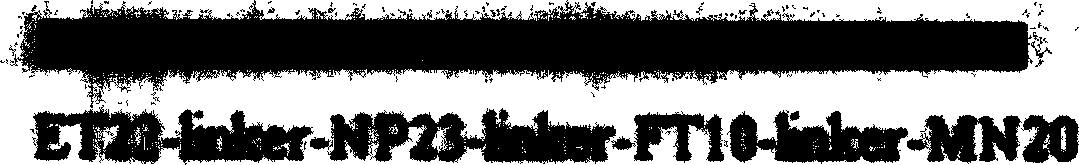SARS-Cov gene vaccine based on epi-position and its contruction
A gene vaccine and epitope technology, applied in the fields of bioengineering and immunology, can solve the problems of inefficient expression of short peptides, achieve high specificity, good expected clinical value, and overcome poor expression efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Construction, Identification and Expression of Epitope-Based SARS-Cov Gene Vaccine
[0059] 1. Construction of epitope-based SARS-Cov gene vaccine
[0060]After software prediction combined with network database prediction, the polypeptide sequence as shown in Table 1 was first determined as the target epitope, according to the mammalian codon reported by Kotsopoulou E. (Journal of Virology; Vol.74, No.10, 4839-4852) After preference optimization, the gene fragments shown in Table 2 were artificially synthesized. After the synthetic fragments were phosphorylated by T4 polynucleotide kinase for 1 hour, they were heated and boiled for 5 minutes, kept warm, cooled and annealed slowly, and then connected in pairs to obtain ab, cd, ef, gh (connected at 16°C for 6 hours). After the cassettes were recovered, abcd and efgh were ligated in pairs again, which were recovered and ligated again, and the target gene fragments with BglII and EcoRI restriction sites at both ...
Embodiment 2
[0068] Example 2 The epitope-based SARS-Cov gene vaccine immunizes mice to induce specific humoral immunity
[0069] Experimental Study of Immune Response
[0070] Experimental animals: 6-8 weeks old female BALB / c(H-2 d ) mice, weighing 16-18 grams, were purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences.
[0071] Experimental grouping and animal immunization: 12 healthy female BALB / c mice each, divided into 2 groups: (1) immunization group with purified pVAON33 empty plasmid vector as control, 6 mice; (2) Purified pVAON33-epis plasmid Immunization group, 6 rats. Intramuscularly immunize female BALB / c mice with mild anesthesia and inject into the tibialis anterior muscle: intraperitoneally inject mice with 0.75% pentobarbital sodium 100-150ul (according to 50ug / g body weight), expose the tibialis anterior muscle, and use a 1ml syringe Insert the needle vertically from the middle of the muscle, insert the needle tip to a depth of ...
Embodiment 3
[0074] Example 3 Epitope-based SARS-Cov gene vaccine immunizes mice to induce specific cells
[0075] Experimental Study of Immune Response
[0076] Experimental animals: 6-8 weeks old female BALB / c(H-2d) mice, weighing 16-18 grams, purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences.
[0077] Experimental grouping and animal immunization: 12 healthy female BALB / c mice each, divided into 2 groups: (1) immunization group with purified pVAON33 empty plasmid vector as control, 6 mice; (2) Purified pVAON33-epis plasmid Immunization group, 6 rats. Intramuscularly immunize female BALB / c mice with mild anesthesia and inject into the tibialis anterior muscle: intraperitoneally inject mice with 0.75% pentobarbital sodium 100-150ul (according to 50ug / g body weight), expose the tibialis anterior muscle, and use a 1ml syringe Insert the needle vertically from the middle of the muscle, insert the needle tip to a depth of 2-3mm, slowly insert th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com