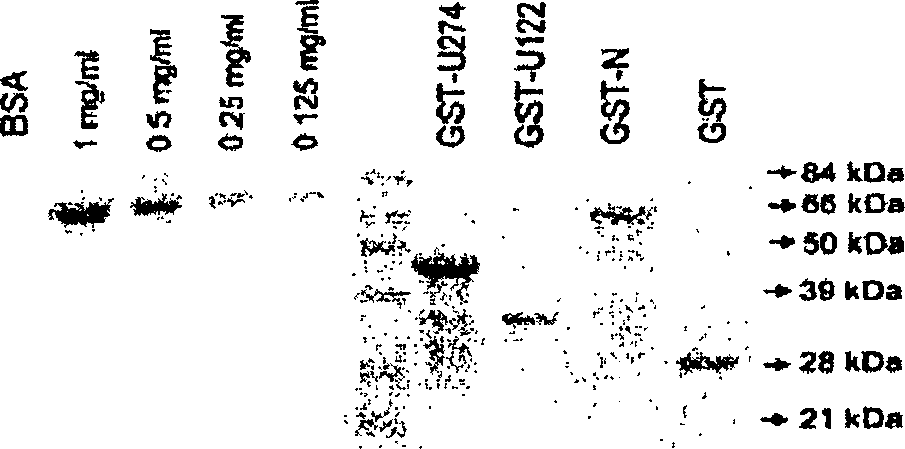Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
153 results about "SARS coronavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Severe acute respiratory syndrome-related coronavirus, sometimes shortened to SARS-CoV, is the virus that causes severe acute respiratory syndrome (SARS). On April 16, 2003, following the outbreak of SARS in Asia and secondary cases elsewhere in the world, the World Health Organization (WHO) issued a press release stating that the coronavirus identified by a number of laboratories was the official cause of SARS. Samples of the virus are being held in laboratories in New York City, San Francisco, Manila, Hong Kong, and Toronto.
Anti-Sars Monoclonal Antibodies
ActiveUS20080081047A1Sugar derivativesViral antigen ingredientsDrug biological activityWestern immunoblot
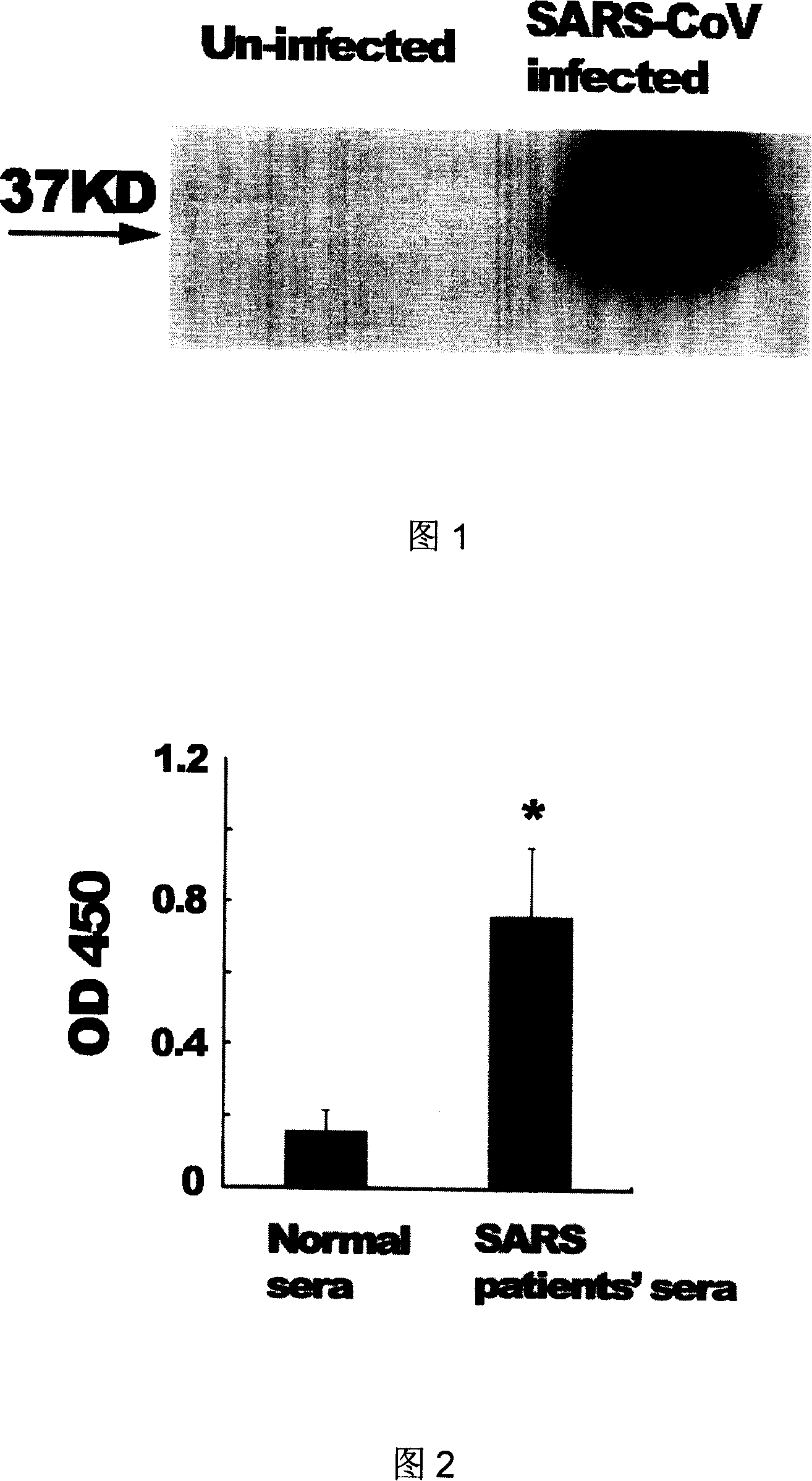
Monoclonal antibody reagents that recognize the SARS-coronavirus (SARS-HCoV) are needed urgently. In this report we describe the development and immunochemical characterisation of mAbs against the SARS-HCoV based upon their specificity, binding requirements, and biological activity. Initial screening by ELISA, using highly purified virus as the coating antigen, resulted in the selection of seventeen mAbs. Five mAbs exhibited Western immunoblot reactivity with the denatured spike protein, of which two demonstrated the ability to neutralize SARS-HCoV in vitro. Another four Western immunoblot-negative mAbs also neutralize the virus. These antibodies will be useful for the development of diagnostic tests, pathogenicity and vaccine studies.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
Vaccine compositions and methods of treating coronavirus infection
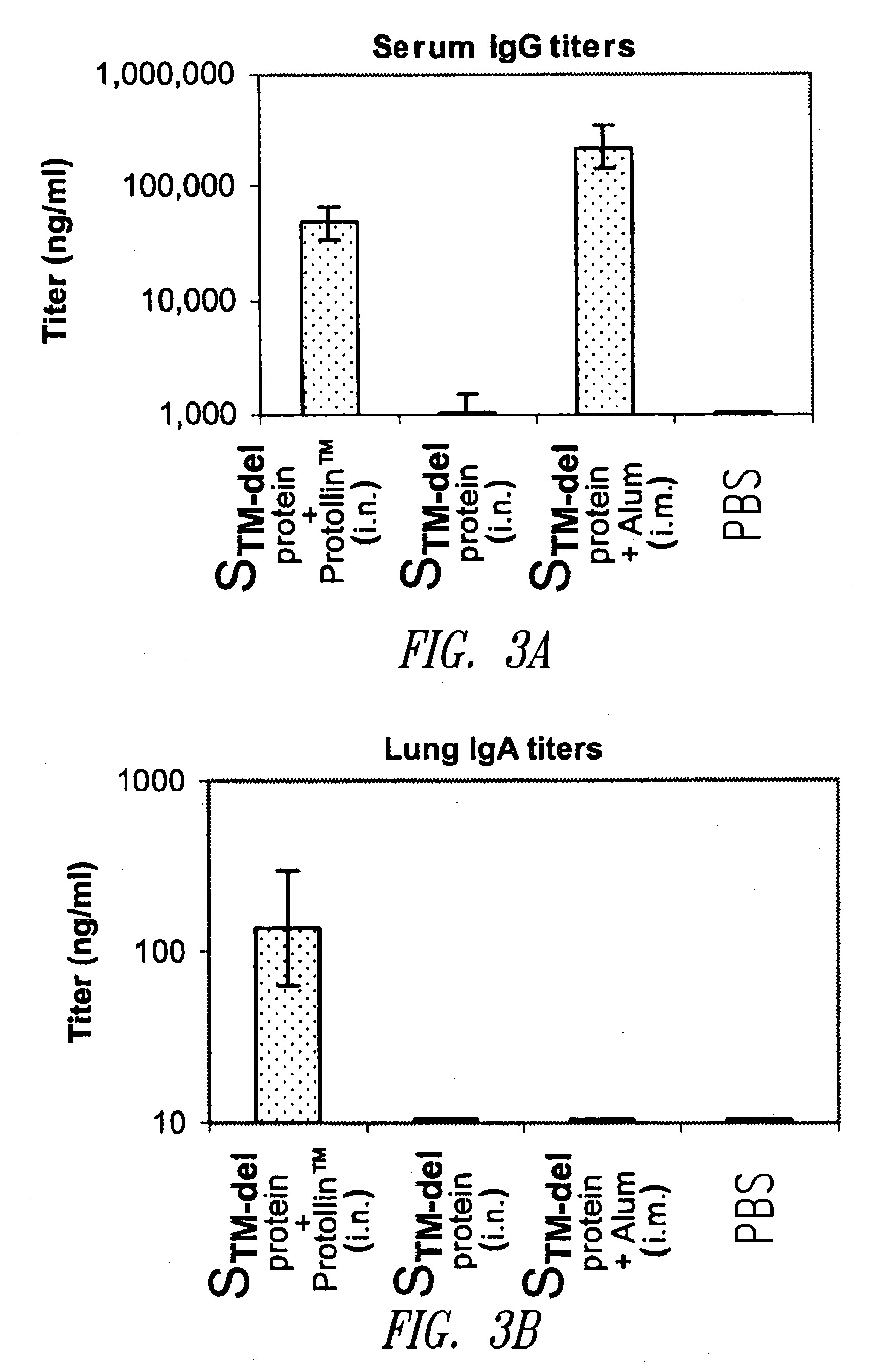
InactiveUS20060286124A1Elicit immune responseSsRNA viruses positive-sensePeptide/protein ingredientsAdjuvantSARS coronavirus
The present disclosure relates to compositions and methods for treating or preventing coronavirus infections. For example, compositions are provided that comprise a coronavirus S protein or N protein, fragment, or variant thereof, capable of eliciting a protective humoral and / or cell-mediated immune response, which compositions are useful for treating or preventing infection by coronavirus, such as the causative agent of SARS. Also, coronavirus S protein and N protein immunogen compositions are provided that include an adjuvant, such as Proteosome or Protollin, which may be used for treating or preventing infection caused by a coronavirus, such as a SARS coronavirus.
Owner:ID BIOMEDICAL CORP LAVAL
Compositions and methods for detecting severe acute respiratory syndrome coronavirus
InactiveUS20050095582A1Lower Level RequirementsReduce rateSsRNA viruses positive-senseMicrobiological testing/measurementSARS coronavirusSevere acute respiratory syndrome coronavirus
The invention provides compositions and methods for detecting the presence of SARS-coronavirus, for screening anti-SARS coronavirus agents and vaccines, and for reducing infection with plus-strand RNA viruses such as SARS-coronavirus.
Owner:DIAGNOSTIC HYBRIDS +1
Peptide-based diagnostic reagents for SARS
InactiveUS20050100883A1Improve quality controlBiocideSsRNA viruses positive-senseSARS coronavirusAssay
The present invention is directed to antigenic peptides and peptide compositions selected from the Membrane glycoprotein (M), the Spike glycoprotein (S), and the Nucleocapsid (N) protein antigens of the SARS coronavirus (SCoV). The present invention is also directed to methods of use of the peptides of the invention, e.g., for the detection of SARS-associated antibodies. Detection methods include enzyme-linked immunosorbent assay (ELISA) or other immunoassay procedures.
Owner:UNITED BIOMEDICAL INC
Methods for determining the presence of sars coronavirus in a sample
InactiveUS20100279276A1High sensitivityHigh mutation rateMicrobiological testing/measurementFermentationSARS coronavirusTest sample
Methods for determining the presence of SARS-CoV in a test sample that include targeting the SARS-CoV 5′ leader sequence or the SARS-CoV 3′ terminal sequence.
Owner:GEN PROBE INC
Polyhydroxy stilbenes compound preparation and uses as drugs for suppressing SARS
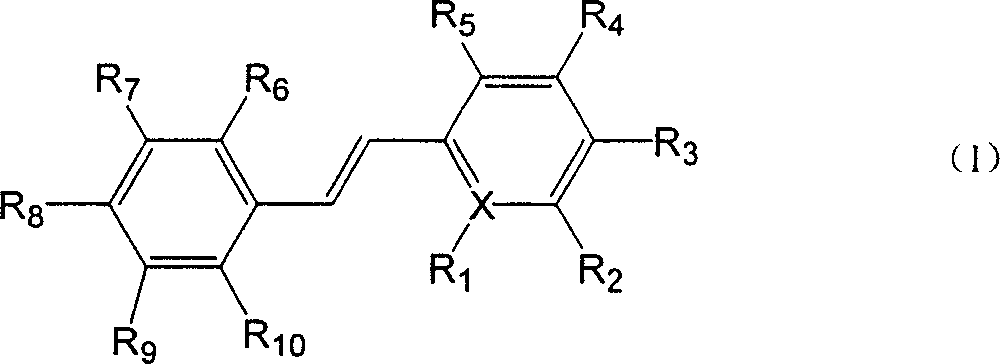
InactiveCN1736986AMeet the requirements of therapeutically effective doseOrganic chemistryHydroxy compound active ingredientsMethyl aldehydeTriethylphosphite
The invention provides a group of polyhydroxy stilbene compounds, their preparing process and use for suppressing and eradicating SARS coronavirus. The preparing process comprises, preparing phosphonic ester by reacting multi-alkyl substituted chloro (bromo) methoxyl or pyridine compounds with triethyl phosphate, then reacting phosphonate ester compound with multi-alkyl (oxy) phenylpyridine methyl aldehyde to obtain multi-alkyl (oxy) stilbene compounds. Finally acting with boron tribromide to obtain polyhydroxy stilbene compounds with substituent groups.
Owner:DALIAN UNIV OF TECH +1
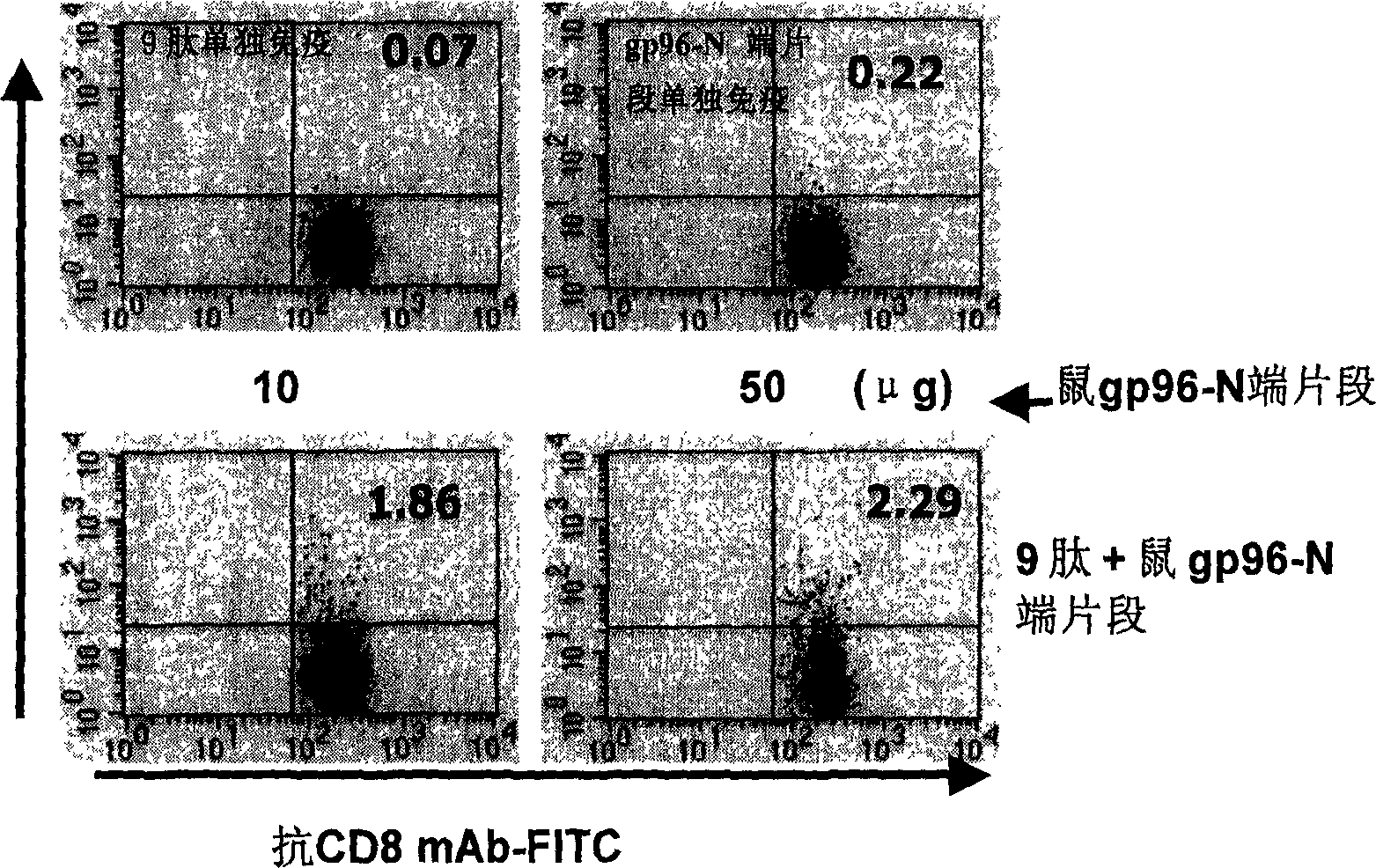
Immunological adjuvant, and its application in preparing vaccine and medicine for anti-virus
InactiveCN1718243AImprove immune activityReach clearAntiviralsAntibody medical ingredientsAnti virusDisease
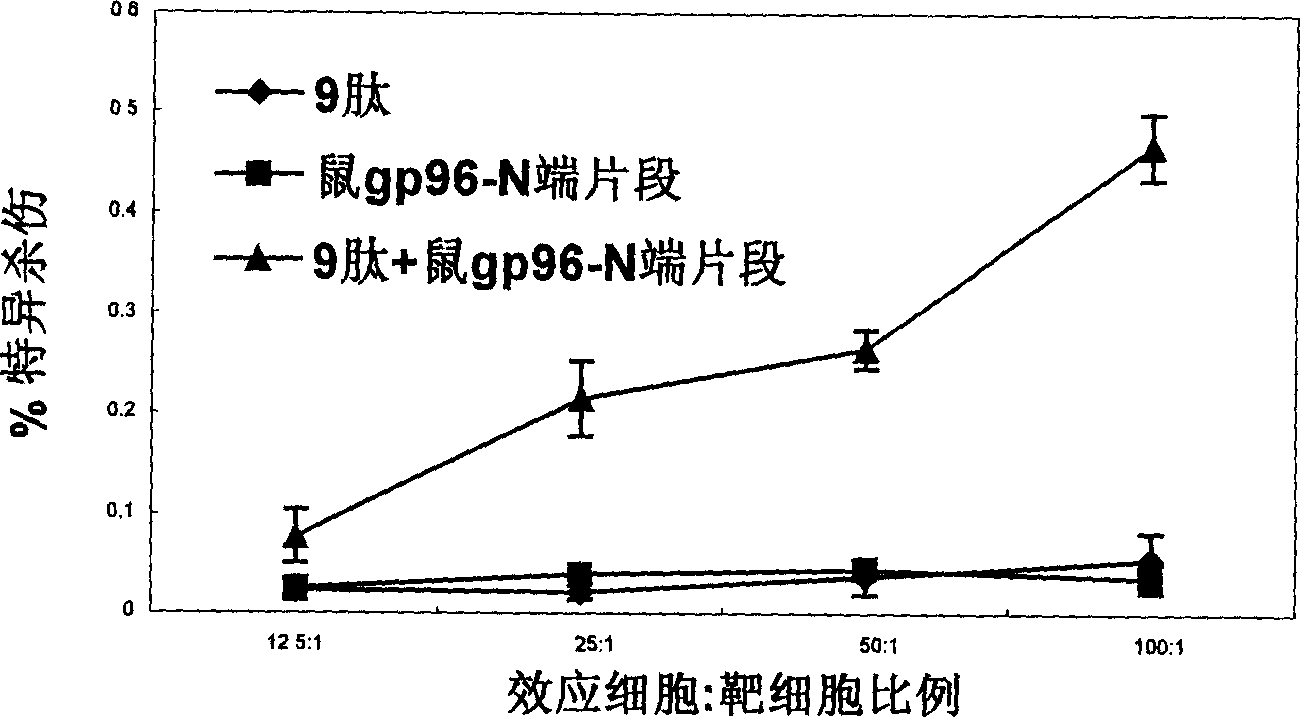
An immunoadjuvant used to prepare the antiviral vaccine or medicine for increasing the immune activity of the antigens for HBV, HCV, SARS coronavirus, fowl influenza virus, etc is a kind of human or animal's novel heat shock proteins gp96, hsp108 and hsp70.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Isolation and characterization of the precursor virus of human sars virus: sars-associated corona virus-like virus
ActiveUS20080069839A1SsRNA viruses positive-senseSugar derivativesViunalikevirusSevere acute respiratory syndrome
The present invention relates to isolation and characterization of a class of isolated novel viruses which is the precursor of the virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). The precursor virus which is a SARS coronavirus-like virus (“SCoV-like virus”) is identified to be morphologically and phylogenetically similar to hSARS virus. The present invention relates to a nucleotide sequence comprising the genomic sequence of the SCoV-like virus. The invention further relates to nucleotide sequences comprising a portion of the genomic sequence of the SCoV-like virus. The invention also relates to the deduced amino acid sequences of the SCoV-like virus. The invention further relates to the nucleic acids and peptides encoded by and / or derived from these sequences and their use in diagnostic methods and therapeutic methods. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences and antibodies directed against polypeptides encoded by the nucleotide sequences. Furthermore, the invention relates to vaccine preparations comprising the SCoV-like virus, including recombinant and chimeric forms of said virus.
Owner:THE UNIVERSITY OF HONG KONG
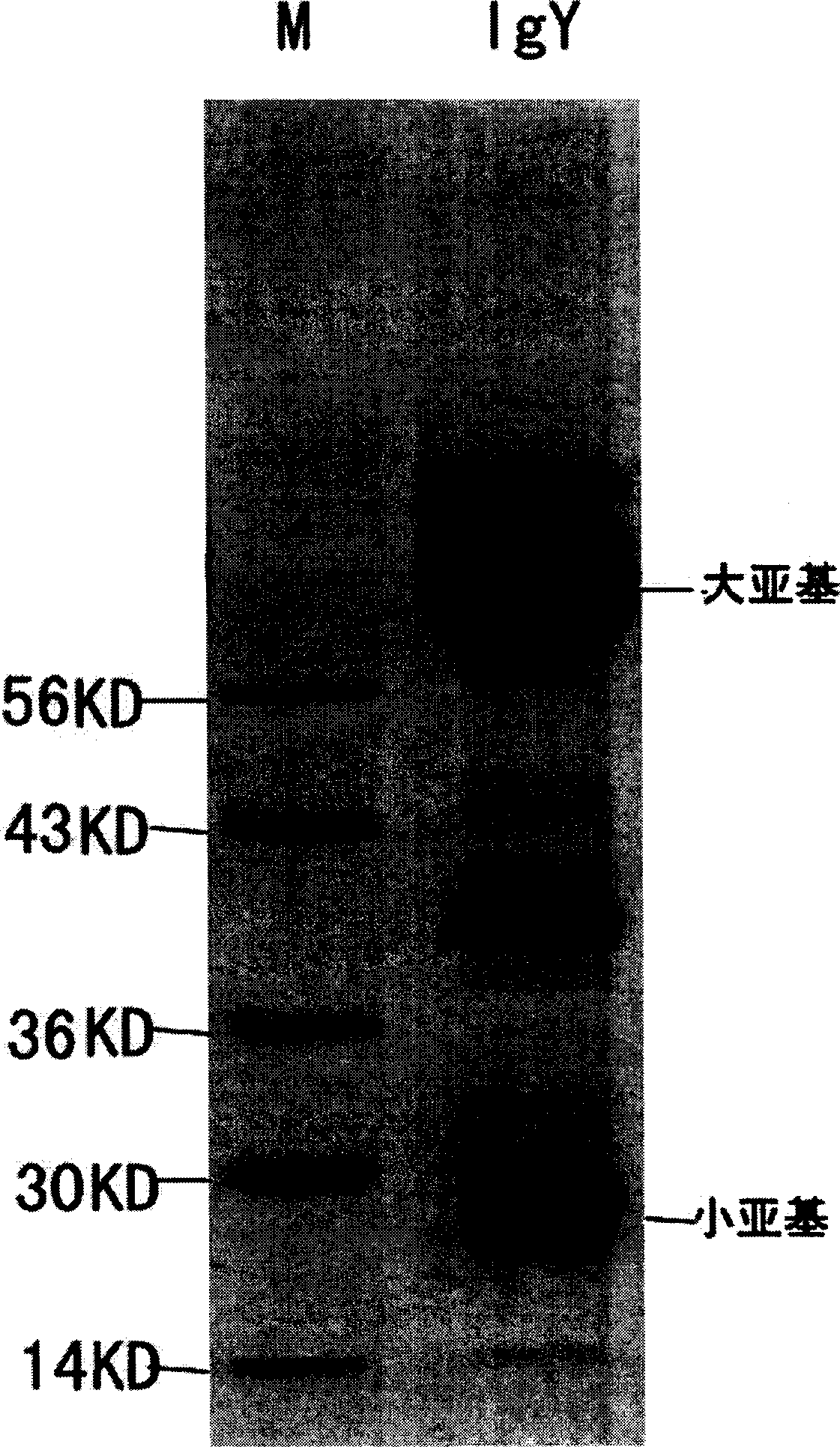
Yolk antibody of anti SARS coronavirus and its preparation method and liquid preparation
InactiveCN1556113AEasy to prepareFast preparation methodEgg immunoglobulinsImmunoglobulins against virusesYolkEpitope
A yolk antibody for SARS coronavirus is prepared through injecting the antigen, which may be recombinant genetic protein S, M, or E,or the antigen epitope for said protein able to represent SARS coronavirus, or the synthetic polypeptide of said protein, etc, in health hen for primary immunizing, booster immunizing, collecting its egg, extracting yolk, and extracting the yolk antibody from it. It can also be prepared to become liquid preparation. It can be used to prevent SARS.
Owner:BIOINFORBODY
Compositions and methods for detecting severe acute respiratory syndrome coronavirus
InactiveUS7129042B2Improve productivityHigh sensitivitySsRNA viruses positive-senseMicrobiological testing/measurementSARS coronavirusSevere acute respiratory syndrome coronavirus
The invention provides compositions and methods for detecting the presence of SARS-coronavirus, for screening anti-SARS coronavirus agents and vaccines, and for reducing infection with plus-strand RNA viruses such as SARS-coronavirus.
Owner:DIAGNOSTIC HYBRIDS +1
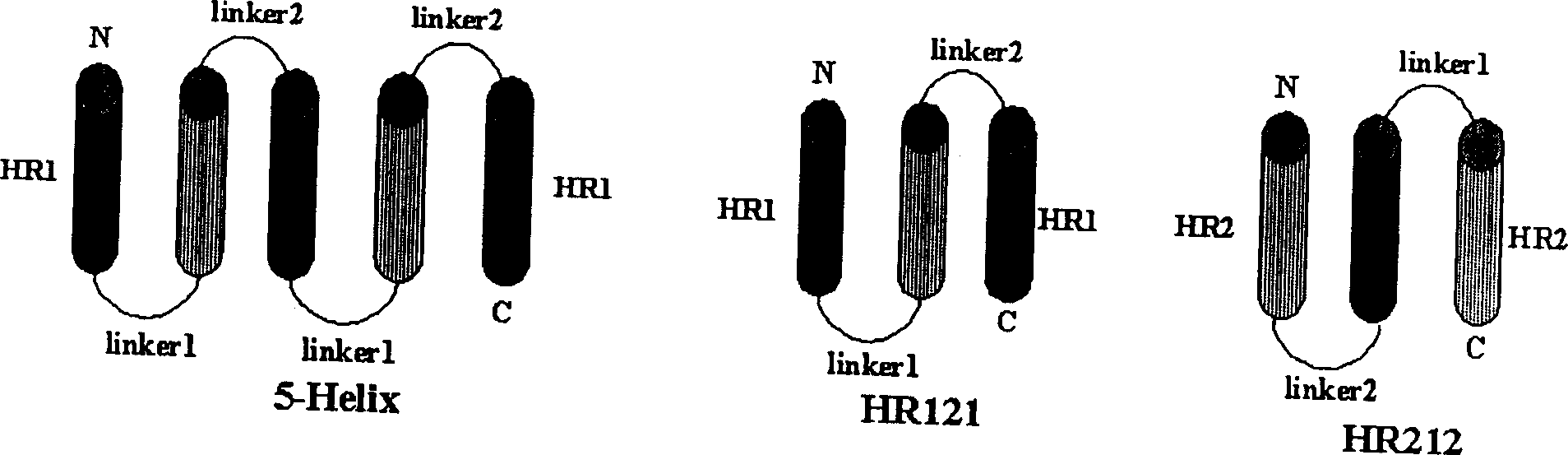
Polypeptide medicine for inhibiting SARS coronavirus, and derivatives and use thereof
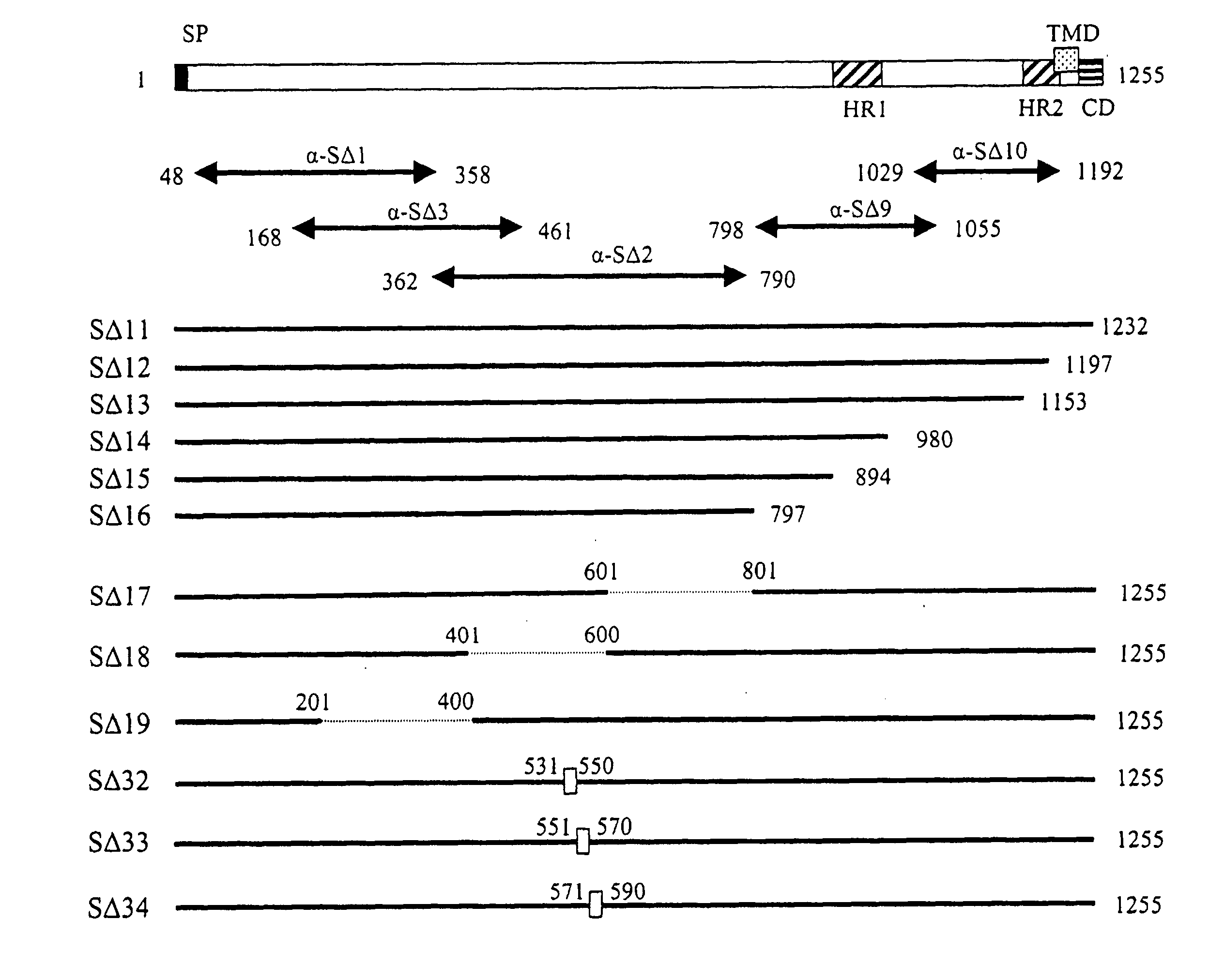
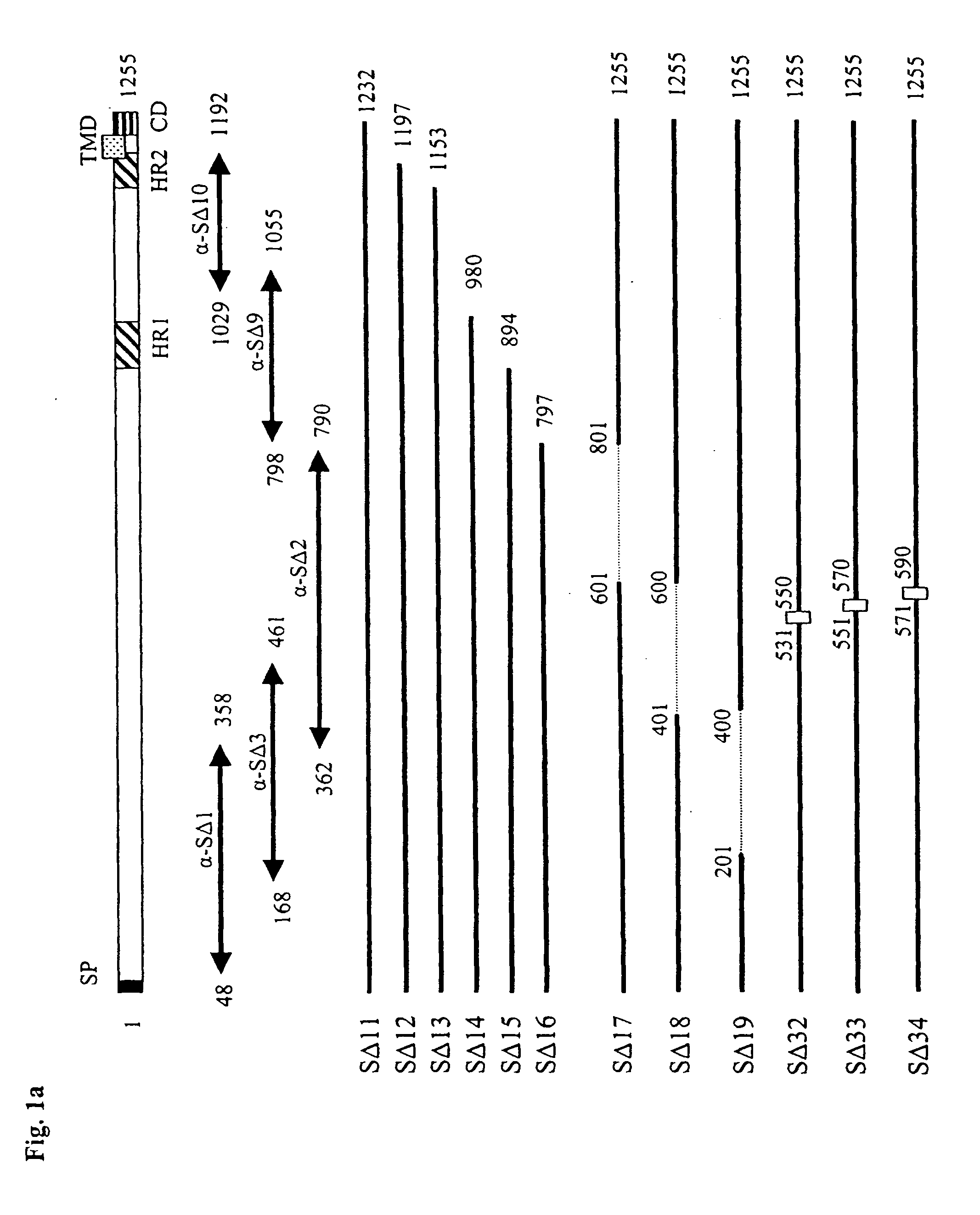
The invention provides a method for blocking SARS coronavirus to infect cell with polypeptide or its derivative. The cistine series of polypeptide comes from two heptad repeat districts HR1 and HR2 of SARS coronavirus spike(S) protein. The invention also provides a method for producing the functional similarity with HR1 and HR2. the invention provides a method for producing HR polypeptide and its derivant with coalescence expression method. All the peptides have rejection capability to SARS.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Fusion proteins of recombinant sars coronavirus structural proteins, their production and uses
InactiveUS20100150923A1SsRNA viruses positive-senseAntibody mimetics/scaffoldsSARS coronavirusStructural protein
Fusion proteins of recombinant SARS coronavirus structural proteins, their production and uses are provided. An optimized SARS coronavirus S protein gene which can be highly expressed in the mammalian cell strains and SARS coronavirus S protein variants comprising deletion, modification or mutation amino acids 318-510 corresponding to SARS coronavirus S protein are also provided.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Method of diagnosing SARS corona virus infection
The present invention relates to methods of detecting the presence or absence of antibody to SARS coronavirus in a sample, based on the discovery that the S, M, E, N and U274 proteins of SARS coronavirus are antigenic. Such methods may be used to diagnose whether a patient has been infected by or exposed to SARS coronavirus. Antibodies directed against S, M, E, N and U274 proteins of SARS are also provided.
Owner:AGENCY FOR SCI TECH & RES
Method and kit for detecting pathogens of infectious diseases
InactiveCN101603096AThe detection process is fastImprove efficiencyMicrobiological testing/measurementMicroorganism based processesSARS coronavirusAvian influenza virus
The invention discloses a method for detecting pathogens of infectious diseases, which possibly exist in a biological sample. The pathogens of the infectious diseases comprise avian influenza virus H5 subtype, avian influenza virus H7 subtype, SARS coronavirus, hanta virus, plague yersinia pestis and bacillus anthracis. The method comprises amplifying a nucleic acid fragment of a biological sample and detection by a probe. The invention also provides a primer for amplification and a probe for detection. The invention also provides a kit including the primer. The method has the advantages of high flexibility, strong specificity, easy operation, wide sample range, the detection for the pathogens of various infectious diseases at the same time and the suitability for early diagnosis of respiratory infectious diseases.
Owner:HAI KANG LIFE
Compositions and methods for determining the presence of SARS coronavirus in a sample
InactiveUS20060134609A1High sensitivityMinimizes reduction in sensitivitySugar derivativesMicrobiological testing/measurementSARS coronavirusTest sample
The present invention relates to oligonucleotides useful for determining the presence of SARS coronavirus in a test sample. The oligonucleotides of the present invention may be incorporated into detection probes, capture probes and amplification oligonucleotides, or used in various combinations thereof.
Owner:GEN PROBE INC
SARS virus antibody detecting method, rapid diagnosis kit and preparation method
InactiveCN1570638AImprove featuresIncreased sensitivityMicrobiological testing/measurementBiological testingSerum igeSARS coronavirus
This invention relates to a detection mode of SARS virus antibody and a preparing method of the box for rapid diagnosis reagent. The checking method includes that (1) putting antihuman Ig antibody marked by colloidal gold in the carrier, and coating detecting thread made of variant antigen of SARS coronavirus and controlling thread made of antipest IgG antibody in the detecting carrier connecting with the marked one. (2) putting human serum in the marked carrier. The box for rapid diagnosis reagent includes checkerboard marked by variant antibody of SARS coronavirus, which comprises water-absorbing layer of adding-kind edge, detecting layer and water-absorbing layer of water-uptake edge. There is antihuman Ig antibody layer between detecting layer and water-absorbing layer of water-uptake edge, and in the detecting layer there is detecting thread made of variant antigen of SARS coronavirus and controlling thread made of antipest IgG antibody coating.
Owner:LANZHOU YAHUA BIOTECH
Sars coronavirus s proteins and uses thereof
InactiveUS20070116716A1SsRNA viruses positive-senseViral antigen ingredientsSARS coronavirusProtein S
The present invention relates to the use of a matured, glycosylated Spike (S) protein of SARS Coronavirus, fragments of the S protein, methods for producing the same, their use in detecting SARS infection, and their use or the use of their corresponding antibodies to treat patients suffering from SARS.
Owner:AGENCY FOR SCI TECH & RES
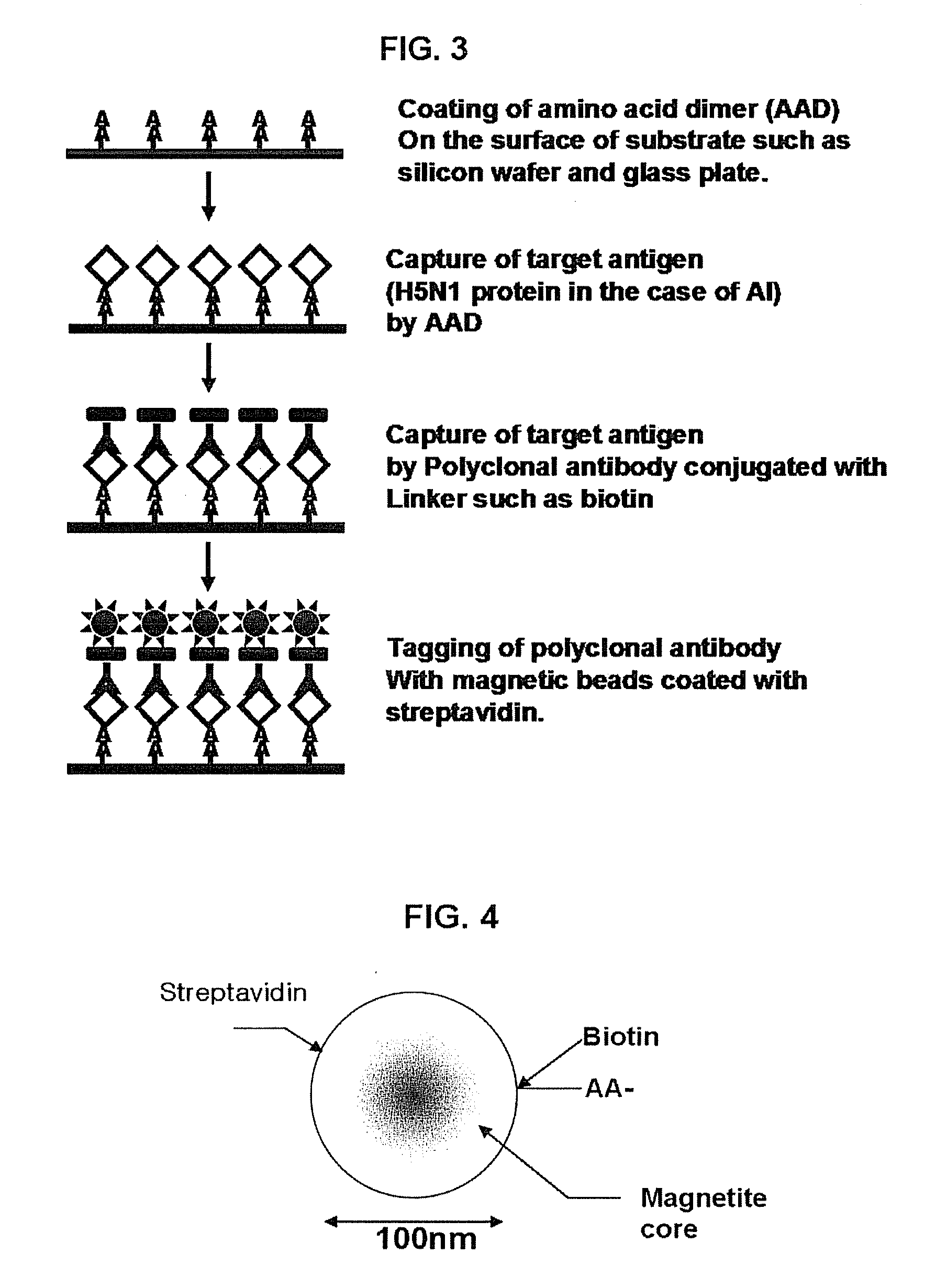
Assay system and methods for detecting SARS-CV
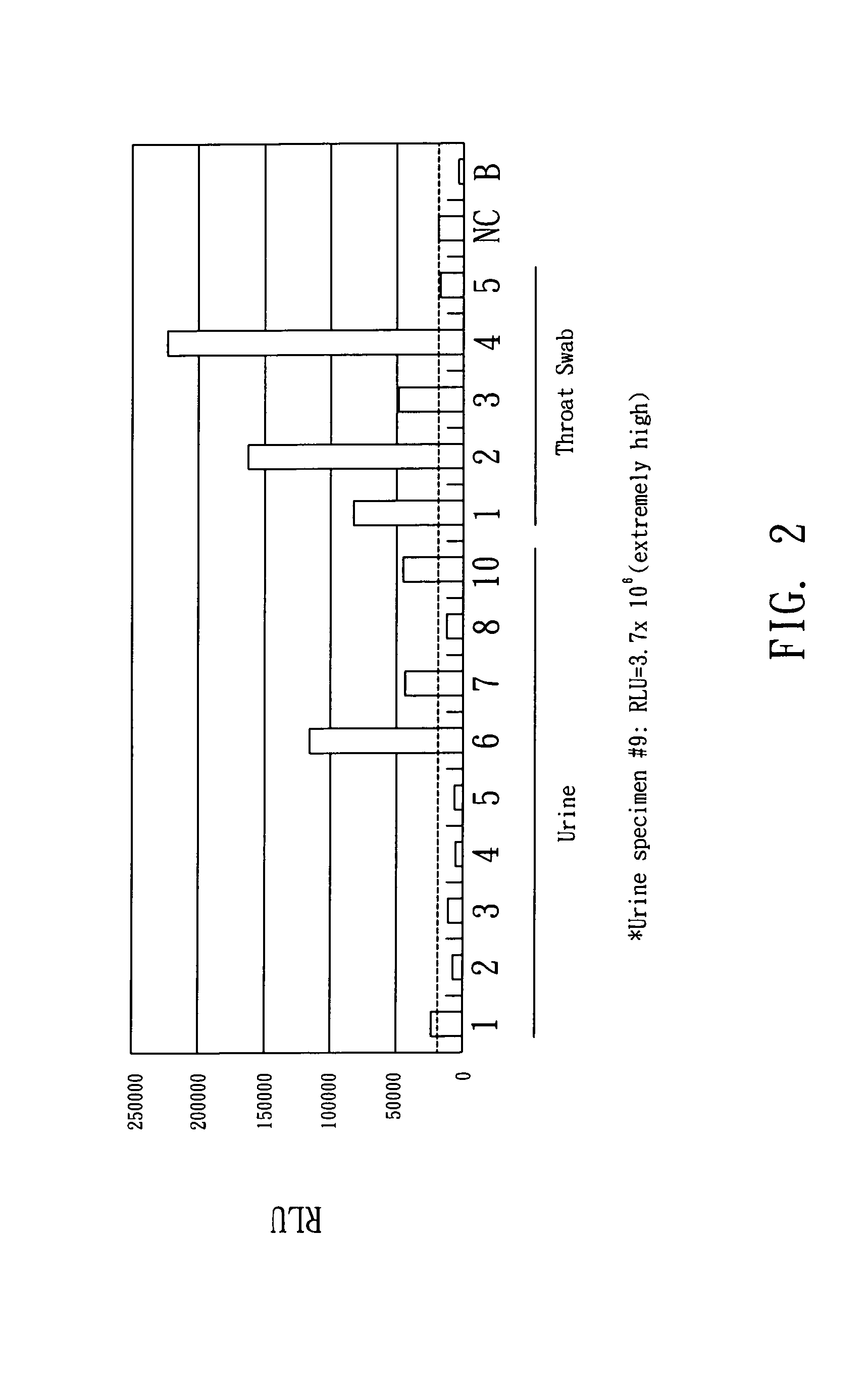
The present invention relates to an assay system and methods for detecting SARS coronavirus (SARS-CV) from the samples (especially for urine) of suspected patient in the control of SARS to provide updated information of prognosis as well as the criteria for discharging a recovered patient from a hospital. The present invention also relates to an apparatus for performing the integration of thermal and magnetic control in the same apparatus to largely reduce the time of hybridization less than 20 minutes and the whole process of SARS-CV detection is less than 5 hours.
Owner:ASIAGEN CORP
Reagent for colloidal gold chromatographic analysis of SARS coronavirus antigen
The present invention relates to one kind of reagent for colloidal gold chromatographic analysis of SARS coronavirus antigen. The reagent consists of coating film, absorbent paper, and glass fiber film coated with gold mark antibody adhered successively together. The present invention is used in colloidal gold chromatographic analysis of SARS coronavirus antigen in hospital, airport, custom, household, etc. Using it can obtain detection result in several minutes and this is favorable to prevent diffusion of epidemic situation.
Owner:GUANGZHOU WONDFO BIOTECH
Triazine compound and application thereof in preparation of antiviral drugs
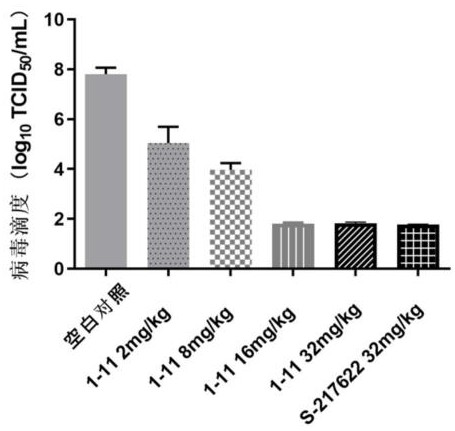
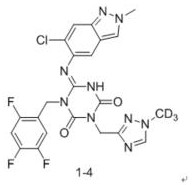
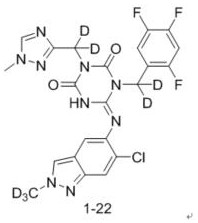
ActiveCN114507221AStrong inhibitory activityIncrease blood concentrationOrganic chemistryAntiviralsViral infectious diseaseSevere acute respiratory syndrome-related coronavirus
The invention relates to a triazine compound with a structure represented by a formula A, or a stereoisomer, a prodrug, an active metabolite or a pharmaceutically acceptable salt, a solvate or a crystal form thereof, a pharmaceutical composition of the triazine compound, the stereoisomer, the prodrug, the active metabolite or the pharmaceutically acceptable salt, the solvate or the crystal form, and a use method of the triazine compound. In addition, the invention also relates to a method for preparing a 3C-like cysteine protease inhibitor or a medicine for treating and / or preventing virus infectious diseases. In particular, the present invention relates to the use for treating viral infectious diseases such as Middle East Syndrome Related Coronavirus (MERS-CoV), Severe Acute Respiratory Syndrome Related Coronavirus (SARS-CoV), Influenza A virus, Influenza B virus, Novel Coronavirus Pneumonia (COVID-19) and the like.
Owner:YAOKANG ZHONGTUO (JIANGSU) PHARMA TECH CO LTD
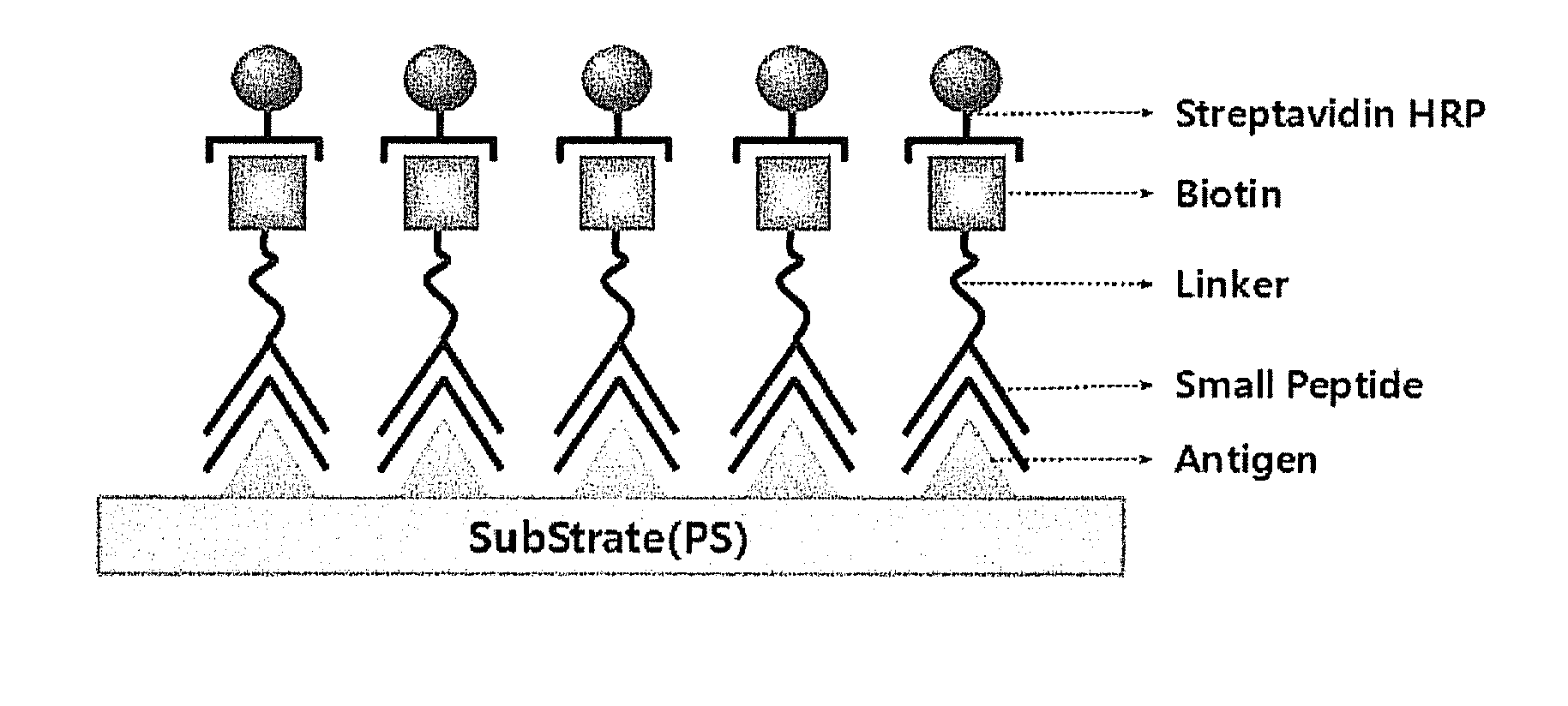
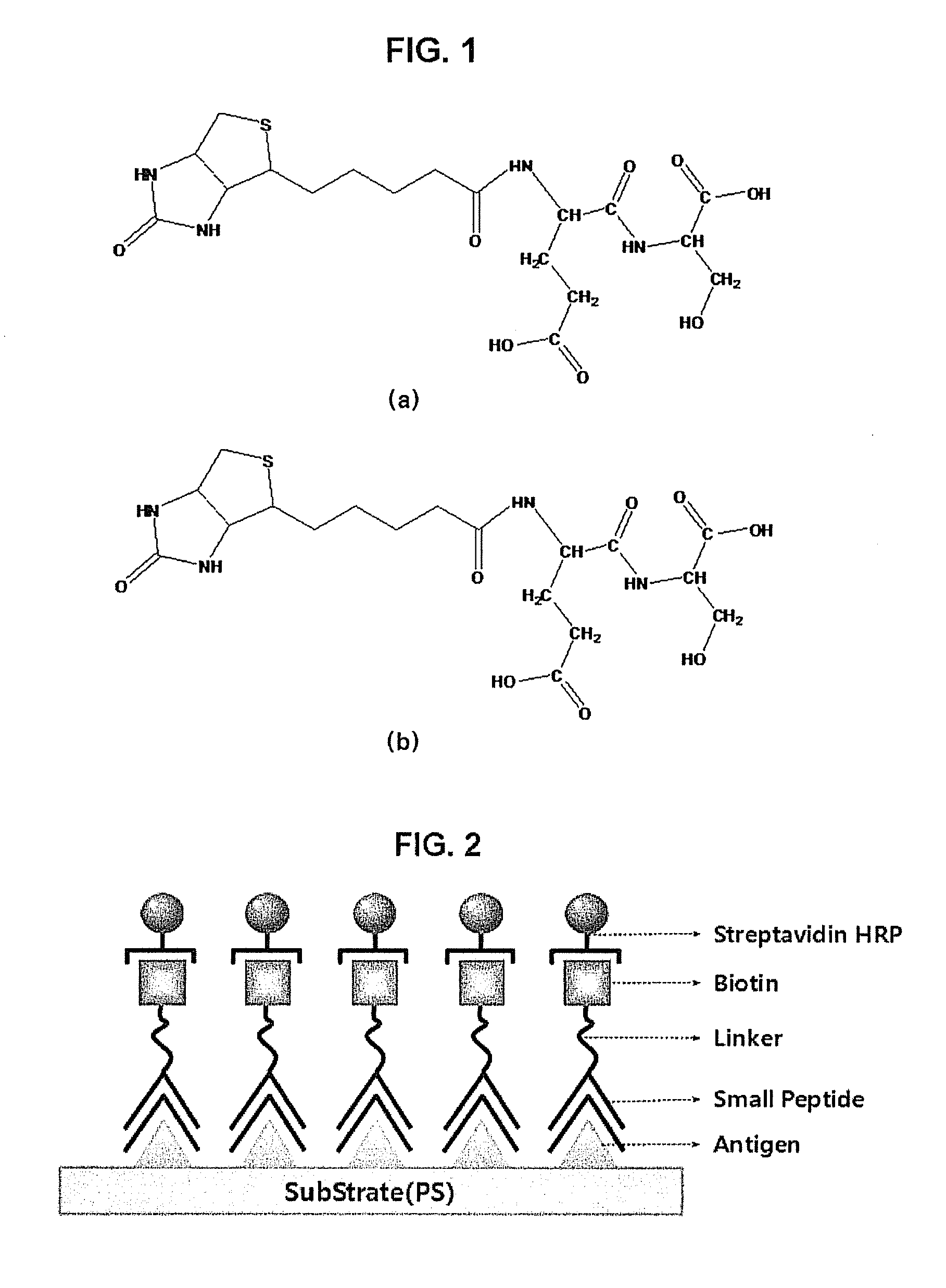
Peptide compounds for detecting or inhibiting sars coronavirus and application thereof
InactiveUS20100304363A1Economical profitInhibit the activity of the SARS coronavirusOrganic active ingredientsPeptide/protein ingredientsDipeptideSARS coronavirus
Disclosed herein are peptide compounds and the application thereof to the detection and inhibition of SARS coronavirus. Composed of dipeptides, the compounds for detecting and inhibiting SARS coronavirus can be readily synthesized and produced at low cost. In addition, they can be stored safely for a long period of time. The dipeptide compounds are useful as inhibitors of SARS coronavirus as well as acting as excellent capturing materials of SARS coronavirus.
Owner:ELECTRONICS & TELECOMM RES INST
Novel coronal virus strain and medicinal use thereof
The present invention relates to a new strain of coronavirus and its application. Said invention utilizes the throat-swabbed cell colture of SARS patient to separate out a new type coronavirus, called by name of SARS coronavirus Fudan I strain. By infecting Vero E 6 cell and patient's serum the indirect FA display is positive, after the influenza virus A and B, RS virus and pathogens of pneumococcus, etc. are excluded, the tests show that said separated coronavirus is a variant strain of new coronavirus. Said strain can be used for providing basis for preparing SARS diagnosis reagent and researchnig and producing medicine for resisting and curing SARS disease.
Owner:FUDAN UNIV
Small molecular inhibiting agent for coronavirus main proteinase, its preparation method and uses
The present invention is serial small molecular inhibitor designed based on the crystal structure of main proteinase of SARS coronavirus and in the structural general expression as shown. The present invention also provides the preparation process of the small molecular inhibitor and its use in preparing medicine for preventing and treating various kinds of coronavirus infection.
Owner:TSINGHUA UNIV +1
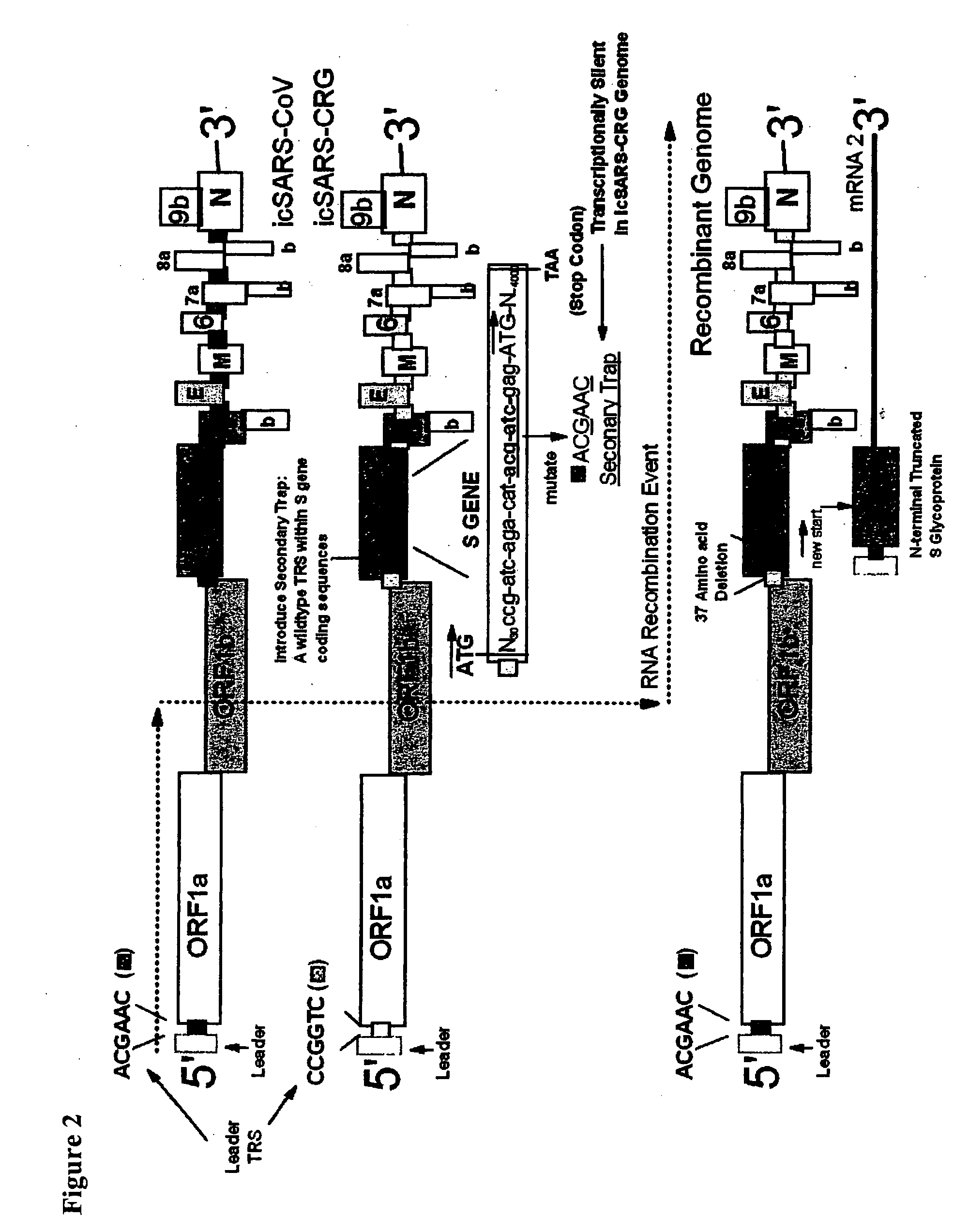
Methods and compositions for infectious cDNA of SARS coronavirus
InactiveUS20060240530A1Avoid infectionSsRNA viruses positive-senseVirus peptidesSARS coronavirusViral vector
The present invention provides a cDNA of a severe acute respiratory syndrome (SARS) coronavirus, recombinant SARS coronavirus vectors, and SARS coronavirus replicon particles. Also provided are methods of making the compositions of this invention and methods of using the compositions as immunogens and / or vaccines and / or to express heterologous nucleic acids.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
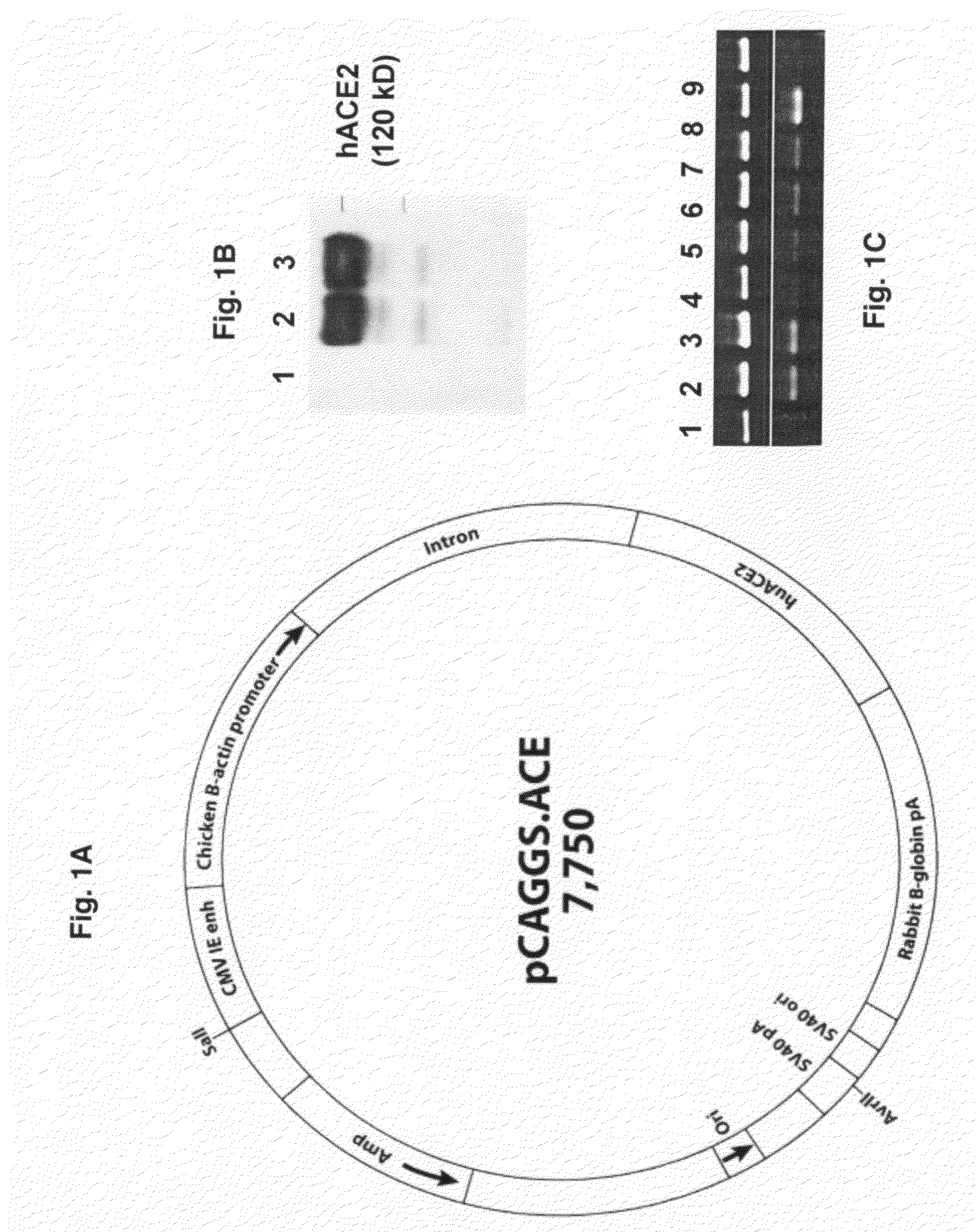
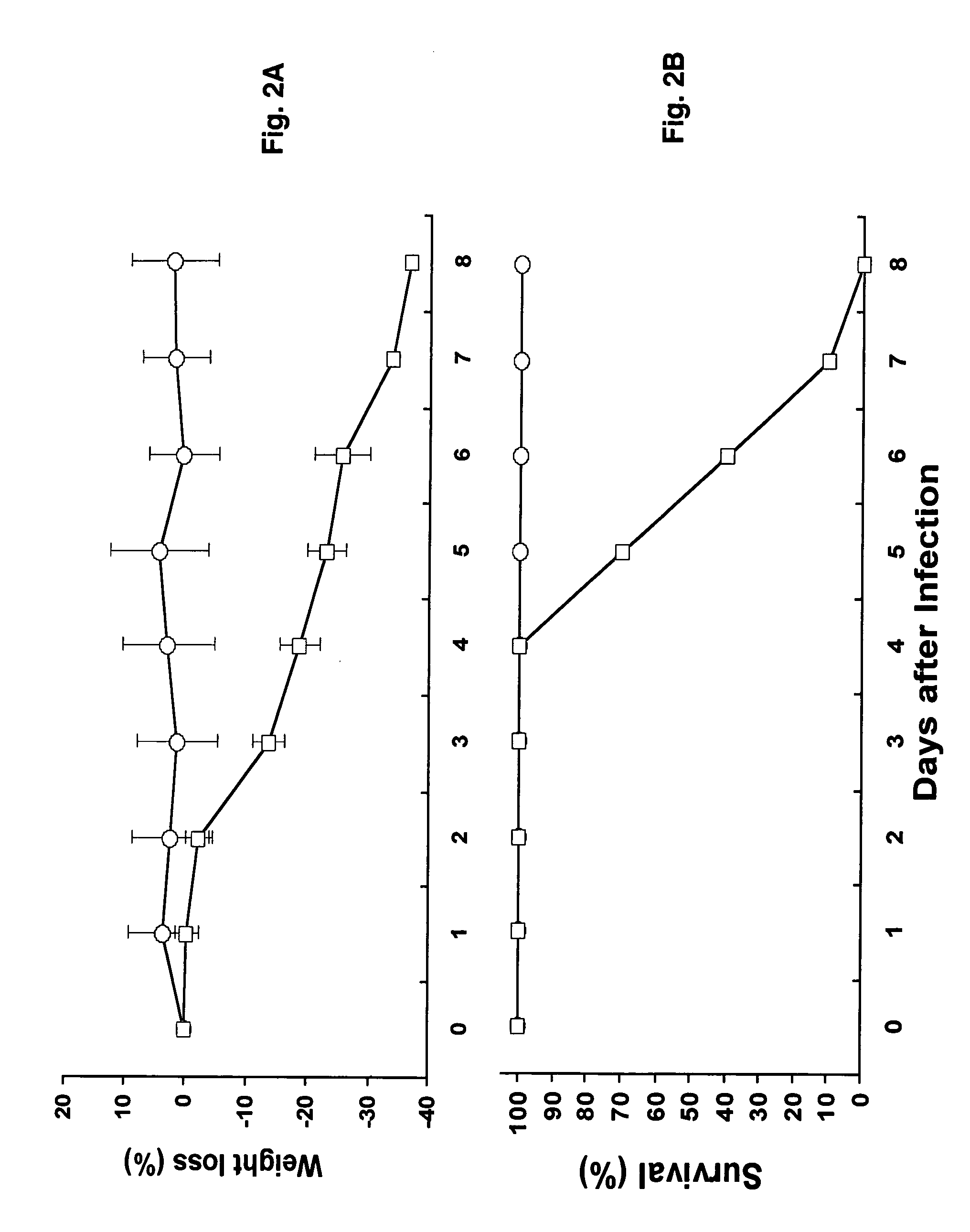
Transgenic Mouse Lines Expressing Human Ace2 and Uses Thereof
InactiveUS20090083865A1Prevents and alleviates symptomShortens courseHydrolasesBiological material analysisClinical manifestationMortality rate
Animal models for severe acute respiratory syndrome-coronavirus infection of humans are needed to elucidate SARS pathogenesis and develop vaccines and antivirals. Transgenic mice were developed expressing human angiotensin-converting enzyme 2, a functional receptor for the virus, under the regulation of a global promoter. A transgenic lineage, designated AC70, was among the best characterized against SARS coronavirus infection, showing weight loss and other clinical manifestations before reaching 100% mortality within 8 days after intranasal infection. Inflammatory mediators were also detected in these tissues, coinciding with high levels of virus replication. In contrast, infected transgene-negative mice survived without showing any clinical illness. The severity of the disease developed in these transgenic mice, AC70 in particular, makes these mouse models valuable not only for evaluating the efficacy of antivirals and vaccines, but also for studying SARS coronavirus pathogenesis and infection by other coronaviruses utilizing human ACE2 for viral entry into cells.
Owner:CHAN TEH SHENG +3
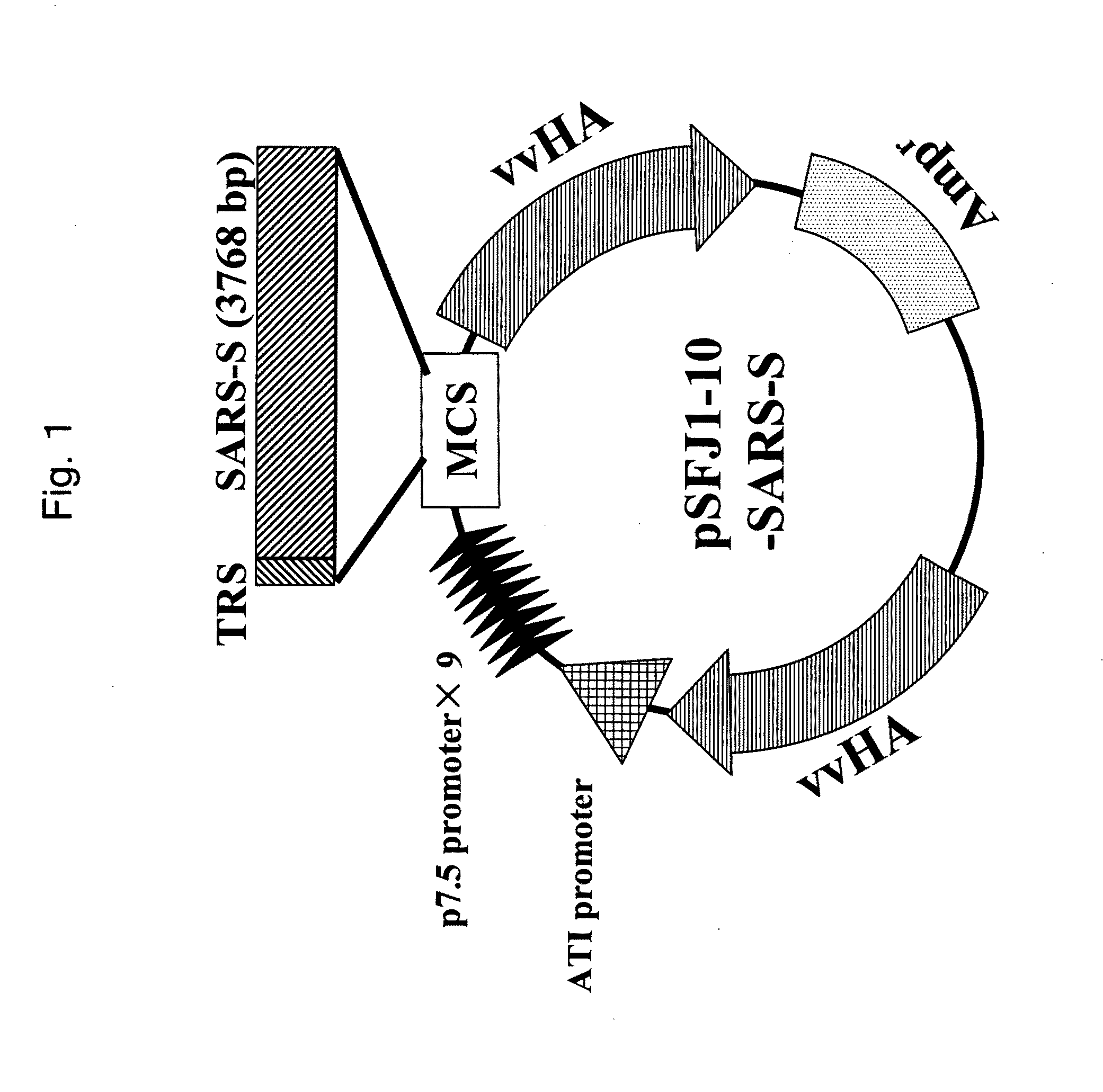
SARS-Cov gene vaccine based on epi-position and its contruction
InactiveCN1657102AReduced risk of infection-enhancing effectsOvercome the shortcomings of weak mutation ability and easy to produce toleranceGenetic material ingredientsAntiviralsSARS coronavirusAutoimmune disease
A SARS-Cov gene vaccine based on epitope is configured from the carrier which is a plasmid able to be used for human body and the target antigen which is several B cell epitopes in the extrinsic B protein antigen of human SARS coronavirus through codon optimizing and genetic engineering. Its preparing process is also disclosed.
Owner:FUDAN UNIV
SARS coronavirus polypeptide antigen and application thereof
InactiveCN101085812AEasy to operateImprove featuresImmunoglobulins against animals/humansDepsipeptidesEpitopeSARS coronavirus
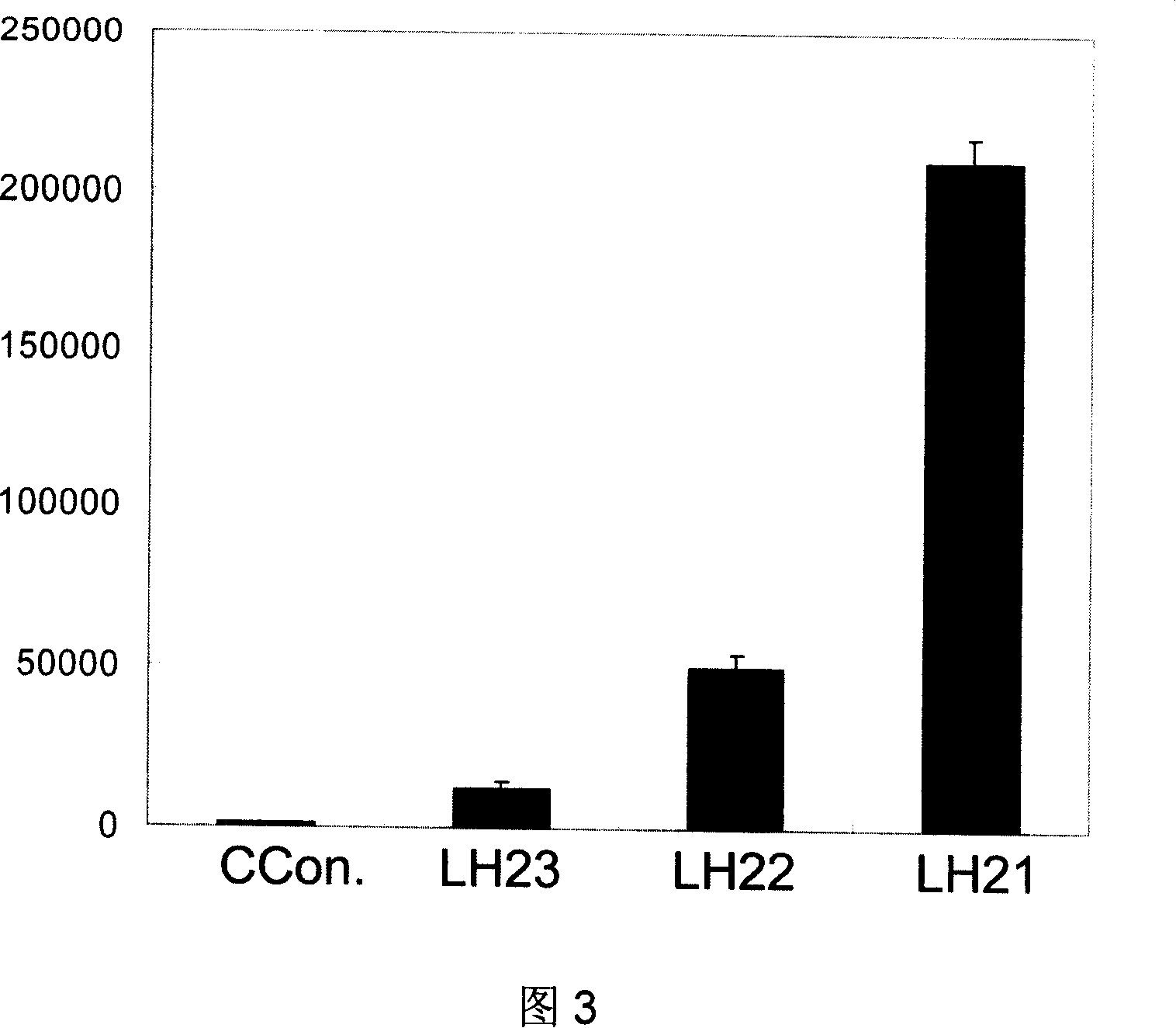
The invention relates to a kind of polypeptide for SARS coronavirus and its application. The amnio acid squence is SEQ ID NO: 1 or its derivant squence. The sifted antigen epitope LH21 is N section of protein coded by ORF3a gene, its immunity is strong, and the clinical diagnosis value is good. The antibody prepared with said epitope can be used to check virus antigen.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Recombinant virus and use thereof
InactiveUS20090214587A1Highly safe in preventing SARS infection and onsetSsRNA viruses positive-senseVectorsSARS coronavirusCoronavirus vaccination
The present invention provides a recombinant virus which is efficacious and highly safe in preventing the onset of SARS infection and a vaccine for SARS coronavirus containing the same. The recombinant virus of the invention can express a SARS coronavirus gene.
Owner:POST GENOME INST CO LTD
Gene vaccine for anti SARS coronal virus and use thereof
InactiveCN1449826AAdvantage preventionAdvantageous therapeuticGenetic material ingredientsAntiviralsNucleotideIn vivo
The present invention discloses a gene vaccine for resisting SARS coronavirus and its application, including eucaryotic expressino plasmid containing total or partial gene fragment of total-length gene sequence of SARS coronavirus S protein. The nucleotide sequence of coded SARS coronavirus S protein can be operatively connected with a promotor, then it can express the complete or partial polypeptide of the coded SARS coronavirus S protein in vivo. The described gene vaccine can be used for immunizing host including human body and rodent, for example mouse to make it produce specific protective body fluid immune and cell immune to resist the infection of SARS coronavirus. Said invention is good in stability, convenient in production and low in cost.
Owner:WUHAN UNIV
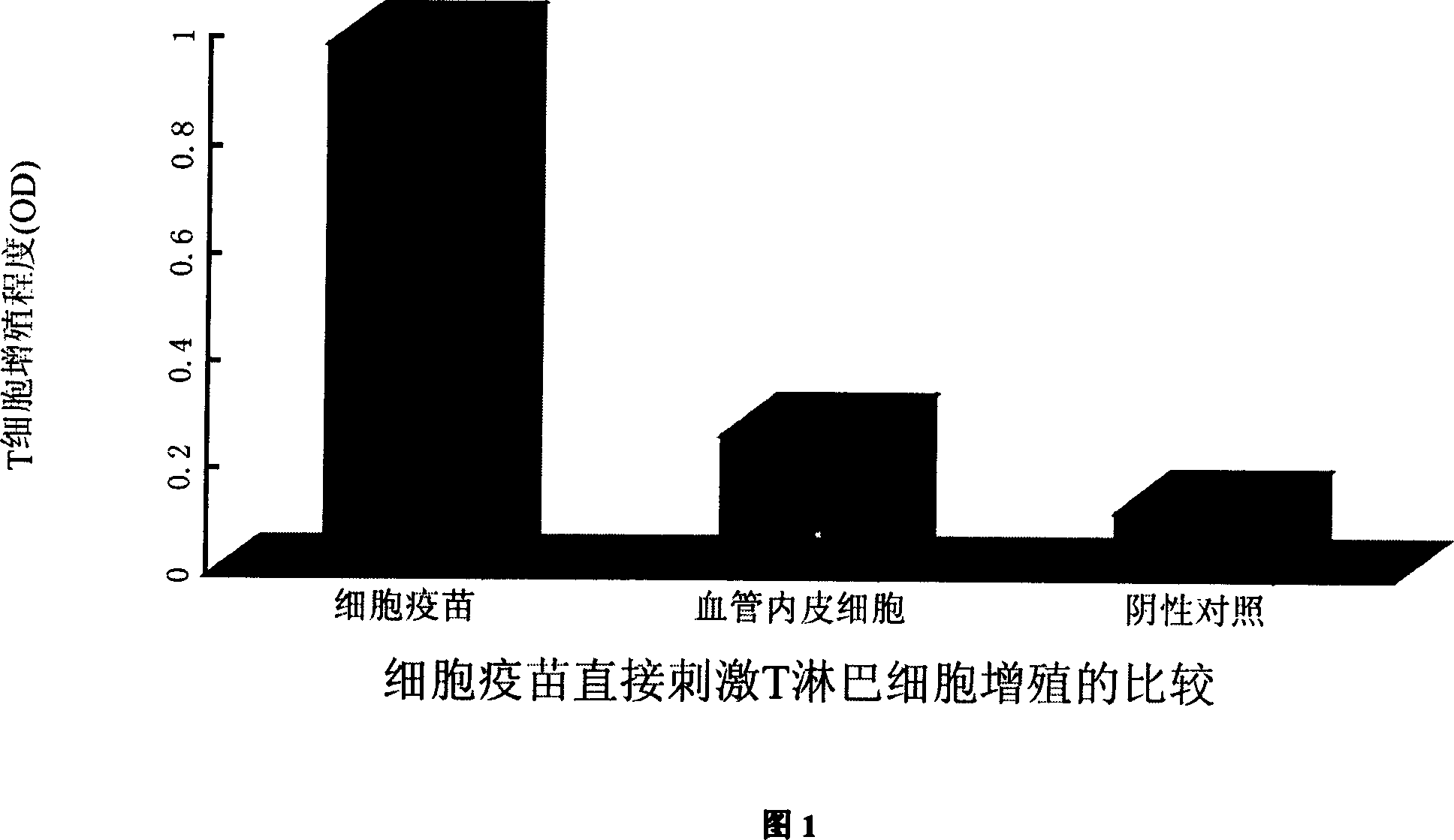
SARS coronary virus resistant cell vaccine and its uses
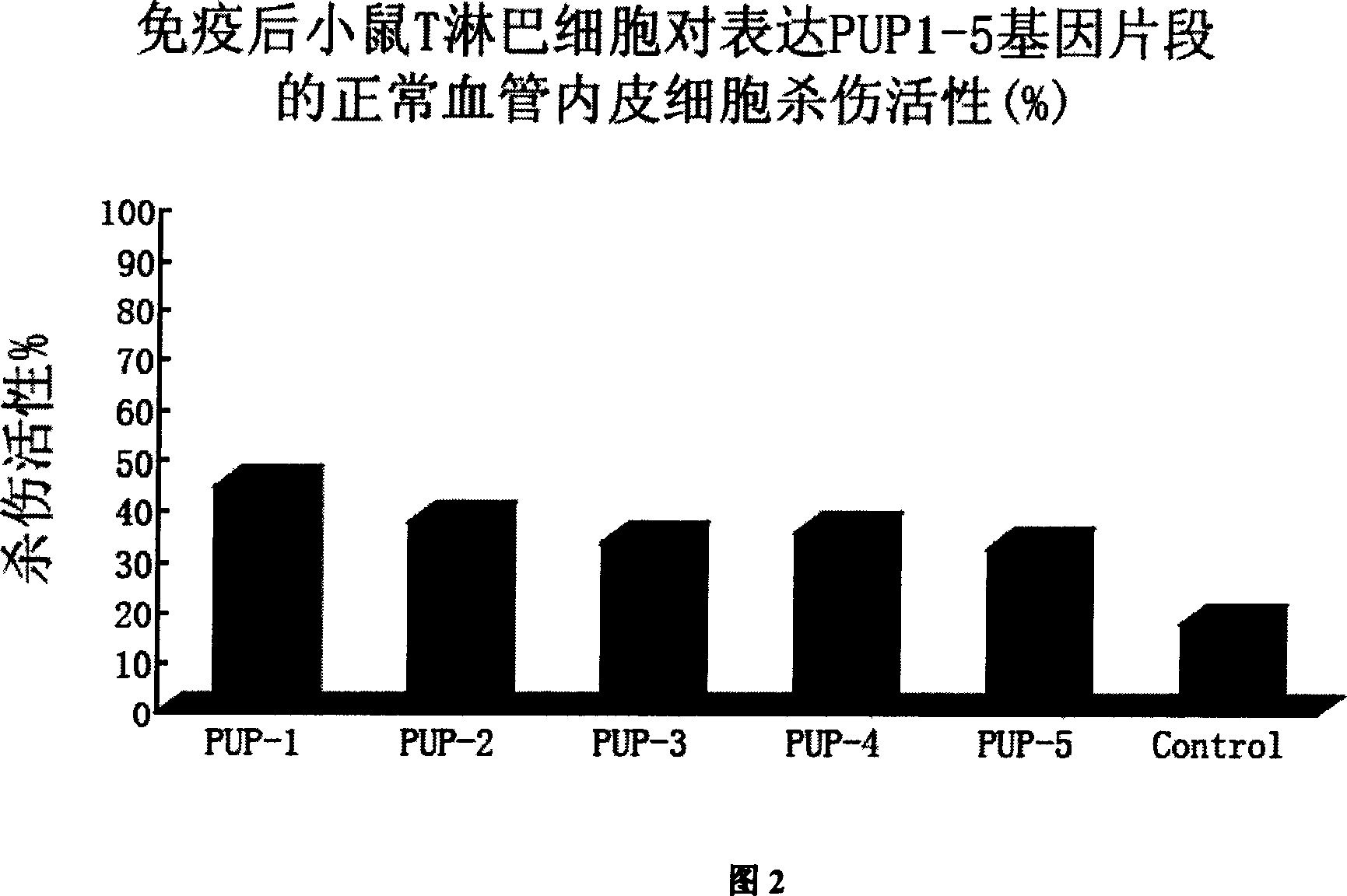
InactiveCN1990042AGood killing effectStrong antigen processing and presentation capabilitiesGenetic material ingredientsAntiviralsHumoral immune reactionBiology
The invention discloses an anti-SARS coronavirus cell vaccine and its application, said cell vaccine (1) containing all or part gene fragment of SARS coronavirus S protein all length gene order and 5 specific eukaryotic expression vector of cDNA fragment; (2) expressing the heterogenetic antigen presenting cells of said SARS coronavirus gene corresponding protei; (3) inactivating the heterogenetic antigen presenting cells expressing SARS coronavirus related protein. Use said cell vaccine for immuning host containing humans and rodents such as mice in order to making them generate protective humoral immunity and cellular immunity and resisting the SARS coronavirus infection which causes atypical pneumonia contagion. The security and stability of the invention are fine, production is convenient and price is low, it can induce the body generating the effective humoral immunity and cellular immunity of SARS coronavirus causing atypical pneumonia at the same time; provide effective and cheap vaccines for treating and preventing SARS.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com