Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63 results about "Intact skin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
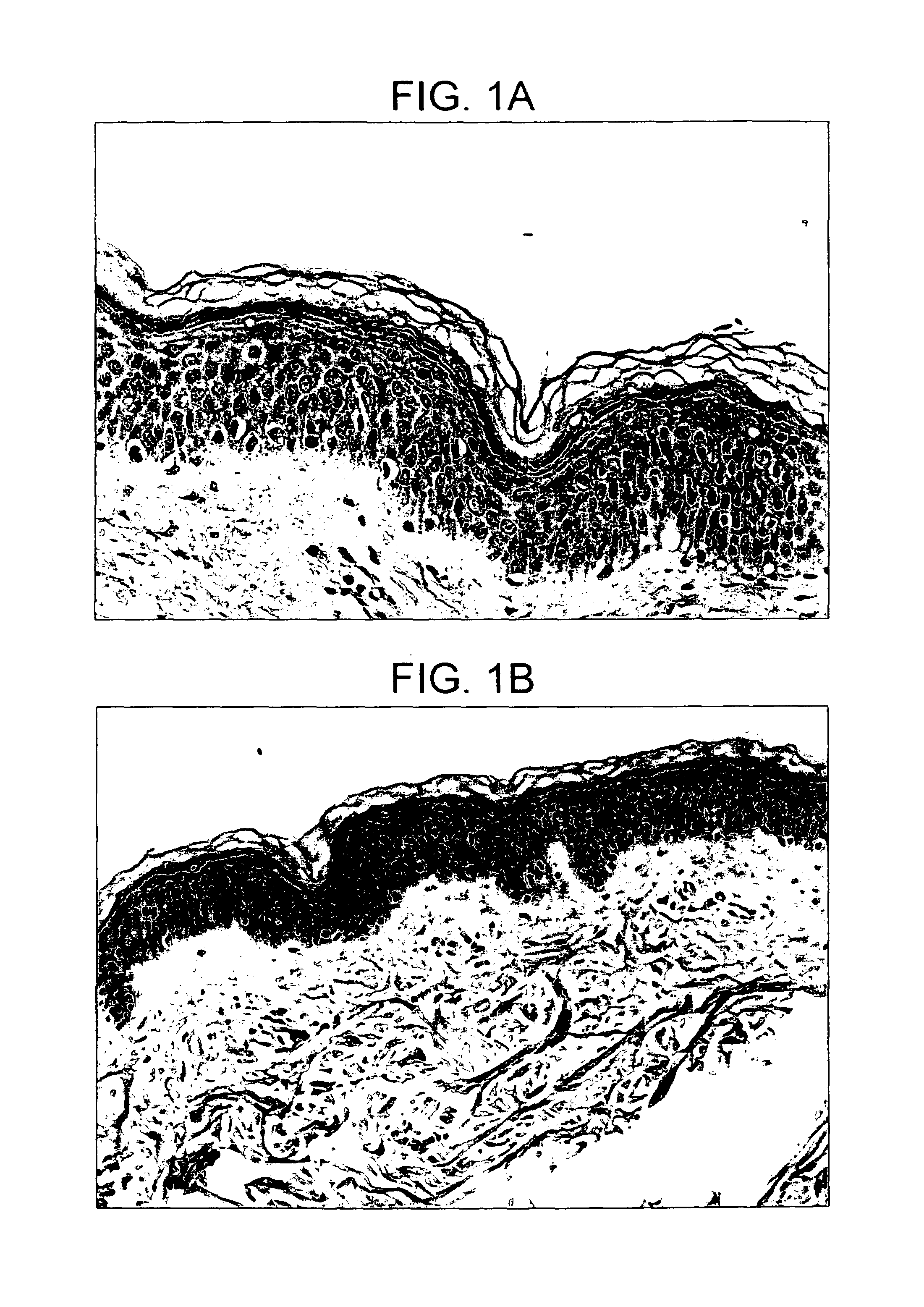
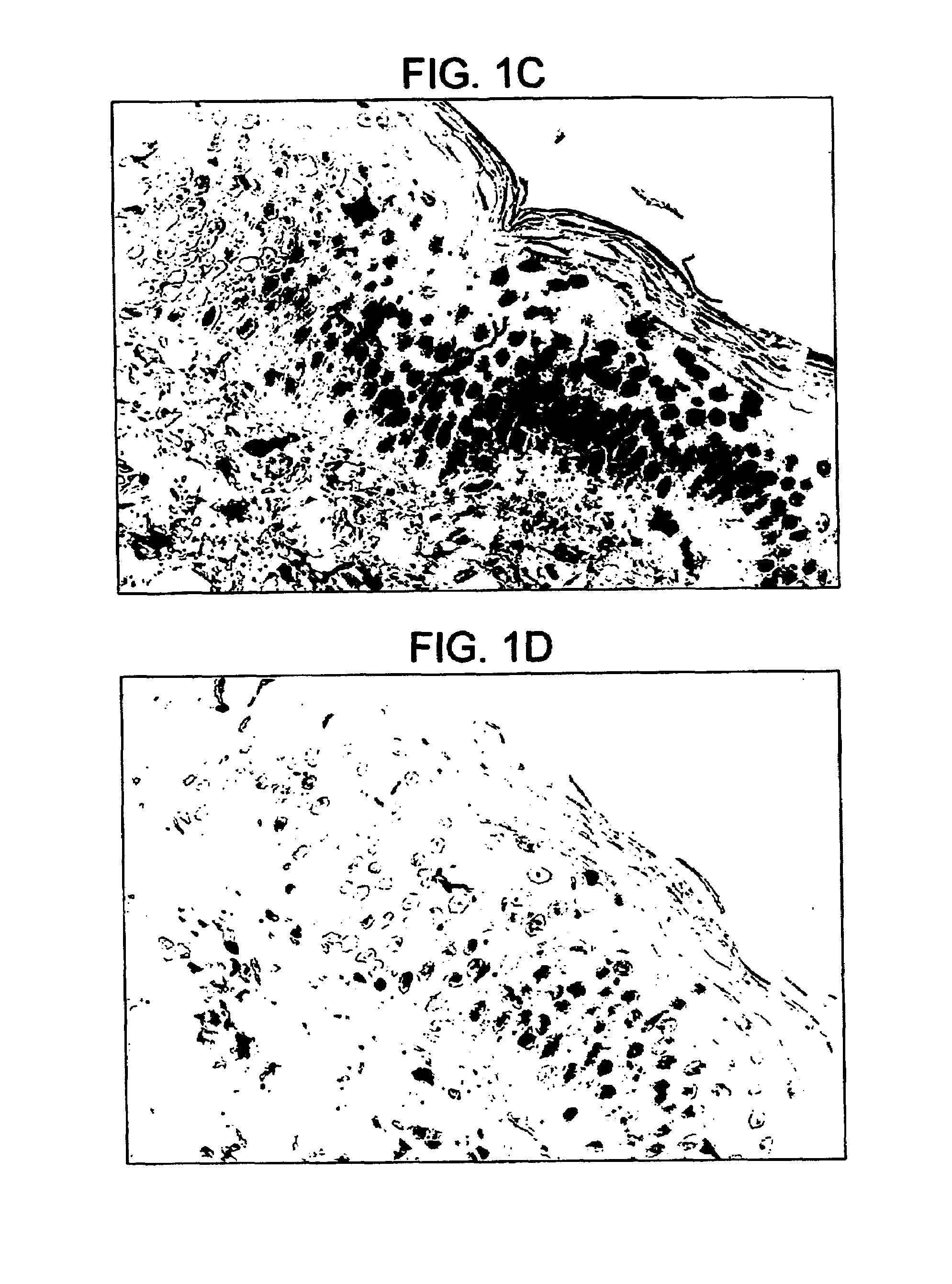
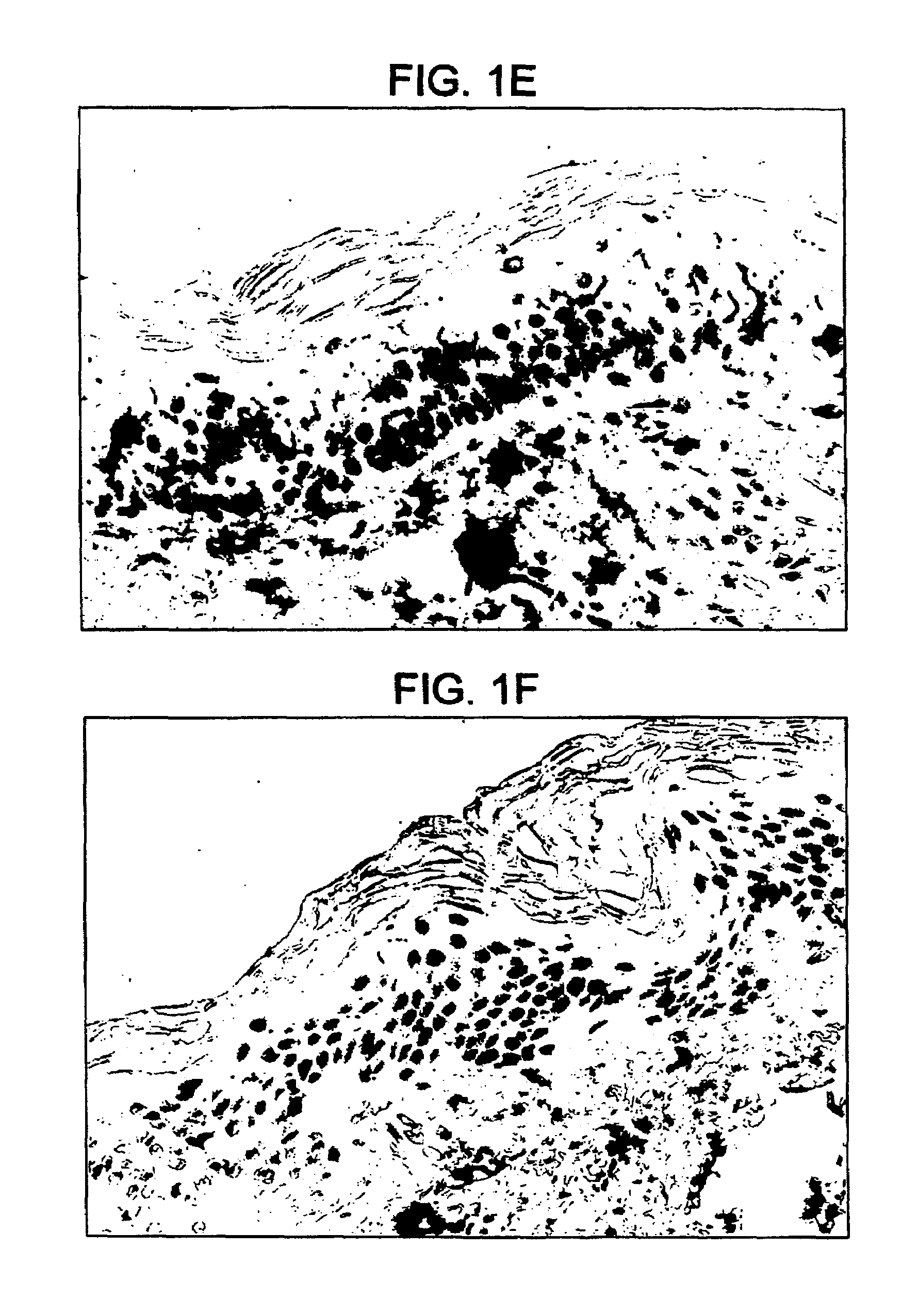
Intact skin. Healthy skin in which there are no breaks, scrapes, cuts, or abnormal openings that allow pathogens to enter.
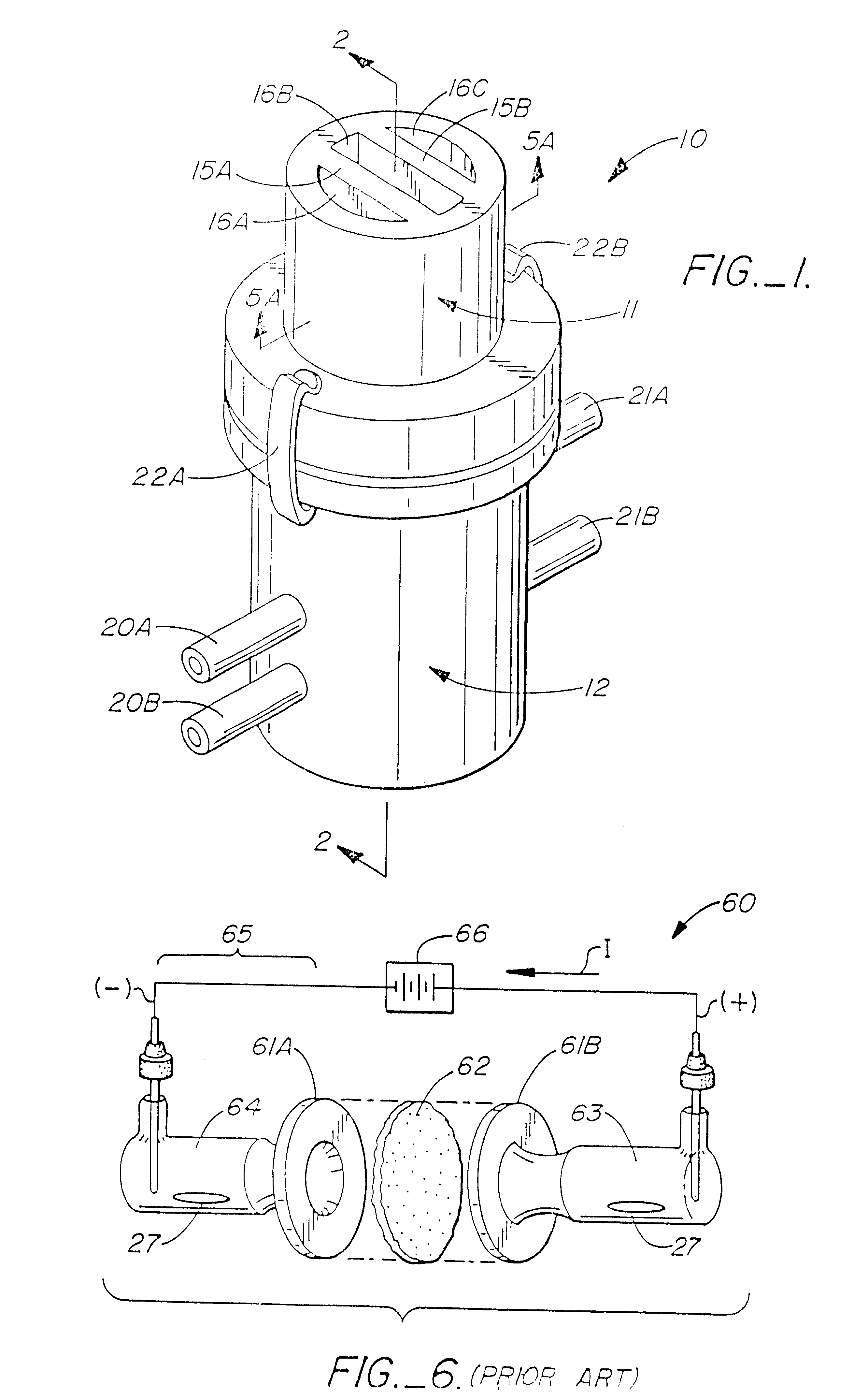
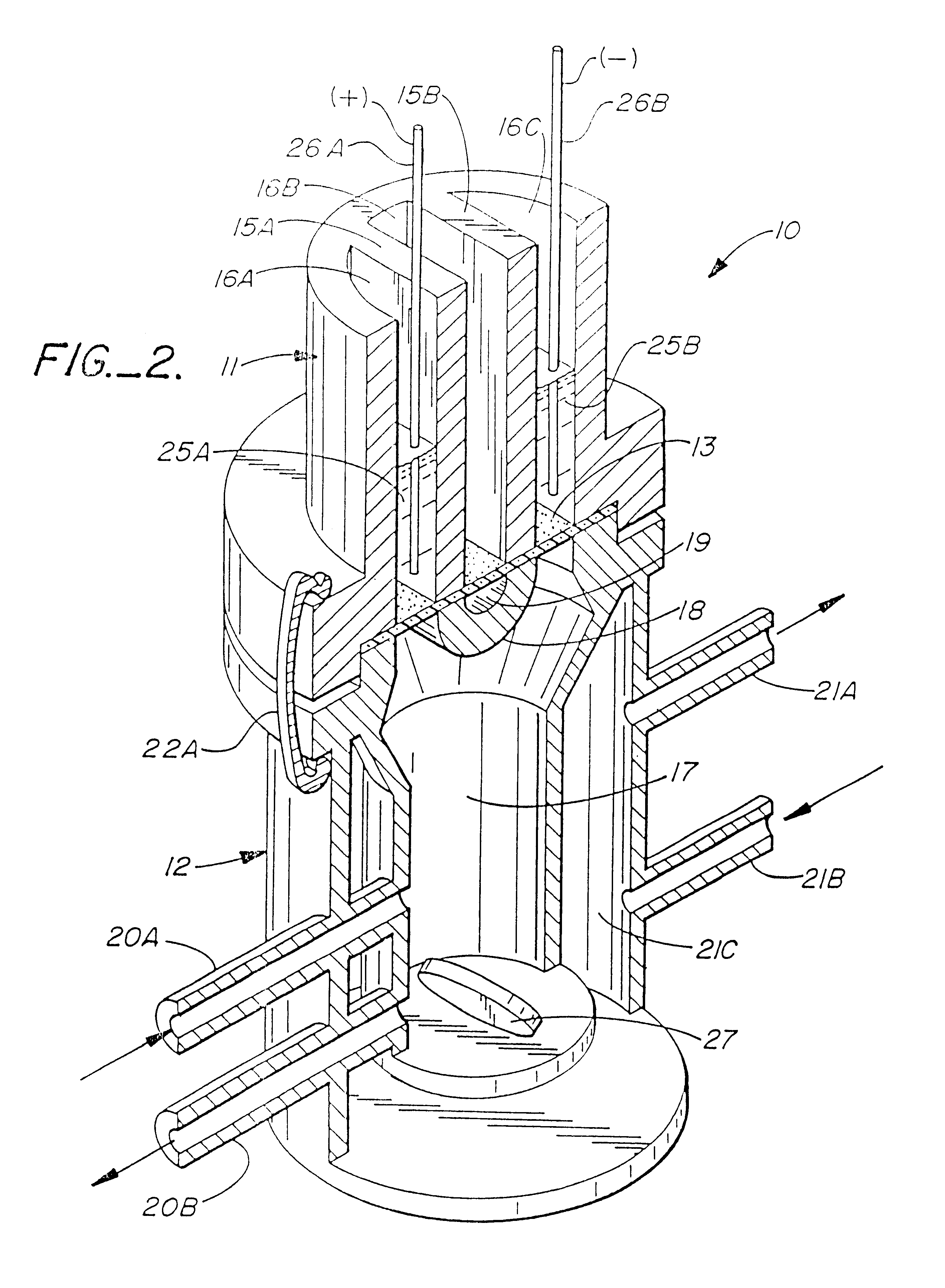
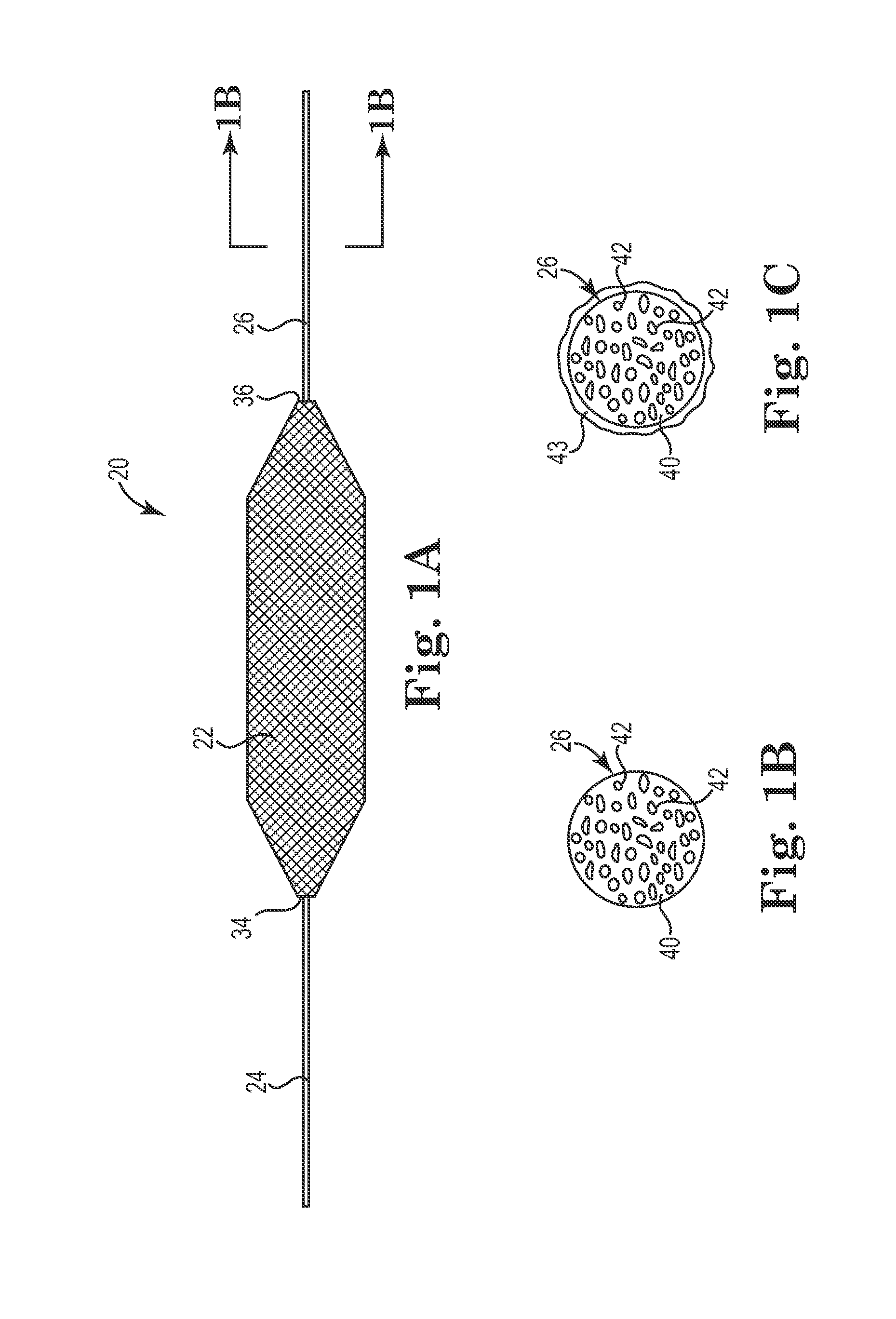
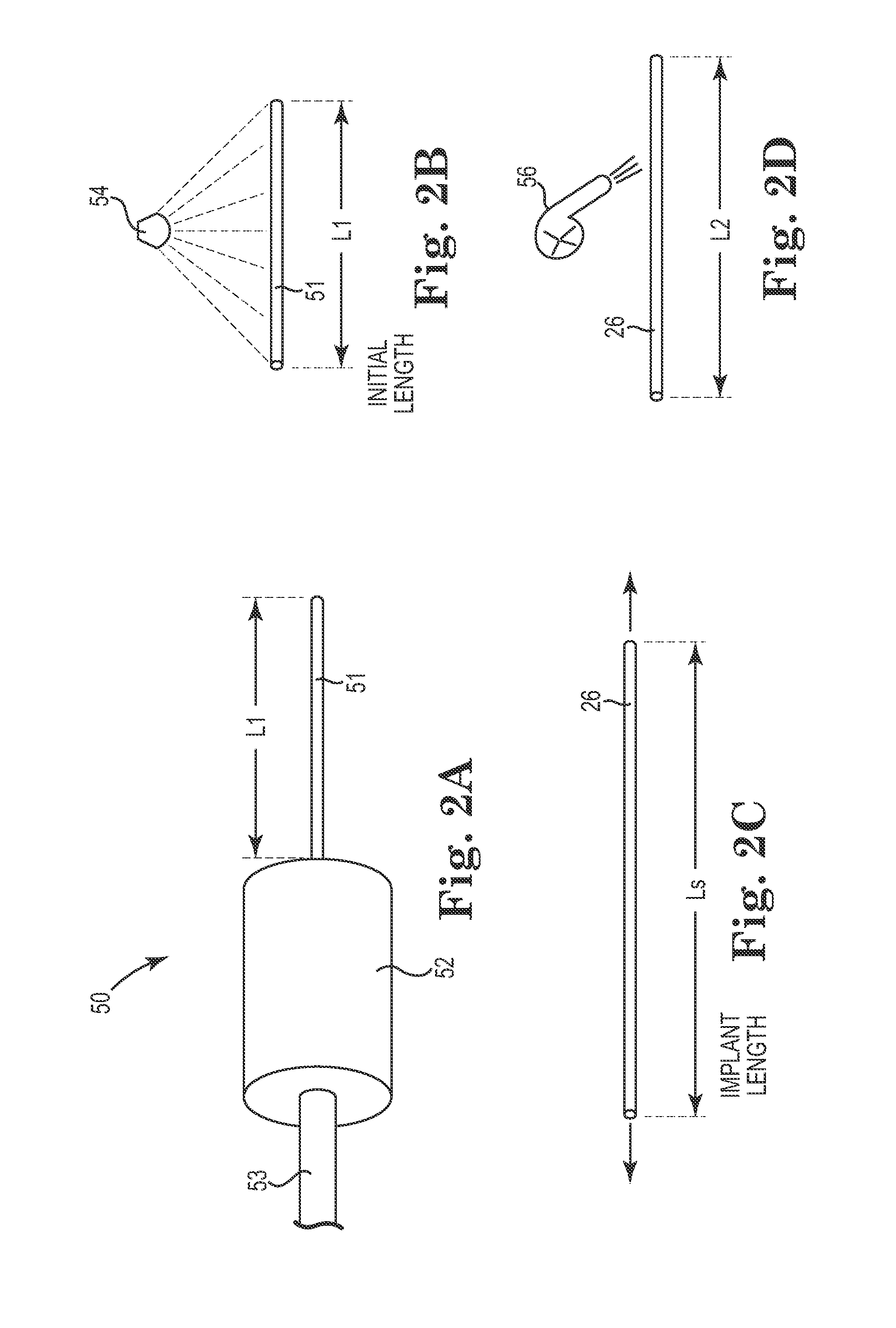
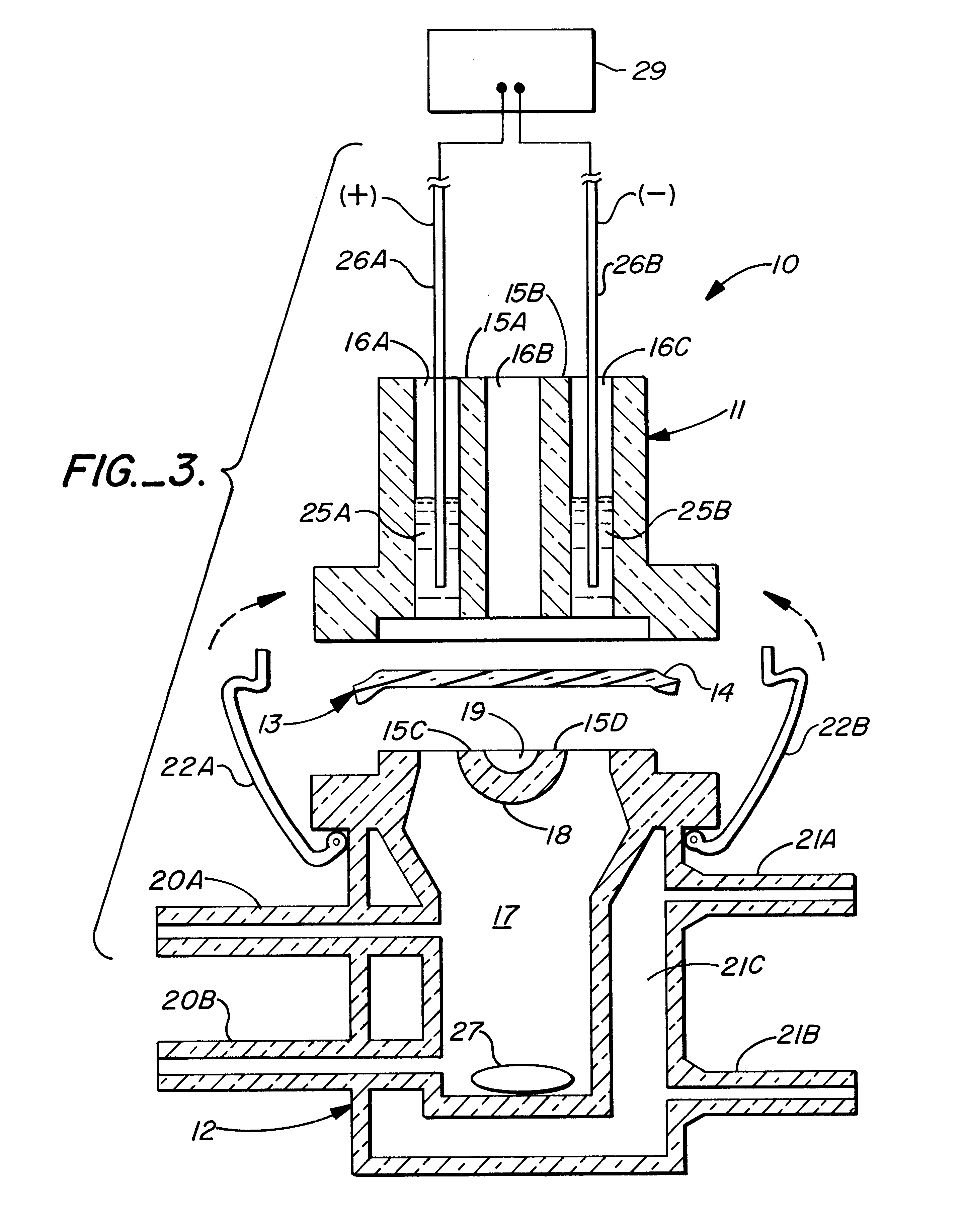
Method for the iontophoretic non-invasive determination of the in vivo concentration level of an inorganic or organic substance
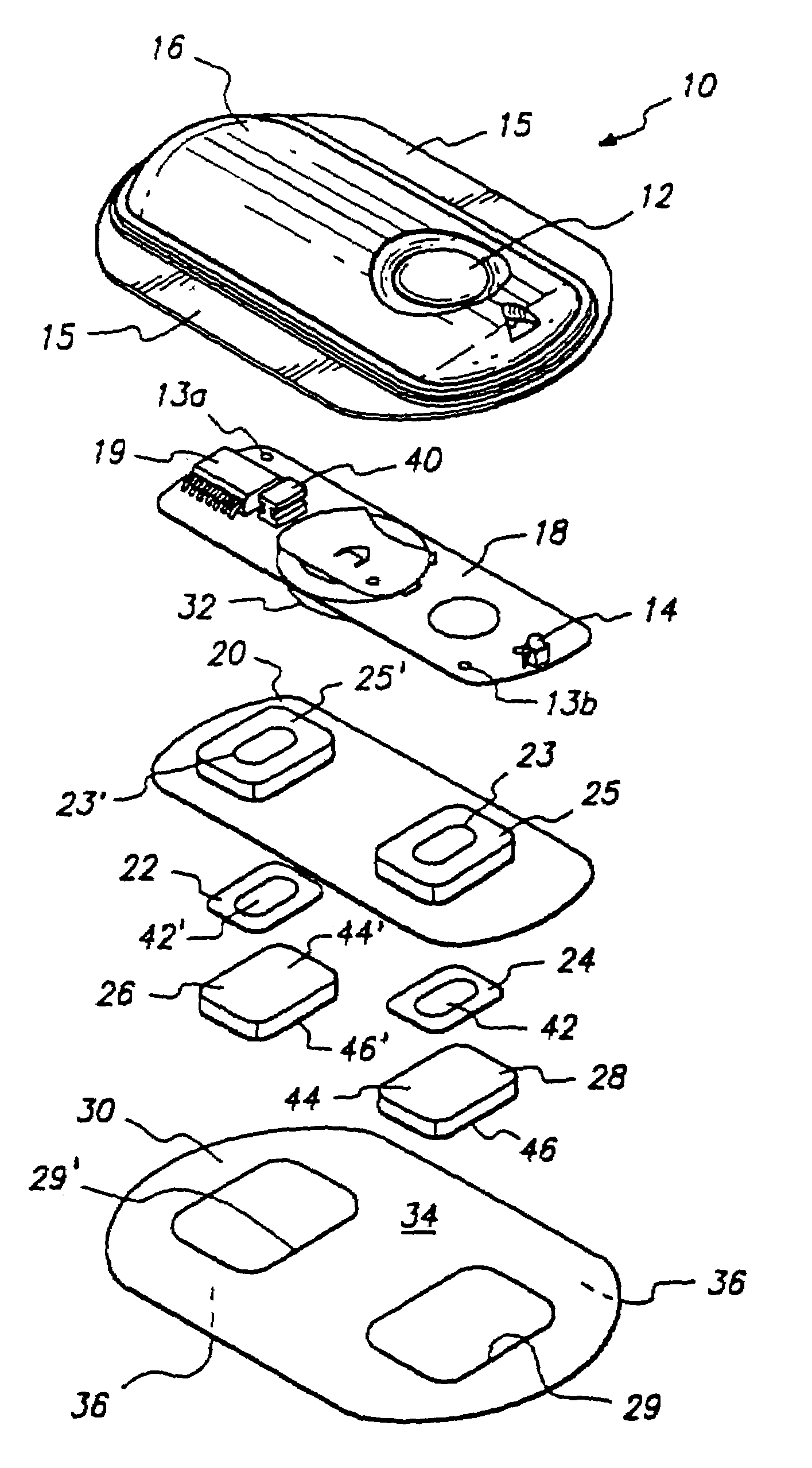
The present invention relates to an vitro device for the removal of ionized substances from a membrane sample without mechanical penetration, which device comprises:(a) a positive electrode;(b) a negative electrode, and(c) electrical insulation between subpart (a) and (b), wherein the positive electrode, and the negative electrode, and electrical insulation are positioned on the same side of the membrane sample.The present invention also relates to a device for the removal of or delivery of ionized substances to a mammal through intact skin or mucosal membrane without mechanical penetration, which device comprises: (a) a positive electrode, (b) a negative electrode, and (c) an electrically insulating material between subpart (a) and (b), wherein the positive electrode, negative electrode and insulating material are physically positioned so that each present a common surface of the device for contact with the same surface of the skin or mucosal membrane of the mammal.The present invention also relates to the use of iontophoresis to determine the level of a charged molecule in a living mammal, and with the use of a feedback mechanism, administer appropriate levels of therapeutic substances.
Owner:RGT UNIV OF CALIFORNIA
Topical compositions and methods for treating pain
InactiveUS6638981B2Avoid painComposition is stableBiocideNervous disorderNR1 NMDA receptorPreventing pain
Topical compositions and methods for treating pain. The invention provides oil-in-water emulsions comprising an antidepressant; an NMDA-receptor antagonists; a lipophilic component; water; and a surfactant. The compositions induce a local-anesthetic effect when topically administered to intact skin thereby treating or preventing pain, for example, neuropathic pain.
Owner:EPICEPT CORP
Sterile, breathable patch for treating wound pain
InactiveUS20030082225A1Not further irritate the wound upon removalBiocideNervous disorderAnesthetic AgentIntact skin
An intradermal patch having a permeable backing coated with a polyvinylpyrrolidone-based hydrogel and containing one or more local anesthetics. The patch is breathable, non-irritating upon application and removal, soothing, and sterile. The patch is useful for treating the pain associated with non-intact skin indications.
Owner:EPICEPT CORP
Systems and methods for topical treatment with nitric oxide
InactiveUS7048951B1Reduce skin irritationBiocideInorganic active ingredientsSandwich likeTransdermal patch
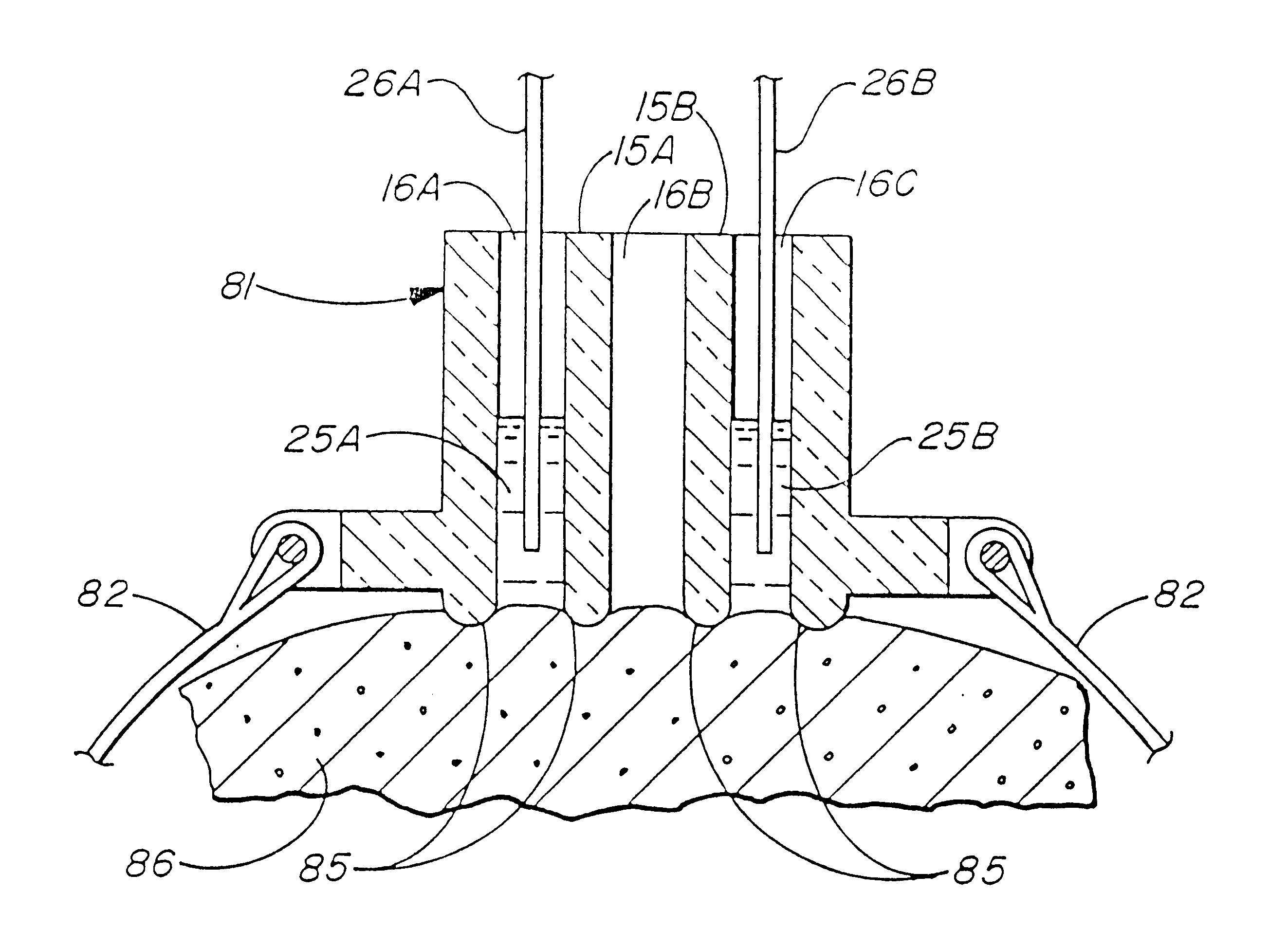
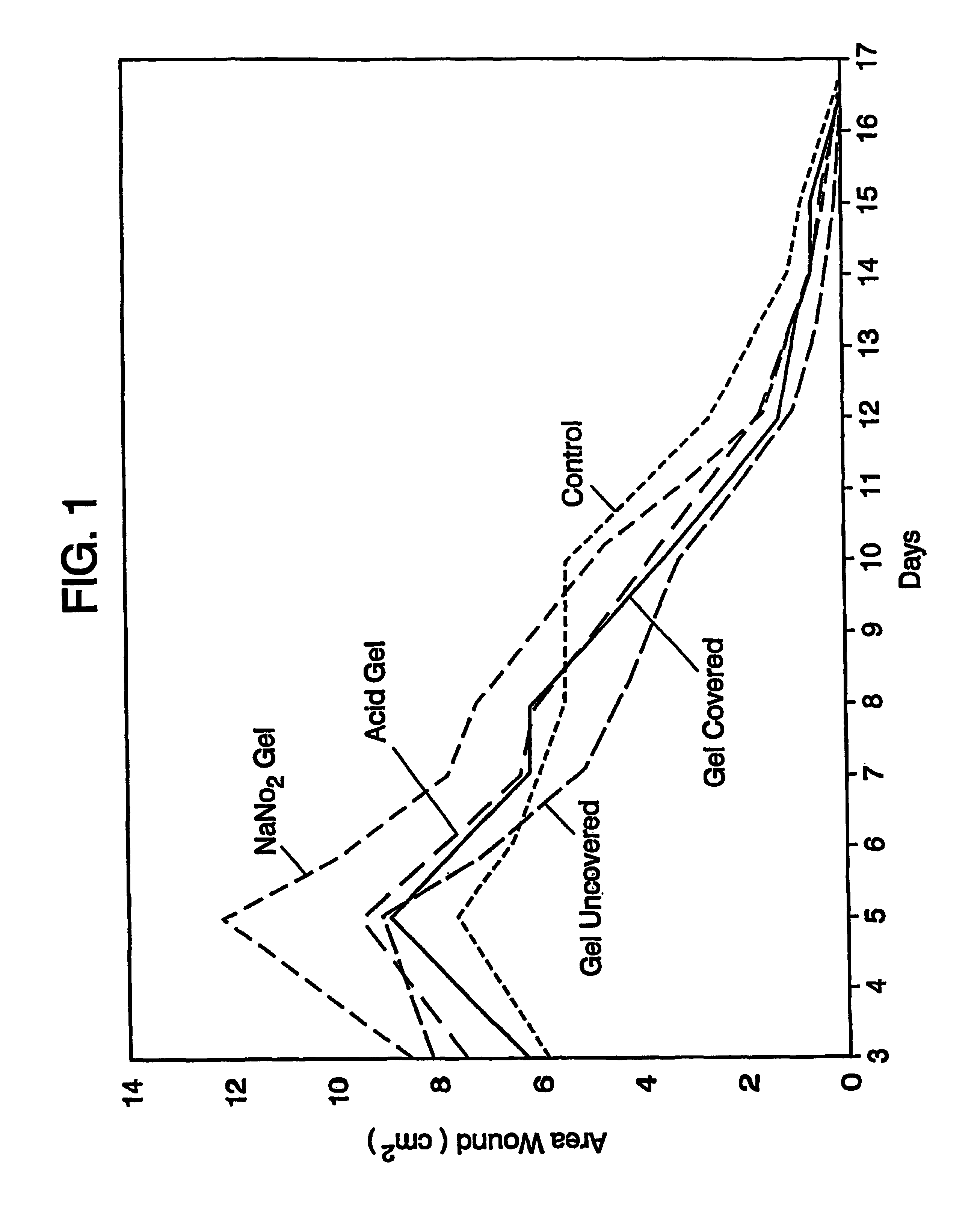
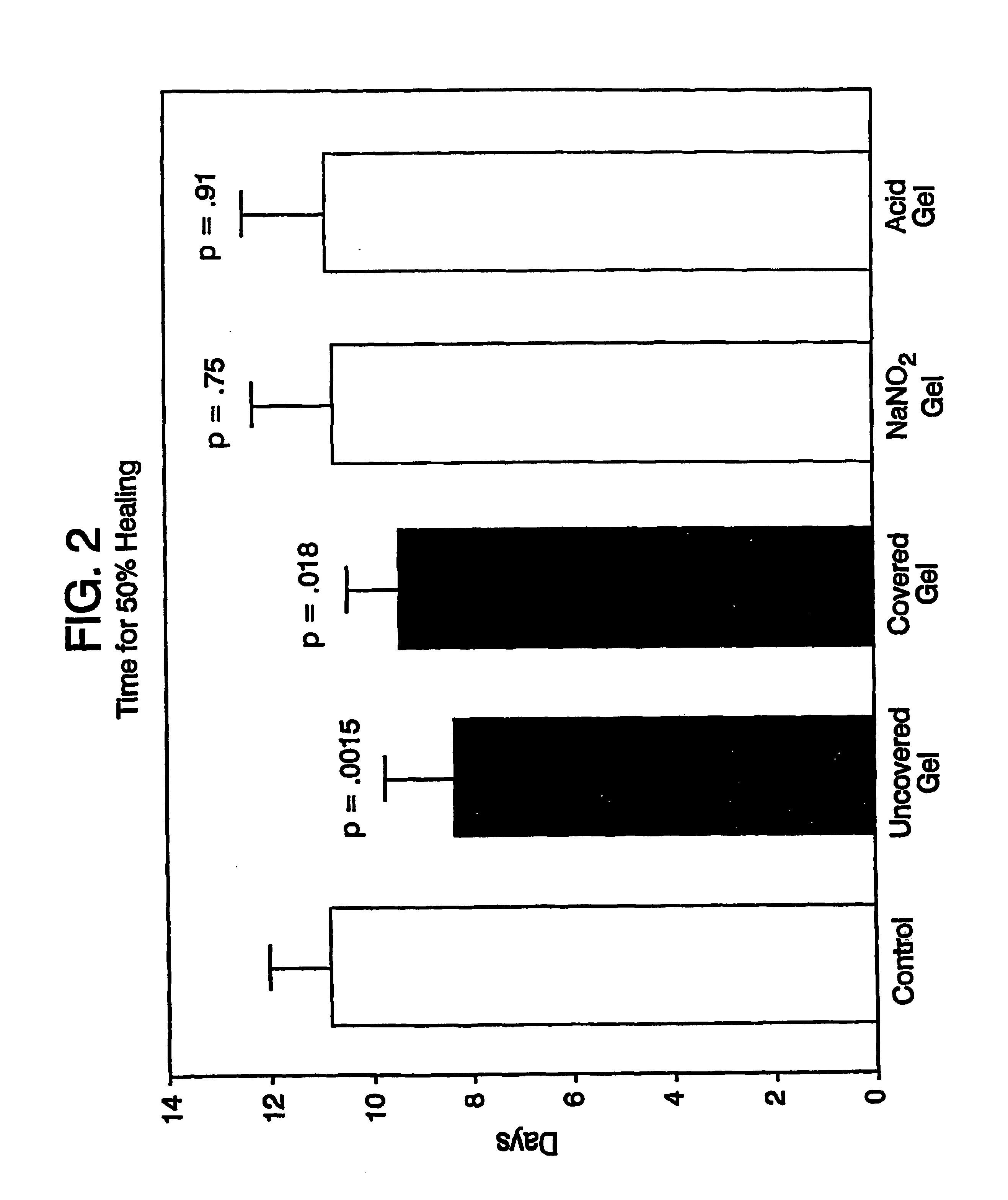
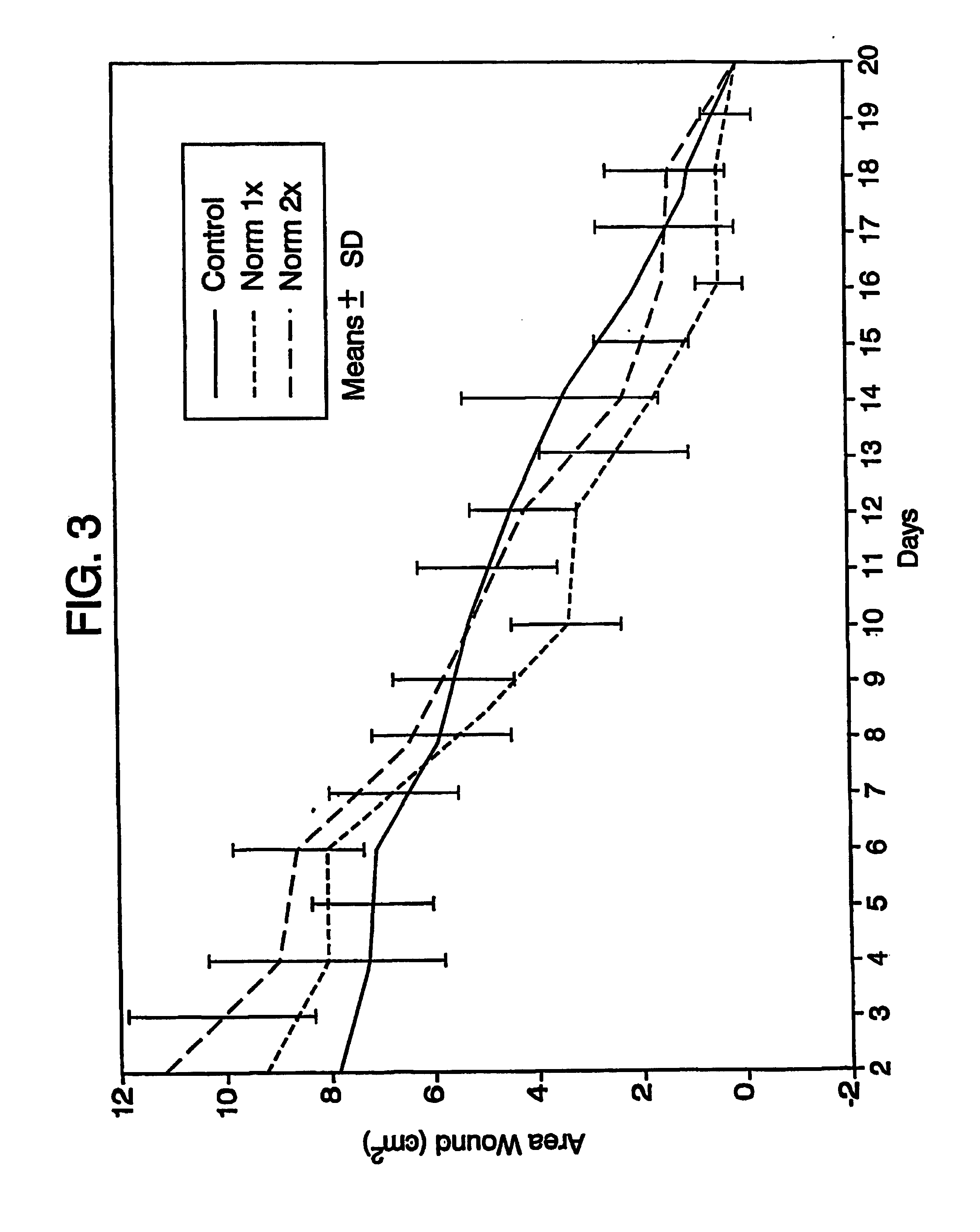
A simple, biocompatible system and procedure for generating nitric oxide (NO) is described. A mixture of powdered sodium nitrite, ascorbic acid, and maleic acid (or another organic acid of adequate strength) immediately generates nitric oxide (NO) on treatment with water. To slow down the NO generation, one may prepare an ointment from a nonaqueous medium (petrolatum, Vaseline™) and the three powdered ingredients, which on being applied topically on the skin will release NO as water permeates through this medium; alternatively, one may convert the aqueous sodium nitrite solution into a gel with hydroxyethylcellulose (or other gel-forming compound) and combine this gel with another gel obtained from aqueous ascorbic and maleic acids with hydroxyethylcellulose for topical application (on intact skin, burns intra-cavity, burns, intra-cavity, etc.). The two gels may be admixed immediately before use (possibly from a single container with separate chambers and dual nozzle, via pushing or squeezing the two gels through the nozzle), or may be applied in sandwich-like fashion (possibly as a transdermal patch) for further slowing down the delivery of NO.
Owner:NITRIC SOLUTIONS
Topical compositions and methods for treating pain
InactiveUS20030082214A1Treating and preventing painAvoid painBiocideNervous disorderPreventing painNR1 NMDA receptor
Topical compositions and methods for treating pain. The invention provides oil-in-water emulsions comprising an antidepressant; an NMDA-receptor antagonists; a lipophilic component; water; and a surfactant. The compositions induce a local-anesthetic effect when topically administered to intact skin thereby treating or preventing pain, for example, neuropathic pain.
Owner:EPICEPT CORP
Fat retraction apparatus and method for using same
InactiveUS20090125013A1Small cell sizeReduce contentSurgical instruments for heatingSurgical instruments using microwavesDiseaseIntact skin
The present invention provides a method and apparatus for non-invasive reduction of excessive fat tissue by externally applying radio frequency (RF) electromagnetic (EM) waves adjusted to specific fat cells absorption frequency and electromagnetic propagation mode. Based on the performed experiments described further, the method and working apparatus has been invented that reduces fat layers without any medication, non-invasively through the intact skin. The invented method and apparatus facilitates the safe fat removal, and may lead to reduction and eradication of obesity and obesity associated diseases.
Owner:SYPNIEWSKI ROZA +1
Transcutaneous immunization without heterologous adjuvant
InactiveUS20060002949A1Increase skin hydrationIncrease local concentrationSsRNA viruses negative-senseAntibacterial agentsDendritic cellSpecific immunity
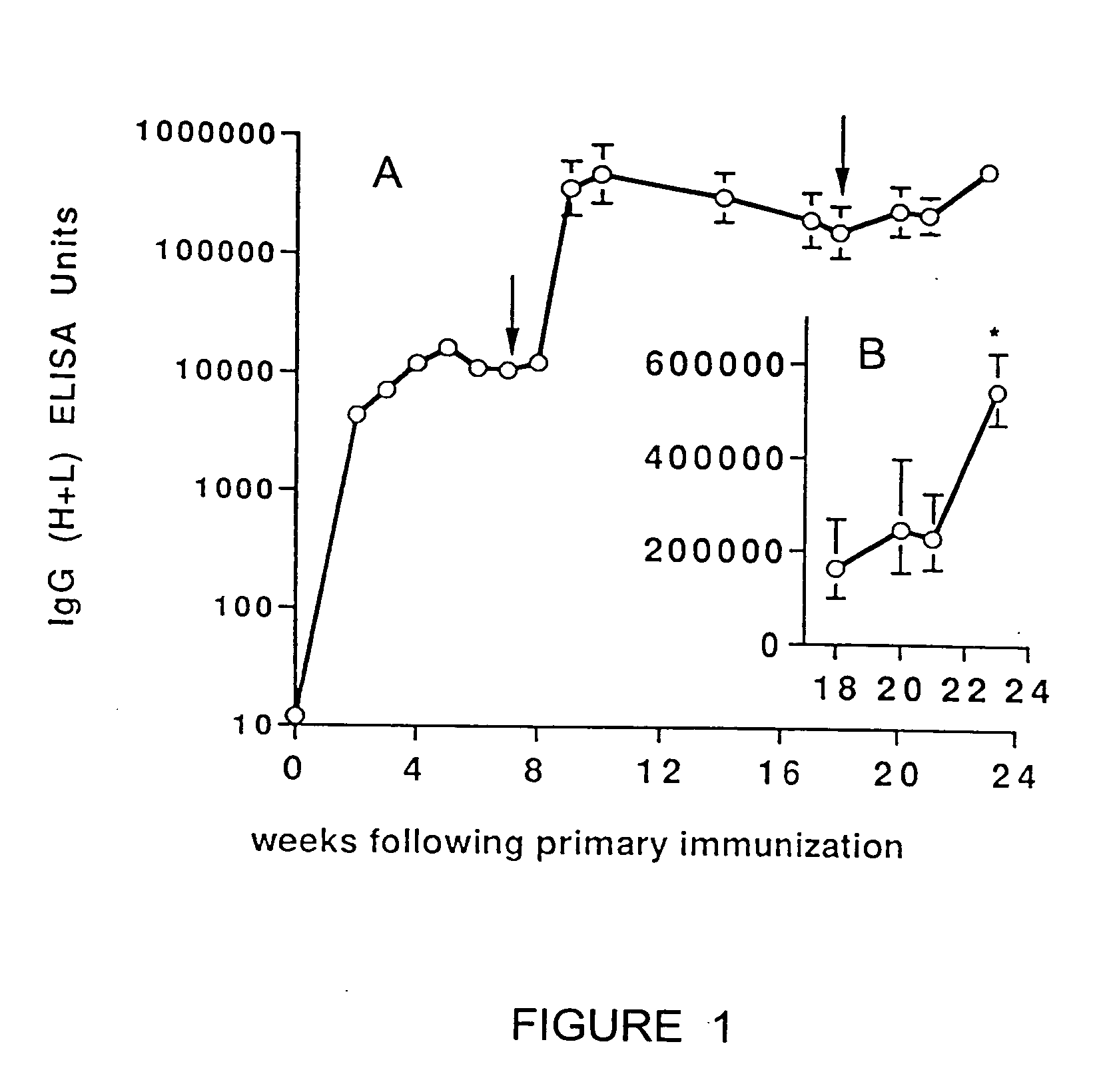
Transcutaneous immunization can deliver antigen to the immune system through the stratum corneum without physical or chemical penetration to the dermis layer of the skin. This delivery system induces an antigen-specific immune response without the use of a heterologous adjuvant. Although perforation of intact skin is not required, superficial penetration or micropenetration of the skin can act as an enhancer; similarly, hydration may enhance the immune response. This system can induce antigen-specific immune effectors after epicutaneous application of a formulation containing one or more antigens. The formulation may initiate processes such as antigen uptake, processing, and presentation; Langerhans cell activation, migration from the skin to other immune organs, and differentiation to mature dendritic cells; contacting antigen with lymphocytes bearing cognate antigen receptors on the cell surface and their stimulation; and combinations thereof. Systemic and / or regional immunity may be induced. Immune responses that provide prophylactic and / or therapeutic treatments are preferred. Antigenic activities in the formulation may be found in the same molecule, two or more different molecules dissociated from each other, or multiple molecules in a complex formed by covalent or non-covalent bonds. For antigens which are proteinaceous, they may be provided in the formulation as a polynucleotide for transcutaneous genetic immunization. Besides simple application of a dry or liquid formulation to the skin, patches and other medical devices may be used to deliver antigen for immunization.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE OFFICE OF THE COMMAND JUDGE ADVOCATE
Adjuvant for transcutaneous immunization
A transcutaneous immunization system delivers antigen to immune cells without perforation of the skin, and induces an immune response in an animal or human. The system uses an adjuvant, preferably an ADP-ribosylating exotoxin, to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a formulation containing antigen and adjuvant to intact skin of the animal or human. The efficiency of immunization may be enhanced by adding hydrating agents (e.g., liposomes), penetration enhancers, or occlusive dressings to the transcutaneous delivery system. This system may allow activation of Langerhans cells in the skin, migration of the Langerhans cells to lymph nodes, and antigen presentation.
Owner:UNITED STATES OF AMERICA
Topical anesthetic composition and method of administration
InactiveUS20050014823A1Minimize occlusionRelieve painBiocidePharmaceutical delivery mechanismWhole bodyIntact skin
The present invention includes a topical anesthetic composition having one or more local anesthetic agents, a penetration enhancer, and an anhydrous, volatile solvent combined to provide a liquid homogenous solution. The present invention may also include water in the topical anesthetic composition as concentration levels low enough to avoid precipitation of the local anesthetic agent and penetration enhancer from solution and undesired enhanced delivery of the local anesthetic agent through the skin or mucosa to the systemic circulation. The topical anesthetic composition can be conveniently sprayed onto intact skin and can be absorbed percutaneously with greater efficacy in order to shorten the time needed for the anesthesia to take effect.
Owner:HAWKINS CHEM
Skin-sctive adjuvants for transcutaneous immuization
InactiveUS20060002959A1Increase skin hydrationEnhance immune responseAntibacterial agentsSsRNA viruses negative-senseDendritic cellSpecific immunity
Transcutaneous immunization can deliver antigen to the immune system through the stratum corneum without physical or chemical penetration to the dermis layer of the skin. This delivery system induces an antigen-specific immune response. Use of skin-active adjuvants is preferred. Although perforation of intact skin is not required, superficial penetration or micropenetration of the skin can act as an enhancer; similarly, hydration may enhance the immune response. This system can induce antigen-specific immune effectors after epicutaneous application of a formulation containing one or more antigen and adjuvant. The formulation may initiate processes such as antigen uptake, processing, and presentation; Langerhans cell activation, migration from the skin to other immune organs, and differentiation to mature dendritic cells; contacting antigen with lymphocytes bearing cognate antigen receptors on the cell surface and their stimulation; and combinations thereof. Systemic and / or regional immunity may be induced; immune responses that result in prophylaxis and / or therapeutic treatments are preferred. Antigen and adjuvant activities in the formulation may be found in the same molecule, two or more different molecules dissociated from each other, or multiple molecules in a complex formed by covalent or non-covalent bonds. For antigens and adjuvants which are proteinaceous, they may be provided in the formulation as a polynucleotide for transcutaneous genetic immunization. Besides simple application of a liquid formulation, patches or other medical devices may be used to deliver antigen for immunization.
Owner:UNITED STATES OF AMERICA
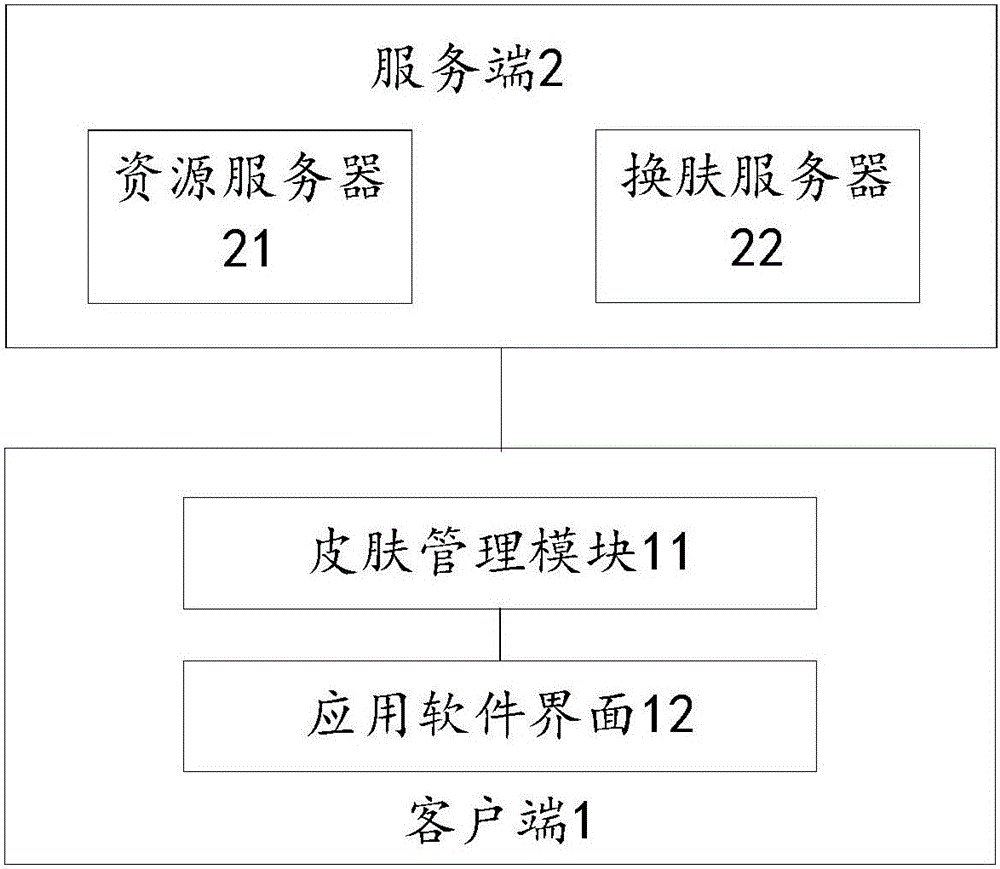
Skin exchanging method and apparatus for application software display interface
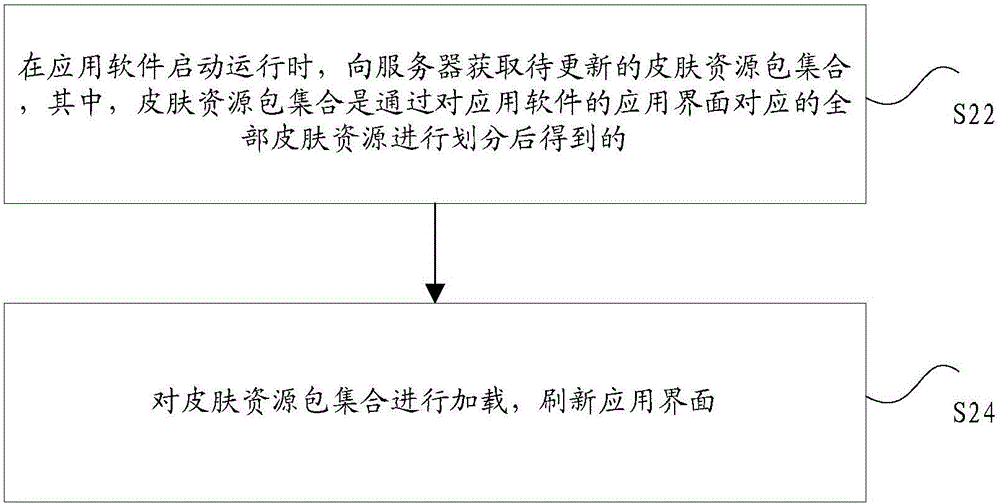
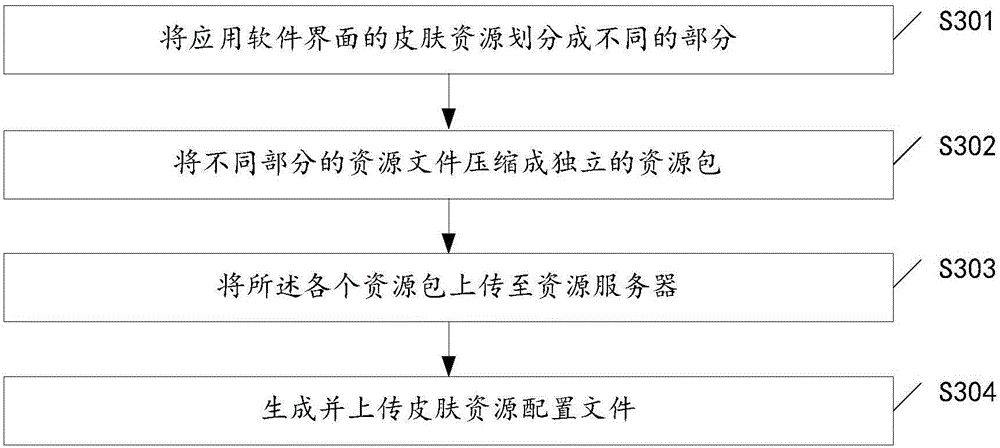
InactiveCN106227512AEfficient managementFlexible managementExecution for user interfacesResource utilizationIntact skin
The invention discloses a skin exchanging method and apparatus for application software display interface. The method comprises steps of acquiring a to-be-updated skin resource package set during start and operation of application software, loading the skin resource package set and refreshing application interface. The skin resource package set is achieved when all skin resources of the application interface of the application software are sorted. By the use of the skin exchanging method, a problem that the whole software interface in relative technology usually has only one skin package file and therefore a user has to re-download the resource during skin update can be solved, so resource utilization rate can be reduced and a technical problem of bandwidth resource can be solved.
Owner:NETEASE (HANGZHOU) NETWORK CO LTD
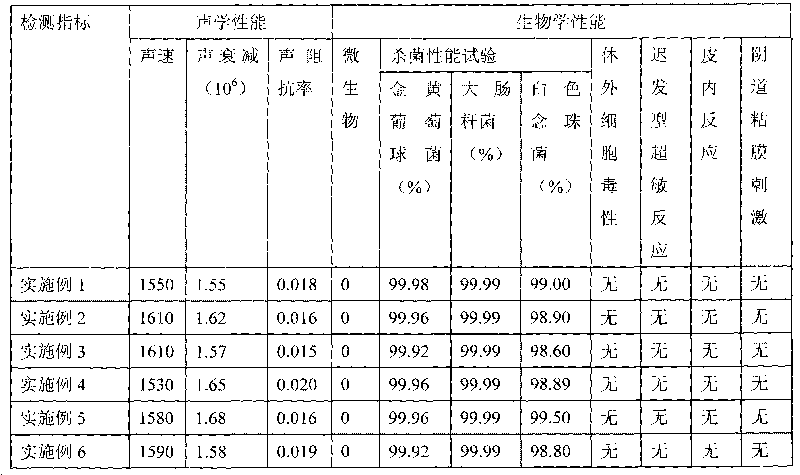
Ultrasonic coupling agent and application thereof
InactiveCN101716354AMeet the needs of testingIn line with biological performanceOrganic active ingredientsInorganic active ingredientsStimulantDisinfectant
The invention discloses an ultrasonic coupling agent and application of the ultrasonic coupling agent in ultrasonic inspection or auxiliary treatment of clinical cavity canals, mucous membranes, non-intact skin and the like in hospital. The ultrasonic coupling agent consists of the following raw materials: safe and nontoxic silver ions or nanometer silver serving as a disinfectant, and bio-compatibility macromolecular materials serving as a viscosity regulator, an anti-discoloration agent, an anti-precipitant agent, and an anti-stimulant agent. The ultrasonic coupling agent meets the requirements on ultrasonic impedance and no corrosion, has the characteristics of disinfection, sterilization, safety, nontoxicity, no stimulation and the like, can effectively avoid cross infection in ultrasonic clinics, can be suitable for detecting the cavity canals, the mucous membranes and the non-intact skin, and has high medical clinical value. The ultrasonic coupling agent has a simple preparation method, and low cost of raw materials.
Owner:GUANGDONG UNIV OF TECH
Method and composition for synergistic topical therapy for neuromuscular pains
InactiveUS20050256187A1Good reliefIncrease painBiocideAntipyreticIntact skinNon steroid anti inflammatory drug
The invention relates to a method and composition for synergistic topical therapy of the symptoms of neuromuscular pains. In this method, for intact skin or open skin, there is used a suitable topical pharmaceutical formulation, which is loaded in a suitable dose relationship with a sodium channel blocker from the class of local anesthetics of the ester or amide type and a substance from the class of non-steroidal anti-inflammatory drugs, and which releases these substances selectively onto or under the skin region. By the simultaneous inhibition of the initial inflammatory pain factors at the cellular level and also of the transmission of neuronal pain impulses in reaction thereto, this therapy achieves pharmacologically more effective alleviation of neuromuscular pain.
Owner:BIONICS PHARMA
Use of penetration enhancers and barrier disruption methods to enhance the immune response of antigen and adjuvant
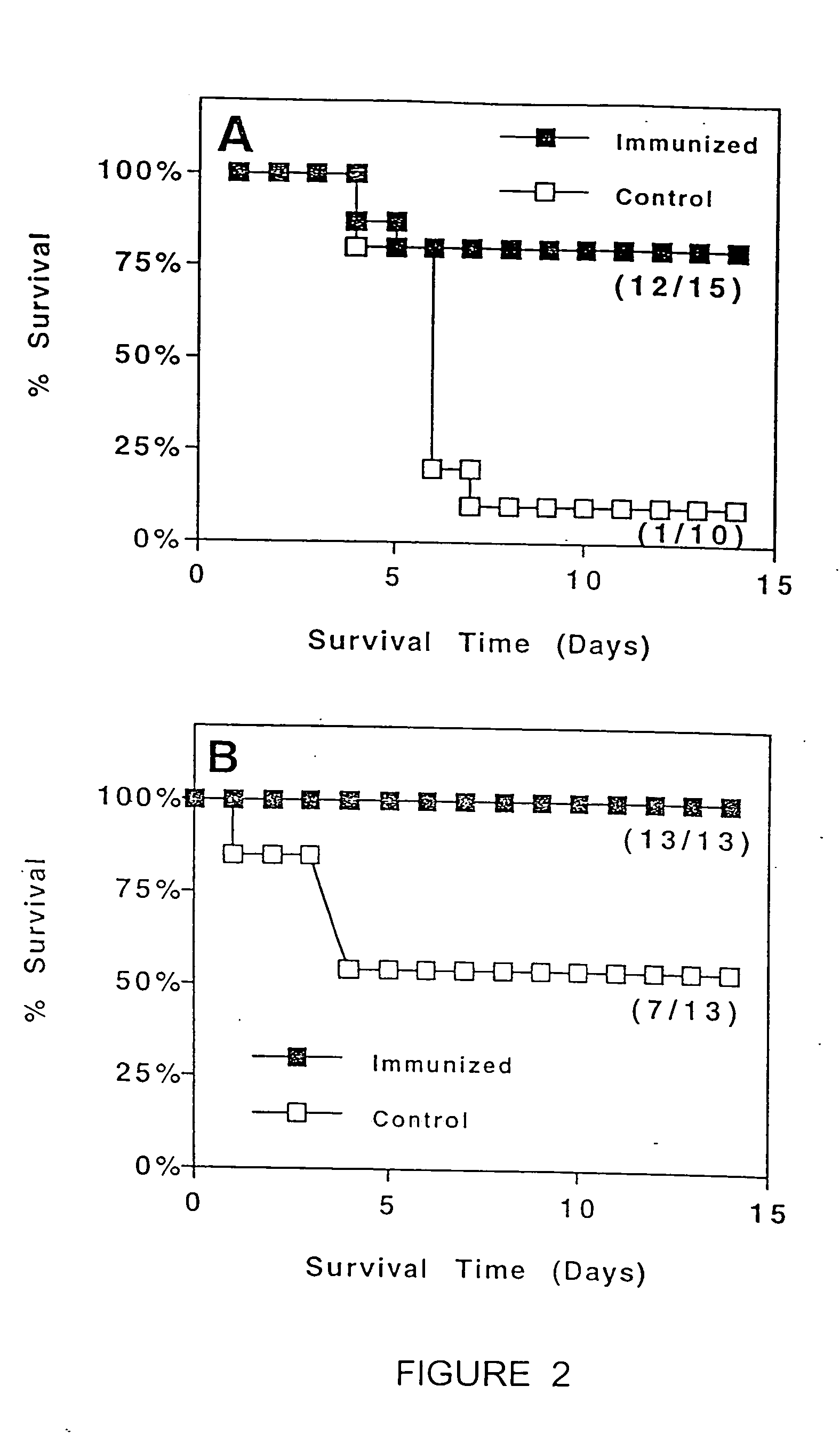
InactiveUS7378097B2Enhance immunizationEnhance vaccinationSsRNA viruses negative-senseBiocideAdjuvantWhole body
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Method of treating incontinence
ActiveUS20110306822A1Reducing incontinenceShorten the lengthSuture equipmentsAnti-incontinence devicesUrethraIntact skin
A method of treating incontinence in a patient includes implanting a support by suspending the support from a pair of connectors attached to tissue thereby supporting a urethra of the patient with an implanted support and a pair of implanted connectors. The method additionally includes evaluating the patient for incontinence post-implantation of the support, and reducing the incontinence of the patient by shortening a length of one of the pair of implanted connectors through intact skin from a location extracorporeal of the patient.
Owner:COLOPLAST AS
Transdermal Method and Patch for Nausea
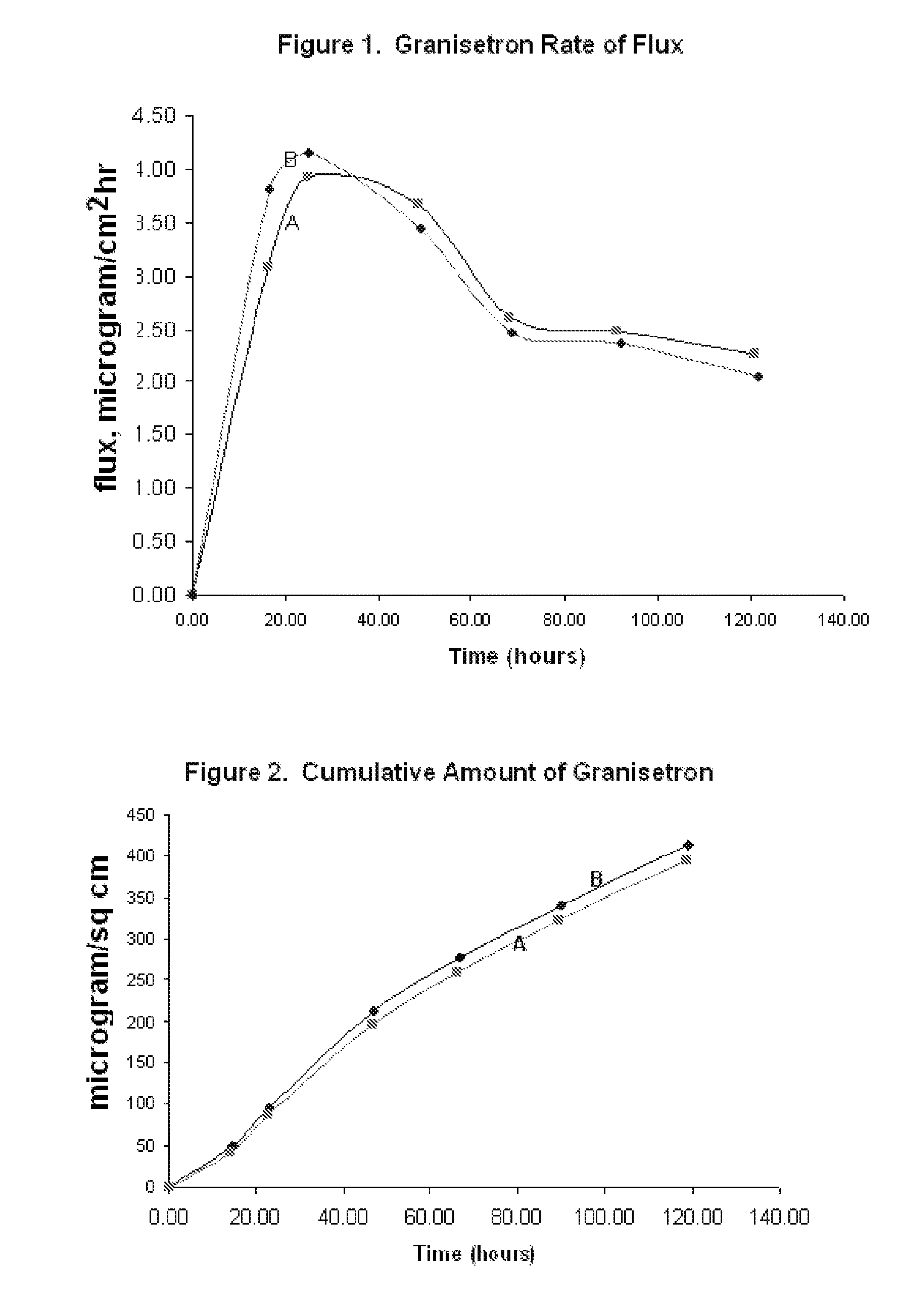
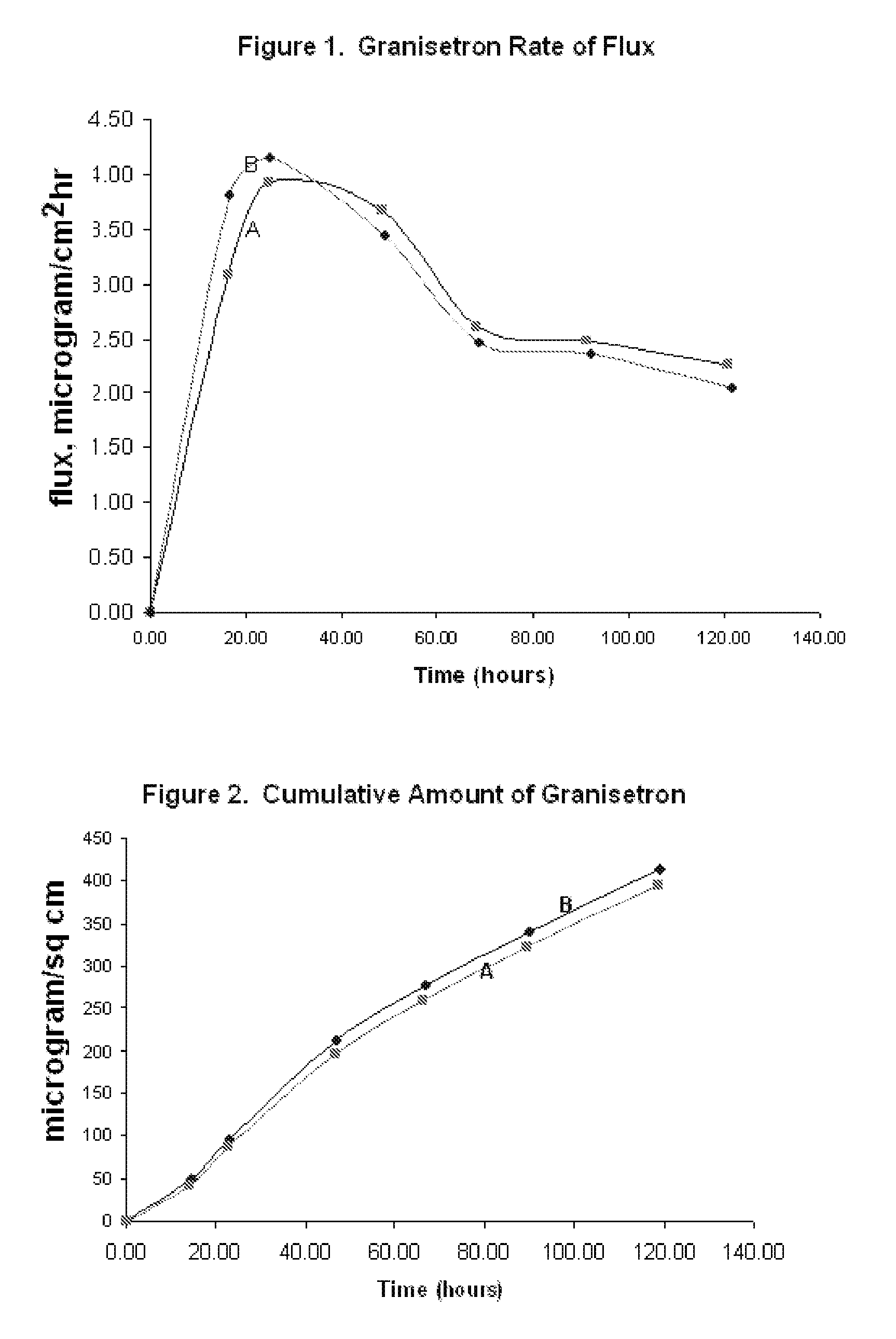
InactiveUS20060263421A1Induce skin irritationLow viscosityBiocideHormonesTransdermal patchIntact skin
Provided, among other things, is a method of treating acute, delayed or anticipatory emesis for a sustained period in an individual, which involves applying to a portion of intact skin on the individual a composition of i. an antiemetically effective amount of a 5-HT3 receptor antagonist; ii. a permeation enhancing amount of permeation enhancer comprising 0.5% to 15% by weight of the skin-contacting layer; and iii. an adhesive, wherein a plasma concentration of the 5-HT3 receptor antagonist in a therapeutically effective range is provided for period of time from an onset time to 12 hours or more after the composition is removed.
Owner:ABEILLE PHARMA
Method for the iontophoretic non-invasive determination of the in vivo concentration level of an inorganic or organic substance
The present invention relates to an vitro device for the removal of ionized substances from a membrane sample without mechanical penetration, which device comprises:(a) a positive electrode;(b) a negative electrode, and(c) electrical insulation between subpart (a) and (b), wherein the positive electrode, and the negative electrode, and electrical insulation are positioned on the same side of the membrane sample.The present invention also relates to a device for the removal of or delivery of ionized substances to a mammal through intact skin or mucosal membrane without mechanical penetration, which device comprises: (a) a positive electrode, (b) a negative electrode, and (c) an electrically insulating material between subpart (a) and (b), wherein the positive electrode, negative electrode and insulating material are physically positioned so that each present a common surface of the device for contact with the same surface of the skin or mucosal membrane of the mammal.The present invention also relates to the use of iontophoresis to determine the level of a charged molecule in a living mammal, and with the use of a feedback mechanism, administer appropriate levels of therapeutic substances.
Owner:RGT UNIV OF CALIFORNIA
Nasal, wound and skin formulations and methods for control of antibiotic-resistant staphylococci and other gram-positive bacteria
Formulations and methods are disclosed which are effective to kill or control bacteria in the nares including gram-positive bacteria strains of S. aureus that are antibiotic resistant (MRSA—methicillin-resistant Staphylococcus aureus. A preferred composition comprises one or more medium-chain alcohols (dodecanol), glycerol monoesters (glycerol monocaprylate or glycerol monolaurate), and / or benzoic acid or benzoic acid analog, in a suitable pharmaceutical carrier, preferably an ointment, along with an odorant compound, preferably eucalyptus oil. The formulations and variations of the formulation may also be used on open wounds or lesions as well as intact skin.
Owner:GUTHERY B EUGENE
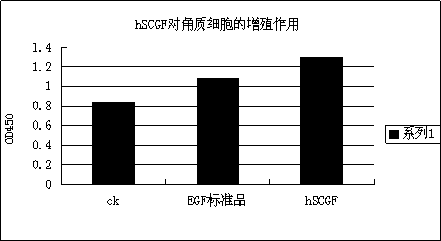
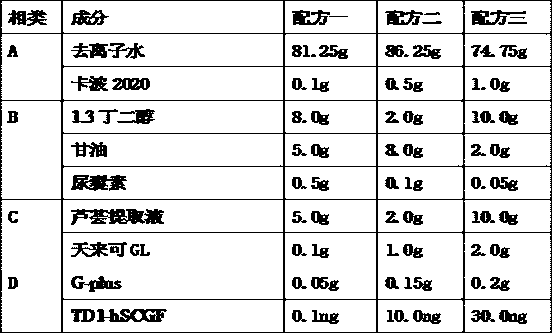
Application of fusion protein in cosmetics
InactiveCN105504066AEasy to pass throughImprove biological activityPolypeptide with localisation/targeting motifCosmetic preparationsMedicineDrug biological activity
The invention provides the application of fusion protein in cosmetics. The fusion protein contains a transdermal peptide and a human stem cell growth factor (hSCGF), and the transdermal peptide is connected to the N end or C end of the hSCGF through a linker peptide or in a covalent mode. The fusion protein can penetrate through intact skin more easily, and high biological activity and skin absorptivity can be maintained.
Owner:GUANGDONG COOWAY BIOTECH CO LTD
Use of penetration enhancers and barrier disruption methods to enhance the immune response of antigen and adjuvant
InactiveUS20080063696A1Enhance immunizationEnhance vaccinationOrganic active ingredientsVirusesAdjuvantWhole body
A transcutaneous immunization system where the topical application of an adjuvant and an antigen or nucleic acid encoding for an antigen, to intact skin induces a systemic or mucosol antibody response. The immune response so elicited can be enhanced by physical or chemical skin penetration enhancement.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Medical disinfecting ultrasound gel composition and preparation method
ActiveCN102580124ALow contact irritationAvoid cross infectionAerosol deliveryEchographic/ultrasound-imaging preparationsPharmacologyCross infections
The invention discloses a medical disinfecting ultrasound gel composition and a preparation method. The Medical disinfected ultrasound gel composition comprises, by mass, 0.1-3% of aldehydes bactericide, 0.3-3% of water-soluble polymer, 0.05-1% of disinfection synergist, 0.05-1% of stabilizing agent, 1-5% of skin moisturizing humectant, a potential of hydrogen (pH) regulating and controlling component and the balance deionized water. Ultrasound gel is not required to be specially disinfected after ultrasonic inspection, can directly kill microorganism on an ultrasonic probe and effectively avoids cross-infection caused by probe contact. The medical disinfecting ultrasound gel composition has low contacting irritation to intact skin, orifices, mucosa, wound and the like, and is non-toxic and free of side effect.
Owner:GUANGDONG UNIV OF TECH
Apparatus and method for preparing cosmeceutical ingredients containing epi-dermal delivery mechanisms
InactiveUS20170181940A1Facilitated DiffusionReduce pressureCosmetic preparationsToilet preparationsRate limitingHypothesis
The skin serves as a barrier that protects the body from the external environment and prevents water loss. This barrier function also prevents most hydrophilic or hydrophobic and large molecular weight ingredients (>500 kDa) from penetrating intact skin. Until recently, methods to increase stratum corneum permeability were generally not effective enough to make the stratum corneum so permeable that the barrier posed by the viable epidermis mattered. However, that has now changed with the development of the present embodiment's physical methods and highly optimized chemical formulations, such that we revisited the permeability of the full epidermis with the example embodiment's constructs and not focus only on the stratum corneum. This example embodiment therefore tests the hypothesis that the viable epidermis offers a significant permeability barrier to both small molecules and macromolecules that becomes the rate limiting step.
Owner:PPP&C INC
Nasal, wound and skin formulations and methods for control of antibiotic-resistant staphylococci and other gram-positive bacteria
Formulations and methods are disclosed which are effective to kill or control bacteria in the nares including gram-positive bacteria strains of S. aureus that are antibiotic resistant (MRSA—methicillin-resistant Staphylococcus aureus. A preferred composition comprises one or more medium-chain alcohols (dodecanol), glycerol monoesters (glycerol monocaprylate or glycerol monolaurate), and / or benzoic acid or benzoic acid analog, in a suitable pharmaceutical carrier, preferably an ointment, along with an odorant compound, preferably eucalyptus oil. The formulations and variations of the formulation may also be used on open wounds or lesions as well as intact skin.
Owner:GUTHERY B EUGENE
Multifunctional sickbed support frame
The invention discloses a multifunctional sickbed support frame. In use, firstly, a medical worker places the support frame directly above a wound of a patient, a pressing plate moves in the oppositedirection, so that the support frame is firmly connected with the sickbed; the medical work reuses a heating pad and an exhaust fan to keep the patient's wound in a constant temperature state so as toreduce the pain of the patient's wound; and at the same time, under the action of a first electric push rod and a second electric push rod, a second slide plate and a third slide plate can allow static distance contact between a cover and the intact skin around the patient's wound according to actual use conditions. The multifunctional sickbed support frame is skillful in structure, is powerful in function, is simple to operate. The patient's wound position can be separated from the cover by using the support frame so as to protect the patient's wound; the recovery progress of the patient isaccelerated, and the workload of the medical staff is reduced.
Owner:芜湖市中医医院
Electrotransport agent delivery apparatus
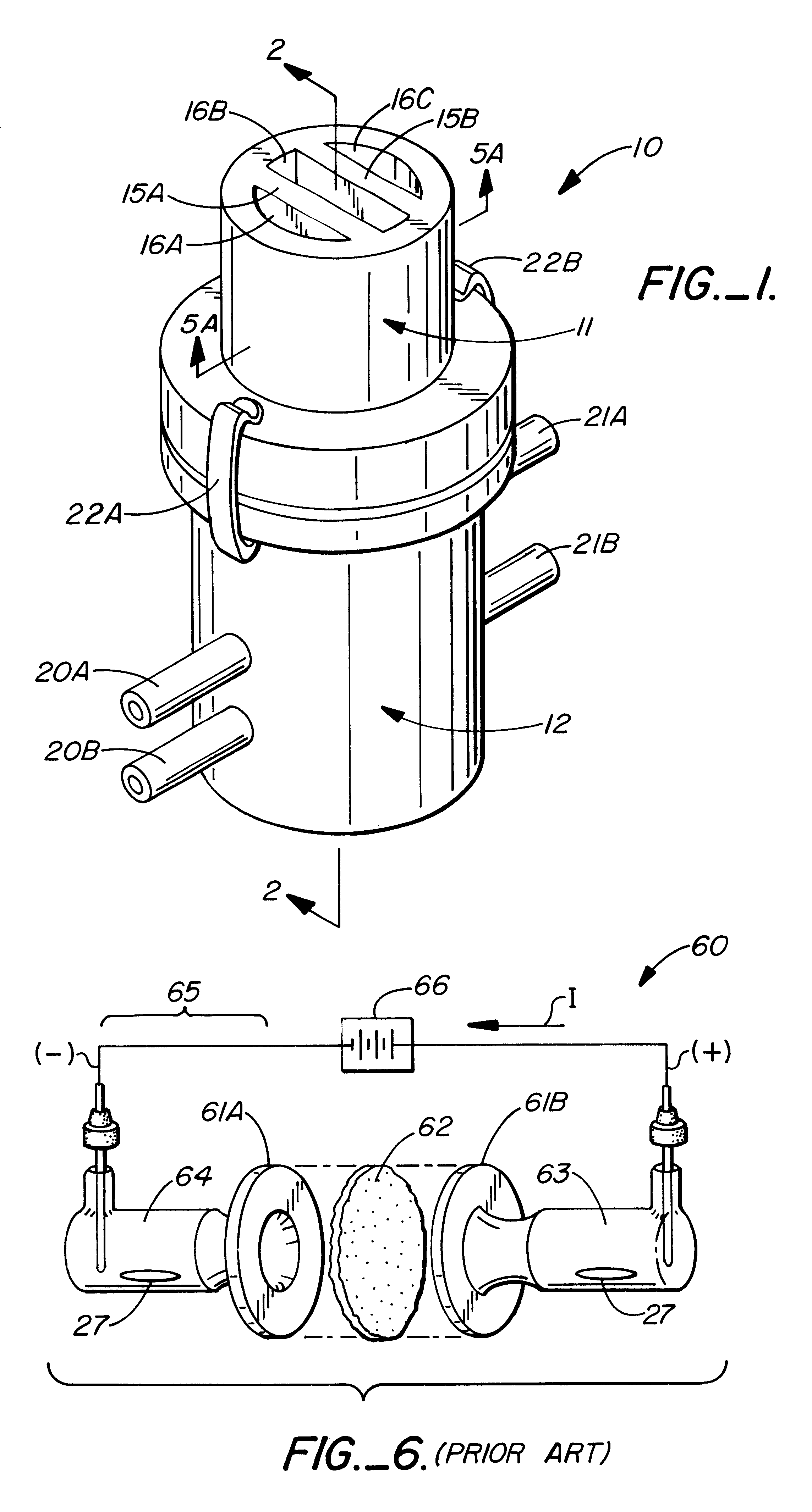
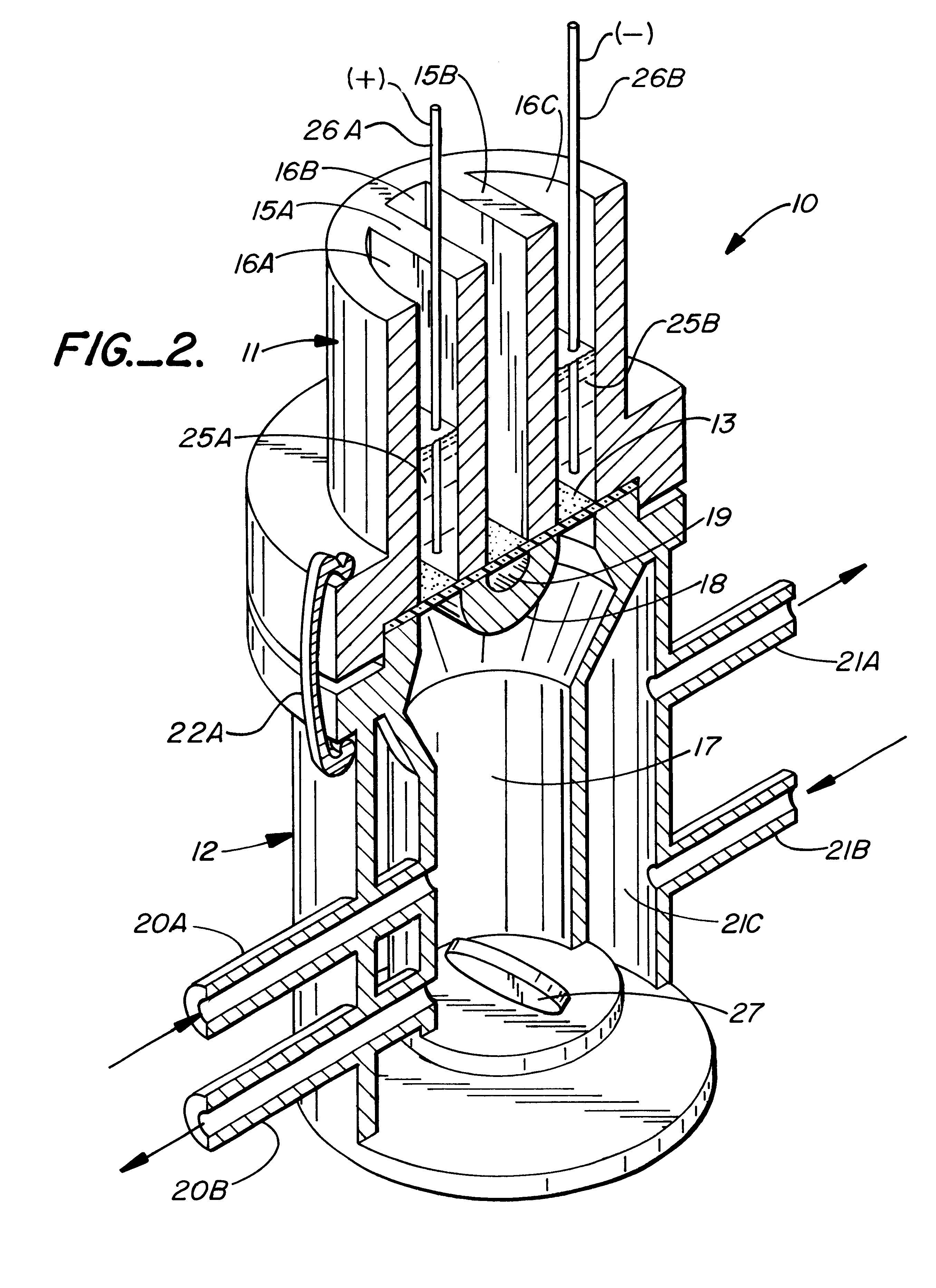
InactiveUS7136698B2Enhancement of electrotransport transdermal agent delivery efficiencyImprove efficiencyElectrotherapyElectricityCritical level
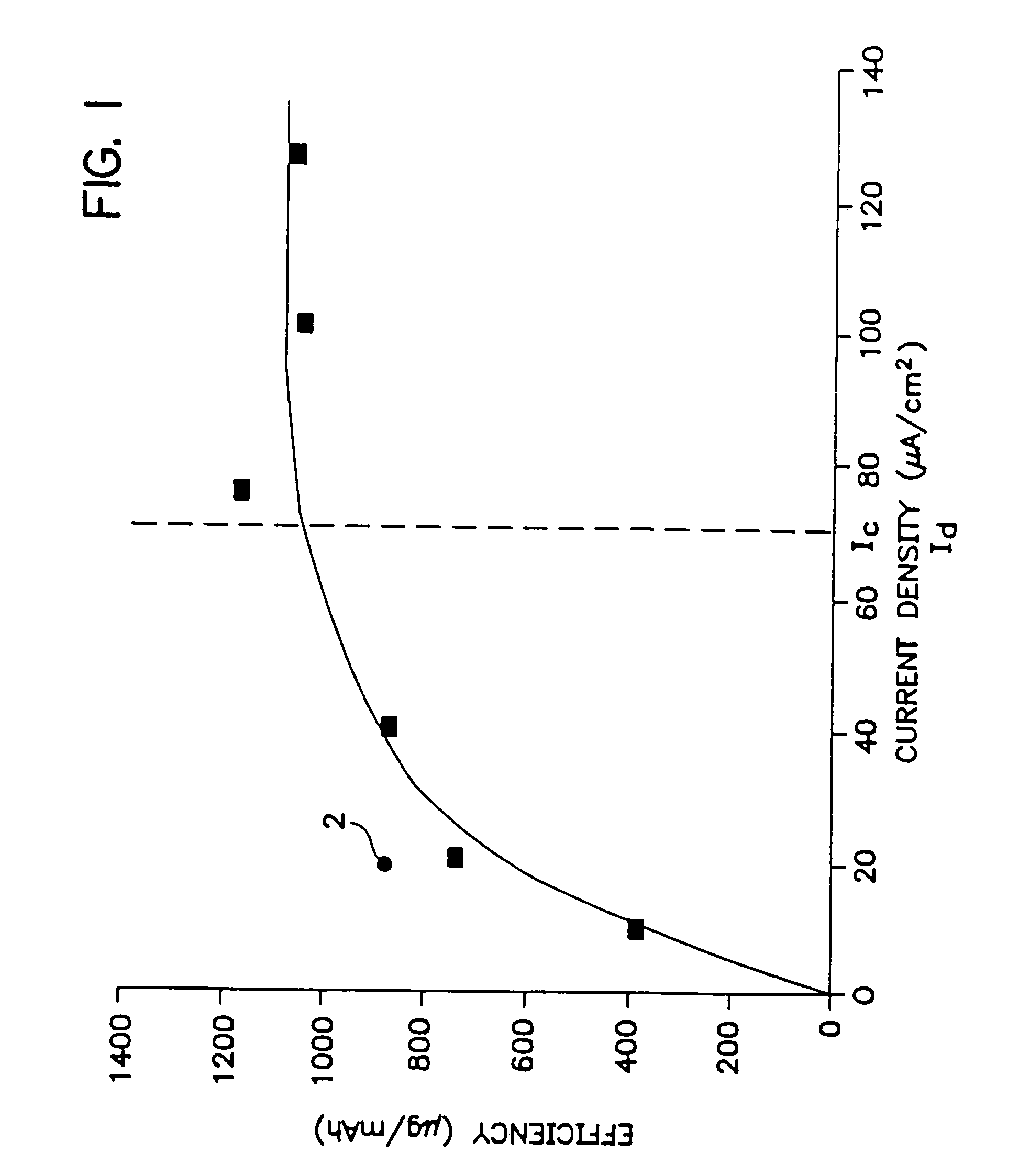
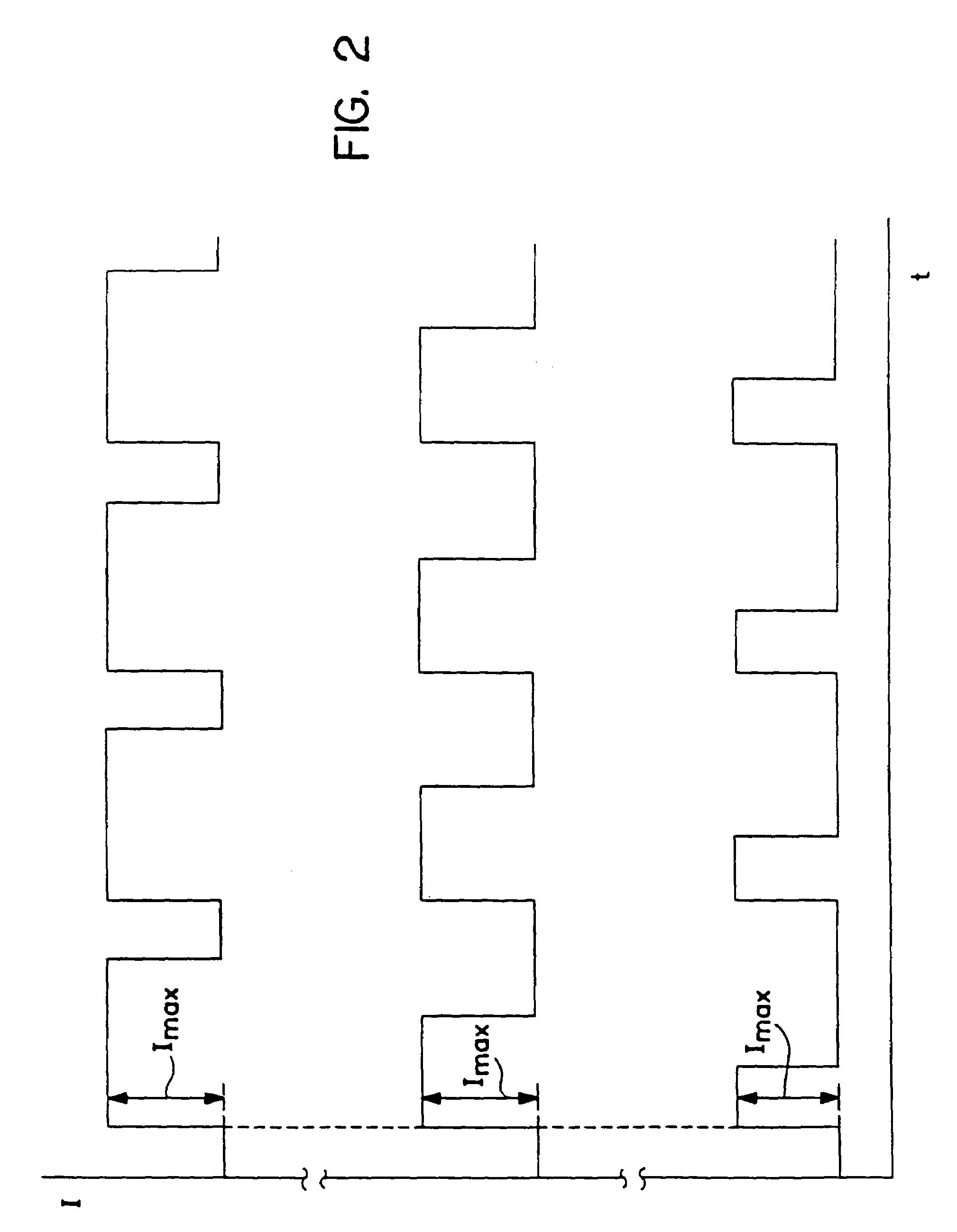
An electrotransport agent delivery device (10) for delivering a therapeutic agent through intact skin, and a method of operating same, is provided. The device applies a pulsing electrotransport current wherein the length of the applied current pulses is at least 5 msec and preferably at least 10 msec. Most preferably, the current pulses have a magnitude above a critical level (Ic) at which the skin is transformed into a higher electrotransport delivery efficiency (E) state.
Owner:ALZA CORP
Compositions and methods for modifying hair color
Compositions and methods for darkening hair of intact skin are presented. Such compositions include defensins in concentrations that are below those that exhibit antimicrobial activity, and can be in the form of a topically applied formulation. Various formulations for such compositions, which can include various pharmaceutically acceptable stabilizers, emollients, and fragrances, are provided.
Owner:MEDICELL TECH LLC
Apparatus and Method for Preparing Cosmeceutical Ingredients Containing Epi-Dermal Delivery Mechanisms
ActiveUS20170172863A1Improve stabilityImprove breathabilityCosmetic preparationsToilet preparationsRate limitingHypothesis
The skin serves as a barrier that protects the body from the external environment and prevents water loss. This barrier function also prevents most hydrophilic or hydrophobic and large molecular weight ingredients (>500 kDa) from penetrating intact skin. Until recently, methods to increase stratum corneum permeability were generally not effective enough to make the stratum corneum so permeable that the barrier posed by the viable epidermis mattered. However, that has now changed with the development of the present embodiment's physical methods and highly optimized chemical formulations, such that we revisited the permeability of the full epidermis with the example embodiment's constructs and not focus only on the stratum corneum. This example embodiment therefore tests the hypothesis that the viable epidermis offers a significant permeability barrier to both small molecules and macromolecules that becomes the rate limiting step.
Owner:PPP&C INC
Natural sugarcane juice processing method
InactiveCN109527307APreserve the flavorRetain nutrientsFood ingredient as antioxidantFood membrane processFiltrationUltrafiltration
The invention relates to a natural sugarcane juice processing method. The natural sugarcane juice processing method comprises the following steps: cutting, washing, squeezing, coarse filtering, blending, ultrafiltration, filling, and refrigerating. The sugarcane with intact skin and no pests and diseases can be cut into small segments, the dirt on skin is washed, and then squeezing and coarse filtration are carried out; the sugarcane juice after coarse filtration is prepared and then subjected to ultrafiltration; the ultrafiltered sugarcane juice is filled into a non-transparent PET bottle andsealed with an aluminum foil; and the packaged sugarcane juice is subjected to low temperature storage. The method can effectively remove microorganisms, inhibits the loss of nutrients, prolongs theshelf life of the sugarcane juice products, ensure the sensory and nutritional quality of the sugarcane juice, and has the advantages of simple operation and low cost; has good market application prospect, and has great significance for extending a sugarcane industry chain and increasing the economic income of sugarcane farmers.
Owner:FUJIAN AGRI & FORESTRY UNIV
Transdermal method and patch for emesis
InactiveUS8246981B2Induce skin irritationLow viscosityHormonesDigestive systemTransdermal patchAdhesive
Provided, among other things, is a method of treating acute, delayed or anticipatory emesis for a sustained period in an individual, which involves applying to a portion of intact skin on the individual a composition ofi. an antiemetically effective amount of a 5-HT3 receptor antagonist;ii. a permeation enhancing amount of permeation enhancer comprising 0.5% to 15% by weight of the skin-contacting layer; andiii. an adhesive.
Owner:ABEILLE PHARMA
Apparatus and method for preparing cosmeceutical ingredients containing epi-dermal delivery mechanisms
InactiveUS20170181937A1Facilitated DiffusionReduce pressureCosmetic preparationsToilet preparationsRate limitingEnvironmental effect
The skin serves as a barrier that protects the body from the external environment and prevents water loss. This barrier function also prevents most hydrophilic or hydrophobic and large molecular weight ingredients (>500 kDa) from penetrating intact skin. Until recently, methods to increase stratum corneum permeability were generally not effective enough to make the stratum corneum so permeable that the barrier posed by the viable epidermis mattered. However, that has now changed with the development of the present embodiment's physical methods and highly optimized chemical formulations, such that we revisited the permeability of the full epidermis with the example embodiment's constructs and not focus only on the stratum corneum. This example embodiment therefore tests the hypothesis that the viable epidermis offers a significant permeability barrier to both small molecules and macromolecules that becomes the rate limiting step.
Owner:PPP&C INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com