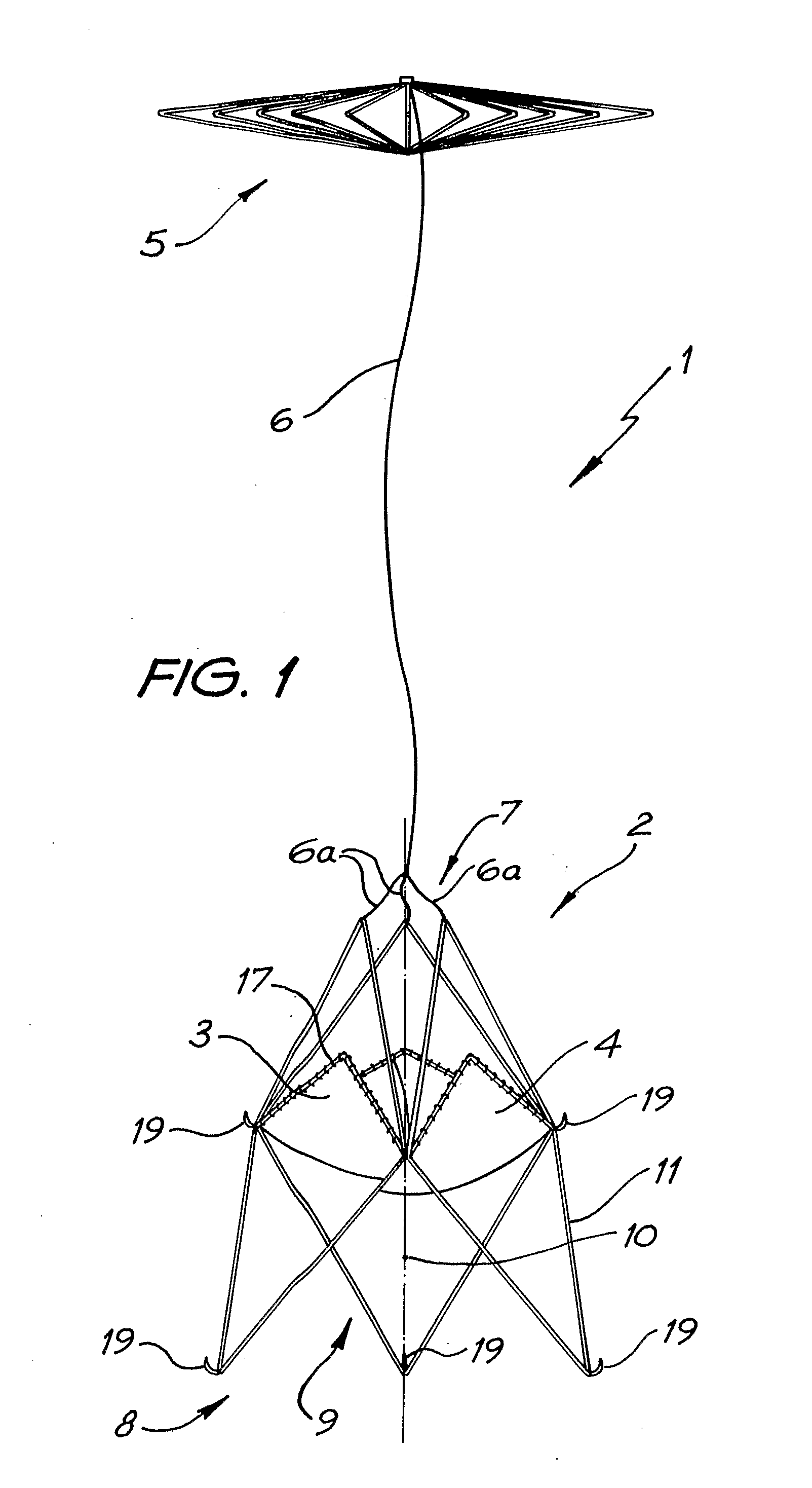
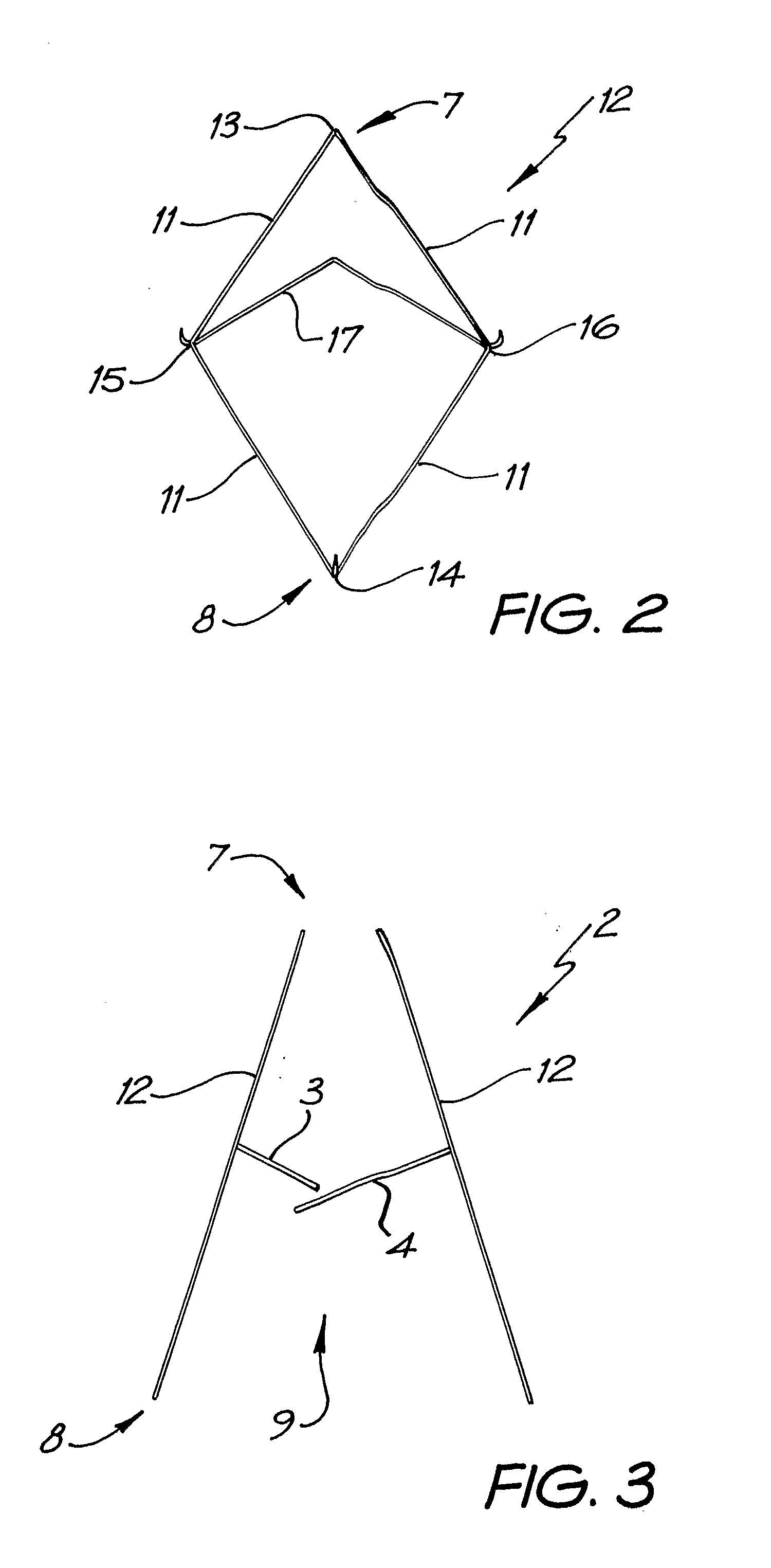
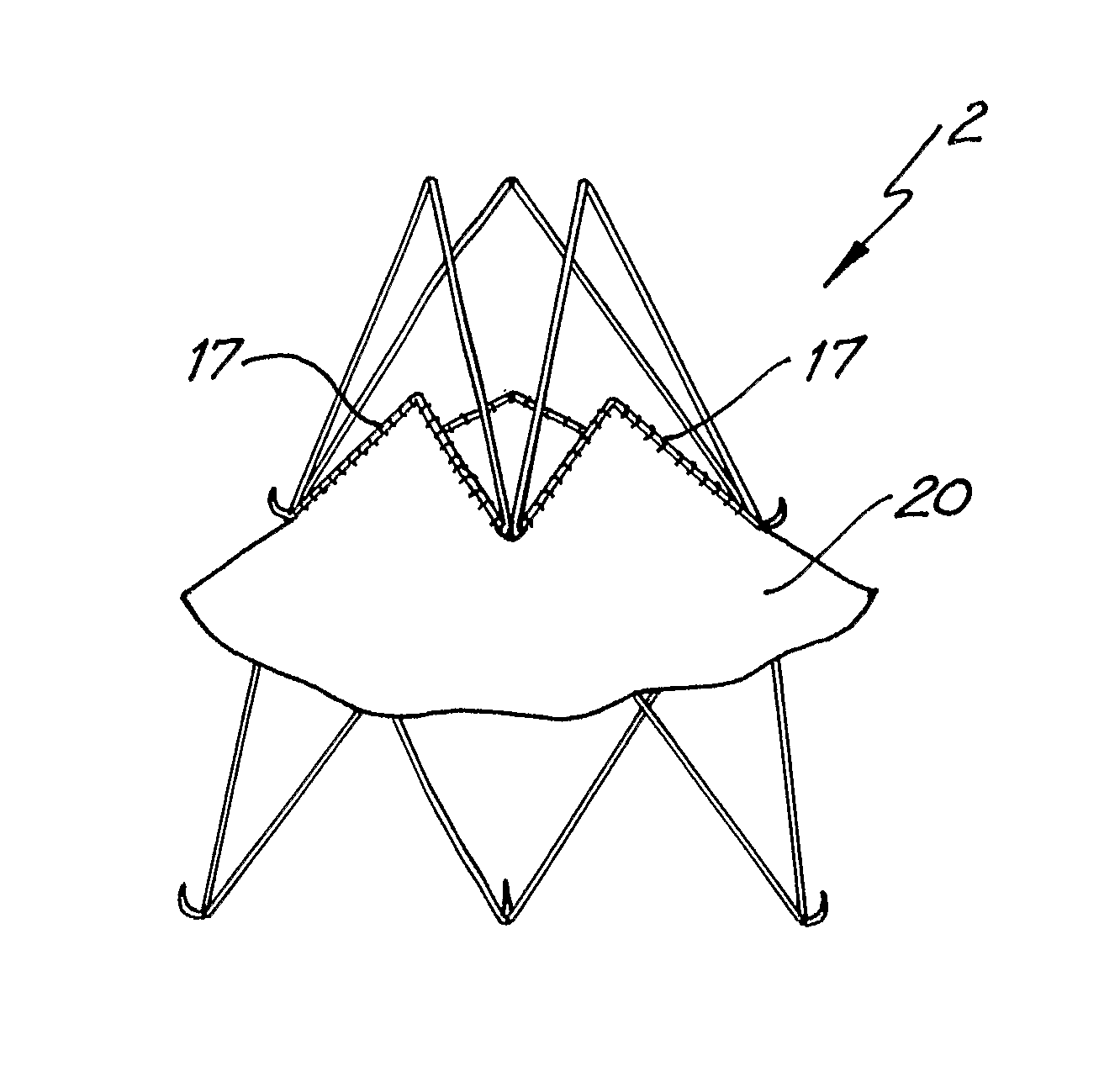
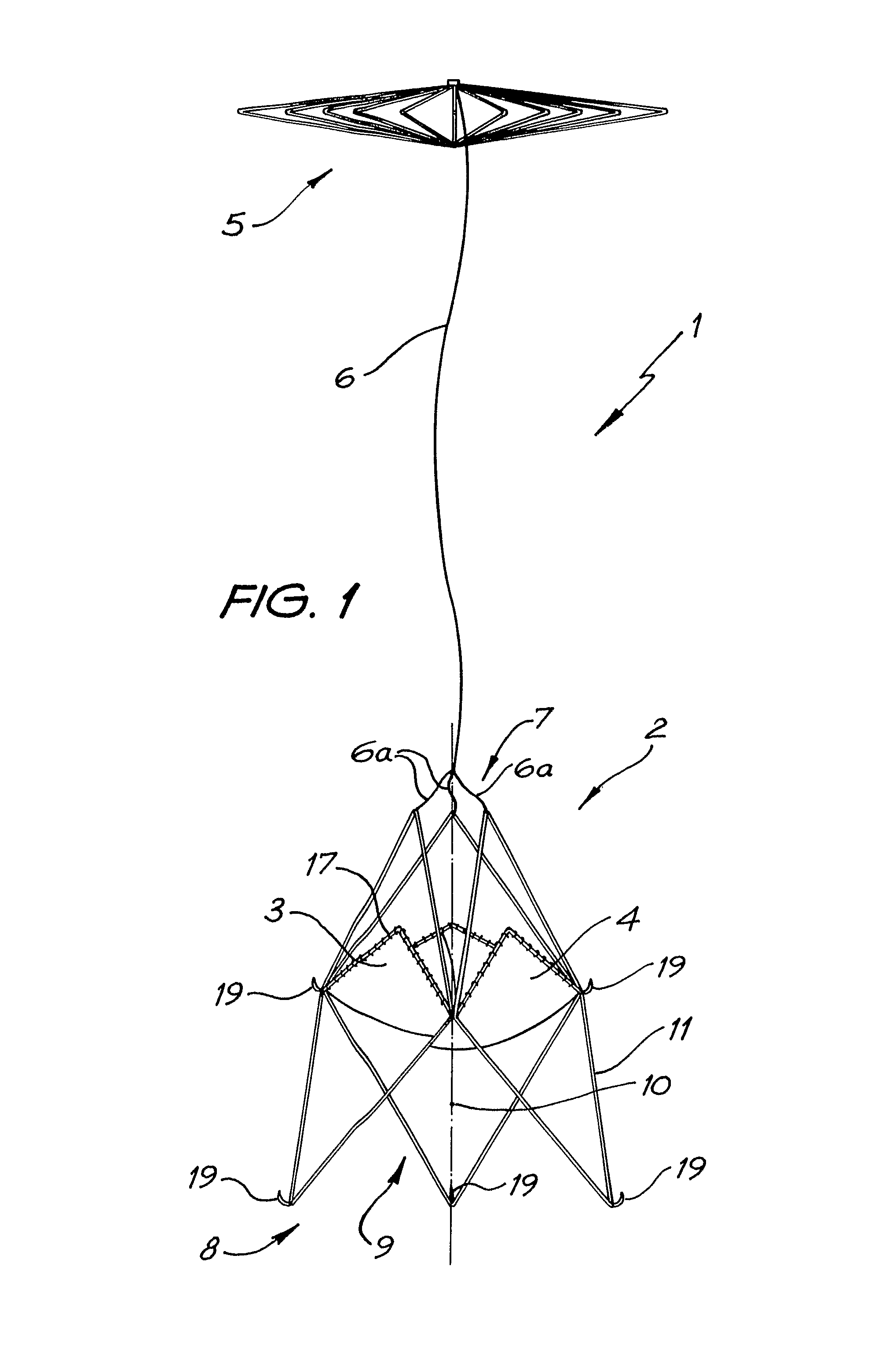
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
201 results about "Atrial septum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
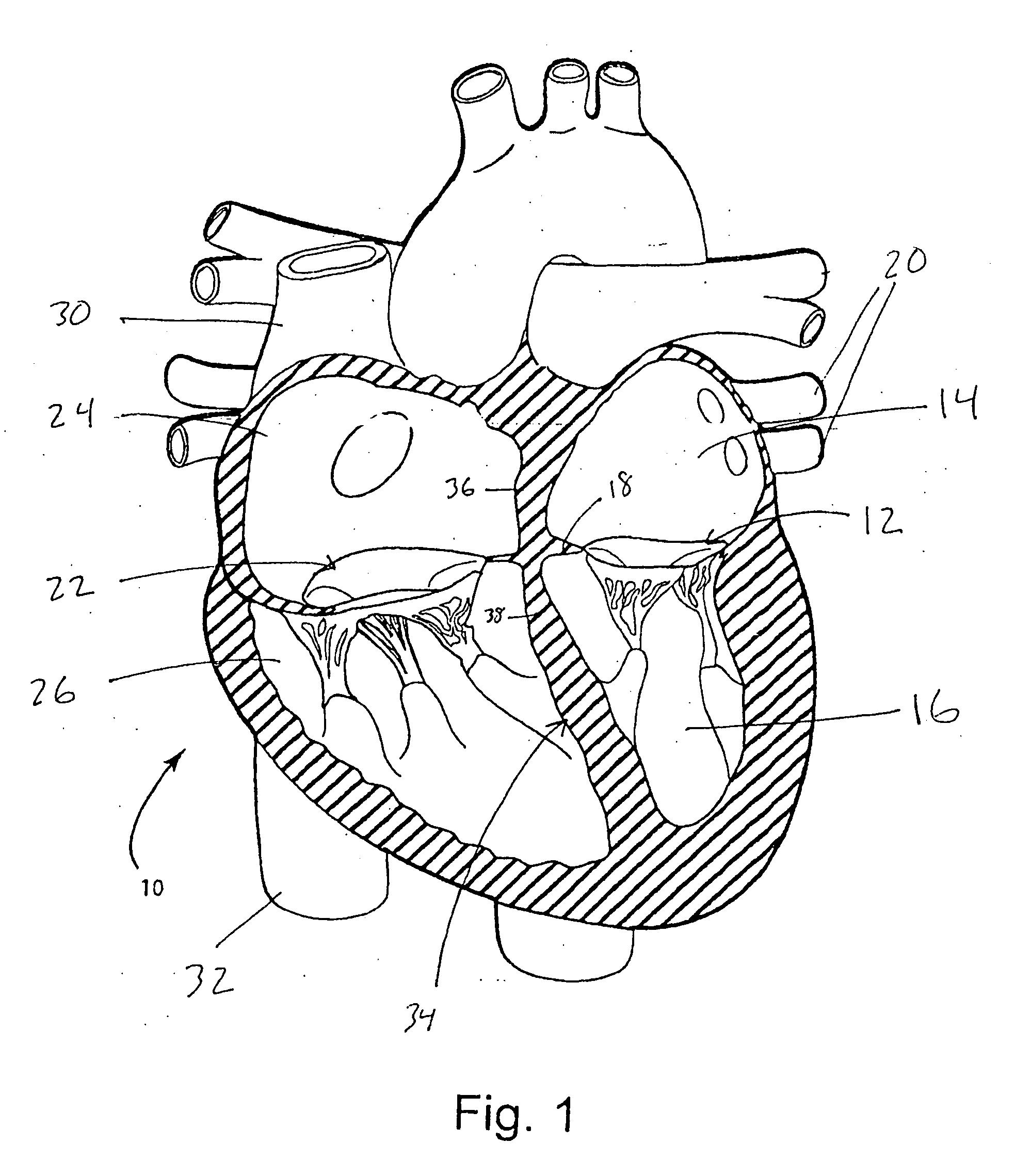
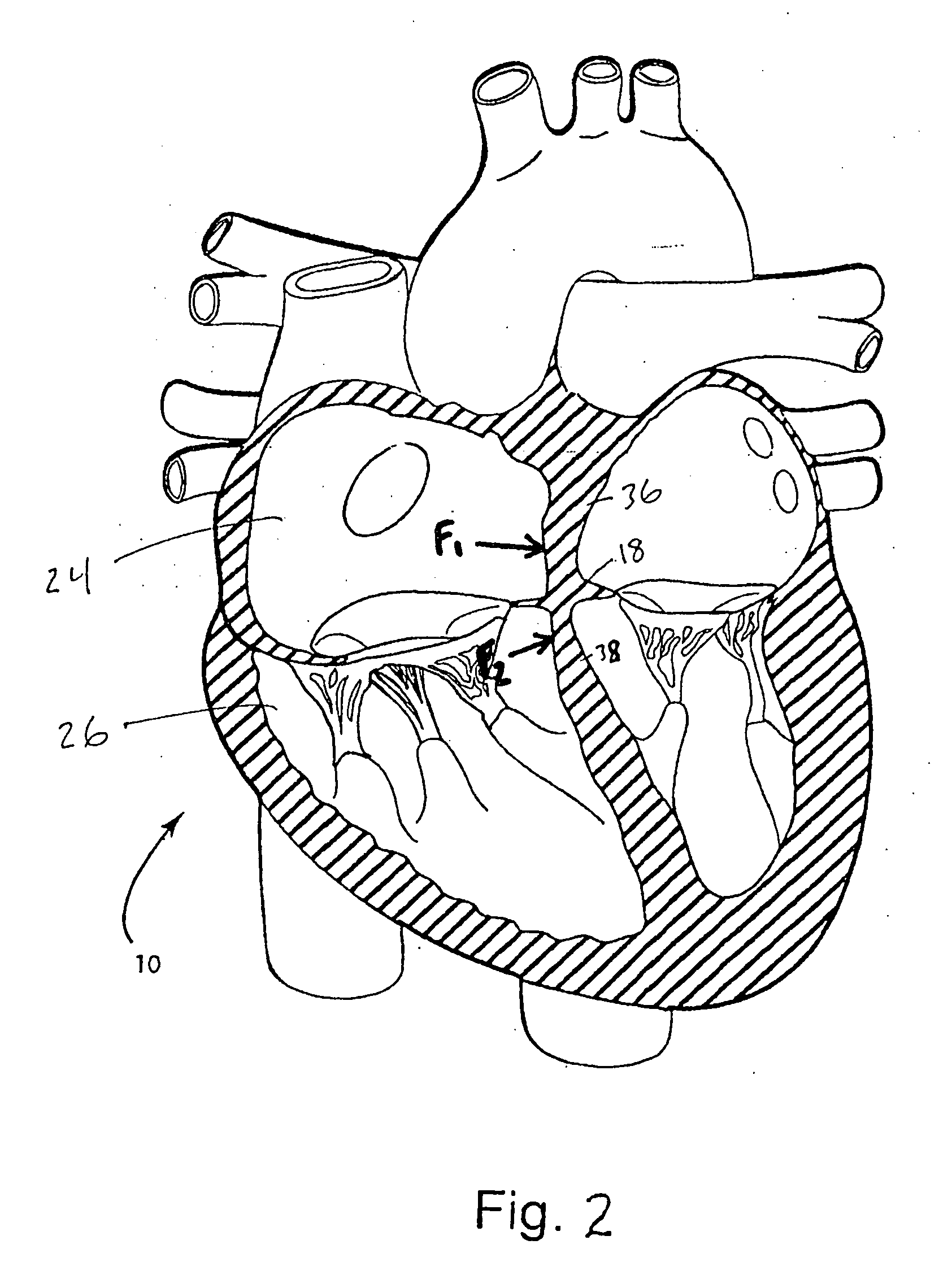
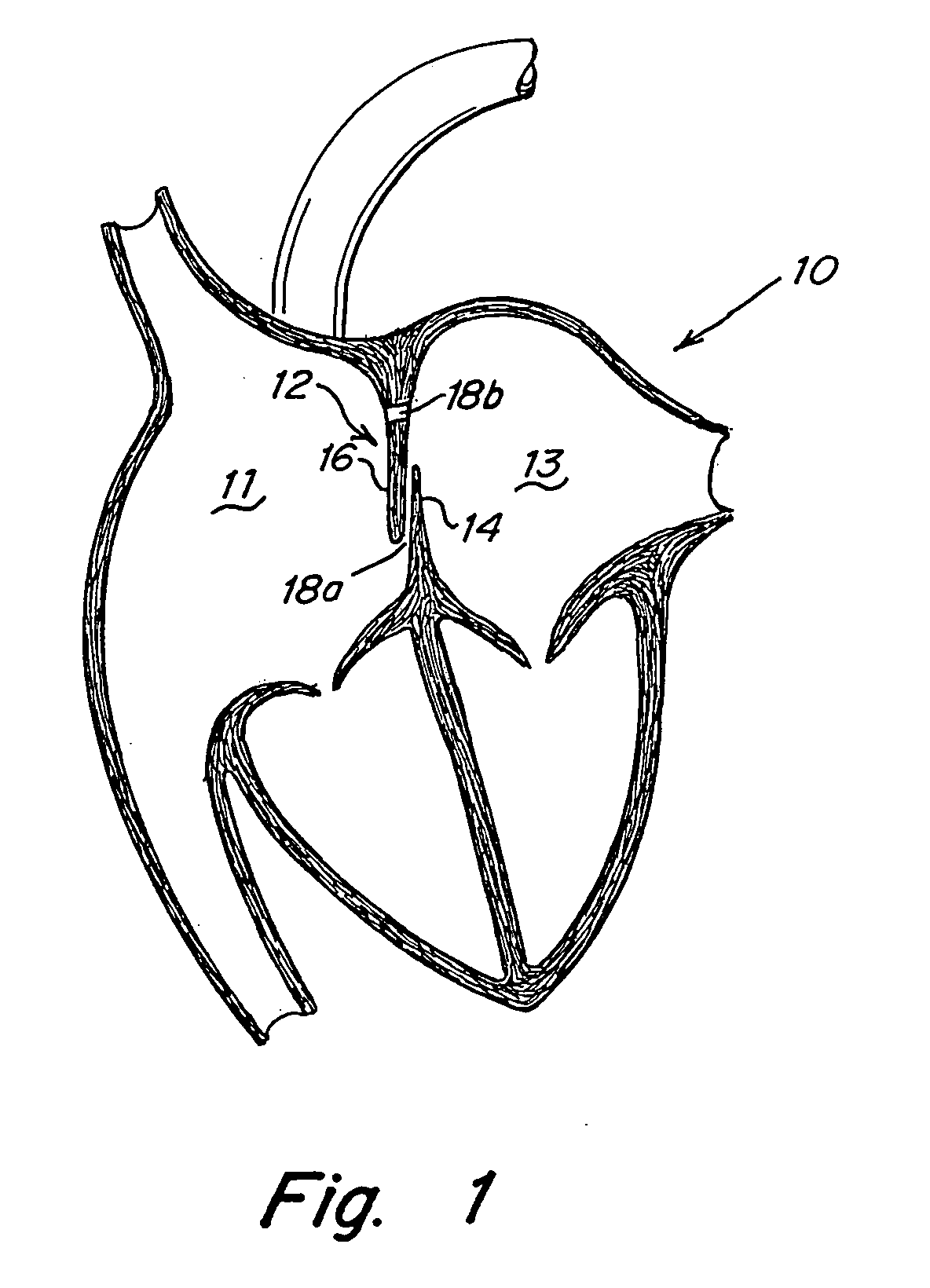
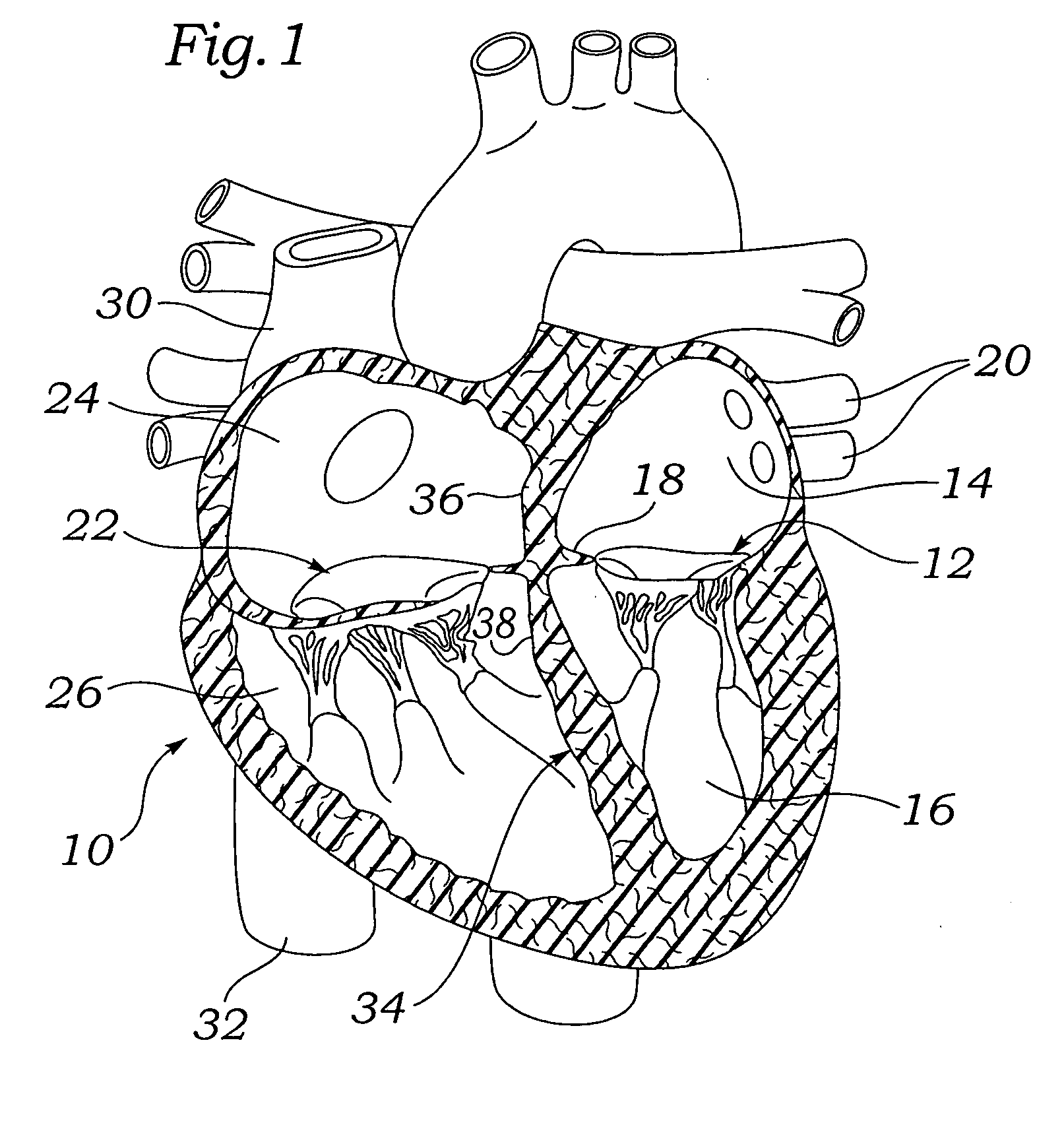
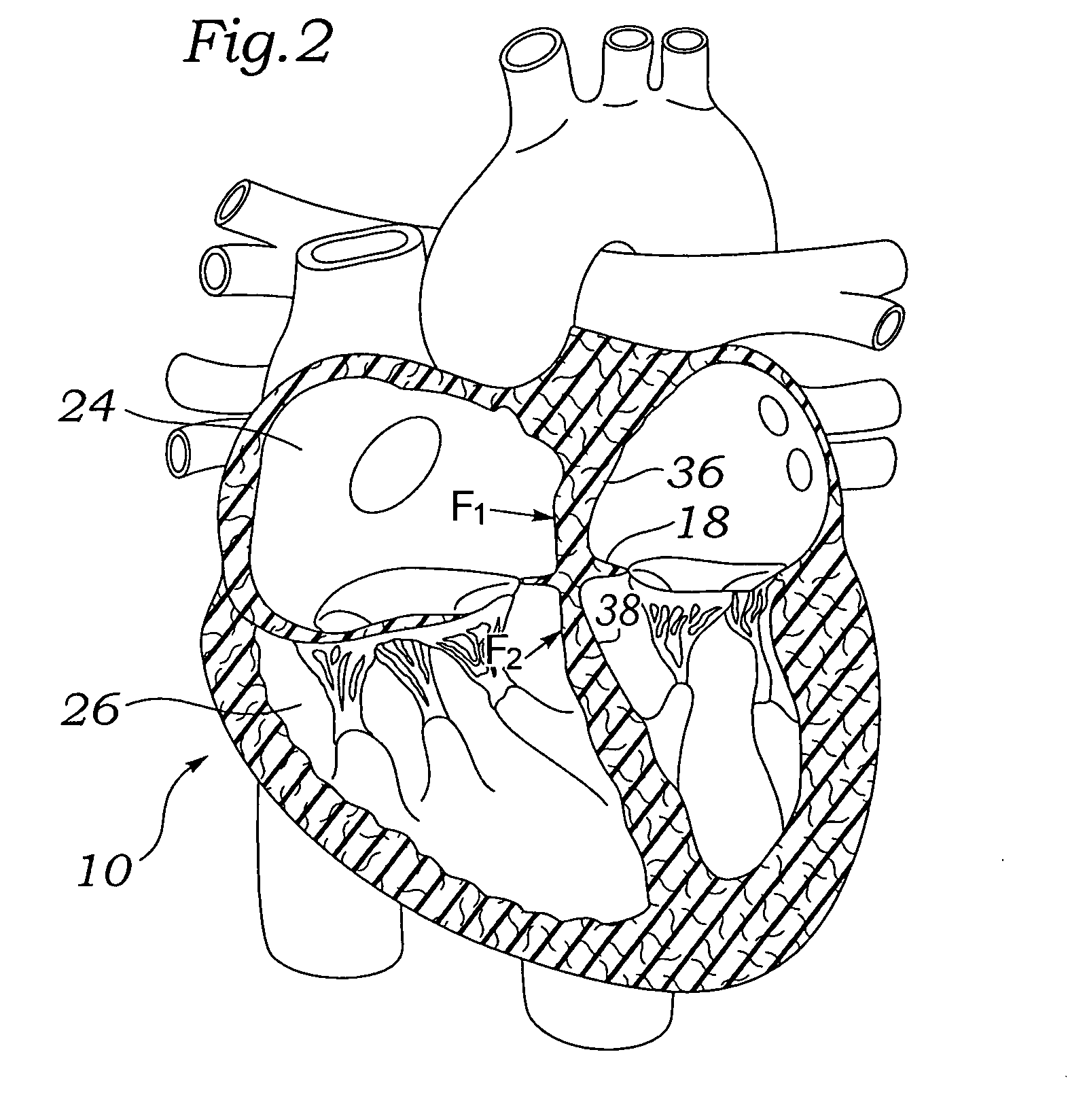
Interatrial septum (septum interatria´le cor´dis) the partition separating the right and left atria of the heart; called also atrial septum. interradicular septum interalveolar septum (def.
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
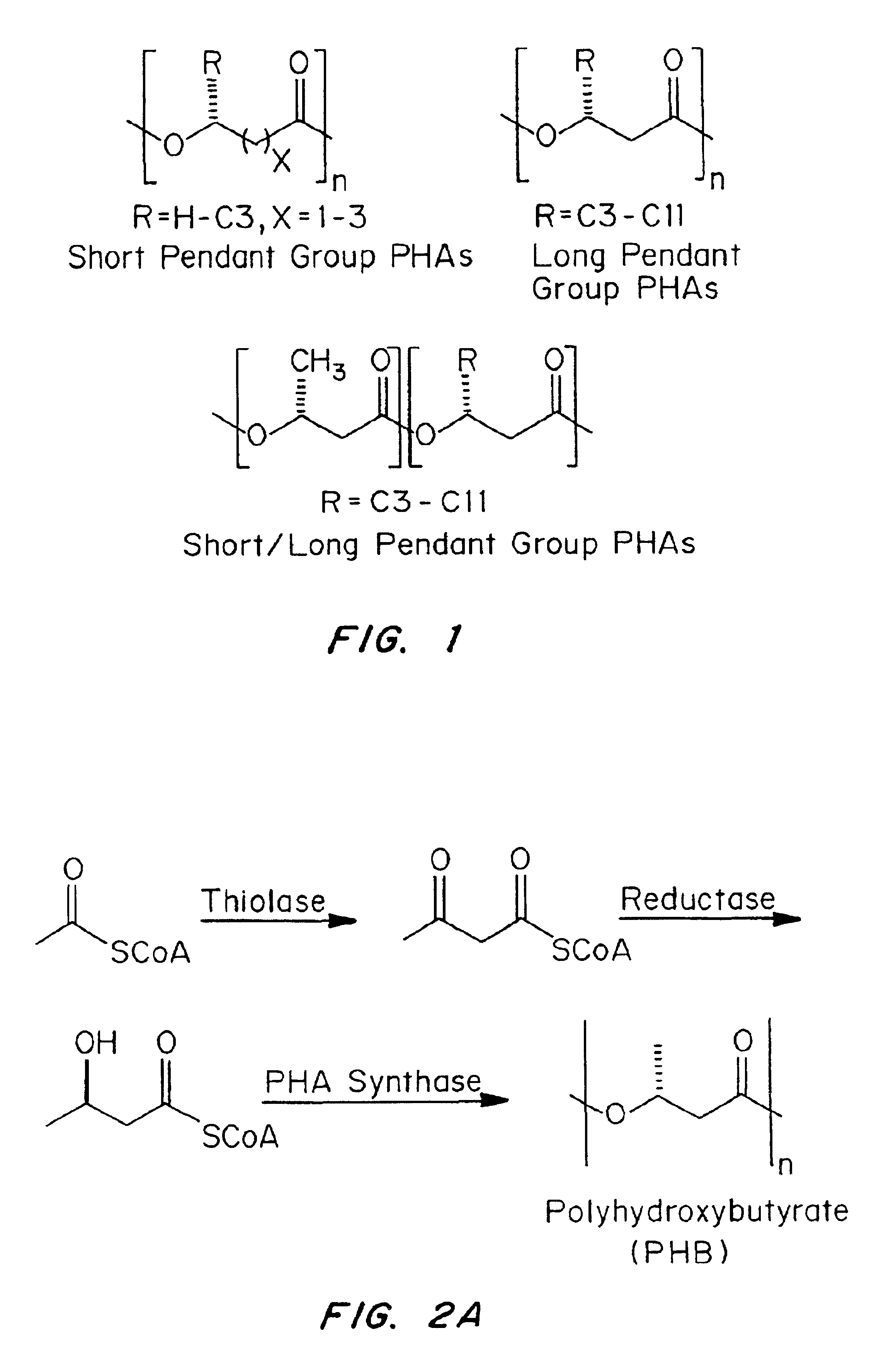
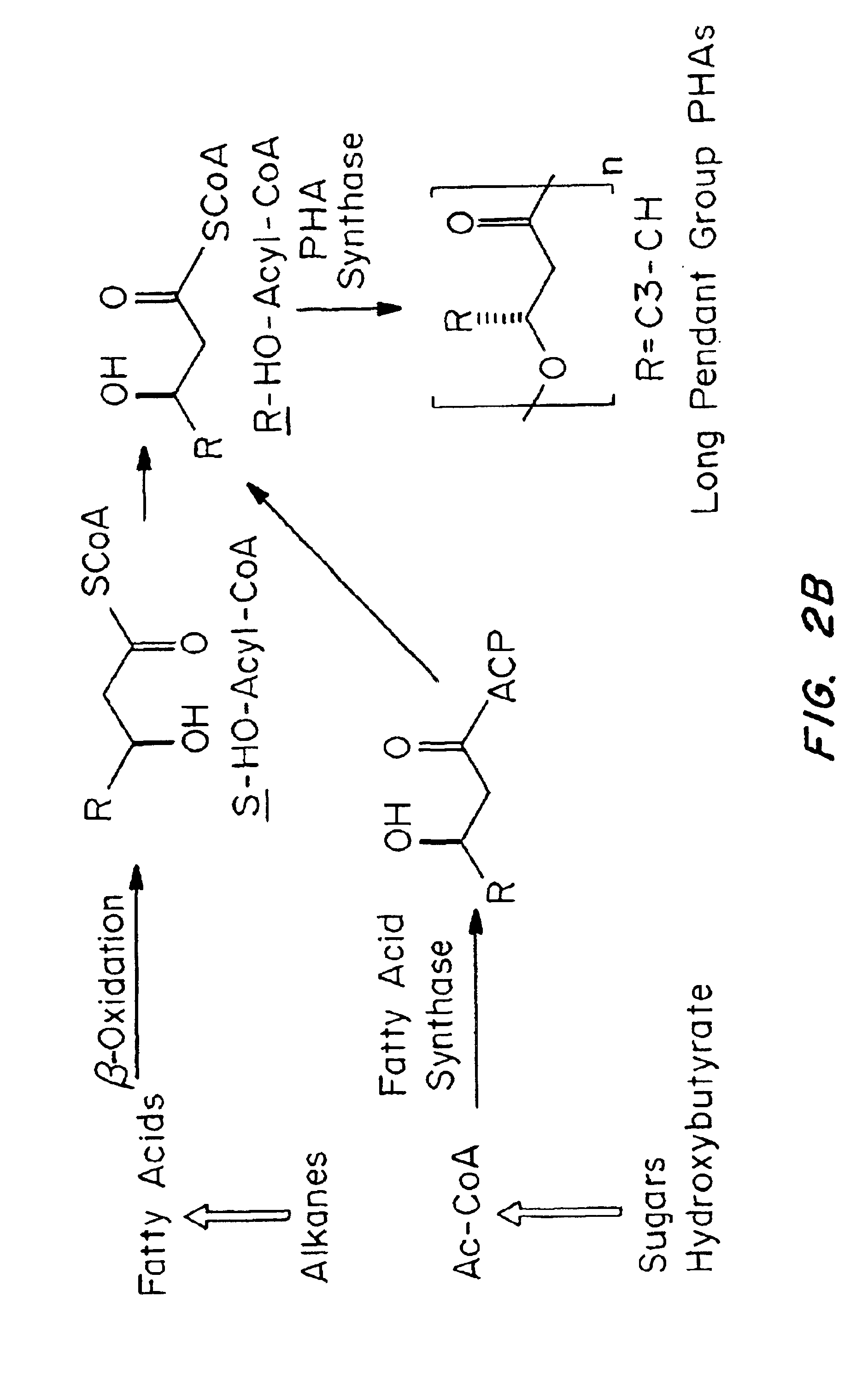
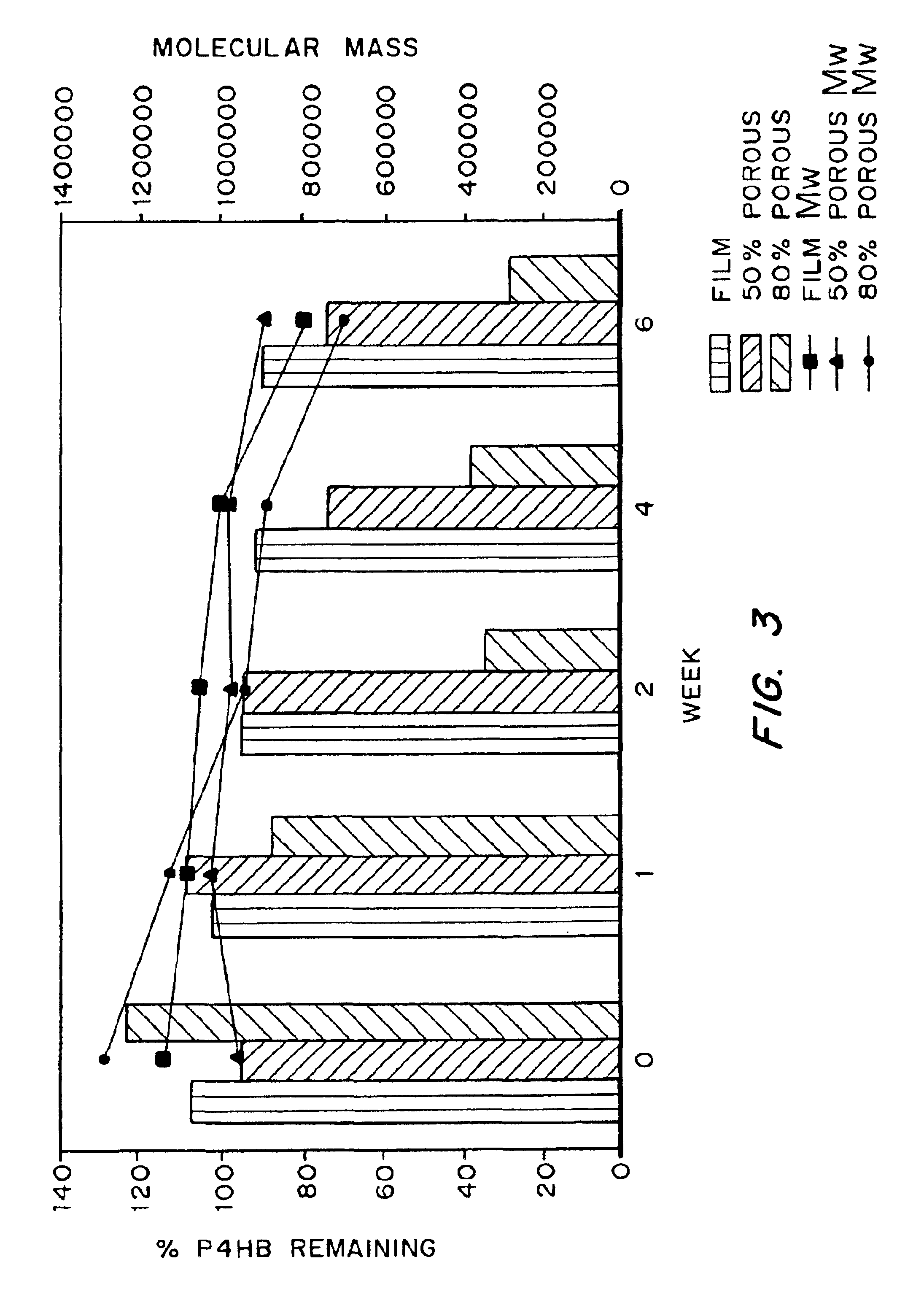
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
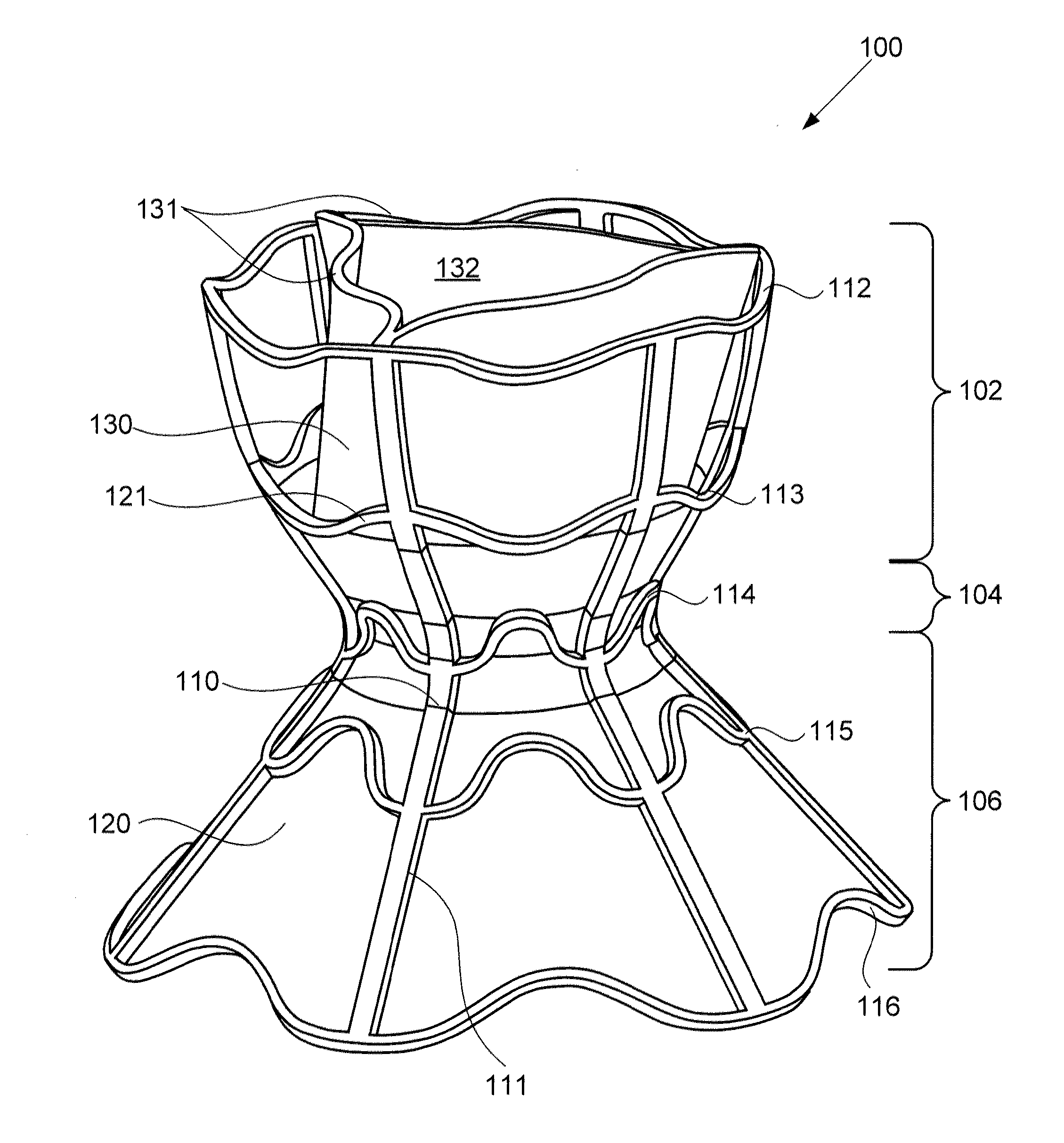
Percutaneous Heart Valve Prosthesis
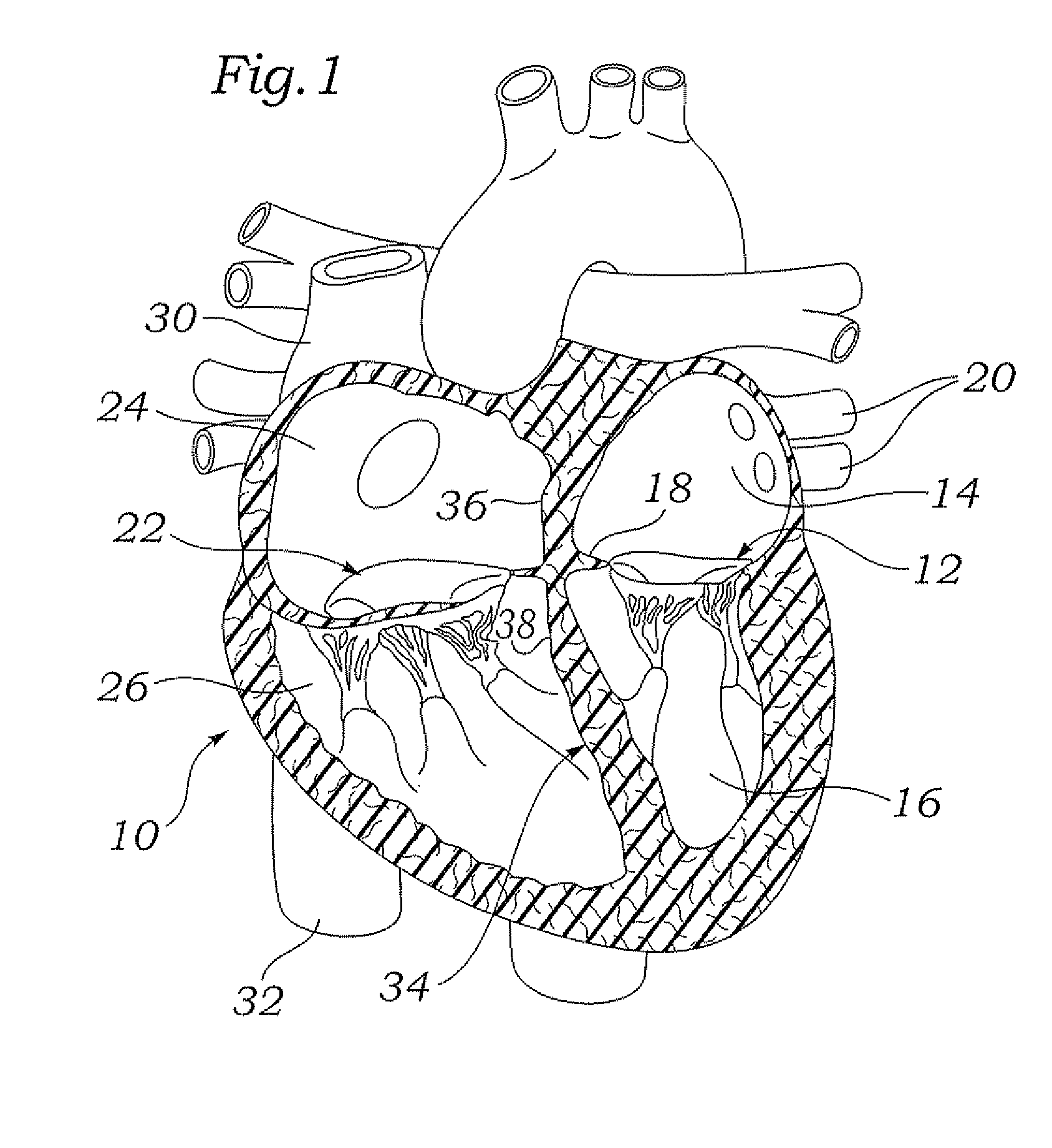
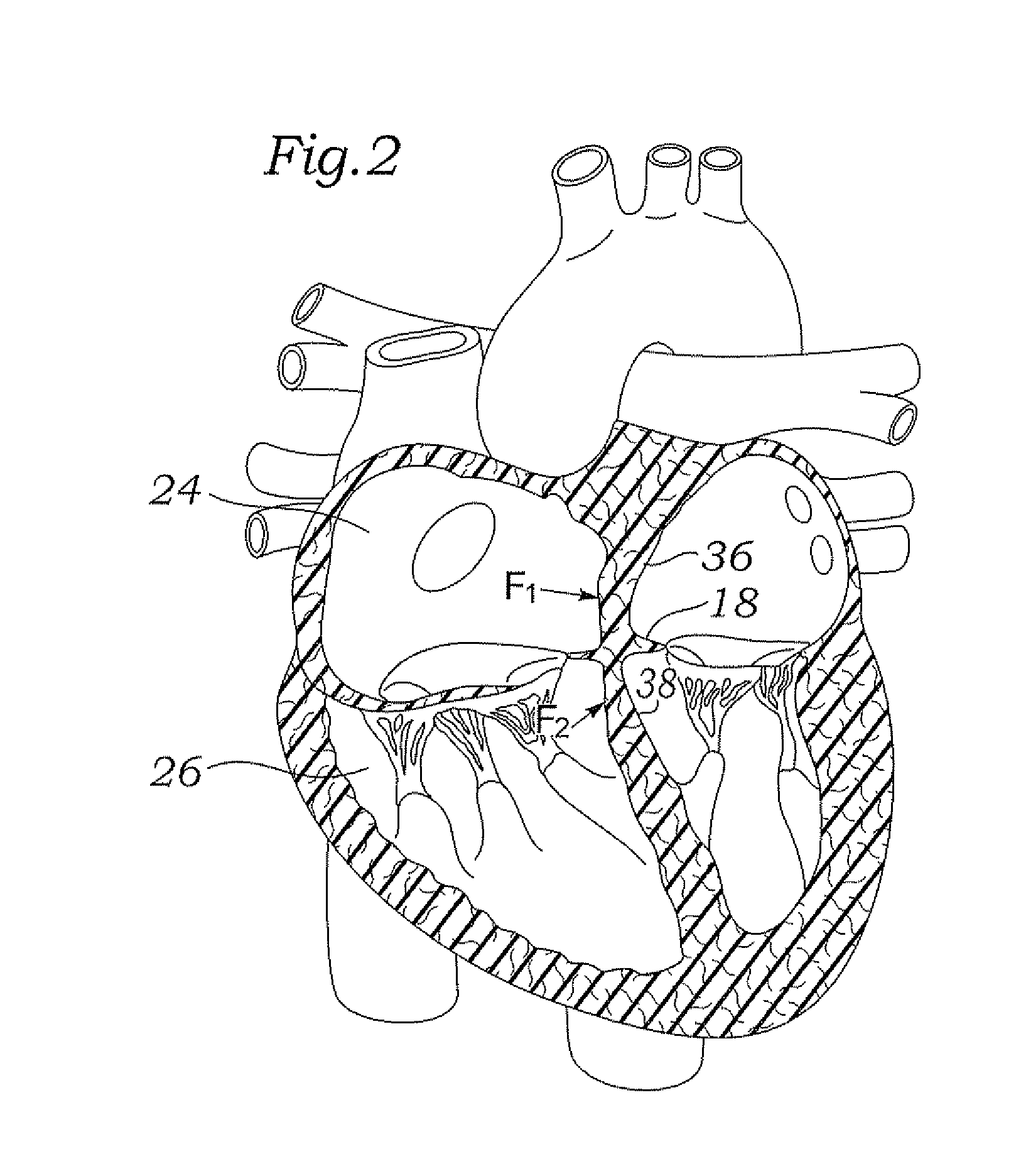
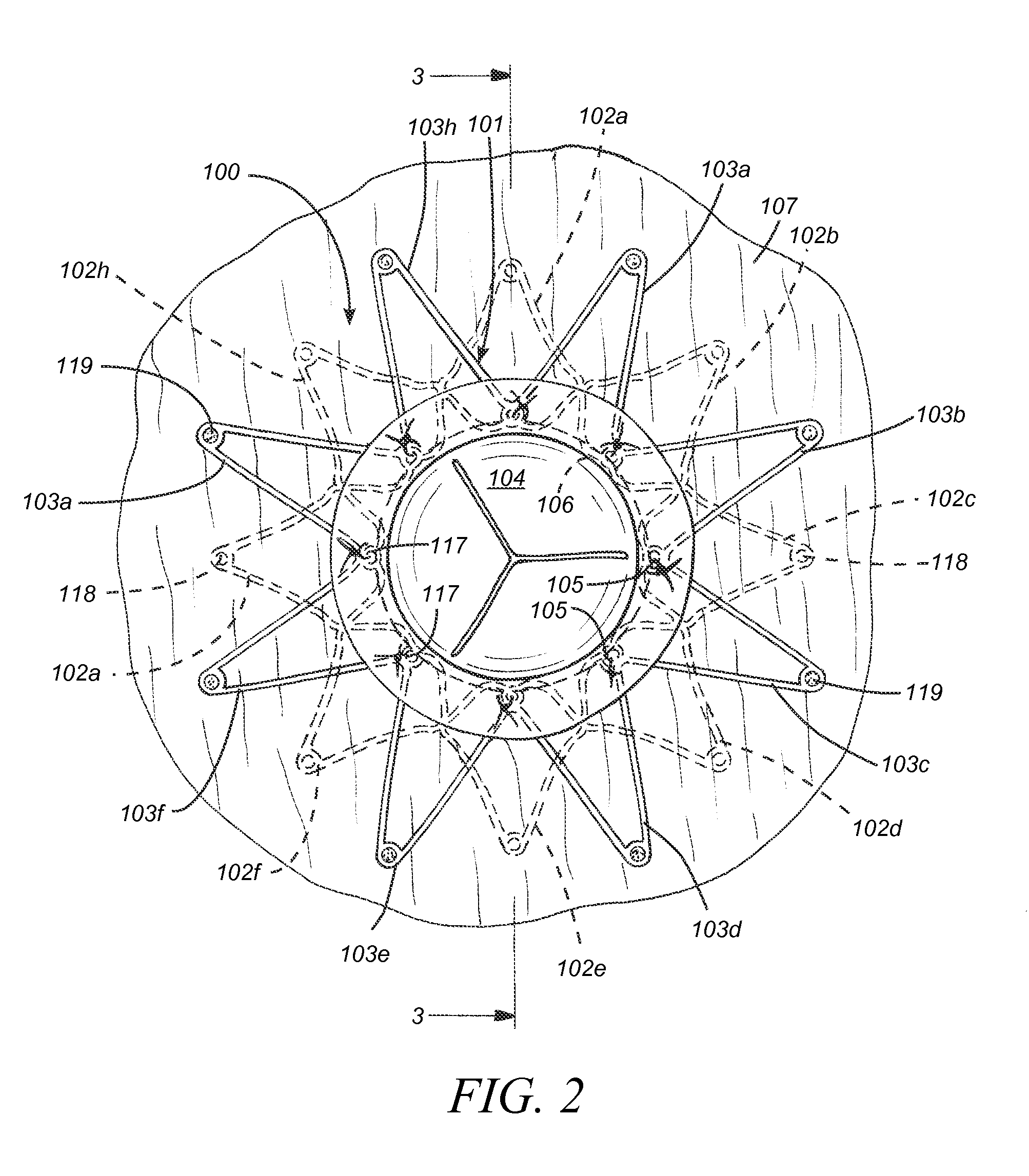
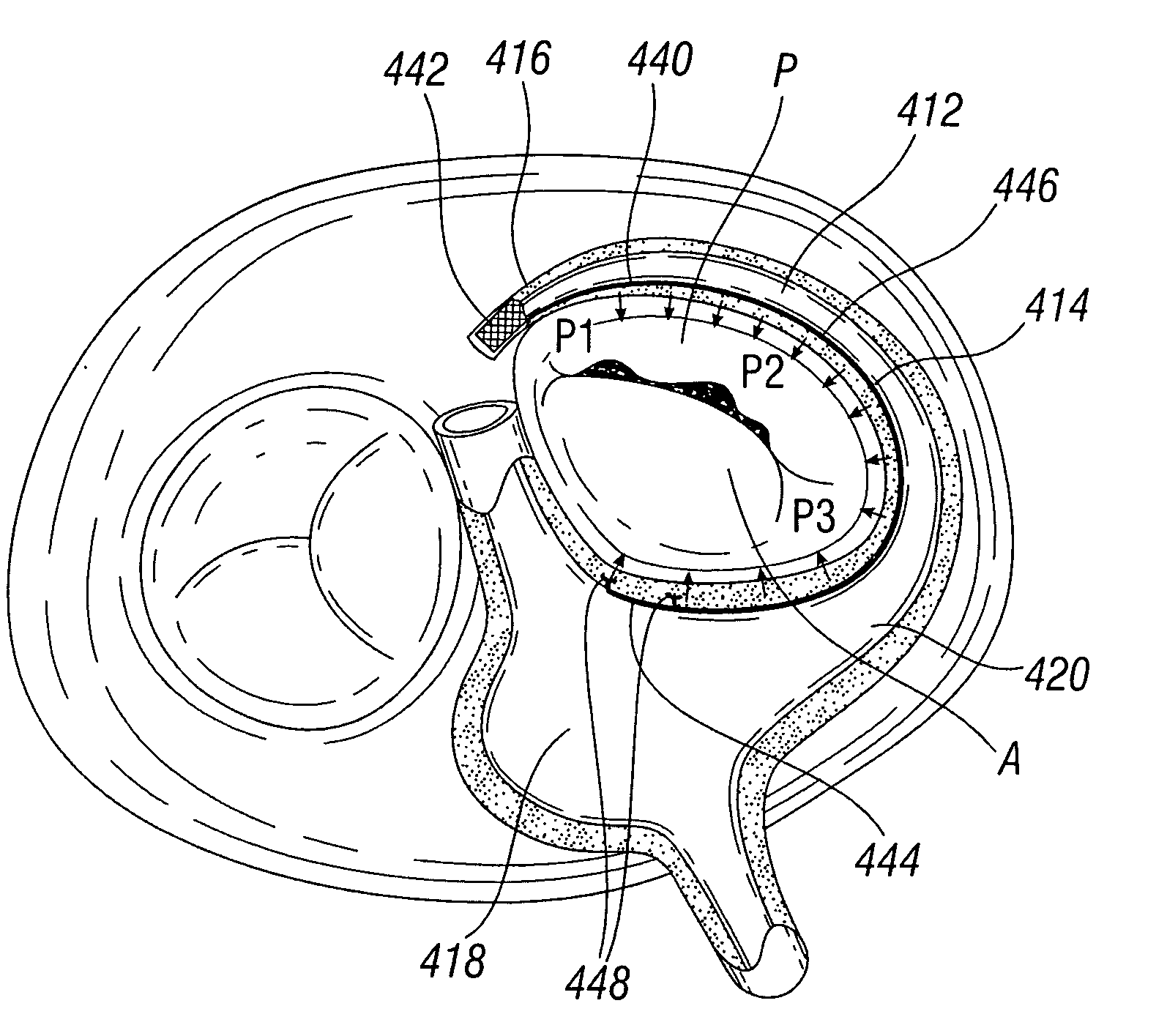
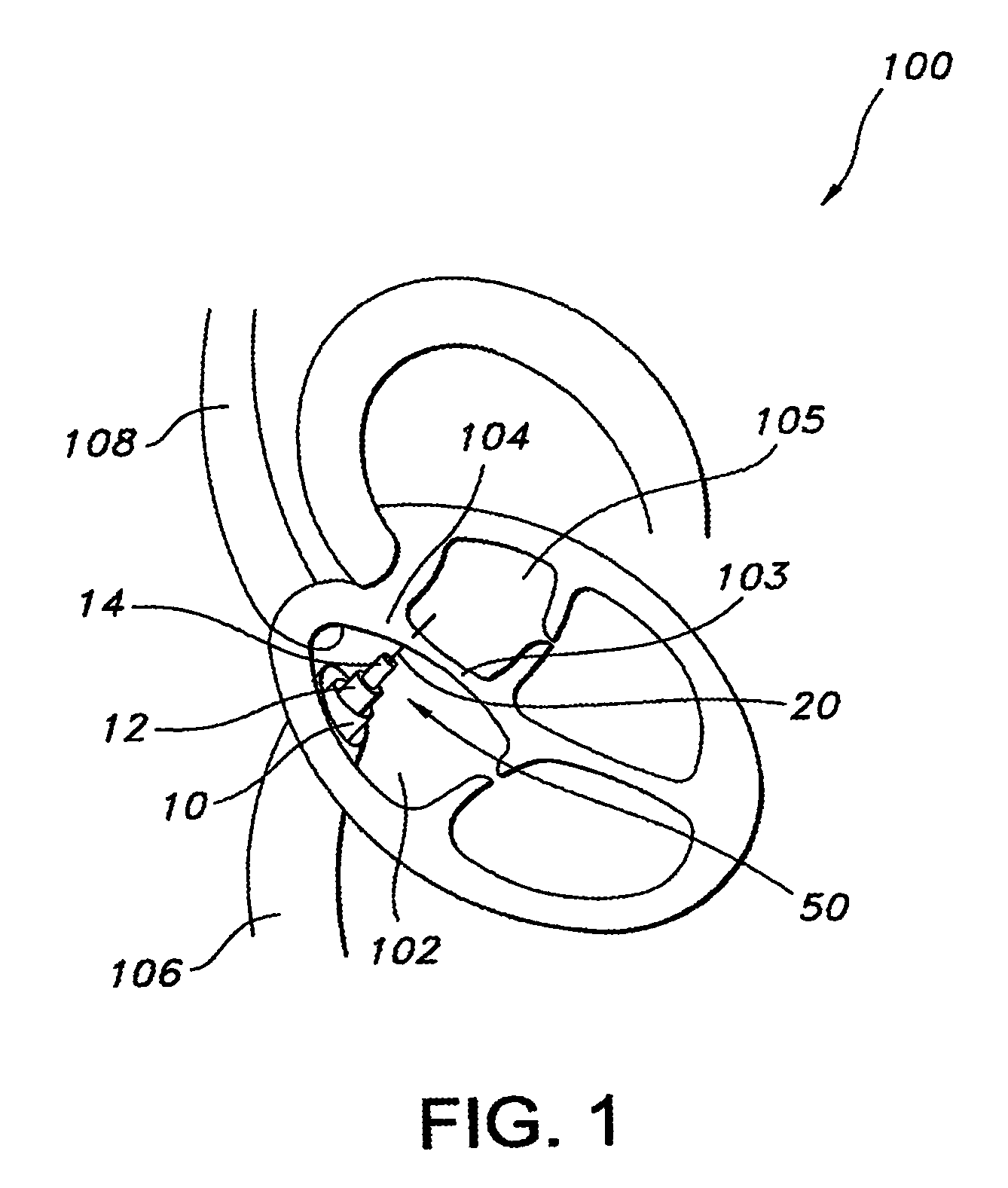
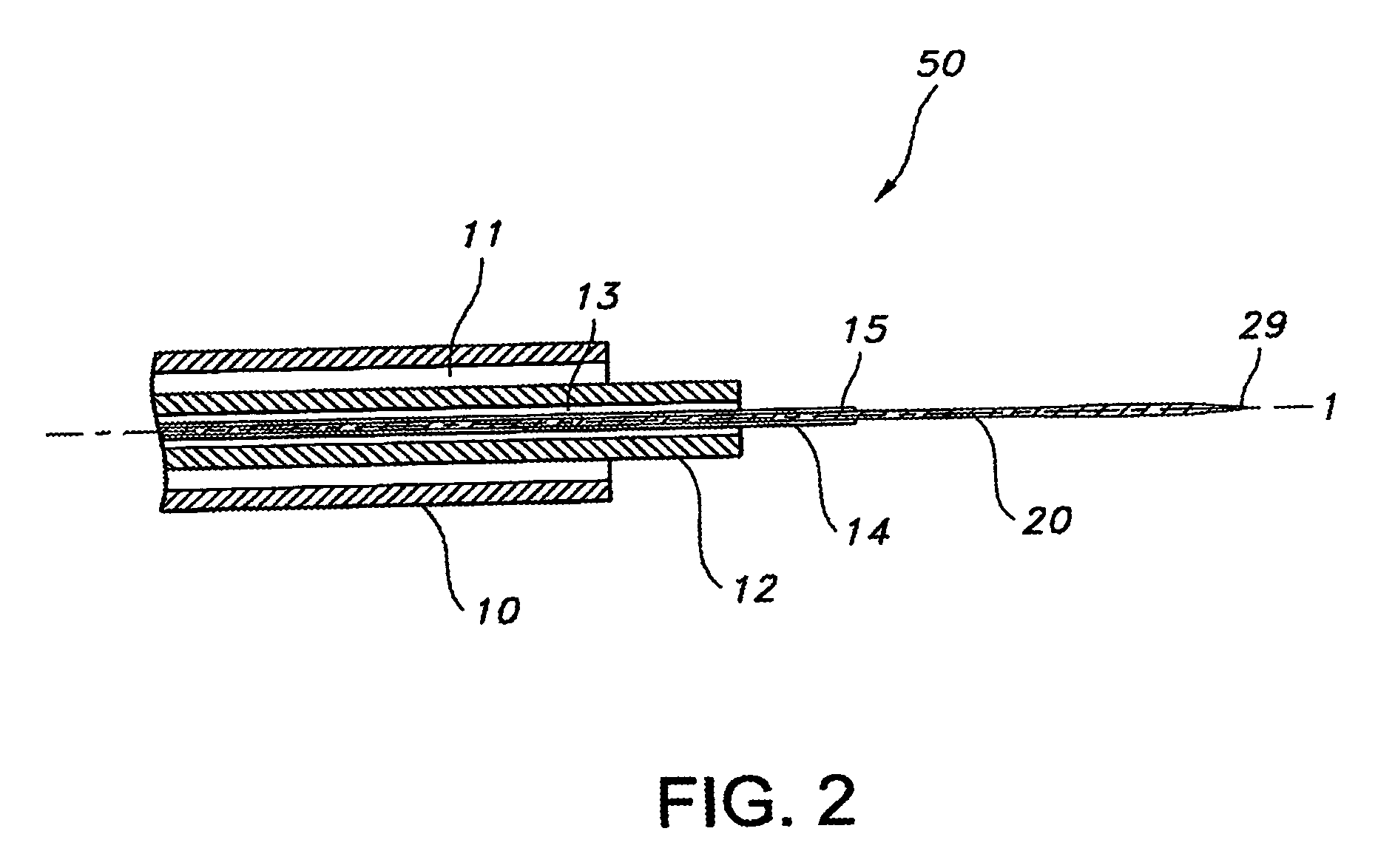
A percutaneous heart valve prosthesis (1) has a valve body (2) with a passage (9) extending between the first and second ends (7, 8) of the valve body (2). The valve body (2) is collapsible about a longitudinal axis (10) of the passage (9) for delivery of the valve body (2) via a catheter (18). One or more flexible valve leaflets (3, 4) are secured to the valve body (2) and extend across the passage (9) for blocking bloodflow in one direction through the passage (9). An anchor device (5), which is also collapsible for delivery via catheter (18), is secured to the valve body (2) by way of an anchor line (6). A failed or failing mitral heart valve (101) is treated by percutaneously locating the valve body (2) in the mitral valve orifice (102) with the anchor device (5) located in the right atrium (107) and engaging the inter-atrial septum (103), such that the taught anchor line (6) acts to secure the valve body (2) within the mitral valve orifice (102).
Owner:PERCUTANEOUS CARDIOVASCULAR SOLUTIONS
Percutaneous heart valve prosthesis
A percutaneous heart valve prosthesis (1) has a valve body (2) with a passage (9) extending between the first and second ends (7, 8) of the valve body (2). The valve body (2) is collapsible about a longitudinal axis (10) of the passage (9) for delivery of the valve body (2) via a catheter (18). One or more flexible valve leaflets (3, 4) are secured to the valve body (2) and extend across the passage (9) for blocking bloodflow in one direction through the passage (9). An anchor device (5), which is also collapsible for delivery via catheter (18), is secured to the valve body (2) by way of an anchor line (6). A failed or failing mitral heart valve (101) is treated by percutaneously locating the valve body (2) in the mitral valve orifice (102) with the anchor device (5) located in the right atrium (107) and engaging the inter-atrial septum (103), such that the taught anchor line (6) acts to secure the valve body (2) within the mitral valve orifice (102).
Owner:PERCUTANEOUS CARDIOVASCULAR SOLUTIONS PTY LTD
Expandable trans-septal sheath
InactiveUS20060135962A1Inhibit bindingAvoid interferenceGuide needlesEar treatmentAccess routeDilator
Disclosed is an expandable transluminal sheath, for introduction into the body while in a first, low cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the vascular system. The access route is through the inferior vena cava to the right atrium, where a trans-septal puncture, followed by advancement of the catheter is completed. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement through the atrial septum into the left atrium. The distal end of the sheath is expanded using a radial dilator. In one application, the sheath is utilized to provide access for a diagnostic or therapeutic procedure such as electrophysiological mapping of the heart, radio-frequency ablation of left atrial tissue, placement of atrial implants, valve repair, or the like.
Owner:ONSET MEDICAL CORP
Device and method for reshaping mitral valve annulus
InactiveUS20070061010A1Improve bindingReduce distanceStentsAnnuloplasty ringsAnterior leafletPosterior leaflet
Owner:EDWARDS LIFESCIENCES CORP
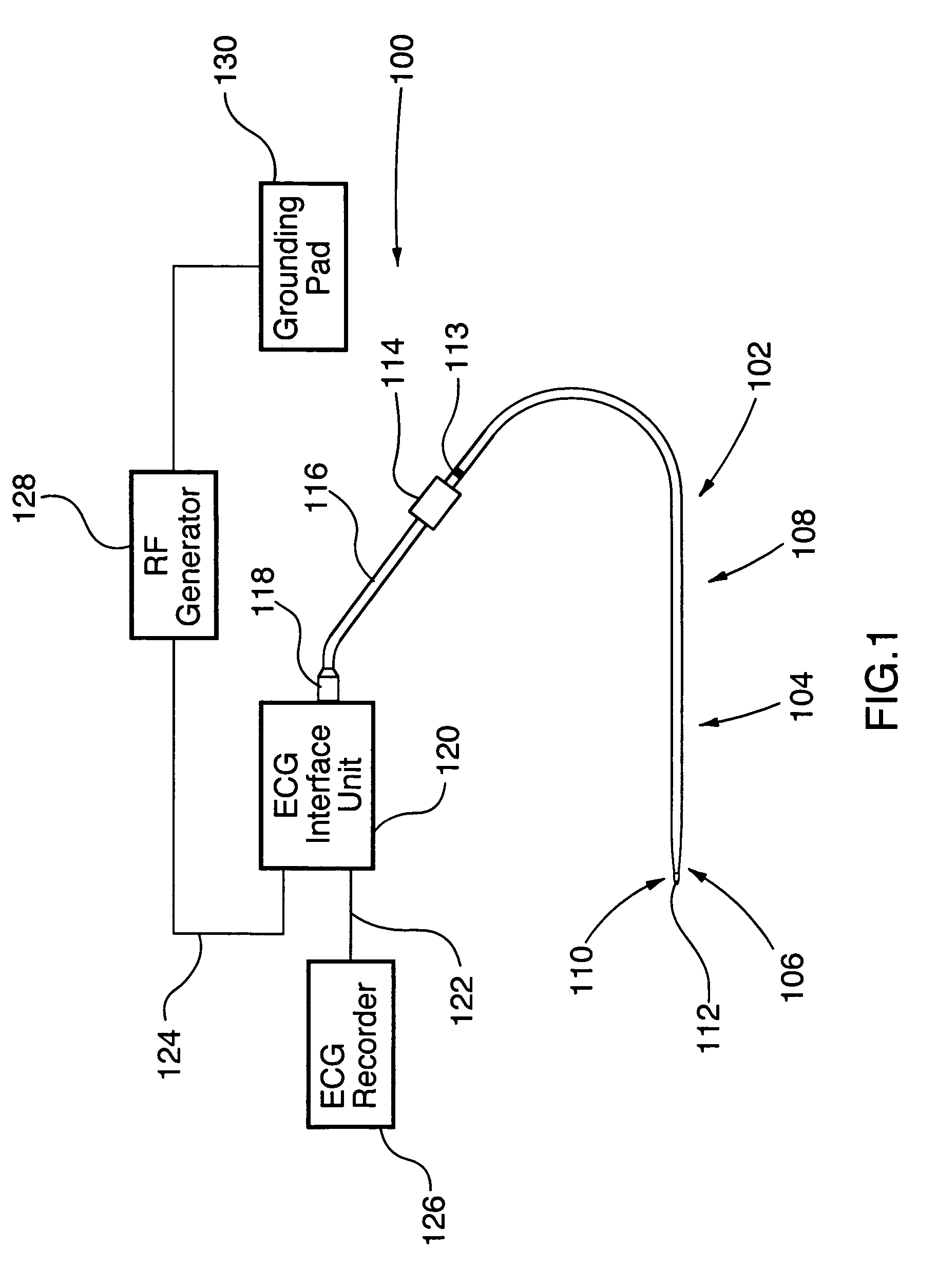
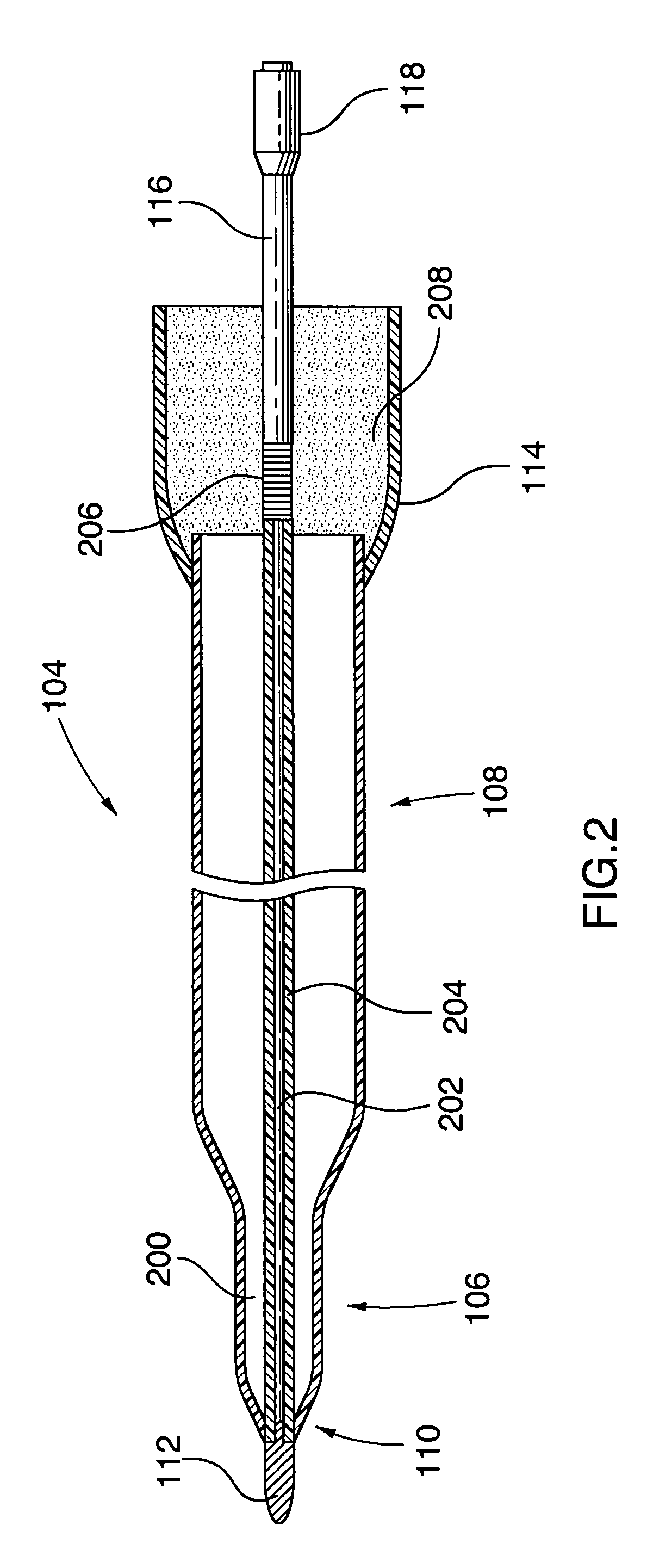
Ablation Therapy System and Method for Treating Continuous Atrial Fibrillation
InactiveUS20080281312A1Limited in amount of energyAvoid the needDiagnosticsSurgical instruments for heatingVeinRf ablation
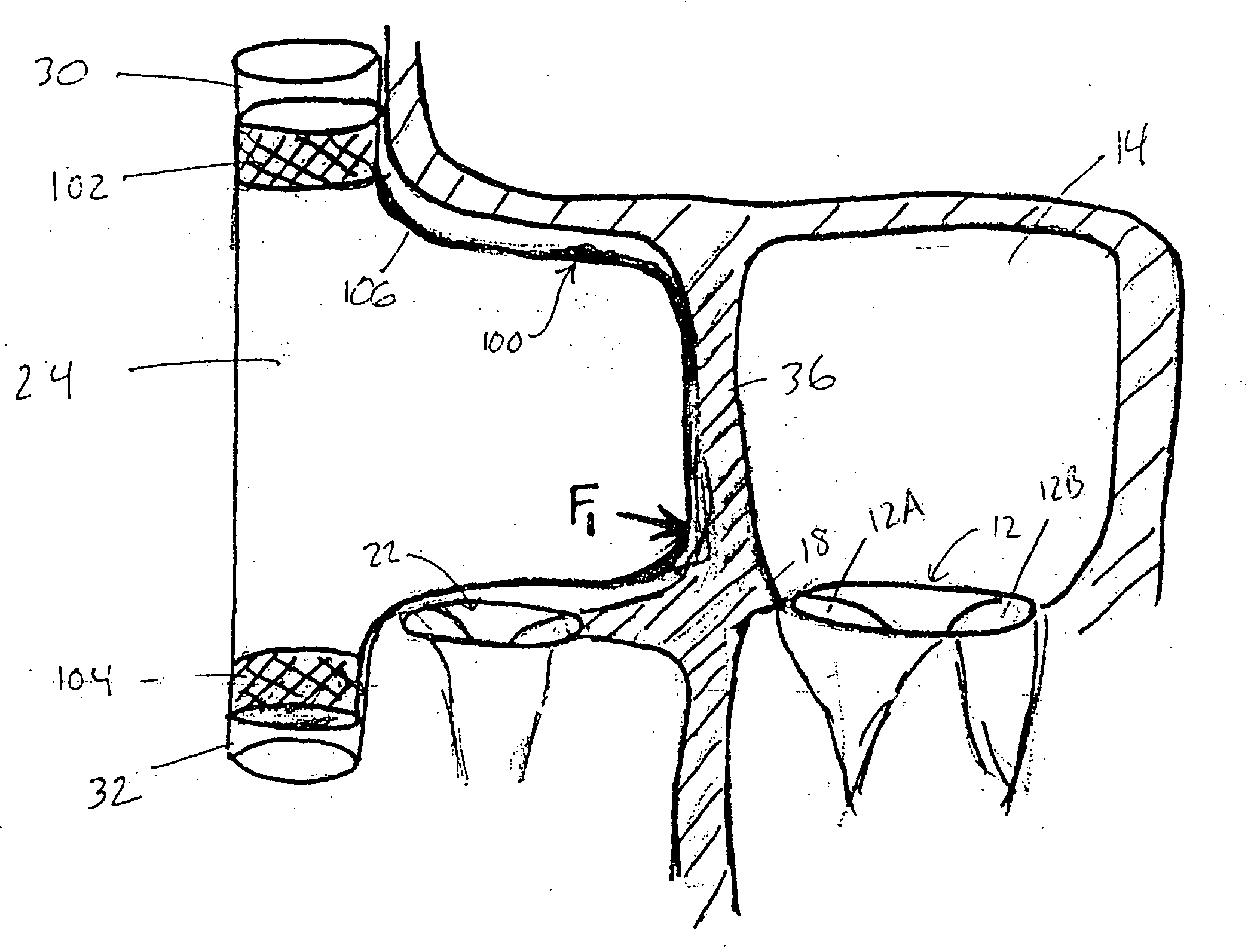
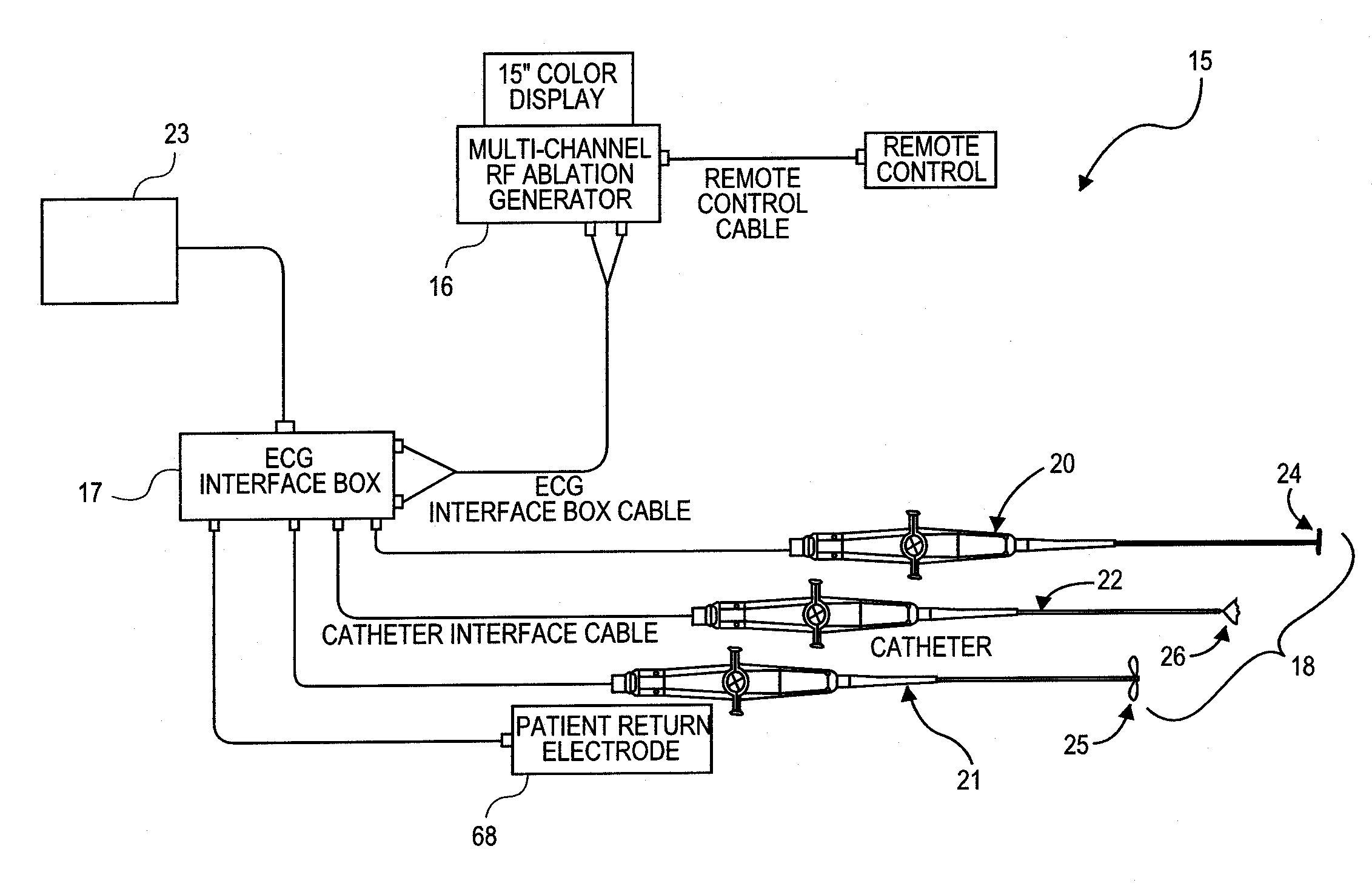
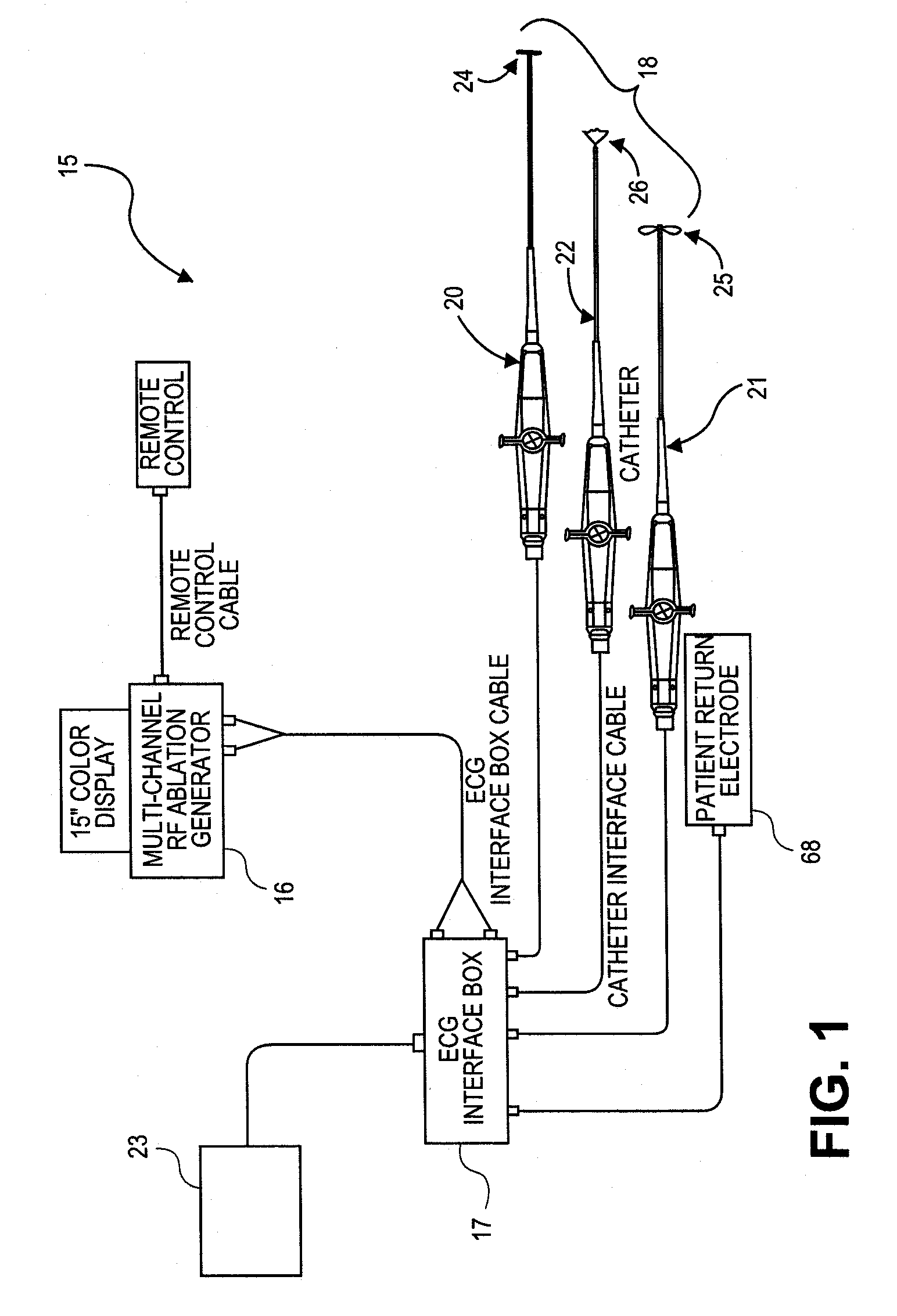
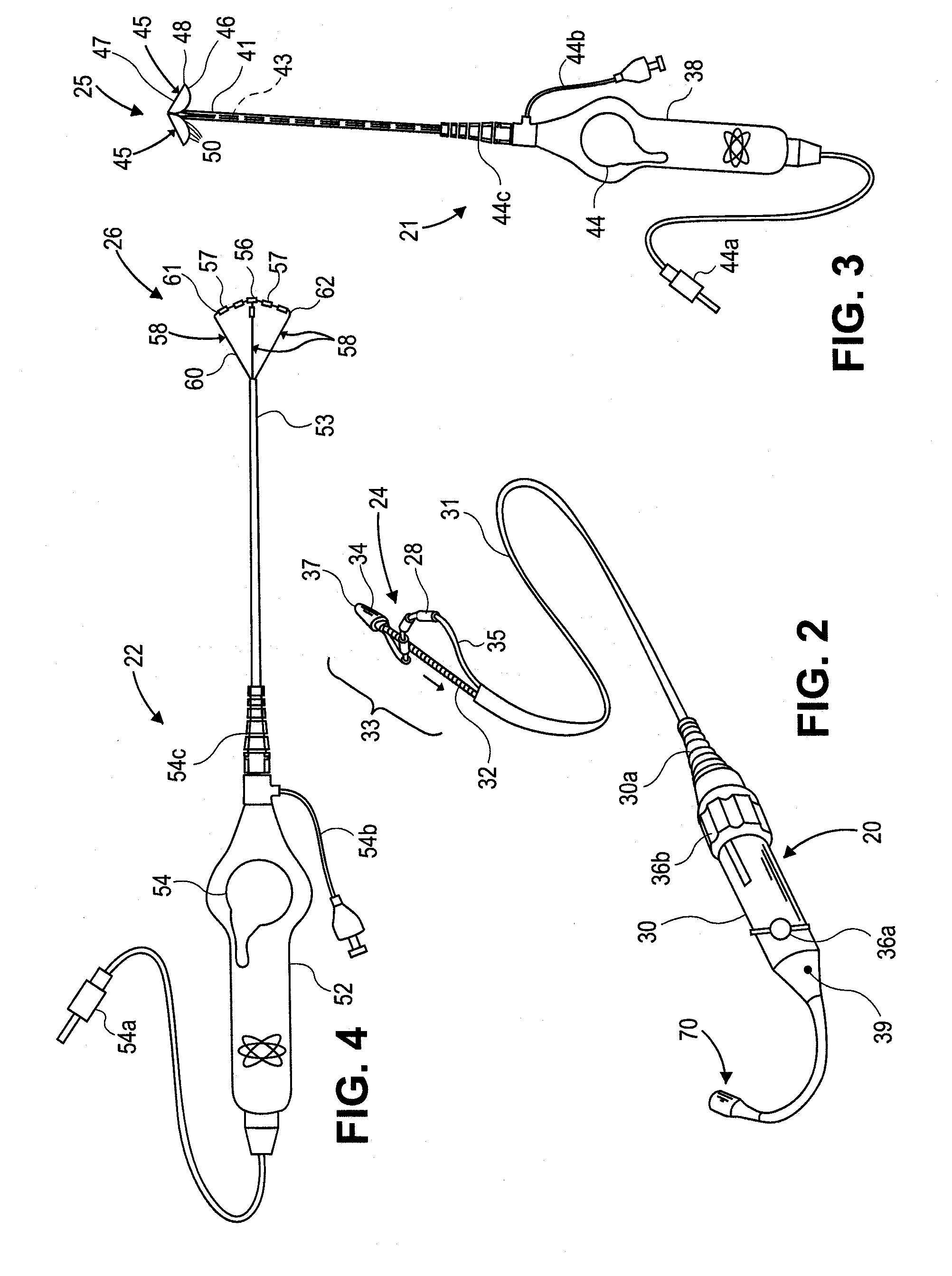
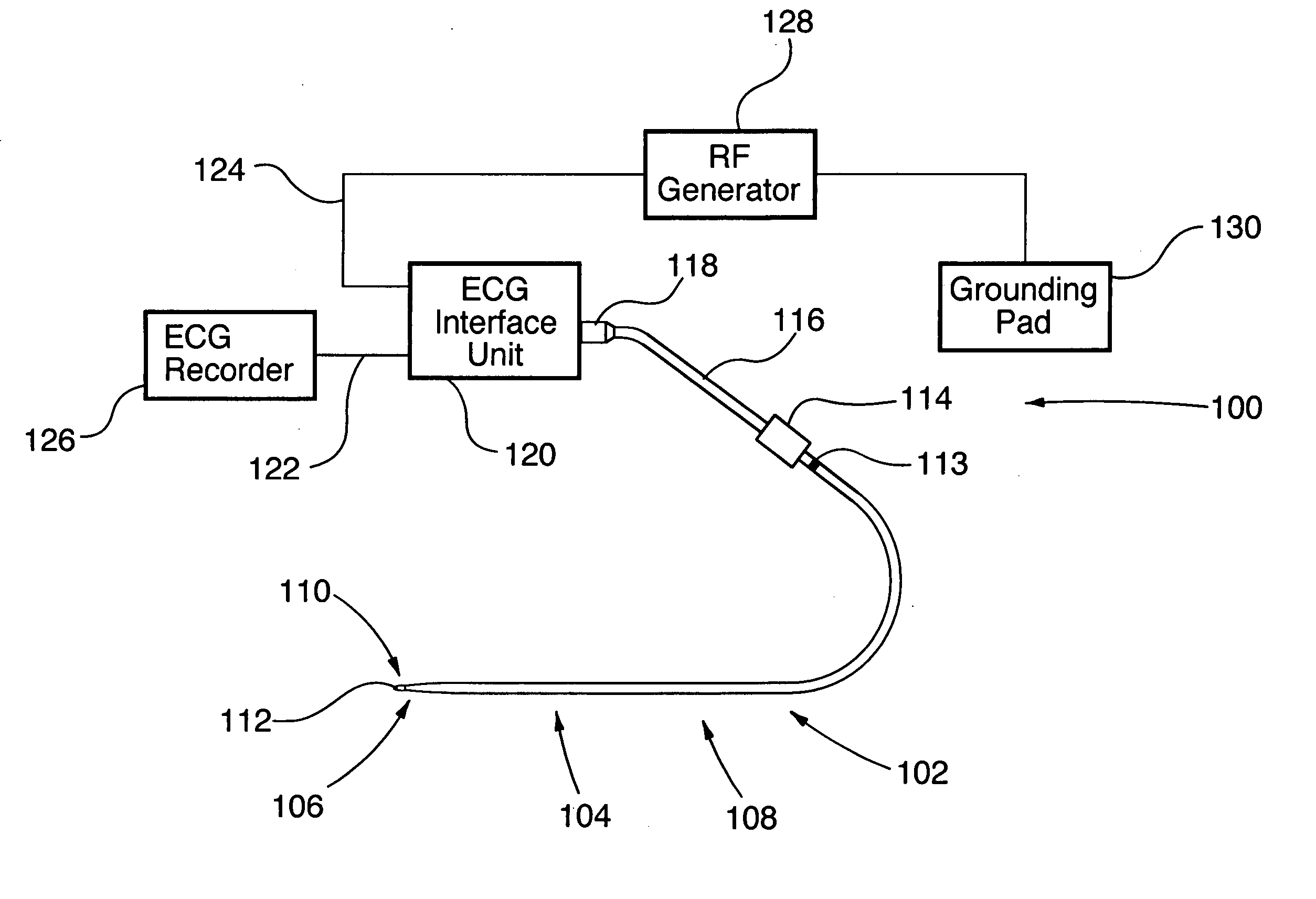
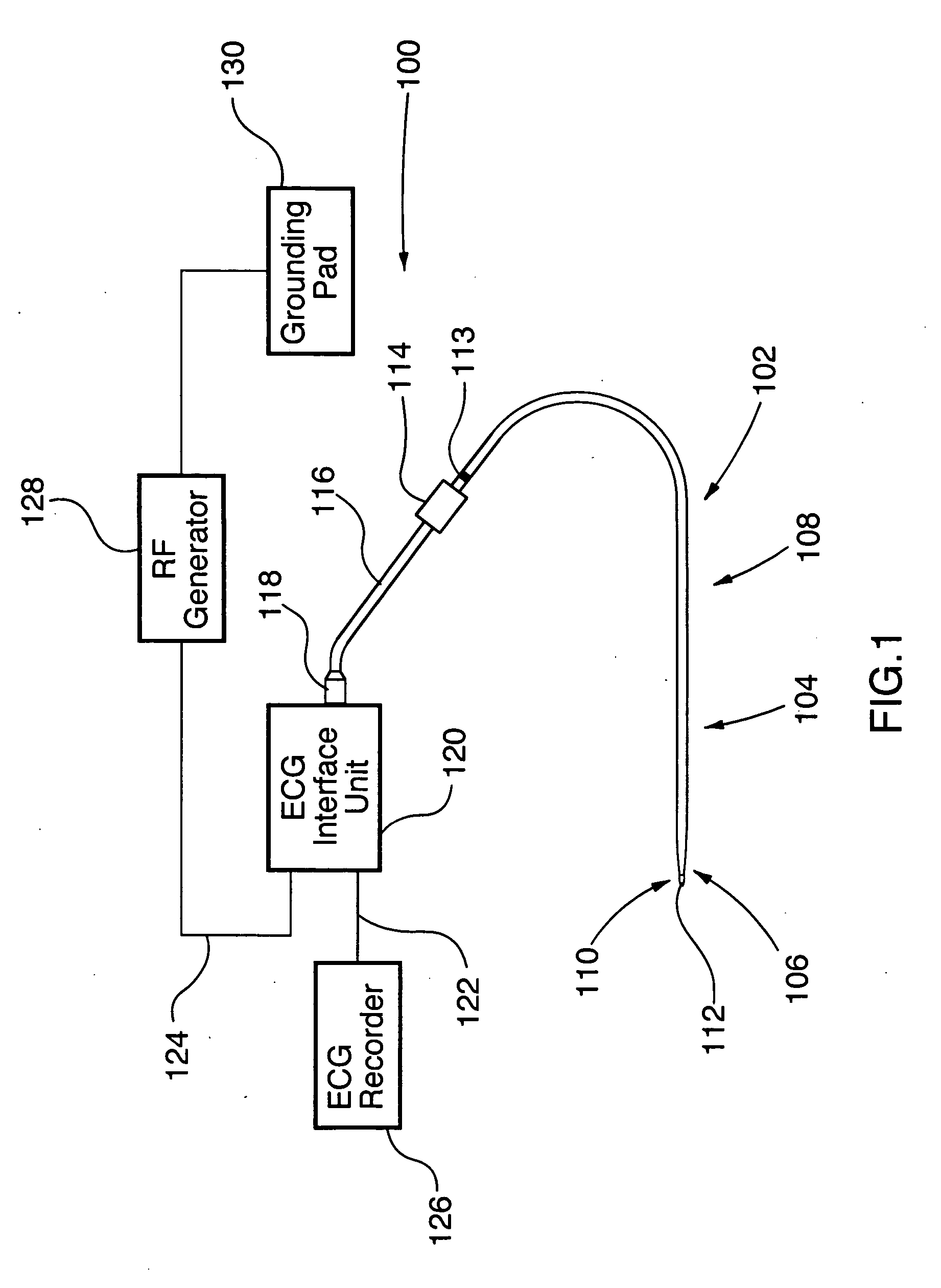
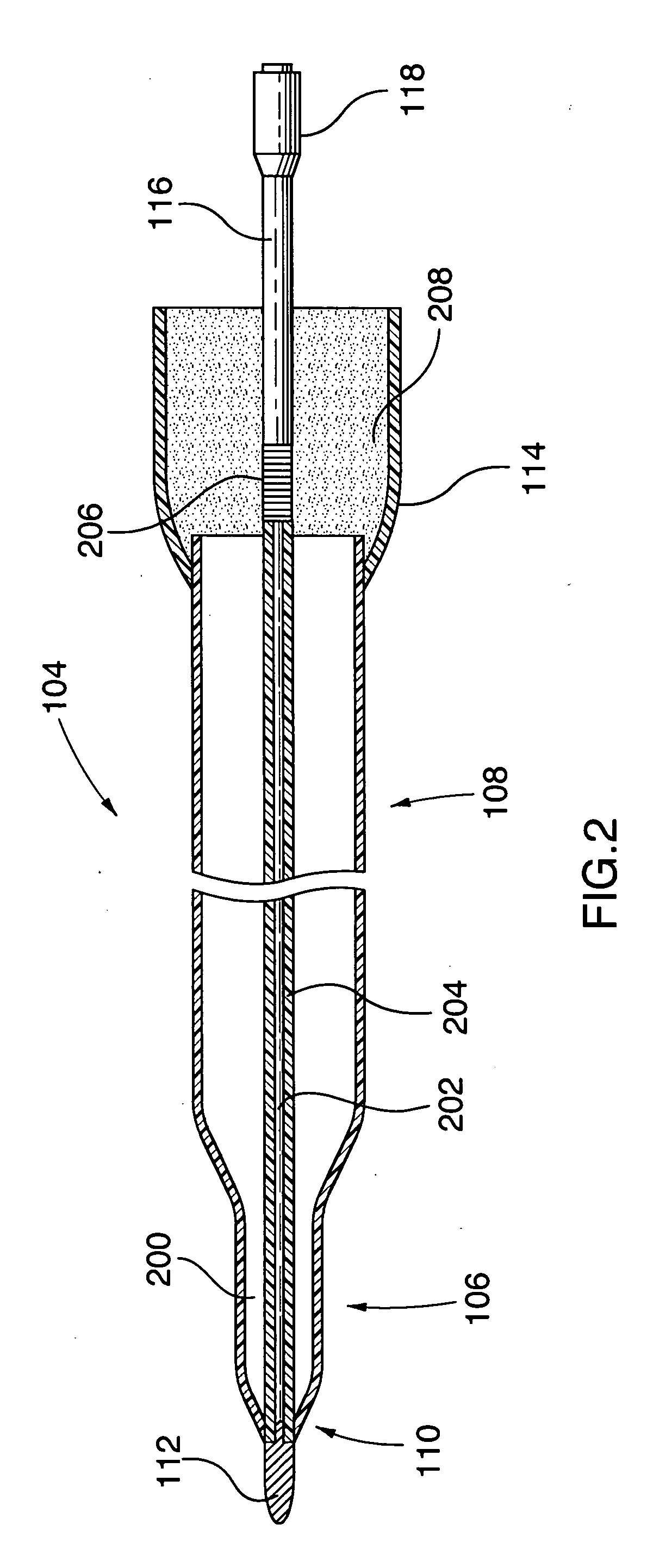
An ablation therapy system and systematic method is provided for treating continuous atrial fibrillation. The therapy system includes a Multi-Channel RF Ablation Generator, an ECG interface, an assembly of at least three ablation catheters, and an ECG interface operably coupling and interfacing the catheters to both an ECG unit and the RF Ablation Generator. The systematic method includes transseptally accessing the Left Atrium (LA) through the septum of the patient's heart, and performing an endocardial pulmonary vein ablation procedure on the pulmonary vein ostial tissue surrounding one or more pulmonary veins in a manner treating aberrant conductive pathways therethrough. After performing the pulmonary vein ablation, the method further includes performing an endocardial atrial septum ablation procedure on the septal tissue in a manner treating aberrant conductive pathways therethrough.
Owner:MEDTRONIC ABLATION FRONTIERS
Tubular patent foramen ovale (PFO) closure device with catch system
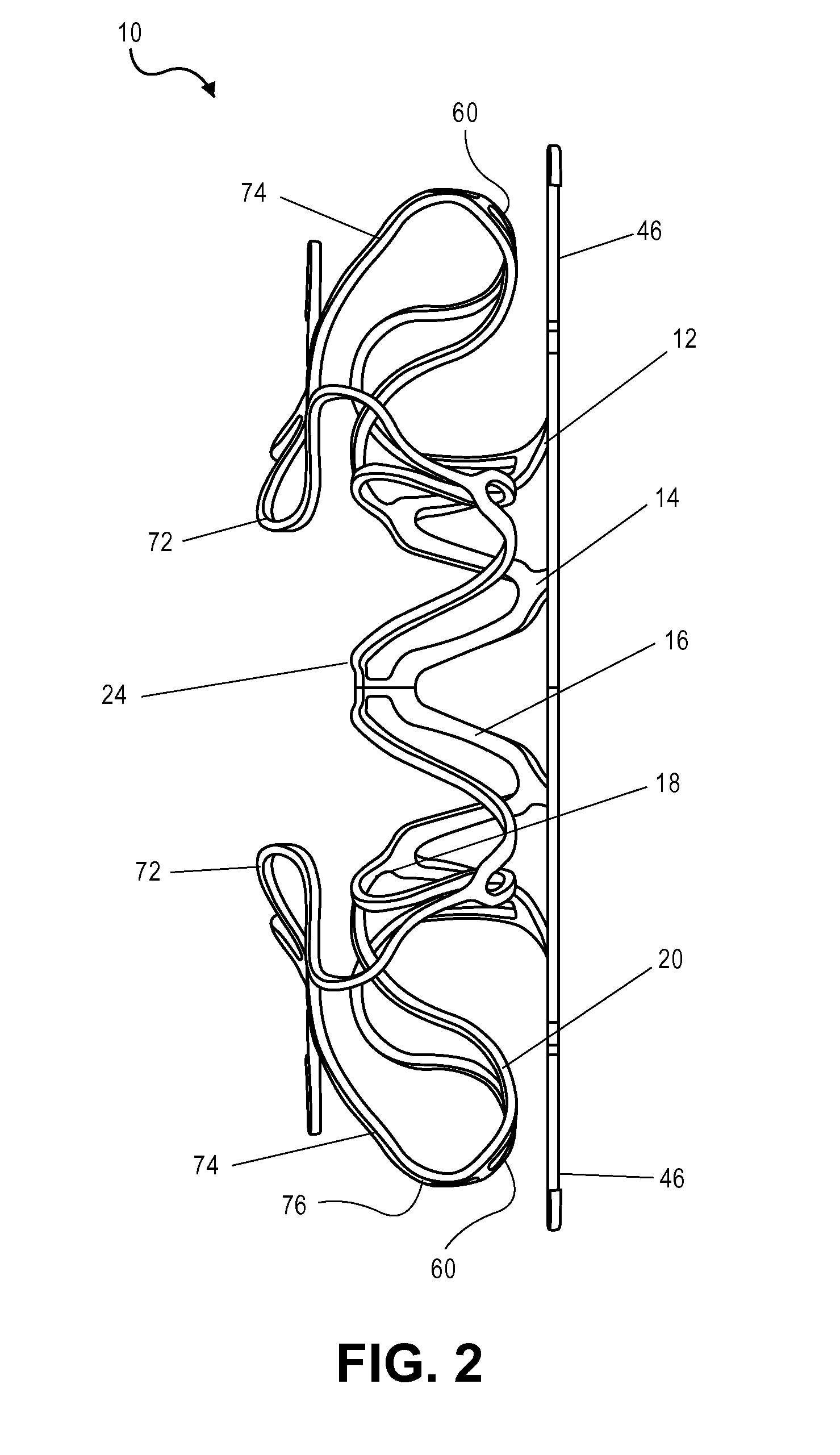
ActiveUS20070010851A1Minimize traumaMinimize distortion to the septal tissue surroundingSurgical veterinaryWound clampsAtrial septal defectsEngineering
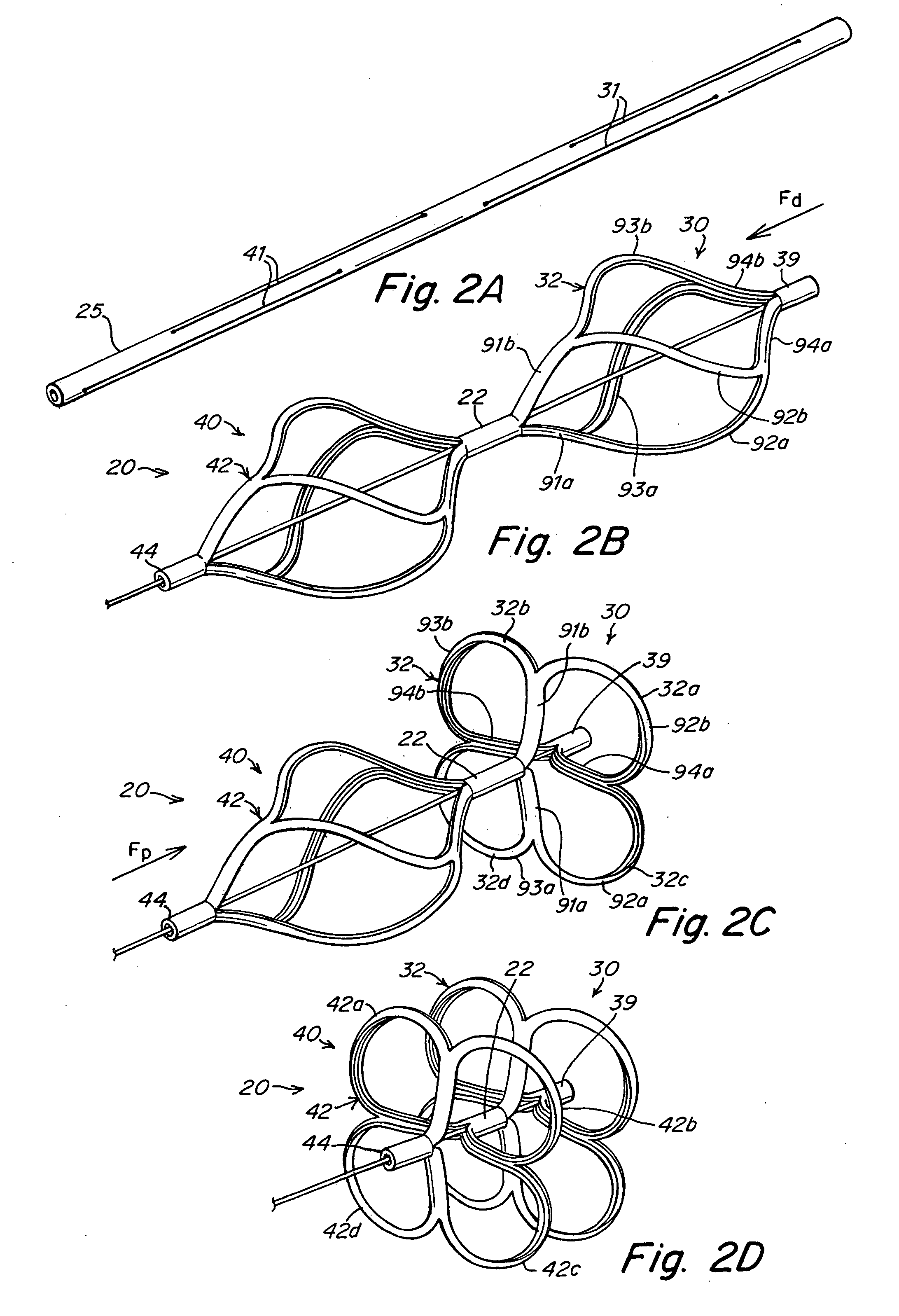
The present invention provides a device for occluding an anatomical aperture, such as an atrial septal defect (ASD) or a patent foramen ovale (PFO). The occluder includes two sides connected by a central tube. The occluder is formed from a tube, which is cut to produce struts in each side. Upon the application of force, the struts deform into loops. The loops may be of various shapes, sizes, and configurations, and, in at least some embodiments, the loops have rounded peripheries. In some embodiments, at least one of the sides includes a tissue scaffold. The occluder further includes a catch system that maintains its deployed state in vivo. When the occluder is deployed in vivo, the two sides are disposed on opposite sides of the septal tissue surrounding the aperture and the catch system is deployed so that the occluder exerts a compressive force on the septal tissue and closes the aperture.
Owner:WL GORE & ASSOC INC
Expandable trans-septal sheath
Owner:ONSET MEDICAL CORP
Method and apparatus for treating heart failure
InactiveUS20050165344A1Prevent cryptogenic strokeReduce stroke occurrenceHeart valvesWound drainsThrombusCatheter
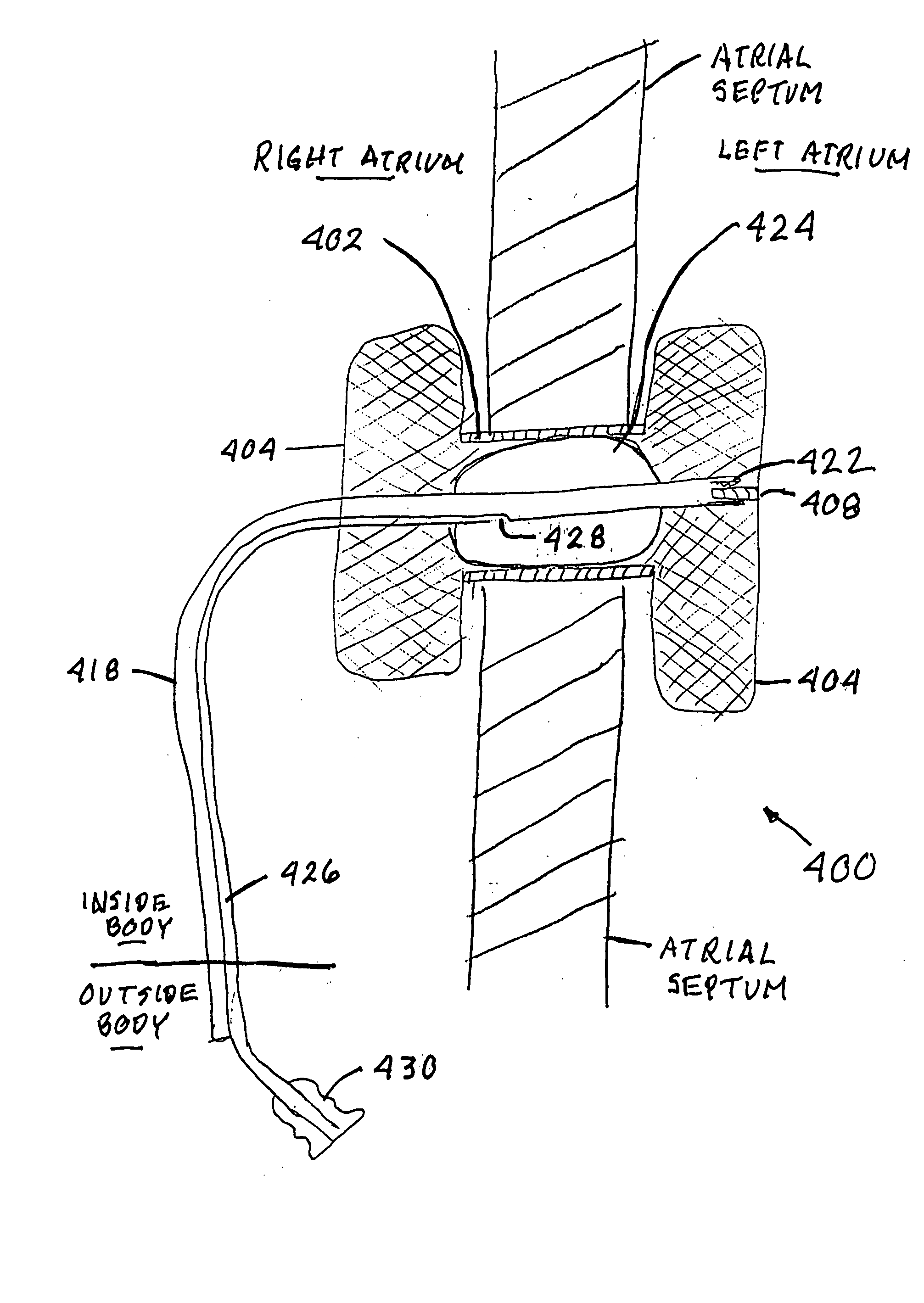
An apparatus for treating heart failure, including a conduit positioned in a hole in the atrial septum of the heart, to allow flow from the left atrium into the right atrium. The conduit is fitted with one or more emboli barriers or one-way valve members, to prevent thrombi or emboli from crossing into the left side circulation.
Owner:BUILDING ADDRESS
Left and right side heart support
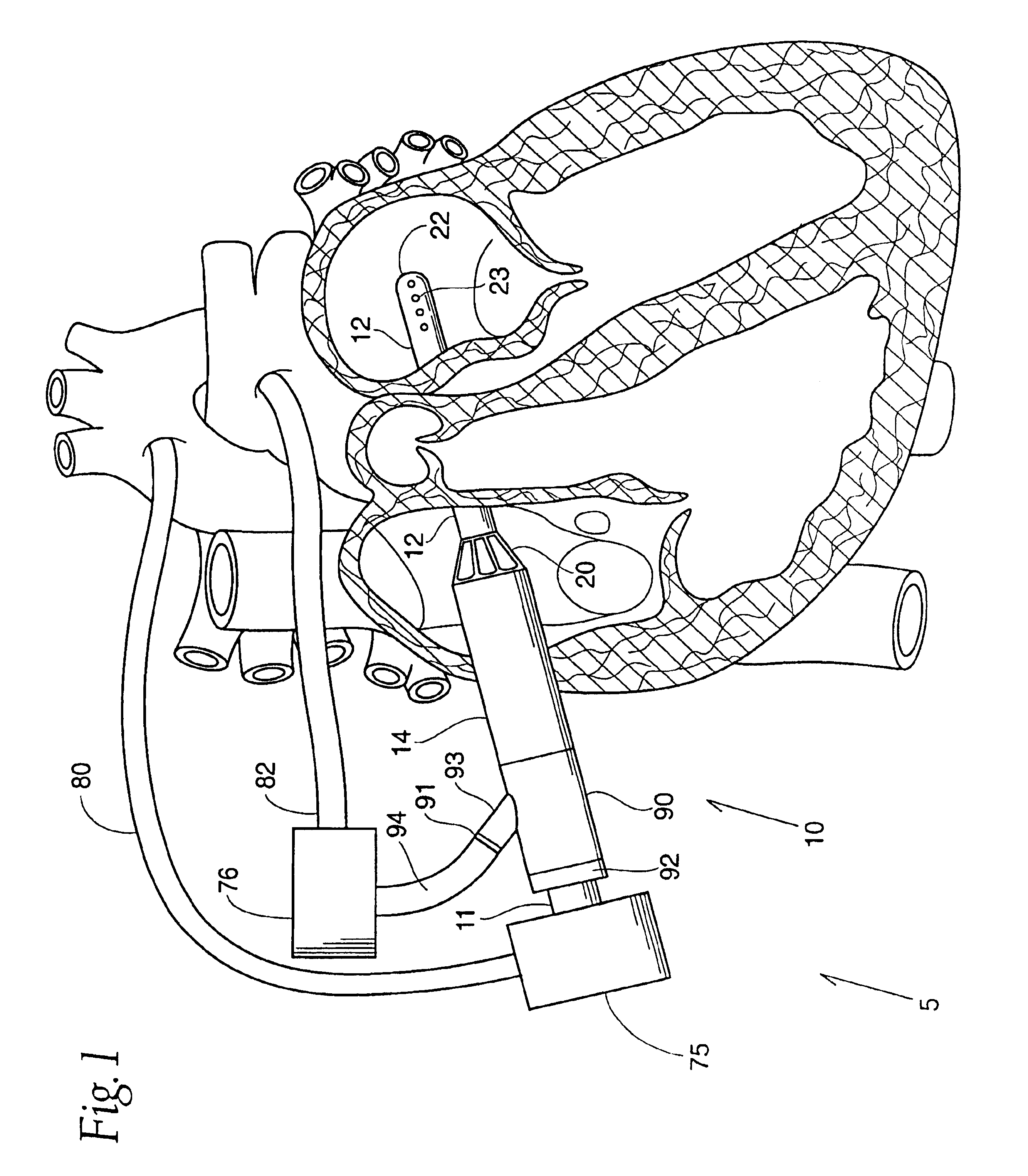
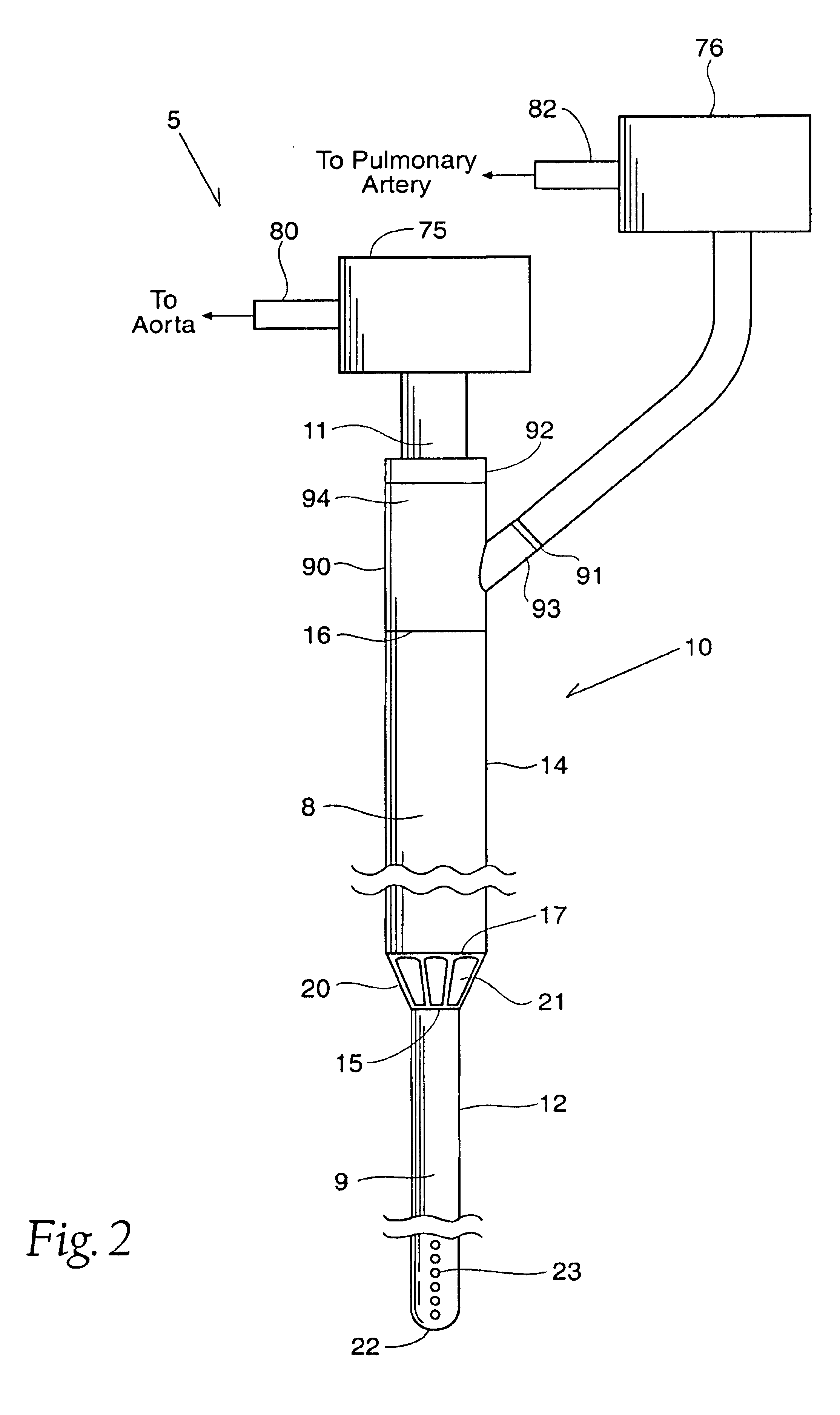
InactiveUS6926662B1Lower the volumeReduce the amount requiredOther blood circulation devicesBlood pumpsOuter CannulaRight atrium
A cannulation system for cardiac support uses an inner cannula disposed within an outer cannula. The outer cannula includes a fluid inlet for placement within the right atrium of a heart. The inner cannula includes a fluid inlet extending through the fluid inlet of the outer cannula and the atrial septum for placement within at least one of the left atrium and left ventricle of the heart. The cannulation system also employs a pumping assembly coupled to the inner and outer cannulas to withdraw blood from the right atrium for delivery to the pulmonary artery to provide right heart support, or to withdraw blood from at least one of the left atrium and left ventricle for delivery into the aorta to provide left heart support, or both.
Owner:MAQUET CARDIOVASCULAR LLC
Expandable trans-septal sheath
ActiveUS20080215008A1Lower potentialLess time-consumingCannulasInfusion syringesAccess routeRight atrium
Disclosed is an expandable transluminal sheath, for introduction into the body while in a first, low cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the vascular system and has utility in the performance of procedures in the left atrium. The access route is through the inferior vena cava to the right atrium, where a trans-septal puncture, followed by advancement of the catheter is completed. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement to the right atrium and through the atrial septum into the left atrium. The distal end of the sheath is subsequently expanded using a radial dilatation device. In an exemplary application, the sheath is utilized to provide access for a diagnostic or therapeutic procedure such as electrophysiological mapping of the heart, radio-frequency ablation of left atrial tissue, placement of left atrial implants, mitral valve repair, or the like.
Owner:ONSET MEDICAL CORP
Surgical perforation device with curve
Owner:BOSTON SCI MEDICAL DEVICE LTD
Devices and methods for the treatment of heart failure
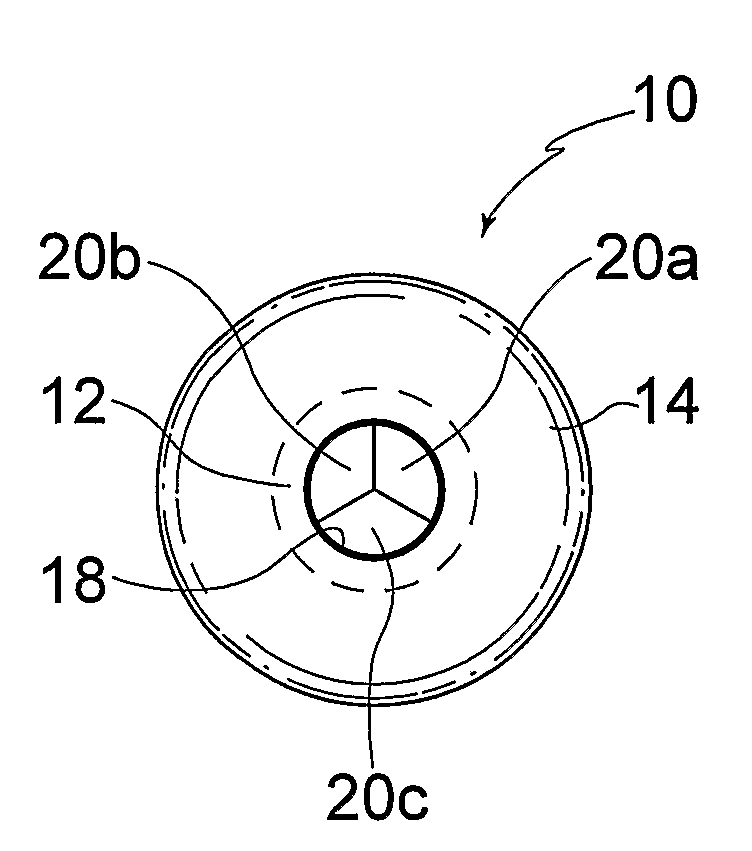
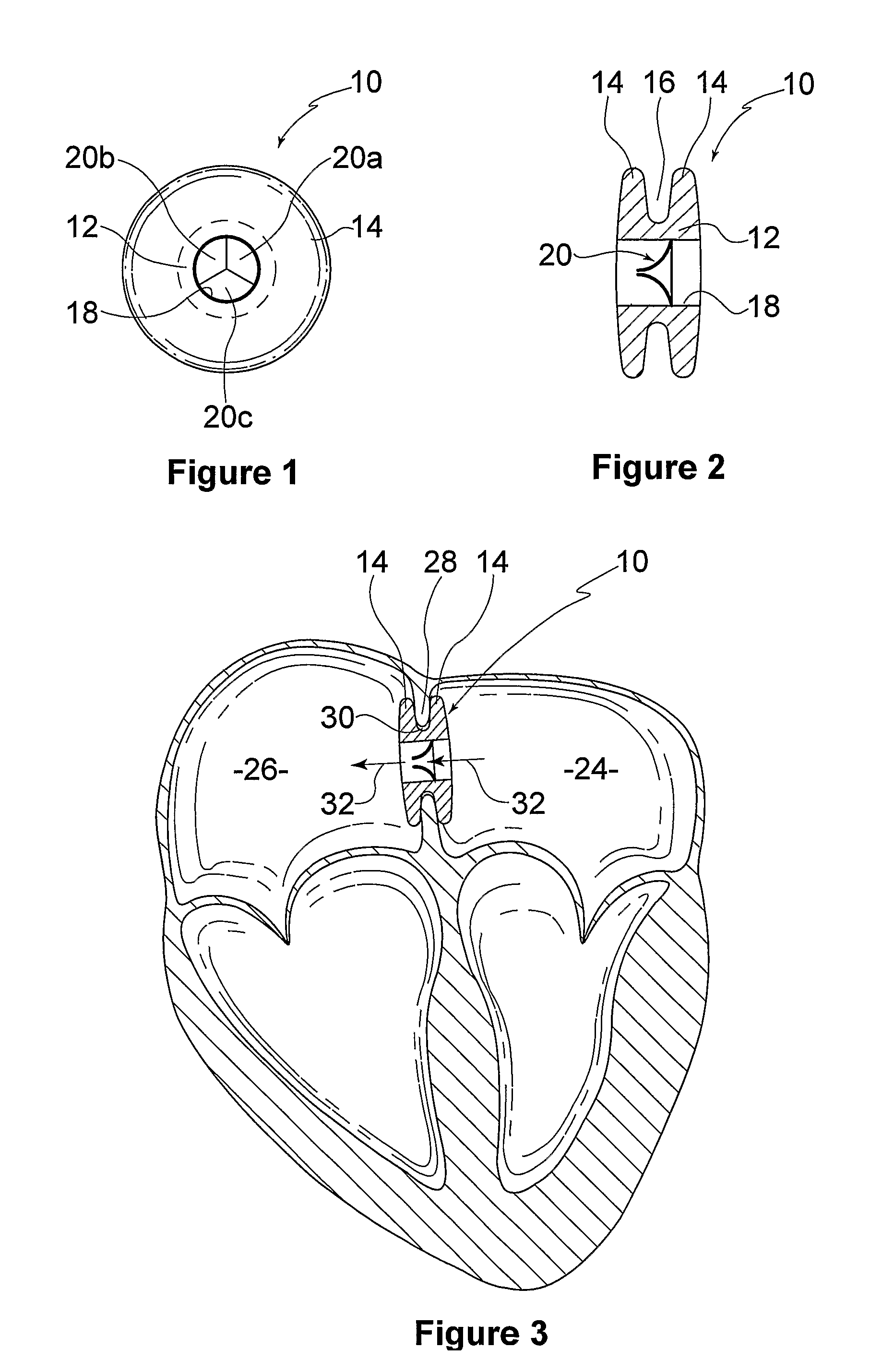
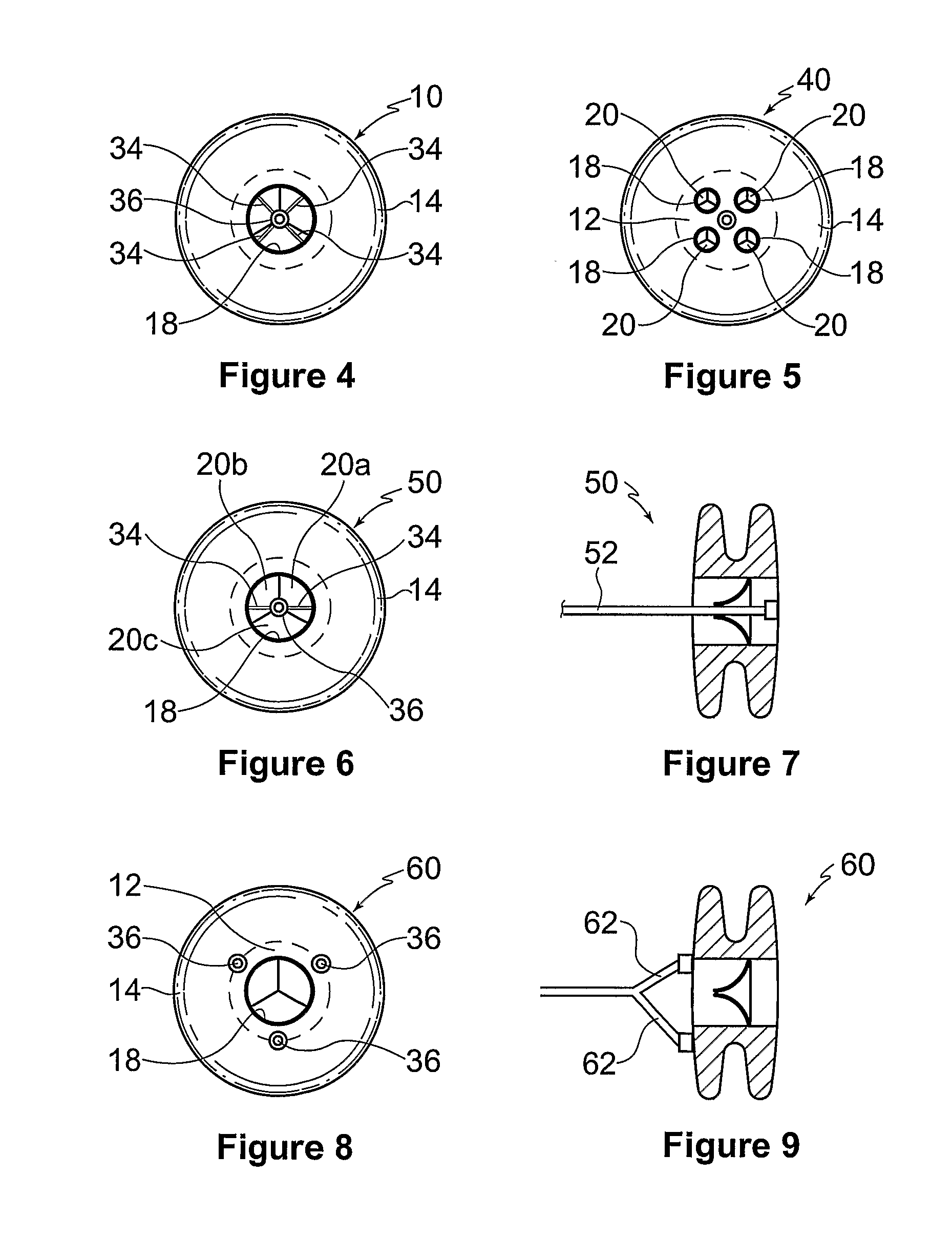
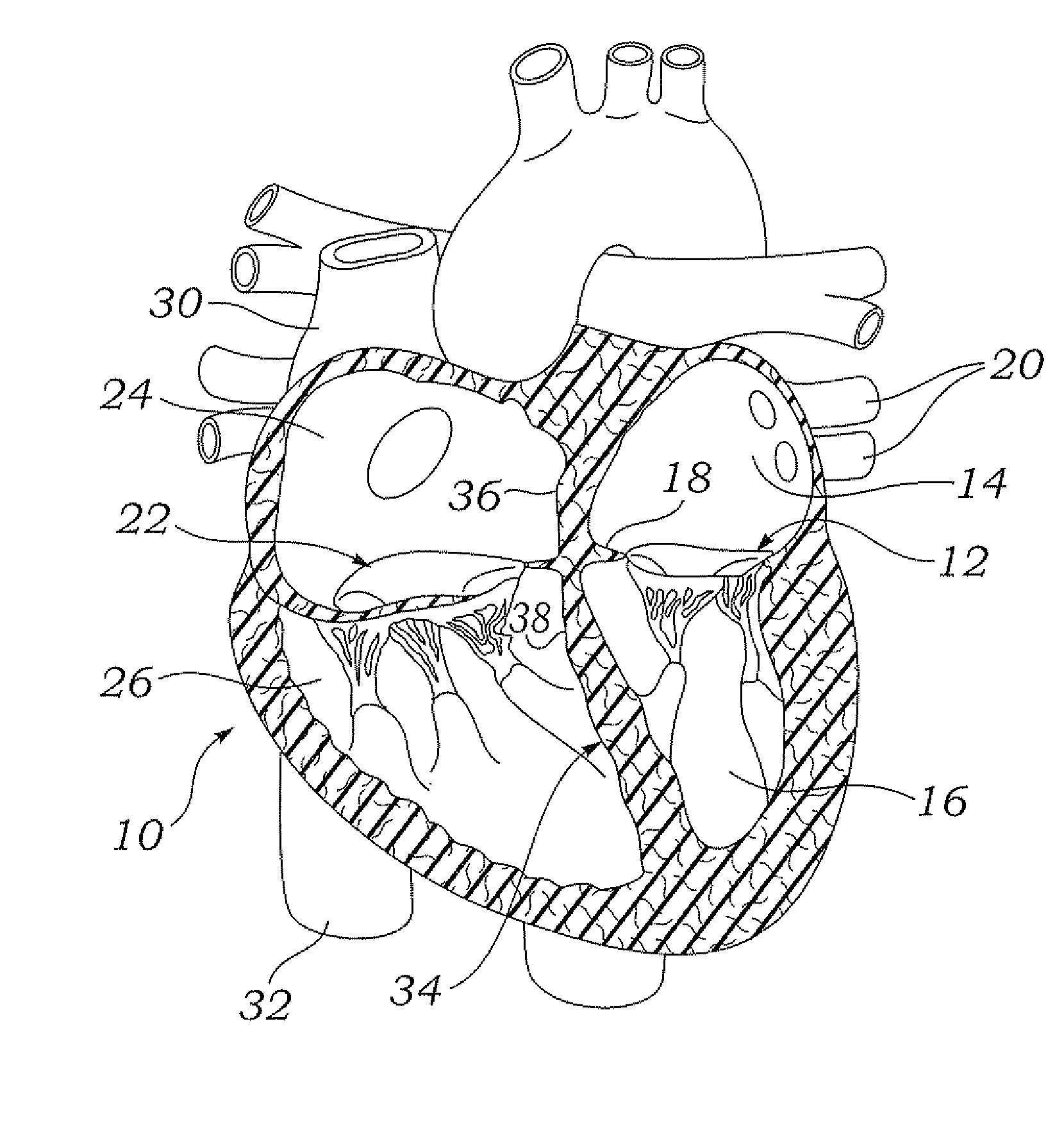
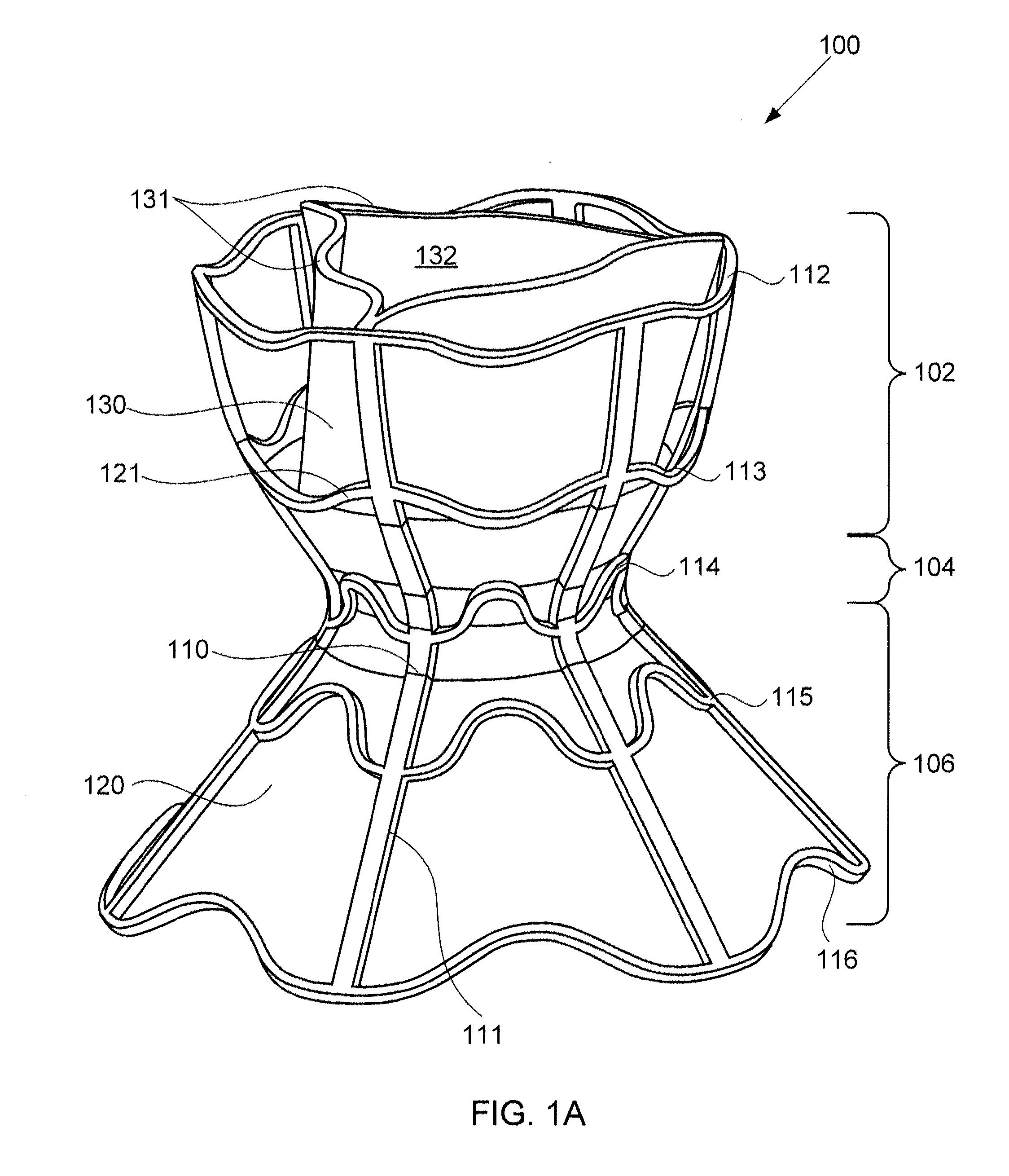
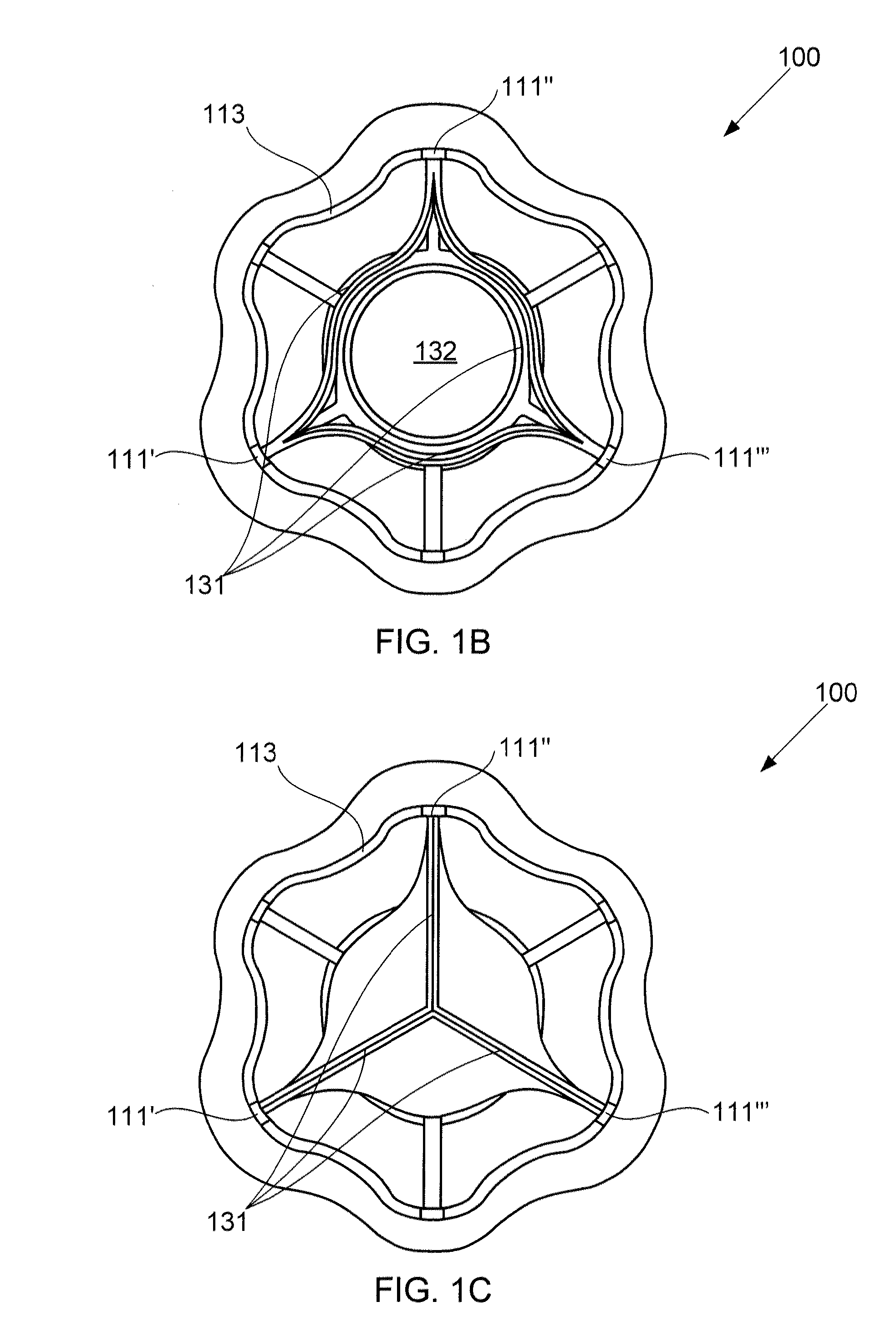
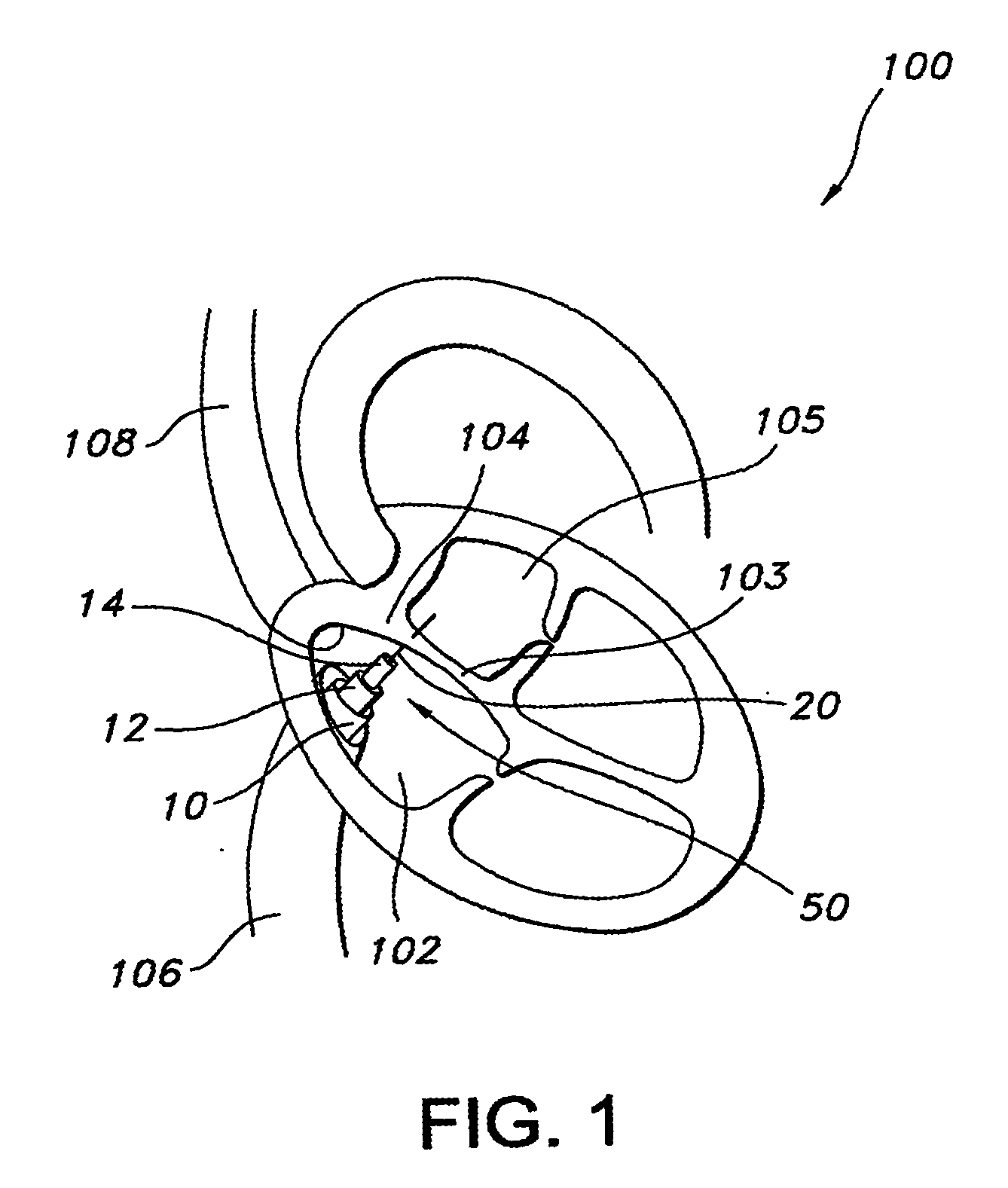
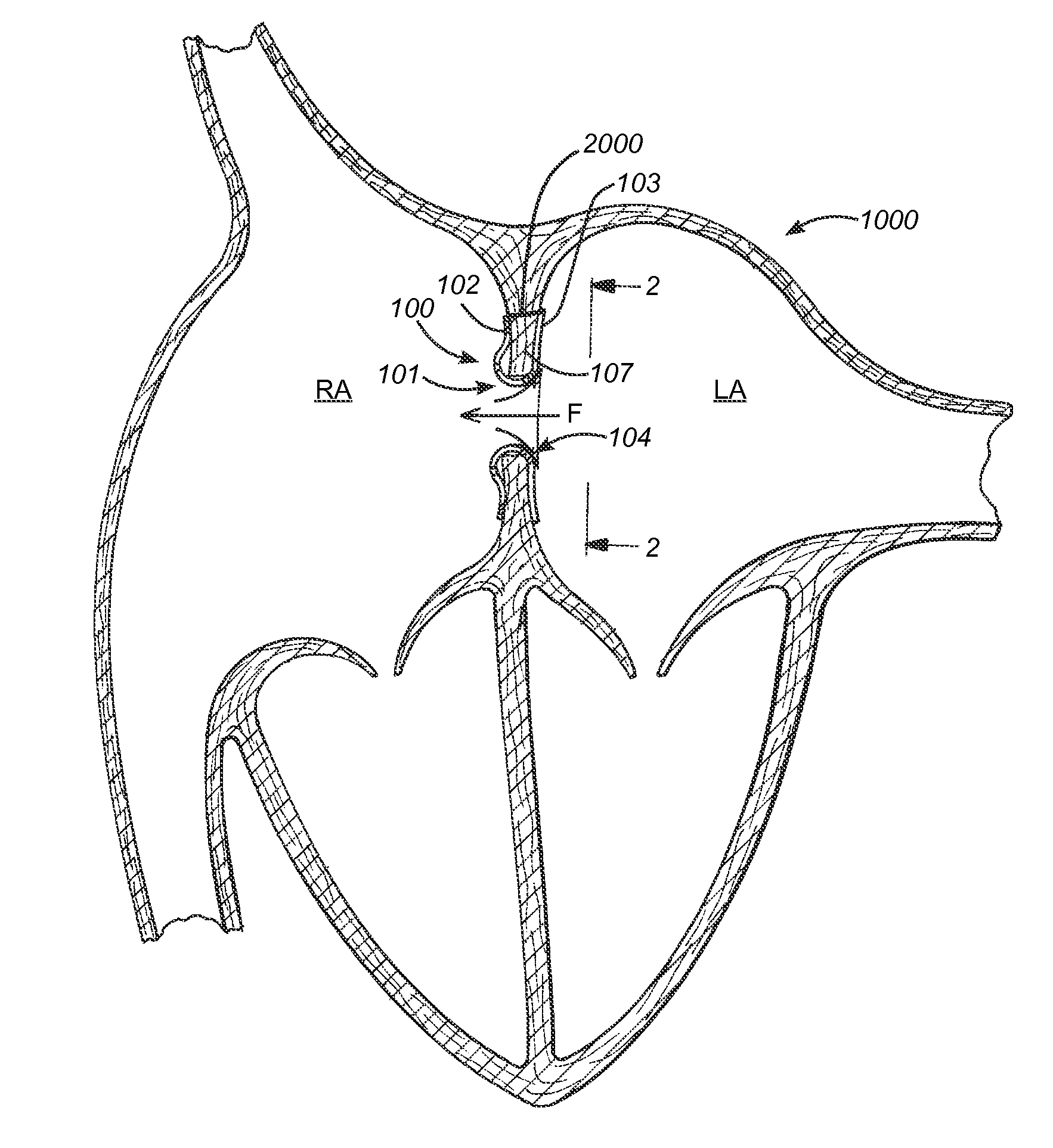
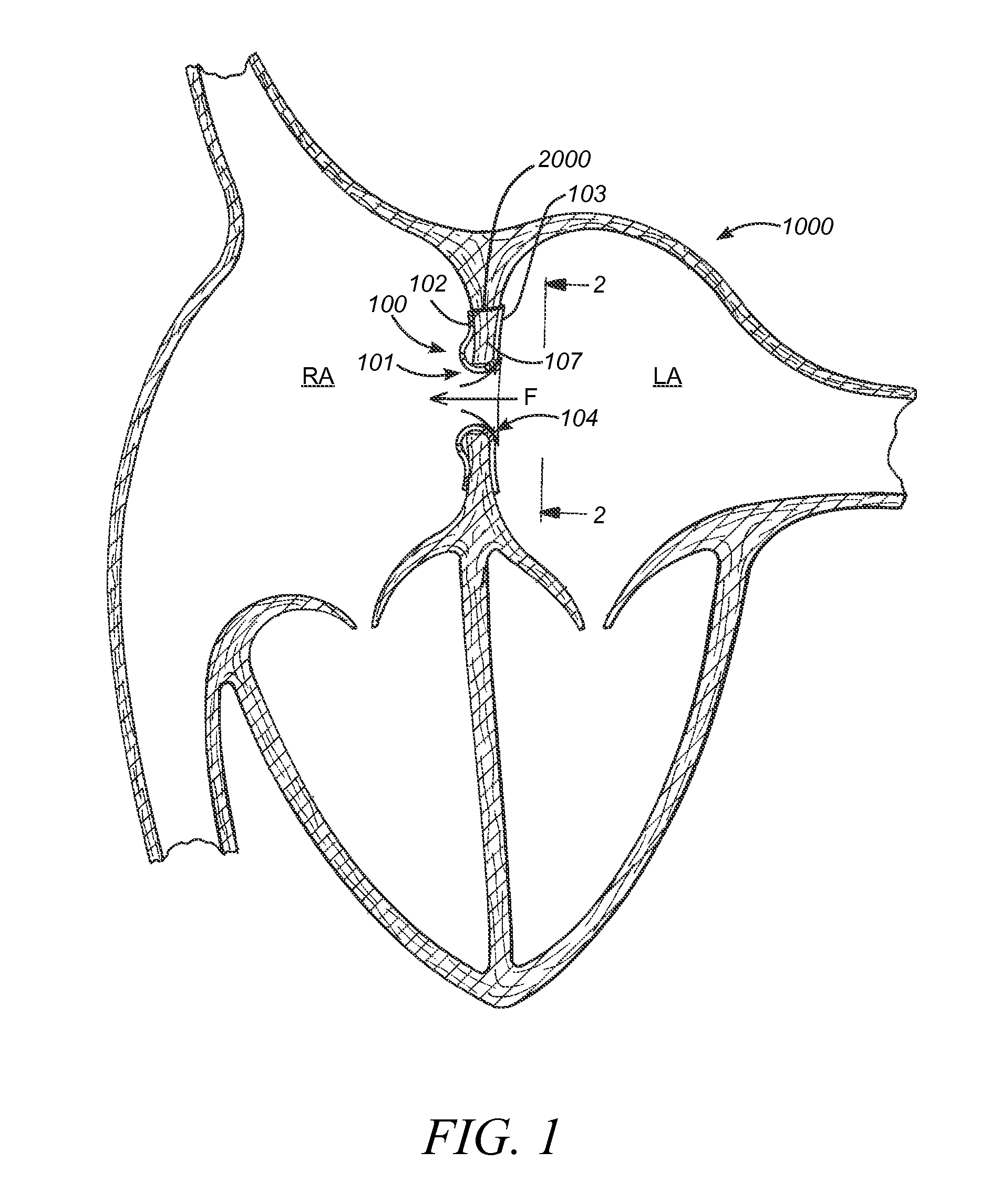
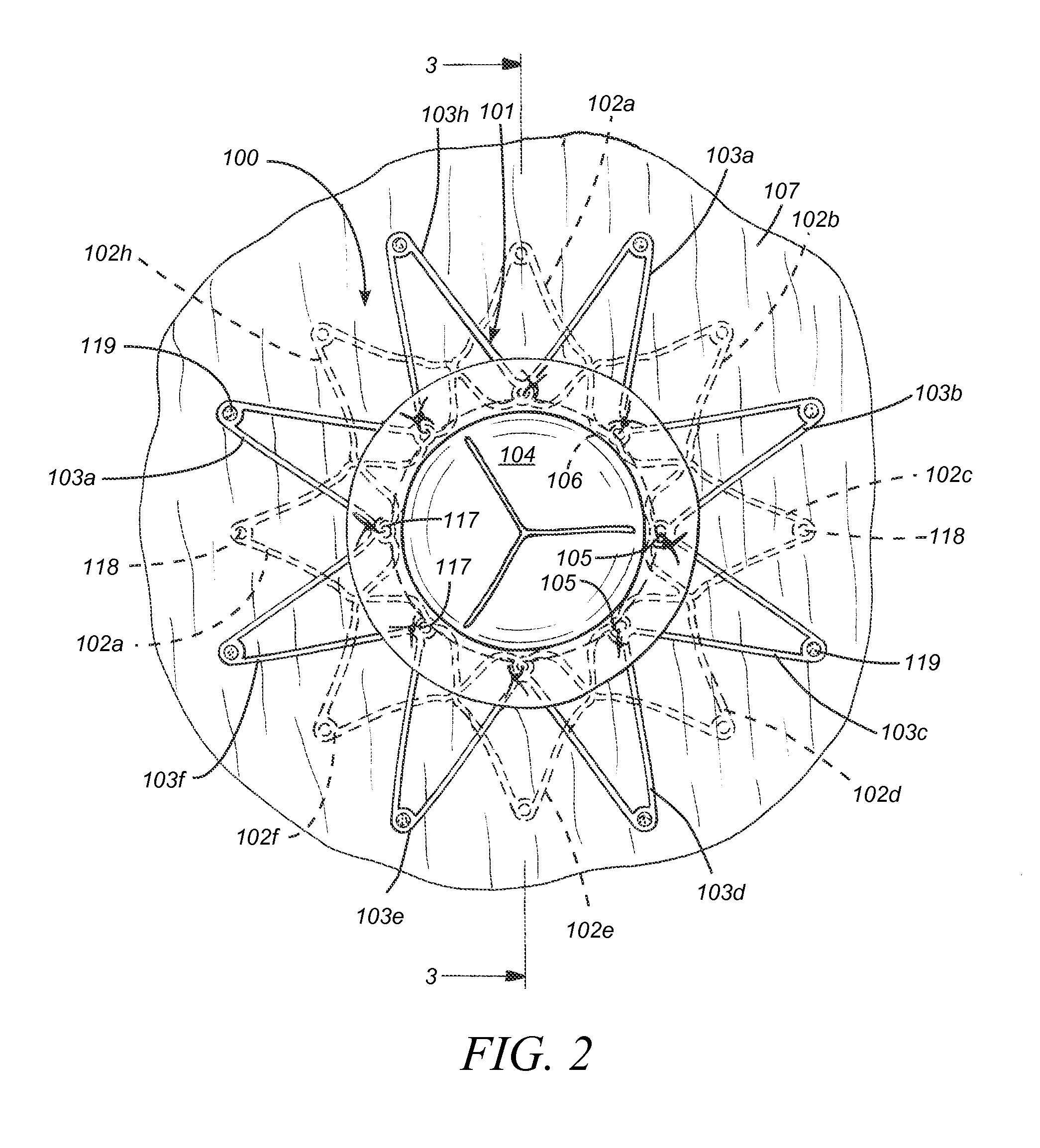
ActiveUS20100057192A1Reduce atrial pressureReduce pressureStentsAnnuloplasty ringsAtrial septumHeart failure
A device (10) for treating heart failure in a patient. The device (10) comprising a body (12), at least one passage (18) through the body (12), at least one one way valve (20) in the passage (18) and a mounting means (14) adapted for mounting the body (12) in an opening provided in the patient's atrial septum. In use, the device (10) is oriented such that, when the patient's left atrial pressure exceeds the patient's right atrial pressure by a predetermined amount, the one way valve(s) (20) opens to allow blood flow through the passage(s) from the left atrium to the right atrium to thereby reduce the left atrial pressure.
Owner:CORVIA MEDICAL
Device and Method for ReShaping Mitral Valve Annulus
ActiveUS20090076586A1Reduce regurgitationImprove bindingStentsDiagnosticsPosterior leafletLeft ventricular size
Devices and methods for reshaping a mitral valve annulus are provided. One preferred device is configured for deployment in the right atrium and is shaped to apply a force along the atrial septum. The device causes the atrial septum to deform and push the anterior leaflet of the mitral valve in a posterior direction for reducing mitral valve regurgitation. Another preferred device is deployed in the left ventricular outflow tract at a location adjacent the aortic valve. The device is expandable for urging the anterior leaflet toward the posterior leaflet. Another preferred device comprises a tether configured to be attached to opposing regions of the mitral valve annulus.
Owner:EDWARDS LIFESCIENCES CORP
Atrial pressure regulation with control, sensing, monitoring and therapy delivery
ActiveUS20120265296A1Minimize damageImprove ventilationStentsHeart valvesInsertion stentCoronary sinus
The present disclosure relates to improved capabilities for stabilizing and regulating atrial pressure with a shunt in the atrial septum or a stent in the coronary sinus. The disclosure also includes sensing, monitoring, drug therapy and control capabilities to provide improved treatment of patients with heart disease and other cardiac related conditions.
Owner:CORVIA MEDICAL
Expandable trans-septal sheath
Disclosed is an expandable transluminal sheath, for introduction into the body while in a first, low cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the vascular system and has utility in the performance of procedures in the left atrium. The access route is through the inferior vena cava to the right atrium, where a trans-septal puncture, followed by advancement of the catheter is completed. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement to the right atrium and through the atrial septum into the left atrium. The distal end of the sheath is subsequently expanded using a radial dilatation device. In an exemplary application, the sheath is utilized to provide access for a diagnostic or therapeutic procedure such as electrophysiological mapping of the heart, radio-frequency ablation of left atrial tissue, placement of left atrial implants, mitral valve repair, or the like.
Owner:ONSET MEDICAL CORP
Surgical perforation device with electrocardiogram (ECG) monitoring ability and method of using ECG to position a surgical perforation device
Owner:BOSTON SCI MEDICAL DEVICE LTD
Devices for reducing left atrial pressure, and methods of making and using same
ActiveUS20120165928A1Reducing left atrial pressureIncrease cardiac outputHeart valvesSurgeryLeft ventricular sizeLeft atrial pressure
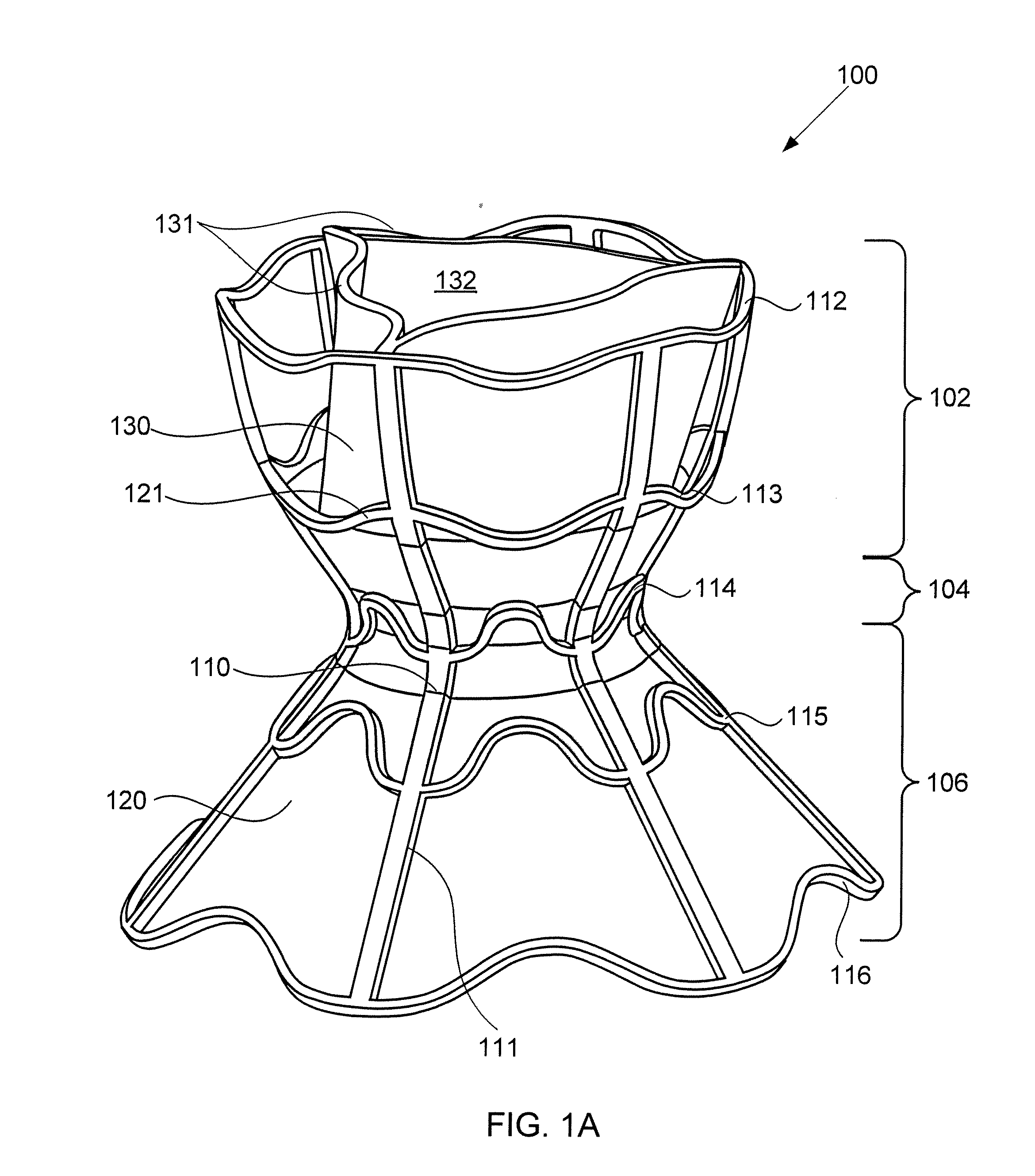
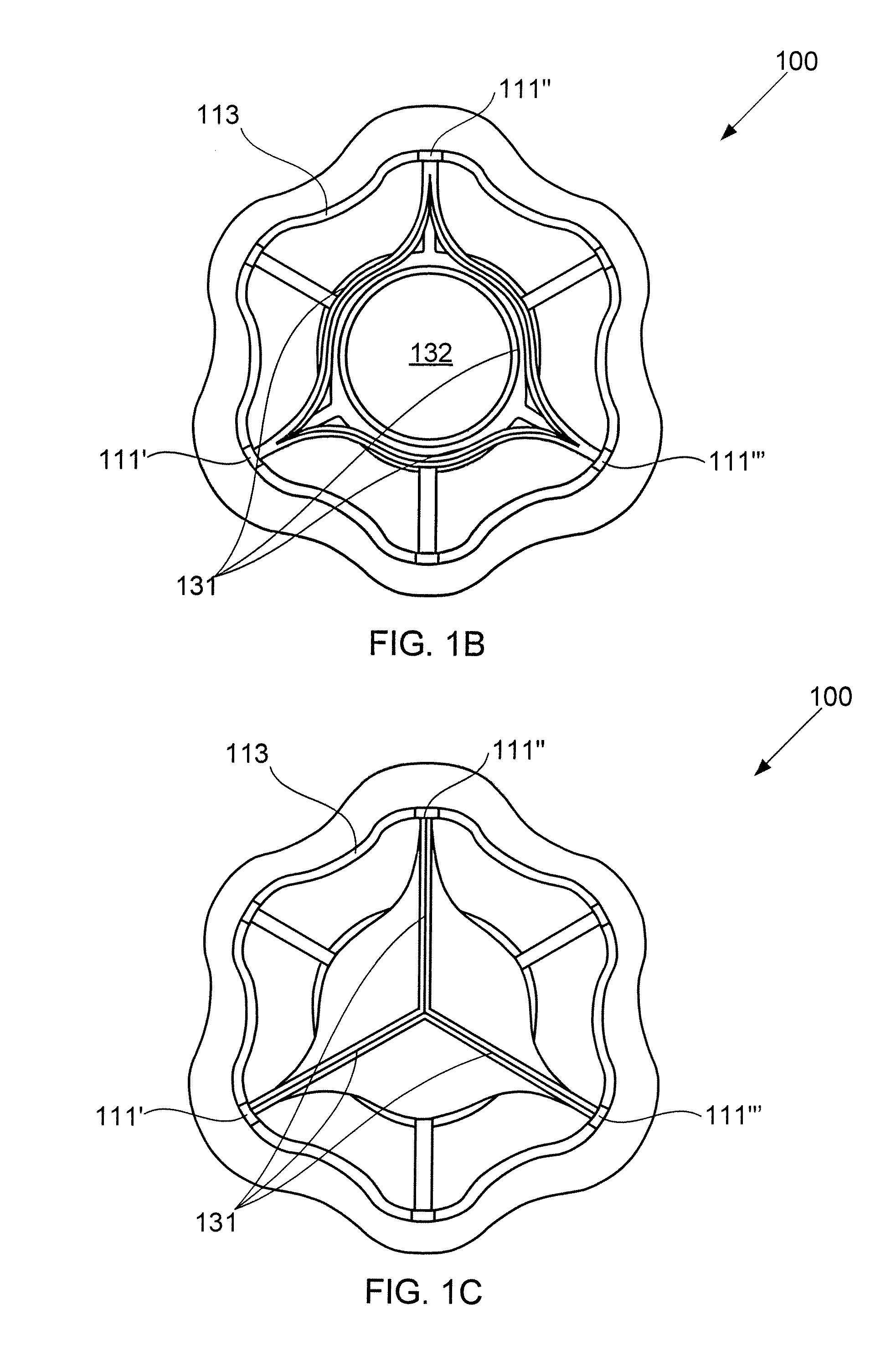
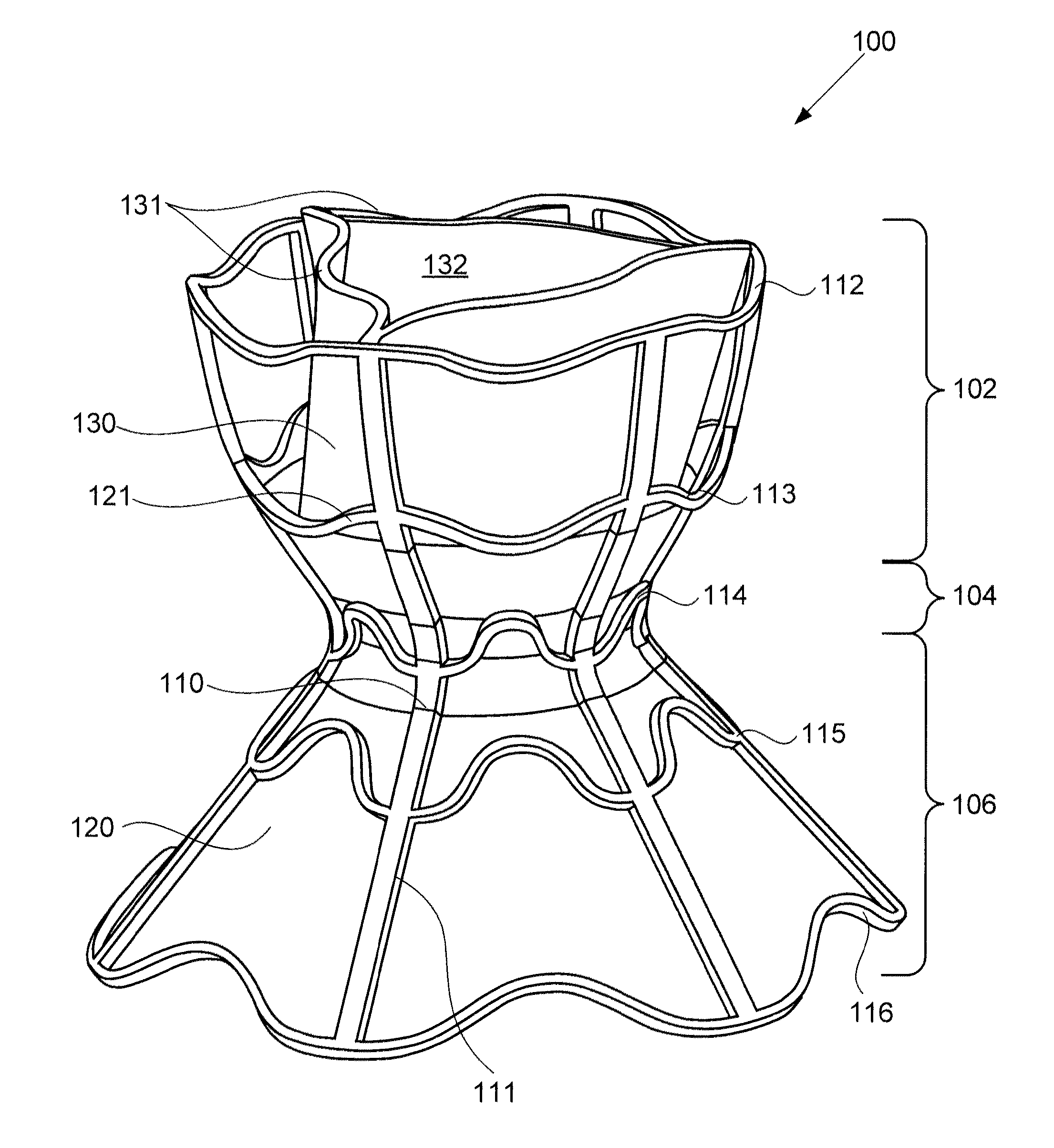
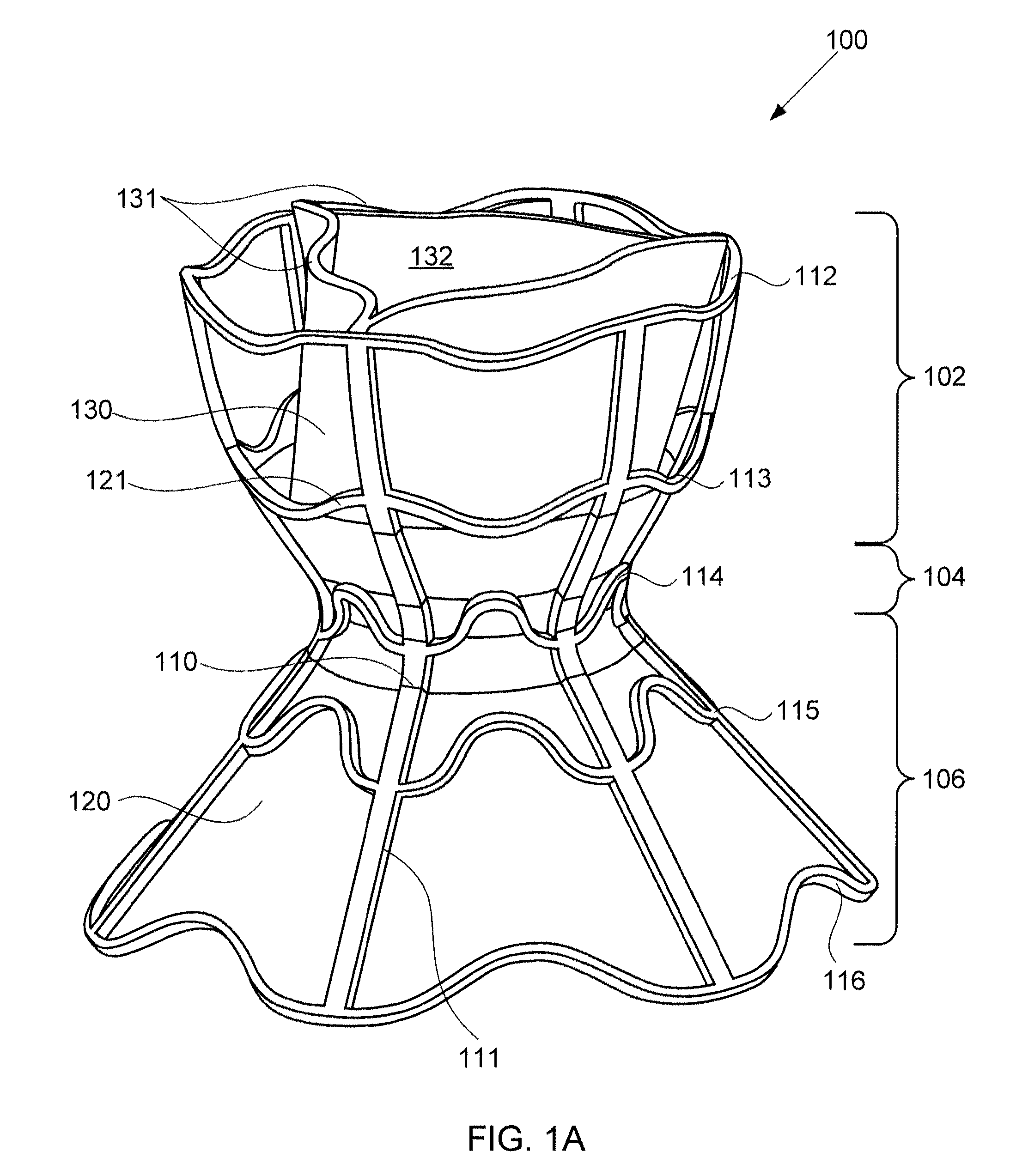
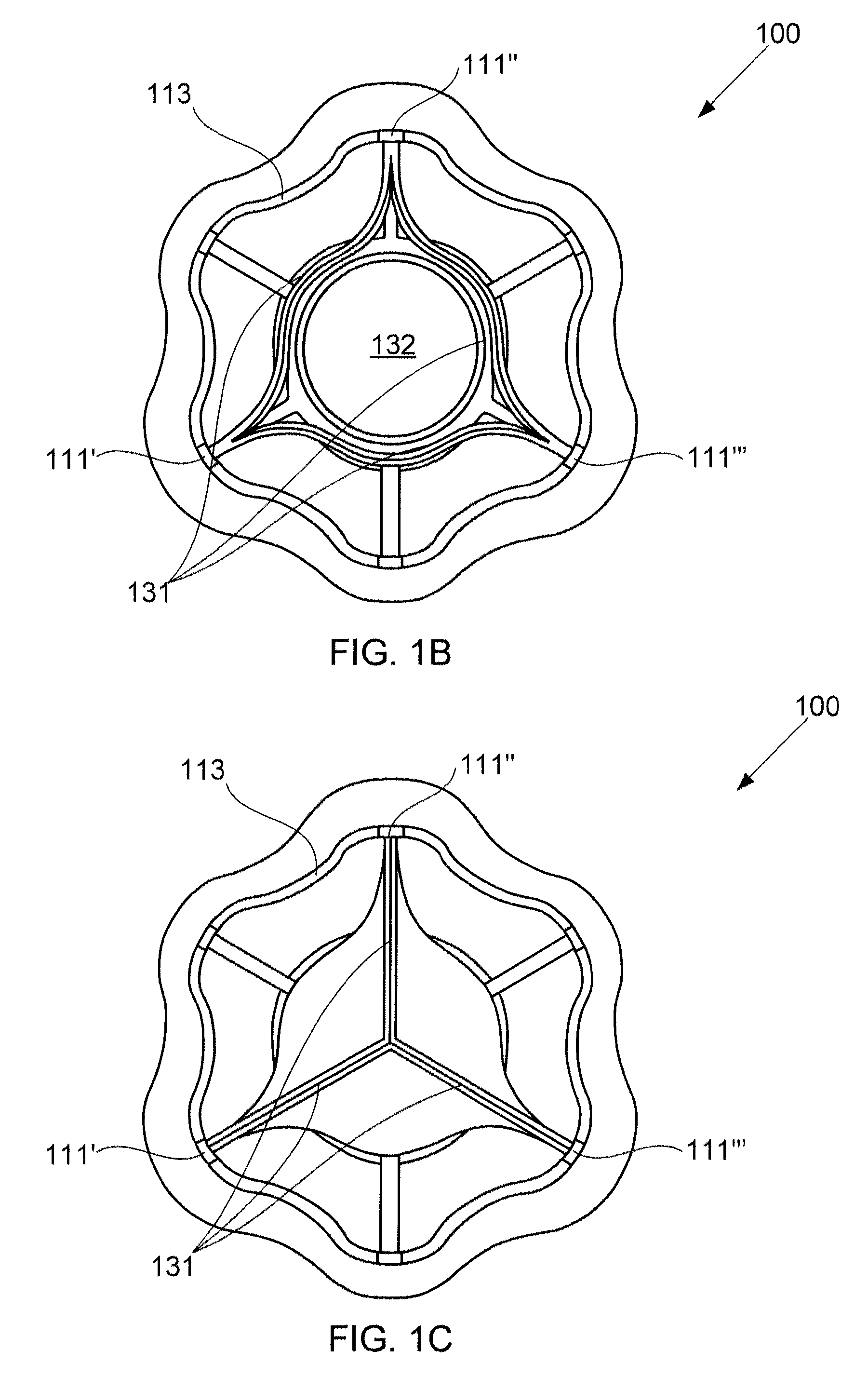
A device for regulating blood pressure between a patient's left atrium and right atrium comprises an hourglass-shaped stent comprising a neck region and first and second flared end regions, the neck region disposed between the first and second end regions and configured to engage the fossa ovalis of the patient's atrial septum; and a one-way tissue valve coupled to the first flared end region and configured to shunt blood from the left atrium to the right atrium when blood pressure in the left atrium exceeds blood pressure in the right atrium. The inventive devices may reduce left atrial pressure and left ventricular end diastolic pressure, and may increase cardiac output, increase ejection fraction, relieve pulmonary congestion, and lower pulmonary artery pressure, among other benefits. The inventive devices may be used, for example, to treat subjects having heart failure, pulmonary congestion, or myocardial infarction, among other pathologies.
Owner:WAVE LTD V
Devices and methods for treating heart failure
A device for implanting into an atrial septum of a patient. In some embodiments, the device has a core region to be disposed in an opening in the atrial septum; a distal retention region adapted to engage tissue on a left atrial side of the septal wall; a proximal retention region adapted to engage tissue on a right atrial side of the septal wall; and a retrieval region comprising a plurality of retrieval members, each retrieval member comprising a connector at a proximal end, the connector being adapted to connect to a delivery system. The device has a delivery configuration and a deployed configuration, the core region, distal retention region and proximal retention region each having a smaller diameter in the delivery configuration than in the deployed configuration, the retrieval member connectors being disposed proximal to and radially outward from the opening in the deployed configuration.
Owner:CORVIA MEDICAL
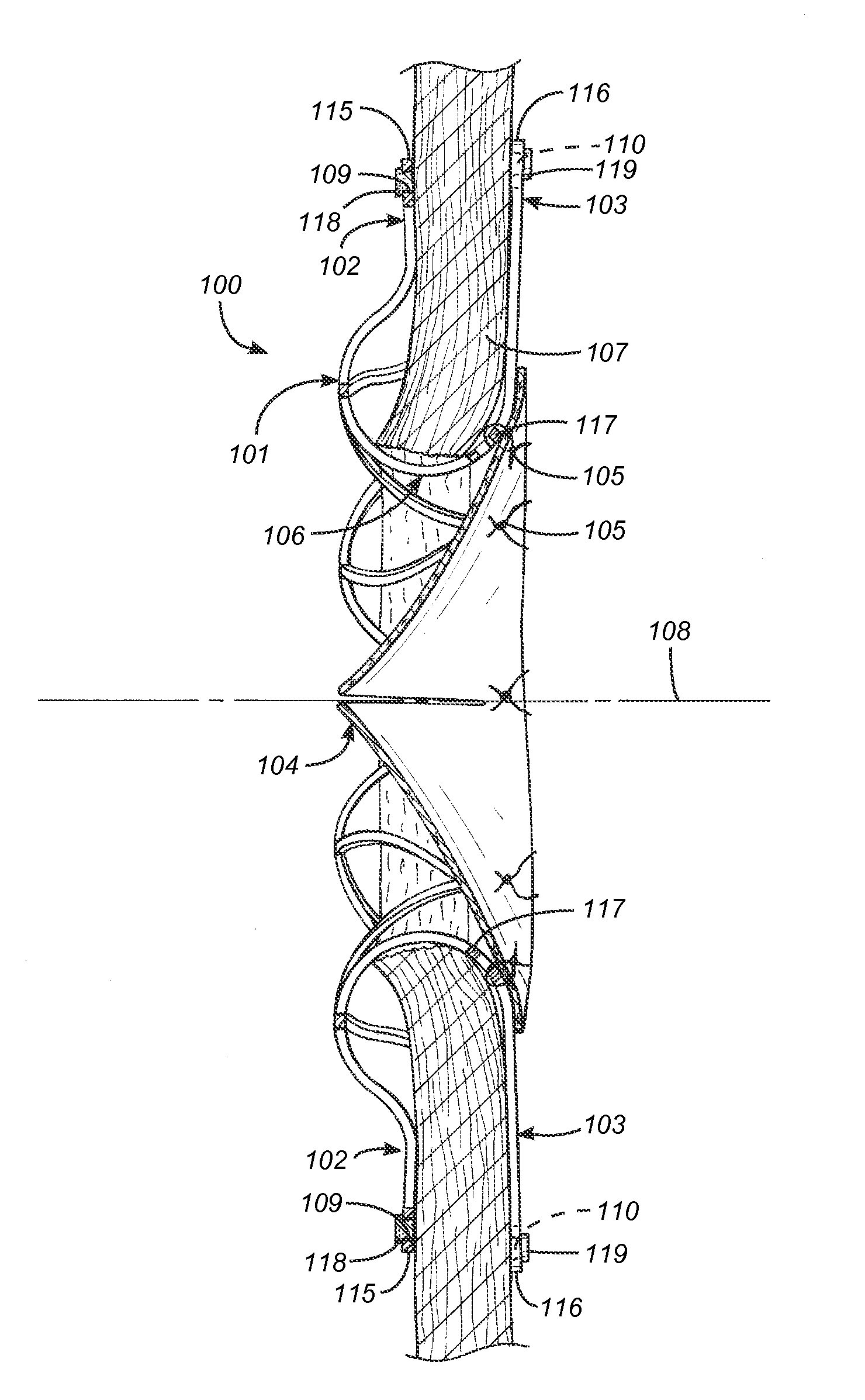
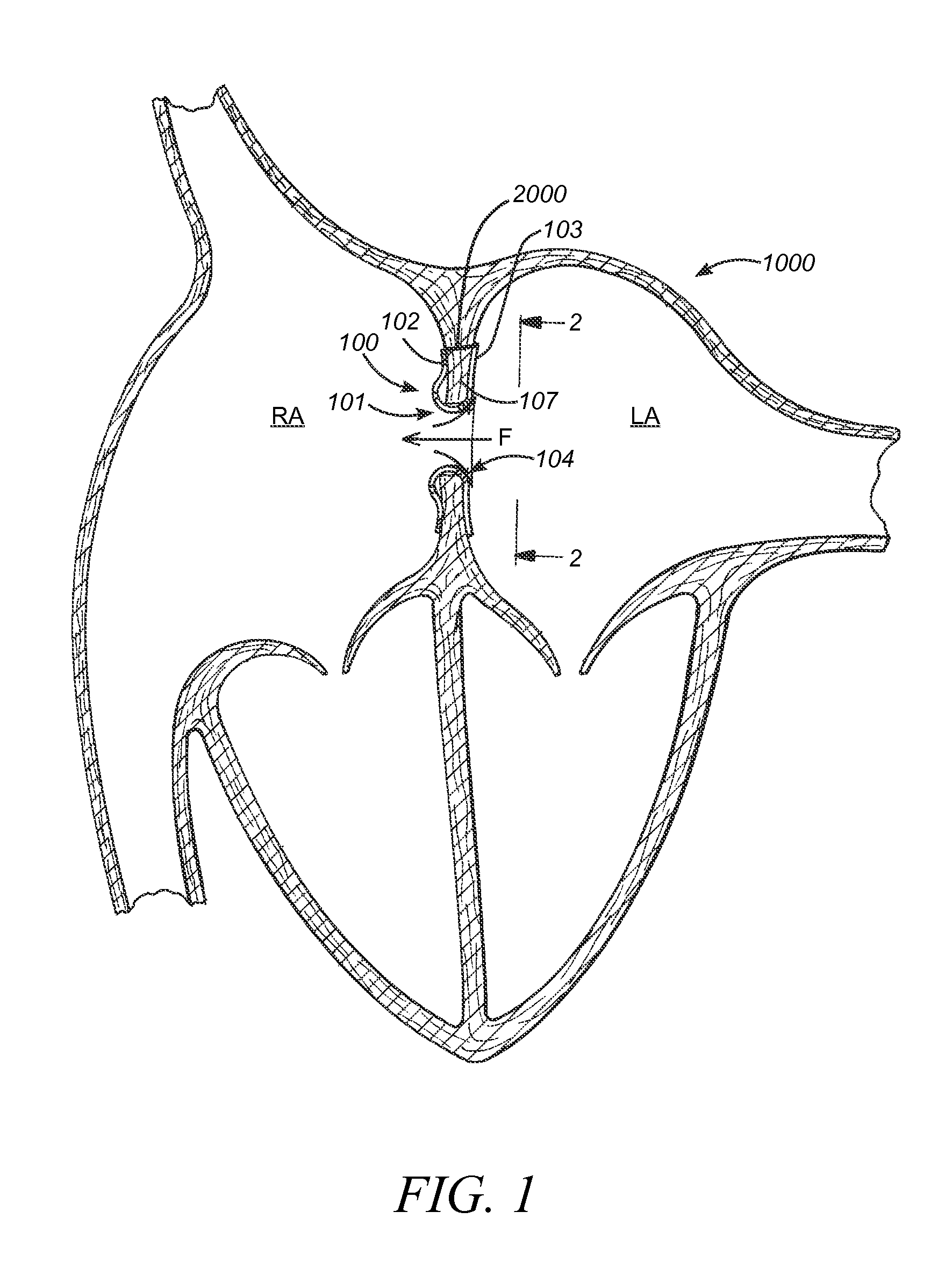
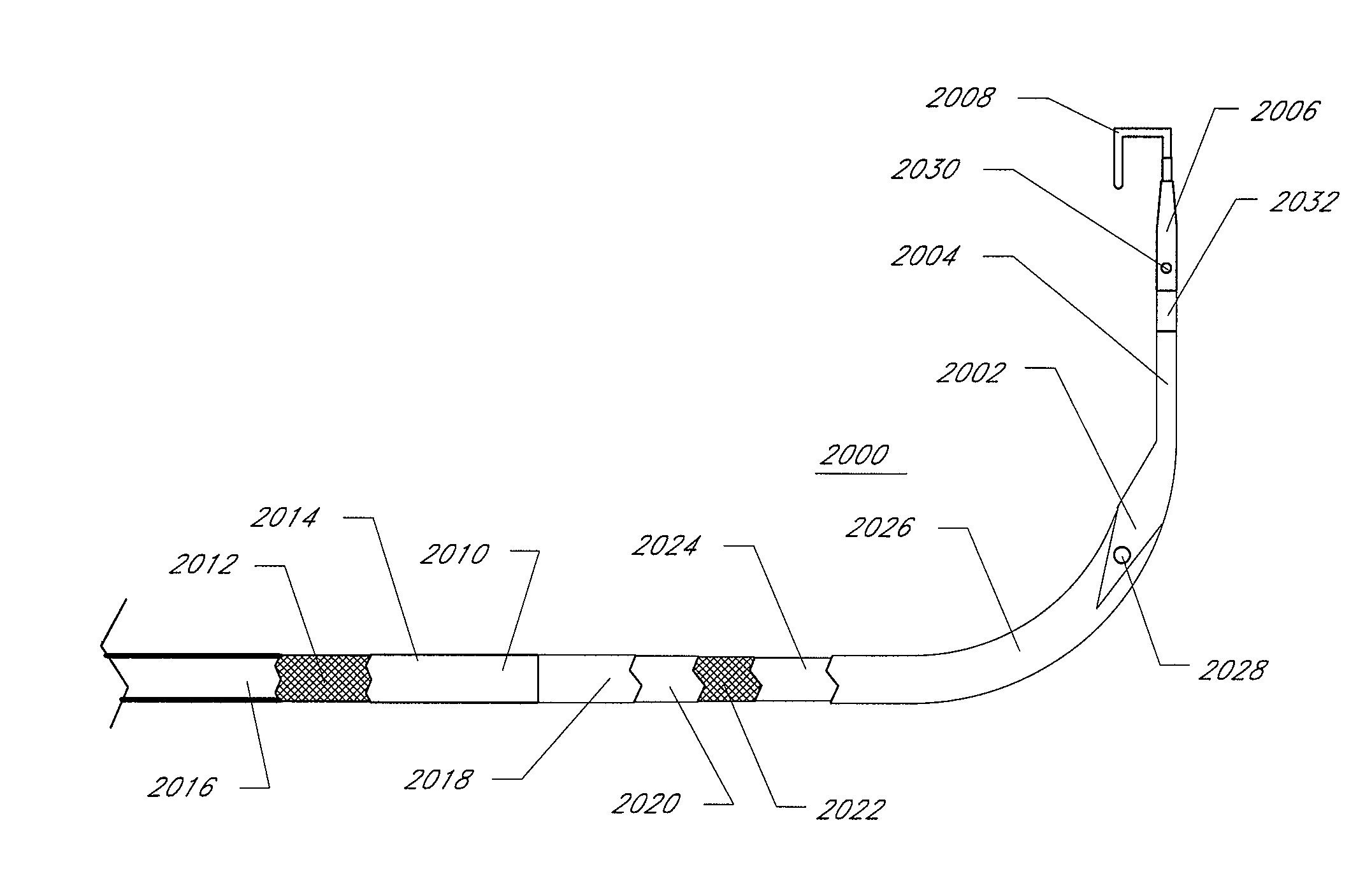
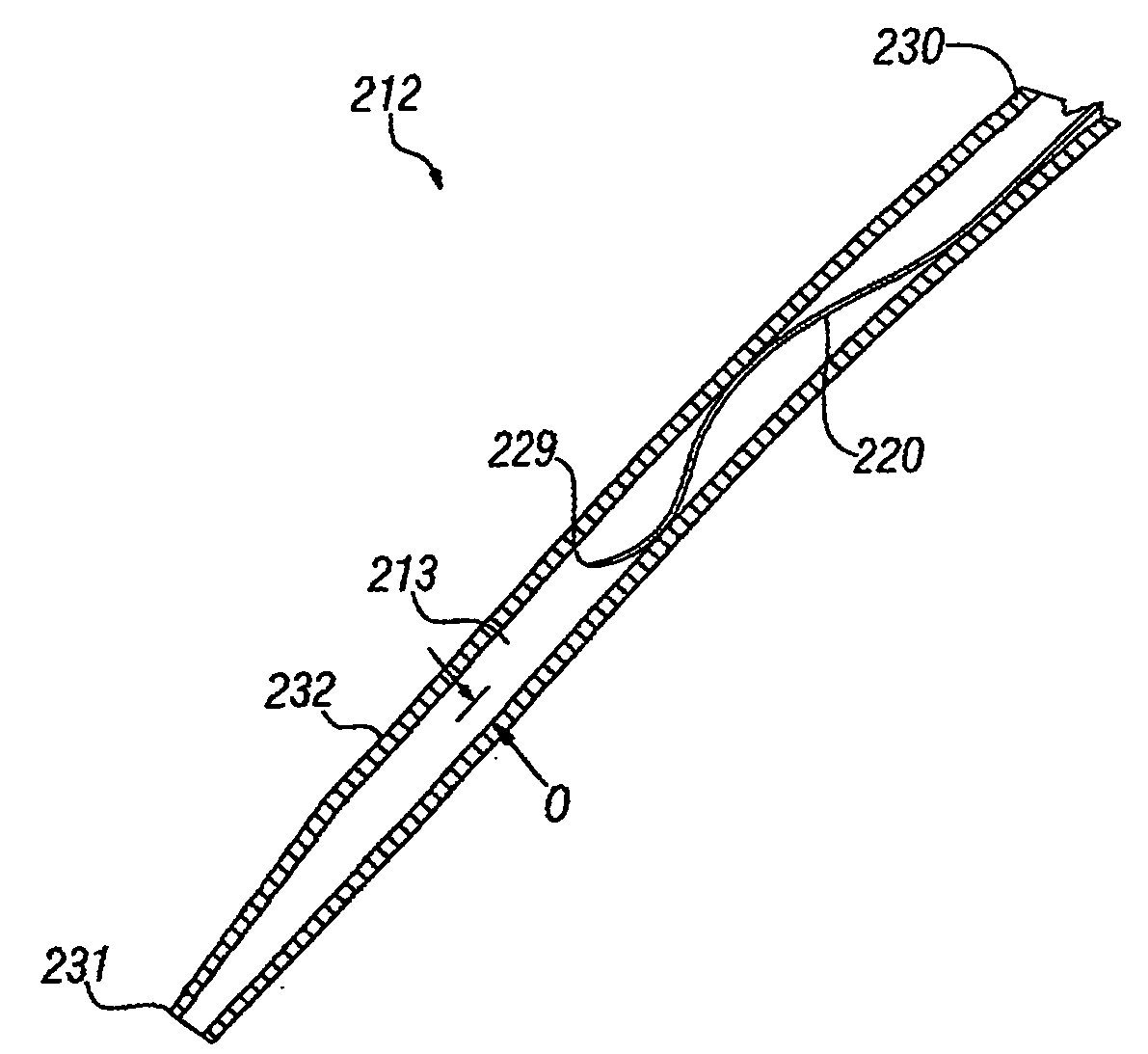
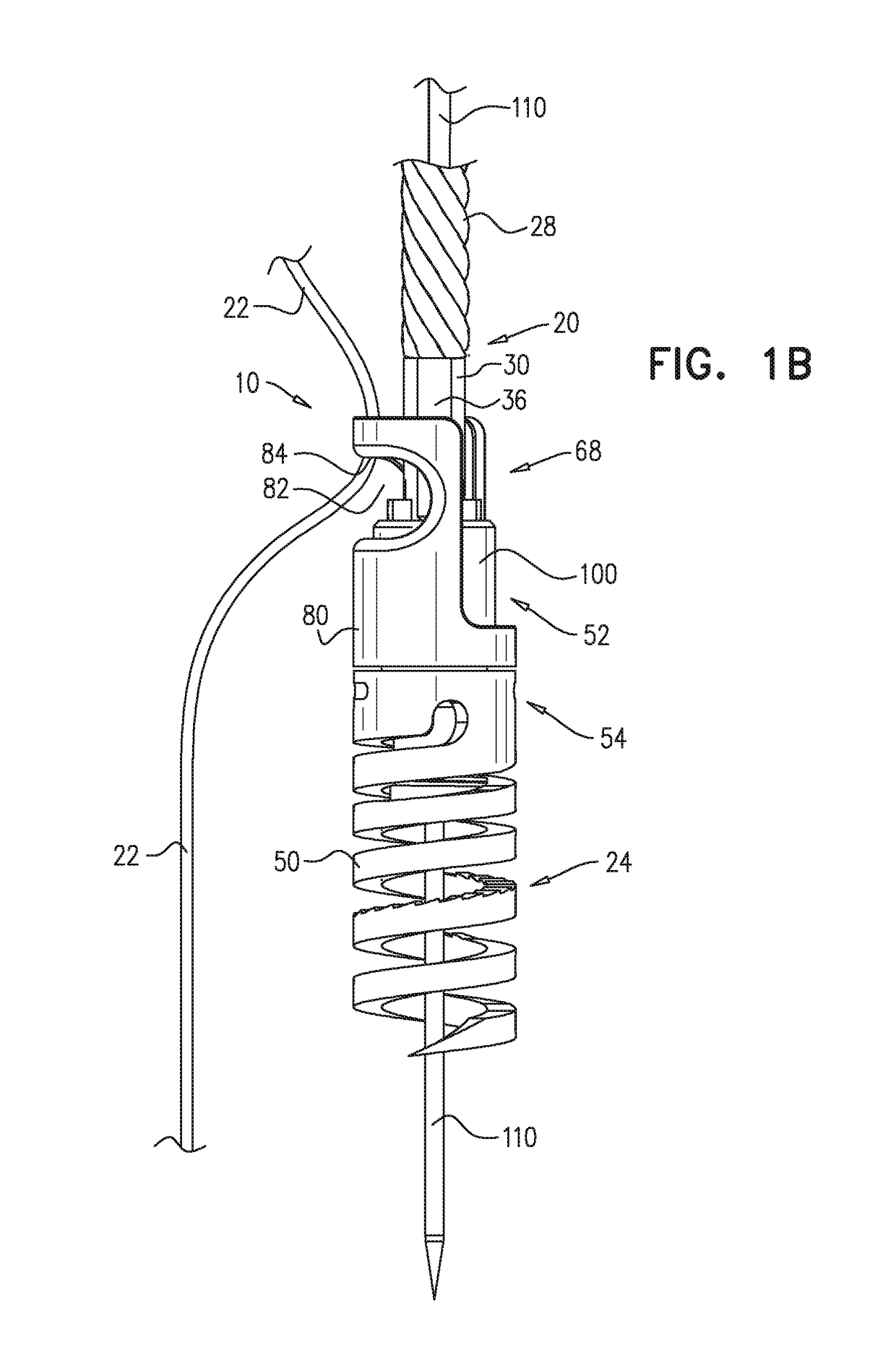
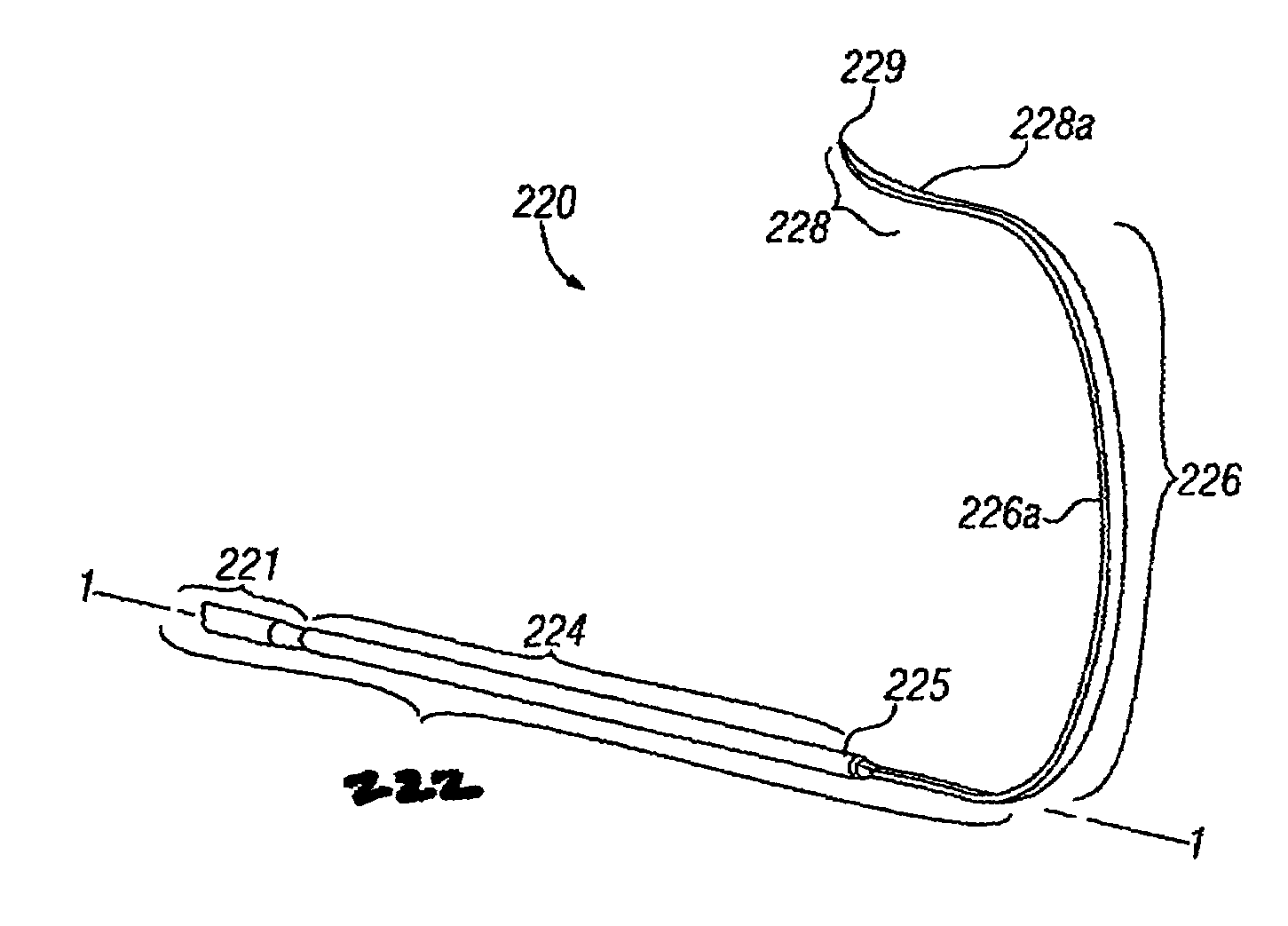
Transseptal guidewire
A transseptal guidewire and methods for perforating the intra-atrial septum of the heart are disclosed. The transseptal guidewire has an elongated body, an end section biased in a curved configuration to define a proximal curve, and a distal section biased in a curved configuration to define a distal curve, the distal curve being oriented in a direction generally opposite that of the proximal curve.
Owner:BOSTON SCI MEDICAL DEVICE LTD
Device and method for mitral valve repair
InactiveUS20100030330A1The implementation process is simpleLess criticalBone implantAnnuloplasty ringsPosterior leafletLeft ventricular size
Devices and methods for reshaping a mitral valve annulus are provided. One device according to the invention is configured for deployment in the right atrium and is shaped to apply a force along the atrial septum. The device causes the atrial septum to deform and push the anterior leaflet of the mitral valve in a posterior direction for reducing mitral valve regurgitation. Another embodiment of a device is deployed in the left ventricular outflow tract at a location adjacent the aortic valve. The device may be expandable for urging the anterior leaflet toward the posterior leaflet. Another embodiment of the device includes a first anchor, a second anchor, and a bridge, with the bridge having sufficient length to reach from the coronary sinus to the right atrium and / or superior or inferior vena cava. In a further embodiment a device includes a middle anchor positioned on the bridge between the distal and proximal anchors.
Owner:EDWARDS LIFESCIENCES CORP
Devices for reducing left atrial pressure having biodegradable constriction, and methods of making and using same
ActiveUS20130030521A1Reducing left atrial pressureIncrease cardiac outputHeart valvesSurgeryLeft ventricular sizeLeft atrial pressure
A device for regulating blood pressure between a patient's left atrium and right atrium comprises an hourglass-shaped stent comprising a neck region and first and second flared end regions, the neck region disposed between the first and second end regions and configured to engage the fossa ovalis of the patient's atrial septum; and a one-way tissue valve coupled to the first flared end region and configured to shunt blood from the left atrium to the right atrium when blood pressure in the left atrium exceeds blood pressure in the right atrium. The inventive device may include a biodegradable material that biodegrades to offset flow changes caused by tissue ingrowth. The inventive device may reduce left atrial pressure and left ventricular end diastolic pressure, and may increase cardiac output, increase ejection fraction, relieve pulmonary congestion, and lower pulmonary artery pressure, among other benefits.
Owner:WAVE LTD V
Surgical perforation device with curve
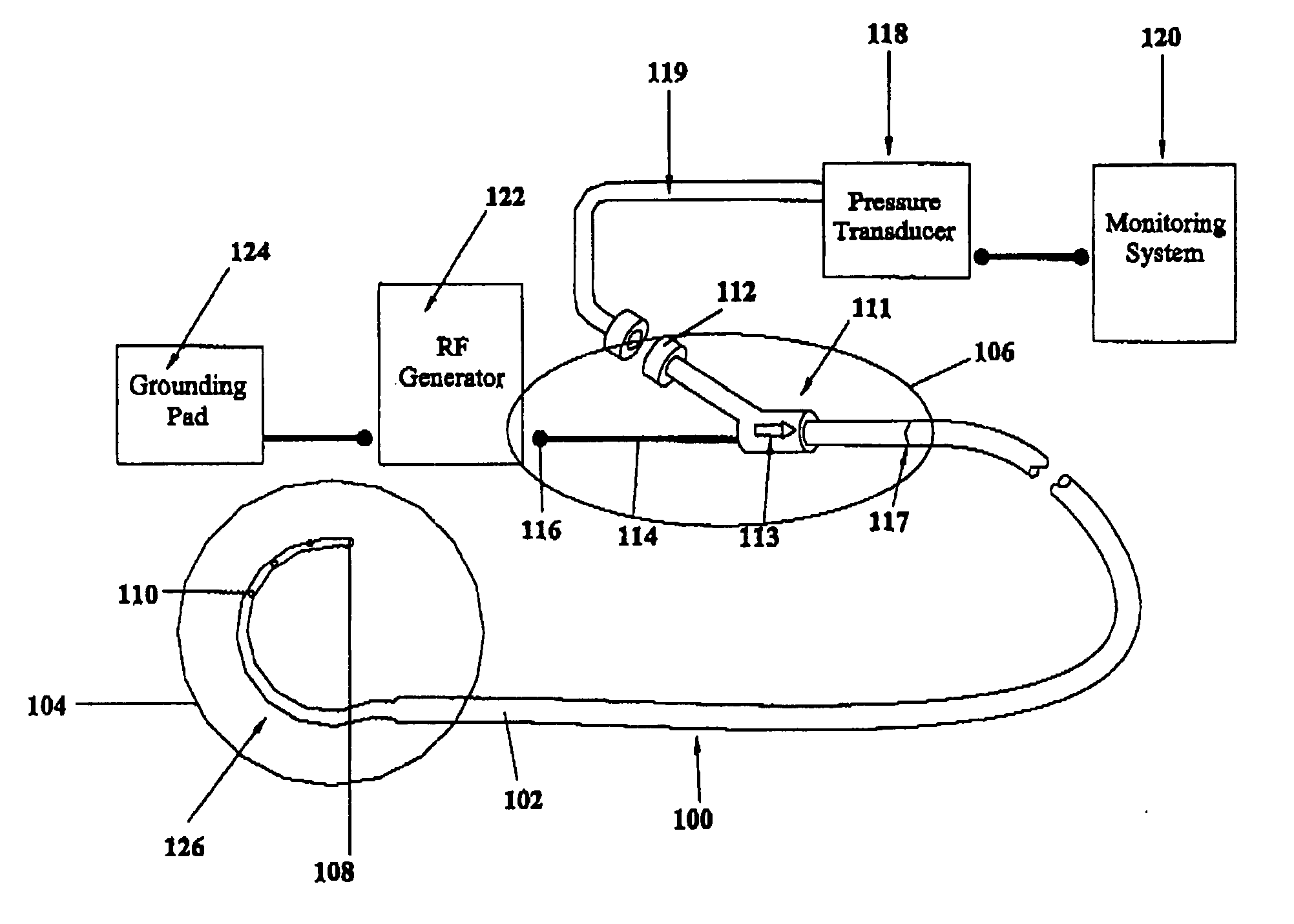
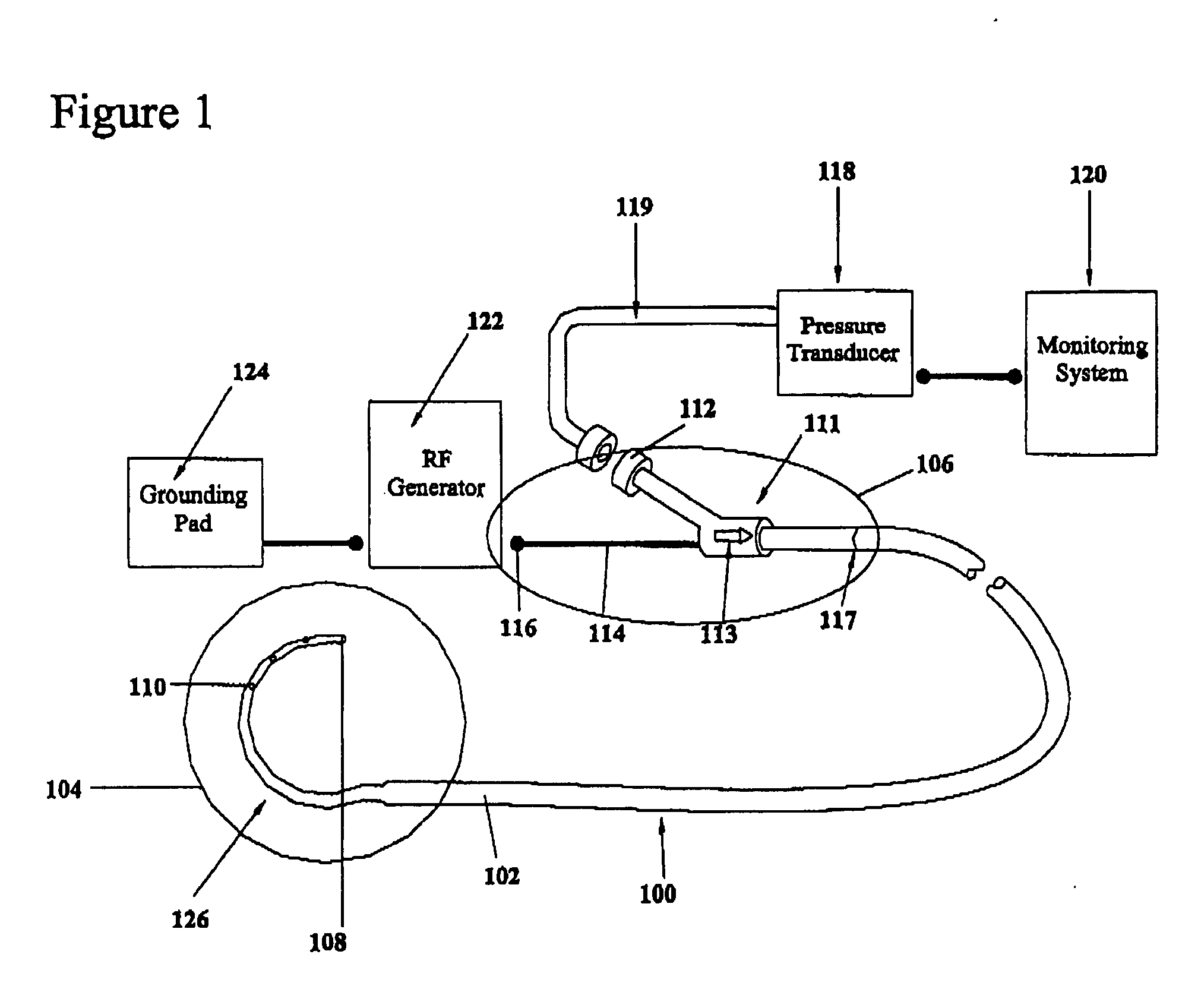
A device for creating a surgical perforation with a functional distal tip for creating a controlled perforation. The functional tip may comprise at least one active electrode for creating the perforation through the application of Radio Frequency (RF) energy. The device is curved in order to decrease the likelihood of injury to structures of a patient such as inadvertent cardiac perforation when used in transseptal perforation procedures. The device is introduced into the right atrium, and the functional tip is then positioned against the atrial septum. Energy is applied to create the perforation. The tip is advanced toward the left atrium to create the perforation and as it advances into the left atrium, the device takes on its curved shape to direct the tip away from cardiac structures. The position of the tip of the device can be determined in response to pressure sensed at the tip and determined by a monitor.
Owner:BOSTON SCI MEDICAL DEVICE LTD
Left and right side heart support
InactiveUS7785246B2Lower the volumeReduce the amount requiredOther blood circulation devicesBlood pumpsOuter CannulaRight atrium
Owner:MAQUET CARDIOVASCULAR LLC
Surgical perforation device with electrocardiogram (ECG) monitoring ability and method of using ECG to position a surgical perforation device
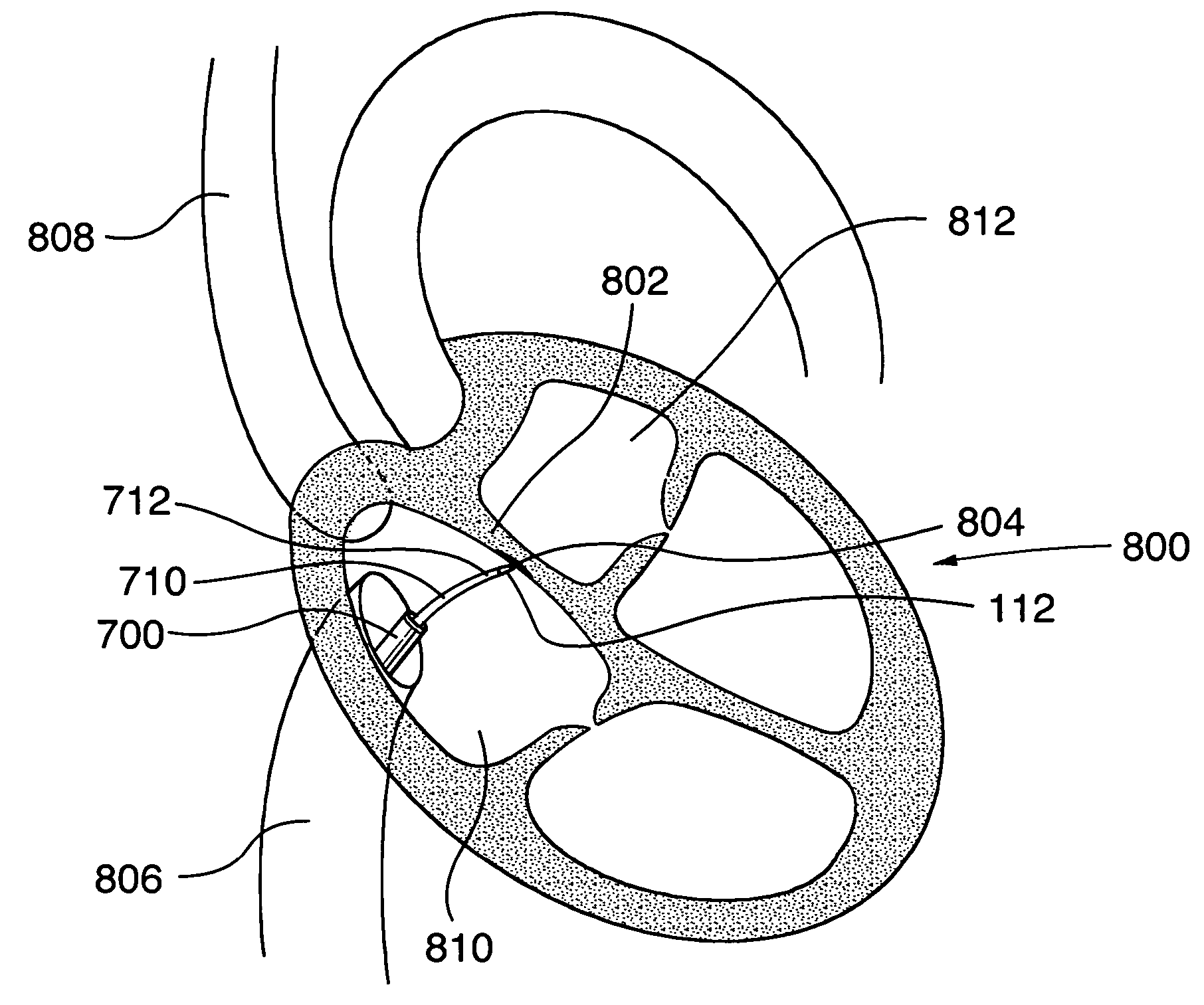
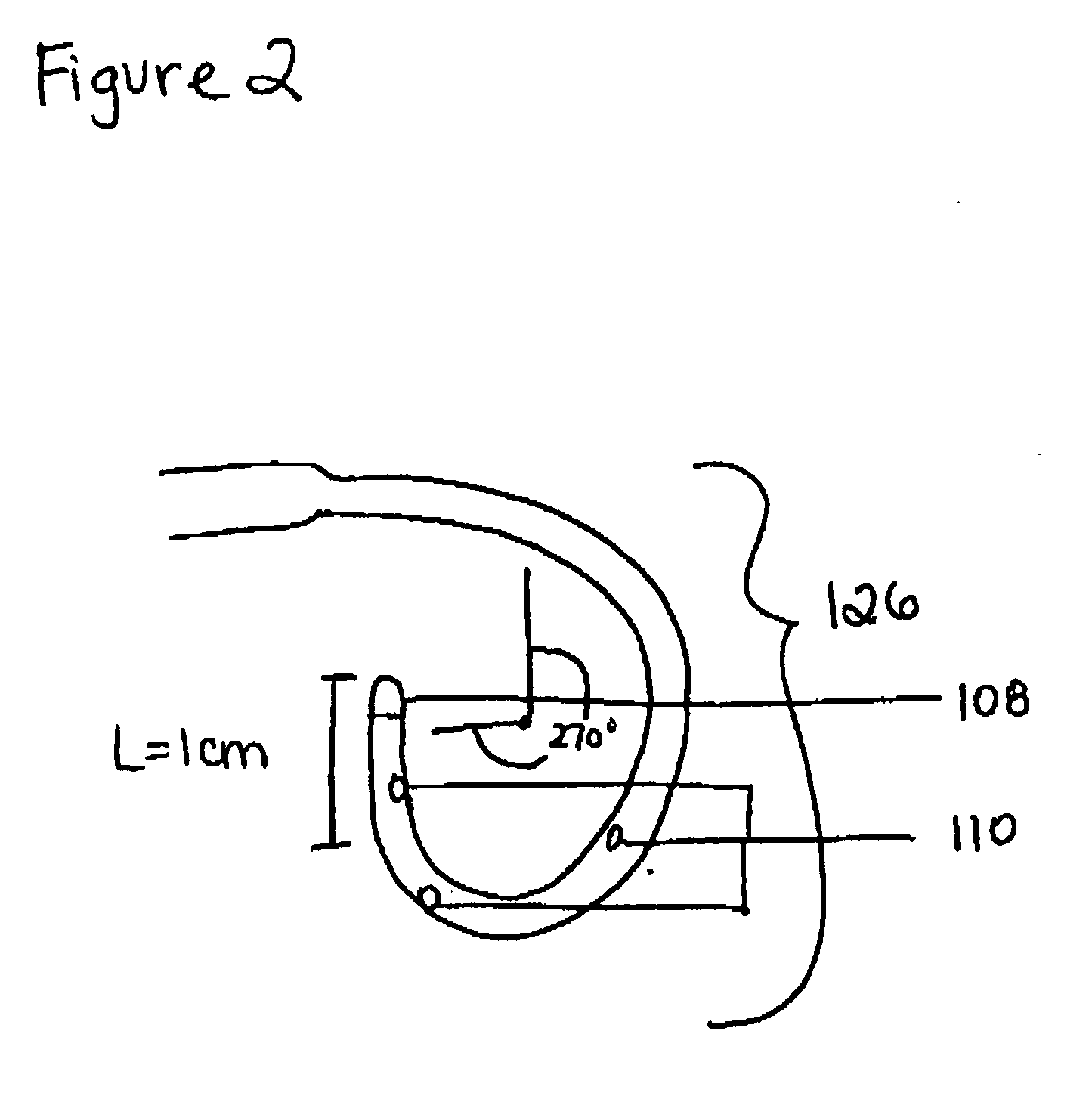
A device with a functional tip containing at least one active electrode capable of creating a controlled perforation in body tissue through the application of energy (e.g. Radio Frequency (RF)) is described. The position of the tip of the device can be determined in response to ECG measured at the tip and determined by a monitor coupled to the device. The device is useful to remove or perforate unwanted tissue in a controlled manner in any location in the body, particularly in the atrial septum for controlled transseptal puncture. In this application, the device is introduced into the right atrium, and the functional tip is then positioned against the atrial septum. ECG is used to locate the region of the fossa ovalis on the atrial septum. Energy is applied to create the perforation and ECG is monitored to determine if the perforation was created in a desired location.
Owner:BOSTON SCI MEDICAL DEVICE LTD
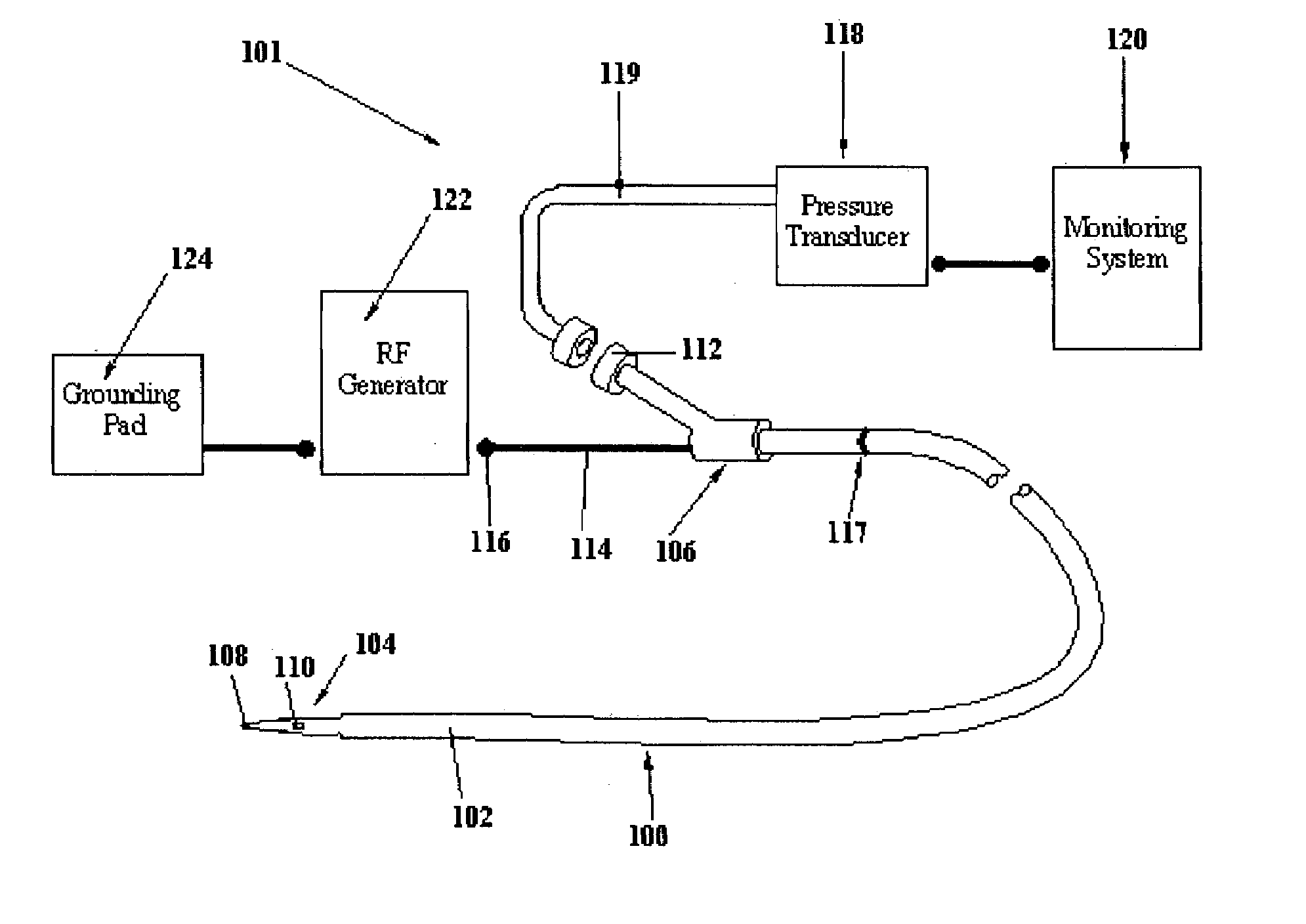
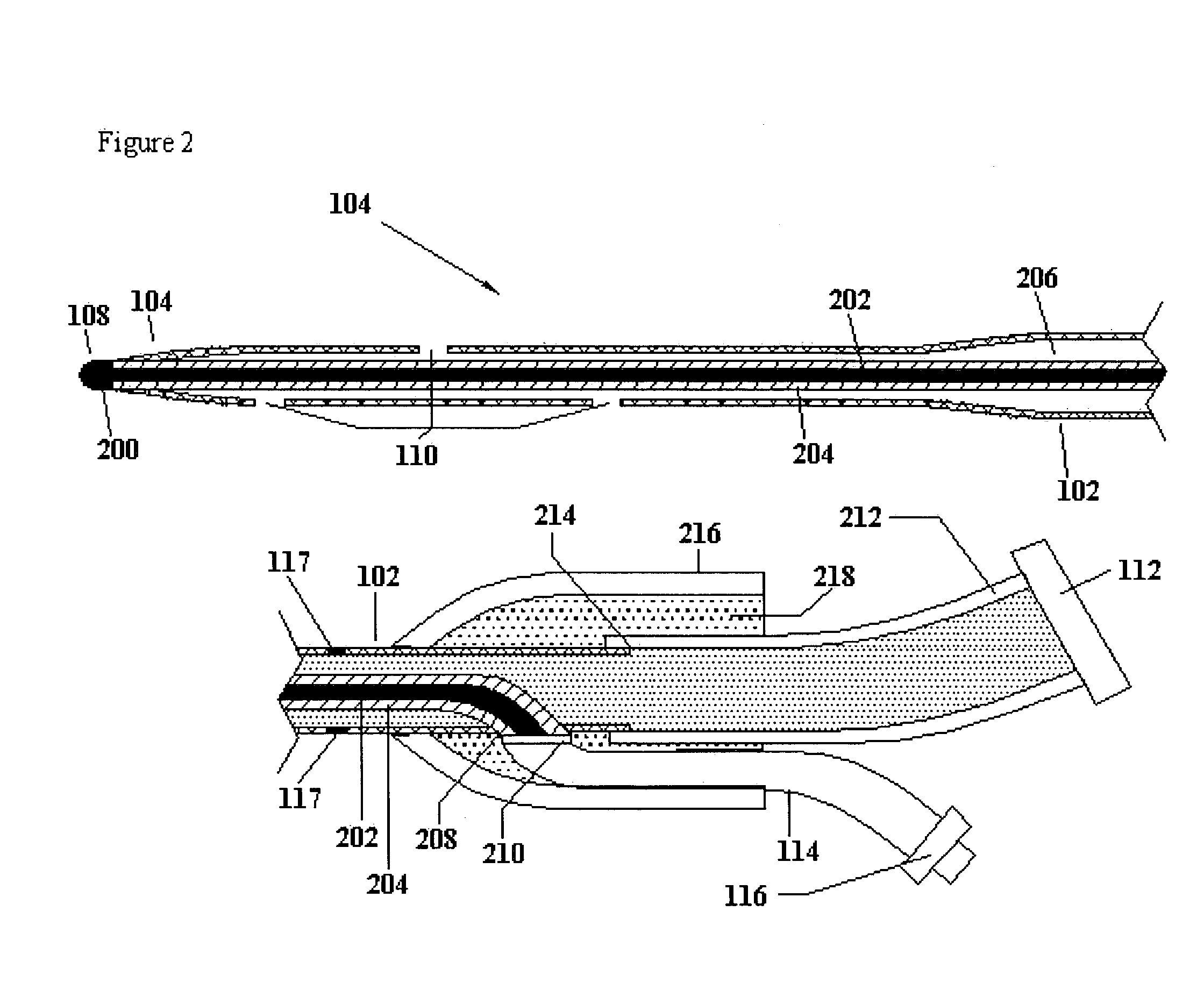
Surgical device with pressure monitoring ability
A device with a functional tip containing at least one active electrode capable of creating a controlled perforation in body tissue through the application of Radio Frequency (RF) energy is described. The position of the tip of the device can be determined in response to pressure sensed at the tip and determined by a monitor. The device is useful to remove or perforate unwanted tissue in a controlled manner in any location in the body, particularly in the atrial septum for controlled transseptal puncture. In this application, the device is introduced into the right atrium, and the functional tip is then positioned against the atrial septum. Energy is applied to create the perforation and pressure is monitored to determine if the perforation was created in a desired location. Other possible applications include the removal of plaque or thrombotic occlusions from diseased vessels.
Owner:BOSTON SCI MEDICAL DEVICE LTD
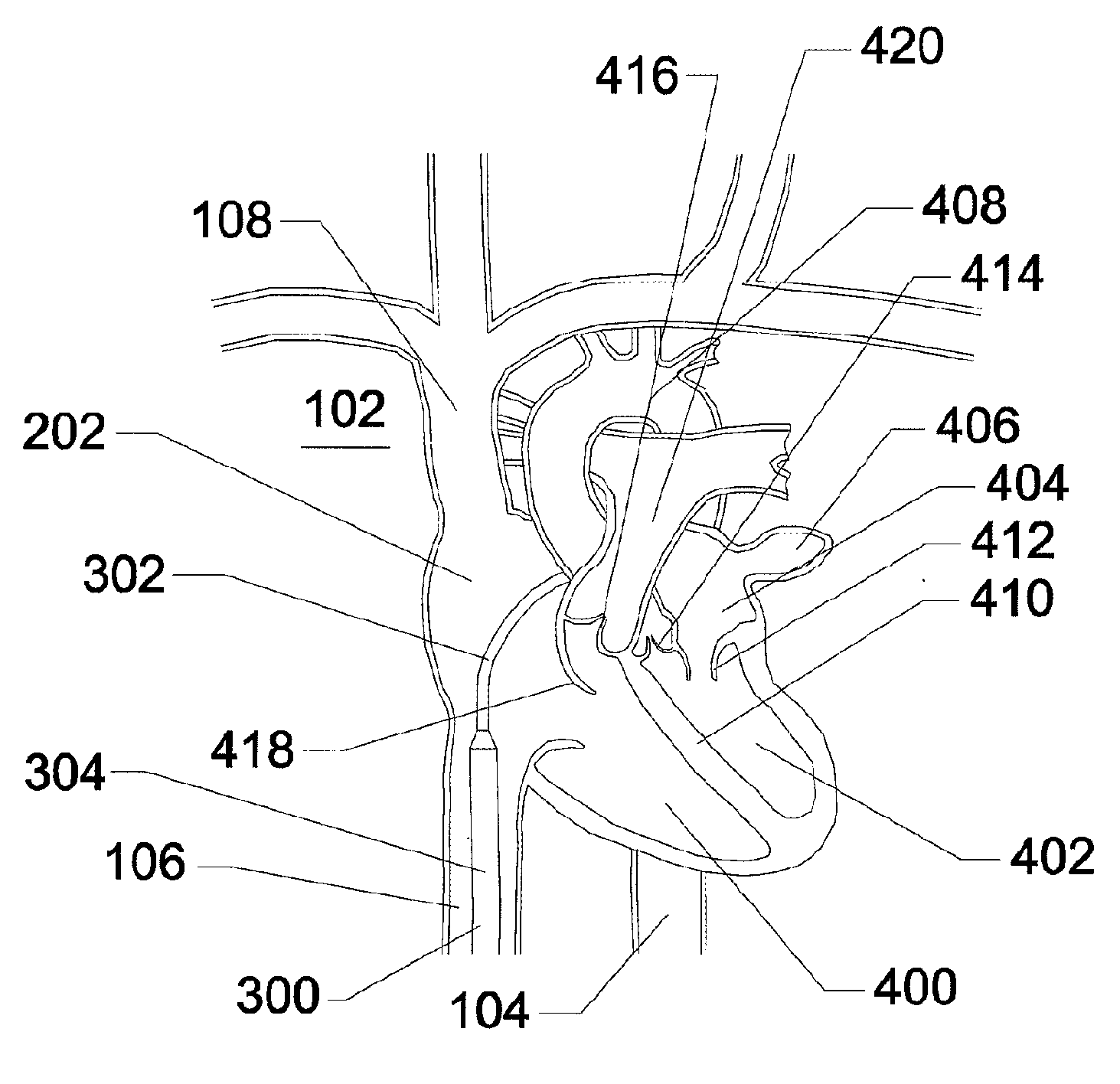
Cardiac tissue cinching
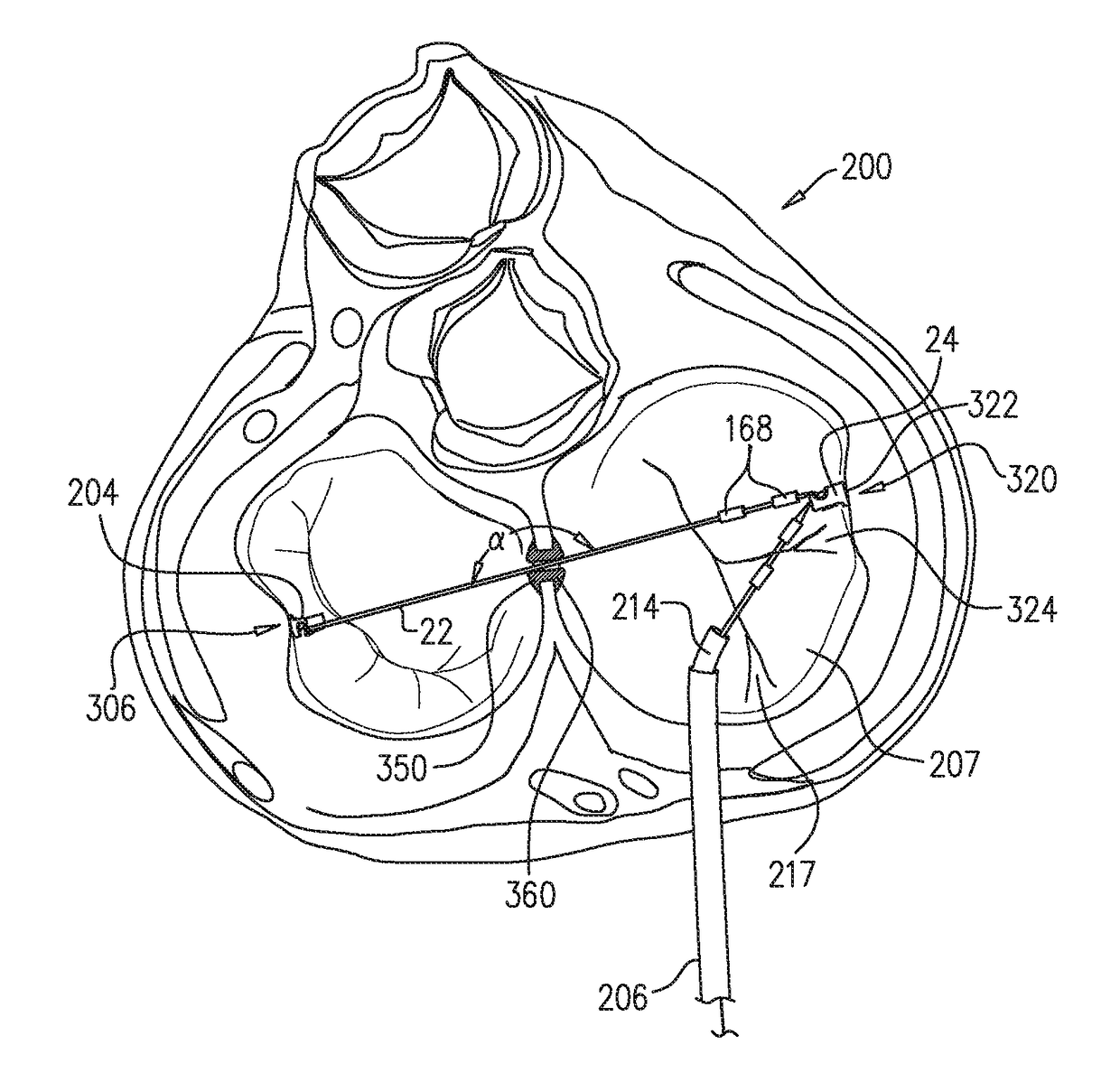
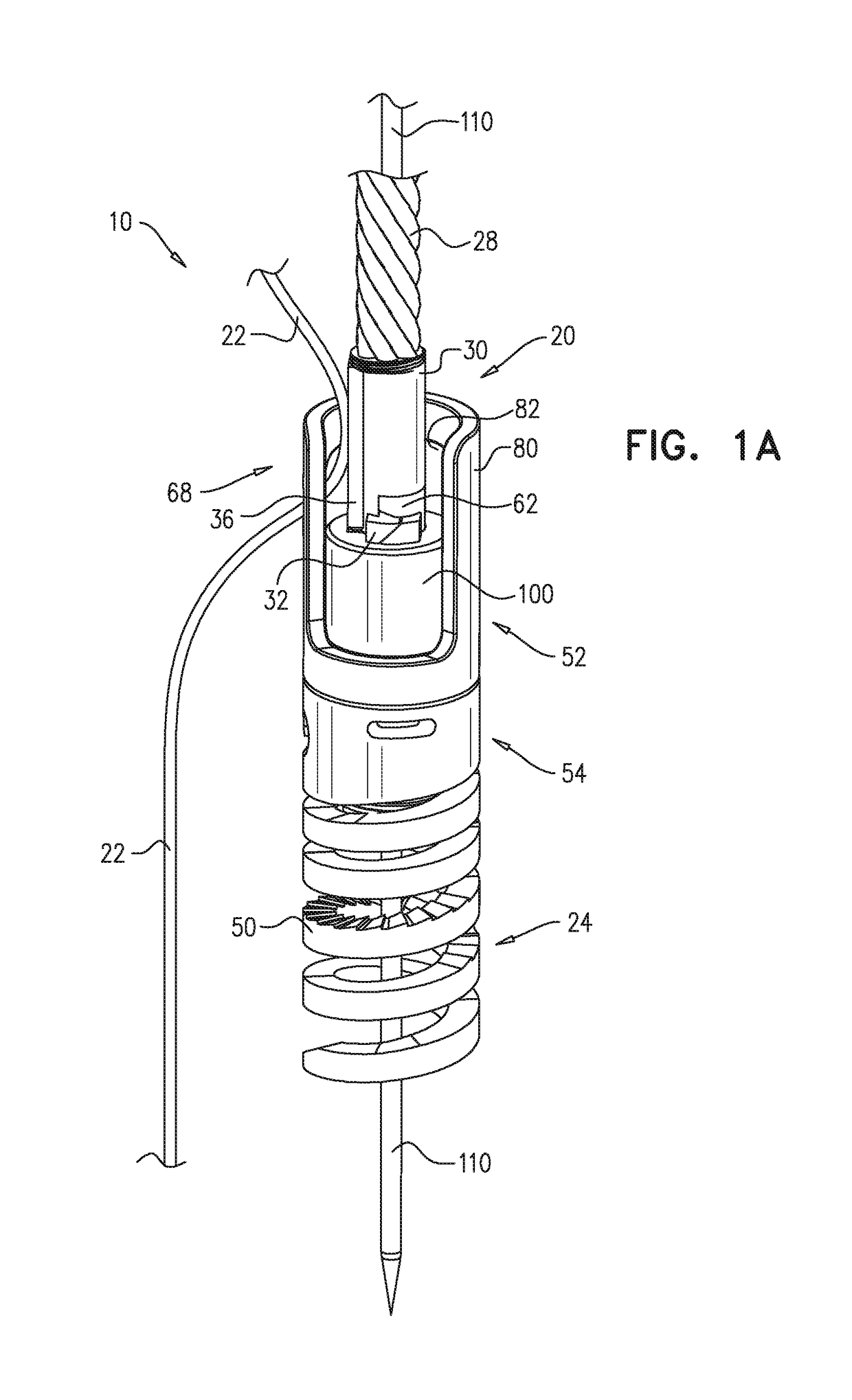
ActiveUS9801720B2Enhanced couplingPrevent slidingSuture equipmentsHeart valvesAtrial cavityRight atrium
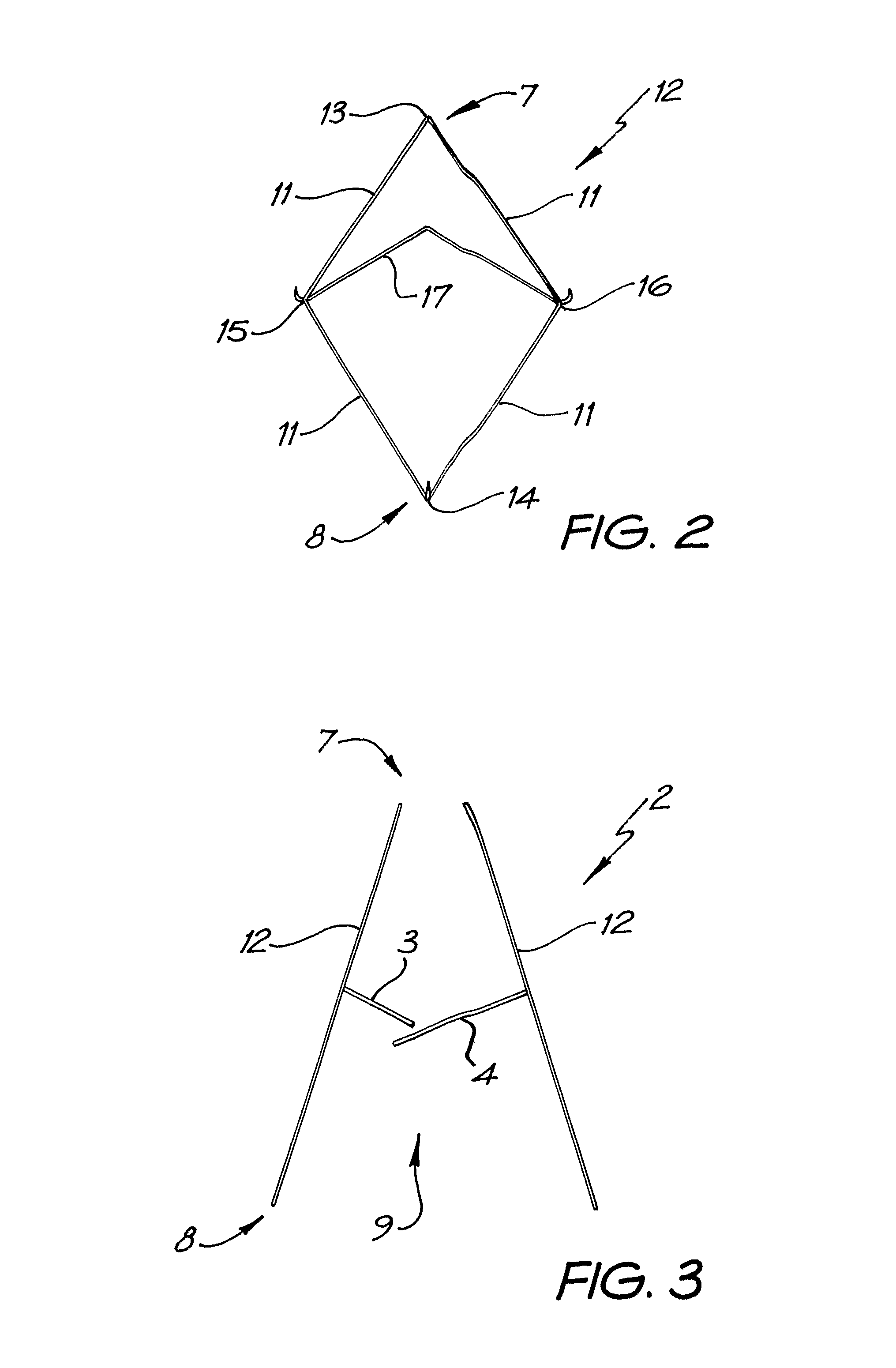
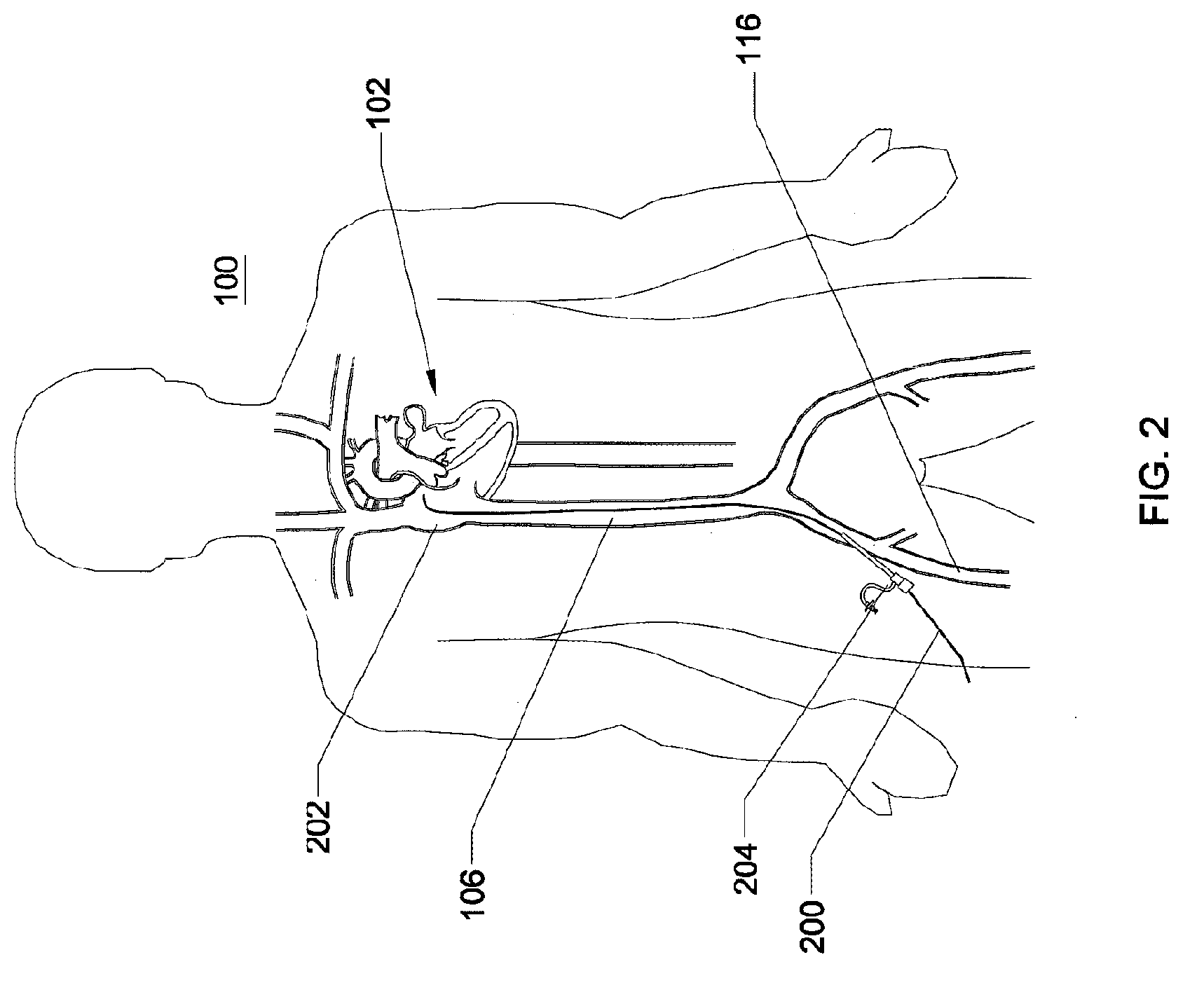
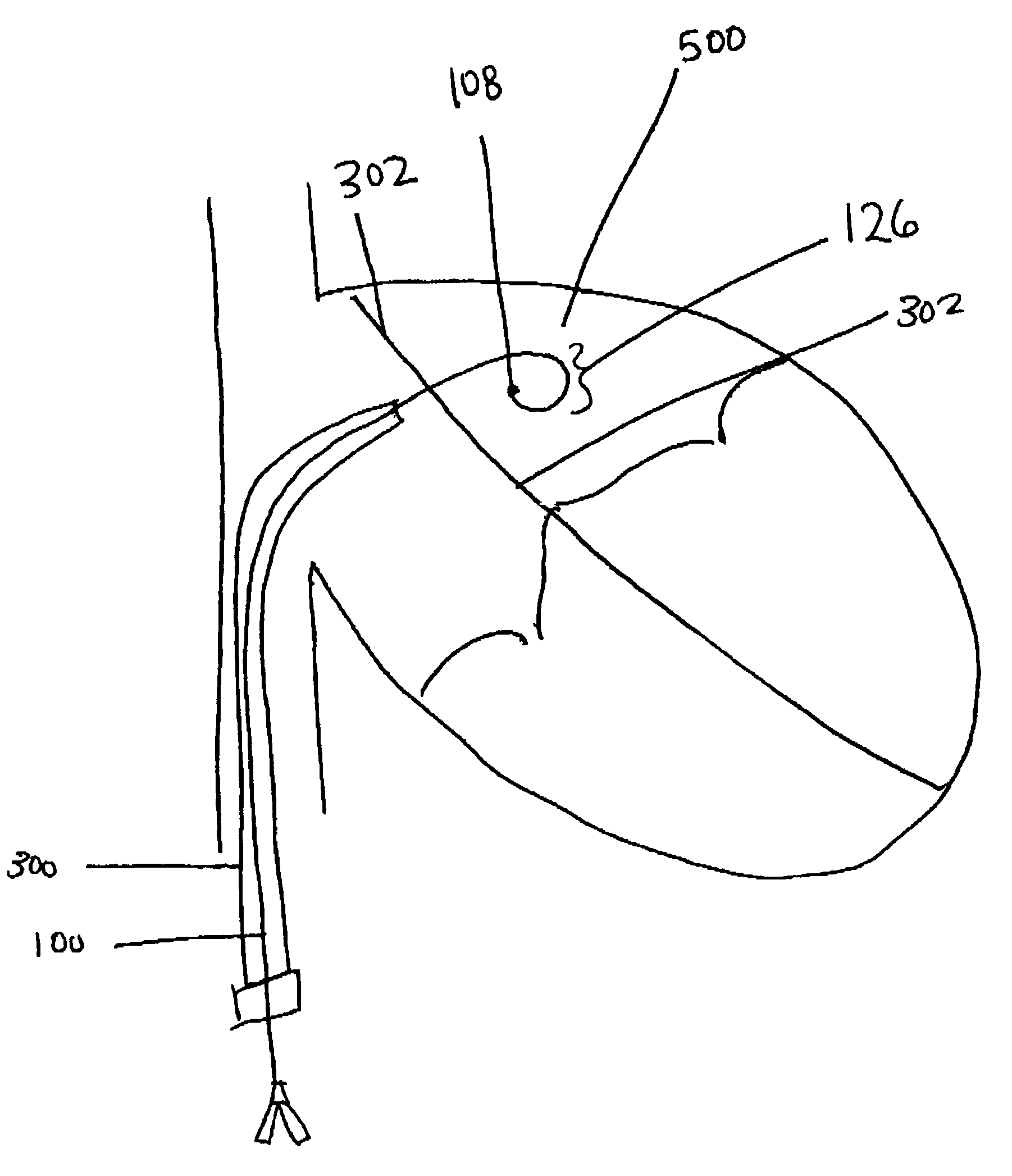
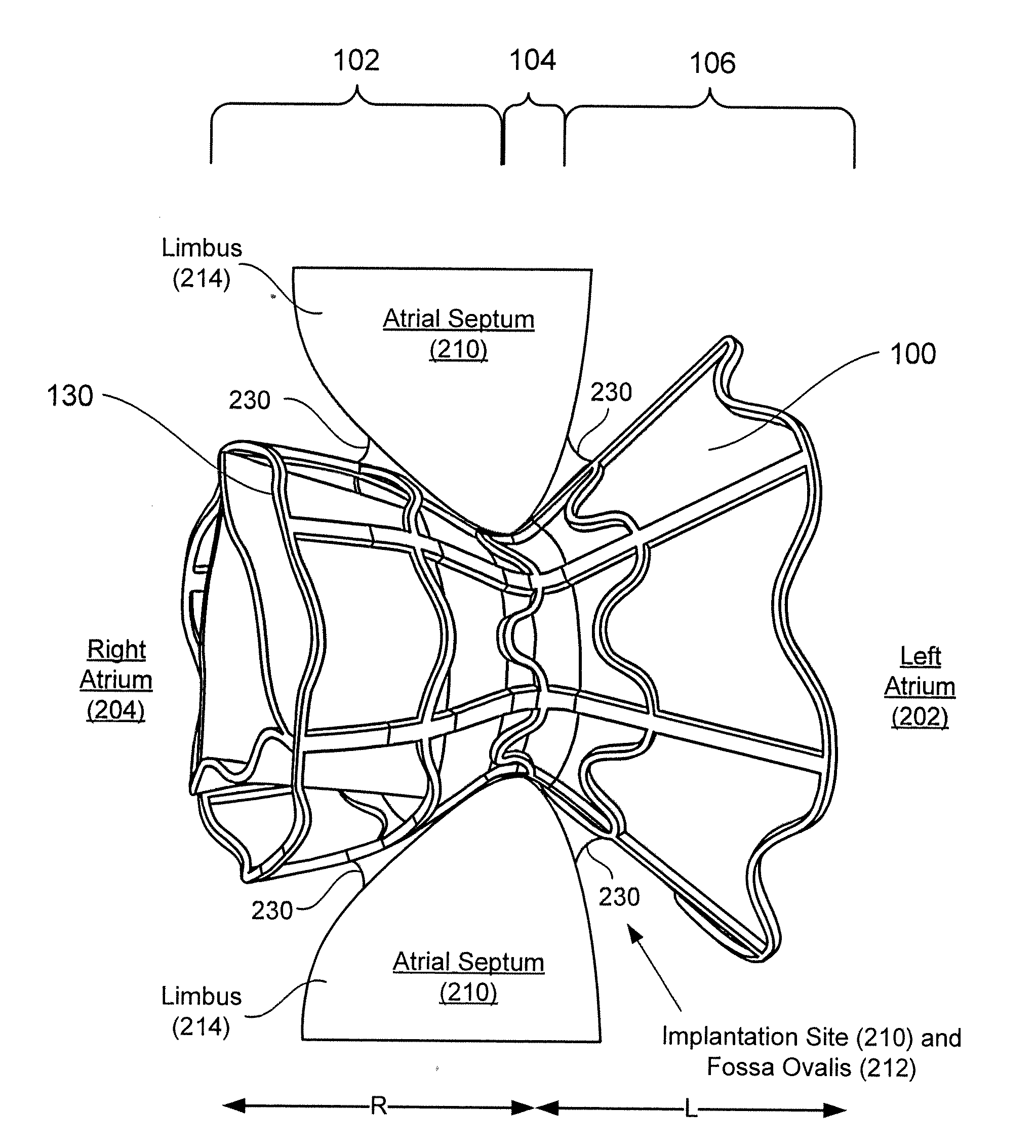
A method is provided including making an opening (300) through an atrial septum (302) at a septal site (304) at least 5 mm from a fossa ovalis (330). A first tissue anchor (204) is endovascularly advanced to a left-atrial site (306) on an annulus of a mitral valve (310) or a wall of a left atrium (308) above the annulus. The first tissue anchor (204) is implanted at the left-atrial site (306). A second tissue anchor (24) is endovascularly advanced to a right-atrial site (320) on an annulus of a tricuspid valve (207) or a wall of a right atrium (200) above the annulus. The second tissue (24) anchor is implanted at the right-atrial site (320). The left-atrial site (306) and the right-atrial site (320) are approximated by tensioning a tether (22) that passes through the opening (300) of the atrial septum (302) and connects the first and the second tissue anchors (204, 24). Other embodiments are also described.
Owner:4TECH INC OXO CAPITAL
Devices, systems and methods to treat heart failure
ActiveUS20100249909A1Relieve symptomsMinimize blood flowAnnuloplasty ringsSurgeryPatients symptomsThrombus
Owner:CORVIA MEDICAL
Devices for reducing left atrial pressure, and methods of making and using same
ActiveUS9034034B2Reduce pressureHigh outputHeart valvesSurgeryLeft ventricular sizeLeft atrial pressure
A device for regulating blood pressure between a patient's left atrium and right atrium comprises an hourglass-shaped stent comprising a neck region and first and second flared end regions, the neck region disposed between the first and second end regions and configured to engage the fossa ovalis of the patient's atrial septum; and a one-way tissue valve coupled to the first flared end region and configured to shunt blood from the left atrium to the right atrium when blood pressure in the left atrium exceeds blood pressure in the right atrium. The inventive devices may reduce left atrial pressure and left ventricular end diastolic pressure, and may increase cardiac output, increase ejection fraction, relieve pulmonary congestion, and lower pulmonary artery pressure, among other benefits. The inventive devices may be used, for example, to treat subjects having heart failure, pulmonary congestion, or myocardial infarction, among other pathologies.
Owner:WAVE LTD V
Transseptal guidewire
A transseptal guidewire and methods for perforating the intra-atrial septum of the heart are disclosed. The transseptal guidewire has an elongated body, an end section biased in a curved configuration to define a proximal curve, and a distal section biased in a curved configuration to define a distal curve, the distal curve being oriented in a direction generally opposite that of the proximal curve.
Owner:BOSTON SCI MEDICAL DEVICE LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com