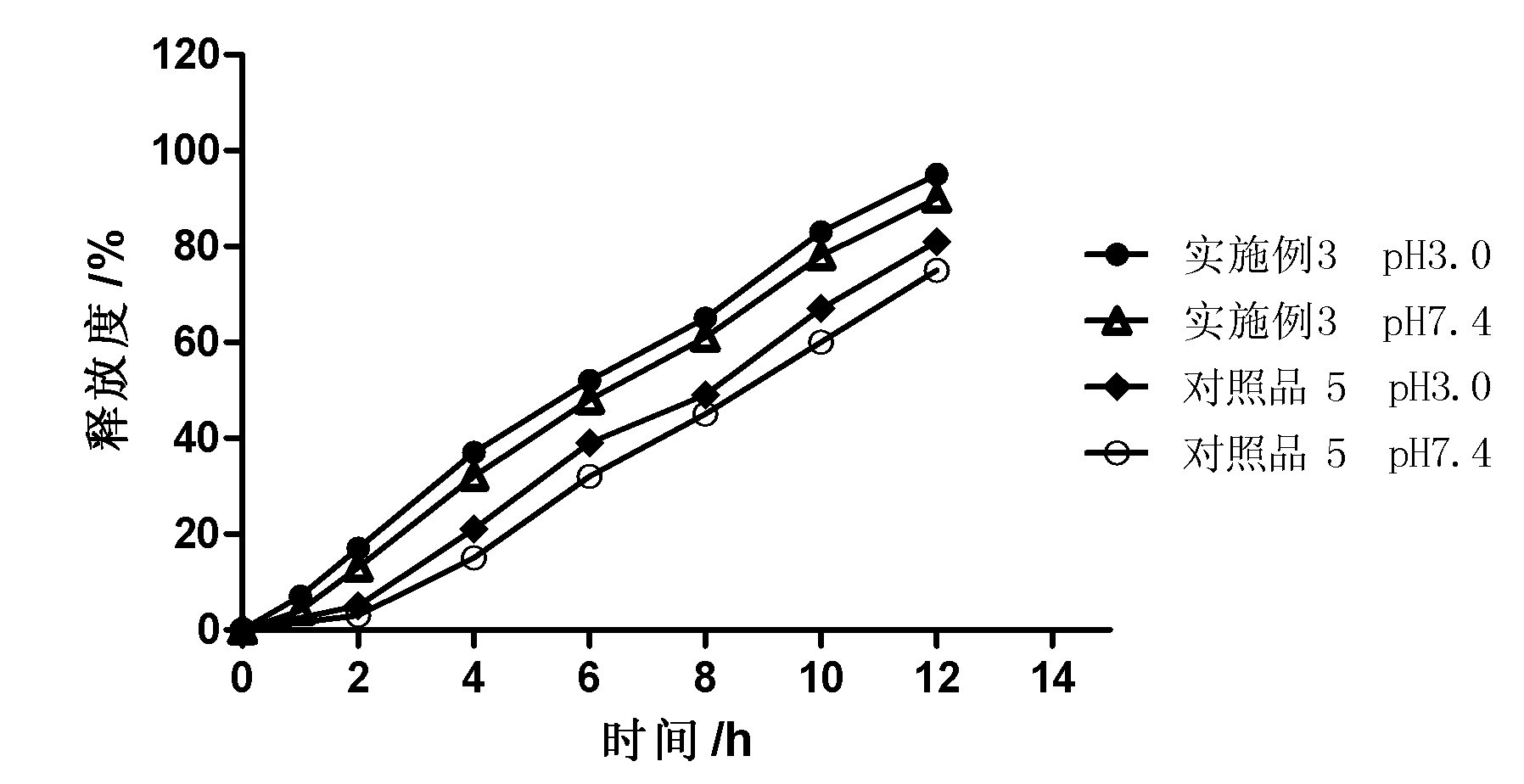
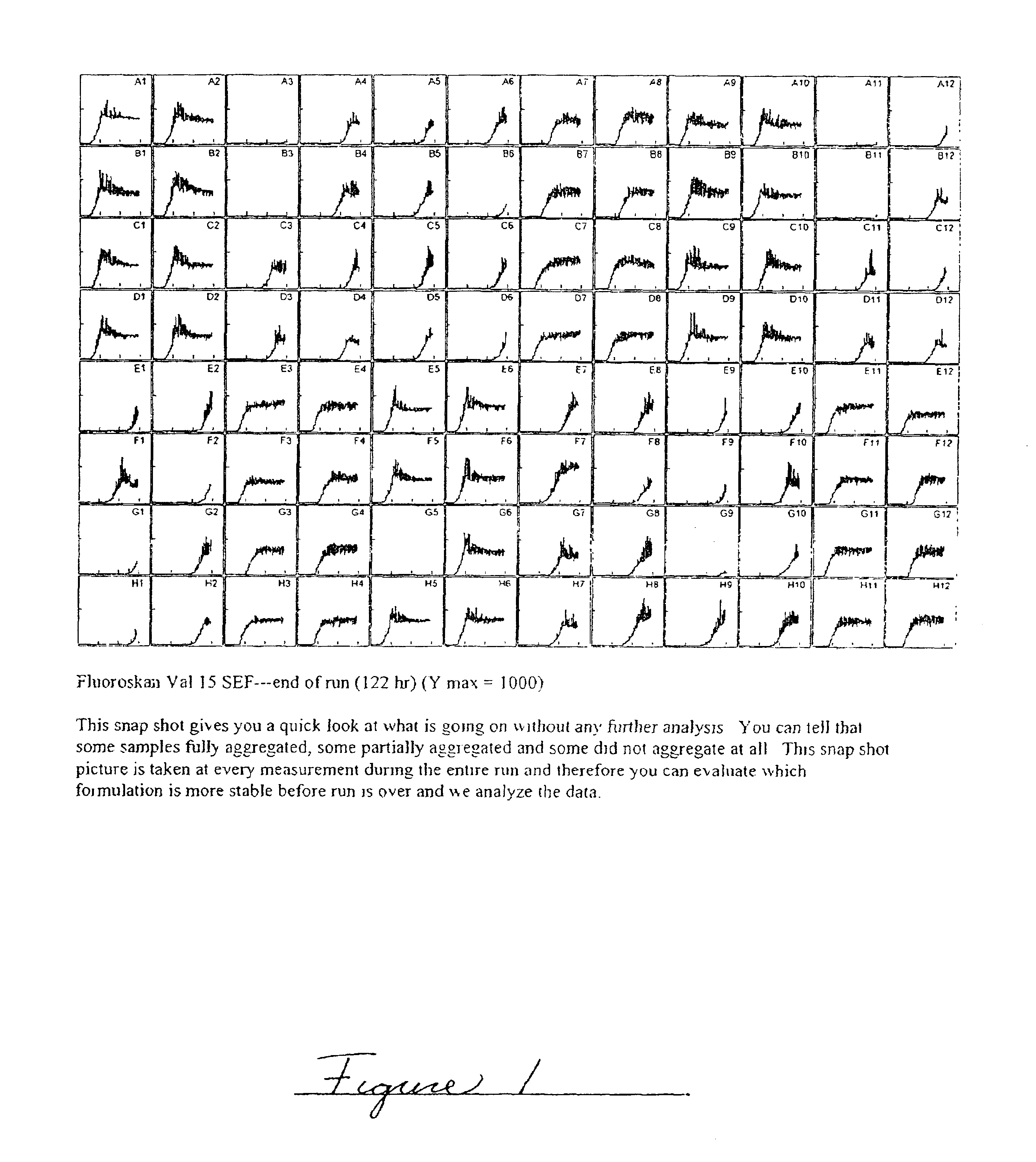
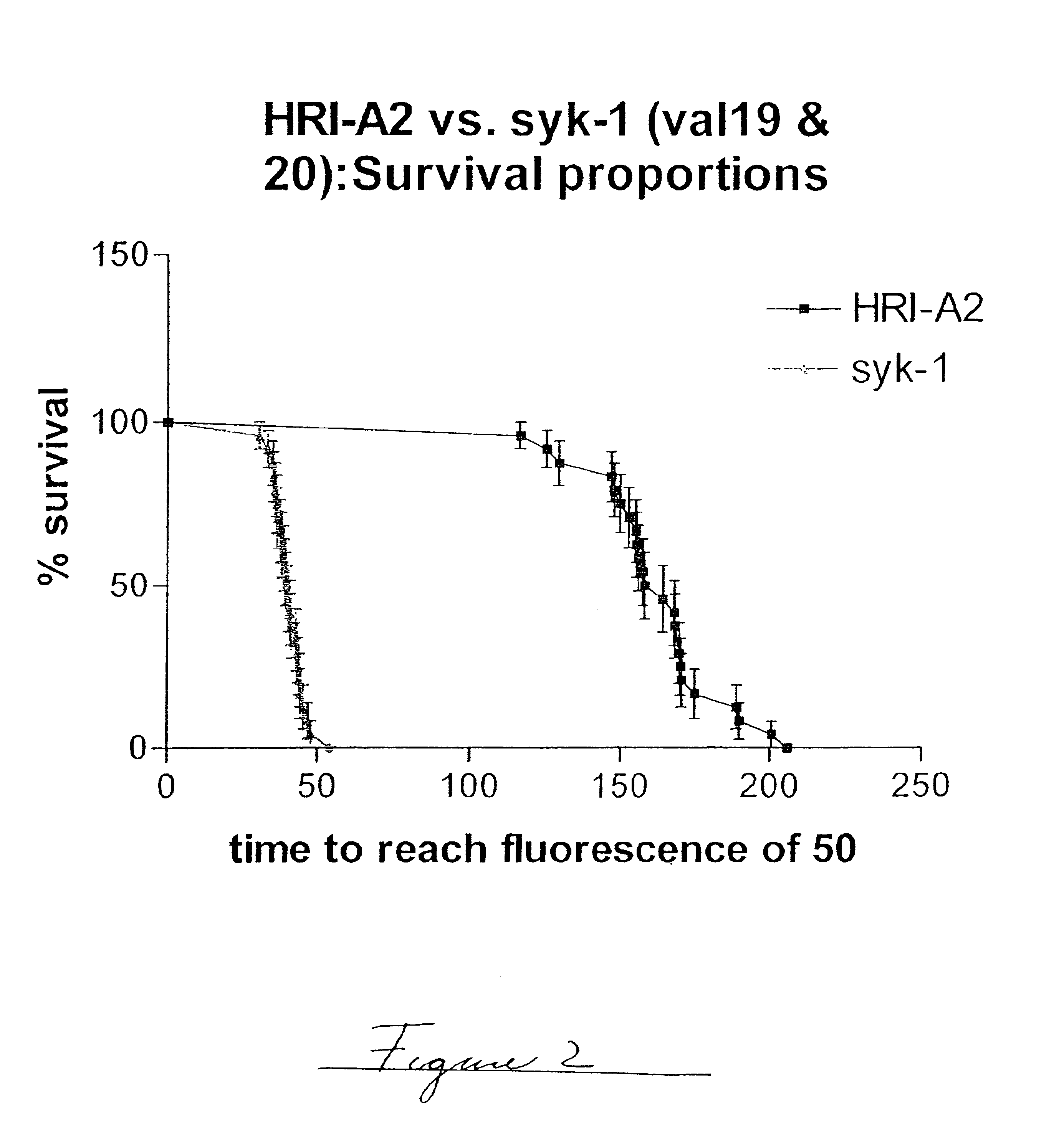
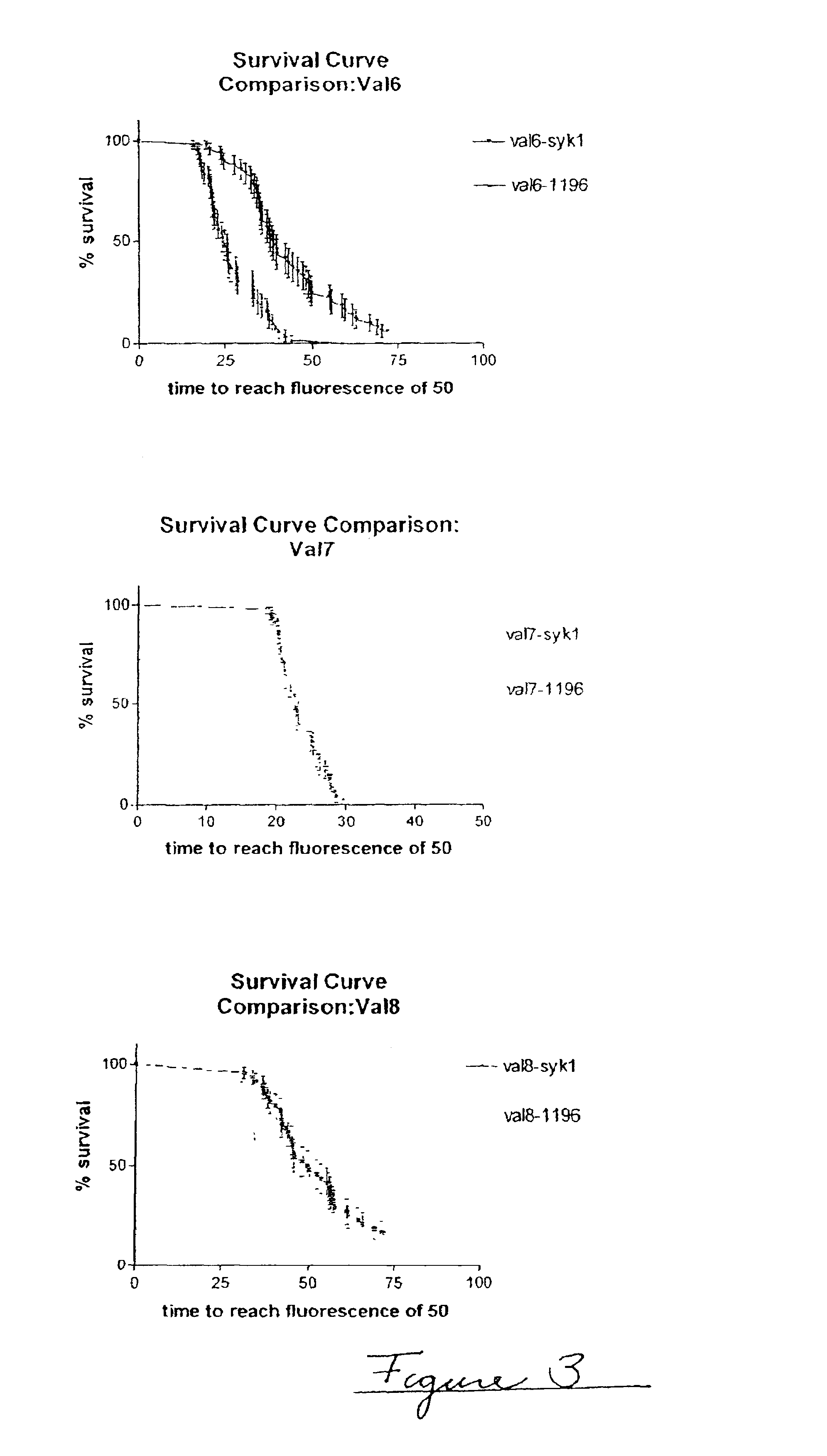
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
13947results about "Inorganic non-active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
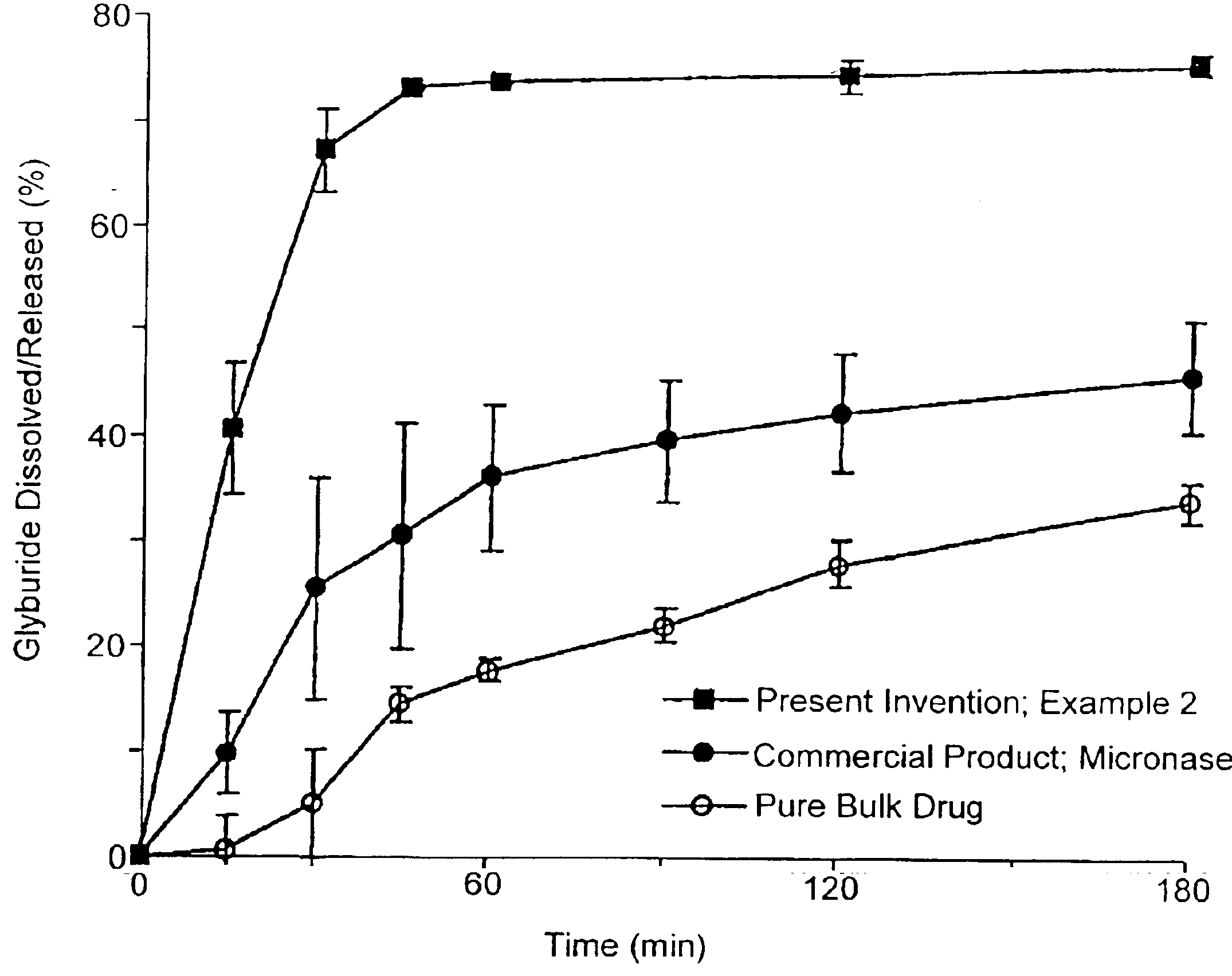
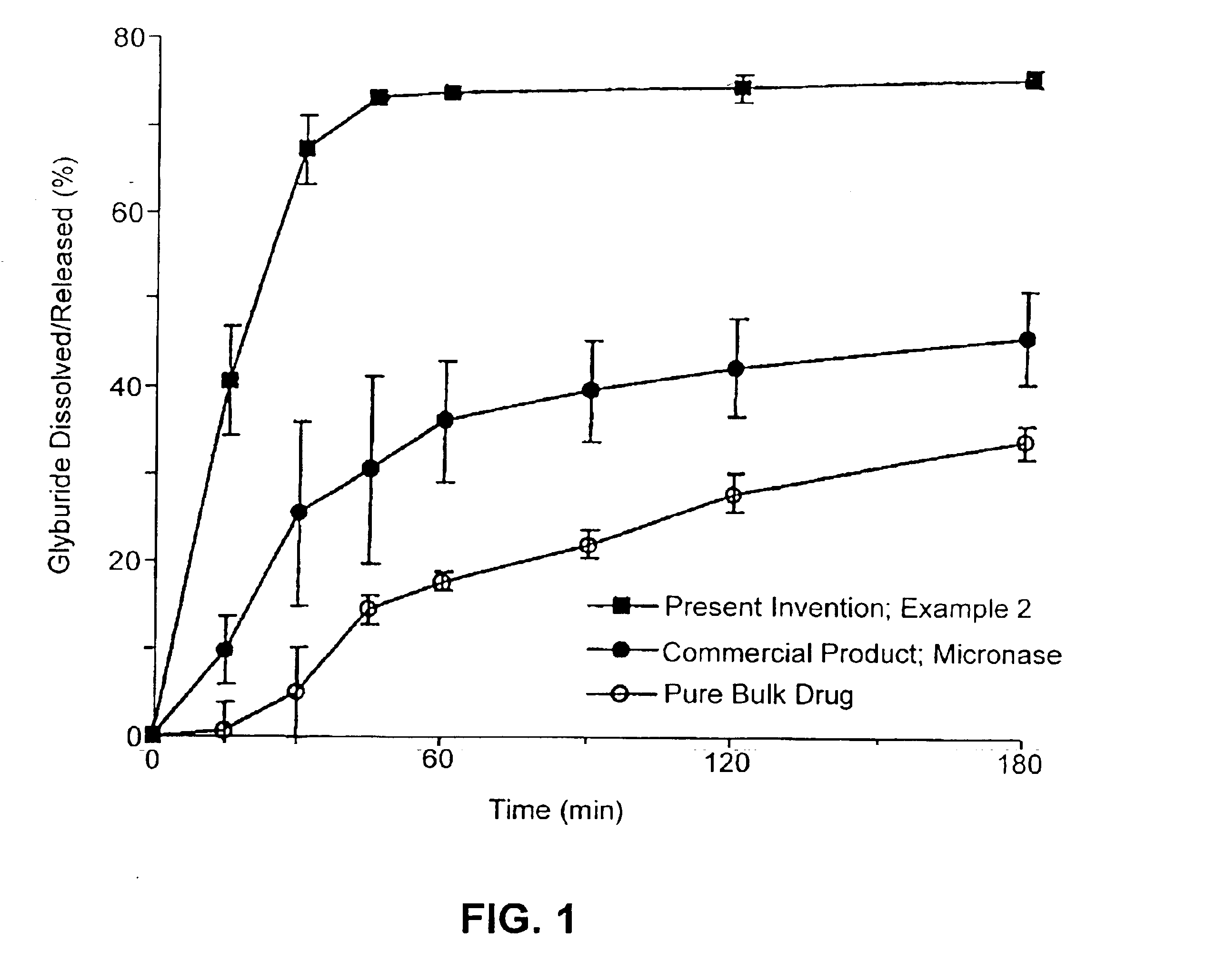
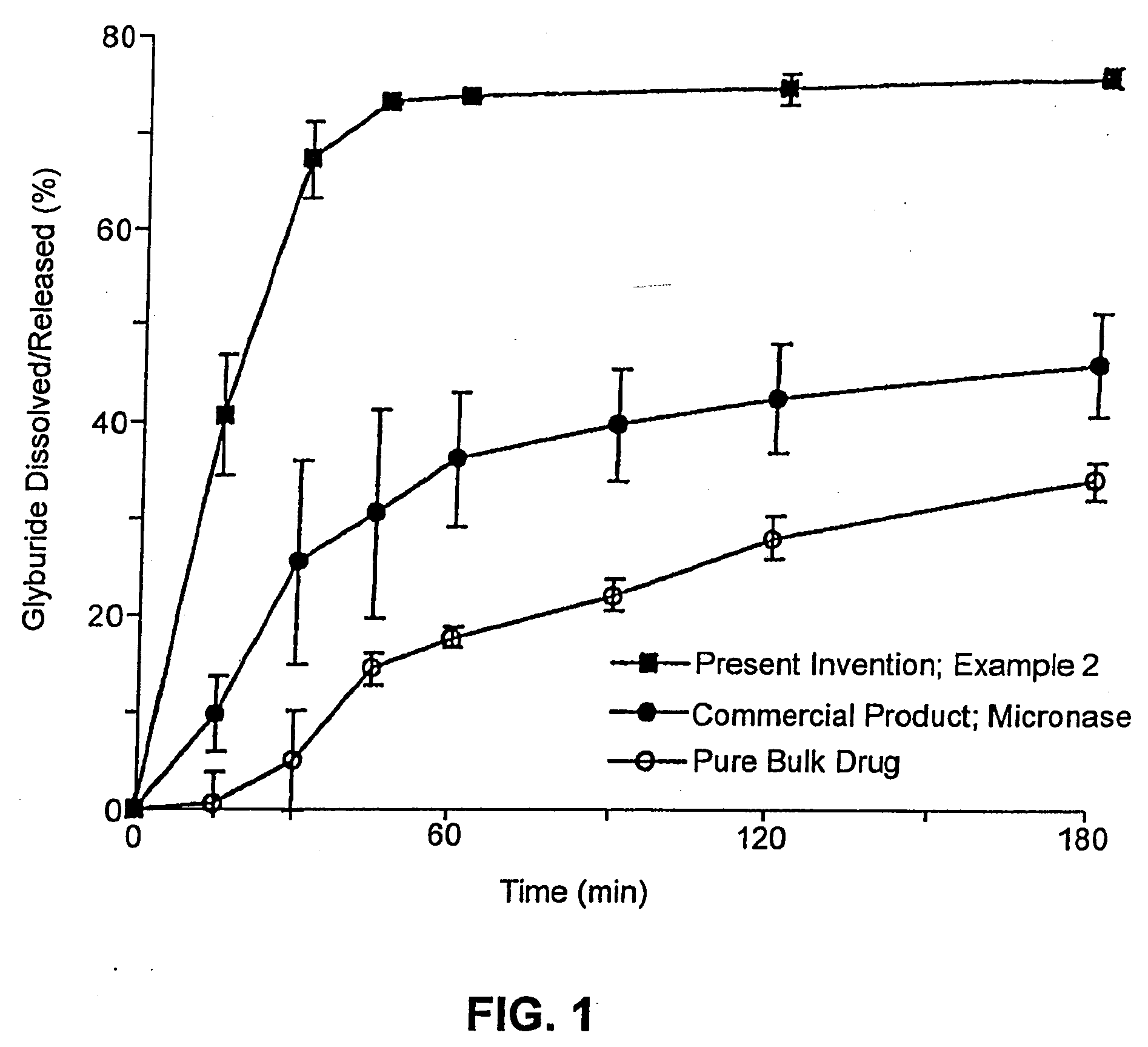
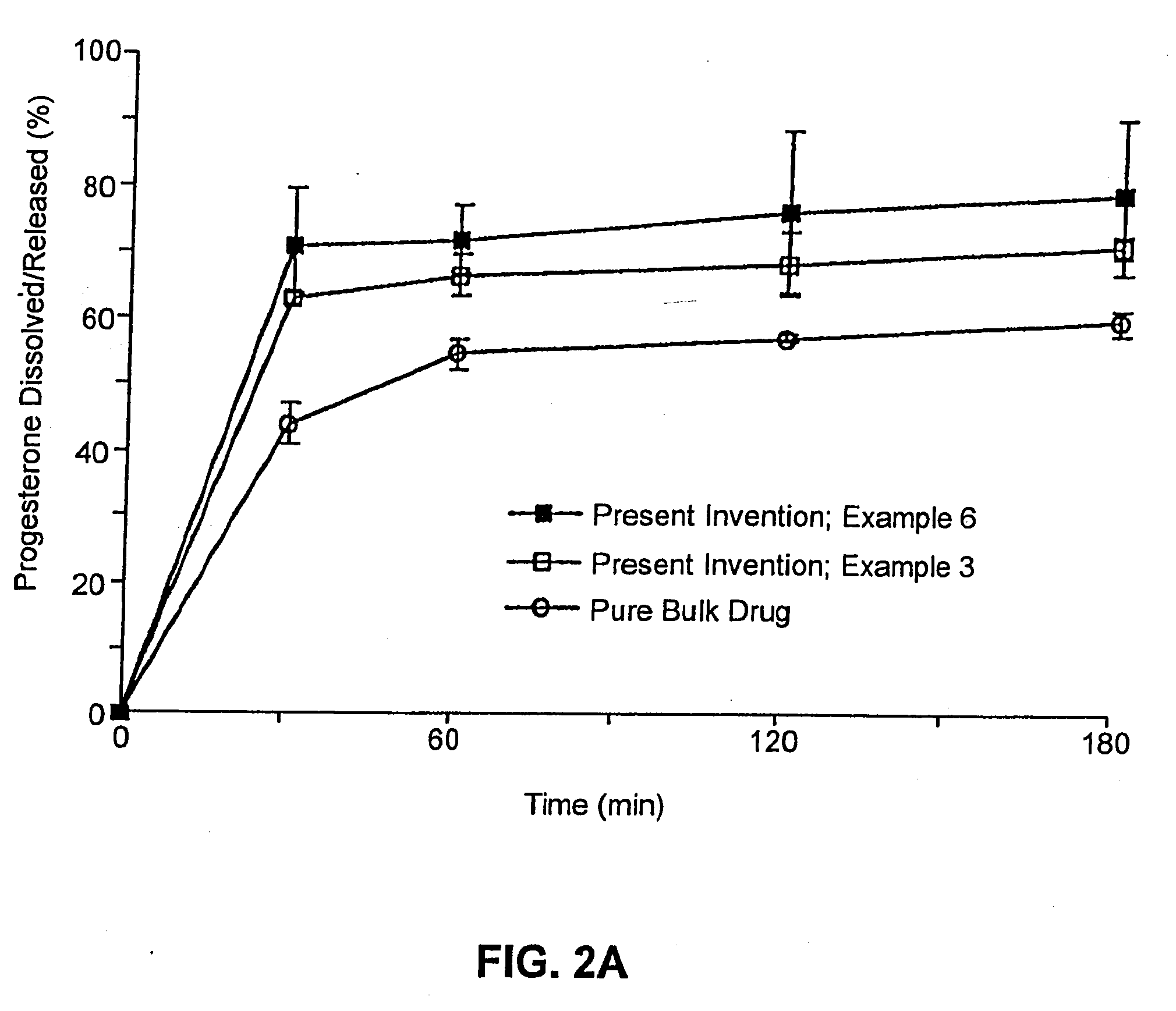
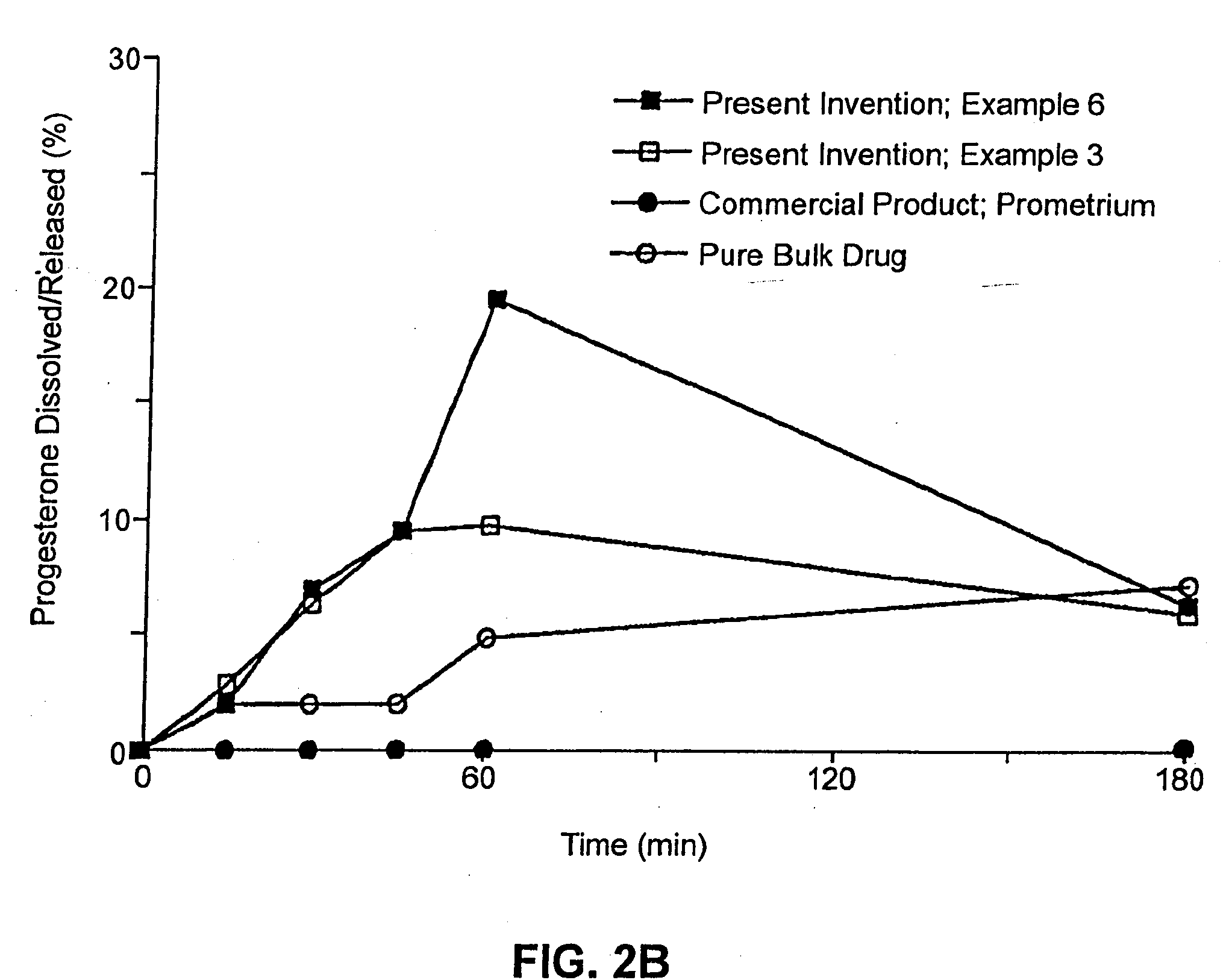
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
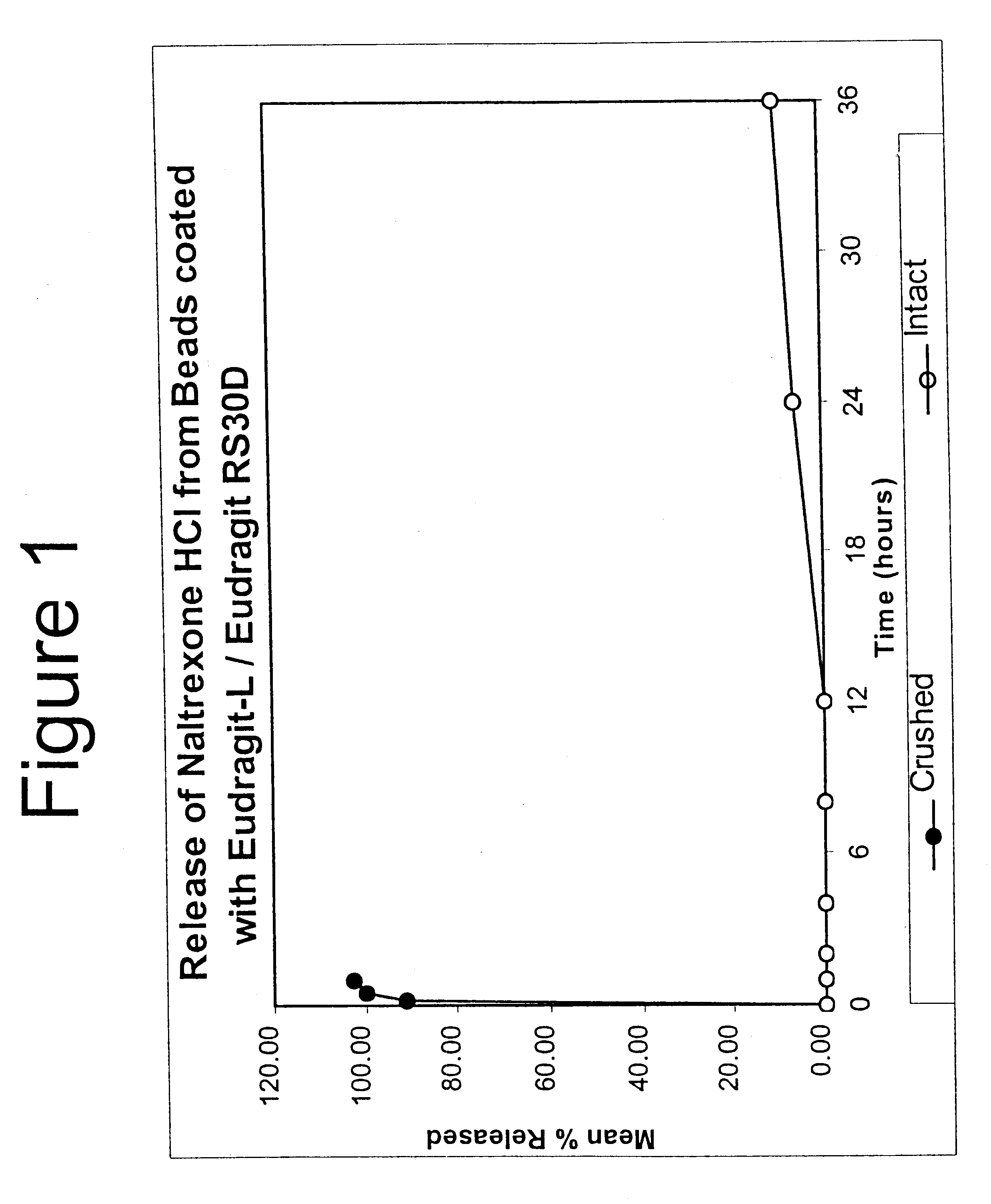
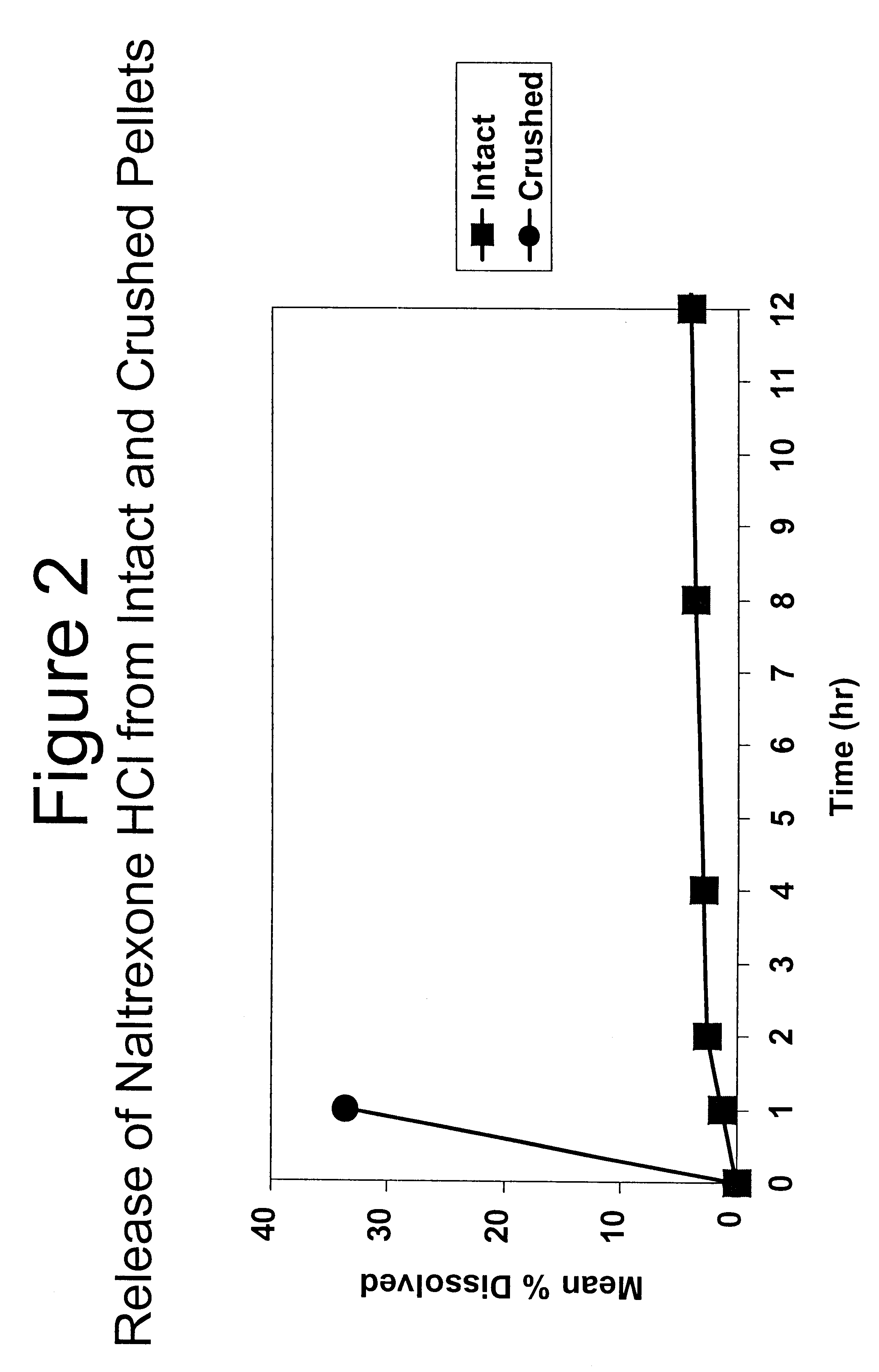
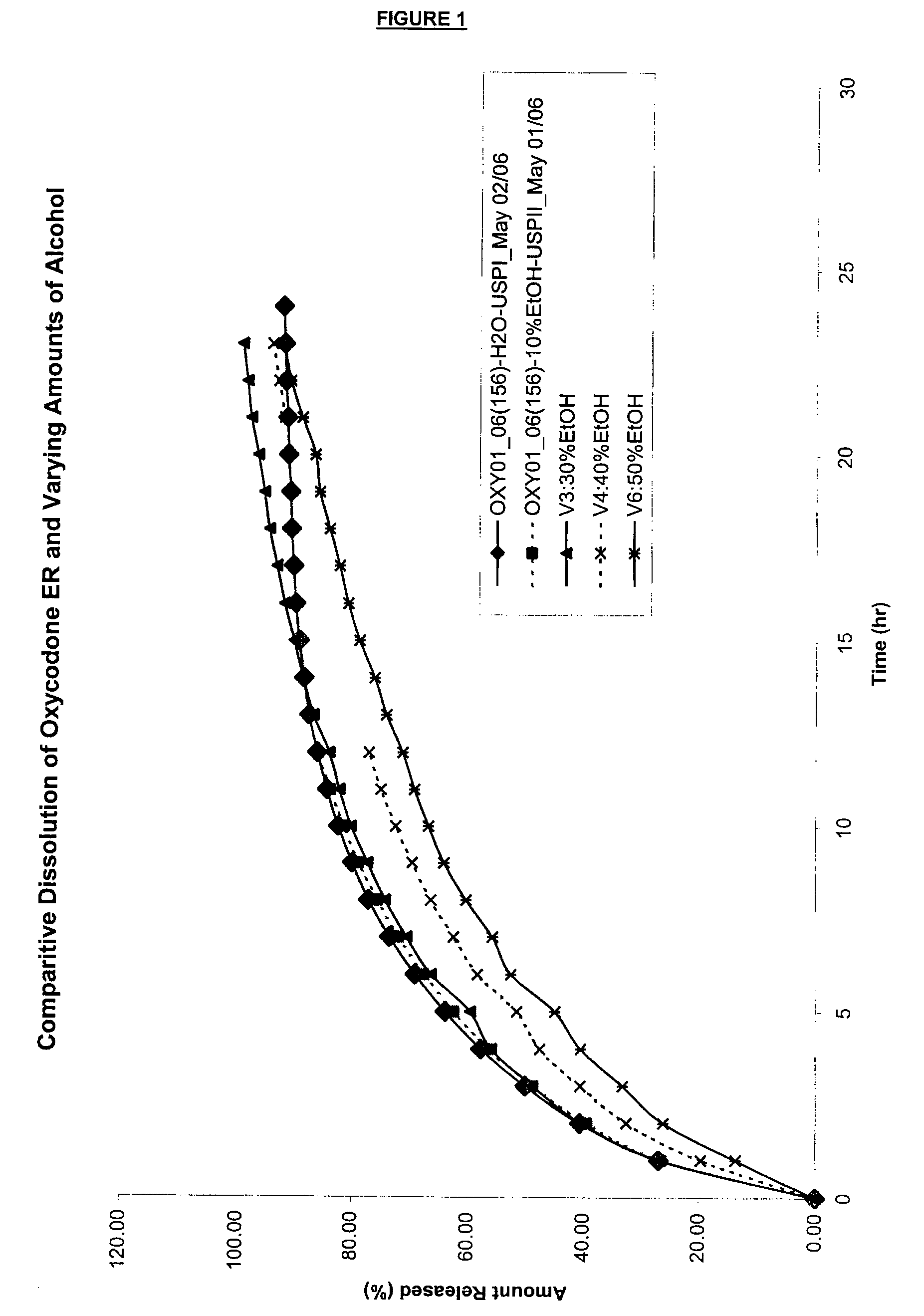
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
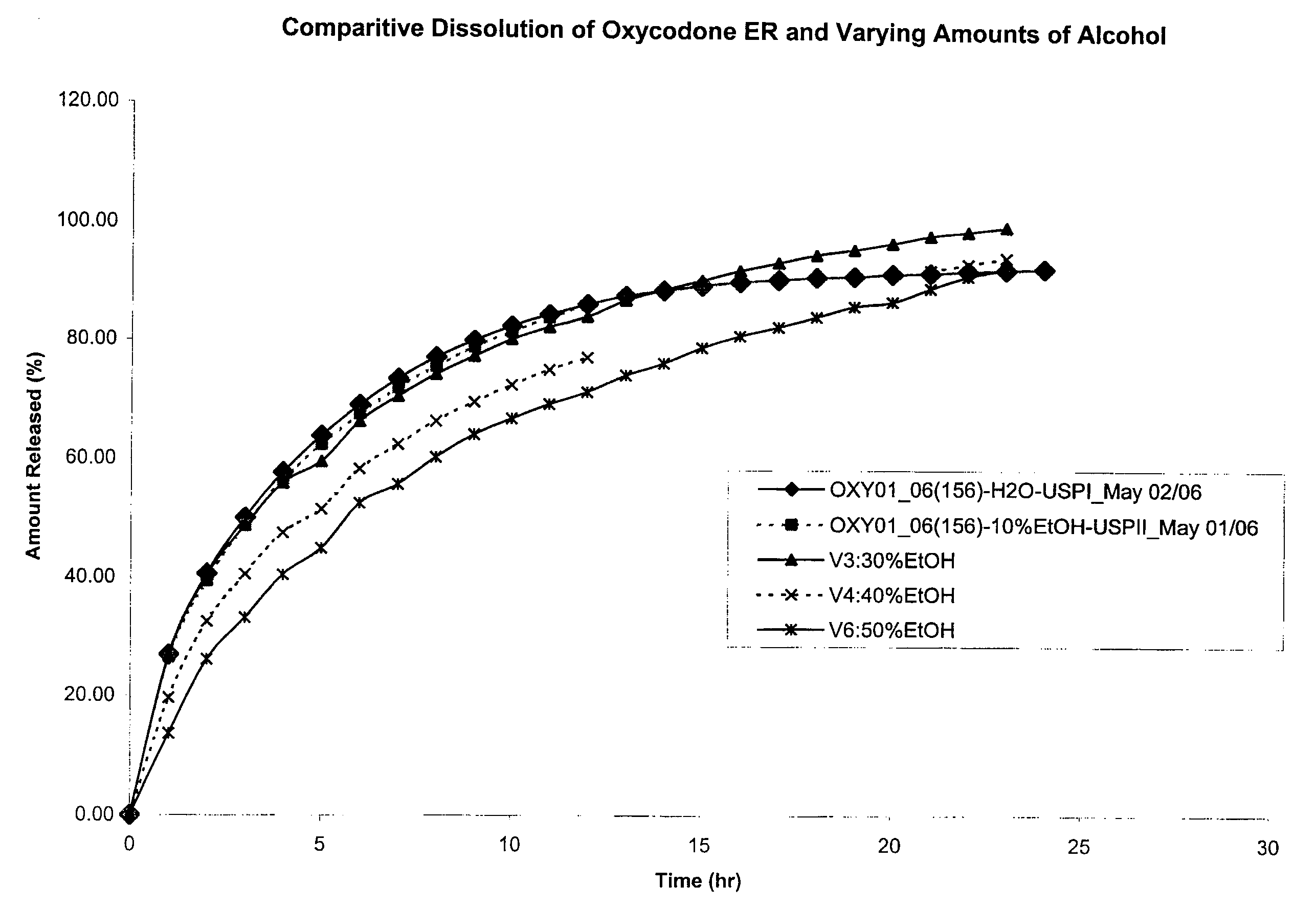
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
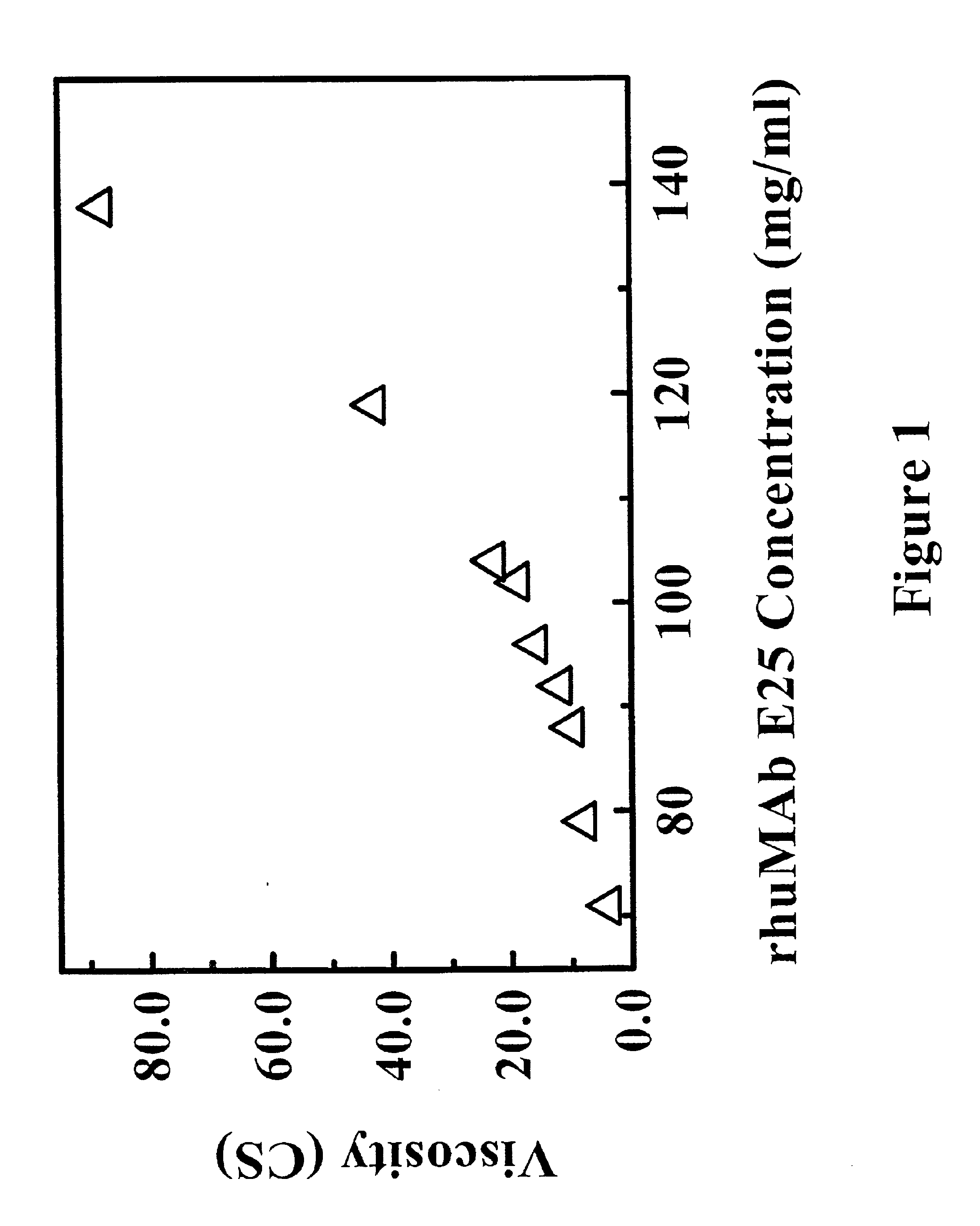
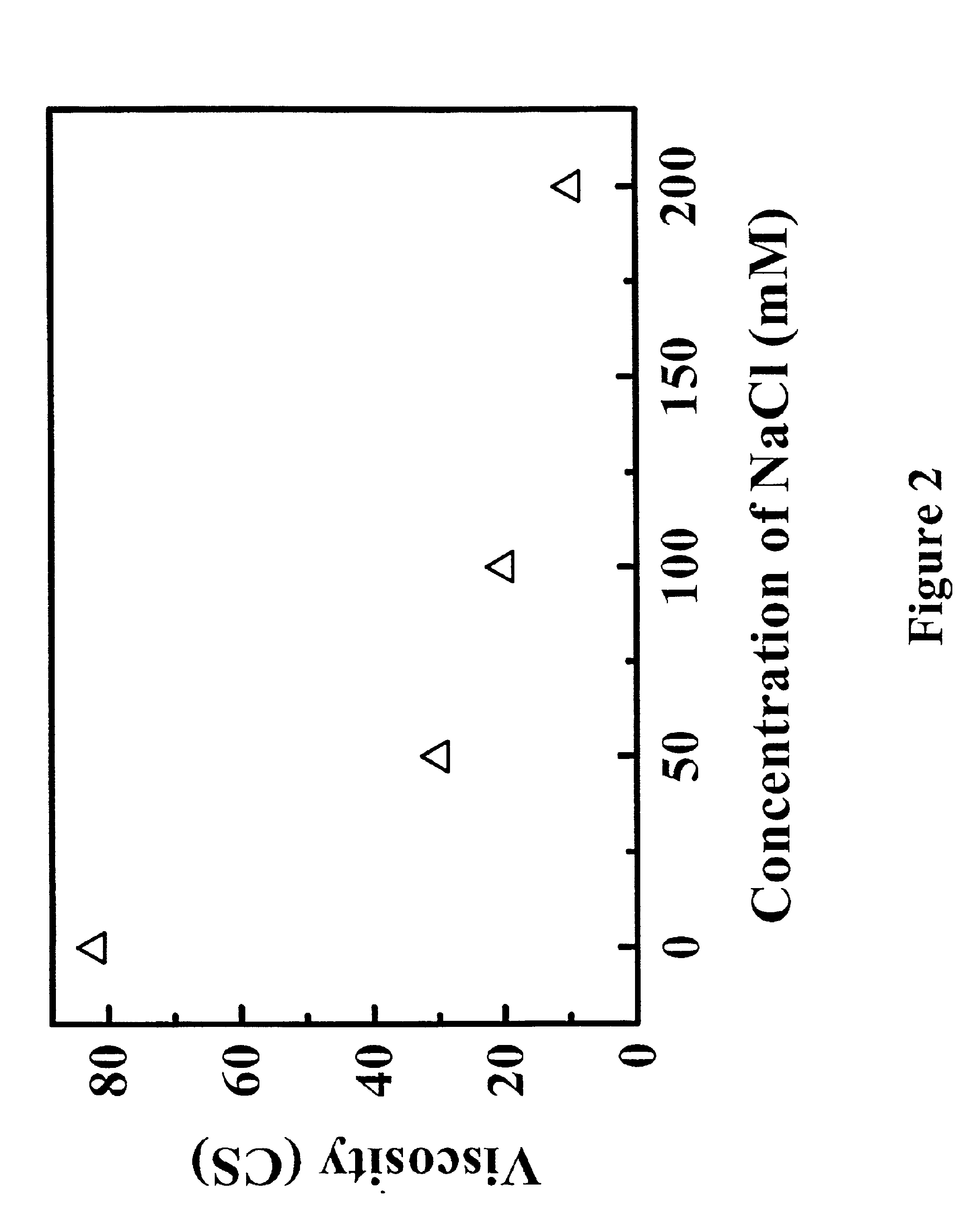
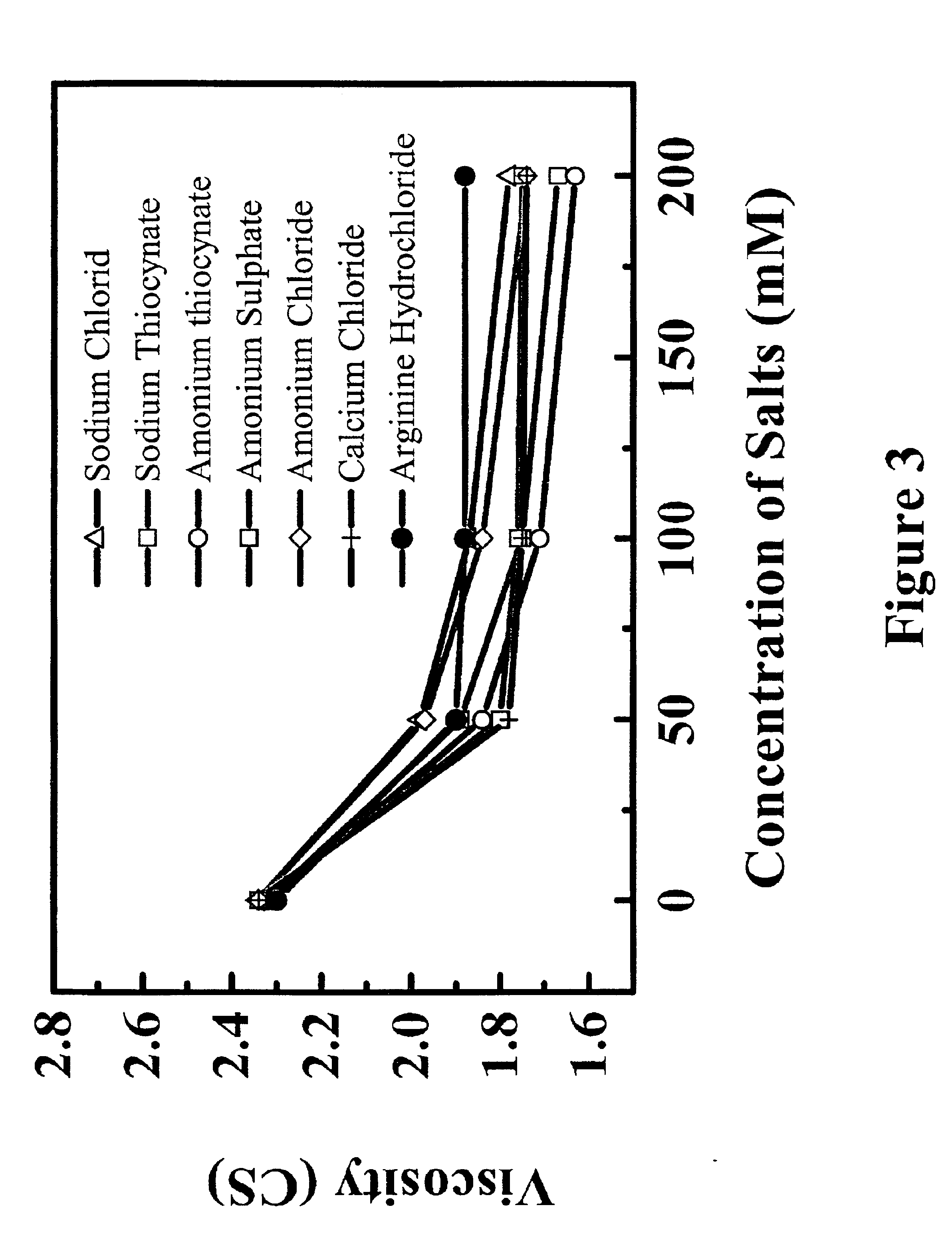
Reduced-viscosity concentrated protein formulations
InactiveUS6875432B2Low viscosityCompromising stability and biological activityPeptide/protein ingredientsInorganic non-active ingredientsConcentration proteinChemistry
The present application concerns concentrated protein formulations with reduced viscosity, which are particularly suitable for subcutaneous administration. The application further concerns a method for reducing the viscosity of concentrated protein formulations.
Owner:GENENTECH INC +1
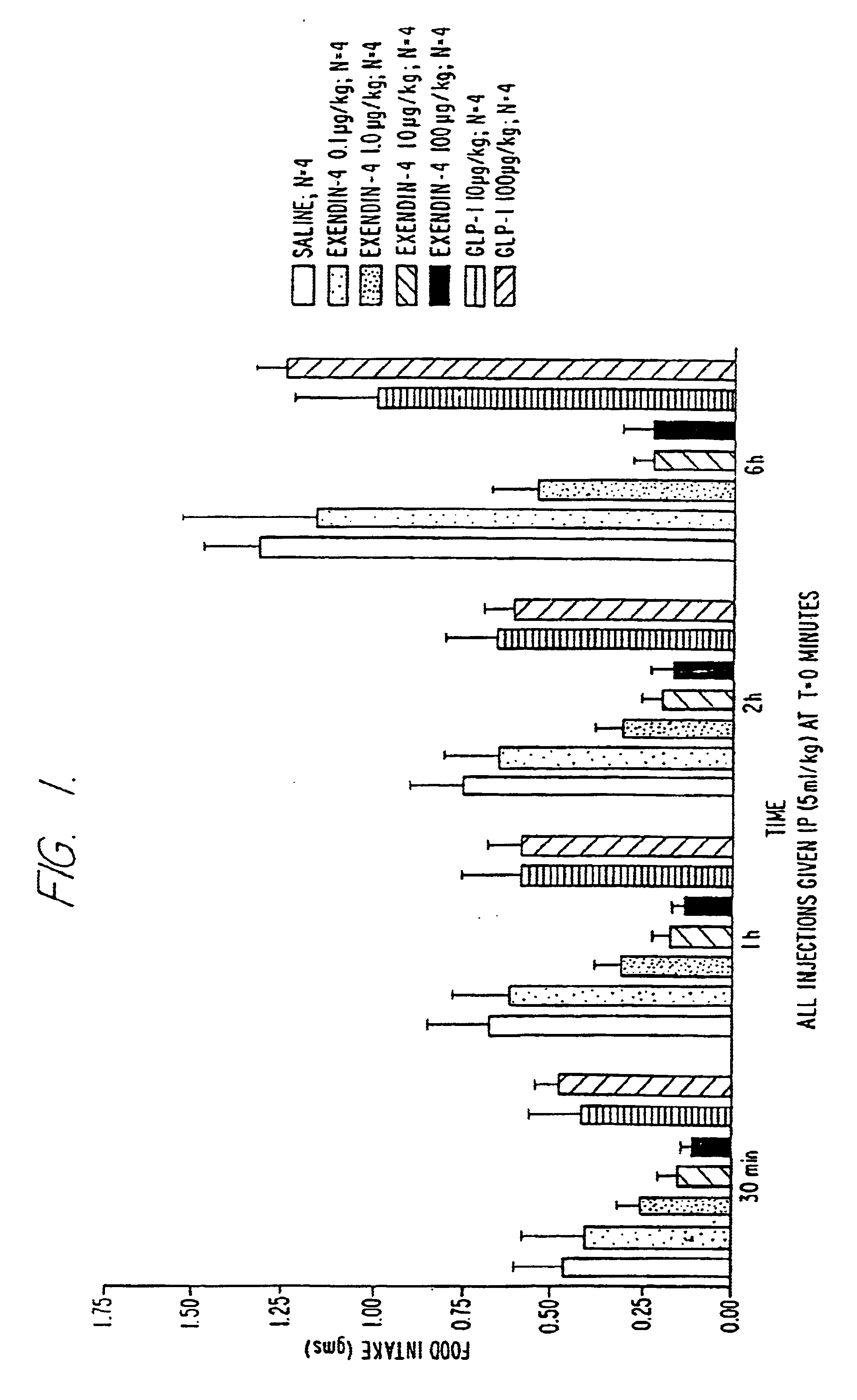
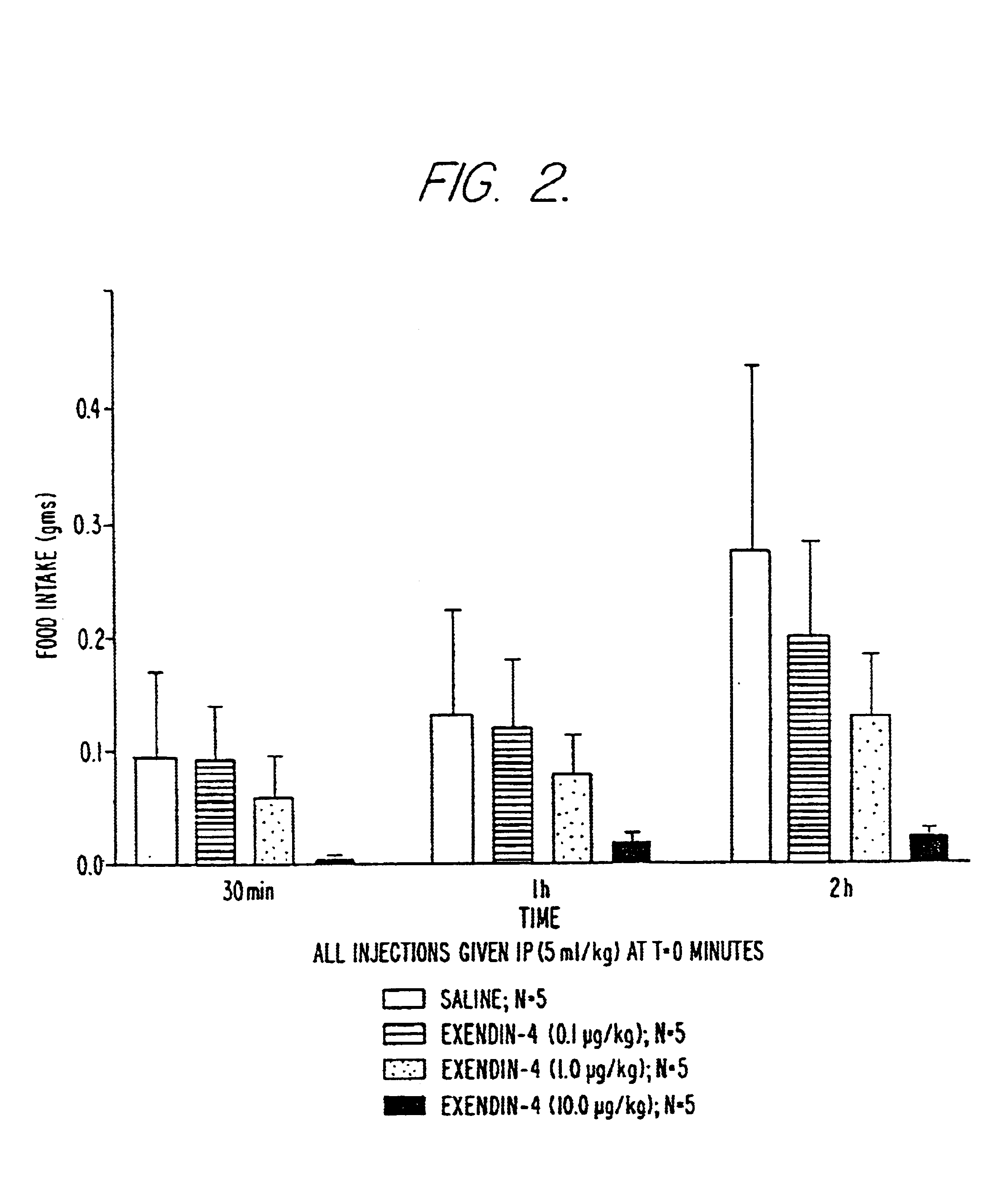
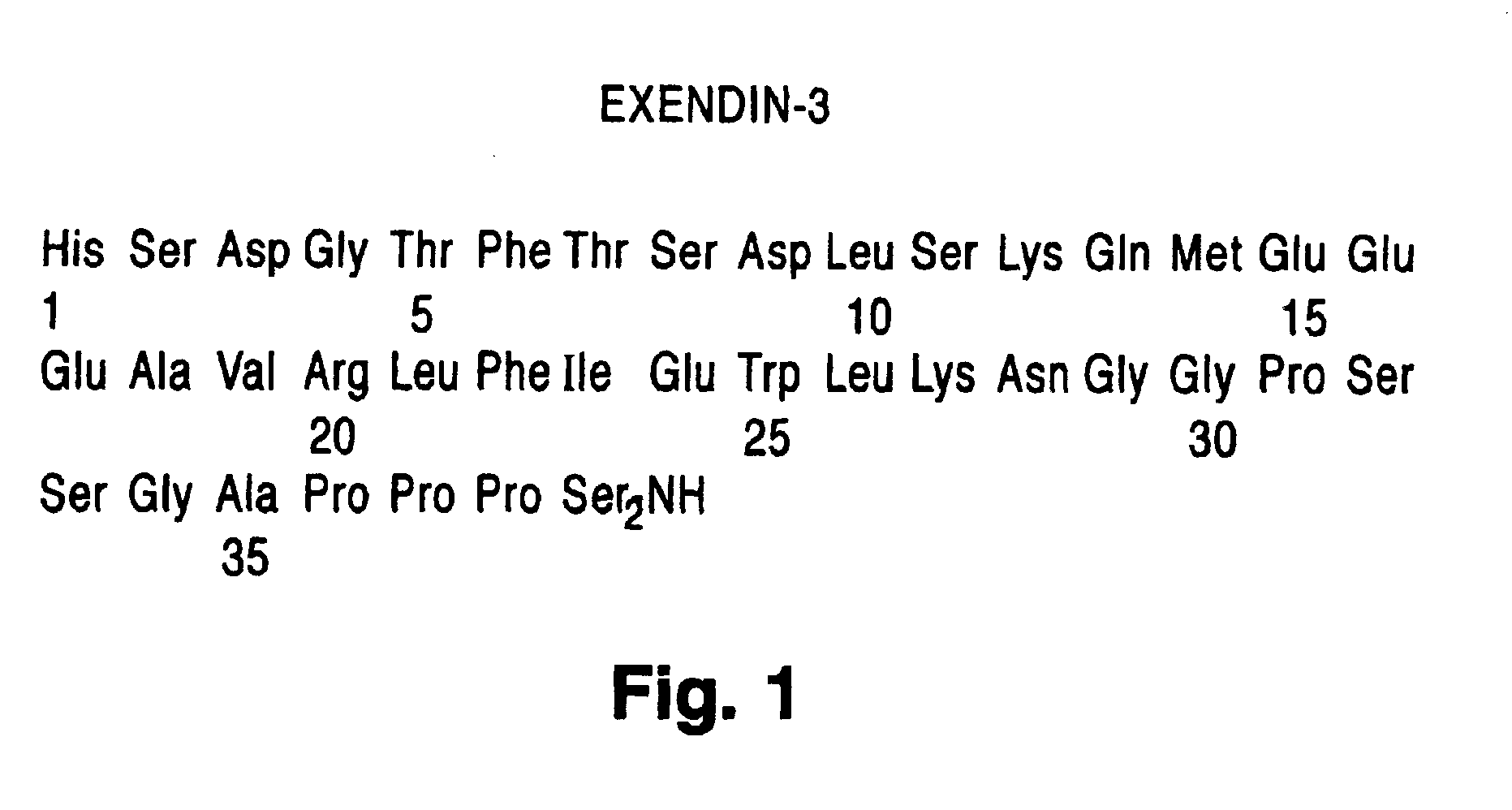
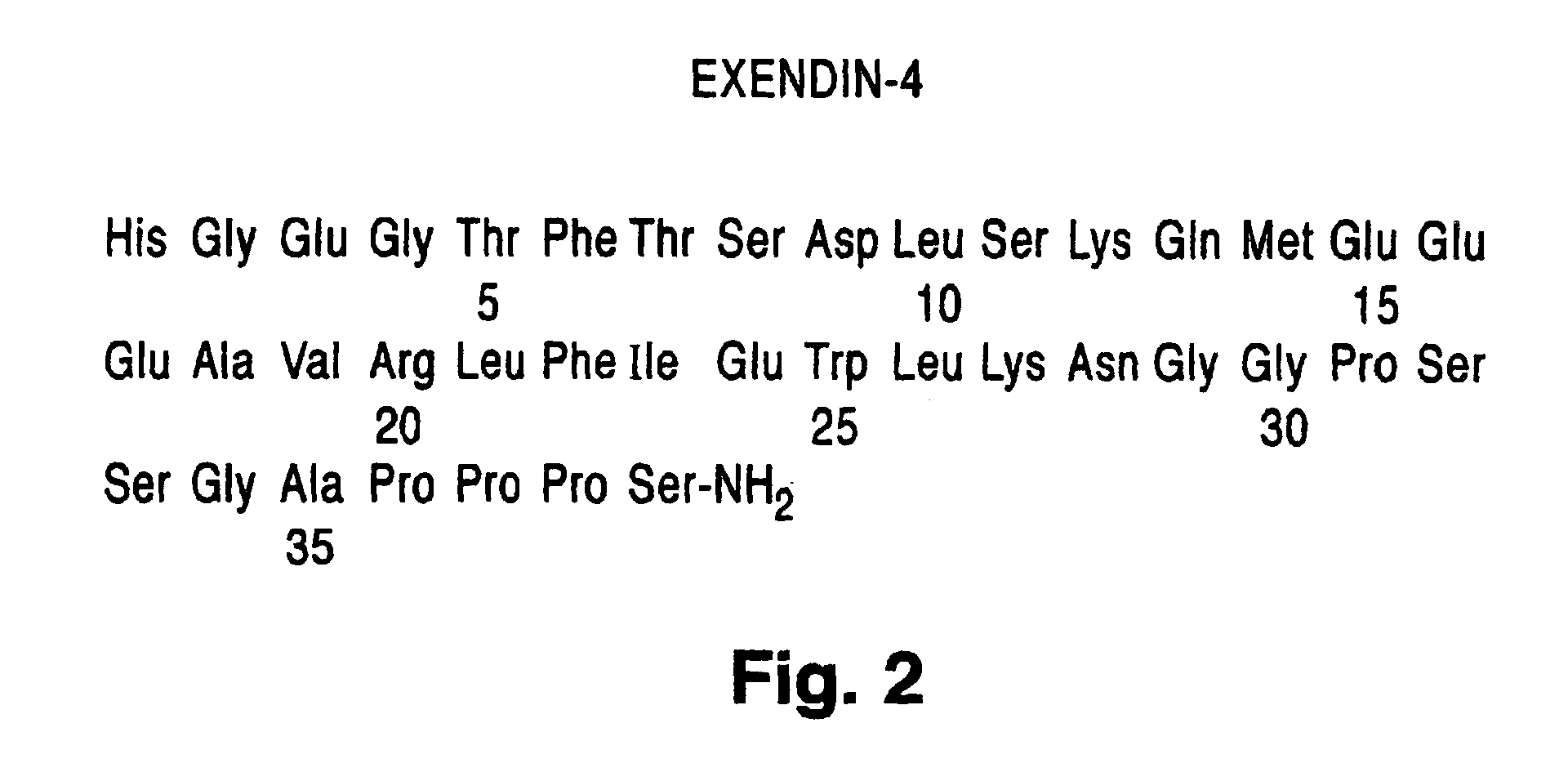
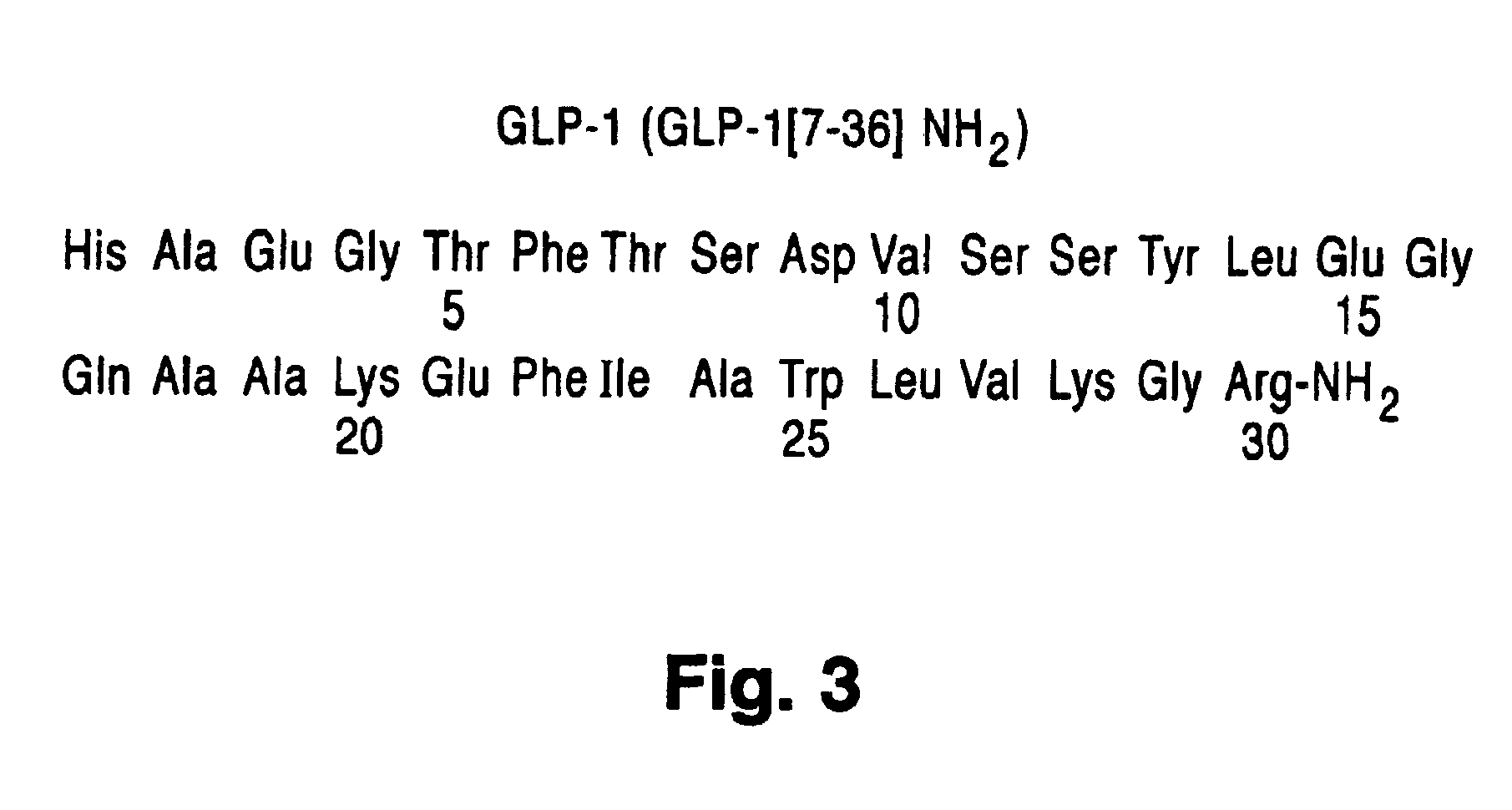
Use of exendins for the reduction of food intake
InactiveUS6956026B2Reduce appetiteReduce cardiac riskPeptide/protein ingredientsPharmaceutical delivery mechanismFeeding disabilityCvd risk
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an exendin or an exendin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. The methods are also useful for lowering the plasma glucose level, lowering the plasma lipid level, reducing the cardiac risk, reducing the appetite, and reducing the weight of subjects. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:ASTRAZENECA PHARMA LP
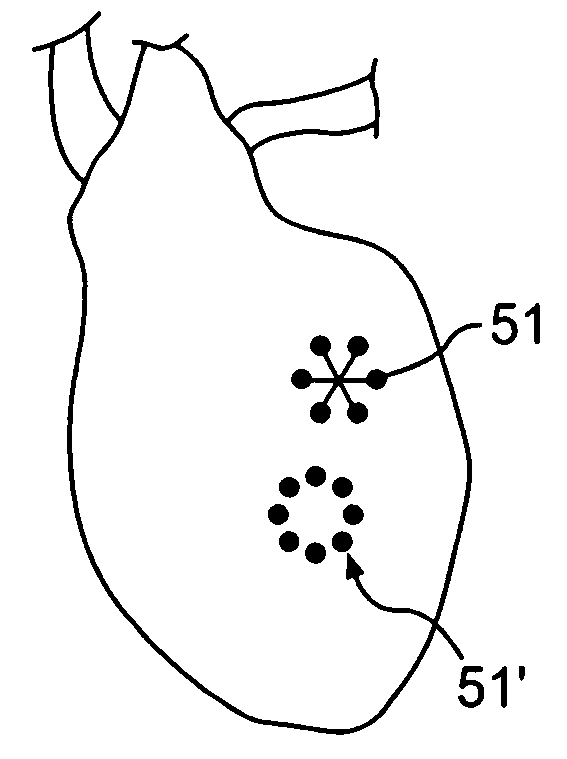
Prevention of myocardial infarction induced ventricular expansion and remodeling
ActiveUS20050080402A1Prevent further deteriorationInhibit swellingSuture equipmentsPowder deliveryCardiac muscleTherapeutic treatment
A method for direct therapeutic treatment of myocardial tissue in a localized region of a heart having a pathological condition. The method includes identifying a target region of the myocardium and applying material directly and substantially only to at least a portion of the myocardial tissue of the target region. The material applied results in a physically modification the mechanical properties, including stiffness, of said tissue. Various devices and modes of practicing the method are disclosed for stiffening, restraining and constraining myocardial tissue for the treatment of conditions including myocardial infarction or mitral valve regurgitation.
Owner:MYOMEND
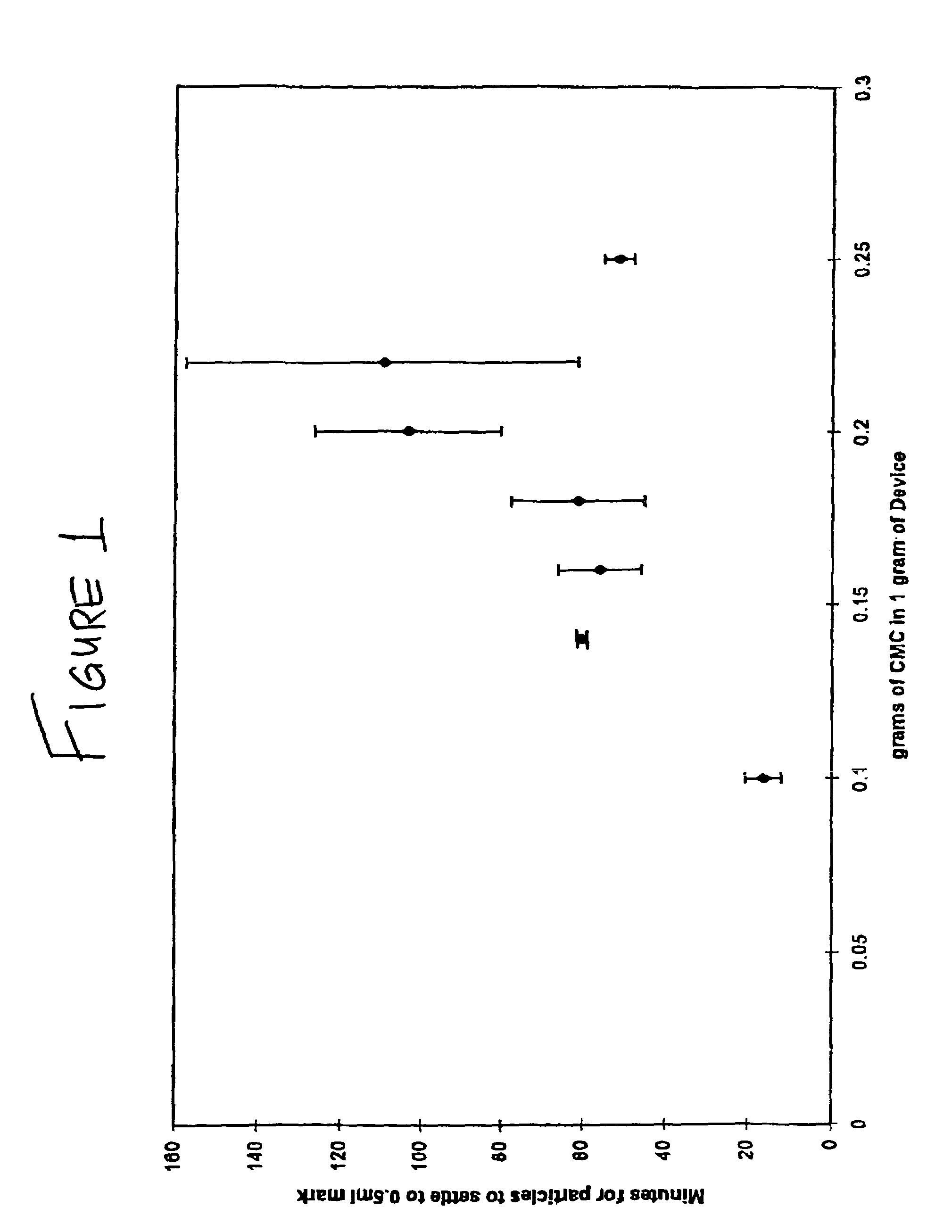
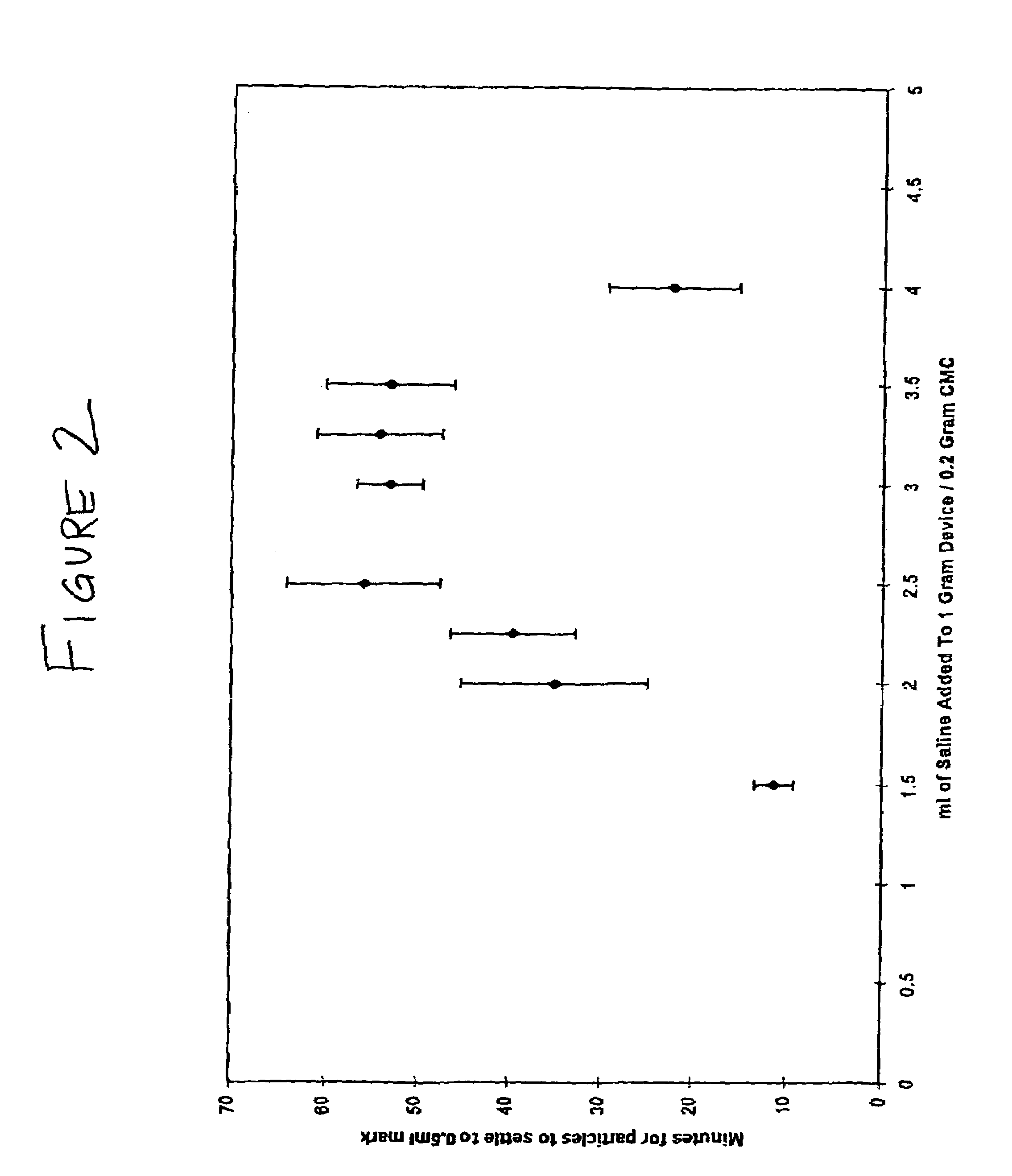
Milled particles
InactiveUS6634576B2Increase incorporationOptimal for incorporationPowder deliveryInorganic non-active ingredientsParticulatesPolymer science
A process for milling a solid substrate in the milling chamber of a dispersion or media mill in the presence of a two or more compositions of milling media bodies is disclosed wherein all milling media bodies contribute to the grinding of the solid substrate and wherein at least one composition of media bodies provides fragments of milling media bodies that are retained with the milled solid substrate particles in the form of a synergetic commixture produced in the milling process. More specifically, a process is disclosed for preparing a synergetic commixture comprising small particles of a solid substrate and small particulates of a first material of a desired size comprising the steps of (a) providing to the milling chamber of a media mill a contents comprising a pre-mix of a solid substrate, a fluid carrier, a plurality of milling bodies of a first material having a fracture toughness Kc1, and a plurality of milling bodies of a second material having a fracture toughness Kc2; (b) operating the media mill to grind the solid substrate and degrade at least a portion of the milling bodies of first material to produce a dispersion in the fluid carrier comprising a synergetic commixture of small particulates of the first material and small particles of the solid substrate having a desired size equal to or less than a size Sp; (c) separating the dispersion from any milling bodies and solid substrate particles having a size larger than Sp; and (d) optionally removing the fluid carrier from the dispersion to form a synergetic commixture free of fluid and comprising the particles and the small particulates, wherein KC2 is greater than KC1.
Owner:RTP PHARMA +1
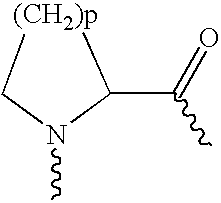
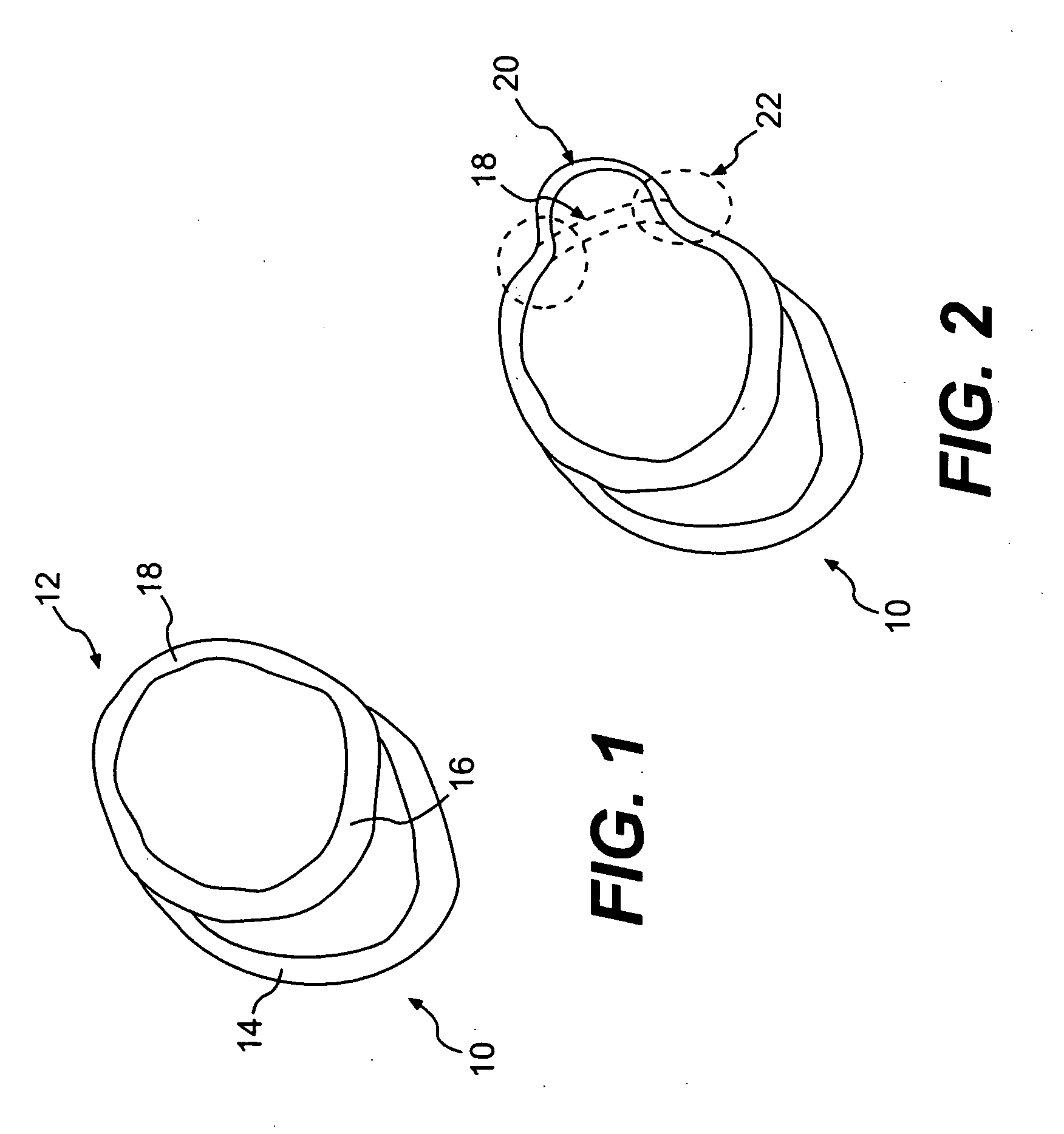
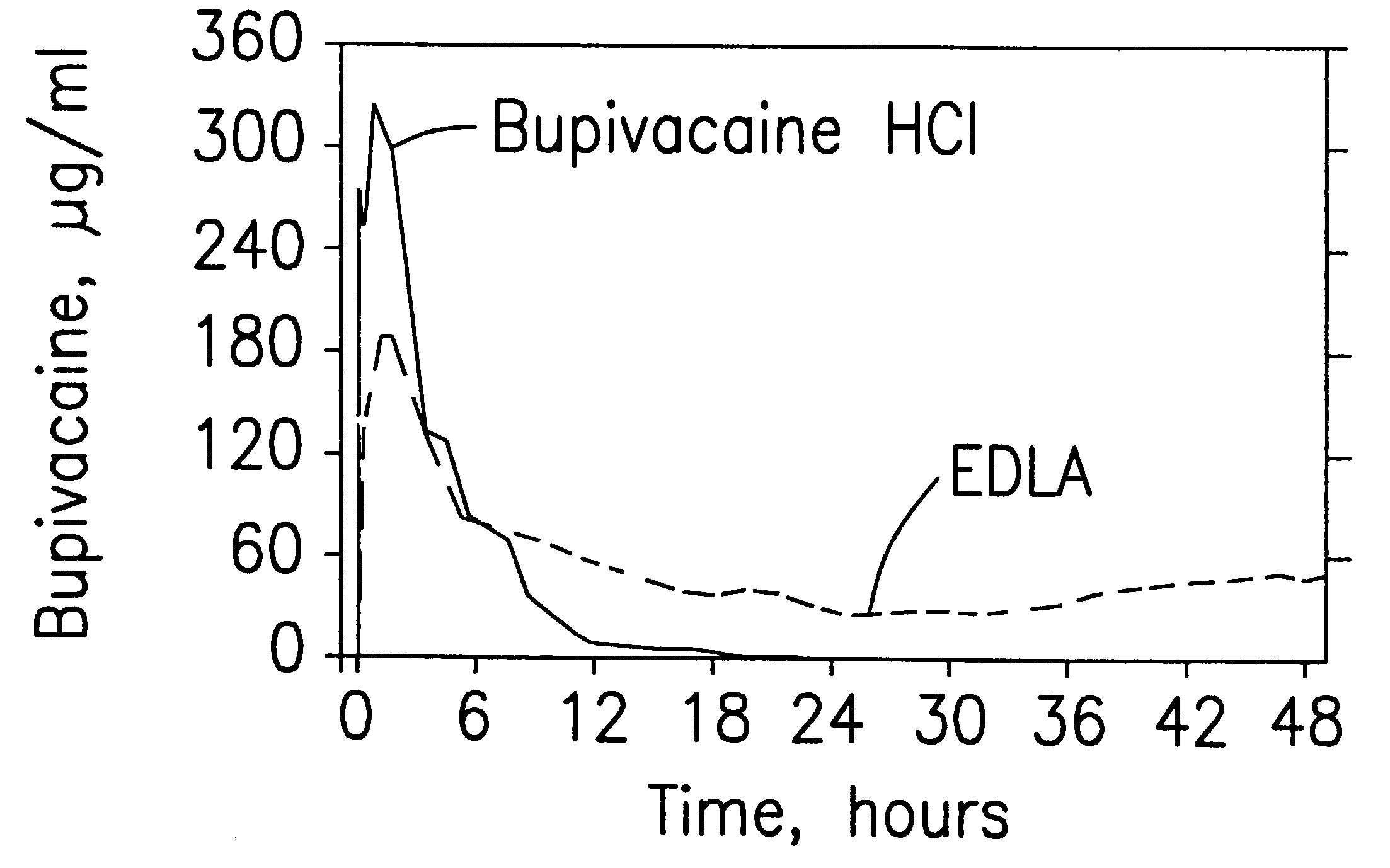
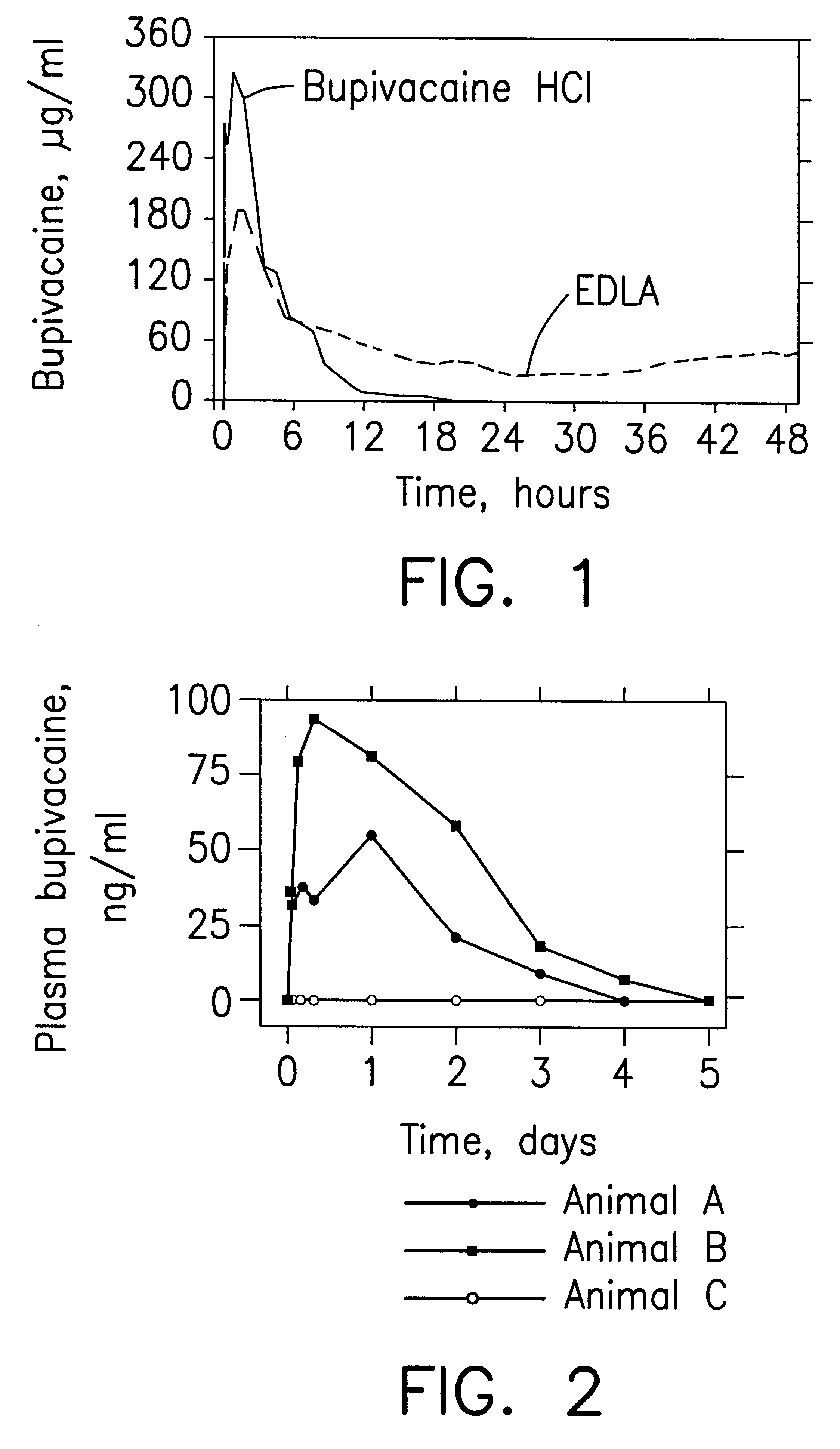
Prolonged anesthesia in joints and body spaces
InactiveUS6248345B1Enhance and prolong local anesthesiaImprovement in administrationInorganic non-active ingredientsAnaestheticsAnesthetic AgentPharmaceutical medicine
Sustained release local anesthetic formulations are administered intra articularly and / or into body spaces / cavities. The formulation is preferably a plurality of injectable microparticles including a local anesthetic and an effective amount of a biocompatible, biodegradable, sustained release material prolonging the release of the local anesthetic and optionally and a pharmaceutically acceptable, i.e., non-toxic, augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable without the augmenting agent.
Owner:PURDUE PHARMA LP
Orally disintegrable tablets
InactiveUS6328994B1Sufficient oral disintegrabilityHigh strengthOrganic active ingredientsPowder deliveryDiseaseOrally disintegrating tablet
An orally disintegrable tablet, of the present invention, which comprises (i) fine granules having an average particle diameter of 400 mum or less, which fine granules comprise a composition coated by an enteric coating layer, said composition having 10 weight % or more of an acid-labile physiologically active substance and (ii) an additive, has superior disintegrability or dissolution in the oral cavity so that it can be used for treatment or prevention of various diseases, as an orally disintegrable tablet capable of being administered to the aged or children and easily administered without water. Also, because the tablet of the present invention contains fine granules having the average particle diameter such that it will not impart roughness in mouth, it can be administered easily without discomfort at the administration.
Owner:TAKEDA PHARMA CO LTD
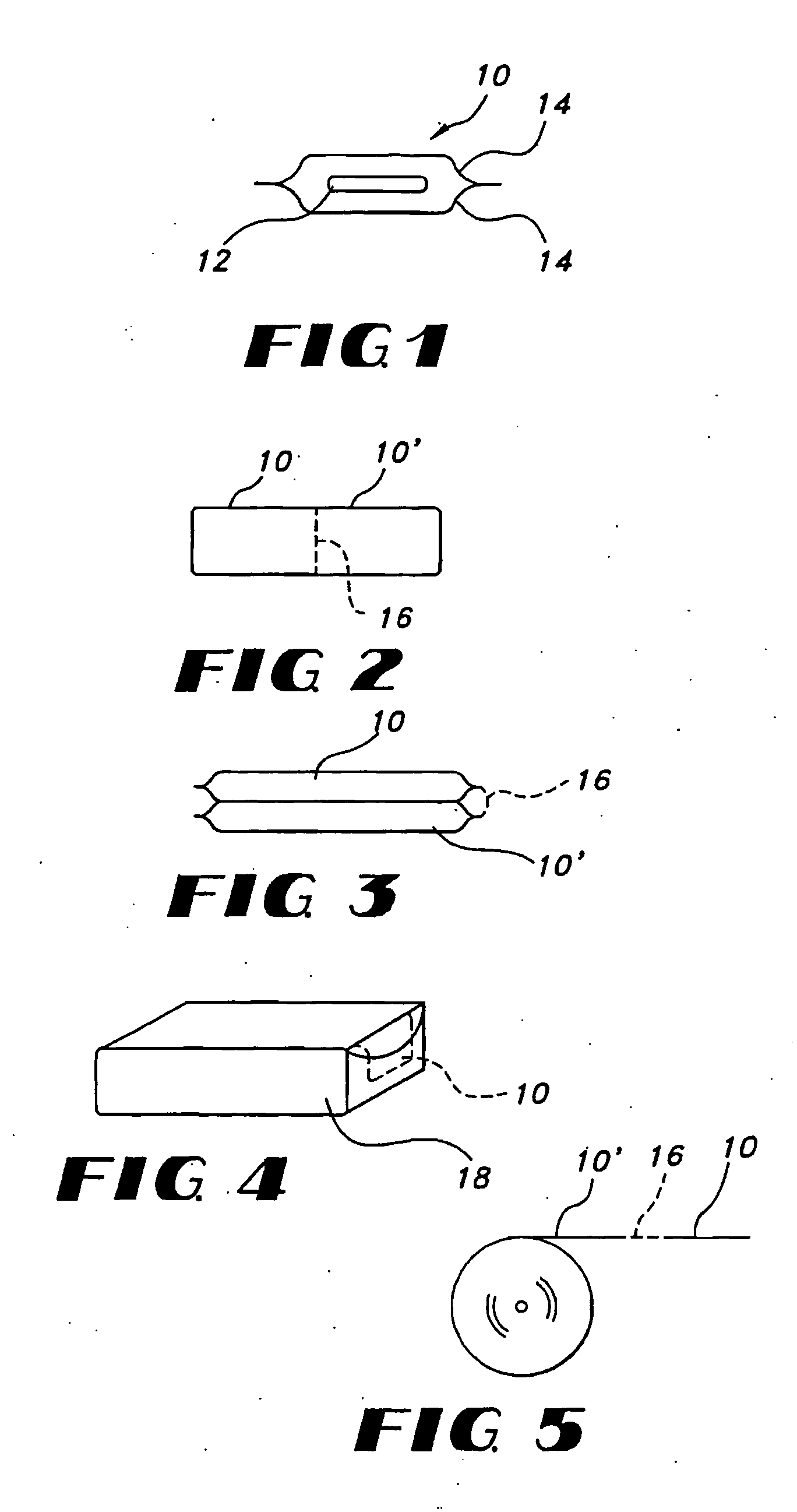
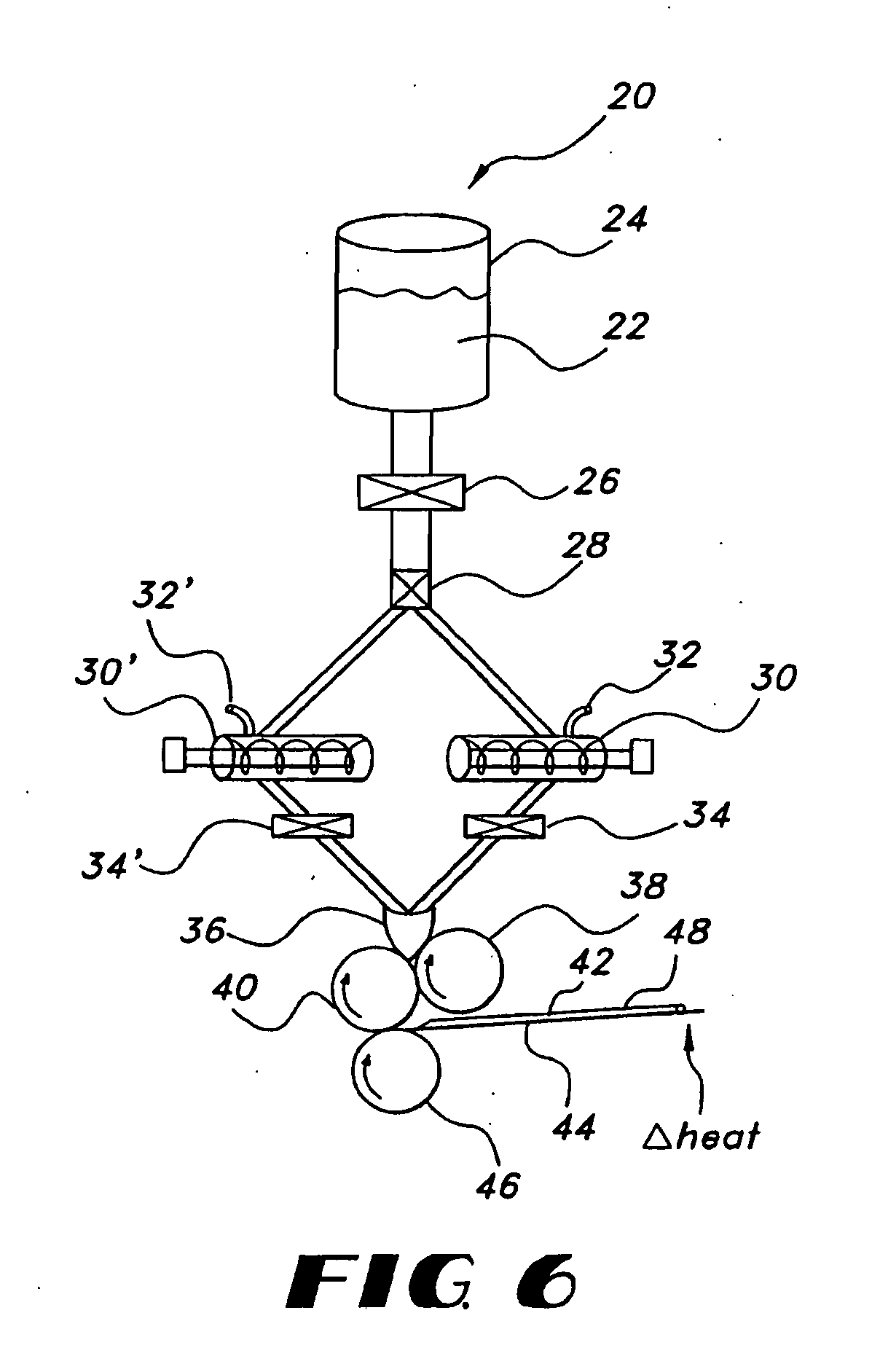
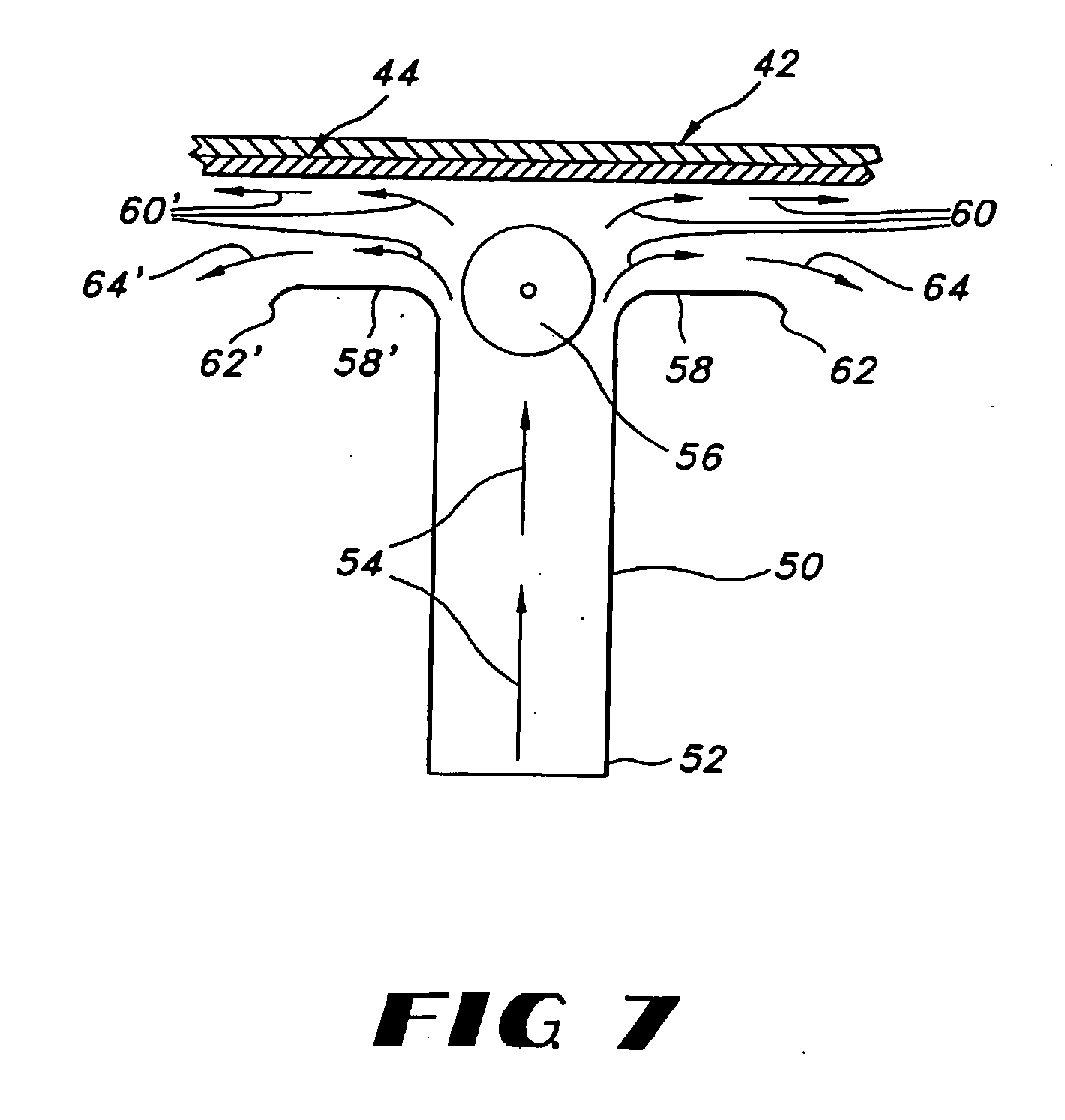
Thin film with non-self-aggregating uniform heterogeneity and drug delivery systems made therefrom
The invention relates to the film products and methods of their preparation that demonstrate a non-self-aggregating uniform heterogeneity. Desirably the films disintegrate in water and may be formed by a controlled drying process, or other process that maintains the required uniformity of the film.
Owner:AQUESTIVE THERAPEUTICS INC
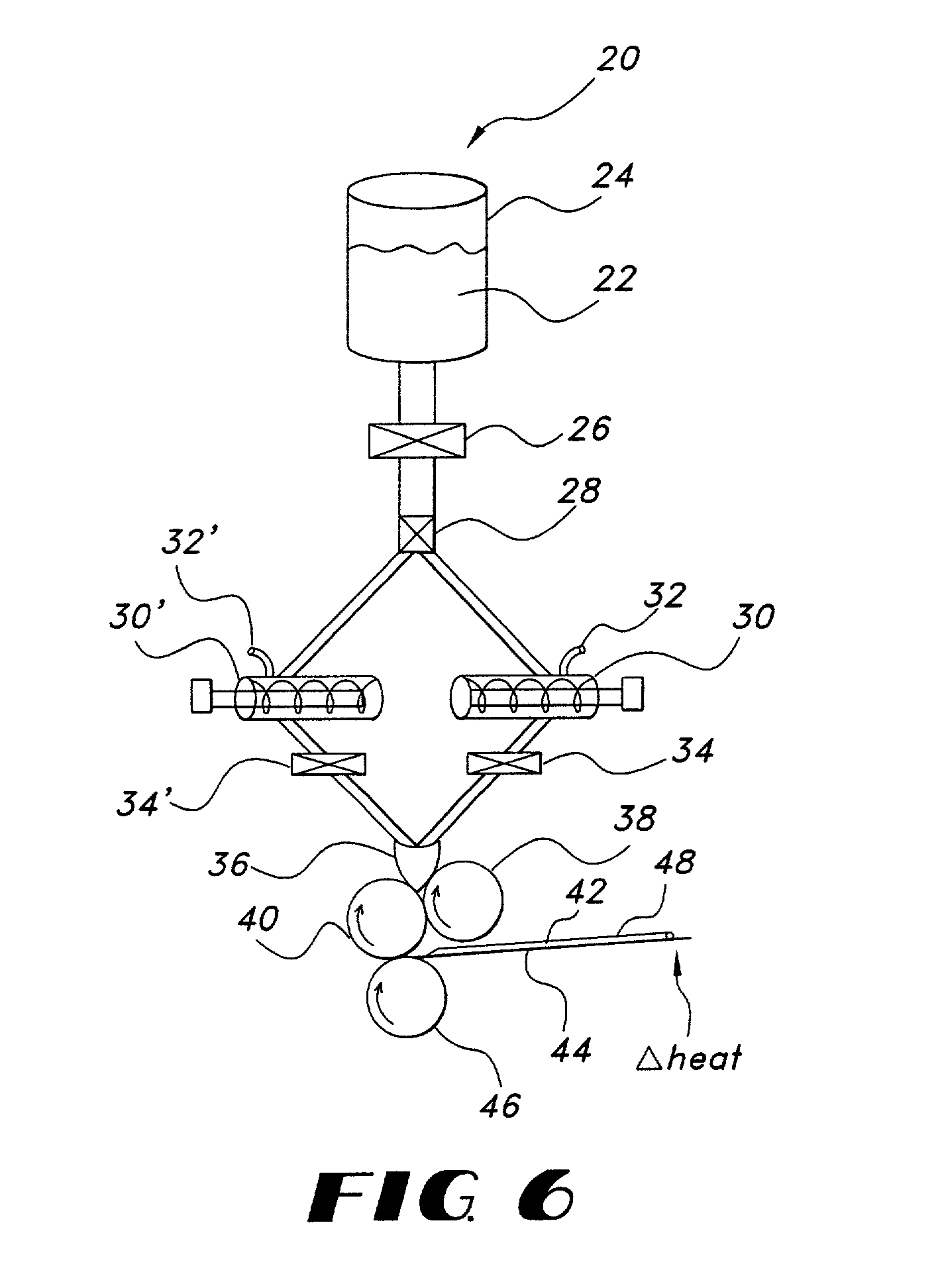
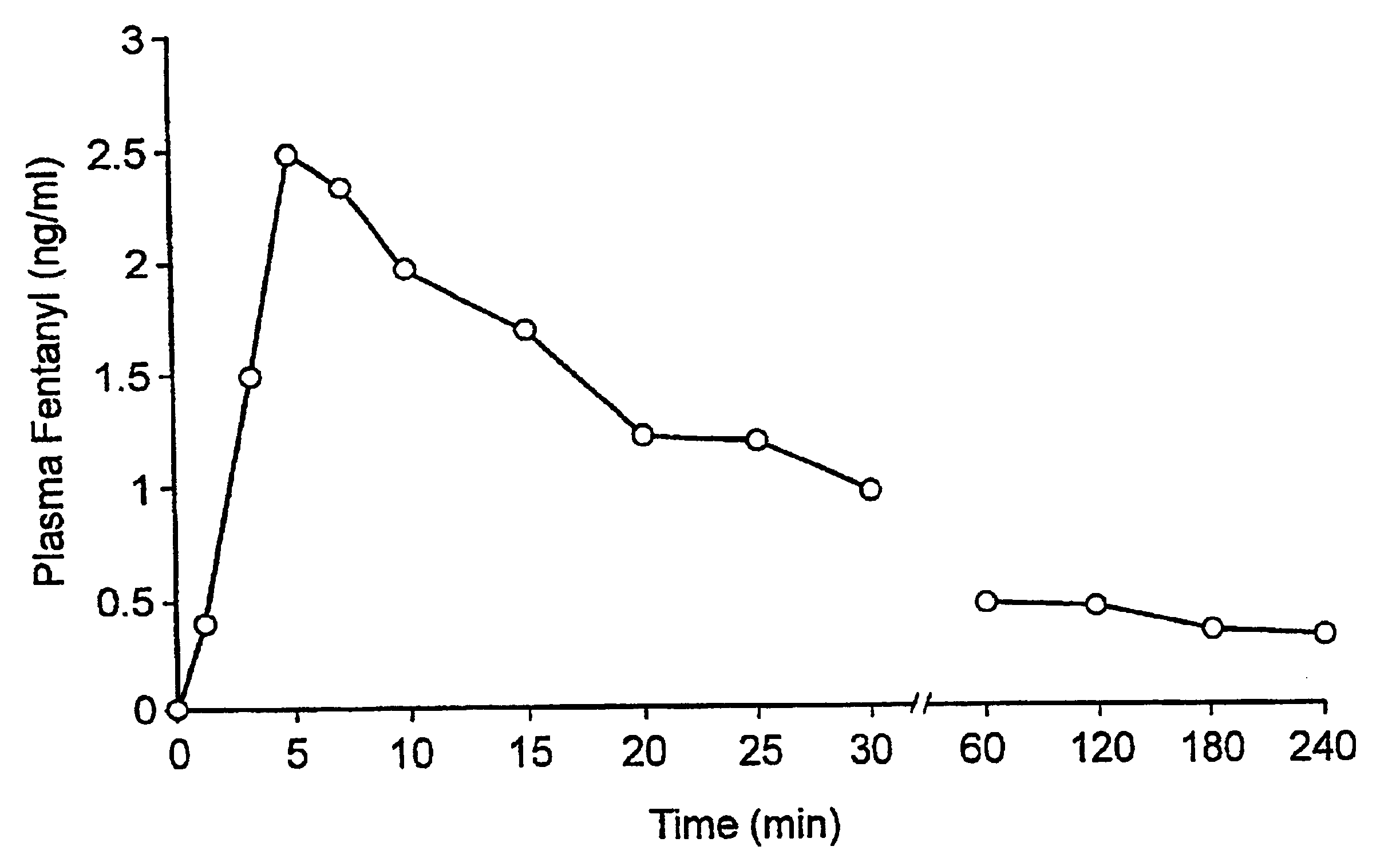
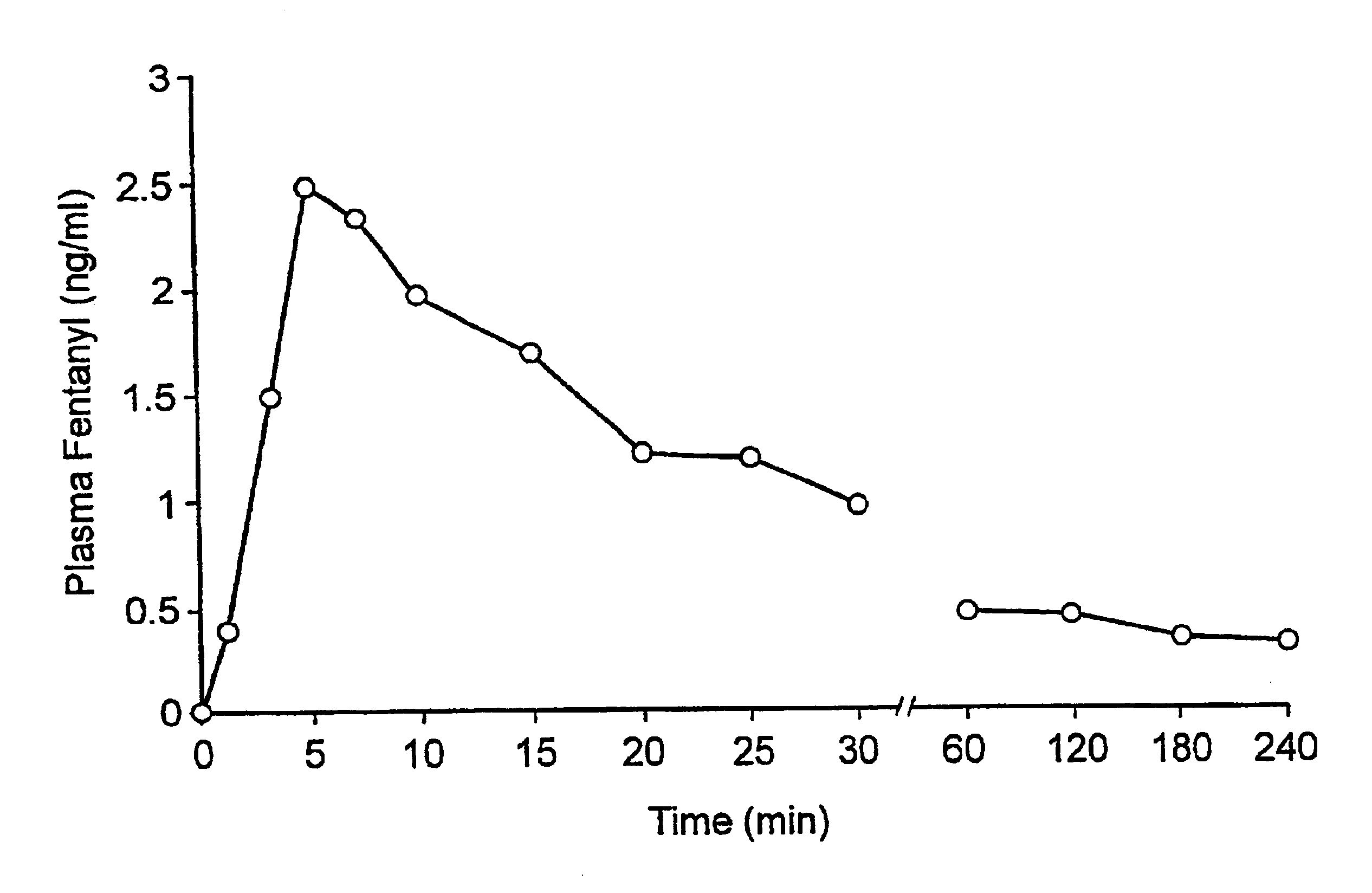
Fentanyl composition for the treatment of acute pain
A pharmaceutical composition for the treatment of acute pain by sublingual administration is described. The composition comprises an essentially water-free, ordered mixture of fentanyl or a pharmaceutically acceptable salt thereof in the form of microparticles which are adhered to the surface of carrier particles which are substantially larger than the particles of fentanyl, and are essentially water-soluble. In a preferred embodiment, the composition also contains a bioadhesion and / or mucoadhesion promoting agent. The invention also relates to the preparation of the composition, and to the use of the composition for the treatment of acute pain.
Owner:DIABAKT AB
Milled particles
InactiveUS20020047058A1Increase incorporationOptimal for incorporationPowder deliveryInorganic non-active ingredientsParticulatesPolymer science
Owner:RTP PHARMA +1
Exendin agonist formulations and methods of administration thereof
InactiveUS6902744B1Slow gastric emptyingLowering plasma glucose levelPowder deliveryPeptide/protein ingredientsGastric emptyingPlasma glucose
Novel exendin and exendin agonist compound formulations and dosages and methods of administration thereof are provided. These compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:ASTRAZENECA PHARMA LP
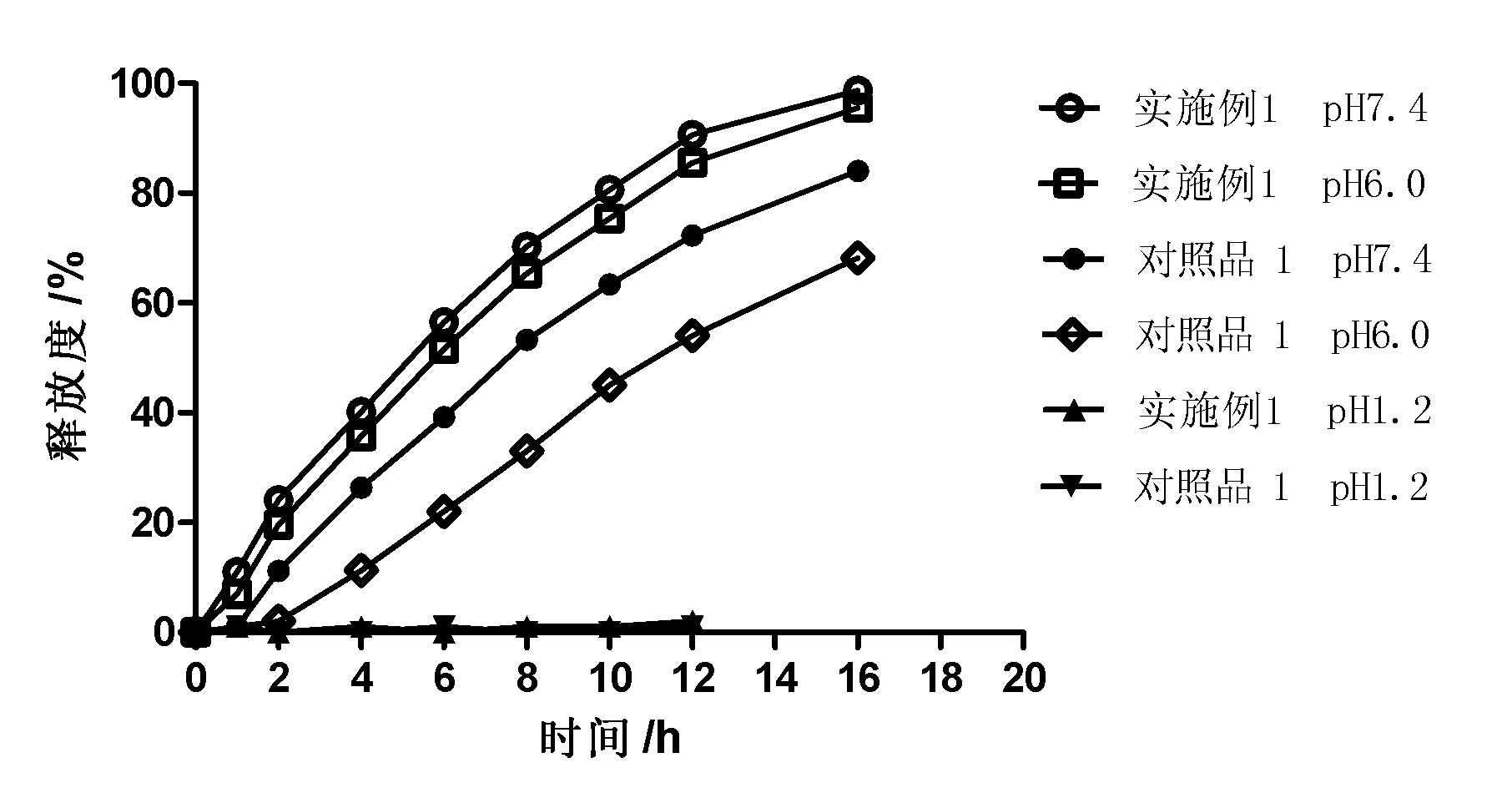
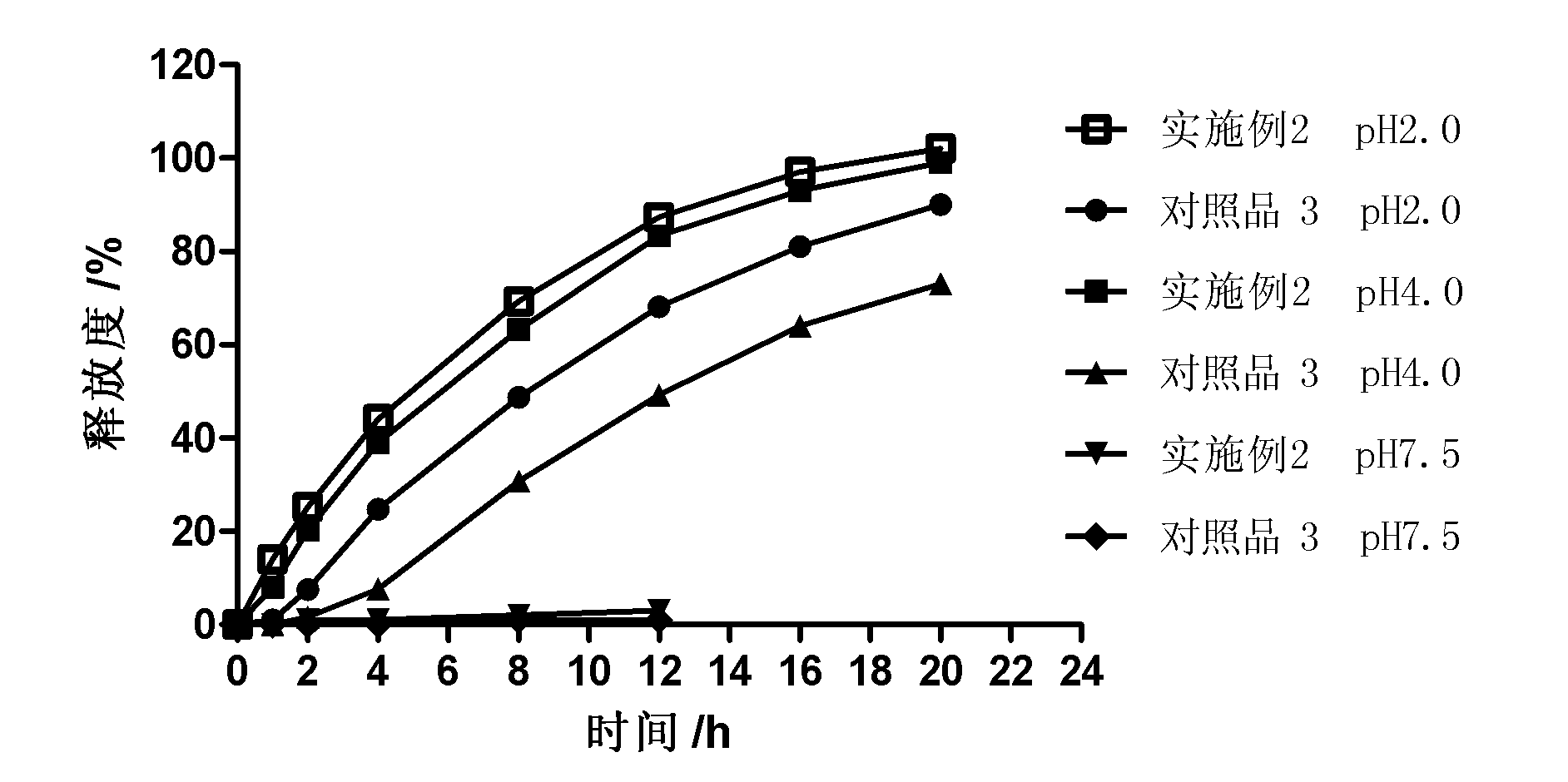
Controlled release preparation
InactiveCN101987081AImprove stabilityRelease impact mitigationInorganic non-active ingredientsSuppositories deliveryParticulatesChemical reaction
The invention discloses a controlled release preparation with improved performance. The controlled release preparation comprises a core containing medicament and a controlled release film covering the outside of the core and being almost insoluble in water as well as stomach and intestines digestive juice. The controlled release film comprises particulate matters of a water soluble medicinal additive, the water-soluble medicinal additive is covered by a polymer film which can be soluble in the stomach and / or intestines digestive juice but almost insoluble in water, the polymer and the medicinal additive can not produce chemical reaction or can produce chemical reaction but do not produce water-insoluble non-gaseous products and the pharmaceutically unacceptable products, and the amount of the polymer is no more 700% of that of the medicinal additive. The invention also discloses a preparation method of the controlled release preparation. The controlled release preparation has the advantages of improved medicament release reproducibility, reduced medicament release lag time, accelerated medicament release and improved bioavailability, can realize located controlled release, delayed controlled release and interval type or pulse type controlled release of the medicament in the gastrointestinal tract, and the like.
Owner:钟术光
Methods of evaluating protein formulation stability and surfactant-stabilized insulin formulations derived therefrom
InactiveUS6737401B2Improve physical stabilityReliable timePeptide/protein ingredientsMicrobiological testing/measurementCell AggregationsProtein aggregation
Embodiments of the invention are directed to a method of estimating the physical stability of a protein formulation. A particular embodiment of the invention places the protein formulation under an agitational stress that causes the protein to aggregate at an accelerated rate. In one embodiment, the change in protein aggregation is monitored spectroscopically using Thioflavin-T. Embodiments of the invention then utilize a survival curve analysis to ascertain the relative physical stability of the different protein formulations under study. This method was used to develop novel surfactant-stabilized insulin formulations in a rapid, cost efficient manner, thus illustrating the utility of the inventive method to the discovery and development of pharmaceutical protein formulations.
Owner:MEDTRONIC MIMIMED INC
Formulation of human antibodies for treating TNF-α associated disorders
ActiveUS8216583B2Improve stabilityAntibacterial agentsPowder deliveryADAMTS ProteinsPharmaceutical formulation
A liquid aqueous pharmaceutical formulation is described which has a high protein concentration, a pH of between about 4 and about 8, and enhanced stability.
Owner:ABBVIE BIOTECHNOLOGY LTD
Interfacial biomaterials
InactiveUS20030185870A1Enough timeExtended half-lifePeptide/protein ingredientsMicrobiological testing/measurementBinding domainBiological organism
An interfacial biomaterial prepared using a plurality of binding agents, each binding agent including a first ligand that specifically binds a non-biological substrate and a second ligand that specifically binds a biological substrate. Also provided is an interfacial biomaterial prepared using a plurality of binding agents, each binding agent including a ligand that specifically binds a non-biological substrate and a non-binding domain that shows substantially no binding to a biological substrate. Also provided are methods for preparing a binding agent, methods for preparing an interfacial biomaterial, and methods for using interfacial biomaterials.
Owner:DUKE UNIV
Combination of Local and Systemic Immunomodulative Therapies for Enhanced Treatment of Cancer
A method for the treatment of cancer comprising administration of a therapeutically effective amount of an intralesional chemoablative pharmaceutical composition, or variant of said composition, in combination with a therapeutically effective amount of a systemic immunomodulatory anticancer agent. A further method for the treatment of cancer comprising administration of a therapeutically effective amount of an intralesional chemoablative pharmaceutical composition, or variant of said composition, in combination with a therapeutically effective amount of a systemic targeted anticancer agent. The present invention is further directed to pharmaceutical compositions for treatment of cancer. The intralesional chemoablative pharmaceutical composition can comprise an IL chemoablative agent comprising primarily a halogenated xanthene.
Owner:PFIZER INC +1
Antimicrobial mesoporous silica nanoparticles
InactiveUS20060018966A1Reduce productionSlow diffusion ratePowder deliveryBiocideMesoporous silicaSilicon dioxide
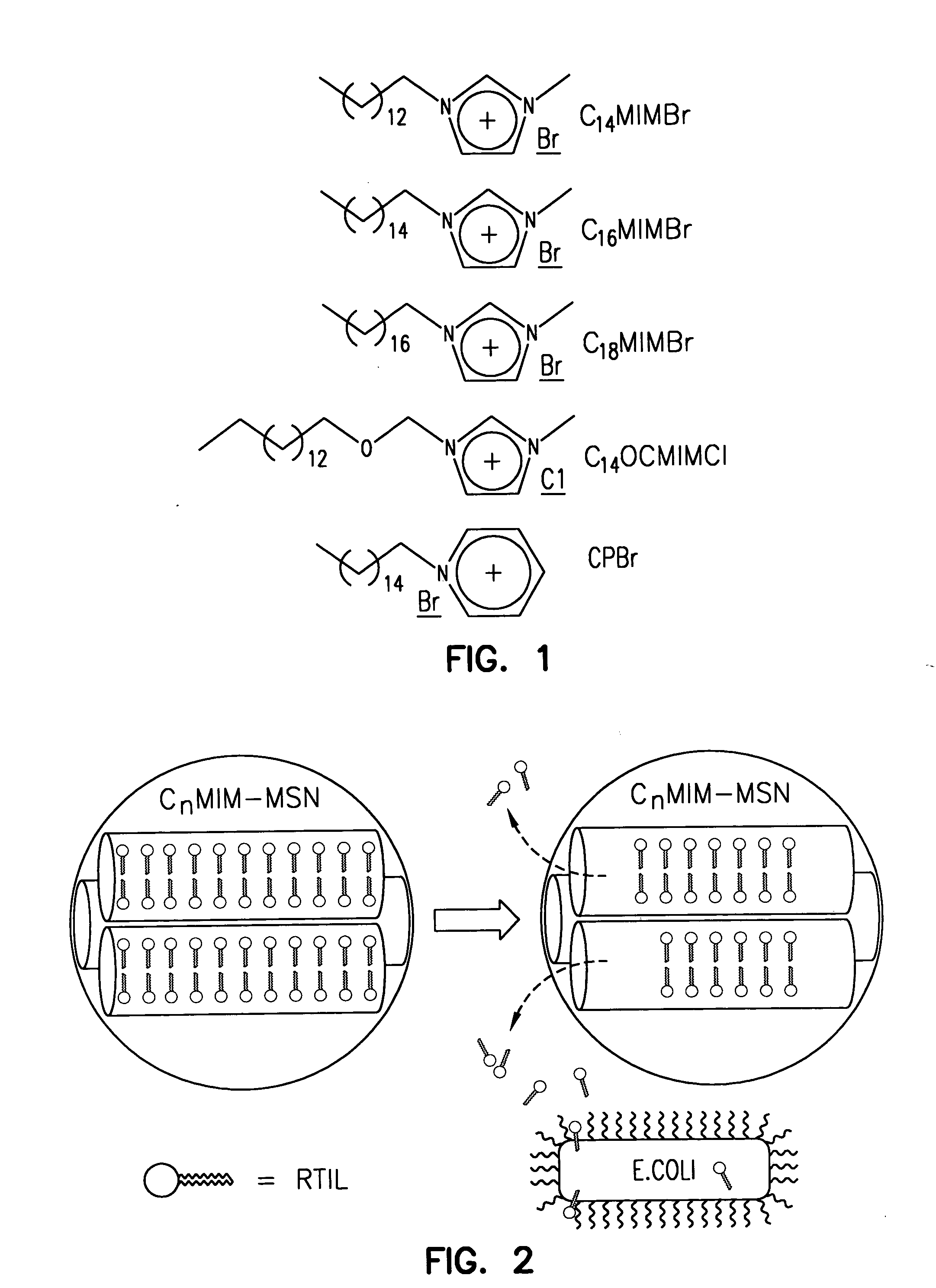
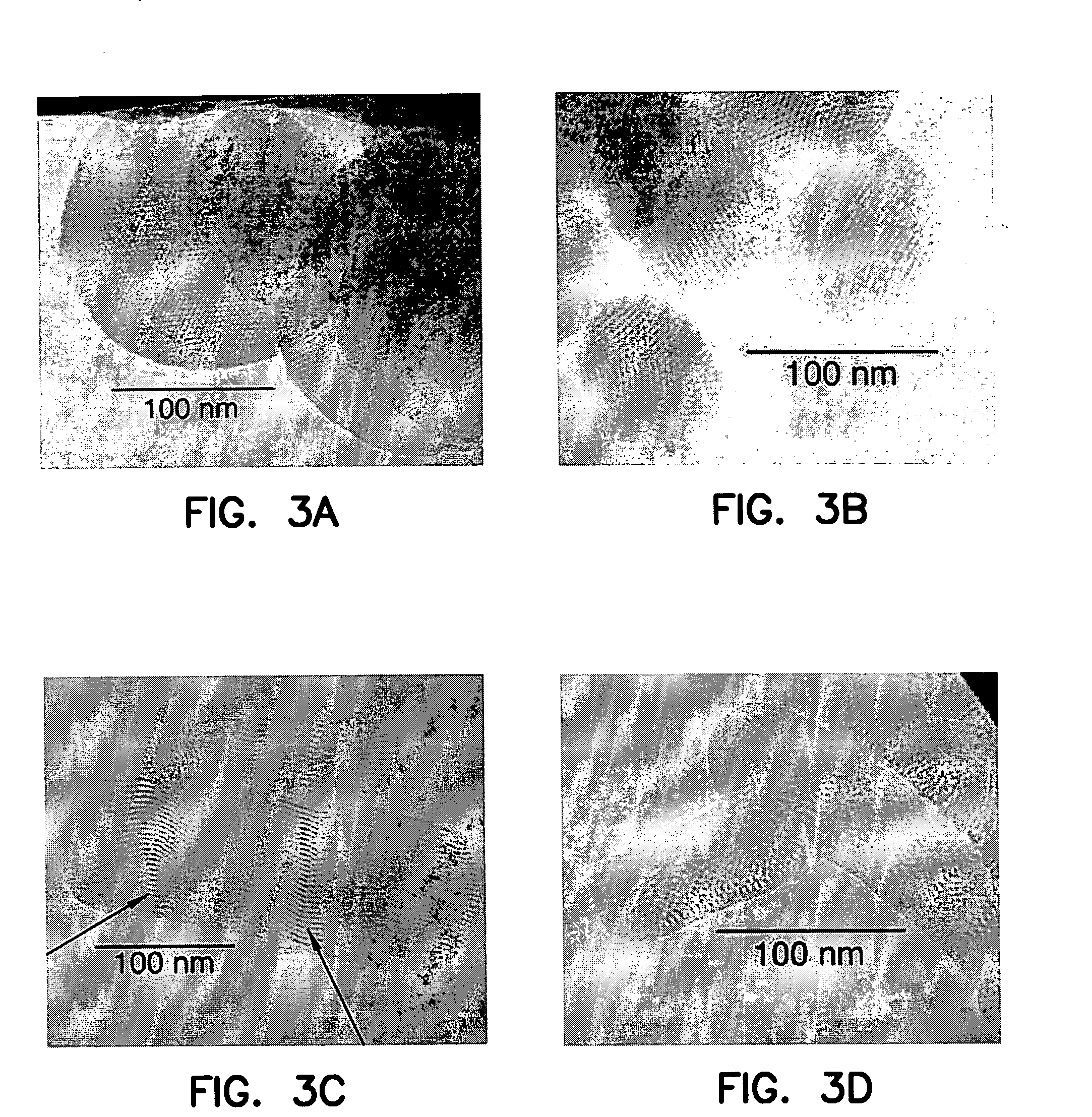
Methods for preparing a series of mesoporous silicates, such as room-temperature ionic liquid (RTIL)-templated mesoporous silicate particles, with various particle morphologies are provided. Methods for preparing silicate particles with antimicrobial agents within the MSN pores is also provided. The particles can be used as controlled-release nanodevices to deliver antimicrobial agents.
Owner:IOWA STATE UNIV RES FOUND
iRNA agents targeting VEGF
ActiveUS20060094032A1Improve propertiesImprove the immunityOrganic active ingredientsSenses disorderVascular endothelial growth factorEndothelium
The features of the present invention relate to compounds, compositions and methods useful for modulating the expression of vascular endothelial growth factor (VEGF), such as by the mechanism of RNA interference (RNAi). The compounds and compositions include iRNA agents that can be unmodified or chemically-modified.
Owner:ALNYLAM PHARM INC
Biofunctional hydroxyapatite coatings and microspheres for in-situ drug encapsulation
InactiveUS20020155144A1Improve relationshipImprove interface strengthPowder deliveryOrganic active ingredientsGene deliverySide effect
Owner:THE UNIV OF BRITISH COLUMBIA
Pharmaceutical compositions comprising substituted benzimidazoles and methods of using same
InactiveUS20050054682A1Improve stabilityDecreased time to therapeutic effectBiocideDispersion deliveryBuffering agentPharmacology
The present invention is directed to, inter alia, pharmaceutical compositions comprising at least one proton pump inhibitor and at least one buffering agent. Compositions of the invention are useful in treating, inter alia, gastric acid related disorders.
Owner:UNIVERSITY OF MISSOURI
Peroxide removal from drug delivery vehicle
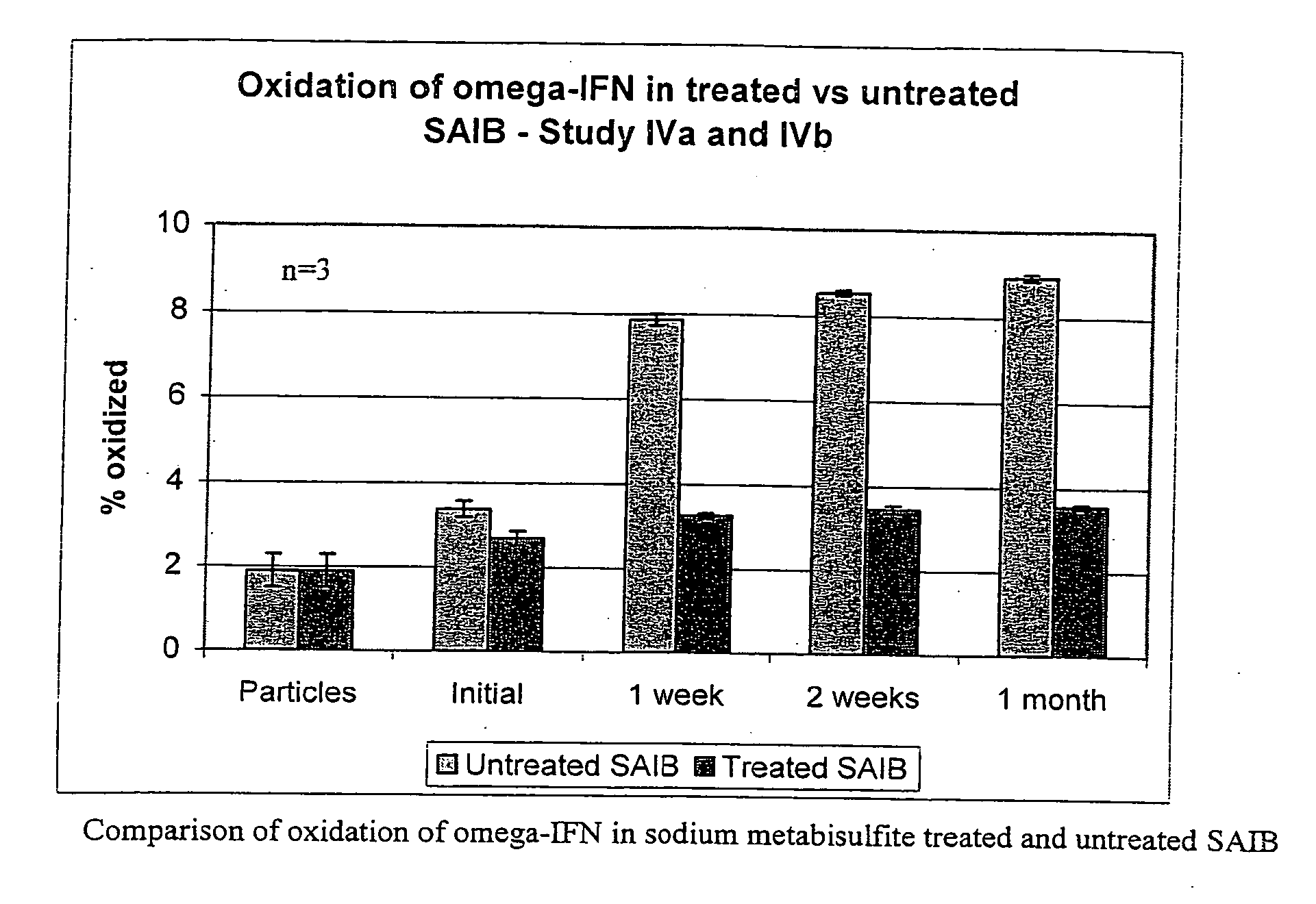
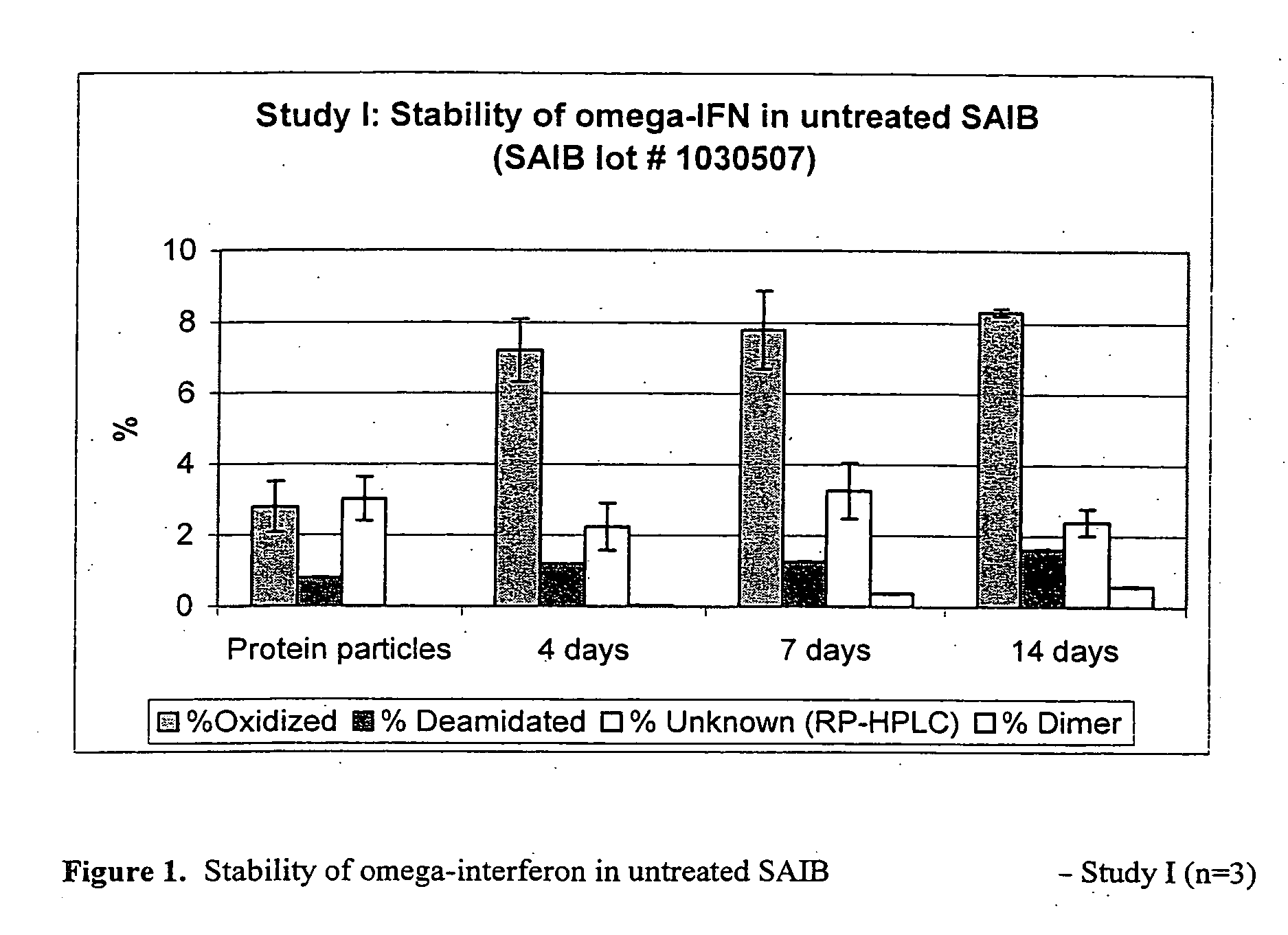
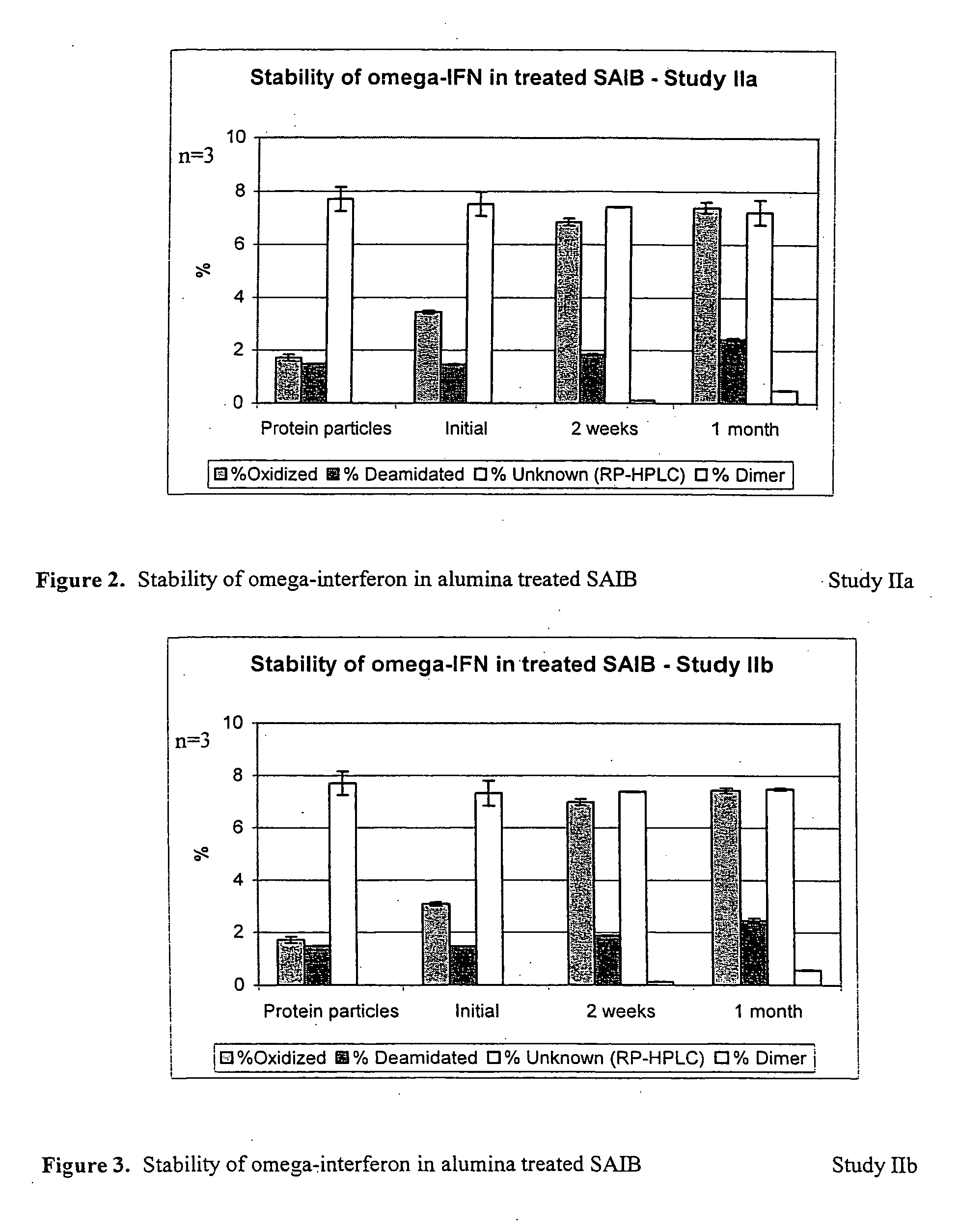
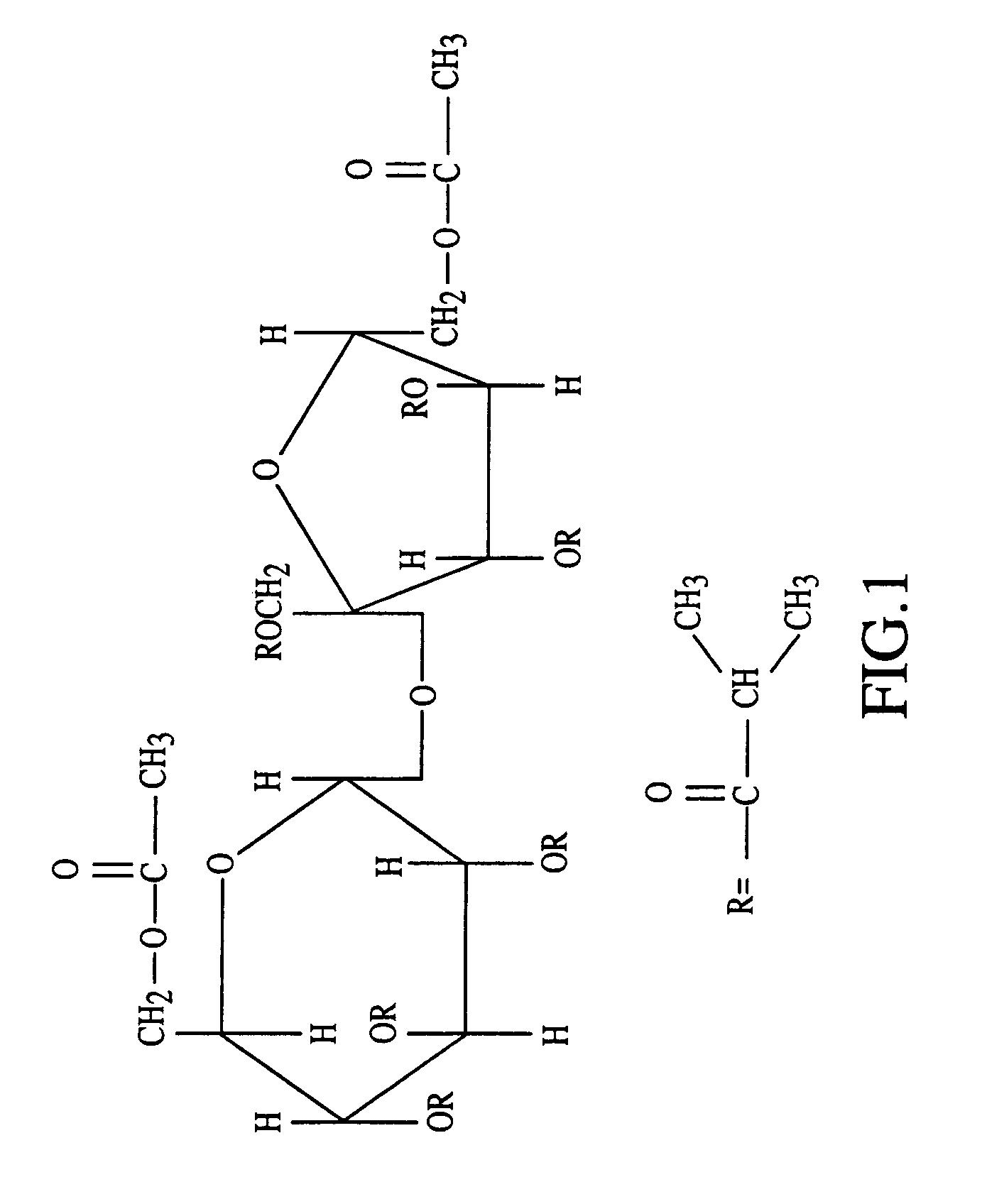
InactiveUS20070027105A1Improve drug stabilityReduced level of peroxideOrganic active ingredientsBiocideMedicineSucrose acetate isobutyrate
Owner:DURECT CORP
Pharmaceutical composition having reduced abuse potential
ActiveUS20090232887A1Efficiently employedReduce decreaseBiocideOrganic active ingredientsControl releaseAdditive ingredient
A pharmaceutical paste composition comprising an active ingredient such as an addictive substance, a controlled release agent, and a pharmaceutically suitable aqueous or non-aqueous carrier. The composition may comprise one or more of a clay, or an oily, waxy, or fatty substance. The composition may be filled into a capsule or other dispensing device. The composition may reduce dose dumping of an active ingredient. Methods of making and using the composition are also described.
Owner:INTELLIPHARMACEUTICS
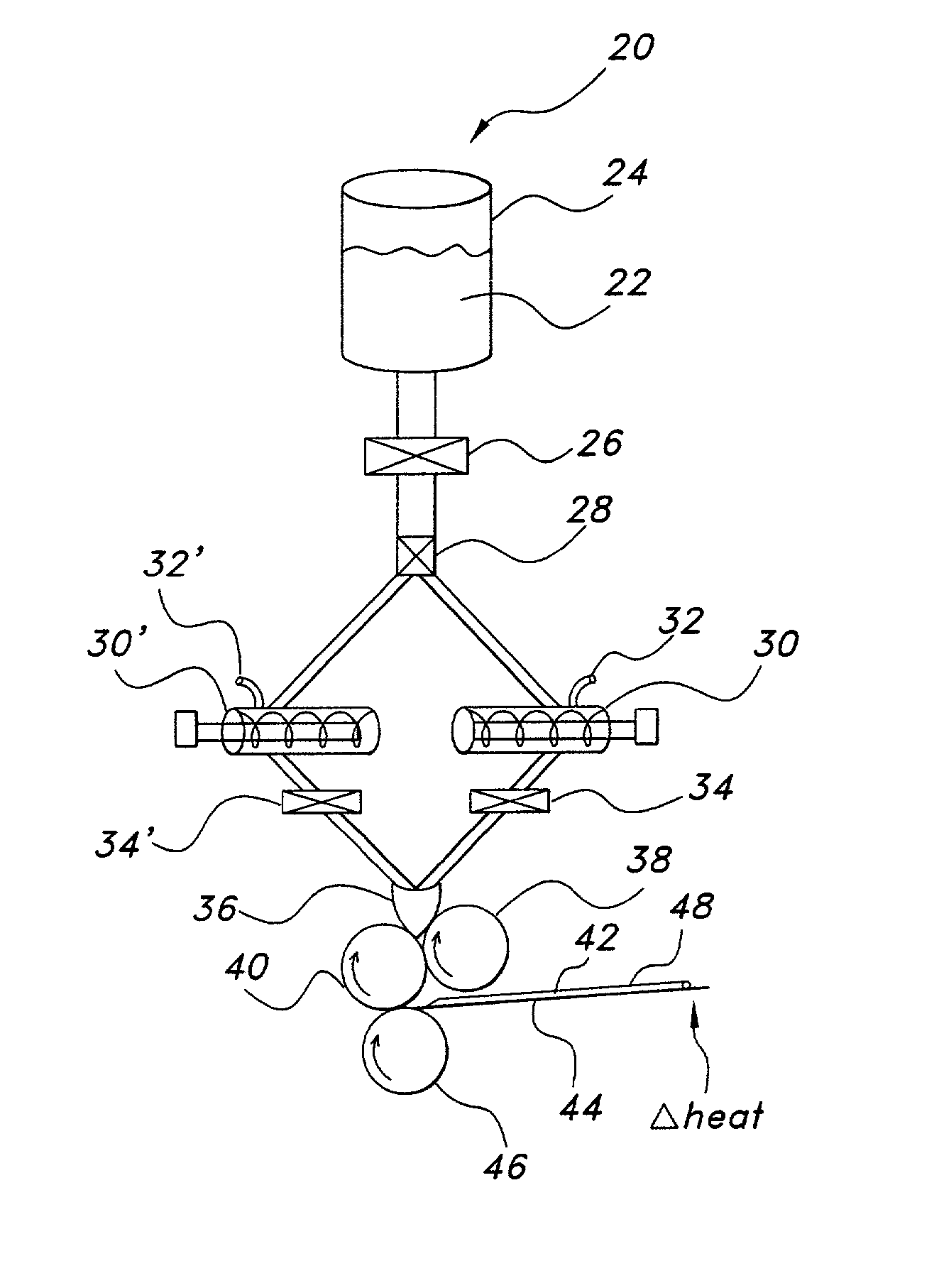
Uniform films for rapid-dissolve dosage form incorporating anti-tacking compositions
The present invention relates to water-soluble films incorporating anti-tacking agents and methods of their preparation. Anti-tacking agents may improve the flow characteristics of the compositions and thereby reduce the problem of film adhering to a user's mouth or to other units of film. In particular, the present invention relates to edible water-soluble delivery systems in the form of a film composition including a water-soluble polymer, an active component selected from cosmetic agents, pharmaceutical agents, vitamins, bioactive agents and combinations thereof and at least one anti-tacking agent.
Owner:AQUESTIVE THERAPEUTICS INC
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS20090074859A1Dissolve fastReduce deliveryAntibacterial agentsPowder deliveryDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Bioceramic compositions
InactiveUS6972130B1Good biocompatibilityEasy to processBiocideInorganic phosphorous active ingredientsDiseaseDelivery vehicle
The present invention provides a synthetic, poorly crystalline apatite (PCA) calcium phosphate containing a biologically active agent and / or cells (preferably tissue-forming or tissue-degrading cells). The compositions provided by the present invention are useful for a variety of in vivo and in vitro applications, including drug delivery (for example, to bony sites, the central nervous system, intramuscular sites, subcutaneous sites, interperitoneal sites, and occular sites) tissue growth (preferably bone or cartilage) osseous augmentation, and methods of diagnosing disease states by assaying tissue forming potential of cells isolated from a host. The invention also provides methods of preparing delivery vehicles, of altering delivery vehicle characteristics, and of delivering biologically active agents to a site. The invention further provides in vitro cell culture systems and cell encapsulation materials. The invention is useful for both medical and veterinary applications.
Owner:LIFE SCI ENTERPRISES
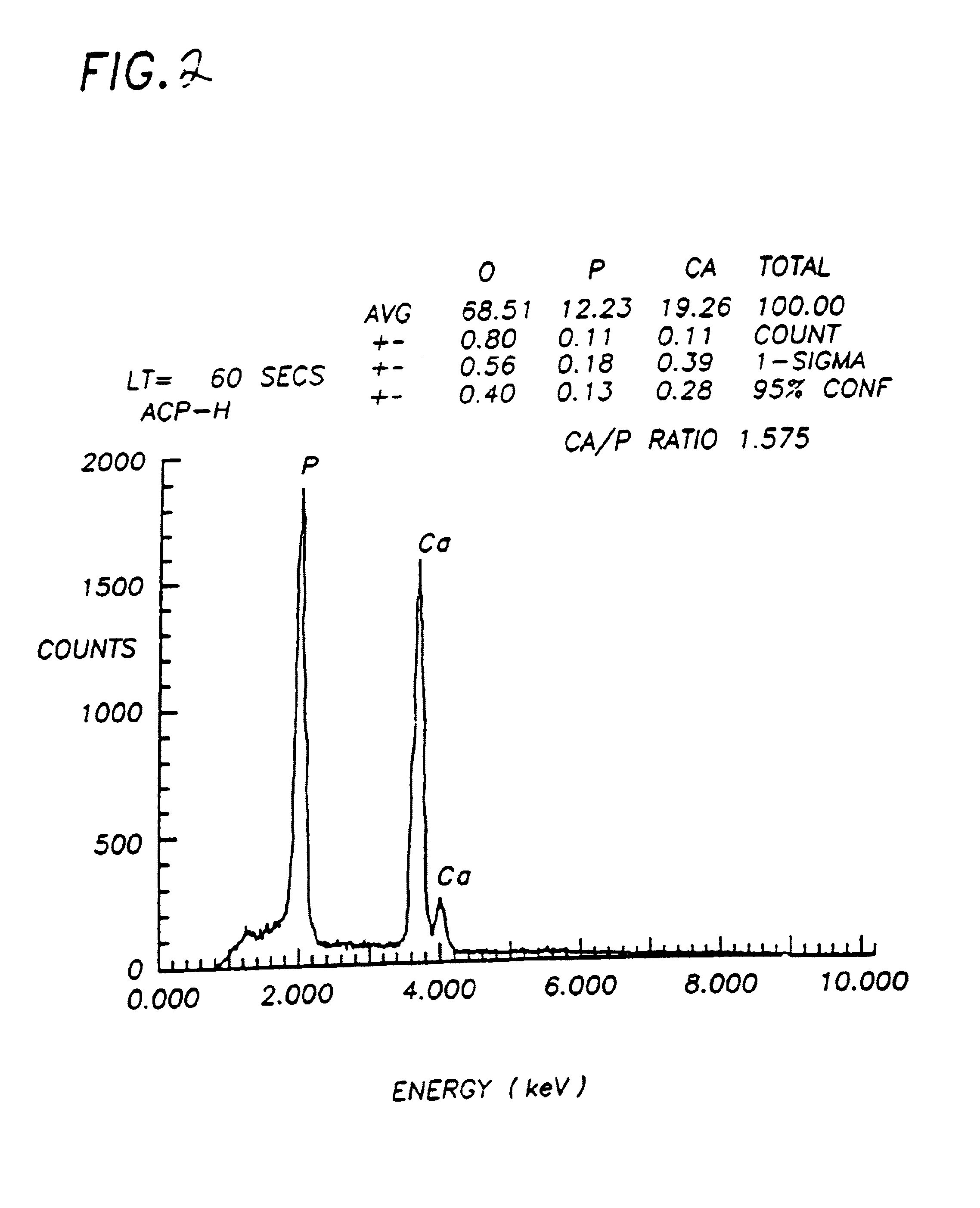
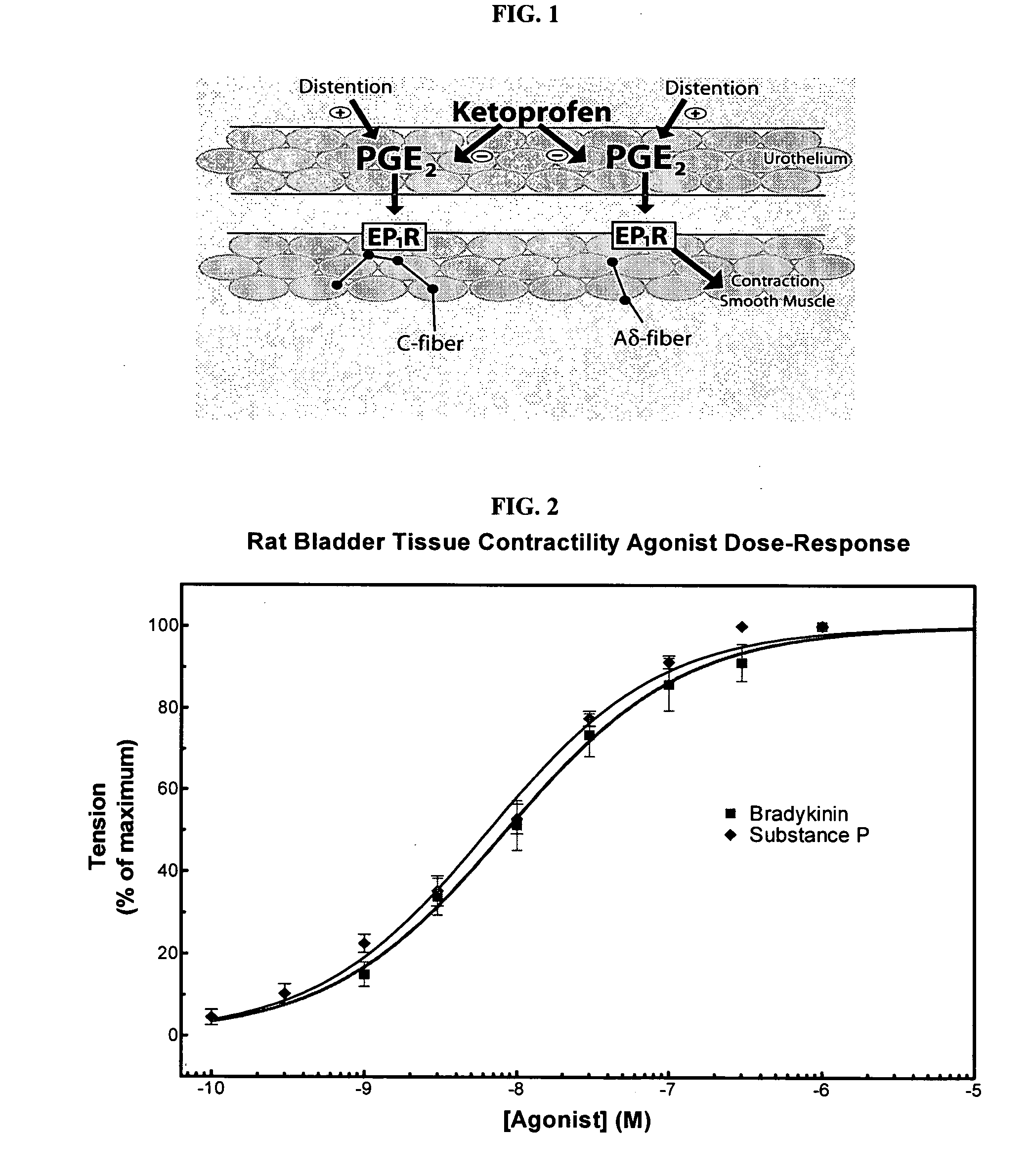
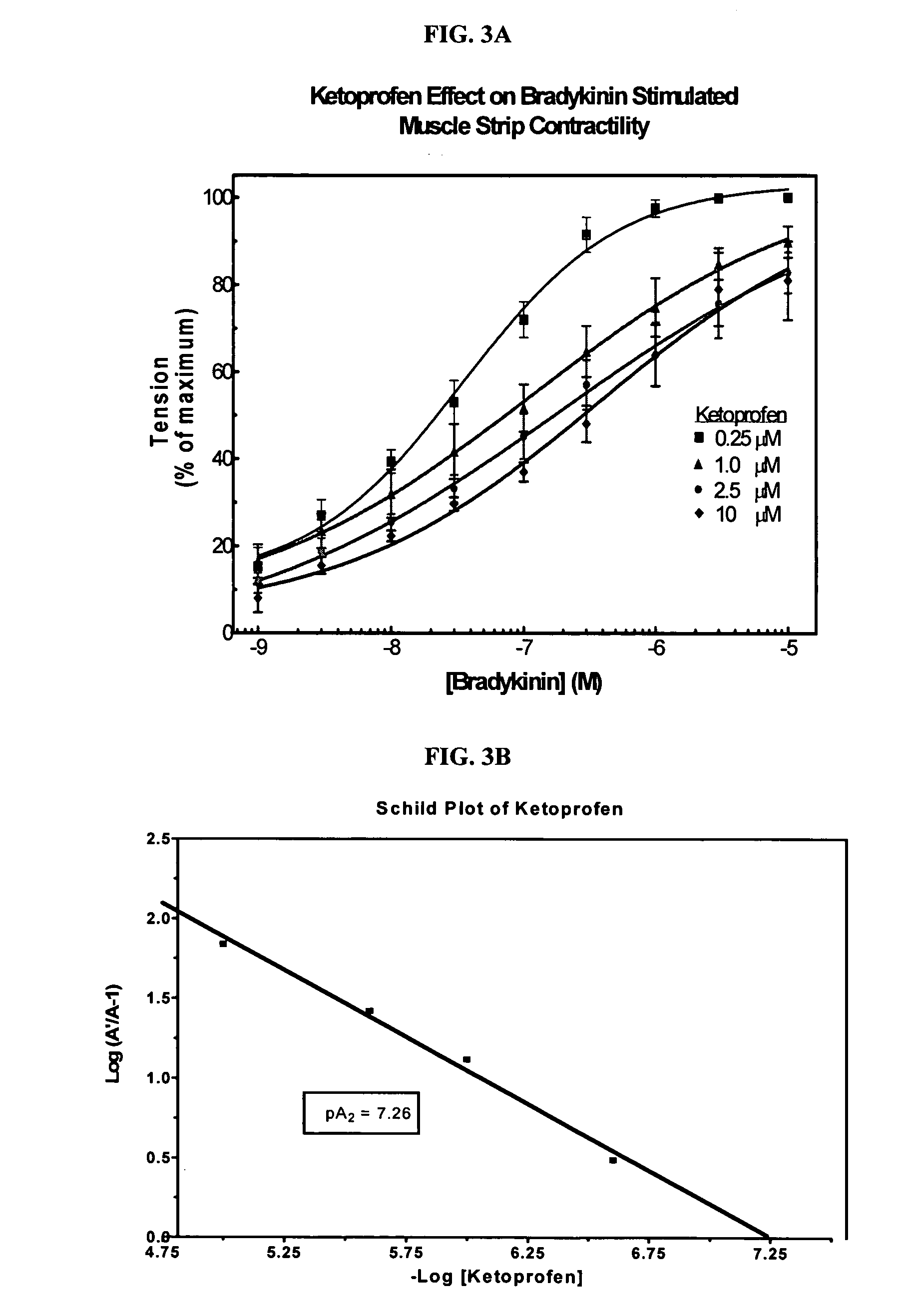
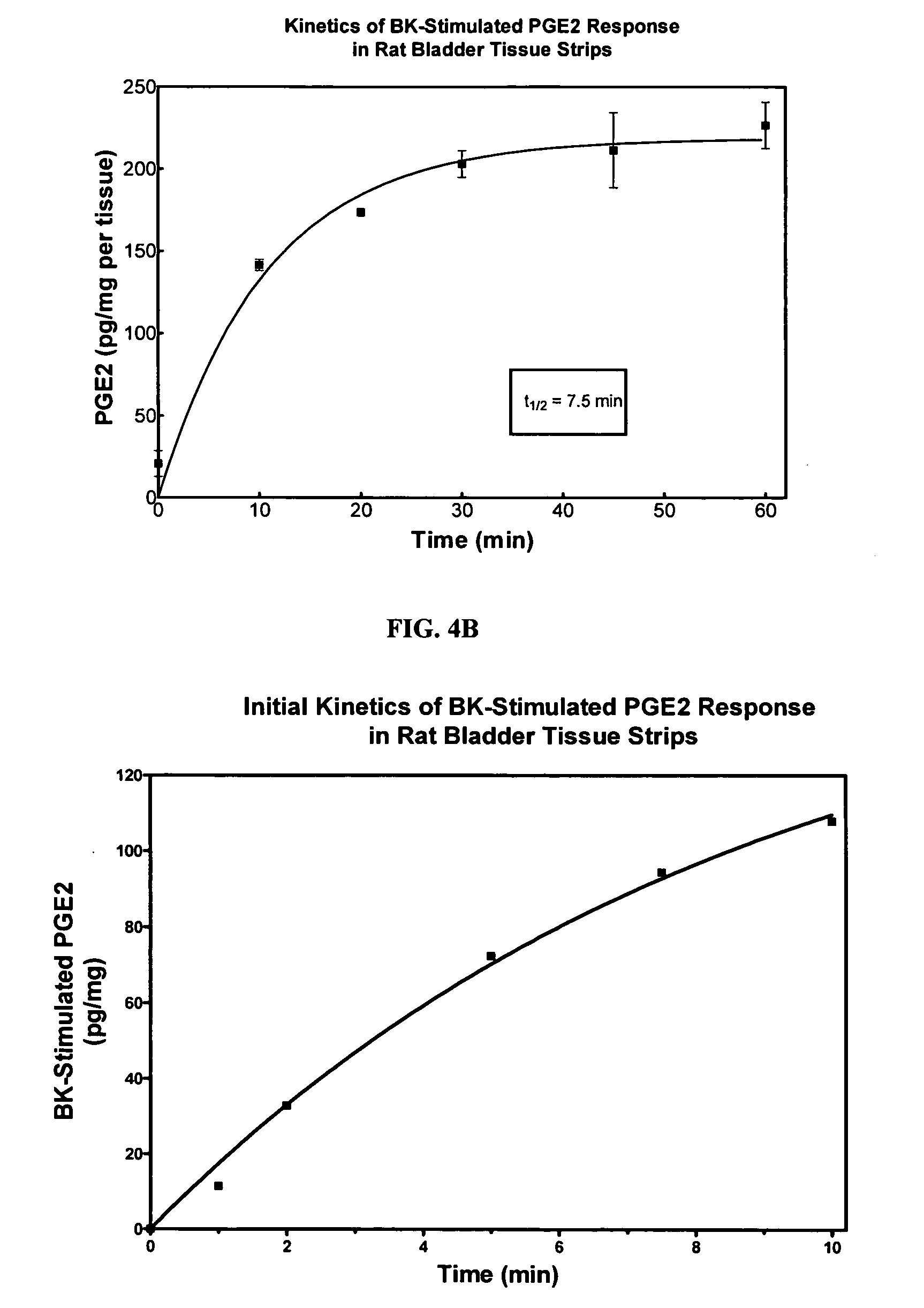
Cyclooxygenase inhibitor and calcium channel antagonist compositions and methods for use in urological procedures
ActiveUS20060263393A1Inhibits pain/inflammation and spasmInhibiting pain/inflammationBiocideNervous disorderNifedipineCyclooxygenase
Compositions of a cyclooxygenase inhibitor and a calcium channel antagonist in a liquid carrier. The composition may be administered the the urinary tract during urological diagnostic, interventional, surgical and other medical procedures. One disclosed composition comprises ketoprofen and nifedipine in a liquid irrigation carrier, and includes a solubilizing agent, stabilizing agents and a buffering agent.
Owner:OMEROS CORP
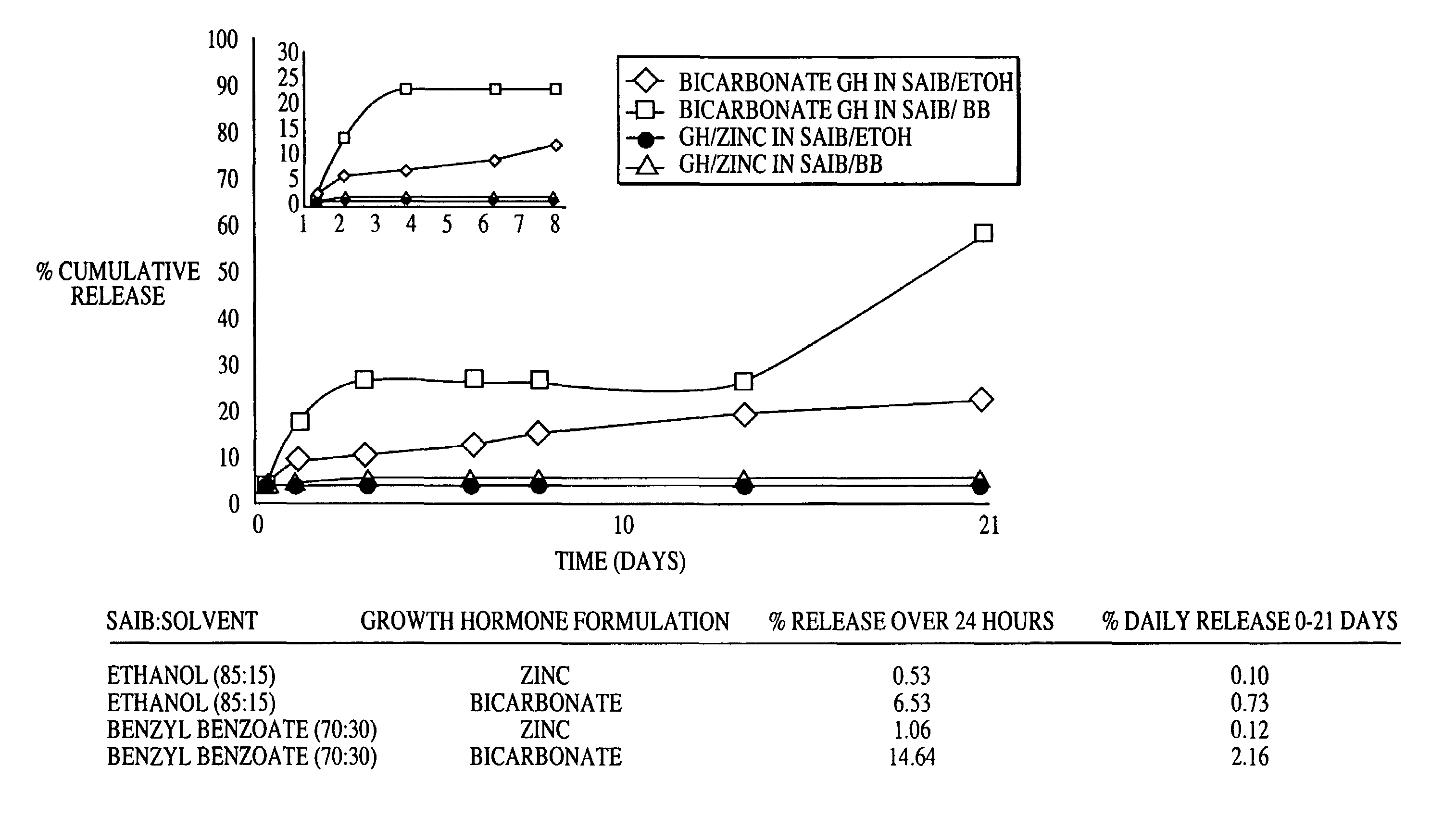
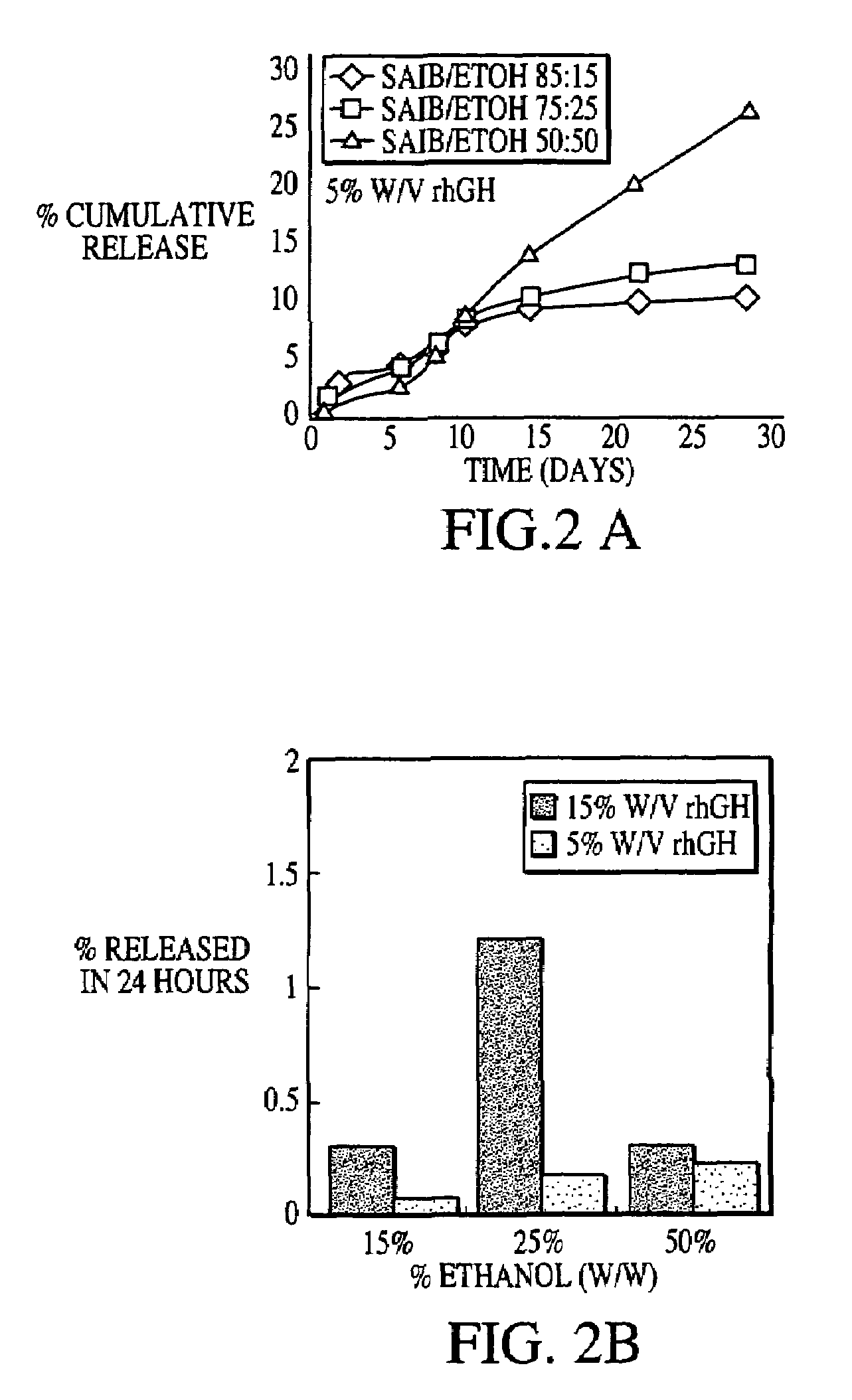
Sustained release formulations
A composition for sustained release comprises a carrier material containing a non-polymeric, non-water soluble liquid material having a viscosity of at least 5,000 cP at 37° C. that does not crytallize neat under ambient physiological conditions, a multivalent metal cation, and growth hormone.
Owner:DURECT CORP
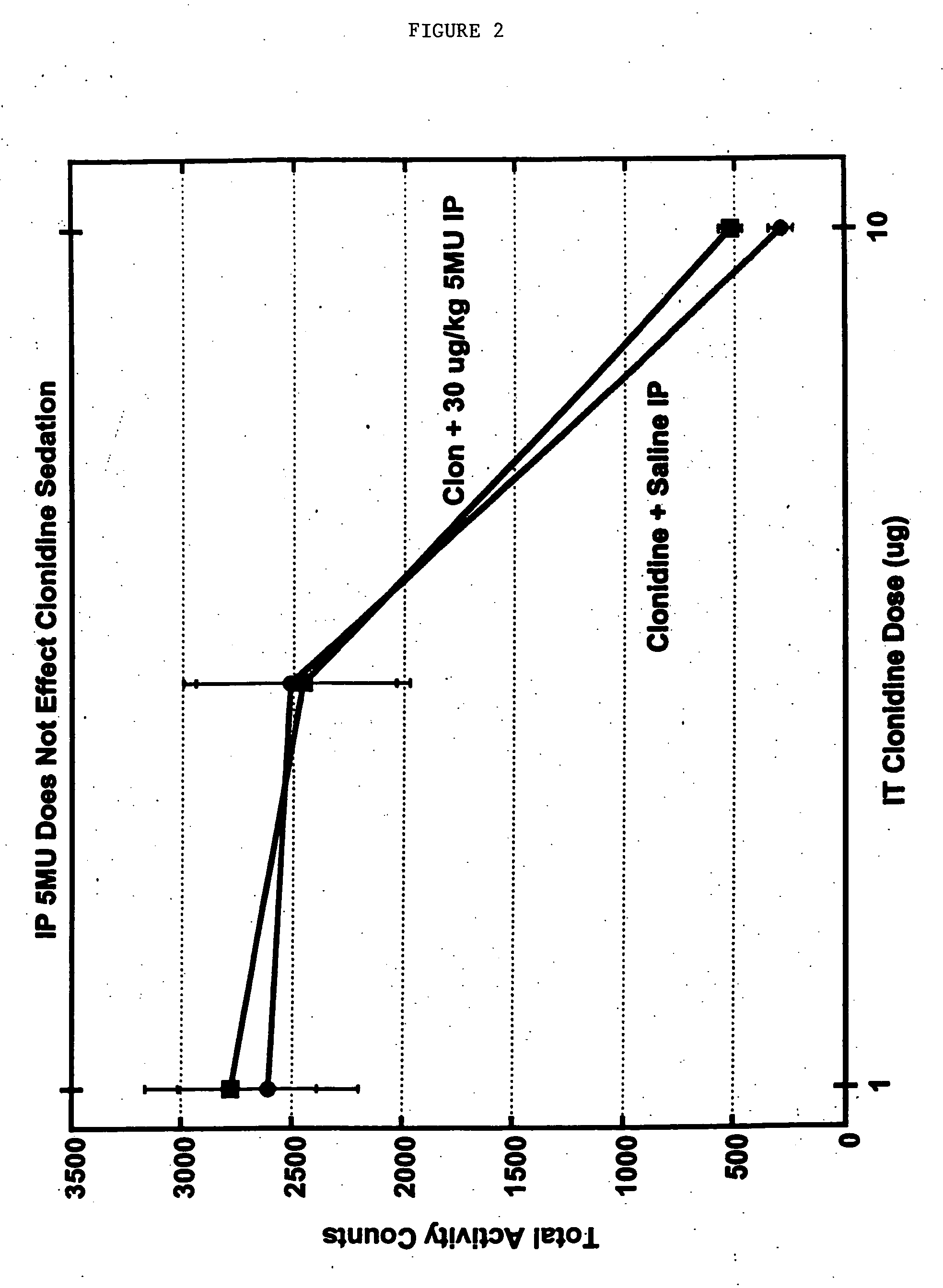
Methods and compositions for the treatment of pain and other alpha 2 adrenergic-mediated conditions
InactiveUS20050058696A1Improve effectivenessHigh activityOrganic active ingredientsSenses disorderAdrenergicSide effect
Owner:ALLERGAN INC
Osteogenic devices and methods of use thereof for repair of endochondral bone and osteochondral defects
InactiveUS7041641B2Avoid undesirable formationOvercome problemsPowder deliveryImpression capsRepair tissueNon union
Disclosed herein are improved osteogenic devices and methods of use thereof for repair of bone and cartilage defects. The devices and methods promote accelerated formation of repair tissue with enhanced stability using less osteogenic protein than devices in the art. Defects susceptible to repair with the instant invention include, but are not limited to: critical size defects, non-critical size defects, non-union fractures, fractures, osteochondral defects, subchondral defects, and defects resulting from degenerative diseases such as osteochondritis dessicans.
Owner:MARIEL THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com