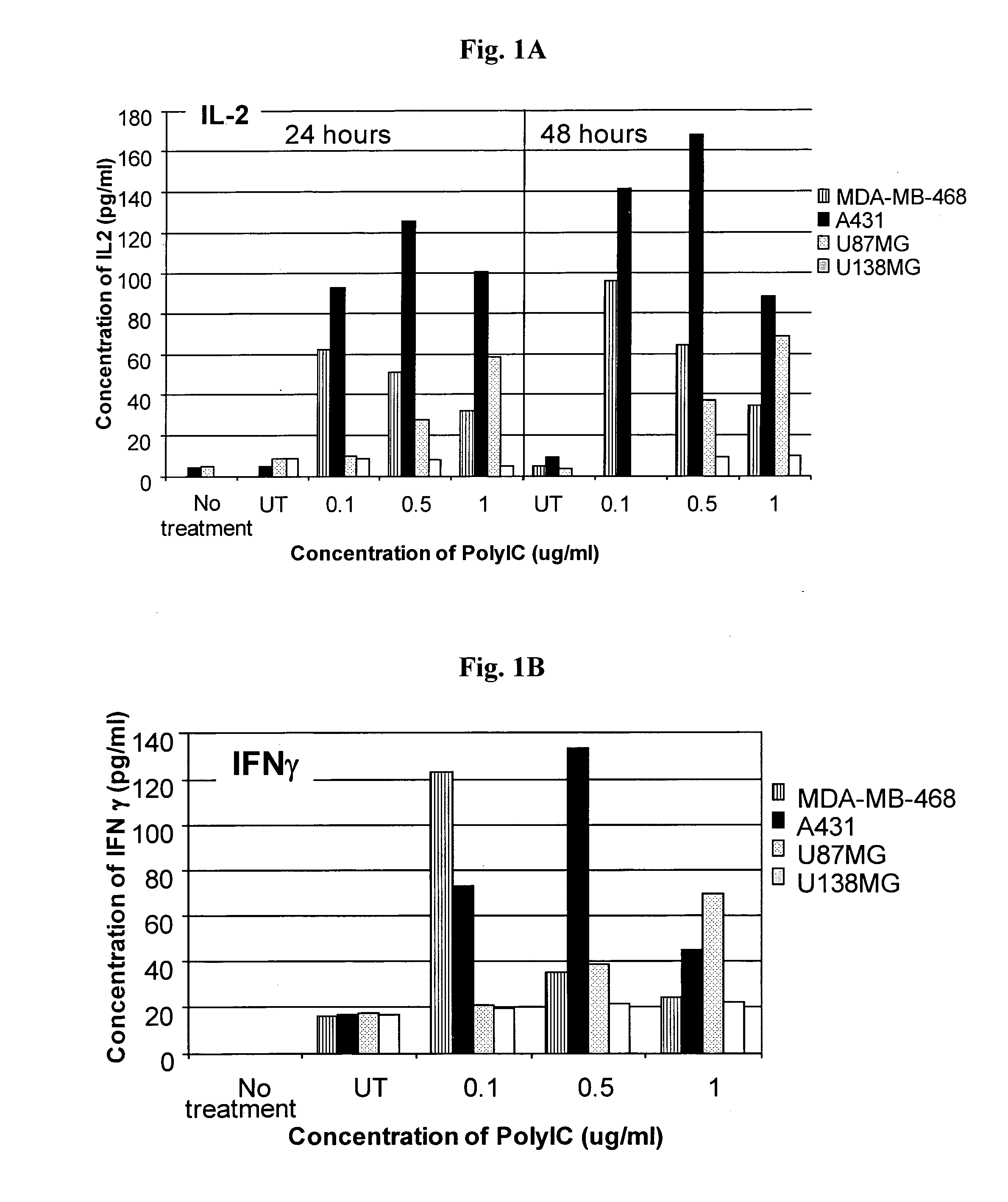
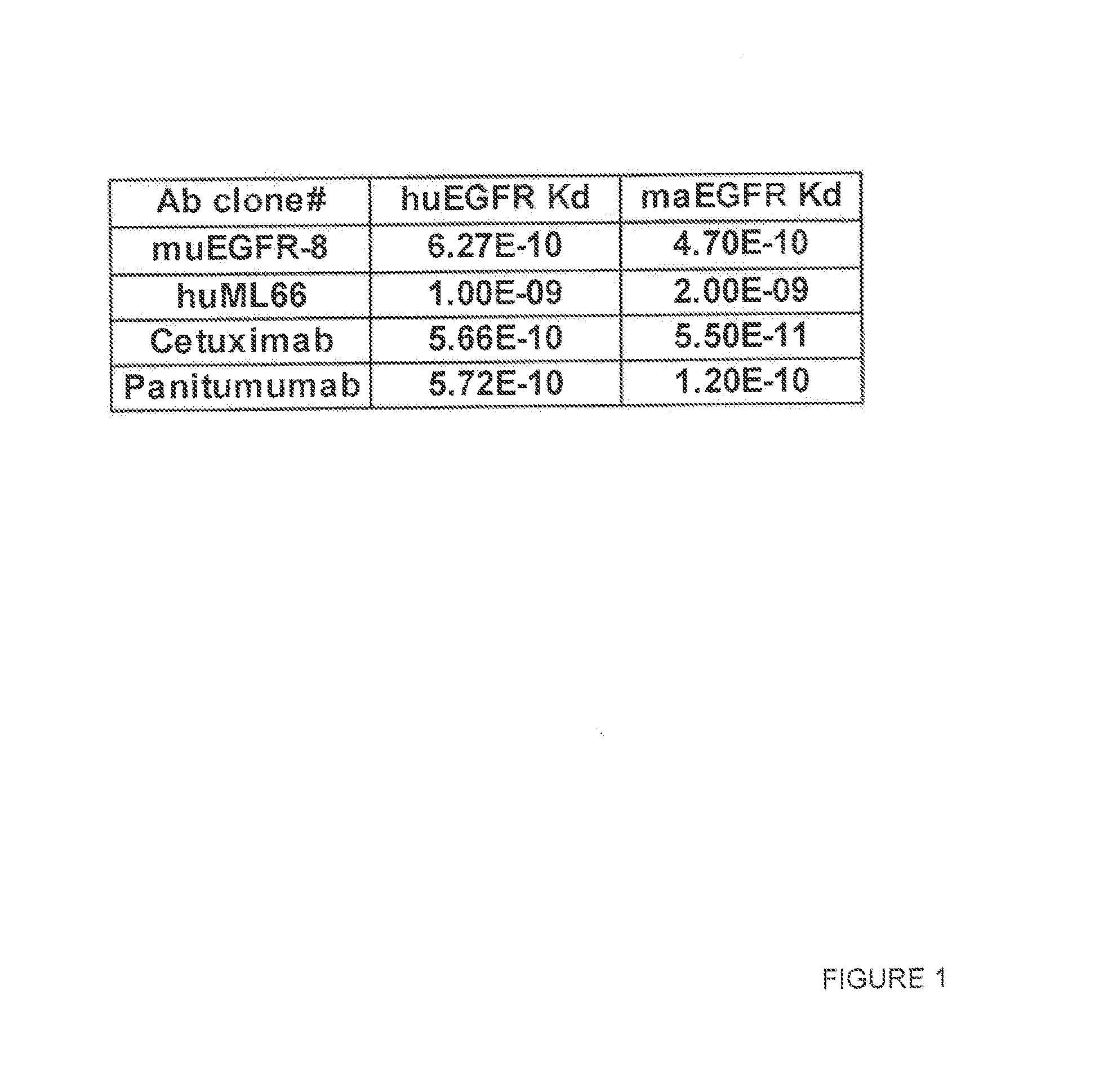
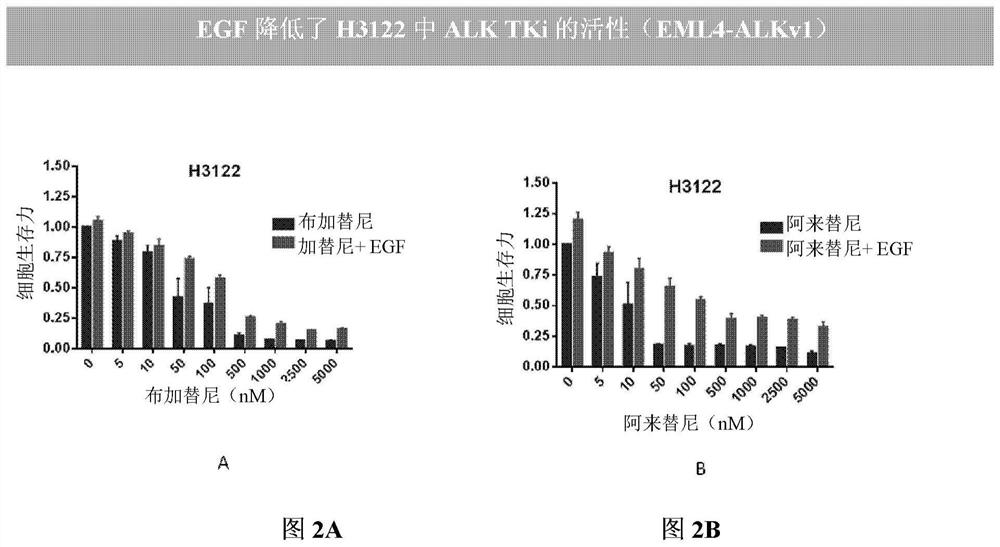
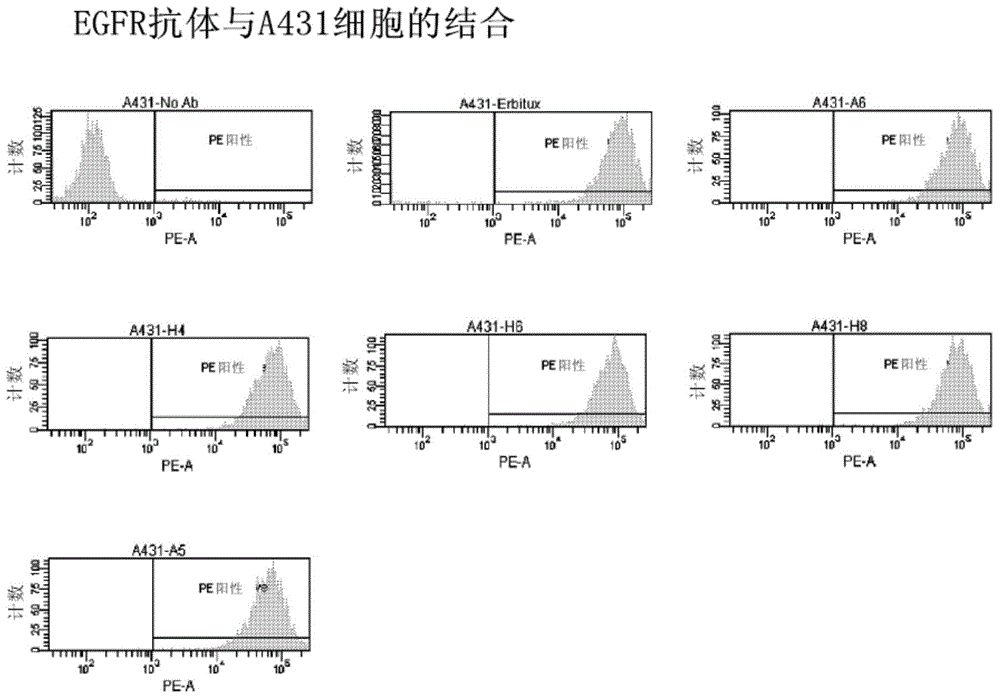
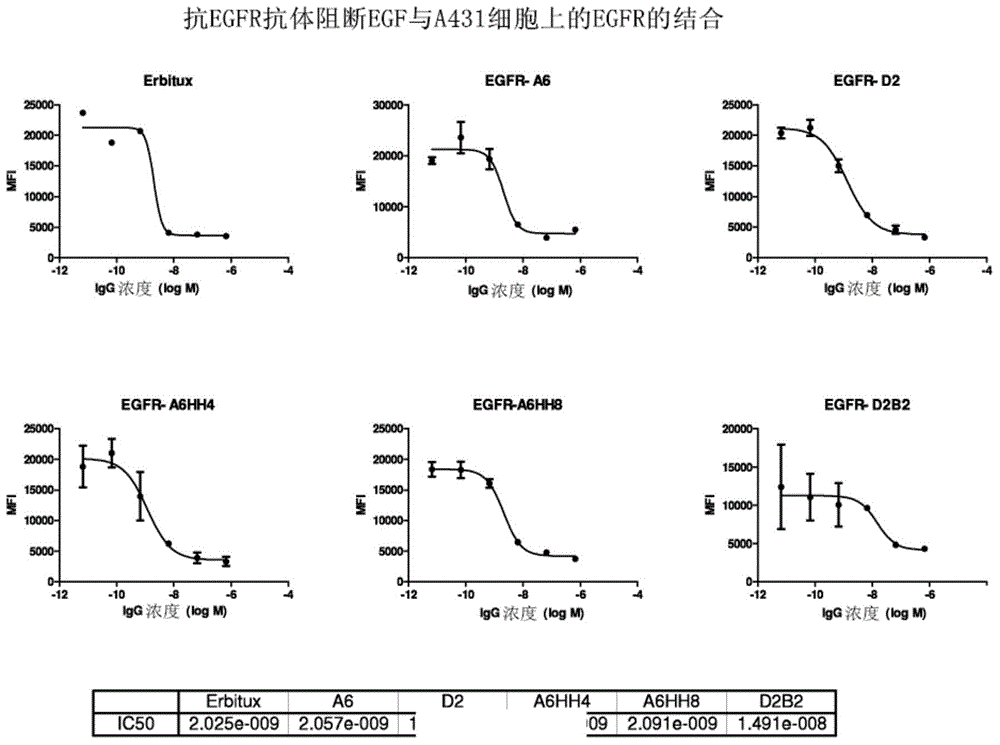
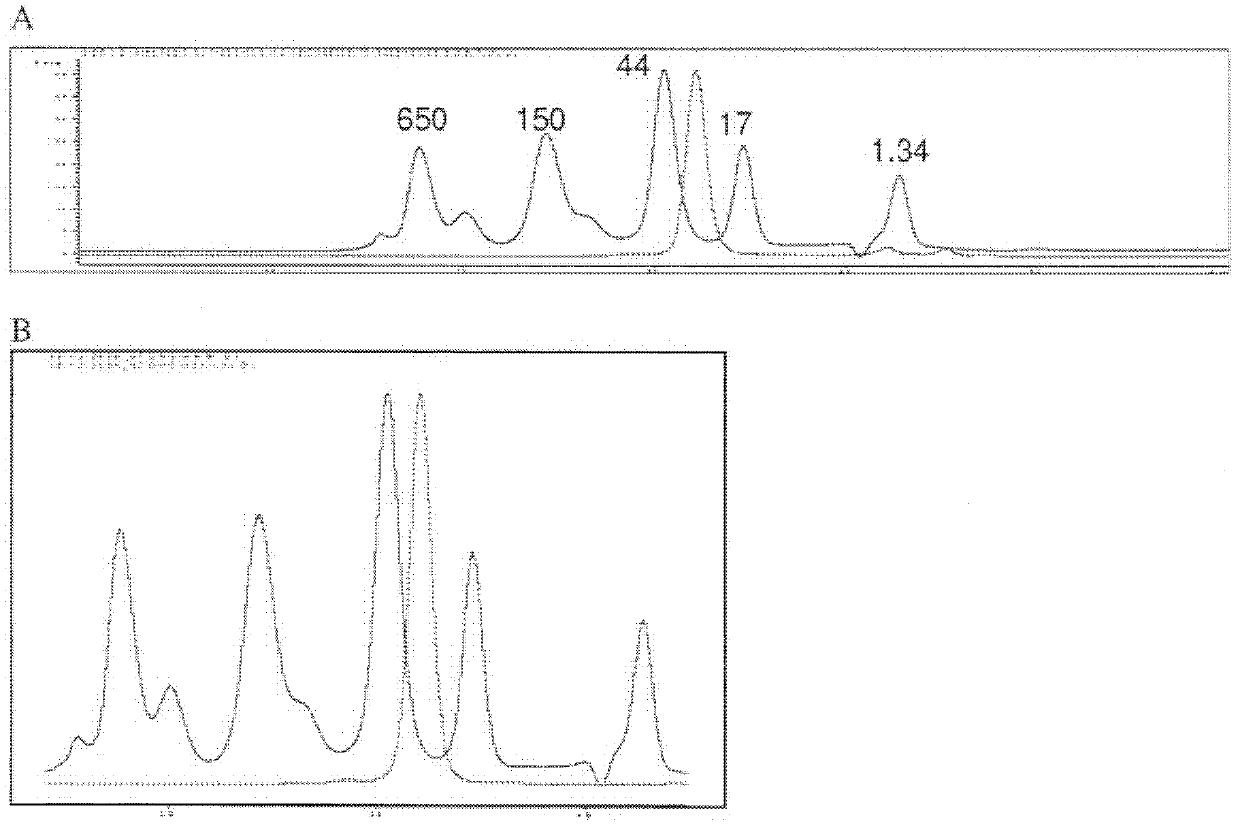
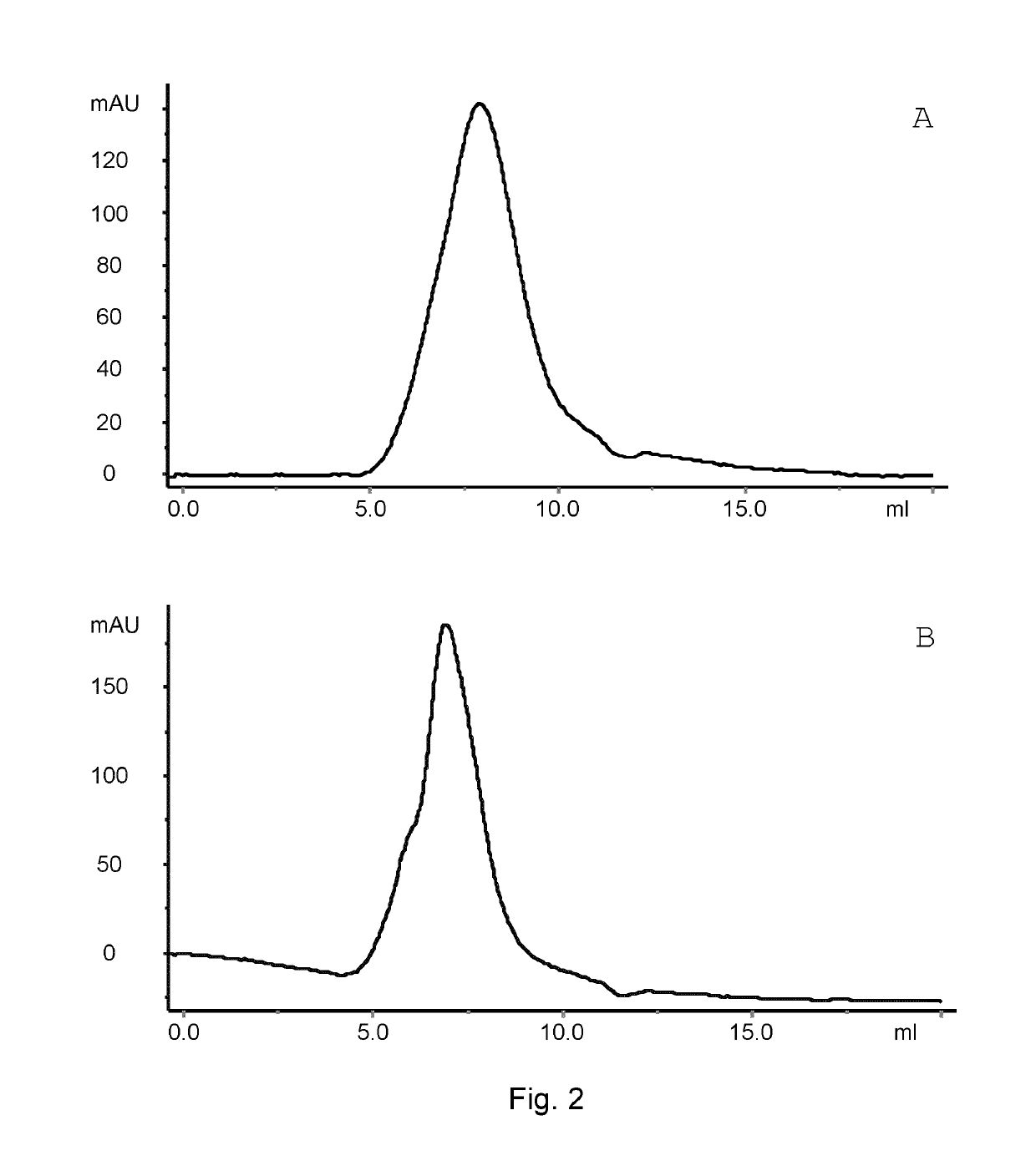
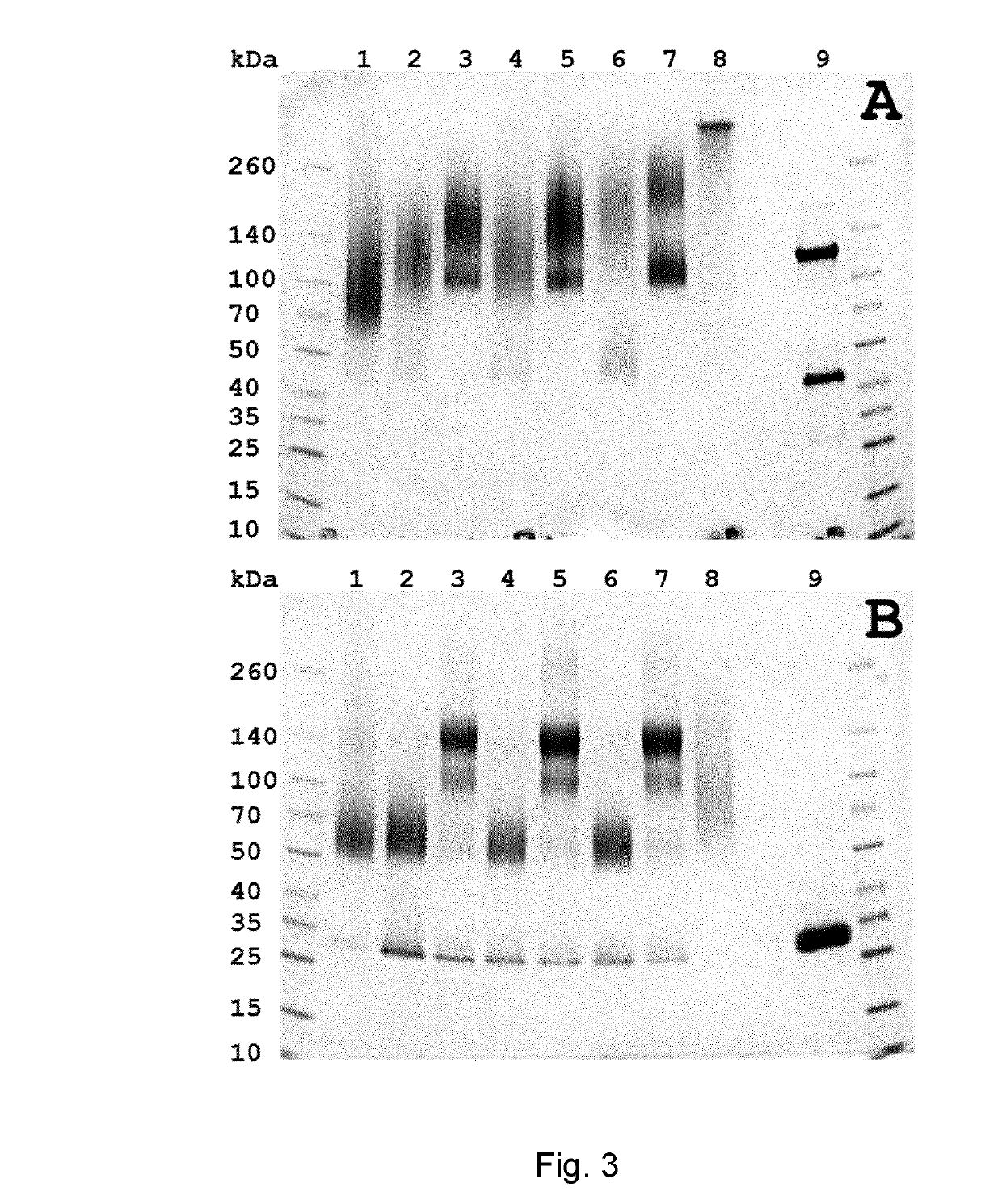
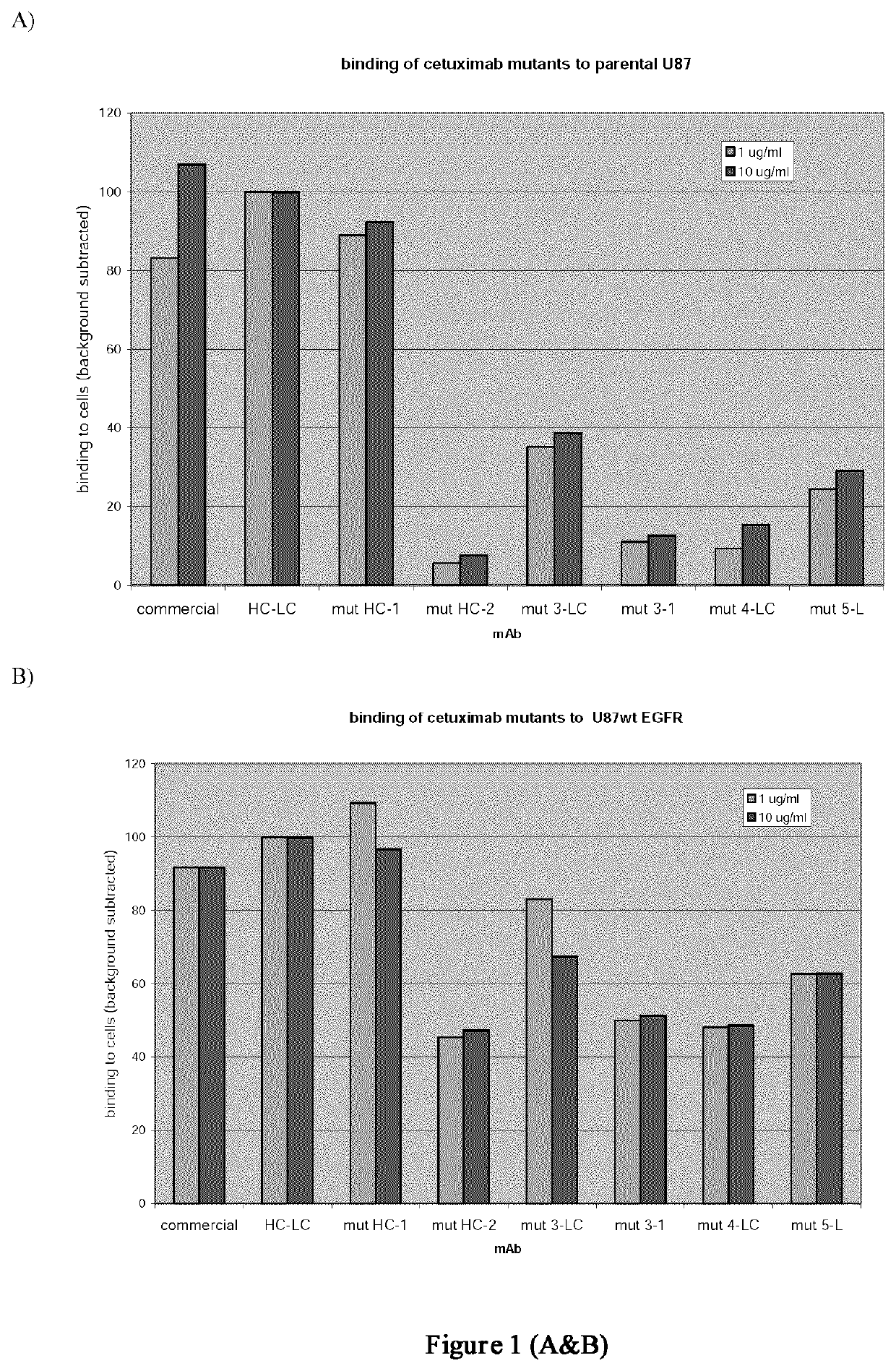
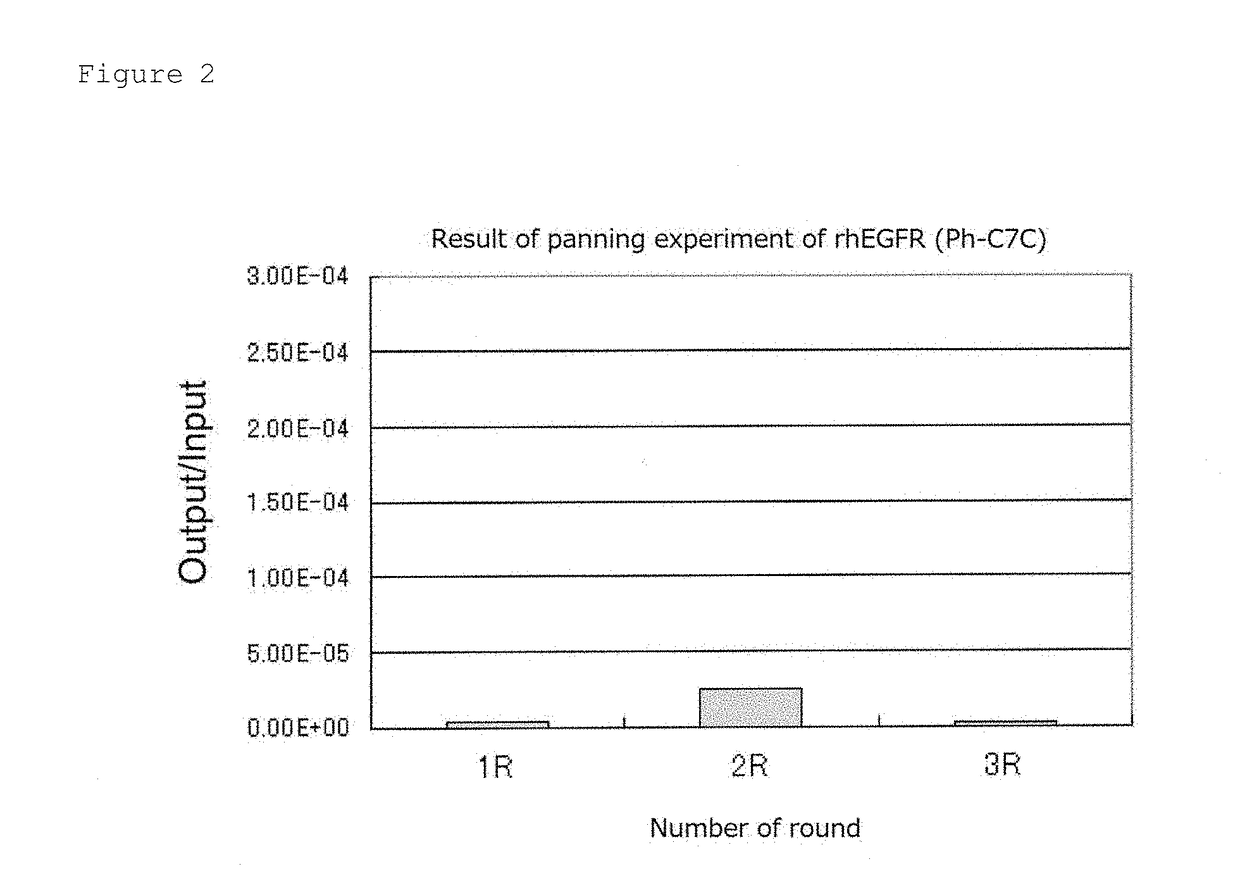
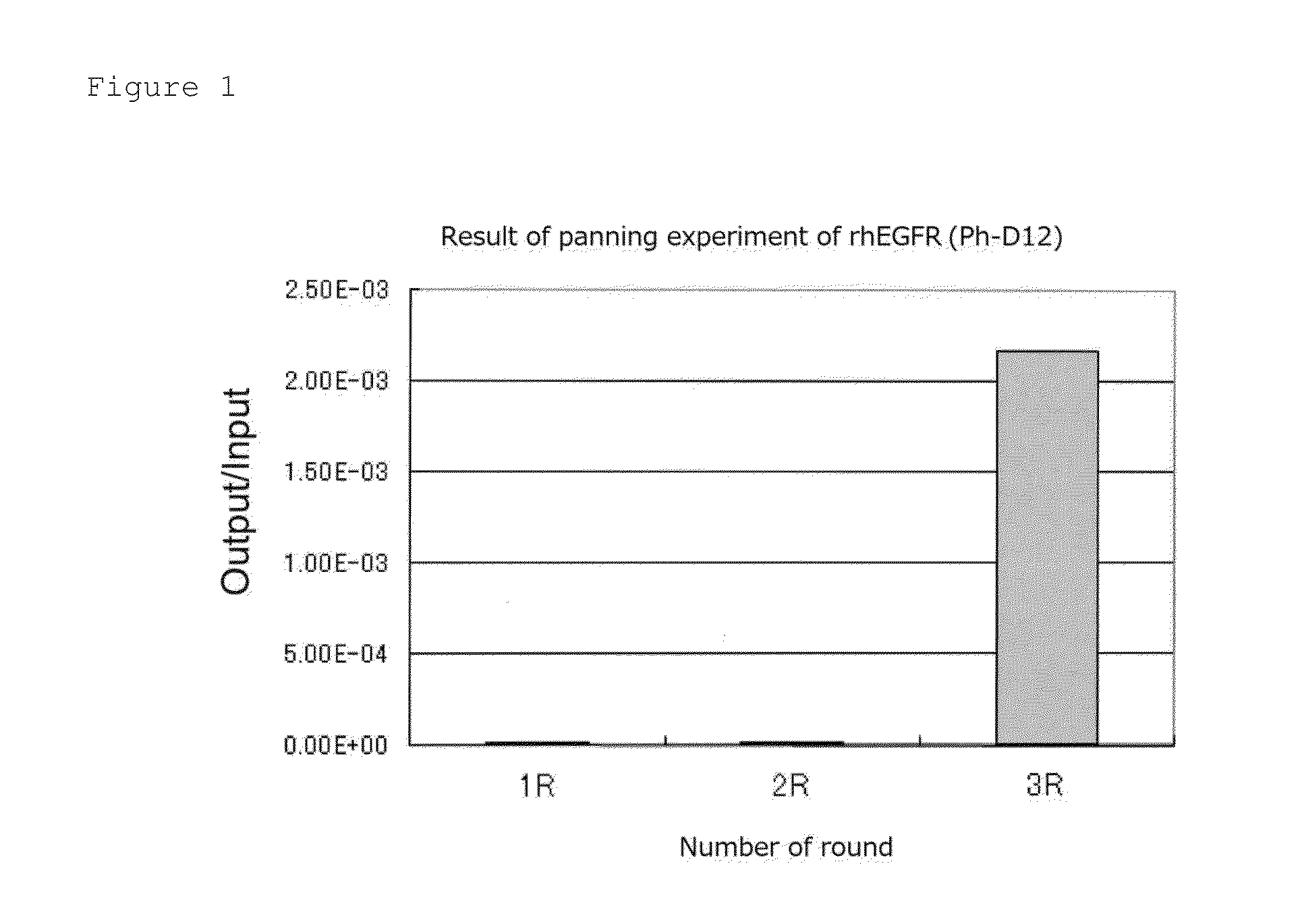
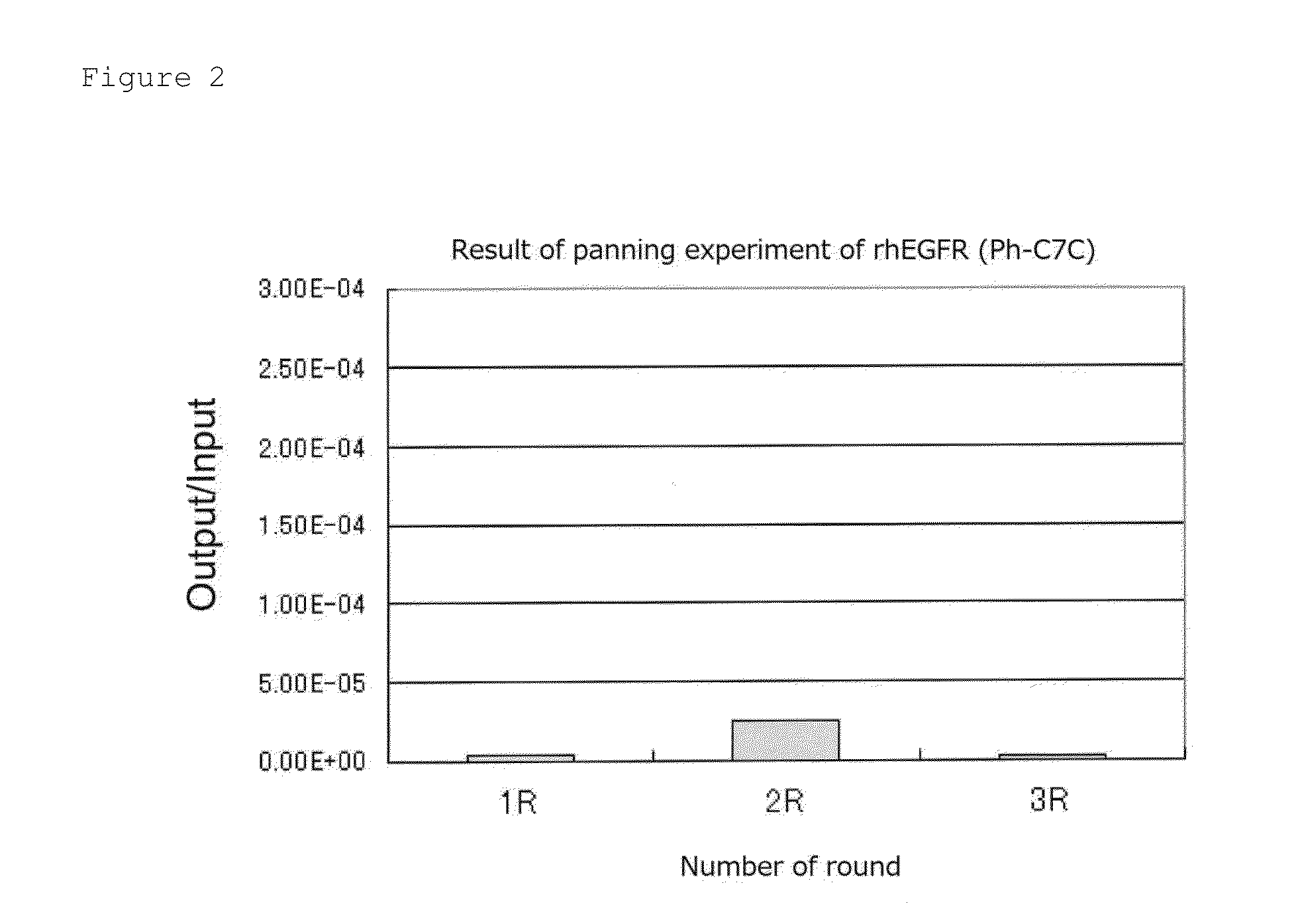
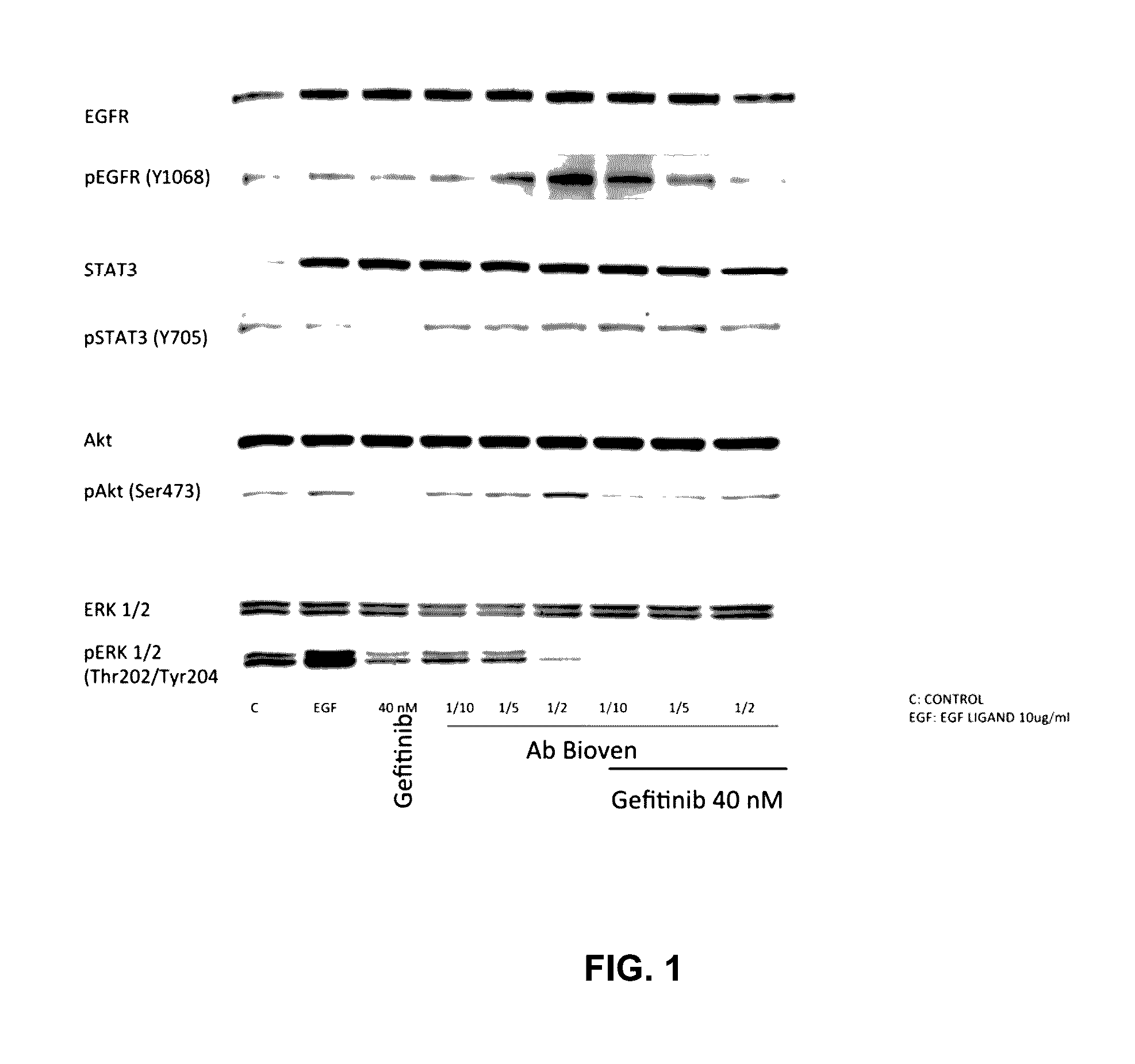
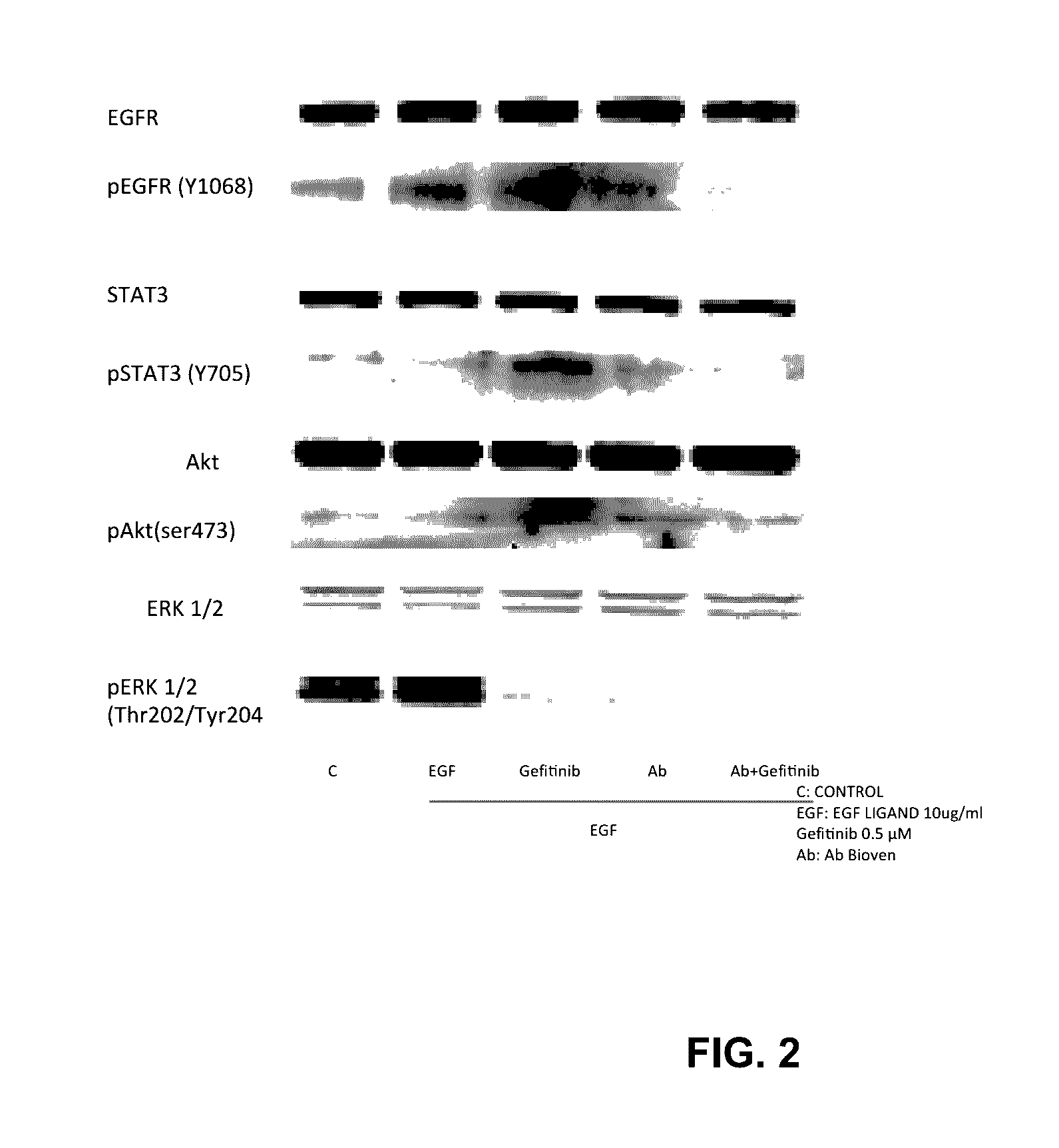
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "EGFR binding" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Egfr-homing double-stranded RNA vector for systemic cancer treatment
ActiveUS20120021006A1Saccharide peptide ingredientsImmunological disordersCancer therapyEGFR binding
An epidermal growth factor receptor (EGFR)-homing vector comprising a double-stranded RNA (dsRNA) molecule with an EGFR-binding peptide or polypeptide, is disclosed for use in combination with immune cells for treatment of cancer overexpressing EGFR.
Owner:TARGIMMUNE THERAPEUTICS AG
Bispecific egfr/igfir binding molecules
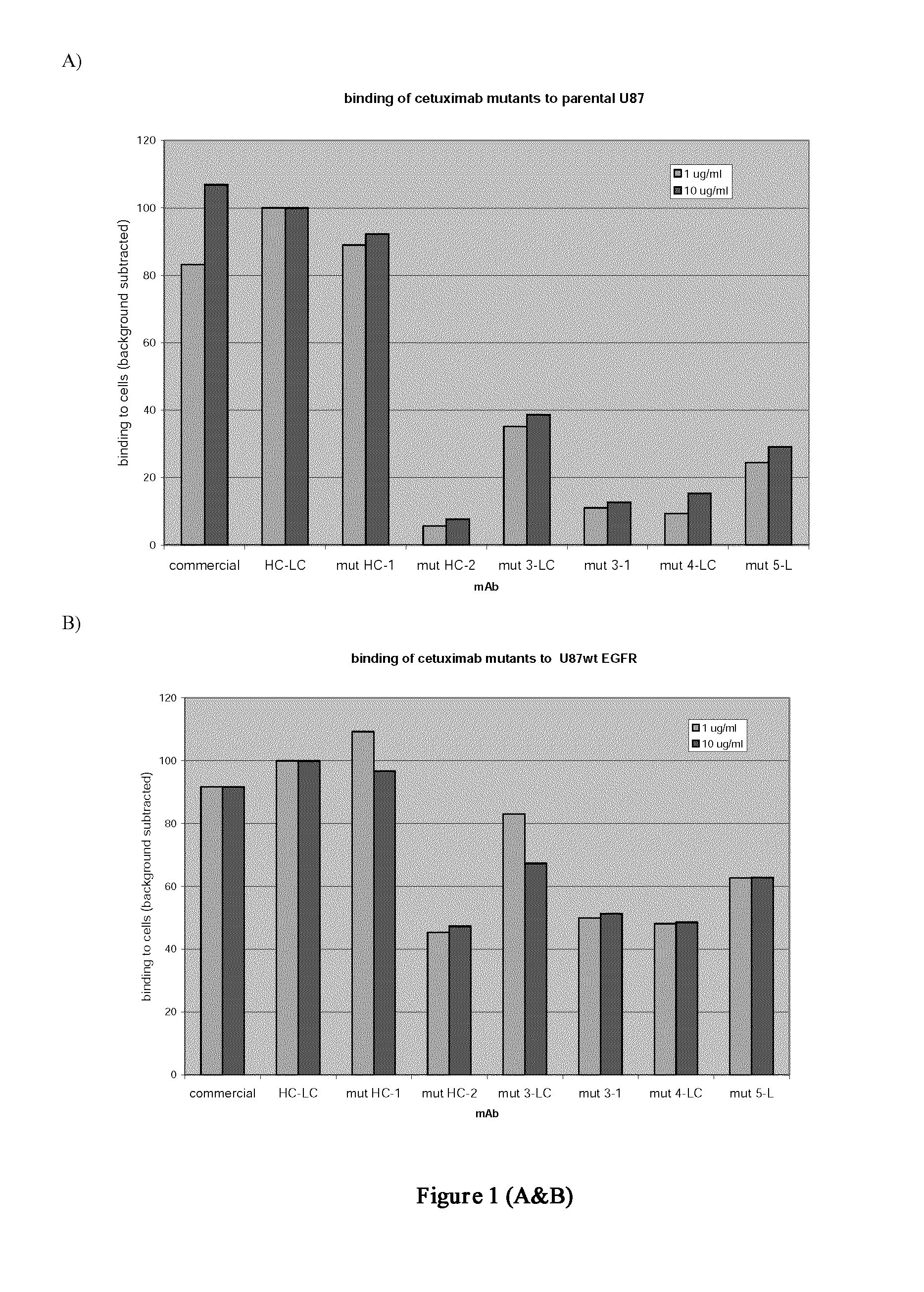
The present invention relates to bispecific molecules comprising an EGFR binding domain and a distinct IGFIR binding domain for use in diagnostic, research and therapeutic applications. The invention further relates to cells comprising such proteins, polynucleotide encoding such proteins or fragments thereof, and vectors comprising the polynucleotides encoding the innovative proteins. Exemplary bispecific molecules include antibody-like protein dimers based on the tenth fibronectin type III domain.
Owner:BRISTOL MYERS SQUIBB CO
Non-antagonistic egfr-binding molecules and immunoconjugates thereof
InactiveUS20140023662A1Immunoglobulins against cell receptors/antigens/surface-determinantsFermentationAnticarcinogenEGFR binding
Novel anti-cancer agents, including, but not limited to, antibodies and immunoconjugates, that bind to EGFR are provided. Methods of using the agents, antibodies, or immunoconjugates, such as methods of inhibiting tumor growth are further provided.
Owner:IMMUNOGEN INC
EGFR-homing double-stranded rna vector for systemic cancer treatment
InactiveCN102325794AImmunoglobulins against cell receptors/antigens/surface-determinantsImmunological disordersGeneralized cancerEGFR binding
An epidermal growth factor receptor (EGFR)-homing vector comprising a double-stranded RNA (dsRNA) molecule with an EGFR-binding peptide or polypeptide, is disclosed for use in combination with immune cells for treatment of cancer overexpressing EGFR.
Owner:YISSUM RES DEV CO OF THE HEBREW UNIV OF JERUSALEM LTD
Novel EGFR binding proteins
ActiveUS20180030098A1Peptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeAnti-EGFR Monoclonal Antibody
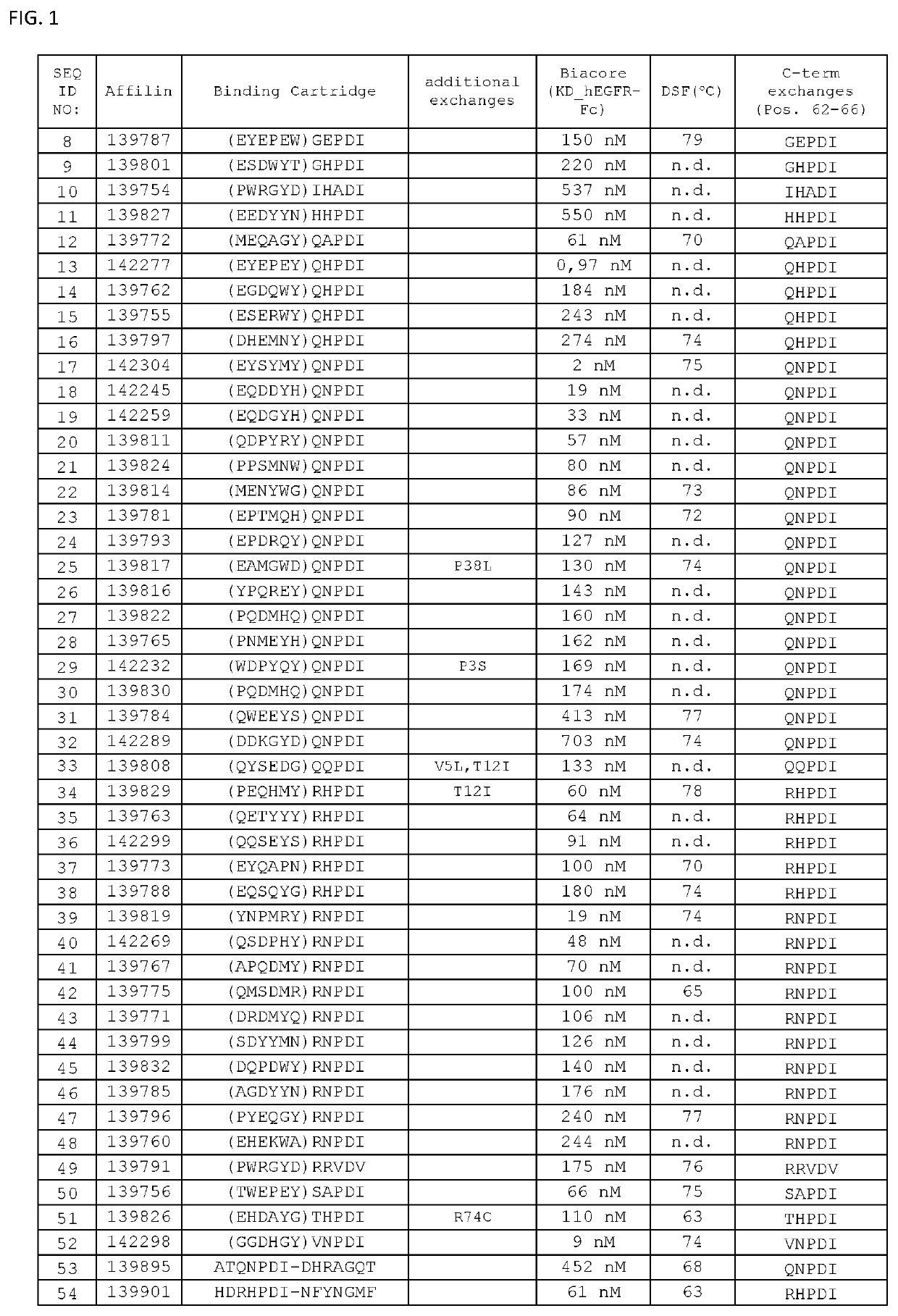
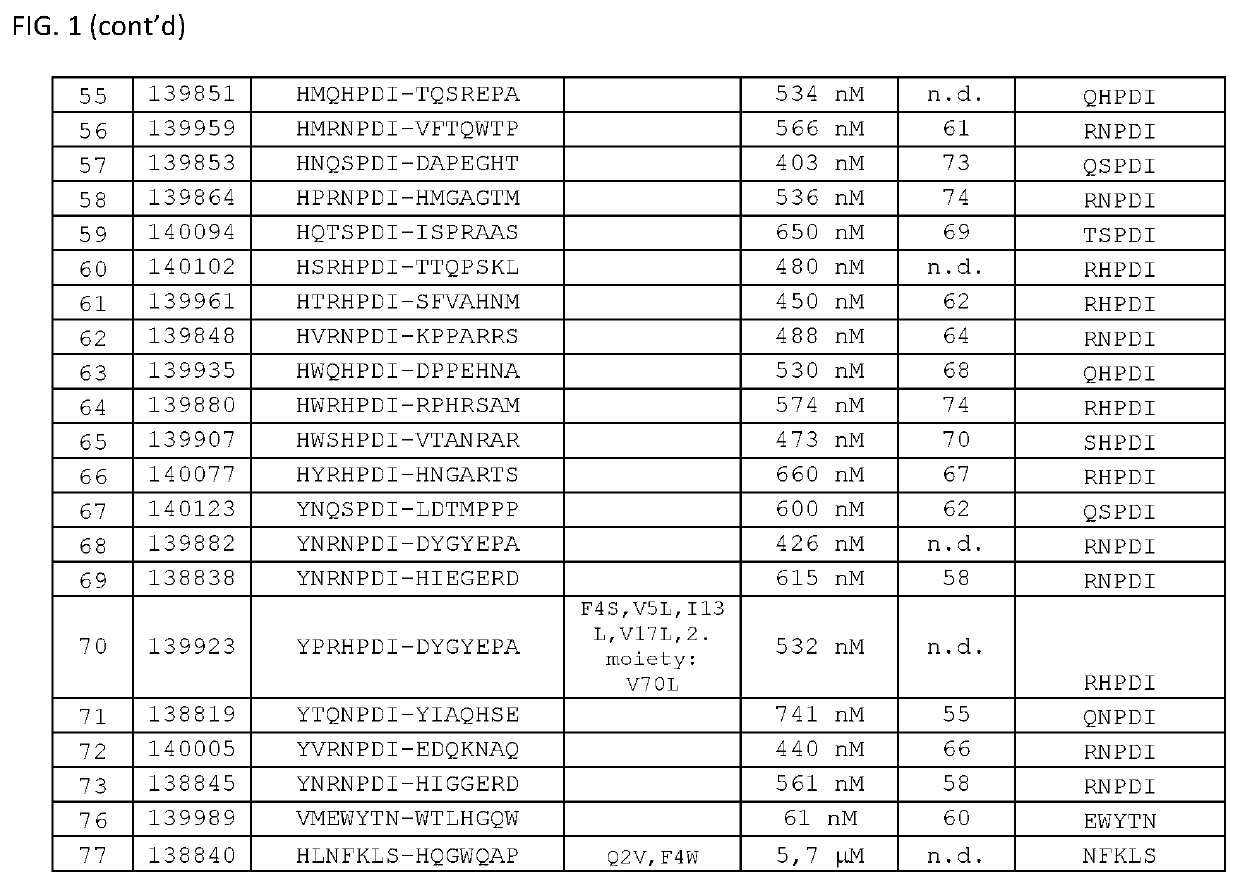
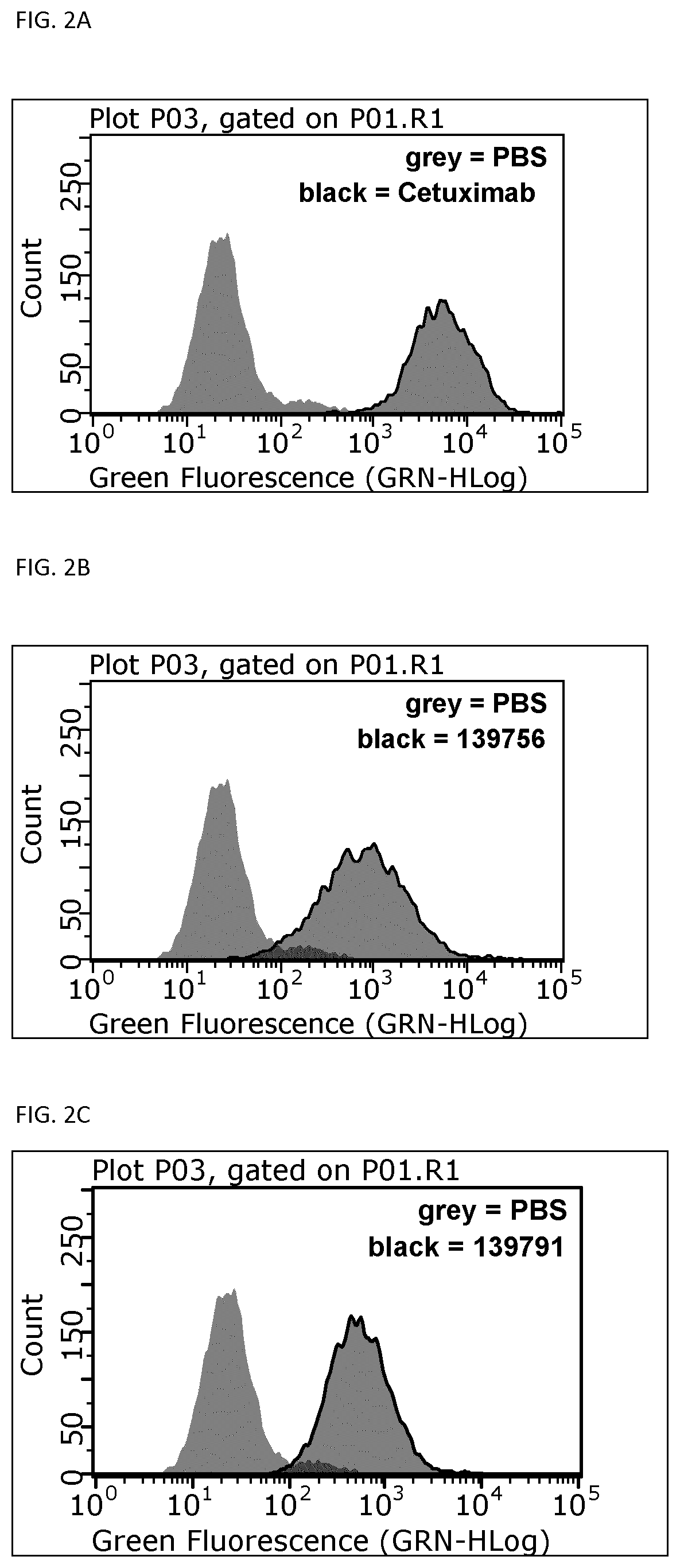
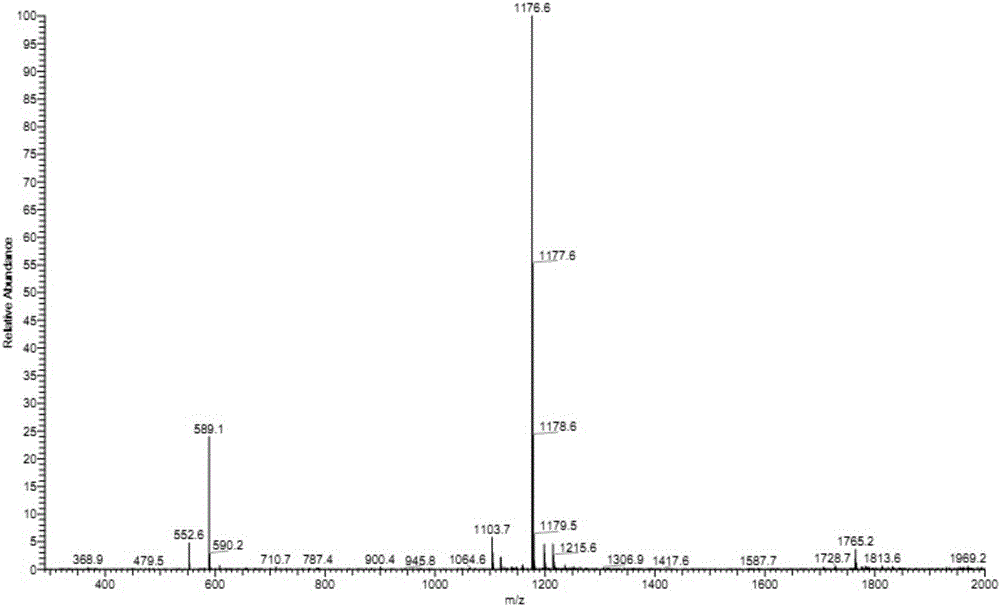
The present invention relates to new EGFR binding molecules based on ubiquitin muteins (Affilin®), preferably Affilin molecules having a characteristic three amino acid residue motif. The invention further refers to EGFR binding molecules that bind to different or non-overlapping epitopes than the anti-EGFR monoclonal antibody Cetuximab. The invention further relates to the use of these EGFR binding proteins in medicine, preferably for use in the diagnosis or treatment of cancer.
Owner:NAVIGO PROTEINS GMBH
EGFR-homing double-stranded RNA vector for systemic cancer treatment
An epidermal growth factor receptor (EGFR)-homing vector comprising a double-stranded RNA (dsRNA) molecule with an EGFR-binding peptide or polypeptide, is disclosed for use in combination with immune cells for treatment of cancer overexpressing EGFR.
Owner:TARGIMMUNE THERAPEUTICS AG
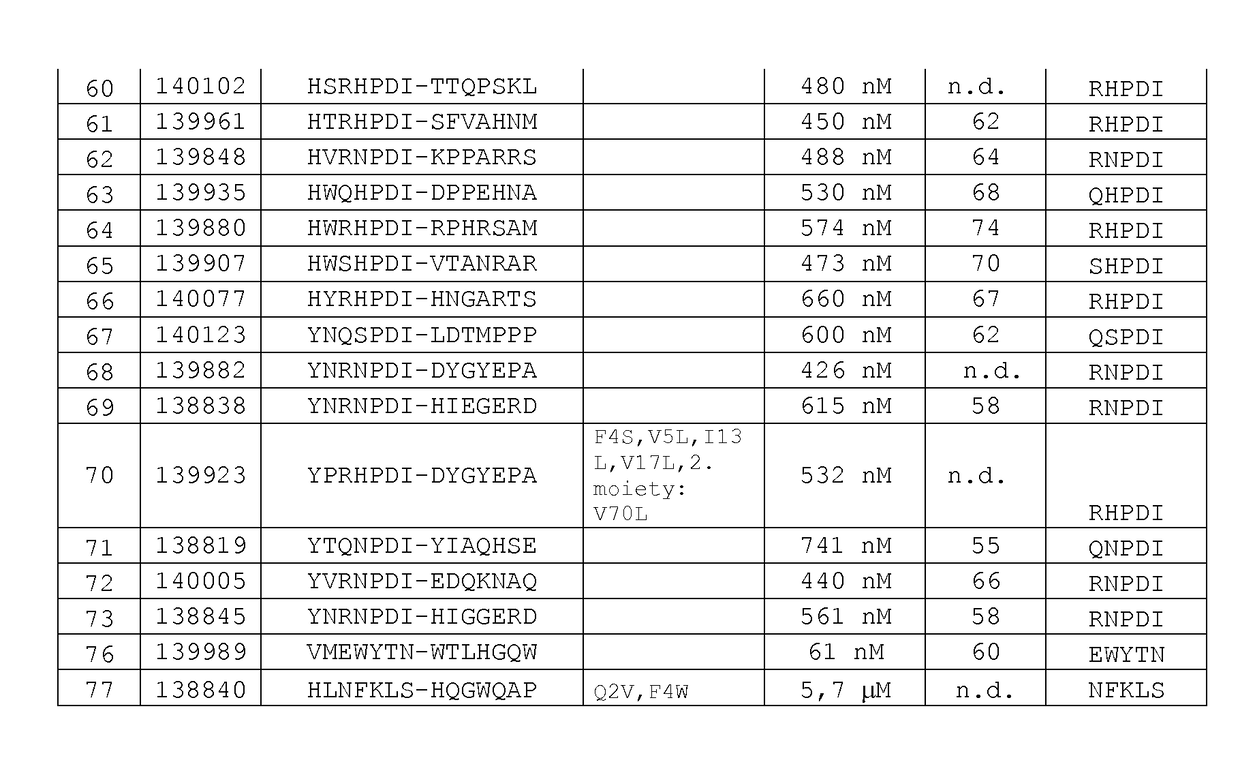
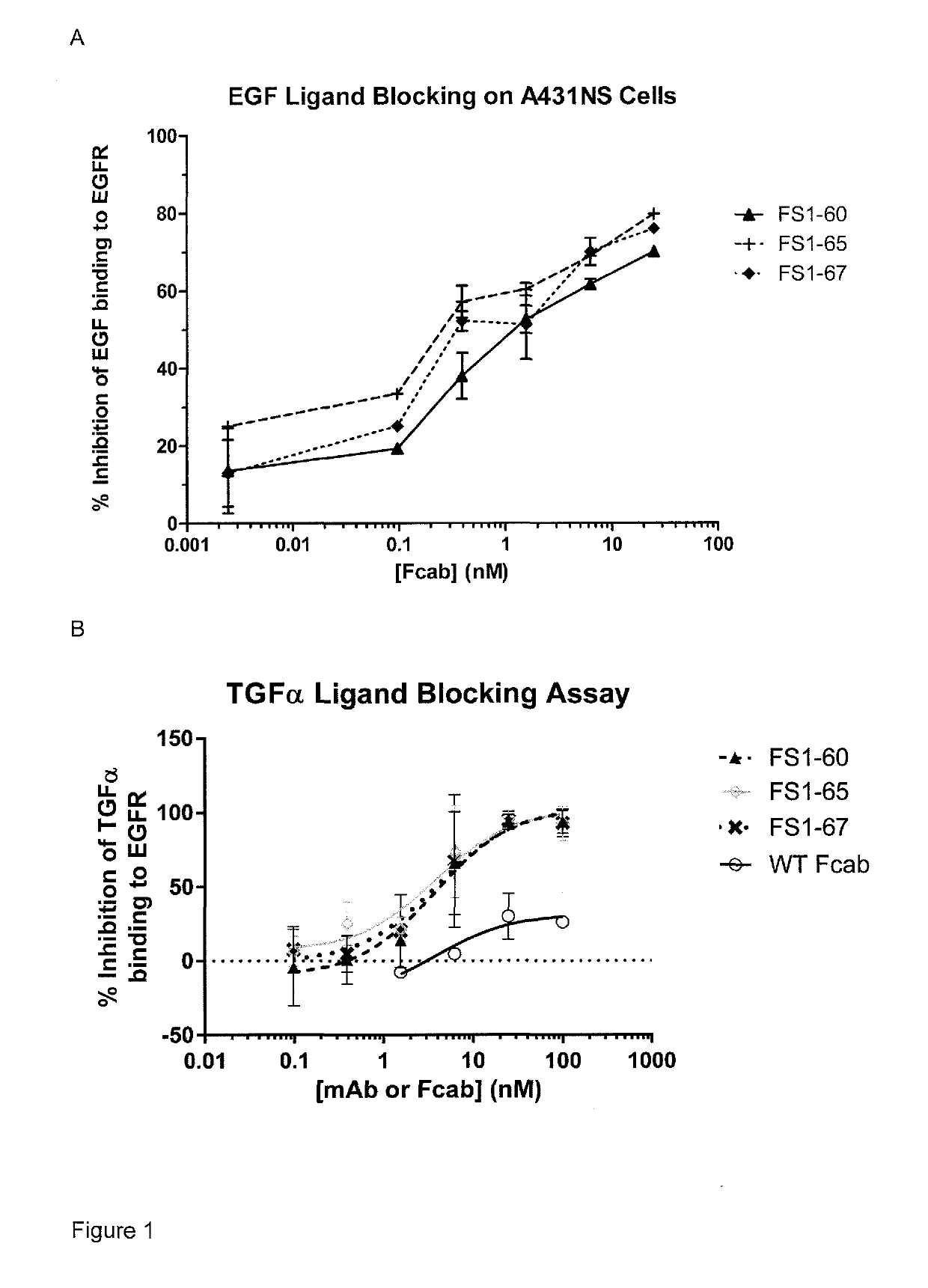
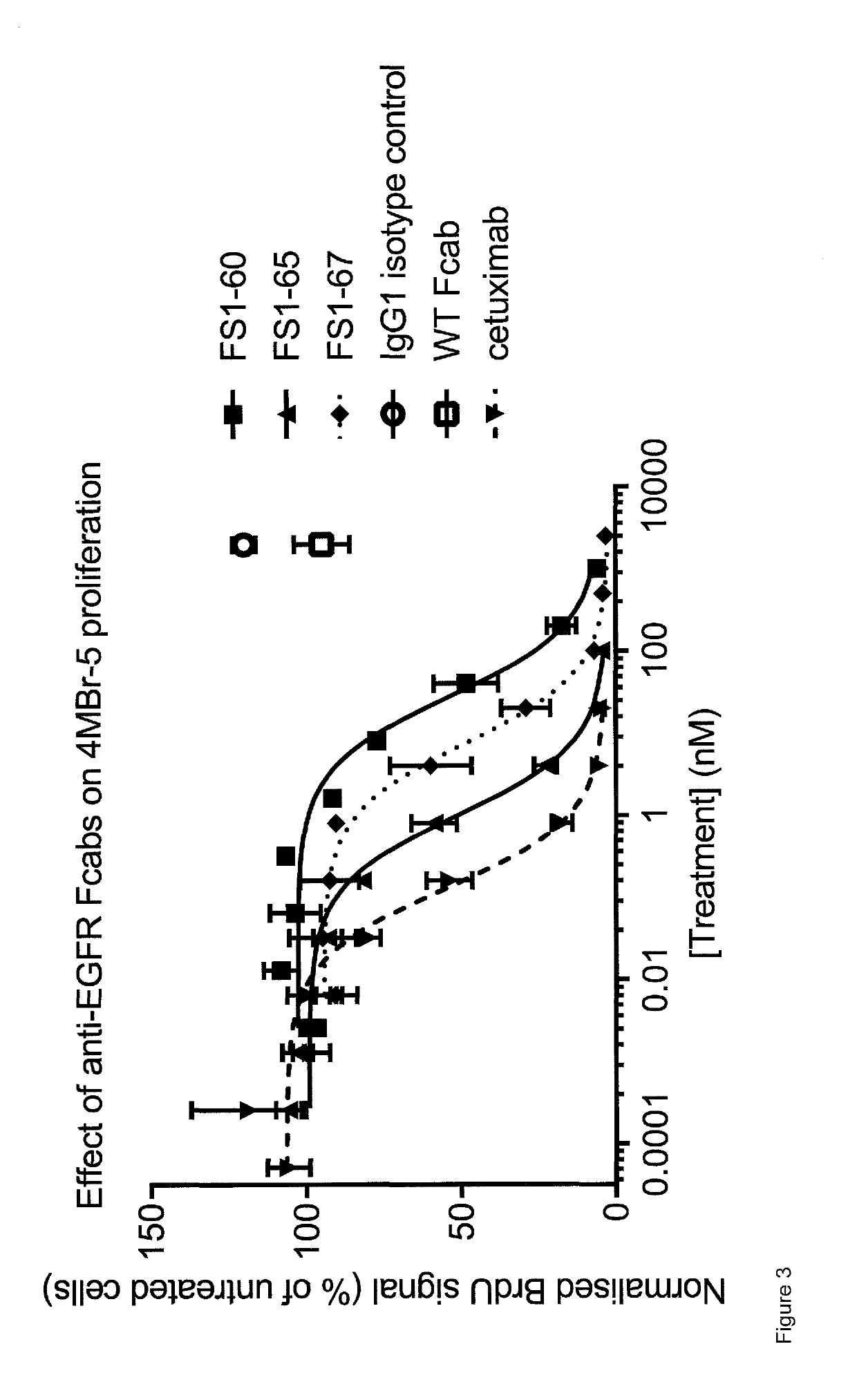
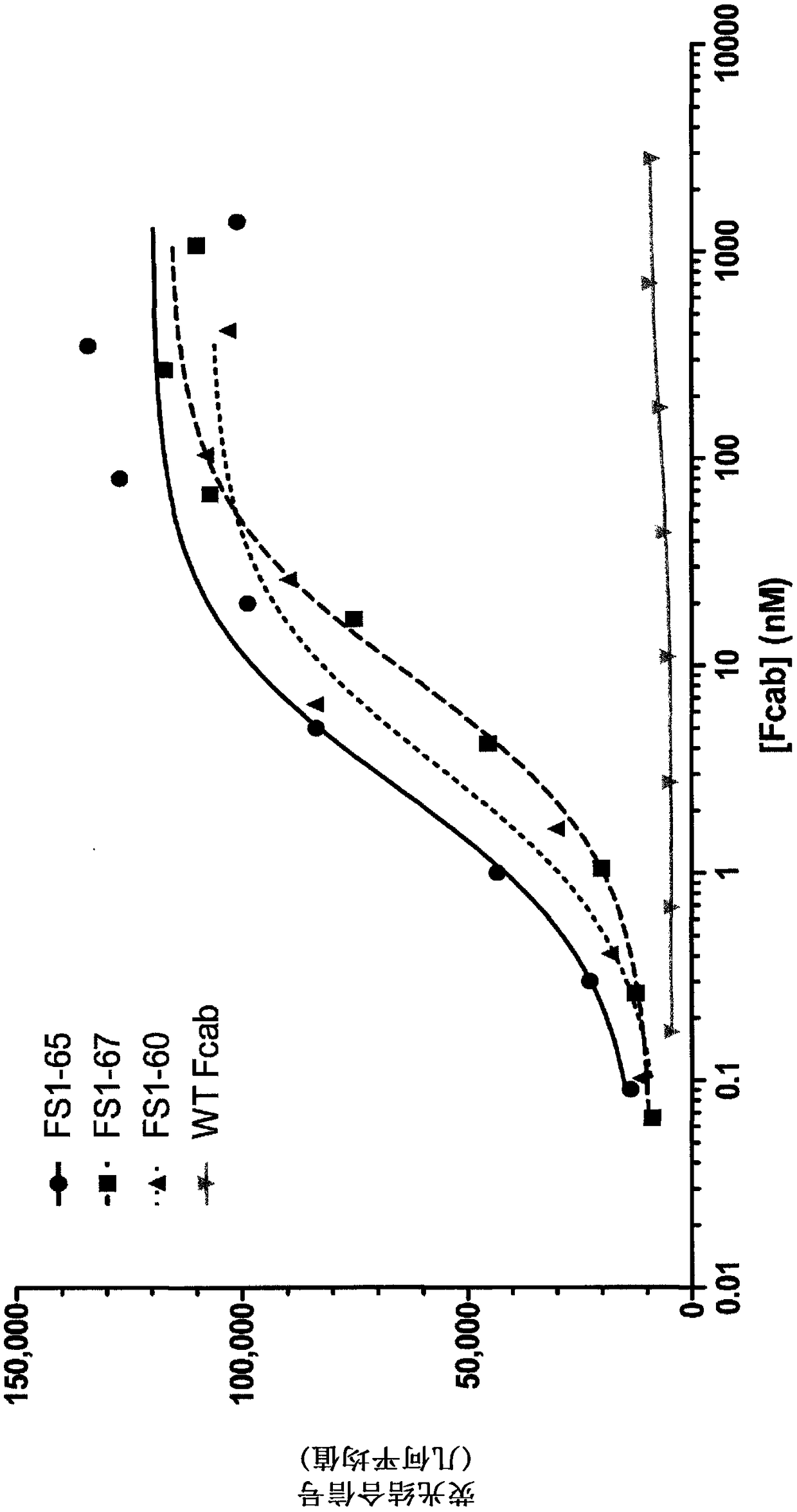
EGFR binding molecules
ActiveUS20190202920A1Good curative effectReduce usageOrganic active ingredientsHybrid immunoglobulinsBinding siteHuman epidermal growth factor receptor
The application relates to specific binding members which bind the human epidermal growth factor receptor (EGFR). The specific binding members preferably comprise an EGFR antigen-binding site which may be located in two or more structural loops of a CH3 domain of the specific binding member. The specific binding members are expected to find application in the treatment of cancers expressing EGFR.
Owner:F STAR THERAPEUTICS LTD
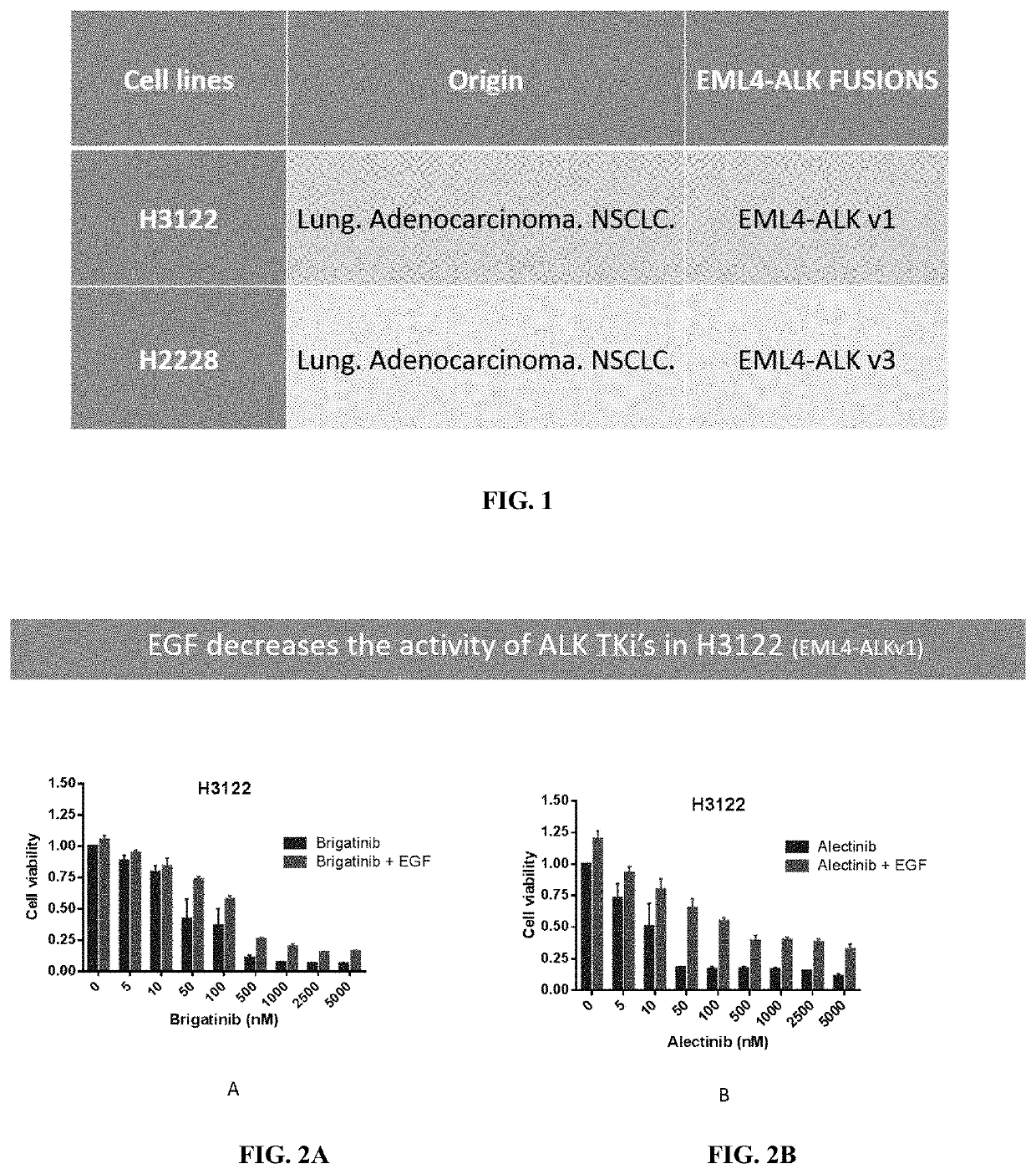
Methods and compositions for inhibition of egf/egfr pathway in combination with anaplastic lymphoma kinase inhibitors
PendingUS20200046690A1Avoid resistanceOrganic active ingredientsImmunoglobulins against growth factorsDosing regimenEgfr pathway
A method of treating patients suffering from cancers driven by deregulated Human Epidermal Growth Factor Receptor (HER1 / Human EGFR) comprising administering to a patient in need of such treatment a flexible and active regimen for combining Anaplastic Lymphoma Kinase Inhibitors (ALK Inhibitors) and anti-EGF antibodies for inhibition of the pathway activated by EGF-EGFR binding (mAb). The anti-EGF antibodies can be produced by active immunization or provided passively by the administration of antibodies that are anti-EGF. The method comprises ALK Inhibitors administered according to a continuous regimen based on an average daily dose in the range of 10 to 250 mg and the mAb is co-administered either actively or passively according to a dosing regimen achieving a therapeutic effective amount repeated thrice, twice or once a week, once in two weeks, once in three weeks or at least once monthly.
Owner:IN3BIO LTD
EGFR binding proteins
ActiveUS10858405B2Peptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeAntiendomysial antibodies
Owner:NAVIGO PROTEINS GMBH
Methods and compositions for inhibition of egf/egfr pathway in combination with tyrosine kinase inhibitors
PendingUS20190358320A1Avoid resistanceOrganic active ingredientsImmunoglobulins against growth factorsDosing regimenEgfr pathway
A method of treating patients suffering from cancers driven by deregulated Human Epidermal Growth Factor Receptor (HER1 / Human EGFR) comprising administering to a patient in need of such treatment a flexible and active regimen for combining a tyrosine kinase inhibitor (TKI) and anti-EGF antibodies for inhibition of the pathway activated by EGF-EGFR binding (mAb). The anti-EGF antibodies can be produced by active immunization or provided passively by the administration of antibodies that are anti-EGF. The method comprises TKI administered according to a continuous regimen based on an average daily dose in the range of 10 to 150 mg and the mAb is co-administered either actively or passively according to a dosing regimen achieving a therapeutic effective amount repeated thrice, twice or once a week, once in two weeks, once in three weeks or at least once monthly.
Owner:IN3BIO LTD
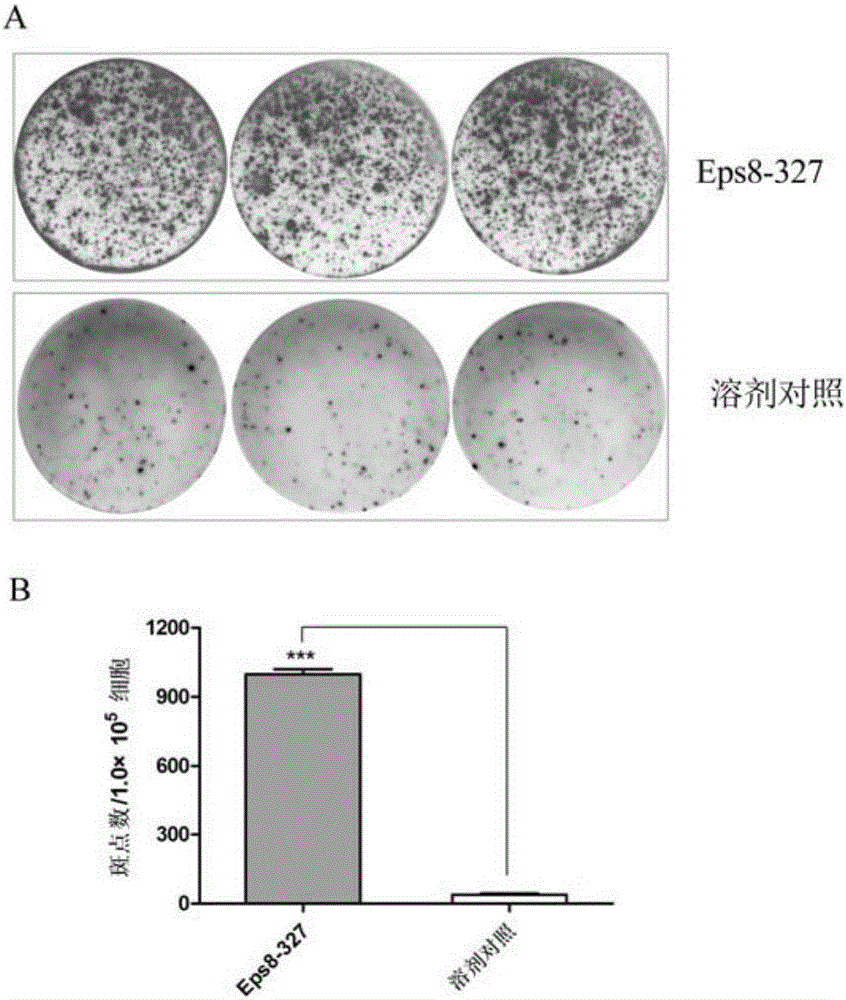
Dual anti-tumor polypeptide based on Eps8-EGFR binding domain
InactiveCN105859865APrevent proliferationTumor rejection antigen precursorsPeptide/protein ingredientsEPS8EGFR binding
The invention relates to a dual anti-tumor polypeptide based on the Eps8-EGFR binding domain. The amino acid sequence of the polypeptide is Glu-Phe-Leu-Asp-Cys-Phe-Gln-Lys-Phe (SEQ ID No:1). The dual anti-tumor polypeptide can induce toxic T cells, effectively kills HLA-A*2402 positive tumors and Eps8 positive tumors, can directly inhibit proliferation of HLA-A*2402 positive tumor cells and Eps8 positive tumor cells, and can be used for preparing dual anti-tumor medicine.
Owner:SOUTHERN MEDICAL UNIVERSITY
Antibodies selective for cells presenting EGFR at high density
ActiveUS20130295086A1Reduce EGFR binding affinity of antibodyMinimizing and avoiding effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody medical ingredientsDiseaseHigh density
Herein described are antibodies to epidermal growth factor receptor (EGFR) having an EGFR binding affinity that is sufficient to kill disease cells presenting EGFR at high density, but is insufficient for binding to normal cells. A therapeutic effect is thus achieved while avoiding adverse events that result from unintended binding to normal cells.
Owner:NAT RES COUNCIL OF CANADA +1
Non-antagonistic EGFR-binding molecules and immunoconjugates thereof
InactiveUS9238690B2Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsAnticarcinogenEGFR binding
Novel anti-cancer agents, including, but not limited to, antibodies and immunoconjugates, that bind to EGFR are provided. Methods of using the agents, antibodies, or immunoconjugates, such as methods of inhibiting tumor growth are further provided.
Owner:IMMUNOGEN INC
Antibodies that bind epidermal growth factor receptor (EGFR)
ActiveUS9944707B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntibody fragmentsHuman epidermal growth factor receptor
There is disclosed compositions and methods relating to or derived from anti-EGFR antibodies. More specifically, there is disclosed fully human antibodies that bind EGFR, EGFR-binding fragments and derivatives of such antibodies, and EGFR-binding polypeptides comprising such fragments. Further still, there is disclosed antibody fragments and derivatives and polypeptides, and methods of using such antibodies, antibody fragments and derivatives and polypeptides, including methods of treating or diagnosing subjects having EGFR-related disorders or conditions.
Owner:SORRENTO THERAPEUTICS INC
EGFR binding molecules
PendingCN109476746AOrganic active ingredientsOrganic chemistry methodsBinding siteHuman epidermal growth factor receptor
The application relates to specific binding members which bind the human epidermal growth factor receptor (EGFR). The specific binding members preferably comprise an EGFR antigen-binding site which may be located in two or more structural loops of a CH3 domain of the specific binding member. The specific binding members are expected to find application in the treatment of cancers expressing EGFR.
Owner:F STAR THERAPEUTICS LTD
Conjugates Comprising an Anti-EGFR1 Antibody
ActiveUS20170112931A1Organic active ingredientsEnergy modified materialsD-GlucopyranoseOxidative cleavage
The present invention relates to a conjugate comprising an anti-EGFR1 antibody or an EGFR binding fragment thereof and at least one dextran derivative, wherein the dextran derivative comprises at least one D-glucopyranosyl unit, wherein at least one carbon selected from carbon 2, 3 or 4 of the at least one D-glucopyranosyl unit is substituted by a substituent of the formula —O—(CH2)n—S—B12H112− wherein n is in the range of 3 to 10; and the dextran derivative is bound to the anti-EGFR antibody or an EGFR1 binding fragment thereof via a bond formed by a reaction between at least one aldehyde group formed by oxidative cleavage of a D-glucopyranosyl unit of the dextran derivative and an amino group of the anti-EGFR1 antibody or an EGFR1 binding fragment thereof.
Owner:TENBORON
Methods and compositions for inhibition of egf/egfr pathway in cobination with anaplastic lymphoma kinase inhibitors
PendingCN112996534AOrganic active ingredientsImmunoglobulins against growth factorsDosing regimenEgfr pathway
A method of treating patients suffering from cancers driven by deregulated Human Epidermal Growth Factor Receptor (HER1 / Human EGFR) comprising administering to a patient in need of such treatment a flexible and active regimen for combining Anaplastic Lymphoma Kinase Inhibitors (ALK Inhibitors) and anti-EGF antibodies for inhibition of the pathway activated by EGF-EGFR binding (mAb). The anti-EGF antibodies can be produced by active immunization or provided passively by the administration of antibodies that are anti-EGF. The method comprises ALK Inhibitors administered according to a continuous regimen based on an average daily dose in the range of 10 to 250 mg and the mAb is co-administered either actively or passively according to a dosing regimen achieving a therapeutic effective amount repeated thrice, twice or once a week, once in two weeks, once in three weeks or at least once monthly.
Owner:ИН3БАЙО ЛТД
Antigen binding proteins that bind EGFR
InactiveCN104918956AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntibody fragmentsAntigen binding
There is disclosed compositions and methods relating to or derived from anti-EGFR antibodies. More specifically, there is disclosed fully human antibodies that bind EGFR, EGFR-binding fragments and derivatives of such antibodies, and EGFR-binding polypeptides comprising such fragments. Further still, there is disclosed antibody fragments and derivatives and polypeptides, and methods of using such antibodies, antibody fragments and derivatives and polypeptides, including methods of treating or diagnosing subjects having EGFR-related disorders or conditions.
Owner:SORRENTO THERAPEUTICS INC
Bispecific EGFR/IGFIR binding molecules
The present invention relates to bispecific molecules comprising an EGFR binding domain and a distinct IGFIR binding domain for use in diagnostic, research and therapeutic applications. The invention further relates to cells comprising such proteins, polynucleotide encoding such proteins or fragments thereof, and vectors comprising the polynucleotides encoding the innovative proteins. Exemplary bispecific molecules include antibody-like protein dimers based on the tenth fibronectin type III domain.
Owner:BRISTOL MYERS SQUIBB CO
Conjugates Comprising An Anti-EGFR1 Antibody
ActiveUS20190240330A1Organic active ingredientsEnergy modified materialsD-GlucopyranoseOxidative cleavage
The present invention relates to a conjugate comprising an anti-EGFR1 antibody or an EGFR binding fragment thereof and at least one dextran derivative, wherein the dextran derivative comprises at least one D-glucopyranosyl unit, wherein at least one carbon selected from carbon 2, 3 or 4 of the at least one D-glucopyranosyl unit is substituted by a substituent of the formula —O—(CH2)”—S—Bi2Hii2− wherein n is in the range of 3 to 10; and the dextran derivative is bound to the anti-EGFR antibody or an EGFR1 binding fragment thereof via a bond formed by a reaction between at least one aldehyde group formed by oxidative cleavage of a D-glucopyranosyl unit of the dextran derivative and an amino group of the anti-EGFR1 antibody or an EGFR1 binding fragment thereof.
Owner:TENBORON
Antibodies selective for cells presenting EGFR at high density
ActiveUS10570211B2Minimizing and avoiding effectReduced and desirably minimal or negligibleImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody medical ingredientsTherapeutic effectEGFR binding
Owner:NAT RES COUNCIL OF CANADA +1
EGFR-binding peptide
ActiveUS9822148B2Specific and high binding propertyPeptide/protein ingredientsBiological material analysisEGFR bindingAmino acid composition
Provided are a substance capable of binding to EGFR specifically with a high affinity and a pharmaceutical agent containing the substance. A peptide which contains at least an amino acid sequence is represented by the formula (1), (2) or (3), as described in the specification, and is composed of 12 to 50 amino acids:(SEQ ID NO: 55)X1-His-X2-X3-Asp-X4-X5-X6-X7-X8-Trp-His(1)(SEQ ID NO: 56)Phe-His-Asp-Trp-X11-Pro-X12-X13-X14-X15-X16-X17(2)(SEQ ID NO: 57)Leu-His-X21-Ser-X22-Trp-Met-X23-X24-X25-X26-X27(3).
Owner:HOYA CORP
Egfr-binding peptide
ActiveUS20150337012A1Specific and high binding propertyInhibit bindingPeptide/protein ingredientsBiological material analysisEGFR bindingAmino acid composition
Provided are a substance capable of binding to EGFR specifically with a high affinity and a pharmaceutical agent containing the substance. A peptide which contains at least an amino acid sequence is represented by the formula (1), (2) or (3), as described in the specification, and is composed of 12 to 50 amino acids:X1-His-X2-X3-Asp-X4-X5-X6-X7-X8-Trp-His(1)Phe-His-Asp-Trp-X11-Pro-X12-X13-X14-X15-X16-X17(2)Leu-His-X21-Ser-X22-Trp-Met-X23-X24-X25-X26-X27(3)
Owner:HOYA CORP
Methods and compositions for inhibition of egf/egfr pathway in combination with tyrosine kinase inhibitors
PendingUS20160333087A1Avoid resistanceOrganic active ingredientsImmunoglobulins against growth factorsEgfr pathwayDosing regimen
A method of treating patients suffering from cancers driven by deregulated Human Epidermal Growth Factor Receptor (HER1 / Human EGFR) comprising administering to a patient in need of such treatment a flexible and active regimen for combining a tyrosine kinase inhibitor (TKI) and anti-EGF antibodies for inhibition of the pathway activated by EGF-EGFR binding (mAb). The anti-EGF antibodies can be produced by active immunization or provided passively by the administration of antibodies that are anti-EGF. The method comprises TKI administered according to a continuous regimen based on an average daily dose in the range of 10 to 150 mg and the mAb is co-administered either actively or passively according to a dosing regimen achieving a therapeutic effective amount repeated thrice, twice or once a week, once in two weeks, once in three weeks or at least once monthly.
Owner:IN3BIO LTD
Cd20- and egfr-binding proteins with enhanced stability
Owner:MASSACHUSETTS INST OF TECH
Conjugates comprising an anti-EGFR1 antibody
ActiveUS10328149B2Organic active ingredientsEnergy modified materialsD-GlucopyranoseOxidative cleavage
The present invention relates to a conjugate comprising an anti-EGFR1 antibody or an EGFR binding fragment thereof and at least one dextran derivative, wherein the dextran derivative comprises at least one D-glucopyranosyl unit, wherein at least one carbon selected from carbon 2, 3 or 4 of the at least one D-glucopyranosyl unit is substituted by a substituent of the formula —O—(CH2)n—S—B12H112− wherein n is in the range of 3 to 10; and the dextran derivative is bound to the anti-EGFR antibody or an EGFR1 binding fragment thereof via a bond formed by a reaction between at least one aldehyde group formed by oxidative cleavage of a D-glucopyranosyl unit of the dextran derivative and an amino group of the anti-EGFR1 antibody or an EGFR1 binding fragment thereof.
Owner:TENBORON
A dual antitumor polypeptide based on the EPS8-EGFR binding domain
InactiveCN105859865BPrevent proliferationTumor rejection antigen precursorsPeptide/protein ingredientsEPS8EGFR binding
The invention relates to a dual anti-tumor polypeptide based on the Eps8-EGFR binding domain. The amino acid sequence of the polypeptide is Glu-Phe-Leu-Asp-Cys-Phe-Gln-Lys-Phe (SEQ ID No:1). The dual anti-tumor polypeptide can induce toxic T cells, effectively kills HLA-A*2402 positive tumors and Eps8 positive tumors, can directly inhibit proliferation of HLA-A*2402 positive tumor cells and Eps8 positive tumor cells, and can be used for preparing dual anti-tumor medicine.
Owner:SOUTHERN MEDICAL UNIVERSITY
Tumor targeted antibodies and method for using the same
Isolated and recombinant antibodies, such as EGFR-binding antibodies. Antibodies can comprising the sequence of an immunoglobulin heavy constant gamma-1 region encoded by a human G1M3 allele. For example, antibodies of the embodiments can comprise an immunoglobulin heavy constant gamma-1 region wherein position 214 is arginine; position 356 is glutamic acid; position 358 is methionine and / or position 431 is alanine. Methods for treating diseases, such as cancer, in a human subject with such antibodies are also provided.
Owner:MUSC FOUND FOR RES DEV
Antibody blocking cetuximab and egfr binding, its kit and hybridoma cell
The invention discloses an antibody for blocking combination of cetuximab and EGFR, a kit and a hybridoma cell thereof. The antibody includes an amino acid sequence expressed by at least one of SEQ NO.:4, SEQ NO.:5 and SEQ NO.:6. The antibody can specifically combine cetuximab and achieve a function of blocking the combination of the cetuximab and the EGFR. The antibody can be used as a positive control for evaluating the immunogenicity of the cetuximab and used for monitoring the sensitivity and specificity of the method. The antibody can also be used for monitoring the sensitivity and specificity of a method for measuring the biological activity of the cetuximab. The invention further discloses a hybridoma cell secreting the antibody and a kit using the antibody. The invention further provides a method for evaluating the neutralization performance of the antibody.
Owner:DRAGONBOAT BIOPHARMACEUTICAL (SHANGHAI) CO LTD
Therapeutic recombinant antibody, its coding gene and application
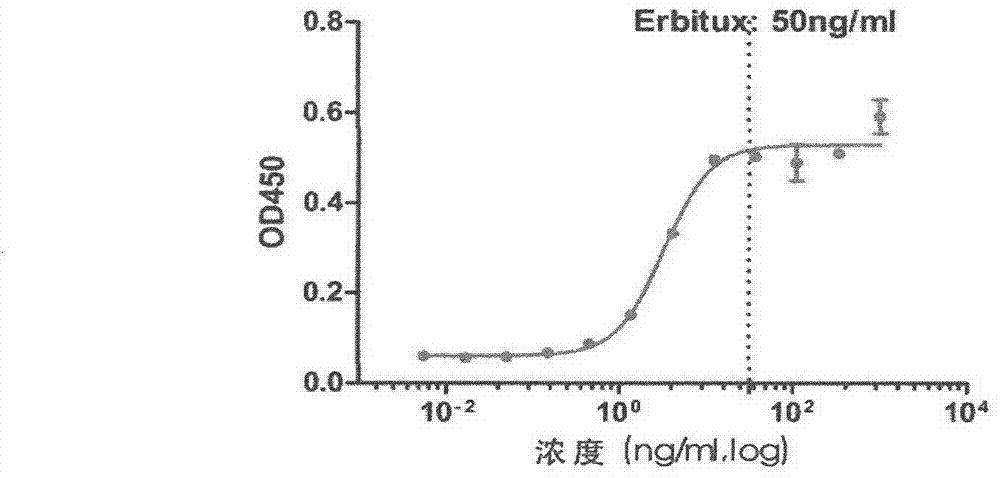
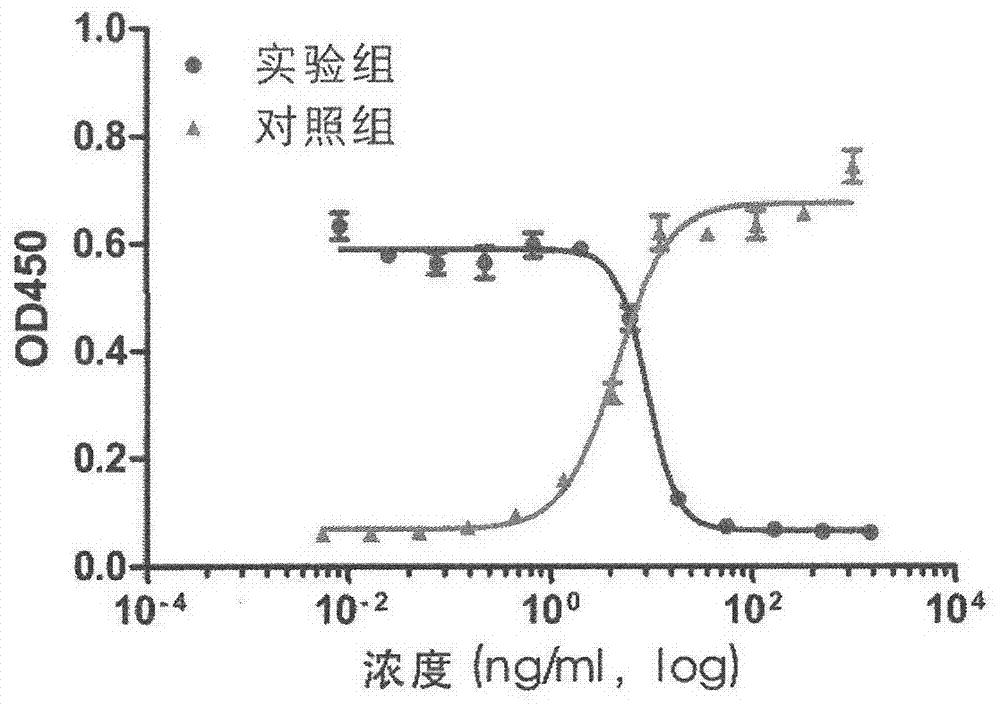
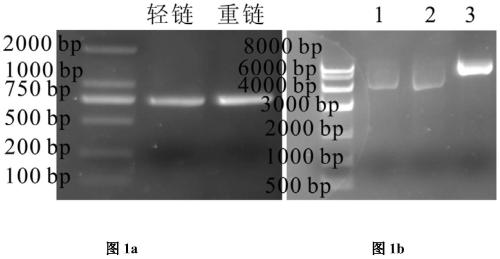
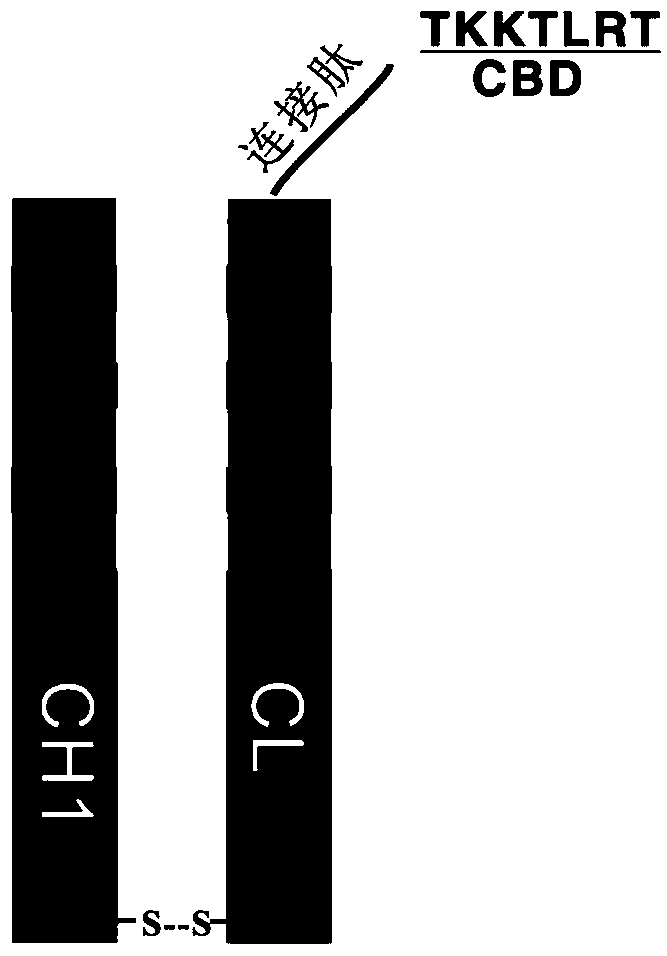
ActiveCN106188306BEfficient killingAchieve sustained releaseAntibody ingredientsPharmaceutical non-active ingredientsAntiendomysial antibodiesOncology
The invention discloses a therapeutic recombinant antibody, and an encoding gene and application thereof. In an excellent embodiment, the therapeutic recombinant antibody is mainly formed by fusion of CBD collagen specific-binding polypeptide and an Fab segment of cetuximab, and contains a light-chain variable region formed by a genetic code with a sequence shown by SEQ ID NO:1 and a heavy-chain variable region which is formed by a genetic code with a sequence shown by SEQ ID NO:2. The therapeutic recombinant antibody disclosed by the invention not only has the specific-binding capacity of collagen but also reserves the EGFR binding capacity of the cetuximab, and besides, the functions of killing tumor cells and suppressing tumor growth of the cetuximab are also maintained; and in addition, the recombinant antibody has high targeting ability and good tumor tissue infiltration capacity in bodies and can realize slow release in tumor tissues, the anti-tumor effects of the therapeutic recombinant antibody are far better than those of a total-length antibody namely the cetuximab, and the therapeutic recombinant antibody has wide application prospects in tumor detection preparations, antitumor medicines and the like.
Owner:DUBU BIOSCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com