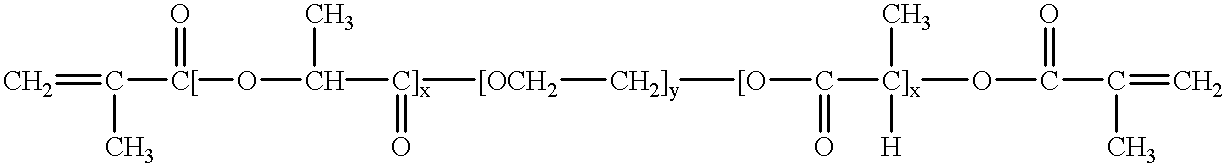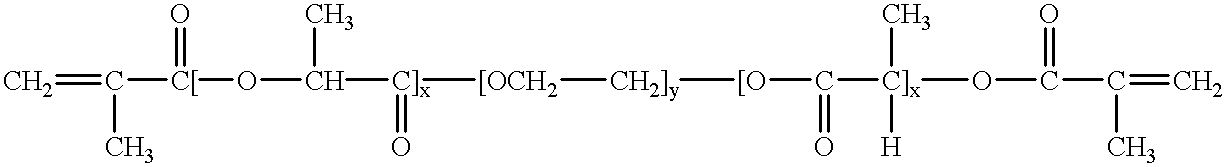Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
791results about "Female contraceptives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
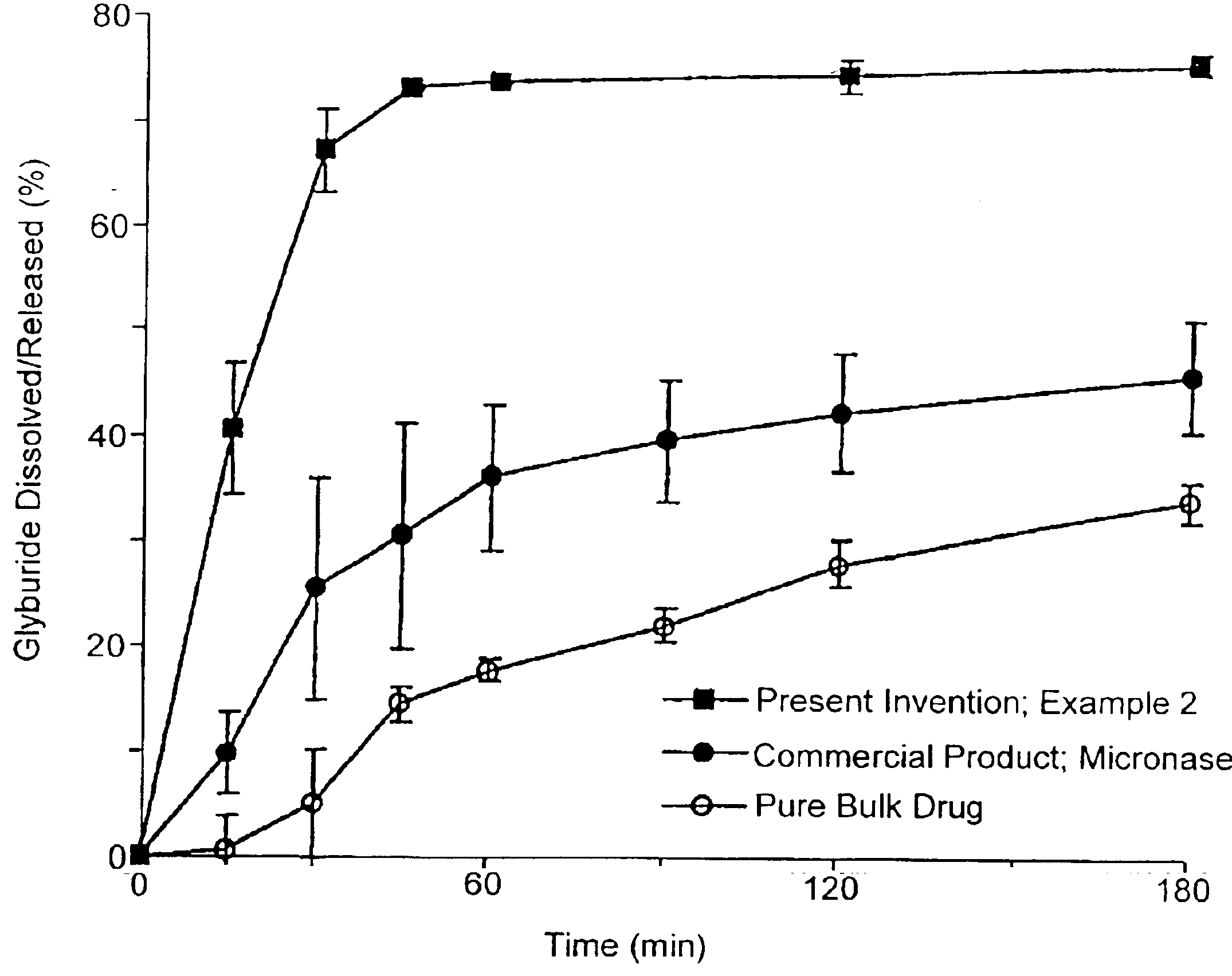
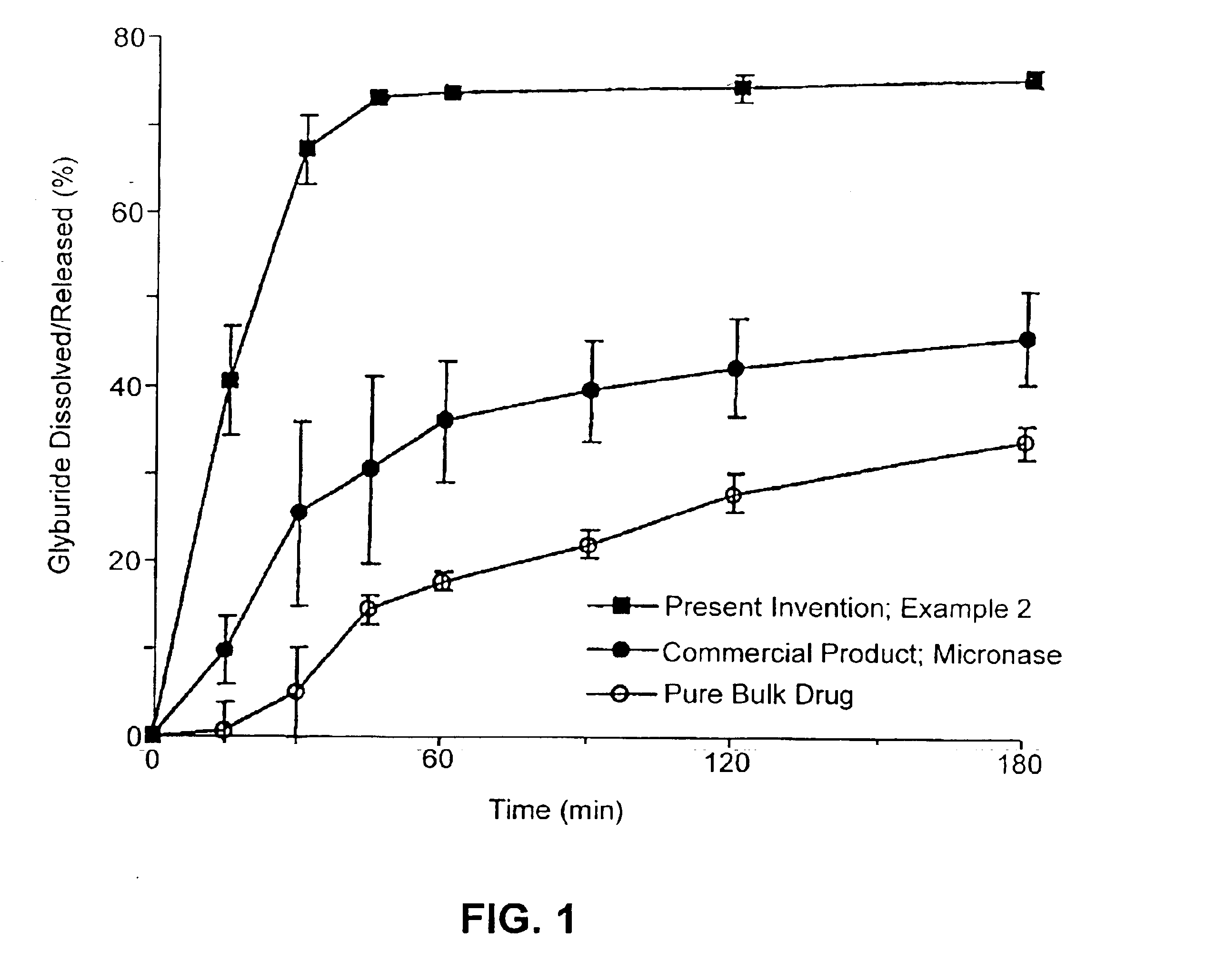
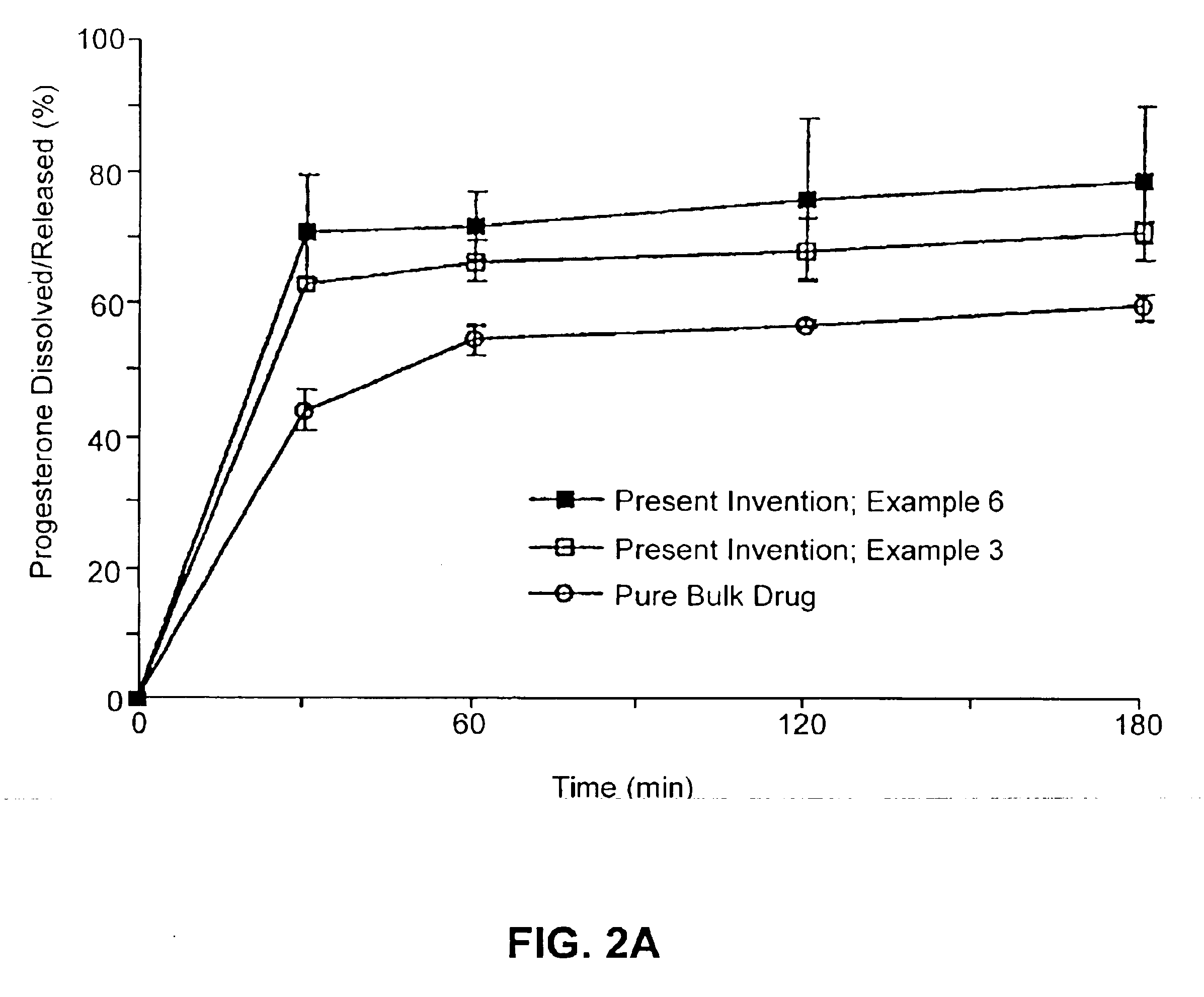
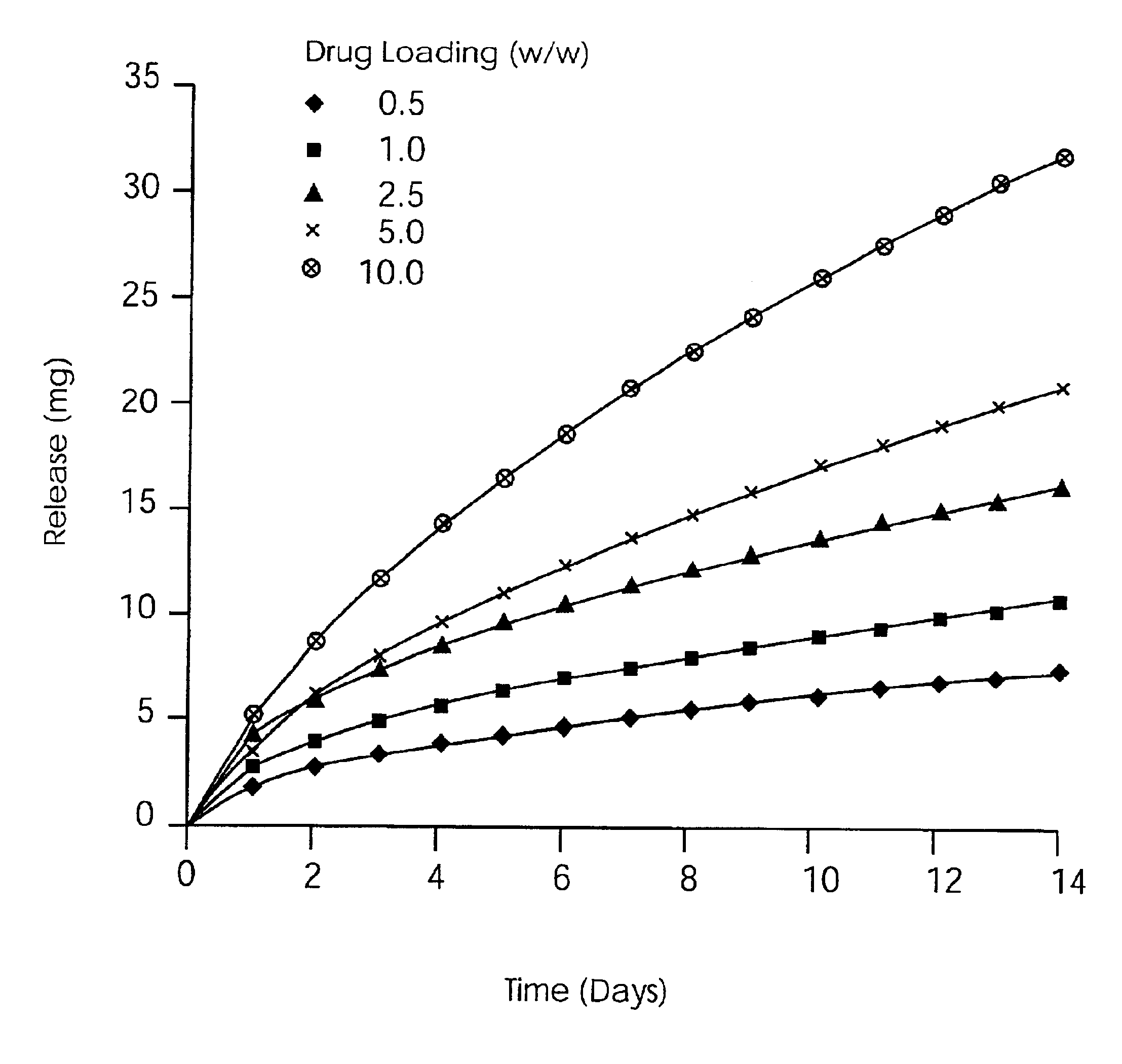
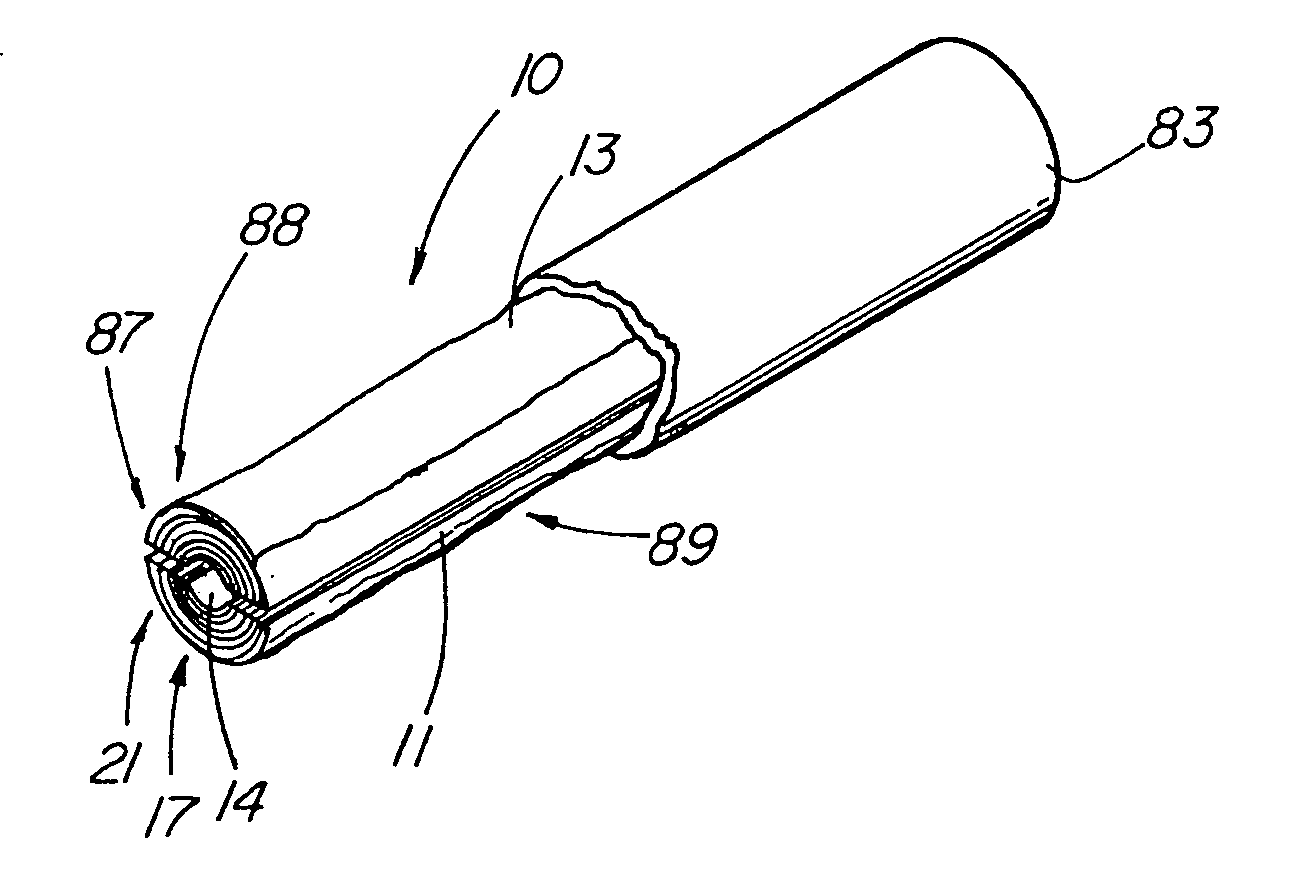
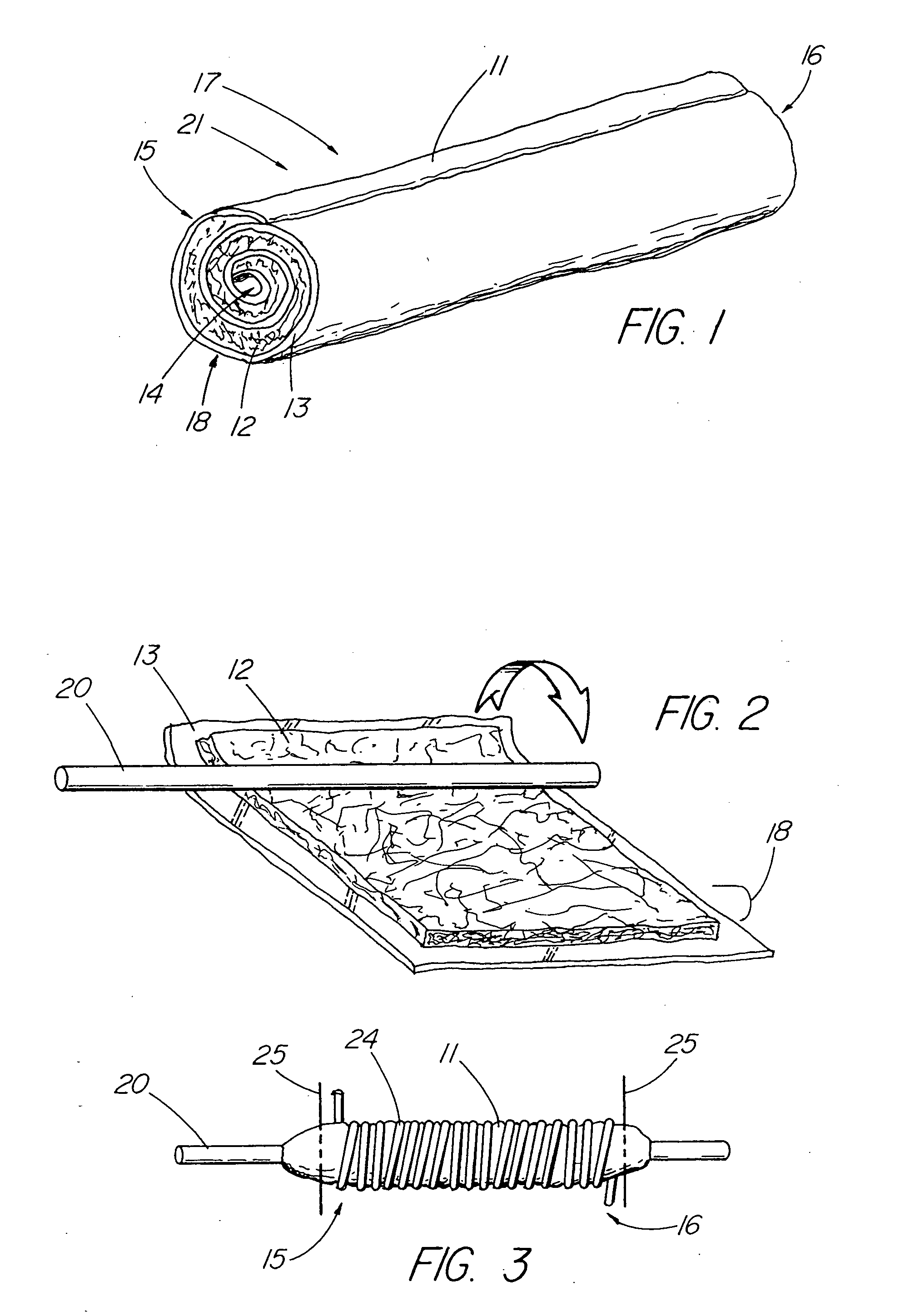
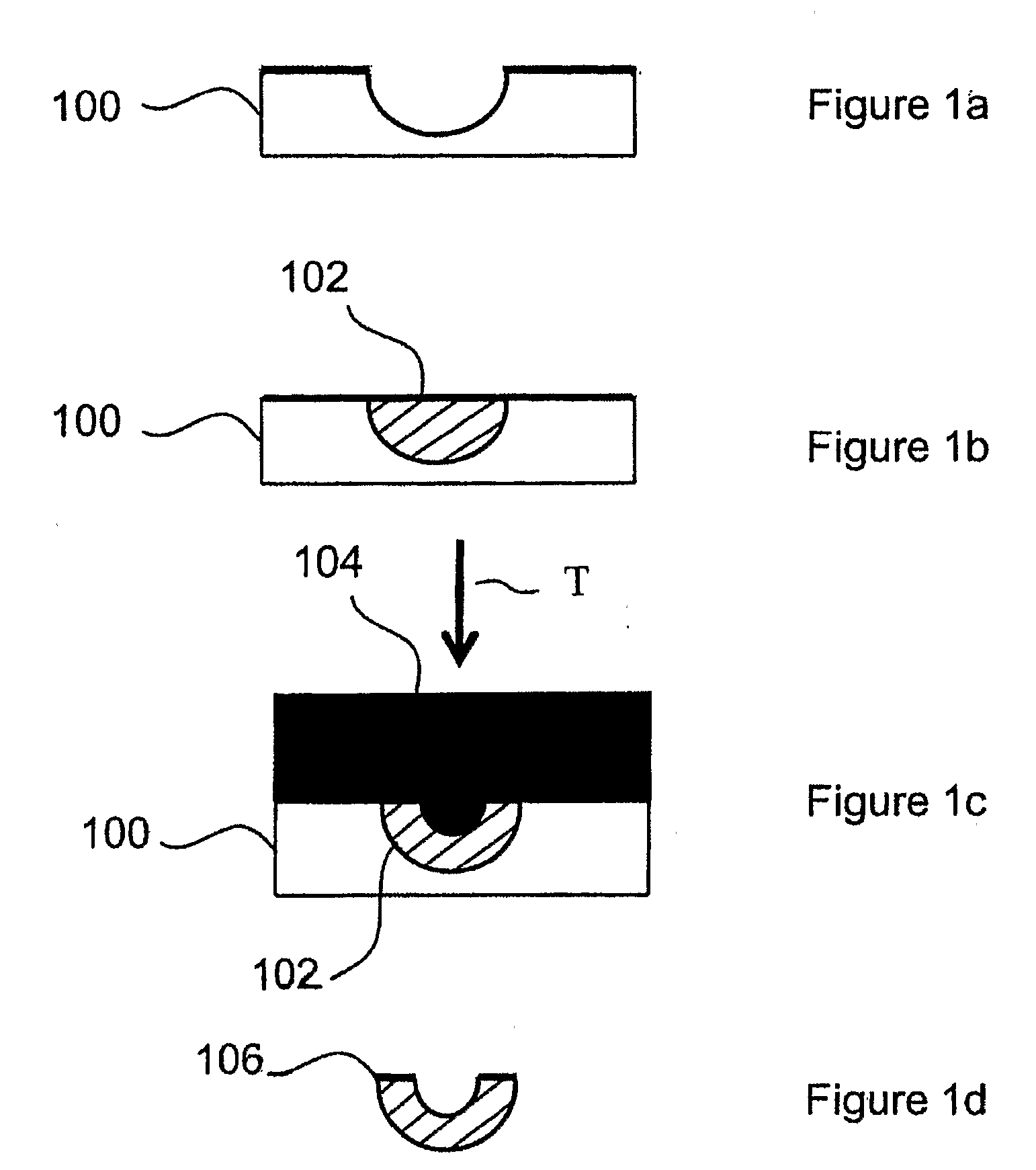
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
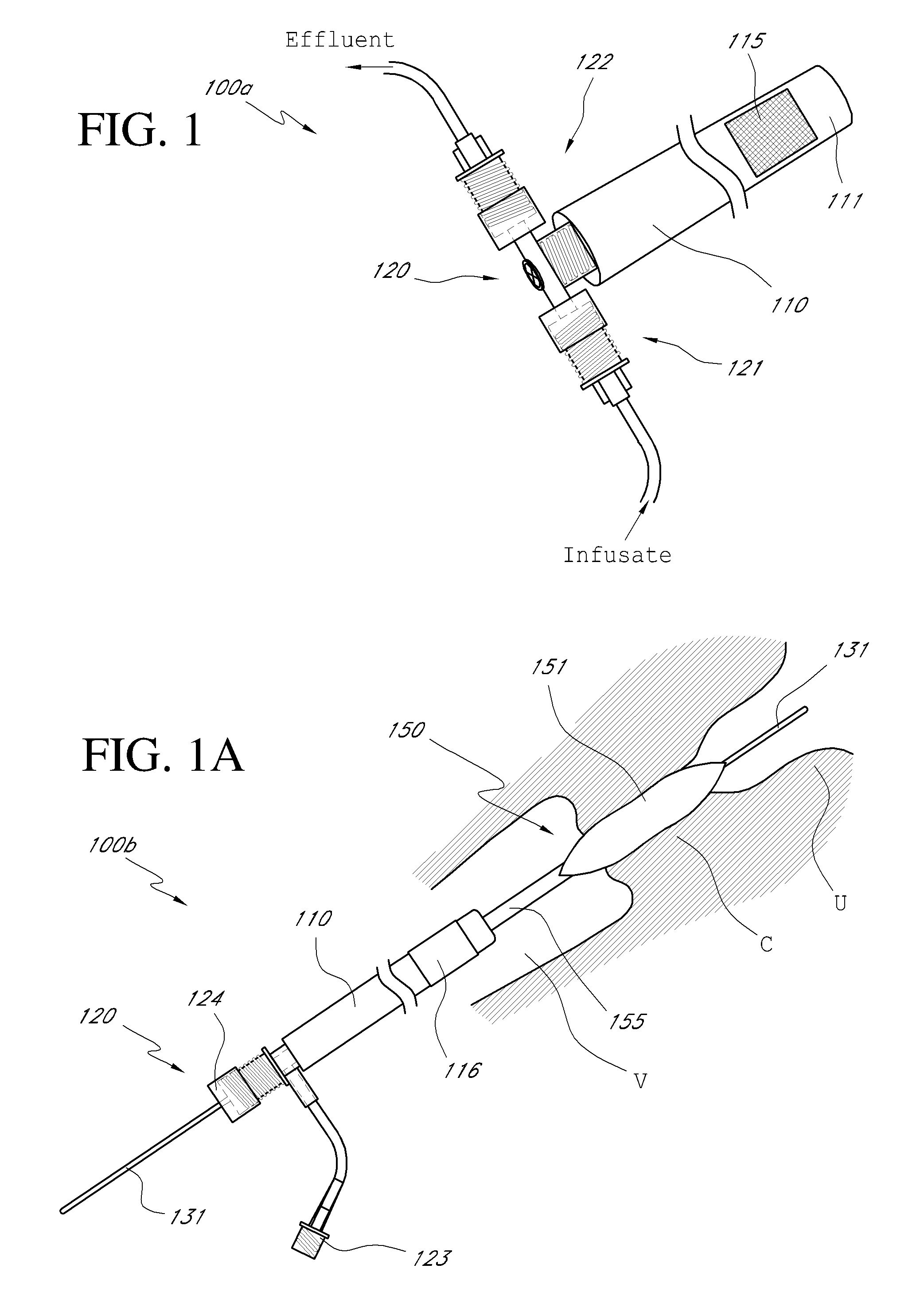
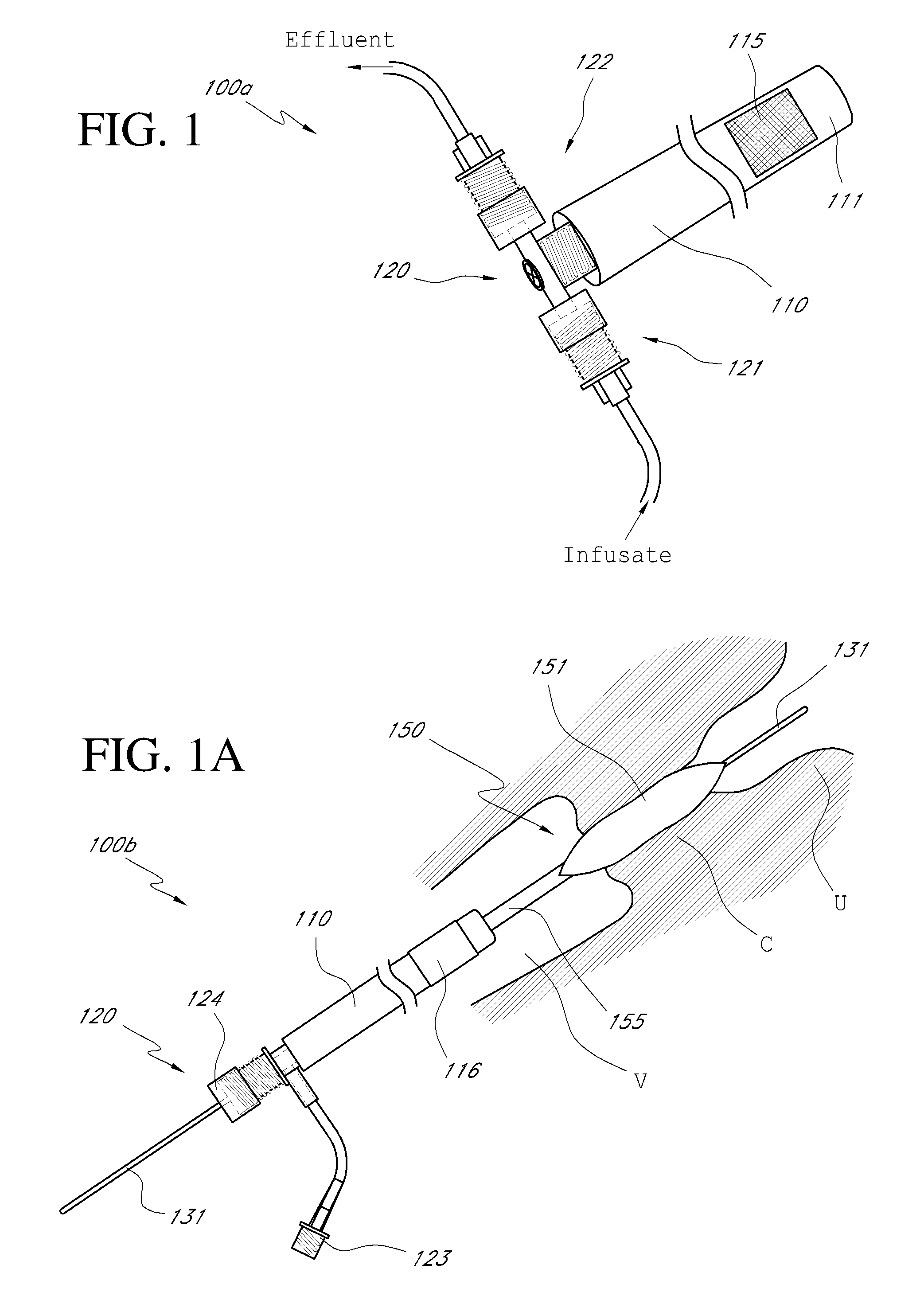
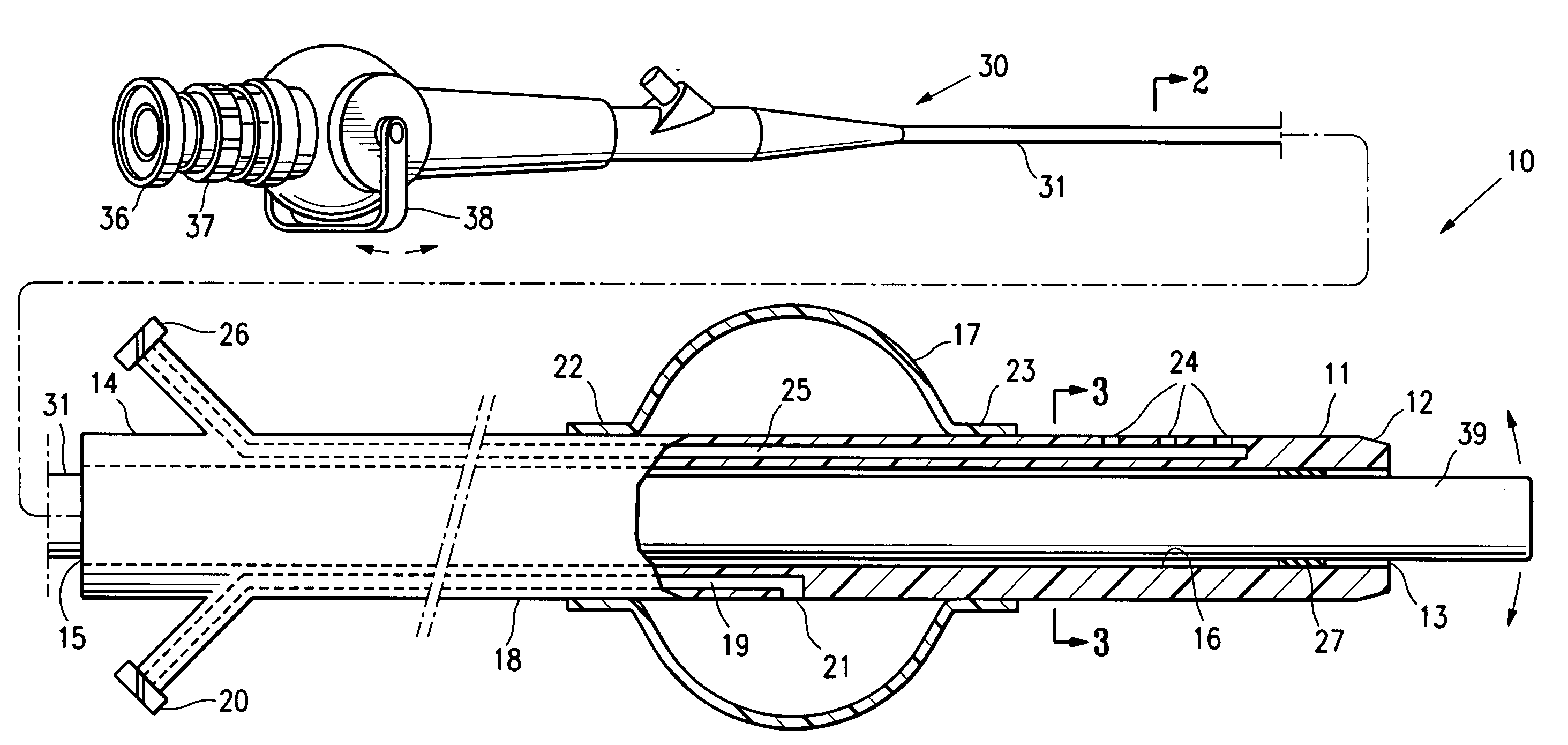
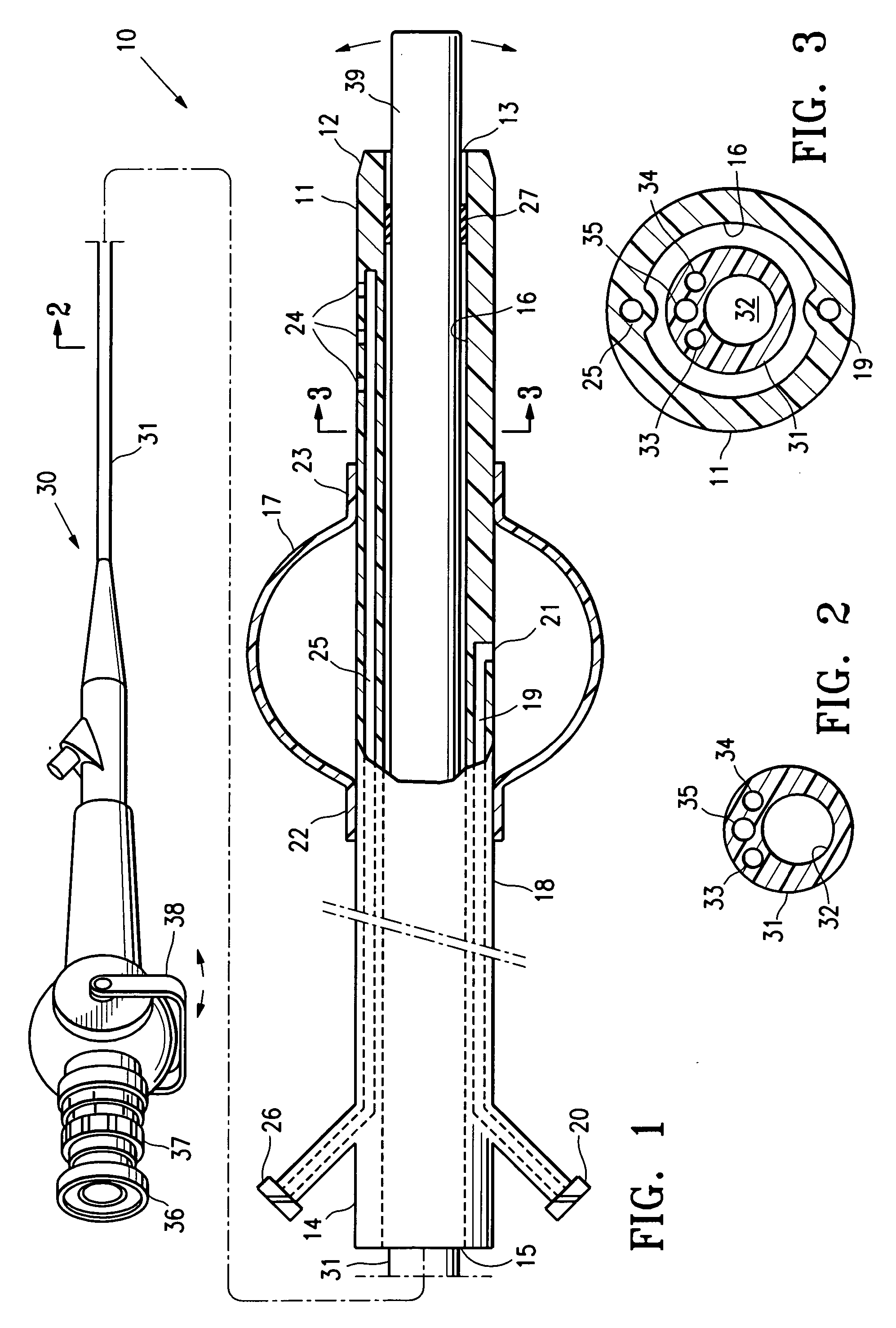
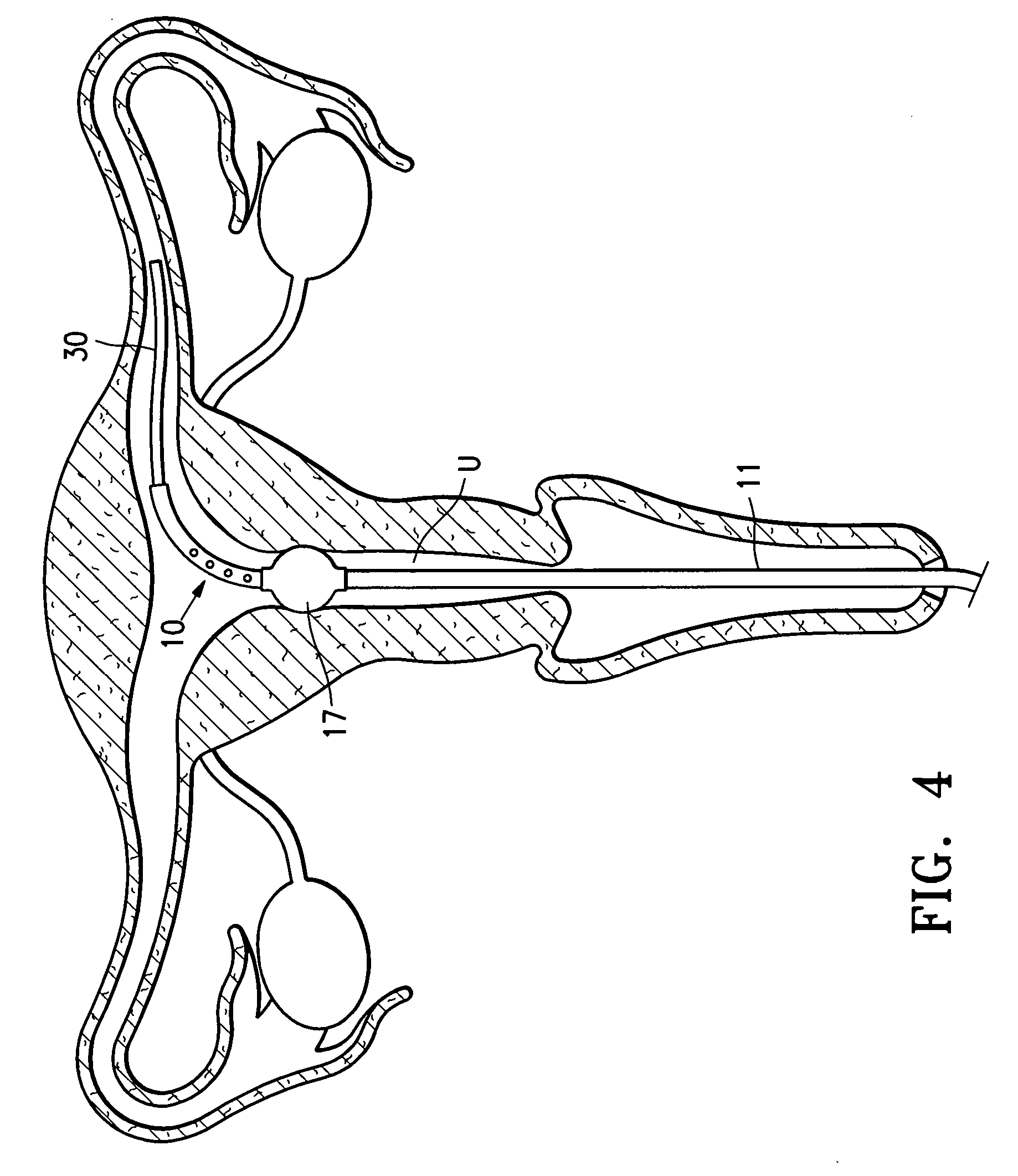
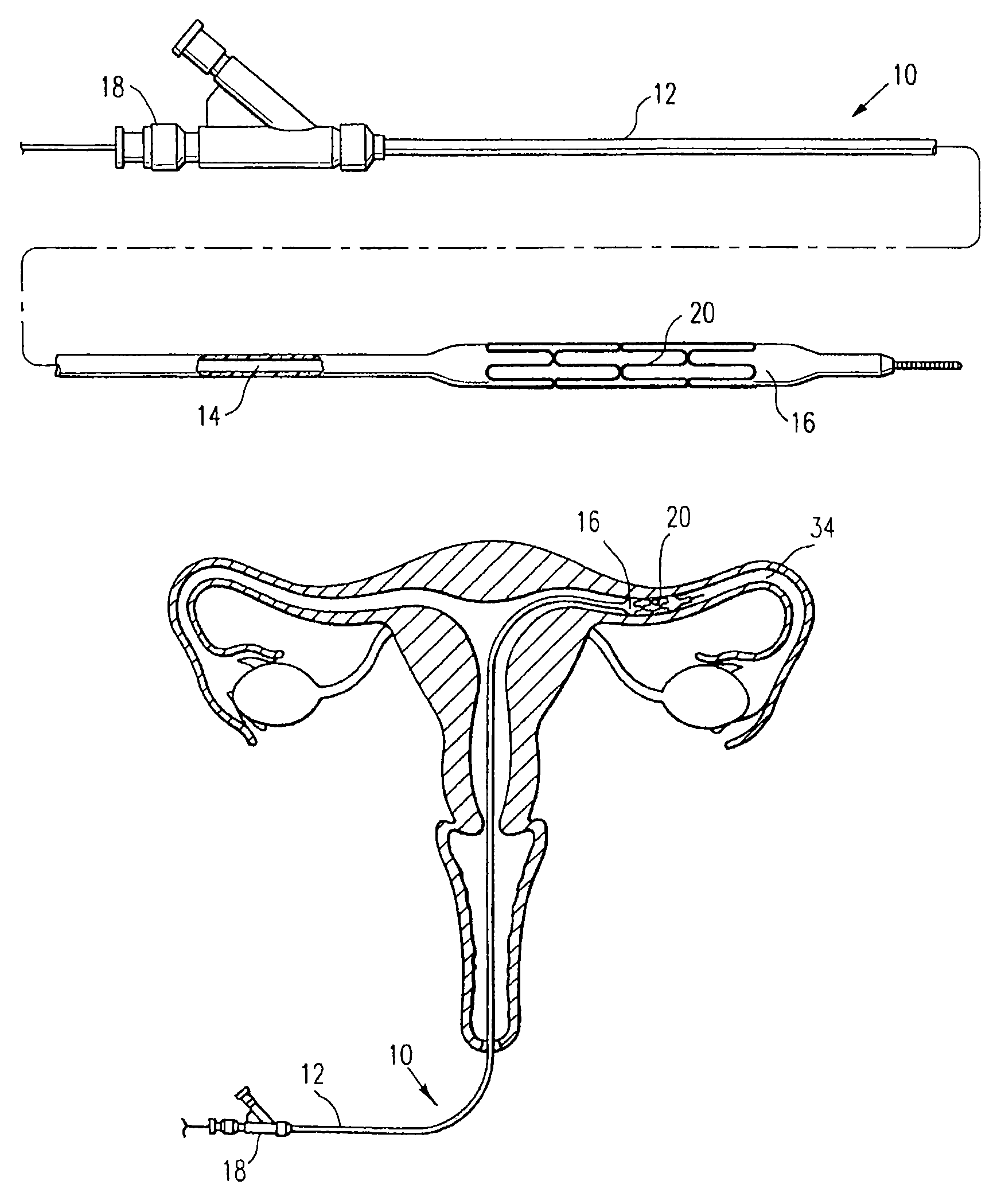
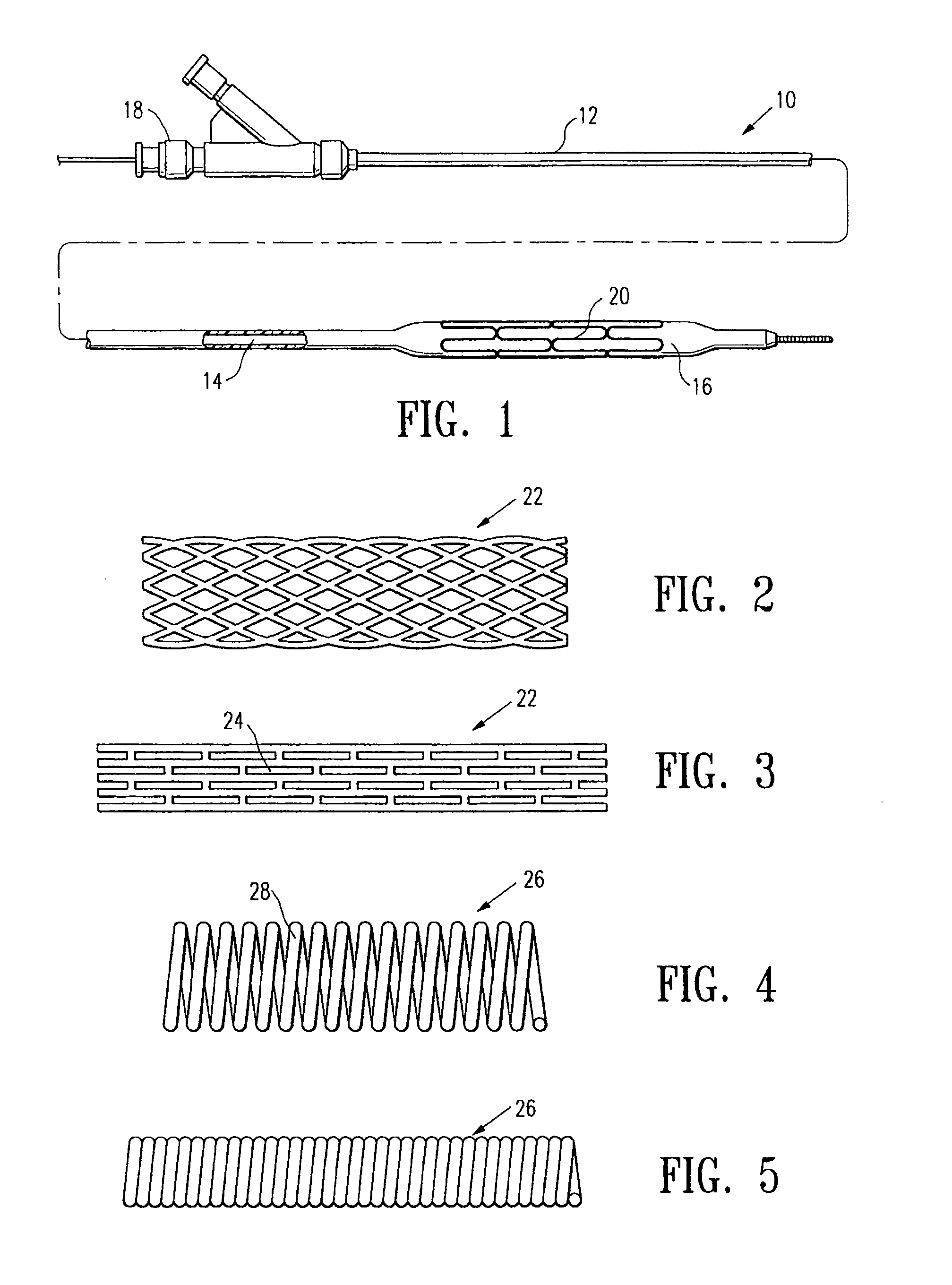
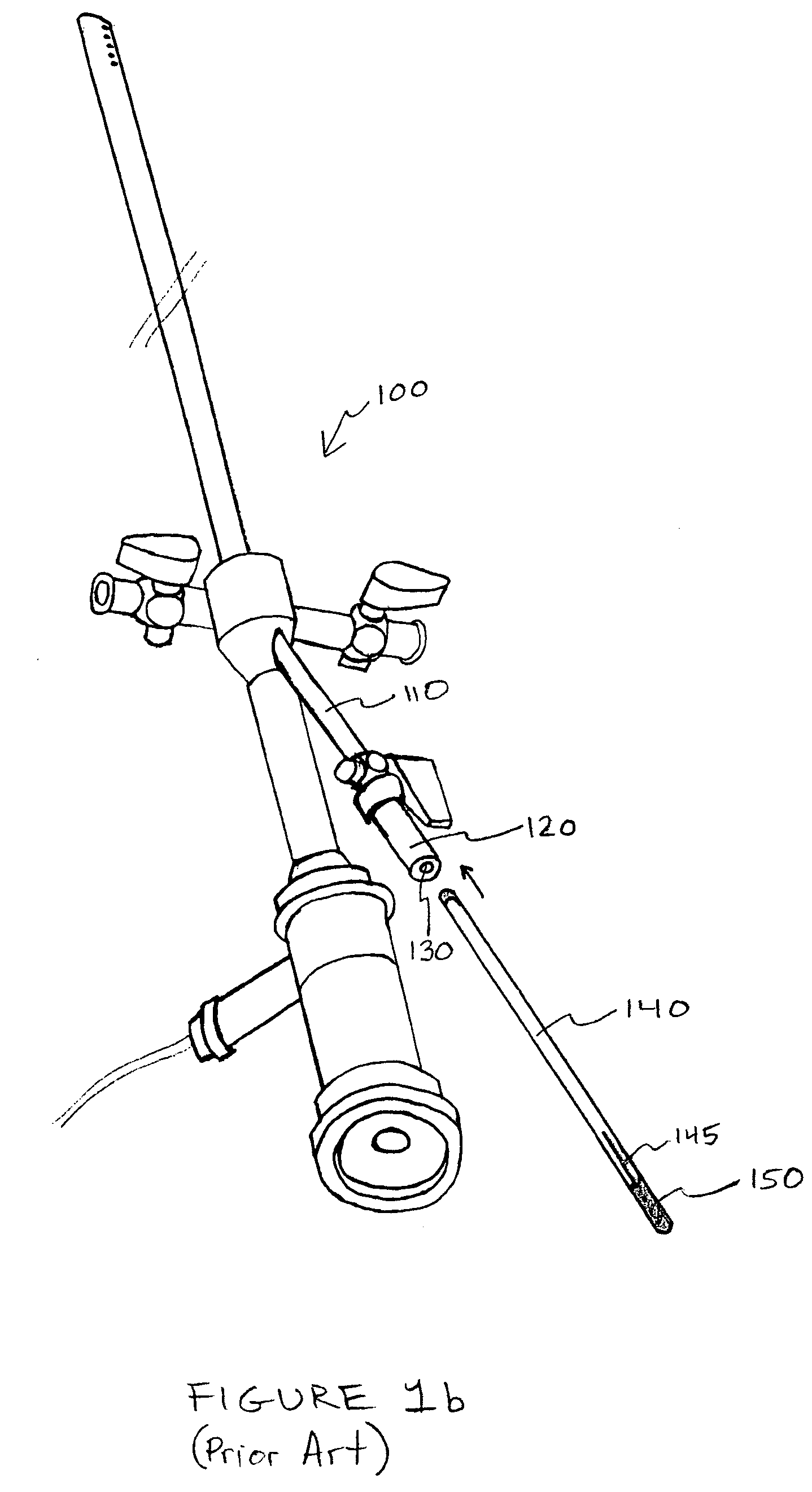
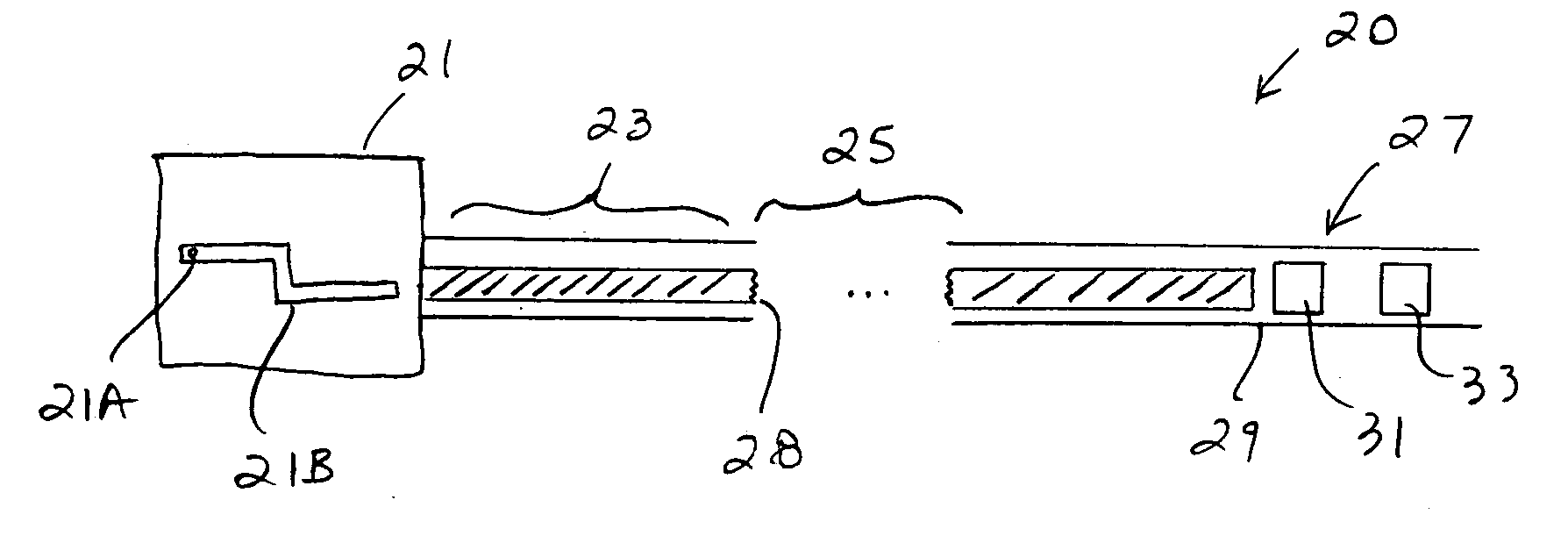
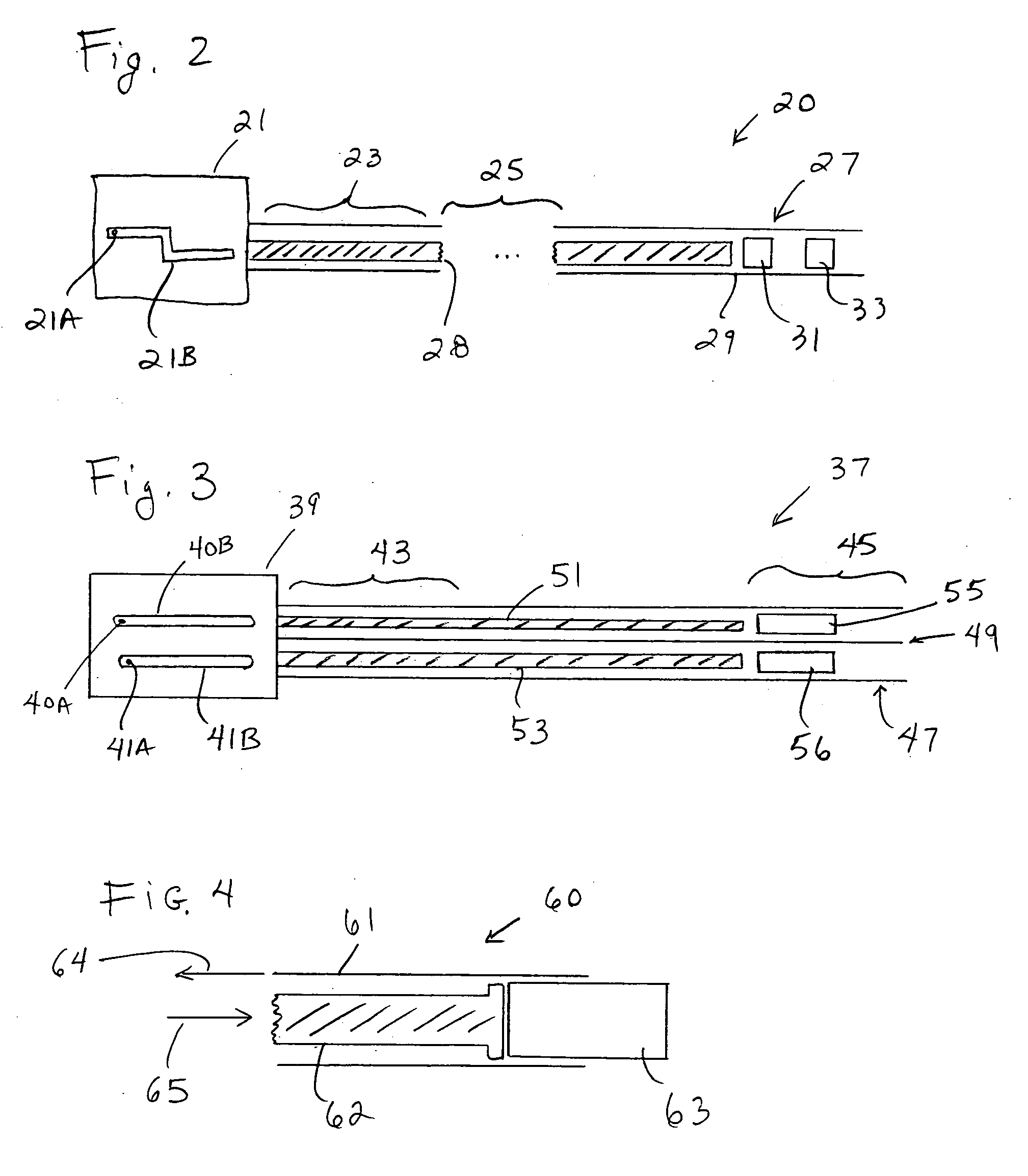
Systems, methods and devices for performing gynecological procedures
ActiveUS20080245371A1Prevent painful and potentially destructive dilation of the cervix is disclosedPrevent painful and potentially destructive dilationMedical devicesEndoscopesProcedural PainGynecology
Systems, methods, apparatus and devices for performing improved gynecologic and urologic procedures are disclosed. The system and devices provide simplified use and reduced risk of adverse events. Patient benefit is achieved through improved outcomes, reduced pain, especially peri-procedural pain, and reduced recovery times. The various embodiments enable procedures to be performed outside the hospital setting, such as in a doctor's office or clinic.
Owner:HOLOGIC INC
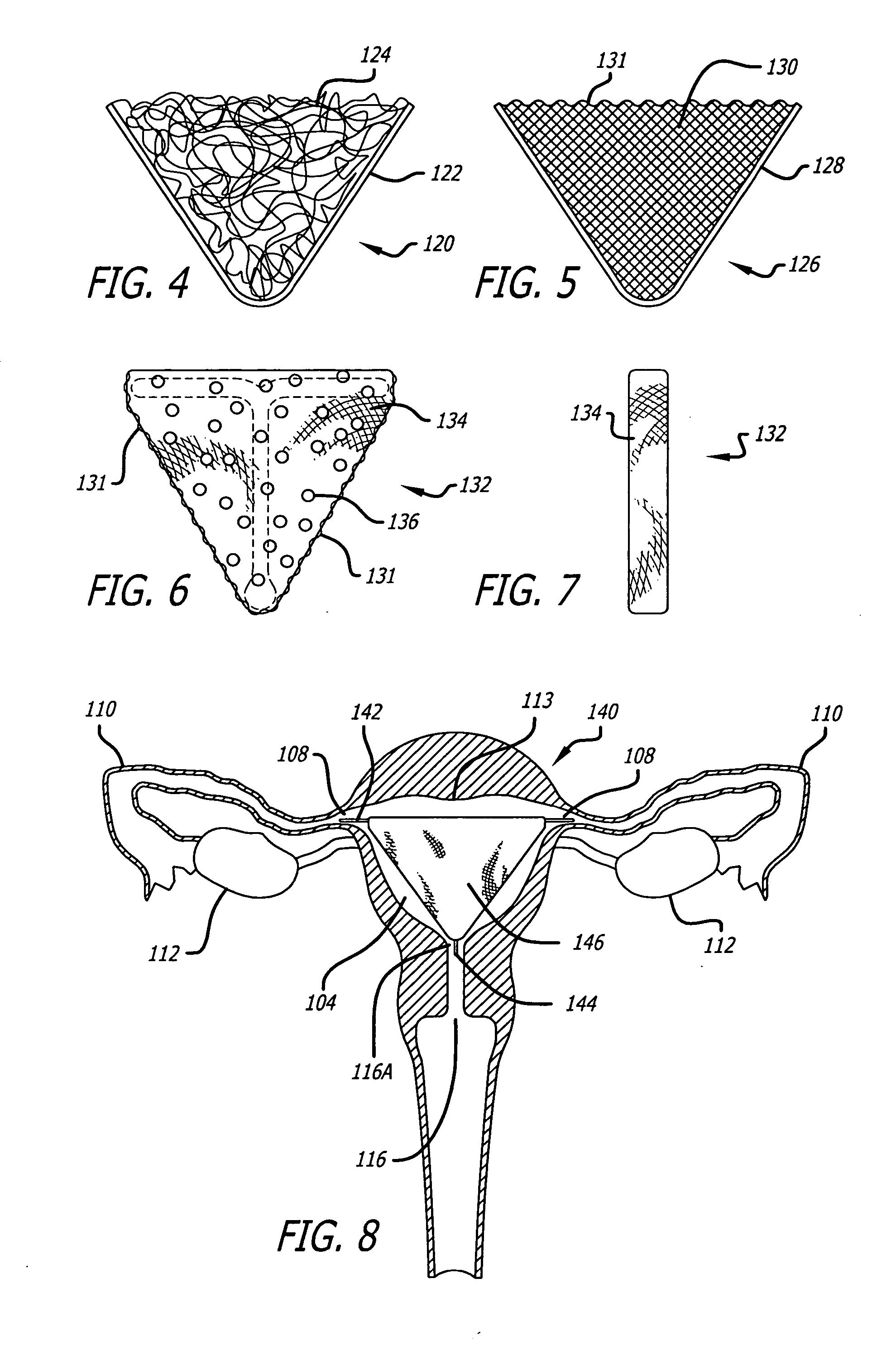
Methods and devices for occluding body lumens and/or enhancing tissue ingrowth
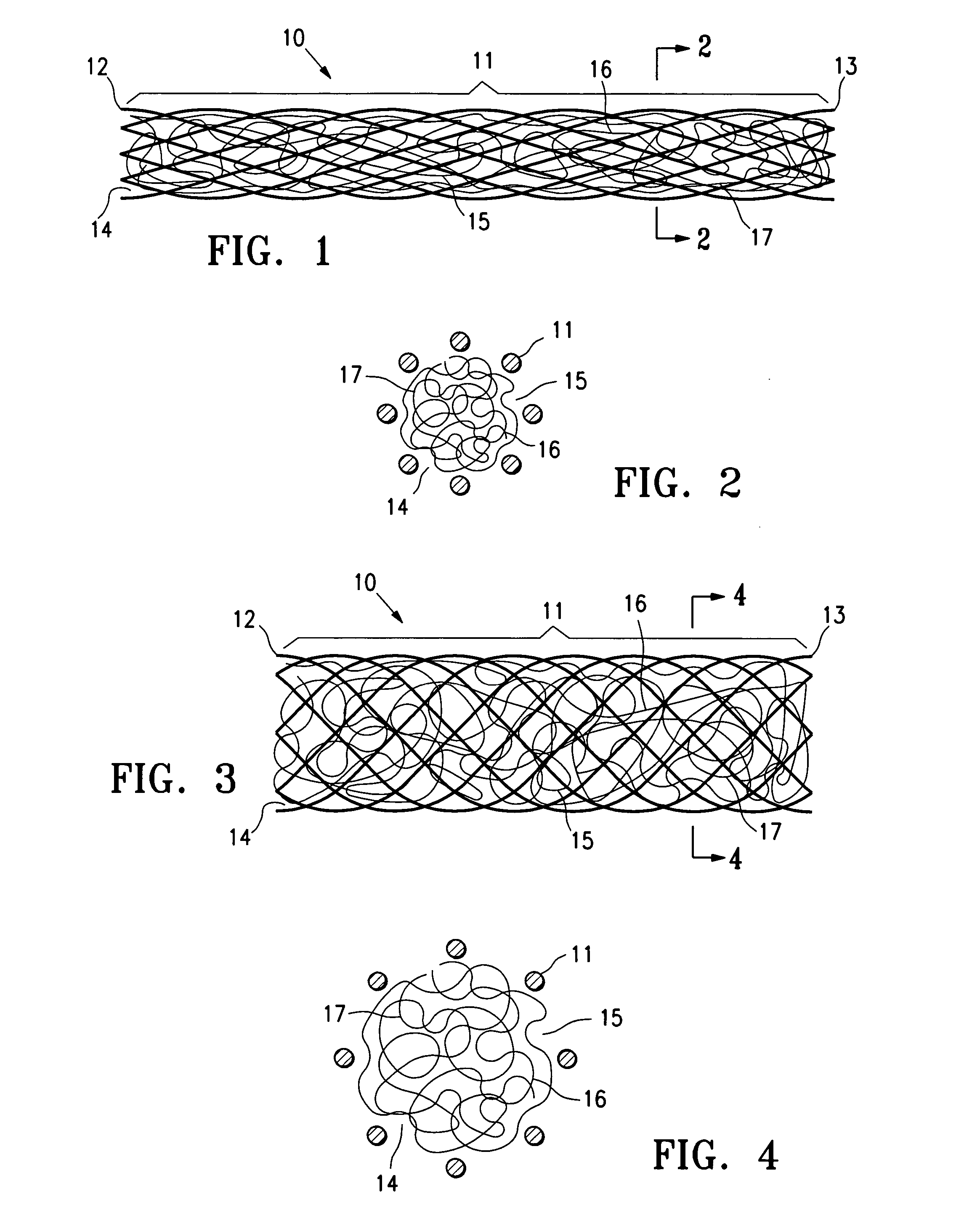
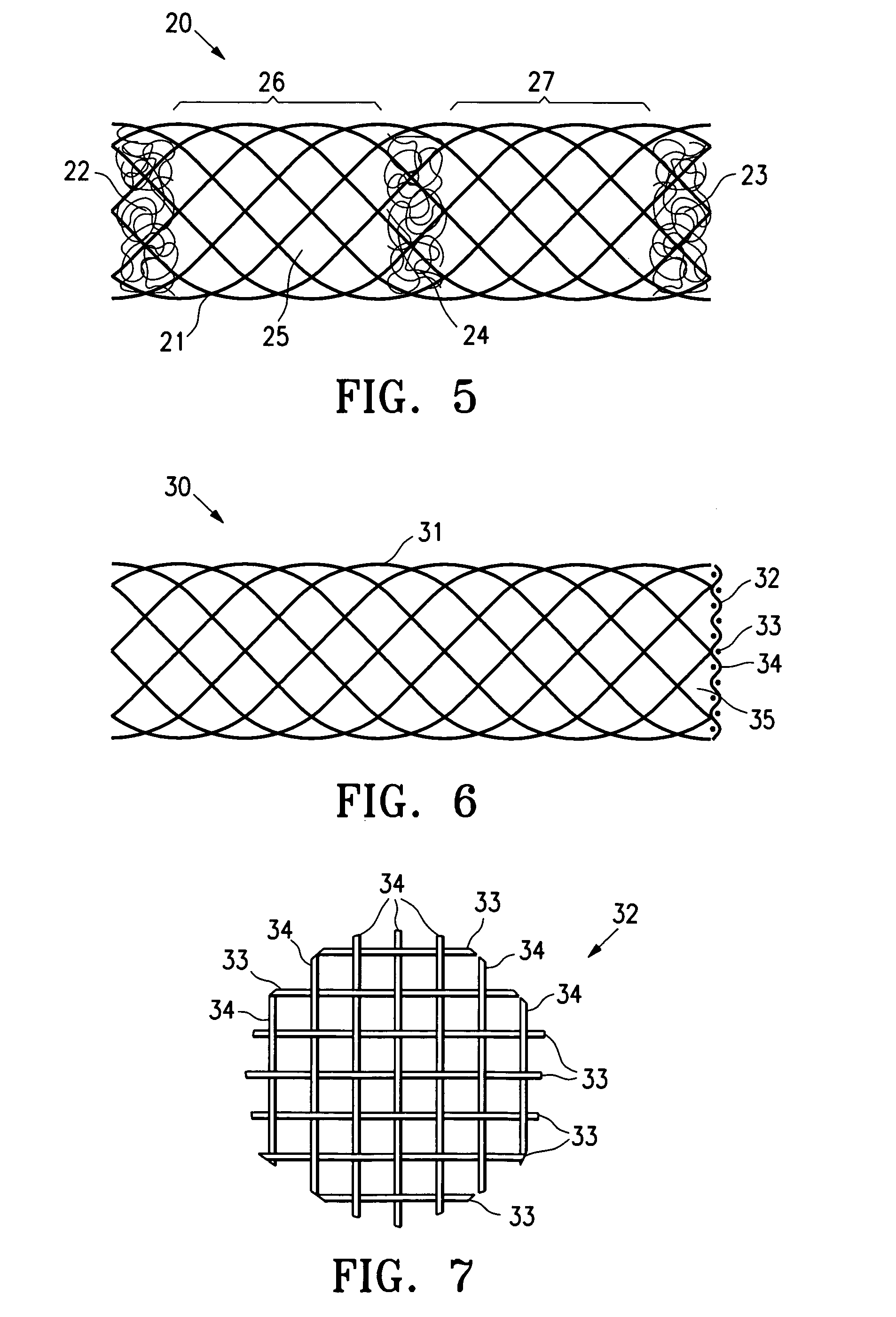
InactiveUS20060009798A1Enhance ingrowthTissue ingrowth is further enhancedStentsFallopian occludersMesh gridEpithelial tissue
The present invention provides devices, methods and systems for the occlusion of various lumens in a body of a patient including devices and methods for enhancing tissue ingrowth, particularly endothelial tissue growth within an occlusive device. The system includes an occlusive device and a delivery device for placing the occlusive device in a body lumen. The occlusive device is generally a tubular member with a mesh member disposed thereon. The occlusive device is configured to be radially expandable along a longitudinal axis of the tubular member and implantable with a delivery catheter such that the occlusive device is in a collapsed state when positioned in the delivery catheter and in an expanded state when positioned in a lumen of a patient. The mesh member of the occlusive device is configured to promote epithelial tissue ingrowth.
Owner:BAYER ESSURE
Compositions used in human treatment
A composition for treatment of a human body comprises a combination of at least one hormone, at least one amino acid, at least one enzyme and / or vitamin, and least one mineral. The relative proportions of the hormone, amino acid, enzyme and mineral in the combination are balanced with respect to each other so as to be present in effective amounts to substantially restore to optimal levels in the body the hormone, amino acid, enzyme and mineral The hormone, amino acid, enzyme and mineral in the combination further operate synergistically to provide both nutrients and command components to enable the body to effectively utilize the nutrients. The invention is also a method of forming a composition for the treatment of a human body.
Owner:COCHRAN TIMOTHY M
Systems, methods and devices for performing gynecological procedures
ActiveUS8528563B2Prevent painful and potentially destructive dilation of the cervix is disclosedPrevent painful and potentially destructive dilationMedical devicesEndoscopesProcedural PainGynecological surgery
Owner:HOLOGIC INC
Bioresorbable hydrogel compositions for implantable prostheses
Crosslinked compositions formed from water-insoluble copolymers are disclosed. These compositions are copolymers having a bioresorbable region, a hydrophilic region and at least two cross-linkable functional groups per polymer chain. Crosslinking of these polymers can be effected in solution in organic solvents or in solvent-free systems. If crosslinking occurs in a humid environment, a hydrogel will form. If crosslinking occurs in a non-humid environment, a xerogel will form which will form a hydrogel when exposed to a humid environment and the resulting crosslinked materials form hydrogels when exposed to humid environments. These hydrogels are useful as components in medical devices such as implantable prostheses. In addition, such hydrogels are useful as delivery vehicles for therapeutic agents and as scaffolding for tissue engineering applications.
Owner:LIFESHIELD SCI
Medical Device Applications of Nanostructured Surfaces
ActiveUS20070282247A1Increase heightPrevent/reduce bio-foulingNanotechElectrotherapyMedicineNanofiber
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices. In one particular embodiment, a method of administering a composition to a patient is disclosed which comprises providing a composition-eluting device, said composition-eluting device comprising at least a first surface and a plurality of nanostructures attached to the first surface, and introducing the composition-eluting device into the body of the patient.
Owner:RGT UNIV OF CALIFORNIA +1
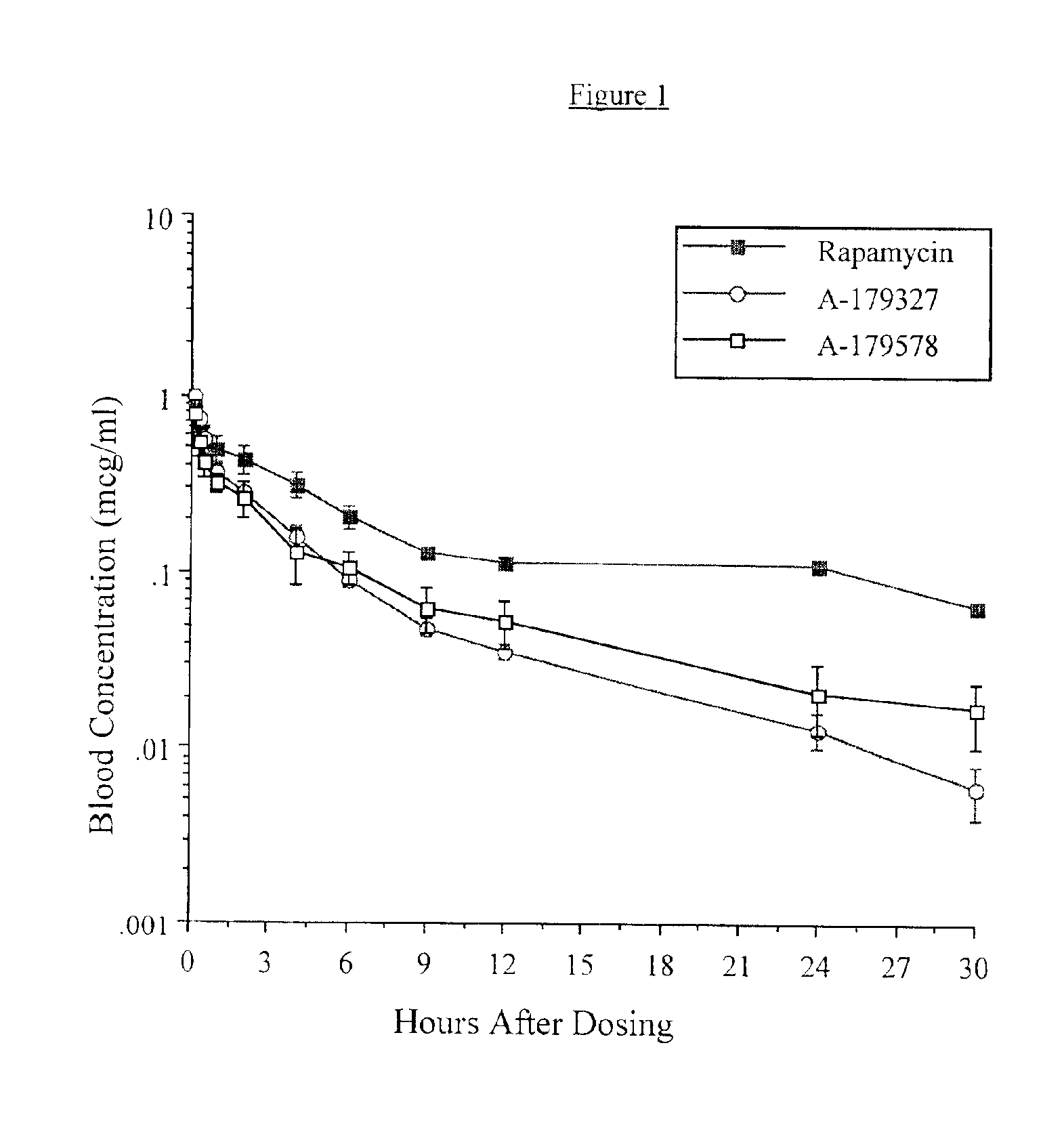
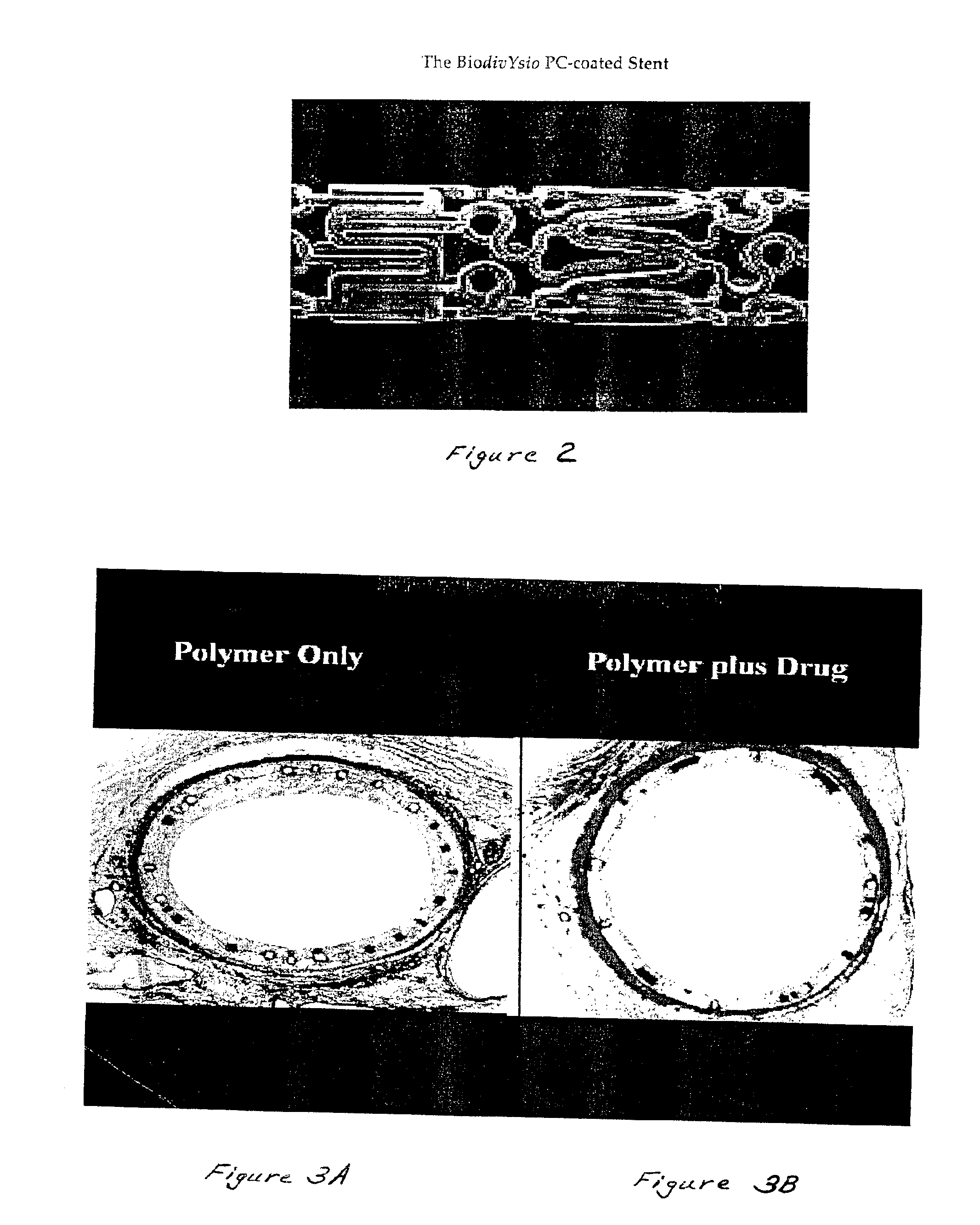
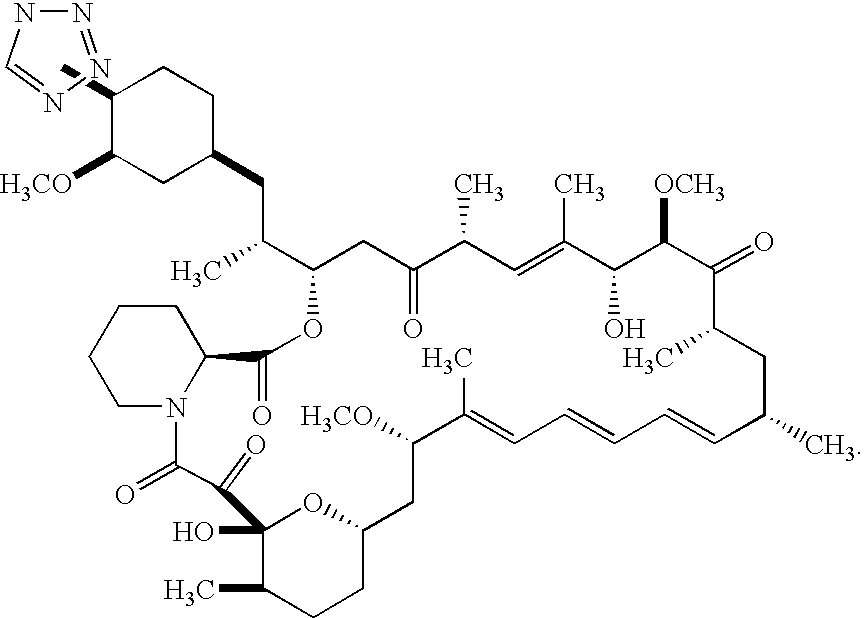
Medical devices containing rapamycin analogs
InactiveUS6890546B2Reduce probabilityReduces restenosis in vasculatureBiocideAntimycoticsArteriovenous graftsMedical treatment
A medical device comprising a supporting structure having a coating on the surface thereof, the coating containing a therapeutic substance, such as, for example, a drug. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to,
Owner:ABBOTT LAB INC
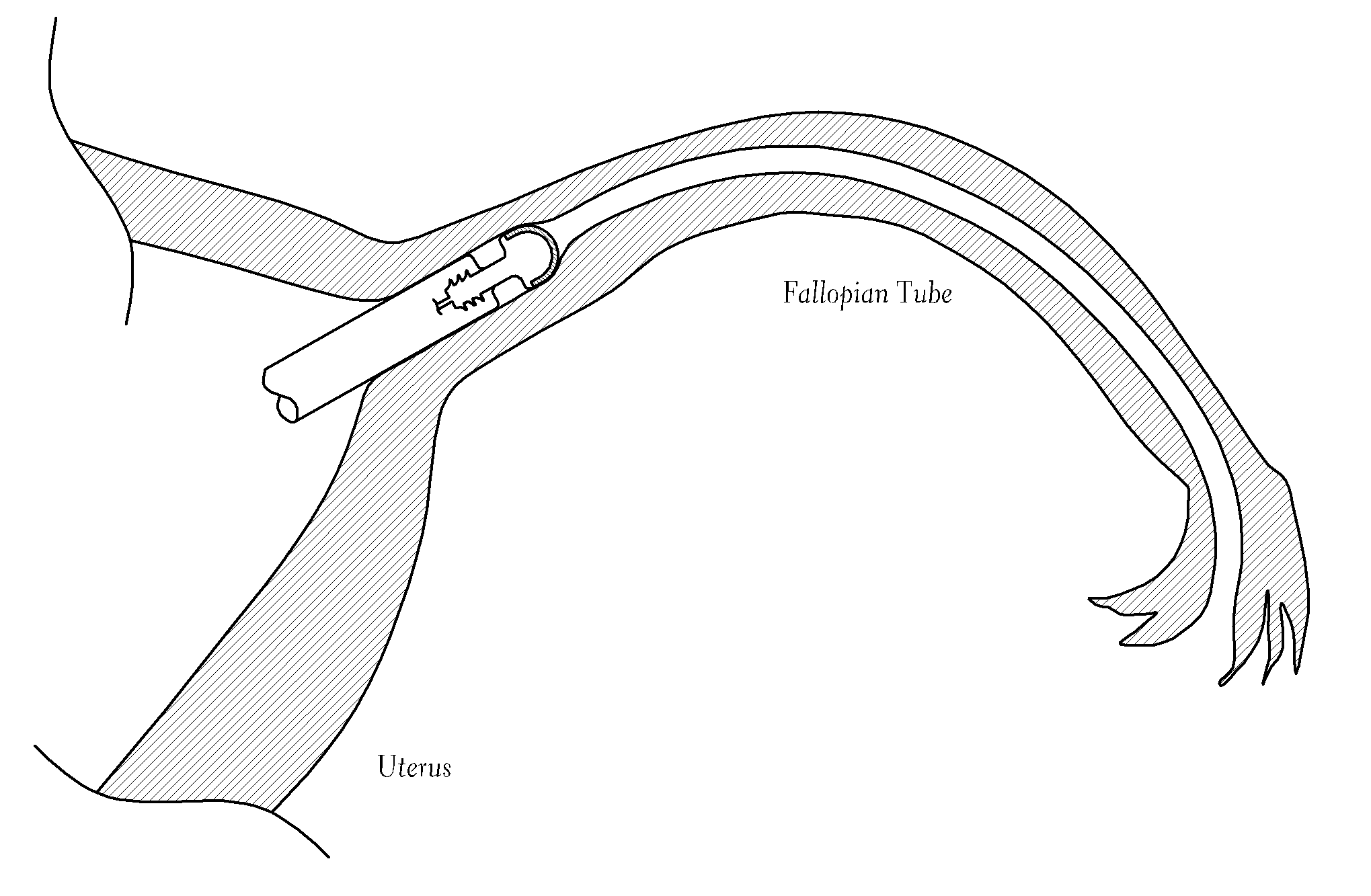
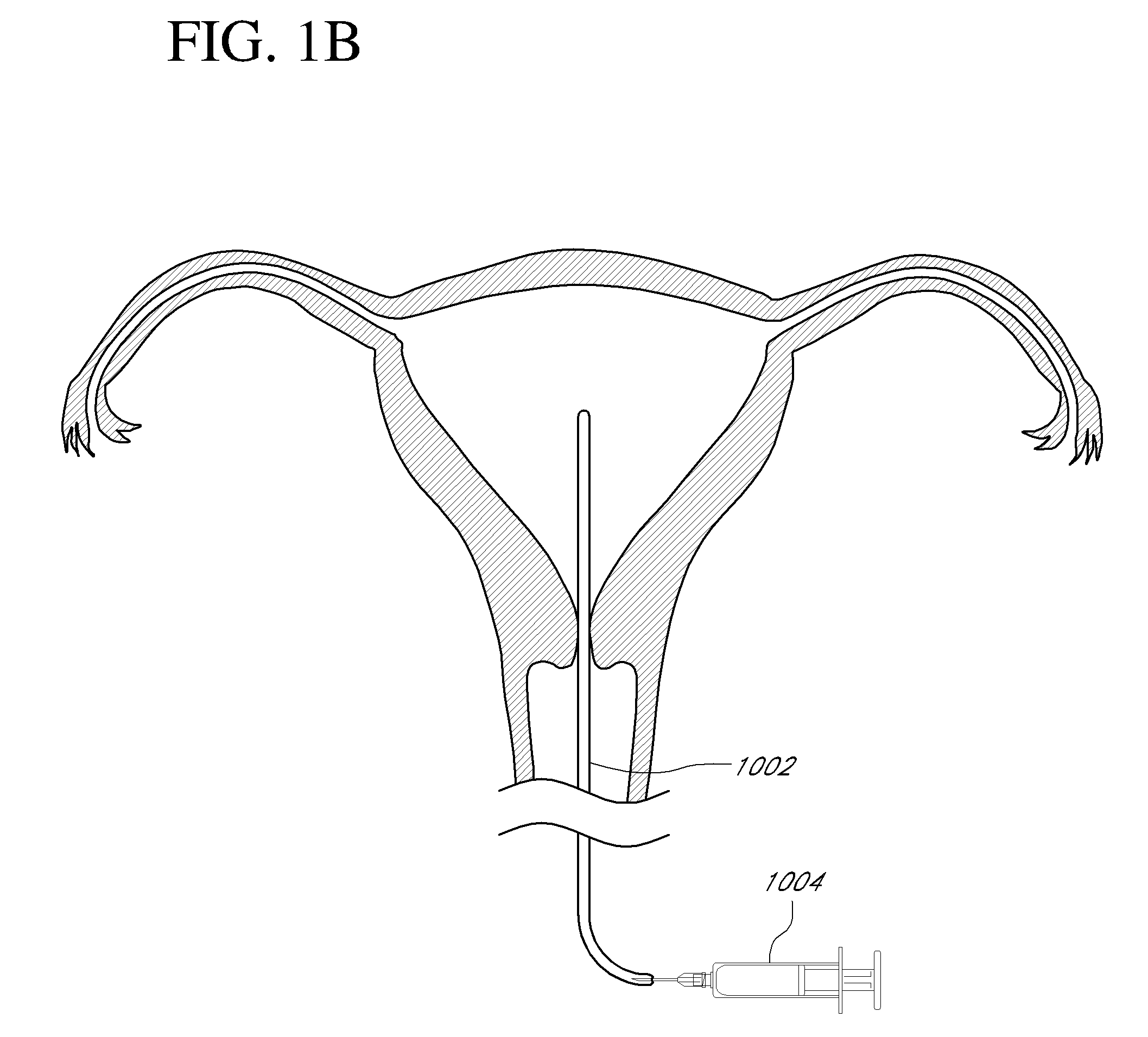
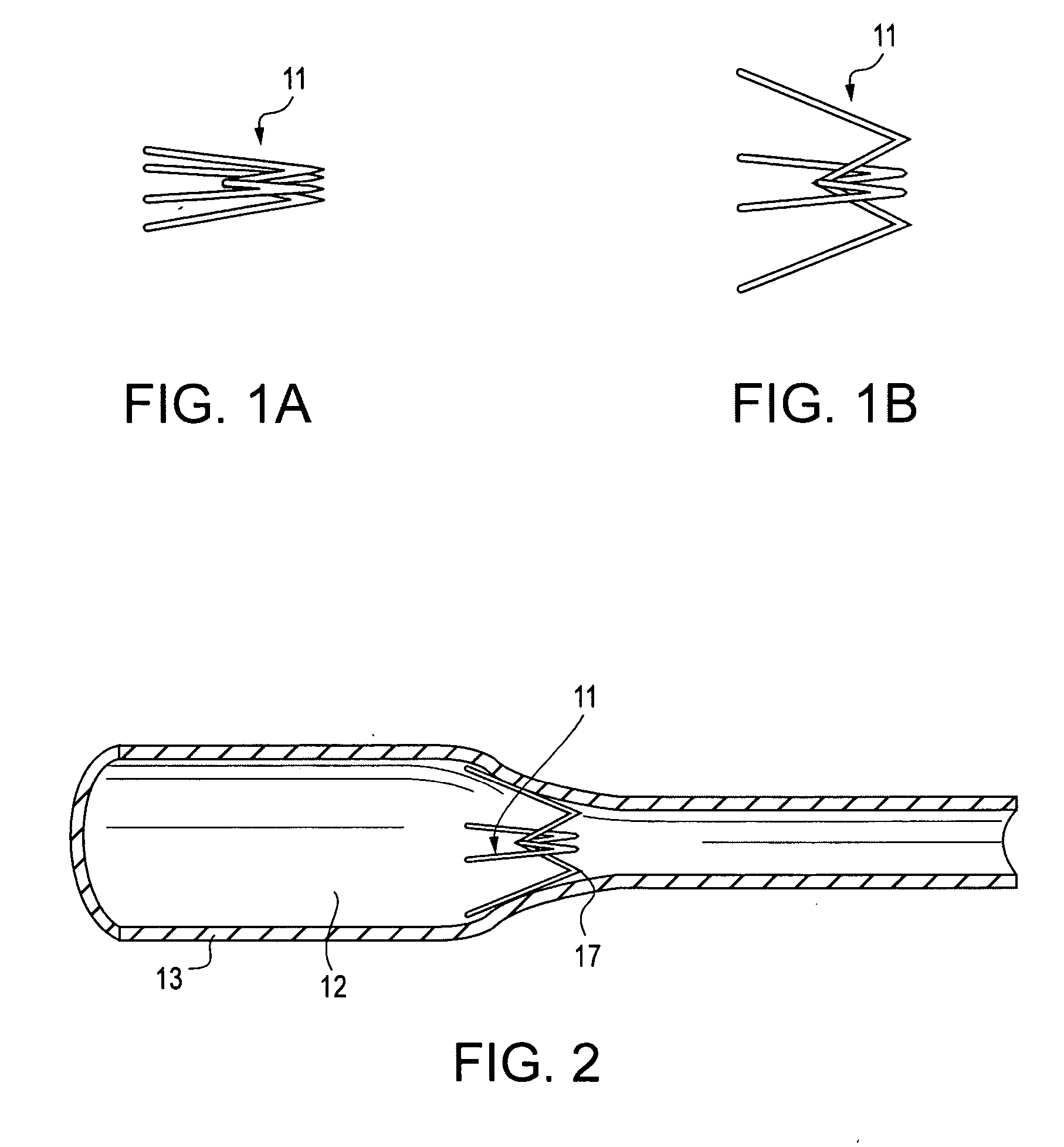
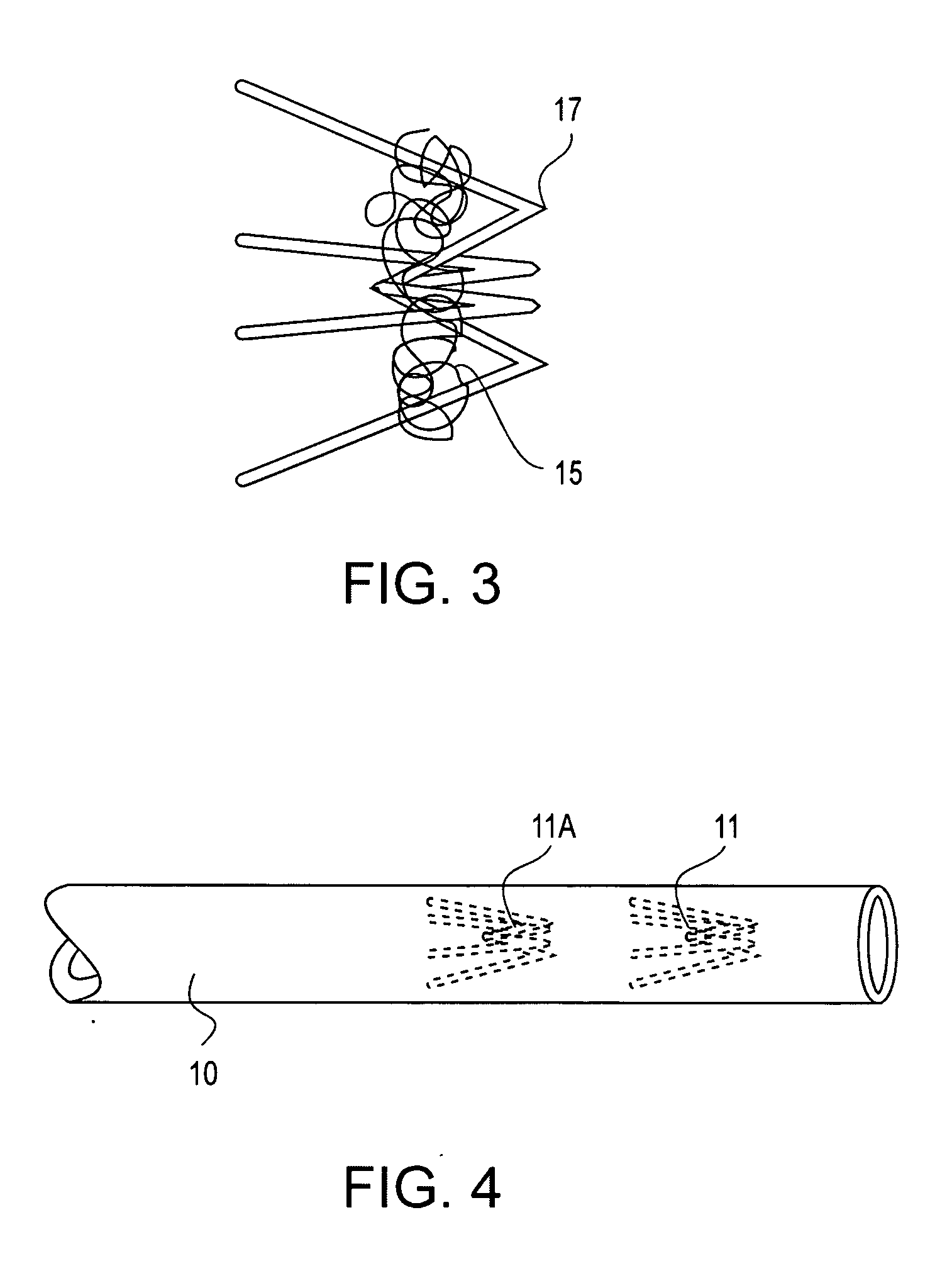
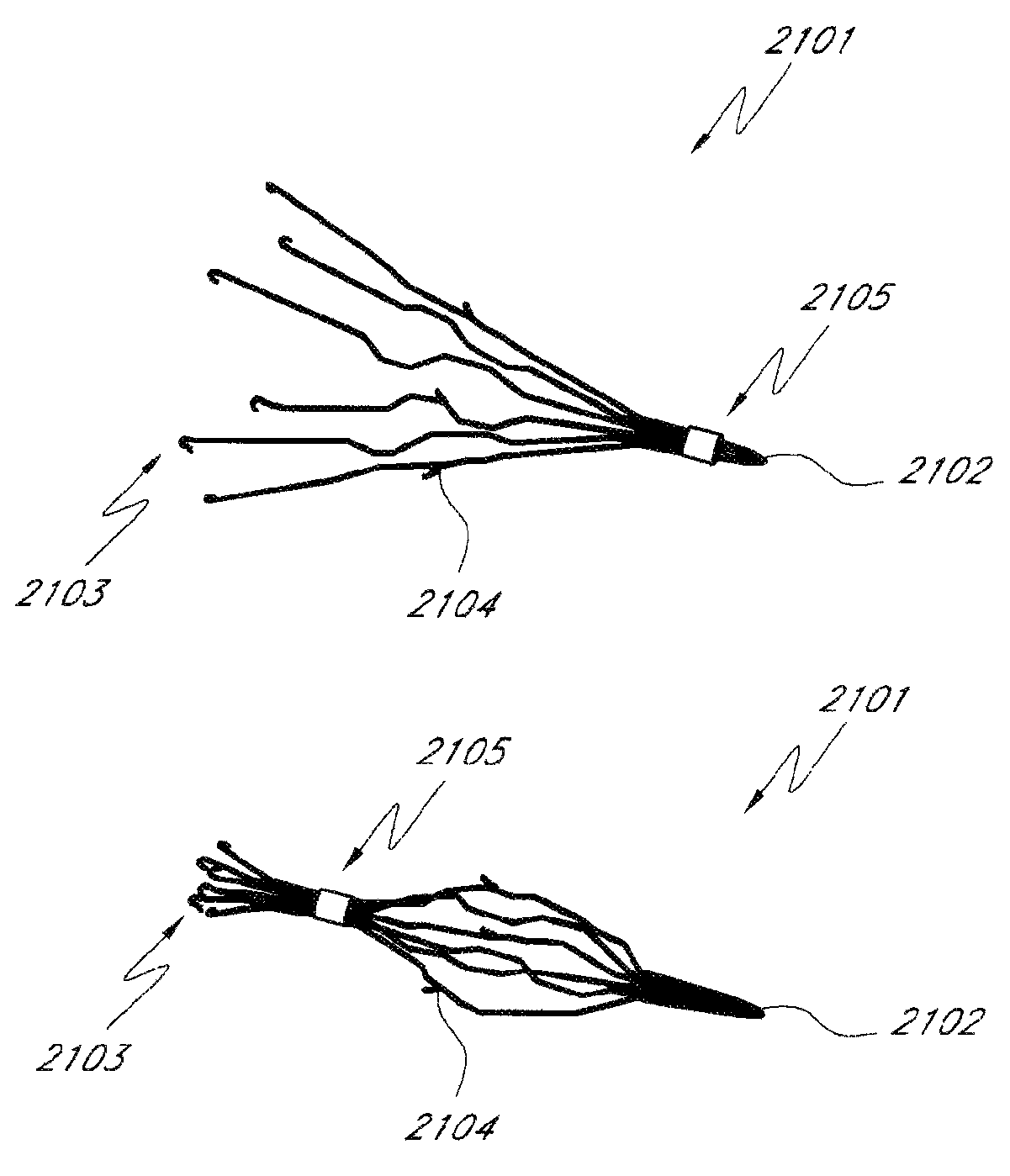
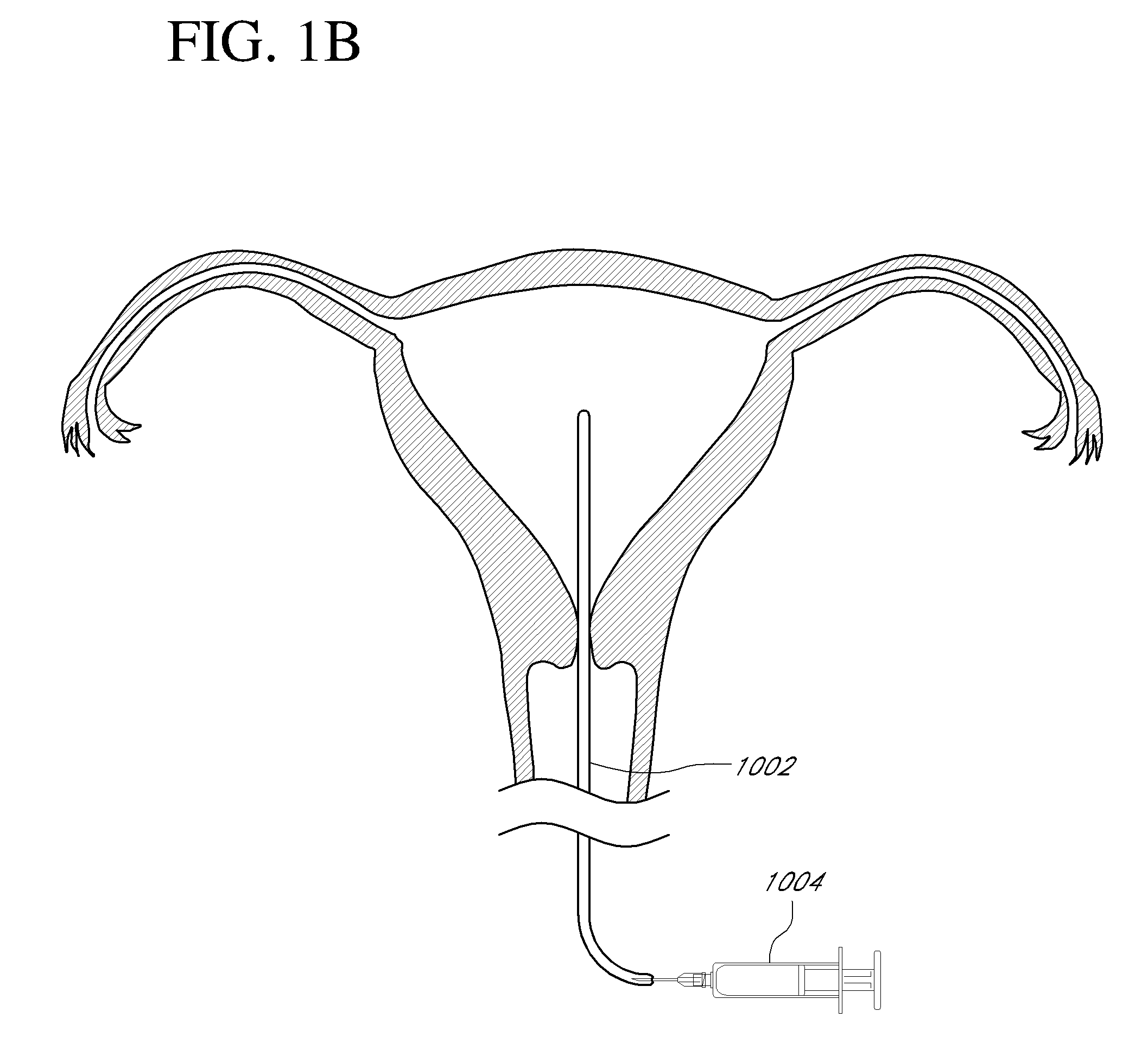
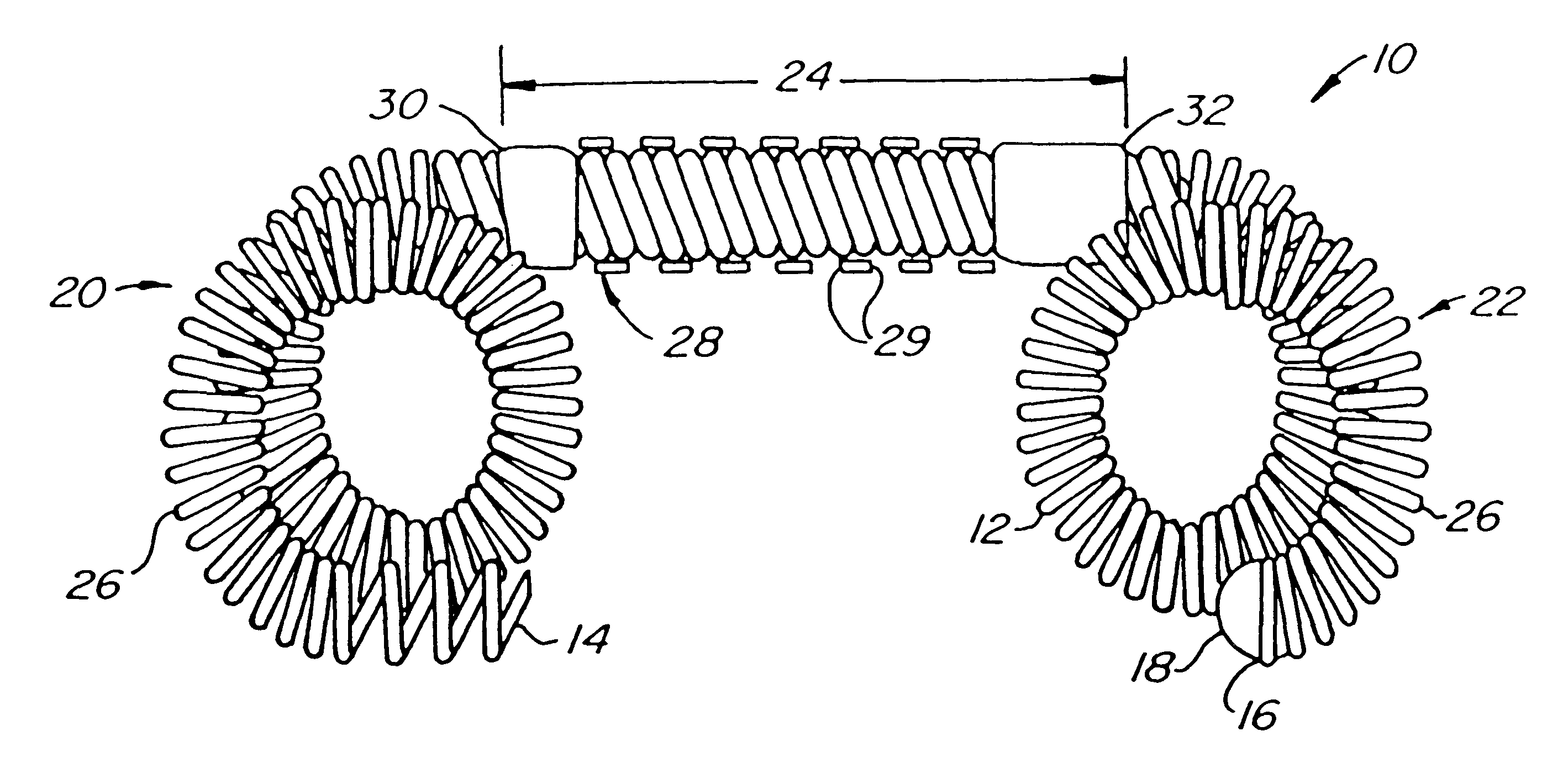
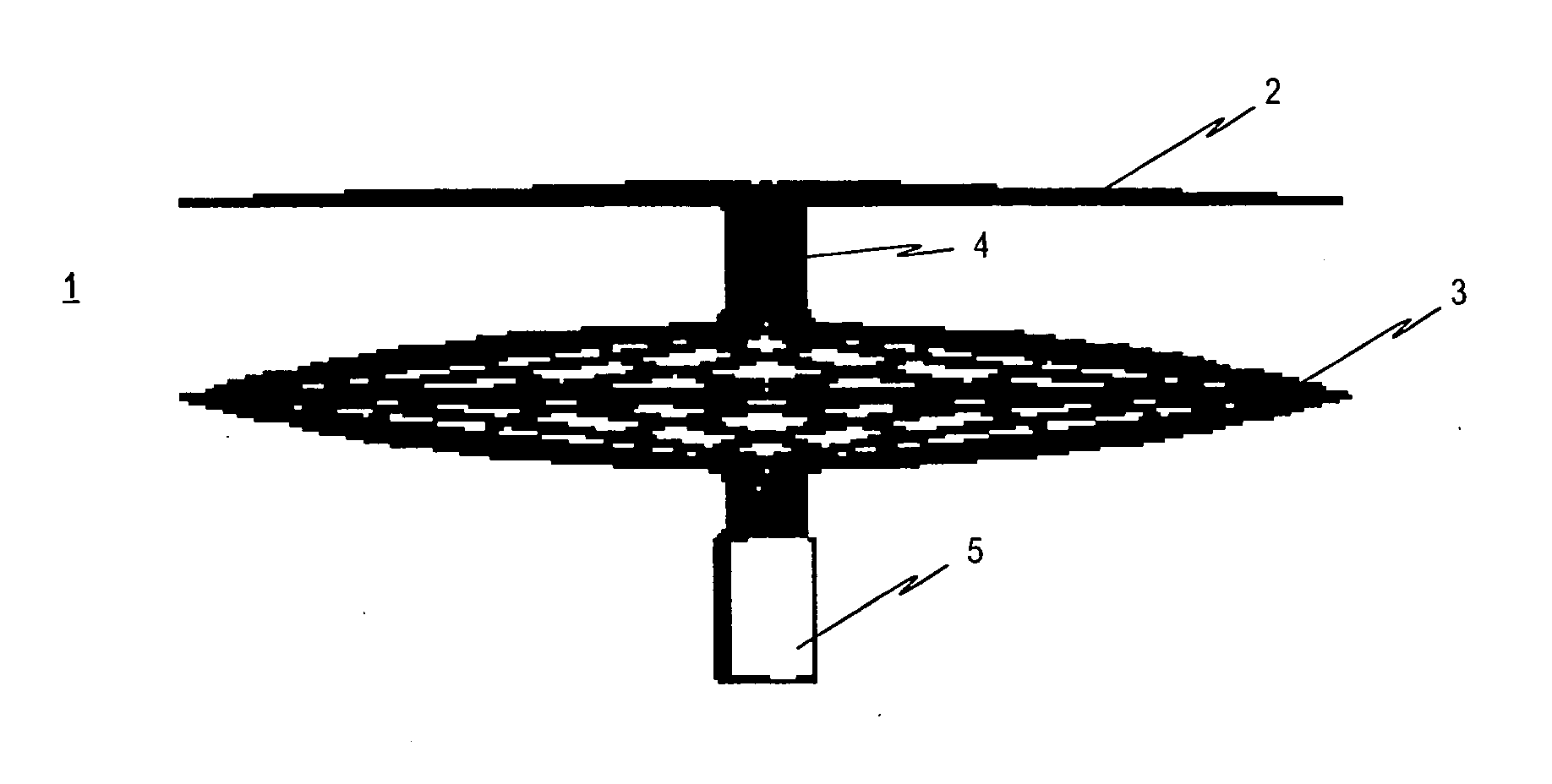
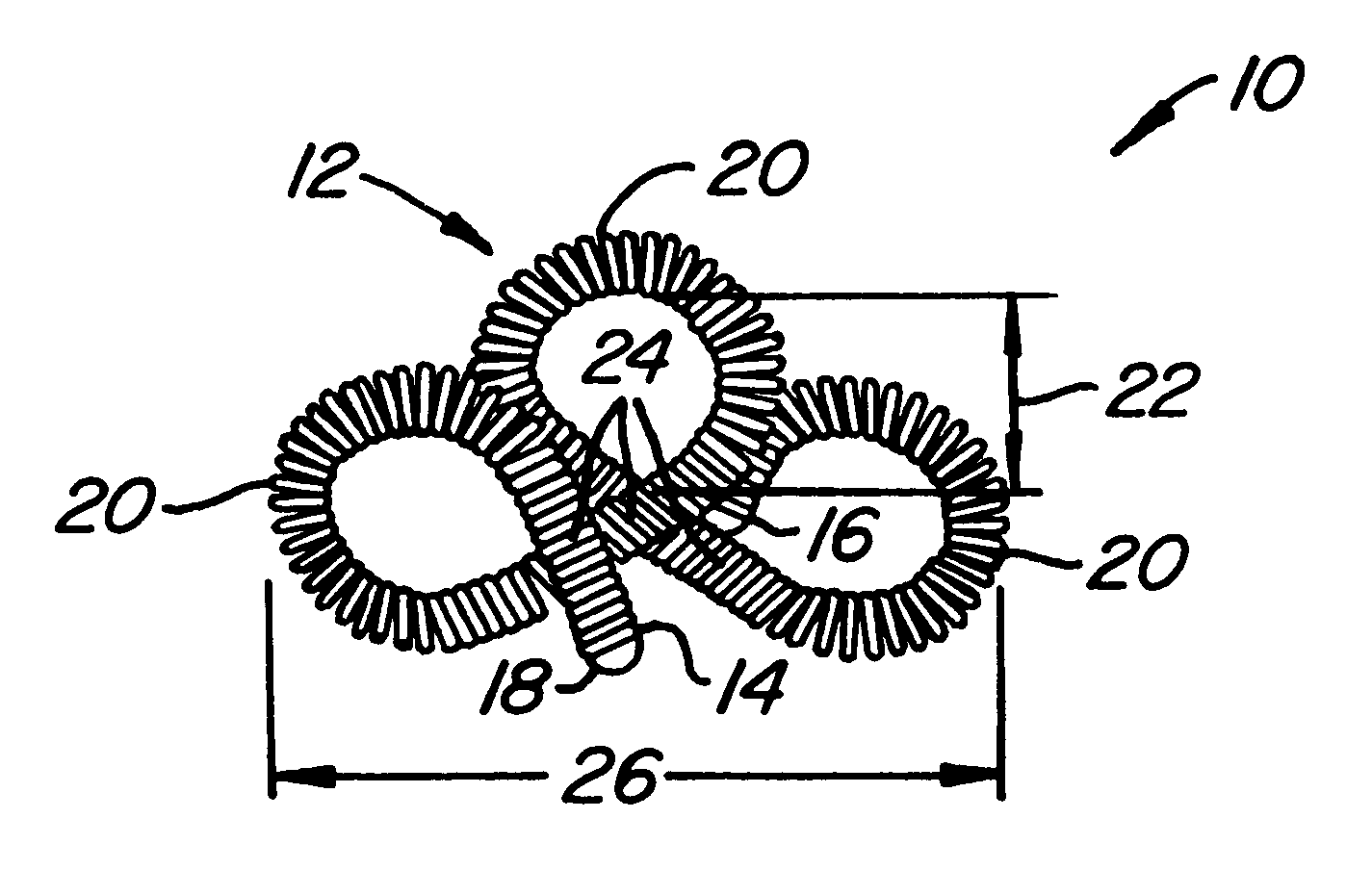
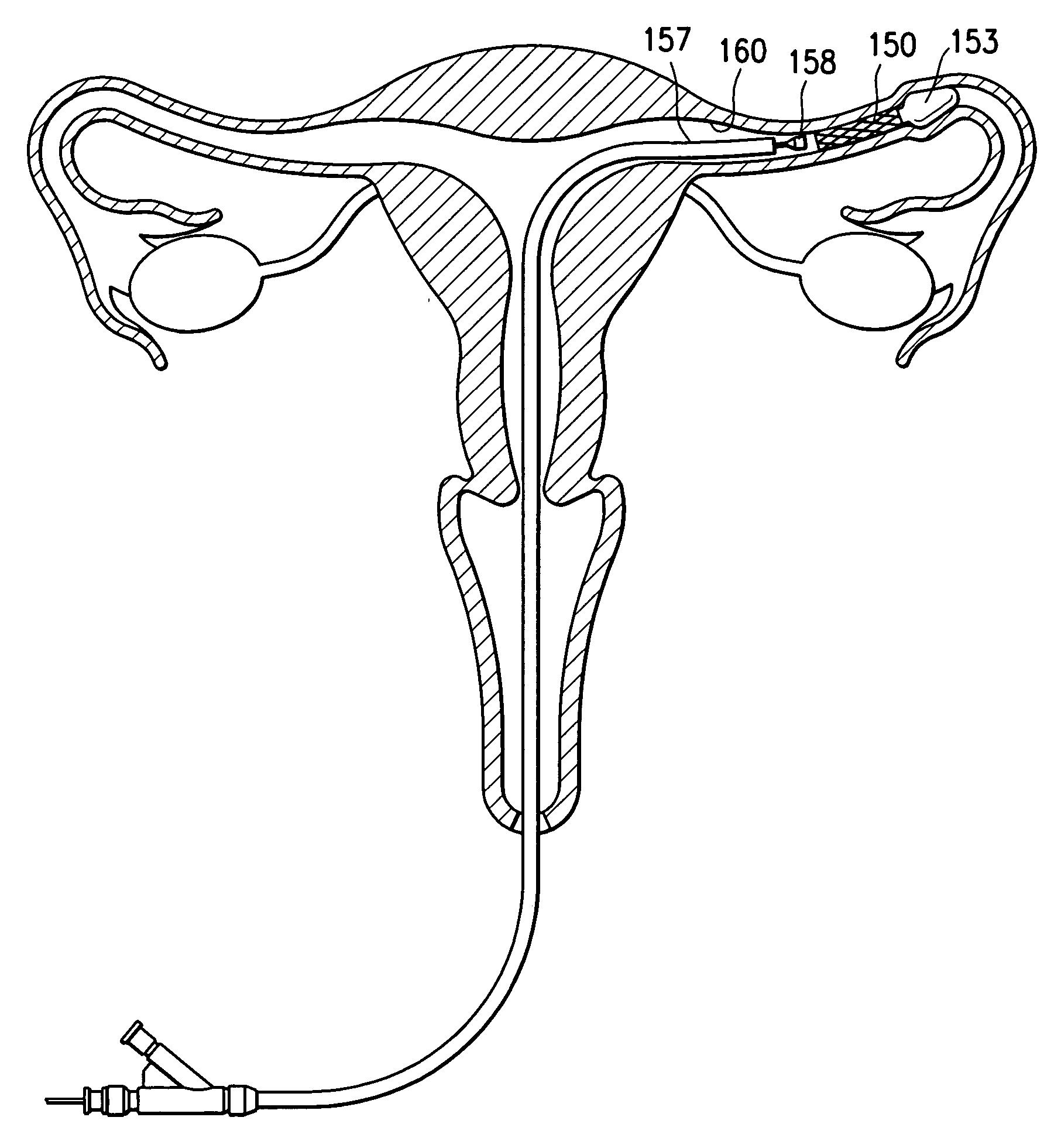
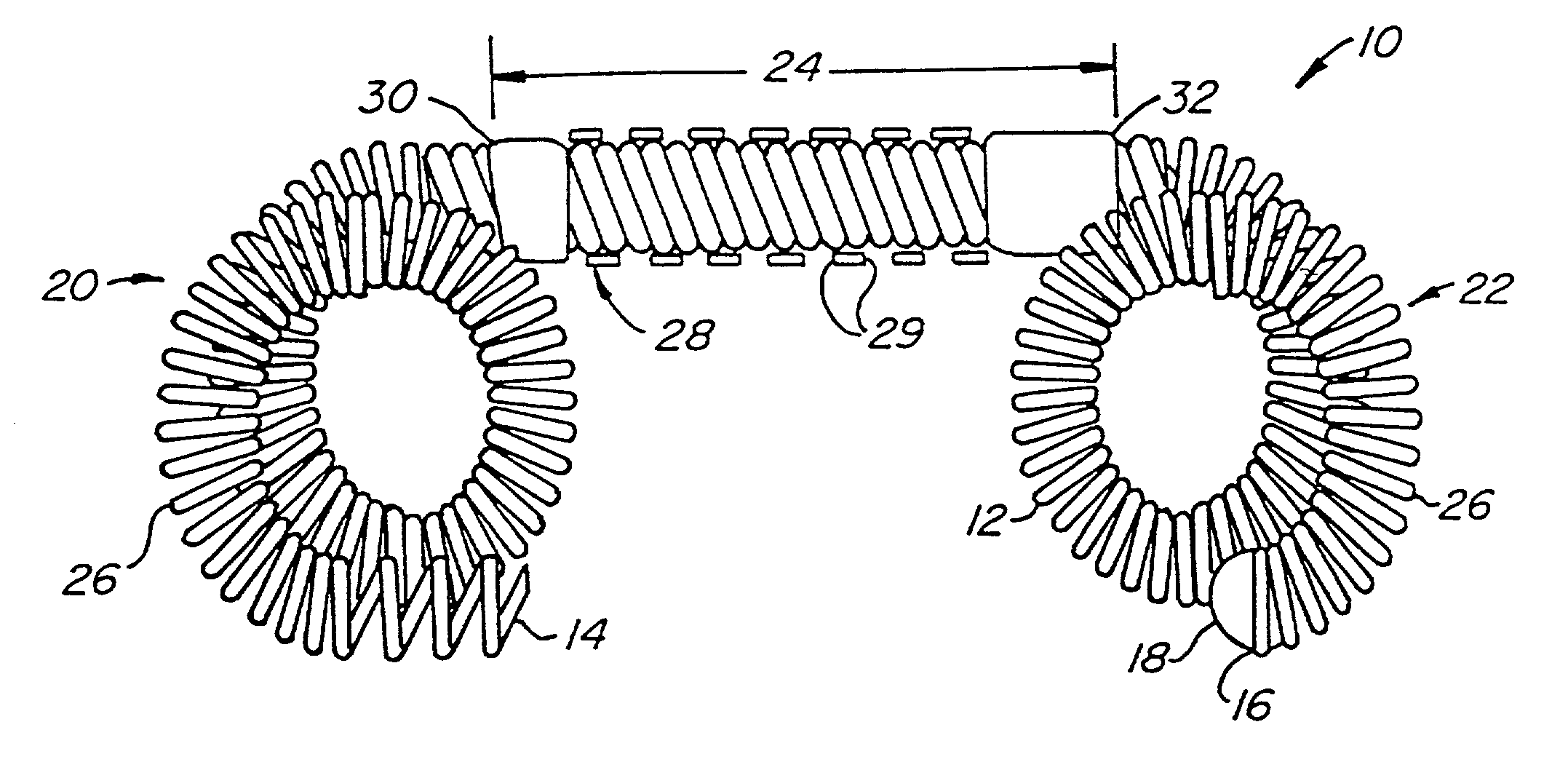
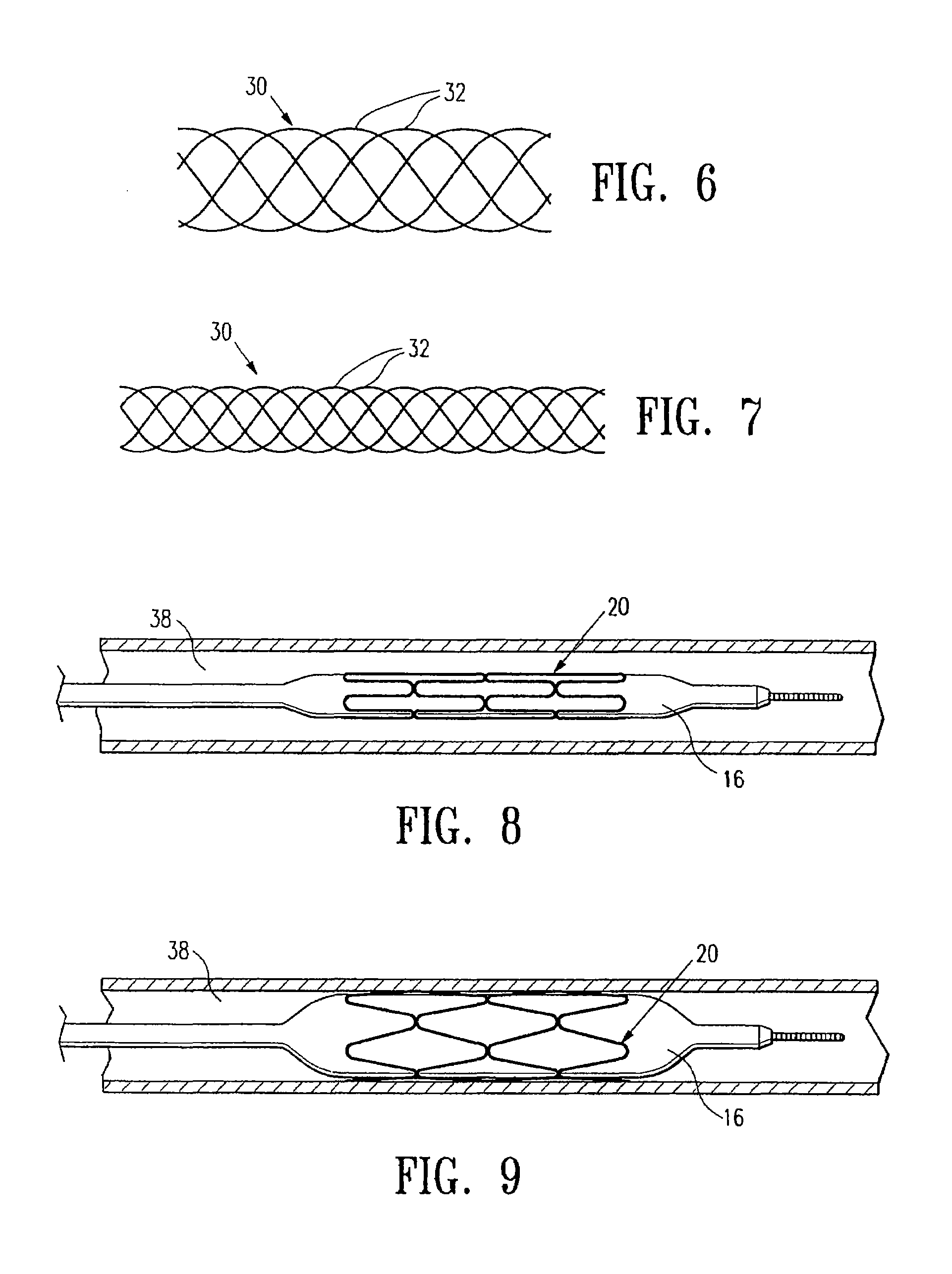
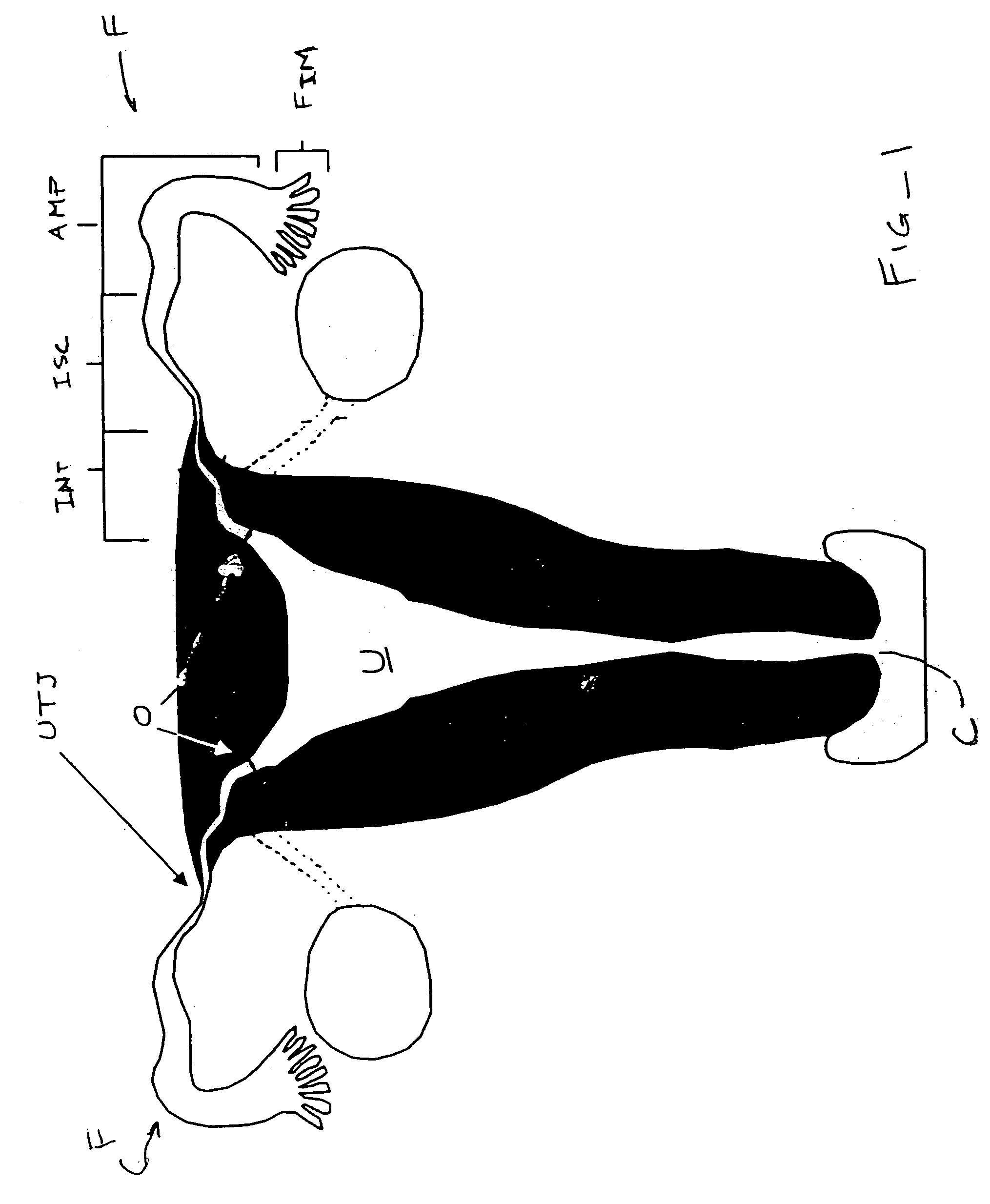
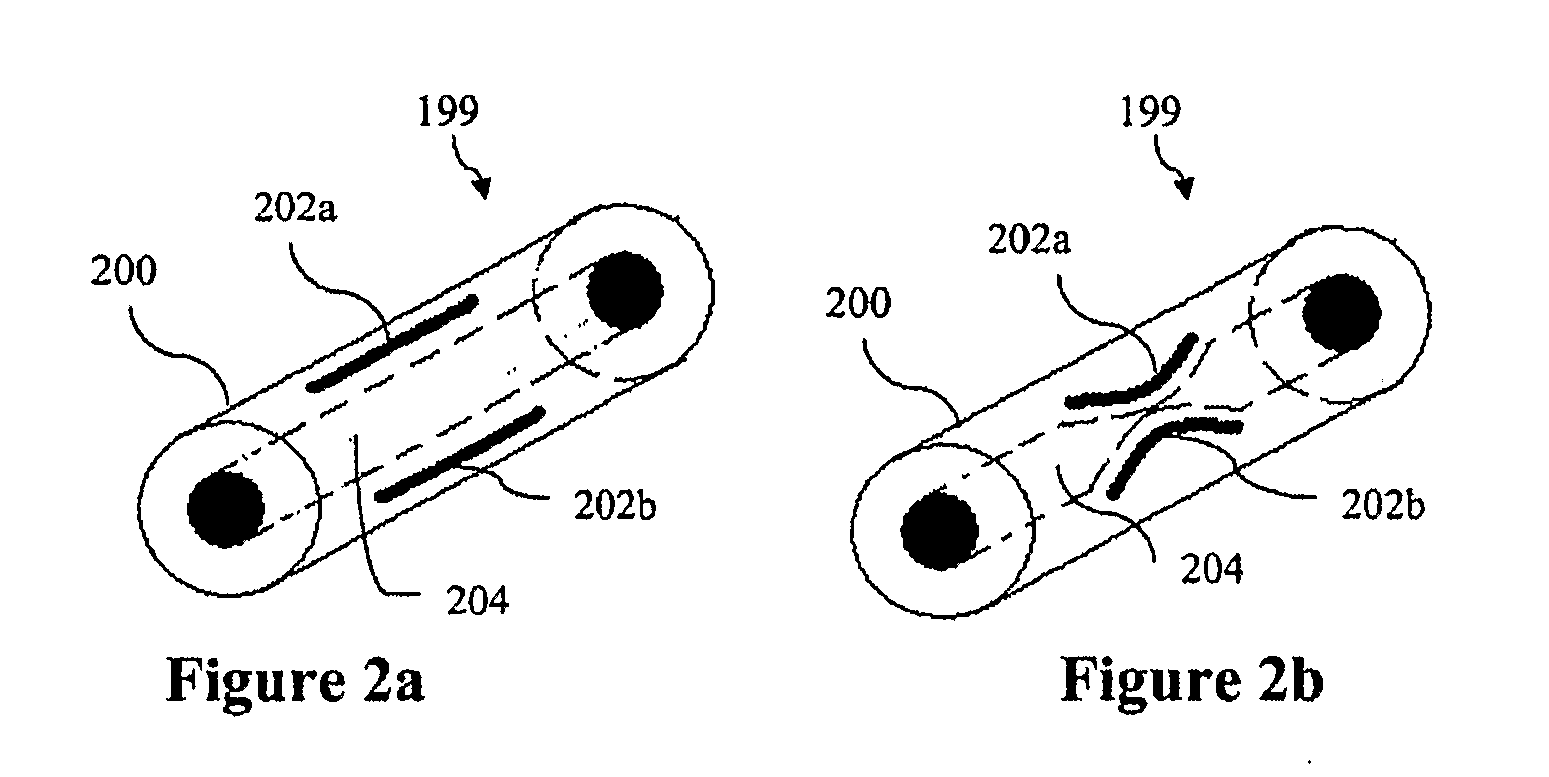
Contraceptive transcervical fallopian tube occlusion devices and methods
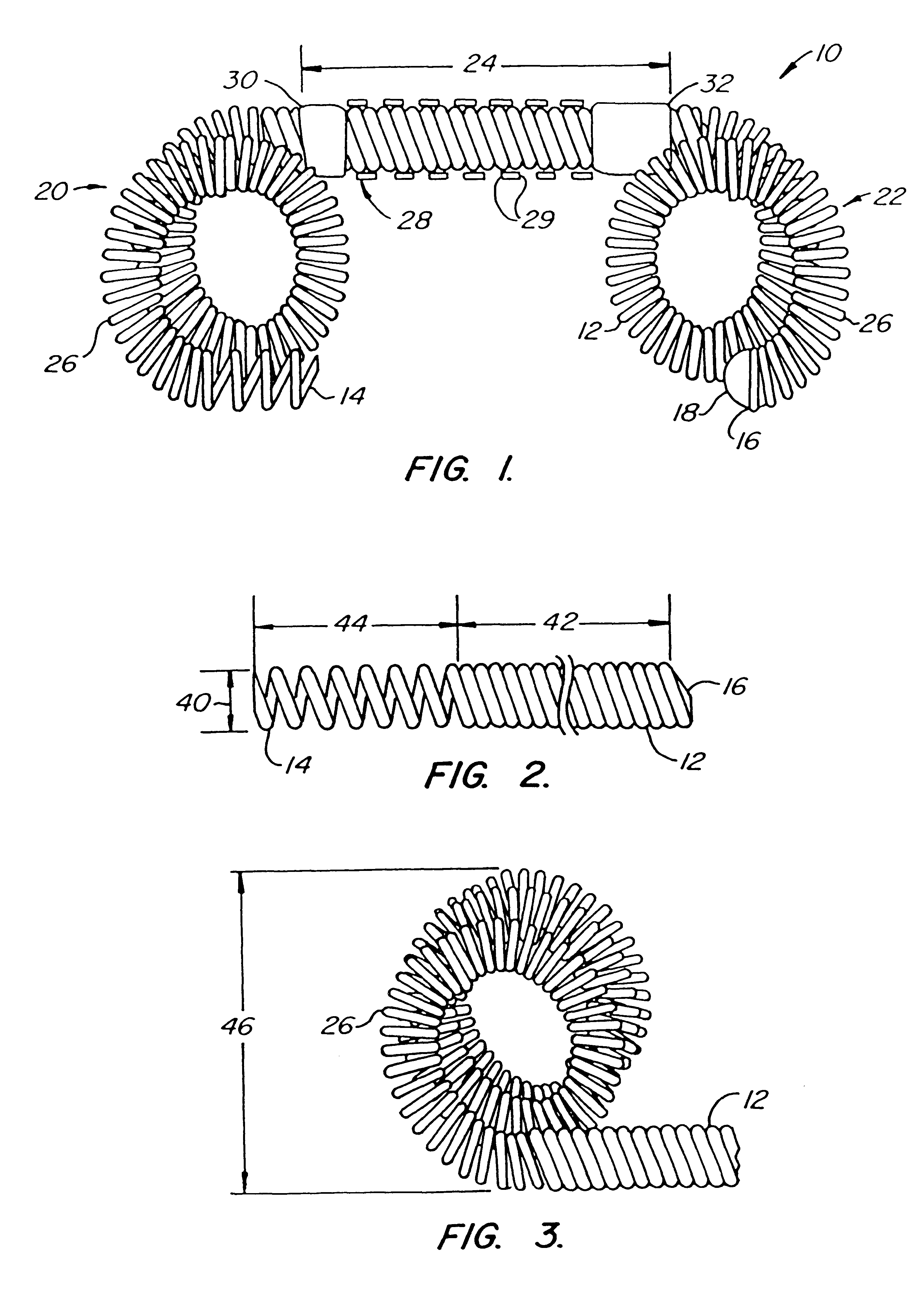
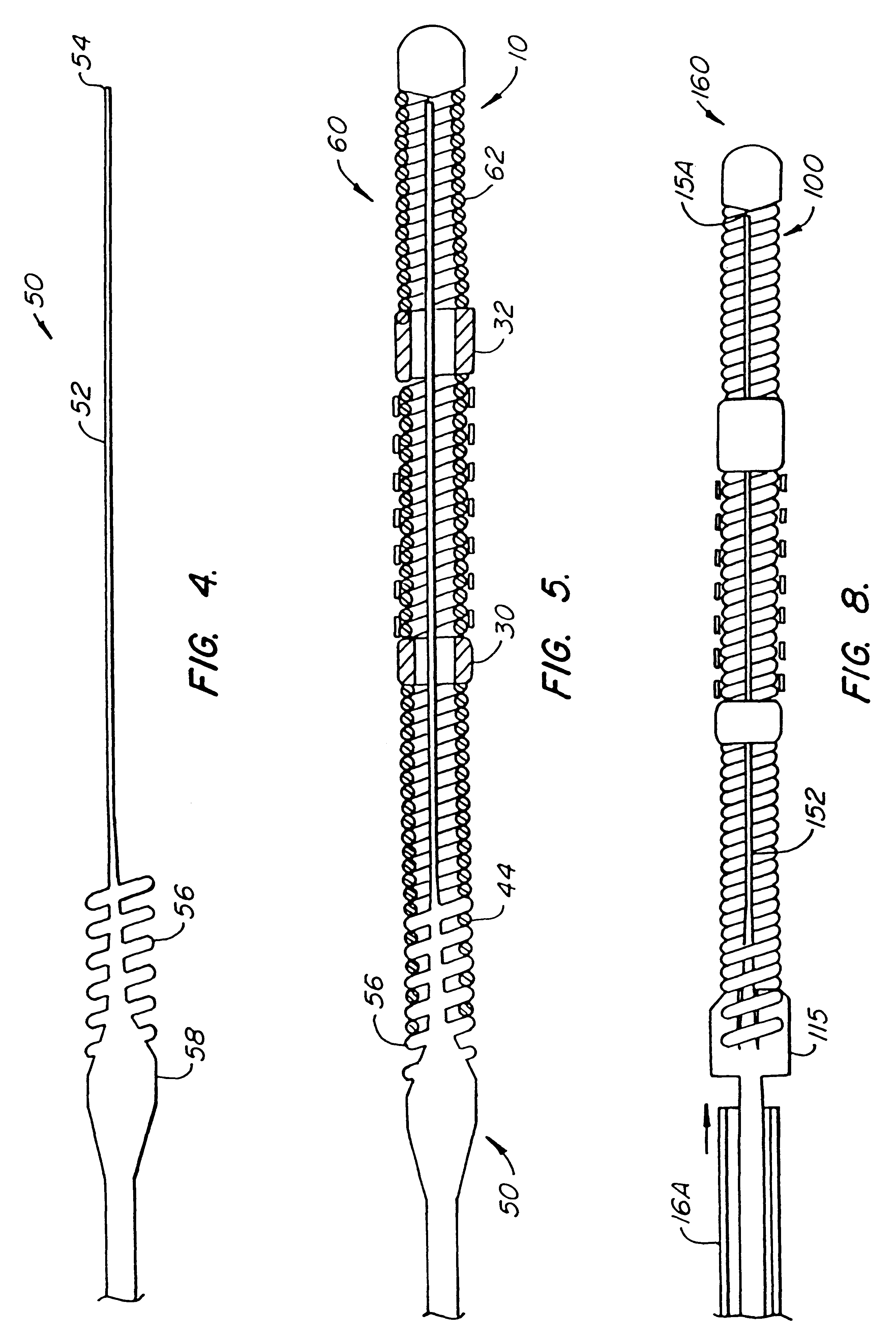
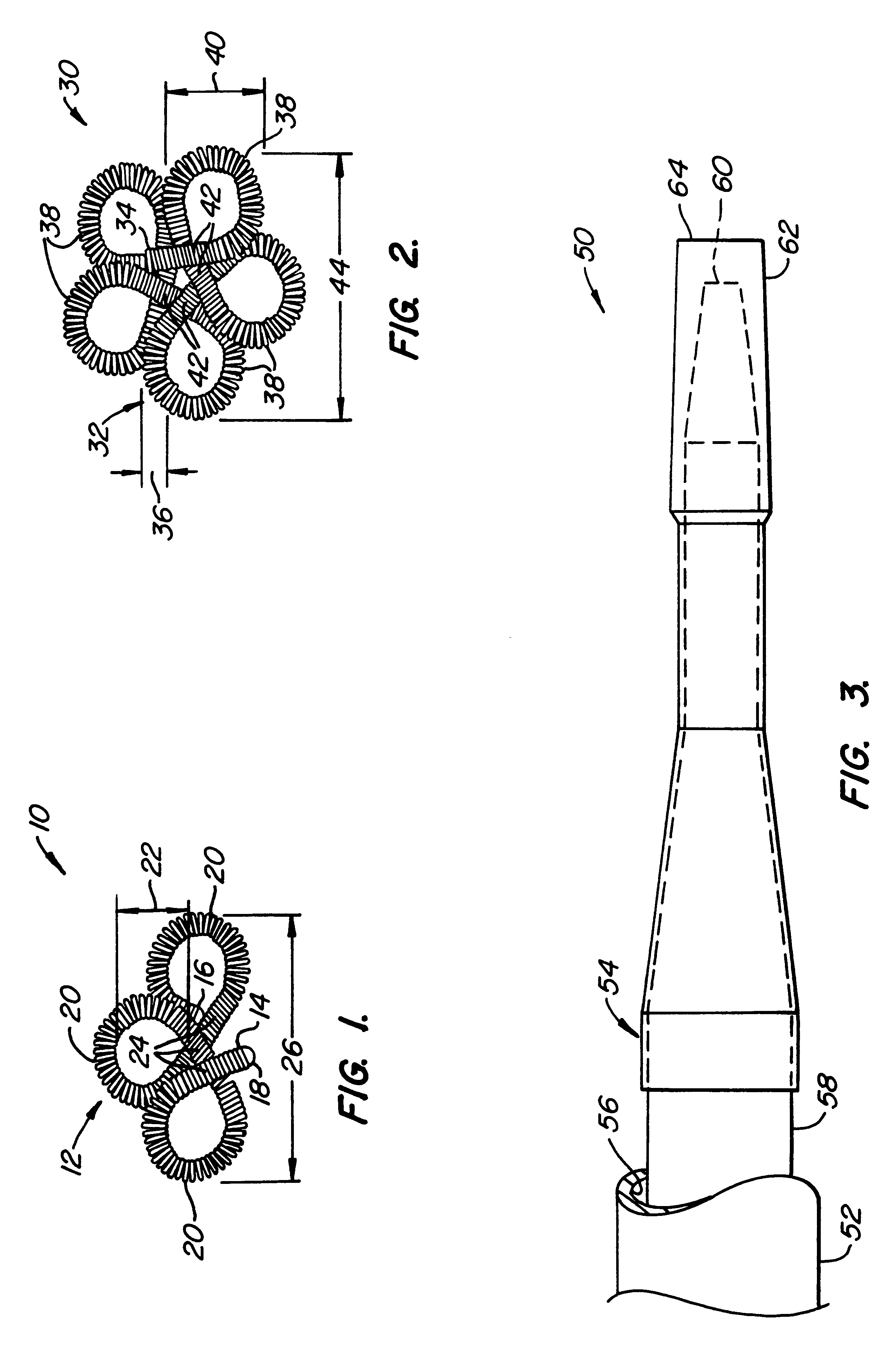
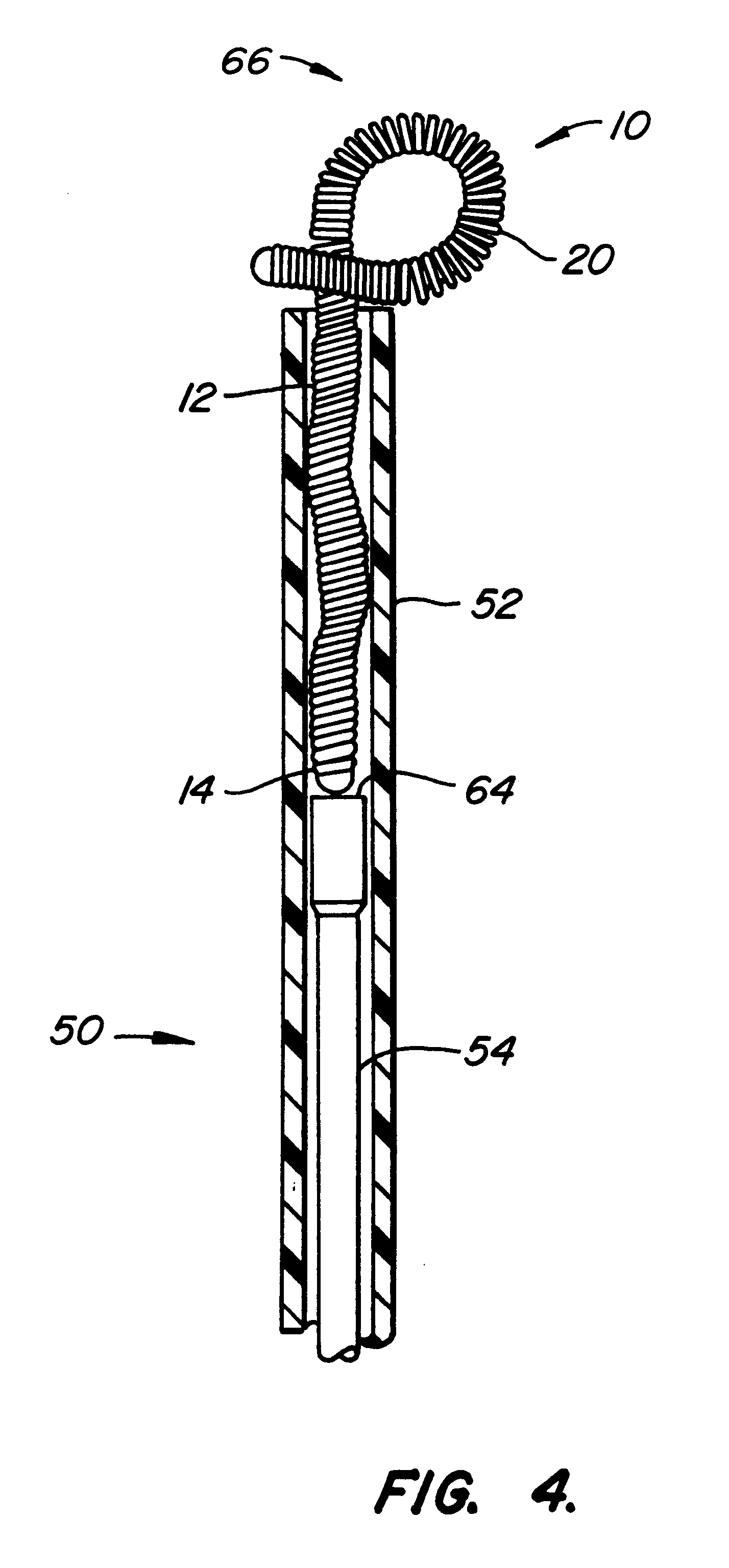
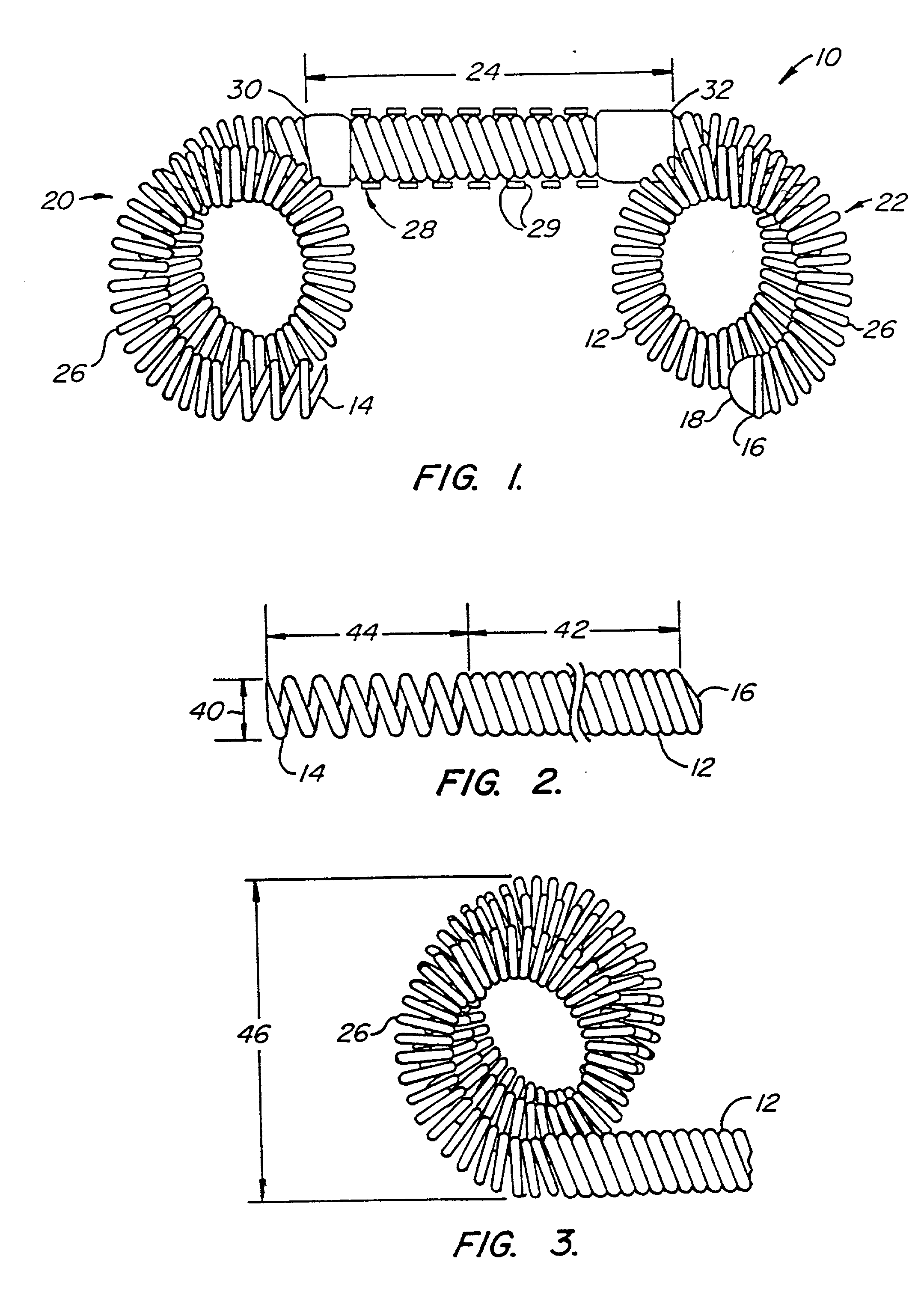
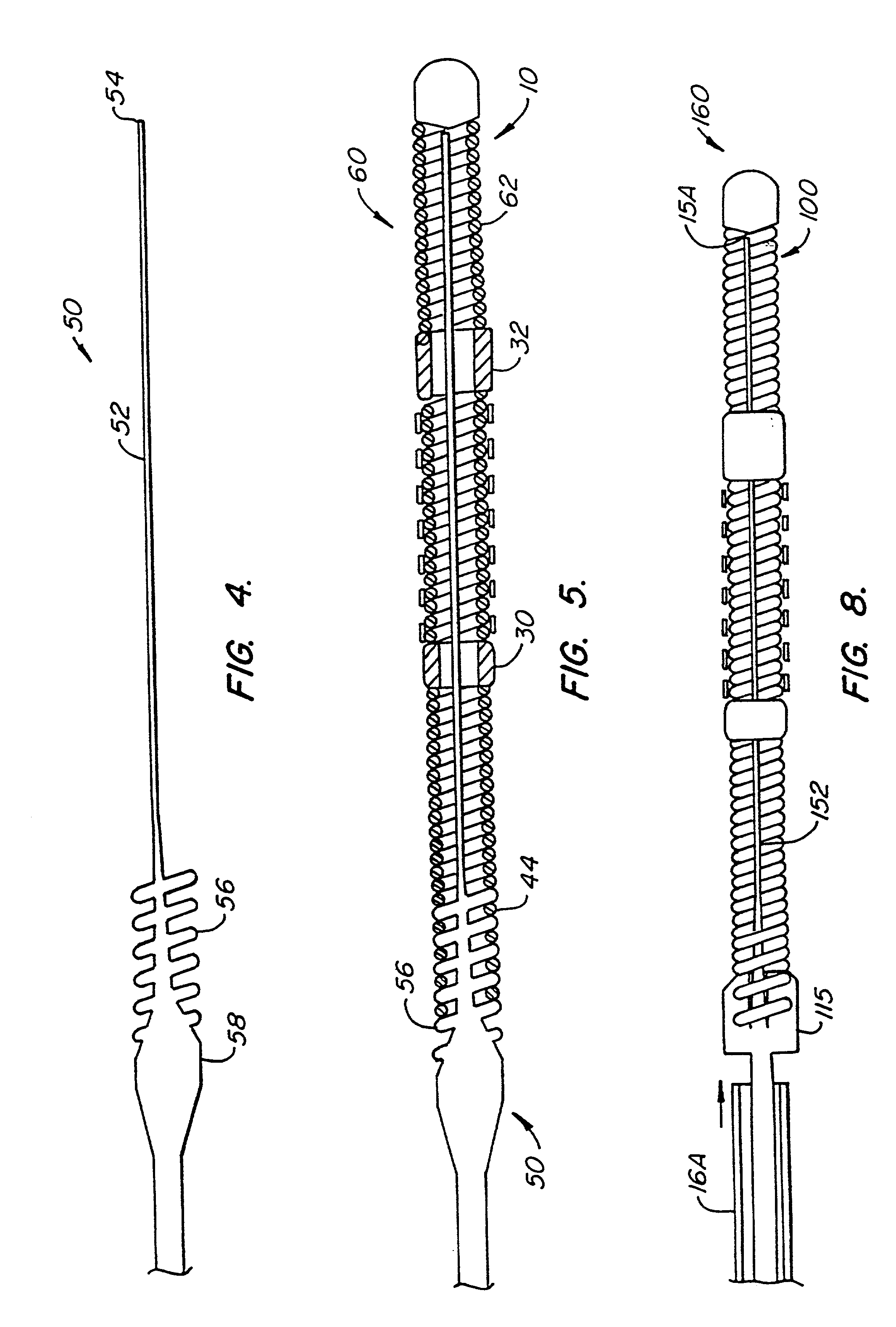
The invention provides intrafallopian devices and non-surgical methods for their placement to prevent conception. The efficacy of the device is enhanced by forming the structure at least in part from copper or a copper alloy. The device is anchored within the fallopian tube by a lumen-traversing region of the resilient structure which has a helical outer surface, together with a portion of the resilient structure which is biased to form a bent secondary shape, the secondary shape having a larger cross-section than the fallopian tube. The resilient structure is restrained in a straight configuration and transcervically inserted within the fallopian tube, where it is released. Optionally, permanent sterilization is effected by passing a current through the resilient structure to the tubal walls.
Owner:BAYER ESSURE
Method and apparatus for creating intrauterine adhesions
InactiveUS20050171569A1Better treat excessive bleedingPromote tissue growthUltrasonic/sonic/infrasonic diagnosticsSuture equipmentsExcessive BleedingGynecology
In general, the present invention contemplates an implantable device for treating excessive bleeding in a body cavity. The device comprises a biocompatible material, for example polyethylene teraphathalate (PET), which is deliverable into the body cavity. The biocompatible material contains an attribute(s) that promotes tissue reaction or growth that results in a tissue response and / or adhesion formation within the body cavity to reduce or stop the excessive bleeding.
Owner:AUB HLDG
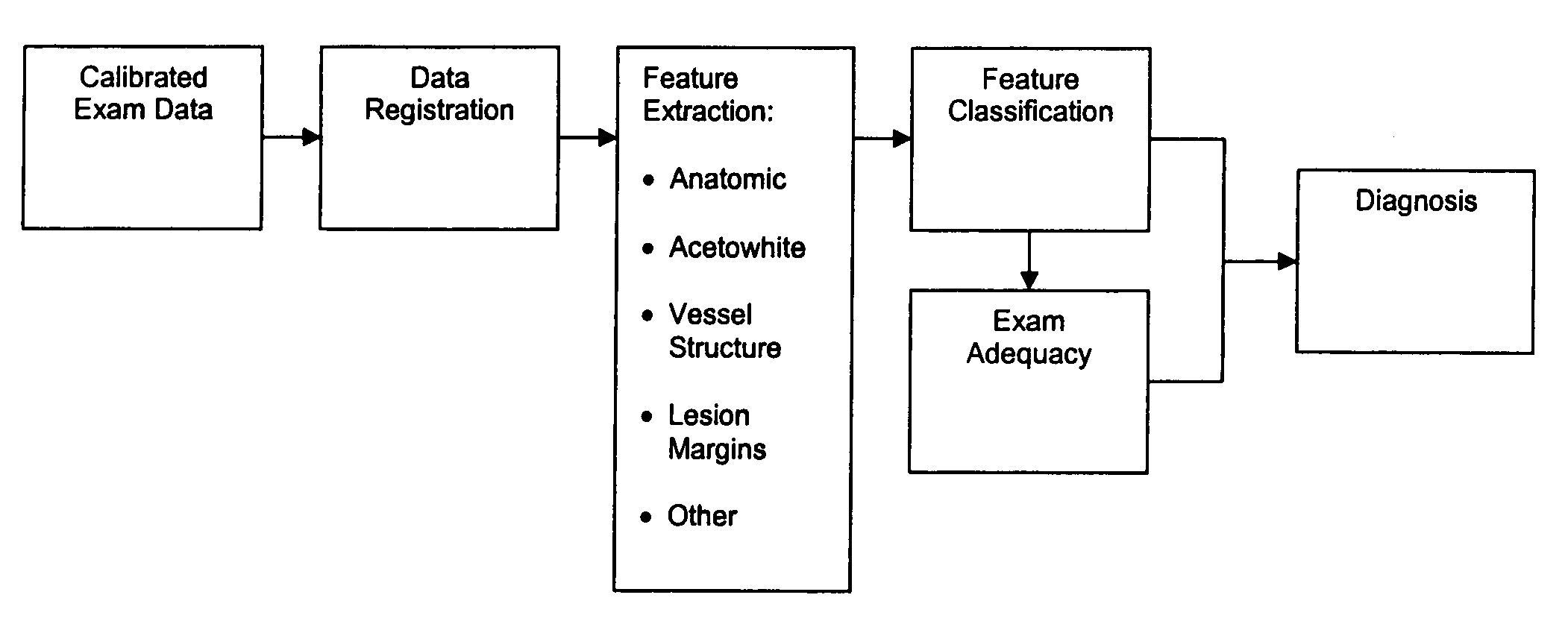
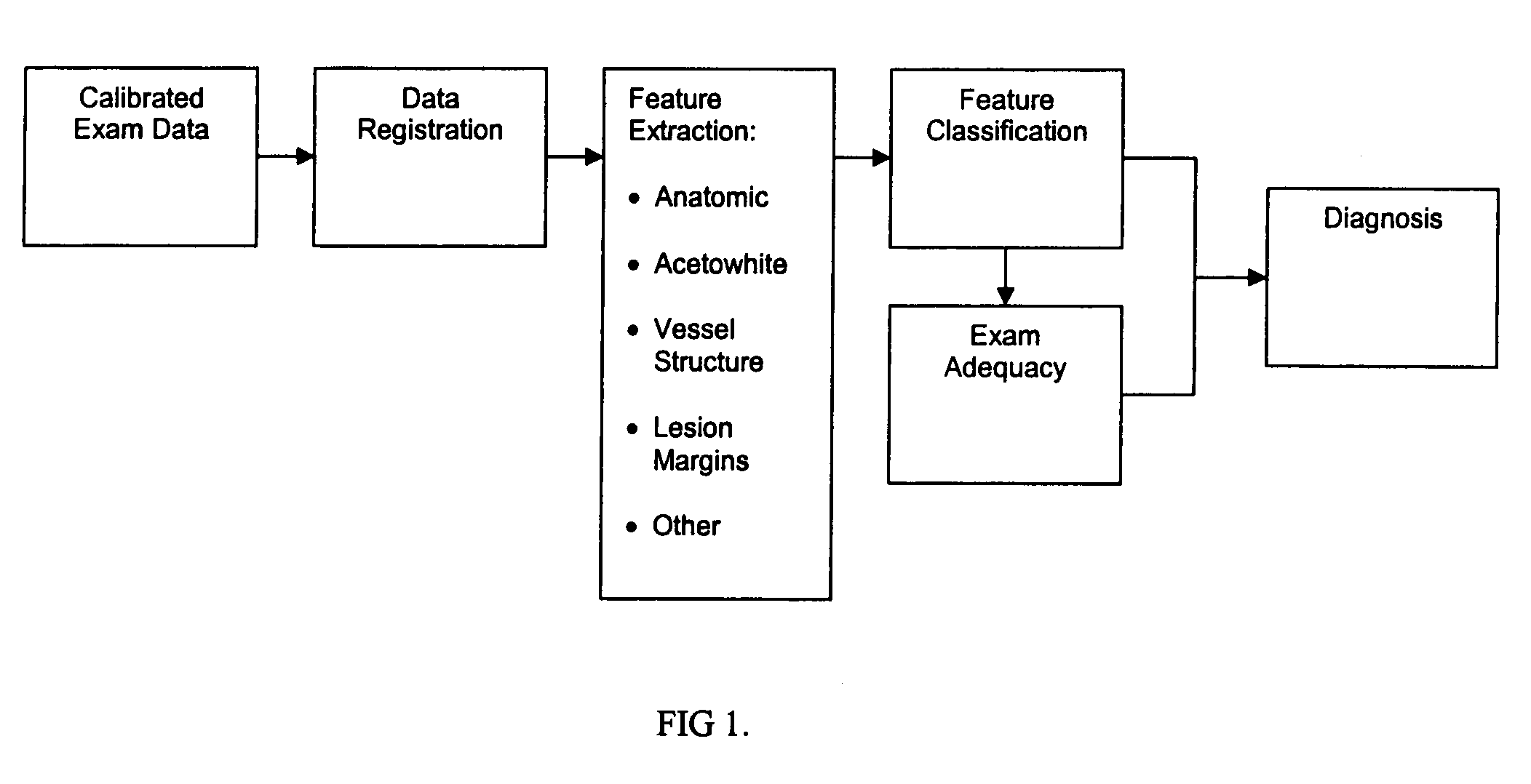
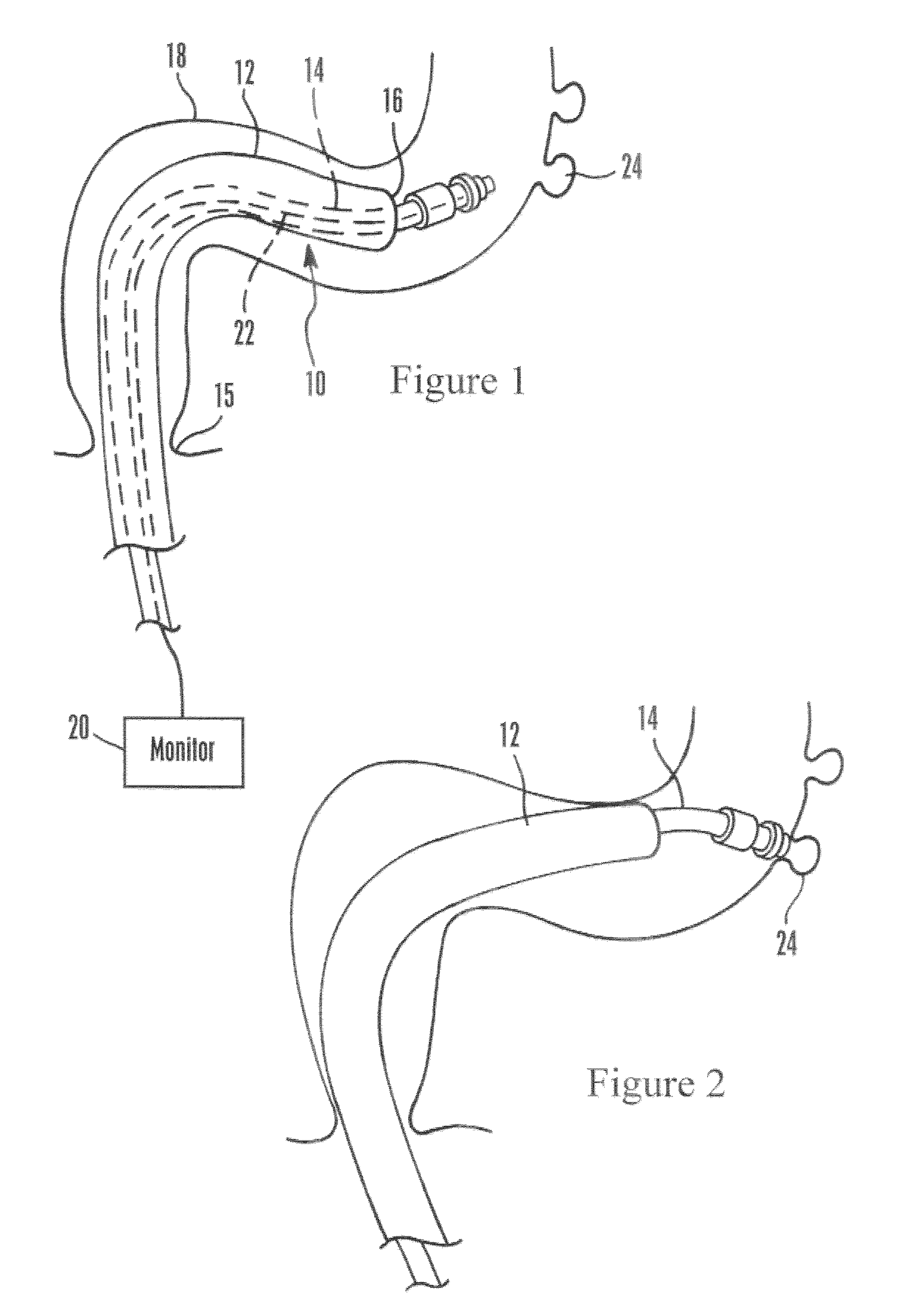
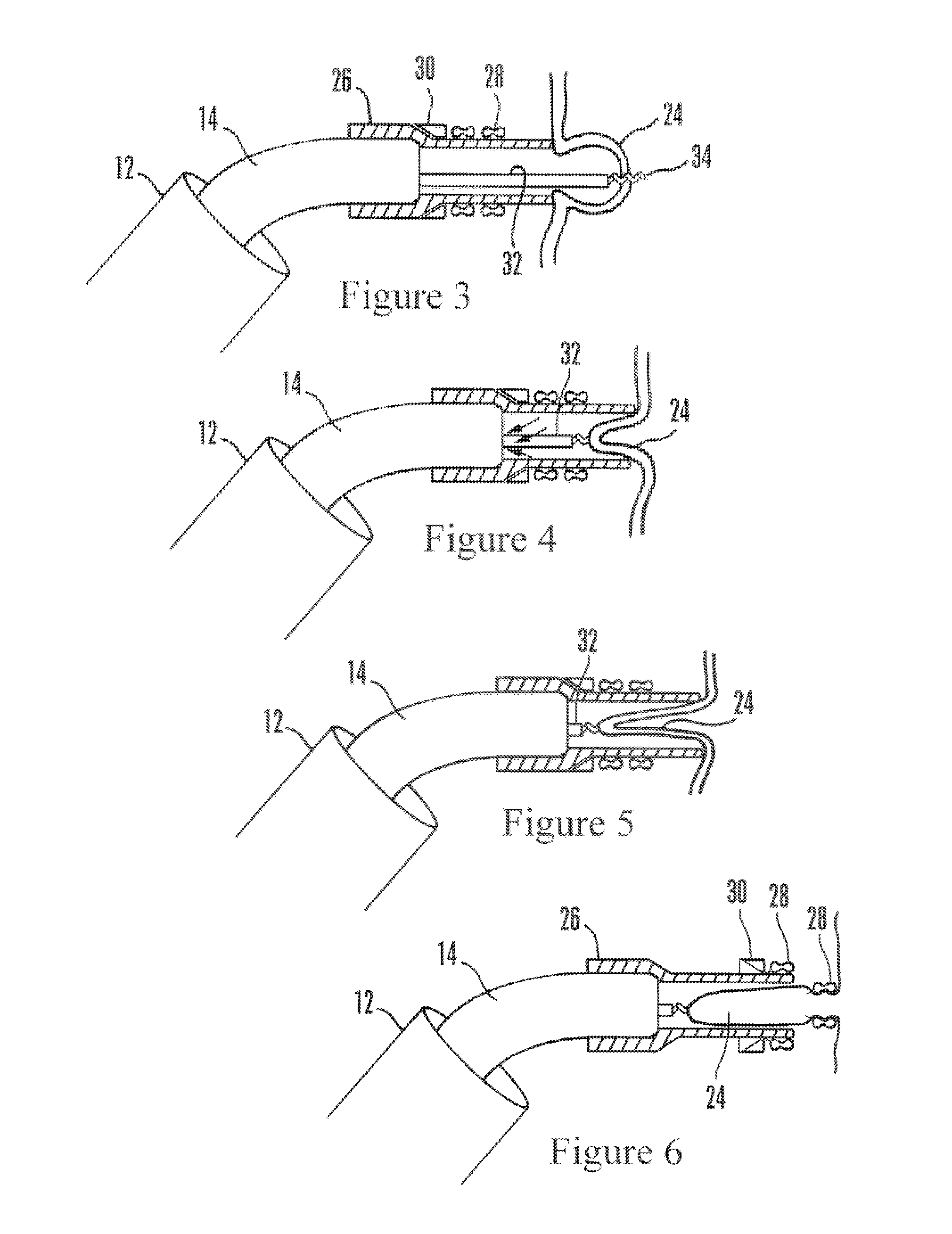
Uterine cervical cancer computer-aided-diagnosis (CAD)
Uterine cervical cancer Computer-Aided-Diagnosis (CAD) according to this invention consists of a core processing system that automatically analyses data acquired from the uterine cervix and provides tissue and patient diagnosis, as well as adequacy of the examination. The data can include, but is not limited to, color still images or video, reflectance and fluorescence multi-spectral or hyper-spectral imagery, coherent optical tomography imagery, and impedance measurements, taken with and without the use of contrast agents like 3-5% acetic acid, Lugol's iodine, or 5-aminolevulinic acid. The core processing system is based on an open, modular, and feature-based architecture, designed for multi-data, multi-sensor, and multi-feature fusion. The core processing system can be embedded in different CAD system realizations. For example: A CAD system for cervical cancer screening could in a very simple version consist of a hand-held device that only acquires one digital RGB image of the uterine cervix after application of 3-5% acetic acid and provides automatically a patient diagnosis. A CAD system used as a colposcopy adjunct could provide all functions that are related to colposcopy and that can be provided by a computer, from automation of the clinical workflow to automated patient diagnosis and treatment recommendation.
Owner:STI MEDICAL SYST
Self-expanding medical occlusion device
InactiveUS20070167980A1Good shape memoryImprove deformation abilitySuture equipmentsEar treatmentMedicineSurgery
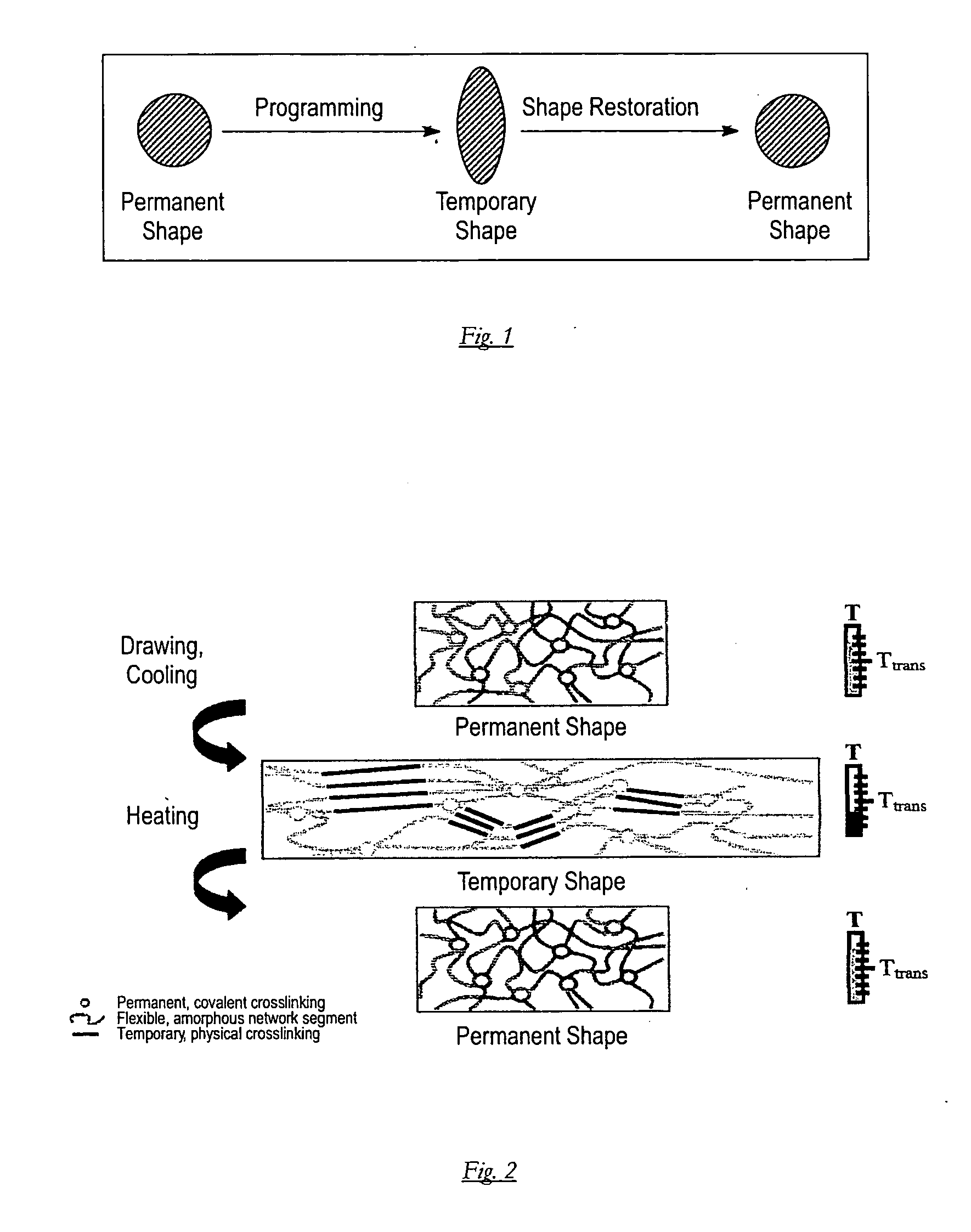
A self-expanding medical occlusion device treats a heart defect in a patient and is inserted into the body in minimally invasive fashion using a catheter system, and includes a braiding of thin threads which exhibits a first preliminarily definable shape as the occlusion device is being inserted into the patient's body and a second preliminarily definable shape in the implanted state, whereby the occlusion device is in a collapsed state in the first shape of the braiding and in an expanded state in the second shape of the braiding. The threads of braiding are composed of a shape memory polymer composite such that braiding deforms from a temporary shape to a permanent shape in consequence of an external stimulus, whereby the temporary shape is given in a first profile form of the braiding and the permanent shape is given in a second profile form of the braiding.
Owner:OCCLUTECH HLDG
Contraceptive transcervical fallopian tube occlusion devices and their delivery
InactiveUS6176240B1Good curative effectLess readily restrainedFallopian occludersFemale contraceptivesObstetricsSalpingostomy
The invention provides intrafallopian devices and non-surgical methods for their placement to prevent conception. The efficacy of the device is enhanced by forming the structure at least in part from copper or a copper alloy. The device is anchored within the fallopian tube by imposing a secondary shape on a resilient structure, the secondary shape having a larger cross-section than the fallopian tube. The resilient structure is restrained in a straight configuration and transcervically inserted within the fallopian tube, where it is released. The resilient structure is then restrained by the walls of the fallopian tube, imposing anchoring forces as it tries to resume the secondary shape.
Owner:BAYER ESSURE
Endoscopic delivery of medical devices
InactiveUS20050288551A1Easy to installFallopian occludersEndoscopesEndoscopic AssemblyMedical device
The invention is directed to an endoscopic assembly having an endoscope, particularly a flexible hysteroscope and an outer sheath disposed about a length of the shaft of the hysteroscope which has an expandable member such as an inflatable balloon for sealing the assembly within a body lumen or cavity. Specifically, the endoscope assembly is configured for delivery of an occlusive contraceptive member to the patient's fallopian tube. The invention is also directed to an endoscope having a driving member for movement of a medical device within the working lumen of an endoscope. In one embodiment the driving member is a friction wheel which engages an elongated medical device disposed within the working channel of the endoscope to effect longitudinal movement of the medical device.
Owner:BAYER ESSURE
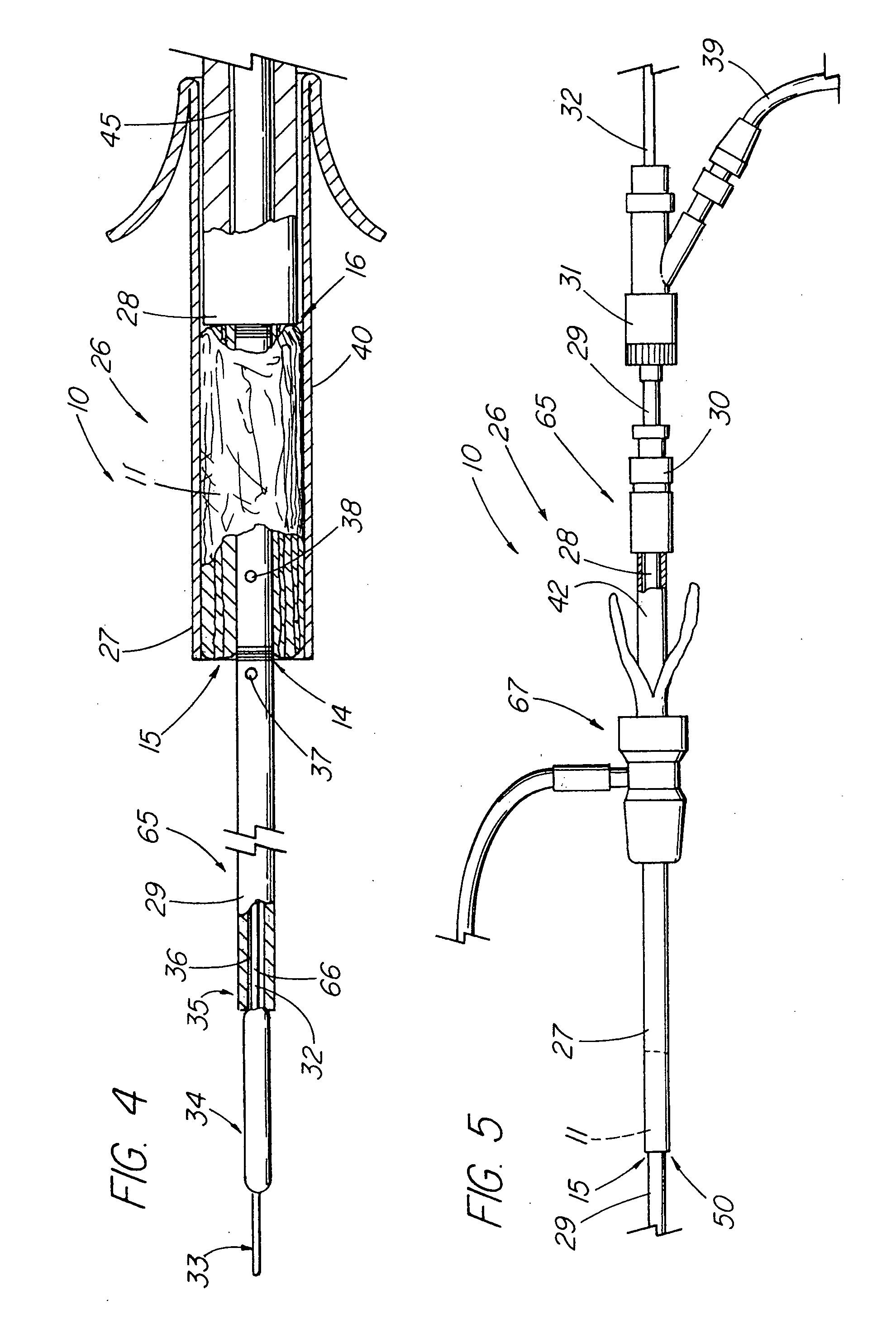
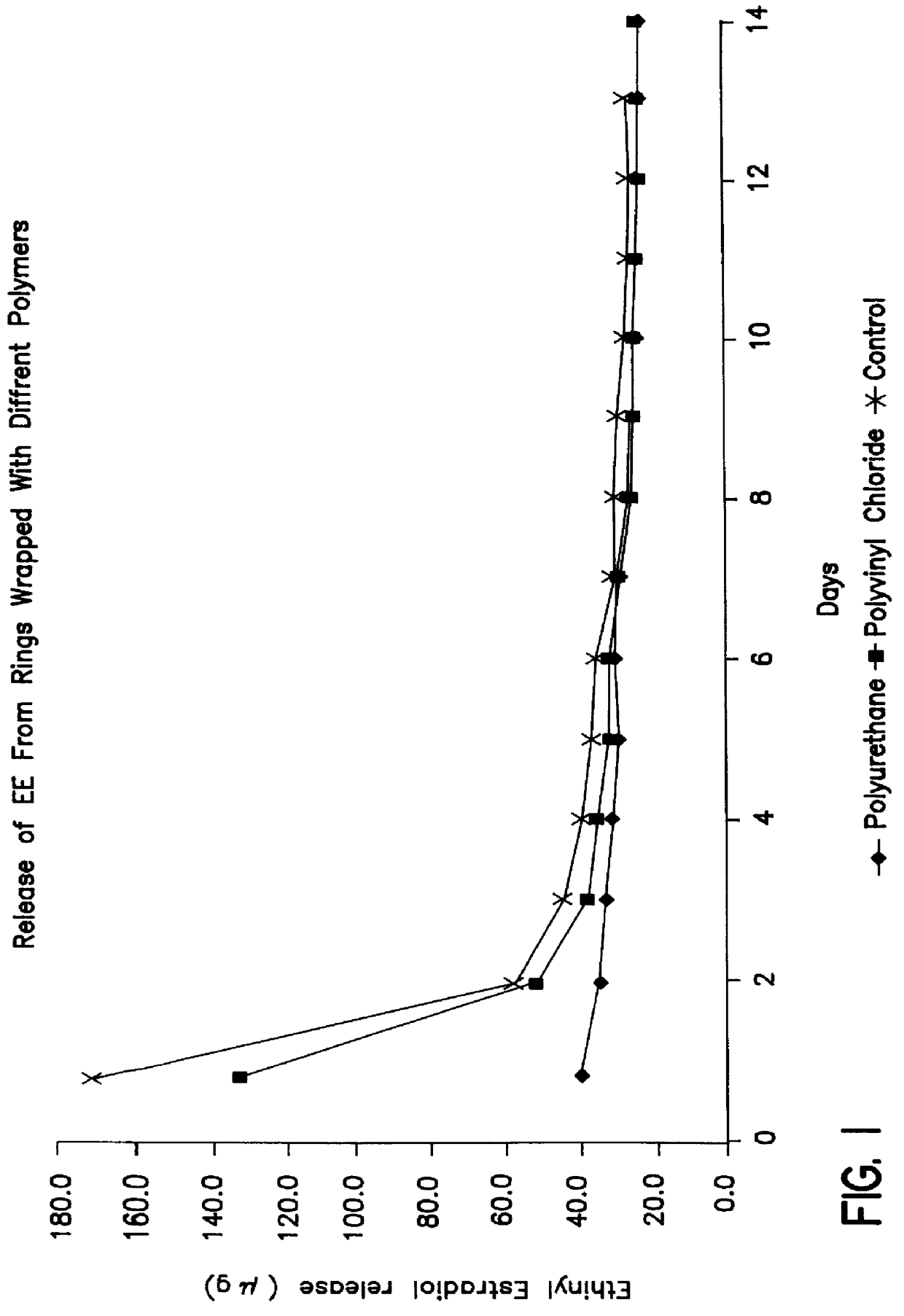
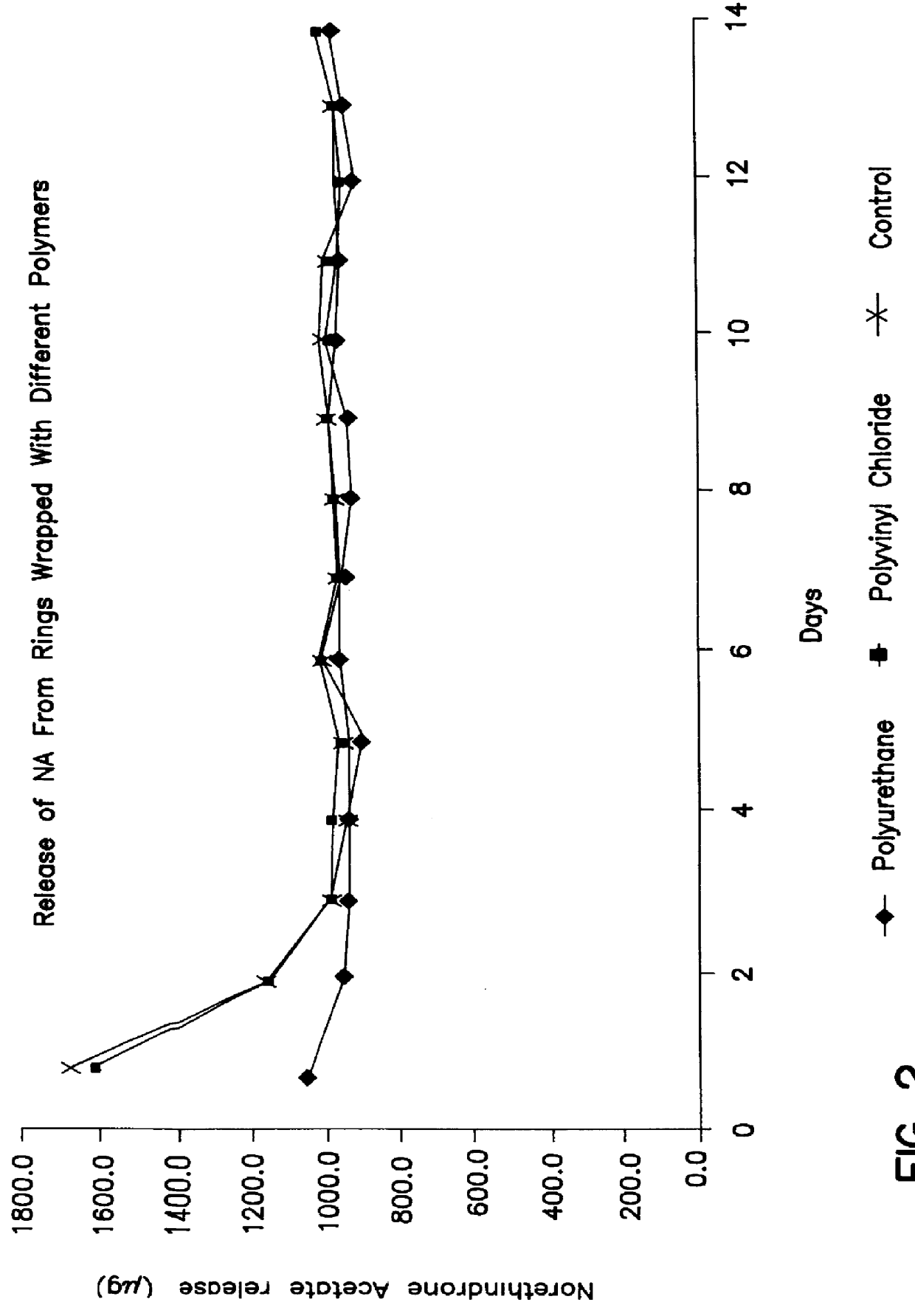
Intravaginal drug delivery devices for the administration of an antimicrobial agent
InactiveUS6951654B2Convenient and high complianceReduce in quantityAntibacterial agentsTetracycline active ingredientsAntimicrobial drugAnti-Microbial Agents
An intravaginal antimicrobial drug delivery device is disclosed having an antimicrobial agent dispersed throughout a biocompatible elastomeric system. Also disclosed is a method of making the antimicrobial drug delivery device.
Owner:APTALIS PHARMA
Bodily lumen closure apparatus and method
ActiveUS20050155608A1Improve sealingPrevent leakageStentsFallopian occludersCell-Extracellular MatrixSource material
An absorbable and expandable closure member used to occlude or exclude a body lumen or cavity, such as a blood vessel, fallopian tube, duct, aneurysmal sac, etc., comprising a closure member comprising one of more sheets of a biomaterial that are rolled, stacked, or folded to form a multilayer construct of a generally cylindrical configuration for deployment through a delivery system, either as a singularly or part of a multiplicity of closure members. The biomaterial is derived from a source material, such as small intestinal submucosa or another remodelable material (e.g., an extracellular matrix) having properties for stimulating ingrowth of adjacent tissue into the biomaterial deployed within the bodily lumen. The closure member is deployed to the bodily lumen from a delivery sheath, cartridge, and / or over a inner guiding member, such as a wire guide or catheter.
Owner:OREGON HEALTH & SCI UNIV +2
Electrically affixed transcervical fallopian tube occlusion devices
InactiveUS6145505AGood curative effectLess readily restrainedFallopian occludersFemale contraceptivesObstetricsSalpingostomy
The invention provides intrafallopian devices and non-surgical methods for their placement to prevent conception. The efficacy of the device is enhanced by forming the structure at least in part from copper or a copper alloy. The device is anchored within the fallopian tube by imposing a secondary shape on a resilient structure, the secondary shape having a larger cross-section than the fallopian tube. The resilient structure is restrained in a straight configuration and transcervically inserted within the fallopian tube, where it is released. The resilient structure is then restrained by the walls of the fallopian tube, imposing anchoring forces as it tries to resume the secondary shape.
Owner:BAYER ESSURE
Solid dose delivery vehicle and methods of making same
The present invention encompasses a solid dose delivery vehicle for ballistic administration of a bioactive material to subcutaneous and intradermal tissue, the delivery vehicle being sized and shaped for penetrating the epidermis. The delivery vehicle further comprises a stabilizing polyol glass loaded with the bioactive material and capable of releasing the bioactive material in situ. The present invention further includes methods of making and using the solid dose delivery vehicle of the invention.
Owner:QUADRANT DRUG DELIVERY
Intravaginal drug delivery devices for the delivery of macromolecules and water-soluble drugs
ActiveUS20090004246A1Good sustained releaseViral antigen ingredientsVirus peptidesIntravaginal administrationPharmacy
An intravaginal drug delivery device comprises a device body comprising a hydrophobic carrier material having at least one channel defining at least one opening to the exterior of said device body, said at least one channel being adapted to receive at least one drug-containing insert; at least one drug-containing insert positioned in said at least one channel, said drug-containing insert capable of releasing a pharmaceutically effective amount of at least one drug suitable for intravaginal administration and containing about 1% to about 70% of at least one water-soluble release enhancer, both the drug and the water-soluble release enhancer dispersed in an insert carrier material; wherein said hydrophobic carrier material and said insert carrier material may be the same or different; and wherein said at least one drug-containing insert is exposed on said exterior of said device body when said intravaginal drug delivery device is in use.
Owner:APTALIS PHARMA
Contraceptive with permeable and impermeable components
InactiveUS20050192616A1Facilitate tissue ingrowthFacilitates tissue ingrowthFallopian occludersCannulasObstetricsTissue ingrowth
An device for occluding a body lumen such as a reproductive lumen which includes an occluding component having an impervious barrier to provide initial occlusion of the body lumen and a permeable body to facilitate tissue ingrowth which provides long term occlusion of the body lumen. The device and the method of using the device is particularly suitable for contraception.
Owner:BAYER HEALTHCARE LLC
Contraceptive transcervical fallopian tube occlusion devices and methods
InactiveUS20020020417A1Good curative effectLess readily restrainedFallopian occludersDiagnosticsPower flowSalpingostomy
The invention provides intrafallopian devices and non-surgical methods for their placement to prevent conception. The efficacy of the device is enhanced by forming the structure at least in part from copper or a copper alloy. The device is anchored within the fallopian tube by a lumen-traversing region of the resilient structure which has a helical outer surface, together with a portion of the resilient structure which is biased to form a bent secondary shape, the secondary shape having a larger cross-section than the fallopian tube. The resilient structure is restrained in a straight configuration and transcervically inserted within the fallopian tube, where it is released. Optionally, permanent sterilization is effected by passing a current through the resilient structure to the tubal walls.
Owner:BAYER ESSURE
Intravaginal drug delivery device
InactiveUS6103256AExcessive quantityEfficient removalPharmaceutical delivery mechanismMedical devicesIntravaginal administrationActive agent
An intravaginal drug delivery device comprising at least one active agent dispersed in a polymer matrix, wherein the concentration of active agent at the outer surface of the device at the time of use is not substantially higher than the concentration of the active agent in the remainder of the device, a method of treatment therewith and a process for its preparation.
Owner:AVENTIS PHARMA SA (US)
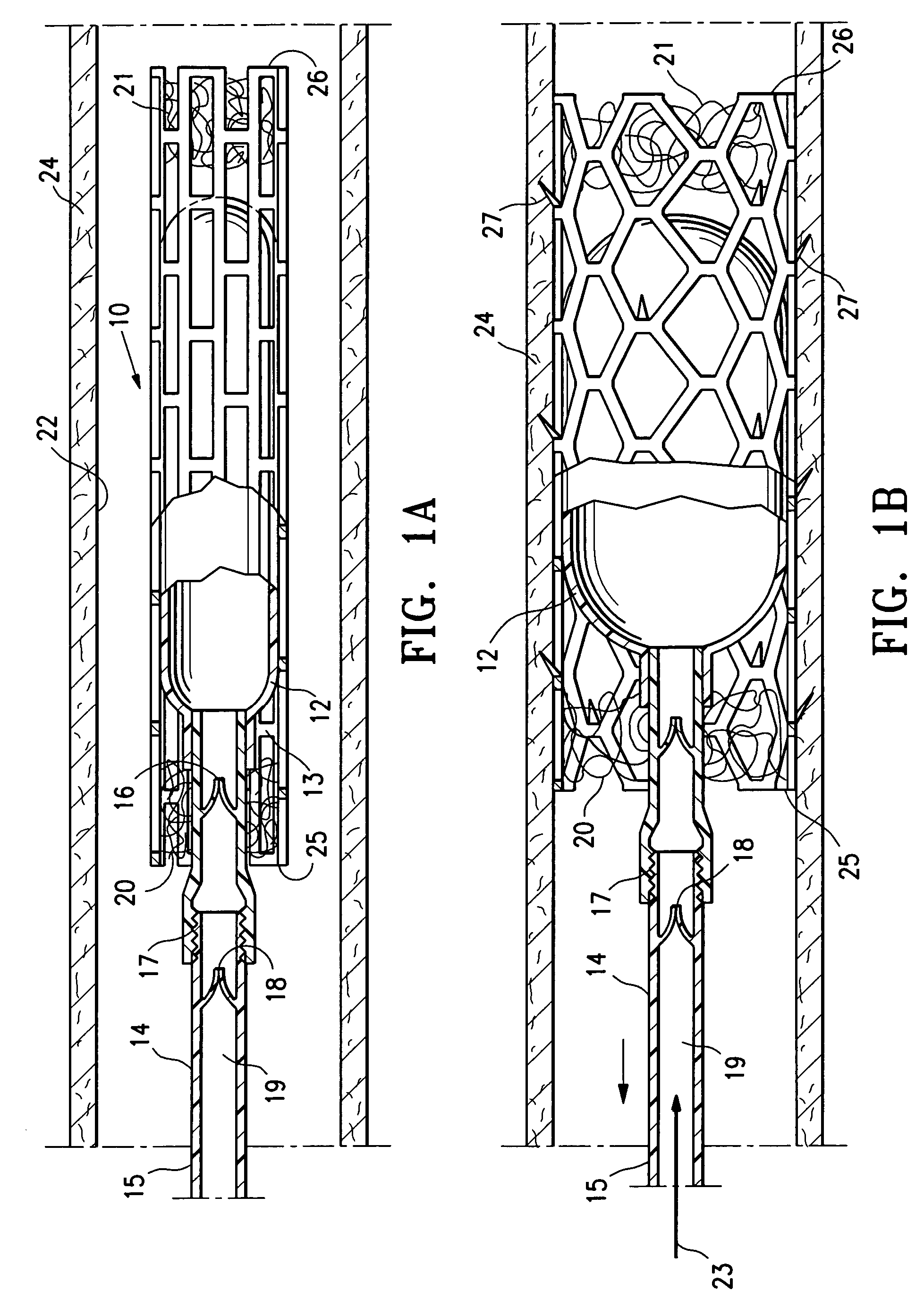
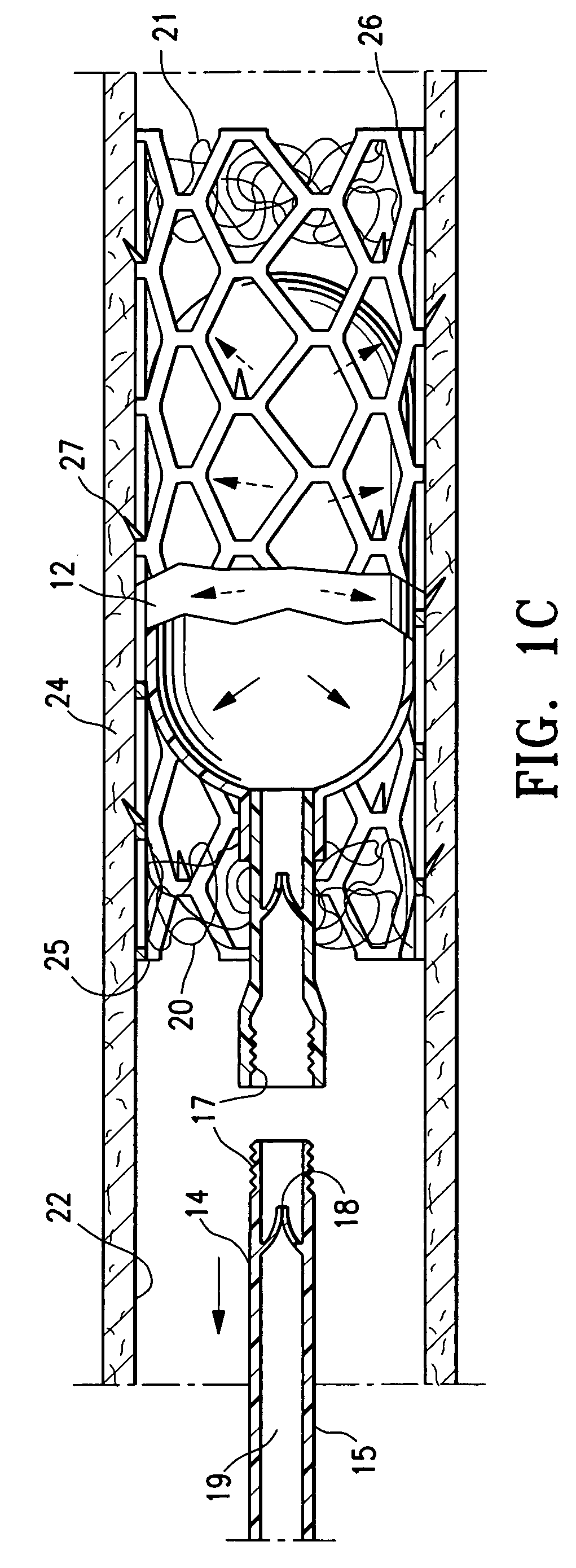
Methods, systems and devices for performing gynecological procedures
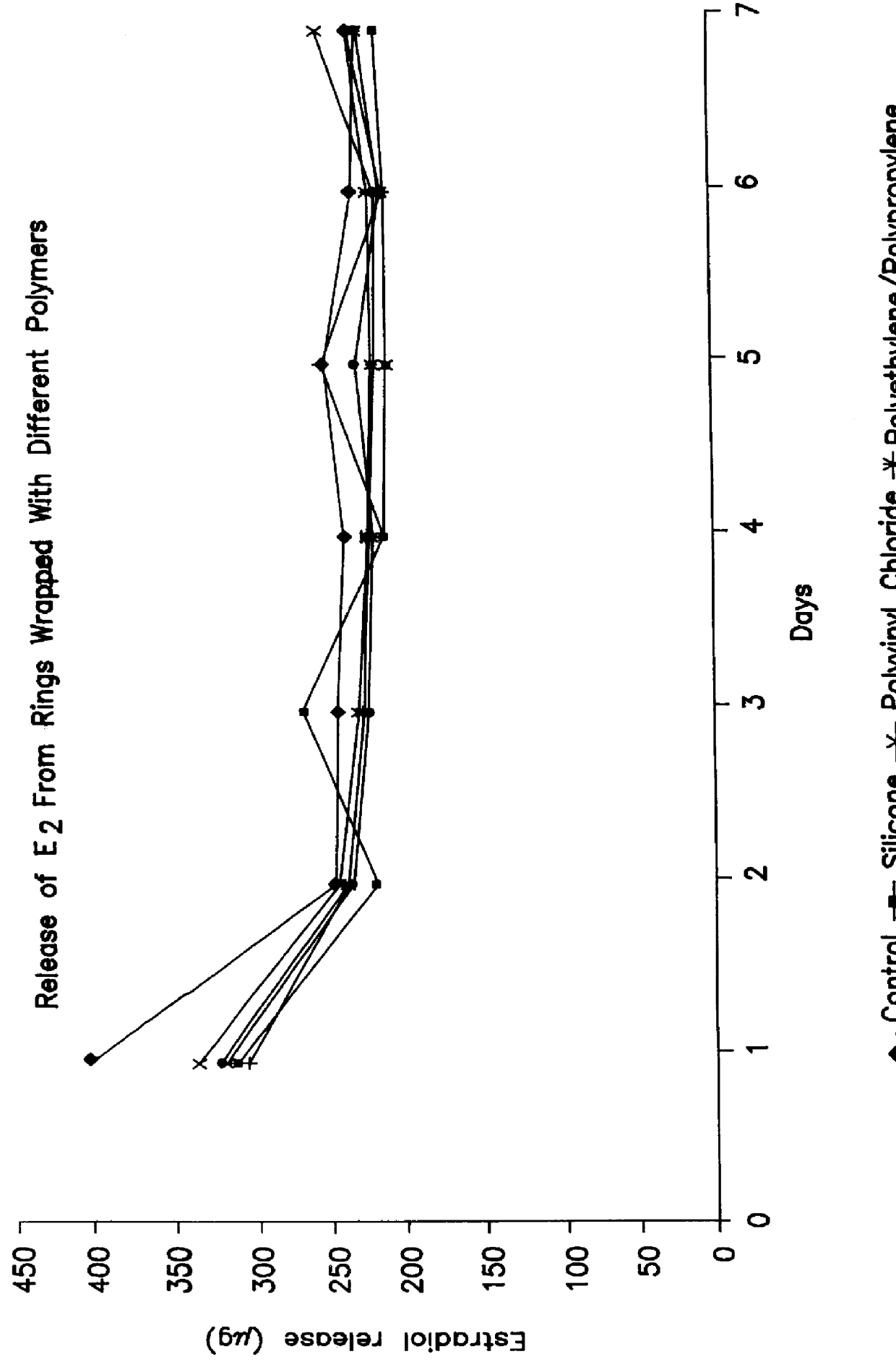
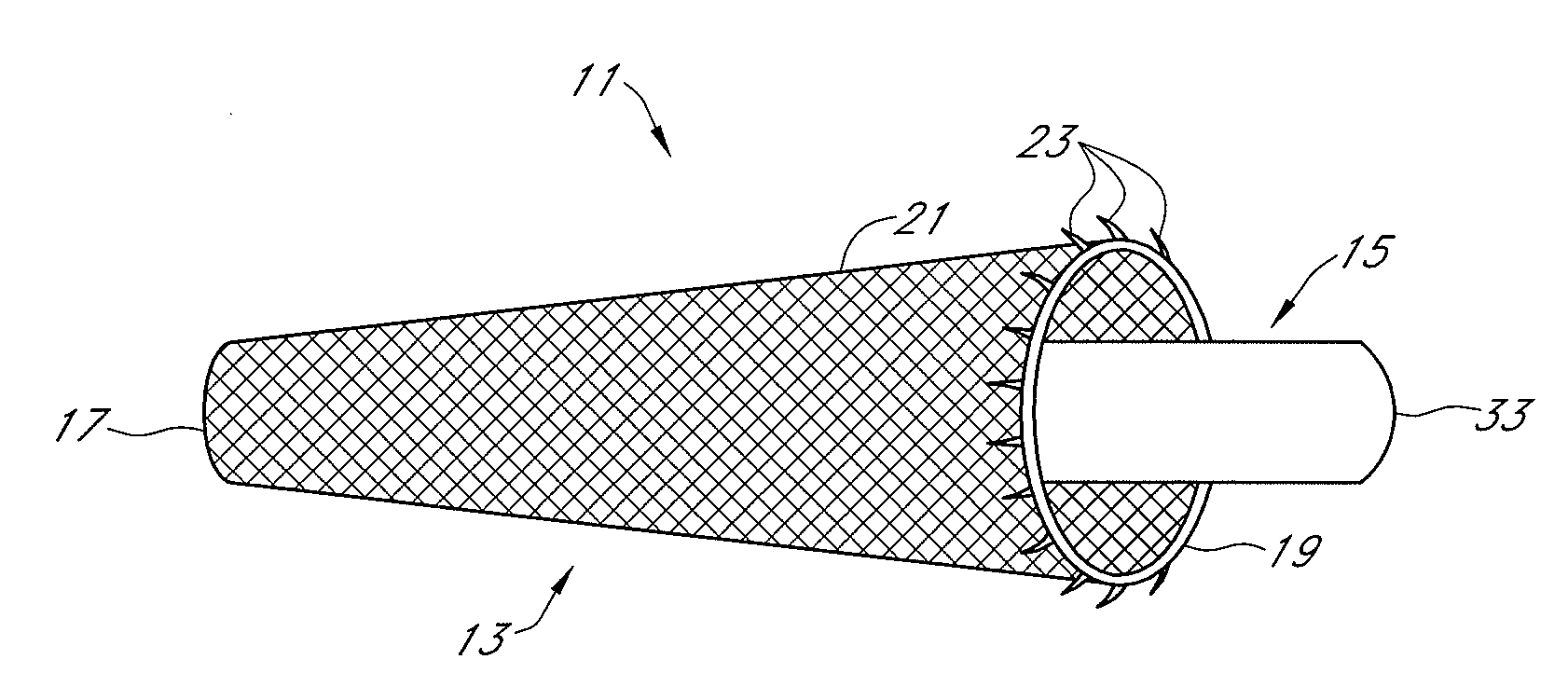
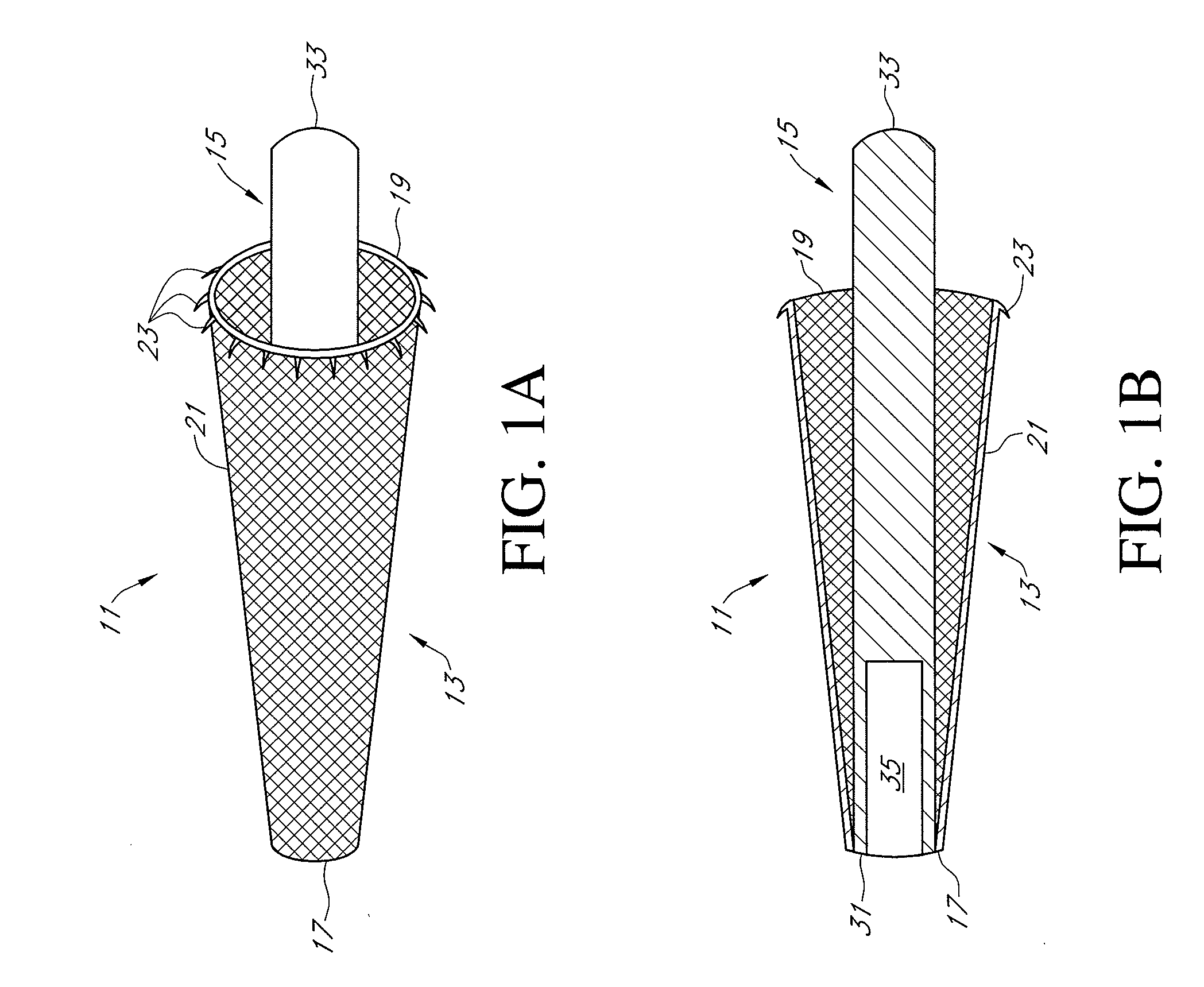
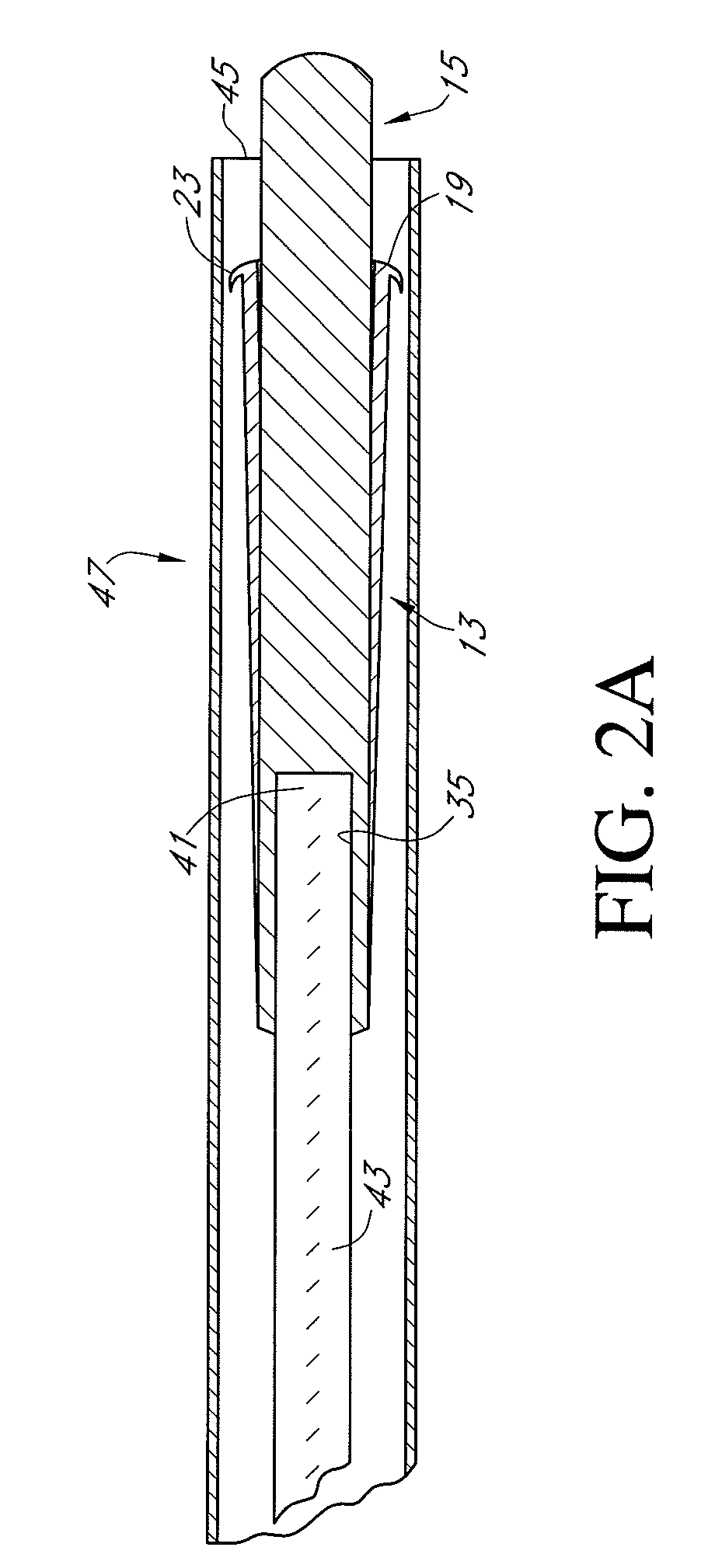
Methods, systems and devices for performing gynecological procedures. According to one embodiment, there is provided a device for occluding a fallopian tube, the device including an outer member and an inner member. The outer member may be a hollow, frusto-conical structure shaped to include an open proximal end, an open distal end, and a side wall. The outer member may be self-expandable such that the distal end is biased radially outwardly. In addition, the side wall may have a porous structure to permit the ingrowth of tissue therethrough. Tines may be provided on the outer surface of the side wall to promote the anchoring of the outer member in a fallopian tube. The inner member, which may be structured to induce scarring, may comprise an elongated fibrous body fixed at its proximal end to the proximal end of the outer member. A bore may extend distally from the proximal end of the inner member to receive a delivery rod. The inner member, which may have pores or interstices to permit the ingrowth of tissue thereinto, is coated or impregnated with a sclerosing agent.
Owner:HOLOGIC INC
Contraceptive system and method of use
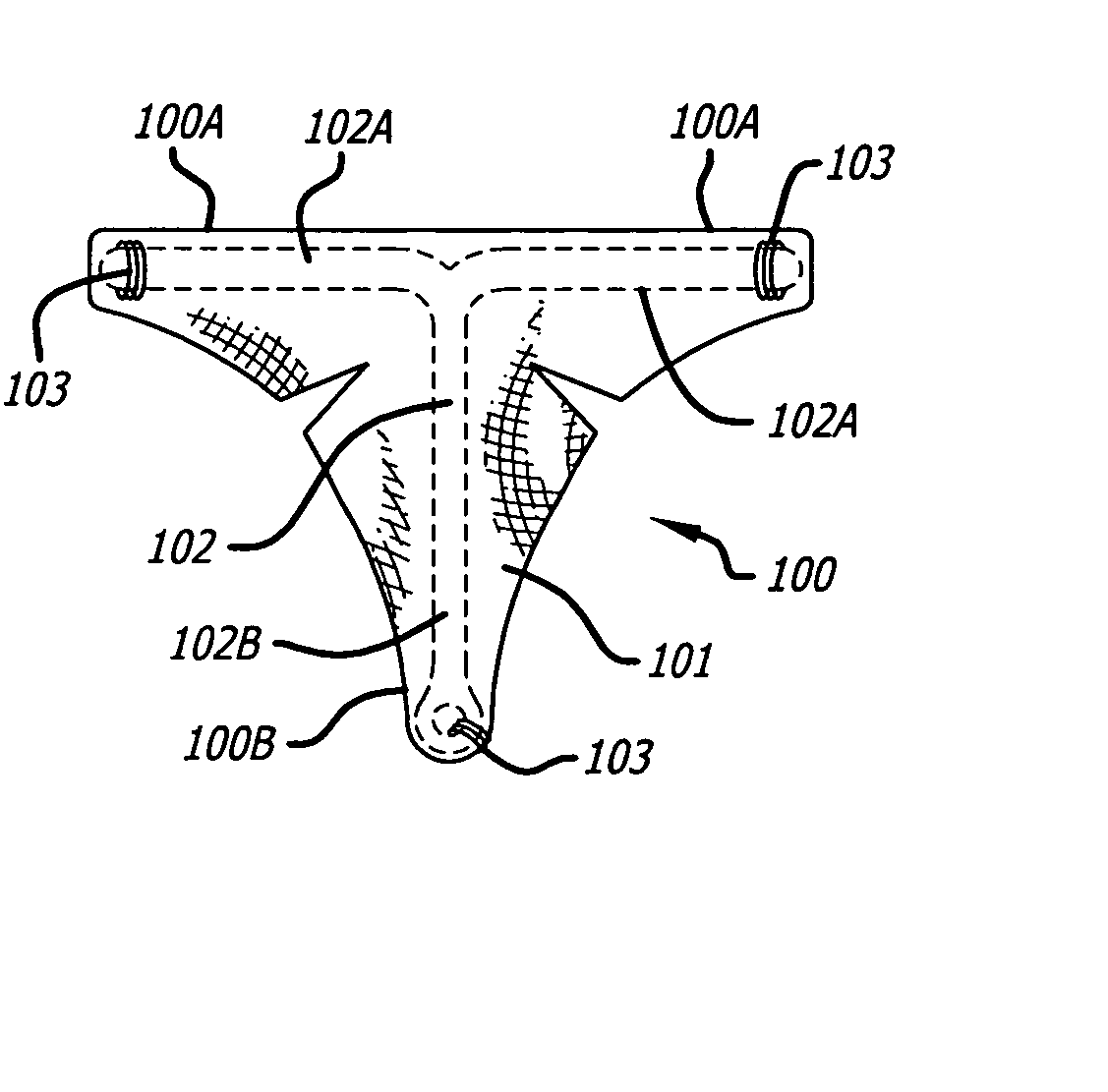
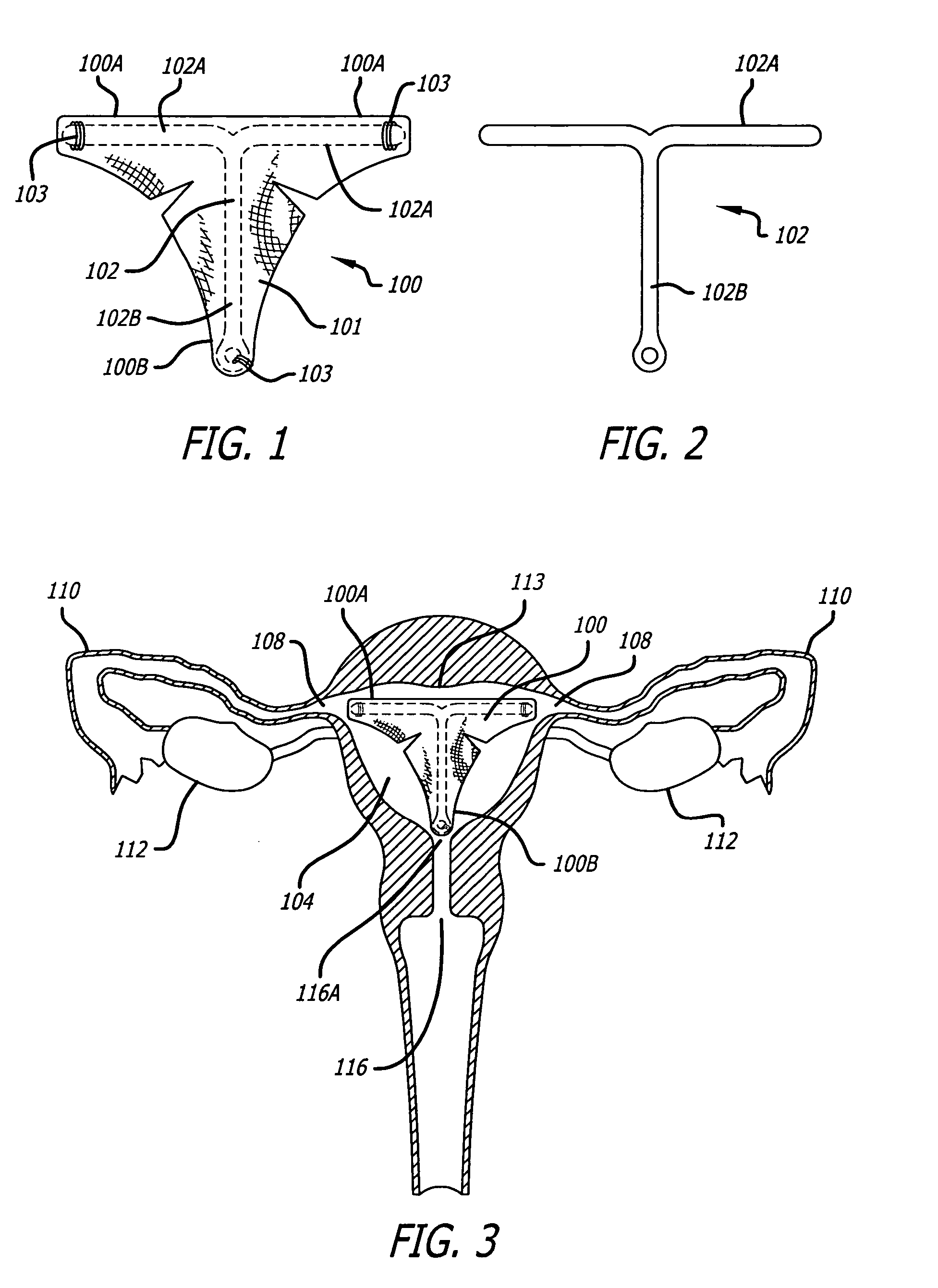
InactiveUS7073504B2Obstruct passageImprove protectionMale contraceptivesFallopian occludersGynecologyBalloon catheter
A device and method of using the device for contraception or sterilization and particularly for reversible contraception by occluding a reproductive lumen to prevent the passage of reproductive cells through the lumen for a desired period of time until the patient wishes to become fertile again and then be reopened. The occluding member preferably comprises a tubular framework formed from a shape memory material configured to be implanted in a reproductive lumen. The occluding member is implanted within a body lumen, secured to the wall of the reproductive lumen and then collapsed to collapse the wall and occlude the lumen. Alternatively, the occluding member may be collapsed upon a solid plug. The closure of the reproductive lumen may be reversed by introducing a balloon catheter and by a series of inflations of the balloon reexpanding the collapsed occluding member or by removing the plug. The occluding member and the plug may be configured to facilitate endothelialization, to provoke an inflammatory responses or to deliver a drug.
Owner:BAYER HEALTHCARE LLC
Enhancing tissue ingrowth for contraception
InactiveUS20050209633A1Facilitate tissue ingrowthAvoid easy placementFallopian occludersFemale contraceptivesMetallic materialsElectric energy
This invention is directed to occluding devices and methods of using such devices for occluding a patient's body lumen, such as a reproductive lumen for contraceptive purposes. The occluding device generally has an occluding component, a first metallic element associated with the occluding component, a second metallic element associated with the occluding component. The first and second metallic elements are configured to generate electrical activity which enhances tissue growth into and / or onto the occluding component to aid in lumen occlusion. In one embodiment the first and second metallic elements are formed of different metallic materials and generate galvanic activity. In a second embodiment electrical power is applies to the first and second metallic elements to generate electrical activity that enhances tissue growth.
Owner:BAYER HEALTHCARE LLC
Minimally invasive surgical stabilization devices and methods
The various embodiments of the present inventions provide stabilization devices and methods for use of the stabilization devices with minimally invasive gynecological procedures such as methods of preventing pregnancy by inserting intrafallopian contraceptive devices into the fallopian tubes
Owner:BAYER HEALTHCARE LLC
Devices and methods for securing tissue
A compression ring to grip and compress body structure such as diverticulum, hemorrhoids, and tissue adjacent a hole. A resilient ring-shaped body defines a compression channel, and one or more axially rigid elongated spikes extend from the body into the channel. The body defines a first axial segment surrounding the compression channel and a second axial segment surrounding the compression channel, with the spike being engaged only with the second axial segment. The first axial segment more tightly compresses the body structure than the second axial segment.
Owner:TENNESSEE MEDICAL INNOVATIONS INC
Medical devices and methods of making and using such devices
InactiveUS20050274384A1Preventing conceptionFallopian occludersInfusion syringesMedical deviceBiomedical engineering
Devices, such as medical devices for inhibiting conception, and methods of using and / or making these devices. In one aspect of the disclosure, a medical device has a delivery system and a first insert, which is removably coupled to the delivery system and which is designed to be deployed within a portion of a first fallopian tube, and a second insert, which is removably coupled to the delivery system and which is designed to be deployed within a portion of a second fallopian tube. Other aspects of the disclosure include, among other things, inserts made from one or more polymers; inserts which are designed to pierce and remain in place; and inserts which are implanted through a fluid delivery system.
Owner:BAYER HEALTHCARE LLC
Biological Vessel Flow Control Devices and Methods
A medical device including a body portion configured and dimensioned to be associated with a vessel of a patient and a responsive component associated with the body portion wherein the responsive component is switchable between a first configuration and a second configuration.
Owner:LIQUIDIA TECH
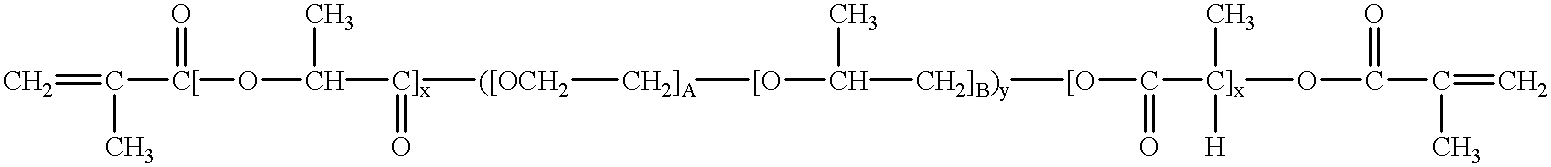
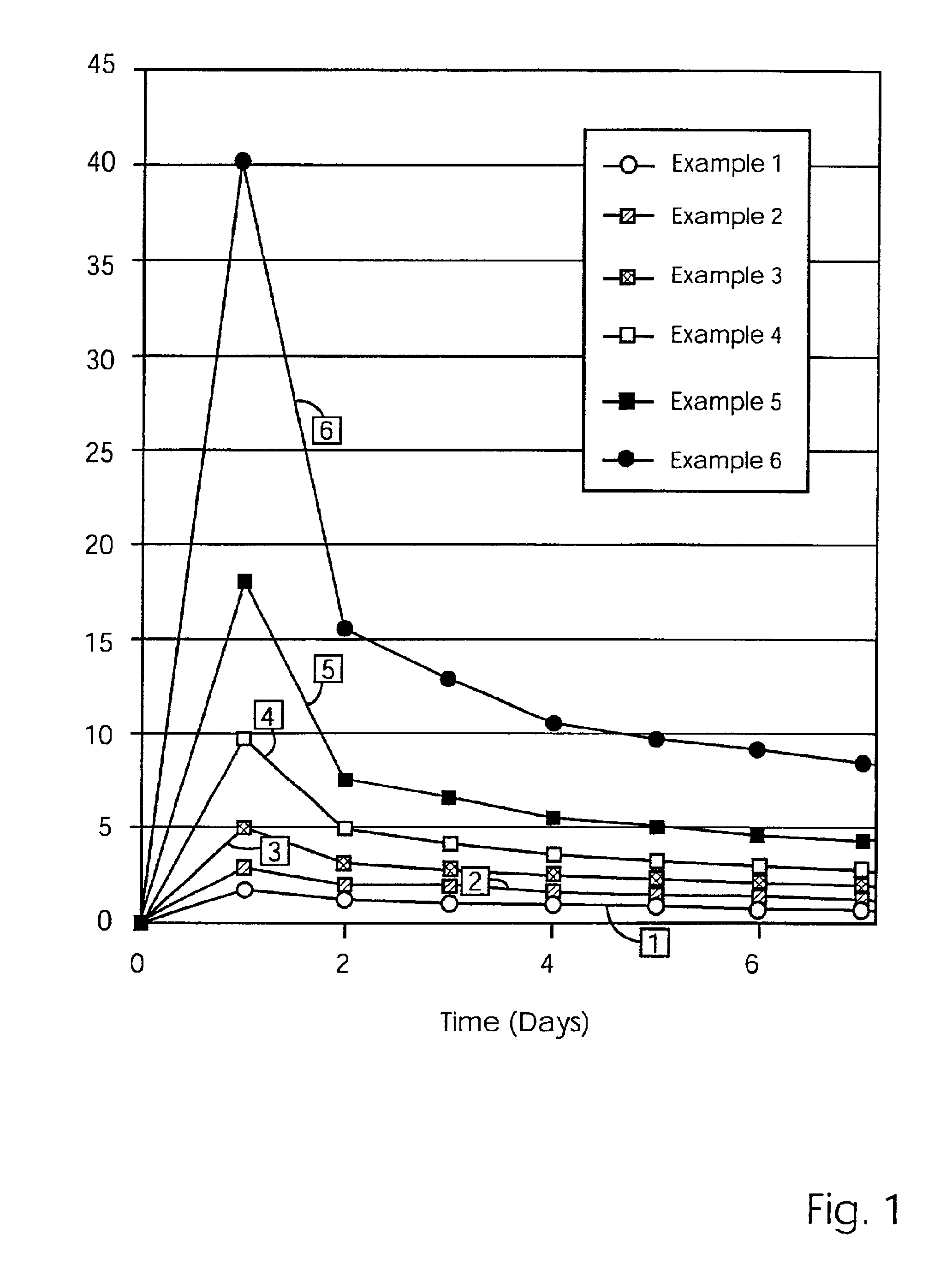
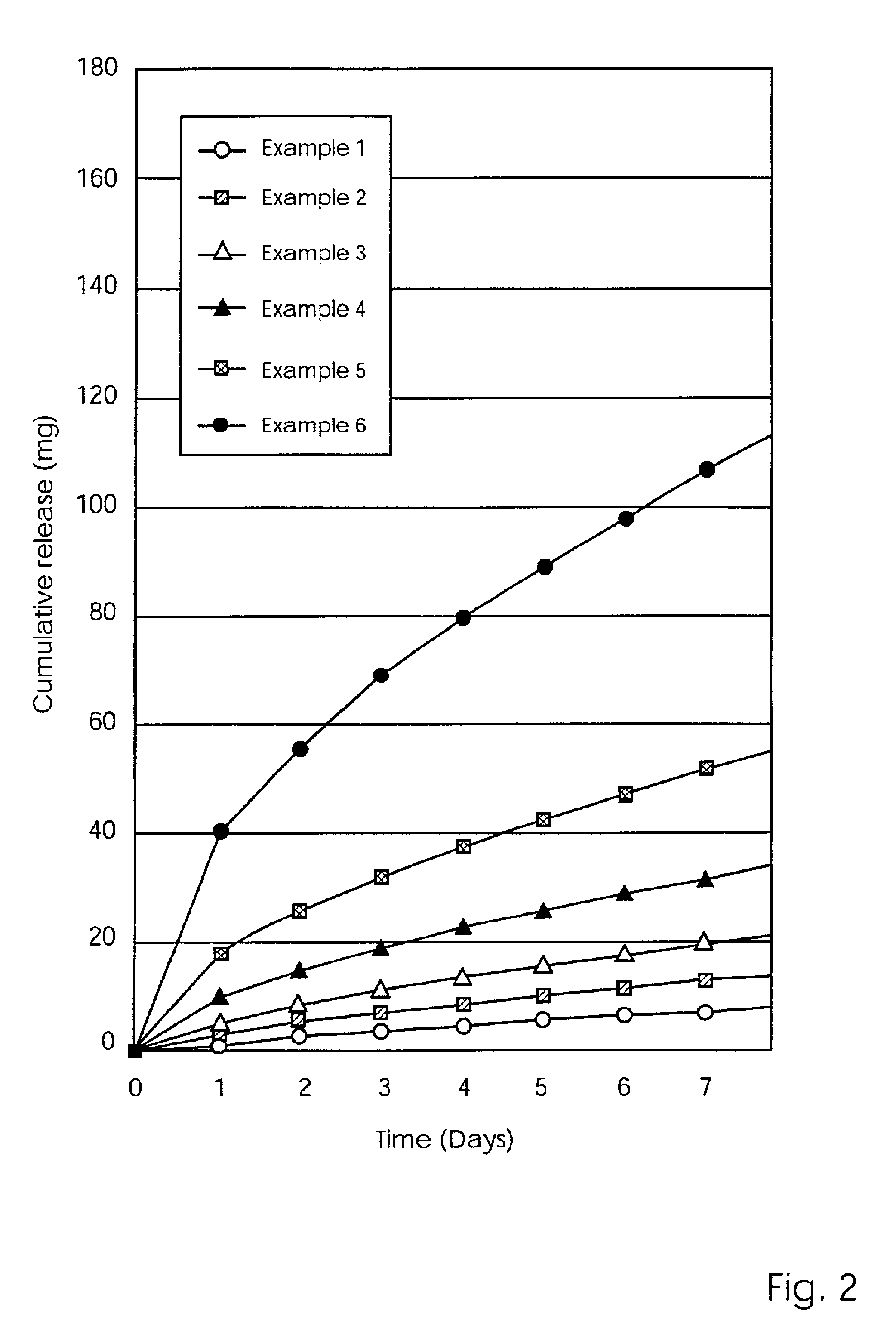
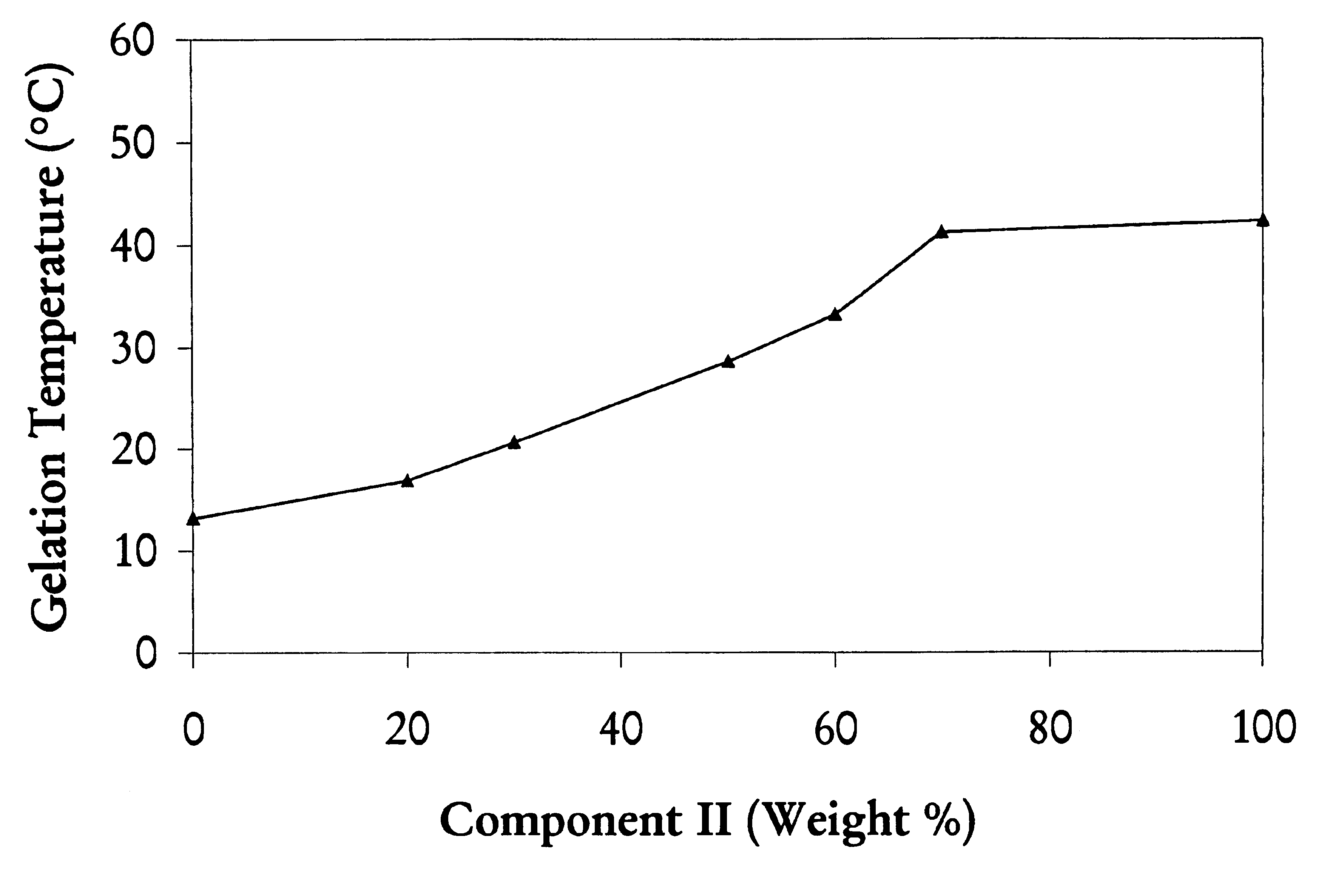
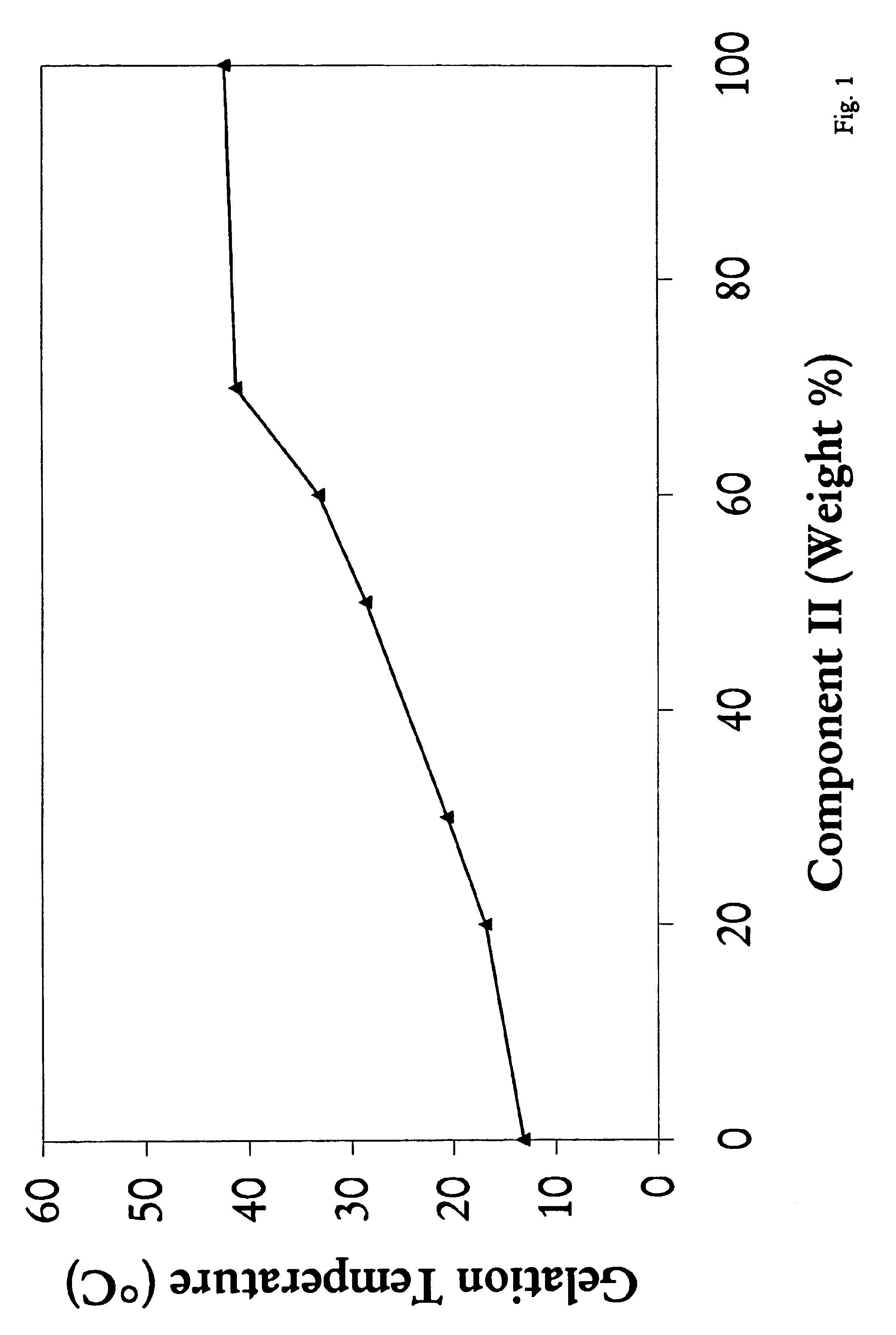
Mixtures of various triblock polyester polyethylene glycol copolymers having improved gel properties
InactiveUS7018645B1Good curative effectMinimize side effectsPowder deliveryPeptide/protein ingredientsPolymer scienceErosion rate
A water soluble, biodegradable reverse thermal gelation system comprising a mixture of at least two types of tri-block copolymer components is disclosed. The tri-block copolymer components are made of a hydrophobic biodegradable polyester A-polymer block and a hydrophilic polyethylene glycol B-polymer block. The drug release and gel matrix erosion rates of the biodegradable reverse thermal gelation system may be modulated by various parameters such as the hydrophobic / hydrophilic component contents, polymer block concentrations, molecular weights and gelation temperatures, and weight ratios of the tri-block copolymer components in the mixture.
Owner:BTG INT LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com