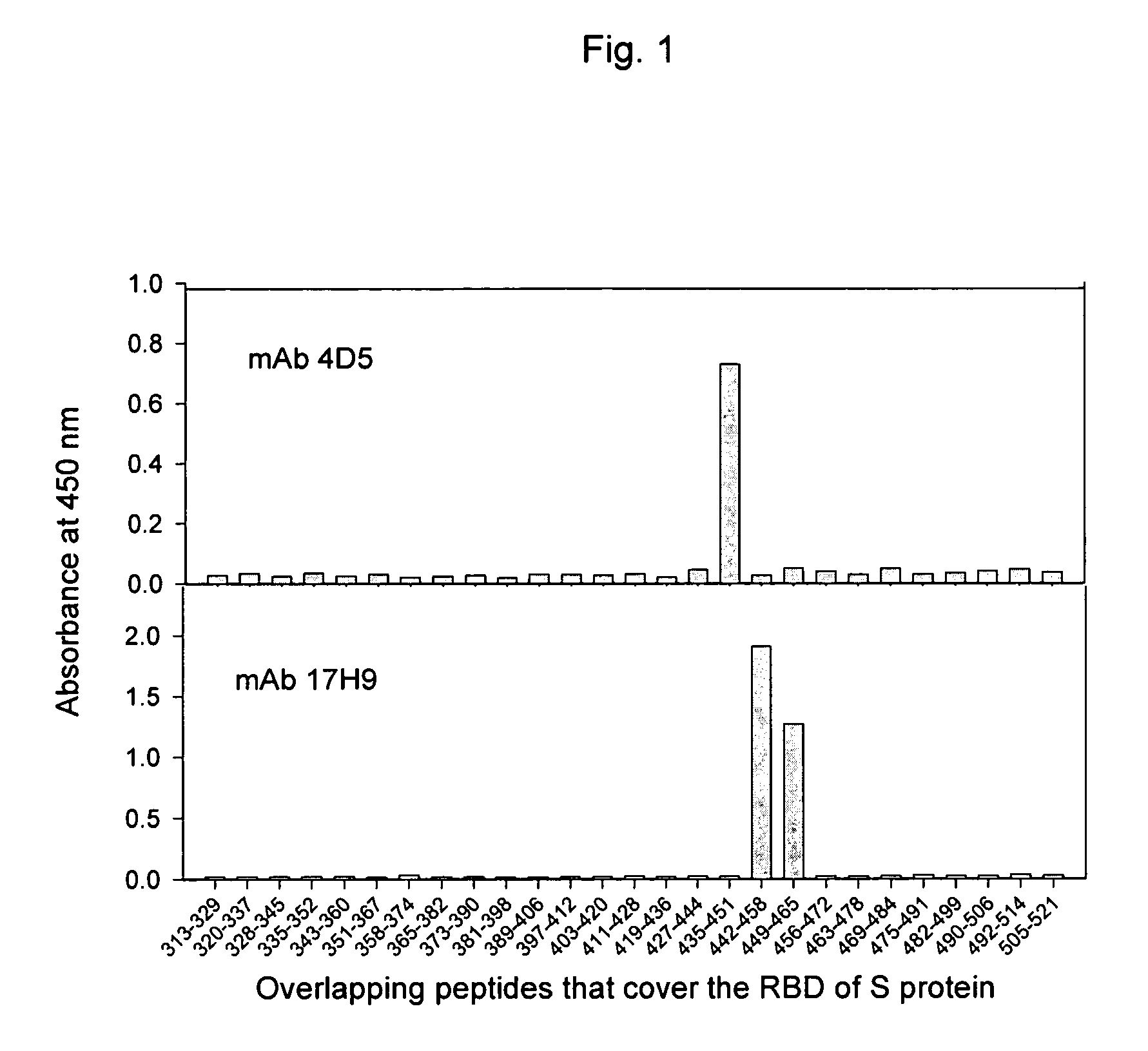
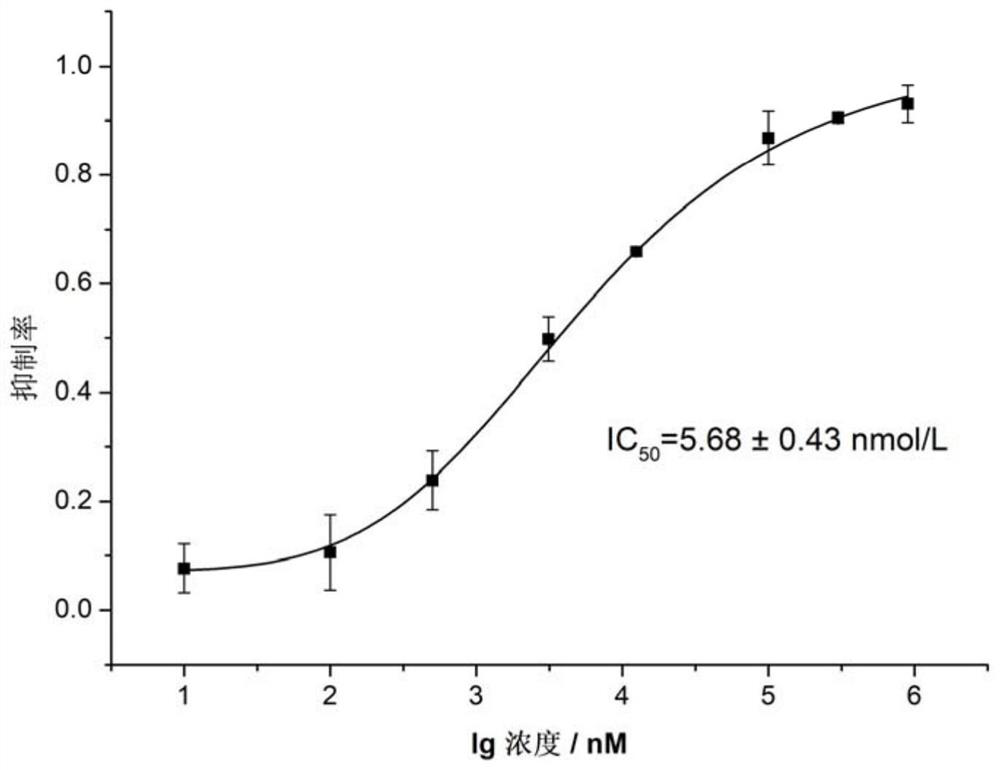
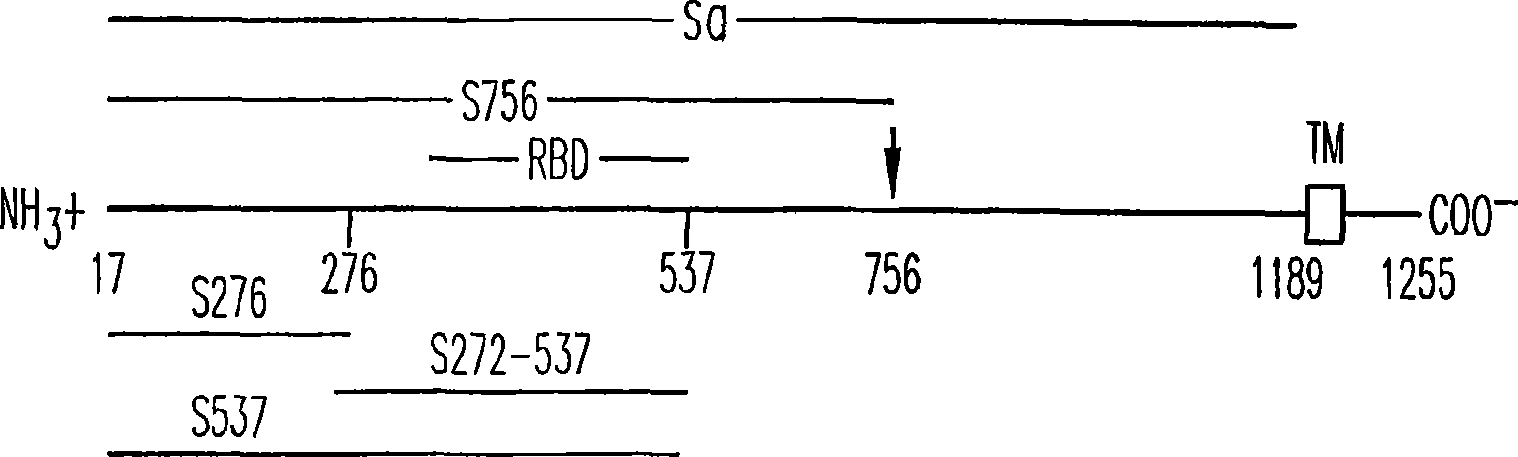
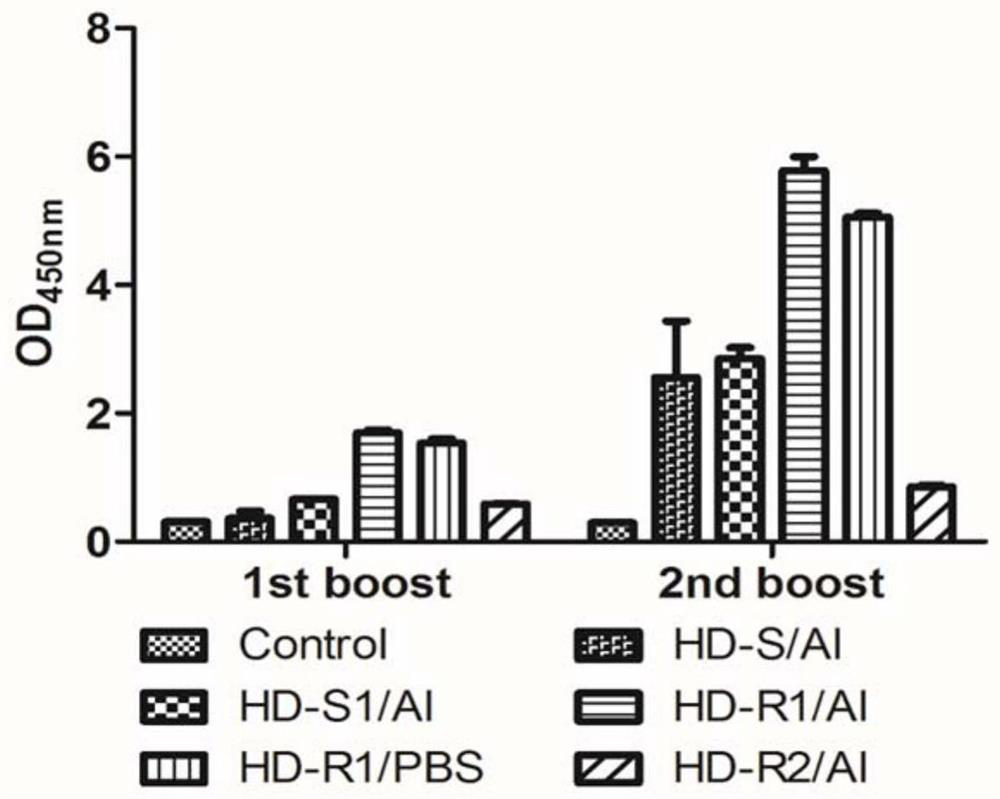
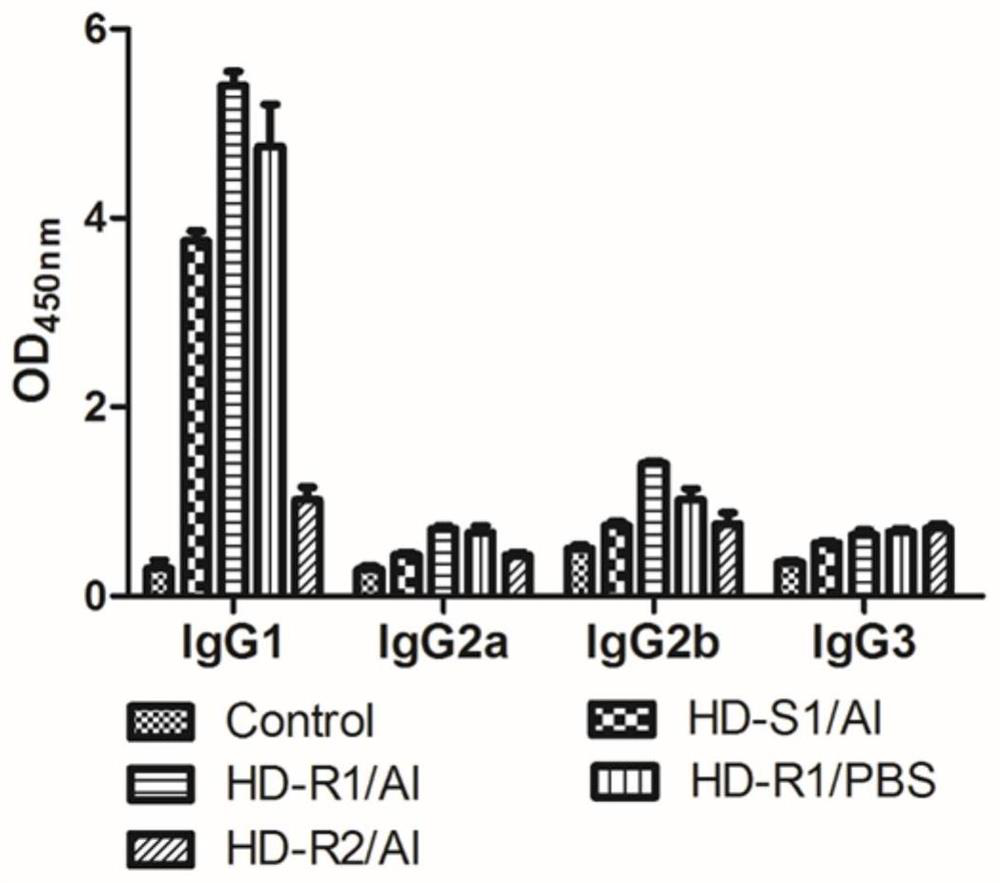
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
306 results about "Spike Protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
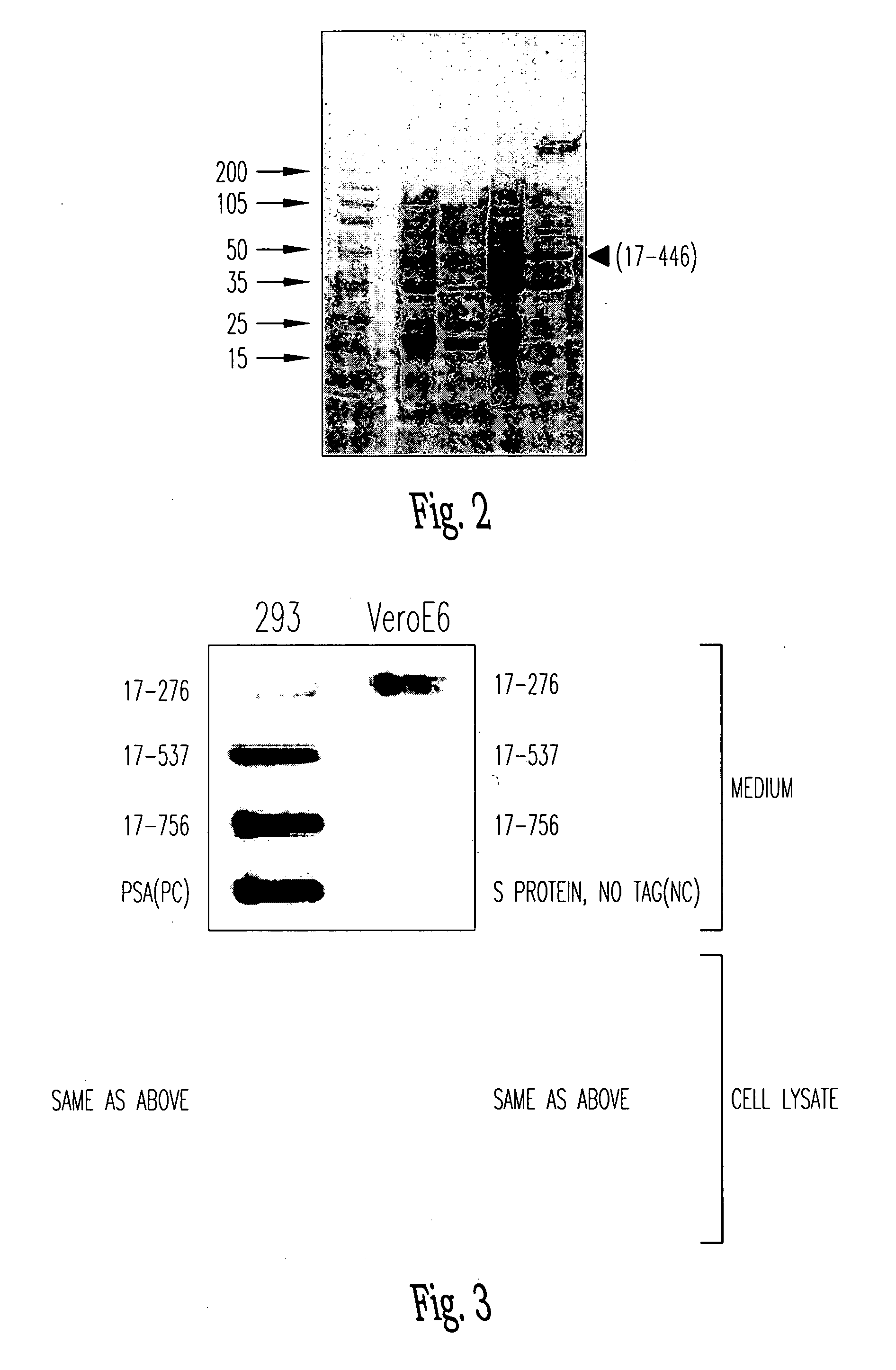
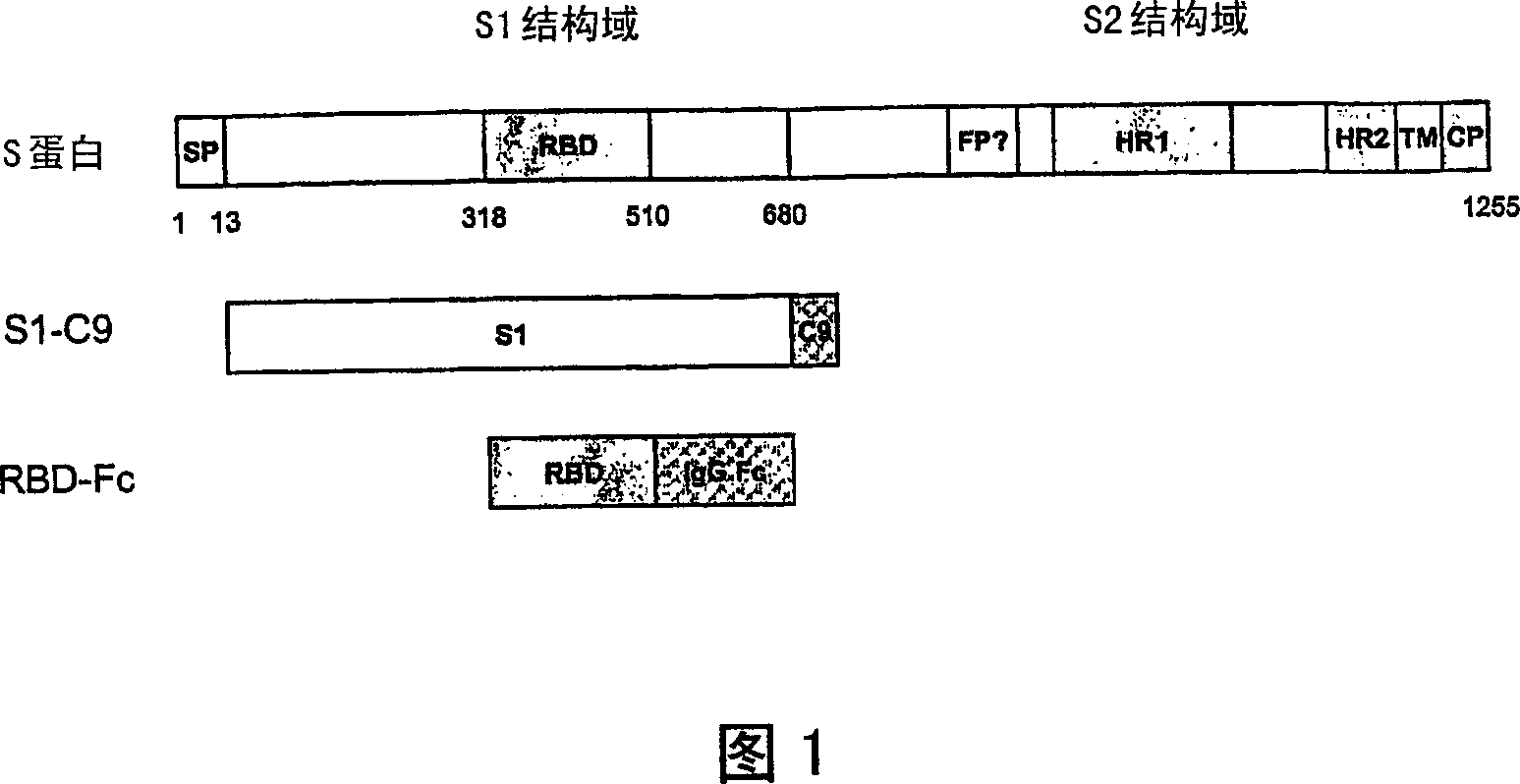
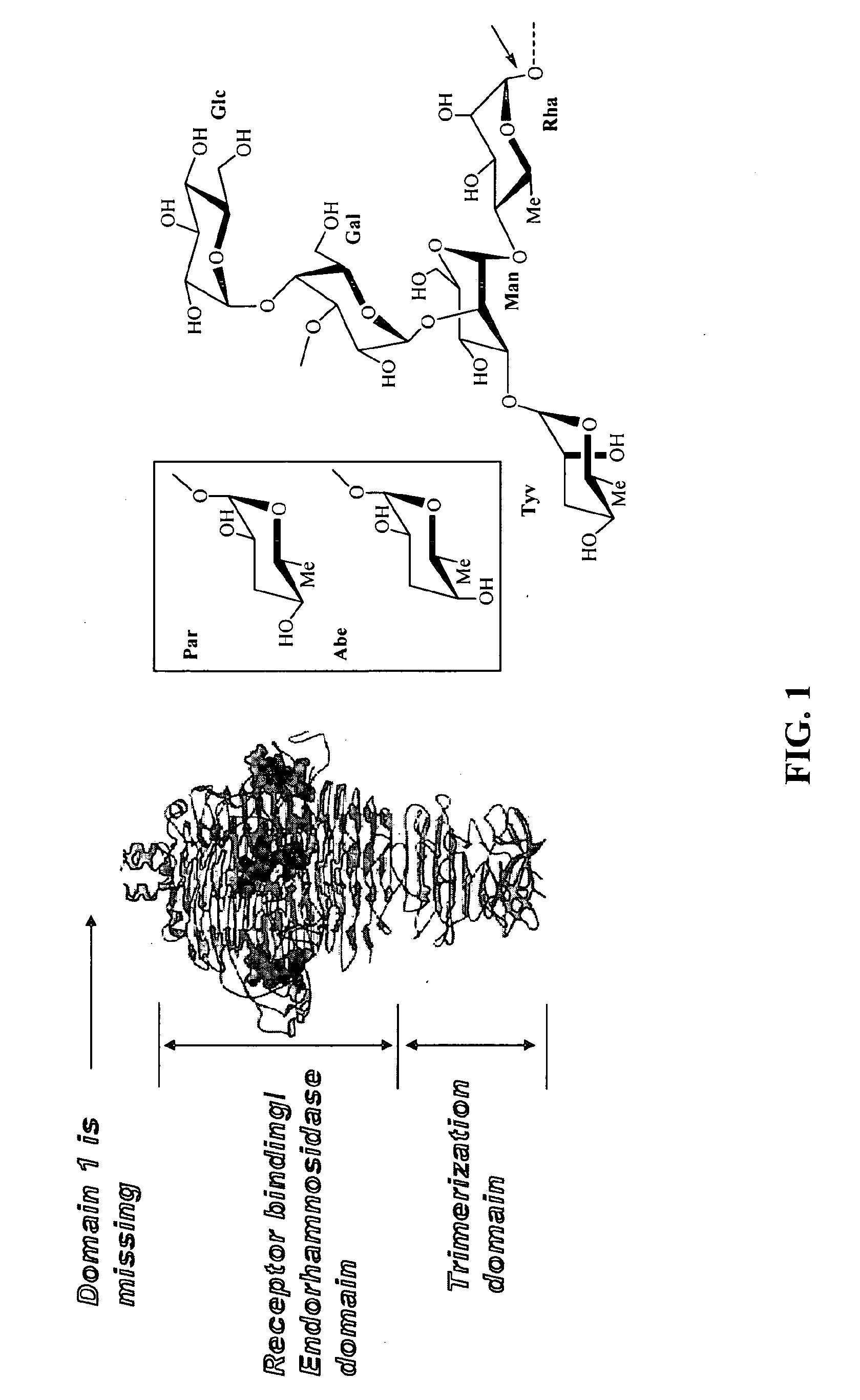
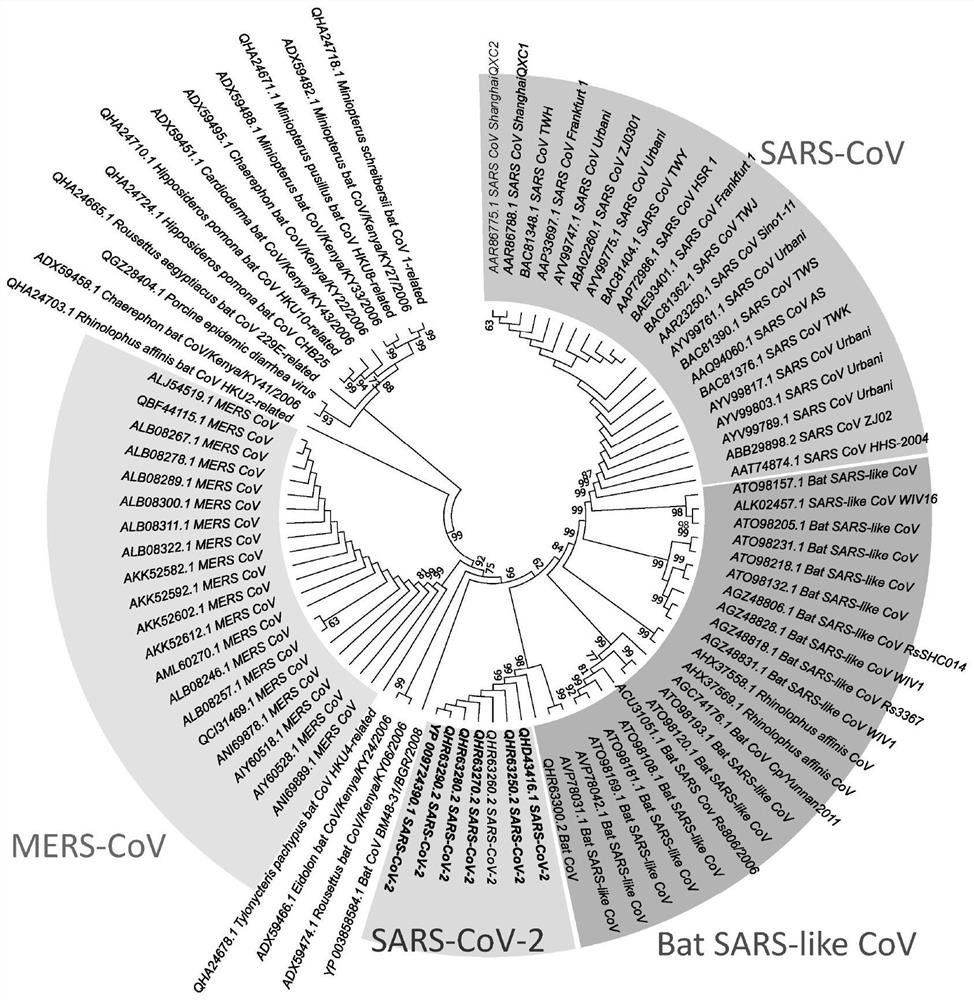
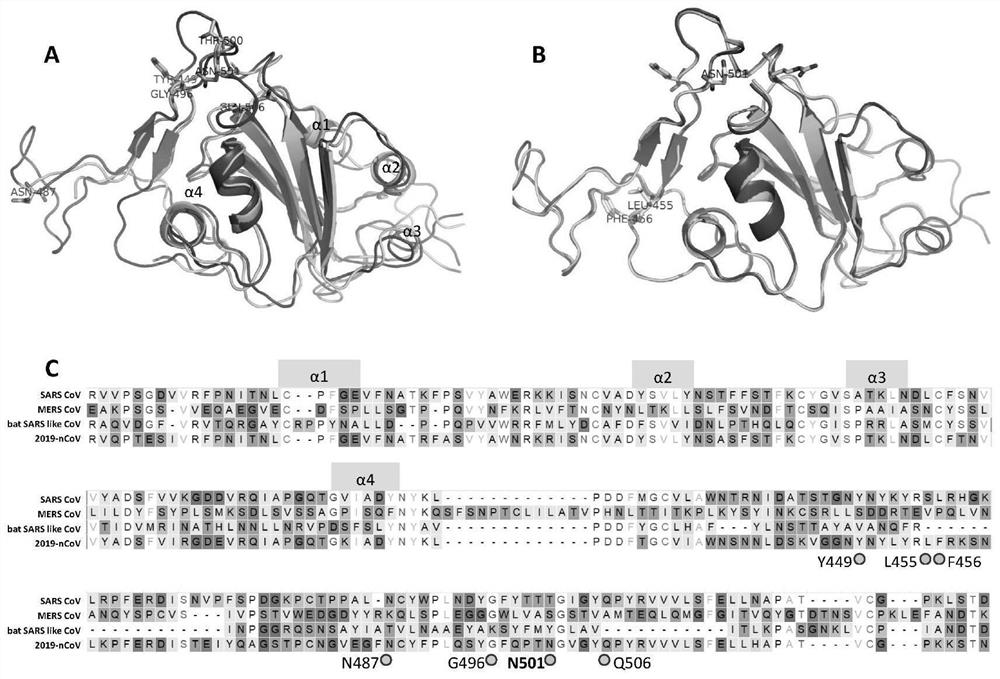
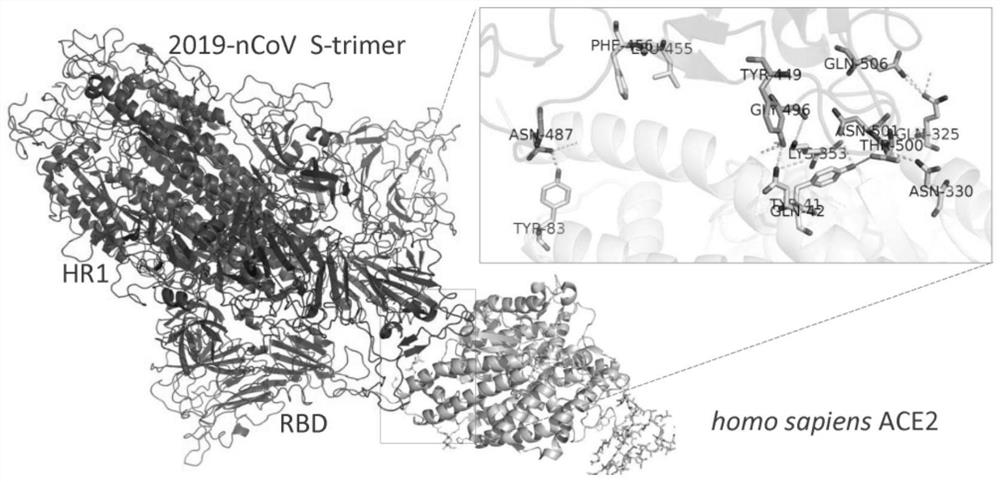
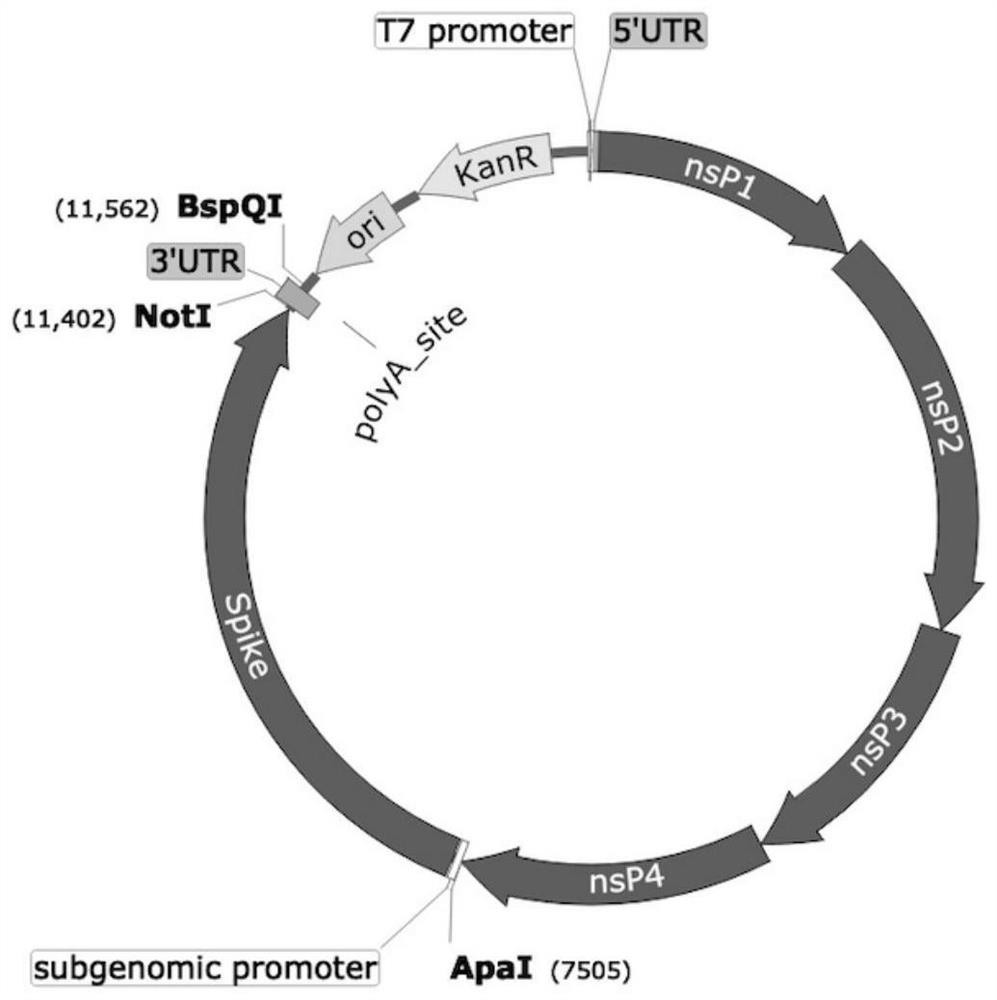
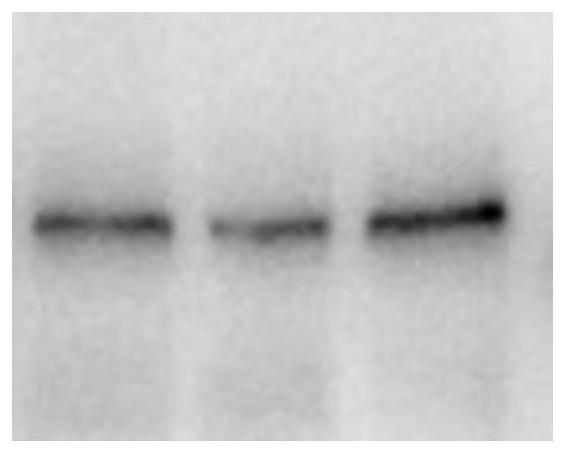
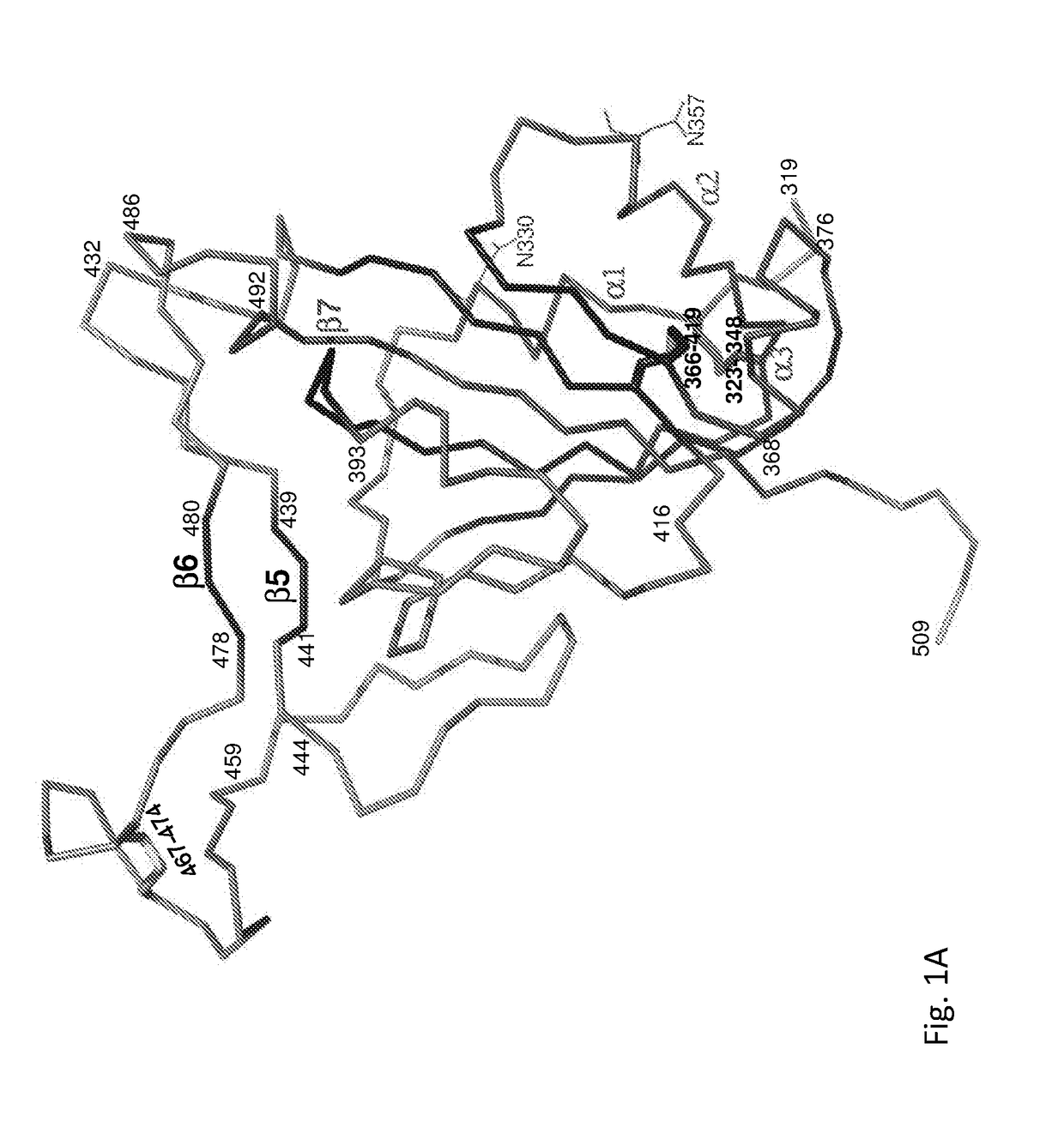
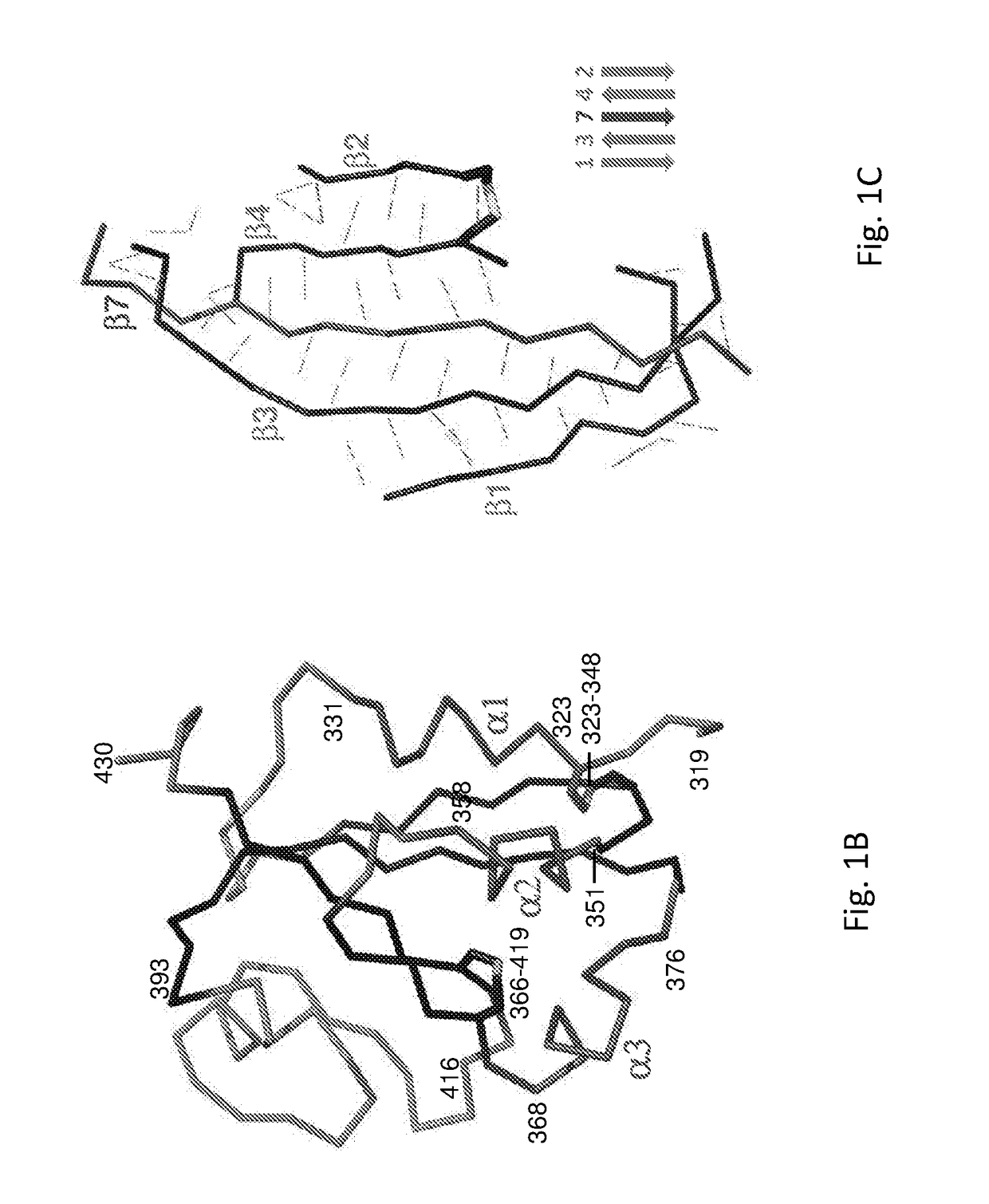
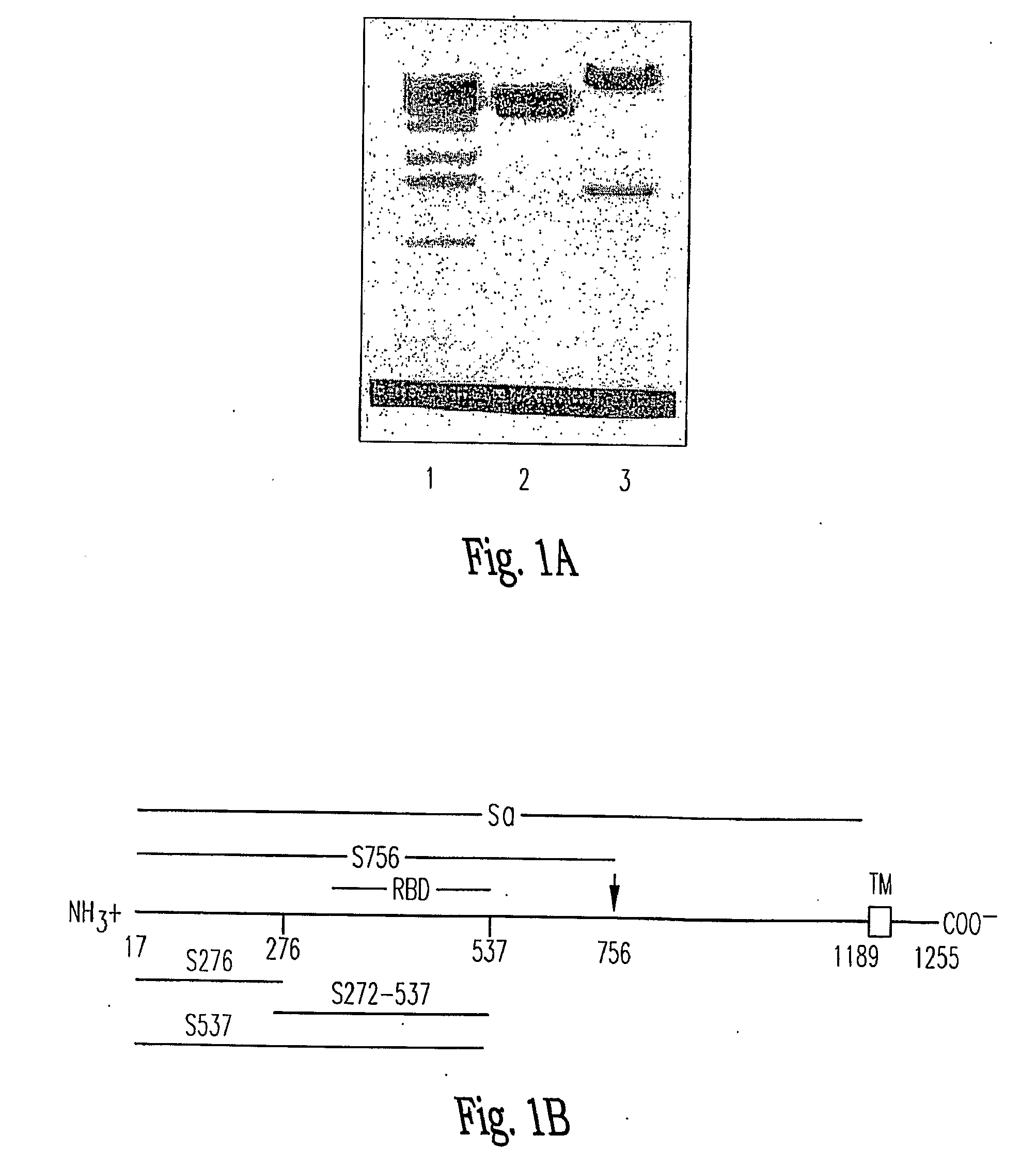
Spike Protein Definition. The spike protein (S protein) is a large type I transmembrane protein ranging from 1,160 amino acids for avian infectious bronchitis virus (IBV) and up to 1,400 amino acids for feline coronavirus (FCoV) (Figure 1).
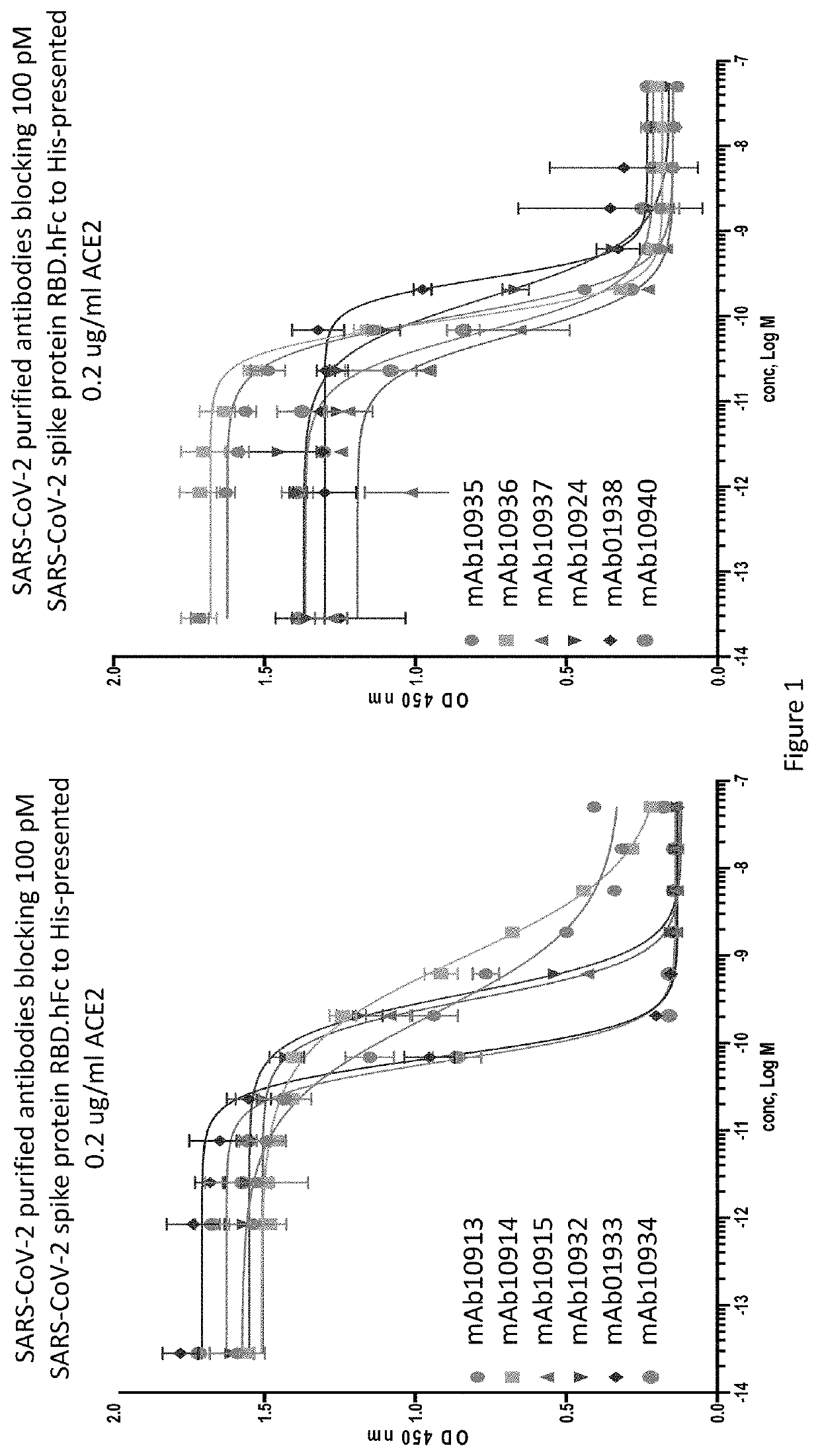
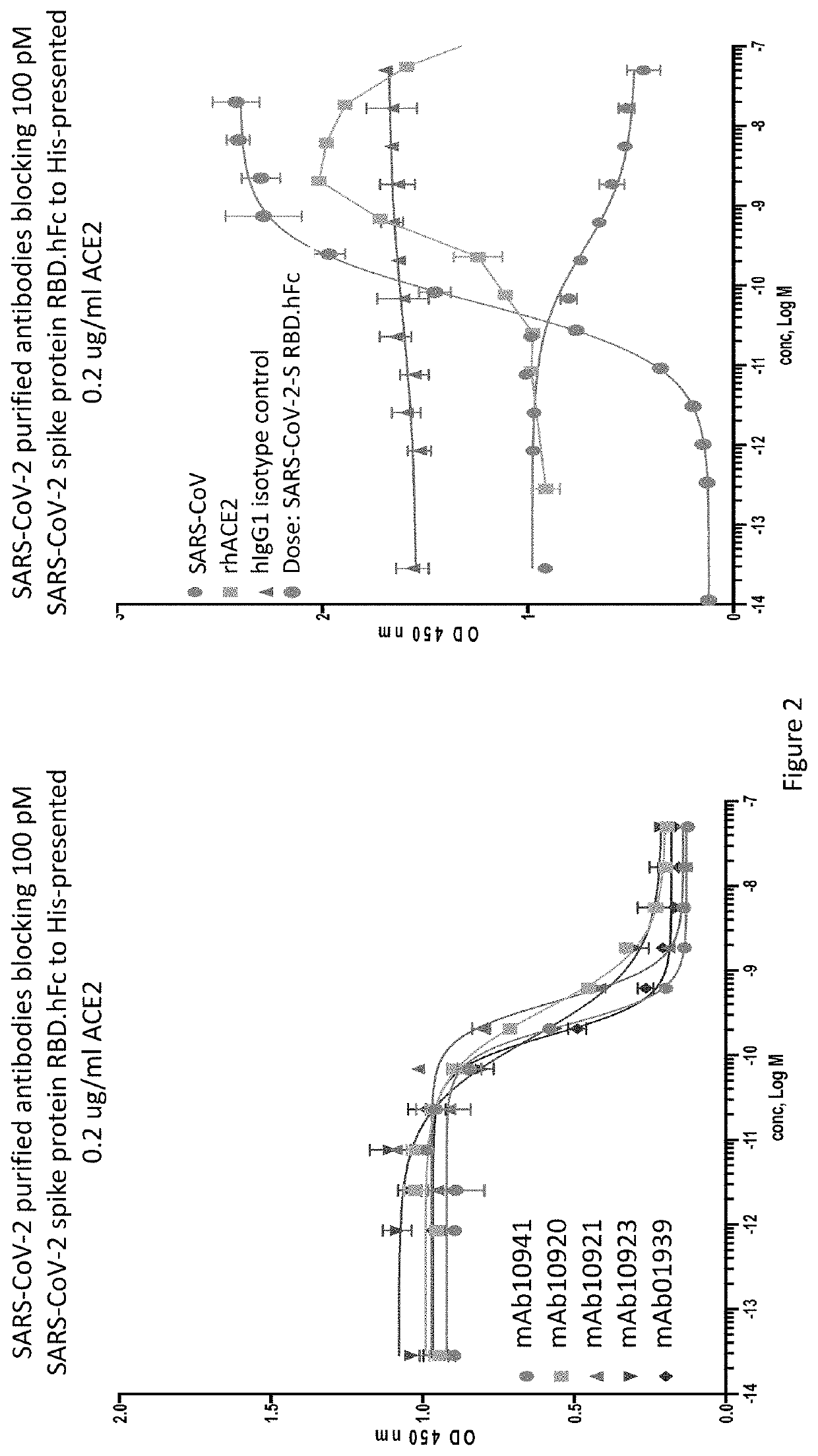
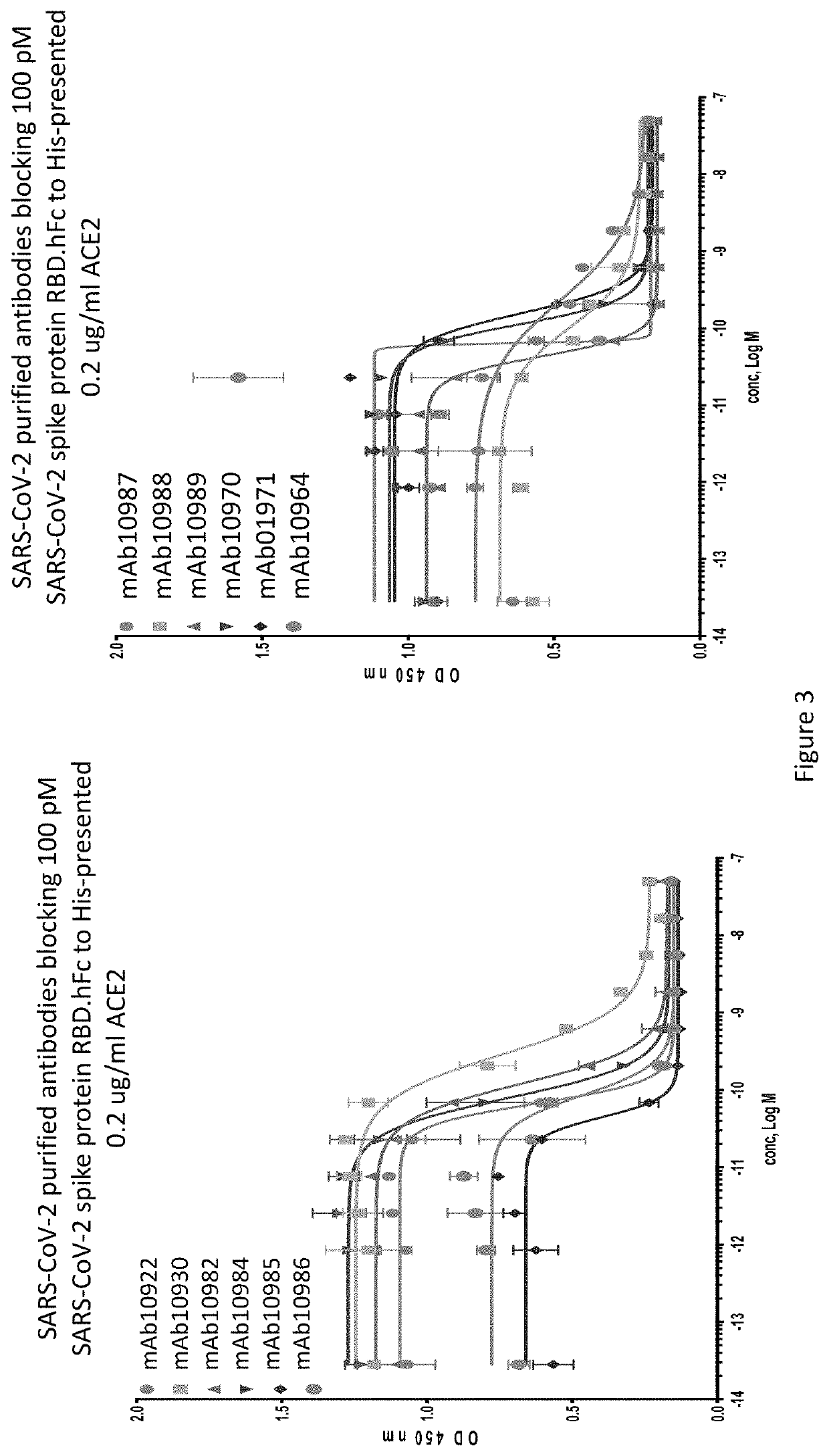
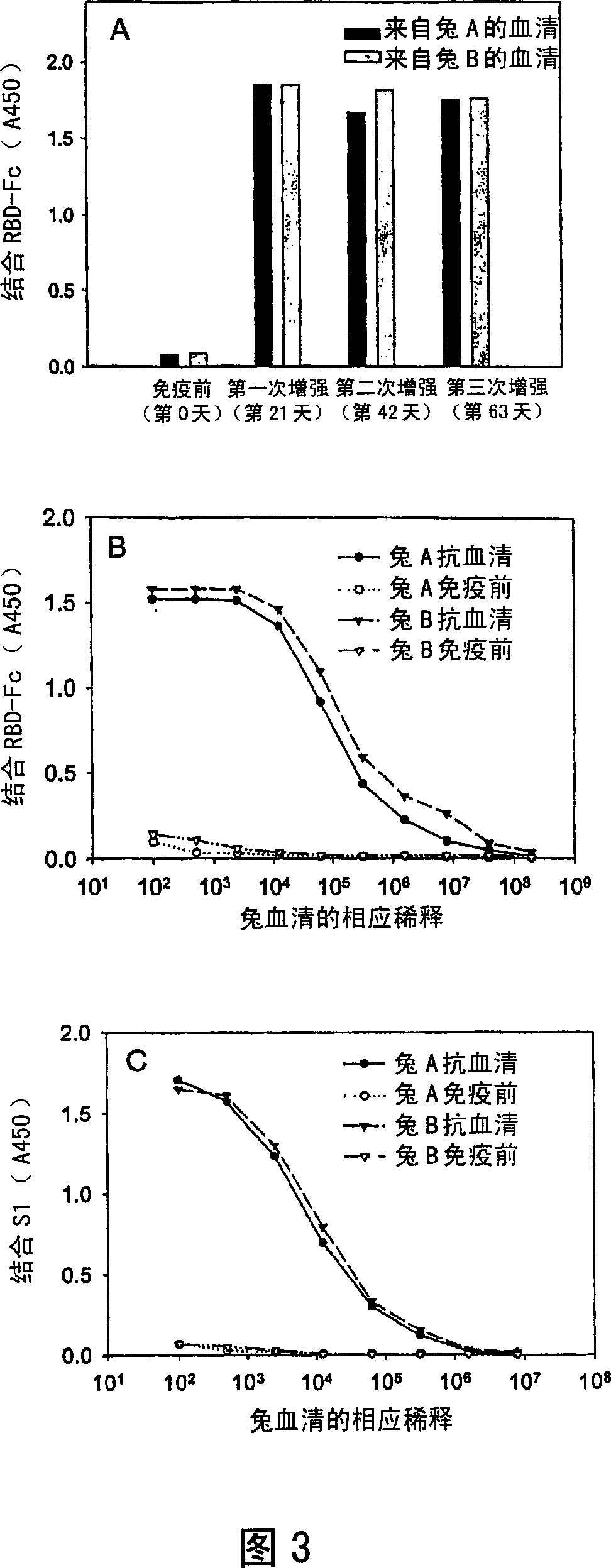
Anti-SARS-CoV-2-spike glycoprotein antibodies and antigen-binding fragments
ActiveUS10787501B1Growth inhibitionControl spreadViral antigen ingredientsImmunoglobulins against virusesAntigenAntiendomysial antibodies
The present disclosure provides antibodies and antigen-binding fragments thereof that bind specifically to a coronavirus spike protein and methods of using such antibodies and fragments for treating or preventing viral infections (e.g., coronavirus infections).
Owner:REGENERON PHARM INC
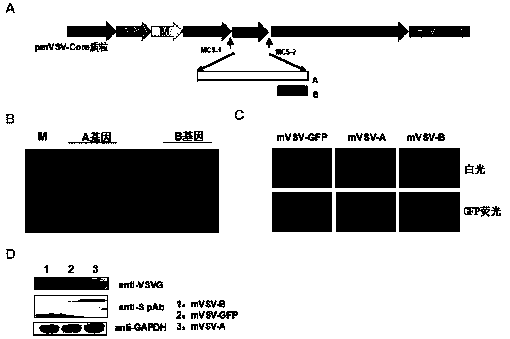
mVSV virus vector and virus vector vaccine, and COVID-19 vaccine based on mVSV mediation
ActiveCN111088283AEnhance immune responseStrong immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousReceptor
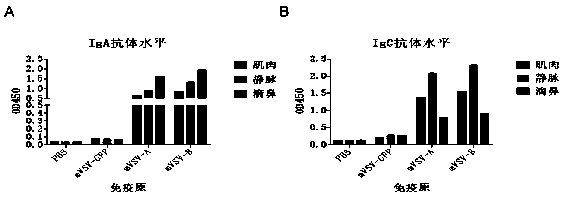
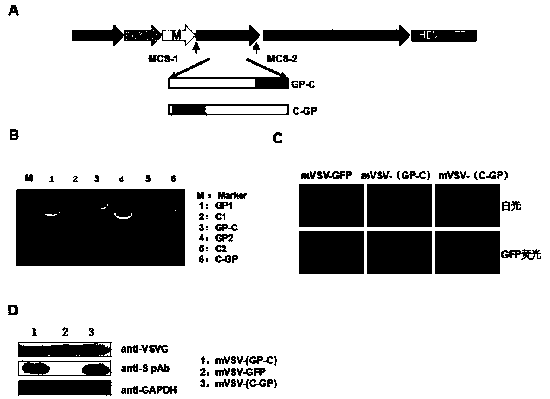
The invention provides an mVSV virus vector, i.e., attenuated mVSV obtained after multiple modification mutations occur to an M protein amino acid site of a wild Indiana strain VSV, and an optimized heterologous antigen gene is preferentially integrated to a double cloning site area of an mVSV packaging core plasmid pmVSV-Core at the same time. The mVSV virus vector vaccine comprises a heterologous antigen gene which fuses or embeds a target virus between G and L genes of an mVSV vector envelope, wherein the antigen gene comprises an enveloped and embedded antigen gene encoding the target virus, an embedded combination antigen gene or a fused antigen gene; the mVSV virus vector is embedded or fused with a dominant antigen of spike protein S of an SARS-CoV-2 pathogen; the dominant antigen is preferably selected from a receptor binding domain of spike protein S, namely RBD; and a COVID-19 vaccine based on mVSV mediation is formed. The vaccine has good prevention or treatment effect on COVID-19 infected people.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
Anti-Sars Monoclonal Antibodies
ActiveUS20080081047A1Sugar derivativesViral antigen ingredientsDrug biological activityWestern immunoblot
Monoclonal antibody reagents that recognize the SARS-coronavirus (SARS-HCoV) are needed urgently. In this report we describe the development and immunochemical characterisation of mAbs against the SARS-HCoV based upon their specificity, binding requirements, and biological activity. Initial screening by ELISA, using highly purified virus as the coating antigen, resulted in the selection of seventeen mAbs. Five mAbs exhibited Western immunoblot reactivity with the denatured spike protein, of which two demonstrated the ability to neutralize SARS-HCoV in vitro. Another four Western immunoblot-negative mAbs also neutralize the virus. These antibodies will be useful for the development of diagnostic tests, pathogenicity and vaccine studies.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
Novel coronavirus (SARS-COV-2) spike protein binding molecule and applications thereof
ActiveCN111647077AAvoid infectionInhibition of amplificationComponent separationMaterial analysis by electric/magnetic meansReceptorIntravenous gammaglobulin
The invention relates to the technical field of medical biology, and specifically discloses a novel coronavirus (SARS-COV-2) spike protein binding molecule and applications thereof. The binding molecule can specifically bind the spike protein of SARS-COV-2 and includes at least one immunoglobulin single variable structural domain. The provided binding molecule can specifically bind the SARS-COV-2-Spike protein and effectively block the binding of the SARS-COV-2-Spike protein and human cell ACE2 receptors, so that the infection process of the SARS-COV-2 on cells can be further blocked, and theinfection and amplification of the SARS-COV-2 can be inhibited.
Owner:SHENZHEN IMMUNOTHERAPY BIOTECH CO LTD
Soluble fragments of the SARS-CoV spike glycoprotein
InactiveUS20060240515A1Easy to produceImprove resistance to degradationSsRNA viruses positive-senseAntibody mimetics/scaffoldsAptamerVaccination
The invention relates to the spike protein from the virus (SARS-CoV) that is etiologically linked to severe acute respiratory syndrome (SARS); polypeptides and peptide fragments of the spike protein; nucleic acid segments and constructs that encode the spike protein, polypeptides and peptide fragments of the spike protein, and coupled proteins that include the spike protein or a portion thereof; peptidomimetics; vaccines; methods for vaccination and treatment of severe acute respiratory syndrome; antibodies; aptamers; and kits containing immunological compositions, or antibodies (or aptamers) that bind to the spike protein.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF US SEC THE DEPT OF
Vaccines and compositions based on S antigen protein of SARS-CoV-2
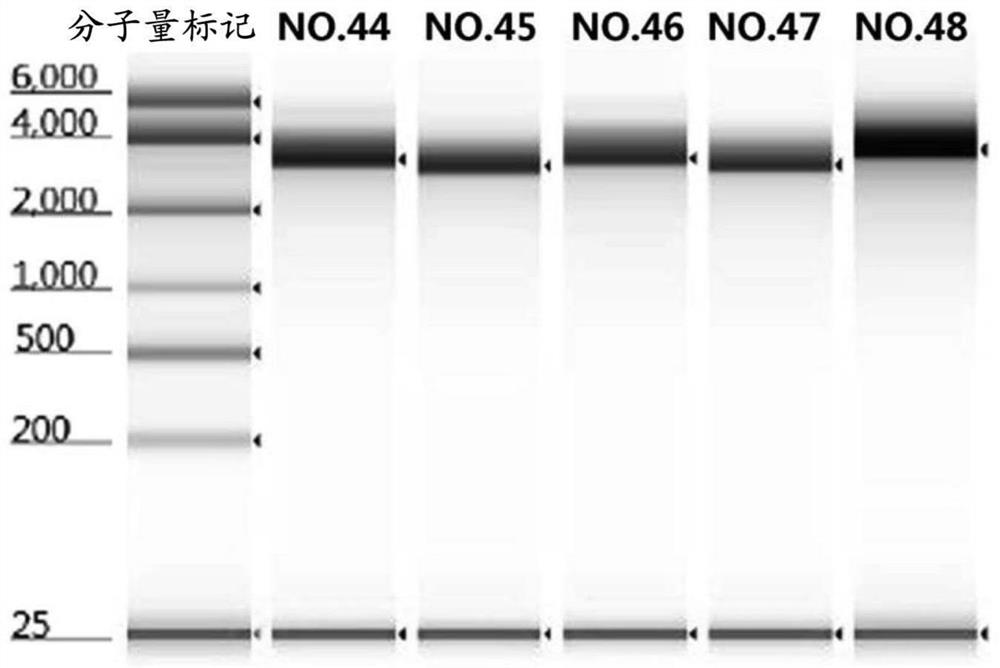
The present disclosure provides vaccines and compositions based on S antigen proteins of SARS-CoV-2, and specifically relates to recombinant SARS-CoV-2 spike proteins (S proteins) or antigenic fragments thereof, and mRNAs encoding the same. The present disclosure also relates to mRNA comprising a sequence selected from any one of SEQ ID NO. 44 to 47. The present disclosure further relates to mRNA-vector particles, such as lipid nanoparticles (LNPs), comprising the aforementioned mRNAs and compositions, such as vaccine compositions.
Owner:GUANGZHOU ARGORNA BIOPHARMACEUTICALS CO LTD
Neutralizing monoclonal antibodies against severe acute respiratory syndrome-associated coronavirus
InactiveUS20060240551A1Reduce the binding forceAvoid infectionAnimal cellsMicrobiological testing/measurementAntigenicitySpike Protein
The present invention provides an isolated antibody capable of binding to the receptor-binding domain of the spike protein of the severe acute respiratory syndrome-associated coronavirus (SARS-CoV) so as to competitively inhibit the binding of the SARS-CoV to host cells. These mAbs or substances can be used: 1) as passive-immunizing agents for prevention of SARS-CoV infection; 2) as biological reagents for diagnosis of SARS-CoV infection; 3) as immunotherapeutics for early treatment of SARS-CoV infection; and 4) as probes for studying the immunogenicity, antigenicity, structure, and function of the SARS-CoV S protein.
Owner:NEW YORK BLOOD CENT
Sars vaccines and methods to produce highly potent antibodies
InactiveCN101098710APeptide preparation methodsAntibody medical ingredientsAntigenSARS-associated coronavirus
This invention provides a vaccine comprising an effective amount of an isolated polypeptide or recombinant protein containing the sequence of the receptor-binding domain in the SARS associated coronavirus spike protein or a functional fragment thereof, or a nucleic acid molecule comprising the sequence of a fragment which encodes the sequence of the receptor-binding domain in the Severe Acute Respiratory Syndrome associated coronavirus spike protein or a functional fragment thereof. This invention provides a composition for increasing the immunogenicity of an antigen comprising an effective amount of an antigen and an IgG Fc domain, its functional fragment, or a substance containing an IgG Fc domain or its functional fragment. The antigen and the IgG Fc may be linked or unlinked. Finally, this invention also provides method for using any of the above compositions for immunization.
Owner:NEW YORK BLOOD CENT
Cell-based systems for producing influenza vaccines
ActiveUS20100021499A1Minimize and prevent virus infectionAvoid componentsSsRNA viruses negative-senseAnimal cellsHemagglutininBinding site
The present invention relates to a cell-based method for producing influenza virus vaccines by enriching the population of surface-bound α2,6-sialic acid receptors on a cell surface, such as on a Chinese Hamster Ovary (CHO) cell surface. The host cell therefore presents numerous binding sites to which an influenza virus can bind via its hemagglutinin spike protein and infect the host cell. In contrast to wild-type CHO cells, the surface of the mutated CHO cells of the present invention contains an enriched population of α2,6-sialic acid receptors which makes the inventive CHO cells highly susceptible to viral infection, and therefore safe, effective, and highly efficient cells for rapidly producing influenza vaccines.
Owner:FLUGEN
Recombinant Spike Protein Subunit Based Vaccine for Porcine Epidemic Diarrhea Virus (PEDV)
The present invention encompasses porcine epidemic diarrhea virus (PEDV) vaccines or compositions. The vaccine or composition may be a vaccine or composition containing PEDV antigens. The invention also encompasses recombinant vectors encoding and expressing PEDV antigens, epitopes or immunogens which can be used to protect porcine animals against PEDV.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Phage receptor binding proteins for antibacterial therapy and other novel uses
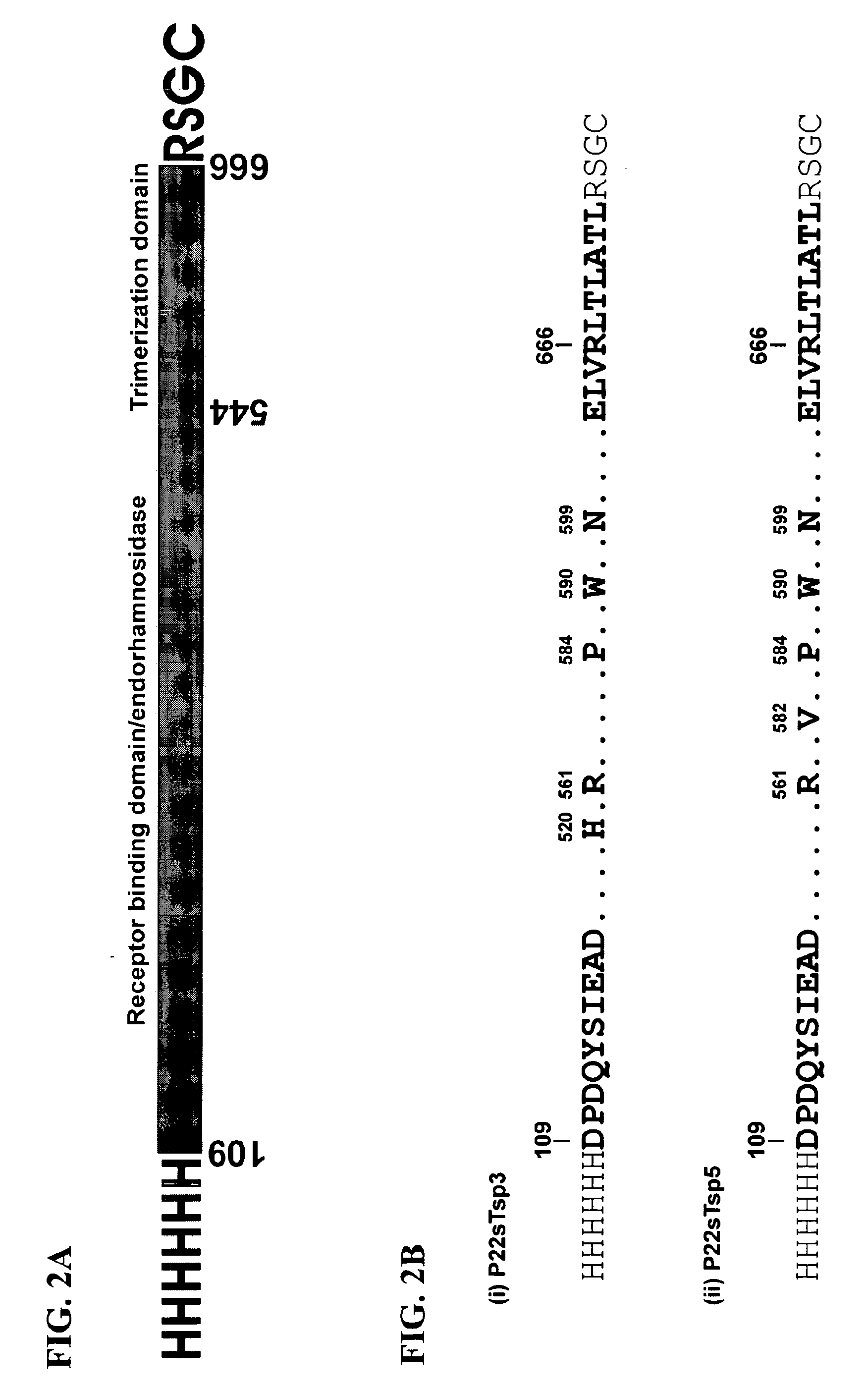
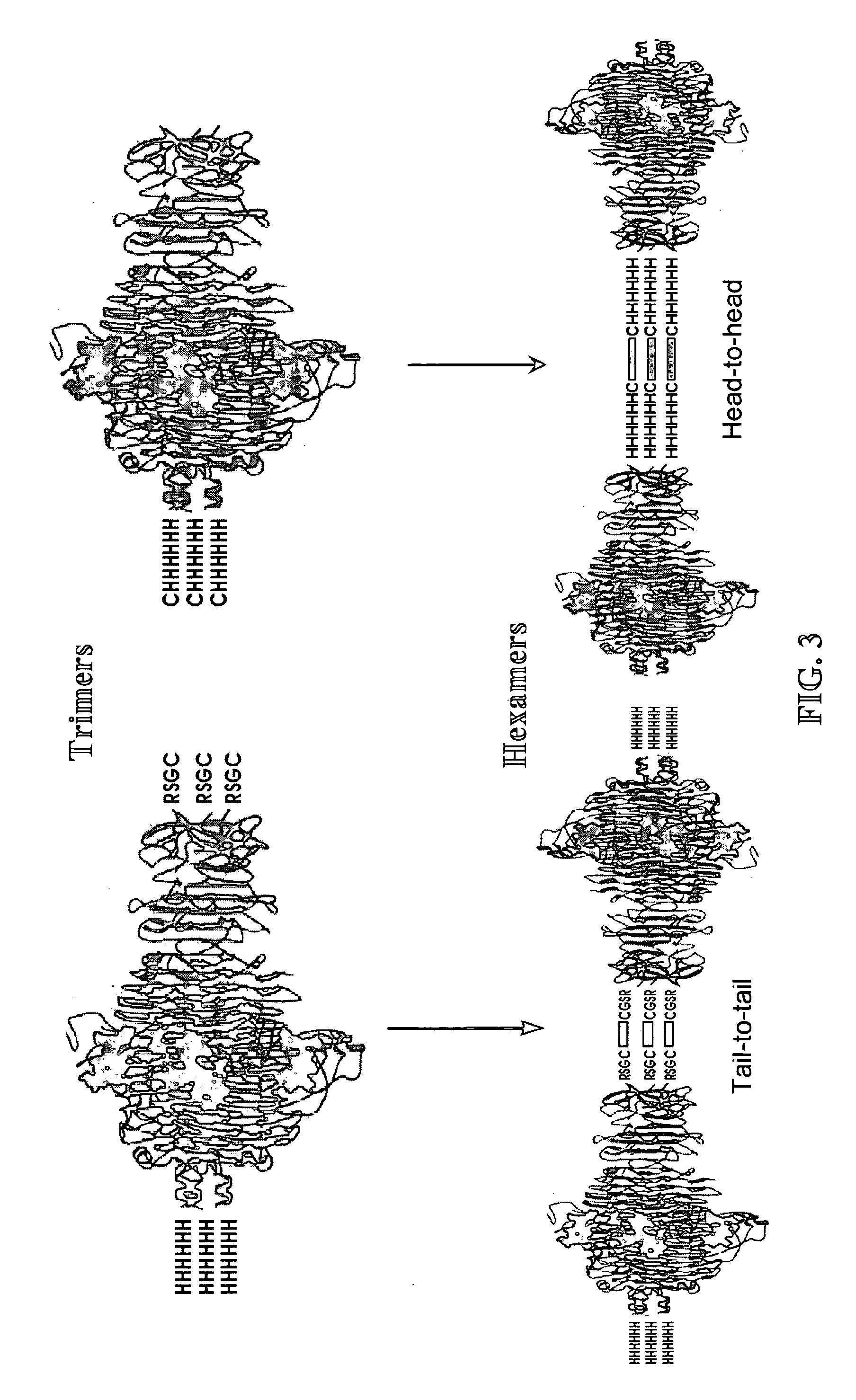
The subject invention relates in part to novel uses of bacteriophage tail spike proteins (TSPs). Some preferred uses are therapeutic uses in animals, such as chickens, against pathogenic bacteria, such as Salmonella. Fragments of the TSPs can also be used according to the subject invention, particularly protein fragments comprising the phage receptor binding domains (PRBDs), which recognize their hosts and facilitate infection. The binding domains are specific to unique surface structures on bacteria and may be used for a variety of applications according to the subject invention. We have shown that by utilizing these PRBDs, it is possible to exploit the long-established evolutionary relationship between bacteria and their viruses (ie bacteriophages) that specifically infect them. The subject invention also relates in part to novel, synthetic forms of tail spike proteins. In some preferred embodiments, these are hexamers.
Owner:DOW AGROSCIENCES LLC +1
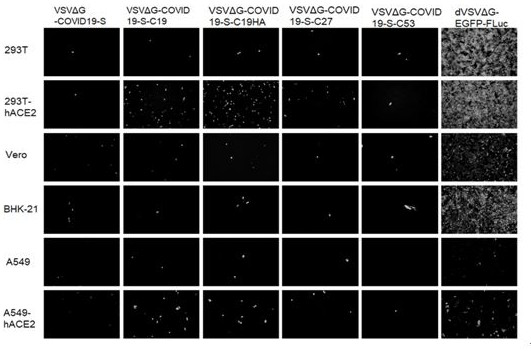
Pseudo-virus packaging system of coronavirus and one-step packaging method
InactiveCN111893097AQuick checkAccurate detectionSsRNA viruses negative-senseSsRNA viruses positive-senseTGE VACCINEScreening tool
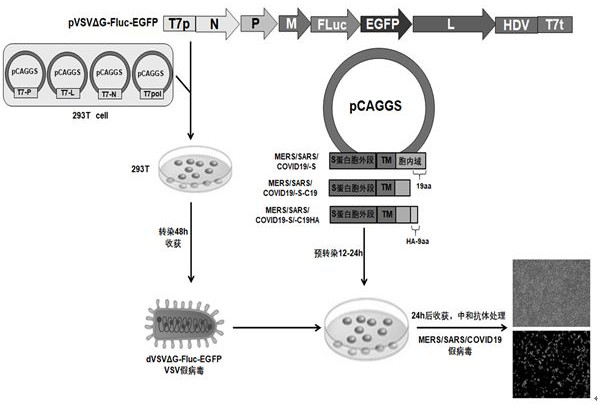
A pseudo-virus packaging system of coronaviruses comprises a vesicular stomatitis virus (VSV) vector with a Fluc and EGFP double reporter gene replacing a GP gene and an assembly cell expressing coronavirus spike protein S. The double reporter gene is selected from luciferase and fluorescent protein, and the luciferase reporter gene is preferably a Fluc gene. The fluorescent protein reporter geneis preferably an EGFP gene, and the assembly cell is preferably 293T. The invention further relates to a one-step packaging method of the packaging system; a pseudo-virus with single-cycle infection,low background value and high titer can be quickly packaged through simple, convenient and rapid operation, and the packaging system has the characteristic of being rapid in detection compared with aslow virus mediated pseudo-virus system; the one-step packaging method can be used for researching COVID-19, SARS, MERS and other coronaviruses and other viruses, provides a powerful screening tool for evaluation of antiviral preparations and vaccine, and has wide application value.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
Novel coronavirus vaccine and application thereof
ActiveCN112266411AHigh titerImprove expression levelSsRNA viruses positive-senseVirus peptidesCoronavirus vaccinationPharmaceutical drug
The invention relates to a novel coronavirus vaccine and application thereof, in particular to a truncated Spike protein, a fusion protein containing the truncated Spike protein, a nucleic acid molecule containing a nucleotide sequence encoding the truncated Spike protein or the fusion protein, and a vector and a host cell containing the nucleic acid molecule. The invention further relates to a pharmaceutical composition containing the truncated Spike protein, fusion protein, nucleic acid molecule or vector.
Owner:BEIJING NORTHLAND BIOTECH
Novel coronavirus SARS-CoV-2 S protein detection method
PendingCN111273006ASolve the problem of inability to effectively detect the new coronavirus SARS-CoV-2S proteinFast preparationImmunoassaysAngiotensin-converting enzymeProtein detection
The invention discloses a novel coronavirus SARS-CoV-2 S protein detection method. SARS-CoV-2 virus depends on spike protein on the surface to be combined with angiotensin converting enzyme 2 on the surface of a cell so as to enter the cell; according to the method, raw materials commonly used in chemiluminescence immunoassay and ACE2 are easy to purchase from the market, the biotin labeling and the alkaline phosphatase labeling are rapid, the reagent for SARS-CoV-2 S protein detection can be rapidly prepared, and the positive coincidence rate, the negative coincidence rate and the total coincidence rate are high compared with the SARS-CoV-2 fluorescence quantitative PCR result.
Owner:SICHUAN ORIENTER BIOLOGICAL TECH
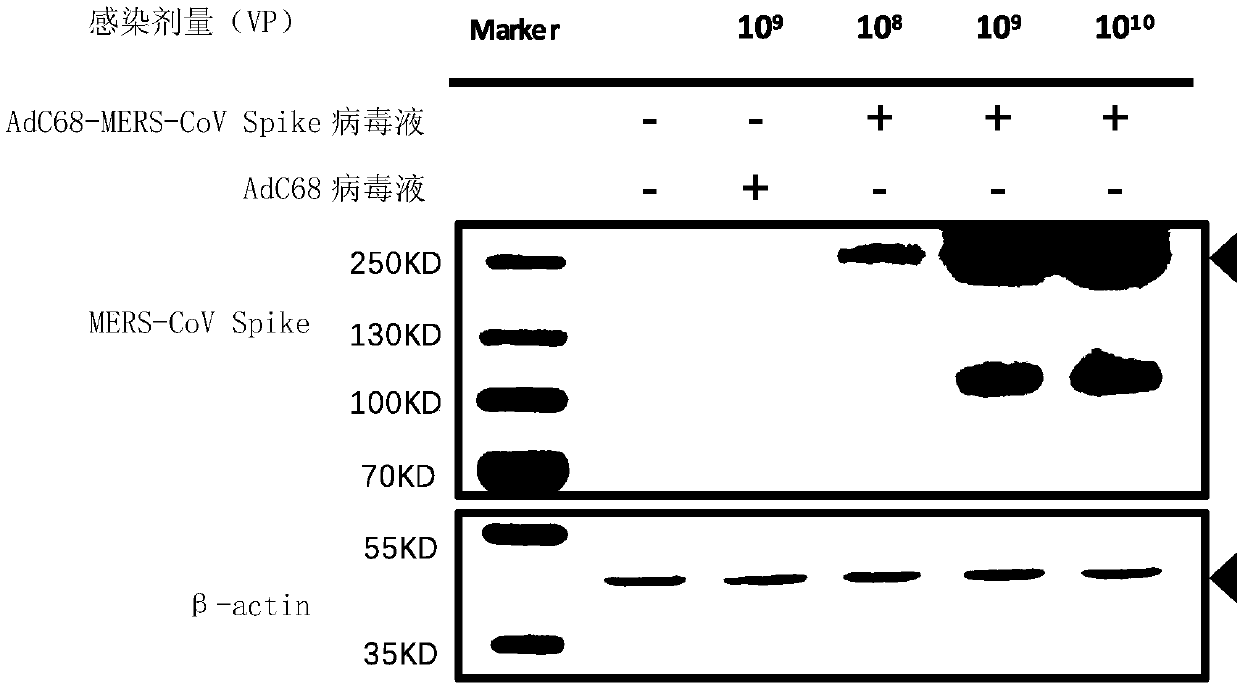
New coronavirus vaccine based on chimpanzee adenovirus type 68 and MERS-CoV full length membrane protein
ActiveCN110616198AAvoiding deficiencies of pre-existing immunityRetain high titersSsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationCell Membrane Proteins
The present invention discloses a new coronavirus vaccine based on chimpanzee adenovirus type 68 and MERS-CoV full length membrane protein. The recombinant adenovirus is obtained by transfecting an adenovirus packaging cell with a recombinant plasmid and then performing cell culture; the recombinant plasmid is obtained by inserting a specific DNA molecule into a delta-E1 region of a chimpanzee adenovirus vector AdC68; the specific DNA molecule has a full-length MERS-CoV Spike protein encoding gene; and the adenovirus packaging cell has an adenovirus E1 gene. The present invention also protectsthe recombinant adenovirus expressing the full-length MERS-CoV Spike protein; and a starting strain of the recombinant adenovirus is chimpanzee adenovirus type 68 or non-replicating chimpanzee adenovirus type 68. The developed vaccine against the new coronavirus MERS-CoV has important theoretical guidance value and broad application prospects, and provides a possibility for radical cure of MiddleEast respiratory syndrome.
Owner:TSINGHUA UNIV +1
Optimized SARS coronary virus spike protein gene
InactiveCN1570115AImprove expression efficiencyImprove efficiencyDepsipeptidesViruses/bacteriophagesMammalFhit gene
The invention discloses an optimized SARS coronary virus spike protein gene, which codes the SARS coronary virus S protein or immunogenicity fragment, and has 77+ / -3% homology with SARS coronary virus S protein coding sequence(such as SEQ ID NO: 1). The invention also provides vector, host cell and vaccine composition containing a optimized S protein coding sequence, whose expression efficiency is higher in mammal cell, So can stimulate the immune response in mammal to SARS.
Owner:陈克勤 +1
Stable polypeptide targeting SARS-CoV-2 spike protein and application of stable polypeptide
ActiveCN112321686AImprove bindingInvasion blocking activityPeptide/protein ingredientsAntiviralsHuman cellFluorescent polarization
The invention provides a stable polypeptide targeting SARS-CoV-2 spike protein. A template amino acid sequence structure of the stable polypeptide is X-(Linker)-CONH-IEEQAKTFLDKFNHEAEDLFYQS-CONH2. Theinvention also provides application of the polypeptide in preparation of a medicine for treating SARS-CoV-2. The invention also provides application of the polypeptide in preparation of a medicine for preventing the SARS-CoV-2 from entering human cells. The polypeptide molecule is combined with the spike protein to block virus from entering the human body by being combined with human ACE2. Experiments of fluorescence polarization detection and the like prove that the polypeptide disclosed by the invention can be well combined with an RBD of the spike protein and has an effect of blocking a virus infection process.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL +1
Nucleic acid for encoding SARS-CoV-2 virus spike protein and application of nucleic acid
ActiveCN112226445ASsRNA viruses positive-senseViral antigen ingredientsTGE VACCINEEquine encephalosis virus
The invention relates to the technical field of biological medicine, in particular to nucleic acid for encoding SARSCoV2 virus spike protein and application of the nucleic acid. Through codon optimization, the invention provides the Spike high-expression novel coronavirus mRNA vaccine based on the TC83Venezuelan Equine Encephalosis Virus (VEEV) in the alpha virus family, and the vaccine can detecta spike antibody after 14 days of inoculation in an animal experiment.
Owner:成都新诺明生物科技有限公司 +1
Monoclonal antibody against RBD region of novel coronavirus SARS-CoV-2 spike protein and application of monoclonal antibody
ActiveCN111995675ANeutralize and reduce toxicityLow toxicityImmunoglobulins against virusesAntiviralsComplementarity determining regionHeavy chain
The invention discloses a monoclonal antibody against a RBD region of novel coronavirus SARS-CoV-2 spike protein and application of the monoclonal antibody. The monoclonal antibody specifically bindsto the RBD region of the novel coronavirus SARS-CoV-2 spike glycoprotein. The monoclonal antibody comprises complementarity determining regions of CDRH1, CDRH2, CDRH3 of a heavy chain variable regionand complementarity determining regions of CDRL1, CDRL2, CDRL3 of a light chain variable region. According to the monoclonal antibody against the RBD region of the novel coronavirus SARS-CoV-2 spike protein, the titer is high, the specificity is higher, efficient expressing can be achieved, the monoclonal antibody can specifically bind to the RBD region of the spike protein on the surface of the novel coronavirus SARS-CoV-2 and can be used for the detection of the ovel coronavirus SARS-CoV-2, certain toxicity of the novel coronavirus can be neutralized and reduced, and the effect of preventingor / and treating the novel coronavirus pneumonia is achieved.
Owner:WEIFANG MEDICAL UNIV
Recombinant baculovirus with surface displaying porcine epidemic diarrhea virus S protein
InactiveCN106085969AImprove Surface DisplayHigh expressionSsRNA viruses positive-senseViral antigen ingredientsSurface displayViral Vaccine
The invention provides a recombinant baculovirus with the surface displaying a porcine epidemic diarrhea virus S protein, and a preparation method thereof. The virus adopts PEDV spike protein S1 gene as antigen gene, the recombinant virus is constructed by using an insect baculovirus vector expression system, and an S1 protein is successfully expressed and displayed on the surface of the virus. The recombinant virus is used to immunize and inoculate mice as a PEDV pseudo-virus vaccine, and serum neutralization test and lymphocyte propagation experiment analysis shows that the recombinant virus can arouse an effective immune protection effect.
Owner:杭州洪晟生物技术股份有限公司
Monoclonal antibody for novel coronavirus virus SARS-CoV-2 spike protein RBD region and application of monoclonal antibody
ActiveCN111995678AHigh potencyStrong specificityImmunoglobulins against virusesAntiviralsComplementarity determining regionHeavy chain
The invention discloses a monoclonal antibody for a novel coronavirus virus SARS-CoV-2 spike protein RBD region and an application of the monoclonal antibody, and belongs to the field of cell immunology and the like. The monoclonal antibody is specifically combined with a novel coronavirus virus SARS-CoV-2 spike glycoprotein RBD region, and comprises complementarity determining regions CDRH1, CDRH2 and CDRH3 of a heavy chain variable region and complementarity determining regions CDRL1, CDRL2 and CDRL3 of a light chain variable region; the monoclonal antibody for the novel coronavirus virus SARS-CoV-2 spike protein RBD region is high in titer and specificity, can be efficiently expressed, can be specifically combined with the spike protein RBD region on the surface of the novel coronavirusvirus SARS-CoV-2, can be used for detecting the novel coronavirus virus SARS-CoV-2, and has a broad application prospect. Certain novel coronavirus virus toxicity can be neutralized and weakened, andthe effect of preventing or / and treating novel coronavirus virus pneumonia is achieved.
Owner:WEIFANG MEDICAL UNIV
A novel sars immunogenic composition
InactiveUS20160376321A1Preventing and delaying onsetRelieve symptomsSsRNA viruses negative-senseBiocideMedicineBinding domain
Embodiments of the disclosure concern immunogenic compositions and methods for treating or preventing Severe acute respiratory syndrome (SARS). The compositions and methods concern a portion of the receptor-binding domain (RBD) of the SARS-CoV spike protein. In at least particular cases, a mutated version of a portion of the RBD is utilized, such as a deglycosylated mutant of the RBD.
Owner:BAYLOR COLLEGE OF MEDICINE
Novel coronavirus COVID-19 antigen fluorescence detection kit and preparation method thereof
The invention relates to the technical field of biology, and provides a novel coronavirus COVID-19 antigen fluorescence detection kit and a preparation method thereof. The kit comprises a bottom plate. A sample pad, a combination pad, a coating film and absorbent paper are laid on the bottom plate. The combination pad contains a fluorescent microsphere labeled novel coronavirus COVID-19 Spike protein monoclonal antibody and a fluorescent microsphere labeled goat anti-chicken antibody. The coating film comprises a detection area coating T and a quality control area coating C. The detection areais coated with a novel coronavirus COVID-19 Spike protein monoclonal antibody of which the epitope is different from that of the novel coronavirus COVID-19 Spike protein monoclonal antibody on the combination pad. The quality control area is coated with a chicken IgY antibody. The kit disclosed by the invention can accurately detect whether the novel coronavirus Spike protein exists in a sample or not, so that on-site rapid detection is realized.
Owner:SHENZHEN ZIJIAN BIOTECH
Coronaviruses epitope-based vaccines
ActiveUS20180334480A1SsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeNeutralizing antibody
Provided are polypeptides derived from the coronaviruses (CoVs) Spike protein (S) characterized by high affinity and specificity the S receptor and its neutralizing antibodies. Further provided are compositions and vaccines, and vaccine-based therapies targeting CoVs, and SARS and MERS viruses in particular.
Owner:RAMOT AT TEL AVIV UNIV LTD
Method for preparing recombinant novel coronavirus Spike protein
ActiveCN112375784AReduce manufacturing costPolypeptide with localisation/targeting motifSsRNA viruses positive-senseSecretion expressionPolynucleotide
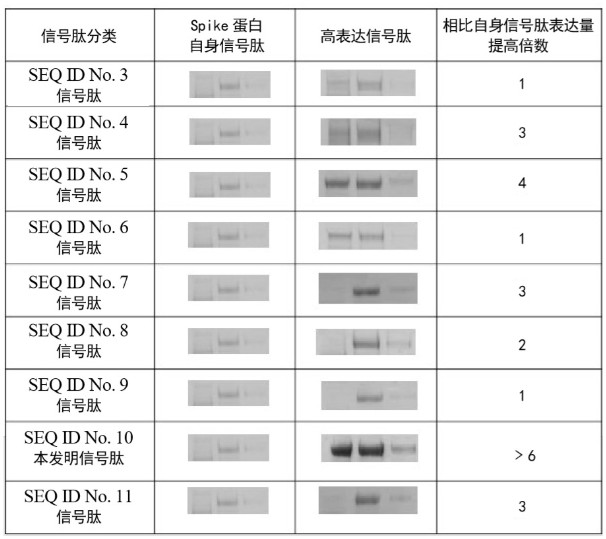
The invention provides a method for preparing a recombinant novel coronavirus Spike protein. The invention firstly provides application of the following polypeptides as a signal peptide in preparationof recombinant novel coronavirus Spike protein: polypeptides composed of the amino acid sequence as shown in SEQ ID No. 10. By adopting a specific signal peptide, an expression vector containing polynucleotide for encoding the recombinant novel coronavirus Spike protein is constructed, and mammalian cells are transfected to secrete and express the recombinant novel coronavirus Spike protein, so that the secretory expression level of the Spike protein in HEK293 cells can be remarkably improved.
Owner:ACROBIOSYSTEMS INC
Application of natural sulfated polysaccharide derived from marine organisms as drug for resisting coronavirus and induced diseases
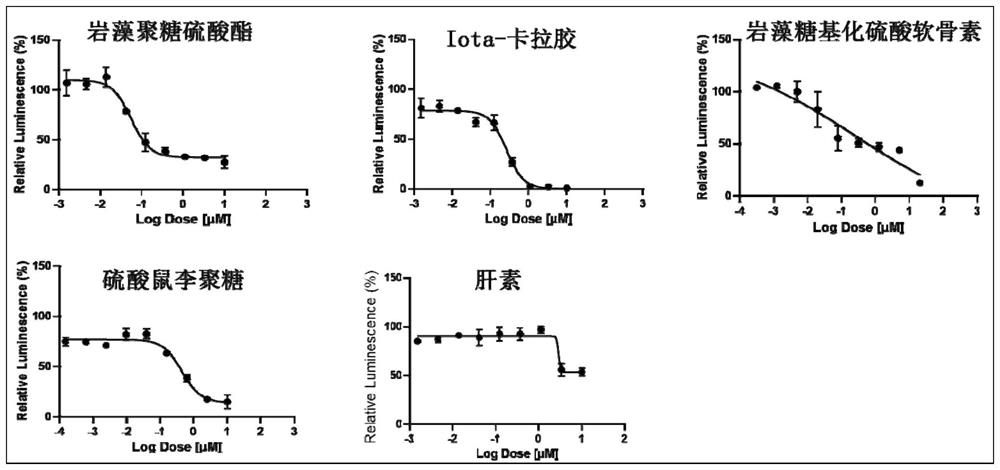
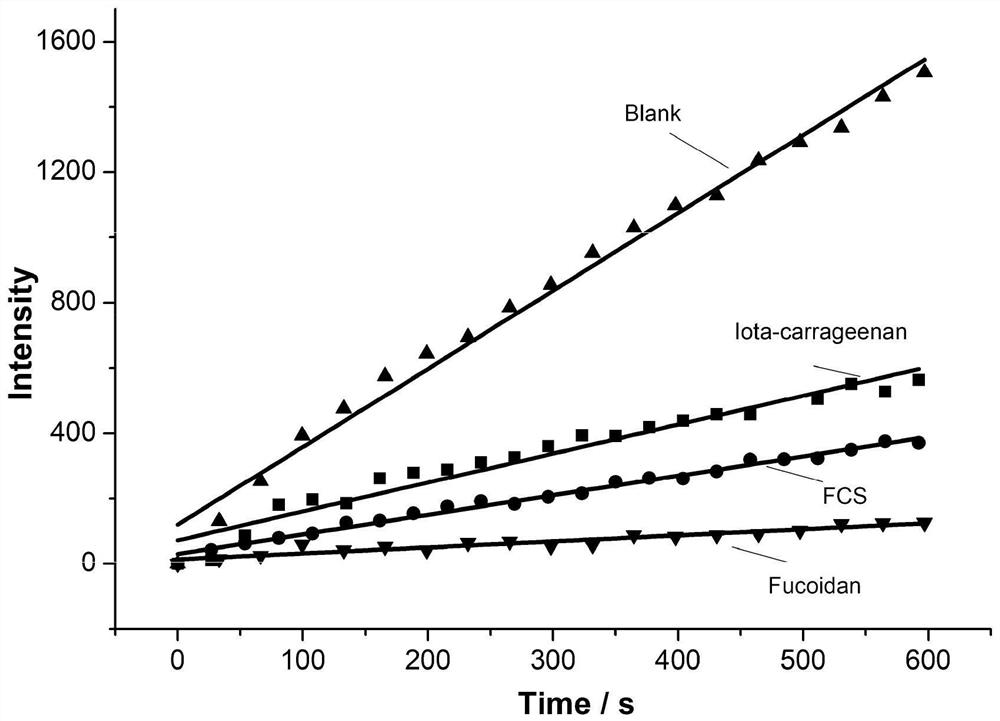
ActiveCN111773240AInhibit bindingInhibition of replicationOrganic active ingredientsAntiviralsSulfated polysaccharidesPharmaceutical drug
Owner:OCEAN UNIV OF CHINA
Soluble fragments of the SARS-cov spike glycoprotein
InactiveCN1852921AEasy to produceGood antigenicityPeptide/protein ingredientsAntibody ingredientsAptamerVaccination
The invention relates to the spike protein from the virus (SARS-CoV) that is etiologically linked to severe acute respiratory syndrome (SARS); polypeptides and peptide fragments of the spike protein; nucleic acid segments and constructs that encode the spike protein, polypeptides and peptide fragments of the spike protein, and coupled proteins that include the spike protein or a portion thereof; peptidomimetics; vaccines; methods for vaccination and treatment of severe acute respiratory syndrome; antibodies; aptamers; and kits containing immunological compositions, or antibodies (or aptamers) that bind to the spike protein.
Owner:THE GOVERNMENT OF THE U S A REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
COVID-19 subunit vaccine as well as preparation method and application thereof
ActiveCN113321739AEasy extractionEasy to purifySsRNA viruses positive-senseViral antigen ingredientsAdjuvantReceptor
Fusion protein RBD-hFc / RBD-His obtained by combining a novel coronavirus (SARS-CoV-2) spike protein receptor binding structural domain (RBD) with a human immunoglobulin hFc domain or His tag through a genetic engineering means is convenient to extract and purify, stable and controllable in quality, short in time consumption and capable of being produced on a large scale. A vaccine composition prepared from the fusion protein and an adjuvant can increase the solubility and stability of a vaccine, enhance the immunogenicity of the vaccine and prolong the half-life period of the vaccine in vivo. The fusion protein and the vaccine composition thereof can inhibit replication and transmission of SARS-CoV-2 wild type and / or variant strains or prevent the SARS-CoV-2 wild type and / or variant strains from settling in a host, so that novel coronavirus pneumonia caused by the SARS-CoV-2 wild type and / or variant strains can be effectively prevented and / or treated.
Owner:广东克冠达医药科技有限公司
Soluble Fragments of The Sars-Cov Spike Glycoprotein
InactiveUS20090304683A1Easy to produceImprove resistance to degradationAntibacterial agentsOrganic active ingredientsVaccinationPeptide fragment
The invention relates to the spike protein from the virus (SARS-CoV) that is etiologically linked to severe acute respiratory syndrome (SARS); polypeptides and peptide fragments of the spike protein; nucleic acid segments and constructs that encode the spike protein, polypeptides and peptide fragments of the spike protein, and coupled proteins that include the spike protein or a portion thereof; peptidomimetics; vaccines; methods for vaccination and treatment of severe acute respiratory syndrome; antibodies; aptamers; and kits containing immunological compositions, or antibodies (or aptamers) that bind to the spike protein.
Owner:GOVERNMENT OF THE US SEC THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Armed chimeric antigen receptor cell targeting coronavirus SPIKE, preparation methods and applications
PendingCN111675765AEffective targetingHigh kill rateAnimal cellsVirusesAmino acidAcid amino sequences
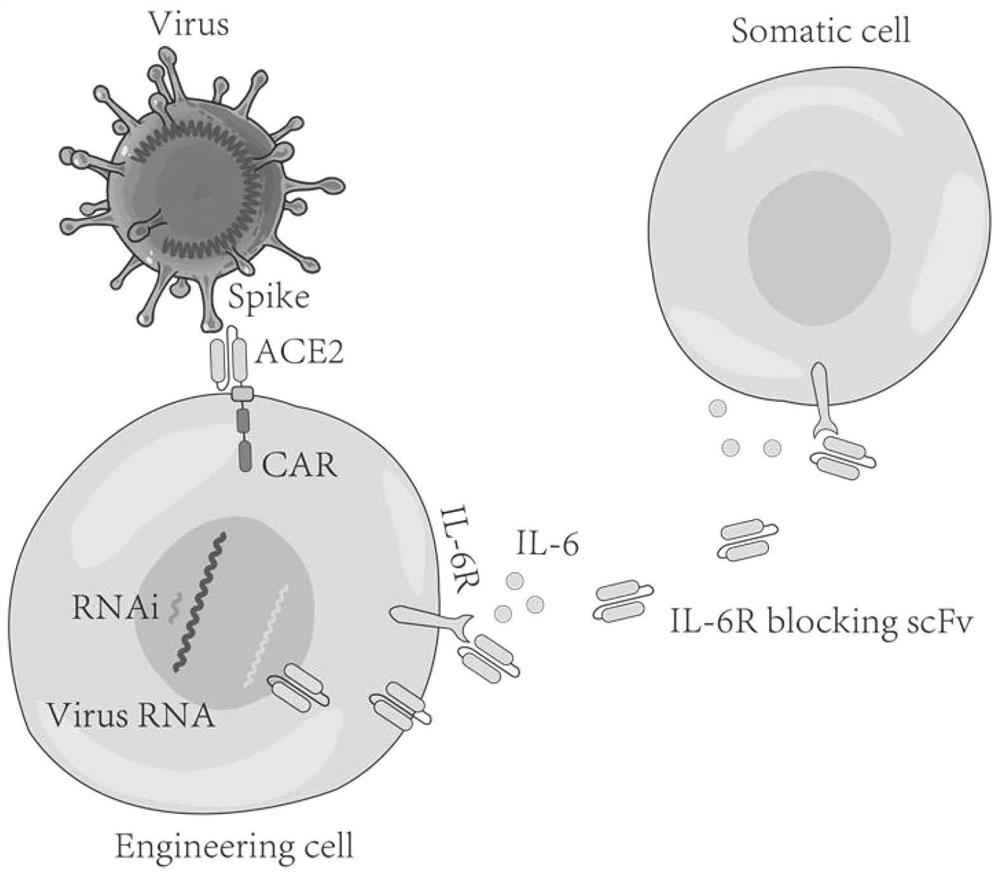
The invention relates to a coding amino acid sequence of an armed chimeric antigen receptor capable of specifically knocking out and targeting the SARS-CoV-2 virus SPIKE protein constructed by SARS-CoV-2 virus RNA polymerase, an immune response cell modified by the amino acid sequence, preparation methods and applications. The recombinant vector of the armed chimeric antigen receptor targeting theSARS-CoV-2 virus SPIKE can be constructed by knocking out the SARS-CoV-2 virus RNA polymerase, and a method using the recombinant vector to modify the immune response cell is also constructed; and the obtained novel functional immune response cell can effectively target and attack various viruses. The preparation method of the immune response cell modified by the armed chimeric antigen receptor targeting the SARS-CoV-2 virus SPIKE is simple in step; and the obtained immune response cell targeting the virus SPIKE and modified by virus replication-dependent RNA polymerase has obvious killing effects on coronavirus.
Owner:NANJING KAEDI BIOTECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com