Antigen binding proteins that bind EGFR
An antibody, fully human antibody technology, applied in the direction of anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, immunoglobulin, etc., can solve the limitation and hinder effective drugs use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
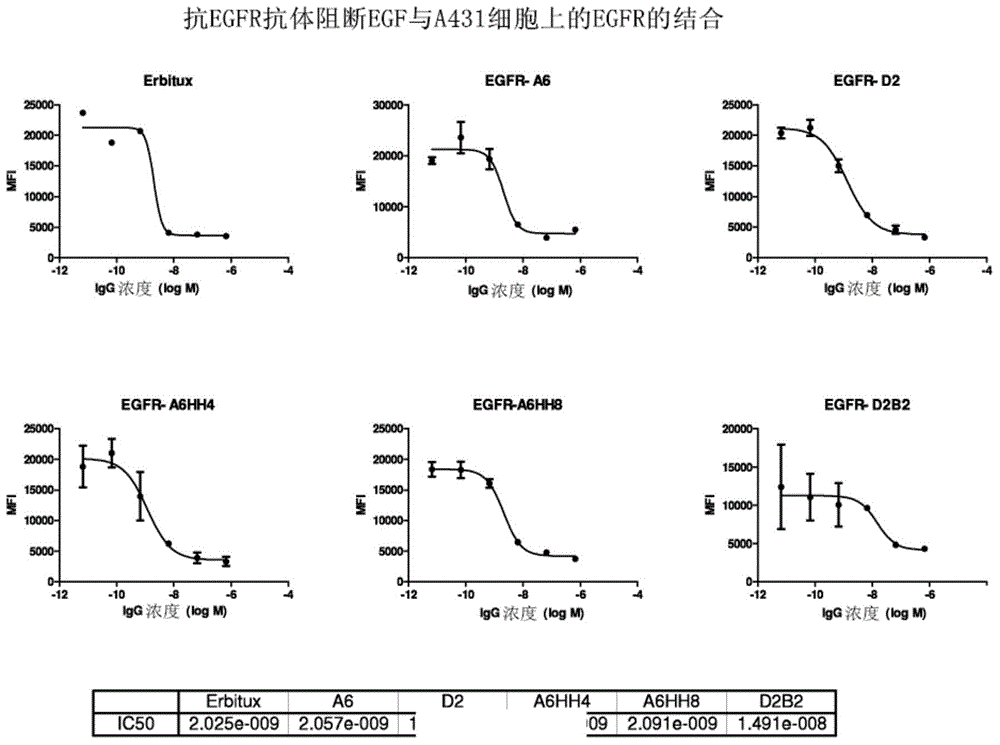
[0151] Full-length IgGs were purified using protein A, and their purity was checked by SDS-PAGE. In terms of binding specificity (compared to soluble EGFR and EGFR-expressing cell lines), binding affinity (Biacore), inhibition of EGF binding to soluble EGFR, EGF-stimulated EGFR phosphorylation and cell proliferation in EGFR-expressing cell lines, Characterization of purified antibodies. Anti-EGFR antibodies were converted to IgGs and expressed in 293 cells. Purification with protein A yielded expression levels in the range of 0.86-5.5 μg / ml. This antibody has complete heavy and light chains. Five of these (A5, A6, B1, B4 and B5) bound strongly to soluble EGFR and EGFR expressed in the cell lines. One antibody (D6) bound moderately to both soluble EGFR and EGFR-expressing cell lines. ELISA analysis showed that the six binding proteins also blocked the binding of ligand EGF to soluble EGFR.
[0152] Three binding proteins (A5, A6 and B5) showed -10% inhibition in EGF-stimul...
Embodiment 2
[0154] This example demonstrates the production of anti-EGFR antibodies. Anti-EGFR antibodies were produced by transient transfection of Chinese hamster ovary subclone S (CHO-S) cells (Life Technologies) with expression vectors containing anti-human EGFR IgG1 heavy chain and light chain structural genes.
[0155] Cell lines were maintained in shake flasks and routinely passaged every 3-4 days using CHO-S-SFMII medium (Life Technologies). Two plasmids were used to generate transient pools: one carrying the heavy chain and the other carrying the light chain. The vector was co-transfected into host cell lines using 25kd linear ethyleneimine (PEI, Polysciences).
[0156] Briefly, PEI was diluted in OptiPro SFM (Life Technologies) and added to plasmid DNA that had been diluted with an equal volume of OptiPro SFM. The PEI / DNA mixture was incubated for 5-10 minutes before being added to the CHO-S cell suspension. Cultures were grown at 37°C for predetermined periods of time and th...
Embodiment 3
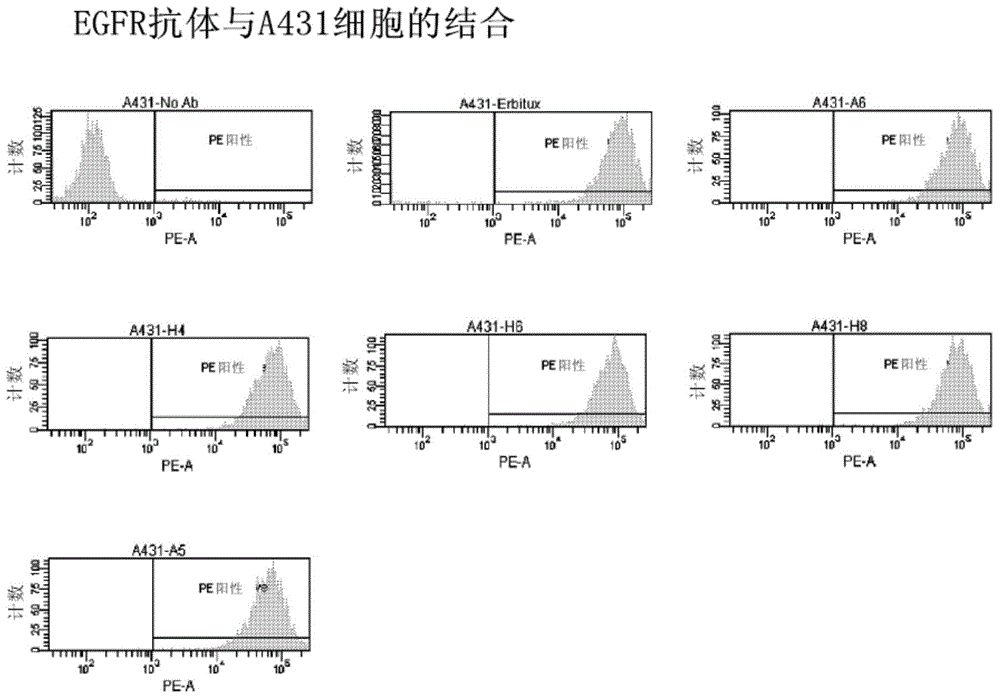
[0158] This example demonstrates cell binding in vitro data for one of the initial screening steps for several antibodies in this disclosure, including A6. This example demonstrates the ability of an anti-EGFR antibody to bind to the EGF receptor expressed on the surface of A431 cells (essential for the antagonistic properties of the antibody), and to compare with commercially available cetuximab (cetuximab ( ))Compare. Here, 100000 A431 cells were aliquoted into a test tube containing 100 μl of FACS Buffer (PBS+2% FBS). Cells were pelleted and resuspended in triplicate in 100 μl FACS Buffer containing 5 μg / ml of the indicated antibody. After culturing for half an hour, the cells were washed once with FACS Buffer, and resuspended in 100 μl of PE-linked goat anti-human IgG (γ-chain specific) secondary antibody (Southern Biotech Cat#2040-09). The cells were cultured again for half an hour, washed once with FACS Buffer, resuspended in 300 μl FACS Buffer, and the median fluores...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com