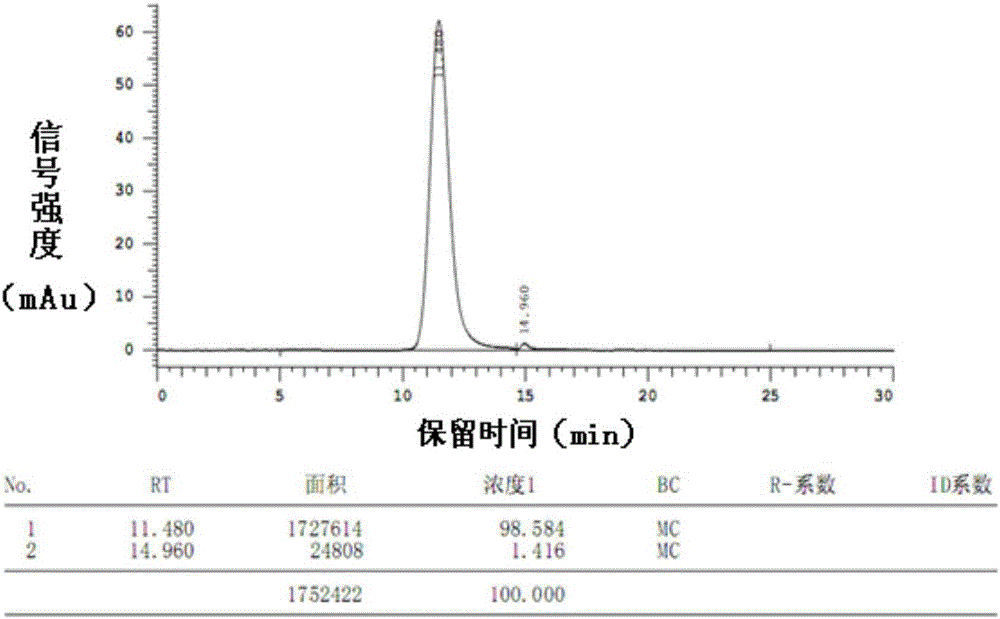
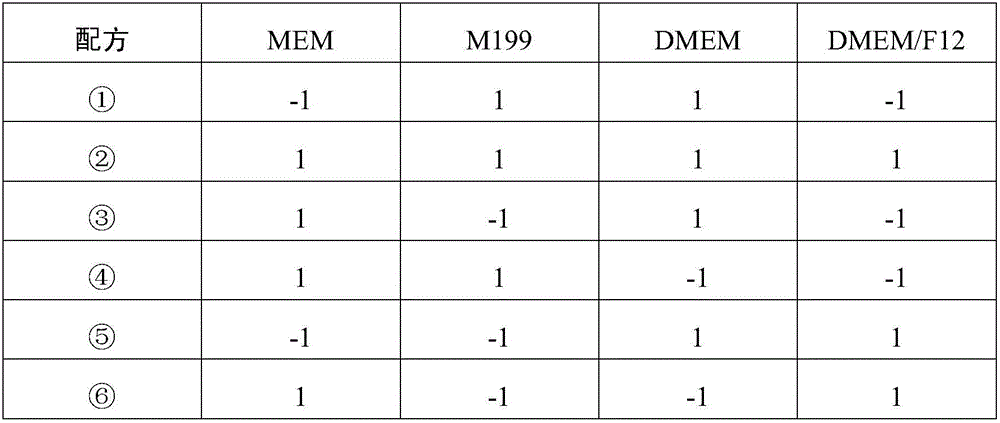
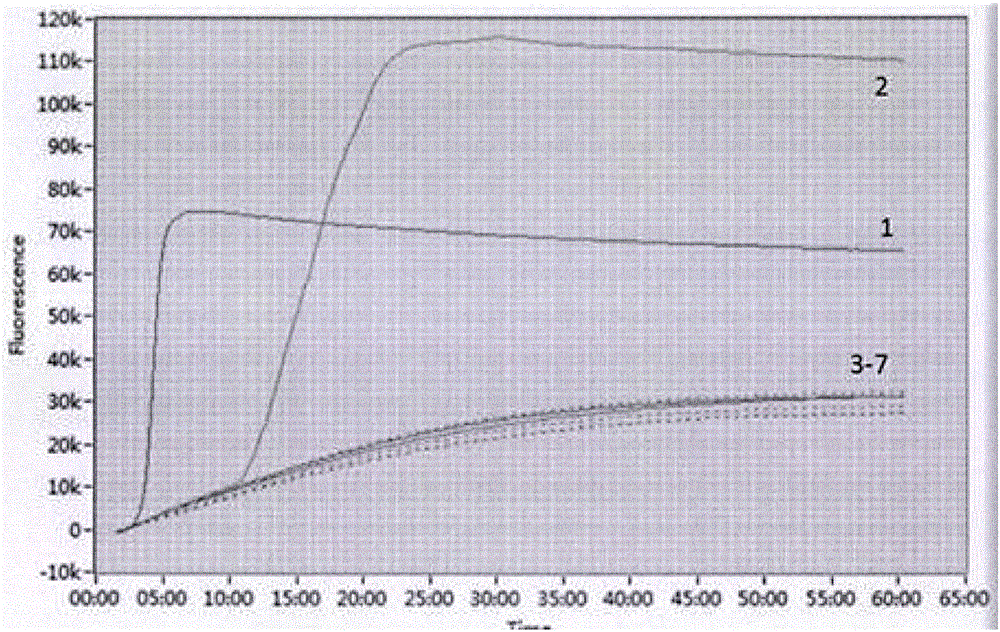
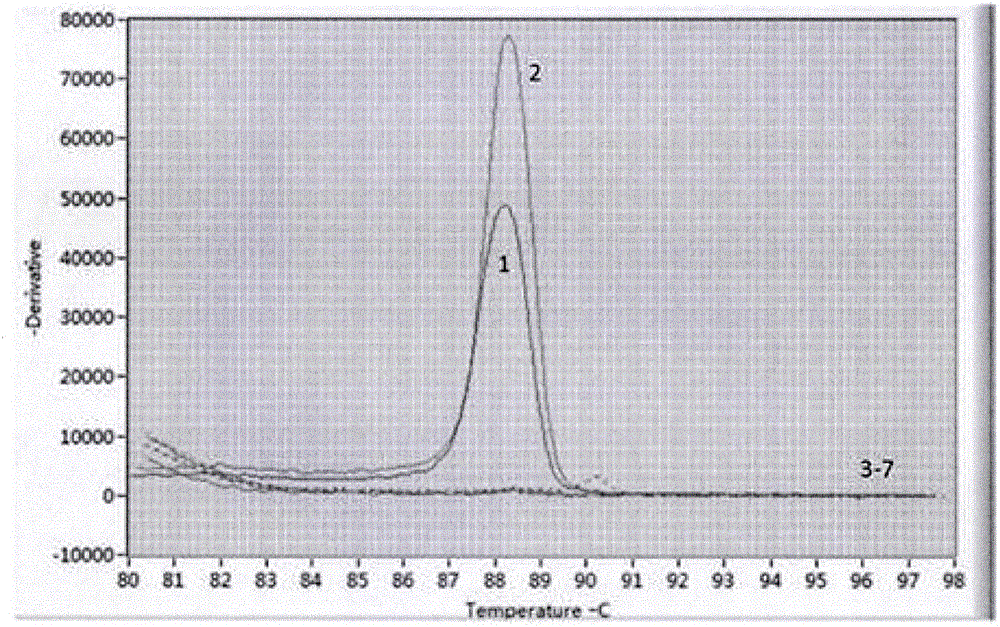
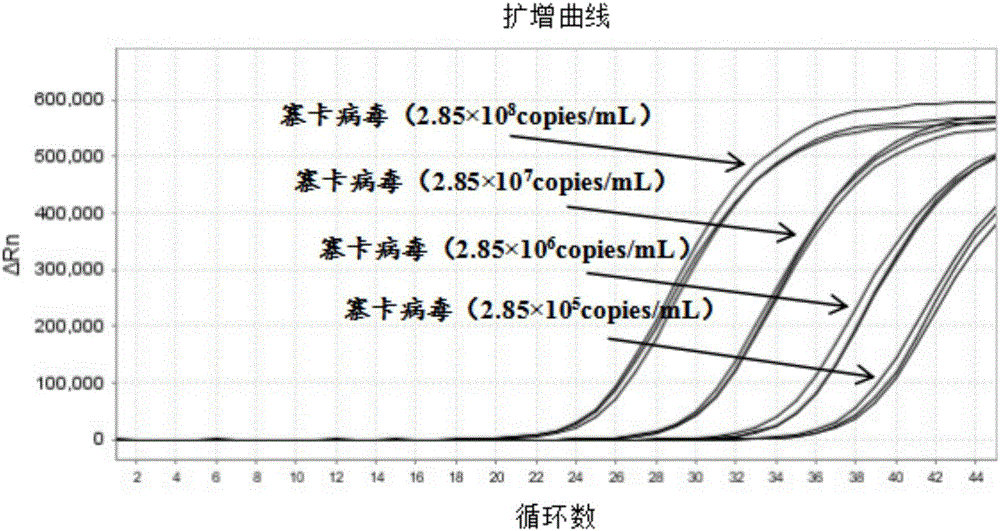
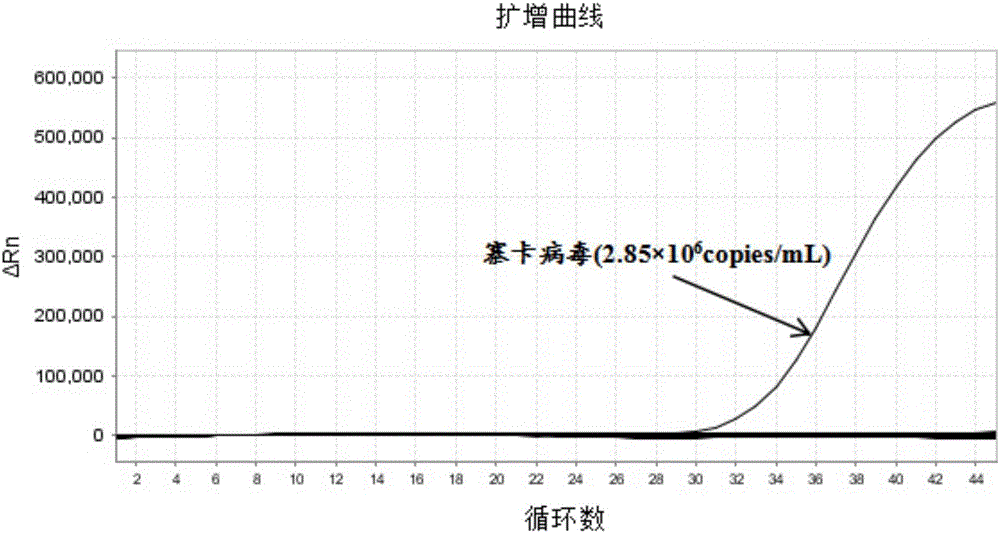
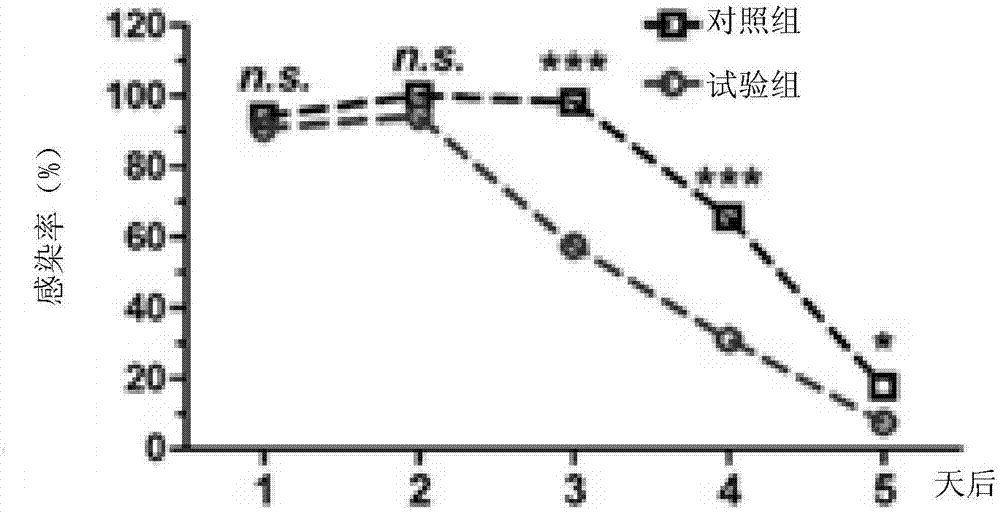
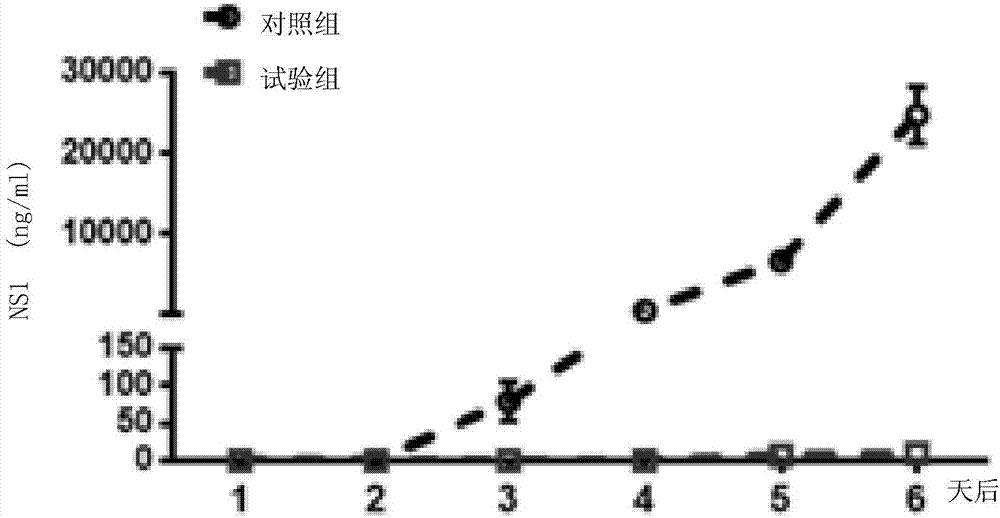
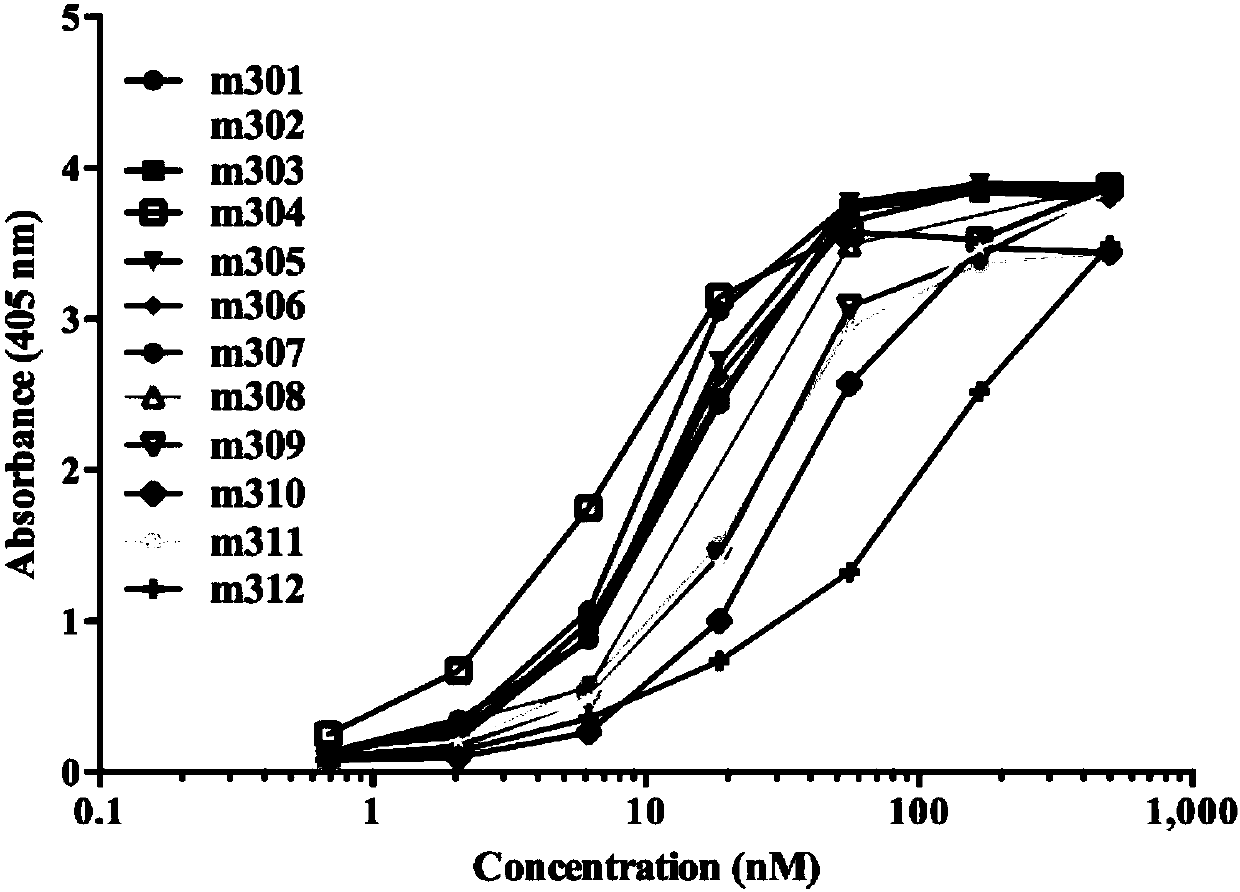
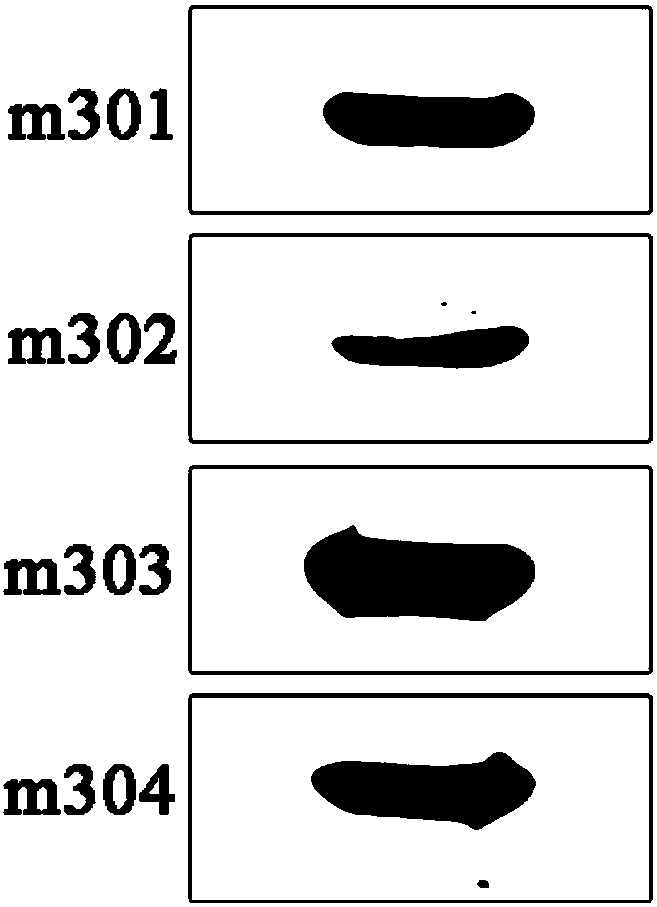
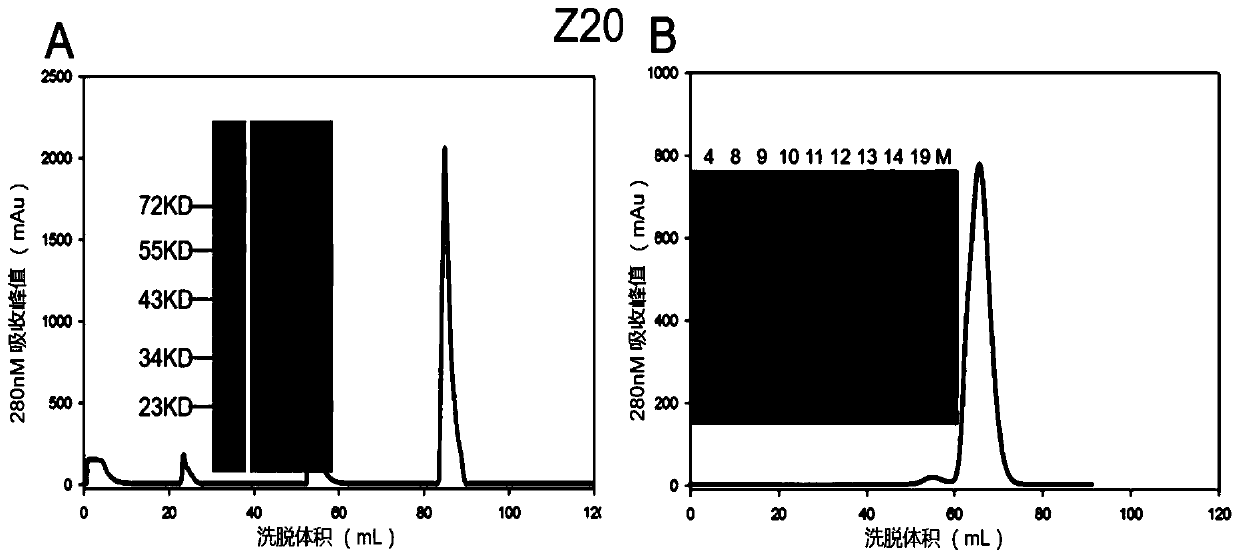
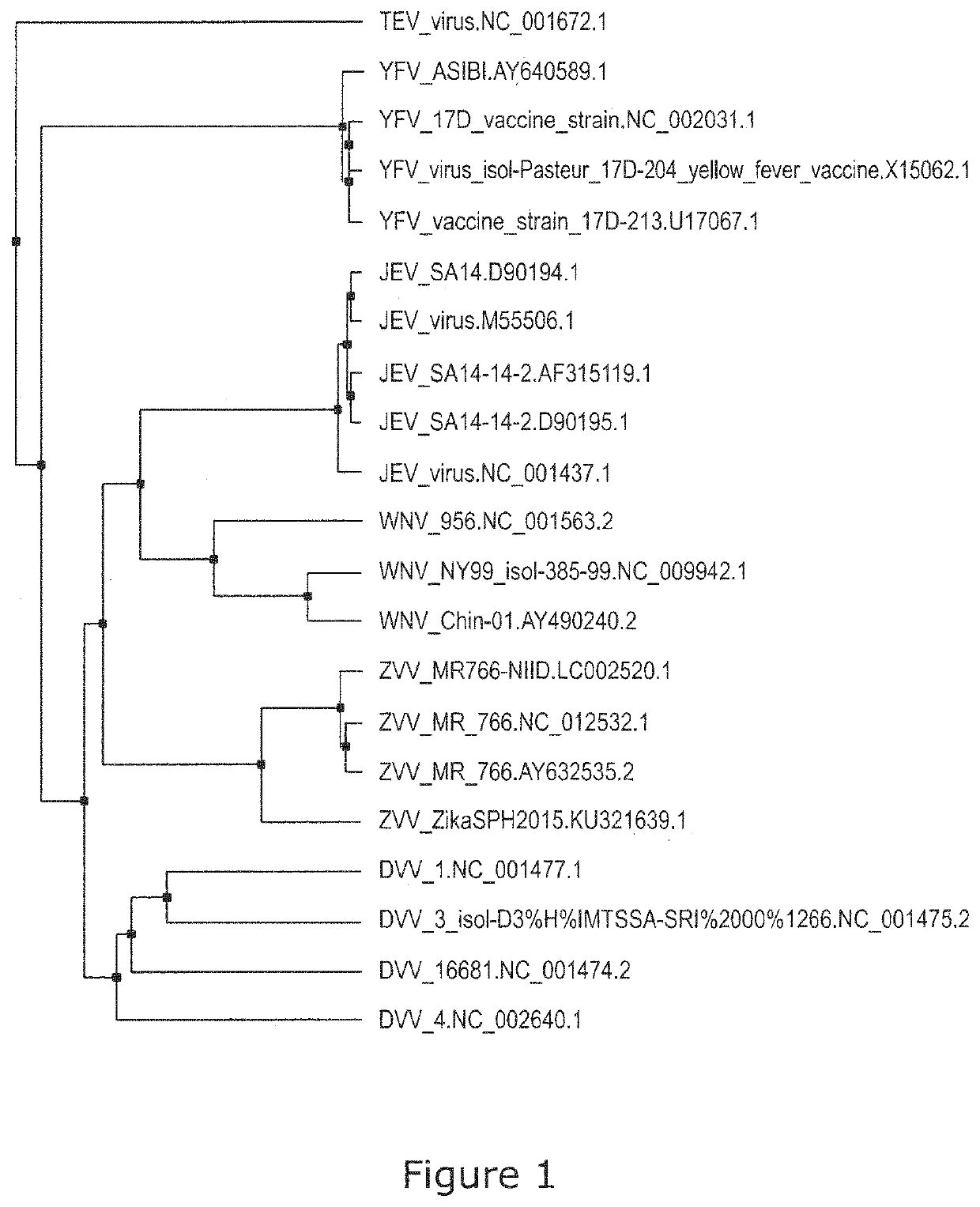
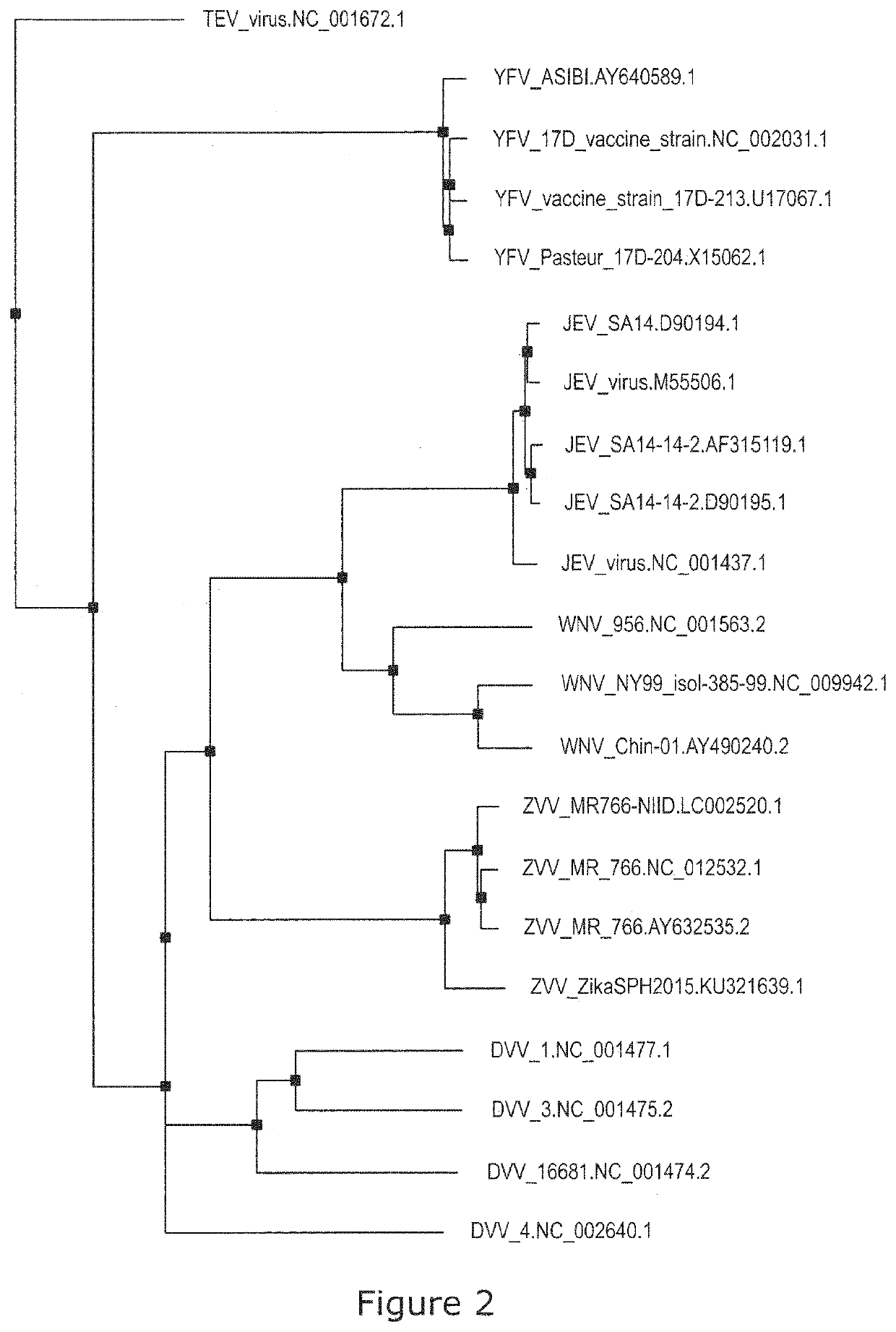
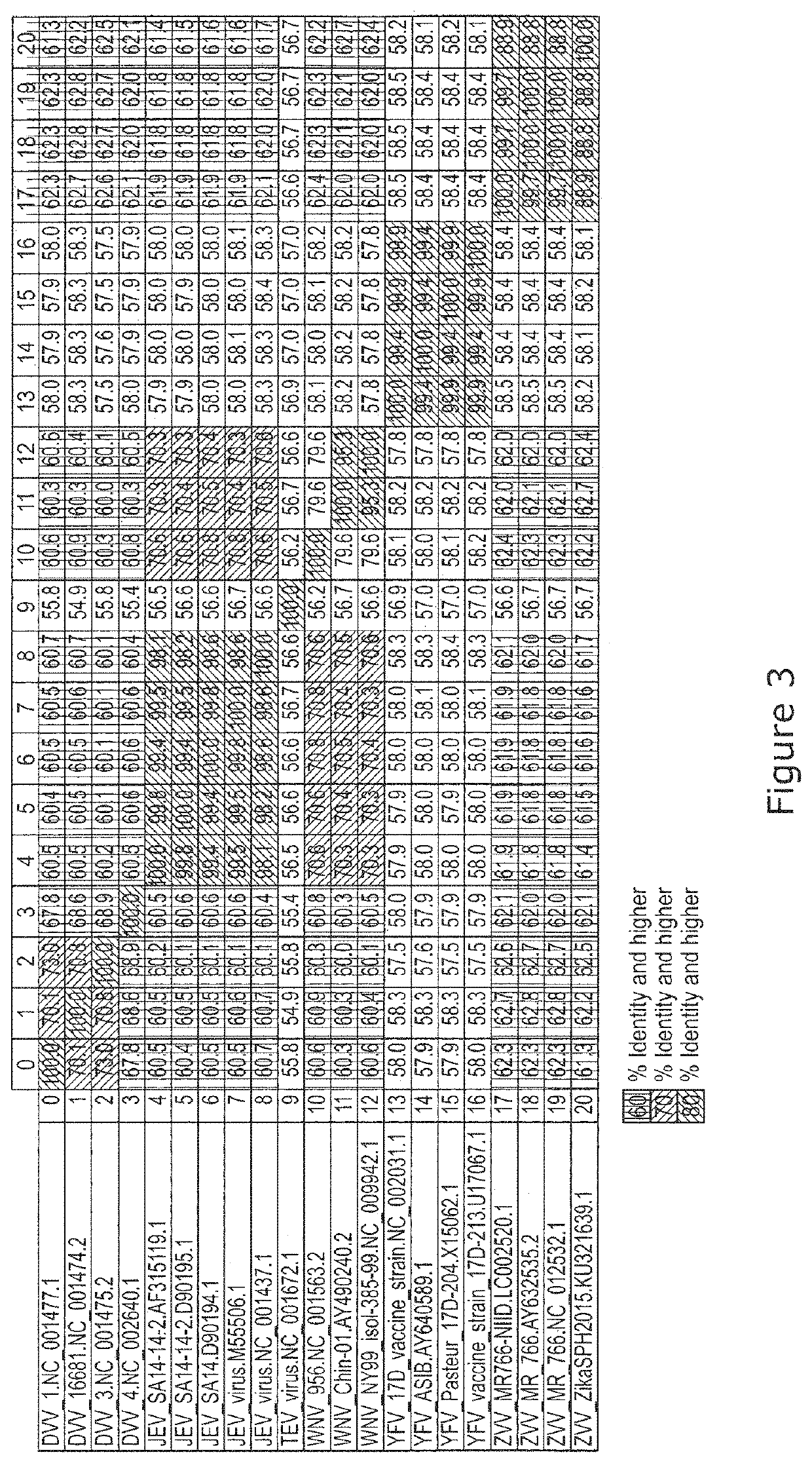
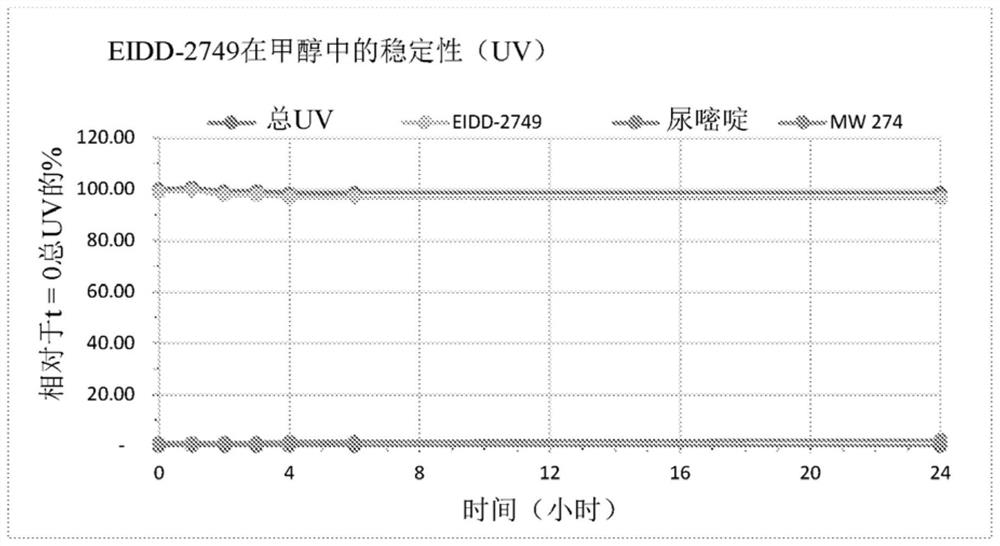
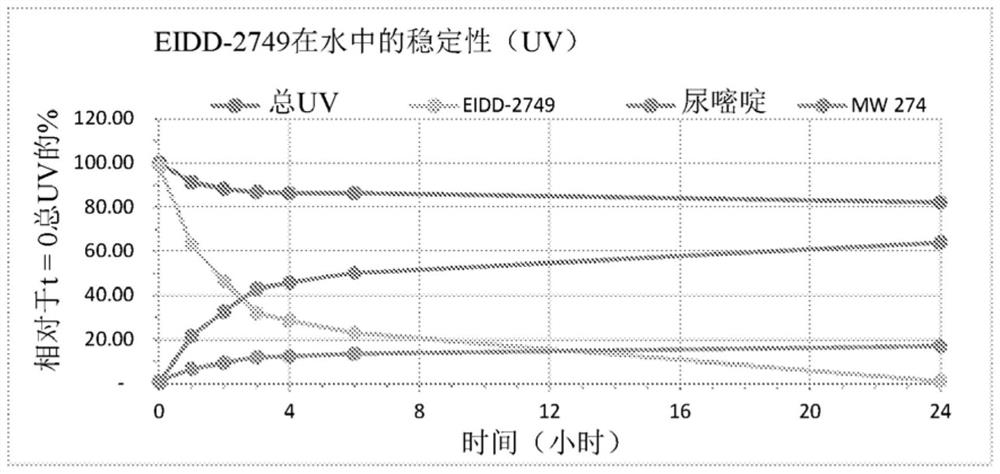
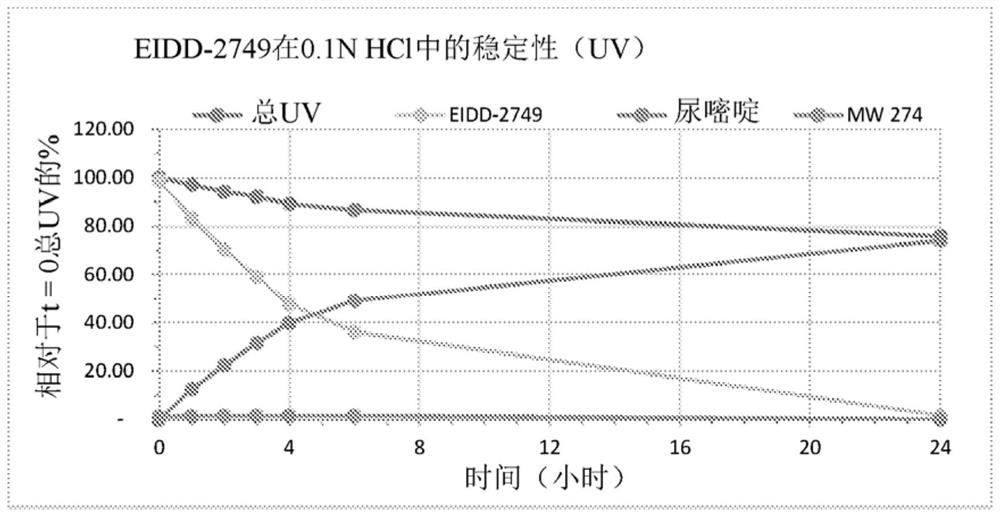
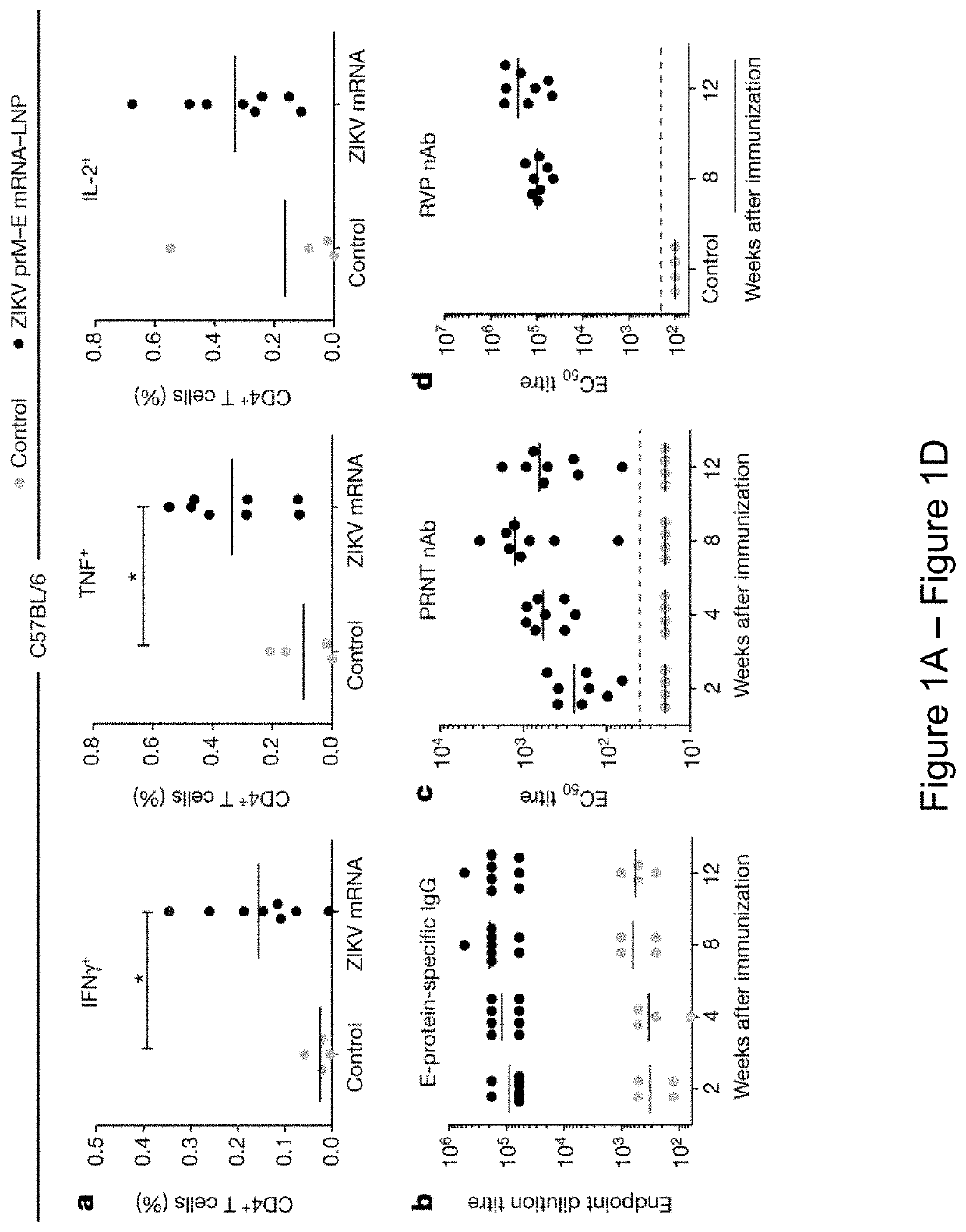
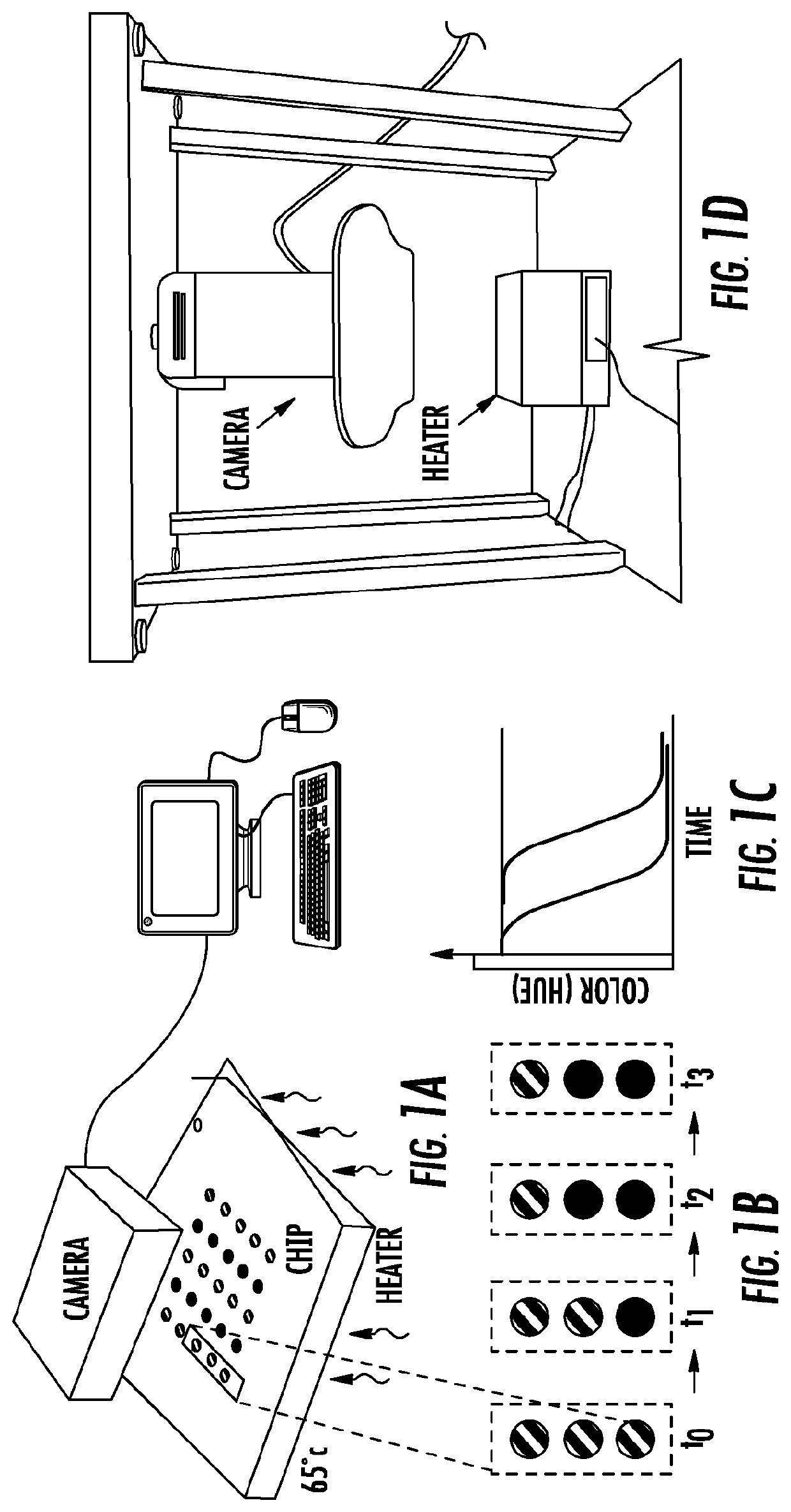
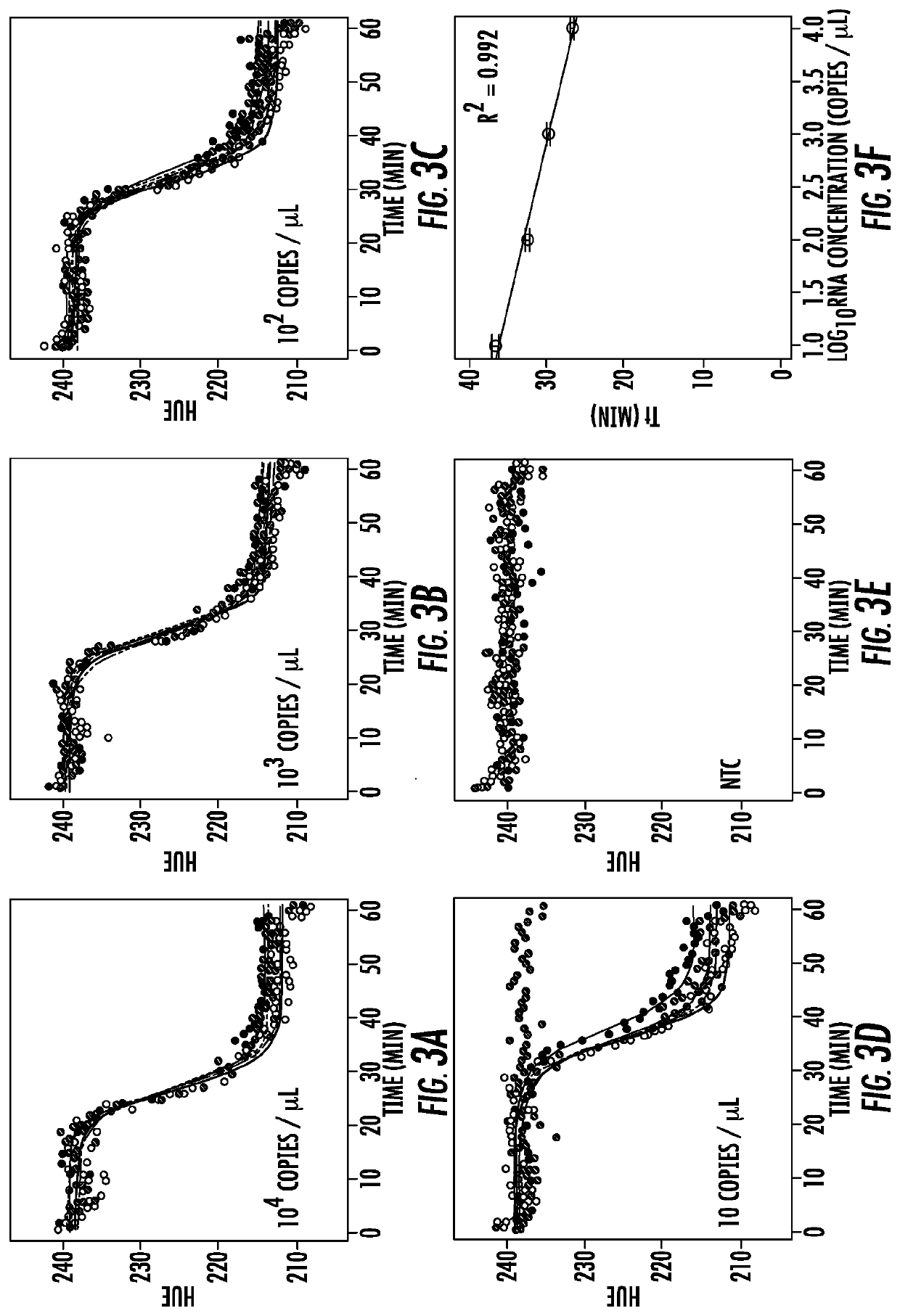
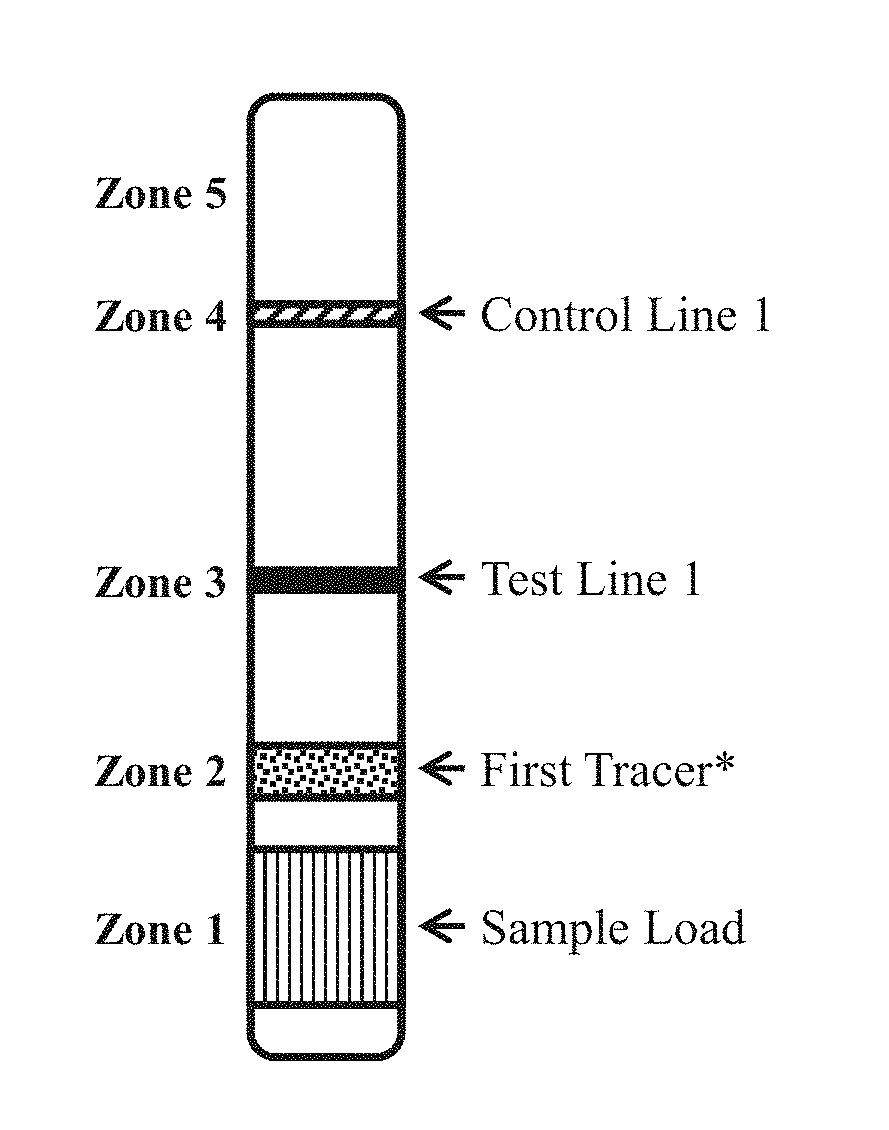
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
366 results about "Zika virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Zika virus (ZIKV) is a member of the virus family Flaviviridae. It is spread by daytime-active Aedes mosquitoes, such as A. aegypti and A. albopictus. Its name comes from the Ziika Forest of Uganda, where the virus was first isolated in 1947. Zika virus is related to the dengue, yellow fever, Japanese encephalitis, and West Nile viruses. Since the 1950s, it has been known to occur within a narrow equatorial belt from Africa to Asia. From 2007 to 2016, the virus spread eastward, across the Pacific Ocean to the Americas, leading to the 2015–16 Zika virus epidemic.
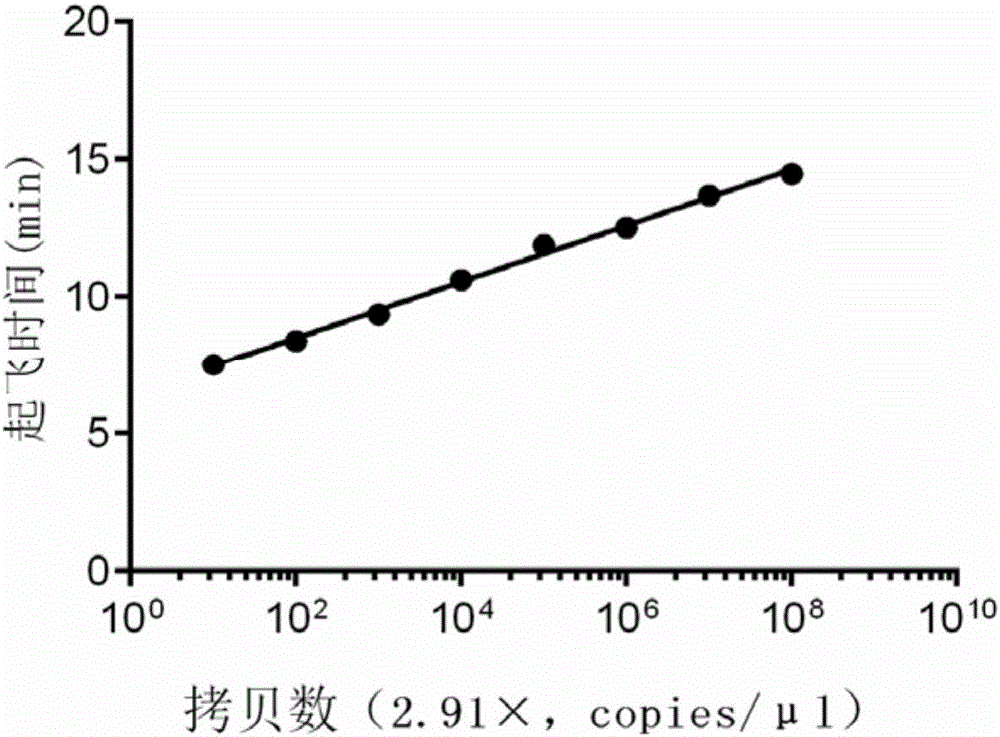
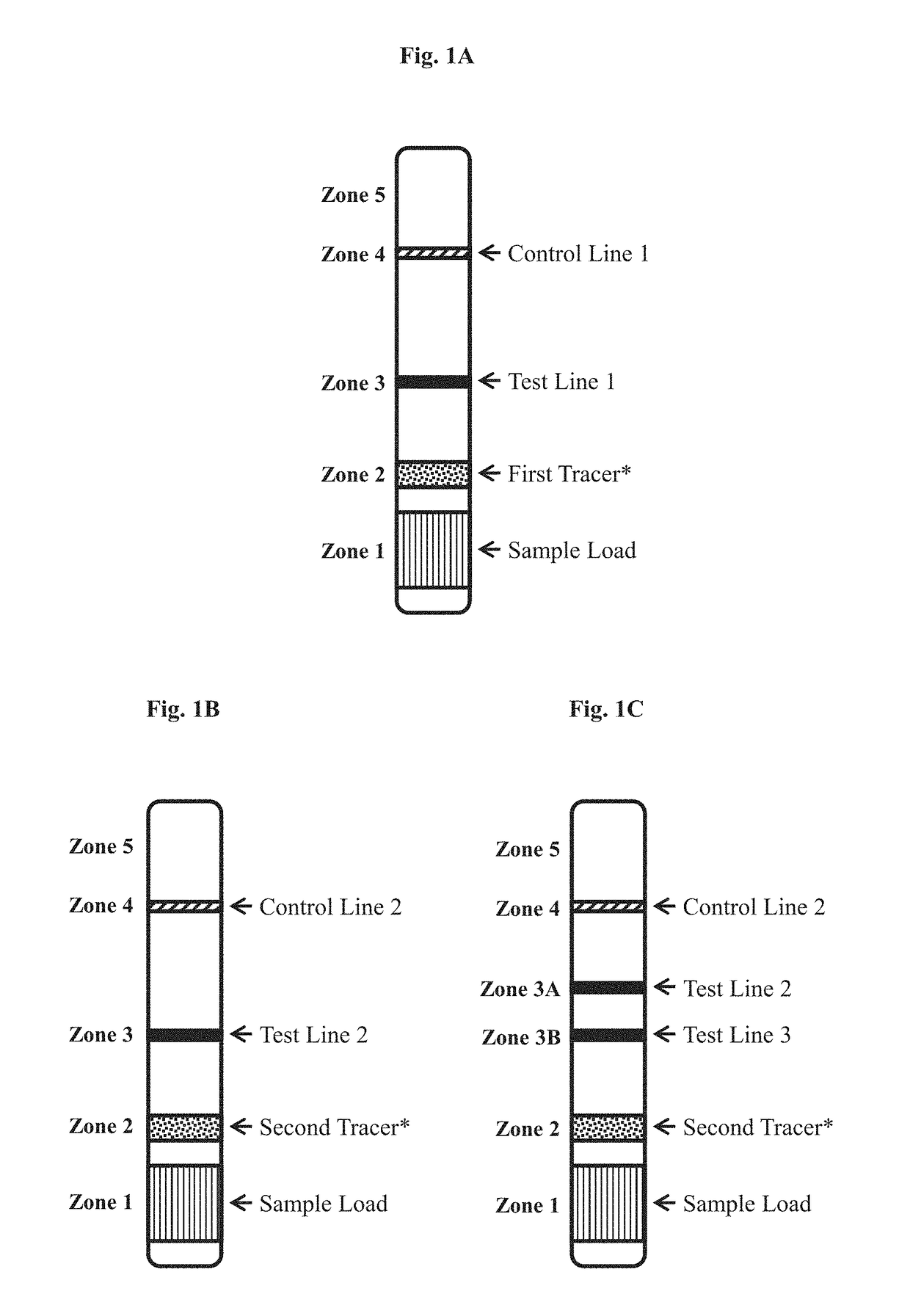
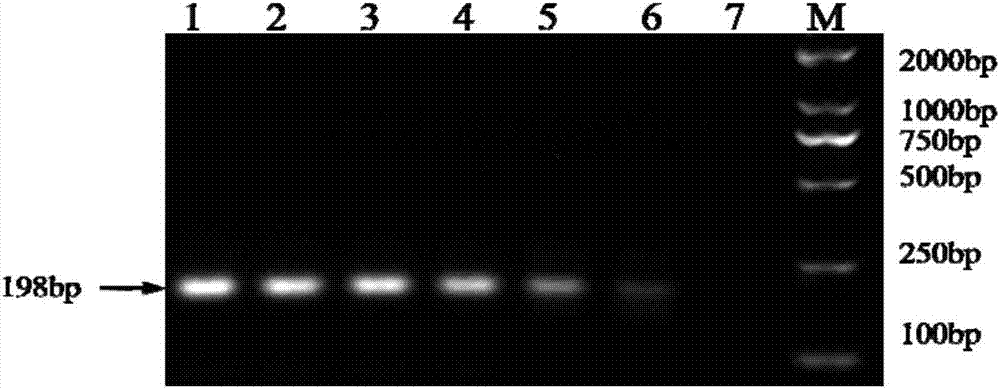
Detection strip for Zika virus detection by means of fast immunochromatography method
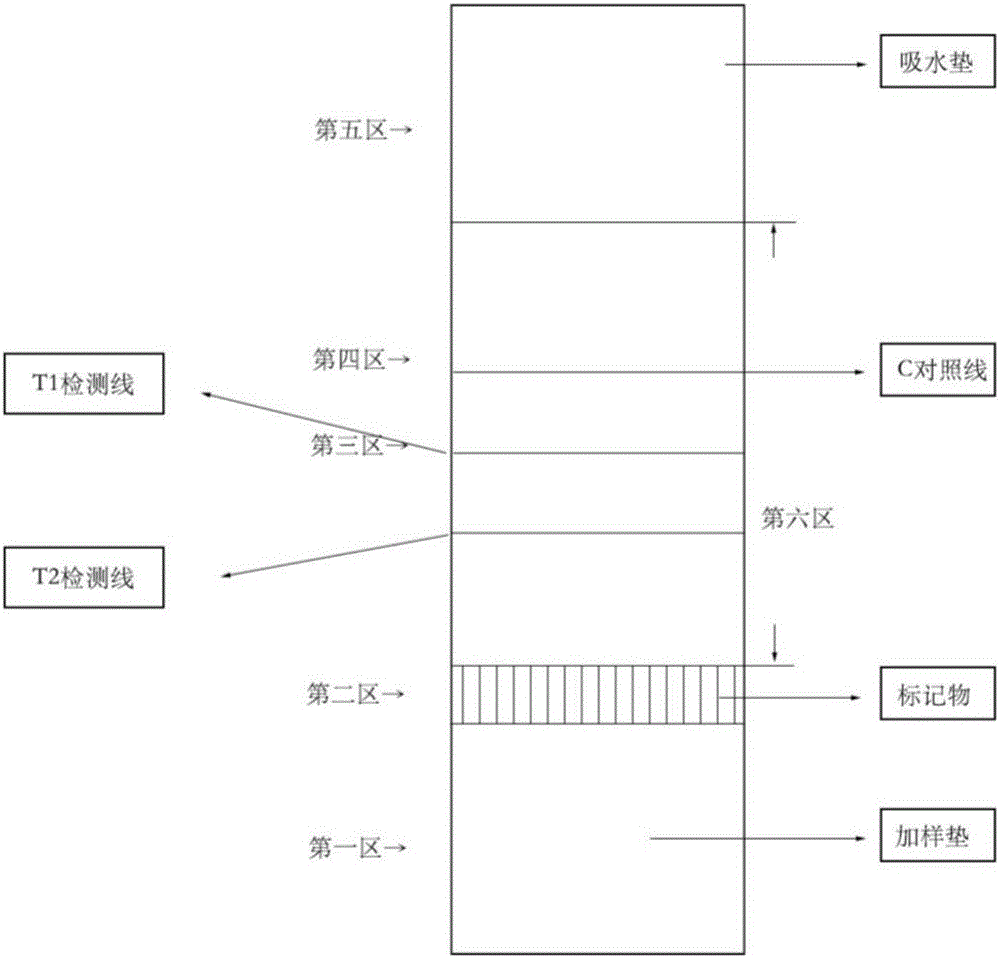
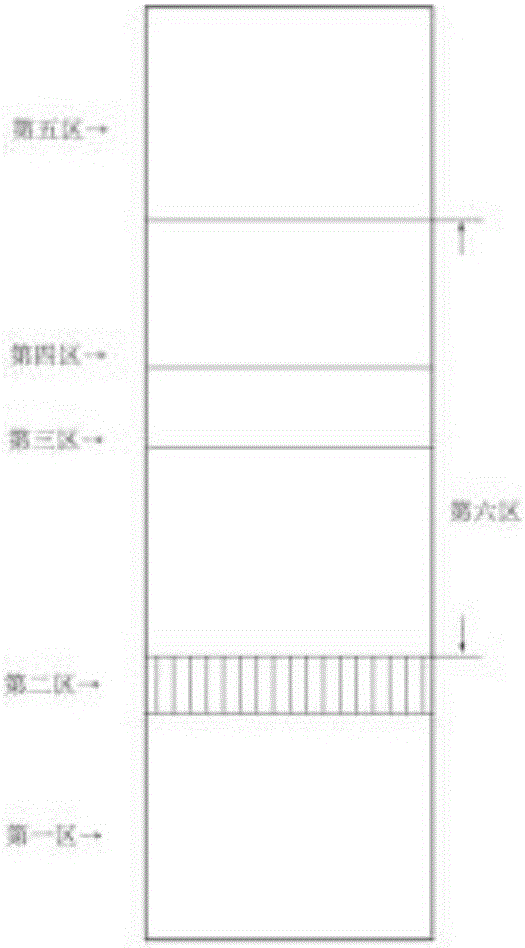
The invention provides a detection strip or kit for Zika virus detection by means of the fast immunochromatography method. The immunodetection strip has six areas. The first area is a sample adding pad; the second area is an immobilized Zika antigen marker area or anti-Zika virus antigen specific antibody marker; the third area is a detection area T and a coated antibody spray area, and the area T is matched with the second area; the fourth area is a contrast area used for immobilization of non-specific antibodies; the fifth area is a water absorber; the sixth area is a nitro fiber chromatography film. According to the detection strip or kit, only a small number of samples to be tested are needed, no equipment is required, a detection result can be obtained within ten minutes, detection is easy and fast, and the detection strip or kit can be purchased from a pharmacy so that patients can conduct detection by themselves.
Owner:卢氏实验室公司 +1
Inactivated Zika virus vaccine
ActiveCN105749268AEase the epidemicReduce the burden onSsRNA viruses positive-senseViral antigen ingredientsZika virusSide effect
The invention provides an inactivated Zika virus vaccine. The inactivated Zika virus vaccine is obtained by: performing ultrafiltration and concentration on Zika virus liquid after inactivation, centrifuging the concentrated virus liquid by adopting a sucrose density zone, performing ion exchange and concentration sterilization on a centrifugal product to obtain a Zika virus vaccine stock solution, diluting the vaccine stock solution until the total protein content is not more than 20mu g / ml, and adding an adjuvant to obtain a vaccine semi-finished product. The method for preparing the vaccine provided by the invention is simple, convenient and easy to operate, the cost is saved, the produced vaccine is suitable for Asian people, a unit dose of the Zika virus liquid is high in immunogenicity, the content of hybrid protein is low, the side effect after injection is small, and the safety is high, so that the vaccine is suitable for vaccination of fertile women before pregnancy, can avoid newborn Brazil microcephaly caused by infection of Zika virus, and is significant in social value and market efficiency.
Owner:SINOVAC RES & DEV
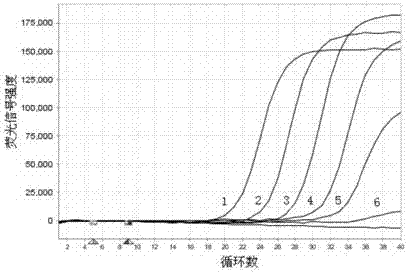
Zika virus loop-mediated isothermal amplification detection kit and using method
InactiveCN106244726AMicrobiological testing/measurementMicroorganism based processesZika virusFluorescence
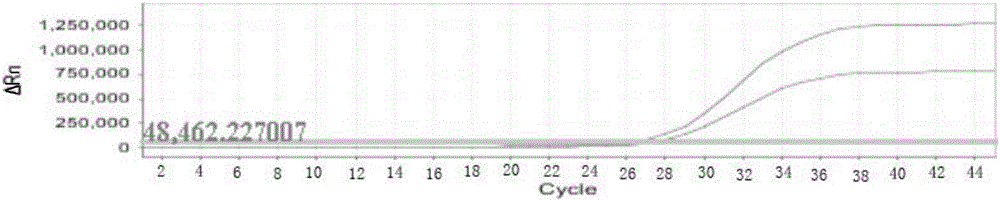
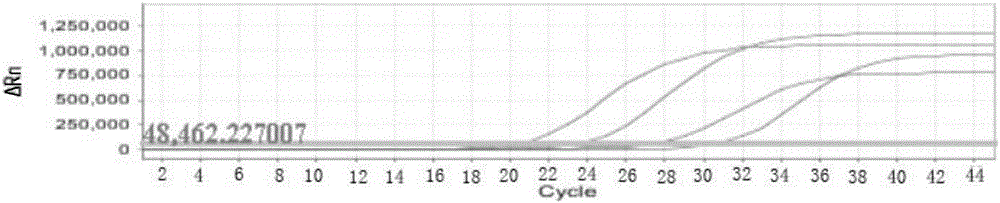
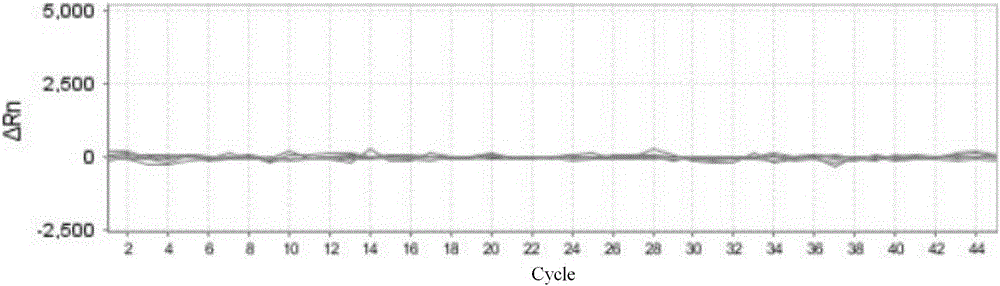
The invention discloses a loop-mediated isothermal amplification kit for detecting Zika viruses and a using method of the kit. The kit is characterized by consisting of a Zika virus envelop protein (E) gene loop-mediated isothermal amplification primer mixed solution, a loop-mediated isothermal amplification reaction pre-mixed solution, a Zika virus E gene positive quality control and a Zika virus E gene negative quality control, and the kit is applicable to the rapid detection of the Zika viruses. The Zika virus E gene loop-mediated isothermal amplification primer group comprises a pair of outer primers (5'-3' sequences are shown as: AAGCACTGGCTGGTTCAC and TCCAGAGCTCCAGCAAGG), a pair of inner primers (5'-3' sequences are shown as: GTGGAGTTCCGGTGTCTGCCAAGGAGTGGTTCCACGACAT and AGAGTTCAAGGACGCACATGCCTGCTCCTTCTTGACTCCCTA) and a pair of loop primers (5'-3' sequences are shown as: CAGCGTGCCAAGGTAATGGA and AAAAGGCAAACTGTCGTGGT). The using method of the kit is characterized in that the real-time rapid diagnosis of the Zika viruses can be achieved by virtue of an isothermal amplification fluorescent detection system. The method is strong in specificity and high in sensitivity; therefore, a convenient and rapid way is provided for the prevention and control of the Zika viruses and for conducing trend investigation and analysis.
Owner:CHINA INSPECTION LAB TECH CO LTD
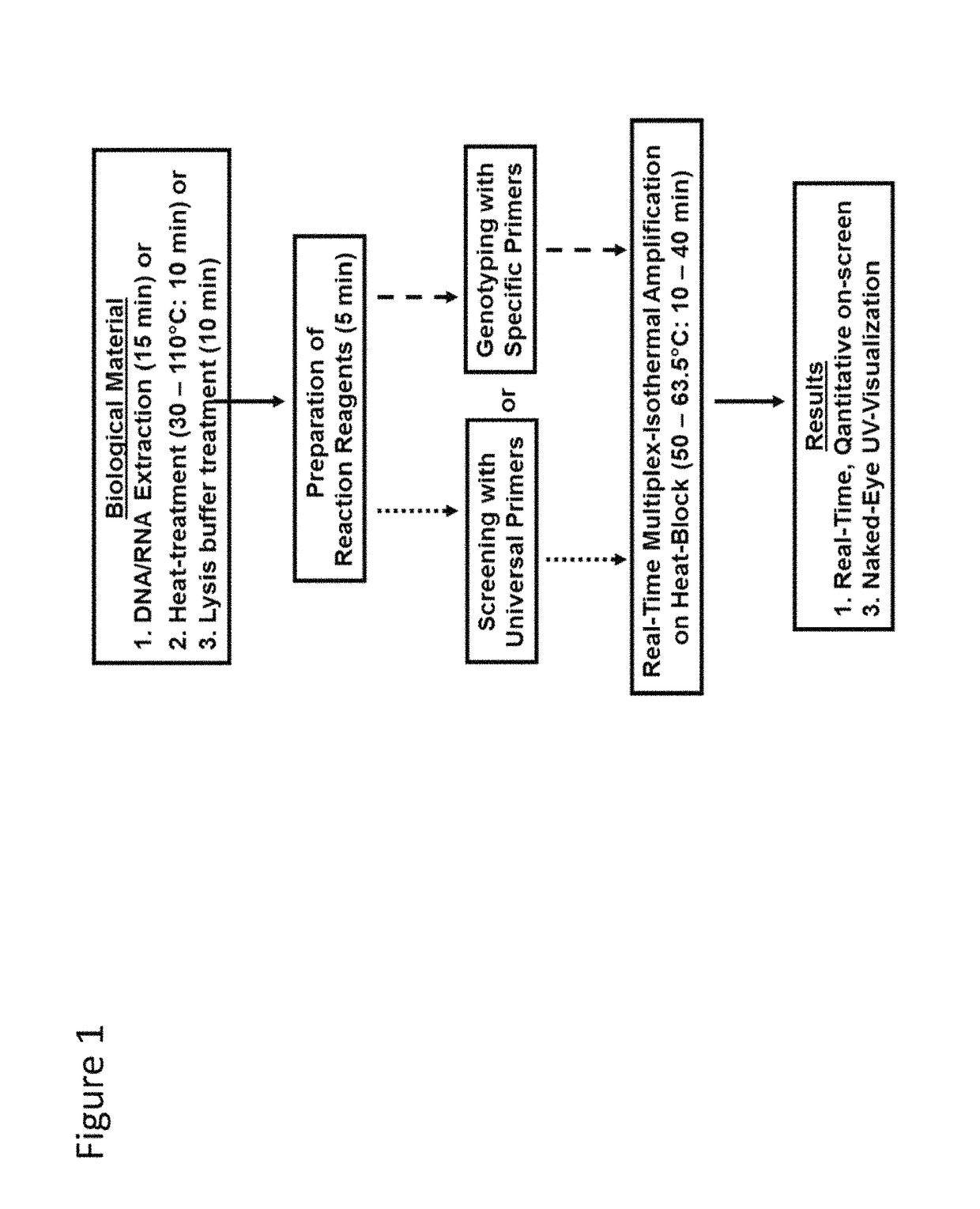
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS20200048722A1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaLoop-mediated isothermal amplification
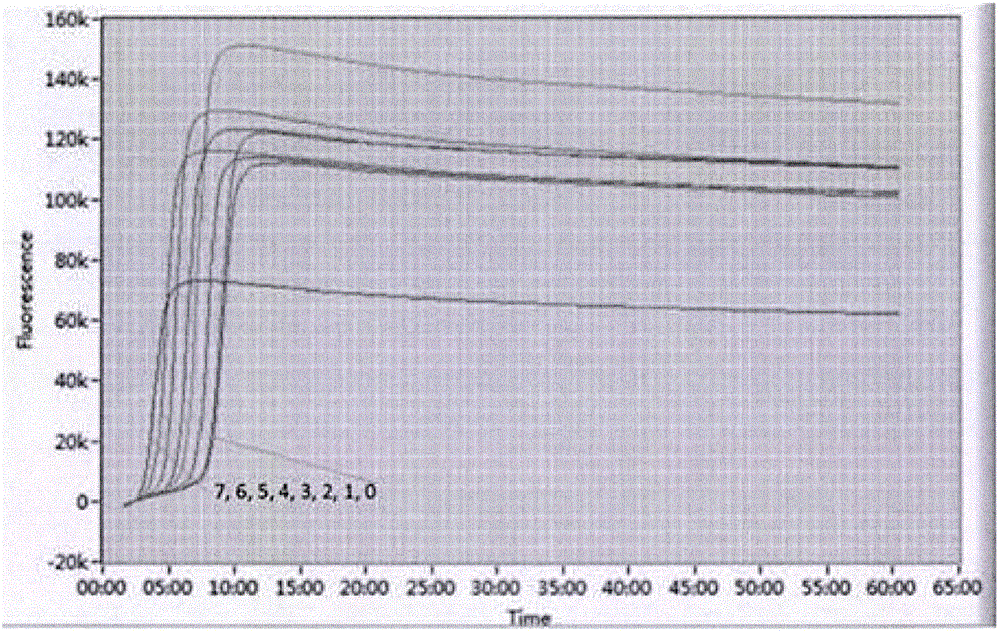
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
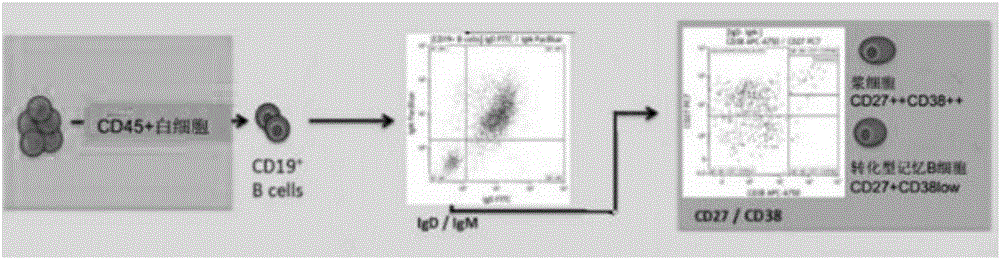
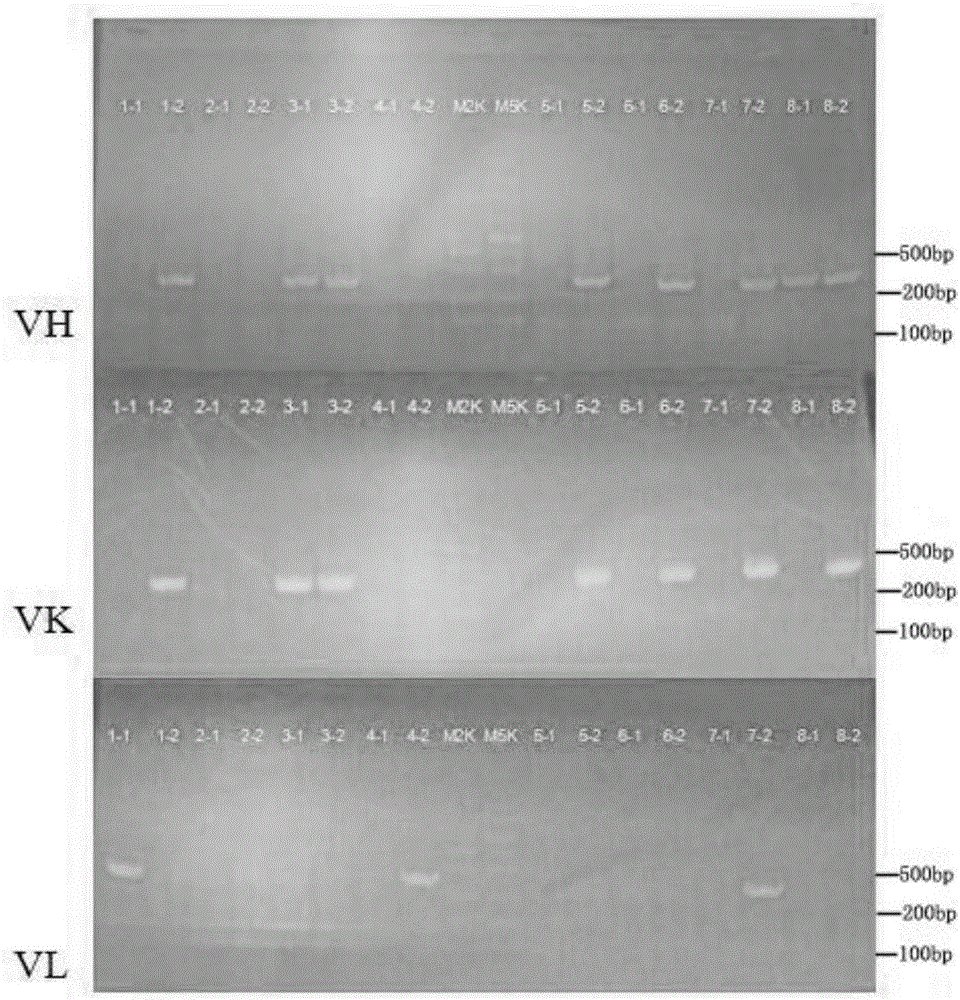
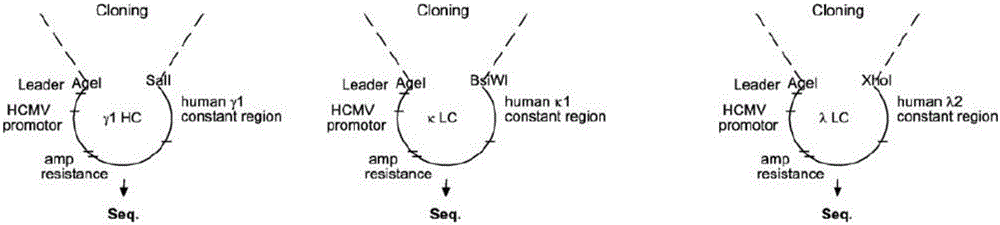
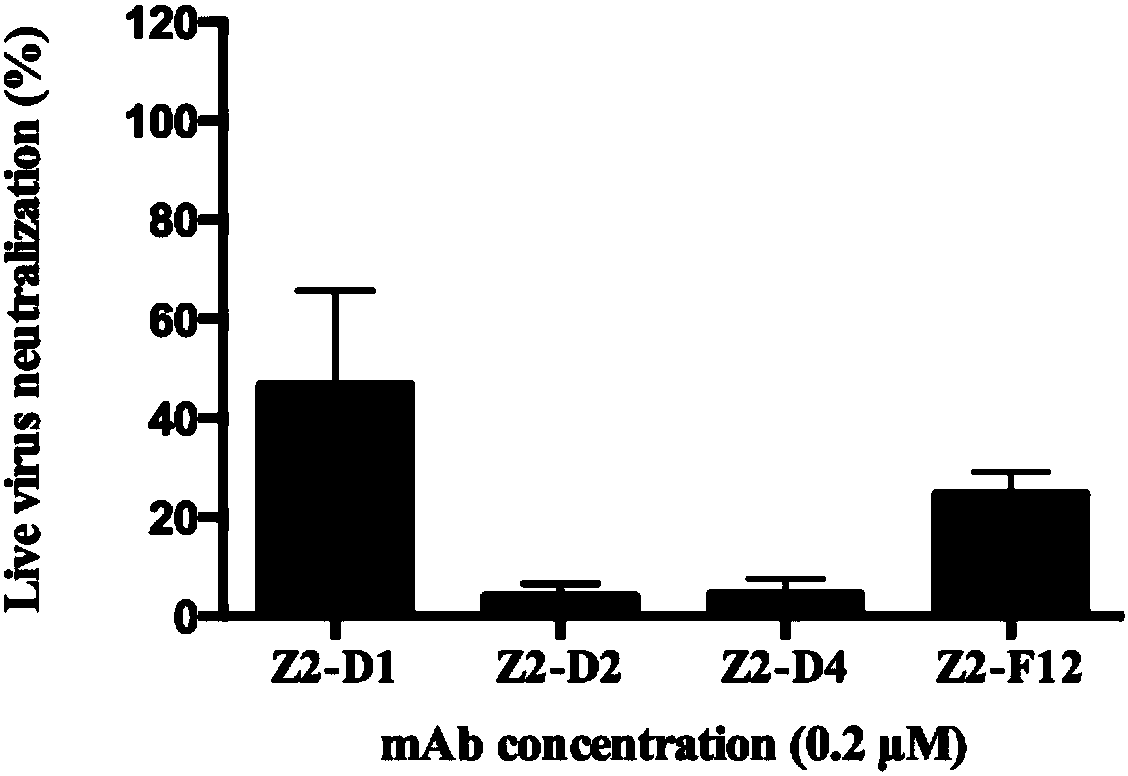
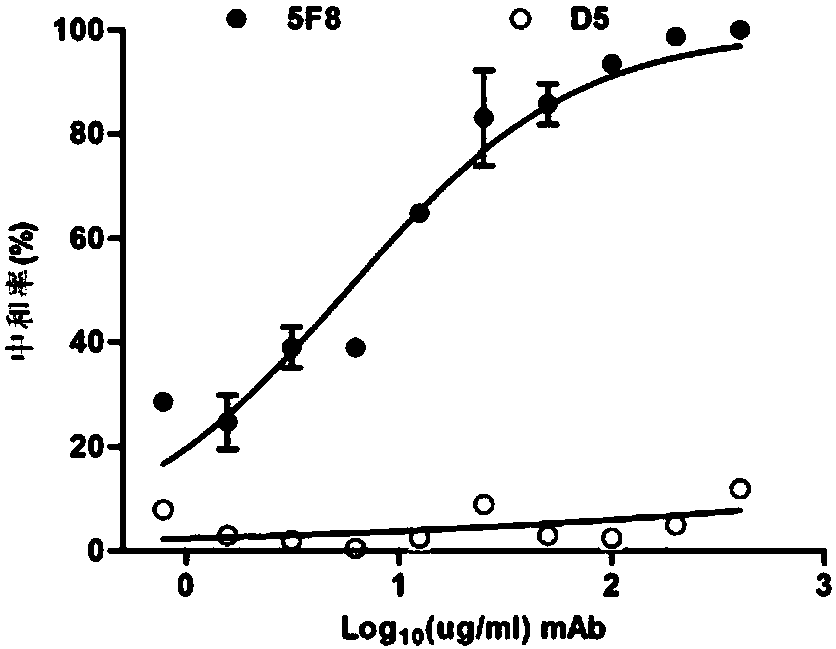
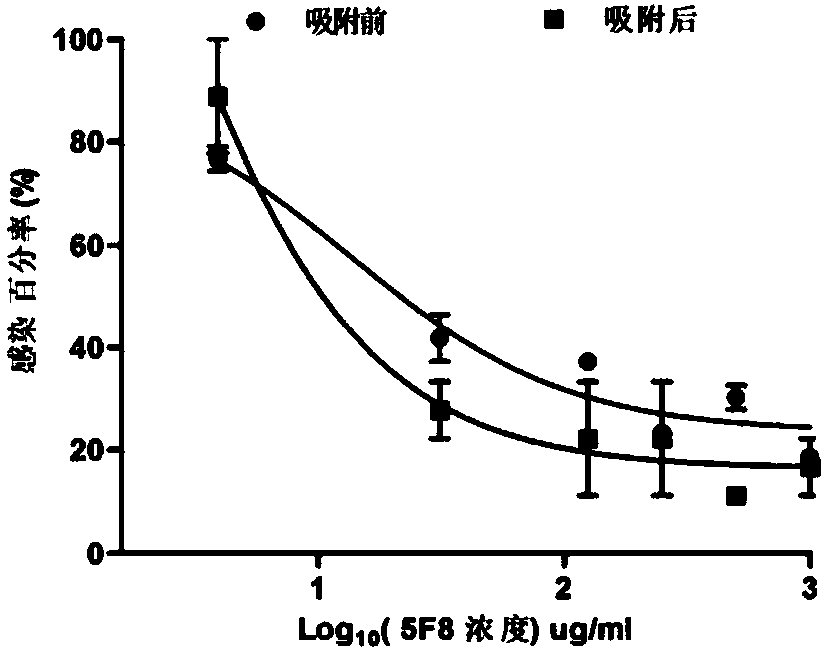
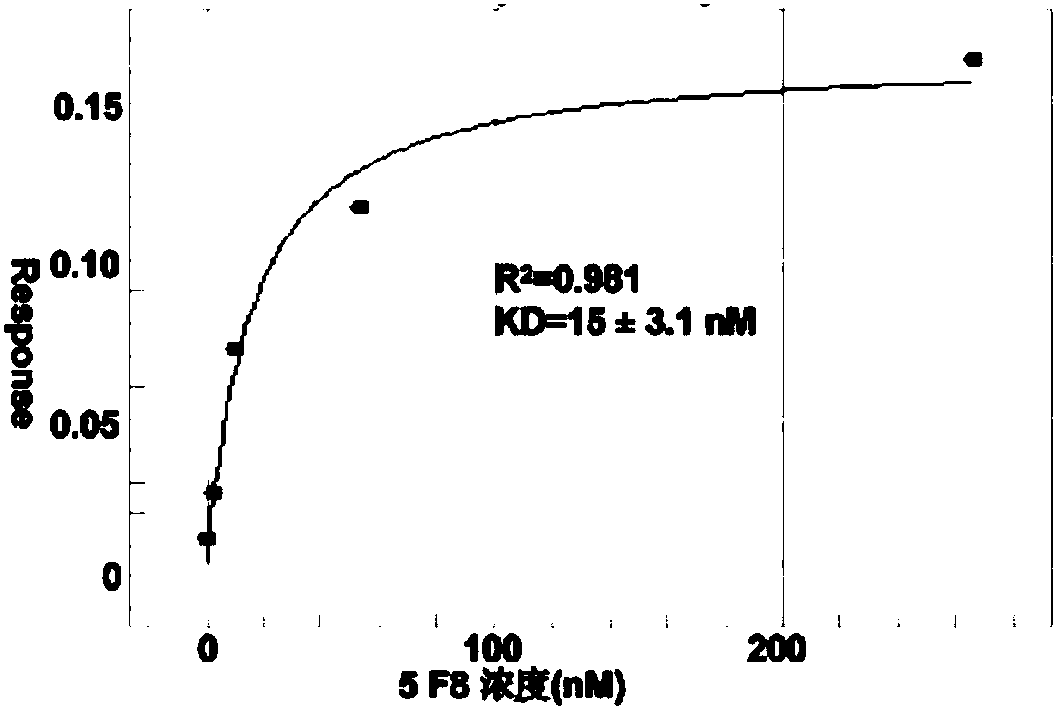
Method for rapidly preparing Zika-virus specific full human monoclonal antibodies and application
InactiveCN106478815AEasy to operateImprove screening efficiencyImmunoglobulins against virusesAntiviralsZika virusPeripheral blood mononuclear cell
The invention belongs to the field of medical biology, and particularly relates to a method for rapidly preparing Zika-virus specific full human monoclonal antibodies and an application. The method for rapidly preparing the Zika-virus specific full human monoclonal antibodies includes the steps of peripheral blood mononuclear cell separating, plasma cell separating, antibody-variable-region-gene PCR amplification, antibody cloning, cotransfection and identifying and the like. The method for rapidly preparing the Zika-virus specific full human monoclonal antibodies is simple and fast and convenient, antigens do not need to be marked, functional antibodies of conformation structural domains which exists in vivo and is difficult to emulate in vitro can be separated, the obtained specific antibodies have important guiding significance on researching of Zika vaccine, and some antibodies can have clinic-treatment application prospects.
Owner:GUANGZHOU EIGHTH PEOPLES HOSPITAL
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS10072309B1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaFluorescence
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
Nucleic acid for detecting Zika virus, real-time fluorescence RPA kit and method
ActiveCN106367533AEasy to useEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationZika virusFluorescence
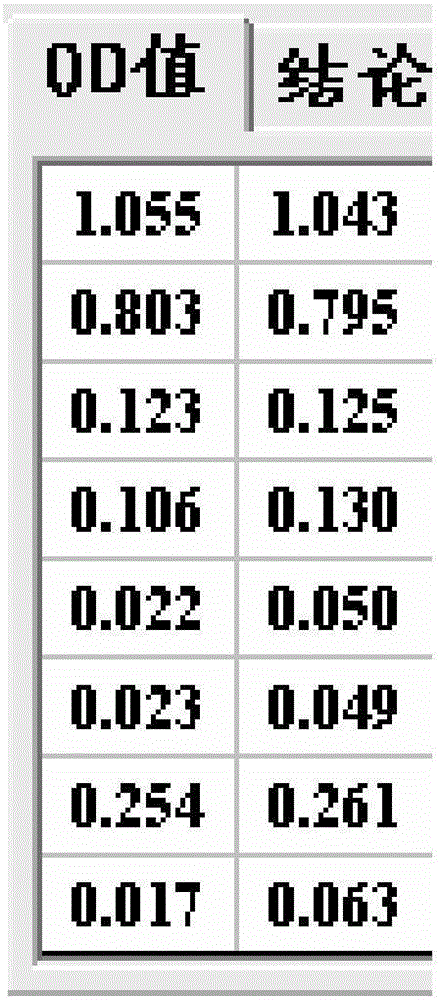
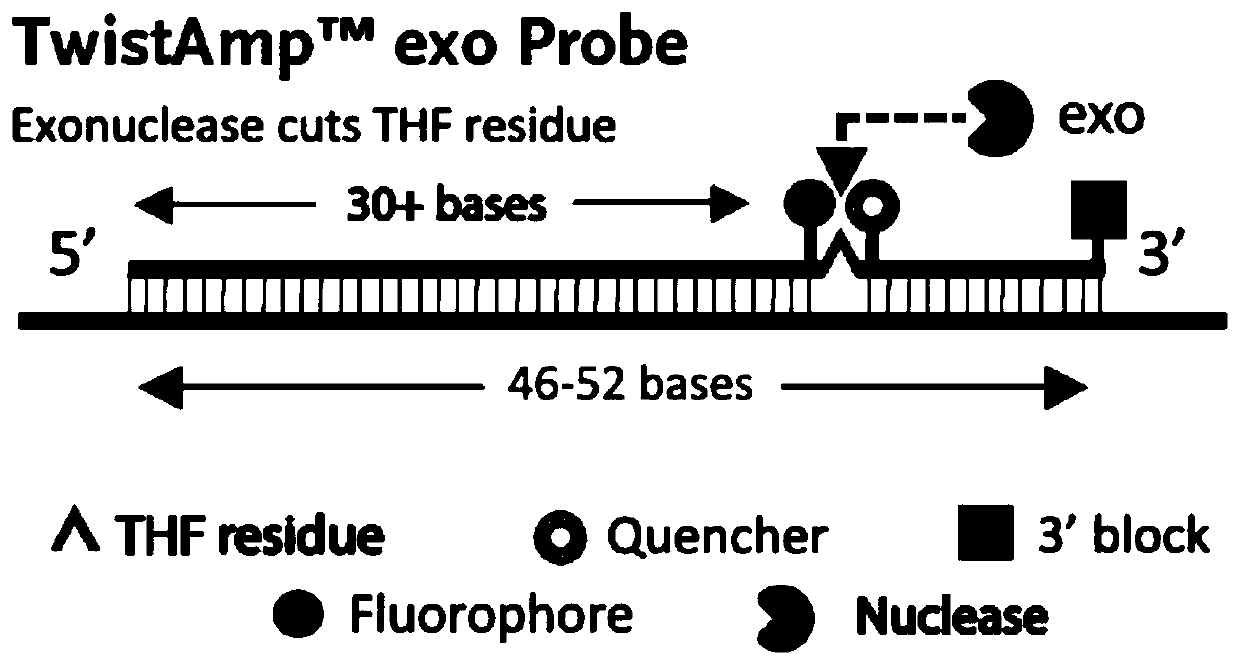
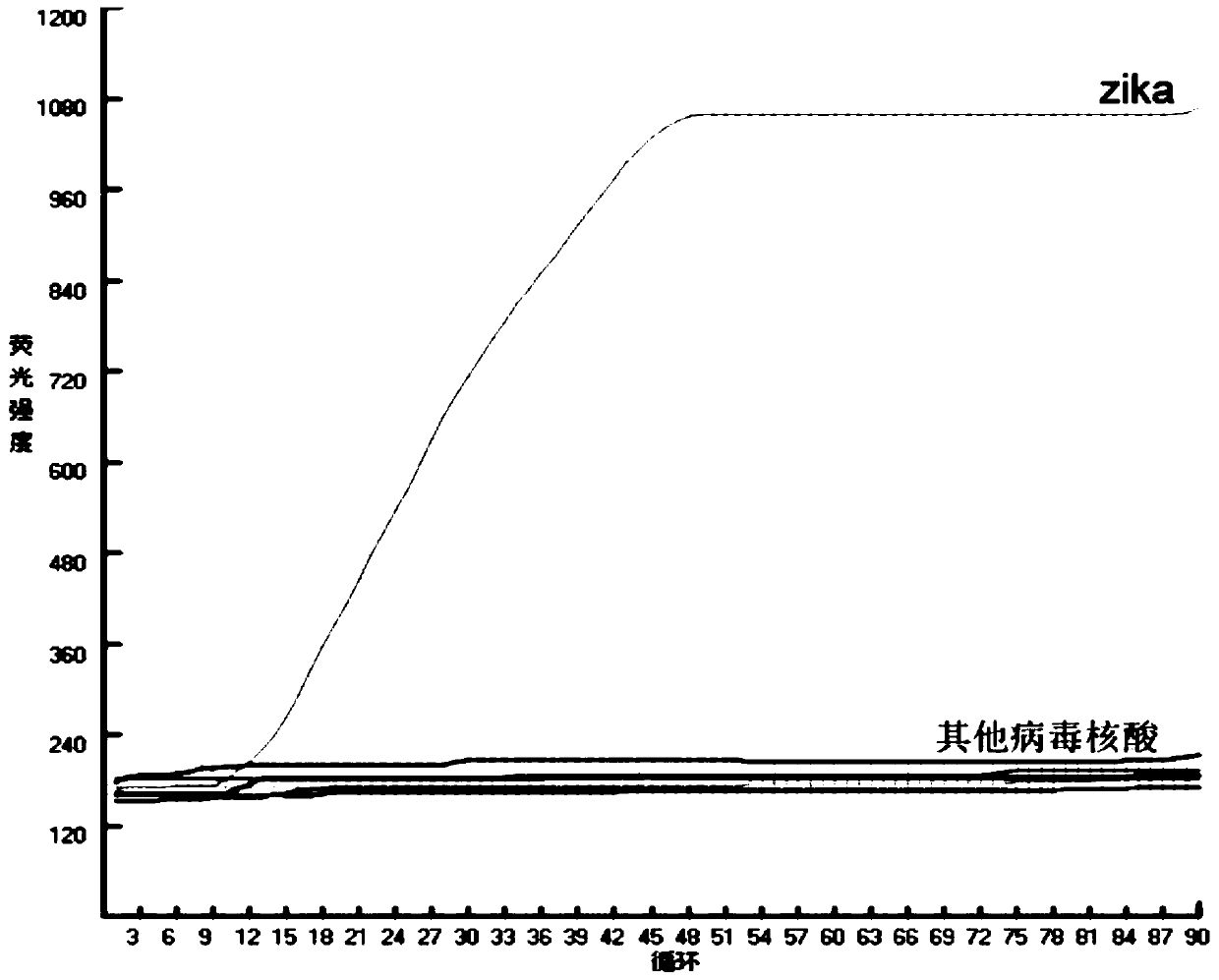
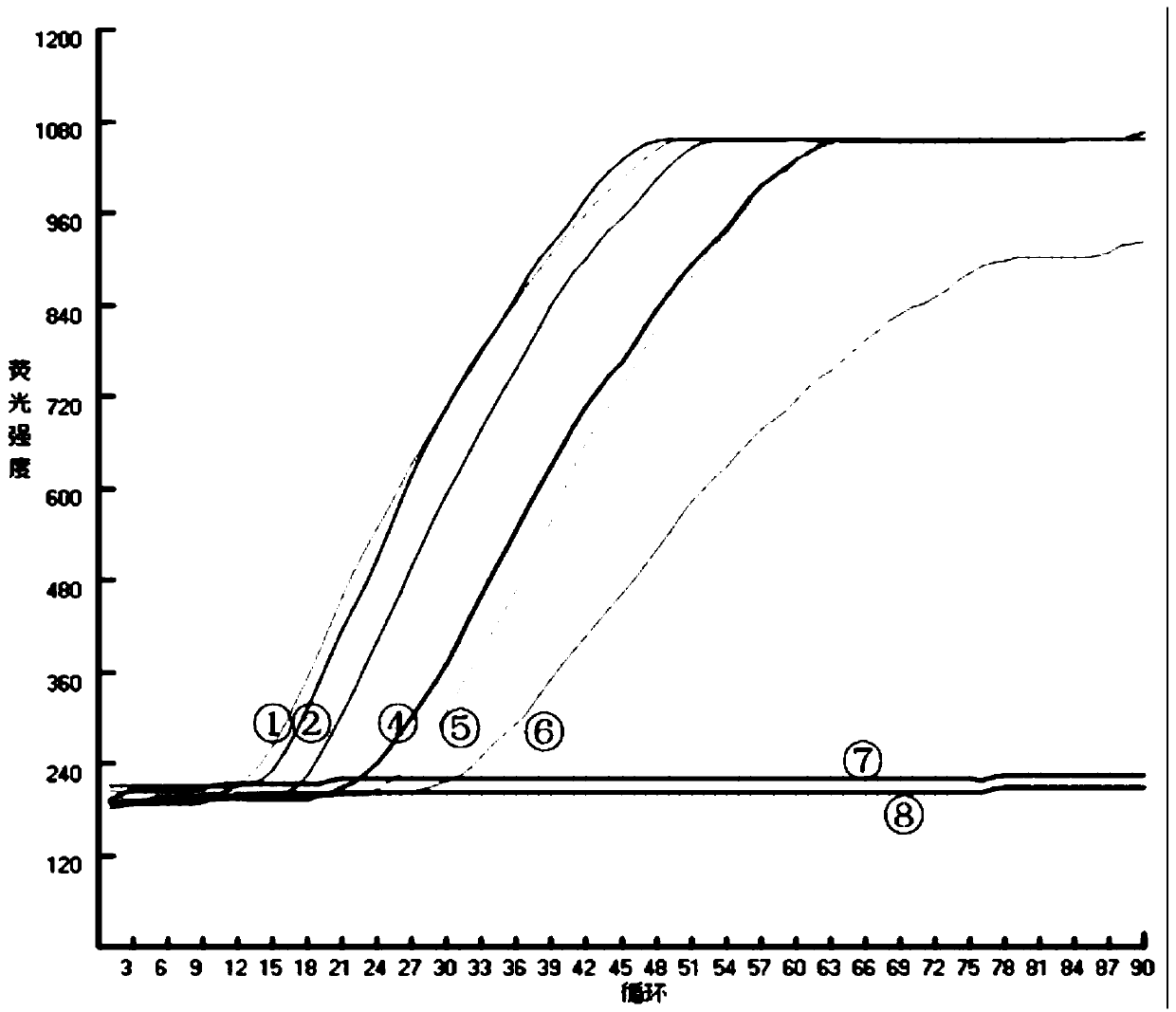
The invention discloses nucleic acid for detecting Zika virus, a real-time fluorescence RPA kit and a detection method thereof. The real-time fluorescence RPA kit is convenient to use, the quantity of reagents adopted is small, the cost is low, the compatibility of required instruments is high, reaction can be performed on a real-time fluorescence PCR instrument and instruments with a fluorescent trapping function, and the greatest advantage of the kit lines in performing isothermal amplication and being capable of detecting fluorescence signals in real time within 10 to 30 min. The detection method greatly simplifies the operation process, reduces steps of repetitive operation, saves time, reduces labor force consumed by repetitive operation, and effectively lowers the cost, and a detection result shows that the method is high in specificity and sensitivity, and rapid and accurate screening of Zika virus is realized.
Owner:淮安市疾病预防控制中心
Kit for flavivirus quick typing and virus load detection
InactiveCN106086242ANo cross reactionAchieve quantitative goalsMicrobiological testing/measurementAgainst vector-borne diseasesZika virusMicroorganism
The invention belongs to the field of microbial molecular detection, and particularly relates to a kit for flavivirus quick typing and virus load detection. The kit for flavivirus quick typing and virus load detection comprises specific primers and probes for flaviviruses, including specific primers and a probe for dengue virus, specific primers and a probe for Zika virus, specific primers and a probe for yellow fever virus, and specific primers and a probe for Chikungunya virus. The kit for flavivirus quick typing and virus load detection has the advantages of high detection speed and accurate detection and quantification results, can simultaneously detect 3 different flavivirus pathogens and 1 togavirus pathogen capable of causing similar clinical symptoms at one time, can detect and diagnose flavivirus-pathogen-infected suspicious cases in time, and enhances the detection accuracy of flavivirus pathogen infection.
Owner:GUANGZHOU EIGHTH PEOPLES HOSPITAL
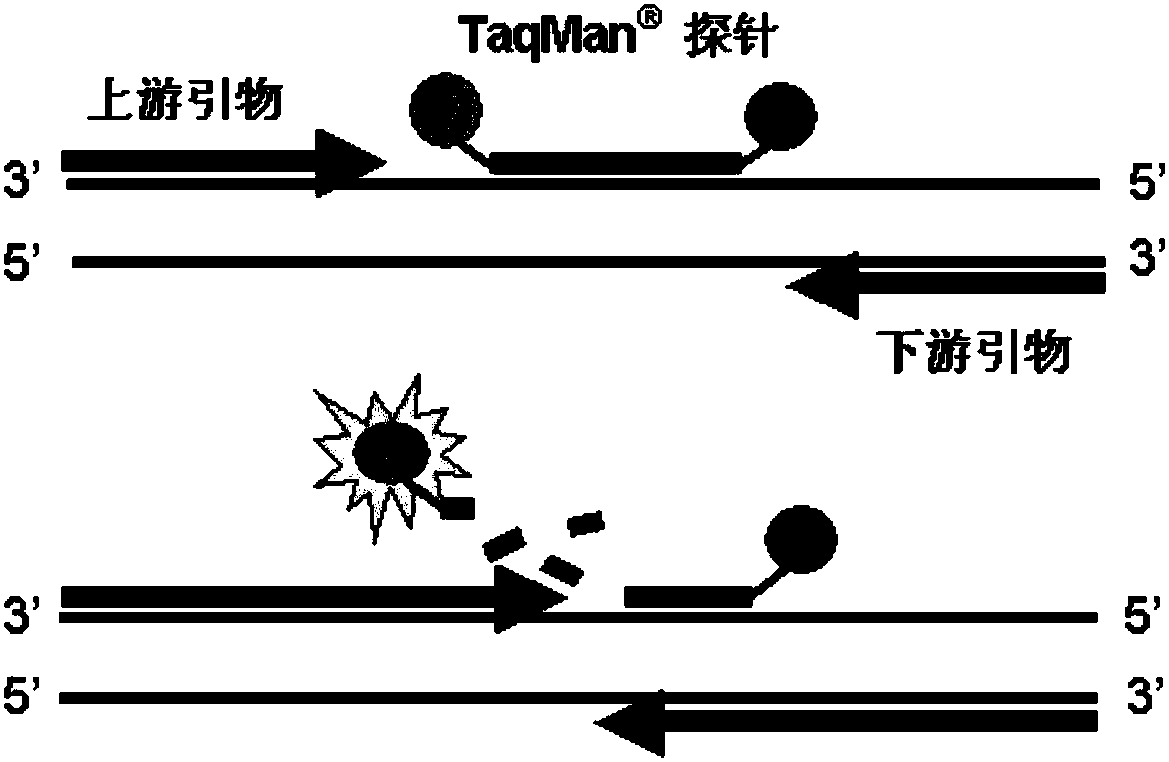
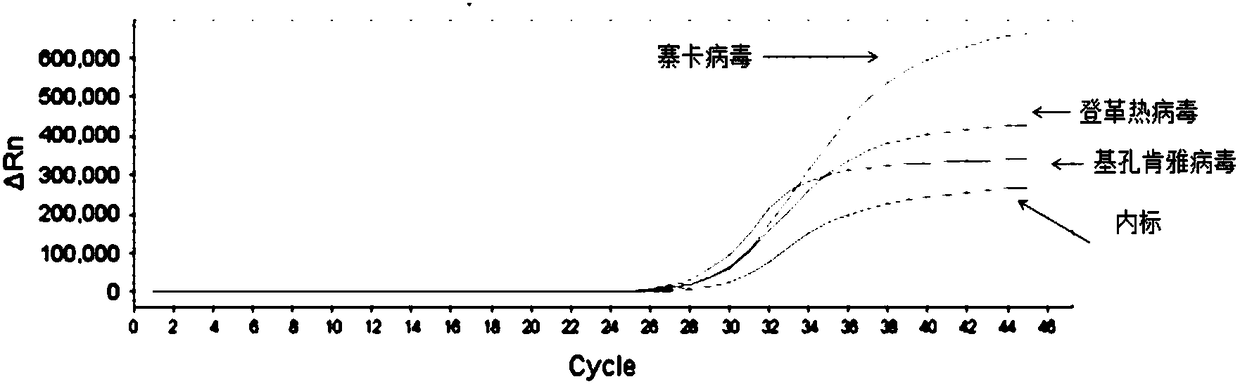
RT-PCR detection method, primer and probe as well as kit of Zika virus, dengue virus and chikungunya virus
InactiveCN106755573AQuick checkAccurate detectionMicrobiological testing/measurementMicroorganism based processesZika virusFluorescence
The invention relates to the technical field of molecular biological detection of an insect-borne infectious disease, and discloses a triple real-time fluorescence RT-PCR detection method, primer and probe as well as kit of Zika virus, dengue virus and chikungunya virus. The primer comprises the sequences of SEQ ID No.1, SEQ ID No.2, SEQ ID No.4, SEQ ID No.5, SEQ ID No.7 and SEQ ID No.8; the probe comprises the sequences of SEQ ID No.3, SEQ ID No.6, and SEQ ID No.9; the kit comprises a primer probe mixed solution, an RT-PCR reaction solution, an enzyme mixed solution, and a positive standard substance. The Zika virus, the dengue virus and the chikungunya virus can be fast detected in the reaction solution of a same tube by use of a triple real-time fluorescence RT-PCR amplification technology by optimizing the reaction solution formula and the primer probe sequences, the single detection is unnecessary; compared with the traditional PCR detection method, the operation step is reduced, whether the reaction solution contains the three virus can be fast and accurately detected, the detection time is shortened, the detection efficiency is improved, and the cost is saved.
Owner:深圳澳东检验检测科技有限公司
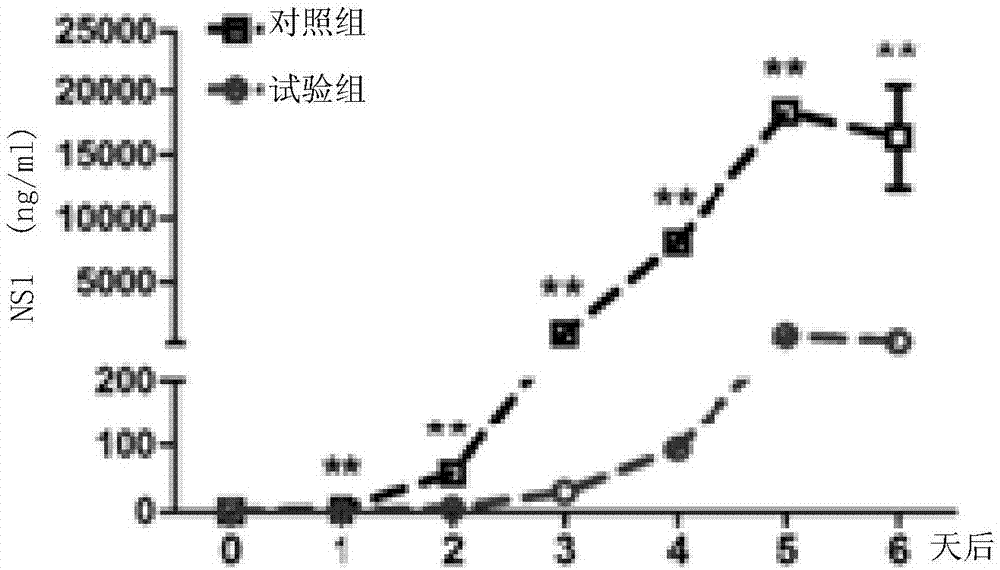
ZIKV(zika virus)-NS1 protein and application of ZIKV-NS1 protein to preparation of ZIKV propagation blocking vaccine
InactiveCN107987136APrevent sudden outbreaksReduce infection rateSsRNA viruses positive-senseViral antigen ingredientsWAS PROTEINZika virus
The invention discloses ZIKV(zika virus)-NS1 protein and application of the ZIKV-NS1 protein to preparation of ZIKV propagation blocking vaccine. The invention provides protein which is named as the ZIKV-NS1 protein; the ZIKV-NS1 protein is protein formed by amino acid sequences shown as sequence 1 in a sequence table. The invention also provides application of the ZIKV-NS1 protein. The application comprises the following steps (e1) or (e2): (e1) ZIKV vaccine preparation; (e2) ZIKV propagation blocking vaccine preparation. The vaccine provided by the invention can block the propagation of ZIKVthrough mosquitos in nature; the infection rate of mosquitos in natural environment is reduced, so that healthy people groups are protected from being infected by ZIKV; the sudden outbreak of the ZIKV is prevented.
Owner:TSINGHUA UNIV
Fully human monoclonal antibodies against Zika virus and application thereof
InactiveCN107586336AAvoid infectionHybrid immunoglobulinsPeptide/protein ingredientsZika virusComplementarity determining region
Belonging to the field of biotechnology, the invention relates to fully human monoclonal antibodies against Zika virus, antigen binding fragments and application thereof. The antibodies provided by the invention are determined by complementary determining region (CDR) specific gene sequences in antibody light chain and heavy chain gene variable regions and are antibodies specifically bound to Zikavirus envelope glycoprotein E (protein E) and effectively expressed in prokaryotic and eukaryotic cells. The antibody CDR region or partial or whole genes can be utilized to transform and produce genetic engineering antibodies of different forms in prokaryotic and eukaryotic cells and any expression system, and can prevent or treat Zika virus related diseases clinically.
Owner:FUDAN UNIV
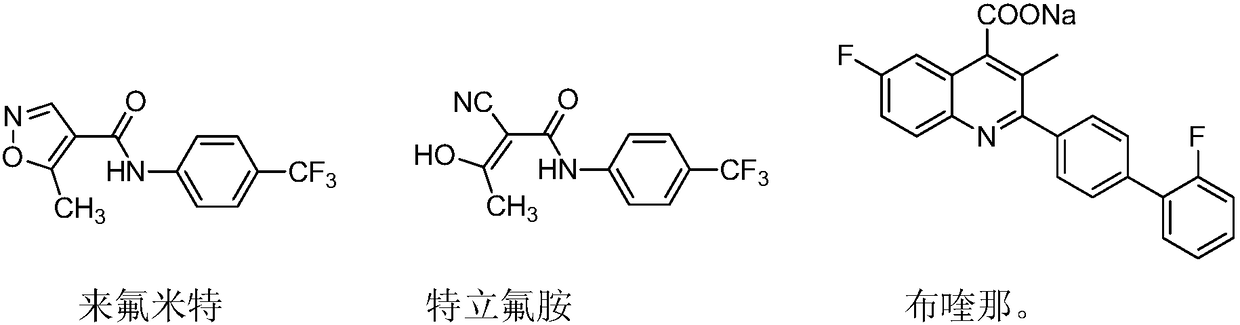
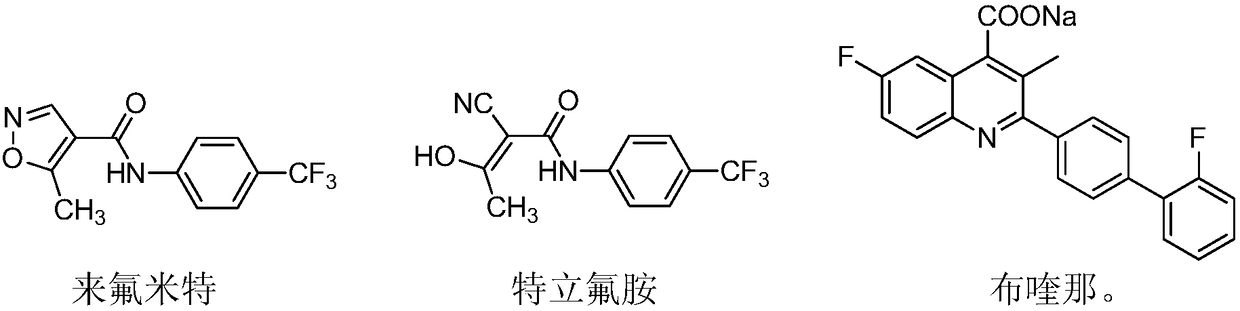
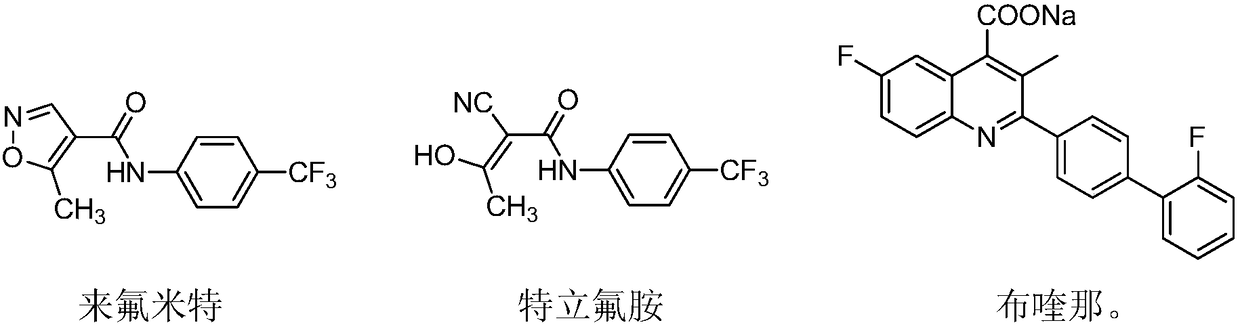
Novel antiviral medicines and application thereof
PendingCN108721281ABroad-spectrum and excellent antiviral activityLow toxicityAntiviralsNitrile/isonitrile active ingredientsLeflunomideRNA virus
The invention discloses application of leflunomide, teriflunomide, brequinar and derivatives thereof to treatment of virus infection, especially RNA virus infection. RNA viruses include but are not limited to influenza viruses, respiratory syncytial viruses, hand, foot and mouth viruses (EV71), dengue viruses (type-2 dengue viruses), Zika viruses and Japanese encephalitis viruses. The medicines have broad-spectrum and excellent antiviral activity and have relatively low toxicity to normal cells.
Owner:EAST CHINA UNIV OF SCI & TECH
Nucleic acid detection kit for Zika virus, dengue fever virus and Chikungunya virus, and application of the kit
ActiveCN108330210ASolve the problem of not being able to detect three viruses simultaneously in one reactionSimple and fast operationMicrobiological testing/measurementAgainst vector-borne diseasesZika virusPositive control
The invention discloses a nucleic acid detection kit for Zika virus, dengue fever virus and Chikungunya virus, and an application of the kit, wherein the kit includes: a RT-PCR reaction solution, an enzyme mixture liquid, a Zika virus / dengue fever virus / Chikungunya virus / endogenous reference gene quadruple reaction solution, a positive control, and a blank control. The kit can simultaneously detect the Zika virus, dengue fever virus and Chikungunya virus in one reaction and solves a problem that an in-vitro detection kit cannot simultaneously detect the Zika virus, dengue fever virus and Chikungunya virus in one reaction tube; besides, the detection kit is high in specificity and sensitivity, has simple operation, excellent repeatability and quick and objection detection results. The invention provides an effective technical means for in-vitro detection of the Zika virus, dengue fever virus and Chikungunya virus.
Owner:GUANGZHOU CENT FOR DISEASE CONTROL & PREVENTION (GUANGZHOU HYGIENE INSPECTION CENT GUANGZHOU CENT FOR FOOD SAFETY RISK SURVEILLANCE & ASSESSMENT INST OF PUBLIC HEALTH OF GUANGZHOU MEDICAL UNIV) +1
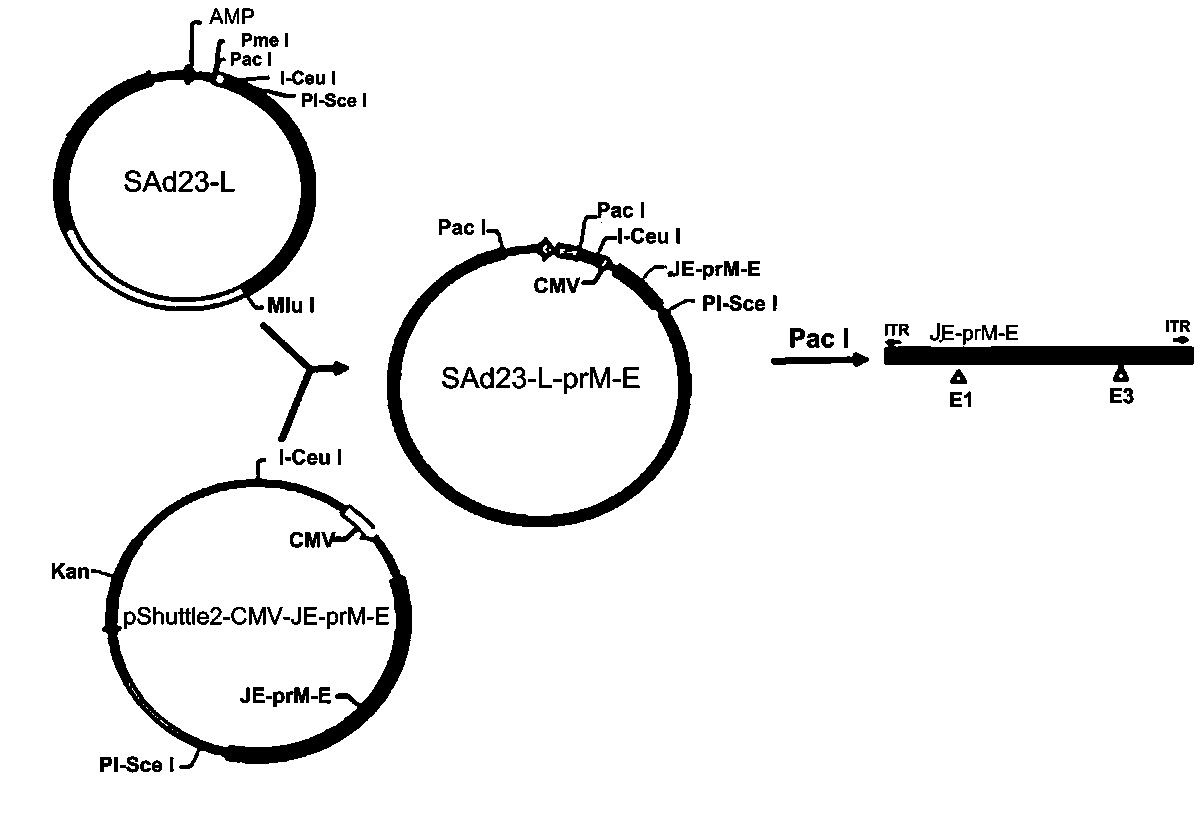
Zika virus vaccine based on replication-defective recombinant adenovirus vector
PendingCN111088271AImprove expression levelImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsNucleotideImmunogenicity
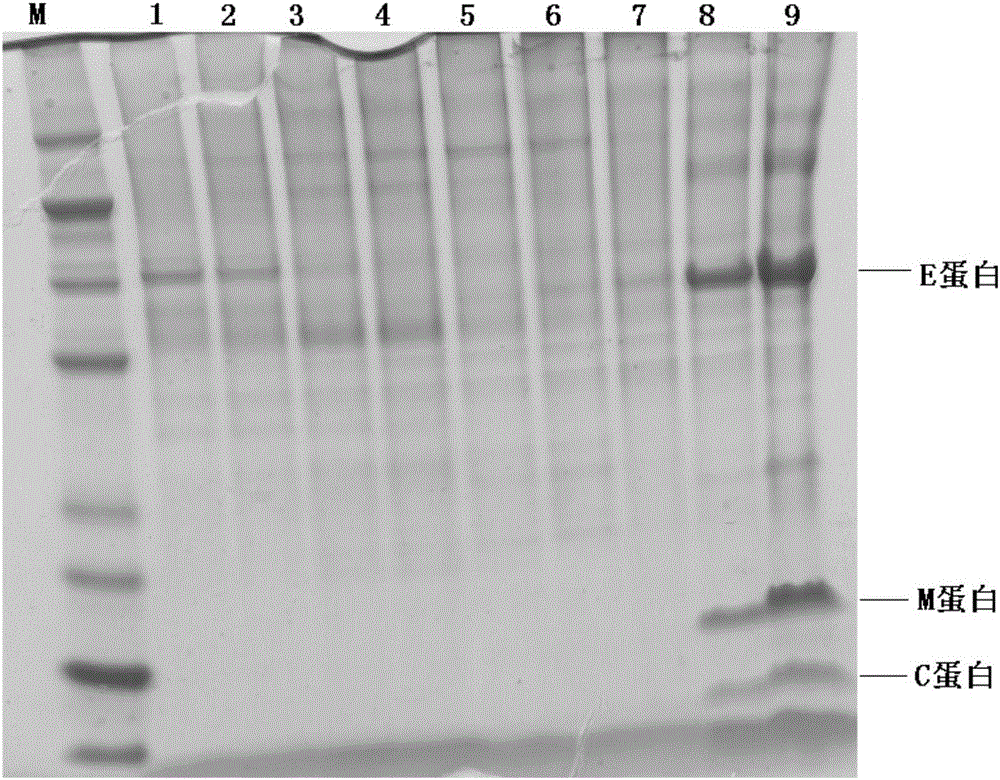
The invention discloses a Zika virus vaccine based on a replication-defective recombinant adenovirus vector. A nucleotide sequence of a JE signal peptide, and an optimized nucleotide sequence encodingZika virus membrane protein prM and envelope glycoprotein E are cloned into a shuttle plasmid vector pShutle2-CMV-Flag to obtain a recombinant shuttle plasmid, so that the expression of foreign proteins is significantly increased, and at the same time immunogenicity of an antigen is improved. Meanwhile, a recombinant adenovirus expression vector SAd23-L is used to escape pre-existing immune responses against common adenovirus vectors, and the humoral and cellular immunity with a higher level can be induced to generate in animals. After the recombinant adenovirus as a Zika virus vaccine is used to immunize animals, the humoral and cellular response to Zika virus is quickly induced to generate, especially a neutralizing antibody with a high level is induced to generate, and the specific cellular response to Zika virus antigen M and E protein is induced to generate in mice. Therefore, the Zika virus vaccine can be used to prevent large-scale outbreak and epidemic of Zika virus.
Owner:广州佰芮慷生物科技有限公司
Human monocolonal antibody with high neutralization activity for Zika virus and application thereof
ActiveCN110172095AFree from attackStrong neutralizing activityImmunoglobulins against virusesAntiviralsAntigenEscherichia coli
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Virus purification
ActiveUS10537630B2Improving immunogenicityHighly cross-protectiveSsRNA viruses positive-senseViral antigen ingredientsYellow fever vaccineZika virus
Described herein are improved purification methods for virus vaccines and compositions. Also described are Zika, Chikungunya, dengue and yellow fever vaccines and methods of producing and administering said vaccines to subjects in need thereof.
Owner:VALNEVA SE
4'-halogen containing nucleotide and nucleoside therapeutic compositions and uses related thereto
Disclosed are halogen containing nucleotide and nucleoside therapeutic compositions and uses related thereto. In certain embodiments, the disclosure relates to the treatment or prophylaxis of viral infections. Such viral infections can include tongaviridae, bunyaviridae, arenaviridae, coronaviridae, flaviviridae, picornaviridae, Eastern, Western, and Venezuelan Equine Encephalitis (EEE, WEE and VEE, respectively), Chikungunya fever (CHIK), Ebola, Influenza, RSV, and Zika virus infections.
Owner:EMORY UNIVERSITY
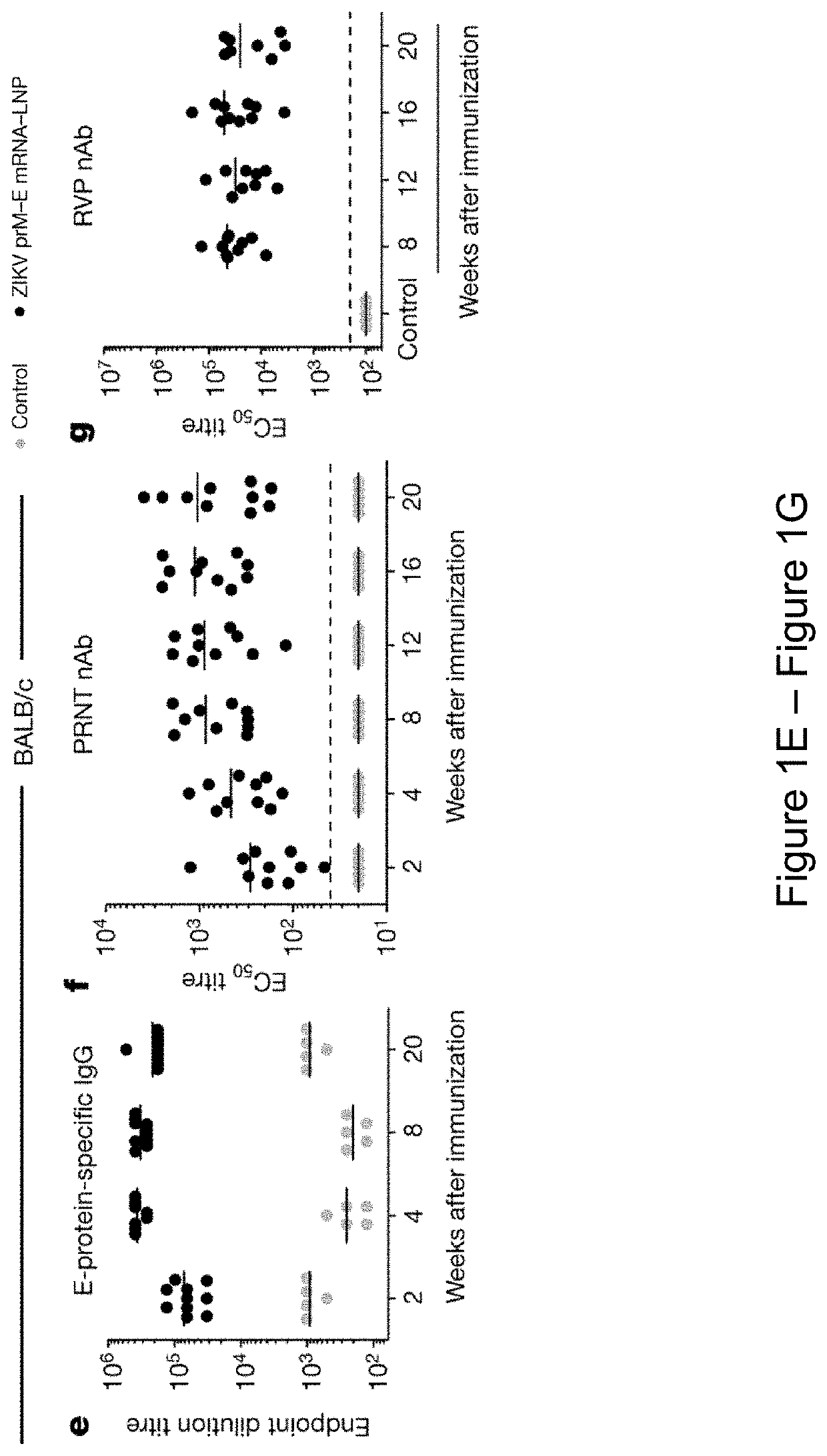
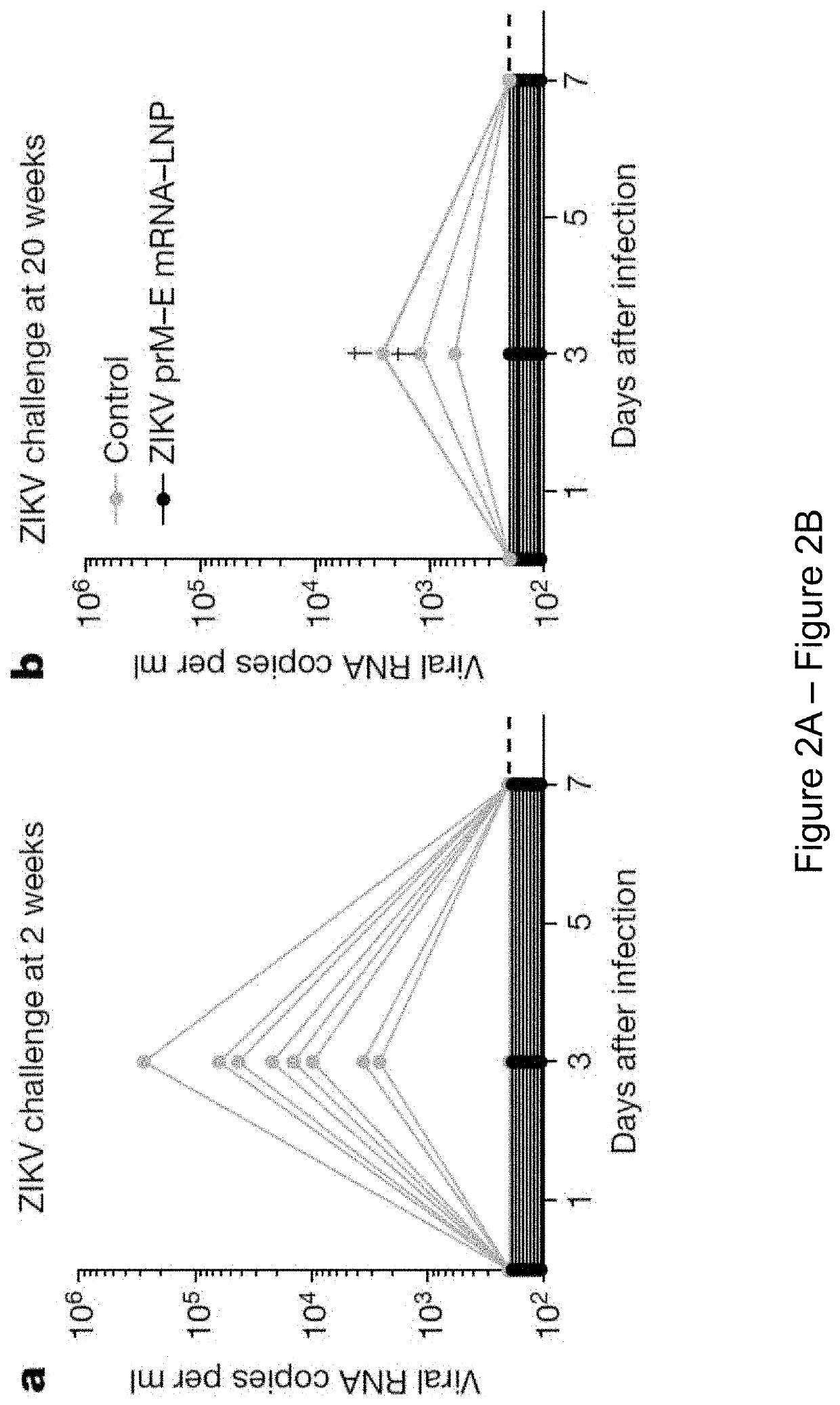
Nucleoside-modified RNA for inducing an immune response against zika virus
The present invention relates to compositions and methods for inducing an adaptive immune response against Zika virus (ZIKV) in a subject. In certain embodiments, the present invention provides a composition comprising a nucleoside-modified nucleic acid molecule encoding a ZIKV antigen, adjuvant, or a combination thereof. For example, in certain embodiments, the composition comprises a vaccine comprising a nucleoside-modified nucleic acid molecule encoding a ZIKV antigen, adjuvant, or a combination thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
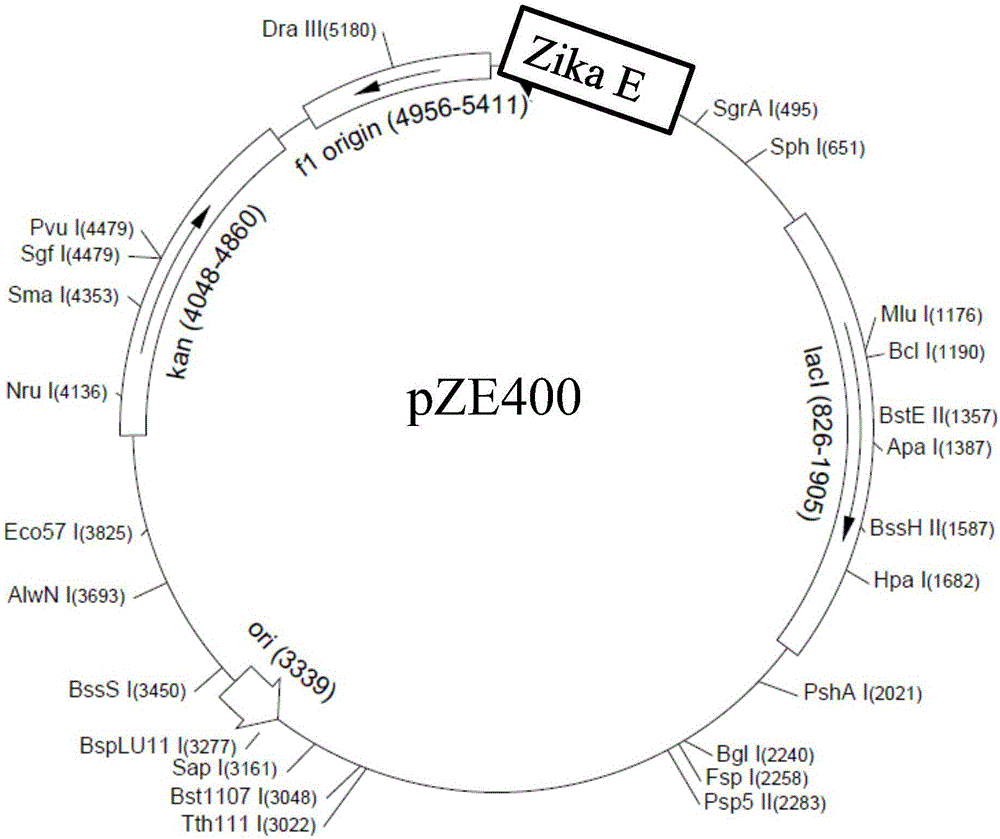
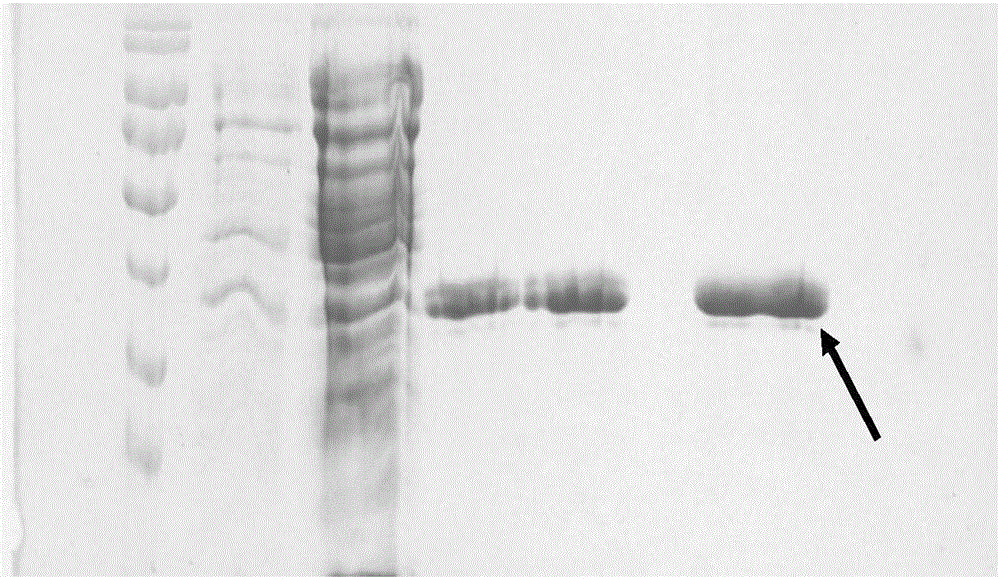
Zika virus antigen and application thereof
ActiveCN106518990AImproving immunogenicityStrong specificitySsRNA viruses positive-senseBacteriaAntigenProtein engineering
The invention provides a Zika virus antigen and application thereof, and belongs to the technical field of protein engineering. DNA sequences corresponding to a protein sequence determined through DNA analysis and protein structure analysis are synthesized, Nde I and Xho I cleavage sites are introduced, a commercialized pET30a plasmid serves as a basic carrier, pZE400 is built, the protein sequence is as shown in SEQ ID NO.1, and the DNA sequences are as shown in SEQ ID NO.2. A protein C end is labeled with a protein band HIS expressed by the expression carrier and Zika virus protein is obtained through affinity chromatography purifying and protein renaturation. The protein is good in immunogenicity and high in specificity, can be used for preparing a Zika virus detection test strip or kit, can also be used for preparing Zika virus vaccines, and is remarkable in market value.
Owner:德诺杰亿(北京)生物科技有限公司
Zika virus real-time fluorescence quantitative RT-RPA detection primers, probes and detection kit
ActiveCN110699491AStrong specificityEfficient identificationMicrobiological testing/measurementDNA/RNA fragmentationZika virusBioinformatics
The invention provides Zika virus real-time fluorescence quantitative RT-RPA detection primers, probes and a detection kit. The kit comprises the primers and the probes for detecting Zika virus basedon a RPA technology, and upstream and downstream primers and probe sequences are respectively shown in SEQ ID NO. 1-3. The real-time fluorescence quantitative RT-RPA technology is adopted for the first time to establish a method for rapidly detecting the Zika virus, and through specificity, sensitivity and stability evaluation, the primers, the probes and the detection kit can be used for clinicalon-site detection, and provides a sensitive and reliable new method for on-site detection of the Zika virus.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
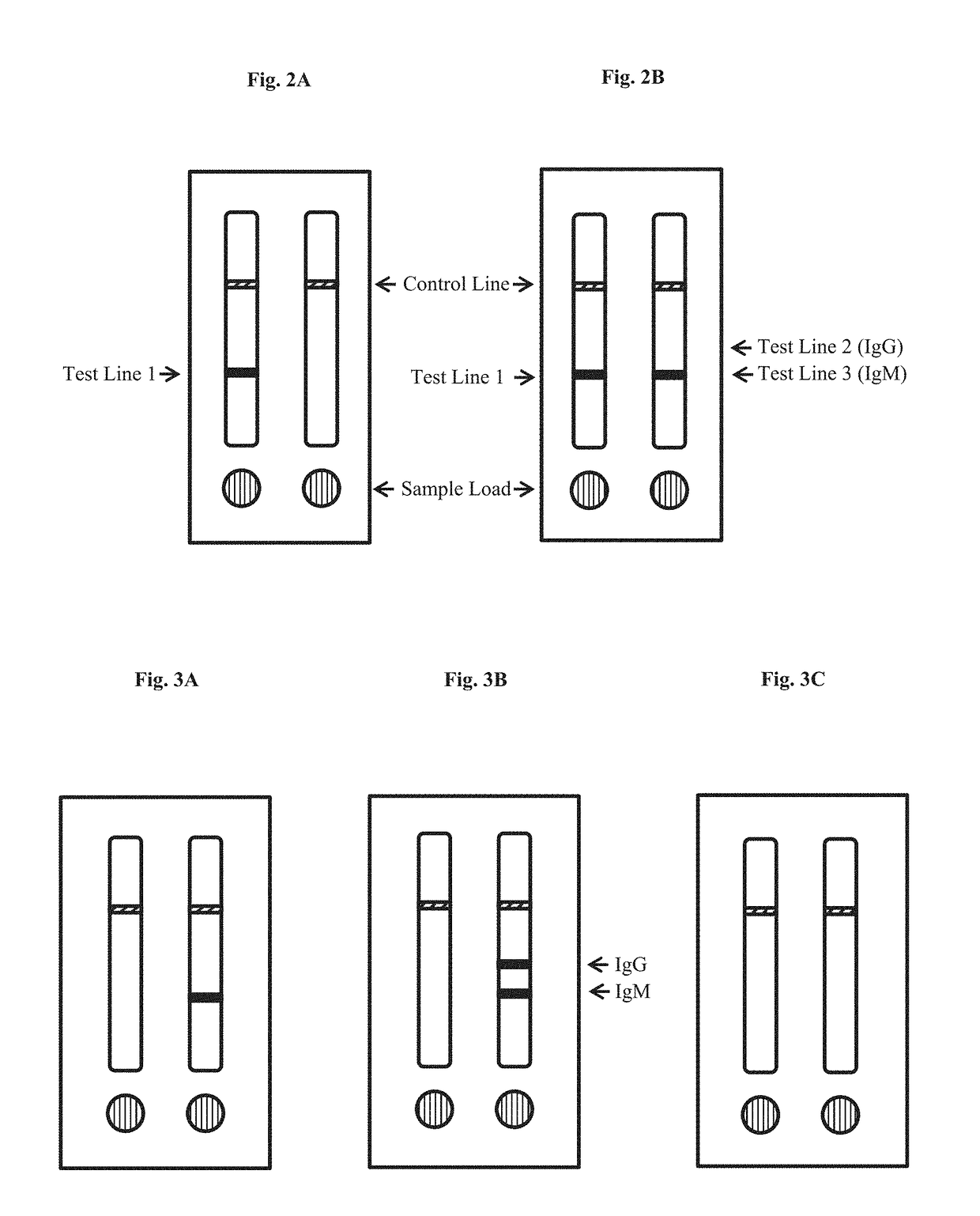
Preparation of Zika virus multi-segment fusion protein and IgG/IgM antibody detection kit
InactiveCN106841601AQuick checkDisease diagnosisAgainst vector-borne diseasesBiotin-streptavidin complexIgm antibody
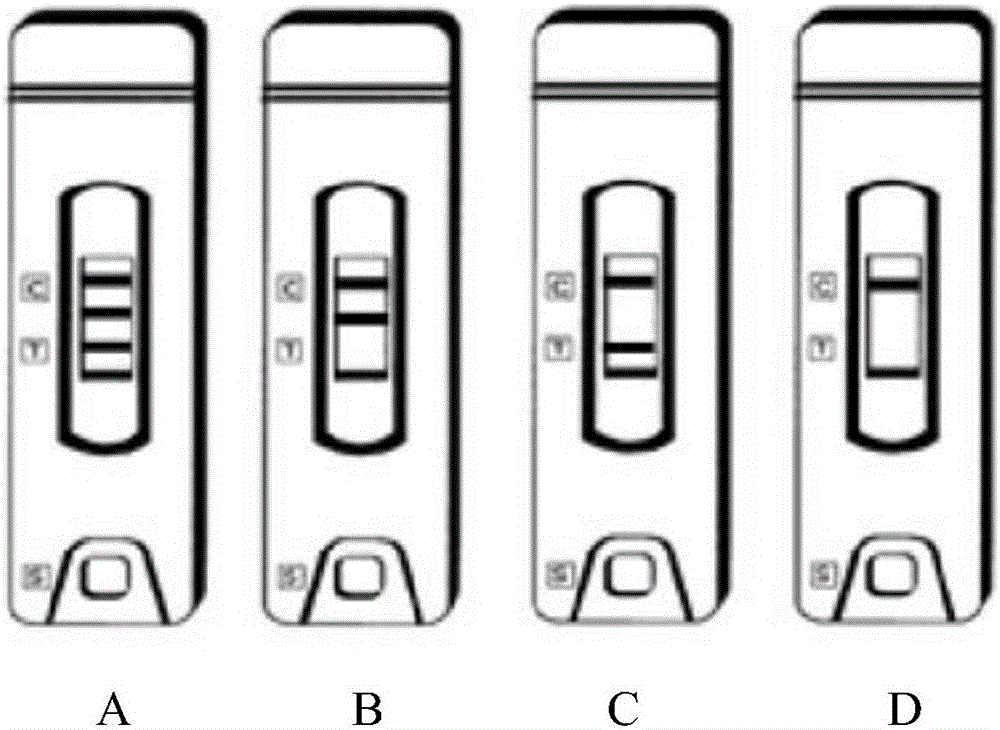
The invention aims at providing a simple and quick Zika virus detection kit. The kit optimally selects fusion expression protein as diagnostic antigen; an anti-human IgG monoclonal antibody A374, an anti-human IgM monoclonal antibody A371 and a biotin-BSA conjugate are respectively coated on a nitrocellulose membrane as a detection line and a quality control line; colloidal gold labeled fusion expression protein and colloidal gold labeled streptavidin and other reagents are matched; and an immunochromatography capture method principle is used for qualitative detection of Zika virus specific IgM antibody and IgG antibody in human serum, thereby realizing quick and specific diagnosis of Zika virus infection.
Owner:GUANGZHOU DARUI BIOTECH
Repair traditional Chinese medicine used for treating blood cell infected by flaviviridae
InactiveCN105920380AEffective preventionEffective therapeuticNervous disorderDigestive systemMedicinal herbsDisease
The invention relates to the field of traditional Chinese medicine, and discloses a repair traditional Chinese medicine used for treating blood cell infected by flaviviridae. The traditional Chinese medicine is prepared from: by weight, herba tubocapsici anomali 30g-80g, cymbopogon citratus 20g-50g, camellia flower 10g-30g, citrus aurantium flower 10g-30g, bitter gourd 20g-50g, mint 20g-50g, phellodendron amurense 10g-30g, cypress leaf 10g-30g, salix leaf 10g-30g, persimmon leaf 15g-35g, perilla nankinensis 10g-20g, radix sophorae flavescentis 30g-60g, eleusine indica 25g-80g, syzygium aromaticum 10g-30g, lonicera japonica 30g-80g, agastache rugosus 20g-50g, portulaca oleracea 10g-30g, glycyrrhiza 5g-15g, chrysanthemum 10g-35g, and foeniculum vulgare 10g-35g. The traditional Chinese medicine has cure effect of the diseases such as dengue, Zika virus, encephalitis, hepatitis and liver cancer infected by flaviviridae spreading, can treat and repair the blood cell from the source, has prophylaxis and treatment effect on flaviviridae diseases, is without any toxicity and side effects, and can improve the cure rate and cure efficiency of patients.
Owner:林家希
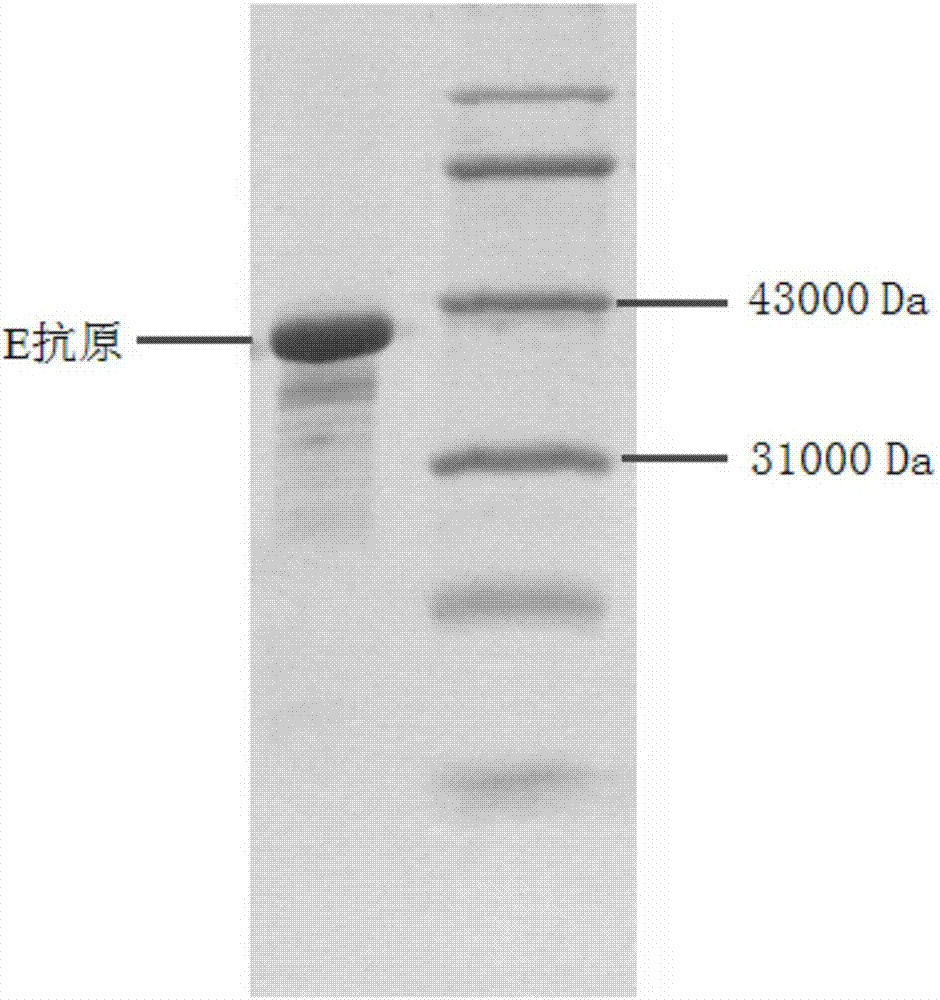
Zika virus (ZIKV) E antigen and application thereof in detecting anti-ZIKV antibody
The invention belongs to the field of immunity detection of an anti-zika virus (ZIKV) antibody, and particularly relates to a ZIKV E antigen and application thereof in detecting the anti-ZIKV antibody. After a ZIKV gene group and the expression protein thereof are analyzed, the E antigen for specifically detecting the anti-ZIKV antibody is found, the anti-ZIKV antibody in human blood is specifically detected by the founded E antigen, and a kit for detecting the anti-ZIKV antibody is prepared. The method for detecting the anti-ZIKV antibody has the advantages that the use of ZIKV with strong infection ability is effectively avoided in the detection process, so that the risk of a whole experiment is decreased; by adopting the technical scheme, the operation is simple and easy, the repeatability is good, and the like; the method can be easily popularized and applied.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Method for detecting Zika virus, Chikungunya virus and Mayaro virus by triple real-time fluorescent quantitative RT-PCR
ActiveCN110305985AImprove universalityStrong specificityMicrobiological testing/measurementMicroorganism based processesZika virusChikungunya fever
The invention relates to the technical field of virus detection, and concretely relates to a method for detecting Zika virus, Chikungunya virus and Mayaro virus by triple real-time fluorescent quantitative RT-PCR. The method combines the probability of occurrence of related mosquito-borne pathogens, the risk of prevalence, and the operability of a laboratory testing procedure, the Mayaro virus isused to replace the dengue virus in an existing common combination detection scheme, and a new detection scheme using Zika virus, Chikungunya virus and Mayaro virus as detection targets is formed. Theinvention provides a specific primer, a probe and a triple real-time quantitative RT-PCR detection method for simultaneously identifying the viral pathogens Zika virus, Chikungunya virus and Mayaro virus which are transmitted by Aedes mosquito and cause similar clinical symptoms of diseases. The method has high specificity and sensitivity, good repeatability, simple and rapid detection, and costsaving, and can complement a traditional detection scheme and has high application value.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Quantitative detection and analysis of target DNA with colorimetric rt-qlamp
PendingUS20200063197A1Image enhancementImage analysisZika virusLoop-mediated isothermal amplification
The present disclosure relates to real-time quantification using loop-mediated isothermal amplification, in particular real-time colorimetric reverse transcription quantitative loop-mediated isothermal amplification (RT-qLAMP). In some embodiments, RT-qLAMP is used to diagnose the presence of and also quantitate the amount of Zika virus in a sample.
Owner:ARIZONA STATE UNIVERSITY
Rapid immunochromatographic lateral flow assay for early zika disease detection
InactiveUS20180238881A1Accurately and rapidly determineAccurately and rapidly infectionDisease diagnosisAgainst vector-borne diseasesZika virusViral infection
The invention relates to a sensitive and specific rapid immunochromatographic lateral flow assay for Zika virus infection. The rapid assay of the invention can determine early, intermediate, and late Zika virus infection status.
Owner:LUSYS LAB
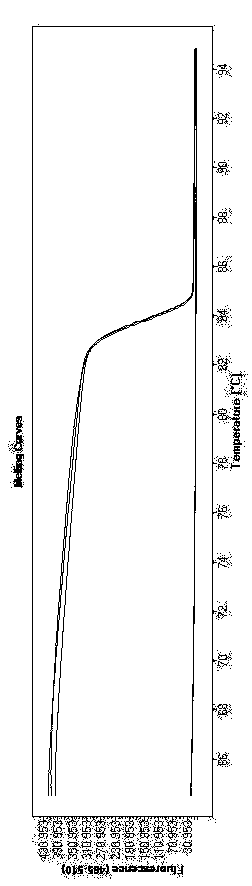
Zika virus detection kit based on high resolution melting analysis and detection method thereof
InactiveCN110229931ADetection sensitivityDetection characteristicsMicrobiological testing/measurementDNA/RNA fragmentationZika virusNucleic acid detection
The invention relates to the field of nucleic acid detection, and discloses a Zika virus detection kit based on high resolution melting analysis and a detection method thereof. The invention is characterized in that specific primers are designed according to Zika virus NS3 gene sequences, Zika virus nucleic acid is detected by using an optimized high resolution melting reaction system, so that theZika virus detection kit is simple in operations, fast in speed and free of the need of post-processing on PCR products and capable of detecting the difference of single base and truly realizing theclosed-tube operation.
Owner:山东国际旅行卫生保健中心
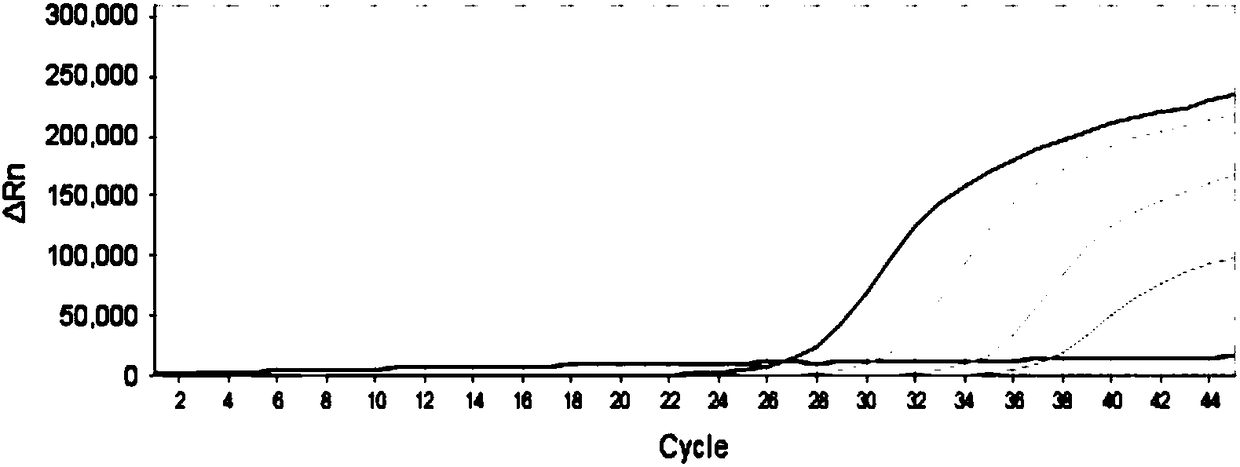
Double fluorescent quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for detecting dengue virus and zika virus
ActiveCN107151711AQuantitatively accurateLow costMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseReference product
The invention provides a double fluorescent quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for detecting dengue virus and zika virus. The kit comprises a quantitative RT-PCR reaction liquid, an enzyme mixed liquid, a primer probe mixed liquid, standard products (DENV and ZIKV), strong-positive reference products (DENV and ZIKV), weak-positive reference products (DENV and ZIKV), and negative reference products. The kit has the advantages that primer probes with high specificity for DENV and ZIKV are designed according to high conservancy of DENV and ZIKV and the polyprotein protein area with smaller difference between types; the dengue virus and the zika virus can be simultaneously detected through one-time reaction in a single tube by a single tube one-step type double real-time fluorescent quantitative RT-PCR; the specificity and sensitivity are high, and the kit can be used for the laboratory emergency diagnosis of epidemic outbreak caused by dengue virus and zika virus, quick screening, clinical diagnosis, and study of epidemiology of fever eruption virus.
Owner:HUZHOU NO 1 PEOPLES HOSPITAL
Monoclonal antibody targeting at Zika virus envelop protein conserved epitope and application thereof
ActiveCN109081868ASensitive and specific recognitionAvoid infectionImmunoglobulins against virusesAntiviralsZika virusViral Vaccine
The invention provides a monoclonal antibody targeting at Zika virus envelop protein conserved epitope and application thereof. specifically, a recombinant expressed Zika virus E-protein immune mouseis used herein to attain a murine monoclonal antibody specific to E-protein. Research results show that the antibody herein is an ideal Zika virus detection antibody and is applicable to the development antibody drugs against Zika virus. Identification of a conserved neutralizing epitope can also guide the development of broad-spectrum Zika virus vaccines.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
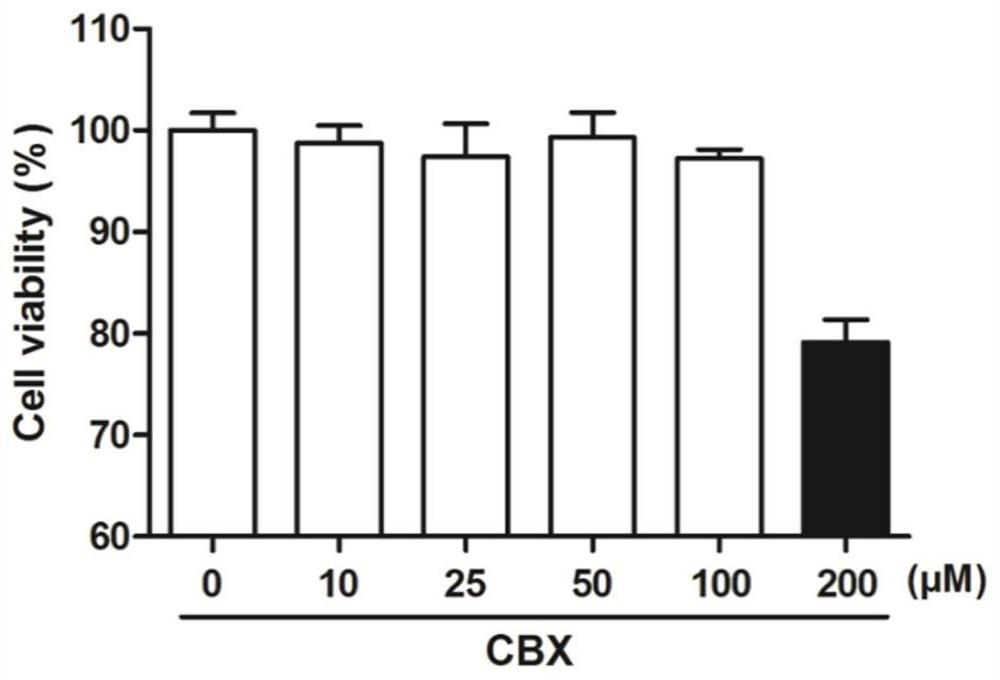
Application of carbenoxolone in preparation of anti-Zika virus medicine
InactiveCN111773228AEnhanced inhibitory effectAbundant resourcesOrganic active ingredientsAntiviralsBrain developmentZika virus
The invention belongs to the technical field of medicine application, and discloses application of carbenoxolone in the preparation of an anti-Zika virus medicine. An in-vitro inhibition experiment ofa Zika virus (ZIVK) proves that carbenoxolone has a good inhibition effect on the ZIVK; by constructing a ZIKV infected animal model, it is discovered that ZIKV causes animal cephalotaxis and brain development of animals infected with ZIKV is obviously delayed, and through administration of carbenoxolone to the animals, the phenomenon of brain development retardation of the ZIKV infected animalscan be significantly improved. Essentially, it is proved that the medicine has wide application prospects in treatment of Zika virus infection diseases. Meanwhile, the carbenoxolone comes from liquorice, and thus is rich in source and convenient to acquire; according to the present invention, the good post-selection drug can be provided for clinical treatment of the diseases caused by the Zika virus, and the good application prospect is provided.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com