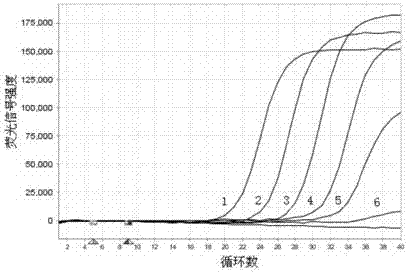
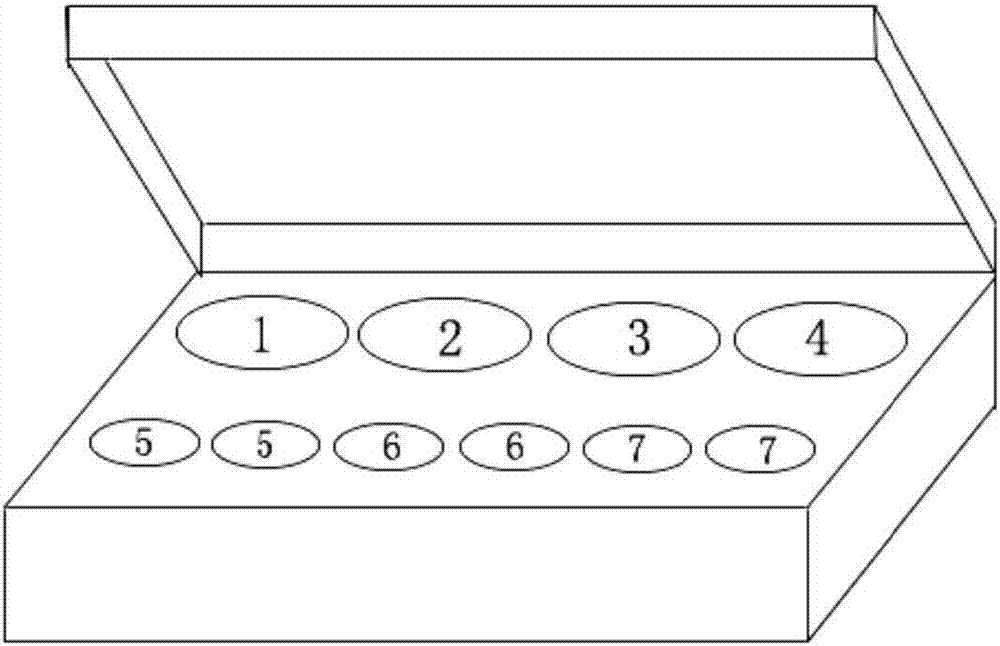
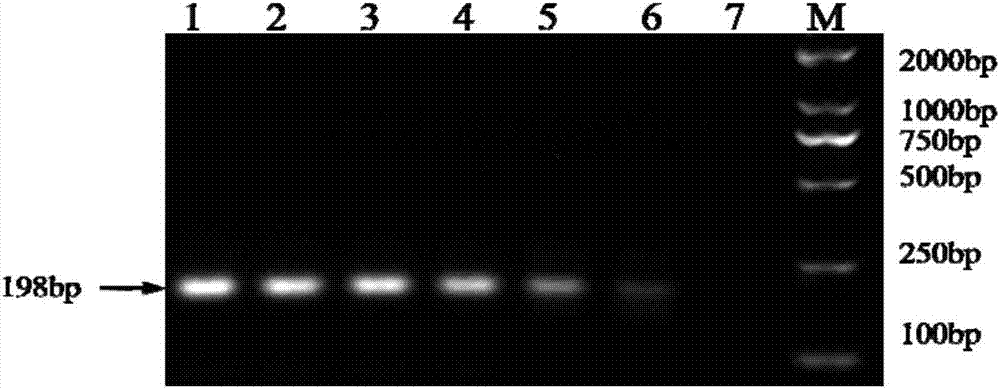
Double fluorescent quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for detecting dengue virus and zika virus
A dual-fluorescence, Zika virus-based technology for cost-saving applications in microbial-based methods, microbial assays/tests, resistance to vector-borne diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A dual real-time fluorescent quantitative RT-PCR detection kit for detecting DENV and ZIKV, comprising: quantitative RT-PCR reaction solution, enzyme mixture, primer-probe mixture, standard (DENV, ZIKV), strong positive control ( DENV, ZIKV), weak positive control (DENV, ZIKV), negative control. The box body of the kit is provided with container holes corresponding to each component of the kit. The box body is provided with container holes, which are respectively used to place the quantitative RT-PCR reaction solution tube (label 1), the enzyme mixed solution tube (label 2), the primer-probe mixed solution tube (label 3), and the negative control tube (label 4). ), standard quality control (including DENV, ZIKV standard substance, a total of 2 tubes, label 5), strong positive control quality control (including DENV, ZIKV positive control substance, a total of 2 tubes, label 6), strong positive control quality control (including DENV, ZIKV positive control substances, a...
Embodiment 2
[0055] 1 Materials and methods
[0056] 1.1 Clinical specimens and viral nucleic acids:
[0057] The positive clinical samples of DENV and ZIKV were provided by Zhejiang Huzhou Center for Disease Control and Prevention and Zhejiang Provincial Center for Disease Control and Prevention. Plasma, serum and / or urine samples from suspected patients in a hospital, and the samples are collected and shipped to the laboratory. In addition, the positive nucleic acids of other viruses related to fever and rash, such as measles virus, rubella virus, Dow virus 71, Coxsackie virus A16 and Coxsackie virus A6, as well as influenza A and B viruses, were obtained from Provided by the Center for Disease Control and Prevention of Huzhou City, Zhejiang Province and the First People's Hospital of Huzhou City, Zhejiang Province.
[0058] 1.2 Primers and probes
[0059] Multiple gene sequences covering DENV subtypes and ZIKV genotypes at home and abroad were downloaded from the NCBI gene bank. Usi...
Embodiment 3
[0086] Detection of clinical samples using this kit: positive clinical samples of DENV (4 copies) and ZIKV (2 copies) were provided by the Huzhou Center for Disease Control and Prevention of Zhejiang Province and the Zhejiang Provincial Center for Disease Control and Prevention, and the suspected samples were from Huzhou, Zhejiang Province A total of 325 plasma, serum and / or urine samples were collected from the First People's Hospital of the city, Huzhou Central Hospital of Zhejiang Province, and several other hospitals in Zhejiang Province. The collected samples were verified by double real-time fluorescent quantitative RT-PCR in this method, and the detection results were as follows: 4 samples were positive for DENV, with a positive rate of 1.2%; 2 samples were positive for ZIKV, with a positive rate of 0.6%. The positive test results were 100% consistent with the reported results.
[0087] Huzhou First People's Hospital
[0088] A Dual Fluorescent Quantitative RT-PCR Ki...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com