Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Chimpanzee adenovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
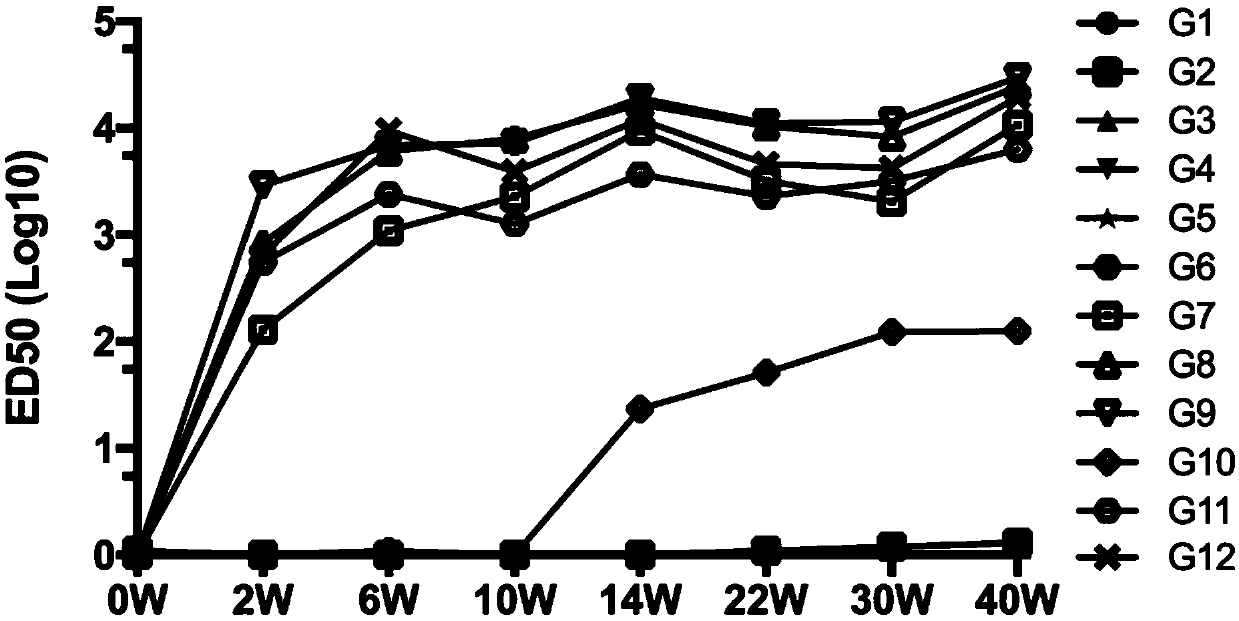
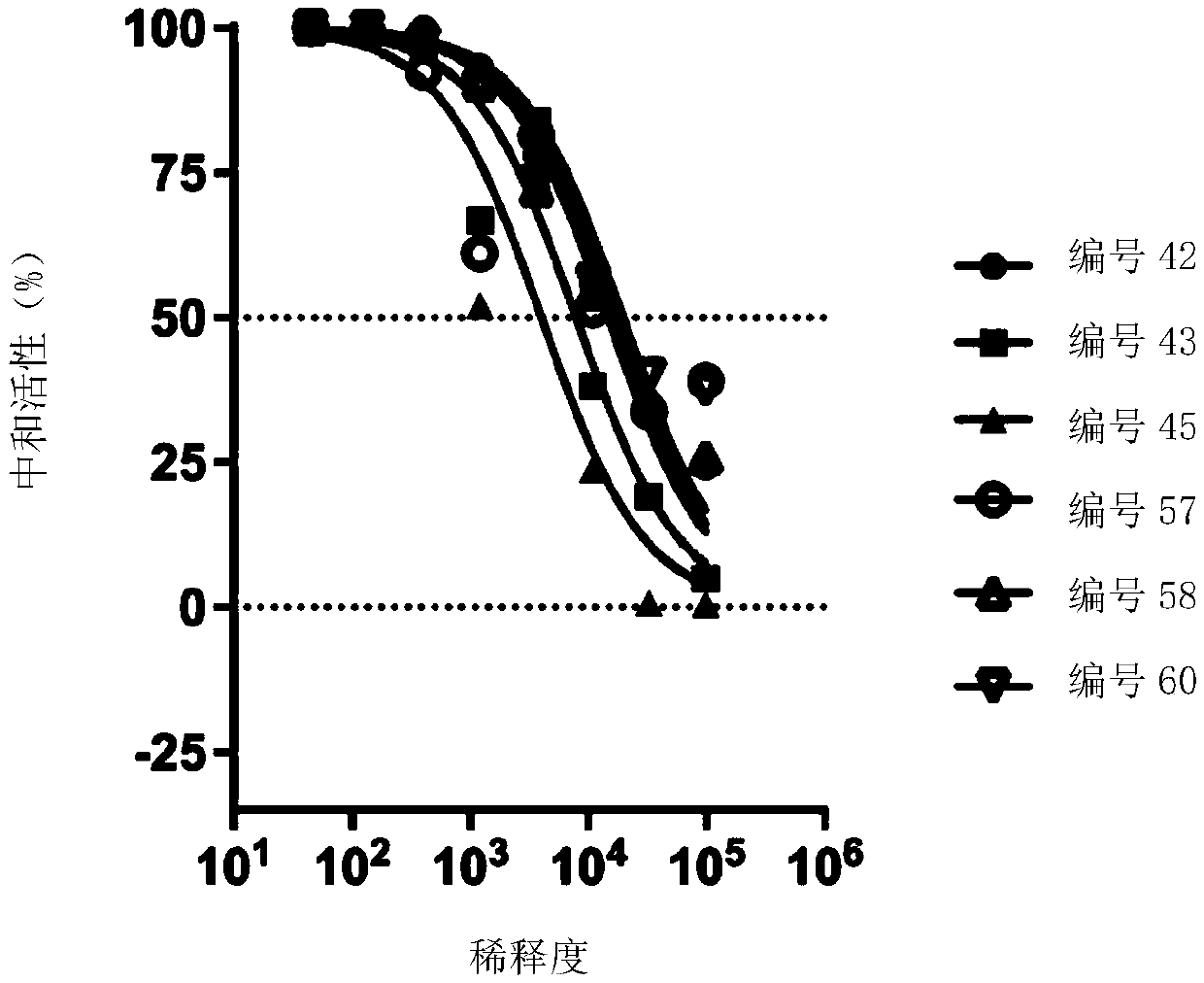
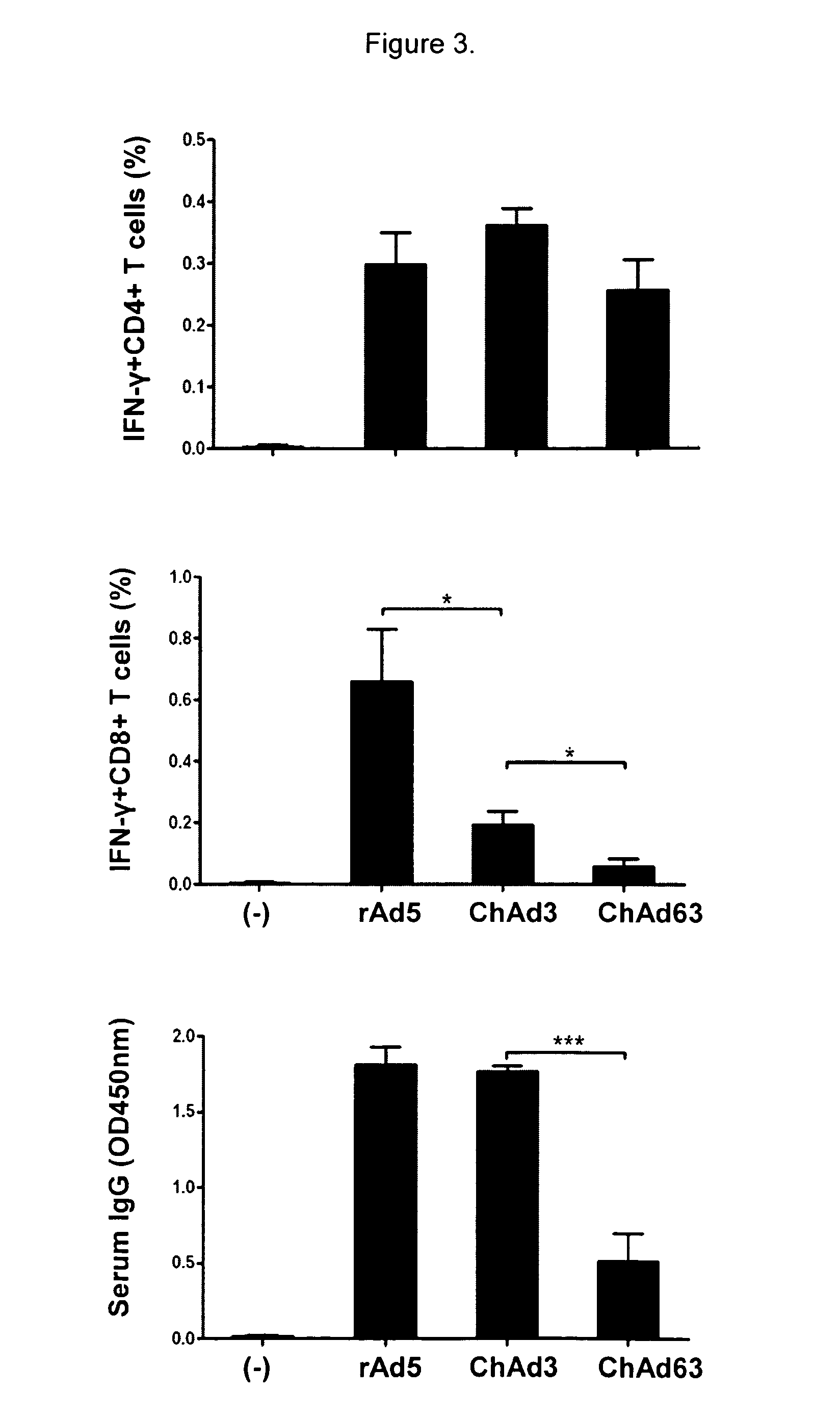
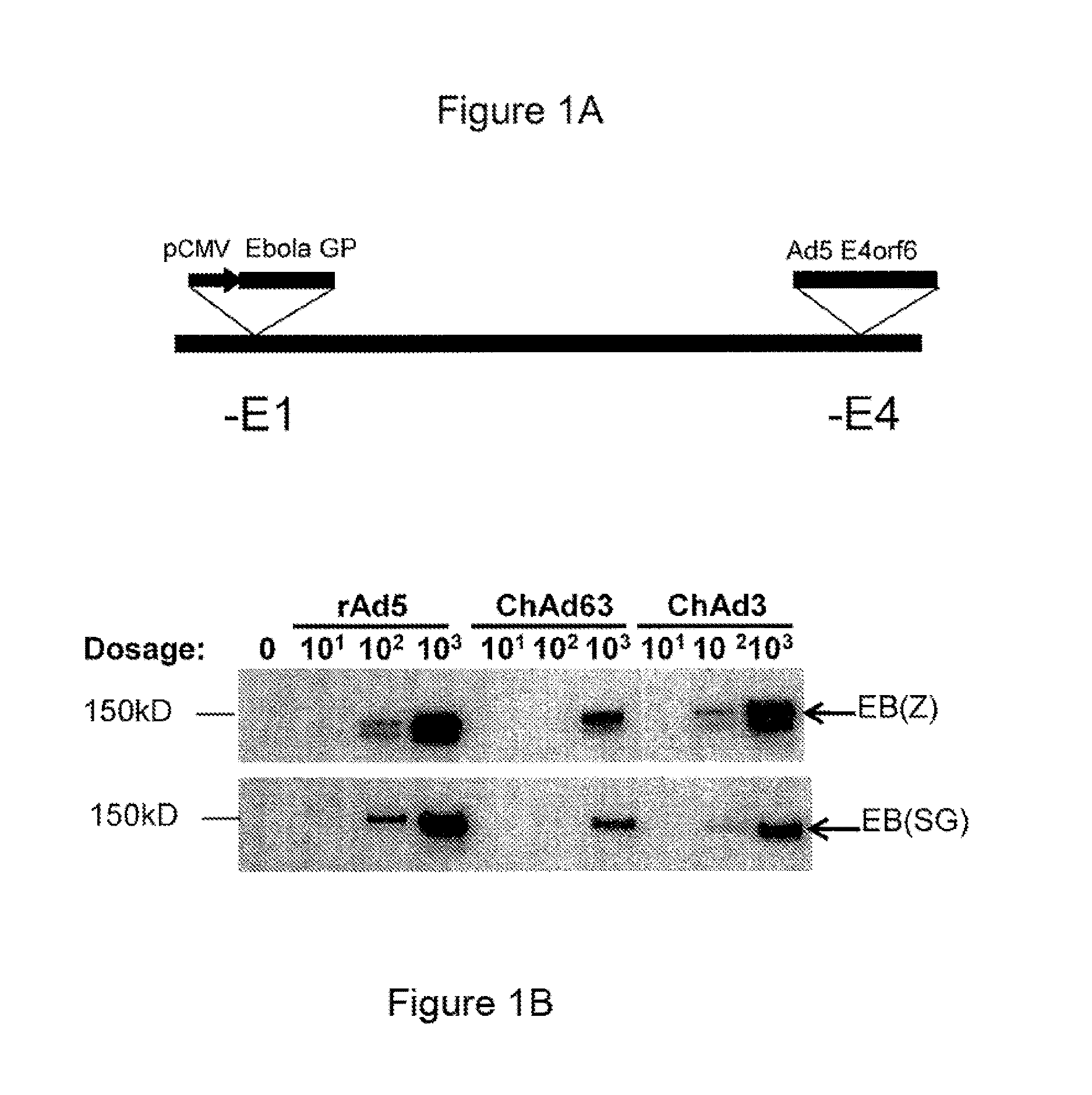
A replication-defective recombinant chimpanzee adenovirus type 3–vectored ebolavirus vaccine (cAd3-EBO), encoding the glycoprotein from Zaire and Sudan species, that offers protection in the ...
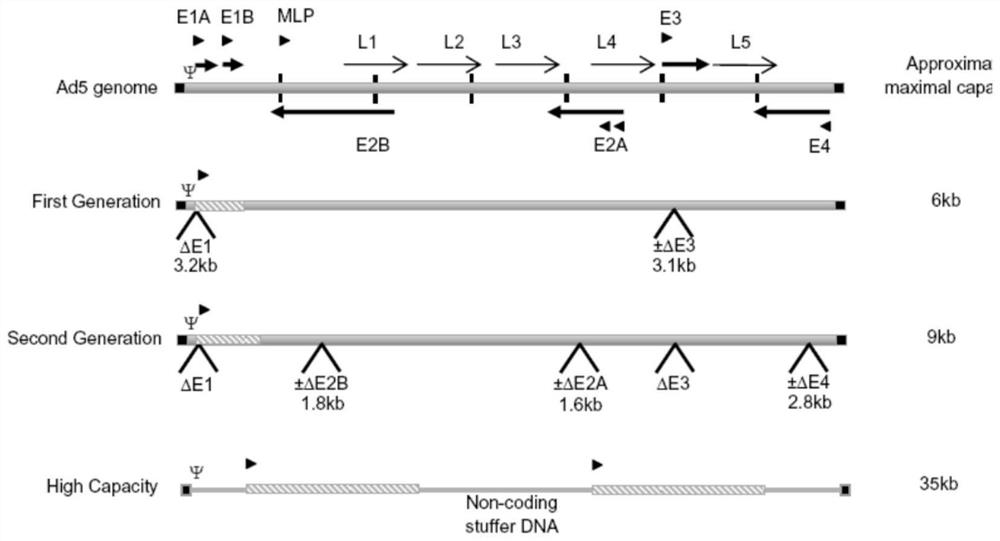
Chimpanzee adenovirus vaccine carriers
The present invention provides recombinant replication-defective adenoviral vectors derived from chimpanzee adenoviruses and methods for generating recombinant adenoviruses in human E1-expressing cell lines. The invention also provides compositions and methods suitable for use for the delivery and expression of transgenes encoding immunogens against which a boosted immune response is desired. The invention further provides methods of generating clinical grade vector stocks suitable for use in humans. In a particular embodiment the invention contemplates the use of vectors comprising transgenes which encode tumor associated antigens in vaccines and pharmaceutical compositions for the prevention and treatment of cancer.
Owner:MSD ITAL
New coronavirus vaccine based on chimpanzee adenovirus type 68 and MERS-CoV full length membrane protein
ActiveCN110616198AAvoiding deficiencies of pre-existing immunityRetain high titersSsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationCell Membrane Proteins
The present invention discloses a new coronavirus vaccine based on chimpanzee adenovirus type 68 and MERS-CoV full length membrane protein. The recombinant adenovirus is obtained by transfecting an adenovirus packaging cell with a recombinant plasmid and then performing cell culture; the recombinant plasmid is obtained by inserting a specific DNA molecule into a delta-E1 region of a chimpanzee adenovirus vector AdC68; the specific DNA molecule has a full-length MERS-CoV Spike protein encoding gene; and the adenovirus packaging cell has an adenovirus E1 gene. The present invention also protectsthe recombinant adenovirus expressing the full-length MERS-CoV Spike protein; and a starting strain of the recombinant adenovirus is chimpanzee adenovirus type 68 or non-replicating chimpanzee adenovirus type 68. The developed vaccine against the new coronavirus MERS-CoV has important theoretical guidance value and broad application prospects, and provides a possibility for radical cure of MiddleEast respiratory syndrome.
Owner:TSINGHUA UNIV +1
Chimpanzee adenoviral vector-based filovirus vaccines
This invention provides vaccines for inducing an immune response and protection against filovirus infection for use as a preventative vaccine in humans. In particular, the invention provides chimpanzee adenoviral vectors expressing filovirus proteins from different strains of Ebolavirus (EBOV) or Marburg virus (MARV).
Owner:UNITED STATES OF AMERICA +1
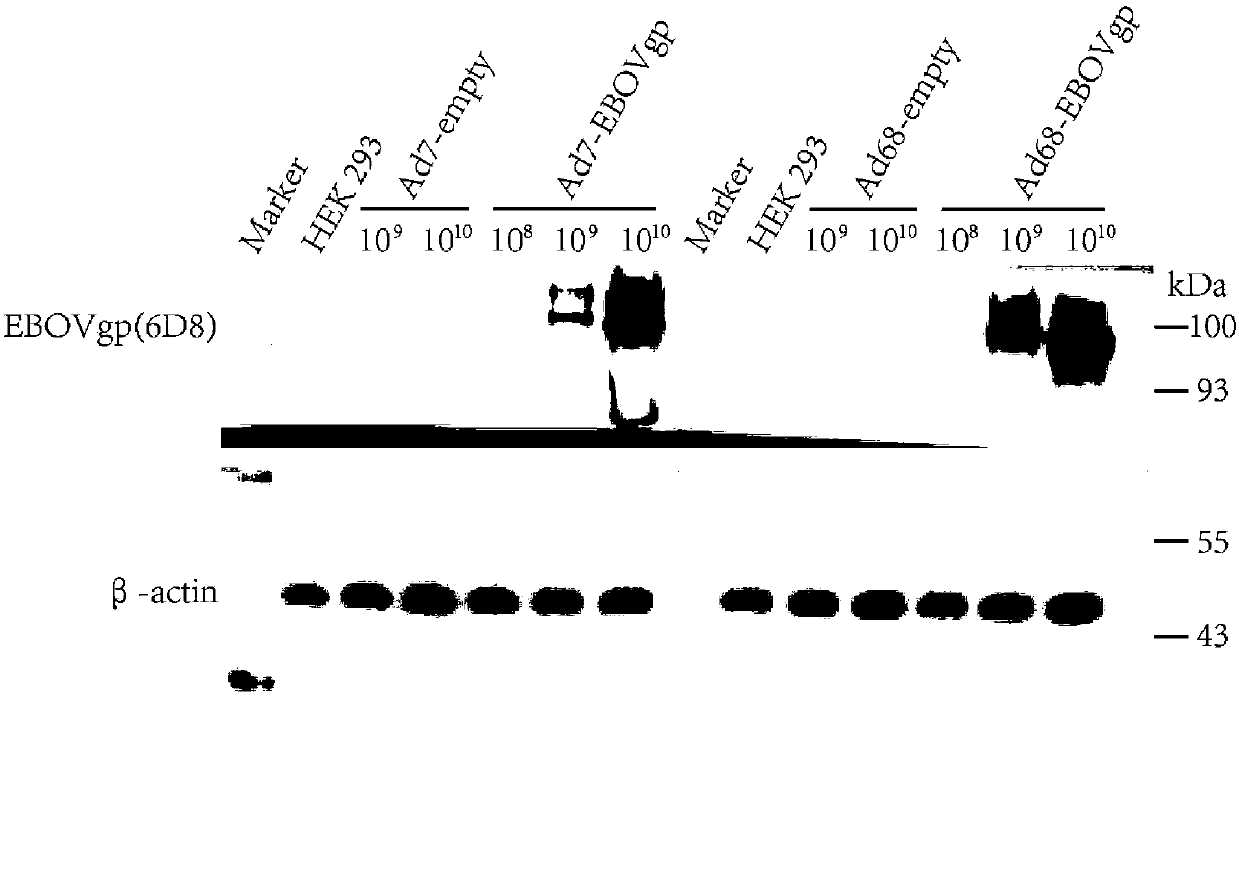
Expression vector based on adenovirus AdC68 and construction method thereof
The invention relates to an expression vector based on an adenovirus AdC68 and a construction method thereof. By improving a construction method, the vaccine vector based on a chimpanzee adenovirus AdC68 genome is constructed. The vaccine vector can be applied to preparation of a vaccine capable of high-efficiency expression and having good immunogenicity.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Expression vector based on adenovirus AdC7 and its construction method
The invention relates to an expression vector based on adenovirus AdC7 and its construction method. An effective strategy is designed for successfully construct the new expression vector based on chimpanzee adenovirus AdC7. The vaccine vector can be applied to the preparation of high expression viral vaccines with good immunogenicity.
Owner:中国科学院上海免疫与感染研究所
Methods for the induction of ebola virus-specific immune responses comprising administering a replication-defective chimpanzee adenovirus vector expressing the ebola virus glycoprotein
ActiveUS9526777B2SsRNA viruses negative-senseViral antigen ingredientsViral glycoproteinMarburg virus
This invention provides vaccines for inducing an immune response and protection against filovirus infection for use as a preventative vaccine in humans. In particular, the invention provides chimpanzee adenoviral vectors expressing filovirus proteins from different strains of Ebola virus (EBOV) or Marburg virus (MARV).
Owner:UNITED STATES OF AMERICA +1
Chimpanzee adenovirus vector based Ebola virus vaccine
The invention relates to a chimpanzee adenovirus vector based Ebola virus vaccine. The chimpanzee adenovirus vector based Ebola virus vaccine is characterized in that replication-defective chimpanzeeadenovirus vector is utilized to build a novel Ebola virus vaccine. The vaccine vector is applicable to preparation of a virus vaccine with efficient expression and high immunogenicity. The Ebola virus vaccine is simple to prepare, low in cost, high in safe, and free from additives.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI +1
Zika virus vaccine based on chimpanzee adenovirus vector and preparation method thereof
ActiveCN107988258AEfficient infectionImprove scalabilitySsRNA viruses positive-senseViral antigen ingredientsZika virusNeutralizing antibody
The invention discloses a zika virus vaccine based on a chimpanzee adenovirus vector and a preparation method thereof, which belong to the field of a biotechnology and virology. A chimpanzee adenovirus AdC 7 is constructed as a replication defective type recombinant expression vector with favorable immunogenicity and hereditary stability, and the novel zika virus vaccine is prepared based on the vector. After mice are immunized through the vaccine, a neutralizing antibody aiming at a zika virus can be effectively induced, so that the mice are protected against being infected, and meanwhile, tissues and organs of the mice can be protected against being damaged.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Respiratory syncytial virus full-length pre-fusion glycoprotein nucleotide sequence, recombinant adenovirus vector and application product thereof
ActiveCN112226444AEasy to prepareRetain high titersSsRNA viruses negative-senseViral antigen ingredientsNucleotideRespiratory syncytial virus (RSV)
The invention provides a respiratory syncytial virus full-length pre-fusion glycoprotein nucleotide sequence, a recombinant adenovirus vector and an application product thereof. The full-length pre-fusion glycoprotein nucleotide sequence comprises four mutation loci including S155C, S190F, V207L and S290C, a transmembrane region and an intracellular region of fusion glycoprotein are reserved, anda sequence table of the full-length pre-fusion glycoprotein nucleotide sequence is shown as a sequence 1. The invention further provides a chimpanzee replication-defective recombinant adenovirus vector pChAd63 or a human replication-defective recombinant adenovirus vector pAd26 containing the full-length pre-fusion glycoprotein nucleotide sequence, an initial strain of the adenovirus vector is chimpanzee adenovirus 63 type or human adenovirus 26 type, adenovirus E1 and E3 regions are deleted, and E4orf6 is replaced with human 5-type adenovirus E4orf6. The invention further provides a product applying the recombinant adenovirus. After the recombinant adenovirus vector is used for immunizing animals, the bodies can be induced to generate strong cellular immunity and humoral immunity reactions in a short time, and the generated antibody has high recognition capability on pre-fusion F and has a good immune effect and an immune protection effect.
Owner:BEIJING JIAOTONG UNIV
Novel adenovirus
There is provided adenoviral vectors encoding a mycobacterial antigen derived from a chimp adenovirus, and to related aspects.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Simian adenovirus and hybrid adenoviral vectors
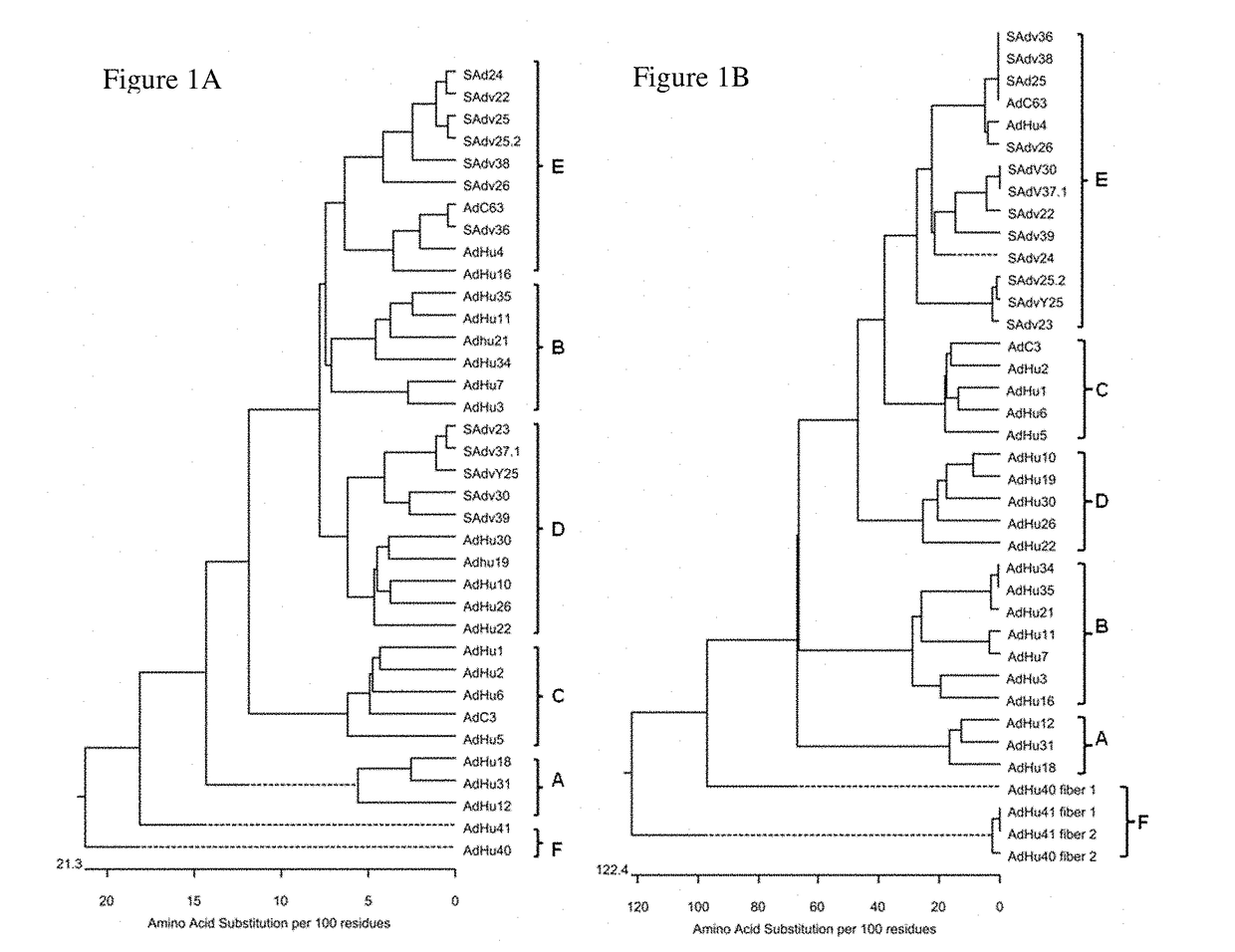
Recombinant adenoviral vectors, immunogenic compositions thereof and their use in medicine, and methods for generating recombinant adenoviral vectors are provided. In particular, the an adenovirus vector having a capsid derived from chimpanzee adenovirus AdY25, wherein the capsid encapsidates a nucleic acid molecule comprising an exogeneous nucleotide sequence of interest are provided.
Owner:OXFORD UNIV INNOVATION LTD
A kind of rabies vaccine and preparation method thereof
The present invention relates to a new rabies vaccine and method for preparation thereof. The present inventor has constructed, on the basis of a vaccine vector of the genome of chimpanzee adenovirus AdC68, a new type of rabies vaccine vector. The present invention also provides a viral vaccine prepared using said vaccine vector, highly expressed, and having good immunogenicity.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI +1
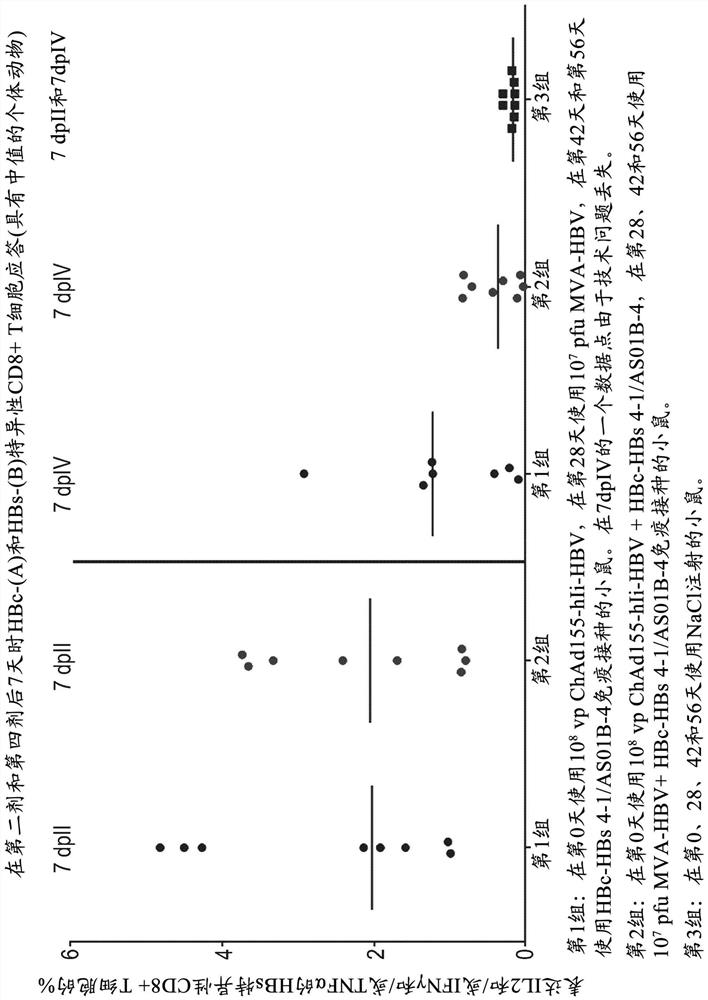
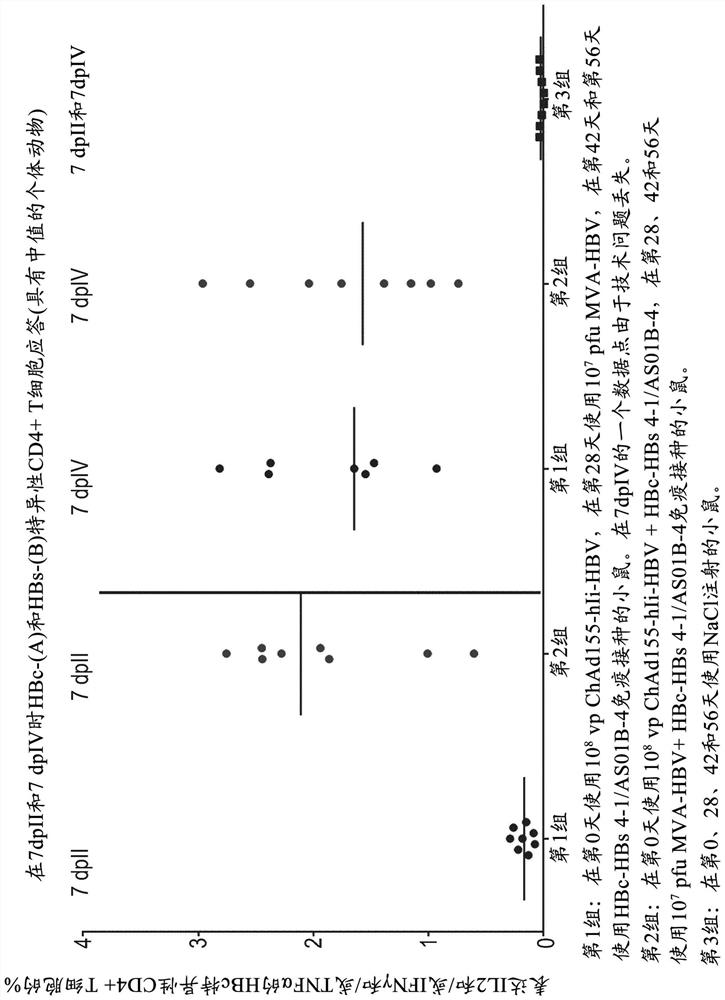
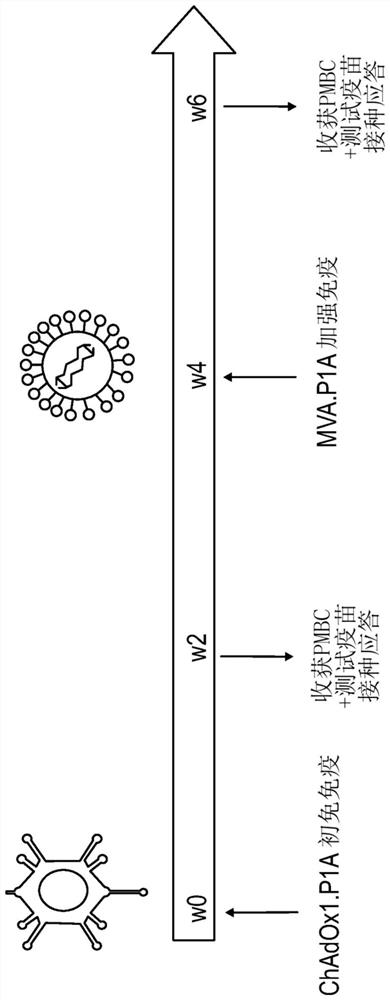
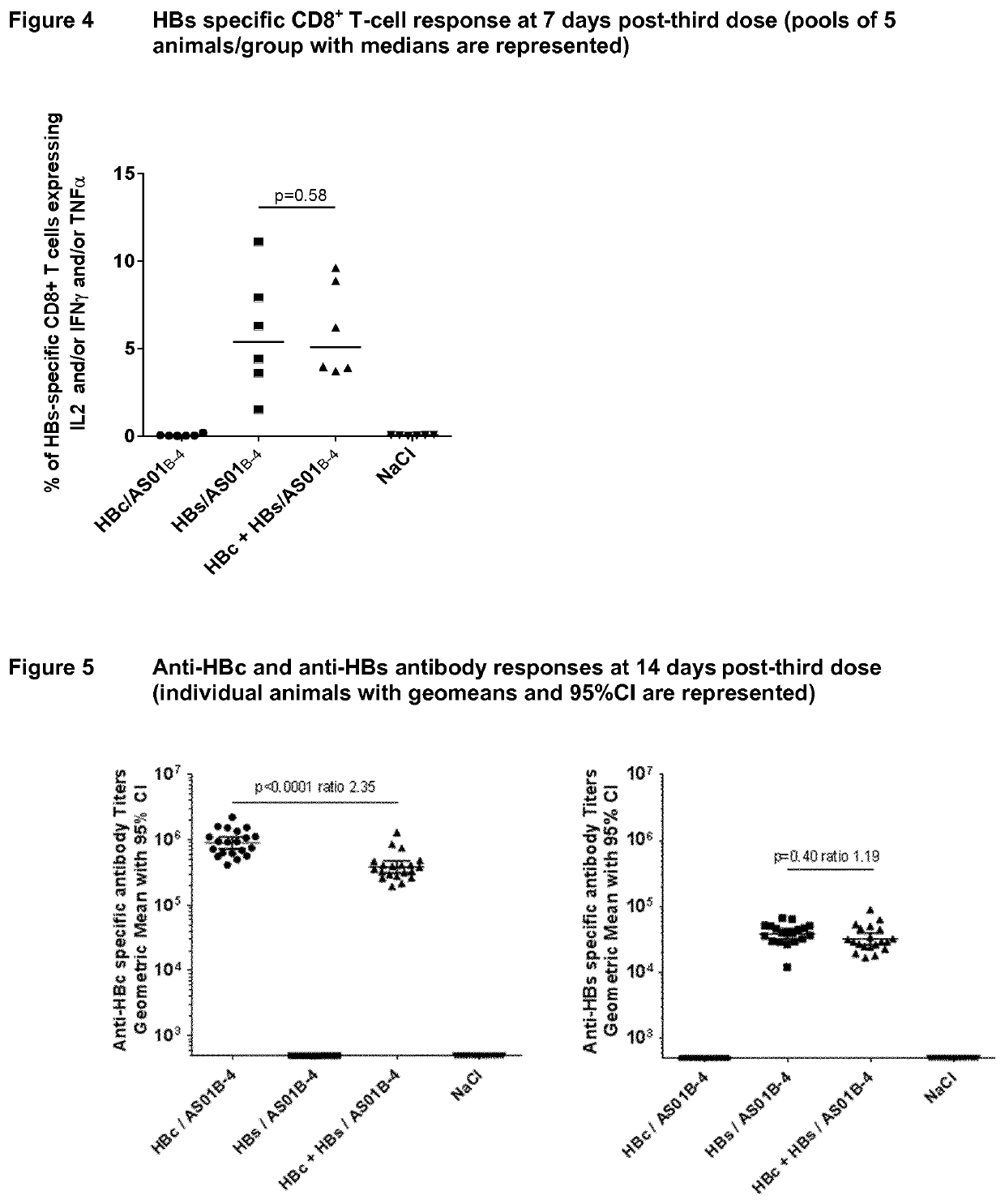
Hepatitis b immunisation regimen and compositions
PendingCN113573730AViral antigen ingredientsWhole-cell/virus/DNA/RNA ingredientsHepatitis B virus core AntigenAdjuvant
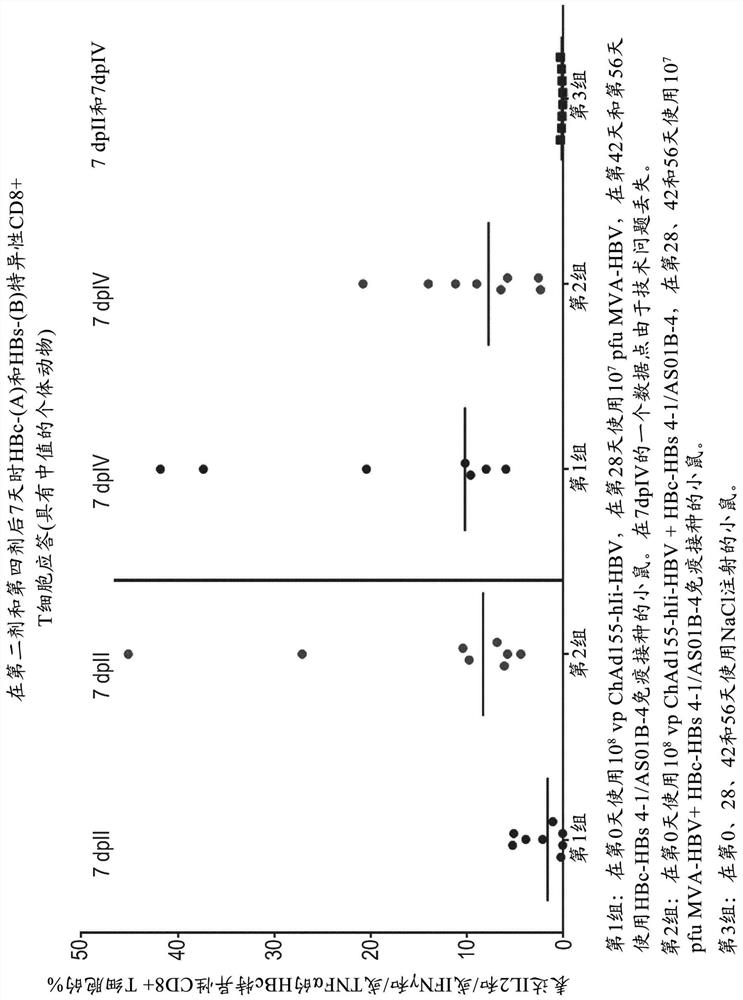
There is provided a method of treating chronic hepatitis B infection (CHB) and / or chronic hepatitis D infection (CHD) in a human, comprising the steps of: a) administering to the human a composition comprising an antisense oligonucleotide (ASO) 10 to 30 nucleosides in length, targeted to a HBV nucleic acid (an HBV ASO); b) administering to the human a composition comprising a replication-defective chimpanzee adenoviral (ChAd) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); c) administering to the human a composition comprising a Modified Vaccinia Virus Ankara (MVA) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); and d) administering to the human a composition comprising a recombinant hepatitis B surface antigen (HBs), recombinant hepatitis B virus core antigen (HBc) and an adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Therapeutic HPV vaccine based on chimpanzee adenovirus vector as well as preparation method and application of therapeutic HPV vaccine
InactiveCN112138150AEasy to makeImprove securityViral antigen ingredientsVirus peptidesVaccine PotencyAdjuvant
The invention discloses a therapeutic HPV vaccine based on a chimpanzee adenovirus vector as well as a preparation method and application of the therapeutic HPV vaccine. The therapeutic HPV vaccine isan immunogenic HPV recombinant adenovirus vaccine obtained by packaging and processing HPV virus vaccine vector plasmids, and the HPV virus vaccine vector is a replication-defective chimpanzee adenovirus vector. The HPV therapeutic vaccine is constructed by using the replication-defective chimpanzee adenovirus vector, the influence of the pre-stored immunity of a human adenovirus vector in a human body on the vaccine efficacy is overcome, and a vaccine product which is simple to prepare, low in cost, good in safety and free of adjuvants is expected to be obtained. The therapeutic HPV vaccinehas good immunogenicity and anti-tumor efficacy, can induce high-level antigen-specific T cell reaction and anti-tumor efficacy in a mouse body through single muscle immunization, and is expected to show good humoral and cellular immunotherapy effect can be embodied in human clinical tests.
Owner:IMMUNE PATH BIOTECHNOLOGY SUZHOU CO LTD
Viral delivery of neoantigens
Disclosed herein are chimpanzee adenoviral vectors that include neo-antigen encoding nucleic acid sequences derived from a tumor of a subject. Also disclosed are nucleotides, cells, and methods associated with the vectors including their use as vaccines.
Owner:GRITSTONE BIO INC
Chimpanzee adenoviral vector-based filovirus vaccines
ActiveUS20170044571A1SsRNA viruses negative-senseViral antigen ingredientsD'Aguilar virusMarburg virus
This invention provides vaccines for inducing an immune response and protection against filovirus infection for use as a preventative vaccine in humans. In particular, the invention provides chimpanzee adenoviral vectors expressing filovirus proteins from different strains of Ebolavirus (EBOV) or Marburg virus (MARV).
Owner:SABIN VACCINE INST
Chimpanzee adenovirus constructs with lyssavirus antigens
The invention provides adenoviral vectors comprising transgenes encoding Lyssaviral antigens. The vectors can be used to produce vaccines for the prophylaxis, amelioration and treatment of diseases caused by Lyssaviral diseases, e.g., rabies.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Norovirus vaccine based on chimpanzee adenovirus vector and preparation method and application thereof
ActiveCN112156178AEasy to makeImprove securitySsRNA viruses positive-senseViral antigen ingredientsAdjuvantNucleotide
The invention discloses a norovirus vaccine based on a chimpanzee adenovirus vector and a preparation method and application thereof. The norovirus vaccine is prepared by the following steps that a nucleotide sequence of a norovirus target protein is inserted into a recombinant chimpanzee adenovirus vector plasmid to construct a recombinant plasmid, and then packaging and processing are carried out to obtain the norovirus recombinant adenovirus vaccine with immunogenicity. According to the norovirus vaccine, the norovirus vaccine is developed for the first time based on the chimpanzee adenovirus vector, and the vaccine cannot be influenced by a pre-stored adenovirus antibody in a human body; the adenovirus vector vaccine is simple to prepare and good in safety, an adjuvant does not need tobe added, and immunization routes such as muscle immunization or oral immunization can be adopted; and the norovirus preventive vaccine is prepared on the basis of the replication-defective chimpanzee adenovirus vector, and a vaccine product which is easy to prepare, low in cost, good in safety, free of adjuvant and wide in application prospect is expected to be obtained.
Owner:IMMUNE PATH BIOTECHNOLOGY SUZHOU CO LTD
Novel adenovirus
There is provided adenoviral vectors encoding a mycobacterial antigen derived from a chimp adenovirus, and to related aspects.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
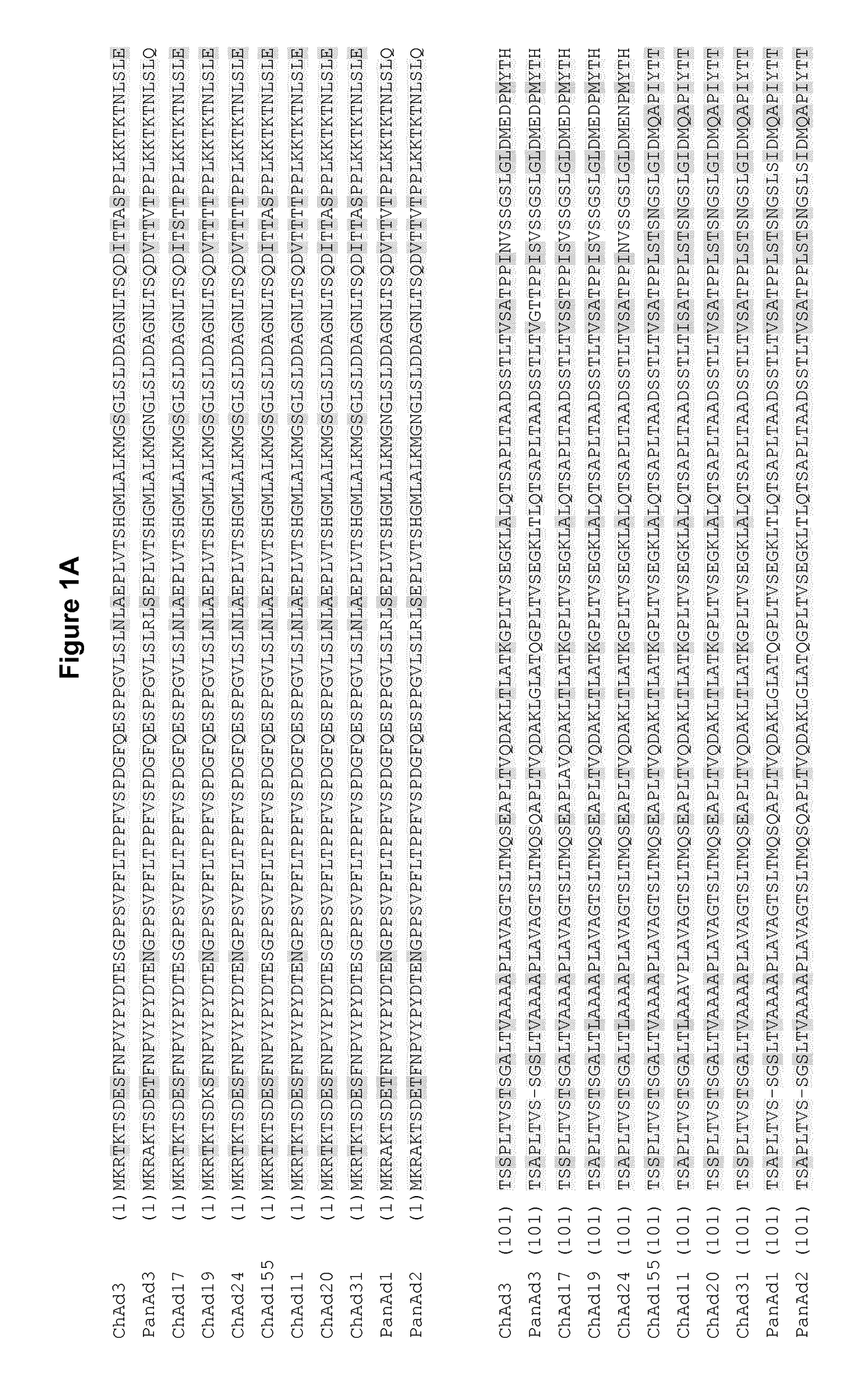
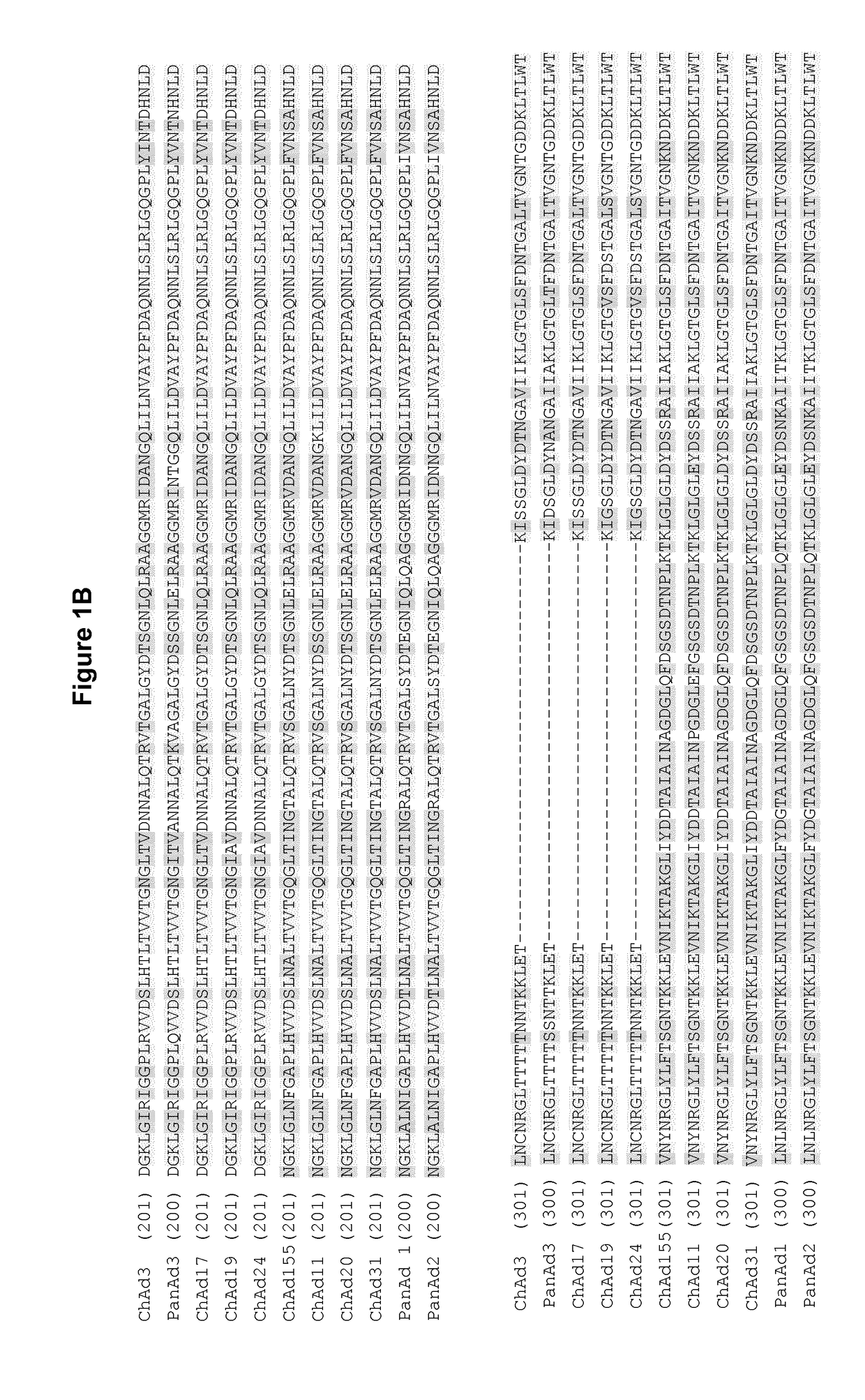
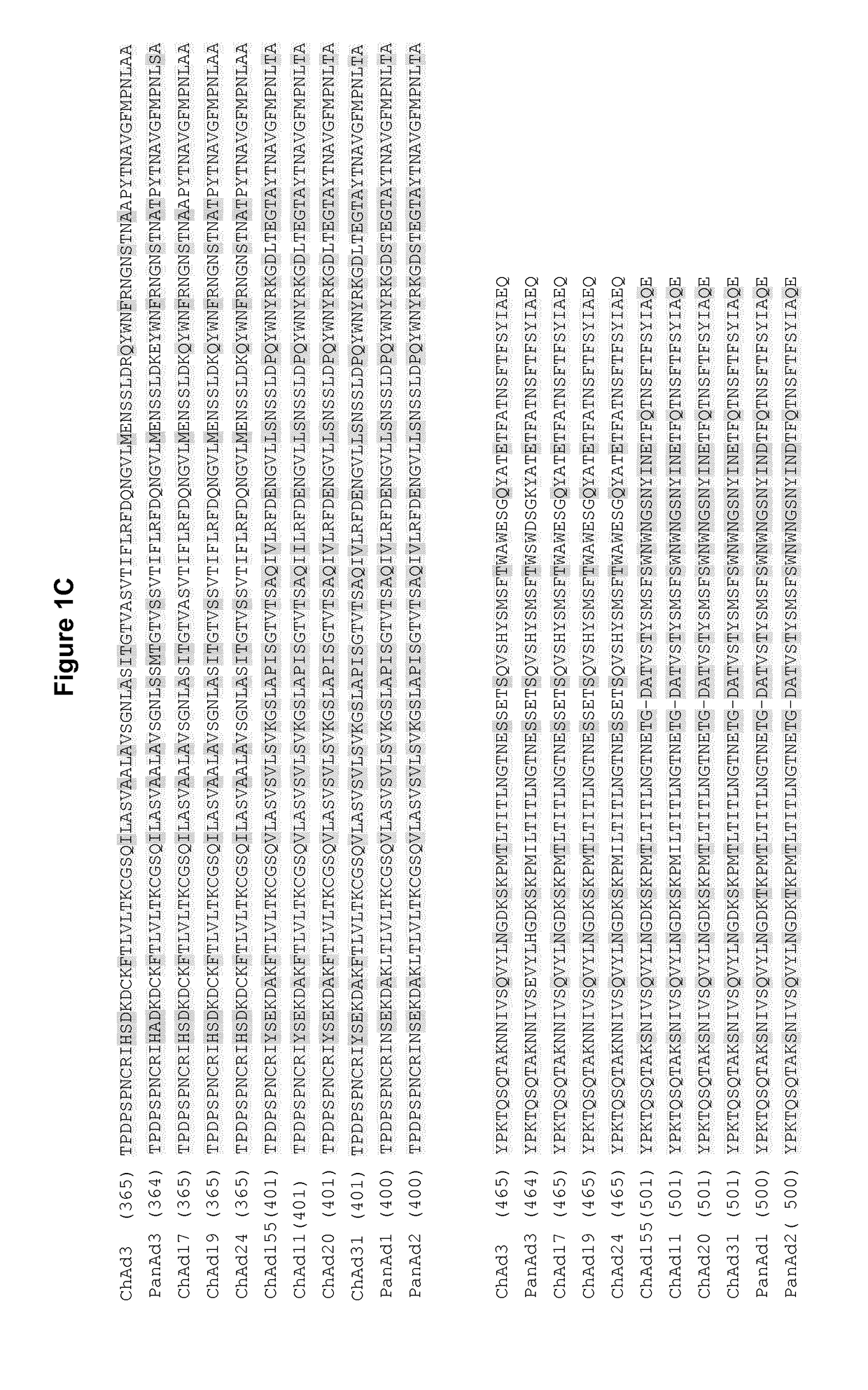
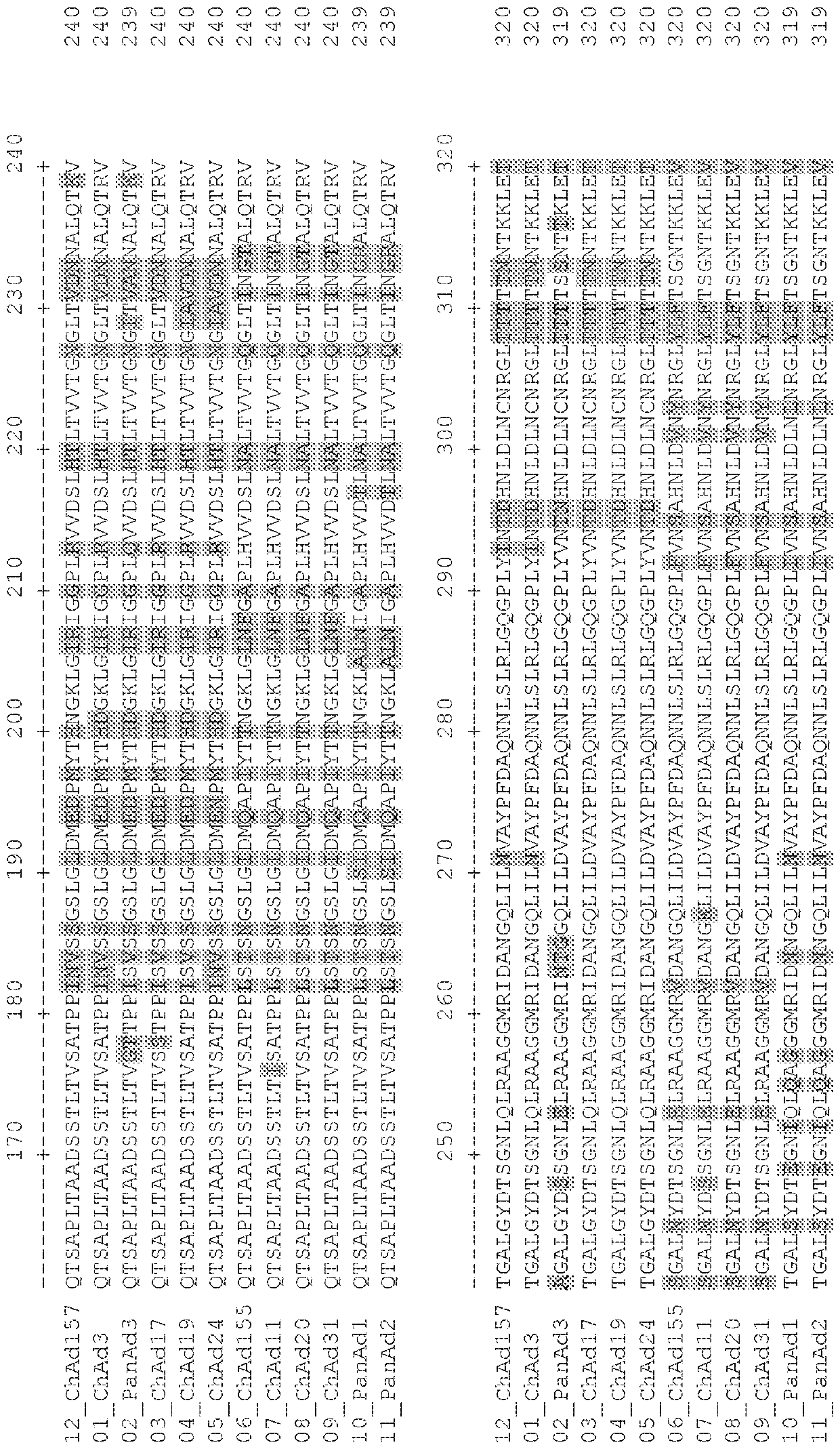
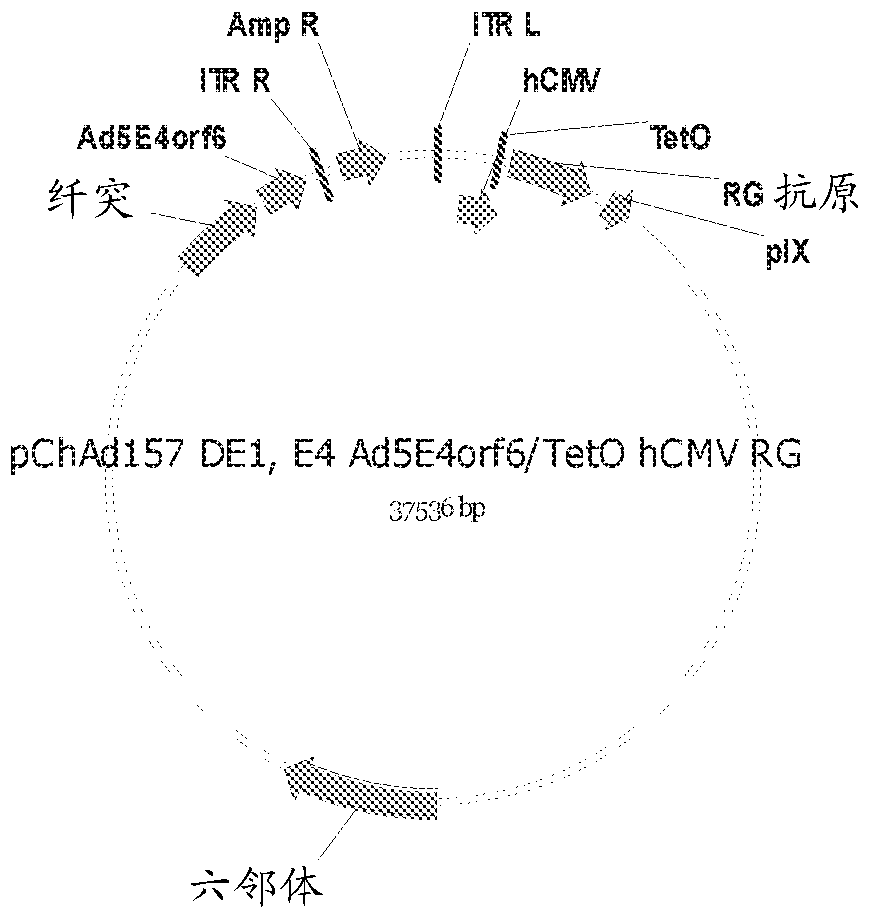
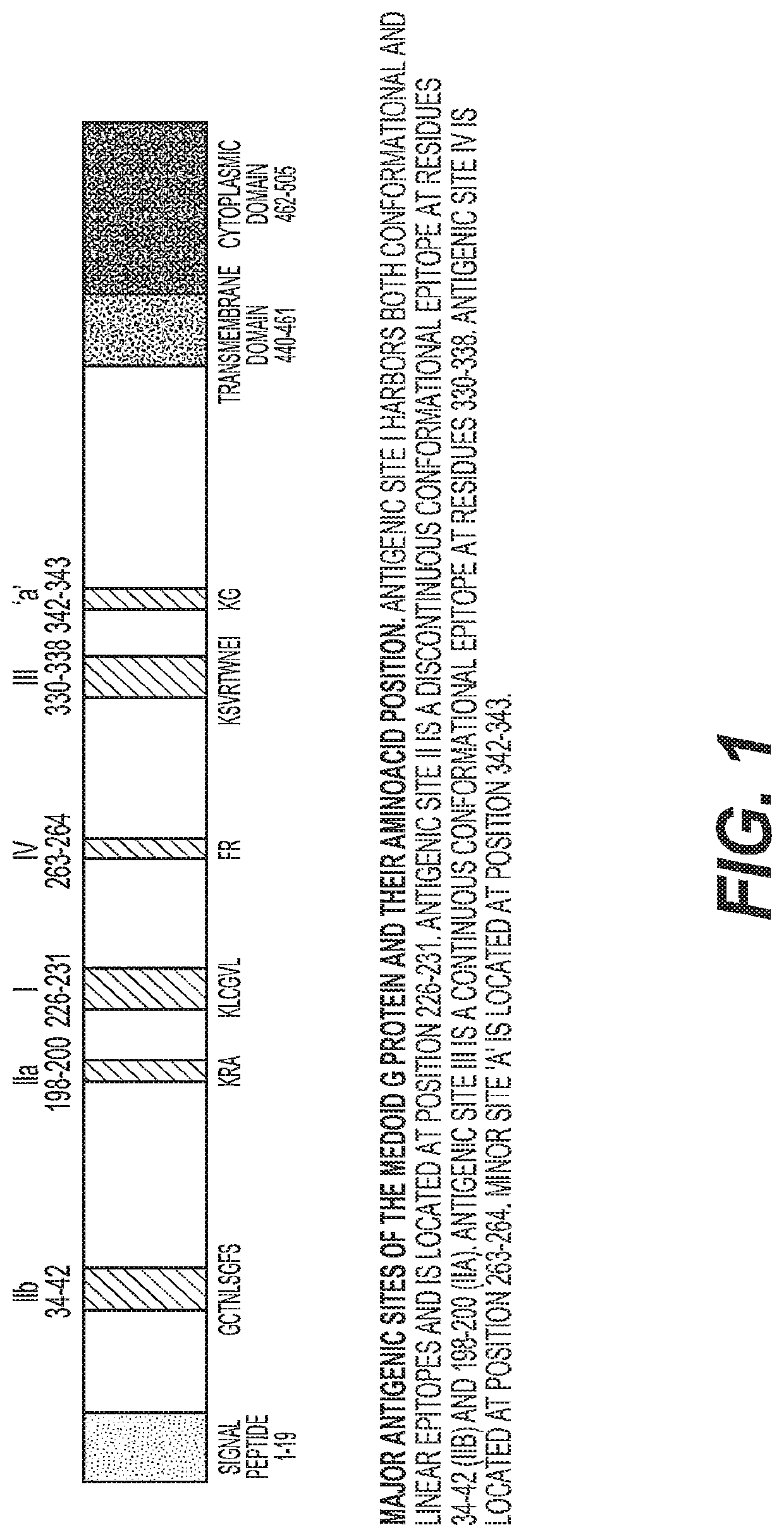
Adenovirus polynucleotides and polypeptides
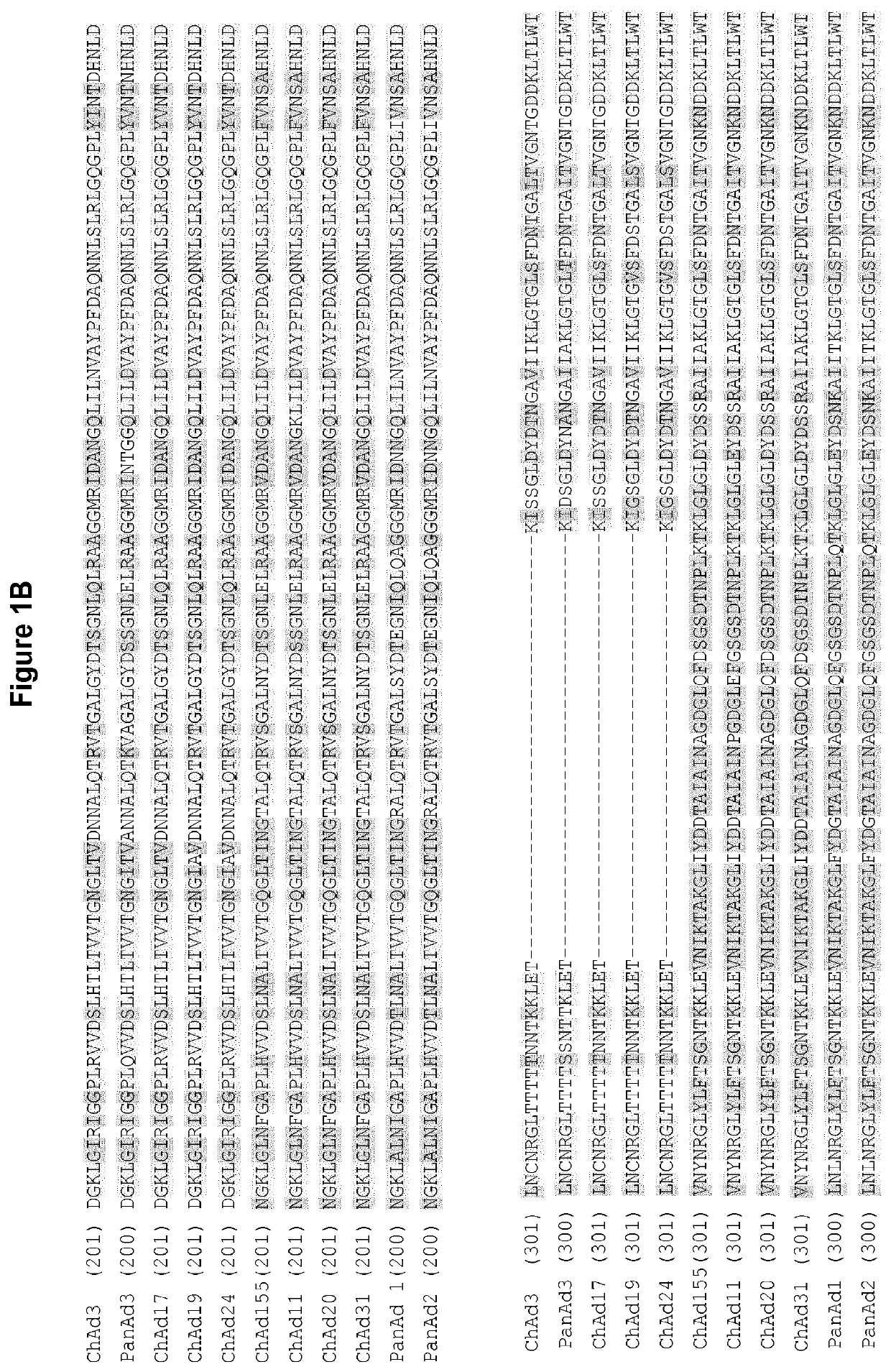
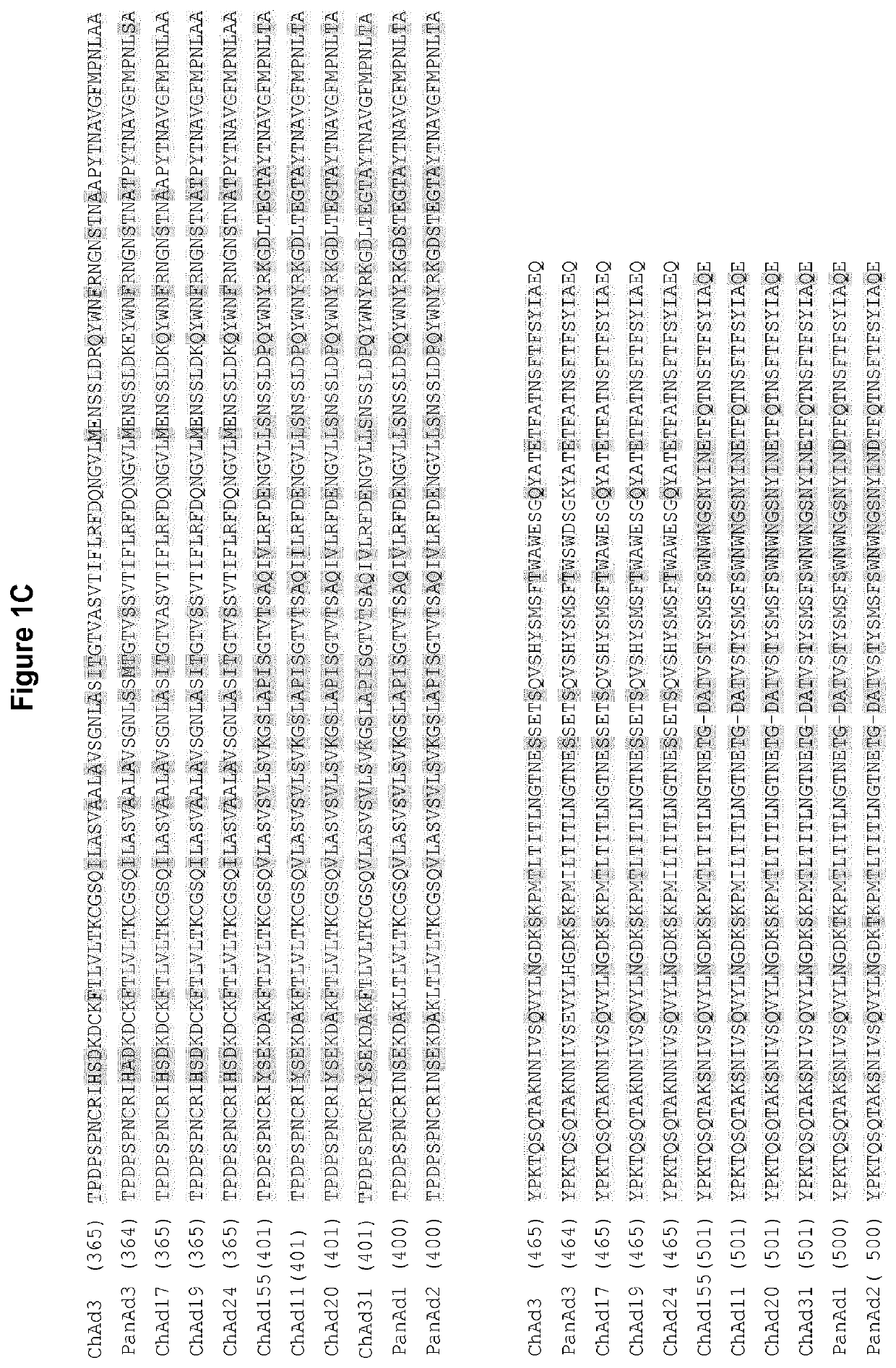
The present invention relates to isolated polynucleotide and polypeptide sequences derived from novel chimpanzee adenovirus ChAd157, as well as to recombinant polynucleotides, vectors, adenoviruses, cells and compositions comprising said polynucleotide and polypeptide sequences.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Hepatitis b immunisation regimen and compositions
There is provided a method of treating chronic hepatitis B infection (CHB) in a human, comprising the steps of: a) administering to the human a composition comprising a replication-defective chimpanzee adenoviral (ChAd) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); b) administering to the human acomposition comprising a Modified Vaccinia Virus Ankara (MVA) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); and c) administering to the human a composition comprising a recombinant hepatitis B surface antigen (HBs), recombinant hepatitis B virus core antigen (HBc) and an adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Adenovirus polynucleotides and polypeptides
The present invention relates to isolated polynucleotide and polypeptide sequences derived from novel chimpanzee adenovirus ChAd157, as well as to recombinant polynucleotides, vectors, adenoviruses, cells and compositions comprising said polynucleotide and polypeptide sequences.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Viral vectors encoding cancer/testis antigens for use in a method of prevention or treatment of cancer
PendingCN112789054APolypeptide with localisation/targeting motifTumor rejection antigen precursorsAdjuvantCancer antigen
Owner:LUDWIG INST FOR CANCER RES LTD
Hepatitis b immunisation regimen and compositions
PendingUS20210069322A1Viral antigen ingredientsVirus peptidesHepatitis B virus core AntigenModified vaccinia Ankara
There is provided a method of treating chronic hepatitis B infection (CHB) in a human, comprising the steps of:a) administering to the human a composition comprising a replication-defective chimpanzee adenoviral (ChAd) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc);b) administering to the human a composition comprising a Modified Vaccinia Virus Ankara (MVA) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); andc) administering to the human a composition comprising a recombinant hepatitis B surface antigen (HBs), recombinant hepatitis B virus core antigen (HBc) and an adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel chimpanzee adenovirus vector as well as construction method and application thereof
PendingCN113897388AAvoid troubleThe construction method is simple and efficientSsRNA viruses positive-senseHydrolasesCoronavirus vaccinationTGE VACCINE
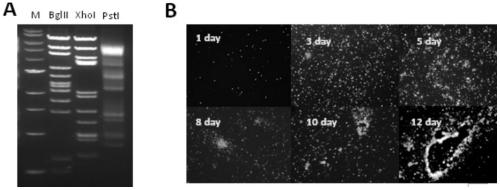
The invention provides a novel chimpanzee adenovirus vector as well as a construction method and application thereof. The novel chimpanzee adenovirus vector has higher virus titer. The invention also provides a method for measuring a virus titer of the chimpanzee adenovirus vector. The chimpanzee adenovirus annular vector is creatively constructed through a shuttle plasmid, E1 and E3 are knocked out through CRISPR / Cas9, and a replication-defective chimpanzee adenovirus vector is constructed. The chimpanzee adenovirus vector does not have a preexisting antibody in people, knockout of the E1 and the E3 is safe, and meanwhile, the chimpanzee adenovirus vector is remarkably different from E1 of human adenovirus type 5 in 293 cells, so that recovery mutation (RCA) can be greatly avoided, and the chimpanzee adenovirus vector is safer. After passage of dozens of generations, the stability of a constructed novel coronavirus vaccine is very high, mutation is not caused, and very strong humoral immunity and cellular response can be induced in a mouse model.
Owner:JIAXING ANYU BIOTECH CO LTD
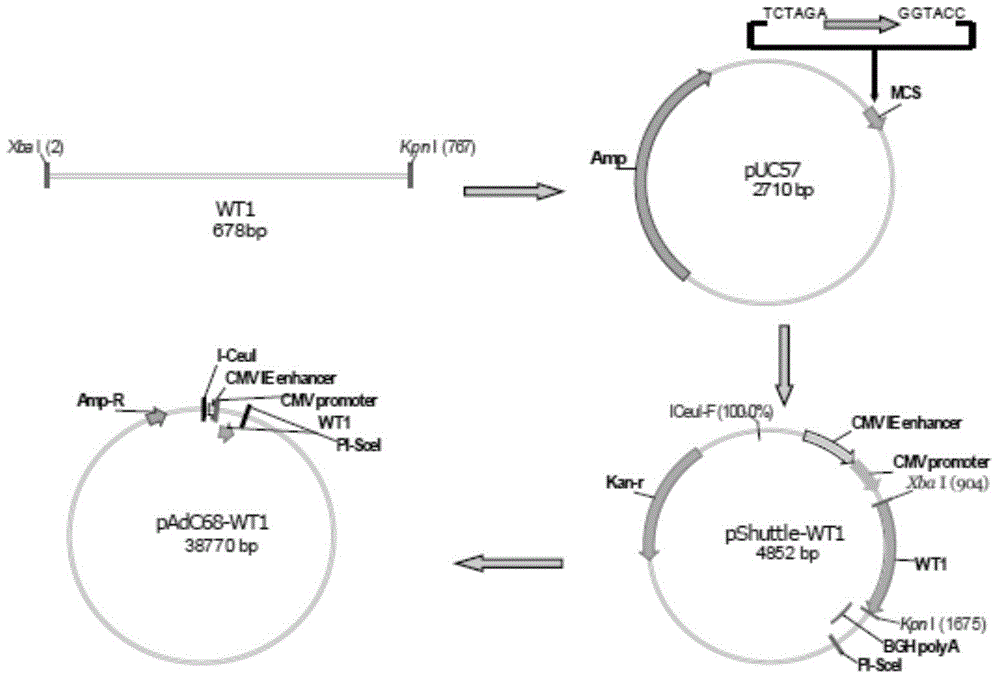
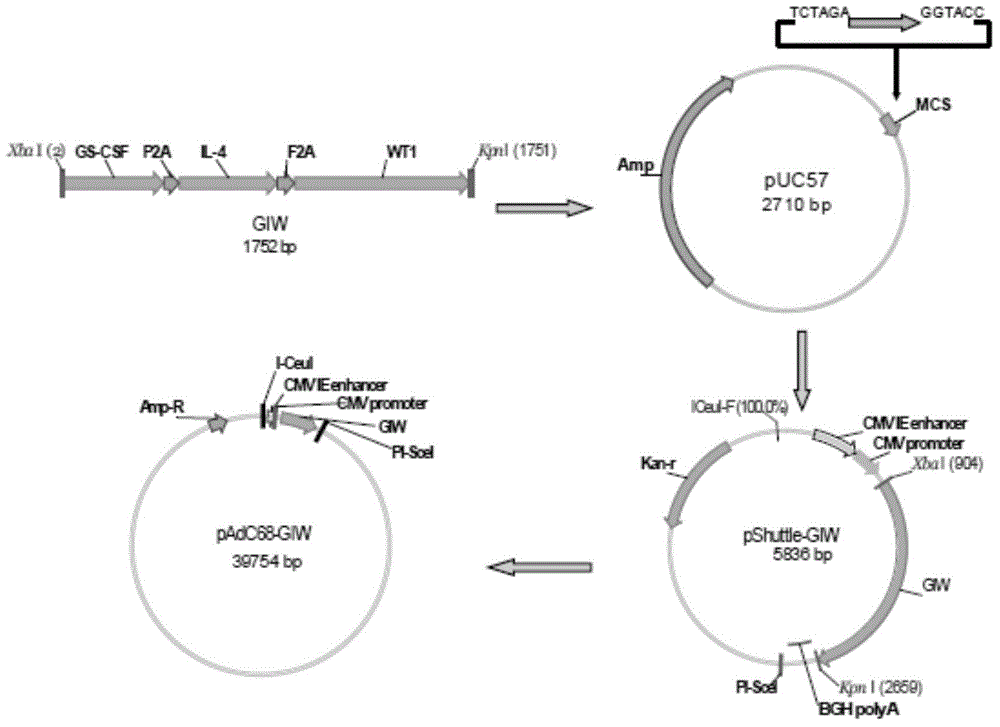
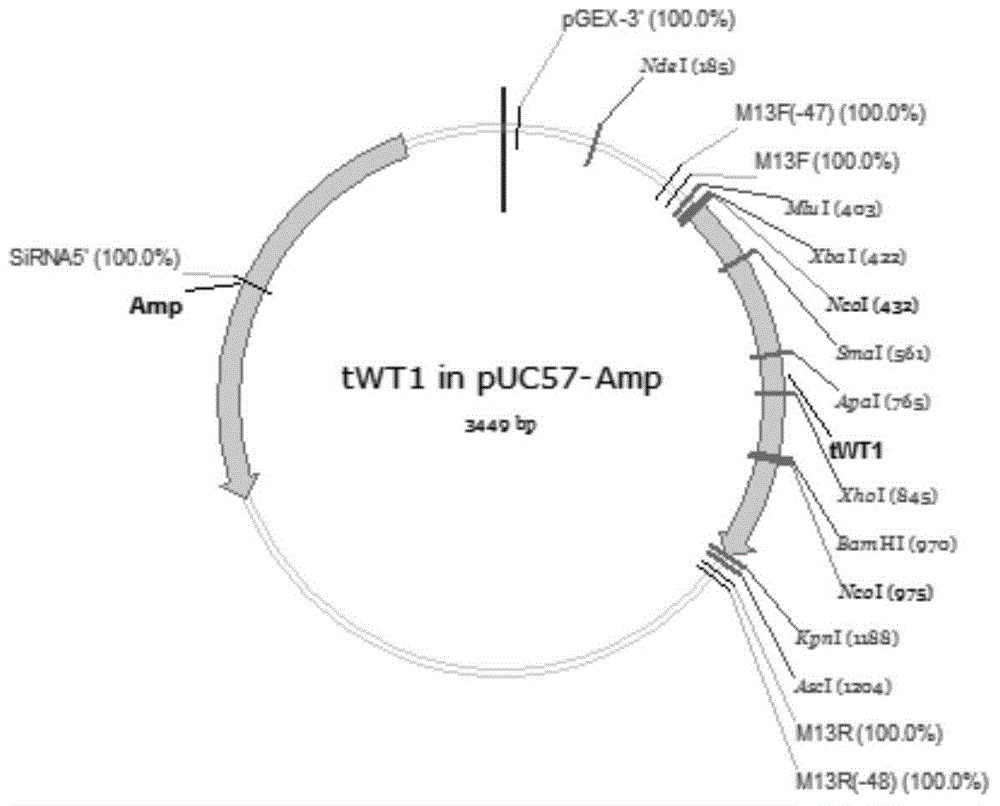
Chimpanzee adenovirus vaccine for preventing and treating highly-expressed WT1 tumor
InactiveCN104784702AProlong lifeGenetic material ingredientsAntibody medical ingredientsCancer researchGene
The invention discloses a chimpanzee adenovirus vaccine for preventing and treating highly-expressed WT1 tumor. A new means for treating breast cancer is provided, a WT1 (Wilm's tumor 1) gene is selected as a target site, a chimpanzee adenovirus is selected as a vector, and a recombinant chimpanzee adenovirus vaccine for expressing WT1 and (GM-CSF)-P2A-(IL-4)-F2A-(WT1) is used for successfully preventing breast cancer and prolonging the lifetime of mice suffering from breast cancer. An experimental foundation is laid for the clinical application of the vaccine, and a prompting effect for the treatment on other tumors is achieved.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Chimpanzee adenovirus constructs with lyssavirus antigens
The invention provides adenoviral vectors comprising transgenes encoding Lyssaviral antigens. The vectors can be used to produce vaccines for the prophylaxis, amelioration and treatment of diseases caused by Lyssaviral diseases, e.g., rabies.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
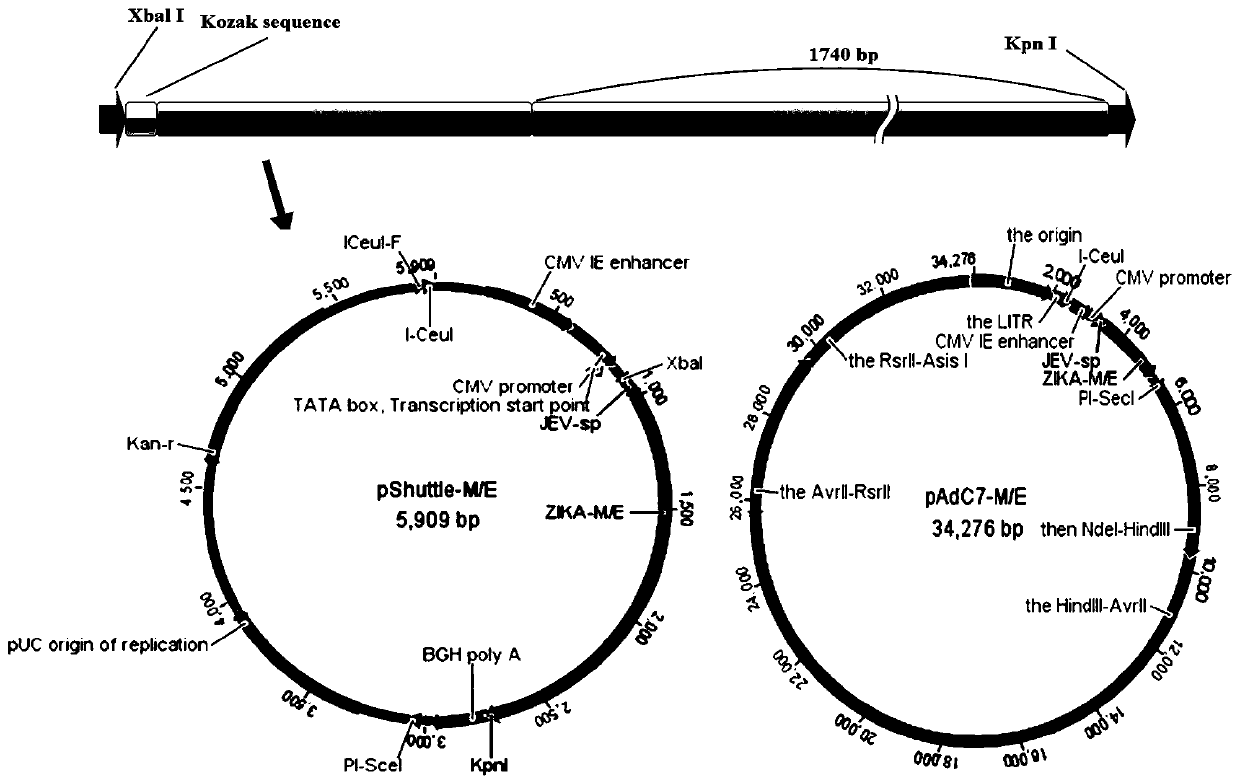
A kind of expression vector based on adenovirus adc7 and its construction method
Owner:中国科学院上海免疫与感染研究所
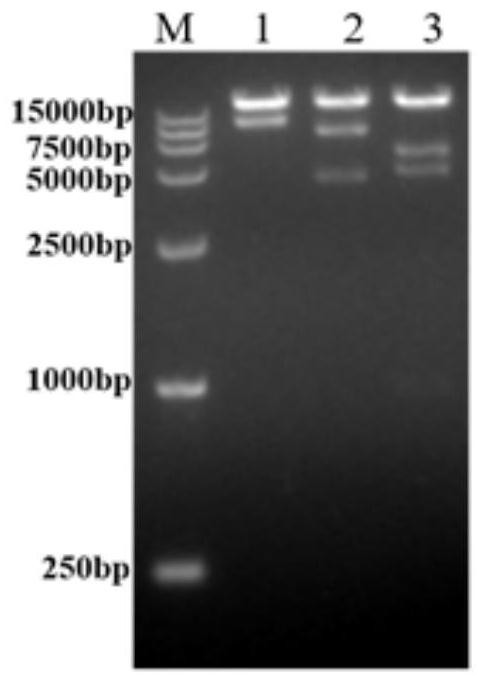
Double antibody sandwich enzyme-linked immunosorbent assay method for detecting chimpanzee adenoviruses AdC68
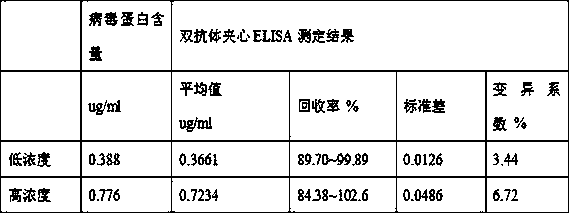
The invention discloses a double antibody sandwich enzyme-linked immunosorbent assay method for detecting chimpanzee adenoviruses AdC68 and belongs to the technical field of immunoassay. The method uses a purified rabbit anti-AdC68 antibody as a coated antibody, purified recombinant ADC68GFP as a standard product, a HRP enzyme-labeled goat anti-adenovirus HEXON antibody as an enzyme-labeled antibody, PBST containing 2% of sheep serum as a sample diluent and a double antibody sandwich method to detect the chimpanzee adenovirus AdC68 antigen, a viral protein, titer and virus particles. The method has high specificity, sensitivity and precision. Compared with the common UV method for measuring virus particles and the plaque method for measuring virus titer, the method has a wide use range andis suitable for on-line detection of the recombinant chimpanzee adenovirus in the purification process. The method can fast estimate the amount of viruses in the preparation of AdC68 and guide the upstream virus culture and downstream purification process.
Owner:天津津斯特生物技术有限责任公司
A kind of Zika virus vaccine based on chimpanzee adenovirus vector and preparation method thereof
ActiveCN107988258BEfficient infectionImprove scalabilitySsRNA viruses positive-senseViral antigen ingredientsZika virusNeutralising antibody
Disclosed are a chimpanzee adenovirus vector-based zika virus vaccine and a preparation method therefor, belonging to the fields of biotechnology and virology. A chimpanzee adenovirus AdC7 is constructed as a replication defective recombinant expression vector. The vector has good immunogenicity and genetic stability, and a novel zika virus vaccine is prepared on the basis of the vector. Neutralizing antibodies against zika virus are effectively induced in mice which have been immunised with the vaccine, thereby protecting the mice from being infected, and also protecting the tissues and organs of the mice from being damaged.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com