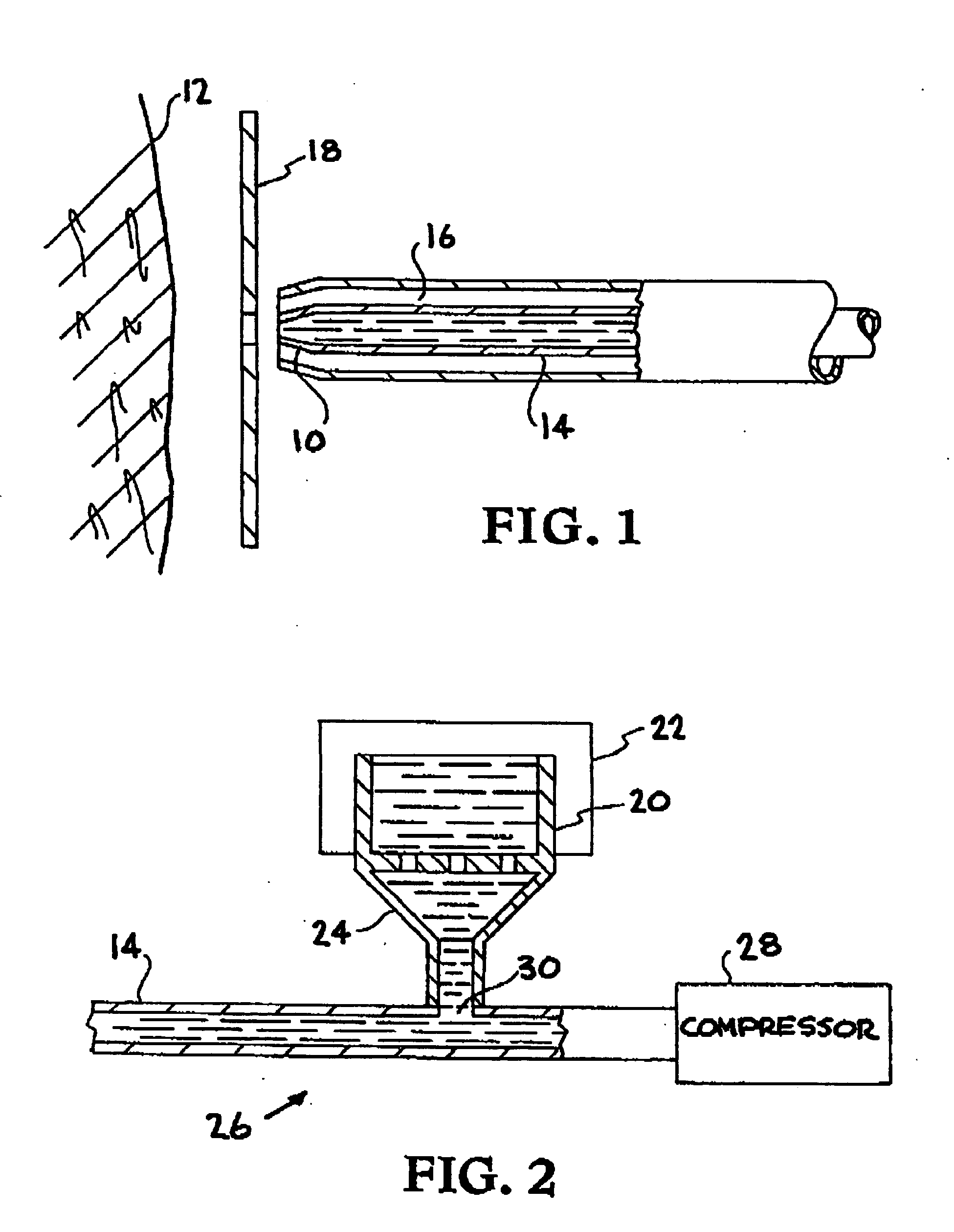
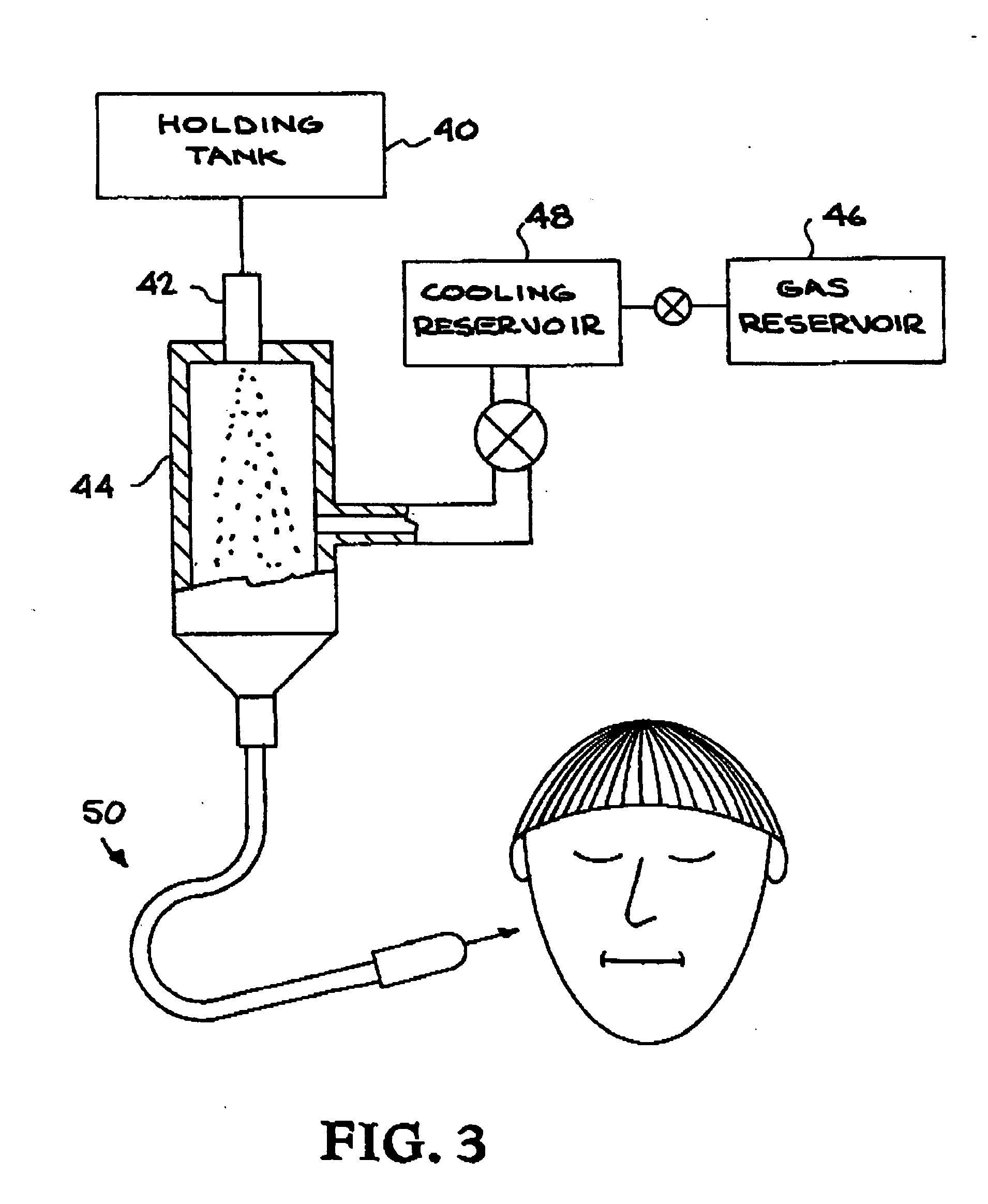
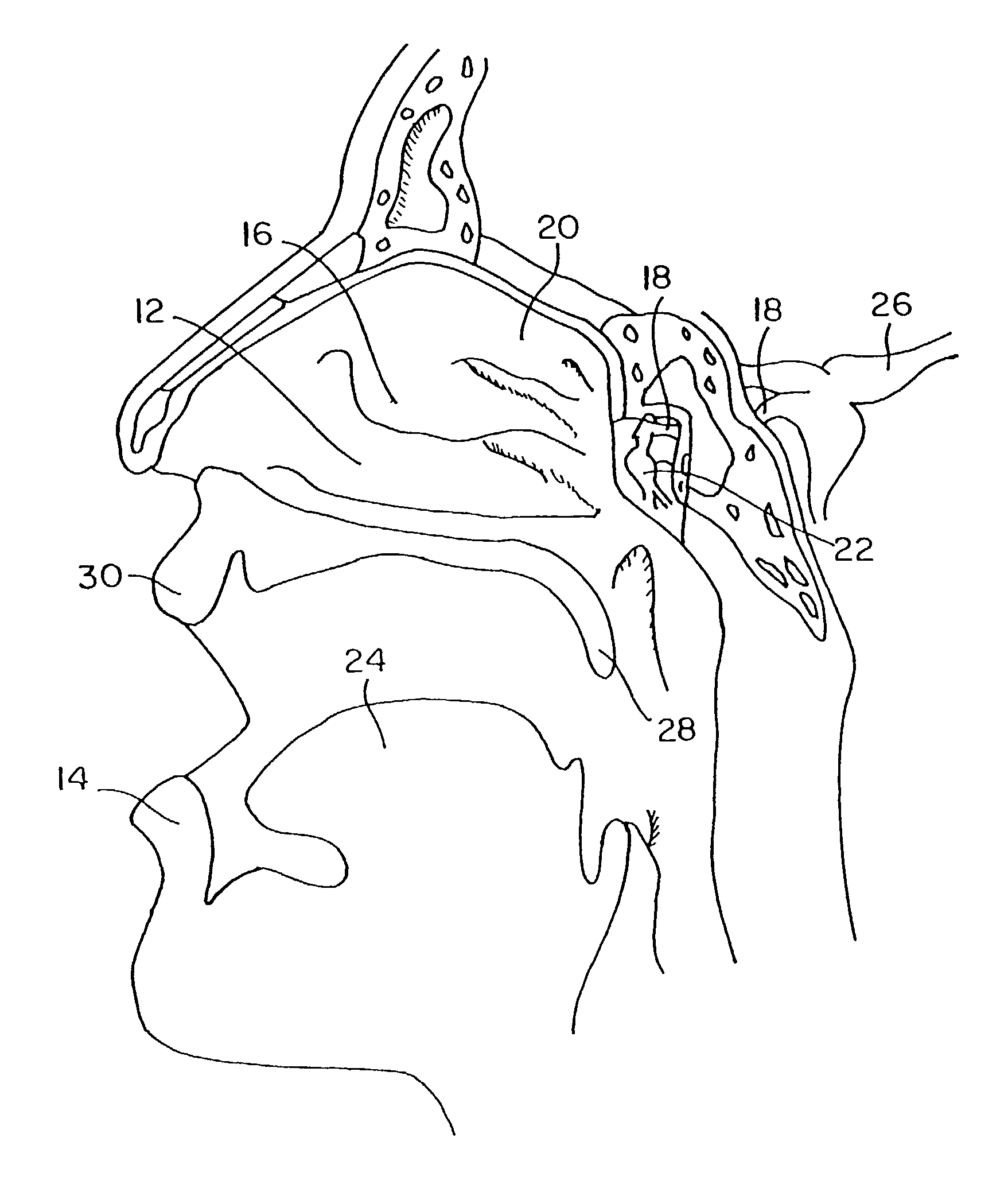
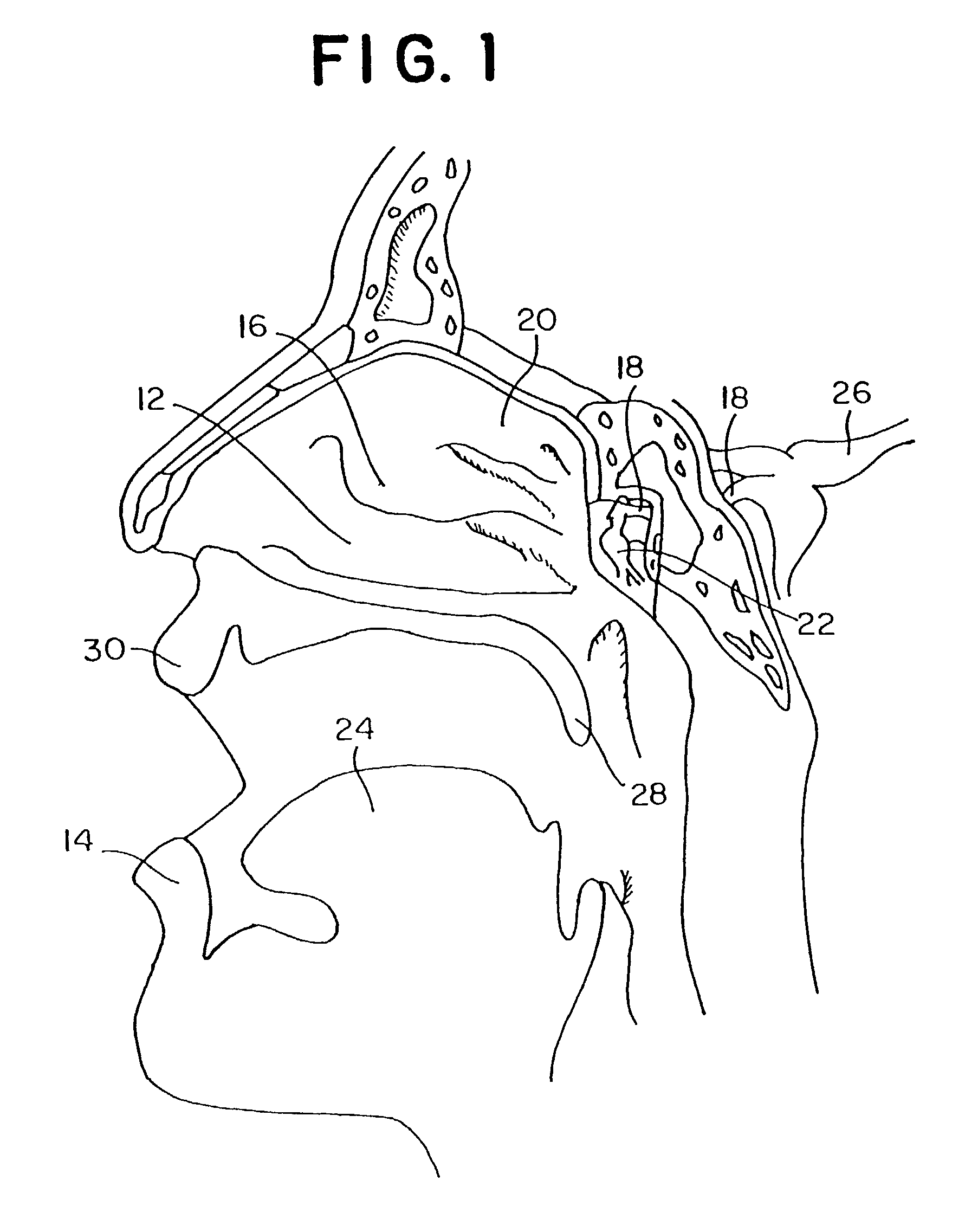
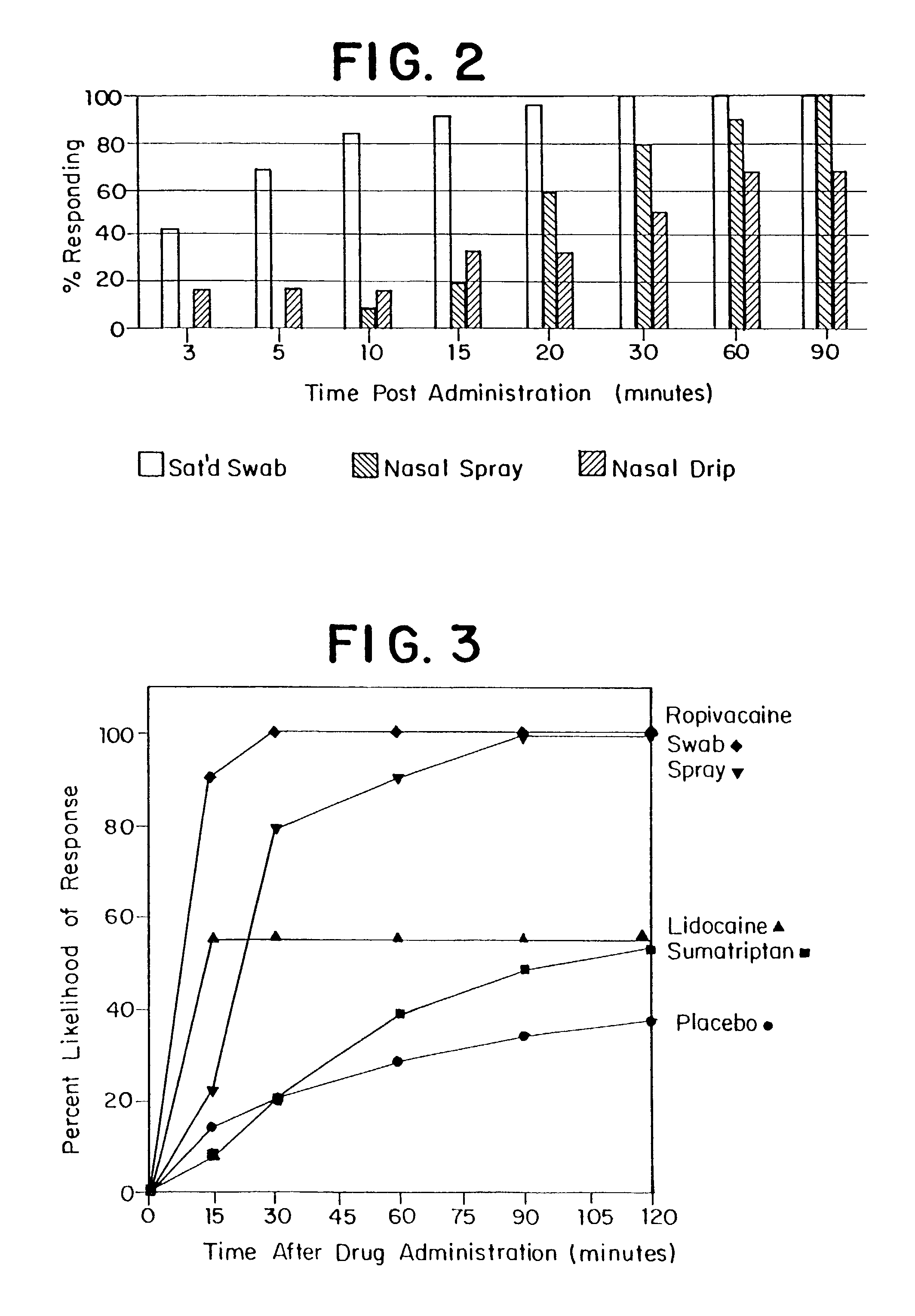
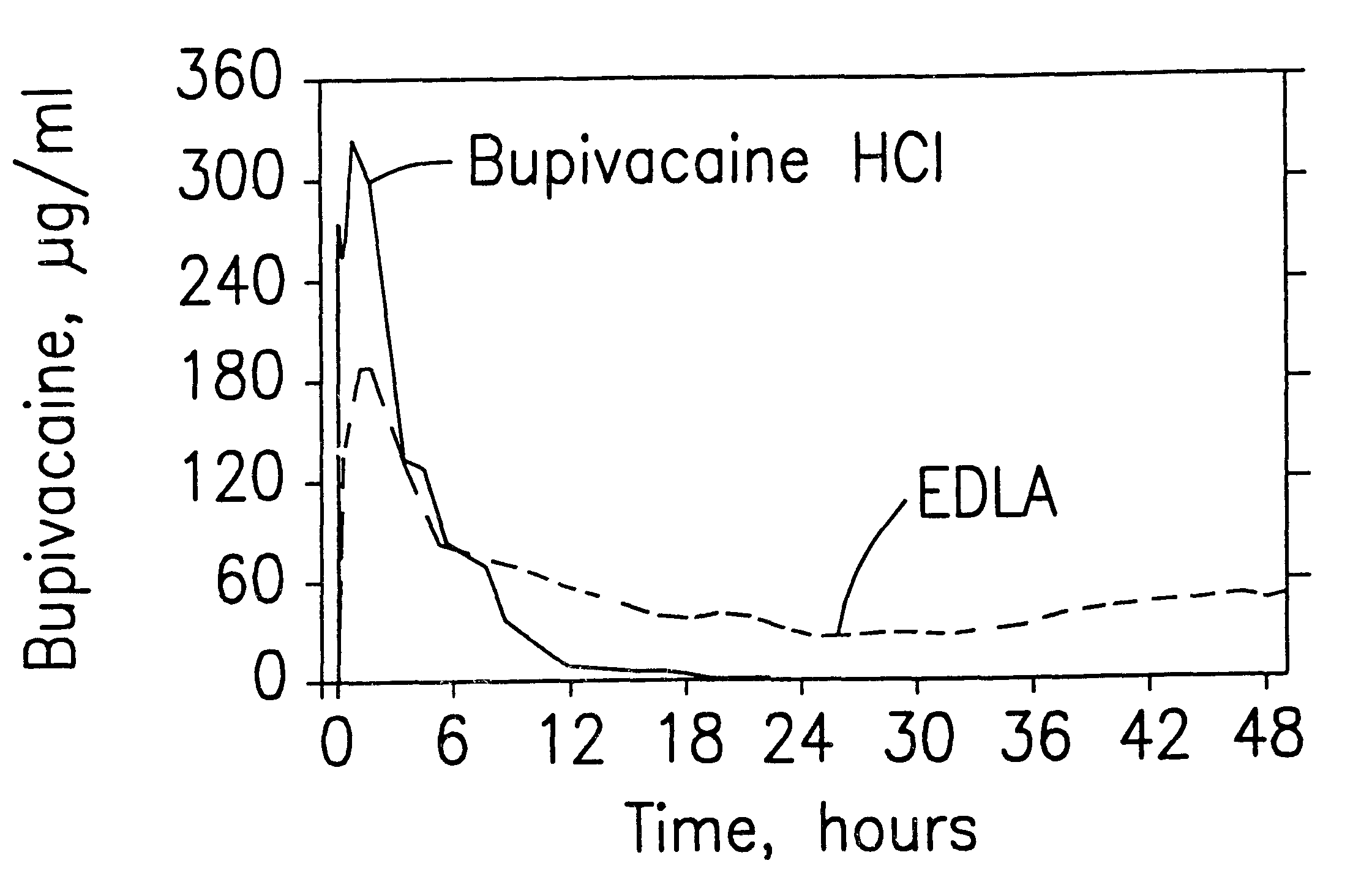
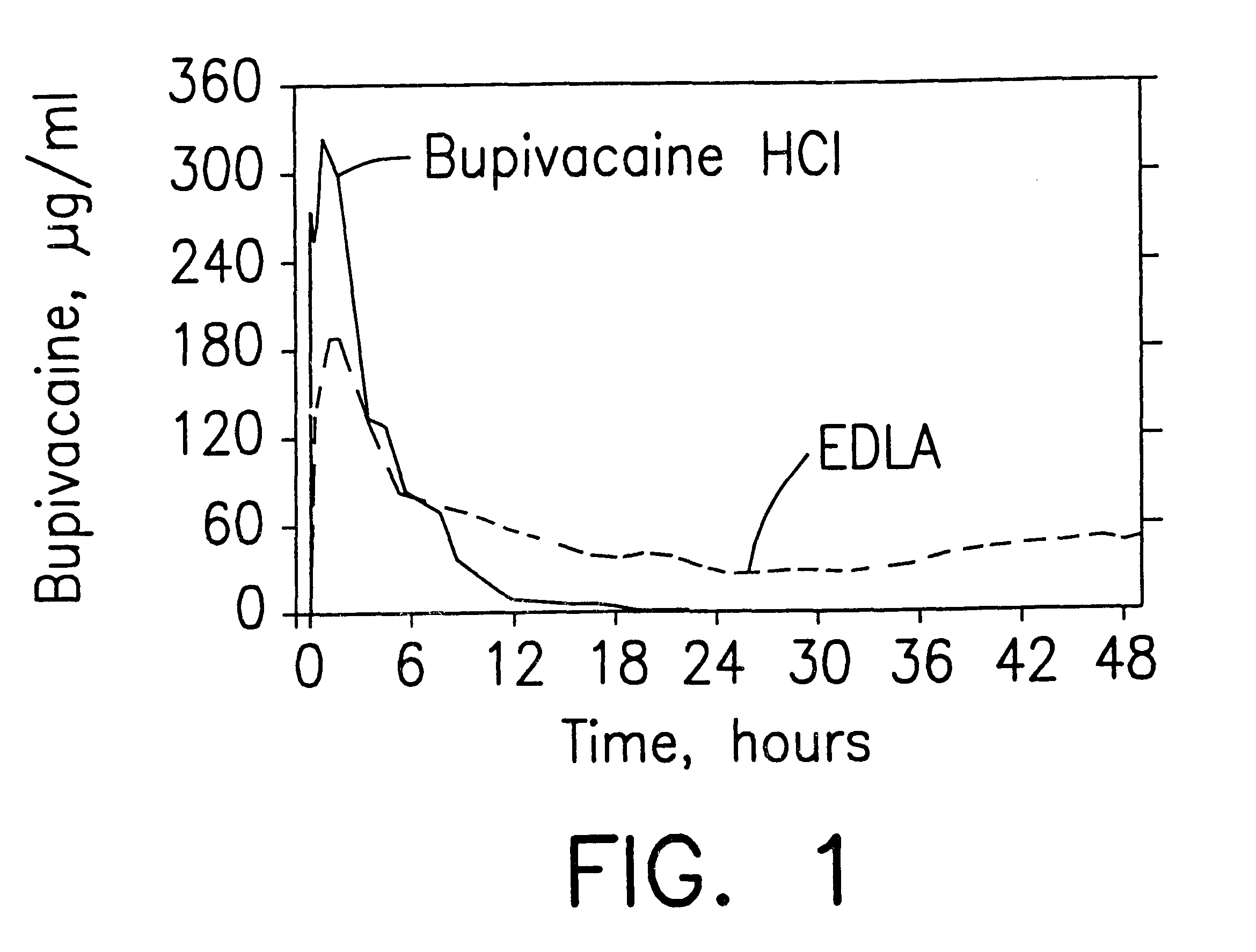
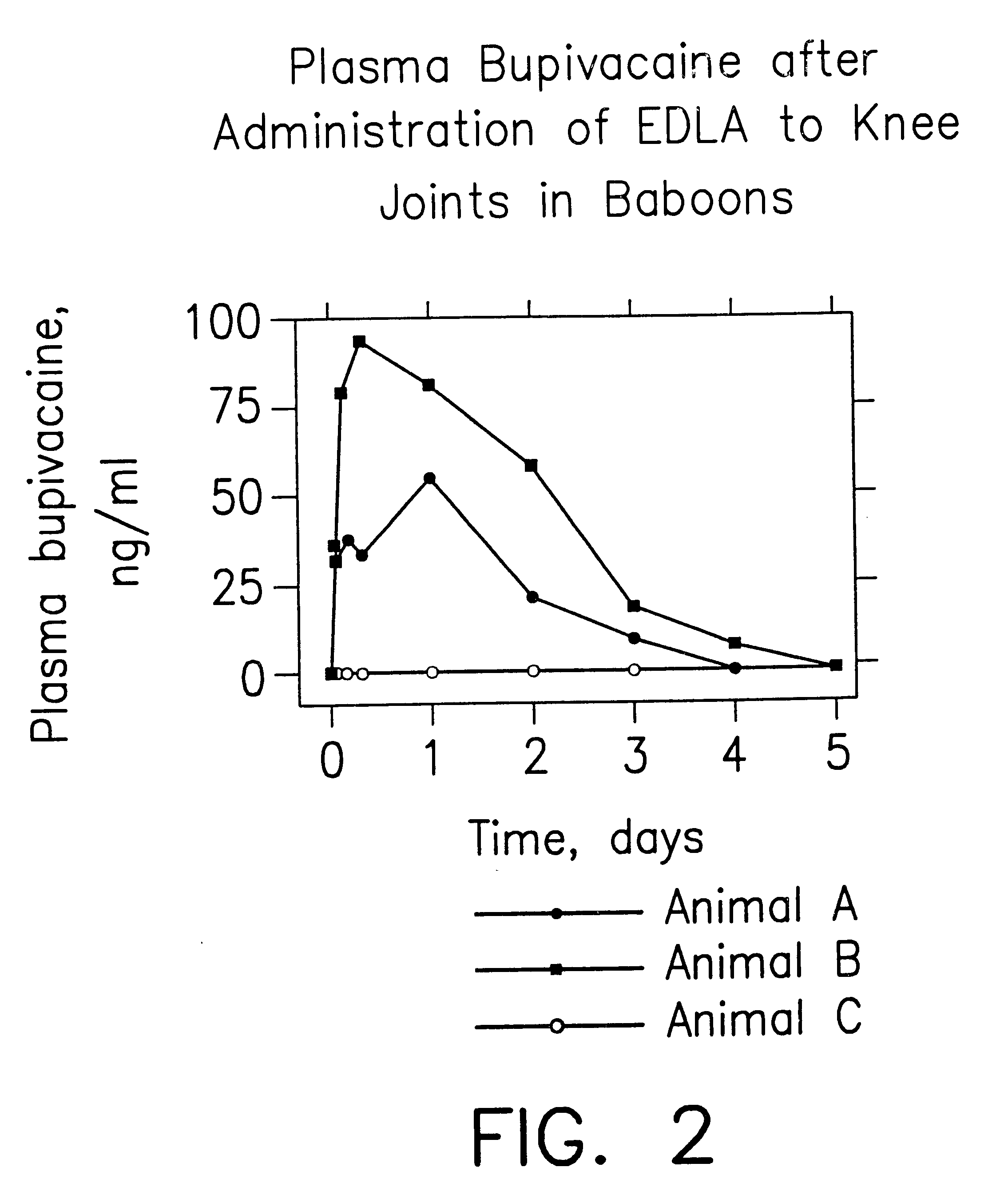
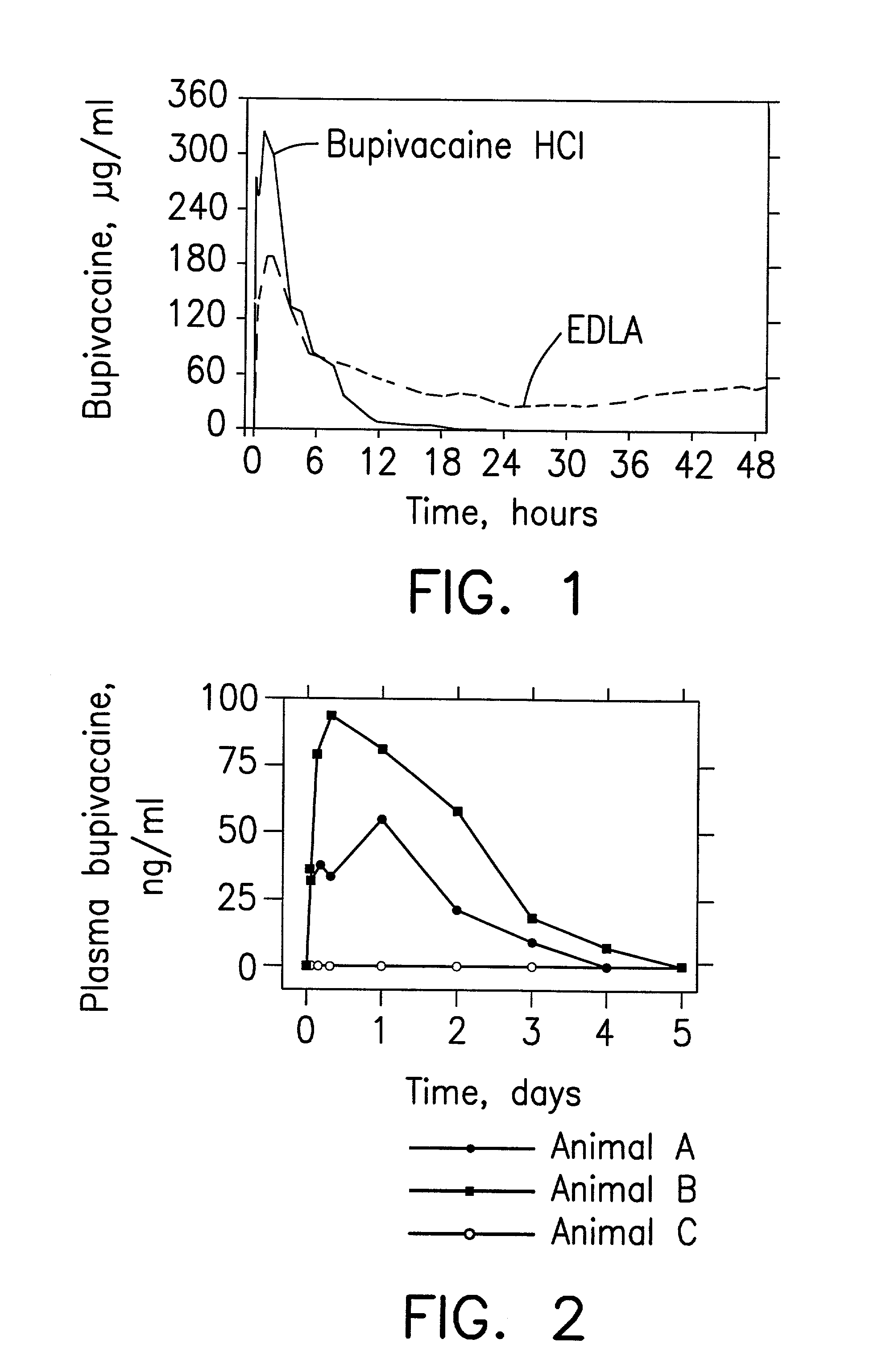
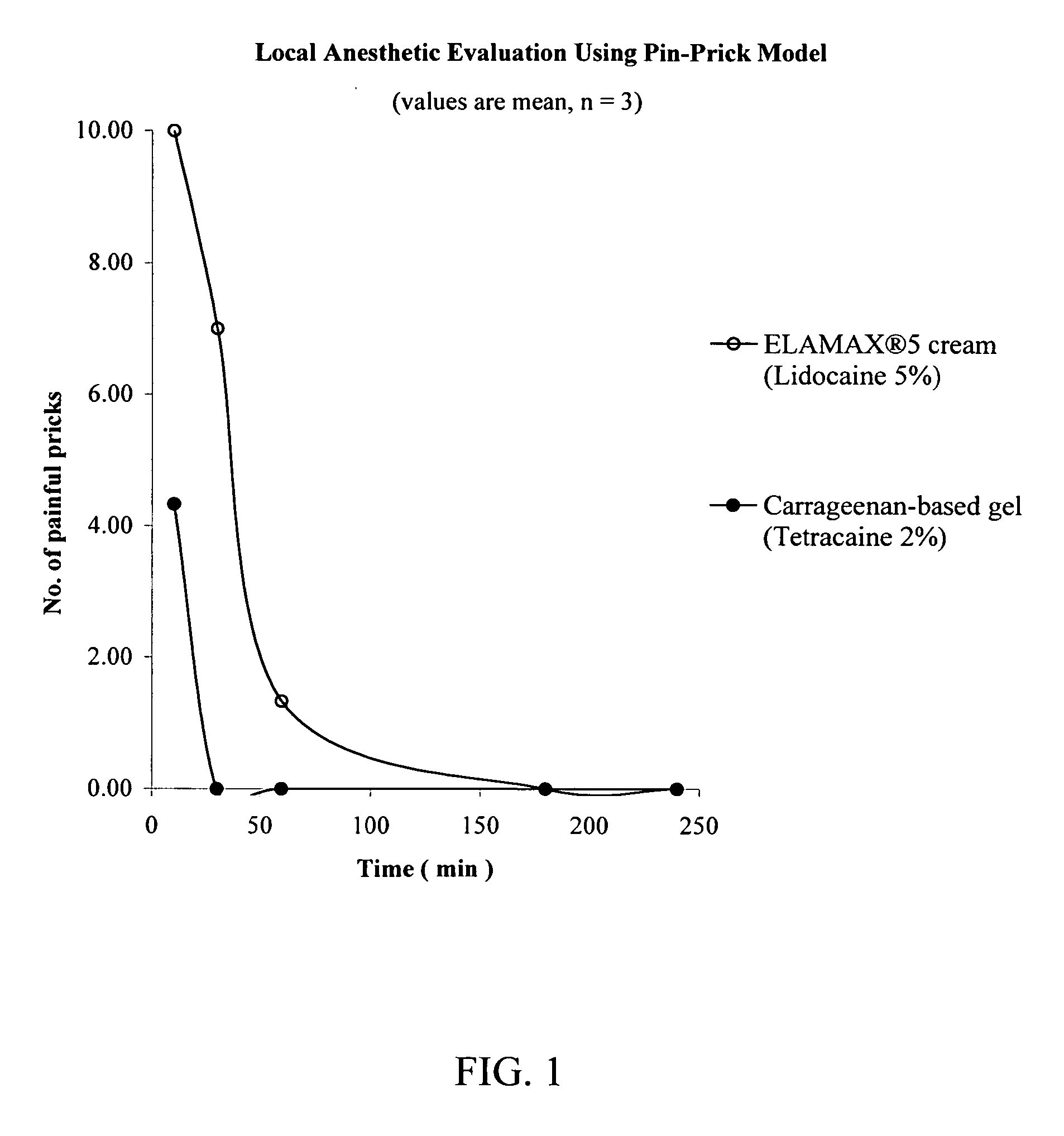
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
356 results about "Topical anesthetic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
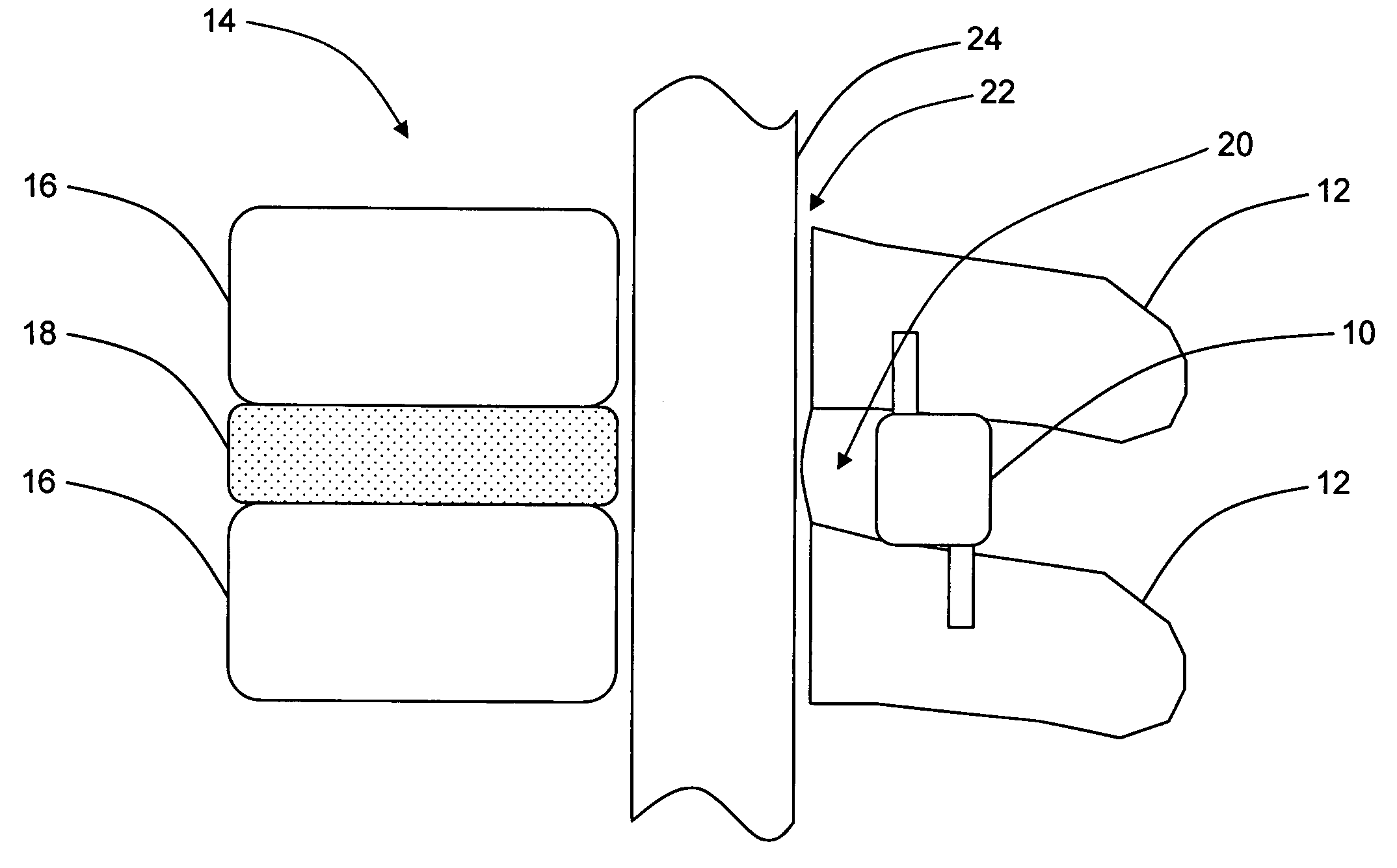
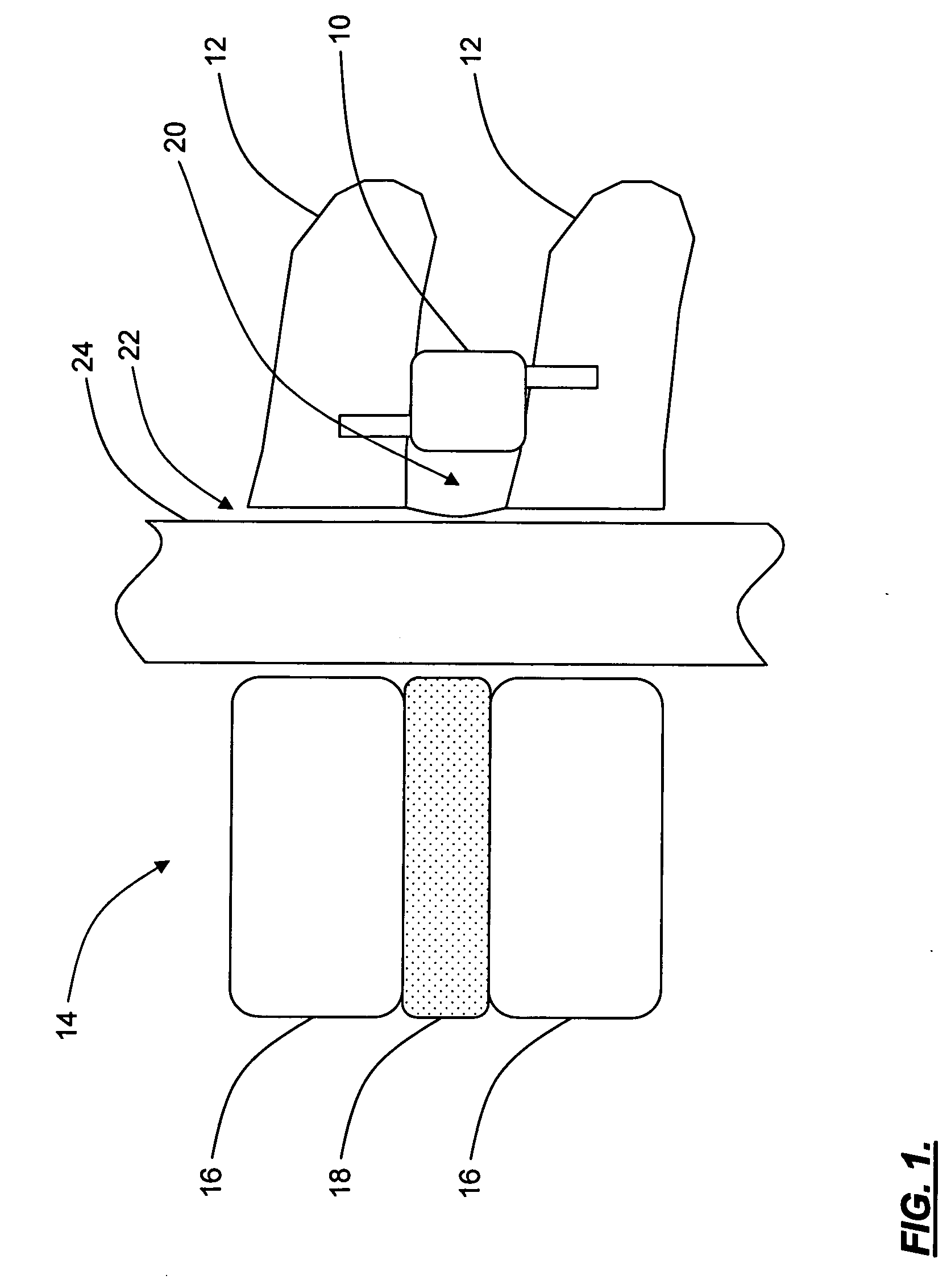
A topical anesthetic is a local anesthetic that is used to numb the surface of a body part. They can be used to numb any area of the skin as well as the front of the eyeball, the inside of the nose, ear or throat, the anus and the genital area. Topical anesthetics are available in creams, ointments, aerosols, sprays, lotions, and jellies. Examples include benzocaine, butamben, dibucaine, lidocaine, oxybuprocaine, pramoxine, proparacaine, proxymetacaine, and tetracaine (also named amethocaine).
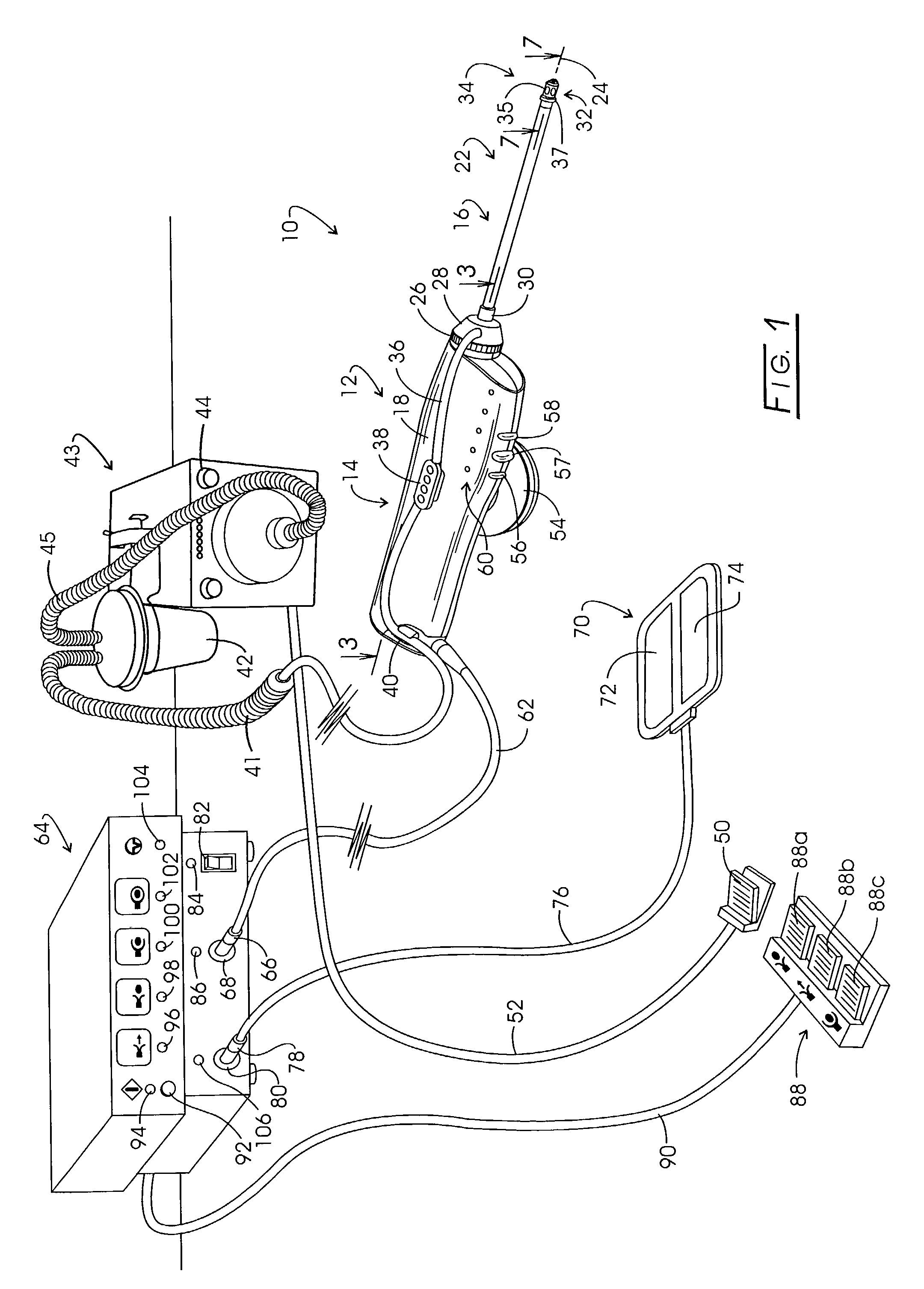
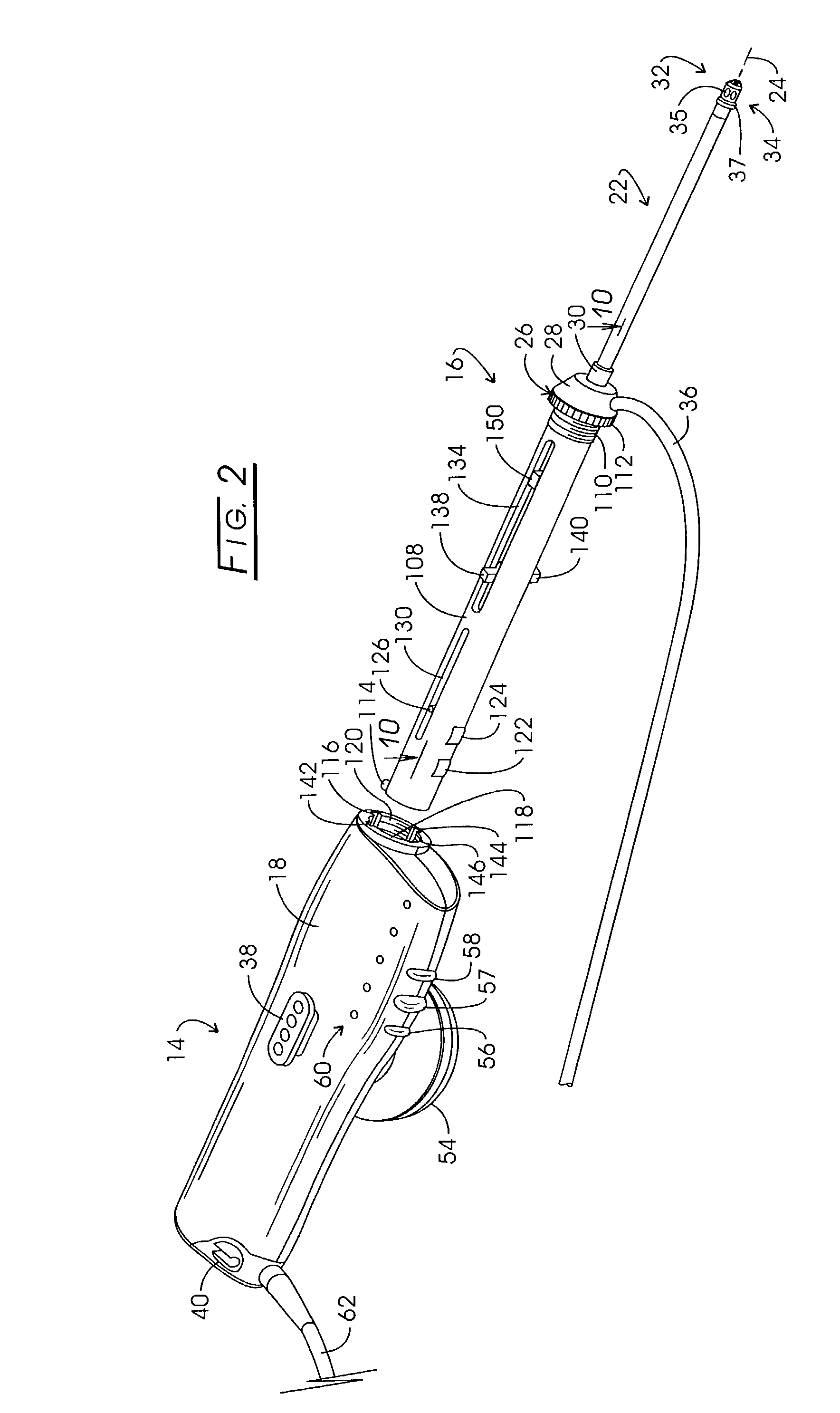
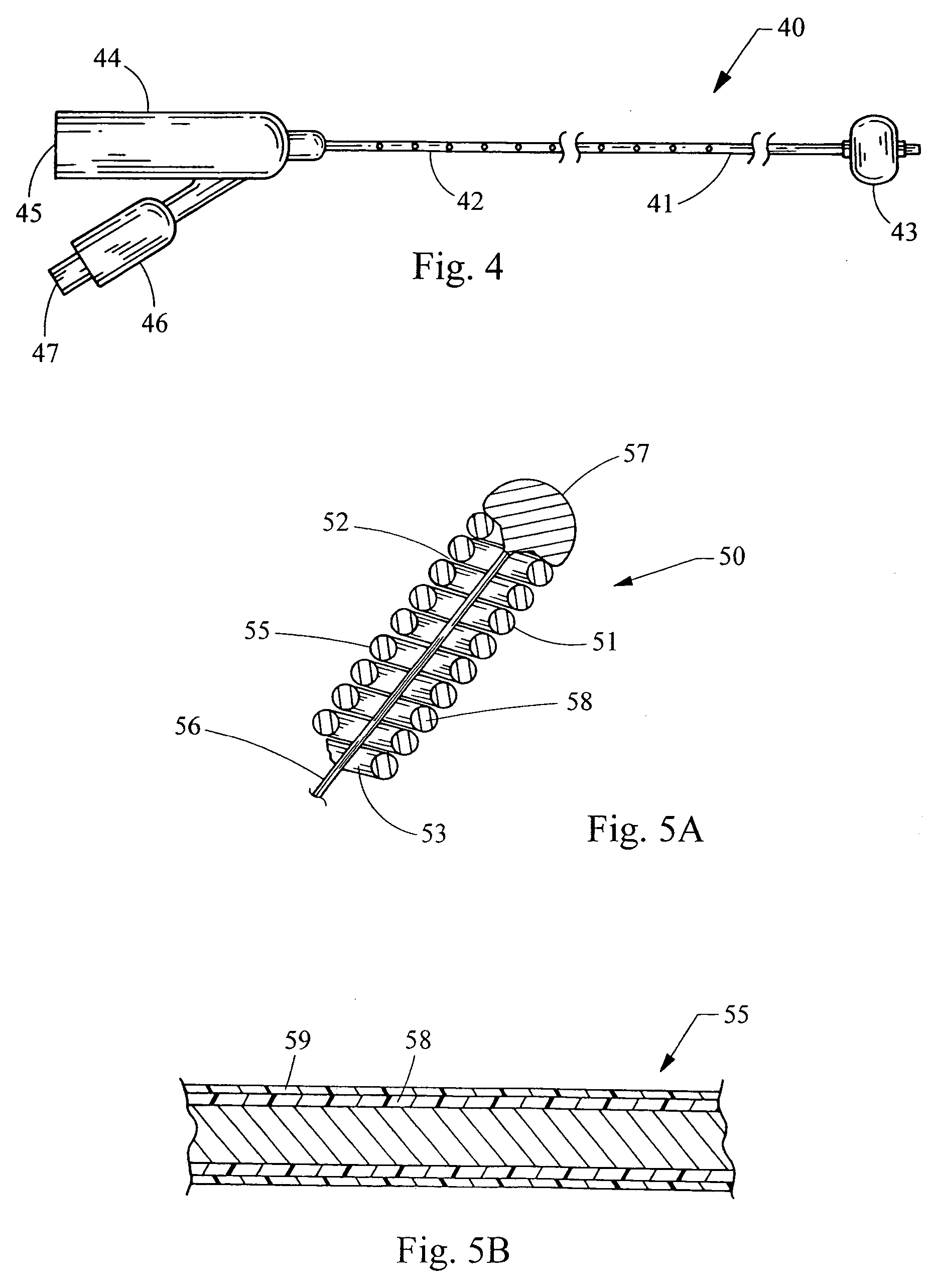
Electrosurgery with infiltration anesthesia
InactiveUS20050267455A1Reliable formationAnaesthesiaSurgical instruments for heatingElectrical resistance and conductanceElectrosurgery
Method for carrying out the recovery of an intact volume of tissue wherein a delivery cannula tip is positioned in confronting adjacency with the volume of tissue to be recovered. The electrosurgical generator employed to form an arc at a capture component extending from the tip is configured having a resistance-power profile which permits recovery of the specimen without excessive thermal artifact while providing sufficient power to sustain a cutting arc. For the recovery procedure, a local anesthetic employing a diluent which exhibits a higher resistivity is utilized and the method for deploying the capture component involves an intermittent formation of a cutting arc with capture component actuation interspersed with pauses of duration effective to evacuate any accumulation or pockets of local anesthetic solution encountered by the cutting electrodes.
Owner:INTACT MEDICAL
Electrosurgery with infiltration anesthesia
InactiveUS7004174B2AnaesthesiaVaccination/ovulation diagnosticsElectrical resistance and conductanceElectrosurgery
Method for carrying out the recovery of an intact volume of tissue wherein a delivery cannula tip is positioned in confronting adjacency with the volume of tissue to be recovered. The electrosurgical generator employed to form an arc at a capture component extending from the tip is configured having a resistance-power profile which permits recovery of the specimen without excessive thermal artifact while providing sufficient power to sustain a cutting arc. For the recovery procedure, a local anesthetic employing a diluent which exhibits a higher resistivity is utilized and the method for deploying the capture component involves an intermittent formation of a cutting arc with capture component actuation interspersed with pauses of duration effective to evacuate any accumulation or pockets of local anesthetic solution encountered by the cutting electrodes.
Owner:COVIDIEN AG
Interspinous distraction devices and associated methods of insertion
InactiveUS20060106397A1Securely holdInternal osteosythesisJoint implantsDistractionMinimally invasive procedures
In various embodiments, the present invention provides a plurality of novel interspinous distraction devices and associated methods of insertion. The interspinous distraction devices of the present invention are designed and configured to effectively treat such conditions as lumbar spinal stenosis and degenerative disc disease. Advantageously, the interspinous distraction devices of the present invention may be inserted through conventional open procedures, typically requiring a relatively large incision and a general anesthetic, or through novel minimally-invasive procedures, typically requiring only a local anesthetic. These novel minimally-invasive procedures and related enabling devices are also disclosed and described herein.
Owner:ZIMMER BIOMET SPINE INC
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Skin resurfacing and treatment using biocompatible materials
InactiveUS20050059940A1Eliminate the problemAvoid infectionSurgeryMedical devicesHuman bodyCarrier fluid
Biocompatible materials are propelled at the skin with sufficient velocity to cause desired resurfacing of skin layers to the desired penetration depth. The materials, such as dry ice or water ice, are harmonious with the human body and thus eliminate foreign body reactions. Various materials may be used in combination, including local anesthetics and vasoconstrictors in solid or liquid form. The biocompatible solid or liquid particles are suspended in a cold carrier fluid and propelled through an insulated delivery system to the surface of the skin. The treatment of diseased skin lesions may be accomplished using the present invention as a drug delivery system.
Owner:PEARL TECHNOLOGY HOLDINGS LLC
Method for directed intranasal administration of a composition
Methods, kits, apparatus, and compositions for inhibiting a cerebral neurovascular disorder, a muscular headache, or cerebral inflammation in a human patient are provided. The methods comprise intranasally administering to the patient a pharmaceutical composition comprising a local anesthetic, and preferably a long-acting local anesthetic ingredient. A composition useful for practicing the methods of the invention is described which comprises at least one local anesthetic in a pharmaceutically acceptable carrier, wherein the composition is formulated for intranasal delivery. Cerebral neurovascular disorders include migraine and cluster headache. Muscular headaches include tension headaches and muscle contraction headaches. A kit comprising the composition and an intranasal applicator and a method of systemically delivering a pharmaceutically active agent to an animal are also included in the invention. Apparatus for directed intranasal administration of the compositions of the invention and for performing the methods of the invention are also described.
Owner:BHL PATENT HLDG
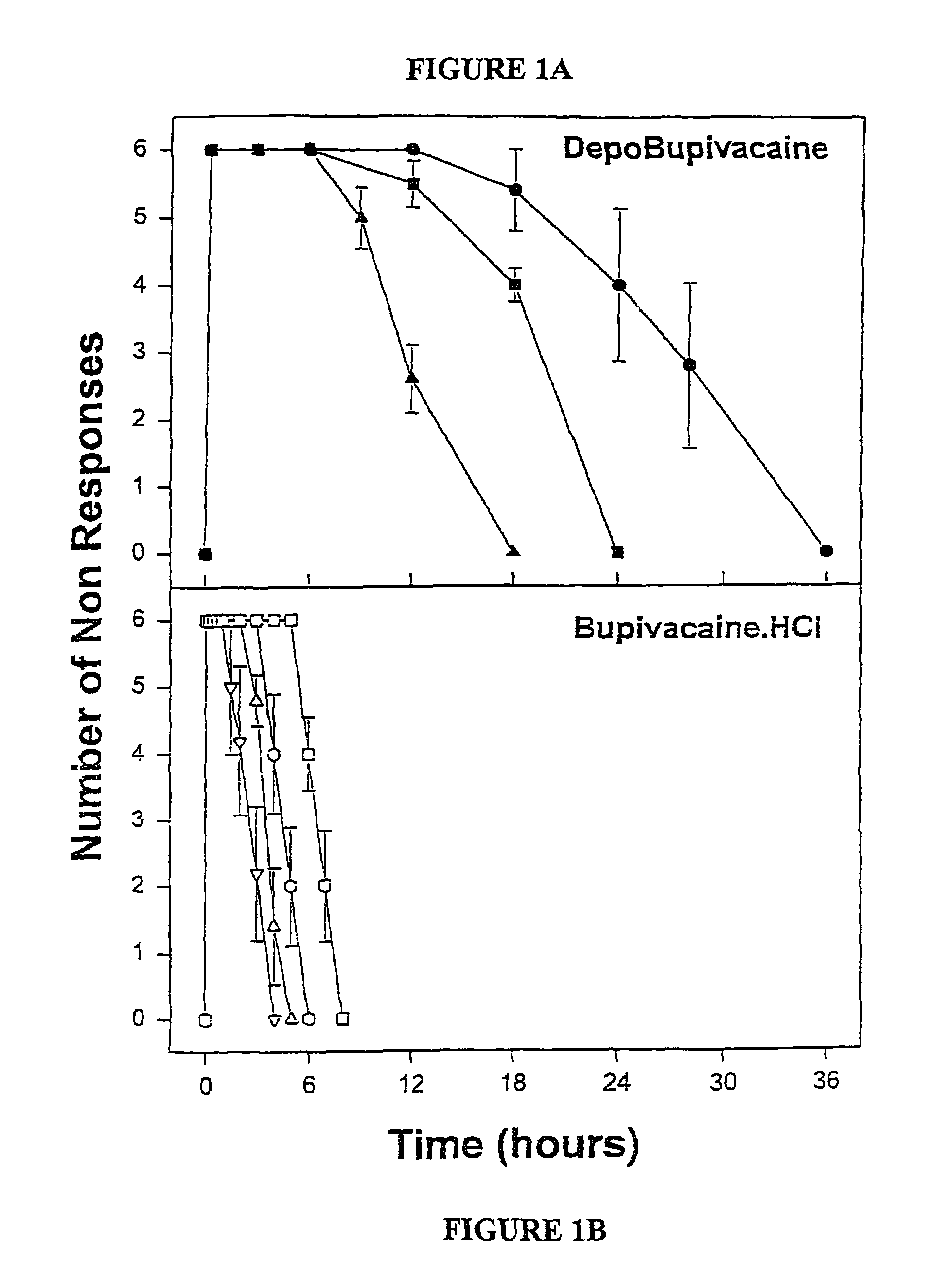
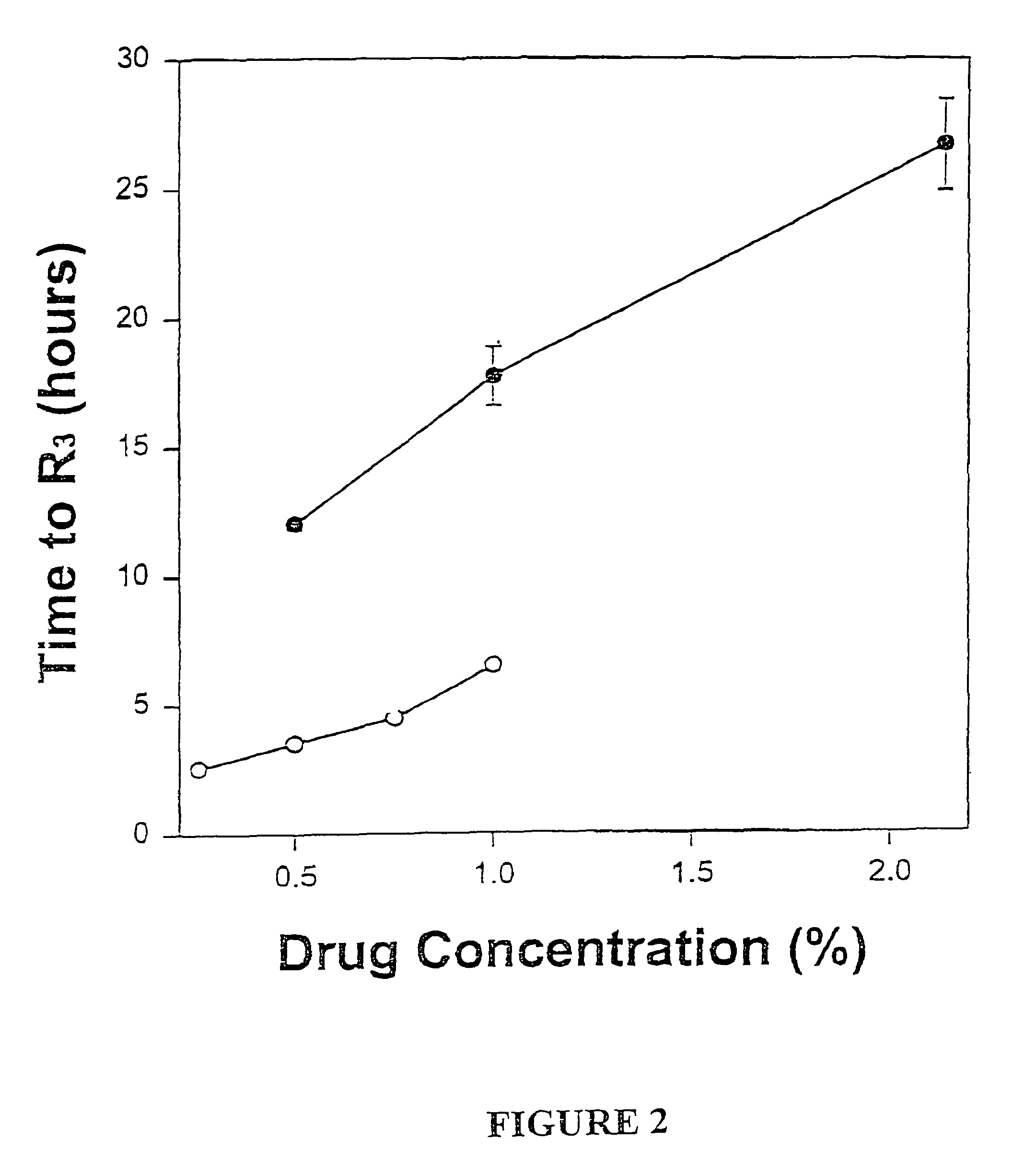
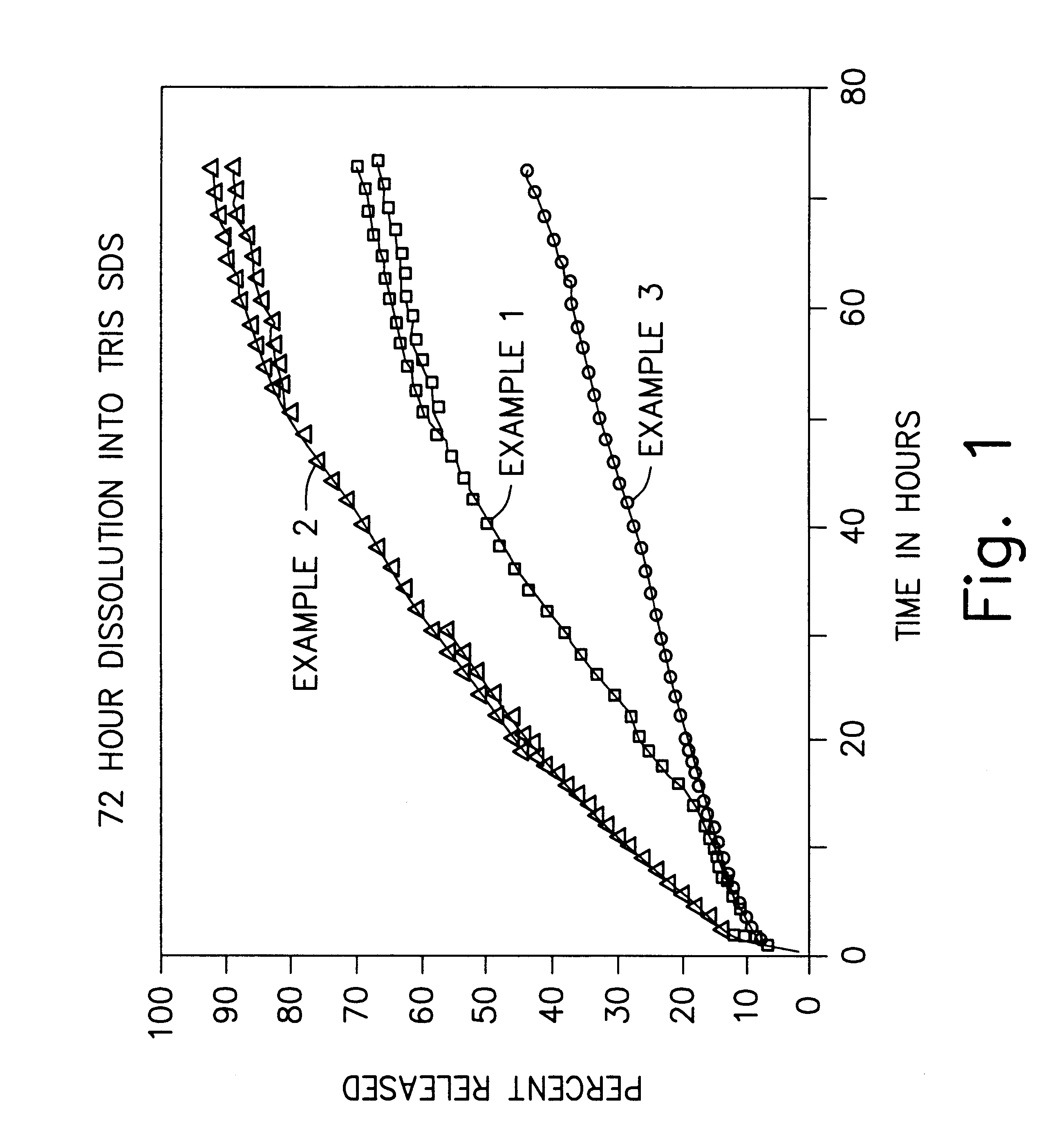
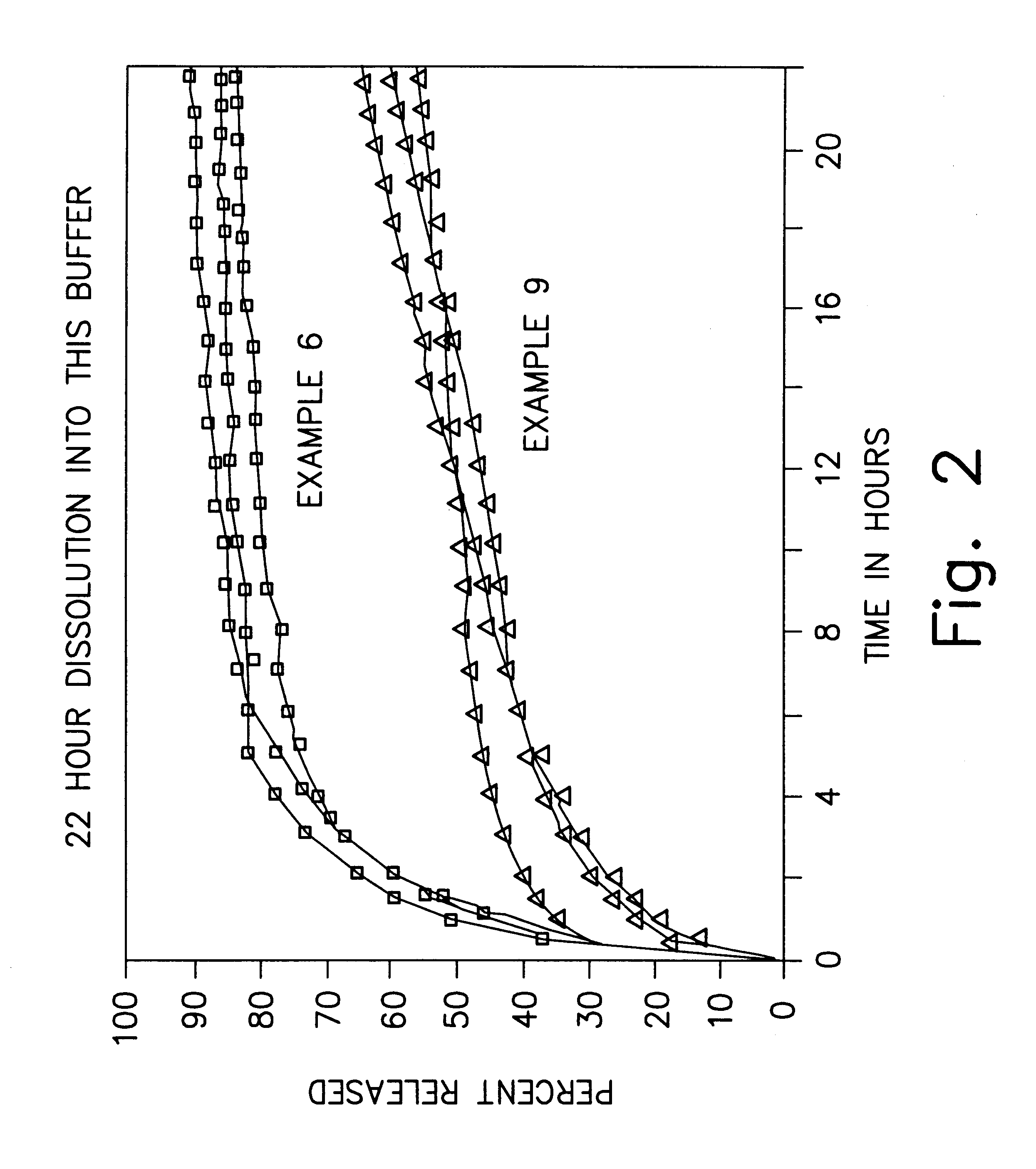
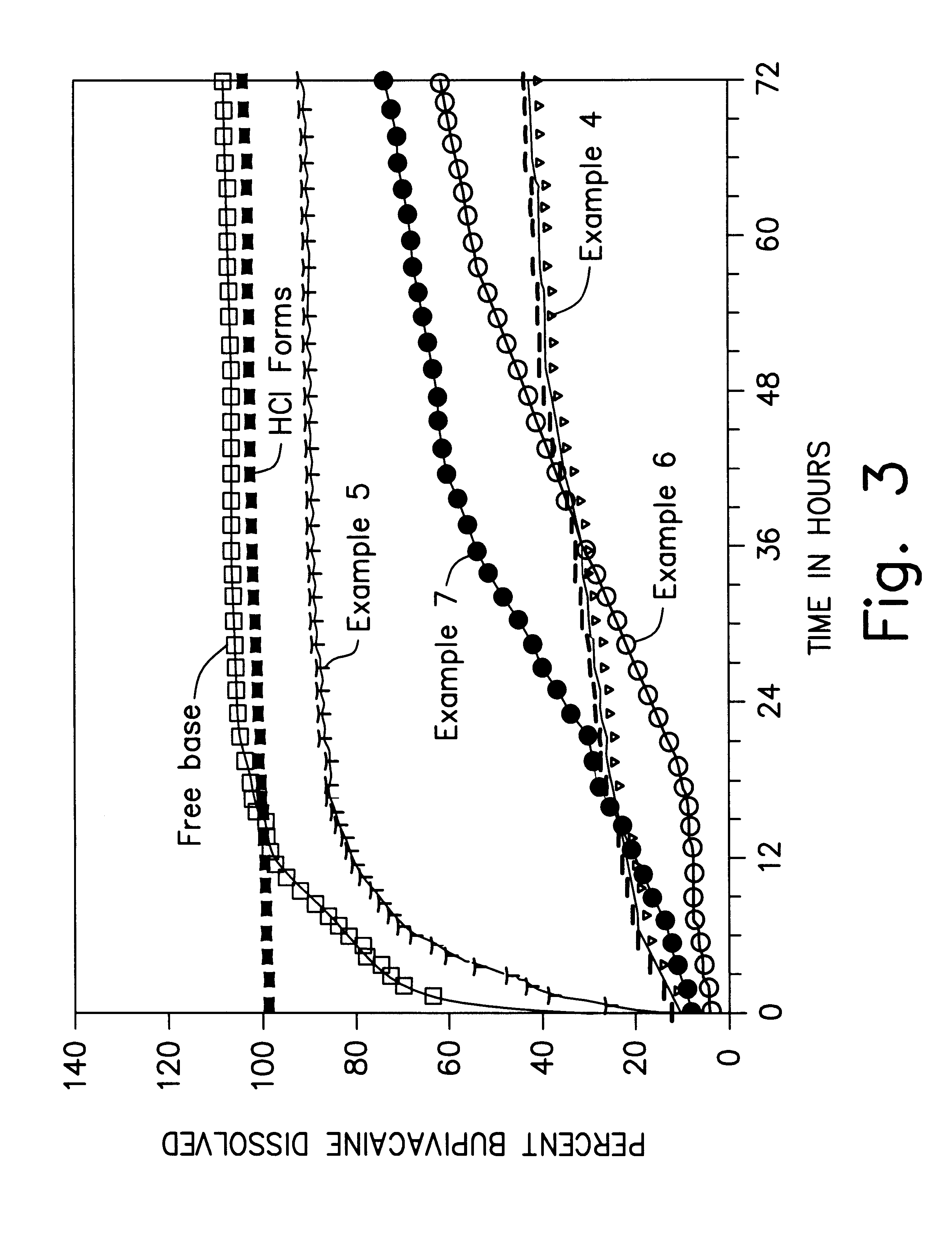
Formulations and methods for providing prolonged local anesthesia
InactiveUS6451335B1Slow in-vitro releaseRelease the local anestheticAnaesthesiaGranular deliveryControlled releaseAnesthetic Agent
A formulation for inducing sustained regional local anesthesia in a patient comprising a substrate comprising a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material prolonging the release of the local anesthetic from the substrate to obtain a reversible local anesthesia when implanted or injected in a patient, and a non-toxic augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the augmenting agent. In preferred embodiments, the controlled release material is a low molecular weight, acid-terminated polymer. A further aspect of the invention is directed to such formulations which release the local anesthetic in two phases, the first a rapid "bolus" to initiate anesthesia and a second, slower release to maintain anesthesia.
Owner:EURO-CELTIQUE SA
Nitric oxide donor composition and method for treatment of anal disorders
A pharmaceutical composition contains a nitric oxide donor and advantageously an optional corticosteroid and / or topical anesthetic. The composition is useful in a method for treating anal disorders such as anal fissure, anal ulcer, hemorrhoidal disease, levator spasm, and so forth, by topical application to or proximate the affected area.
Owner:STRAKAN INT S A R L
Apparatus and method for the treatment of stress urinary incontinence
An apparatus and method for the treatment of stress urinary incontinence. The apparatus includes a suburethral sling having an adjustment member for adjusting the tension of the sling both during the procedure and post-procedure. The method includes using a needle to simultaneously implant the sling and to deliver a local anesthetic in the groin area while implanting the sling.
Owner:BOSTON SCI SCIMED INC
Sustained-release liposomal anesthetic compositions
InactiveUS8182835B2High acceptabilityImprove encapsulationInorganic non-active ingredientsAnaestheticsHalf-lifeMaximum tolerated dose
Owner:PACIRA PHARMA INC
Methods for providing safe local anesthesia
InactiveUS6699908B2Reduce riskImproved therapeutic indexBiocidePeptide/protein ingredientsAnesthetic AgentControlled release
Methods and formulations for inducing substantially safer local anesthesia in a patient are provided. The methods comprise administering, to a patient in need thereof, a substrate containing a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material to safely obtain a reversible nerve blockade when implanted or injected in a patient.
Owner:PURDUE PHARMA LP
Topical compositions and methods for treating pain and inflammation
InactiveUS20060194759A1Easy to transportEnhance pain-relieving and anti-inflammatory propertyBiocideHydroxy compound active ingredientsMentholHydrocortisone
A topical composition and method for treating pain and inflammation by administering an effective amount of a topical composition comprising an anti-inflammatory steroid such as hydrocortisone, a topical anesthetic such as lidocaine, menthol, and a medically acceptable carrier into which the forgoing are incorporated. A chondroprotective agent can also be added.
Owner:EIDELSON STEWART G
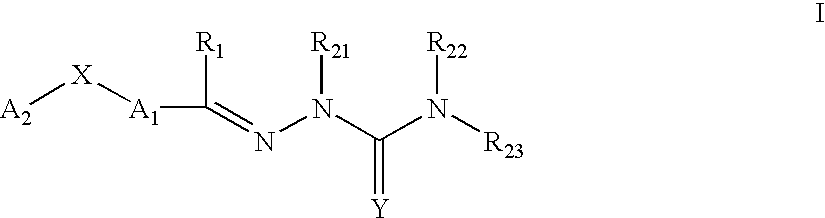
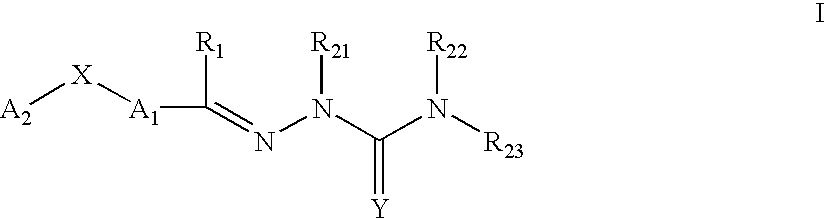
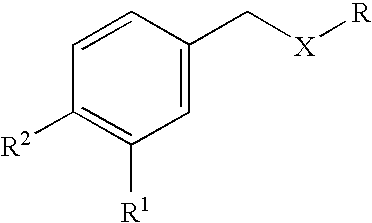
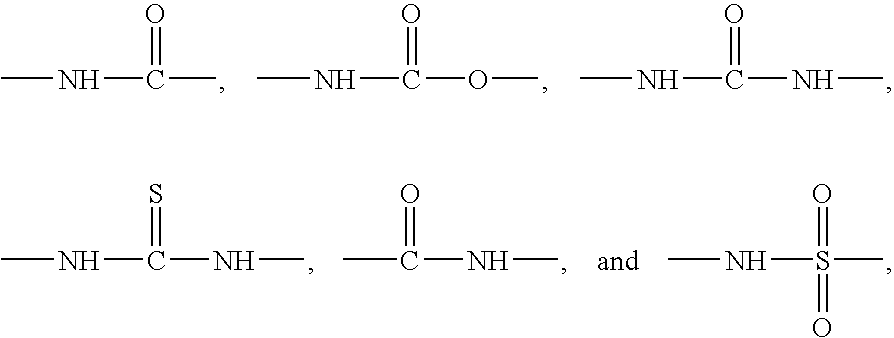
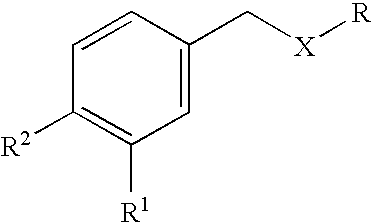
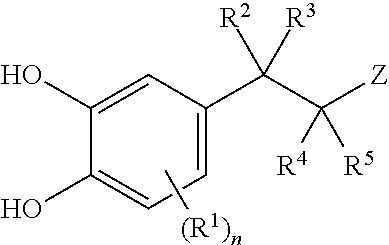
Carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones and the use thereof
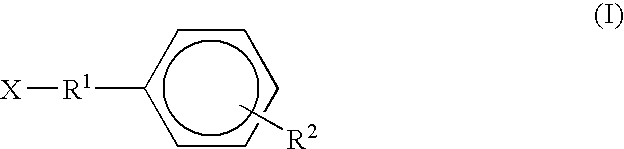
This invention is related to carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones represented by Formula I: ##STR1## or a pharmaceutically acceptable salt or prodrug thereof, wherein: Y is oxygen or sulfur; R.sub.1, R.sub.21, R.sub.22 and R.sub.23 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl; or R.sub.22 and R.sub.23, together with the N, form a heterocycle; A.sub.1 and A.sub.2 are independently aryl, heteroaryl, saturated or partially unsaturated carbocycle or saturated or partially unsaturated heterocycle, any of which is optionally substituted; X is one or O, S, NR.sub.24, CR.sub.25 R.sub.26, C(O), NR.sub.24 C(O), C(O)NR.sub.24, SO, SO.sub.2 or a covalent bond; where R.sub.24, R.sub.25 and R.sub.26 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl. The invention also is directed to the use of carbocycle and heterocycle substituted semicarbazones and thiosemicarbazones for the treatment of neuronal damage following global and focal ischemia, for the treatment or prevention of neurodegenerative conditions such as amyotrophic lateral sclerosis (ALS), for the treatment and prevention of otoneurotoxicity and eye diseases involving glutamate toxicity and for the treatment, prevention or amelioration of pain, as anticonvulsants, and as antimanic depressants, as local anesthetics, as antiarrhythmics and for the treatment or prevention of diabetic neuropathy and urinary incontinence.
Owner:COCENSYS
Pharmaceutical dental formulation for topical application of metronidazole benzoate, chlorhexidine gluconate and local anesthetic
Pharmaceutical dental gel preparation comprising of metronidazole benzoate, chlorhexidine gluconate, and local anesthetic as the active ingredient; glycol as the solvent medium; a carboxyvinyl polymer, cross-linked polymer of acrylic acid copolymerized with polyalkylsucrose as a gelling agent.
Owner:J B CHEM & PHARMA
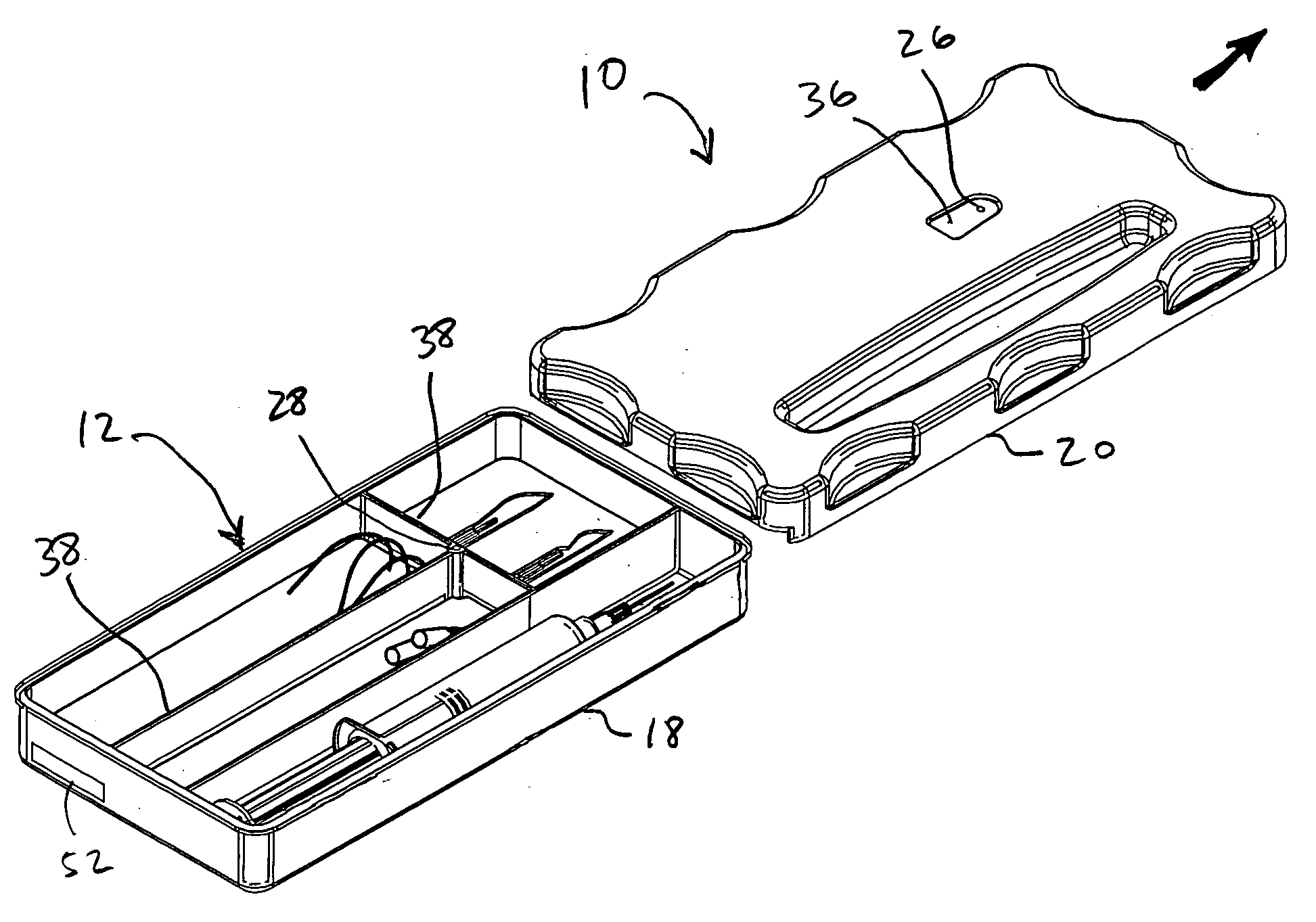
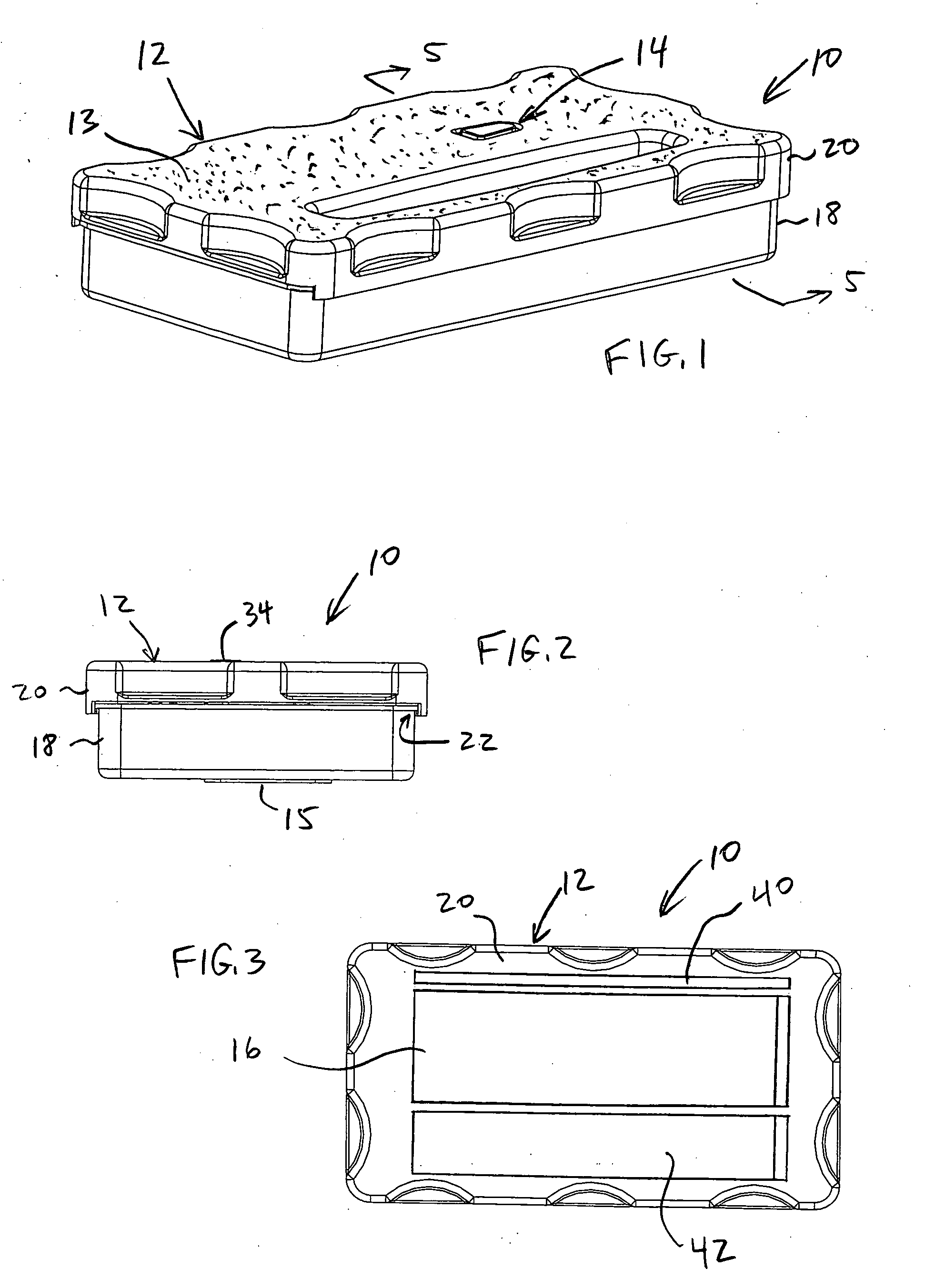
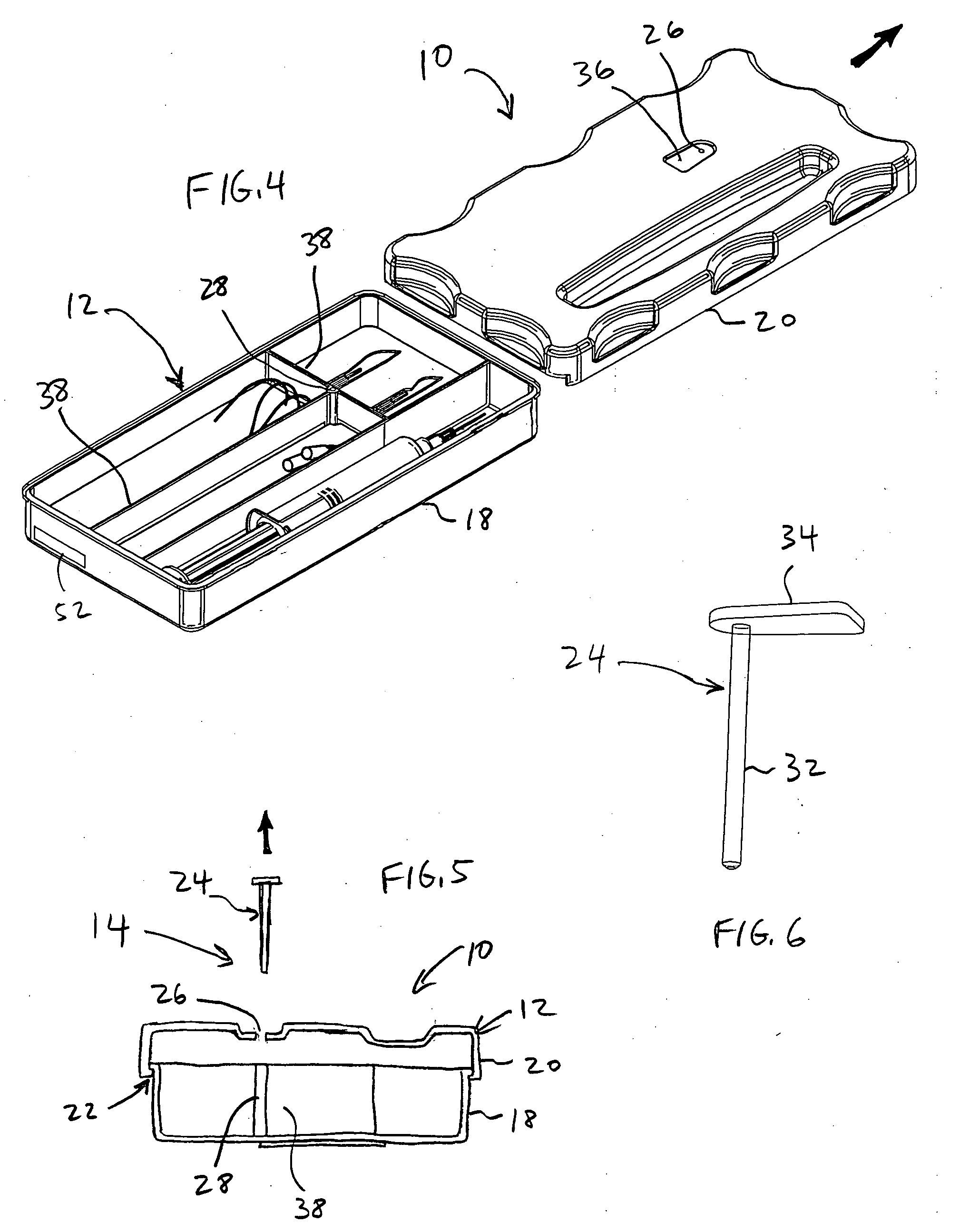
System and method for preventing wrong-site surgeries
ActiveUS20060096877A1Preventing wrong-site surgeryPreventing wrong-site surgeriesSurgical furnitureDispensing apparatusMedical recordLocking mechanism
A container holds at least one surgical implement, has a lock mechanism, and has a signature label that impedes access to the surgical implement until the correct surgical site is confirmed. A method of using the container includes the steps of confirming the correct surgical site, signing the label and removing it from the container, placing the label in the medical record, unlocking the container, removing the implement, and beginning the surgery, wherein the surgical team is forced to pause to confirm the correct surgical site before starting the surgery. Preferably, the container top may be removed and placed between the surgeon and surgical technician to define a no-hands “neutral zone” to avoid being stuck by the sharps. Also, the container preferably includes compartments for storing used sharps and / or a local anesthetic-loaded syringe, and the top may be replaced and secured for safely disposing of the sharps after the surgery.
Owner:STARTBOX
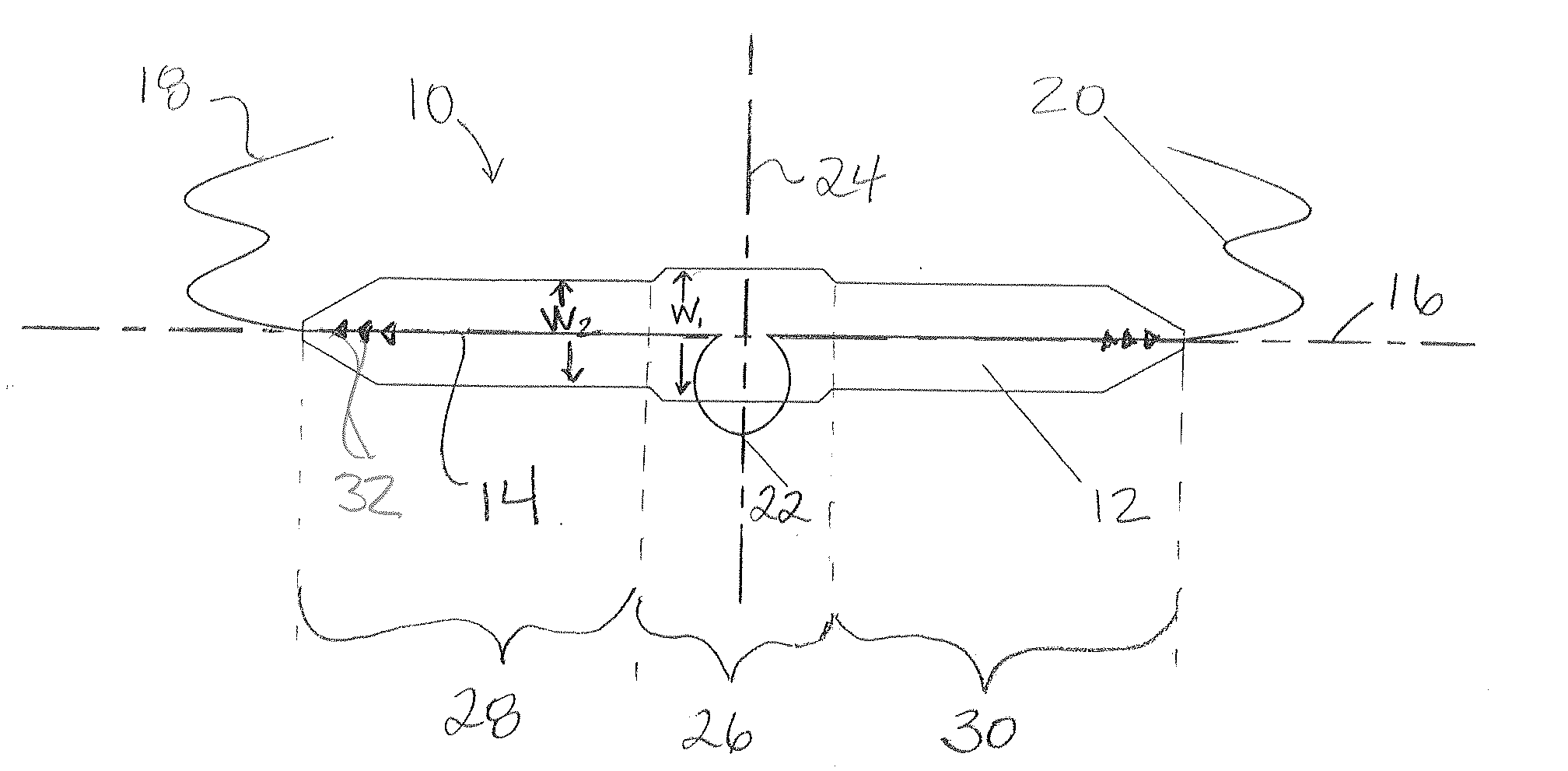
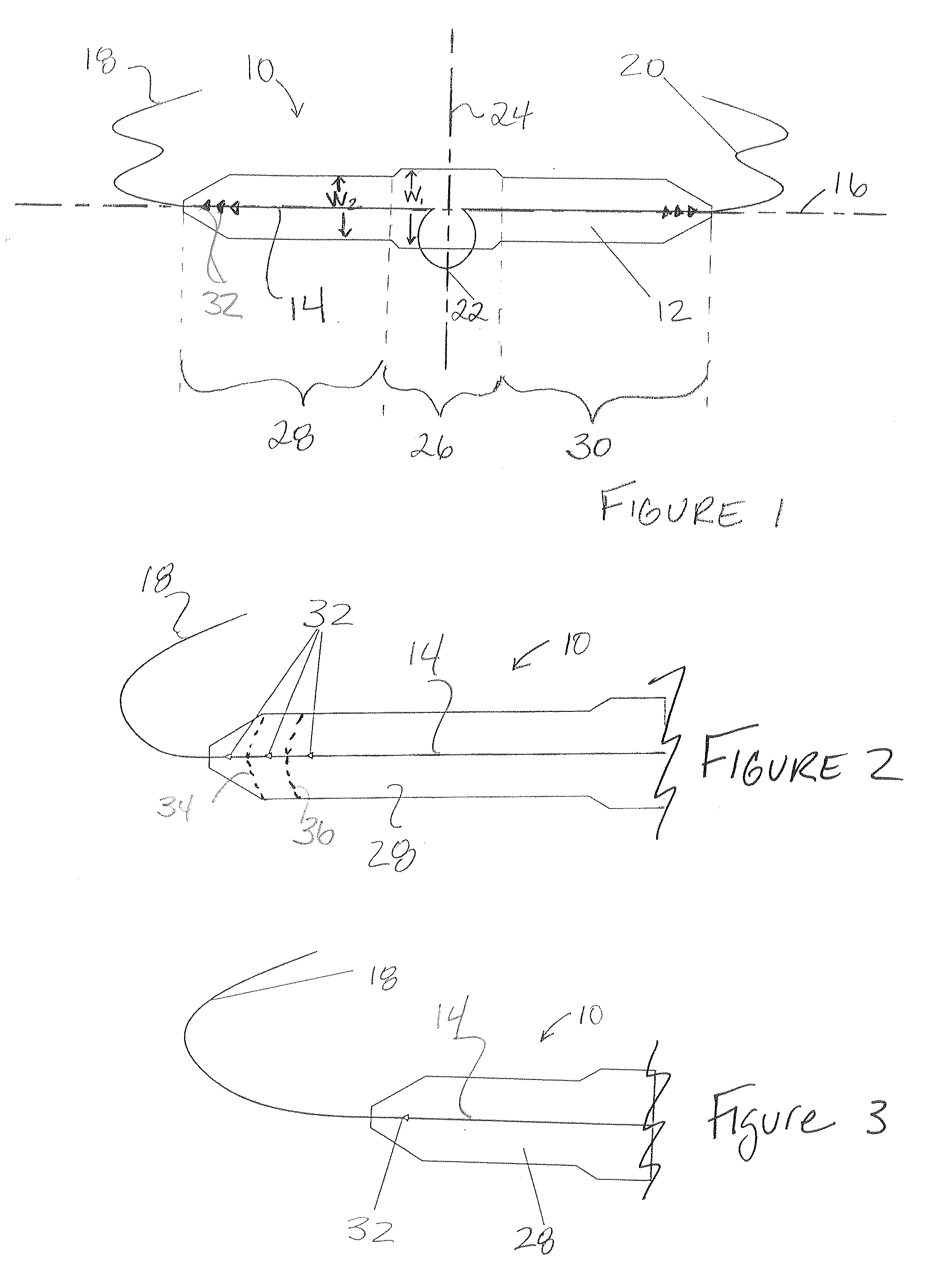
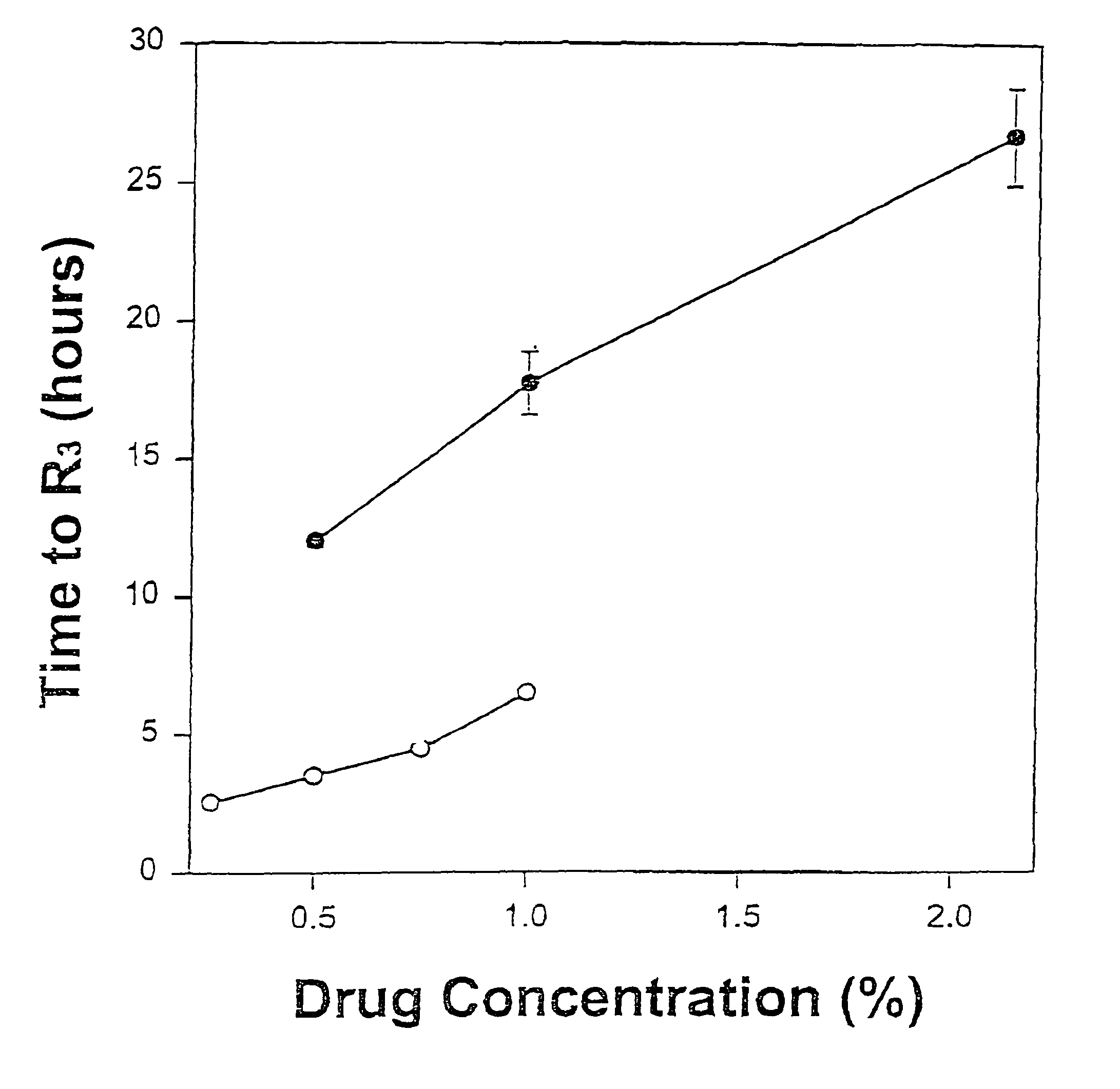
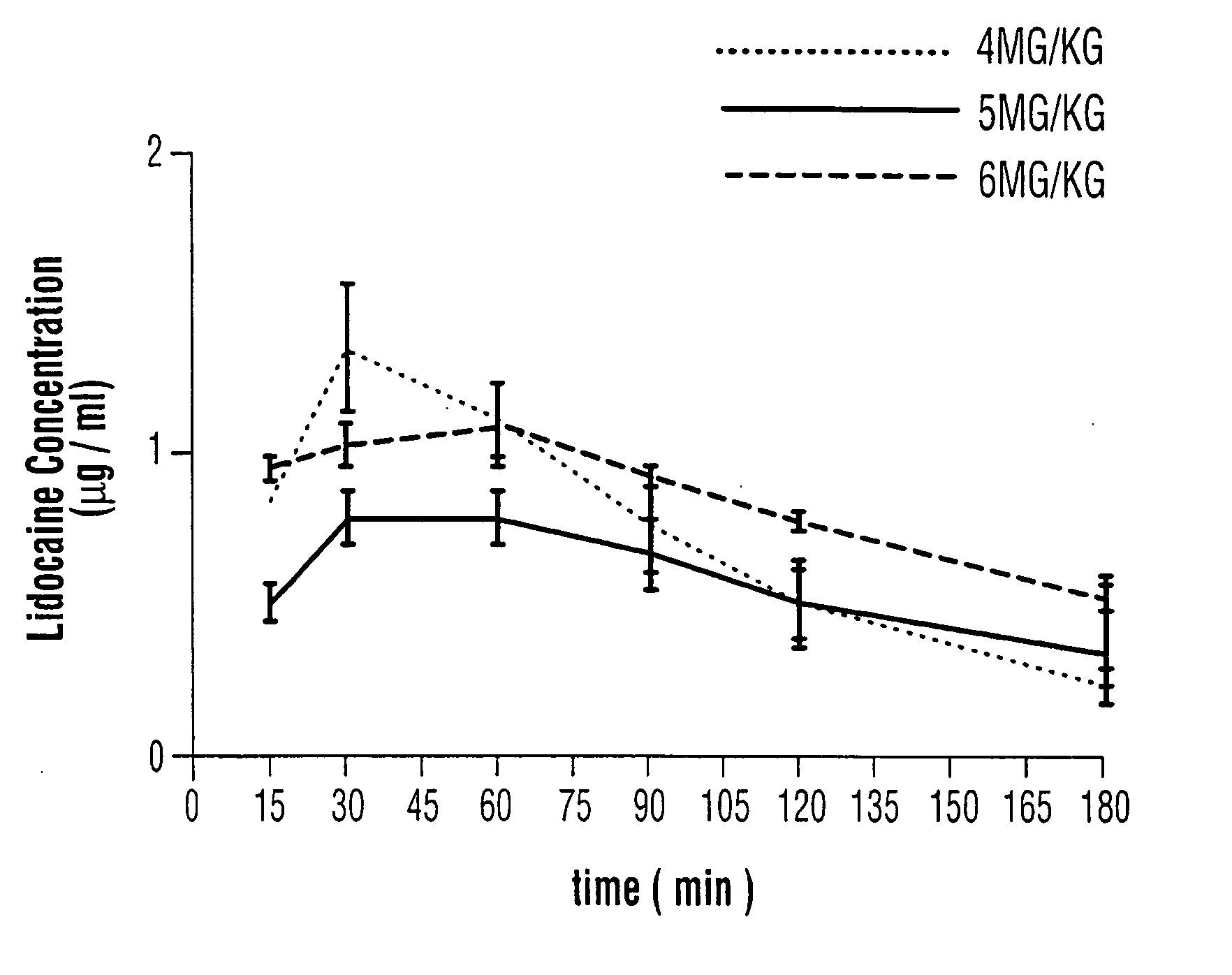
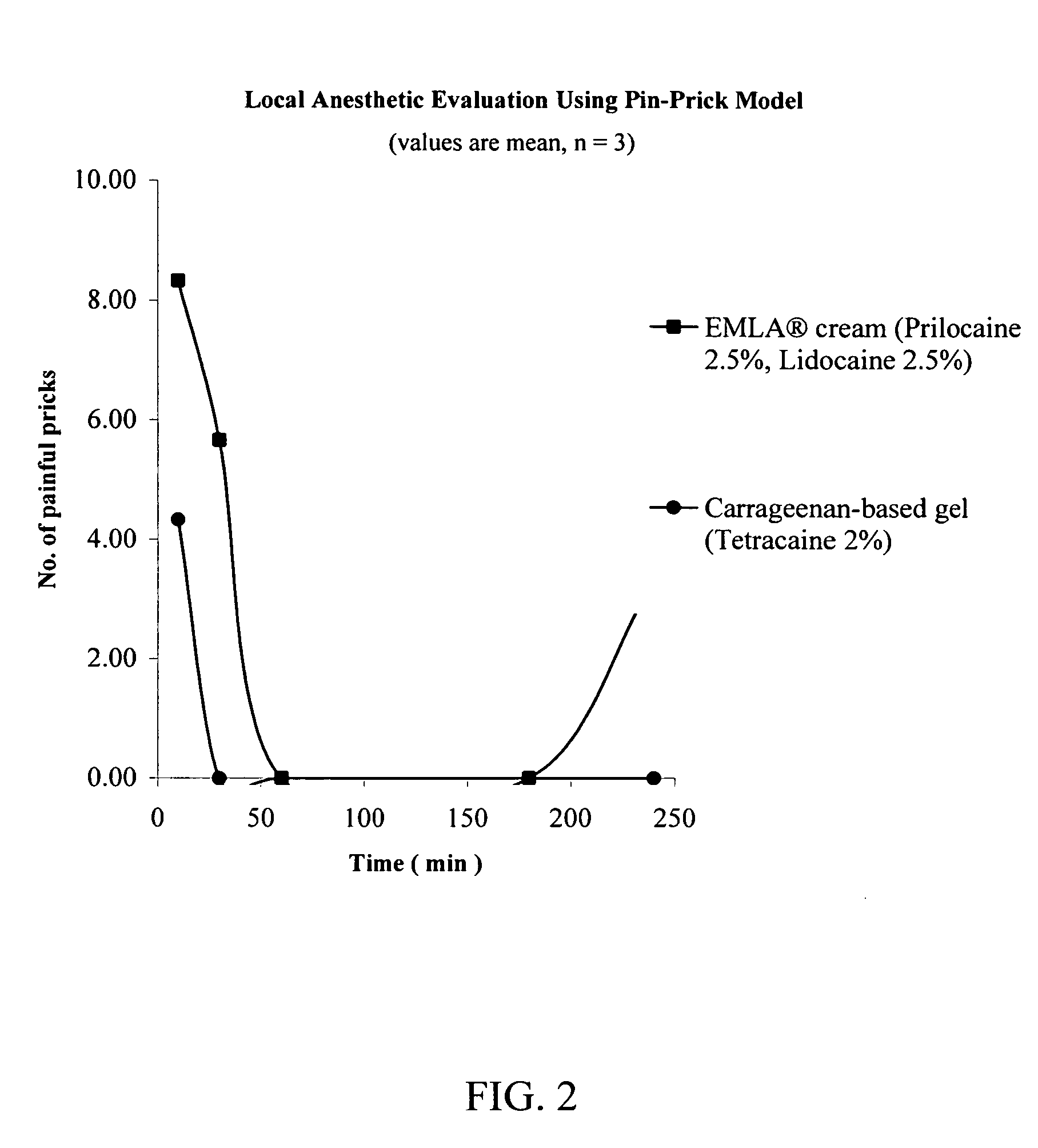
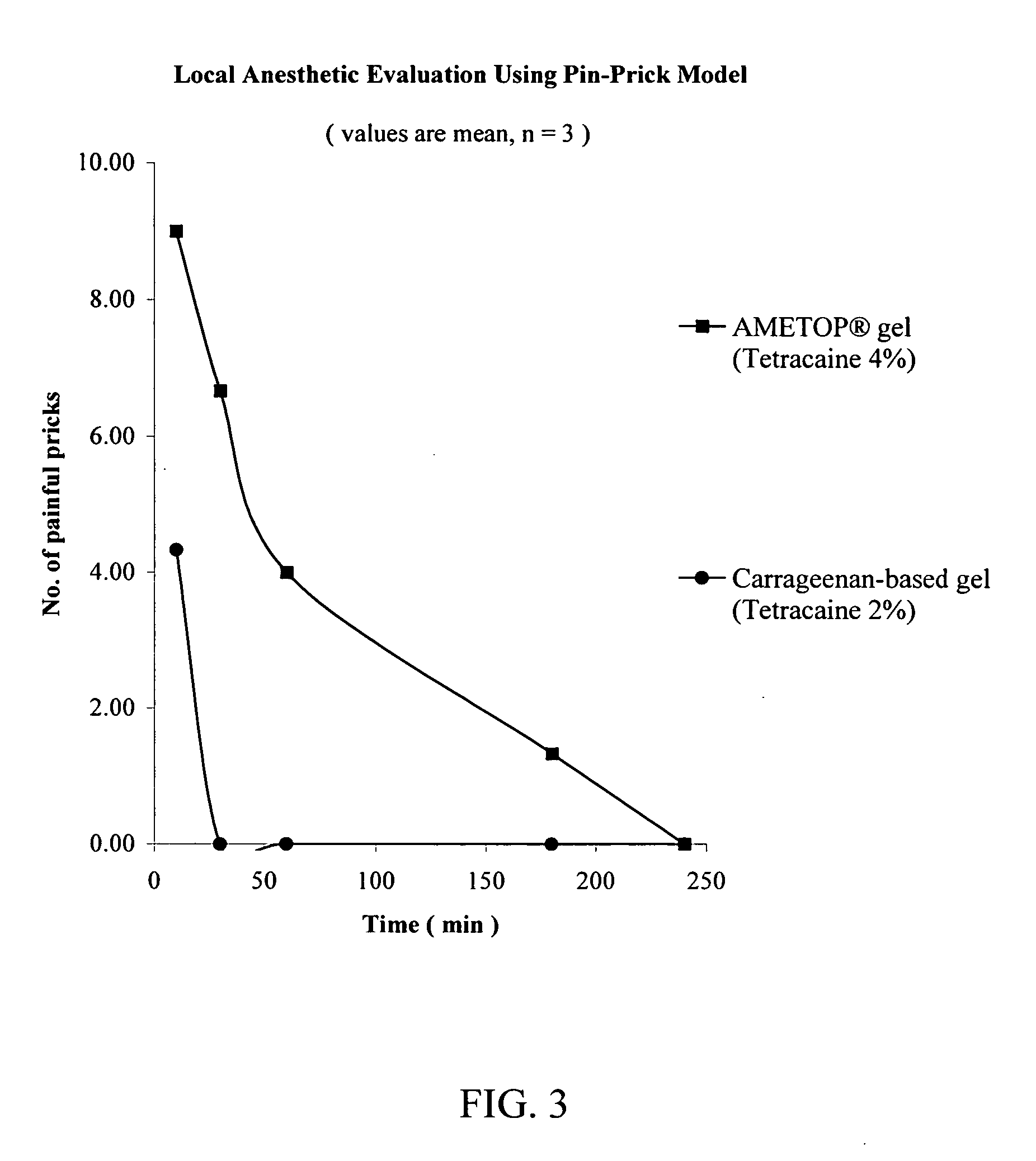
Topical anesthetic for rapid local anesthesia and method of applying a topical anesthetic
The invention relates to a drug delivery system for the topical administration of anesthetic agents. For example, a topical anesthetic for rapid local anesthesia is provided. The topical anesthetic includes an anesthetic, volatile and non-volatile solvents, and an optional thickener. In addition, a method is taught for applying the topical anesthetic to the face of a patient without occlusion. The anesthetic is applied topically to an area for injection such that the dermatological procedure (cosmetic injections) can be performed in fifteen minutes.
Owner:JUVENTIO
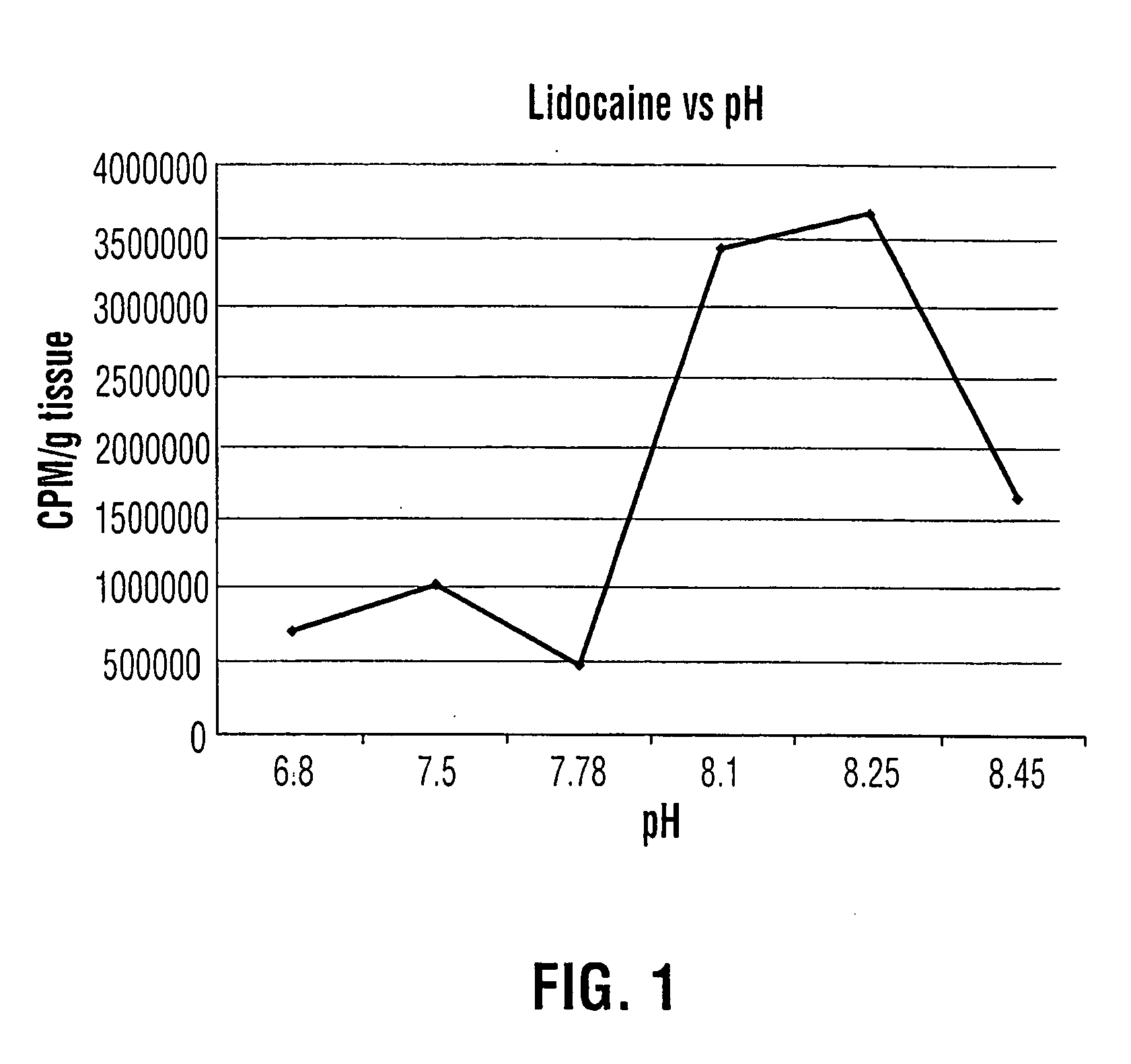
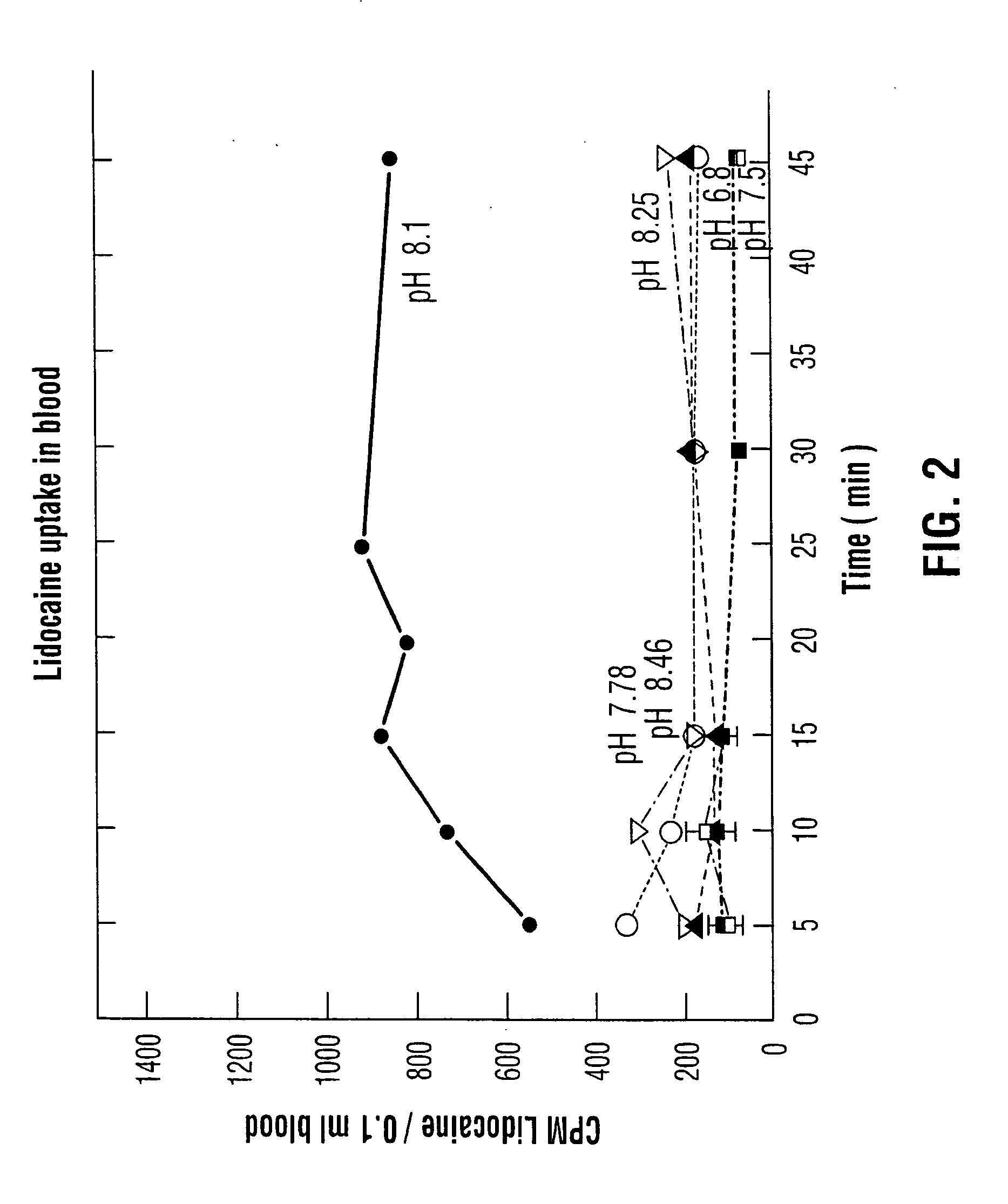
Topical anesthesia of the urinary bladder
InactiveUS20050238733A1Reduce concentrationReduce absorptionBiocideInorganic active ingredientsSodium bicarbonateCystoscopy
An aqueous solution of local anesthetic is instilled into the urinary bladder in sufficient concentration with the addition of an alkalinizing agent such as sodium bicarbonate to elevate the intra-vesical pH to approximately 8.0. The combination is left in situ in the bladder for at least fifteen minutes to allow time for absorption of the base form of the local anesthetic. This method provides safe and effective topical anesthesia to allow pain-free cystoscopic biopsy and cautery of bladder lesions such as bladder cancer, and provides a means to treat inflammatory conditions of the bladder such as chronic interstitial cystitis and acute bacterial cystitis.
Owner:HENRY RICHARD
Injectable biodegradable polymer compositions for soft tissue repair and augmentation
InactiveUS20100260703A1Pharmaceutical delivery mechanismUrinary disorderInjectable polymersActive agent
Methods for soft tissue repair and / or augmentation using injectable, biodegradable polymers are described herein. In one embodiment, the polymer compositions are liquid or pastes at room temperature. In a preferred embodiment, the polymer composition contains liquid or pasty hydroxy fatty acid-based copolyesters, polyester-anhydrides, or combinations thereof. The viscosity of the polymers increases upon contact with bodily fluid to form a solid or semisolid implant suitable for soft tissue repair and / or augmentation. In another embodiment, the polymer composition contains particles of a polymer stereocomplex. One or more active agents may be incorporated into the polymer compositions. Suitable classes of active agents include local anesthetics, anti-inflammatory agents, antibiotics, analgesics, growth factors and agents that induce and / or enhance growth of tissue within the filled cavity or control the growth of a certain type of tissue, and combinations thereof. The polymer compositions may also contain one or more additives or excipients that modify the physical and / or mechanical properties of the polymer. The polymer compositions are typically administered by injection. The injectable polymers can be used for a variety of soft tissue repair and augmentation procedures.
Owner:POLYGENE LTD
Implantable medical device with analgesic or anesthetic
An implantable medical device such as a catheter with a controlled-release outer layer including a pharmacologically active ingredient for helping to relieve pain associated with the implantation of the device. The device includes a base material with an outer bioactive material layer including, for example, an analgesic or a local anesthetic.
Owner:VANCE PROD INC D B A COOK UROLOGICAL INC
Formulations for the treatment of pain
Formulations and methods are provided for the treatment of pain, and neuropathic pain in particular. The formulations are eutectic mixtures of a capsaicinoid and a local anesthetic agent and / or an anti-pruritic agent.
Owner:ACORDA THERAPEUTICS INC
Transdermal anesthetic and vasodilator composition and methods for topical administration
A composition for topical application comprising a therapeutically effective amount of a topical anesthetic, a safe and effective amount of a pharmaceutically acceptable topical vasodilator and a pharmaceutically acceptable carrier and a method of administering the composition to a mammal are disclosed.
Owner:SAMUELS PAUL J +1
Topical anesthetic formulation
The topical medicament gel formulation of the present invention includes an anesthetic, an anti-microbial, an oxidant, a nutrient, a diuretic, an opioid, an anti-emetic, an anti-seizure drug, and a non-steroidal anti-inflammatory drug (NSAID), USP in a molecular, as opposed to a salt form, as the active ingredient. Additional constituents illustratively include a skin penetration enhancer and a gelling agent. This invention deals with problems commonly associated with topical application of local medicaments such as: slow onset of action; need for occlusion; and rapid loss of effect due to rapid systemic dispersion. The invention permits enhanced penetration of the medicament and thereby allows for a lesser total dosage of pharmaceutically active ingredient. The use of a lesser total dosage also decreases systemic toxicity.
Owner:WEPFER SCOTT
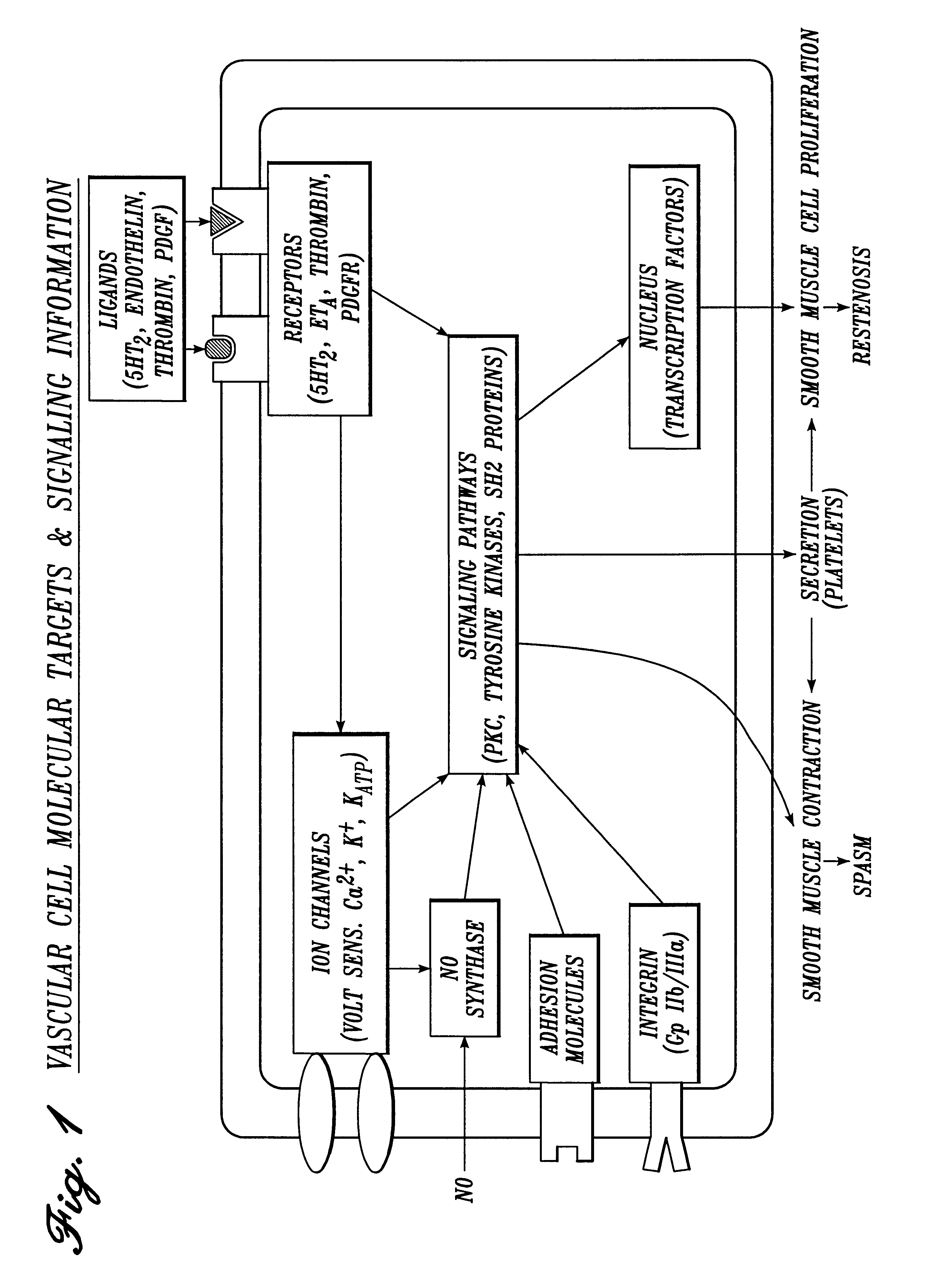
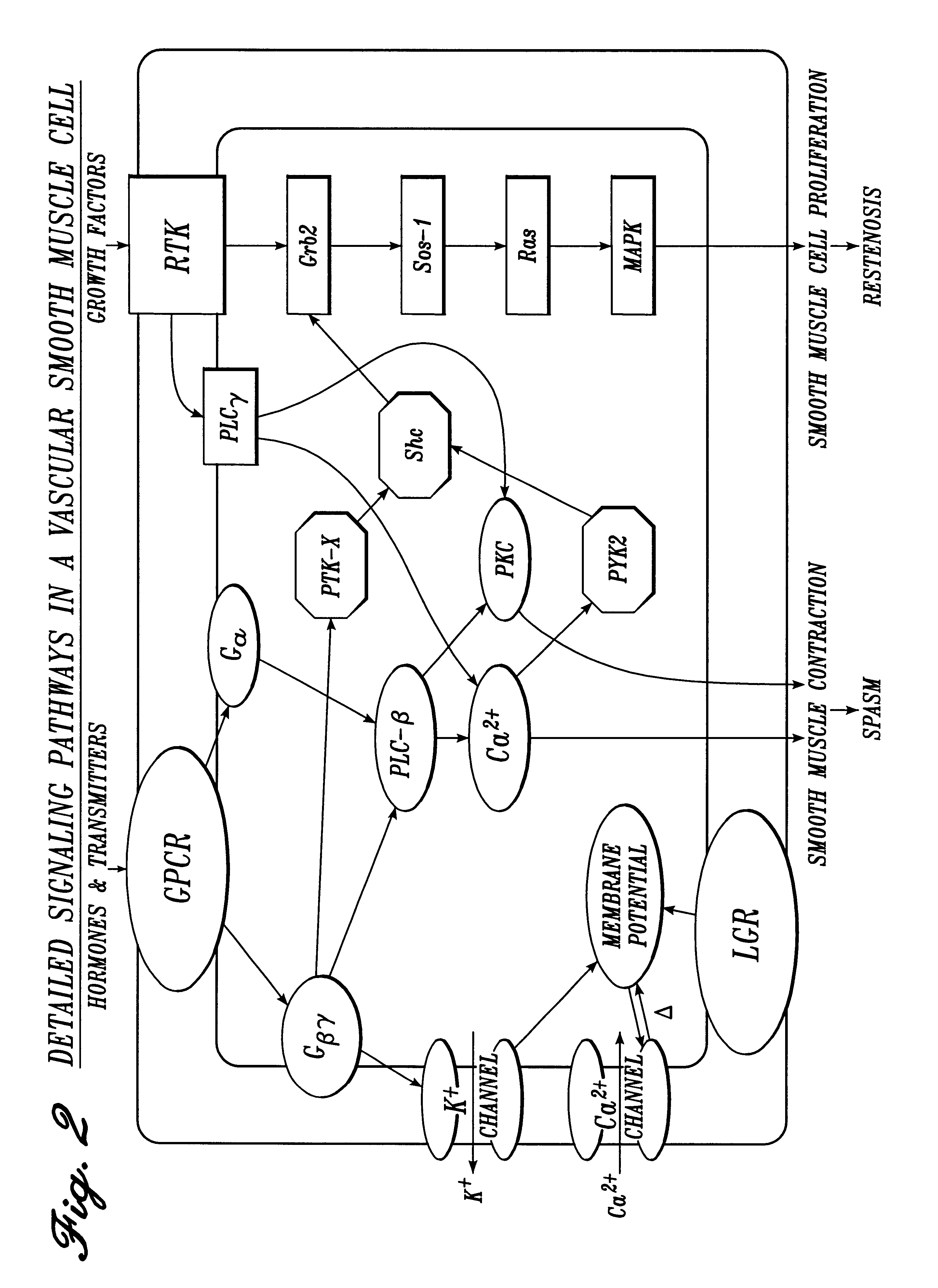
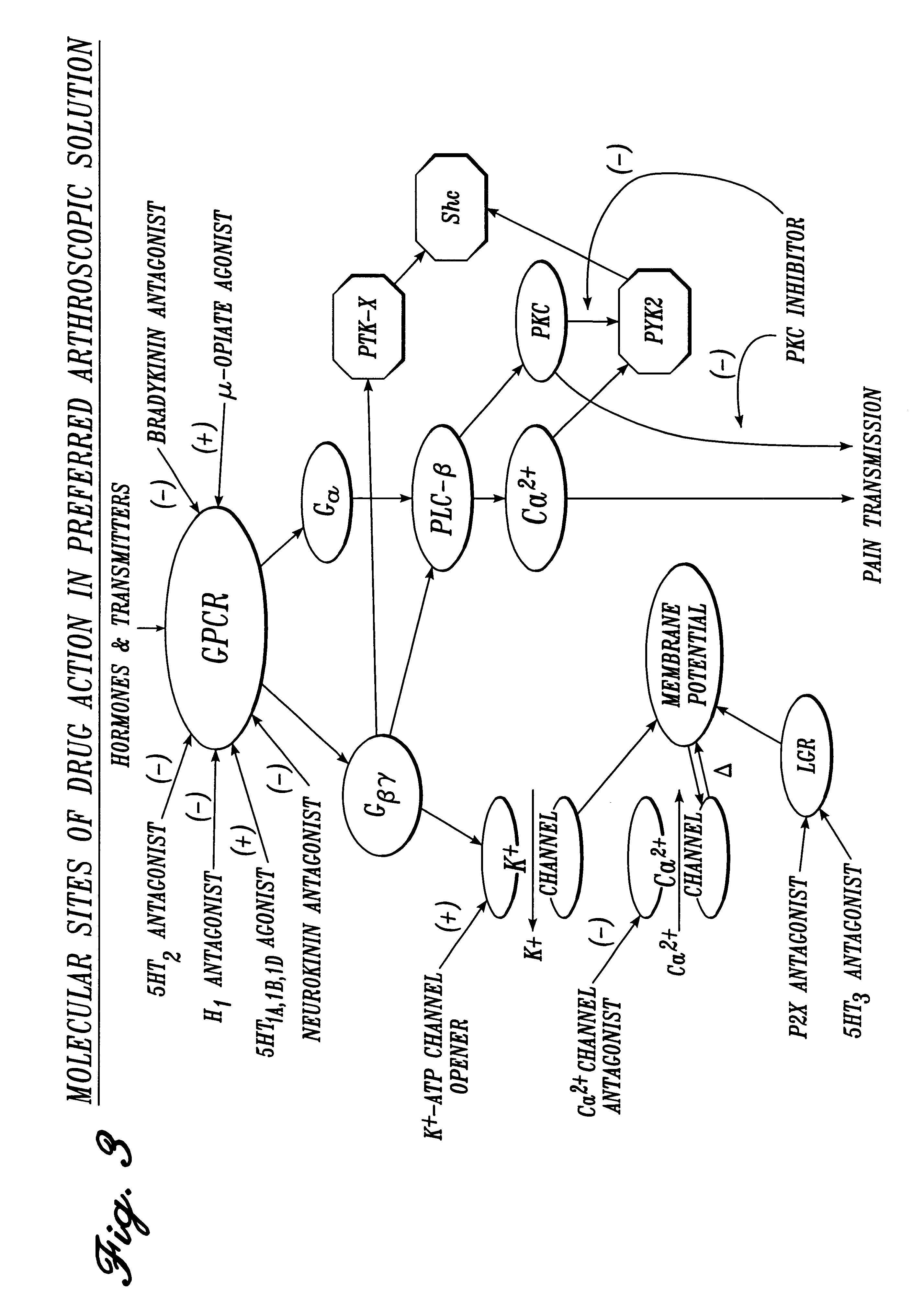
Irrigation solution and method for inhibition of pain, inflammation, spasm and restenosis
InactiveUS6413961B1Limit their usefulnessLow costBiocideNervous disorderPercent Diameter StenosisMuscle spasm
A method and solution for perioperatively inhibiting a variety of pain, inflammation, spasm and restenosis processes resulting from cardiovascular or general surgical, therapeutic and diagnostic procedures. The solution preferably includes multiple pain and inflammation inhibitory agents, including at least one local anesthetic agent, and spasm inhibitory agents at dilute concentration in a physiologic carrier, such as saline or lactated Ringer's solution. Specific preferred embodiments of the solution of the present invention for use in cardiovascular and general vascular procedures also include anti-restenosis agents.
Owner:OMEROS CORP
Prolonged anesthesia in joints and body spaces
InactiveUS20020054915A1Enhance and prolong local anesthesiaGood effectPeptide/protein ingredientsAnaestheticsAnesthetic AgentMicroparticle
Sustained release local anesthetic formulations are administered intra articularly and / or into body spaces / cavities. The formulation is preferably a plurality of injectable microparticles including a local anesthetic and an effective amount of a biocompatible, biodegradable, sustained release material prolonging the release of the local anesthetic and optionally and a pharmaceutically acceptable, i.e., non-toxic, augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable without the augmenting agent.
Owner:PURDUE PHARMA LP
Therapeutic 1,2,3,6-tetrahydropyrimidine-2-one compositions and methods therewith
Owner:CRAGMONT PHARMA
Compositions and delivery systems for administration of a local anesthetic agent
InactiveUS20050152957A1Good effectLocal effectBiocideAdhesive dressingsActive agentHydrophobic polymer
A pharmaceutical composition is provided for topical administration of a local anesthetic agent. The composition comprises (a) a therapeutically effective amount of a local anesthetic agent and (b) a pharmaceutically acceptable, nonliposomal carrier comprised of a monohydric alcohol, a penetration enhancer, and polymer, which may be a hydrophilic polymer, a hydrophobic polymer or a combination thereof. The composition can be in the form of a gel, or it may form a film following application to a patient's body surface and evaporation of the monohydric alcohol. The composition provides rapid onset of local anesthesia as well as penetration of the active agent into the skin. Methods and drug delivery systems for administration of local anesthetic agents are also provided.
Owner:CORIUM INT
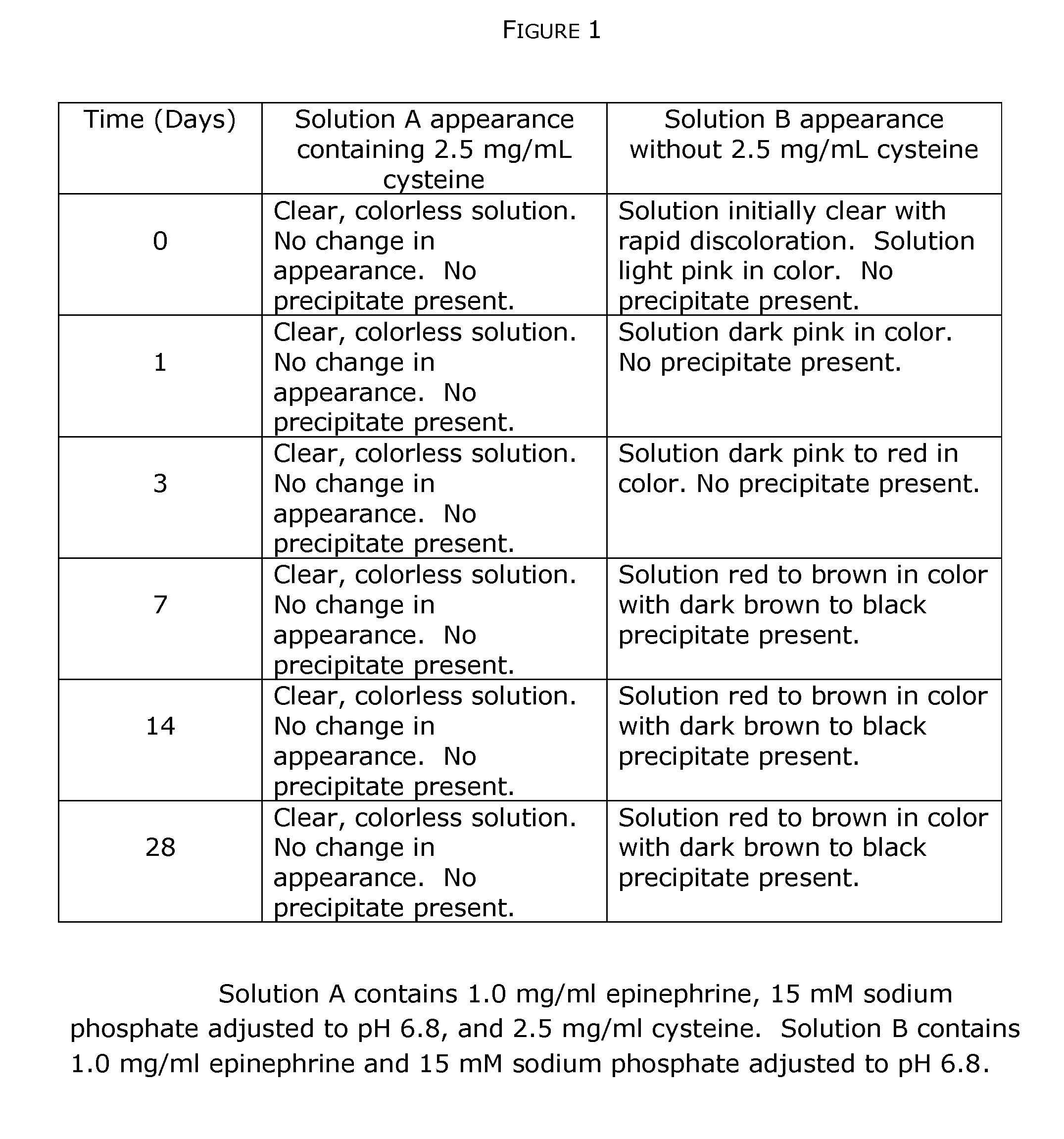
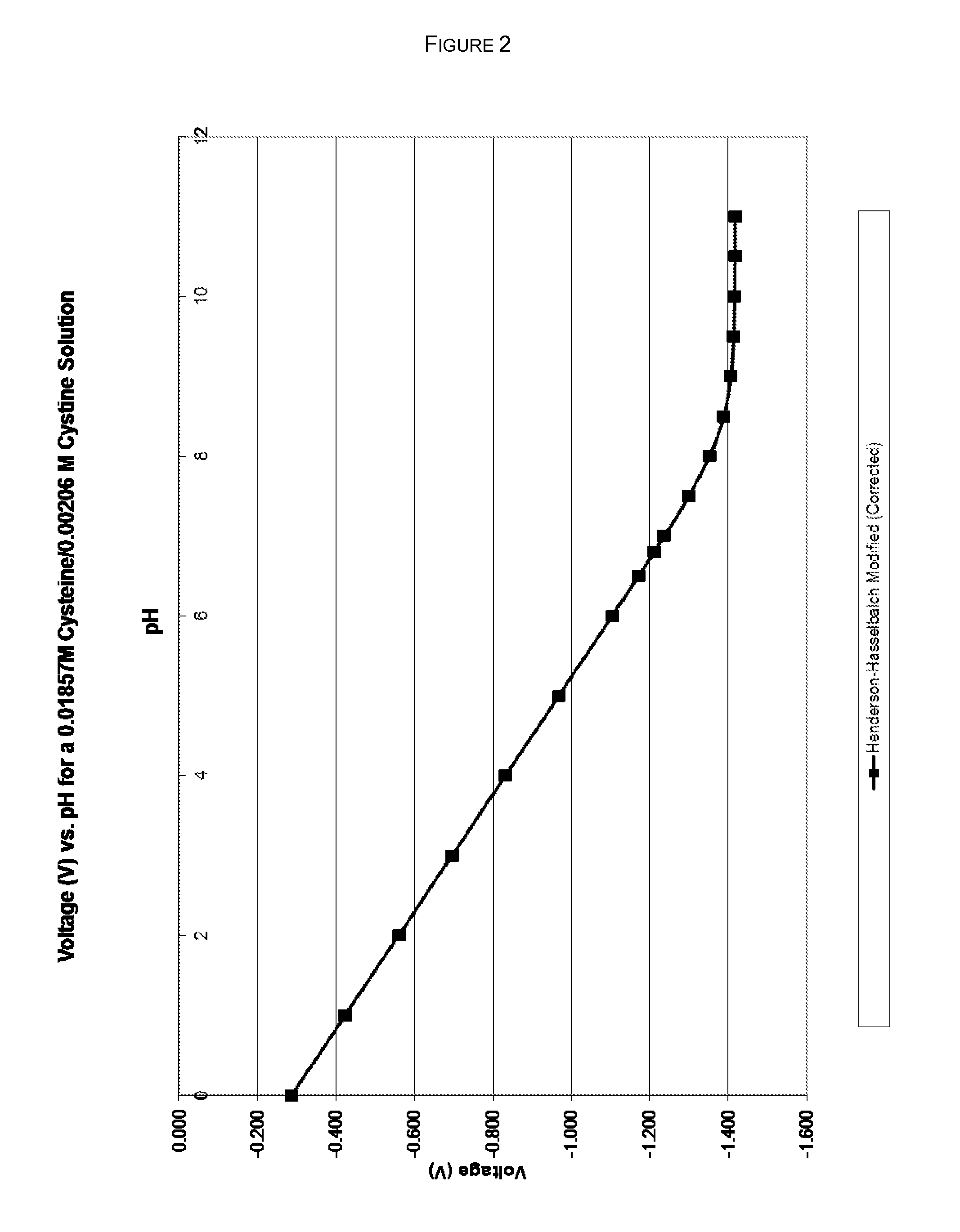
Stabilization of quinol composition such as catecholamine drugs
InactiveUS20120029085A1Stabilizing compoundReduced form requirementsBiocidePharmaceutical delivery mechanismCatecholaminePh buffering
Compositions and methods are provided for obtaining stabilized quinol compositions, such as catecholamine drugs (e.g., epinephrine solutions), and also for obtaining stable pharmaceutical formulations that comprise a stabilized quinol composition and a second pharmacologically active component such as a local anesthetic or other active drug ingredient having a reversibly protonated amine group. Stability is achieved through the inclusion of an appropriately selected pH buffer and a thiol agent, based on redox and pH buffering principles including pKa of the buffer and of the reversibly protonated amine group.
Owner:MACKAY JON
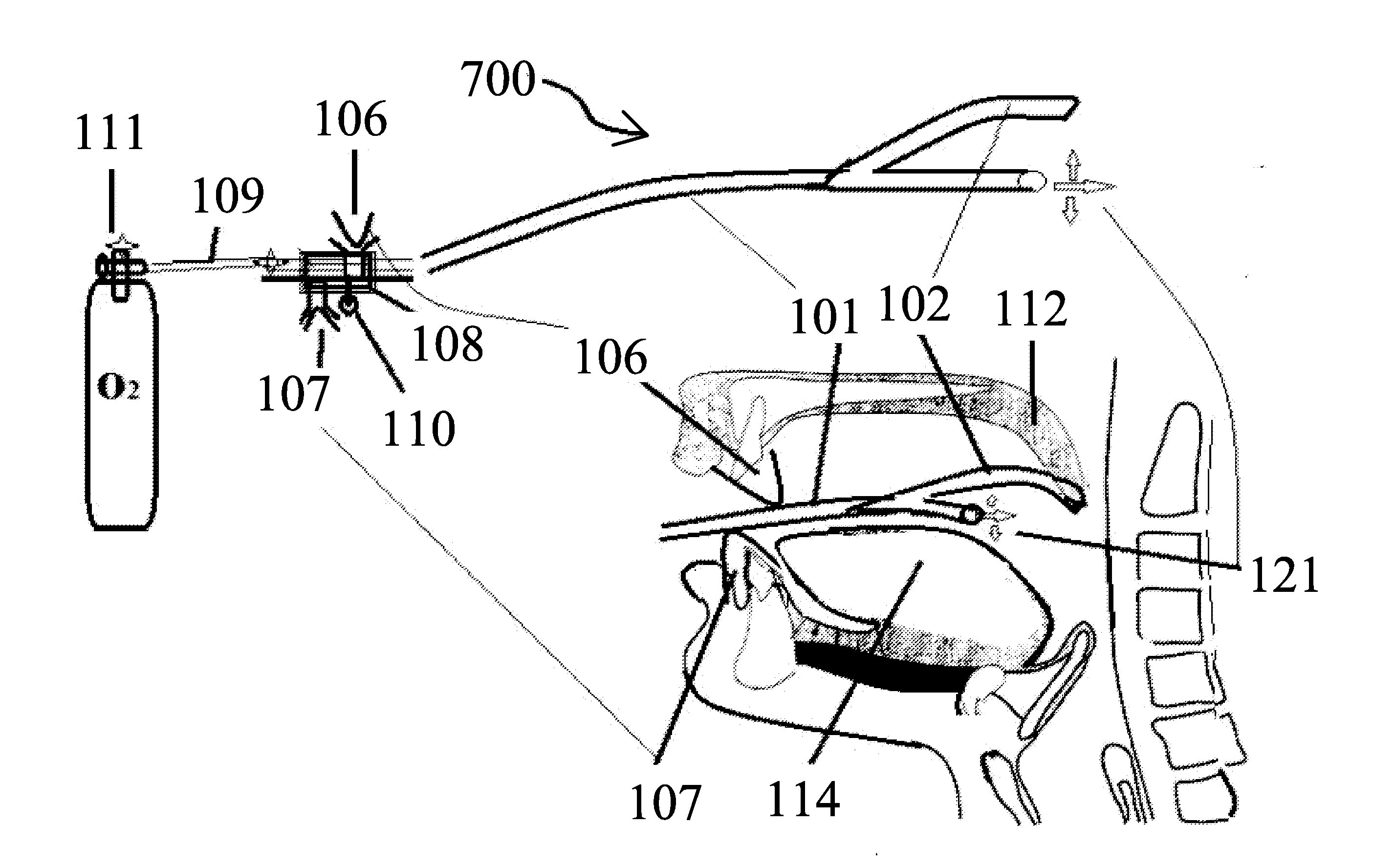
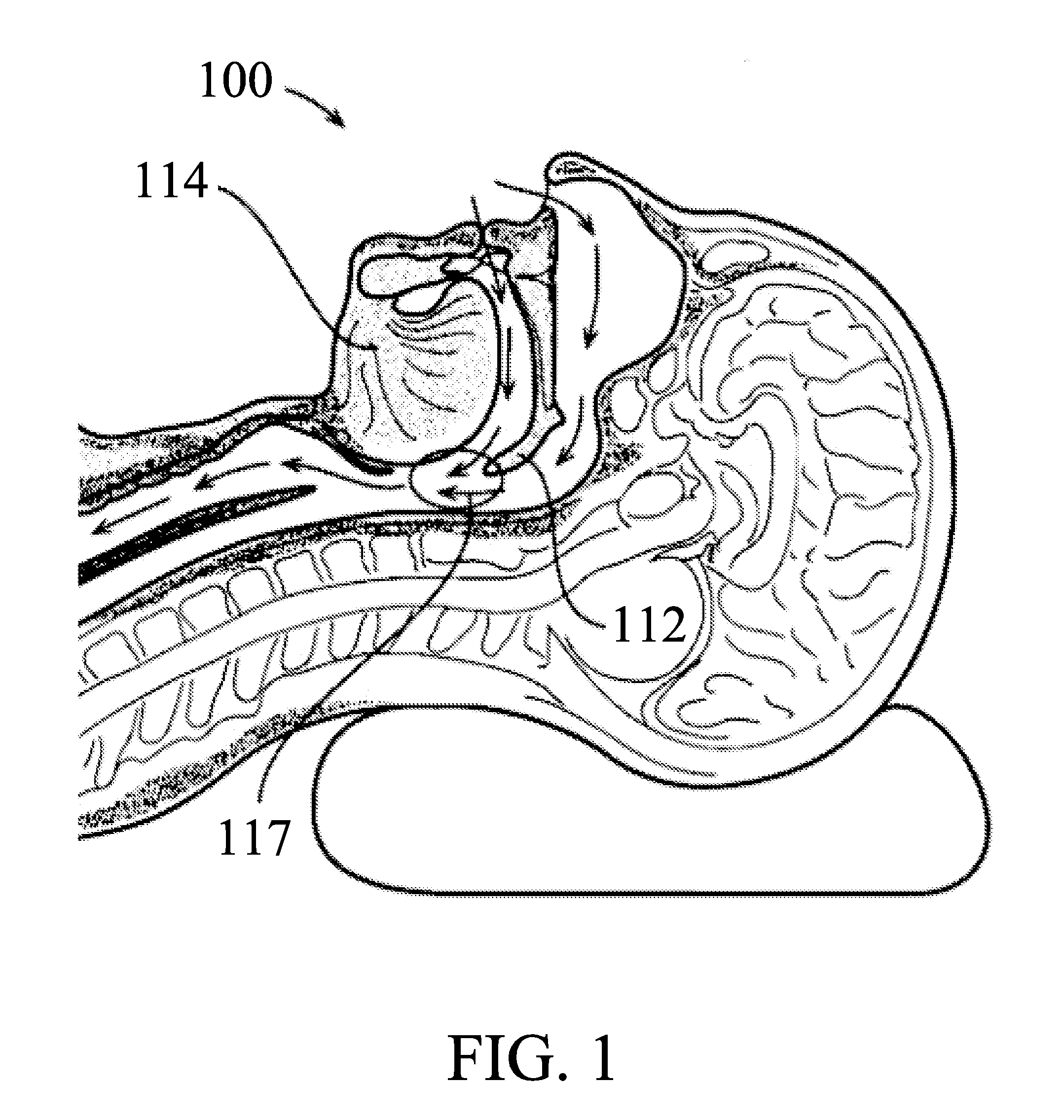
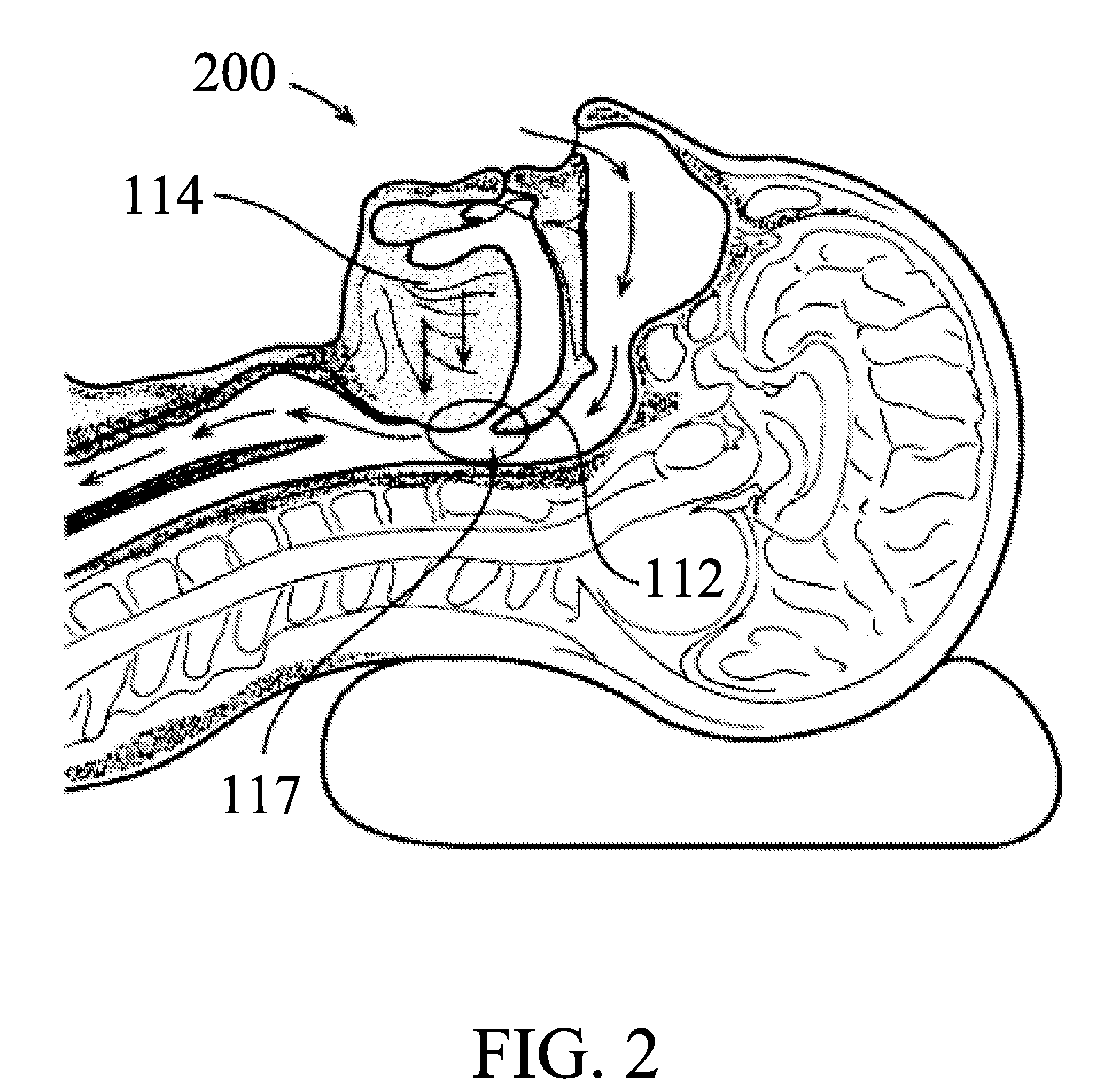
Snoring and obstructive sleep apnea prevention and treatment device
InactiveUS20120234332A1Reduce air turbulencePromotes nasalRespiratorsHead electrodesDiseaseHyoid bone
A anti-snoring and anti-obstructive sleep apnea apparatus has a plate or metal frame tongue shelf splint to prevent the flaccid tongue falling back, a palate shelf splint projection to elevate the soft palate and prevents its vibration, incisors teeth receptacles or pockets sockets for jaw displacer to hold the mandible moved forwards, and to prevent it falling back held between the bite block, catheter-tubing to administer oxygen supplementation from the external source which reduces air turbulence and promotes nasal breathing, a submental suprahyoid muscle stimulator placed below the tongue, and above the mucous membrane. An injection port is provided to administer any therapeutic agents and local anesthetics to reduce the sensitivity of the tongue and oral cavity mucus membrane lining to the foreign objects. This device is used to deliver therapeutic agents to prevent and to treat halitosis and various diseases.
Owner:SHANTHA TOTADA R
Preparation for Iontophoresis
InactiveUS20090163597A1Low absorption rateStable materialBiocideAntipyreticElectrical driveBiomedical engineering
A preparation used for iontophoresis in order to absorb a physiologically active substance via the skin or mucosa using electrical driving force and the preparation containing a local anesthetic, epinephrine or its salt, water and chlorobutanol.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Methods and compositions for treatment of inflammatory disease
InactiveUS7112578B2Promote absorptionProtect normal developmentBiocidePeptide/protein ingredientsArthritisCytokine
Compositions useful for treating inflammatory diseases including arthritis are disclosed which comprise cetyl myristoleate compounds or related compounds and at least one compound useful for treatment of inflammatory disease, such as tetracycline compounds, Cox-2 inhibitors, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, local anaesthetics, chelating agents, matrix metalloprotease inhibitors, inhibitors of inflammatory cytokines, glucosamine, chondroitin sulfate and collagen hydrolysate. Also disclosed are pharmaceutical compositions and methods of treatment for inflammatory disease and local inflammation and dermal irritation. Also disclosed are compositions including tetracycline and at least one compound useful for treatment of inflammatory disease.
Owner:LEVIN BRUCE H
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com