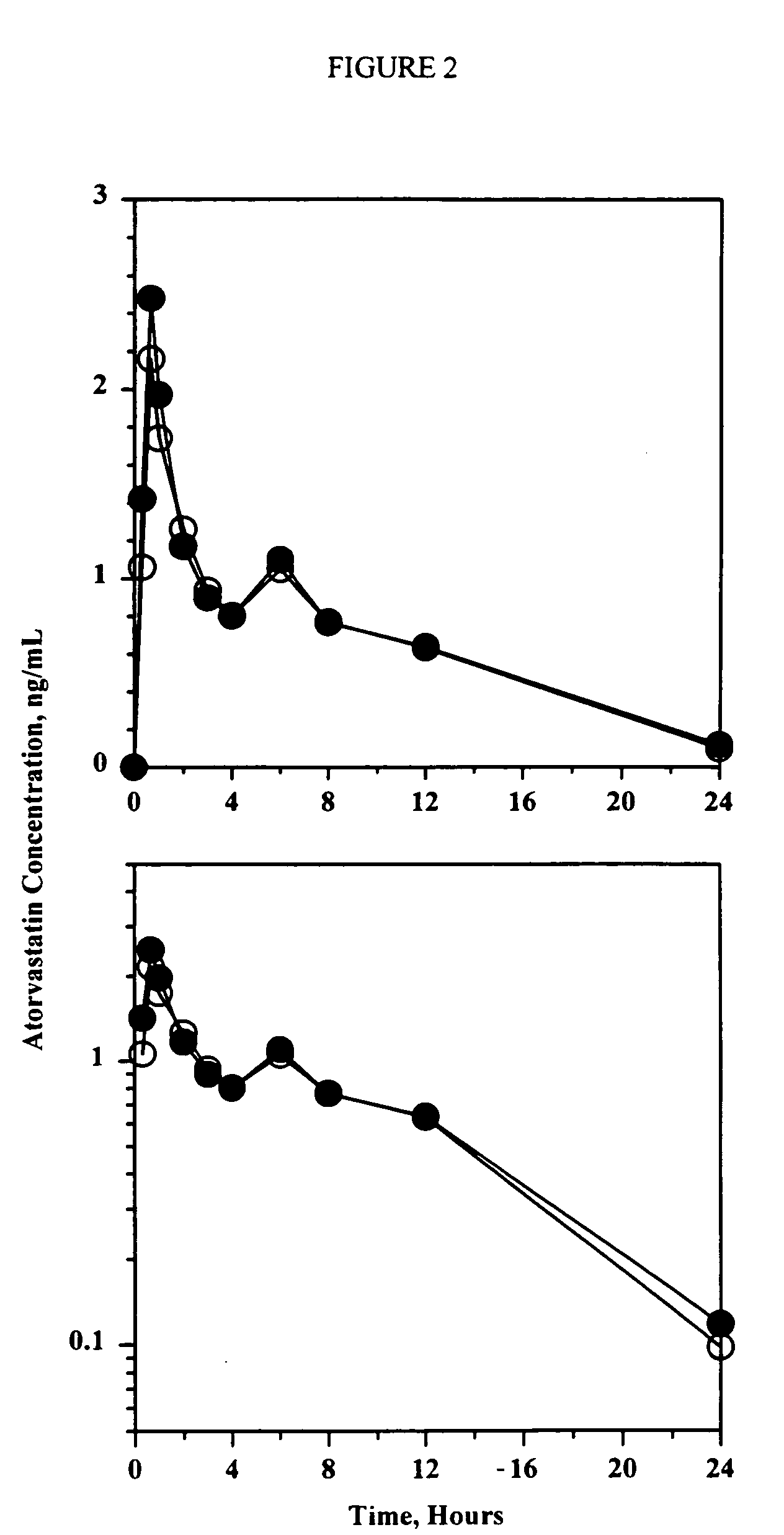
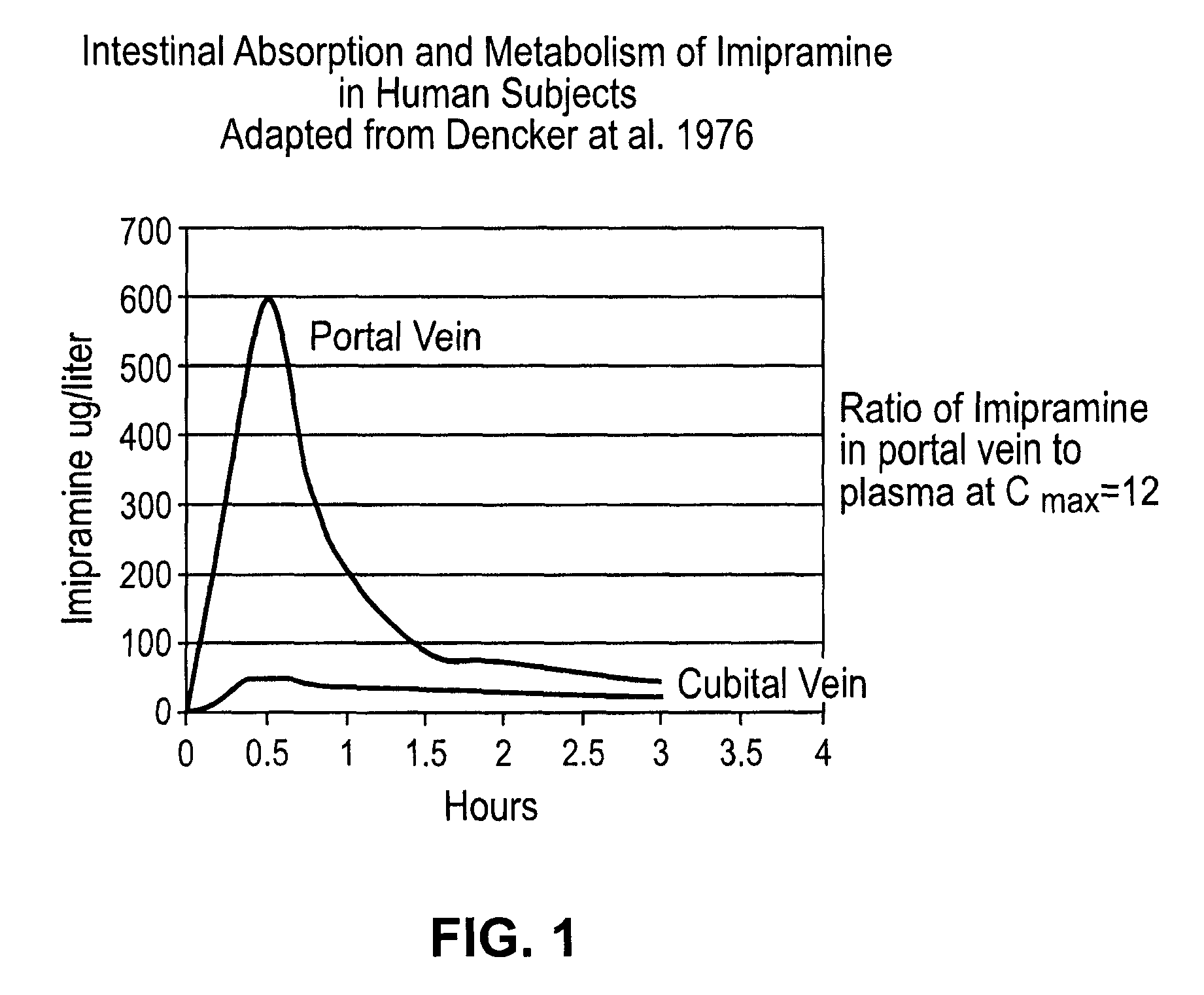
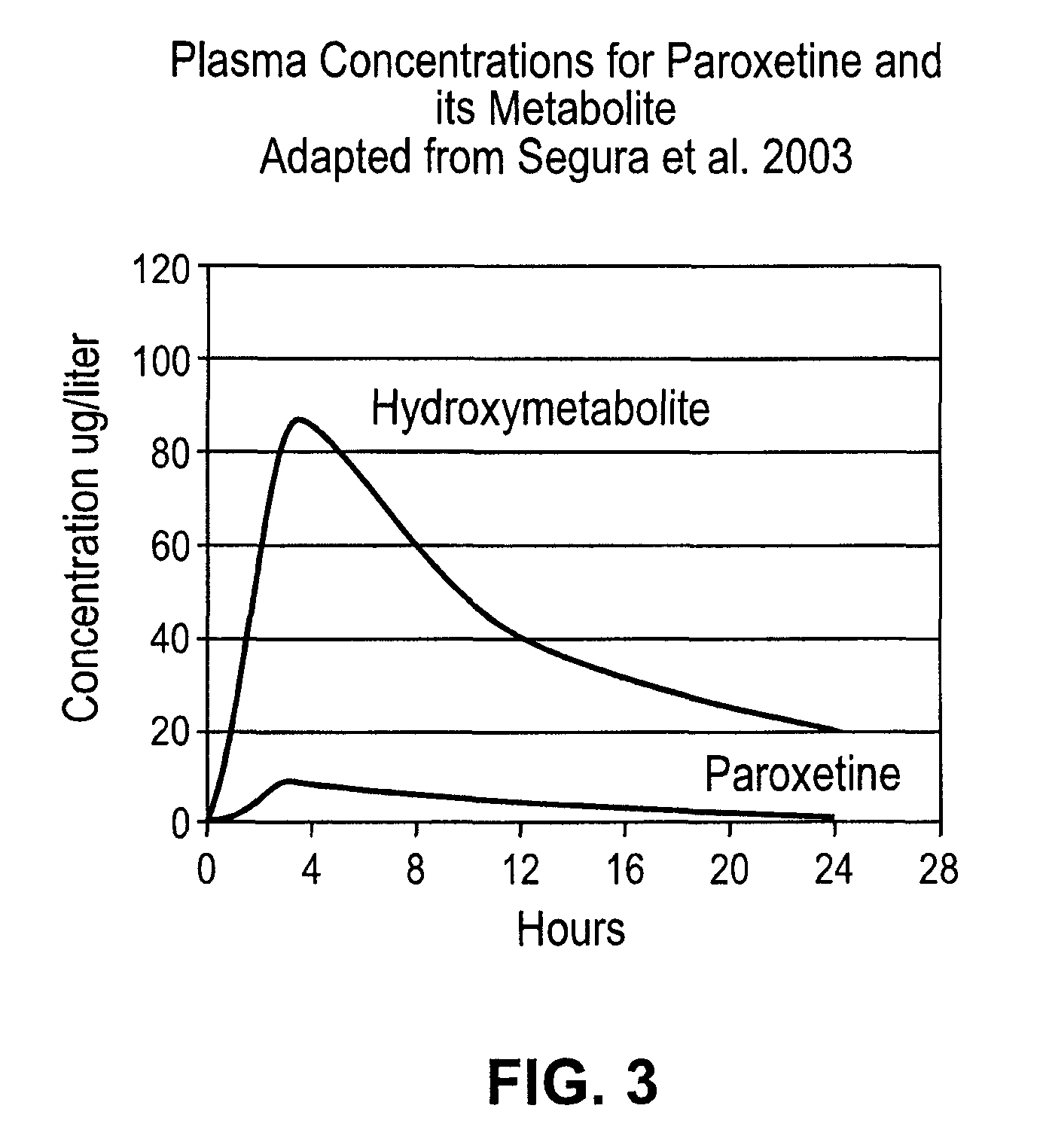
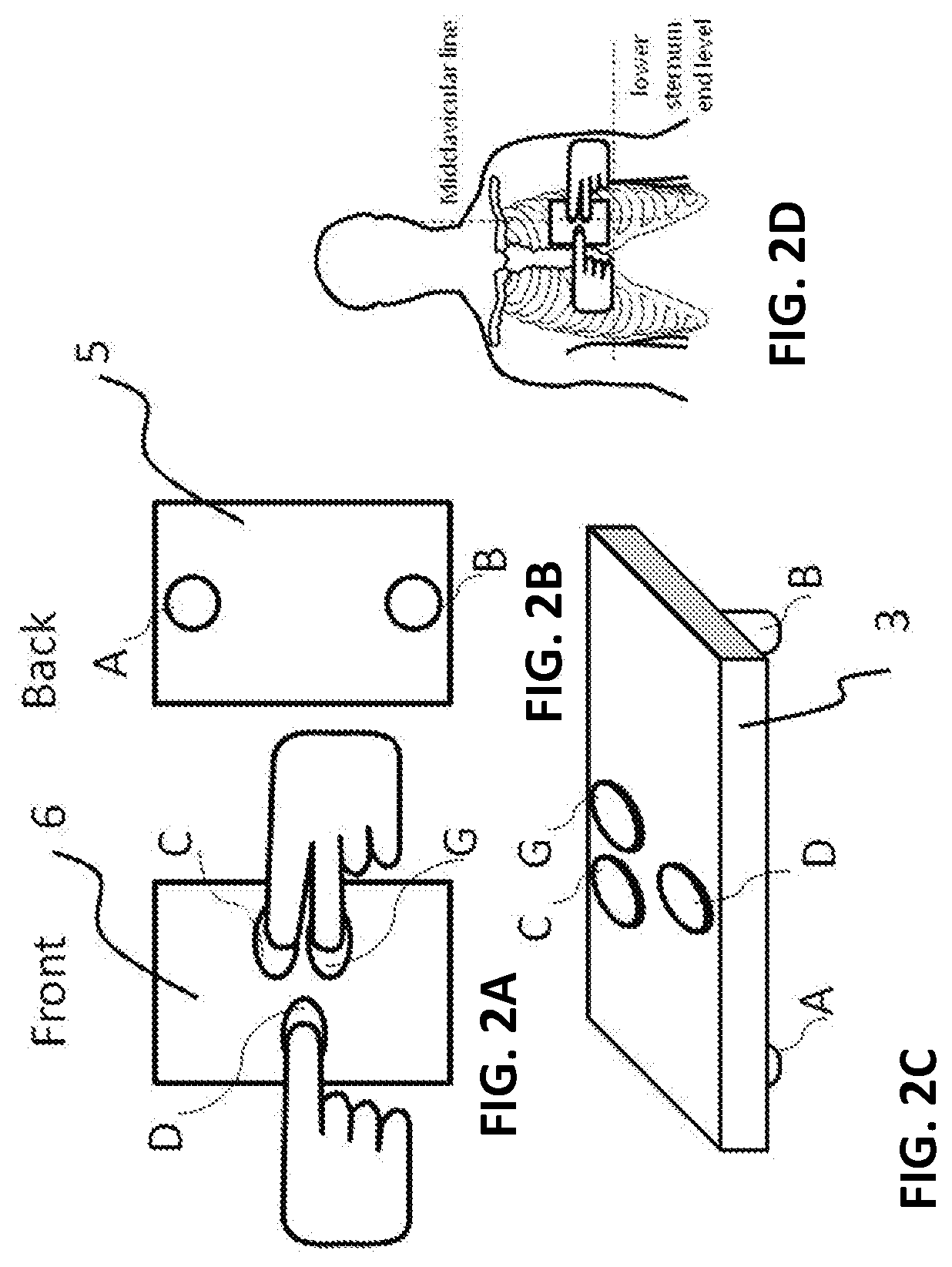
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Cardiac risk" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
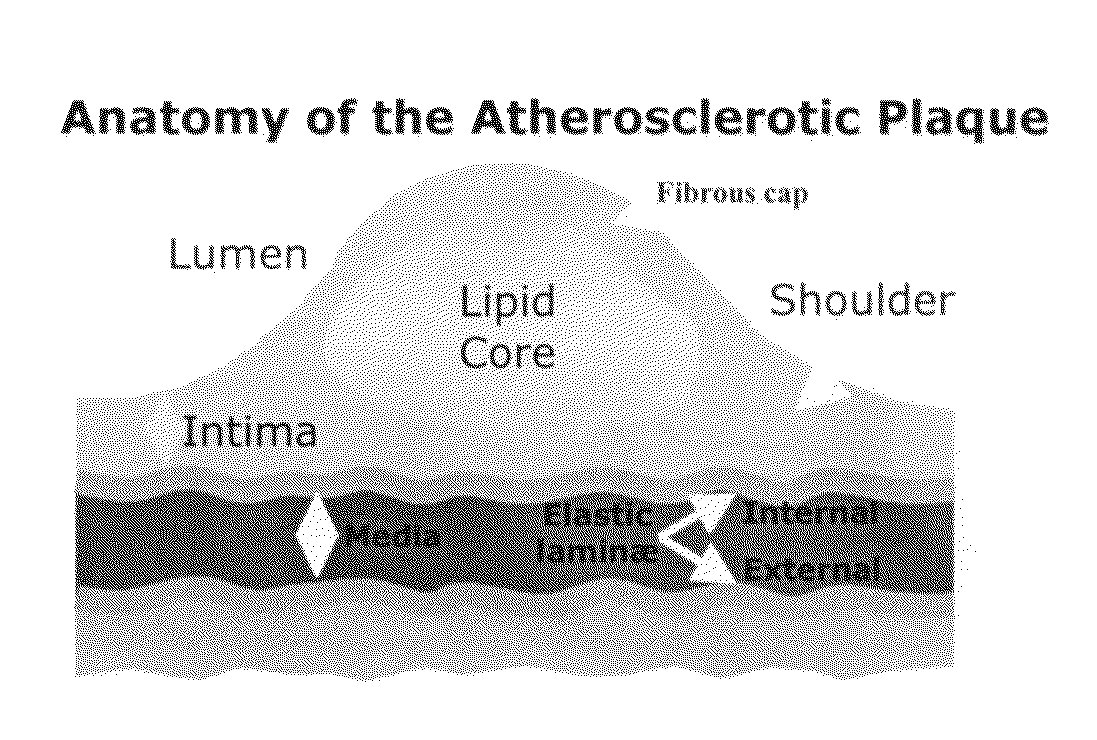
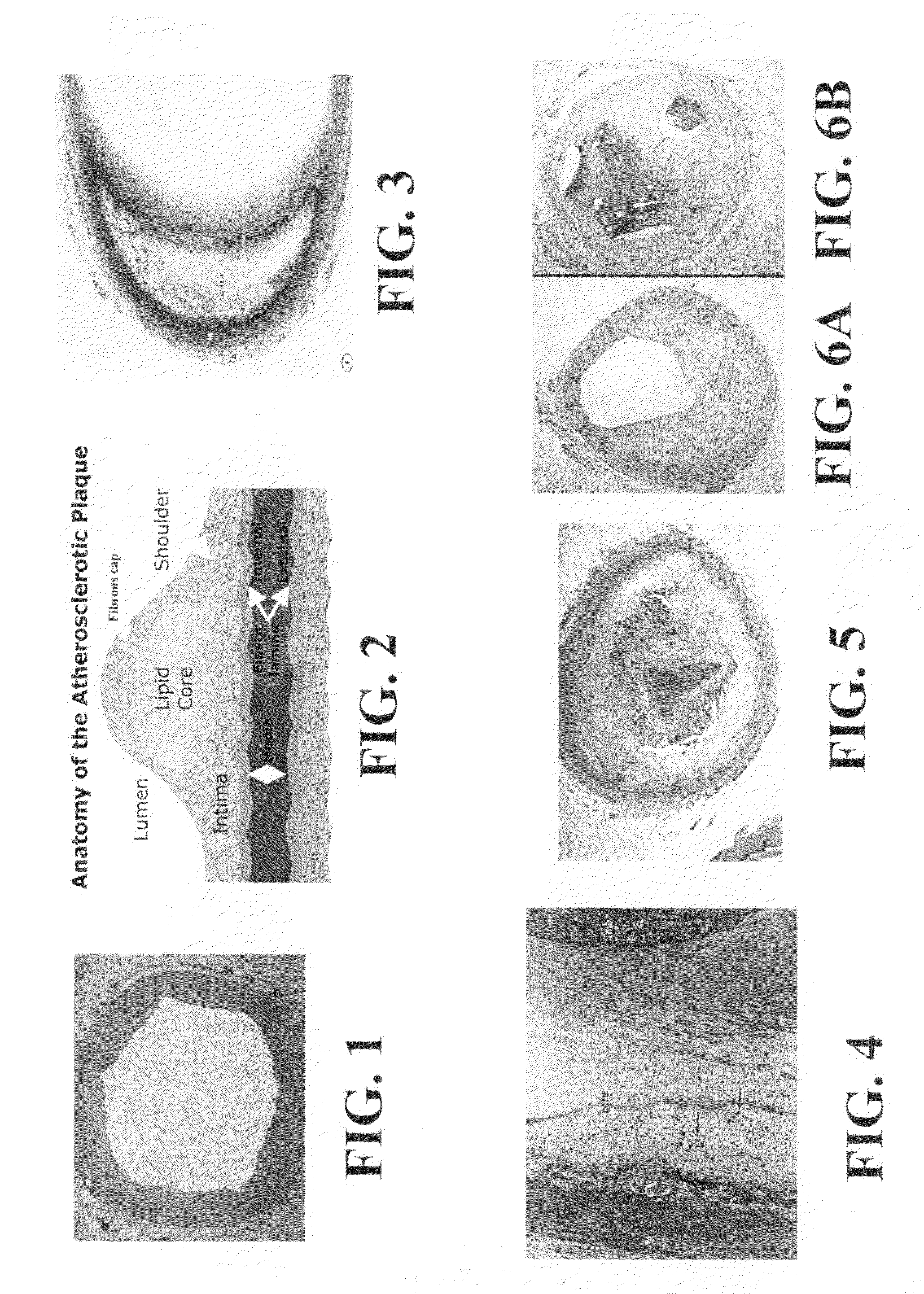
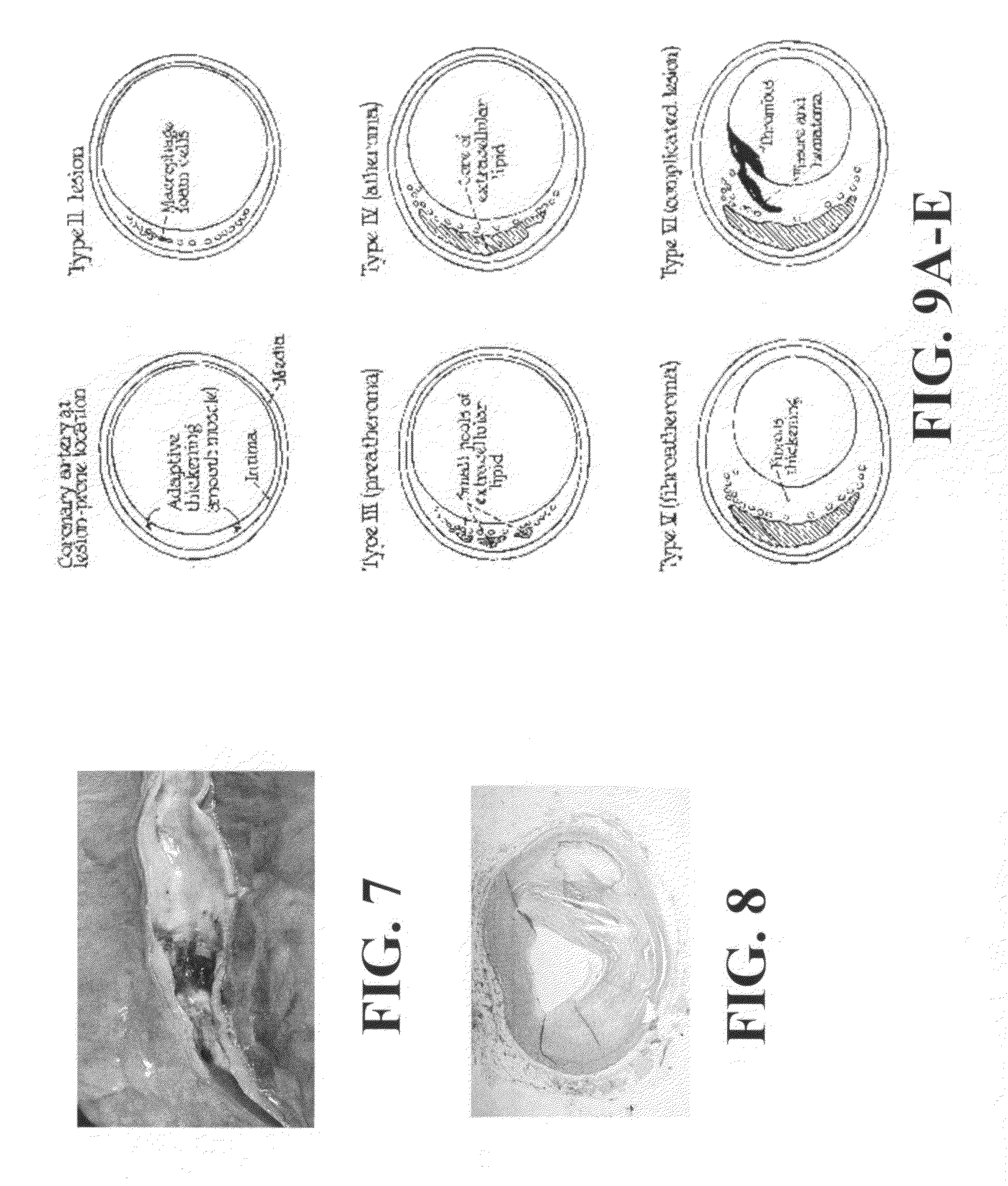
Scoring Method for Imaging-Based Detection of Vulnerable Patients
InactiveUS20100278405A1Enhance coronary risk assessmentImprove risk assessmentMedical simulationHealth-index calculationMedicineScore method
A new cardiac risk factors are disclosed along with method for deriving the components of the factors, for developing the factors and for using the factors. Methods for computing pericardial fat and abdominal fat are also disclosed as well as methods for motion compensation.
Owner:KAKADIARIS IOANNIS A +1
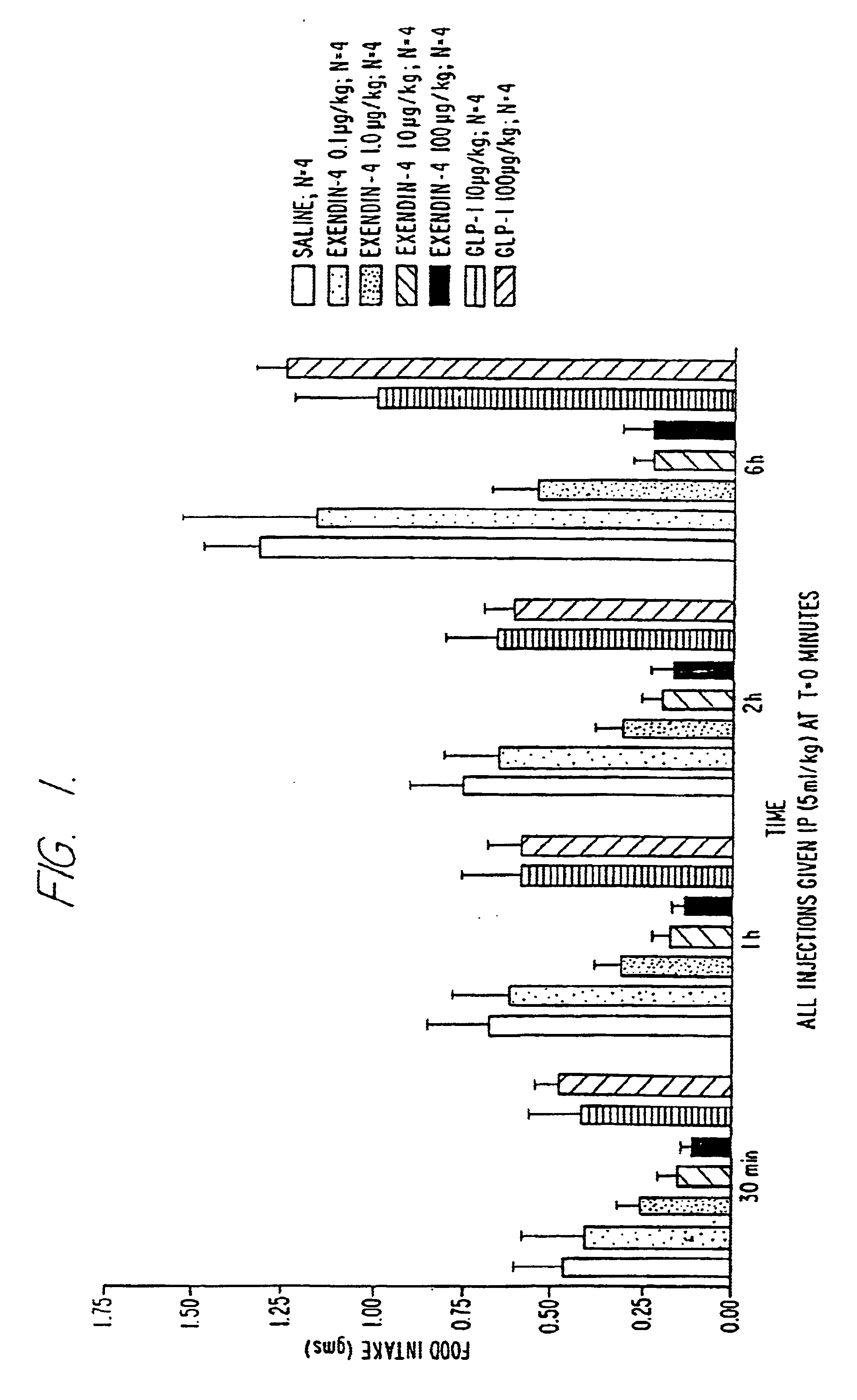
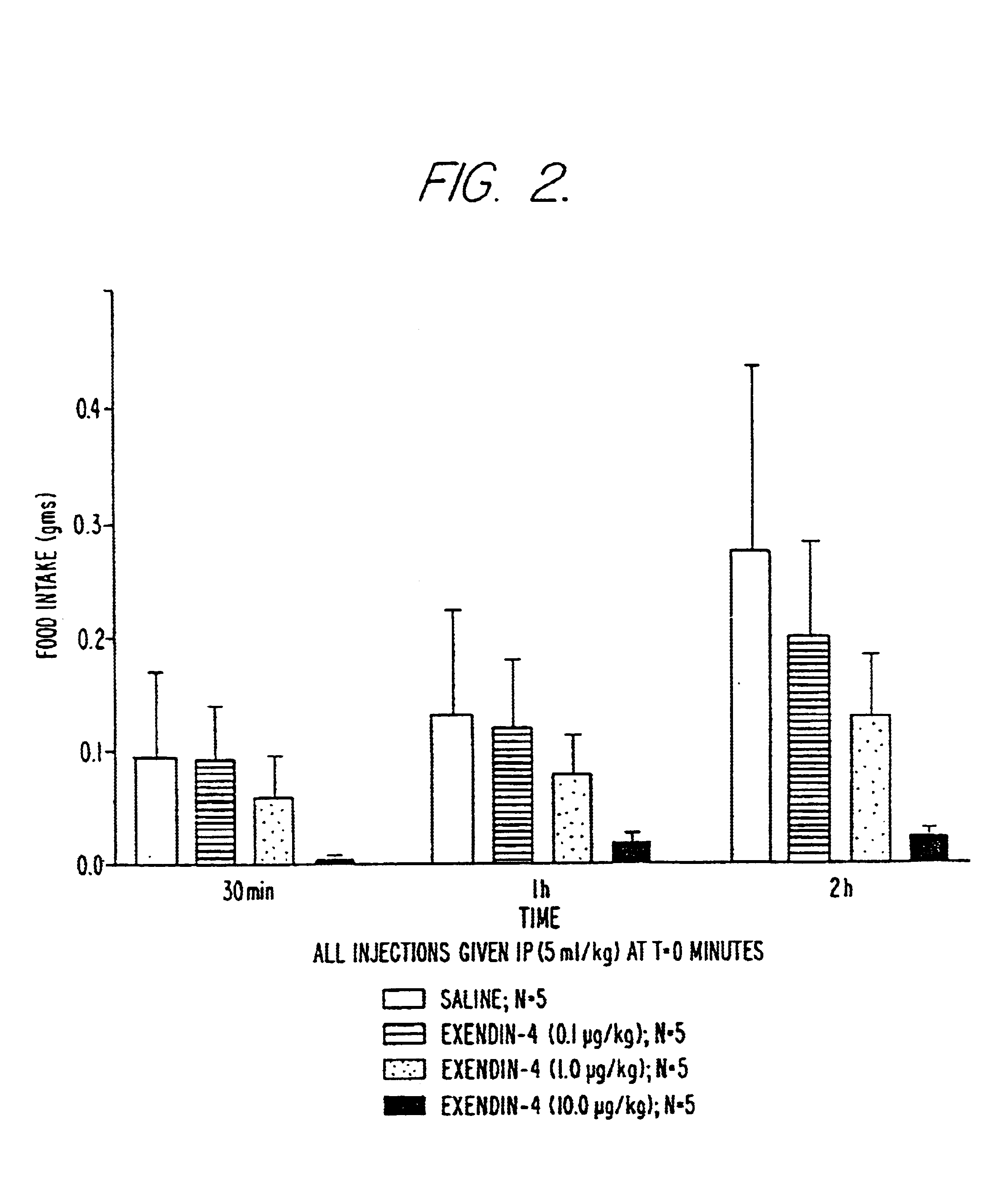
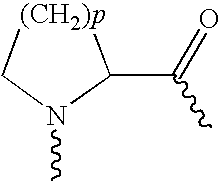
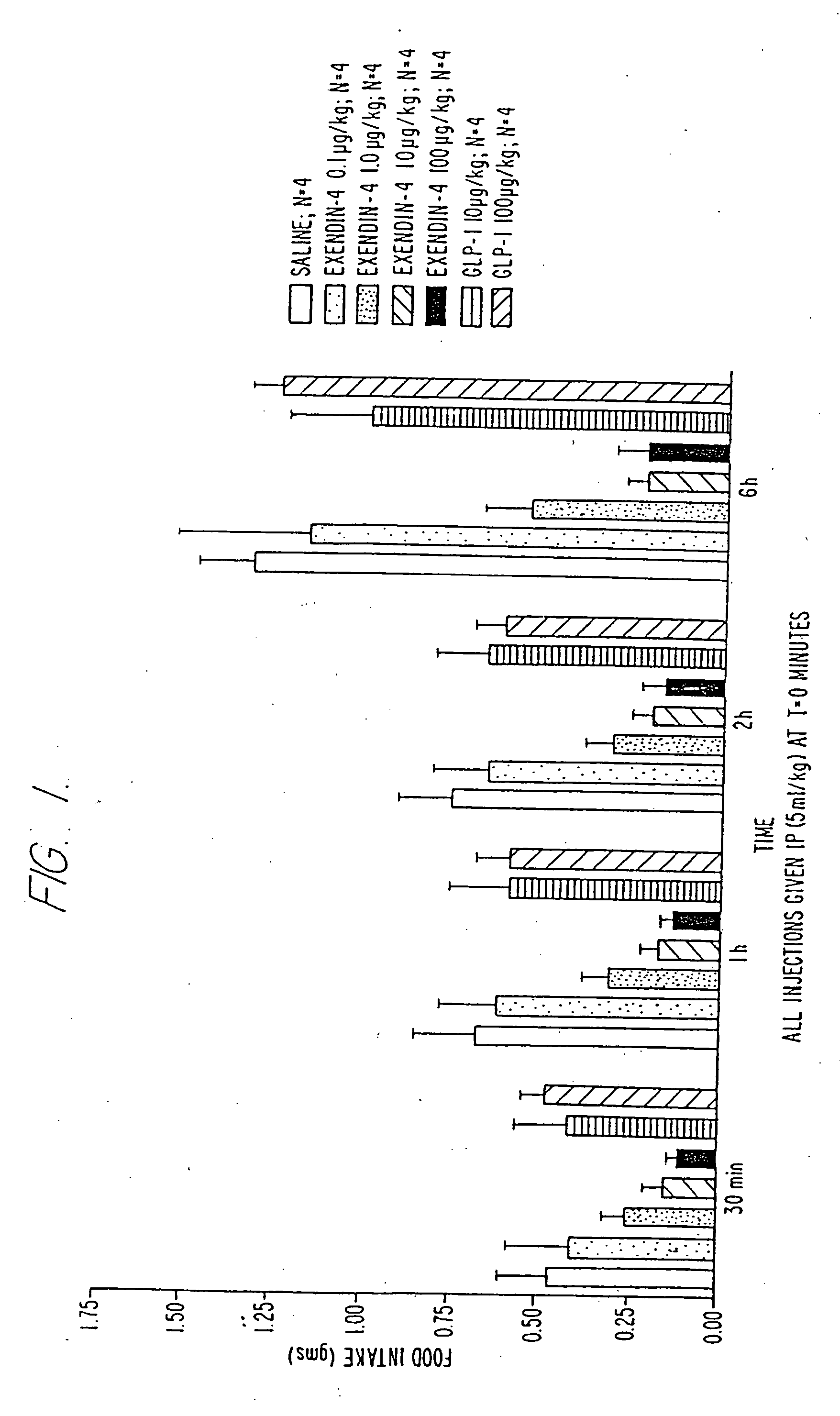
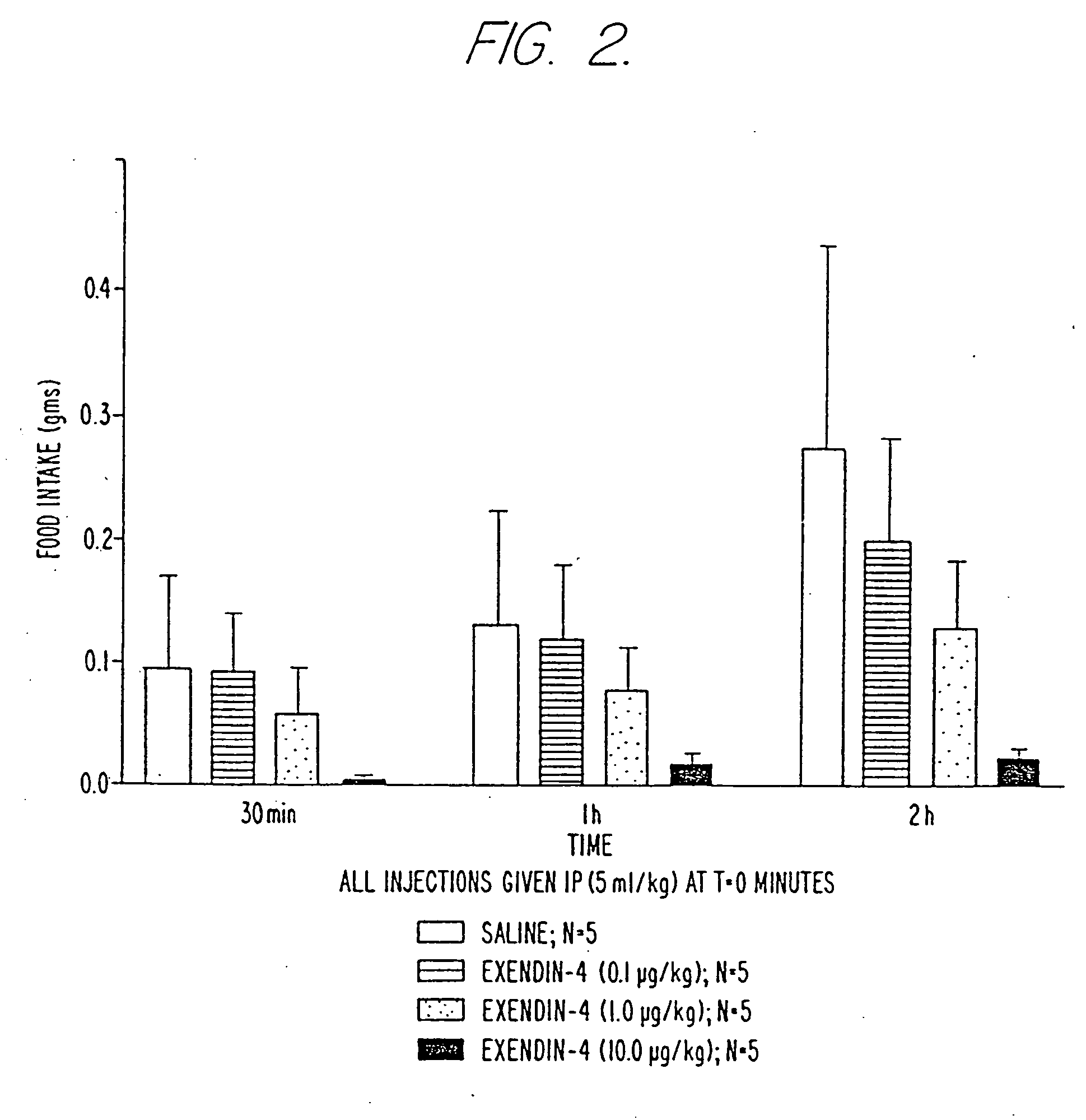
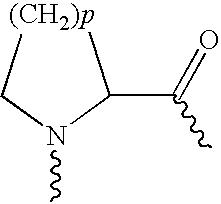
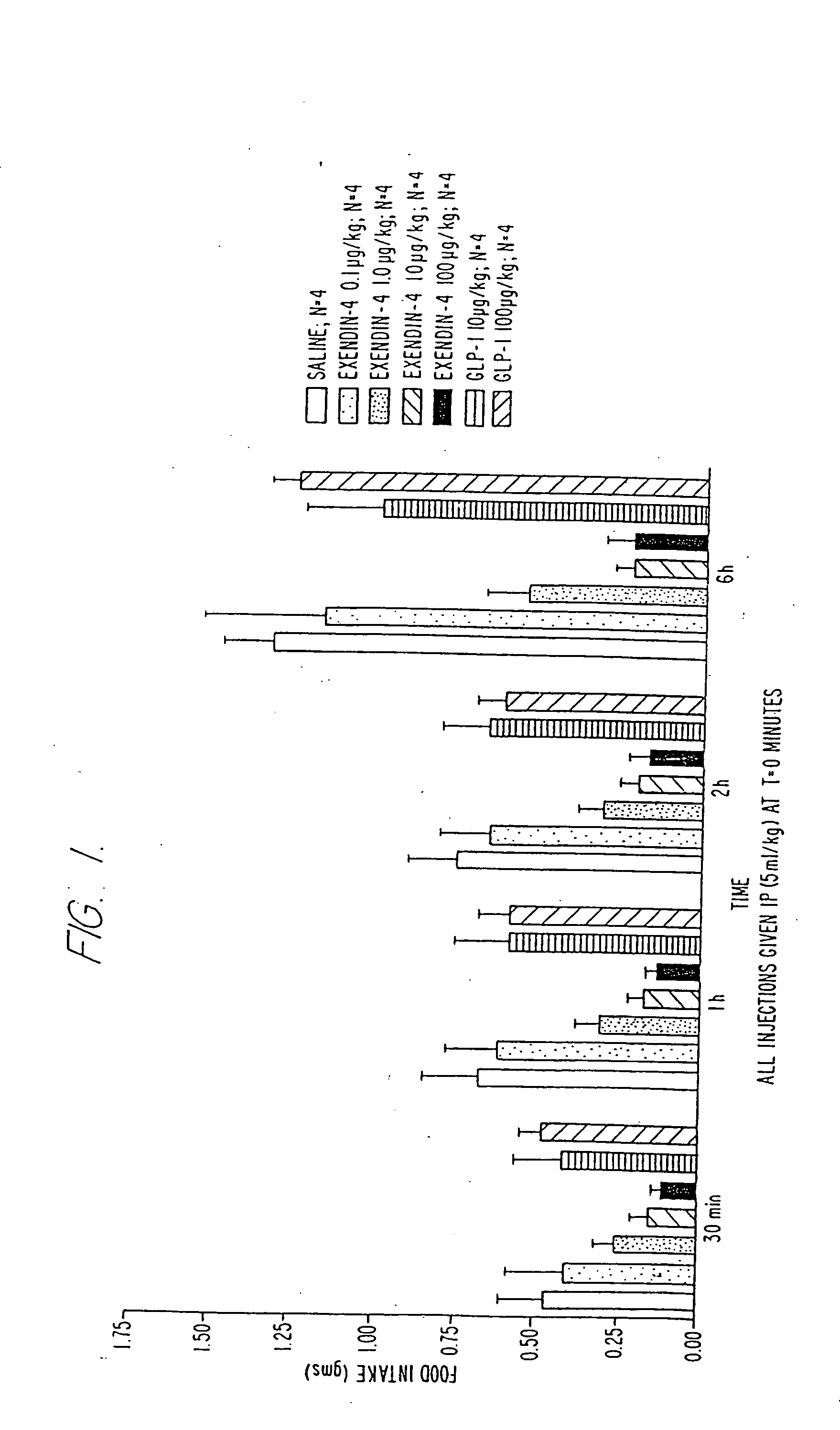
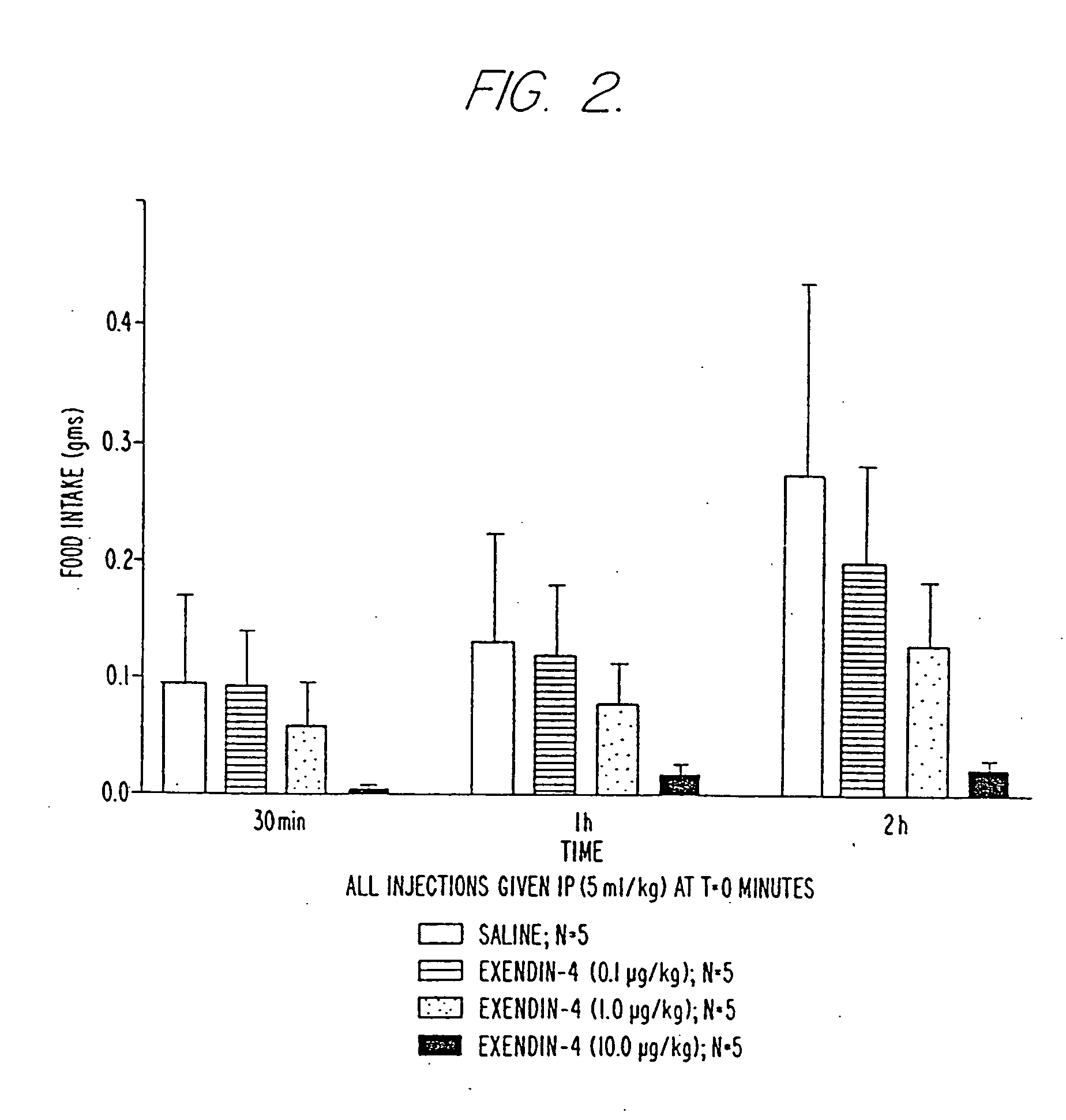
Use of exendins for the reduction of food intake
InactiveUS6956026B2Reduce appetiteReduce cardiac riskPeptide/protein ingredientsPharmaceutical delivery mechanismFeeding disabilityCvd risk
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an exendin or an exendin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. The methods are also useful for lowering the plasma glucose level, lowering the plasma lipid level, reducing the cardiac risk, reducing the appetite, and reducing the weight of subjects. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:ASTRAZENECA PHARMA LP
Methods of computing pericardial and abdominal fat and methods for motion compensation
ActiveUS20170337343A1Enhance coronary risk assessmentImprove risk assessmentMedical simulationHealth-index calculationPericardiumPhysical therapy
A new cardiac risk factors are disclosed along with method for deriving the components of the factors, for developing the factors and for using the factors. Methods for computing pericardial fat and abdominal fat are also disclosed as well as methods for motion compensation.
Owner:UNIV HOUSTON SYST
Cardiovascular pulse wave analysis method and system
InactiveUS20140249424A1Easy to useComputing time to provide the pulse analysis results is negligibleHealth-index calculationCatheterDiseaseFactor base
Factor retrieving is a major approach for pulse wave analysis. Stiffness index and cardiac output are widely used factors for cardiac risk detection. Research has been done on clinical pulse wave data which are collected by pulse oximeter. The result shows that collected factors have a positive correlation with certain cardiac risks. Some adjustments have been applied on the algorithms that increase the significance. In addition to the factor based analysis, other signal processing techniques for pulse waveforms are included such as bispectrum estimation, Wavelet transform, and weighted dynamic time warping. Bispectrum estimation and Wavelet transform have meaningful features of pulse waveforms with some special shapes. Weighted dynamic time warping compares the similarity of waveforms. It also includes medical significance into the calculation by adjusting the weight vector. This algorithm has higher accuracy when providing more samples to compare. The factor based analysis and waveform analysis compose an analytic model which can be used for risk evaluation, classification and disease detection.
Owner:UNIVERSITY OF WINNIPEG
Medical risk assessment method and program product
ActiveUS7306562B1Physical therapies and activitiesData processing applicationsGuidelineRisk classification
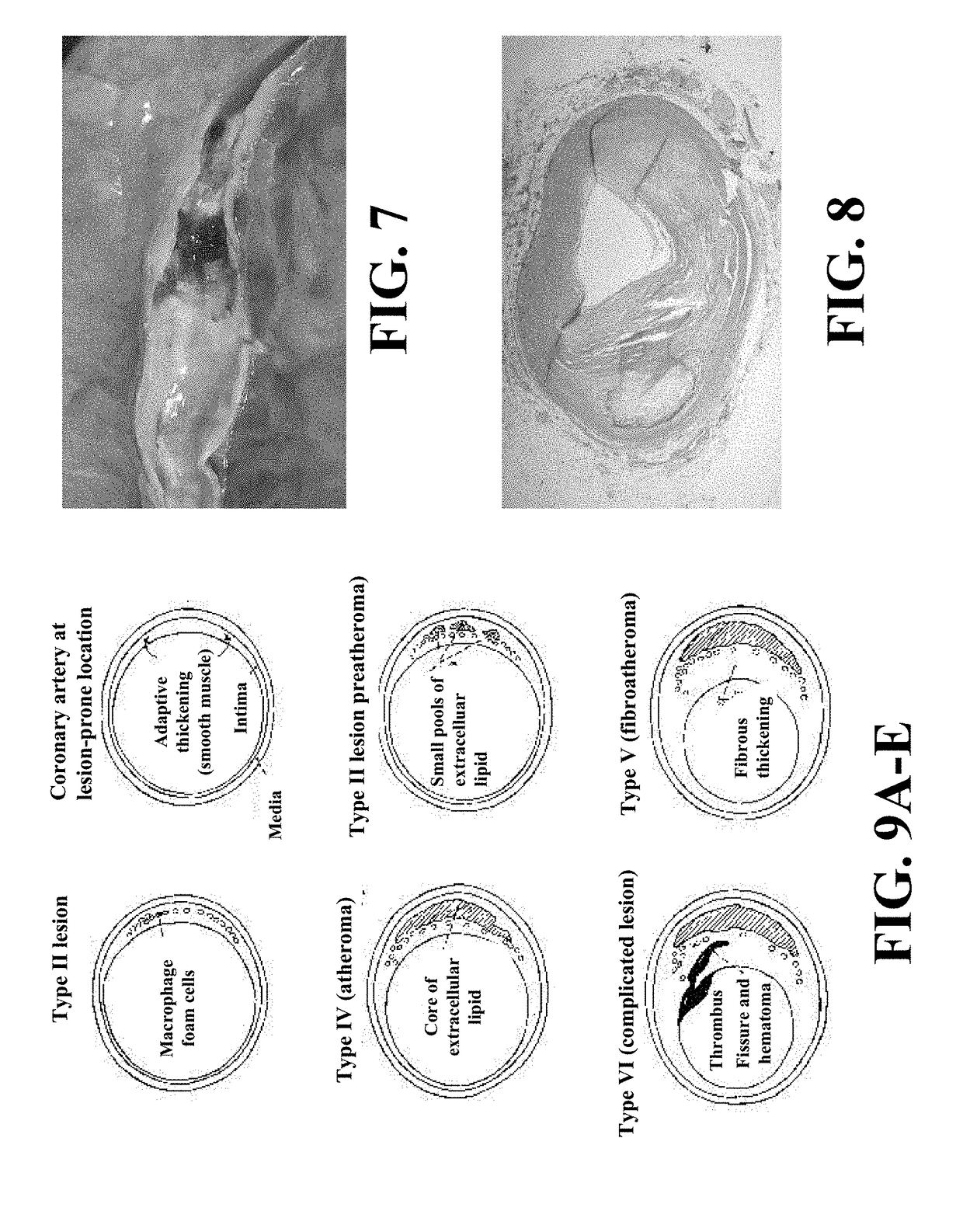
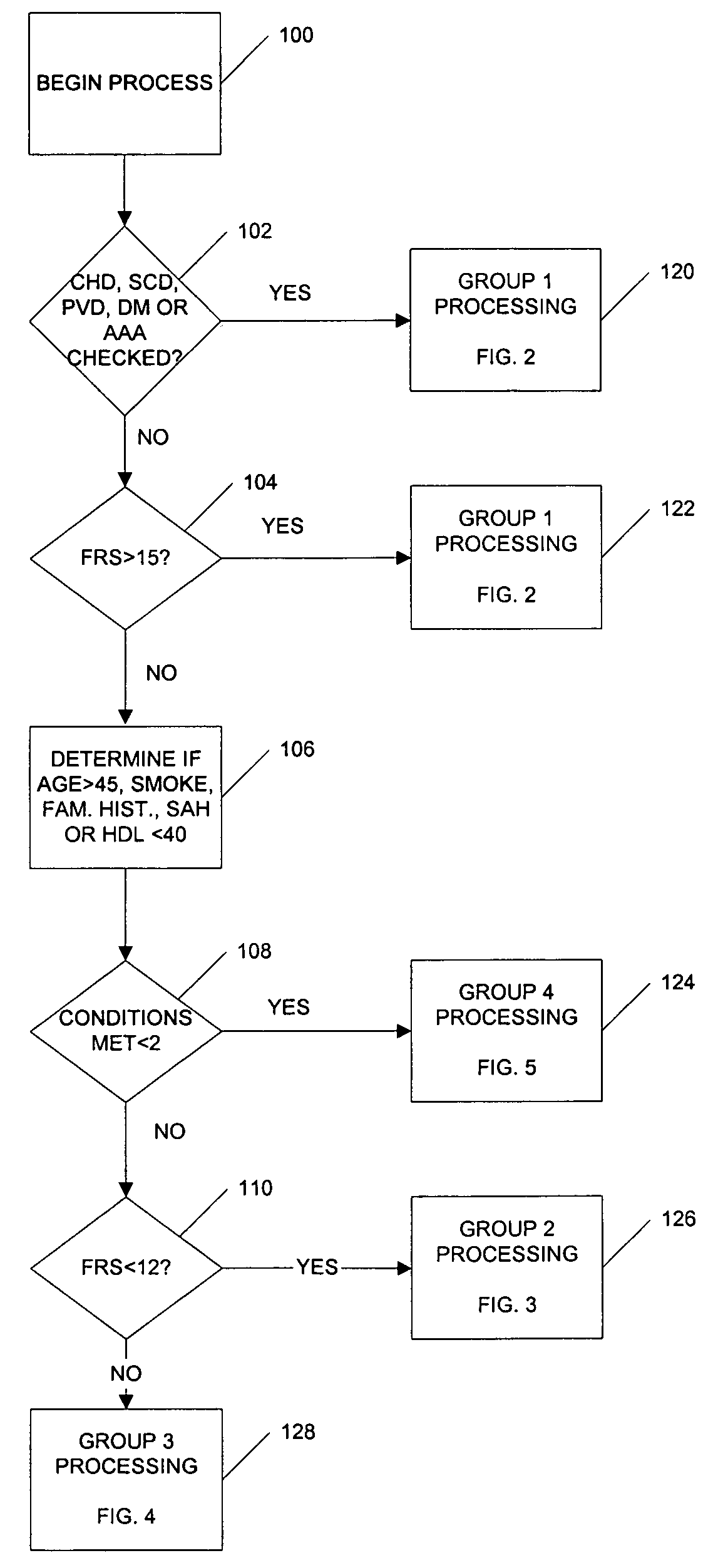
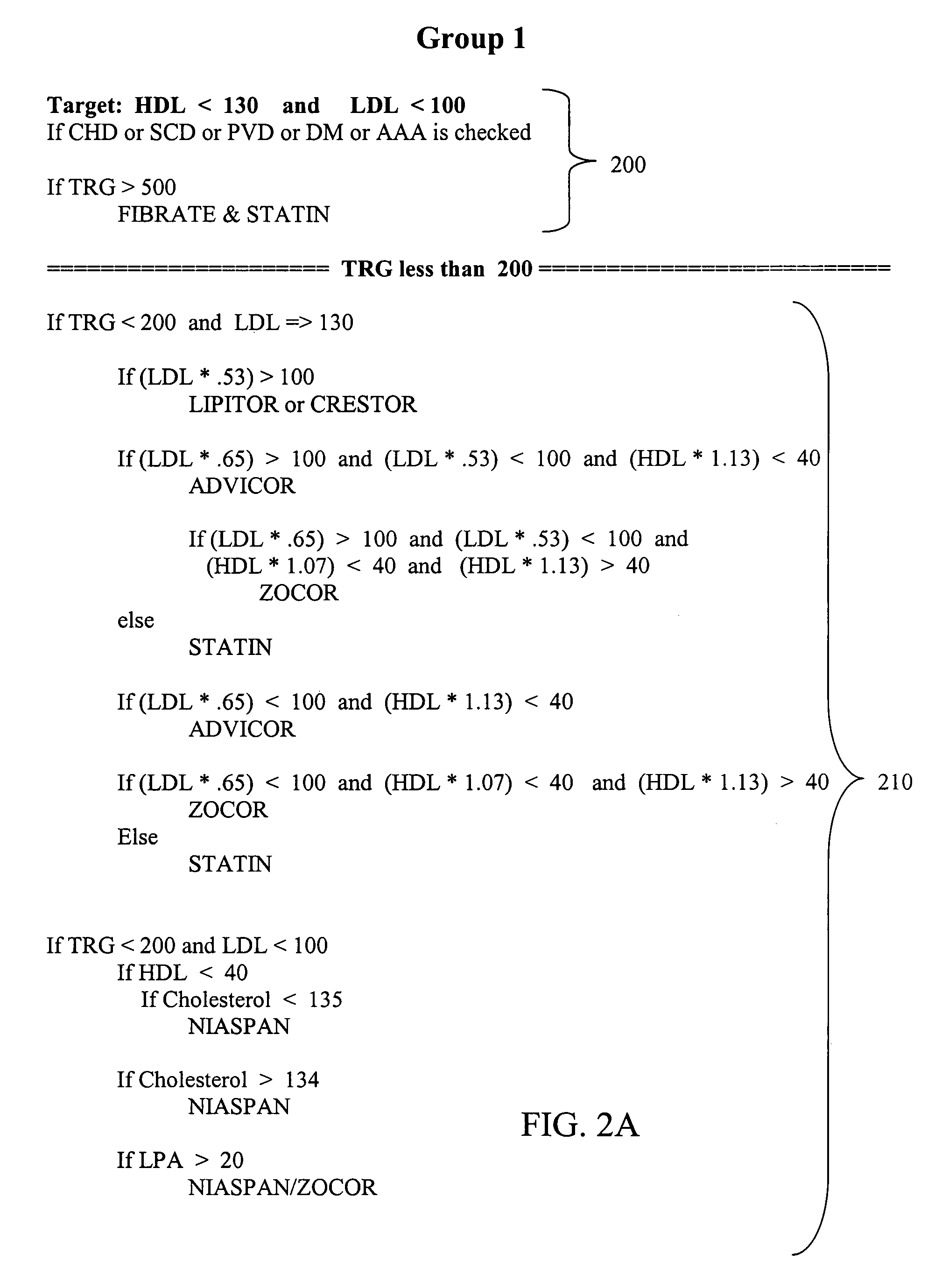
A medical risk assessment method and computer program product resident on a computer or a hand-held device that allows a physician to determine the best strategy for primary and secondary cardiovascular disease prevention utilizing current guidelines and published medical literature. The computer program product evaluates a number of risk factors to determine specific recommendations for an individual patient, including Framingham risk scoring (FRS), pertinent medical history, individual lipid panel and advanced lipoprotein profiling, patient laboratory test results, and published literature on the effects of anti-lipid medicines on plasma concentration and / or composition of lipoprotein molecules and clinical outcomes. The risk assessment method establishes a cardiovascular treatment therapy strategy for a patient by determining a cardiac risk classification group, determining a cardiovascular treatment therapy based on the patient's lipoprotein profile and the patient's cardiac group risk classification, and presenting the cardiovascular treatment therapy for the patient to a medical practitioner on a patient evaluation display.
Owner:MEDICAL SOFTWARE
Health monitoring and diagnostic device and network-based health assessment and medical records maintenance system
InactiveUS20100169123A1Low costReduce inconvenienceDigital data processing detailsAnalogue secracy/subscription systemsMedical recordEmergency medicine
A health monitoring and diagnostic device (LIFESTREAM cholesterol meter) configured as a self-contained testing and diagnostic unit in a clam-shell type case. One side of the case includes a spring-loaded finger stick and a compartment for carrying one or more packages of disposable items including a test strip, a needle for the finger stick, and an alcohol swipe. The other half of the case includes a test strip reader, a key pad, and a liquid crystal display. The meter reads a test strip carrying a droplet of blood and receives additional diagnostic information from the patient, such as age, gender, weight, and family history of heart disease. Within minutes, the meter displays test results, including total cholesterol levels. The meter also displays additional diagnostic results, such as the patient's “cardiac age,” recommended weight loss, and a cardiac risk assessment. The meter also works in connection with a network-based comprehensive health analysis and reporting system. The meter writes patient data to a smartcard. This patient data typically includes patient identification information, the test results, the diagnostic information, and the diagnostic results. A computer station reads the smartcard and establishes a network connection with a health report server over the Internet. The computer then downloads the patient data to the health report server, which prepares a comprehensive health report. Within minutes, this report is transmitted back to the computer station, where it is printed out and delivered to the patient.
Owner:POLYMER TECH SYST INC
Smartcard Accessed Secure Electronic Data Storage System
InactiveUS20090282192A1Low costReduce inconvenienceComplete banking machinesFinanceNetwork connectionThe Internet
A health monitoring and diagnostic device (LIFESTREAM cholesterol meter) configured as a self-contained testing and diagnostic unit in a clam-shell type case. One side of the case includes a spring-loaded finger stick and a compartment for carrying one or more packages of disposable items including a test strip, a needle for the finger stick, and an alcohol swipe. The other half of the case includes a test strip reader, a key pad, and a liquid crystal display. The meter reads a test strip carrying a droplet of blood and receives additional diagnostic information from the patient, such as age, gender, weight, and family history of heart disease. Within minutes, the meter displays test results, including total cholesterol levels. The meter also displays additional diagnostic results, such as the patient's “cardiac age,” recommended weight loss, and a cardiac risk assessment. The meter also works in connection with a network-based comprehensive health analysis and reporting system. The meter writes patient data to a smartcard. This patient data typically includes patient identification information, the test results, the diagnostic information, and the diagnostic results. A computer station reads the smartcard and establishes a network connection with a health report server over the Internet. The computer then downloads the patient data to the health report server, which prepares a comprehensive health report. Within minutes, this report is transmitted back to the computer station, where it is printed out and delivered to the patient.
Owner:ORANGEHOOK INC
Pharmaceutical compositions containing exendins
InactiveUS20050215469A1Reduce appetiteReduce cardiac riskPeptide/protein ingredientsMetabolism disorderBlood plasmaInsulin resistance
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an exendin or an exendin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. The methods are also useful for lowering the plasma glucose level, lowering the plasma lipid level, reducing the cardiac risk, reducing the appetite, and reducing the weight of subjects. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:ASTRAZENECA PHARMA LP
Use of exendins and agonists thereof for lowering plasma lipid
InactiveUS20050101537A1Reduce appetiteReduce cardiac riskPeptide/protein ingredientsMetabolism disorderBlood plasmaInsulin resistance
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an exendin or an exendin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. The methods are also useful for lowering the plasma glucose level, lowering the plasma lipid level, reducing the cardiac risk, reducing the appetite, and reducing the weight of subjects. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:AMYLIN PHARMA INC
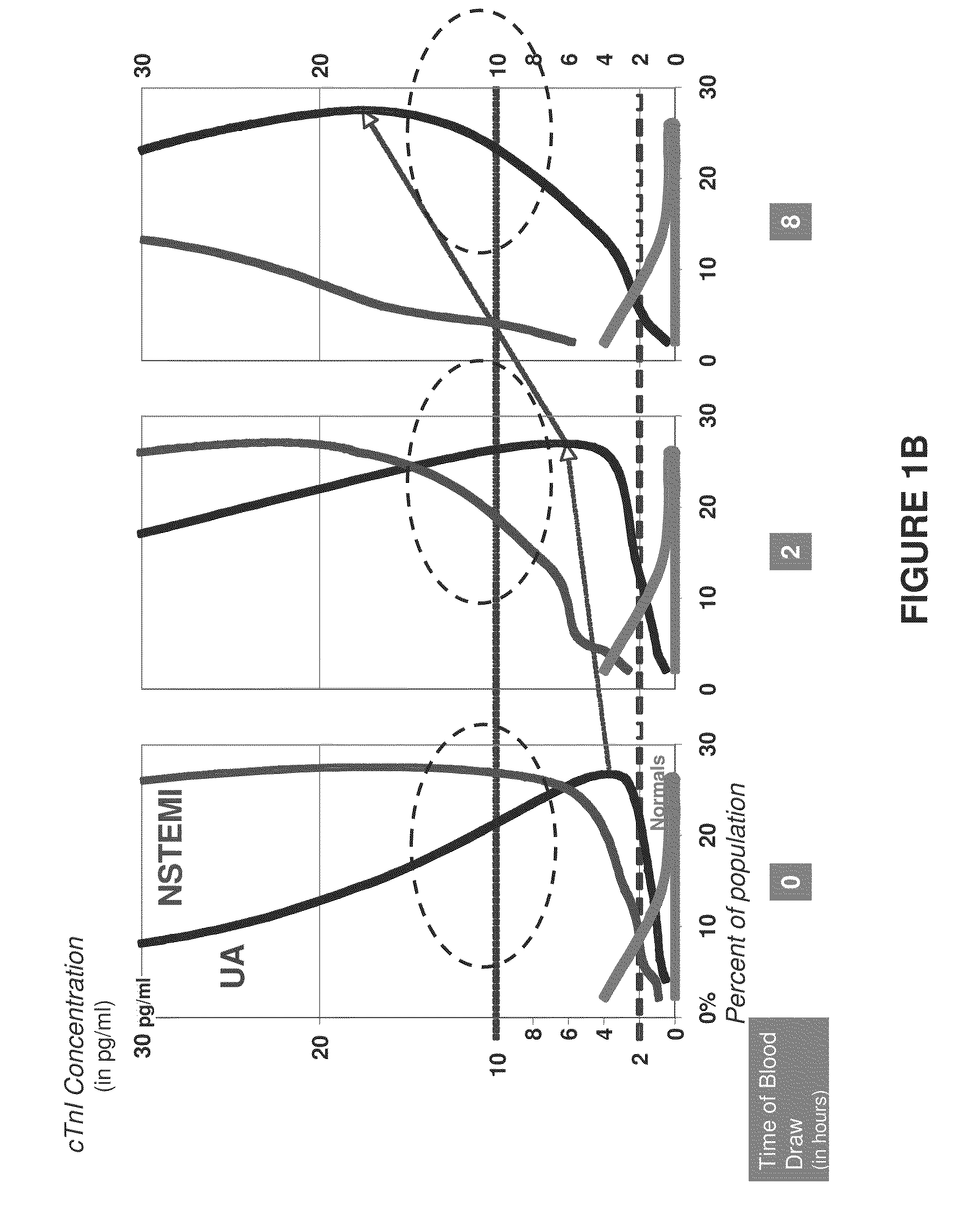
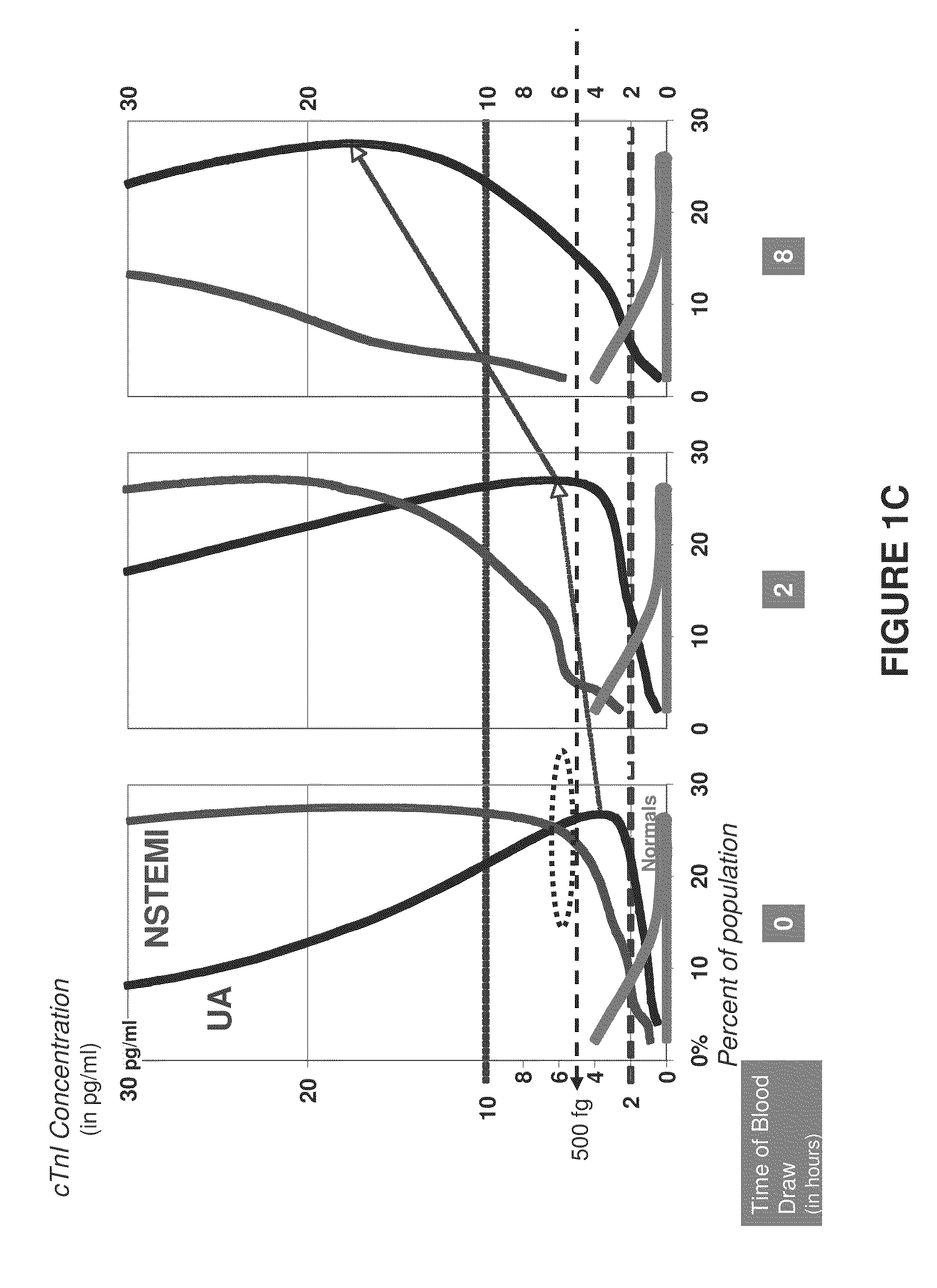
Methods and assays to assess cardiac risk and ischemia
InactiveUS20090233312A1Easy to detectImprove the level ofDisease diagnosisBiological testingPhysiological fluidIschemia
The invention provides methods and apparatus to assess cardiac risk and ischemia by detecting or analyzing cardiac troponin levels. Also provided are methods to detect low levels of cardiac troponin in physiological fluid samples.
Owner:NANOSPHERE INC
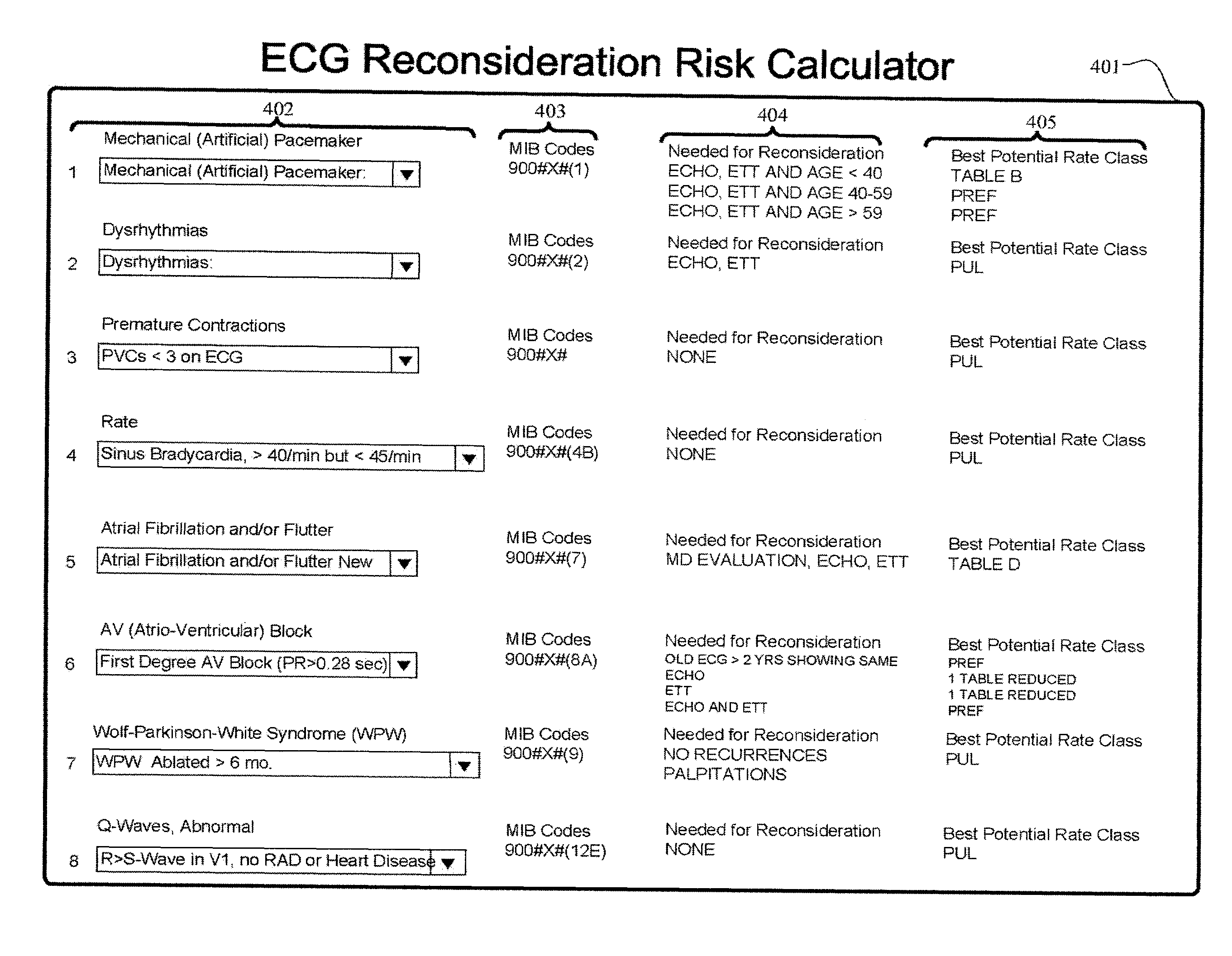
Systems and methods of automating reconsideration of cardiac risk
Systems, methods, and computer-readable media to automate reconsideration of a cardiac risk category associated with issuance of a life insurance policy may provide an indication of a best potential risk category or rate class that would be available if at least one action is completed by a life insurance applicant.
Owner:USAA
Combination therapy comprising amlodipine and a statin compound
InactiveCN1268054AReduce riskMetabolism disorderPharmaceutical delivery mechanismCombined Modality TherapySecondary hyperlipidemia
This invention relates to pharmaceutical combinations of amlodipine or a pharmaceutically acceptable acid addition salt thereof and statins or pharmaceutically acceptable salts thereof, kits containing such combinations and methods of using such combinations to treat subjects suffering from angina pectoris, atherosclerosis, combined hypertension and hyperlipidemia and to treat subjects presenting with symptoms of cardiac risk, including humans. This invention also relates to additive and synergistic combinations of amlodipine or a pharmaceutically acceptable acid addition salt thereof and statins or pharmaceutically acceptable salts thereof whereby those additive and synergistic combinations are useful in treating subjects suffering from angina pectoris, atherosclerosis, combined hypertension and hyperlipidemia and those subjects presenting with symptoms of cardiac risk, including humans.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
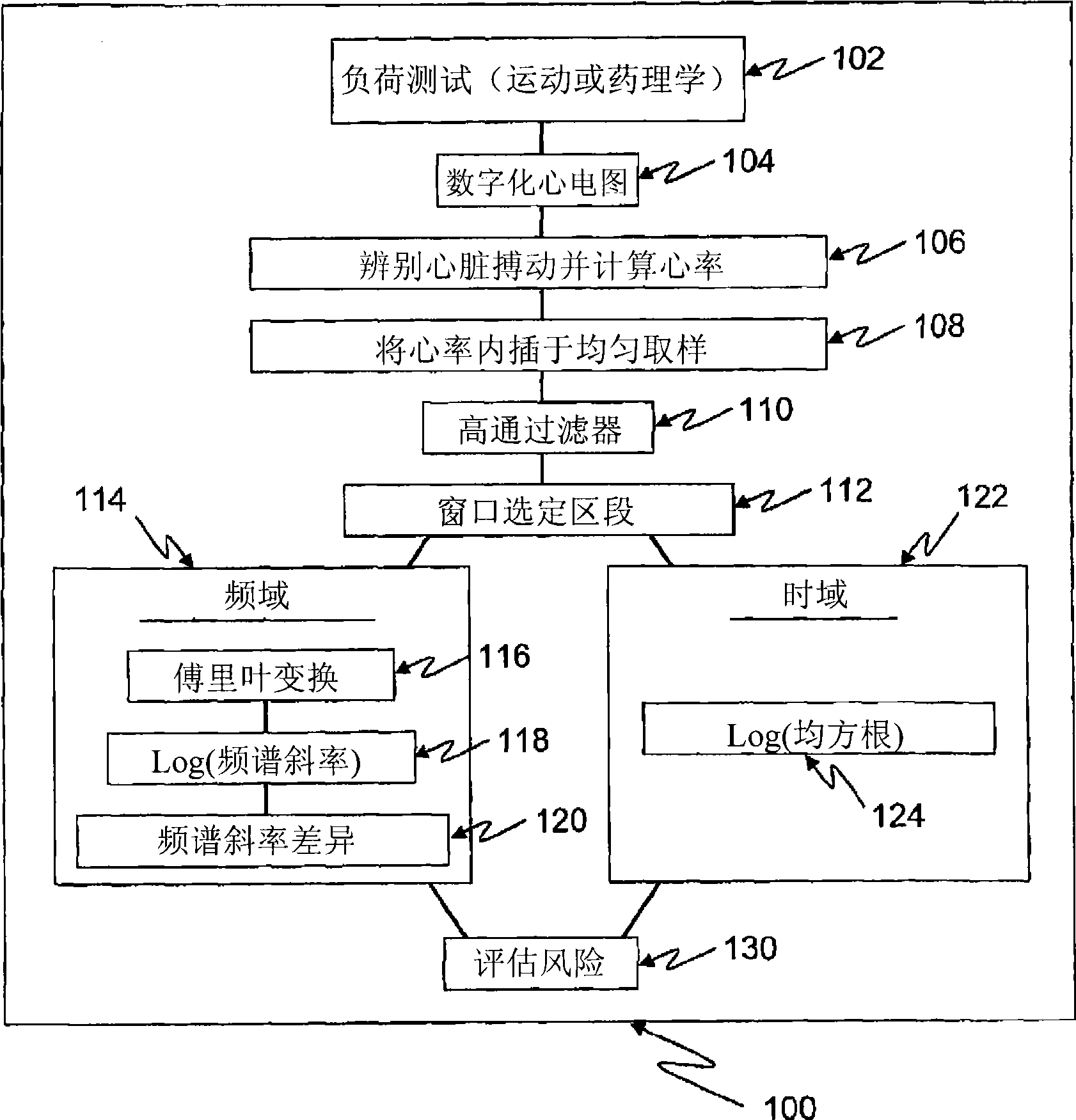
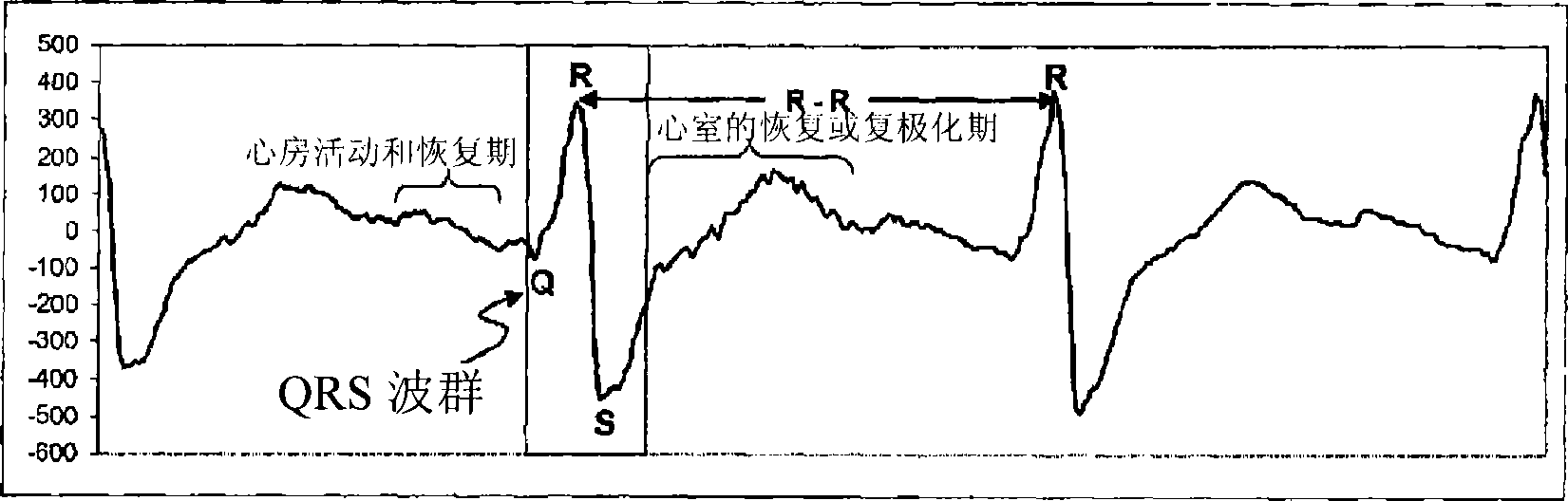
Methods for quantifying the risk of cardiac death using exercise induced heart rate variability metrics
Methods and apparatus for assessing cardiac risks in a specific patient. One embodiment of a method in accordance with the invention comprises providing heart rate activity of a specific patient including a windowed time series relating to heart rate variability during a heart rate test. This method further includes determining a frequency domain value based on energy values of frequency bands of the heart rate variability in the windowed time series, and / or determining an aggregate power for a frequency band of the windowed time series. This method further includes assessing the risk of a cardiac event based on the frequency value and / or the aggregate power.
Owner:CARDIAC SCIENCE CORP
Noncardiotoxic pharmaceutical compounds
The present invention relates to novel noncardiotoxic compounds and pharmaceutical compositions useful in the treatment of a variety of disorders including the treatment of depression, allergies, psychoses, cancer and gastrointestinal disorders. In particular, the present invention describes pharmaceutical compositions that mitigate life-threatening arrhythmias such as torsade de pointes. Torsade de pointes is a particular cardiac problem associated with many therapeutic agents and has been implicated as a possible cause of sudden death, particularly in those individuals with a past history of disturbances of cardiac rhythm, myocardial infarction, congenital repolarization abnormalities and cardiac risk factors such as hyperlipidemia and age. This arrhythmia is a variant of paroxysmal ventricular tachycardia associated with a prolonged QTc interval or prominent U waves on the ECG. Torsade de pointes is potentially lethal because it can progress to ventricular fibrillation, life-threatening arrhythmias or precipitate sudden death.
Owner:B& G PARTNERS
Method and system for the detection of cardiac risk factors
ActiveUS8257967B2Quick checkMaterial nanotechnologyBioreactor/fermenter combinationsSensor arrayMulti analyte
A system for the rapid characterization of multi-cardiovascular risk factor analyte fluids, in one embodiment, includes a light source, a sensor array, and a detector. The sensor array is formed from a supporting member, in which a plurality of cavities may be formed. A series of chemically sensitive particles, in one embodiment, are positioned within the cavities. The particles may produce a signal when a receptor, coupled to the particle, interacts with the cardiovascular risk factor analyte and the particle-analyte complex is visualized using a visualization reagent. Using pattern recognition techniques, the analytes within a multi-analyte fluid may be characterized. In an embodiment, each cavity of the plurality of cavities is designed to capture and contain a specific size particle. Flexible projections may be positioned over each of the cavities to provide retention of the particles in the cavities.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Patient risk evaluation
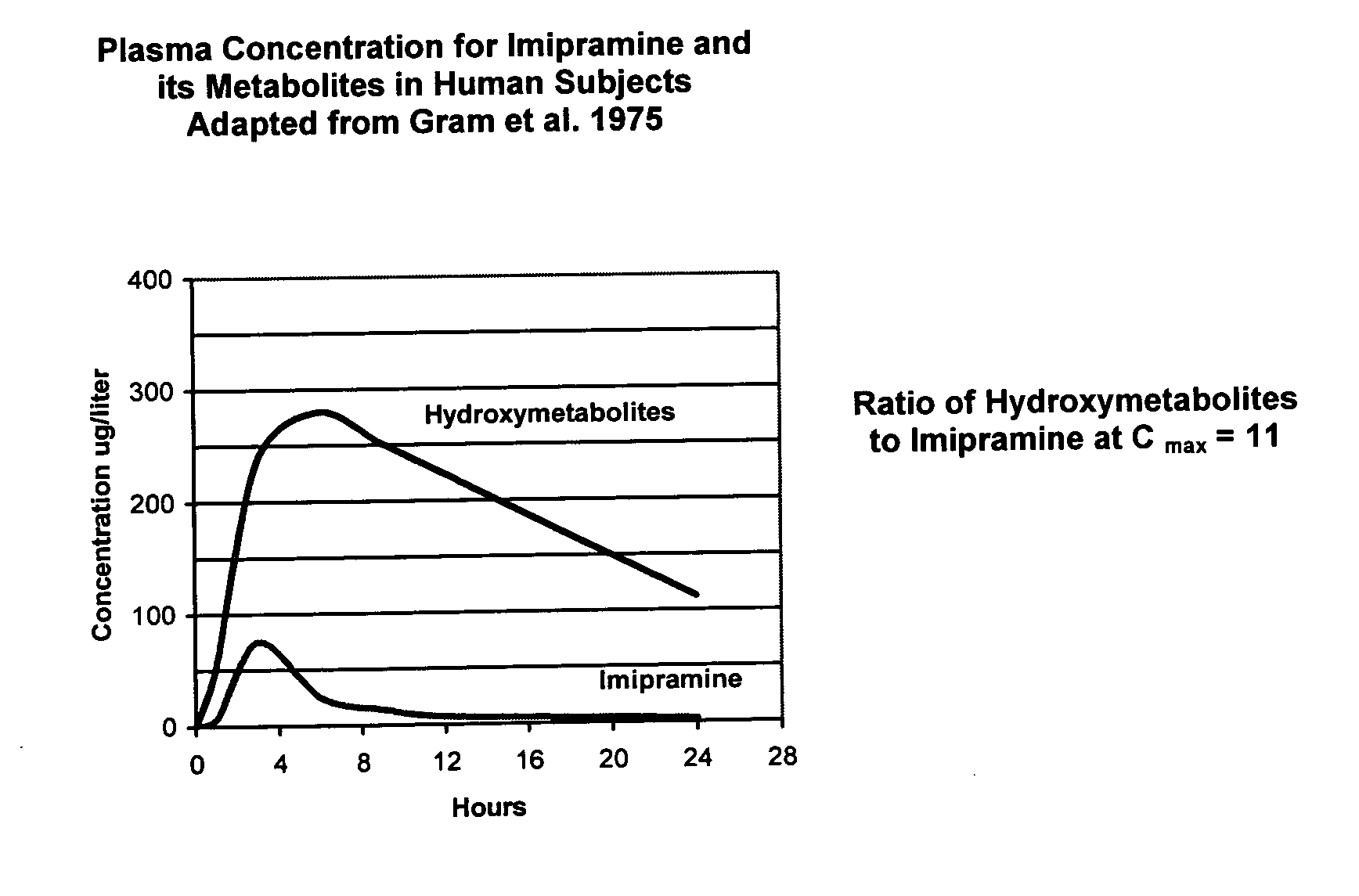
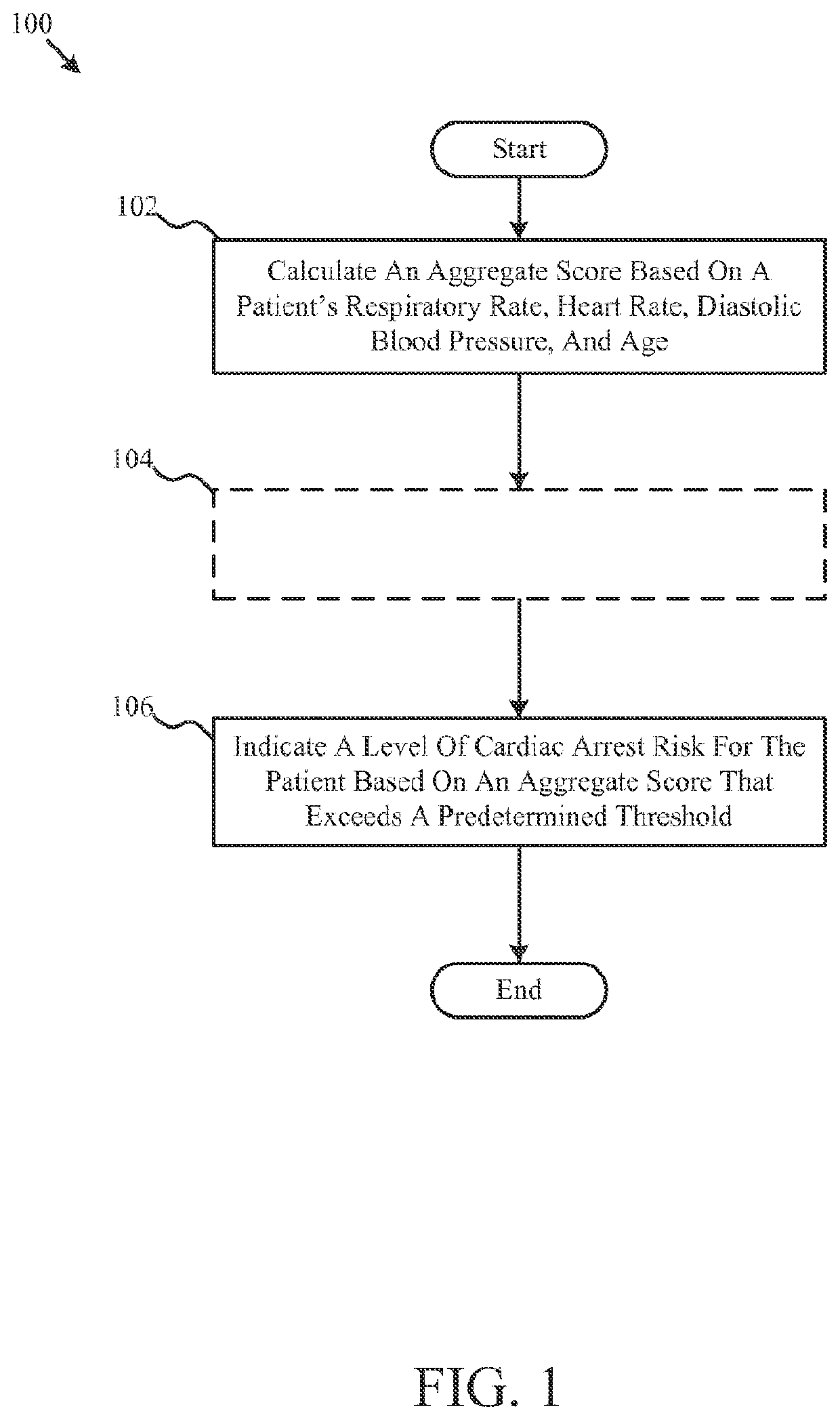
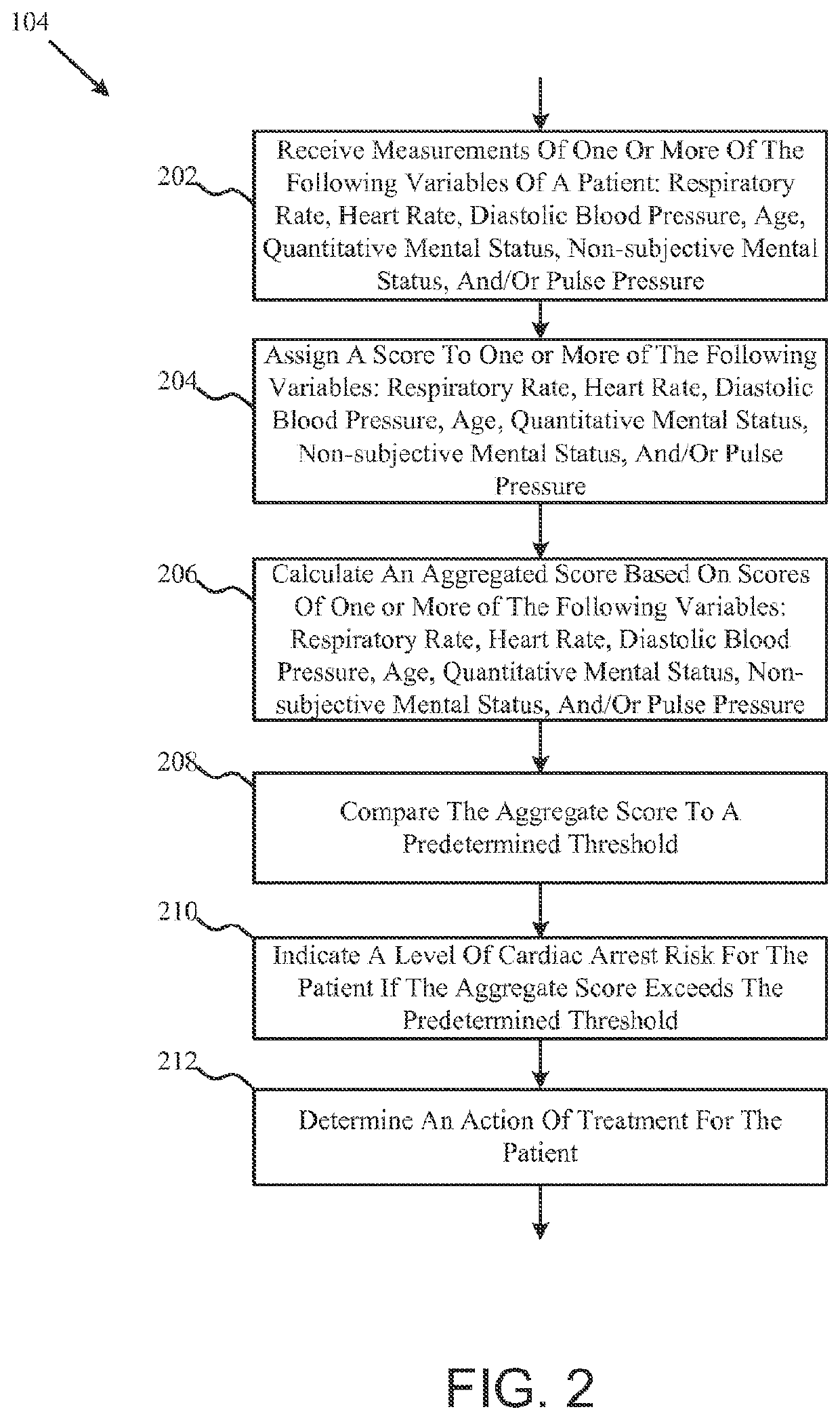
ActiveUS11410777B2Facilitates efficient allocation of resourceHealth-index calculationEvaluation of blood vesselsBreath rateEmergency medicine
This disclosure includes various embodiments of apparatuses, systems and methods for evaluating a patient's cardiac risk and / or mental status. Cardiac risk evaluation may be based (e.g., only) on the patient's respiratory rate, heart rate, diastolic blood pressure, age, and / or mental status. An aggregate score, which is indicative likelihood of the patient's cardiac risk, may be calculated based (e.g., only) on the patient's respiratory rate, heart rate, diastolic blood pressure, age, and / or mental status. If the calculated aggregate score exceeds a predetermined threshold, the patient may be identified as having a critical cardiac risk, and actions may be taken to treat the patient. The cardiac risk evaluation may be based further on the patient's mental status, where the patient's mental status may be evaluated based on a game with visual indicators.
Owner:UNIVERSITY OF CHICAGO
Therapeutic combination
InactiveUS20060223865A1Reduce riskShorten the progressBiocidePharmaceutical delivery mechanismSecondary hyperlipidemiaAngina
This invention relates to pharmaceutical combinations of amlodipine or a pharmaceutically acceptable acid addition salt thereof and atorvastatin or a pharmaceutically acceptable salt thereof, kits containing such combinations and methods of using such combinations to treat subjects suffering from angina pectoris, atherosclerosis, combined hypertension and hyperlipidemia and to treat subjects presenting with symptoms of cardiac risk, including humans. This invention also relates to additive and synergistic combinations of amlodipine and atorvastatin whereby those synergistic combinations are useful in treating subjects suffering from angina pectoris, atherosclerosis, combined hypertension and hyperlipidemia and those subjects presenting with symptoms of cardiac risk, including humans.
Owner:BUCH JAN +1
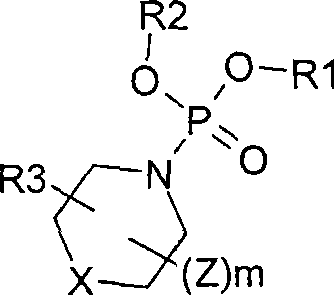
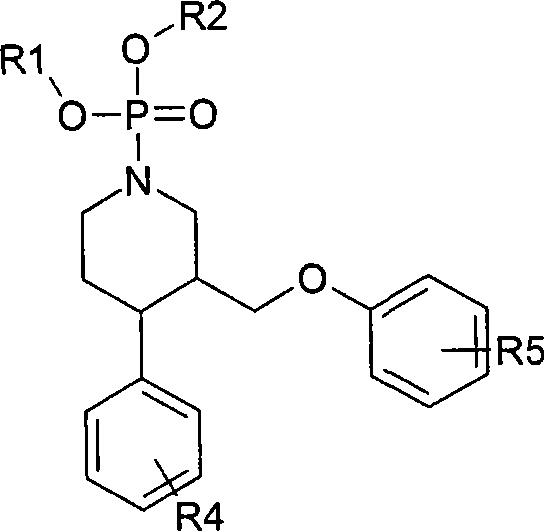
Cardiac risk stratification by nos1ap genotyping
InactiveUS20110177503A1High riskElectrocardiographyMicrobiological testing/measurementGenotypeTagging SNP
A risk-conferring genetic modifier is found in a large LQTS cohort. A NOS1AP tag SNP genotype provides an additional clinical assessment, which helps assess risk and choice of therapeutic strategies in LQTS as well as other conditions such as Brugada Syndrome, and catecholaminergic polymorphic ventricular tachycardia (CPTV).
Owner:FOND SALVATORE MAUGERI
Pharmaceutical compositions of amlodipine and atorvastatin
InactiveUS20050107446A1Lower levelLow level of degradation product and impurityBiocideMetabolism disorderSecondary hyperlipidemiaAmlodipine
A pharmaceutical composition comprising two components: (a) one component comprising a granulation of atorvastatin or pharmaceutically acceptable salts thereof and a carrier including an alkalizing agent that forms a pH greater than 5; and (b) a second component comprising amlodipine or pharmaceutically acceptable salts thereof and a carrier excluding an alkalizing agent that forms a pH greater than 5, wherein the two components are combined to form a final composition for a solid dosage form is described as well as methods to prepare the compositions, kits for containing such compositions, and a method of treating angina pectoris, atherosclerosis, combined hypertension and hyperlipidemia and / or hypercholesterolemia, and symptoms of cardiac risk using a therapeutically effective amount of the pharmaceutical composition.
Owner:ALANI LAMAN +3
Noncardiotoxic pharmaceutical compounds
The present invention relates to novel noncardiotoxic compounds and pharmaceutical compositions useful in the treatment of a variety of disorders including the treatment of depression, allergies, psychoses, cancer and gastrointestinal disorders. In particular, the present invention describes pharmaceutical compositions that mitigate life-threatening arrhythmias such as torsade de pointes. Torsade de pointes is a particular cardiac problem associated with many therapeutic agents and has been implicated as a possible cause of sudden death, particularly in those individuals with a past history of disturbances of cardiac rhythm, myocardial infarction, congenital repolarization abnormalities and cardiac risk factors such as hyperlipidemia and age. This arrhythmia is a variant of paroxysmal ventricular tachycardia associated with a prolonged QTc interval or prominent U waves on the ECG. Torsade de pointes is potentially lethal because it can progress to ventricular fibrillation, life-threatening arrhythmias or precipitate sudden death.
Owner:B& G PARTNERS
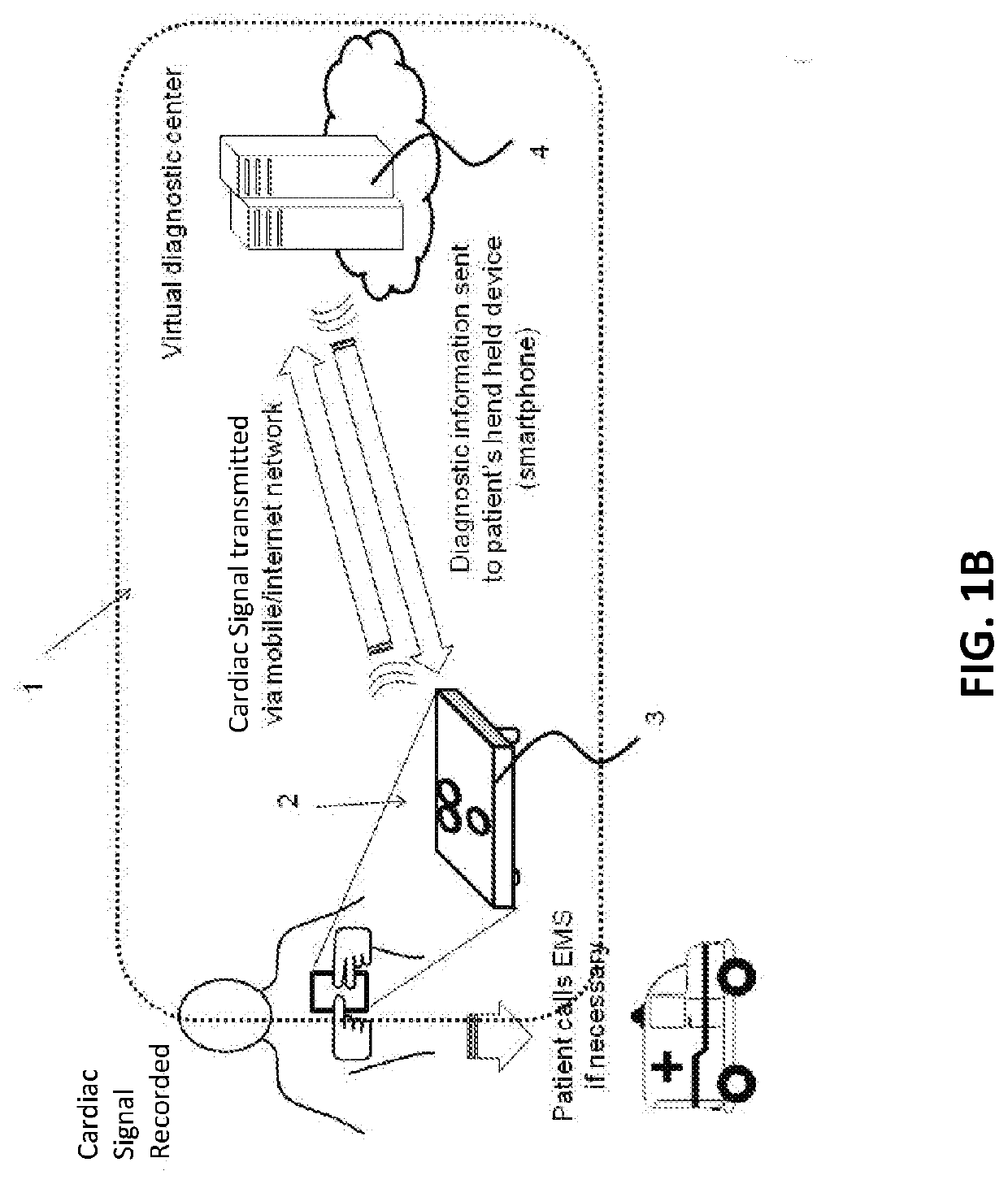
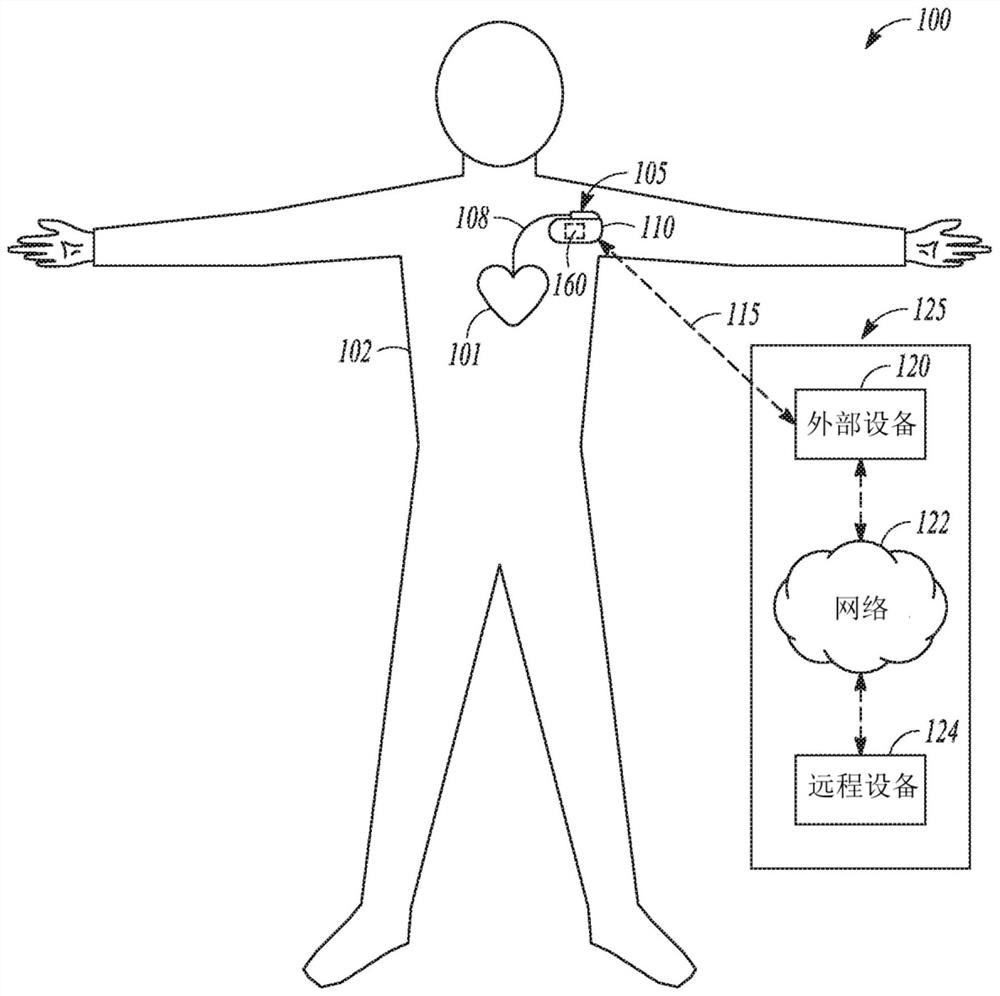
Hand held device for automatic cardiac risk and diagnostic assessment
ActiveUS20220015679A1Easy to useStored dataHealth-index calculationDiagnostic recording/measuringHand heldEmergency medicine
Method and apparatus for performing automatic cardiac diagnosis. The apparatuses described herein may be handheld devices which enables self-recording of cardiac signals by the patient, including entering relevant data by patients regarding their cardiac history, including cardiac disease risk factors, and / or current conditions and symptoms. Based on recorded cardiac signals, cardiac risk factors and the current symptoms, the apparatus may calculates a cardiac risk score and may provide simplified diagnostic information and actionable instructions to the patient.
Owner:HEARTBEAM INC
Method for risk stratification in stable coronary artery disease
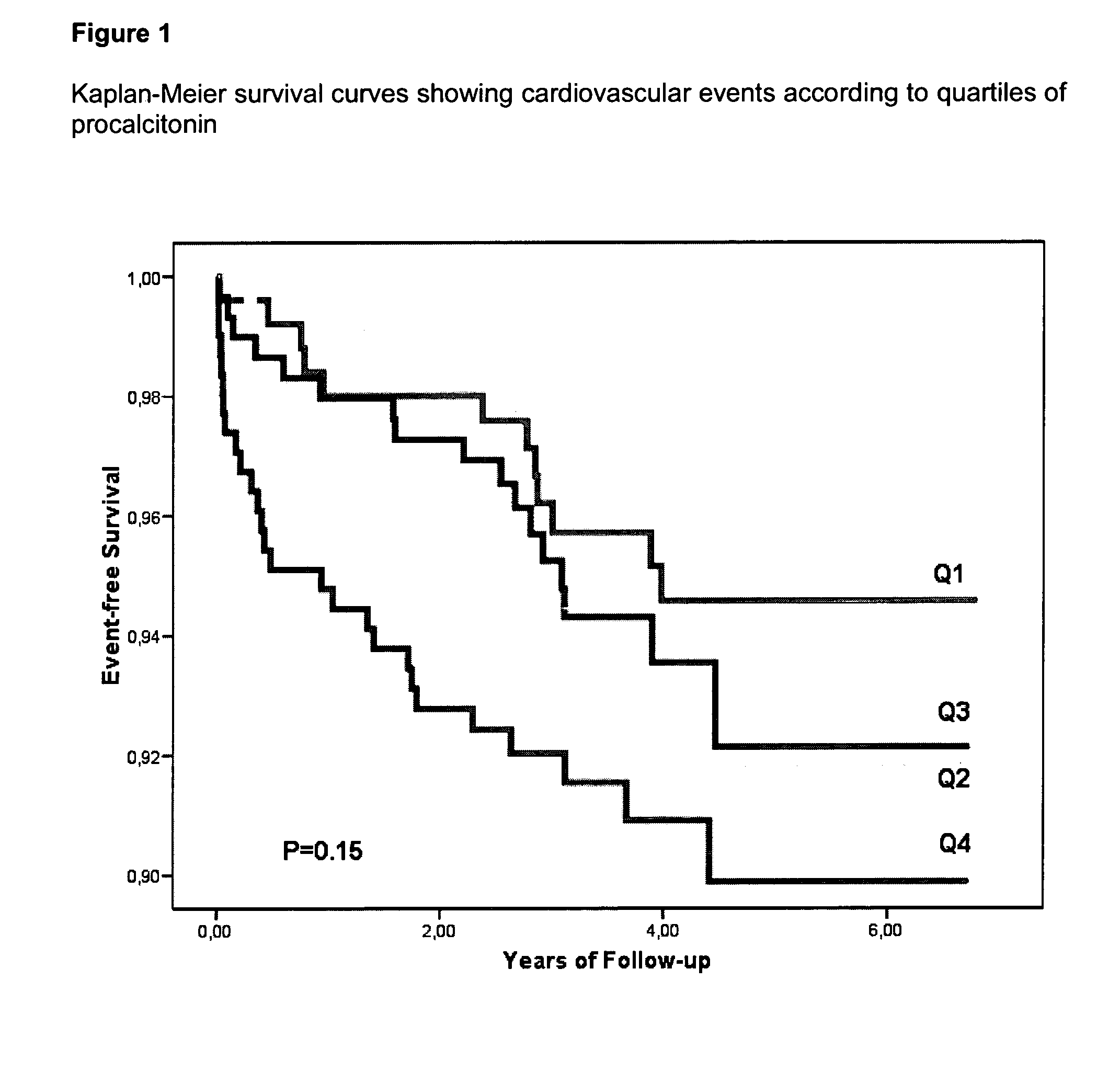
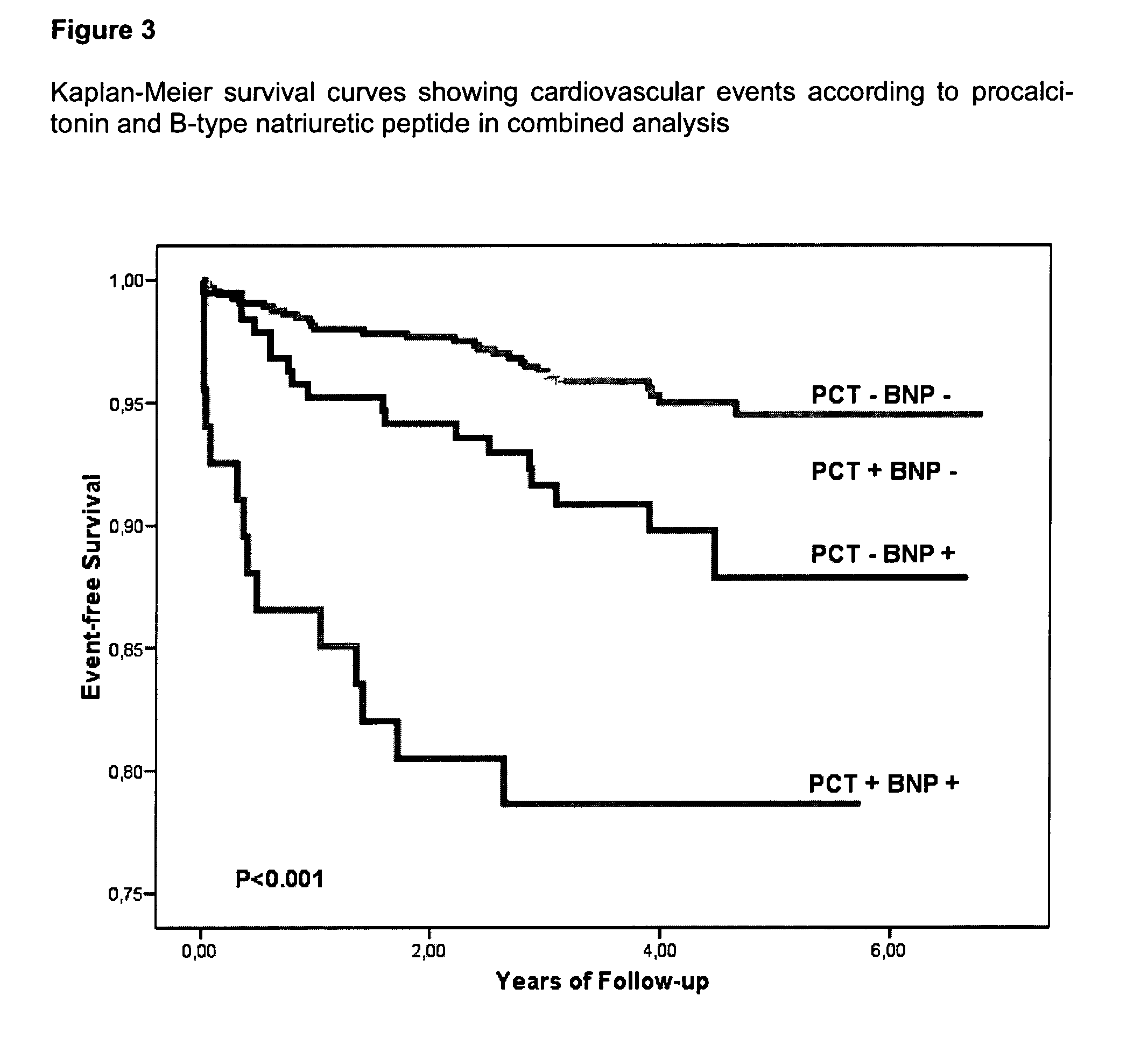
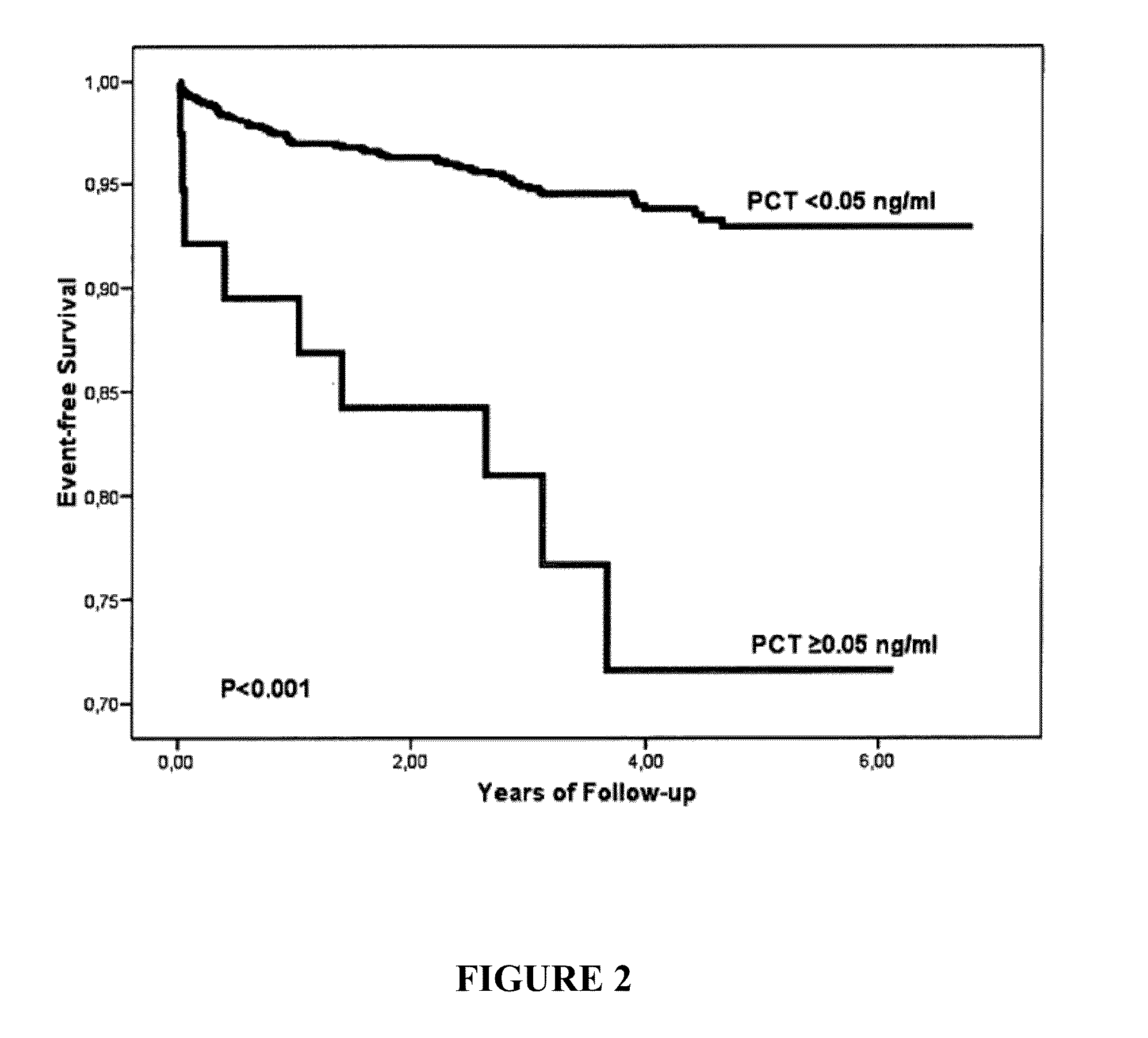
An in vitro method for the risk stratification of patients with stable arteriosclerosis, especially stable coronary artery disease, is disclosed wherein the concentration of procalcitonin is determined in the circulation of such patients using a highly sensitive PCT assay, and wherein within the range of PCT concentrations in the typical normal range of healthy individuals cutoff values are defined which distinguish groups of individual patients with stable arteriosclerosis in accordance with personal cardiac risk, and patients are allotted to one of said risk groups on the basis of their individual PCT concentrations.
Owner:BRAHMS GMBH
Mutual prodrugs of amlodipine and atorvastatin
This invention relates to mutual prodrugs of amlodipine and atorvastatin and to pharmaceutical compositions thereof. This invention also relates to methods of treating angina pectoris, atherosclerosis, and hypertension and hyperlipidemia in a mammal using those prodrugs and compositions. This invention also relates to methods of managing cardiac risk in a mammal, including humans, presenting with symptoms of cardiac risk by administering those prodrugs and compositions.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Method for risk stratification in stable coronary artery disease
An in vitro method for the risk stratification of patients with stable arteriosclerosis, especially stable coronary artery disease, is disclosed wherein the concentration of procalcitonin is determined in the circulation of such patients using a highly sensitive PCT assay, and wherein within the range of PCT concentrations in the typical normal range of healthy individuals cutoff values are defined which distinguish groups of individual patients with stable arteriosclerosis in accordance with personal cardiac risk, and patients are allotted to one of said risk groups on the basis of their individual PCT concentrations.
Owner:BRAHMS GMBH
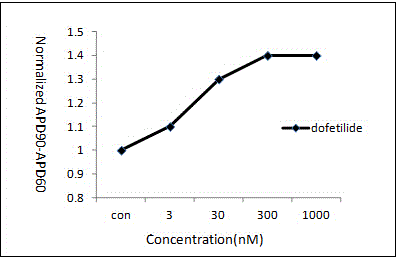
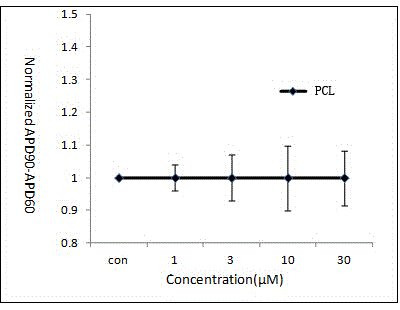
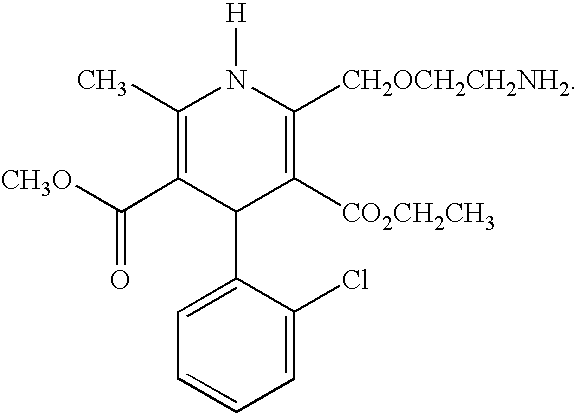
Pharmaceutical pre-clinical cardiac risk assessment method
InactiveCN104951870AImprove screening efficiencyImprove accuracyResourcesTriangulationPharmaceutical care
The invention provides a pharmaceutical pre-clinical cardiac risk assessment method. The method includes the steps of design of action potential frequency, recording of action potential, and recording of a manual patch clamp for the hERG (human ether-Alpha-go-go-related gene) channel. The method has the advantages that the risk of pharmaceutical cardiac arrhythmias is assessed by means of frequency dependence, action potential triangulation and hERG blocking dynamics, screening efficiency and accuracy of pharmaceuticals is greatly improved, and pharmaceutical clinical cardiac arrhythmias is less false positive.
Owner:NANTONG PHARMACORE LABS MEDICAL TECH
Systems and methods of automating reconsideration of cardiac risk
Systems, methods, and computer-readable media to automate reconsideration of a cardiac risk category associated with issuance of a life insurance policy may provide an indication of a best potential risk category or rate class that would be available if at least one action is completed by a life insurance applicant.
Owner:USAA
Therapeutic combination
InactiveUS20060270717A1Shorten the progressReduce riskBiocidePharmaceutical delivery mechanismSecondary hyperlipidemiaAngina
This invention relates to pharmaceutical combinations of amlodipine or a pharmaceutically acceptable acid addition salt thereof and atorvastatin or a pharmaceutically acceptable salt thereof, kits containing such combinations and methods of using such combinations to treat subjects suffering from angina pectoris, atherosclerosis, combined hypertension and hyperlipidemia and to treat subjects presenting with symptoms of cardiac risk, including humans. This invention also relates to additive and synergistic combinations of amlodipine and atorvastatin whereby those synergistic combinations are useful in treating subjects suffering from angina pectoris, atherosclerosis, combined hypertension and hyperlipidemia and those subjects presenting with symptoms of cardiac risk, including humans.
Owner:BUCH JAN
Systems and methods for detecting worsening heart failure
ActiveCN109069060AUltrasonic/sonic/infrasonic diagnosticsElectrotherapyHeart failure cellDetector circuits
Systems and methods for detecting worsening cardiac conditions such as worsening heart failure events are described. A system may include sensor circuits to sense physiological signals and signal processors to generate from the physiological signals first and second signal metrics. The system may include a risk stratifier circuit to produce a cardiac risk indication. The system may use at least the first signal metric to generate a primary detection indication, and use at least the second signal metric and the risk indication to generate a secondary detection indication. The risk indication may be used to modulate the second signal metric. A detector circuit may detect the worsening cardiac event using the primary and secondary detection indications.
Owner:CARDIAC PACEMAKERS INC
Noncardiotoxic pharmaceutical compounds
InactiveCN101432003AOrganic active ingredientsGroup 5/15 element organic compoundsDiseaseCardiac problems
The present invention relates to novel noncardiotoxic compounds and pharmaceutical compositions useful in the treatment of a variety of disorders including the treatment of depression, allergies, psychoses, cancer and gastrointestinal disorders. In particular, the present invention describes pharmaceutical compositions that mitigate life-threatening arrhythmias such as torsade de pointes. Torsade de pointes is a particular cardiac problem associated with many therapeutic agents and has been implicated as a possible cause of sudden death, particularly in those individuals with a past history of disturbances of cardiac rhythm, myocardial infarction, congenital repolarization abnormalities and cardiac risk factors such as hyperlipidemia and age. This arrhythmia is a variant of paroxysmal ventricular tachycardia associated with a prolonged QTc interval or prominent U waves on the ECG. Torsade de pointes is potentially lethal because it can progress to ventricular fibrillation, life-threatening arrhythmias or precipitate sudden death.
Owner:唐纳德·L·巴尔博
Systems and methods for detecting worsening heart failure
ActiveCN109069060BUltrasonic/sonic/infrasonic diagnosticsElectrotherapyDetector circuitsHeart disease
Systems and methods for detecting a worsening cardiac condition, such as a worsening heart failure event, are described. The system can include a sensor circuit for sensing a physiological signal and a signal processor for generating a first signal metric and a second signal metric from the physiological signal. The system can include a risk stratifier circuit for generating a cardiac risk indication. The system may generate a primary detection indication using at least the first signal metric and generate a secondary detection indication using at least the second signal metric and the risk indication. The risk indication may be used to modulate the second signal metric. A detector circuit may use the primary detection indication and the secondary detection indication to detect an exacerbated cardiac event.
Owner:CARDIAC PACEMAKERS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com