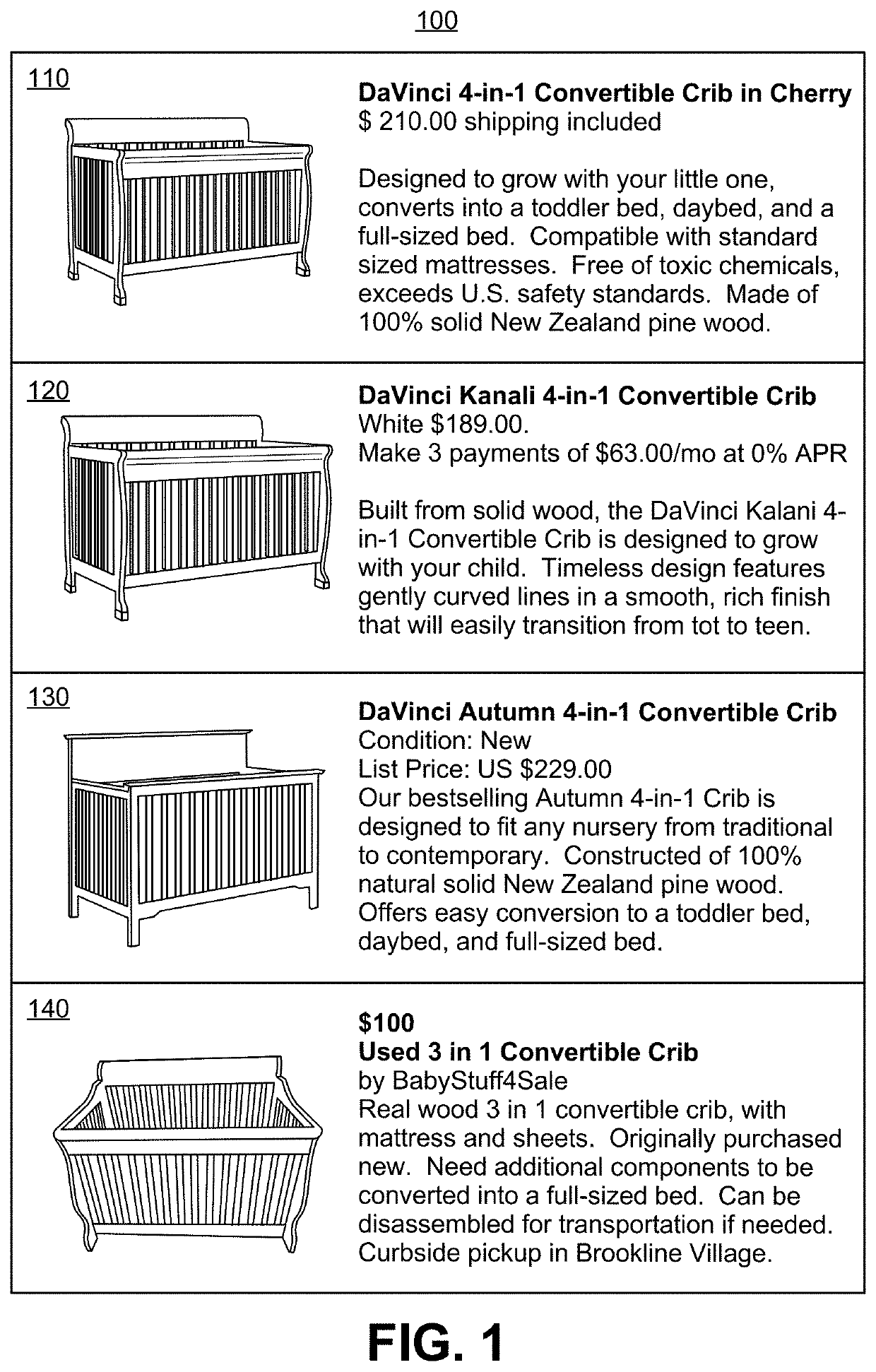
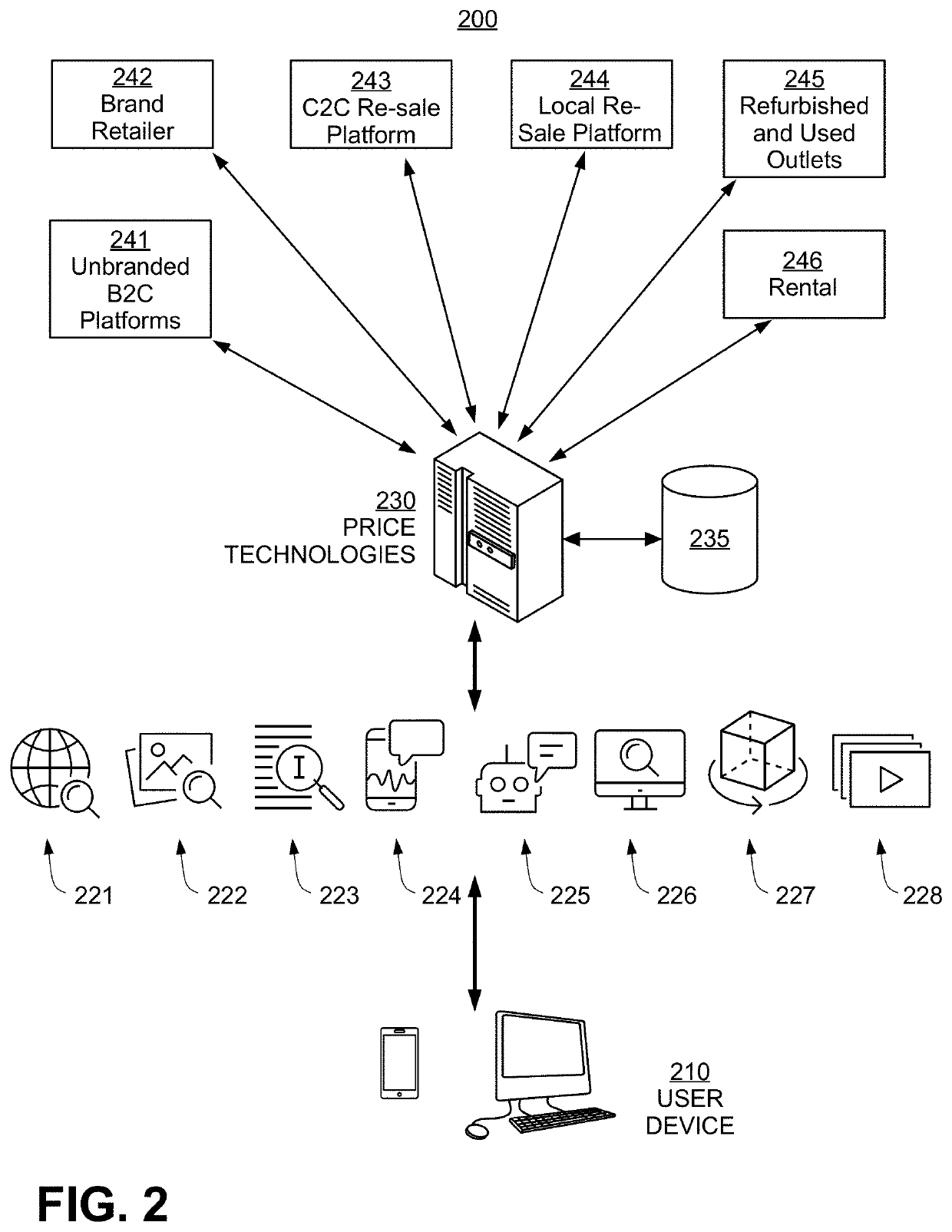
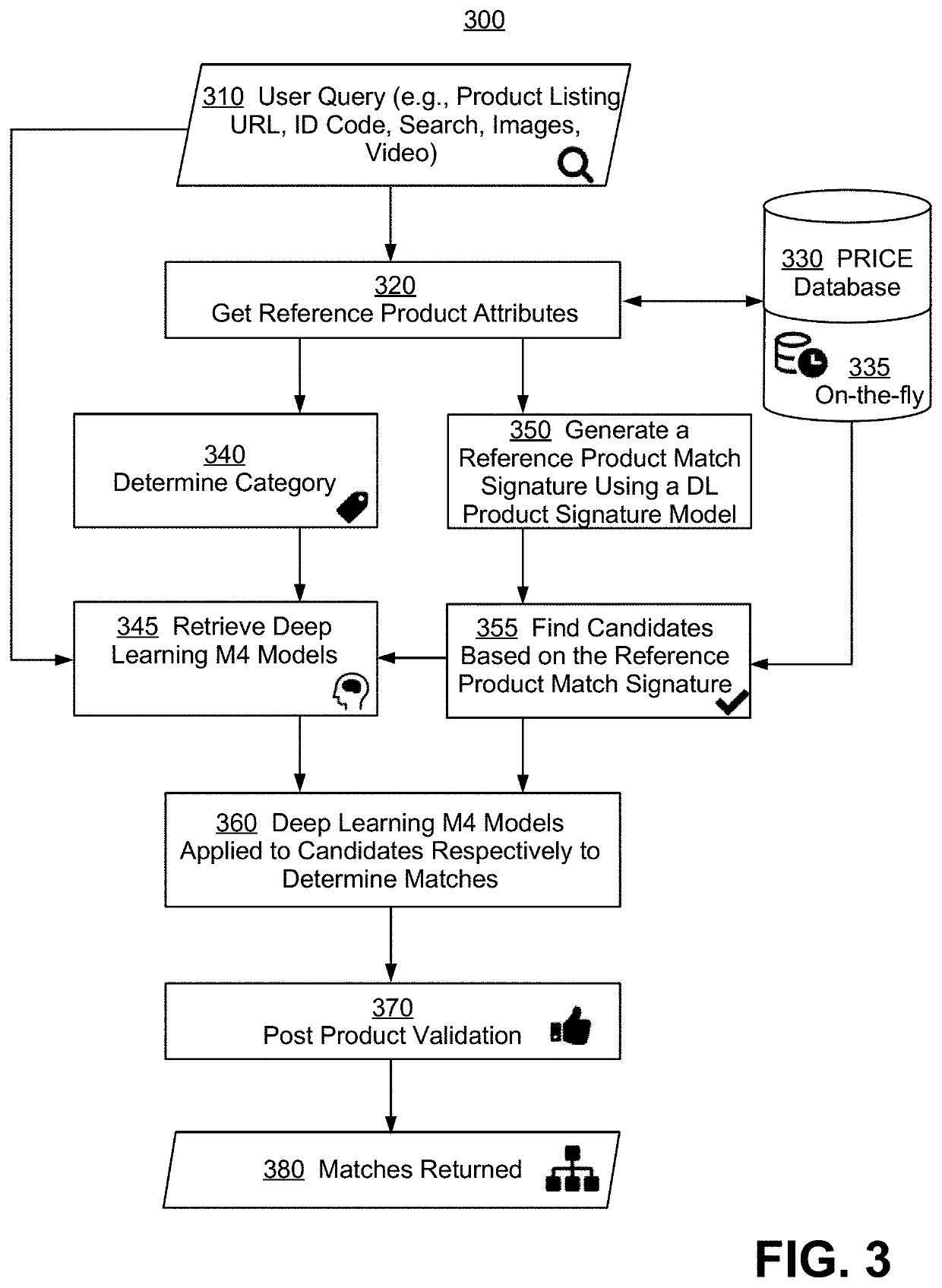
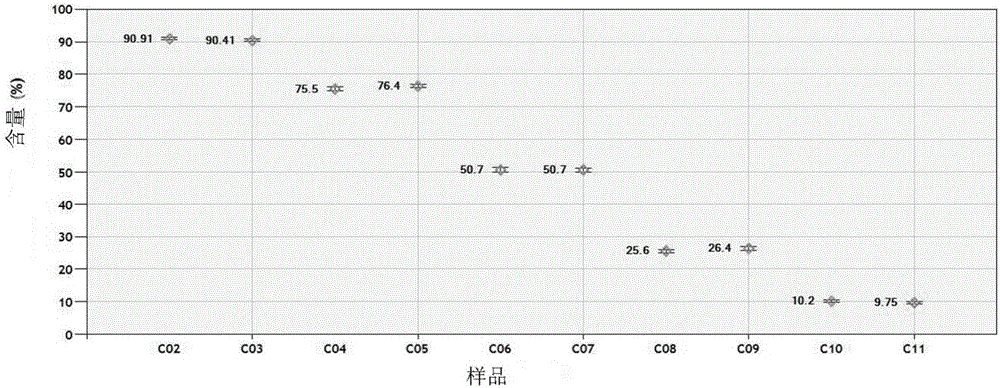
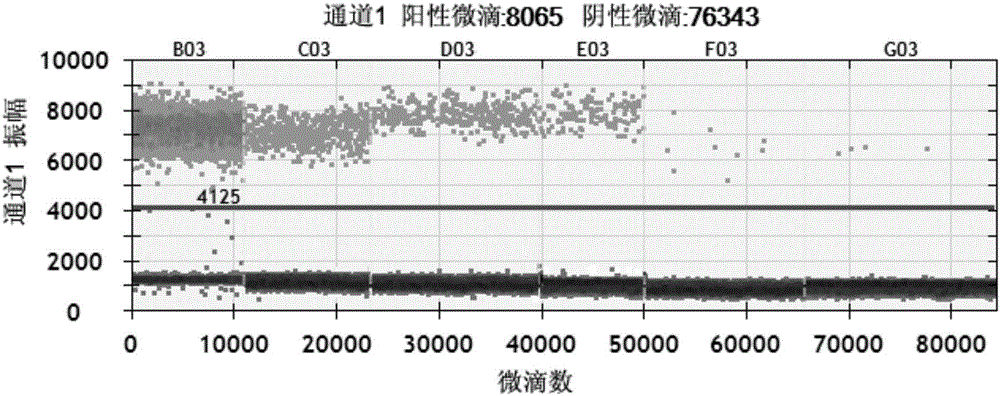
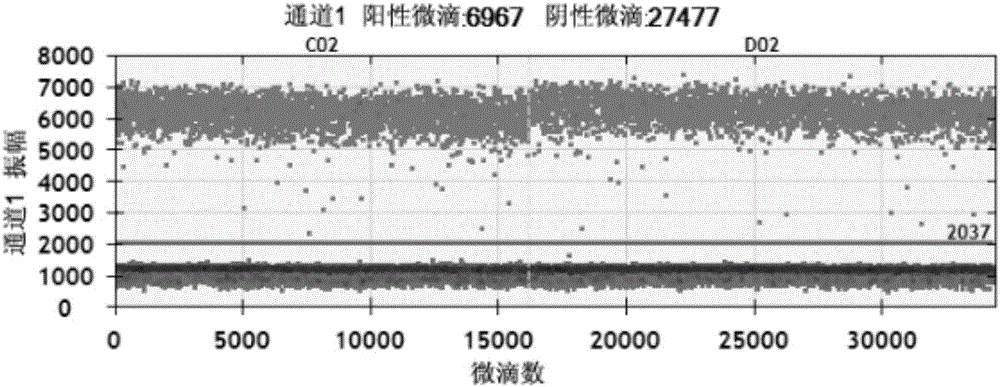
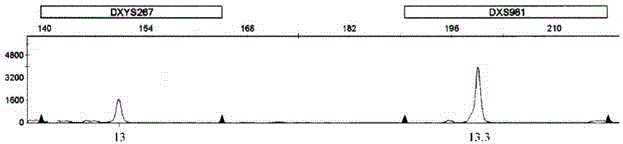
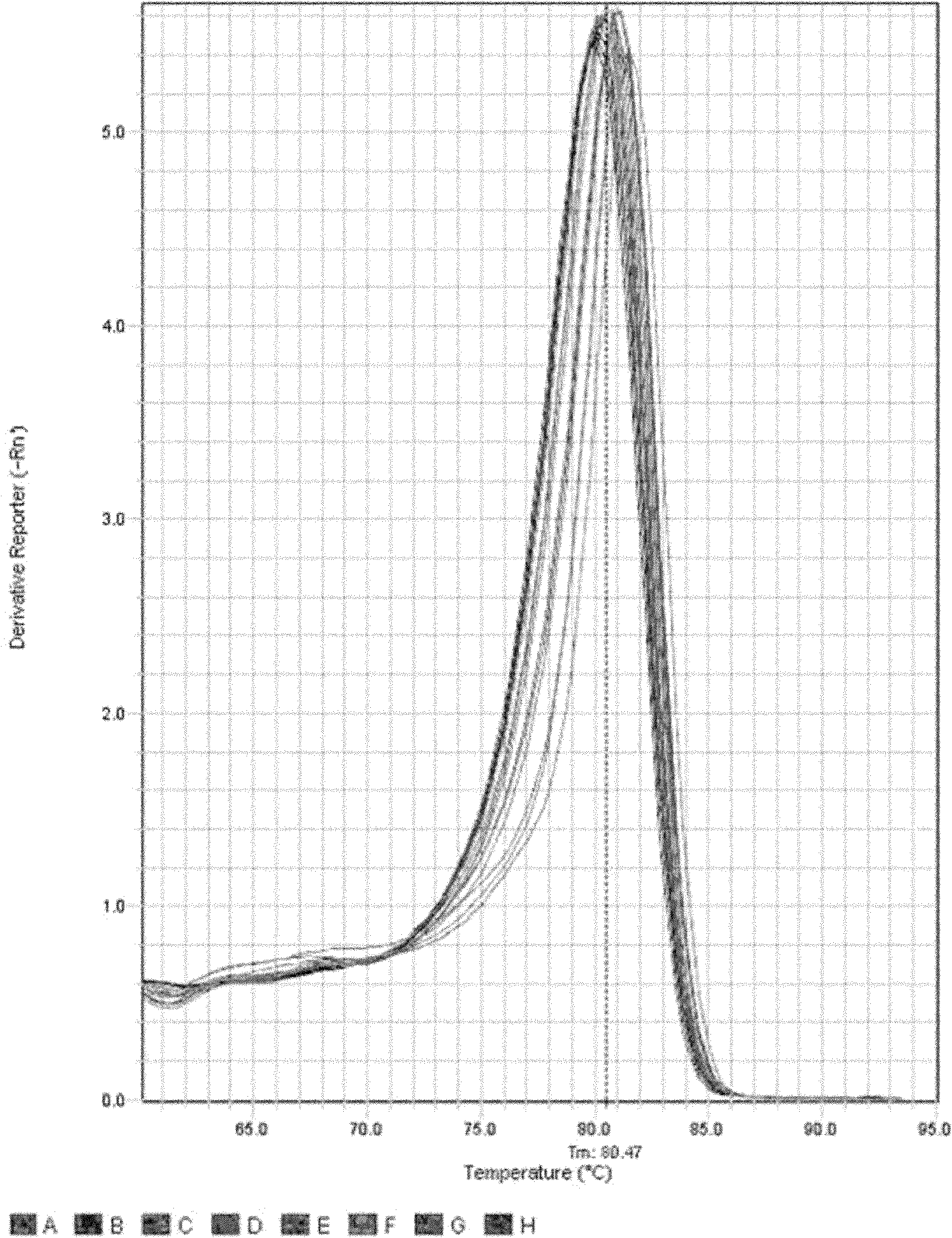
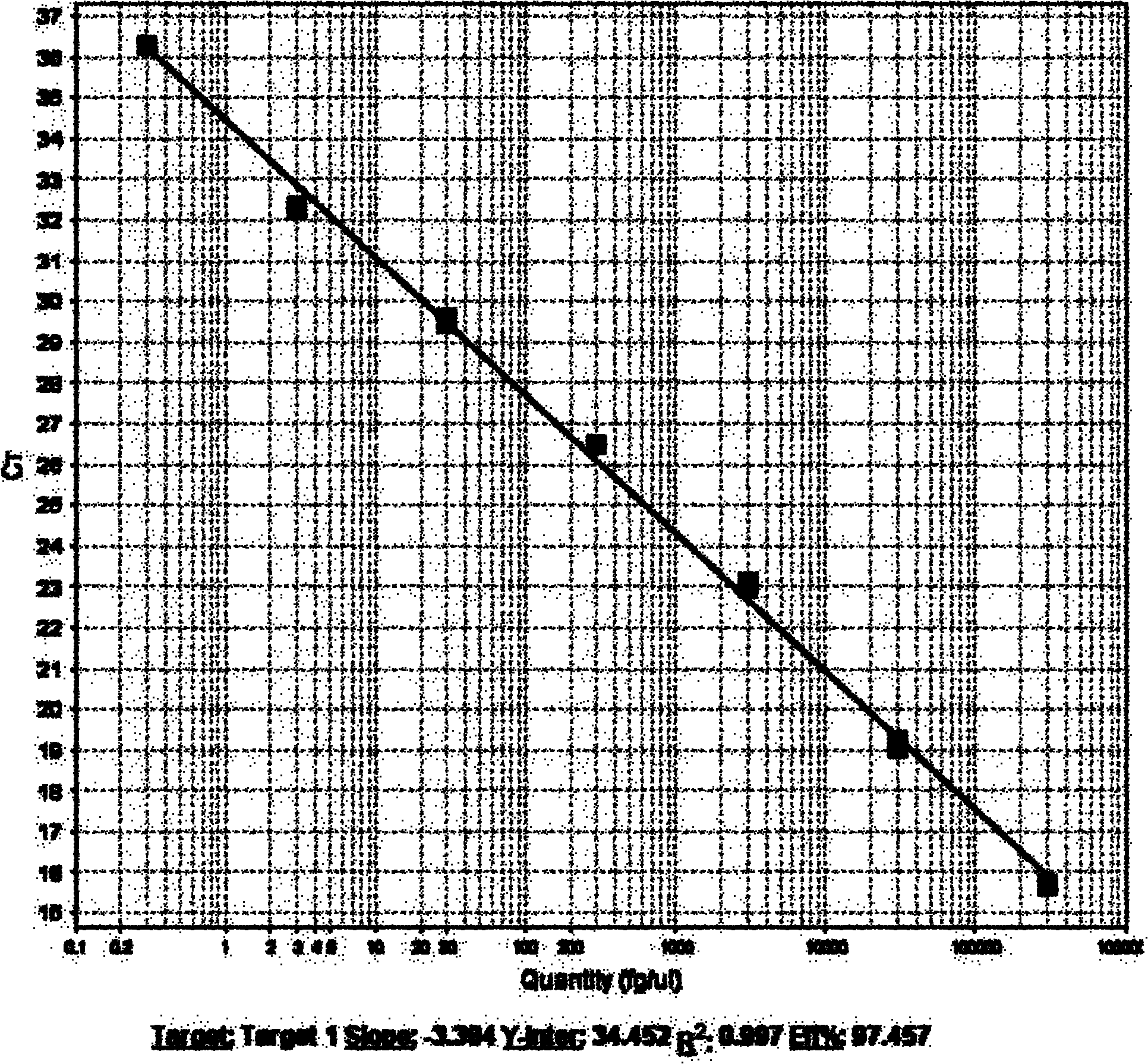
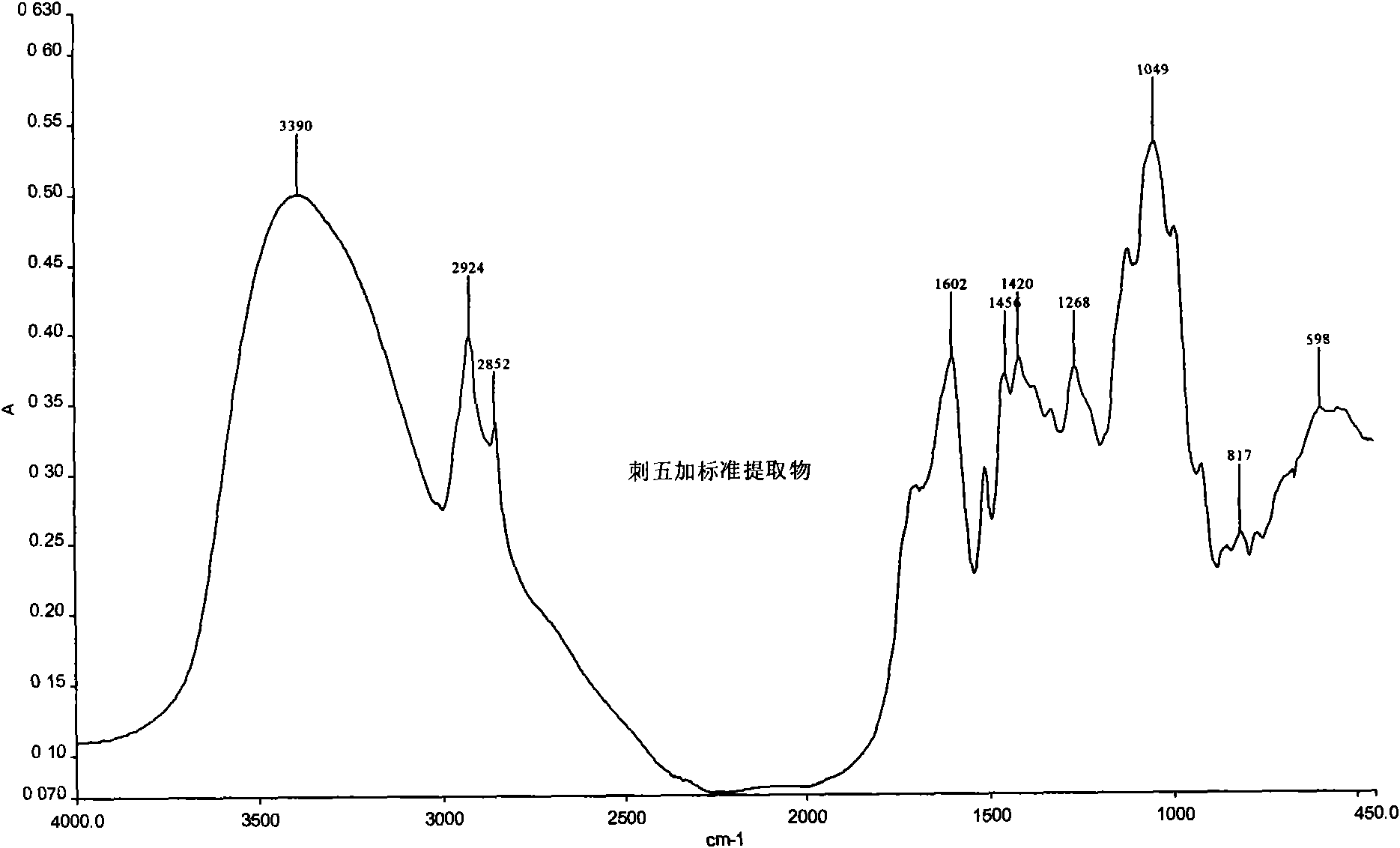
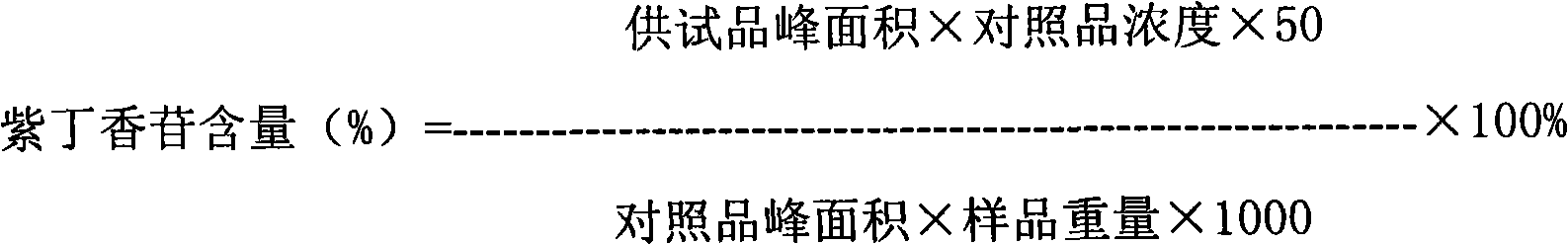
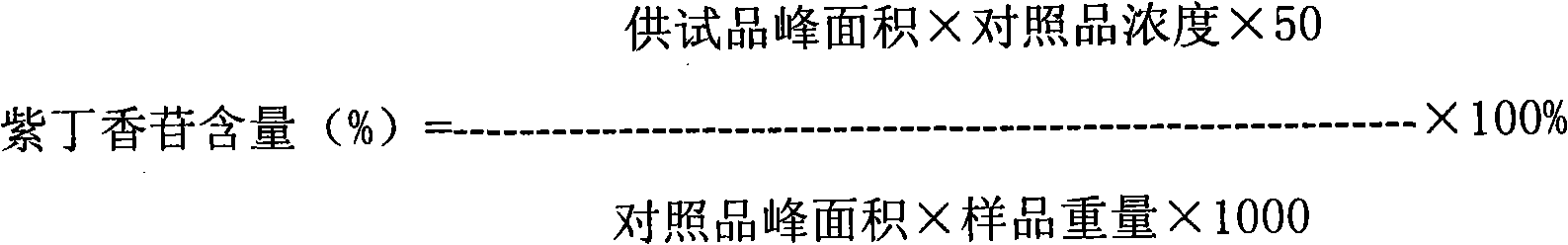
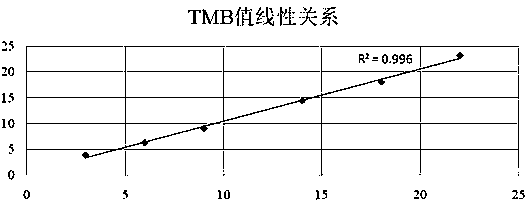
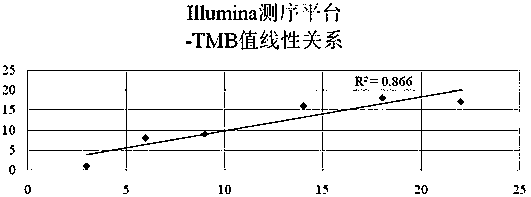
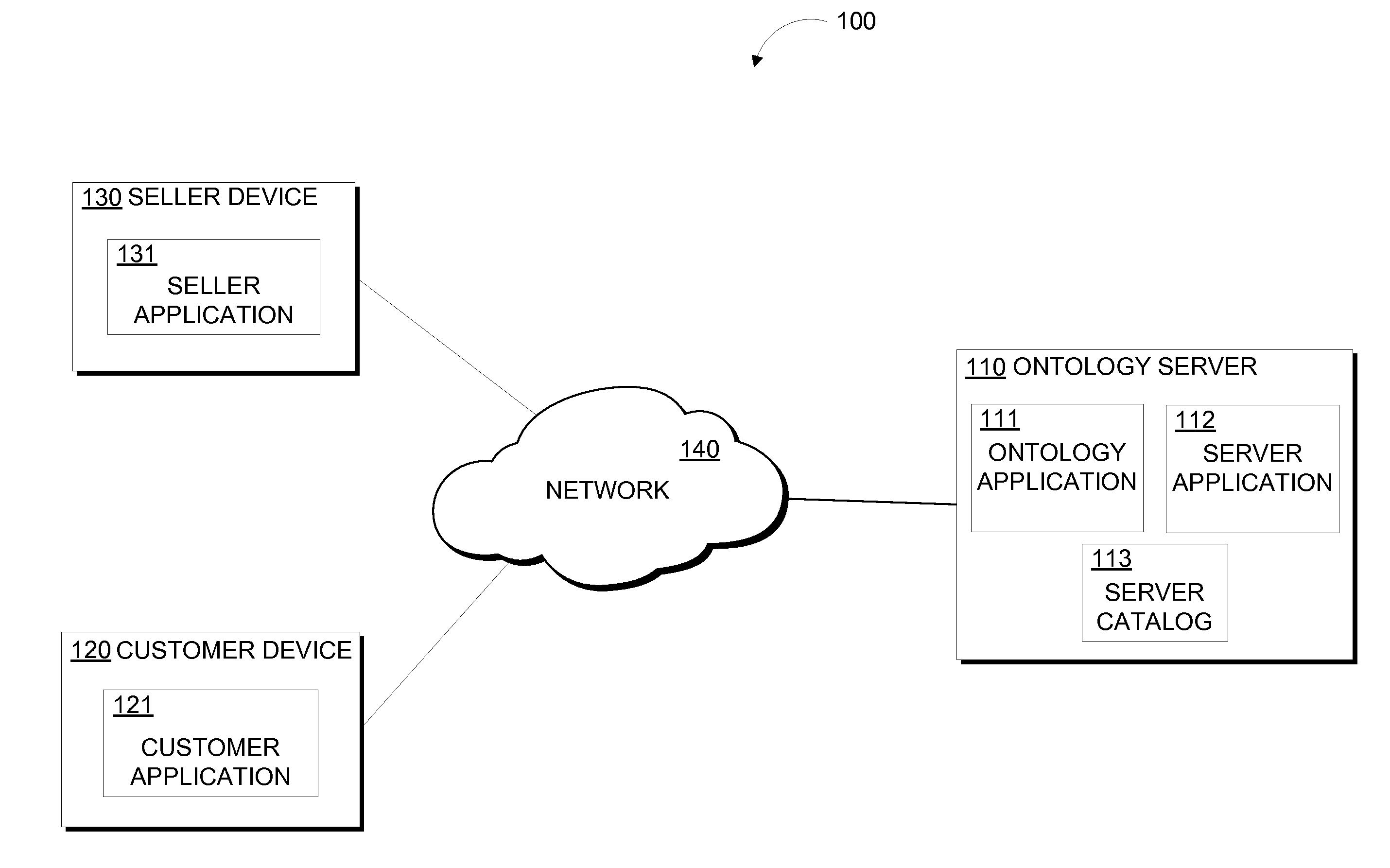
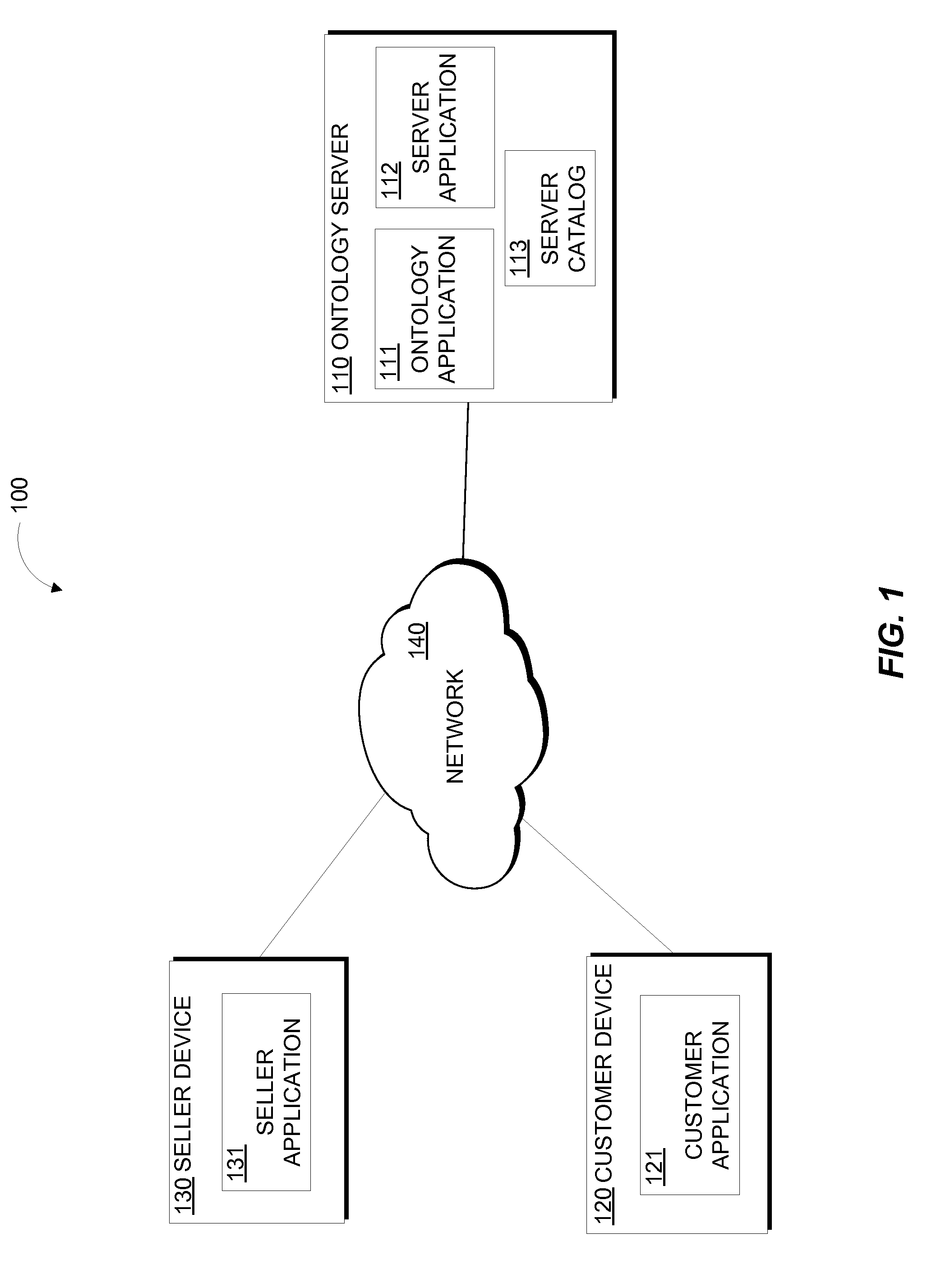
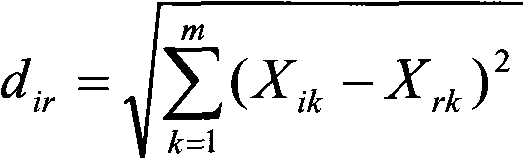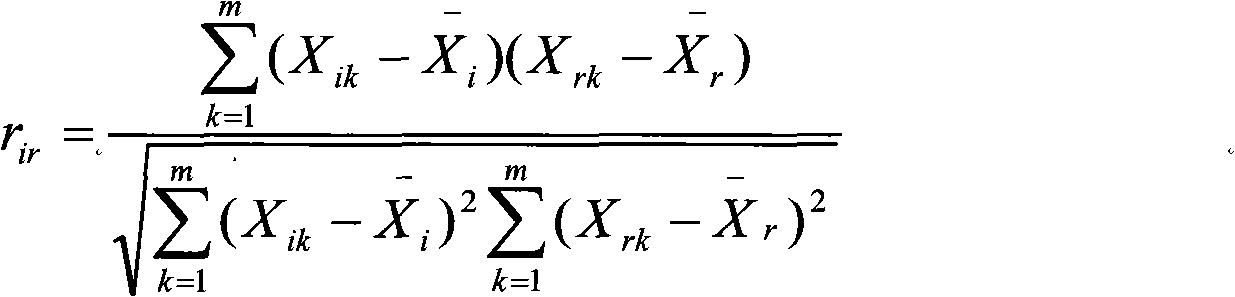Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
292 results about "Reference product" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
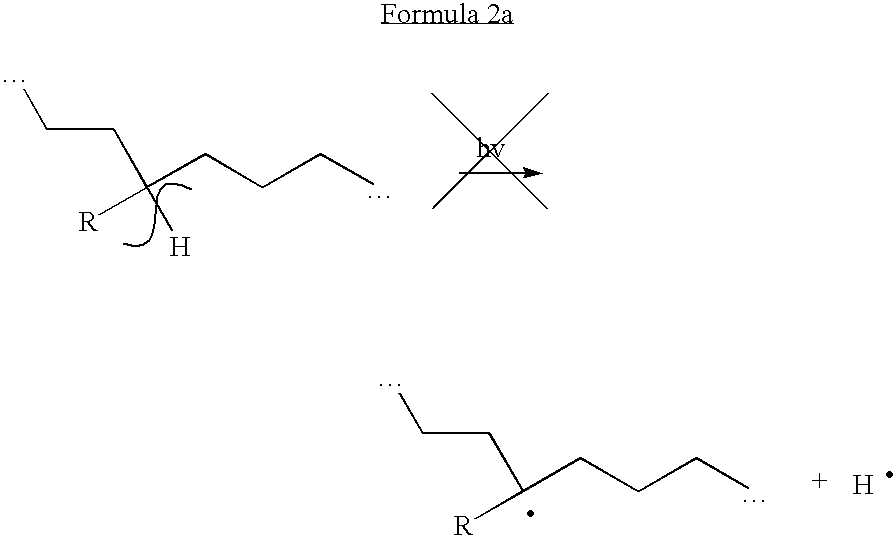
A reference product is the single biological product, already approved by FDA, against which a proposed biosimilar product is compared. A reference product is approved based on, among other things, a full complement of safety and effectiveness data.
Method and apparatus for distribution of fashion and seasonal goods
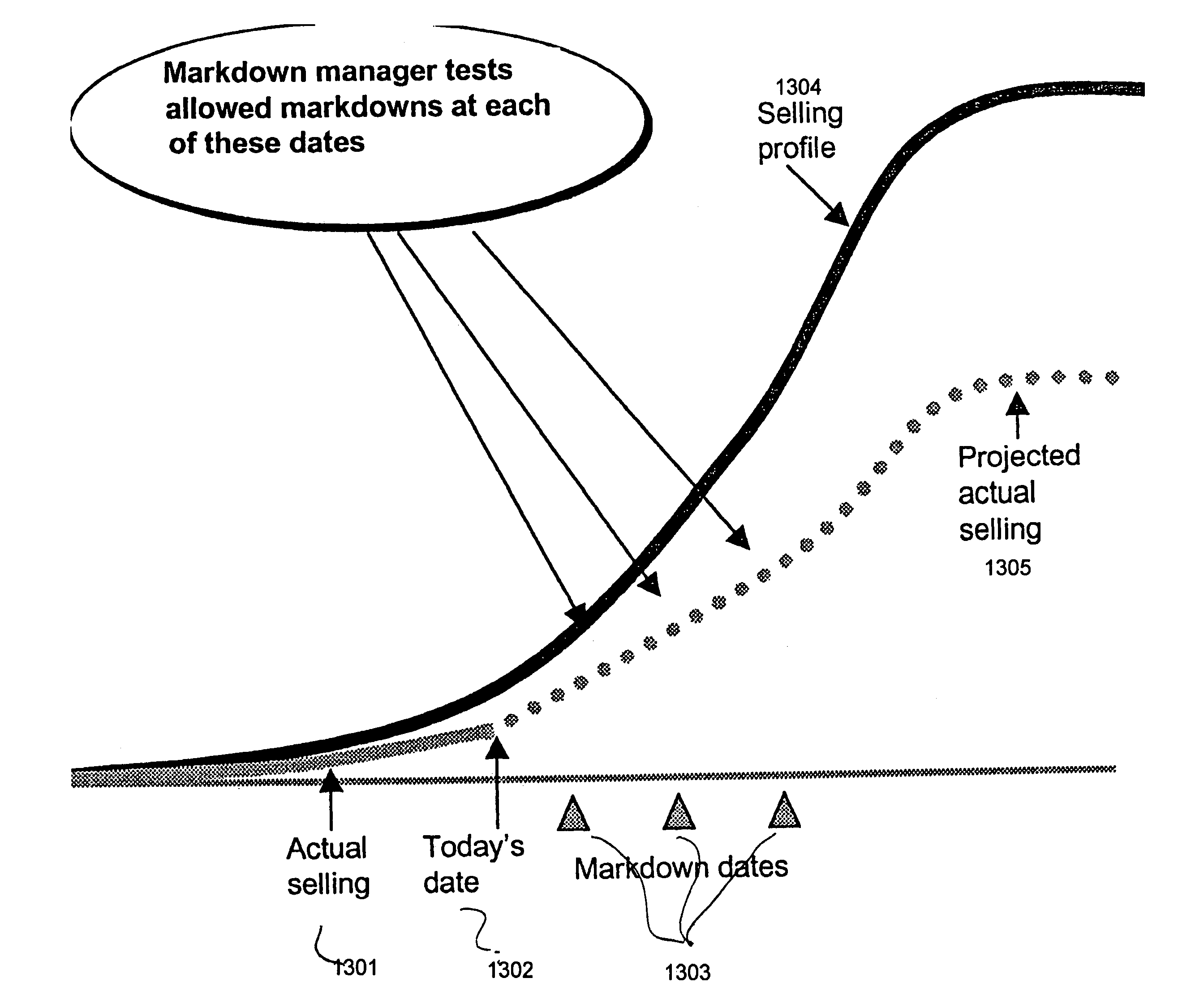
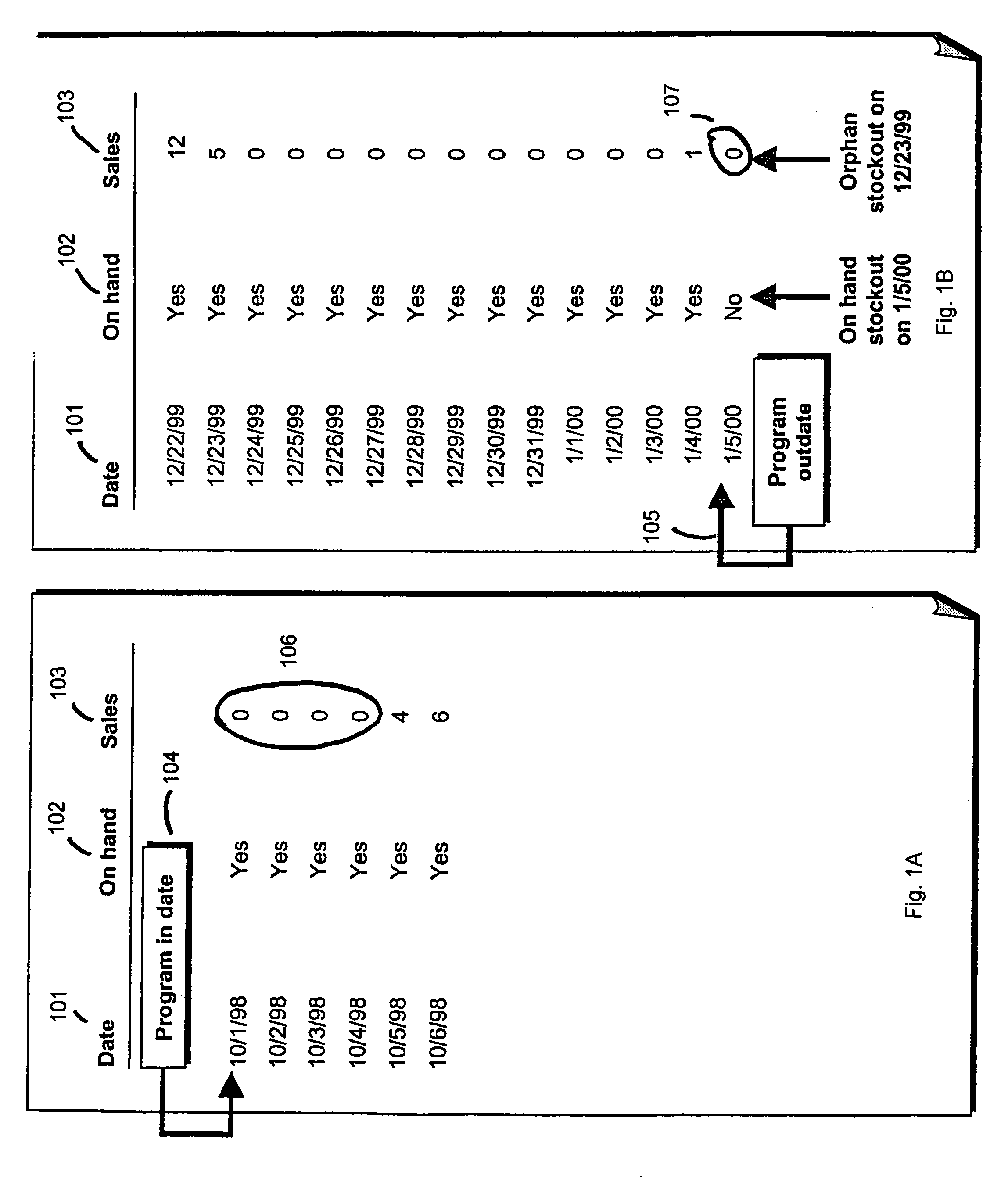
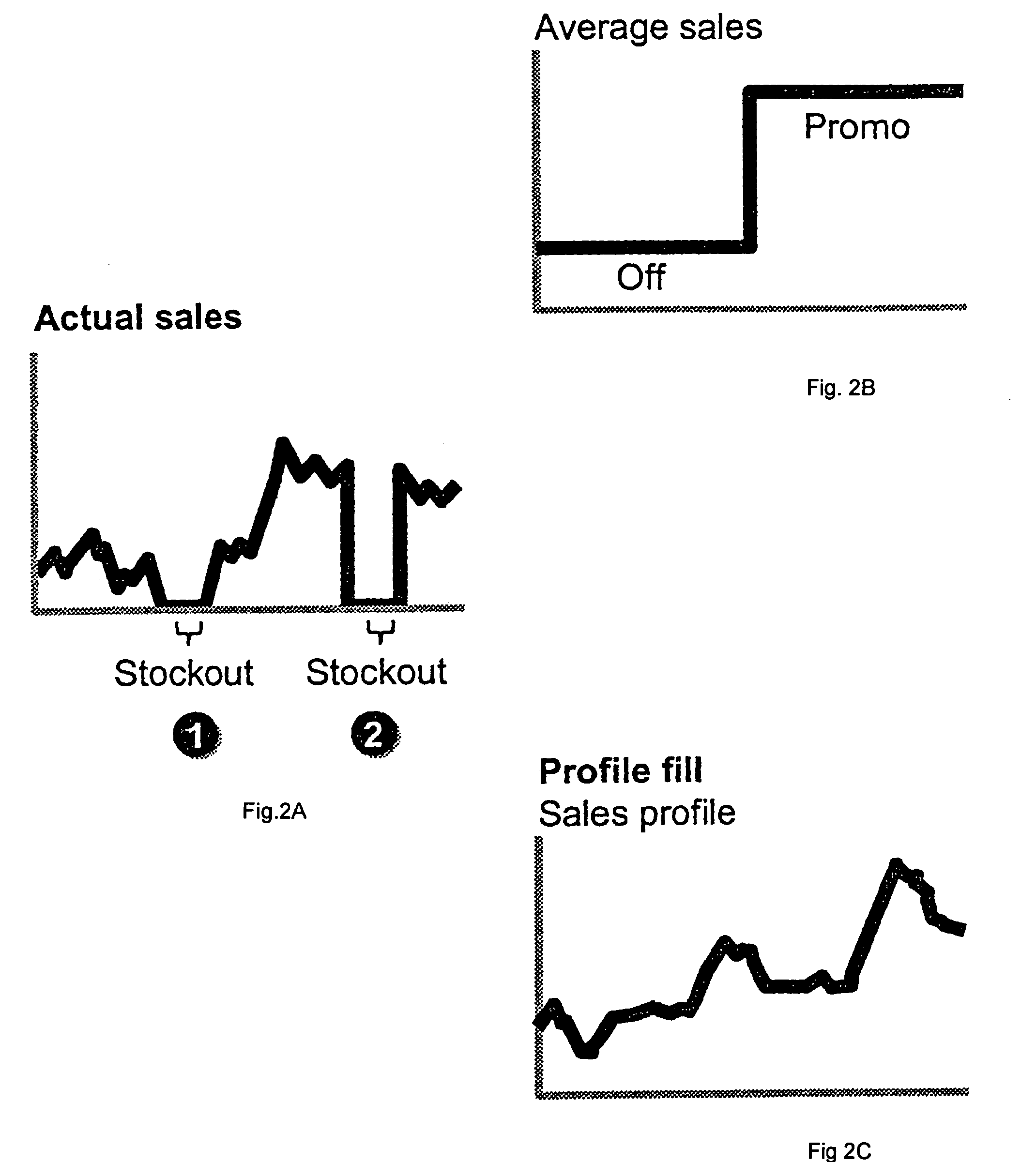
One embodiment practicing aspects of the present invention provides a computer-implemented method for adjusting a reference selling profile for a reference product, comprising retrieving one or more reference selling profiles corresponding to daily or more frequent historical data for one or more reference products, and adjusting the reference selling profiles to correct for one or more promotions which impacted the historical data. Other embodiments and aspects provide for determining location distribution shares, projecting sales, determining distribution quantities, comparing alternative markdown scenarios, etc.
Owner:BLUEFIRE SYST
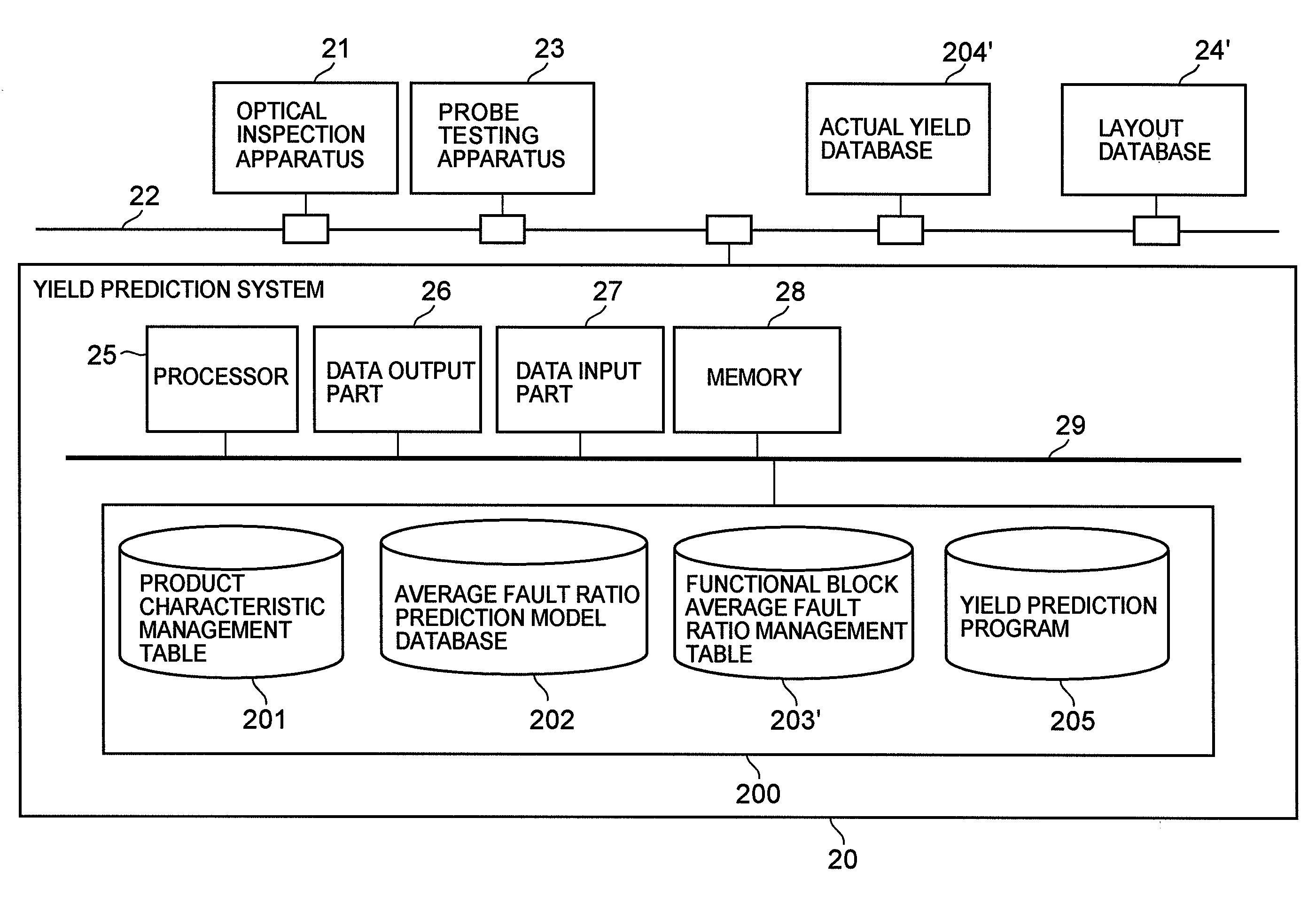
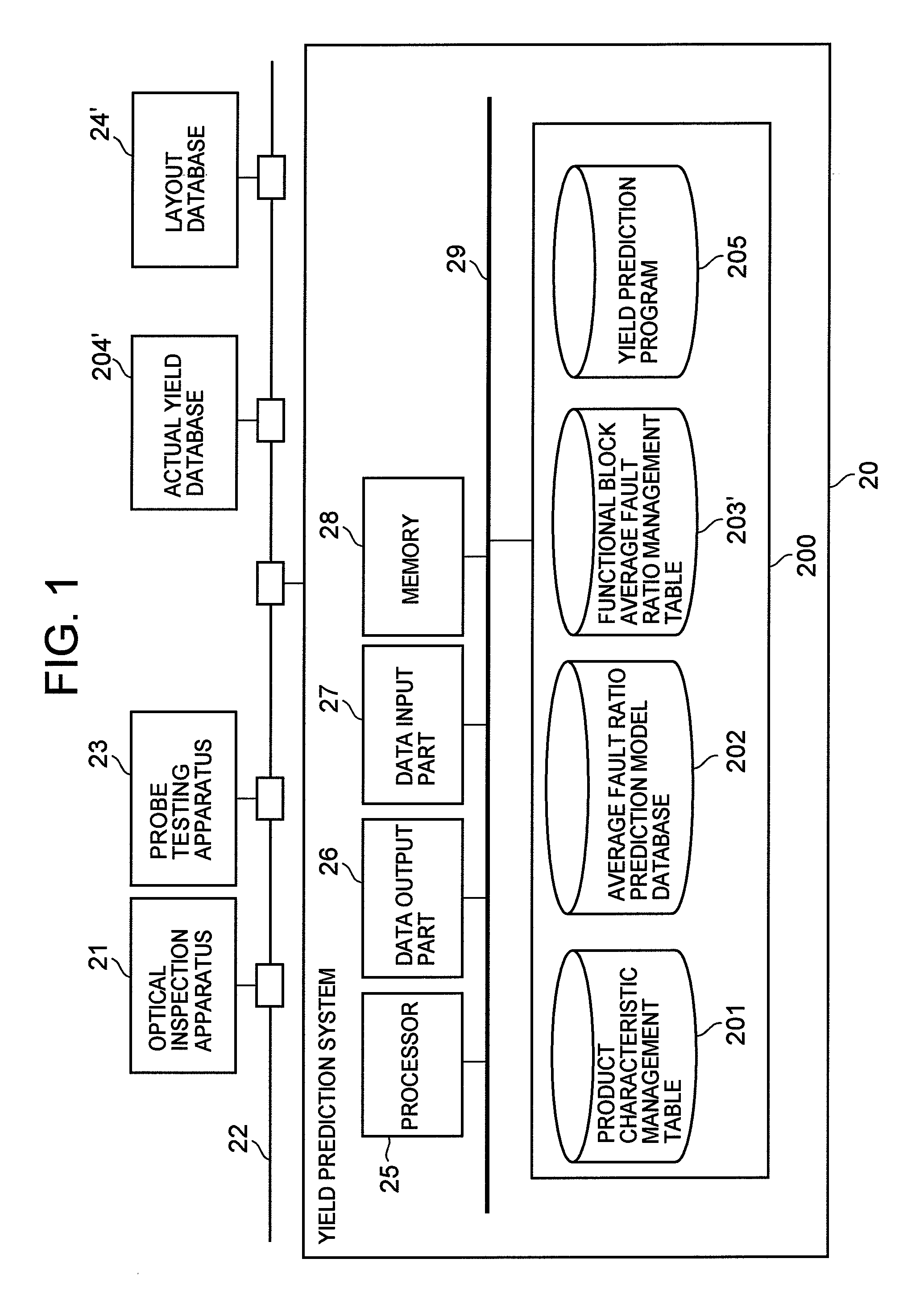
Semiconductor device yield prediction system and method
InactiveUS20080140330A1Predict yieldAccurate predictionSemiconductor/solid-state device testing/measurementComputer controlElectricityReference product
An average fault ratio is calculated from product characteristics of a product as a target of yield prediction, in order to predict yield accurately in the course of manufacturing the prediction target product.With respect to a reference product, whose wiring pattern is different from the prediction target product but manufactured by the same manufacturing process, a monthly electric fault density is calculated from actually measured data. Respective average fault ratios are obtained from product characteristics of the prediction target product and the reference product. A monthly electric fault density of the prediction target product is obtained by multiplying the monthly electric fault density of the reference product by the ratio of the average fault ratios. The yield is calculated by using the monthly electric fault density of the month in which a yield prediction target lot of the prediction target product was processed.
Owner:HITACHI LTD
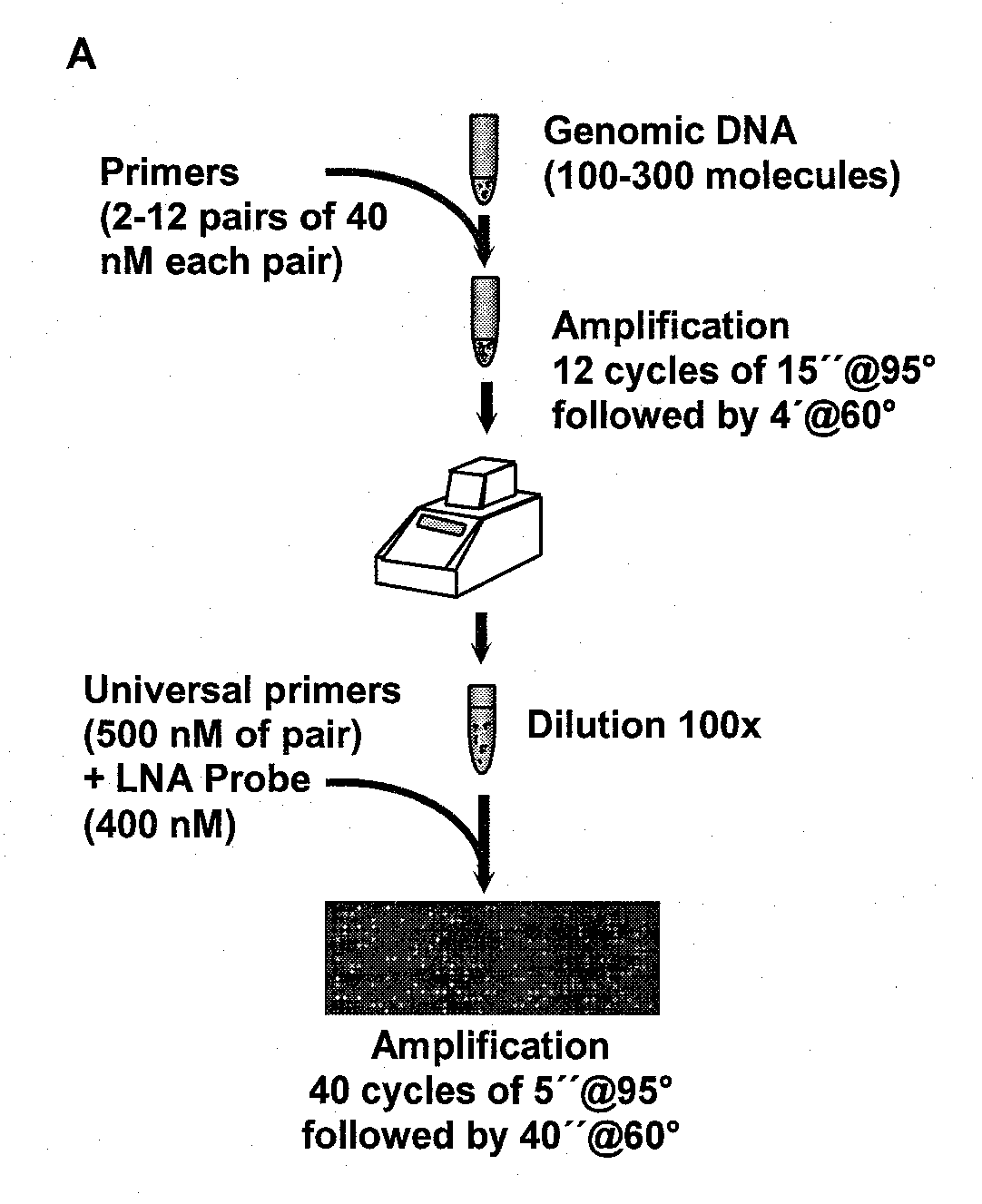
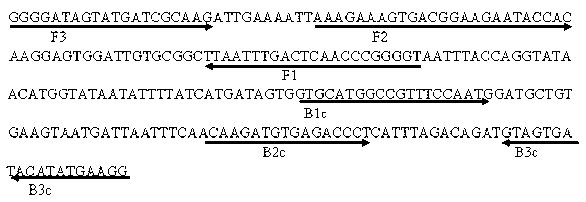
Multiplex Amplification for the Detection of Nucleic Acid Variations
InactiveUS20130022973A1High background noiseHigh cross reactivityMicrobiological testing/measurementFluorescence/phosphorescenceReference productBioinformatics
Owner:THE UNIV OF BRITISH COLUMBIA +1
Quantitative detection kit of hepatitis B virus (HBV) nucleic acid
ActiveCN103642941AAvoid PCR false negativesHighly conservativeMicrobiological testing/measurementMagnetic beadReference product
The invention discloses a quantitative detection kit of a hepatitis B virus (HBV) nucleic acid applied to the field of biomedical clinic diagnosis. The kit comprises a paramagnetic particle method extraction kit and an HBV nucleic acid amplification kit, wherein the paramagnetic particle method extraction kit comprises a pyrolysis binding solution, a rinsing solution, an eluant and magnetic bead liquid; the HBV nucleic acid amplification kit comprises an HBV-PCR (Polymerase Chain Reaction) reaction solution, an enzyme mixed solution, an HBV-interior label, HBV quantitative reference products 1-4, a negative quality product, a clinical positive quality product and a strong positive quality product. The quantitative detection kit is simple, convenient and fast in operation, low in cost, high in detection sensitivity, good in repeatability, high in conservative property of primer and probe, and strong in specificity, and covers different subtypes or variants of the hepatitis B virus, improvement of the accuracy and the specificity of the hepatitis B detection is facilitated, an efficient interior label system is led in, the problems such as reciprocal inhibition, interference and the like caused by simultaneous amplification of a target gene and the interior label are solved, the overall PCR amplification process can be effectively monitored, and a false negative result is avoided.
Owner:东北制药集团辽宁生物医药有限公司
Lipoprotein phospholipase A2 assaying reagent and preparation method thereof
ActiveCN103033629ASimple and fast operationRaw materials are easy to getBiological testingPhospholipase A2Reference product
The invention relates to a lipoprotein phospholipase A2 assaying reagent and a preparation method thereof, and aims to ensure the characteristics of high reagent accuracy and convenience in preparation method. The invention adopts the technical scheme that the lipoprotein phospholipase A2 assaying reagent comprises the following components: a, a lipoprotein phospholipase A2 reagent 1; b, a lipoprotein phospholipase A2 reagent 2; and c, a liquid lipoprotein phospholipase A2 reference product; the preparation method comprises the following steps: (1) for the lipoprotein phospholipase A2 reagent 1: uniformly mixing; (2) for the lipoprotein phospholipase A2 reagent 2: (1) taking a suspension, (2) reacting with a mixture, (3) obtaining the suspension, (4) regulating the concentration, (5) adding the suspension in step (3) into the solution obtained in the substep (4), (6) reacting, (7) adding ethanol amine, and (8) performing centrifugal treatment; and (3) for the liquid LP-PLA2 (lipoprotein phospholipase A2) reference product: mixing according to a formulation, arranging according to contents, or adding a purified product in the mixed solution.
Owner:YESEN BIOTECH SHANGHAI
Information feed in-line purchasing system
ActiveUS20140236762A1Payment architectureBuying/selling/leasing transactionsPaymentReference product
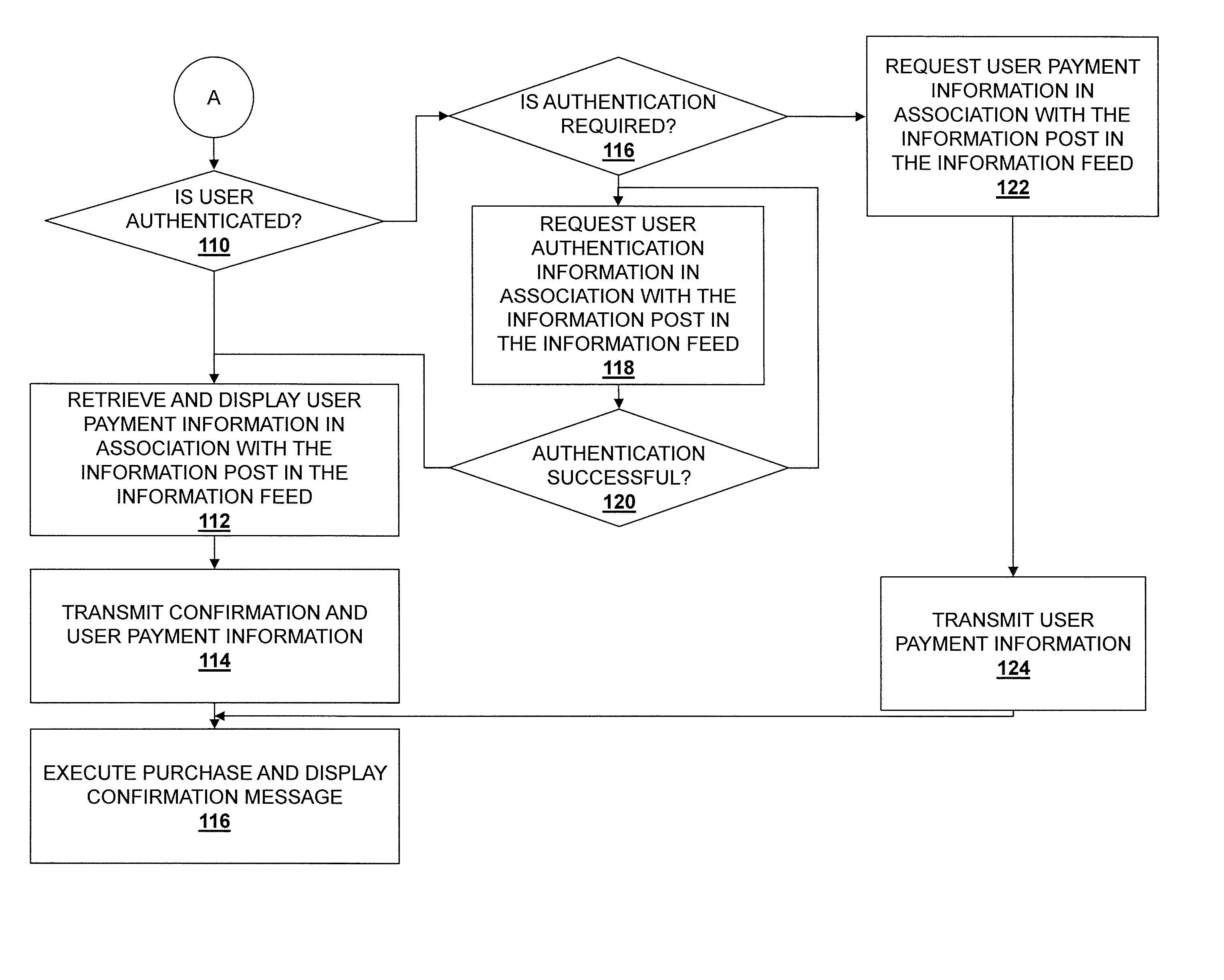
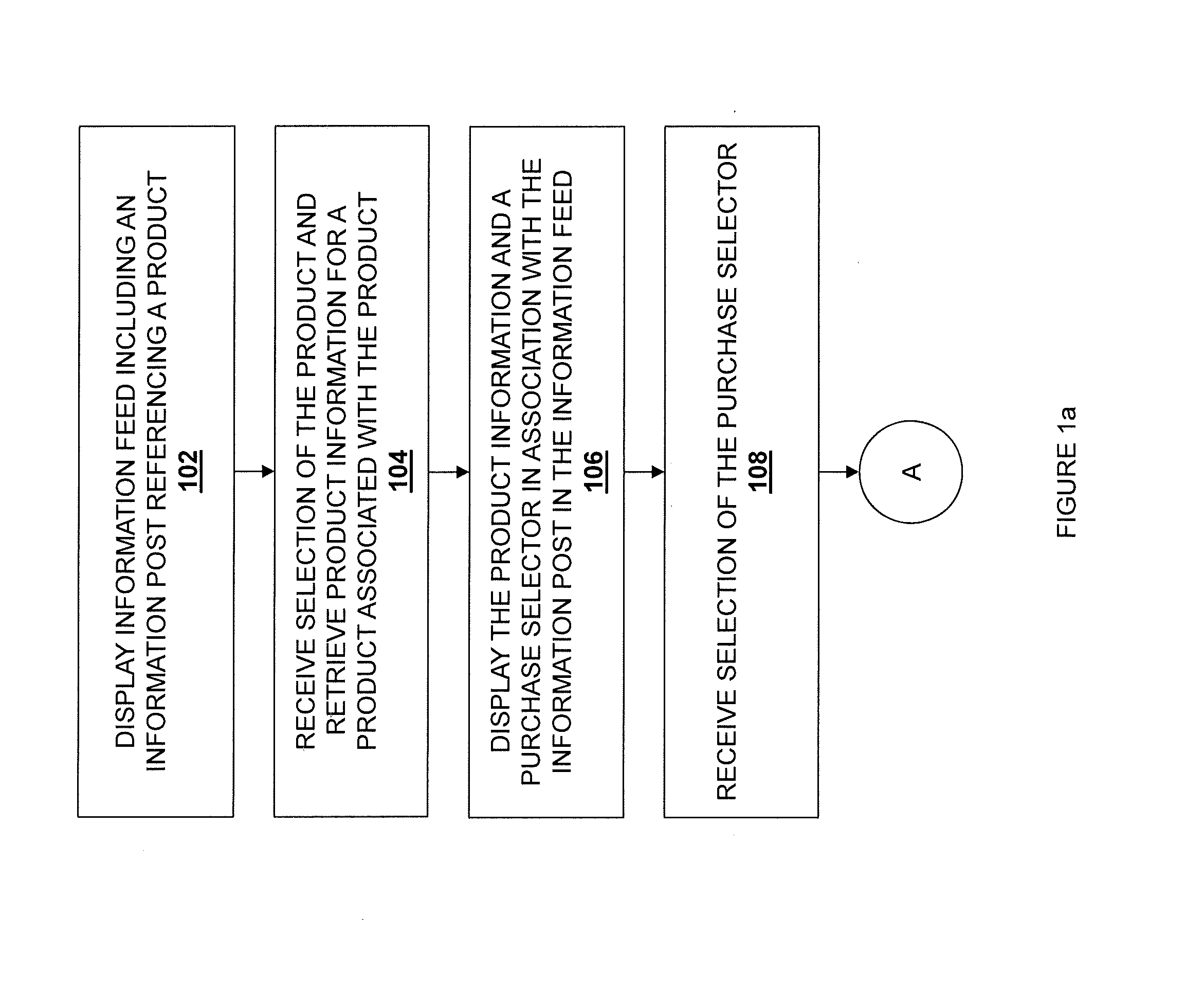
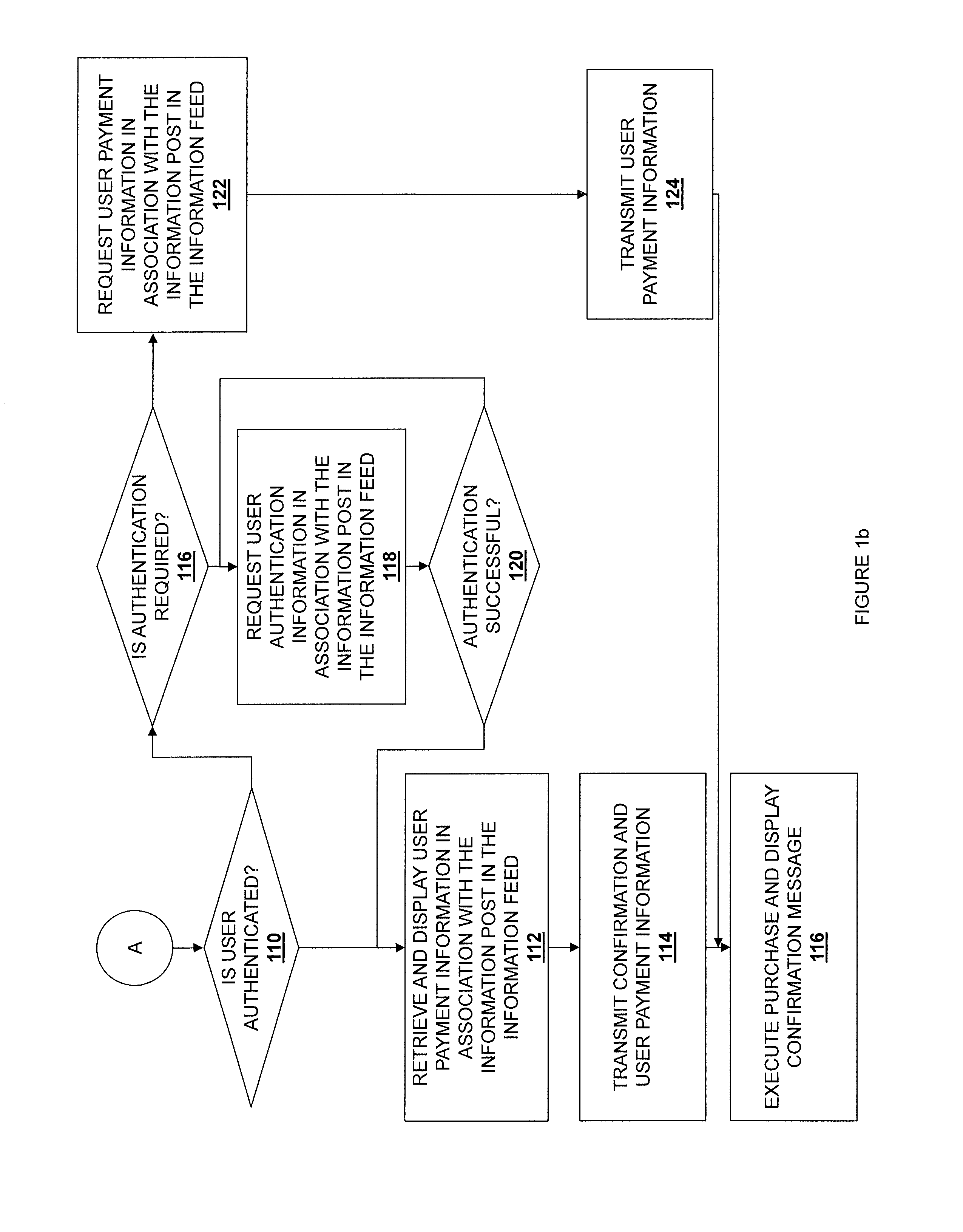
Embodiments described herein disclose a system and method for providing an improved user experience for purchasing a product within an information feed. An information feed is displayed which includes an information post having a product link or otherwise referencing a product. A selection of the product link or the referenced product is received, and product information for a product associated with the product link or referenced product is retrieved. The product information and a purchase selector are displayed within the information feed, in association with the information post. A selection of the purchase selector is received. User payment information is retrieved and displayed in association with the information post in the information feed. User payment information is transmitted, based on a received confirmation from a user. The purchase is then executed, and a confirmation message may be displayed.
Owner:PAYPAL INC
Systems and methods for deep learning model based product matching using multi modal data
ActiveUS10949907B1Character and pattern recognitionBuying/selling/leasing transactionsData classReference product
Methods and systems for generating a list of products each matching a reference product are disclosed. A user query is first received, and multi-modal attribute data for the reference product are determined, with each data mode being a type of product characterization having a modality selected from a text data class, categorical data, a pre-compared engineered feature, audio, image, and video. Next, a first list of candidate products is determined based on a product match signature, and a second list of candidate products is generated from the first, wherein for at least one given candidate product, a deep learning multi-modal matching model is selected to determine whether a match is found. Lastly, the second list is filtered to remove outliers and to generate the list of matching products. Also disclosed are benefits of the new methods and systems, and alternative embodiments of the implementation.
Owner:PRICE TECH INC
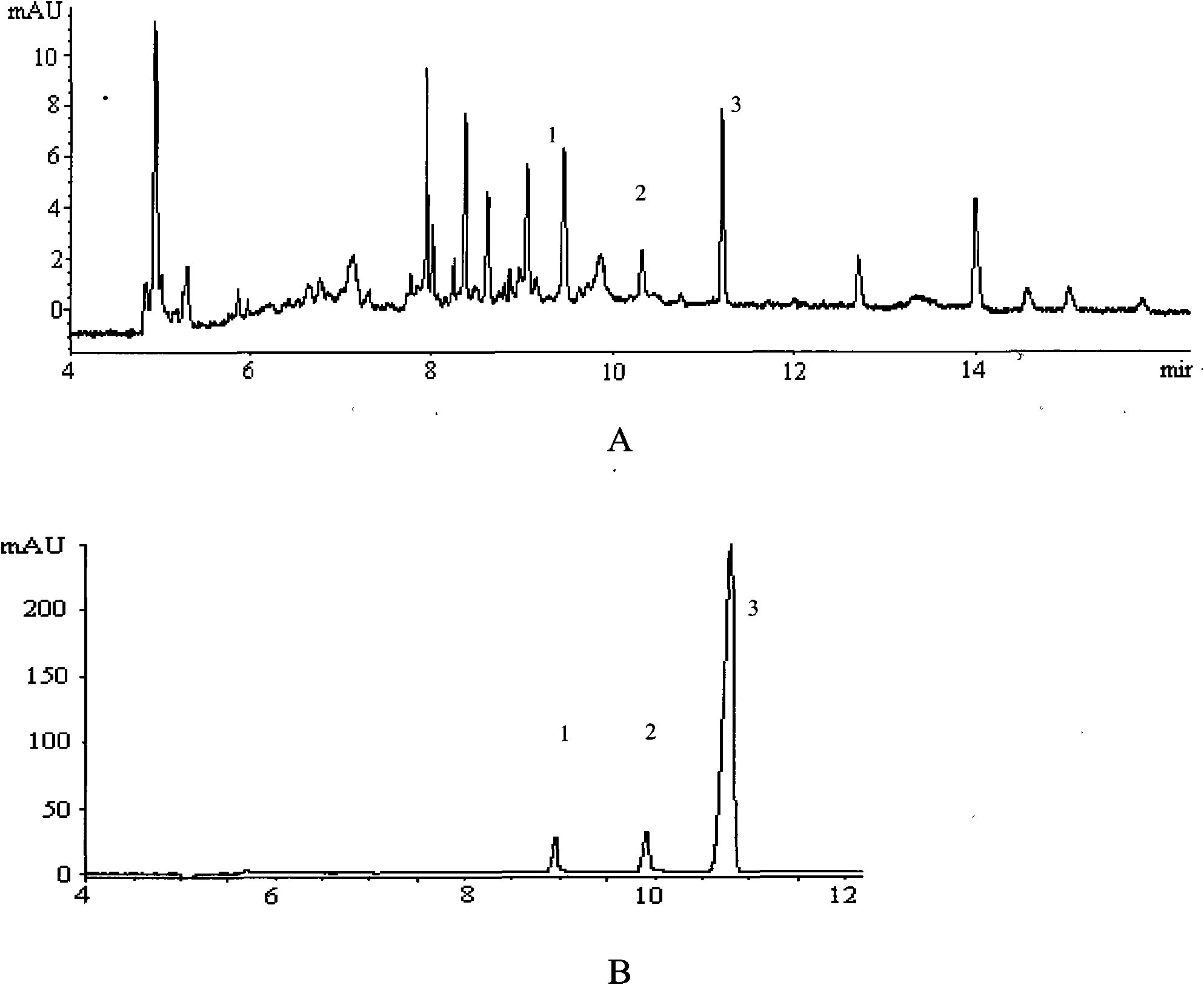
Method for simultaneously determining contents of chlorogenic acid, caffeic acid in dandelion preparation by HPLC
InactiveCN101324546AHigh recovery rateHigh sensitivityComponent separationChlorogenic acidHplc method
The invention relates to a method for simultaneously measuring the content of chlorogenic acid and caffeic acid in a dandelion preparation by utilizing the HPLC method. The measurement method can effectively solve the problem that the chlorogenic acid and the caffeic acid are taken as the index components to simultaneously carry out the content measurement in order to lay the foundation for the quality standard of the dandelion preparation. The specific technical proposal comprises the following steps: respectively preparing a sample solution to be measured and a reference product solution, gradient-eluting with the RP-HPLC method by taking the methanol-phosphoric acid water as the mobile phase and measuring the content of the chlorogenic acid and the caffeic acid. The method has the advantages of convenience, reliability, rapidness, high sensitivity, good repeatability and high recovery rate, takes the chlorogenic acid and the caffeic acid as the index components, lays the foundation for the quality standard establishment of dandelion and the preparation thereof and provides more scientific basis.
Owner:HENAN UNIV OF CHINESE MEDICINE
Quality analysis method of compound Ganmaoling tablets
ActiveCN101961430ASave inspection timeSave inspection costComponent separationAntiinfectivesChlorogenic acidReference product
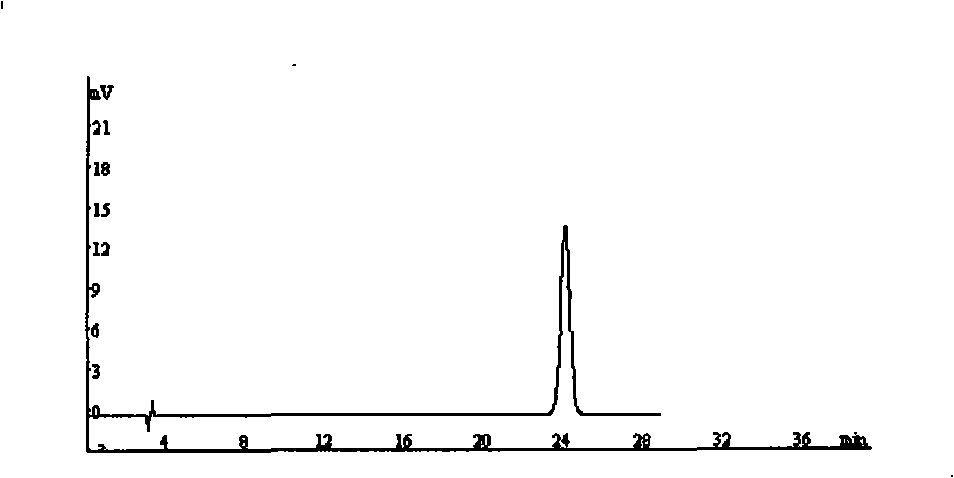
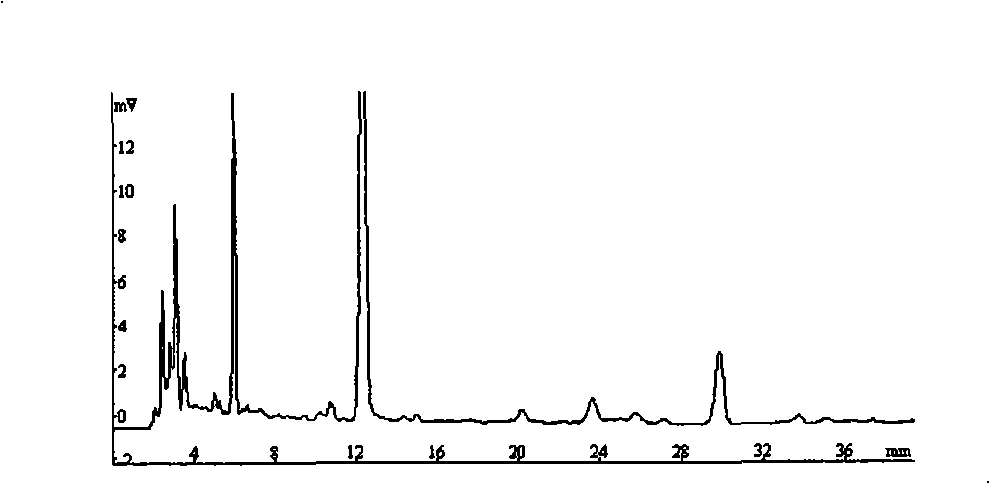
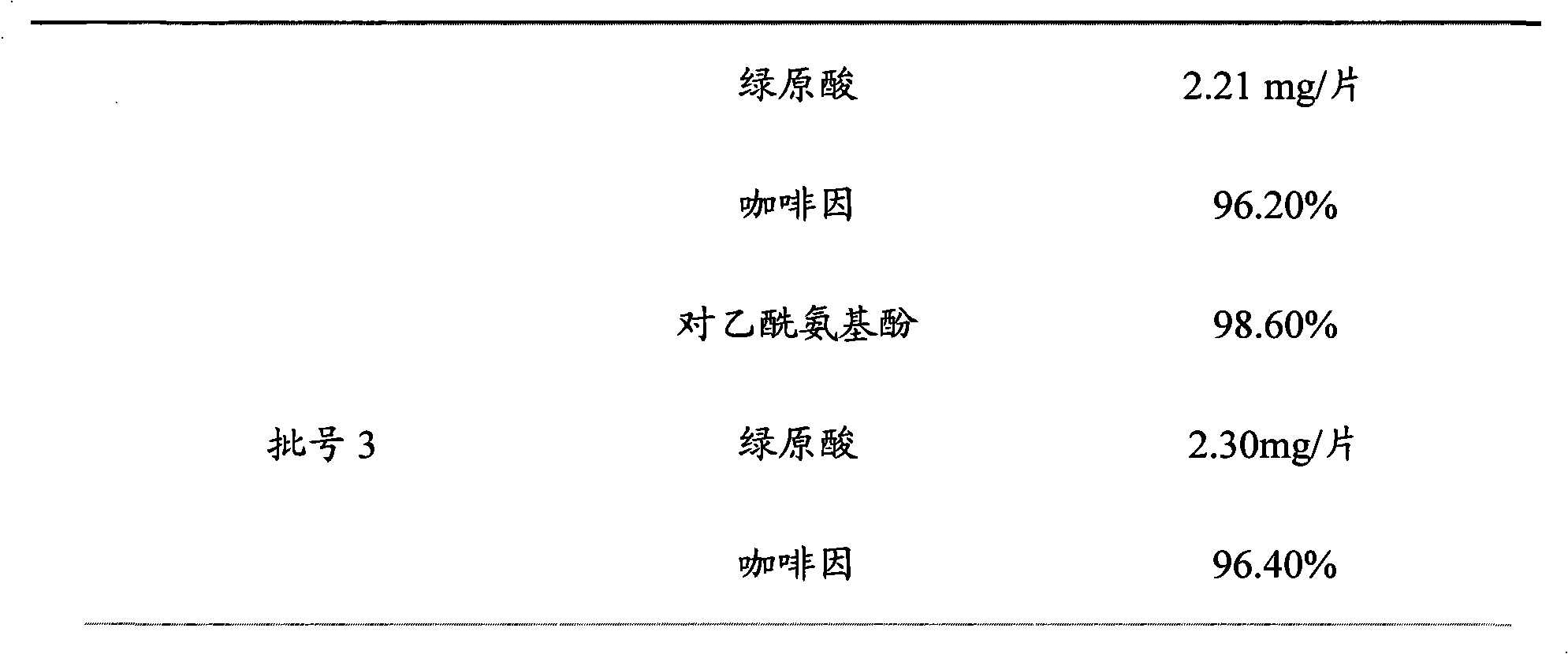
The patent refers to the field of 'pharmaceutical preparations'. The invention discloses a quality analysis method of compound Ganmaoling tablets. In the method, high-performance liquid chromatography (HLPC) is used for measure the chlorogenic acid content, acetaminophen content and caffeine content of the compound Ganmaoling tablets, test product solution is prepared and reference product solution is prepared and measured. The quality analysis method of the invention can detect the acetaminophen, chlorogenic acid and caffeine components under the same color spectrum condition at the same time, so the test time and cost are saved and the working efficiency is improved; the test method has high sensitivity, high separating degree; the basic line is stable; a negative reference is not affected; and the accuracy, repeatability, linearity and stability of the test method meet scientific research and production requirements, and the detection method is suitable for promotion and application.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
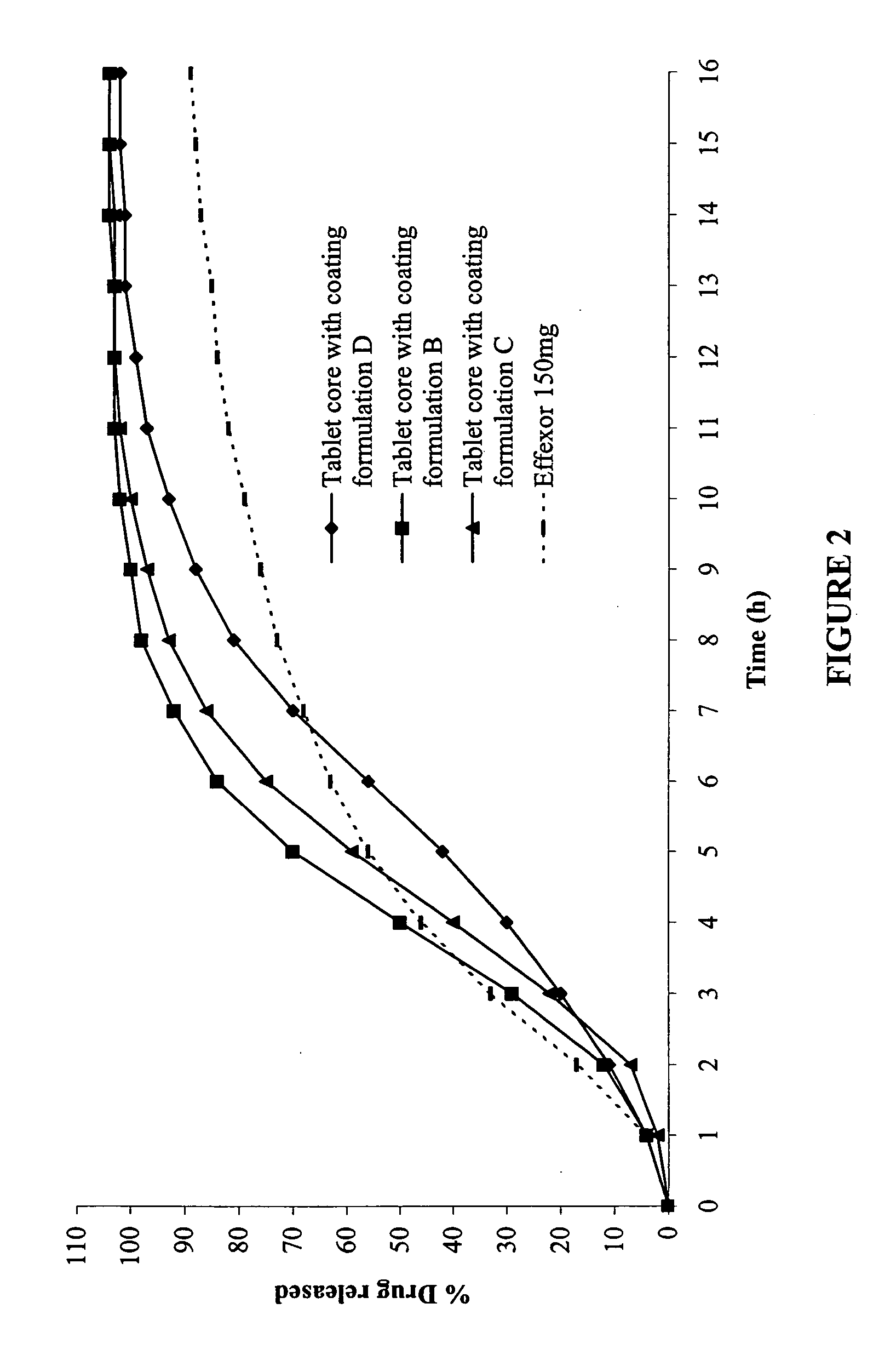
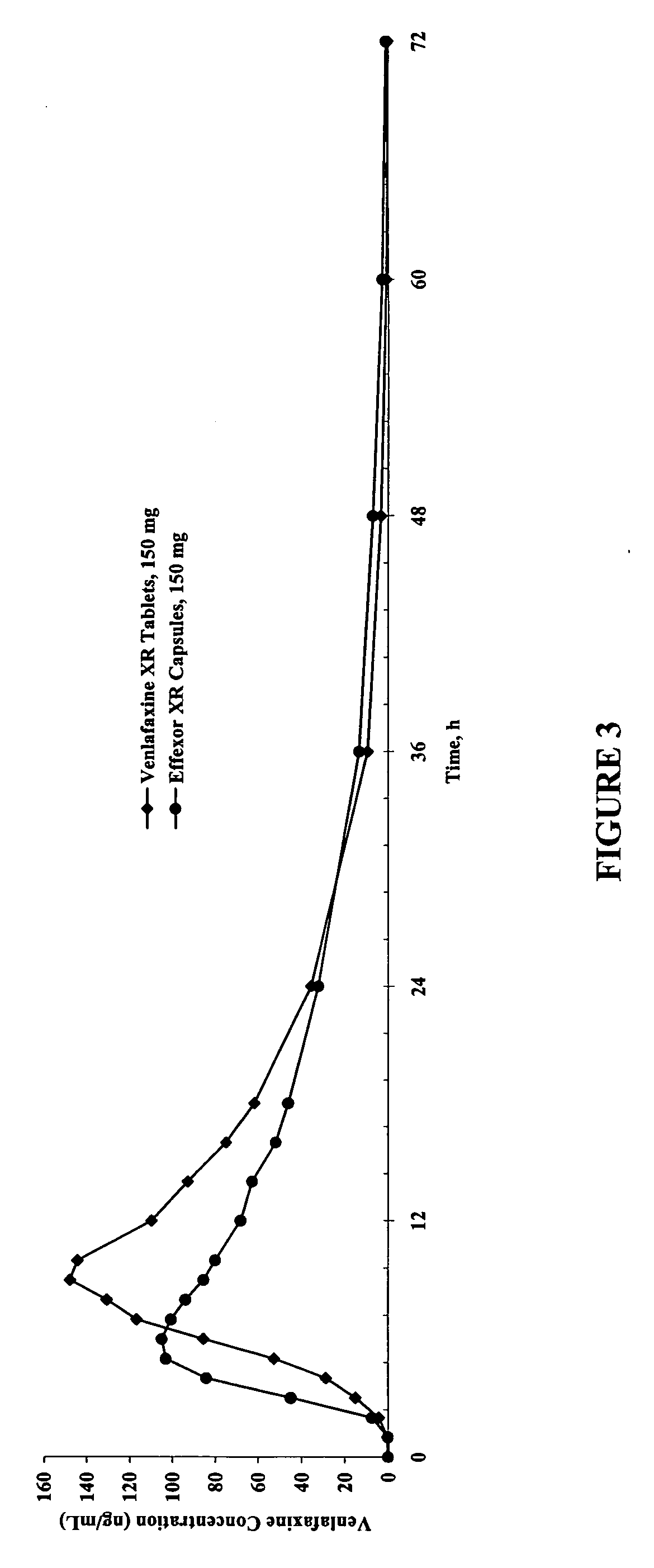
Modified-release compositions of at least one form of venlafaxine
InactiveUS20050244498A1Reduce morbidityReduce releaseBiocideAnimal repellantsControlled releaseOral medication
The present invention relates to a modified release composition of at least one form of venlafaxine, which is an enhanced absorption delayed controlled release composition for oral administration suitable for once daily dosing. The composition comprises a core comprising at least one form of venlafaxine selected from the group consisting of venlafaxine, an active metabolite of venlafaxine, a pharmaceutically acceptable salt of venlafaxine, a pharmaceutically acceptable salt of an active metabolite of venlafaxine, and combinations thereof, and a pharmaceutically acceptable excipient. The composition further comprises a modified release coating which substantially surrounds the core. The compositions of the invention provide enhanced absorption delayed controlled release of the at least one form of venlafaxine such that the combined geometric mean ratio of the composition of the invention to the reference product for the AUC0-t or the Cmax for venlafaxine and its active metabolite O-desmethylvenlafaxine is greater than 2 after first administration of the composition under fed or fasting conditions.
Owner:BIOVAIL LAB INT SRL
FFPE reference product for gene detection and preparation method and application of FFPE reference product
PendingCN109628595AHigh simulationAchieve accuracyMicrobiological testing/measurementDNA/RNA fragmentationMutation frequencyReference product
The invention discloses an FFPE reference product for gene detection and a preparation method and application of the FFPE reference product. The preparation method comprises the following steps that S1, cell culture is performed on a tumor cell line, and cell pellets are collected; S2, formalin fixation is performed on the cell pellets, then sepharose gel wraps the cell pellets to form cell aggregates, and the cell aggregate are prepared into cell paraffin blocks; S3, genomic DNA of the tumor cell line in the cell paraffin blocks is extracted, and genetic mutation frequency determination is performed on the genomic DNA of the tumor cell line; S4, the genomic DNA of the tumor cell line containing a target mutation site is mixed into a DNA mixture of a target mutation frequency, and the DNAmixture is the FFPE reference product for gene detection. According to the technical scheme, formalin fixation and paraffin wrapping are performed on the cells cultured by tumor cell line, so that thesituation of clinical samples can be better simulated.
Owner:ZHENYUE BIOTECHNOLOGY JIANGSU CO LTD
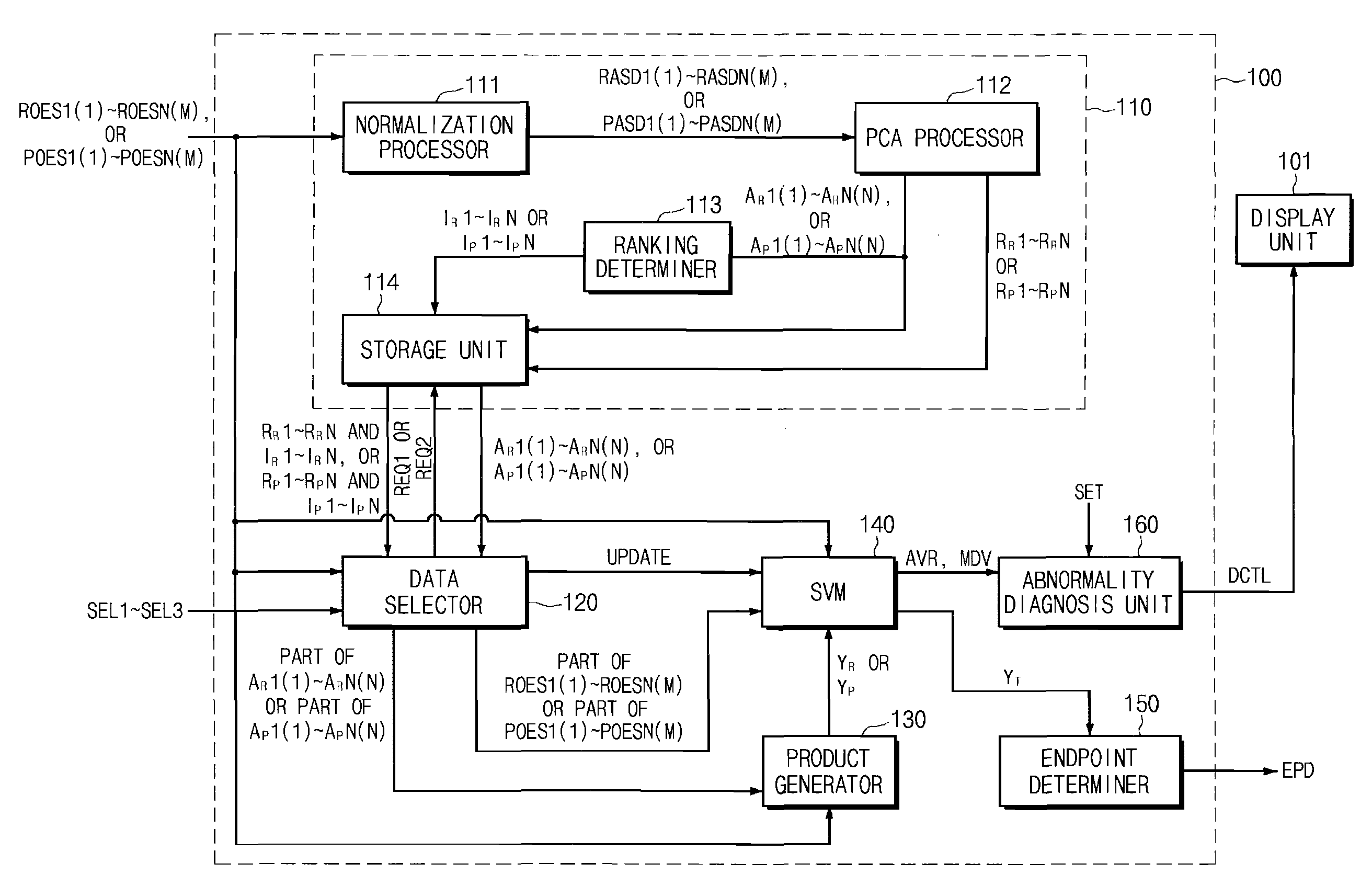
Endpoint Detection Device For Realizing Real-Time Control Of Plasma Reactor, Plasma Reactor With Endpoint Detection Device, And Endpoint Detection Method
InactiveUS20090029489A1Solve the detection speed is slowRealize real-time controlLiquid surface applicatorsElectric discharge tubesMultiplexerReference product
An endpoint detection device, a plasma reactor with the endpoint detection device, and an endpoint detection method are provided. The endpoint detection device includes an OES data operation unit, a data selector, a product generator, an SVM, and an endpoint determiner. The OES data operation unit processes reference OES data by normalization and PCA. The data selector selects part of the linear reference loading vectors and selects part of the selected linear reference loading vectors. The product generator outputs at least one reference product value. The SVM performs regression and outputs a prediction product value. The endpoint determiner detects a process wafer etch or deposition endpoint and outputs a detection signal.
Owner:DMS CO LTD
Herpes virus type I PCR (polymerase chain reaction) fluorescence quantitative rapid test kit and method
ActiveCN102337355AGuaranteed credibilityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesReference productFluorescence
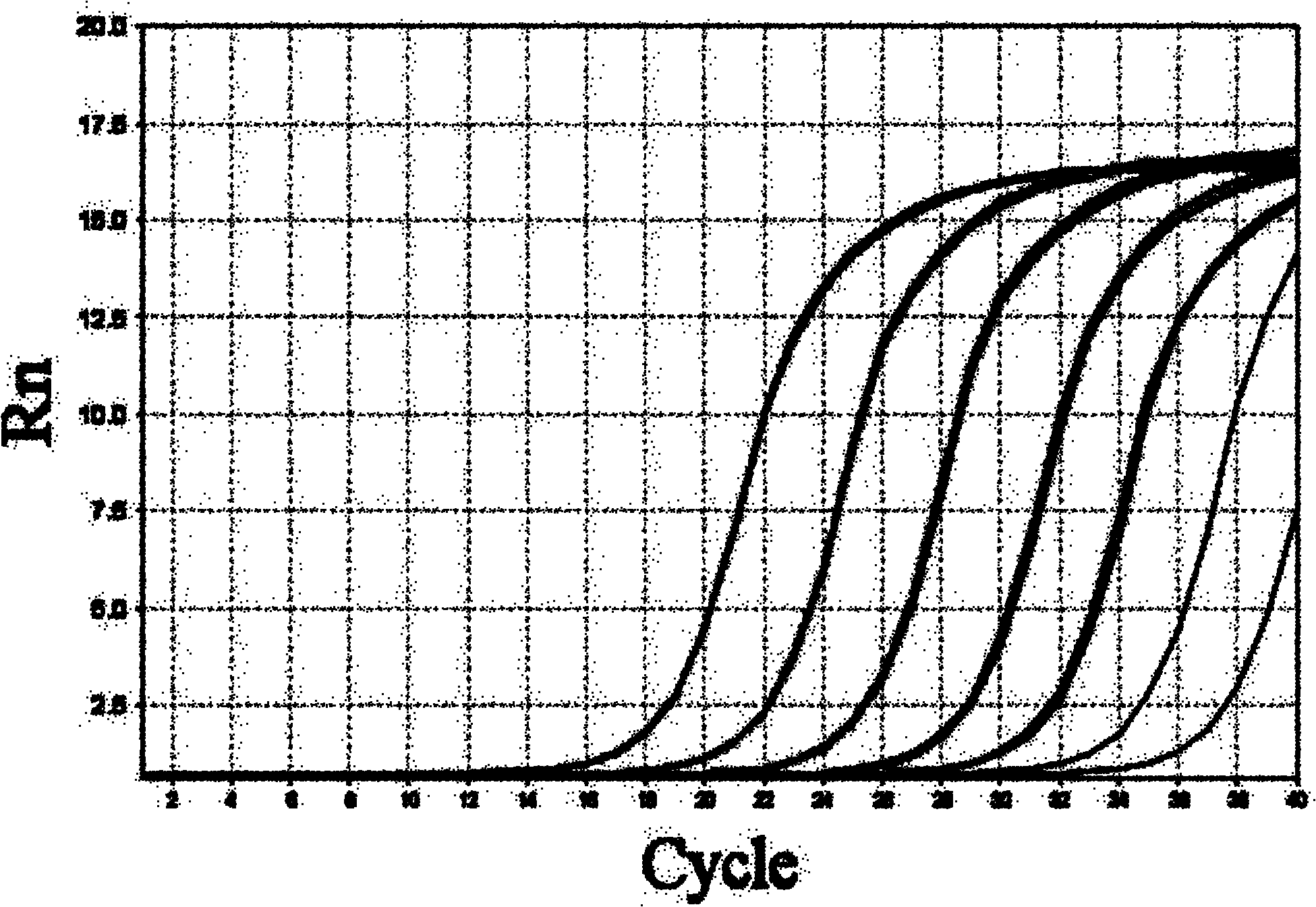
The invention aims at providing a herpes virus type I PCR (polymerase chain reaction) fluorescence quantitative rapid test kit which can detect the specific nucleic acid sequence of pure herpes virus type I in a clinical sample and further achieve the purpose of rapidly judging the existence of the pure herpes virus type I. In order to realize the purpose, the technical scheme of the invention isas follows: the herpes virus type I PCR fluorescence quantitative rapid test kit provided by the invention comprises a DNA (deoxyribonucleic acid) extraction solution, a PCR reaction solution, a DNA polymerase, a positive quality control product, a weak positive quality control product, a negative quality control product and a quantitative reference product, wherein the PCR reaction solution comprises primers and a fluorescent probe, and the primers are divided into an upstream primer and a downstream primer. The herpes virus type I PCR fluorescence quantitative rapid test kit has the beneficial effect of filling in the blank of a fluorescence PCR kit for clinically detecting the pure herpes virus type I (HSV I) in China. Furthermore, a Taqman core technology platform and an arabidopsis thaliana internal control system are utilized for detecting the pure herpes virus type I (HSV I), so that the herpes virus type I PCR fluorescence quantitative rapid test kit has the advantages of highsensitivity, high specificity, stability, timeliness, convenience in operation and the like.
Owner:泰普生物科学(中国)有限公司
Kit for in vitro detection of anti-cyclic citrullinated peptide (CCP) antibody and preparation method thereof
InactiveCN102323402ANo biological toxicitySignificant technological progressMaterial analysisAnti ccp antibodiesImmunosorbents
The invention belongs to the field of biological engineering, and provides a kit for in vitro detection of an anti-cyclic citrullinated peptide (CCP) antibody, which comprises a detection plate, a CCP antigen, a colloidal gold-labeled recombinant gold staphylococcus aureus A protein conjugate, a confining liquid, a washing liquid, a positive reference product and a negative reference product. The invention also provides a preparation method of the kit for the in vitro detection of the anti-CCP antibody. The kit adopts an indirect method immunosorbent assay principle to detect the anti-CCP antibody in a human serum, the CCP antigen is coated on a nitrocellulose membrane to be made into a solid-phase antigen for capturing the anti-CCP antibody in the human serum, and then colloidal gold is labeled on staphylococcus aureus A protein (SPA) to form the colloidal gold conjugate as a tracer; and if the detected human serum contains the anti-CCP antibody, then a solid phase antigen-anti-CCP antibody-colloid gold conjugate is formed and has red spots. The invention can be applied to the auxiliary diagnosis of rheumatoid arthritis.
Owner:上海精臻生物科技有限公司
Quantitative detection method of bovine-derived and porcine-derived components based on droplet digital PCR (polymerase chain reaction) as well as primer, probe and kit
InactiveCN106676189AOvercoming a series of shortcomings of real-time fluorescent quantitative PCRStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceReference product
The invention relates to the field of molecular biology and discloses a quantitative detection method of bovine-derived and porcine-derived components based on droplet digital PCR (polymerase chain reaction) as well as a primer and probe combination and a corresponding kit used by the method. The sequences of the primer and the probe are SEQ ID NO. 1-6. The method is applied to quantitative detection of bovine components in beef or beef products doped with pork; the defect that real-time fluorescence PCR quantitative detection needs to rely on a standard product is overcome; the method is capable of calculating the accurate content of one certain meat component by accurately measuring nucleic acid copy numbers of two meat components under the condition without any standard product or reference product; the method has extremely important practical significance for cracking down on counterfeit goods in the market and safeguarding the rights of consumers.
Owner:珠海出入境检验检疫局检验检疫技术中心
Kit for detecting aneuploidy of five human chromosomes through monotube multiple amplification
ActiveCN103555849AHigh detection sensitivityStrong specificityMicrobiological testing/measurementTrisomy 13 SyndromeReference product
The invention relates to a kit for detecting the STR (Short Tandem Repeat) genetype of human chromosomes 21, 18 and 13 and sex chromosomes, and particularly relates to a QF-PCR (Quantitative Fluorescence-Polymerase Chain Reaction) kit for detecting the number of the chromosomes 21, 18 and 13 and the sex chromosomes by adopting five-color fluorescence labeling monotube fast multiple amplification and mainly for diagnosing 21 trisomy syndrome, 18 trisomy syndrome, 13 trisomy syndrome and the aneuploid abnormality of the sex chromosomes. The kit comprises a primer mixture, a hot start C-Taq enzyme, an amplification reaction solution, a positive quality control product, a negative reference product, a fluorescence interior label Siz-500 and an allelic gene typing standard substance. Compared with the traditional antenatal diagnosis method, the kit disclosed by the invention can realize high-flux, fast, reliable and standardized detection.
Owner:AGCU SCIENTECH +1
Leptin detection kit
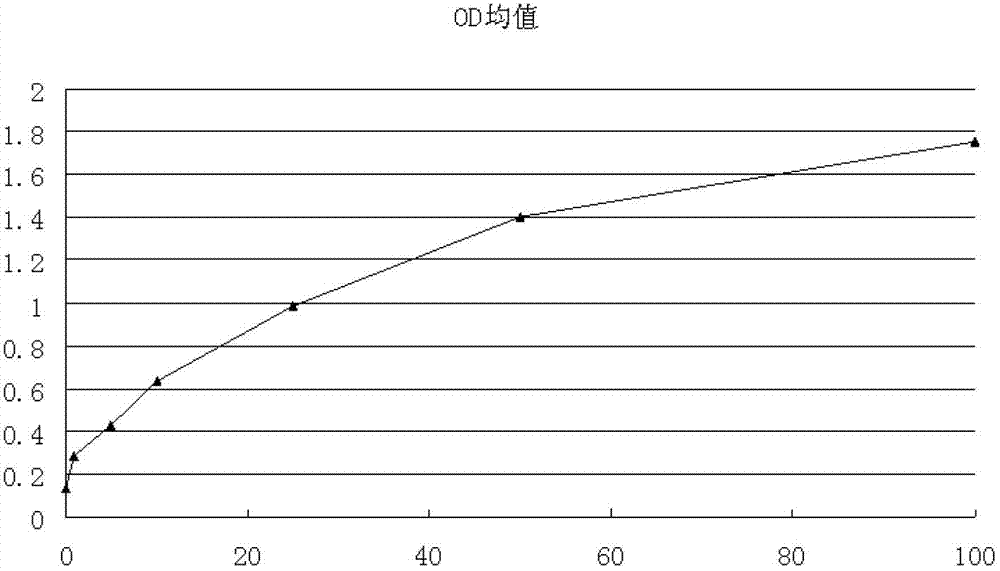
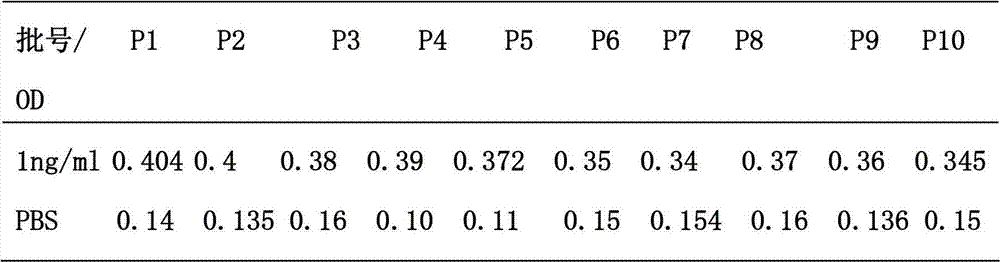
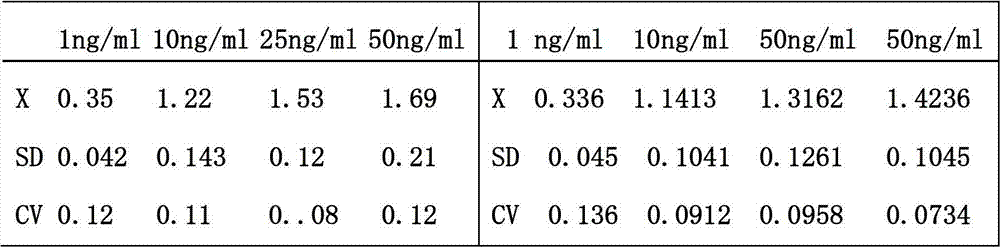
The invention relates to a leptin detection kit. The leptin detection kit comprises a leptin reference product, an antibody-coated microtiter plate, an enzyme-labeled anti-leptin, a washing solution, a color development solution and a stop solution, wherein the leptin reference product is of a recombinant human leptin polypeptide antigen, and the antibody-coated microtiter plate and the enzyme-labeled anti-leptin are of recombinant human leptin polypeptide monoclonal antibodies. The detection principle of the leptin detection kit disclosed by the invention is as follows: the recombinant human leptin polypeptide monoclonal antibody is taken as the antibody-coated microtiter plate, a serum sample to be detected is added, the corresponding enzyme-labeled antibody is further added, a compound of the coating antibody, the serum sample to be detected and the enzyme-labeled antibody is generated, the color development solution is added for color development, then the stop solution is further added for stopping, the residual solution is washed away by the washing solution, the light absorption value of the serum sample to be detected is determined, and the leptin content in the serum sample to be detected can be obtained by comparing with a standard curve.
Owner:ANHUI ANKE BIOTECHNOLOGY (GRP) CO LTD
Kit for detecting DNA residues of CHO cell and using method thereof
ActiveCN102181530AHigh sensitivityReliable Quality Control EvidenceMicrobiological testing/measurementFluorescenceReference product
The invention relates to a kit for detecting deoxyribose nucleic acid (DNA) residues of a Chinese hamster ovary (CHO) cell and a using method thereof. The kit comprises DNA extracting solution, polymerase chain reaction (PCR) amplification reaction liquid, a DNA quantitative reference product of a CHO cell genome, a negative quality control product, a positive quality control product and DNA diluent. In the kit, EvaGreen is used as a fluorescent dye, and the DNA of the CHO cell genome is detected by a real-time quantitative PCR technology; and products such as a treatment protein medicament, a recombinant vaccine, a monoclonal antibody and the like from the CHO cell can be accurately and quantitatively detected.
Owner:SHANGHAI HENLIUS BIOTECH INC
Panax root extract detection method
InactiveCN102048777AImprove accuracyHigh sensitivityNervous disorderDigestive systemReference productSyringin
The invention relates to a medicament detection method, in particular to a panax root extract detection method. The detection method comprises: measuring the content of a syringin component; and comparing the intermediate infrared one-dimensional spectrum of the panax root extract and the intermediate infrared one-dimensional spectrum of a reference product.
Owner:TIANJIN TASLY MORDEN TCM RESOURCES
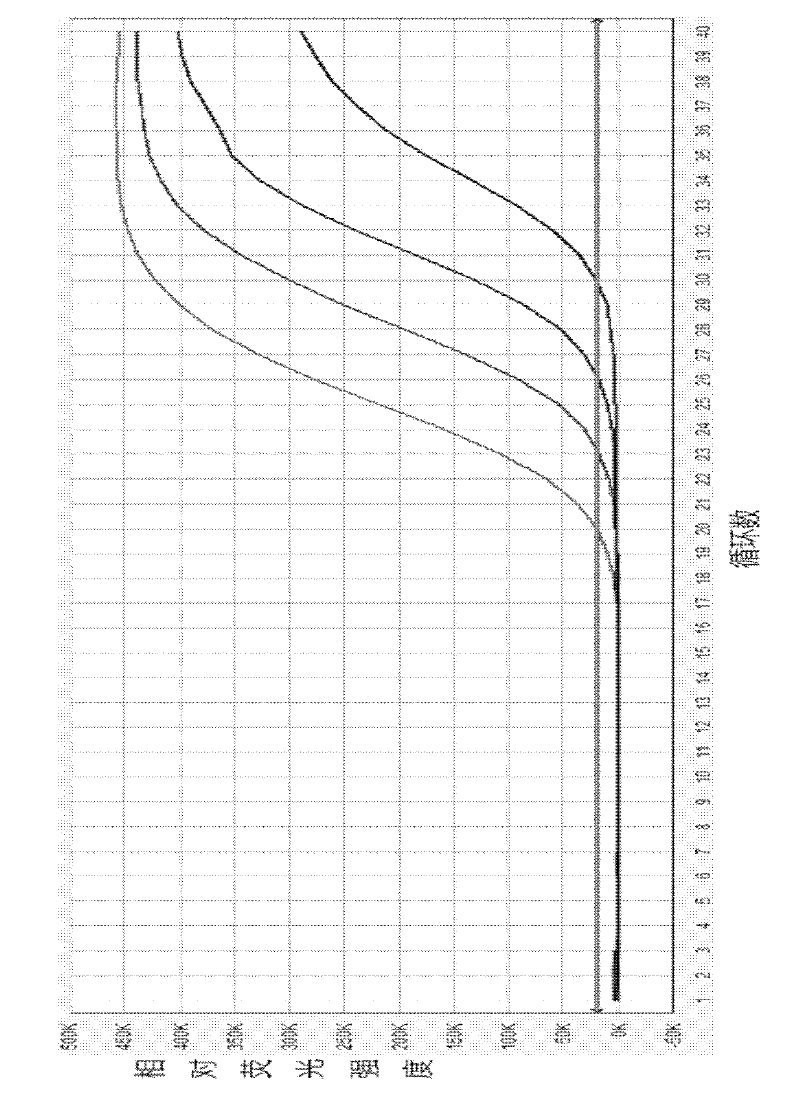
Method for acquiring reference product yield of new product on production line
InactiveCN102110584AAccurate Inherent Defect DensitySemiconductor/solid-state device manufacturingProduction lineReference product
The invention provides a method for acquiring the reference product yield of a new product on a production line. The method comprises the following steps of: introducing yield screening, overkill rate compensation and a defect density correction coefficient; substituting in a defect density formula to obtain a defect density correction formula; and calculating to acquire the intrinsic defect density of the production line so as to accurately acquire the reference product yield of the new product on the production line.
Owner:SEMICON MFG INT (SHANGHAI) CORP +1
Fluorescent standard card and test method for calibration and quality control of fluorescent immunoassay analyzer
InactiveCN109633189ALinear dependencies can be assessedImprove consistencyFluorescence/phosphorescenceReference productEngineering
The invention discloses a fluorescent standard card for calibration and quality control of a fluorescent immunoassay analyzer. The fluorescent standard card comprises a fluorescent standard card bodyand a fluorescent detecting unit. The fluorescent detecting unit is made of a fluorescent solution, a photocurable fluorescent material, fluorescent printing ink or fluorescent ink. Compared with theuse of a quality control product, reference product or standard product, the fluorescent standard card has the characteristics of stable luminescent properties, being not easy to decay with time, highline width precision, uniform spatial distribution of fluorescent substances, linearity of fluorescent substance amount and fluorescent intensity and great preparation consistency. The invention further discloses a test method. The method and parameters of a fluorescent standard card evaluation device are established. The method has the advantages of complete evaluation content and easy operation, is a systematic measurement and evaluation scheme, is beneficial for calibration and quality control of the fluorescent immunoassay analyzer, and improves the detection accuracy of the fluorescent immunoassay analyzer. The traceability scheme of each method can reliably ensure that the properties of the fluorescent standard card are strictly controlled during production and use.
Owner:SHENZHEN KINGFOCUS BIOMDICAL ENG CO LTD
Thermoplastic Material With Adjustable Useful Lifetime, Method For Their Manufacture And Products Thereof
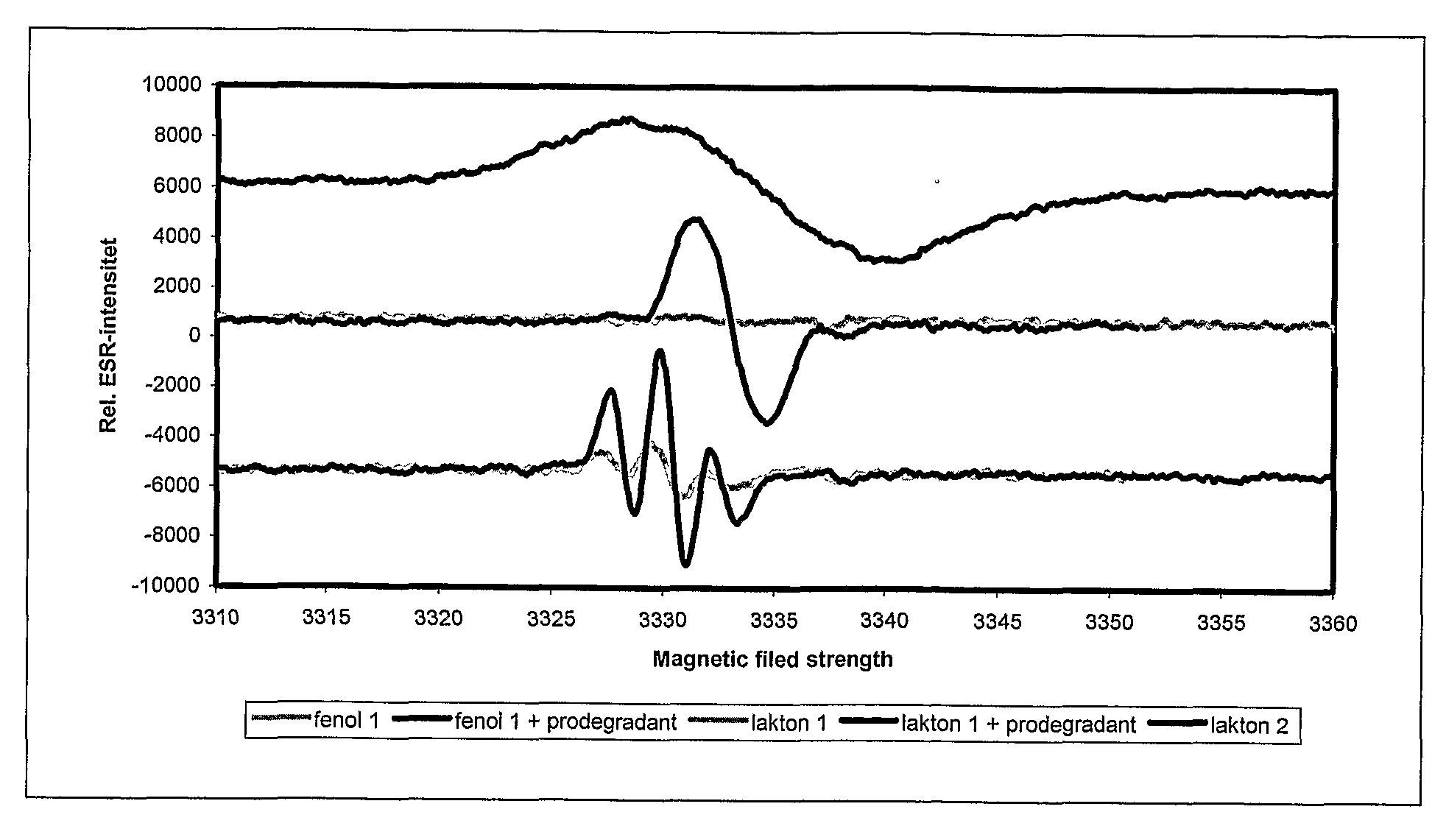
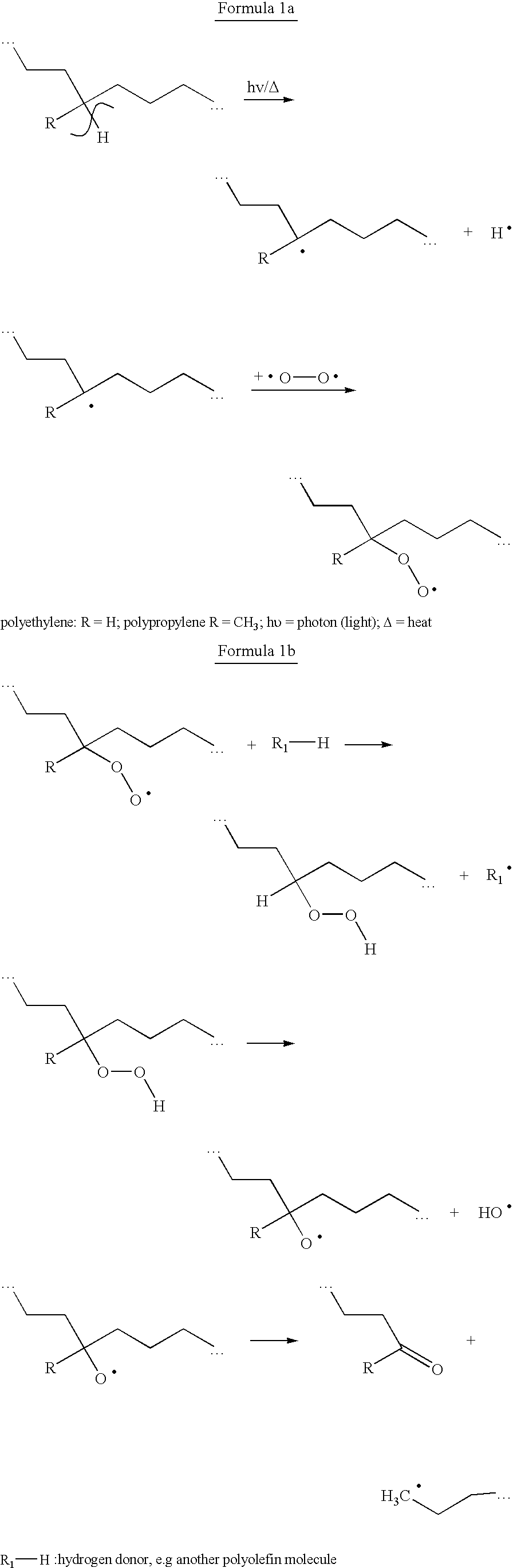
Method and mixture for the manufacture of thermoplastic materials with good processibility and adjustable lifetime, comprising at least one oxidizing promoting agent (prodegradant) and at least one stabilizer. The prodegradant is a fat-soluble metal compound manufacturable by allowing a metal salt to react with a fat-soluble organic compound in a process involving a suitable oxidizing. The end product has an oxidizing ability with respect to a certain reduction agent that is higher than the oxidizing ability of a reference product manufactured from the same metal salt and the same fat-soluble organic compound without the use of oxidizing agent. A stabilizer with suitable process stability and long-term stability is used in combination with the prodegradant. The invention also concerns products manufactured by the method.
Owner:NOR X IND
Dissolution rate detection method for simvastatin
InactiveCN103076410AEasy to detectQuick checkComponent separationSustained Release TabletSodium phosphates
The invention provides a dissolution rate detection method for simvastatin, which comprises the following steps: 1) preparation of dissolution medium: dissolving sodium phosphate dibasic dehydrate and sodium dodecyl sulfate in water, stirring, and adjusting the pH value to 6.95-7.005, so as to obtain the dissolution medium; 2 sample solution preparation: adding a nicotinic acid / simvastatin sustained release tablet in the dissolution medium obtained in the step 1), performing a dissolution experiment at the paddle rotating speed of 50 rounds / minute by adopting the paddle method and a tablet sedimentation method, so as to obtain the sample solution; 3) contrast solution preparation: weighing a simvastatin reference product, and adding into a mobile phase for dissolving, so as to obtain the contrast solution; 4) detection: taking the contrast solution and sample solutions at all dissolution time points, recording the chromatogram and analyzing; and 5) calculating the dissolution rate. The dissolution rate detection method can objectively reflect dissolution release action of the simvastatin, and can detect the dissolution rate of the simvastatin simply, conveniently, quickly and accurately, and can accurately detect the quality of the agent.
Owner:JIANG SU PHARMAMAXCORP
LAMP visual rapid detection kit of silkworm pathogenic micro spore worms and detection method thereof
ActiveCN103131773AGuaranteed FeaturesGuaranteed detection effectMicrobiological testing/measurementMicroorganism based processesBiotechnologySpore
The invention relates to the technical field of biology, and discloses a LAMP visual rapid detection kit of silkworm pathogenic micro spore worms and a detection method of the LAMP visual rapid detection kit of the silkworm pathogenic micro spore worms. The kit comprises four LAMP primers, deoxyribonucleic acid (DNA) lysate liquid, LAMP reaction liquid, positive reference products, negative reference products and color development liquid to form a detection reaction system. Under the constant temperature of 60 DEG C to 65 DEG C, formwork DNA is rapidly amplified, developed dye is added to the formwork DNA, and identification results are observed through naked eyes under natural light. If the color of reaction products changes into green, the fact that samples to be tested contain the silkworm pathogenic micro spore worms is confirmed, and if the color of reaction products changes into orange, the fact that the samples to be tested do not contain the silkworm pathogenic micro spore worms is confirmed. The kit can detect current various silkworm pathogenic micro spore worms fast and flexibly, is easy to operate, and low in cost, and needs no special or complex instruments, and reaction results are easy to judge, and specificity is strong. The kit can meet the urgent needs of disease surveillance, on-site emergency situation and the detection of production samples, and can be widely popularized and applied easily.
Owner:SOUTH CHINA AGRI UNIV
Multiplex amplification for the detection of nucleic acid variations
InactiveCN102782158AMicrobiological testing/measurementLibrary screeningReference productNucleic acid
Owner:THE UNIV OF BRITISH COLUMBIA +1
Method for constructing Bidens parviflor willd medicinal material capillary electrophoresis fingerprint and application thereof
InactiveCN101890066AImprove the quality evaluation systemOvercome costsComponent separationDigestive systemHplc fingerprintReference product
The invention provides a method for constructing a Bidens parviflor willd medicinal material capillary electrophoresis fingerprint and an application thereof. The construction method comprises the steps of preparing a test sample solution, preparing a reference solution and analyzing capillary electrophoresis, wherein the reference product is one or more of salicylic acid, rutin or hyperin. The invention radicates the construction method of the Bidens parviflor willd medicinal material fingerprint, can obtain the Bidens parviflor willd medicinal material fingerprint by the method, improve the quality evaluation system of the Bidens parviflor willd medicinal material, overcome the defects of damaged chromatographic columns, large used amount of toxic organic solvents, high analysis cost and polluted environment in the HPLC fingerprint, and provide theory and practice basis for the Bidens parviflor willd medicinal material and overall and effective quality control of crude slices thereof.
Owner:深圳市药品检验所
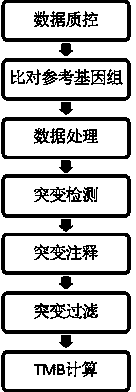
Tumor mutation burden standard substance, and preparation method and kit thereof
ActiveCN111118167AAccurate measurementFit closelyMicrobiological testing/measurementProteomicsPaired samplesReference product
The invention provides a tumor mutation burden standard substance, and a preparation method and application thereof. The tumor mutation burden standard substance is obtained by mixing extracted genomeDNA of 6 pairs of paired cell lines according to a certain proportion. Through whole exon sequencing, a gradient reference substance is sequenced; and a tumor mutation burden value of the corresponding gradient reference substance is calculated through a specific biological information algorithm. For a detection limit reference product, a tumor cell sample is diluted by a normal cell sample in paired samples; and a droplet digital PCR method is used for verifying the dilution proportion for ensuring the mixing to conform to the expectation, so that the complexity of clinic samples and the specificity and sensitivity of an inspection and testing system are simulated to a maximum degree. The product can be used for evaluating the performance of tumor mutation burden detection products, andcan also be used for algorithm optimization or detection flow process optimization of the tumor mutation burden. The tumor mutation burden standard substance is applicable to high-flux sequencing strategies of whole exon and targeted genome or exome subsets.
Owner:菁良科技(深圳)有限公司
Product recommendations over multiple stores
InactiveUS20160092960A1Digital data information retrievalBuying/selling/leasing transactionsSocial mediaReference product
Embodiments of the present invention disclose a method, computer program product, and system for identifying matching products relative to a reference product. A reference product is identified from a received product query and a query is generated based on the reference product. A generated query comprises of an ontology, at least one word appearing in a title of the reference product, and a set of key words appearing in social media data associated with the reference product. A database is searched using the generated query to find matching product sets and the results are returned and filtered. Results are filtered by calculating a relationship score between the reference product and one or more matching products in the set of matching products, and / or by filtering a subset of the set of matching products based on a customer profile. The filtered subset of results are communicated to a recipient.
Owner:IBM CORP
Indometacin sustained release tablet, preparation method thereof, as well as release controlling method and standard
InactiveCN103099795AThe production process is simple and controllableReduce the amount requiredOrganic active ingredientsAntipyreticIndometacinReference product
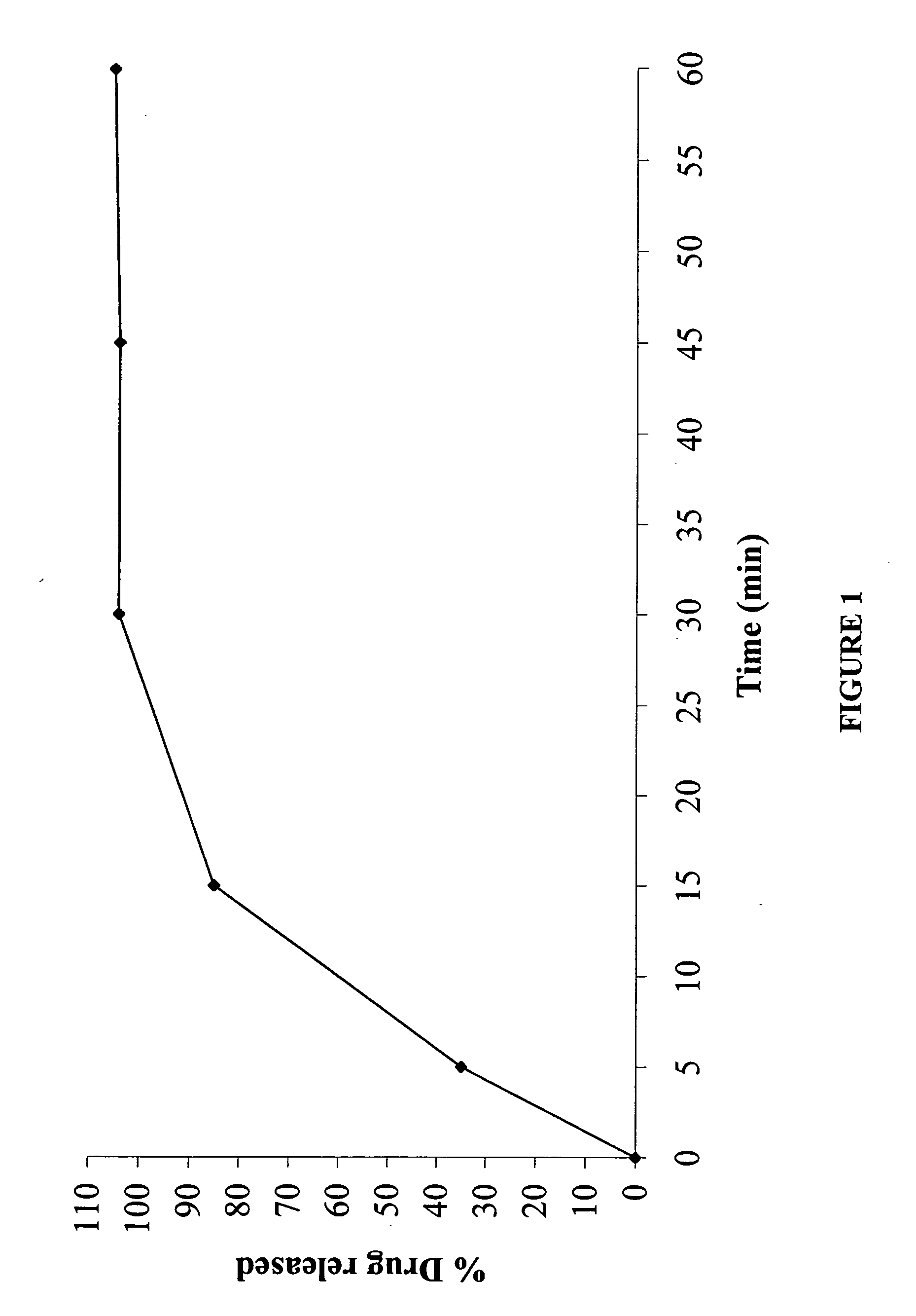
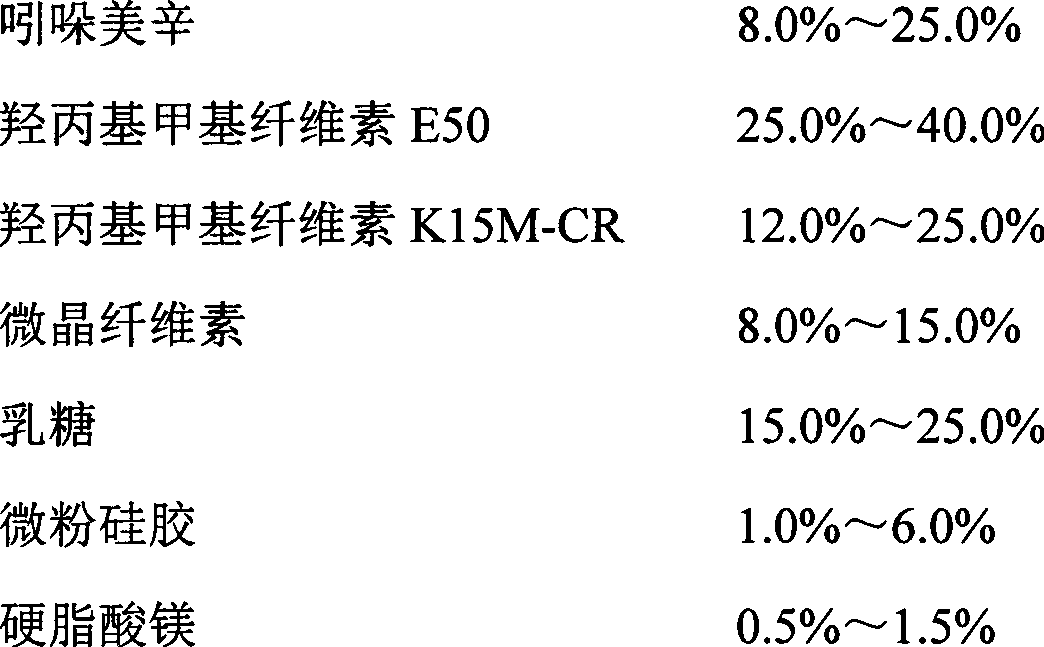
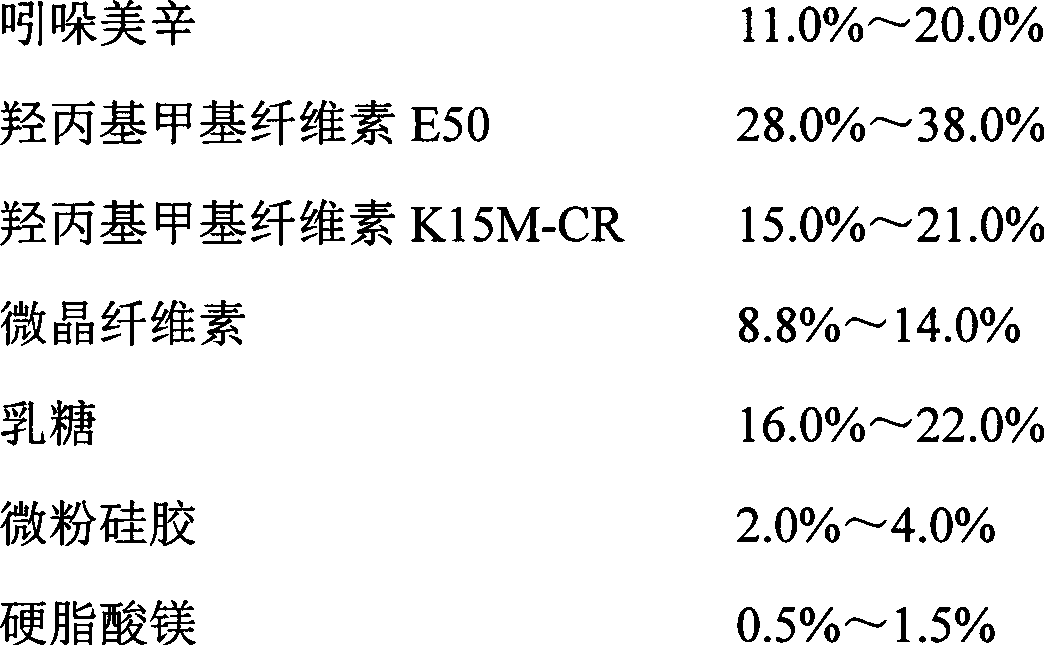
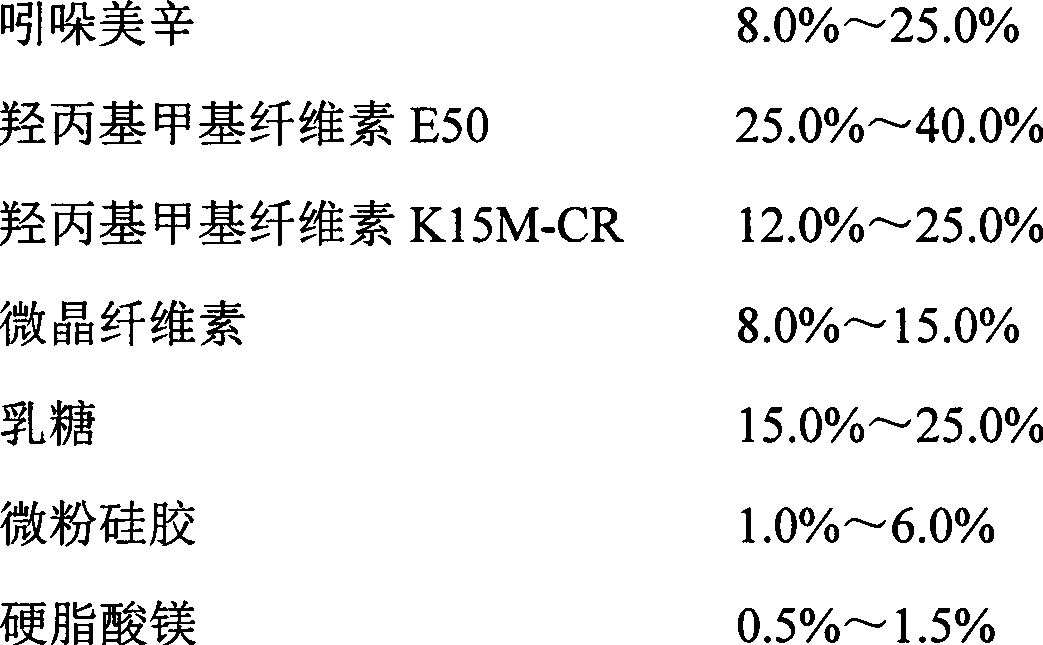
The invention relates to an indometacin sustained release tablet, a preparation method thereof, as well as a release controlling method and standard. The indometacin sustained release tablet is prepared from the following components in percentage by weight: 8.0-25.0% of indometacin, 25.0-40.0% of hydroxypropyl methyl cellulose, 12.0-25.0% of microcrystalline cellulose K15M-CR, 8.0-15.0% of microcrystalline cellulose, 15.0-25.0% of lactin, 1.0-6.0% of gum acacia and 0.5-1.5% of magnesium stearate. A full-powder direct compression process is adopted; the production process is simplified; the methoxyl content of prepared mid-product methoxyl is 8.2-18%; and the propoxyl content is 2.6-7.8%. The quality of the indometacin sustained release tablet disclosed by the invention reaches the standard; a reference product has release similarity; mass control is carried out before production; rework can be reduced; the production cost is saved; the energy consumption is reduced; and the production efficiency is improved.
Owner:深圳国源国药有限公司
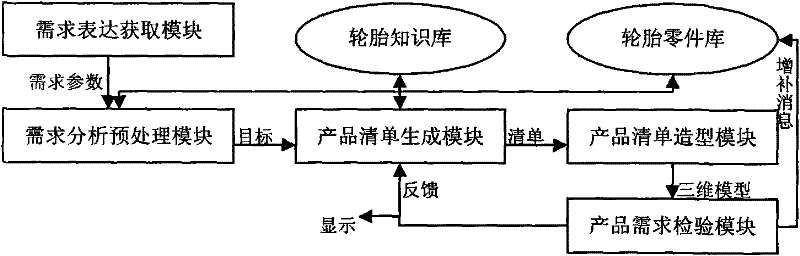
Development and design platform for digitalized model of three-dimensional product by CAD (Computer Aided Design)
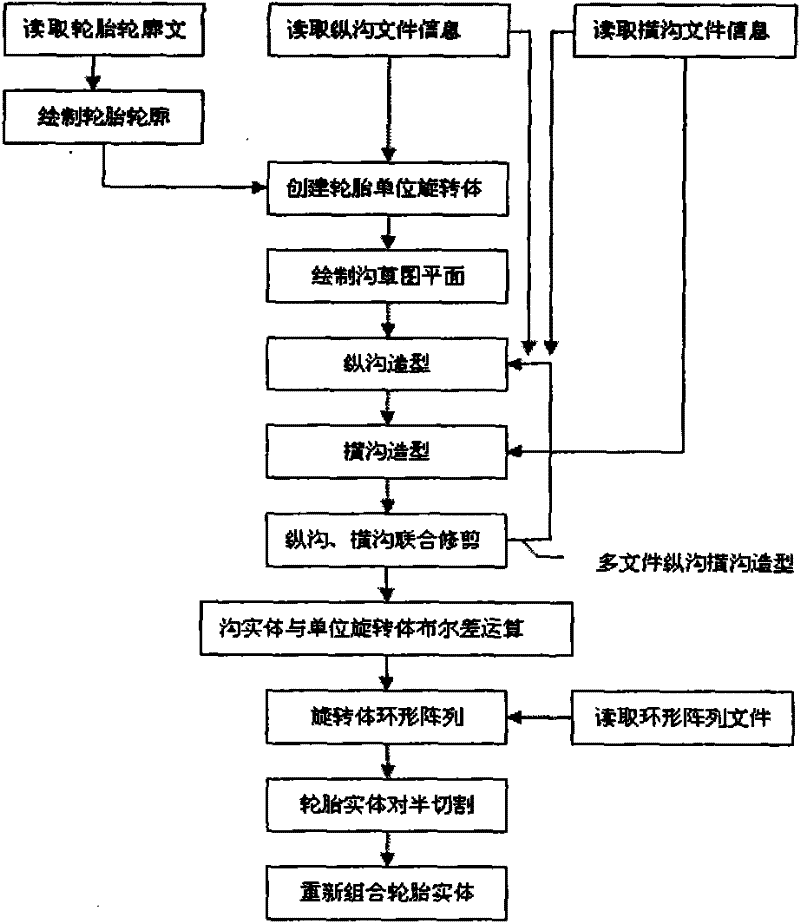
The invention provides a development and design platform for digitalized model of three-dimensional product by CAD, comprising: a demand representation acquiring module, a product knowledge library, a product part library, a demand analysis preprocessing module, a product list generating module, a product demand inspecting module and the like; the development and design platform forms a feasible product list with reference to the product knowledge library and the product part library by analyzing demand information, and obtains accurate three-dimensional model of tire products by means of processing in CAD software according to the product list, thus quick design of tire is implemented.
Owner:TONGJI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com