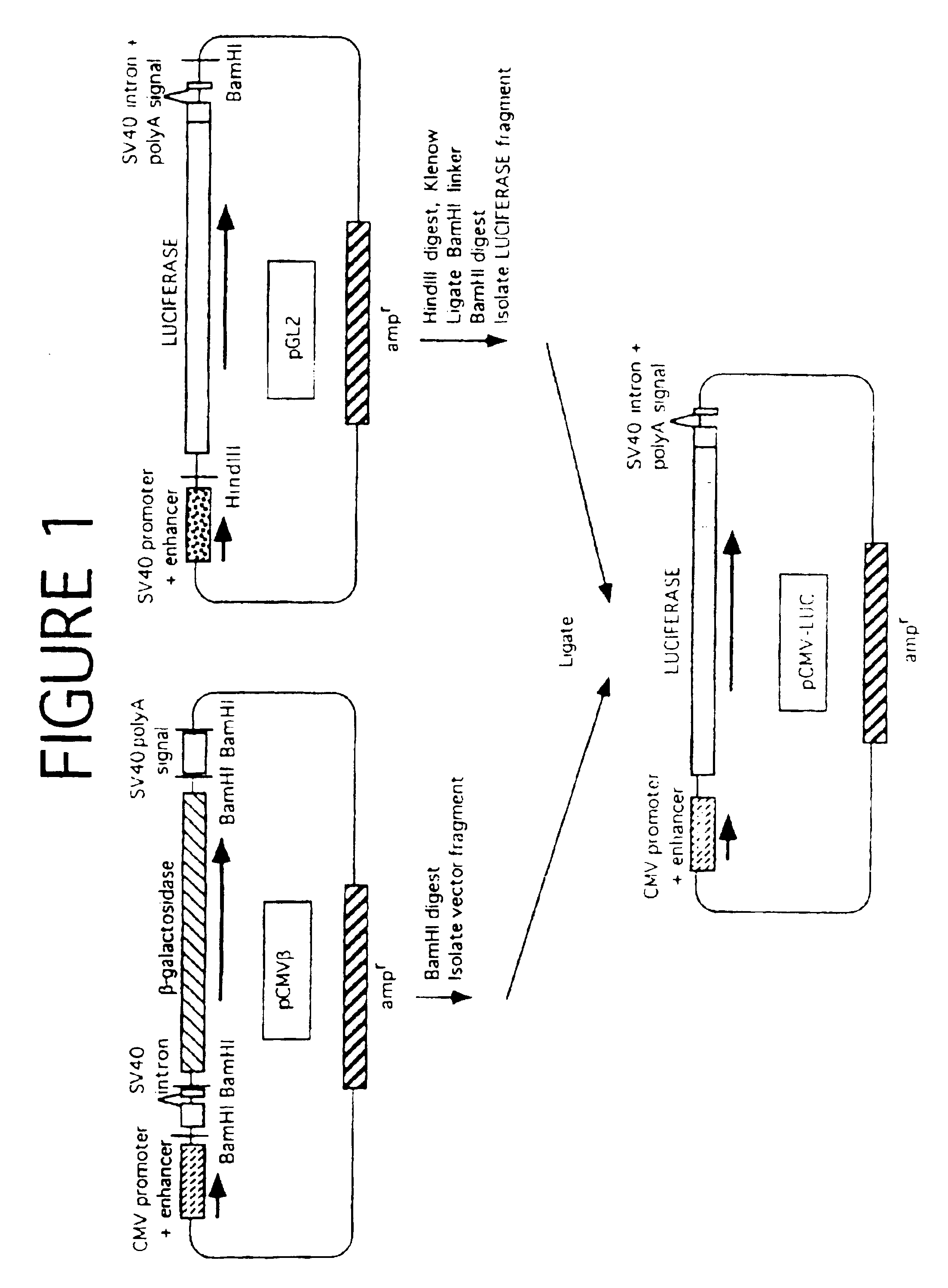
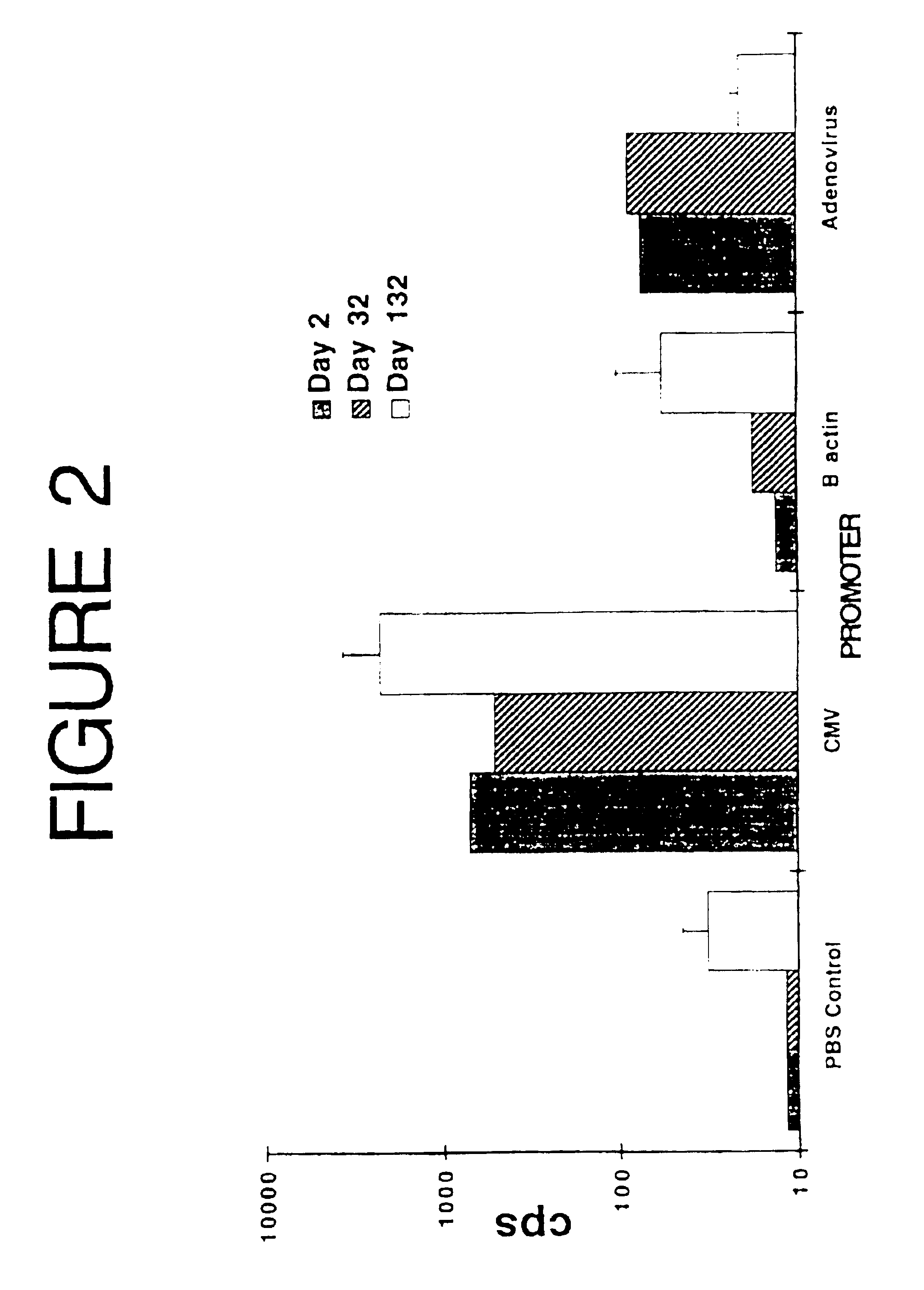
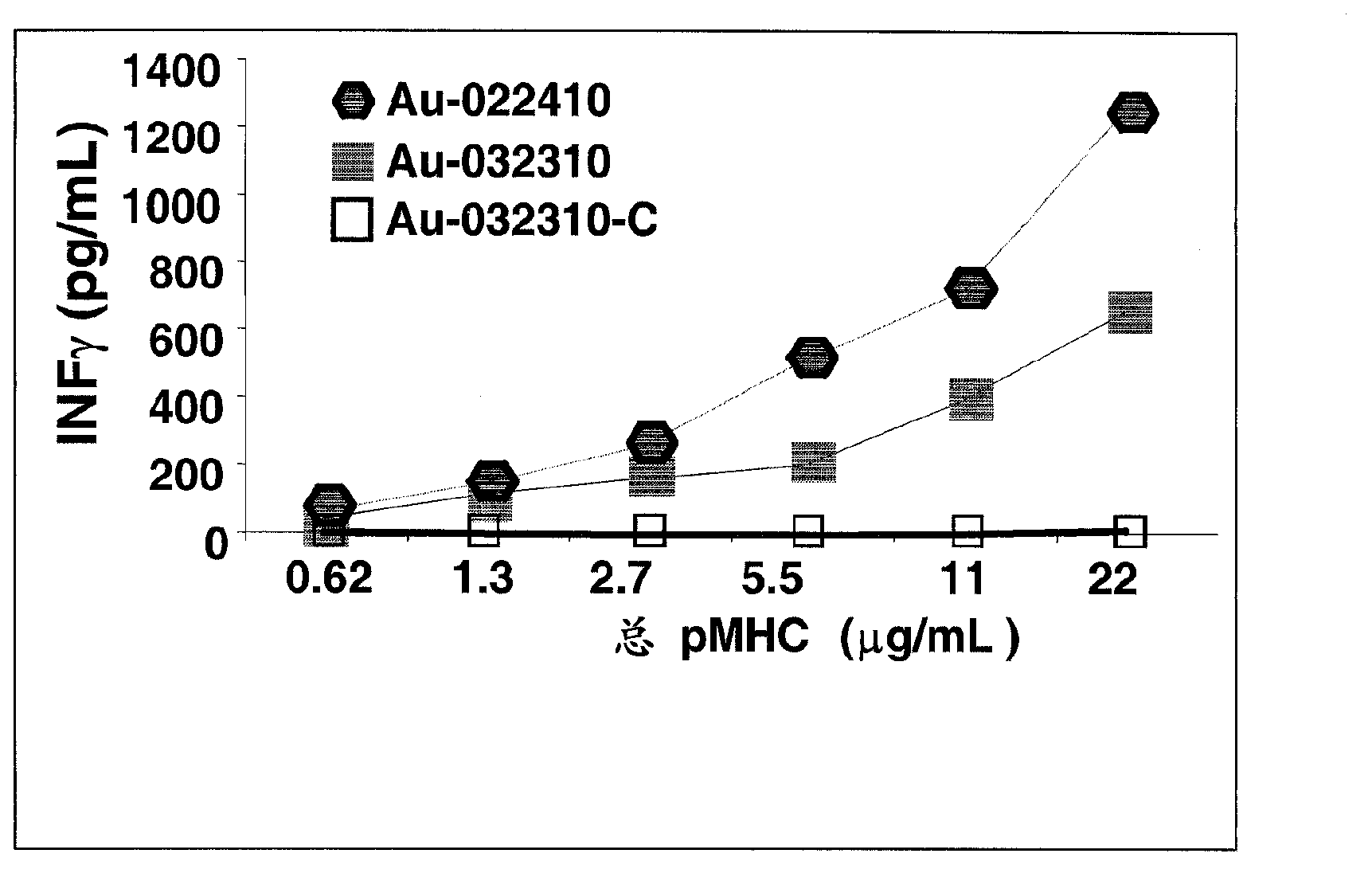
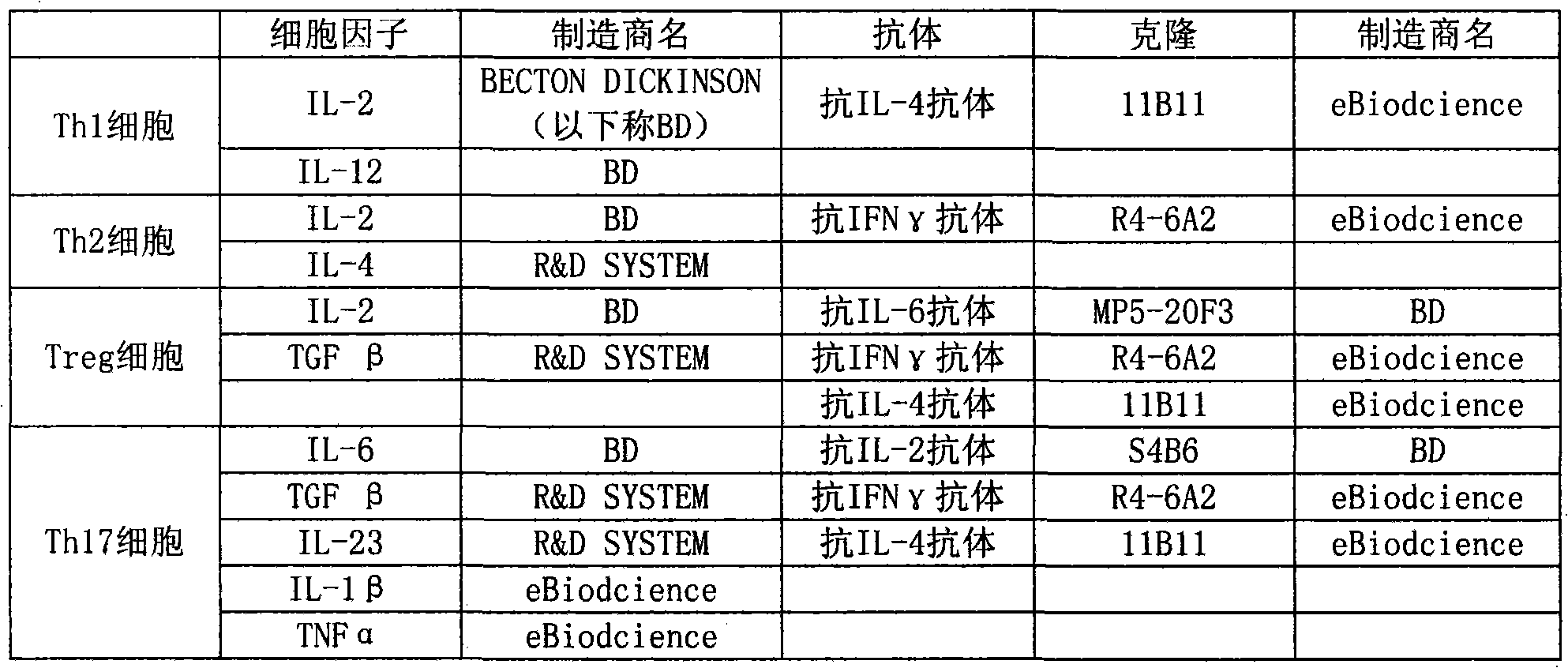
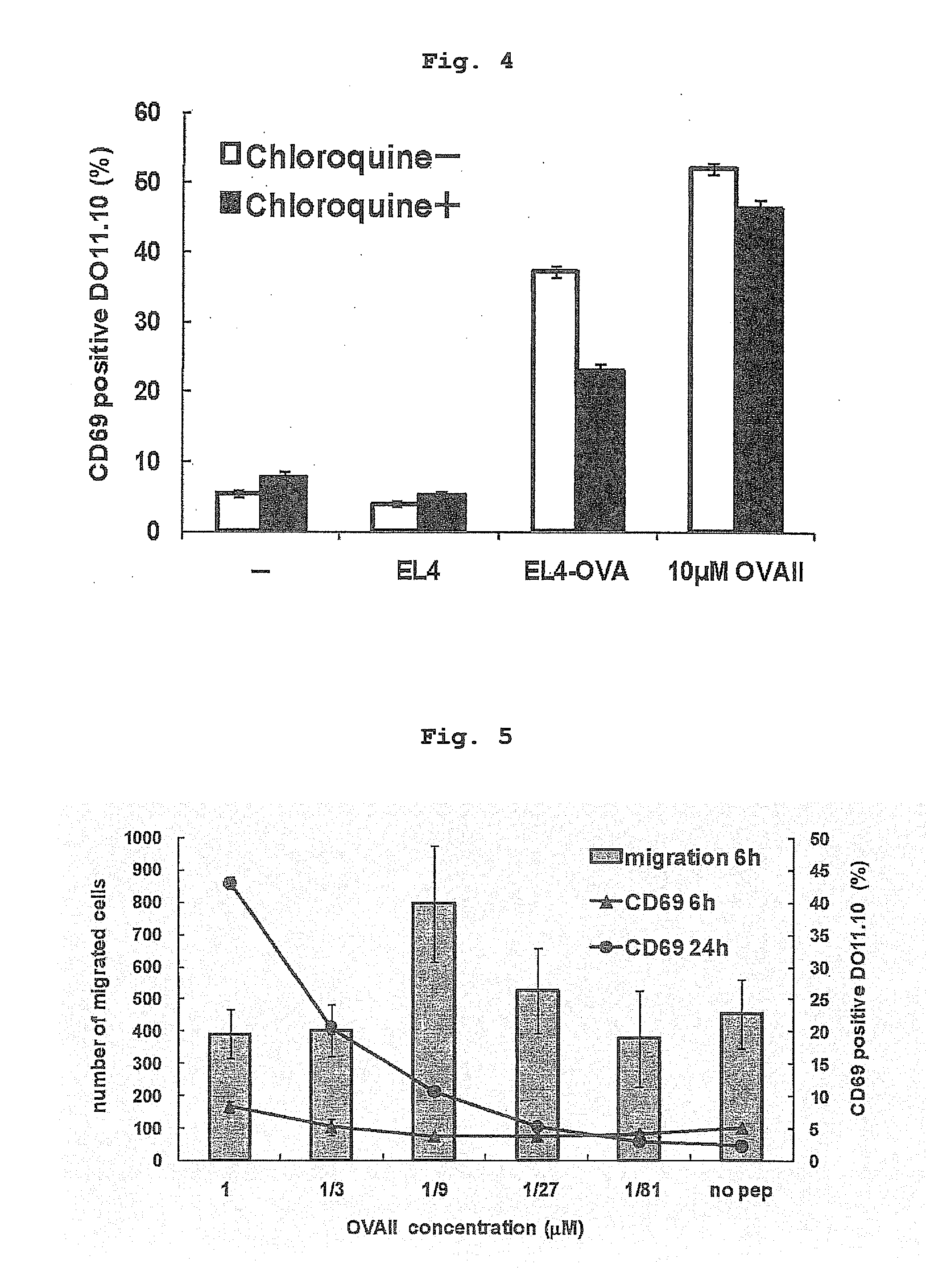
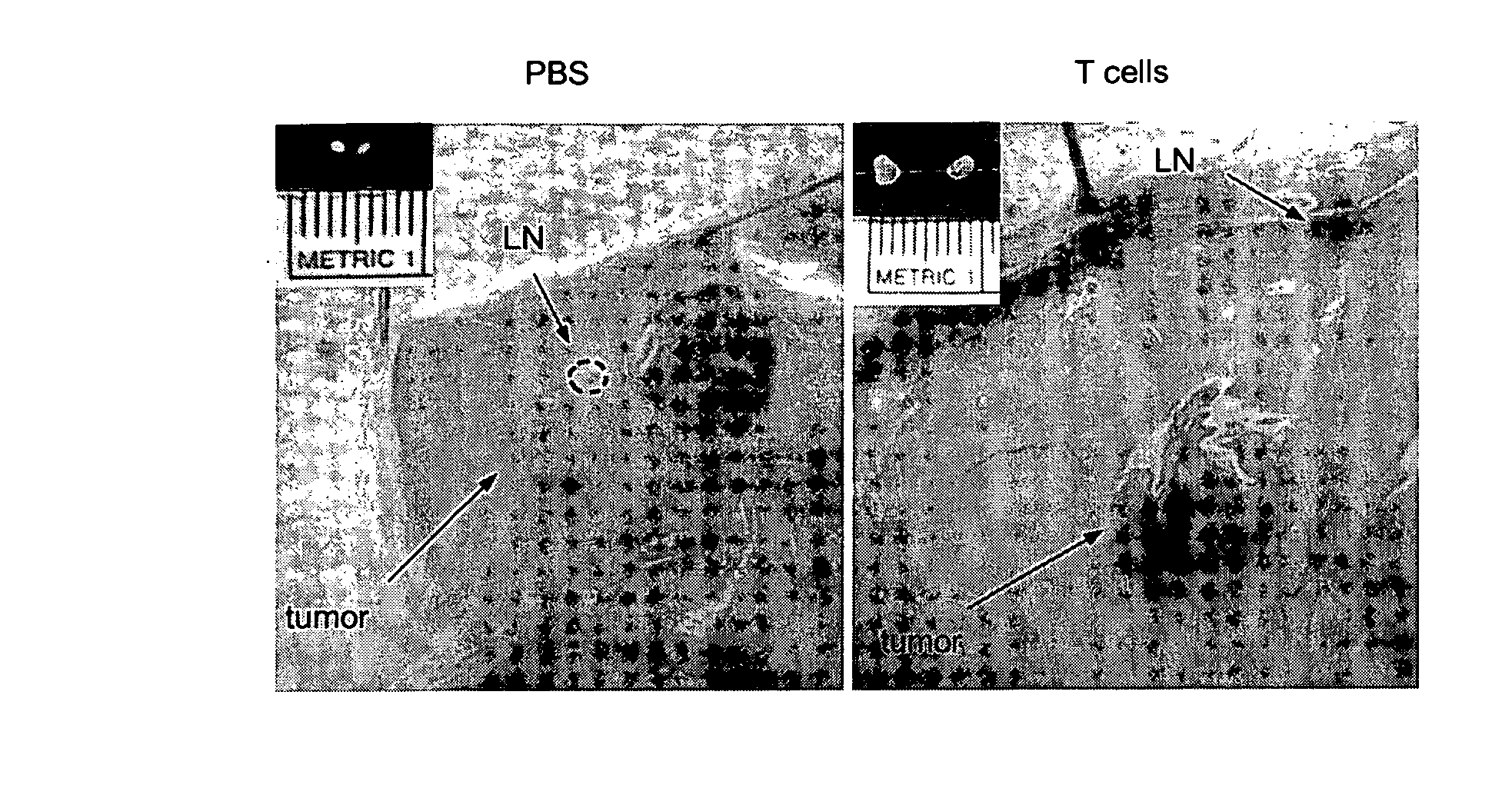
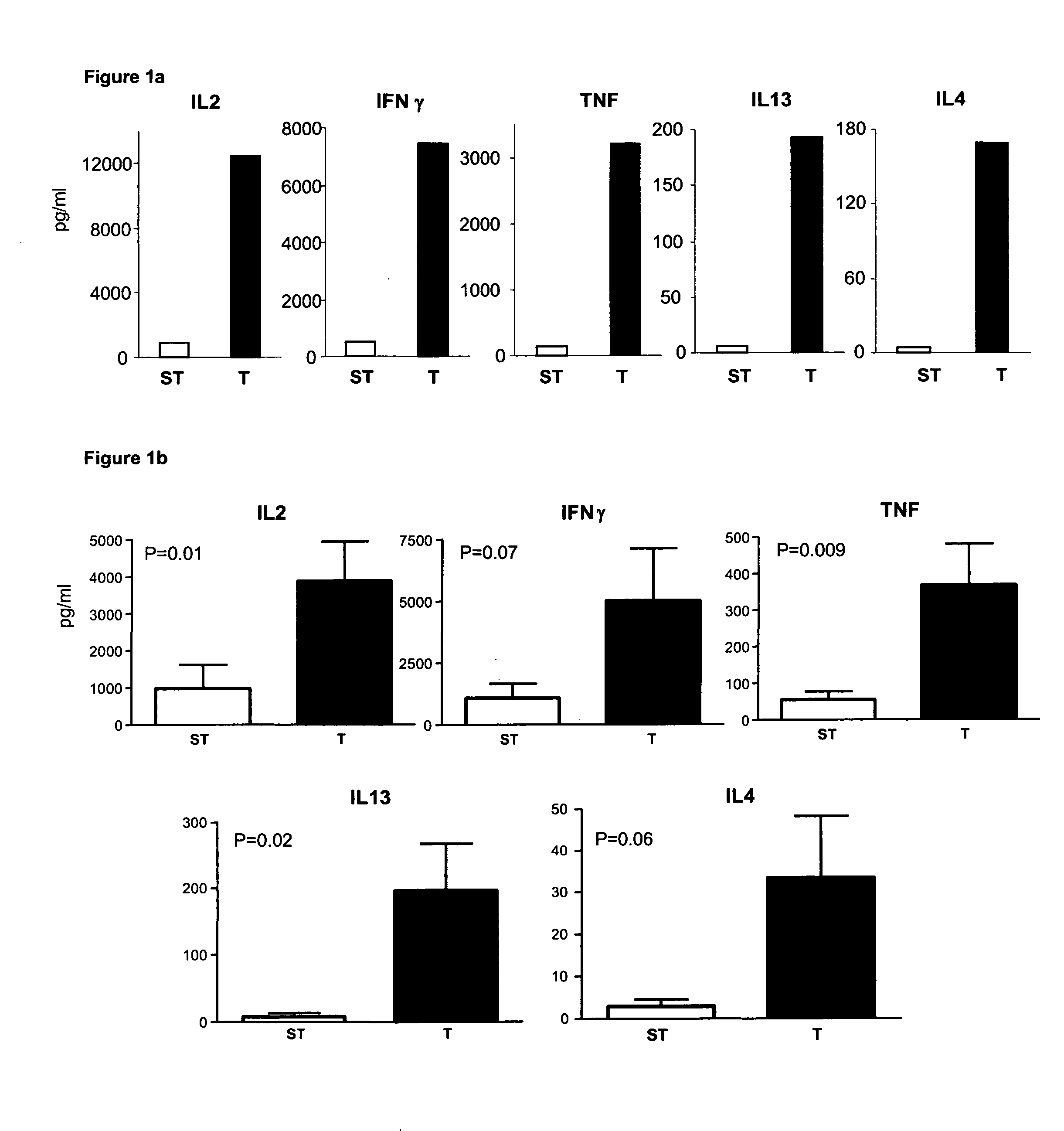
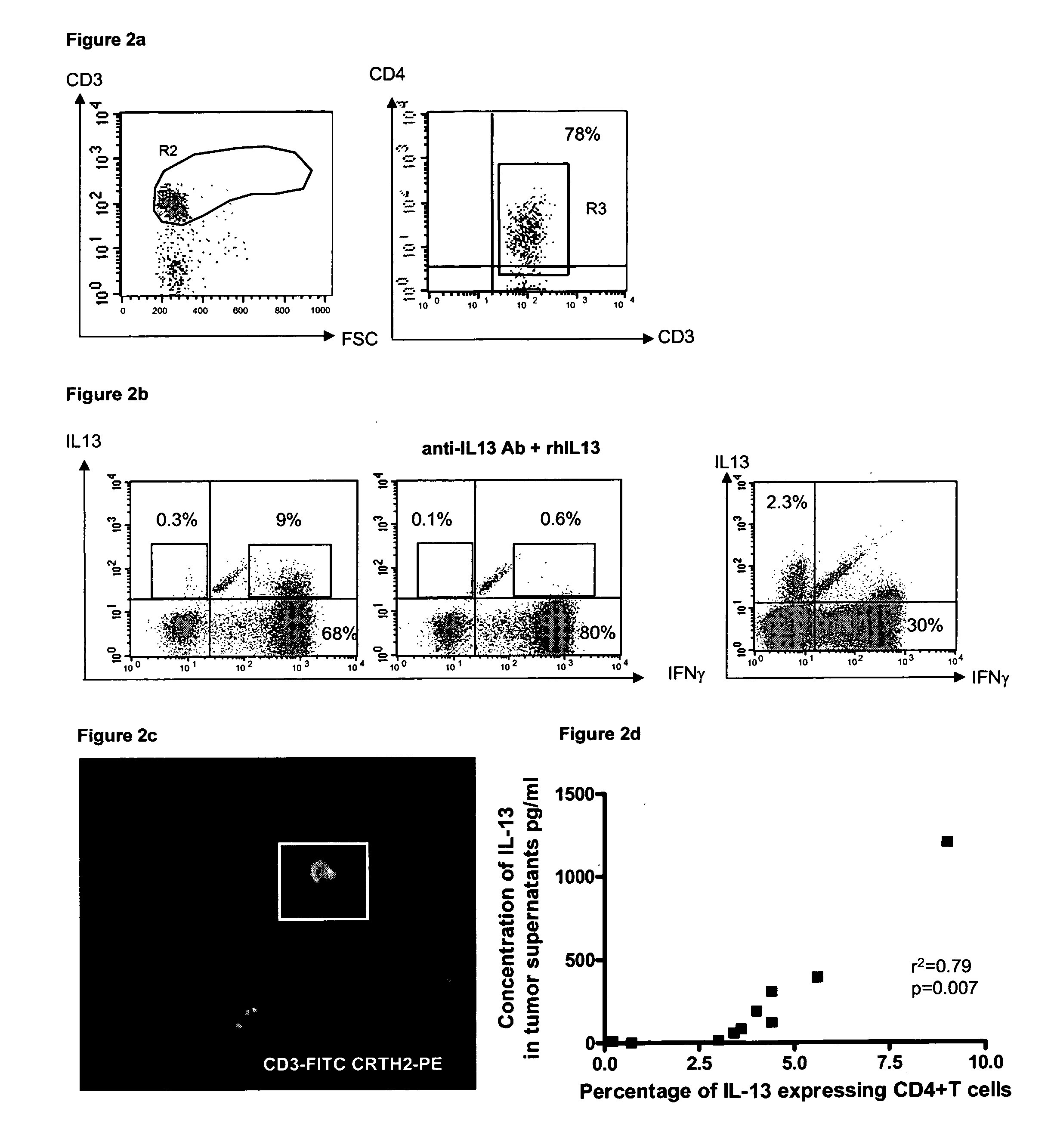
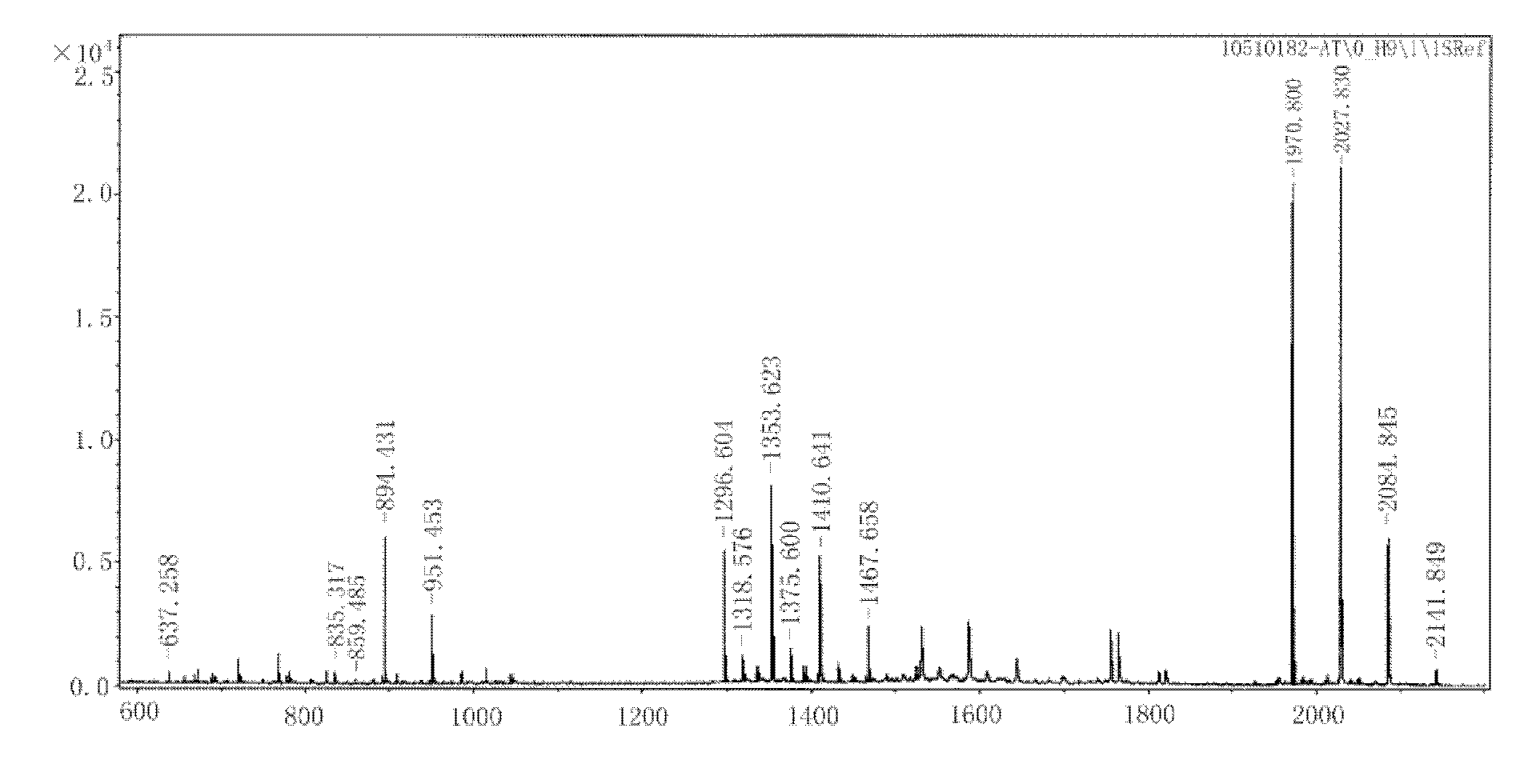
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
127 results about "Helper t-cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
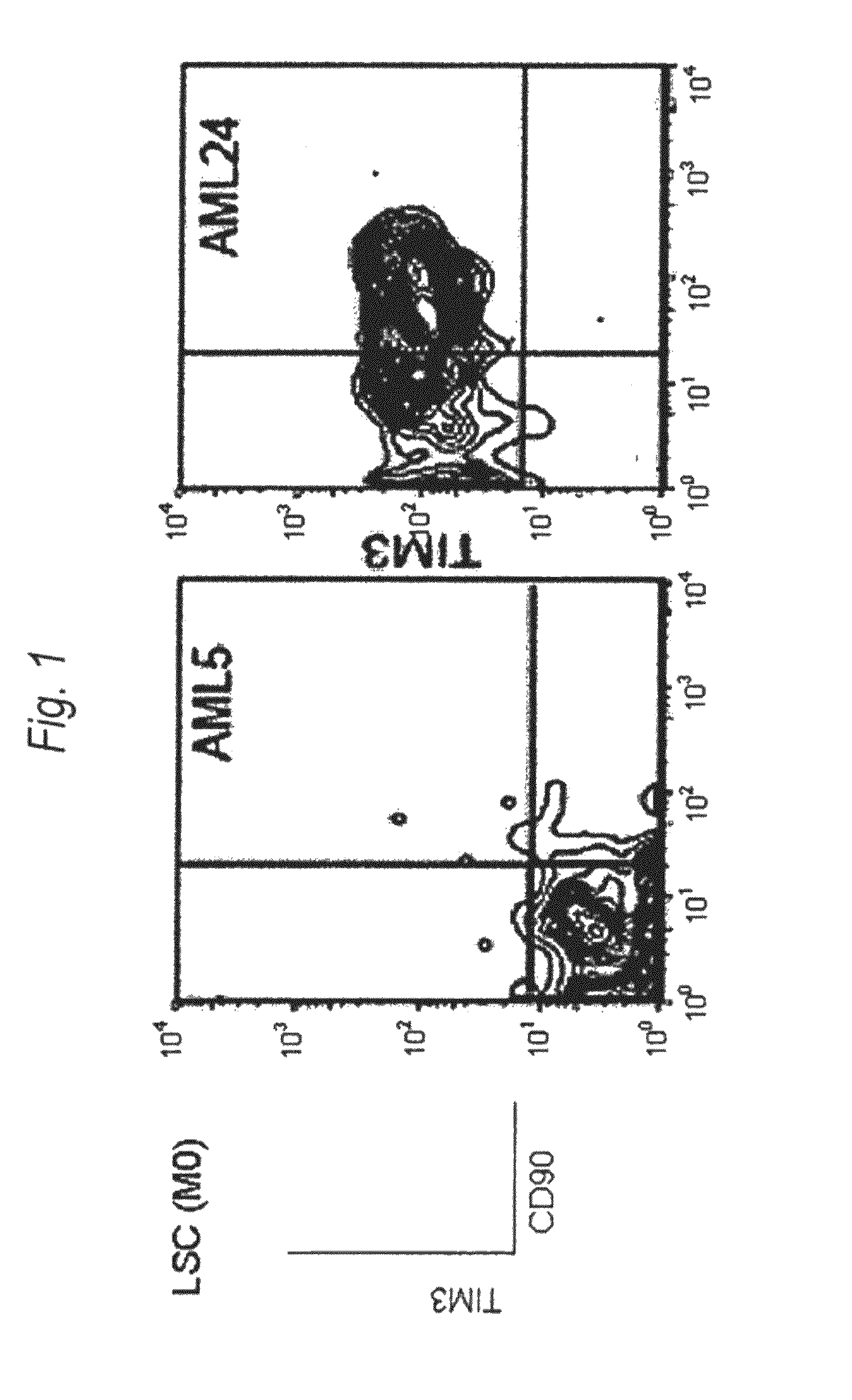
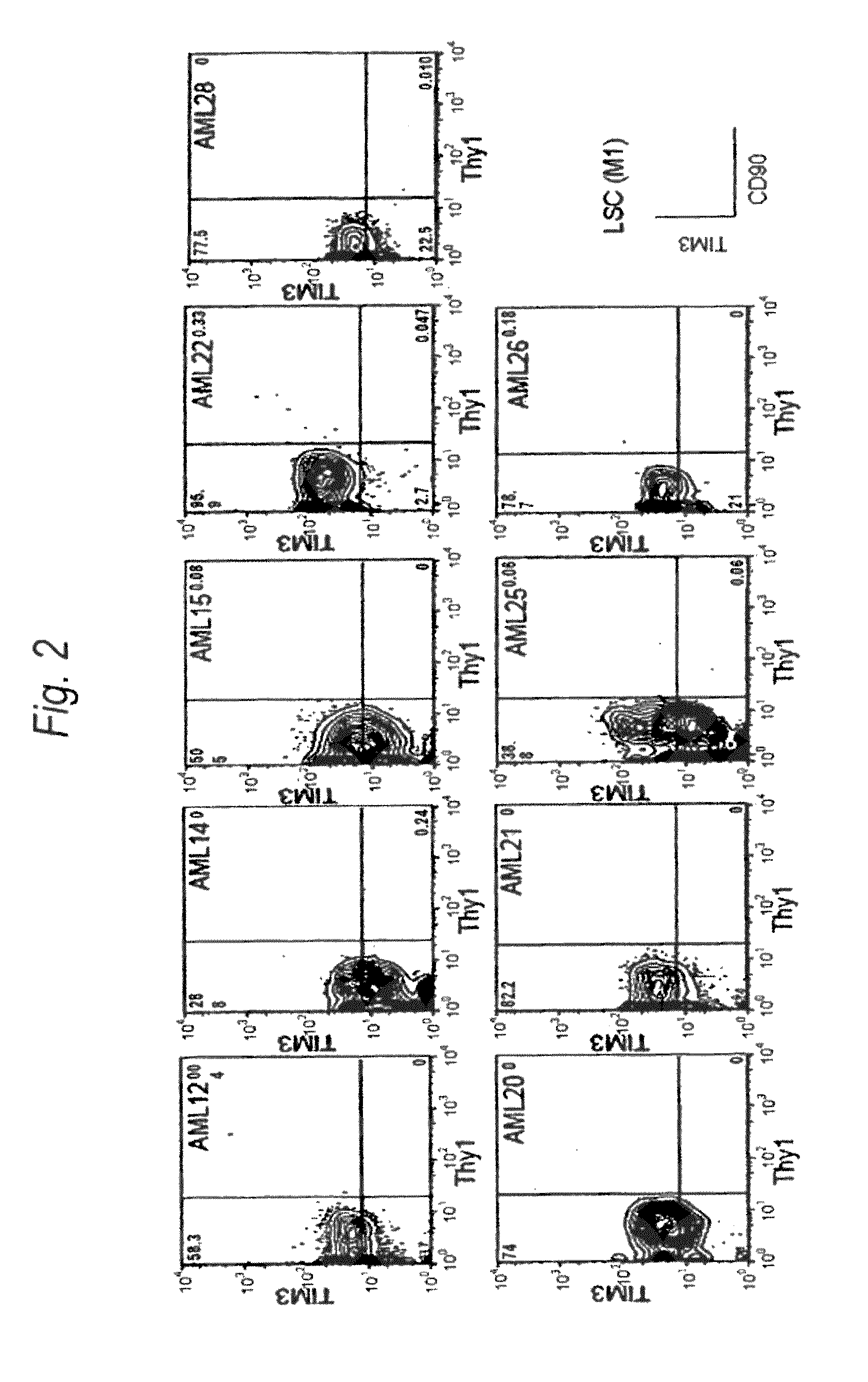
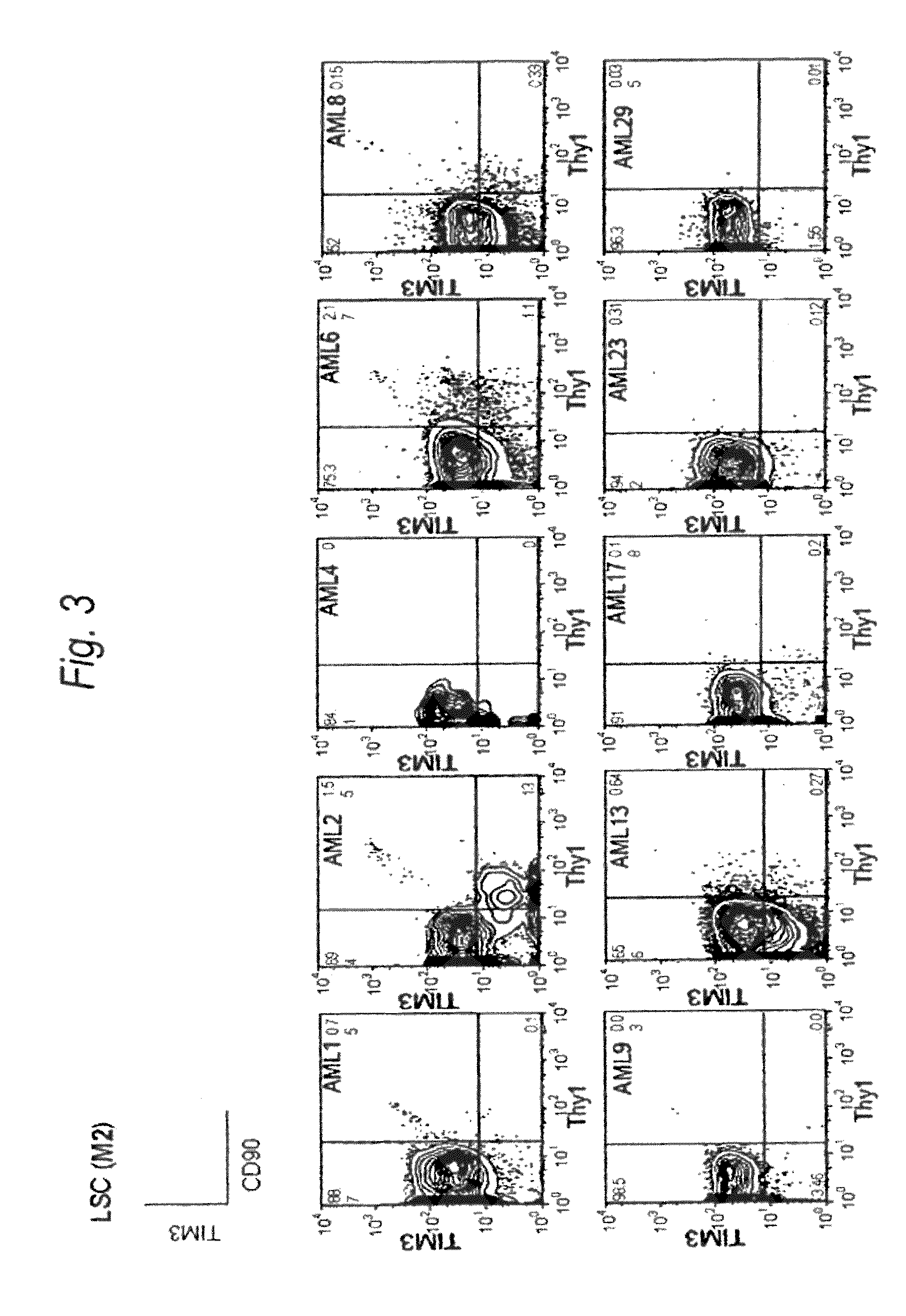
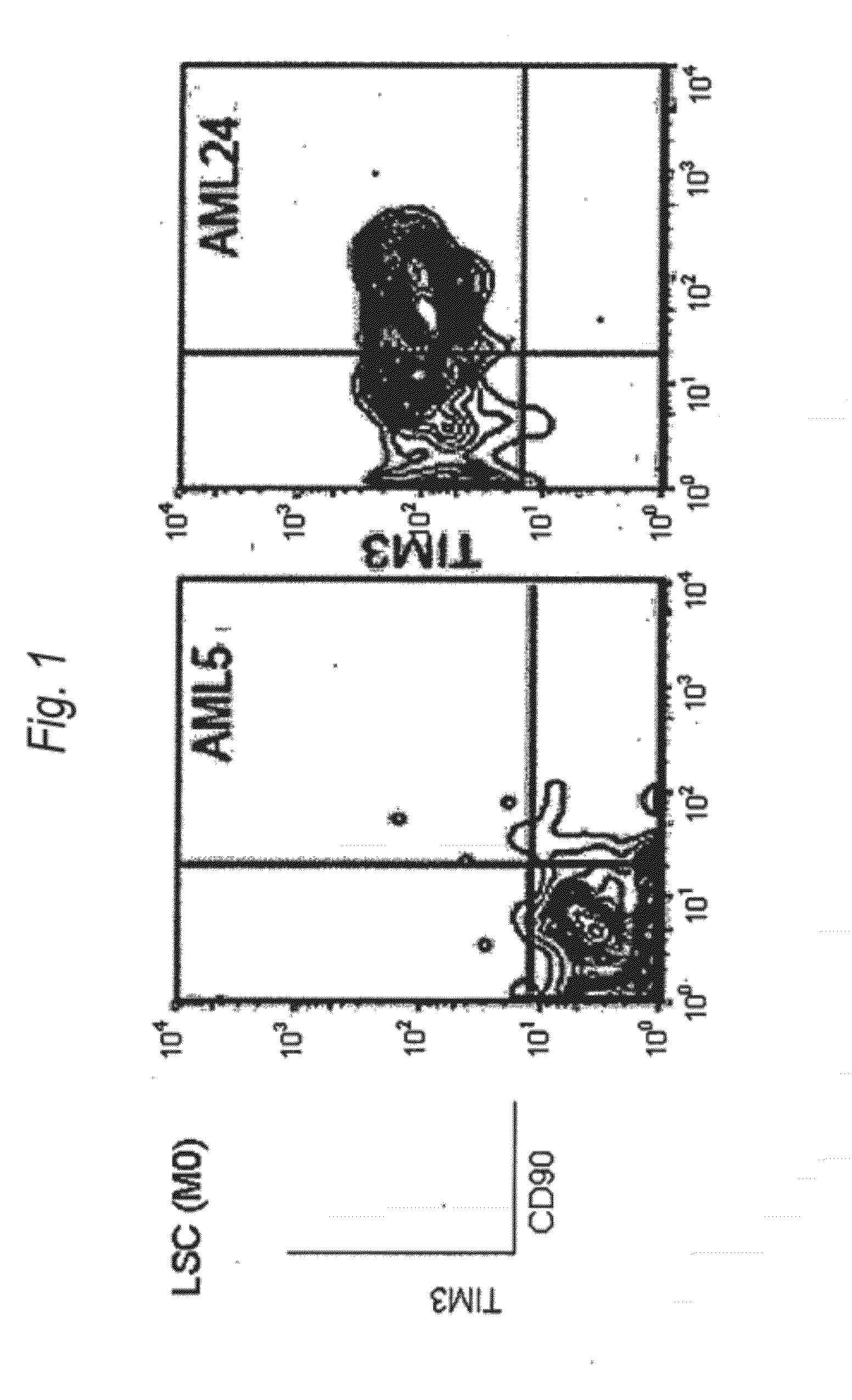
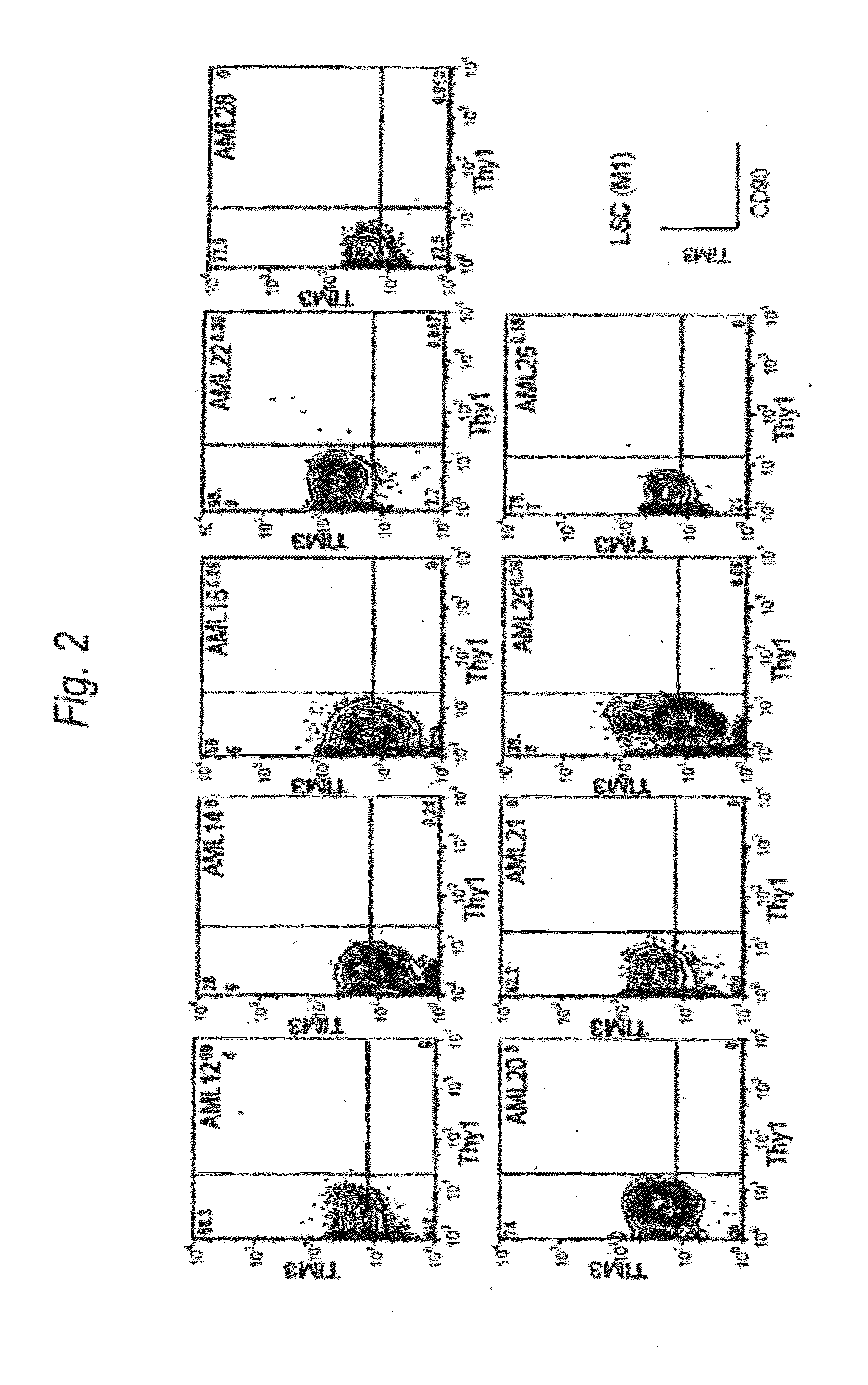
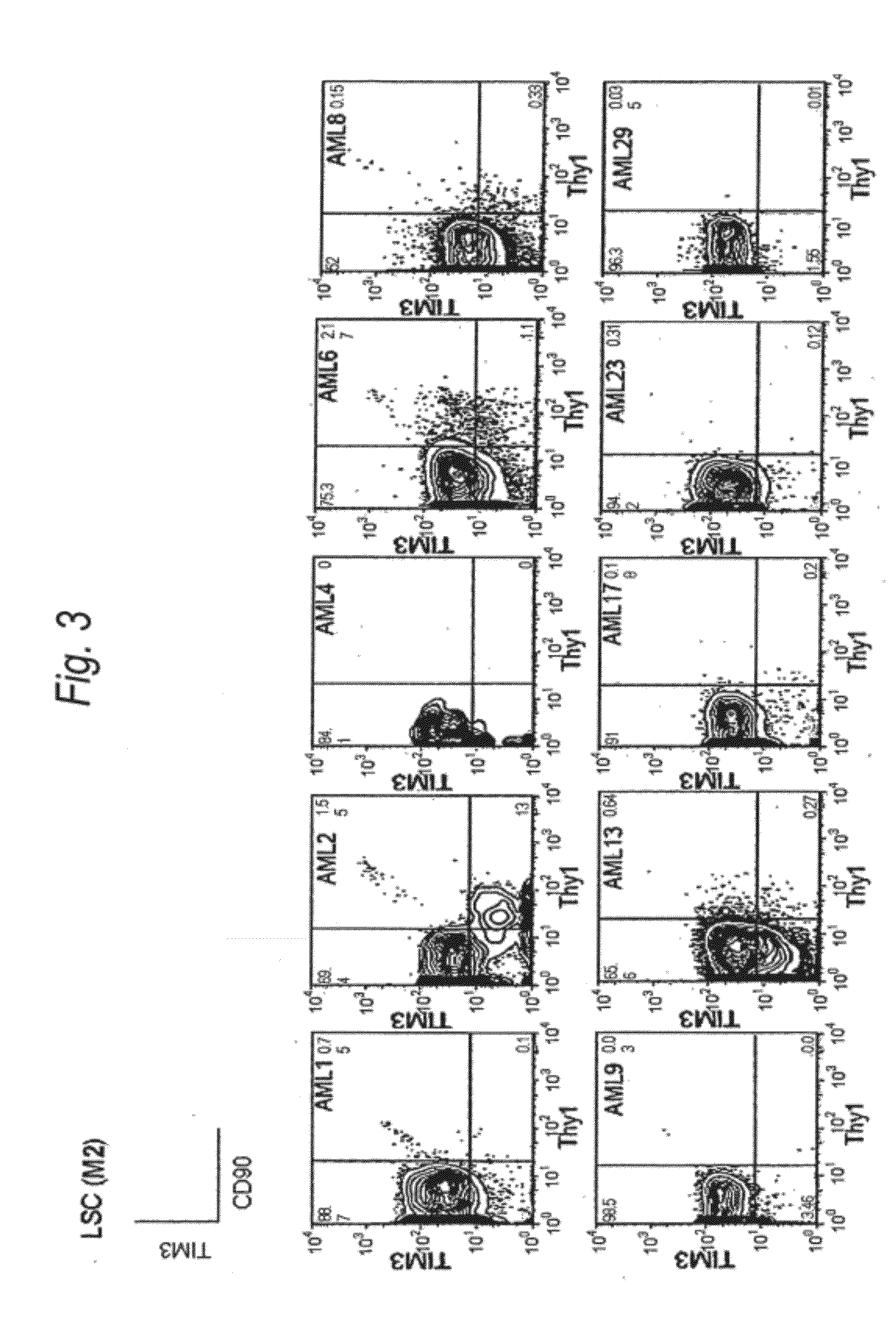
Method for treatment of blood tumor using anti-TIM-3 antibody
Disclosed is a therapeutic method comprising administering a TIM-3 antibody to a subject who is suspected to be suffering from blood tumor and in whom TIM-3 has been expressed in a Lin(−)CD34(+)CD38(−) cell fraction of bone marrow or peripheral blood or a subject who has been received any treatment for blood tumor. Also disclosed is a composition for preventing or treating blood tumor, which comprises a TIM-3 antibody as an active ingredient. Conceived diseases include those diseases which can be treated through the binding or targeting of the TIM-3 antibody to blood tumor cells (AML cells, CML cells, MDS cells, ALL cells, CLL cells, multiple myeloma cells, etc.), helper T cell (e.g., Th1 cells, Th17 cells), and antigen-presenting cells (e.g., dendritic cells, monocytes, macrophages, and cells resembling to the aforementioned cells (hepatic stellate cells, osteoclasts, microglial cells, intraepidermal macrophages, dust cells (alveolar macrophages), etc)), all of which are capable of expressing TIM-3. The diseases for which the therapeutic use is to be examined include blood diseases in which the expression of TIM-3 is observed in bone marrow or peripheral blood, particularly blood tumor.
Owner:KYOWA HAKKO KIRIN CO LTD +1
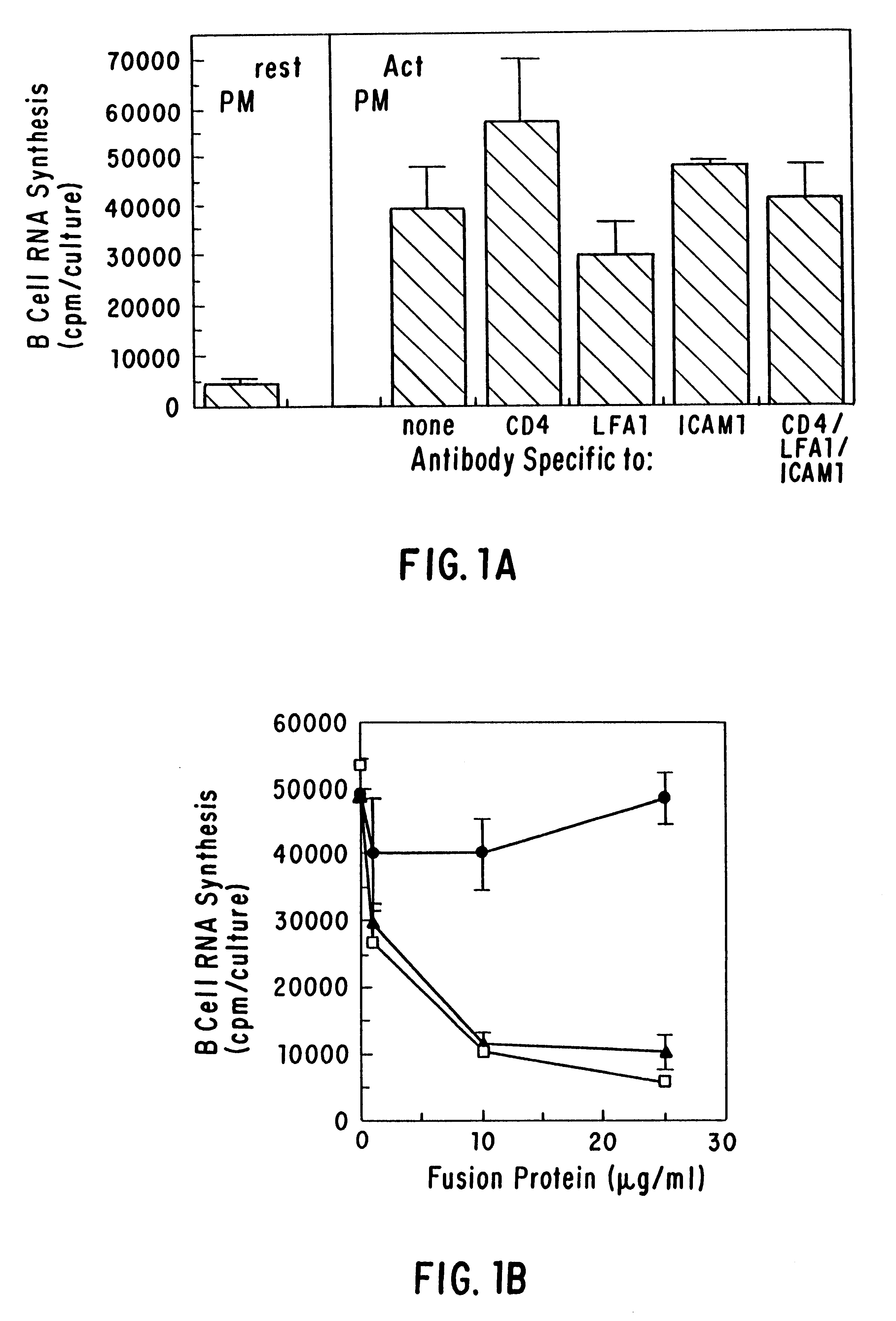
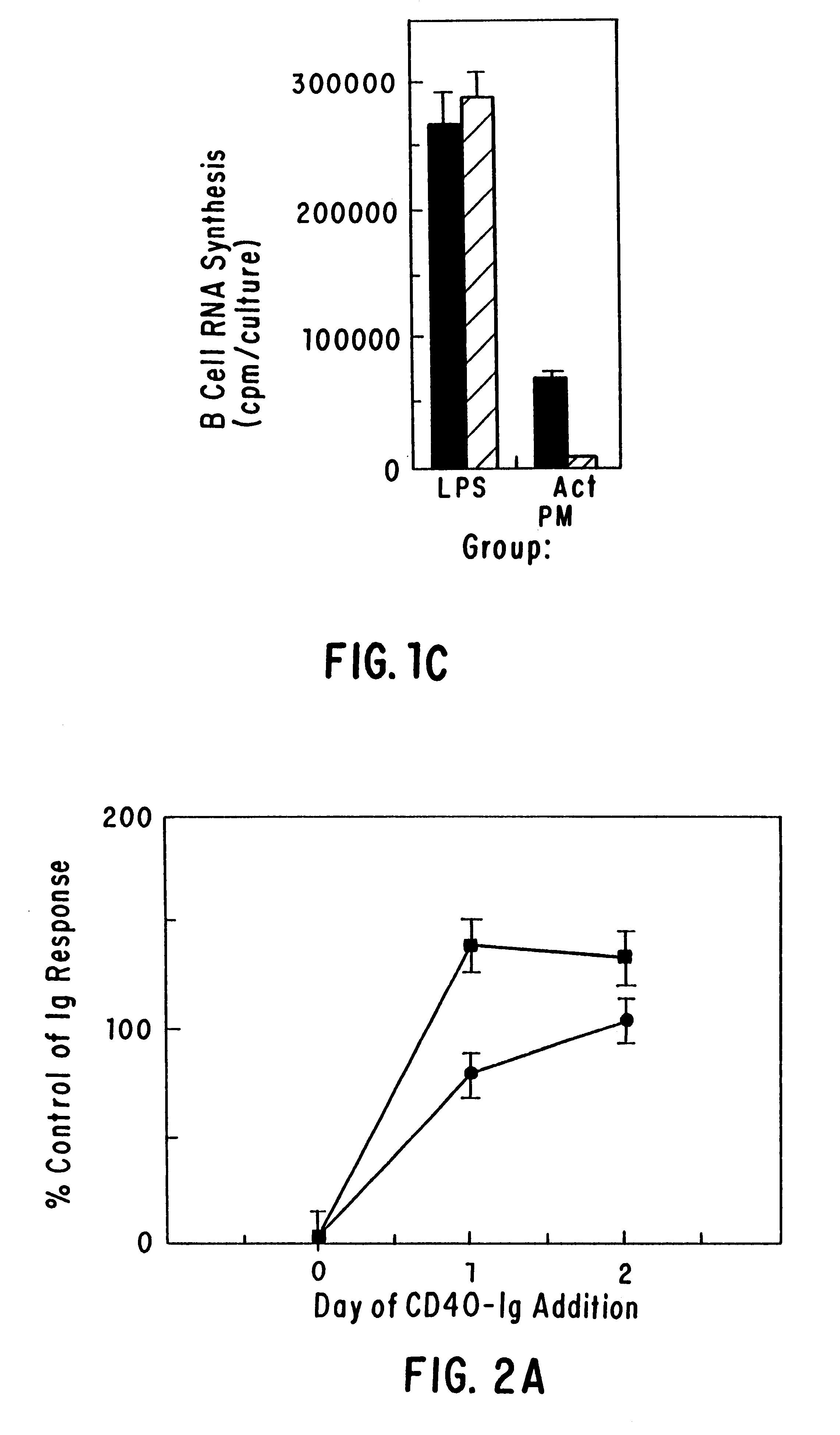
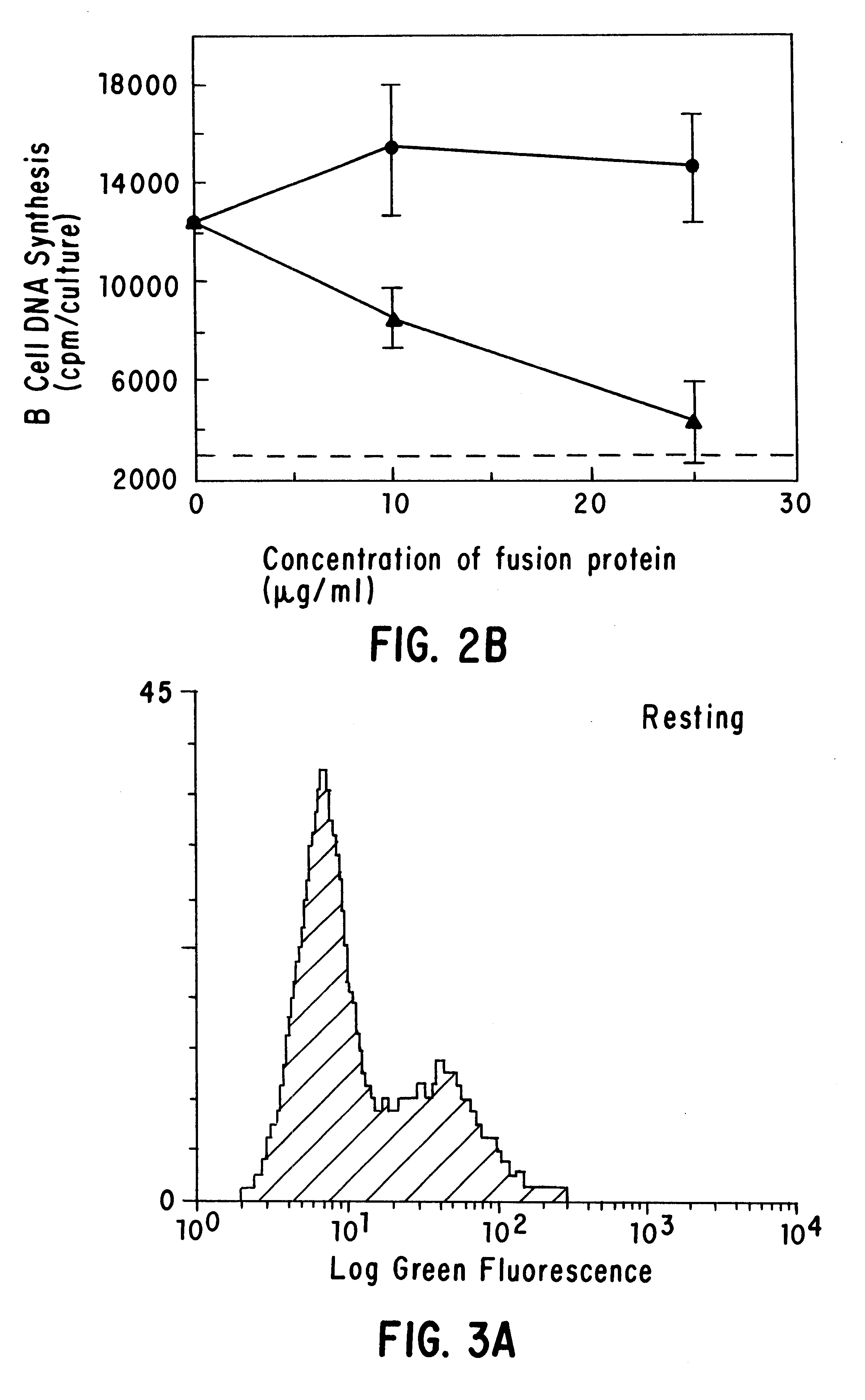
CD40 receptor ligands
InactiveUS6472510B1Analysis impossiblePeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseCell membrane
The present invention relates to a counter-receptor, termed CD40CR, for the CD40 B-cell antigen, and to soluble ligands for this receptor, including fusion molecules comprising at least a portion of CD40 protein. It is based, at least in part, on the discovery that a soluble CD40 / immunoglobulin fusion protein was able to inhibit helper T-cell mediated B-cell activation by binding to a novel 39 kD protein receptor on helper T-cell membranes. The present invention provides for a substantially purified CD40CR receptor; for soluble ligands of CD40CR, including antibodies as well as fusion molecules comprising at least a portion of CD40 protein; and for methods of controlling B-cell activation which may be especially useful in the treatment of allergy or autoimmune disease.
Owner:BRISTOL MYERS SQUIBB CO
Immunogenic peptide composition comprising measles virus Fprotein Thelper cell epitope (MUFThl-16) and N-terminus of β-amyloid peptide
InactiveUS6906169B2Increase the gapHigh cross reactivityNervous disorderPeptide/protein ingredientsFibrilDisease patient
The present invention relates to a composition comprsing a peptide immunogen useful for the prevention and treatment of Alzheimer's Disease. More particularly, the peptide immunogen comprises a main functional / regulatory site, an N-terminal fragment of Amyloid β (Aβ) peptide linked to a helper T cell epitope (Th) having multiple class II MHC binding motifs. The peptide immunogen elicit a site-directed immune response against the main functional / regulatory site of the Aβ peptide and generate antibodies, which are highly cross-reactive to the soluble Aβ1-42 peptide and the amyloid plaques formed in the brain of Alzheimer's Disease patients. The antibodies elicited being cross reactive to the soluble Aβ1-42 peptide, promote fibril disaggregation and inhibit fibrillar aggregation leading to immunoneutralization of the “soluble Aβ-derived toxins”; and being cross-reactive to the amyloid plaques, accelerate the clearance of these plaques from the brain. Thus, the composition of the invention comprising the peptide immunogen is useful for the prevention and treatment of Alzheimer's Disease.
Owner:UNITED NEUROSCIENCE LIMITED
Gelled immunomodulating topical compositions and a method of treating warts and other human papilloma virus skin infections
Topical drug compositions of this invention contain delayed type contact sensitizing haptens in a unique non-flowable, non-toxic, non-volatile, anhydrous gel composition to achieve retained site application on warts and other human papilloma virus (HPV) skin infections. The preferred gelled compositions contain, but are not limited to, the sensitizing haptens, squaric acid dibutylester and diphenylcyclopropenone in optimized blends of Polysorbate 80, Isopropyl myristate uniquely gelled with Polyoxyl 40 stearate to form a penetrant of keratinized epitheliiuim of warts for direct application wherein virucidal pharlacologic action is induced by Th-1 cell mediated immune responses with resultant releases of CD4 helper T cells, CD8 killer T cells and cytokines to attack the human papilloma viruses. The commonly used vehicles with these contact sensitizers are acetone, petrolatum, or water containing emulsion creams which do not have the capacity to penetrate the keratinized wart surfaces and are therefore minimally effective in treating warts.
Owner:HAPTEN PHARMA
Method for treatment of blood tumor using Anti-tim-3 antibody
ActiveUS20120100131A1Use to establishBiological material analysisAntibody ingredientsDiseaseCell Fraction
Disclosed is a therapeutic method comprising administering a TIM-3 antibody to a subject who is suspected to be suffering from blood tumor and in whom TIM-3 has been expressed in a Lin(−)CD34(+)CD38(−) cell fraction of bone marrow or peripheral blood or a subject who has been received any treatment for blood tumor. Also disclosed is a composition for preventing or treating blood tumor, which comprises a TIM-3 antibody as an active ingredient. Conceived diseases include those diseases which can be treated through the binding or targeting of the TIM-3 antibody to blood tumor cells (AML cells, CML cells, MDS cells, ALL cells, CLL cells, multiple myeloma cells, etc.), helper T cell (e.g., Th1 cells, Th17 cells), and antigen-presenting cells (e.g., dendritic cells, monocytes, macrophages, and cells resembling to the aforementioned cells (hepatic stellate cells, osteoclasts, microglial cells, intraepidermal macrophages, dust cells (alveolar macrophages), etc)), all of which are capable of expressing TIM-3. The diseases for which the therapeutic use is to be examined include blood diseases in which the expression of TIM-3 is observed in bone marrow or peripheral blood, particularly blood tumor.
Owner:KYOWA HAKKO KIRIN CO LTD +1
Tolerance-induced targeted antibody production
InactiveUS20070036809A1Growth inhibitionOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthTolerance induction
The present invention provides methods for directing the immune response of an animal towards immunologically weak or rare antigens such as tumor antigens. The methods combine subtractive immunization with hyperimmunization and result in the controlled or directed production of target-specific antibodies, helper T cells (CD4+-T lymphocytes) and cytotoxic T cells (CD8+-T lymphocytes). Also provided by the present invention are untransformed and transformed cell lines, and growth media necessary to grow the untransformed cell line in a differentiated state. Monoclonal antibodies which react with different neoplastic cell lines and hybridomas producing such antibodies are also provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
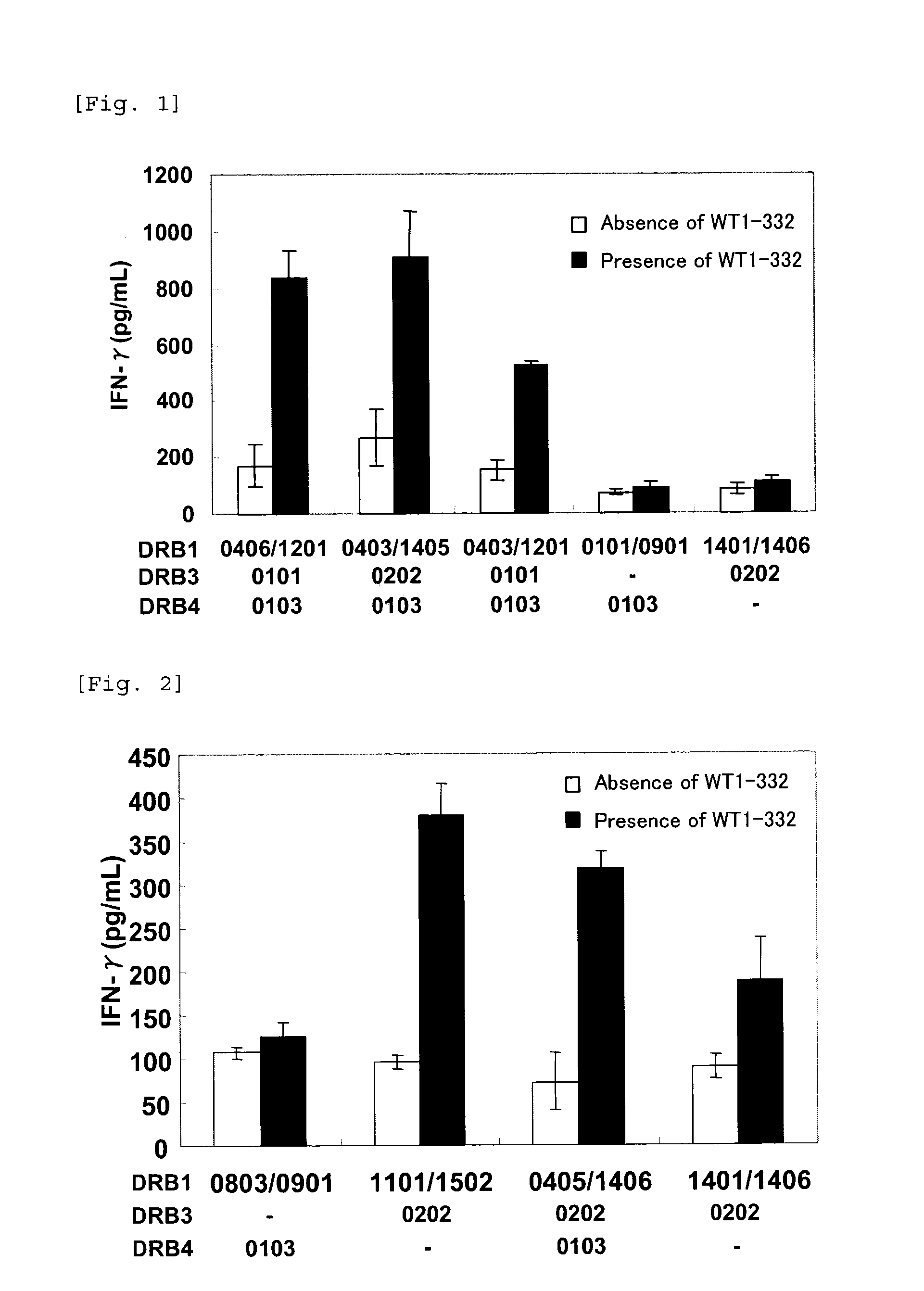
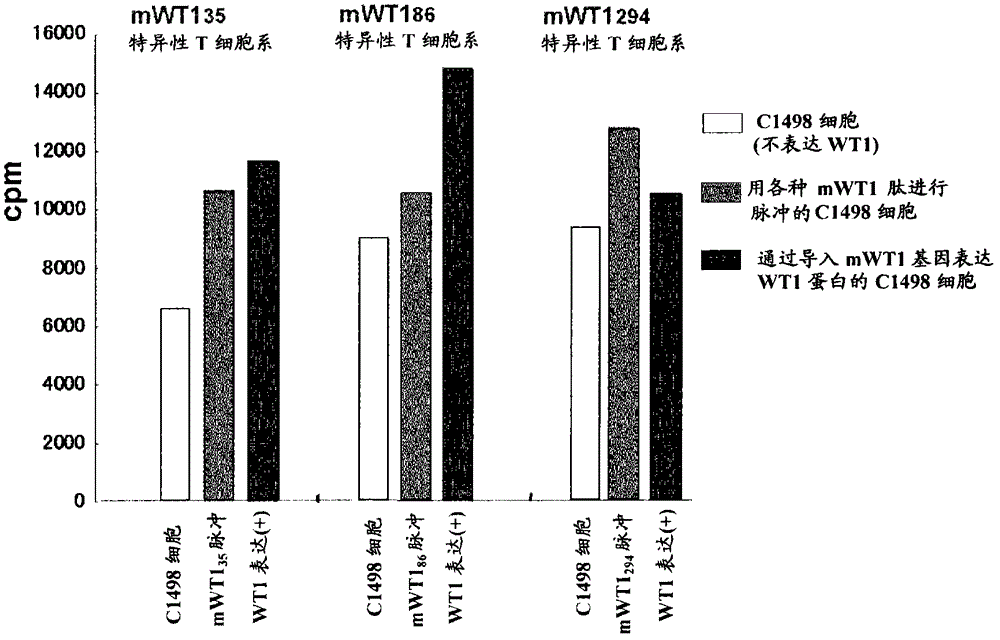
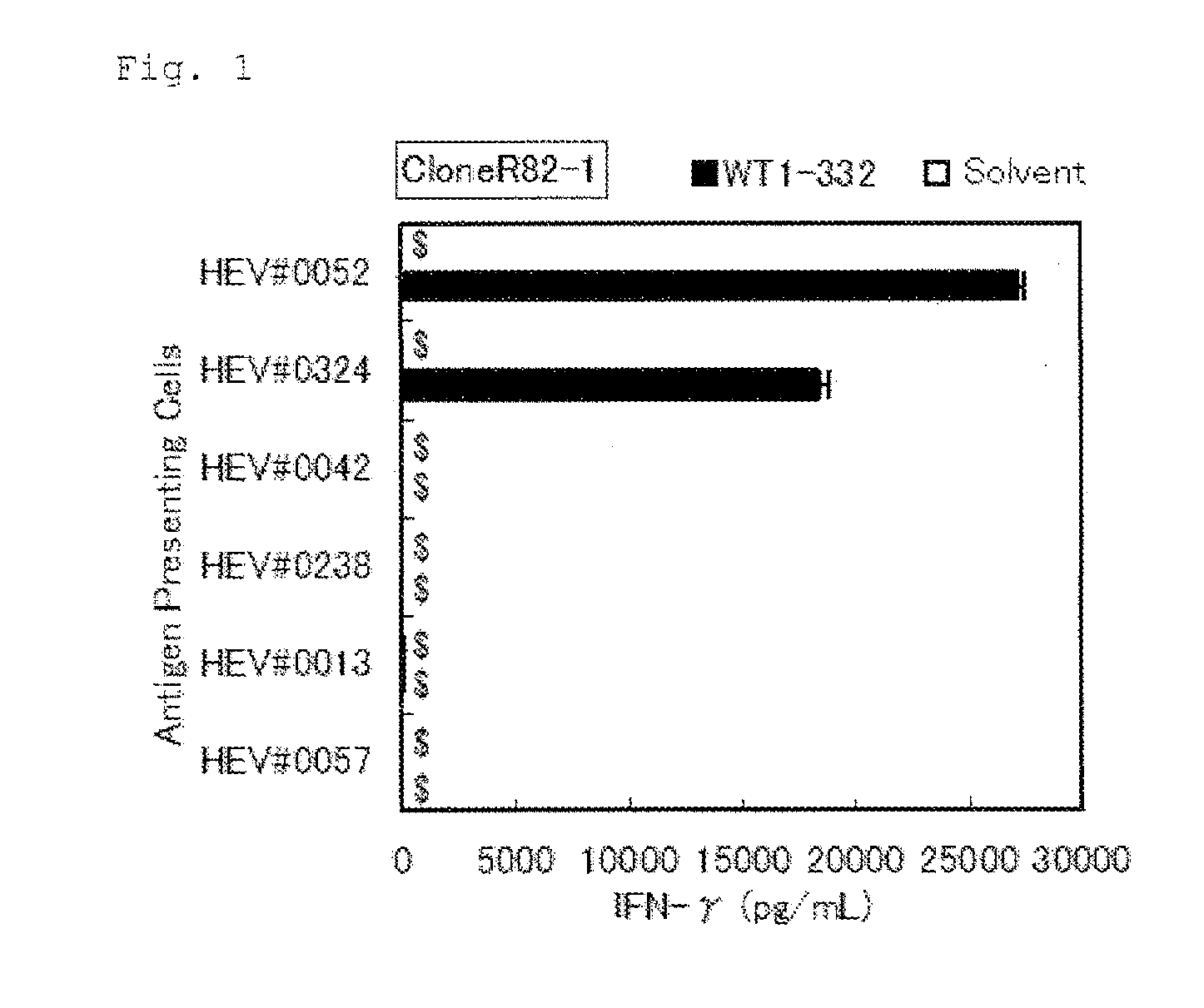
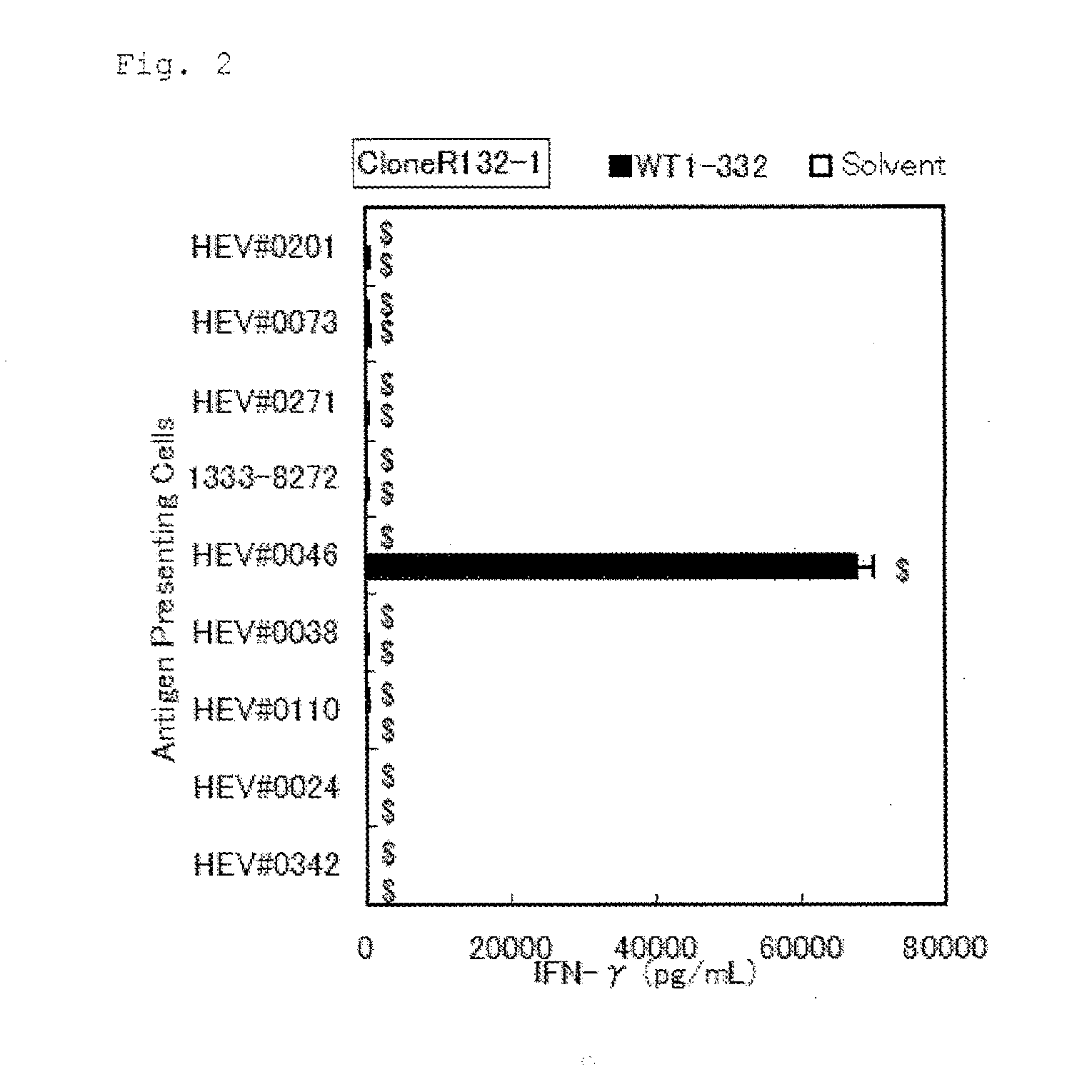
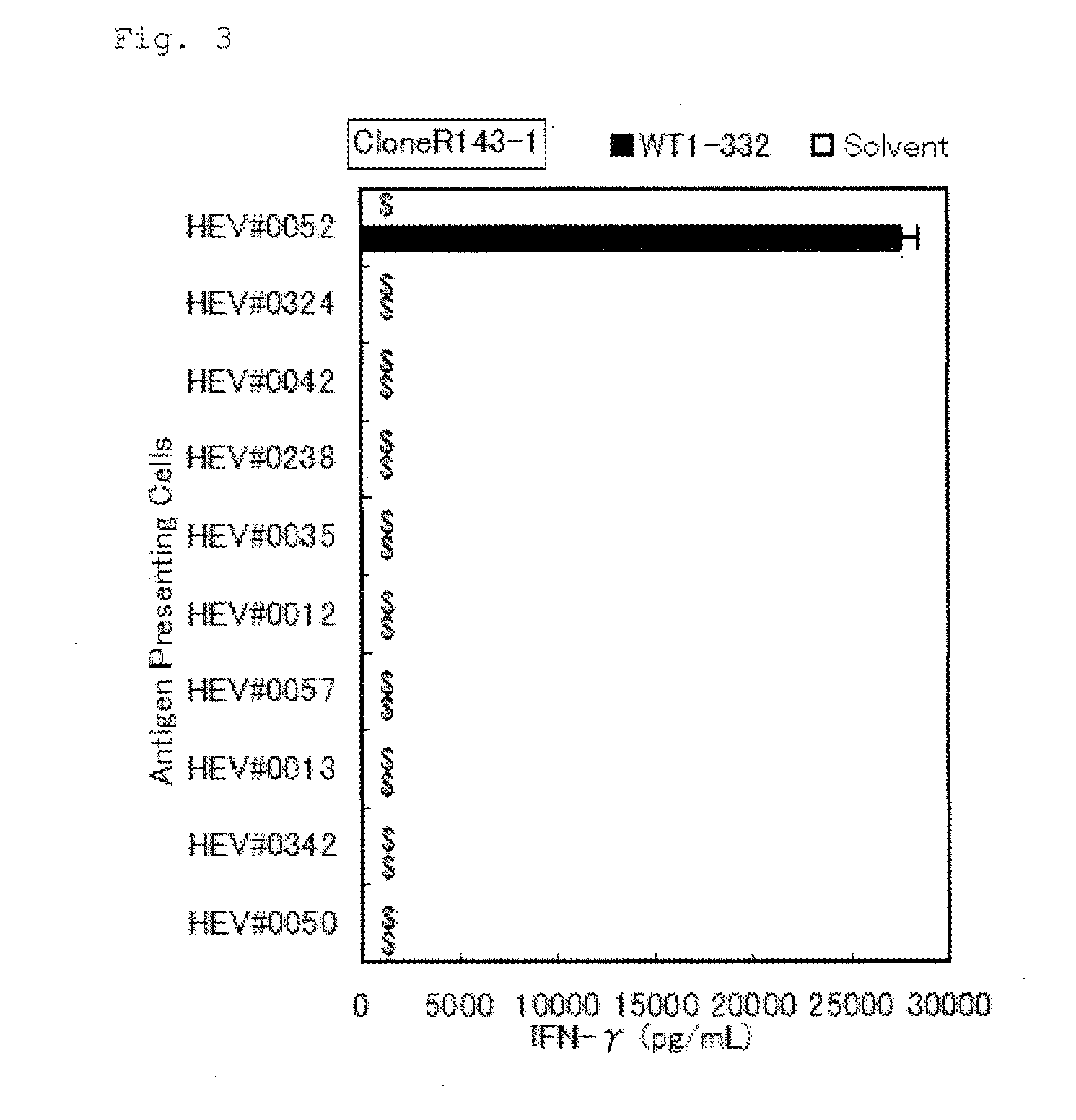
Method for activating helper t cell
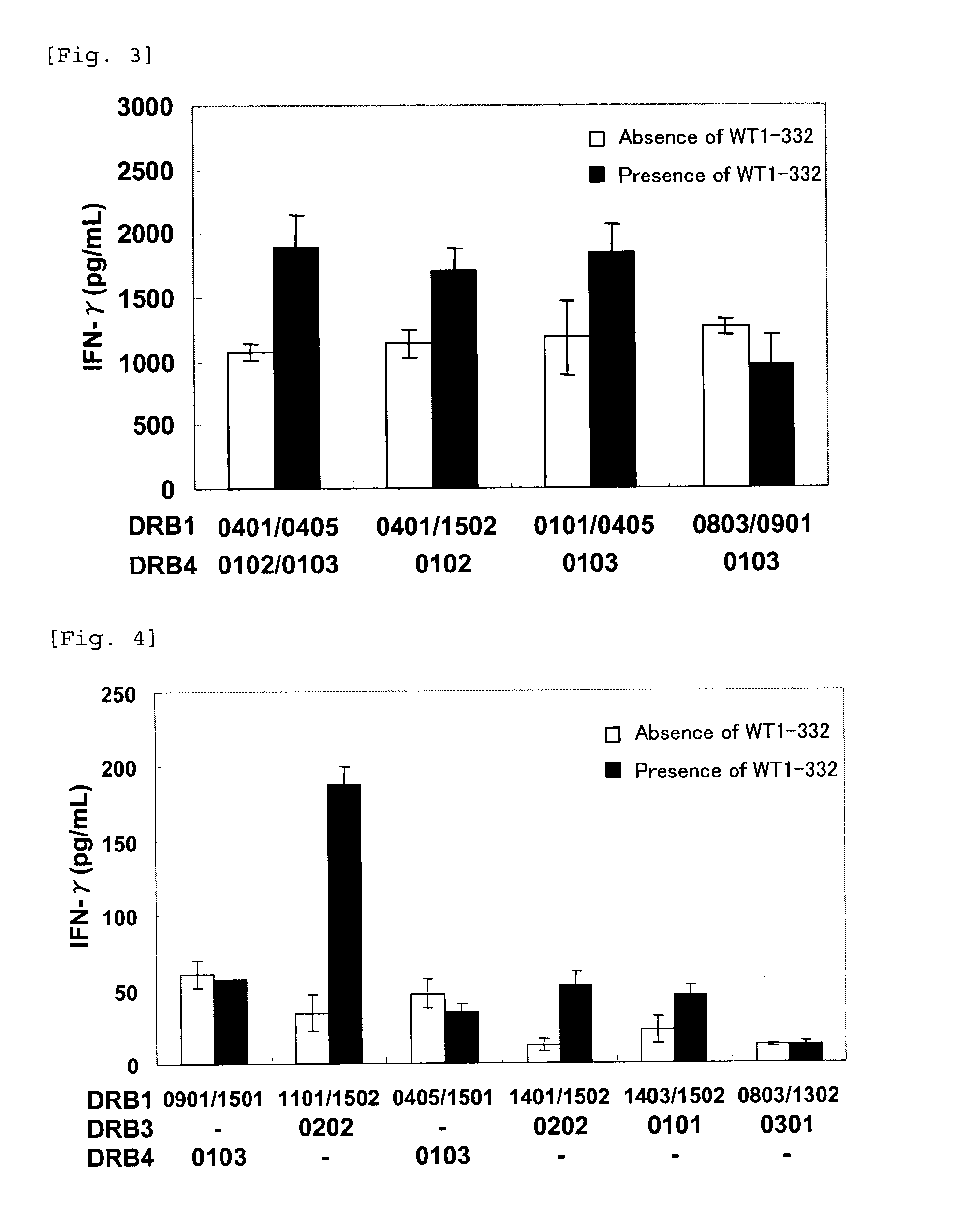
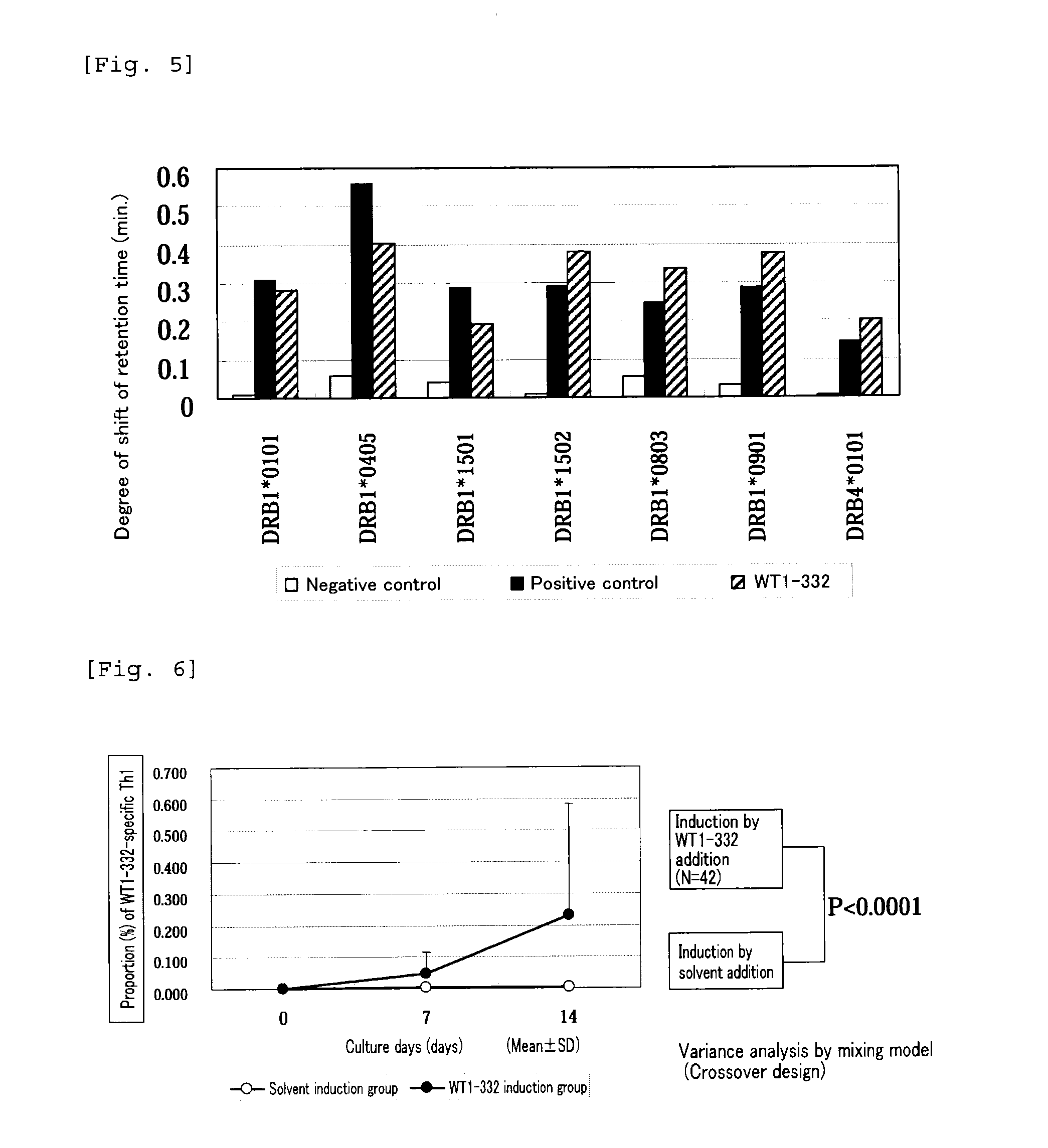
The present invention relates to a method for activating helper T cells, which includes the step of activating helper T cells by adding a WT1 peptide to antigen presenting cells, wherein the WT1 peptide has the ability to bind to any MHC class II molecule of an HLA-DRB1* 0101 molecule, an HLA-DRB1* 0401 molecule, an HLA-DRB1* 0403 molecule, an HLA-DRB1* 0406 molecule, an HLA-DRB1* 0803 molecule, an HLA-DRB1* 0901 molecule, an HLA-DRB1* 1101 molecule, an HLA-DRB3* 0202 molecule, an HLA-DRB4* 0101 molecule, an HLA-DPB1* 0201 molecule or an HLA-DPB1* 0301 molecule, and the like.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Cancer antigen helper peptide
ActiveCN102803487AEfficient activationOrganic active ingredientsTumor rejection antigen precursorsAntigenCancer antigen
Disclosed are: a WT1 peptide which comprises an amino acid sequence composed of contiguous amino acid residues derived from a WT1 protein and can bind to an MHC class-II molecule to induce a WT1-specific helper T cell; a pharmaceutical composition containing the peptide; and others.
Owner:INT INST OF CANCER IMMUNOLOGY INC
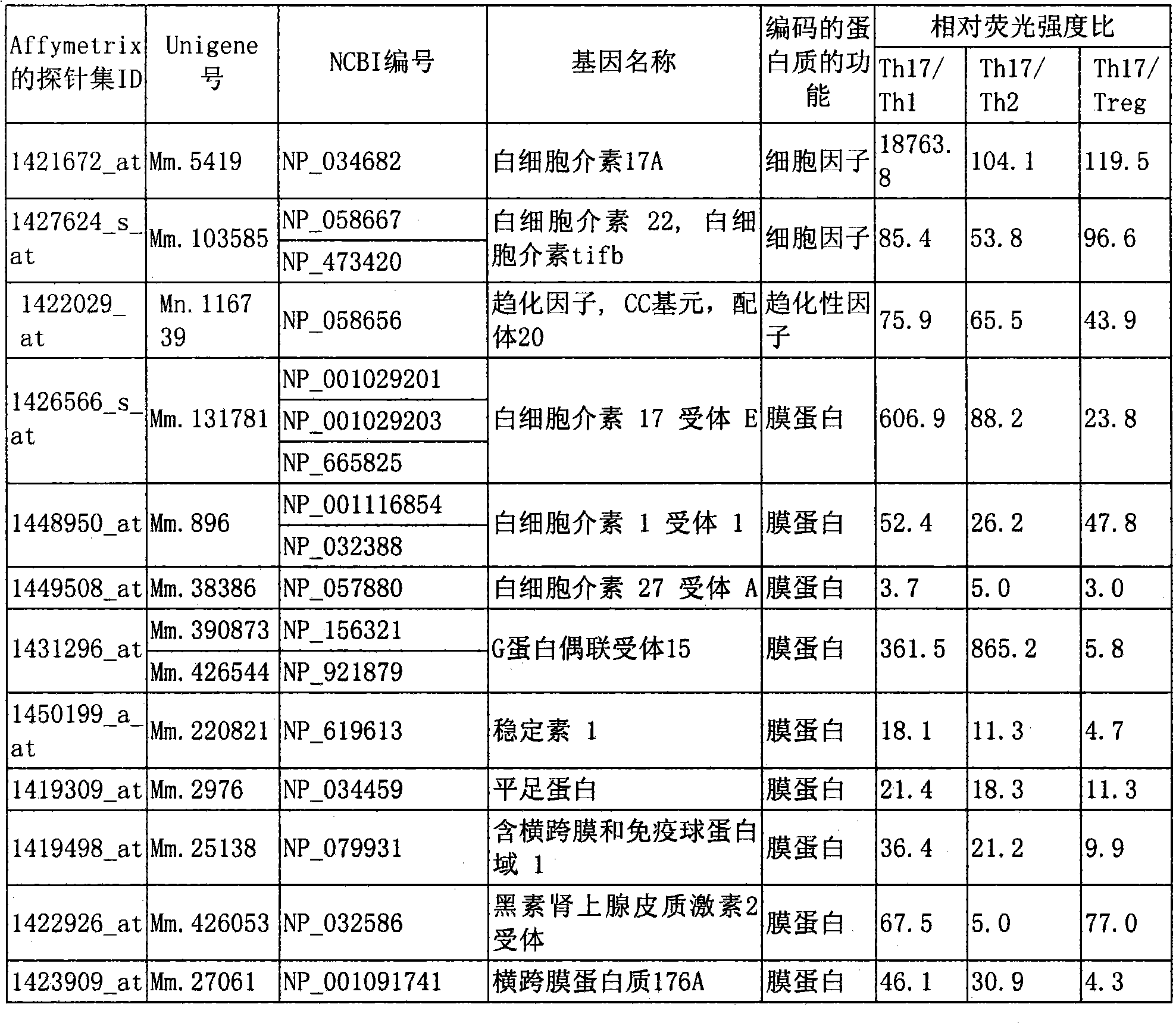
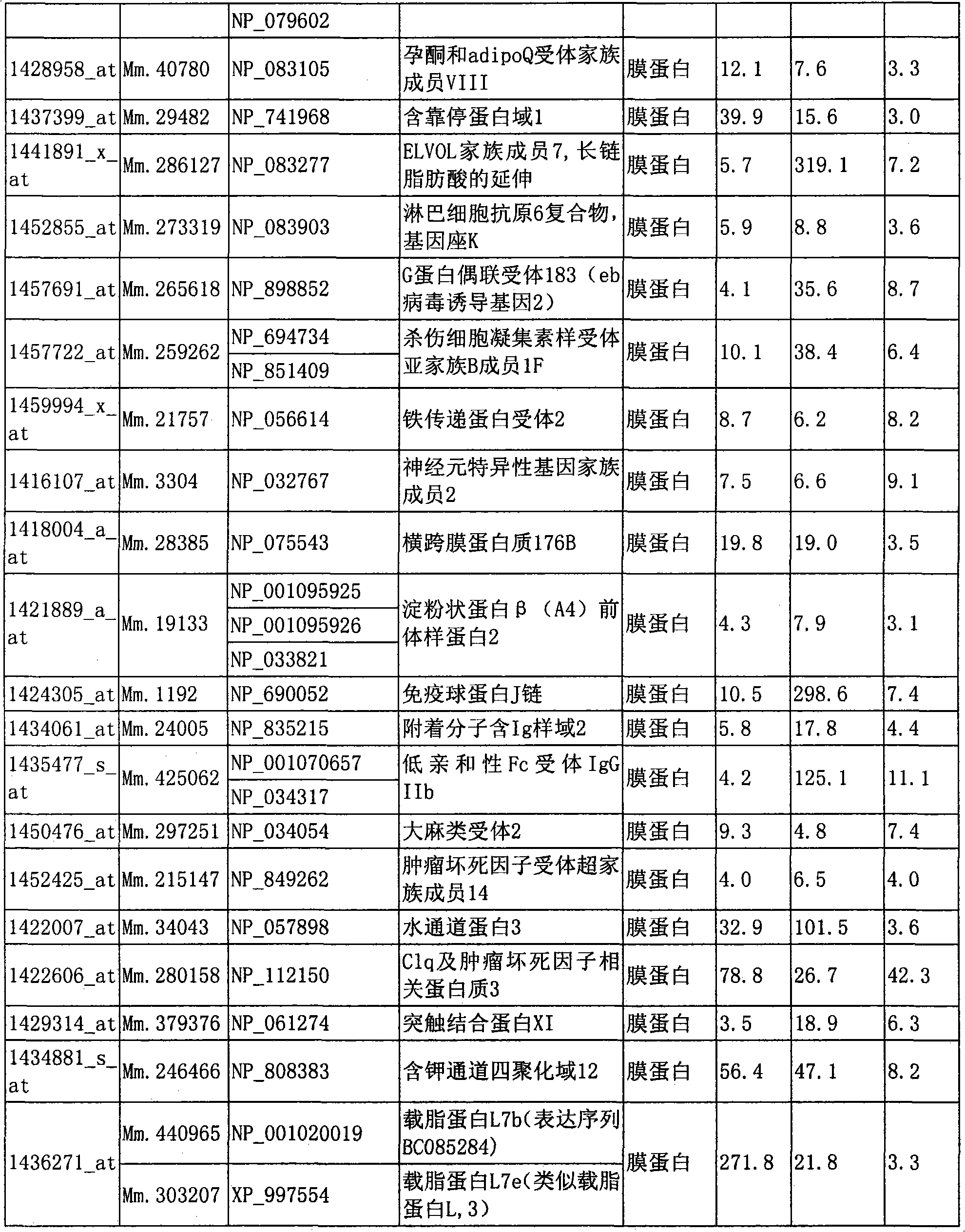
Marker for detecting il-17-producing helper t cell, and method for detecting il-17-producing helper t cell
InactiveUS20110136113A1Potential morbiditySugar derivativesMicrobiological testing/measurementSpecific detectionPolynucleotide
Disclosed is at least one polynucleotide marker or protein marker which enables the specific detection of an IL-17-producing helper T cell (a Th17 cells). Also disclosed is a method for detecting a Th17 cell, which is characterized by comprising detecting the occurrence of the above-mentioned at least one marker.
Owner:SYSMEX CORP
Method for activating helper t cell
The present invention relates to a method for activating helper T cells, which includes the step of activating helper T cells by adding a WT1 peptide to antigen presenting cells, wherein the WT1 peptide has the ability to bind to an MHC class II molecule selected from HLA-DRB1*08:02 molecule, an HLA-DRB1*13:02 molecule, an HLA-DRB1*14:03 molecule, an HLA-DRB1*14:05 molecule, an HLA-DQB1*03:02 molecule, and an HLA-DQB1*04:01 molecule.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Live vaccine for human immunodeficiency virus
InactiveUS7189402B1Bacterial antigen ingredientsAntibody mimetics/scaffoldsSalmonella wienHIV Proteins
The present invention discloses development of a model live vaccine for HIV, using an attenuated strain of Salmonella engineered to surface express specific HIV proteins and testing of this vaccine in mice. There are provided two recombinant plasmids, containing the Lpp-OmpA genes required for surface exposure, followed by the genes for the HIV-1 proteins, Reverse Transcriptase or Transactivating protein (Tat). These plasmids are electroporated into an attenuated strain of Salmonella, and antigen expression is verified. These live vaccines are then used to orally inoculate mice and the vaccinated mice are tested for fecal IgA response and helper T cell response specific for the HIV antigens.
Owner:RES DEVMENT FOUND
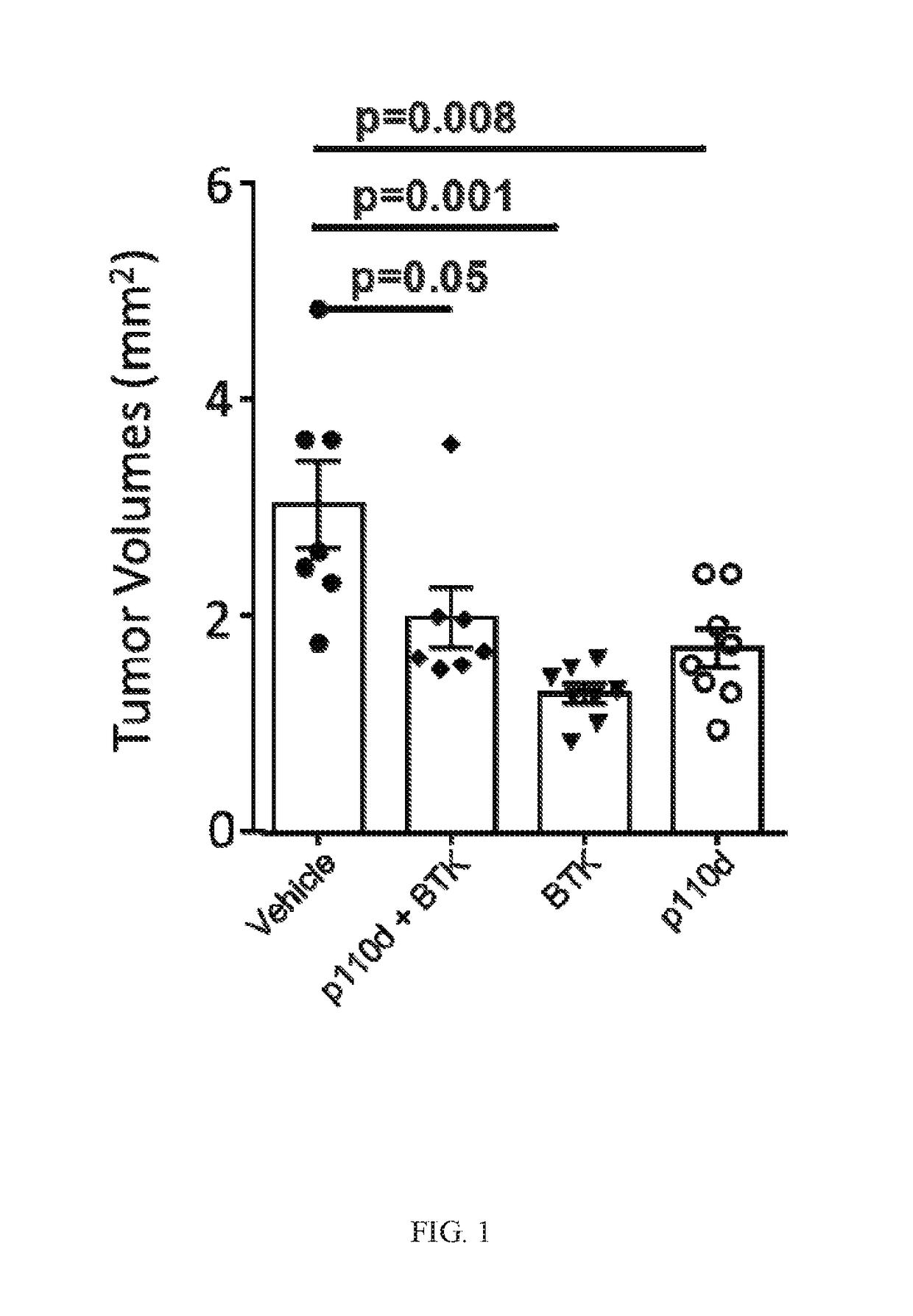
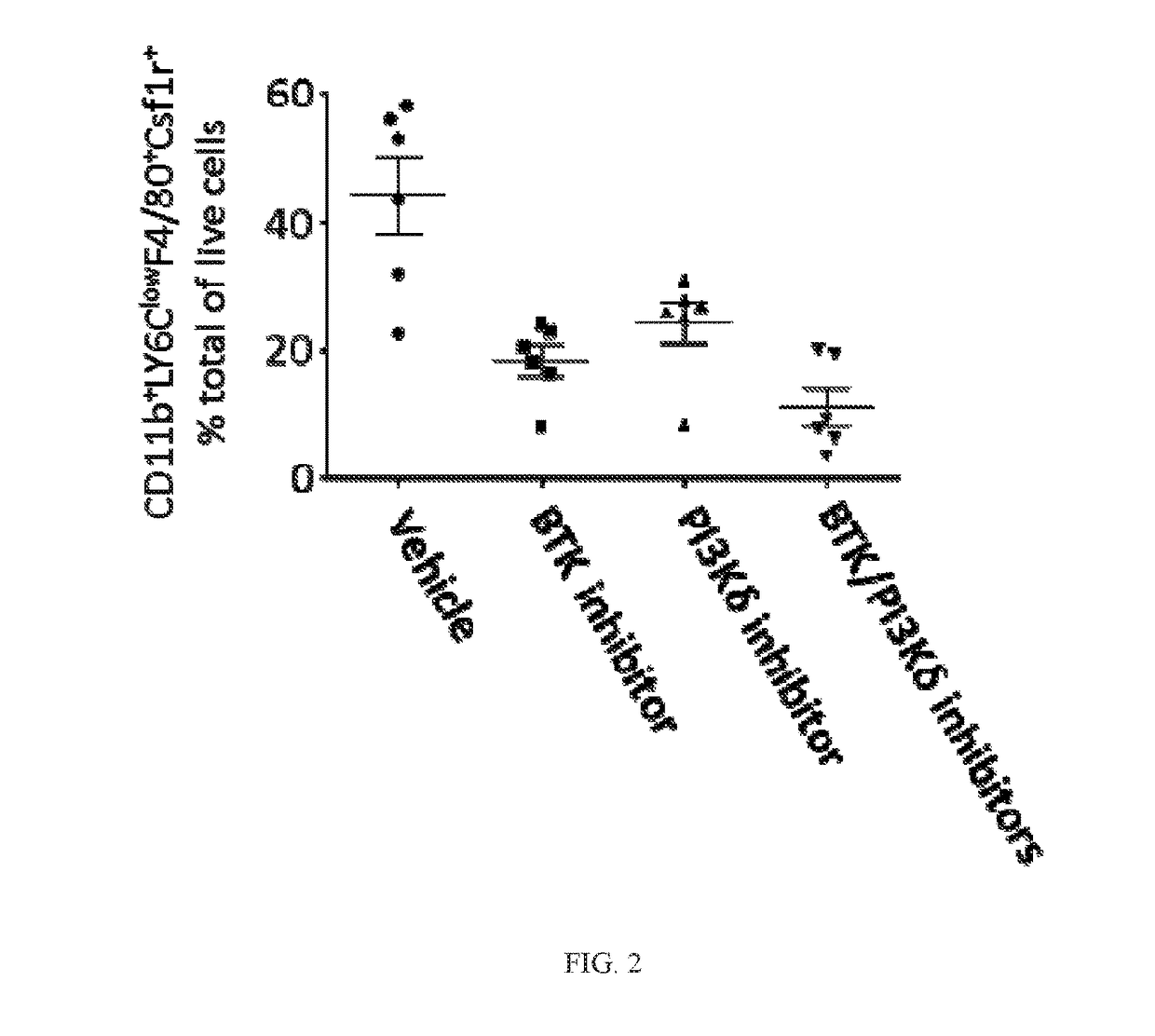
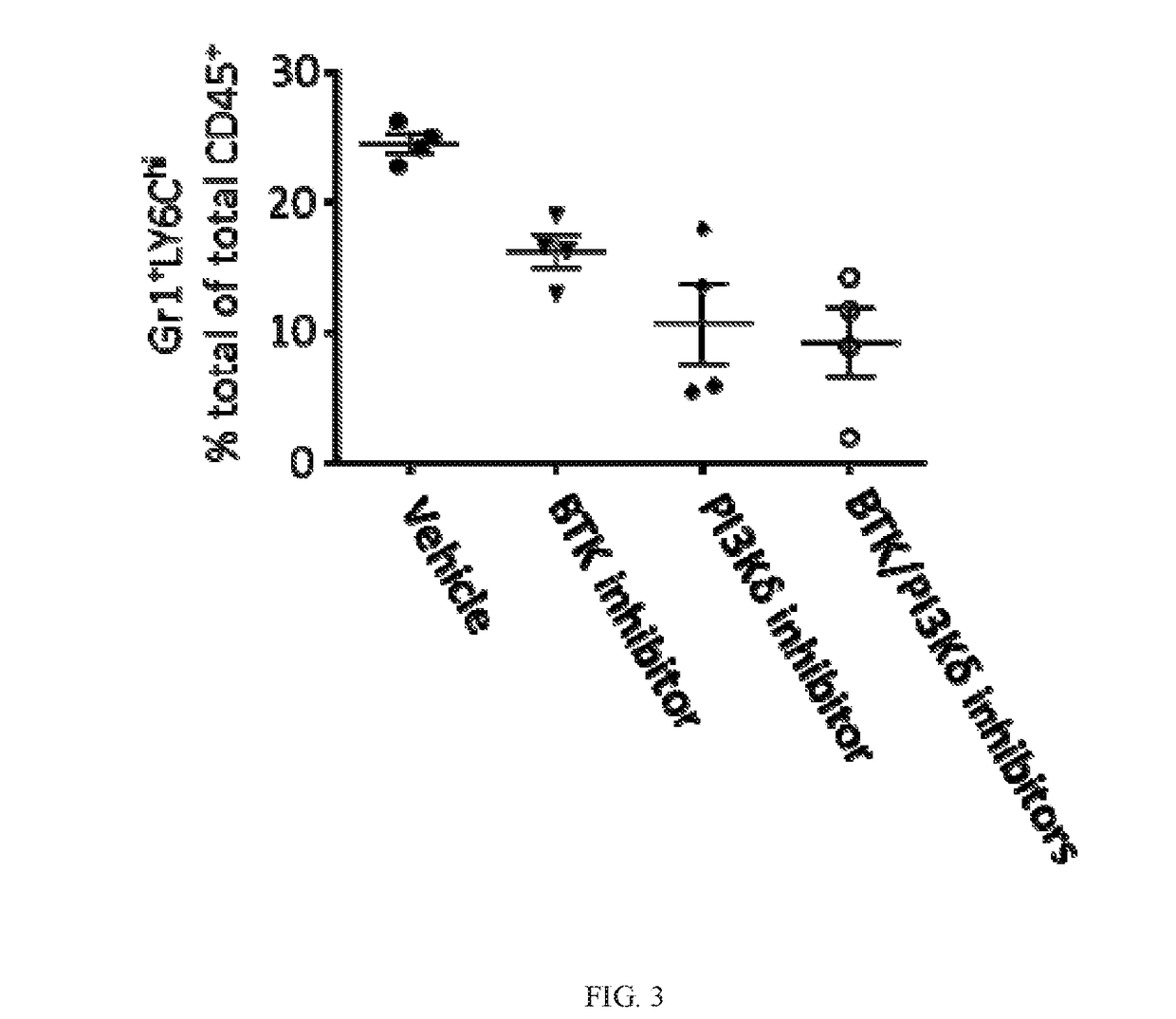
BTK Inhibitors to Treat Solid Tumors Through Modulation of the Tumor Microenvironment
InactiveUS20170231995A1Improve developmentOrganic active ingredientsAntineoplastic agentsRegulatory T cellDendritic cell
In certain embodiments, the invention includes therapeutic methods of using a BTK inhibitor to treat solid tumor cancers by modulation of the tumor microenvironment, including macrophages, monocytes, mast cells, helper T cells, cytotoxic T cells, regulatory T cells, natural killer cells, myeloid-derived suppressor cells, regulatory B cells, neutrophils, dendritic cells, and fibroblasts.
Owner:ACERTA PHARMA BV
Marker for detection of il-17-producing helper t-cell, and method for detection of il-17-producing helper t-cell
Disclosed is a polynucleotide marker or a protein marker for use in the specific detection of an IL-17-producing helper T-cell (a Th17 cell). Also disclosed is a method for detecting a Th17 cell, which is characterized by detecting the occurrence of the polynucleotide marker or the protein marker.
Owner:SYSMEX CORP
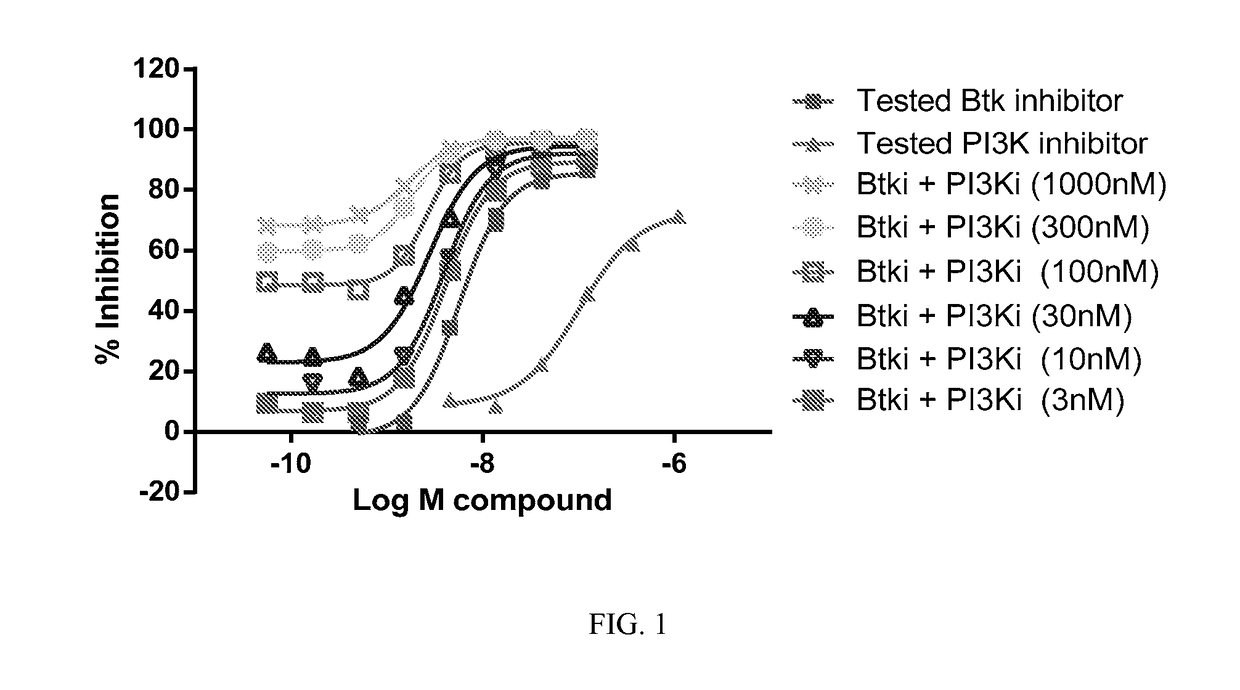
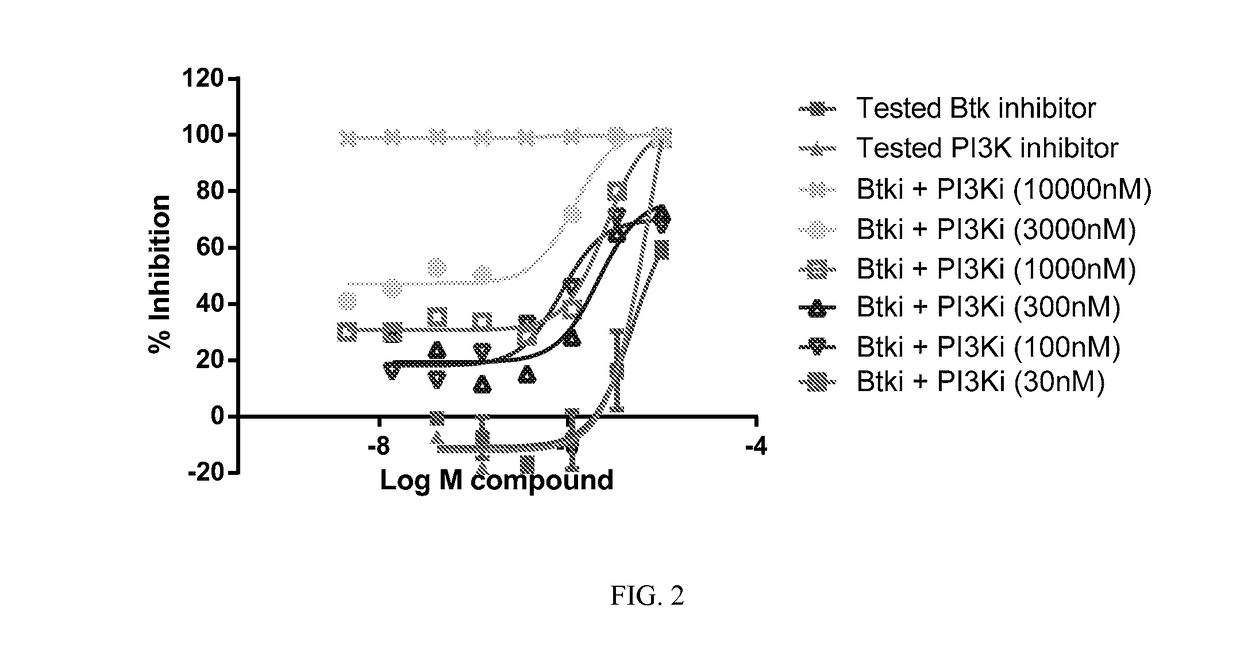
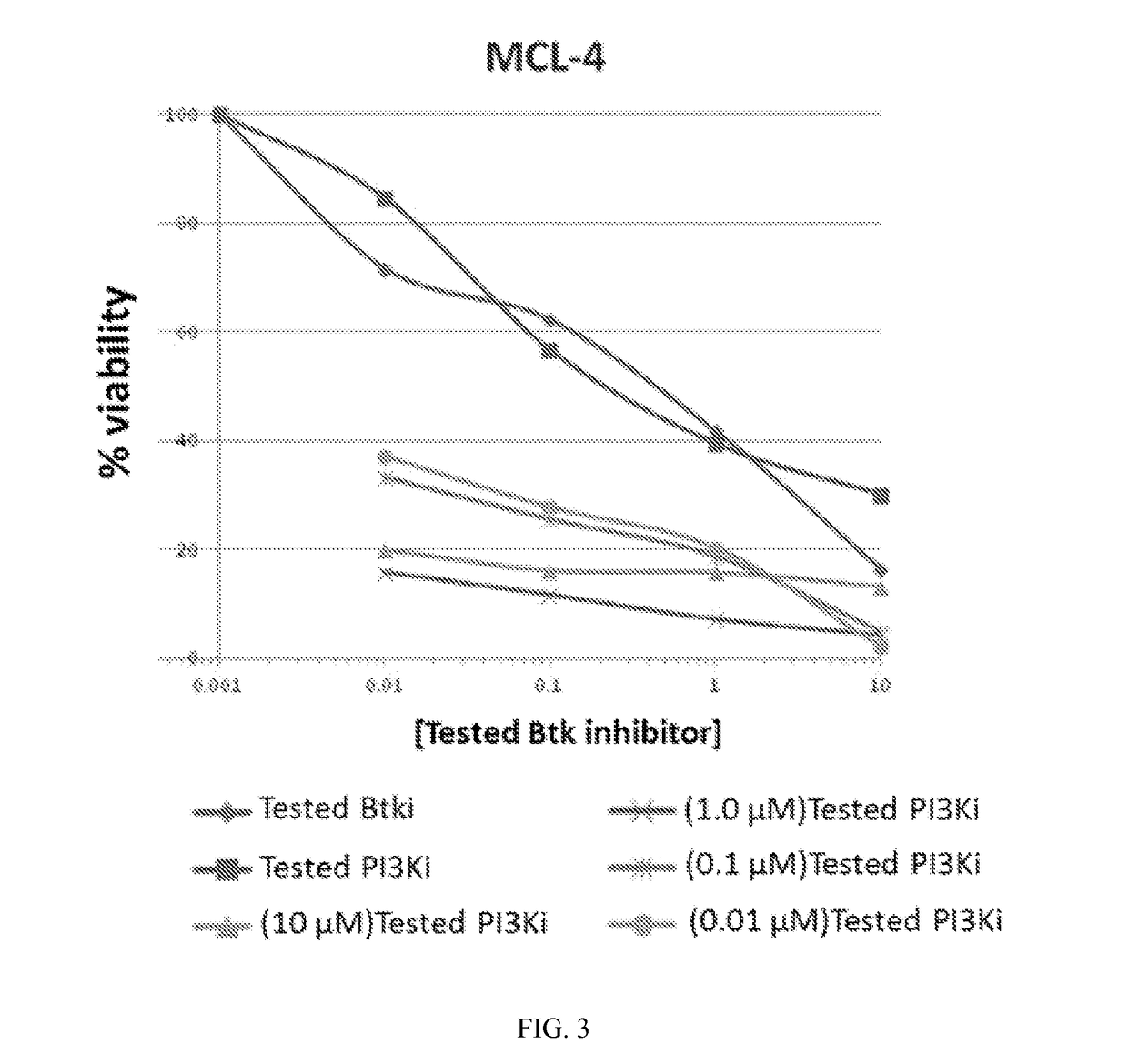
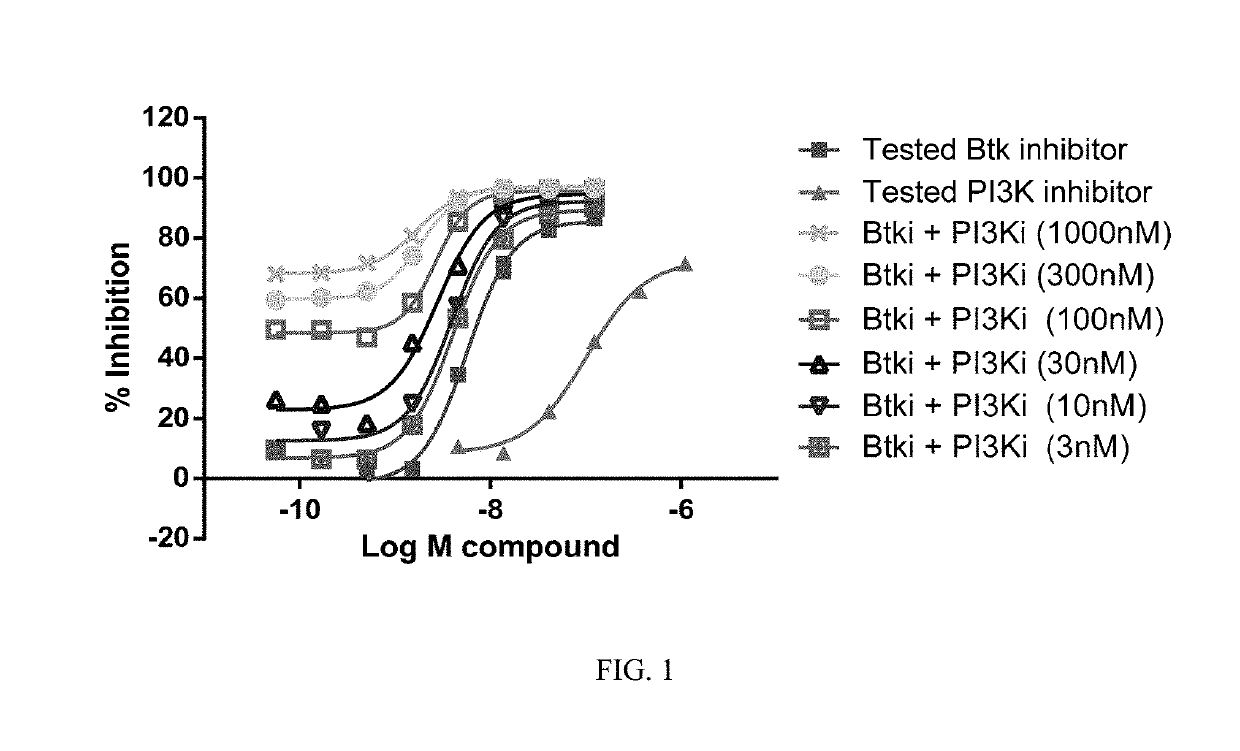
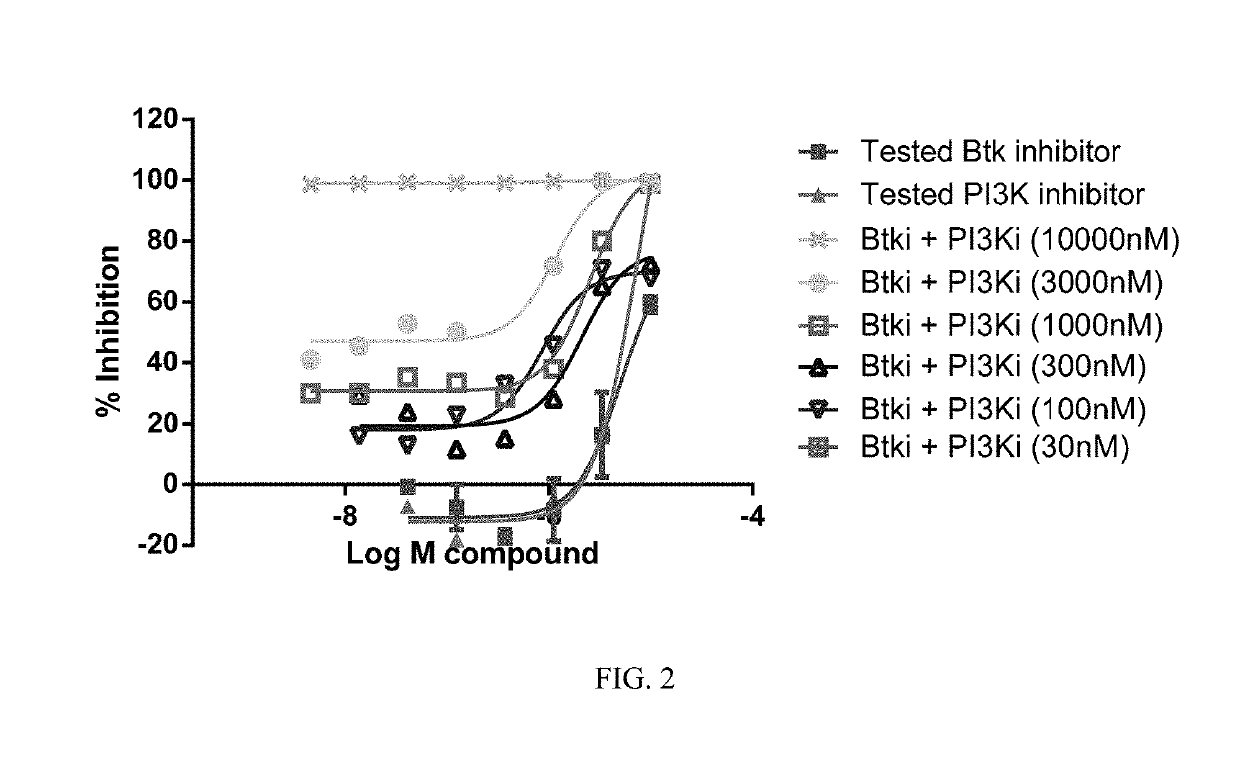
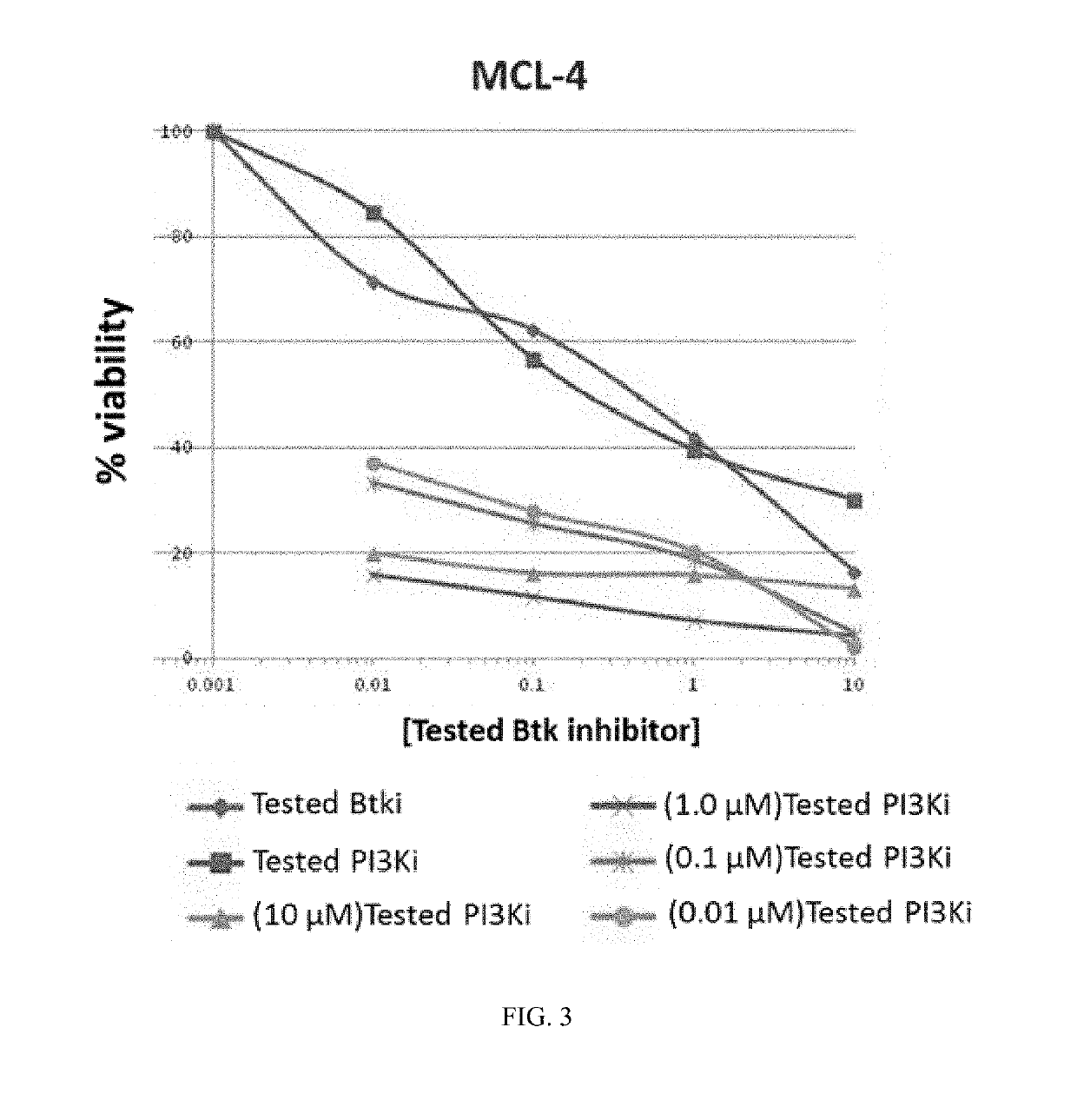
Therapeutic Combination of PI3K Inhibitor and a BTK Inhibitor
ActiveUS20170266191A1Antineoplastic agentsHeterocyclic compound active ingredientsRegulatory T cellDendritic cell
In some embodiments, the invention includes a therapeutic combination of a phosphoinositide 3-kinase (PI3K) inhibitor, including PI3K inhibitors selective for the γ- and δ-isoforms and selective for both γ- and δ-isoforms, and a Bruton's tyrosine kinase (BTK) inhibitor. In some embodiments, the invention includes therapeutic methods of using a BTK inhibitor and a PI3K-δ inhibitor to treat solid tumor cancers by modulation of the tumor microenvironment, including macrophages, monocytes, mast cells, helper T cells, cytotoxic T cells, regulatory T cells, natural killer cells, myeloid-derived suppressor cells, regulatory B cells, neutrophils, dendritic cells, and fibroblasts.
Owner:ACERTA PHARMA BV
Plasmid-based vaccine for treating atherosclerosis
InactiveUS6846808B1Effective transcriptionGenetic material ingredientsAntibody ingredientsB-Cell EpitopesAtheroma
A plasmid-based vaccine is provided herein based on the combination of DNA segments coding for one or more B cell epitopes of CETP and one or more broad range helper T cell epitopes. Administration of the plasmids as a vaccine to a vertebrate subject provides an immune response to the subject's endogenous CETP and modulation of CETP activity, leading to prevention or reversal of various manifestations of heart disease. The vaccines provide an advantageous strategy for the prevention or treatment of atherosclerosis.
Owner:CELLDEX THERAPEUTICS INC
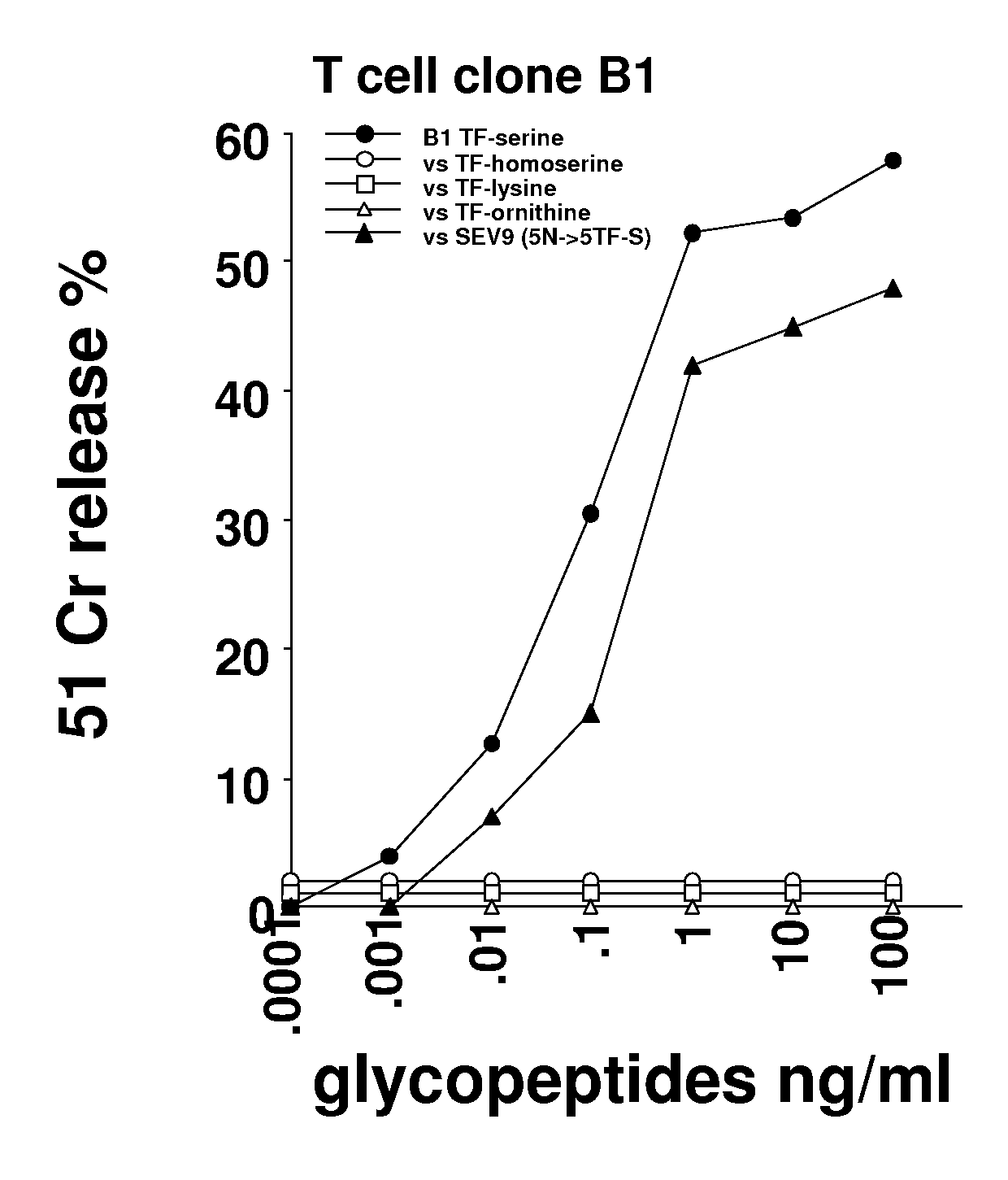
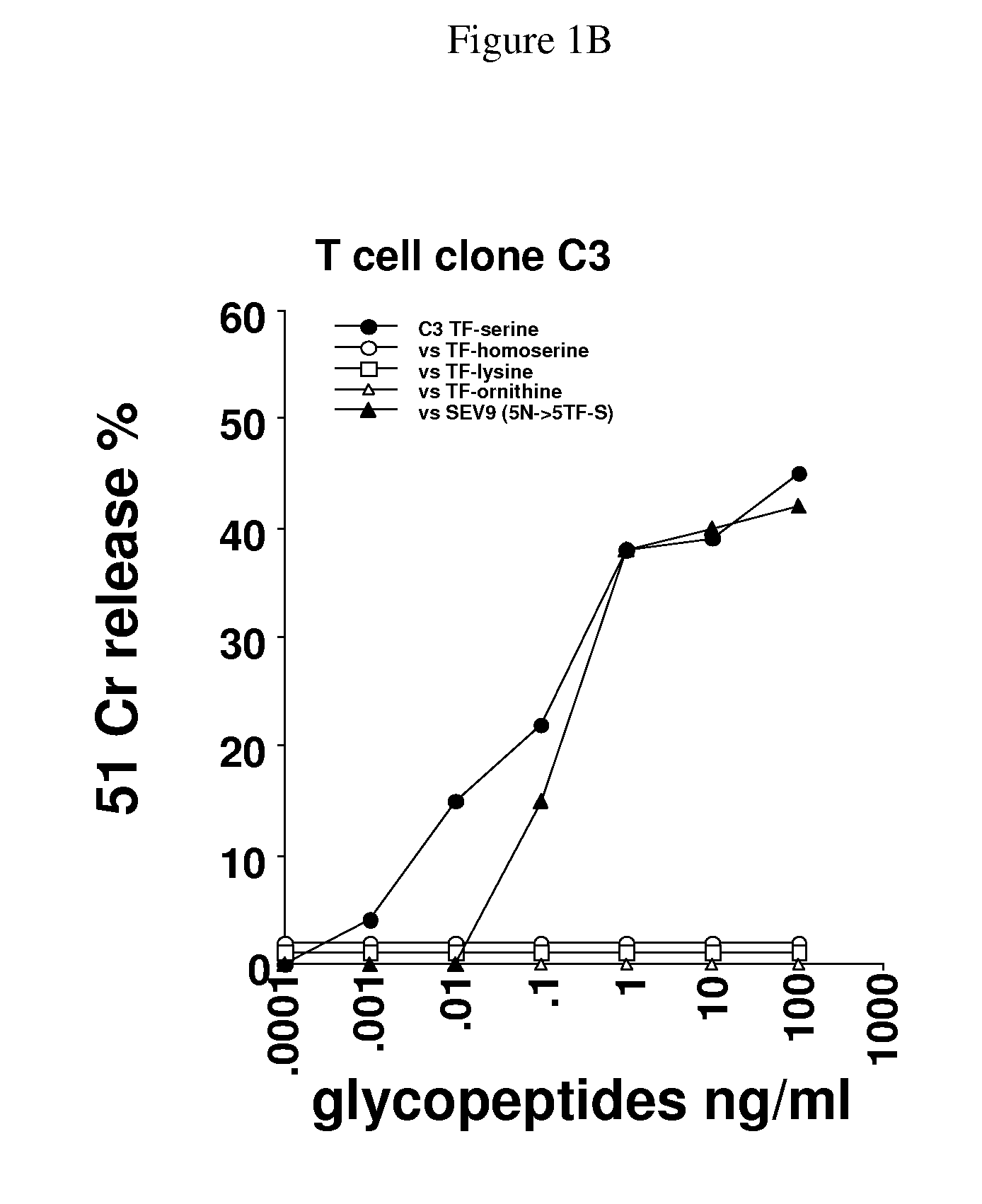
Glycopeptides and methods of making and using them
ActiveUS20110045046A1Guaranteed monitoring effectGenerate efficientlyPowder deliveryImmunoglobulins against animals/humansEpitopeMajor histocompatibility
In one embodiment, the invention provides glycopeptides (or carbohydrate-peptide conjugates) comprising TACAs that direct against (e.g., bind specifically to) cytotoxic T lymphocytes (CTLs) or helper T cells for, e.g., CTL- or T-helper-based immunotherapy of carcinomas, and methods for making and using the glycopeptides of the invention. In one embodiment, the invention provides novel glycopeptides comprising tumor-derived carbohydrate or tumor-derived epitopes that specifically bind to major histocompatibility (MHC) class I molecules on cytotoxic T lymphocytes (CTLs) or MHC molecules on helper T cells, and methods for using same, e.g., as a vaccine, including a pan-cancer vaccine.
Owner:RGT UNIV OF CALIFORNIA
Synthetic peptide vaccines for foot-and-mouth disease
InactiveCN101353373ASsRNA viruses positive-senseViral antigen ingredientsWild speciesImmune profiling
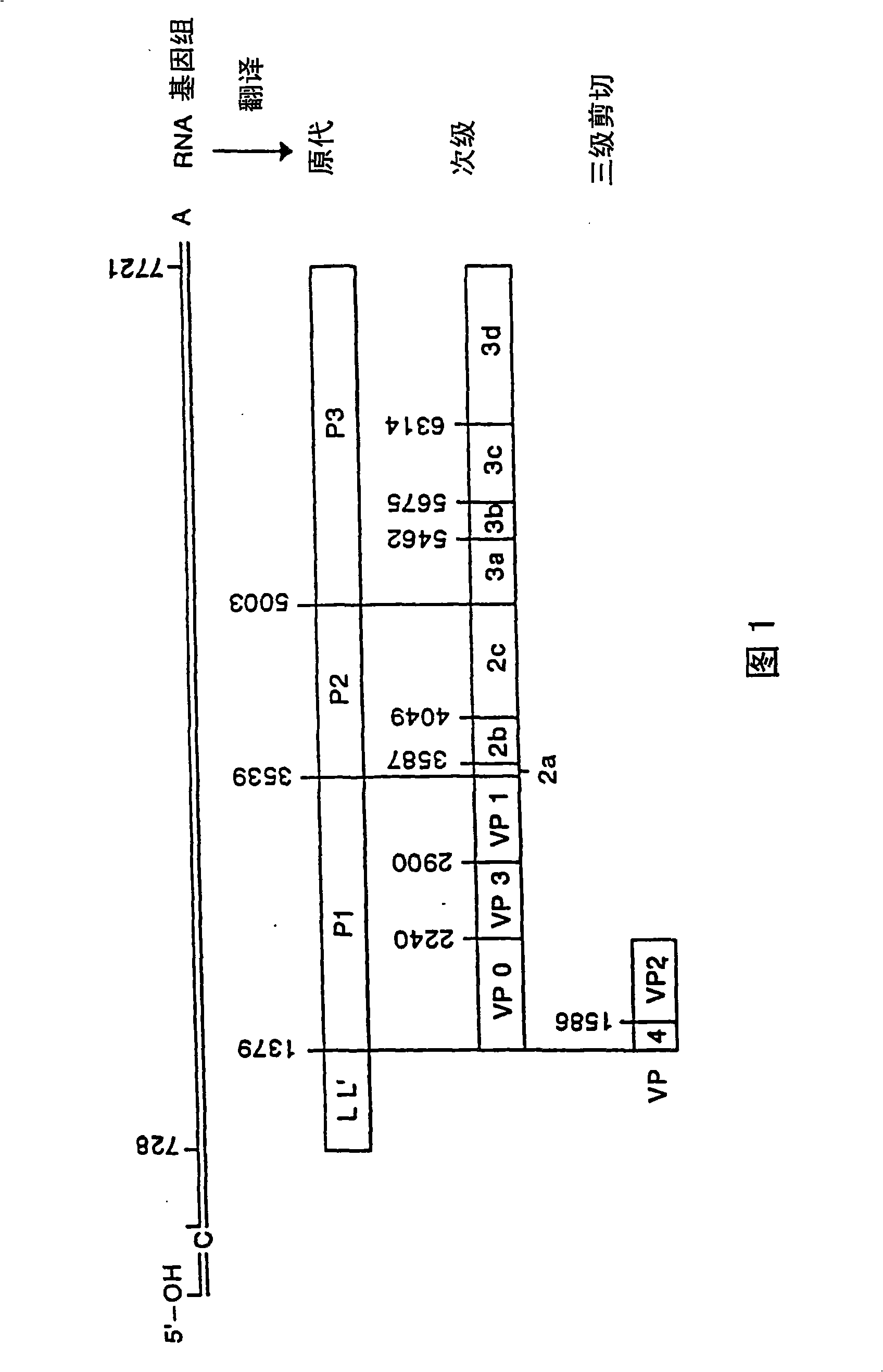
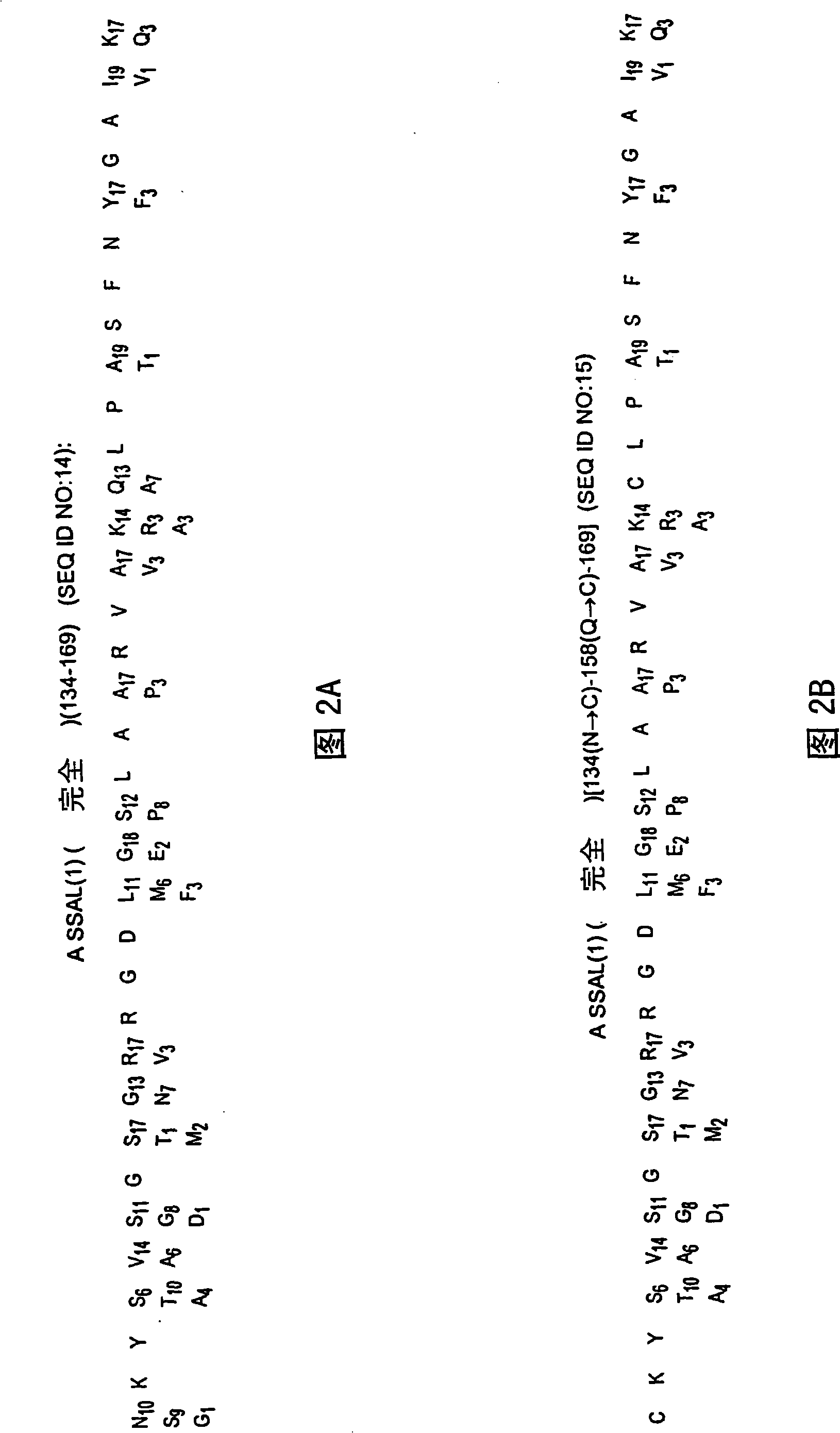
The present invention relates to the use of a peptide composition as an immunogen, with each peptide contained therein comprising a target antigenic site derived from the VP1 capsid protein of Foot-and-Mouth Disease Virus (FMDV). The antigenic site is covalently linked to a helper T cell epitope and, preferably, to other immunostimulatory sequences, preferably by conventional peptide bond(s) through direct synthesis, for the prevention of FMDV infection and eradication of Food-and-Mouth Disease (FMD). More particularly, the present invention relates to the use of such peptide composition as an immunogen to elicit the production in animals including swine, cattle, sheep, goets and susceptible wild species, of high titer polyclonal antibodies that can effectively neutralize, in vitro, multiple strains or serotypes of FMDV, and to the use of such composition as a vaccine to prevent, and / or reduce the incidence of, FMDV infection regardless of serotype, and thus affect the eradication of FMD. The present invention also relates to the peptides used in the compositions, and to immunoassays and / or diagnostic kits containing one or more of these peptides, and methods of diagnosing FMDV inmammals using such materials.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
Compositions and methods for the prevention and treatment of cancer
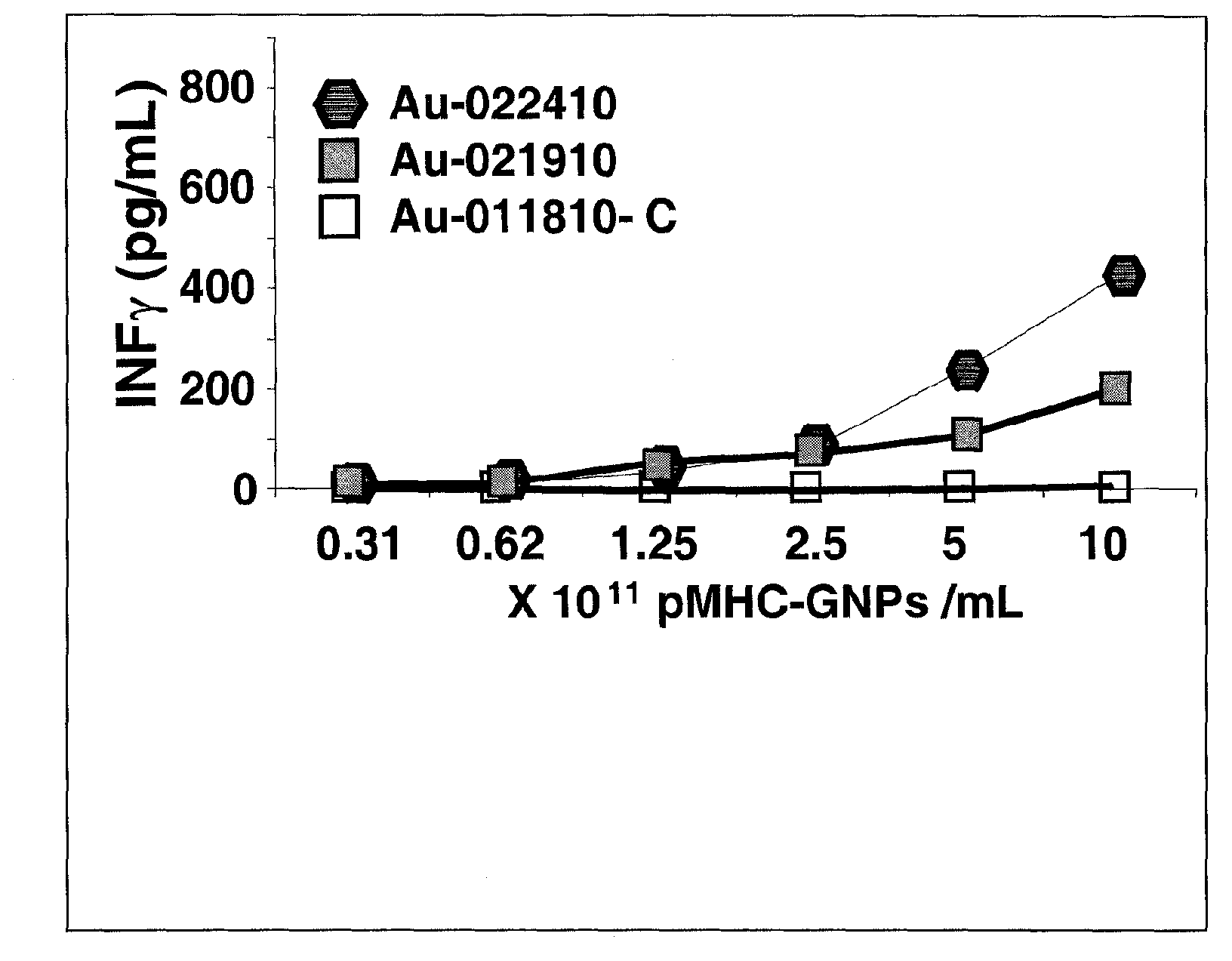
Conventional cancer immunotherapy falls short at efficiently expanding T cells that specifically target cancerous cells in numbers sufficient to significantly reduce the tumor size or cancerous cell number in vivo. To overcome this limitation, provided herein are nanoparticles coated with MHC class I and / or class II molecules presenting tumor-specific antigens and co-stimulatory molecules and their use to expand antigen-specific anti-tumorigenic T cellsto levels not achieved in current immunotherapeutic techniques. These antigen-specific anti-tumorigenic T cells include cytotoxic T cells, effector T cells, memory T cells, and helper T cells that are necessary to initiate and maintain a substantial immune response against metastatic or non- metastatic cancerous, pre-cancerous, or neoplastic cells in vivo.; The present invention describes a systemic approach to targeting cancerous or pre-cancerous cells that are circulating cells, as in lymphomas, migratory metastatic cells, and solid tumors.
Owner:UTI LLP
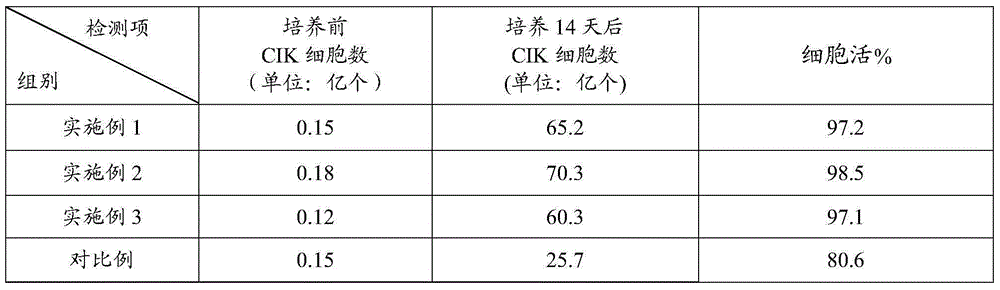
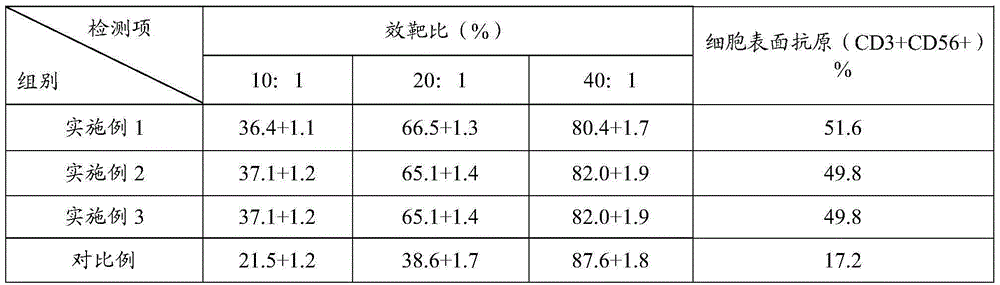
CIK (cytokine-induced killer) cell culture fluid, CIK cell culture method and application of lentinan in CIK cell culture
The invention relates to the biotechnology field, in particular to CIK cell culture fluid, a CIK cell culture method and application of lentinan in CIK cell culture. The CIK cell culture fluid comprises interferon-gamma, CD3 stimulated monoclonal antibodies, interleukin-2, the lentinan and serum-free basal culture media. When the CIK cell culture fluid with the lentinan is used for CIK cell culture, PBMCs (peripheral blood mononuclear cells) can generate lymphocyte activation factors and release helper T cell factors to promote CIK cell multiplication, so that obtained CIK cells have large amplification multiple, high killing activity and high cell surface antigen content. Experiment results show that after 14-day CIK cell culture by the CIK cell culture fluid, the amplification multiple of the CIK cells is larger than 300, in-vitro killing rate (effector-target ratio being 40:1) of the CIK cells is larger than (80+ / -2)%, and the number of CIK cell surface antigens (CD3+ and CD56+) is larger than 48%.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Marker for detection of IL-17-producing helper T-cell, and method for detection of IL-17-producing helper T-cell
InactiveCN101960021AMicrobiological testing/measurementDisease diagnosisSpecific detectionPolynucleotide
Disclosed is a polynucleotide marker or a protein marker for use in the specific detection of an IL-17-producing helper T-cell (a Th17 cell). Also disclosed is a method for detecting a Th17 cell, which is characterized by detecting the occurrence of the polynucleotide marker or the protein marker.
Owner:SYSMEX CORP
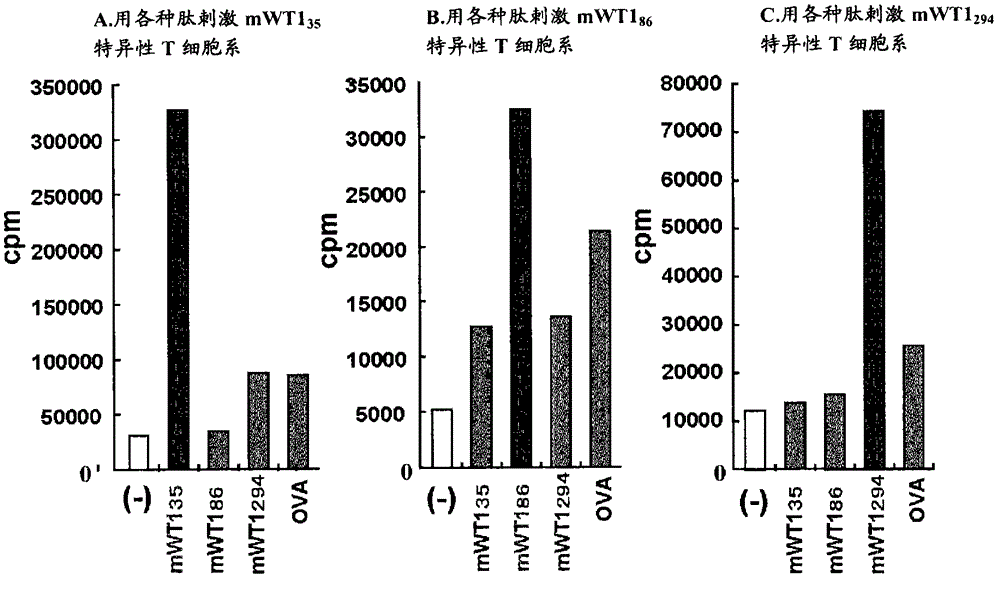
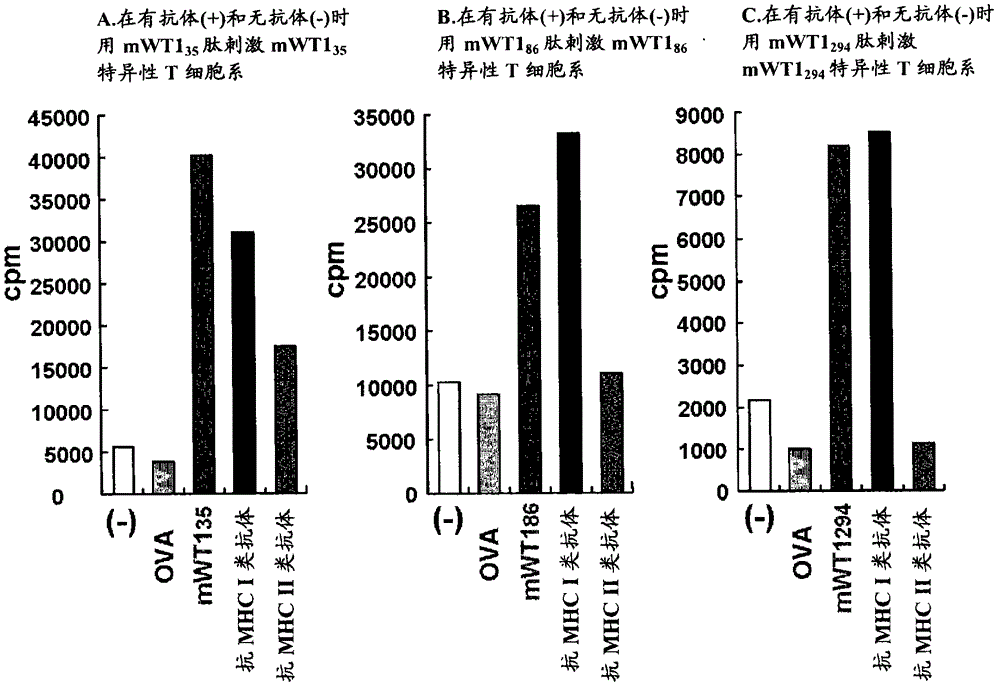
Modification of helper t cell-inducing polypeptide
InactiveUS20140341939A1Efficient inductionInduced more efficientlyNervous disorderTumor rejection antigen precursorsTumor antigenCancer research
The present invention provides a tumor antigen-specific Th-inducing polypeptide capable of efficient antigen presentation, and an antitumor agent using same.
Owner:NAT UNIV CORP KOCHI UNIV
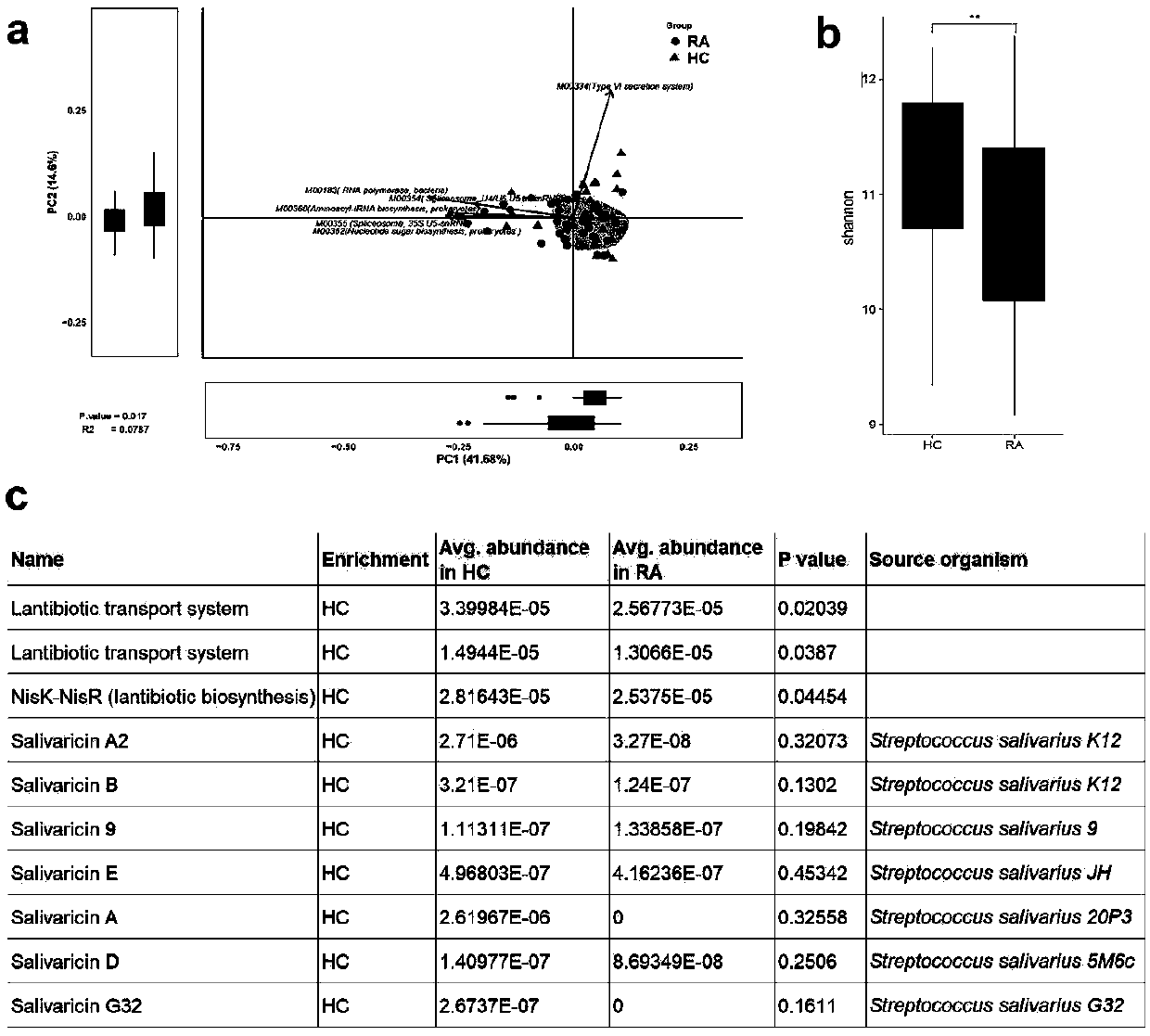
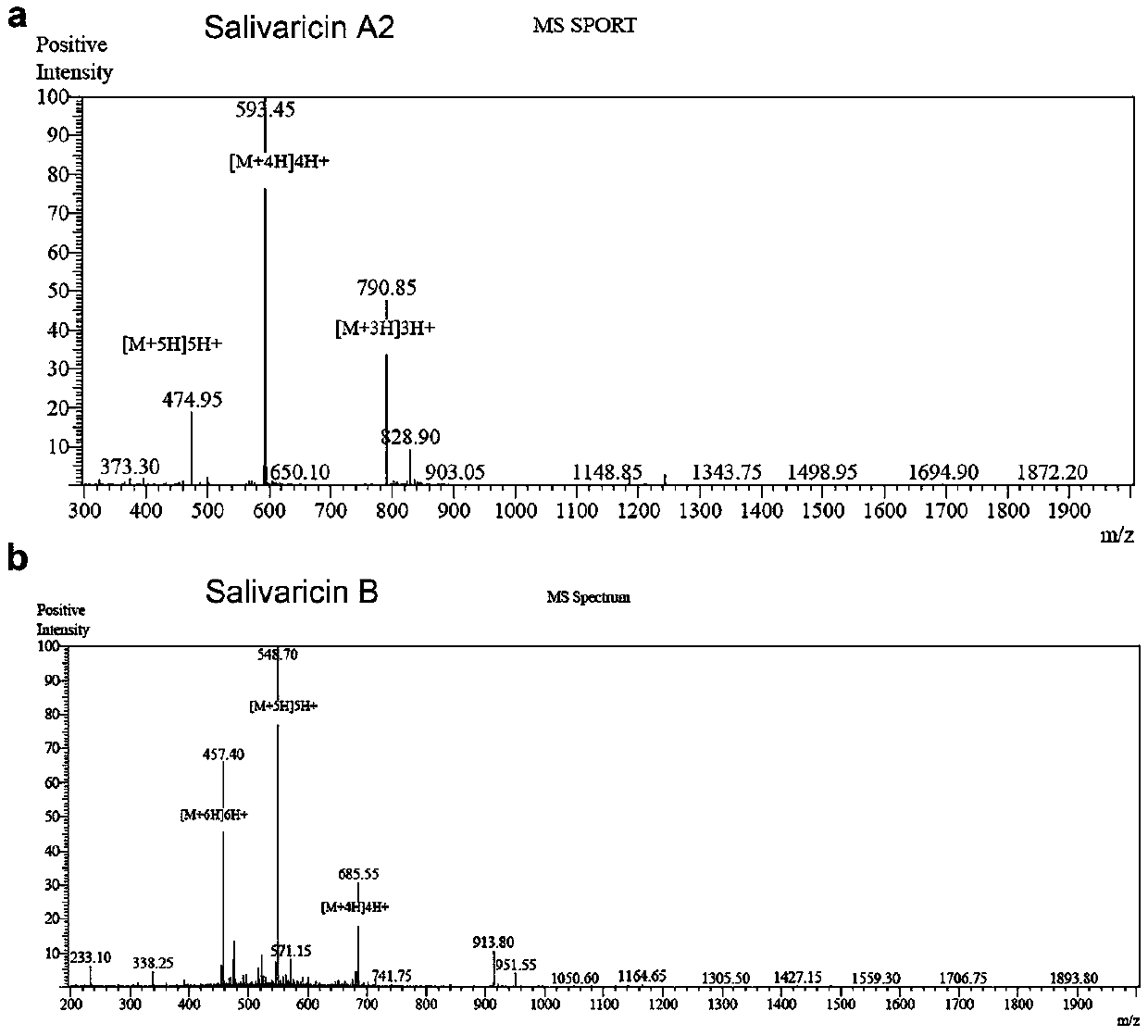
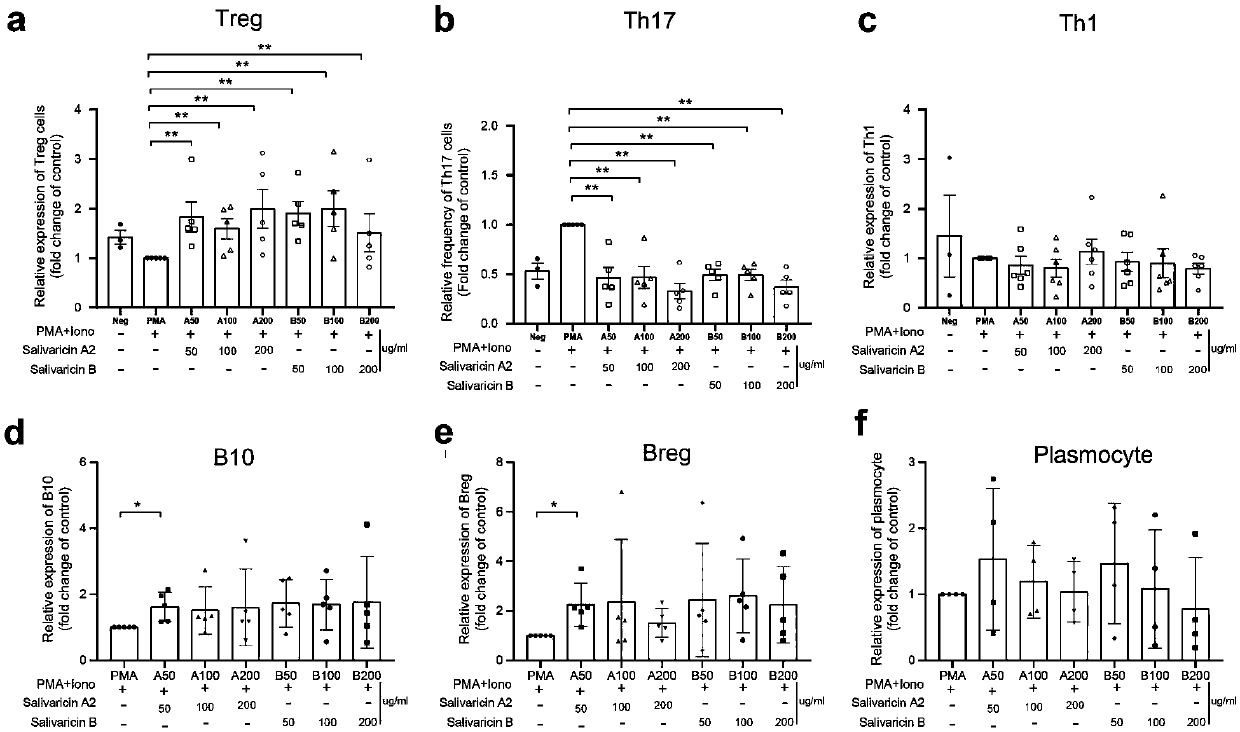
Application of salivaricin in preparation of medicines for preventing and/or treating autoimmune diseases
ActiveCN109675017APromote secretionRegulate immune balancePeptide/protein ingredientsImmunological disordersImmunologic disordersAutoimmune condition
The invention provides application of salivaricin in preparation of medicines for preventing and / or treating autoimmune diseases, belonging to the technical field of treatment of the autoimmune diseases. The salivaricin is an antibacterial peptide synthesized and secreted by streptococcus salivarius and plays an important role in maintenance of the local micro-ecological balance of the mucosa. Thesalivaricin can significantly promote the secretion of an anti-inflammatory cytokine IL-10 and inhibit the expression of the pro-inflammatory cytokines IL-17, IL-21 and TNF-alpha. Furthermore, the salivaricin can up-regulate the proportion of anti-inflammatory cell regulatory T (Treg) cells, and down-regulates the proportion of pro-inflammatory cell follicular helper T cells and helper T-17 cellsin immune cell subsets. The salivaricin can relieve an immune imbalance state by inhibiting an immune response, reestablishes the immune balance, and provides a new treatment method for the autoimmune diseases such as rheumatoid arthritis.
Owner:PEOPLES HOSPITAL PEKING UNIV
Compositions and methods for the treatment of cancer
InactiveUS20070237763A1Improve immunityImprove responsePeptide/protein ingredientsAntibody ingredientsAbnormal tissue growthLymphatic Spread
The present invention includes compositions and methods for the treatment of cancers by controlling the type of immune response mounted against the tumor, and more particularly, the treatment of tumors with cytokine antagonists to change the type of helper T cell response and inhibition of angiogenesis, prevention of formation of metastasis and prevention of formation of tumor stroma.
Owner:BAYLOR RES INST
Recombinant beta-amyloid peptide B cell epitope polypeptide chimeric antigen and preparation method and application thereof
ActiveCN102180971AStimulate immune responseImprove applicabilityNervous disorderPeptide/protein ingredientsProkaryotic expressionB-Cell Epitopes
The invention discloses a recombinant beta-amyloid peptide B cell epitope polypeptide chimeric antigen and a preparation method and application thereof. The recombinant polypeptide chimeric antigen is a polypeptide antigen Abeta1-15 comprising 5-6 serial beta-amyloid peptide B cell epitopes or a dipolymer constituted by the polypeptide antigen. The recombinant polypeptide chimeric antigen further comprises auxiliary Pan-DR Helper T Cell Epitopes (PADRE) or further comprises toxin segment carrier molecules. In the invention, genes of targeted beta-amyloid peptide B cell epitope polypeptide chimeric antigen are synthesized manually, expression is performed by using a prokaryotic expression system, and a purified recombinant polypeptide chimeric antigen protein can be taken as a subunit vaccine for immunoprophylaxis and treatment of Alzheimer's disease.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Vaccine preparation for cancer treatment
InactiveCN103957930AHigh cancer treatment effectTumor rejection antigen precursorsAntibody mimetics/scaffoldsVaccine antigenCD8
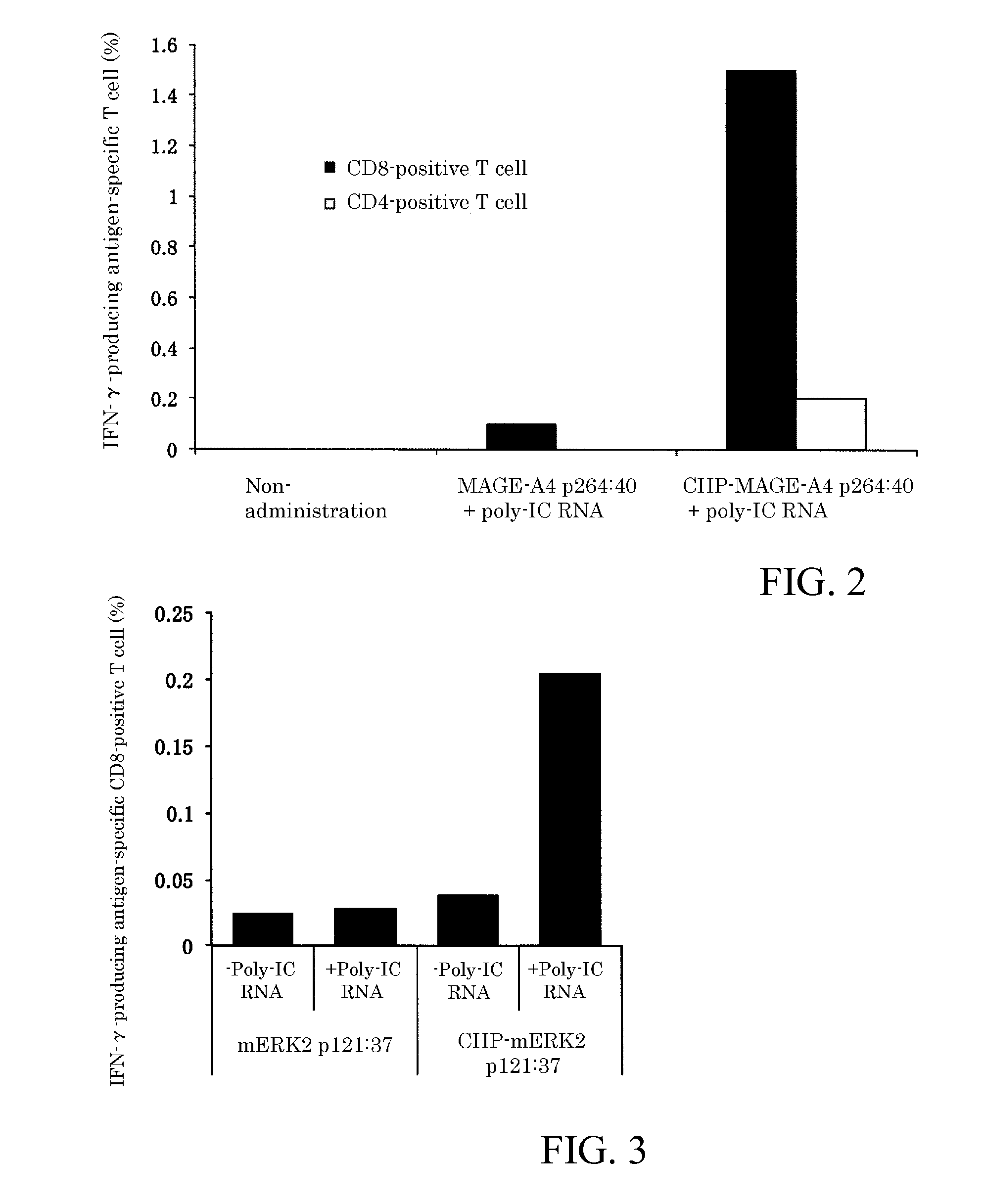
An object of the invention is to provide a method of clearly deriving killer T cells and helper T cells in a cancer treatment vaccine in which the vaccine antibody is a synthetic long peptide which is derived from a tumor-specific antigen protein and / or pathogen-derived protein and which simultaneously contains a CD8-positive, cytotoxic T-cell recognizing epitope and a CD4-positive, helper T-cell recognizing epitope peptide. By simultaneously dosing an immunopotentiating agent with a conjugate of a hydrophobized polysaccharide (the antigen delivery system) to the synthetic long peptide, it is possible to achieve clear derivation of antigen-specific killer T-cells and helper T-cells from the cancer treatment vaccine in which the synthetic long peptide is the antigen.
Owner:MIE UNIVERSITY
Vaccine preparation for cancer treatment
InactiveUS20140322344A1High encapsulation efficiencyTumor rejection antigen precursorsAntibody mimetics/scaffoldsCD8Tumor-specific antigen
A vaccine preparation for treating cancer includes a complex of a hydrophobized polysaccharide and at least one synthetic long peptide derived from a tumor-specific antigenic protein and / or a pathogen-derived antigenic protein. The at least one synthetic long peptide contains at least one CD8+ cytotoxic T-cell recognition epitope and at least one CD4+ helper T-cell recognition epitope. The complex is simultaneously administered to the patient with at least one immunopotentiating agent.
Owner:MIE UNIVERSITY +1
Human oocyte zona pellucida protein multi-epitope chimeric peptide antigen and preparation method thereof
InactiveCN101696239AMicroorganism based processesAntibody medical ingredientsEscherichia coliPeptide antigen
The invention belongs to the technical field of immunology and bioengineering and particularly relates to human oocyte zona pellucida protein multi-epitope chimeric peptide (ZPCP) and a preparation method and an application thereof. A ZPCP molecule designed by the invention is combined with three proteins of huZP (huZP1, huZP2 and huZP3), nine linear B cell epitopes (BCE) and four wide-adaptability helper T cell epitopes (TCE) and comprises a section of pig ZP4 (pZP4), N terminal 25 peptide and a beta turn forming peptide (GPSL). The ZPCP molecule has the advantages that an amino acid residue sequence of the ZPCP molecule or a DNA coding sequence thereof can be highly expressed in a escherichia coli; a targeting expression protein with the electrophoresis uniformity higher than 95 percent can be conveniently obtained; the ZPCP molecule can generate a high level anti ZPCP antibody in an induction way in a rabbit body as an immunogen, including anti each embedded BCE antibody which can be detected in serum.
Owner:SHANGHAI INST OF PLANNED PARENTHOOD RES +2
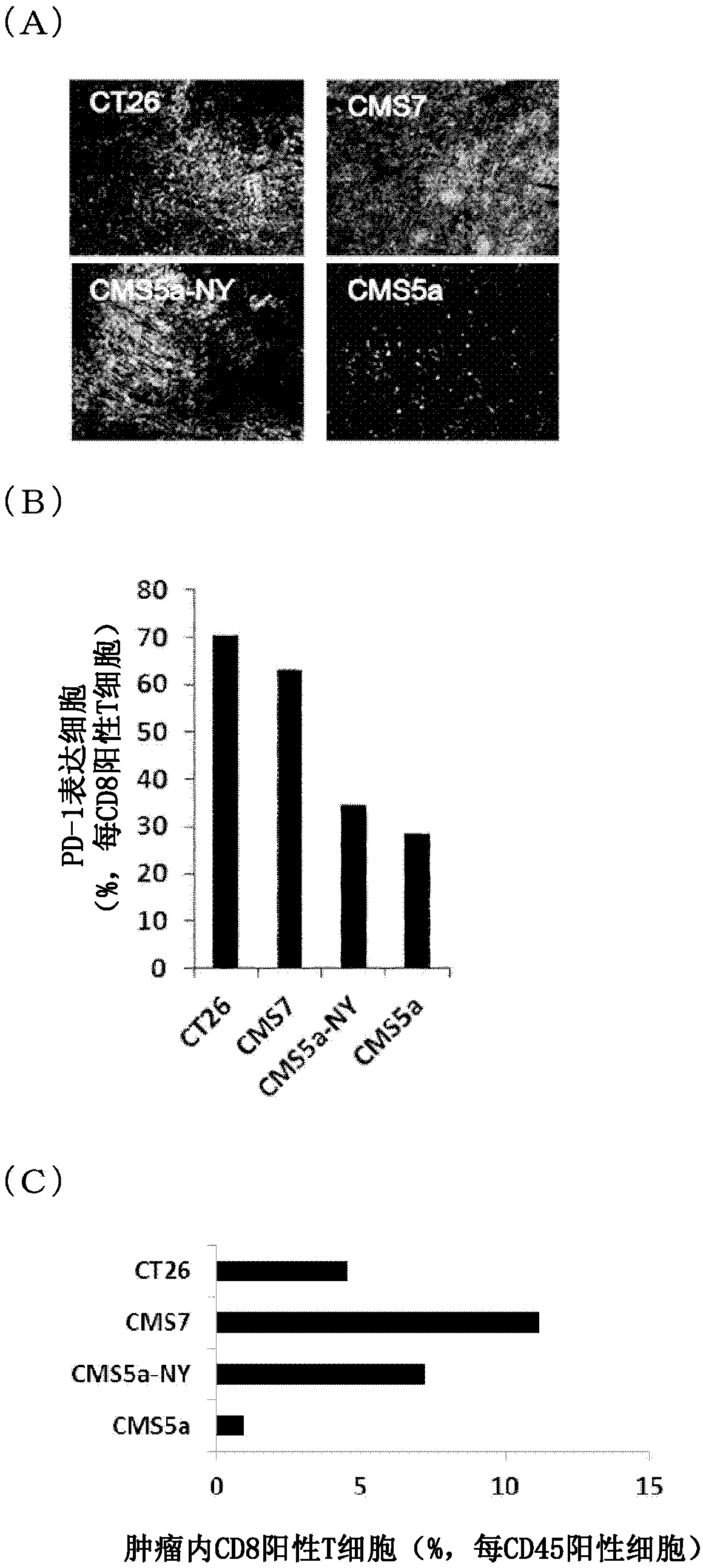
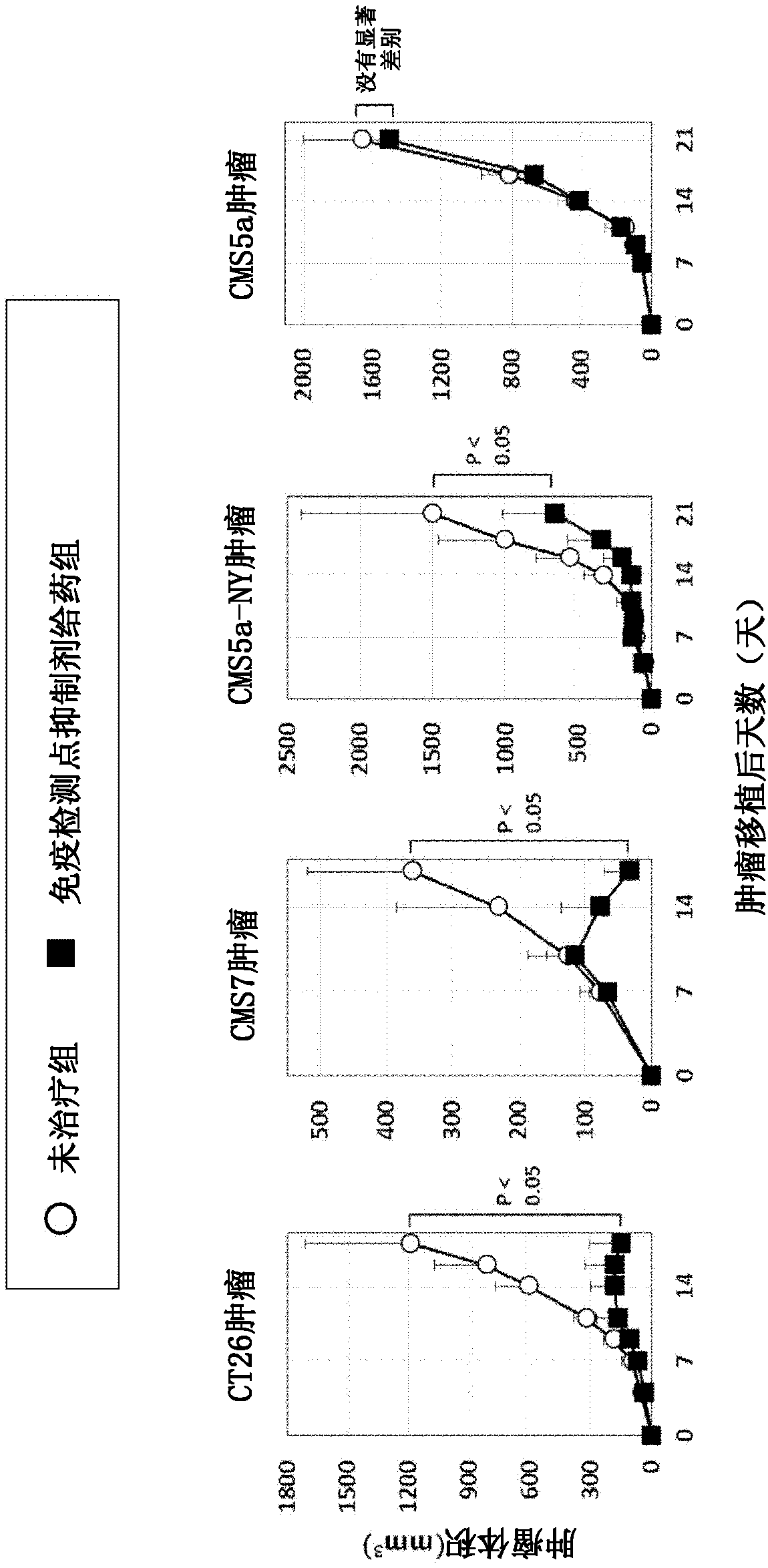
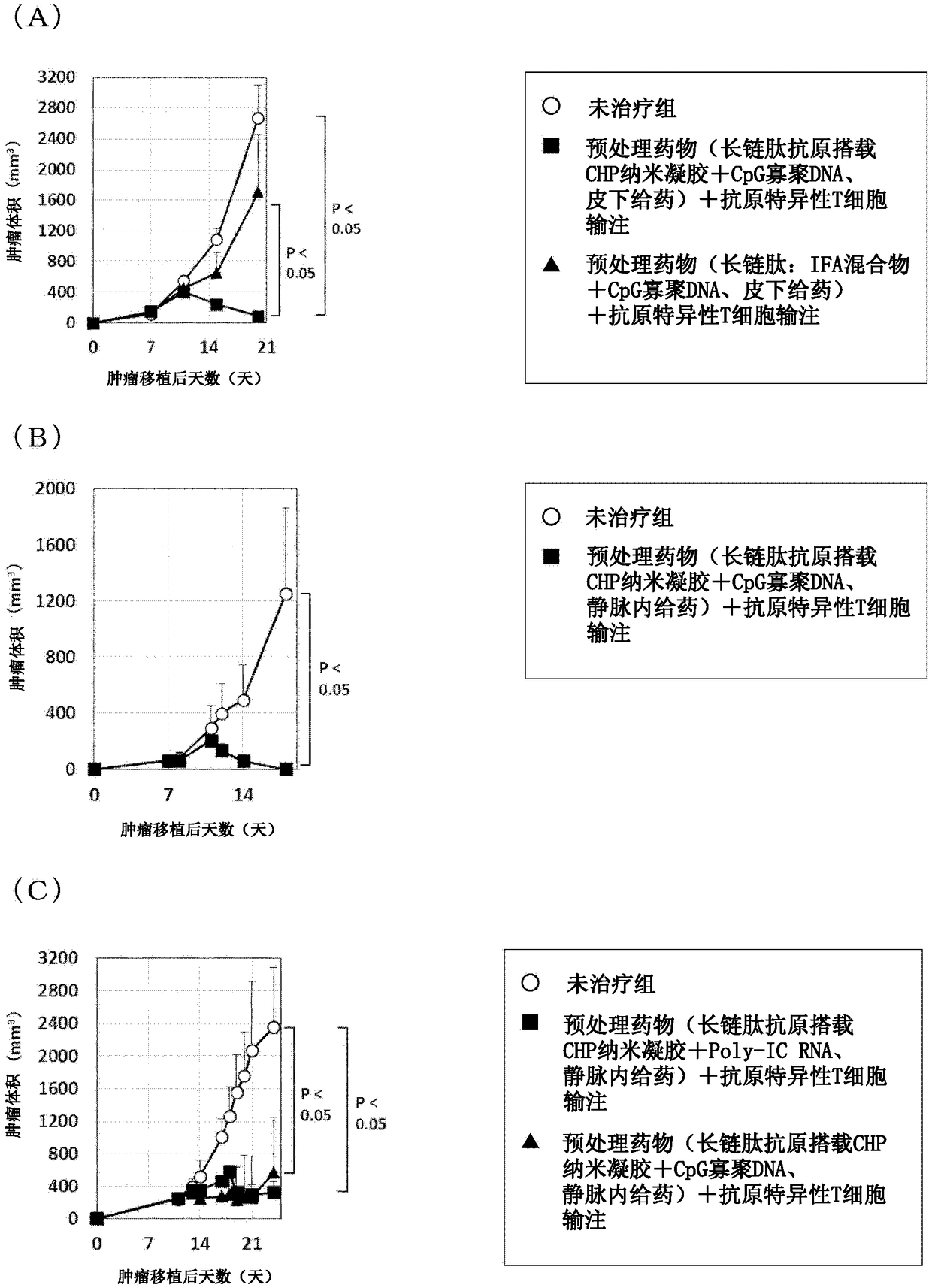
Pretreatment drug for t cell infusion therapy for immune-checkpoint inhibitor-resistant tumor
To provide a technique relating to a therapy for an immune-checkpoint inhibitor-resistant tumor. The problem can be solved by a pharmaceutical composition which is intended to be administered prior tothe administration of T cells specific to an antigen in a T cell infusion therapy for an immune-checkpoint inhibitor-resistant tumor, said pharmaceutical composition containing an antigen-encapsulated nano gel, wherein the antigen-encapsulated nano gel comprises a long-chain peptide antigen or a protein antigen each of which is encapsulated in a hydrophobized polysaccharide nano gel, and the long-chain peptide antigen or the protein antigen contains a CD8-positive cytotoxic T cell-recognizing epitope and a CD4-positive helper T cell-recognizing epitope both originated from the aforementionedantigen.
Owner:MIE UNIVERSITY +1
Therapeutic combination of PI3K inhibitor and a BTK inhibitor
ActiveUS10328080B2Antineoplastic agentsHeterocyclic compound active ingredientsRegulatory T cellDendritic cell
Owner:ACERTA PHARMA BV
Application of autovaccine preparation containing TGF beta 1
InactiveCN101780272AAvoid it happening againEliminate side effectsGenetic material ingredientsAntiinfectivesAdjuvantRegulatory T cell
The invention discloses application of autovaccine preparation containing TGF beta 1. Adjuvant is added in the autovaccine preparation to prepare medicinal preparation for treating continuous pathogen infection of livestock. The vaccine preparation is fusion protein obtained by the way that part or total of the TGF beta 1 or part or total of mutant or total of similar gene sequences is recombined with at least one helper T cell capable of improving immunogenicity or the gene sequence of carrier protein to construct eukaryon or pronucleus expression plasmid and transform host cell for expression, or is directly prepared by eukaryon expression plasmid obtained by construction. The vaccine preparation can be directly used for preparing medicinal preparation for treating the continuous pathogen infection of livestock, induce domestic animals to generate antibody capable of neutralizing self TGF beta 1, and remove immunodepression function caused by too high level of TGF beta 1 in continuous process of infection; simultaneously, the vaccine preparation can depress the generation of inducible regulatory T cell at the persistent infection part of an organism, thus being beneficial to breaking the immune tolerance state of in-vivo pathogen by the organism and promoting the removing function of the organism on the continuously infectious pathogen.
Owner:HUNAN AGRICULTURAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com