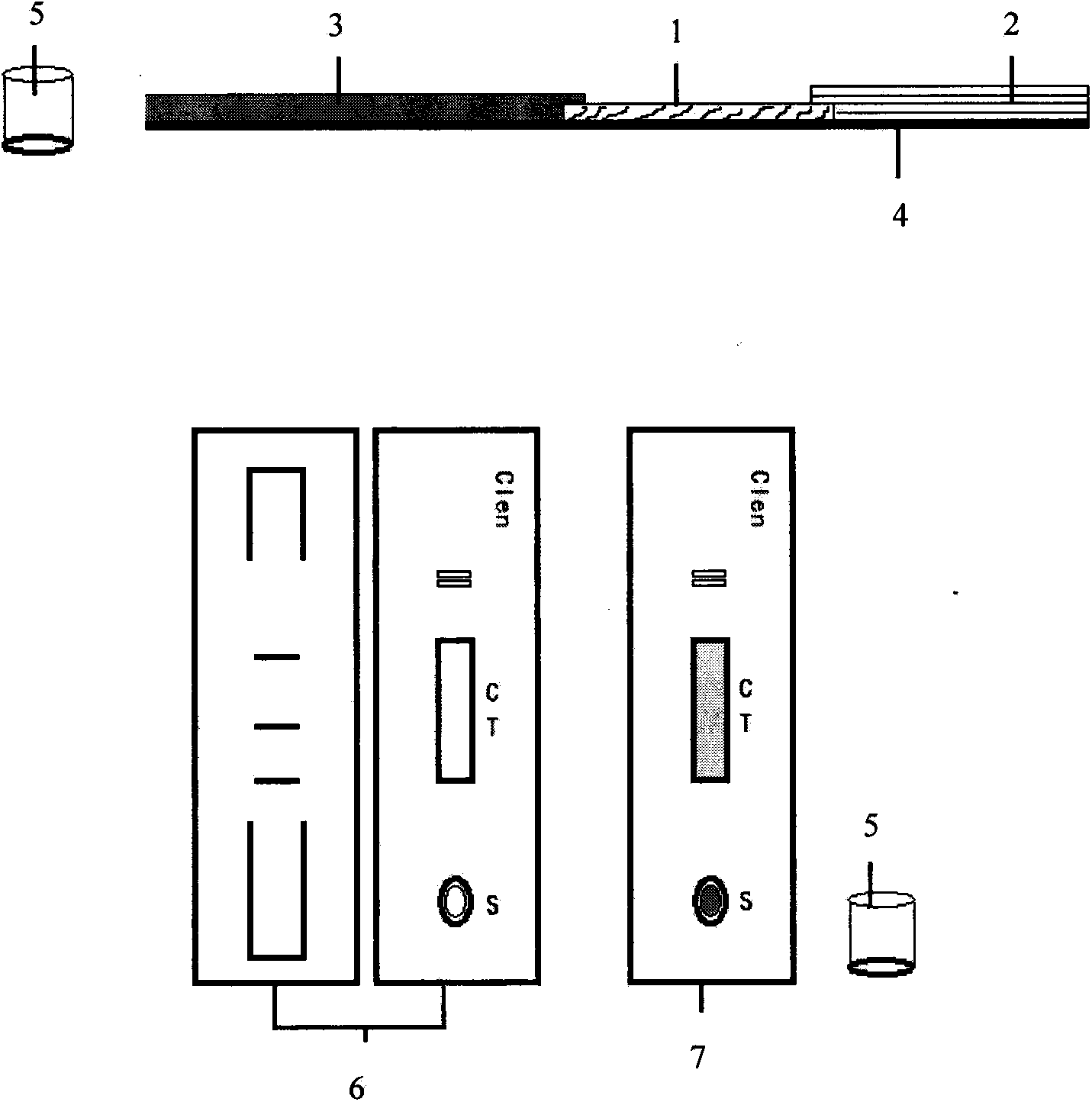
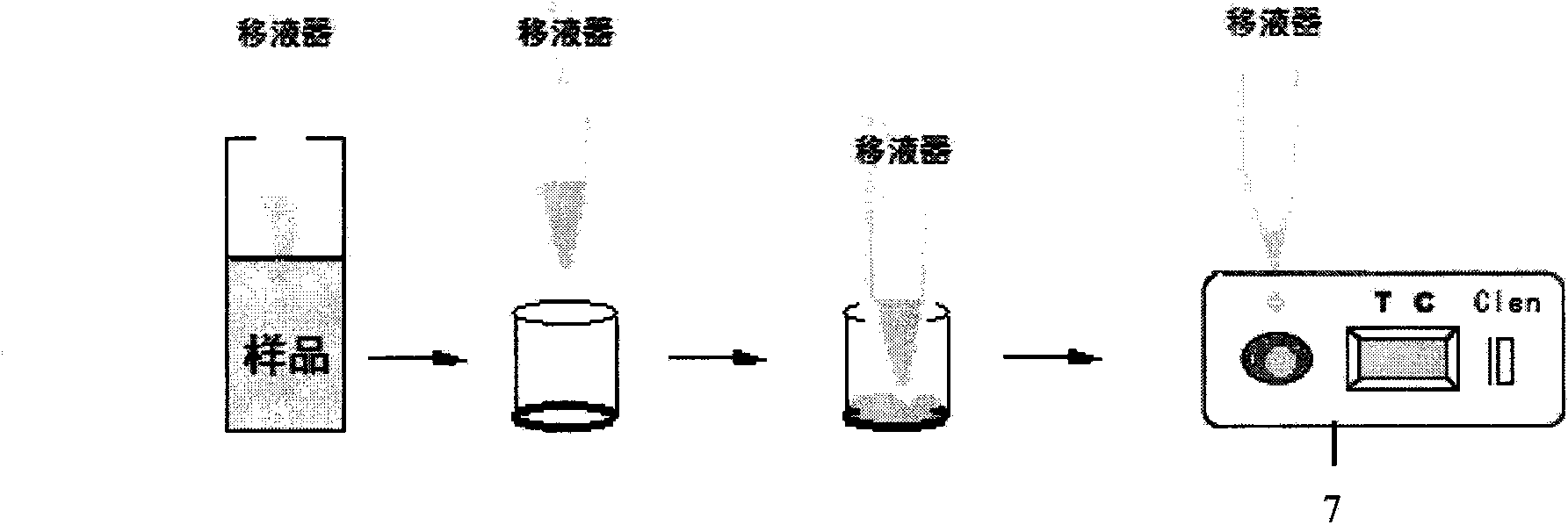
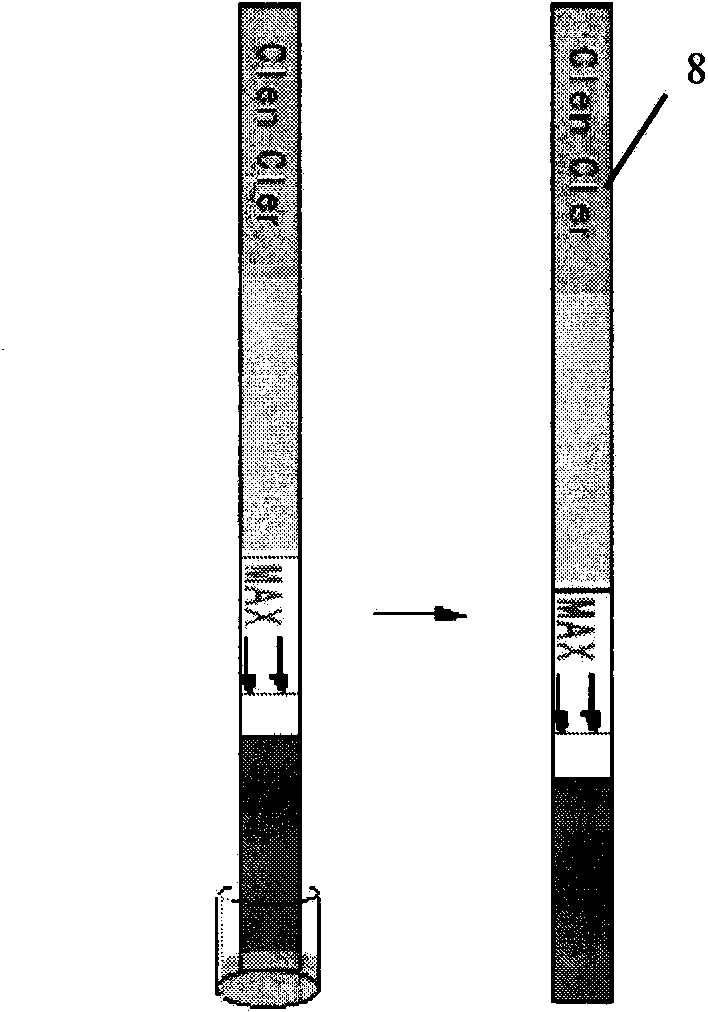
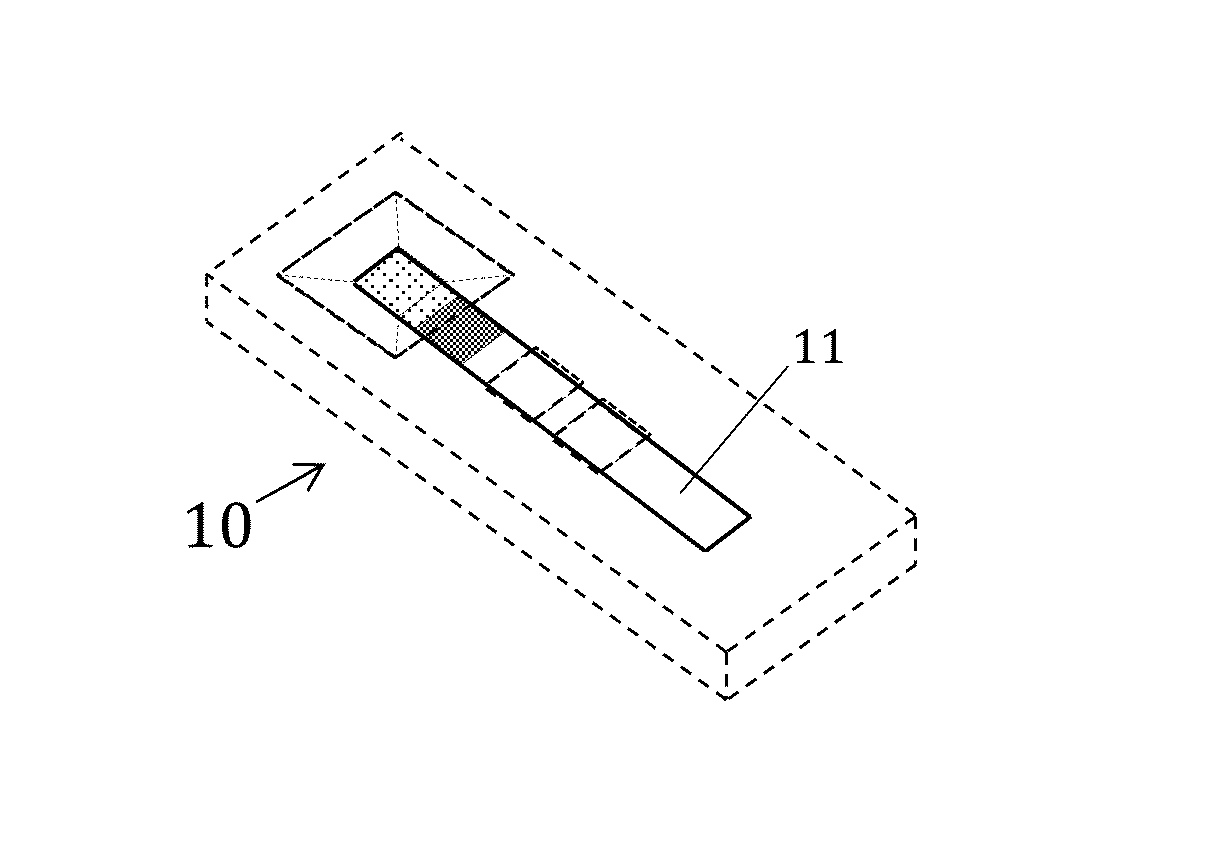
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
336 results about "Immunochromatographic Assays" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
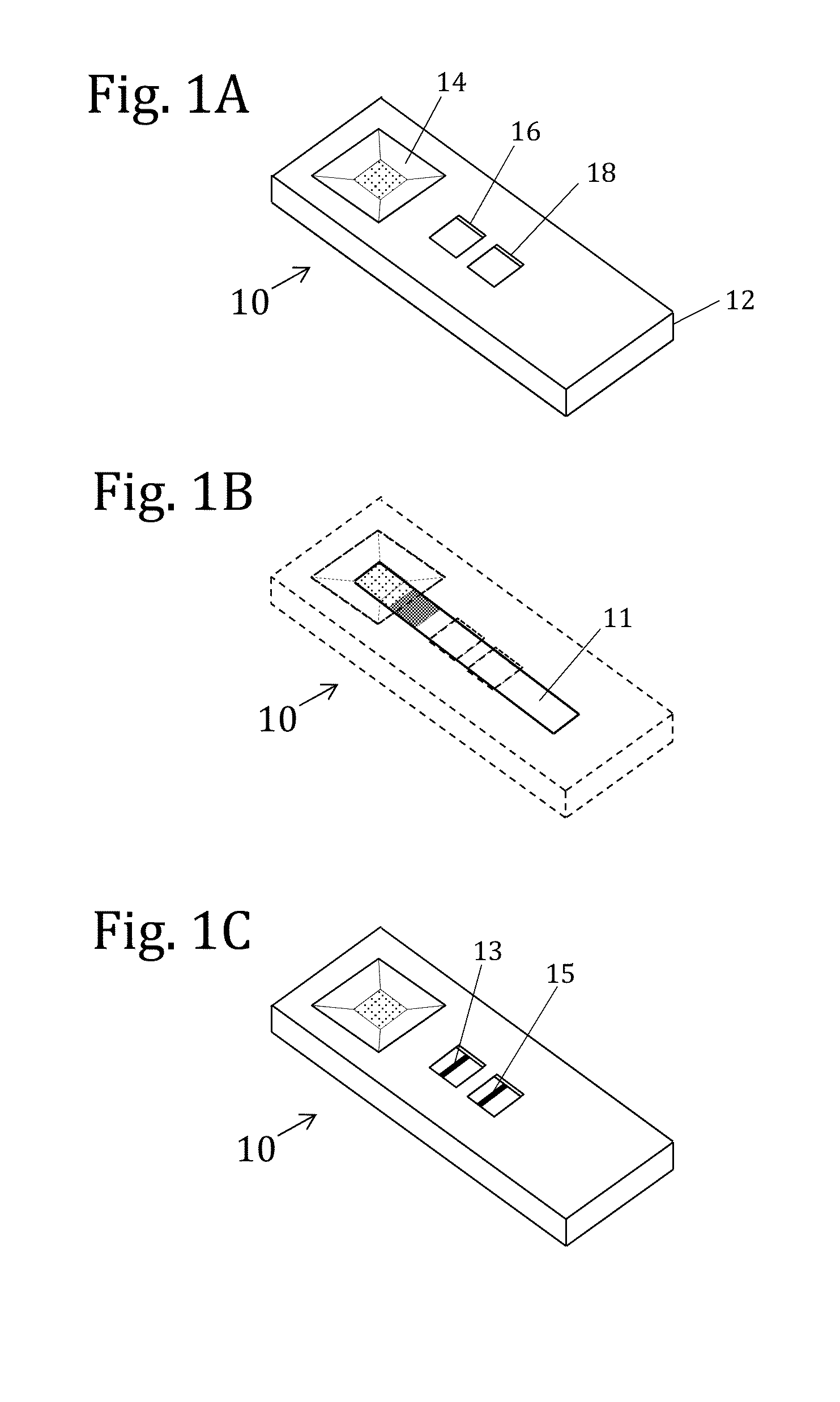
Electrochemical membrane strip biosensor
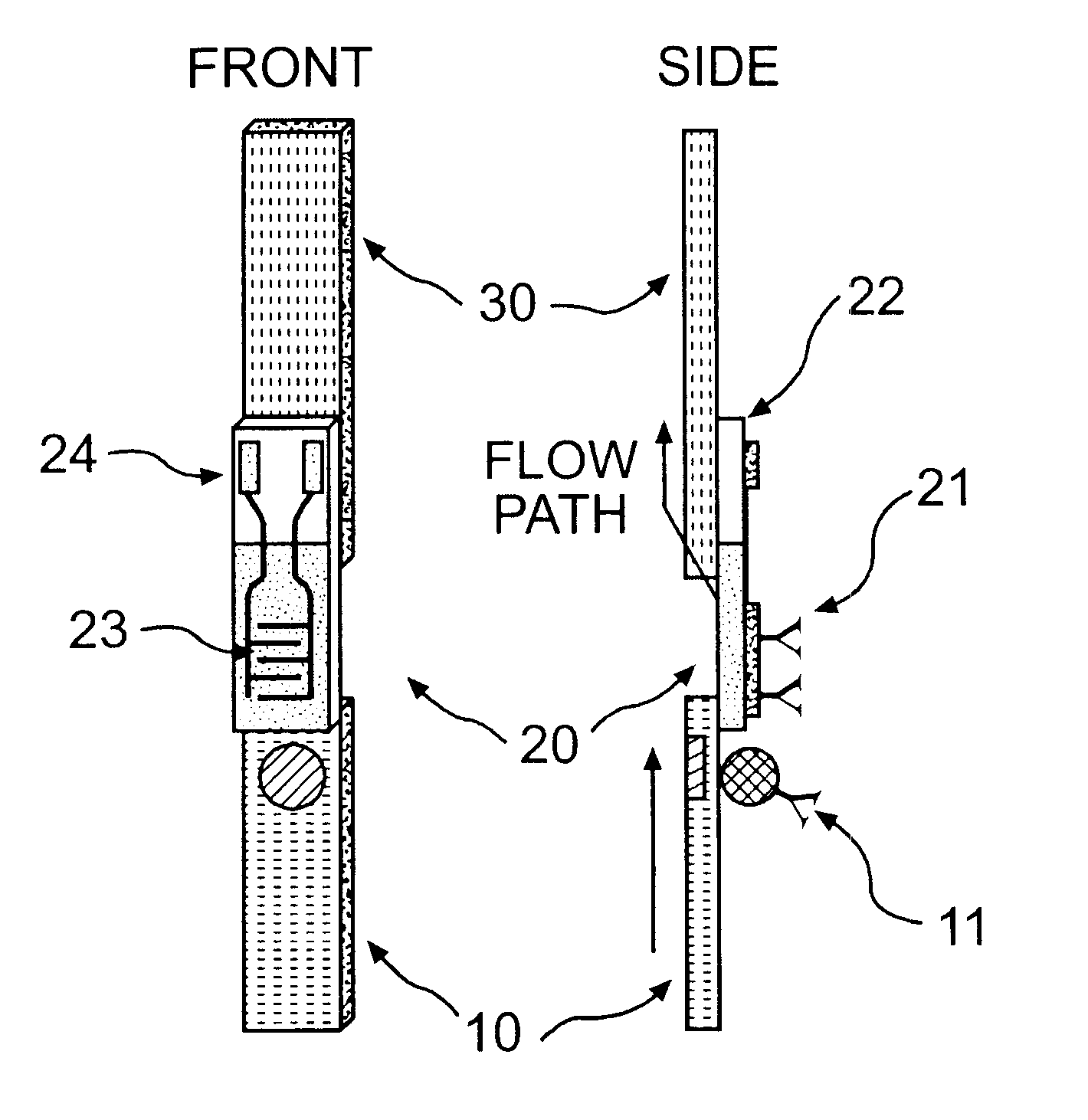
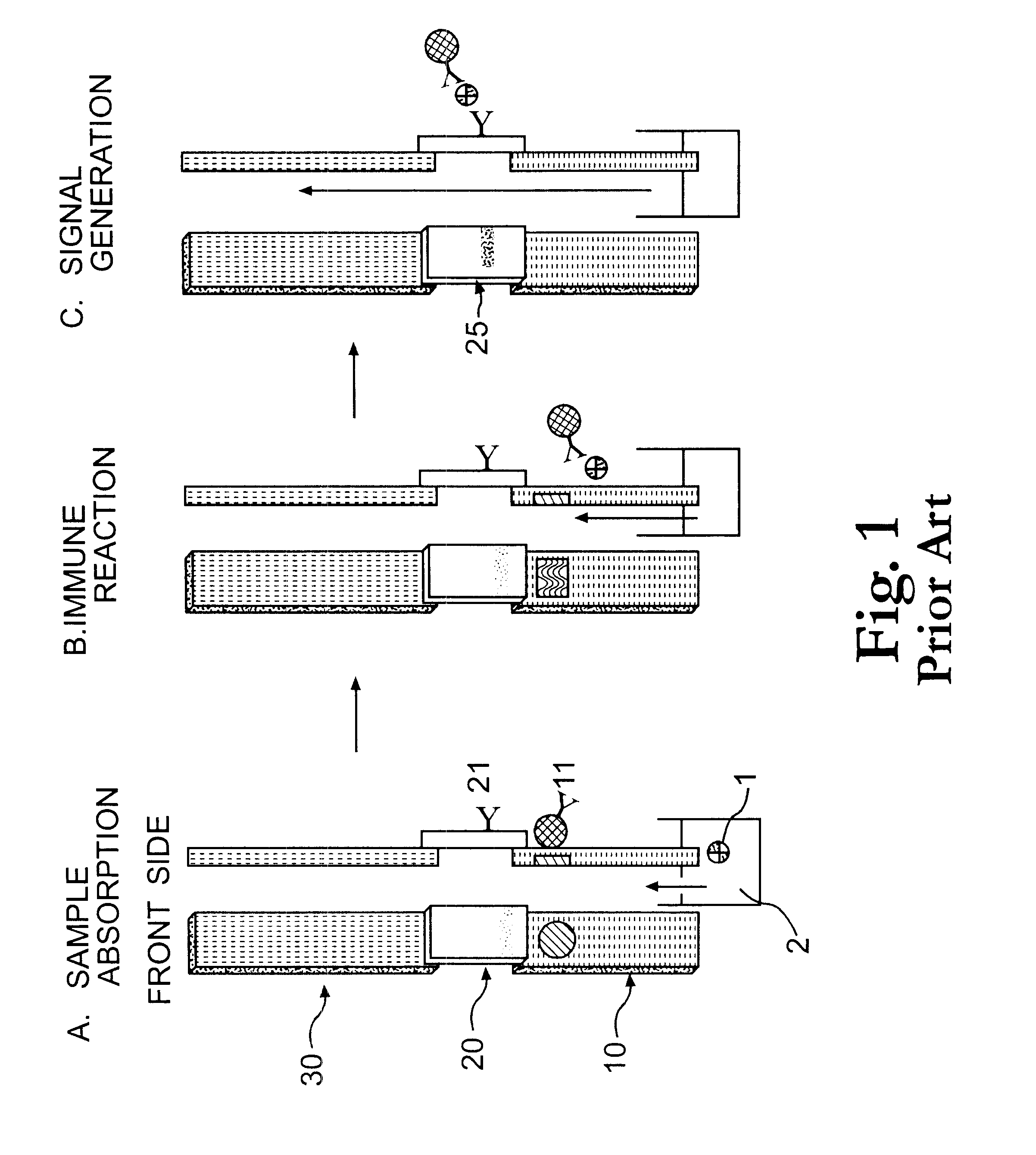
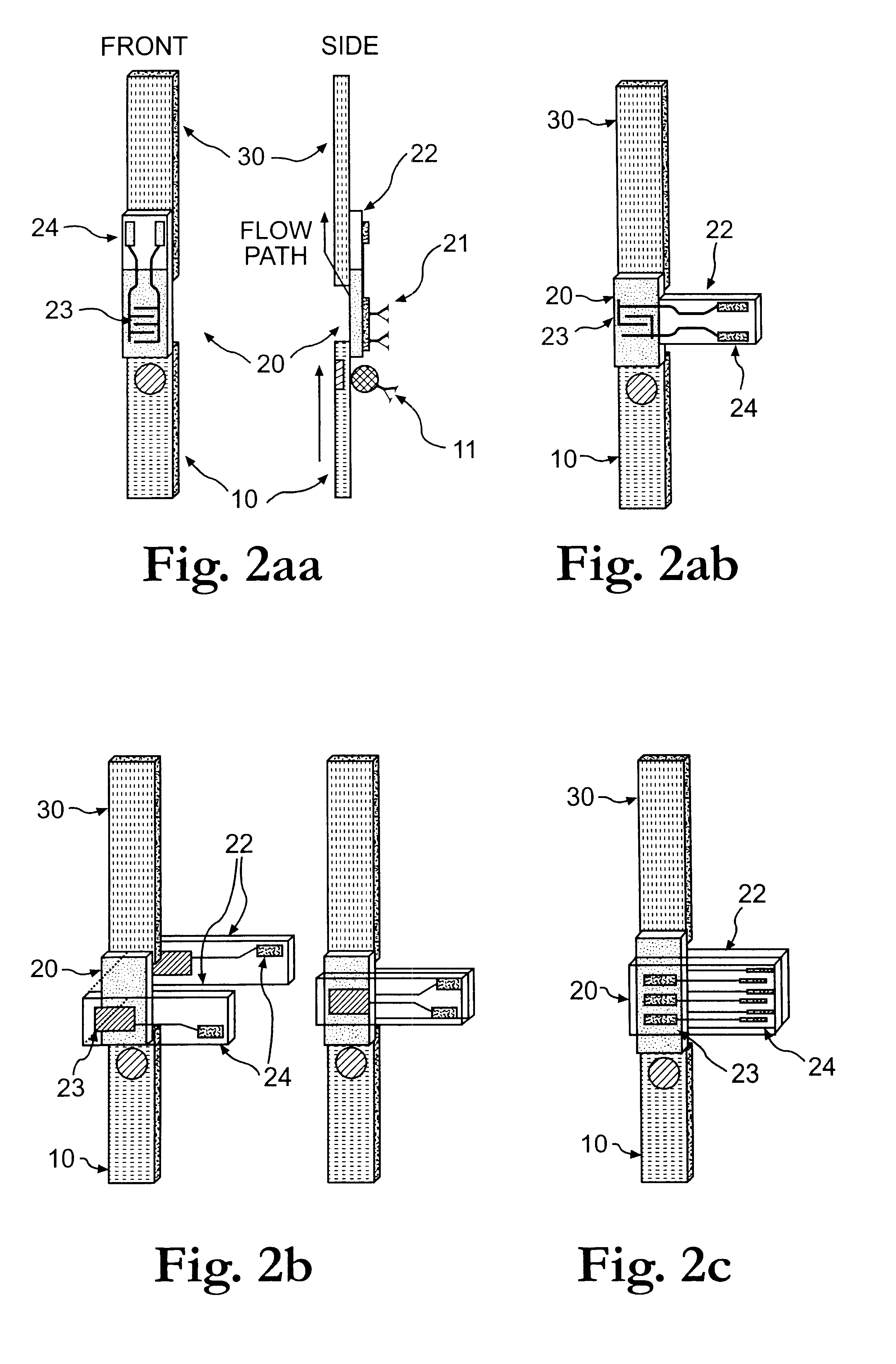
The present invention is directed to the development of a biosensor based on the immuno-chromatographic method that can provide an assay speed and convenience required for point-of-care (the doctor's office and emergency room) testing or home-version diagnosis. Though certain physical symptoms, such as pregnancy and ovulation, or bacterial infection may be identified by a qualitative analysis for the presence of indicating substances, most analytes for clinical investigation demand their concentrations known in specimens. Therefore, the inventors of the present invention have developed a novel biosensor by combining the immuno-chromatographic method and the electric conductivity detection technology so that on-site quantitative determination at the points of care or at home may be carried out.
Owner:BIODIGIT LAB CORP
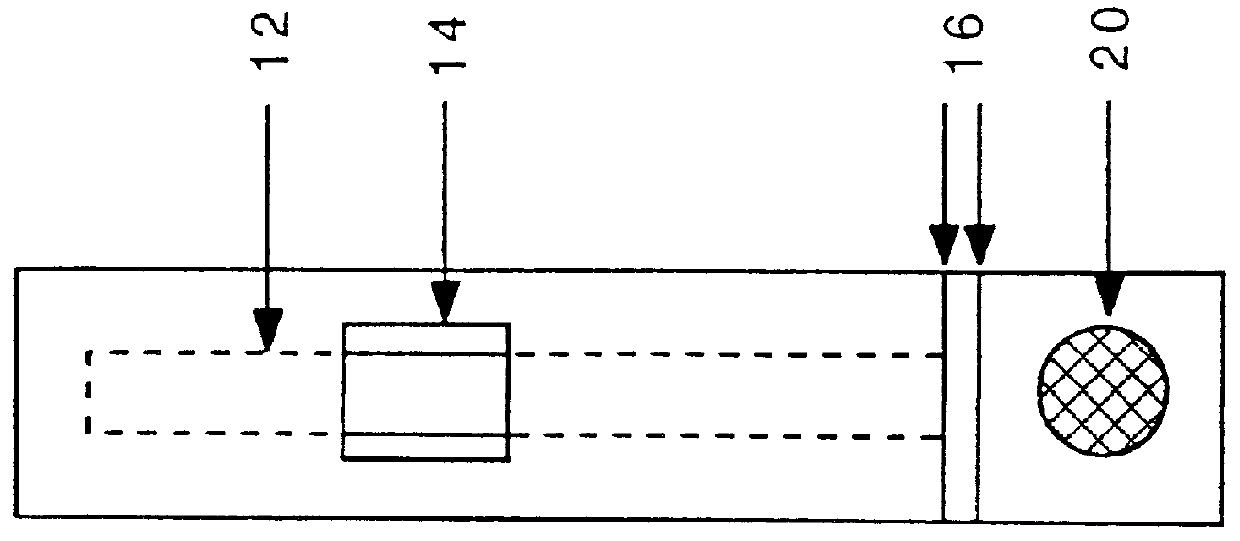
Apparatus, method and article to perform assays using assay strips
ActiveUS20100045789A1Material analysis by observing effect on chemical indicatorLocal control/monitoringControl signalControl line
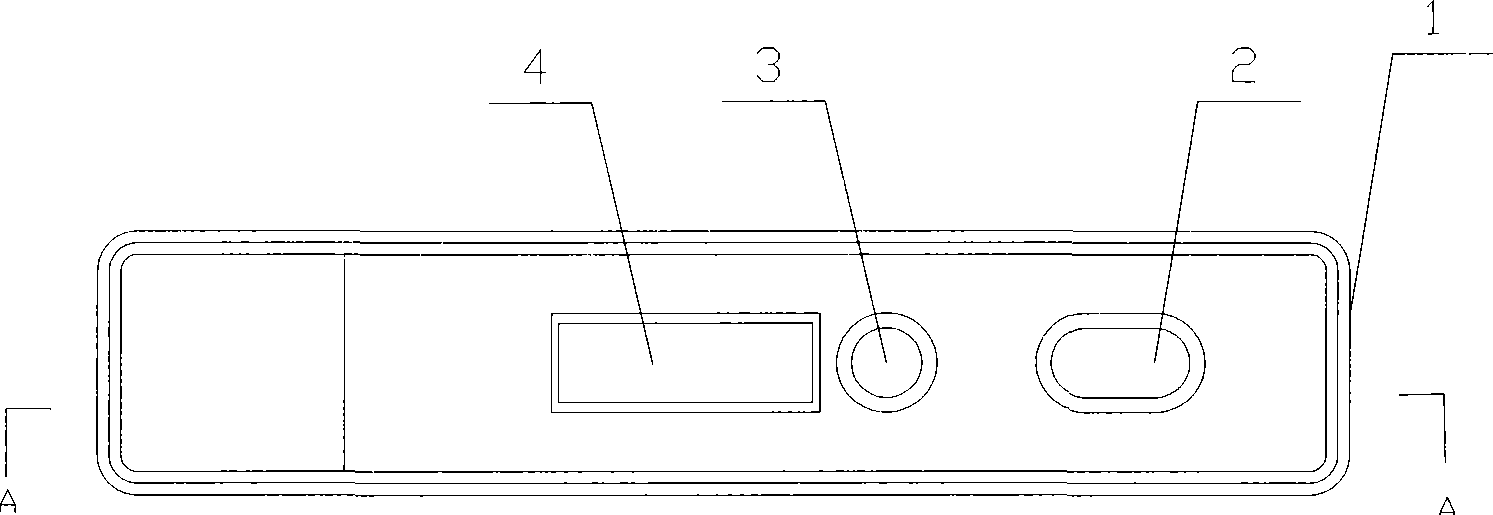
An assay system includes an optical imager to acquire high resolution images of assay strips (e.g., lateral flow immunochromatographic test strips) and performs image processing to identify individual assay strips and determine results for each assay strip, by quantifies the presence or absence of test signal line(s) and control signal line(s). Assay strips may be in a holder or carrier contained in a specimen container also holding a specimen. The assay system automatically logs all results and data to a database that stores a high resolution image of the original immunochromatographic assay, the values of test line(s) and control line(s), and the test result. A user interface directs an end user through operation.
Owner:GENPRIME
Human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and preparation method and application thereof
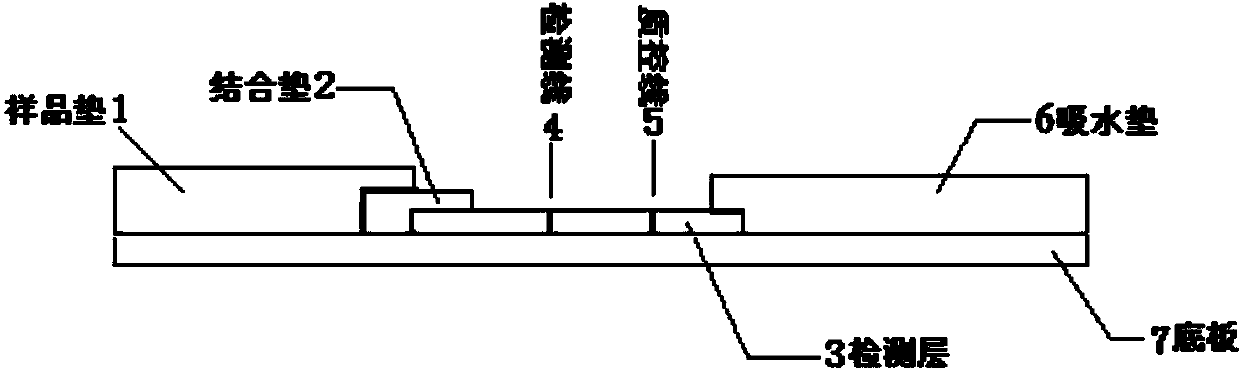
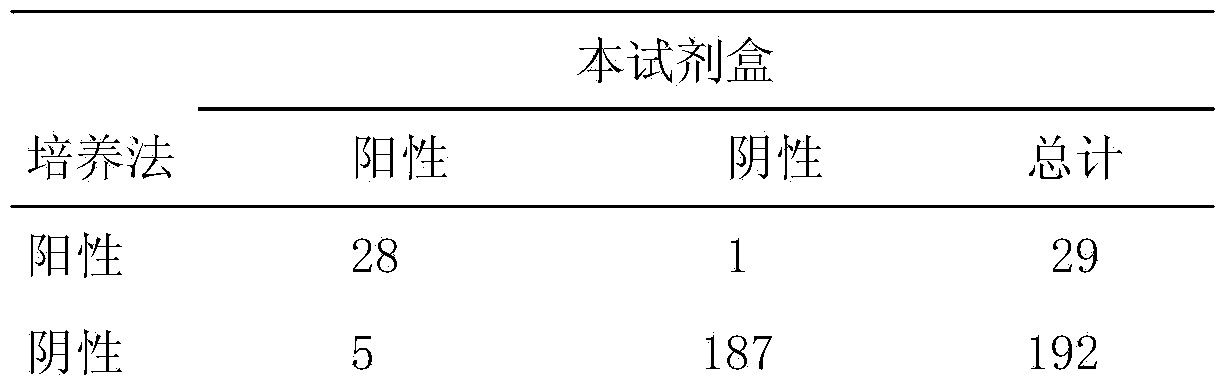
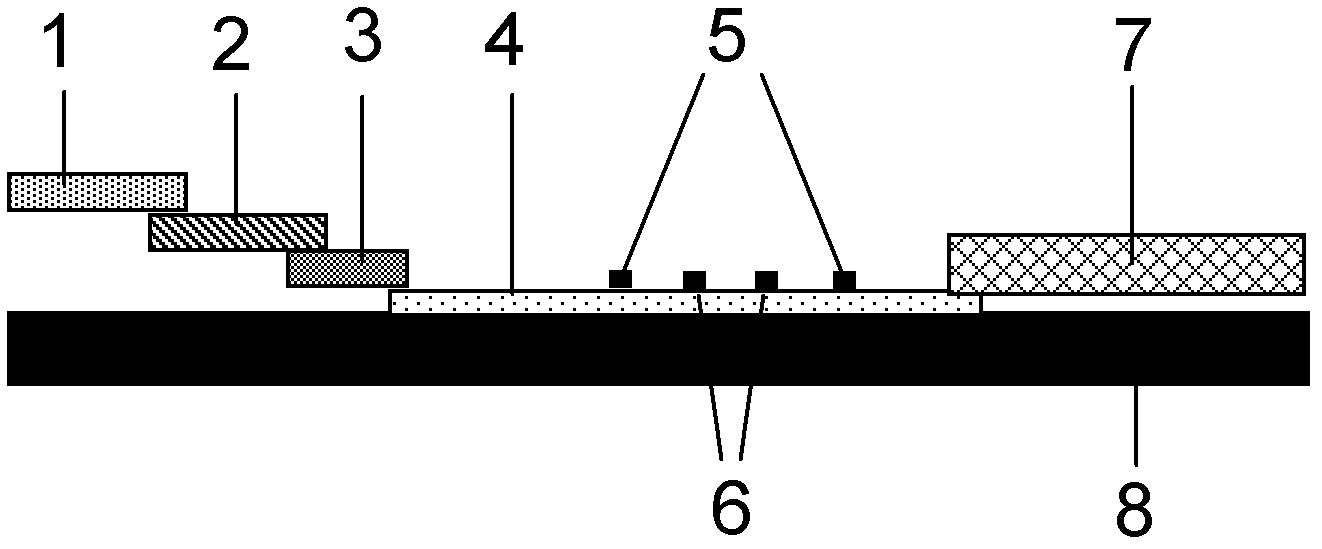
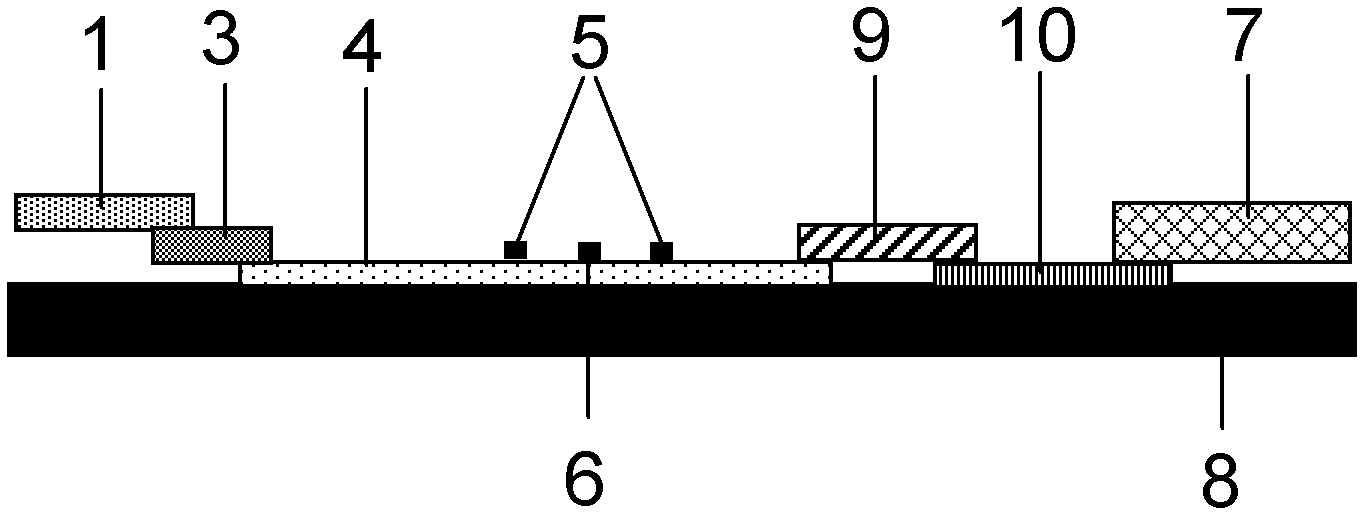
The invention provides a human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and a preparation method and application thereof. The assay kit comprises a detection card and a silver-stained sensitivity-enhanced pad, wherein the detection card is composed of a bottom plate, a sample pad, an absorbent pad, a conjugate pad and a detection layer; the conjugate pad is coated with a colloidal gold-marked polyclonal antibody mixture of colloidal gold marked rabbit anti-human mycoplasma pneumoniae P1 protein and P30 protein; the detection layer is composed of a solid phase nitrocellulose membrane with a detection line and a quality control line; the detection layer is bonded on the bottom plate, the conjugate pad and the absorbent pad are partially overlapped with the detection layer respectively and are bonded with the detection layer and the bottom plate respectively; the sample pad and the conjugate pad are partially overlapped to be bonded with the conjugate pad and the bottom plate respectively; and the silver-stained sensitivity-enhanced pad consists of a AgNO3 pad and a restoring pad. The human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit can effectively improve the detection sensitivity of the human mycoplasma pneumoniae, has the strong specificity and has the high application value in the aspects of clinical diagnosis of human mycoplasma pneumoniae, etiology identification, epidemiological investigation and the like.
Owner:HUBEI UNIV OF TECH +1
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165ASensitive quantitative detection fastRealize detectionMaterial analysisCritical illnessLinear range
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Lateral flow diagnostic assay reader with radial cassette
InactiveUS20050208593A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteEngineering
The present invention provides a method and field portable apparatus for analyte detection using lateral flow immunochromatographic assays, where a single test sample may be simultaneously applied to multiple assays.
Owner:ARIZONA BOARD OF REGENTS ACTING FOR & ON BEHALF OF NORTHERN ARIZONA UNIV
Sensitive immunochromatographic assay
ActiveUS7175992B2Easy to detectCertain constraintBioreactor/fermenter combinationsAnalysis using chemical indicatorsAnalyteCapillary action
Methods for quantitatively measuring the amount of an analyte of interest in a fluid sample, and kits useful in the methods, are disclosed. The methods involve providing a solid phase apparatus comprising a membrane having an application point, a sample capture zone, and a control capture zone, where the sample capture region is between the contact region and the control capture zone; and providing a sample collection apparatus comprising a population of analyte binding particles or a population of analyte coated particles. In the assays, a fluid sample is introduced into the sample collection apparatus, and the resultant mixture is applied to the application point of the membrane. The fluid allows transport components of the assay by capillary action to and through the sample capture zone and subsequently to and through the control capture zone. The amount of analyte in the fluid sample is related (e.g., either directly or inversely) to a corrected particle amount, which can be determined, for example, as a ratio of the amount of particles in the sample capture zone and the amount of particles in the control capture zone.
Owner:RESPONSE BIOMEDICAL CORP
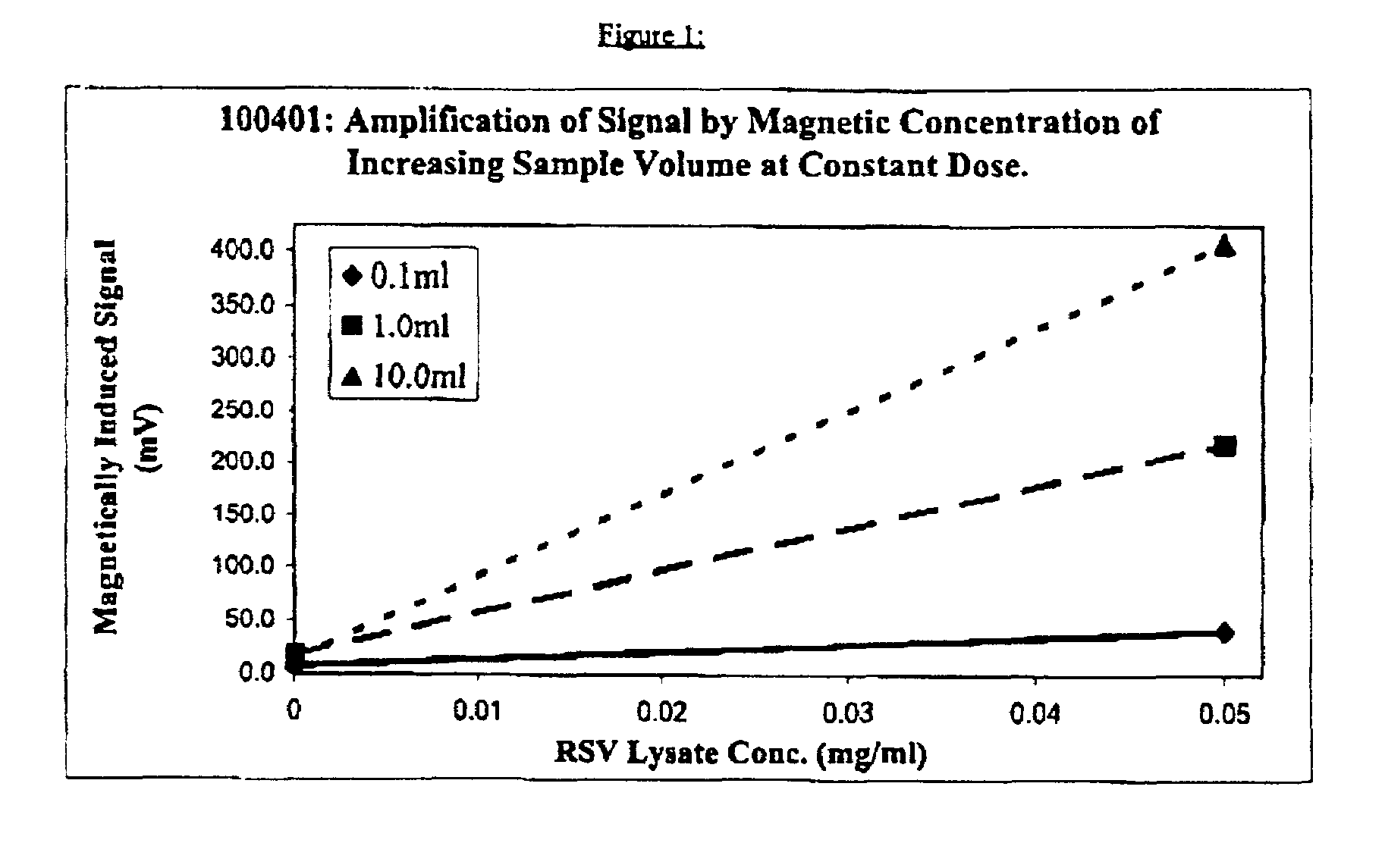
Process for (A) separating biological/ligands from dilute solutions and (B) conducting an immunochromatographic assay thereof employing superparamagnetic particles throughtout
InactiveUS7018849B2Highly effectiveBioreactor/fermenter combinationsBiological substance pretreatmentsMean diameterSuperparamagnetism
Superparamagnetic (“SPM”) subunits of 1–30 nm average mean diameter (e.g. ferro fluid) subparticles are treated with a magnetically noninterfering substance capable of coating and covering them (e.g, BSA) and they spontaneously form agglomerates of about 100 nm to about 450 nm or higher average mean diameter and are then used to form complexes with target biological ligands such as viruses, contained in large volumes of liquid. The complexes are subjected to the gradient intensity of a strong magnetic field, and excess liquid is removed, where upon an immunochromatographic assay is conducted to determine the identity and / or amount of target ligand present, in which operation SPM particles that bonded to the ligand function as tags for ligand detection.
Owner:ABBOTT DIAGNOSTICS SCARBOROUGH INC
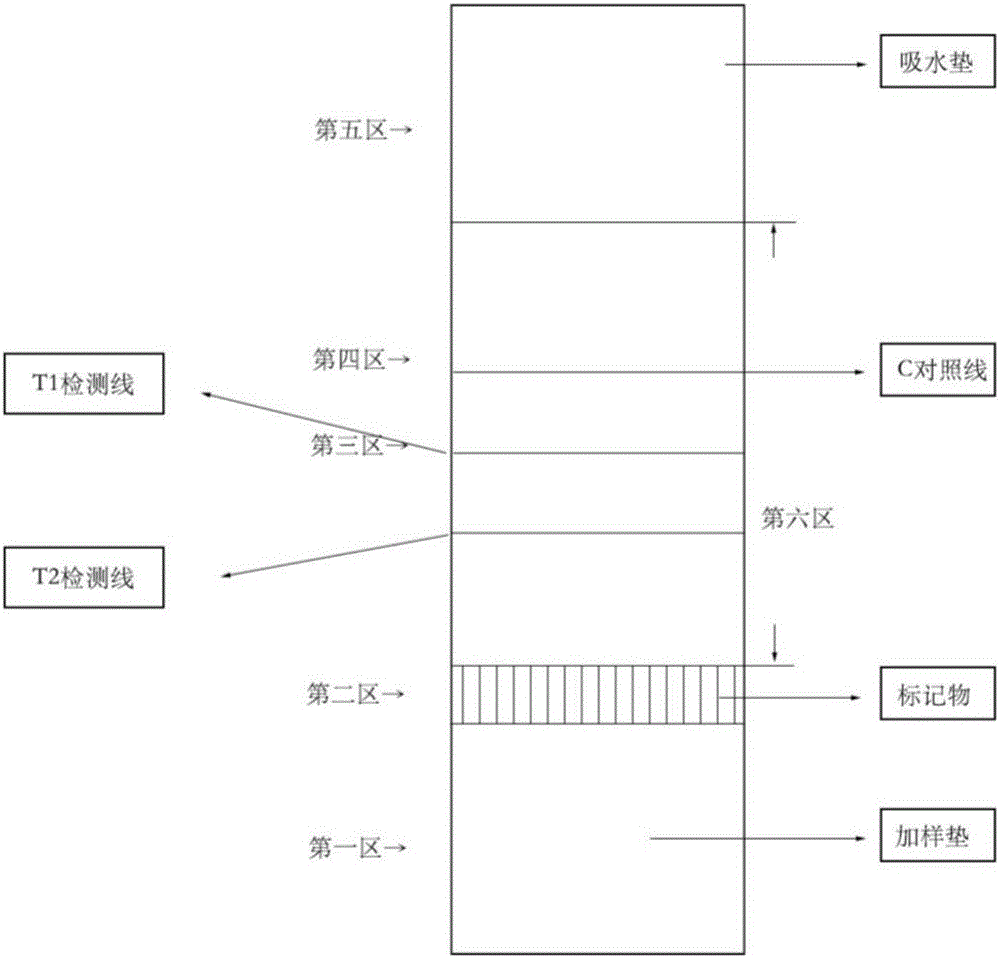
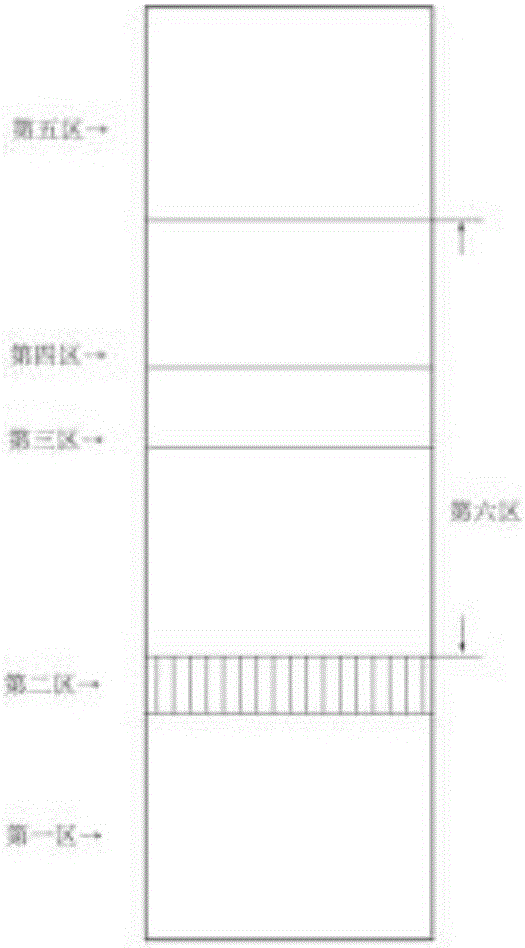
Detection strip for Zika virus detection by means of fast immunochromatography method
The invention provides a detection strip or kit for Zika virus detection by means of the fast immunochromatography method. The immunodetection strip has six areas. The first area is a sample adding pad; the second area is an immobilized Zika antigen marker area or anti-Zika virus antigen specific antibody marker; the third area is a detection area T and a coated antibody spray area, and the area T is matched with the second area; the fourth area is a contrast area used for immobilization of non-specific antibodies; the fifth area is a water absorber; the sixth area is a nitro fiber chromatography film. According to the detection strip or kit, only a small number of samples to be tested are needed, no equipment is required, a detection result can be obtained within ten minutes, detection is easy and fast, and the detection strip or kit can be purchased from a pharmacy so that patients can conduct detection by themselves.
Owner:卢氏实验室公司 +1
Methods of use of one step immunochromatographic device for Streptococcus A antigen
InactiveUS6979576B1Reduce the numberLess manipulationBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenReagent
A method to determine the presence or absence of Streptococcus Group A antigen in a sample, comprising the following steps: extracting the antigen from the sample in an assay chamber with two or less extraction reagents, wherein the two reagents may be added to the assay chamber in no particular sequence; introducing a lateral flow immunochromatographic assay device into the extraction reagents containing the extracted antigen without further addition of reagents or manipulation of the sample; forming an antigen-indicator labeling reagent complex; and determining the presence or absence of the antigen in the sample by the presence or absence of a signal formed by the binding of the antigen-indicator labeling reagent complex to an indicator capture reagent specific for said antigen-indicator labeling reagent complex.
Owner:SEKISUI DIAGNOSTICS
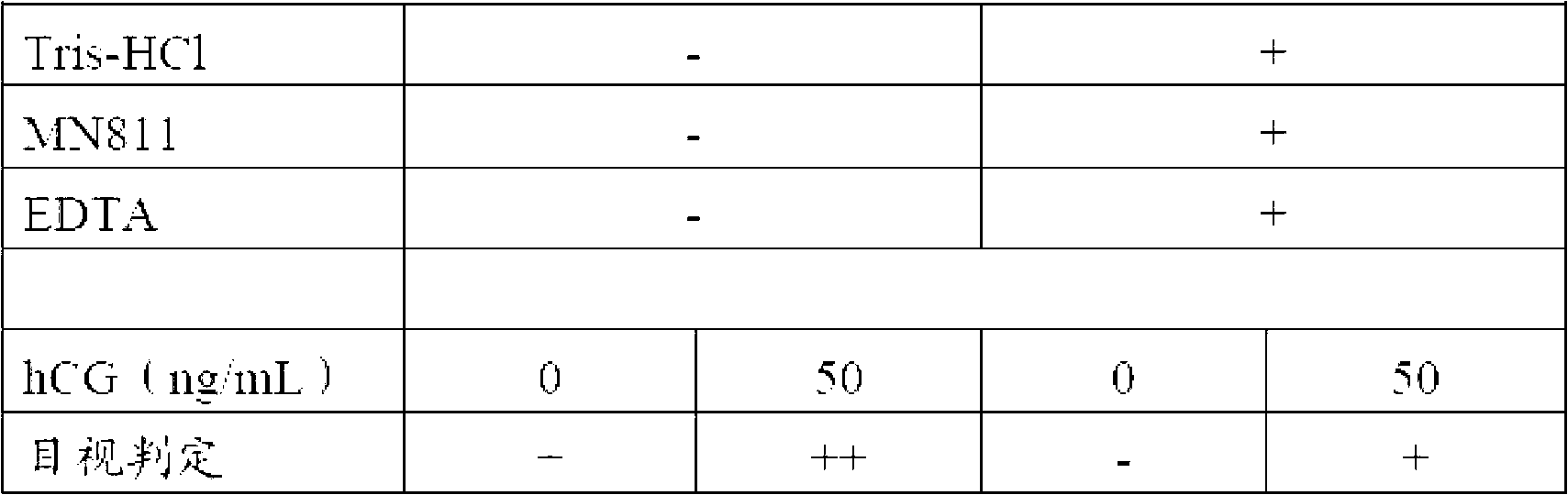
Reagent composition for immunochromatography
InactiveCN102713625ASuppresses non-specific reactionsThe result is accurateBiological testingBuffer solutionReagent
Provided are a sample diluent or a reagent composition for immunochromatography, wherein it is possible to inhibit nonspecific reactions compared to prior art in which an object to be detected was detected using an immunochromatography device. Also provided are an immunochromatography device which uses said reagent composition or diluent and which exerts high performance and sensitivity, and a detection kit which uses said reagent composition or diluent and with which it is possible to swiftly and easily perform examinations. A reagent composition for immunochromatography, a developer for immunochromatography, or a sample diluent for immunochromatography, which contain a buffer solution, a chelating agent, and a nonionic surfactant, are used when detecting an object to be detected in a sample by means of the immunochromatography method. As a consequence, it is possible to inhibit nonspecific reactions.
Owner:TANAKA PRECIOUS METAL IND
Reagent for quantitative detection of Beta-receptor stimulant through Europium chelate latex time-resolved immunochromatographic assay
InactiveCN104090248AHigh sensitivityHigh quantitative sensitivityFluorescence/phosphorescenceMagnitude/direction of magnetic fieldsCelluloseReceptor
Disclosed is a reagent for quantitative detection of a Beta-receptor stimulant through Europium chelate latex time-resolved immunochromatographic assay. The reagent includes test card or test strip which includes a nitrocellulose membrane, absorbent paper, a sample pad and a PVC bottom plate, and a microporous container. A test sample firstly dissolves out Eu3+ fluorescent latex particles which are attached in the microporous container and marked with an anti-Beta-receptor-stimulant small-molecule antibody and after sufficient mixing, the test sample reacts completely with the marker and then a reaction liquid is dropped to the test card or test strip to carry out immunochromatography and at the same time, an immune competition reaction with a Beta-receptor-stimulant small molecule and BSA conjugate which envelopes the nitrocellulose membrane is carried out and five minutes later, the test card or the test strip is inserted into a fluorescent reading meter to measure a fluorescent value so as o obtain a test result. The method is high in sensitivity and quantitative and integrates the advantages of simple and convenient operation and rapidness, the method is applied to rapid detection of veterinary drug residuals such as the Beta-receptor stimulant and the like in food and raw materials on sites of production fields such as plantation, cultivation, animal husbandry and food processing and the like.
Owner:ROHI BIOTECH
System and method for spatiotemporally analyzed rapid assays
ActiveUS20140065647A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteData set
The invention relates to methods of reliably and quantitatively determining the amount of an analyte of interest in a fluid sample using a flow-induced assay, such as an immunochromatographic assay, in which spatiotemporal measurements are recorded during the course of the assay reaction, generating a spatiotemporal dataset, and subsequently analyzed. The invention also relates to a system incorporating instruments for recording spatiotemporal datasets (spatiotemporal data recorders), devices comprised of flow-induced assays configured for analysis on a spatiotemporal recorder, and programs for analyzing the recorded spatiotemporal datasets.
Owner:MAMENTA EDWARD L
Test bar and method for quantitatively detecting micromolecular compound in sample
The invention provides a test bar and a method for quantitatively detecting a micromolecular compound in a sample. The detection is performed by adopting the test bar containing fluorescence emulsion particles and a micromolecular compound antibody on a binding pad and using a time resolution fluorescence immunochromatographic assay, and a specific value is compared with a standard curve, so that whether the micromolecular compound exists or not is determined or the content of the micromolecular compound is detected. The test bar can be used for quantitatively detecting the micromolecular compound in the sample, can be used for greatly eliminating the non-specific combination so that the signal to noise ratio of a detection signal is remarkably improved, and test bar has the advantages of rapidness, high sensitivity, high specificity and the like.
Owner:SHANGHAI VENTURE BIOTECH CO LTD
Integrated packaging holder device for immunochromatographic assays in flow-through or dipstick formats
InactiveUS6087185ABioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteUrine cotinine level
The prevent invention relates to an integrated package-holder assay devices for detecting the presence of analyte in a sample. The device serves the dual roles of supporting and protecting an immunochromatographic assay. The device is compatible with any immunochromatographic assay format. The assay can be performed in a single apparatus for use in a laboratory or a field setting. In a specific example, the assay device is a nylon membrane formatted for an immunochromatographic assay for cotinine sealed between transparent adhesive tape and a stiff plastic strip. White tape placed over the plastic strip defined a window for observing the assay results.
Owner:SEREX
Method for fast testing human ABO/Rh/MN blood type and kit
ActiveCN101603967AQuick, easy and accurate identificationEasy to operateBiological testingRed blood cellGroup A - blood
The invention relates to a method for fast testing human ABO / Rh / MN blood type and a kit used in the method. The method and the testing kit follow the principle of immunochromatography and realize the blood type test by observing whether residual red substances resulted from the agglutination of erythrocytes exist or not after the antigen antibody reaction of blood type substances. The method has simple operation and can avoid various defects in present ordinary blood type testing methods. In the method, the loading amount of tested blood sample is no more than 10 microliter, and the whole test process lasts no more than 2 minutes.
Owner:INTEC PROD INC
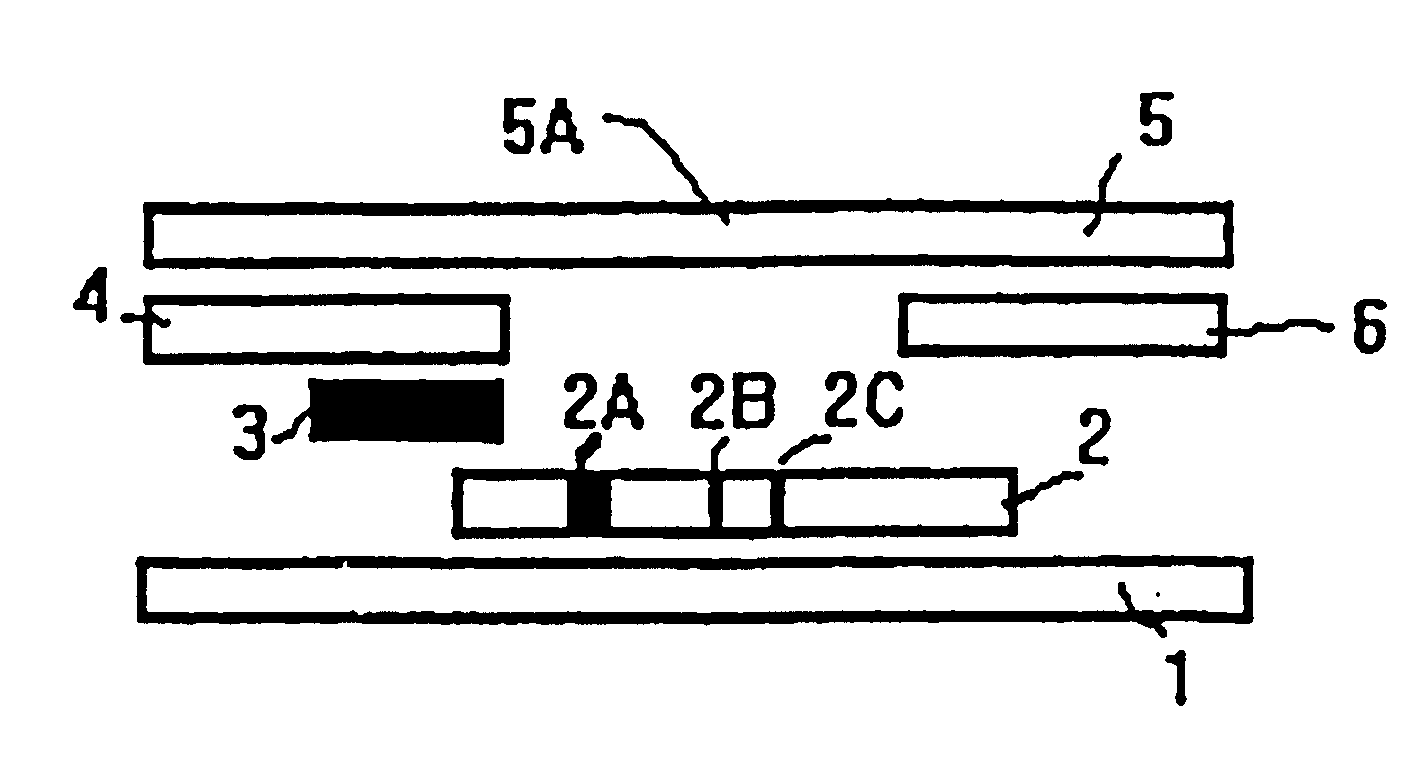
Human C-reactive protein colloidal gold immunochromatographic assay quantitative test paper
InactiveCN101887063AEasy to operateImprove detection efficiencyMaterial analysis by observing effect on chemical indicatorBiological testingAdhesiveColloid
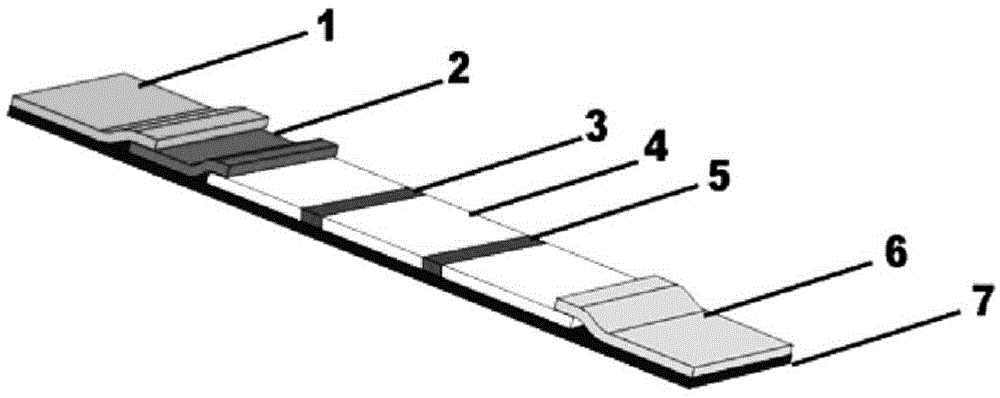
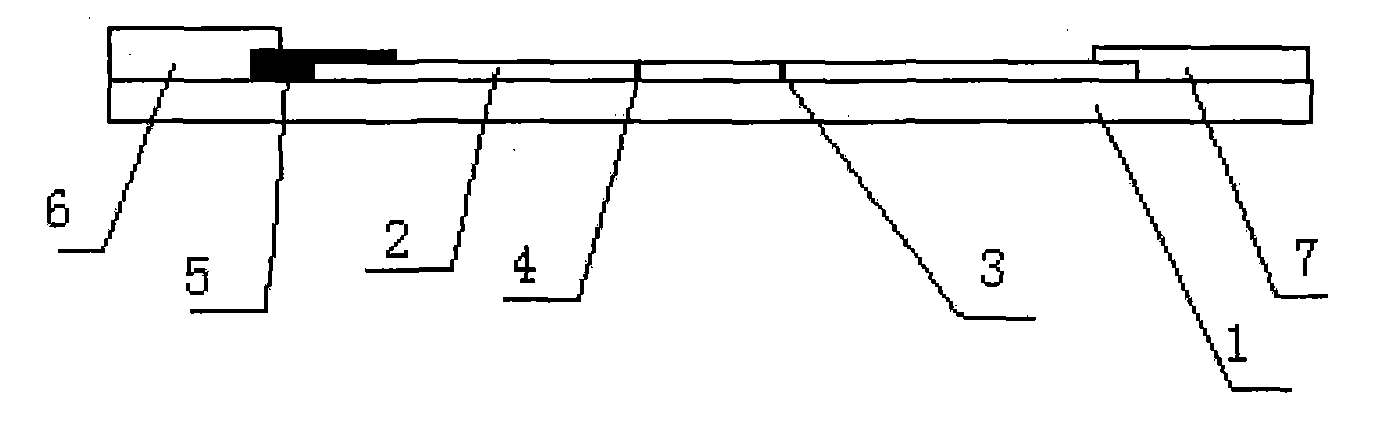
The invention relates human C-reactive protein (CRP) colloidal gold immunochromatographic assay quantitative test paper, which comprises a sample pad, a bonding pad, a nitrocellulose membrane and absorbent paper mutually lapped on a bottom plate with an adhesive in turn. A colloidal gold-labeled CRP antibody complex is coated on the bonding pad; and a detection line and a quality control line are bonded on the nitrocellulose membrane, and are antibody-enveloped lines. During detection, the samples to be detected contact the lower end of the test paper and are subjected to chromatography along the test paper; if the quality control line and a quantitative line do not develop, the test is ineffective; if only the quality control line develops, the CRP concentration is lower than the lowest detection concentration; and if both the quality control line and the quantitative line become red, the content of the CRP is read through a quantitative detection device. The test paper is characterized in that the quality control line and the quantitative line are respectively arranged from the lower end to the upper end of the test paper along the sample chromatography direction.
Owner:沈鹤柏
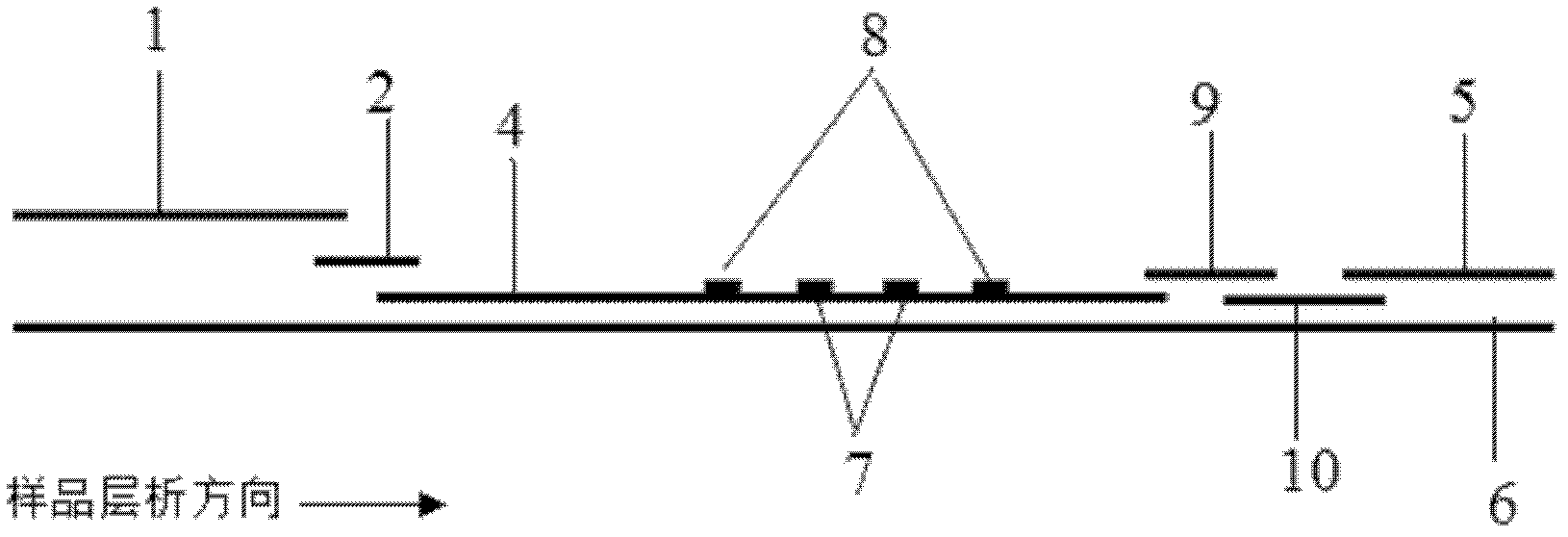
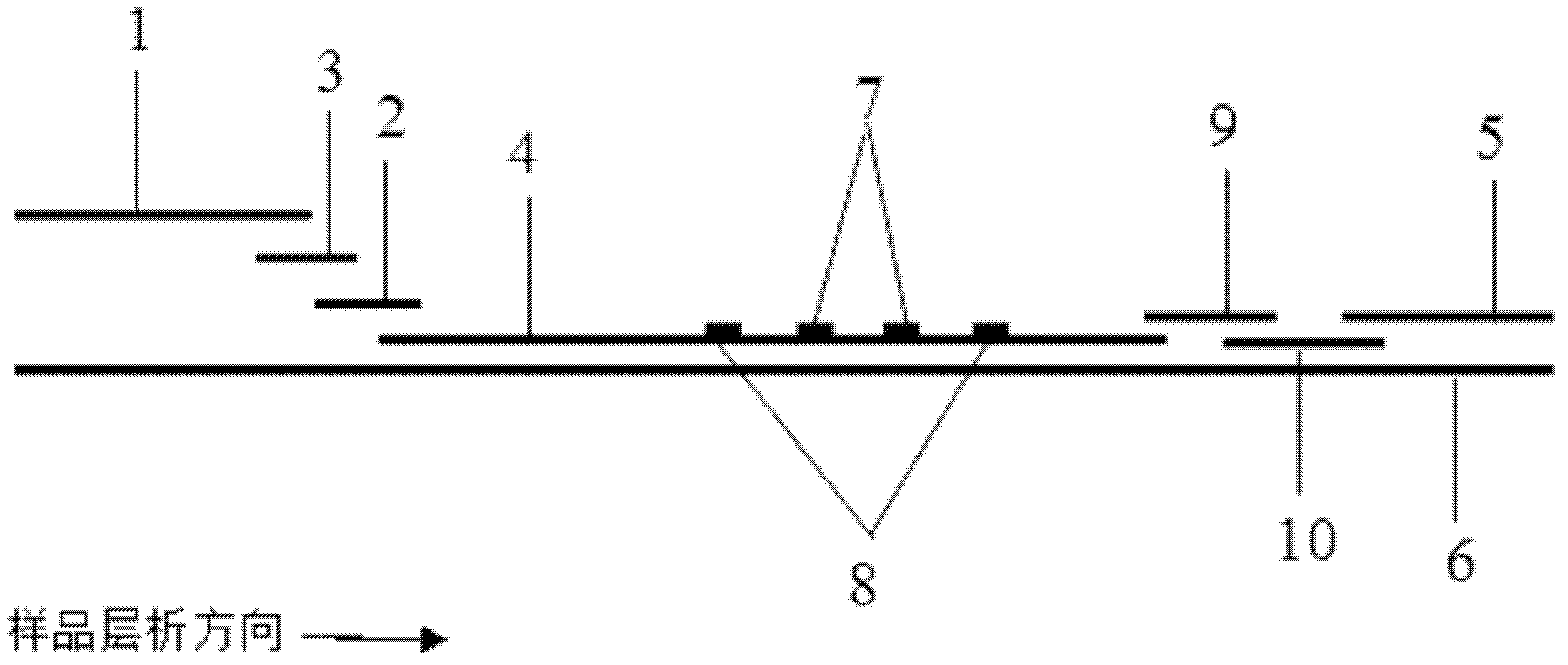
Rapid test for glycated albumin in saliva
InactiveUS20060270060A1Accurate assessmentBioreactor/fermenter combinationsBiological substance pretreatmentsMeasuring instrumentPlasma Albumin
This invention describes a rapid immunochromatographic assay for measuring the ratio of glycated albumin to total albumin in saliva. Patients with diabetes have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. Saliva albumin is derived from plasma albumin and therefore contains glycated and non-glycated albumin fractions that can be measured. The ratio of glycated albumin to total albumin in saliva will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period. The test is performed using a disposable strip that contains the testing reagents and the results are measured in a measuring instrument that automatically reads, calculates and displays the final result. The results of tests performed over a period of time are stored in the instrument's memory and presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:SMITH HENRY JOHN
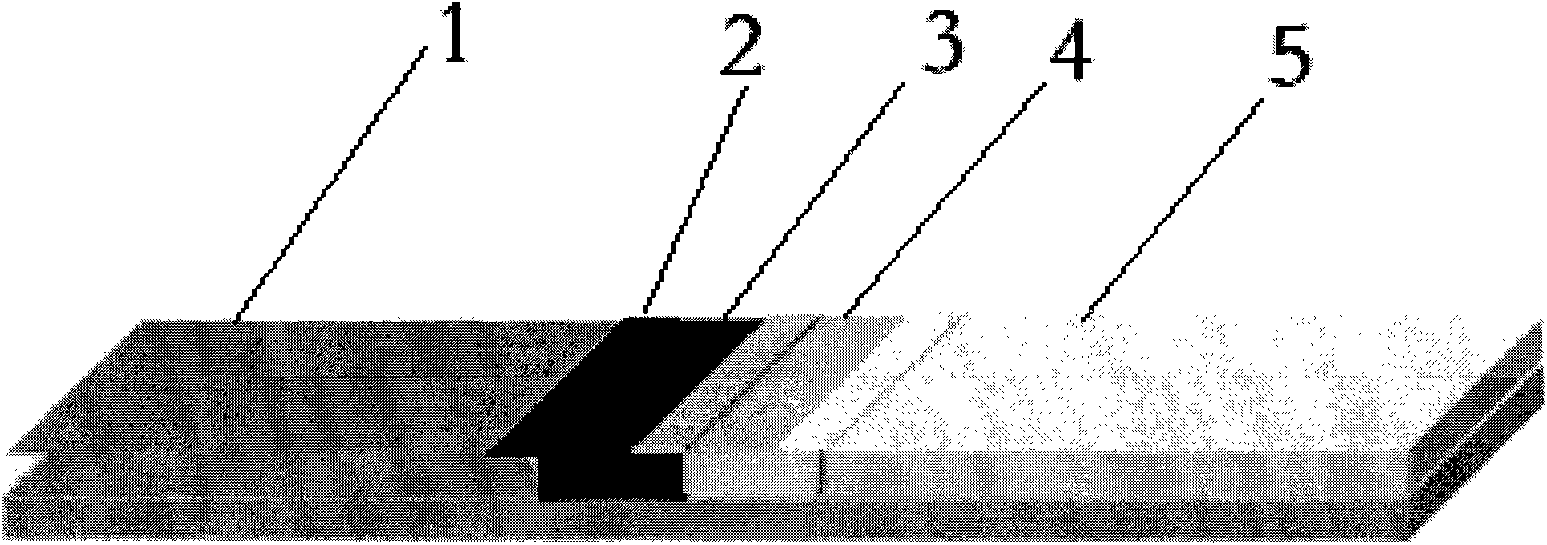
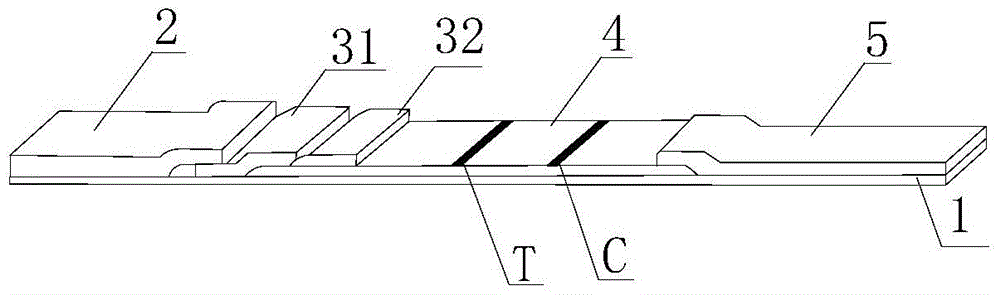
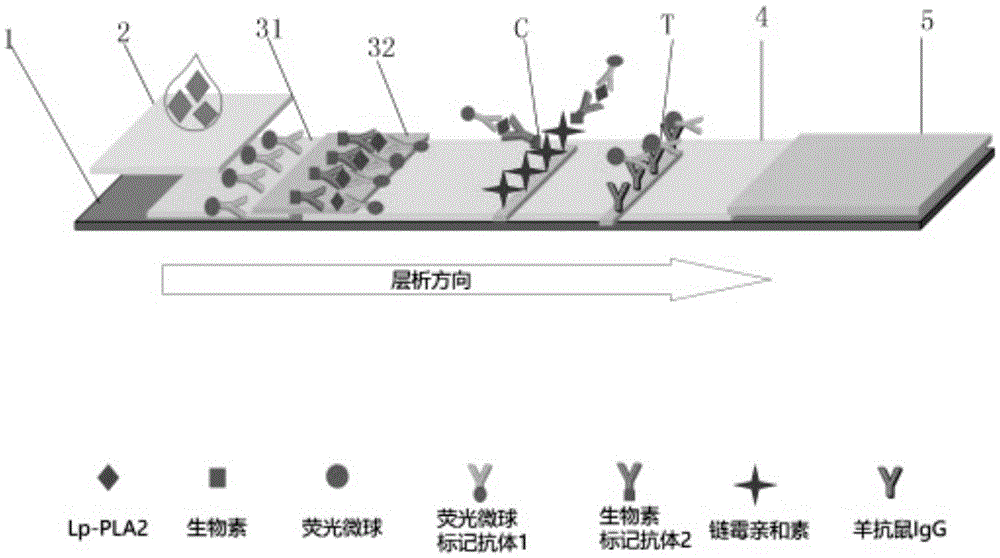
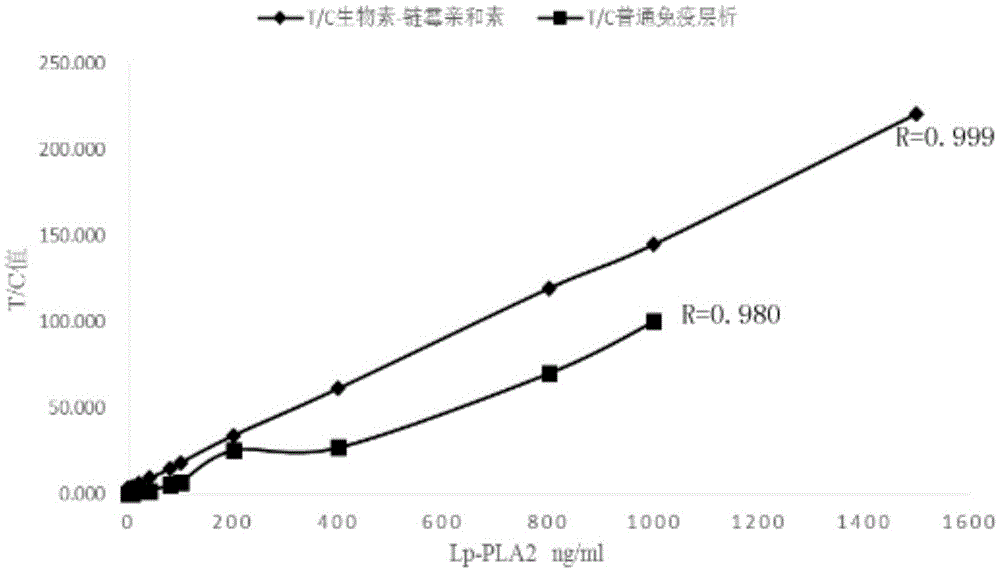
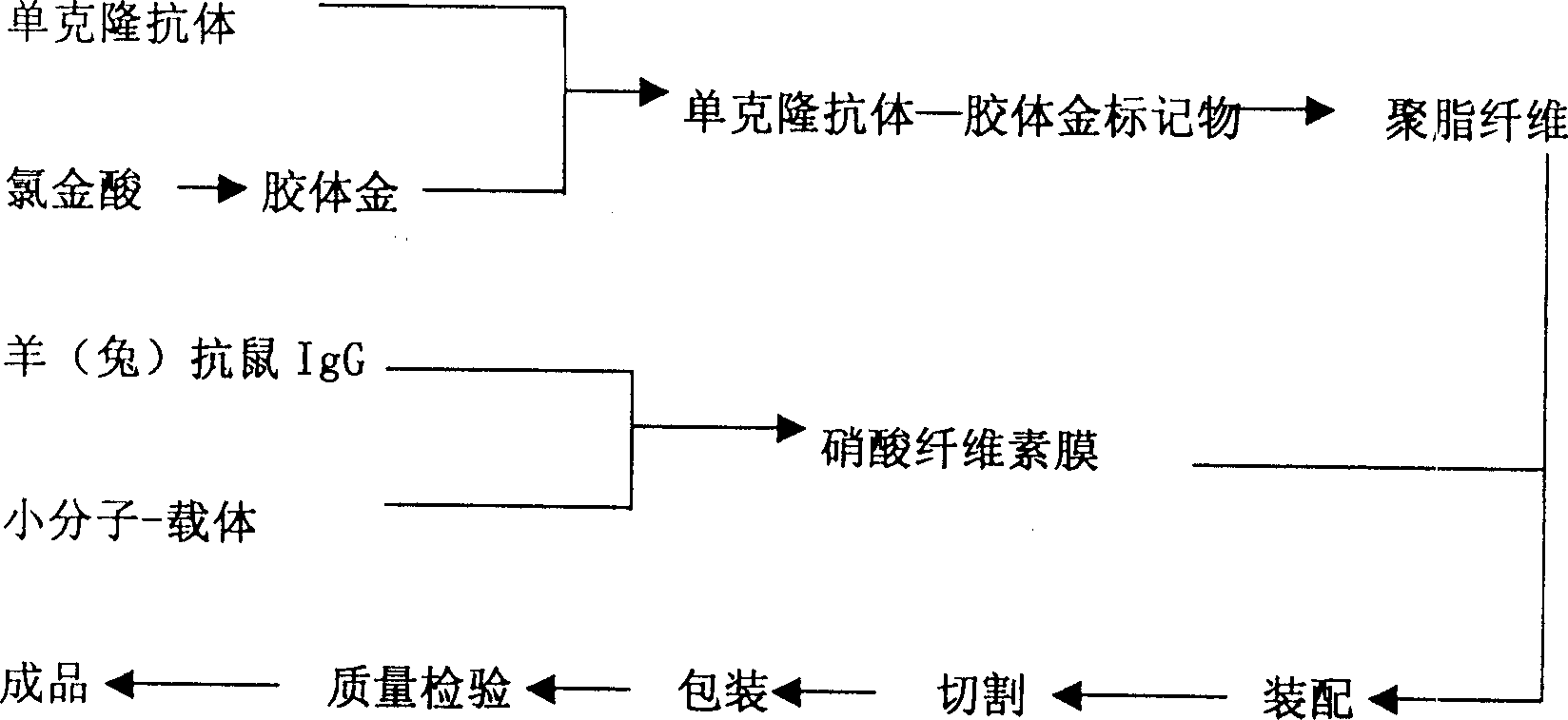
Human Lp-PLA2 biotin-streptavidin fluorescence immunochromatographic assay card and preparation method thereof
ActiveCN105652008AInhibitory activityAvoid the phenomenon that is not easy to preserve)Biological material analysisStreptolydiginMonoclonal antibody
Owner:GUANGZHOU WEIMI BIOLOGICAL SCI & TECH
Use of colloidal gold immunological chromatography in detection of aquatic, agricutural and livestock products
InactiveCN1409113AStrong specificityIncreased sensitivityBiological testingProduct inspectionAquatic product
A colloidal gold immunity chromatograph to be used for the inspection of aquatic product, agricultural product and livestock product belongs to the technical field of biological medicine. The presentinvention with high sensitiivty and high specificity and be widely used in the departments of inspection and epidemic prevention to overcome the problems such as short of inspecting means and limitation by the conditions existed in the product inspection for the quatic, and livestock agricultural prdoucts.
Owner:张少恩
Tumor marker colloidal gold immunochromatographic assay quantitative detection test paper and method for preparing same
InactiveCN101900733ARealize quantitative detectionEasy to operateMaterial analysisHuman tumorAntibody conjugate
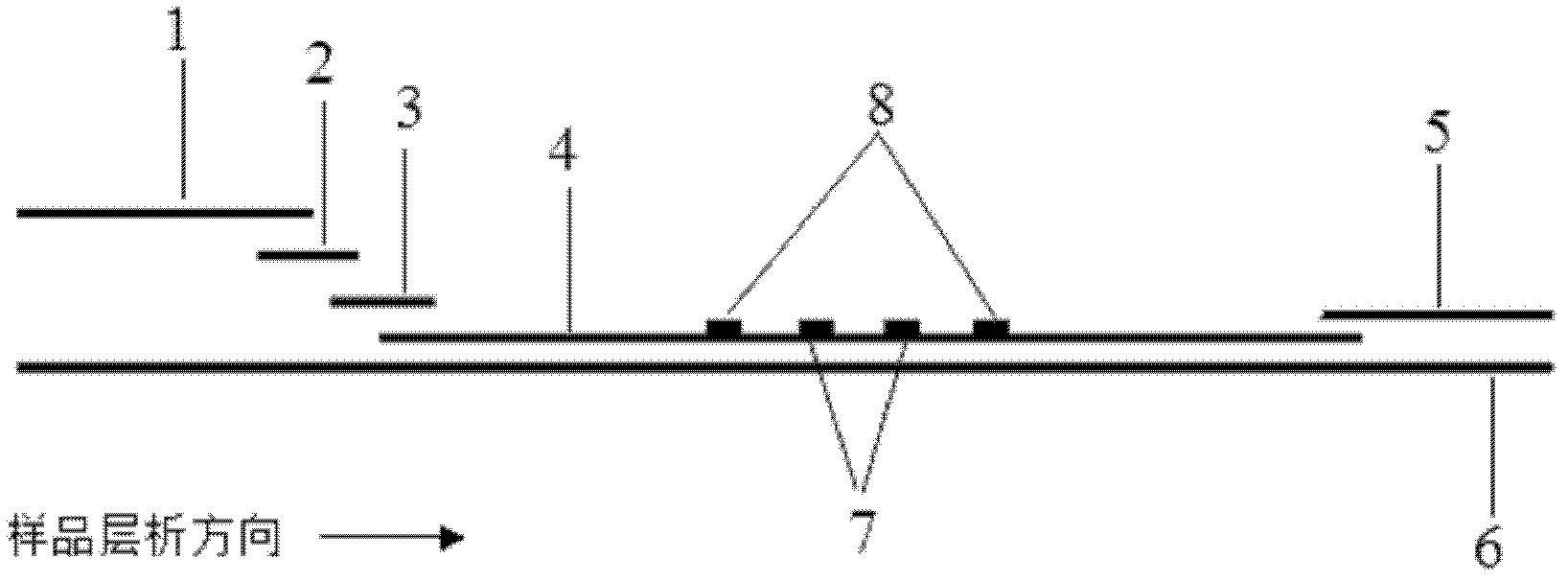
The invention discloses tumor marker colloidal gold immunochromatographic assay quantitative detection test paper and a method for preparing the same. The test paper comprises a sample pad, a gold-labeled antibody conjugate film, a nitrocellulose membrane, a water absorbing cushion and a reaction support, wherein the nitrocellulose membrane of which the end close to the sample pad is used as a starting end and the end close to the water absorbing cushion is used as a tail end is enveloped by five antibody strips in turn, namely T1, T2, T3 and T4 detection strips of mouse anti-human tumor marker monoclonal antibody I and a C quality control strip of goat anti-mouse IgG respectively; and the gold-labeled antibody conjugate film is a film coated with a colloidal gold-labeled mouse anti-human tumor marker monoclonal antibody II. The test paper and the method realize rapid and accurate quantitative detection of the tumor marker and thus meet the requirement of rapid detection of cancer surveying and screening, family cancer prognosis monitoring and the like.
Owner:CENT SOUTH UNIV
Measurement kit and an immunochromatography method
ActiveUS20090181470A1Clear measurement resultReduce distanceAnalysis using chemical indicatorsEnzymologyEngineeringBackground noise
Owner:FUJIFILM CORP
Sensitization detection method of colloidal gold immunity chromatography and use thereof
A sensitivity raising detection method of colloid gold immunity chromatography method comprises: when using general colloid gold immunity chromatography to check object sample, adding an object sample, adding signal amplifying agents of 10 to 80ul between the gold label antibody layer and the detection line; processing the detection line obtained by general colloid gold immunity chromatography method; reacting under room temperature for 1 to 10min; if the object sample contains antigen, the detection carrier is formed with a detection line and a control line as clear black or grey black color, to represent positive property, or if the object sample not contains antigens, the control line is black or grey black color, and the detection line has not color, to represent negative property. The method can significantly improve the sensitivity of colloid gold immunity chromatography reagent kit on the detection of biological macromolecules, having fast color development, lower background interference compared with general silver stain method, wide application prospect and high development value.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Quick test paper for detecting enterovirus and method for preparing same
ActiveCN101650366AFacilitate on-site screening workStrong specificityMaterial analysisCelluloseEnterovirus
The invention discloses a quick test paper for detecting enterovirus and a method for preparing the same. The test paper detects enterovirus EV71 and Coxsackievirus A16 viruses in a sample by the immunochromatography adopting marking by colorful particles. The quick test strip is formed by sequentially overlapping and bonding a sample adsorption liquid layer, a colorful particle storing pad, a cellulose nitrate membrane and a water adsorption board on a bottom board, wherein the colorful particle storing pad is coated with a monoclonal antibody with a colorful particle mark; and the cellulose nitrate membrane is provided with a detection line sprayed by the monoclonal antibody of the EV71 and / or Coxsackievirus A16 and a control line sprayed by the polyclonal antibody of anti-mouse IgG. The test paper can quickly detects the EV71 and Coxsackievirus A16 viruses in the detected sample at the same time or respectively, finds the epidemic situation caused by the virus infection as early as possible and has the advantages of being easily operated, quickly getting results, avoiding special operators and the like.
Owner:万志静 +1
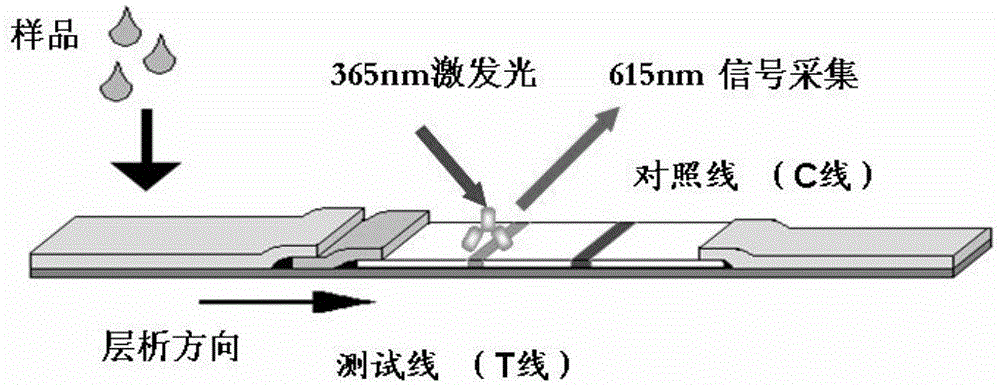
Fluorescence immunochromatographic assay method for quantitatively detecting heart fatty acid binding protein and kit for quantitatively detecting same
ActiveCN102520194ASolve the backgroundSolve the signal indistinguishableBiological testingBlood plasmaBiology
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
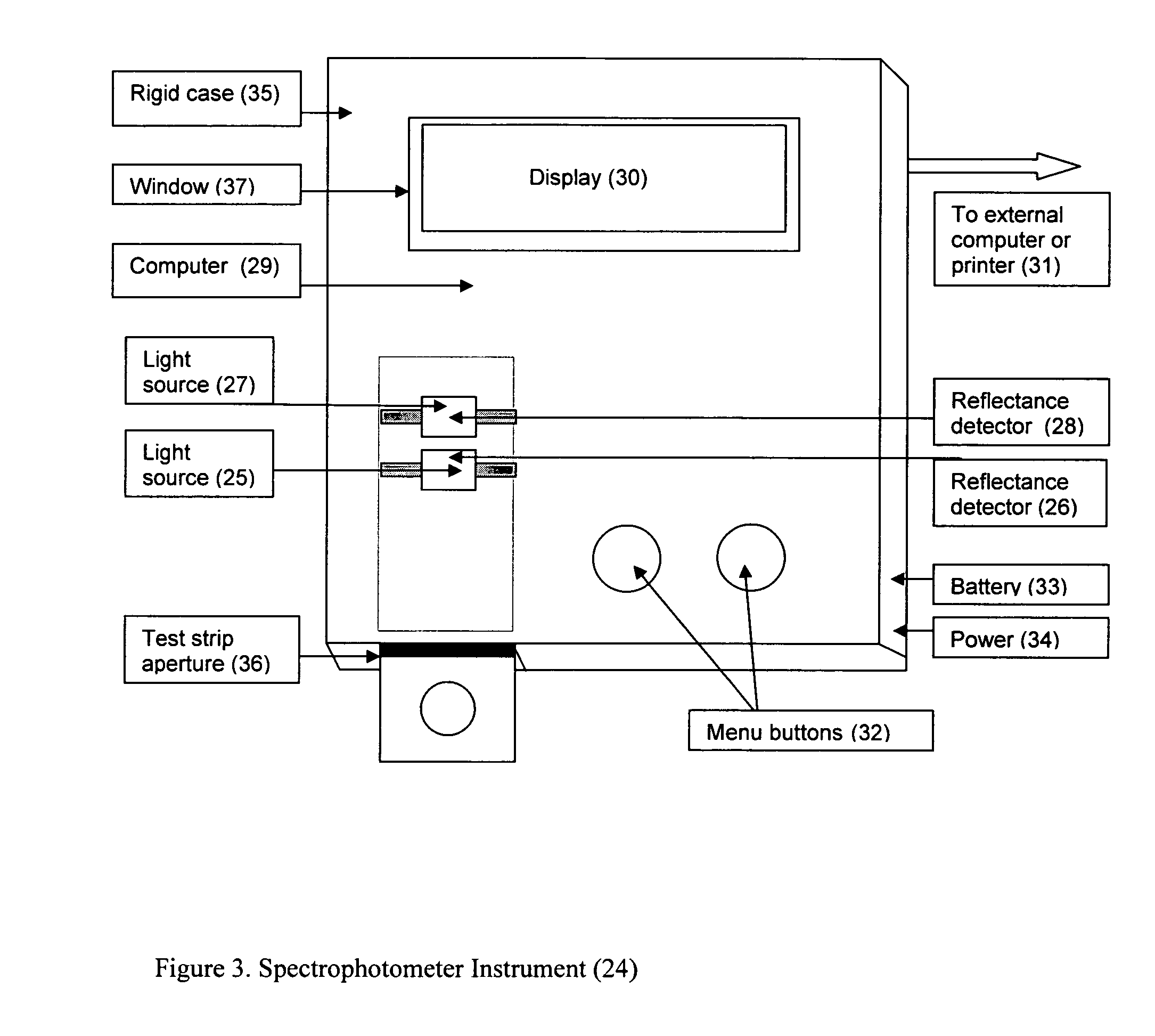
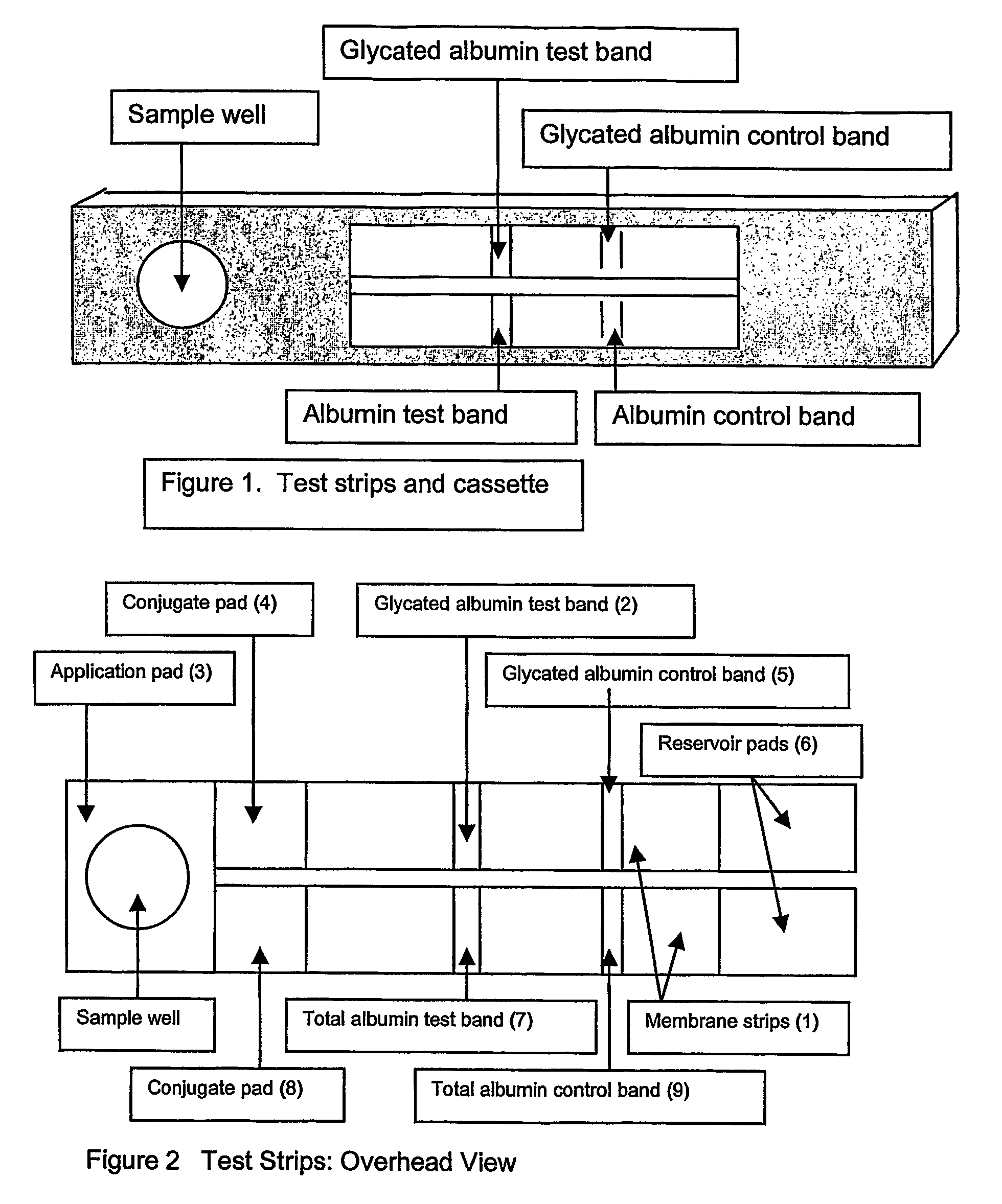
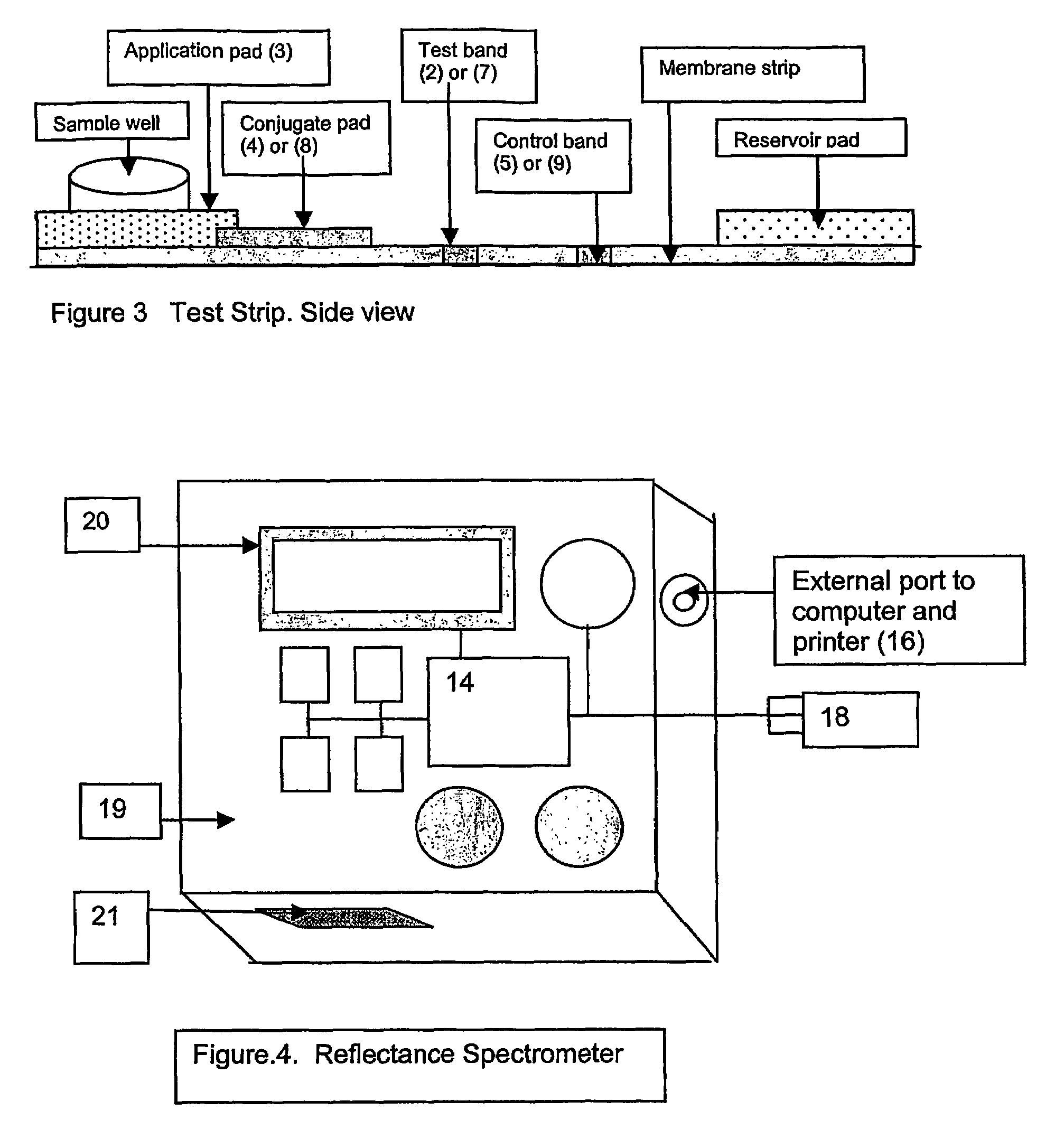
Rapid test for glycated albumin
ActiveUS7659107B2Bioreactor/fermenter combinationsBiological substance pretreatmentsALBUMIN TESTSingle sample
A rapid immunochromatographic assay system is provided for measuring the amount of glycated albumin in a blood sample relative to the total level of albumin in the sample. The assay system is comprised of a disposable cassette that contains the test strips and testing reagents, and a measurement device that automatically reads, calculates and displays the test results over a period of time. The test cassette contains two test strips that are used to measure glycated albumin and total albumin respectively. The strips are contiguous beneath the single sample application well so that the same sample is tested simultaneously by both test strips. Part of the sample will migrate thru the glycated albumin test strip where it will react with the glycated albumin test reagents to yield a glycated albumin result, while part of the sample will migrate thru the total albumin test strip where it will react with the total albumin test reagents to yield a total albumin result. The test cassette is placed within a measuring device such as a reflectance spectrometer or fluorometer, that reads, calculates and expresses the result as the percentage of glycated albumin relative to total albumin in the sample. The results of successive testing that are performed over a period of time are stored in the instrument's memory and displayed in a numerical or graphical format so that the individual's glycated albumin levels can be monitored over time.
Owner:MEDYTOX SOLUTIONS
Fluorescence immunochromatographic assay and kit for quantitatively detecting N-terminal pro brain natriuretic peptide
ActiveCN102565423ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceDiseaseN-terminal pro-Brain Natriuretic Peptide
Owner:SHENZHEN KANGMEI BIOTECH
Sensitive immunochromatographic assay
InactiveUS7691595B2Easy to detectCertain constraintBioreactor/fermenter combinationsBiological substance pretreatmentsAssayAnalyte
Methods for quantitatively measuring the amount of an analyte of interest in a fluid sample, and kits useful in the methods, are disclosed. The methods involve providing a solid phase apparatus comprising a membrane having an application point, a sample capture zone, and a control capture zone, where the sample capture region is between the application point and the control capture zone; and providing a sample collection apparatus comprising a population of analyte binding particles or a population of analyte coated particles. In the assays, a fluid sample is introduced into the sample collection apparatus, and the resultant mixture is applied to the application point of the membrane. The fluid allows transport components of the assay by capillary action to and through the sample capture zone and subsequently to and through the control capture zone. The amount of analyte in the fluid sample is related (e.g., either directly or inversely) to a corrected particle amount, which can be determined, for example, as a ratio of the amount of particles in the sample capture zone and the amount of particles in the control capture zone.
Owner:RESPONSE BIOMEDICAL CORP
Fluorescence immunochromatographic assay method of serum amyloid protein A and kit
InactiveCN105675879AHigh sensitivity in vitroImprove accuracyBiological testingFluorescenceAmyloid A Protein
The invention relates to a fluorescence immunochromatographic method of serum amyloid protein A and a detection kit thereof. The method includes the steps of preparing a probe fixing pad, preparing an immunochromatographic test strip, preparing a sample diluting liquid and detecting a sample. The probe fixing pad is prepared by mixing serum amyloid protein A monoclonal antibody fluorescent latex particles and goat-anti-rabbit IgG fluorescent latex particles, and diluting the mixture with a gold diluent and spraying the liquid onto glass fibers. A detection line is coated with serum amyloid protein A monoclonal antibody and a control line is coated with goat-anti-rabbit IgG to prepare the immune-chromatographic test strip. The detection kit includes an immune-chromatographic detection card including a PVC lining plate, a sample pad, the probe fixing pad, a nitrocellulose membrane and a water absorbing paper. The probe fixing pad is prepared by mixing and drying the serum amyloid protein A monoclonal antibody fluorescent latex particles and the goat-anti-rabbit IgG fluorescent latex particles, so that the detection kit is high in detection sensitivity and accuracy, is simple in operation and is low in cost.
Owner:SUZHOU BIONANOTECH CO LTD
Preparation method of multifunctional test paper for early pregnancy
ActiveCN103777002ASimplify detection stepsThe test result is accurateDisease diagnosisBiological testingPolyesterCellulose
The invention discloses a preparation method of a multifunctional test paper for early pregnancy. The preparation method comprises the steps: preparing a nitrocellulose membrane; labeling and curing a polyester fiber strip; and assembling and cutting. A human chorionic gonadotropin beta core segment and a human chorionic gonadotropin regular molecule in urine are detected by applying a principle of a double-antibody sandwich method and an immunochromatographic method, detection steps are simple, a result can be observed within 5 min, and a common user can detect the result, and the detection result is more accurate. Through comparing color depth of a first detection line and a second detection line, whether to be pregnant and whether to be normally pregnant are determined, and ectopic gestation and threatened abortion are subjected to risk assessment.
Owner:NANTONG EGENS BIOTECH
Test strip and method for fast quantitative detection of human chorionic gonadotropin (HCG)
InactiveCN102890157AAvoid the hook effectAvoid troubleBiological testingHook effectMonoclonal antibody
The invention relates to a test strip and a method for fast quantitative detection of human chorionic gonadotropin (HCG). The test strip comprises a sample loading pad, a marker pad, an NC membrane, a sample suction pad and a plastic plate. The sample loading pad, the marker pad, the NC membrane and the sample suction pad are orderly adhered to the plastic plate. The marker pad is coated with colloidal gold-marked mouse anti-human HCG-beta monoclonal antibodies. The NC membrane is coated with a detection line T composed of mouse anti-human HCG-alpha antibodies and a quality control line C composed of goat anti-mouse IgG antibodies. On the NC membrane, a detection line T side close to the marker pad is provided with a sample loading indication line for indicating a sample loading position. Through loading of a sample on the NC membrane, the test strip can solve the problem that the existing detection reagent produces a hook effect because of too high HCG content, and can realize quantitative detection of HCG in blood or urine by a matched immunochromatographic assay interpretoscope.
Owner:天津中新科炬生物制药股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com