Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Human herpes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human herpesvirus 1 (HHV-1): A herpes virus that causes cold sores and fever blisters in and around the mouth. Here is a depiction of a typical fever blister caused by HHV-1: In rare cases, as when the immune system is severely compromised, this virus can cause infection of the brain (encephalitis). HHV-1 is also known as herpes simplex type .
Quantitation method of virus
InactiveUS20110053145A1Sure easyHigh degreeMicrobiological testing/measurementBiological material analysisQuantitative determinationContinuous evaluation
The present invention relates to a method of quantitatively determining the number of human herpesvirus (HHV) collected from a body fluid and a kit for performing the method. Conventionally, a trained technician has been required to accurately quantitatively determine a number of HHV collected from a body fluid. The method of the present invention is a novel method of quantitative determination that enables measurement of a number of HHV in a body fluid to be simply, accurately, and efficiently determined. The method of the present invention can enable continuous evaluation of the number of HHV in body fluids and, therefore, can be applied to quantitative evaluation of the accumulation of fatigue.
Owner:JAPAN TOBACCO INC +1
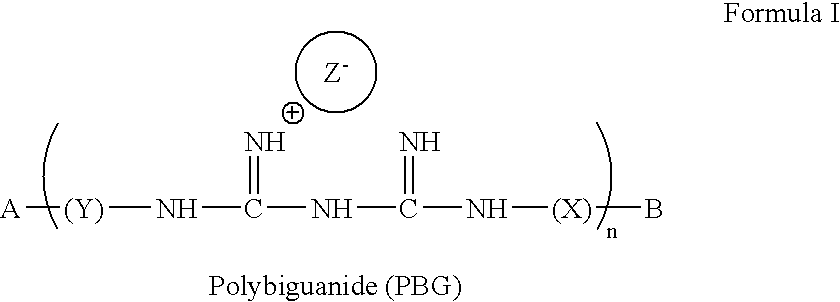
Method for the treatment or prevention of virus infection using polybiguanide-based compounds
InactiveUS20040009144A1High activityBroaden applicationAntibacterial agentsBiocideDiseasePathogenic microorganism
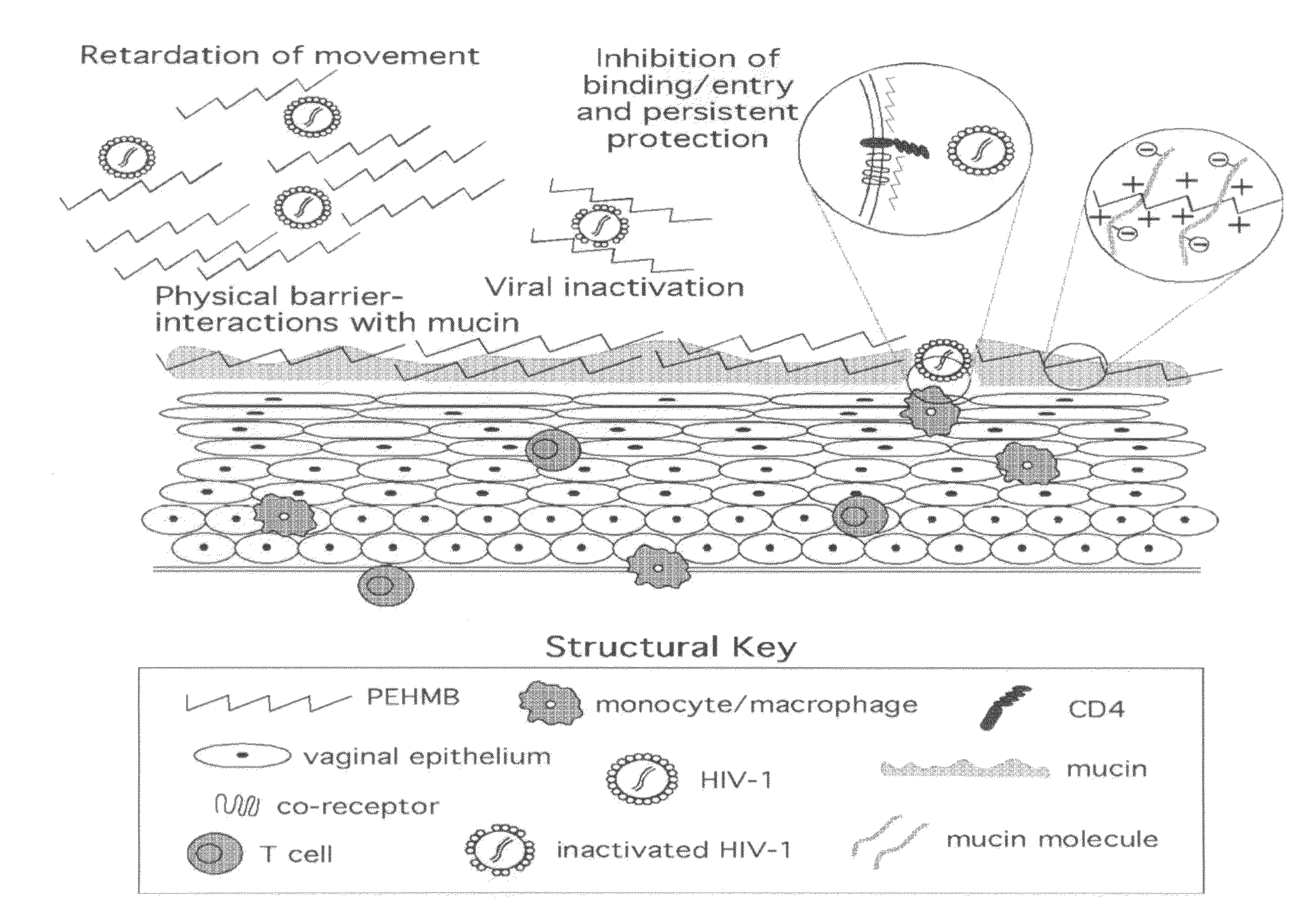
An inexpensive, easily available, and convenient method of treating or preventing a virus infection is provided. The present invention relates to a method for the treatment or prevention of virus infections using polybiguanide-based compounds administering a therapeutically effective amount of a compound or a pharmaceutically acceptable salt thereof. The invention relies on the unique biochemical reaction in which polybiguanide-based compounds interfere with the spread of virus within or between organisms. The compositions and formulations described in the present invention are effective means to reduce the infectivity of the human immunodeficiency virus type 1 (HIV-1), and human herpes simplex viruses, and also to kill the causative organisms of many other sexually transmitted diseases (STDs).
Owner:NOVAFLUX INC
Fluorescent quantitation PCR (polymerase chain reaction) detection kit for four conventional herpesvirus hominises
InactiveCN103820573AMicrobiological testing/measurementMicroorganism based processesFluoProbesHerpesvirus hominis
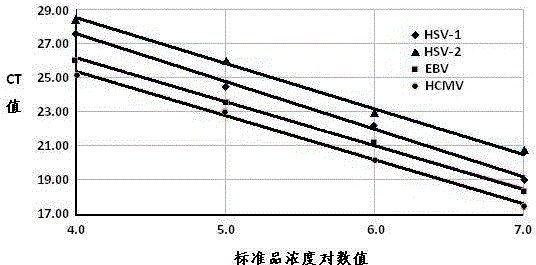
The invention provides a real-time fluorescent quantitation PCR (polymerase chain reaction) kit for simultaneously detecting conventional herpesvirus hominises HSV (herpes simplex virus)-1, HSV-2, EBV and HCMV (human cytomegalovinis). The kit consists of quantitation PCR reaction liquid, quantitation reaction liquid, an HSV-1 standard substance, an HSV-2 standard substance, an EBV standard substance, an HCMV standard substance, a negative reference substance, an HSV-1 positive reference substance, an HSV-2 positive reference substance, an EBV positive reference substance, an HCMV positive reference substance, a specification and a kit body. The kit disclosed by the invention adopts a real-time fluorescent quantitation PCR technology and two specific fluorescent probes in the same reaction system; the detection range covers the four most conventional herpesvirus hominises, so that the four herpesvirus hominises in a sample can be simultaneously subjected to parting detection; real-time and accurate quantitation can be realized; the need for early, quick and accurate diagnosis can be met; a powerful basis is supplied to epidemiological investigation and immediate formulation of a targeted treating scheme.
Owner:ZHEJIANG UNIV
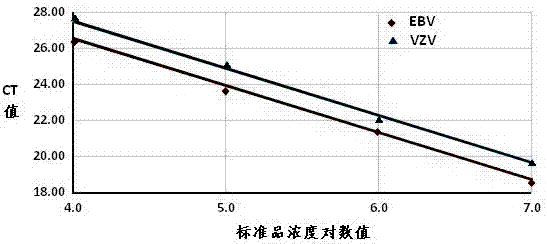
Detection kit for human herpes viruses EBV and VZV
The invention provides a detection kit for human herpes viruses EBV and VZV. The detection kit comprises quantitative PCR reaction solution, an EBV standard, a VZV standard, an EBV positive control, a VZV positive control, a negative control, instructions and the kit body, the quantitative PCR reaction solution comprises PCR buffer, MgCl2, dNTPs, heat-resistant DNA polymerase, an EBV forward amplification primer, an EBV reverse amplification primer, a VZV forward amplification primer, a VZV reverse amplification primer, a EBV fluorescent probe and a VZV fluorescence probe. The human herpes viruses EBV and VZV can be detected in one step by using real-time quantitative PCR and two-color fluorescent probes according to the detection kit, synchronous diagnosis of EBV and VZV infections is realized, positive virus can be accurately quantified in real time, urgent need for accurate diagnosis of EBV and VZV infections in the early clinical diagnosis is met and a basis is provided for timely definitive therapy of EBV and VZV infections.
Owner:ZHEJIANG UNIV
Reagent for rapidly detecting and quantifying subtypes of human herpes viruses and kit
ActiveCN106834543AStrong specificityImprove accuracyMicrobiological testing/measurementMicroorganism based processesClinical diagnosisReagent
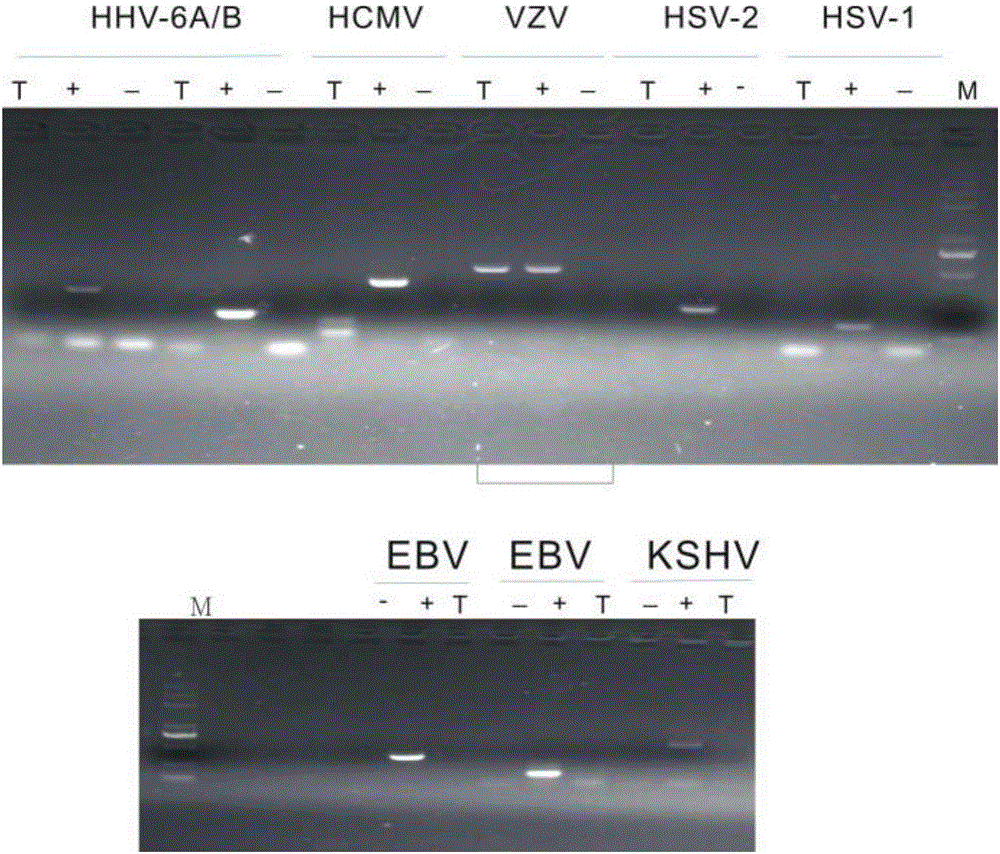
The invention discloses a reagent for rapidly detecting various subtypes of human herpes viruses. The reagent comprises forward and reverse primers specifically amplifying HSV-1 RL2, HSV-2 UL28, VZV ORF26, EBV EBNA1, HCMV IE, HHV-6A or HHV-6B U22, HHV-6A or HHV-6B IE, HHV-7 U95 and KSHV ORF72 and a corresponding internal reference standard. The invention also discloses a kit which contains the detection reagent and is used for rapidly detecting various subtypes of the human herpes viruses and also discloses a reagent which contains the detection reagent and is used for rapidly quantifying the various subtypes of the human herpes viruses as well as a detection quantification kit. The reagent disclosed by the invention can rapidly and effectively identify and diagnose the various subtypes on clinical samples in early stage of infection and with different sources, also can conveniently and accurately carry out virus copy number quantitative calculation and has high clinical diagnosis use value.
Owner:FUDAN UNIV
Triple detection kit for human herpes viruses HSV-1, HSV-2 and HCMV
InactiveCN103773898AMeet the needs of clinical diagnosisMeet early infectionMicrobiological testing/measurementFluoProbesBioinformatics
The invention provides a triple detection kit for human herpes viruses HSV-1, HSV-2 and HCMV. The triple detection kit is composed of a quantitative PCR (Polymerase Chain Reaction) reaction liquid, an HSV-1 standard substance, an HSV-2 standard substance, an HCMV standard substance, a negative reference substance, an HSV-1 positive reference substance, an HSV-2 positive reference substance, an HCMV positive reference substance, a specification and a box, wherein the quantitative PCR reaction liquid contains a PCR buffer solution, MgC12, dNTPs (deoxyriboNucleoside Tri-Phosphates), a heat-resisting DNA polymerase, an upstream amplification primer, a downstream amplification primer, an HSV-1 fluorescent probe, an HSV-2 fluorescent probe and an HCMV fluorescent probe. The kit is capable of realizing simultaneous typing detection on the common human herpes viruses HSV-1, HSV-2 and HCMV in a sample by virtue of the real-time quantitative PCR technique and the specific fluorescent probes of three colors; the kit is capable of quantifying positive viruses accurately in real time, so that the production cost and the detection cost can be saved and the detection efficiency can be improved; besides, the kit is capable of meeting diagnosis requirements and helpful for formulating a targeting treatment scheme timely.
Owner:ZHEJIANG UNIV
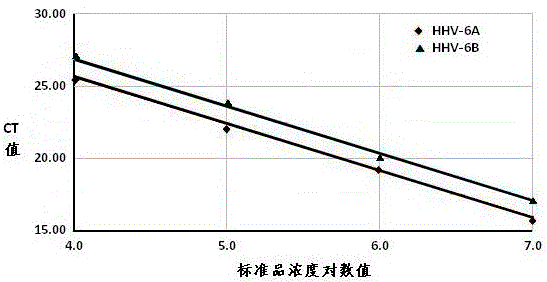
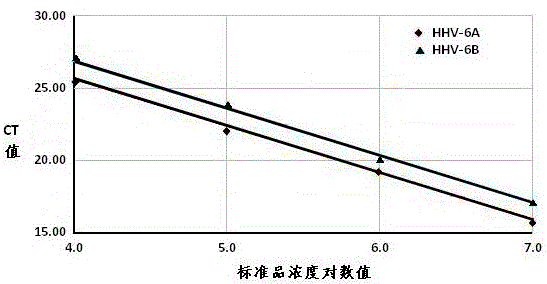
Real-time fluorescent quantitation PCR (polymerase chain reaction) parting detection kit for human herpesvirus-6
The invention provides a real-time fluorescent quantitation PCR (polymerase chain reaction) parting detection kit for human herpesvirus-6 (HHV-6). The real-time fluorescent quantitation PCR parting detection kit consists of quantitation PCR reaction liquid, an HHV-6A standard substance, an HHV-6B standard substance, an HHV-6A positive reference substance, an HHV-6B positive reference substance, a negative reference substance, a specification and a kit body, wherein the quantitation PCR reaction liquid contains a PCR buffer solution, MgCl2, dNTPs, heat-resistant DNA polymerase, an upstream amplification primer, a downstream amplification primer, an HHV-6A fluorescent probe and an HHV-6B fluorescent probe. By the adoption of a real-time fluorescent quantitation PCR technology and a double-color fluorescent probe, the kit disclosed by the invention can detect two subtypes of the HHV-6 by a one-step method; the HHV-6A and HHV-6B in the sample can be simultaneously parted; a positive virus subtype can be accurately quantified in real time; the urgent need of early and acute parting diagnosis on the HHV-6 infection can be met; a basis is supplied to epidemiological investigation and targeted treatment on the HHV-6 infection.
Owner:ZHEJIANG UNIV
Detection and quantification of human herpes viruses
InactiveUS7338761B2Rapid and sensitiveRapid and sensitive methodSugar derivativesMicrobiological testing/measurementClinical settingsInformatics
In clinical settings as well as in a drug development context, human herpes viruses can be detected, and even quantified, by the use of a real time PCR-based assay. An informatics analysis of existing gene sequences from different HHV types or strains is used to identify a target segment within a gene. A probe oligonucleotide and at least two primer oligonucleotides are then designed for selectively directing the amplification, in the course of a single amplification reaction, of the target segment of a particular HHV type or strain. This method is capable of an unprecedented level of discrimination among the following HHV types and strains: HHV1, drug resistant HHV1, HHV2, drug resistant HHV2, HHV3, HHV4a, HHV4b, HHV5, HHV6a, HHV6b, HHV7, and HHV8.
Owner:VIGEN LAB
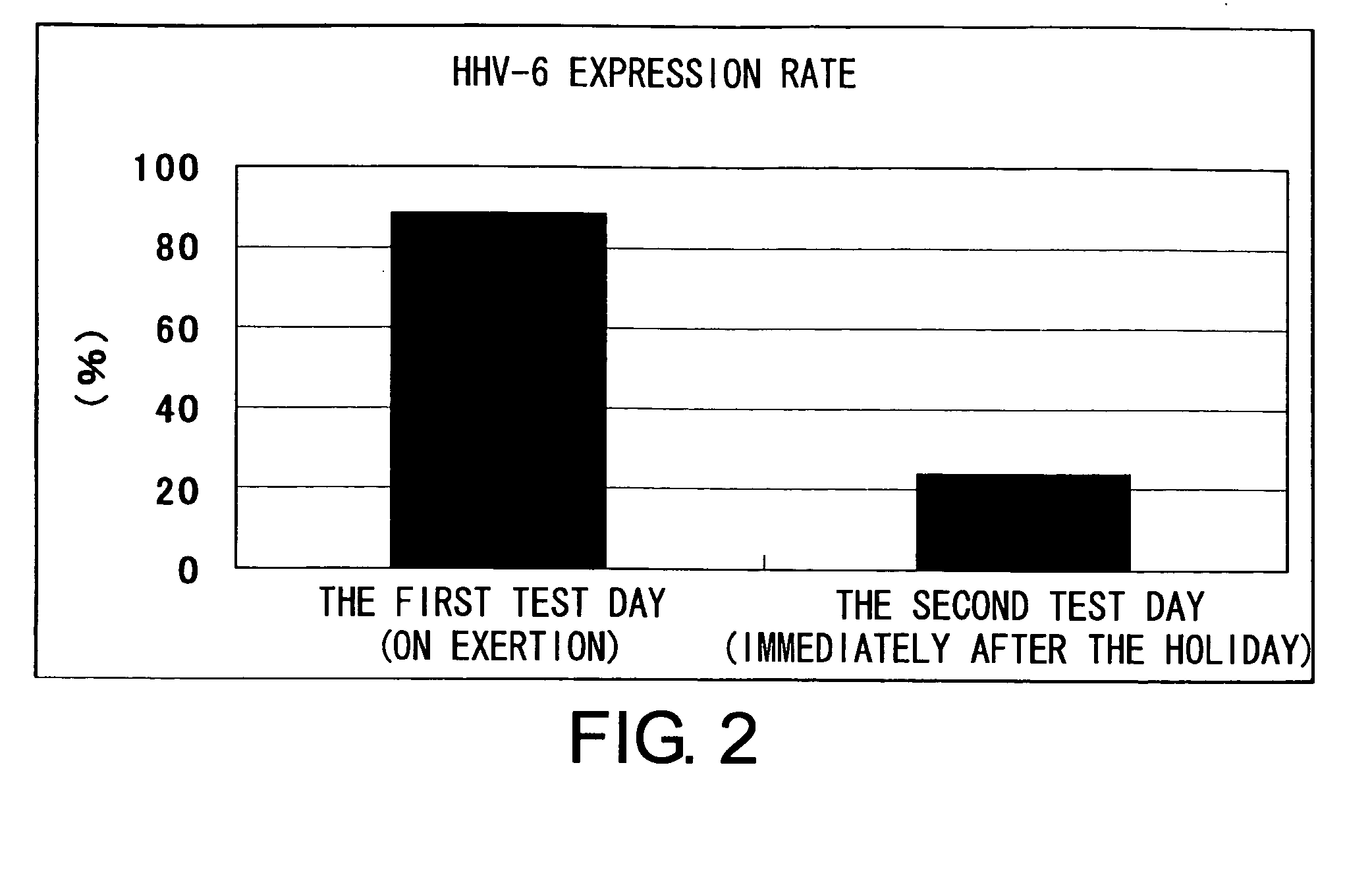
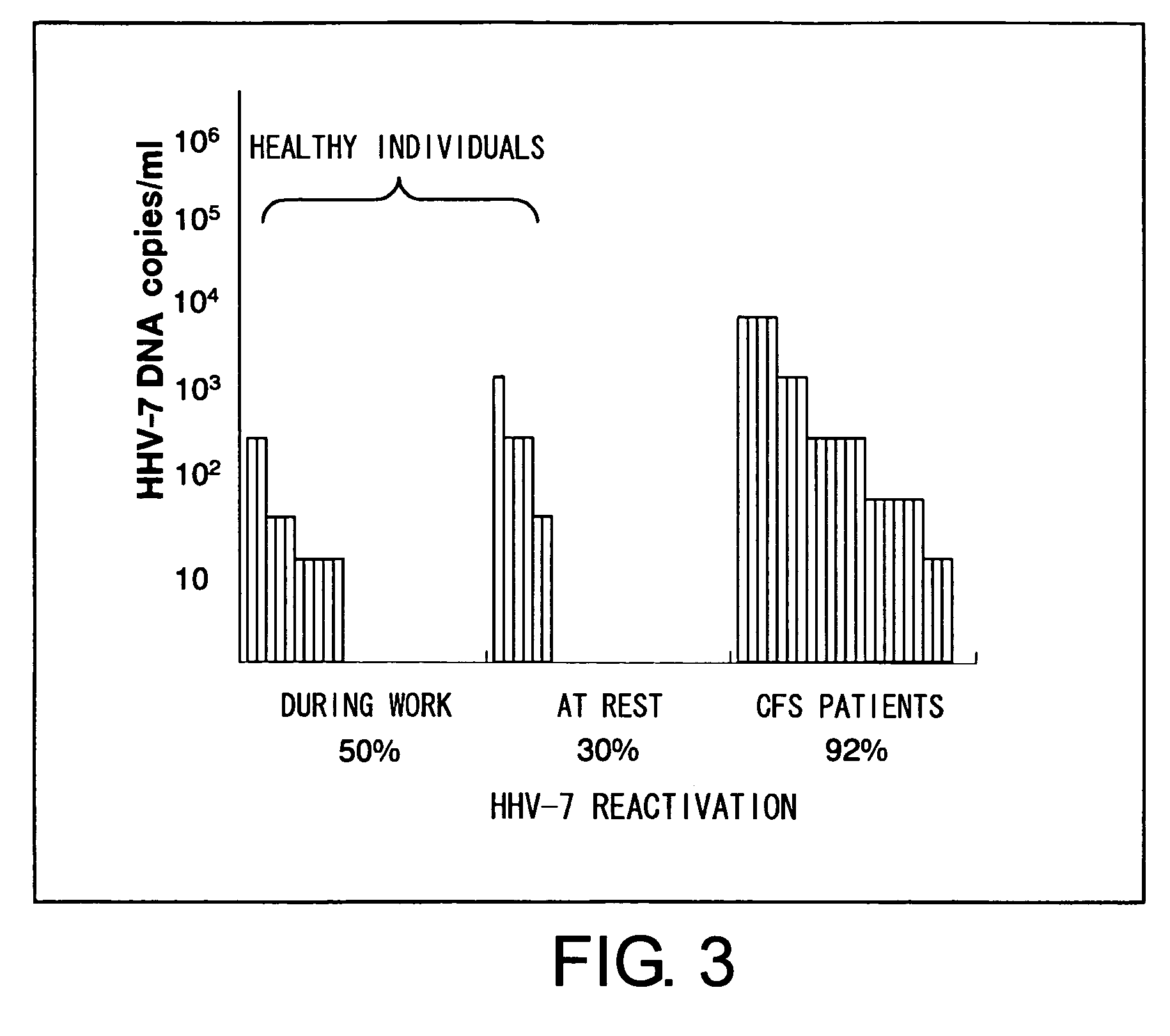
Methods for assessing fatigue level and applications thereof
InactiveUS7824888B2Cause painCause to testMicrobiological testing/measurementDisease diagnosisMedicineBody fluid
Level of fatigue that accompanies everyday life or a disease can be simply, easily, and quantitatively assessed by obtaining a body fluid from a test subject and measuring the amount of human herpesvirus in the body fluid. Furthermore, the anti-fatigue potency of anti-fatigue substances and anti-fatigue food products can be measured.
Owner:VIRUS IKAGAKU KENKYUSHO
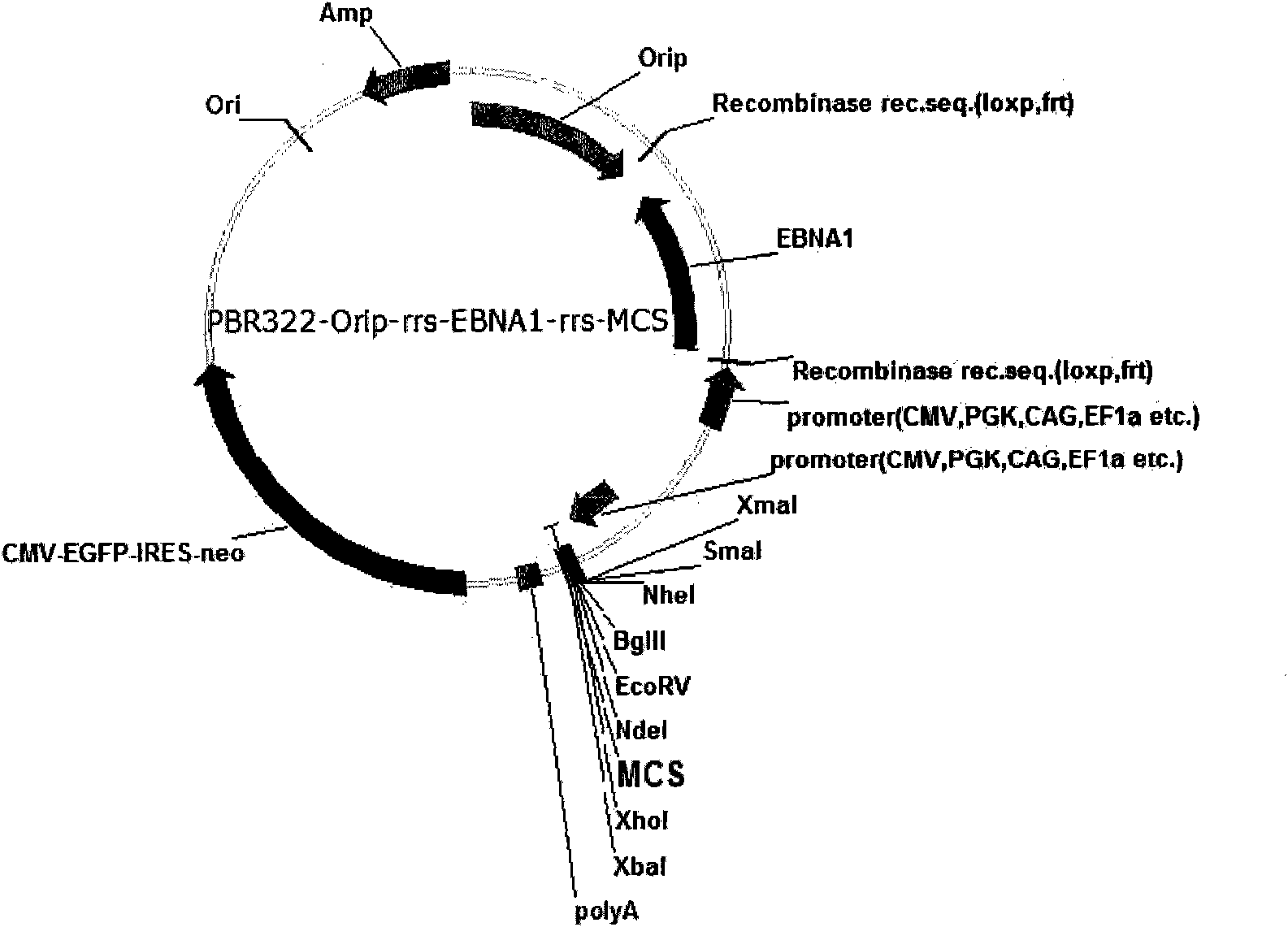
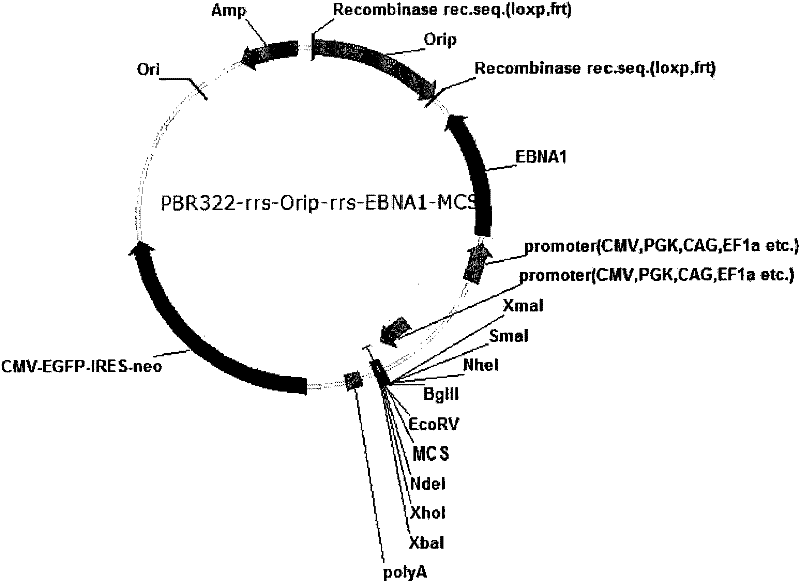
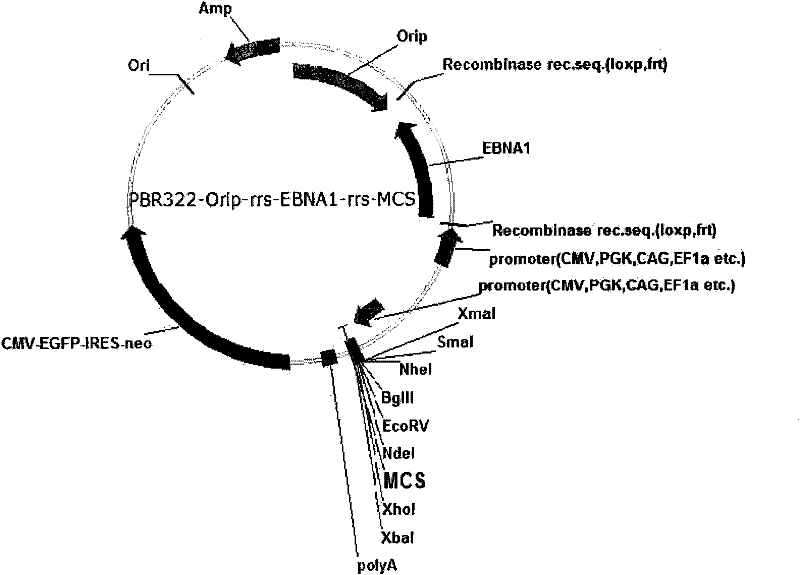
Expression system for nonintegrated, long-time and erasable expression vector
ActiveCN101955977AStable expressionControlled deletionFermentationGenetic engineeringReprogrammingRecognition sequence
The invention provides an expression system for a nonintegrated, long-time and erasable expression vector. The expression vector of the expression system adopts human herpes virus replication origin (Orip), an EBNA-1 expression box is introduced, and both ends of the Orip, both end of EBN-1 or both ends of Orip-EBNA-1 are provided with recombinase recognition sequences. The expression vector is not integrated in a cell genome, can stably express for a long time, can controllably erase vector plasmid by instantaneously inducing (or expressing) recombinase from a hose cell, can be applied to most kinds of cells by using a strong initiator and can distinguish a cell with the vector plasmid from a cell without the vector plasmid by using a real-time marker. The expression system can widely applied to mammal cell expression and biomedicine engineering for realizing cell reprogramming by expressing a foreign gene.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Recombinant virus vector for gene introduction in lymphocyte
It is intended to provide a recombinant herpesvirus; a process for producing the same; and a pharmacological composition comprising a recombinant herpesvirus. Further, it is intended to provide a vector comprising a herpesvirus genome gene and a BAC vector sequence; a cell comprising such a vector; a fragment capable of homologous recombination with herpesvirus genome; and a nucleic acid cassettecomprising a BAC vector sequence. The objects have been attained by the development of a process for producing a recombinant herpesvirus in which use is made of a BAC vector sequence.
Owner:THE RES FOUND FOR MICROBIAL DISEASES OFOSAKA UNIV
A topical antiviral composition containing a local anesthetic and method of making the same
The present invention provides a novel pharmaceutical composition and method of making the same. The composition comprises an effective amount of an antiviral agent and a local anesthetic. The antiviral drug can be sorivudine. The local anesthetic can be lidocaine or its pharmaceutically acceptable salt. Herpes virus infections in humans can be caused by different human herpes viruses, the most common being herpes simplex virus types 1 and 2 (HSV1 and HSV2) and varicella-zoster virus (VZV).
Owner:NAL PHARM LTD
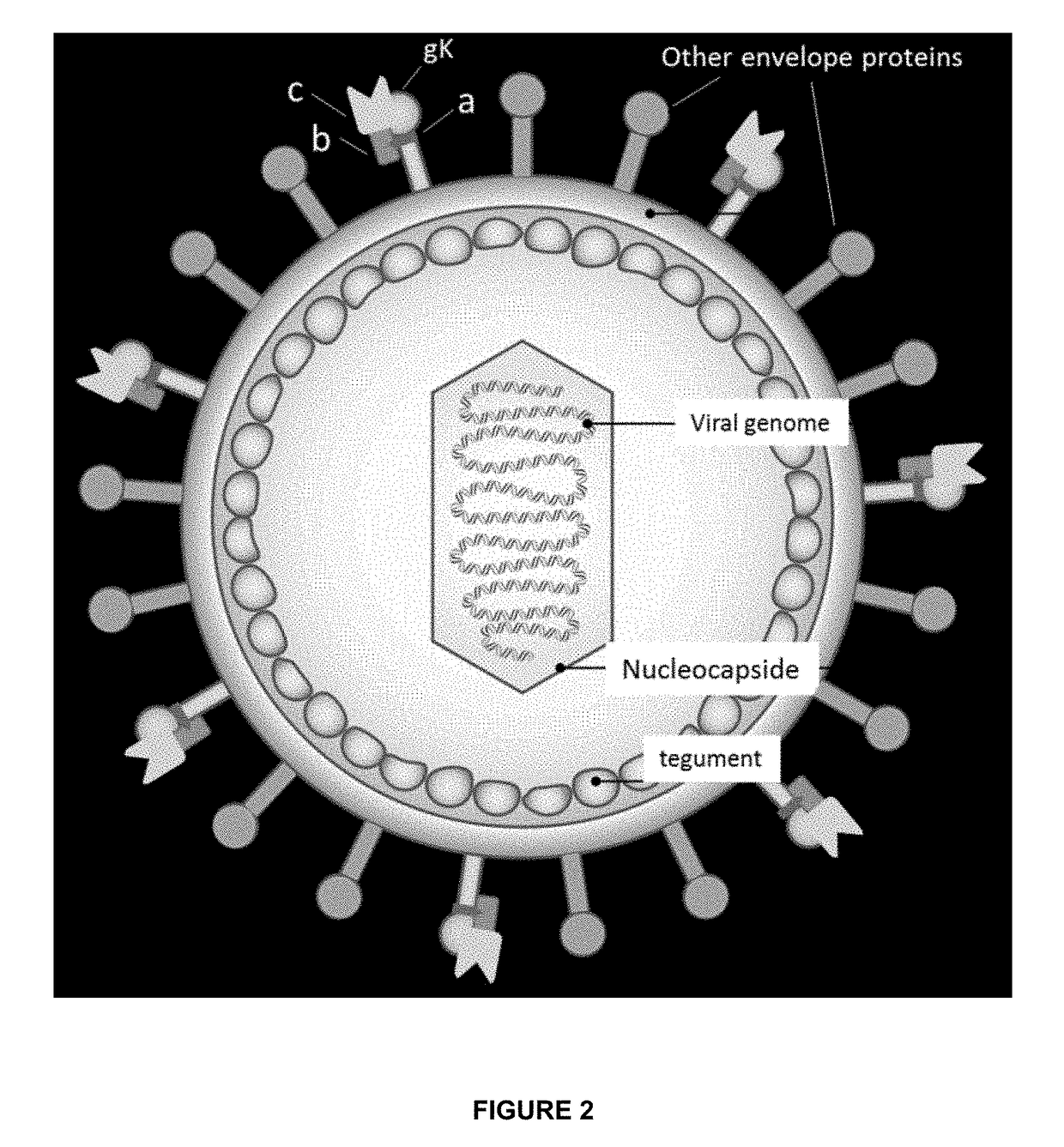
Oncolytic viruses & methods of use thereof
InactiveUS20170319639A1Valid choiceAvoid undesirable off-target effectPeptide/protein ingredientsAntibody mimetics/scaffoldsOncolytic adenovirusWild type
Modified and improved oncolytic viruses (and methods of use thereof) are disclosed. More particularly, modified and oncolytic human herpesviruses (and methods of use thereof) are disclosed, which include a modified amino acid sequence that includes a deletion in a region that represents the amino terminus of glycoprotein K. The modified and oncolytic human herpesviruses include a deletion of amino acid residues 31-68, relative to the wild type amino acid sequence of glycoprotein K in human herpesviruses. Isolated vector constructs that encode such oncolytic viruses are also disclosed. In addition, methods for using such oncolytic viruses to treat cancers are disclosed.
Owner:VIROGIN BIOTECH CANADA LTD
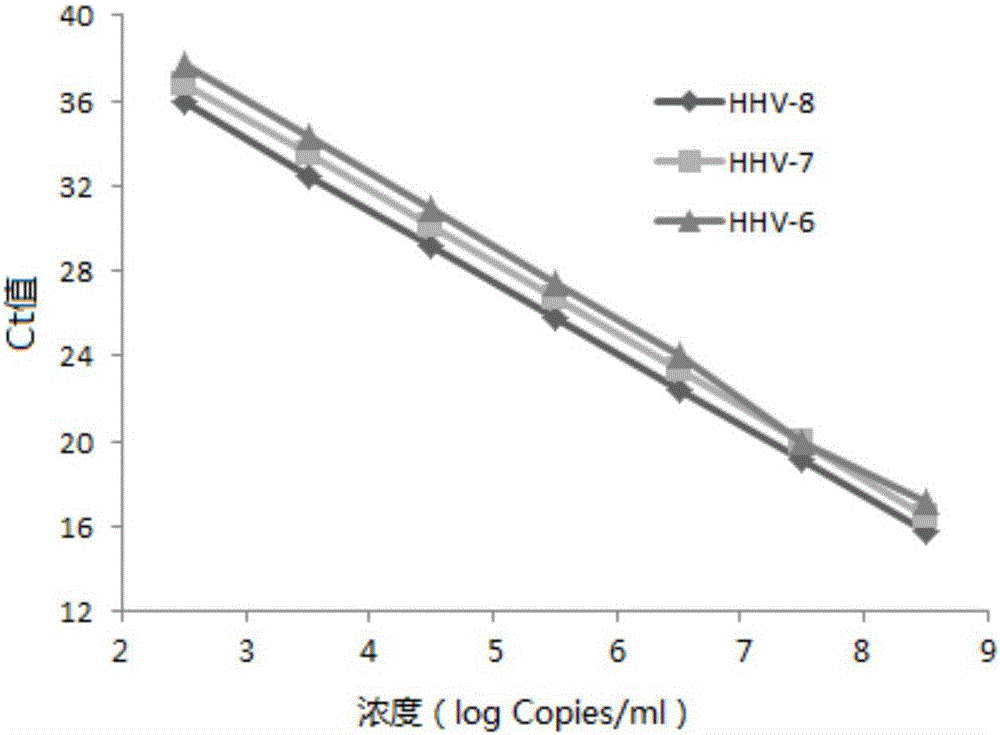
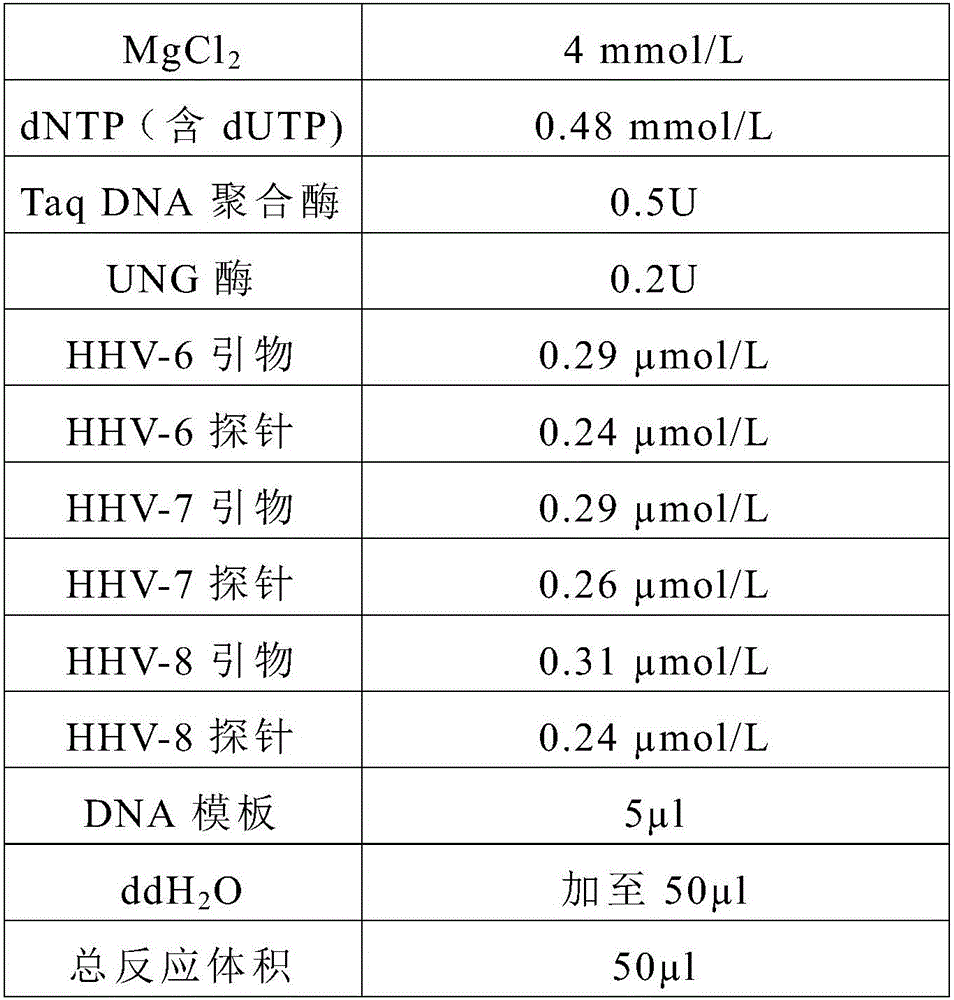
Primer, probe and kit for synchronously detecting human herpes virus types 6, 7 and 8
ActiveCN106119424ALittle interactionStrong specificityMicrobiological testing/measurementMicroorganism based processesVirus typeSynchronous detection
The invention discloses a primer, a probe and a kit for synchronously detecting human herpes virus types 6, 7 and 8. By selecting a new target gene, redesigning a primer and a kit, and optimizing a reaction system, the false positive rate of clinical synchronous detection on human herpes viruses is greatly reduced, the primer, the probe and the kit are good in sensitivity, good in specificity and short in detection time, and the technique can be applied to clinical application and popularization.
Owner:湖南光谷创新医疗器械公共服务平台有限公司
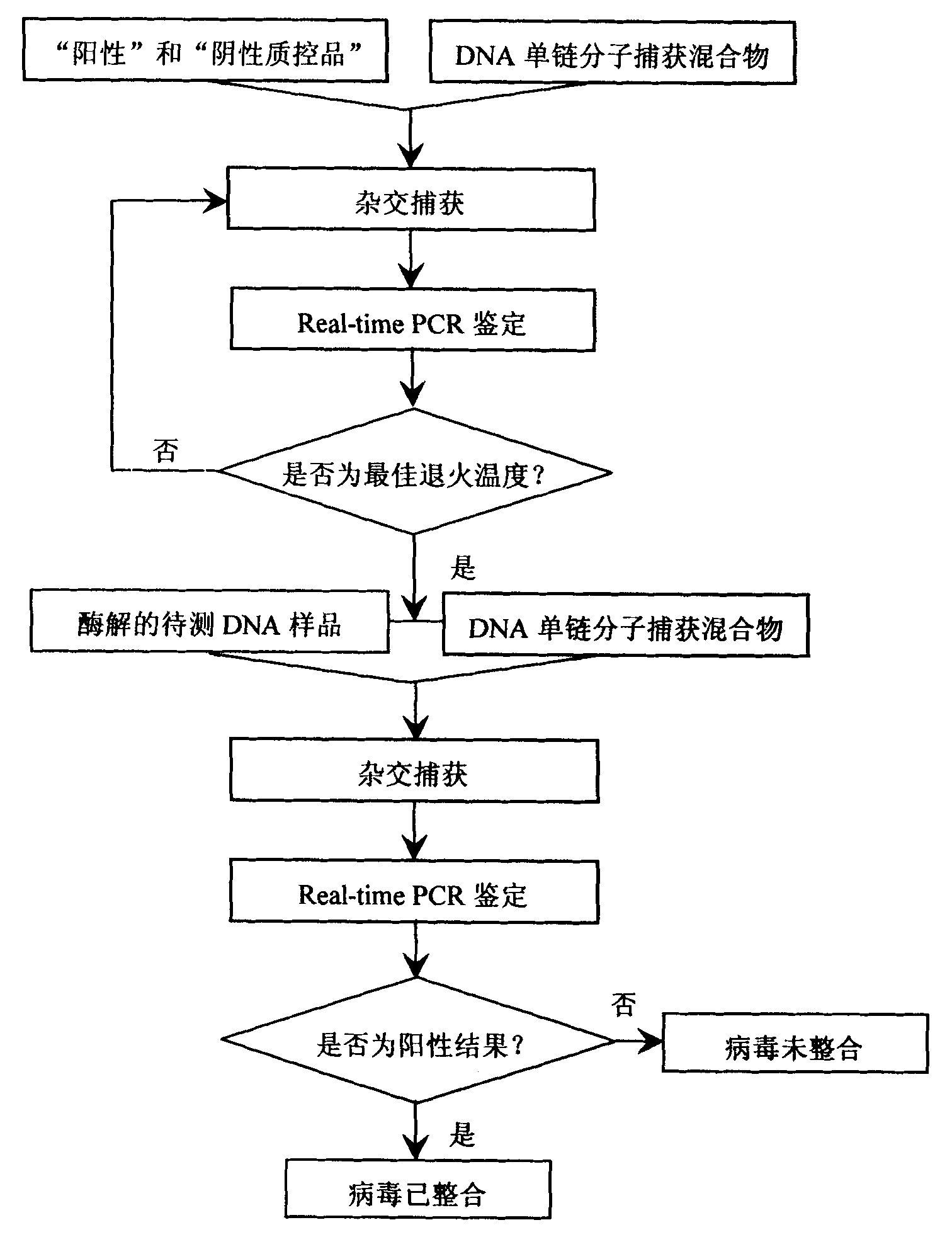
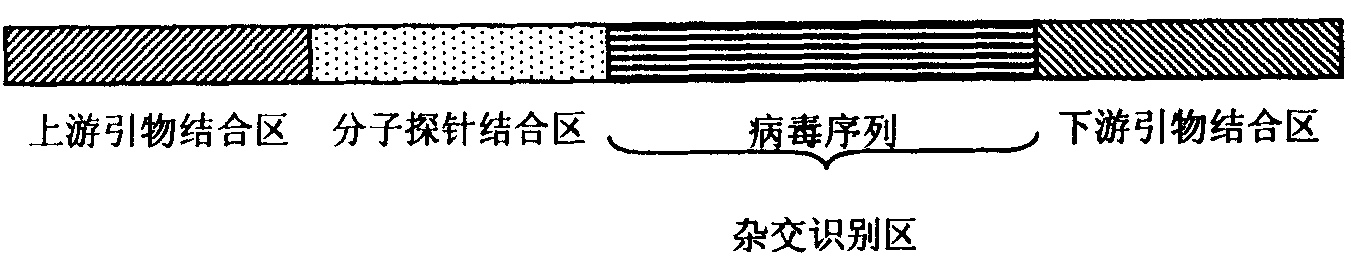
Kit for detecting integrated viruses of genome in hybrid capture
InactiveCN101974652AMicrobiological testing/measurementMicroorganism based processesHuman DNA sequencingBiomedicine
The invention belongs to the field of biomedicine, in particular to a kit for detecting integrated viruses of a genome in hybrid capture. The kit comprises the following main components: (1) a solid phase medium fixed with a capture mixture of human genome DNA (Deoxyribonucleic Acid) single-chain molecules, (2) positive and negative quality control materials for identifying the hybrid capture efficiency and specificity, and (3) a primer pair and a molecular probe for carrying out real-time PCR (Polymerase Chain Reaction) identification to the captured mixture of the target DNA molecules, obtained by capture. By utilizing all or partial components in the invention, a complete integrated genome virus detection kit or an integrated genome virus detection kit with the type specificity can be assembled. The obtained virus detection kit can be used for detecting and identifying the integrated state of the genome in a certain common integrated and oncogenic viruses, such as human hepatitis B viruses, human papilloma viruses, Epstein-Barr viruses and human herpes simplex viruses, in tissue or cytology specimens.
Owner:何以丰
Method for the treatment or prevention of virus infection using polybiguanide-based compounds
An inexpensive, easily available, and convenient method of treating or preventing a virus infection is provided. The present invention relates to a method for the treatment or prevention of virus infections using polybiguanide-based compounds administering a therapeutically effective amount of a compound or a pharmaceutically acceptable salt thereof The invention relies on the unique biochemical reaction in which polybiguanide-based compounds interfere with the spread of virus within or between organisms. The compositions and formulations described in the present invention are effective means to reduce the infectivity of the human immunodeficiency virus type 1 (HIV-1), and human herpes simplex viruses, and also to kill the causative organisms of many other sexually transmitted diseases (STDs).
Owner:NOVAFLUX INC
Method for the treatment or prevention of virus infection using polybiguanide-based compounds
An inexpensive, easily available, and convenient method of treating or preventing a virus infection is provided. The present invention relates to a method for the treatment or prevention of virus infections using polybiguanide-based compounds administering a therapeutically effective amount of a compound or a pharmaceutically acceptable salt thereof. The invention relies on the unique biochemical reaction in which polybiguanide-based compounds interfere with the spread of virus within or between organisms. The compositions and formulations described in the present invention are effective means to reduce the infectivity of the human immunodeficiency virus type 1 (HIV-1), and human herpes simplex viruses, and also to kill the causative organisms of many other sexually transmitted diseases (STDs).
Owner:NOVAFLUX INC
Polymerase chain reaction (PCR) detection kit for chromosome-integrated herpesvirus hominis 6
ActiveCN103757136AQuick checkEasy to operateMicrobiological testing/measurementIntracellularHerpesvirus hominis
The invention provides a polymerase chain reaction (PCR) detection kit for chromosome-integrated herpesvirus hominis 6. The detection kit comprises a deoxyribonucleic acid (DNA) extracting agent, a PCR reagent tube ingredient, negative control and positive control. By adopting the PCR detection kit, the blank of domestic CI-HHV-6 detection is compensated, whether the sample DNA contains a specific amplification band or not at 178bp is detected by the kit, whether the sample cell is injected with herpesvirus hominis 6 or not can be judged, and the PCR detection kit is simple in operation, high in sensitivity and strong in specificity.
Owner:NANJING MEDICAL UNIV
Human herpes virus hominis pyrolysis, reproduction and activation host marker and purpose thereof
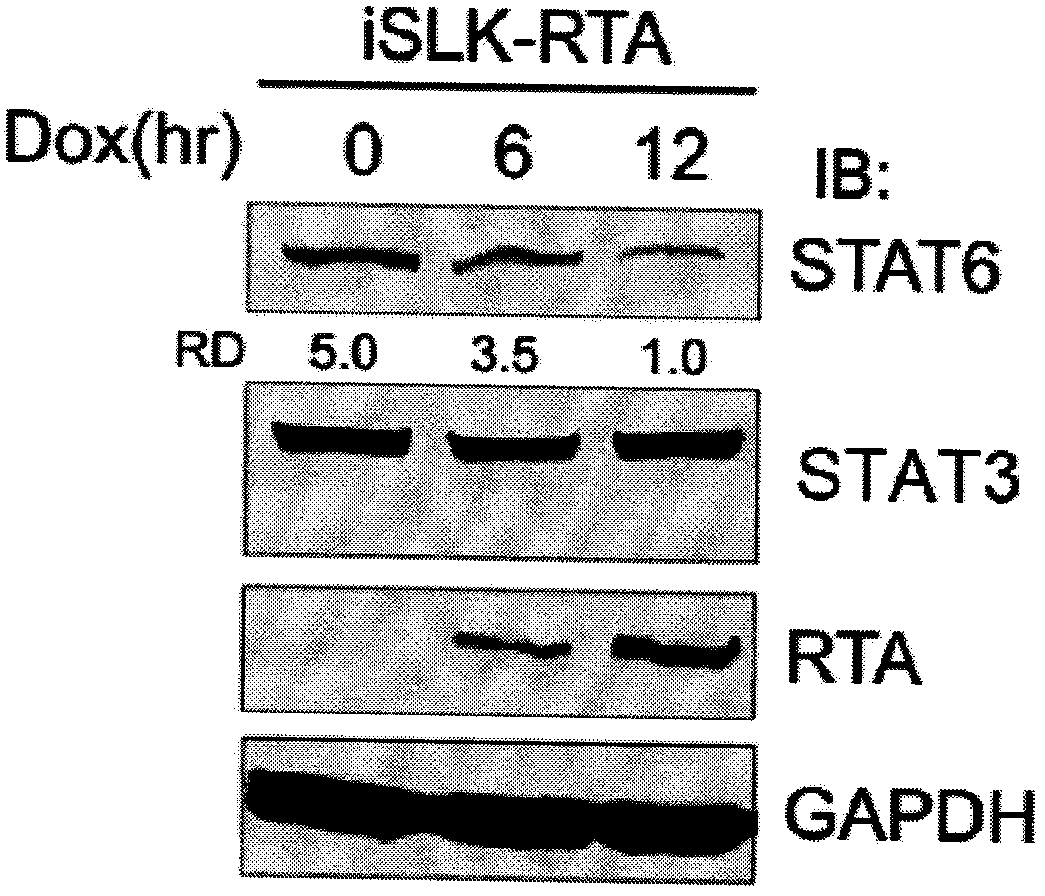
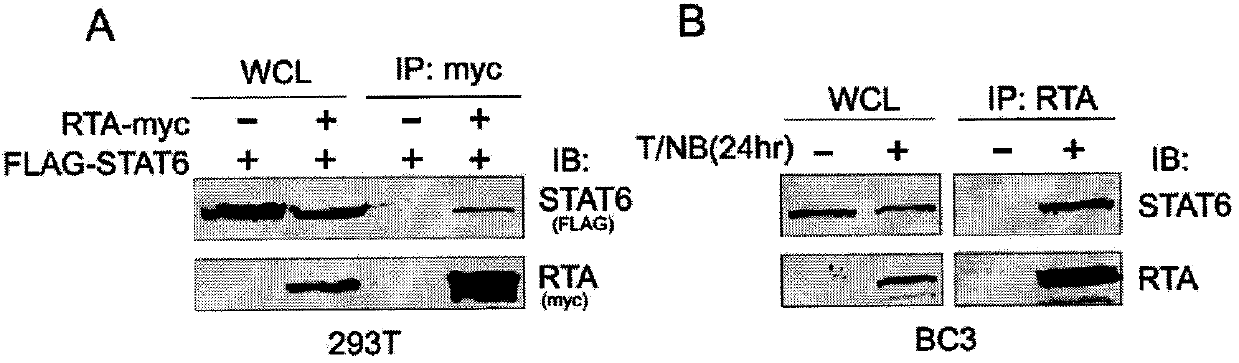
The invention belongs to the technical field of biomedicine, and relates to a human herpes virus hominis pyrolysis, reproduction and activation detection marker, in particular to the purpose of a signal transducer and activator of transcription 6 (Signal transducer and activator of transcription 6,STAT6) as the human herpes virus hominis pyrolysis, reproduction and activation detection marker. Target protein-STAT6 which is consistently regulated during human herpes virus pyrolysis and reproduction is obtained from the host angle, the experiment result shows that the STAT6 protein is significantly reduced in the infection course of Kaposi sarcoma herpesvirus (KSHV), and the STAT6 protein can be used as the marker for detecting pyrolysis, reproduction and activation of various herpes viruses, can be further used as a common target for diagnosing and treating herpes virus relevant tumor diseases, and can be further used for preparing products for preventing, detecting and treating human herpes virus hominis infection.
Owner:FUDAN UNIV
Methods for assessing fatigue level and applications thereof
InactiveUS20110020789A1Cause painCause to testMicrobiological testing/measurementDisease diagnosisDiseaseDaily living
Level of fatigue that accompanies everyday life or a disease can be simply, easily, and quantitatively assessed by obtaining a body fluid from a test subject and measuring the amount of human herpesvirus in the body fluid. Furthermore, the anti-fatigue potency of anti-fatigue substances and anti-fatigue food products can be measured.
Owner:VIRUS IKAGAKU KENKYUSHO
Human herpesvirus type 6 real-time fluorescent quantitative PCR typing detection kit
The invention provides a real-time fluorescent quantitation PCR (polymerase chain reaction) parting detection kit for human herpesvirus-6 (HHV-6). The real-time fluorescent quantitation PCR parting detection kit consists of quantitation PCR reaction liquid, an HHV-6A standard substance, an HHV-6B standard substance, an HHV-6A positive reference substance, an HHV-6B positive reference substance, a negative reference substance, a specification and a kit body, wherein the quantitation PCR reaction liquid contains a PCR buffer solution, MgCl2, dNTPs, heat-resistant DNA polymerase, an upstream amplification primer, a downstream amplification primer, an HHV-6A fluorescent probe and an HHV-6B fluorescent probe. By the adoption of a real-time fluorescent quantitation PCR technology and a double-color fluorescent probe, the kit disclosed by the invention can detect two subtypes of the HHV-6 by a one-step method; the HHV-6A and HHV-6B in the sample can be simultaneously parted; a positive virus subtype can be accurately quantified in real time; the urgent need of early and acute parting diagnosis on the HHV-6 infection can be met; a basis is supplied to epidemiological investigation and targeted treatment on the HHV-6 infection.
Owner:ZHEJIANG UNIV
Expression system for nonintegrated, long-time and erasable expression vector
ActiveCN101955977BStable expressionControlled deletionGenetic engineeringFermentationRecombinaseBiomedicine
The invention provides an expression system for a nonintegrated, long-time and erasable expression vector. The expression vector of the expression system adopts human herpes virus replication origin (Orip), an EBNA-1 expression box is introduced, and both ends of the Orip, both end of EBN-1 or both ends of Orip-EBNA-1 are provided with recombinase recognition sequences. The expression vector is not integrated in a cell genome, can stably express for a long time, can controllably erase vector plasmid by instantaneously inducing (or expressing) recombinase from a hose cell, can be applied to most kinds of cells by using a strong initiator and can distinguish a cell with the vector plasmid from a cell without the vector plasmid by using a real-time marker. The expression system can widely applied to mammal cell expression and biomedicine engineering for realizing cell reprogramming by expressing a foreign gene.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Method For The Simultaneous Detection And Quantification Of Epstein-Barr Virus, Cytomegalovirus, Human Herpesvirus 6, Human Herpesvirus 7 and Kaposi's Sarcoma Virus Using A Multiplex, Real-Time Polymerase Chain Reaction
PendingUS20210032710A1Microbiological testing/measurementMaterial analysisMultiplexHuman herpesvirus type 7
The present invention relates to a method for the multiple and simultaneous detection and quantification of any combination of beta and gamma genera human herpesviruses: Epstein-Barr virus (EBV), Cytomegalovirus (CMV), Human herpesvirus type 6 (HHV6), Human Herpesvirus type 7 (HHV7) an d Kaposi's Sarcoma Virus (KSV) and the beta-actin human endogenous gene by DNA amplification reaction using the multiplex polymerase chain reaction in real time.
Owner:HOSPITAL INFANTIL DE MEXICO FEDERICO GOMEZ
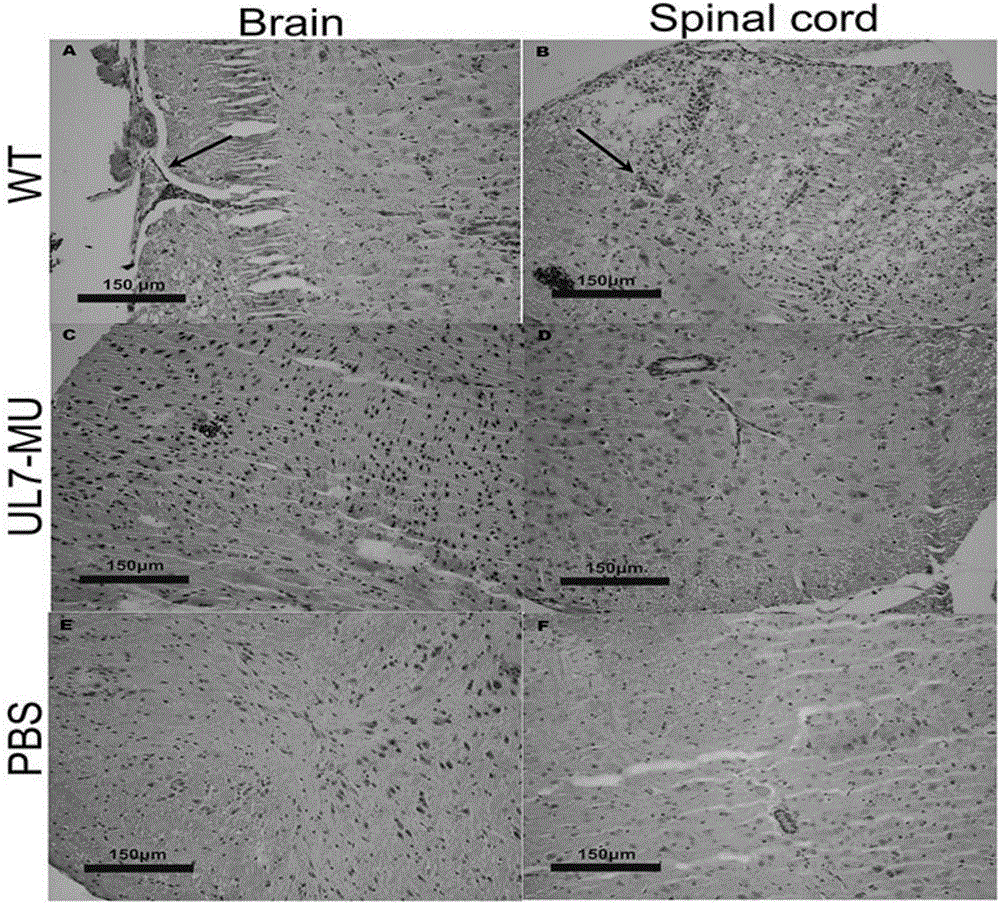
Human herpes simplex type-I virus UL-7 gene mutation attenuated strain as well as construction method and applications thereof
InactiveCN105950573AReduce capacityPerfect technologyInactivation/attenuationMicroorganism based processesUltrasound attenuationGenetic engineering
The invention belongs to the fields of genetic engineering and genetic modification, and in particular relates to a human herpes simplex type-I virus UL-7 gene mutation attenuated strain as well as a construction method and applications of the human herpes simplex type-I virus UL-7 gene mutation attenuated strain. With the adoption of the technical scheme, the technical process and validation index for preparing the HSVI UL-7 mutation attenuated strain are perfected. After the UL-7 mutation attenuated strain is obtained, the corresponding detection confirmation is carried out on the attenuation features of the UL-7 mutation attenuated strain. Meanwhile, the corresponding study is made on the application possibility of the mutant strain virus as the HSVI attenuated live vaccine.
Owner:康普润(苏州)生物科技有限公司
Clinical assays for the detection and typing of human herpesviruses
InactiveUS7348145B2Quick checkTest cycle time superiorSugar derivativesMicrobiological testing/measurementHeterologousHeteroduplex
The present invention provides methods of unambiguously identifying a human herpesvirus in a sample. The assays, which allow for the detection and typing of all ten human herpesviruses, involve multiplex PCR assays using consensus primers to amplify conserved regions of the herpesvirus DNA. A dot blot / chemiluminescence assay and real time PCR assay ideal for clinical setting were disclosed. A heteroduplex mobility assay suitable for uses in research laboratory was also presented.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
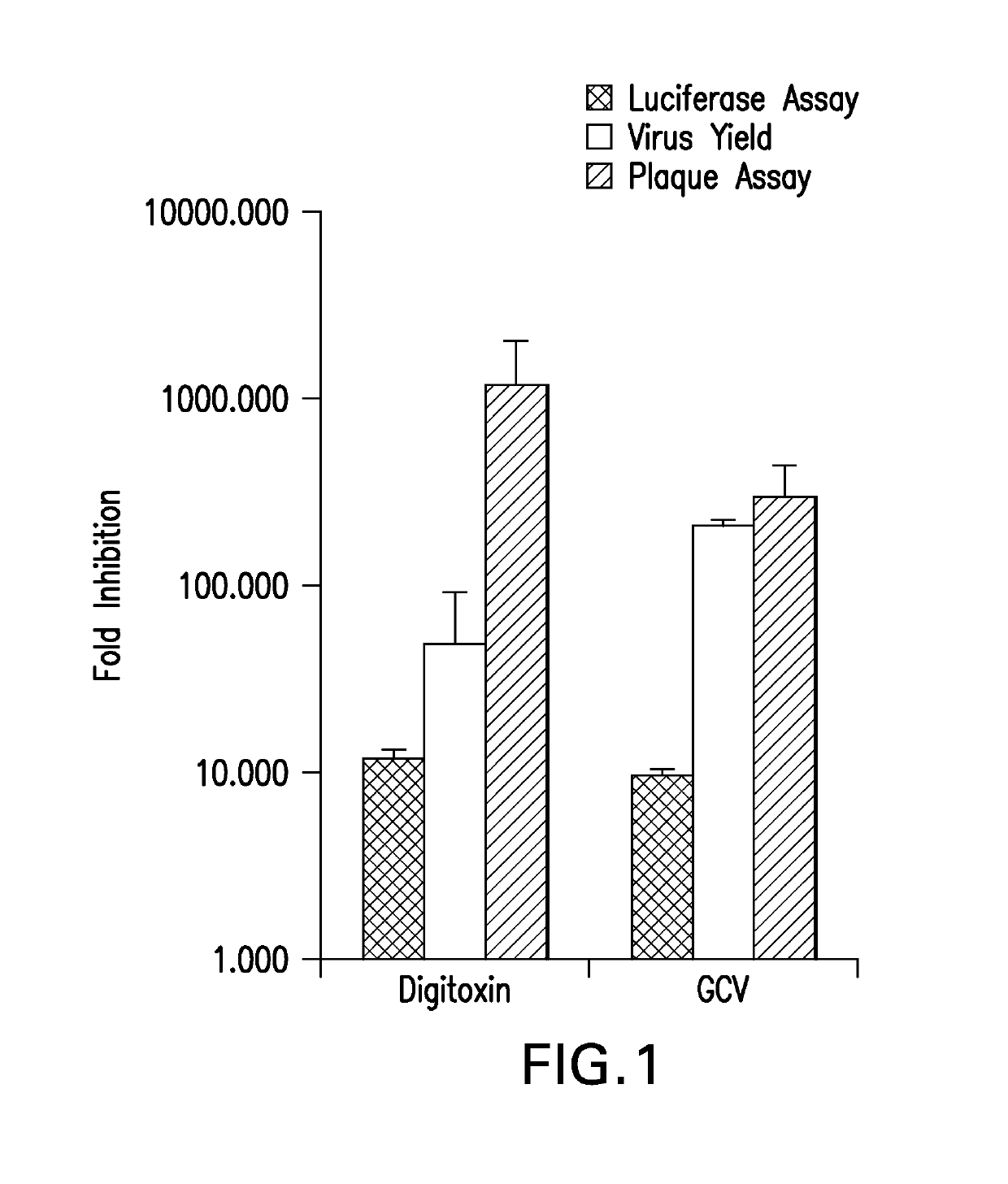
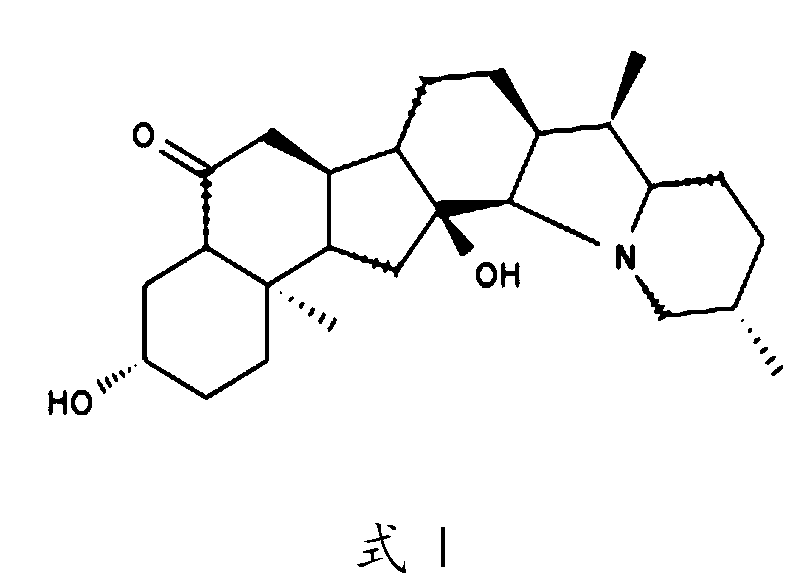
Cardiac glycoside analogs and their use in methods for inhibition of viral infection
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
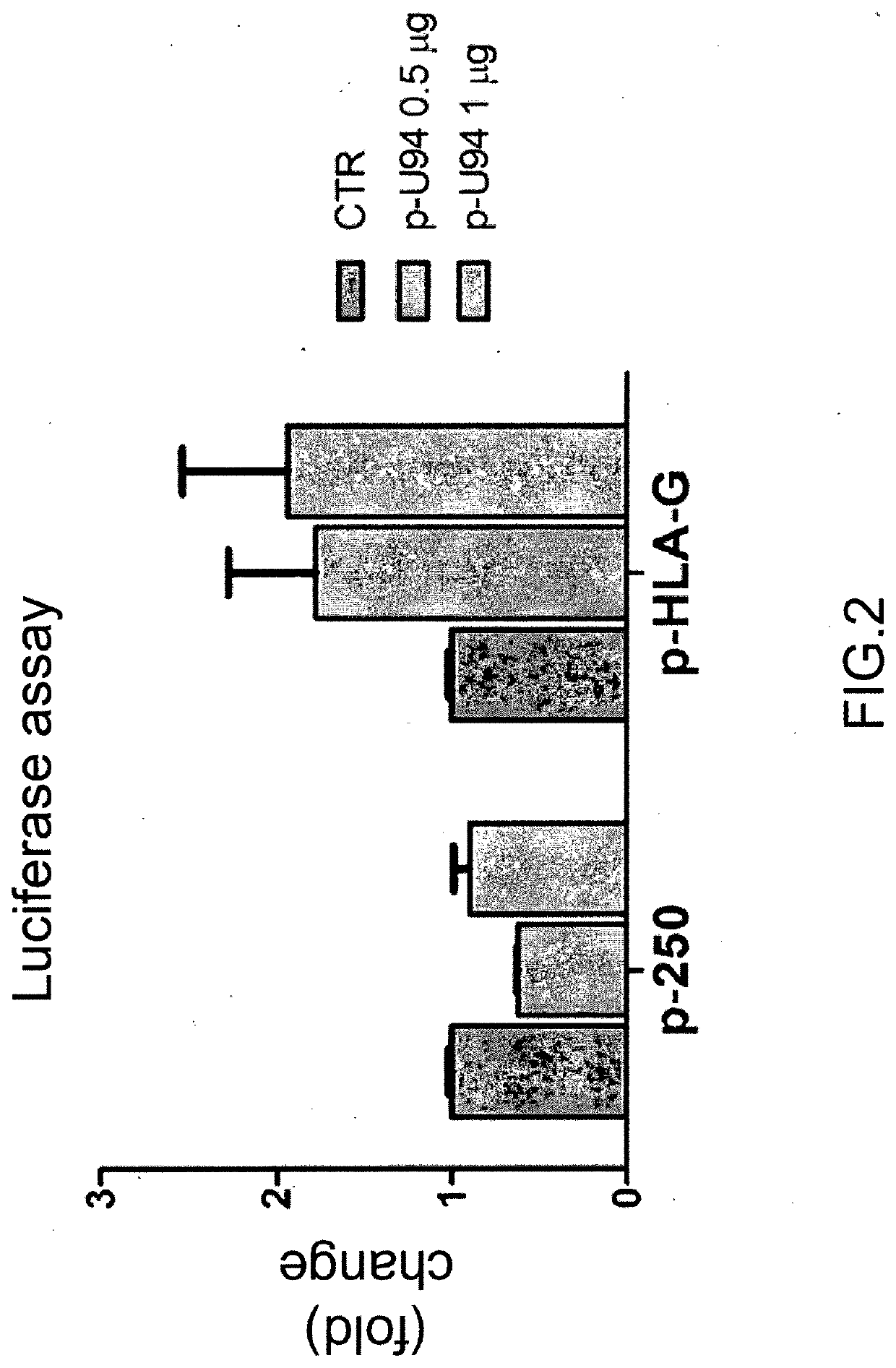
Use of the u94 molecule of human herpesvirus 6 and derivatives thereof to increase or induce the expression of the hla-g molecule
A method of enhancing or inducing the expression of the HLA-G molecule by human cells is provided. The method includes administering the human Herpesvirus 6 U94 molecule or a derivative thereof, or a gene expression vector including and expressing the human Herpesvirus 6 U94 gene to a patient in need thereof or to an in vitro cell, tissue, or organ culture.
Owner:FOND U BONINO E M S PULEJO
PCR (Polymerase Chain Reaction) primer group for detecting human herpes virus 6B type in human body fluid and droplet type digital PCR kit thereof
PendingCN114807452ARich in genetic informationReduce background noiseMicrobiological testing/measurementMicroorganism based processesDiseaseConserved sequence
The invention discloses a PCR (Polymerase Chain Reaction) primer group for detecting human herpes virus type 6B in human body fluid and a droplet type digital PCR kit thereof. A specific primer and an MGB hydrolysis probe are designed on the basis of a conserved sequence of a human herpes virus 6B genome, so that the microdroplet type digital PCR kit is obtained, free DNA in body fluid of a patient suffering from blood system diseases after being treated by chimeric antigen receptor T cells is extracted, HHV-6B DNA is detected through the microdroplet type digital PCR kit, and the detection sensitivity is high. The method has important guiding significance for assisting clinical understanding of HHV-6B infection conditions, identification of infection of other pathogens and identification of CAR-T cell-related encephalopathy syndromes.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Application of pimbidone in the preparation of medicaments for preventing and/or treating human herpesvirus infection
ActiveCN106265658BImprove survival rateStrong antiviral activityOrganic active ingredientsAntiviralsMedicineDeath cause
Owner:JIANGSU KANION PHARMA CO LTD
Topical antiviral composition containing a local anesthetic and method of making the same
The present invention provides a novel pharmaceutical composition and method of making the same. The composition comprises an effective amount of an antiviral agent and a local anesthetic. The antiviral drug can be sorivudine. The local anesthetic can be lidocaine or its pharmaceutically acceptable salt. Herpes virus infections in humans can be caused by different human herpes viruses, the most common being herpes simplex virus types 1 and 2 (HSV1 and HSV2) and varicella-zoster virus (VZV).
Owner:NAL PHARM LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com