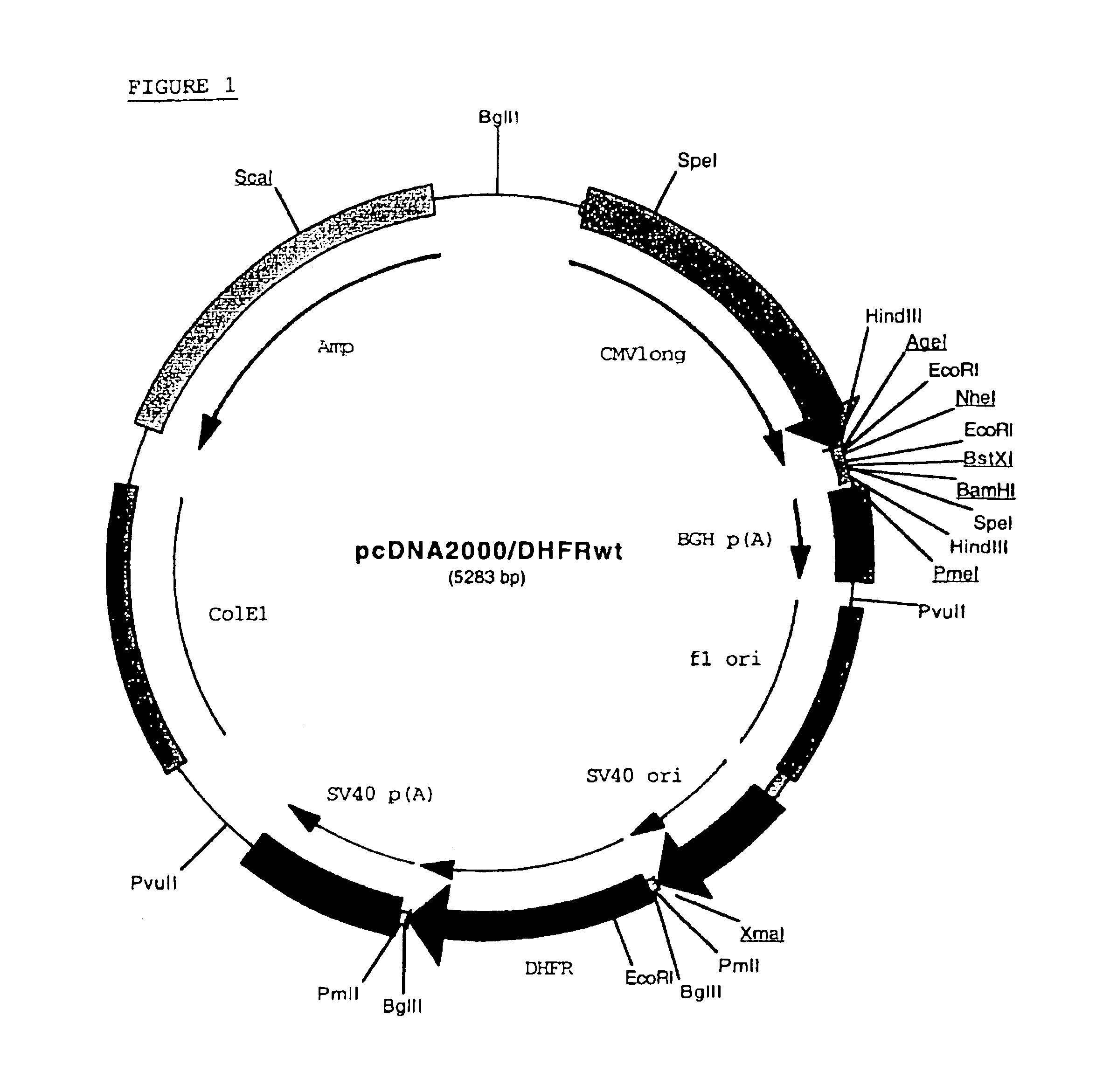
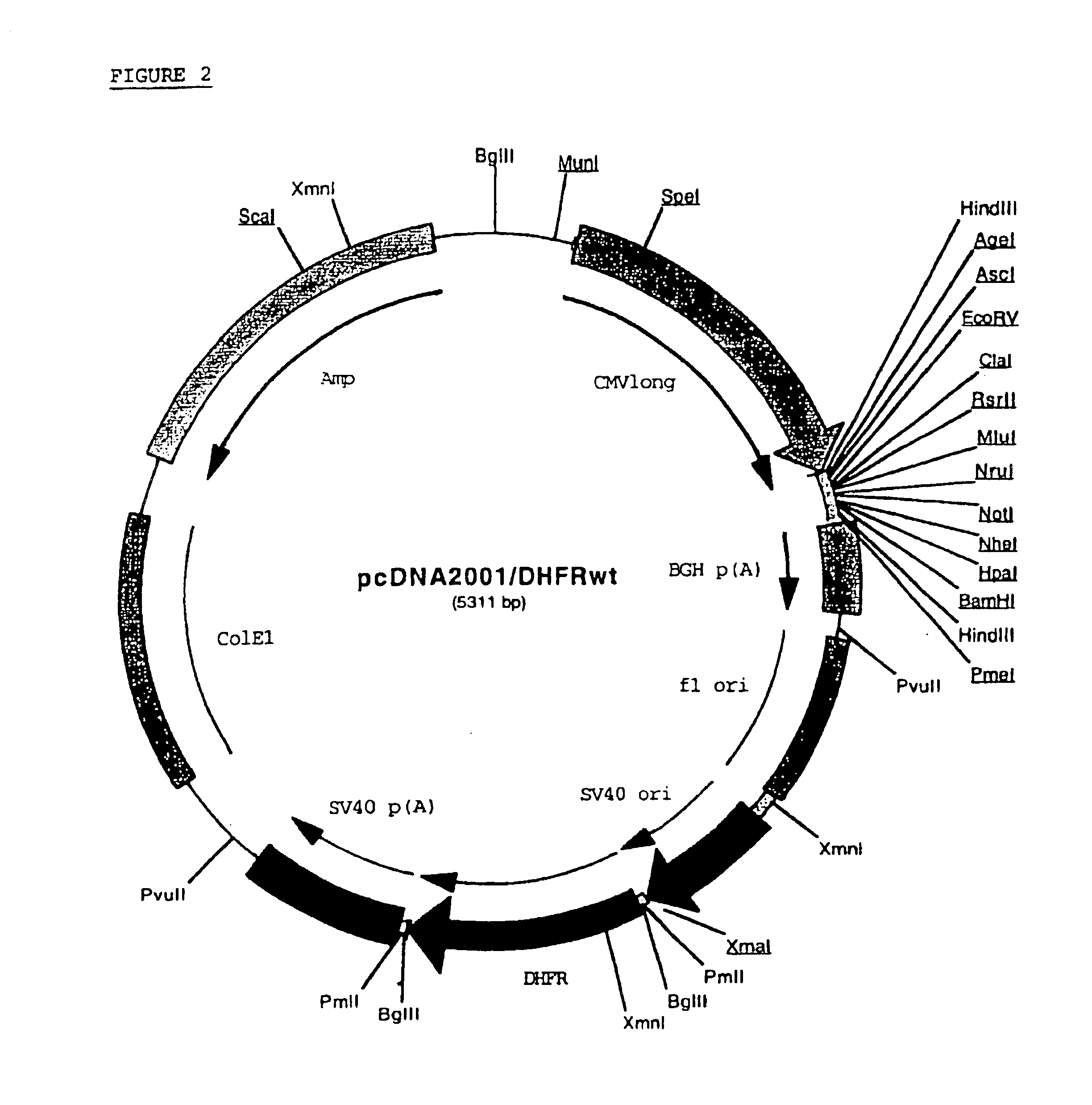
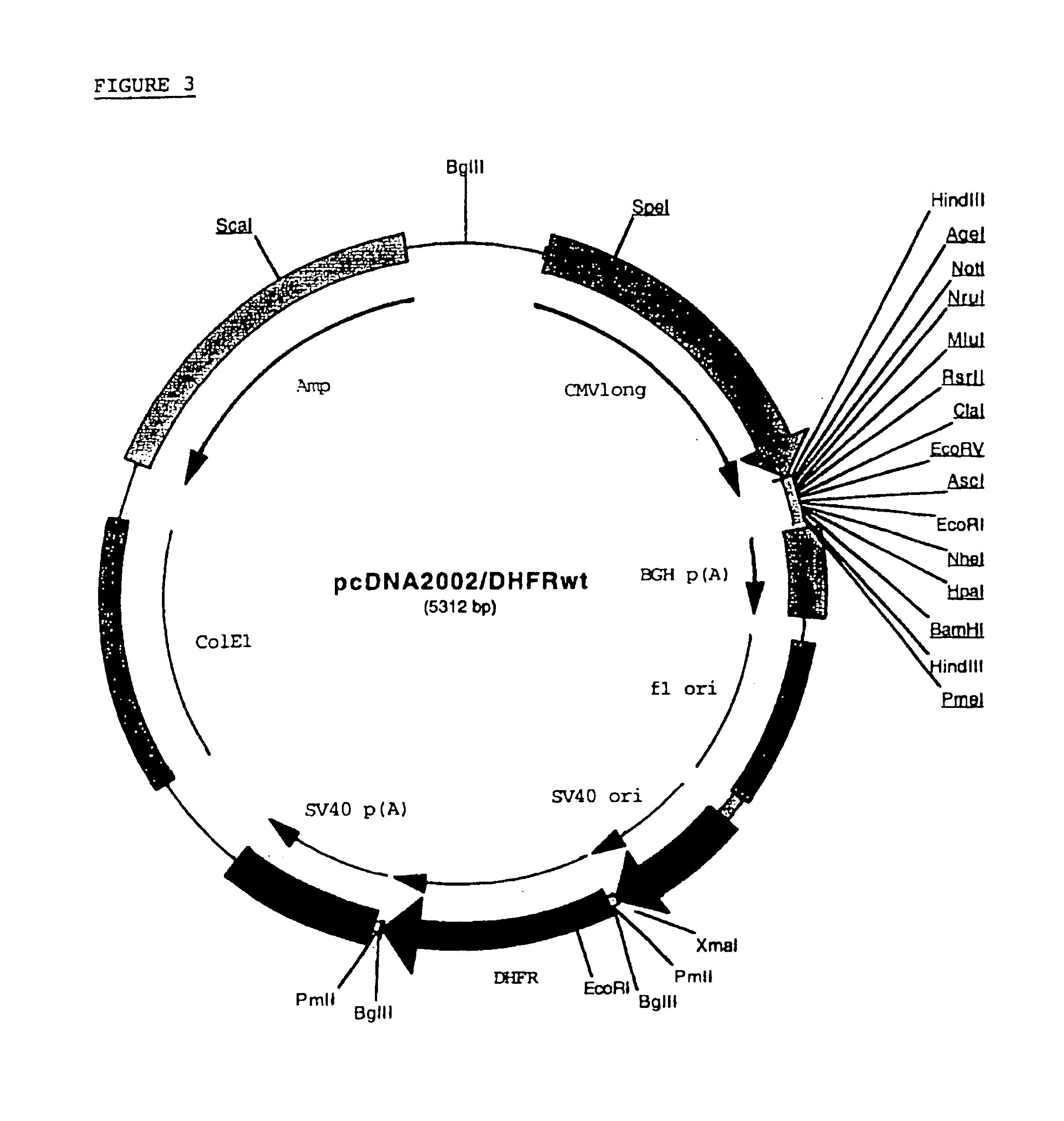
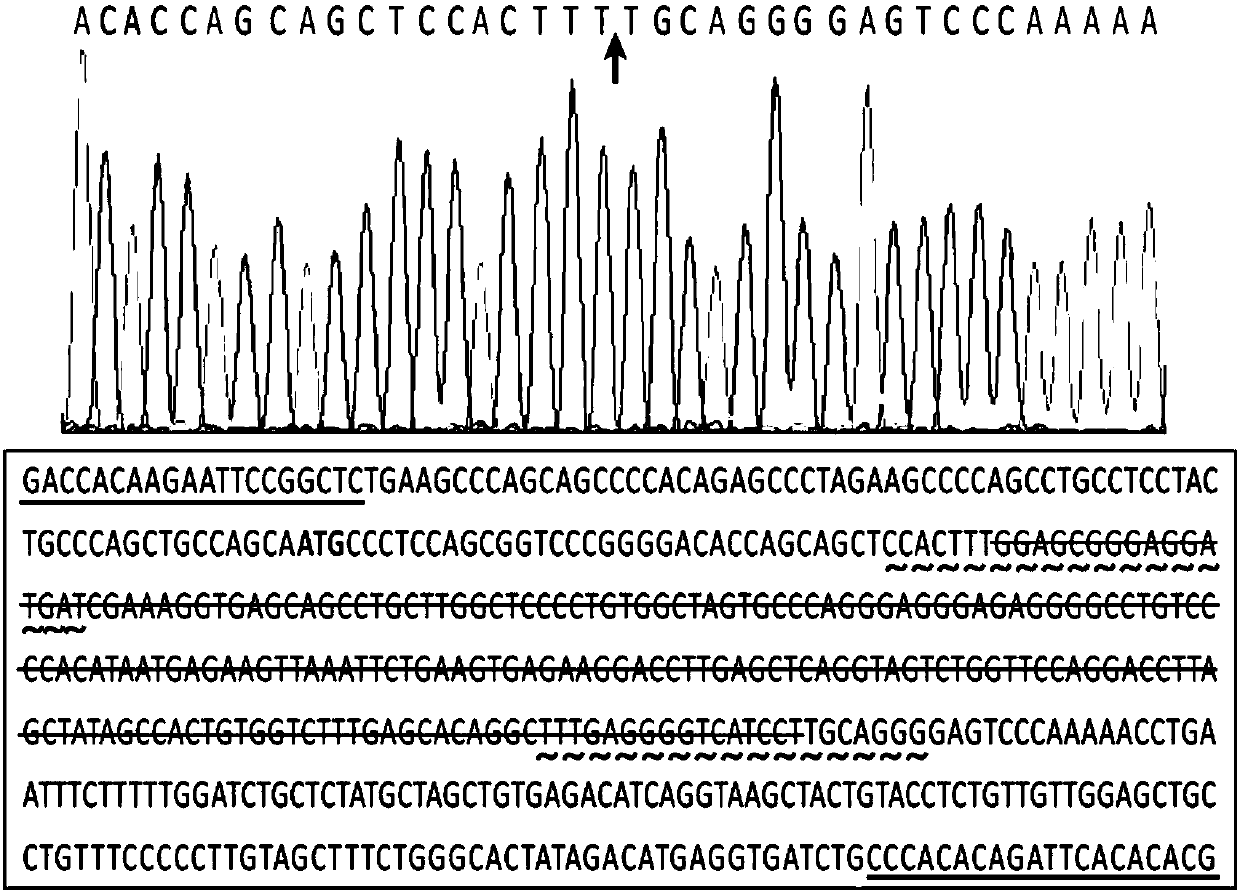
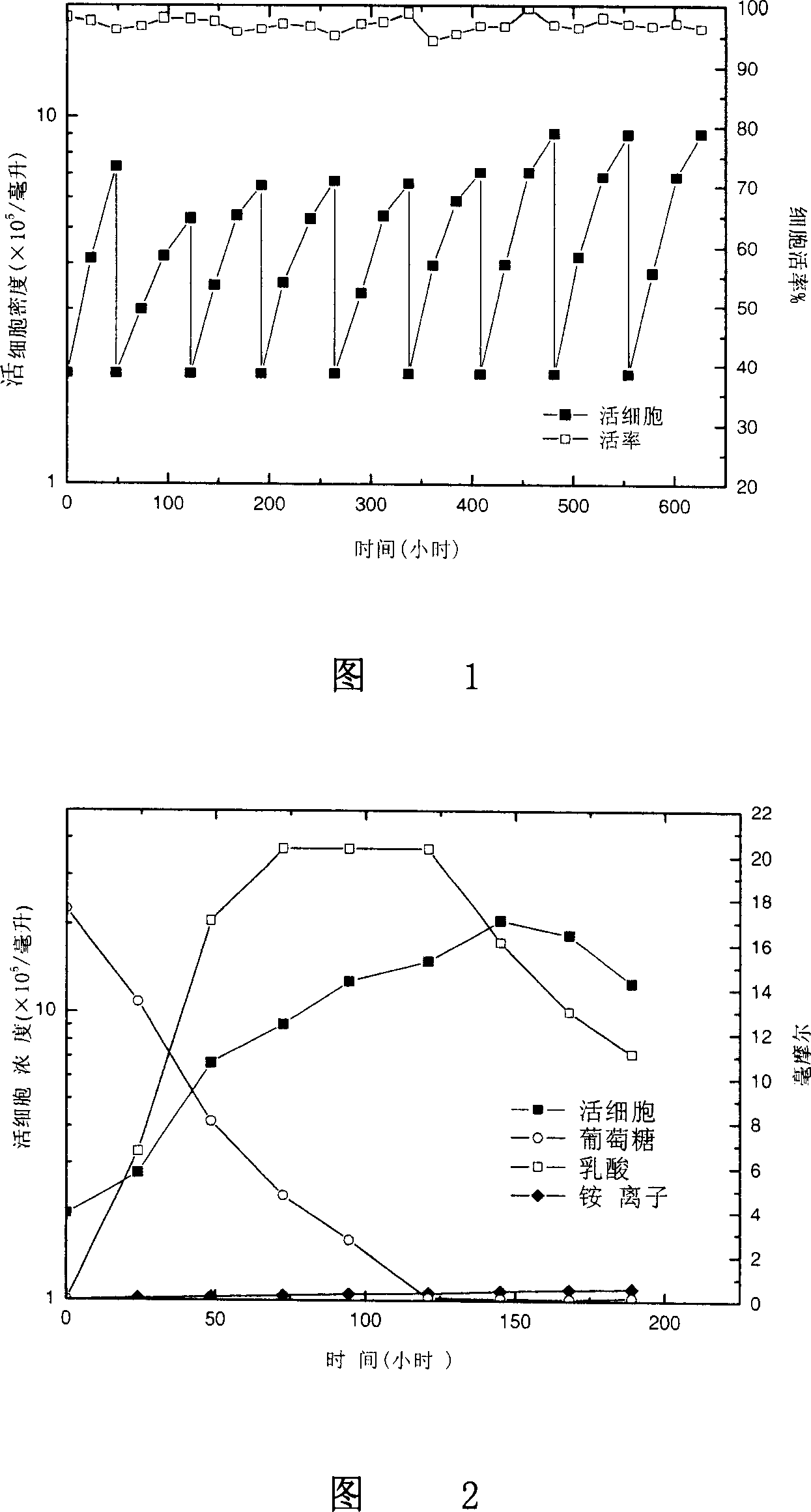
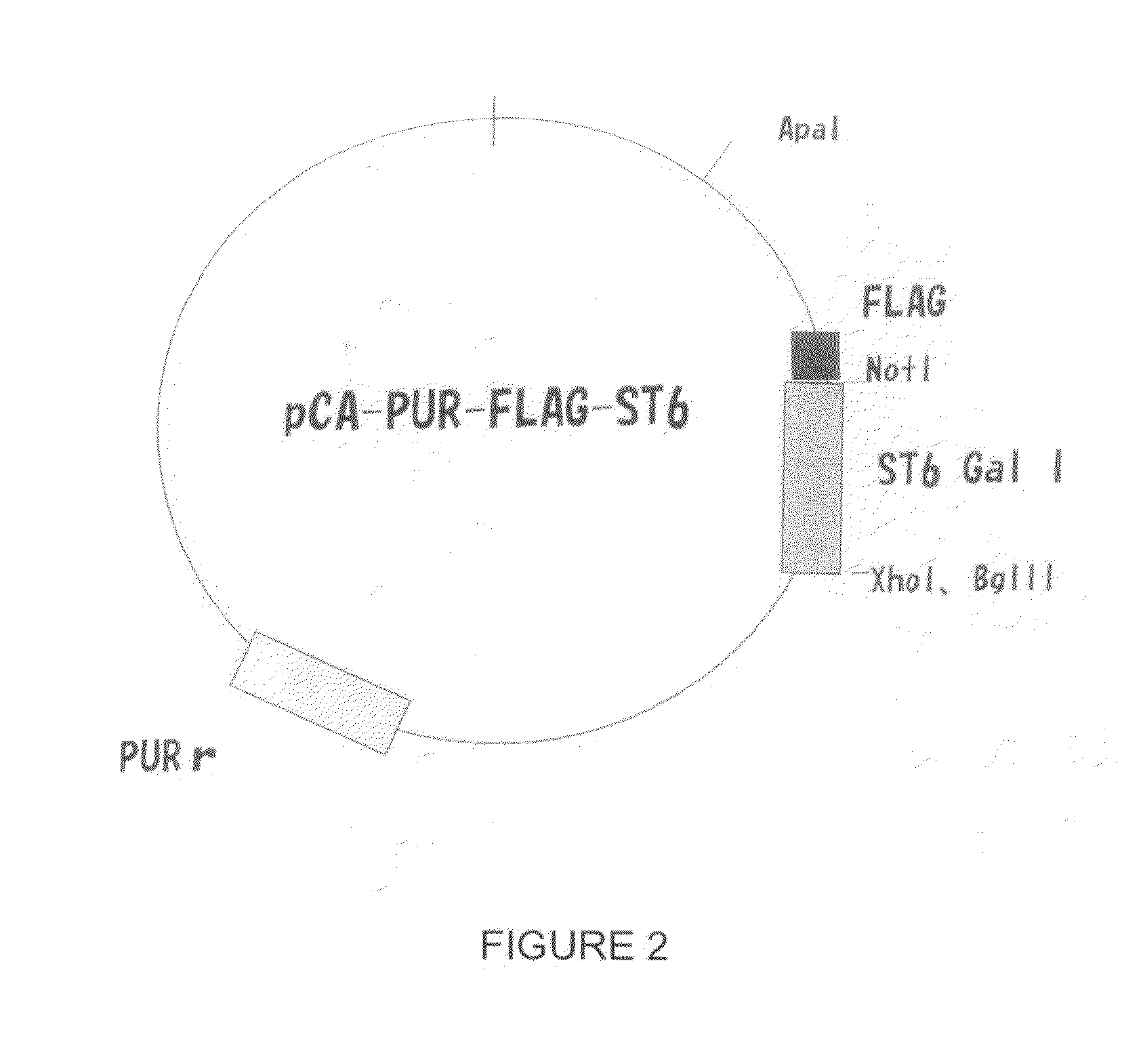
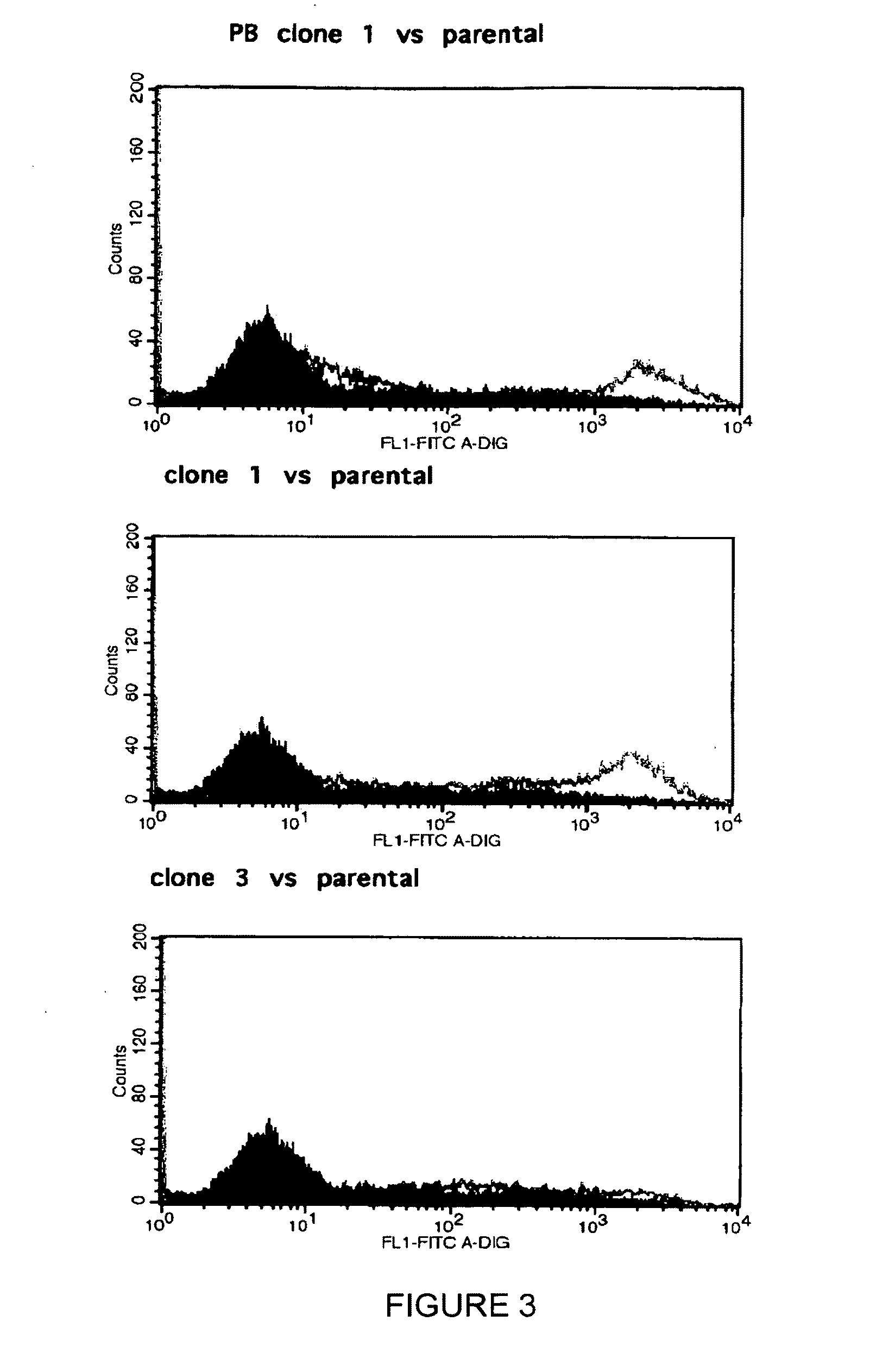
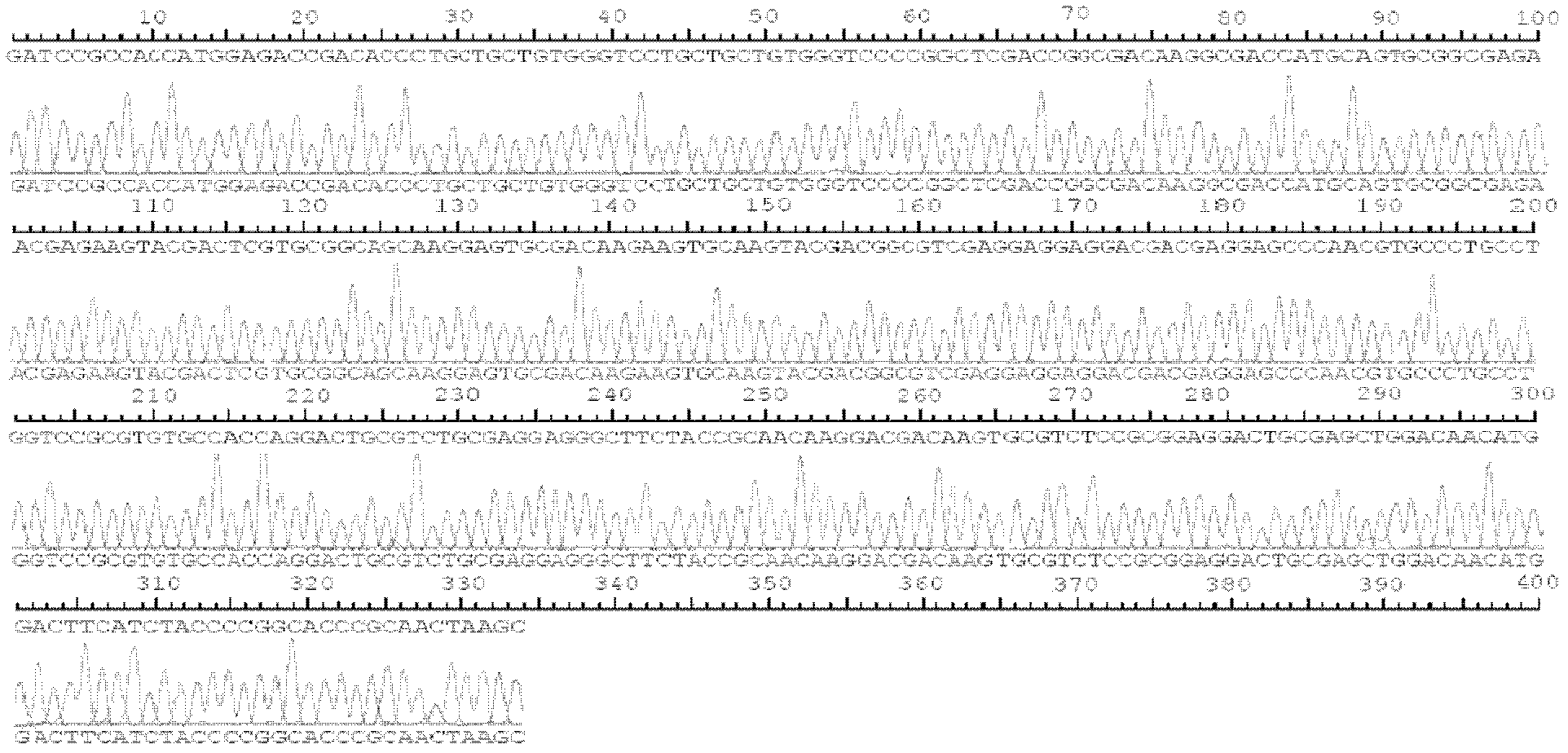
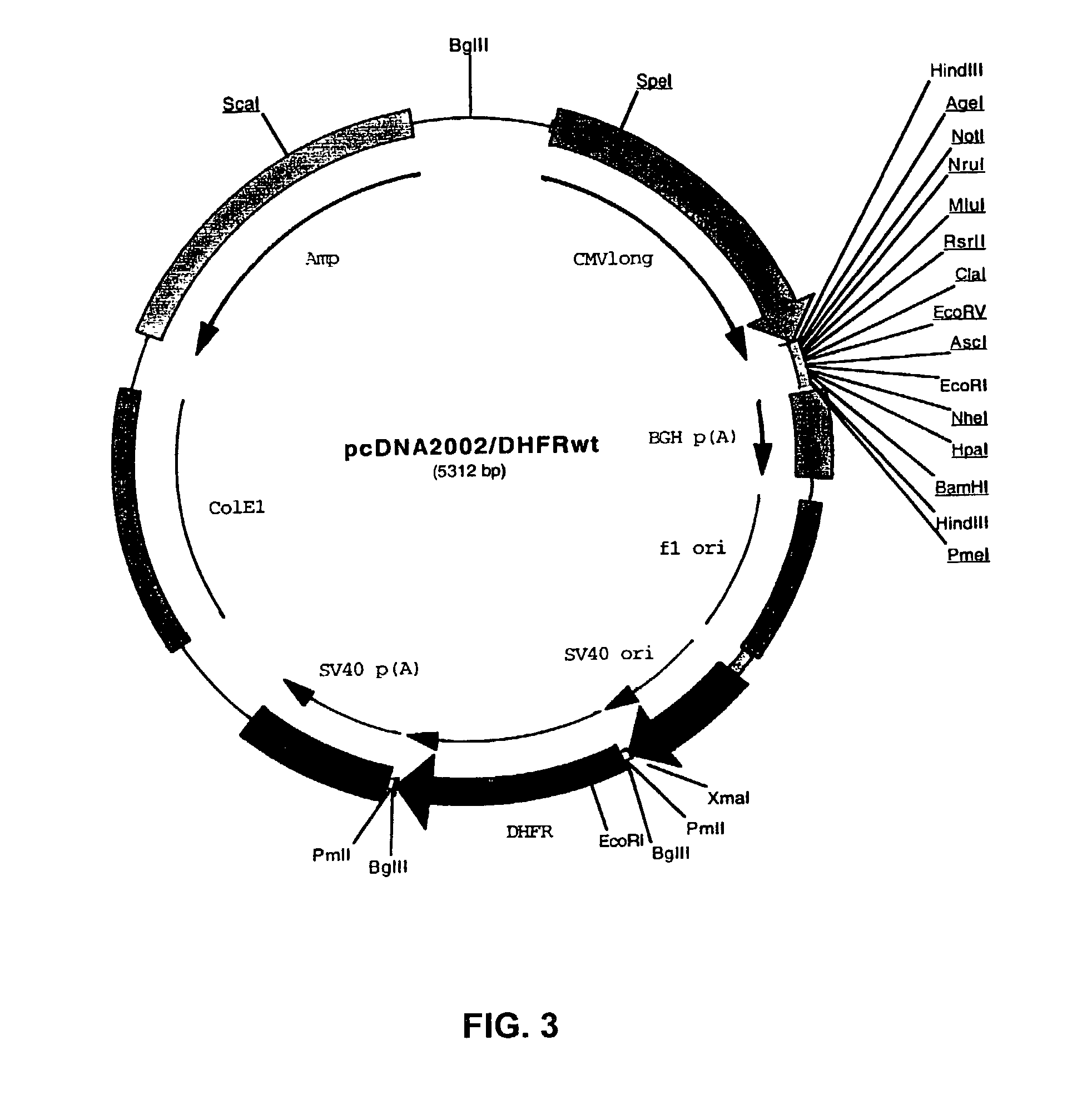
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
244 results about "Chinese hamster" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chinese hamsters (Cricetulus griseus) are rodents in genius Cricetulus of the subfamily Cricetidae that originated in the deserts of northern China and Mongolia. They are distinguished by an abnormally long tail relative to other hamsters, whose tails are stubby. Chinese hamsters are primarily nocturnal however for small periods they will stay up throughout the day. They tend to become aggressive if kept in enclosures which are inhabited by other hamsters or are too small.
Recombinant protein production in a human cell
InactiveUS6855544B1Easy to handleLarge-scale (continuous) productionSsRNA viruses negative-senseSugar derivativesHamsterHuman cell
Methods and compositions for the production of recombinant proteins in a human cell line. The methods and positions are particularly useful for generating stable expression of human recombinant proteins of interest that are modified post-translationally, for example, by glycosylation. Such proteins may have advantageous properties in comparison with their counterparts produced in non-human systems such as Chinese Hamster Ovary cells.
Owner:JANSSEN VACCINES & PREVENTION BV
DNA Transmethylase defective CHO (Chinese hamster ovary) cell line and preparation method and application thereof
InactiveCN107828738AImprove expression levelIncrease productionTransferasesStable introduction of DNAInstabilityA-DNA
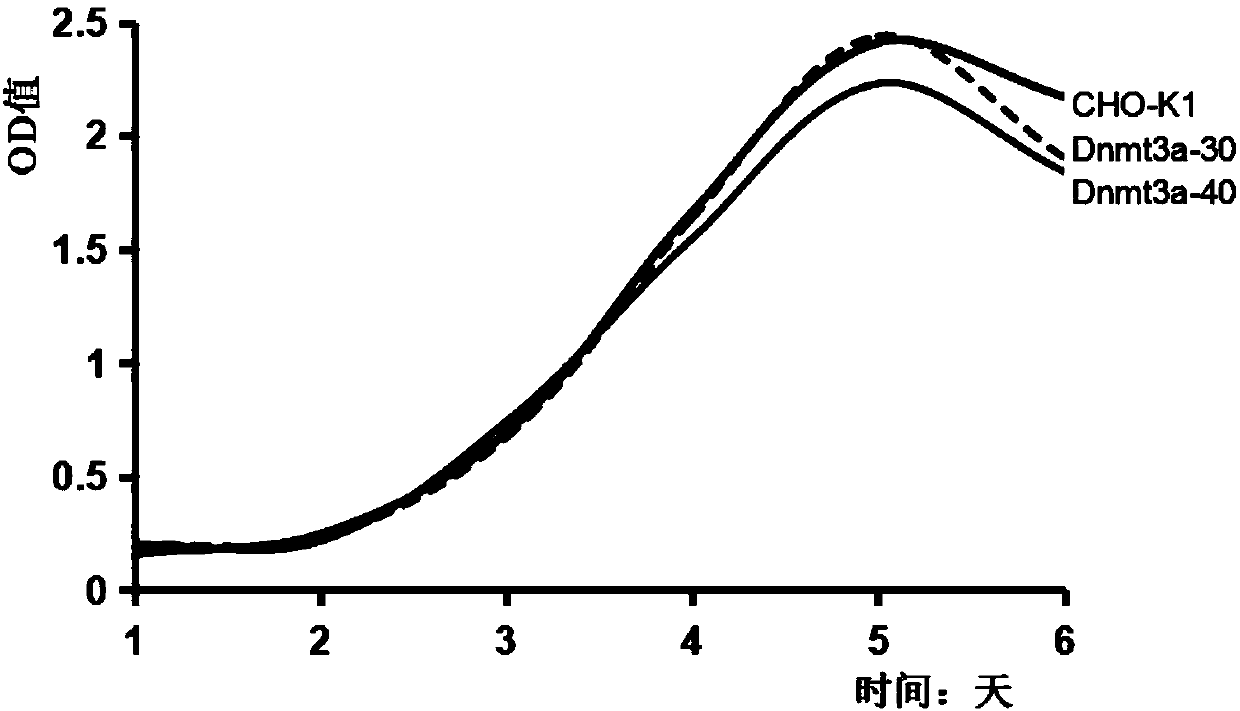
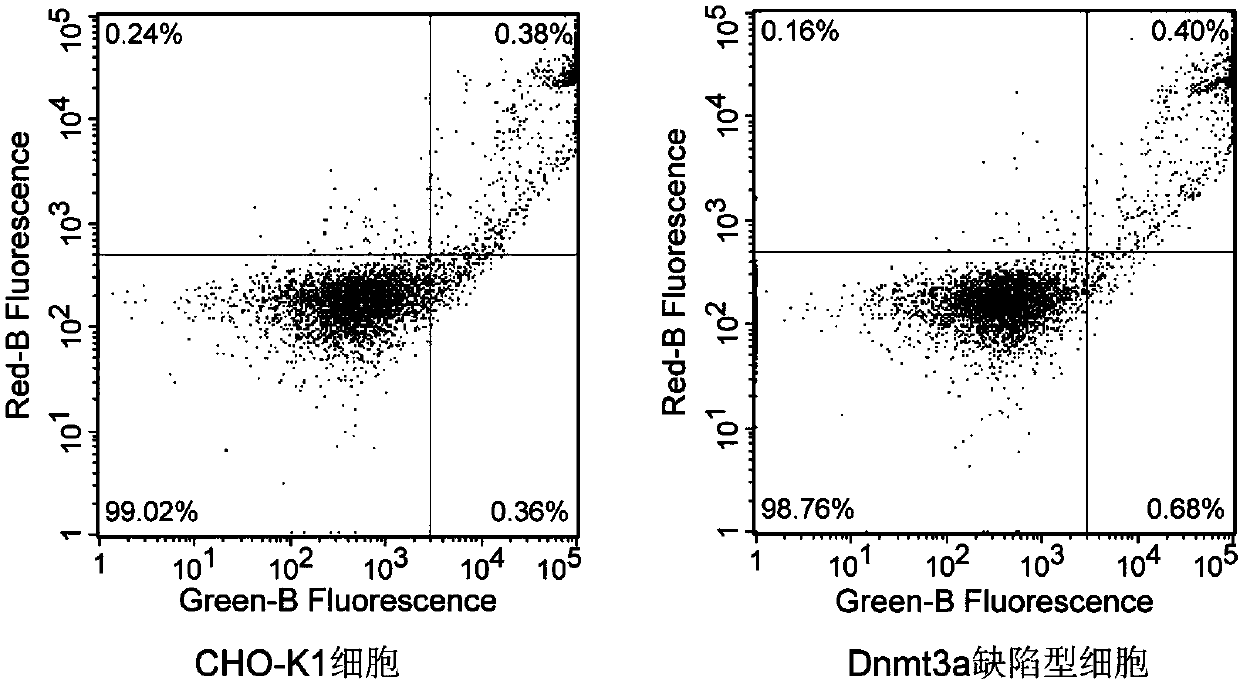
The invention relates to a DNA transmethylase defective CHO (Chinese hamster ovary) cell line and a preparation method and application thereof and belongs to the technical field of gene engineering. DNA Transmethylase Dnmt3a gene of CHO cells is knocked off by means of CRISPR / Cas9 gene editing technique, and screening and identifying are performed to obtain DNA transmethylase Dnmt3a defective CHOcells; the cells are transfected with a eukaryotic expression vector, stably expressed recombinant CHO cell strains are screened, and accordingly a novel CHO cell expression system based on DNA transmethylase deficiency is established. The recombinant gene CHO cell expression system is established via host CHO cell genetic modifications, expression level of recombinant proteins can be significantly increased, the problem of recombinant protein expression instability is solved, and recombinant protein expression stability is improved.
Owner:XINXIANG MEDICAL UNIV +1
CHO (Chinese hamster ovary) cell strain with high-efficiency expression of CD2V protein of African swine fever (ASF)
ActiveCN110078801AHigh expressionEasy to purifyVirus peptidesMicroorganism based processesAfrican swine feverChinese hamster
The invention provides CD2V protein of African swine fever (ASF) capable of being expressed with high-efficiency in a CHO (Chinese hamster ovary) cell strain. The amino acid sequence of the CD2V protein is shown in SEQ ID NO:4; a recombinant plasmid constructed by the invention is used for expressing the CD2V protein of an African swine fever virus in CHO cells; the invention further provides a recombinant CHO cell strain prepared by transfecting the CHO cells through the recombinant plasmid, the recombinant CHO cell strain can be used to prepare the CD2V protein, and the prepared protein canbe used for differential diagnosis of the African swine fever. According to the cell strain with the expression of the CD2V protein of the African swine fever, the expression quantity is high, purification is easy, the cell strain can be used for the differential diagnosis, and a solid foundation is laid for the production of subunit vaccines and diagnostic reagents of the African swine fever.
Owner:YEBIO BIOENG OF QINGDAO
Serum-free medium for mammalian cell
The invention discloses a taming method of non-serum culture medium for mammal cell, which contains cross tumour cell, Chinese hamster oophoron cell (CHO) and 293-cell.
Owner:上海中科伍佰豪生物工程有限公司
Cell-based systems for producing influenza vaccines
ActiveUS20100021499A1Minimize and prevent virus infectionAvoid componentsSsRNA viruses negative-senseAnimal cellsHemagglutininBinding site
The present invention relates to a cell-based method for producing influenza virus vaccines by enriching the population of surface-bound α2,6-sialic acid receptors on a cell surface, such as on a Chinese Hamster Ovary (CHO) cell surface. The host cell therefore presents numerous binding sites to which an influenza virus can bind via its hemagglutinin spike protein and infect the host cell. In contrast to wild-type CHO cells, the surface of the mutated CHO cells of the present invention contains an enriched population of α2,6-sialic acid receptors which makes the inventive CHO cells highly susceptible to viral infection, and therefore safe, effective, and highly efficient cells for rapidly producing influenza vaccines.
Owner:FLUGEN
Chinese hamster ovary genetic engineering cell line for performing high-level secretory expression on AcAPc2
InactiveCN102321588AAdaptableExtended half-lifeVector-based foreign material introductionForeign genetic material cellsHigh level expressionHamster
The invention discloses a Chinese hamster ovary (CHO) genetic engineering cell line for performing high-level secretory expression on AcAPc2 and relates to a CHO cell line for performing high-level secretory expression on the AcAPc2. The invention aims to solve the problems that the expression level of an exogenous gene product expressed by using CHO is low at present and the industrialized production cannot be realized. The CHO genetic engineering cell line for performing high-level secretory expression on the AcAPc2 is obtained by the following step of: screening dihydrofolate reductase-deficient CHO cells which are taken as host cells, and amplifying to obtain the cell line for performing stable and high-level expression on the AcAPc2. The CHO cell line can perform high-level expression on the AcAPc2; the expression level can be up to 10mg / L.72h; and the CHO cell line can be industrially produced. The cell line is used for resisting blood coagulation and treating tumor, septicaemiaand the like.
Owner:HEILONGJIANG UNIV
Fusion protein for treating diabetes and preparation method for fusion protein
InactiveCN102558362ACan stimulate secretionReduce physical burdenPeptide/protein ingredientsMetabolism disorderPancreatic hormoneChinese hamster
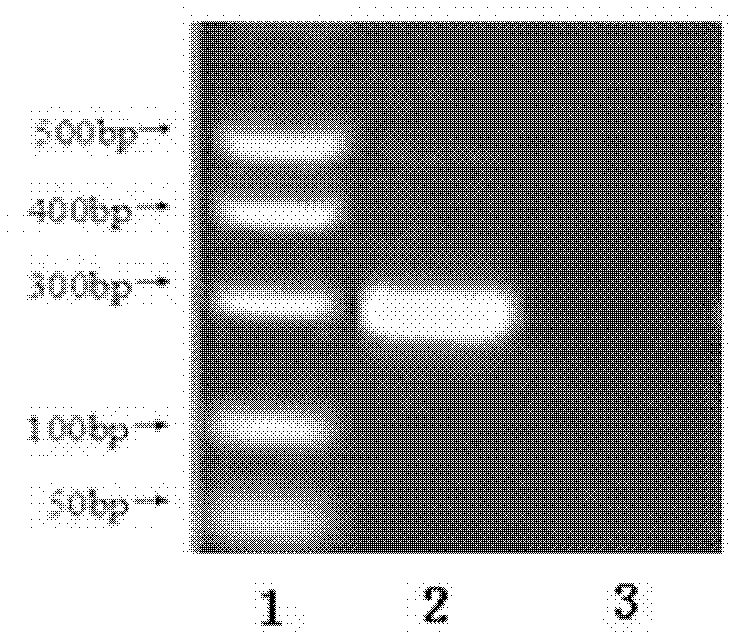
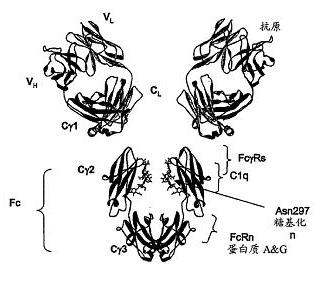
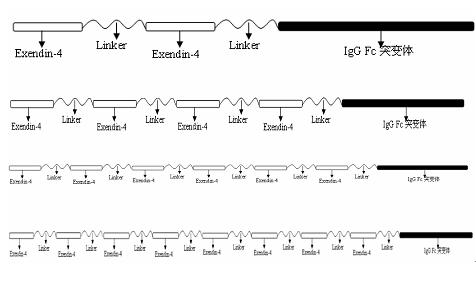
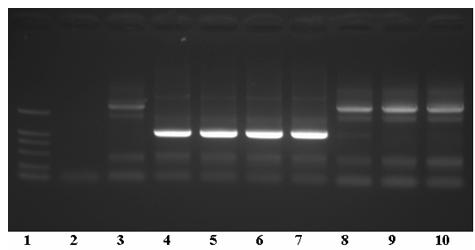
The invention relates to the technical field of medicines for treating diabetes, in particular to a fusion protein for treating diabetes and a preparation method for the fusion protein. The fusion protein is recombined by 2 to 8 Exendin-4-Linkers and a human IgGFc mutant; Linker is a flexible peptide fragment; and the human IgGFc mutant is an IgG1Fc, IgG2Fc or IgG4Fc mutant. The invention also discloses the preparation method for the fusion protein. The recombined fusion protein obtained by the steps of performing high-level expression in Chinese hamster ovary (CHO) cells, affiliating, and performing ion exchange and molecular sieve chromatography has the bioactivities of stimulating the secretion of insulin and inhibiting glucagon generated after dinner from being released which are possessed by Exendin-4, also has the characteristics of prolonging the half-life period of the Exendin-4 in serum, is used for treating type I and type II diabetes, can effectively reduce the psychological and physiological burden of patients, is safe in use and high in practicability, and can be massively produced and sold.
Owner:DONGGUAN JINLANG BIOTECH
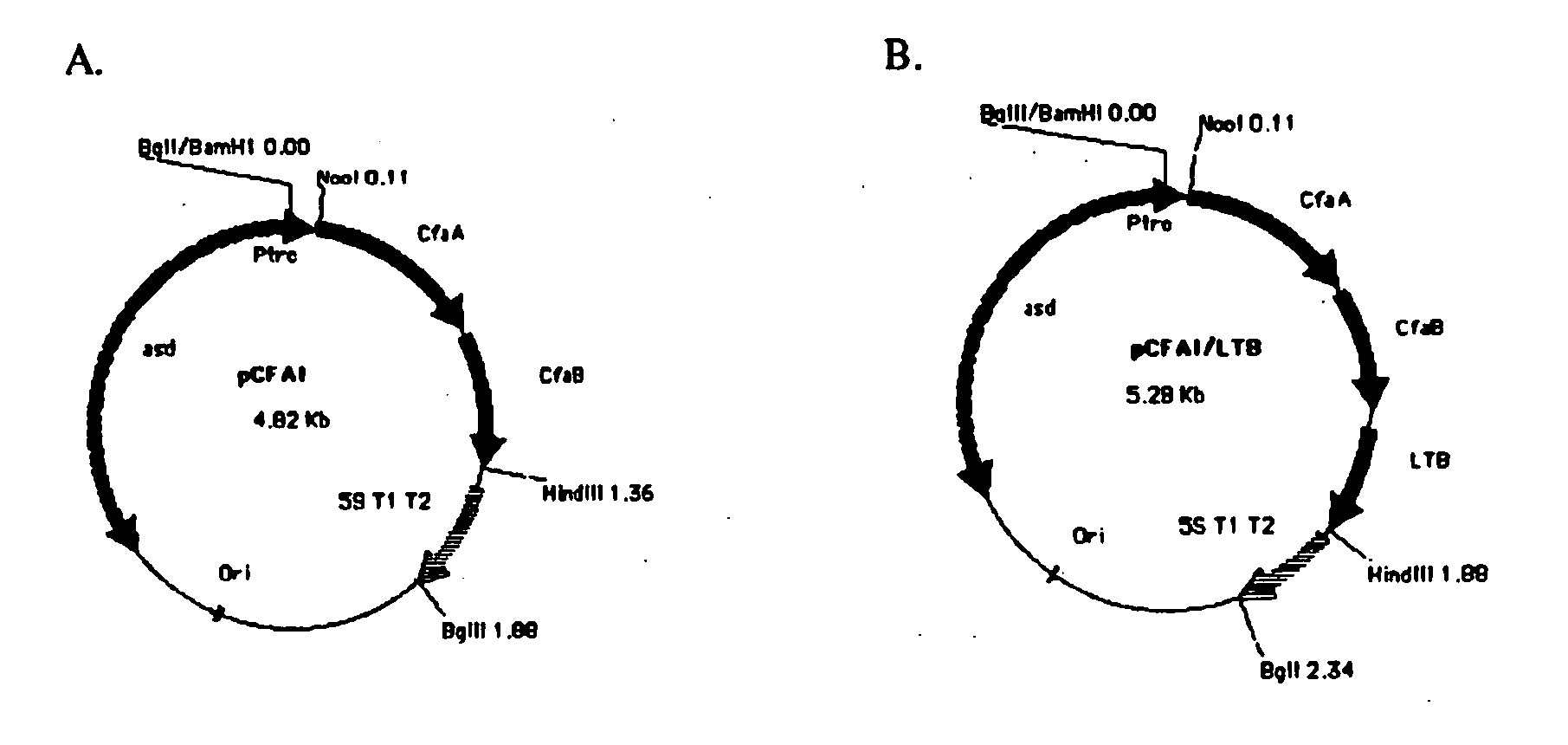
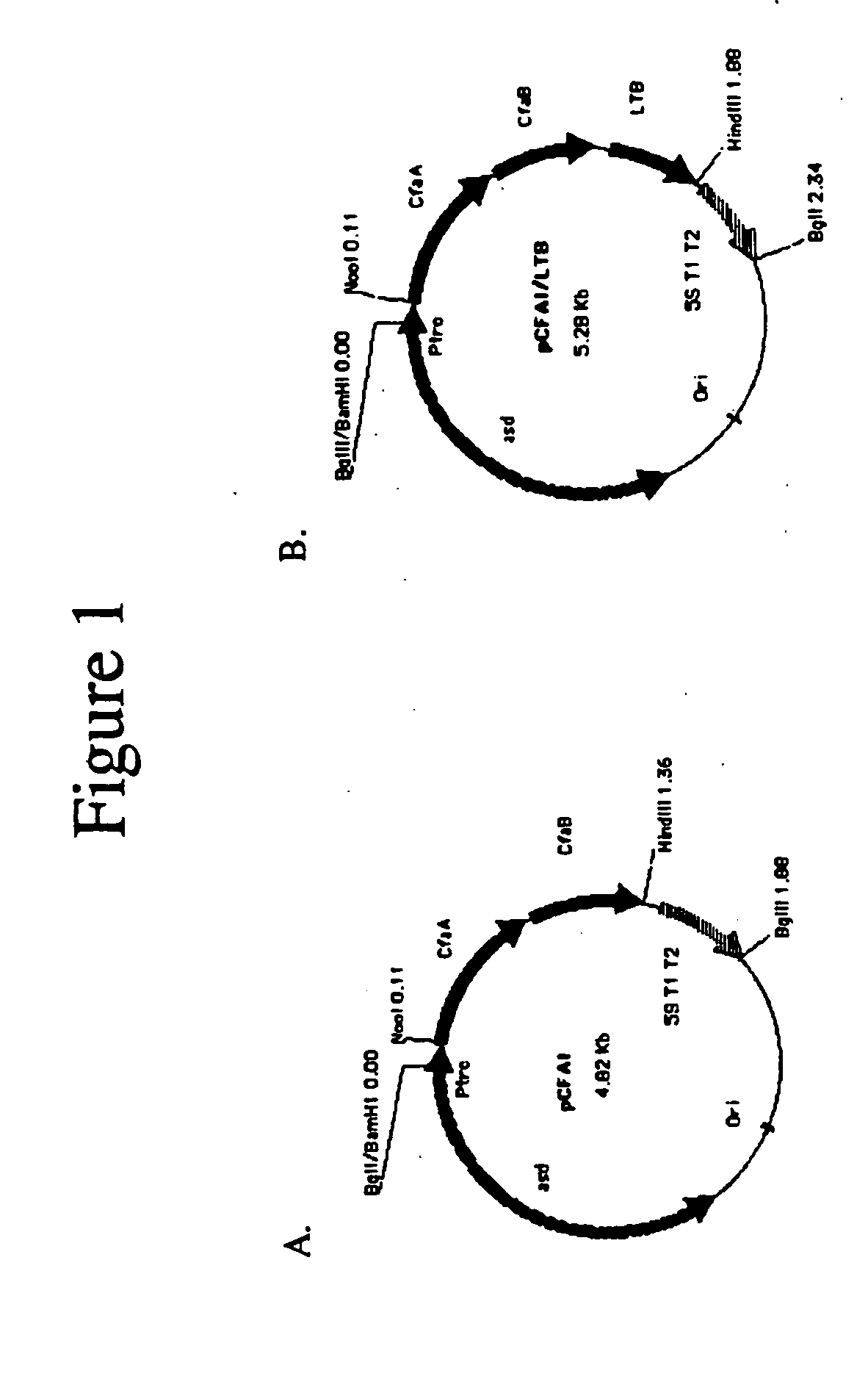
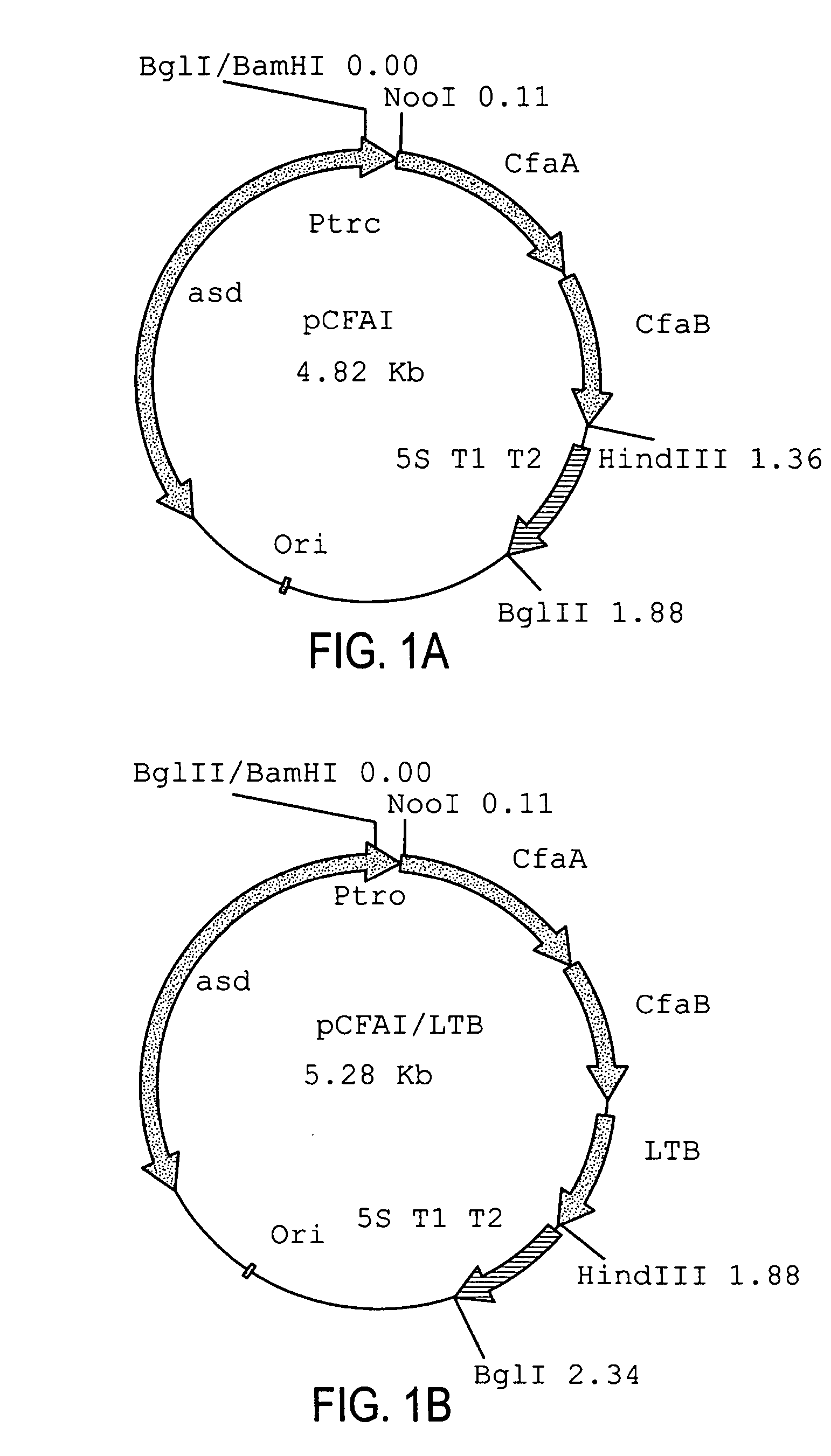
Construction of live attenuated Shigella vaccine strains that express CFA/I antigens (cfaB and CfaE) and the B subunit of heat-labile enterotoxin (LTB) from enterotoxigenic E.coli
ActiveUS20070237791A1Reduce intrusionBacterial antigen ingredientsBacteriaHeterologousMucosal Immune Responses
With the goal of creating a combination vaccine against Shigella and other diarrheal pathogens we have constructed a prototype vaccine strain of Shigella flexneri 2a (SC608) that can serve as a vector for the expression and delivery of heterologous antigens to the mucosal immune system. SC608 is an asd derivative of SC602, a well-characterized vaccine strain, which has recently undergone several phase 1 and 2 trials for safety and immunogenicity. Using non-antibiotic asd-based plasmids, we have created novel constructs for the expression of antigens from enterotoxigenic E. coli (ETEC), including CFA / I (CfaB and CfaE) and the B-subunit from heat-labile enterotoxin (LTB) in Shigella vaccine strain SC608. Heterologous protein expression levels and cellular localization are critical to immune recognition and have been verified by immunoblot analysis. Following intranasal immunization (SC608(CFAI) and SC608(CFAI / LTB) of guinea pigs, serum IgG and IgA immune responses to both the Shigella LPS and ETEC antigens can be detected by ELISA. In addition, ELISPOT analysis for ASCs from cervical lymph nodes and spleen showed similar responses. All vaccine strains conferred high levels of protection against challenge with wild-type S. flexneri 2a using the Sereny test. Furthermore, serum from guinea pigs immunized with SC608 expressing CfaB and LTB contained antibodies capable of neutralizing the cytological affects of heat-labile toxin (HLT) on Chinese Hamster Ovary (CHO) cells. These initial experiments demonstrate the validity of a multivalent invasive Shigella strain that can serve as a vector for the delivery of pathogen-derived antigens.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Gonad cell amplification culture medium of Chinese hamster and uses thereof
InactiveCN101195816AImprove consistencyImprove stabilityVertebrate cellsArtificial cell constructsHamsterChinese hamster
The invention relates to a culture medium and the usage thereof, in particular to the Chinese hamster ovary cell enlarging culture medium and the usage thereof. The invention belongs to the biological product field. The Chinese hamster ovary cell enlarging non-protein culture medium of the invention, wherein comprises minimal medium, and also comprises microelement, Transferrin and Insulin subsistent. A serum-containing routine culture medium can be directly replaced with the serum free culture medium of the Chinese hamster ovary cells, and the Chinese hamster ovary cells are not required to adapt process.
Owner:天津百若克医药生物技术有限责任公司
Culture method for highly expressing erythropoietin in serum-free medium and cho cells
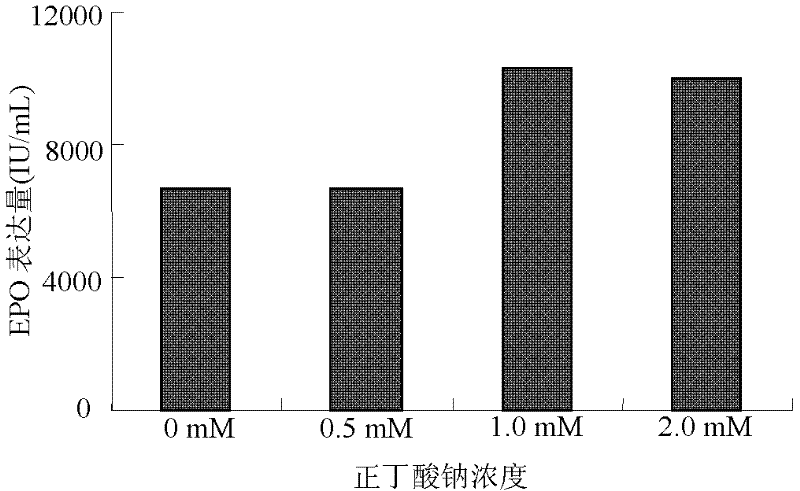
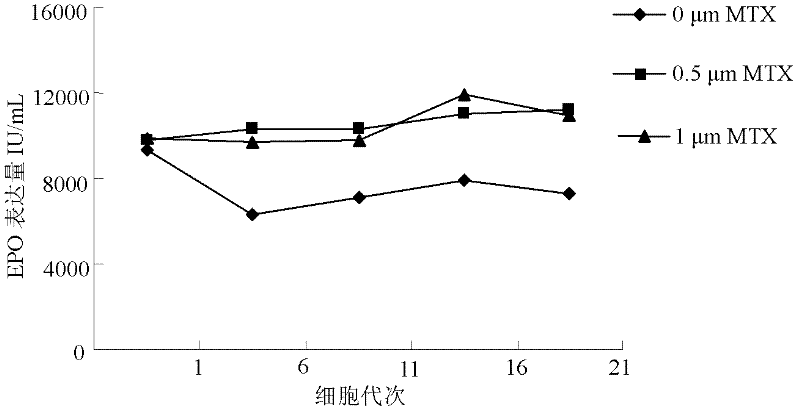
ActiveCN102268402AIncreased glycosylationIncrease the proportionMicroorganism based processesArtificial cell constructsCulture fluidChinese hamster
The invention discloses a serum free medium for expressing erythropoietin in CHO (Chinese hamster ovary) cells, and a culture method for high expression of erythropoietin in CHO cells. The serum free medium contains additives D-glucose and sodium butyrate, and also contains one or more of D-galactose, D-mannitose and N-acetylglucosamine. The culture method comprises the steps of: performing serumculture on activated seed cells by using a serum medium, and then performing serum free culture by the serum free medium. By adopting the medium and culture method disclosed by the invention, recombinant human erythropoietin protein of high and stable expression can be obtained, and the glycosylation degree of erythropoietin is improved, i.e. the specific gravity of EPO (erythropoietin) sialic acid is improved. As high as 1.0*107IU recombinant human erythropoietin can be obtained from one liter of culture fluid by the culture method provided by the invention, the expression is stable, and theculture method is simple and suitable for large-scale production.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
Recombinant protein production in a human cell
Owner:JANSSEN VACCINES & PREVENTION BV
CHO (Chinese Hamster Ovary) cell strain of efficiently-expressed recombinant human nerve growth factor and construction method thereof
ActiveCN102586190AImprove stabilityLow costMicroorganism based processesFermentationHuman bodyEmbryo
The intervention relates to a CHO (Chinese Hamster Ovary) cell strain of an efficiently-expressed recombinant human nerve growth factor and a construction method thereof and belongs to the field of biotechnology. The preservation number of the cell strain is CGMCC No. 4541, and the recombinant human nerve growth factor (rhNGF) with biological activity can be stably expressed. The invention also discloses a method for constructing the CHO cell strain; and through cell transfection, gene amplification and subcloning screening carried out on an amplified cell line, an efficiently-expressed monoclonal cell strain suitable for serum-free suspension culture is obtained. The invention simultaneously discloses a recombinant human nerve growth factor sequence secretly expressed by the CHO cell strain; and the secrete recombinant human nerve growth factor has good biological activity, so that PC12 multiplication can be kept outside a human body, PC12 cell differentiation is induced, and the chick embryo dorsal root ganglion nerve fiber growth is stimulated to lay a good foundation for the large-scale industrialization preparation of the recombinant human nerve growth factor.
Owner:北京福睿君安科技有限公司
Recombinant human-like collagen protein-human cell growth factor fusion protein and preparation method and application thereof
The invention discloses a recombinant human-like collagen protein-human original cell growth factor fusion protein and a preparation method and application thereof. The prepared fusion protein is provided with a special fibrous structure and good biological tissue compatibility, and can promote proliferation and differentiation of epidermis cells, endothelial cells and fibroblast cells. The preparation method comprises the following steps: obtaining encoded DNA sequence of the fusion protein, reconstructing and recombining fusion protein expressed by an expression vectors in escherichia coli, pichia pastoris or chinese hamster ovary cells. The fusion protein comprises a first zone and a second zone, wherein the first zone has at least 85% of sequence homology with human collagen protein and has the function of the human collagen protein, and the second zone has at least 85% of human original cell growth factor and has the function of the human original cell growth factor; connecting peptide is arranged between the first zone and the second zone; the general formula of the connecting peptide is (GGGS)n; preferably, the amino acid sequence of the fusion protein is SEQ ID NO. 15.
Owner:浙江肽源生物科技有限公司
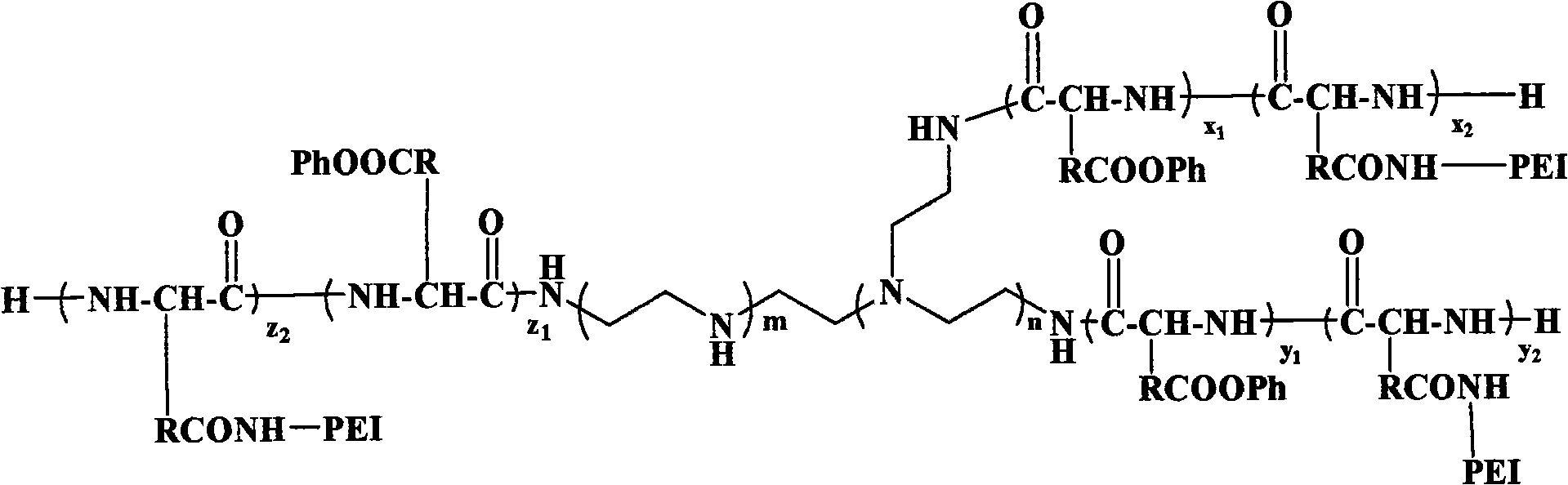
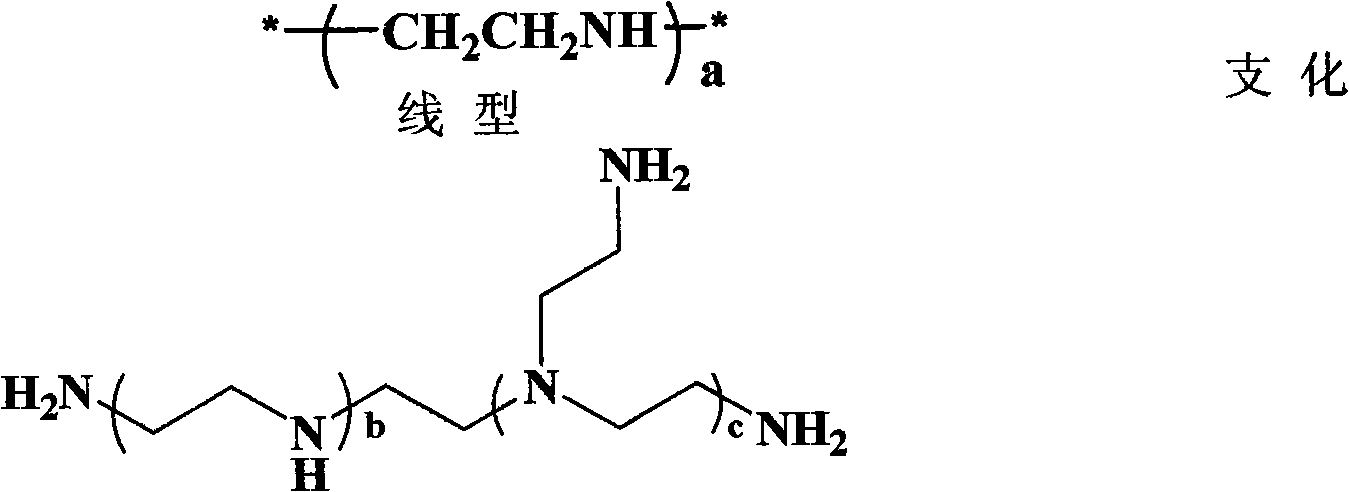
Multi-arm polyamino acid (ester) grafted polyethyleneimine copolymer, preparation method and application in gene delivery
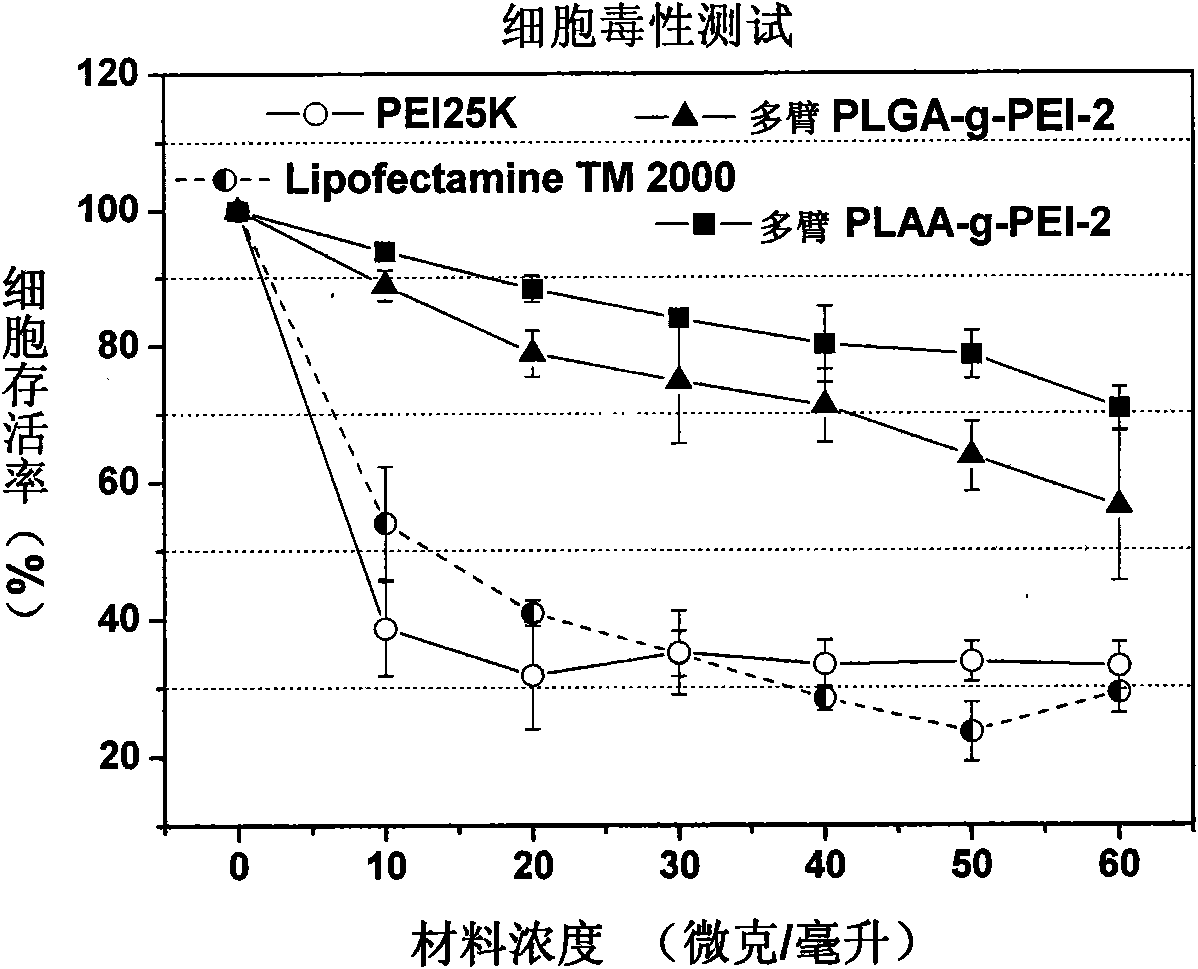
InactiveCN101575416ALow toxicityHigh transfection efficiencyOther foreign material introduction processesVector-based foreign material introductionGene deliveryCytotoxicity
The invention relates to a multi-arm polyamino acid (ester) grafted polyethyleneimine copolymer, a preparation method and application in gene delivery. The method includes the steps of: dissolving polyamine initiator in organic solvent and initiating the ring opening polymerization of alpha-amino acid-N-carboxylic acid anhydride protected by phenmethyl under anhydrous and oxygen free conditions to obtain the multi-arm polyamino acid (ester); and then causing the full or partial aminolysis reaction to occur between benzyl ester at the lateral chain of the multi-arm polyamino acid (ester) and amino group of polyethyleneimine to form the grafted copolymer. The polymer is a novel high-efficiency polycation gene vector, integrates the properties of polyethyleneimine and polyamino acid and is high in transfection efficiency, and the highest transfection efficiency to the mediate luciferase plasmid of Chinese Hamster Ovary epithelial cell is ten times than that of the Lipofectamine2000 of the commercial transfection reagent in US Invitrogen biomax under the same conditions; cytotoxicity is small; and cell survival rate is over 80% within the best transfection rate range, thus having broad application prospect.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
CHO (Chinese hamster ovary) cell serum-free medium supporting high expression of product
ActiveCN109337861APromote generationHigh densityCulture processArtificial cell constructsSerum free mediaHydrolysate
The invention discloses a CHO (Chinese hamster ovary) cell serum-free medium. The culture medium comprises main components such as amino acids, inorganic salt components, vitamins, trace elements, yeast hydrolysates, albumin and the like. The CHO cell serum-free medium disclosed by the invention does not contain any serum components and animal-derived components, and is increased in affinity compared with that of other serum-free mediums. Cells can be directly inoculated into the serum-free medium from a serum culture medium or other serum-free mediums and grow normally, thus eliminating a cumbersome domestication process. By adopting the CHO cell serum-free medium, the growth density and the viability of CHO cells are improved, the tolerance of the cells therein is increased, the growth platform maintenance time is prolonged, most importantly, the ability of CHO engineering cells to express foreign proteins in the CHO cell serum-free medium is improved, and the product yield is greatly enhanced.
Owner:YOCON BIOLOGY TECH CO
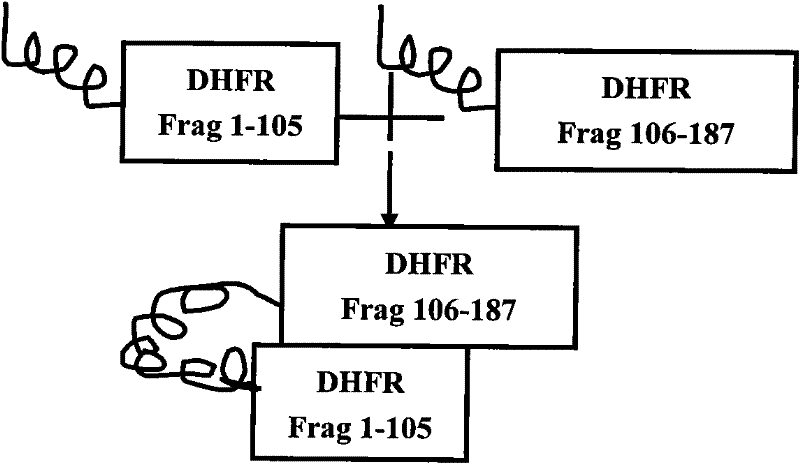
A co-transfection eukaryotic expression vector for dhfr complementary expression and its preparation method and application
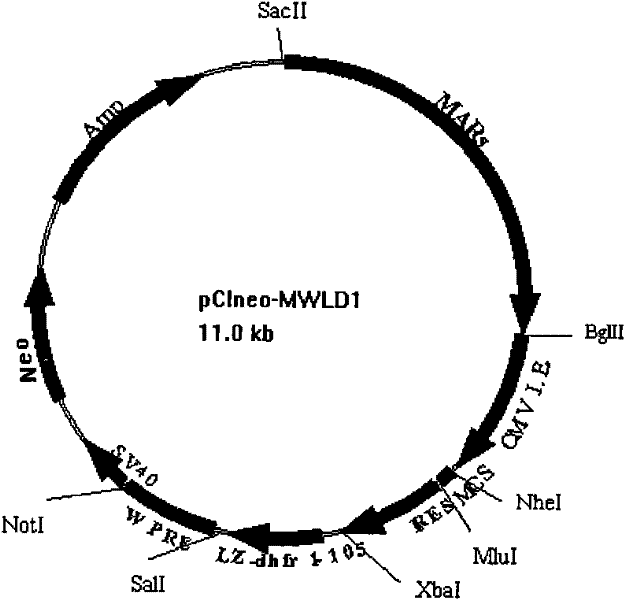
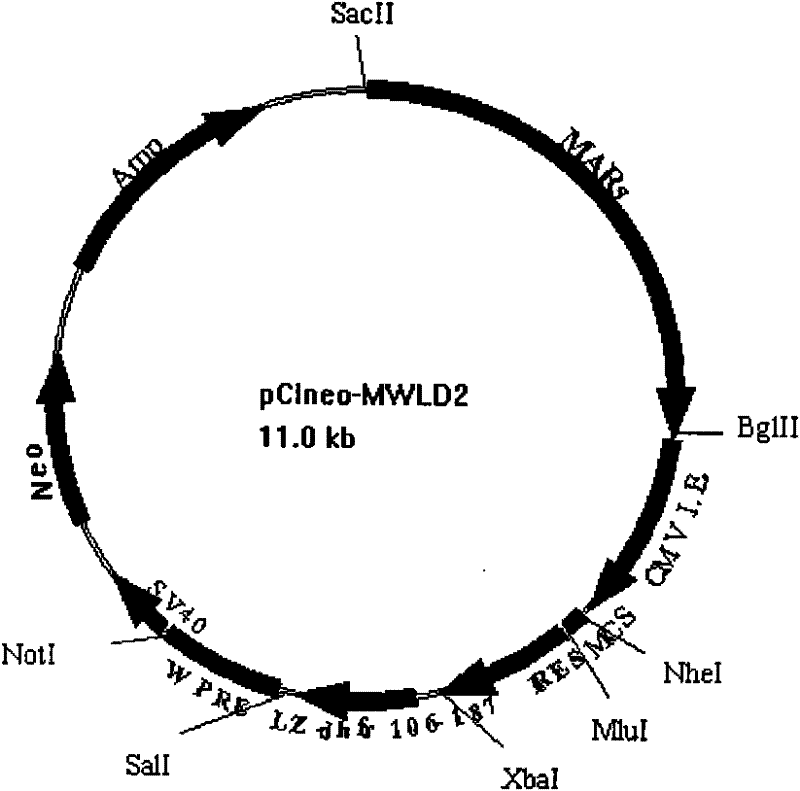
ActiveCN102277380AFermentationVector-based foreign material introductionProtein targetWoodchuck hepatitis virus
The invention relates to an efficient eukaryotic expression vector capable of effectively expressing an antibody and other target proteins and a preparation method and an application thereof, belonging to the technical field of biotechnology. The vector is formed by inserting a nuclear matrix adhering zone, a marmot hepatitis virus post-transcriptional control sequence, a ribosome entry site sequence, a dhfr1-105aa gene segment or a dhfr106-187aa gene segment on a pCI-neo plasmid. According to the invention, the defects that time and energy are wasted when the traditional mammalian cells are expressed and screened and target protein expression level is low are overcome, and a vector transfection CHO (Chinese Hamster Ovary) cell applying the vector can obtain a monoclonal cell strain capable of stably and efficiently expressing antibodies or fusion proteins in a short time, thus the vector provided by the invention has good application value for efficient expression and industrialization of recombinant protein.
Owner:QILU PHARMA
CHO (Chinese hamster ovary) cell culture technology capable of reducing content of acidic variants
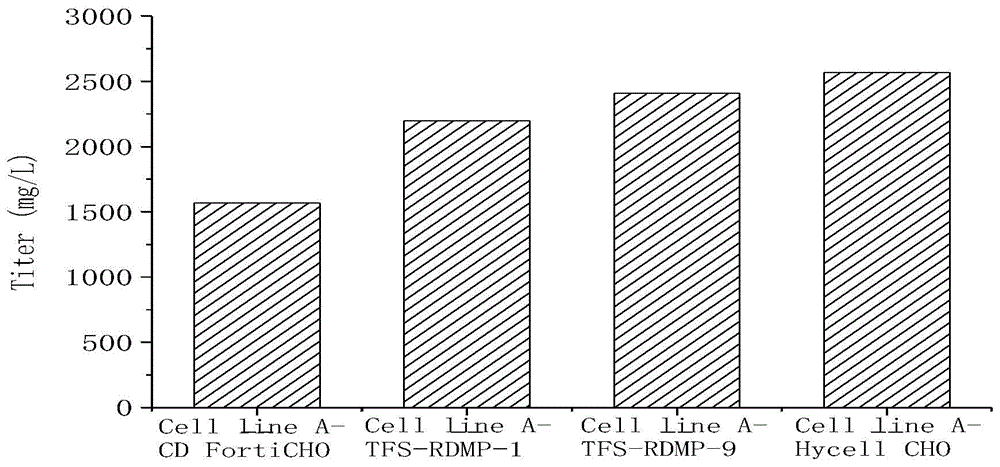
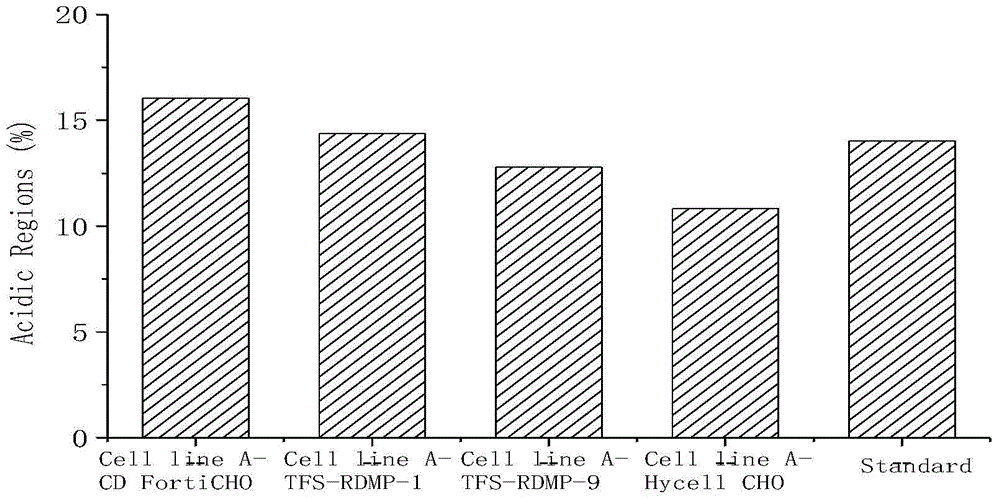
ActiveCN104560882AImprove qualityEasy to operateForeign genetic material cellsBiotechnologyEconomic benefits
The invention relates to the field of cell culture, and discloses a CHO (Chinese hamster ovary) cell culture technology capable of reducing content of acidic variants. According to the invention, different basal culture media and concentrated culture media are added in the culture process of the CHO cell technology to increase the expression quantity of recombinant protein and reduce the acid peak level and further increase the quality, so that the technology is applicable to mass production and commercial application of antibody medicines and has better economic benefit.
Owner:SUNSHINE LAKE PHARM CO LTD
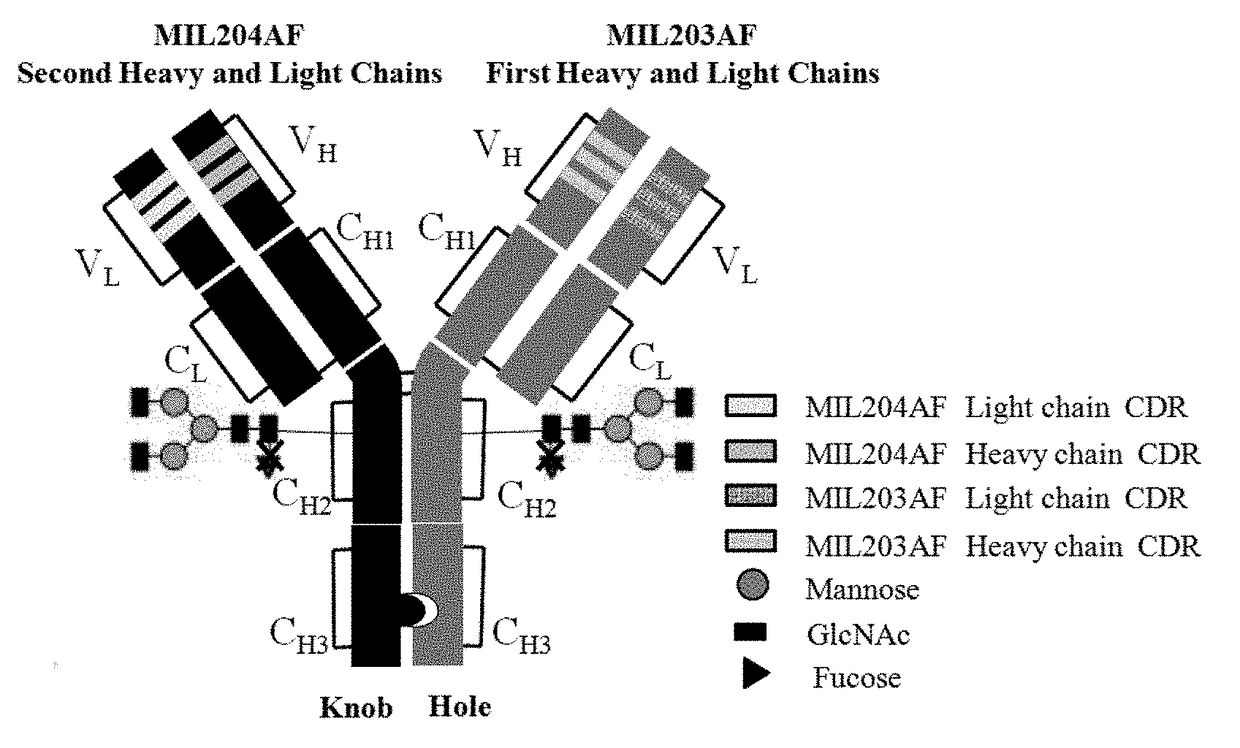
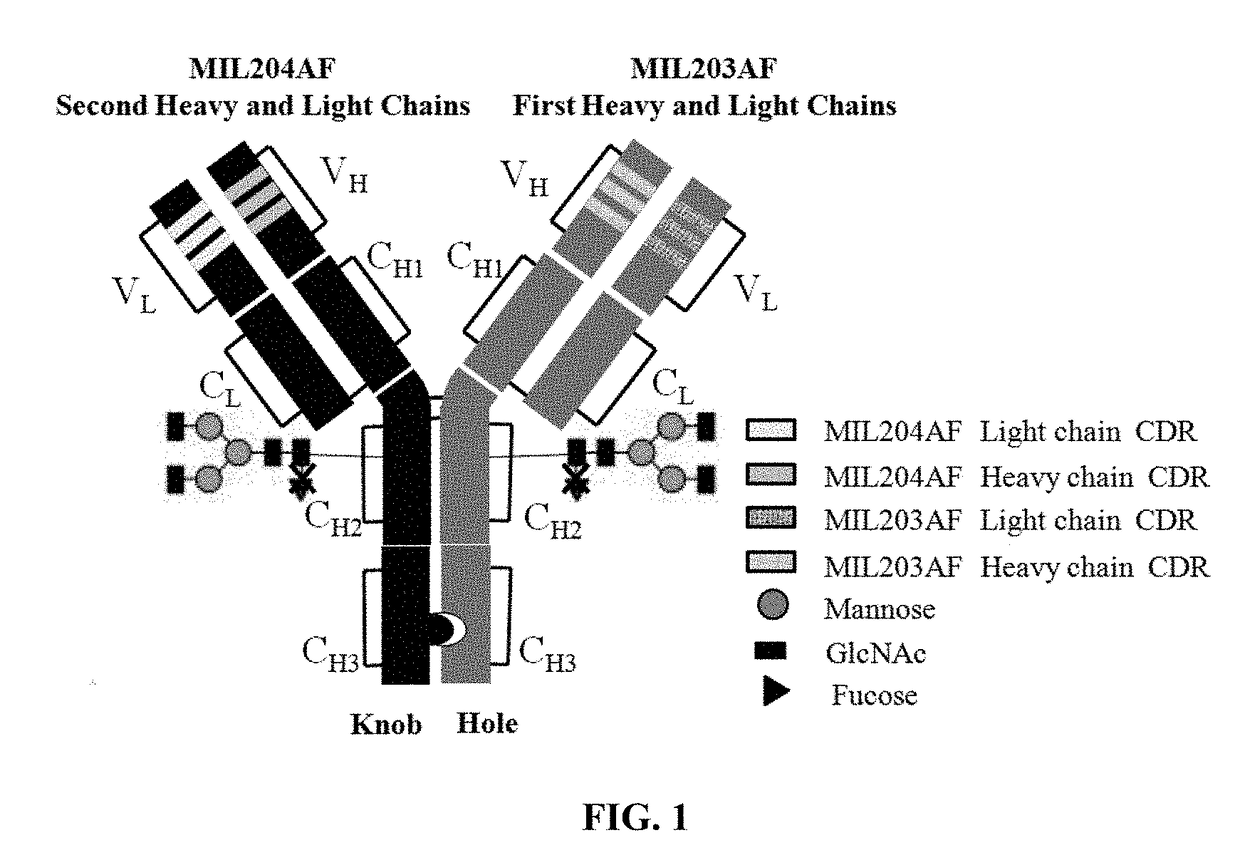
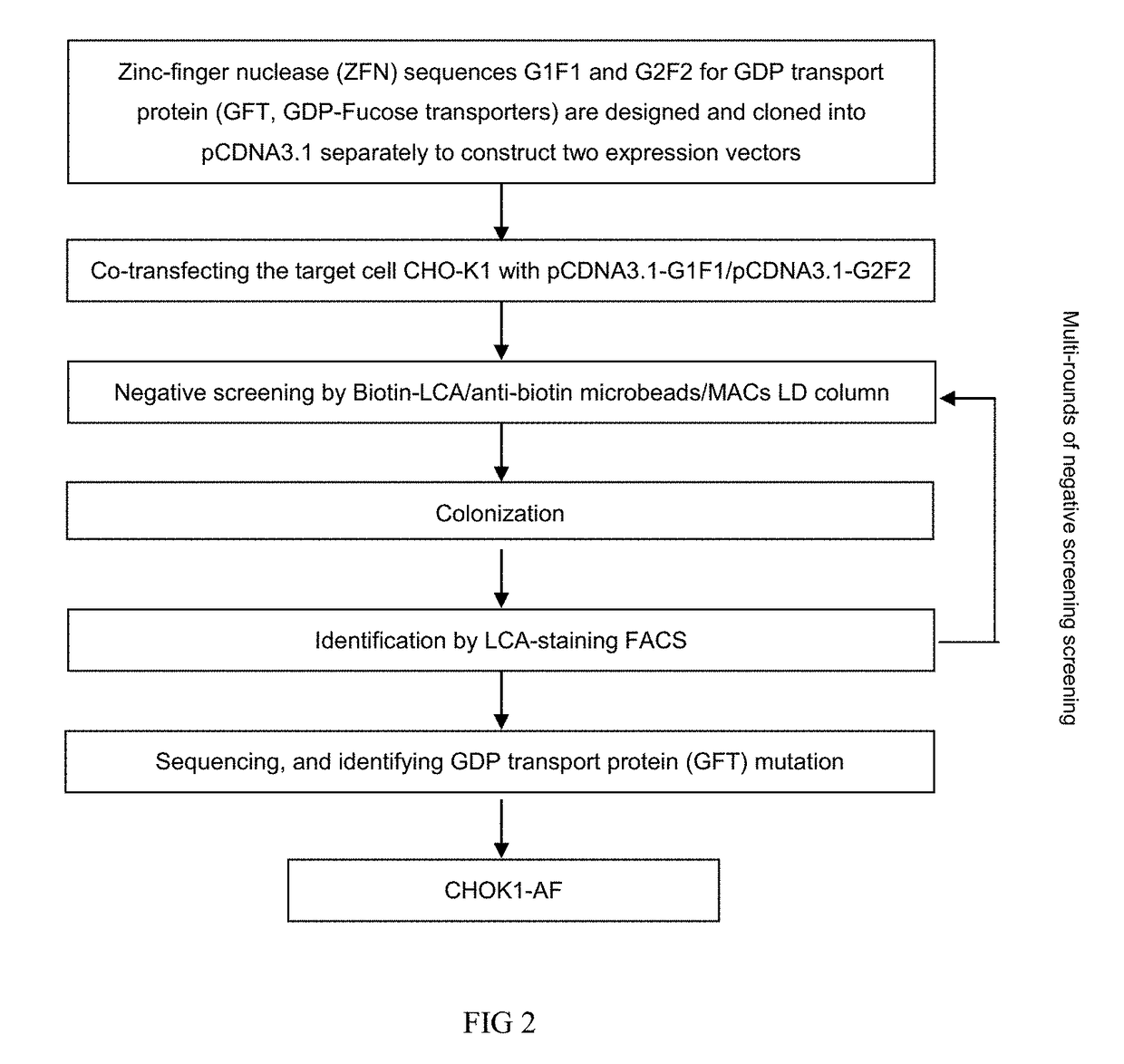
Bispecific Anti-her2 antibody
The present invention relates to humanized bispecific anti-HER2 antibodies that comprise one antigen binding site containing variable regions of heavy and light chain of trastuzumab, and another antigen binding site containing variable regions of heavy and light chain of pertuzumab. The bispecific anti-HER2 antibodies is effective for treating cancer, such as breast cancer, gastric cancer, or ovarian cancer. Preferred bispecific anti-HER antibodies of the present invention are afucosylated antibodies. The present invention also relates to Chinese Hamster ovary (CHO) mutant cell line that has a dysfunctional Slc35C1 gene, which is the only dysfunctional gene in the mutant that affects glycan regulation.
Owner:BEIJING MABWORKS BIOTECH
Chinese hamster ovary genetic engineering cell line capable of efficiently expressing Ancylostoma caninumanticoagulant peptide 5 (AcAP5) in secretion mode
A Chinese hamster ovary genetic engineering cell line capable of efficiently expressing Ancylostoma caninumanticoagulant peptide 5 (AcAP5) in secretion mode relates to a Chinese hamster ovary (CHO) cell line capable of efficiently expressing the AcAP5 in secretion mode. The cell line resolves the problem that at present, expression amount is low by using CHO to express exogenous gene products, and requirements of industrial production cannot be met. The Chinese hamster ovary genetic engineering cell line for the efficient secretion expression AcAP5 utilizes dihydrofolic acid reductase defect type Chinese hamster ovary cells as host cells, and the cell line capable of stably and efficiently expressing AcAP5 is obtained after the host cells are screened and amplified. The CHO cell line can efficiently express the AcAP5, can be produced in industrialization mode and is used for resisting blood coagulation and thrombus. Furthermore, expression amount can reach to 12 mg / L / 72h.
Owner:HEILONGJIANG UNIV
High-expression vector for animal cells
It is intended to provide an overexpression vector which makes it possible to easily acquire a high-level producing strain with the use of mammalian cells, in particular, Chinese hamster ovary cells (CHO cells) as a host. Namely, an expression vector capable of inducing high productivity of a genetically modified protein in animal host cells which comprises a promoter strongly inducing expression, a multicloning site for gene integration and a polyadenylation signal sequence, in this order starting from the upstream, and a drug-resistance gene having no promoter functioning in animal cells in the downstream thereof.
Owner:IMMUNO JAPAN
Kit for detecting DNA residues of CHO cell and using method thereof
ActiveCN102181530AHigh sensitivityReliable Quality Control EvidenceMicrobiological testing/measurementFluorescenceReference product
The invention relates to a kit for detecting deoxyribose nucleic acid (DNA) residues of a Chinese hamster ovary (CHO) cell and a using method thereof. The kit comprises DNA extracting solution, polymerase chain reaction (PCR) amplification reaction liquid, a DNA quantitative reference product of a CHO cell genome, a negative quality control product, a positive quality control product and DNA diluent. In the kit, EvaGreen is used as a fluorescent dye, and the DNA of the CHO cell genome is detected by a real-time quantitative PCR technology; and products such as a treatment protein medicament, a recombinant vaccine, a monoclonal antibody and the like from the CHO cell can be accurately and quantitatively detected.
Owner:SHANGHAI HENLIUS BIOTECH INC
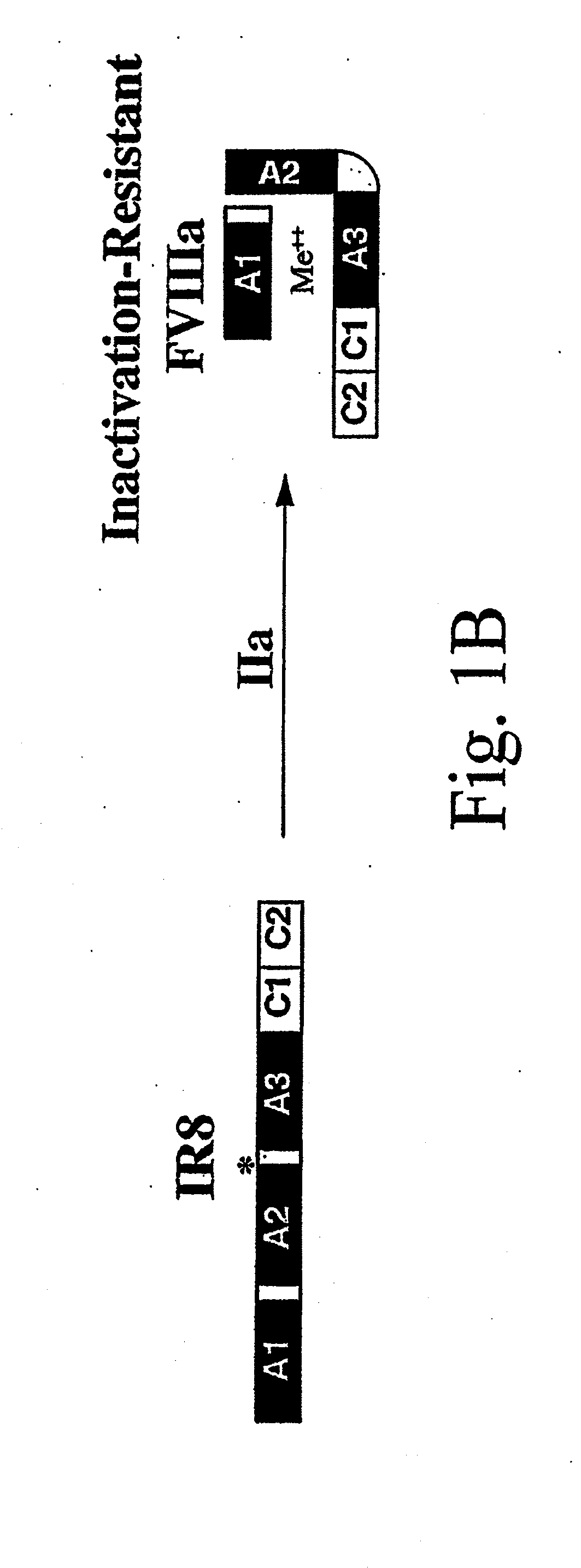
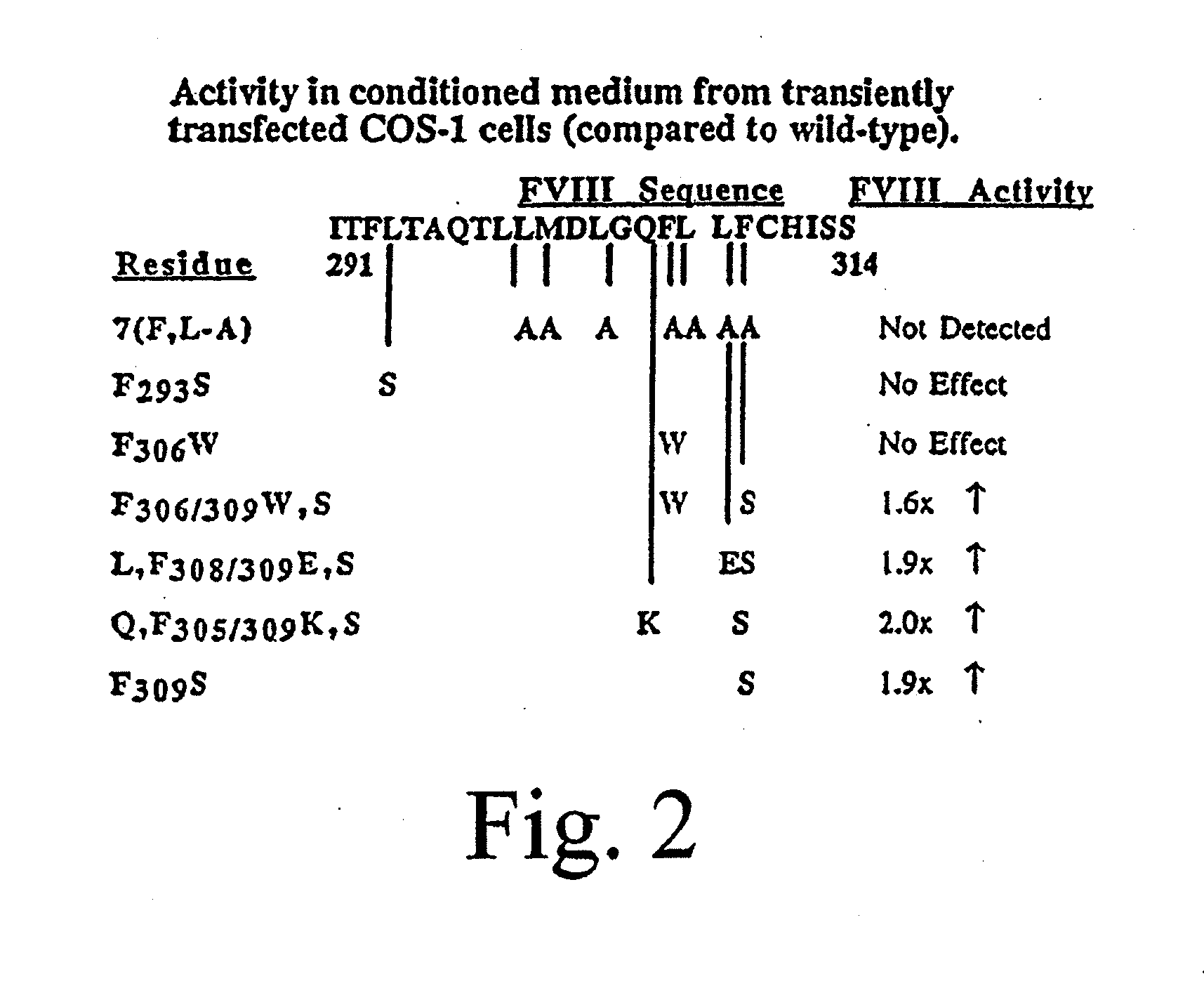
Method of producing factor viii proteins by recombinant methods
InactiveUS20120028900A1High expressionFacilitates secretionOrganic active ingredientsFungiElongation factorChinese hamster
Provided herein are methods and compositions for producing Factor VIII proteins. Such methods include introducing into a cell a nucleic acid molecule encoding a Factor VIII protein operably linked to a promoter, wherein the promoter is characterized by the ability to produce commercially viable Factor VIII protein; and incubating the cell under conditions for producing commercially viable Factor VIII protein. Also provided are nucleic acid molecules which encode a Factor VIII protein operably linked to a Chinese hamster elongation factor 1-α (CHEF1) promoter, which may be used in the methods provided herein.
Owner:CNJ HLDG +1
Chinese hamster ovary culture medium as well as preparation method and application thereof
InactiveCN102021139ACulture regulationCultivate delivery and controlTissue cultureAdditive ingredientCell culture media
The invention discloses a Chinese hamster ovary culture medium as well as a preparation method and application thereof. The culture medium comprises ascorbic acid, pyridoxine hydrochloride, vitamin B12, nicotinamide, riboflavin, thiamine chloride, choline chloride, folic acid, calcium pantothenate, sodium pyruvate, reduced glutathione, inositol, biotin, hypoxanthine, putrescine, lipoic acid, linoleic acid, thymine, glucose, HEPES (2-hydroxyethyl) buffer solution, dextran sulphate, Pluronic-F68, various amino acids and salts thereof and various inorganic salts. The culture medium has low cost, and the component does not contain animal origin ingredients, does not contain protein and has definite chemical ingredients. All ingredients are cooperated and blended, and the Chinese hamster ovary culture medium can satisfy the basic growth requirement of cells, and can effectively regulate, transfer and control the culture of cells so as to realize good high-density cell culture. Compared with the culture effect of the existing culture medium, the culture effect of culture medium disclosed in the invention is at least equivalent to even superior to the existing culture medium.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method for replication and transcription activator (Rta) protein and application of Rta protein to nasopharynx cancer detection reagent
The invention discloses a preparation method for a replication and transcription activator (Rta) protein and the application of the Rta protein to a nasopharynx cancer detection reagent and relates to a medical diagnosis reagent. The preparation method disclosed by the invention comprises the following steps of: 1, constructing a recombinant expression vector by taking a BRLF1 full-length gene as an exogenous gene; 2, transfecting: transfecting the recombinant expression vector into an eukaryotic expression system to obtain a positive transfected cell; and 3, expressing and purifying: culturing the positive transfected cell so as to enable the positive transfected cell to express an interest protein, and separating and purifying the interest protein, wherein the eukaryotic expression system refers to a Chinese hamster ovary (CHO) cell. The Rta protein prepared by the method disclosed by the invention is used for detecting nasopharynx cancer; the sensitivity of the Rta protein is 96 percent (288 / 300), and the specificity of the Rta protein is 96.7 percent (290 / 300). The sensitivity and the specificity are superior to those of antigens respectively prepared by a prokaryotic expression system and a pichia expression system, and the sensitivity and the specificity on clinical early diagnosis on the nasopharynx cancer are greatly improved.
Owner:同昕生物技术(北京)有限公司
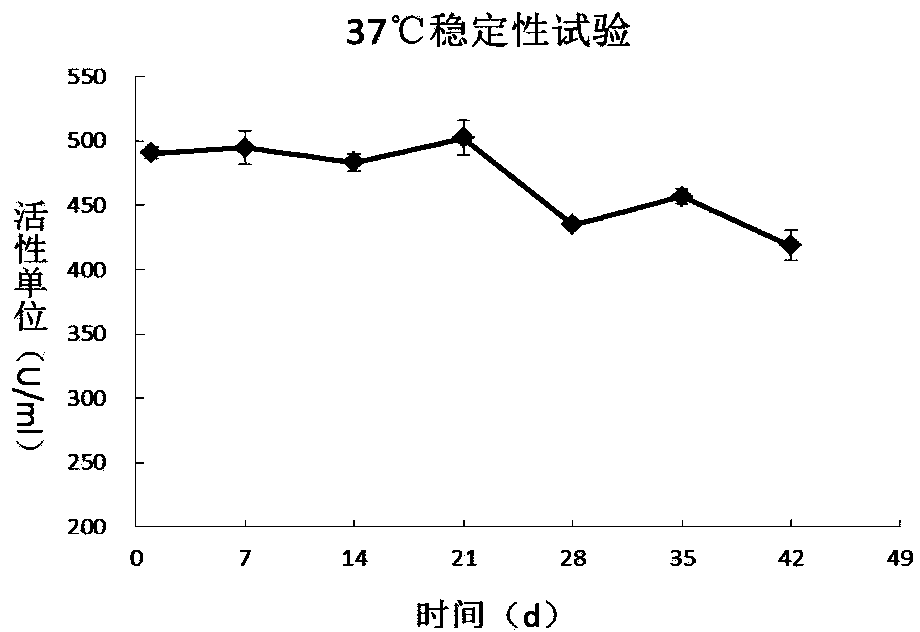
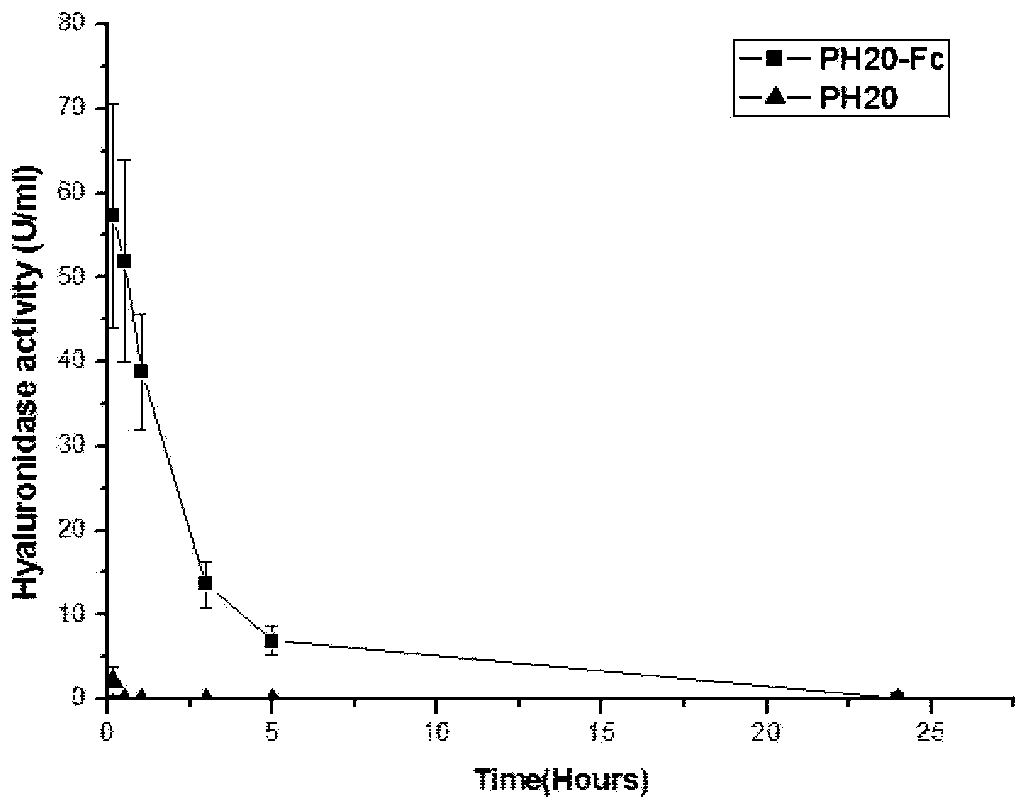
Recombinant long-acting human hyaluronidase, and encoding gene, production method and application thereof
The invention discloses a recombinant long-acting human hyaluronidase. The recombinant long-acting human hyaluronidase is a fusion protein PH20-HSA or PH20-IgFc of a human hyaluronidase PH20 extracellular part and a human protein (albumin HSA fragment or immunoglobulin IgG2Fc fragment), and the amino acid sequences of the PH20-HSA and the PH20-IgFc are represented by SEQ ID No. 4 and SEQ ID No. 5 respectively, and have the advantages of high in-vivo safety and long half-life. The invention also discloses an encoding gene, an expression vector, a host cell, a production method and a stable preparation of the recombinant long-acting human hyaluronidase. The achievement of industrial level and the obtaining of purified products with active energy are realized through expressing by using GC-rich high expression system and through producing Chinese hamster cells (CHO). The invention further discloses an application of the recombinant long-acting human hyaluronidase in cooperative dosage and the production of micro-molecular hyaluronic acid.
Owner:QINGDAO HUINUODE BIOLOGICAL TECH CO LTD
CHO (Chinese hamster ovary) cell serum-free protein-free culture medium and preparation method thereof
InactiveCN106190950AGrow fastClear chemical compositionCulture processArtificial cell constructsMinor elementHydrolysate
The invention relates to preparation of a cell culture medium, and particularly provides preparation of a novel serum-free protein-free defined-chemical-component cell culture medium. The culture medium contains multiple amino acids, vitamins, inorganic salts, minor elements, carbohydrates and other supplementary factors. The culture medium is free of any extract or hydrolysate. The culture medium is free of any animal-derived component. The culture medium can well support in-vitro suspension culture of CHO (Chinese hamster ovary) cells, and satisfies the demands for CHO cell culture. The culture medium contains defined chemical components, and is low in cost.
Owner:BEIJING SL PHARMA +2
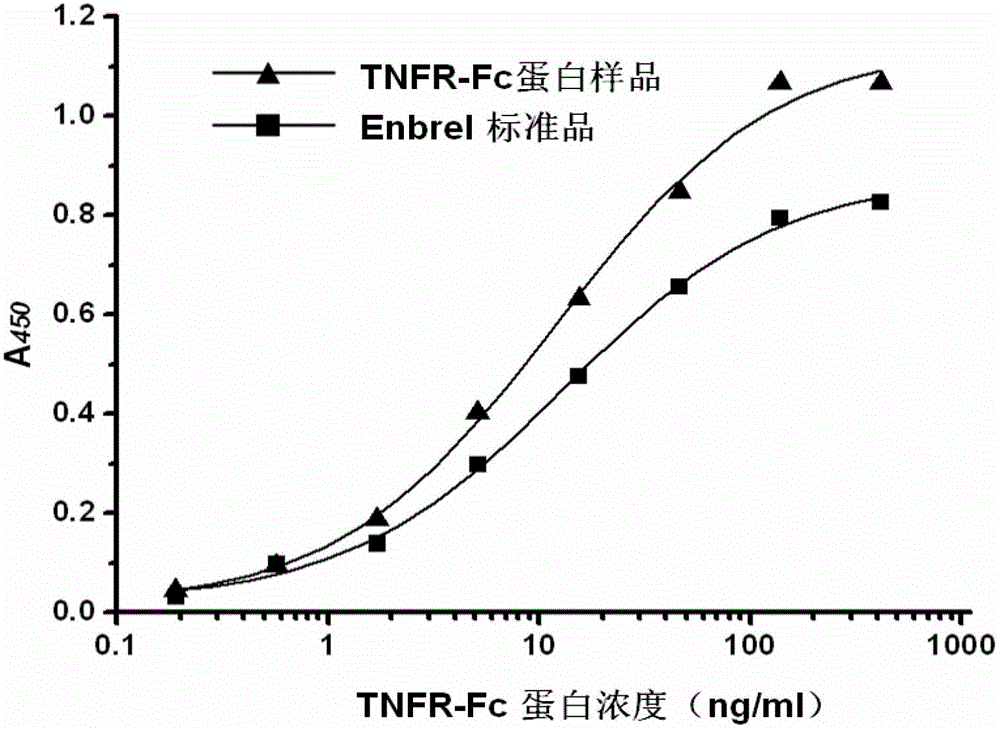
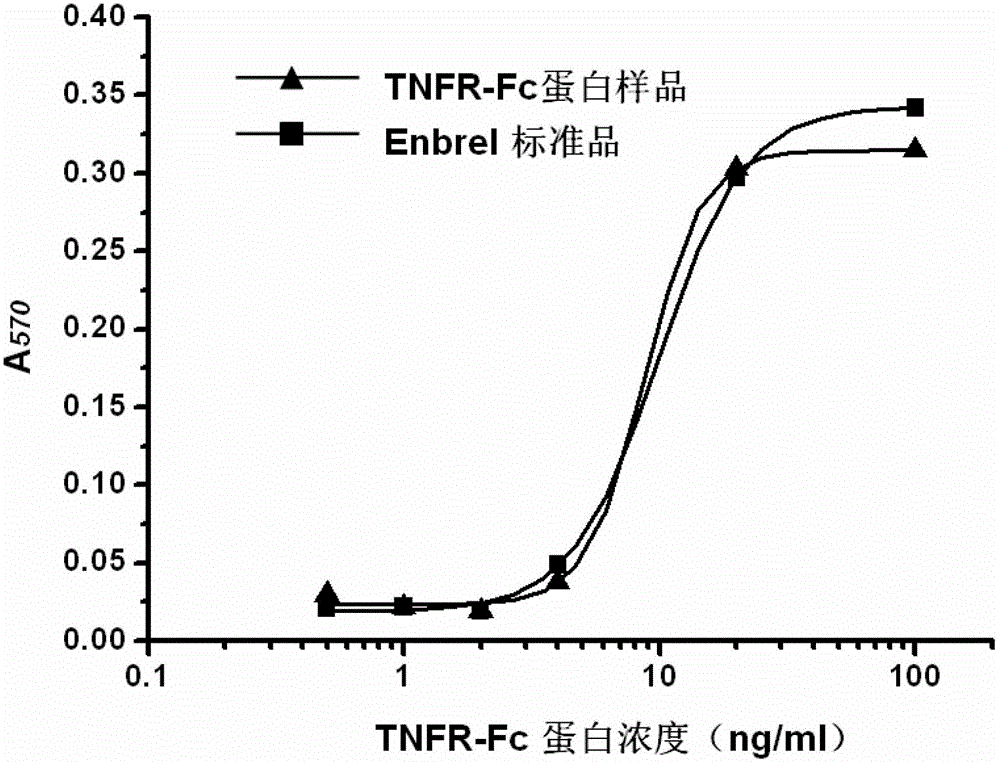
Gene for coding recombinant human TNFR-Fc fusion protein and application of gene
ActiveCN102911958AHigh affinityEasy to operateFermentationVector-based foreign material introductionProtein targetFhit gene
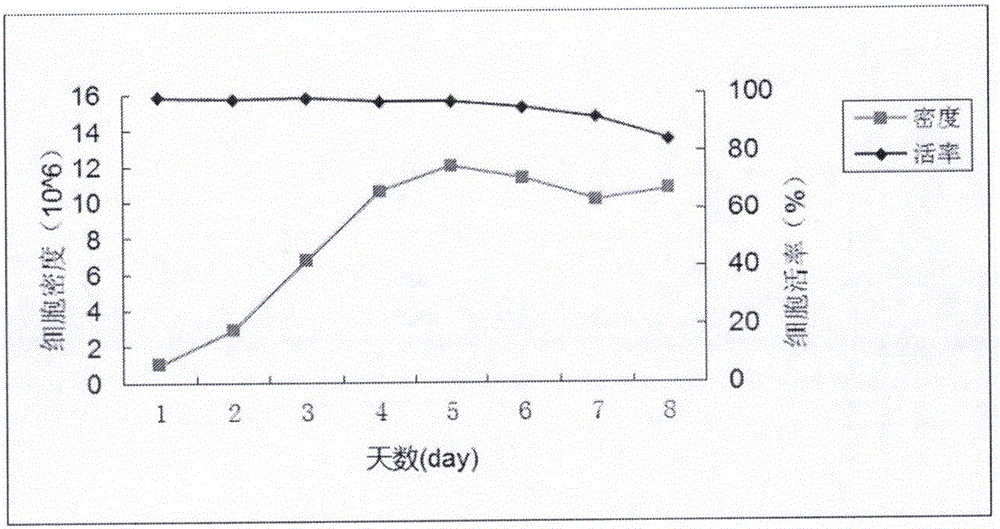
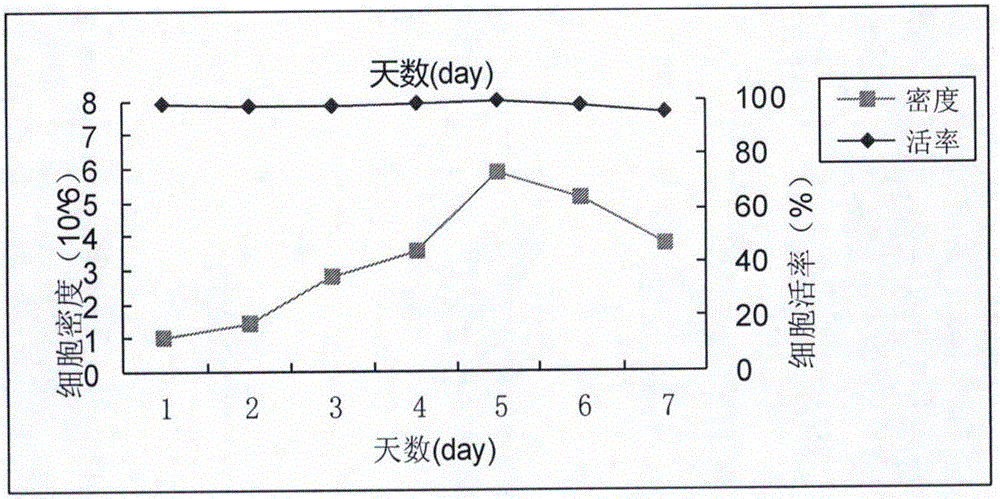
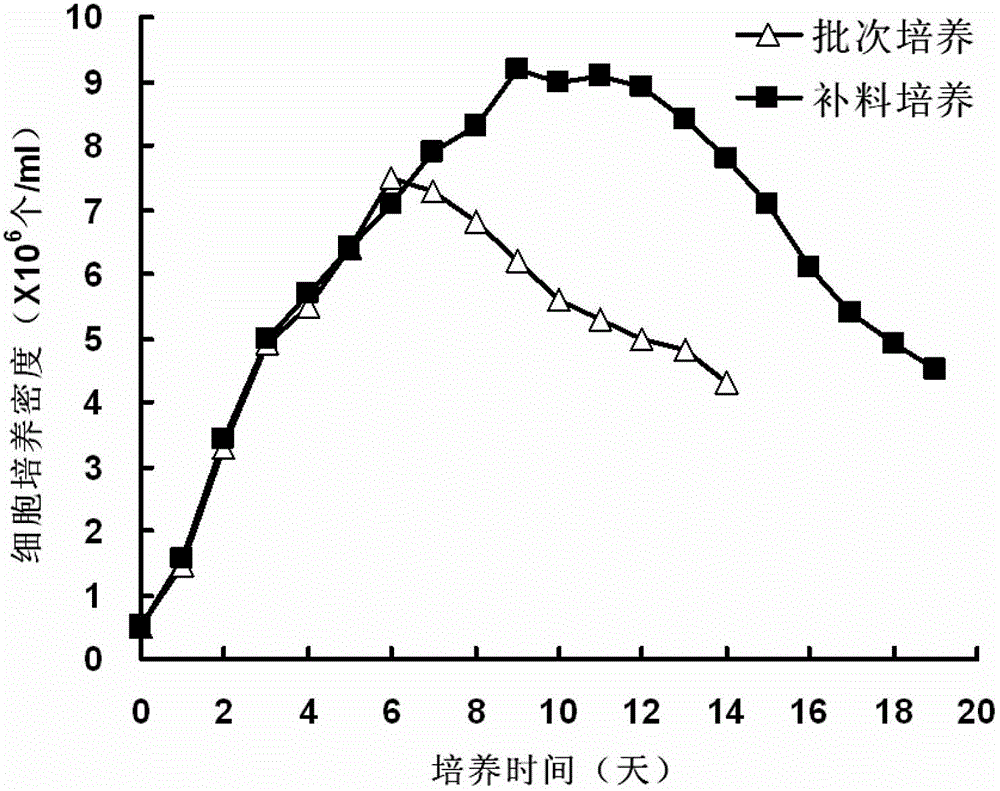
The invention discloses a gene for coding a recombinant human TNFR-Fc fusion protein and an application of the gene. The gene for coding the recombinant human TNFR-Fc fusion protein as shown in SEQ ID NO.1 is obtained by optimized screening of codons. The gene has high expression index in a CHO (Chinese Hamster Ovary) cell, and the expressed protein has high affinity with TNFR. The gene is transferred into the CHO cell to obtain a cell for expressing the recombinant human TNFR-Fc fusion protein. The CHO cell into which the gene is transferred is fermented by using a disposable reactor, the operation is simple, and the obtained protein quantity is higher in comparison with the conventional fermentation tank; and especially after a supplemented medium is added, the cell growth time can be prolonged, the expression level can be increased, the production cost can be reduced, and a high-purity target protein can be obtained.
Owner:JINAN UNIVERSITY +1
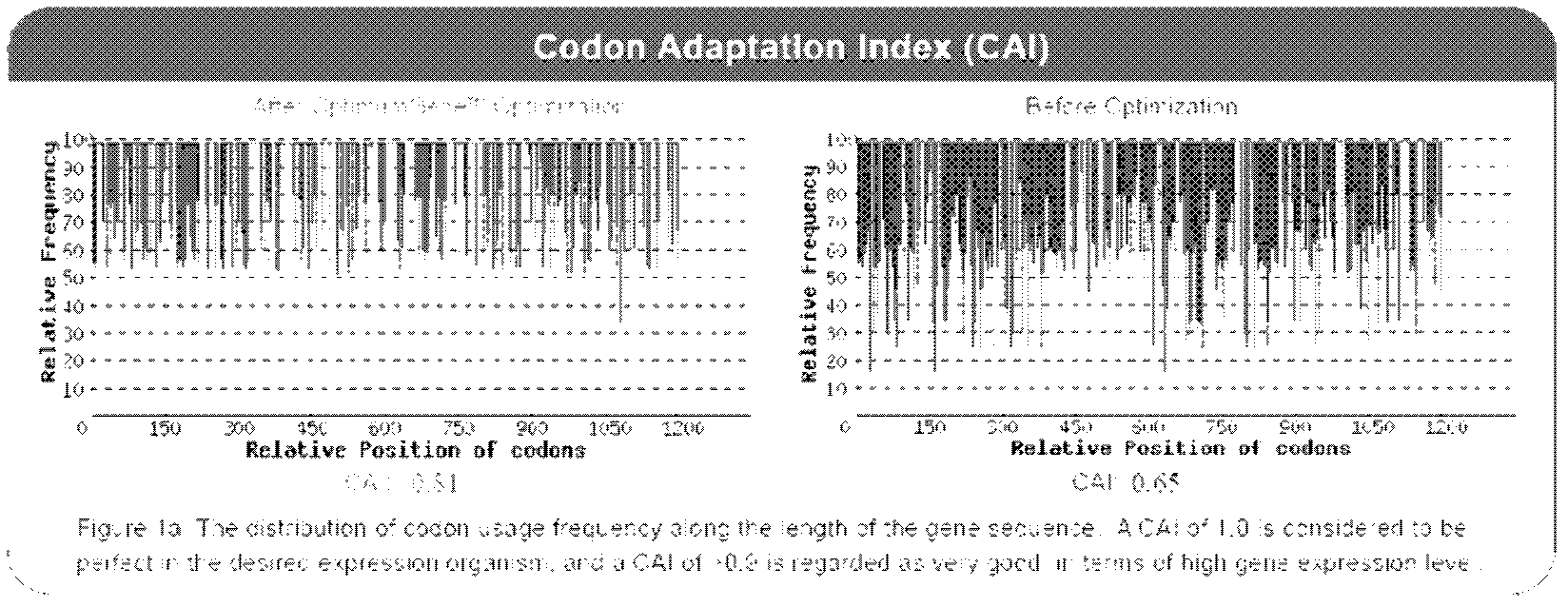
Codon optimized fatty acid desaturase gene sequence and application thereof
InactiveCN102533807AEfficient productionReduce production processFermentationVector-based foreign material introductionControl cellChinese hamster
The invention relates to a codon optimized fatty acid desaturase (FatI for short) gene sequence and application thereof. A eukaryotic expression vector is constructed, and is transfected in Chinese hamster ovary (CHO), and a cell strain which stably expresses FatI is obtained through G418 screening; through genome polymerase chain reaction (PCR) and Southern Blot determination, an exogenous gene is integrated into a genome, and the exogenous gene can be transcribed into mRNA in the cell; a n-3 / n-6 value of a cell of the successfully transfected CHO cell line is improved by 8.47 percent than that of a control cell; and therefore, the FatI gene sequence can promote n-6 fatty acid in the cell to be converted into n-3 fatty acid.
Owner:QINGDAO AGRI UNIV
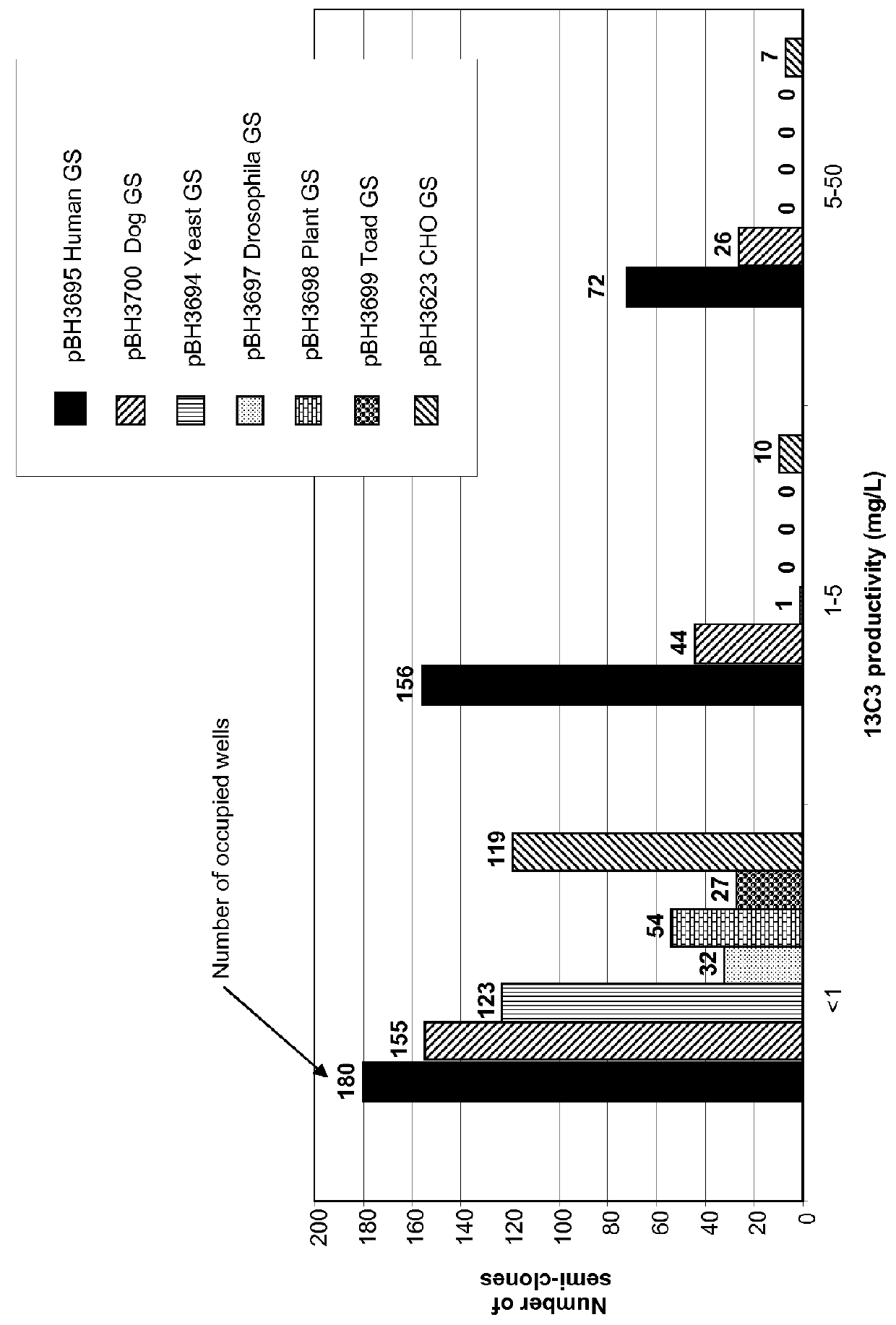
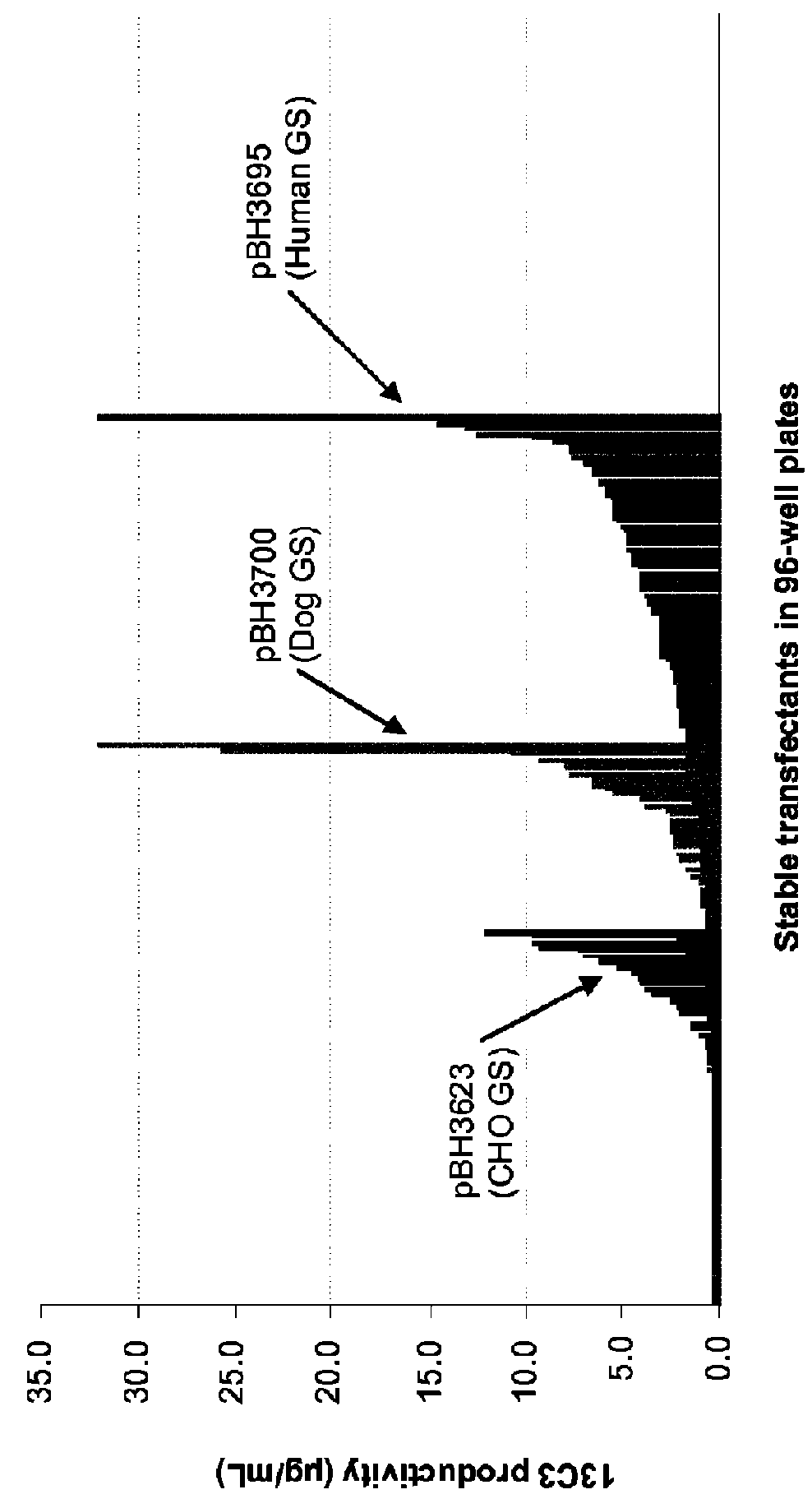
CHO expression system
The present invention is within the field of industrial protein production. The inventors have designed and constructed a new expression system comprising an expression vector coding for a glutamine synthetase of human or dog origin, and a CHO cell line. More specifically, the invention pertains to a combination of (i) a DNA vector suitable for production of a recombinant protein, wherein said vector comprises a sequence coding for a glutamine synthetase, and (ii) a Chinese Hamster Ovary (CHO) cell line, wherein said GS comprises a sequence at least 94.5% identical to the sequence of SEQ ID NO: 1 or to the sequence of SEQ ID NO: 2.
Owner:SANOFI SA
Processes and compositions for transfecting Chinese hamster ovary (CHO) cells
InactiveUS8076139B1Efficient deliveryEasy to producePowder deliveryMicroencapsulation basedHamsterAqueous medium
The present invention generally relates to processes and compositions for the transfection of Chinese hamster ovary (CHO) cells. More specifically, the present invention relates to processes for the transfection of CHO cells suspended in an aqueous medium using a transfection composition containing nucleic acid and linear polyethyleneimine.
Owner:LEINCO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com