Herpes virus type I PCR (polymerase chain reaction) fluorescence quantitative rapid test kit and method
A detection kit and fluorescence quantitative technology, applied in the direction of microorganism-based methods, microorganism measurement/inspection, biochemical equipment and methods, etc., can solve the problems of poor sensitivity, achieve convenient operation, high sensitivity and specificity, and ensure credibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
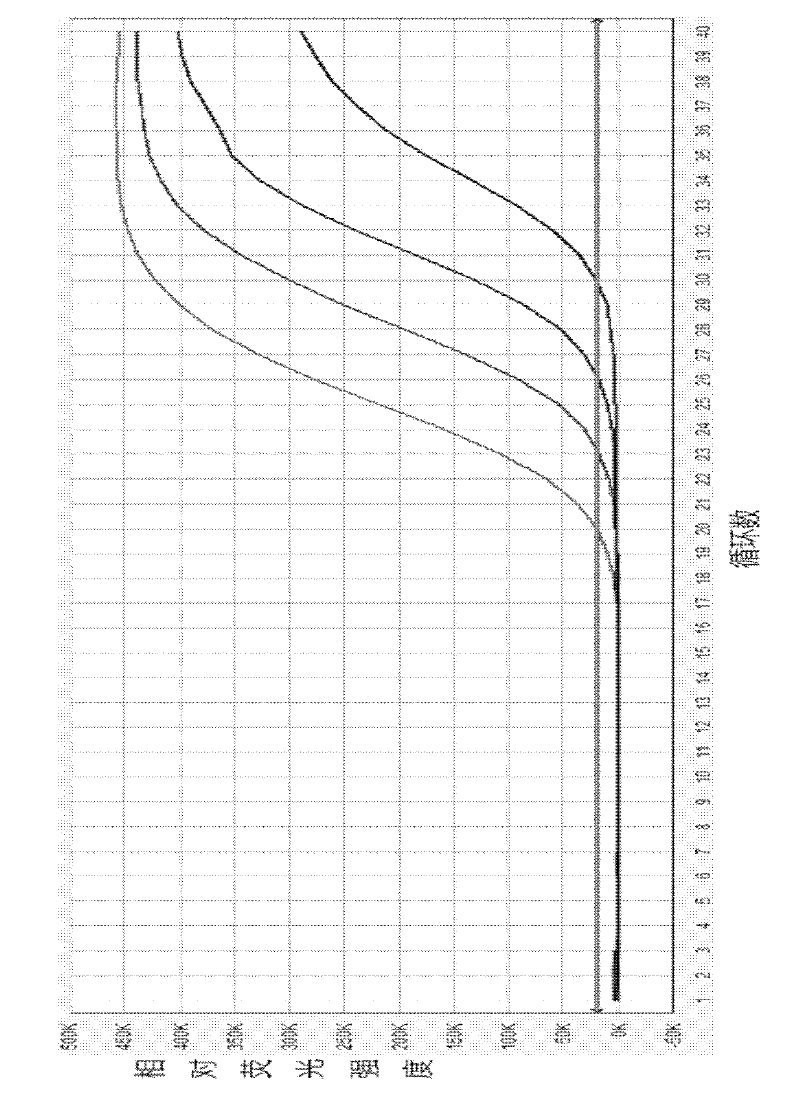
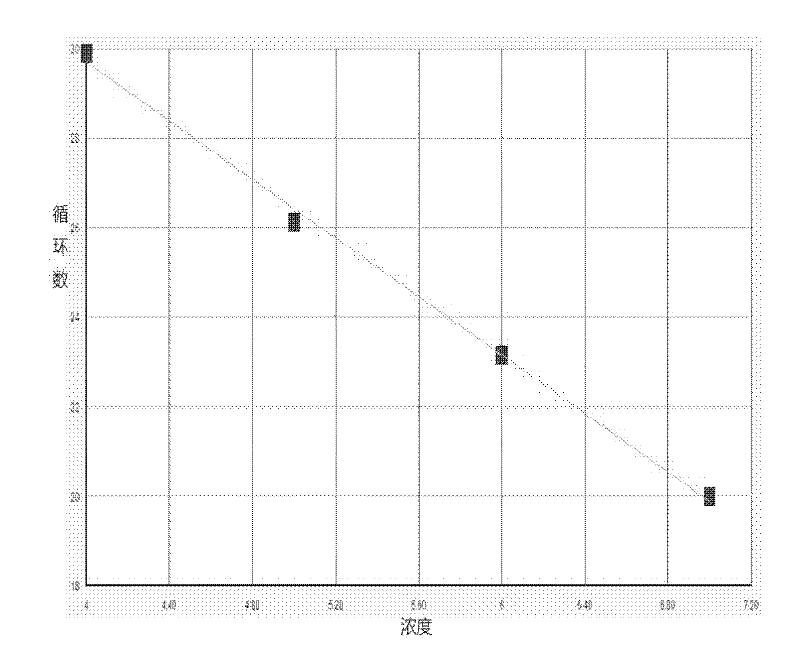
Image
Examples
Embodiment 1
[0034] Table 1: Kit Composition
[0035]
[0036]
[0037] Preparation of basic reagents:
[0038] 1.1 Tris: analytically pure, product from a qualified supplier, content 99.7%, infrared qualified, pH (5% water) 10.3-10.9, moisture 0.3%, melting point 167-171°C, qualified absorption system, highest content of impurities qualified.
[0039] 1.2 NaOH: Analytical pure, the product of Beijing Chemical Reagent Factory or a qualified supplier.
[0040] 1.3TritonX100: brown oily liquid, soluble in cold water, and has strong foaming properties.
[0041] 1.4 EDTA: analytically pure, a product from qualified suppliers, white crystalline powder, soluble in water, acidic in solution, insoluble in alcohol, content not less than 99.5%, qualified in aqueous solution reaction and complexing force test , the highest impurity content is qualified.
[0042]1.5 Purified water: use pure water from Taipu Bioscience Co., Ltd., and then treat it with a Milli-Q Biocel pure water machine from...
Embodiment 2
[0056] Embodiment 2 The use of kit of the present invention
[0057] 1. Reagent preparation (reagent preparation area)
[0058] 1. Take out the DNA extraction solution and set aside.
[0059] 2. After determining the number n of reaction tubes to be performed (number of samples + negative + strong positive quality control + weak positive quality control + 4 quantitative reference substances), take out the HSV-I PCR reaction solution and mix n×44μl HSV- I-PCR reaction solution, add n×1μl DNA polymerase system into a centrifuge tube and vortex to mix well. After instantaneous centrifugation, dispense 45μl into each PCR reaction tube, cover the tube cap and transfer to the sample addition area, avoiding Put it in the refrigerator at 4°C for later use.
[0060] 3. Transfer the reference substance and quantitative reference substance to the sample processing area, and put them in a 4°C refrigerator for later use.
[0061] 2. Sample processing (sample processing area)
[0062] 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com