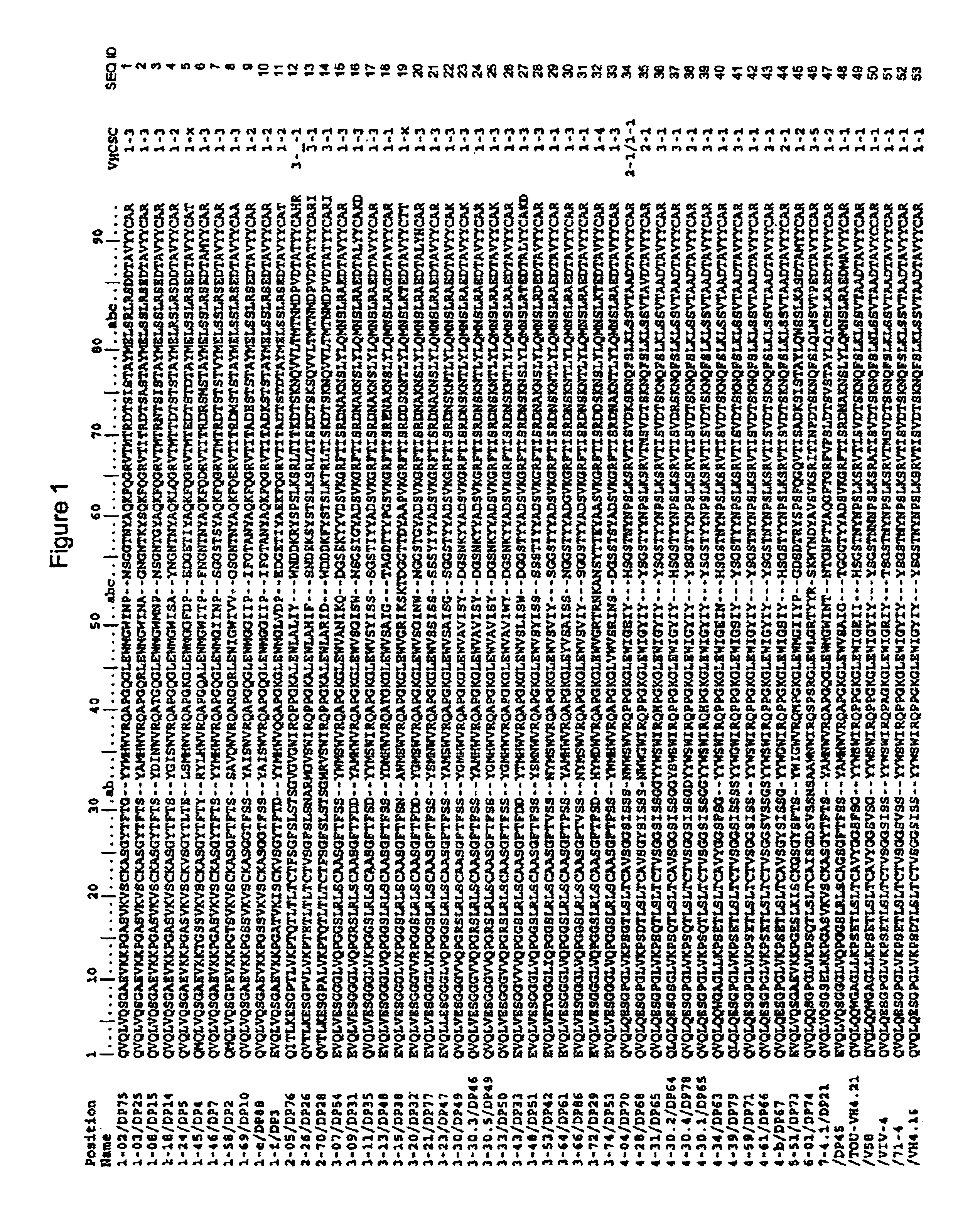
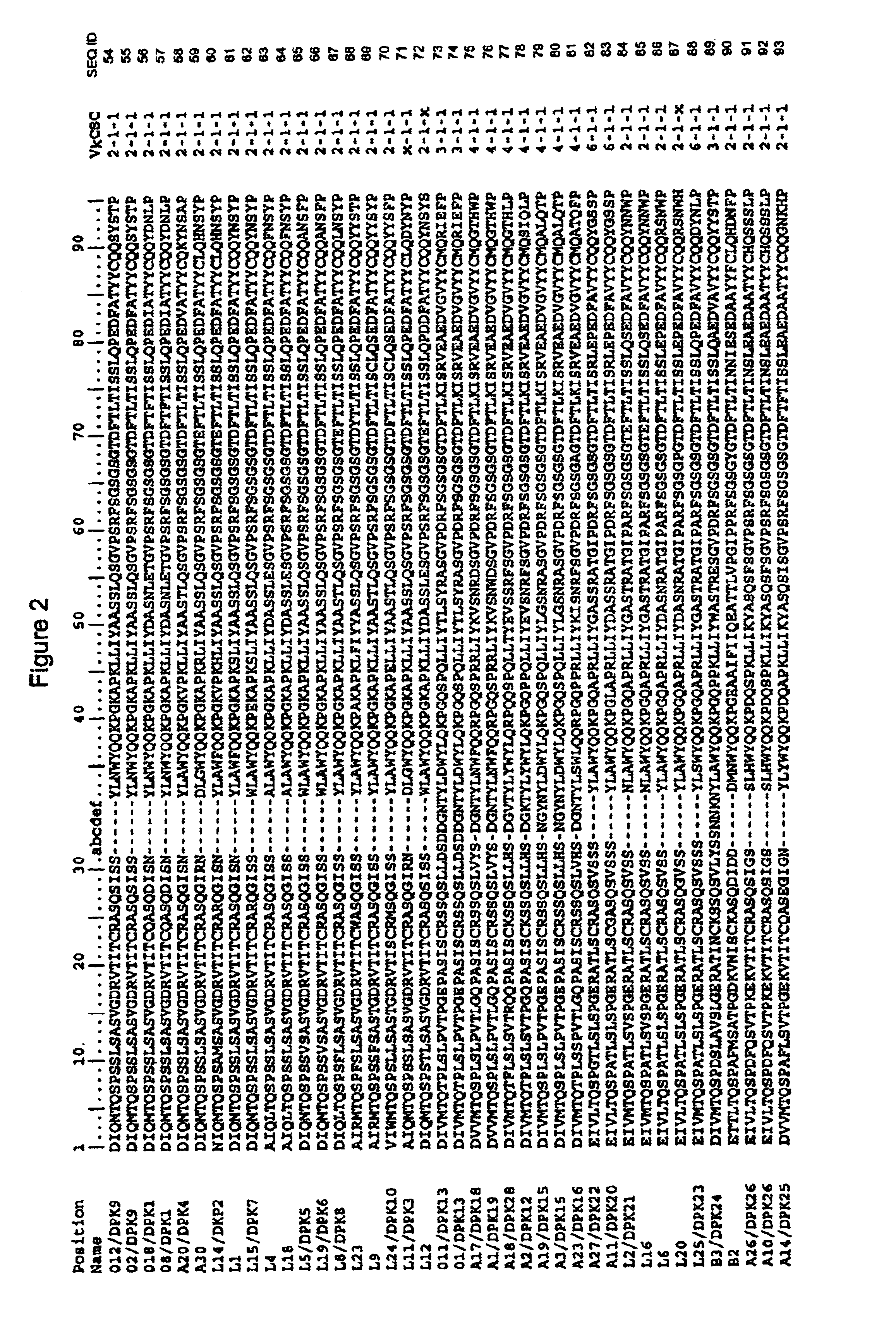
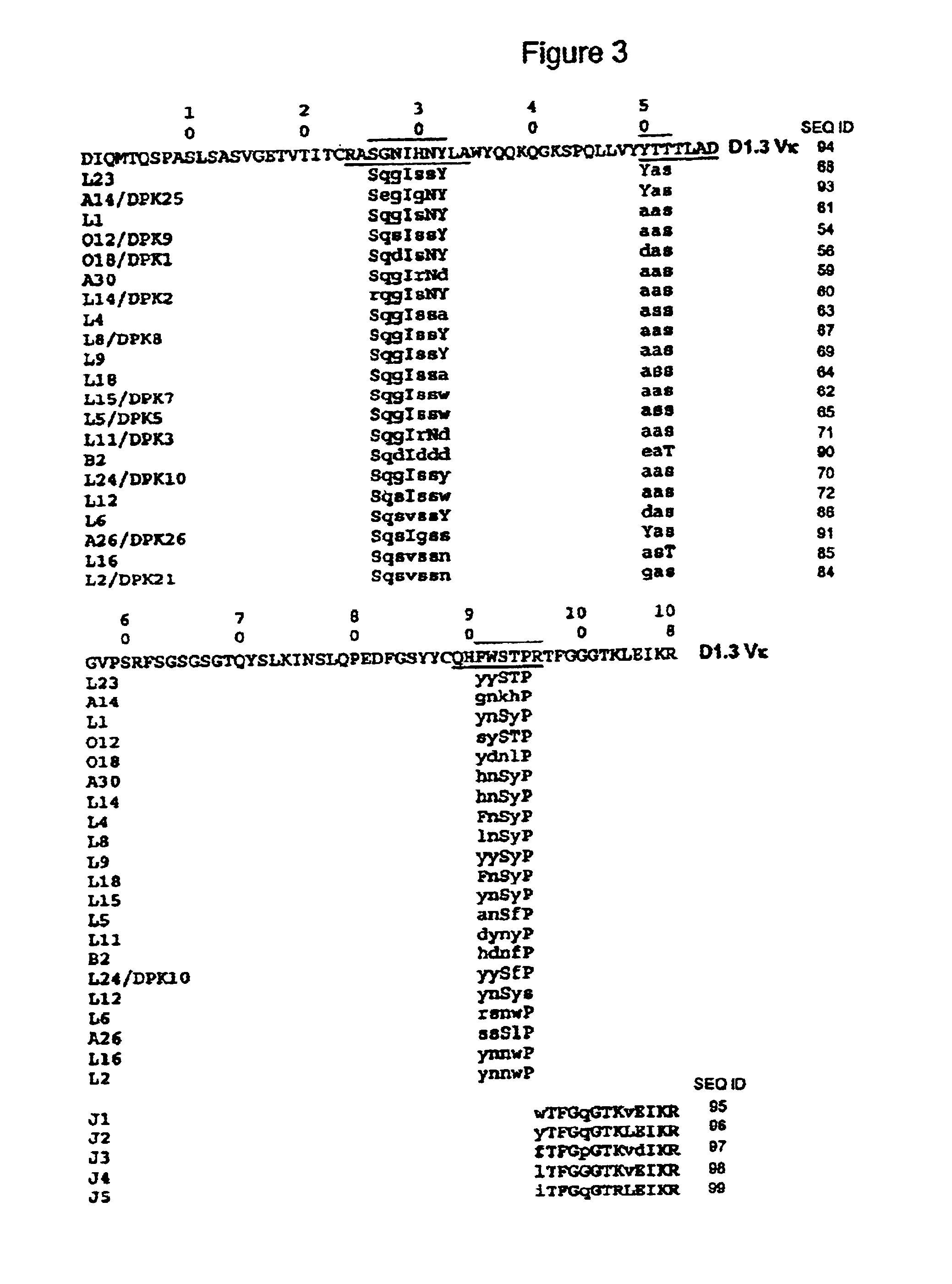
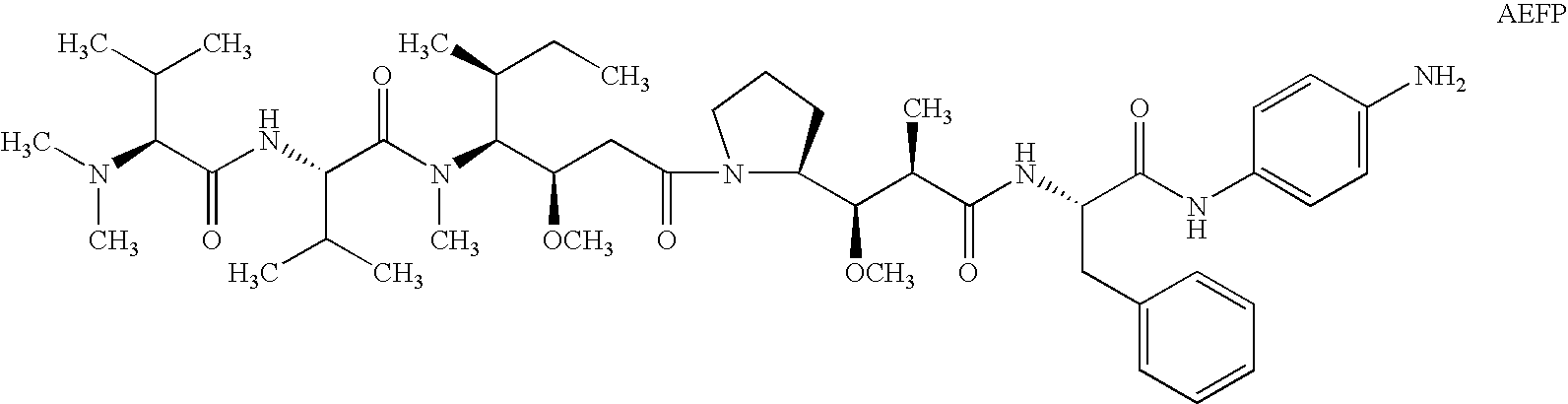
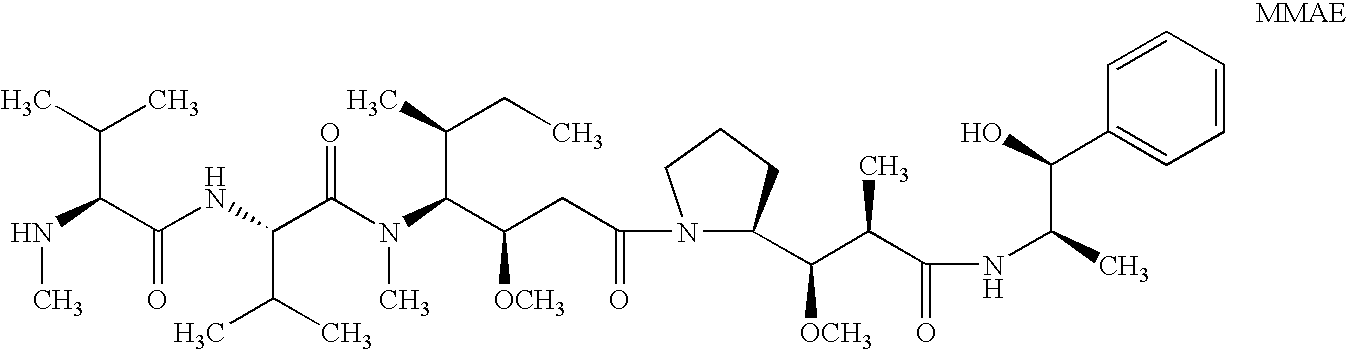
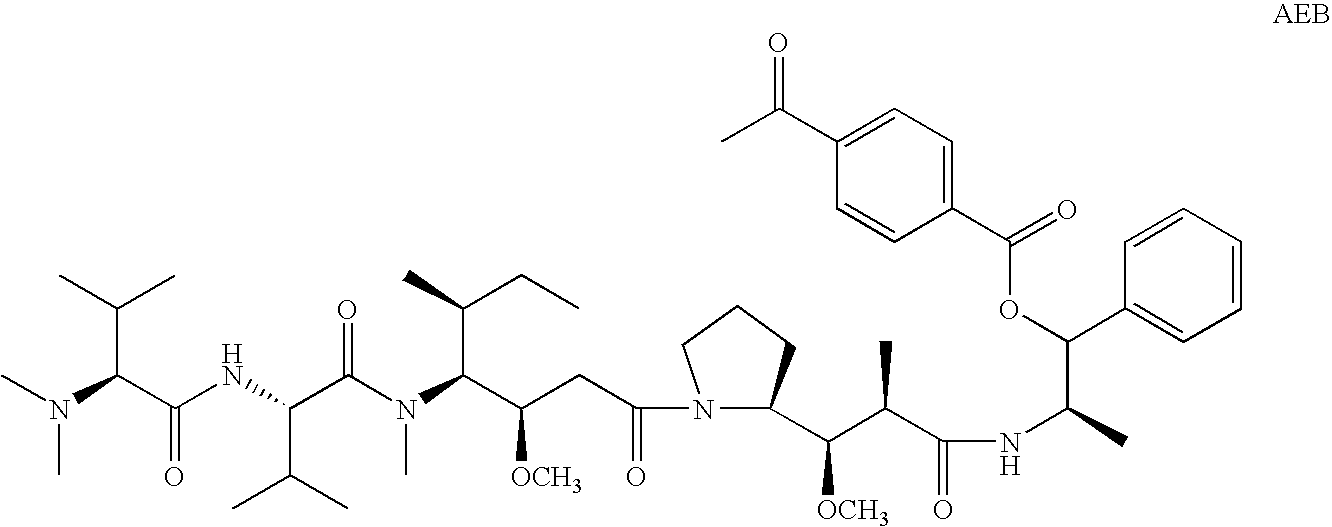
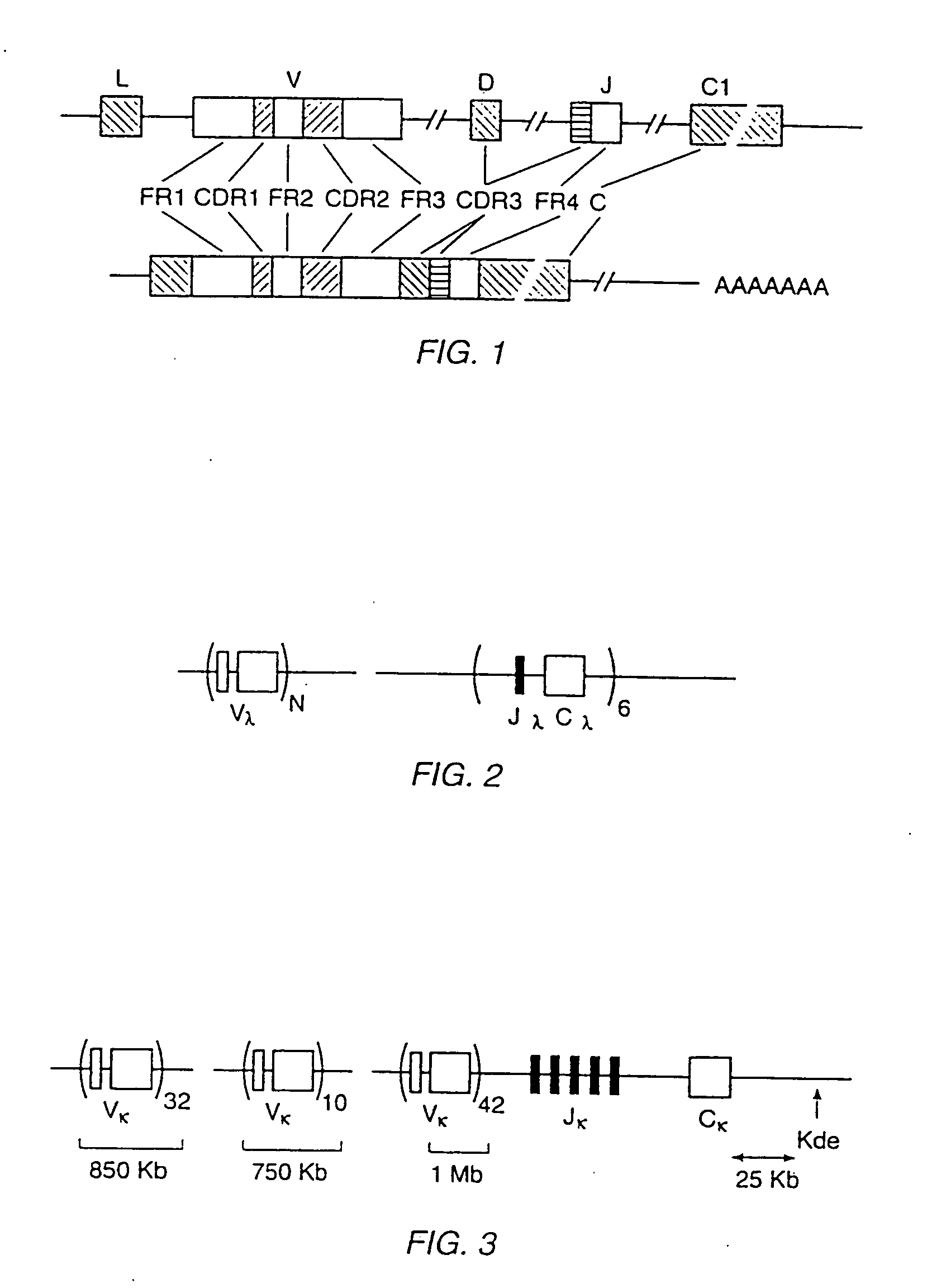
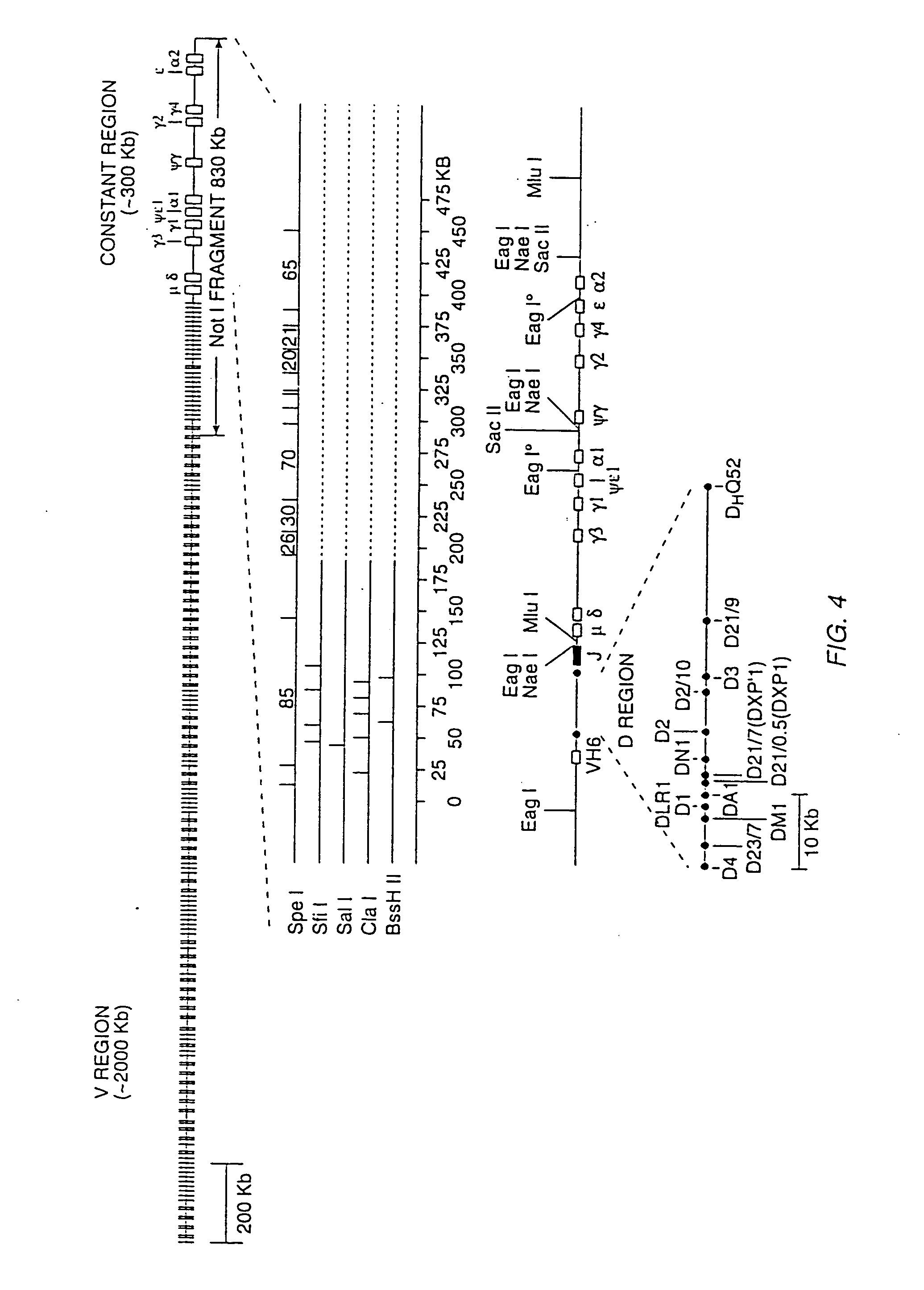
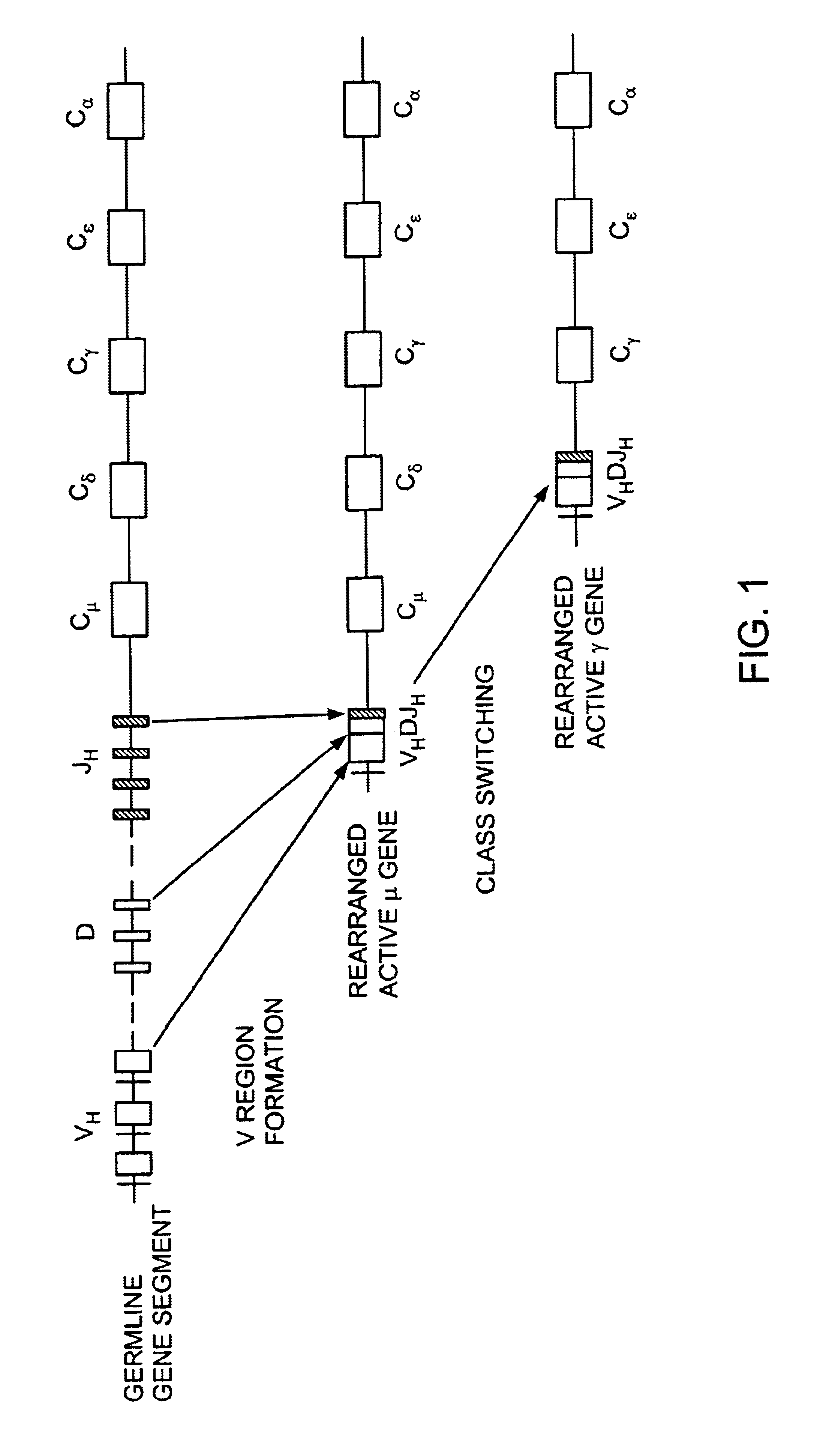
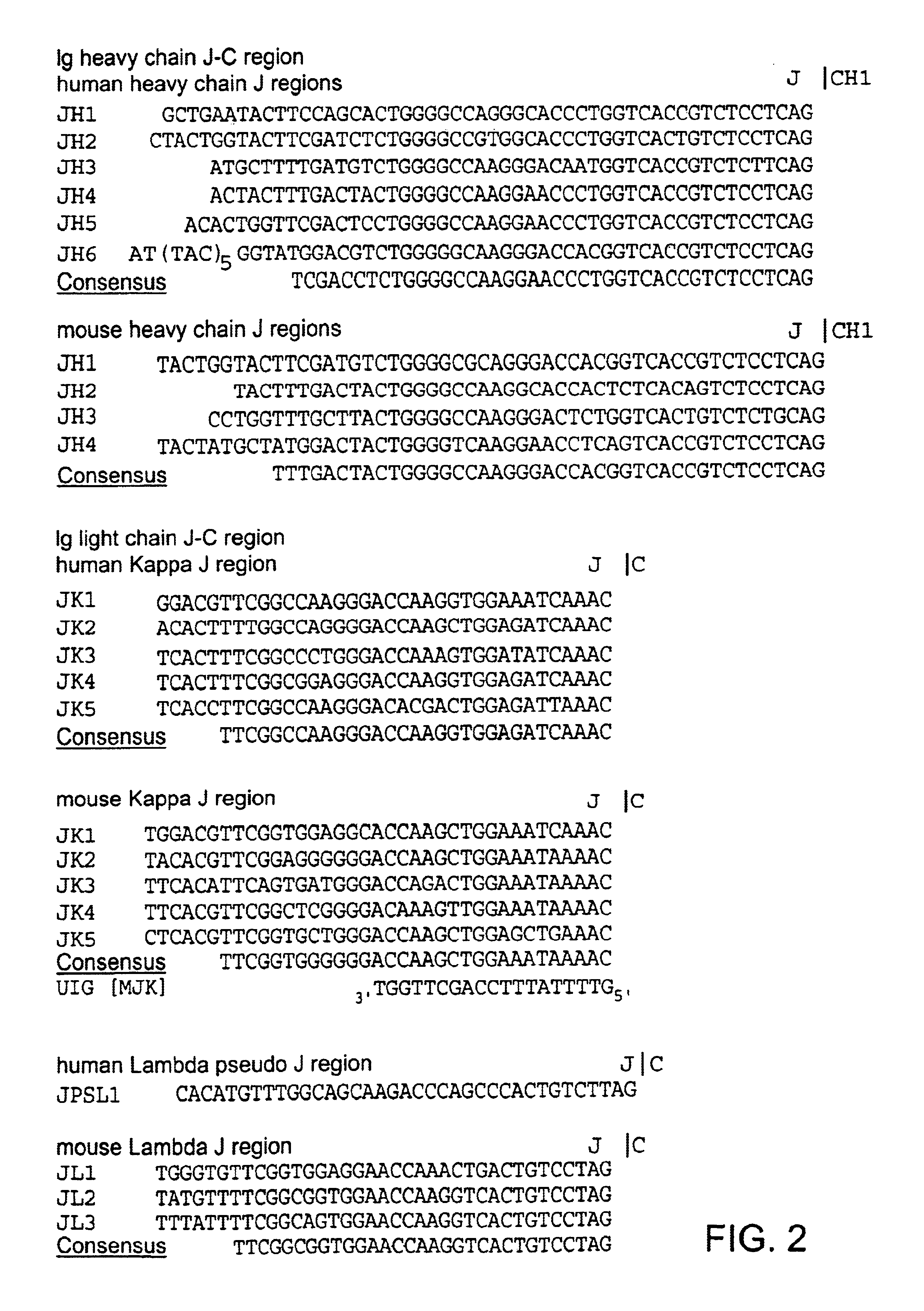
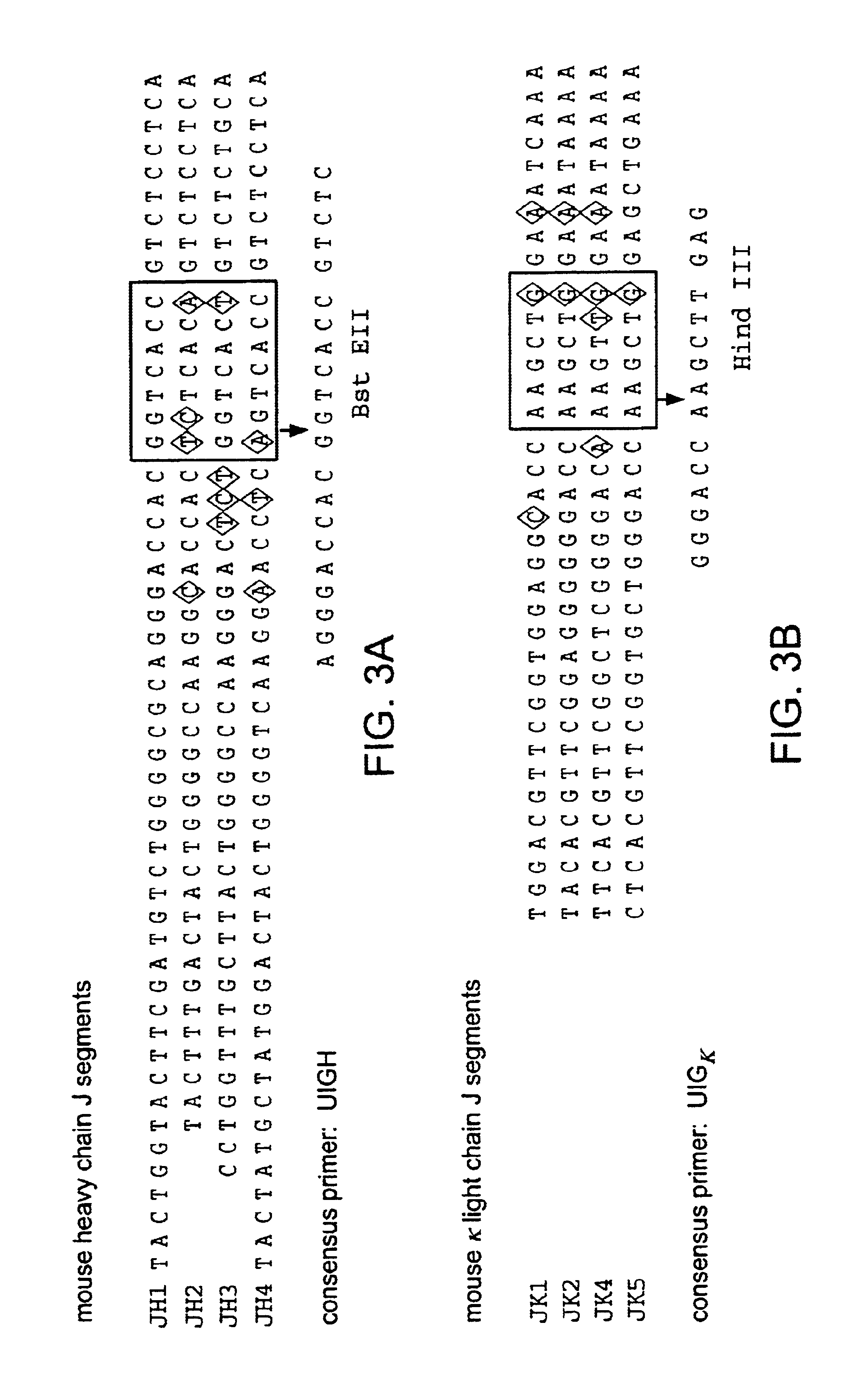
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
417 results about "Chimeric antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chimeric Antibodies. A chimeric antibody is a hybrid substance combining antibodies and parts of antibodies with the potential to track down and illuminate remote and microscopic tumors. It is less easily rejected by the body's immune system than the ordinary monoclonal antibody.
Super humanized antibodies
InactiveUS6881557B2Antibody mimetics/scaffoldsAnalogue computers for chemical processesHuman sequenceHumanized antibody
Disclosed herein are methods for humanizing antibodies based on selecting variable region framework sequences from human antibody genes by comparing canonical CDR structure types for CDR sequences of the variable region of a non-human antibody to canonical CDR structure types for corresponding CDRs from a library of human antibody sequences, preferably germline antibody gene segments. Human antibody variable regions having similar canonical CDR structure types to the non-human CDRs form a subset of member human antibody sequences from which to select human framework sequences. The subset members may be further ranked by amino acid similarity between the human and the non-human CDR sequences. Top ranking human sequences are selected to provide the framework sequences for constructing a chimeric antibody that functionally replaces human CDR sequences with the non-human CDR counterparts using the selected subset member human frameworks, thereby providing a humanized antibody of high affinity and low immunogenicity without need for comparing framework sequences between the non-human and human antibodies. Chimeric antibodies made according to the method are also disclosed.
Owner:ARROWSMITH TECH
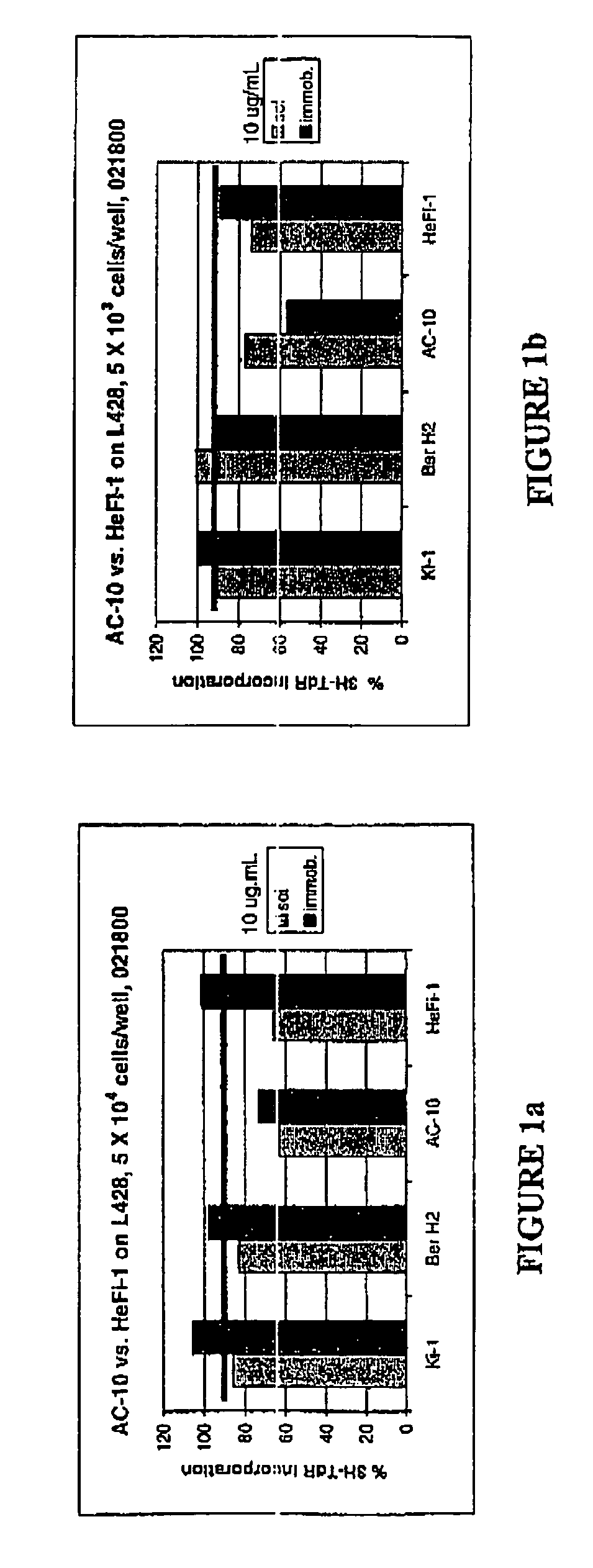
Recombinant anti-CD30 antibodies and uses thereof
InactiveUS20040018194A1Nervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseChemotherapeutic drugs
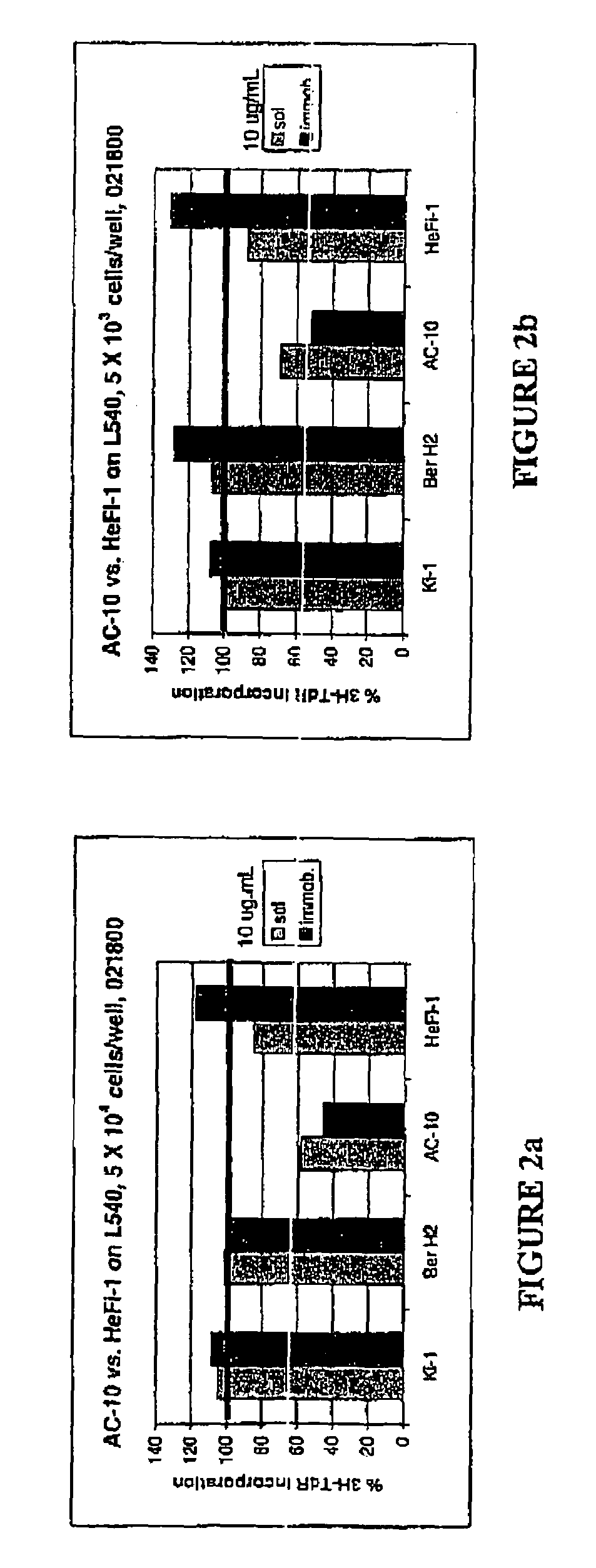
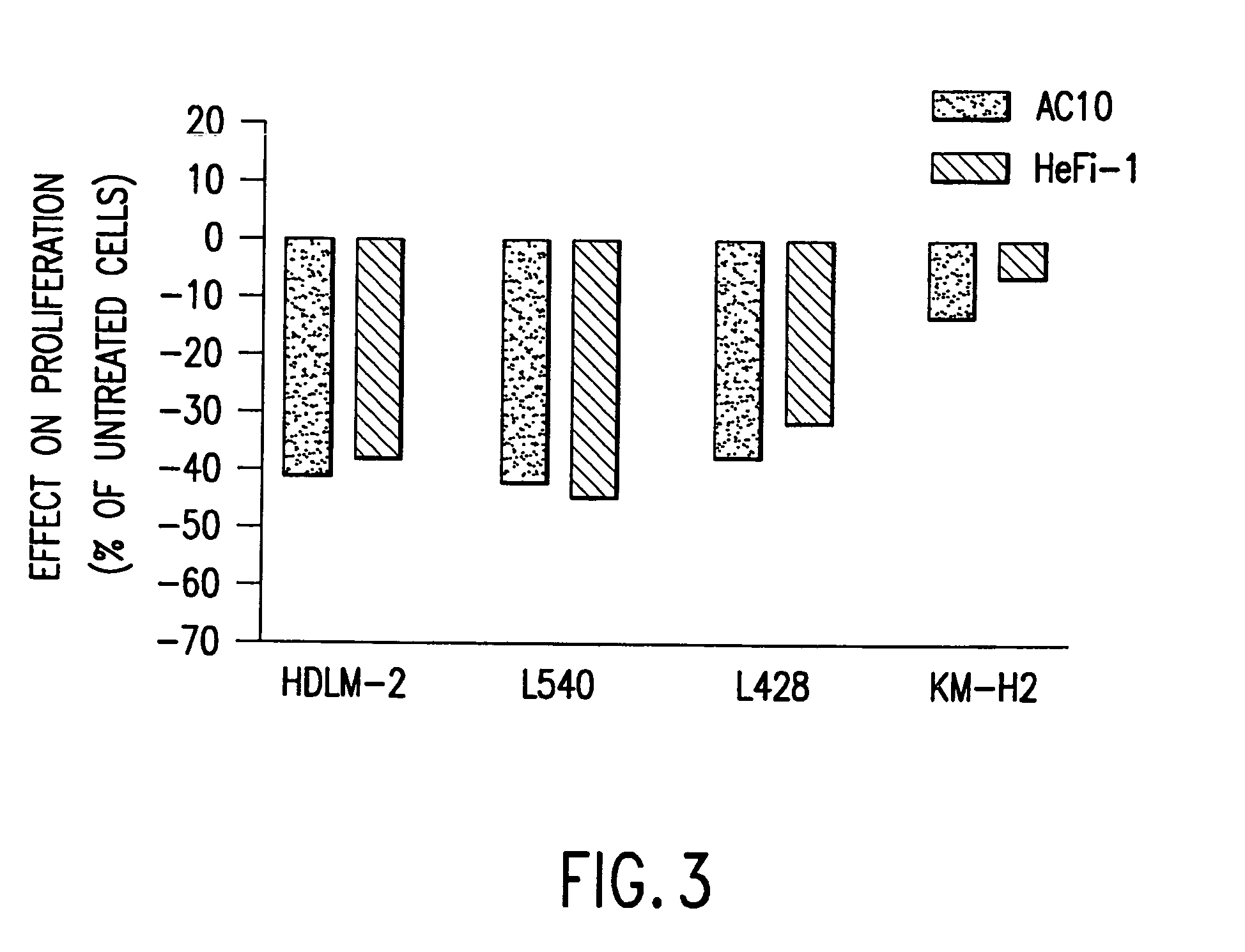
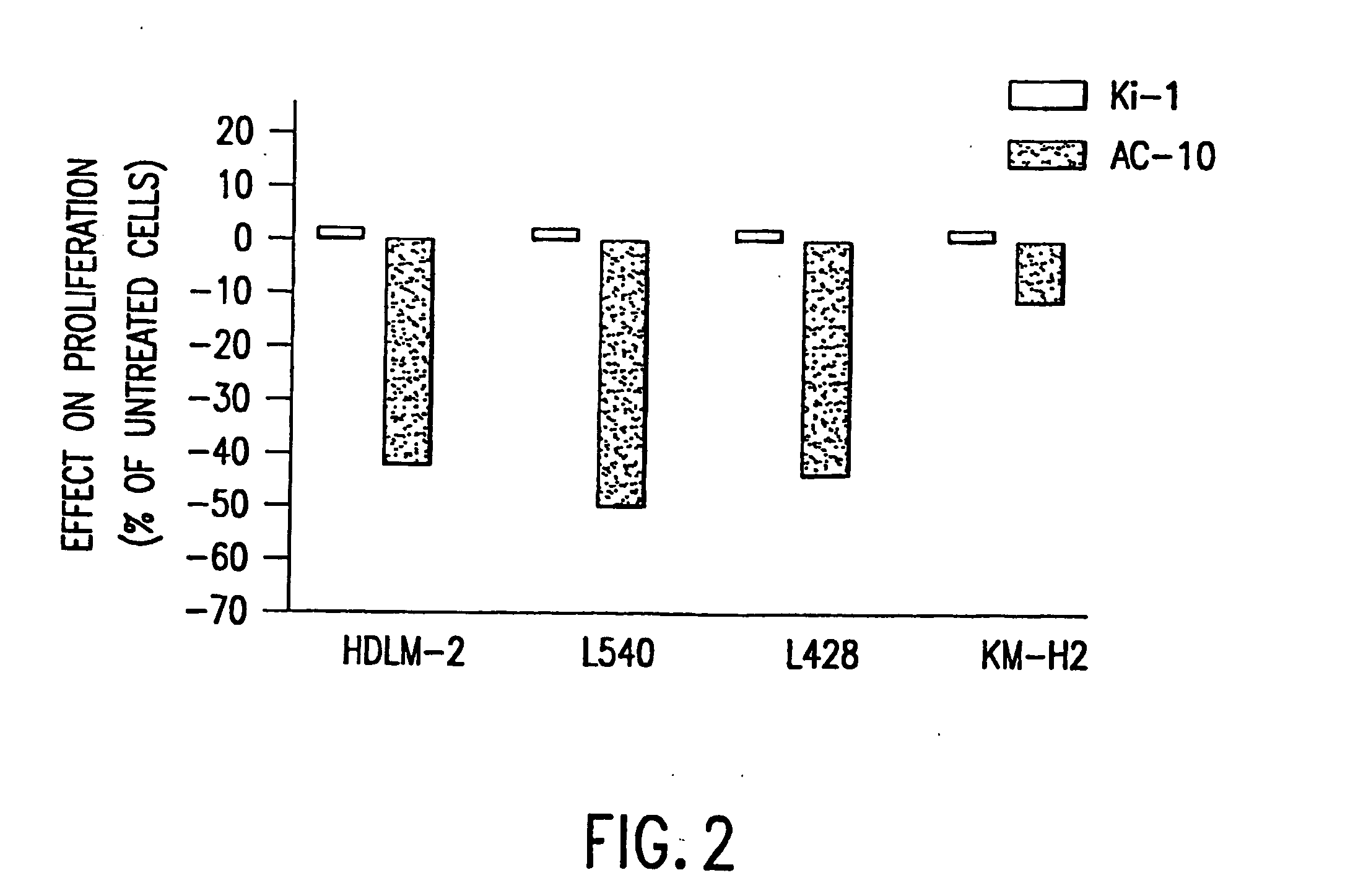
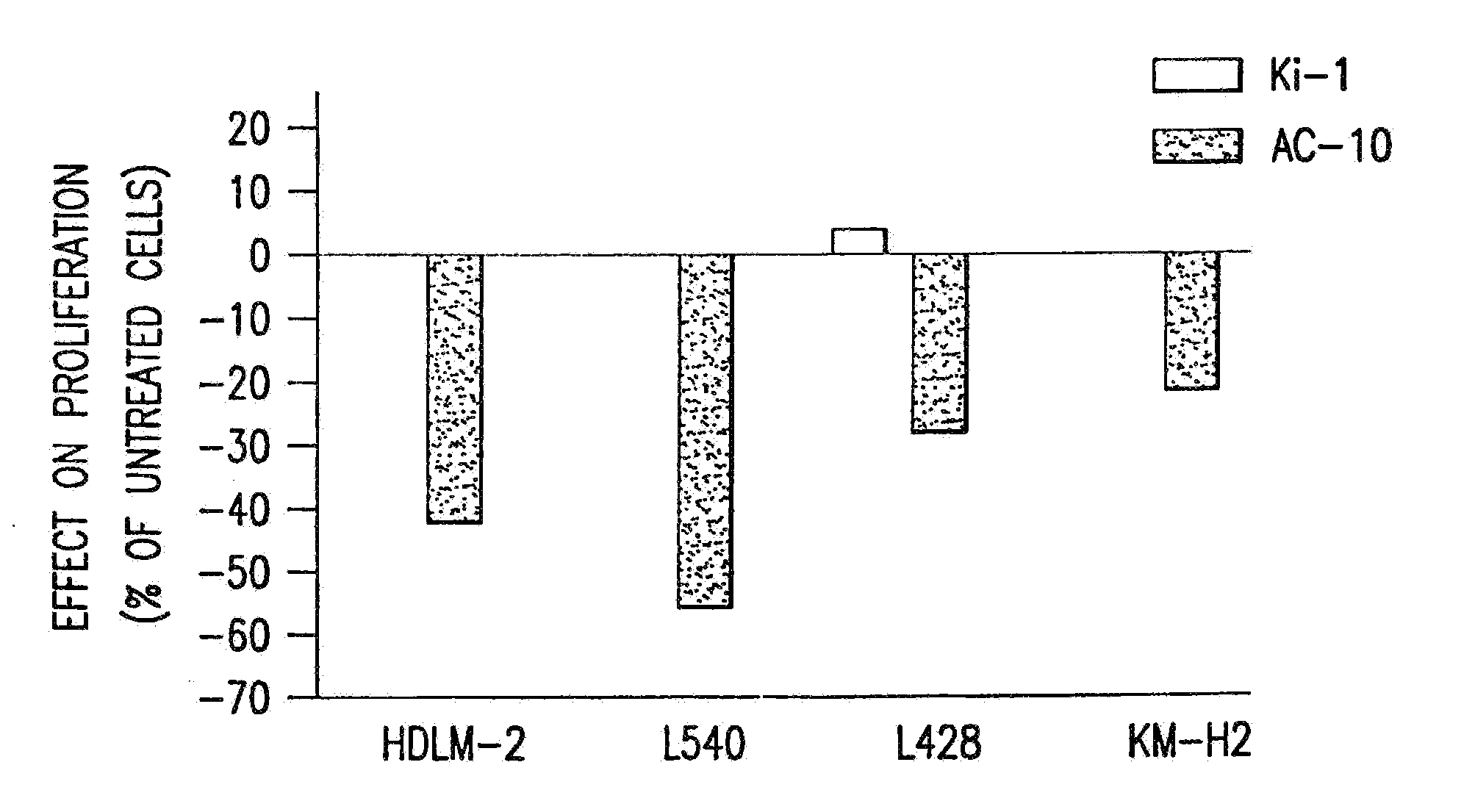
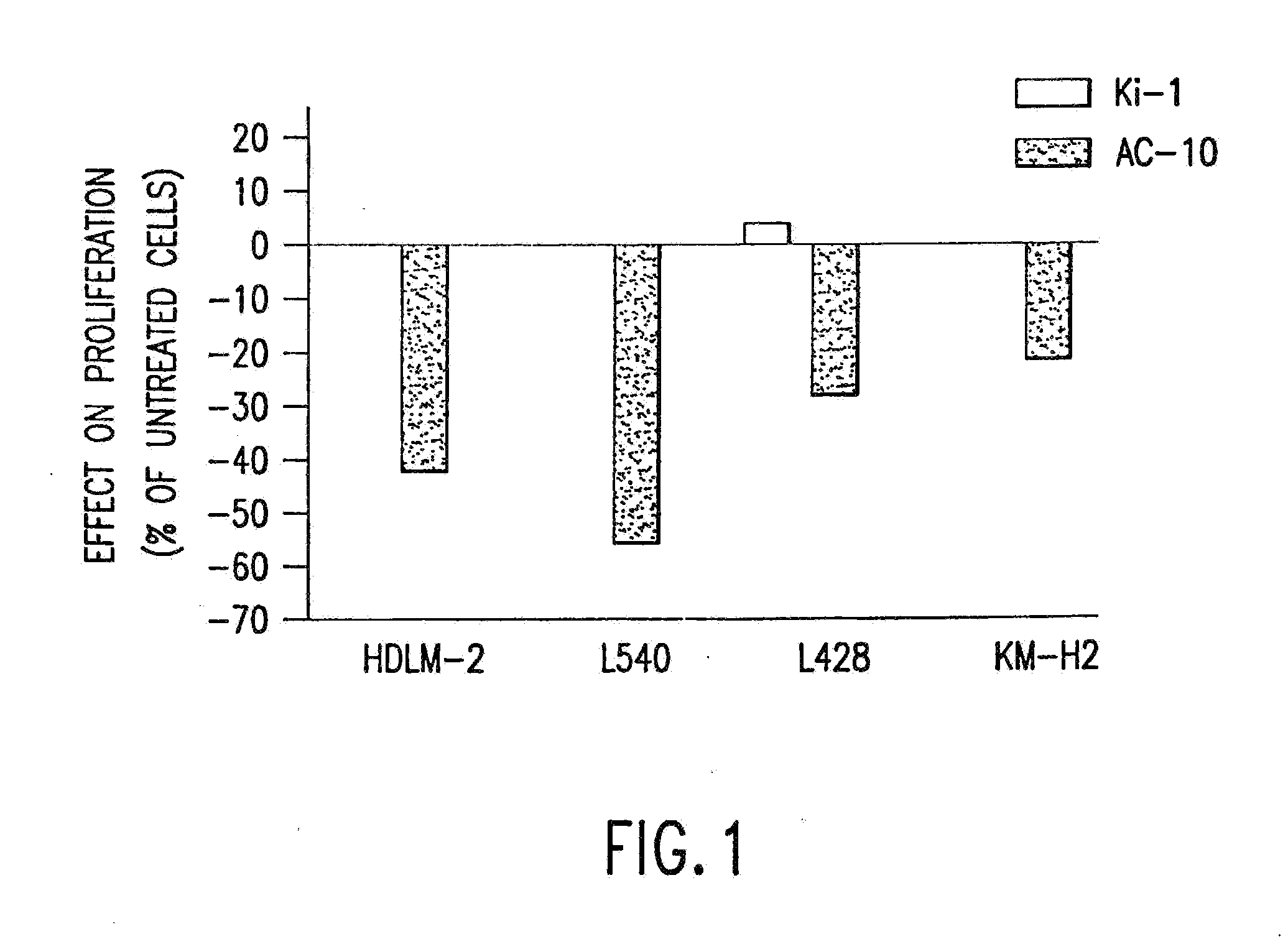
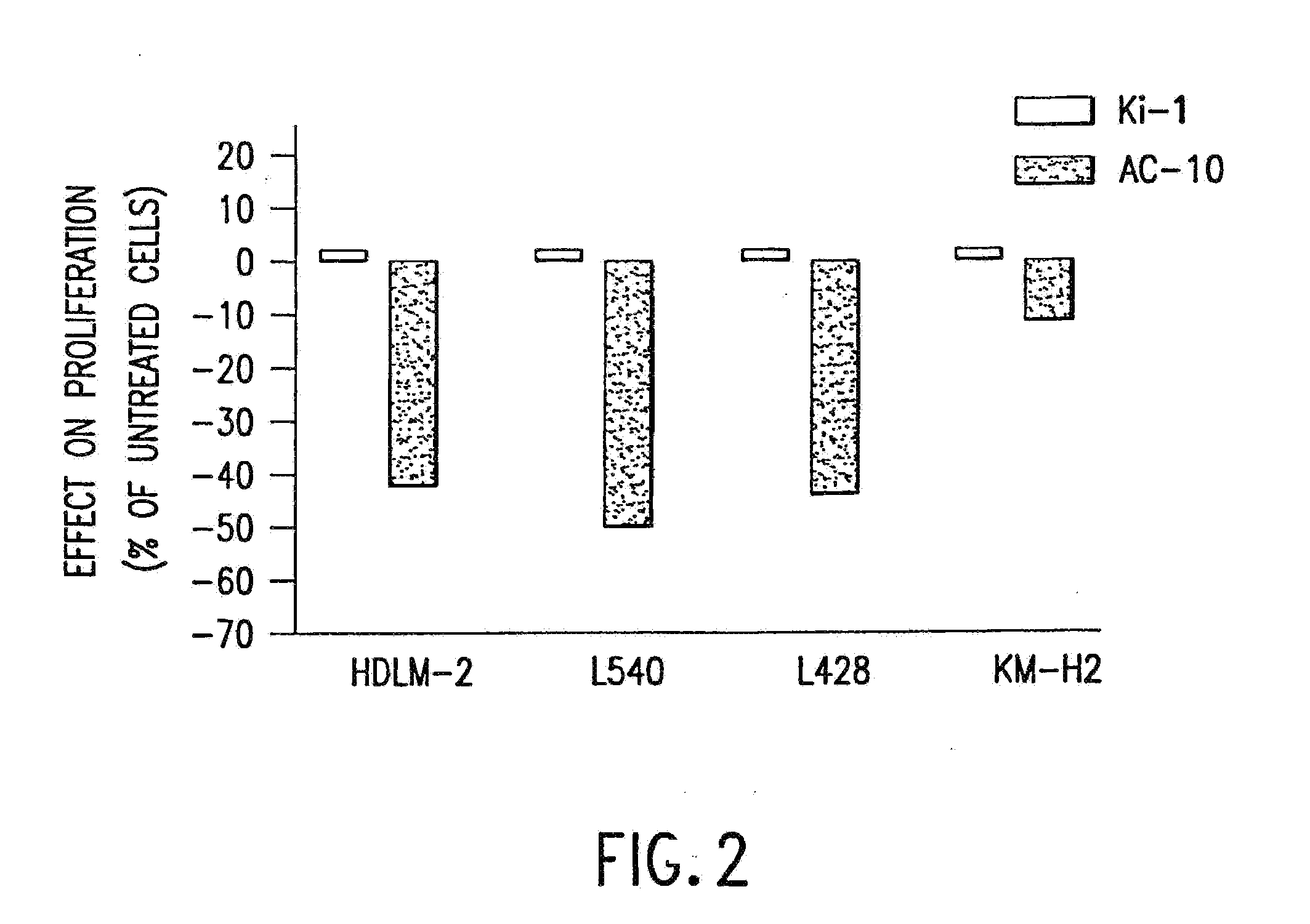
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's disease cells in the absence of effector cells or complement. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEATTLE GENETICS INC
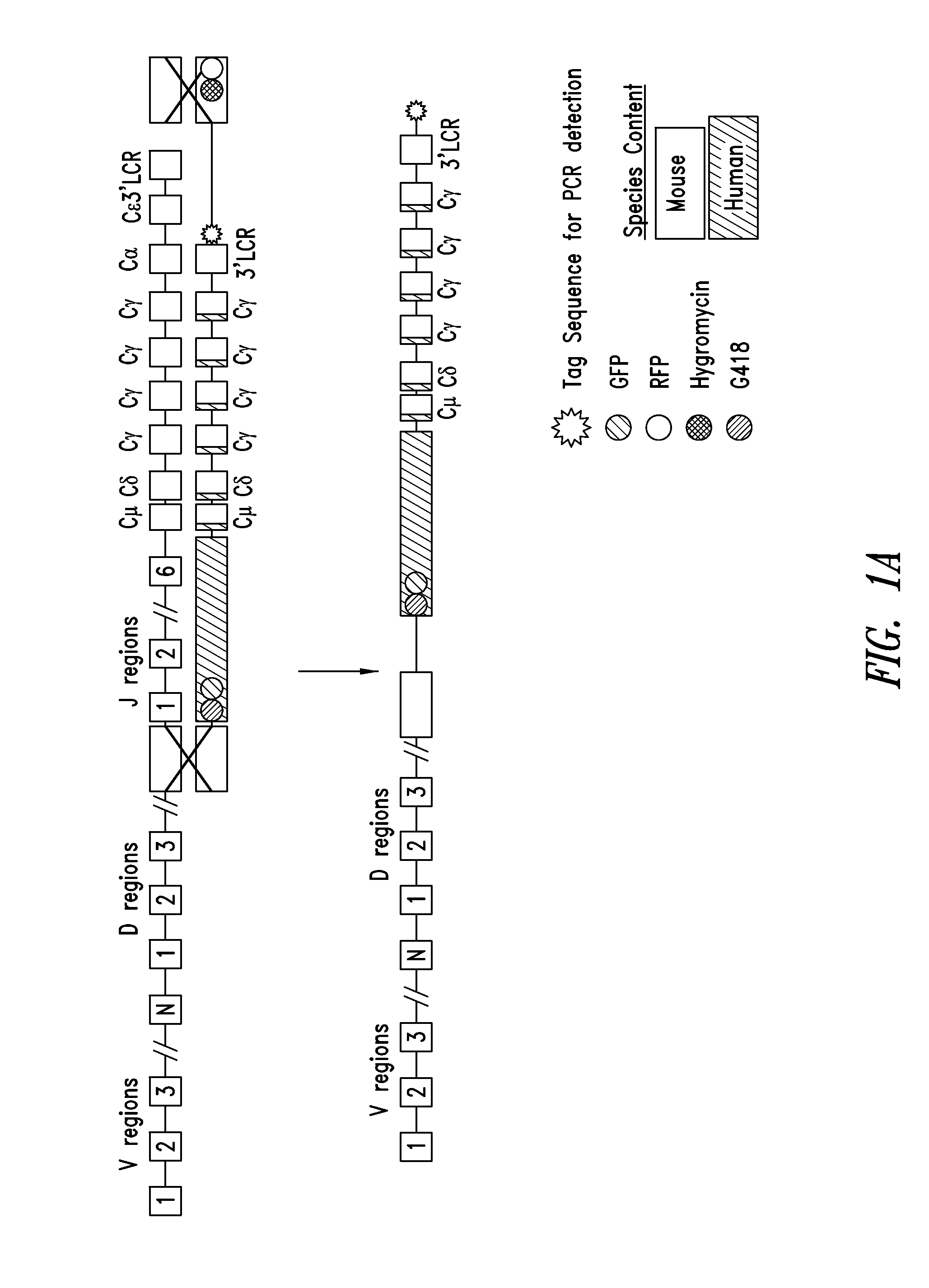
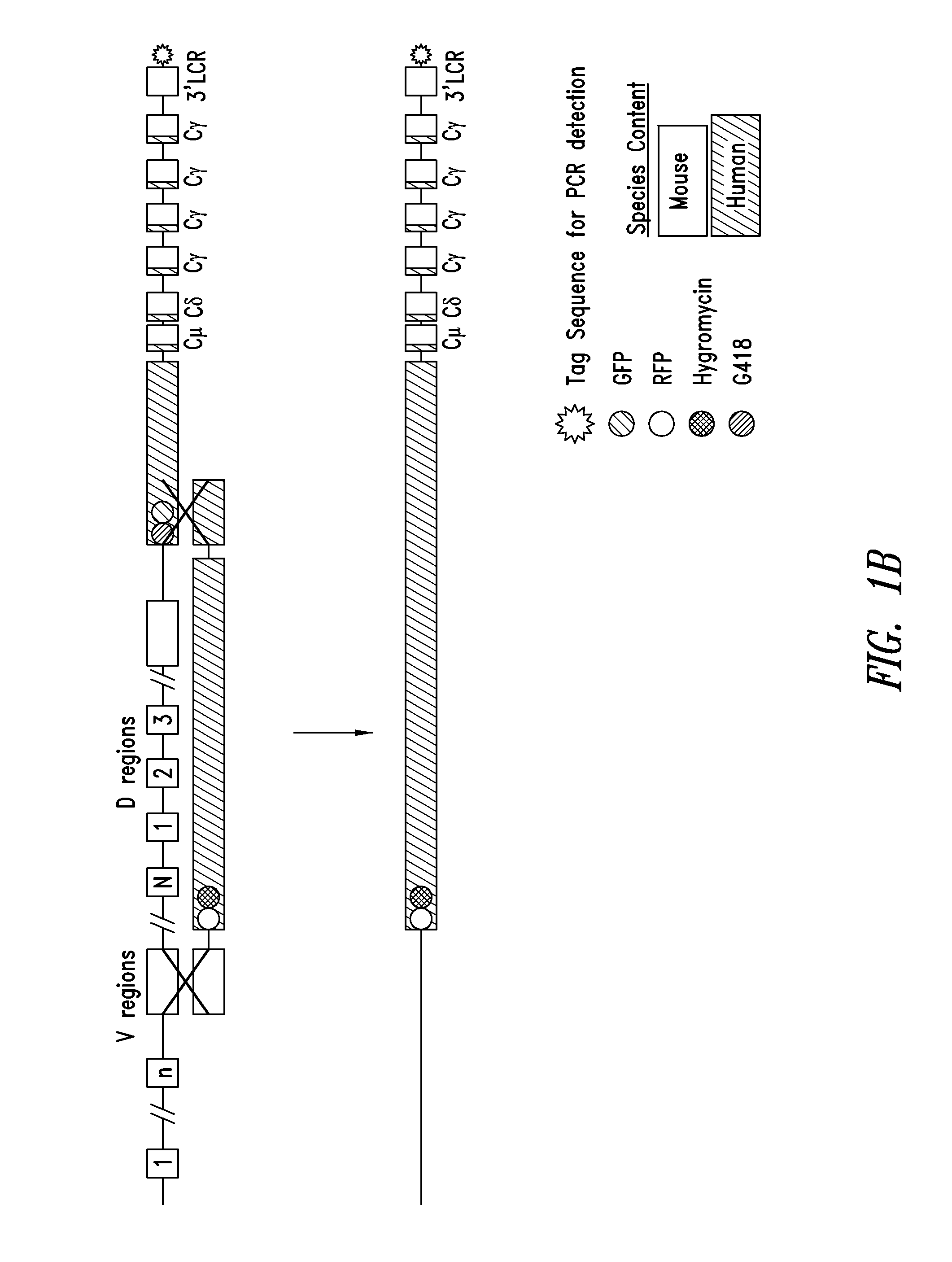
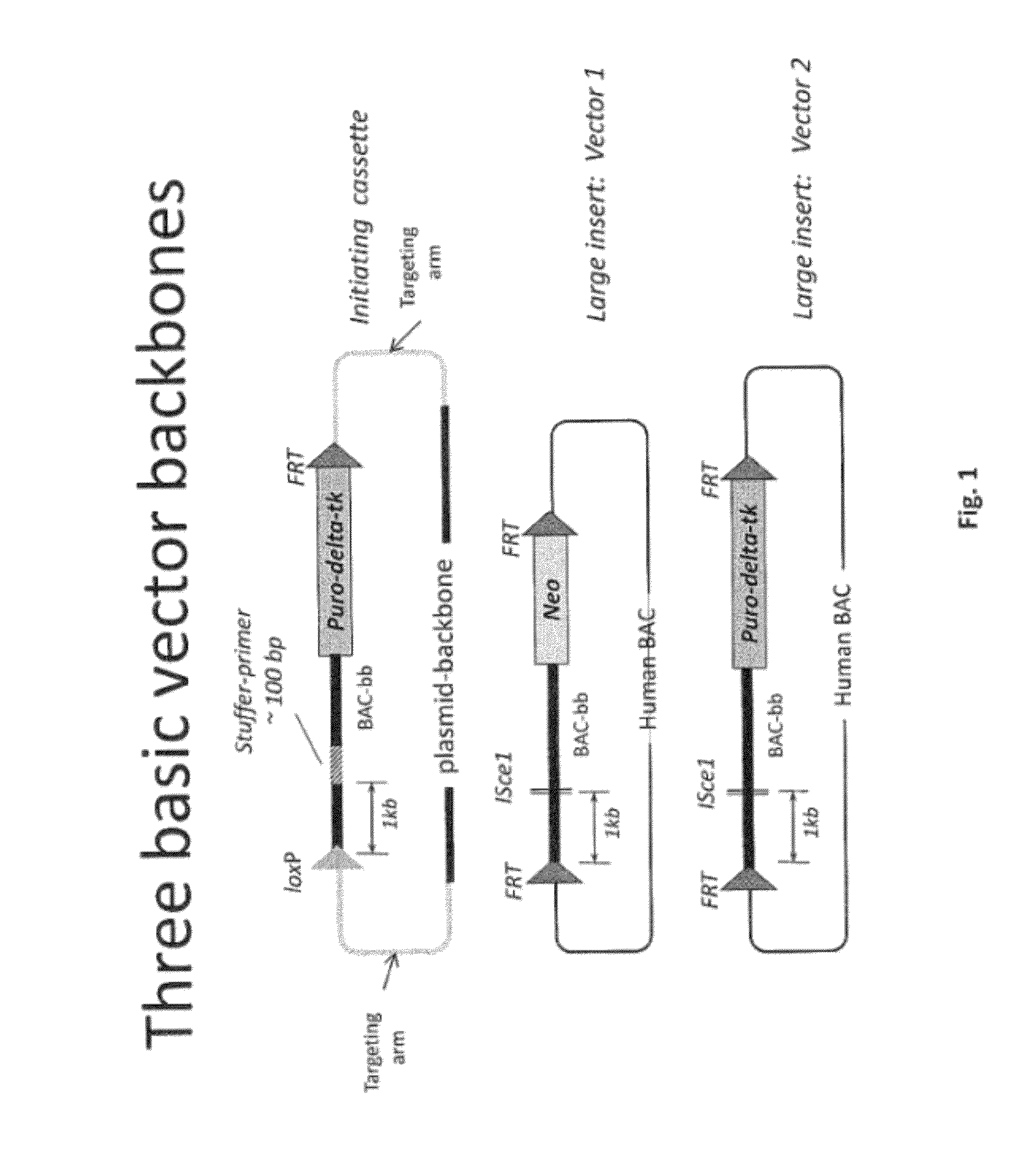
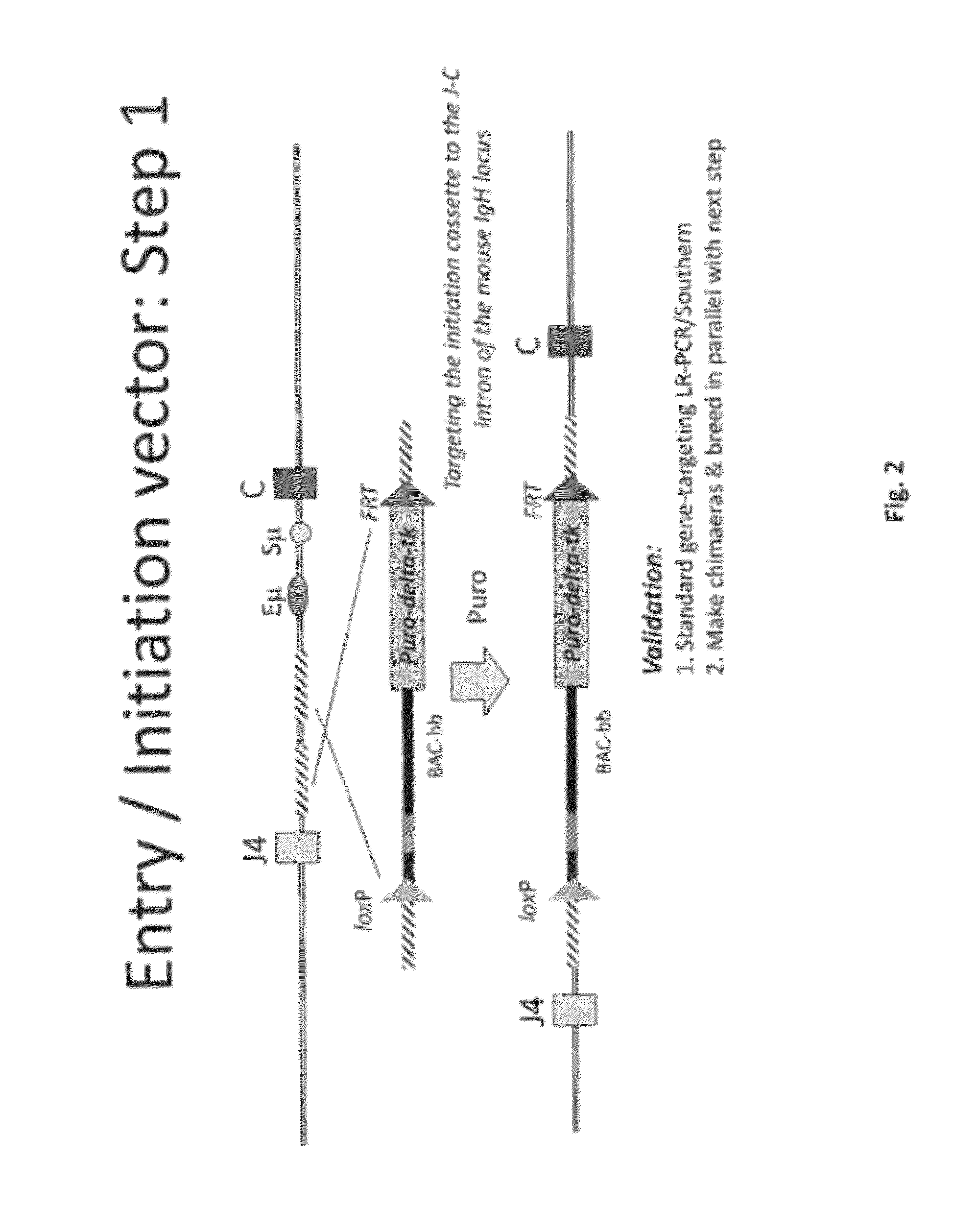
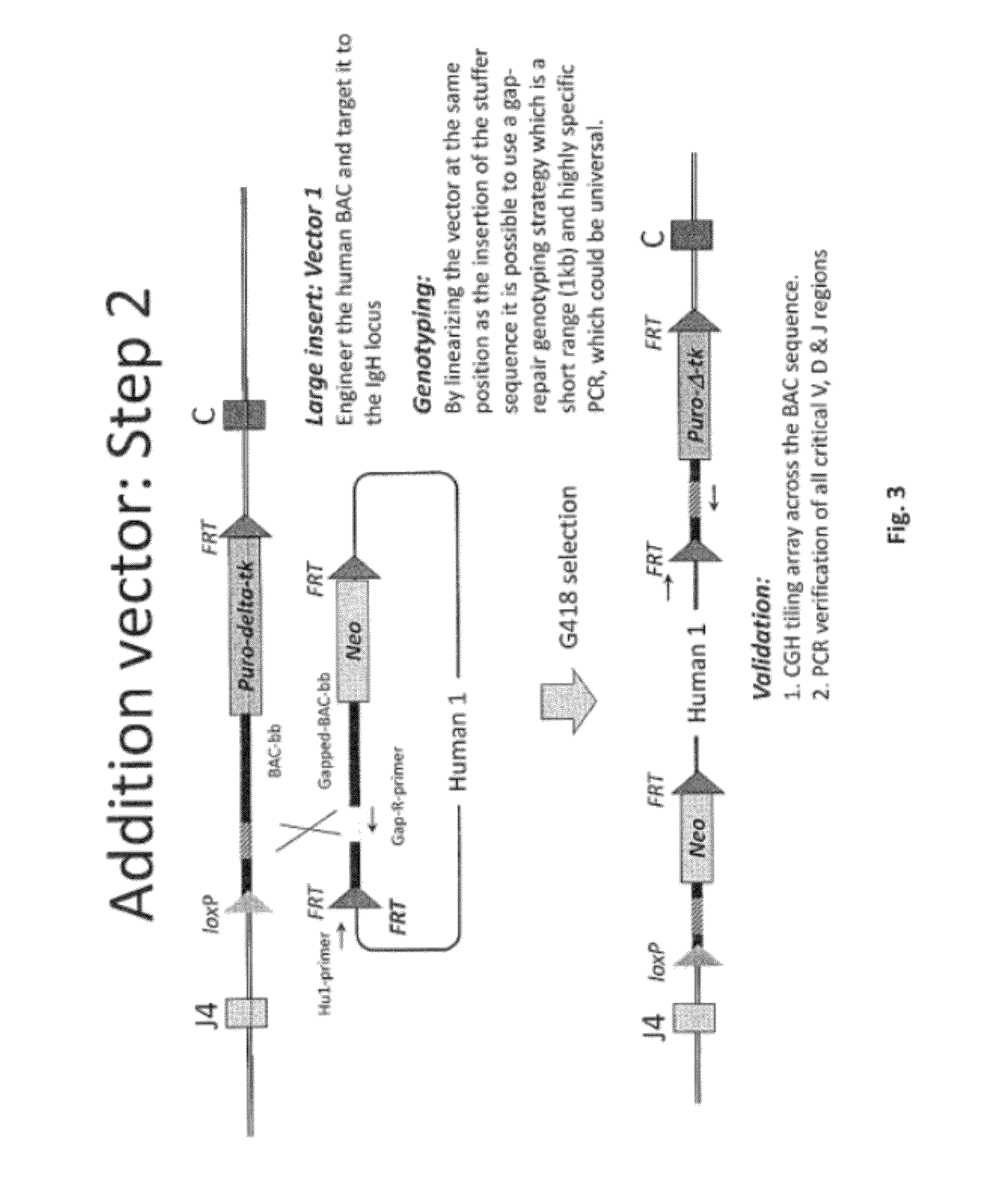
Transgenic non-human animals for producing chimeric antibodies
InactiveUS20060015957A1Inhibit expressionEasy to switchImmunoglobulinsGenetic engineeringAntigenHuman animal
The invention relates to transgenic non-human animals capable of producing heterologous antibodies and methods for producing human sequence antibodies which bind to human antigens with substantial affinity.
Owner:GENPHARM INT INC
Chimeric antibody with specificity to human B cell surface antigen
A chimeric antibody with human constant region and murine variable region, having specificity to a 35 kDA polypeptide (Bp35(CD20)) expressed on the surface of human B cells, methods of production, and uses.
Owner:ROYALTY PHARMA FINANCE TRUST
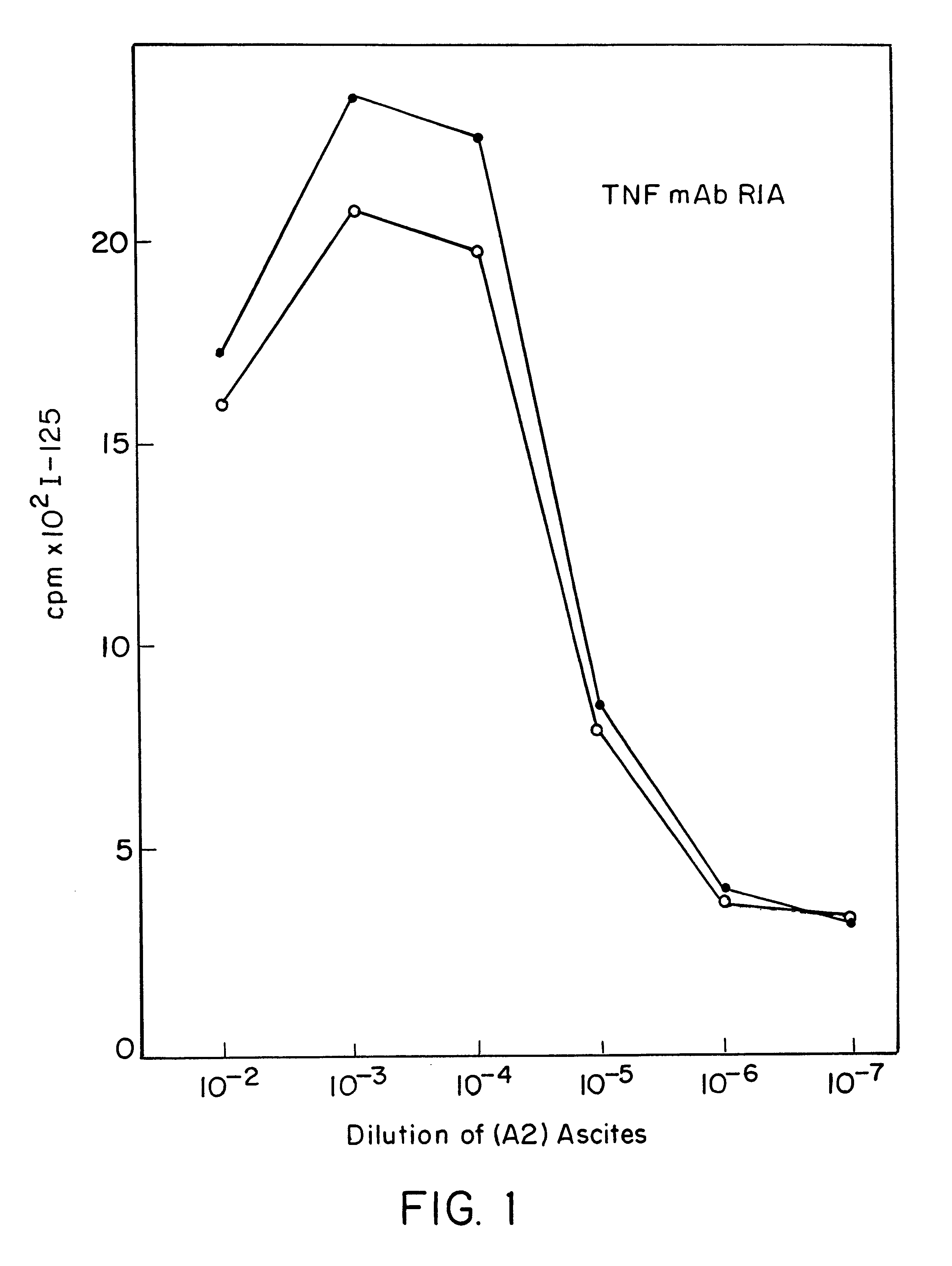
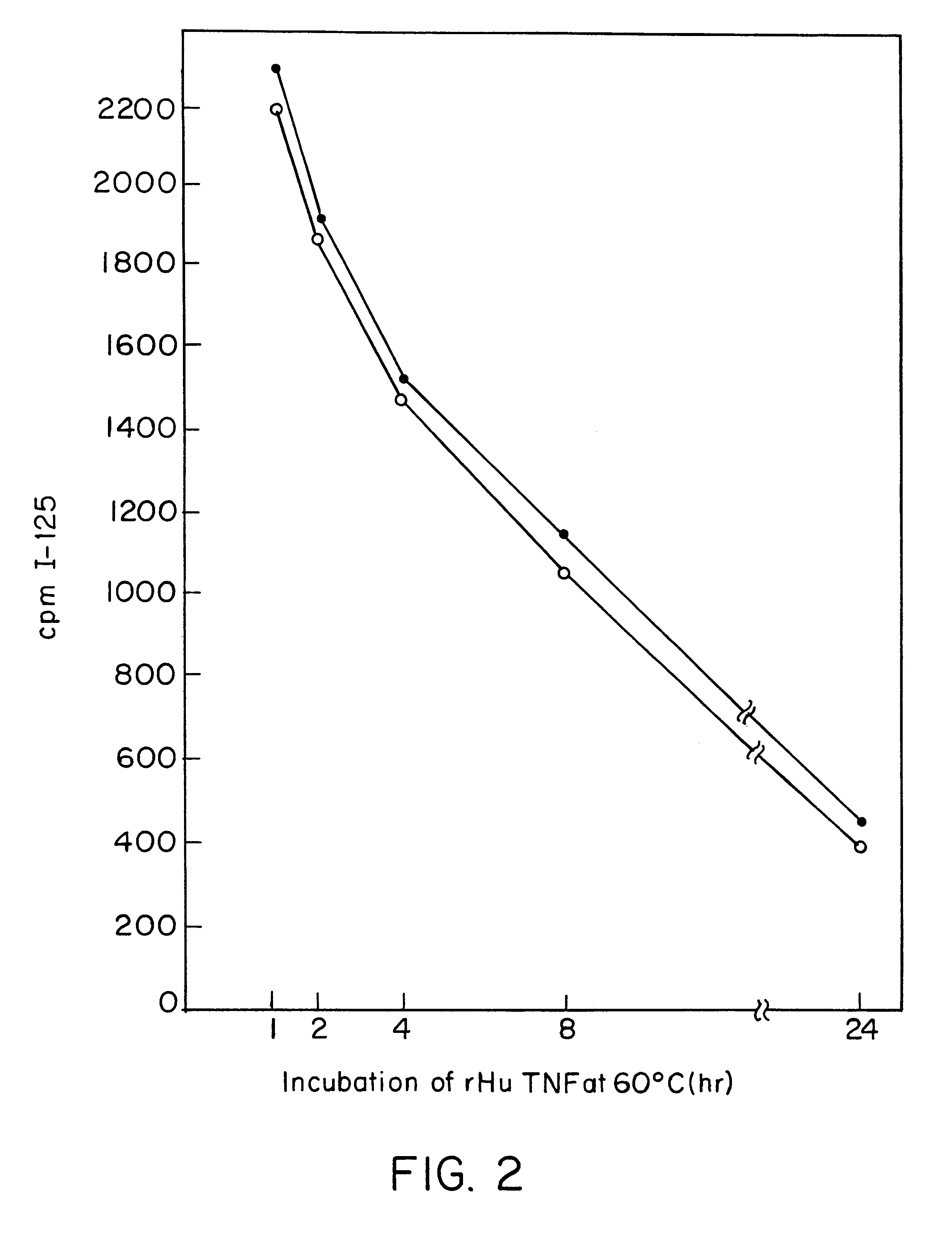
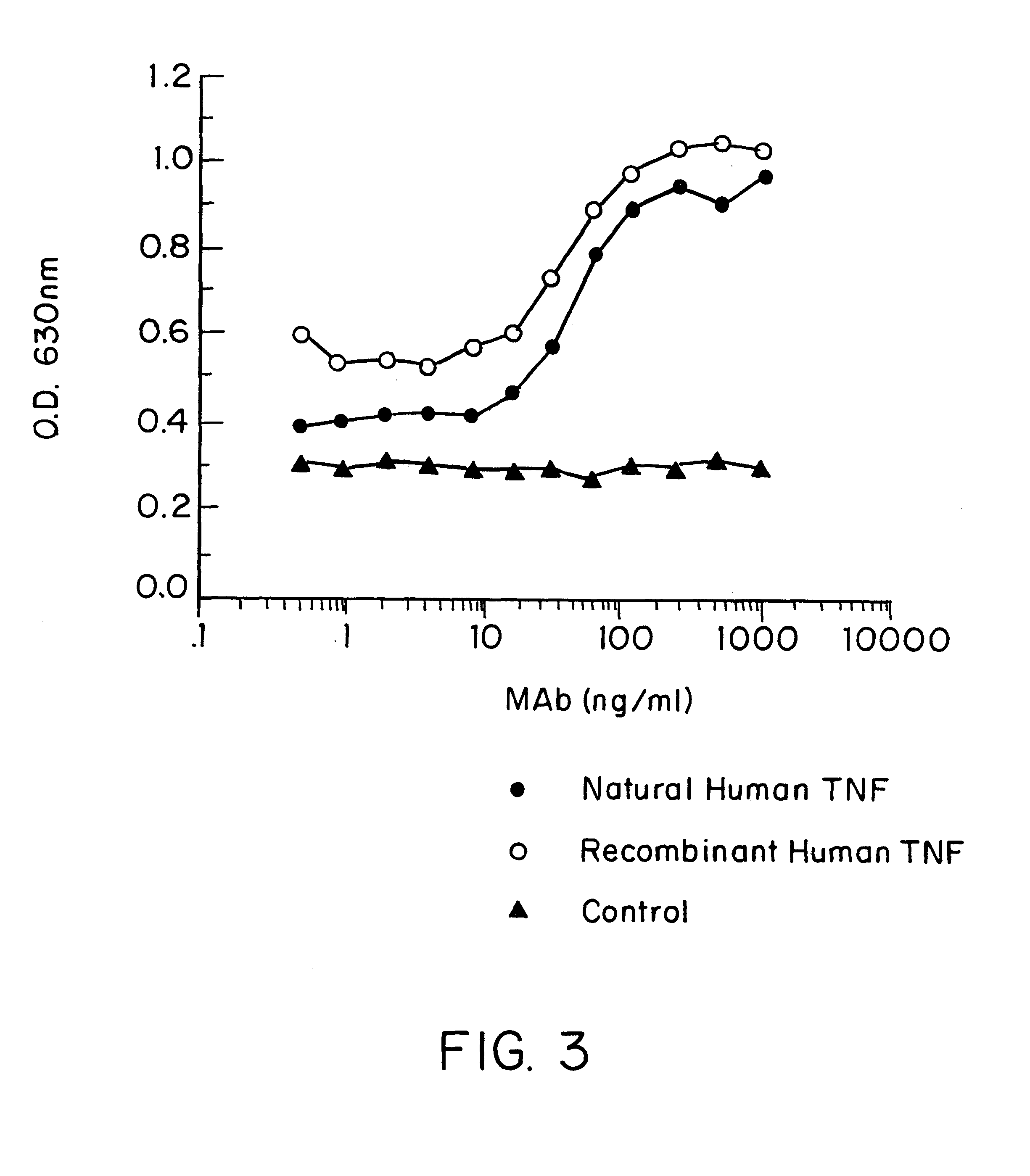
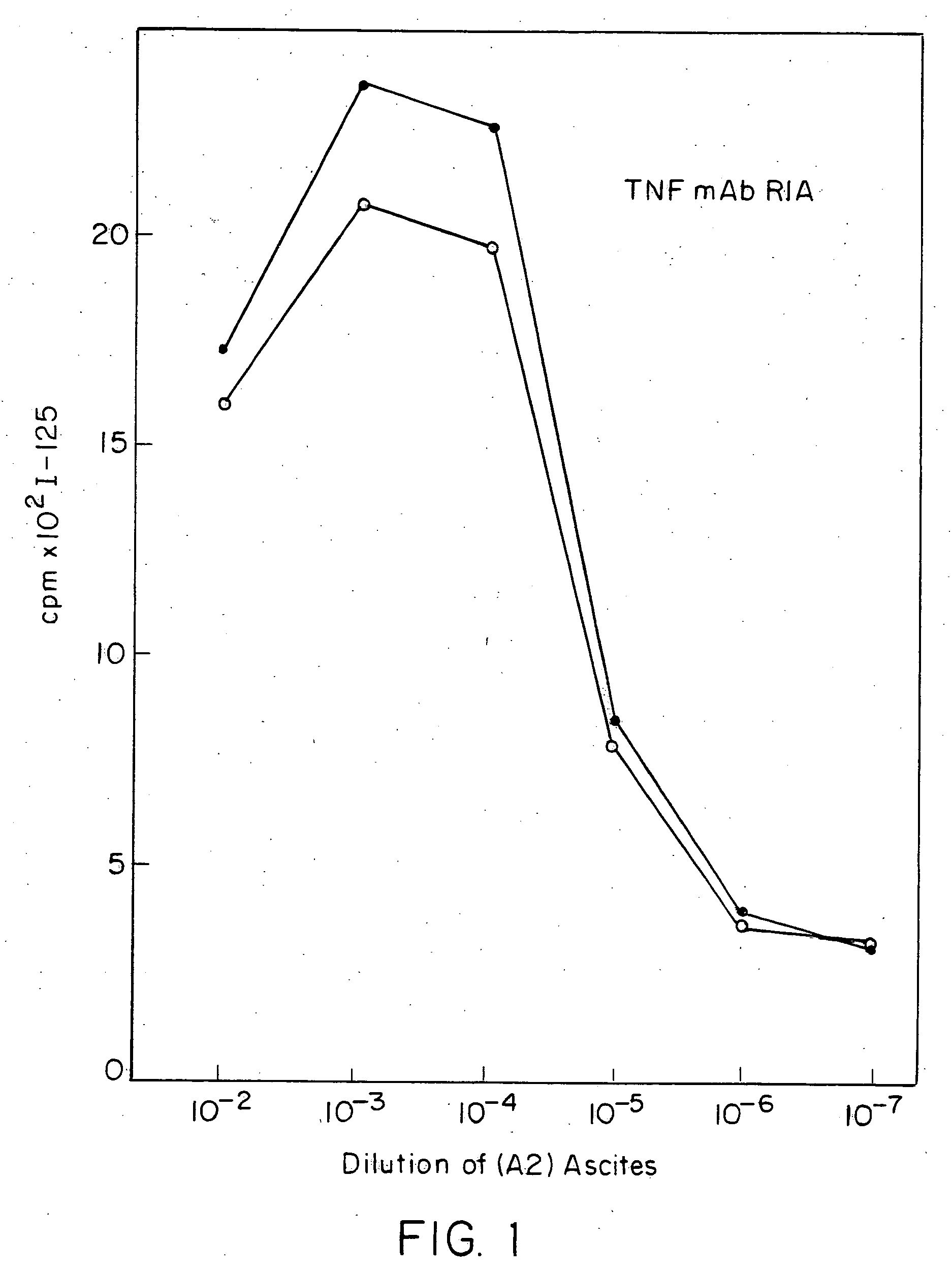
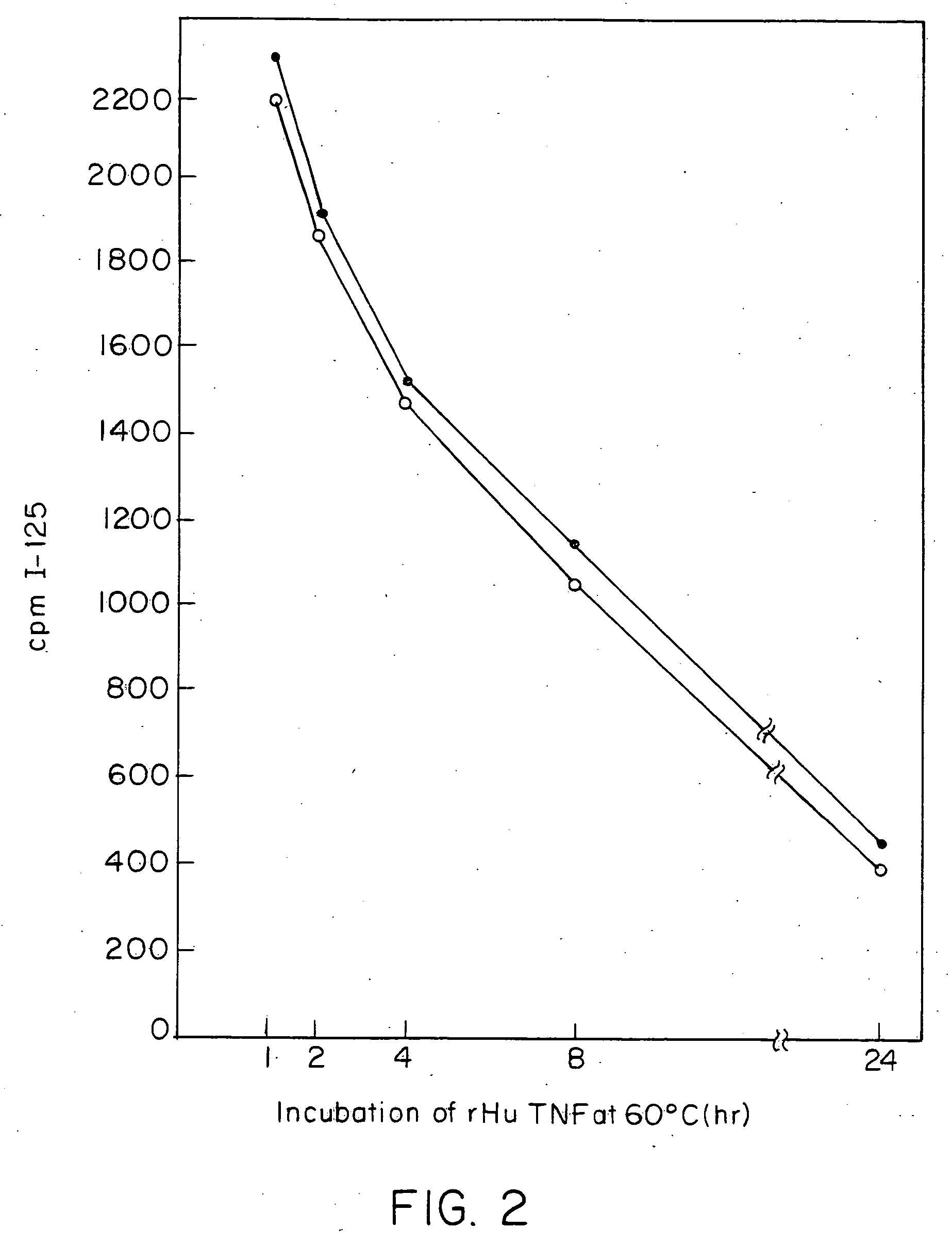
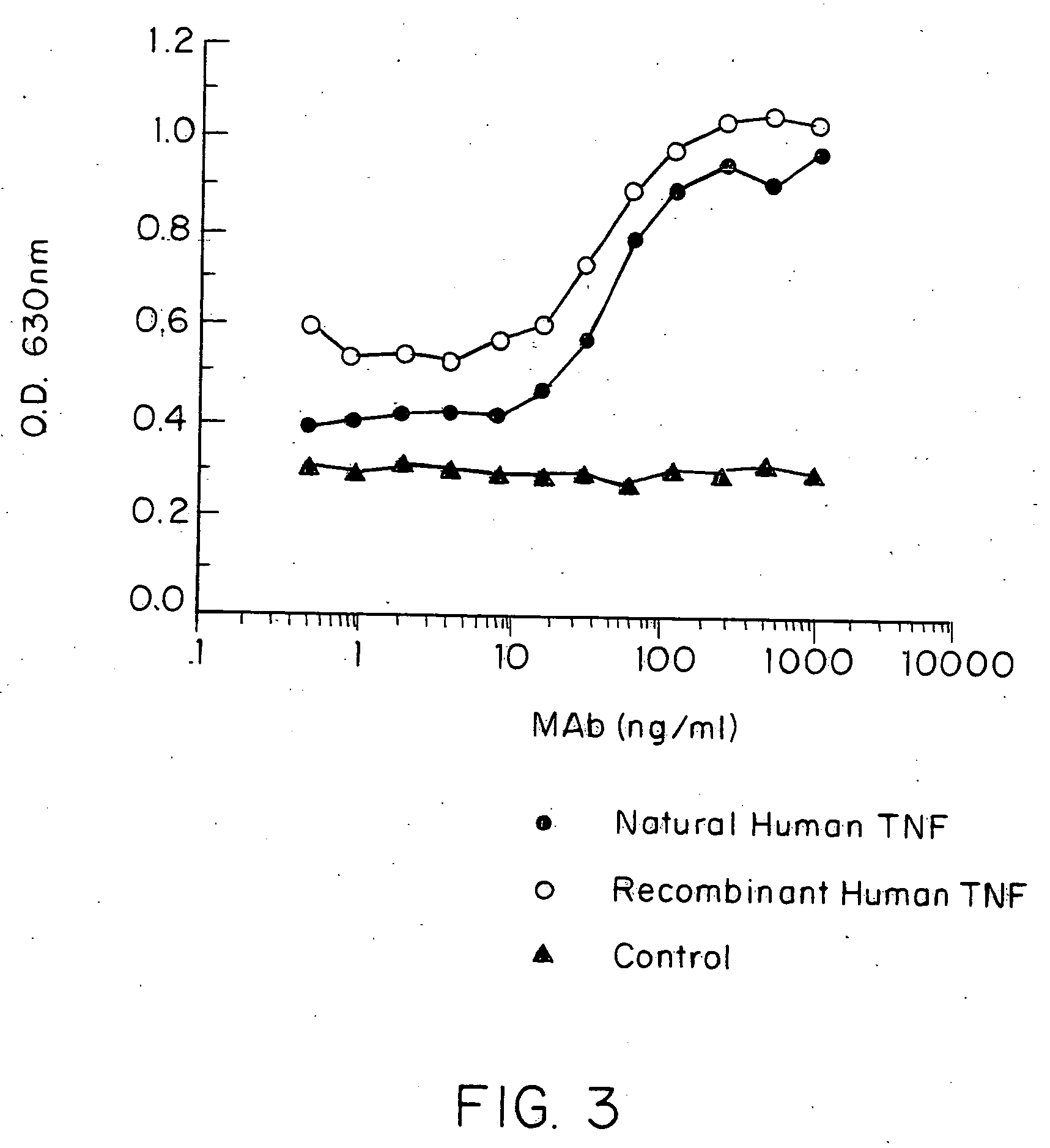
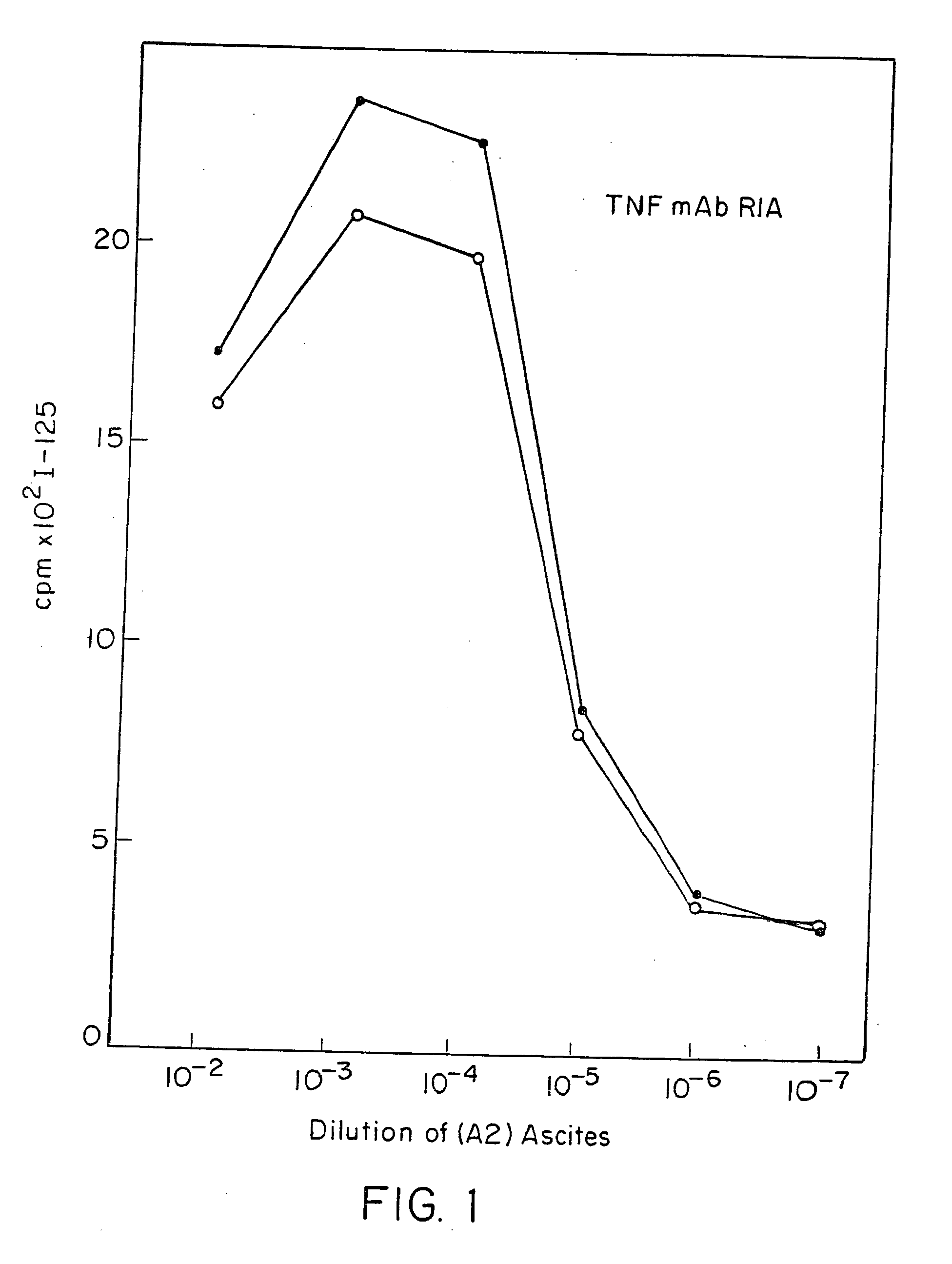
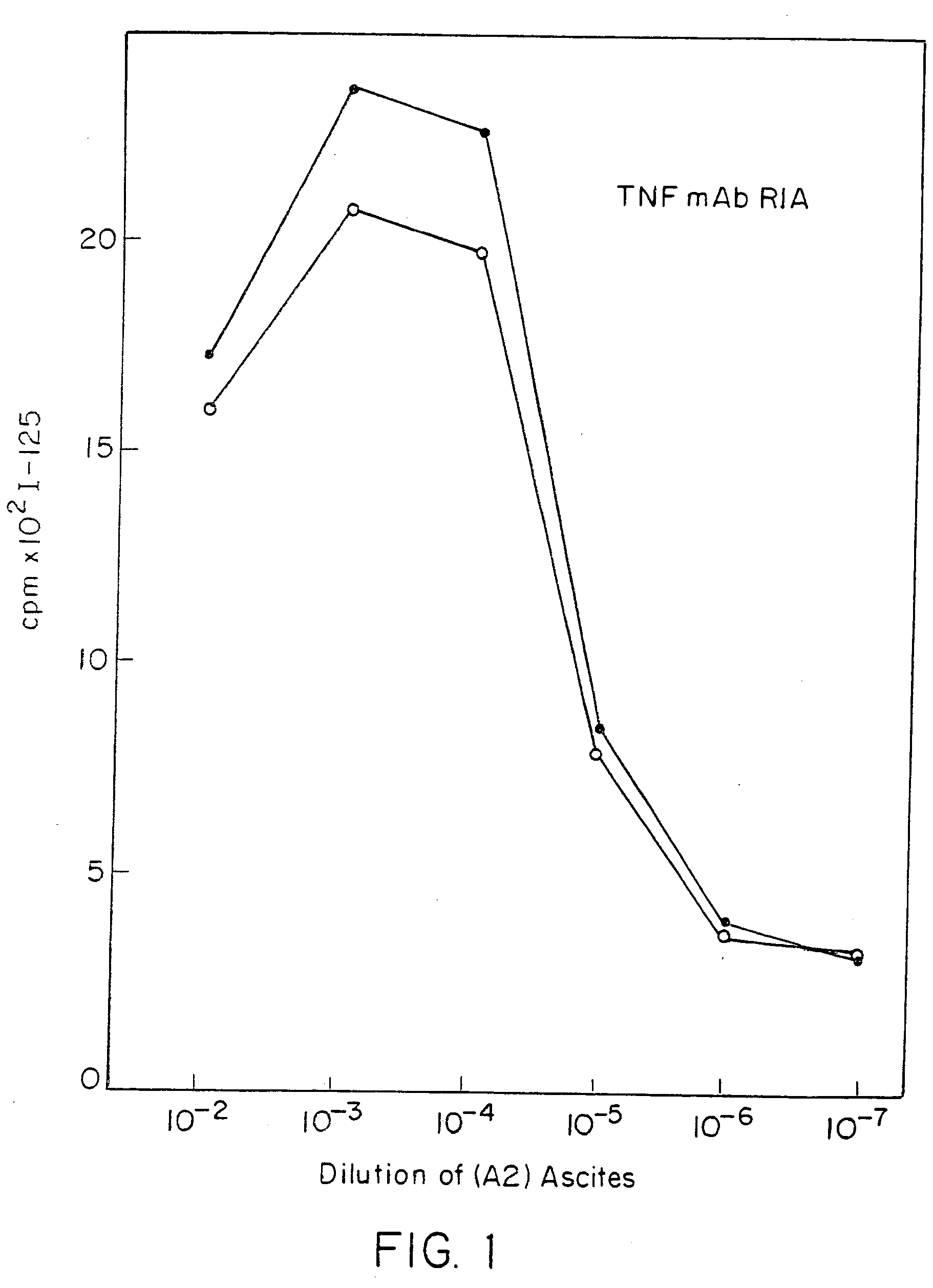
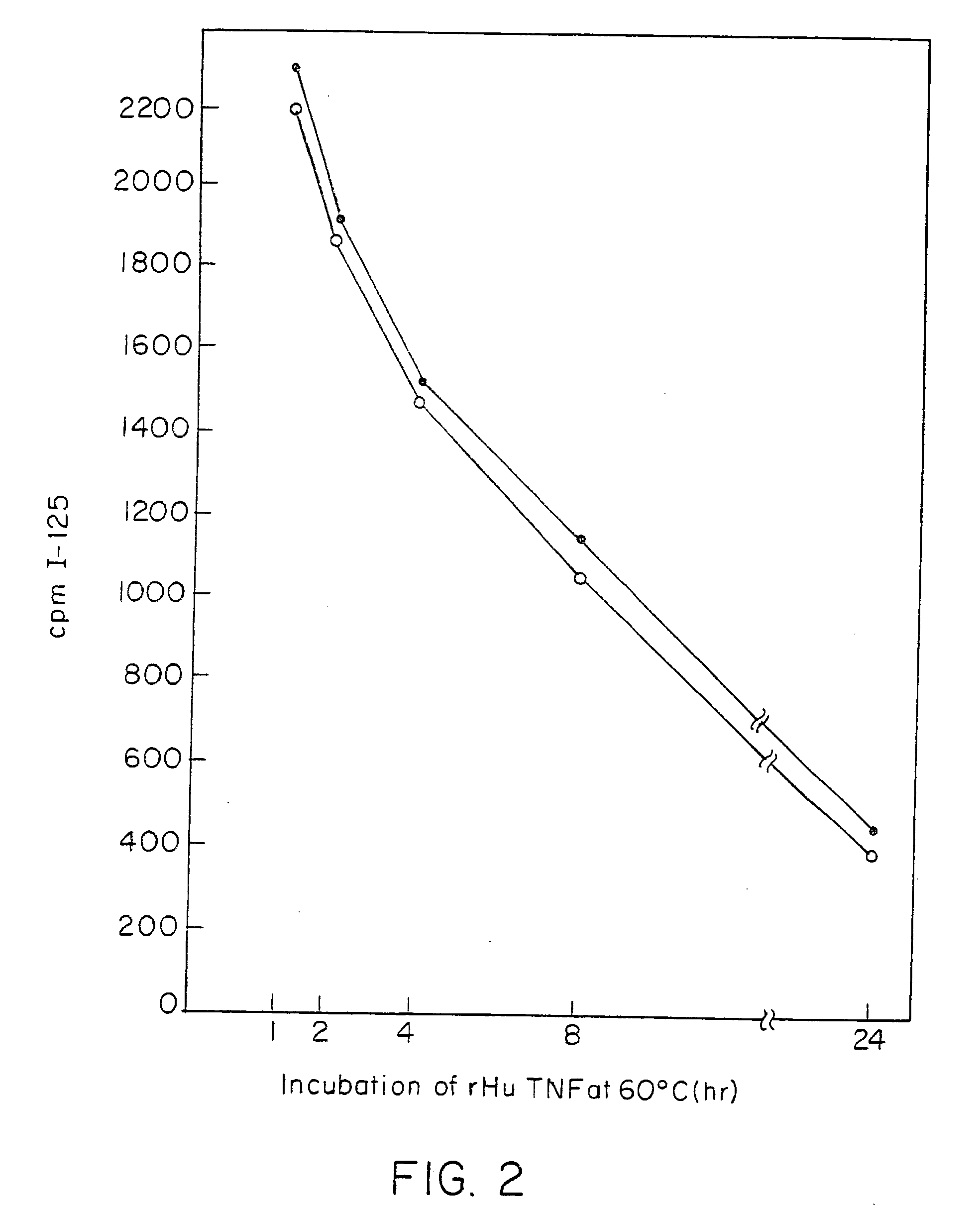
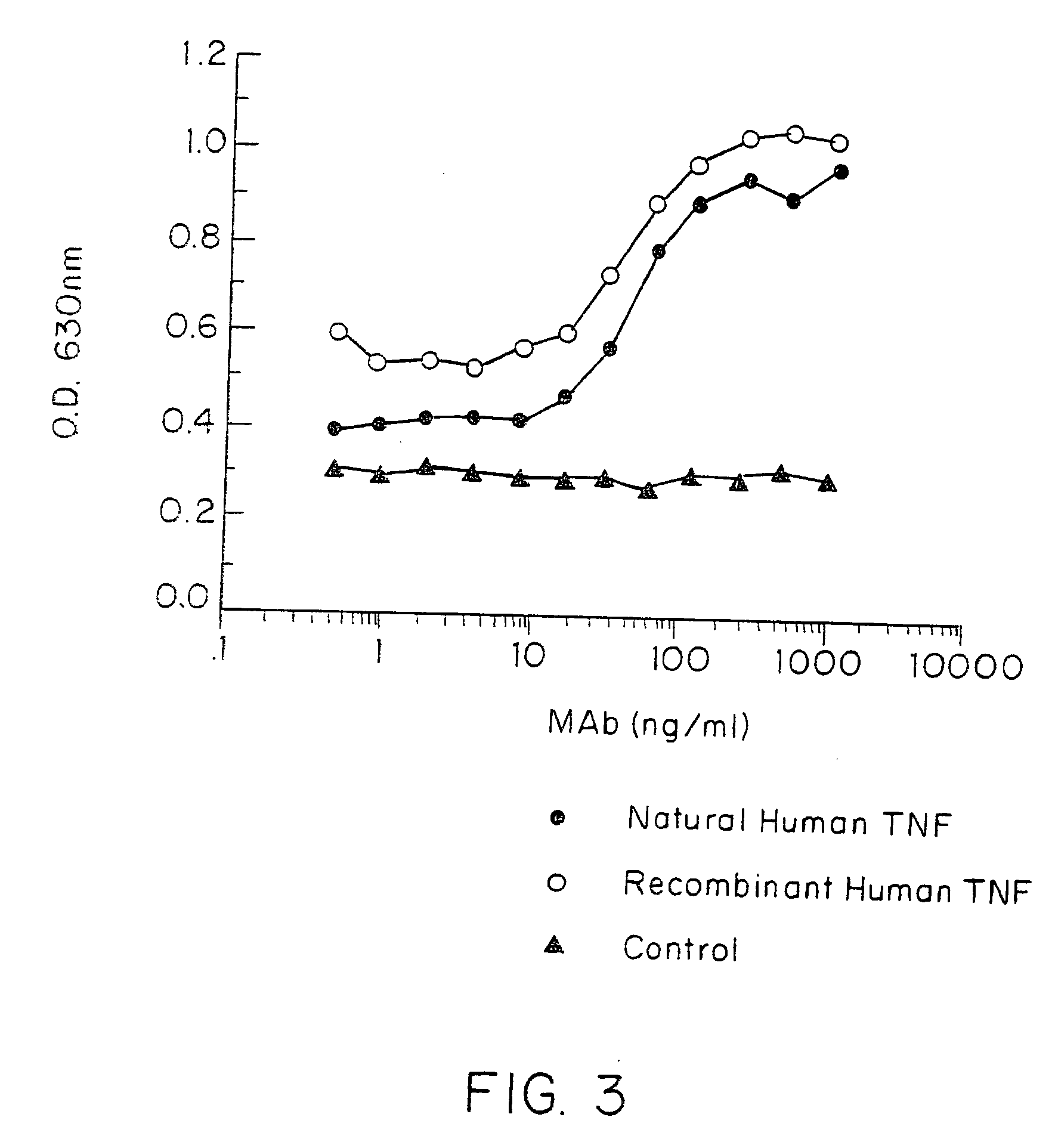
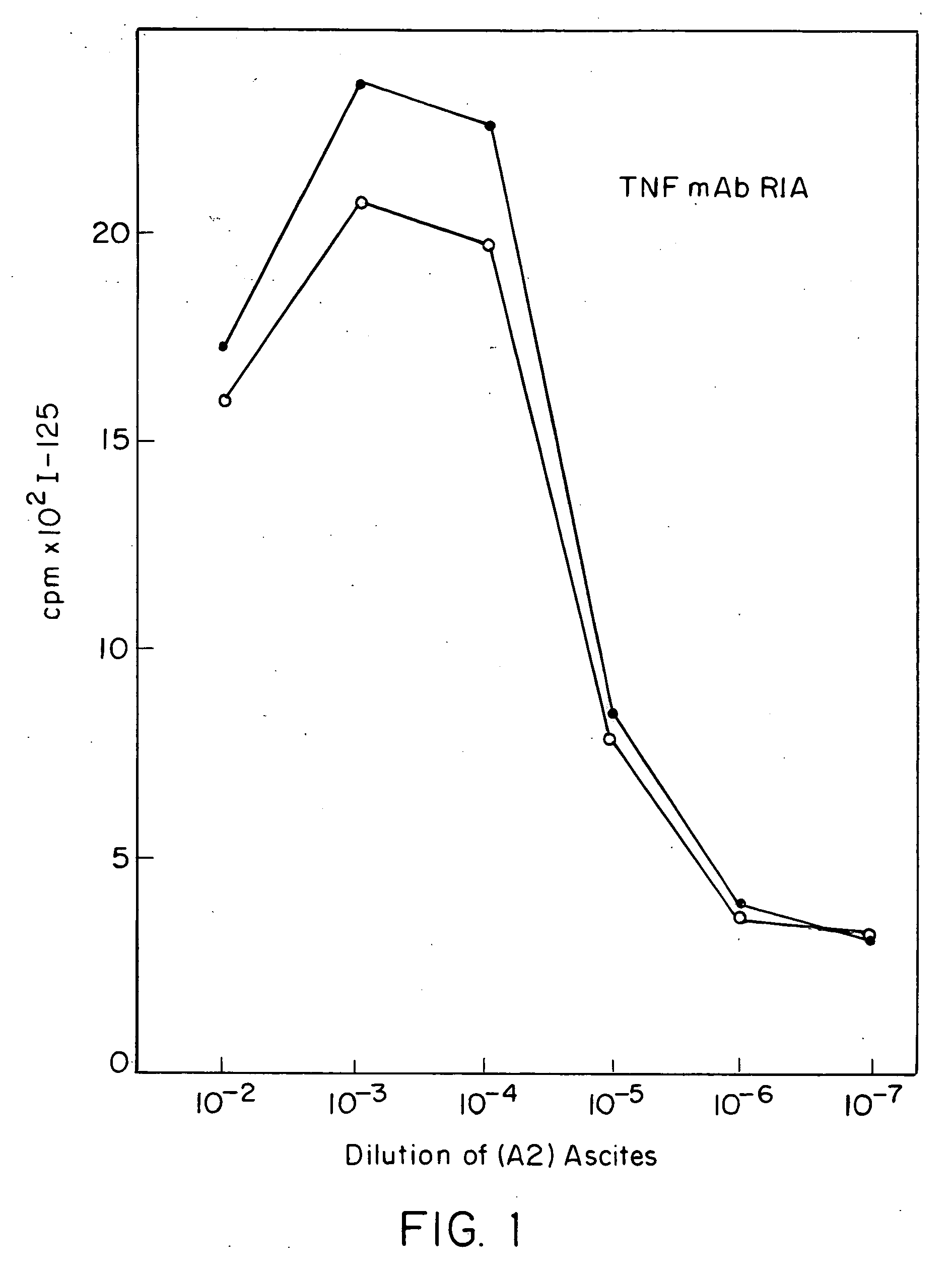
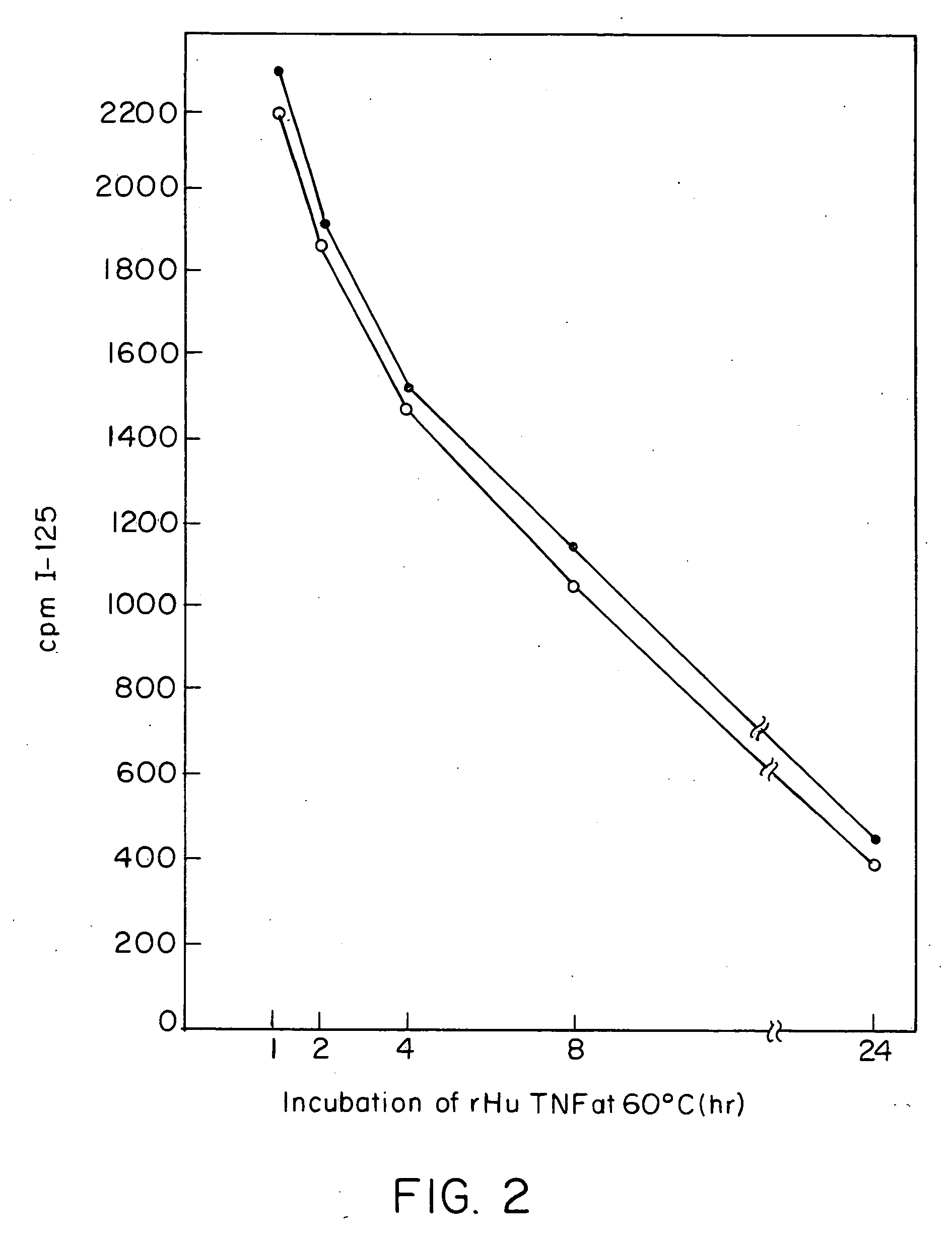
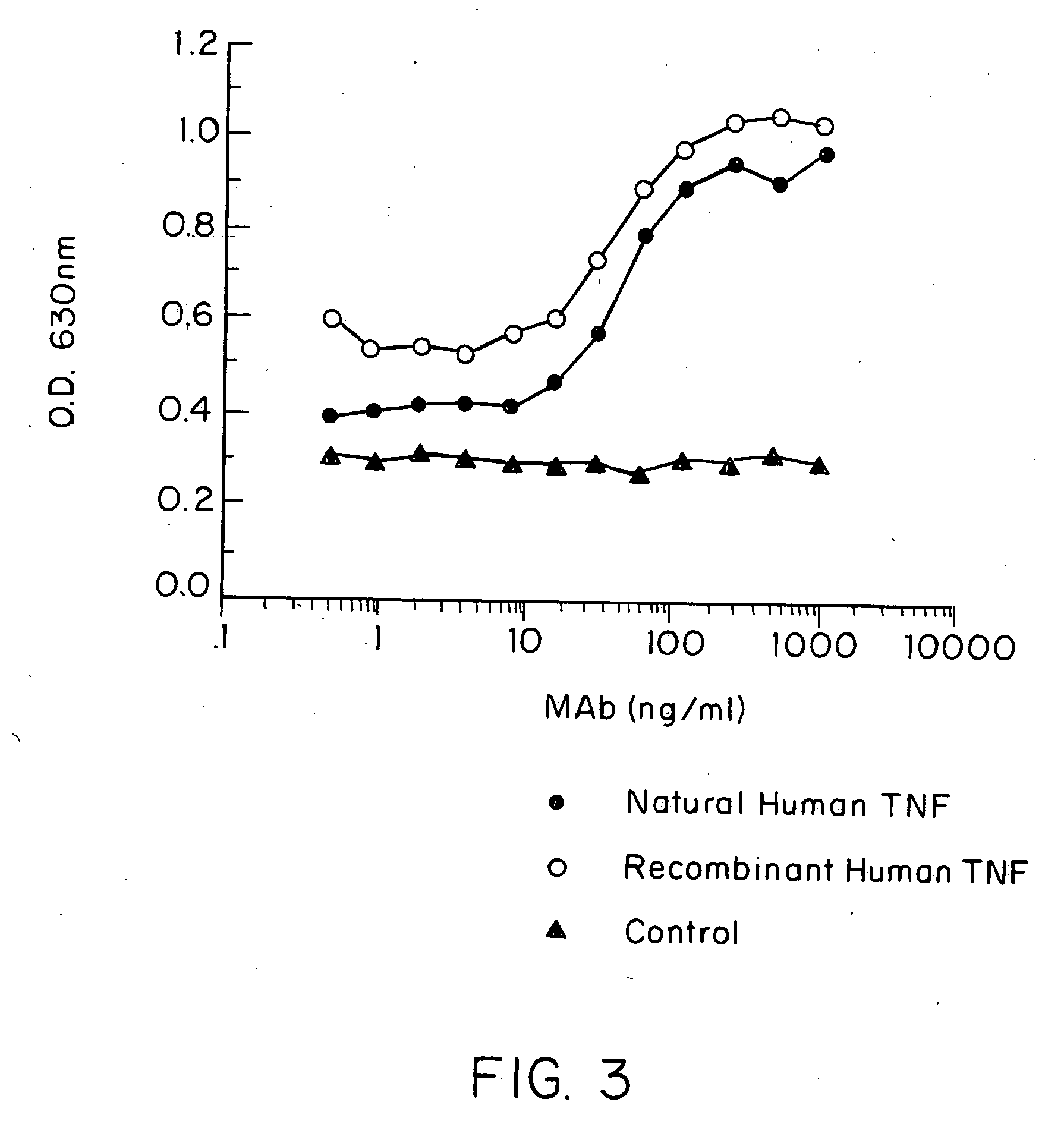
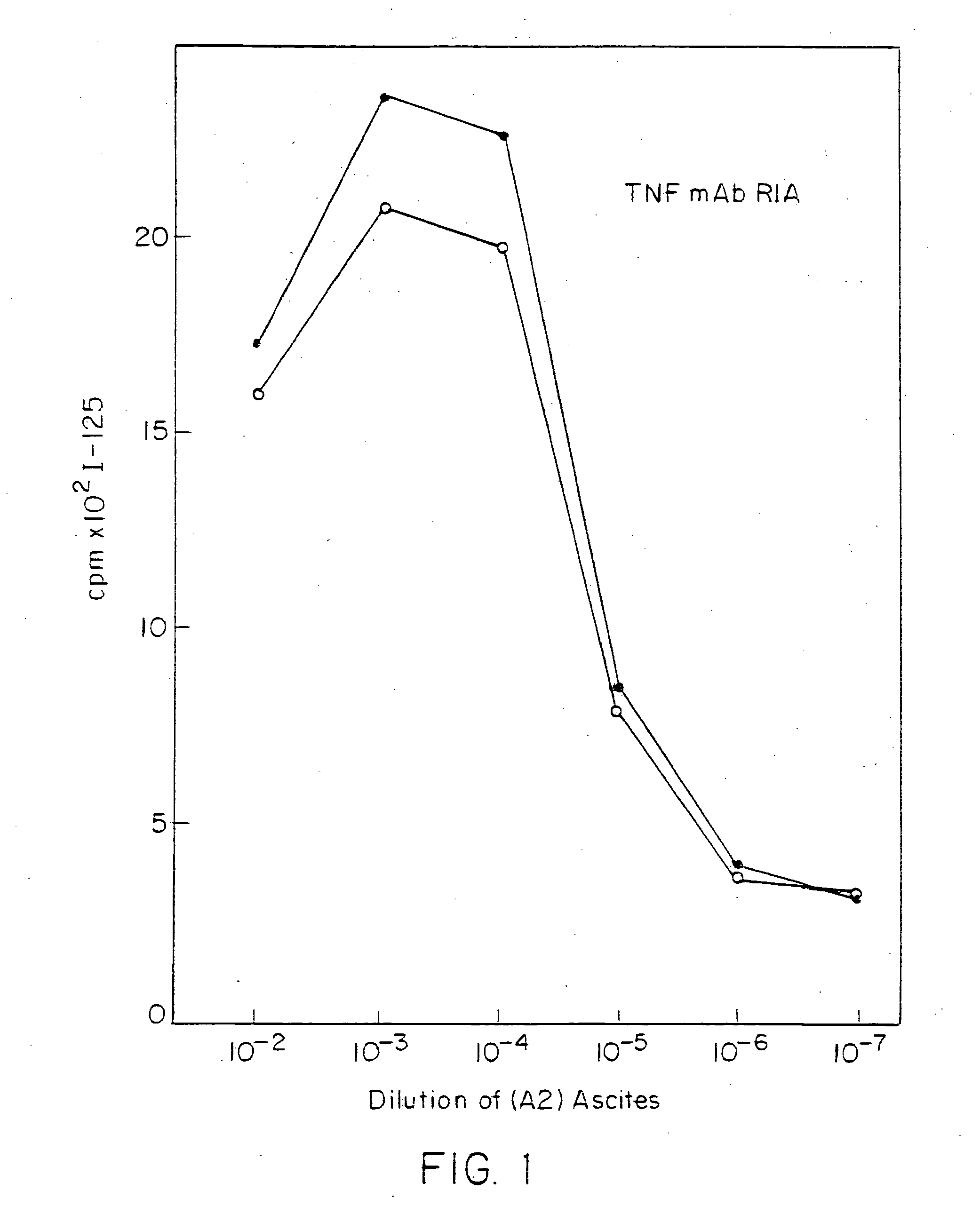
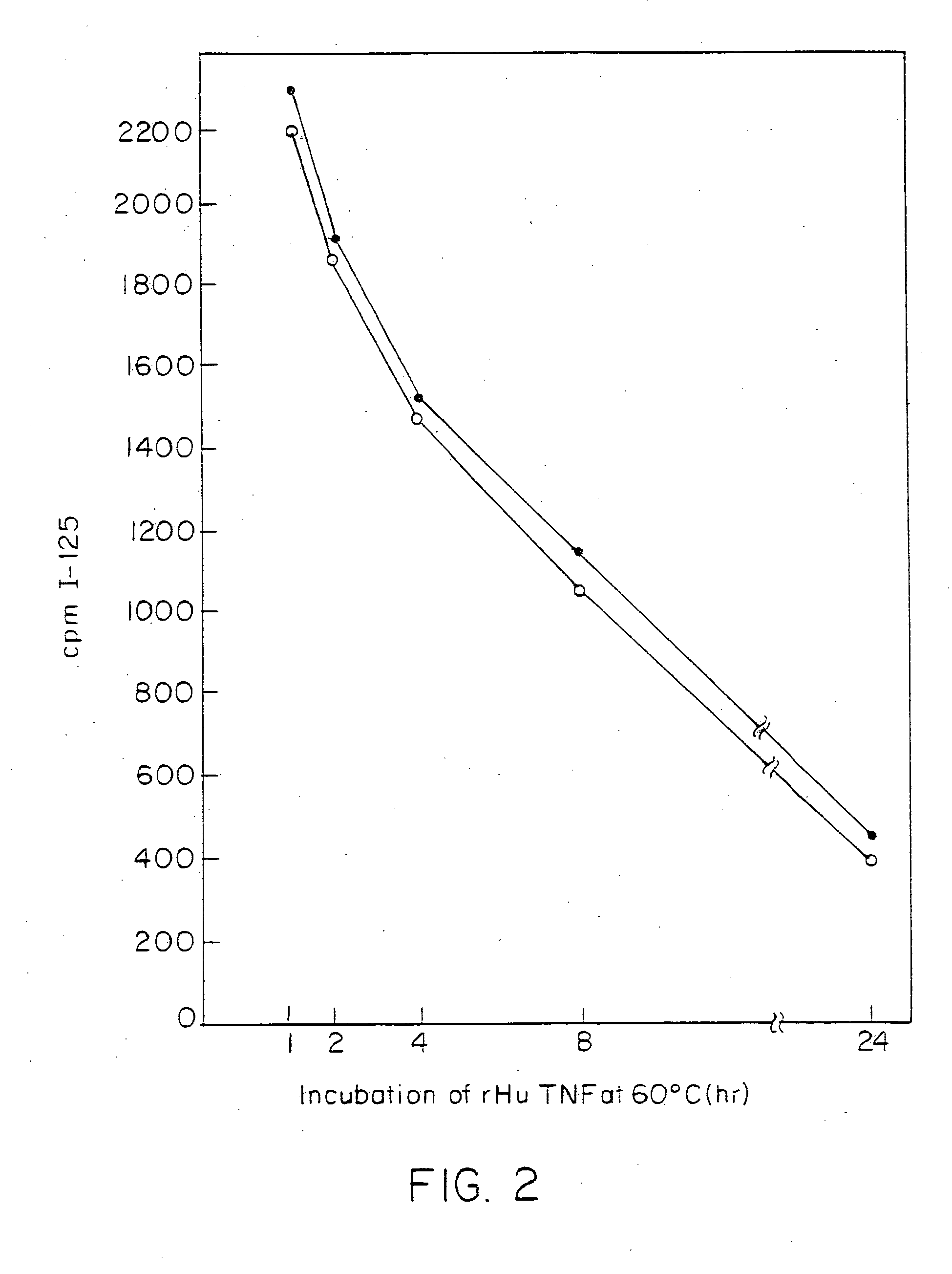
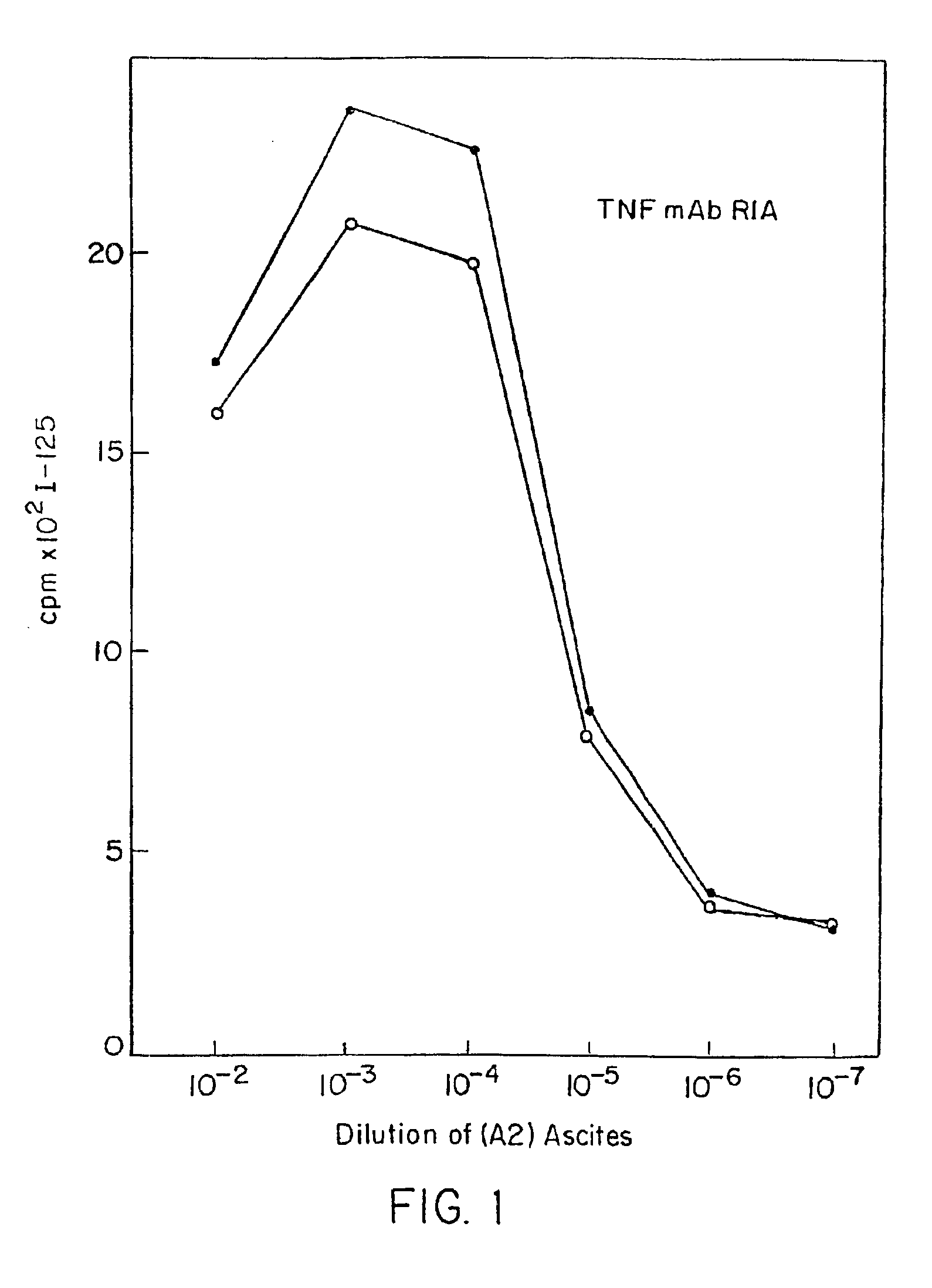
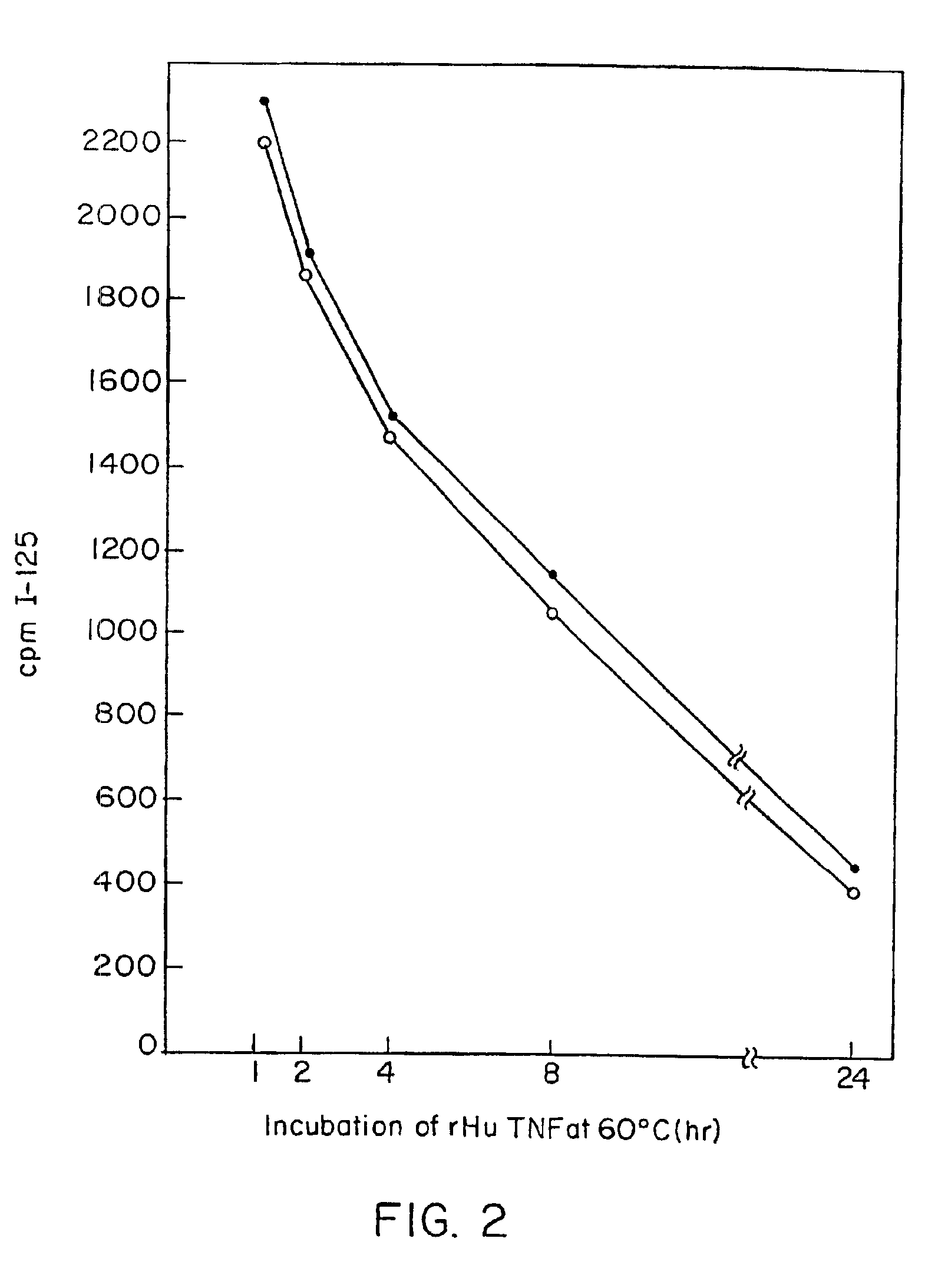
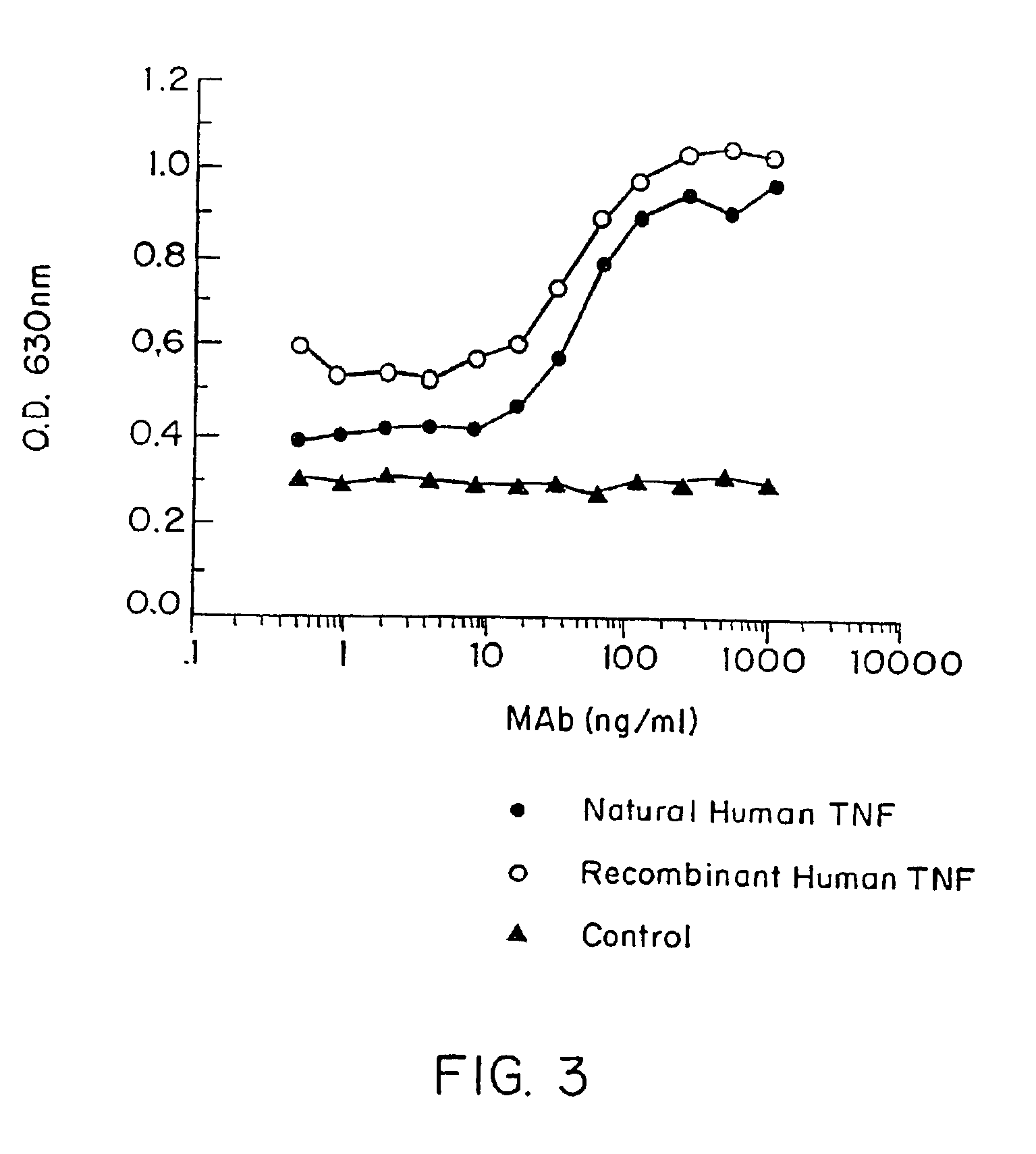
Anti-TNF antibodies and peptides of human tumor necrosis factor
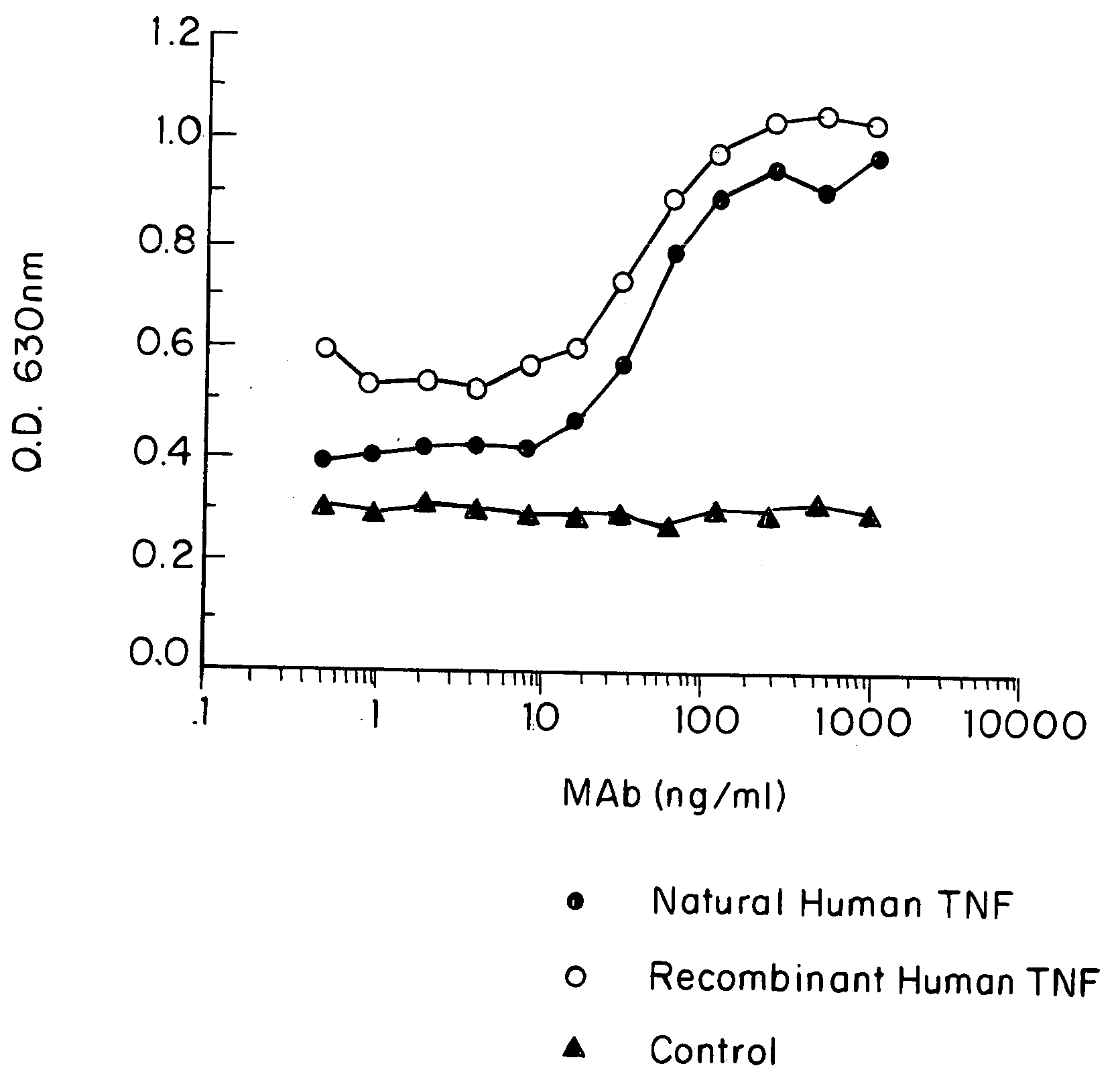
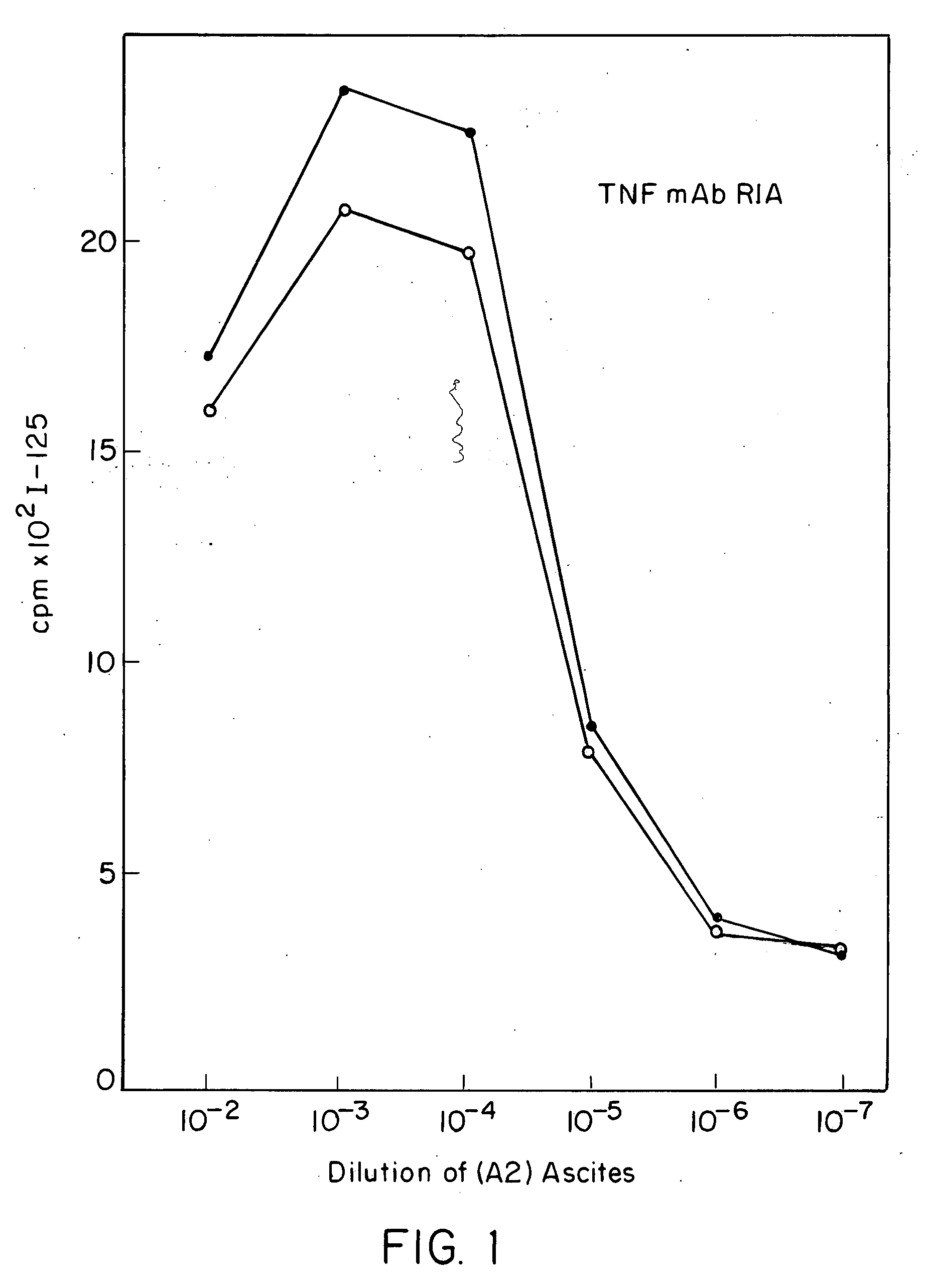
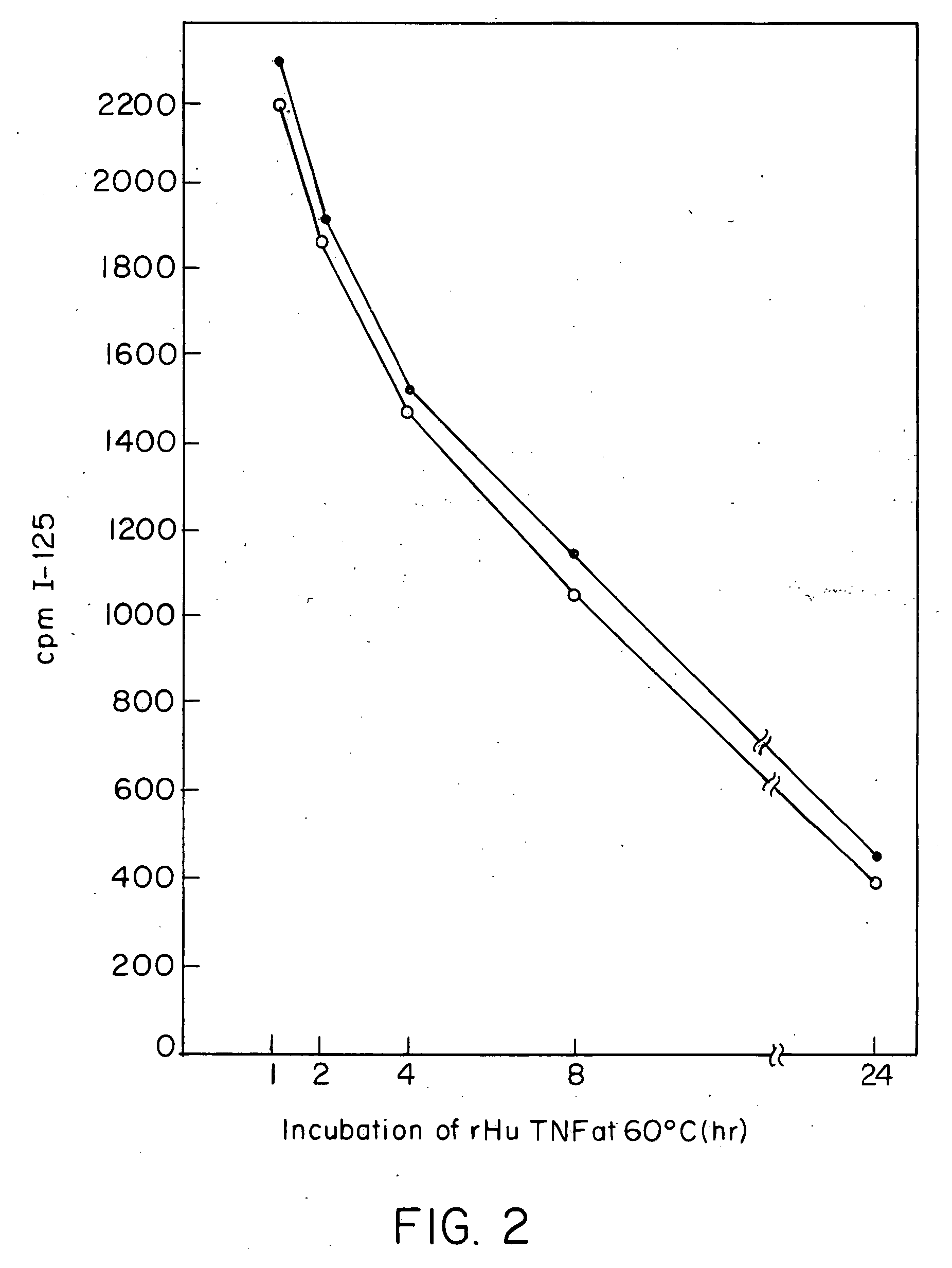
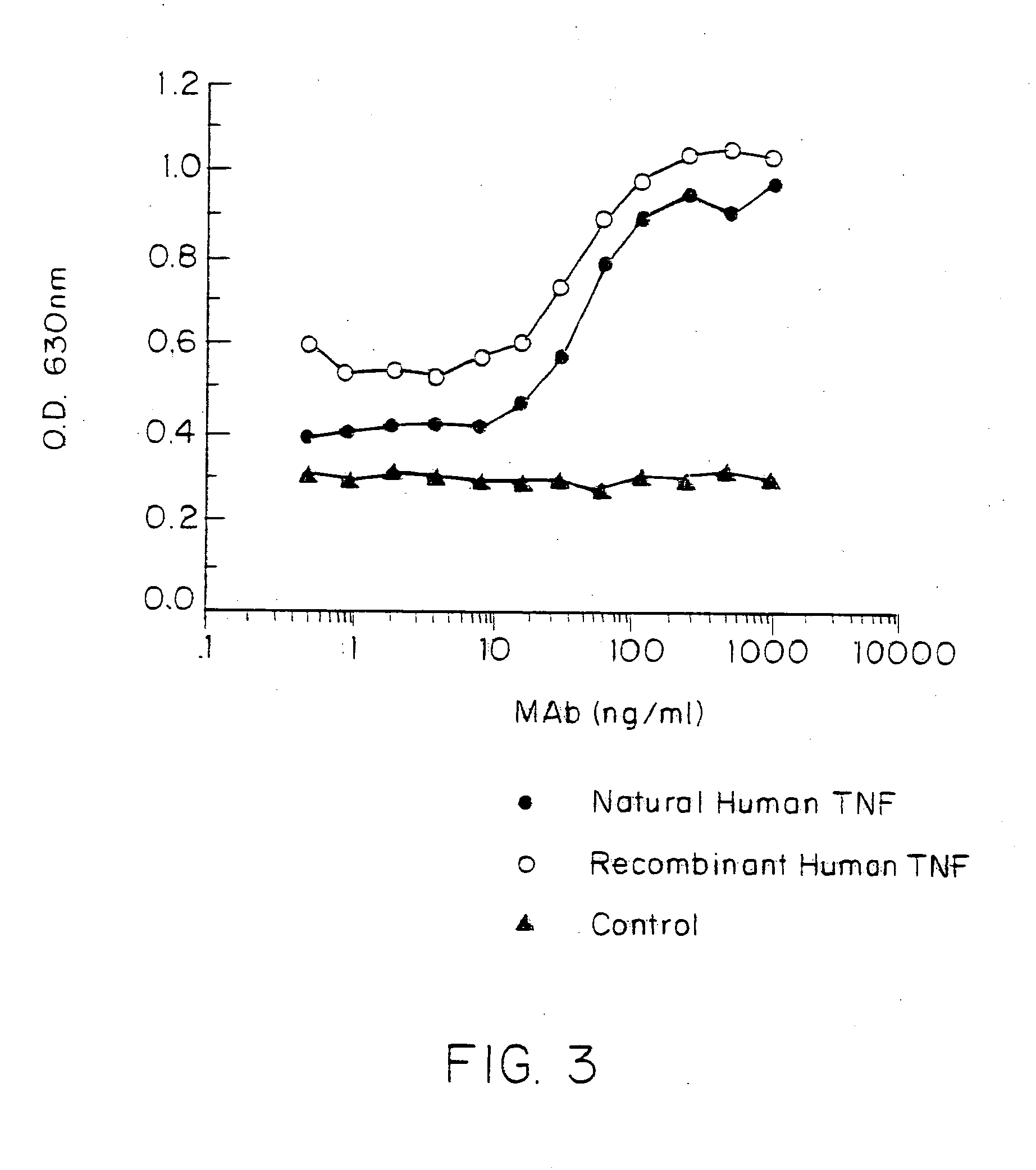
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-alpha (TNFalpha) and are useful in vivo diagnosis and therapy of a number of TNFalpha-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV +1
Recombinant anti-CD30 antibodies and uses thereof
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's Disease cells. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEAGEN INC
Pharmaceutical composition for treatment of diseases caused by IL-6 production
Pharmaceutical compositions for prevention or treatment of diseases caused by interleukin-6 production, comprising an antibody to interleukin-6 receptor (IL-6R antibody). As the IL-6R antibody, an antibody of animals other than the human such as mice, rats, etc., a chimeric antibody between these and a human antibody, a reshaped human antibody, etc. may be used. The pharmaceutical compositions are useful for prevention or treatment of diseases caused by interleukin-6 production such as plasmacytosis, anti-IgGl-emia, anemia, nephritis, etc.
Owner:KISHIMOTO TADAMITSU +2
Bivalent IgY antibody constructs for diagnostic and therapeutic applications
InactiveUS20070141049A1Improve the immunityIncrease contentImmunoglobulins against virusesAntibody ingredientsHeavy chainMammal
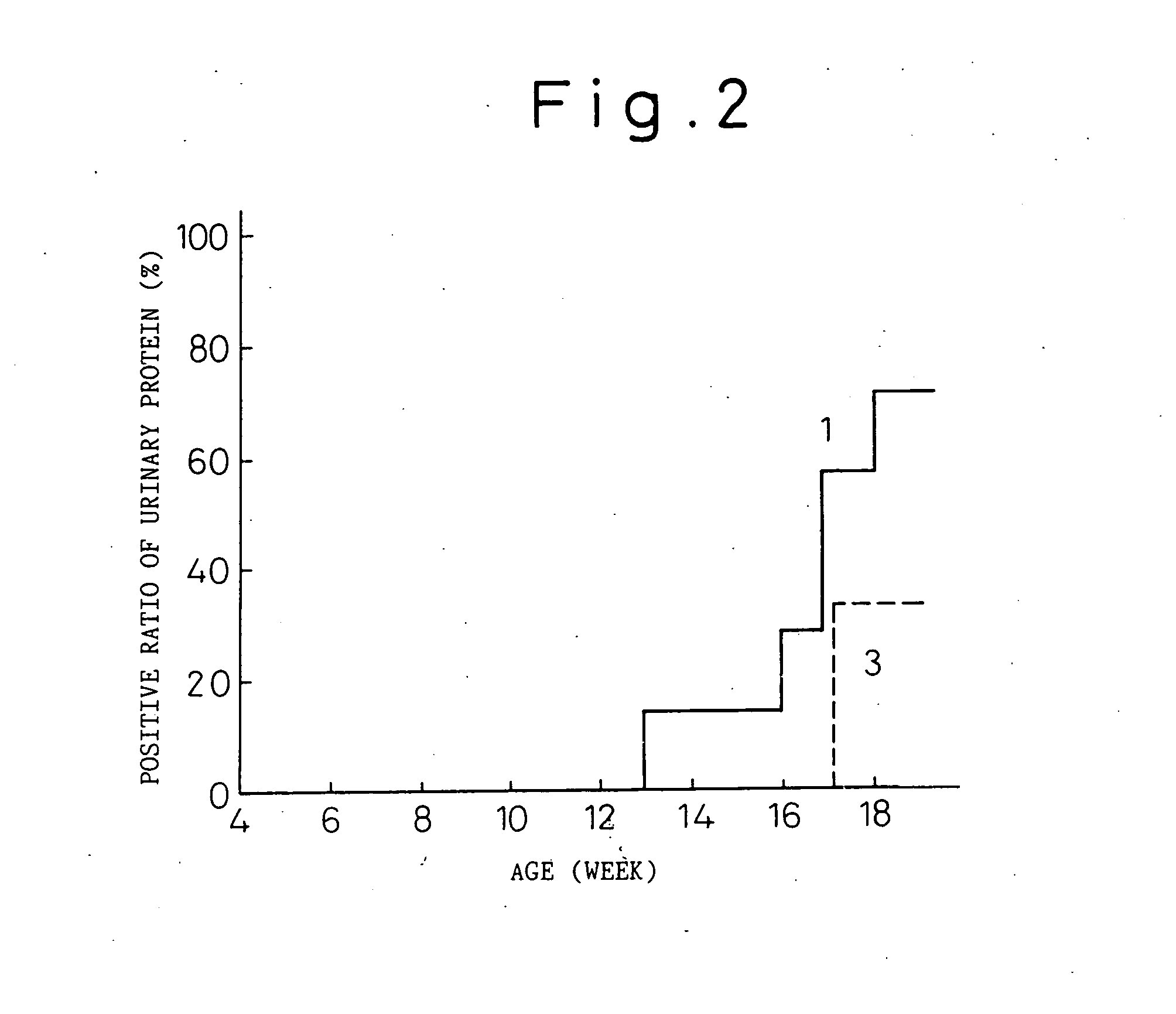
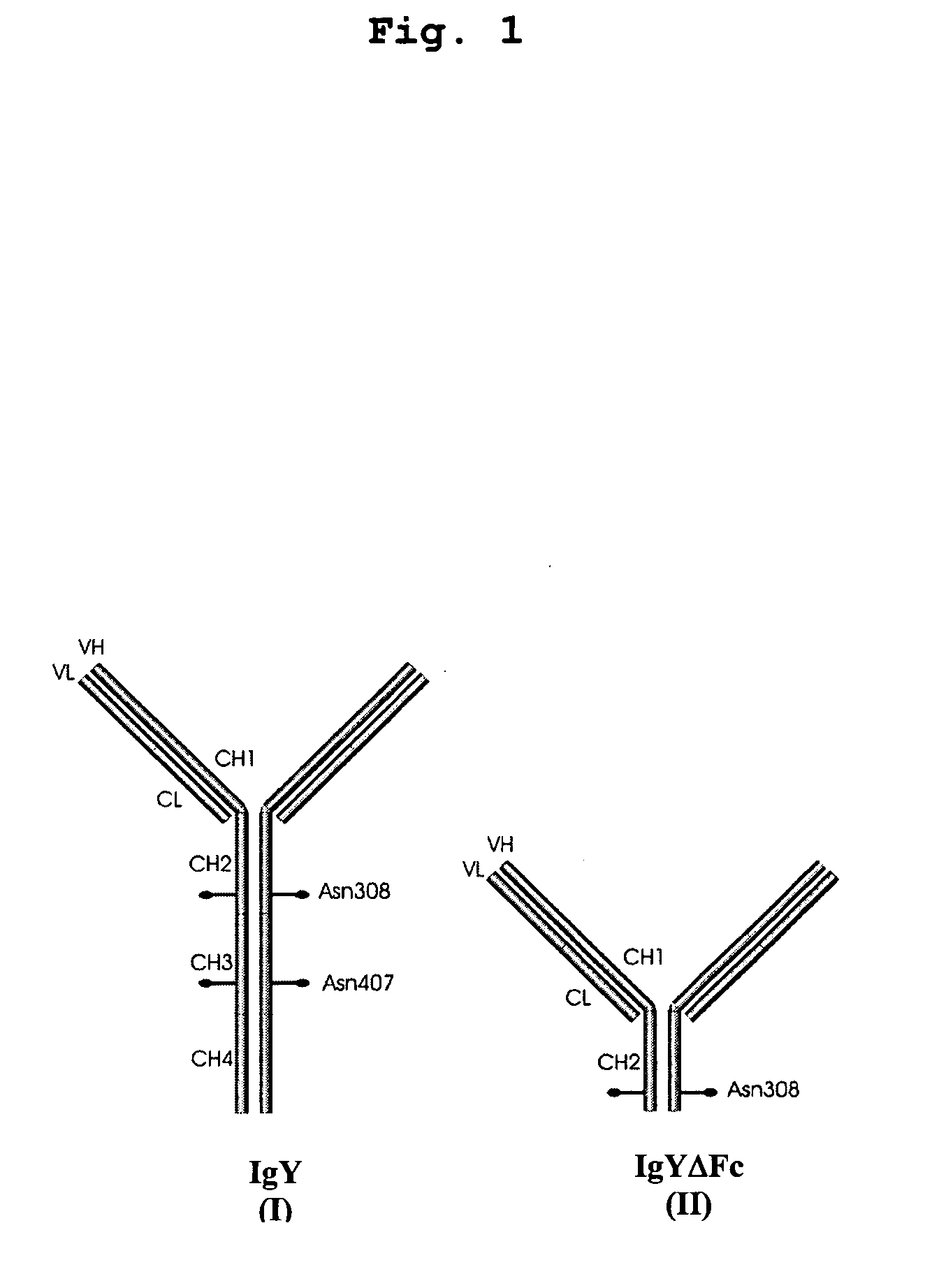
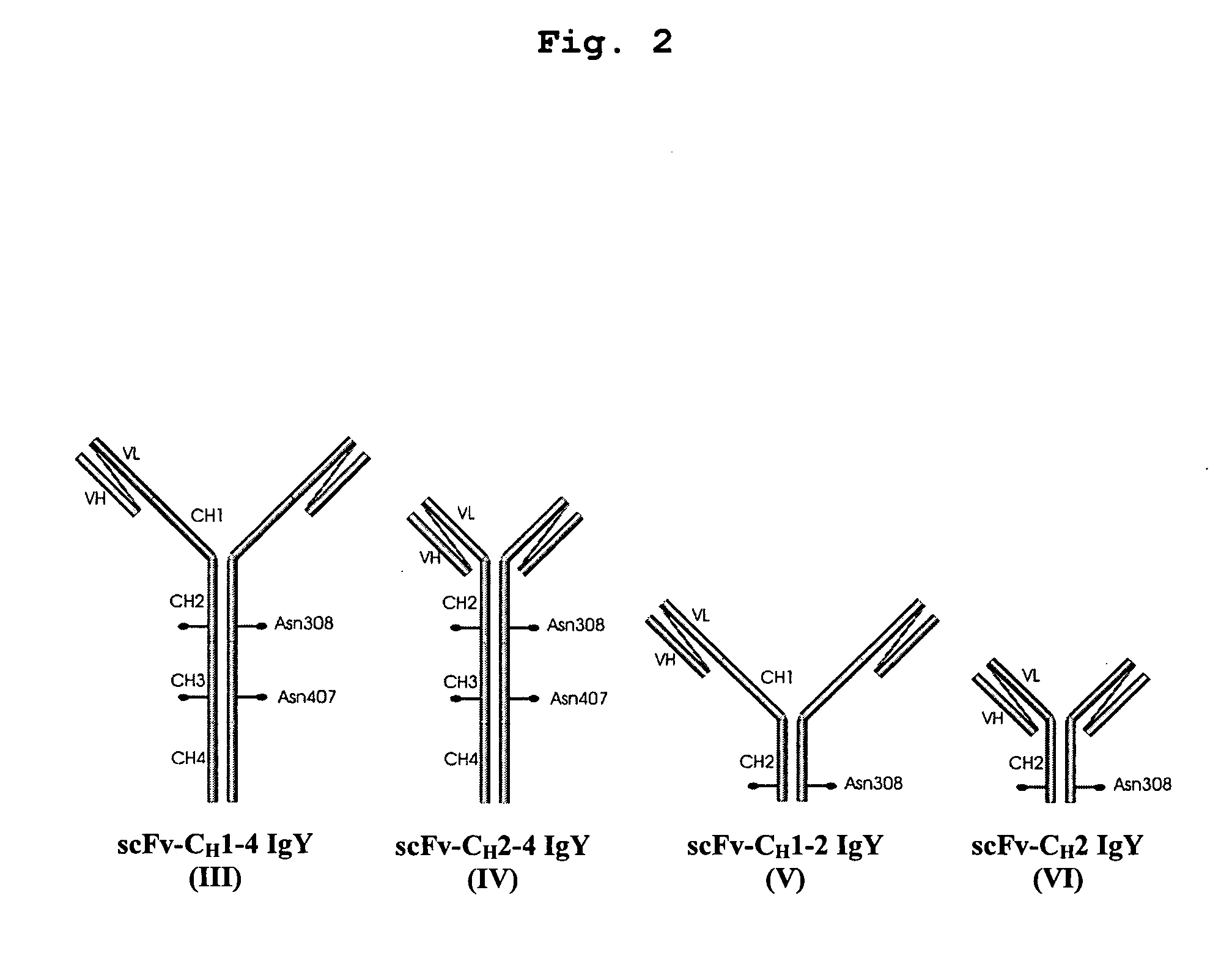
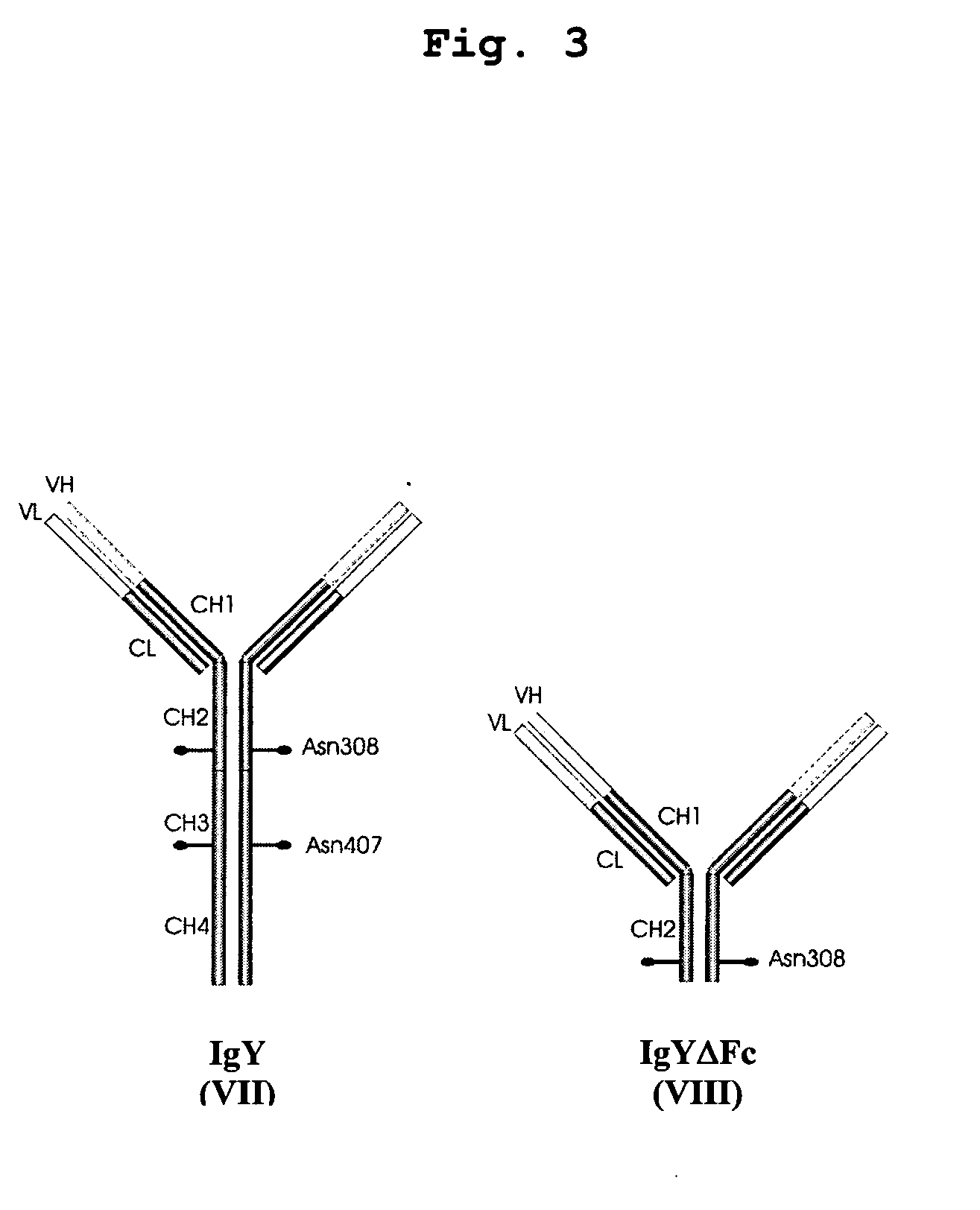
This invention relates to the field of recombinant antibody technology. It provides novel recombinant IgY antibody constructs for diagnostic and therapeutical applications. The bivalent antibody constructs display a heterotetrameric or homodimeric format stabilized by disulfide bonds. The constant heavy chain domains CH2-CH4 are partly or completely of avian origin, whereas the VH, VL, CL, and CH1 domains as well as the hinge region may be of avian origin or derived from any other species. The invention allows to combine the advantages of IgY antibodies with those of established mammalian monoclonal antibodies. IgY antibody constructs comprising nonglycosylated IgY constant heavy chain domains allow to reduce unwanted interactions with C-type lectins, e.g., in human sera. Furthermore, chimeric IgY antibody containing mammalian VH, VL, CL, and CH1 domains as well as a mammalian hinge region provide a higher molecular stability than IgY antibodies in acidic conditions and, thereby, are especially suited for peroral therapeutic applications.
Owner:PLS DESIGN
Anti-TNF antibodies and peptides of human tumor necrosis factor
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:CENTOCOR
Methods of treating ankylosing spondylitis using anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20050249735A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV +1
Recombinant Anti-Cd30 Antibodies and Uses Thereof
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's disease cells in the absence of effector cells or complement. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEATTLE GENETICS INC
Methods of treating ankylosing spondylitis using anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20080025976A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:LE JUNMING +6
Anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20060018907A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV +1
Methods of treating seronegative arthropathy with anti-TNF antibodies
InactiveUS20070298040A1Inhibit biological activityHigh affinityAntibody ingredientsImmunoglobulinsSeronegative arthropathyHuman tumor
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFαx-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV
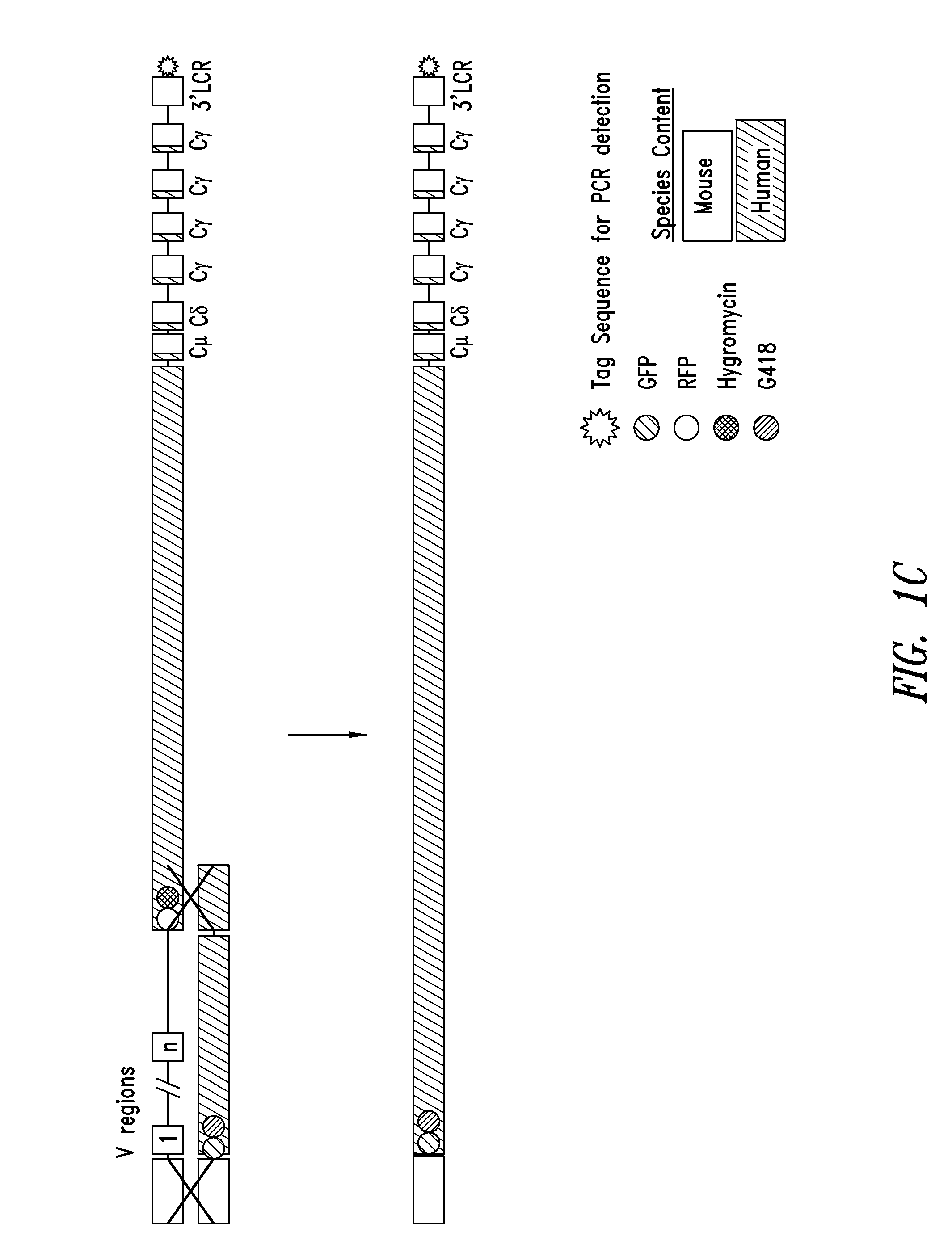
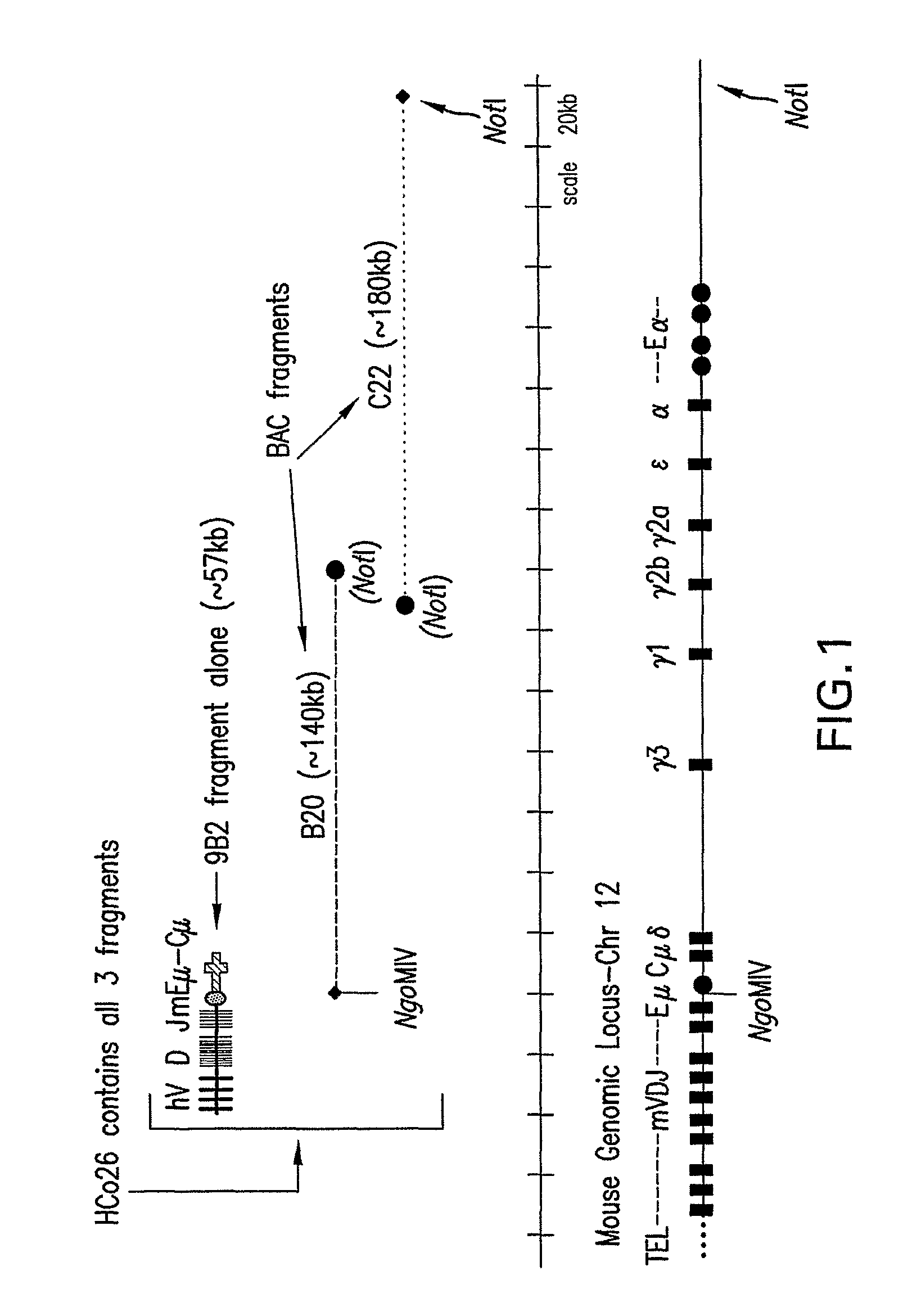
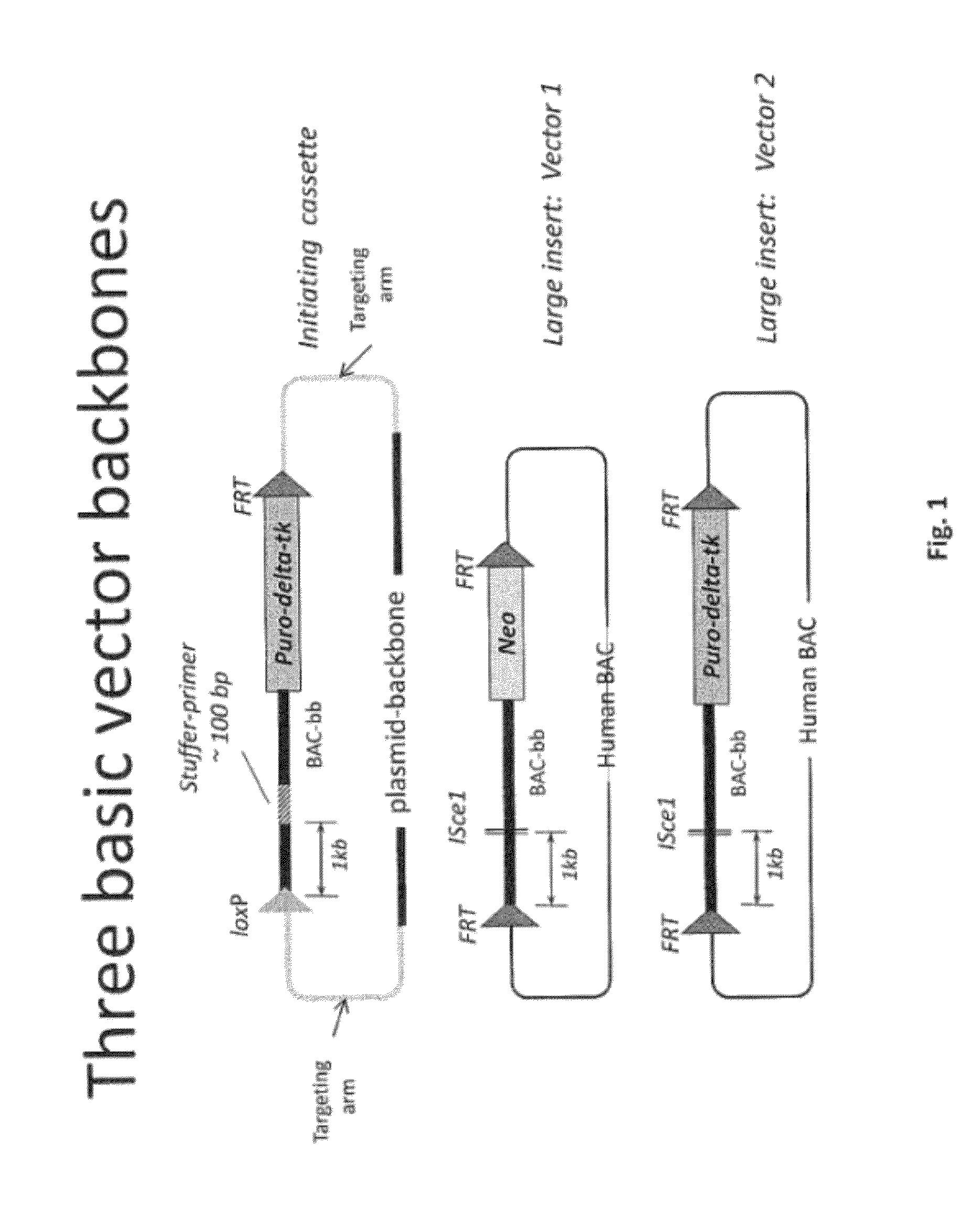
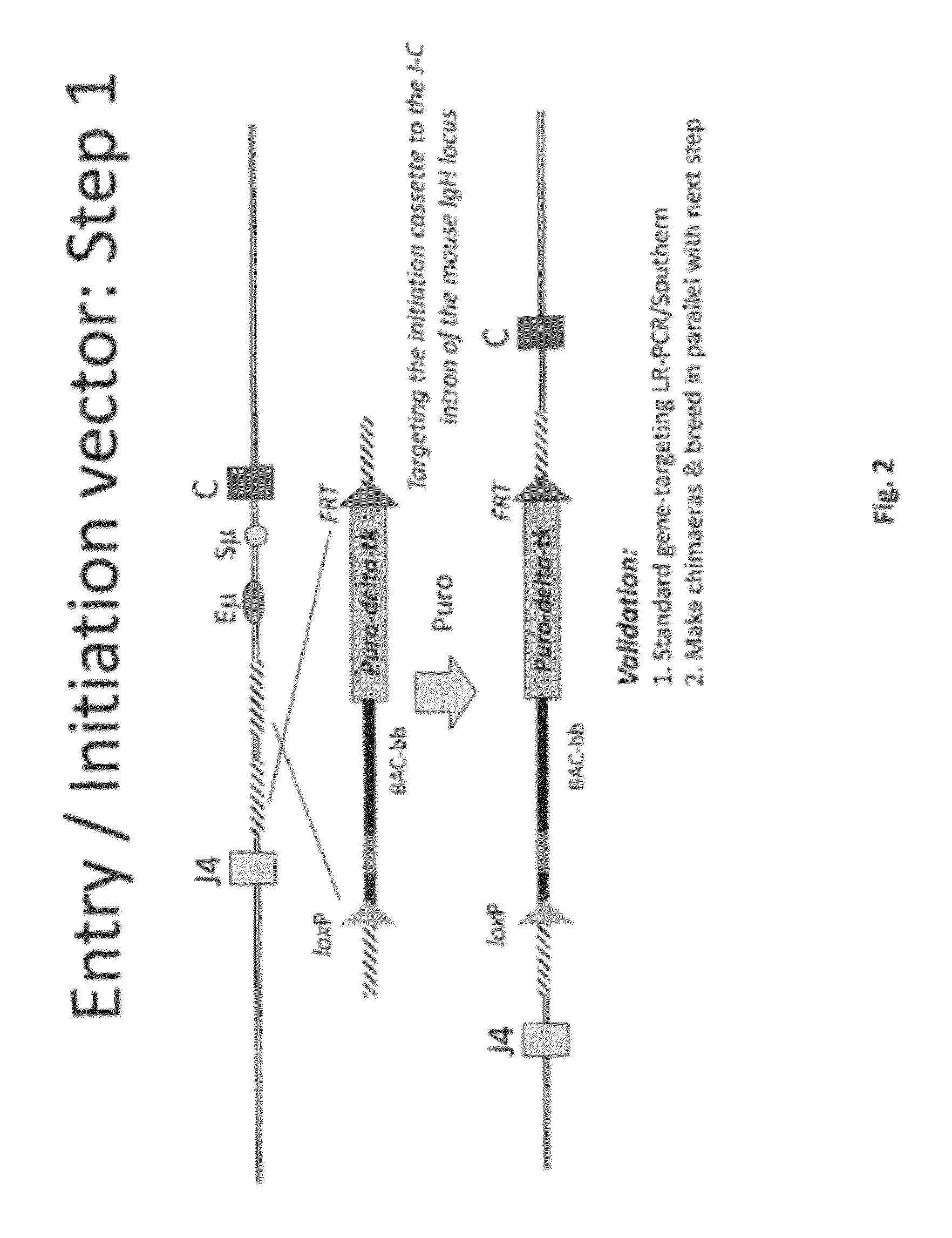
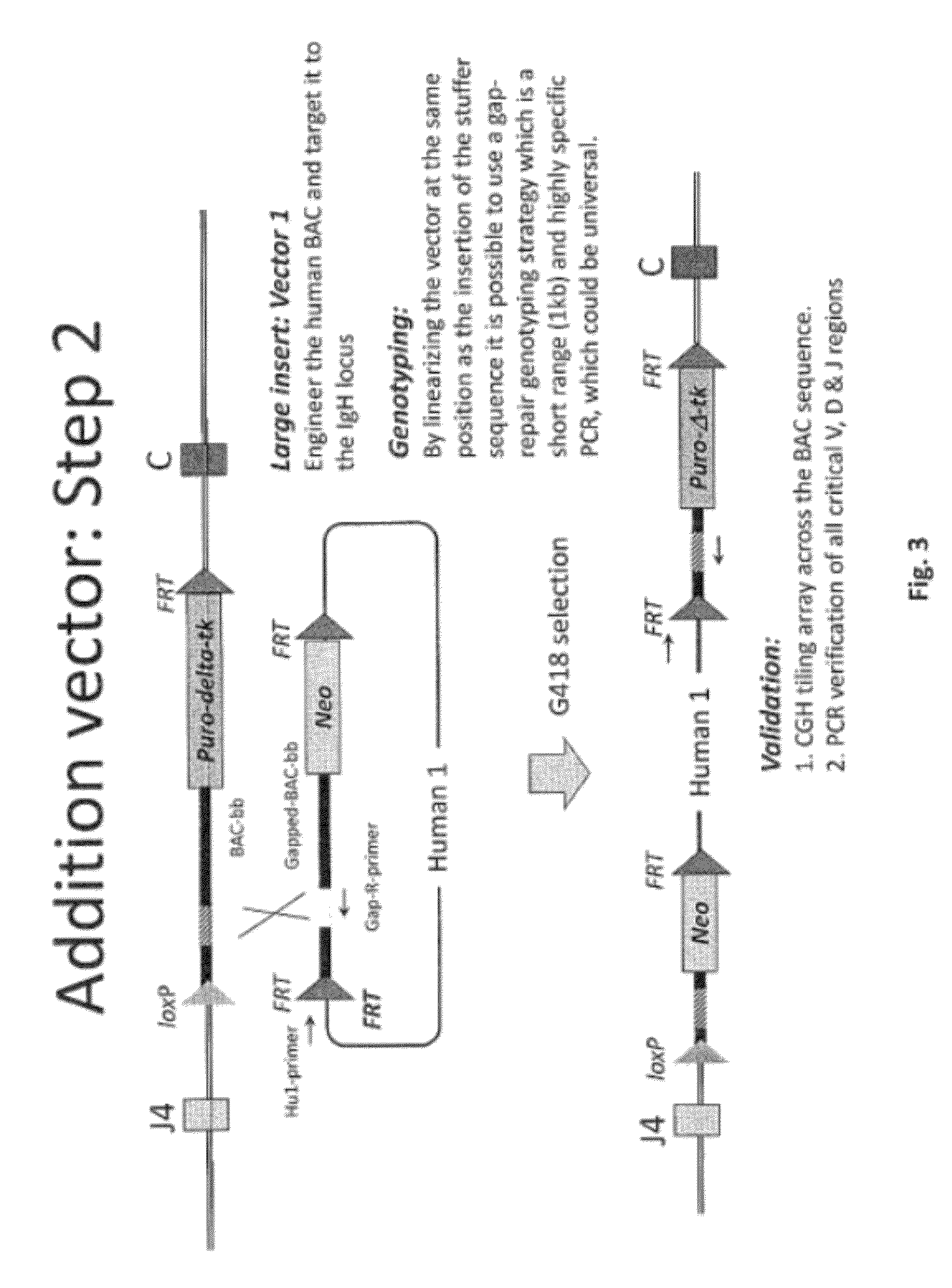
Non-human mammals for the production of chimeric antibodies
ActiveUS20110236378A1Improve representationEfficiently rearrangedPeptide librariesSugar derivativesMammalHumanized antibody
The invention provides knock-in non-human cells and mammals having a genome encoding chimeric antibodies and methods of producing knock-in cells and mammals. Certain aspects of the invention include chimeric antibodies, humanized antibodies, pharmaceutical compositions and kits. Certain aspects of the invention also relate to diagnostic and treatment methods using the antibodies of the invention.
Owner:ABLEXIS LLC
Transgenic animals expressing chimeric antibodies for use in preparing human antibodies
ActiveUS7910798B2Improved trafficking developmentStrengthen associationSugar derivativesImmunoglobulins against cytokines/lymphokines/interferonsTransgenesisIn vivo
The invention provides transgene constructs for expressing chimeric antibodies, and transgenic non-human host animals carrying such constructs, wherein the chimeric antibodies comprise human variable regions and constant regions of the non-human transgenic host animal. The presence of immunoglobulin constant regions of the host animal allows for generation of improved antibodies in such transgenic host animals. Subsequently, the chimeric antibodies can be readily converted to fully human antibodies using recombinant DNA techniques. Thus, the invention provides compositions and methods for generating human antibodies in which chimeric antibodies raised in vivo in transgenic mice are used as intermediates and then converted to fully human antibodies in vitro.
Owner:ER SQUIBB & SONS INC
Anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20070196373A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:LE JUNMING +6
Transgenic non-human animals for producing heterologous and chimeric antibodies
The invention relates to transgenic non-human animals capable of producing heterologous antibodies and methods for producing human sequence antibodies which bind to human antigens with substantial affinity.
Owner:GENPHARM INT INC
Animal models and therapeutic molecules
The invention discloses methods for the generation of chimaeric human-non-human antibodies and chimaeric antibody chains, antibodies and antibody chains so produced, and derivatives thereof including fully humanised antibodies; compositions comprising said antibodies, antibody chains and derivatives, as well as cells, non-human mammals and vectors, suitable for use in said methods.
Owner:KIMAB LTD
Expression of heterologous multi-domain proteins in yeast
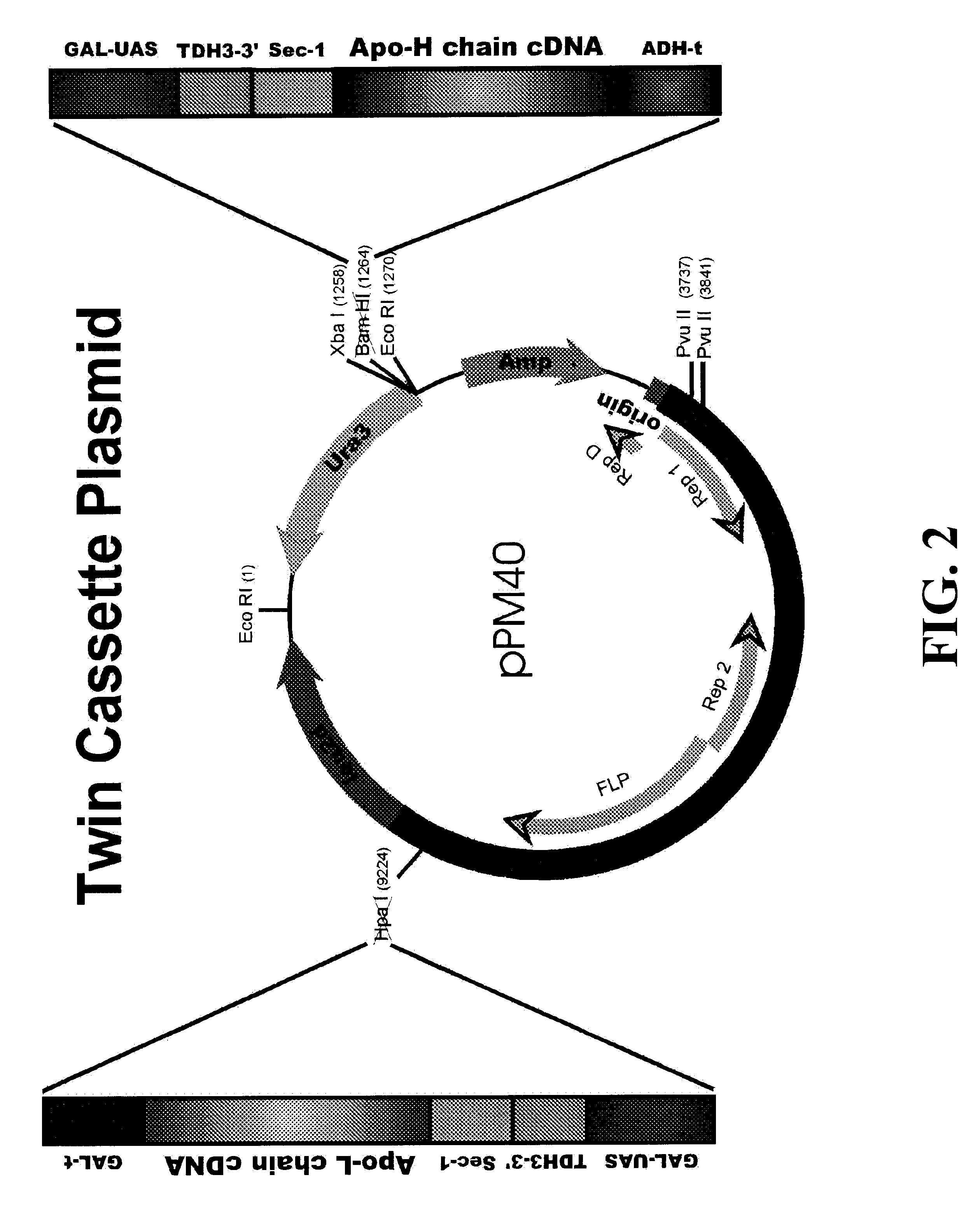
InactiveUS6358733B1Increase productionCost effective productionSugar derivativesAntibody mimetics/scaffoldsYeastSingle-Chain Antibodies
This invention demonstrates the utility of a yeast expression system for the expression of functional heterologous multi-domain proteins in yeast. The yeast expression system allows for the inclusion of a plurality of (up to three) modular expression cassettes which may encode multiple polypeptide chains of a heterologous multi-domain protein on a single plasmid (Twin Cassette). Because multiple polypeptide chains may be encoded for by the expression cassettes of the present invention in a single vector, the system can produce equivalent amounts of the multiple polypeptide chains, thereby enhancing the yield of a functional heterologous multi-domain protein. For example, functional monoclonal antibodies (MAbs) comprising a heavy chain and a light chain of an immunoglobulin (IgG), and functional immunotoxins comprising an antibody domain and an oxidase toxin may be produced using the Yeast expression system of the present invention. In addition, functional single chain antibodies, antibody fragments and chimeric antibodies may also be produced.
Owner:APOLIFE
Recombinant Anti-cd30 antibodies and uses thereof
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's disease cells in the absence of effector cells or complement. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEATTLE GENETICS INC
Animal models and therapeutic molecules
The invention discloses methods for the generation of chimaeric human—non-human antibodies and chimaeric antibody chains, antibodies and antibody chains so produced, and derivatives thereof including fully humanised antibodies; compositions comprising said antibodies, antibody chains and derivatives, as well as cells, non-human mammals and vectors, suitable for use in said methods.
Owner:KIMAB LTD
Human chimeric antibody specific for the ganglioside GD3
InactiveUS6437098B1Easy to produceEasy constructionPeptide/protein ingredientsAntibody mimetics/scaffoldsGanglioside GD3Chimeric antibody
A humanized chimera antibody, a pharmaceutical composition comprising a humanized chimera antibody and a pharmaceutically acceptable carrier, and a method of treating cancer which comprises administering to a patient a pharmaceutically acceptable amount of the humanized chimera antibody, are disclosed.
Owner:KYOWA HAKKO KIRIN CO LTD
Antibodies specific to KDR and uses thereof
InactiveUS20050214860A1Inhibit tumor growthCompound screeningVirusesSingle-Chain AntibodiesAngiogenesis growth factor
The invention provides an immunoglobulin molecule which binds KDR with an affinity comparable to human VEGF, and that neutralizes activation of KDR. Immunoglobulin molecules include monovalent single chain antibodies, multivalent single chain antibodies, diabodies, triabodies, antibodies, humanized antibodies and chimerized antibodies. The invention further provides nucleic acid molecules that encode these immunoglobulin molecules. The invention also provides a method of making the immunoglobulin molecules mentioned above. The invention further provides a method of neutralizing the activation of KDR, a method of inhibiting angiogenesis in a mammal and a method of inhibiting tumor growth in a mammal with such immunoglobulin molecules.
Owner:ZHU ZHENPING +1
Specific binding proteins and uses thereof
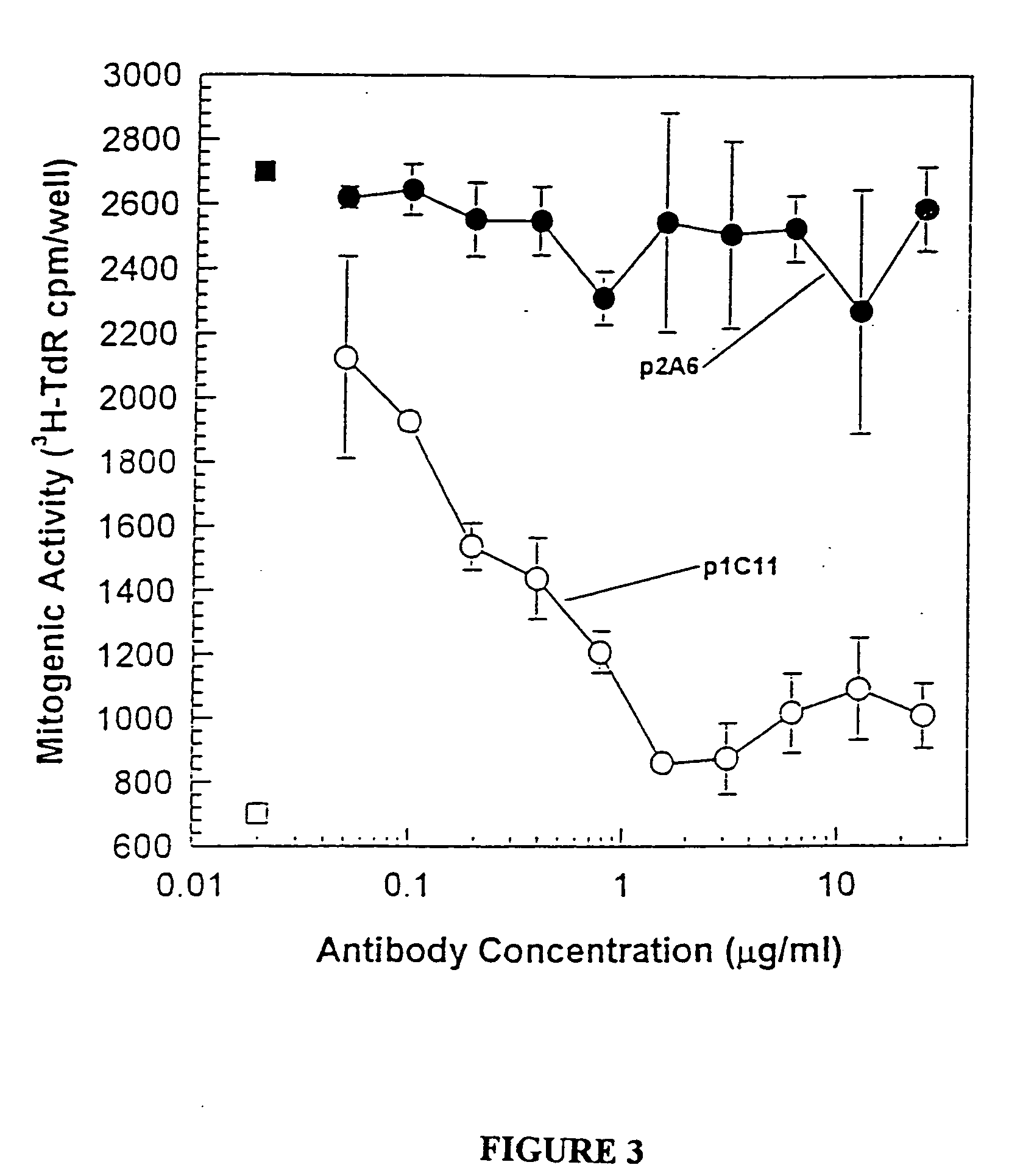
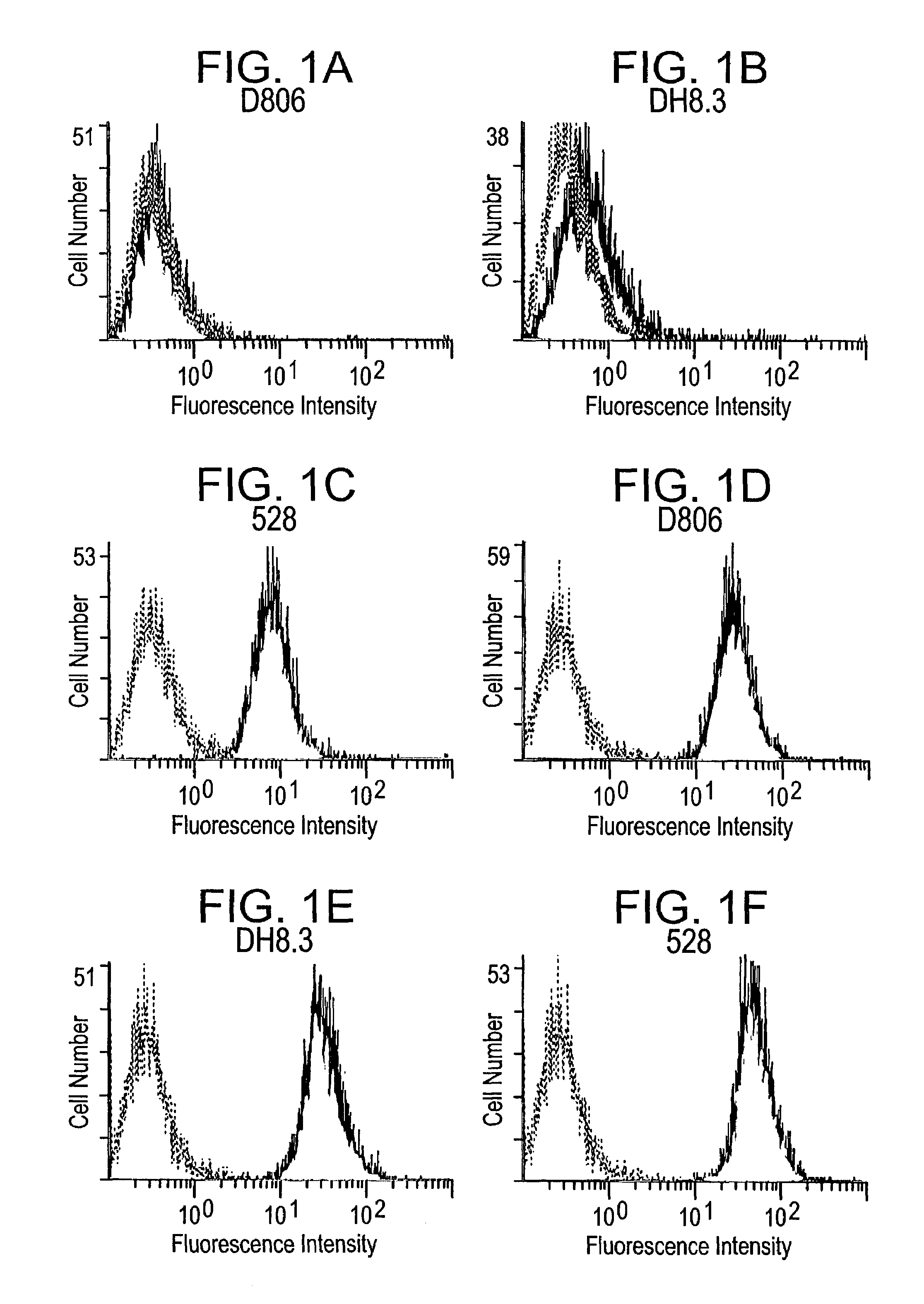
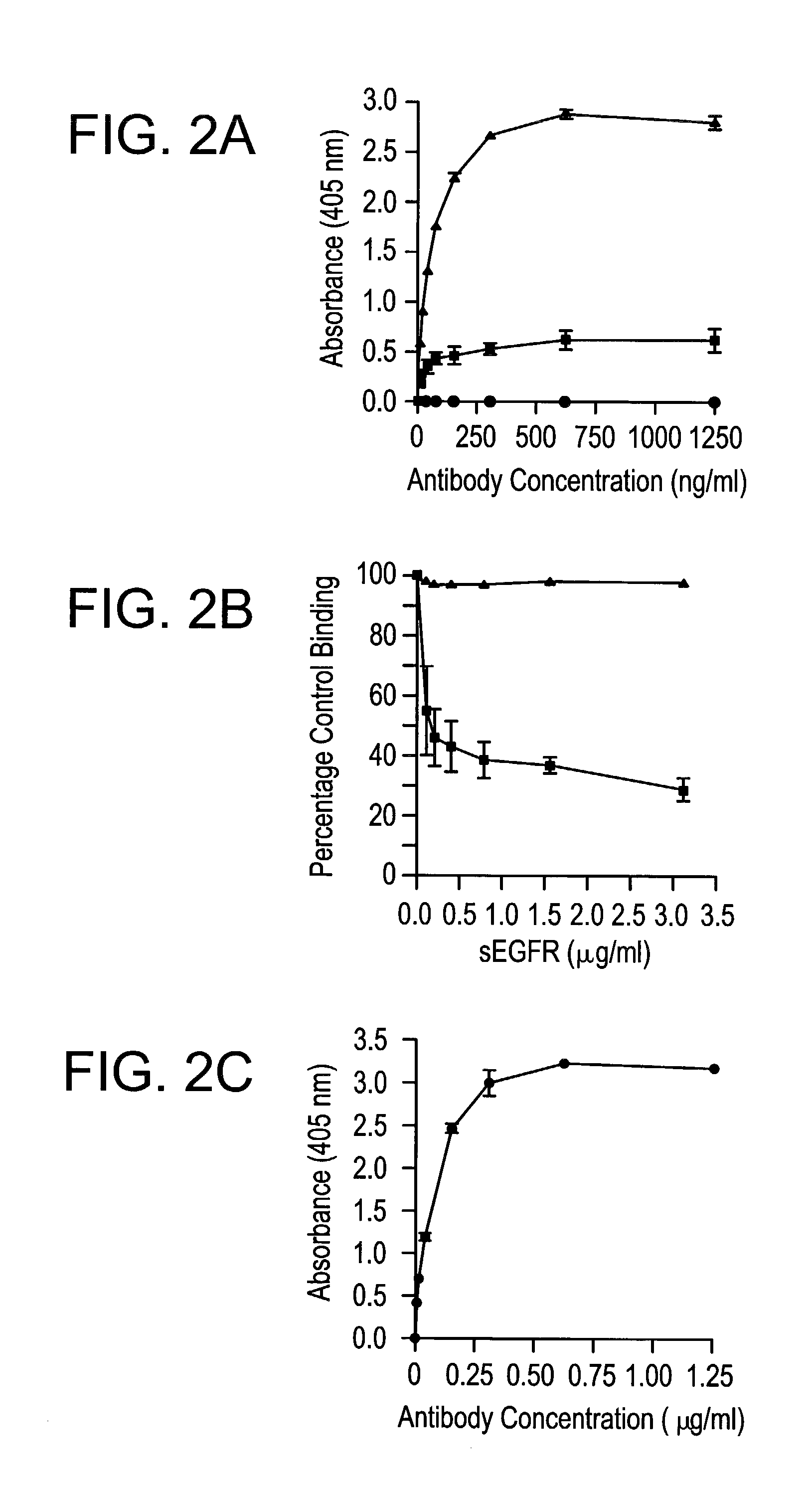
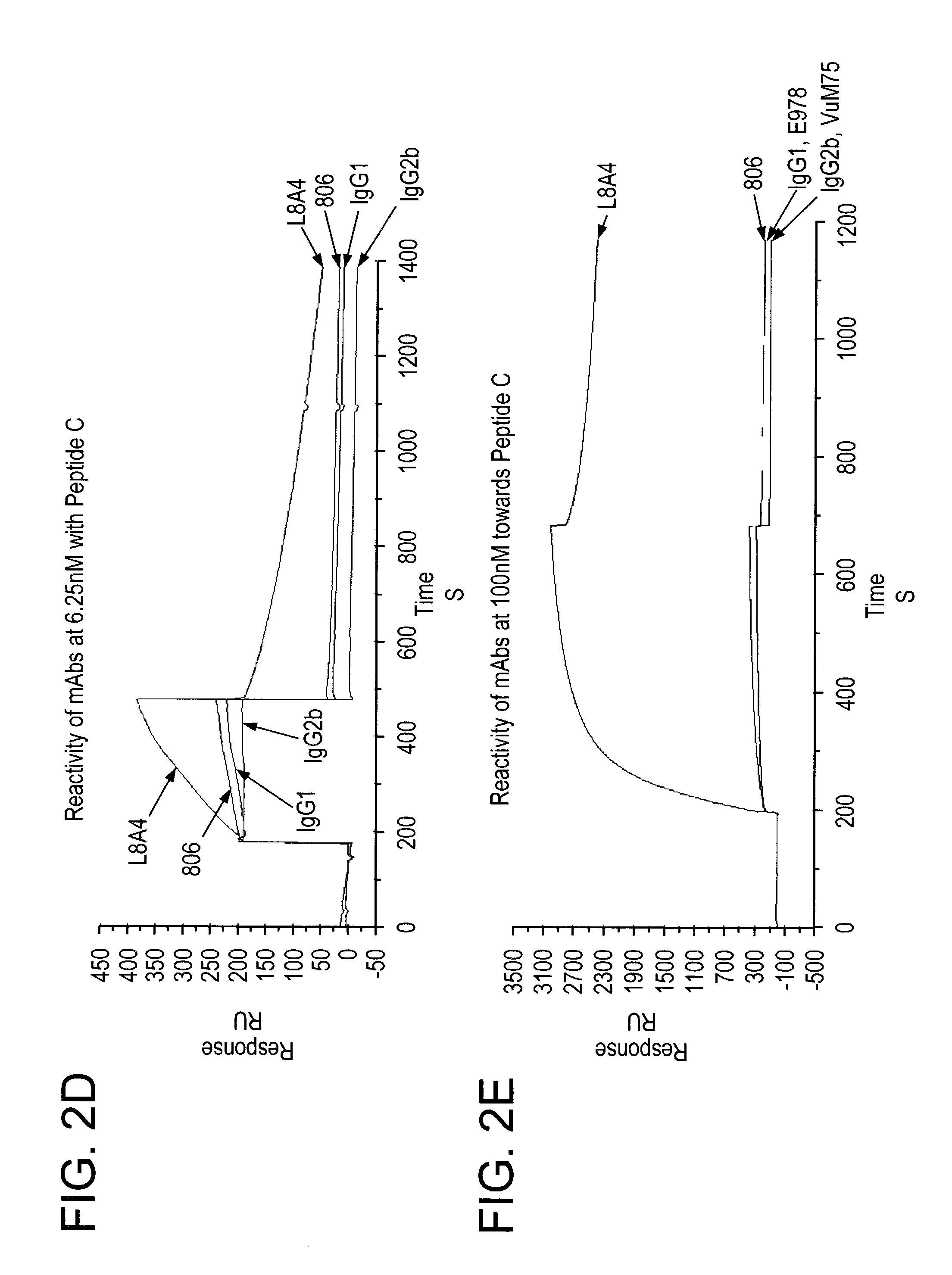
The invention relates to specific binding members, particularly antibodies and active fragments thereof, which recognize an aberrant post-translationally modified, particularly an aberrant glycosylated form of the EGFR. The binding members, particularly antibodies and fragments thereof, of the invention do not bind to EGFR on normal cells in the absence of amplification of the wild-type gene and are capable of binding the de2-7 EGFR at an epitope which is distinct from the junctional peptide. Antibodies of this type are exemplified by the novel antibody 806 whose VH and VL sequences are illustrated as SEQ ID NOs: 2 and 4 and chimeric antibodies thereof as exemplified by ch806.
Owner:LUDWIG INST FOR CANCER RES LTD
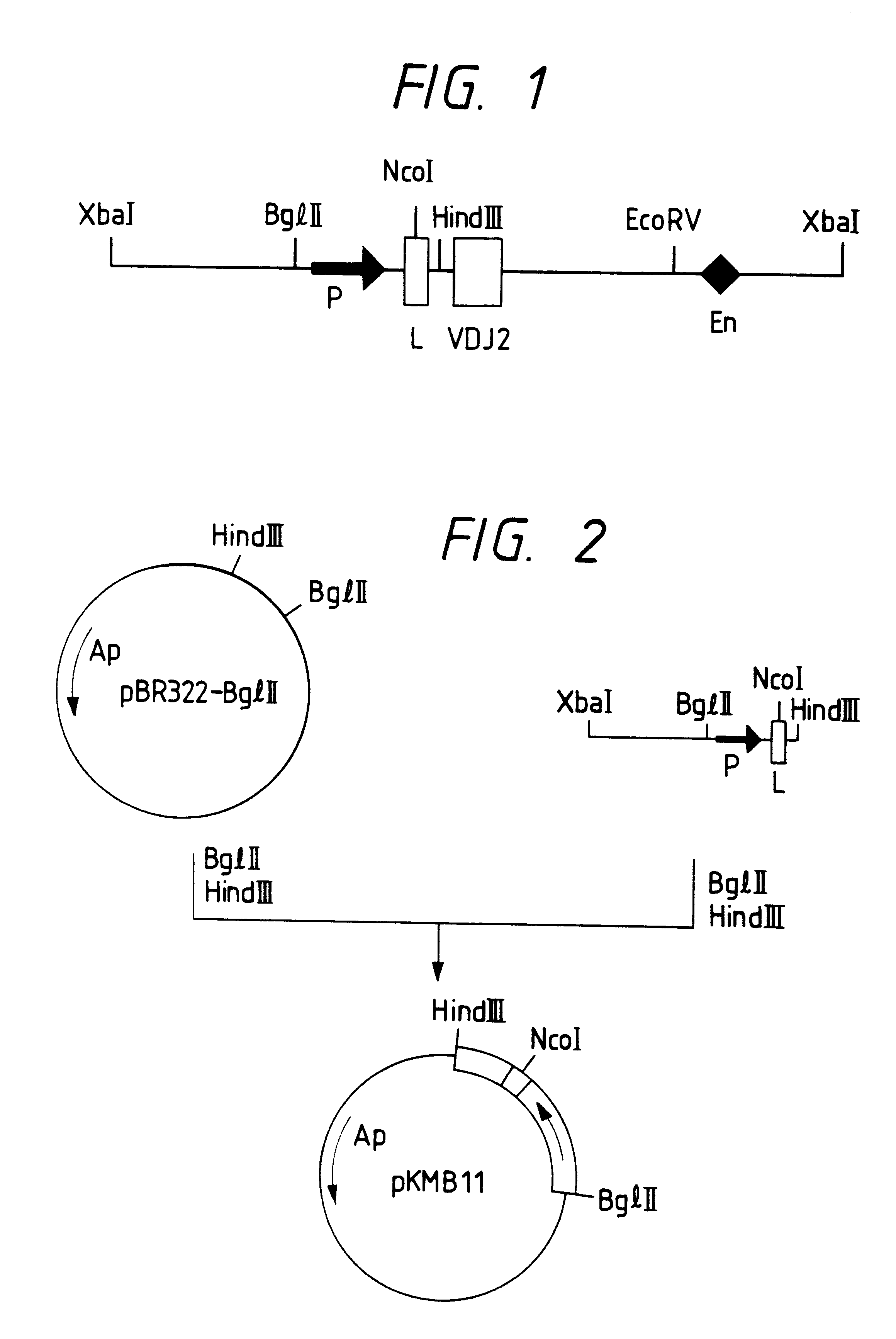
Chimeric antibodies
InactiveUS6020153ALow percentage and absence of bindingAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainIn vivo
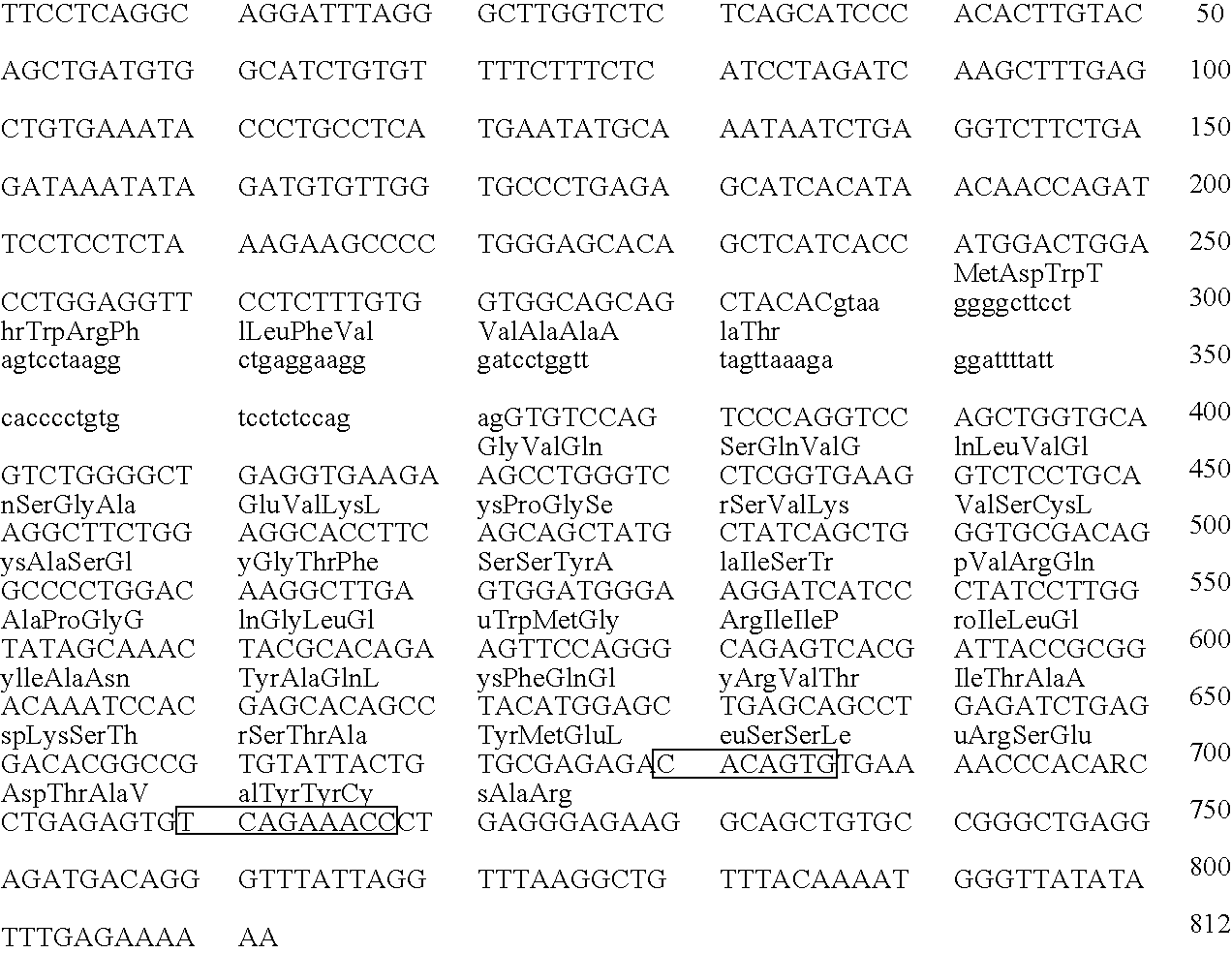
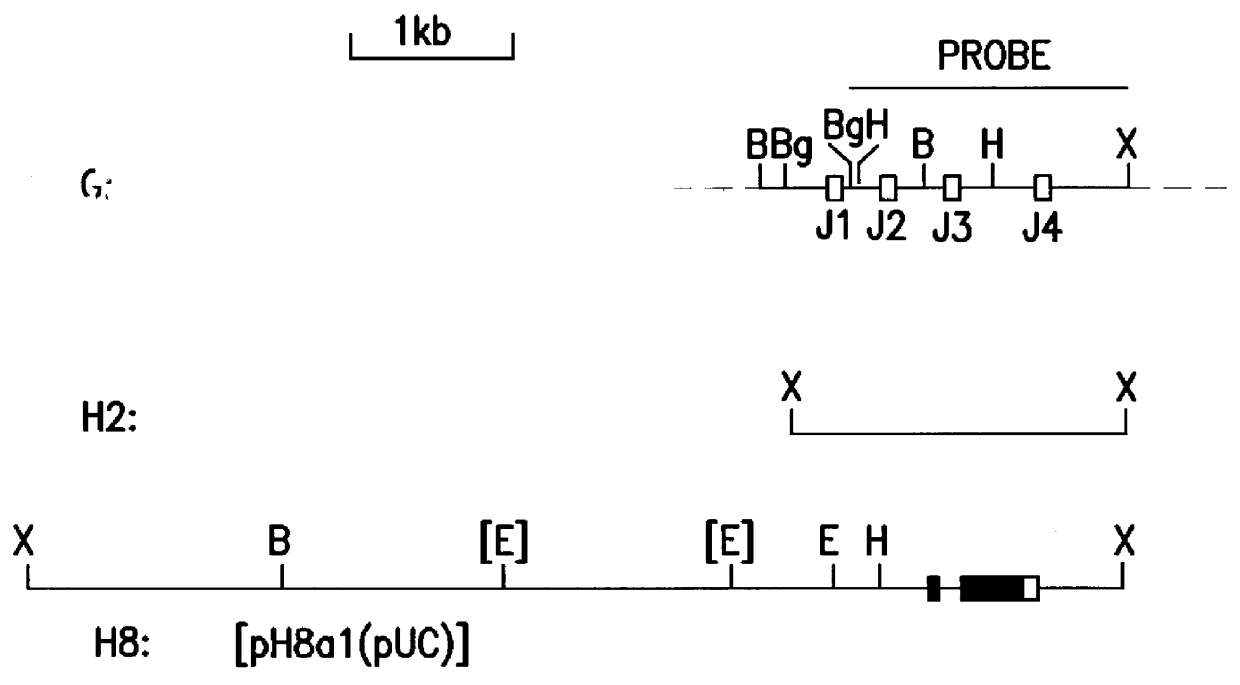
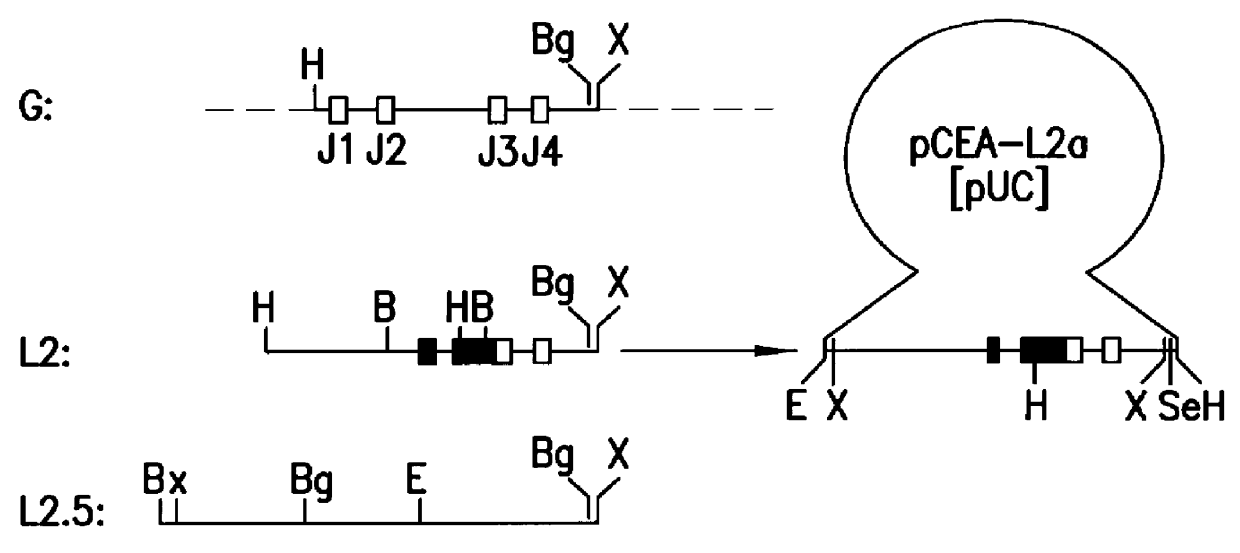
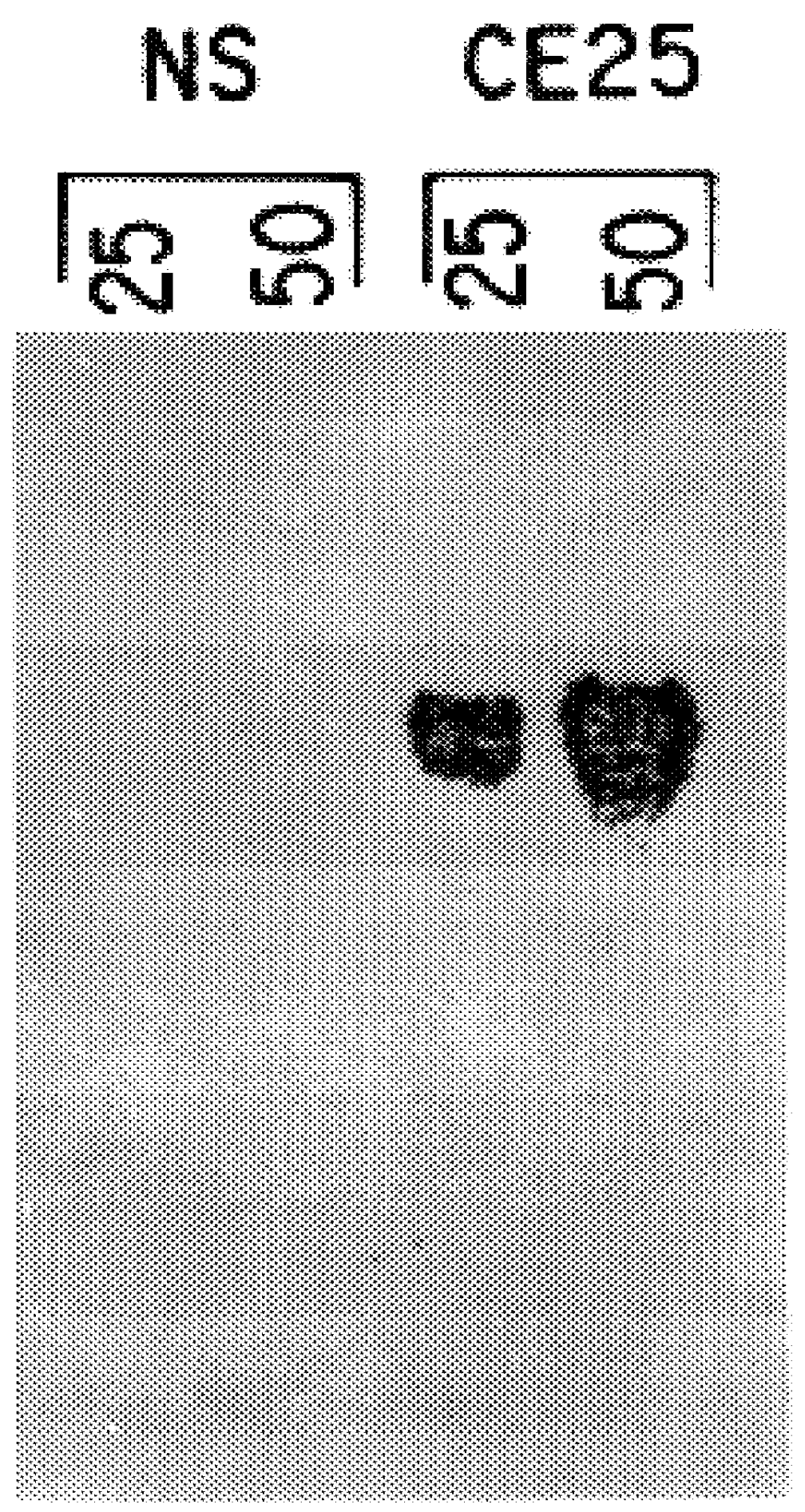
The invention relates to murine / human chimeric monoclonal antibodies with high specificity to and affinity for human carcinoembryonic antigen (CEA), derivatives thereof, processes for the preparation of these antibodies and their derivatives, DNAs coding for heavy and light chains of these antibodies, processes for the preparation of said DNAs, mammalian cell lines that produce and secrete the antibodies and processes for the preparation of said cell lines. The chimeric antibodies and their derivatives are used for clinical purposes in vitro and in vivo, especially for the diagnosis of cancer, for localization and in vivo imaging of tumors, for therapy, e.g. site-directed delivery of cytotoxins, and similar purposes. The invention also concerns test kits and pharmaceutical compositions containing said chimeric monoclonal antibodies and / or derivatives thereof.
Owner:CIBA GEIGY CORP
Chimeric antibody with specificity to human B cell surface antigen
A chimeric antibody with human constant region and murine variable region, having specificity to a 35 kDA polypeptide (Bp35(CD20)) expressed on the surface of human B cells, methods of production, and uses.
Owner:ROBINSON RANDY +2
Compositions and methods for treating coagulation related disorders
InactiveUS20060159675A1Initiate and prolong such disorderRelieve symptomsImmunoglobulins against blood coagulation factorsAntibacterial agentsDiseaseTissue factor
Disclosed are methods for preventing or treating sepsis, a sepsis-related condition or an inflammatory disease in a mammal. In one embodiment, the method includes administering to the mammal a therapeutically effective amount of at least one humanized antibody, chimeric antibody, or fragment thereof that binds specifically to tissue factor (TF) to form a complex in which factor X or factor IX binding to the complex is inhibited and the administration is sufficient to prevent or treat the sepsis in the mammal. The invention has a wide spectrum of useful applications including treating sepsis, disorders related to sepsis, and inflammatory diseases such as arthritis.
Owner:GENENTECH INC
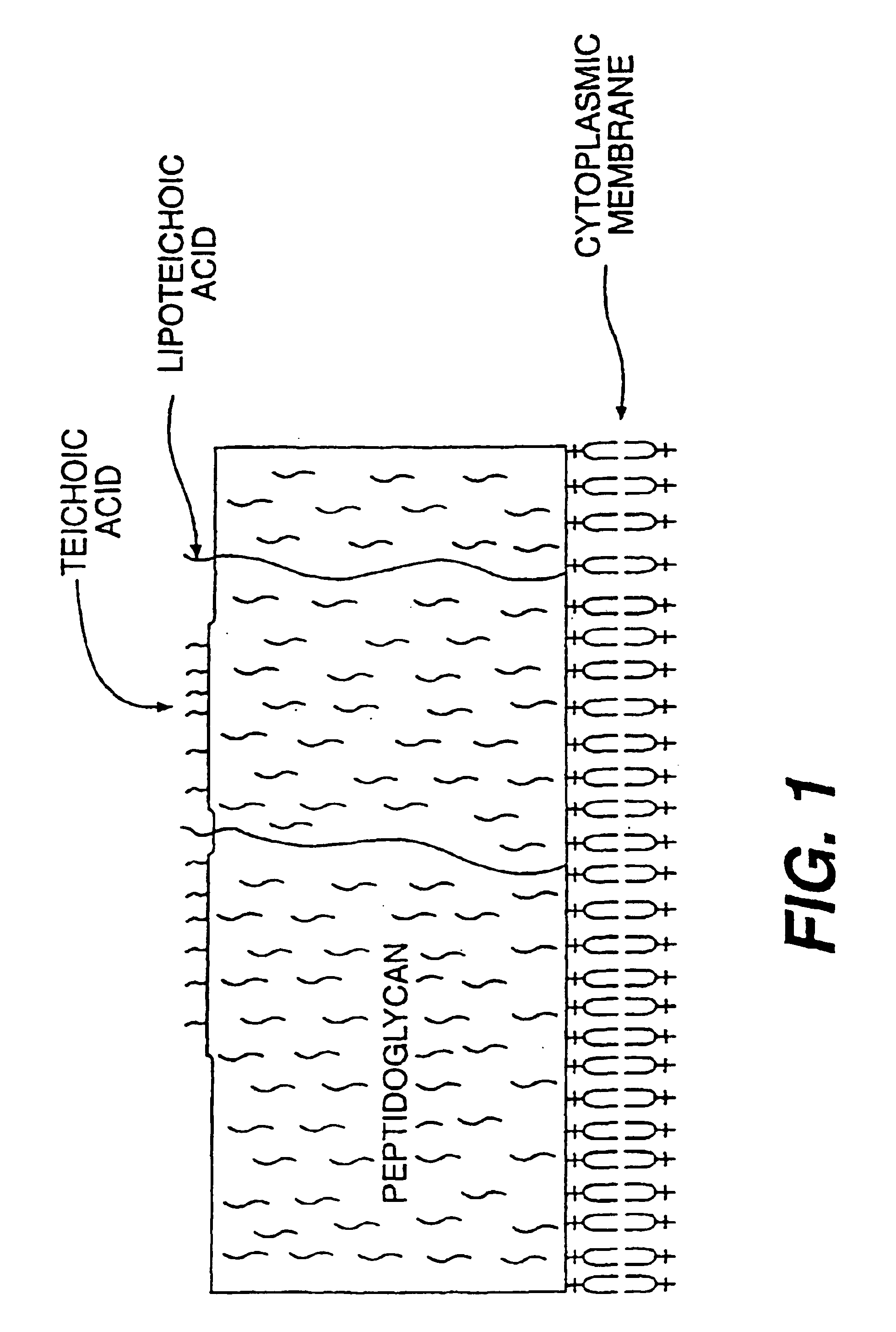
Opsonic and protective monoclonal and chimeric antibodies specific for lipoteichoic acid of gram positive bacteria
InactiveUS6939543B2Enhance phagocytosis and killing of the bacteriaIncrease infectionAntibacterial agentsHybrid immunoglobulinsBacteroidesBinding site
The present invention encompasses monoclonal and chimeric antibodies that bind to lipoteichoic acid of Gram positive bacteria. The antibodies also bind to whole bacteria and enhance phagocytosis and killing of the bacteria in vitro and enhance protection from lethal infection in vivo. The mouse monoclonal antibody has been humanized and the resulting chimeric antibody provides a previously unknown means to diagnose, prevent and / or treat infections caused by gram positive bacteria bearing lipoteichoic acid. This invention also encompasses a peptide mimic of the lipoteichoic acid epitope binding site defined by the monoclonal antibody. This epitope or epitope peptide mimic identifies other antibodies that may bind to the lipoteichoic acid epitope. Moreover, the epitope or epitope peptide mimic provides a valuable substrate for the generation of vaccines or other therapeutics.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC +1
Anti-idiotypic anti-TNF antibodies and related immunoassay methods
Anti-TNF antibodies and anti-TNF peptides, specific for tumor necrosis factor (TNF) are useful for in vivo diagnosis and therapy of a number of TNF-mediated pathologies and conditions, as well as polynucleotides coding for anti-TNF murine and chimeric antibodies, peptides, methods of making and using the antibody or peptides in immunoassays and immuno-therapeutic approaches are provided, where the anti-TNF peptide is selected from a soluble portion of TNF receptor, an anti-TNF antibody or structural analog thereof.
Owner:NEW YORK UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com