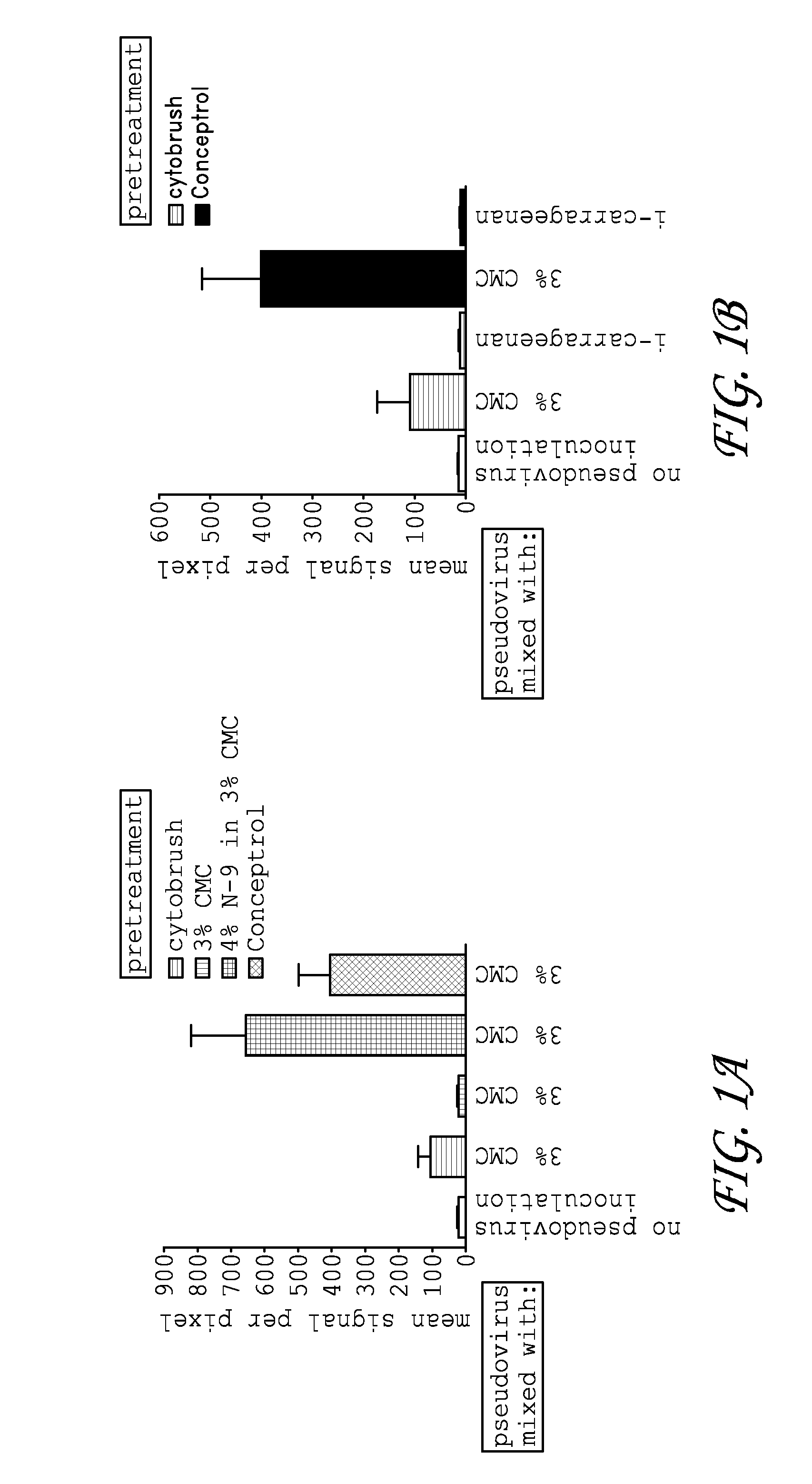
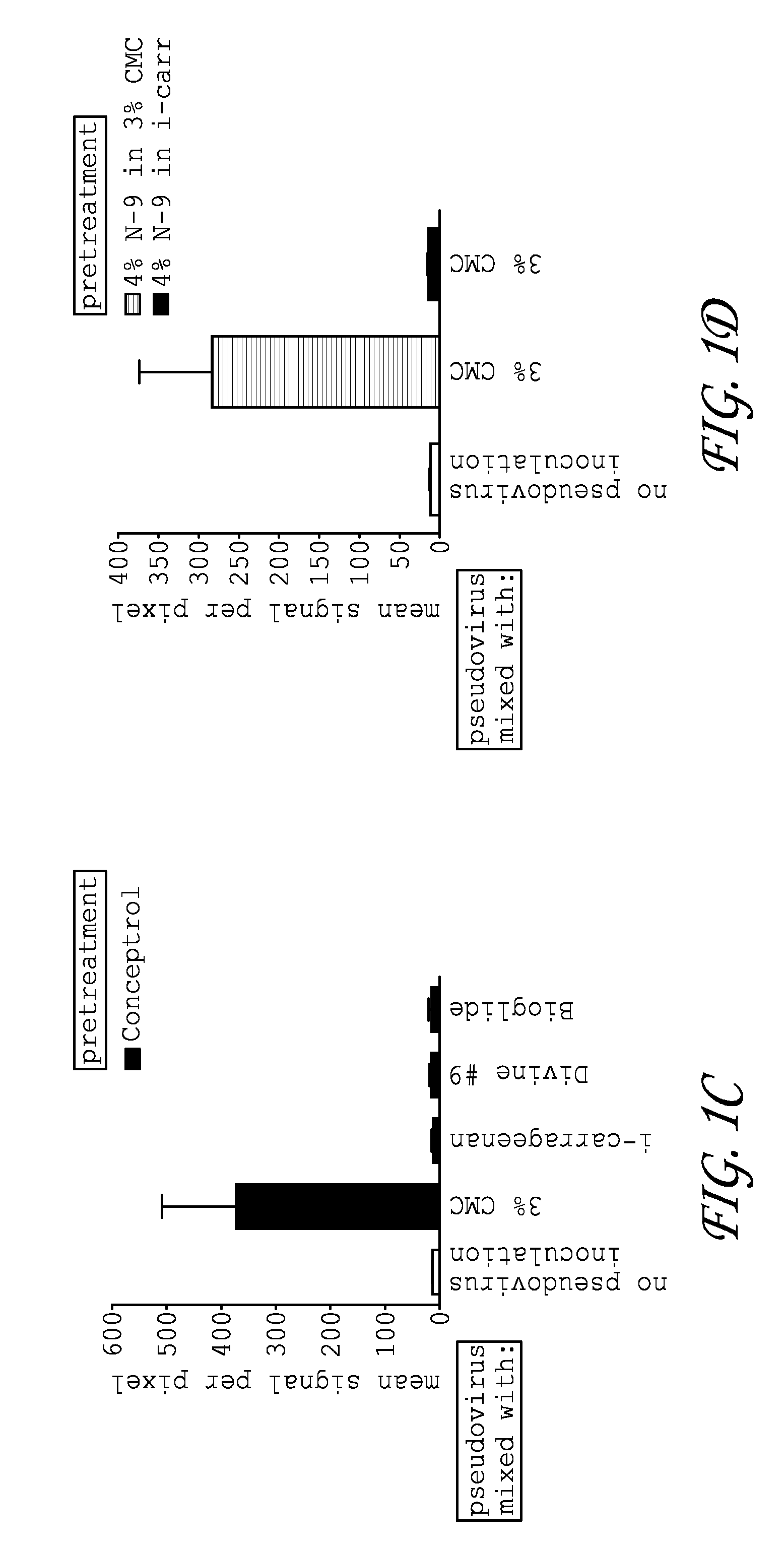
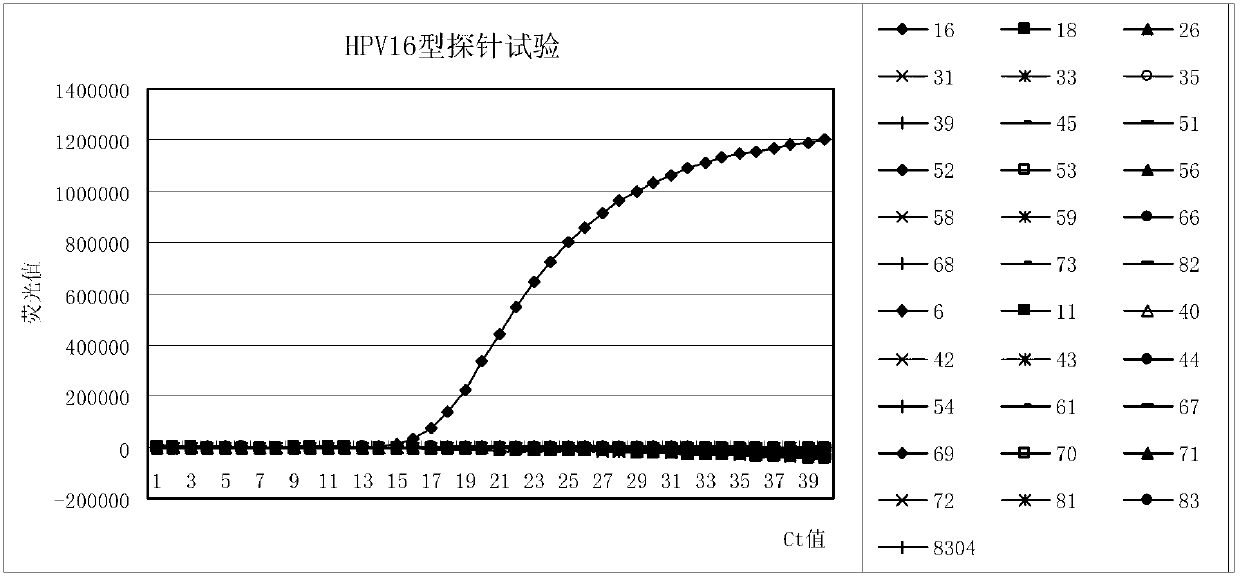
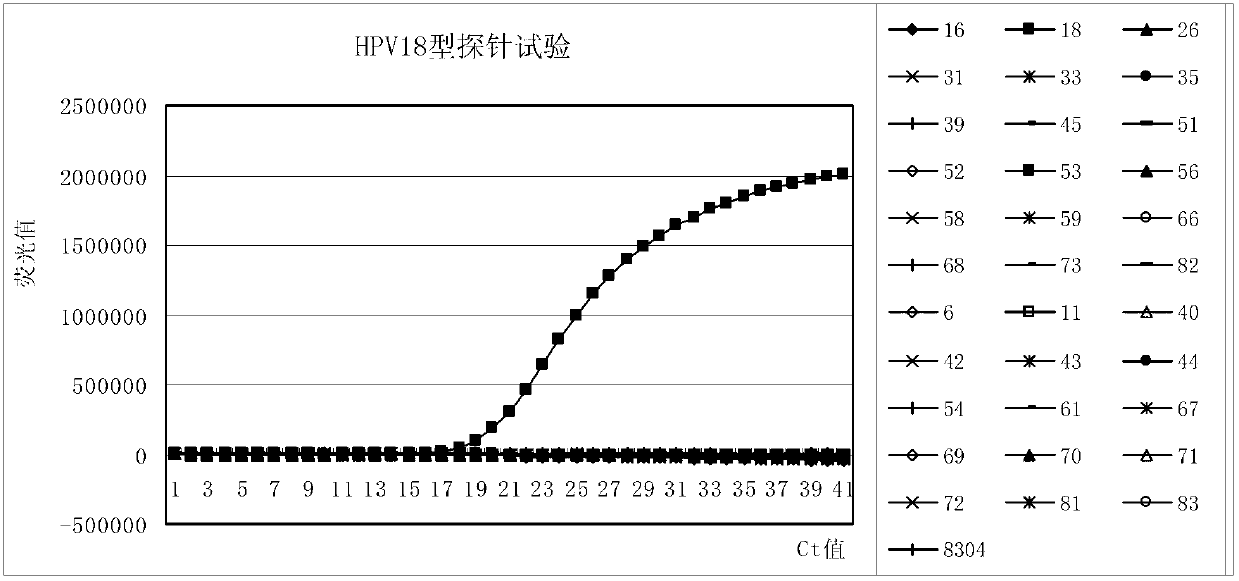
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
149 results about "Papilloma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A benign epithelial tumor that forms on the skin or mucous membrane.
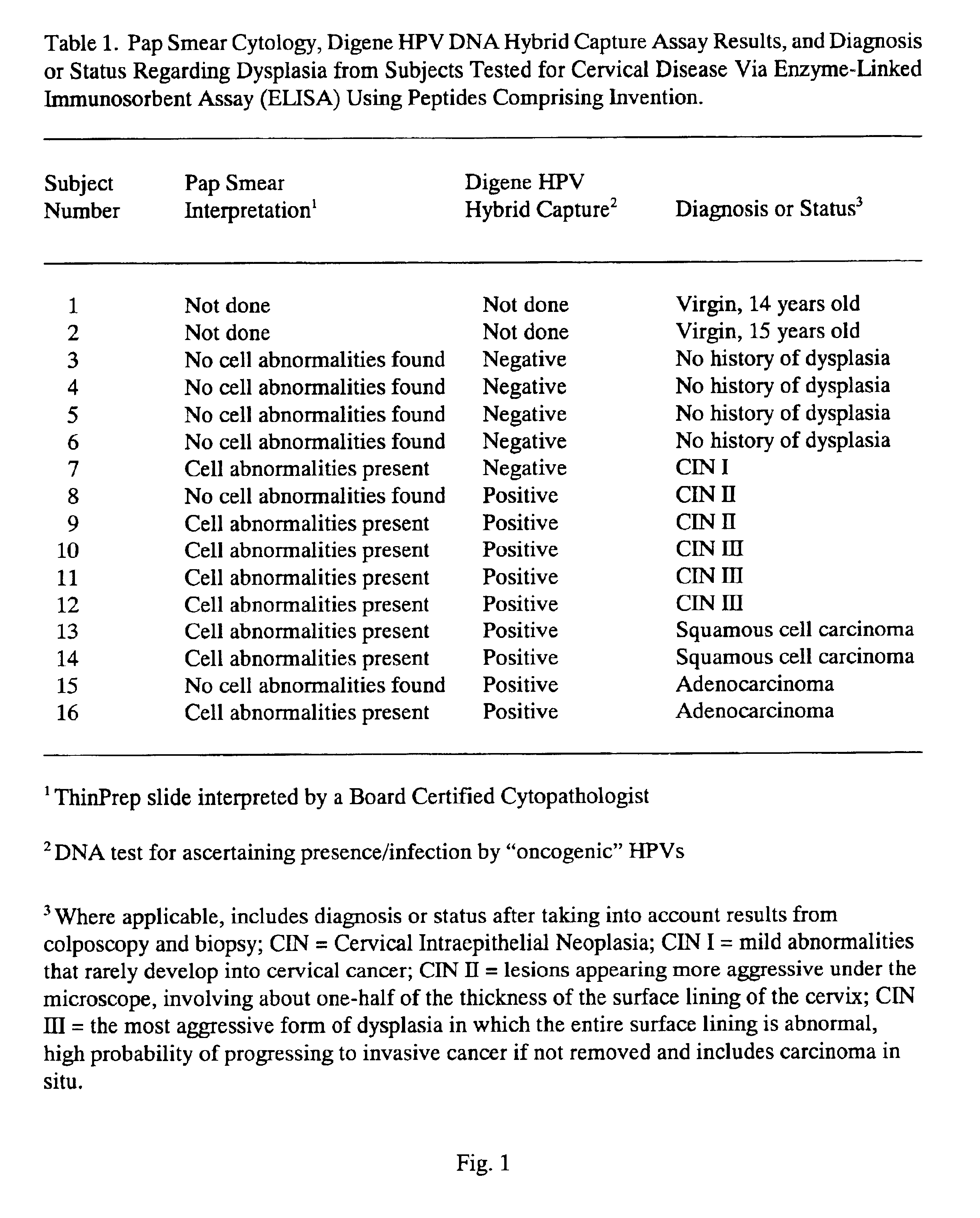
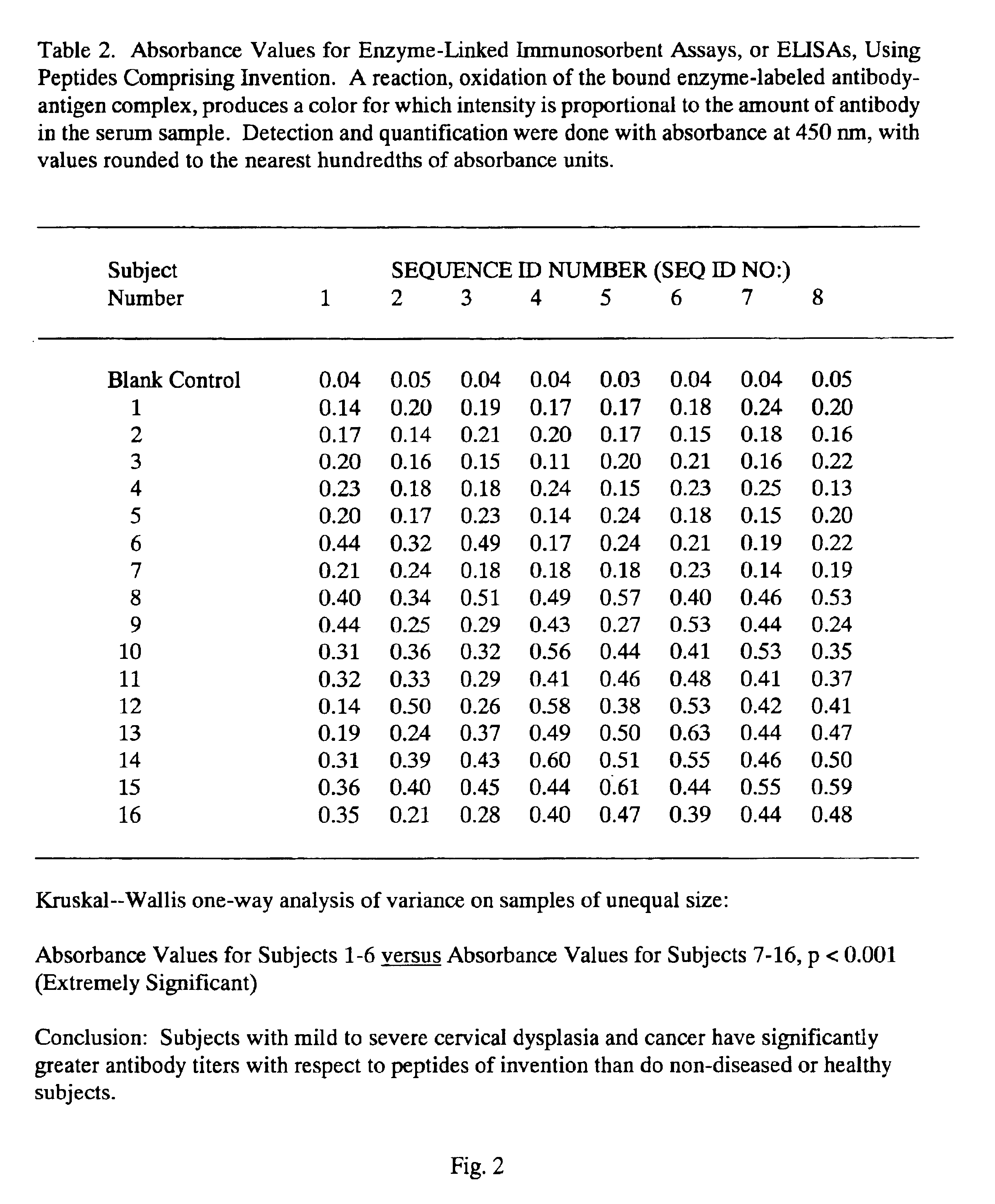
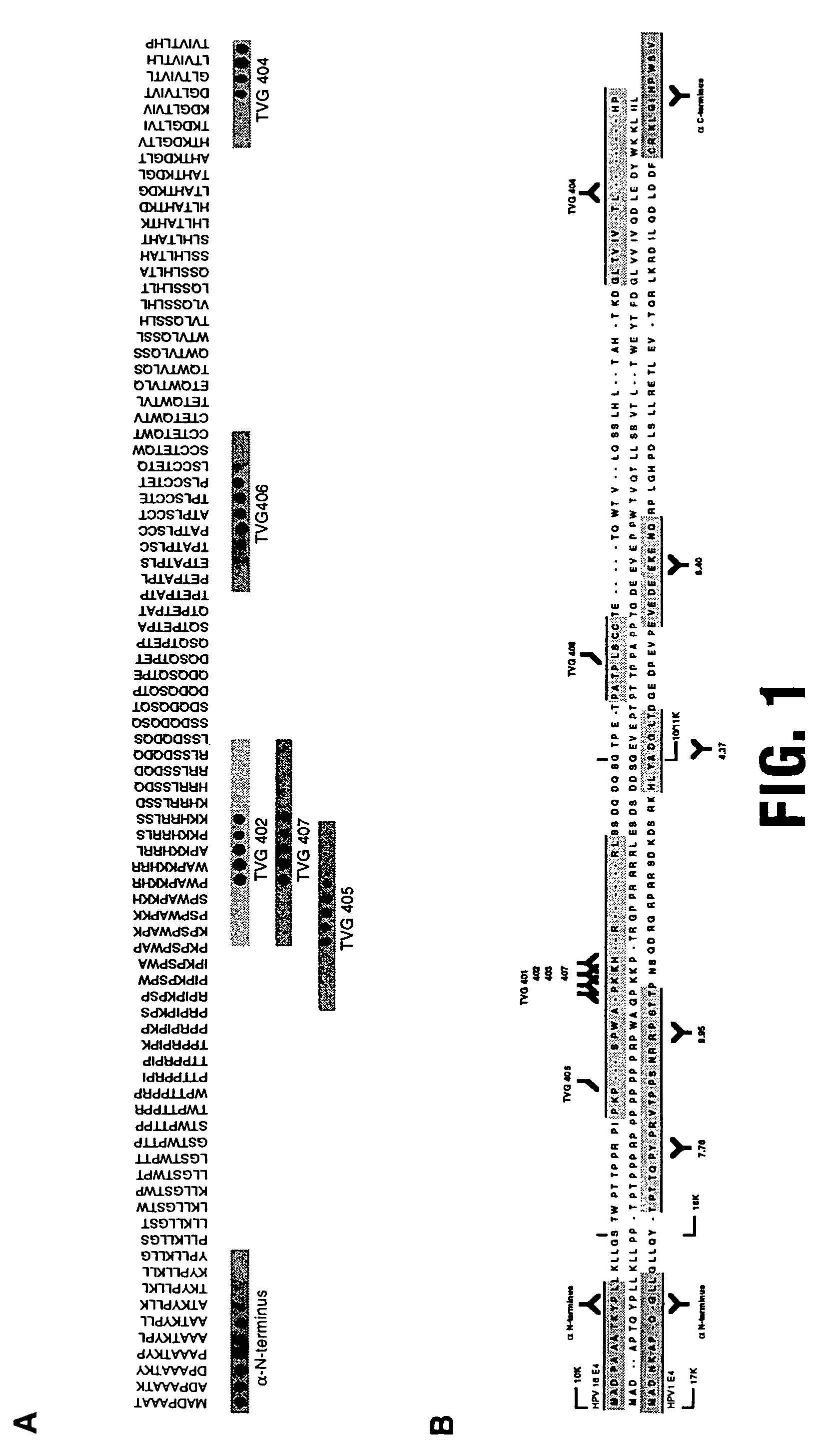
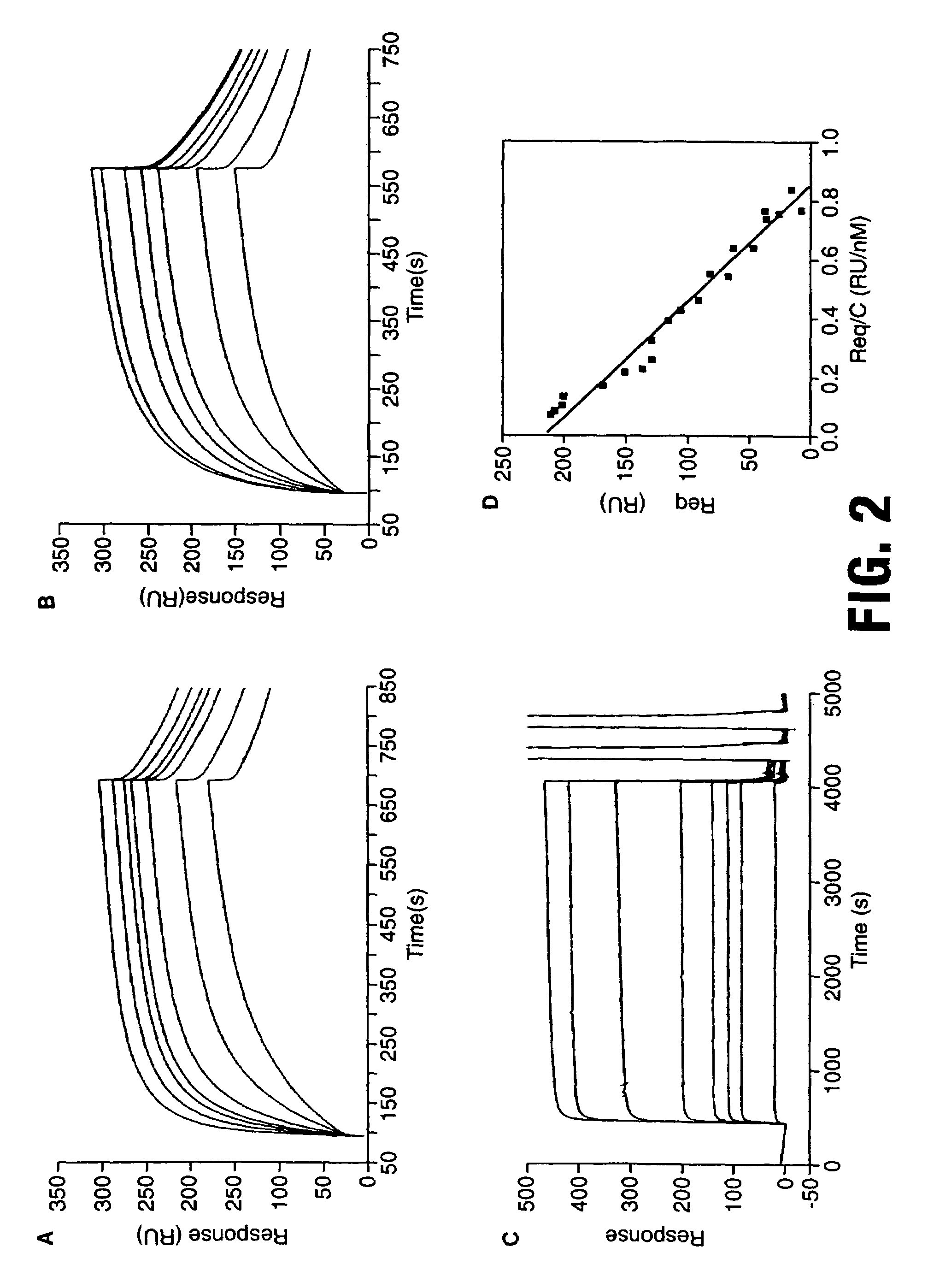
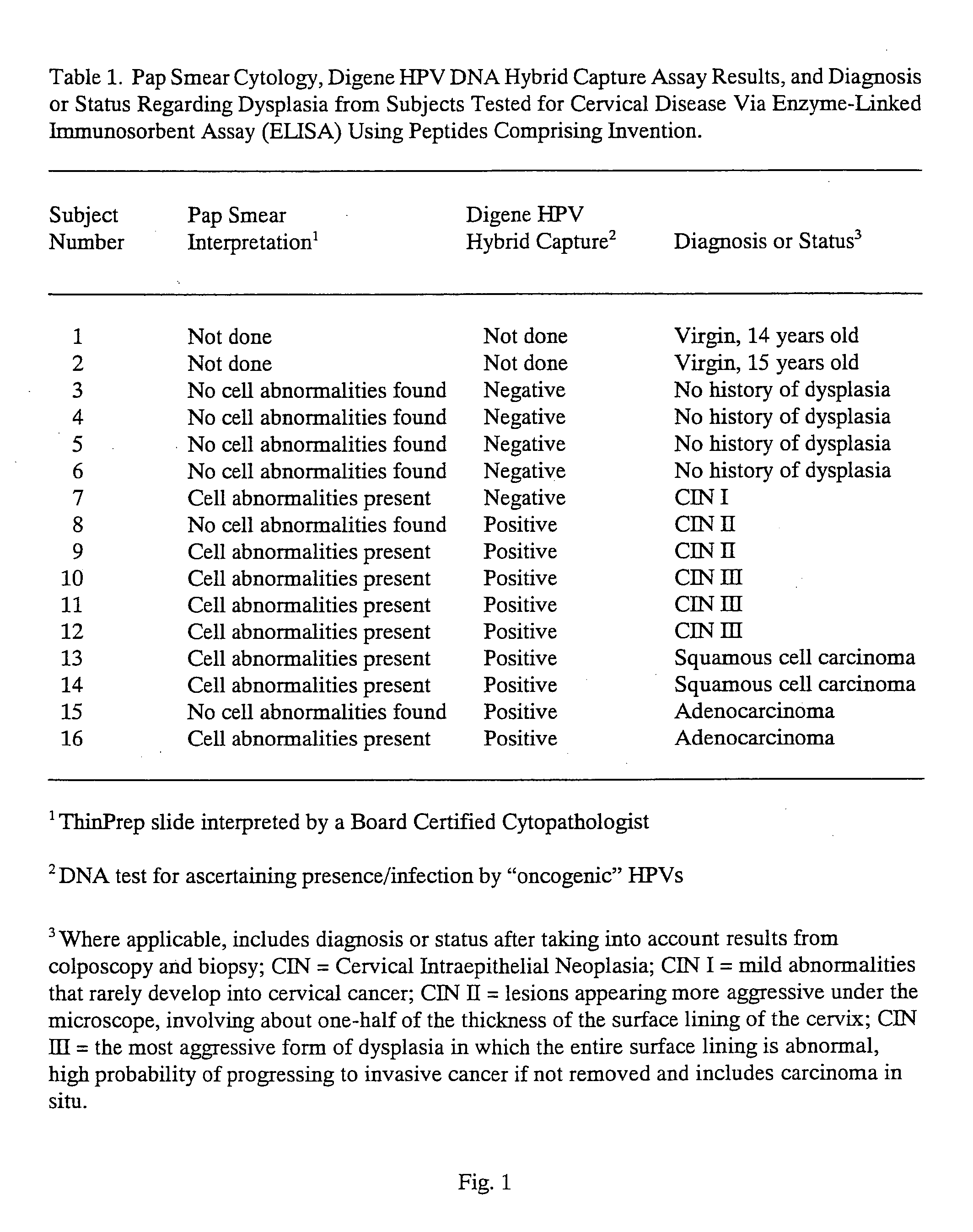
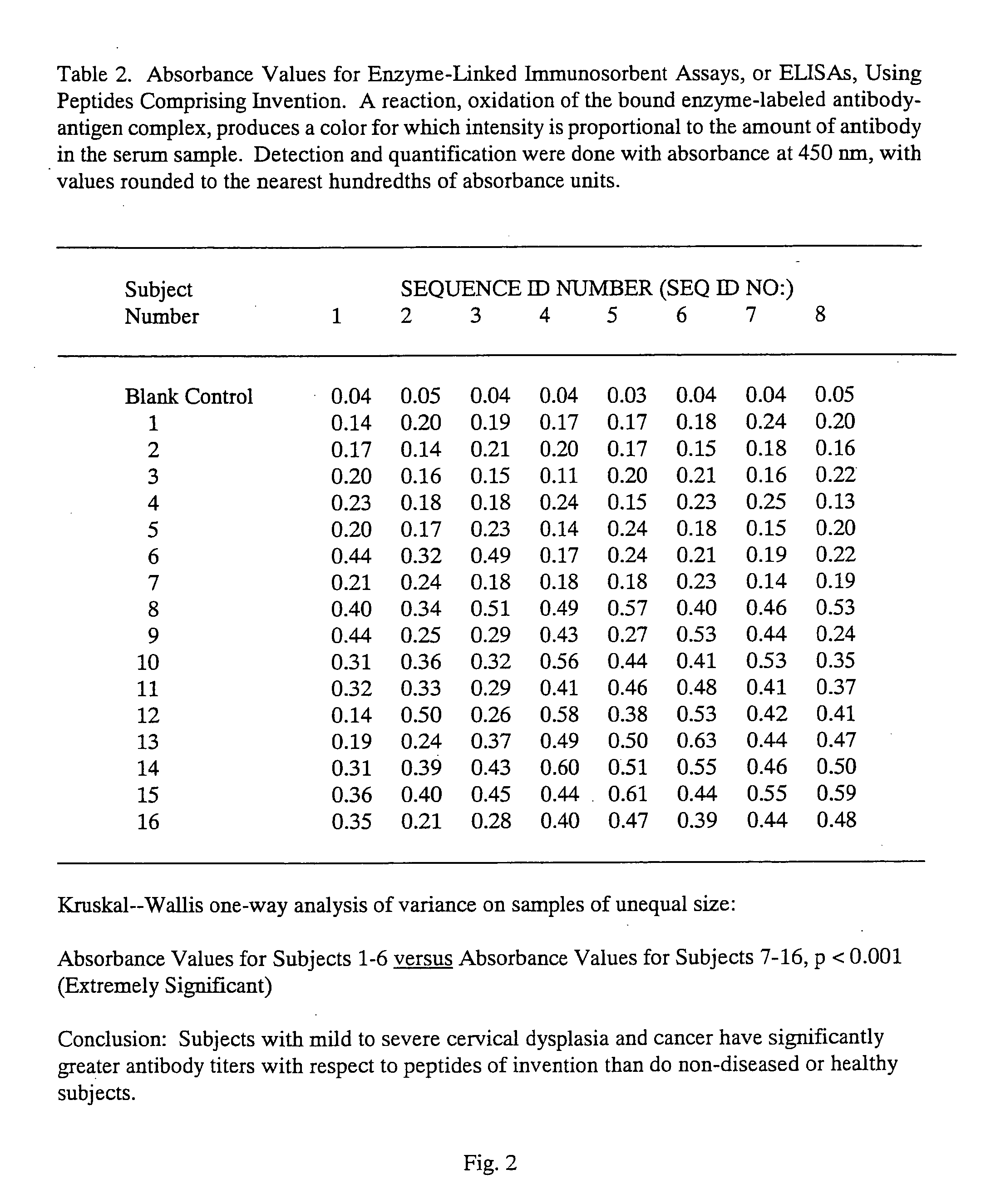
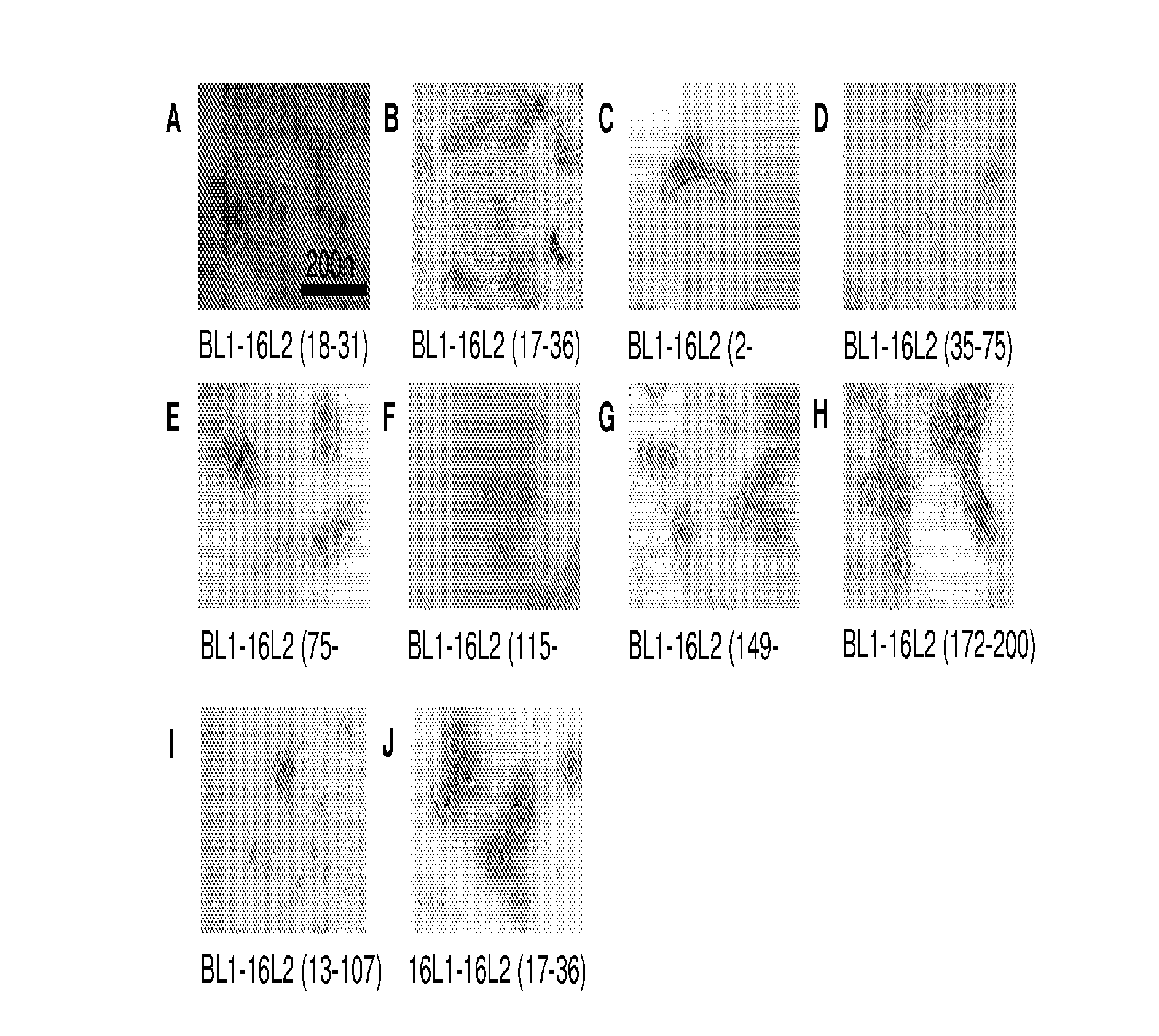
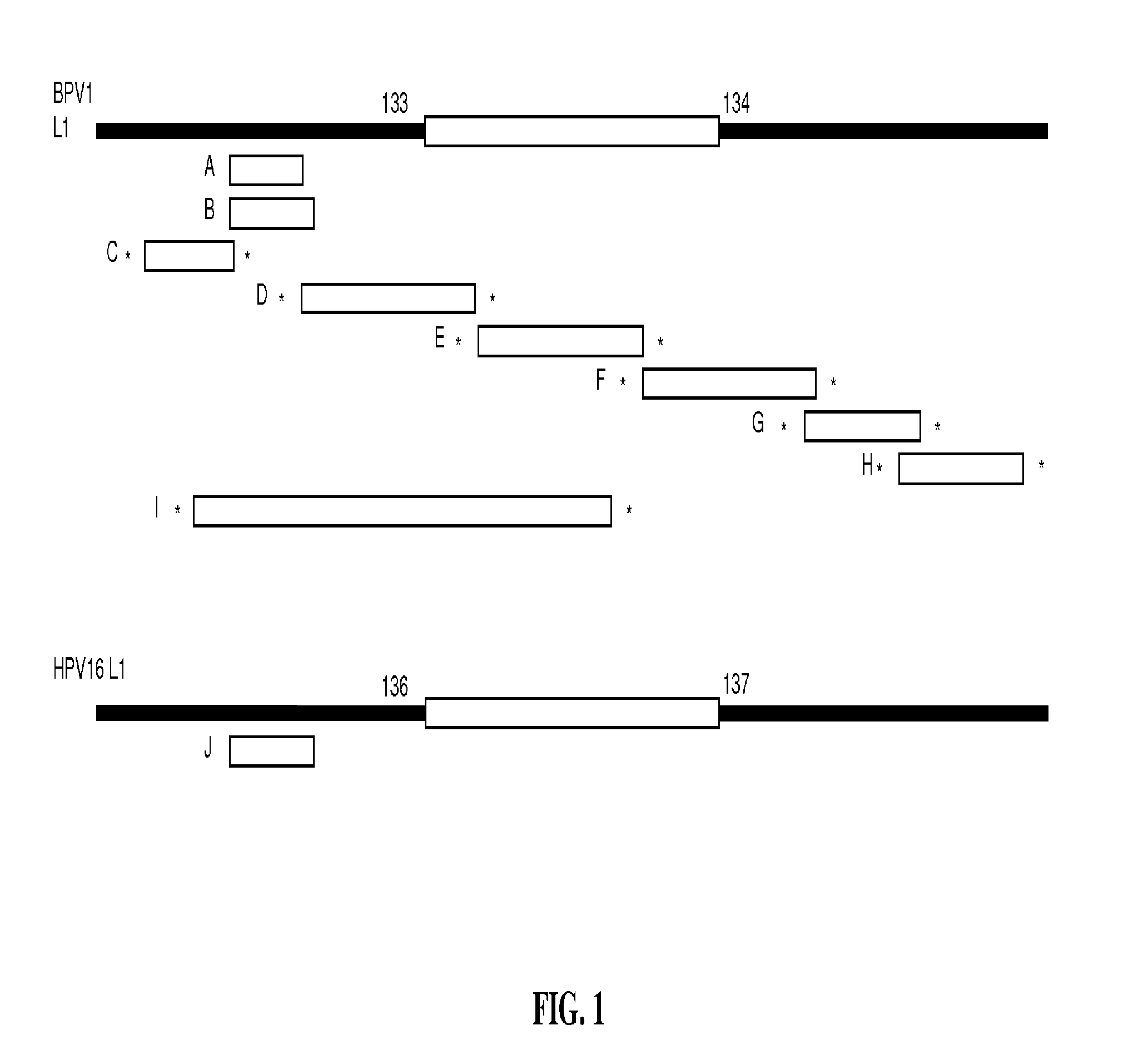
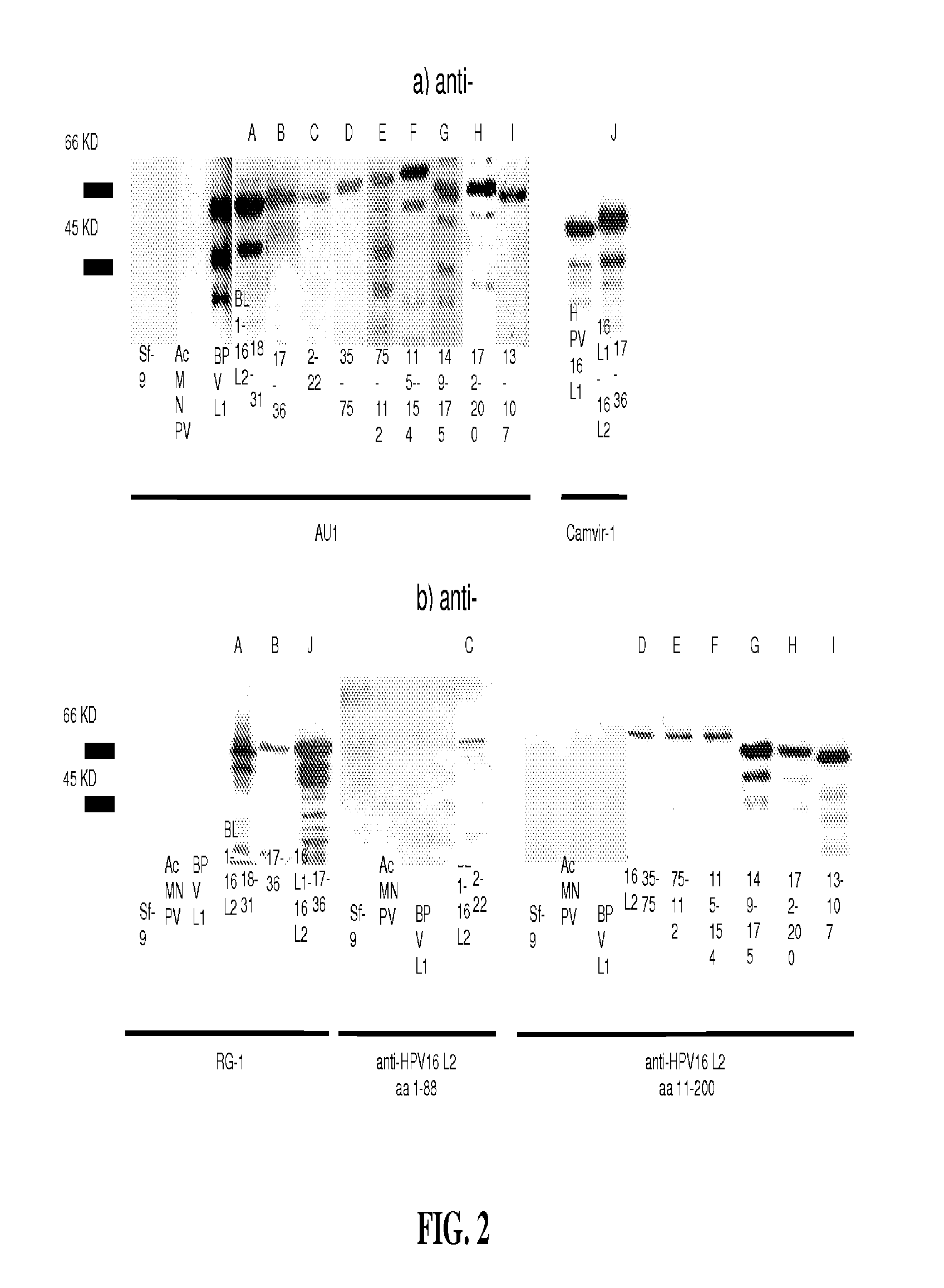
Peptides from the E2, E6, and E7 proteins of human papilloma viruses 16 and 18 for detecting and/or diagnosing cervical and other human papillomavirus associated cancers
InactiveUS6933123B2Simple and rapid and and more testMicrobiological testing/measurementVirus peptidesCysteine thiolateTryptophan
Owner:HU YAO XIONG
Virion Derived Protein Nanoparticles For Delivering Diagnostic Or Therapeutic Agents For The Treatment of Alopecia
InactiveUS20130012566A1Overcomes shortcomingSimple methodOrganic active ingredientsPeptide/protein ingredientsDiseaseTherapeutic intent
This invention relates to a transdermal delivery system for treating skin related diseases employing papilloma-derived protein nanoparticles to deliver drugs to the keratinocytes and basal membrane cells for the treatment of alopecia. The current invention presents an effective method for delivering small molecule nucleic acids to the epidermal cells.
Owner:AURA BIOSCI
Traditional Chinese medicine for treating breast diseases
InactiveCN102151325AAnthropod material medical ingredientsAntineoplastic agentsMyrrhFritillaria thunbergii
The invention provides traditional Chinese medicine for treating breast diseases, which is formed by mixing Chinese angelica, red paeony root, peach kernels, red flower, salvia miltiorrhiza, zedoary turmeric oil, rhizoma corydalis, szechuan lovage rhizome, lumbricus, pangolin scales, uniflower swisscentaury root, fritillaria thunbergii, spina gleditsiae, cowherb seeds, angelica root, mongolian snakegourd root, concha ostreae, pericarpium citri reticulatae viride, nutgrass galingale rhizome, tangerine seeds, radix bupleuri, dandelion, rhizoma pleionis, fructus forsythiae, astragalus root, codonopsis pilosula, liquorice, frankincense, myrrh, poria cocos and oldenlandia diffusa. The traditional Chinese medicine has the effects of invigorating Qi and enriching the blood, invigorating the blood circulation and softening the hard lumps, activating the meridians and detoxicating, soothing the depressed livers and regulating the flow of Qi, softening and resolving the hard lumps, invigorating the spleens and reducing the phlegm, strengthening the healthy energy and eliminating the pathogenic factors and can be used for treating hyperplasia of mammary gland, acute or chronic mastitis, breast fibroadenoma, intraductal papilloma, breast lump, breast scrofula, breast carbuncle and breast cancer.
Owner:苑学
Methods for generating immunity to antigen
InactiveUS20050226888A1Tumor rejection antigen precursorsTumor specific antigensInfectious agentTumor antigen
Provided are methods of generating an immune response to an antigen. The method comprises priming an individual by administering an expression vector encoding the antigen. The vectors comprises a transcription unit encoding a secretable fusion protein, the fusion protein containing an antigen and CD40 ligand. Administration of a fusion protein containing the antigen and CD40 ligand is used to enhance the immune response above that obtained by vector administration alone. The invention methods may be used to generate an immune response against cancer expressing a tumor antigen such as a mucin or human papilloma viral tumor antigen and to generate an immune response against an infectious agent. Also provided is a method for simultaneously producing the expression vector and the fusion protein.
Owner:MICROVAX +1
Papillomavirus pseudoviruses for detection and therapy of tumors
ActiveUS20100135902A1Ultrasonic/sonic/infrasonic diagnosticsCompounds screening/testingCancer cellCancer therapy
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Primer, probe and kit for fluorescence PCR (Polymerase Chain Reaction) detection of 18 high-risk human papilloma viruses
ActiveCN102994651AMicrobiological testing/measurementFluorescence/phosphorescenceHuman papillomavirus DNAFluorescence
The invention discloses a primer, a probe and a kit for fluorescence PCR (Polymerase Chain Reaction) detection of 18 types of high-risk human papilloma viruses. Different typing kits are detected by adding different specificity probes; the DNAs (Deoxyribose Nucleic Acids)) of 18 types of common high-risk human papilloma viruses internationally recognized and closely related to the cervical cancer can be detected once; and typing detection is carried out on HPV (Human Papilloma Virus)16 and 18. The application provides 6 universal primers and 18 specific molecular beacon probes. The DNAs of the 18 types of common high-risk human papilloma viruses can be amplified by the 6 universal primers; and meanwhile, the 18 probes are the specific molecular beacon probes designed by aiming at the 18 high-risk types; different types of probes are added according to the detection need and combined as the kits aiming at the detection need of the different high-risk types; and at the same time, the added probes are marked by different report genes, so that the purpose of carrying out typing detection on the high-risk HPV is achieved.
Owner:英科新创(苏州)生物科技有限公司
Papillomavirus pseudoviruses for detection and therapy of tumors
ActiveUS8394411B2Compounds screening/testingUltrasonic/sonic/infrasonic diagnosticsCancer cellCancer therapy
Owner:UNITED STATES OF AMERICA
Gelled immunomodulating topical compositions and a method of treating warts and other human papilloma virus skin infections
Topical drug compositions of this invention contain delayed type contact sensitizing haptens in a unique non-flowable, non-toxic, non-volatile, anhydrous gel composition to achieve retained site application on warts and other human papilloma virus (HPV) skin infections. The preferred gelled compositions contain, but are not limited to, the sensitizing haptens, squaric acid dibutylester and diphenylcyclopropenone in optimized blends of Polysorbate 80, Isopropyl myristate uniquely gelled with Polyoxyl 40 stearate to form a penetrant of keratinized epitheliiuim of warts for direct application wherein virucidal pharlacologic action is induced by Th-1 cell mediated immune responses with resultant releases of CD4 helper T cells, CD8 killer T cells and cytokines to attack the human papilloma viruses. The commonly used vehicles with these contact sensitizers are acetone, petrolatum, or water containing emulsion creams which do not have the capacity to penetrate the keratinized wart surfaces and are therefore minimally effective in treating warts.
Owner:HAPTEN PHARMA
Screening for papilloma viruses
InactiveUS7135281B2Microbiological testing/measurementImmunoglobulins against virusesHuman papilloma virus infectionMalignancy
The invention relates to a method of screening for precursor lesions which can lead to cervical malignancy, methods of detecting and typing human papilloma virus infections, and reagents of use in these methods.
Owner:MEDICAL RESEARCH COUNCIL
Pharmaceutical use of medicine composition containing zedoary turmeric oil
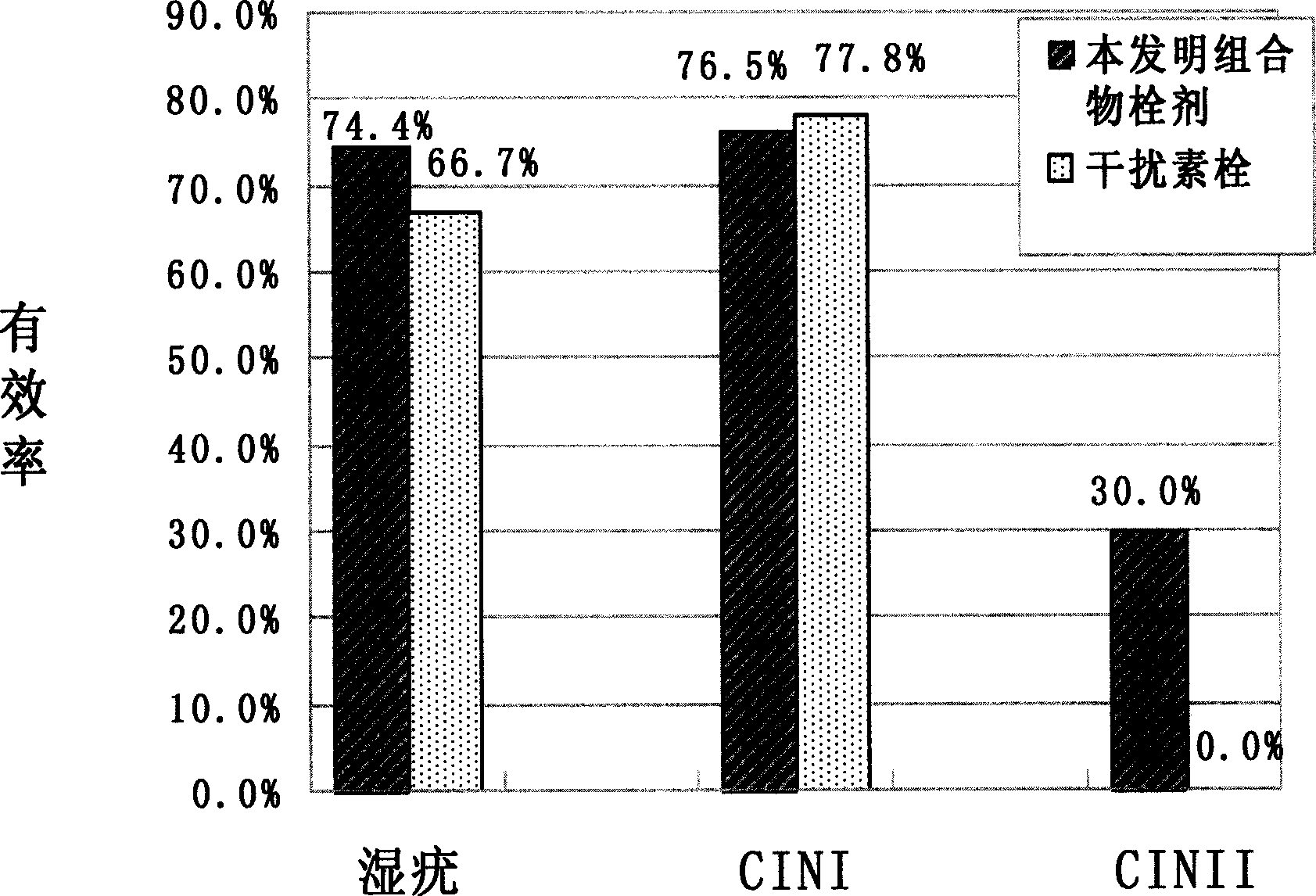
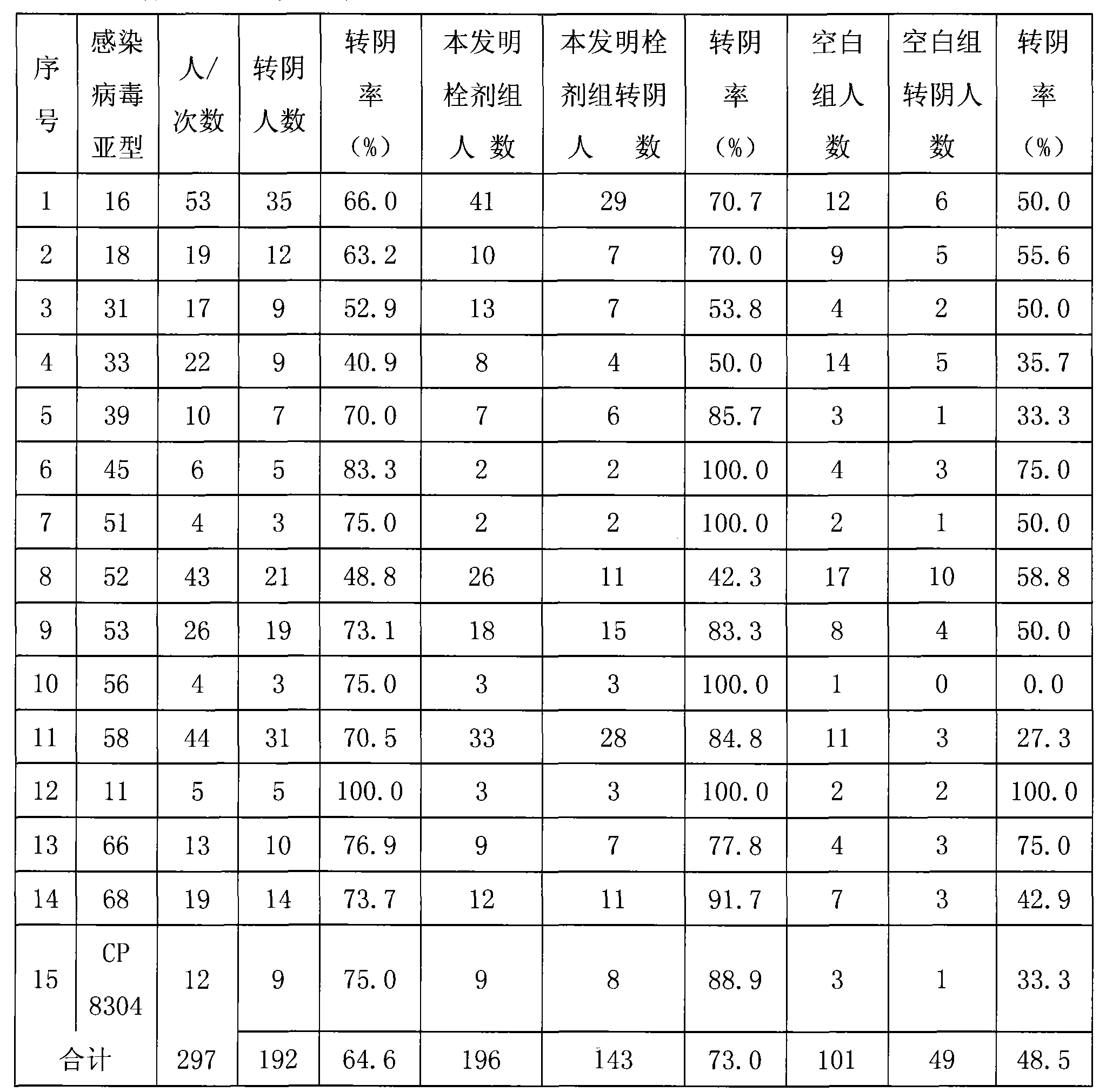
The invention relates to the pharmaceutical application of a medicament combination containing oil of zedoary turmeric. The medicament combination has new pharmaceutical application for preventing and treating various diseases, such as various HPV virus subtype infections, intraepithelial lesion(isl), papilloma and cervical carcinoma, and the like, in particular to high-risk HPV subtype infections. The medicament is prepared into various conventional formulations in the category of pharmaceutics, and the medicament combination can significantly prevent and treat multiple related diseases of HPV infection by clinical verification.
Owner:HAINAN BIKAI PHARM CO LTD
Tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor)
InactiveCN107034235AIncrease lethalityImprove homingGenetically modified cellsMammal material medical ingredientsTumor targetEGFR Antibody
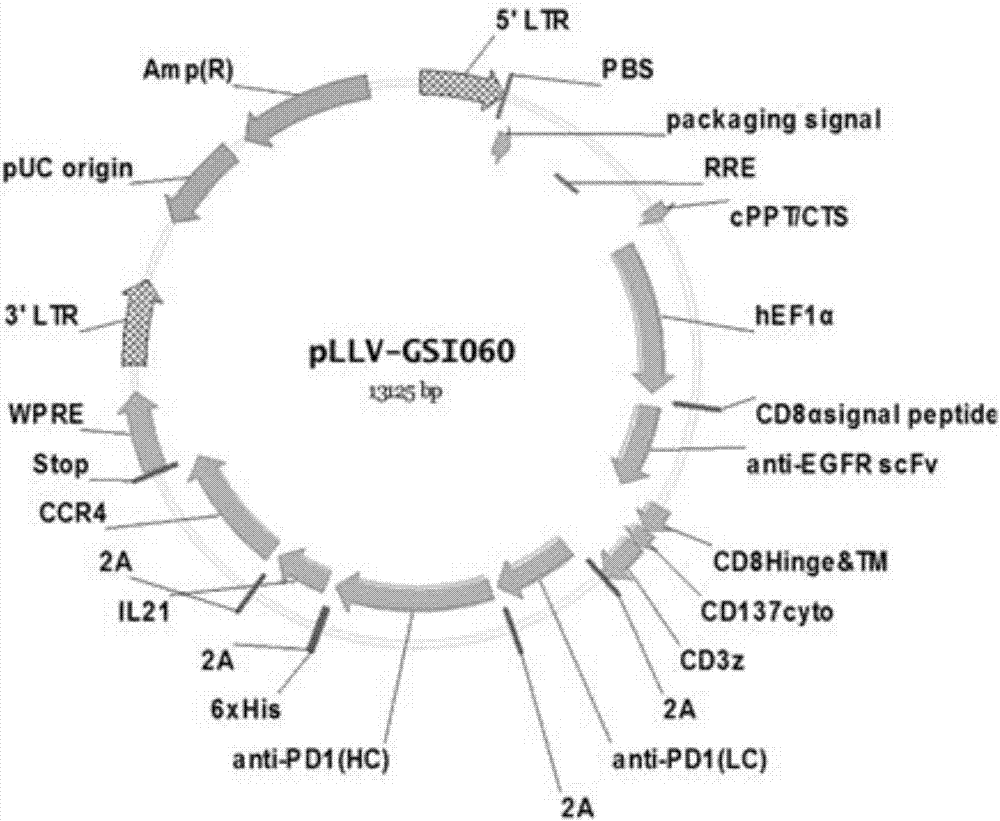
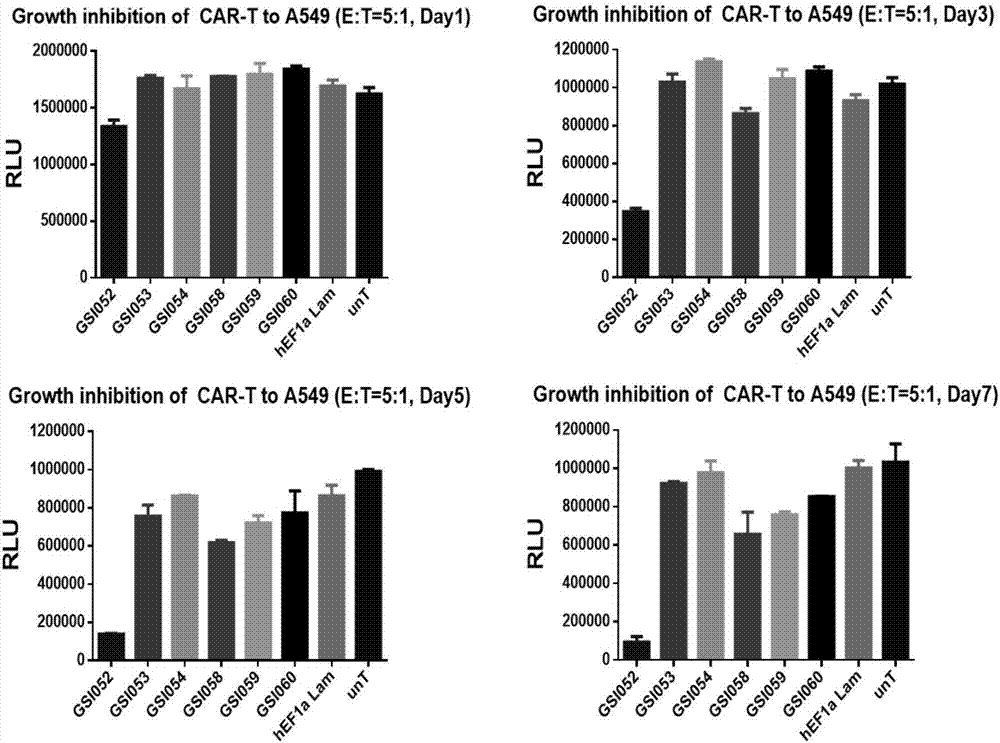
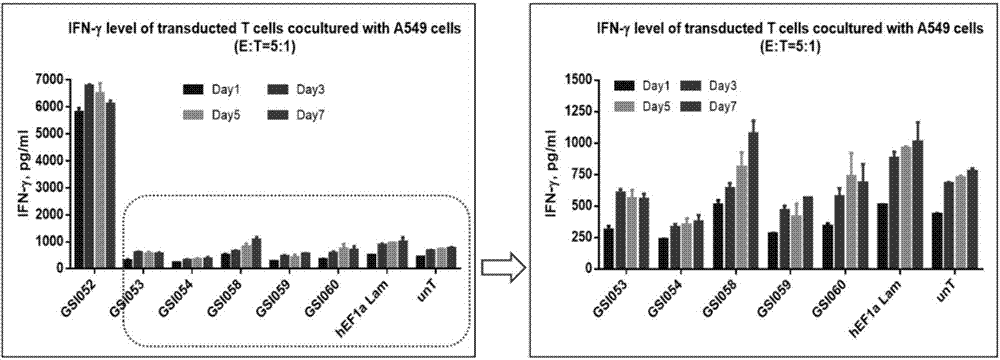
The invention discloses a tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor) and also discloses a plasmid vector for implementing the method. In combination with fourth-generation CAR-T (chimeric antigen receptor T) cells targeting at PD-1 and EGFR, a lentiviral vector is used as a CAR-T vector basic structure, and truncated EGFR antibody is selected as a CAR core to give tumor targeted enrichment to play, tumor-killing effect is given to play in conformity with overexpressed immune checkpoint inhibitor PD-1 monoclonal antibody; by constructing the fourth-generation CAR-T vector for co-expressing various regulatory factors such as IL21, CCR4 and Bcl2, the killing, homing and persistent proliferating abilities of CAR-T cells are improved. EP-CAR T (esophageal papilloma chimeric antigen receptor T) cells are treated by transducing patient's autologous T-lymphocytes in vitro, amplifying suitably and transducing back to the patient's body via autologous transfusion, and no reports on similar designs of CAR-T cells are provided at present.
Owner:尹荣
Method and detector for identifying subtypes of human papilloma viruses
InactiveUS20050175989A1Rapid and reliable detectionRapid and reliable and identificationMicrobiological testing/measurementGeneOligonucleotide
A detector for detecting and simultaneously diagnosing at least one subtype of human papilloma viruses (HPV) contained in a biological sample is provided. The detector comprises: a carrier, a plurality of micro-dots immobilized on the carrier, wherein each micro-dot is for identifying one particular HPV subtype, and the HPV subtype is one selected from a group consisting of 39 different HPV subtypes; and at least one oligonucleotide sequence contained in each the micro-dot that is specific to the one particular HPV subtype, wherein the at least one oligonucleotide sequence serves as a detection probe that hybridizes specifically with an L1 gene sequence of the one particular HPV subtype to form a hybridization complex as a detection indicator, so that each micro-dot identifies one particular HPV subtype via a corresponding oligonucleotide of the one particular HPV subtype, and thereby detecting and simultaneously identifying subtypes of human papilloma viruses.
Owner:KING CAR FOOD IND
Method for detecting human papilloma virogene type
ActiveCN101435002AReduce usageAvoid false positivesMicrobiological testing/measurementMaterial analysis by electric/magnetic meansThroughputHpv genotypes
The invention provides a method for detecting the HPV genotypes, with the gene to be detected being one or several types of the type 17 HPV, comprising the following steps: according to the variant sites of the selected HPV genotype universal primer sequence to be detected, an amplification primer aiming at each type is designed; the specific extension primer of each type is designed; (2) PCR amplification is conducted; (3) SAP enzyme treatment is conducted; (4) extension reaction is conducted, among the extending products and the extending primers, the difference of the molecular weight among the extending products of each type is not less than 9D; (5) resin is used to purify extension reaction products; and (6) mass spectrometry detection is conducted, and the type of HPV gene to be detected is determined. By using the method, the invention solves the problems of some of existing detection methods that the typing can not be realized, the multiple infections can not be detected, the accuracy is limited, the throughput is low, the cost is high, and the stability of reaction may be affected as the probe is RNA.
Owner:BGI SHENZHEN CO LTD
Combined measles-human papilloma vacine
The present invention relates to combined vaccines against measles and human papilloma virus (HPV). In particular, the invention relates to recombinant measles virus vectors containing heterologous nucleic acid encoding single or several antigens derived from HPV, preferably, the major capside antigen L1, the minor capside antigen L2, the early gene E6 and the early gene E7 oncoproteins of HPV type 16, and optionally of types 18, 6 and 11. In a first embodiment, prophylactic vaccines are generated expressing HPV antigens, preferably L1 and / or L2 such that they induce a potent long-lasting immune response in mammals, preferably humans, to protect against HPV and MV infection. In another embodiment, therapeutic vaccines are generated expressing E6 and E7 proteins, and optionally L1 and L2, such that they induced strong immune responses will resolve persistent HPV infections at early or late stages, including HPV-induced cervical carcinoma. In a preferred embodiment, the combined vaccines are easy to produce on a large scale and can be distributed at low cost.
Owner:CADILA HEALTHCARE LTD
Method, oligonucleotide and kit for detecting high-risk HPV (human papilloma viruses)
ActiveCN105603121AReduce usageGuaranteed SensitivityMicrobiological testing/measurementMicroorganism based processesTrue positive ratePhylogenetic tree
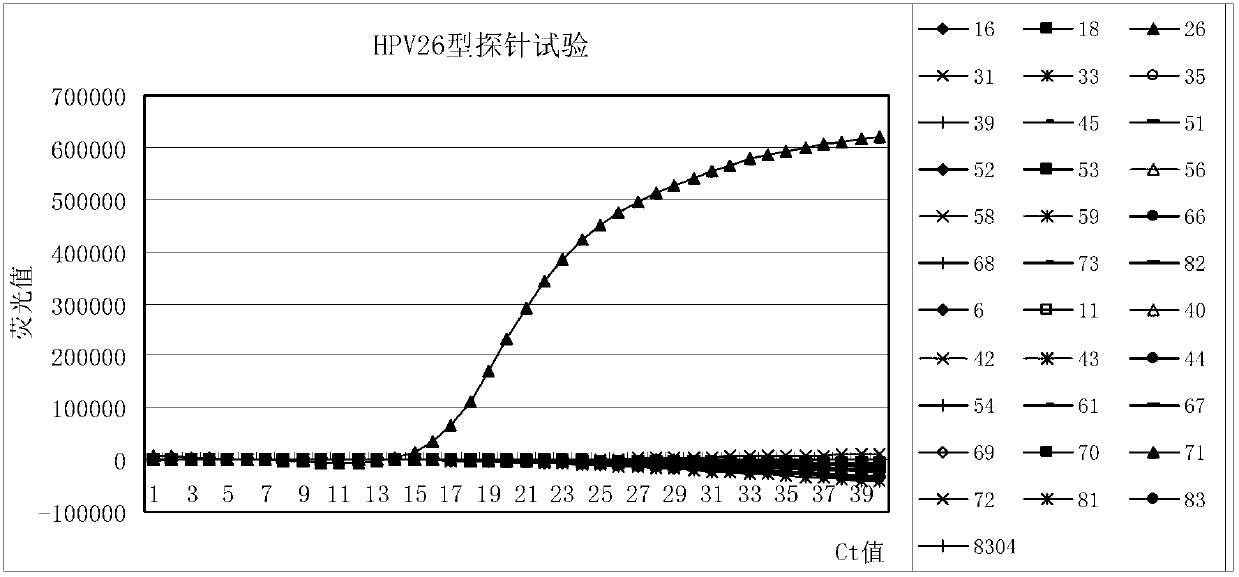
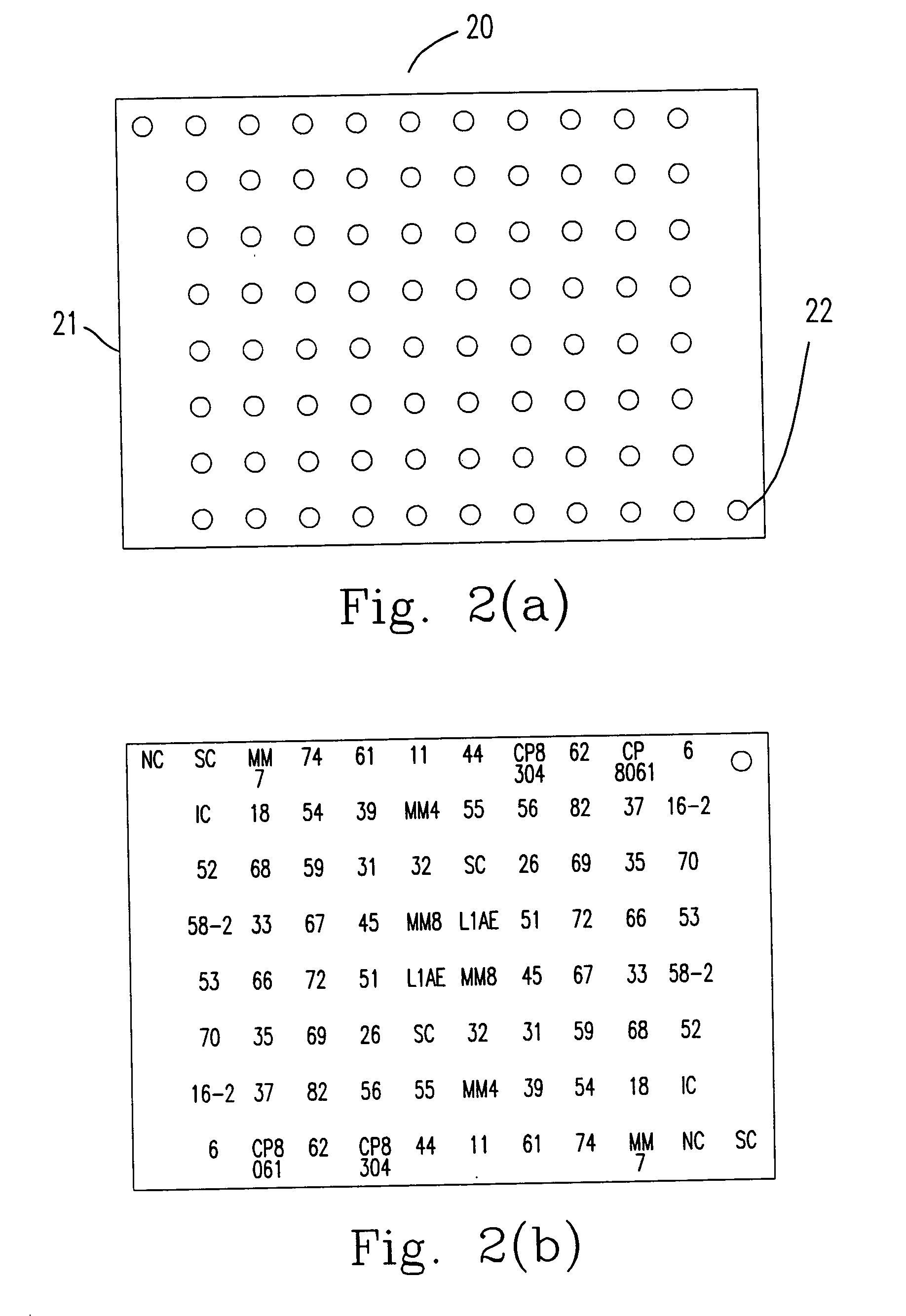
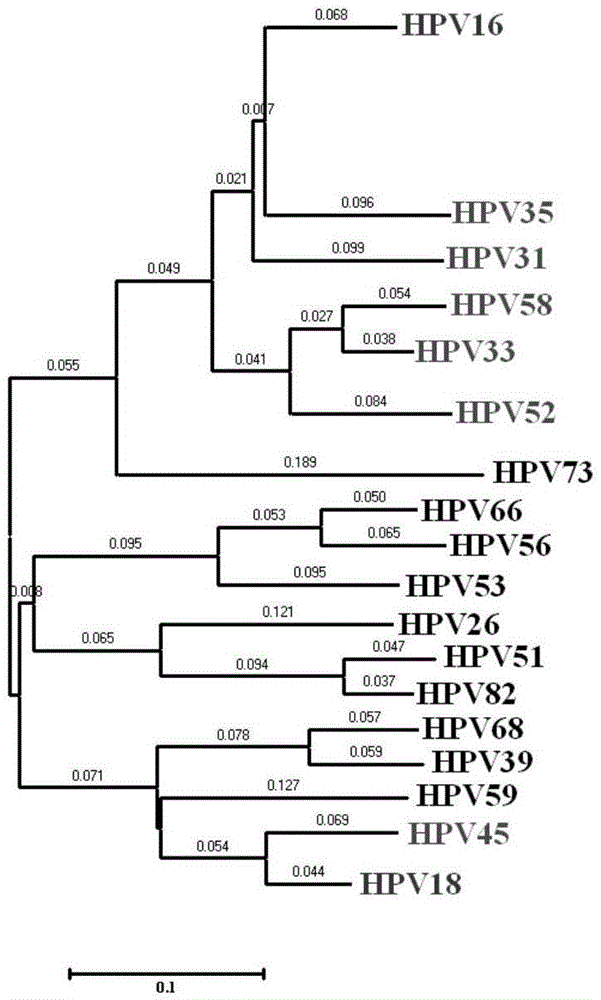
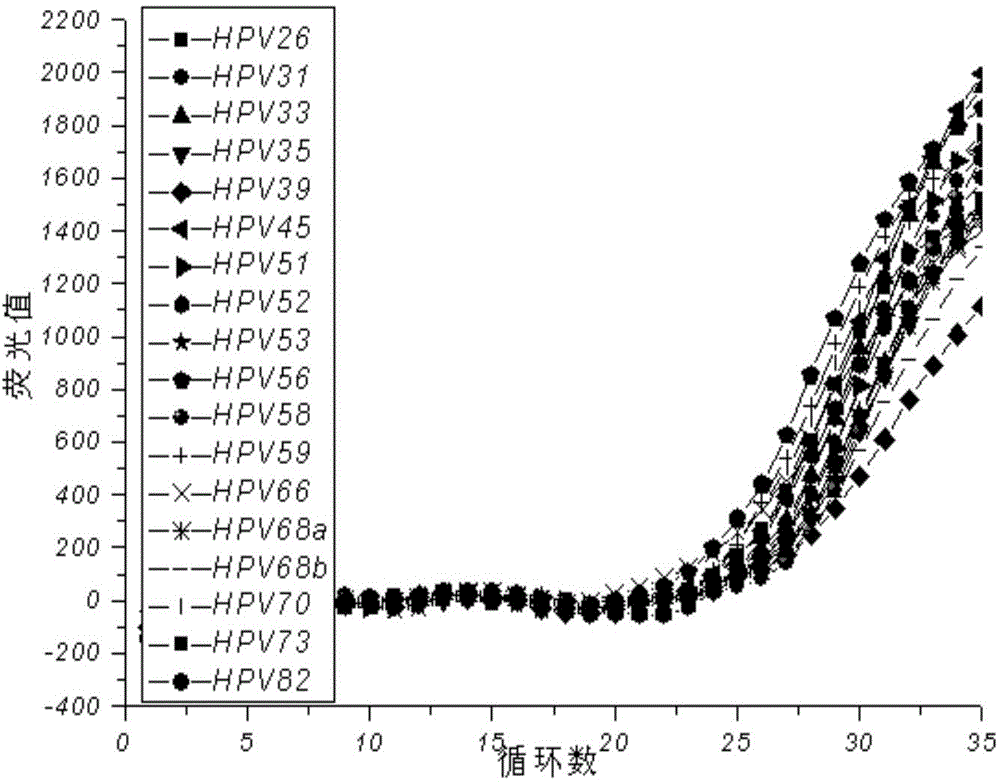
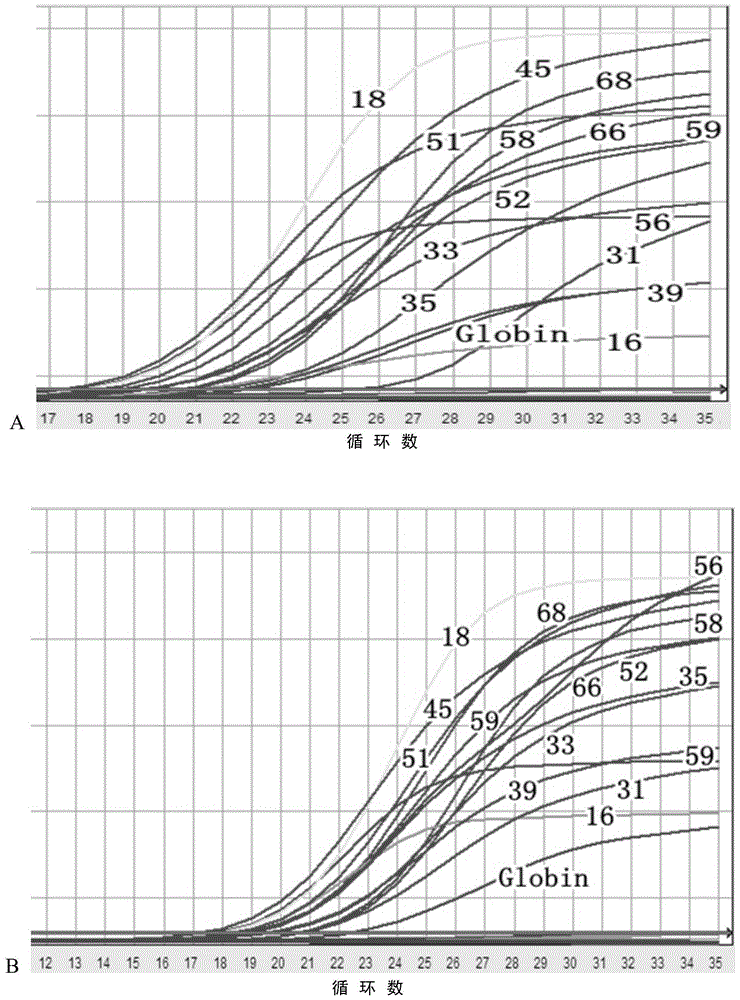
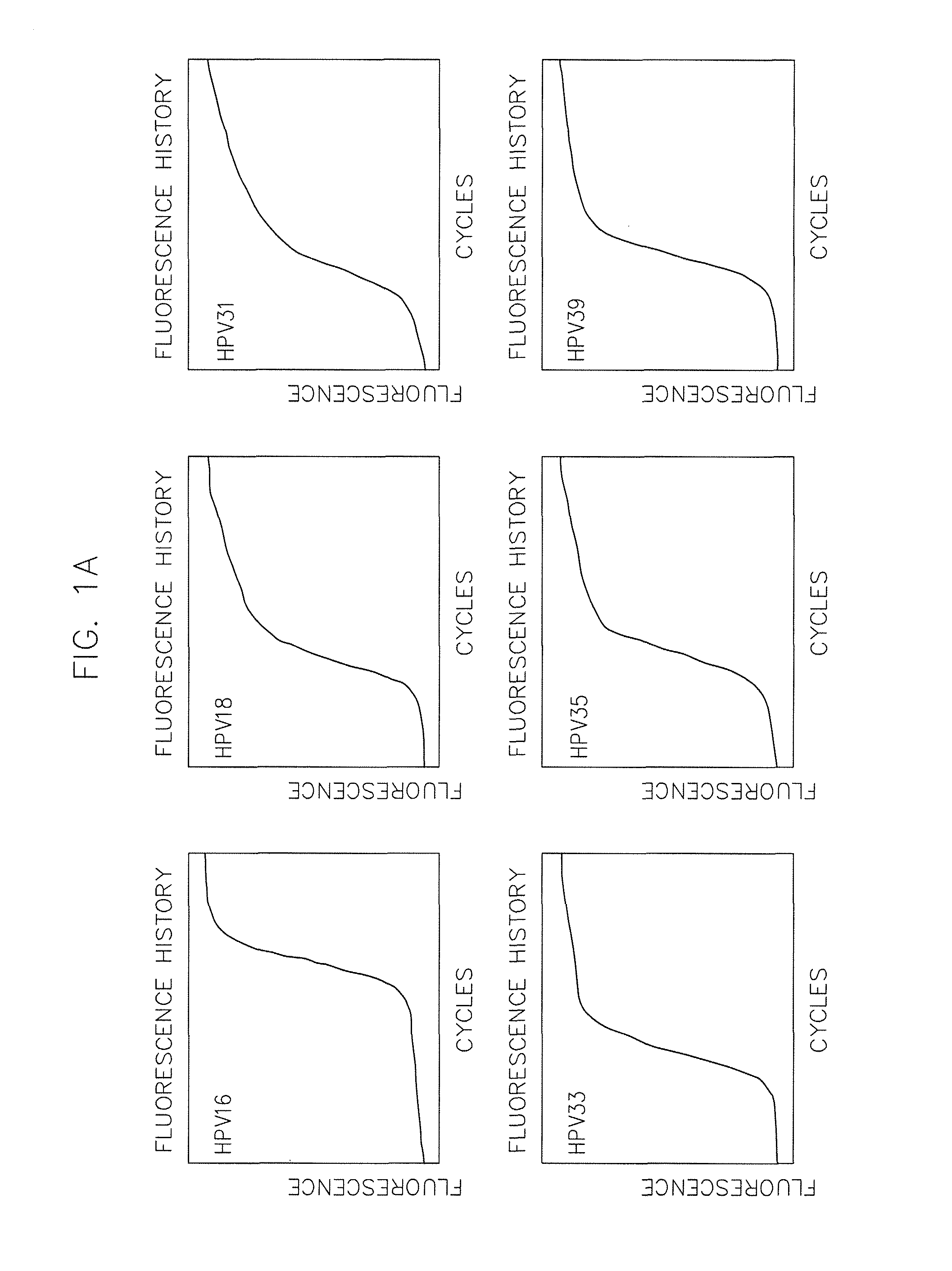
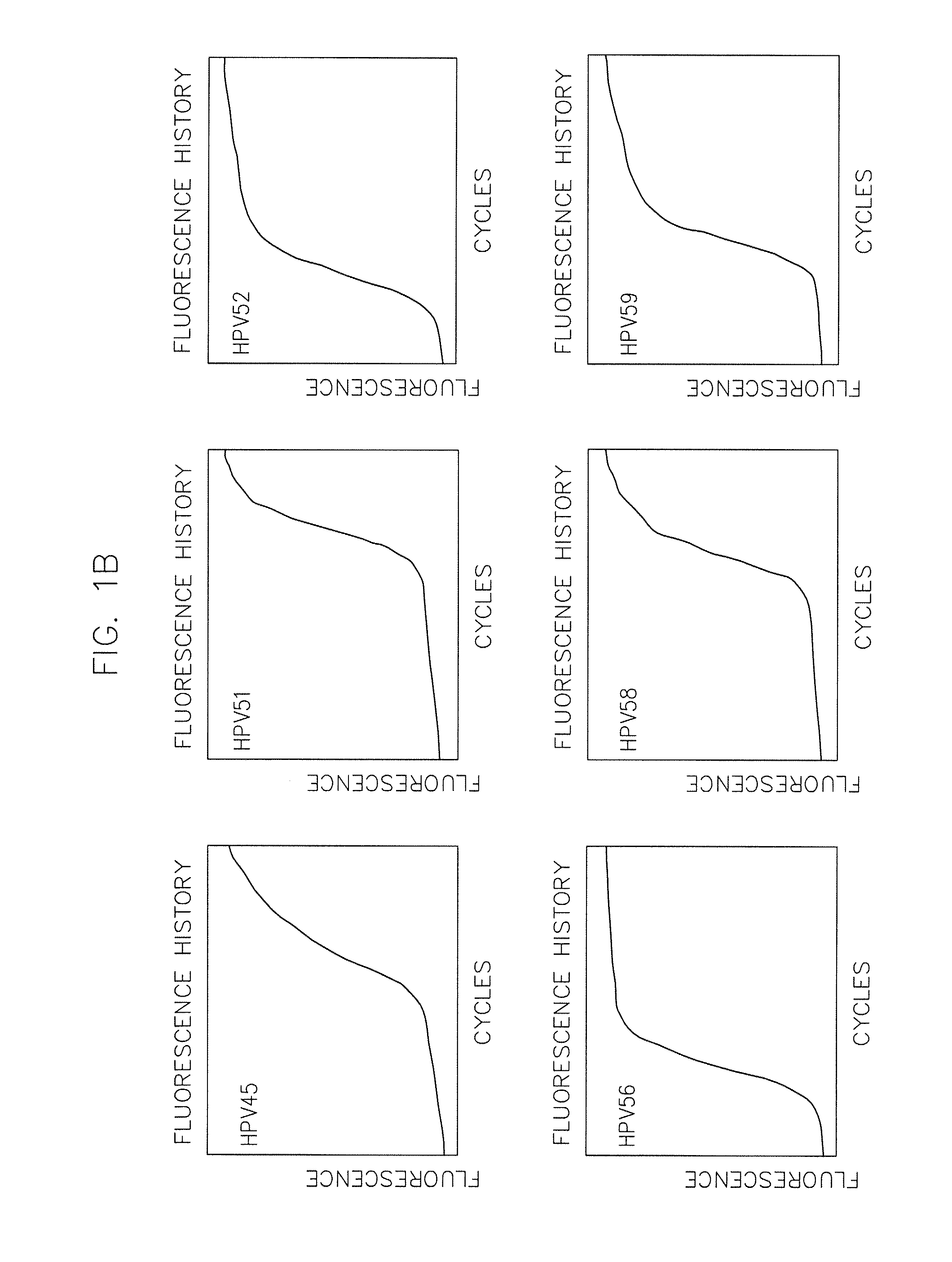
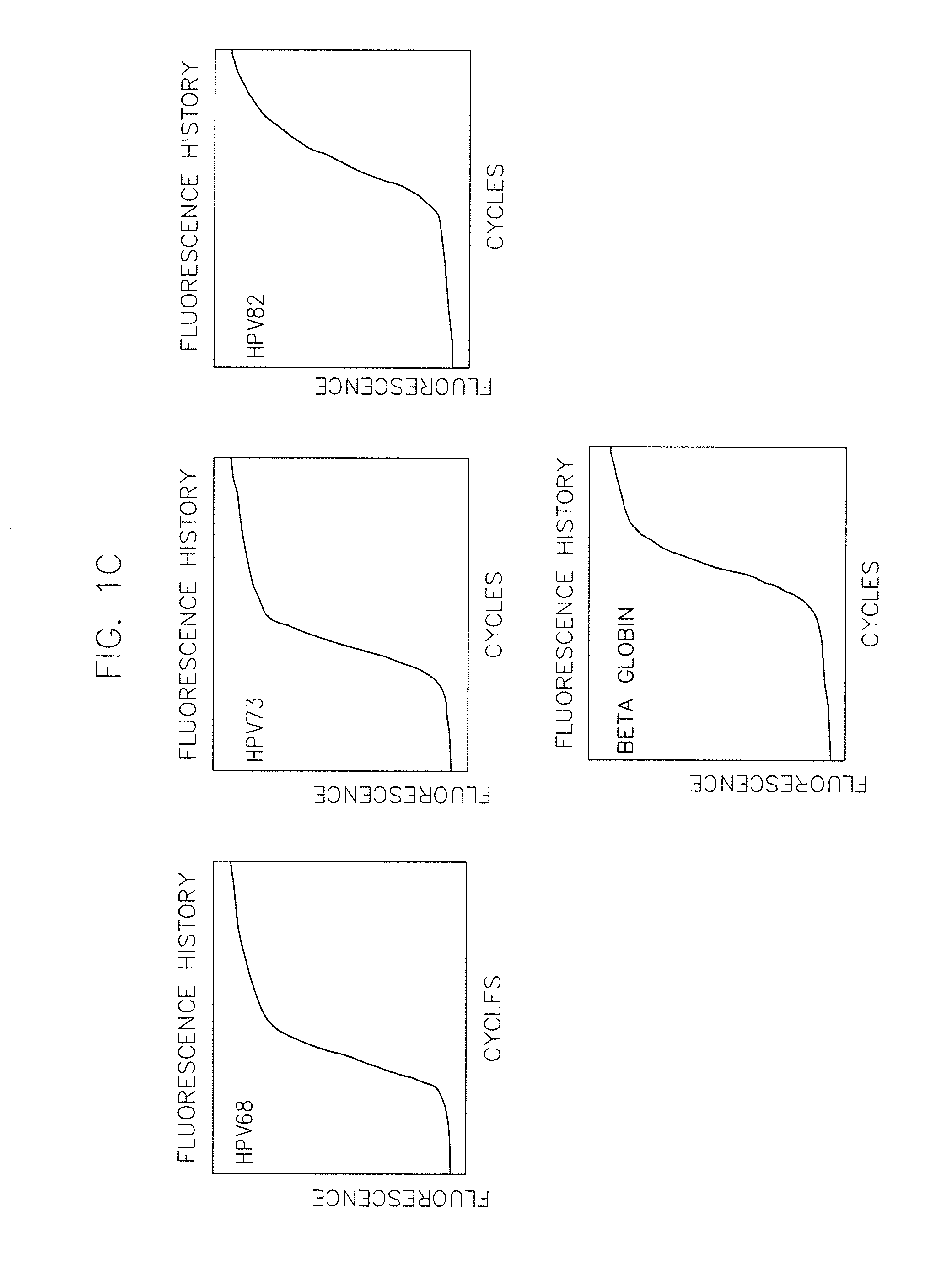
The invention provides a method, oligonucleotide and kit for detecting common high-risk HPV (human papilloma viruses). A fluorescence PCR (polymerase chain reaction) technology is adopted, HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 26, 53, 66, 73 and 82 which possibly exist in a sample are subjected to initial detection and type identification. By the method, oligonucleotide and kit for detecting the common high-risk HPV, 18 high-risk types can be detected simultaneously, and the HPV 16 and 18 can be subjected to genetic typing simultaneously. On the basis of 18 types of high-risk HPV and affinity of other types on a phylogenetic tree, a high-risk HPV primer and a probe are designed, a background fluorescence value is reduced remarkably while detection accuracy, sensitivity and specificity are guaranteed, detection flux is increased remarkably, and detection cost is greatly reduced.
Owner:ACON BIOTECH (HANGZHOU) CO LTD
Method and detector for identifying subtypes of human papilloma viruses
InactiveUS20070031827A1Rapid and reliable detection and identificationRapid and reliable detectionMicrobiological testing/measurementFermentationGeneOligonucleotide
A detector for detecting and simultaneously diagnosing at least one subtype of human papilloma viruses (HPV) contained in a biological sample is provided. The detector comprises: a carrier, a plurality of micro-dots immobilized on the carrier, wherein each micro-dot is for identifying one particular HPV subtype, and the HPV subtype is one selected from a group consisting of 39 different HPV subtypes; and at least one oligonucleotide sequence contained in each the micro-dot that is specific to the one particular HPV subtype, wherein the at least one oligonucleotide sequence serves as a detection probe that hybridizes specifically with an L1 gene sequence of the one particular HPV subtype to form a hybridization complex as a detection indicator, so that each micro-dot identifies one particular HPV subtype via a corresponding oligonucleotide of the one particular HPV subtype, and thereby detecting and simultaneously identifying subtypes of human papilloma viruses.
Owner:LIN CHING YU +13
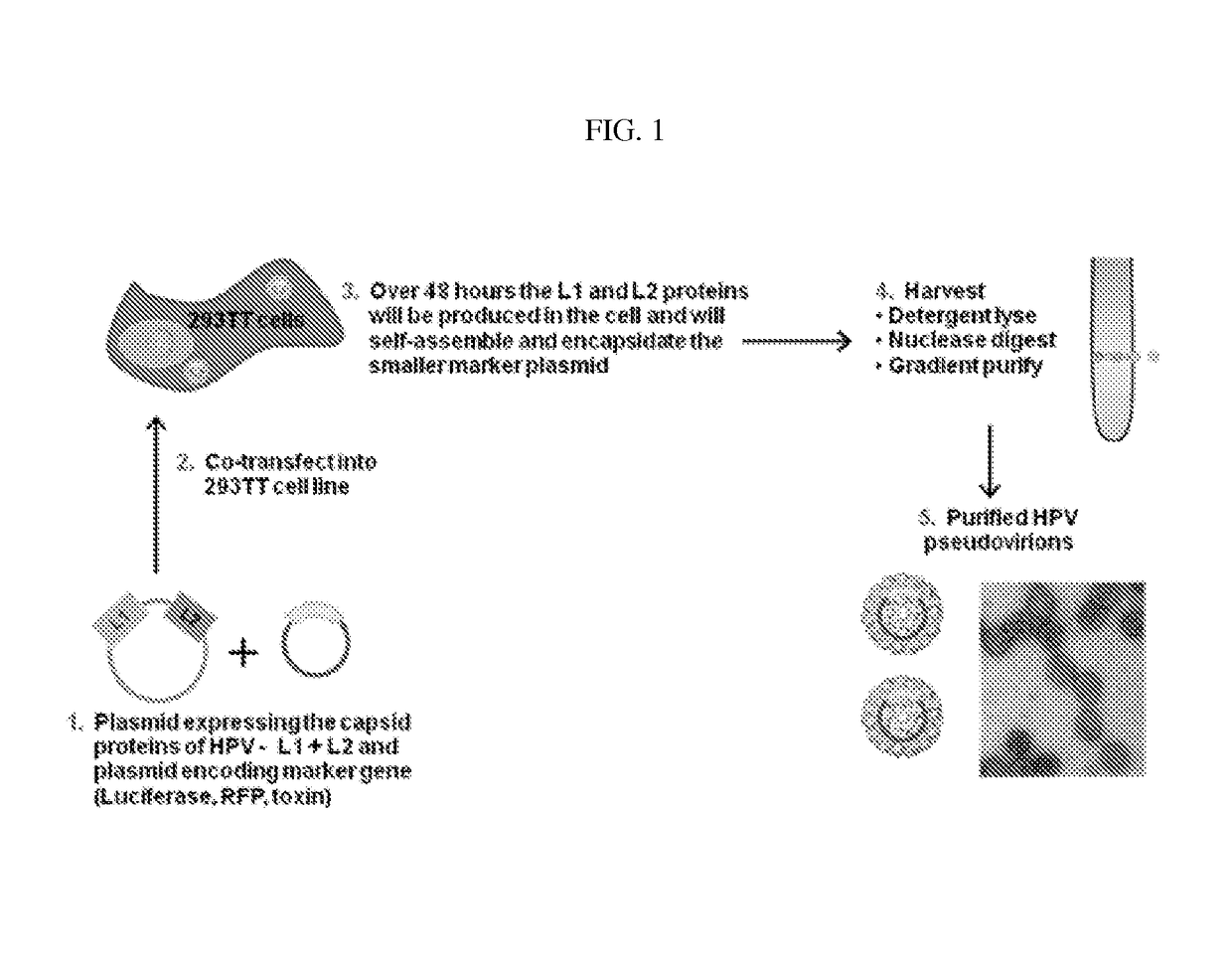
Papilloma pseudovirus and preparation
The invention involves a papilloma pseudovirus that can induce immune response after oral intake as well as its preparation. It is characterized in that HPV or BPV pseudovirus are made by disrupting HPV-VLP or BPV-VLP, mixing them with plasmids (plasmids or DNA vaccine), and reassembling them into the pseudoviruses (VLPs with plasmids inside). Oral administration of the pseudoviruses will result in delivery to mucosal and systemic lymphoid tissues and induce immune responses for disease prevention and treatment. The pseudovirus induces stronger immune response than DNA vaccines. Additionally, the pseudovirus can be applied in gene therapy by bringing the therapeutic genes into lymphoid tissues in the human body.
Owner:LOYOLA UNIV OF CHICAGO
Detection kit, detection system and detection method for 19 high-risk human papilloma viruses (HPVs)
ActiveCN104818342AHigh sensitivityImprove featuresMicrobiological testing/measurementMicroorganism based processesRisk typeBioinformatics
The invention discloses a detection kit, detection system and detection method for 19 high-risk HPVs. The 19 high-risk HPVs comprise HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73 and 82. According to the invention, a plurality of general primers are employed for amplification of target fragments, and specific fluorescence probes are used for real-time detection of the amplified fragments; since the general primers are employed, the primer amount of the system and complexity of the system are reduced, cost is saved, and all the high risk types are detected through combined action of 10 primers; and 20 specific fluorescence probes are used to distinguish different types. With the detection kit, detection system and detection method provided by the invention, the 19 high-risk HPVs can be detected in a reaction tube; high sensitivity and good specificity are obtained; operation is simple and fast; and for patients, screening cost is saved.
Owner:AMOY DIAGNOSTICS CO LTD
Methods for generating immunity to antigen
InactiveUS8828957B2Tumor rejection antigen precursorsPeptide/protein ingredientsInfectious agentTumor antigen
Provided are methods of generating an immune response to an antigen. The method comprises priming an individual by administering an expression vector encoding the antigen. The vectors comprises a transcription unit encoding a secretable fusion protein, the fusion protein containing an antigen and CD40 ligand. Administration of a fusion protein containing the antigen and CD40 ligand is used to enhance the immune response above that obtained by vector administration alone. The invention methods may be used to generate an immune response against cancer expressing a tumor antigen such as a mucin or human papilloma viral tumor antigen and to generate an immune response against an infectious agent. Also provided is a method for simultaneously producing the expression vector and the fusion protein.
Owner:MICROVAX LLC +1
Virion-derived nanospheres for selective delivery of therapeutic and diagnostic agents to cancer cells
ActiveUS9700639B2Easy to detectIncrease contrastPowder deliveryPeptide/protein ingredientsCancer cellDiagnostic agent
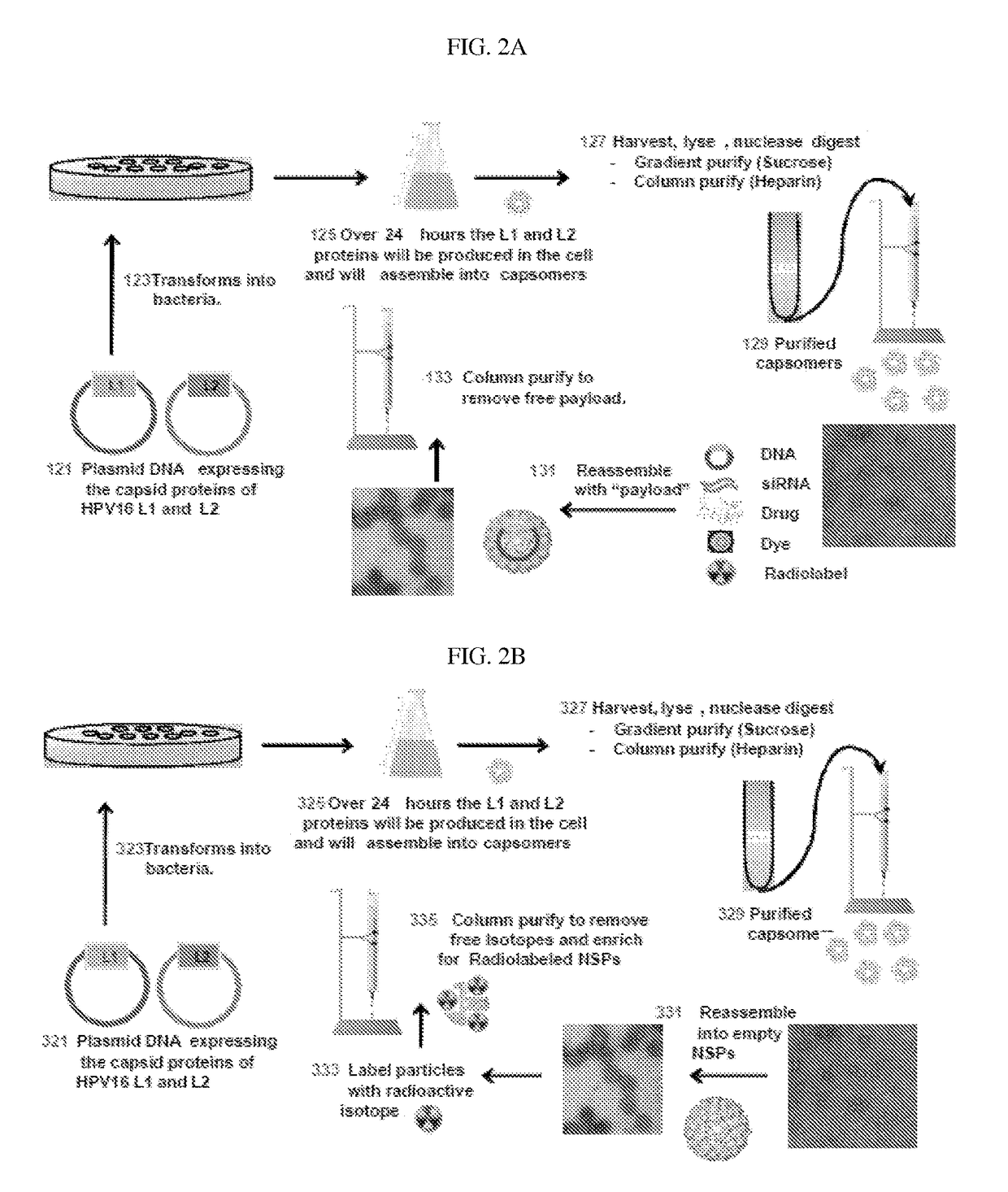
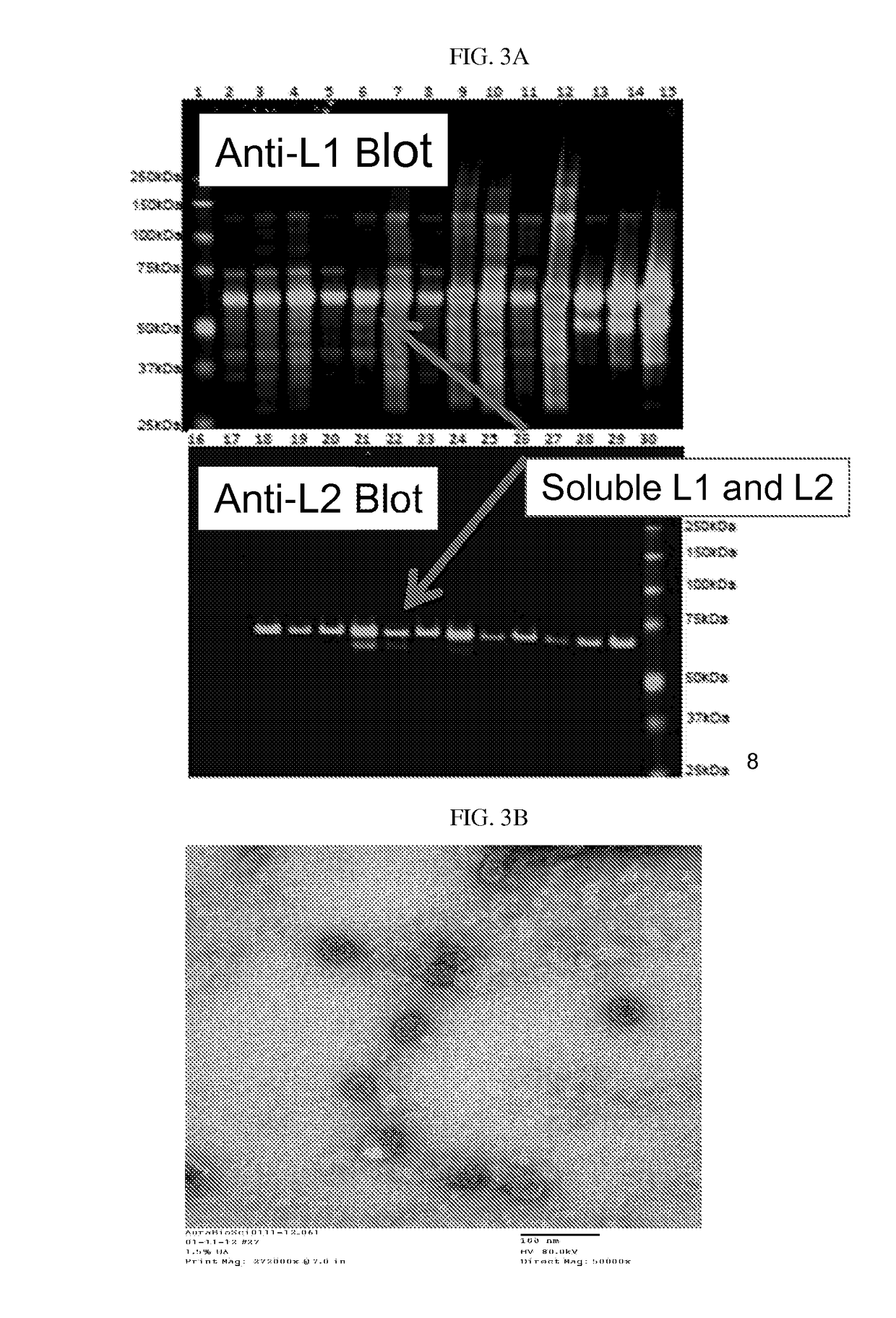
The invention relates to methods for producing papilloma-derived nanosphere particles that contain therapeutic, diagnostic, or other agents. The invention also provides nanosphere particle preparations that are useful for selectively delivering therapeutic, diagnostic, and / or other agents to cancer cells of subjects without eliciting a serotype-specific immunogenic response in the subjects.
Owner:AURA BIOSCI
Reagent kit for detecting and typing high-risk type human papilloma viruses and application of reagent kit
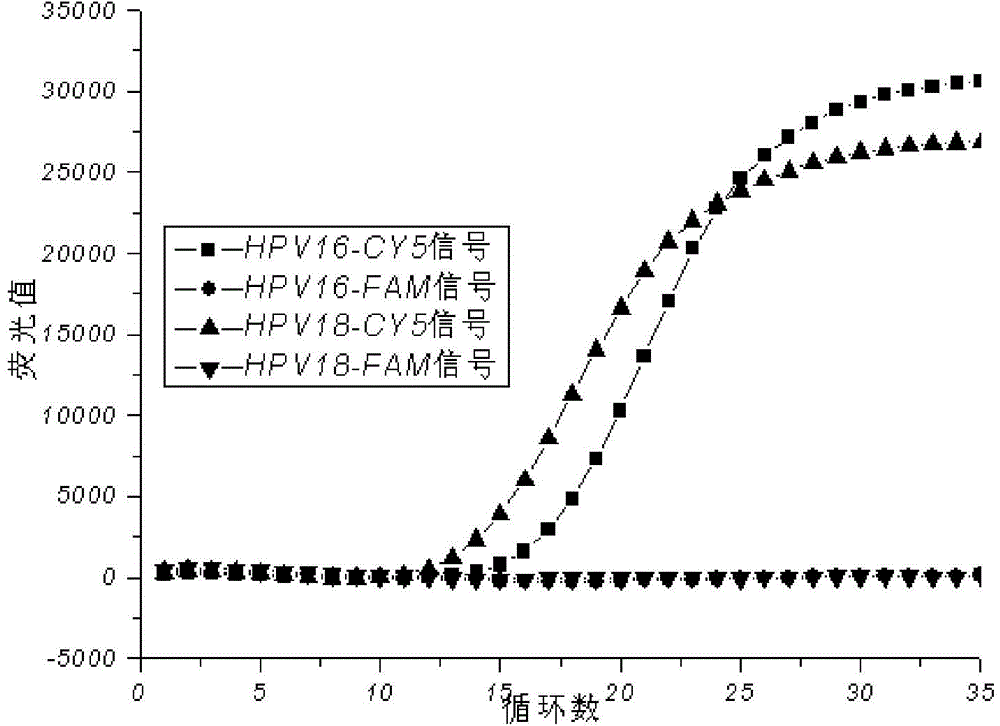
The invention relates to a reagent kit for detecting and typing high-risk type human papilloma viruses and application of the reagent kit, and discloses a detection reagent particularly applicable to diagnosing the high-risk type human papilloma viruses (PHV) and typing the human papilloma viruses HPV16 and PHV18.The reagent kit and the method have the advantages that the detection reagent is obtained by means of reasonable design and optimization, is excellent in specificity and high in amplification efficiency and sensitivity when used for PCR (polymerase chain reaction) amplification, diversified high-risk type viruses can be simultaneously detected by the detection reagent, and detection programs are simple and are easy to implement; PCR amplification reaction programs are further optimized by an inventor, and accordingly the amplification efficiency further can be improved.
Owner:SHANGHAI TELLGEN LIFE SCI CO LTD
Primers and method for detecting and typing human papilloma viruses in esophagi
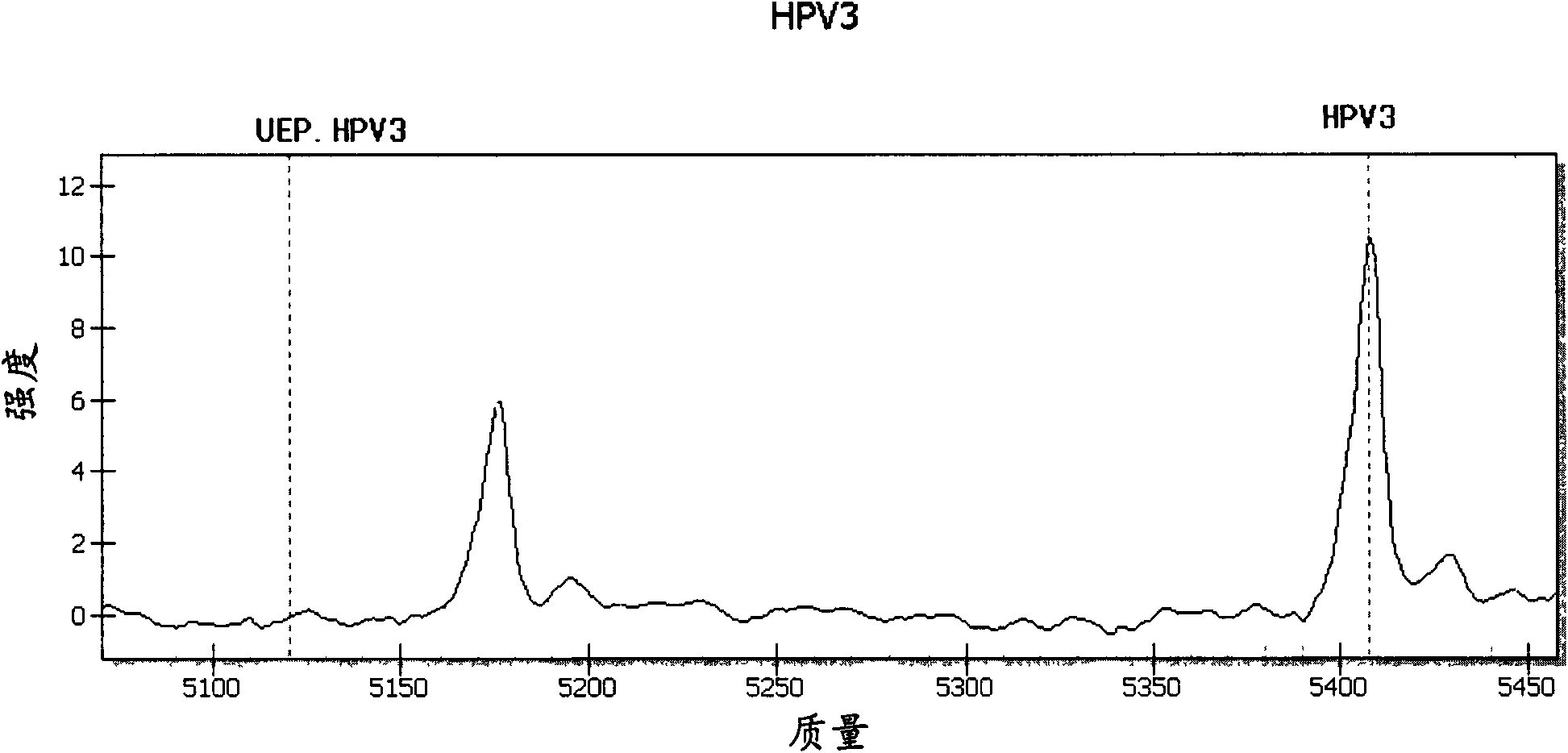
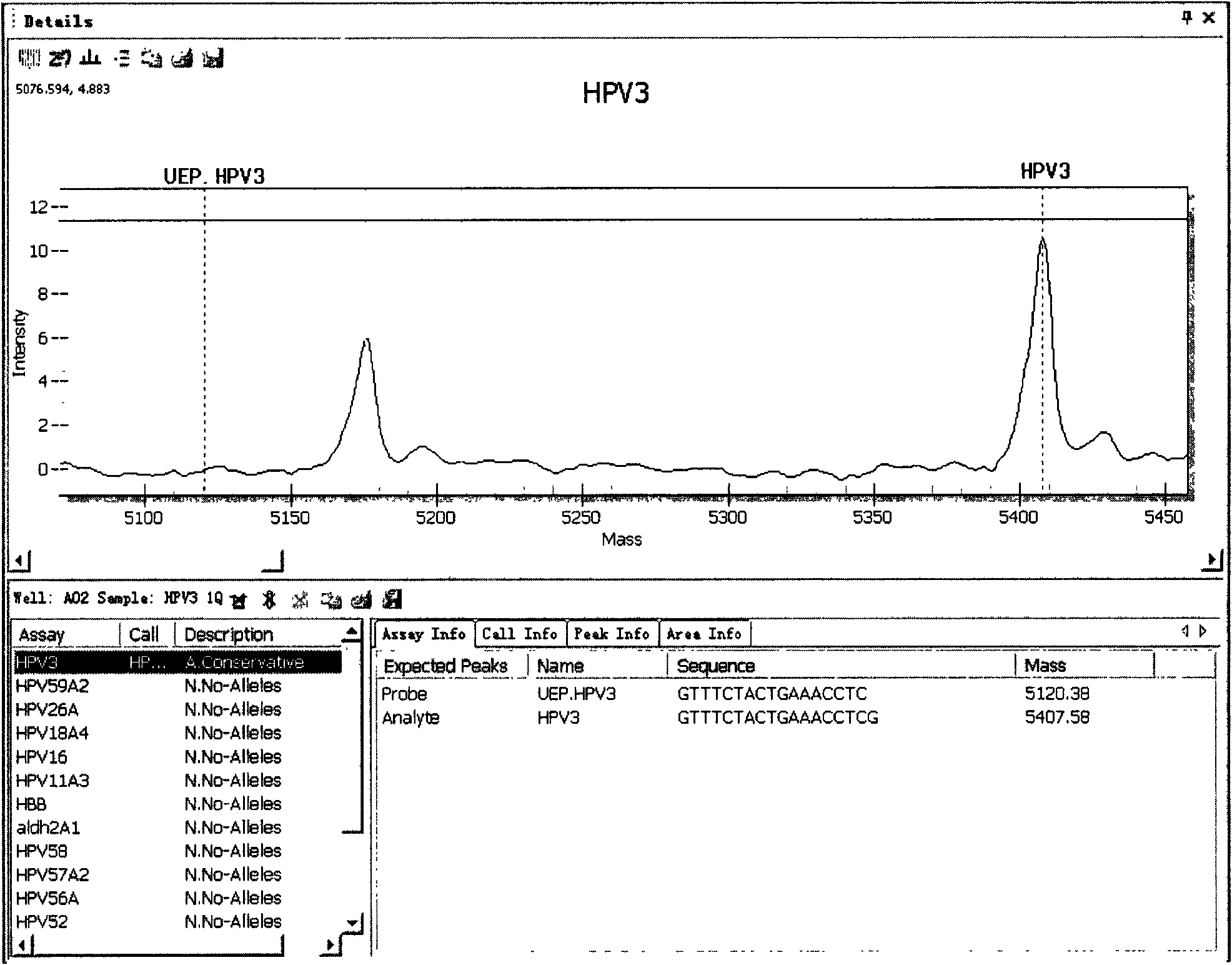
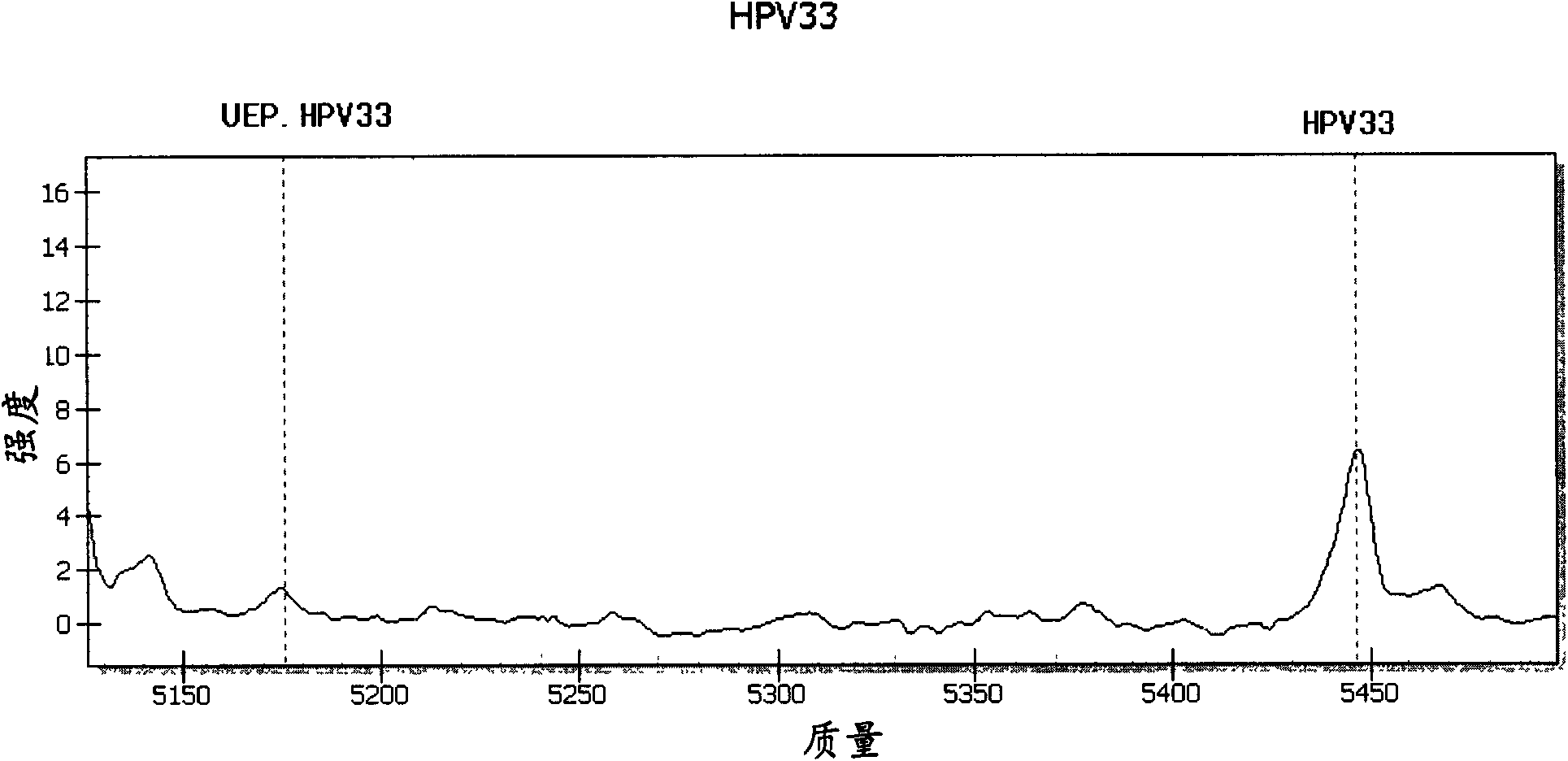
ActiveCN101942440AMicrobiological testing/measurementMaterial analysis by electric/magnetic meansTime-of-flight mass spectrometryTyping
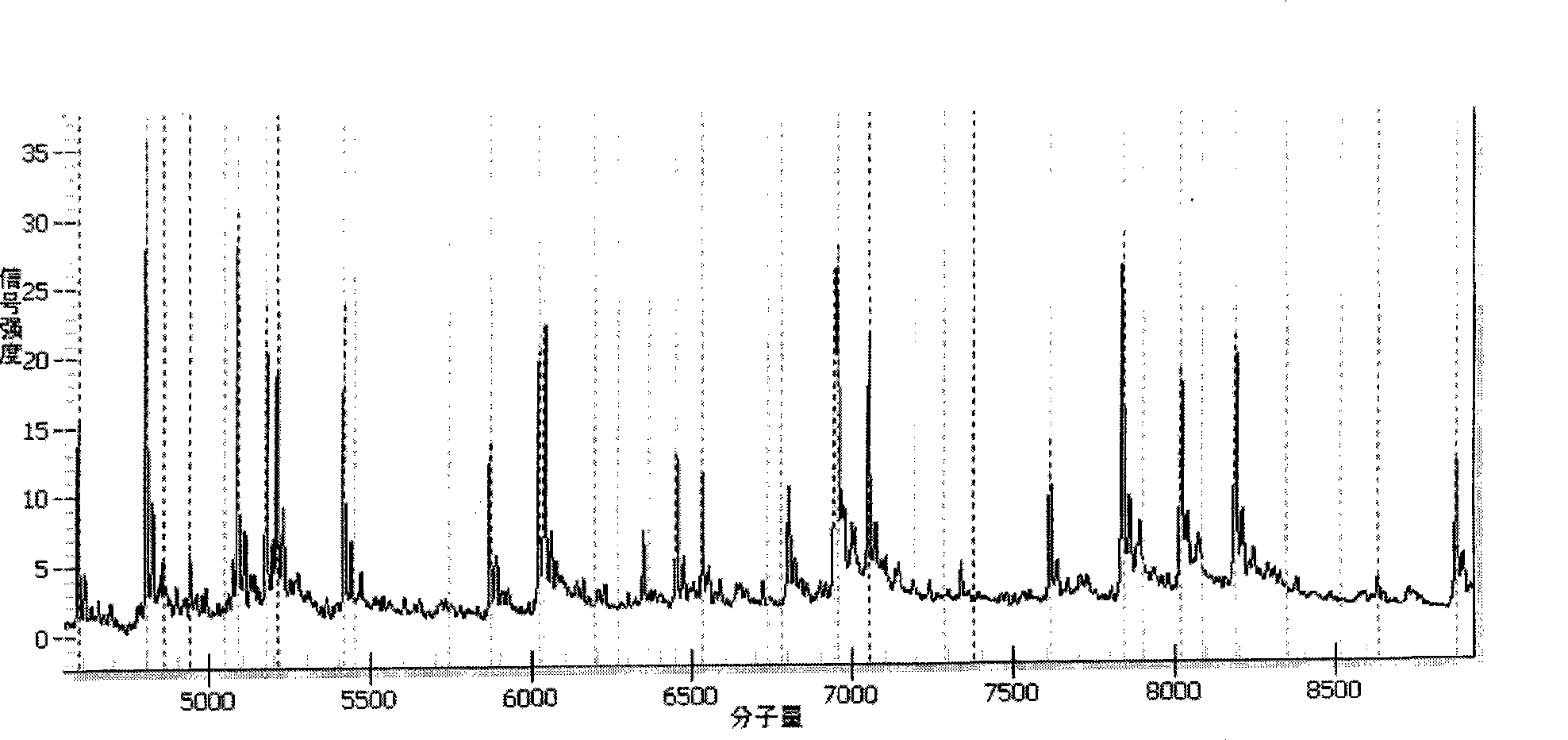
The invention relates to primers and a method for detecting and typing human papilloma viruses (HPV) infecting esophageal cells (such as esophageal mucosa cells). In particular, the invention designs a set of brand-new primers (including amplification primers and extension primers) based on the mass spectrometry detection technology of a matrix assisted laser desorption / ionization time-of-flight mass spectrometry system (MALDI-TOF-MS), thus realizing detection and typing of 15 types infecting the esophageal cells.
Owner:BGI GENOMICS CO LTD
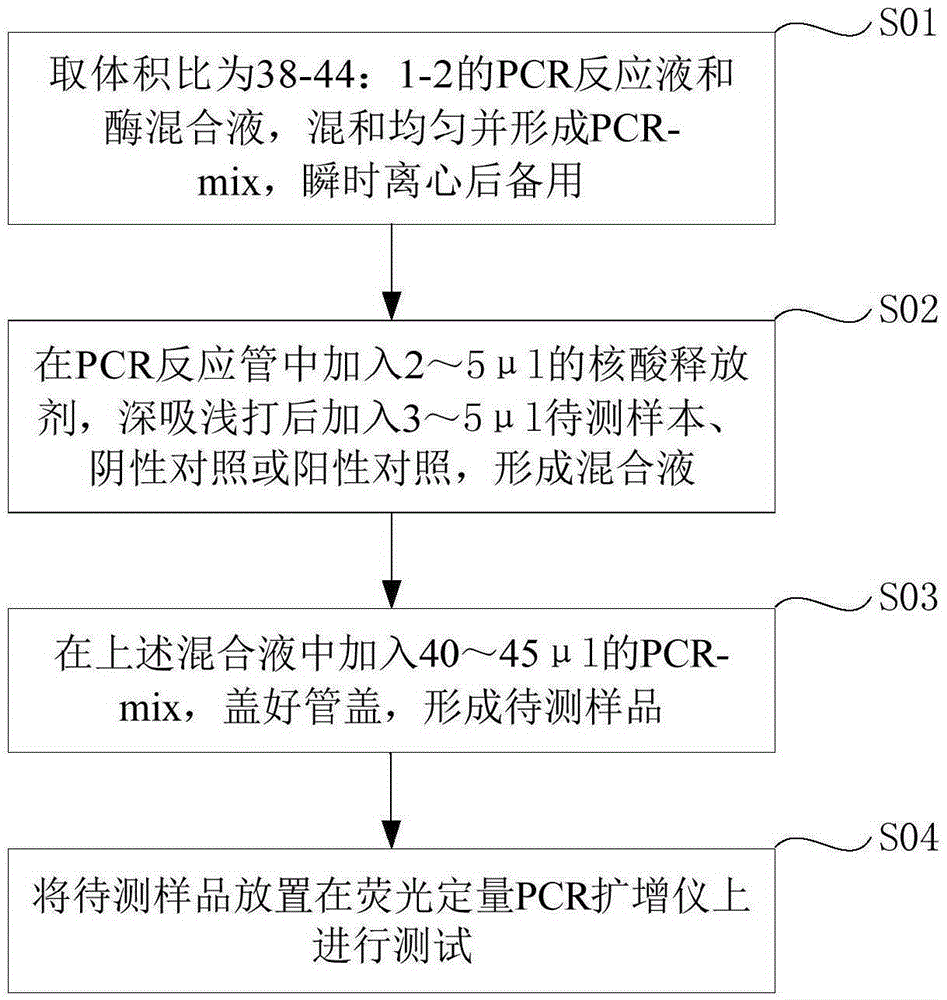
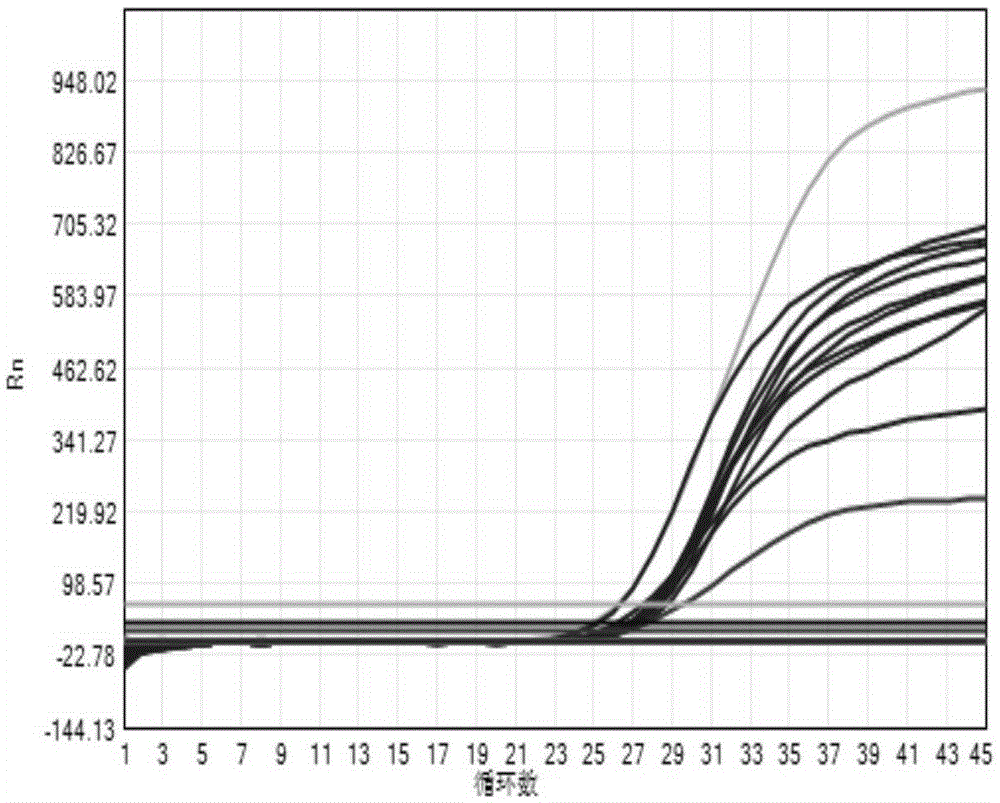
Fluorescent quantitation PCR (Polymerase Chain Reaction) detection kit for human papilloma viruses and application method of fluorescent quantitation PCR detection kit
ActiveCN105385786AHigh sensitivityEasy to operateMicrobiological testing/measurementMicroorganism based processes3-deoxyribosePolynucleotide
The invention provides a fluorescent quantitation PCR (Polymerase Chain Reaction) detection kit for human papilloma viruses. The detection kit comprises a PCR reaction solution, wherein the PCR reaction solution comprises a PCR buffering solution, deoxyribonucleoside triphosphate, upstream and downstream primers used for amplifying target polynucleotide, and a probe for detecting the target polynucleotide. The fluorescent quantitation PCR detection kit for the human papilloma viruses, provided by the invention, has very good specificity and has the advantages that the operation is rapid, the method is simple and convenient and the sensitivity is high; meanwhile, an interior label, which is increased in a reaction system, can be used for effectively preventing detection from being false negative. The fluorescent quantitation PCR detection kit for the human papilloma viruses, provided by the invention, adopts a rapid nucleic acid releasing method when DNAs (Deoxyribose Nucleic Acid) are extracted; the method is simple, convenient and rapid, and heating is not needed.
Owner:SANSURE BIOTECH INC
Papilloma pseudo-virus and preparation
The invention involves a papilloma pseudo-virus that can induce immune response after oral intake as well as its preparation. It is characterized in that HPV or BPV pseudo-virus are made by disrupting HPV-VLP or BPV-VLP, mixing them with plasmids (plasmids or DNA vaccine), and reassembling them into the pseudo-viruses (VLPs with plasmids inside). Oral administration of the pseudo-viruses will result in delivery to mucosal and systemic lymphoid tissues and induce immune responses for disease prevention and treatment. The pseudo-virus induces stronger immune response than DNA vaccines. Additionally, the pseudo-virus can be applied in gene therapy by bringing the therapeutic genes into lymphoid tissues in the human body.
Owner:LOYOLA UNIV OF CHICAGO
Combined primer for amplification and typing of human papilloma virogenes and application of combined primer
InactiveCN104561372AAchieve accurate typingFew stepsMicrobiological testing/measurementMicroorganism based processesForward primerTyping
The invention discloses a combined primer for the amplification and typing of human papilloma virogenes. The combined primer is divided into a combined forward primer and a combined reverse primer; the combined forward primer is composed of three parts, namely a joint part P2 sequence, a tag sequence and a forward primer sequence in order; the combined reverse primer is composed of a reverse primer sequence and a joint part P1 sequence in order. The invention also discloses a kit for the amplification and typing of the human papilloma virogenes. The invention also discloses the applications of the combined primer and the kit. The combined primer has real flux, and is low in cost and capable of accurately typing HPV. The type of HPV can be accurately determined by using a plurality of primers (6 forward primers and 5 reverse primers) for amplification and comparing the sequence information of the amplification product with an HPV database; as a result, a basis is provided for clinical diagnosis and treatment scheme selection.
Owner:南京普东兴生物科技有限公司
Peptides from the E7 protein of human papilloma viruses 16 and 18 for detecting and/or diagnosing cervical and other human papilloma virus associated cancers
InactiveUS20050221295A1Simple and rapid and and more testMicrobiological testing/measurementVirus peptidesCysteine thiolateTryptophan
An isolated protein sequence or peptide from the E2, E6 or E7 early coding region of human papillomavirus (HPV) that is soluble in an aqueous medium, and characterized by a relative paucity of tryptophan, methionine and cysteine residues, and a relative abundance of glycine and asparagine residues. Also disclosed are isolated protein sequences or peptides from the E2, E6 or E7 early coding regions of HPV 16 and 18 and methodologies for detecting or diagnosing cancer or cellular abnormalities. Detection or diagnosis of Cancer or cellular abnormalities may include detecting or diagnosing pre-cancerous or pre-malignant conditions, cervical dysplasia, cervical carcinoma, koilocytosis, hyperkeratosis, intraepithelial lesions, and other cancers. A methodology for detecting or diagnosing cancer or cellular abnormalities comprises the steps of (1) reacting a sample of body fluid or tissue with isolated protein sequences or peptides; (2) forming an antibody-peptide complex; and (3) detecting the antibody-peptide complex.
Owner:HU YAO XIONG
Papillomavirus-like particles (VLP) as broad spectrum human papillomavirus (HPV) vaccines
ActiveUS20120093821A1Induce high-titer anti-L1 antibody levelsImprove toleranceFungiVirusesSurface displayHuman papillomavirus
This invention relates, e.g., to a virus-like particle (VLP) composition assembled from a chimeric polypeptide comprising a papilloma virus (e.g., human papillomavirus, or HPV) L1 major capsid protein, into which is inserted a surface-displayed peptide comprising a neutralizing epitope of a papillomavirus L2 protein. Vaccine compositions comprising the VLP are described, as well as methods for inducing an immune response (e.g., vaccinating) a subject against papilloma virus, using the VLP, and kits comprising the VLP, for carrying out a method of the invention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Enclosures housing cell-coated supports for treating tumors
InactiveUS7056503B2Bioreactor/fermenter combinationsPowder deliveryAbnormal tissue growthTherapeutic protein
The present invention relates to devices, systems and methods for treating tumors. In particular, the present invention relates to enclosures housing cell-coated supports for promoting regression of tumors, such as cancerous tumors, papillomas, and warts. In preferred embodiments, the present invention provides methods of promoting tumor regression employing enclosures secreting therapeutic proteins.
Owner:RGT UNIV OF MICHIGAN
Biomarker for breast cancer
ActiveCN103547923AHigh precisionImmunoglobulins against cell receptors/antigens/surface-determinantsBiological testingIntraductal breast papillomaMammary gland structure
Owner:J PHARMA
Method of simultaneous detection and typing of human papilloma viruses
Owner:PHYSICIAN REFERENCE LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com