Zika virus vaccine based on chimpanzee adenovirus vector and preparation method thereof
A Zika virus and adenovirus technology, applied in microorganism-based methods, viruses/phages, botanical equipment and methods, etc., can solve the problems of technical bottlenecks, restricted use, etc., and achieve a simple production process, low toxicity, and easy expansion. Enhancement and purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
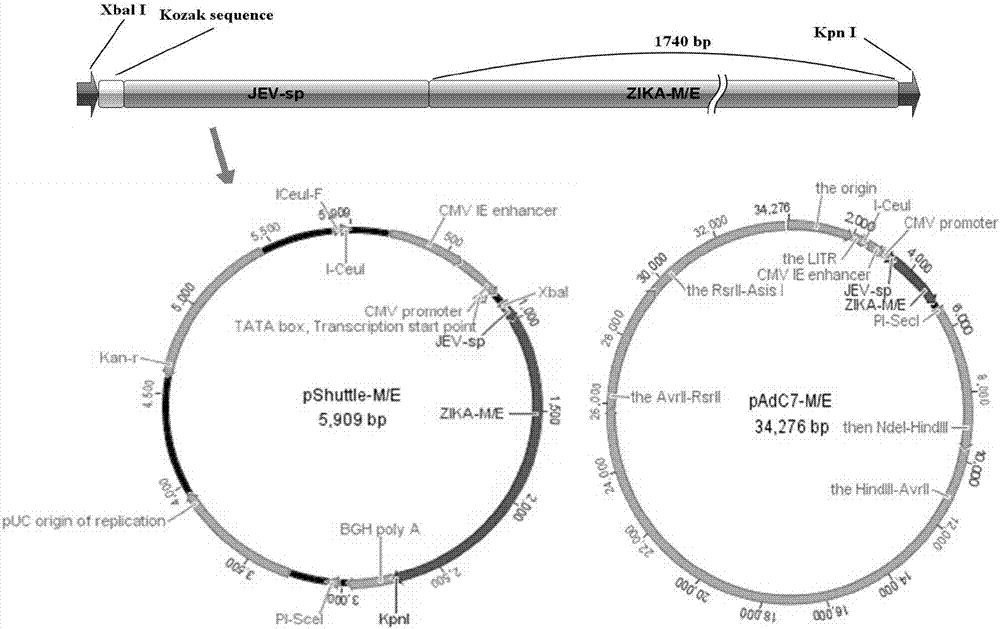
[0061] Example 1, construction of pAdC7-M / E vector
[0062] Acquisition of the target gene: the signal peptide sequence derived from JEV is used for protein expression, the M / E gene sequence is derived from the FSS13025 virus strain, the signal peptide and the M / E gene sequence are codon optimized, and the optimized signal peptide sequence is shown in SEQ ID NO As shown in .1, the optimized M / E gene sequence is shown in SEQ ID NO.2. After being synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd., it was cloned into the pCAGGS vector to obtain pCAGGS-M / E.
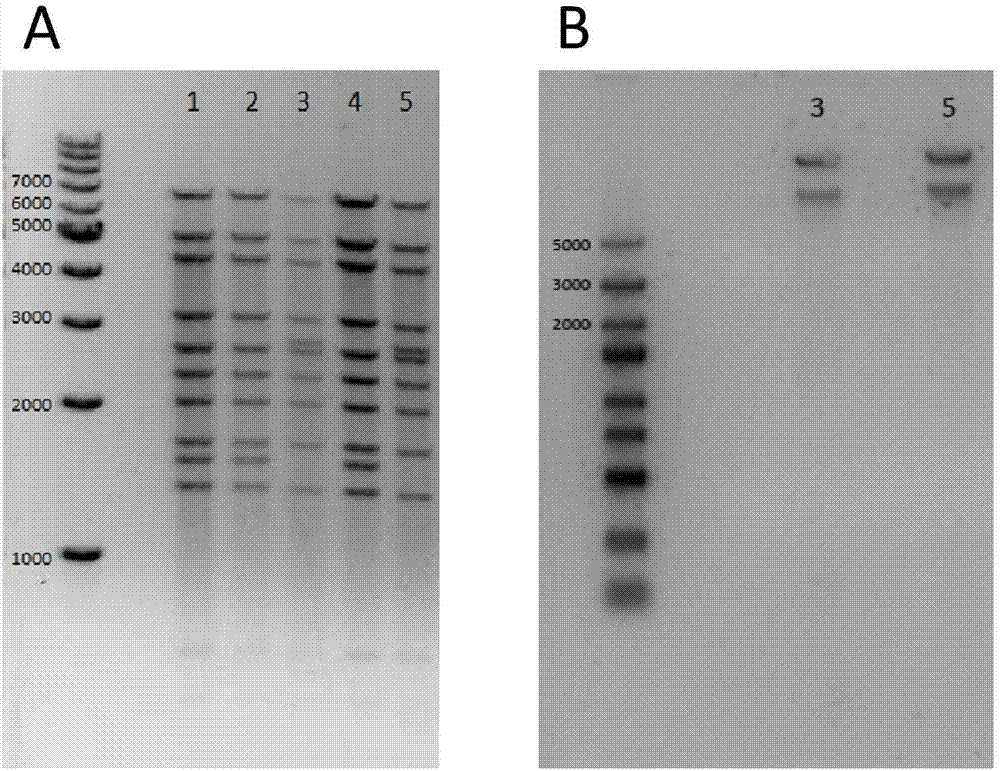
[0063]The acquisition of the recombinant adenovirus shuttle vector: using pCAGGS-M / E as a template, design a forward primer to access the XbaI restriction site and Kozak sequence (GCCACC), design a reverse primer to access the KpnI restriction site, use High-fidelity DNA polymerase is used for PCR, and the PCR product is subjected to gel electrophoresis, and then recovered and purified with a kit. Both the PCR product a...
Embodiment 2
[0065] Embodiment 2 Packaging AdC7-M / E
[0066] After linearizing pAdC7-M / E with PacI enzyme and inactivating it, transfected HEK 293 cells, plaques could be seen in about 7 days ( image 3 ), indicating that the adenovirus has been successfully packaged, wait another 2-3 days for all the cells to fall off, and collect the cells and supernatant in a cryopreservation tube.
Embodiment 3
[0067] Example 3 Western blot detection of protein expression
[0068] Spread HEK293T cells on a 12-well plate one day in advance, and add 10 9 , 10 8 , 10 7 For vpAdC7-M / E, the cells and supernatant were collected after 48 hours, and GAPDH was used as an internal reference to detect the expression of E protein by Western blot. It was found that in the cells and supernatant, the amount of E protein was positively correlated with the scale of the added adenovirus ( Figure 4 A, B).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com