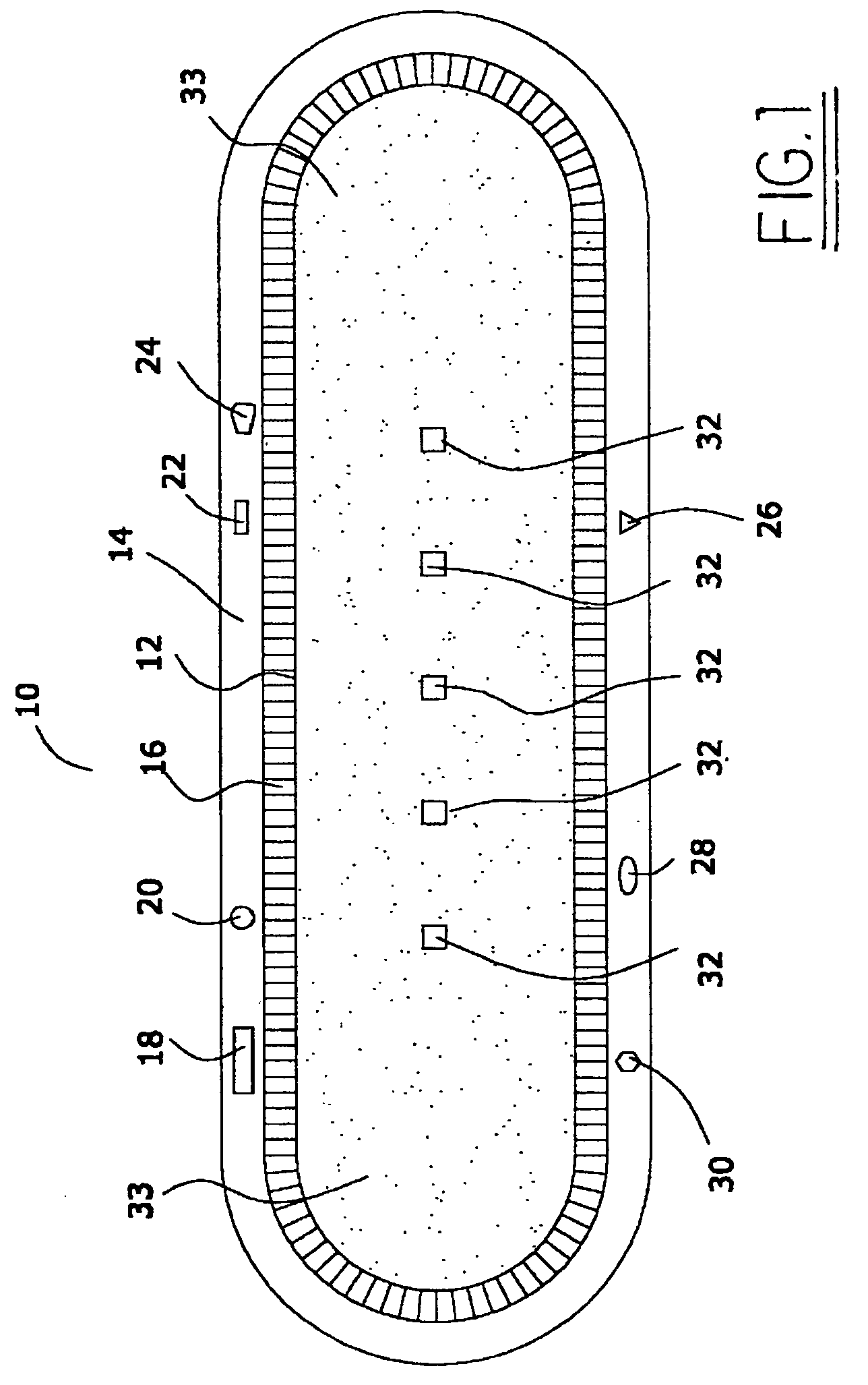
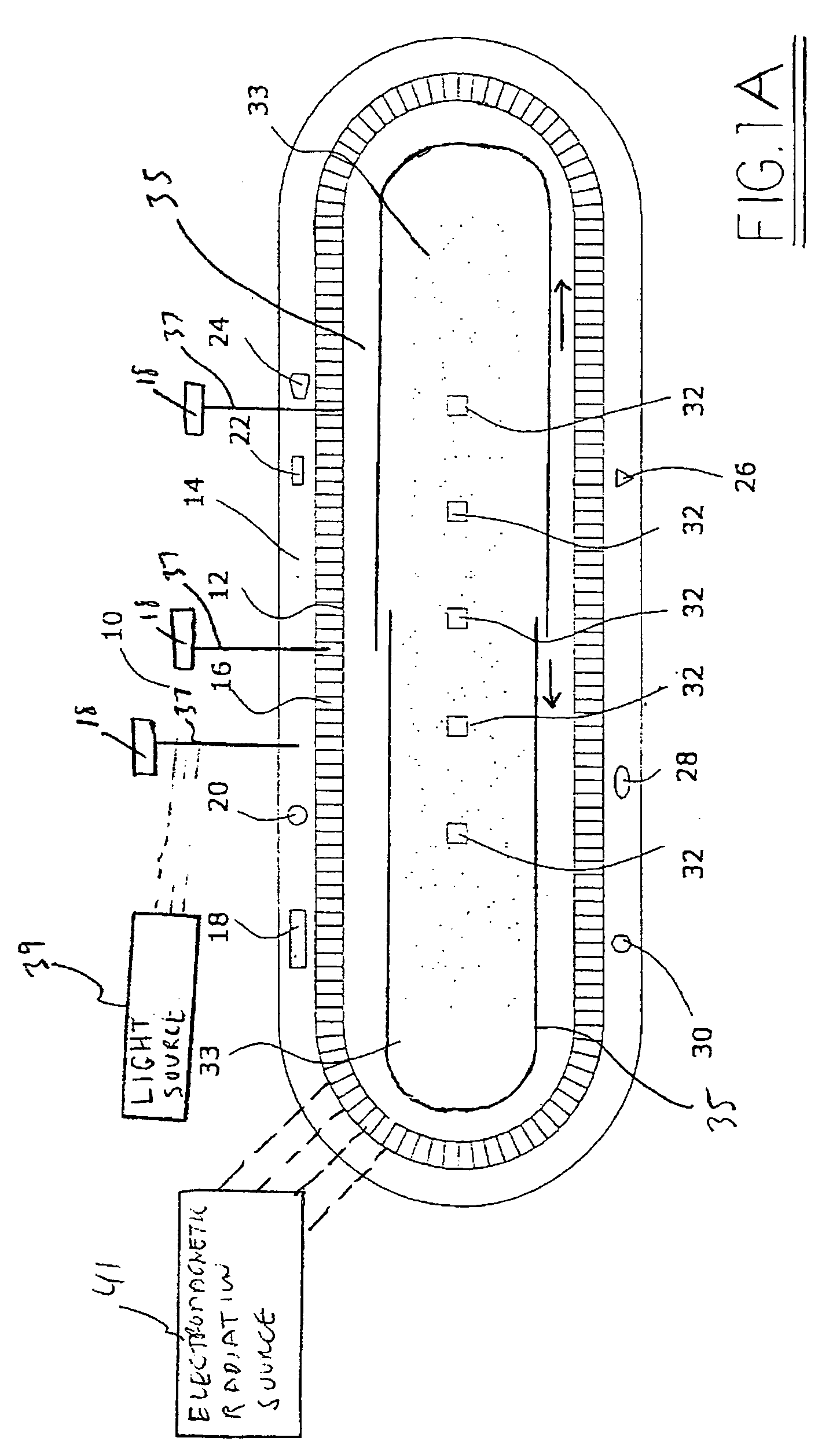
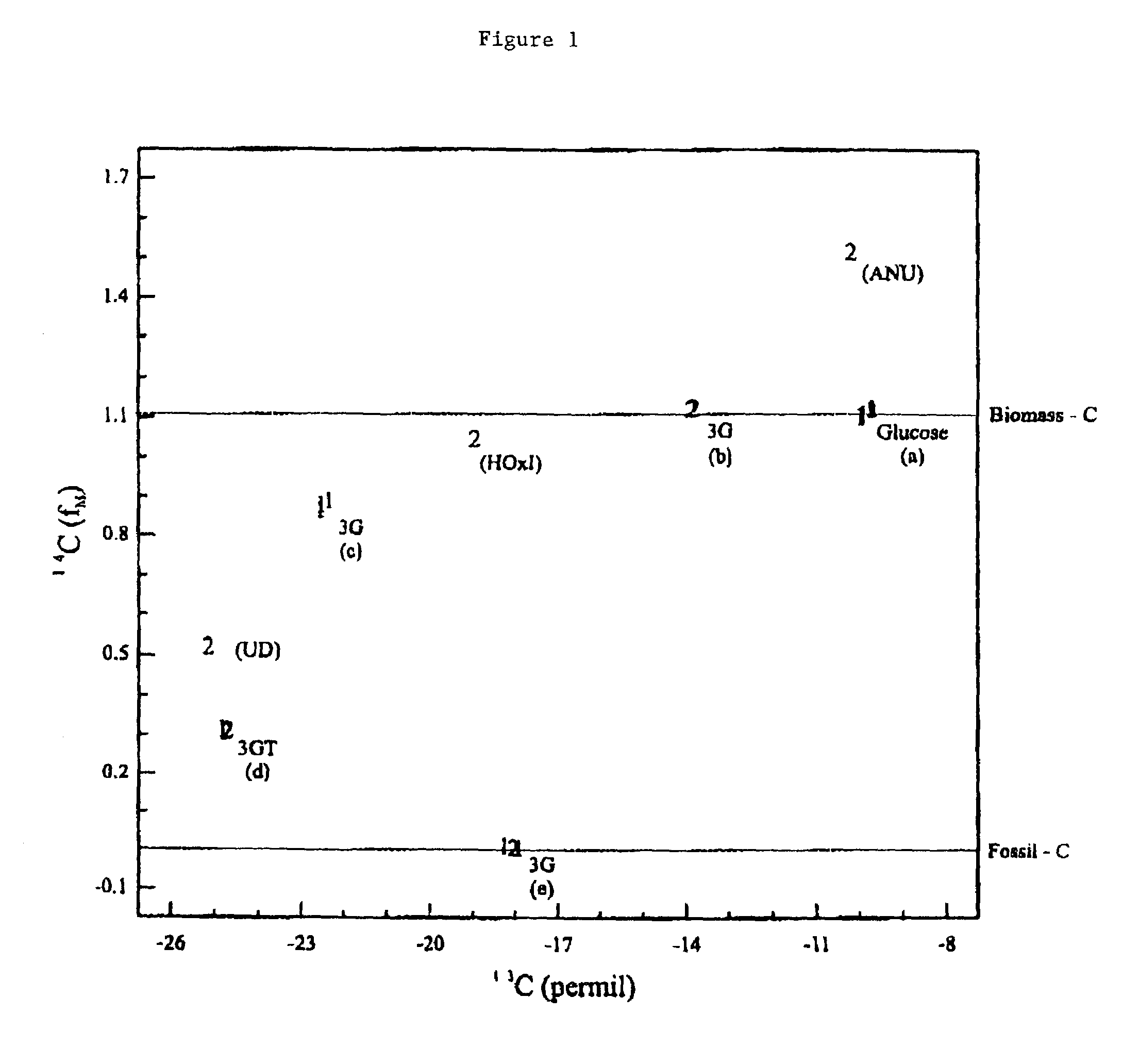
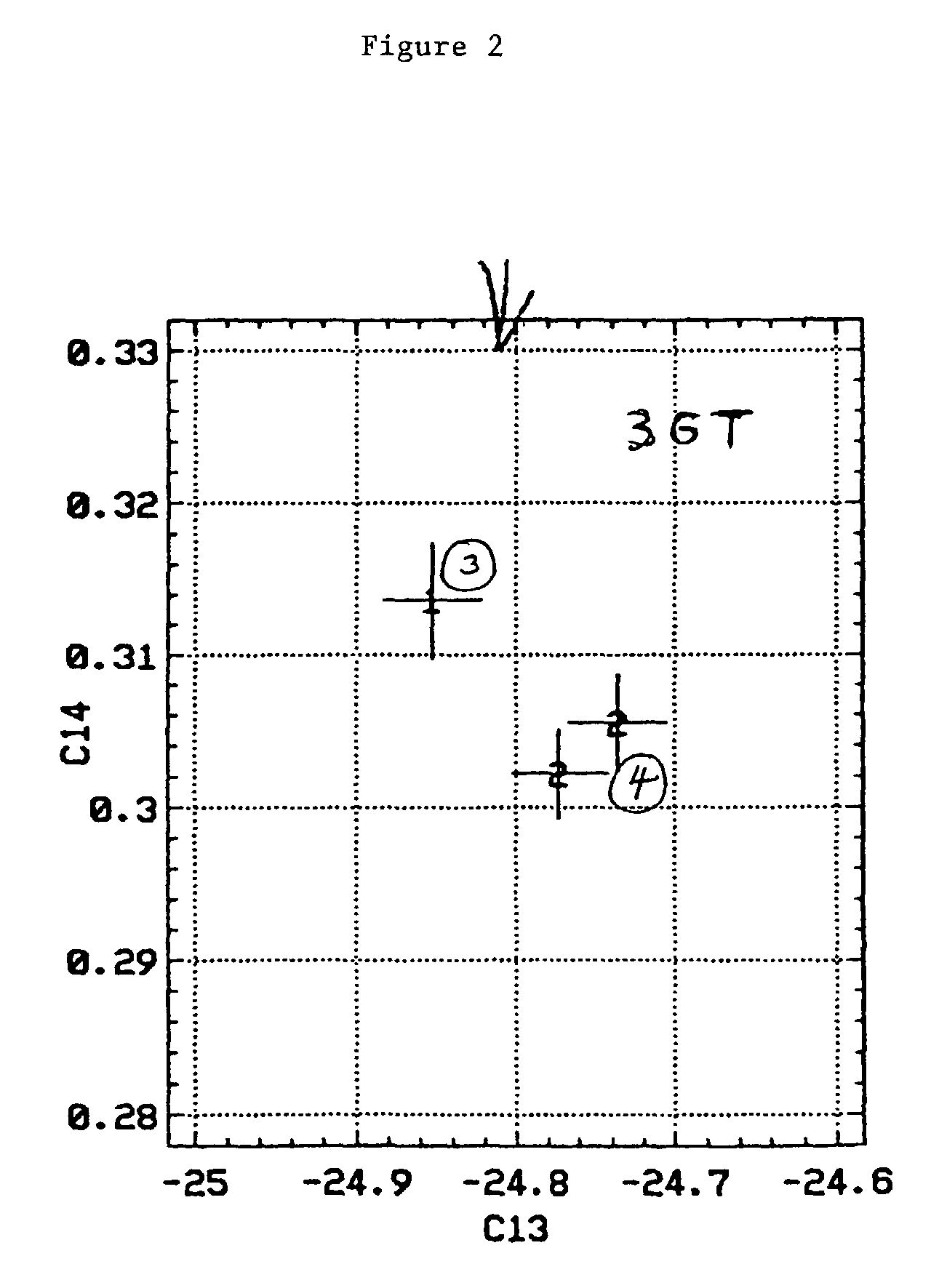
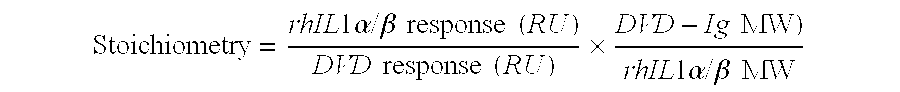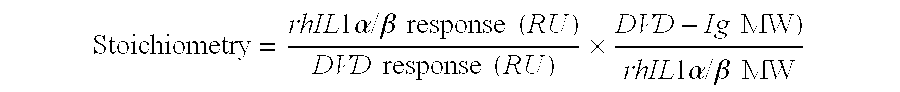Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3561results about "In-vivo radioactive preparations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Wound closure material
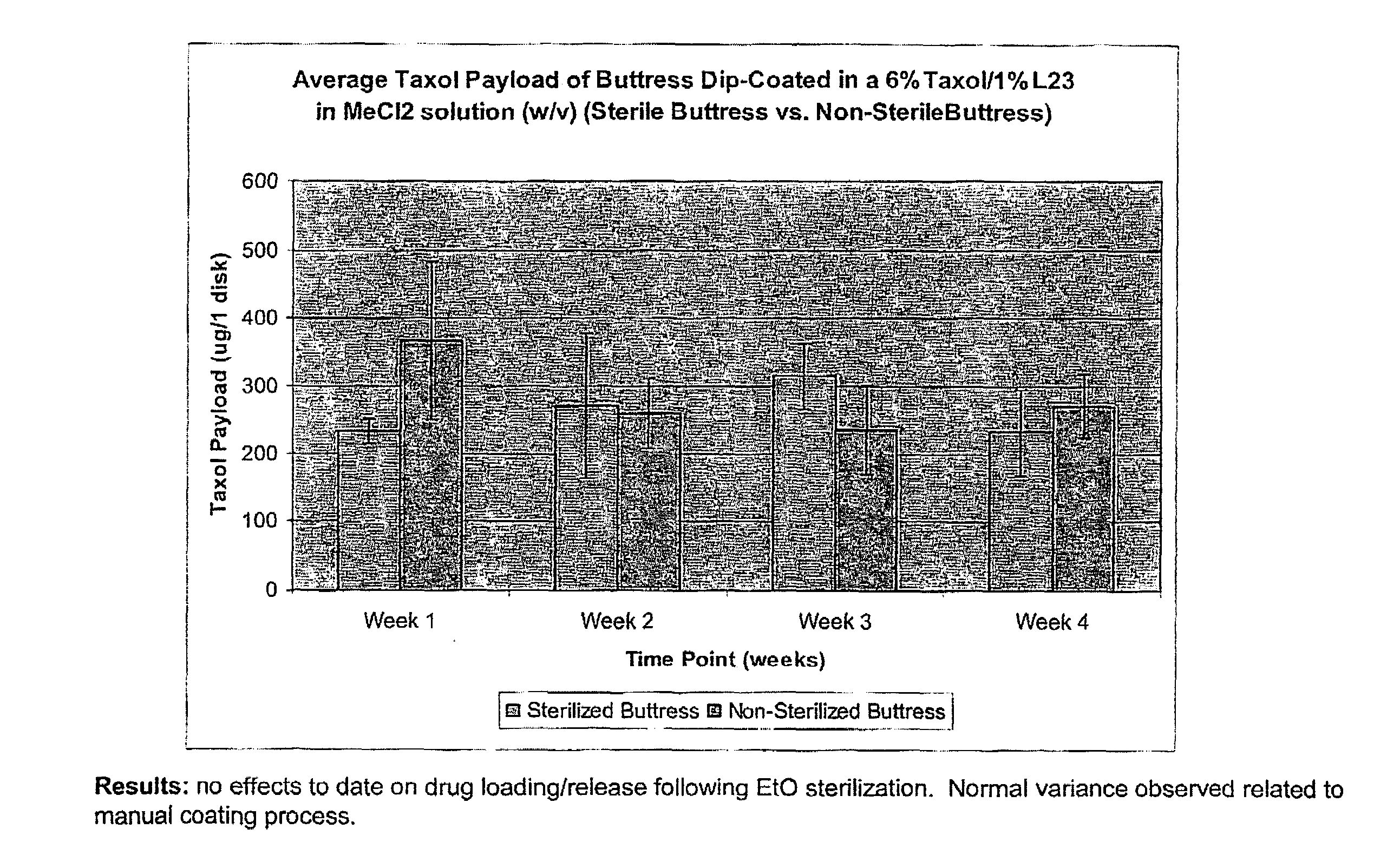
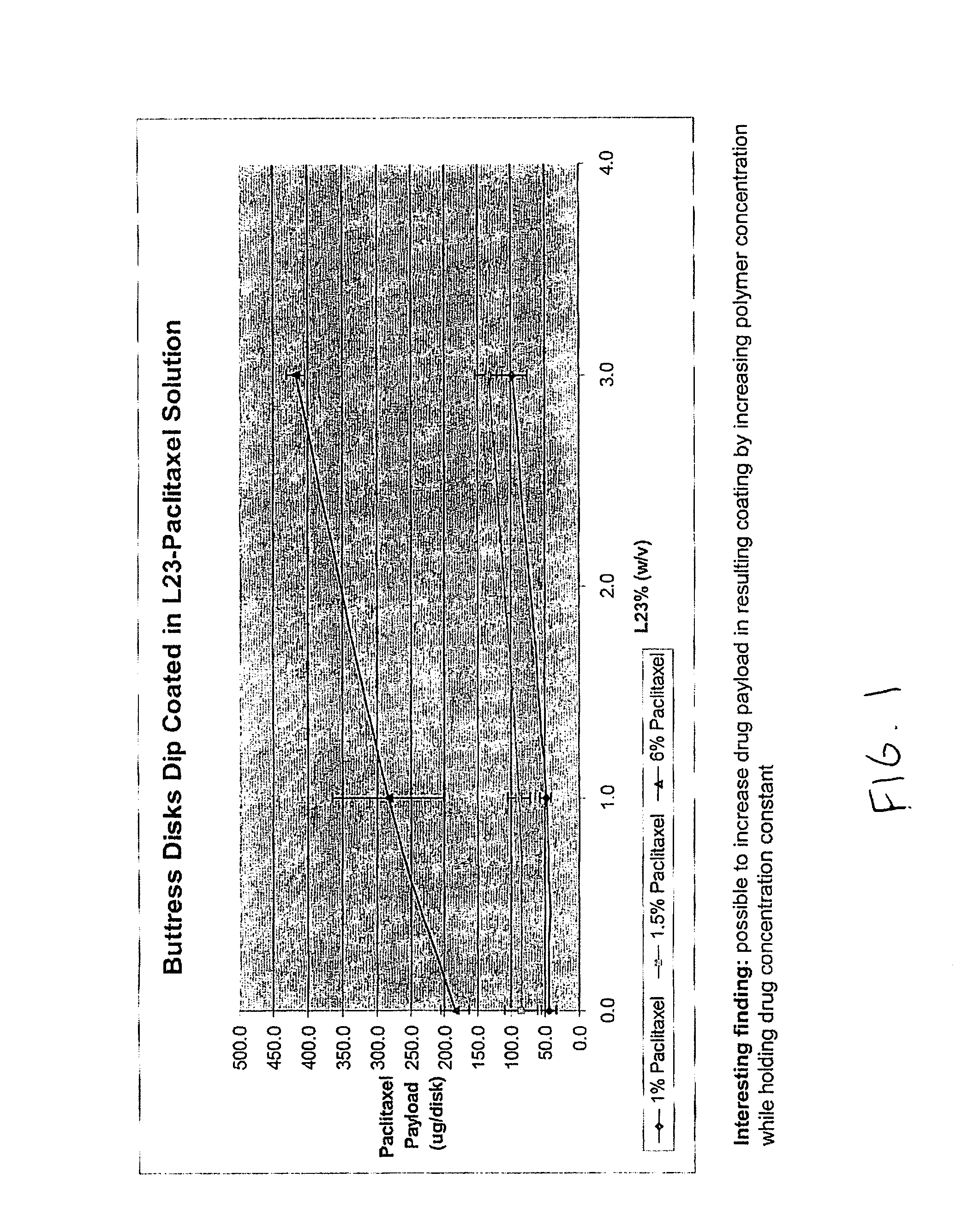
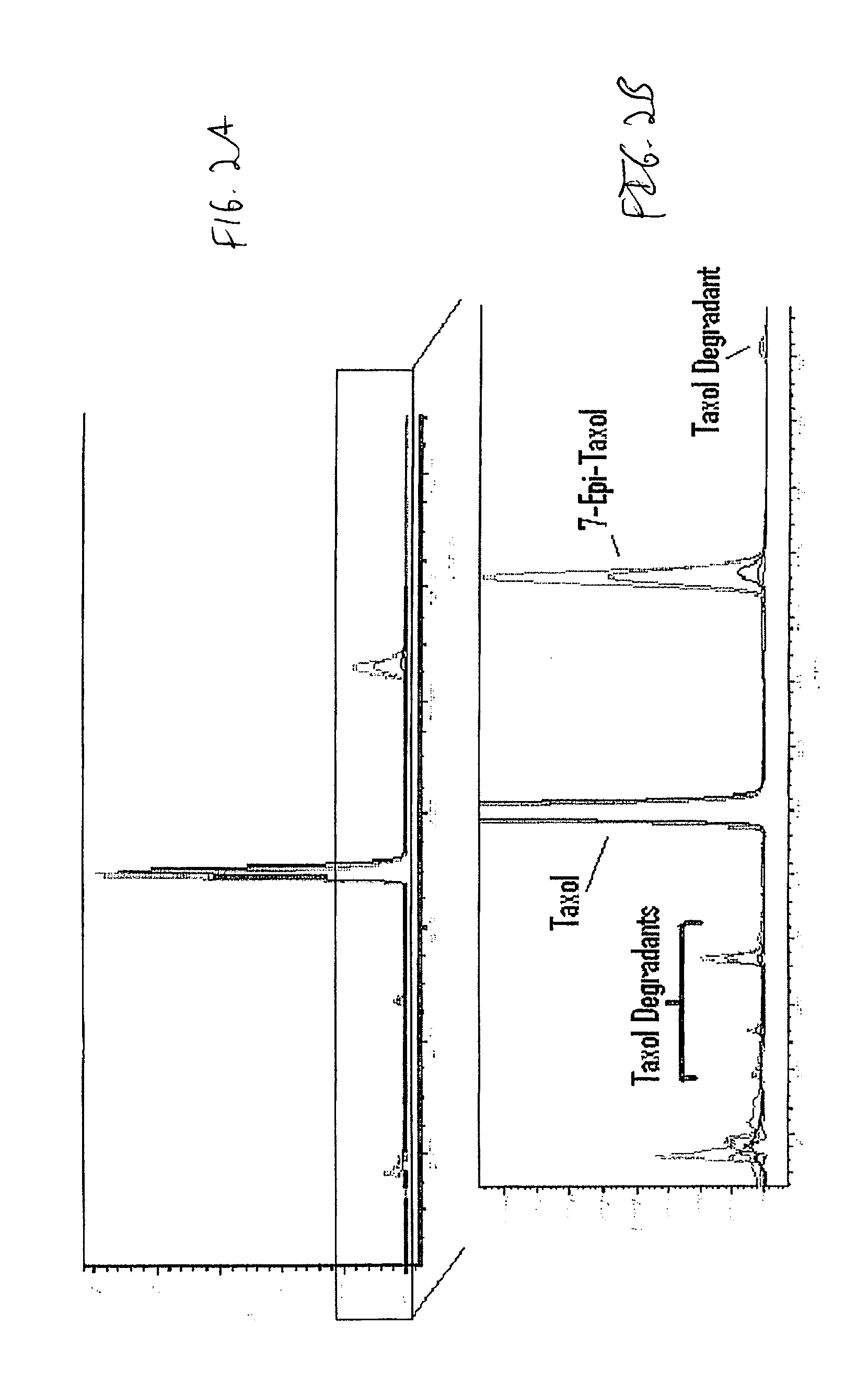
InactiveUS20100076489A1Prevent leakageSuture equipmentsHeavy metal active ingredientsButtressBiomedical engineering
Articles are provided having no orientation or a multi-directional orientation. Such articles may be in the form of films, ribbons, sheets, and / or tapes and may be utilized as buttresses with a surgical stapling apparatus or as reinforcing means for suture lines. The articles may be produced with a polymeric material having an agent, such as a chemotherapeutic agent or a radiotherapeutic agent, incorporated therein or applied as a coating thereon.
Owner:TYCO HEALTHCARE GRP LP
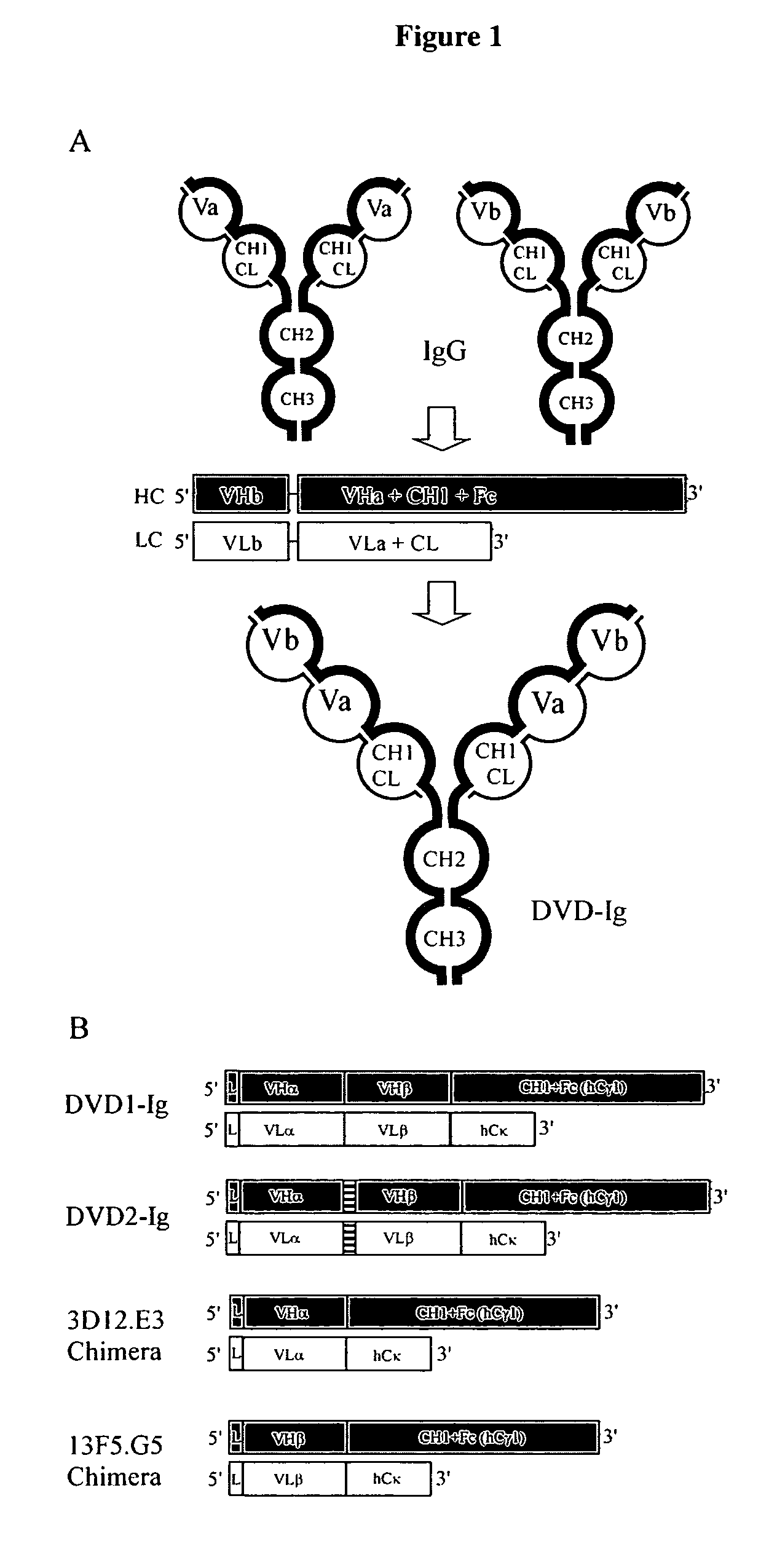
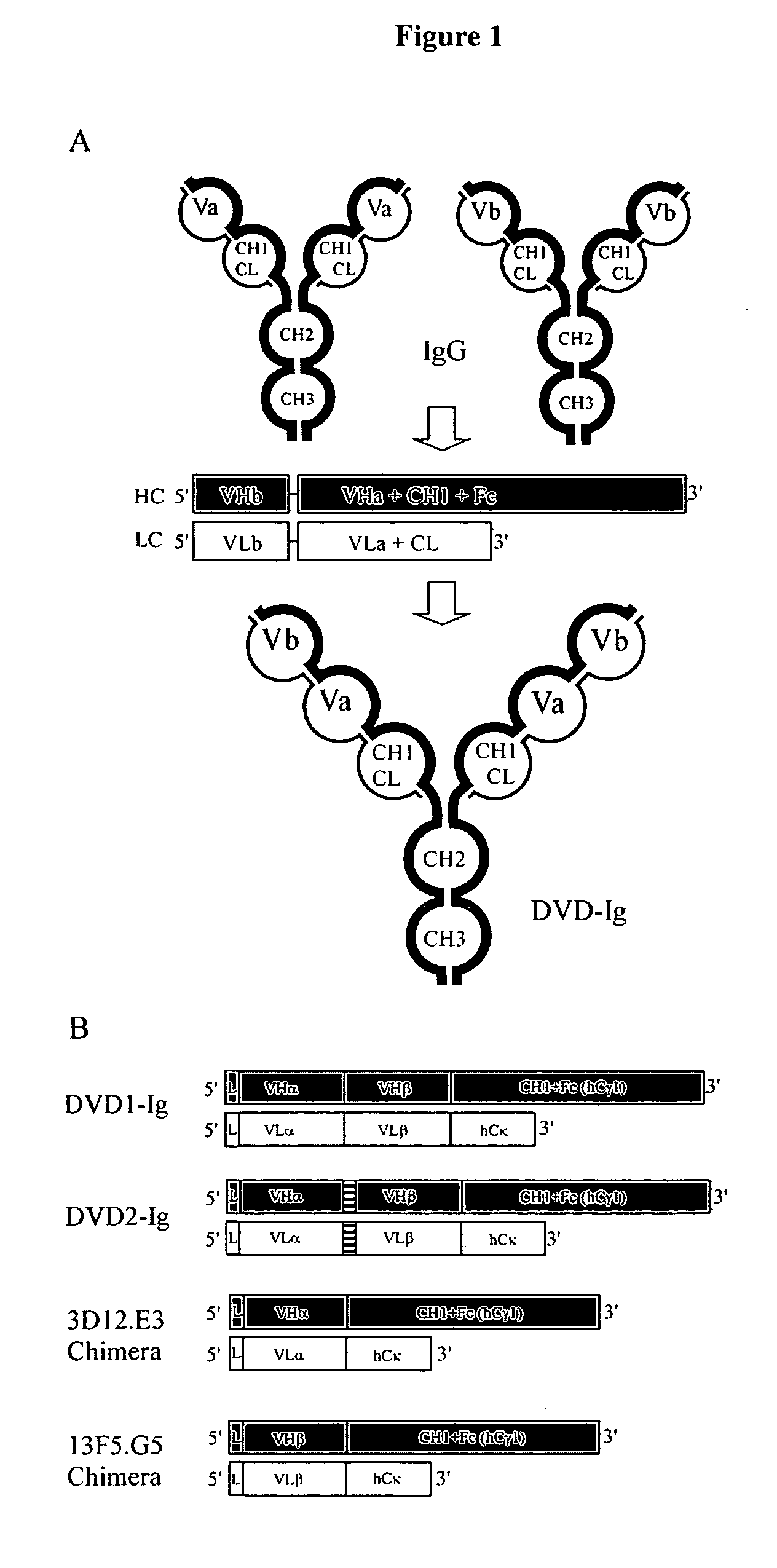
Dual variable domain immunoglobulin and uses thereof
Owner:ABBVIE INC
Dual variable domain immunoglobulin and uses thereof
Owner:ABBVIE INC
Method and apparatus for EMR treatment
InactiveUS6997923B2Improve cooling effectUltrasound therapyIn-vivo radioactive preparationsTherapeutic treatmentLength wave
A method and apparatus are provided for performing a therapeutic treatment on a patient's skin by concentrating applied radiation of at least one selected wavelength at a plurality of selected, three-dimensionally located, treatment portions, which treatment portions are within non-treatment portions. The ratio of treatment portions to the total volume may vary from 0.1% to 90%, but is preferably less than 50%. Various techniques, including wavelength, may be utilized to control the depth to which radiation is concentrated and suitable optical systems may be provided to concentrate applied radiation in parallel or in series for selected combinations of one or more treatment portions.
Owner:PALOMAR MEDICAL TECH +1
Cysteine engineered anti-MUC16 antibodies and antibody drug conjugates
ActiveUS7723485B2In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkDrug compound
Cysteine engineered anti-MUC16 antibodies are engineered by replacing one or more amino acids of a parent anti-MUC16 antibody with non cross-linked, reactive cysteine amino acids. Methods of design, preparation, screening, and selection of the cysteine engineered anti-MUC16 antibodies are provided. Cysteine engineered anti-MUC16 antibodies (Ab) are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered anti-MUC16 antibody-drug conjugates having Formula I:Ab-(L-D)p Iwhere p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Method and kit for imaging and treating organs and tissues
InactiveUS6331175B1High resolutionStrong specificityElectrotherapyNanomedicineMammalHypoplastic genitalia
Provided are methods and compositions for detecting and treating normal, hypoplastic, ectopic or remnant tissue, organ or cells in a mammal. The method comprises parenterally injecting a mammalian subject, at a locus and by a route providing access to above-mentioned tissue or organ, with an composition comprising antibody / fragment which specifically binds to targeted organ, tissue or cell. The antibody / fragment may be administered alone, or labeled or conjugated with an imaging, therapeutic, cytoprotective or activating agent.
Owner:IMMUNOMEDICS INC
Multivalent immunoglobulin-based bioactive assemblies
ActiveUS7527787B2Efficacious for arrestingInhibition formationPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseDiagnostic agent
Owner:IBC PHARMACEUTICALS INC
Targeted and high density drug loaded polymeric materials
ActiveUS20060002852A1Increase molecular densityHigh densityPowder deliveryBiocideAntigenWound dressing
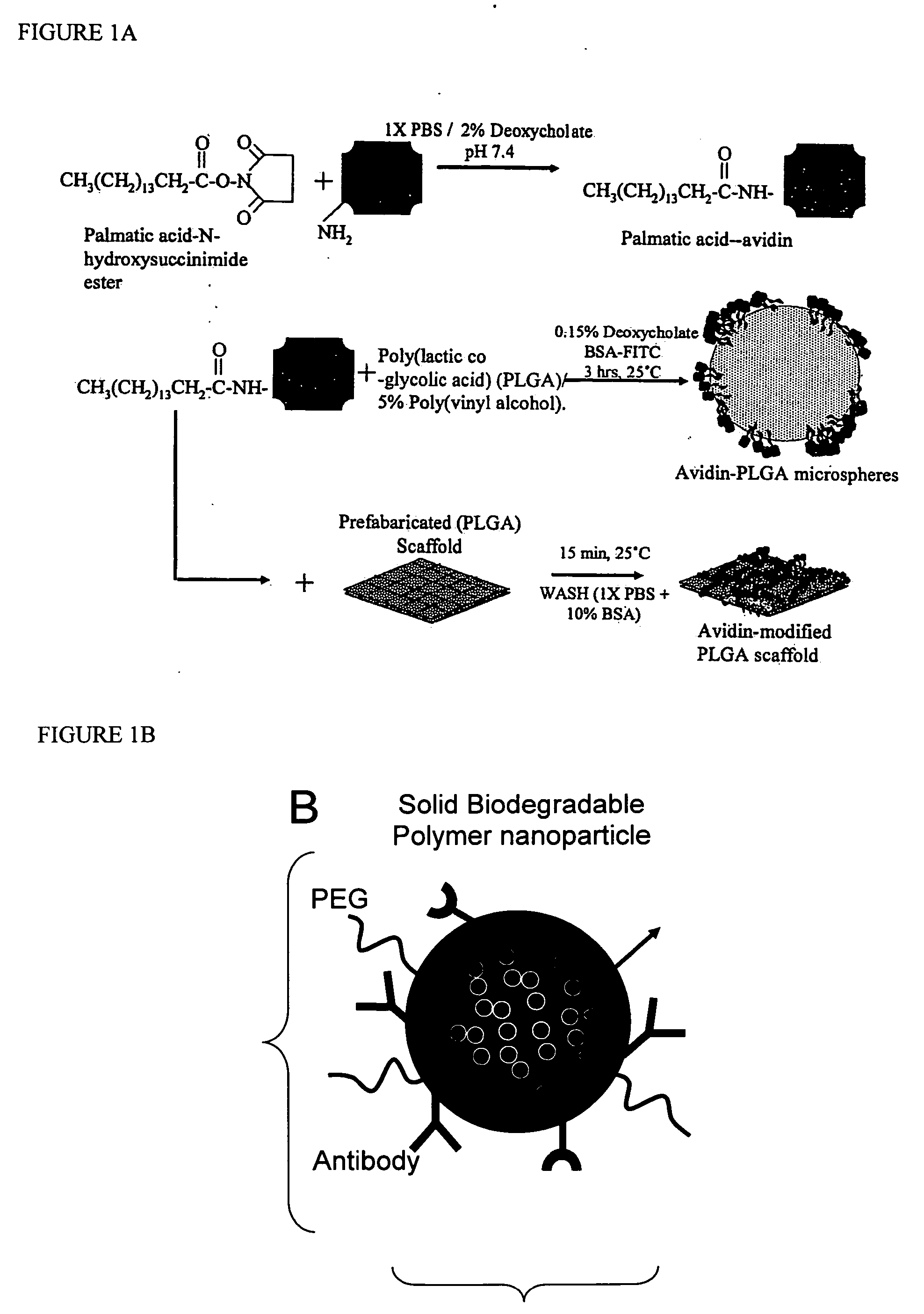
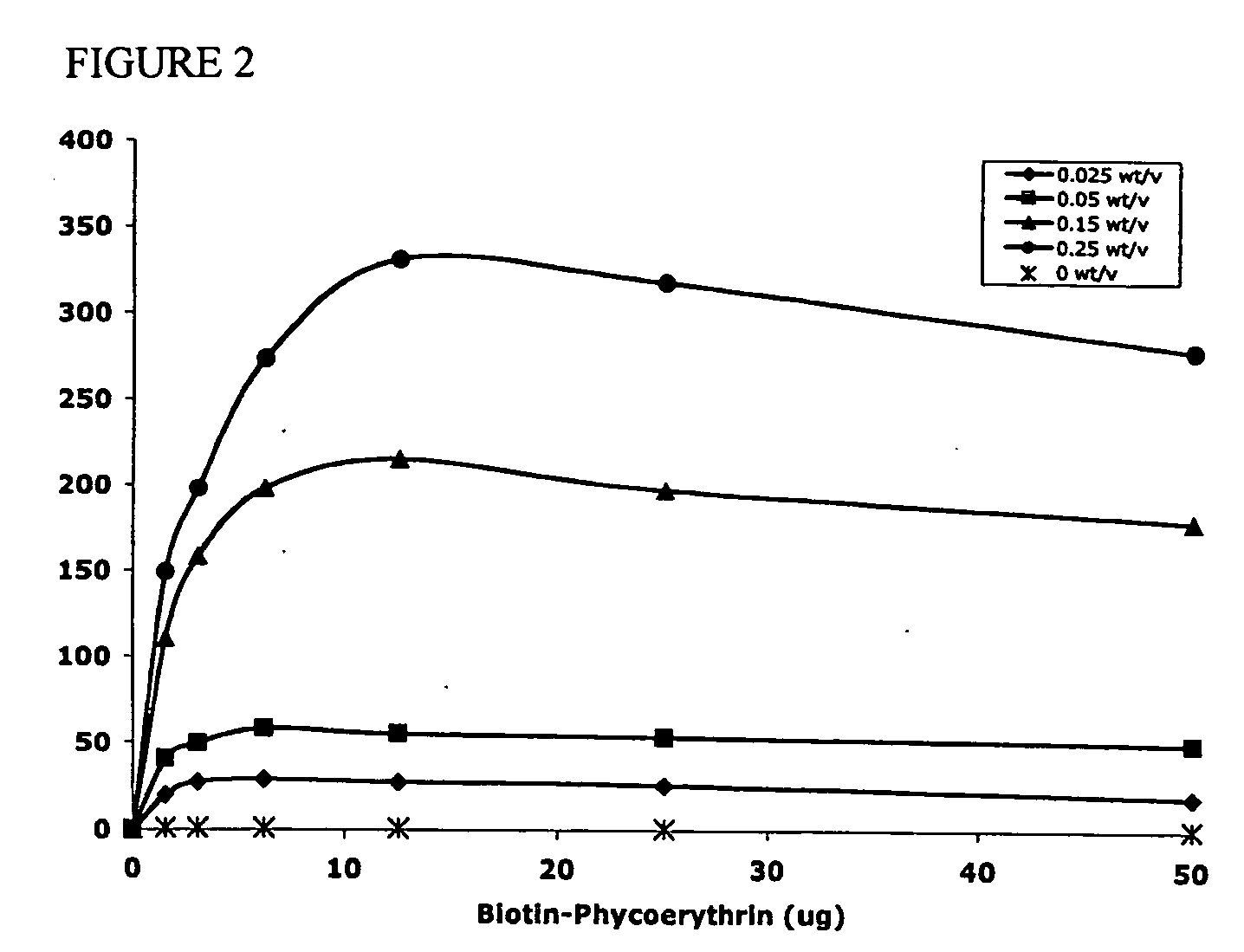
Polymeric delivery devices have been developed which combine high loading / high density of molecules to be delivered with the option of targeting. As used herein, “high density” refers to microparticles having a high density of ligands or coupling agents, which is in the range of 1000-10,000,000, more preferably between 10,000 and 1,000,000 ligands per square micron of microparticle surface area. A general method for incorporating molecules into the surface of biocompatible polymers using materials with an HLB of less than 10, more preferably less than 5, such as fatty acids, has been developed. Because of its ease, generality and flexibility, this method has widespread utility in modifying the surface of polymeric materials for applications in drug delivery and tissue engineering, as well other other fields. Targeted polymeric microparticles have also been developed which encapsulate therapeutic compounds such as drugs, cellular materials or components, and antigens, and have targeting ligands directly bound to the microparticle surface. Preferred applications include use in tissue engineering matrices, wound dressings, bone repair or regeneration materials, and other applications where the microparticles are retained at the site of application or implantation. Another preferred application is in the use of microparticles to deliver anti-proliferative agents to the lining of blood vessels following angioplasty, transplantation or bypass surgery to prevent or decrease restenosis, and in cancer therapy. In still another application, the microparticles are used to treat or prevent macular degeneration when administered to the eye, where agents such as complement inhibitors are administered.
Owner:YALE UNIV
Methods and compositions for generating bioactive assemblies of increased complexity and uses
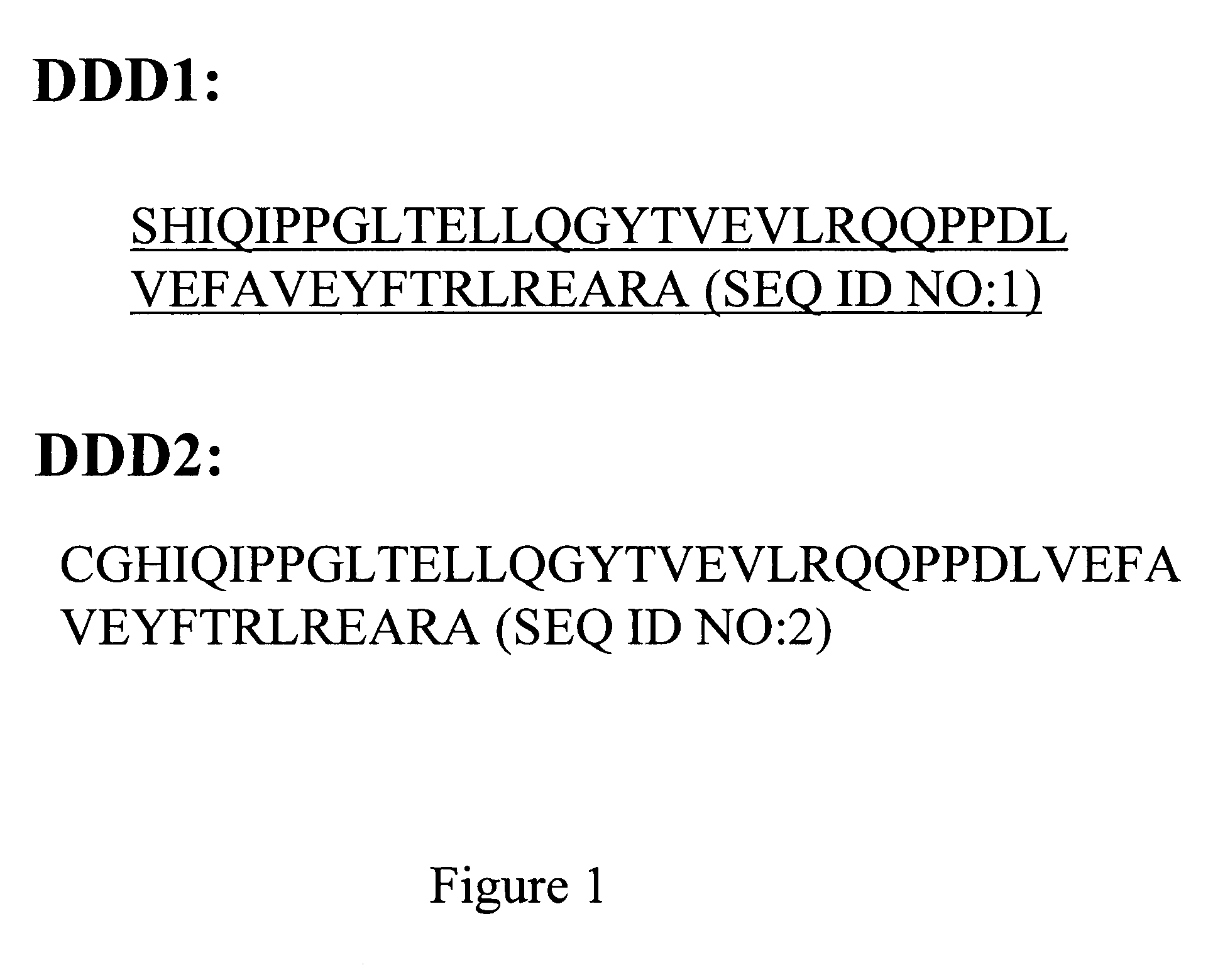
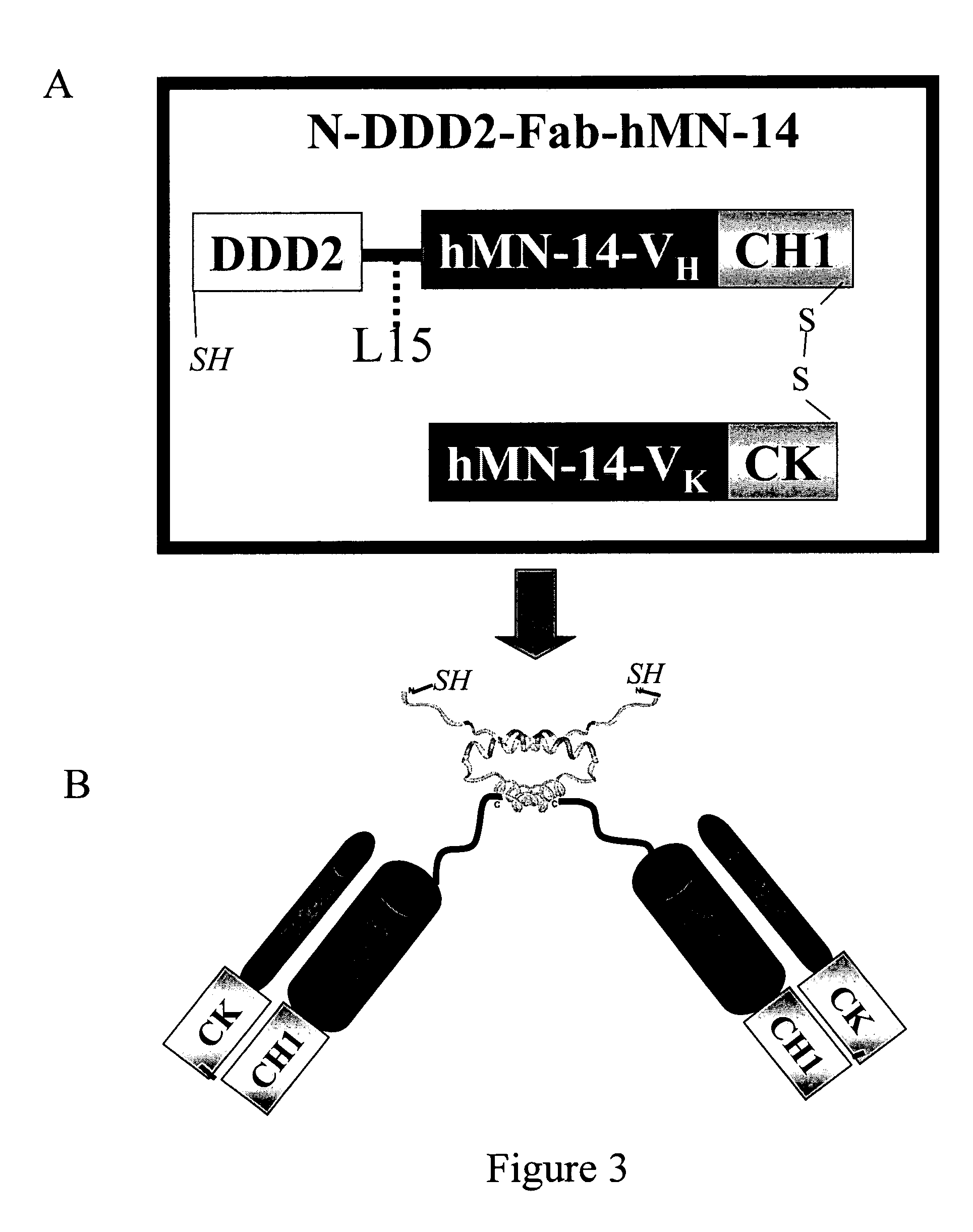
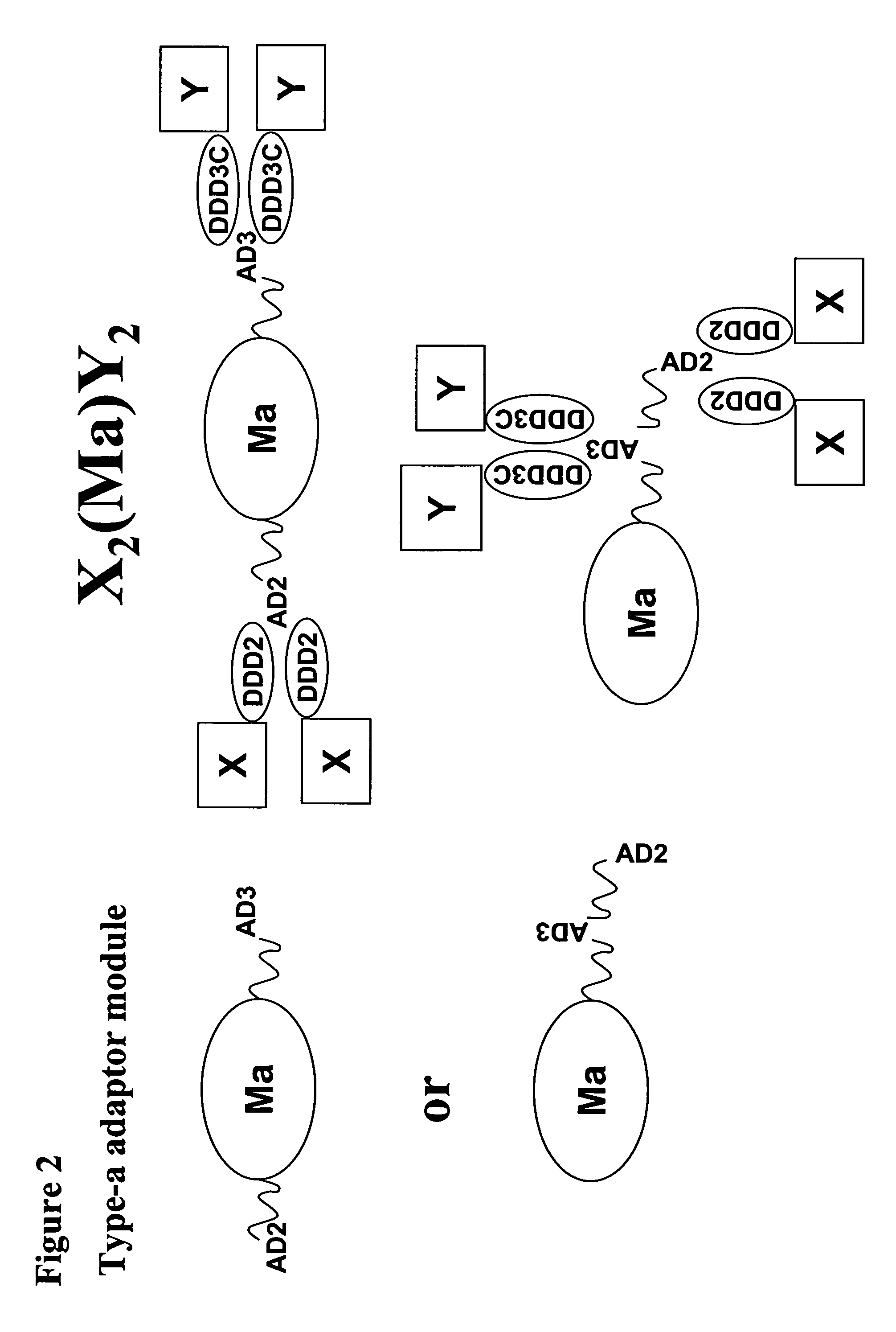
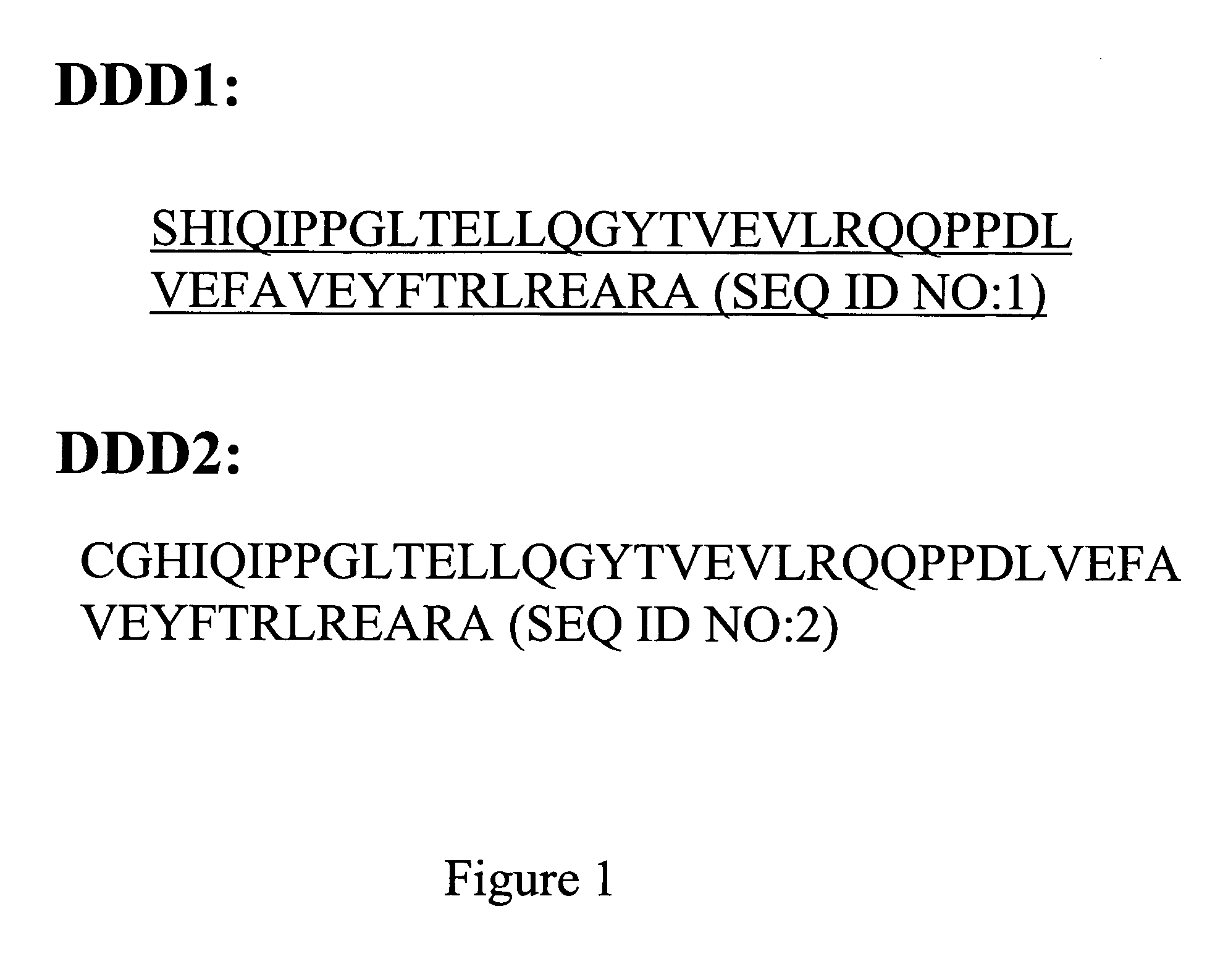
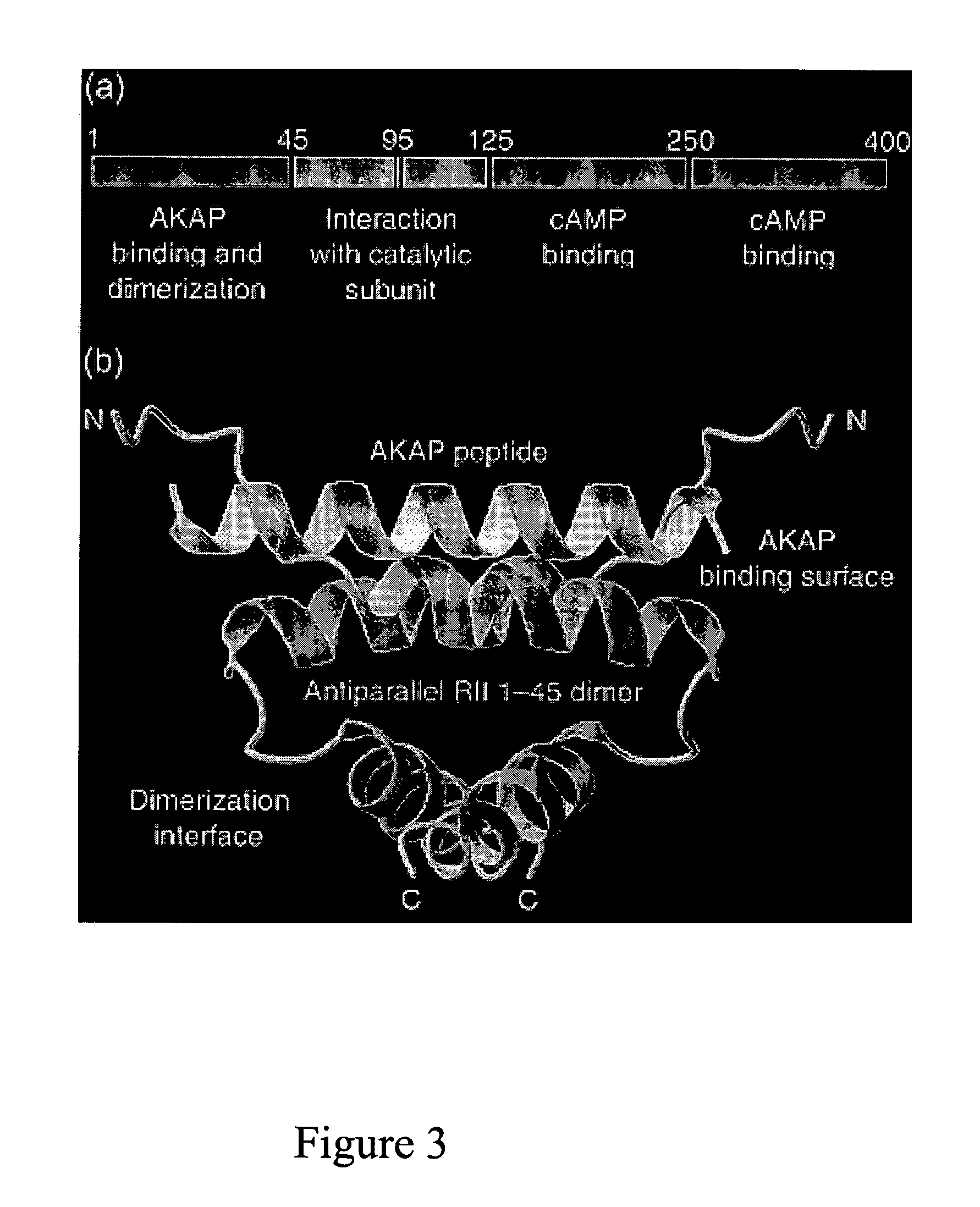
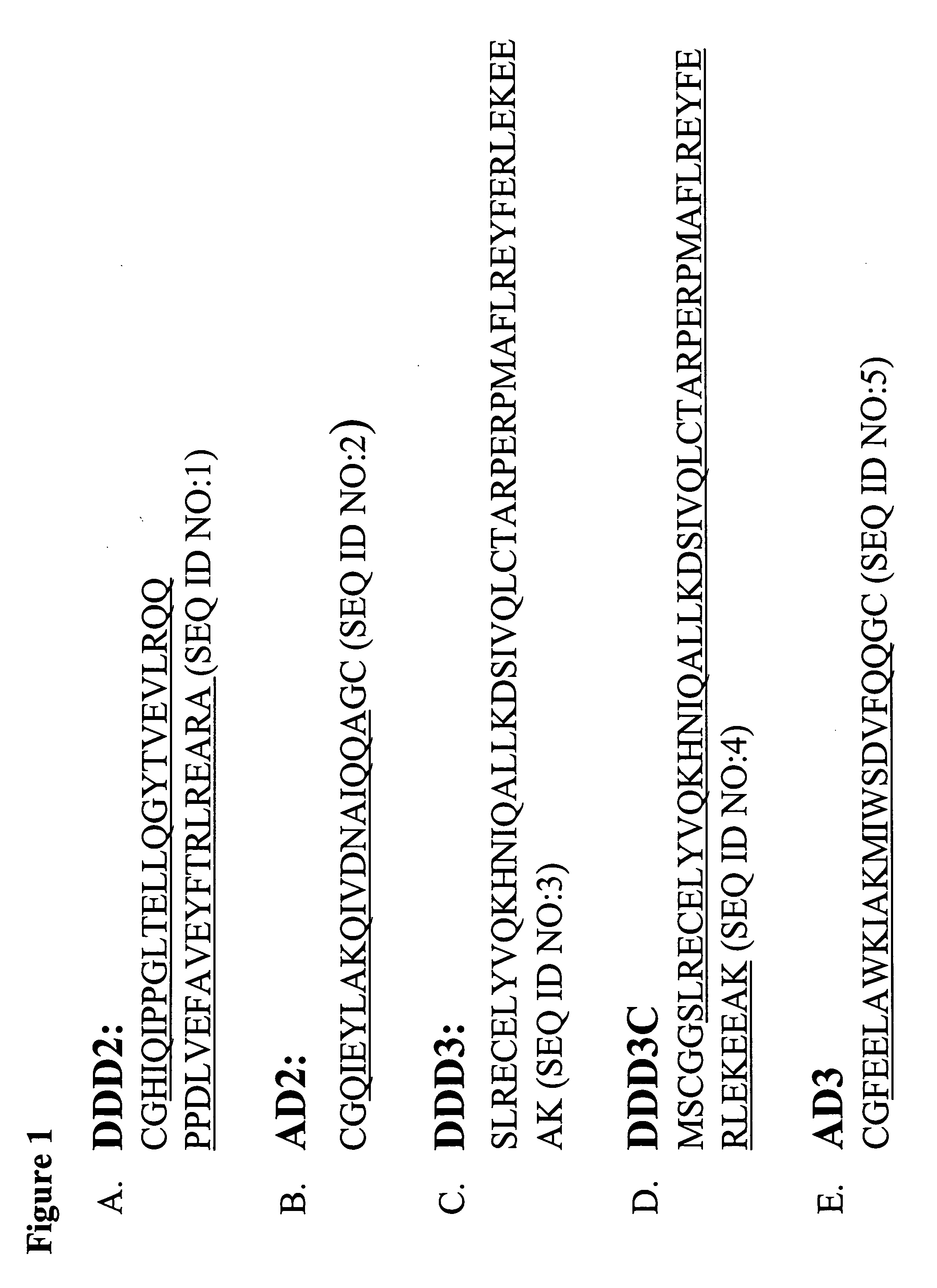
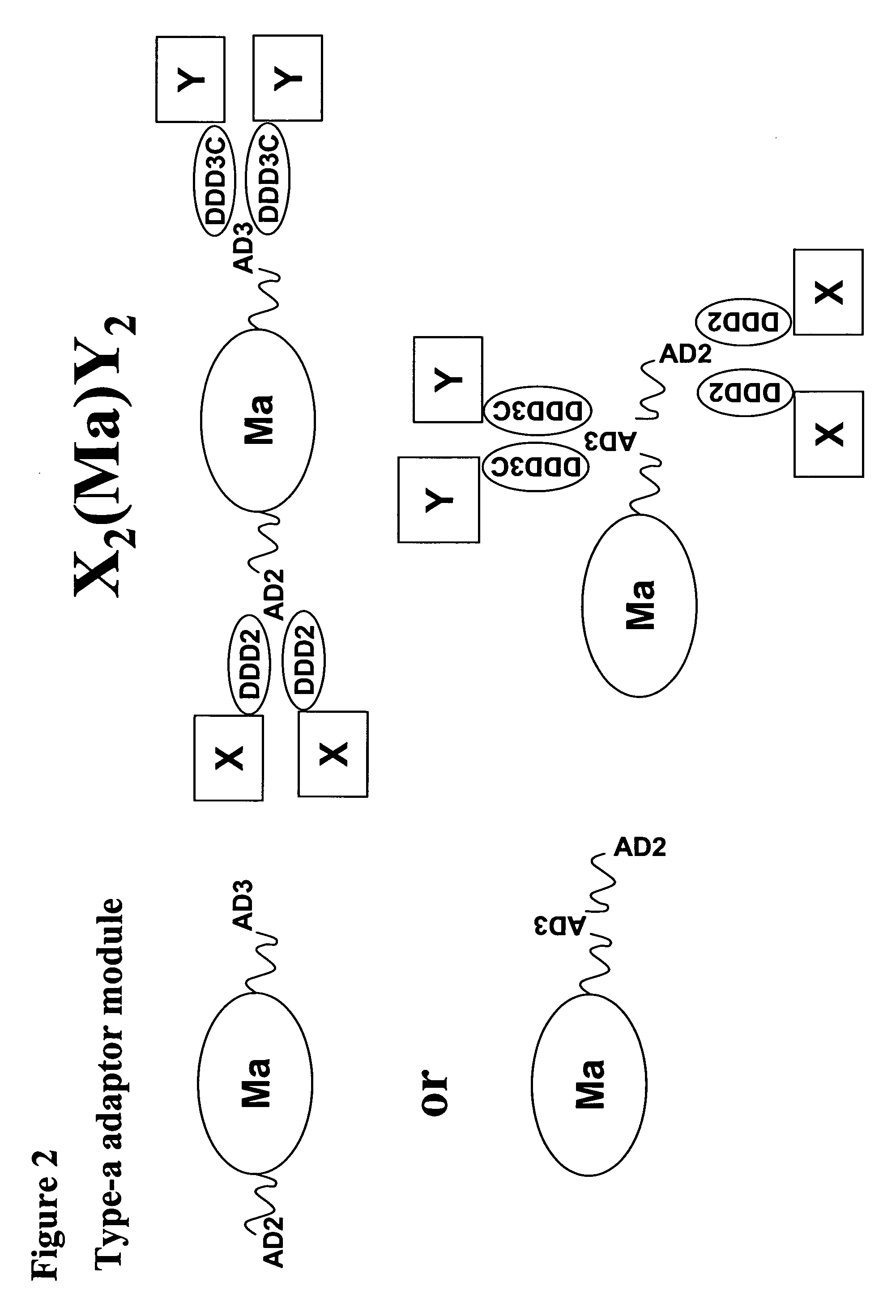
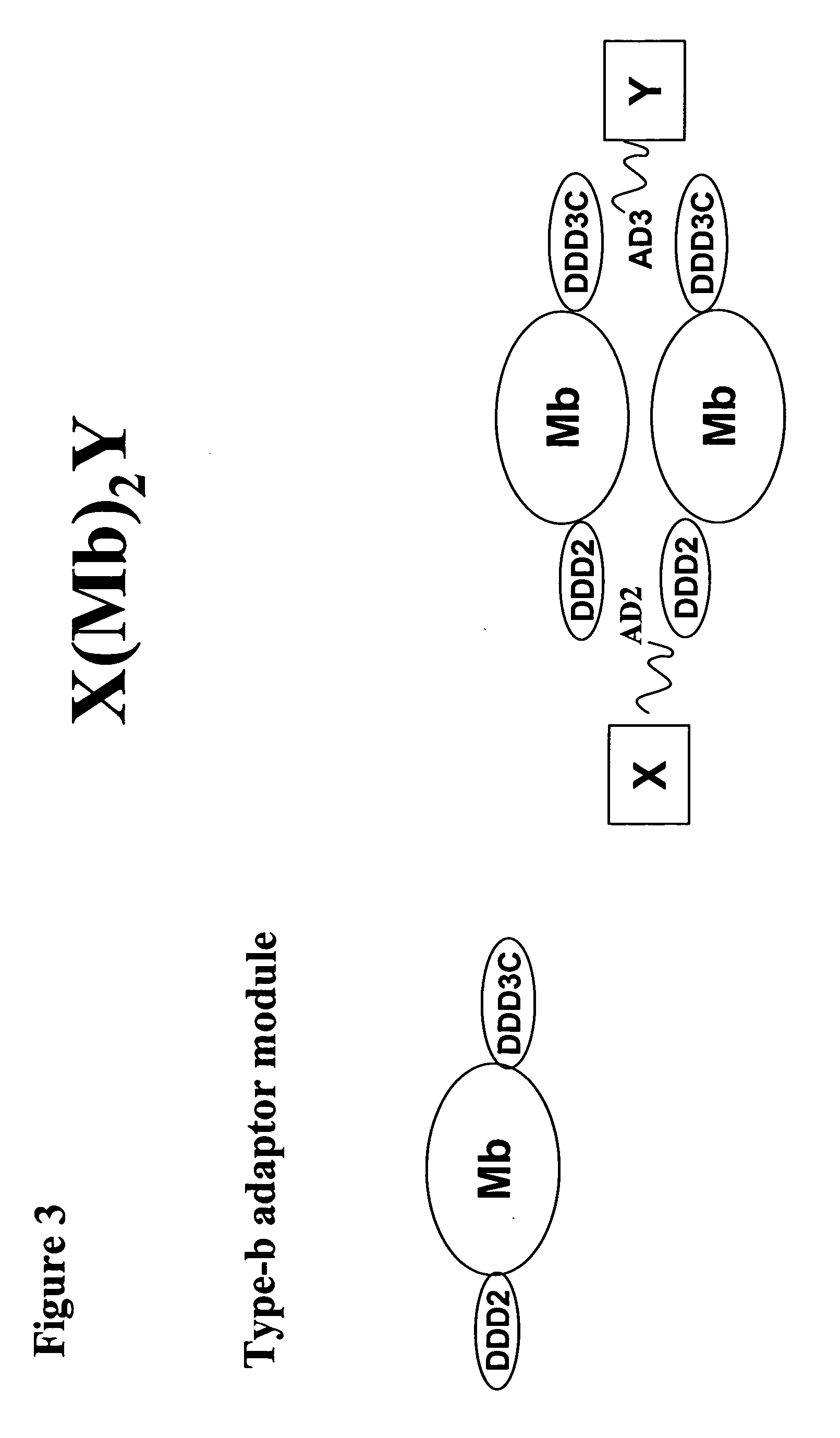
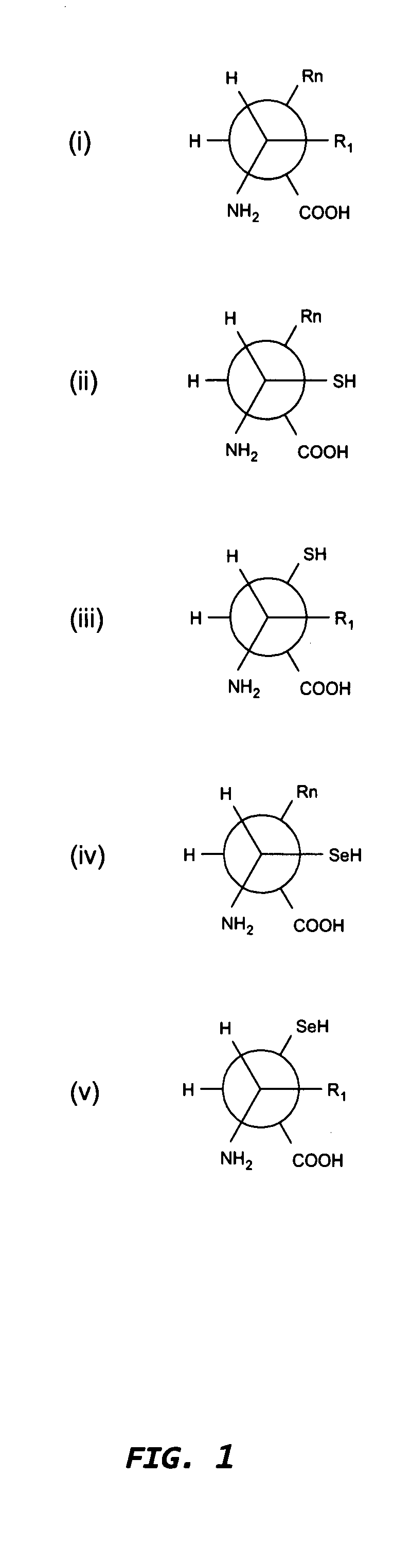
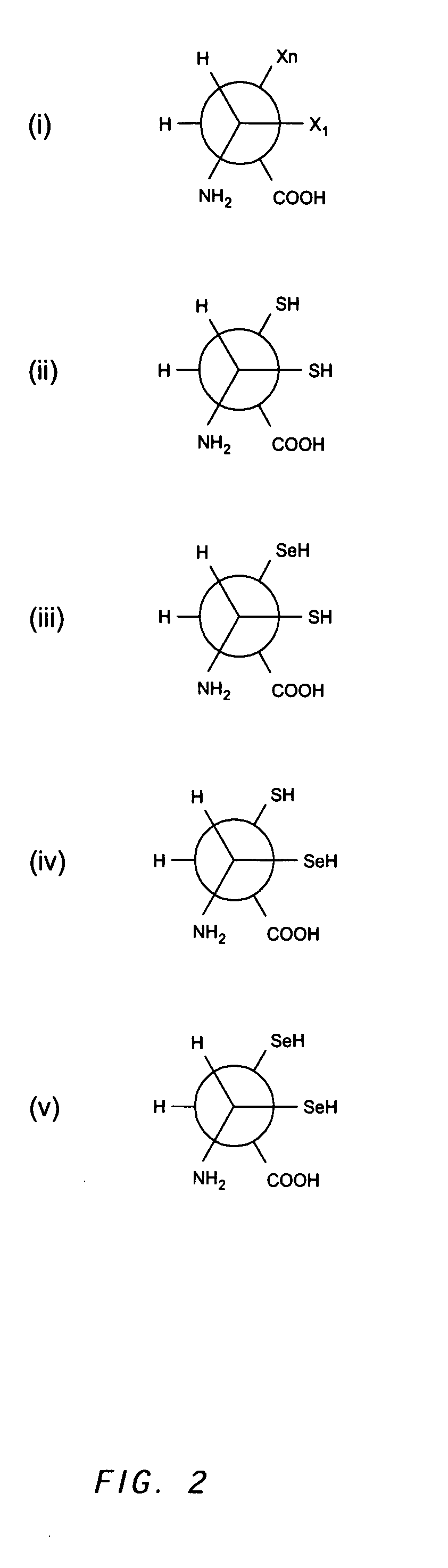
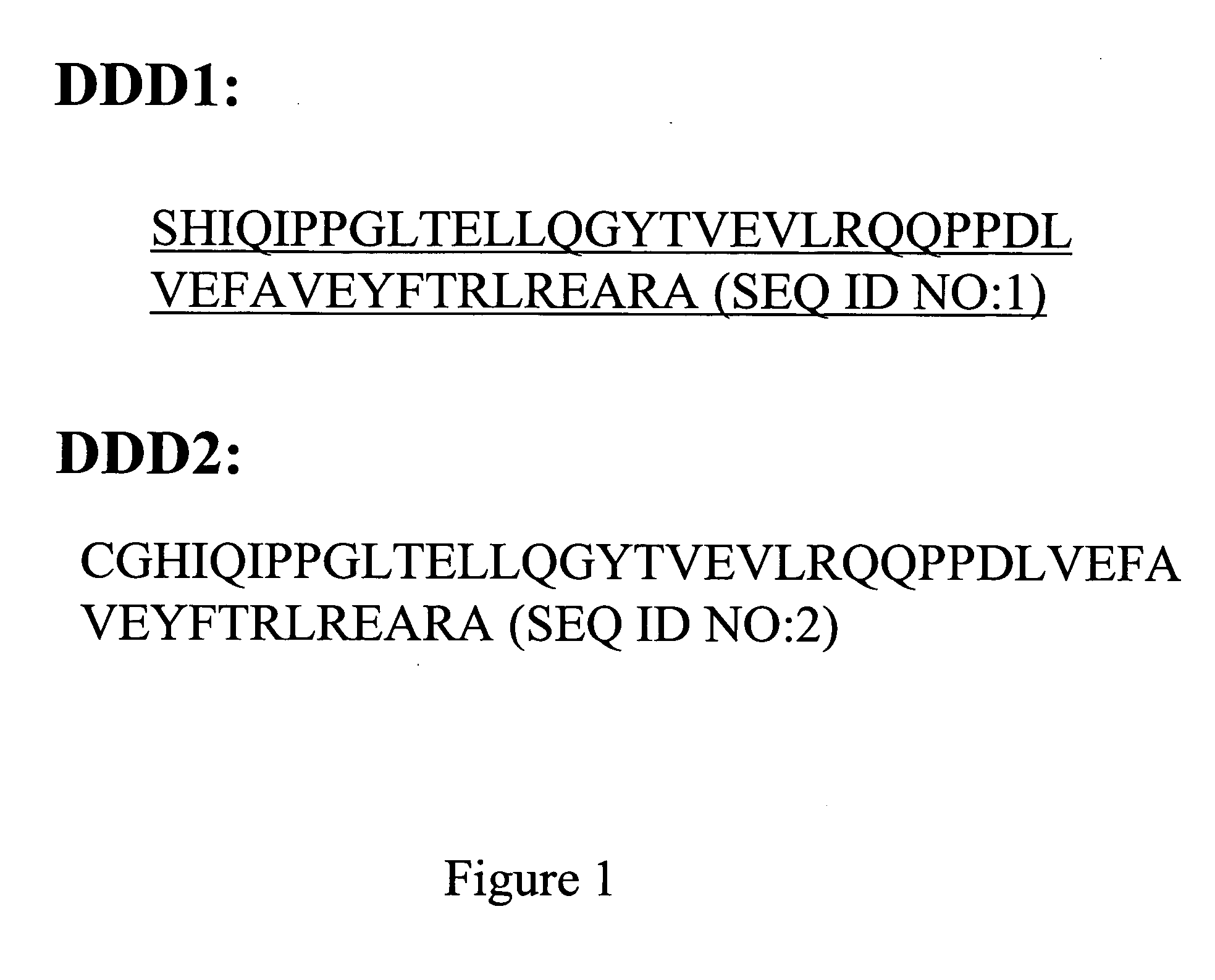
The present invention concerns methods and compositions for making and using bioactive assemblies of defined compositions, which may have multiple functionalities and / or binding specificities. In particular embodiments, the bioactive assembly is formed using dock-and-lock (DNL) methodology, which takes advantage of the specific binding interaction between dimerization and docking domains (DDD) and anchoring domains (AD) to form the assembly. In various embodiments, one or more effectors may be attached to a DDD or AD sequence. Complementary AD or DDD sequences may be attached to an adaptor module that forms the core of the bioactive assembly, allowing formation of the assembly through the specific DDD / AD binding interactions. Such assemblies may be attached to a wide variety of effector moieties for treatment, detection and / or diagnosis of a disease, pathogen infection or other medical or veterinary condition.
Owner:IBC PHARMACEUTICALS INC
Stably tethered structures of defined compositions with multiple functions or binding specificities
Owner:IBC PHARMACEUTICALS INC
Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules
ActiveUS7404969B2Reduce deliveryAntibacterial agentsOrganic active ingredientsLipid formationMolecular composition
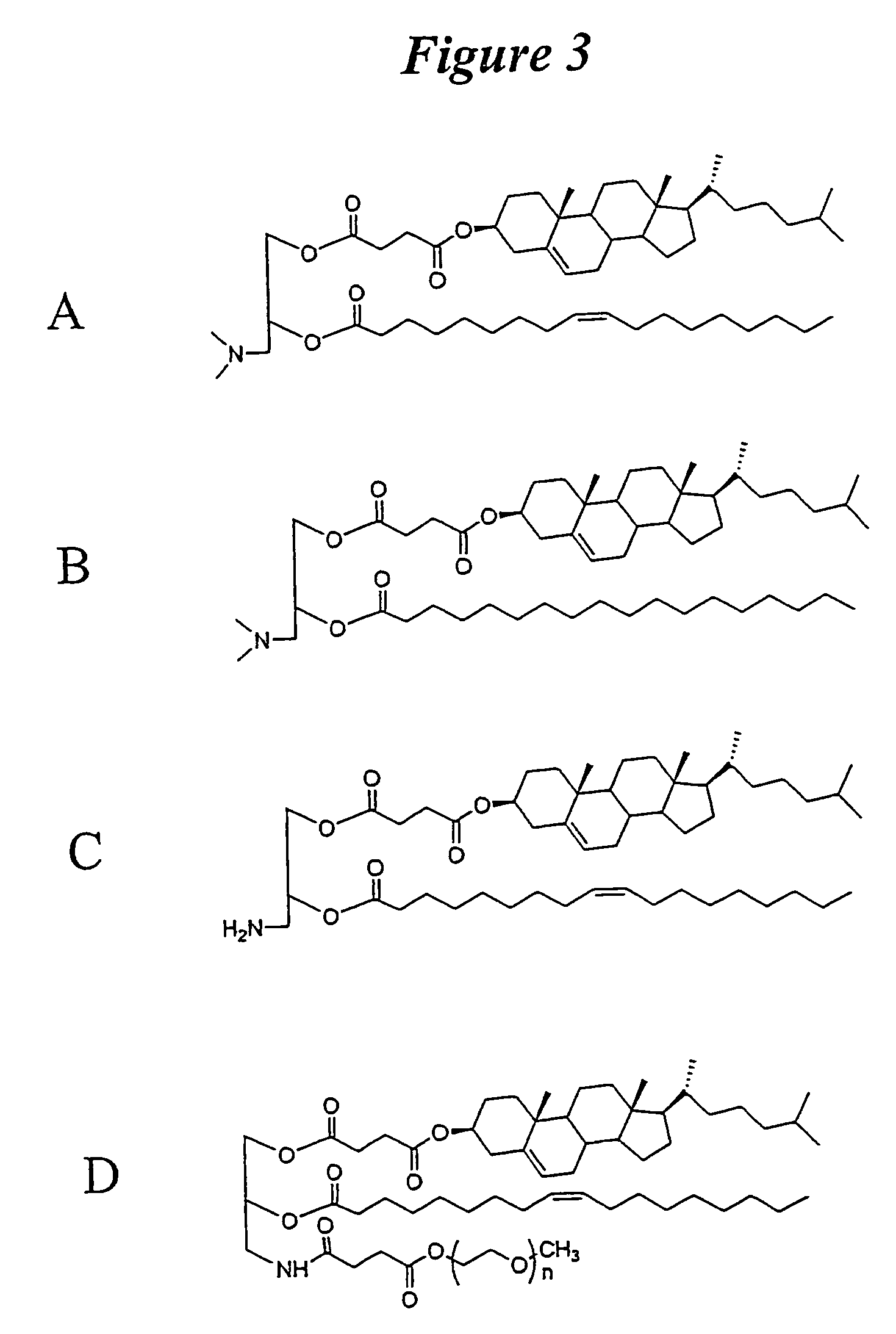
The present invention relates to novel cationic lipids, transfection agents, microparticles, nanoparticles, and short interfering nucleic acid (siNA) molecules. Specifically, the invention relates to novel cationic lipids, microparticles, nanoparticles and transfection agents that effectively transfect or deliver short interfering nucleic acid (siNA). The compositions described herein are generally referred to as formulated molecular compositions (FMC) or lipid nanoparticles (LNP).
Owner:SIRNA THERAPEUTICS INC
Biphenyl-pyrazolecarboxamide compounds
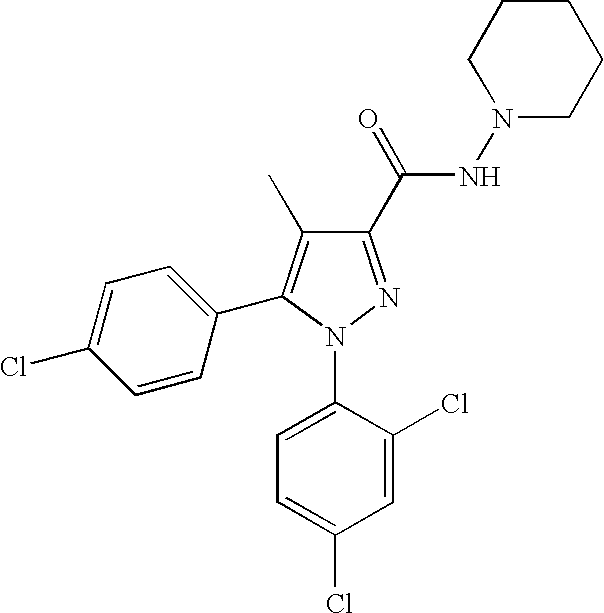
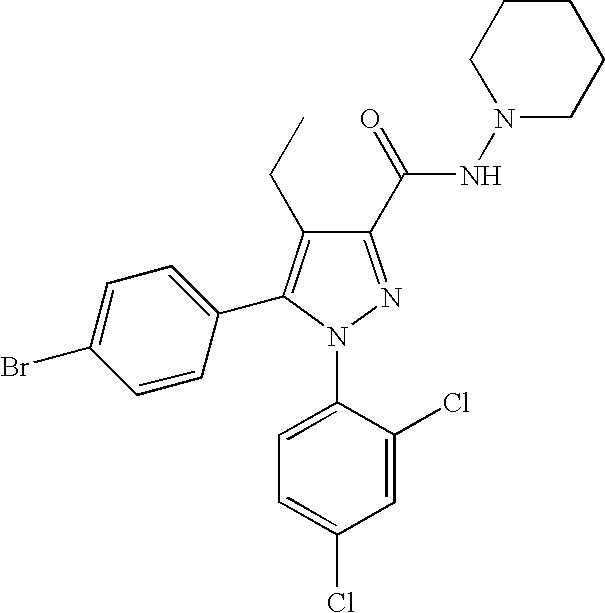
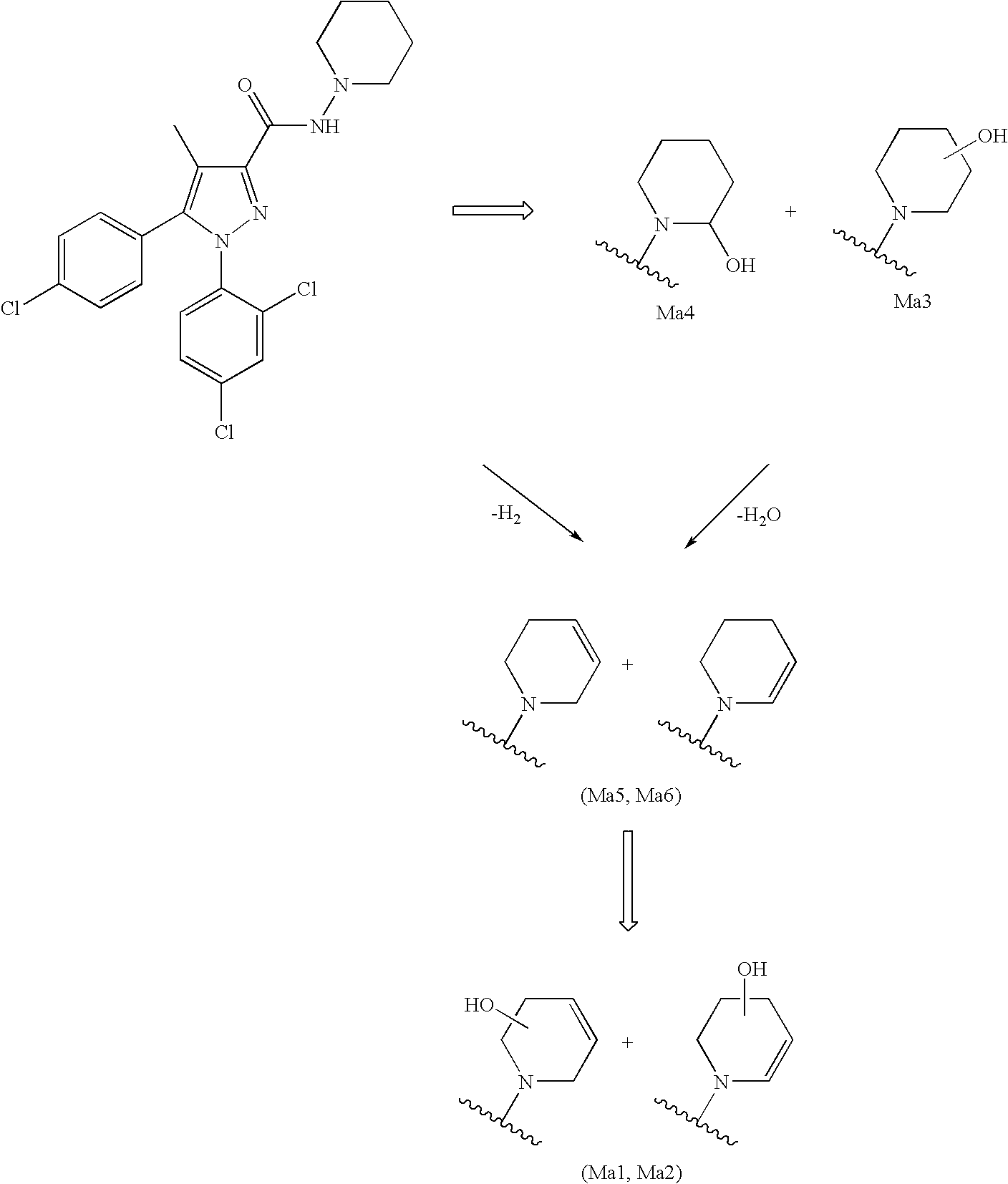
The present invention relates to biphenyl-pyrazole compounds and in particular biphenyl-pyrazolecarboxamides. The invention further provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions beneficially treated by antagonism or inverse agonism of the CB1 receptor, such as obesity, smoking cessation, and normalization of blood lipid composition.
Owner:SUN PHARMA IND INC
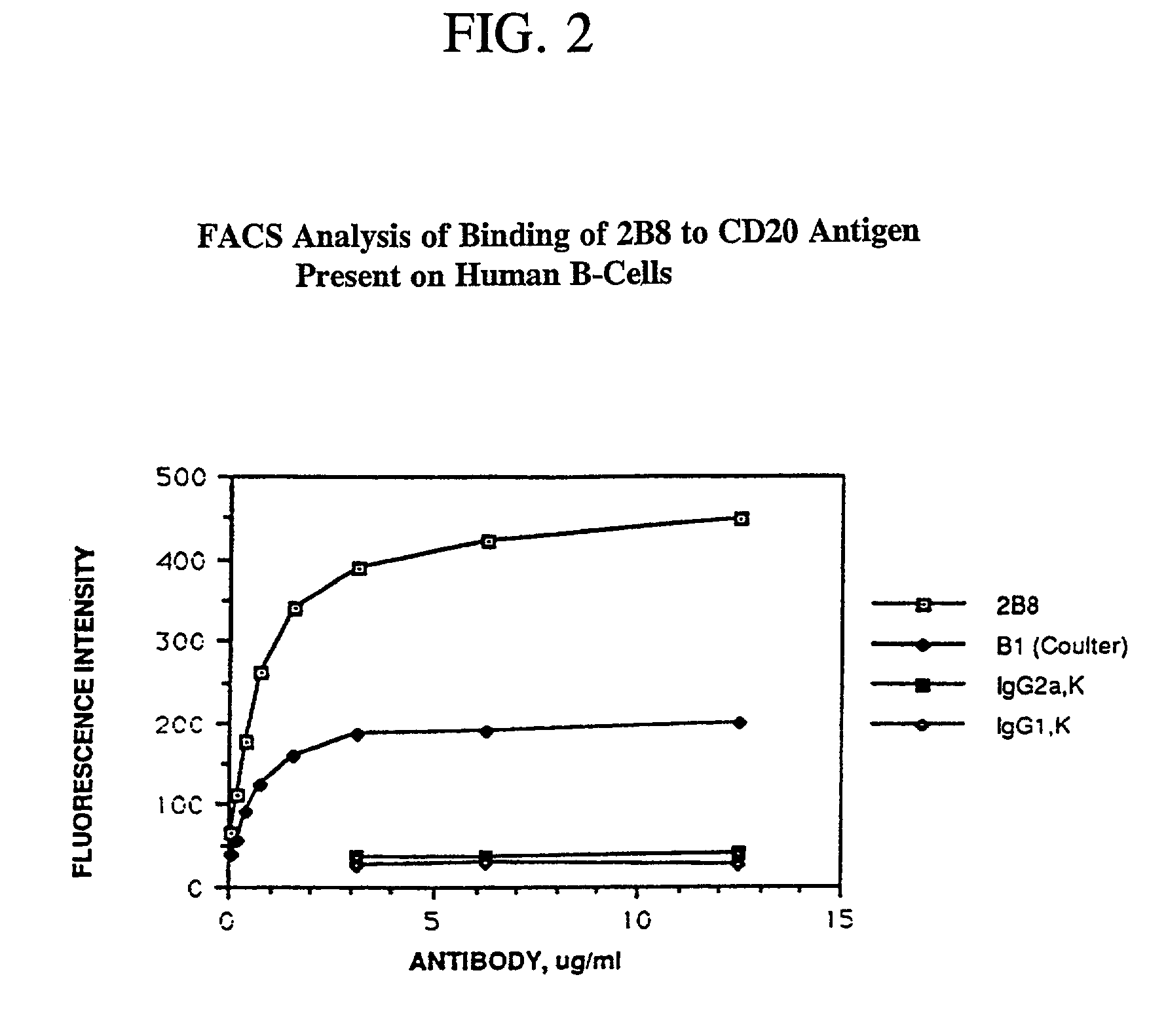
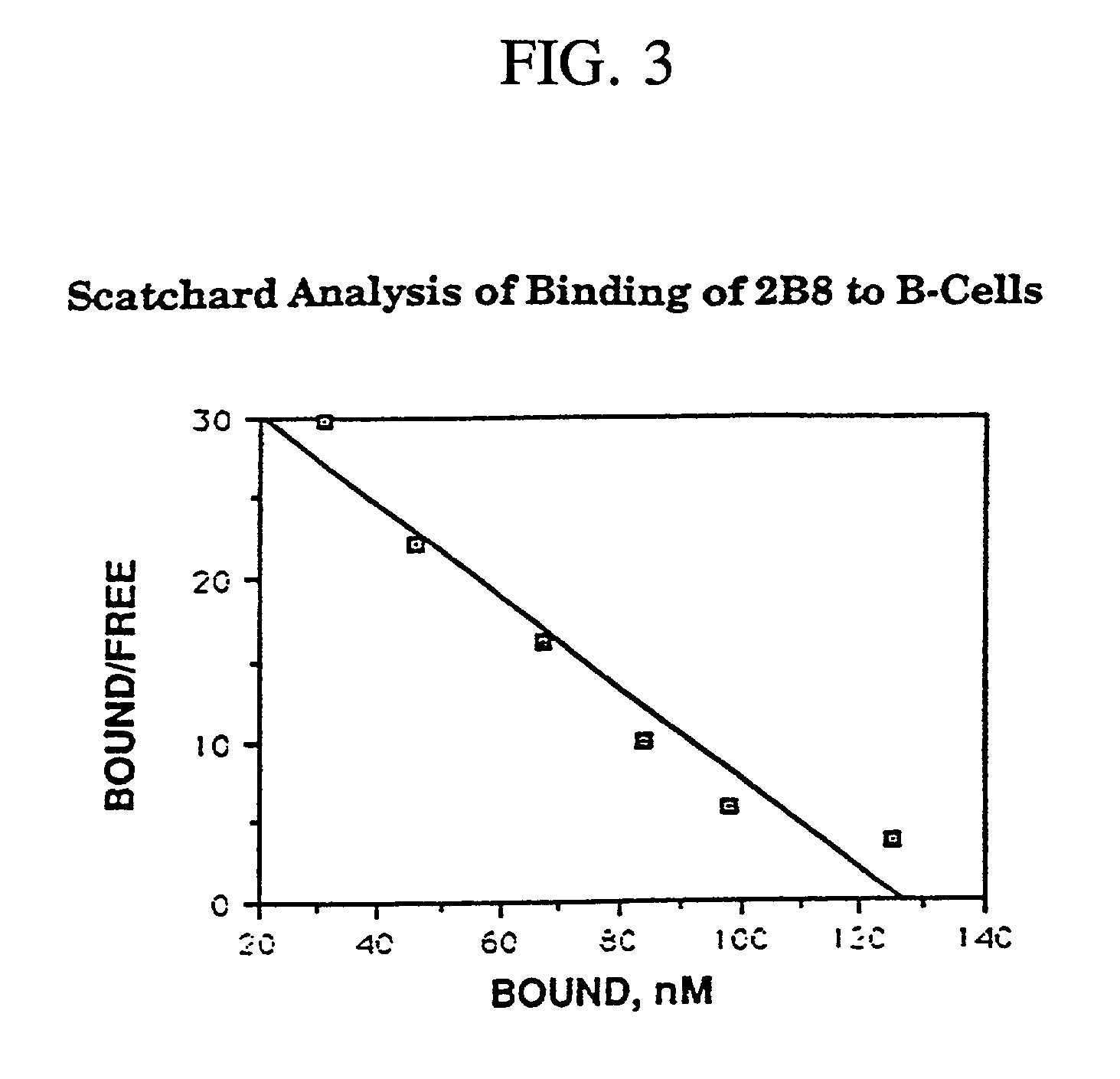
Chimeric antibody with specificity to human B cell surface antigen
A chimeric antibody with human constant region and murine variable region, having specificity to a 35 kDA polypeptide (Bp35(CD20)) expressed on the surface of human B cells, methods of production, and uses.
Owner:ROYALTY PHARMA FINANCE TRUST
Medical device with low magnetic susceptibility
InactiveUS20050079132A1Batteries circuit arrangementsElectrotherapyMagnetic susceptibilityCoherence length
An assembly with a substrate, nanomagnetic material and magetoresistive material. The nanomagnetic material has a saturation magentization of from about 2 to about 3000 electromagnetic units per cubic centimeter; and it contains nanomagnetic particles with an average particle size of less than about 100 nanometers. The average coherence length between adjacent nanomagnetic particles is less than 100 nanometers.
Owner:BIOPHAN TECH
Bioconversion of a fermentable carbon source to 1,3-propanediol by a single microorganism
A new polypropylene terephthalate composition is provided. The polypropylene terephthalate is comprised of 1,3-propanediol and terephthalate. The 1,3-propanediol is produced by the bioconversion of a fermentatble carbon source, preferable glucose. The resulting polypropylene terephthalate is distinguished from petrochemically produced polymer on the basis of dual carbon-isotopic fingerprinting which indicates both the source and the age of the carbon.
Owner:EI DU PONT DE NEMOURS & CO +1
Multivalent immunoglobulin-based bioactive assemblies
ActiveUS20070140966A1Prevent further clot formationHigh affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiagnostic agentAutoimmune condition
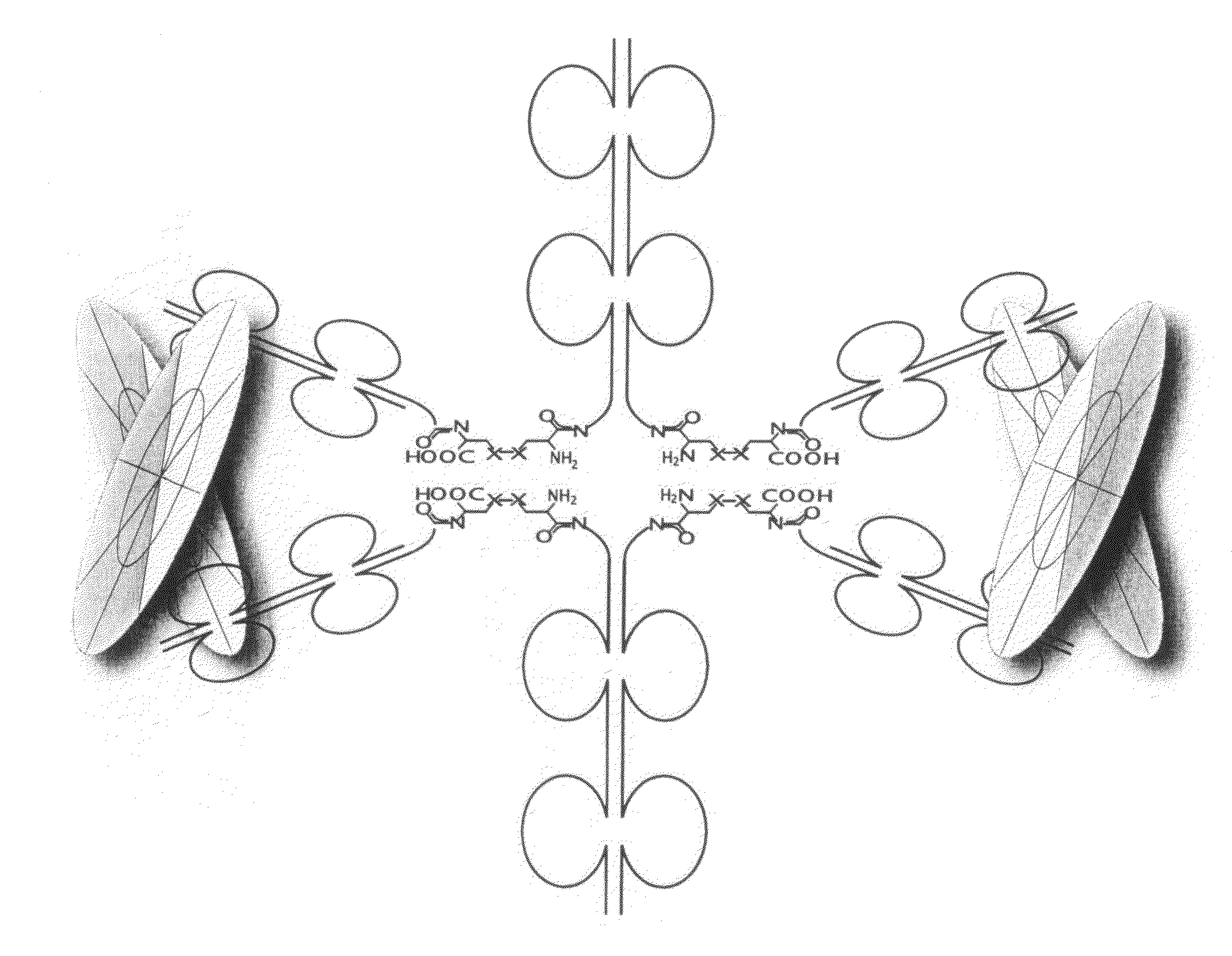
The present invention concerns methods and compositions for stably tethered structures of defined compositions, which may have multiple functionalities and / or binding specificities. Preferred embodiments concern hexameric stably tethered structures comprising one or more IgG antibody fragments and which may be monospecific or bispecific. The disclosed methods and compositions provide a facile and general way to obtain stably tethered structures of virtually any functionality and / or binding specificity. The stably tethered structures may be administered to subjects for diagnostic and / or therapeutic use, for example for treatment of cancer or autoimmune disease. The stably tethered structures may bind to and / or be conjugated to a variety of known effectors, such as drugs, enzymes, radionuclides, therapeutic agents and / or diagnostic agents.
Owner:IBC PHARMACEUTICALS INC
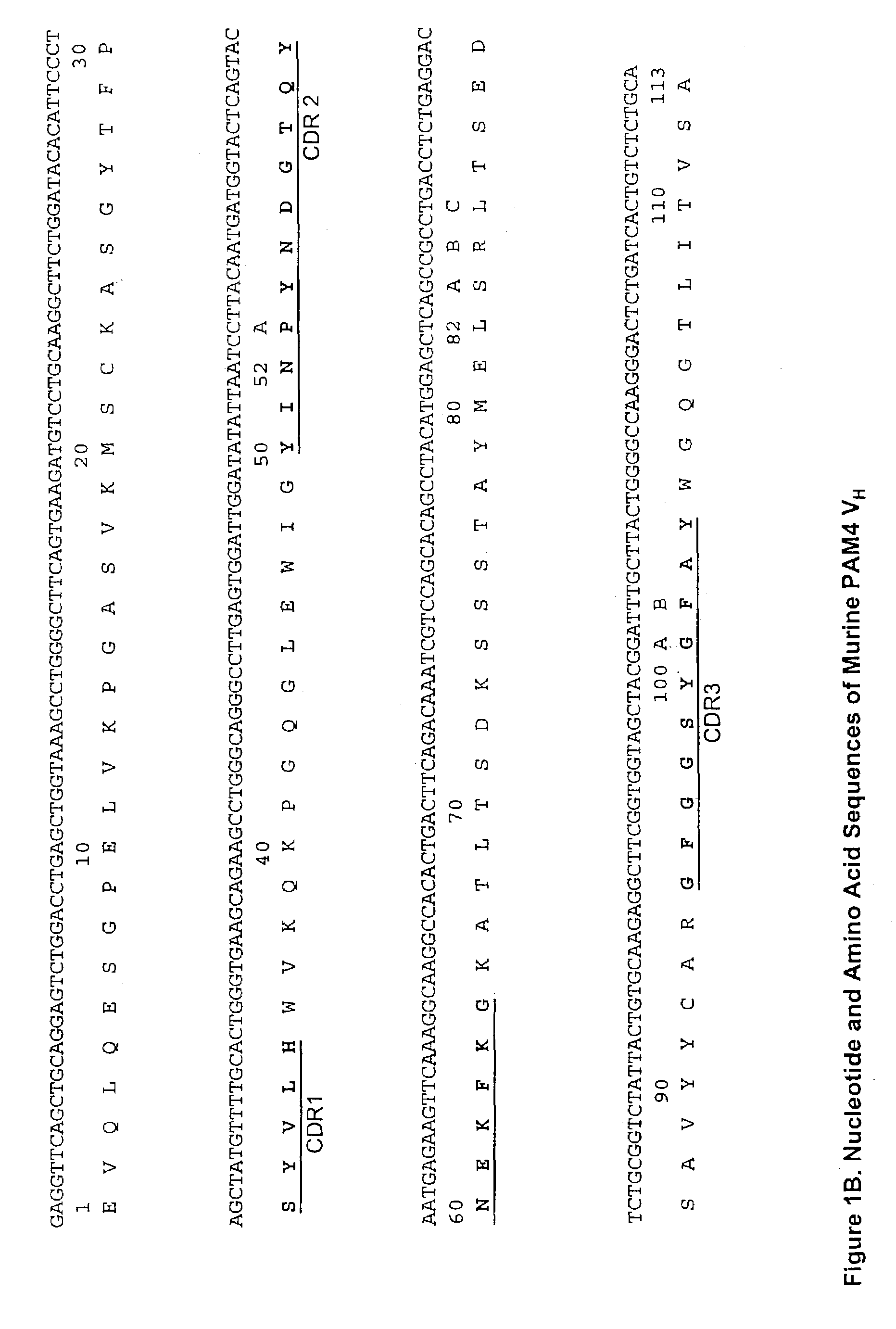
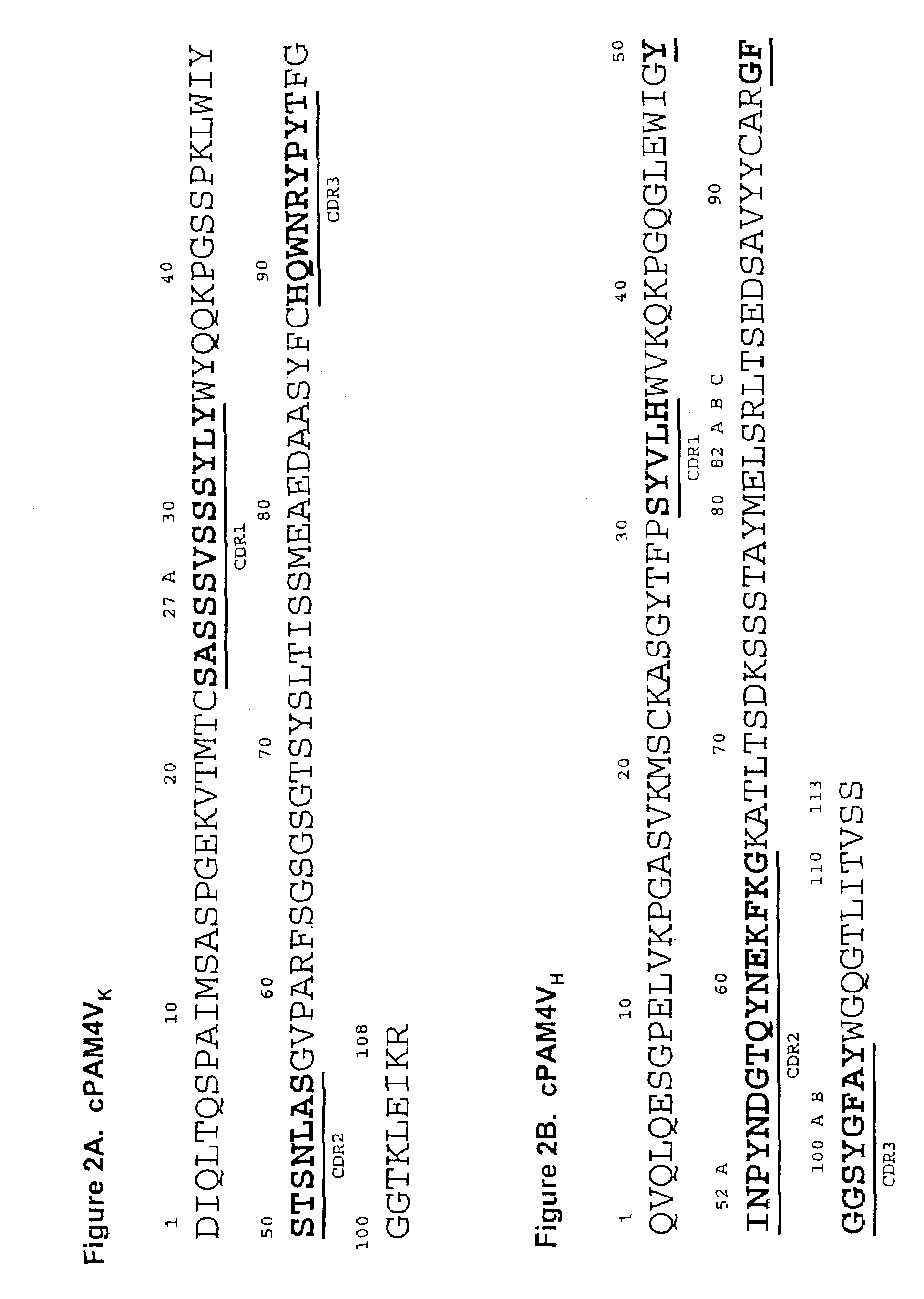
Monoclonal antibody hPAM4
This invention relates to monovalent and multivalent, monospecific antibodies and to multivalent, multispecific antibodies. One embodiment of these antibodies has one or more identical binding sites where each binding site binds with a target antigen or an epitope on a target antigen. Another embodiment of these antibodies has two or more binding sites where these binding sites have affinity towards different epitopes on a target antigen or different target antigens, or have affinity towards a target antigen and a hapten. The present invention further relates to recombinant vectors useful for the expression of these functional antibodies in a host. More specifically, the present invention relates to the tumor-associated antibody designated PAM4. The invention further relates to humanized and human PAM4 antibodies, and the use of such antibodies in diagnosis and therapy.
Owner:IMMUNOMEDICS INC
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20020012665A1Avoiding and decreasing and resistanceOrganic active ingredientsIn-vivo radioactive preparationsFactor iiBiological activation
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN(R).
Owner:BIOGEN INC
Production and use of novel peptide-based agents for use with bi-specific antibodies
The present invention relates to a bi-specific antibody or antibody fragment having at least one arm that is reactive against a targeted tissue and at least one other arm that is reactive against a linker moiety. The linker moiety encompasses a hapten to which antibodies have been prepared. The antigenic linker is conjugated to one or more therapeutic or diagnostic agents or enzymes. The invention provides constructs and methods for producing the bispecific antibodies or antibody fragments, as well as methods for using them.
Owner:IMMUNOMEDICS INC
Antibody to the human OX40 receptor
InactiveUS7550140B2Antibacterial agentsOrganic active ingredientsReceptor for activated C kinase 1Nucleic acid
The present invention provides binding molecules, such as human binding molecules, that bind to and stimulate the human OX40-receptor. The invention also provides nucleic acids encoding such binding molecules. Methods for producing such binding molecules are also provided by the present invention. The binding molecules and nucleic acids are useful in the stimulation of human T-cells and can be used to enhance antigen-specific immune responses.
Owner:JANSSEN VACCINES & PREVENTION BV
Detection of antigens via oligonucleotide antibody conjugates
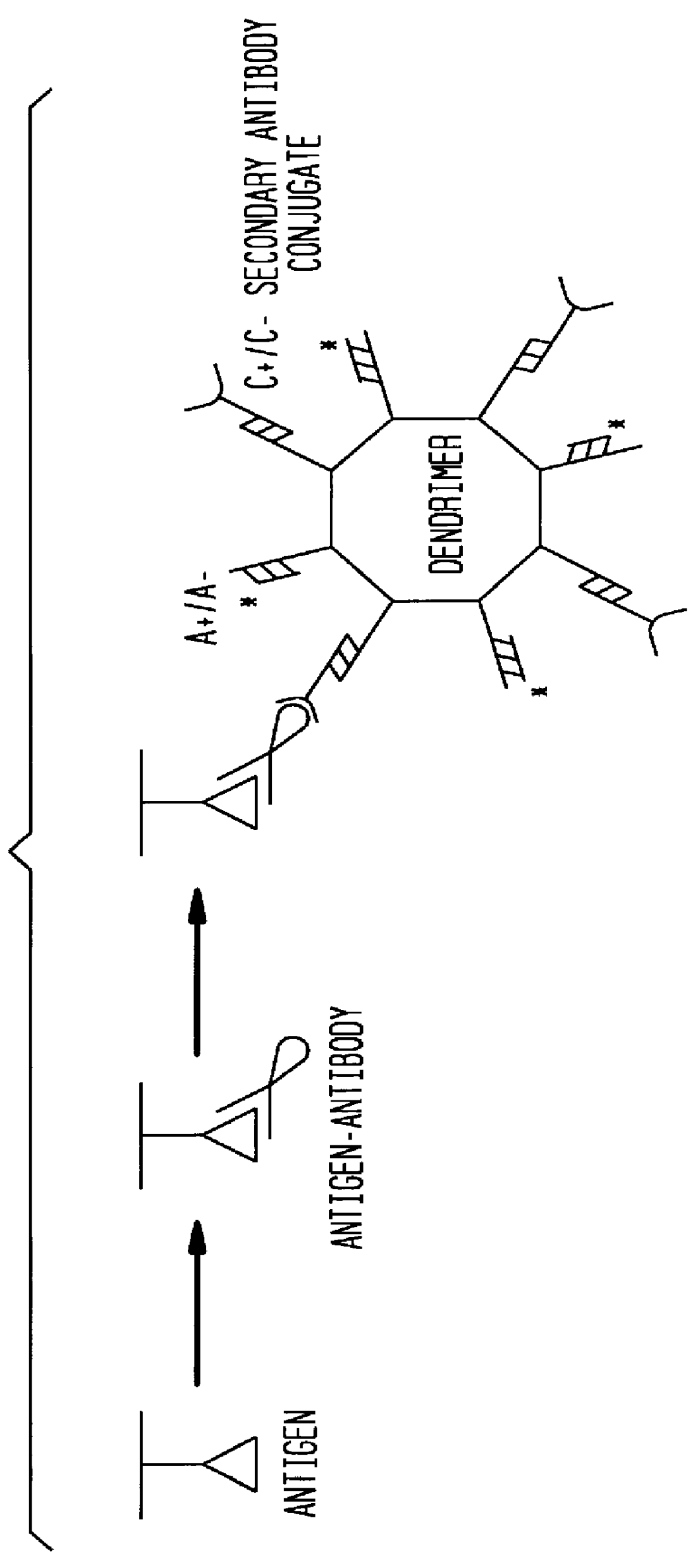
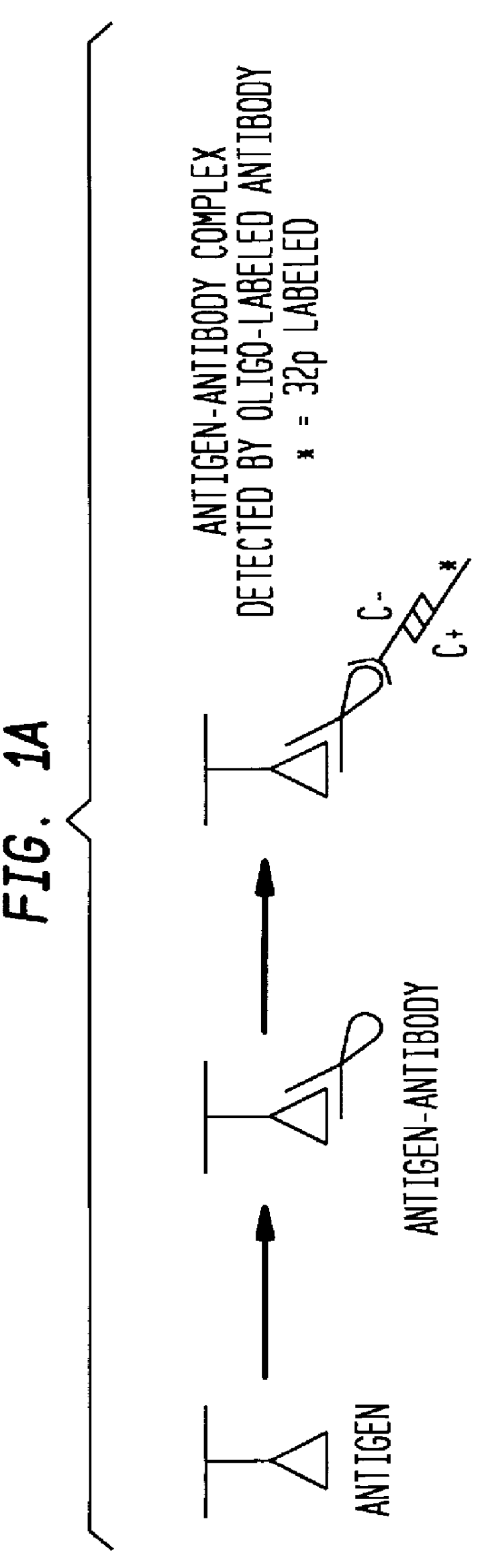
InactiveUS6117631AHigh detection sensitivityEnhancing observed signalIn-vivo radioactive preparationsSugar derivativesDendrimerAntibody conjugate
The present invention provides a method of detecting antigens, which comprises immobilizing an antigen to a solid support and contacting the solid support with a means for hybridizing a labeled dendrimer to the antibody, through an oligonucleotide complexed thereto. A directly oligonucleotide labeled primary antibody or an oligonucleotide labeled secondary antibody may be employed, and a conventionally labeled dendrimer can subsequently be hybridized to the oligonucleotide through one or more of the outer arms of the dendrimer. The present invention offers the advantage over conventional methods of antigen detection by providing multiple label molecules per antigen, thereby enhancing the observed signal associated with the label.
Owner:GENISPHERE LLC
Methods and compositions for generating bioactive assemblies of increased complexity and uses
The present invention concerns methods and compositions for making and using bioactive assemblies of defined compositions, which may have multiple functionalities and / or binding specificities. In particular embodiments, the bioactive assembly is formed using dock-and-lock (DNL) methodology, which takes advantage of the specific binding interaction between dimerization and docking domains (DDD) and anchoring domains (AD) to form the assembly. In various embodiments, one or more effectors may be attached to a DDD or AD sequence. Complementary AD or DDD sequences may be attached to an adaptor module that forms the core of the bioactive assembly, allowing formation of the assembly through the specific DDD / AD binding interactions. Such assemblies may be attached to a wide variety of effector moieties for treatment, detection and / or diagnosis of a disease, pathogen infection or other medical or veterinary condition.
Owner:IBC PHARMACEUTICALS INC
Hybrid immunoglobulins with moving parts
Hybrid immunoglobulins containing moving parts are provided as well as related compositions and methods of use and methods of production. In addition, analogous genetic devices are provided as well as related compositions and methods of use and methods of production.
Owner:BIOMOLECULAR HLDG LLC
Stably tethered structures of defined compositions with multiple functions or binding specificities
ActiveUS20060228300A1Reduce exposureReduce deliveryAntibacterial agentsSenses disorderAntibody fragmentsBinding peptide
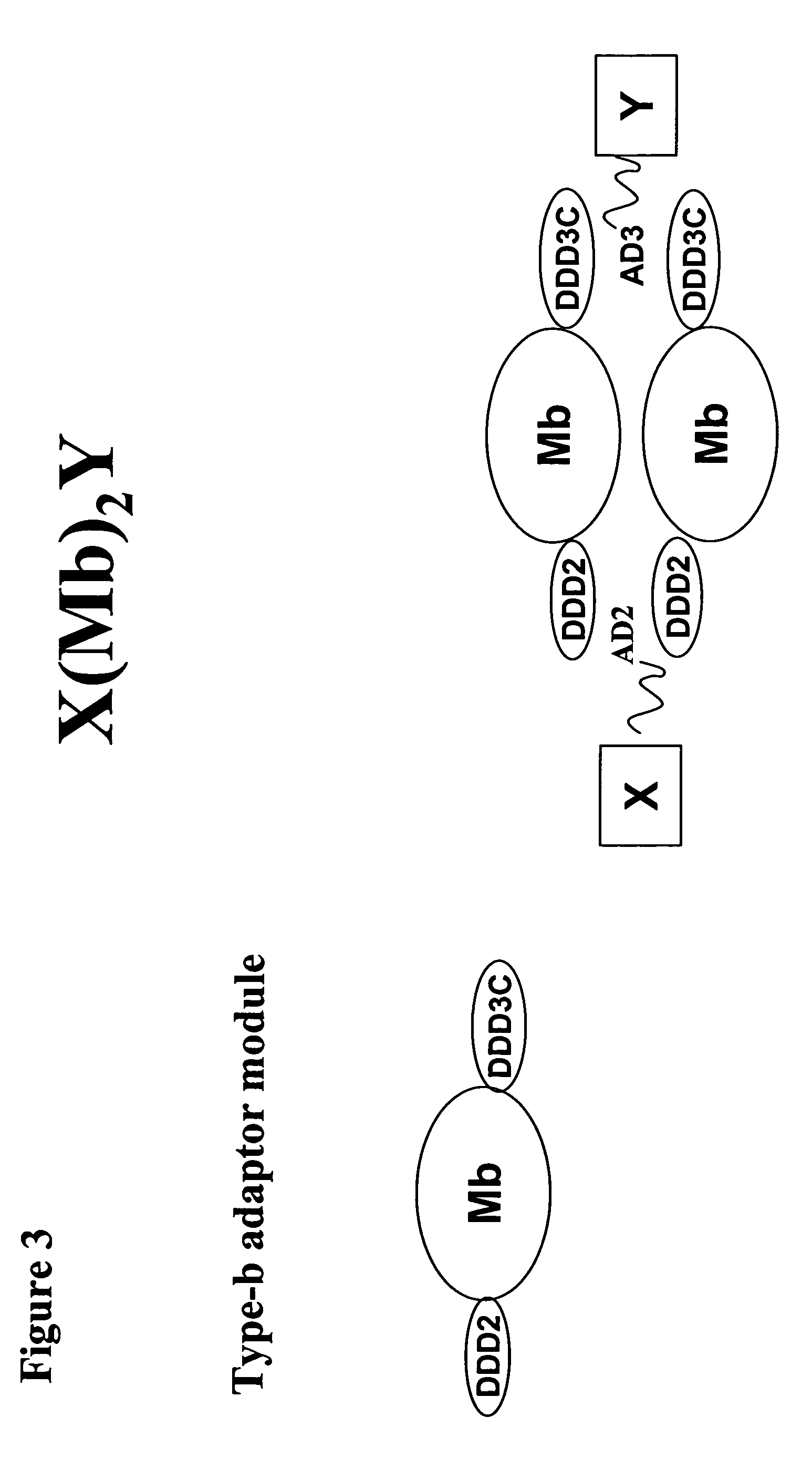
The present invention concerns methods and compositions for stably tethered structures of defined compositions with multiple functionalities and / or binding specificities. Particular embodiments concern stably tethered structures comprising a homodimer of a first monomer, comprising a dimerization and docking domain attached to a first precursor, and a second monomer comprising an anchoring domain attached to a second precursor. The first and second precursors may be virtually any molecule or structure, such as antibodies, antibody fragments, antibody analogs or mimetics, aptamers, binding peptides, fragments of binding proteins, known ligands for proteins or other molecules, enzymes, detectable labels or tags, therapeutic agents, toxins, pharmaceuticals, cytokines, interleukins, interferons, radioisotopes, proteins, peptides, peptide mimetics, polynucleotides, RNAi, oligosaccharides, natural or synthetic polymeric substances, nanoparticles, quantum dots, organic or inorganic compounds, etc. The disclosed methods and compositions provide a simple, easy to purify way to obtain any binary compound attached to any monomeric compound, or any trinary compound.
Owner:IBC PHARMACEUTICALS INC
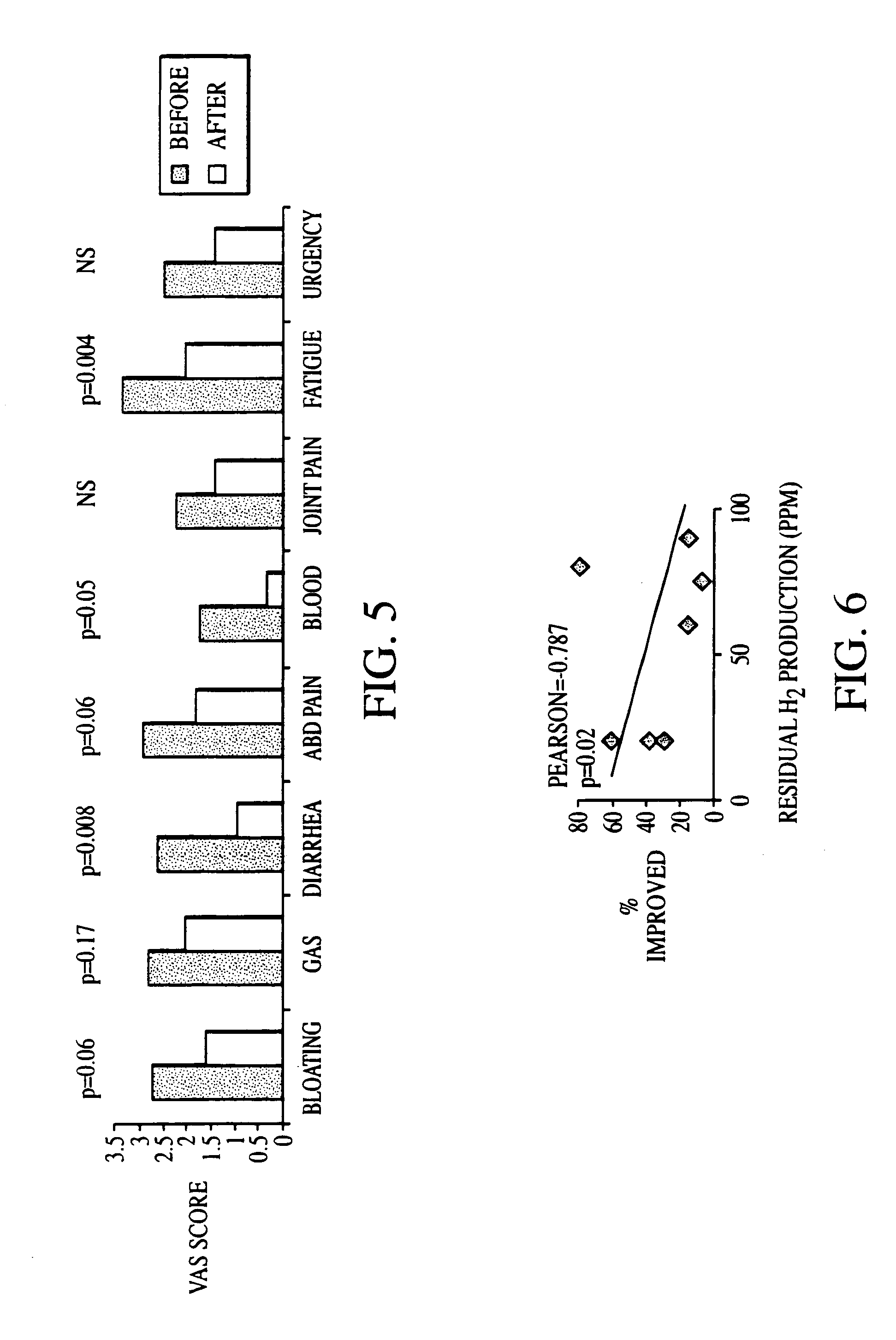
Methods of diagnosing and treating small intestinal bacterial overgrowth (SIBO) and SIBO-related conditions
InactiveUS7048906B2Prevent further growthReduced magnitudeAntibacterial agentsCompounds screening/testingImmunologic disordersPhysiology
Disclosed is a method of treating small intestinal bacterial overgrowth (SIBO) or a SIBO-caused condition in a human subject. SIBO-caused conditions include irritable bowel syndrome, fibromyalgia, chronic pelvic pain syndrome, chronic fatigue syndrome, depression, impaired mentation, impaired memory, halitosis, tinnitus, sugar craving, autism, attention deficit / hyperactivity disorder, drug sensitivity, an autoimmune disease, and Crohn's disease. Also disclosed are a method of screening for the abnormally likely presence of SIBO in a human subject and a method of detecting SIBO in a human subject. A method of determining the relative severity of SIBO or a SIBO-caused condition in a human subject, in whom small intestinal bacterial overgrowth (SIBO) has been detected, is also disclosed.
Owner:CEDARS SINAI MEDICAL CENT
Radiolabeling kit and binding assay
InactiveUS20020102208A1No reduction in immunoreactivityNegligible lossIn-vivo radioactive preparationsDepsipeptidesTherapeutic antibodyAssay
Antibody binding assays and radiolabeling kits are disclosed for radiolabeling and testing therapeutic antibodies in the commercial setting. In particular, the kits are designed for making and evaluating radiolabeled anti-CD20 conjugates to be used for the treatment and imaging of B cell lymphoma tumors. All kit reagents are sterile and are designed to achieve a high level of antibody radiolabeling and product stability with results which are highly reproducible.
Owner:BIOGEN INC
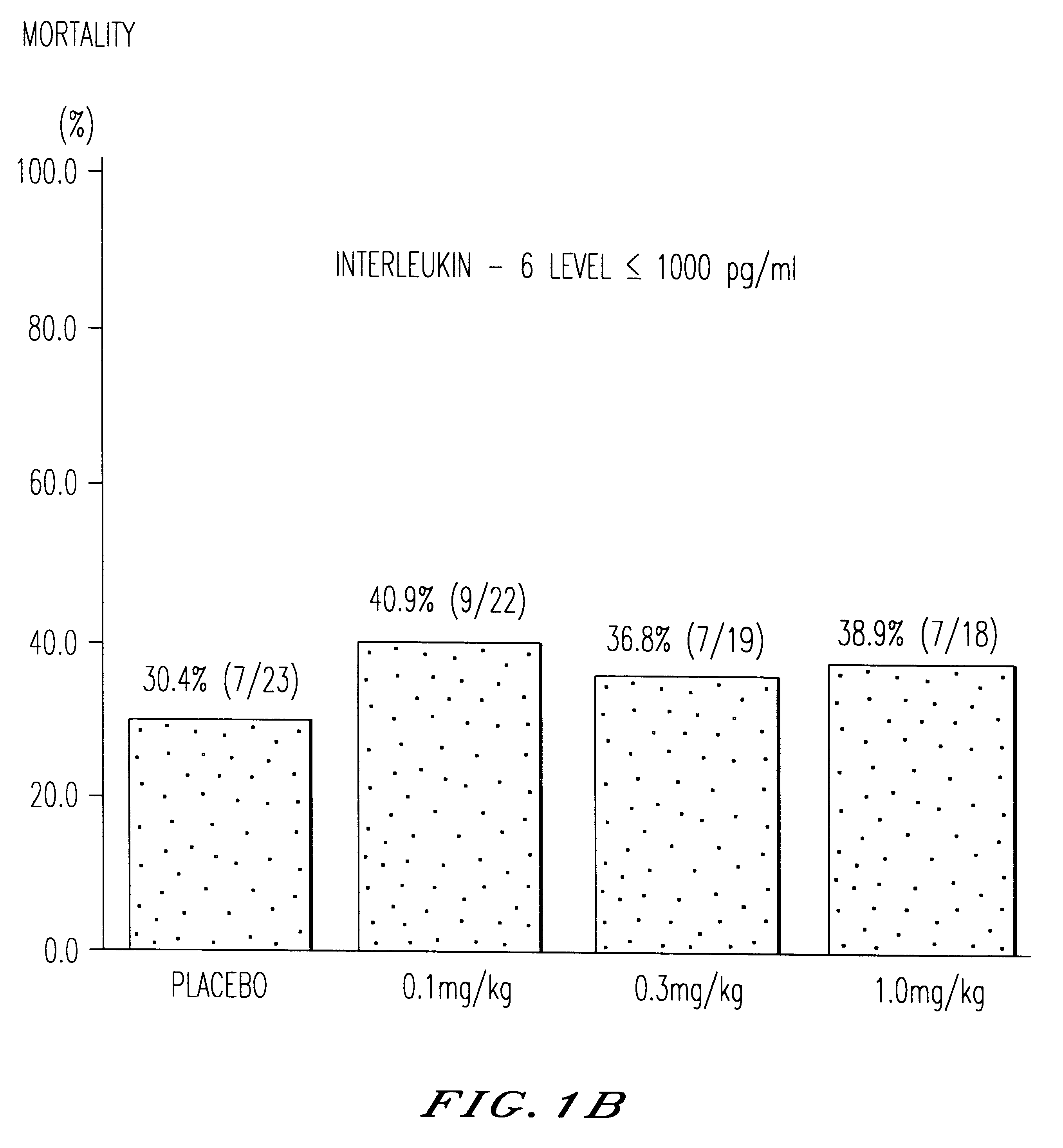
Use of anti-TNF antibodies as drugs for the treatment of disorders with an elevated serum level of interleukin-6
The invention relates to the use of TNF antagonists for producing drugs for the treatment of disorders characterized by elevated serum levels of interleukin-6.
Owner:BASF SE +1
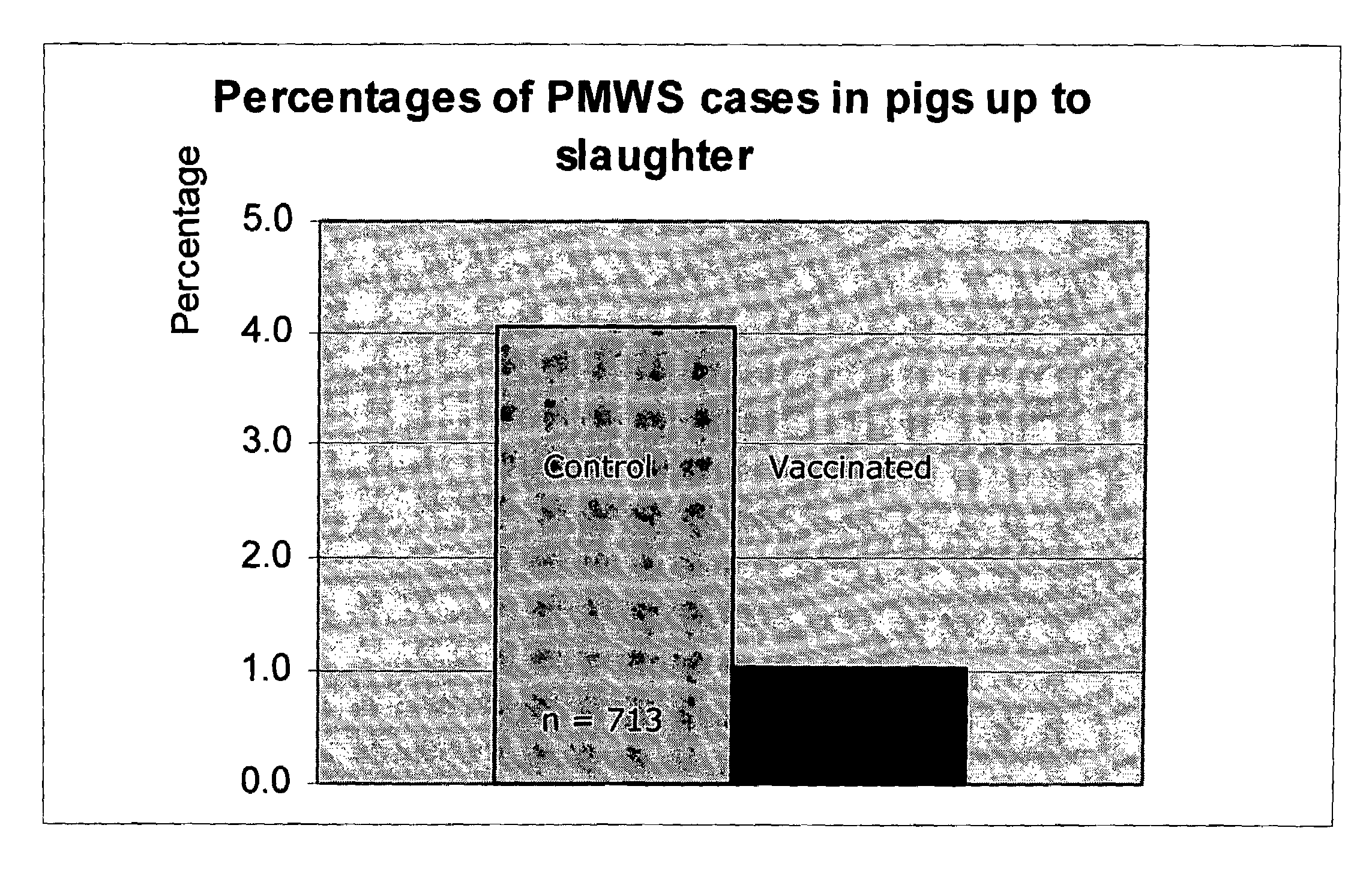
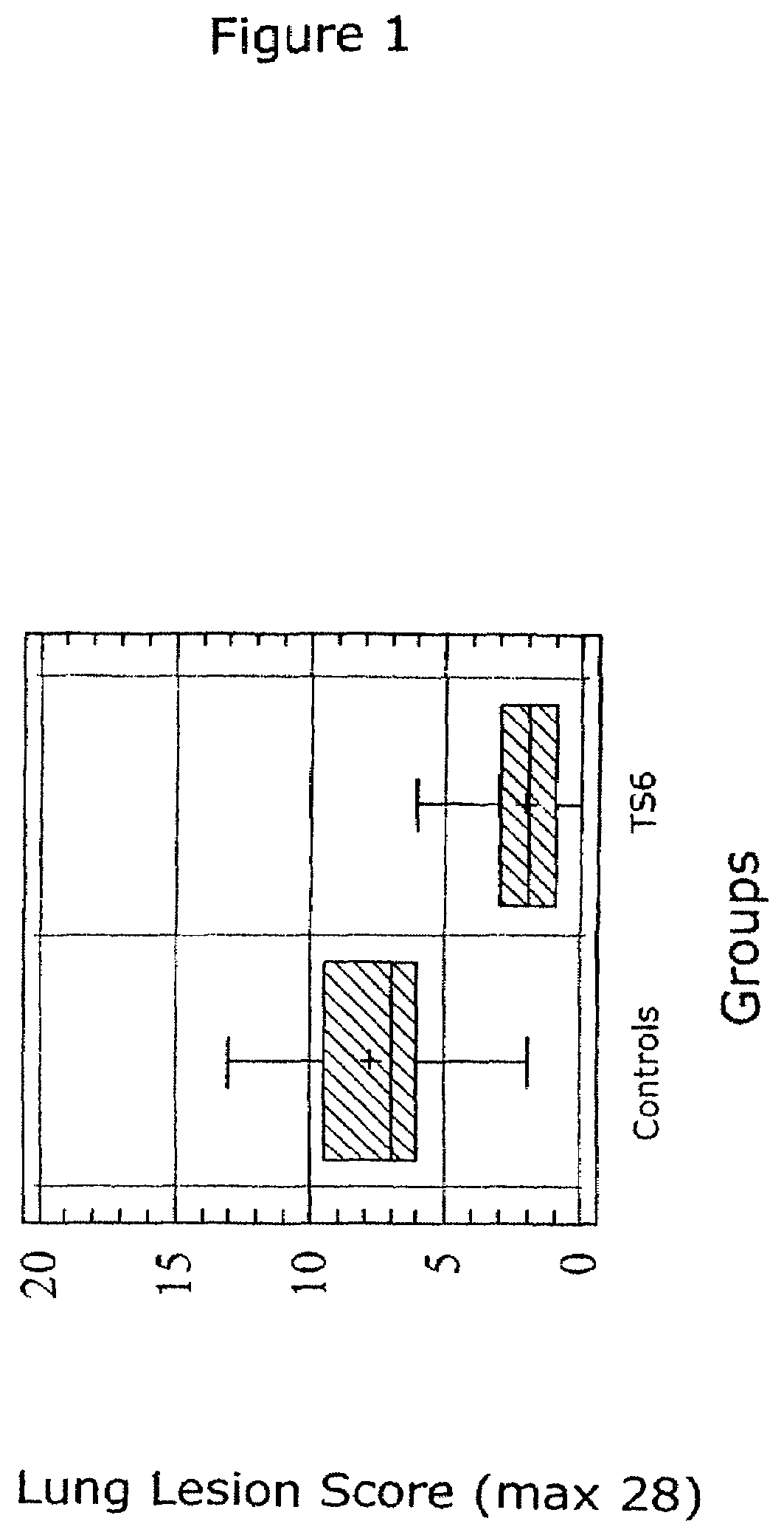
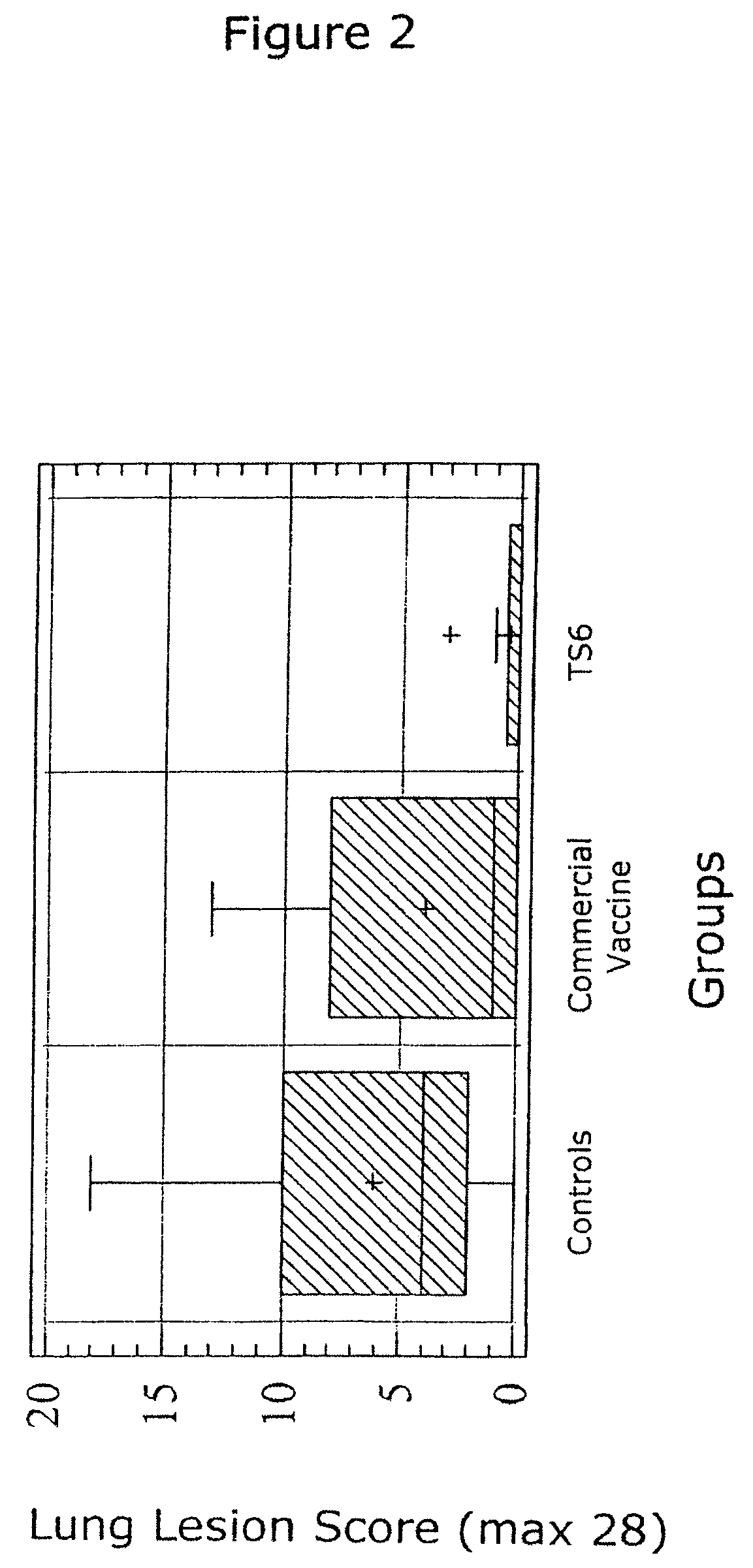
Vaccine formulations
ActiveUS7371395B2Improve stabilityStable and safe and easily administrableSsRNA viruses negative-senseAntibacterial agentsPlasmidBacilli
Owner:MERIAL INC
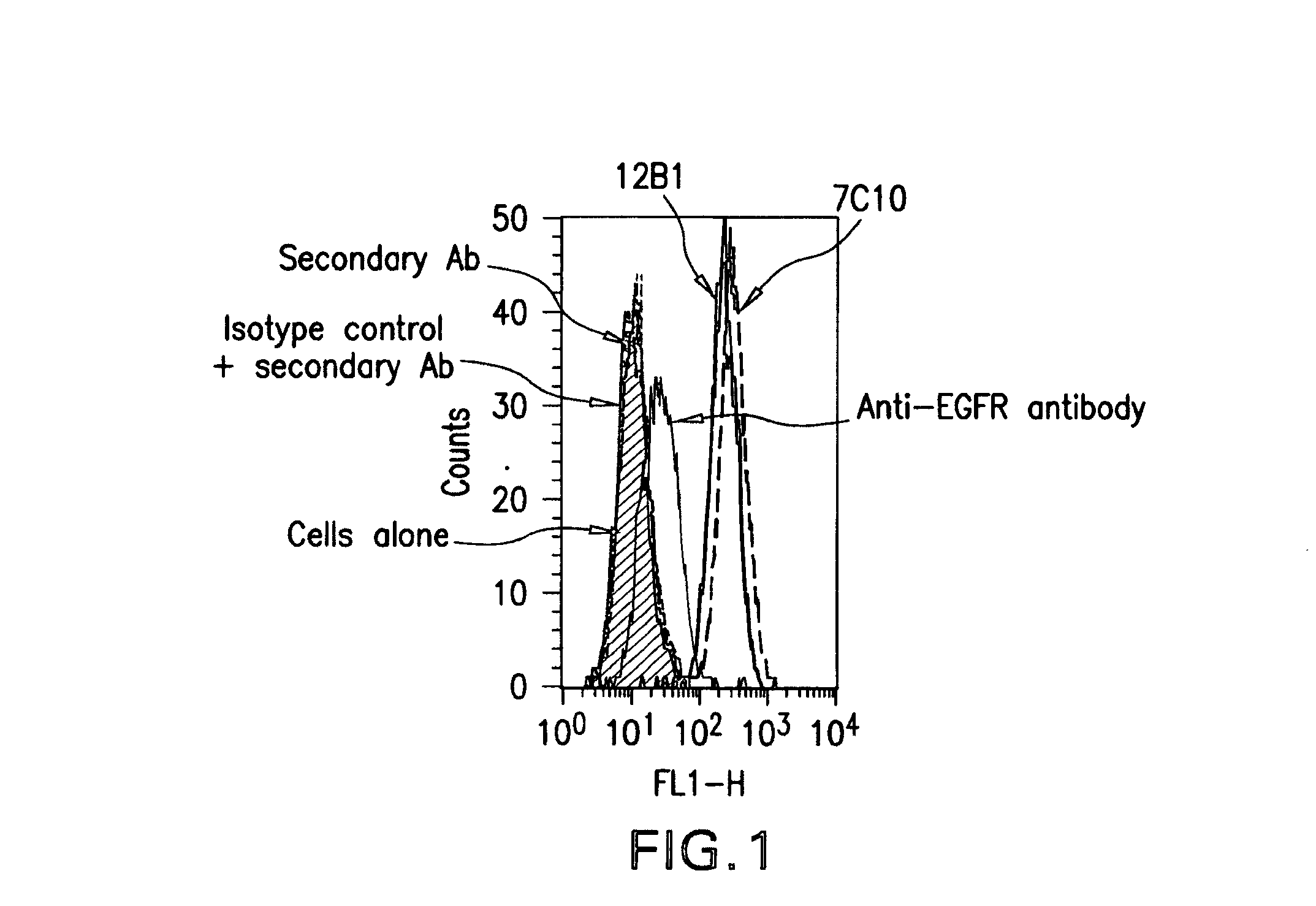
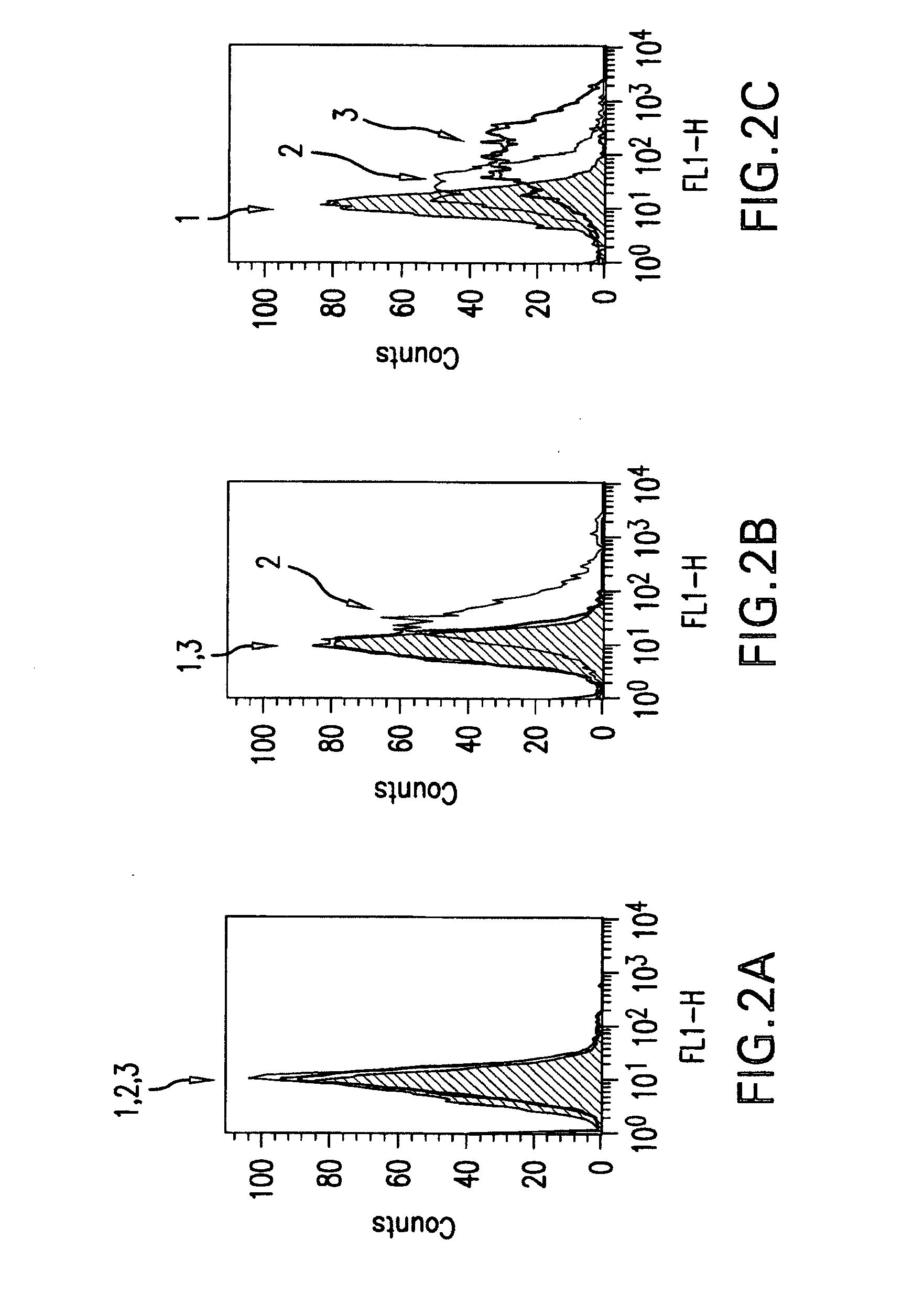
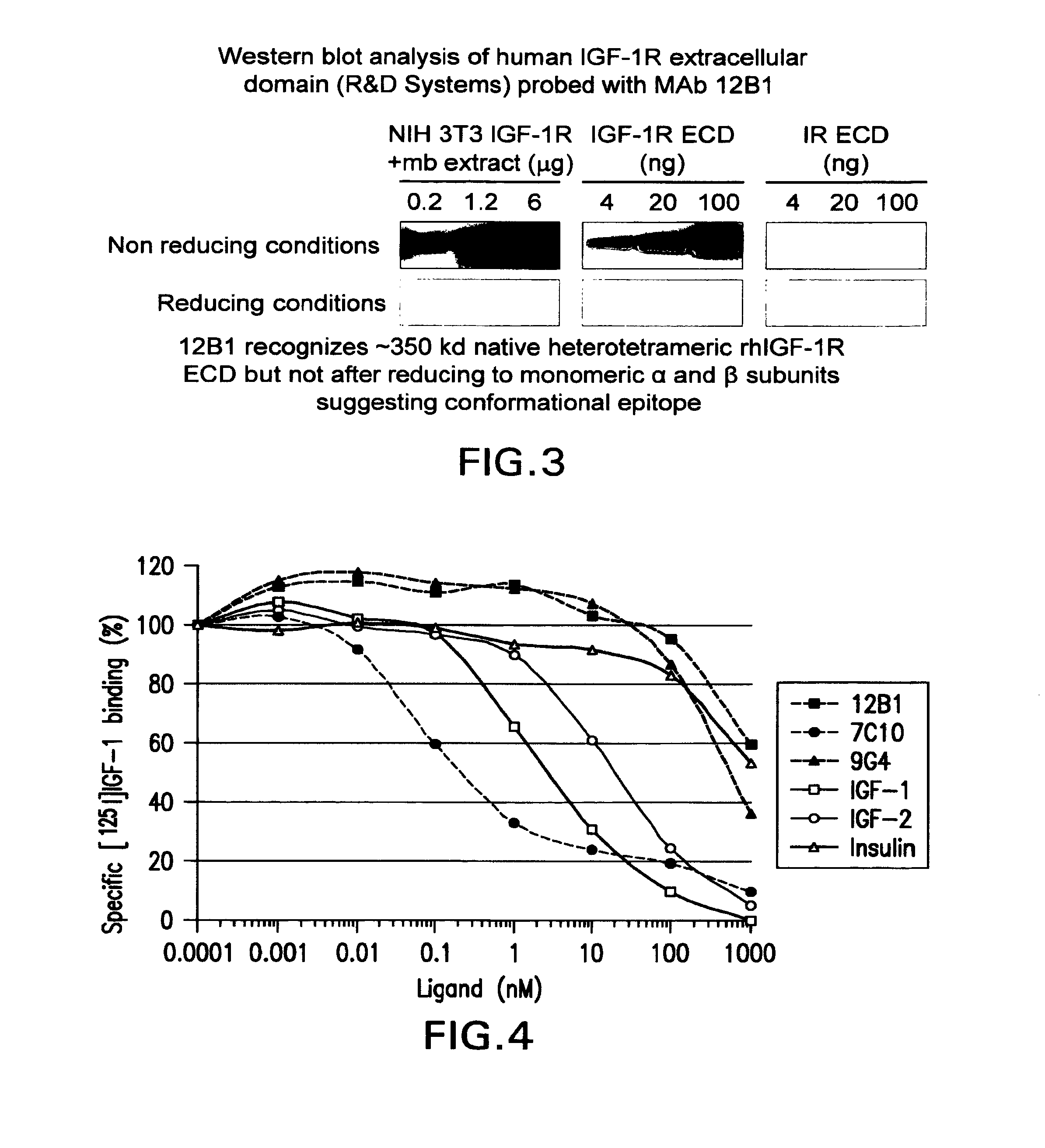
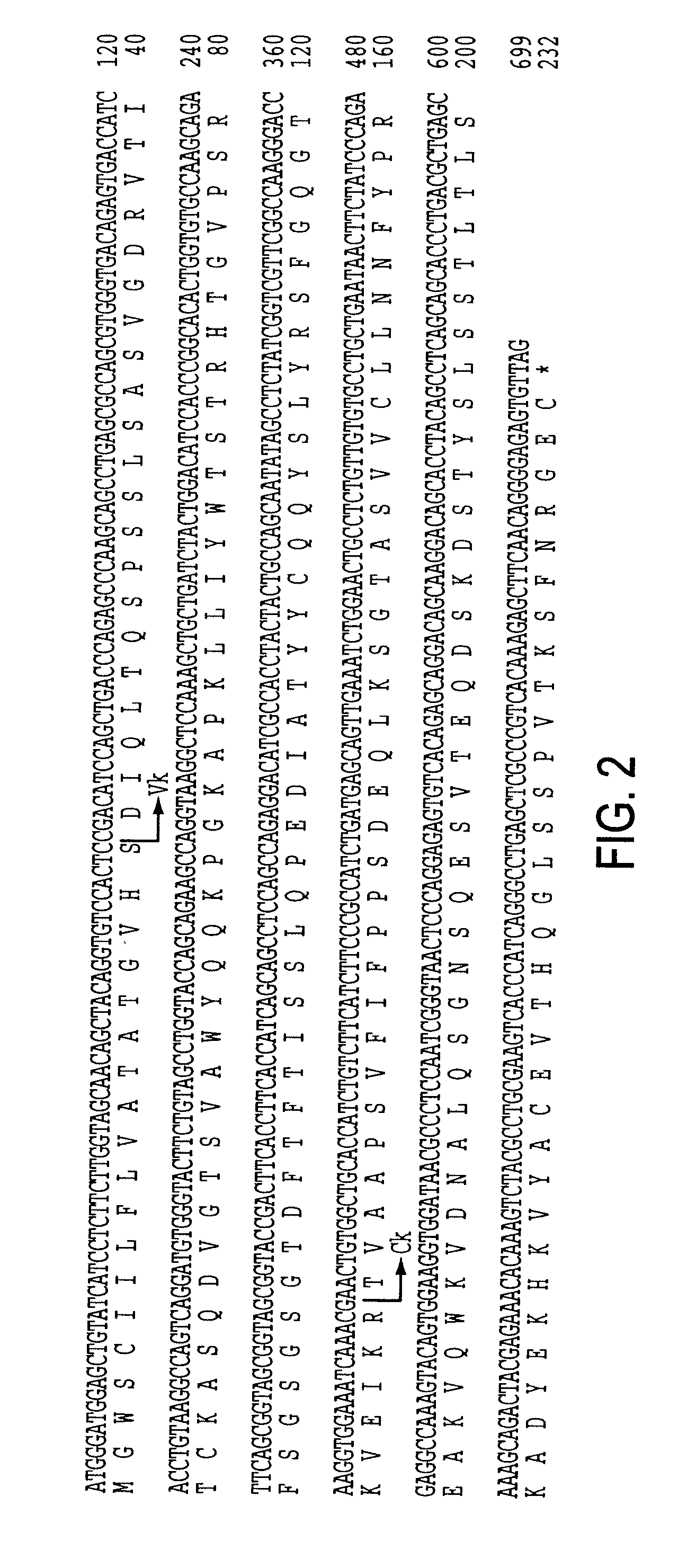
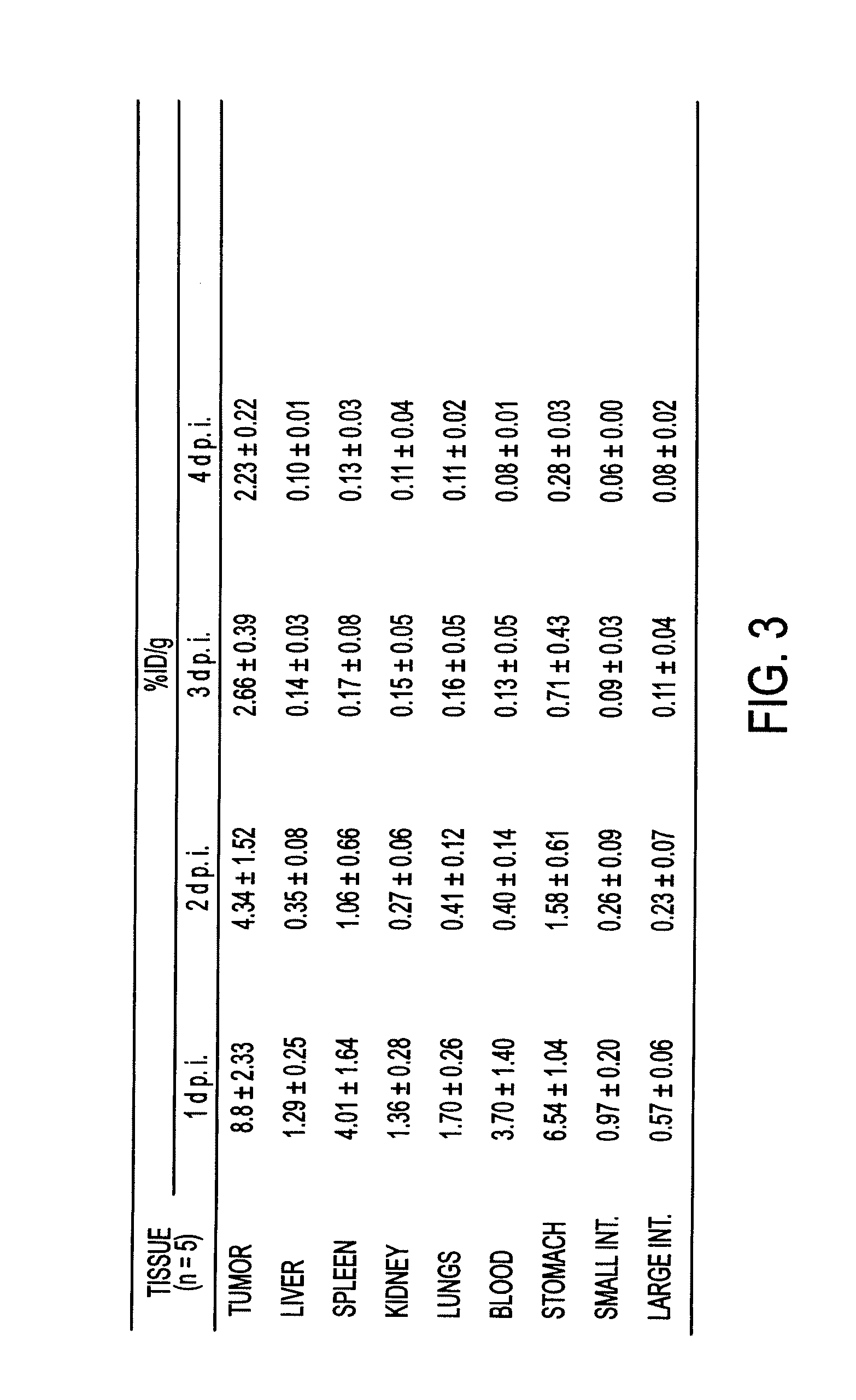
Igf-1r specific antibodies useful in the detection and diagnosis of cellular proliferative disorders
InactiveUS20130084243A1High affinityUseful in detectionAnimal cellsIn-vivo radioactive preparationsDiseaseSingle-Chain Antibodies
The present invention relates to mammalian antibodies, designated 12B1 and antigen-binding portions thereof that specifically bind to insulin-like growth factor I receptor (IGF-IR), preferably human IGF-IR. Also included are chimeric, bispecific, derivatized, single chain antibodies derived from the antibodies disclosed herein. Nucleic acid molecules encoding the mammalian antibodies as well as methods of use thereof are also disclosed. Also included are pharmaceutical compositions comprising these antibodies and methods of using the antibodies and compositions thereof for treatment and diagnosis of pathological hyperproliferative oncogenic disorders associated with expression of IGf-1R.
Owner:GOETSCH LILIANE +4
Bispecific Antibody Point Mutations for Enhancing Rate of Clearance
A mutant bispecific antibody that includes (a) a human hinge constant region from IgG having one or more amino acid mutations in the CH2 domain, (b) two scFvs; and (c) two Fvs has been constructed. This type of antibody displays enhanced clearance, which has been found to be particularly useful in the context of pre-targeting methods.
Owner:IMMUNOMEDICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com