Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1864results about How to "Lower potential" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Instrument introducer
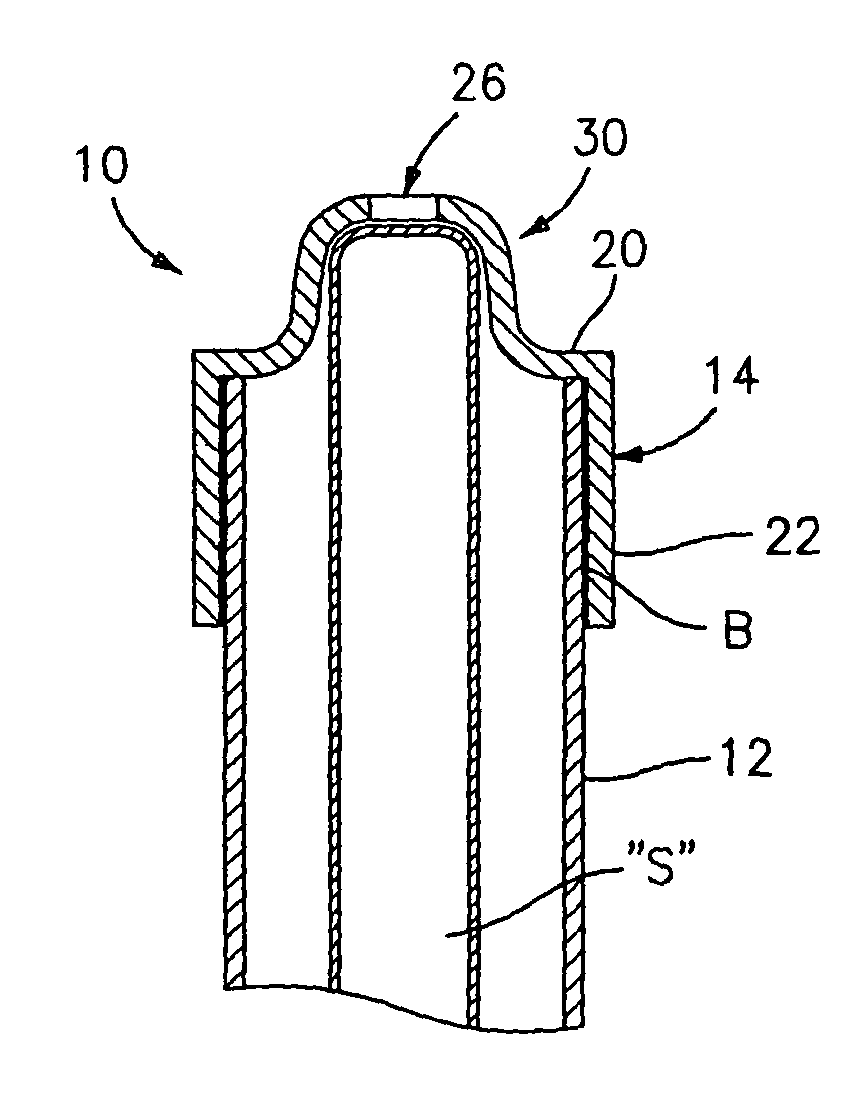
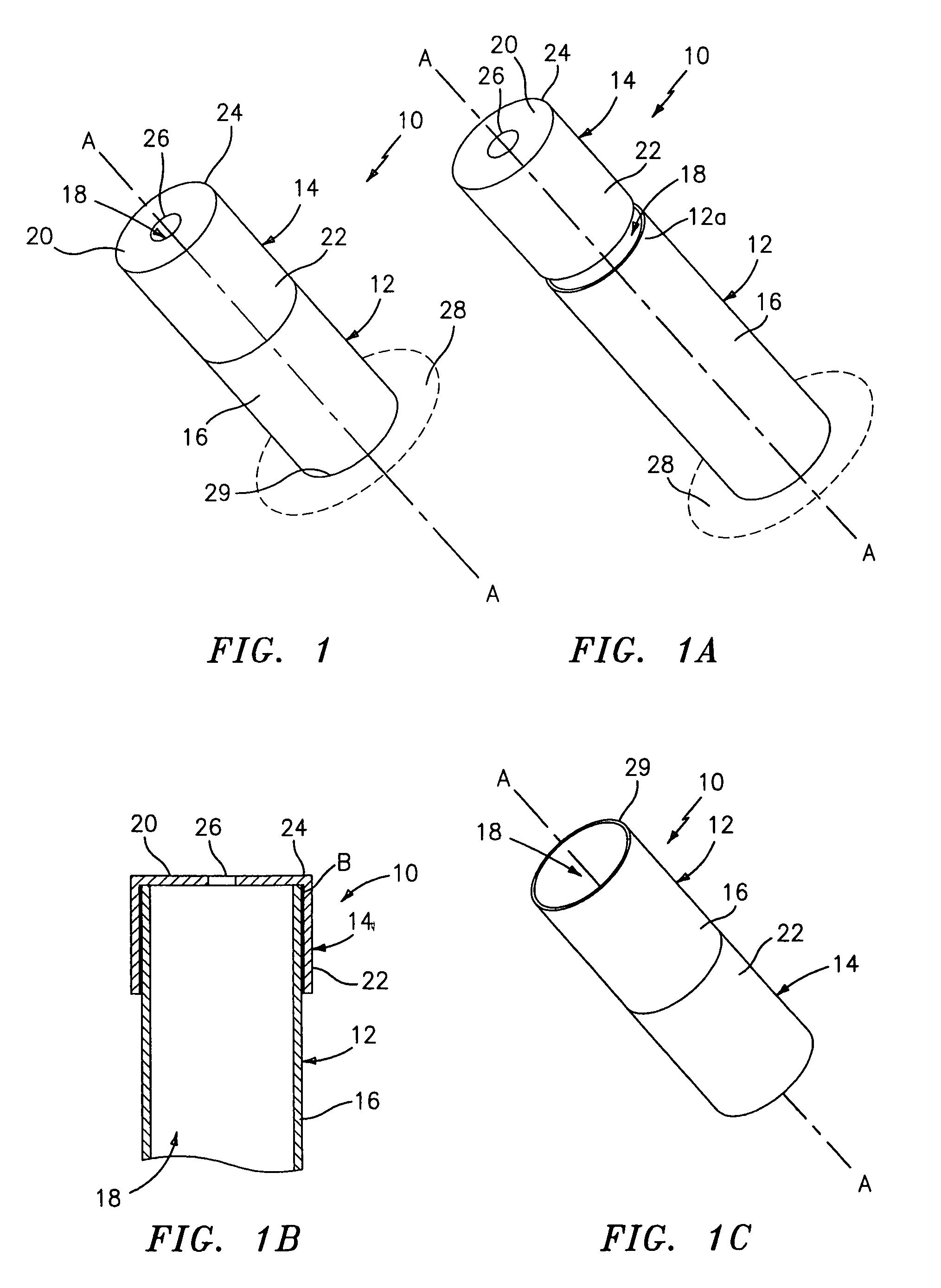
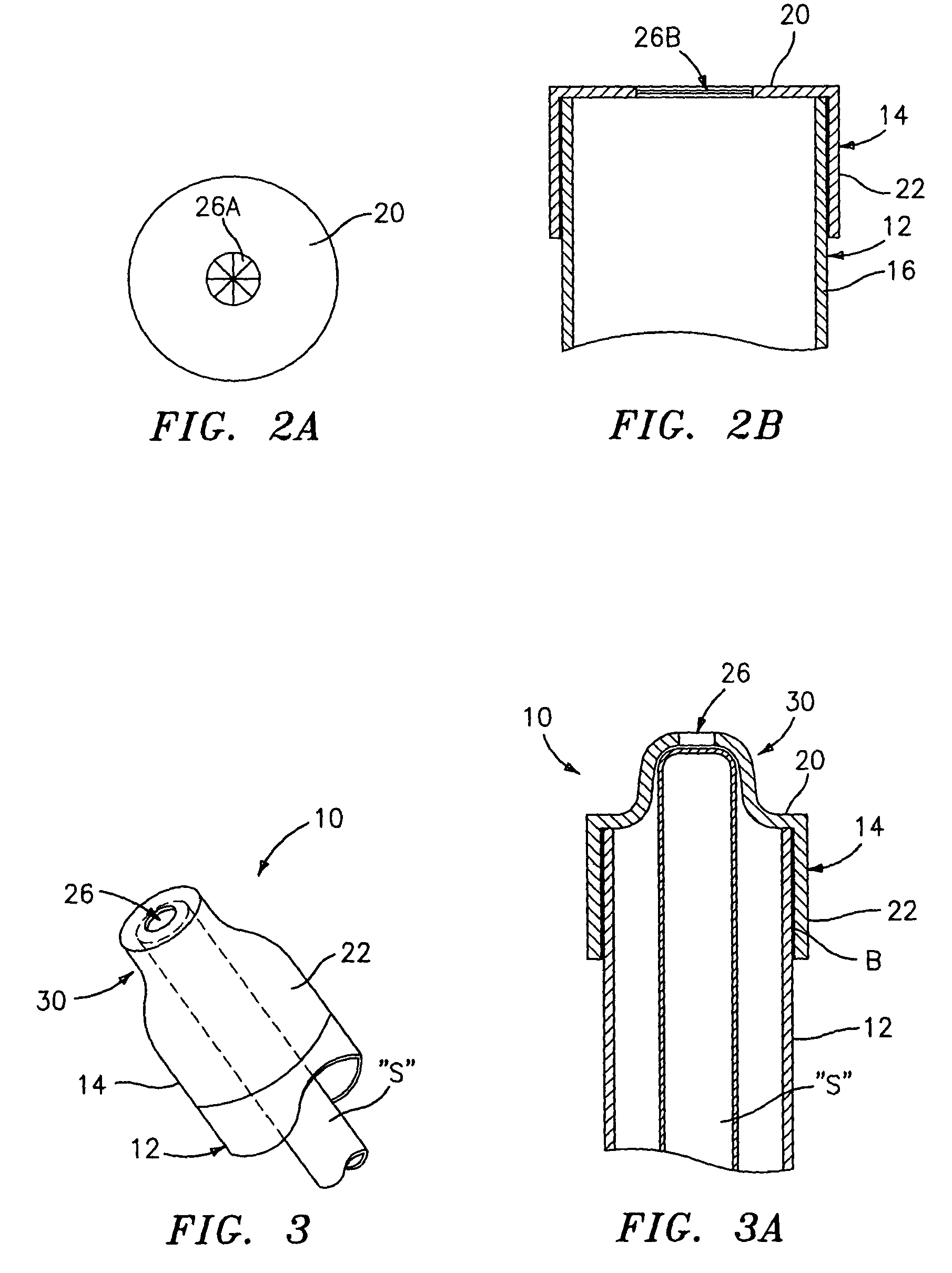
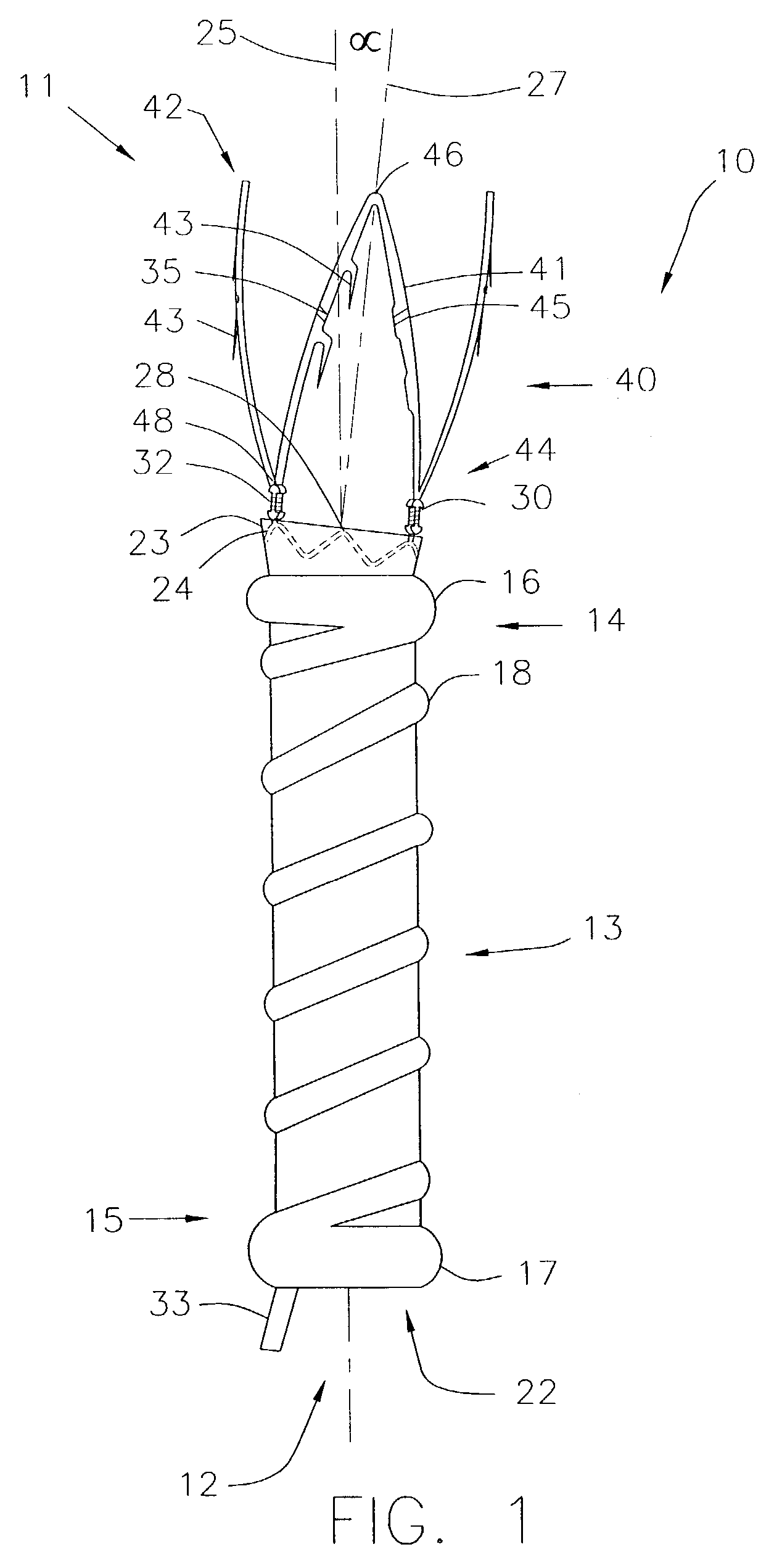
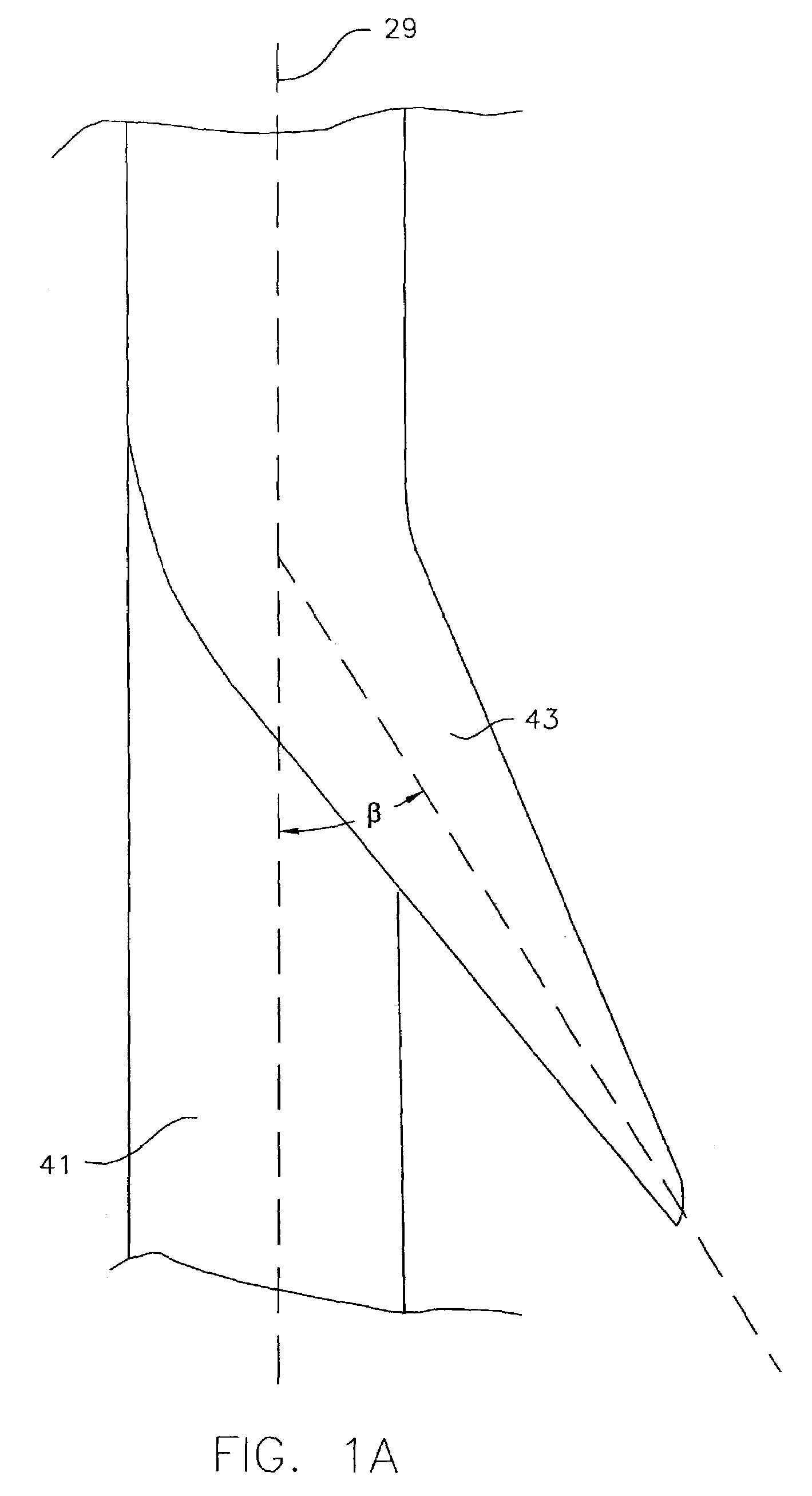
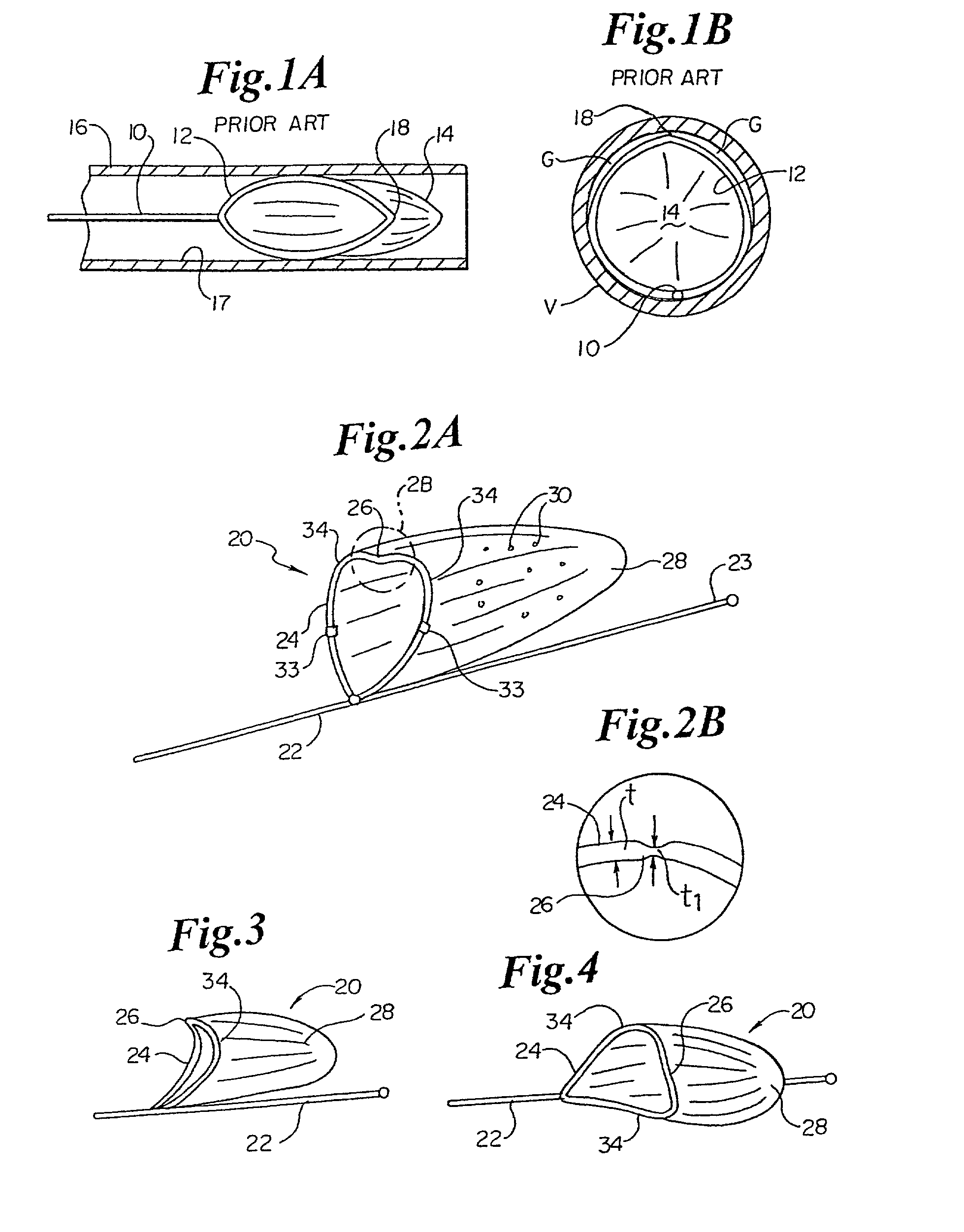
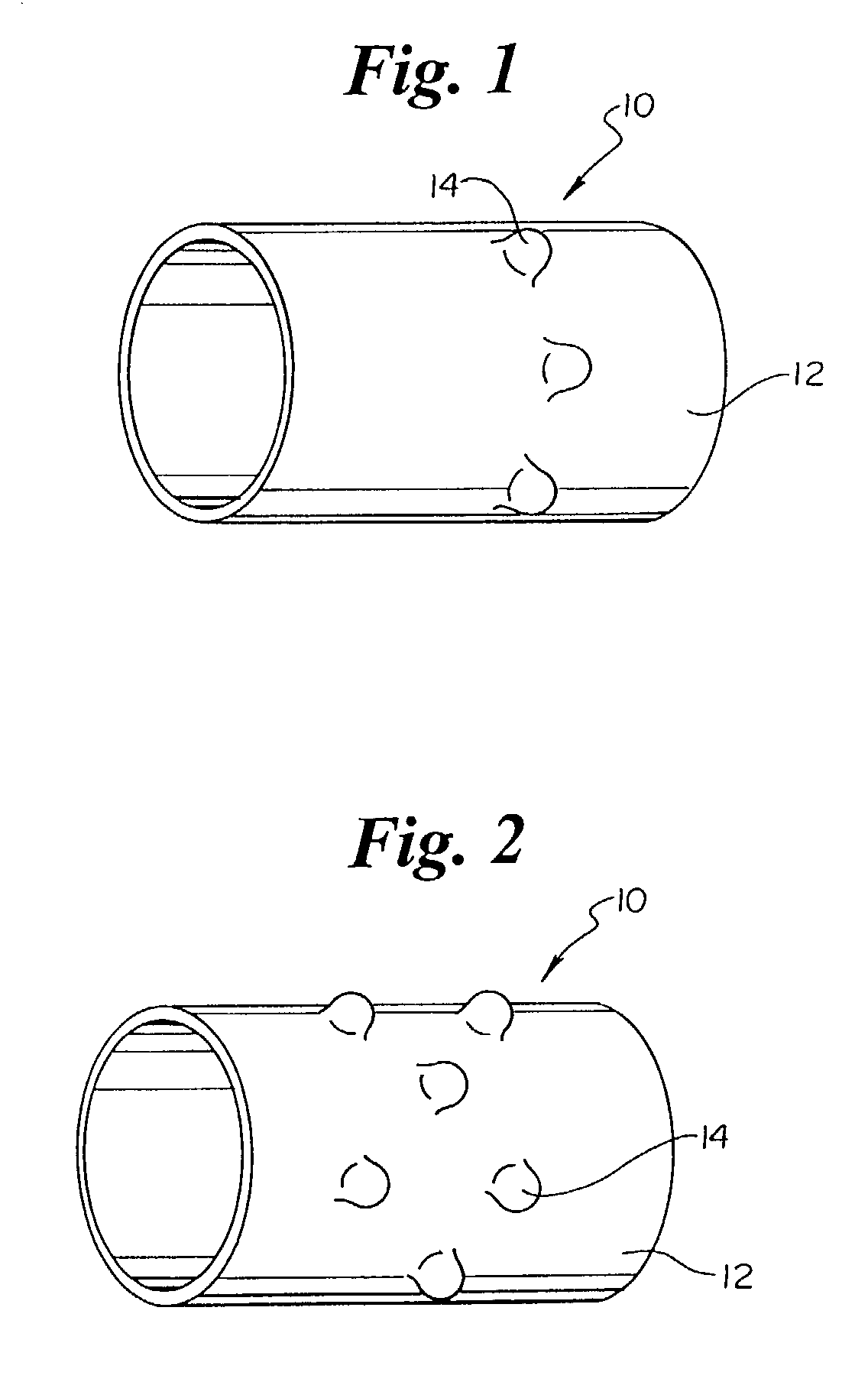
InactiveUS7846149B2Easy to insertLower potentialInternal osteosythesisCannulasDistal portionSurgical device
Instrument introducers are disclosed which facilitate the insertion of a surgical instrument into a cavity or a body of a patient. In one embodiment, the instrument introducer includes a hollow elongate cylindrical body including a distal end portion terminating in a distal edge and a proximal end portion, the cylindrical body defining a central longitudinal axis, and an elastomeric cap secured to the distal end portion of the cylindrical body, the cap including a distal end wall having an outer terminal edge and an annular side wall depending from the outer terminal edge thereof. The distal end wall includes an aperture formed therein, wherein a center of the aperture is coaxially aligned with the central longitudinal axis.
Owner:TYCO HEALTHCARE GRP LP
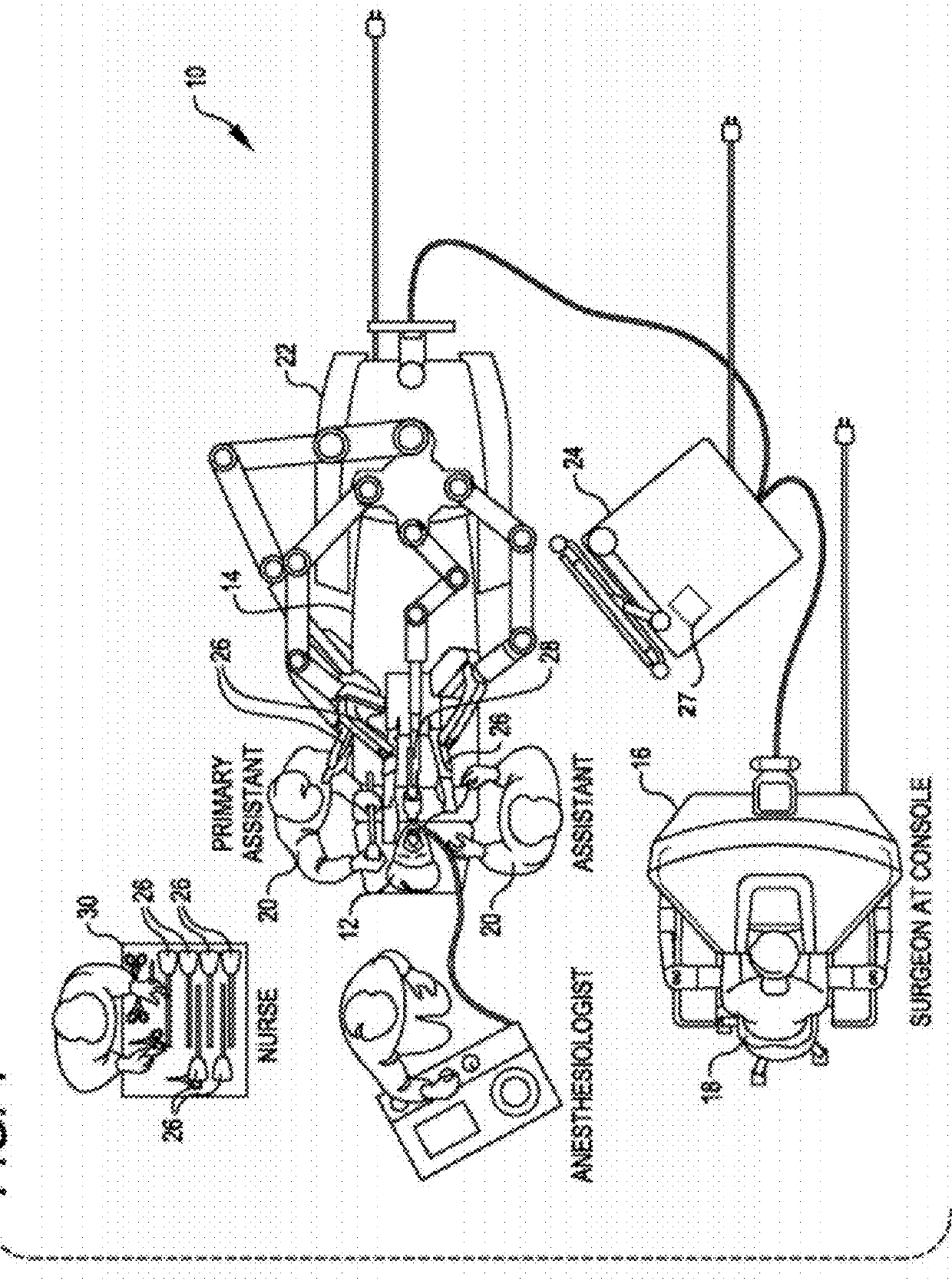
Methods and systems for detecting clamping or firing failure
ActiveUS9226750B2Lower potentialHigh strengthInternal osteosythesisDiagnosticsActuatorControl theory
Systems and methods are provided for detecting failure in clamping of a material and / or firing of a staple into a clamped material and indicating such failure to a user on a user interface. The system and methods are particularly suited for use with end effectors having closing and / or firing mechanisms coupled to an actuator. By monitoring a driving parameter of an actuator that effects the clamping and / or firing, the systems and methods provide an indication of failure in response to the monitored drive parameter. In some embodiments, an indication of failure is output when the monitored drive parameter is outside an acceptable range of desired driving parameters during clamping and / or firing. The disclosed systems and methods are particularly beneficial when used for minimally invasive surgery.
Owner:INTUITIVE SURGICAL OPERATIONS INC +1
Methods and systems for detecting staple cartridge misfire or failure
ActiveUS9393017B2Reduce the amount requiredShorten the lengthDiagnosticsSurgical robotsEngineeringUser interface
Owner:INTUITIVE SURGICAL OPERATIONS INC
High frequency interconnect structures, electronic assemblies that utilize high frequency interconnect structures, and methods of operating the same
ActiveUS20120306587A1Lower potentialMultiple-port networksFrequency measurement arrangementEngineeringElectronic assemblies
High frequency interconnect structures, electronic assemblies that utilize high frequency interconnect structures, and methods of operating the same. The high frequency interconnect structures include a plurality of dielectric waveguides and are configured to communicatively connect a plurality of transmitters with a plurality of receivers and to convey a plurality of signals therebetween. The plurality of signals may include a plurality of electromagnetic waves and may have a frequency of at least 200 GHz. The high frequency interconnect structures further may be configured to decrease a potential for crosstalk between a first signal that is conveyed by a first dielectric waveguide of the plurality of dielectric waveguides and a second signal that is conveyed by a second dielectric waveguide of the plurality of dielectric waveguides, such as through control of a passband of the first dielectric waveguide relative to the second dielectric waveguide and / or the use of a crosstalk mitigation structure.
Owner:FORMFACTOR INC
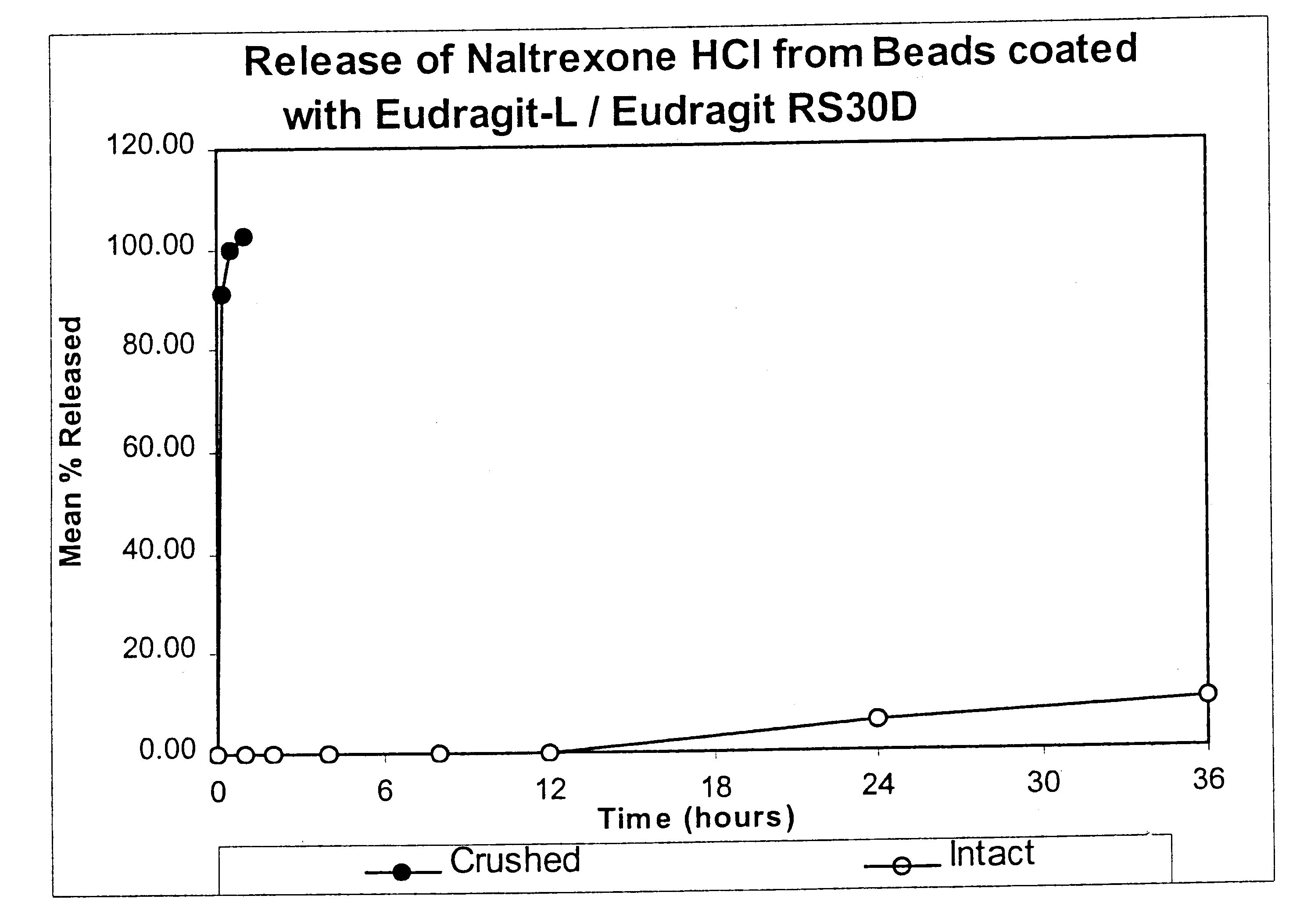
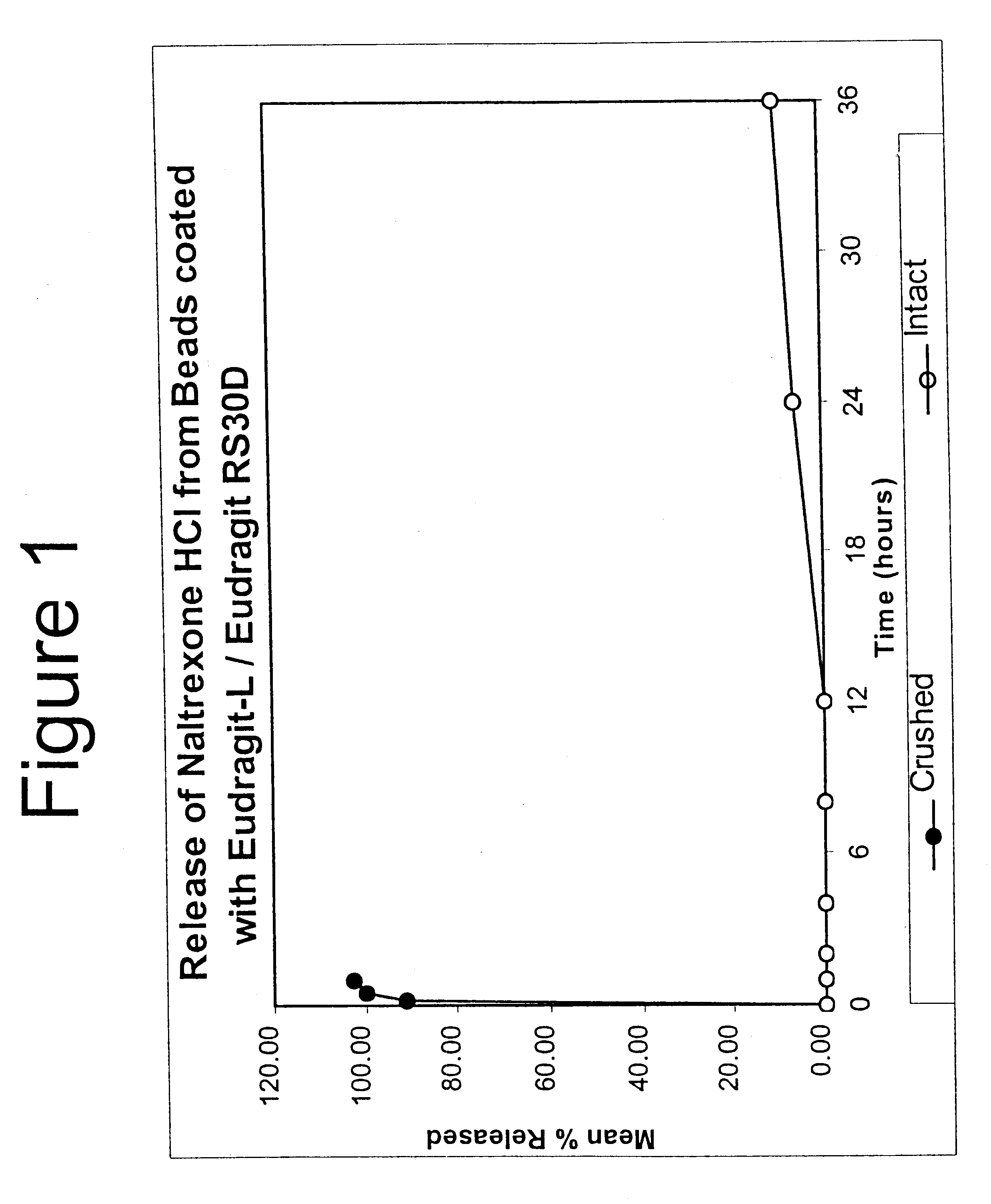
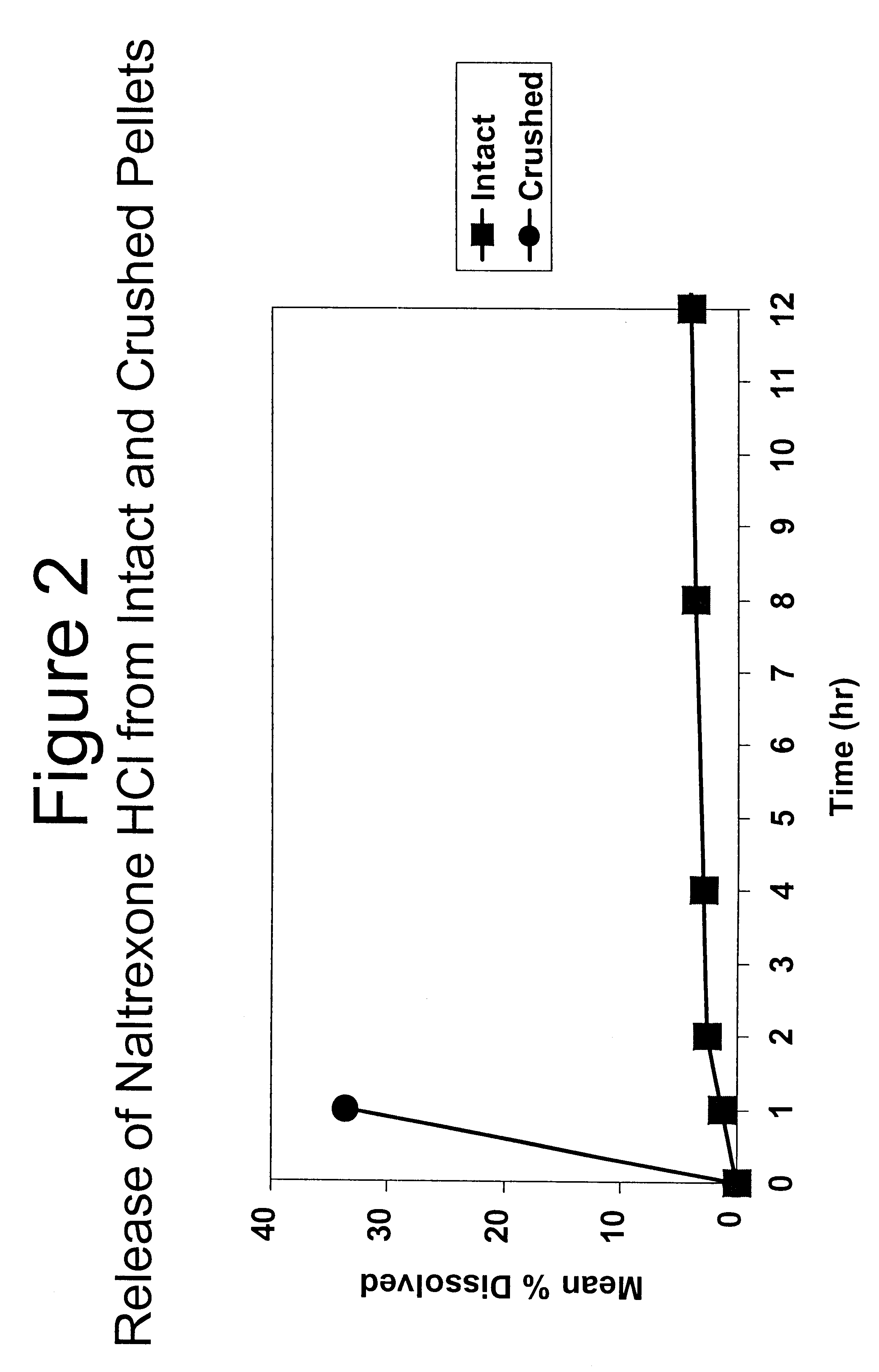
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
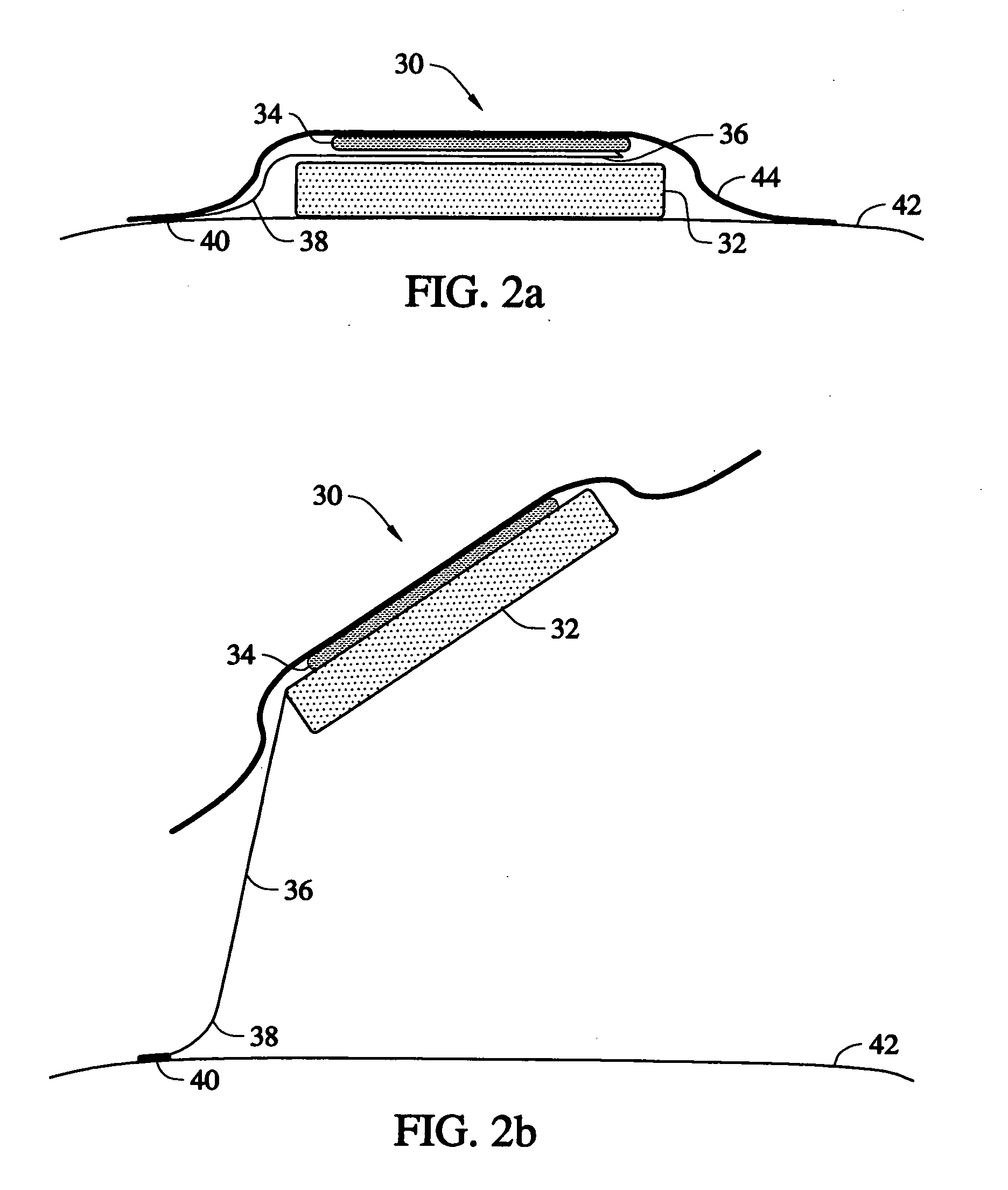
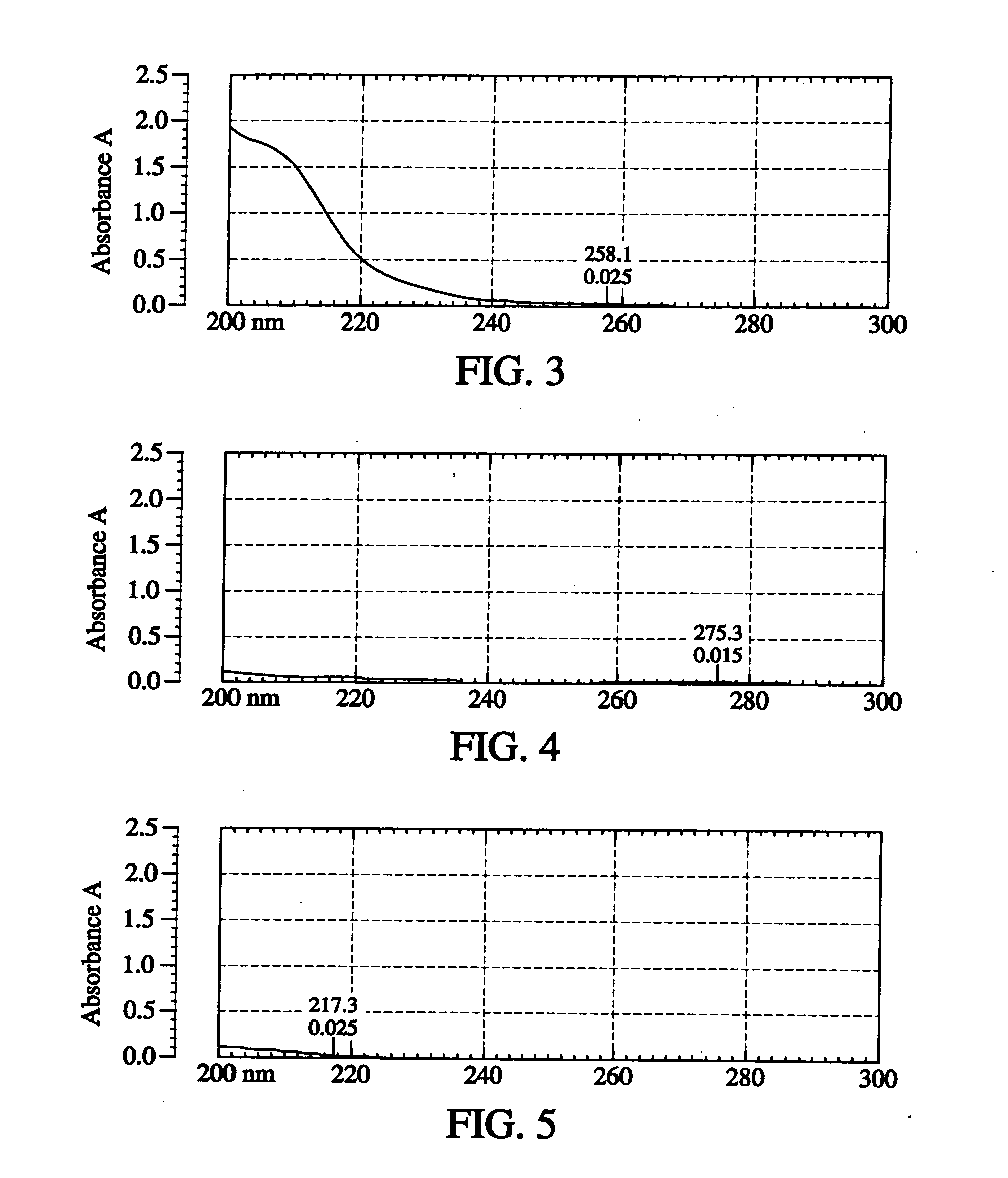
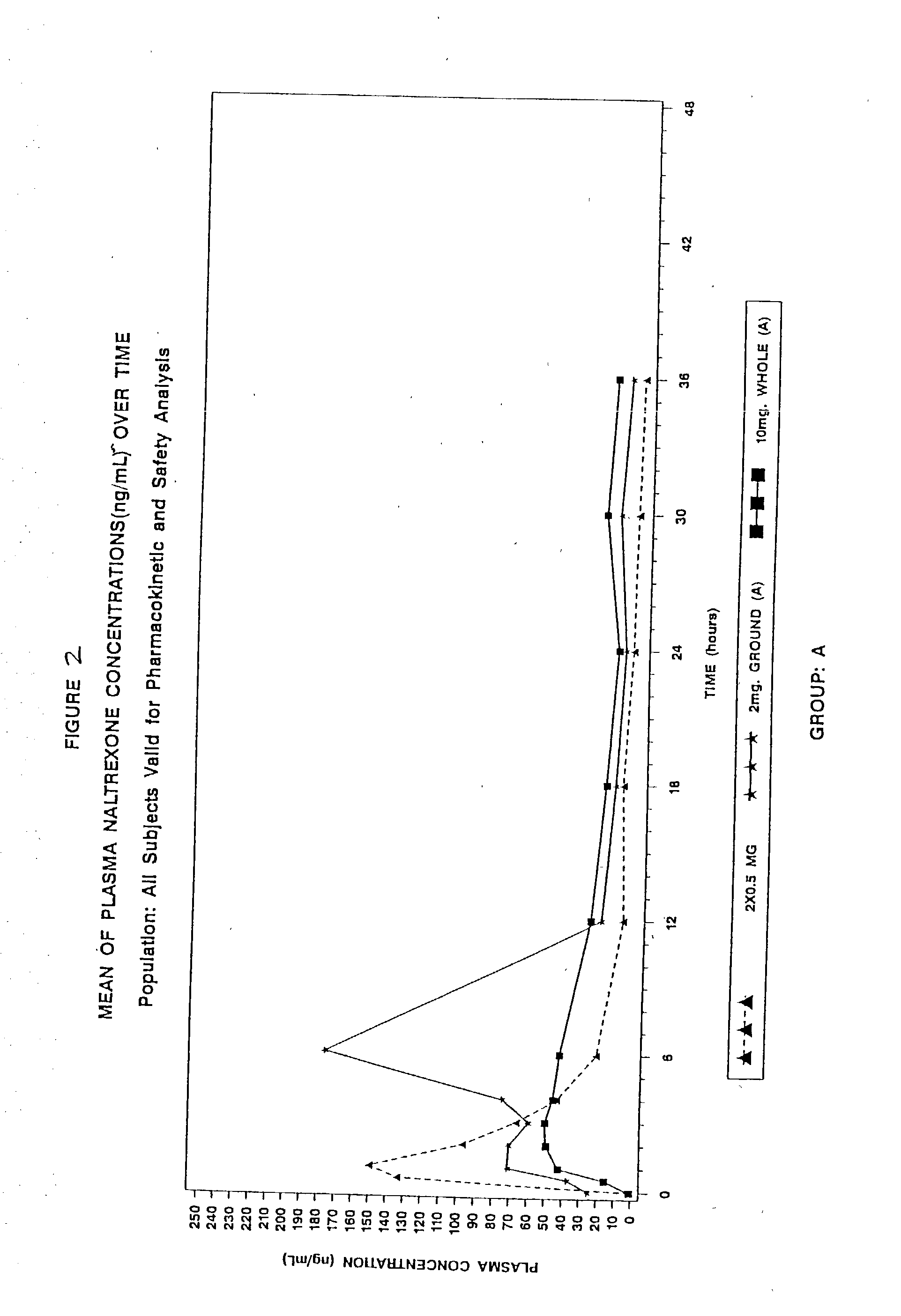
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Surgical access system and related methods
ActiveUS20050149035A1Increase the number ofStructural damageSurgeryJoint implantsDistractionNerve structure
A surgical access system including a tissue distraction assembly and a tissue retraction assembly, both of which may be equipped with one or more electrodes for use in detecting the existence of (and optionally the distance and / or direction to) neural structures before, during, and after the establishment of an operative corridor to a surgical target site. Some embodiments of the surgical access system may be particularly suited for establishing an operative corridor to a surgical target site in the spine. Such an operative corridor may be established through the retroperitoneal space and the psoas muscle during a direct lateral, retroperitoneal approach to the spine.
Owner:NUVASIVE
Device and method for continuously shuffling and monitoring cards
InactiveUS6588751B1Reducing eliminating numberLow costSpeech analysisCard gamesPlaying cardHandling system
The present invention provides an apparatus and method for moving playing cards from a first group of cards into a second group of cards, wherein the second group of cards is randomly arranged or shuffled. The apparatus comprises a card receiver for receiving the first group of cards, a single stack of card-receiving compartments generally adjacent to the card receiver, the stack generally vertically movable, an elevator for moving the stack, a card-moving mechanism between the card receiver and the stack for moving cards one at a time into a selected one of the compartments, another card moving mechanism for moving cards from one of the compartments to a second card receiver and a microprocessor that controls the card-moving mechanisms and the elevator. A count of cards within specified areas of the card handling system is maintained and card handling is halted and all cards counted by adding a count of all cards not within the specified areas to the total of cards counted within the specified areas.
Owner:BALLY GAMING INC
Methods and systems for detecting staple cartridge misfire or failure
ActiveUS20120248167A1Reduce the amount requiredShorten the lengthStapling toolsDiagnosticsSurgical stapleEngineering
Systems and methods are provided for detecting misfire of a surgical staple cartridge and for indicating such misfire to a user on a user interface. An actuation force applied to a staple cartridge is measured and compared to a threshold actuation force. In response to the comparison, a controller determines if there has been a misfire of the surgical staple cartridge. A determination that misfire has occurred is made when the measured actuation force is greater than a maximum threshold force or less than a minimum threshold force, and under such circumstances, the controller terminates the staple firing sequence.
Owner:INTUITIVE SURGICAL OPERATIONS INC
Method and apparatus relating to electronic smoking-substitute devices
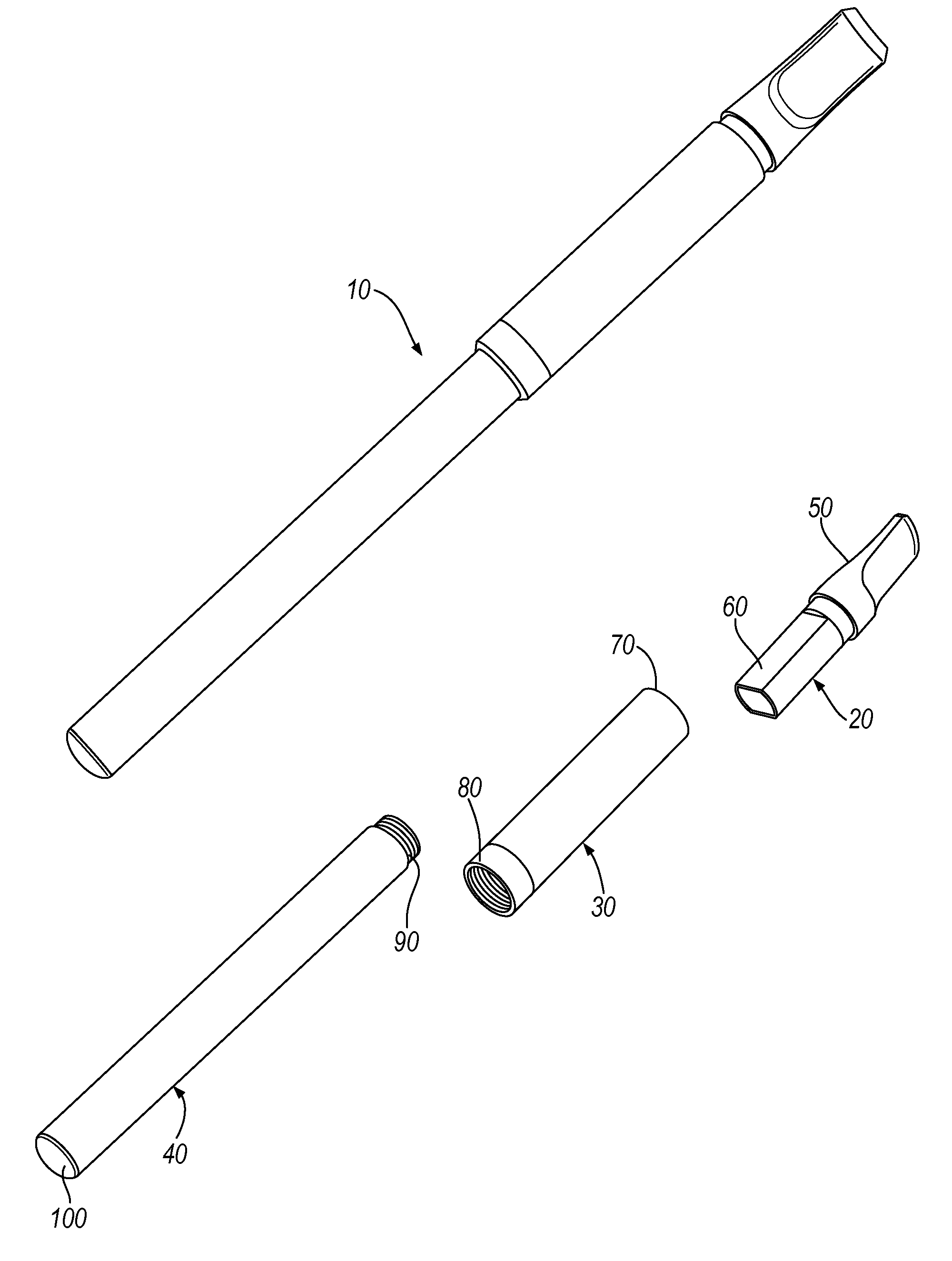
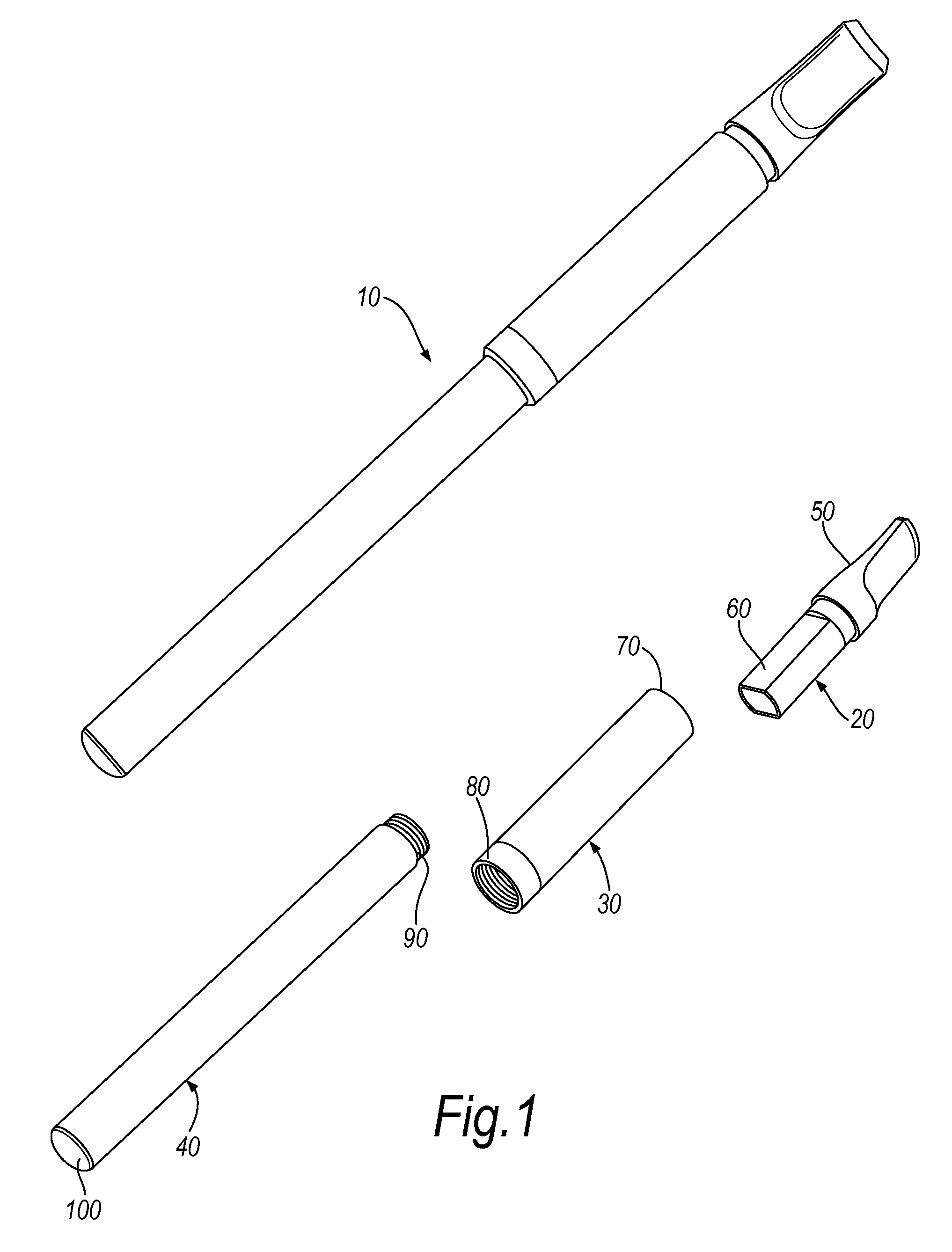
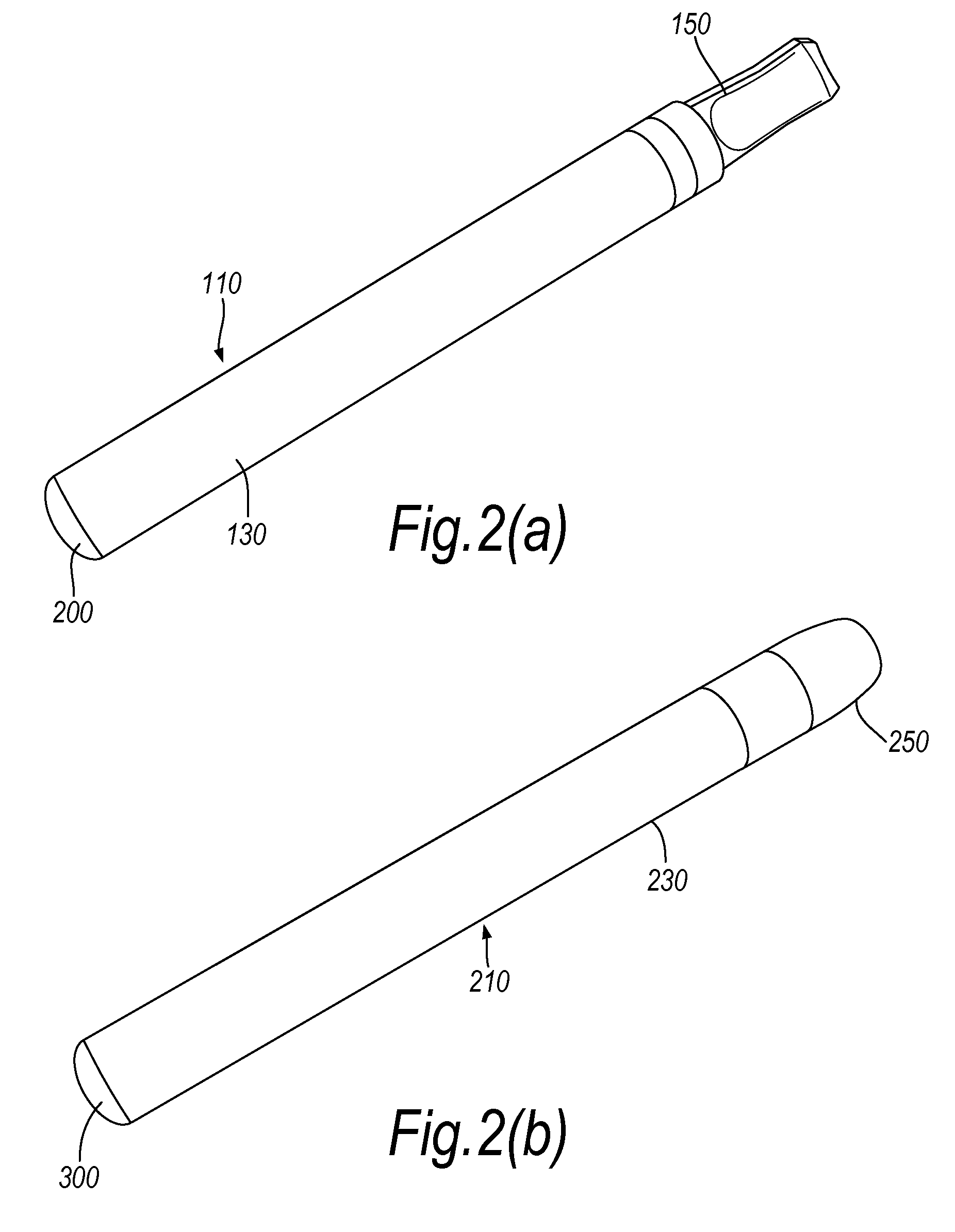
InactiveUS20100031968A1Increase the effective areaSmall batteryTobacco devicesEngineeringHeating element
An electronic smoking-substitute device 110 includes a tube containing a reservoir 310 containing a liquid. The liquid includes a substance to be inhaled by the user, for example a nicotine dilution. The device also has a heating element 320, the heating element is a coil and is in direct contact with the reservoir 310. A power source 340 is arranged to power the heating element.
Owner:XL DISTRIBUTORS
Advanced endovascular graft
This invention is a system for the treatment of body passageways; in particular, vessels with vascular disease. The system includes an endovascular graft with a low-profile delivery configuration and a deployed configuration in which it conforms to the morphology of the vessel or body passageway to be treated as well as various connector members and stents. The graft is made from an inflatable graft body section and may be bifurcated. One or more inflatable cuffs may be disposed at either end of the graft body section. At least one inflatable channel is disposed between and in fluid communication with the inflatable cuffs.
Owner:BOSTON SCI CORP
Methods and systems for the manufacture of layered three-dimensional forms
ActiveUS20050017394A1Lower potentialEasy to controlConfectionerySweetmeatsParticulatesBiological activation
New methods and systems for manufacturing a three-dimensional form, comprising steps of providing a plurality of particulates; contacting the particulates with an activation agent; contacting particulates having the activation agent with a binder material that is activatable by the activation agent; at least partially hardening the binder for forming a layer of the three-dimensional form; and repeating these steps to form the remainder of the three-dimensional form. Following sequential application of all required layers and binder material to make the form, the unbound particles are appropriately removed (and optionally re-used), to result in the desired three-dimensional form. The invention also contemplates a novel method for preparing a form, where unbound particulates free of binder material are re-claimed.
Owner:EXONE
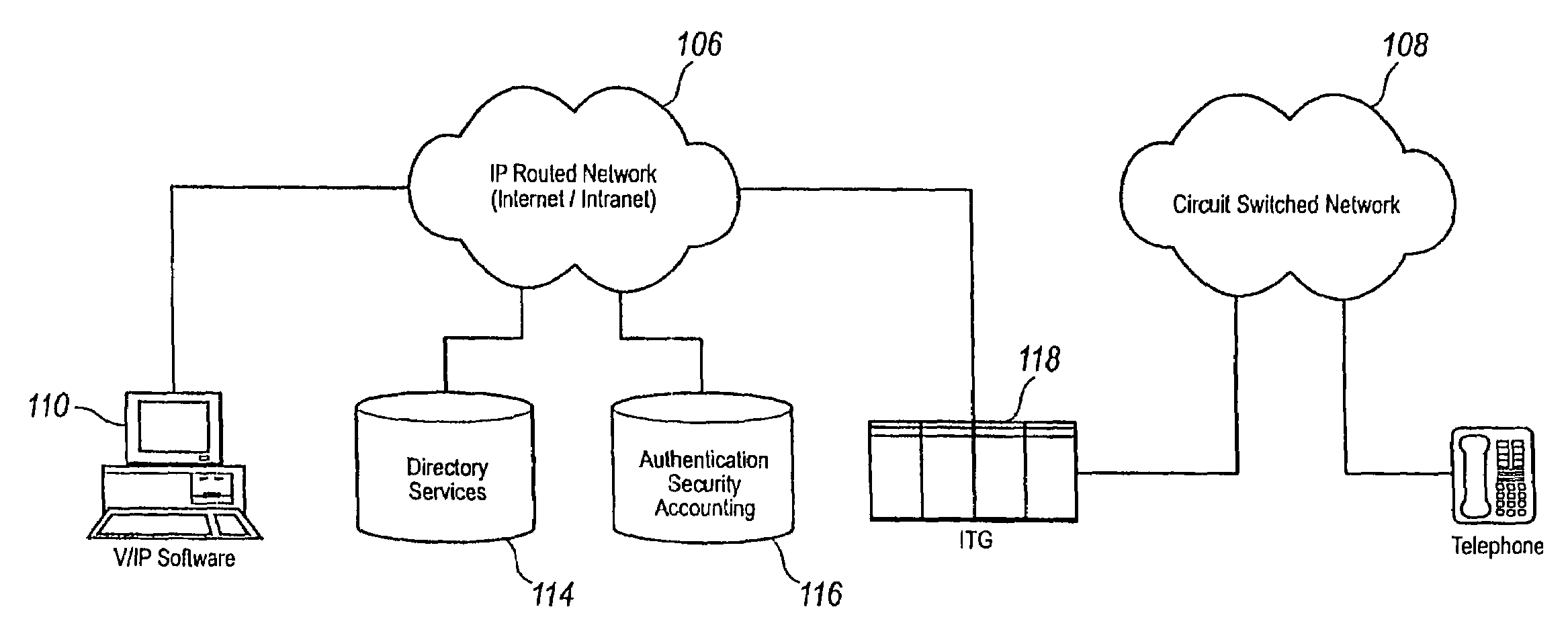
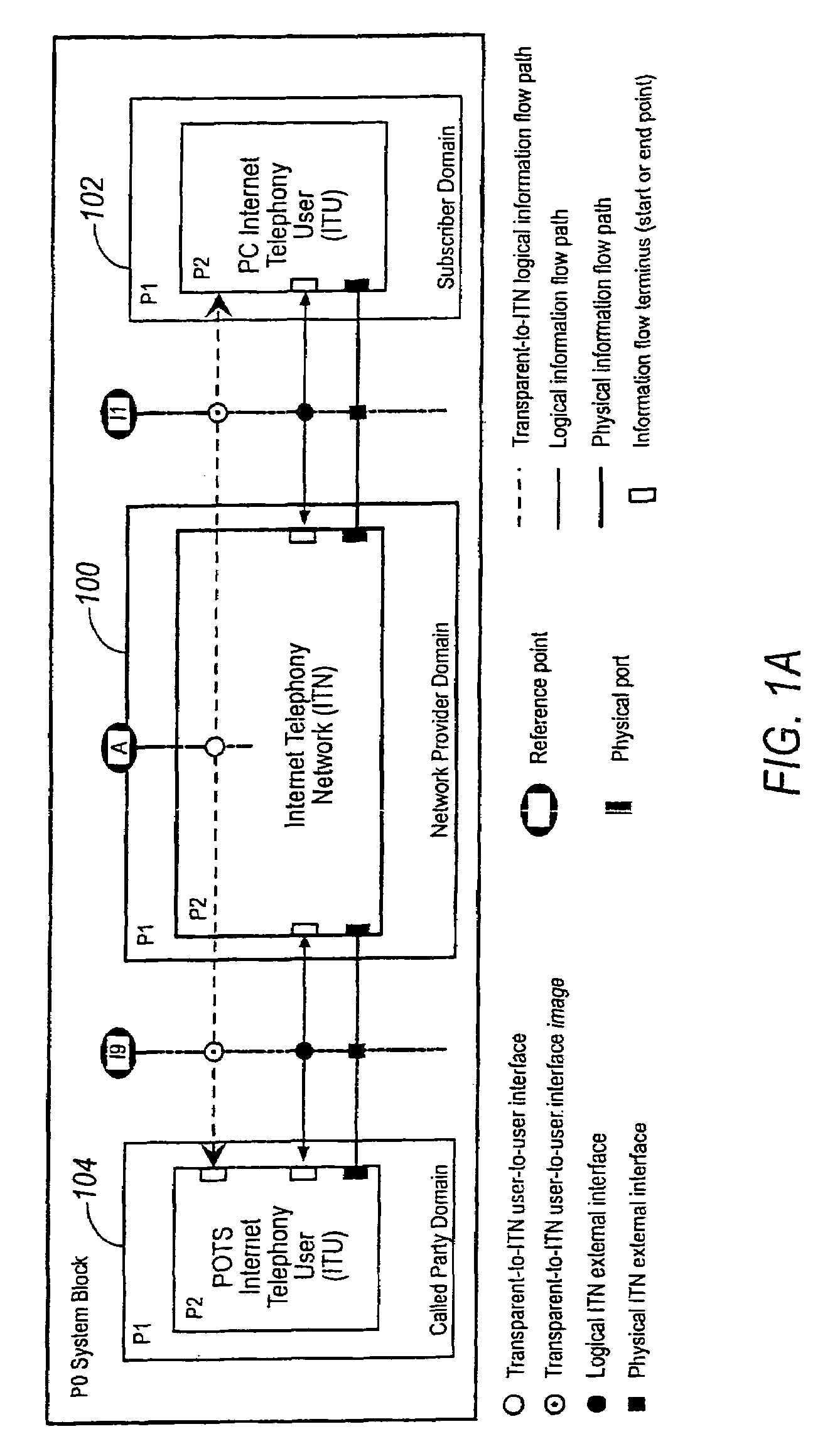
Network session management
InactiveUS7948968B2Easy accessPrevent overrunningInterconnection arrangementsMetering/charging/biilling arrangementsTime informationThe Internet
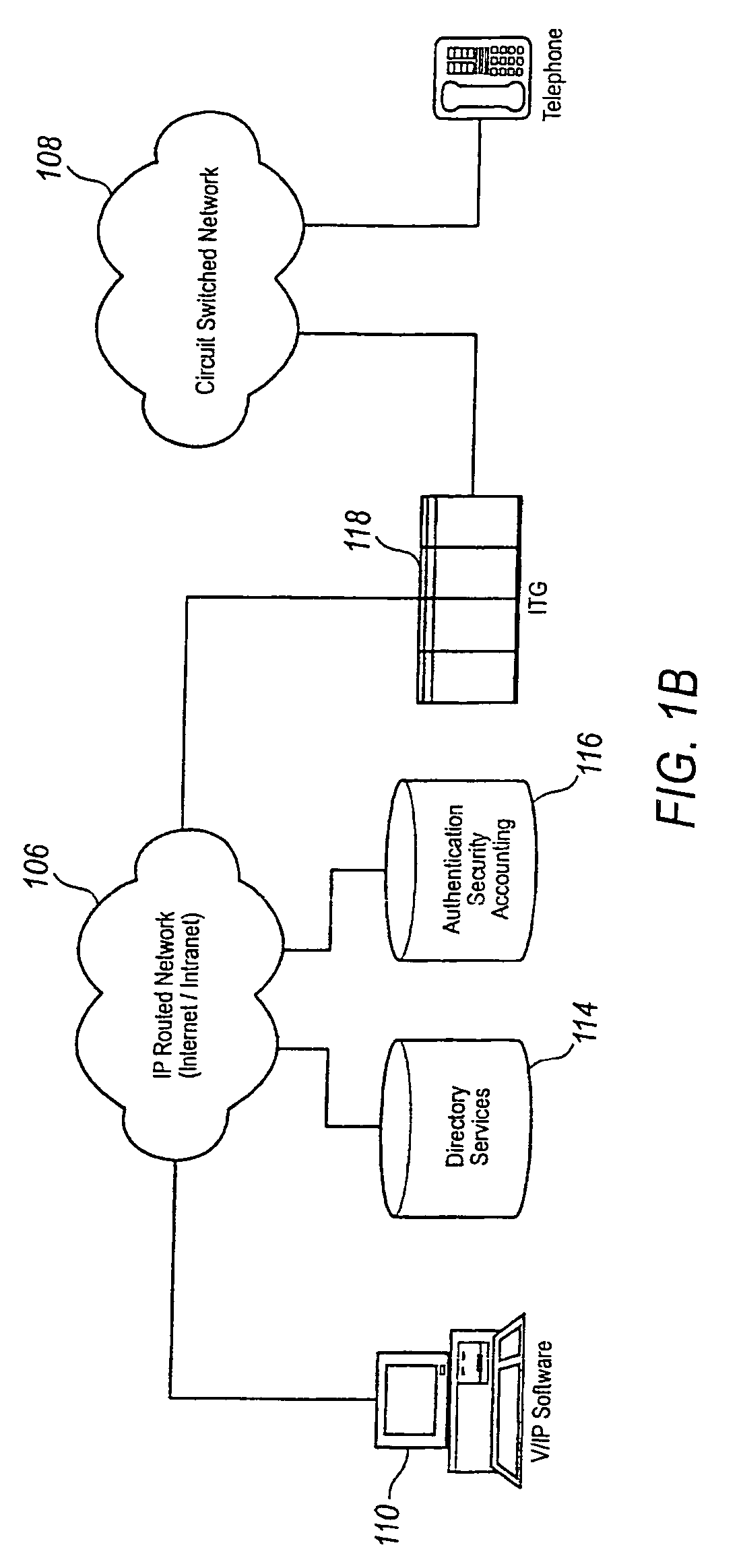
A communication system providing telephony communication across combined circuit switched and packet switched networks, such as a telephone network and the Internet, which are connectable to terminals, such as telephones and computers, for selective communication therebetween. The communication system includes an authorization and account control object in the packet switched network, multiple gateways between the circuit switched and packet switched networks providing controlled connectivity between those networks, and an information retrieval object in the packet switched network, wherein the authorization and account control object maintains a substantially real time record of user accounts and usage, and the information and retrieval object provides substantially real time retrieval of selected information from the authorization and account control object. The retrieval object provides on demand to terminals which provide authentication for access to an identified account information regarding that account. The information regarding the account is substantially real time information including information with respect to communications in progress, which are chargeable to the account which has been authenticated. The authorization and account control object is preferably a unitary logical object having distributed instances thereof handling multitudinous accounts of widely separated terminals. The retrieval object provides isolation of the authorization and account control object permitting simultaneous multitasking by the authorization and account control object and the retrieval object respectively.
Owner:VERIZON PATENT & LICENSING INC
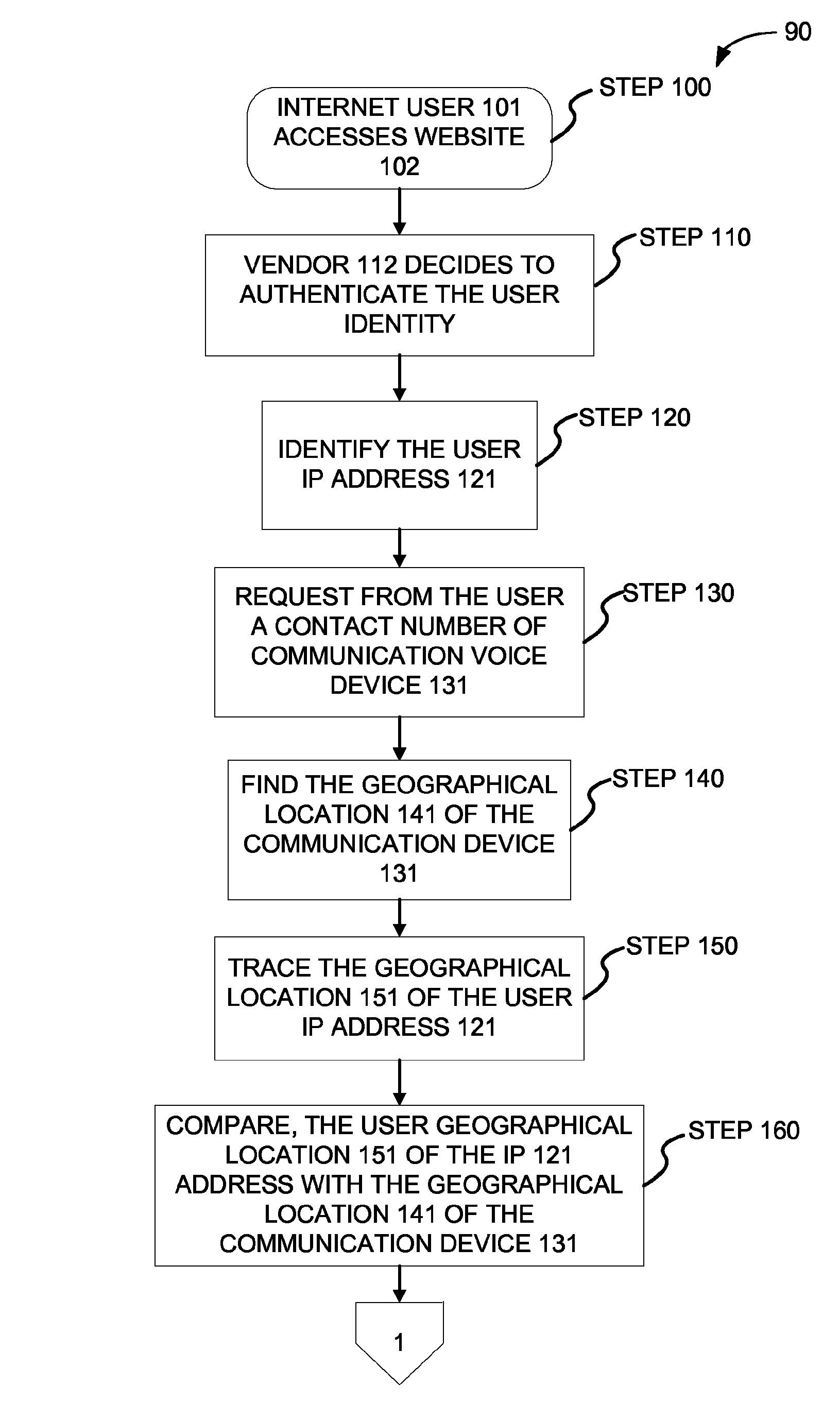
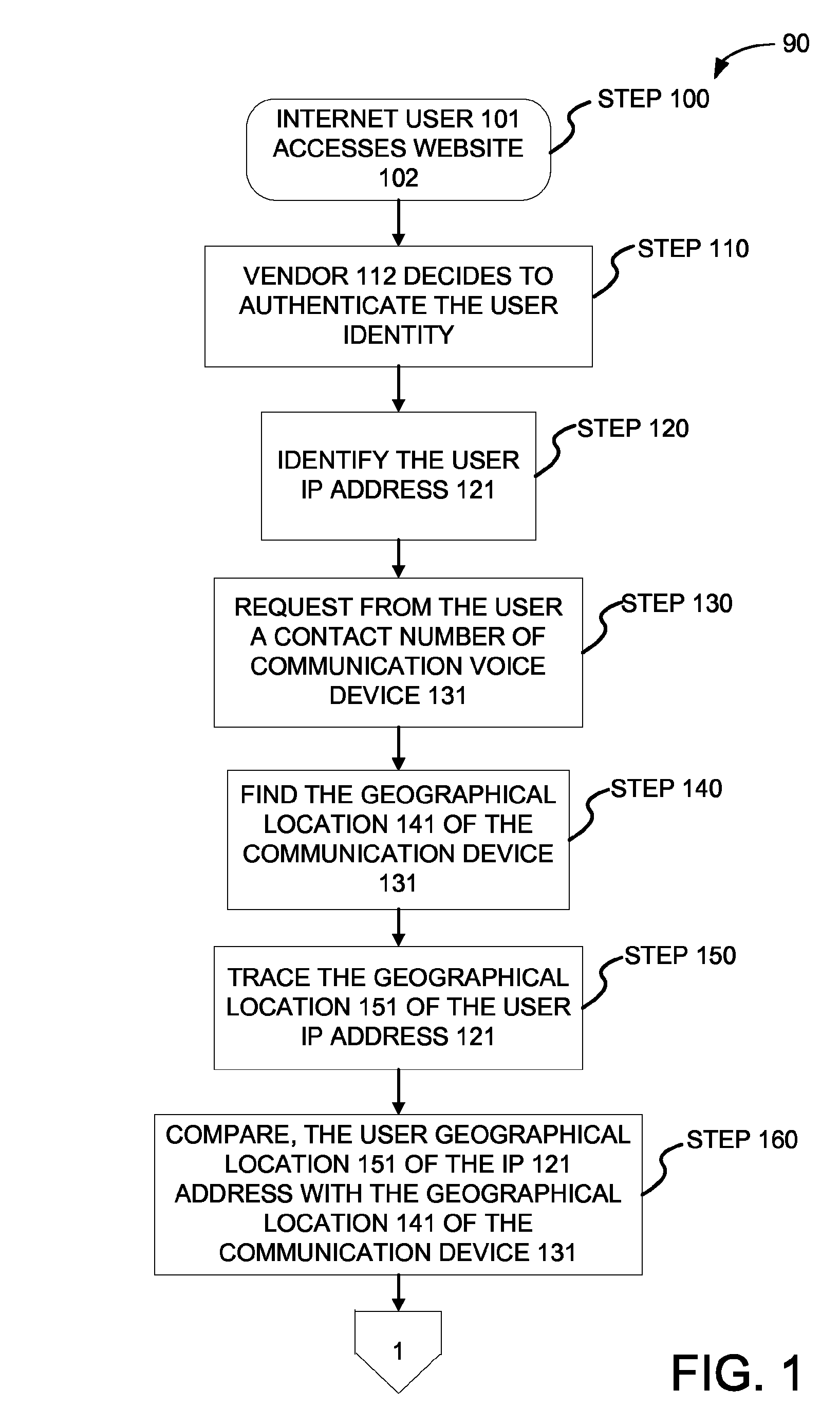
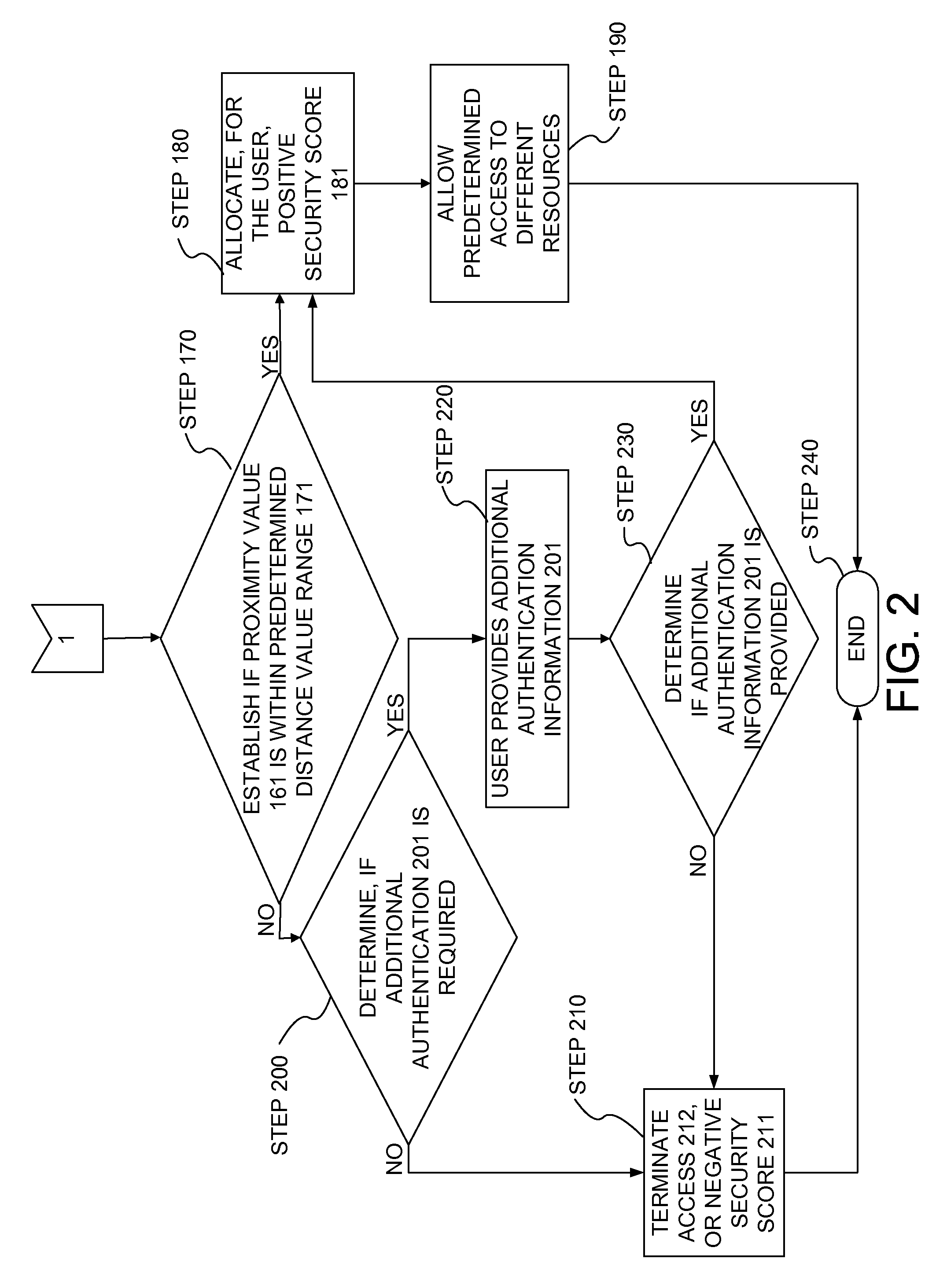
Method and system for authenticating internet user identity
InactiveUS8656458B2Lower potentialDigital data processing detailsSpecial service for subscribersIp addressInternet users
Owner:HEFFEZ GUY
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
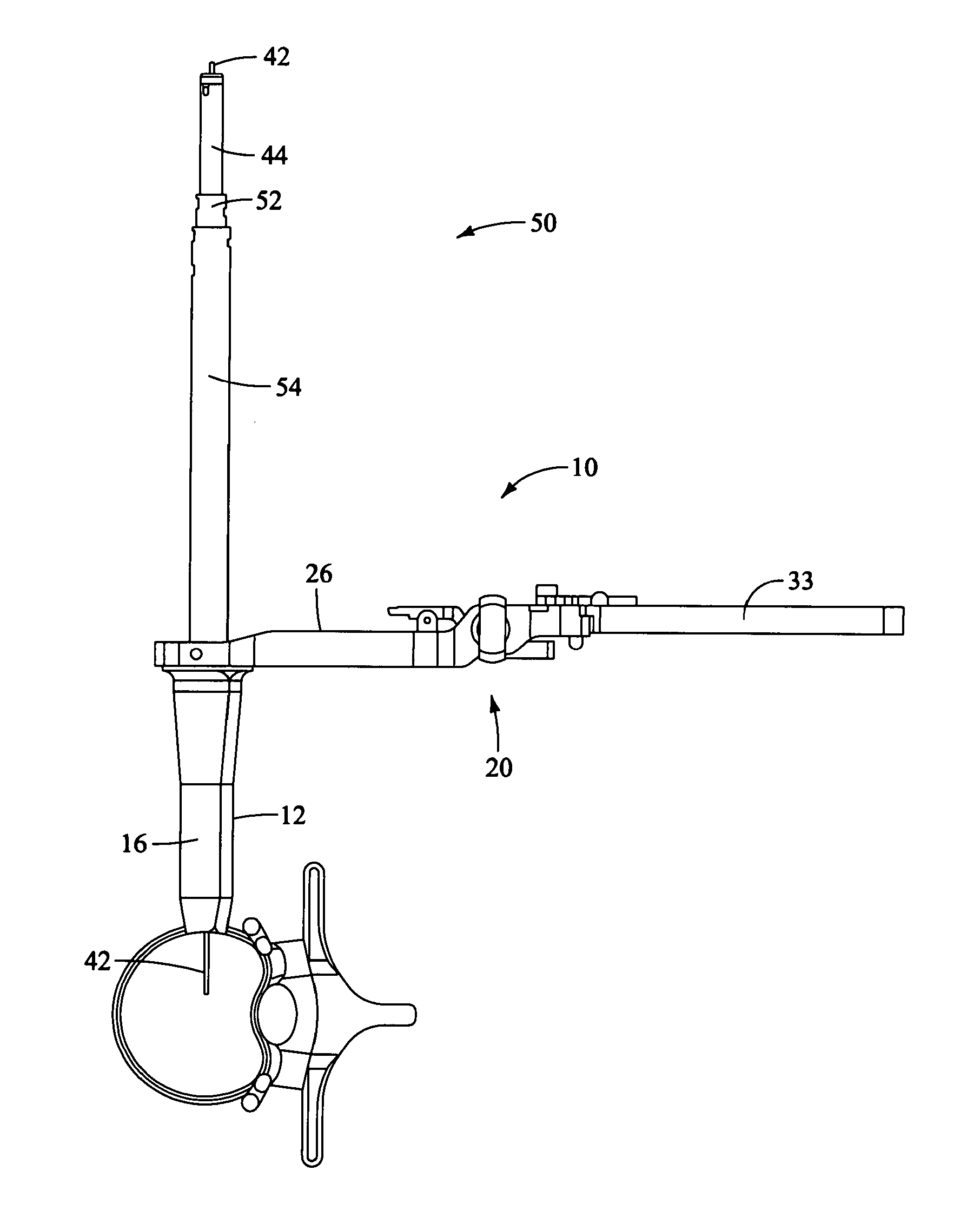
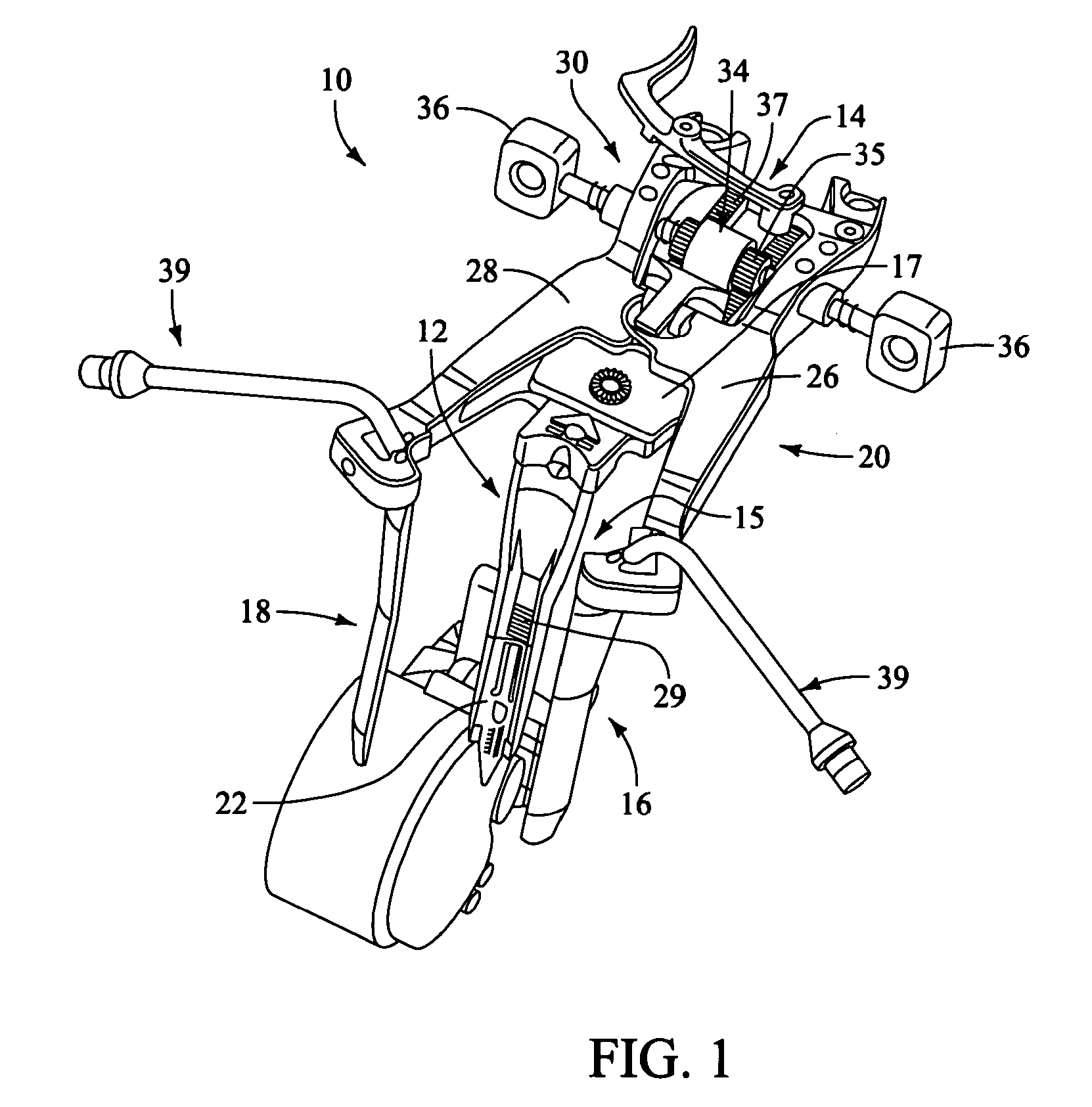
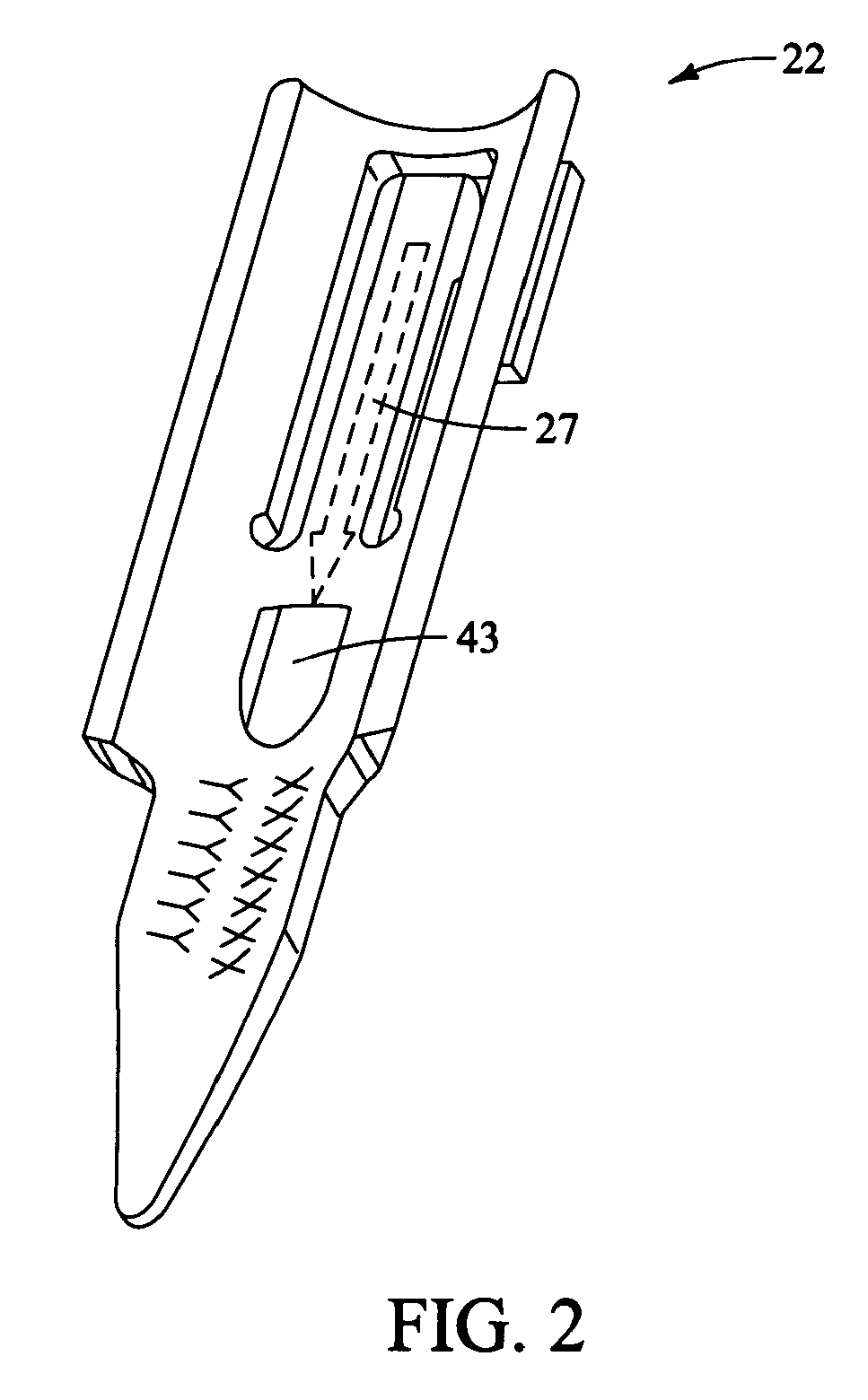
Distraction instrument and method for distracting an intervertebral site
InactiveUS20070123904A1Minimize impactReduce amountSpinal implantsOsteosynthesis devicesDistractionCircular disc
A distraction instrument includes a drive rod, a driver mechanism coupled to the drive rod, and a pair of arms linked to the driver mechanism. A method of implanting an artificial disc or a fusion implant in an intervertebral site includes preparing the intervertebral site, actuating a gear mechanism of a distraction instrument to distract the intervertebral site, and inserting at least a core of the artificial disc or fusion implant into the intervertebral site.
Owner:DEPUY SPINE INC (US)
Vascular device for emboli and thrombi removal and methods of use
InactiveUS7306618B2Permit useFacilitate percutaneous introductionBalloon catheterInfusion syringesThrombusVascular device
Apparatus and methods are provided for use in filtering emboli from a vessel and / or performing thrombectomy and embolectomy, wherein a vascular device disposed on a guidewire comprises a support hoop disposed from a suspension strut. Alternately, a support hoop having an articulation region may be directly connected to a region proximate the distal end of the guidewire. A blood permeable sac is affixed to the support hoop to form a mouth of the blood permeable sac. The support hoop is disposed obliquely relative to the longitudinal axis of the guidewire and is capable of being properly used in a wide range of vessel diameters. The vascular device collapses during removal to prevent material from escaping from the sac. A delivery sheath and introducer sheath for use with the vascular device of the present invention are also provided.
Owner:INCEPT LLC
Exendin agonist formulations and methods of administration thereof
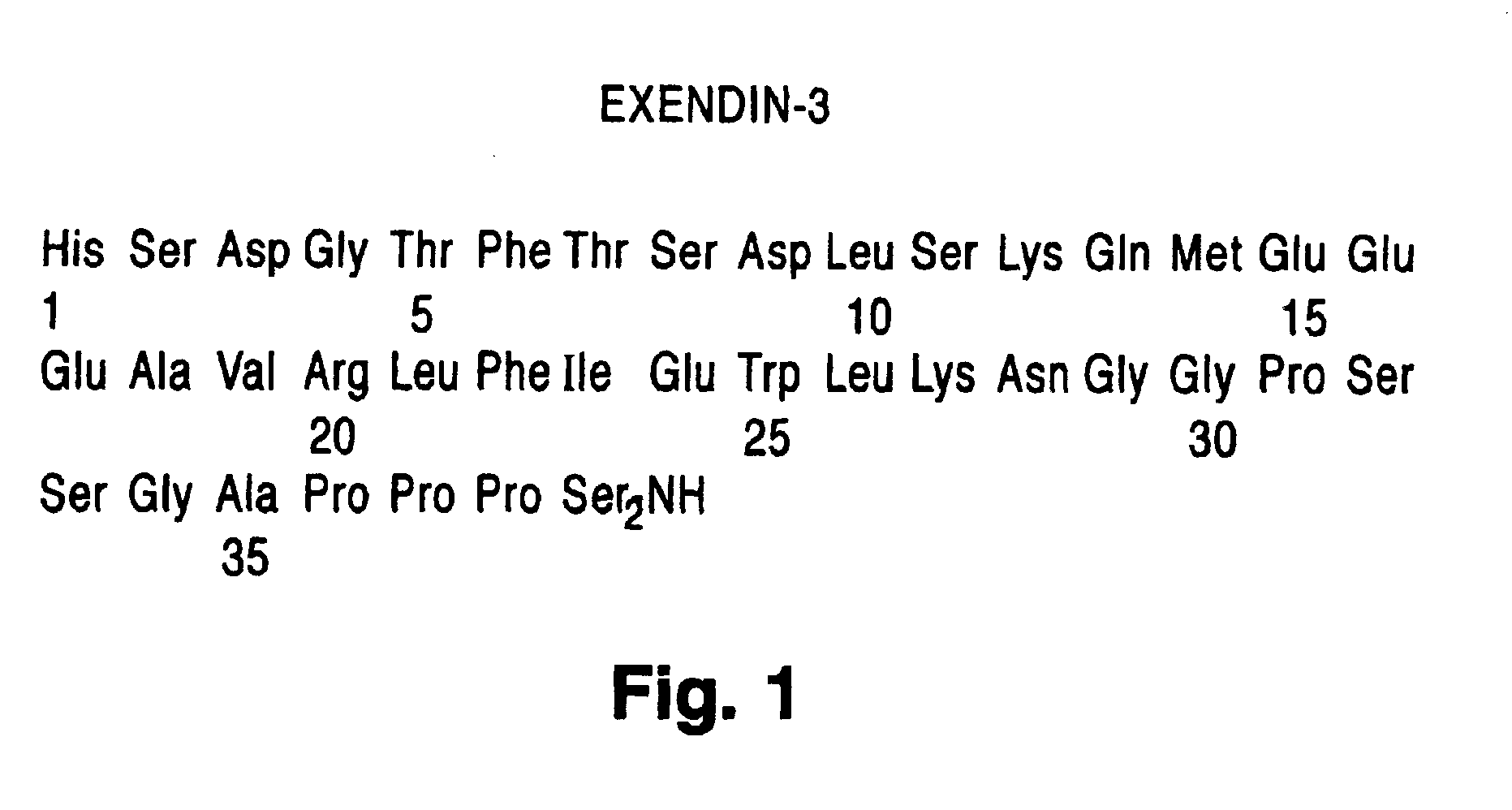
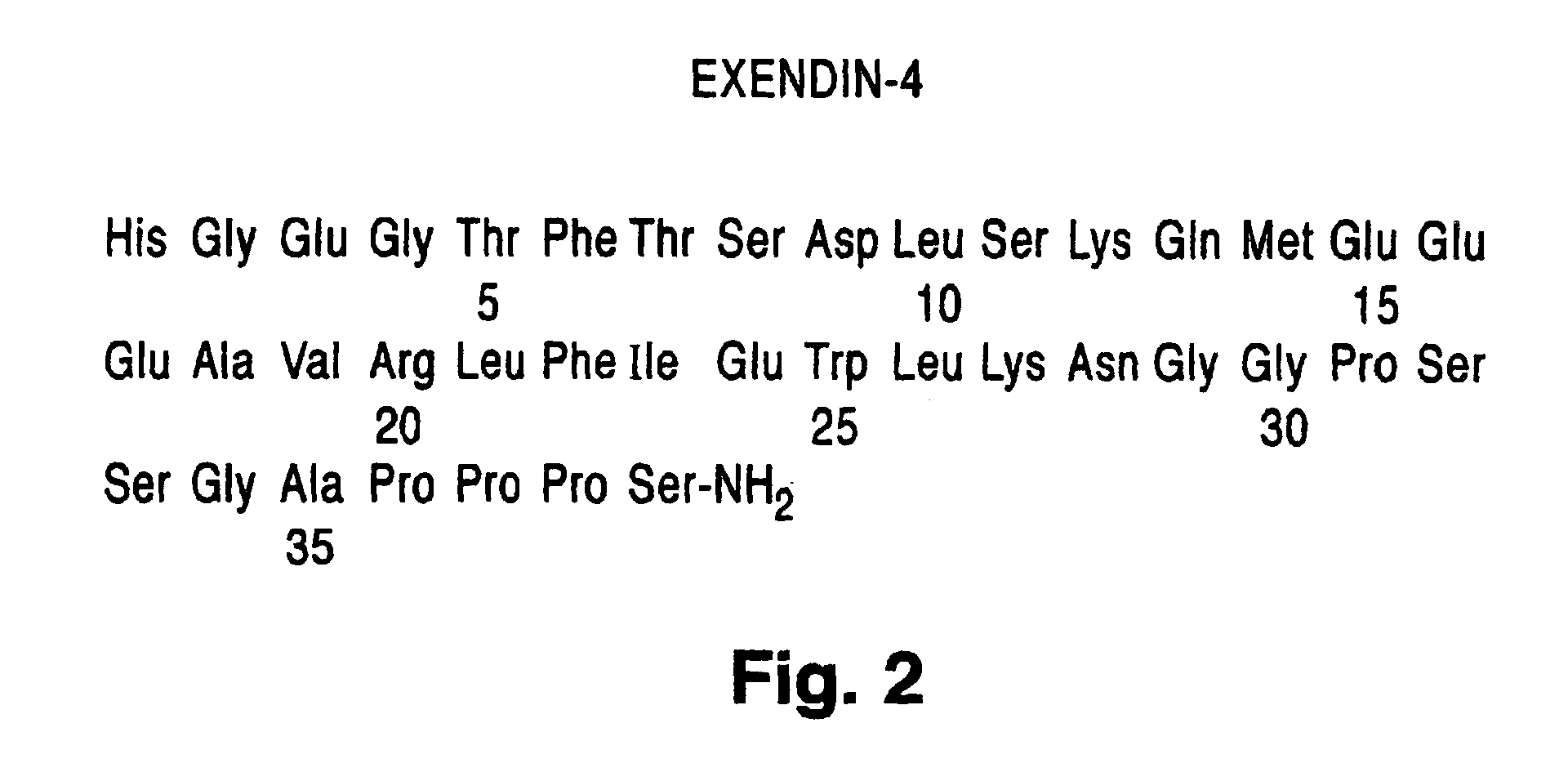
InactiveUS6902744B1Slow gastric emptyingLowering plasma glucose levelPowder deliveryPeptide/protein ingredientsGastric emptyingPlasma glucose
Novel exendin and exendin agonist compound formulations and dosages and methods of administration thereof are provided. These compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:ASTRAZENECA PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS7144587B2Reducing abuse potential of dosage formLower potentialPowder deliveryPill deliveryOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
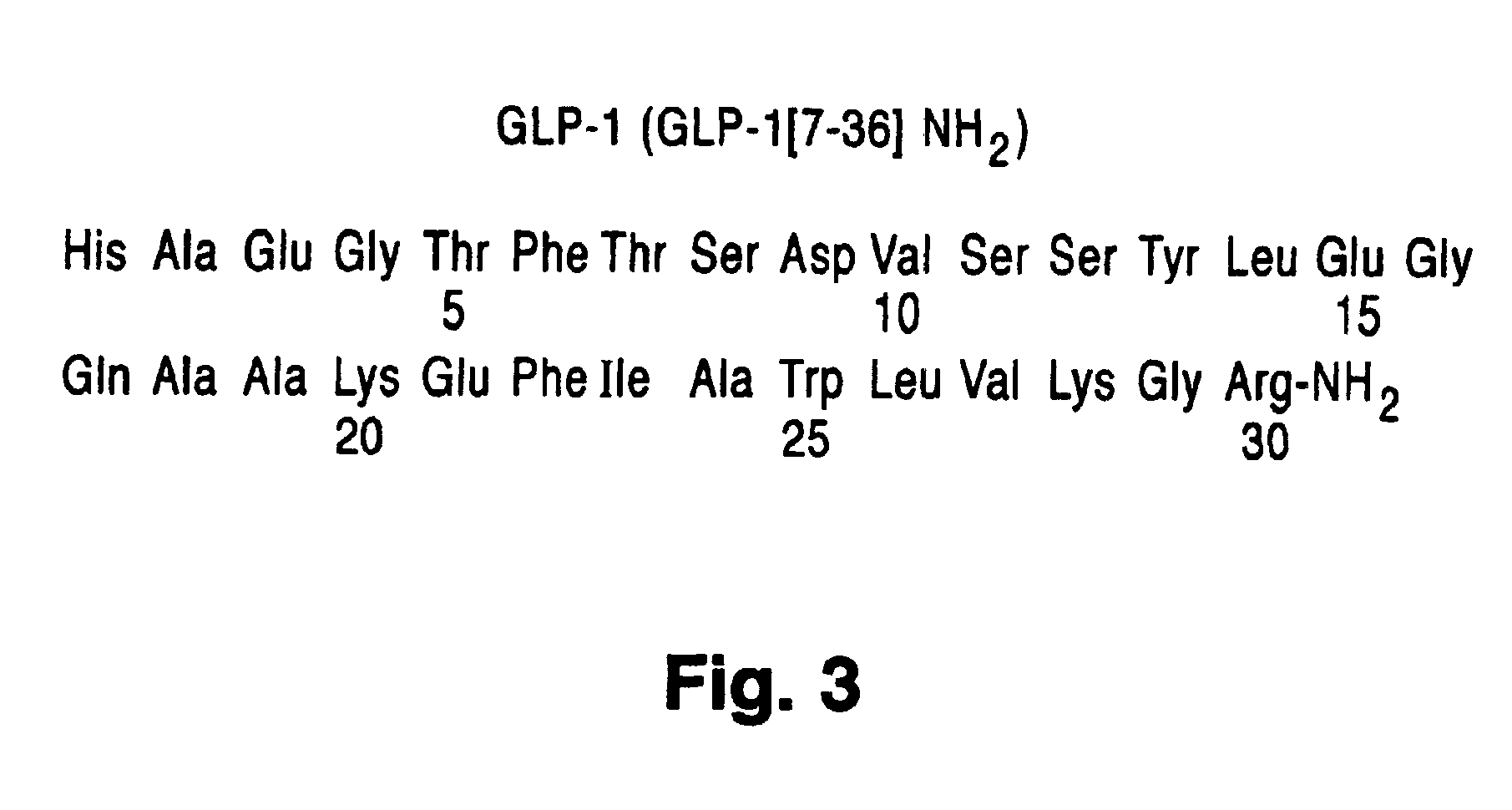
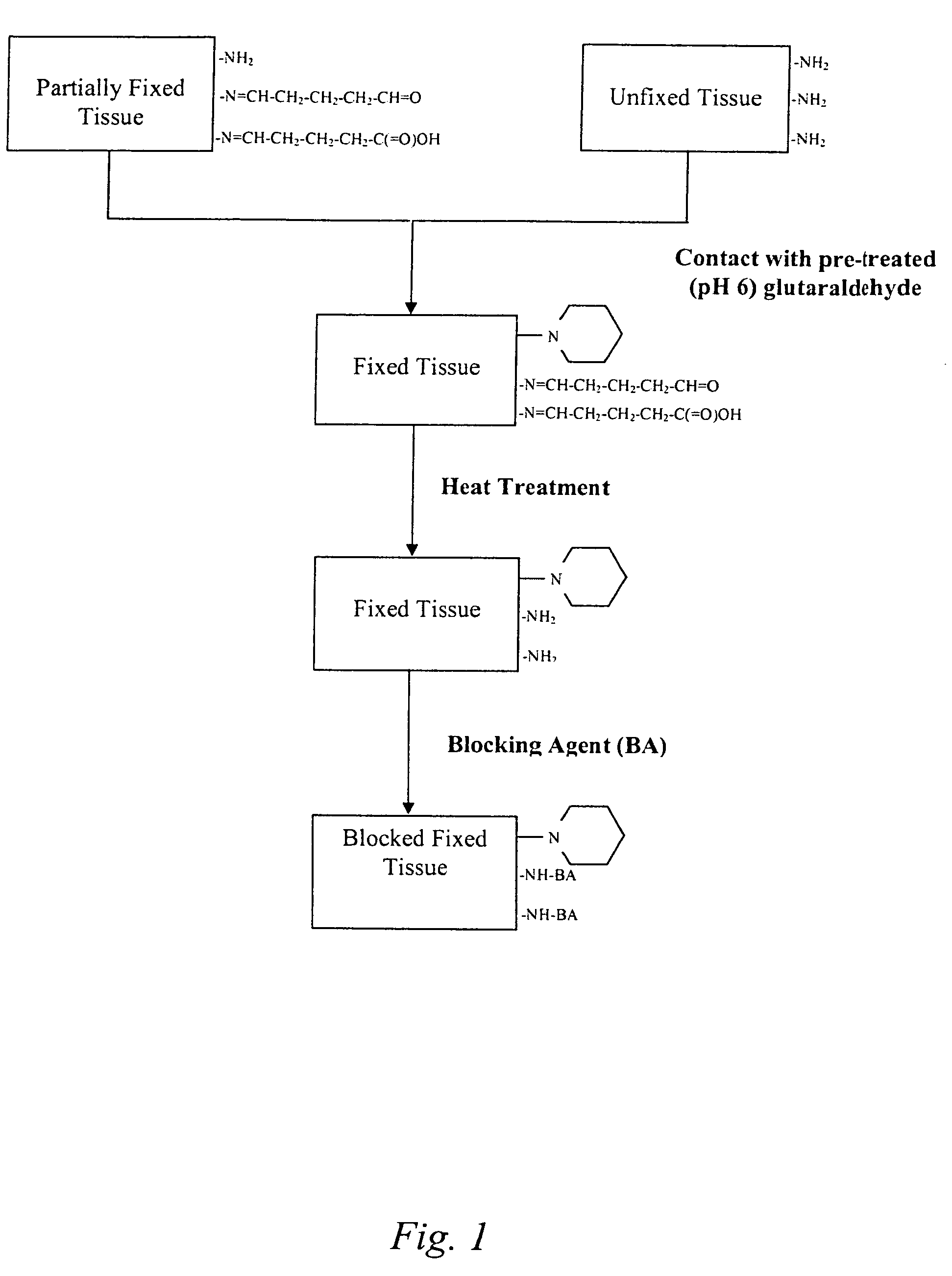
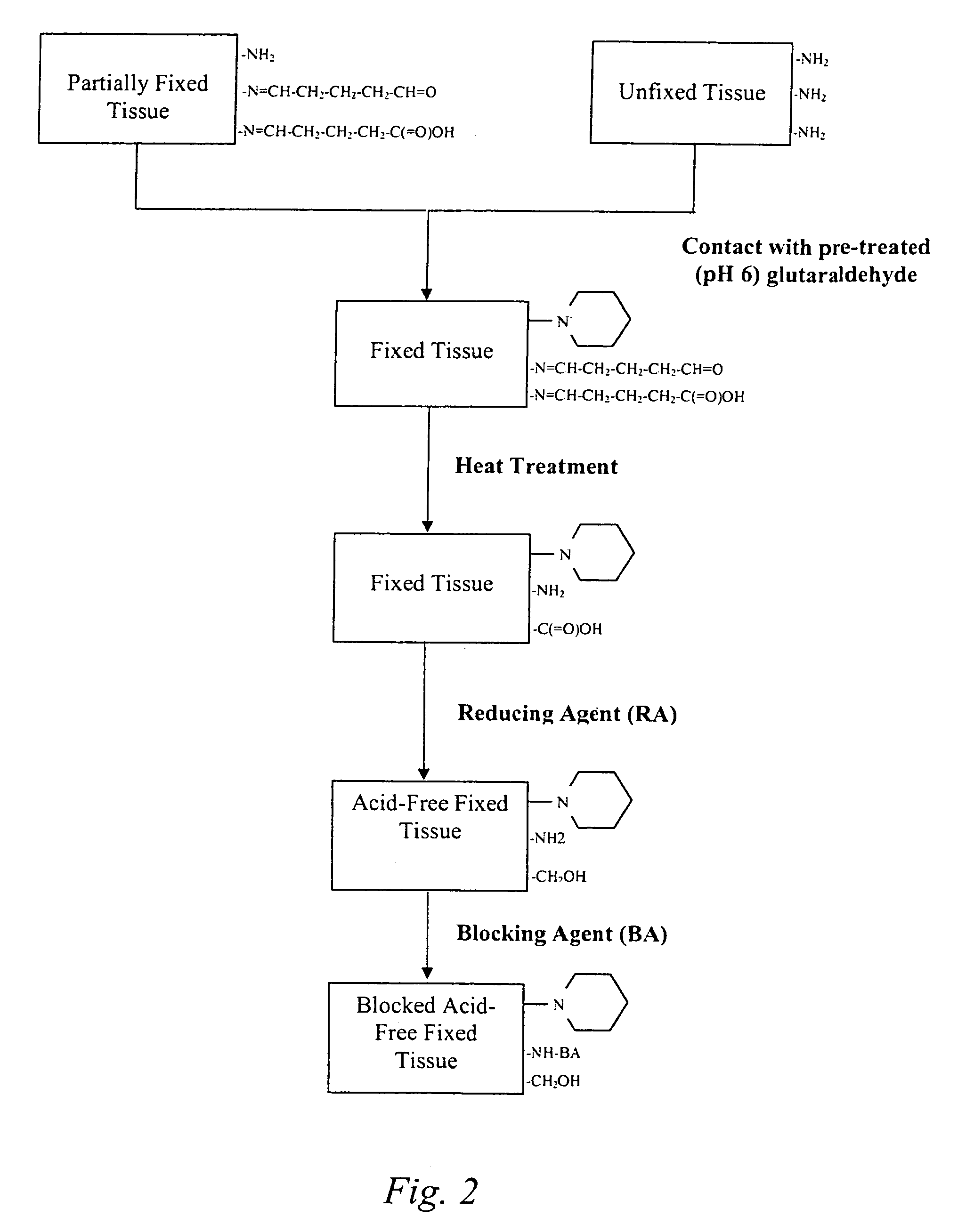
Treatment of bioprosthetic tissues to mitigate post implantation calcification
The present invention provides methods for treating tissue to inhibit post-implant calcification of a biological tissue. In one method of this invention, a tissue is immersed in or otherwise contacted with a pretreated glutaraldehyde solution, i.e., a heat-treated or pH-adjusted glutaraldehyde solution. The tissue may be partially fixed with glutaraldehyde prior to, after, or concurrently with the step of contacting the tissue with the pretreated gluteraldehyde. Contact with the pretreated gluteraldehyde produces free amine groups on the tissue, which are subsequently blocked by contacting the crosslinked tissue with a blocking agent. In another embodiment, a tissue is contacted with either a non-pretreated glutaraldehyde or a pH-adjusted glutaraldehyde solution for a period of time sufficient to crosslink the tissue. The crosslinked tissue is then treated with a reducing agent that reduces aldehyde and carboxylic acid groups on the fixed tissue.
Owner:EDWARDS LIFESCIENCES CORP
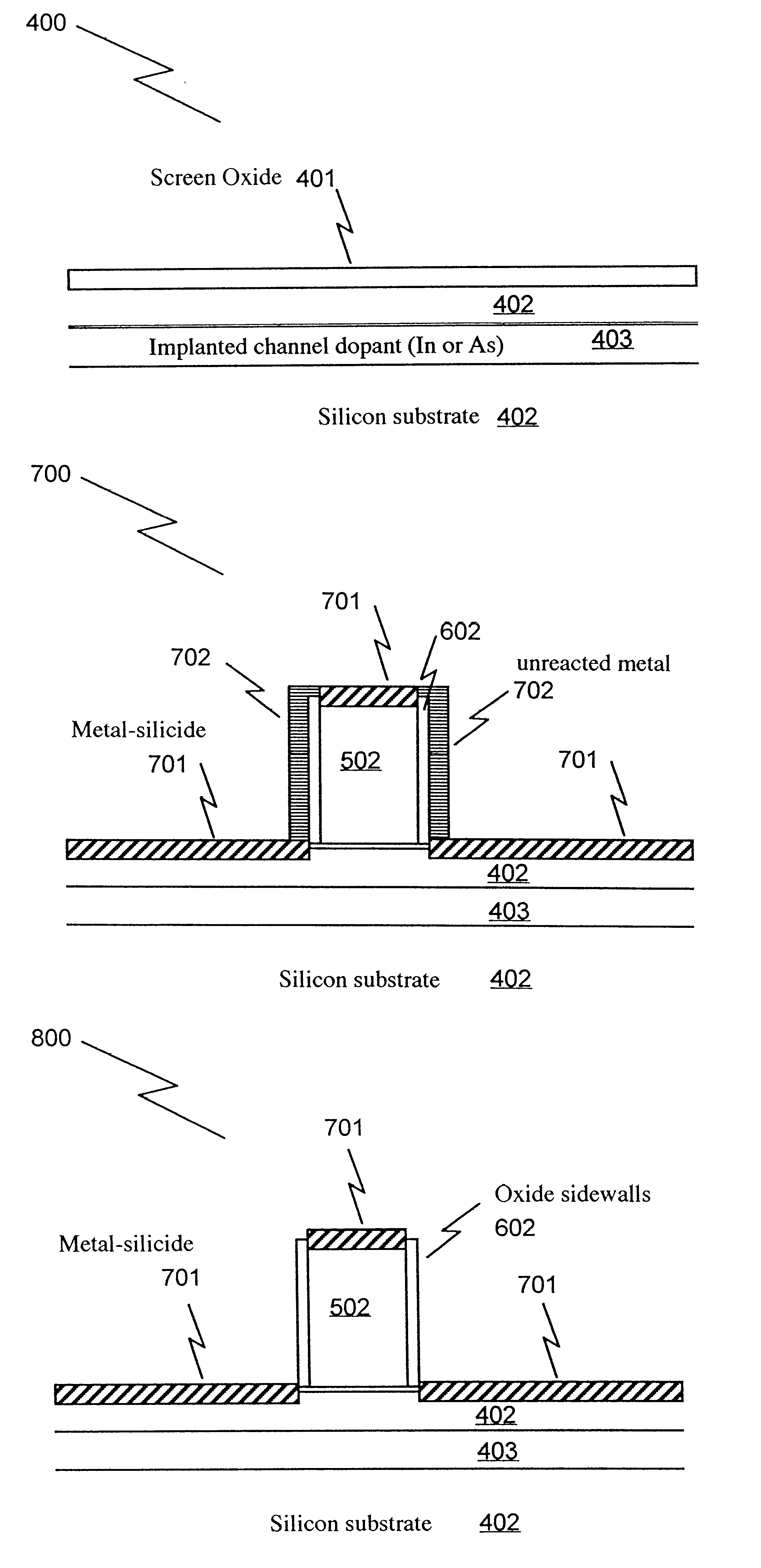
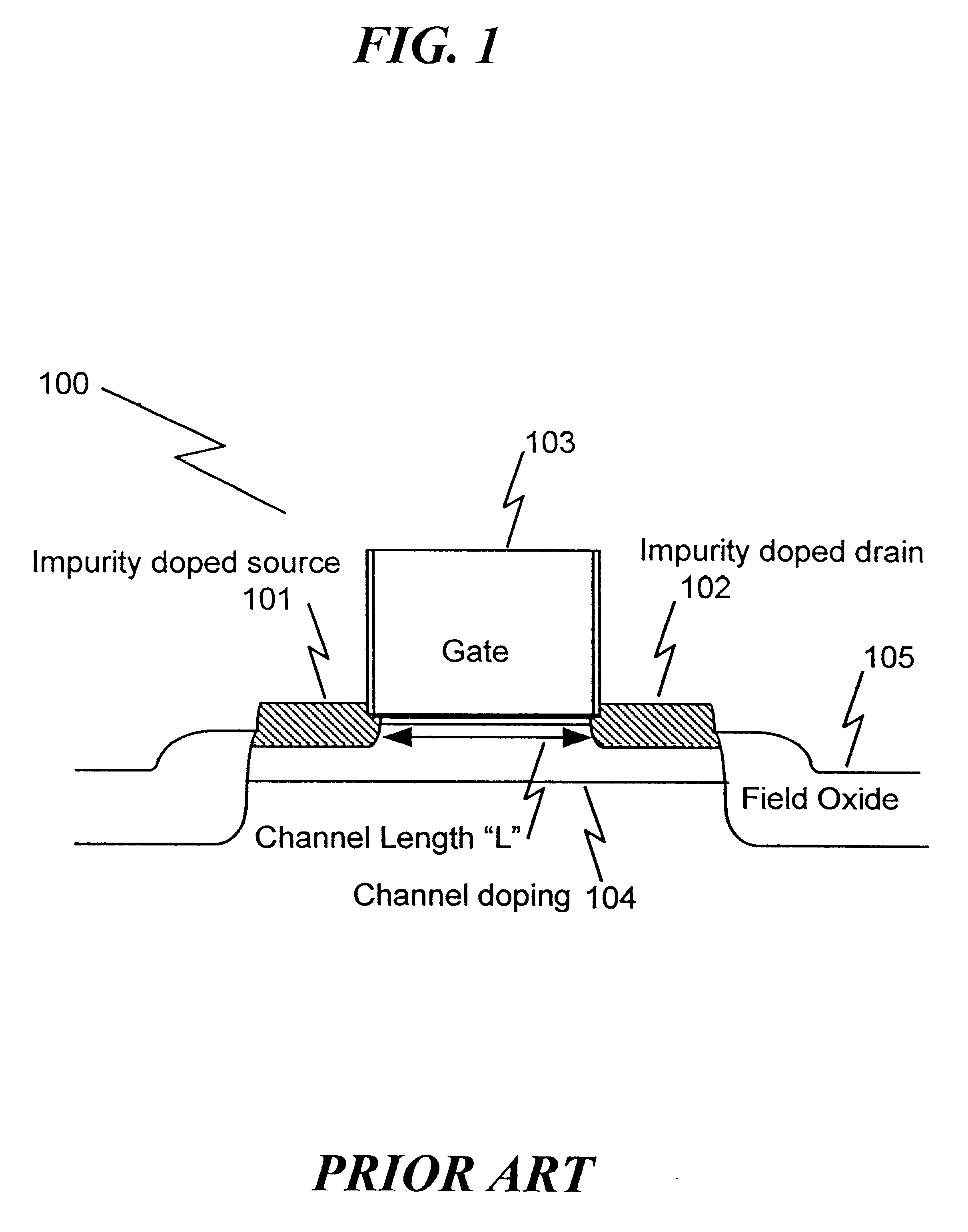
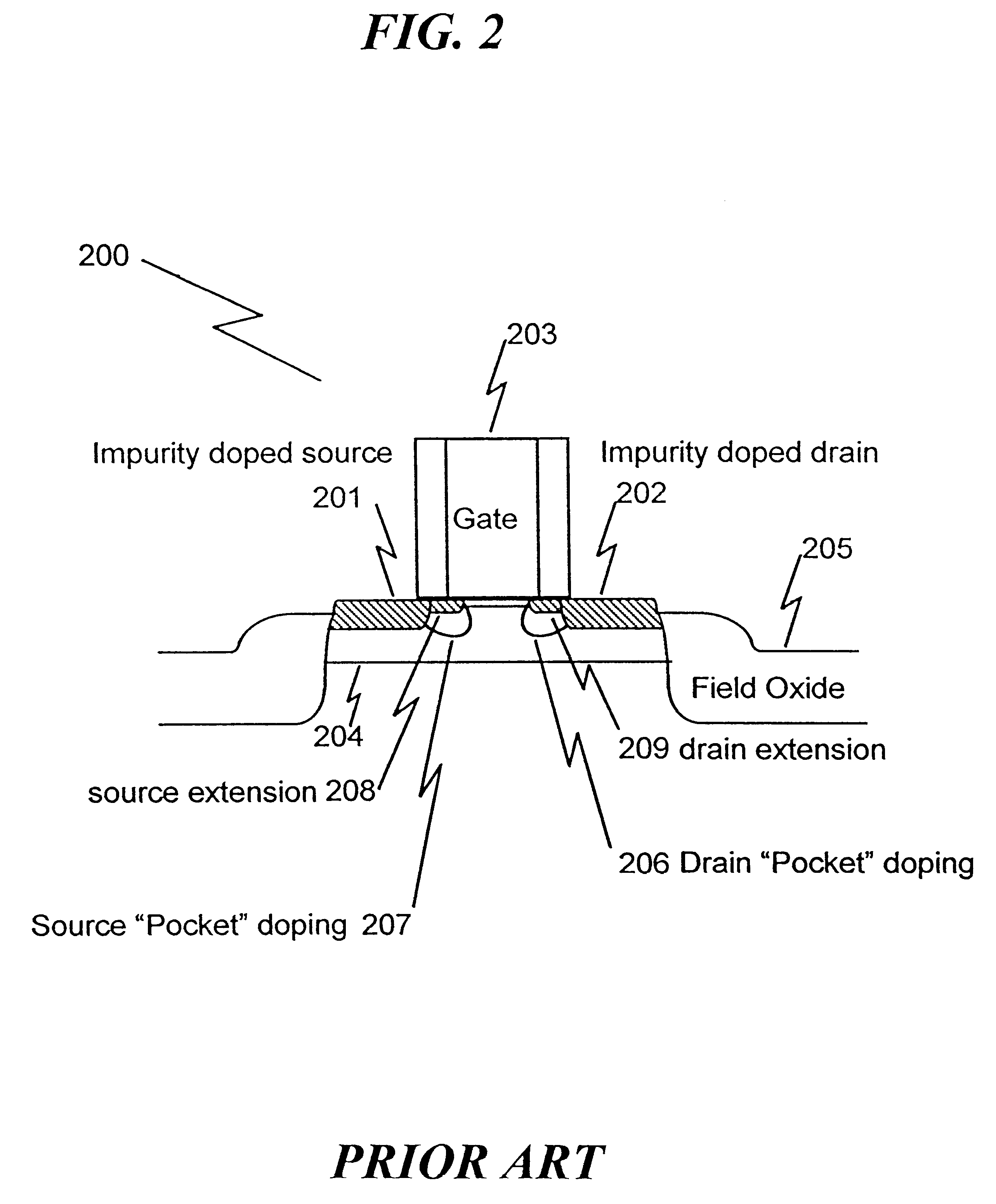
Method of manufacturing a short-channel FET with Schottky-barrier source and drain contacts
InactiveUS6303479B1Without add parasitic capacitanceReduce manufacturingTransistorSolid-state devicesEngineeringDopant
The present invention Is a fabrication method for a short-channel Schottky-barrier field-effect transistor device. The method of the present invention includes introducing channel dopants into a semiconductor substrate such that the dopant concentration varies in the vertical direction and is generally constant in the lateral direction. A gate electrode is formed on the semiconductor substrate, and source and drain electrodes are formed on the substrate to form a Schottky or Schottky-like contact to the substrate.
Owner:AVOLARE 2 LLC
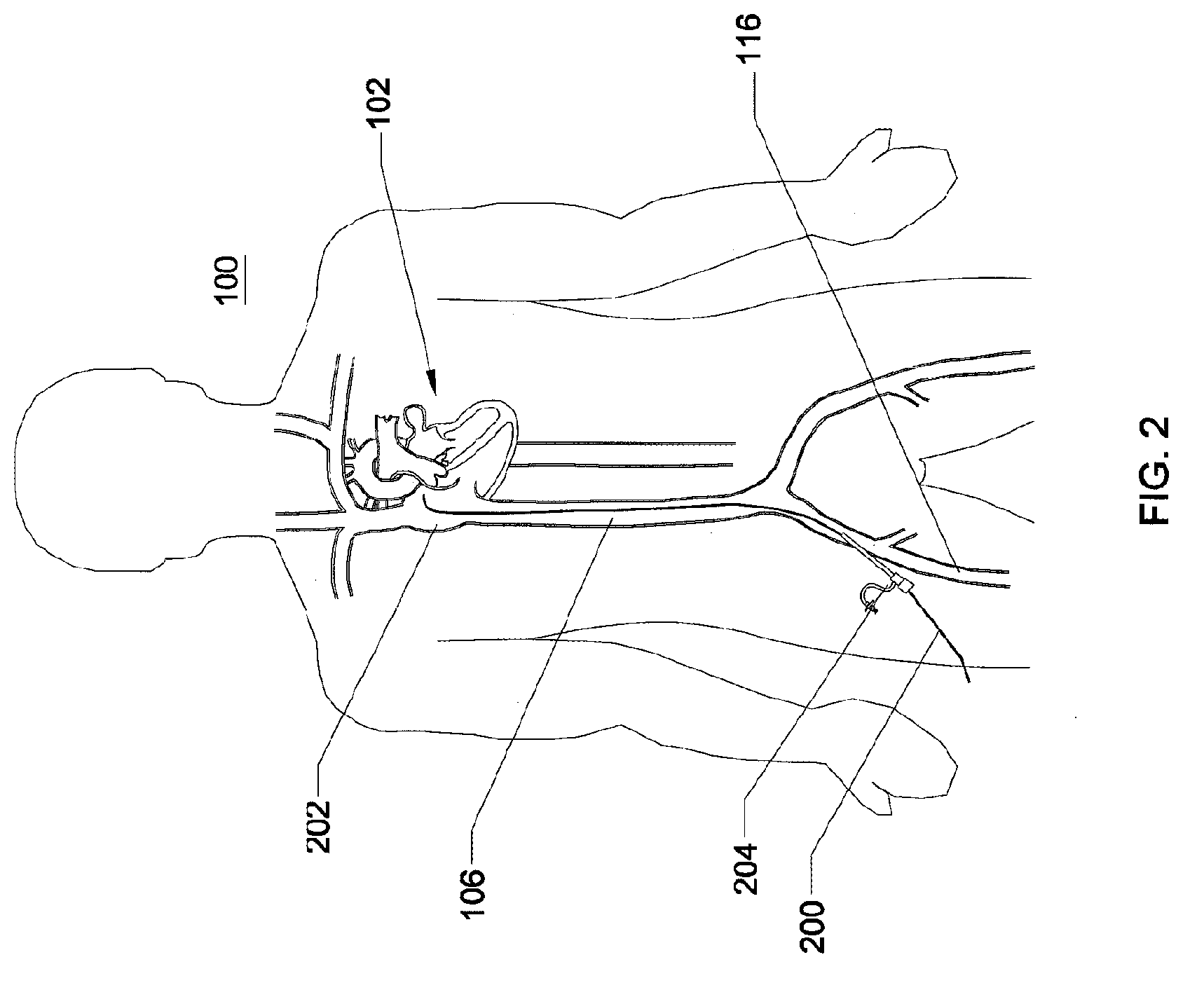
Expandable trans-septal sheath
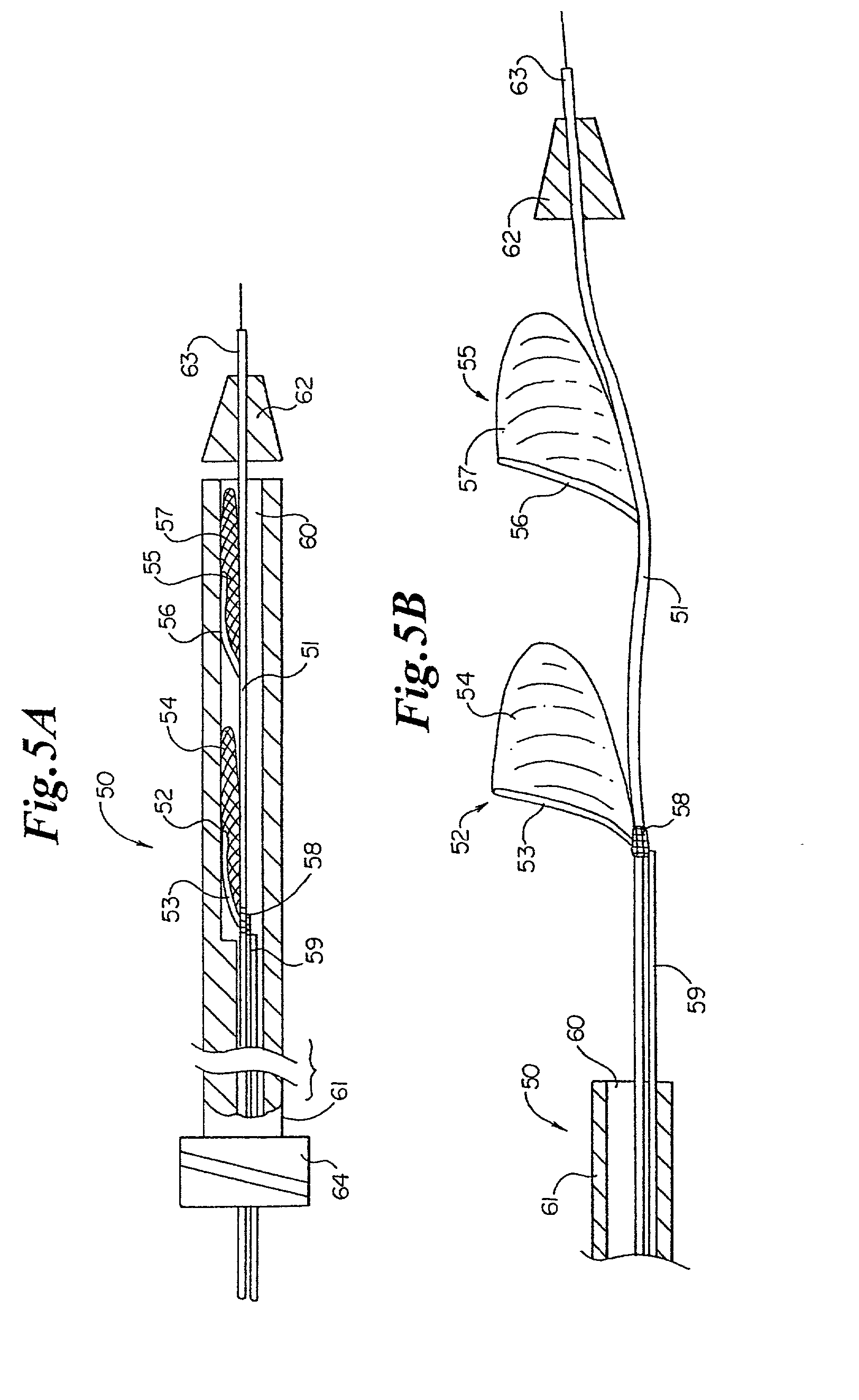
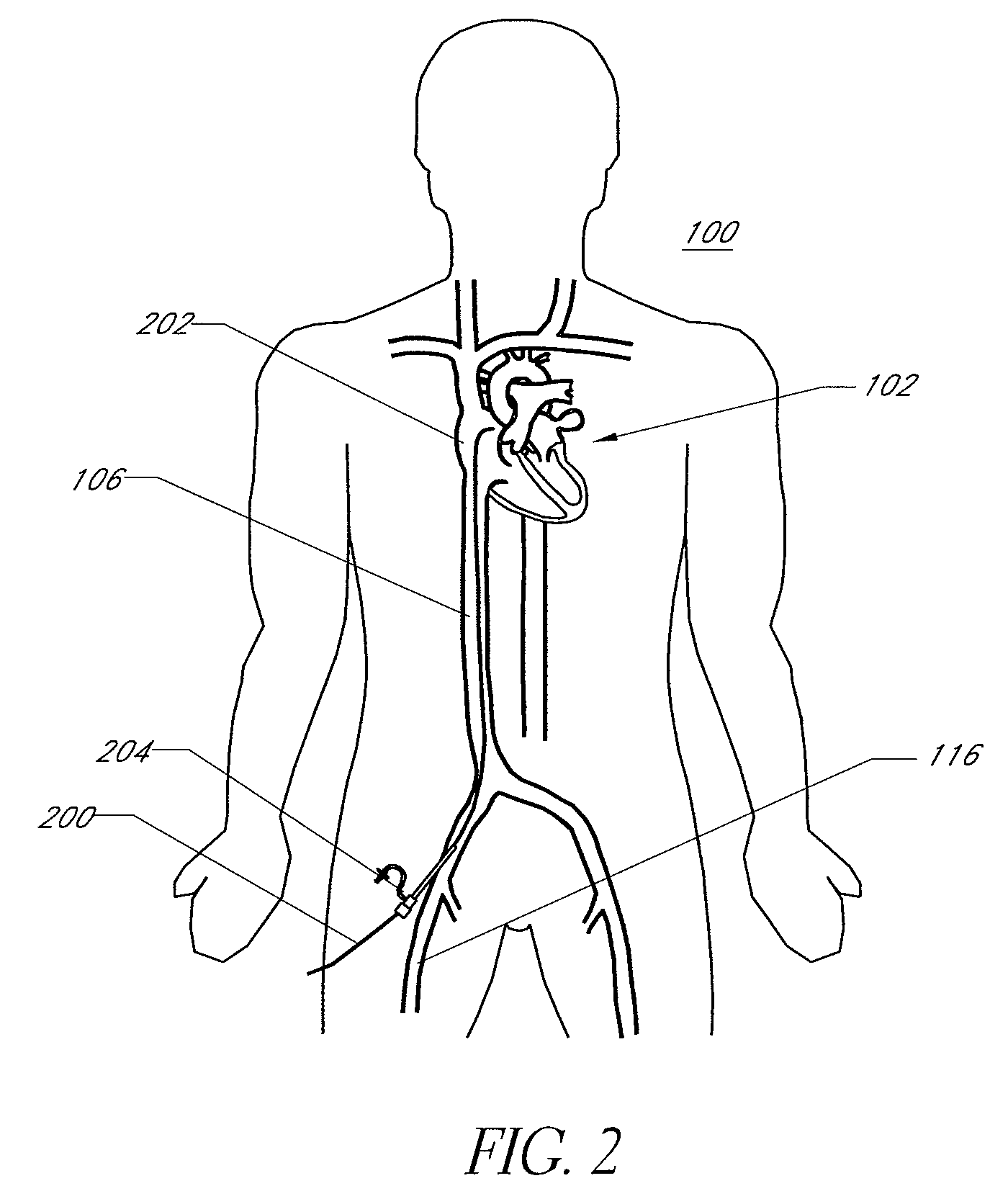
ActiveUS20080215008A1Lower potentialLess time-consumingCannulasInfusion syringesAccess routeRight atrium
Disclosed is an expandable transluminal sheath, for introduction into the body while in a first, low cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the vascular system and has utility in the performance of procedures in the left atrium. The access route is through the inferior vena cava to the right atrium, where a trans-septal puncture, followed by advancement of the catheter is completed. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement to the right atrium and through the atrial septum into the left atrium. The distal end of the sheath is subsequently expanded using a radial dilatation device. In an exemplary application, the sheath is utilized to provide access for a diagnostic or therapeutic procedure such as electrophysiological mapping of the heart, radio-frequency ablation of left atrial tissue, placement of left atrial implants, mitral valve repair, or the like.
Owner:ONSET MEDICAL CORP
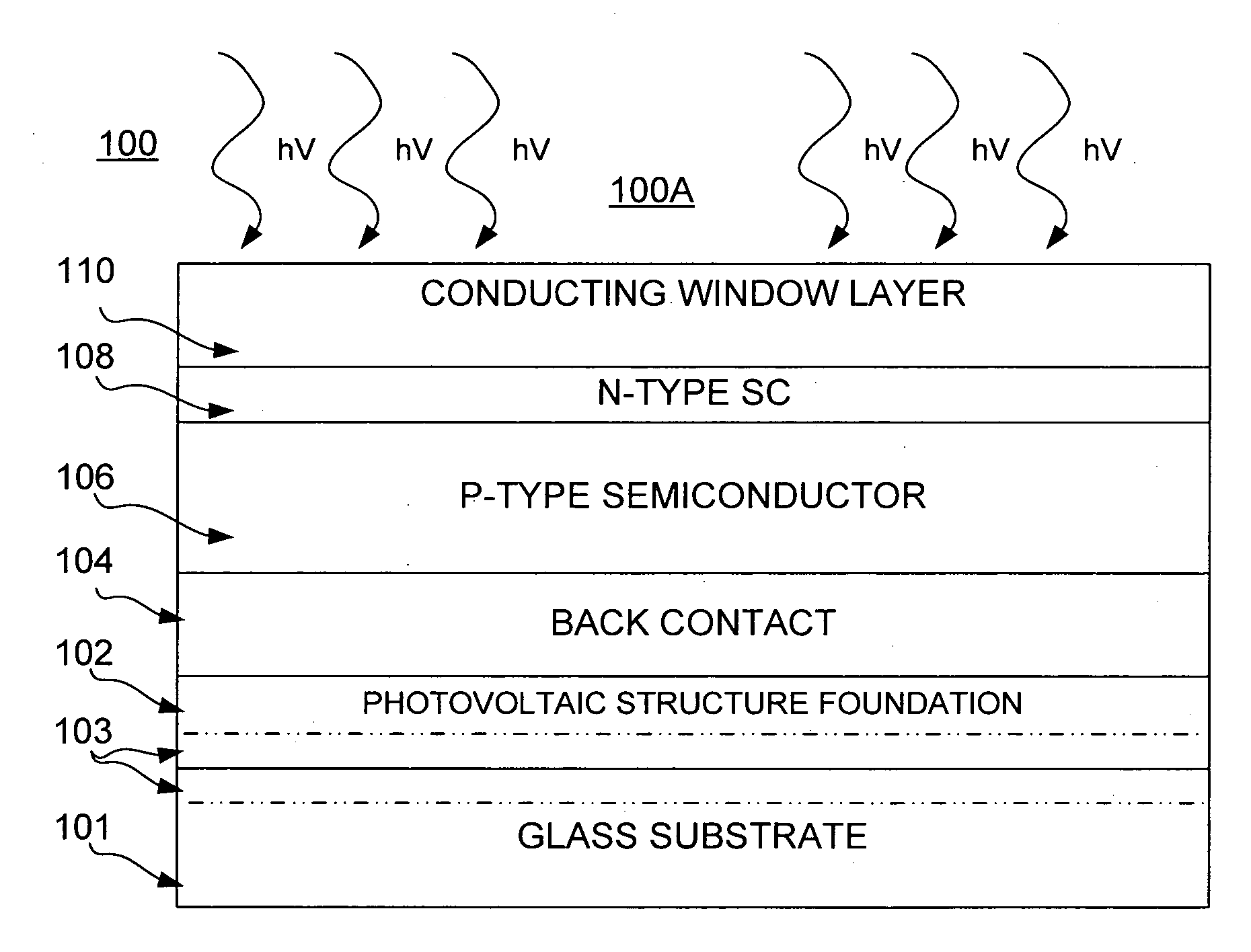
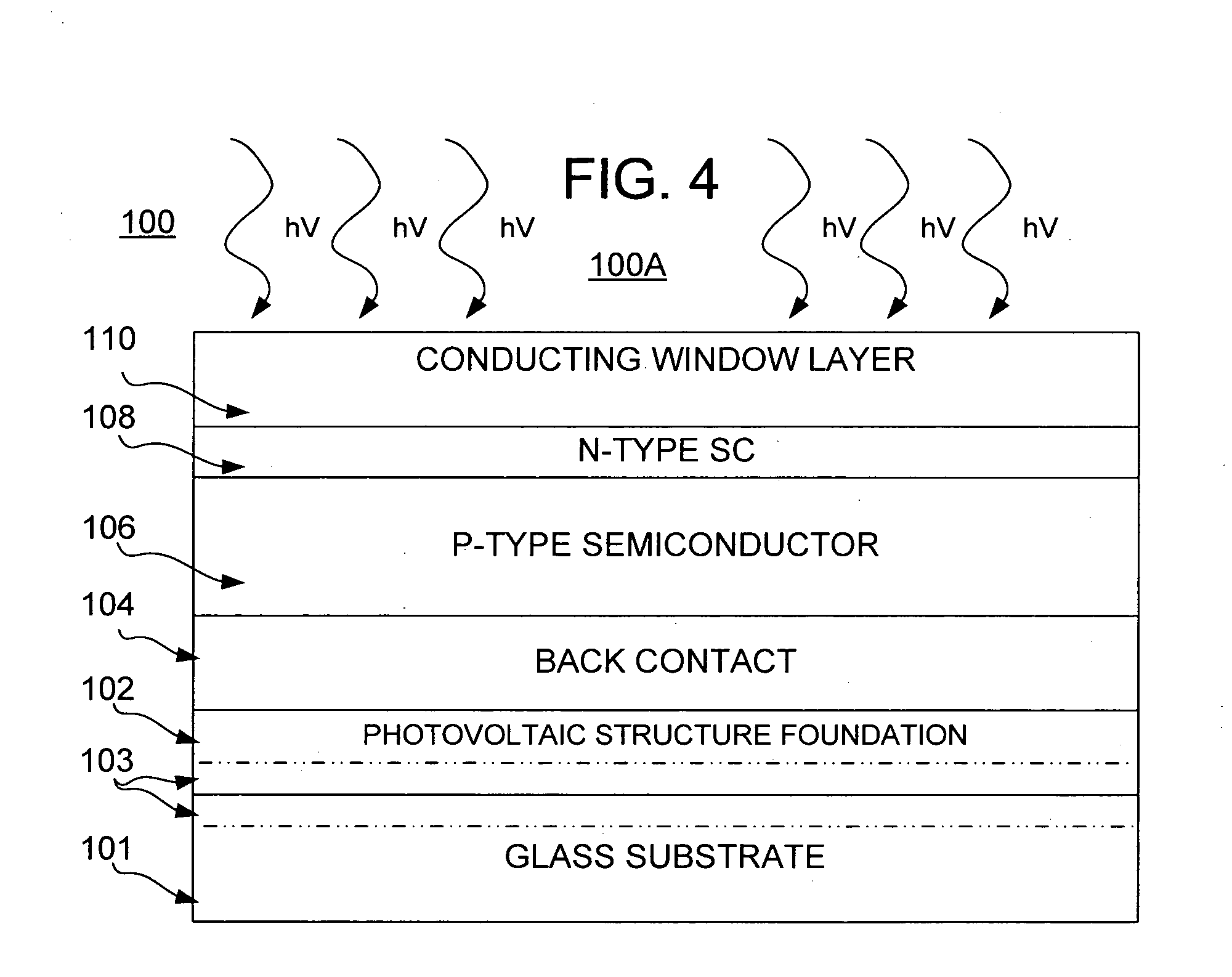
Thin film photovoltaic structure
InactiveUS20070277874A1Faster throughputReduce needSemiconductor/solid-state device manufacturingPhotovoltaic energy generationAnodic bondingVoltage
Systems and methods of production of a photovoltaic device include creating on a donor semiconductor wafer an exfoliation layer and transferring the exfoliation layer to an insulator substrate. One or more finishing processes may be performed before and / or after transferring the exfoliation layer, such as to create a plurality of photovoltaic structure layers. Production of the photovoltaic device further may include subjecting the donor semiconductor wafer to an ion implantation process to create the exfoliation layer, bonding the exfoliation layer to the insulator substrate, and separating the exfoliation layer from the donor semiconductor wafer. Transferring may include forming an anodic bond via electrolysis, such as through the application of heat, pressure and voltage to the exfoliation layer and the insulator structure.
Owner:CORNING INC
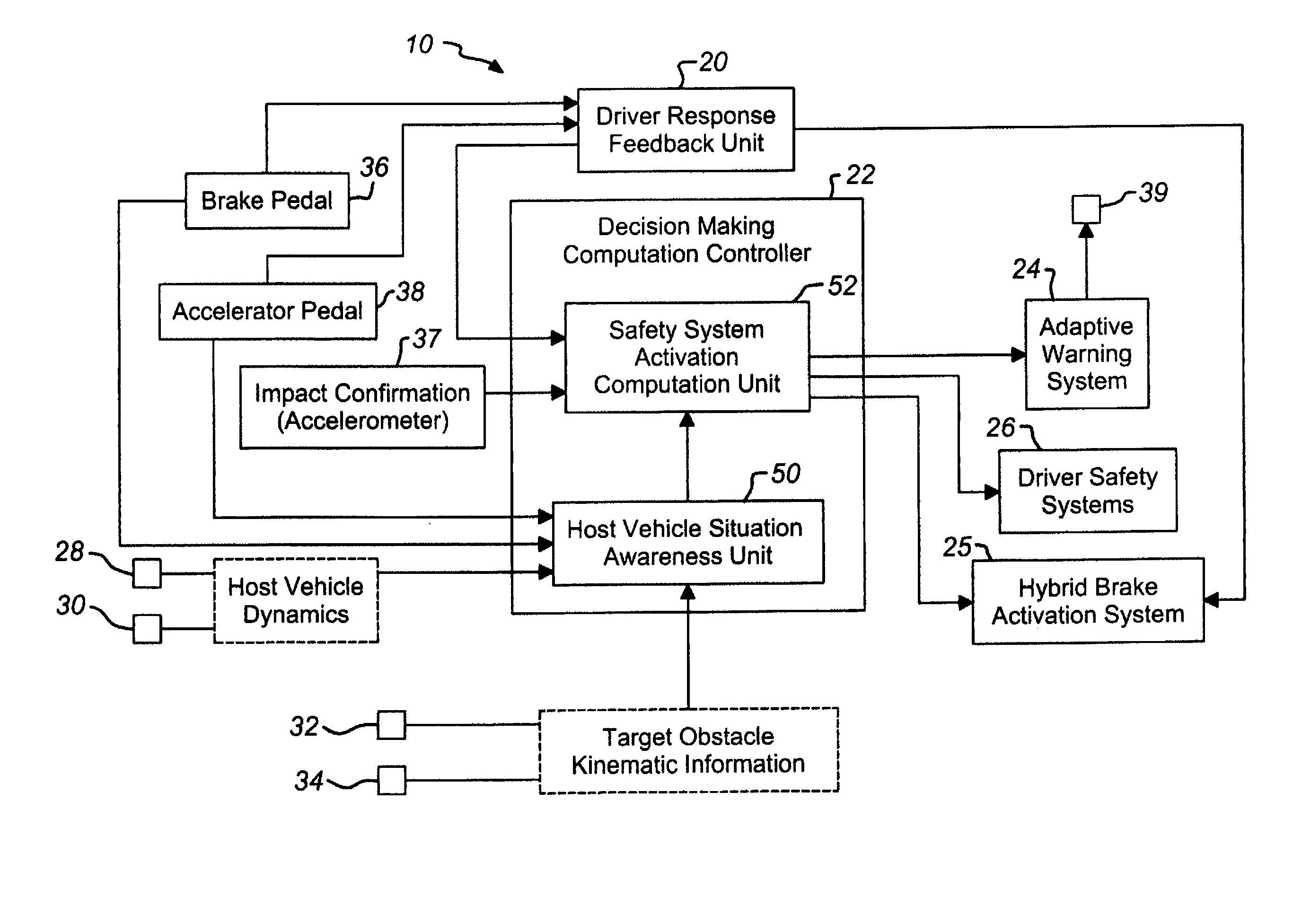
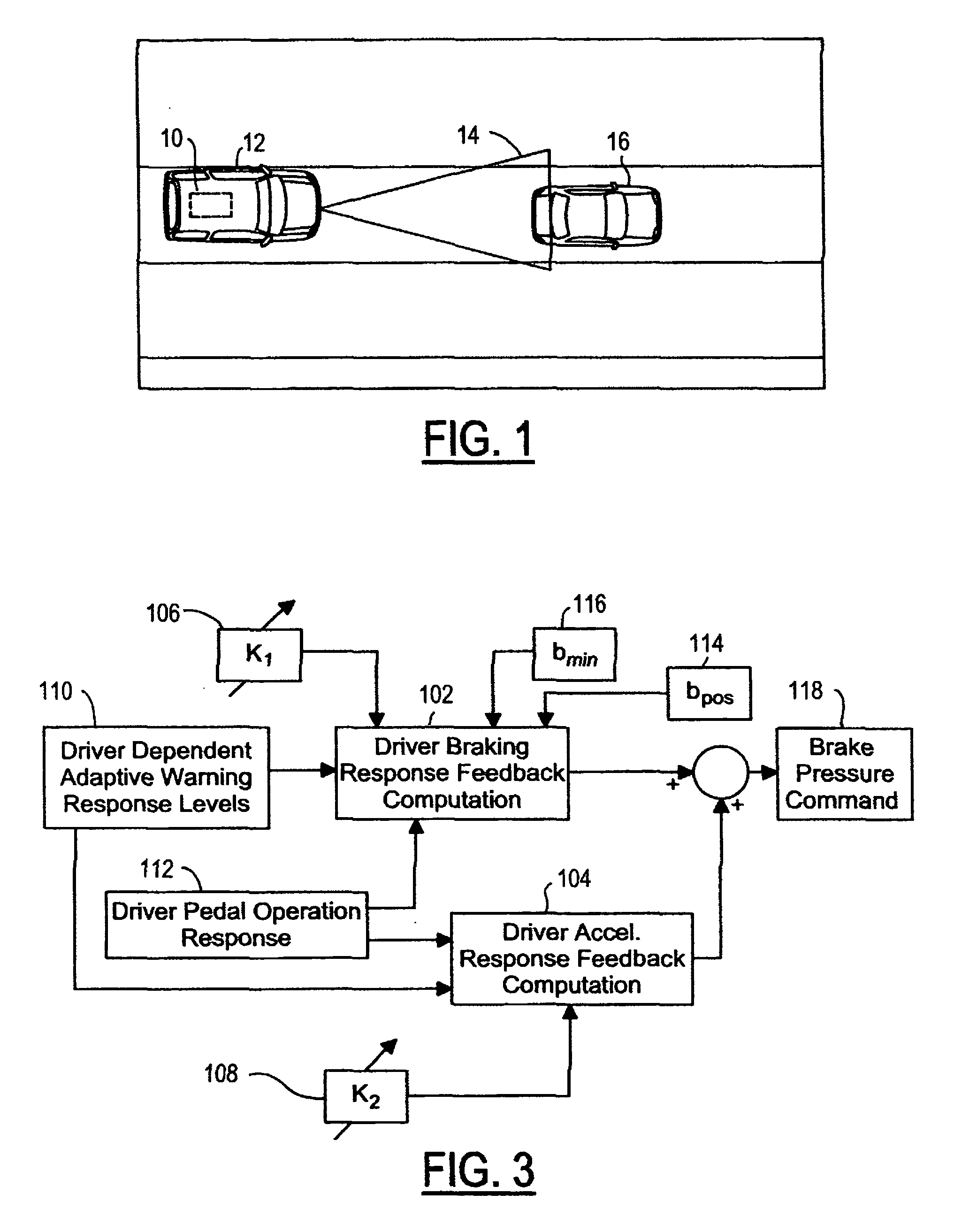
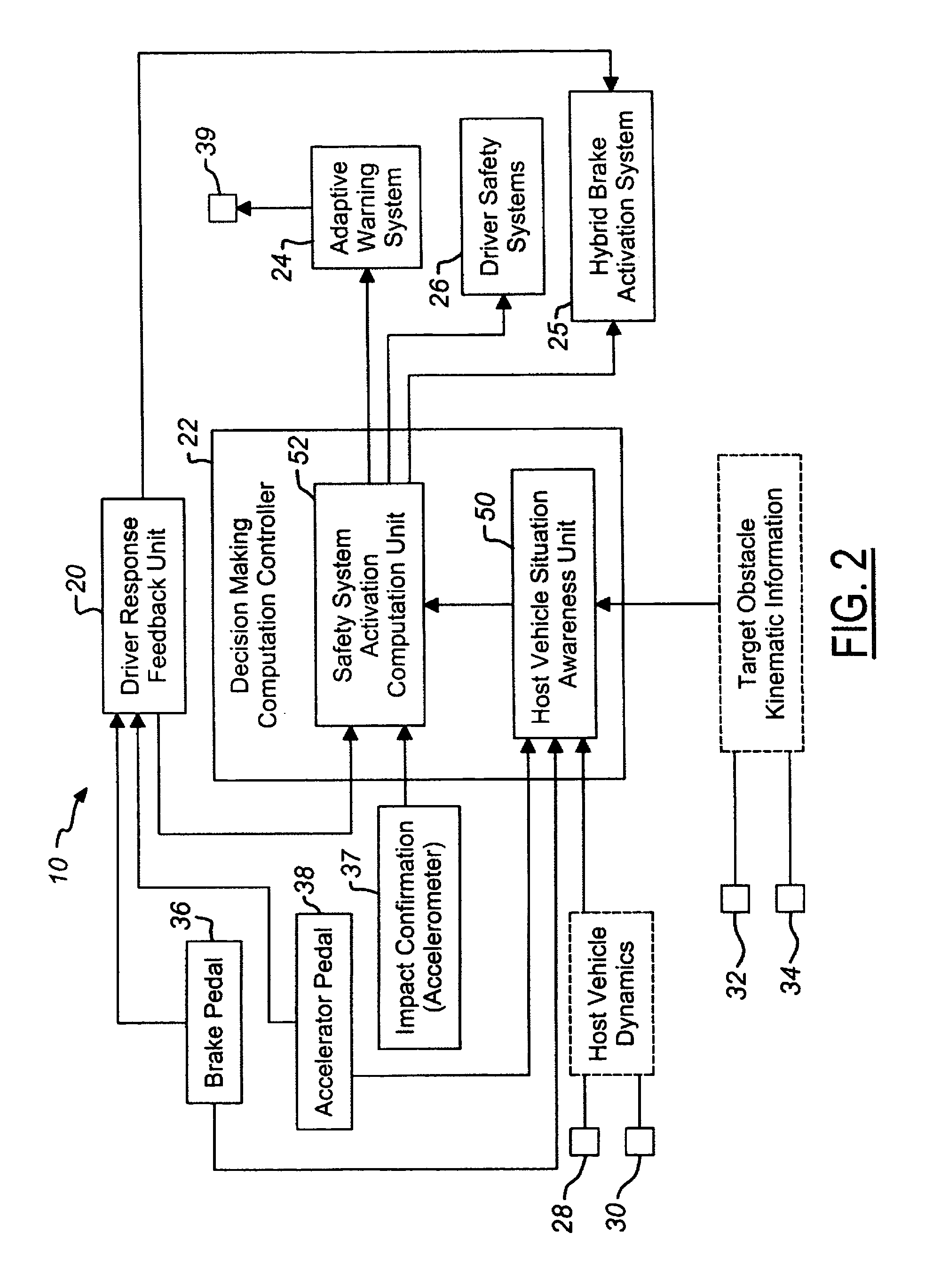
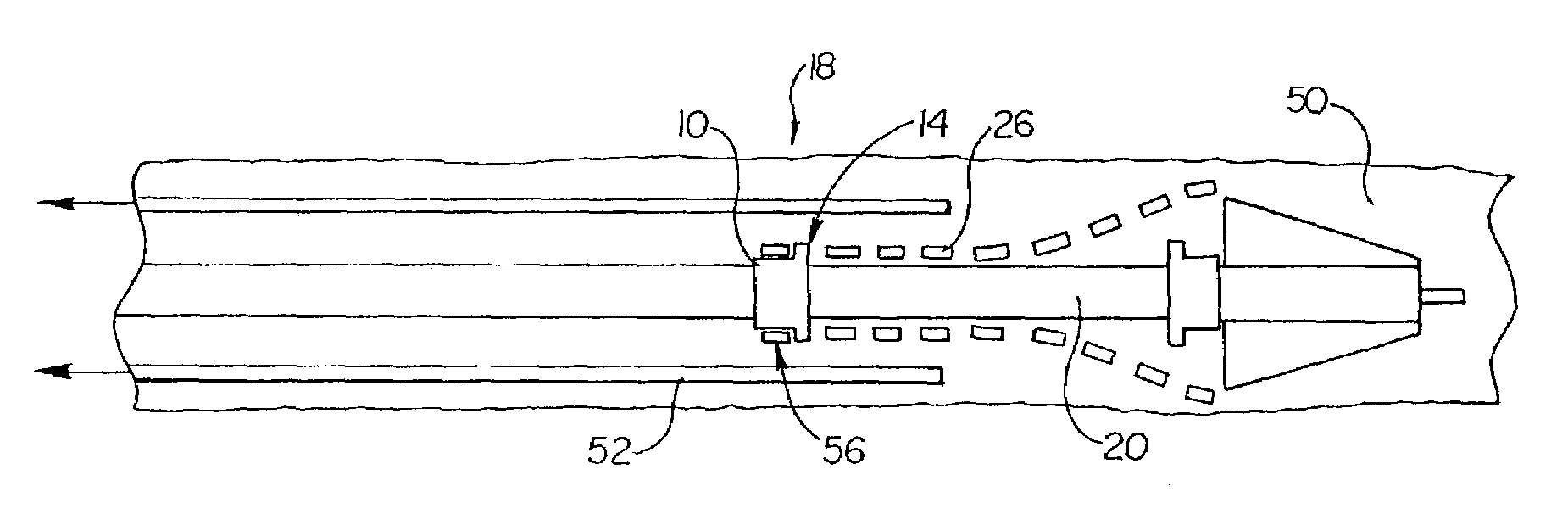
Vehicle pre-impact sensing and control system with driver response feedback
InactiveUS7253724B2Improve reliabilityLower potentialAnti-collision systemsExternal condition input parametersControl systemEngineering
A vehicle pre-impact sensing and control system generates tailored adaptive warning signals as a function of driver vehicle use. The tailored signals are used in a vehicle controller for determining appropriate driver warning or safety device activations.
Owner:FORD GLOBAL TECH LLC
Stent delivery system with securement and deployment accuracy
ActiveUS7473271B2Improve accuracyReduces occurrence and/or severityStentsBlood vesselsBody regionCatheter device
A method and apparatus for reducing the longitudinal aspect of the catheter to stent force comprises at least one grip member for use with a stent delivery system. The grip engages a stent in the unexpanded state prior to delivery of the stent by retracting a stent retaining sheath. The grip comprises a body region having an outer diameter, a first end and a second end. The outer diameter of the first end is greater than the outer diameter of the second end. The grip is at least partially constructed from a polymeric material.
Owner:BOSTON SCI SCIMED INC
Opioid agonist formulations with releasable and sequestered antagonist
Disclosed are oral dosage forms, comprising (i) a therapeutically effective amount of an opioid agonist; (ii) an opioid antagonist in releasable form; and (iii) a sequestered opioid antagonist which is not released when the dosage form is administered intact, and methods thereof.
Owner:PURDUE PHARMA LP
Expandable trans-septal sheath
Disclosed is an expandable transluminal sheath, for introduction into the body while in a first, low cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the vascular system and has utility in the performance of procedures in the left atrium. The access route is through the inferior vena cava to the right atrium, where a trans-septal puncture, followed by advancement of the catheter is completed. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement to the right atrium and through the atrial septum into the left atrium. The distal end of the sheath is subsequently expanded using a radial dilatation device. In an exemplary application, the sheath is utilized to provide access for a diagnostic or therapeutic procedure such as electrophysiological mapping of the heart, radio-frequency ablation of left atrial tissue, placement of left atrial implants, mitral valve repair, or the like.
Owner:ONSET MEDICAL CORP
Abuse-resistant amphetamine compounds
InactiveUS7105486B2Reduced activityRelease is diminished and eliminatedOrganic active ingredientsPeptide/protein ingredientsChemical MoietyDisease
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Surgical access system and related methods
ActiveUS20100130827A1Increase the number ofStructural damageElectrotherapySurgeryDistractionSurgical operation
A surgical access system including a tissue distraction assembly and a tissue retraction assembly, both of which may be equipped with one or more electrodes for use in detecting the existence of (and optionally the distance and / or direction to) neural structures before, during, and after the establishment of an operative corridor to a surgical target site. Some embodiments of the surgical access system may be particularly suited for establishing an operative corridor to a surgical target site in the spine. Such an operative corridor may be established through the retroperitoneal space and the psoas muscle during a direct lateral, retroperitoneal approach to the spine.
Owner:NUVASIVE
Abuse potential reduction in abusable substance dosage form
ActiveUS20050163717A1Lower potentialReducing the potential for abuseBiocideNervous disorderSubstance abuserMedicine
The potential for substance abuse involving residual amounts of abusable substances remaining in used skin-worn patches is reduced by the provision of a system and method for combining the abusable substance with a separate anti-abuse substance agent as part of a removal or disposal procedure.
Owner:VERDE ENVIRONMENTAL TECH INC
Sequestered antagonist formulations
InactiveUS20030157168A1Decrease in potentialLower potentialBiocideNervous disorderOpioid antagonistOral medication
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the mean Cmax of the antagonist after single dose oral administration of the dosage form after tampering to the mean Cmax of antagonist after single dose oral administration of an intact dosage form is at least 1.5:1.
Owner:PURDUE PHARMA LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com