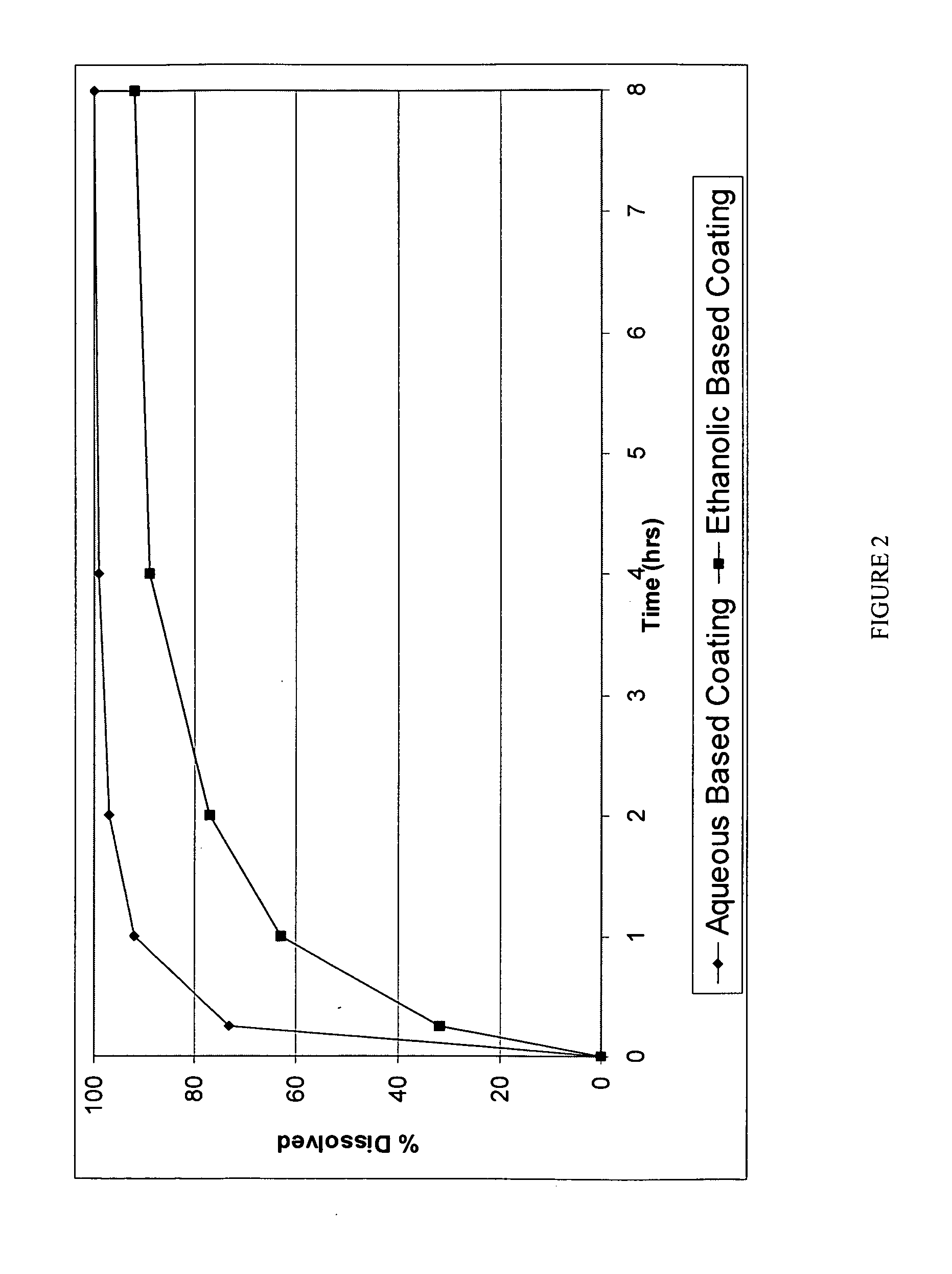
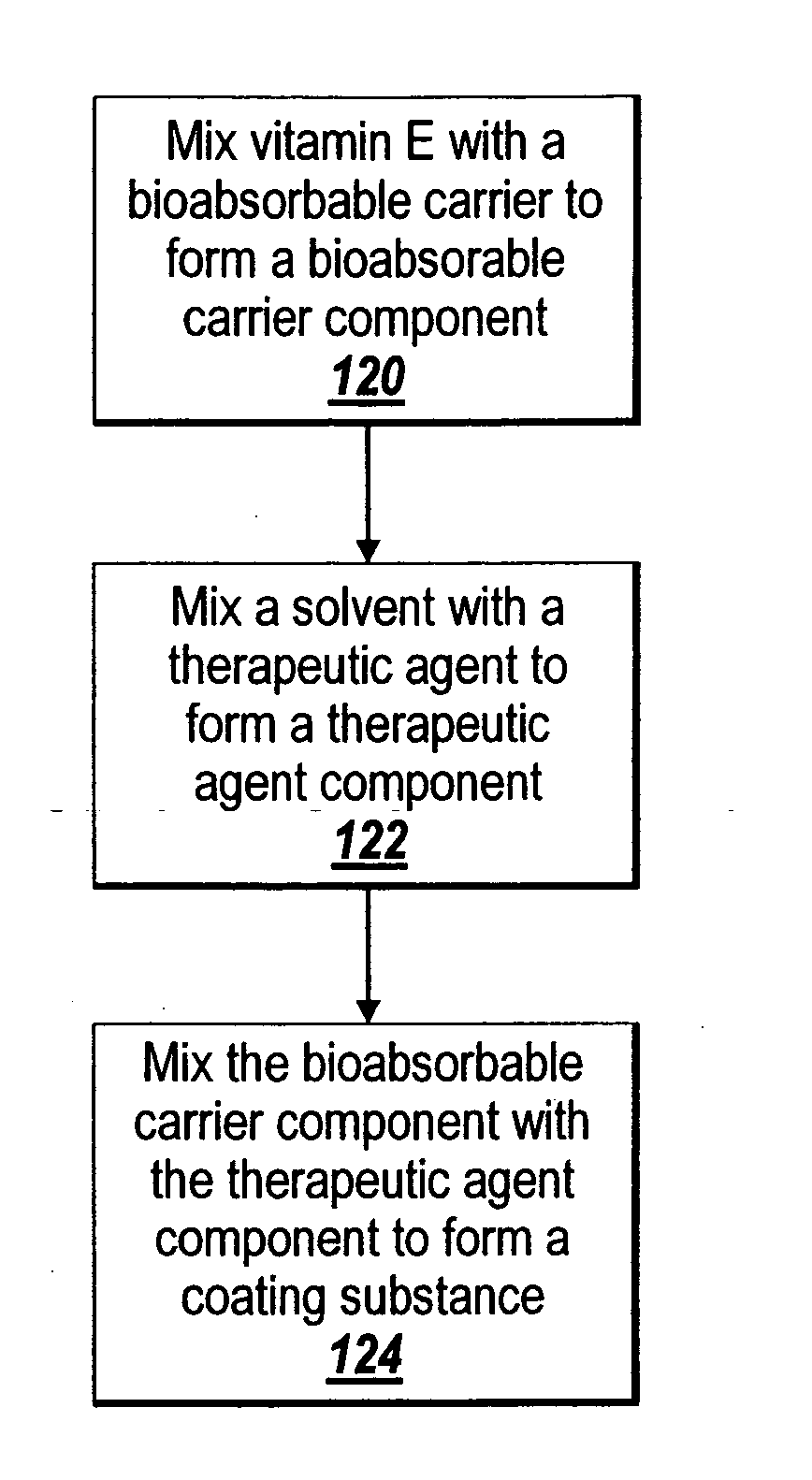
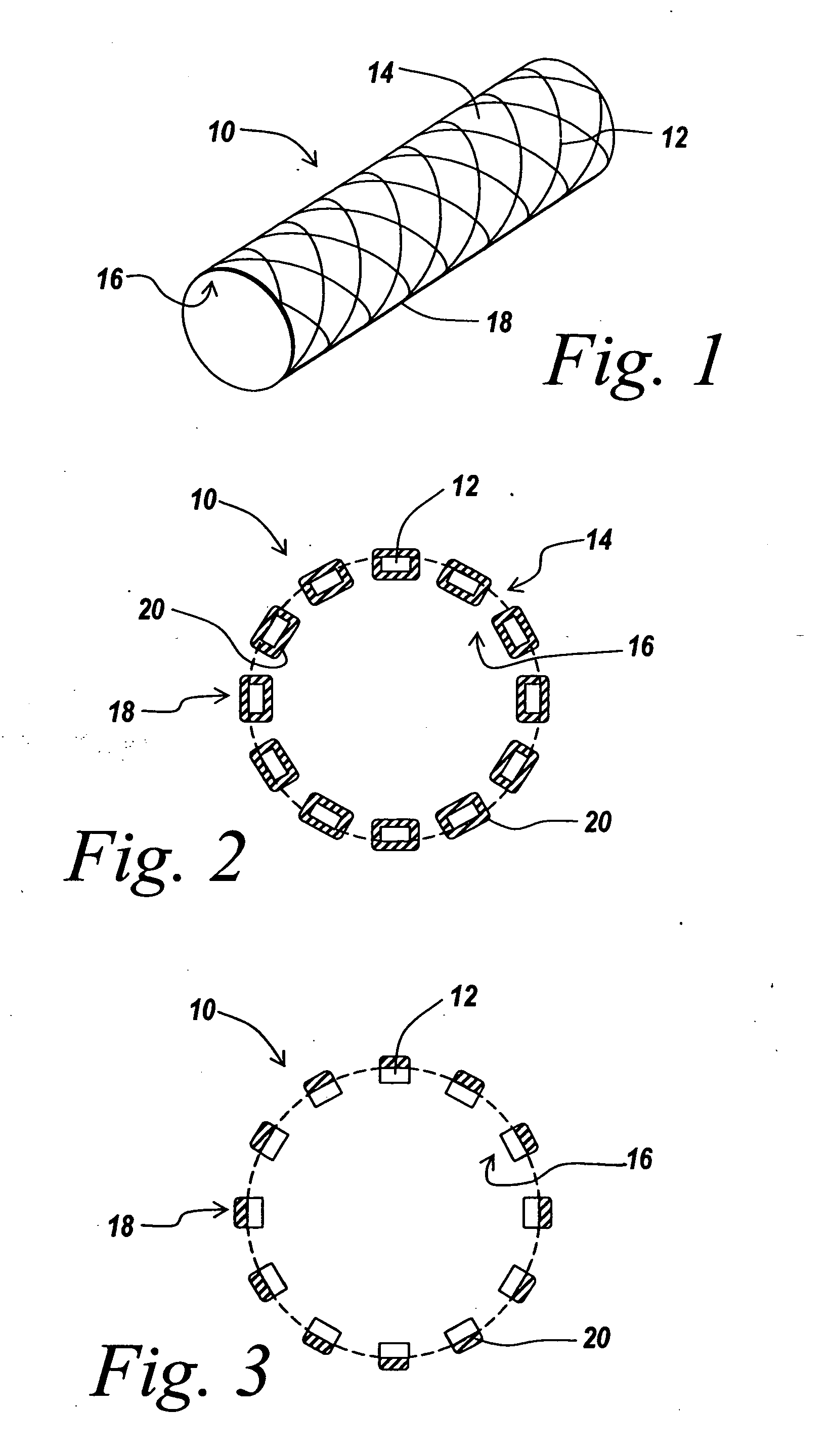
InactiveUS20060067977A1Increases rate of solvent evaporationRaise transfer toAntipyreticAnalgesicsInsertion stentSolvent
A method and apparatus for the provision of a coating for application to a medical device results in a medical device having a bio-absorbable coating. The coating includes a bio-absorbable carrier component. In addition to the bio-absorbable carrier component, a therapeutic agent component and solvent can also be provided. The solvent is removed from the coating before the coating is applied to the medical device. The coated medical device is implantable in a patient to effect controlled delivery of the coating, including the therapeutic agent, to the patient.
Owner:ATRIUM MEDICAL
Powders for inhalation
InactiveUS6045828AControl cohesivenessGood estimateBiocideOrganic active ingredientsLipid formationInhalation
PCT No. PCT / SE95 / 01560 Sec. 371 Date Mar. 20, 1996 Sec. 102(e) Date Mar. 20, 1996 PCT Filed Dec. 20, 1995 PCT Pub. No. WO96 / 19199 PCT Pub. Date Jun. 27, 1996A proliposome powder, said powder comprising in a single phase discrete particles of a biologically active component together with a lipid or mixture of lipids having a phase transition temperature of below 37 DEG C. and a process for the manufacture of a proliposome powder for inhalation.
Owner:ASTRAZENECA AB
Anti-misuse microparticulate oral pharmaceutical form
InactiveUS20070224129A1Avoid misusePrevent misuse by short liquid extraction and/or crushingOrganic active ingredientsDrug compositionsPublic healthMicroparticle
The present invention relates to solid microparticulate oral pharmaceutical forms whose composition and structure make it possible to avoid misuse of the pharmaceutical active principle (AP) they contain. The object of the present invention is to prevent solid oral drugs from being misappropriated for any use other than the therapeutic use(s) officially approved by the competent public health authorities. In other words, the object is to avoid the voluntary or involuntary misuse of solid oral drugs. The invention relates to a solid oral pharmaceutical form which is characterized in that it contains anti-misuse means, in that at least part of the AP it comprises is contained in coated microparticles for modified release of the AP, and in that the coated microparticles of AP have a coating layer (Ra) which assures the modified release of the AP and simultaneously imparts crushing resistance to the coated microparticles of AP so as to avoid misuse.
Owner:FLAMEL IRELAND
Topical dermal anaesthetic
A liquid composition applied transdermally for relief of pain comprising alcohol in an amount by weight of about 57 to about 91 percent; glycerin in an amount by weight of about 1 to about 12 percent; an analgesic agent in an amount by weight of about 2 to about 28 percent, the analgesic agent comprising a derivative of salicylic acid; methylsulfonylmethane in an amount by weight of about 0.02 to 5 percent; and emu oil in an amount by weight of about 0.01 to 3 percent, the liquid composition permeating skin to relieve pain. The composition further comprising, as an additional feature, aloe vera in an amount by weight of at least about 0.05 percent and having an amount by weight of about 0.05 to 4 percent. The composition features transdermal pain relief such that a patient can apply the analgesic agent directly to an area of pain without such side effects as stomach irritation which is normally associated with aspirin. The composition may be sprayed or rolled directly onto the painful area. Because of the unique formula, the composition is safe to vital internal organs, requires no mixing before use, and is shelf stable for marketing purposes.
Owner:VELTRAN LP
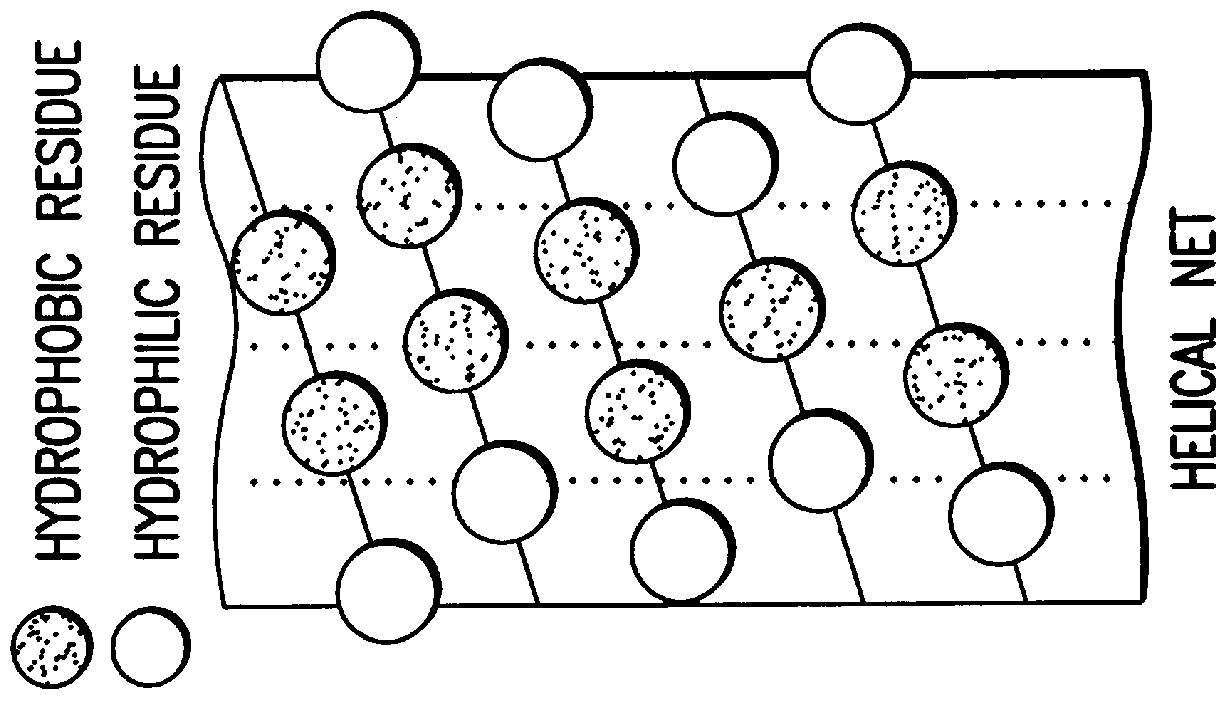
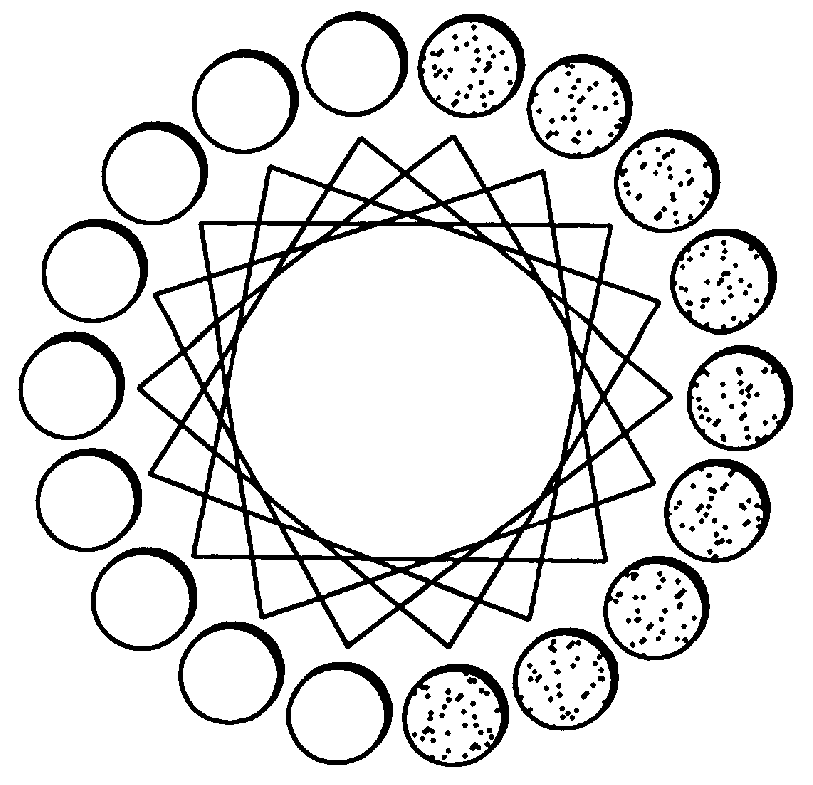
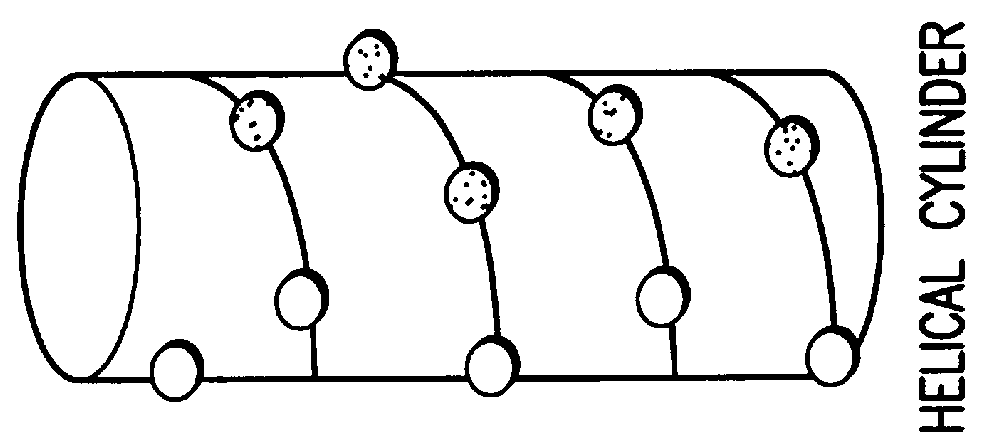
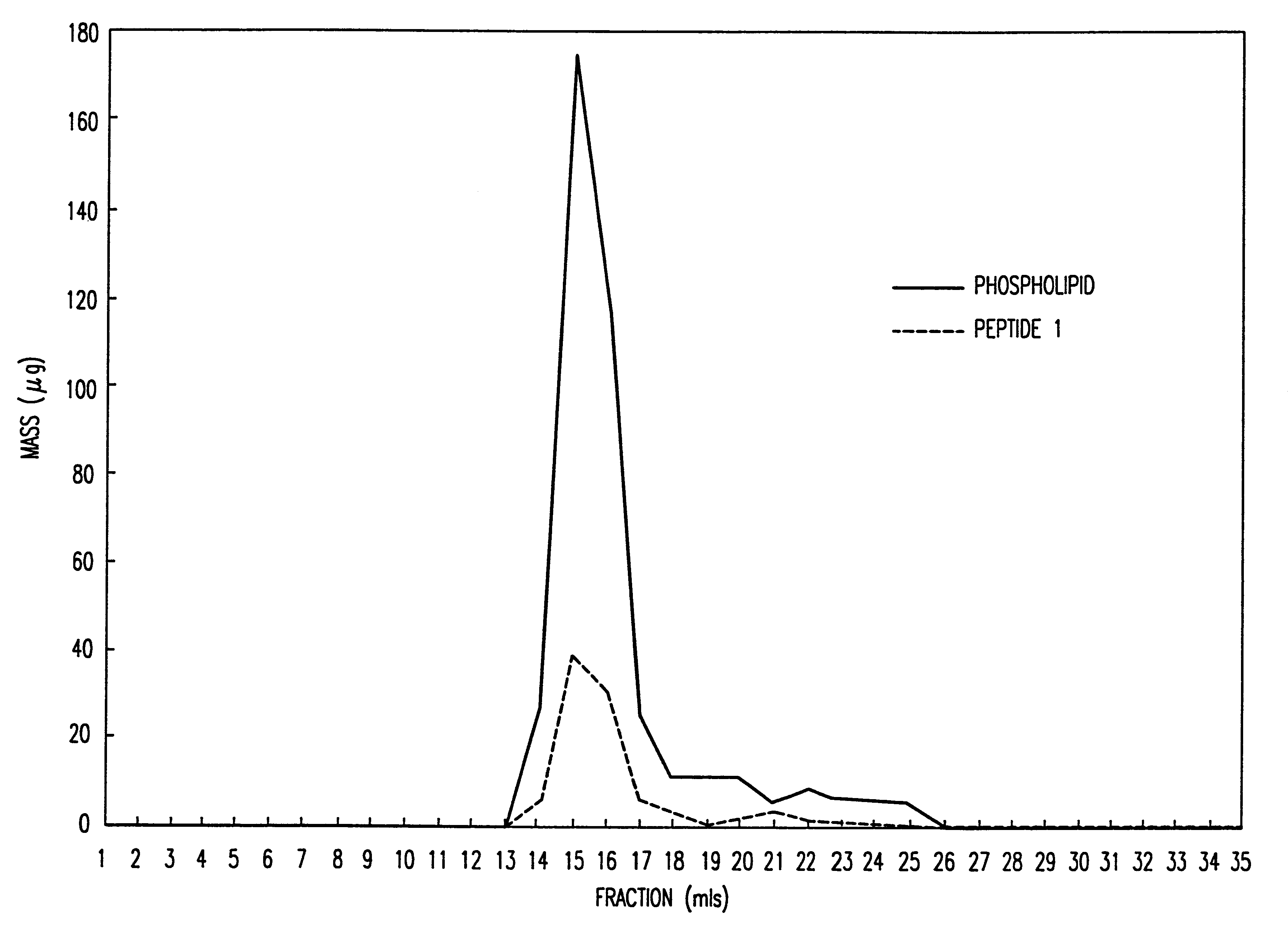
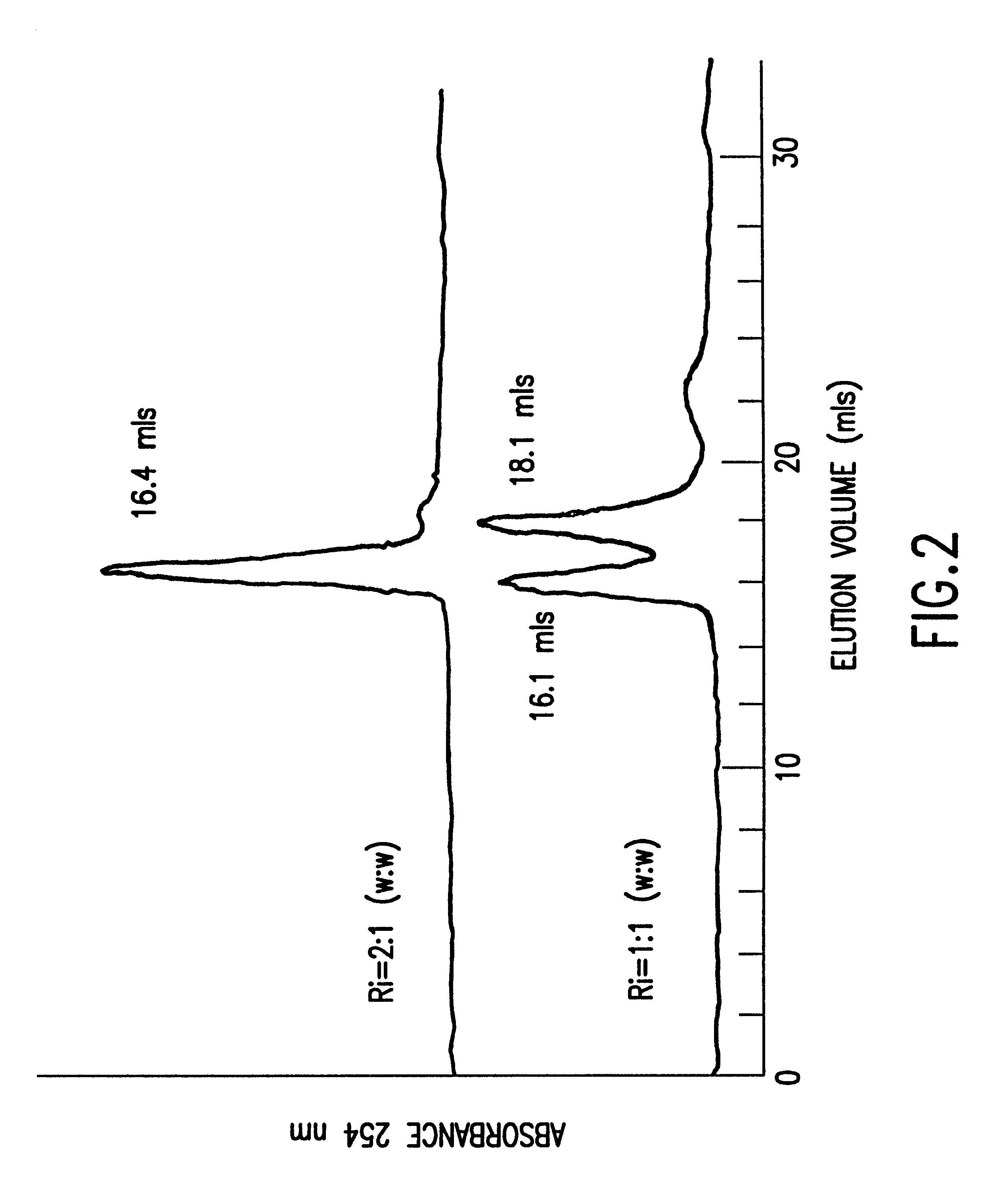
Peptide/lipid complex formation by co-lyophilization
The invention relates to the formation of peptide / lipid vesicles and complexes through the co-lyophilization of peptides, preferably that are able to adopt an amphipathic alphahelical conformation, and one or more lipids. A single solution which solubilizes both the peptides and lipids or two separate solutions may be lyophilized.
Owner:ESPERION THERAPEUTICS
Compositions capable of facilitating penetration across a biological barrier
InactiveUS20050232981A1Effectively translocatingIncrease in translocationAntibacterial agentsOrganic active ingredientsDiseaseMedicine
This invention relates to novel penetrating compositions including one or more effectors included within a water soluble composition, immersed in a hydrophobic medium. The invention also relates to methods of treating or preventing diseases by administering such penetrating compositions to affected subjects.
Owner:CHIASMA INC
Alcohol-free transdermal analgesic composition and processes for manufacture and use thereof
InactiveUS7052715B2Reduced shelf lifeImprove permeabilityOrganic active ingredientsBiocideAlkaneAlcohol free
The instant invention is directed toward a dermal delivery system composition comprising an aqueous base vehicle including American Emu oil, Isopropyl Palmitate (PROTACHEM IPP), PEG-8 (a polyethylene glycol available under the tradename PROTACHEM 400), methylsulfonylmethane (MSM) and SEPIGEL 305 (a combination including polyacrylamide / C13–C14 Iso-paraffin and Laureth-7), in combination with an analgesic composition, such as ibuprofen, and to processes for the manufacture and use thereof.
Owner:ALL NATURAL FMG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com