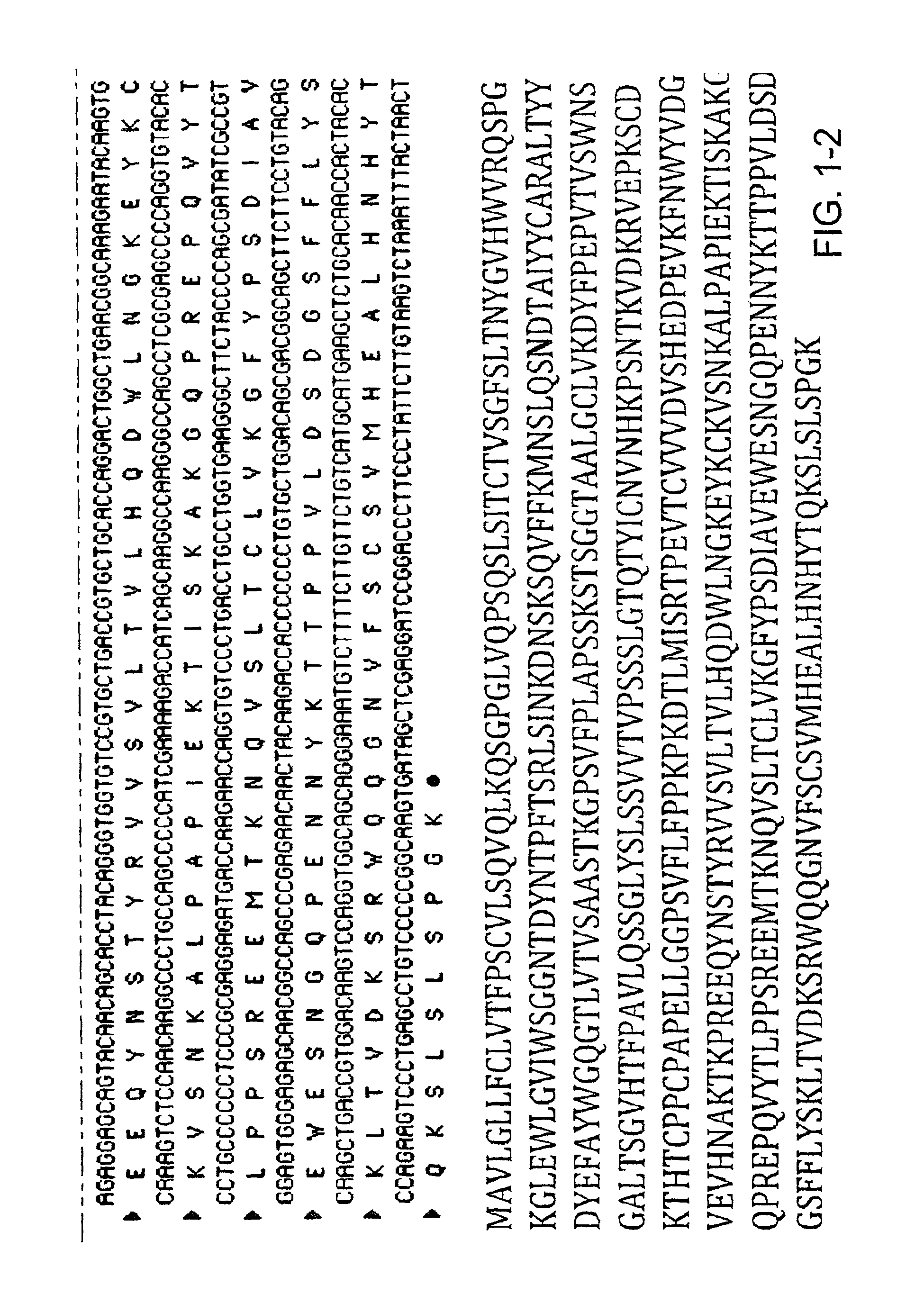
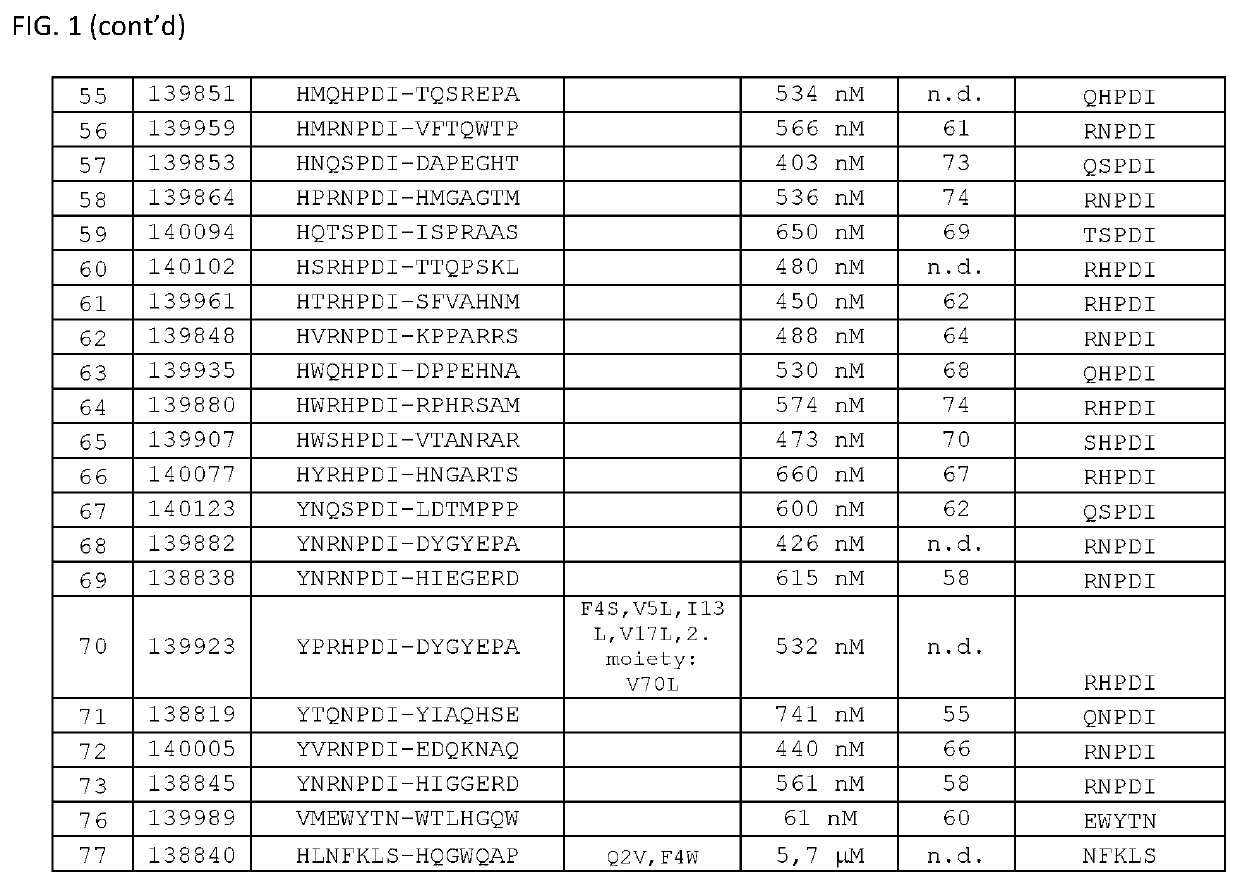
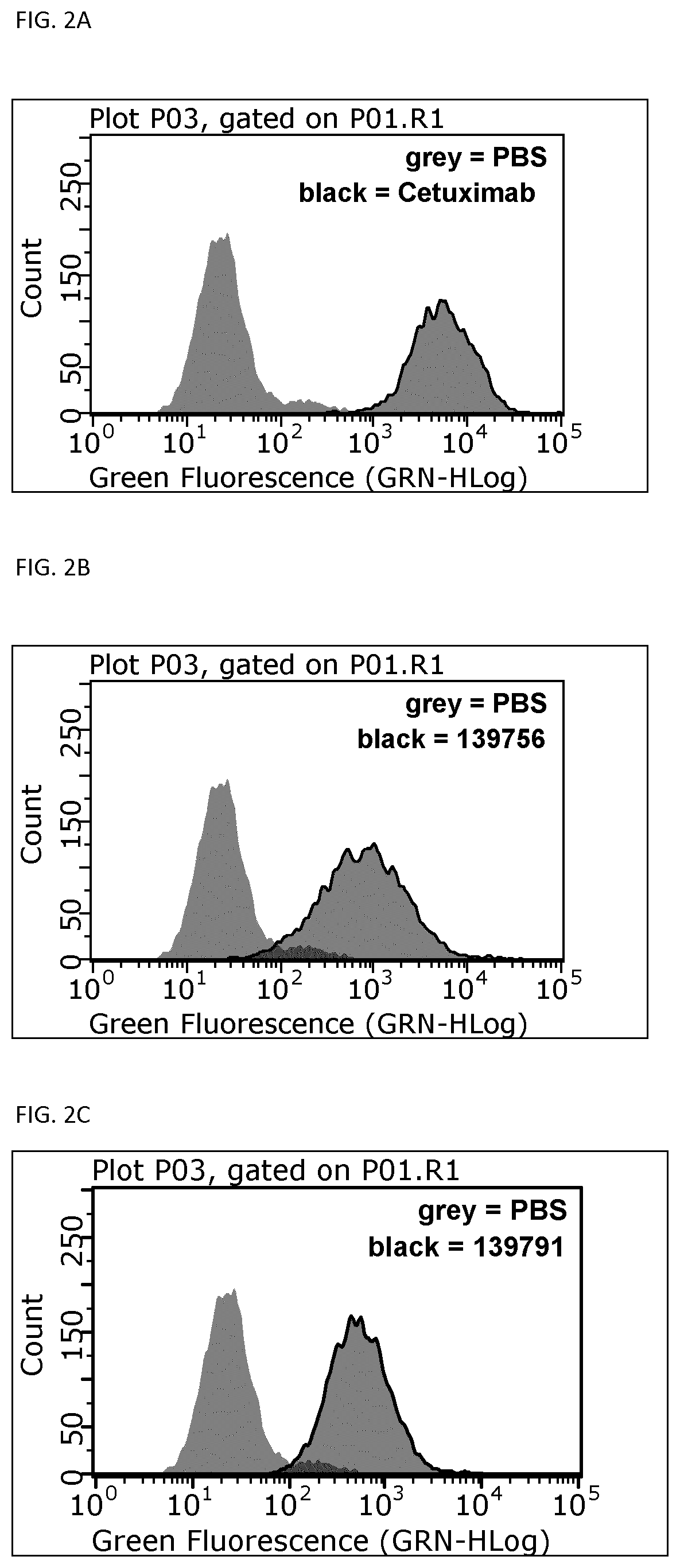
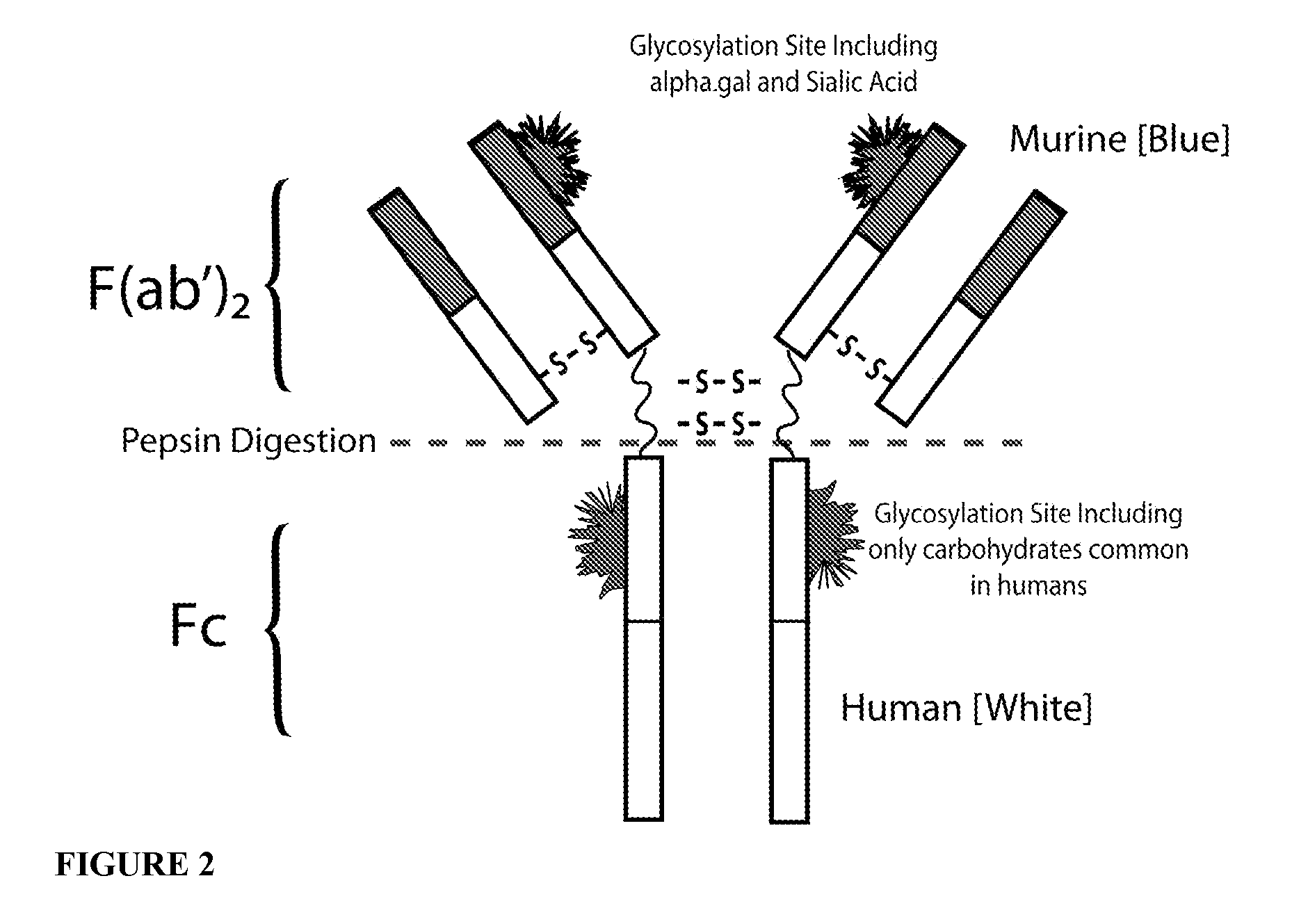
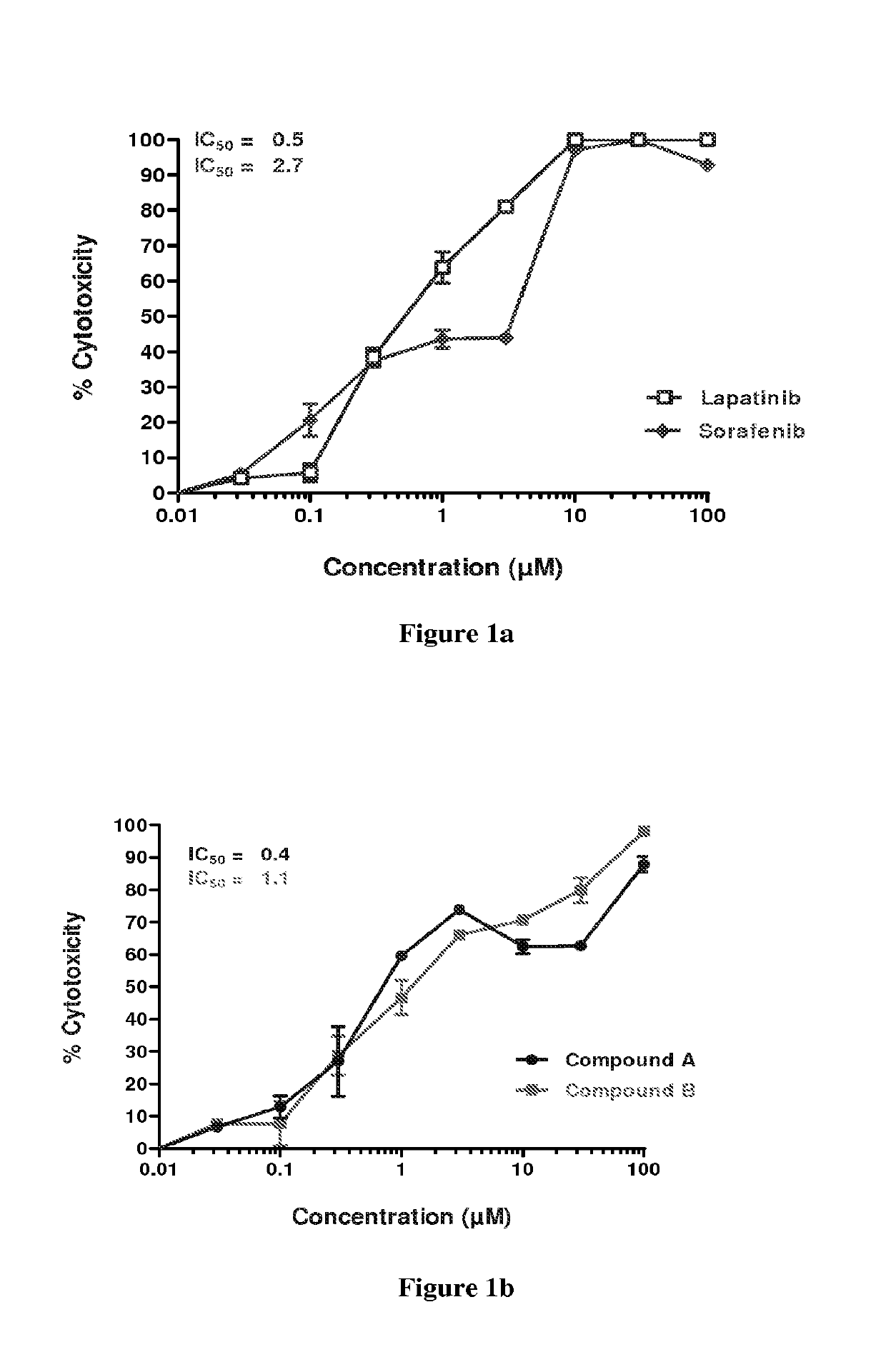
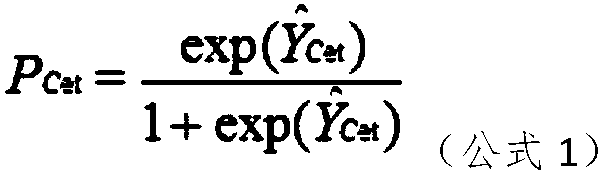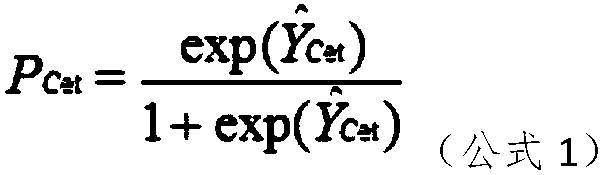Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
95 results about "Cetuximab" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cetuximab is used to treat a certain type of cancer of the colon (large intestine) or rectum that has spread to other parts of the body. This medication is also used to treat head and neck cancer.
Combination methods and compositions
InactiveUS20110223241A1BiocideCarbohydrate active ingredientsBevacizumab InjectionTherapeutic effect
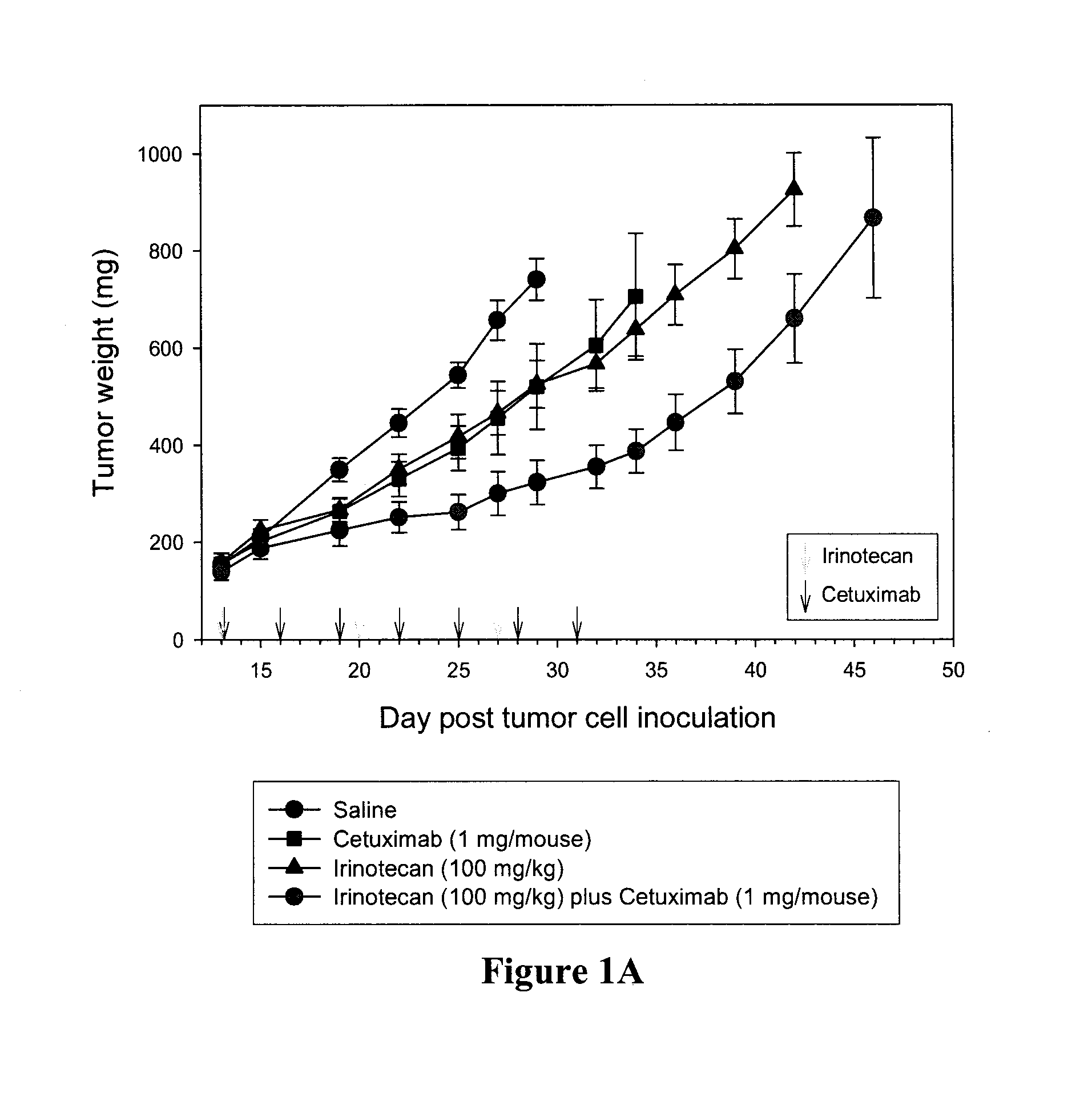
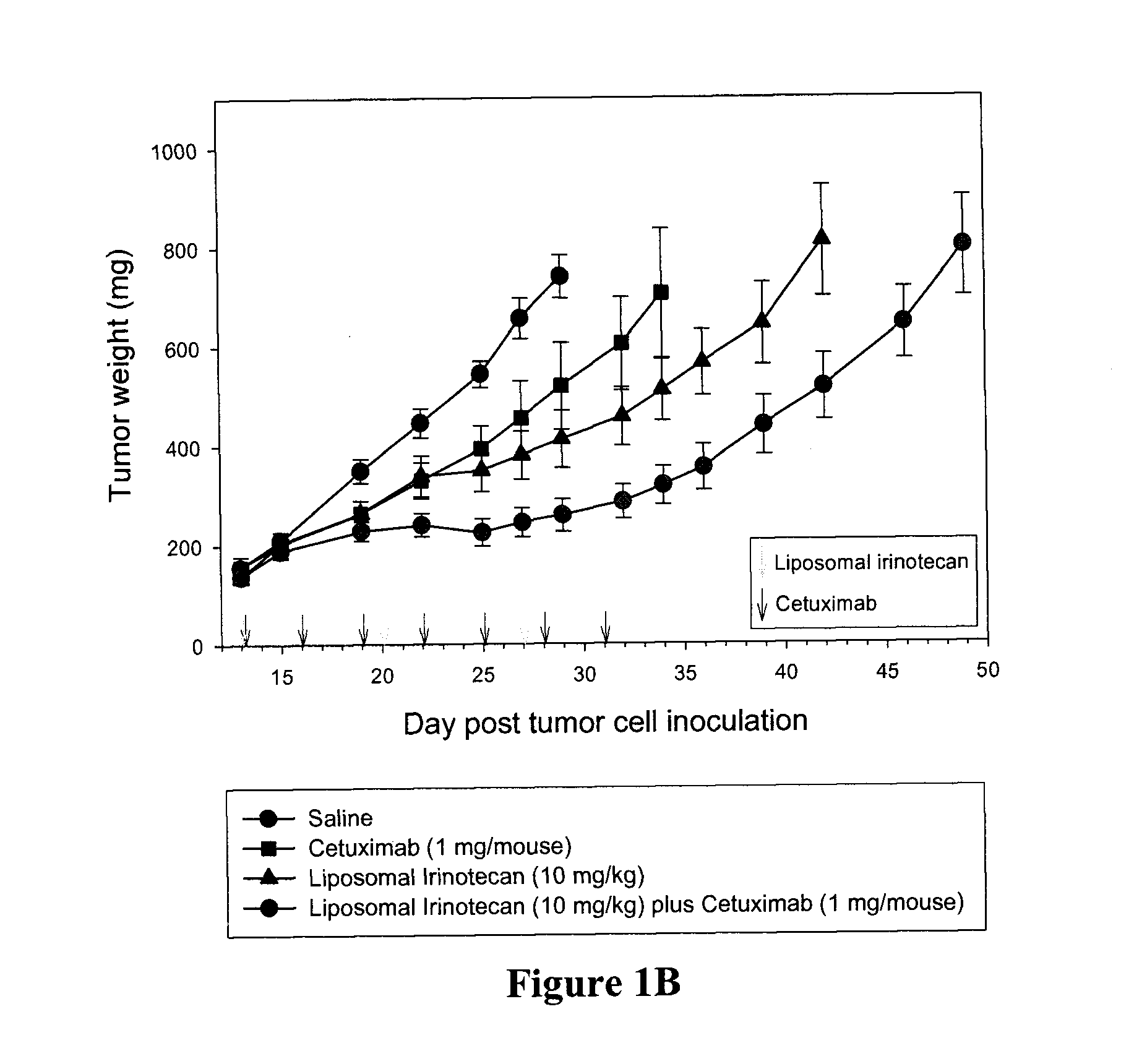
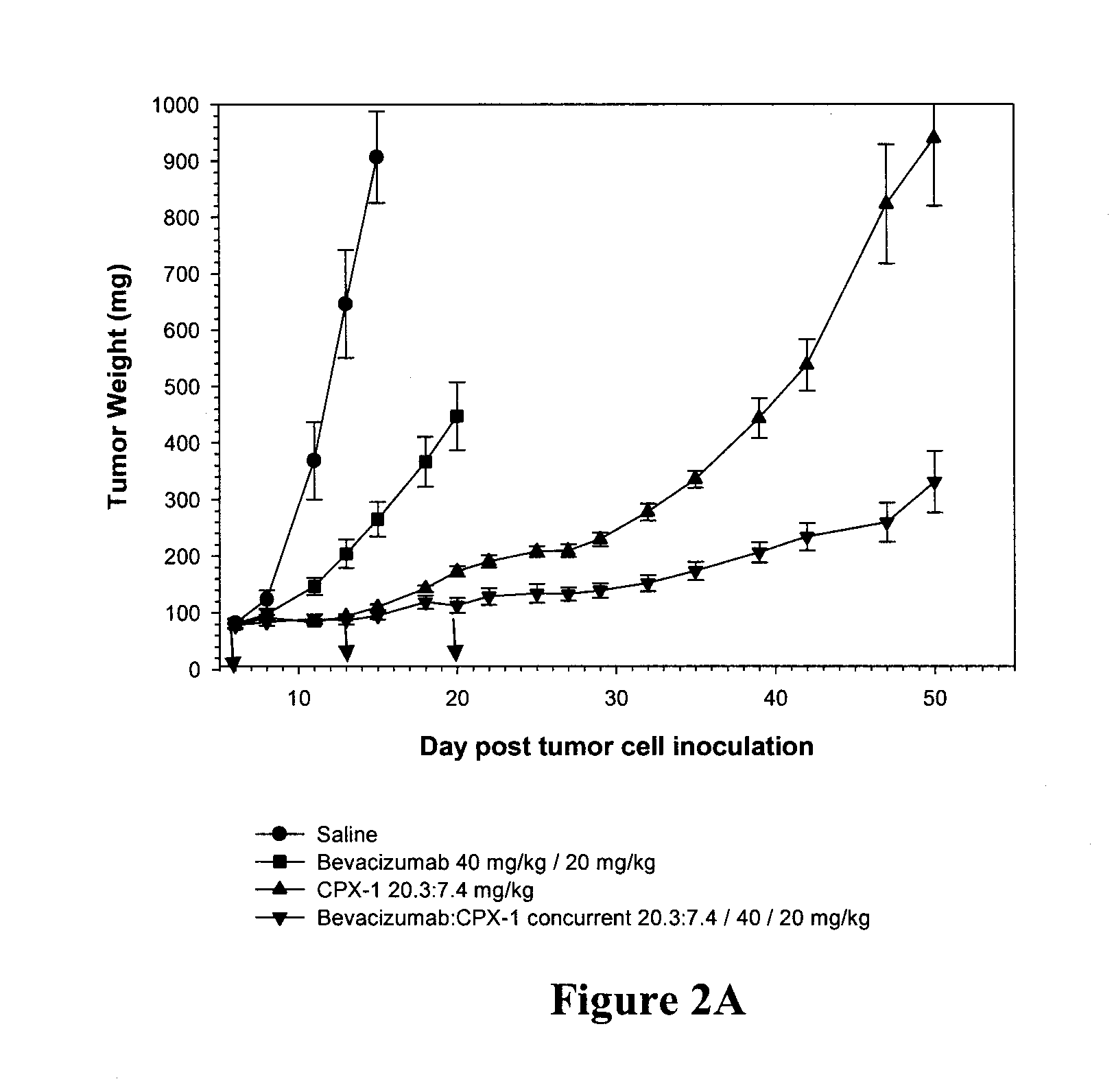
Compositions which comprise a liposomal water-soluble camptothecin and optionally a liposomal fluoropyrimidine in combination with a vascular epithelial growth factor (VEGF) inhibitor such as cetuximab or an epidermal growth factor receptor (EGFR) inhibitor such as bevacizumab are useful in achieving enhanced therapeutic effects for the treatment of cancer.
Owner:CELATOR PHARMA INC
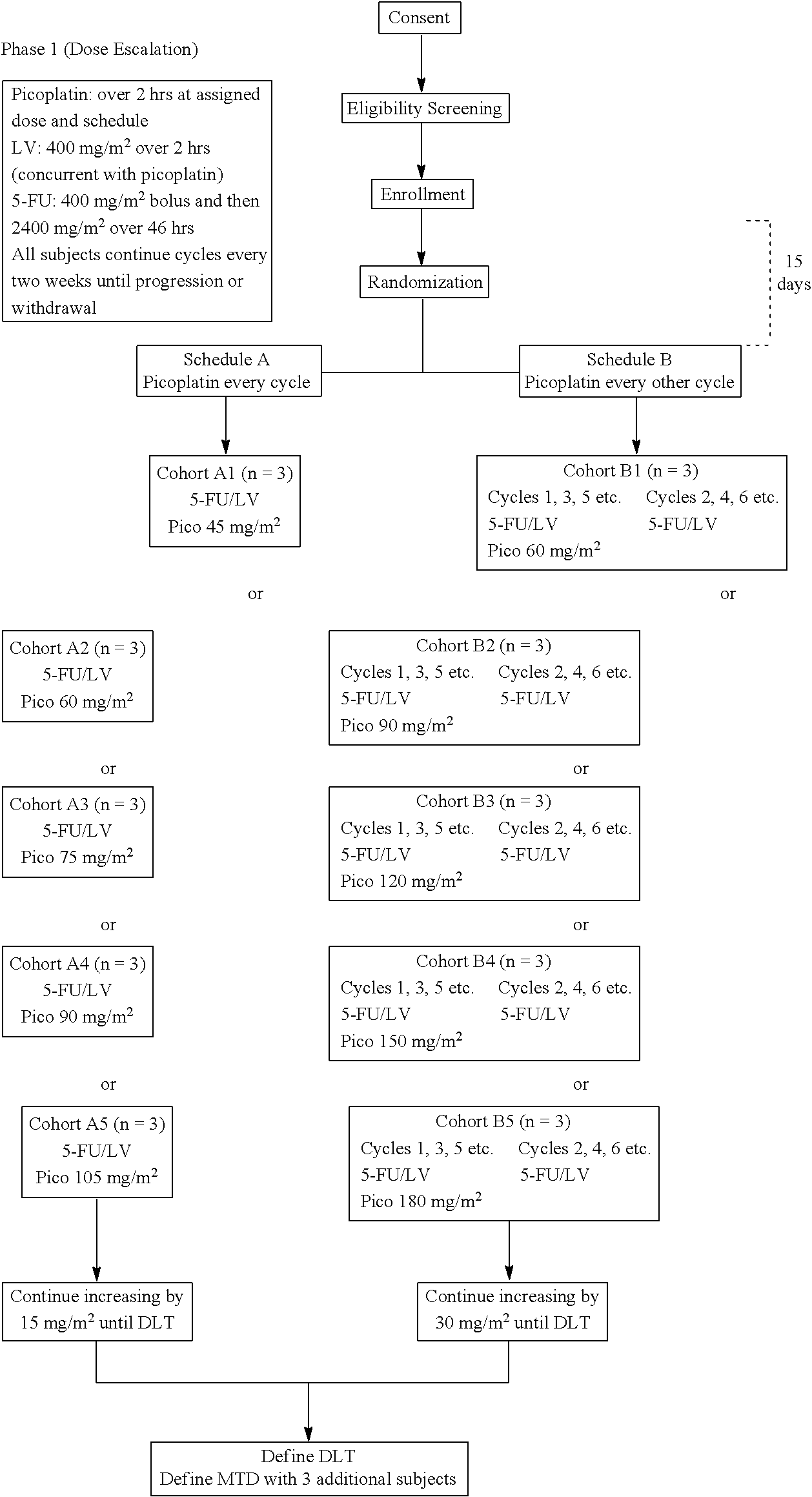
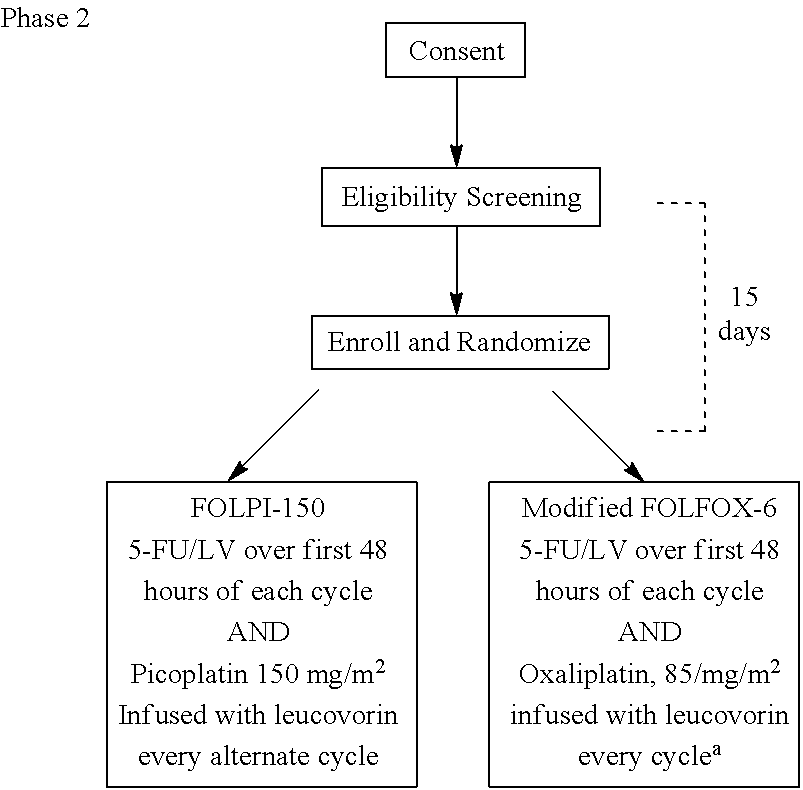
Use of picoplatin and cetuximab to treat colorectal cancer
The invention provides a method of treatment of metastatic colorectal cancer by administration of the anti-cancer platinum drug picoplatin in conjunction with cetuximab, 5-FU, and leucovorin in a variety of treatment regimens. The invention also provides a use of picoplatin in conjunction with cetuximab, 5-FU, and leucovorin for treatment of metastatic colorectal cancer. The invention further provides kits adapted for administration of picoplatin in conjunction with cetuximab. Also, methods for determining dosage regimens for patients afflicted with a cancer comprising EGFR are provided.
Owner:PONIARD PHARMA INC
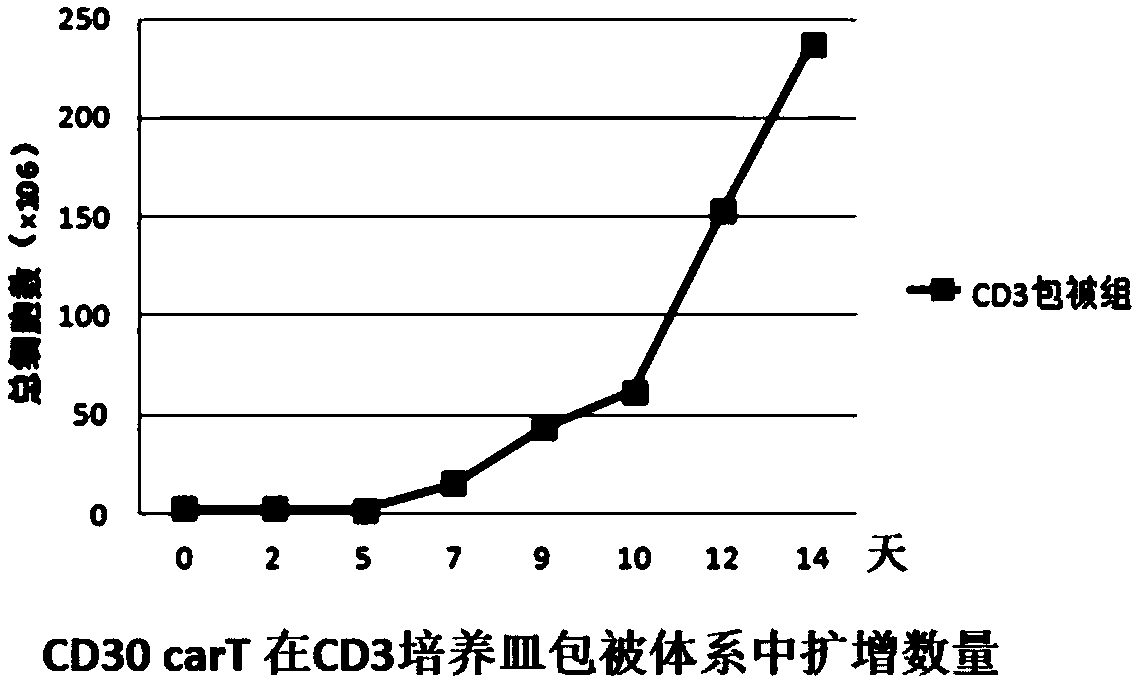
Transgenic T cell of targeted CD30 antigen as well as preparation method and application of transgenic T cell
InactiveCN107759699ADisinhibitionFunction increaseHydrolasesAntibody mimetics/scaffoldsAntigenPrimary cell
The invention discloses a transgenic T cell of a targeted CD30 antigen. The transgenic T cell is a primary cell which is integrated with a gene shown as SEQ ID NO:2 and encoding the targeted CD30 antigen, and knocks out a PD1 gene and / or CTLA4 gene, or is a primary cell containing a recombinant lentivirus expression vector (including a gene which is shown as SEQ ID NO:2 and encodes the targeted CD30 antigen and shRNA of a targeted PD1 gene or / and shRNA of a targeted CTLA4 gene); the primary cell is CD4+T cell or CD8+T cell. A preparation method comprises the following steps: firstly, carryingout lentivirus infection on the CD4+T cell or the CD8+T cell; secondly, mixing gRNA, CRISPR-cas9mRNA and HDR, and carrying out electroporation recombination on the T cell to obtain a finished product.According to the transgenic T cell disclosed by the invention, a recognition sequence of an EGFR (Epidermal Growth Factor Receptor) is introduced in carT construction; if necessary, a carT cell can be eliminated by using EGFR monoclonal antibody Cetuximab, the PD1 gene and the CTLA4 gene are knocked out or silenced, inhibition of the gene to the carT cell is eliminated, and the function of overcoming a tumor microenvironment and inhibiting immune cells by the carT cell are enhanced.
Owner:YINFENG BIOLOGICAL GRP
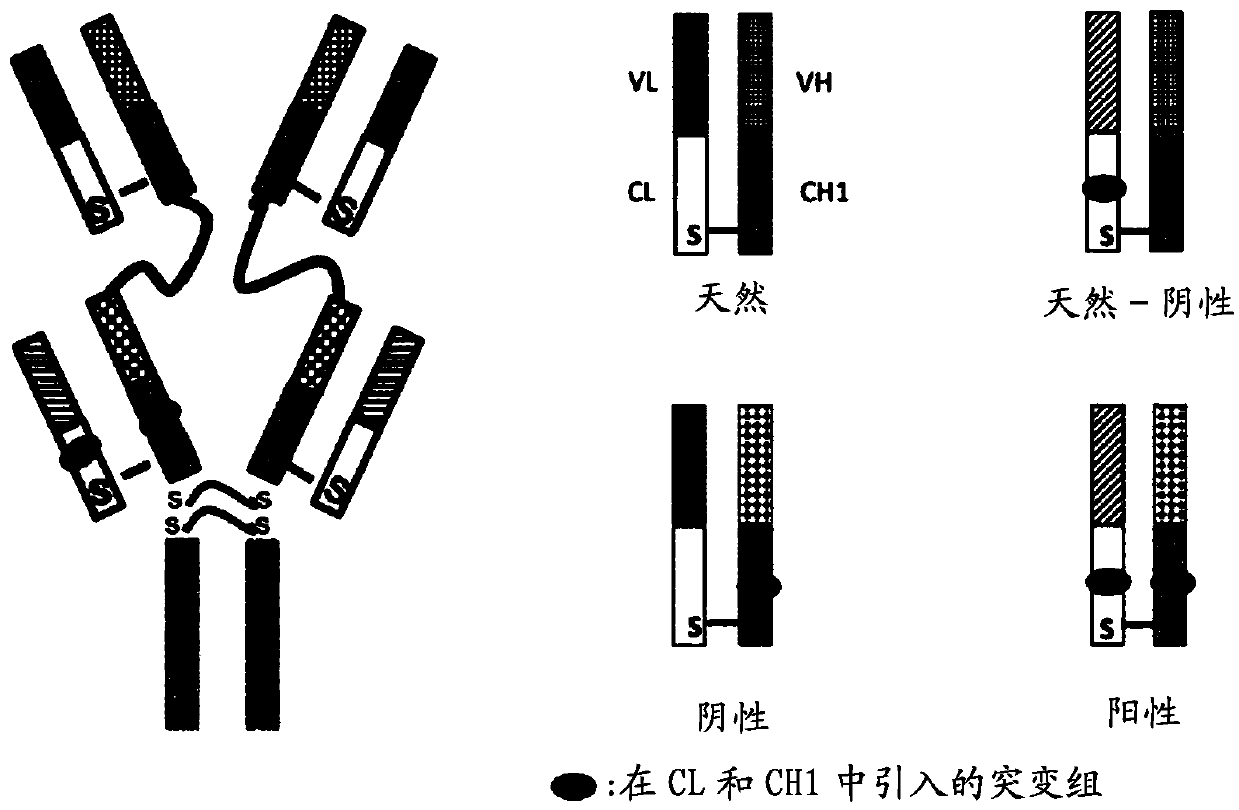
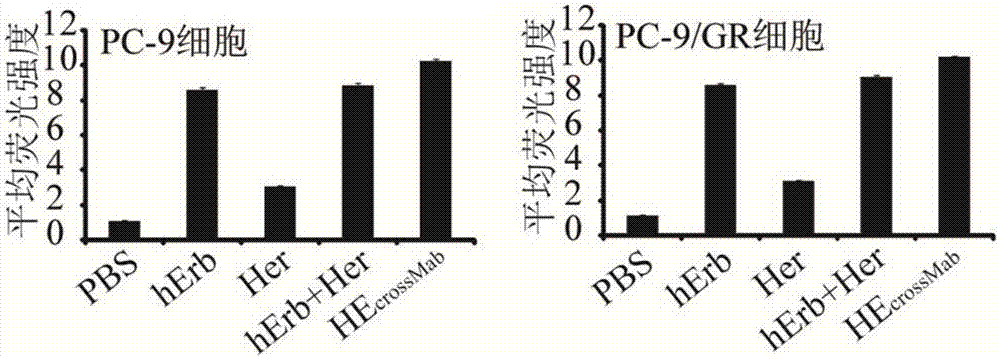
Bispecific antibodies targeting EGFR and HER2
ActiveCN109563166AHybrid immunoglobulinsPeptide/protein ingredientsAntiendomysial antibodiesCetuximab
The present disclosure relates to bispecific antibodies targeting EGFR and HER2, and methods for the production of these antibodies. The bispecific antibodies consist of one complete antibody on whichtwo VH-VL chains are attached via a linker to each NH terminal region of both VH chains of the antibody. The bispecific antibodies constructed use the amino acid sequences of the heavy chain (VH) andthe light chain (VL) variable regions of two monoclonal antibodies targeting EGFR and HER2, namely cetuximab and trastuzumab, respectively
Owner:BIOMUNEX PHARMA +3
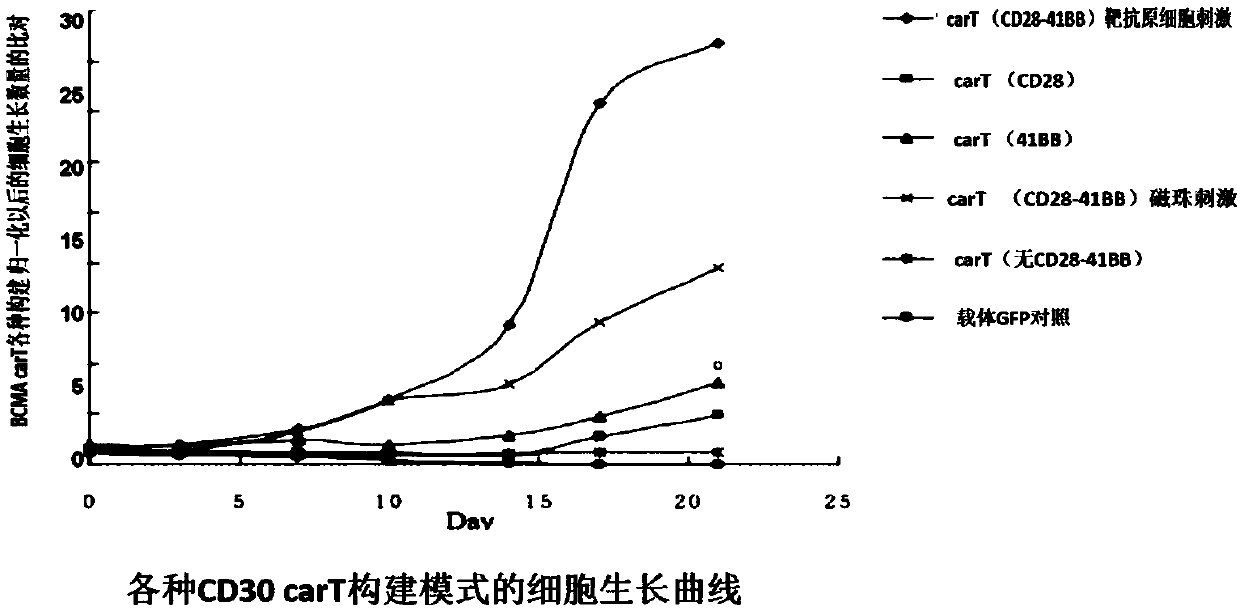
Myeloma BCMA antigen-targeted transgenic T cell, and preparation method and application thereof
InactiveCN107827989ADisinhibitionKnockout efficiency is lowHydrolasesAntibody mimetics/scaffoldsAntigenAntigen receptors
The invention discloses a gene for encoding anti-BCMA chimeric antigen receptor. The nucleotide sequence of the gene is represented by SEQ ID NO:2. The invention also discloses a recombinant expression vector containing the gene, and a myeloma BCMA antigen-targeted transgenic T cell. The transgenic T-cell is a primitive cell containing the recombinant expression vector and knocked out of a PD1 gene or / and a CTLA4 gene, or is a primitive cell with the chromosome being integrated with the gene for encoding anti-BCMA chimeric antigen receptor and knocked out of tbe PD1 gene or / and the CTLA4 gene.A preparation method of the transgenic T-cell comprises the following steps: mixing of gRNA, CRISPR-cas9 mRNA and HDR mix, and electrotransformation recombination of the T cell. The invention furtherdiscloses an application of the myeloma BCMA antigen-targeted transgenic T cell in the preparation of drugs for treating multiple myeloma. In the construction process of carT of, a recognition sequence of EGFR is introduced, EGFR monoclonal antibody Cetuximab is used to eliminate a carT cell if necessary, and PD1 and CTLA4 genes are knocked out to relieve the inhibition effect of the PD1 and CTLA4 genes on the carT cell and enhance the overcoming effect of the carT cell on the inhibition of the tumor microenvironment on immune cell functions.
Owner:YINFENG BIOLOGICAL GRP +1
FCgamma POLYMORPHISMS FOR PREDICTING DISEASE AND TREATMENT OUTCOME
The invention provides compositions and methods for determining the likelihood of successful treatment with Cetuximab or other equivalent. The methods comprise determining the genomic polymorphism present in a predetermined region of the FcγRIIa gene at amino acid position 131 and / or alternatively the FcγRIIIa gene at amino acid position 158.
Owner:UNIV OF SOUTHERN CALIFORNIA
Anti-HER3 antibody, preparing method thereof and applications of the antibody
ActiveCN105367657AHigh affinityLow immunogenicityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsImmunogenicityChimeric antibody
The invention provides an anti-HER3 antibody, a preparing method thereof and applications of the antibody and particularly provides a novel anti-HER3 antibody. The antibody is high in affinity with HER3 molecules and can specifically combine an antigen molecule. In particular, a human-mouse chimeric antibody effectively reduces immunogenicity of a mouse antibody. By combined application of the anti-HER3 antibody and cetuximab, activity of the cetuximab is significantly improved, and the using amount of the cetuximab is reduced.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Novel EGFR binding proteins
ActiveUS20180030098A1Peptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeAnti-EGFR Monoclonal Antibody
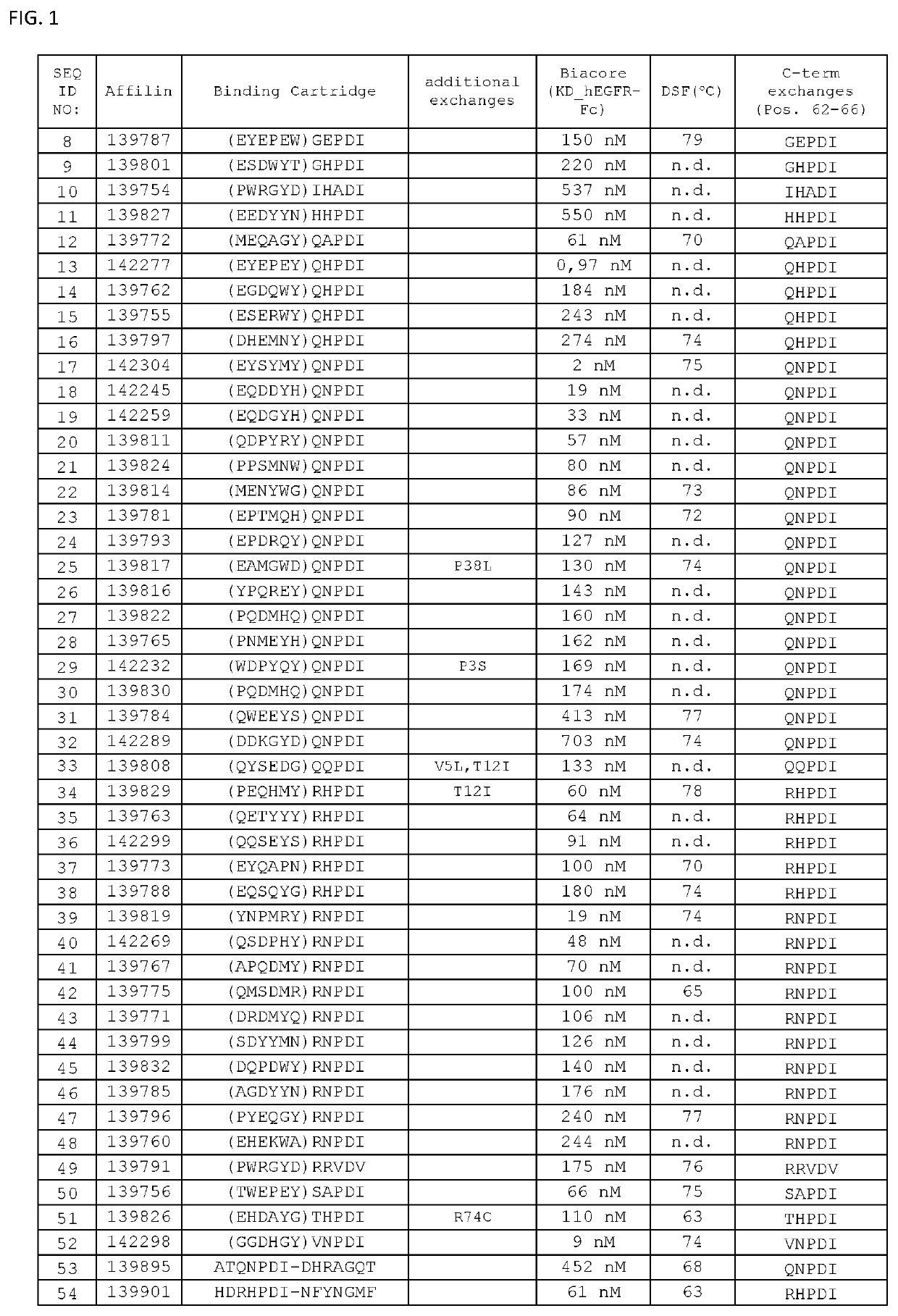
The present invention relates to new EGFR binding molecules based on ubiquitin muteins (Affilin®), preferably Affilin molecules having a characteristic three amino acid residue motif. The invention further refers to EGFR binding molecules that bind to different or non-overlapping epitopes than the anti-EGFR monoclonal antibody Cetuximab. The invention further relates to the use of these EGFR binding proteins in medicine, preferably for use in the diagnosis or treatment of cancer.
Owner:NAVIGO PROTEINS GMBH
Cetuximab with modified glycosylation and uses thereof
InactiveUS20160002330A1Milk immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsCetuximabMethods of production
In one aspect, the disclosure relates to antibodies with altered glycosylation patterns, methods of production of said antibodies, and methods of use thereof. In some embodiments, the antibody is cetuximab.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
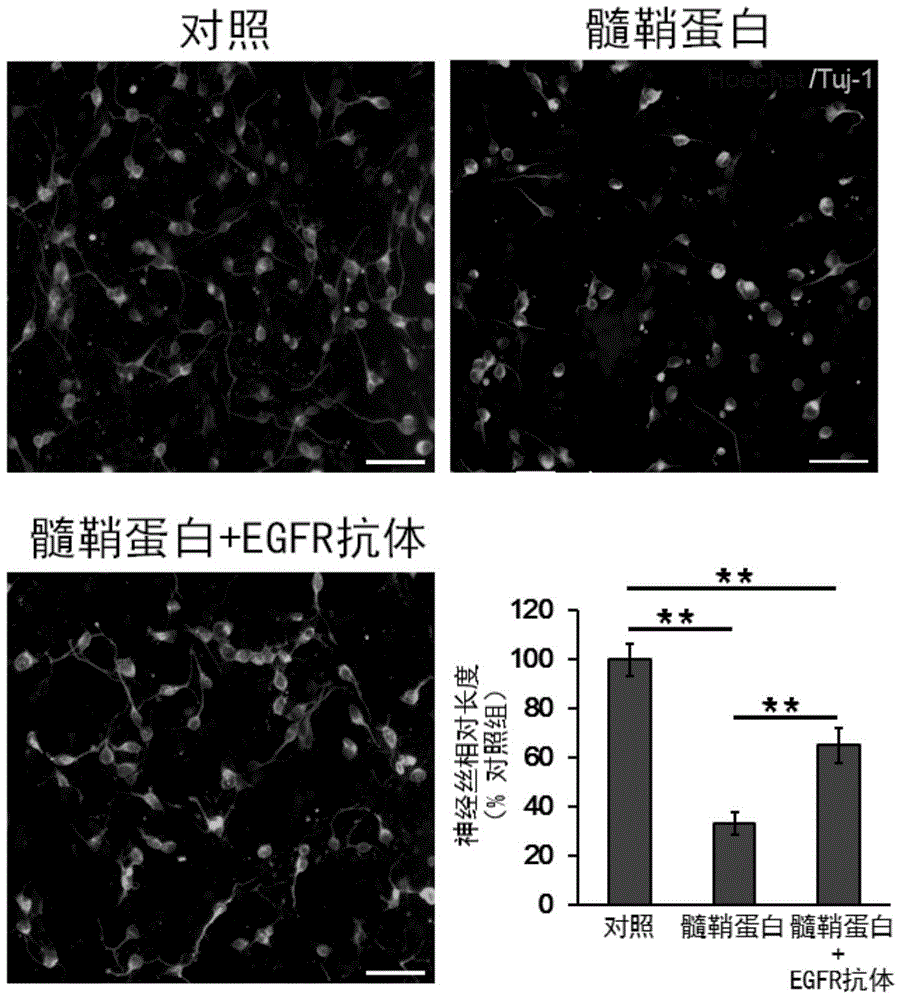
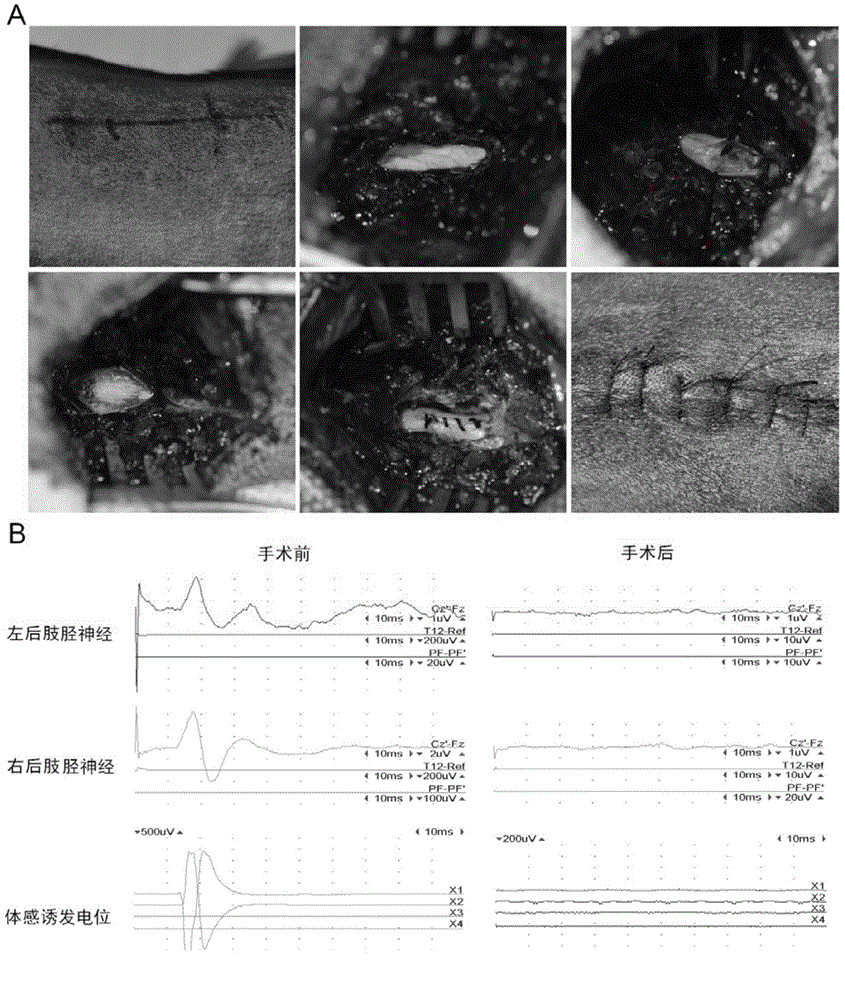
Application of cetuximab and collagen scaffold loaded with cetuximab to preparation of drug for repairing spinal cord injury
The invention discloses the application of cetuximab and a collagen scaffold loaded with cetuximab to preparation of a drug for repairing spinal cord injury, and also provides a nerve regeneration collagen scaffold material loaded with cetuximab. The preparing method comprises the steps of 1, making the nerve regeneration collagen scaffold material absorb cetuximab to obtain the nerve regeneration collagen scaffold material containing cetuximab; 2, incubating the nerve regeneration collagen scaffold material loaded with cetuximab to obtain the drug. Tests prove that both cetuximab and the collagen scaffold loaded with cetuximab can repair spinal cord injury, promote nerve regeneration and / or reduce spinal cord injury part gliocyte proliferation, and have great practical guiding significance in application to clinical spinal cord injury repair in future.
Owner:WUQI BIOMEDICAL TECH (JIANGSU) CO LTD
Transgenic T cell of targeted CD19 antigen as well as preparation method and application of transgenic T cell
InactiveCN107759700ALow immunogenicityFunction increaseAntibody mimetics/scaffoldsStable introduction of DNAPrimary cellCetuximab
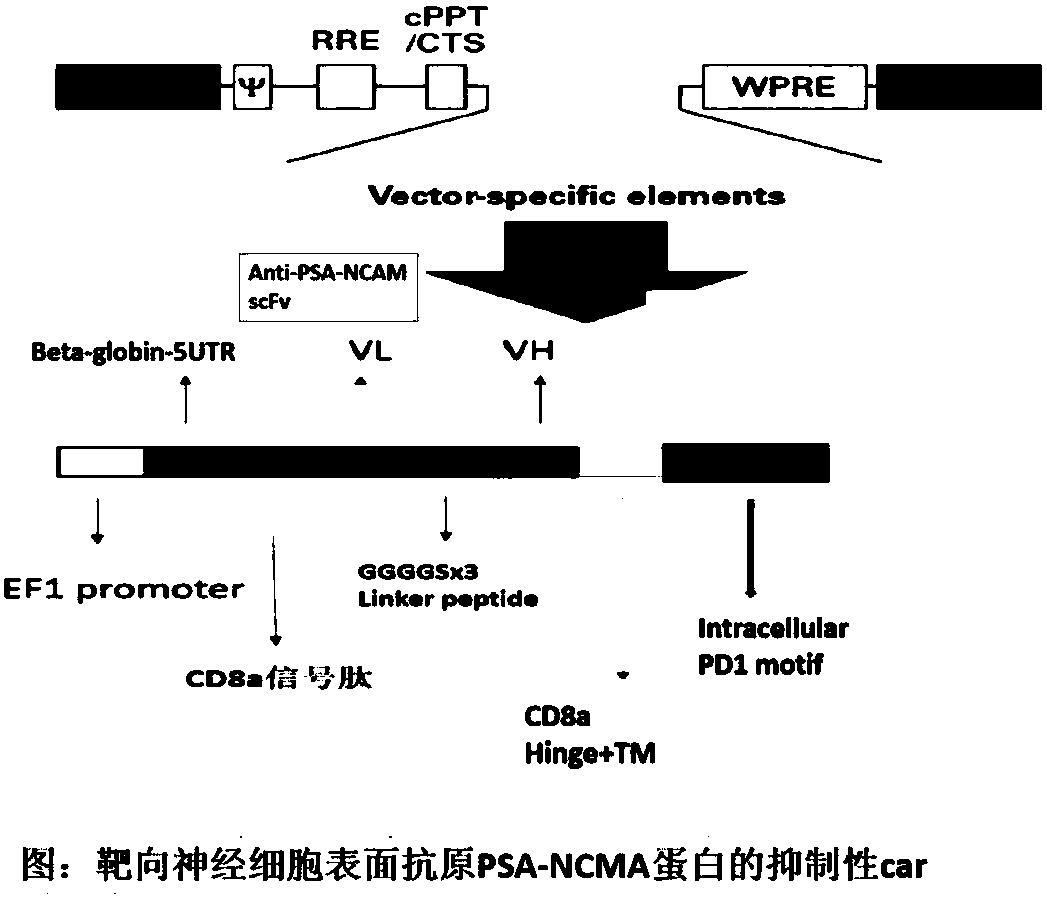
The invention discloses a transgenic T cell of a targeted CD19 antigen. The transgenic T cell is a primary cell which is integrated with a gene shown as SEQ ID NO:8 and encoding the targeted CD19 antigen in chromosome, and knocks out a PD1 gene and / or CTLA4 gene, or is a primary cell which is integrated with a gene shown as SEQ ID NO:2 and encoding a targeted PSA-NCAM receptor ,and a gene shown asSEQ ID NO:8 and encoding the targeted CD19 antigen in the chromosome; the primary cell is CD4+T cell or CD8+T cell. The invention also discloses application of the transgenic T cell of the targeted CD19 antigen in preparation of a medicine for treating malignant B cell lymphoma. According to the transgenic T cell disclosed by the invention, a recognition sequence of an EGFR (Epidermal Growth Factor Receptor) is introduced in carT construction; if necessary, a carT cell can be eliminated by using EGFR monoclonal antibody Cetuximab, the PD1 gene and the CTLA4 gene are silenced or knocked out, inhibition of the gene to the carT cell is eliminated, and the function of overcoming a tumor microenvironment and inhibiting immune cells by the carT cell are enhanced.
Owner:YINFENG BIOLOGICAL GRP
Mathematical model for predicting therapeutic effect of colorectal cancer liver metastasis
InactiveCN107622800AHigh feasibilityPromote conversionMedical simulationLymphatic SpreadMathematical model
The present invention discloses a mathematical model for predicting a therapeutic effect of colorectal cancer liver metastasis. The model is Pcet=exp(Y<^>Cet) / (1+exp(Y<^>Cet)), wherein, Y<^>Cet=1.516-0.165XA-0.726XC-1.140XE-0.944XL-0.477XR+0.821XP. According to the mathematical model for predicting the therapeutic effect of colorectal cancer liver metastasis, after screening a population sensitiveto cetuximab, the objective response rate is increased significantly, and thus the model can be used for the prediction of a treatment mode well.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Triptolide antibody conjugates
PendingUS20210023238A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCancer cellAntiendomysial antibodies
The antibody-drug conjugates provided herein including embodiments thereof, include the compound triptolide attached to a cancer-specific antibody (e.g., cetuximab) and are, inter alia, useful as highly effective anti-cancer therapeutics. The conjugates provided herein are capable of targeting cancer cells and thereby specifically deliver triptolide to the cancer cell.
Owner:CITY OF HOPE
Methods of predicting responsiveness of a cancer to an agent and methods of determining a prognosis for a cancer patient
Owner:DUKE UNIV
Targeting EGFR and HER2 double-specific antibody and application thereof
InactiveCN107325184AImprove target specificityPreserve affinityHybrid immunoglobulinsAntibody ingredientsHeavy chainBispecific antibody
The invention discloses a targeting EGFR and HER2 double-specific antibody and an application thereof. An antigen bonding zone thereof is composed of humanized cetuximab and herceptin. According to the invention, a bioengineering technology is utilized to construct a double-specific antibody for simultaneously targeting and combining with EGFR and HER2 antigens. The invention provides DNA sequences of two heavy chains and two light chains of the antibody and expression host cells thereof. The invention also protects the application of the double-specific antibody for preparing drugs for non-small cell lung cancers. The double-specific antibody has an important application value for treating the non-small cell lung cancers.
Owner:ANHUI UNIVERSITY
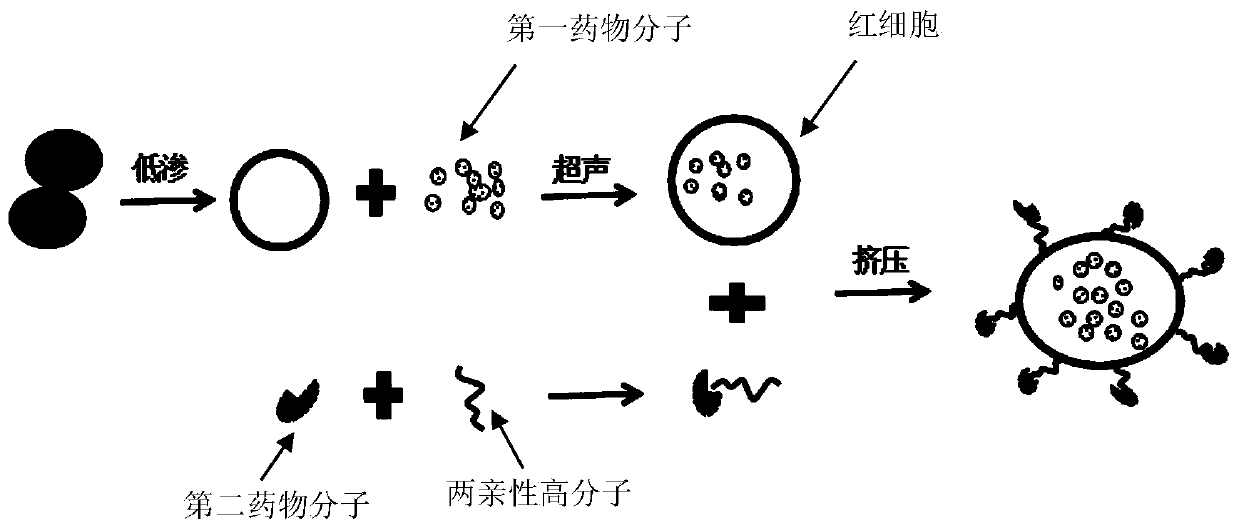
Dual drug-loaded red blood cell(RBC) carrier, preparation method and application thereof
ActiveCN110279672ASimple preparation processRaw materials are economically availableOrganic active ingredientsNervous disorderSide effectTherapeutic effect
The invention discloses a dual drug-loaded RBC carrier and preparation method and application of the dual drug-loaded RBC carrier. The dual drug-loaded RBC carrier comprises RBC, first drug molecules encapsulated in RBCs, and second drug molecules coupled to the surfaces of RBCs. The first drug molecules may be paclitaxel (PTX) or the like, and the second drug molecules may be cetuximab (Cet) or the like. The dual drug-loaded RBC carrier disclosed by the invention utilizes RBC to simultaneously load two therapeutic molecules of the first drug molecules and the second drug molecules, and reduces drug resistance risks and toxic and side effects caused by drugs through entrapment of the RBC carrier on the one hand to increase the safety of administration, and further can exert better therapeutic effects on different problems through two different functions of drug loading on the other hand. Furthermore, the dual drug-loaded RBC carrier disclosed by the invention is simple in preparation process, economical and readily available in raw materials, does not need to rely on complicated equipments, and is easy to realize industrial production.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
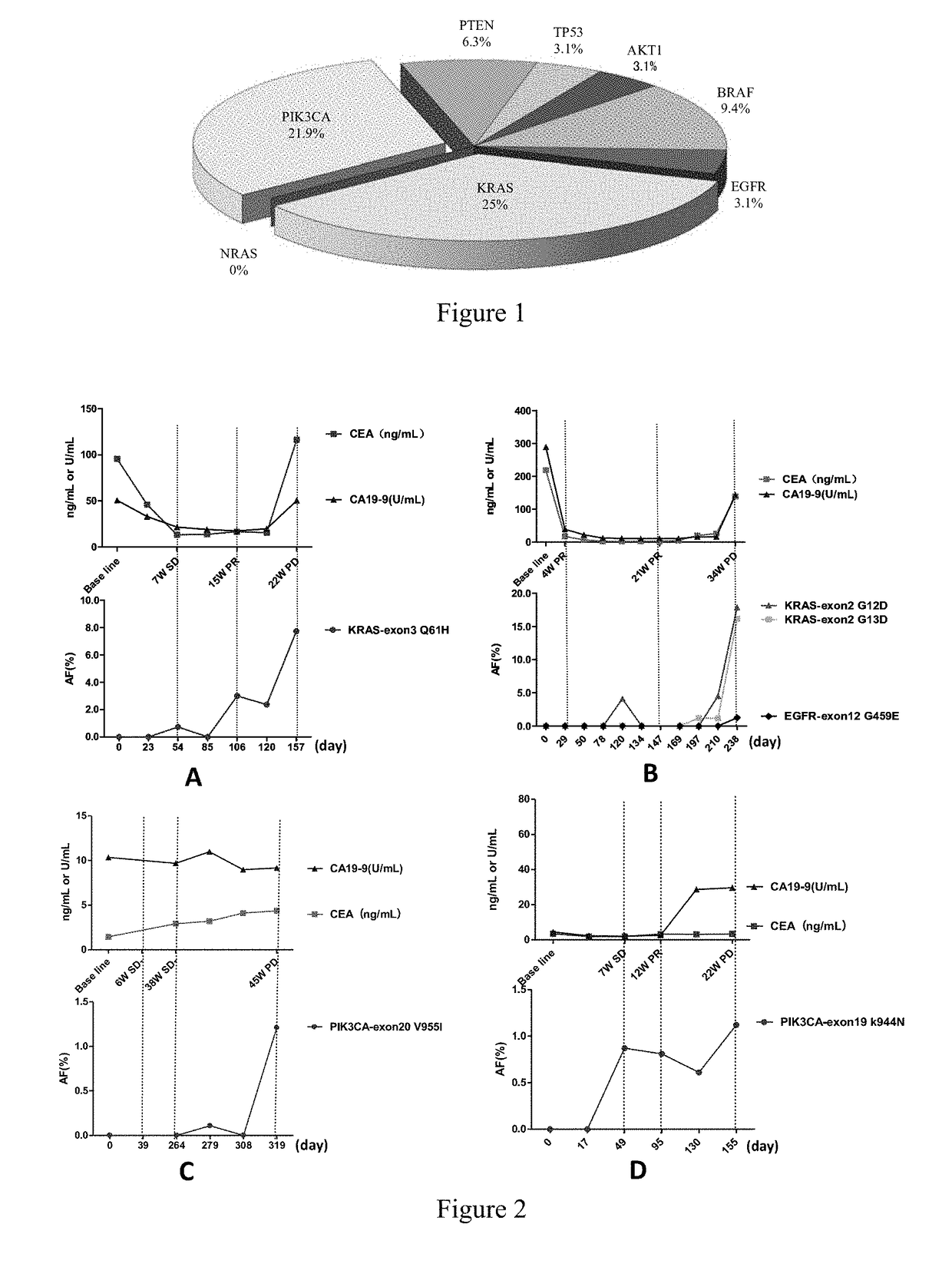
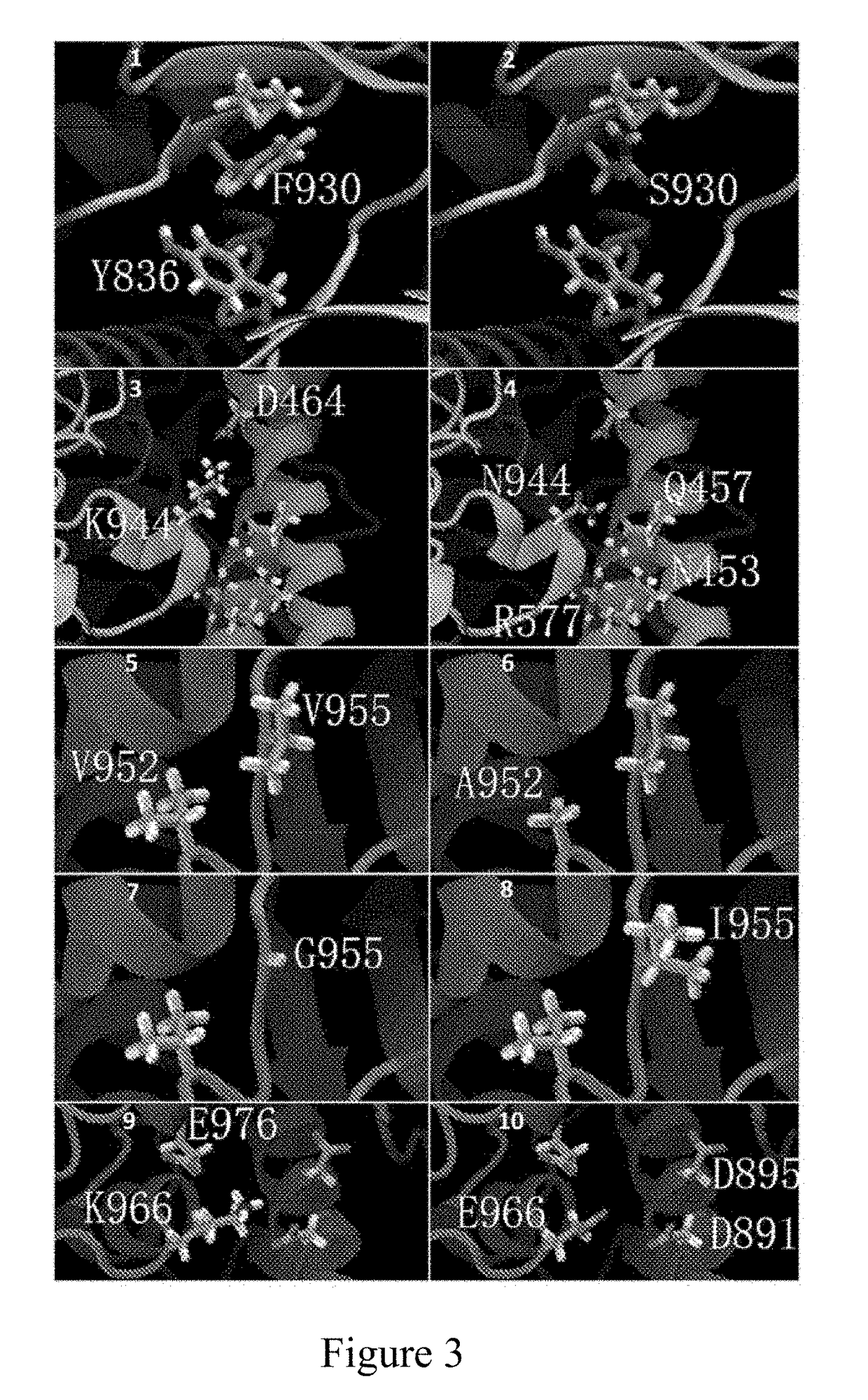
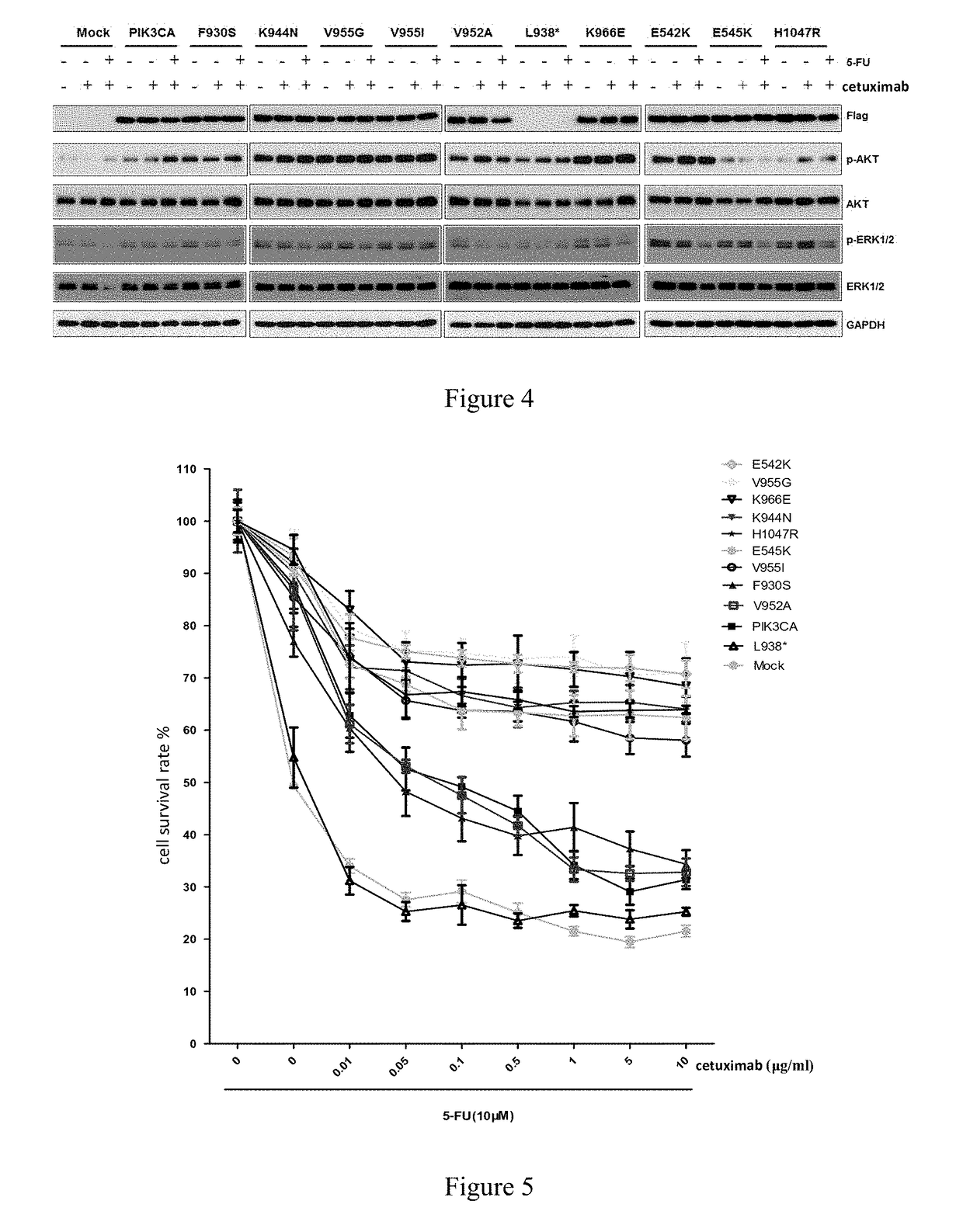
Pik3ca novel mutations detection for diagnosis of acquired cetuximab resistance in metastatic colorectal cancer patients
Disclosed is a kit for detecting drug resistance of cetuximab in the treatment of metastatic colorectal cancer. The kit comprises a substance used for detecting gene mutations in Exon 19 of the PIK3CA gene, and may further comprise a specification recording the following contents: if Exon 19 in the PIK3CA gene of a patient with metastatic colorectal cancer as a subject to be tested, who is intended to receive cetuximab treatment or is receiving cetuximab treatment and does not have drug resistance, has at least one of K944N, F930S, V955G, V955I, and K966E mutations, the subject to be tested will develop drug resistance or will be a candidate to develop drug resistance when receiving or continuing to receive cetuximab for treating metastatic colorectal cancer.
Owner:AFFILIATED HOSPITAL CHINA ACADEMY OF MILITARY MEDICAL SCI
Graphene oxide modified cetuximab as well as preparation method and application of graphene oxide modified cetuximab
InactiveCN106474483AStable in natureReduce stimulationAntibody ingredientsPharmaceutical non-active ingredientsCetuximabBlood vessel
The invention discloses graphene oxide modified cetuximab as well as a preparation method and application of graphene oxide modified cetuximab. The graphene oxide modified cetuximab comprises cetuximab and graphene oxide, which are connected with each other by virtue of a non-covalent bond, the graphene oxide is two-dimensional nano graphene oxide, and the particle size of the graphene oxide is less than 0.22 micrometer. According to the graphene oxide modified cetuximab, the cetuximab and the graphene oxide are connected with each other by virtue of a non-covalent bond to form a compound antibody, so that a biological reaction can be greatly enhanced. Compared with cetuximab which only has a slight inhibitory action in vitro, the cetuximab can directly inhibit or kill and wound glioma cells. Meanwhile, by adopting the two-dimensional nano graphene oxide of which the particle size is less than 0.22 micrometer, the graphene oxide modified cetuximab is stable in property, and the stimulation to peripheral blood vessels is reduced at the same time.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Method for treating tumors based on adenovirus vector carrier expressed full-length cetuximab
InactiveCN106282232AImmunoglobulins against cell receptors/antigens/surface-determinantsUnknown materialsMonoclonal antibodyEffective treatment
The invention relates to a method for treating tumors based on adenovirus vector carrier expressed full-length cetuximab. By adopting the method, an adnovirus vector is first adopted to successfully and efficiently express a cetuximab monoclonal antibody. The obtained monoclonal antibody is good in biological activity and can realize an effective treatment effect for animals.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
A pulmonary inhaled chitosan-based nano targeting polymer particles and its production method thereof
ActiveCN106265607AUniform particle size distributionGood biocompatibilityPowder deliverySpray deliveryBiocompatibility TestingPhospholipid
This invention provides a pulmonary inhaled chitosan-based nano targeting polymer particle which consists chitosan or 1-5 parts of its derivatives; 1-5 parts of sodium tripolyphosphate; 0.01-0.03 parts of cetuximab, phospholipid or 1-3 parts of its derivatives, 1-5 parts of hydroxypropyl-Beta-cyclodextrin and 1-6 parts of excipient. This invention also provides the preparation method of the polymer particle. The particle diameter of this pulmonary inhaled chitosan-based nano targeting polymer particle prepared in this invention is 2-10Mum with uniform distribution. The loaded particle diameter of this pulmonary inhaled chitosan-based nano targeting polymer particle is 50-201nm with good biocompatibility. The particle prepared in this invention improves the stability and the ability for cell phagocytosis. The phagocytic rate of A549 cell reaches 50-60% in 2hours which means an increase of bioavailability. This particle is easy to deposit at lung and resolve at a short time which helps particles work in lung with retention rate of 50-70%.
Owner:WEIFANG MEDICAL UNIV
FCgamma POLYMORPHISMS FOR PREDICTING DISEASE AND TREATMENT OUTCOME
The invention provides compositions and methods for determining the likelihood of successful treatment with Cetuximab or other equivalent. The methods comprise determining the genomic polymorphism present in a predetermined region of the FcγRIIa gene at amino acid position 131 and / or alternatively the FcγRIIIa gene at amino acid position 158.
Owner:UNIV OF SOUTHERN CALIFORNIA
Anti-EGFR (epidemic growth factor receptor) humanized antibody L2-H3 and coding gene and application thereof
InactiveCN102153650BExcellent antigen binding activityImprove bindingFungiBacteriaHumanized antibodyCetuximab
The invention discloses an anti-EGFR (epidemic growth factor receptor) humanized antibody L2-H3 and a coding gene and application thereof. The antibody is formed by a light chain and a heavy chain, wherein the heavy chain is formed by connection of a heavy chain variable region and a heavy chain constant region; the amino acid sequence of the heavy chain variable region is shown in the 1st-145th positions in the SEQ ID NO:3; the heavy chain constant region is the heavy chain constant region of a humanized antibody IgG1; and the amino acid sequence of the light chain is shown in the SEQ ID NO:1. The experimental results prove that the antibody has good binding activity and capability of inhibiting growth and migration of the tumor cells; and the affinity of the common anti-EGFR human-mouthchimeric antibody, namely cetuximab in the domestic and foreign markets is 1.1*10<9>M. The humanized antibody disclosed by the invention can better bind with the EGFR, thus ensuring the anti-tumor effect of the humanized antibody. By adopting a method for preparing the antibody, the light chain and the heavy chain can be simultaneously expressed, the expression ratio of the light chain to the heavy chain is closer to 1:1, and the mutually matched double-chain antibody with higher ratio is generated. In conclusion, the antibody and the preparation method have broad application prospects in thefield of tumor prevention and / or treatment.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Protein modification open tubular column and application of protein modification open tubular column to monoclonal antibody charge isomer separation
ActiveCN106198780AInhibition of adsorptionEasy to separateComponent separationStationary phaseMonoclonal antibody
The invention discloses a protein stationary phase modification open tubular column and application of the protein stationary phase modification open tubular column to monoclonal antibody charge isomer separation. The open tubular column is prepared through the following steps that PDDA is fixed on the inner wall of a capillary tube by an electrostatic self-assembly method; then, protein is adsorbed on the PDDA surface through electrostatic self assembly; the protein stationary phase modification open tubular column is obtained. The open tubular column shows specific selectivity on monoclonal antibody charge isomers. Seven kinds of different-form charge isomers of cetuximab are successfully separated; two alkaline isomers and one acid isomer of the rituximab and two alkaline charge isomers and four acid isomers of trastuzumab are successfully separated from main peaks.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Colorectal cancer cetuximab monoclonal antibody anti-drug trace amount DNA mutation detection method and device
ActiveCN108588201AImprove applicabilityComprehensive Mutation InformationBioreactor/fermenter combinationsBiological substance pretreatmentsMutation detectionMonoclonal antibody
The invention discloses a colorectal cancer cetuximab monoclonal antibody anti-drug trace amount DNA mutation detection method and a colorectal cancer cetuximab monoclonal antibody anti-drug trace amount DNA mutation detection device. The method comprises the following steps: extracting free DNA from a sample; constructing a library by using the free DNA; enriching the library; capturing the enriched library by using a capture probe to obtain captured DNA; sequencing the captured DNA to obtain the sequencing result; and comparing the sequencing result with a reference sequence and detecting amutation site. By the method, low-initial-quantity DNA enriched capture sequencing mutation detection is realized.
Owner:ZHEJIANG PROVINCIAL PEOPLES HOSPITAL +2
Anti-EGFR (epidemic growth factor receptor) humanized antibody L2-H3 and coding gene and application thereof
InactiveCN102153650AExcellent antigen binding activityImprove bindingFungiBacteriaHuman mouthHumanized antibody
The invention discloses an anti-EGFR (epidemic growth factor receptor) humanized antibody L2-H3 and a coding gene and application thereof. The antibody is formed by a light chain and a heavy chain, wherein the heavy chain is formed by connection of a heavy chain variable region and a heavy chain constant region; the amino acid sequence of the heavy chain variable region is shown in the 1st-145th positions in the SEQ ID NO:3; the heavy chain constant region is the heavy chain constant region of a humanized antibody IgG1; and the amino acid sequence of the light chain is shown in the SEQ ID NO:1. The experimental results prove that the antibody has good binding activity and capability of inhibiting growth and migration of the tumor cells; and the affinity of the common anti-EGFR human-mouth chimeric antibody, namely cetuximab in the domestic and foreign markets is 1.1*10<9>M. The humanized antibody disclosed by the invention can better bind with the EGFR, thus ensuring the anti-tumor effect of the humanized antibody. By adopting a method for preparing the antibody, the light chain and the heavy chain can be simultaneously expressed, the expression ratio of the light chain to the heavy chain is closer to 1:1, and the mutually matched double-chain antibody with higher ratio is generated. In conclusion, the antibody and the preparation method have broad application prospects in the field of tumor prevention and / or treatment.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Human colorectal cancer cetuximab drug-resistant cell line and application thereof
ActiveCN108795868AIncrease production capacityMicrobiological testing/measurementCulture processTreatment durationMedicine
The invention discloses a human colorectal cancer cetuximab drug-resistant cell line, and belongs to the field of biomedical technology. The human colorectal cancer cetuximab drug-resistant cell linehas the advantages that a human colorectal cancer cell line NCI-H508 is treated by the cetuximab of different concentration, and the drug concentration for survival of 50% of cells is used as the initial concentration to treat the cells for a long time, until the cell NCI-H508 can normally grow under the concentration; the cell NCI-H508 is continued to treat for a long time by a gradual increasingmethod, the treatment duration is six months, and finally the human colorectal cancer cetuximab drug-resistant cell line NCI-H508 / C225 which can quickly grow under the treatment function of the cetuximab can be finally obtained; the human colorectal cancer cetuximab drug-resistant cell line can be used for analyzing the phenotypic change of morphology and biology after drug resistance of human colorectal cancer cetuximab, studying the molecule mechanism of the human colorectal cancer cetuximab drug resistance and the reversing drug resistance method, screening other anti-tumor drugs, findingthe tumor drug-resistant markers, and screening and evaluating the novel antitumor drugs and the like, and the scientific and research value and the production and application value are higher.
Owner:JIANGSU CANCER HOSPITAL
EGFR binding proteins
ActiveUS10858405B2Peptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeAntiendomysial antibodies
Owner:NAVIGO PROTEINS GMBH
IgE Antibodies to Chimeric or Humanized IgG Therapeutic Monoclonal Antibodies as a Screening Test for Anaphylaxis
ActiveUS20100120058A1A large amountBiological material analysisImmunoglobulinsSerum igeMonoclonal antibody
The present invention provides an assay for detecting serum IgE antibody levels to cetuximab and to other proteins. The present invention further provides a method for predicting whether a subject will respond adversely to cetuximab treatment. The present further provides a method for detecting sensitivity to compounds comprising galactose-alpha-1,3-galactose.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Synergistic pharmaceutical combination for the treatment of squamous cell carcinoma of head and neck
The present invention relates to a pharmaceutical combination for use in the treatment of squamous cell carcinoma, comprising a CDK inhibitor selected from the compounds of formula (I);or a pharmaceutically acceptable salt thereof and one or more antineoplastic agents selected from sorafenib, lapatinib, erlotinib, cisplatin, 5-fluorouracil, docetaxel or cetuximab or a pharmaceutically acceptable salt thereof. The said pharmaceutical combination exhibits synergy when used in the treatment of squamous cell carcinoma of head and neck (SCCHN). The invention also relates to a pharmaceutical composition comprising the said combination and a method for the treatment of squamous cell carcinoma of head and neck (SCCHN), using a therapeutically effective amount of said combination.
Owner:PIRAMAL ENTERPRISES LTD
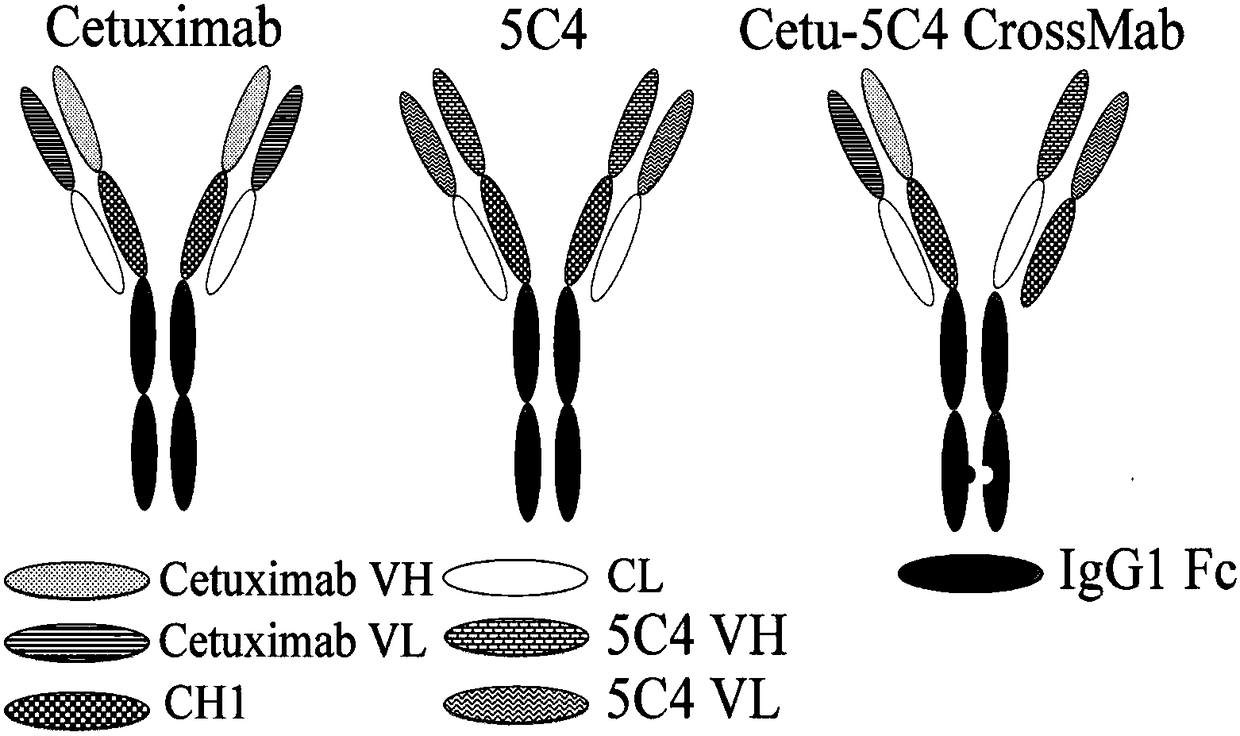
EGFR/PD-1 double-targeted antibody, preparation method and application thereof
The invention provides an epidermal growth factor receptor / programmed death-1 (EGFR / PD-1) double-targeted antibody, a preparation method thereof and an application thereof in preparation of anti-tumordrugs. The EGFR / PD-1 double-targeted antibody is prepared by taking a Cetuximab antibody and a 5C4 antibody as parents and adopting a knob-into-hole technology. The double-targeted antibody has a better anti-tumor effect than the combined use of the Cetuximab antibody and the 5C4 antibody, and has a huge application prospect.
Owner:GENERAL HOSPITAL OF PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com