Application of carbenoxolone in preparation of anti-Zika virus medicine
A technology of Zika virus and carbenoxolone is applied in the application field of carbenoxolide in the preparation of anti-Zika virus drugs, which can solve the problem of lack of drugs that effectively inhibit Zika virus, and achieves improvement of brain development retardation phenomenon and resources Rich and convenient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
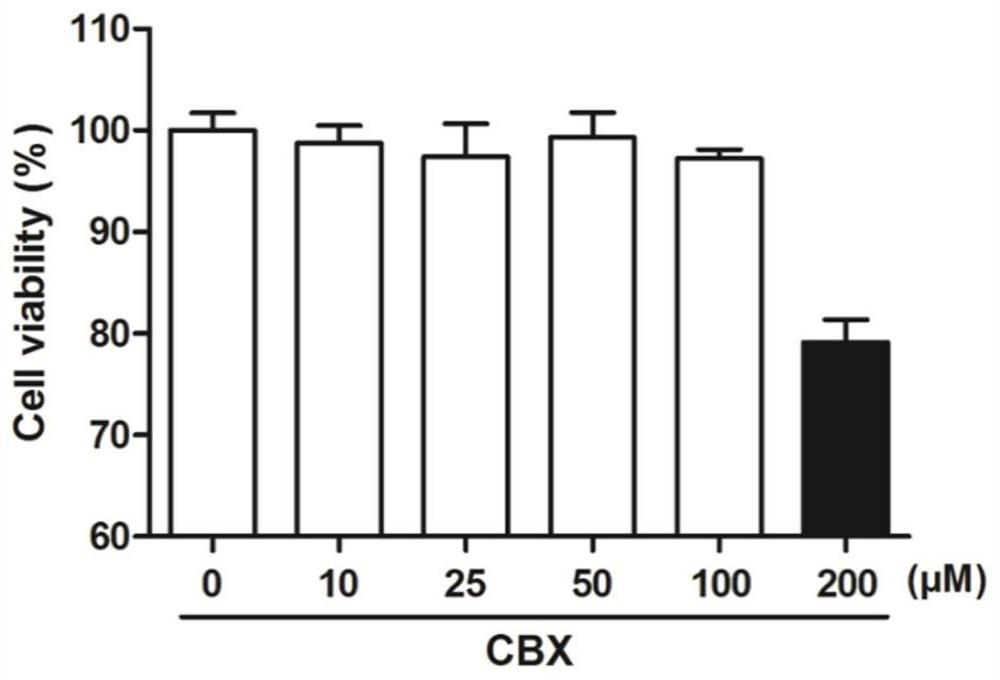
[0044] Embodiment 1 carbenoxic acid is to the toxicity experiment of A549 cell
[0045] Include the following steps:
[0046] 1. Inoculation of A549 cells: Use DMEM medium containing 5% fetal bovine serum to prepare a single cell suspension, and inoculate 2000-3000 cells per well into a 96-well cell culture plate, with an inoculation volume of 100 μl per well.
[0047] 2. Culture A549 cells: at 37°C, 5% CO 2 Under culture conditions, culture for 1 day.
[0048] 3. Add carbenoxic acid: discard the DMEM medium in each well, add 100 μl to each well and dilute with DMEM medium containing 5% fetal bovine serum to the corresponding concentration (10 μM, 25 μM, 50 μM, 100 μM, 200 μM) 100 μl of DMEM medium containing 5% fetal bovine serum without drugs was added to control wells.
[0049] 4. Coloring: After 24 hours of culture, add 10 μl of MTT solution to each well, and incubate at 37°C, 5% CO 2 Continue to incubate under the culture conditions for 4 hours, then terminate the cul...
Embodiment 2
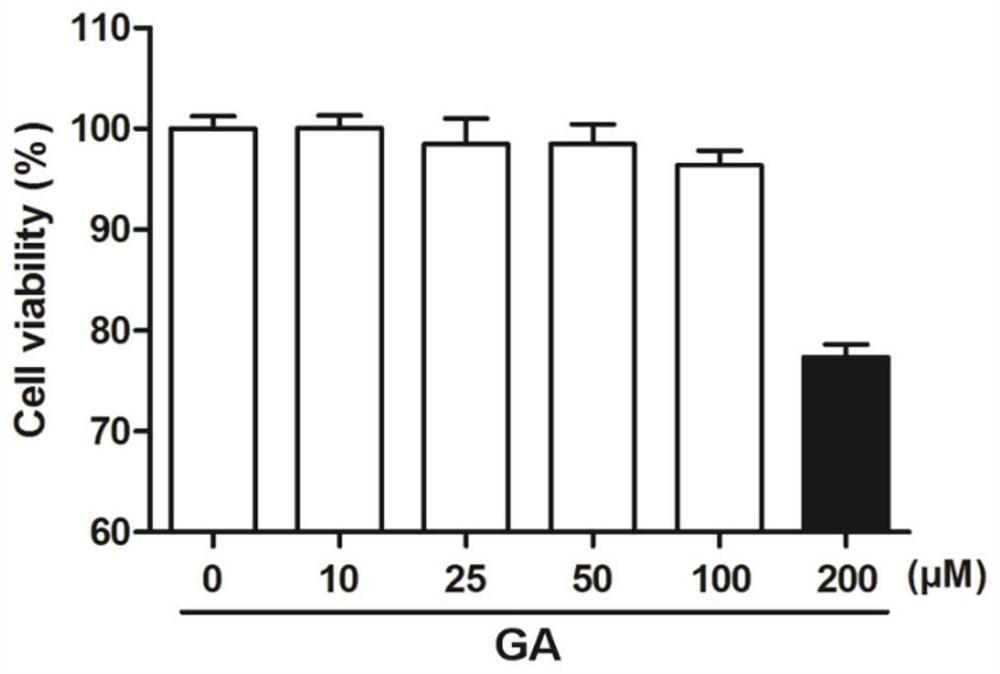
[0054] Example 2 Toxicity Experiment of Glycyrrhizin to A549 Cells
[0055] Include the following steps:
[0056] 1. Inoculation of A549 cells: Use DMEM medium containing 5% fetal bovine serum to prepare a single cell suspension, and inoculate 2000-3000 cells per well into a 96-well cell culture plate, with an inoculation volume of 100 μl per well.
[0057] 2. Culture A549 cells: at 37°C, 5% CO 2 Under culture conditions, culture for 1 day.
[0058] 3. Add glycyrrhizin: discard the DMEM medium in each well, add 100 μl to each well and dilute to the corresponding concentration (10 μM, 25 μM, 50 μM, 100 μM, 200 μM) with DMEM medium containing 5% fetal bovine serum Glycyrrhizin (GA), 100 μl of drug-free 5% fetal bovine serum DMEM medium was added to the control well.
[0059] 4. Coloring: After 24 hours of culture, add 10 μl of MTT solution to each well, and incubate at 37°C, 5% CO 2 Continue to incubate under the culture conditions for 4 hours, then terminate the culture, di...
Embodiment 318
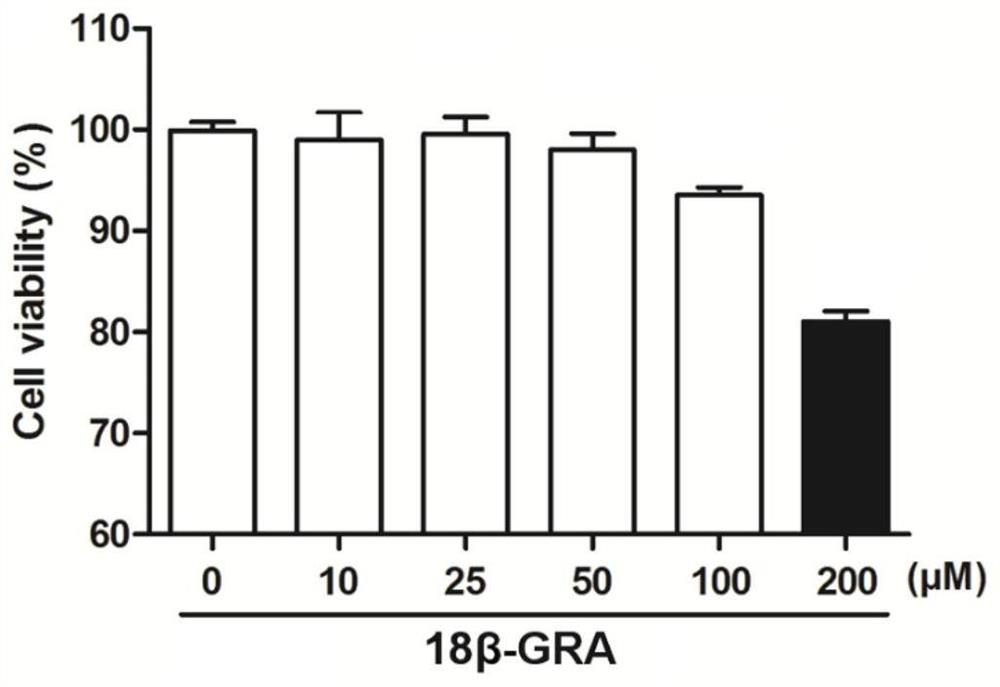
[0065] Example 3 Toxicity test of 18β-glycyrrhetinic acid on A549 cells
[0066] Include the following steps:
[0067] 1. Inoculation of A549 cells: Use DMEM medium containing 5% fetal bovine serum to prepare a single cell suspension, and inoculate 2000-3000 cells per well into a 96-well cell culture plate, with an inoculation volume of 100 μl per well.
[0068] 2. Culture A549 cells: at 37°C, 5% CO 2 Under culture conditions, culture for 1 day.
[0069] 3. Add 18β-glycyrrhetinic acid: discard the DMEM medium in each well, add 100 μl to each well and dilute to corresponding concentration (10 μM, 25 μM, 50 μM, 100 μM, 200 μM) of 18β-glycyrrhetinic acid (18β-GRA), and 100 μl of DMEM medium containing 5% fetal bovine serum without drug was added to the control well.
[0070] 4. Coloring: After 24 hours of culture, add 10 μl of MTT solution to each well, and incubate at 37°C, 5% CO 2 Continue to incubate under the culture conditions for 4 hours, then terminate the culture, dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com