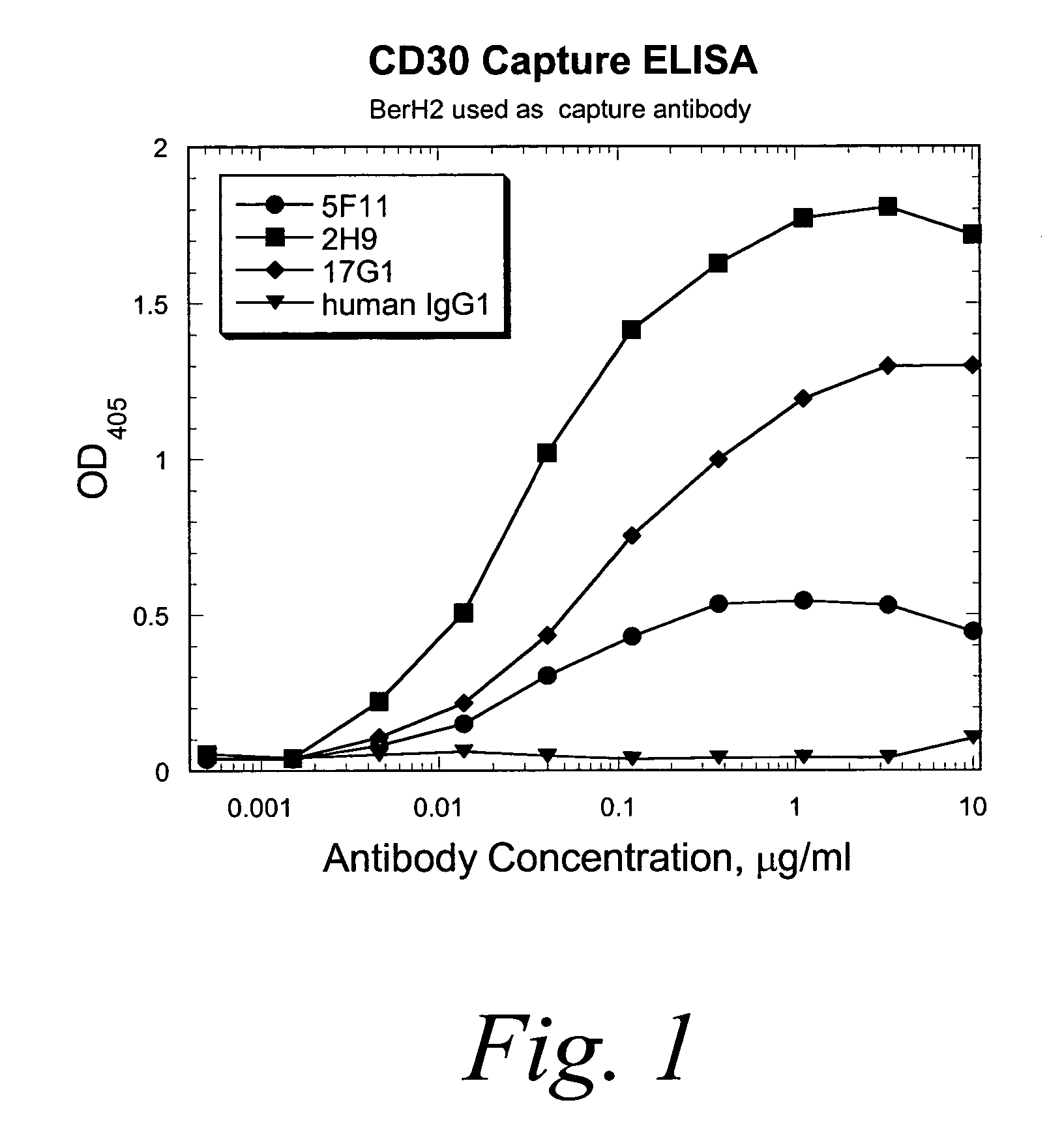
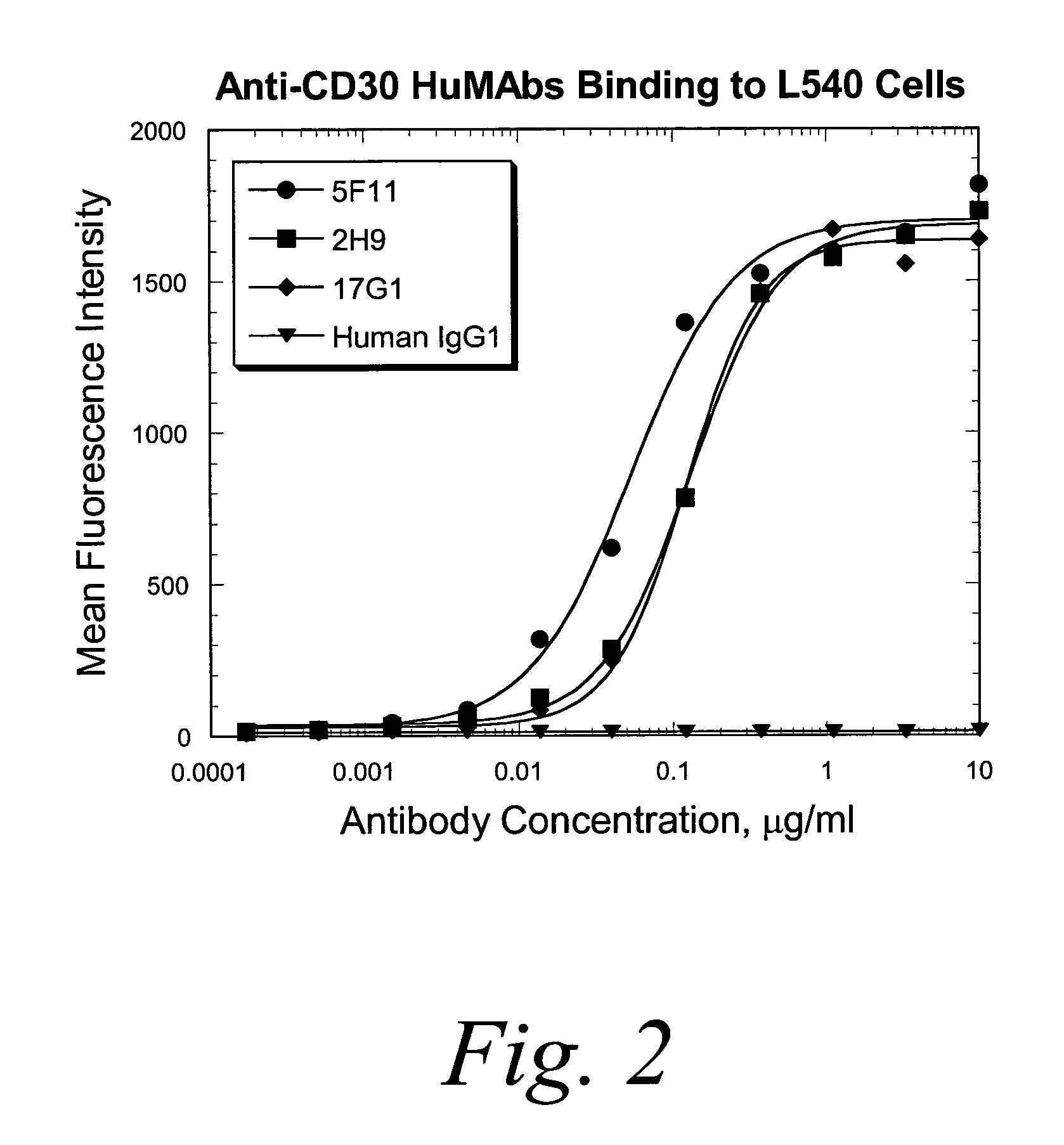
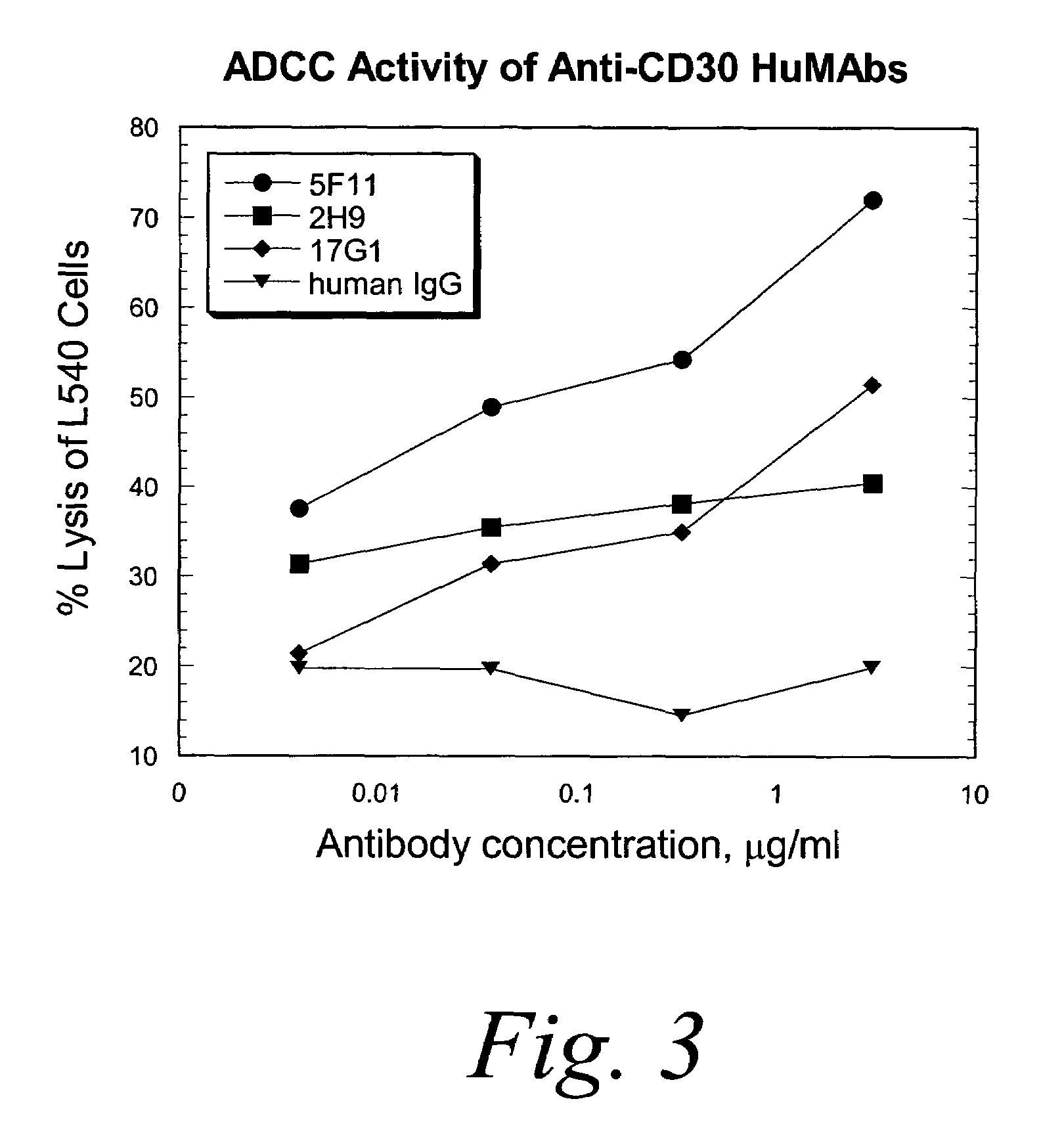
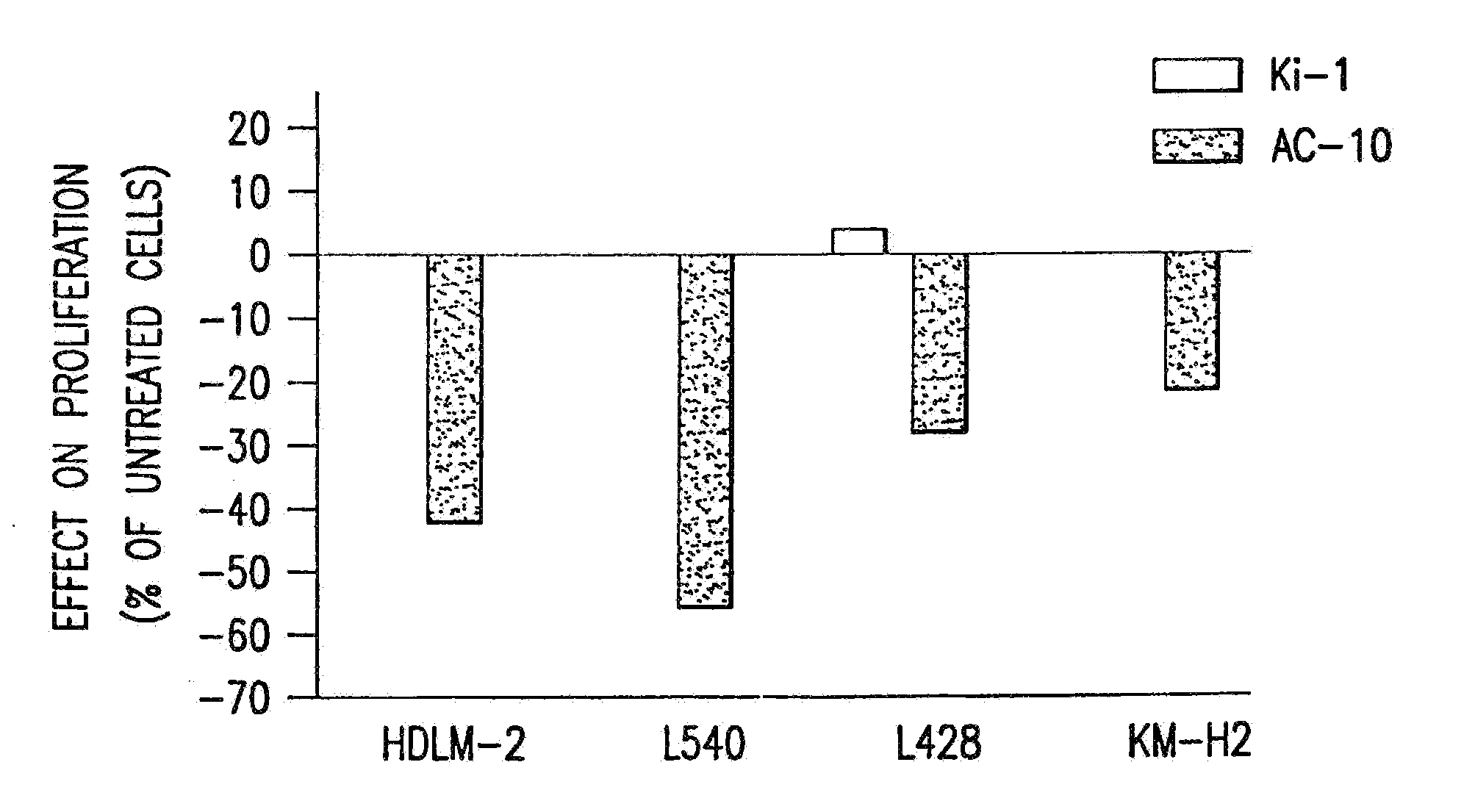
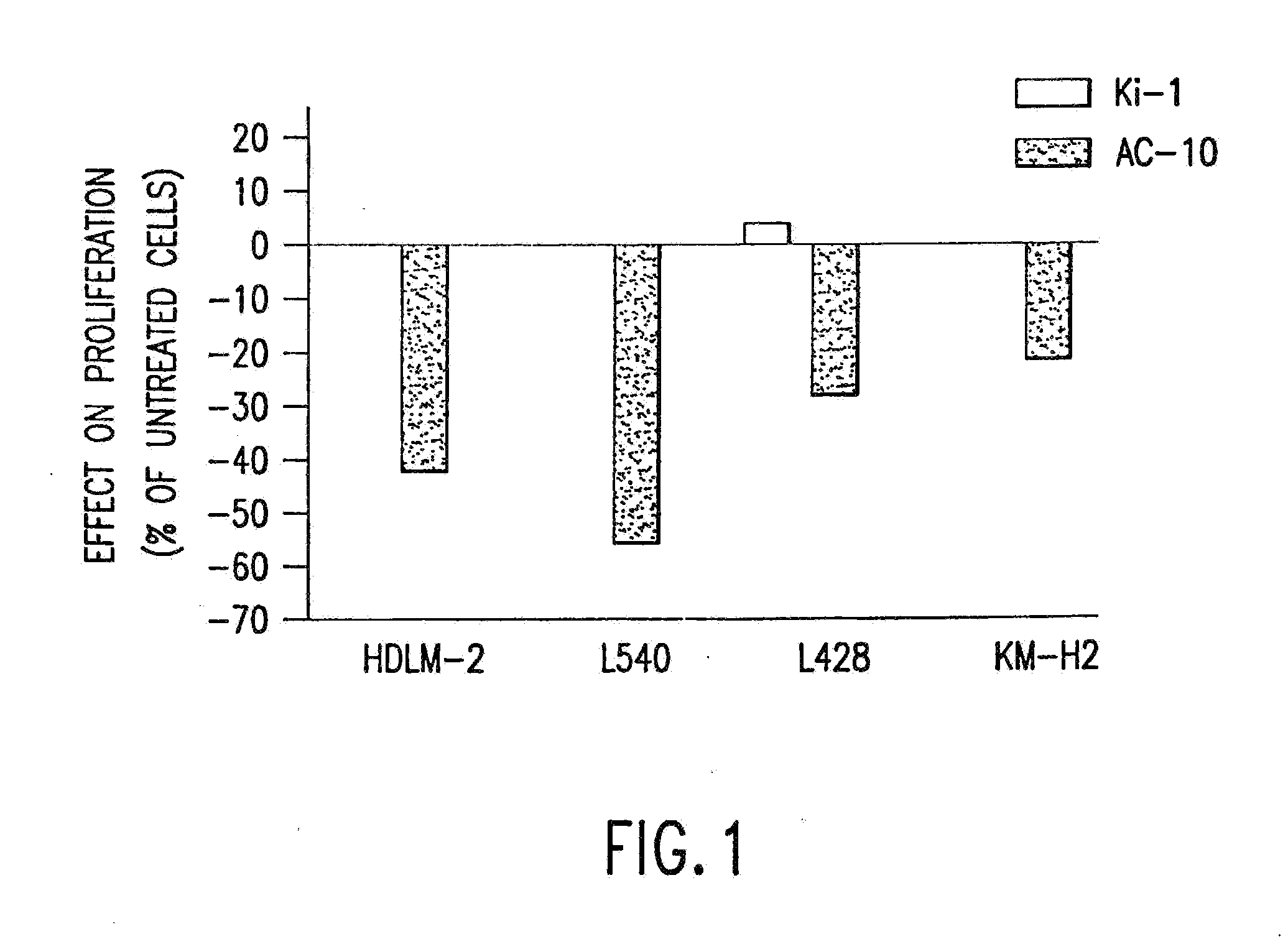
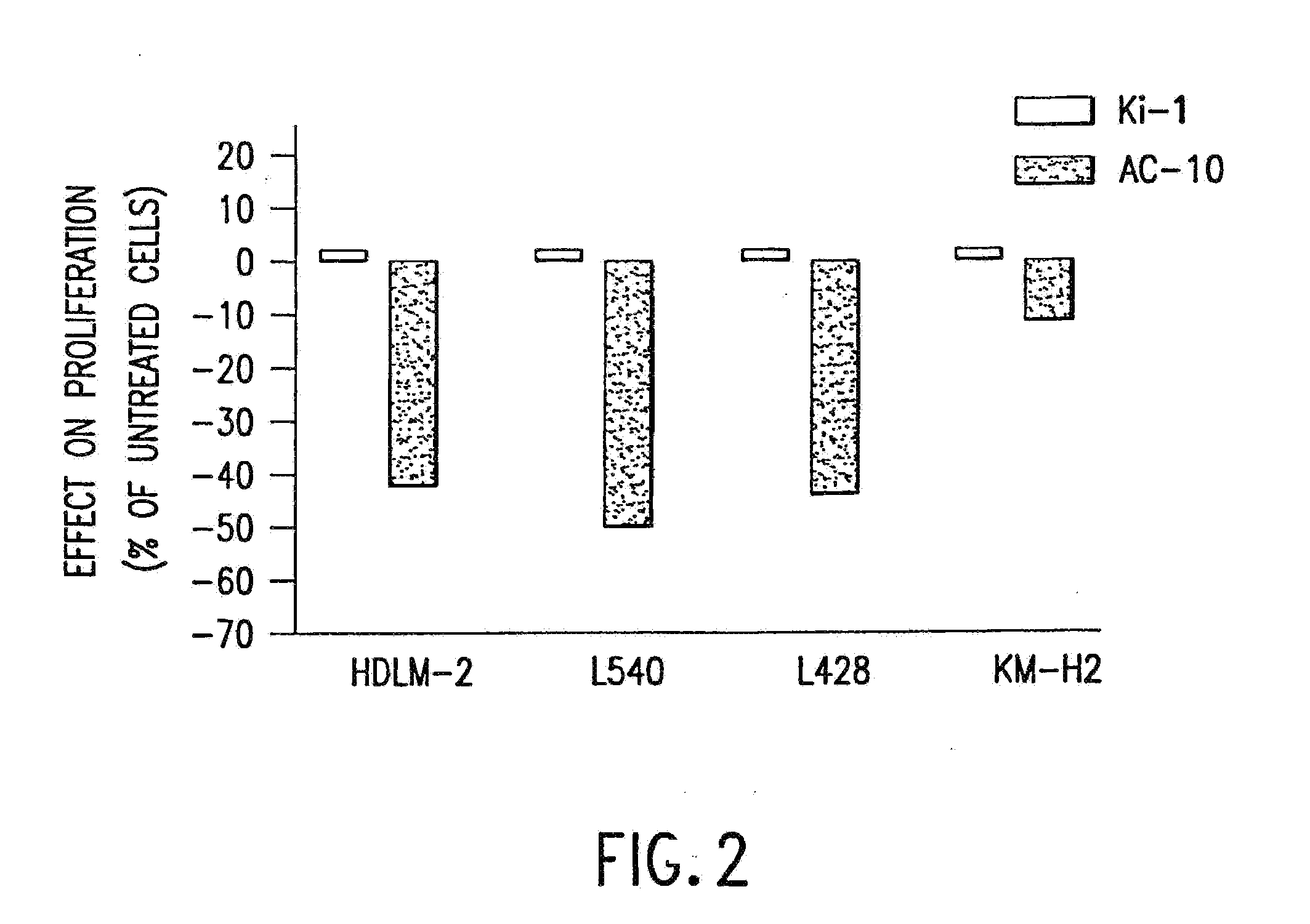
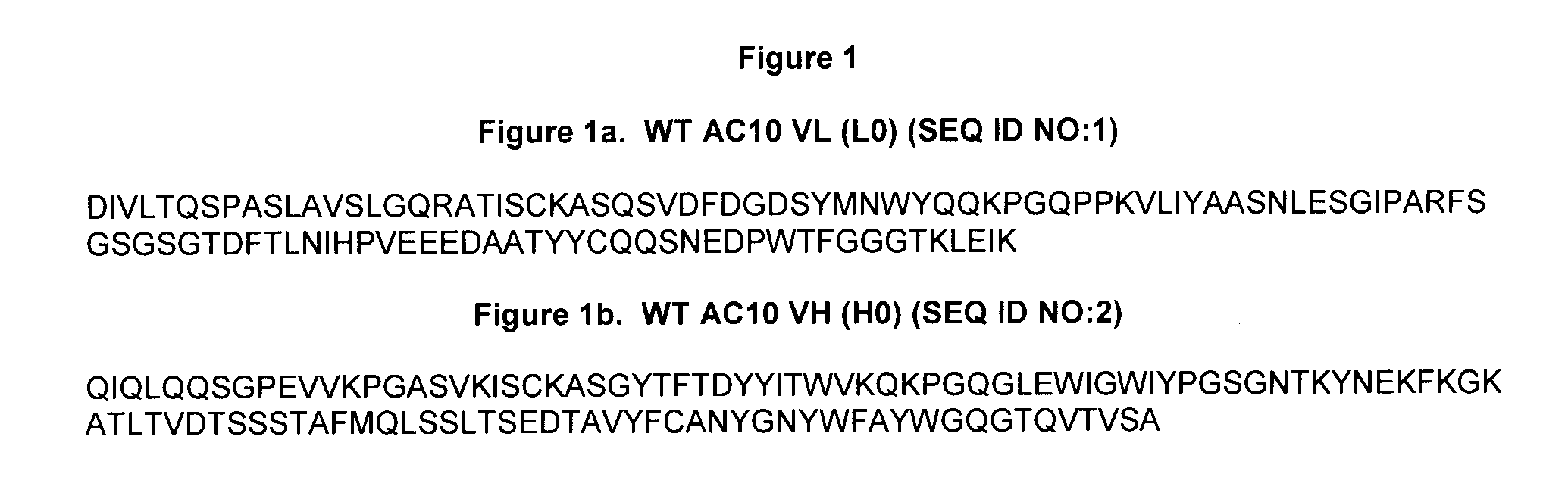
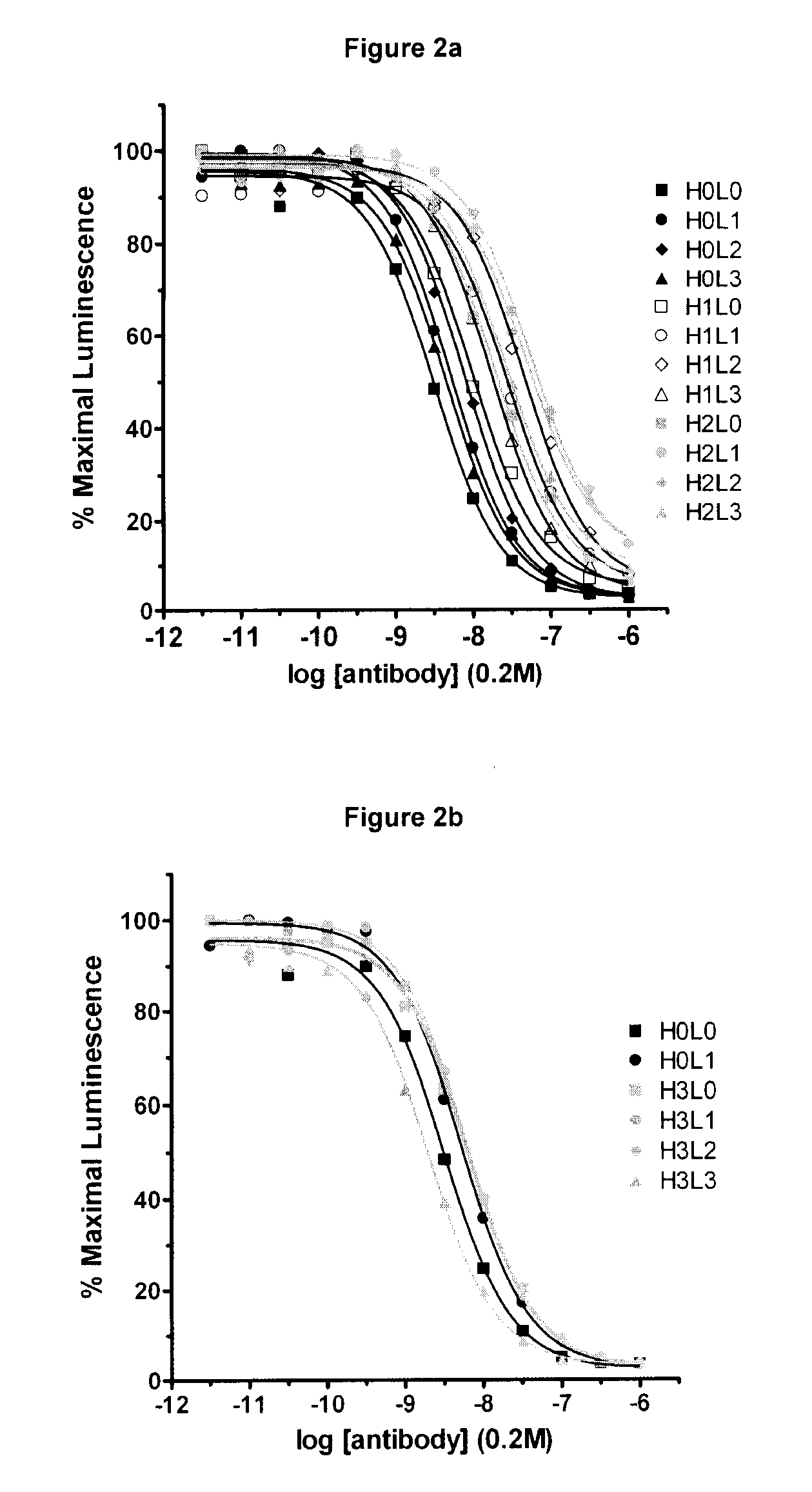
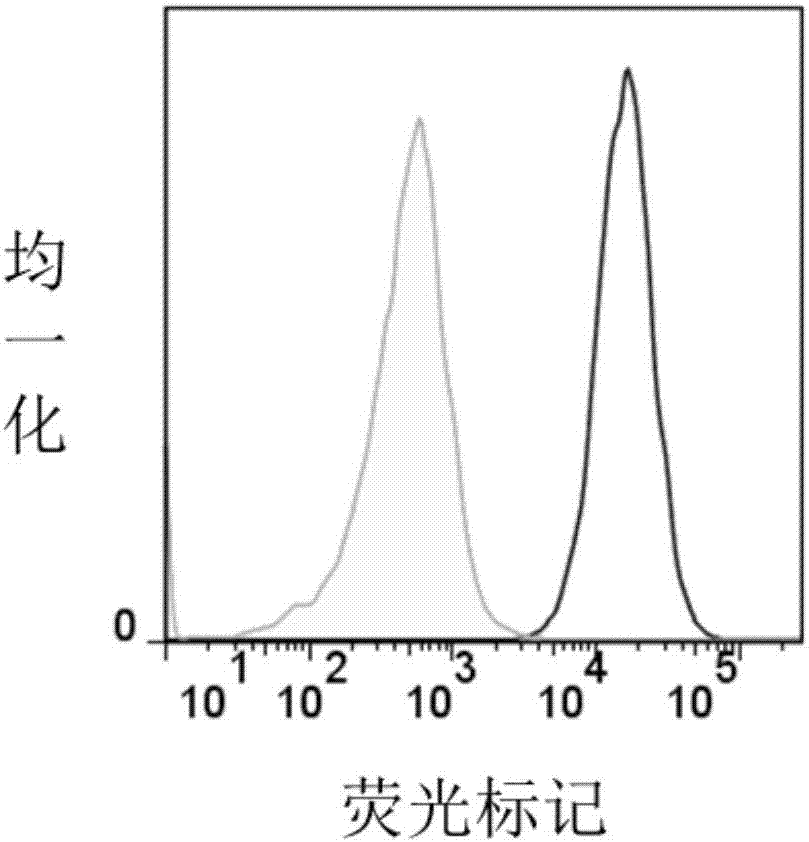
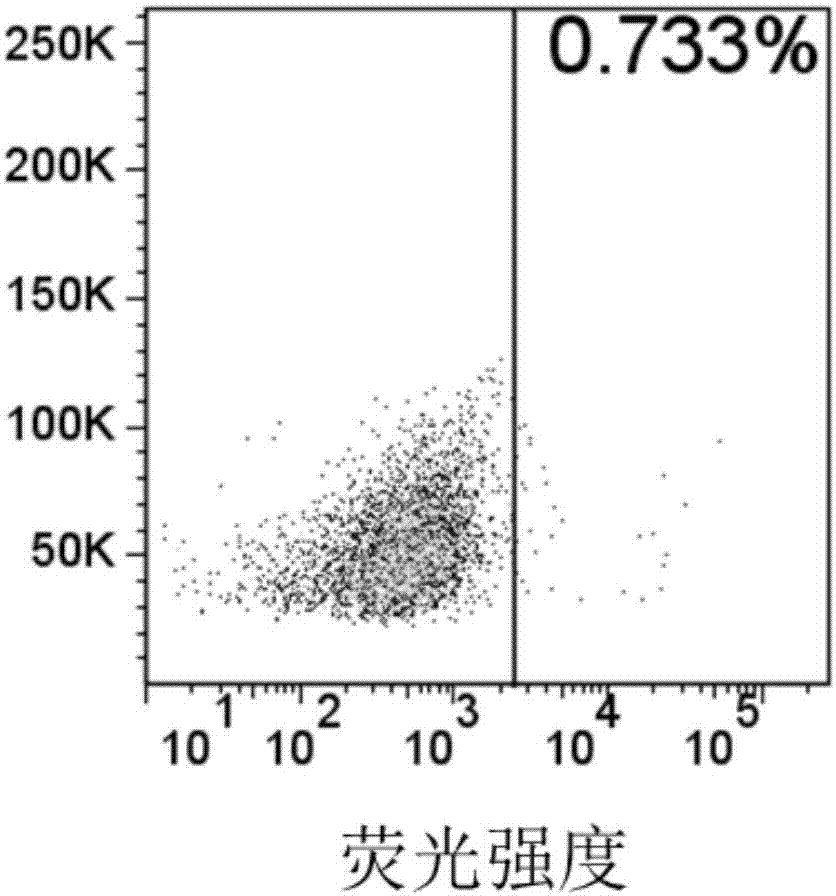
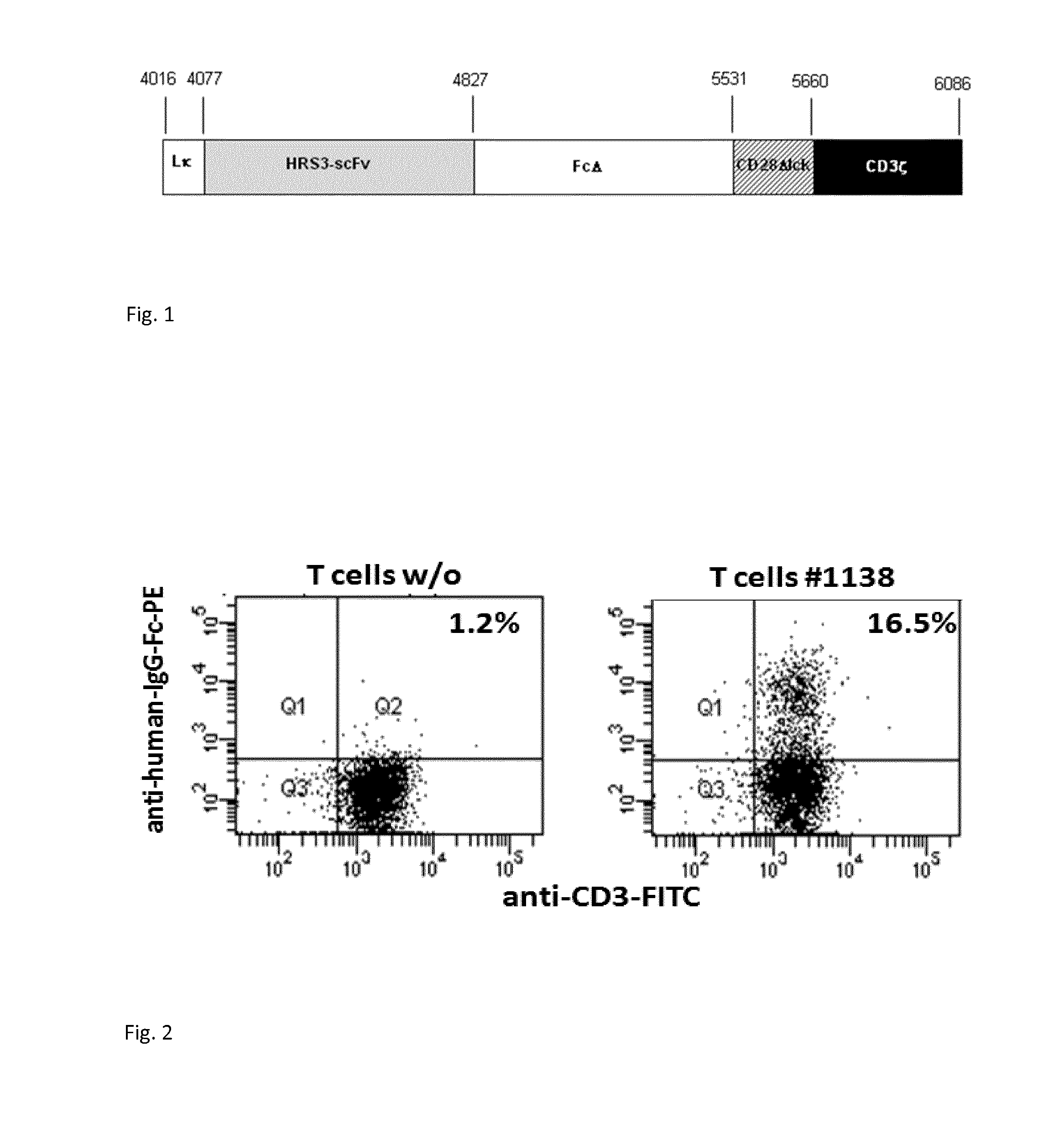
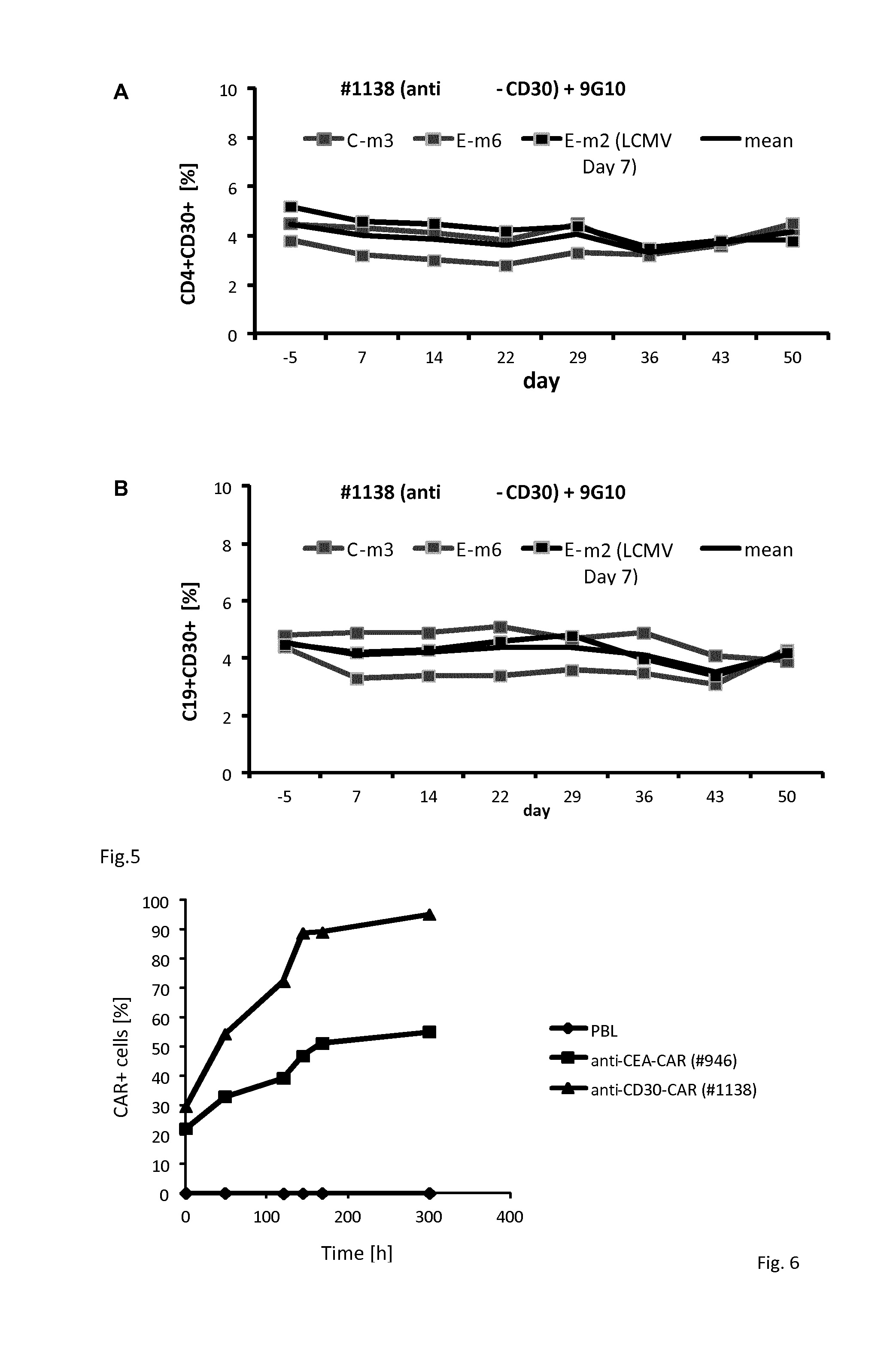
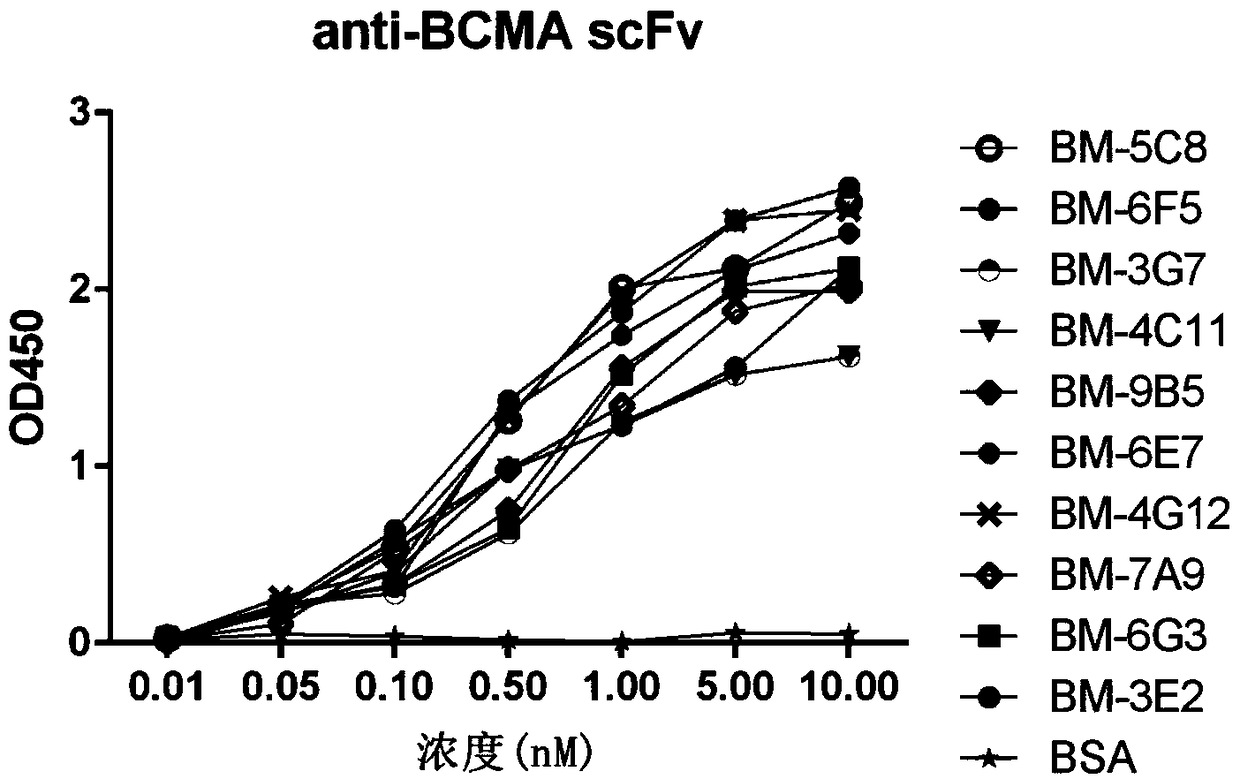
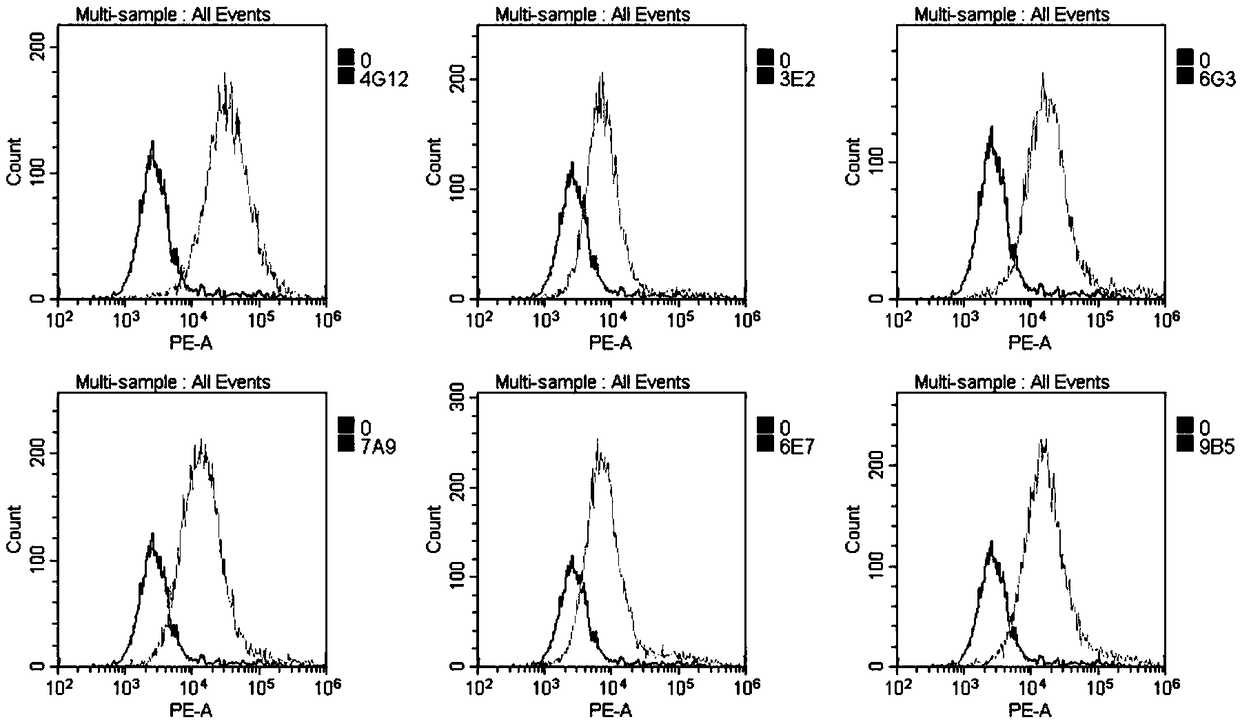
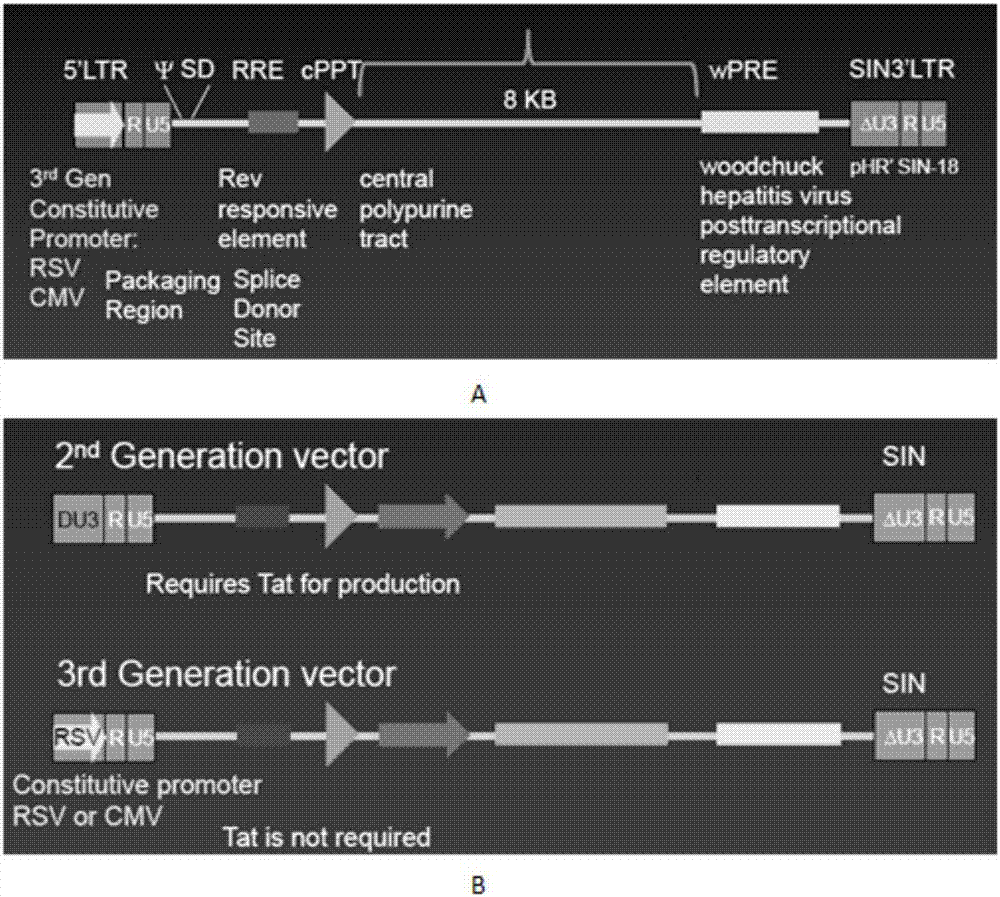
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
104 results about "CD30" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CD30, also known as TNFRSF8, is a cell membrane protein of the tumor necrosis factor receptor family and tumor marker.
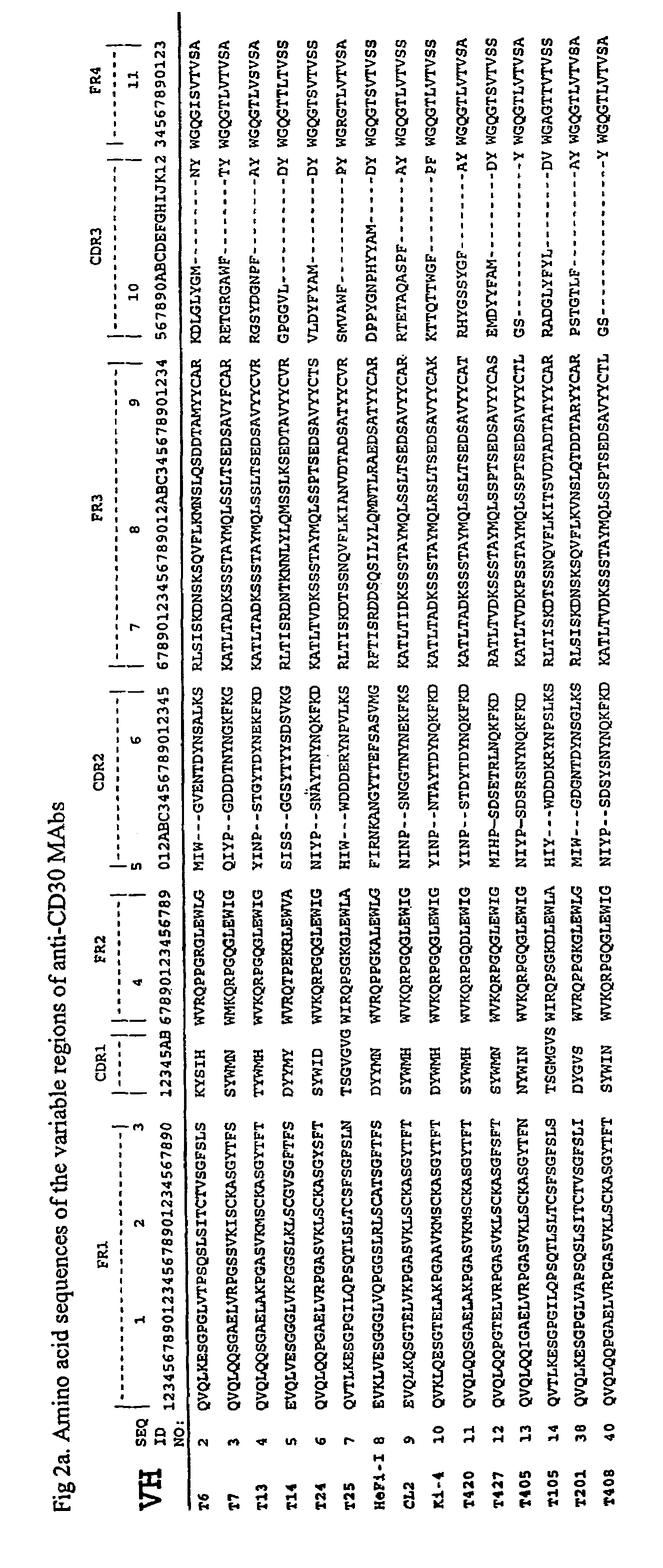
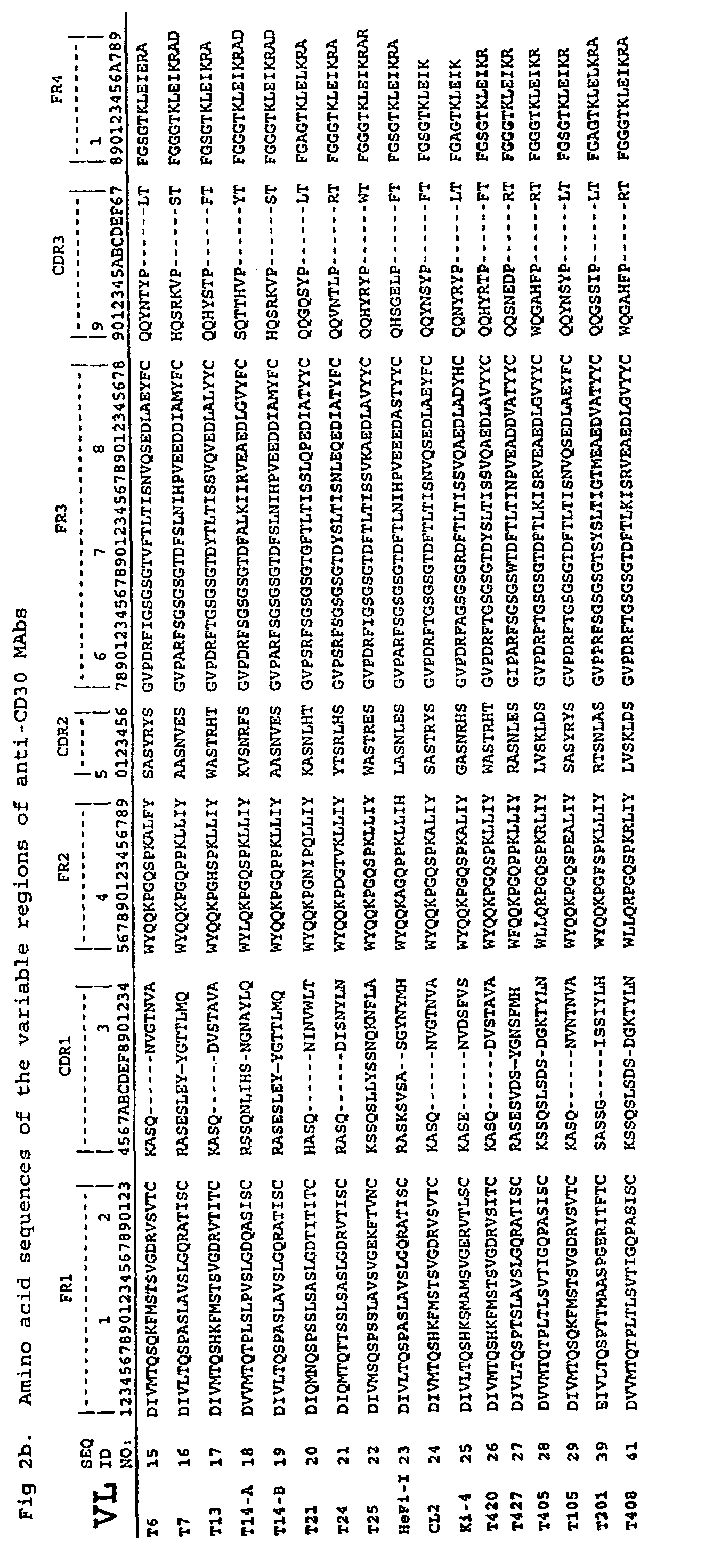
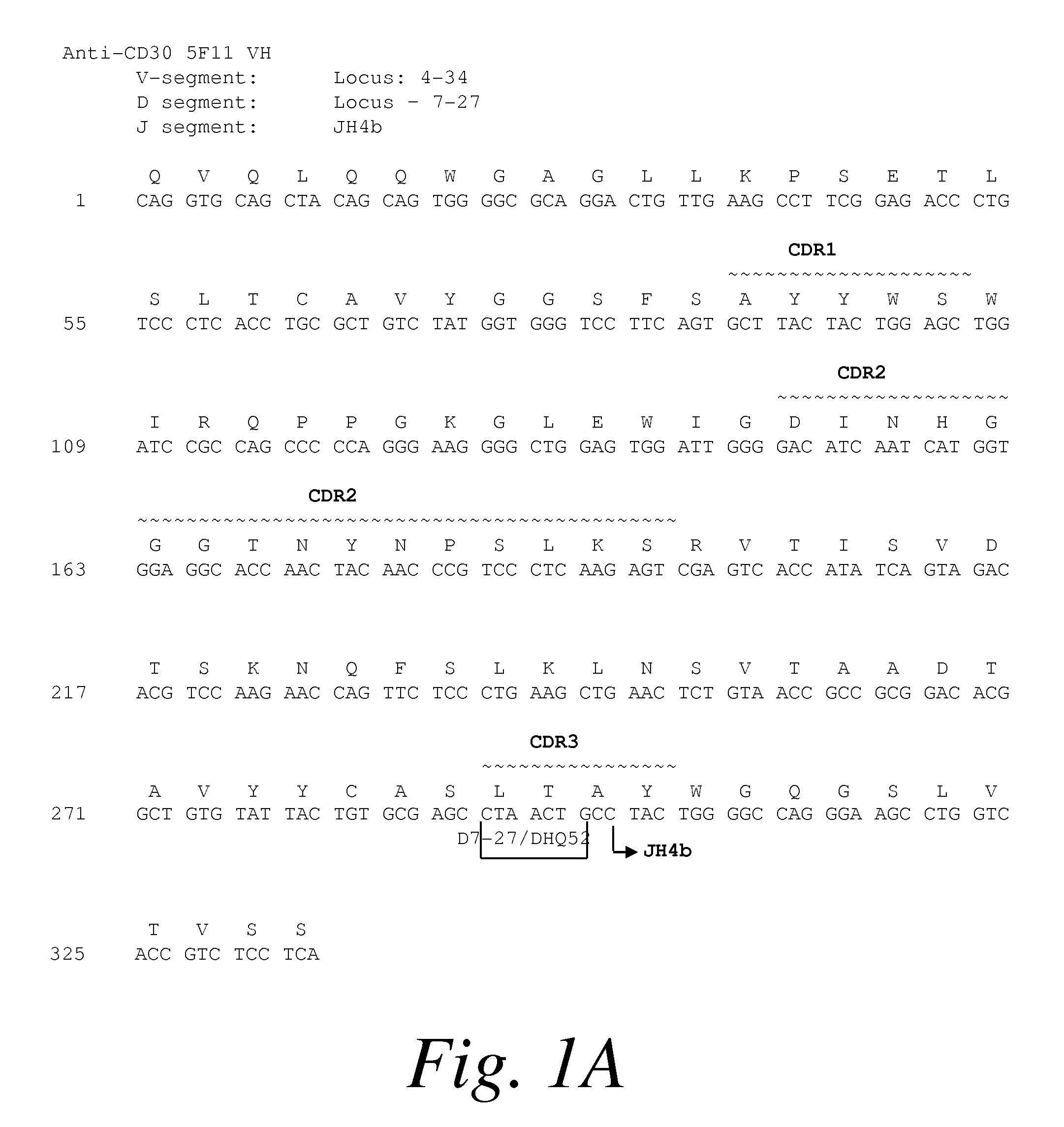
Recombinant anti-CD30 antibodies and uses thereof
InactiveUS20040018194A1Nervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseChemotherapeutic drugs
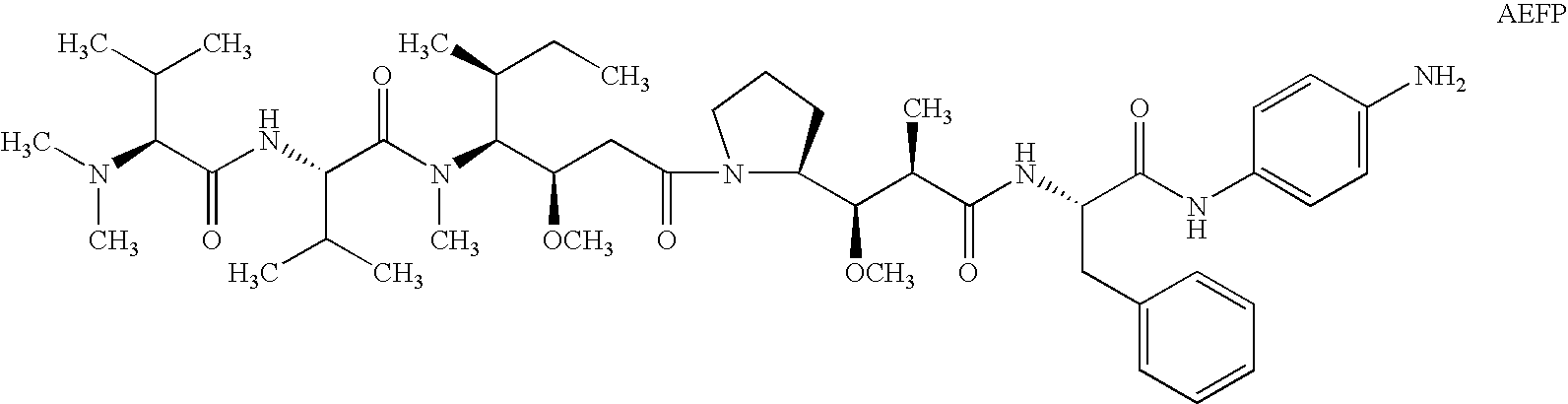
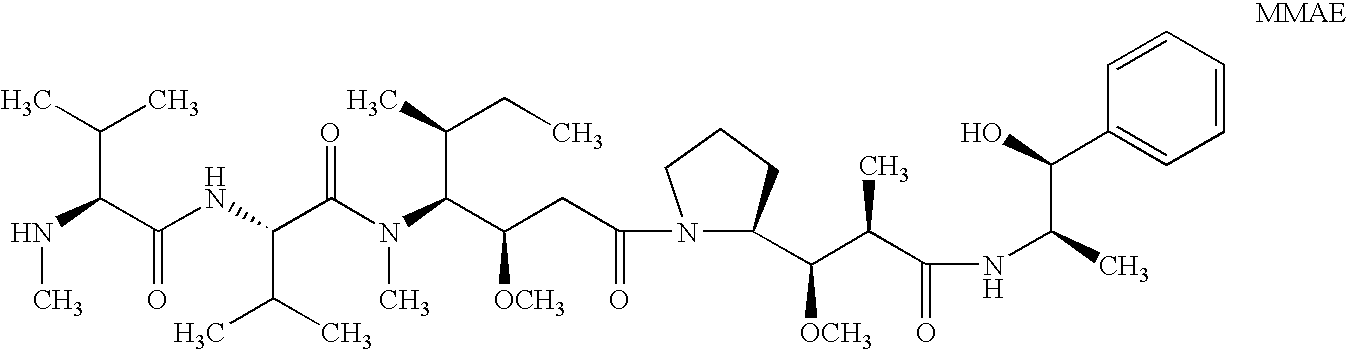
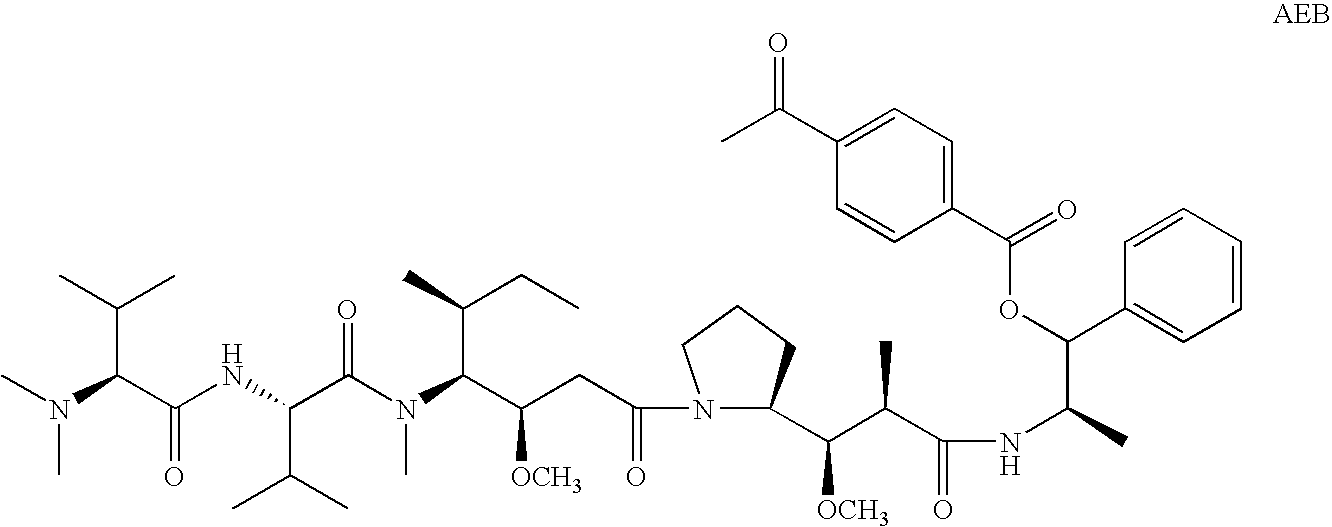
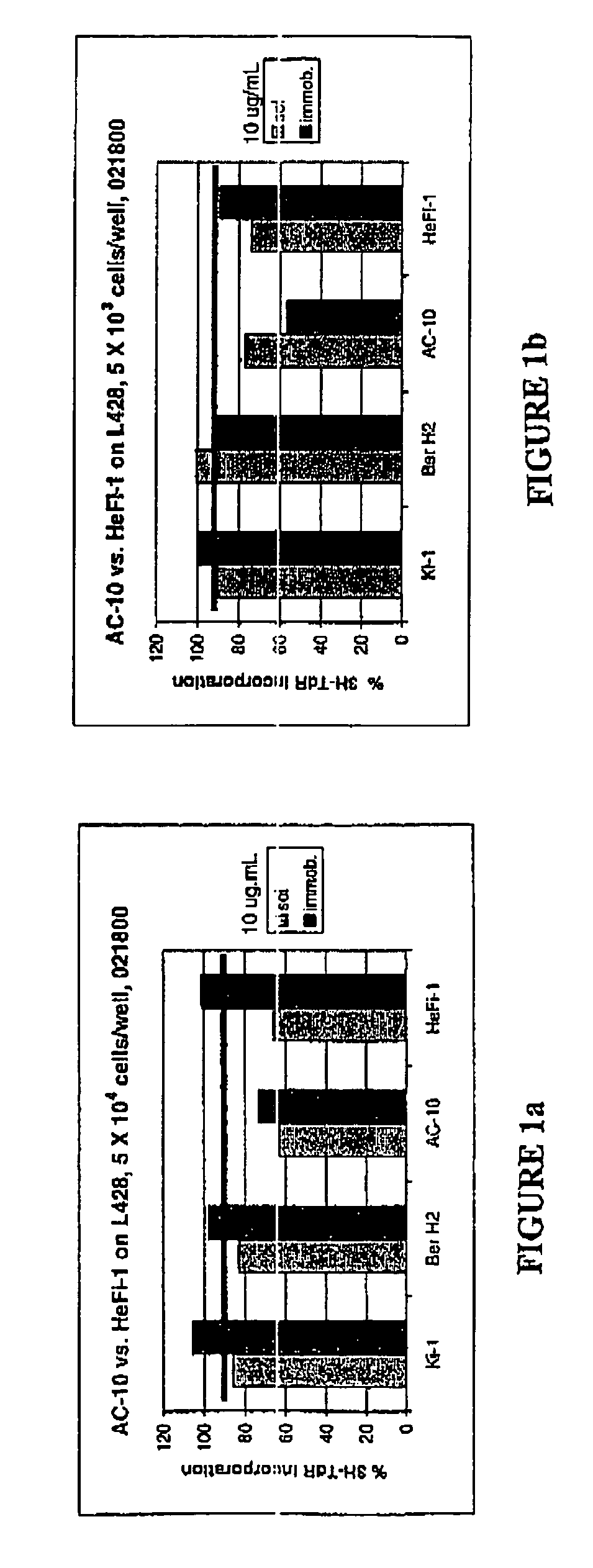
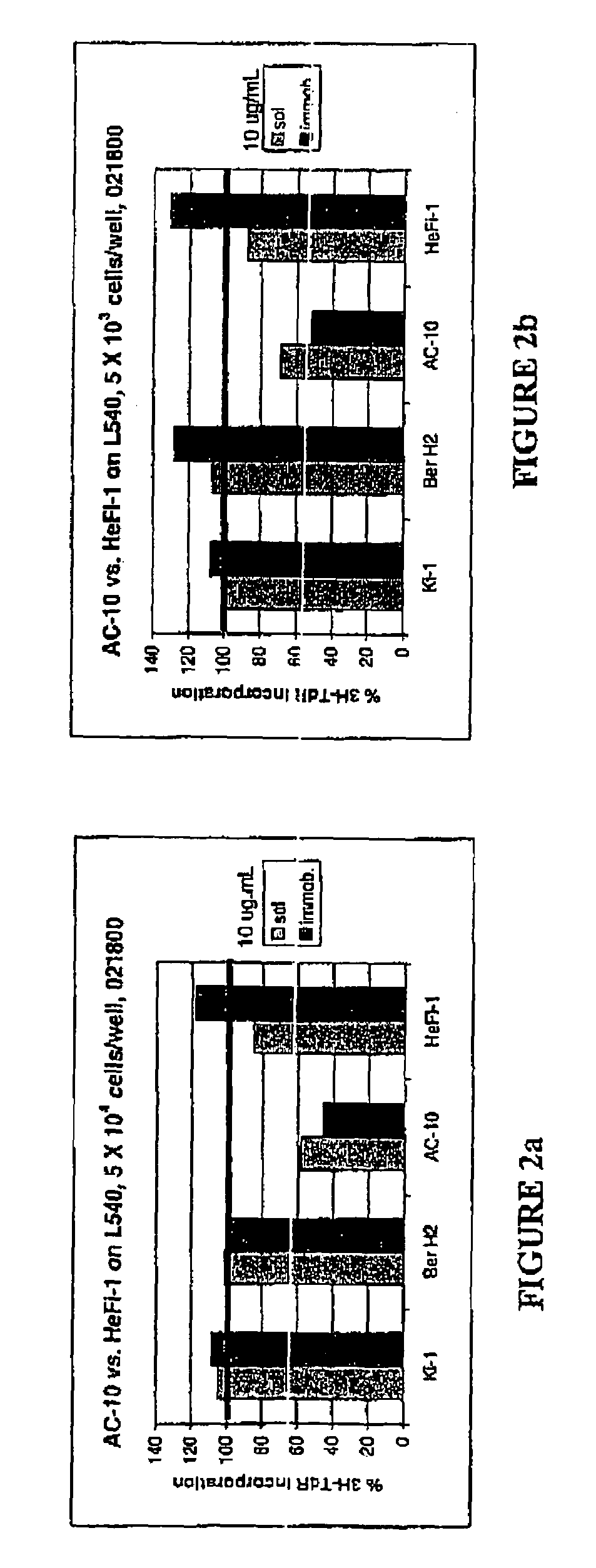
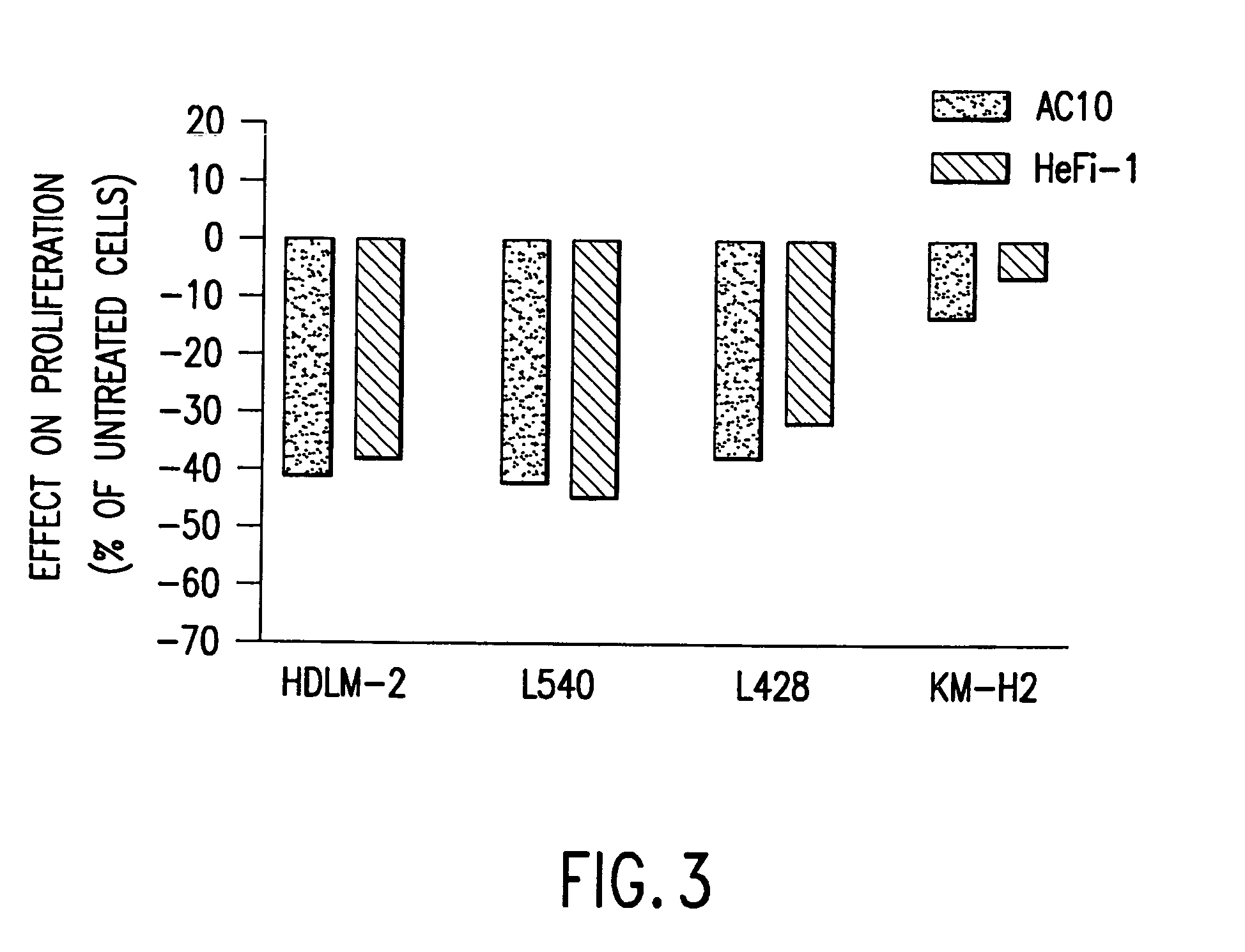
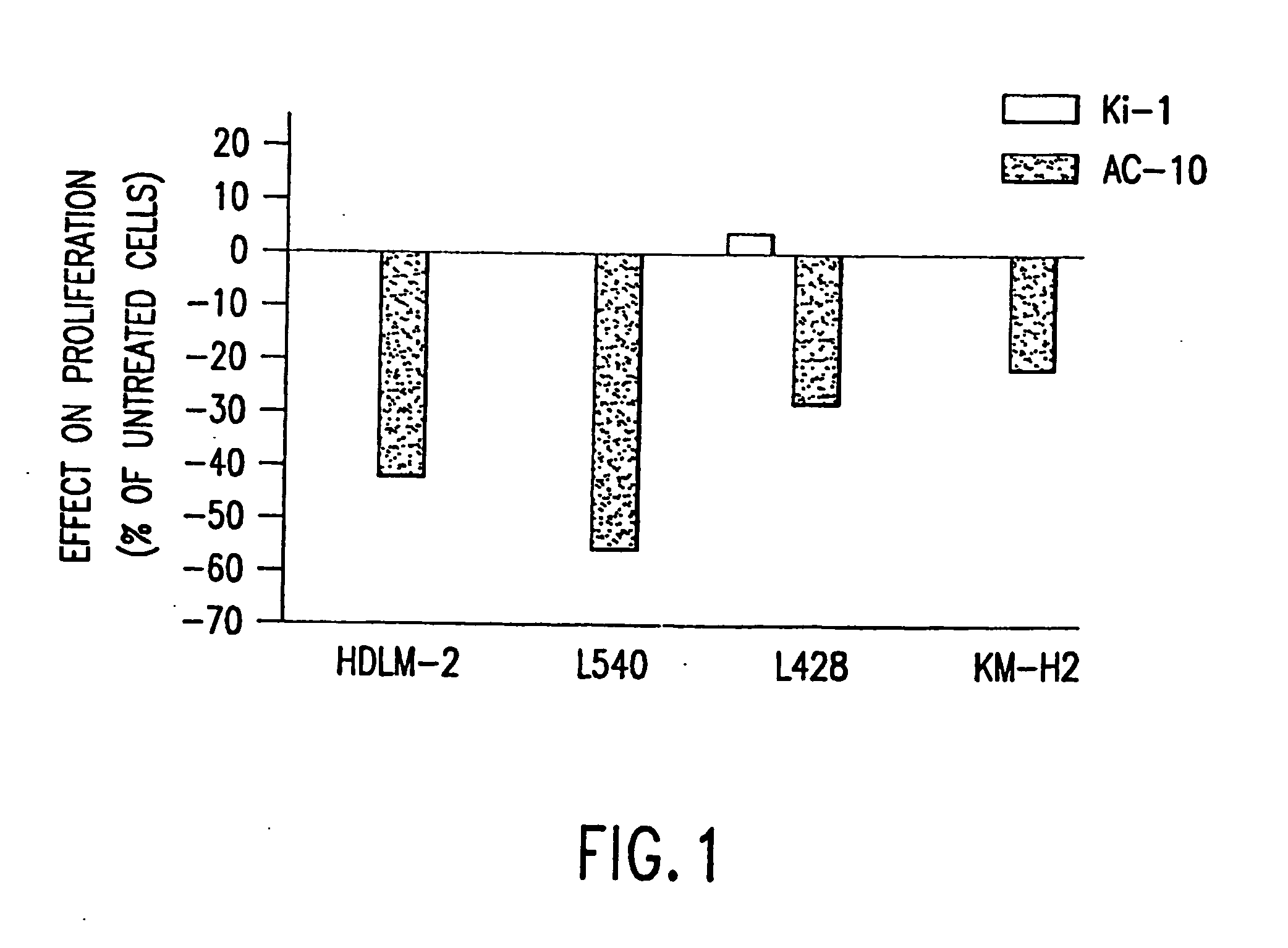
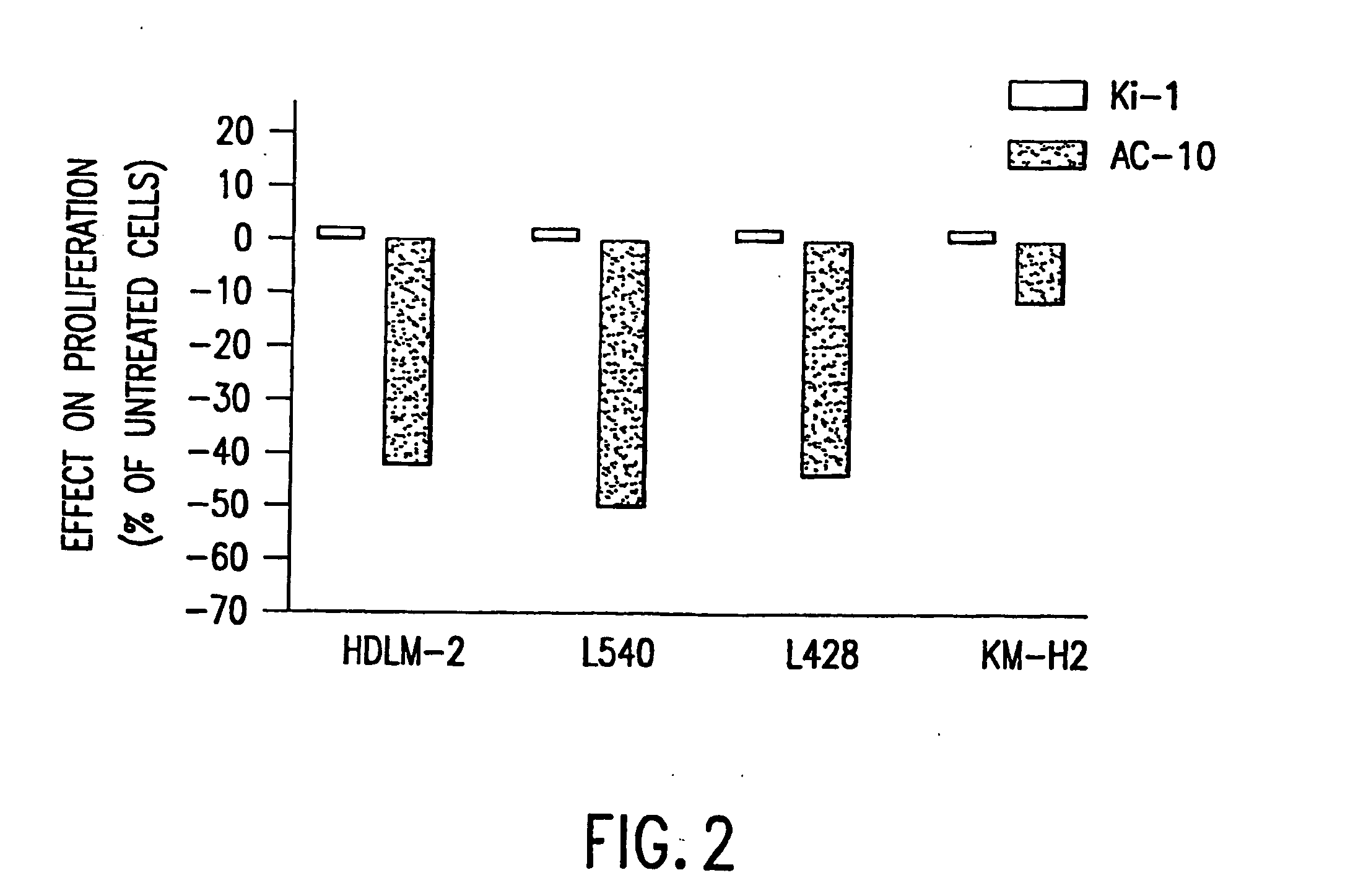
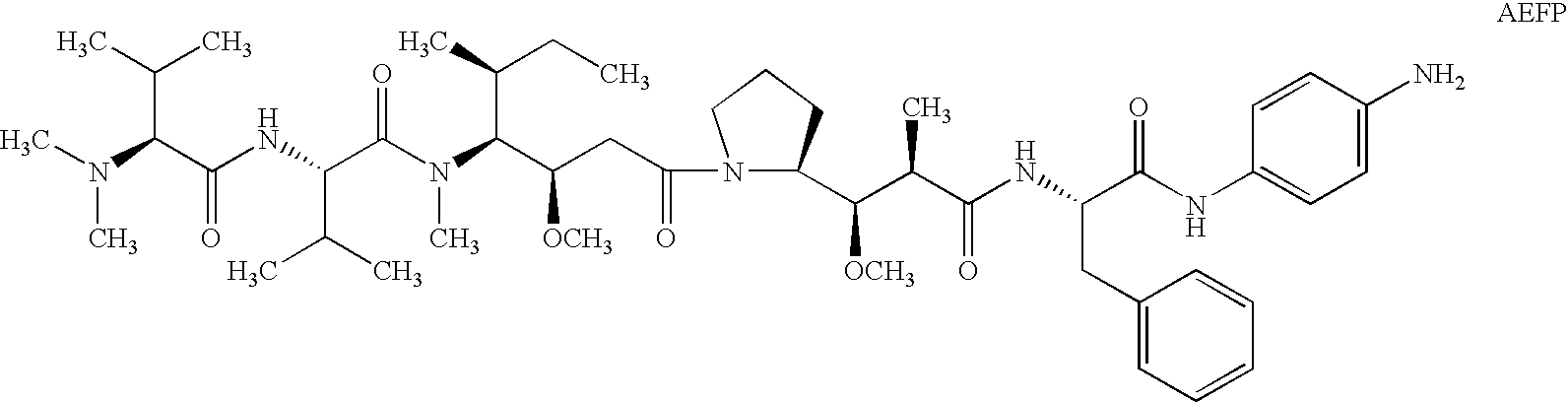
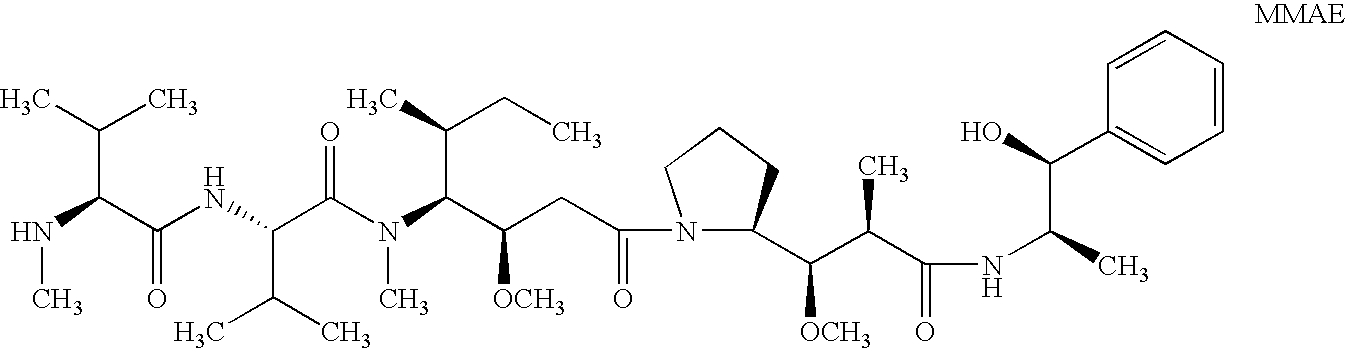
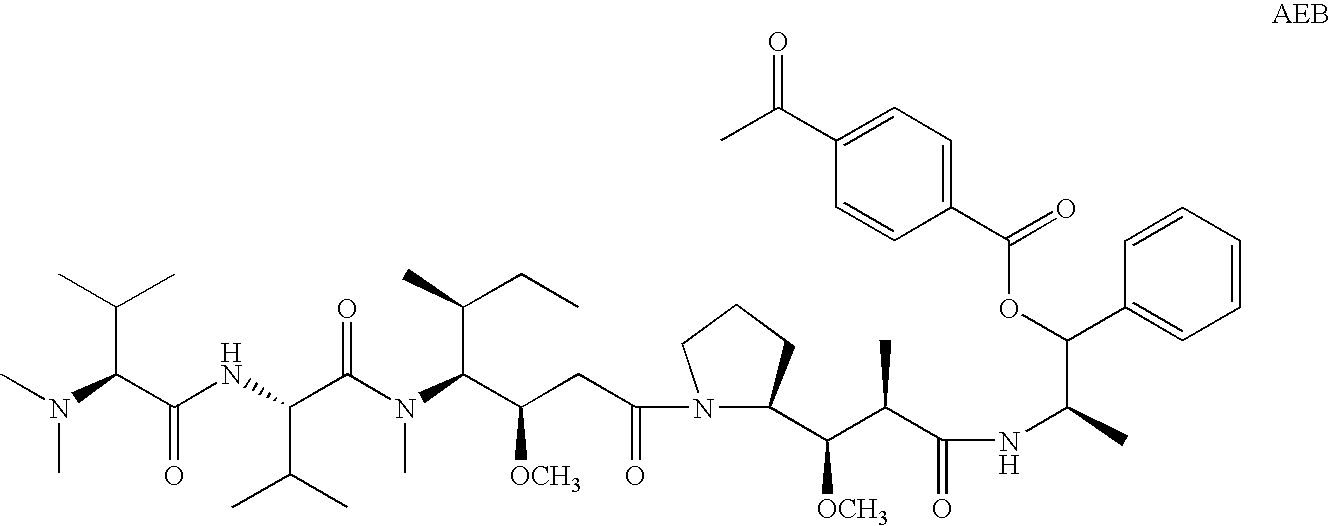
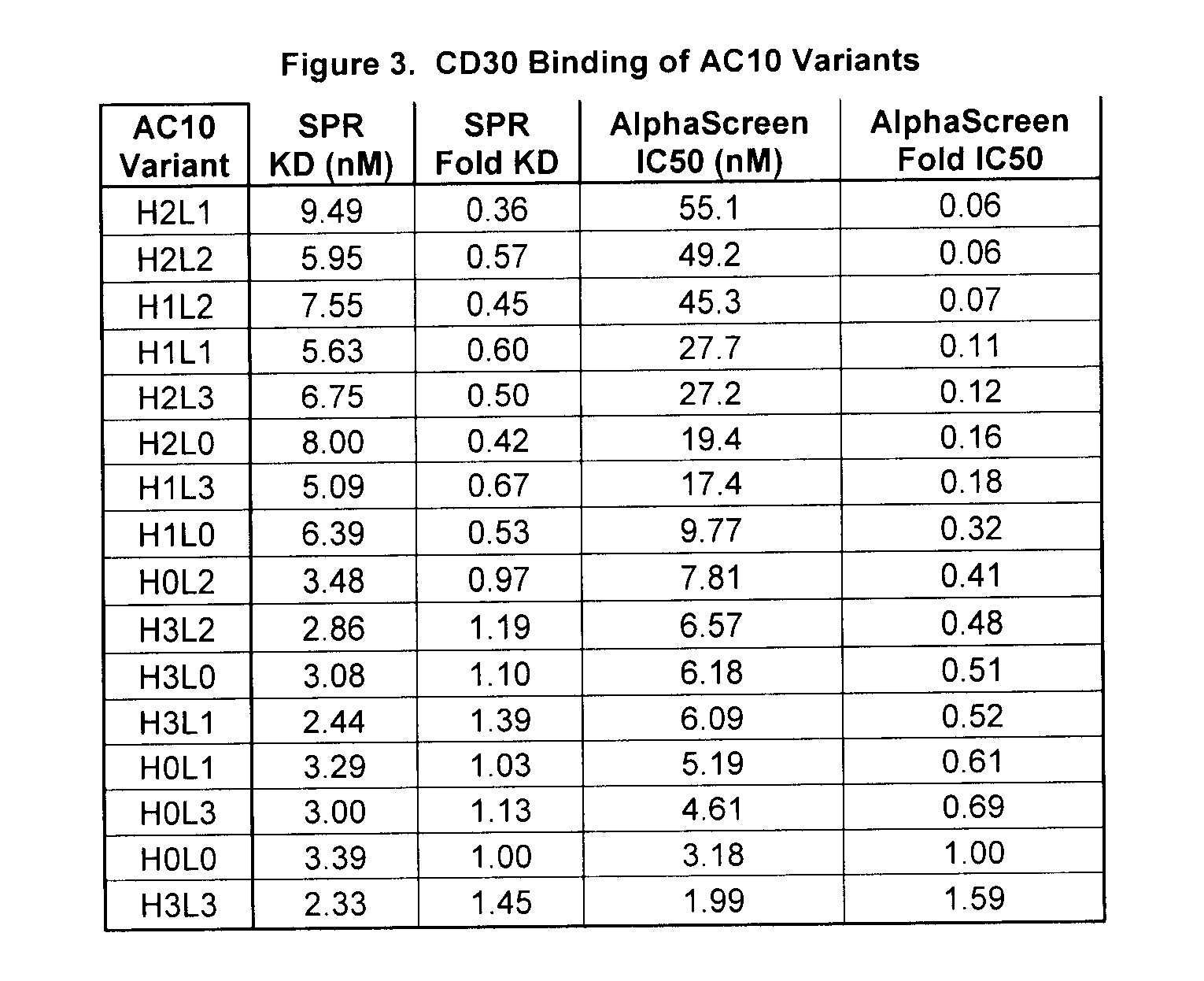
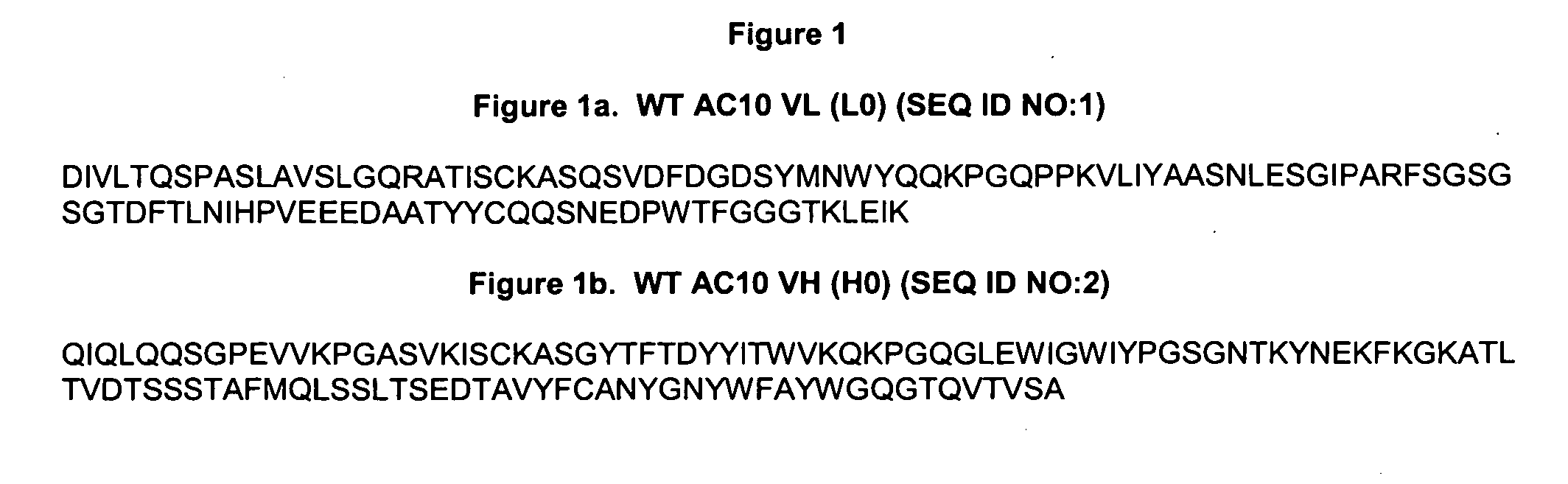
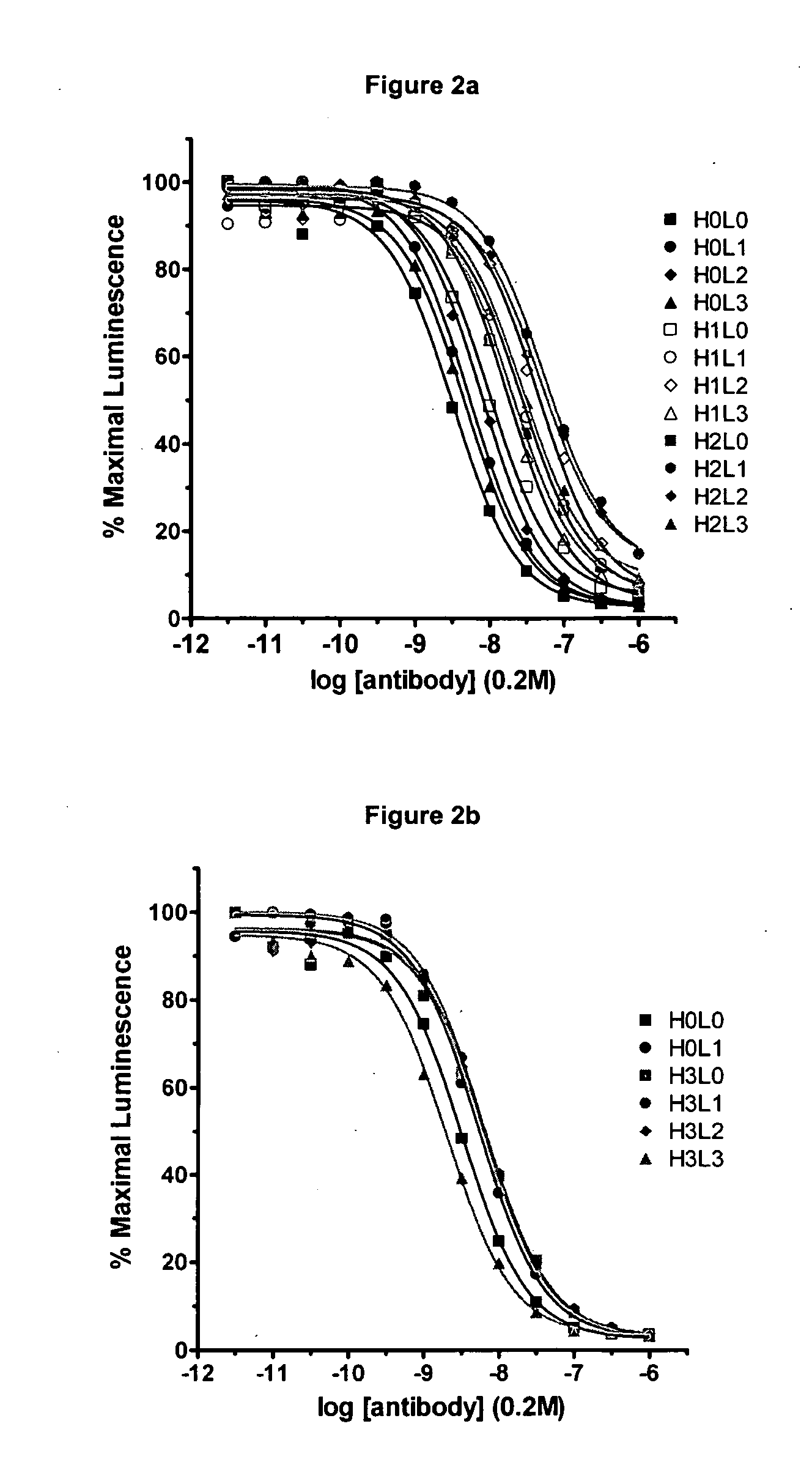
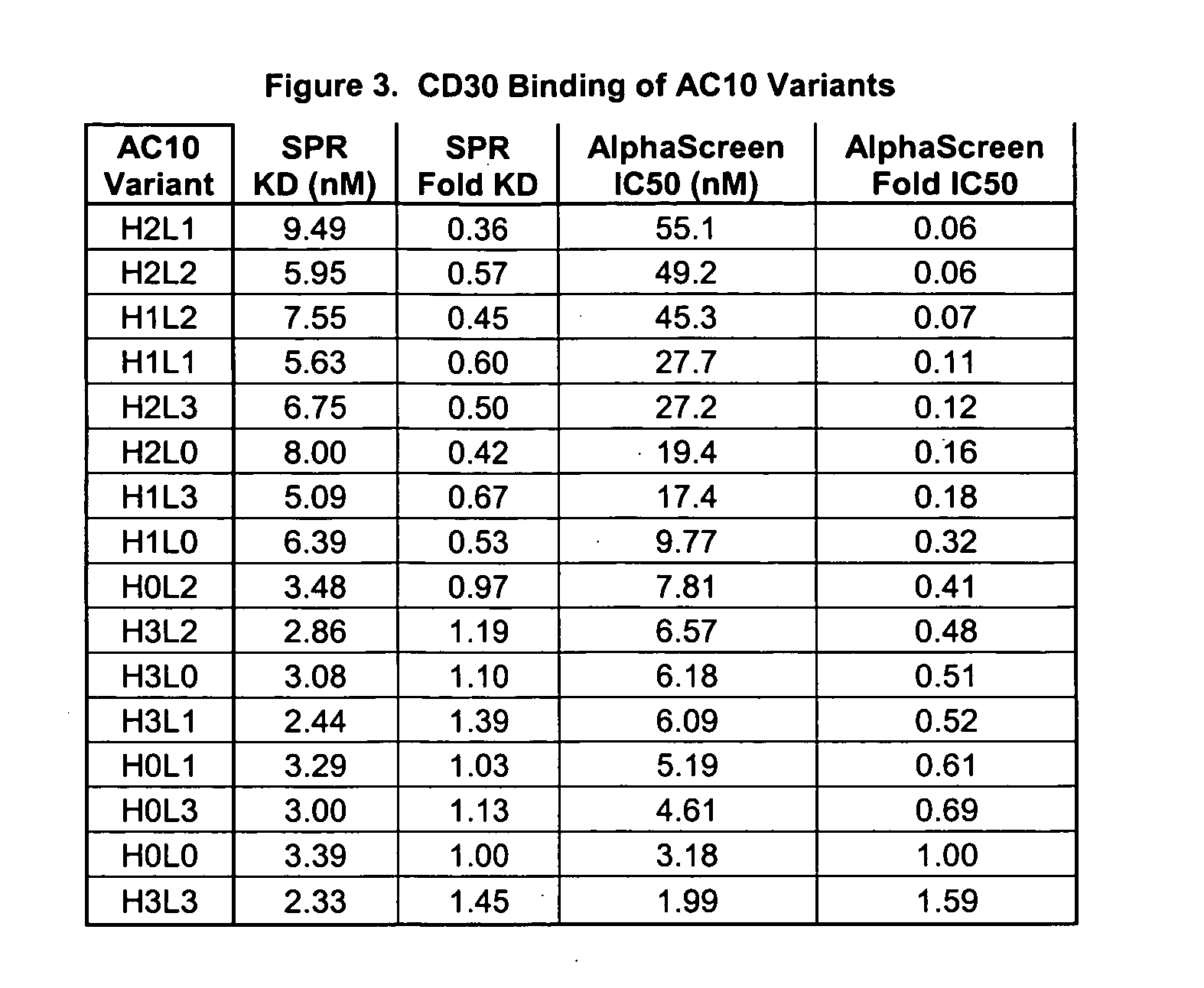
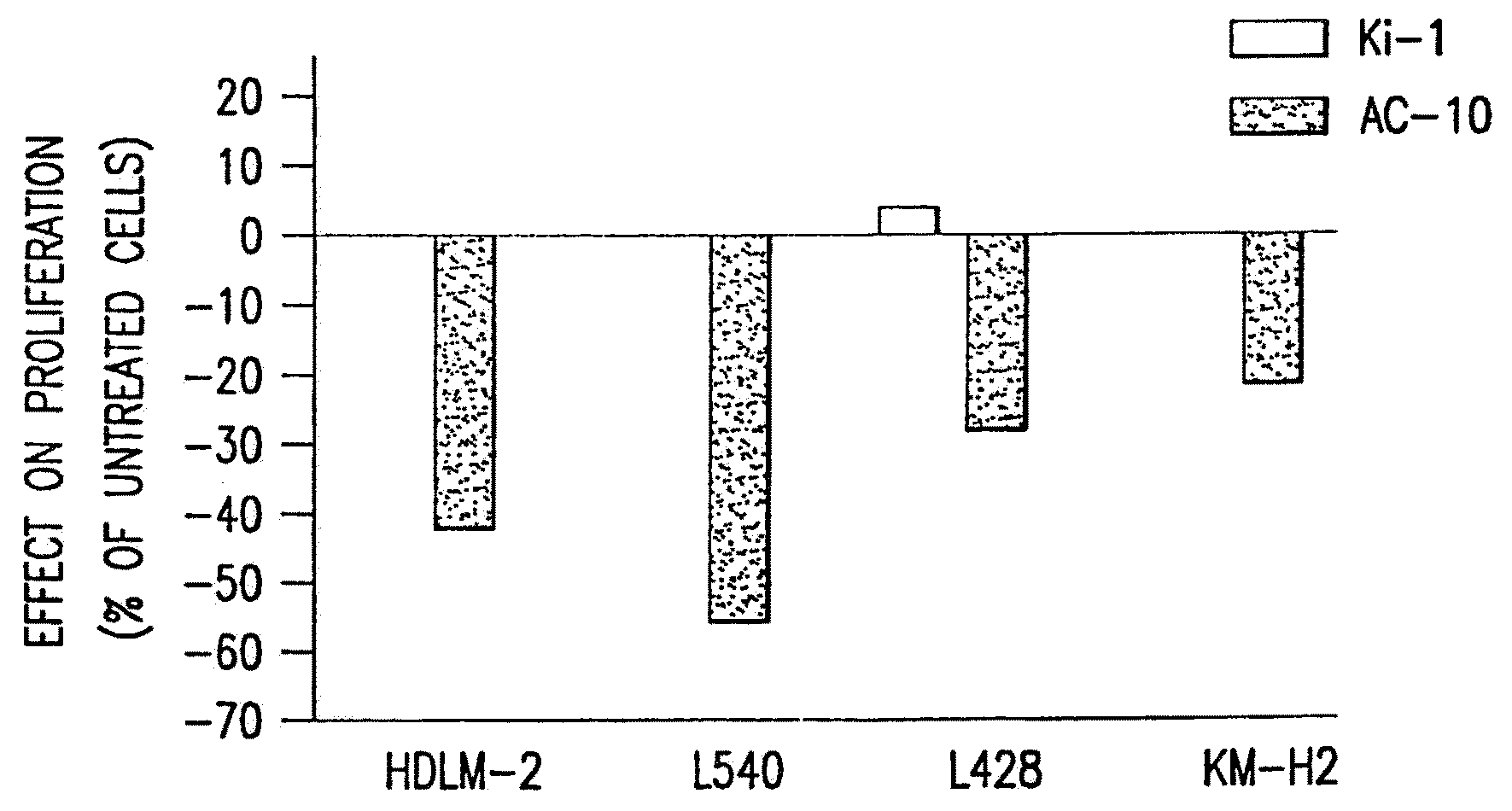
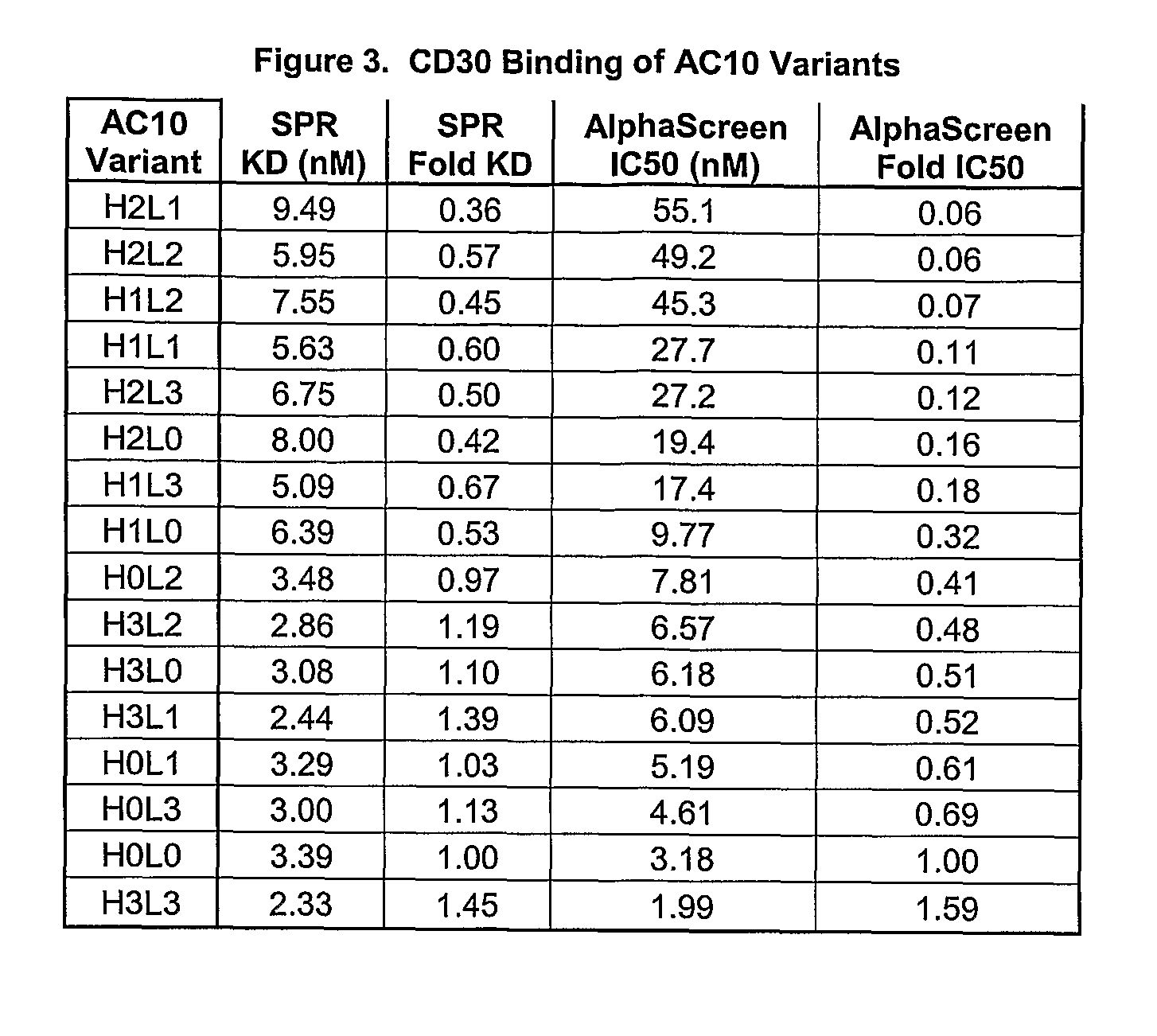
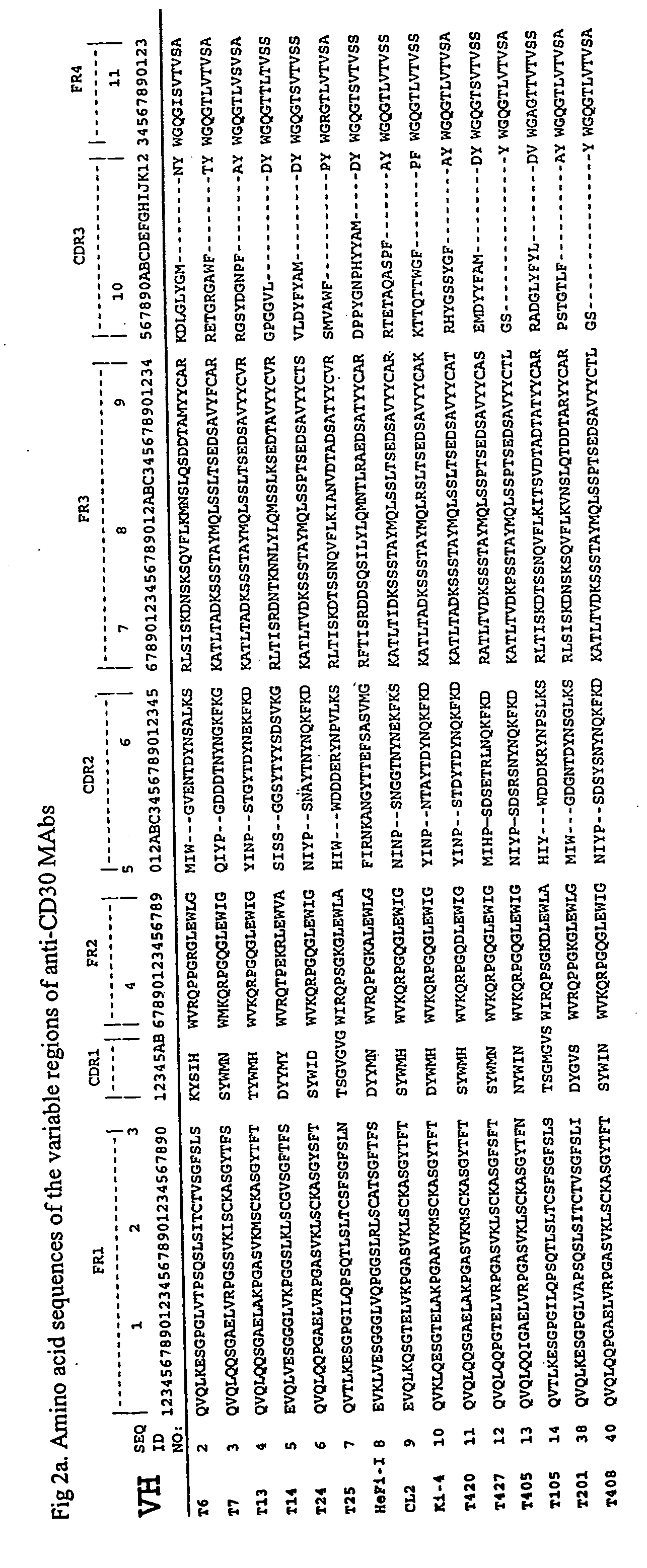
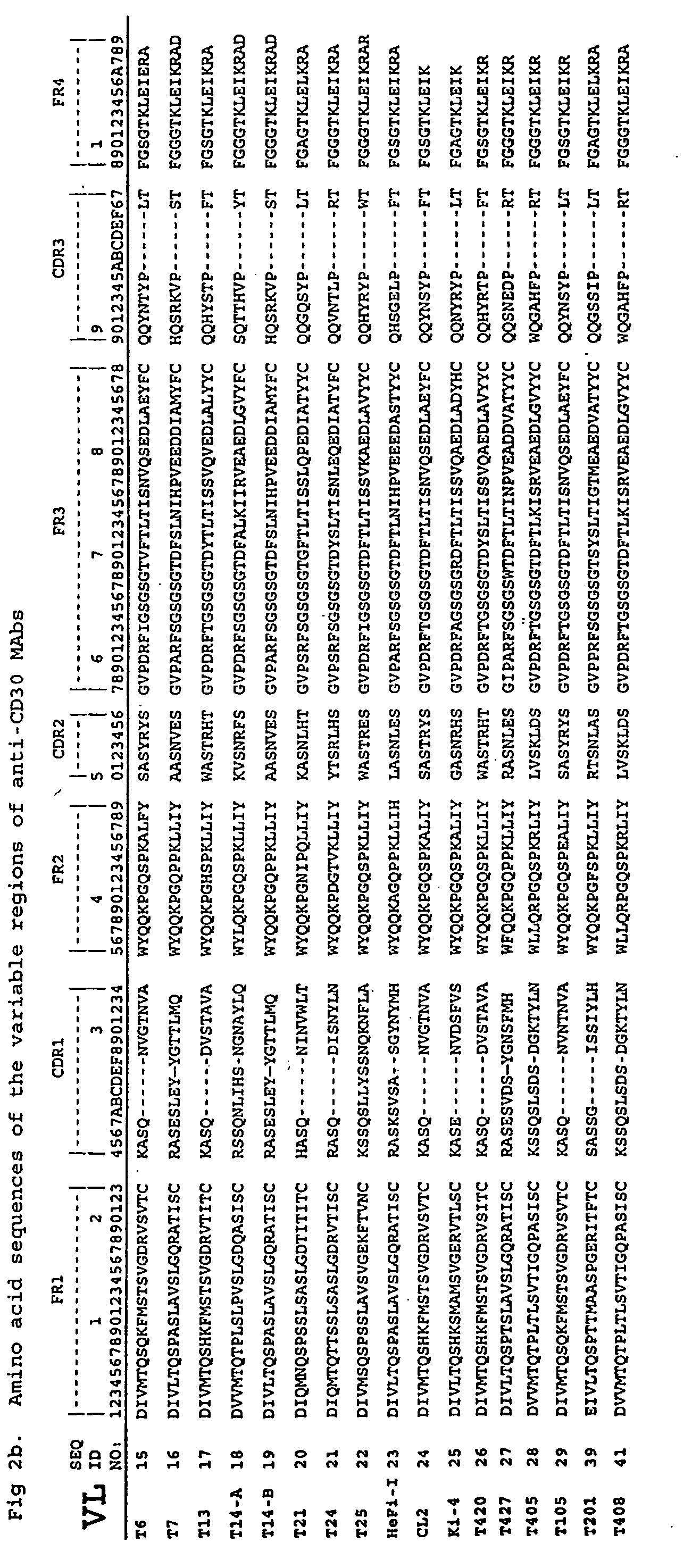
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's disease cells in the absence of effector cells or complement. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEATTLE GENETICS INC
Recombinant anti-CD30 antibodies and uses thereof
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's Disease cells. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEAGEN INC
Recombinant Anti-Cd30 Antibodies and Uses Thereof
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's disease cells in the absence of effector cells or complement. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEATTLE GENETICS INC
Treatment of immunological disorders using anti-dc30 antibodies
InactiveUS20050123536A1Enhancing cytotoxicEnhancing cytostatic effectOrganic active ingredientsSenses disorderDiseaseAntibody conjugate
The present invention relates to methods for the treatment of immunological disorders other than cancer, comprising administering proteins characterized by their ability to bind to CD30 and exert a cytostatic or cytotoxic effect on an activated lymphocyte. Such proteins include monoclonal antibodies AC10 and IleFi1. AC10 and HeFi-1 derivatives, and antibodies that compete with AC10 and HeFi-1 for binding to CD30. Other such proteins include multivalent anti-CD30 antibodies and anti-CD30 antibodies conjugated to cytotoxic agents. Treatment modalities with antibodies of the invention are also provided.
Owner:SEATTLE GENETICS INC
Human monoclonal antibodies against CD30
ActiveUS7387776B2Good curative effectInhibit growth/mediate killingAntipyreticAnalgesicsV(D)J recombinationBispecific antibody
Isolated human monoclonal antibodies which bind to CD30 (e.g., human CD30) are disclosed. The human antibodies can be produced in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are derivatives of the human antibodies (e.g., bispecific antibodies and immunoconjugates), pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:ER SQUIBB & SONS INC
Recombinant Anti-cd30 antibodies and uses thereof
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's disease cells in the absence of effector cells or complement. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEATTLE GENETICS INC
Optimized Anti-CD30 antibodies
InactiveUS20070166309A1Reduced fucosylationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD30Antiantibody
Owner:XENCOR INC
Optimized anti-CD30 antibodies
InactiveUS20070148171A1Reduced fucosylationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD30Antiantibody
Owner:XENCOR
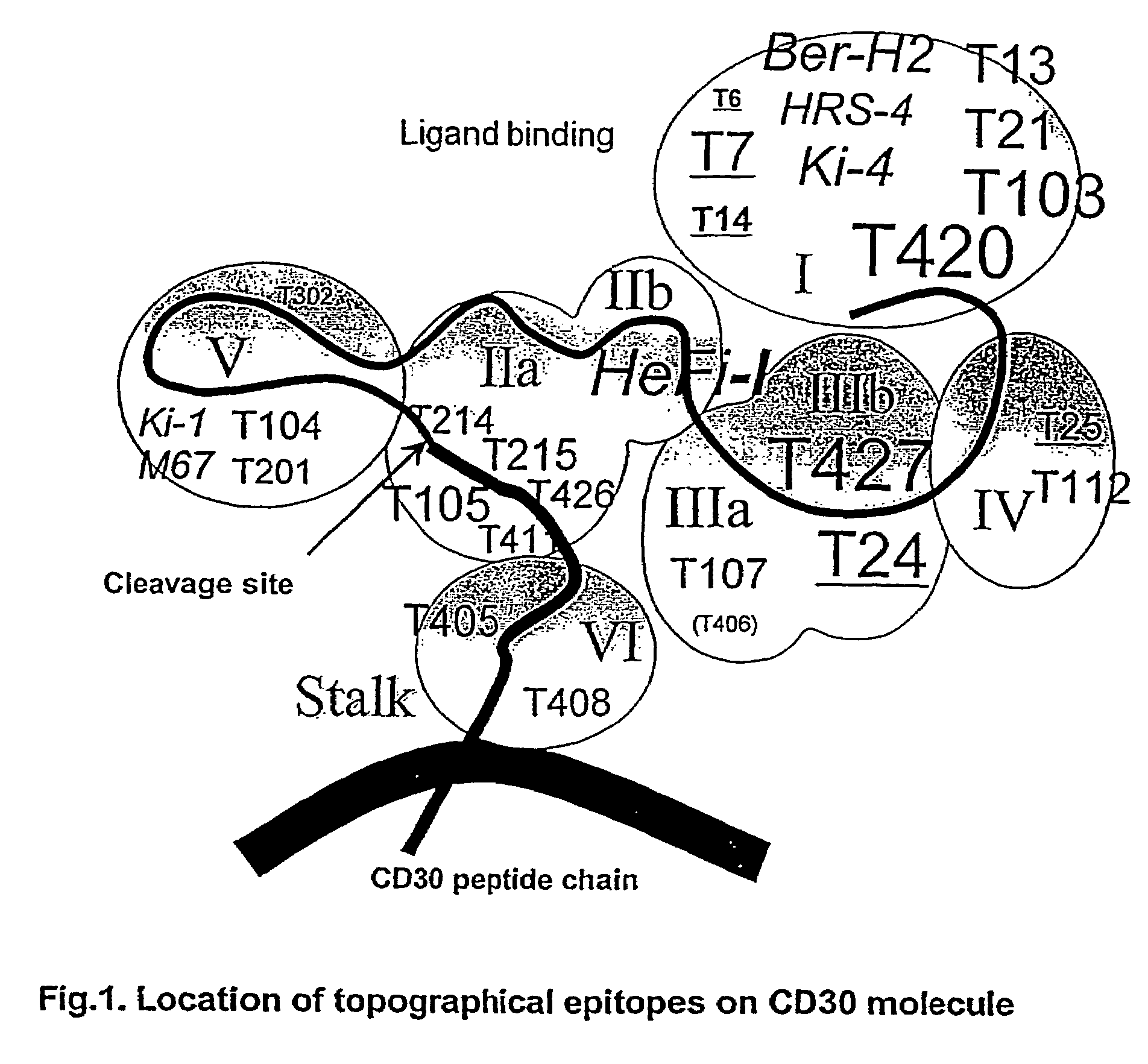
Anti-CD30 stalk and anti-CD30 antibodies suitable for use in immunotoxins
InactiveUS7470775B2Low immunogenicityGrowth inhibitionAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseCancer cell
Owner:UNITED STATES OF AMERICA
Immunoconjugates with improved efficacy for the treatment of diseases
InactiveUS20070196274A1Improve therapeutic efficacyHigh detection sensitivityIn-vivo radioactive preparationsImmunoglobulins against animals/humansAntibody conjugateCD11a
The invention provides therapeutic or diagnostic antibodies with modified N— or C-terminal sequences that are enriched with lysine or tyrosine residues. These lysine or tyfosine residues can be used to couple radioisotopes, cytotoxic agents, or detectable labels. The increased stoichiometric ratios of these agents in the antibody conjugates lead to improved therapeutic efficacy or enhanced detection sensitivity. Non-limiting examples of antibodies suitable for the present invention include anti-CD22, anti-ErbB2, anti-VEGF, anti-EGFR, anti-VEGFR, anti-Her-3, anti-Her-4, anti-CEA, anti-CTLA-4, anti-CD4, anti-CD3, anti-CD20, anti-TNF-a, anti-CD11a, anti-Lewis Y antigen, anti-TrailR, anti-IL2R, anti-CD30, anti-CD146, anti-CD147, anti-alpha V integrin beta, anti-CD19, anti-GD2, anti-3H11, anti-EBV, anti-HIV, anti-HBV, anti-HCV, and other disease-specific antibodies.
Owner:WELSON PHARMA
Monoclonal antibodies against cd30 lacking in fucosyl and xylosyl residues
InactiveUS20120276086A1Strong cytotoxicityArtificial cell constructsImmunoglobulins against cell receptors/antigens/surface-determinantsFucosylationMonoclonal antibody 14G2A
The invention pertains to anti-CD30 antibodies that lack fucosyl and xylosyl residues. The antibodies of the invention exhibit increased antibody-dependent cellular cytotoxicity (ADCC) activity, including the ability to lyse CD30-expressing cell lines that are not lysed by the fucosylated and xylosylated form of the antibodies. The invention also provides host cells that express the anti-CD30 antibodies that lack fucosyl and xylosyl residues, wherein the host cells are deficient for a fucosyltransferase and a xylosyltransferase. Methods of using the antibodies to inhibit the grown of CD30+ cells, such as tumor cells, are also provided.
Owner:BIOLEX THERAPEUTICS INC +2
Method of treatment of th2-mediated conditions using optimized Anti-cd30 antibodies
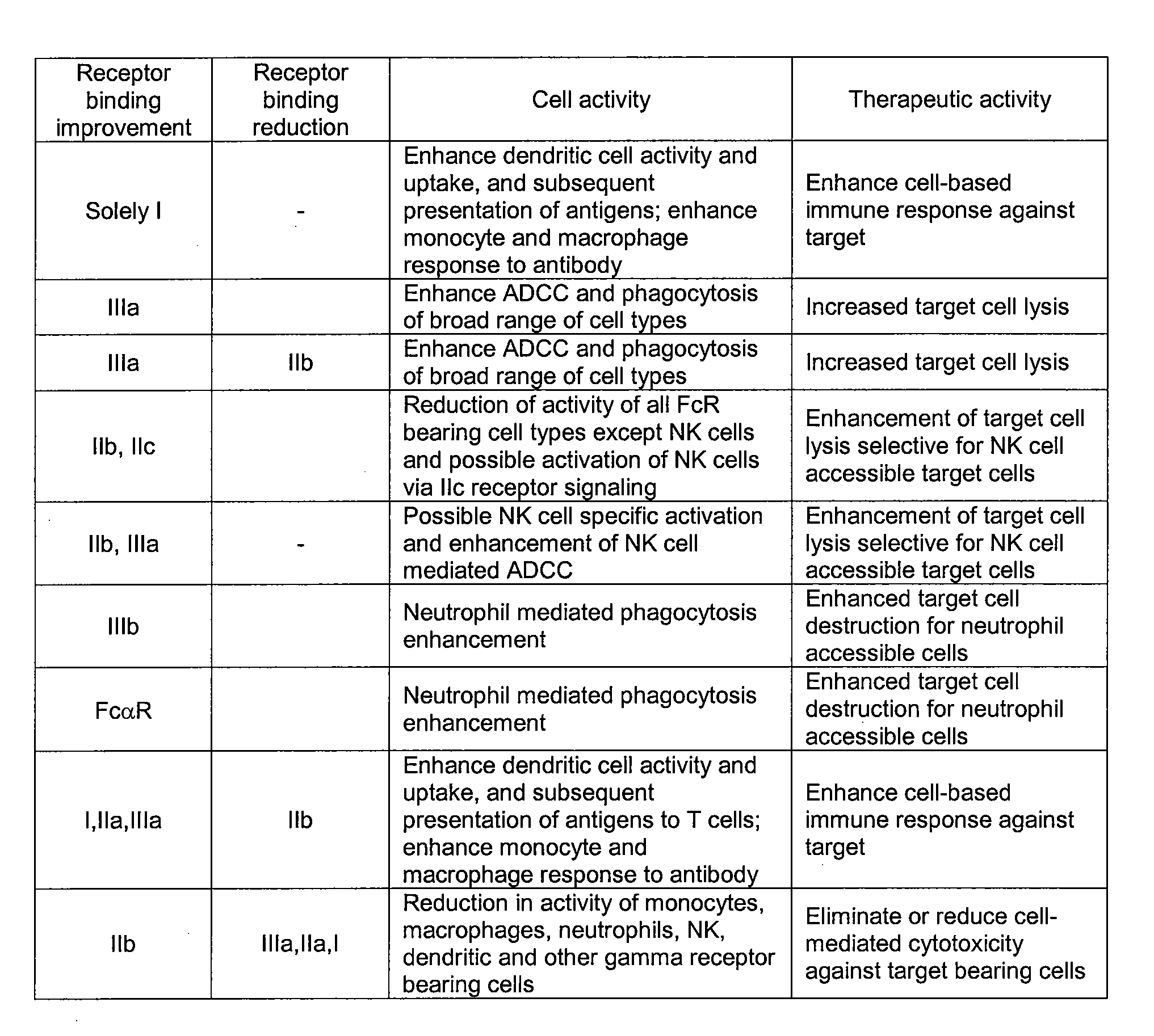
A method of treating Th2-mediated conditions, e.g., atopic conditions, e.g., atopic asthma and / or atopic dermatitis, using effective amount of an antibody that targets CD30, where the antibody has at least one modification relative to a parent antibody and the antibody binds with altered affinity to an FcγR or alters effector function as compared to the parent antibody.
Owner:XENCOR
Recombinant Anti-cd30 antibodies and uses thereof
InactiveUS20080317747A1Nervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseChemotherapeutic drugs
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's disease cells in the absence of effector cells or complement. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEATTLE GENETICS INC
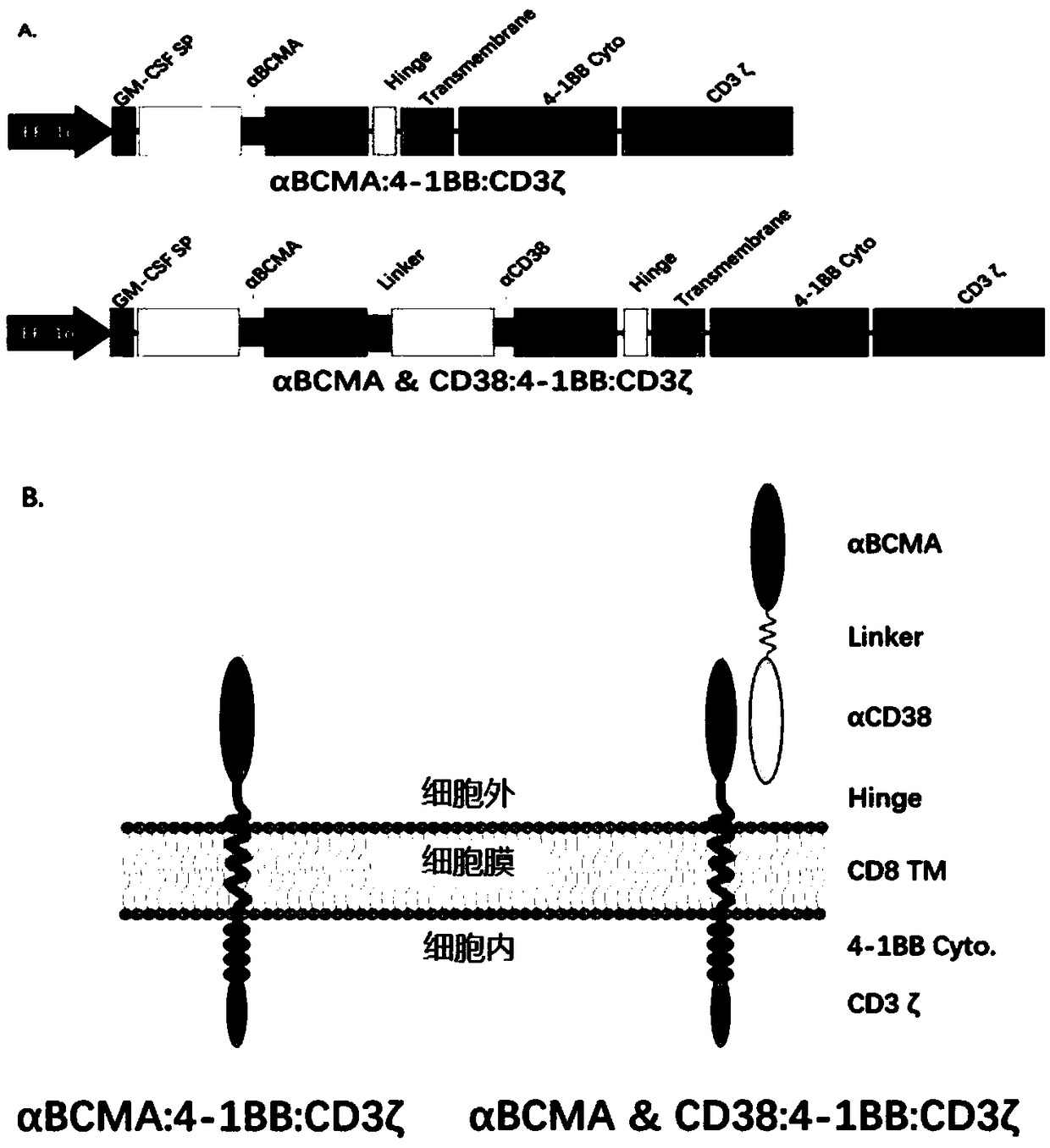
Embedded antigen receptor based on CD30 and application thereof
ActiveCN107312097AHigh expressionImprove immunityAntibody mimetics/scaffoldsMammal material medical ingredientsSingle-Chain AntibodiesAntigen receptors
The invention relates to an embedded antigen receptor based on CD30 and application thereof, and particularly relates to a building method of an embedded antigen receptor T (CAR-T) cell technology based on a tumor specific target site CD30 and application thereof in anti-tumor treatment. The embedded antigen receptor is prepared by connecting an antigen binding structural domain, a transmembrane structural domain, a costimulatory signal conducting region and a CD3 zeta signal conducting structural domain in series, wherein the antigen binding structural domain is combined with tumor-surface antigen, and the tumor-surface antigen is CD30. By adopting the embedded antigen receptor, specific gene transformation on a single chain antibody of the tumor-surface antigen CD30 is carried out, and because of the transformed antibody, the binding force of the antigen-antibody is stronger, and the mutation difficultly occurs; compared with other embedded antigen receptors and other tumor antigens, the embedded antigen receptor has a better effect, and the expression quantity of the target site is high, so that an immunization effect of CAR-T cells is enhanced, and a treatment effect on the CAR-T cells is enhanced.
Owner:SHENZHEN GENO IMMUNE MEDICAL INST
Anti cd30 chimeric antigen receptor and its use
ActiveUS20160200824A1Minimize side effectsIncreased persistenceAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellAntigen receptors
In a first aspect, the present disclosure relates to genetically modified T-cells having a chimeric antigen receptor for use in adoptive cell therapy for treating CD30+cancer in a subject need thereof. In particular, the present disclosure relates to a T-cell containing a specific chimeric antigen receptor being toxic to CD30+cancer cells while being non-toxic to CD30+non-cancer cells. In a further aspect, the present disclosure relates to a specific chimeric antigen receptor and the nucleic acid molecule encoding the receptor as well vectors and cells containing the same. Finally, the present disclosure relates to the use of the chimeric antigen receptor for use in improving persistence and amplification of lymphocyte containing the same and the use of specific peptides for improving persistence and amplification of genetically modified lymphocytes expressing the same.
Owner:CHMIELEWSKI MARKUS +2
Transgenic T cell of targeted CD30 antigen as well as preparation method and application of transgenic T cell
InactiveCN107759699ADisinhibitionFunction increaseHydrolasesAntibody mimetics/scaffoldsAntigenPrimary cell
The invention discloses a transgenic T cell of a targeted CD30 antigen. The transgenic T cell is a primary cell which is integrated with a gene shown as SEQ ID NO:2 and encoding the targeted CD30 antigen, and knocks out a PD1 gene and / or CTLA4 gene, or is a primary cell containing a recombinant lentivirus expression vector (including a gene which is shown as SEQ ID NO:2 and encodes the targeted CD30 antigen and shRNA of a targeted PD1 gene or / and shRNA of a targeted CTLA4 gene); the primary cell is CD4+T cell or CD8+T cell. A preparation method comprises the following steps: firstly, carryingout lentivirus infection on the CD4+T cell or the CD8+T cell; secondly, mixing gRNA, CRISPR-cas9mRNA and HDR, and carrying out electroporation recombination on the T cell to obtain a finished product.According to the transgenic T cell disclosed by the invention, a recognition sequence of an EGFR (Epidermal Growth Factor Receptor) is introduced in carT construction; if necessary, a carT cell can be eliminated by using EGFR monoclonal antibody Cetuximab, the PD1 gene and the CTLA4 gene are knocked out or silenced, inhibition of the gene to the carT cell is eliminated, and the function of overcoming a tumor microenvironment and inhibiting immune cells by the carT cell are enhanced.
Owner:YINFENG BIOLOGICAL GRP
Compositions and methods for treating inflammatory lung disease
The invention provides a method of modulating a T cell immune response by modulating DR3 function in the T cell, wherein the T cell response causes a symptom of inflammatory lung disease. The invention also provides a method of treating a reactive airway disease in an animal subject by administering to the subject an agent which modulates at least one functional activity of CD30. The invention additionally provides a method for treating an inflammatory lung disease by administering an agent that decreases the activity of DR3 or CD30, whereby IL-13 expression is decreased.
Owner:UNIV OF MIAMI +1
Chimeric antigen receptor as well as gene and recombinant expression vector, engineered CD30-targeted NKT cell and application thereof
InactiveCN105859890AEnhance specific killing activityProlong survival timeMammal material medical ingredientsFermentationHinge regionTherapeutic effect
The invention discloses a chimeric antigen receptor as well as a gene and a recombinant expression vector, an engineered CD30-targeted NKT cell and an application thereof. The chimeric antigen receptor is CD30ScFv-CD8-CD137-CD3 zeta, which comprises CD30ScFv, a hinge region and a transmembrane region of CD8, an intracellular signal structural domain of CD137, and an intracellular signal structural domain of CD3 zeta which are connected in series connection. The chimeric antigen receptor CD30ScFv-CD8-CD137-CD3 zeta modified NKT cell is used for treating CD30-positive Hodgkin lymphoma and non-Hodgkin's lymphoma; the chimeric antigen receptor has a good and specific killing activity for lymphoma cells, and has a certain treatment effects for CD30-positive Hodgkin lymphoma patients who have been treated for many times without obvious curative effects (such as CD30 monoclonal antibody combined with cytotoxin medicines or radio isotope therapy and the like).
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI
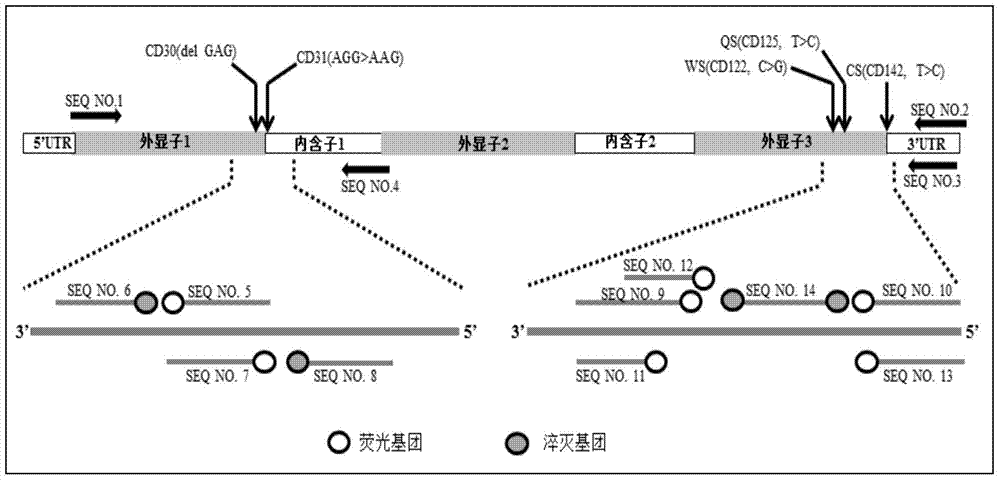
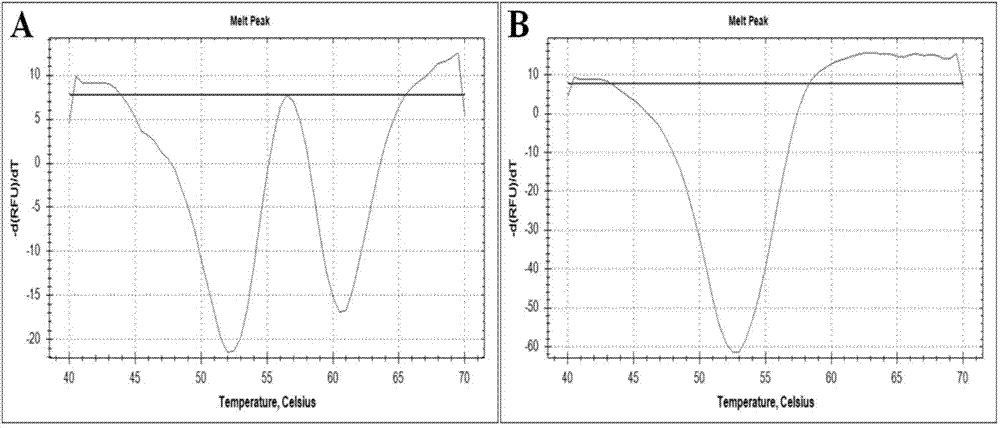
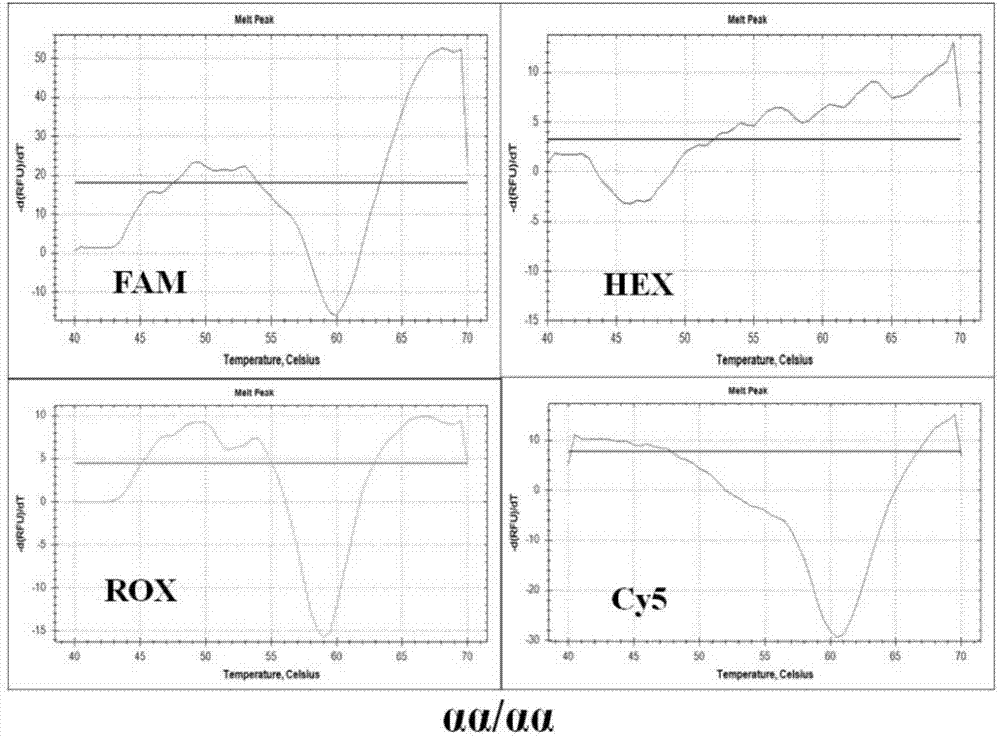
Nested asymmetric PCR reagent kit for detecting alpha 2 globin gene point mutation
ActiveCN103898213AImprove featuresGood repeatabilityMicrobiological testing/measurementFluorescenceWild type
The invention discloses a nested asymmetric PCR reagent kit for detecting alpha 2 globin gene point mutation. The reagent kit comprises a nested asymmetric PCR amplification primer, a fluorescent probe, a quenching probe, an LADNA polymerase and the like. The alpha-thalassemia point mutation genotype of a detected sample is detected by virtue of specific amplification of five point mutation destination fragments WS, QS, CS, CD30 and CD31 of the human alpha 2 globin gene and molecular hybridization and unwinding analysis of a specific fluorescent probe. The reagent kit has good sensitivity and accuracy to wild type and mutation type of alpha-thalassemia five point mutation positions WS, QS, CS, CD30 and CD31, has good repeatability and stability, and is totally suitable for clinical detection of alpha-thalassemia point mutation.
Owner:SOUTHERN MEDICAL UNIVERSITY
Duplex-specific chimeric antigen receptor molecule and application thereof to tumor therapy
ActiveCN109503716AIncreased persistenceImprove targetingPeptide/protein ingredientsMammal material medical ingredientsSequence signalCD20
Owner:CELLYAN THERAPEUTICS WUHAN CO LTD
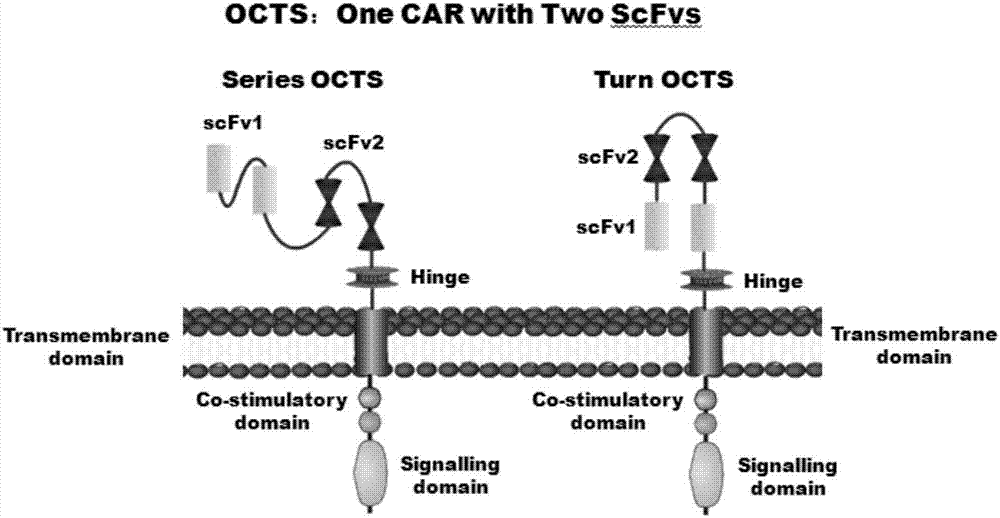
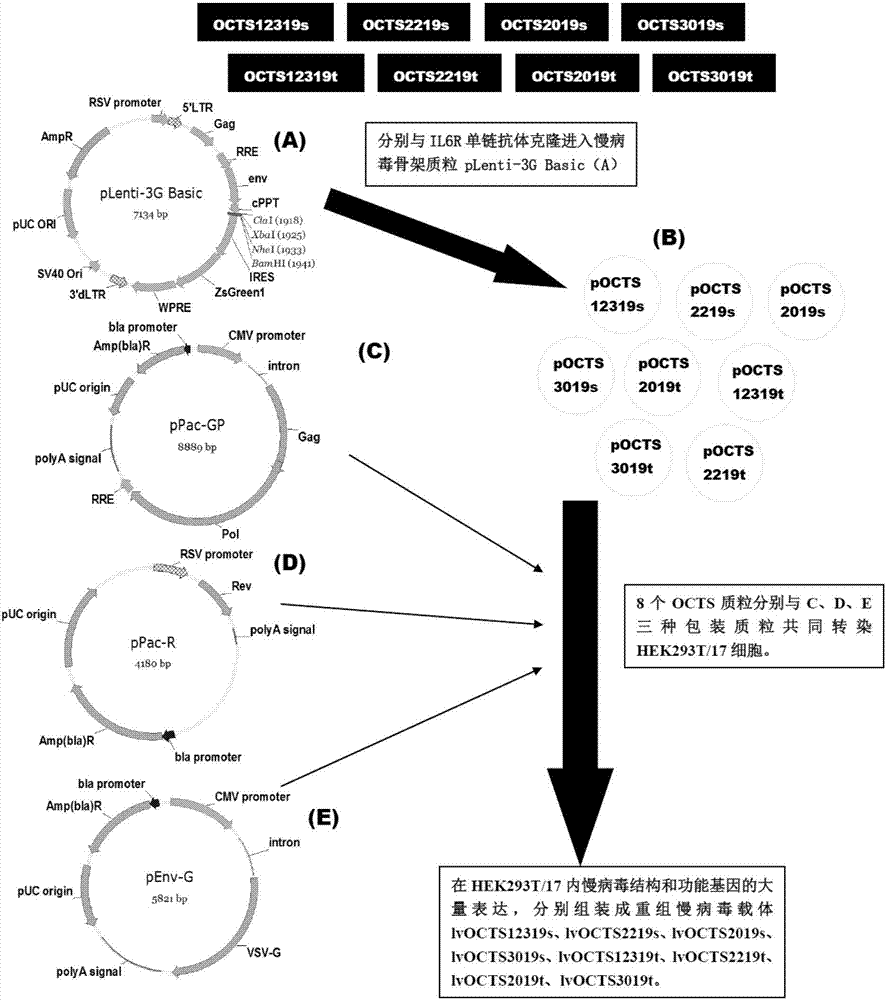
Lymphoblastic leukemia CAR-T (Chimeric Antigen Receptor-T Cell Immunotherapy) therapy carrier based on OCTS (One CAR with Two SeFvs) technology as well as constructing method and application of lymphoblastic leukemia CAR-T therapy carrier
ActiveCN107245500AExpand the scope of recognitionAvoid batch cultureMammal material medical ingredientsNGF-receptor/TNF-receptor superfamilySequence signalCD20
The invention discloses a lymphoblastic leukemia CAR-T (Chimeric Antigen Receptor-T Cell Immunotherapy) therapy carrier based on an OCTS (One CAR with Two SeFvs) technology. The lymphoblastic leukemia CAR-T therapy carrier comprises a lentivirus skeleton plasma, a human EF1alpha promoter, an OCTS chimeric receptor structural domain and an IL6R single-chain antibody, wherein the OCTS chimeric receptor structural domain comprises a CD8 leader chimeric receptor signal peptide and two groups of single-chain antibodies; the first group of single-chain antibodies is selected from any one of the following four groups of single-chain antibodies: a CD20 single-chain antibody light chain VL and a CD20 single-chain antibody heavy chain VH, a CD22 single-chain antibody light chain VL and a CD22 single-chain antibody heavy chain VH, a CD30 single-chain antibody light chain VL and a CD30 single-chain antibody heavy chain VH, and a CD123 single-chain antibody light chain VL and a CD123 single-chain antibody heavy chain VH; and the second group of the single-chain antibodies is a CD19 single-chain antibody light chain VL and a CD19 single-chain antibody heavy chain VH, an antibody Inner-Linker, a single-chain antibody Inter-Linker, a CD8-Hinge chimeric receptor linker, a CD8 Transmembrane chimeric receptor transmembrane zone, a TCR (T Cell Receptor) chimeric receptor T cell activation domain and a chimeric receptor co-stimulator zone. Besides, the invention discloses a constructing method for the carrier and application of the carrier to preparation of a drug for treating lymphoblastic leukemia.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Multiplexed method for diagnosing classical hodgkin lymphoma
InactiveUS20150226743A1Good precisionEnhanced informationLibrary screeningAntibody medical ingredientsSingle sampleCD20
A method of analyzing a biological sample suspected of having classic Hodgkin lymphoma, comprising (1) detecting, in a single sample, for the expression of at least two biomarkers selected from CD30, CD15, CD20, CD45, CD3, Pax-5, CD79A, BOB1 and OCT-2; and (b) analyzing the sample based on the presence, absence and / or expression level of the at least two biomarkers. Also provided is a method wherein all the nine biomarkers are analyzed on a single sample. Further provided are method for diagnosing classical Hodgkin lymphoma, as well as system and kit that comprise the means for executing the novel methods.
Owner:GE HEALTHCARE BIO SCI CORP
Human monoclonal antibodies against CD30
ActiveUS20100322920A1Inhibit growth/mediate killingMore therapeutically effective and usefulAntipyreticAnalgesicsV(D)J recombinationBispecific antibody
Isolated human monoclonal antibodies which bind to CD30 (e.g., human CD30) are disclosed. The human antibodies can be produced in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are derivatives of the human antibodies (e.g., bispecific antibodies and immunoconjugates), pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:ER SQUIBB & SONS INC
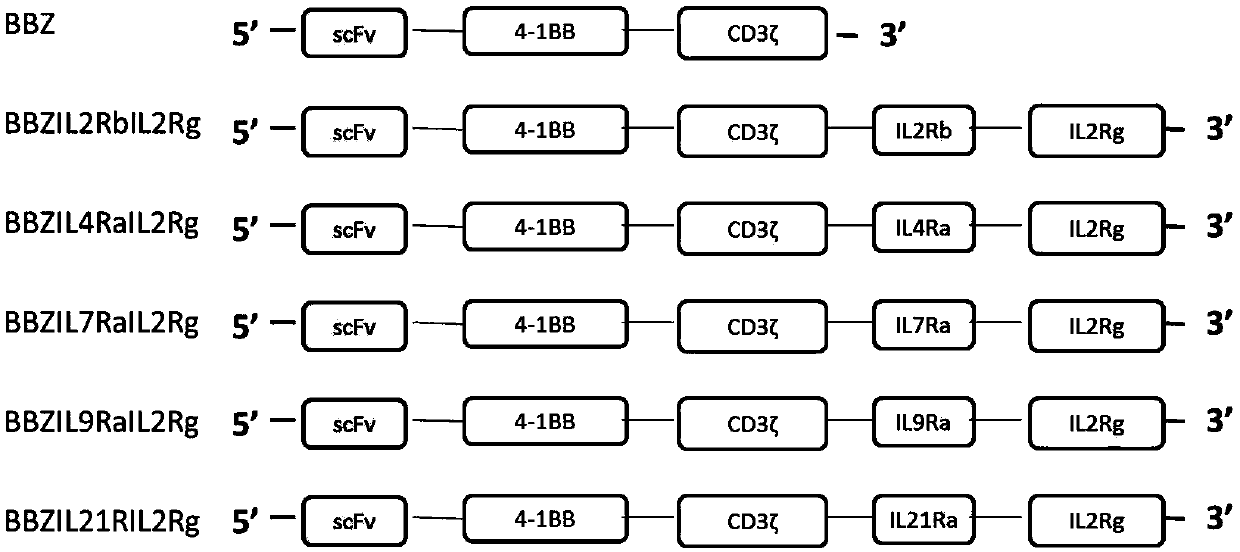
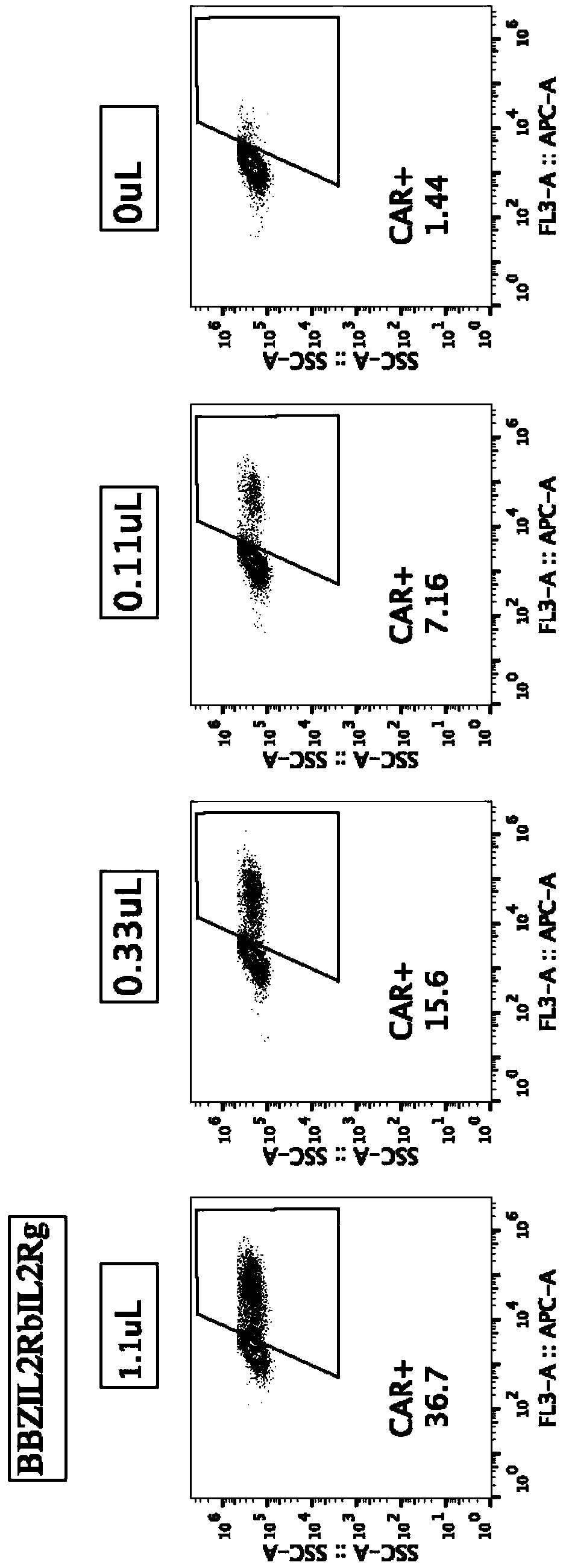
Chimeric antigen receptor (CAR) containing third signal receptor and application of CAR
ActiveCN110615843AIncrease lethalityImprove acceleration performanceVirusesPeptide/protein ingredientsSide effectTumor targeting
The invention relates to a chimeric antigen receptor (CAR) containing a third signal receptor. The structure of the CAR is scFv(X)-(Y)CD3zeta-MN, wherein X comprises a tumor-targeting antibody or a ligand and receptor capable of being specifically combined with tumors, Y is an intracellular region of a costimulatory receptor, the costimulatory receptor is selected from ICOS, CD28, CD27, HVEM, LIGHT, CD40L, 4-1BB, OX40, DR3, GITR, CD30, TIM1, SLAM, CD2 and CD226, M is an intracellular region of a cytokine receptor of a gamma chain family, the cytokine receptor is selected from IL2Ra, IL2Rb, IL4Ra, IL7Ra, IL9Ra, IL15Ra and IL21Ra, and N is an IL2Rg intracellular region. The invention further relates to a CAR-T cell constructed from a recombinant expression vector of the CAR and a preparationmethod and application of the CAR-T cell. Through the CAR-T cell, the tumor killing ability and the amplification ability are significantly improved, the CAR-T cell contains the third signal receptor, the third signal receptor has the potential function of effect enhancement and only has the effect on the CAR-T cell, and the risk of causing immune side effects is reduced.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
Monoclonal antibodies against cd30 lacking in fucosyl and xylosyl residues
InactiveUS20090175886A1Strong cytotoxicityHybrid immunoglobulinsDepsipeptidesFucosylationMonoclonal antibody
Owner:BIOLEX THERAPEUTICS INC +1
Anti CD30 chimeric antigen receptor and its use
ActiveUS10808035B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAdoptive cellular therapyCancer cell
In a first aspect, the present disclosure relates to genetically modified T-cells having a chimeric antigen receptor for use in adoptive cell therapy for treating CD30+cancer in a subject need thereof. In particular, the present disclosure relates to a T-cell containing a specific chimeric antigen receptor being toxic to CD30+cancer cells while being non-toxic to CD30+non-cancer cells. In a further aspect, the present disclosure relates to a specific chimeric antigen receptor and the nucleic acid molecule encoding the receptor as well vectors and cells containing the same. Finally, the present disclosure relates to the use of the chimeric antigen receptor for use in improving persistence and amplification of lymphocyte containing the same and the use of specific peptides for improving persistence and amplification of genetically modified lymphocytes expressing the same.
Owner:CHMIELEWSKI MARKUS +2
Anti-cd30 stalk and anti-cd30 antibodies suitable for use in immunotoxins
InactiveUS20060193771A1Low immunogenicityGrowth inhibitionAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseCancer cell
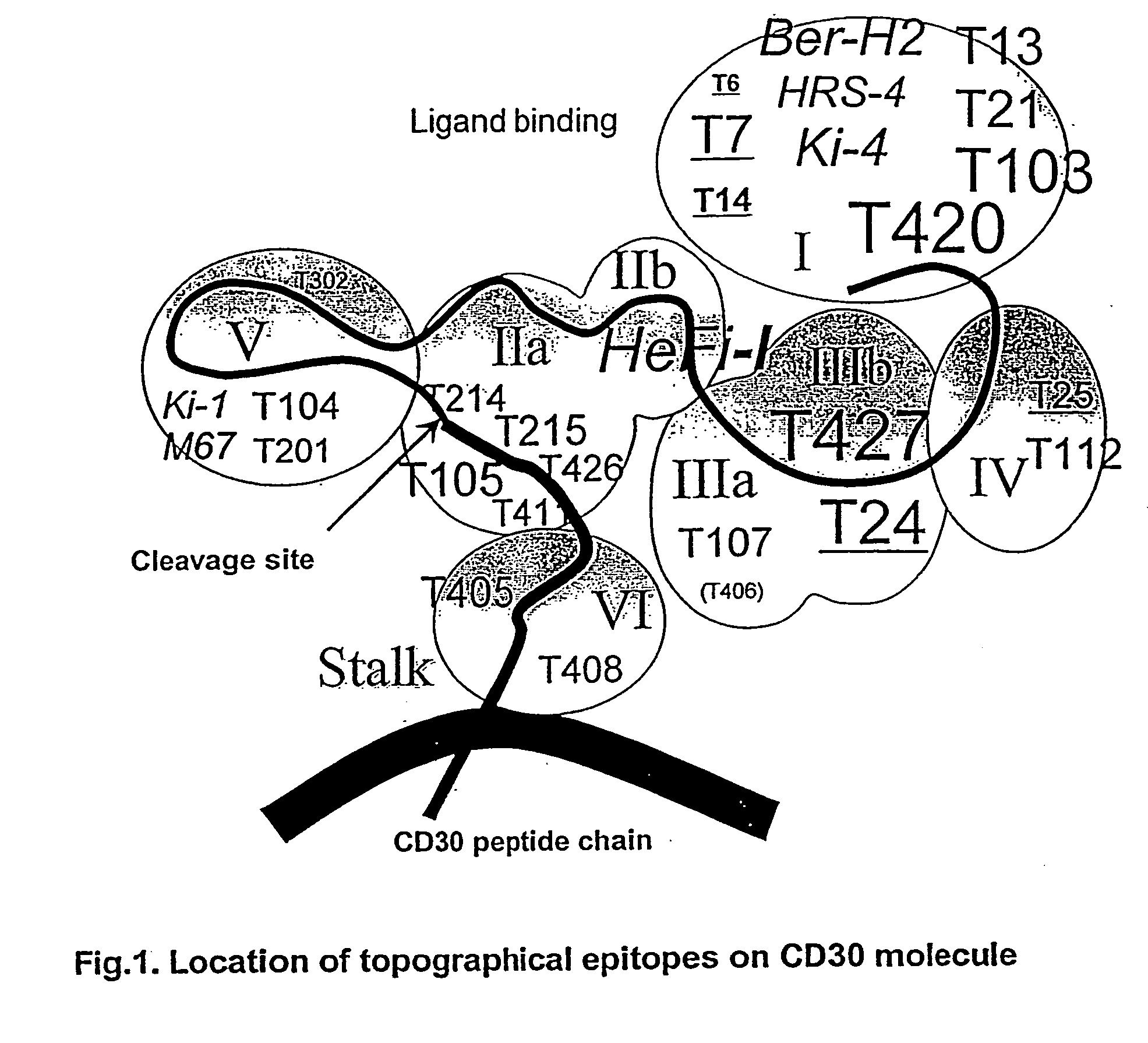
CD30 is a receptor expressed on cells of Hodgkin's disease and certain leukemias. The extracellular portion of CD30 is cleaved, releasing a form known as sCD30. The invention relates in part to the discovery that a residual, extracellular “stalk” of CD30 remains after cleavage of sCD30. The stalk provides an advantageous and previously unrecognized target for immunotoxins. The invention provides antibodies that bind to the CD30 stalk or to epitopes destroyed upon the cleavage of CD30 which results in the stalk. The invention further provides new anti-CD30 antibodies that form effective immunotoxins and are particularly suitable for making disulfide stabilized Fv (“dsFv”)-immunoconjugates. The dsFv immunoconjugates can be used as reagents to label CD30-expressing cancer cells or to inhibit the growth of CD30-expressing cancer cells. Moreover, the invention provides anti-CD30 antibodies that activate complement-dependent cytotoxicity.
Owner:UNITED STATES OF AMERICA
Anti-CD30 chimeric antigen receptors
ActiveUS10815301B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsPharmaceutical drug
Chimeric antigen receptors (CARs) that specifically bind to and immunologically recognize CD30 are disclosed. Related nucleic acids, recombinant expression vectors, host cells, populations of cells, and pharmaceutical compositions relating to the CARs are also disclosed. Methods of treating or preventing a condition in a mammal, wherein the condition is cancer, are also disclosed.
Owner:UNITED STATES OF AMERICA
Car-cd30 t cells for treatment of cd30+ tumors
PendingCN112261950AEfficient removalBuild long-term immune memoryPolypeptide with localisation/targeting motifImmunoglobulin superfamilyOncologyT cell
The present invention concerns a third generation of CAR-CD30 T cells for treatment of CD30+ Tumors such as lymphoid malignancies, leukemia, solid tumors.
Owner:OSPEDALE PEDIATRICO BAMBINO GESU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com