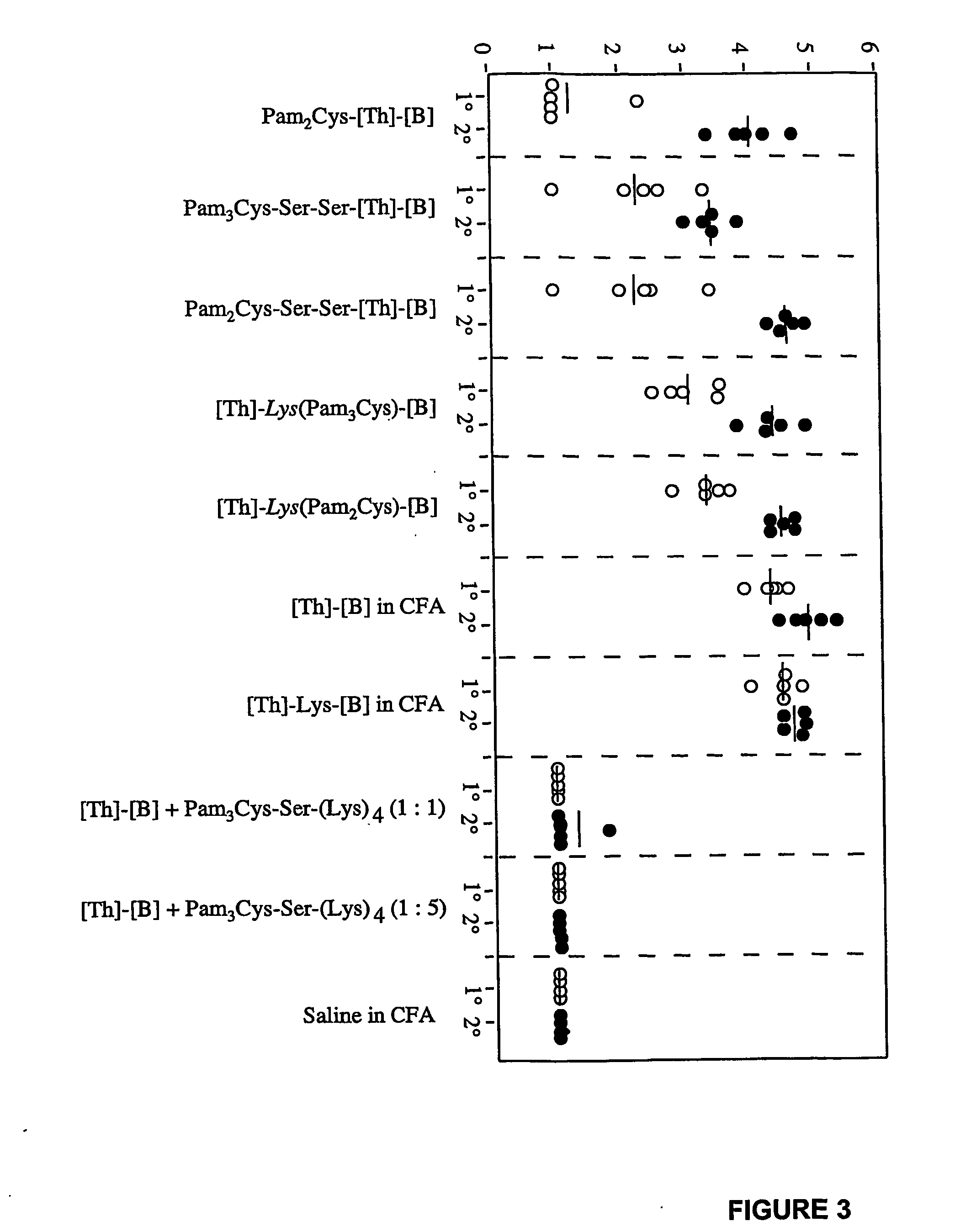
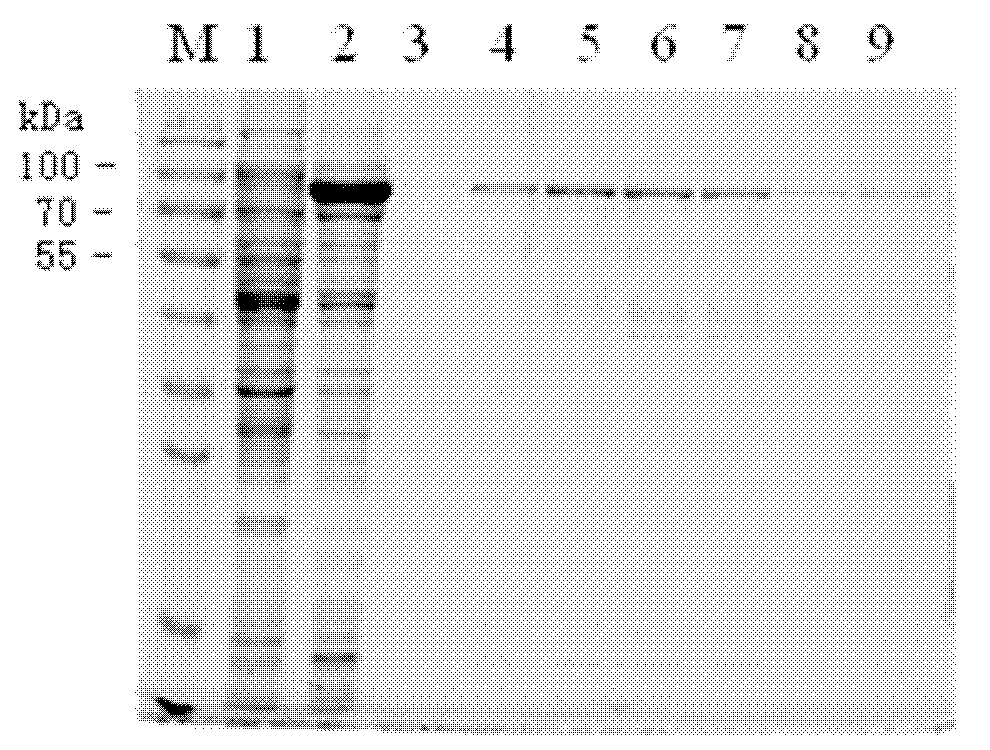
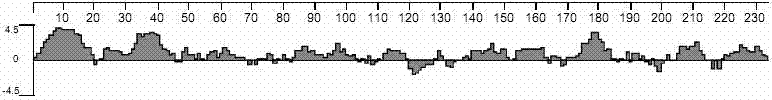
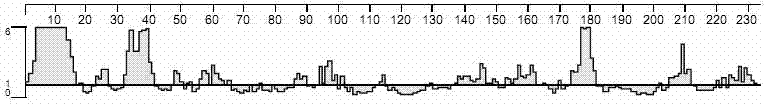
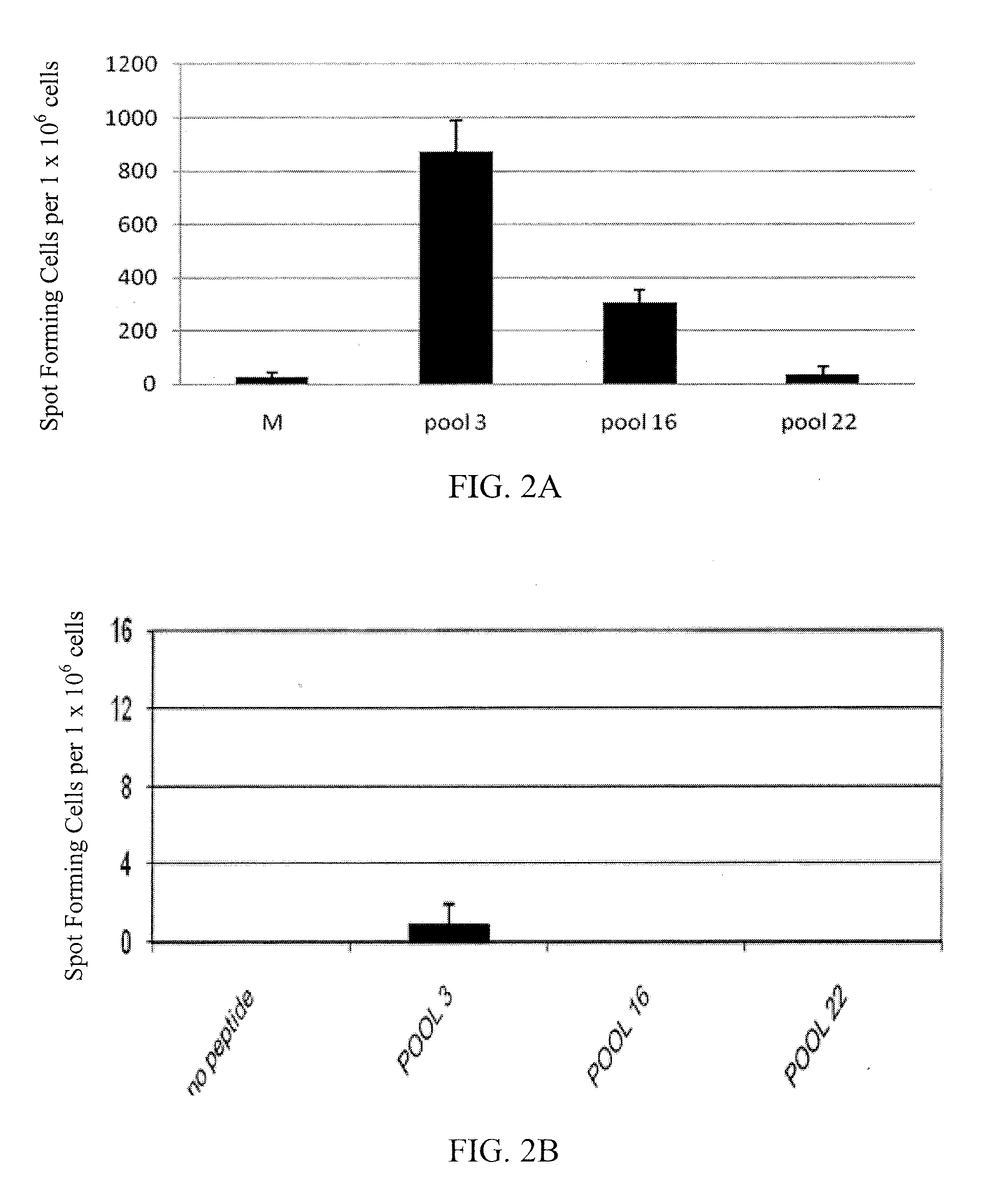
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
305 results about "B-Cell Epitopes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
B cell epitopes consist of groups of amino acids that lie close together on the protein surface and that determine antigenicity1. There are two main classifications of B cell epitopes: Linear, or continuous, epitopes are defined by the primary amino acid sequence of a particular region of a protein.
Novel multi-oligosaccharide glycoconjugate bacterial meningitis vaccines
InactiveUS20010048929A1Inhibition effectWeight increaseAntibacterial agentsPeptide/protein ingredientsSerotypeTumor antigen
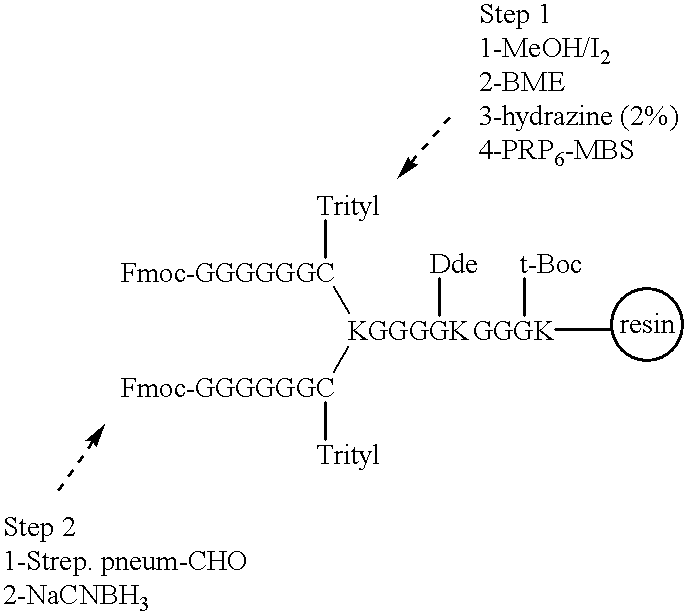
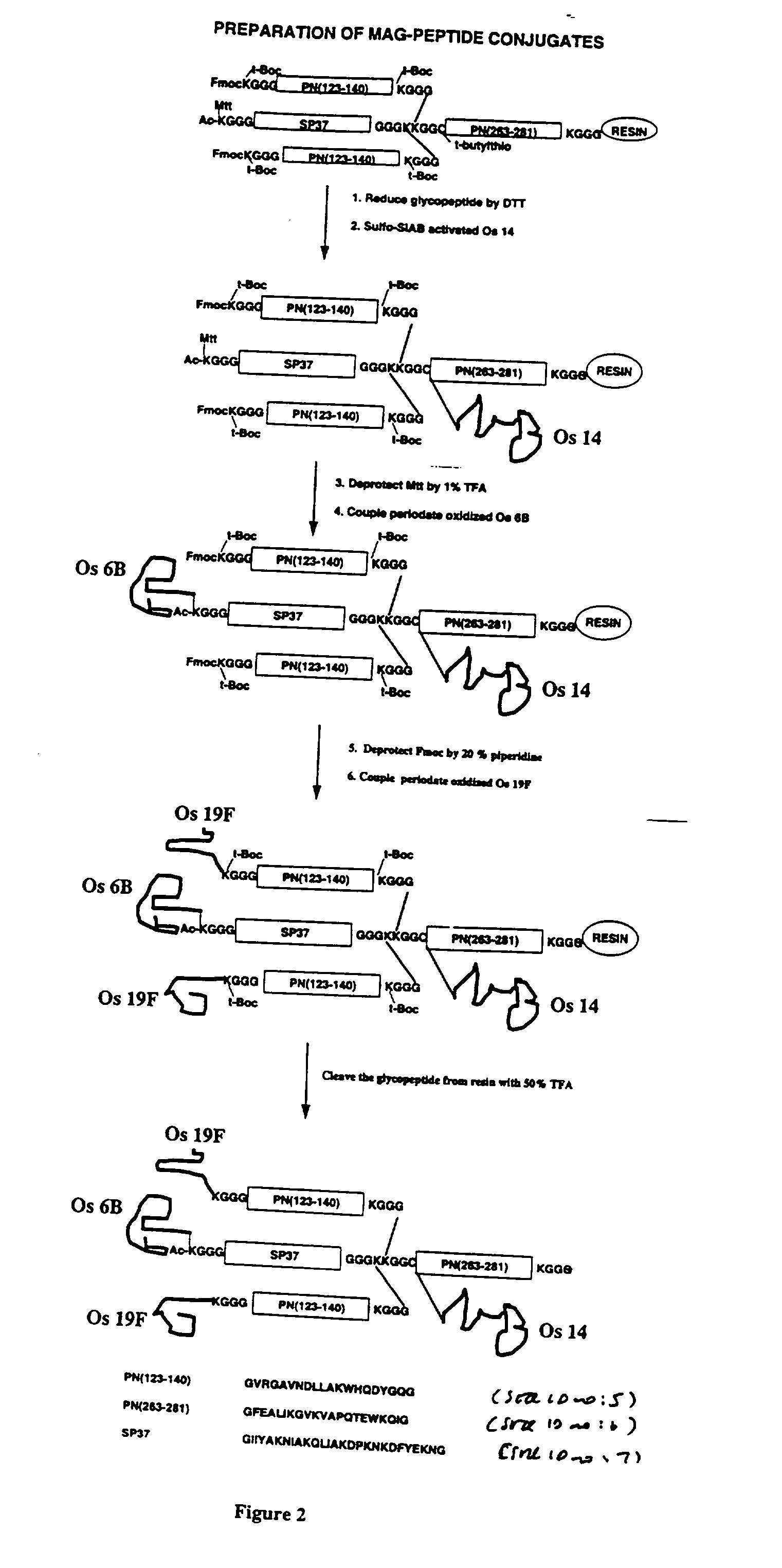
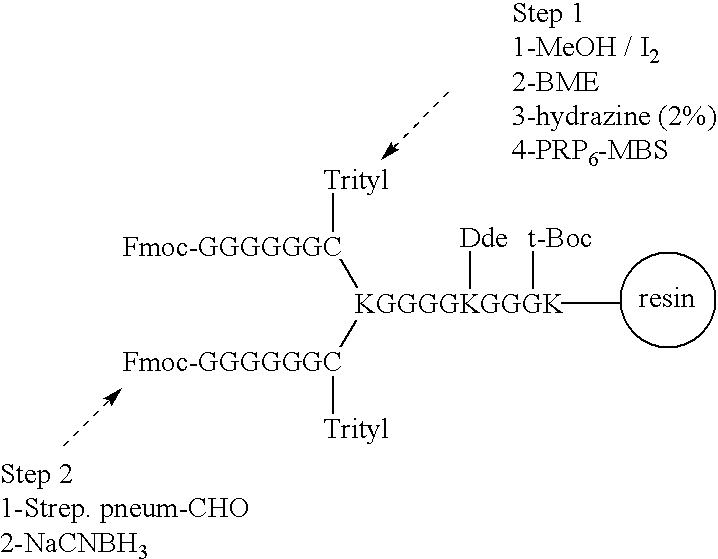
Multivalent immunogenic molecules comprise a carrier molecule containing at least one functional T-cell epitope and multiple different carbohydrate fragments each linker to the carrier molecule and each containing at least one functional B-cell epitope. The carrier molecule inputs enhanced immunogenicity to the multiple carbohydrate fragments. The carbohydrate fragments may be capsular oligosaccharide fragments from Streptococcus pneumoniae, which may be serotypes 1, 4, 5, 6B, 9V, 14, 18C, 19F or 23F, or Neisseria meningitidis, which may be serotype A, B, C, W-135 or Y. Such oligosaccharide fragments may be sized from 2 to 5 kDa. Alternatively, the carbohydrate fragments may be fragments of carbohydrate-based tumor antigens, such as Globo H, LeY or STn. The multivalent molecules may be produced by random conjugation or site-directed conjugation of the carbohydrate fragments to the carrier molecule. The multivalent molecules may be employed in vaccines or in the generation of antibodies for diagnostic application.
Owner:CONNAUGHT LAB
Framework-patched immunoglobulins
InactiveUS7321026B2Reduced and eliminated immunogenicityIncrease flexibilityAntipyreticAnalgesicsB-Cell EpitopesVaccine Immunogenicity
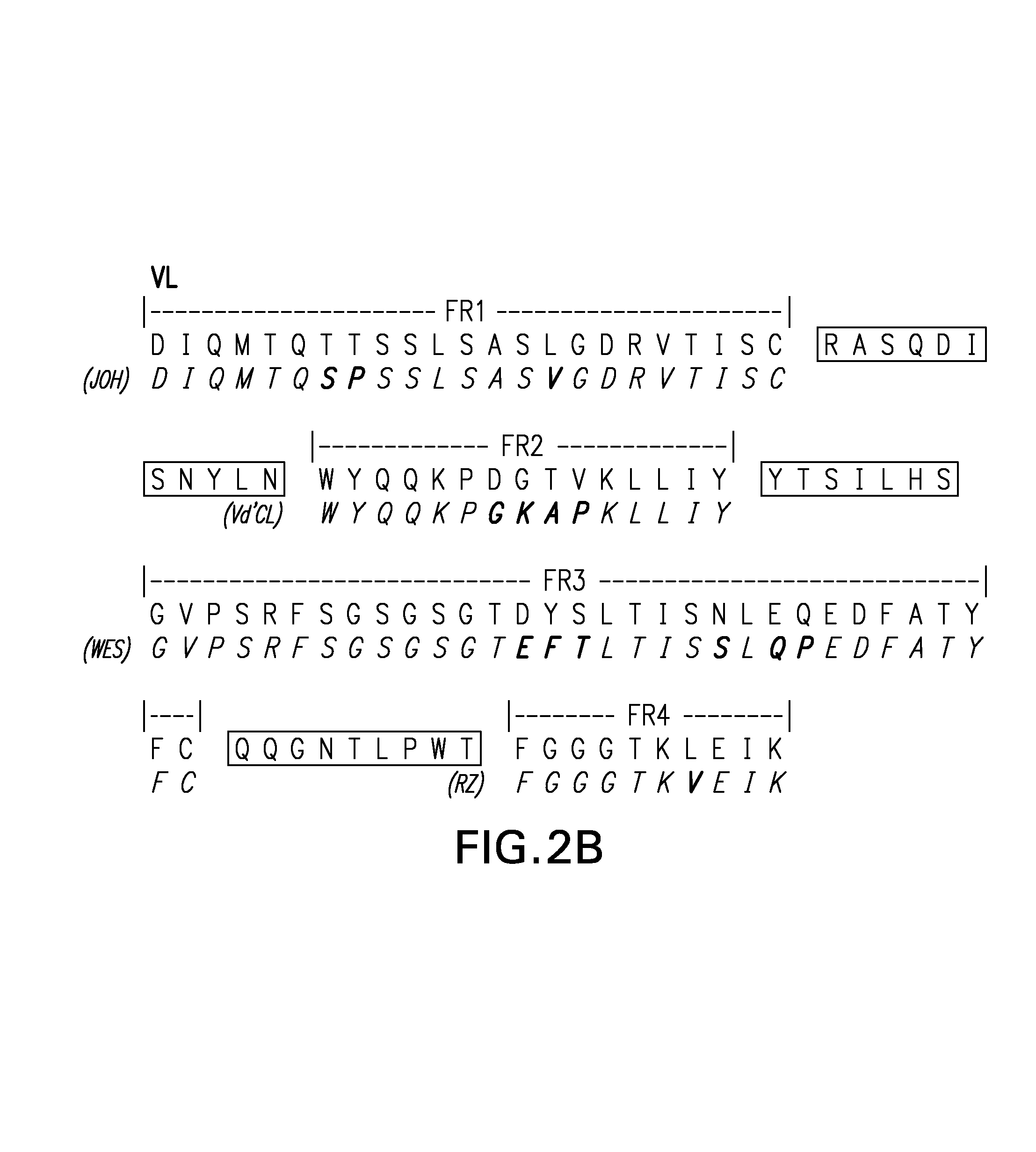
Framework (FR)-patching is a novel approach to modify immunoglobulin for reducing potential immunogenicity without significant alterations in specificity and affinity. Unlike previous described methods of humanization, which graft CDRs from a donor onto the frameworks of a single acceptor immunoglobulin, we patch segments of framework (FR1, FR2, FR3, and FR4), or FRs, to replace the corresponding FRs of the parent immunoglobulin. Free assortment of these FRs from different immunoglobulins and from different species can be mixed and matched into forming the final immunoglobulin chain. A set of criteria in the choice of these FRs to minimize or eliminate the need to reintroduce framework amino acids from the parent immunoglobulin for patching is described. The approach gives greater flexibility in the choice of framework sequences, minimizes the need to include parent framework amino acids, and, most importantly, reduces the chances of creating new T- and B-cell epitopes in the resultant immunoglobulin.
Owner:SKYTECH TECH
Multi oligosaccharide glycoconjugate bacterial meningitis vaccines
InactiveUS6656472B1Inhibition effectWeight increaseAntibacterial agentsAntipyreticSerotypeTumor antigen
Owner:AVENTIS PASTEUR LTD
Bovine germline D-genes and their application
ActiveUS7196185B2Large capacitySugar derivativesMicrobiological testing/measurementDiseaseImmunocompetence
The present invention relates to a bovine VDJ cassette (BF1H1) that provides the novel ability to develop chimeric immunoglobulin molecule capable of incorporating both linear T cell epitope(s) (CDR1H and CDR2H) as well as conformational B cell epitope(s) (exceptionally long CDR3H). Further, multiple epitopes can be incorporated for development of multivalent vaccine by replacing at least a portion of an immunoglobulin molecule with the desired epitope such that functional ability of both epitope(s) and parent VDJ rearrangement is retained. The antigenized immunoglobulin incorporating both T and B epitopes of interest is especially useful for development of oral vaccines for use in humans apart from other species including cattle. The long CDR3H in BF1H1 VDJ rearrangement originates from long germline D-genes. The novel bovine germline D-genes provide unique molecular genetic marker for sustaining the D-gene pool in cattle essential for immunocompetence via selective breeding. D-gene specific DNA probe permits typing and selection of breeding cattle stock for maximum gemline D gene pool for better health and disease prevention. The bovine D-genes are unique to cattle and, therefore, provide sensitive and specific forensic analytical tool using molecular biology techniques to determine tissues suspected of bovine origin.
Owner:KAUSHIK AZAD KUMAR +2
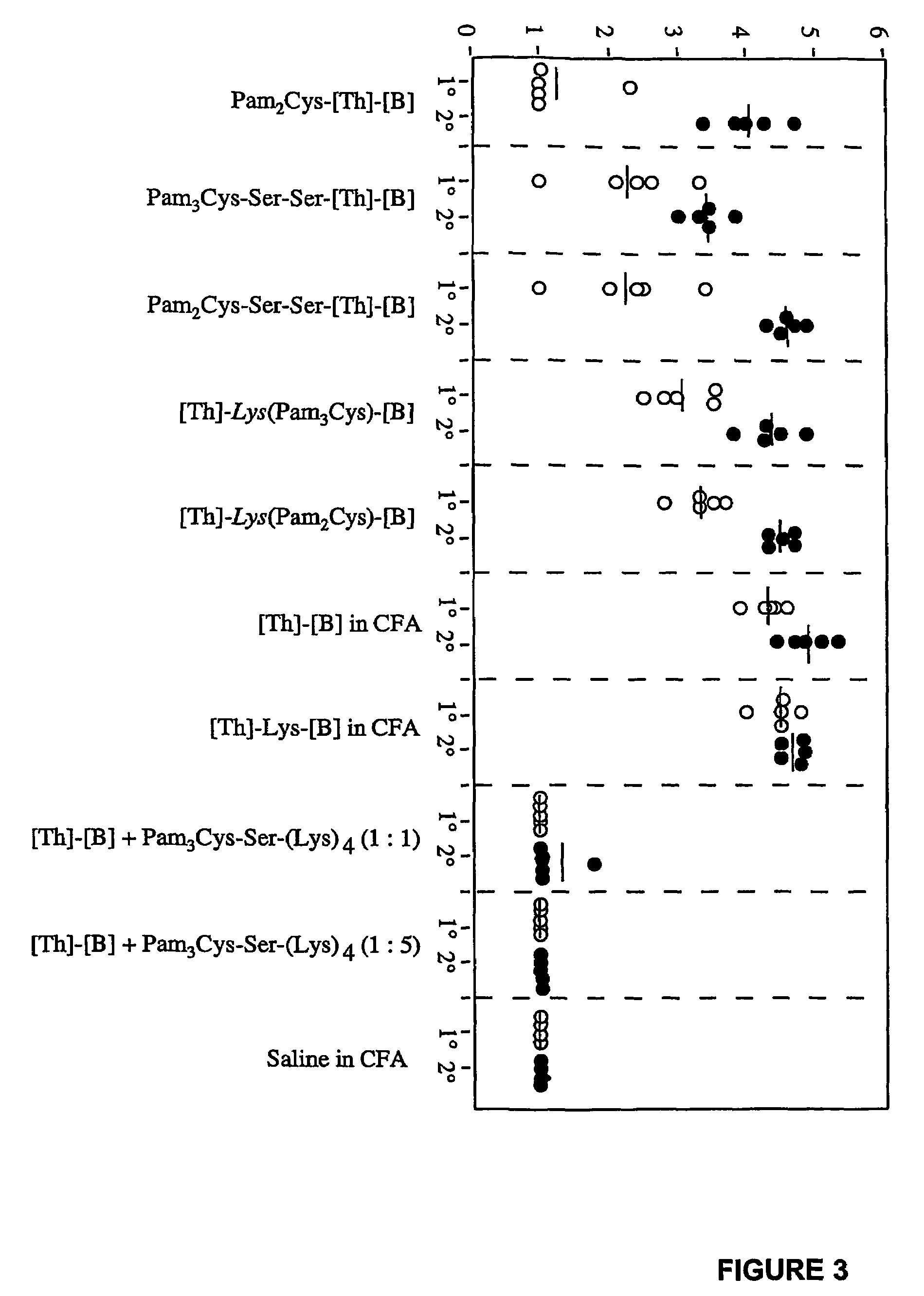
Immunogenic lipopeptides comprising T-helper and B-cell epitopes
InactiveUS7569225B2Increase profitEasy to specifyAntibacterial agentsBiocideSynthetic ImmunogensVaccination
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and B cell epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular antigens. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the B cell epitope or within the T-helper epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
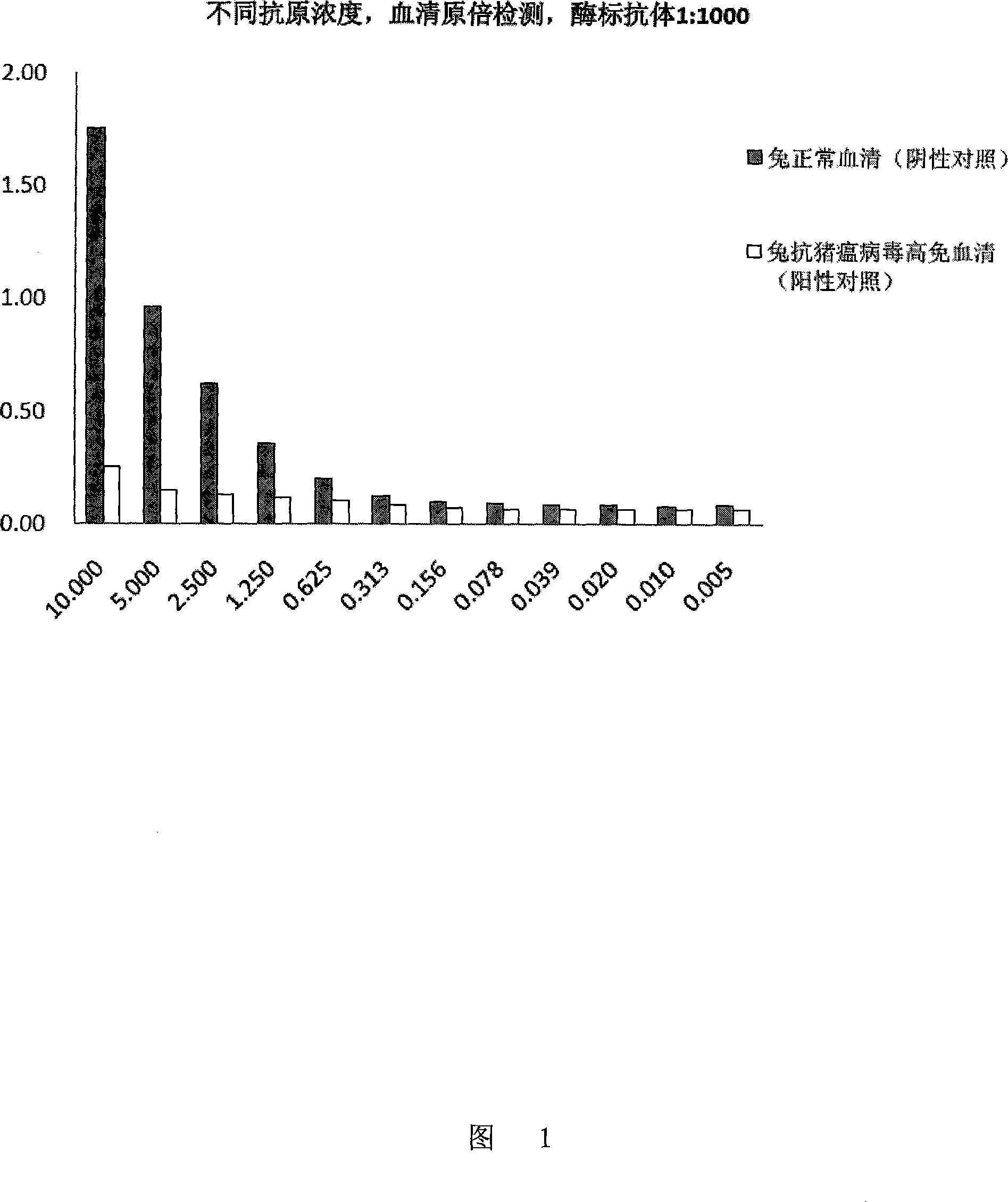
Method for detecting pig plague virus specific antibody and its ELISA reagent kit
InactiveCN101144818AGuaranteed specificityGuaranteed Differential DiagnosisMaterial analysis by observing effect on chemical indicatorElisa kitSwine Fever Virus
The invention discloses a method for detecting the specific antibody of classical swine fever virus and a special ELISA kit thereof. The kit includes a classical swine fever virus antigen and an enzyme-labeled classical swine fever virus single-epitope specific antibody; the said swine fever virus antigen is a polypeptide containing one or more than one amino acid residue sequence described in sequence 1. The detection reagent of the classical swine fever virus specific antibody of the present invention can carry out effective detection to the classical swine fever virus specific antibody by solid-phase antigen competition ELISA (blocking method); The B cell epitope ensures the differential diagnosis, and the high degree of conservation of the epitope among various strains ensures the specificity of detection.
Owner:TSINGHUA UNIV +2
Mutated parvovirus structural proteins as vaccines
ActiveUS20160153992A1Easily be screened and producedHigh affinityAntibacterial agentsCompound screeningAntigenHeterologous
The present invention is related to a method for identifying a parvovirus mutated structural protein capable of specifically binding to a binder for an antigen, a parvovirus mutated structural protein which comprises at least one B-cell epitope heterologous to the parvovirus, a multimeric structure comprising the protein, a nucleic acid encoding the protein, a virus or cell comprising the protein, a method of preparing the protein, a medicament comprising the protein, nucleic acid or multimeric structure and its use.
Owner:MEDIGENE
Foot-and-mouth disease genetic engineering mixed epitope vaccine and preparation method thereof
ActiveCN103007273AGood immune protectionUniform response levelAntiviralsAntibody medical ingredientsGenetic engineeringPolyinosinic Acids
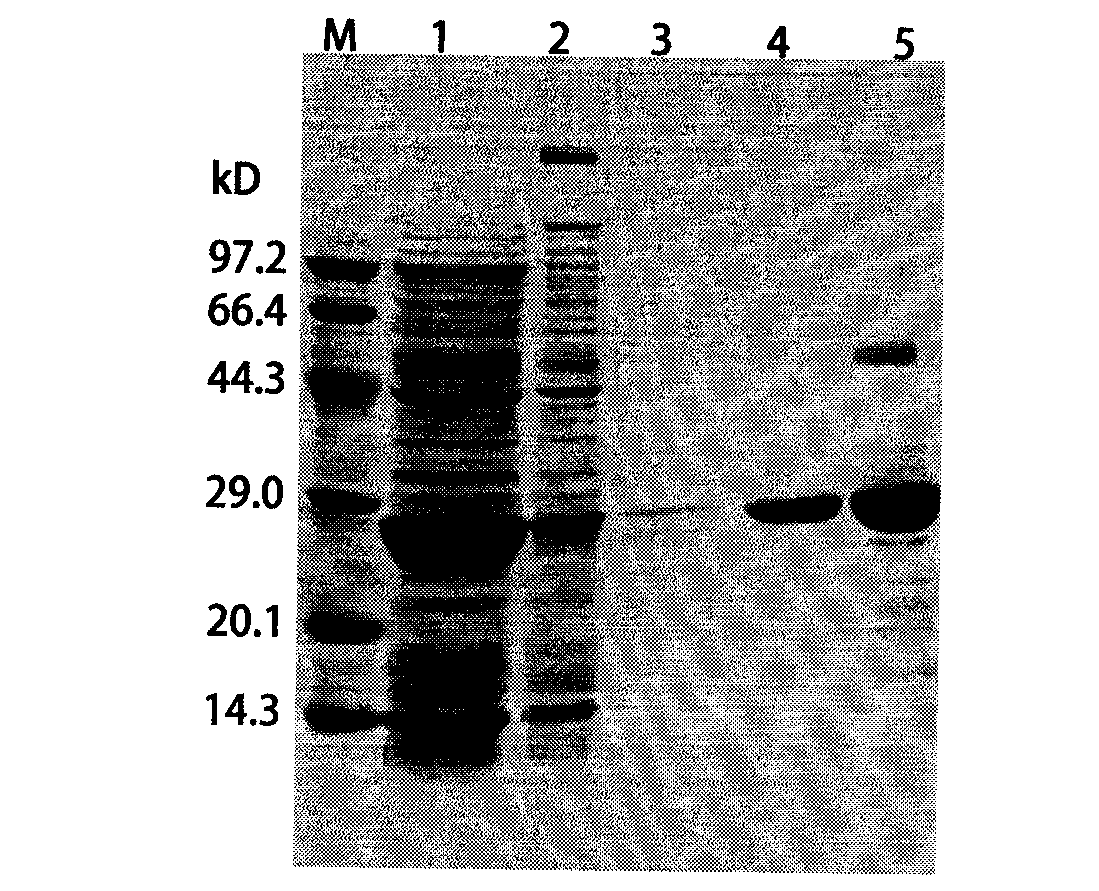
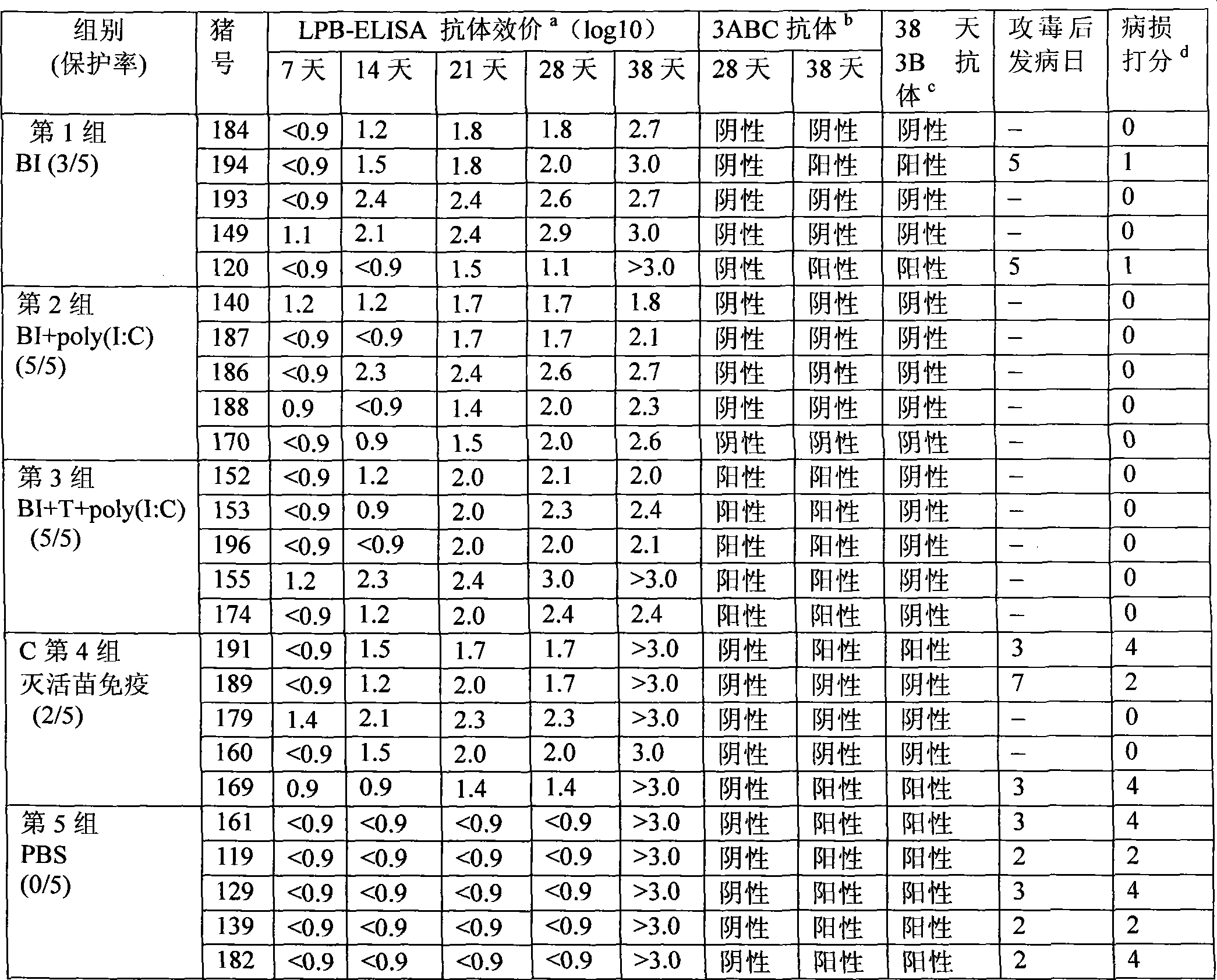
The invention discloses a foot-and-mouth disease genetic engineering mixed epitope vaccine and a preparation method thereof. The vaccine consists of the following four parts: a serial B cell epitope recombinant protein BI consisting of main neutralizing epitops of O-type foot-and-mouth disease viruses in Cathay, Transasia and Mya 98 pedigrees with a gene sequence of SEQ ID NO:1 and an amino acid sequence of SEQ ID NO:2, a T-cell epitope recombinant protein TI consisting of serial connection of universal T-cell epitope and a plurality of foot-and-mouth disease virus specific T-cell epitopes with a gene sequence of SEQ ID NO:3 and an amino acid sequence of SEQ ID NO:4, Toll-like receptor 3 agonist-polyinosinic acid-polycytidysic acid and / or Toll-like receptor7 / 8 agonist-R848 serving as immunopotentiator, and 201 oil adjuvant. When being used for immunizing a pig, the BI and TI mixed epitope vaccine prepared by utilizing the method can produce a protective immunization effect the same as or better than that of an inactivated influenza virus Vaccines, and has a cross protection effect to viruses of the three pedigrees, so that the vaccine is a novel immune-enhanced O-type foot-and-mouth genetic engineering mixed epitope vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Chimeric peptides as immunogens, antibodies thereto, and methods for immunization using chimeric peptides or antibodies
The invention provides a chimeric peptide or mixture of chimeric peptides that can be formulated as an immunizing composition and used in a method for immunization of a mammal against an internal peptide cleavage product derived from a precursor or mature protein, for which the peptide cleavage product and the precursor or mature protein are self molecules. The chimeric peptide or peptides have an end-specific B cell epitope from a naturally-occurring internal peptide cleavage product of a precursor or mature protein, as a free N- or C-terminus, fused with or without spacer residues to a T helper cell epitope derived from a living source different from that of the internal peptide cleavage product.
Owner:COLLATERAL AGENTS
B cell epitope peptide segment of amino-terminal pro-brain natriuretic peptide and applications thereof
ActiveCN102887943AHigh purityHigh potencyMicroorganism based processesTissue cultureSpecific antibodyAtrial natriuretic peptide
The invention belongs to the field of medicine, particularly relates to a diagnostic technique of heart failure, and specifically relates to a B cell epitope peptide segment of amino-terminal pro-brain natriuretic peptide. An amino acid sequence is shown in SEQ ID NO:4 or SEQ ID NO:5. The prepared specific antibody can be used as a diagnostic reagent of heart failure. The amino-terminal pro-brain natriuretic peptide prepared by the invention is high-purity monomer recombinant protein of the amino-terminal pro-brain natriuretic peptide. The protein can be used for immunogen and screening collagen prepared from an antibody, and can be simultaneously used as a calibrator when the amino-terminal pro-brain natriuretic peptide is built to carry out quantitative detection. A monoclonal antibody prepared by immune mice at the B cell epitope peptide segment of the amino-terminal pro-brain natriuretic peptide has the advantages of high purity (SDS-PAGE detection purity greater than 96%), high valence (the valence of ELISA up to 1:512000), good specificity, mass preparation, and the like.
Owner:重庆业为基生物科技有限公司
Therapeutic Antibodies, Antibody Fragments and Antibody Conjugates
InactiveUS20090010929A1Prevent increase in bacterial countReduce bacteria countAntibacterial agentsBiocideDiseaseTherapeutic antibody
The present invention provides compositions, including pharmaceutical compositions, comprising an amount of an antibody, antibody fragment or antibody conjugate sufficient to treat Group A streptococcus (GAS) infection or complication thereof in a subject or a disease or complication associated with GAS infection in a subject wherein said antibody, antibody fragment or antibody conjugate binds immunospecifically to a B-cell epitope of GAS M-protein. The present invention also provides methods for the prophylactic or therapeutic treatment of infection by GAS and complications thereof comprising administering the compositions to a subject in need thereof.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
A group of antigen epitope polypeptide and uses thereof
InactiveCN101492495AViral antigen ingredientsMicroorganism based processesCarrier proteinB-Cell Epitopes
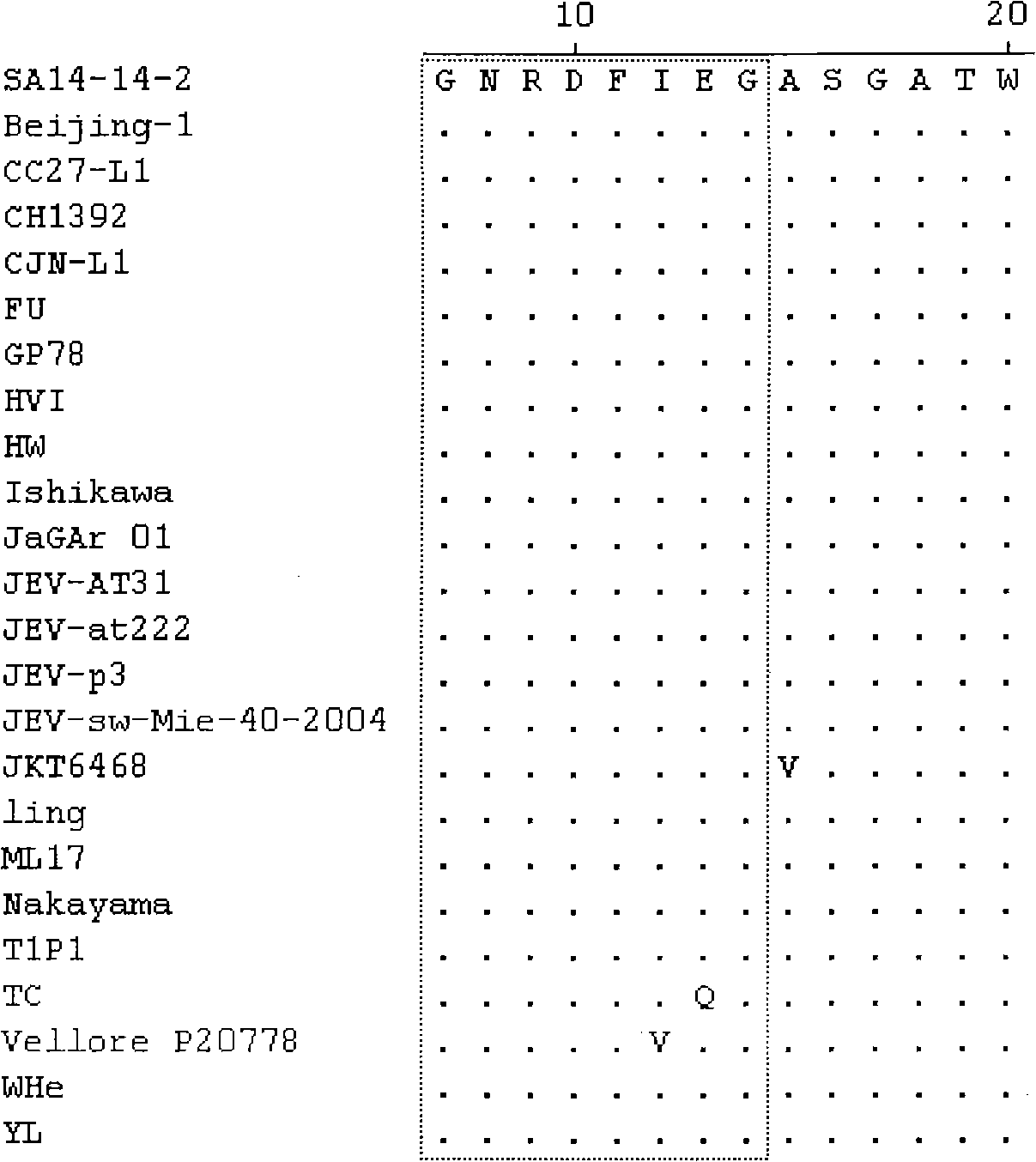
The invention discloses a set of epitope polypeptides, particularly relates to a neutralizing B cell epitope sequence of encephalitis B virus E protein, and further discloses application of controlling and diagnosing encephalitis B virus by these epitopes. The amino acid sequences of the epitope polypeptides in the invention are respectively any amino acid sequence of SEQ ID NO: 64, SEQ ID NO: 79, SEQ ID NO: 95, SEQ ID NO: 108, SEQ ID NO: 124 and SEQ ID NO: 139. After the epitope polypeptides in the invention are coupled or fused with carrier proteins to be expressed as immunogenic or vaccine immuno animal organism, neutralizing antibodies aiming at JEV can be generated, and the JEV can be neutralized in vivo or in vitro so as to prevent virus from infecting animal organism. The epitope polypeptides in the invention or the junctional complex thereof can be used as reagents for detecting encephalitis B virus antibodies or encephalitis B virus polypeptide antibodies.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for screening wheat varieties with low celiac sprue toxicity based on T cell epitopes
InactiveCN103675291AExtraction is fast, effective and comprehensiveHigh detection sensitivityElectrophoretic profilingPreparing sample for investigationStainingScreening method
A method for screening wheat varieties with low celiac sprue toxicity based on T cell epitopes comprises the following steps: separating and extracting wheat gluten protein in two steps, performing SDS-PAGE electrophoresis combined with PageBlue TM staining method to identify the gluten protein, detecting celiac sprue T cell epitopes caused by the gluten protein through immunoblotting and other steps. According to the invention, T cell epitopes-based screening method is adopted to screen out the wheat varieties with low celiac sprue toxicity, so that safe and reliable low gluten food can be supplied for celiac sprue patients from the origin of raw materials, and the safety risk of the celiac sprue patients is reduced.
Owner:NANCHANG UNIV
Novel immunogenic lipopeptides comprising t-helper and b-cell epitopes
InactiveUS20070066534A1Promote maturityEnhance antigen presentationAntibacterial agentsBiocideSynthetic ImmunogensDiamino acid
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and B cell epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular antigens. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the B cell epitope or within the T-helper epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Specific B cell epitope polypeptide of NS1 protein of encephalitis B virus and use thereof
ActiveCN102206249AStrong specificityReduce false positive ratePeptidesGenetic engineeringMolecular ImmunologyScreening method
The invention discloses the sequence of a specific B cell epitope polypeptide of an NS1 protein of encephalitis B virus and the use of the epitope polypeptide in the diagnosis of encephalitis B virus and a screening method of specific B cell epitope polypeptide of NS1 protein of encephalitis B virus, and belongs to the field of molecular immunology. The amino sequence of the epitope polypeptide is represented by SEQ ID No.1 or SEQ ID No.2. The specific B cell epitope synthetic polypeptide of the JEV NS1 protein, the coupling antigen of the synthetic epitope polypeptide and the epitope fusion expression protein can be used for specifically detecting a JEV NS1 protein antibody generated in body which is immunized and infected. The epitope polypeptide can be used for diagnosis of JEV infection, evaluation on immunity effect and identification and diagnosis of immunity of inactivated vaccine and natural infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Porcine circovirus II-type (PCV2) epitope peptide vaccine and preparation method thereof
ActiveCN103536912AStrong specificityEasy to saveViral antigen ingredientsAntiviralsDiseaseCircovirus
The invention relates to a porcine circovirus II-type (PCV2) epitope peptide vaccine and a preparation method of the vaccine. The vaccine contains three b cell epitopes, the lysine of the vaccine is four-branch peptide with the same core matrix structure epitope monomer and is connected with a general T ancillary cell (Th) epitope in series, and the vaccine has the molecular weight of 13kDa; the epitope peptide is named PCV CP98-156-228; after being vaccinated, a mice has stronger immune response, and a high potency virus neutralizing antibody can be generated. The epitope peptide vaccine has the advantages of being low in price, safe, high in specificity and easy to store and apply, and plays an important role in preventing and controlling the porcine circovirus disease. The PCV2 epitope peptide vaccine is prepared by four steps including predicting and screening epitope peptide, designing the epitope peptide vaccine, synthesizing, purifying and authenticating the epitope peptide as well as preparing the epitope peptide vaccine; the method is easy in raw material obtaining, lower in cost and easy to control, and has better operability, thus being suitable for being popularized and applied.
Owner:CHONGQING UNIV OF TECH +2
Polypeptide vaccine of anti Asiatic I virus of foot-and-mouth disease and its preparing method
The invention is a kind of polypeptide vaccine and the manufacturing method which can resist Asia one foot and mouth disease virus. The vaccine is prepared by that DNA array of B , T cell form location amino acid on VP1 and VP4 contact with coding form location albumen alone, or link with macromolecule of the carrier coding schedule location merged protein. The invention uses chemical method to synthesize the peptide gene of B cell and T cell form location on coding Asia1 type VP1 and VP4, and they are contacted, the gene is inserted with particle carrier alone or connecting with the carrier molecular, and transferred into bacteria express, and yeasted and gets the product.
Owner:FUDAN UNIV
Chimeric protein, virus-like particle and application thereof
ActiveCN104693310AImproving immunogenicityFast and Efficient CarryBacteriaViral antigen ingredientsCircovirusVirus-like particle
The invention provides a chimeric protein, a virus-like particle and application thereof and belongs to the field of molecular biology. The chimeric protein is prepared by the following steps: replacing one, two or three of four sites in a porcine circovirus 2 type Cap protein by a main B cell epitope of a porcine O-shaped foot-and-mouth disease virus VP1 protein to obtain 58th-66th amino acid residues, 72nd-94th amino acid residues, 122nd-147th amino acid residues and 162nd-197th amino acid residues. The chimeric protein can form the chimeric virus-like particle after soluble expression, shows the main B cell epitope of the porcine O-shaped foot-and-mouth disease virus VP1 protein on the surface of the chimeric virus-like particle, has very good immunogenicity to both PCV2 and FMDV and can generate a high antibody to the PCV2 and the FMDV, by once immune.
Owner:江苏省苏农科技术转移中心有限公司
Gene cloning of polyepitope antigen of hepatitis C virus and its coding sequence
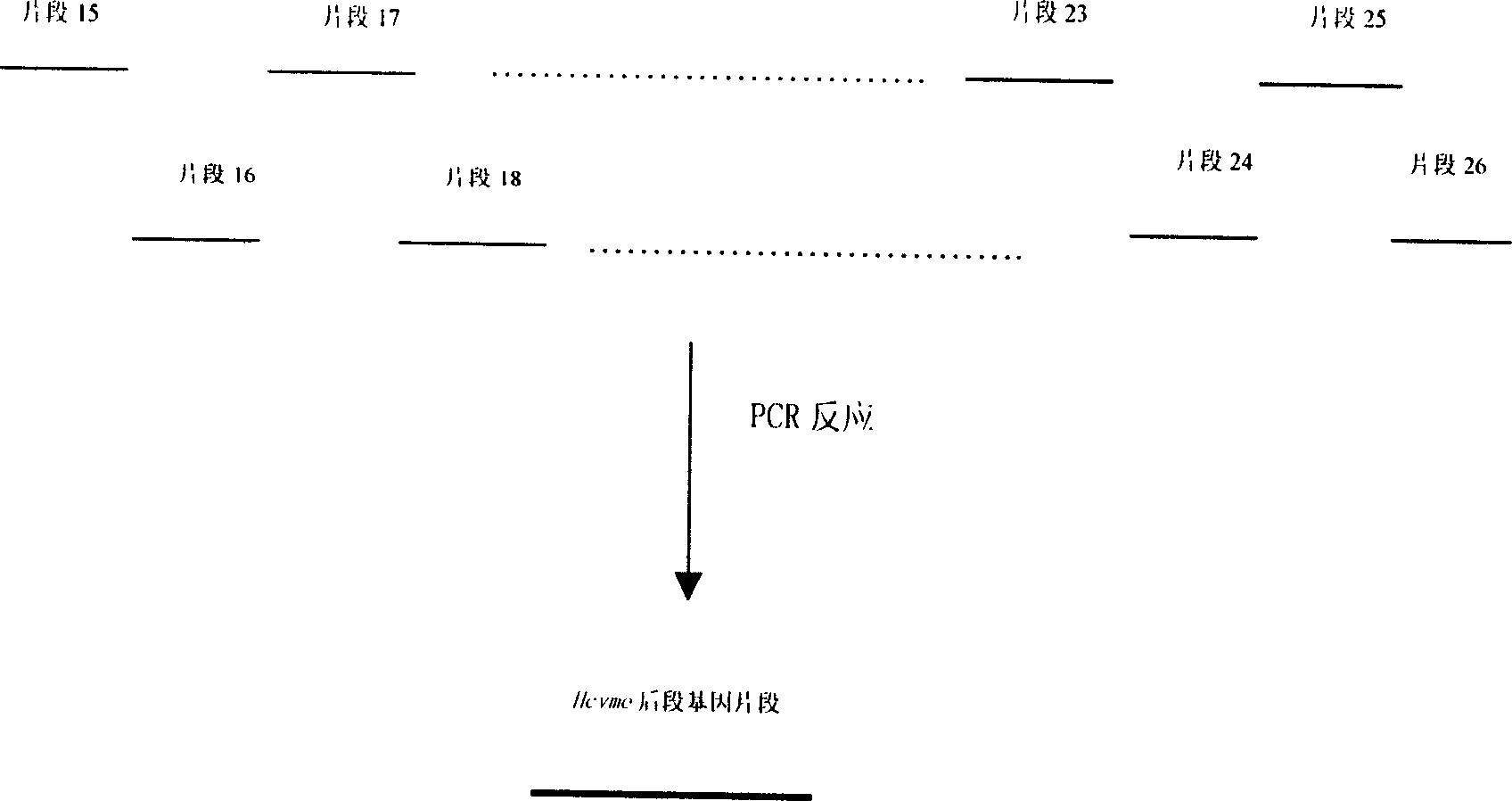
InactiveCN1609213AAvoid the disadvantage of being prone to mutationValid submissionGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCtl epitopeGene clone
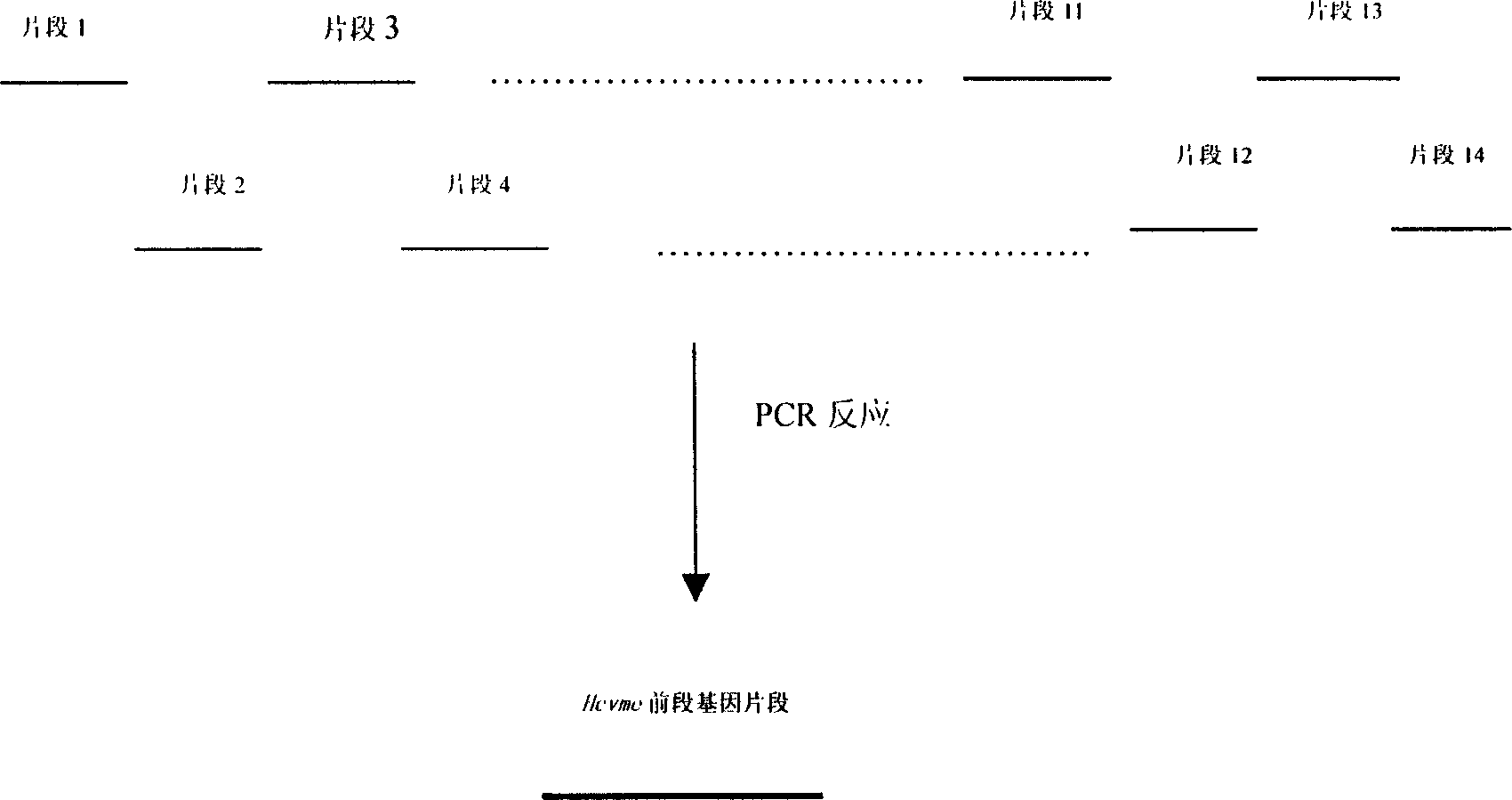
The present invention relates to biomedicine engineering technology, and is the gene cloning of polyepitope antigen of hepatitis C virus hcvme and the coded polyepitope antigen HCVME. The hcvme includes 9 epitope genes of HVR1 simulating B cell and 4 epitope genes of T cell in E2 region of HCV genome, and the 4 epitope genes of T cell includes 2 of conservative CTL epitope in region C, 1 of conservative CTL epitope in region NS3 and 1 of conservative Th epitope in region NS3. Serial tests show that hcvme has high cross reaction with karyogamy expressed product GST-HCVME of gst gene and positive hepatitis C antibody serum, and the GST-HCVME immunized male rabbit serum has high distinction frequency on natural HVR1 synthetic peptide. Therefore, hcvme and its expressing product HCVME may be used in preparing nucleic acid vaccine and polypeptide vaccine for hepatitis C and the test reagent for HCV antigen and antibody.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
O-type aftosa synthetic peptide vaccine
ActiveCN101659695AImprove protectionFree from attackAntiviralsPeptide preparation methodsChemical synthesisPeptide vaccine
The invention provides O-type aftosa synthetic peptide vaccine, and in particular relates to polypeptide or polypeptide polymer thereof used in the vaccine as well as the vaccine containing the polypeptide or the polypeptide polymer thereof and a preparation method thereof. The polypeptide has amino acid sequences shown in SEQ ID No.1, SEQ ID No.2 and SEQ ID No.3. The O-type aftosa synthetic peptide vaccine carries out chemical synthesis of potential antigen site peptide segments by carrying out sequencing of domestic aftosa epidemic strains to study the variation of the main antigen sites ofaftosa and combining with computer assistant to carry out antigen site analytical prediction. Candidate polypeptide antigens are screened out by carrying out large numbers of animal experiments and aftosa virus antigen sites are optimized according to the screening result; and T cell epitope and B cell epitope are effectively combined to improve the immune effects of the polypeptide antigens. TheO-type aftosa synthetic peptide vaccine can effectively cope with the antigen variation of aftosa virus and has ideal biosafety and easy large-scale synthesis, thereby having a good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Epitope peptide H362 of HN protein in peste des petits ruminants virus (PPRV), and determination, preparation method and application thereof
ActiveCN107216372AStrong green fluorescenceGood reactogenicitySsRNA viruses negative-senseViral antigen ingredientsF proteinHN Protein
The invention relates to an epitope peptide H362 of an HN protein in PPRV, and determination, a preparation method and application thereof. The amino acid sequence of the epitope peptide is H362: <362>EANWVVPSTDVRDL<375>. The invention detects reactogenicity of a monoclonal antibody and PPRV and specificity of the monoclonal antibody; according to detection results, the monoclonal antibody has good reactogenicity to rPPRV-HN-F protein; immunoinformatic technology is cooperatively used for predicating the B cell epitope of the HN protein; an aminated ELISA plate is employed for detecting candidate epitopes and the monoclonal antibody 10E3, and the epitope peptide H362 corresponding to 10E3 is determined; and determination of the epitope peptide lays a theoretical foundation for preparation of epitope vaccine antigens and diagnostic reagent antigens for PPRV.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Pseudomonas exotoxin A with less immunogenic B cell epitopes
ActiveUS9206240B2Treat and prevent cancerPolypeptide with localisation/targeting motifPeptide/protein ingredientsPseudomonas exotoxinBiology
The invention provides a Pseudomonas exotoxin A (PE) comprising an amino acid sequence having a substitution of one or more of amino acid residues E420, D463, Y481, L516, R563, D581, D589, and K606, wherein the amino acid residues are defined by reference to SEQ ID NO: 1. The invention further provides related chimeric molecules, as well as related nucleic acids, recombinant expression vectors, host cells, populations of cells, and pharmaceutical compositions. Methods of treating or preventing cancer in a mammal, methods of inhibiting the growth of a target cell, methods of producing the PE, and methods of producing the chimeric molecule are further provided by the invention.
Owner:UNITED STATES OF AMERICA
Helicobacter pylori multivalent epitope vaccine and preparation method thereof
ActiveCN105169381ASmall molecular weightBiological toxicity avoidanceBacteriaDigestive systemEscherichia coliBiology
The present invention provides a Helicobacter pylori multivalent epitope vaccine, wherein the activity is a polypeptide, and the polypeptide comprises urease subunit A, urease subunit B, adhesin HpaA, heat shock protein HSP60 advantage Th, B cell epitope or segment, neutrophil activating protein NAP and cholera toxin subunit B. According to the present invention, the artificial gene is synthesized through the gene synthesis technology, and comprises the gene sequences of urease subunit A, urease subunit B, adhesin HpaA, heat shock protein HSP60 advantage Th, B cell epitope or segment and neutrophil activating protein NAP, the artificial gene is coupled to cholera toxin subunit B gene to form a fusion gene, the fusion gene is expressed through escherichia coli, and protein purification is performed to obtain the multivalent epitope vaccine; and the multivalent epitope vaccine can stimulate the body to produce the T cell immune response and the antibody humoral immunoresponse against urease, adhesin HpaA, heat shock protein HSP60 and neutrophil activating protein NAP, and can be used for prevention and treatment of Helicobacter pylori infection-related diseases.
Owner:NINGXIA MEDICAL UNIV
SARS-Cov gene vaccine based on epi-position and its contruction
InactiveCN1657102AReduced risk of infection-enhancing effectsOvercome the shortcomings of weak mutation ability and easy to produce toleranceGenetic material ingredientsAntiviralsSARS coronavirusAutoimmune disease
A SARS-Cov gene vaccine based on epitope is configured from the carrier which is a plasmid able to be used for human body and the target antigen which is several B cell epitopes in the extrinsic B protein antigen of human SARS coronavirus through codon optimizing and genetic engineering. Its preparing process is also disclosed.
Owner:FUDAN UNIV
Pseudomonas exotoxin A with less immunogenic T cell and/or B cell epitopes
ActiveUS9346859B2Treat and prevent cancerPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsPseudomonas exotoxinPseudomonas
The invention provides a Pseudomonas exotoxin A (PE) comprising an amino acid sequence having a substitution of one or more B-cell and / or T-cell epitopes. The invention further provides related chimeric molecules, as well as related nucleic acids, recombinant expression vectors, host cells, populations of cells, and pharmaceutical compositions. Methods of treating or preventing cancer in a mammal, methods of inhibiting the growth of a target cell, methods of producing the PE, and methods of producing the chimeric molecule are further provided by the invention.
Owner:UNITED STATES OF AMERICA
Helicobacter Pylori urease B subunit Th epitope peptide, its coding DNA, vaccine and uses
InactiveCN1807452AHas the effect of clearing HpImproving immunogenicityAntibacterial agentsPeptide/protein ingredientsPylorusHelicobacter pylori gastritis
The invention provides three Th cell epitope peptide and code DNA of pylorus spirillum urease B subunit, also providing a bacterin of B cell epitope which contains the triepitope peptide and an additional urease B subunit. Among them, the epitope peptide is the polypeptide which has one of the amino acid residue sequences as follows: 1) hasing amino acid sequence of sequence 2, sequence3 and sequence 7 in sequence table; 2) replacing, deleting or appending the amino acid sequence of sequence 2, sequence3 and sequence 7 in sequence table by one or several amino acid residue to form derivanting polypeptide. The coded DNA is one of the following sequences: 1) hasing the ribonucleotide sequence of sequence 8, sequence 9 and sequence sequence 10 table. 2) Hasing the ribonucleotide sequence of same coded product with sequence 8, sequence 9 and sequence10 in sequence table. The epitope vaccine which contains the polypeptide of this invention has the protein vaccine of amino acid sequence in sequence 12 of sequence table. The vaccine or medication which is made by using the polypeptide of the invention as active component can clean out the infection caused by pylorus spirillum and has wide application prospect in medical realm.
Owner:ARMY MEDICAL UNIV
Hemorrhagic bacillus coli of intestine 0157:H7 shiga toxin IIB epitope peptide and uses thereof
InactiveCN101314616AAntibacterial agentsPeptide/protein ingredientsIntestinal structureBacillus coli
The invention belongs to the pharmaceutical biotechnology field, and relates to enterohemorrhagic E.coli O157:H7 Shiga toxin IIB epitope peptide and the application thereof. Four preferred polypeptides are derived from B-cell epitope of the subunit (Stx2A1) of EHEC O157:H7 Shiga toxin IIB epitope peptide. The product can be applied for preparing pharmaceuticals for the diagnosis and treatment of EHEC O157 infection and complications thereof.
Owner:ARMY MEDICAL UNIV
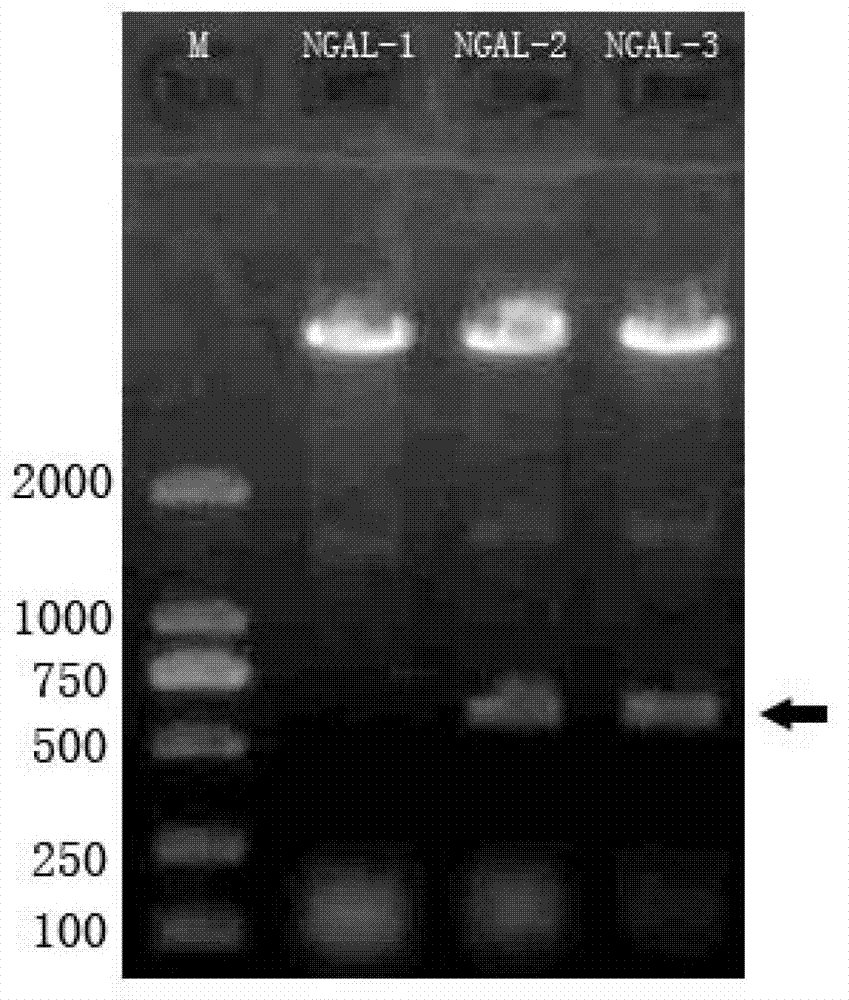
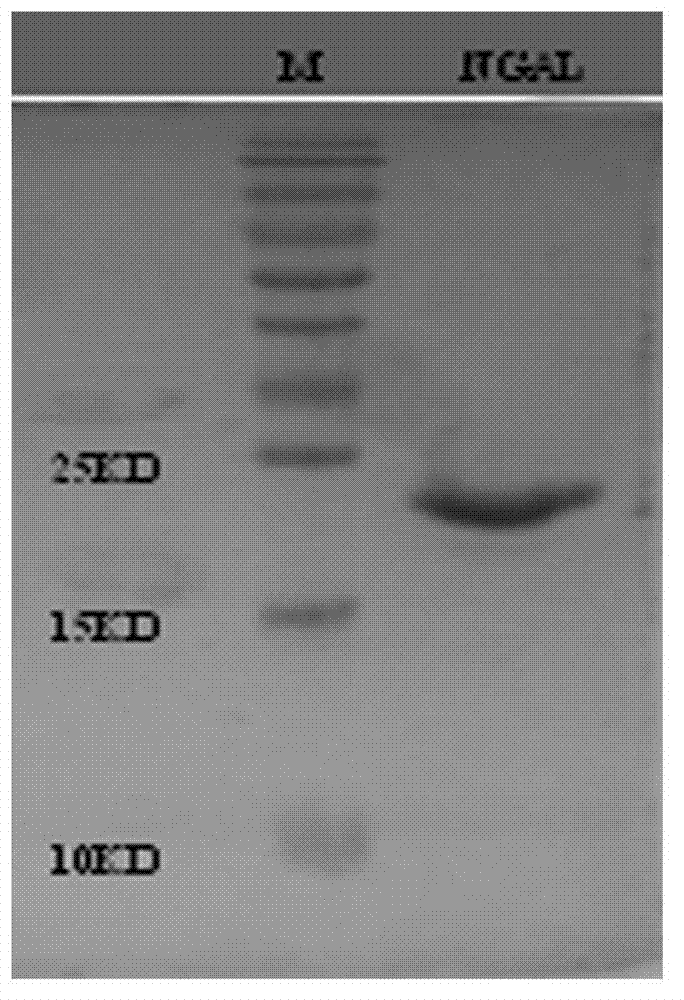
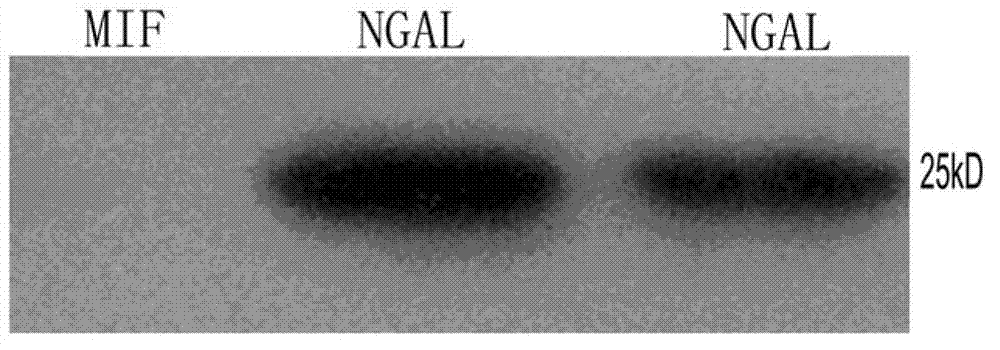
B cell epitope peptide of human neutrophil gelatinase associated lipocalin and application thereof
ActiveCN102775473AHigh purityHigh potencyImmunoglobulins against animals/humansMicroorganism based processesImmune complex depositionNGAL Protein
The invention belongs to the medical field and particularly relates to an acute kidney injury diagnosis technology, a B cell epitope peptide of human NGAL (Neutrophil Gelatinase Associated Lipocalin) and a hybridoma cell prepared by same, and an application of the B cell epitope peptide and a specific antibody thereof and an immune complex of the specific antibody in preparing diagnostic reagent for acute kidney injury. The B cell epitope peptide prepared by the invention immunizes a monoclonal antibody prepared by adopting a rat and has the advantages of high purity (The SDS (Sodium Dodecyl Sulfate)-PAGE (PolyAcrylamide Gel Electrophoresi) detection purity is more than 96 percent), high valence (the ELISA (Enzyme-Linked Immuno Sorbent Assay) valence reaches 1:256000), good specificity, mass preparation and the like; and through the monoclonal antibody and a polyclonal antibody prepared by the B cell epitope peptide, the B cell epitope peptide can be used for detecting the content of the NGAL in the urine of a patient, for example, by adopting a double antibody sandwich ELISA reaction mode, a double antibody sandwich structure formed by an enzyme labeled human anti-NGAL monoclonal antibody, an enzyme labeled plate package anti-NGAL polyclonal antibody and a measured sample NGAL antigen is detected.
Owner:重庆业为基生物科技有限公司
Monoclonal antibody BTV8-VP2-3E11 resistant to bluetongue virus serum 8 type VP2 protein, B-cell epitope peptide identified thereby and application
The invention discloses a monoclonal antibody BTV8-VP2-3E11 resistant to bluetongue virus 8 type (BTV8) VP2 protein, B-cell epitope peptide identified thereby and application, and belongs to the field of BTV8 control. A selected hybridoma cell strain capable of stably secreting a BTV8-VP2 protein resistant monoclonal antibody is stored with a microbial preservation number of CGMCC (China General Microbiological Culture Collection Center) No.7004. The experiment results show that the monoclonal antibody BTV8-VP2-3E11 secreted by the hybridoma cell strain can perform idiosyncratic reaction with the BTV8-VP2 protein but not reacts with the VP2 proteins of other serum types. The monoclonal antibody BTV8-VP2-3E11 and the BTV8-VP2 protein virus specific conserved B cell epitope peptide identified by the a monoclonal antibody can be prepared into an agent used for diagnosing BTV8 infection, thus laying a good foundation for creating serology differential diagnosis methods for BTV8 and other types of serum.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Application of B cell epitope peptide of human PCT (Procalcitonin) and monoclonal antibody of B cell epitope peptide
ActiveCN102643332AHigh purityHigh potencyTissue cultureImmunoglobulins against hormonesAssayB-Cell Epitopes
The invention belongs to the field of immunology in medicines and particularly relates to application of a B cell epitope peptide of a human PCT (Procalcitonin) and a monoclonal antibody of the B cell epitope peptide. The epitope peptide is shown as SEQ ID NO:3 and can be used for preparing a hybridoma cell and secreting a corresponding monoclonal antibody. The monoclonal antibody can be used for preparing a diagnostic reagent for detecting the procalcitonin and has the advantages of purity as high as 98 percent, valence as high as 1:512000, favorable specificity, capability of being prepared in a large batch and the like. The monoclonal antibody and a polyclonal antibody which are prepared by the invention can be used for detecting the content of the PCT in the blood of a patient, for example, a double antibody sandwich ELISA (Enzyme-Linked Immuno Sorbent Assay) reaction mode, namely enzyme-labelled anti-human PCT antibody and an elisa plate to used to coat an anti-PCT polyclonal antibody and a measured sample PCT antigen to form a double antibody sandwich structure for measuring.
Owner:重庆业为基生物科技集团有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com