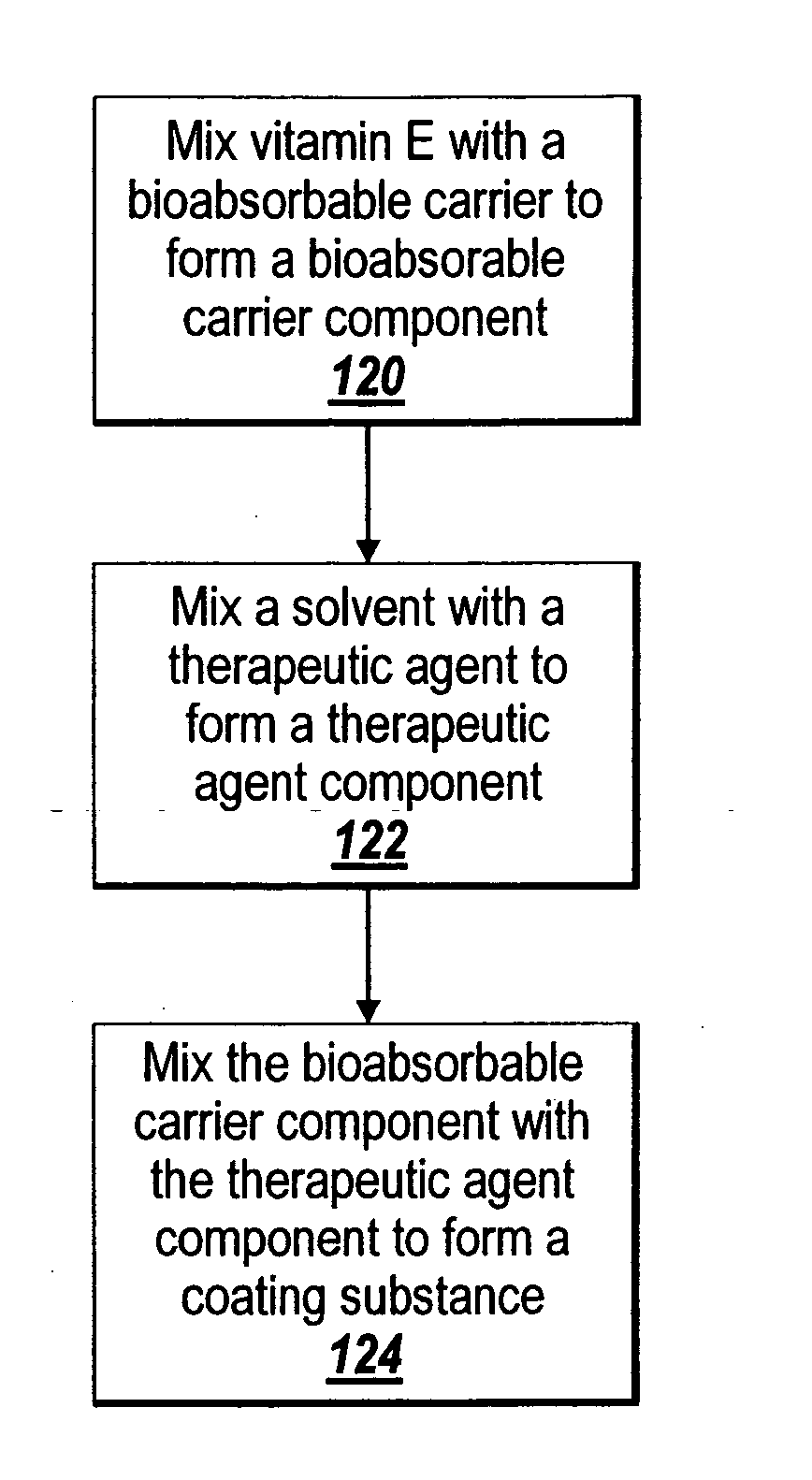
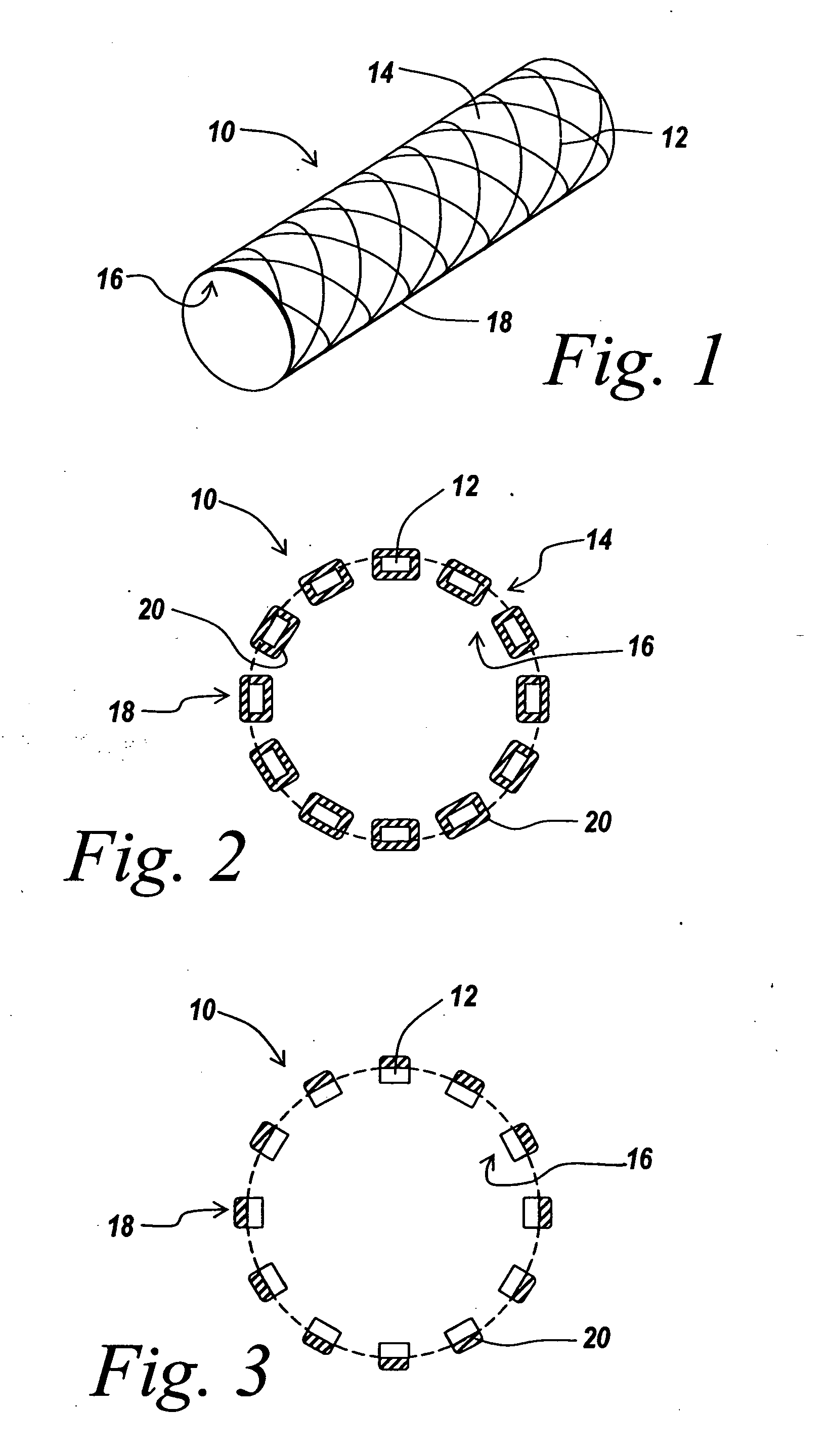
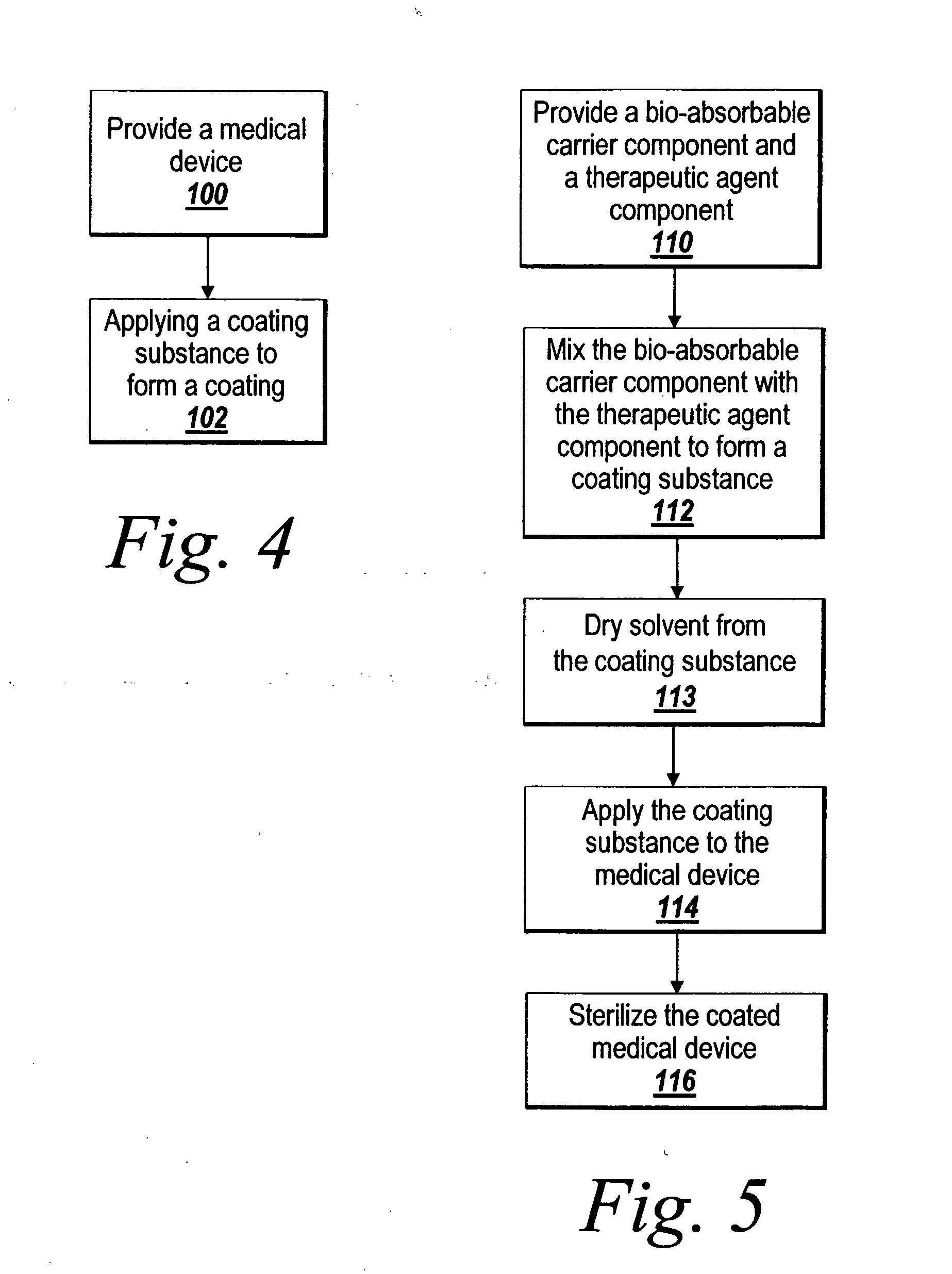
Pre-dried drug delivery coating for use with a stent
a drug delivery and pre-dried technology, applied in the field of coatings, can solve the problems of oil utilization, unsatisfactory effects, and large doses of therapeutic agents, and achieve the effects of increasing the drying rate of the coating material, accelerating the transfer of solvent, and increasing the solvent evaporation ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0117] A fish oil:vitamin E coating was mixed at a ratio of 50:50.1.5 grams of this mixture was blended with 1.5 grams of ethanol. When placed in a bell jar vacuum (50 to 60 mTorr) in a weigh pan (19.63 cm2 area), it took 24 hours to completely remove the ethanol. When placed in a Petri dish (62.07 cm2 area), it took 4 hours to completely remove the ethanol under similar vacuum conditions. When the same formulation was placed in the rotating fixture (FIG. 6B) and the surface area was continually refreshed, it took 1 hour to remove the ethanol under similar vacuum conditions. The removal of ethanol was confirmed by FTIR.
example 2
[0118] A fish oil:vitamin E coating was mixed at a ratio of 50:50. 1.5 grams of this mixture was blended with 1.5 grams of nMP. When placed in a bell jar vacuum (50 to 60 mTorr) in a weigh pan (19.63 cm2 area), the nMP could not be removed even after 120 hours of vacuum. When placed in a Petri dish (62.07 cm2 area), it took 96 hours to completely remove the nMP under similar vacuum conditions. When the same formulation was placed in the rotating fixture (FIG. 6B) and the surface area was continually refreshed, it took 30 hours to remove the nMP under similar vacuum conditions. The removal of NMP was confirmed by FTIR.
example 3
[0119] A 30:70 fish oil:vitamin E formulation was prepared. A rapamycin pro-drug was dissolved in ethanol and 1.9169 grams of the drug solution was added to 1.0751 grams of the coating formulation. This mixture was placed in the rotating fixture (FIG. 6B) and the surface area was continually refreshed. The final drug concentration was 24.26% and was confirmed by HPLC analysis. The ethanol solvent was completely removed after 24 hours of operation. The removal of the ethanol was confirmed by FTIR analysis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com