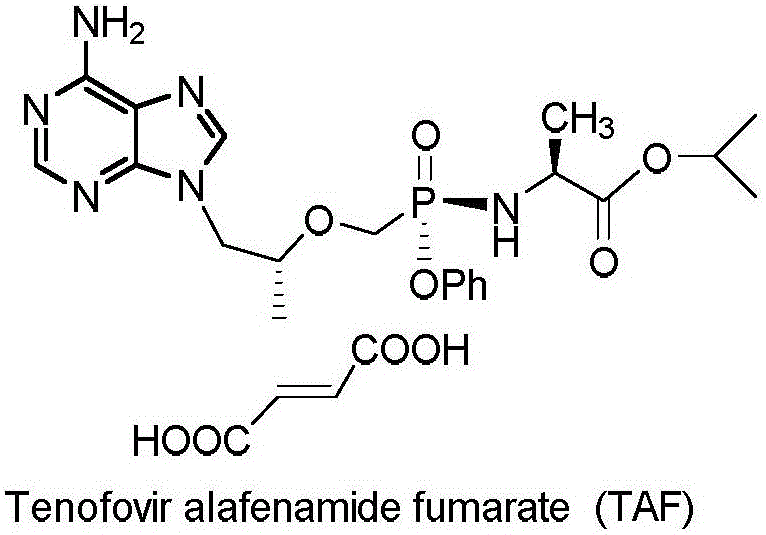
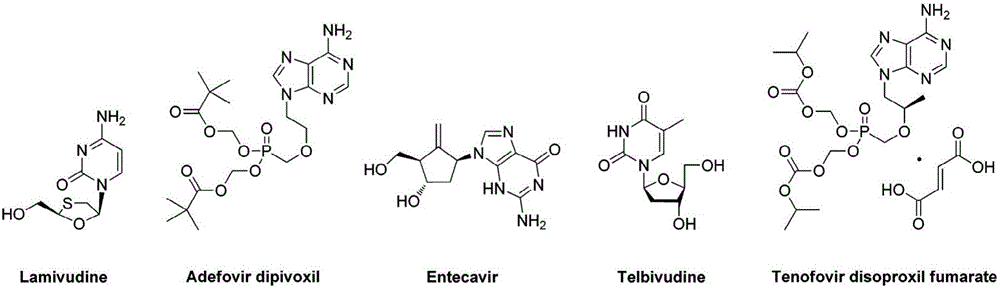
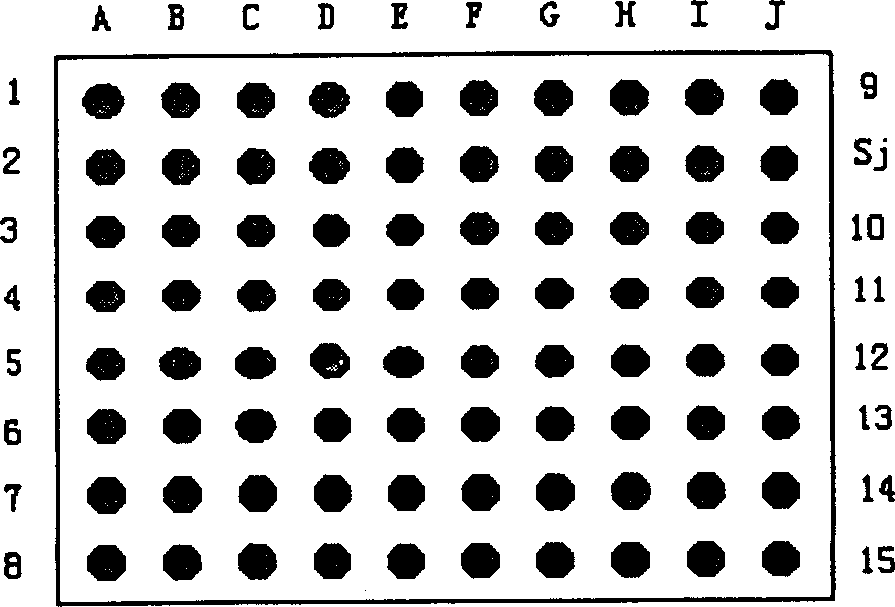Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
650 results about "Infection disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infection is the invasion of an organism's body tissues by disease-causing agents, their multiplication, and the reaction of host tissues to the infectious agents and the toxins they produce. Infectious disease, also known as transmissible disease or communicable disease, is illness resulting from an infection.
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
Piggybac transposon-based vectors and methods of nucleic acid integration
InactiveUS20090042297A1Sugar derivativesOther foreign material introduction processesPiggybac transposonInfection disease
Disclosed herein are compositions comprising integrating enzymes that can deliver nucleic acids to a target DNA. Additionally, the methods of using the compositions disclosed herein relate to treatments for a variety of infections, conditions, and genetic disorders.
Owner:VANDERBILT UNIV
Cysteine protease inhimbitors
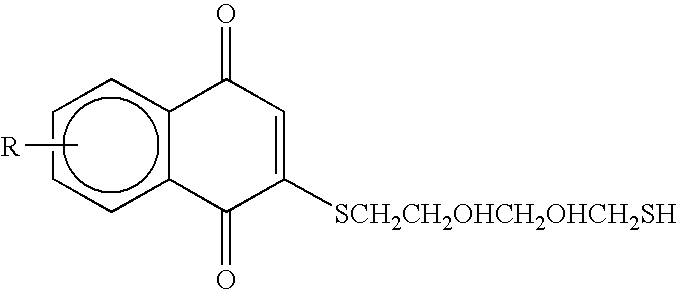
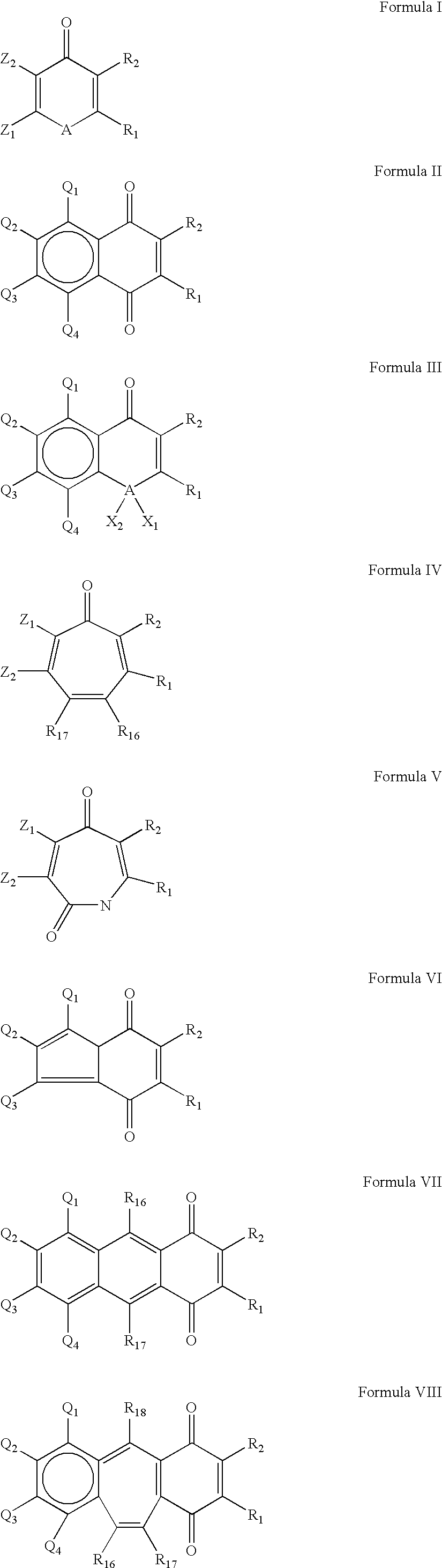
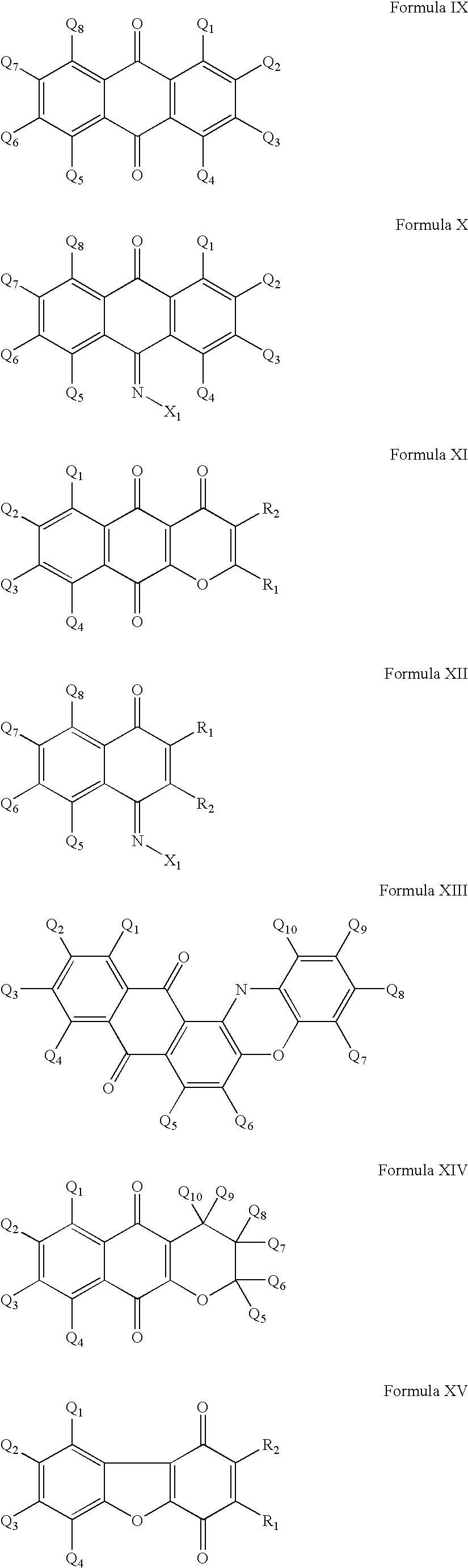
Compounds having quinone and quinone analogs useful for pharmaceutical preparations have now been found which inhibit cysteine proteases, in particular, caspases and 3C-cysteine proteases. The cysteine protease inhibitors of the present invention can be identified by their mode of action in disrupting the ability of cysteine proteases and, in particular, caspases to cleave a peptide chain. These compounds are useful in inhibiting cysteine protease or cysteine protease-like proteins and for treating infections diseases or physiopathological diseases or disorders attributed to the presence of excessive or insufficient levels of cysteine proteases.
Owner:ARAD DORIT +6
Intravenous immunoglobulin composition
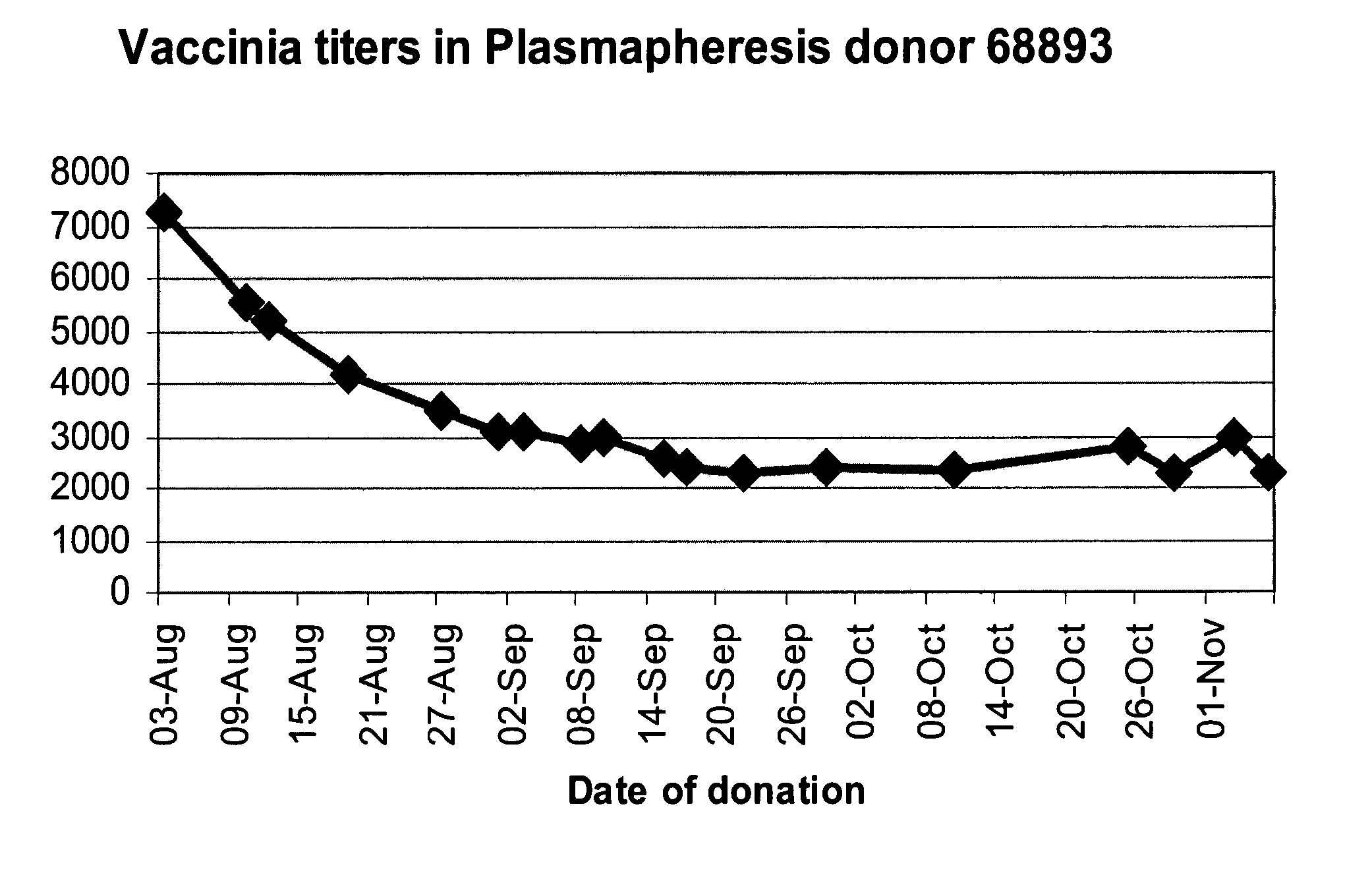
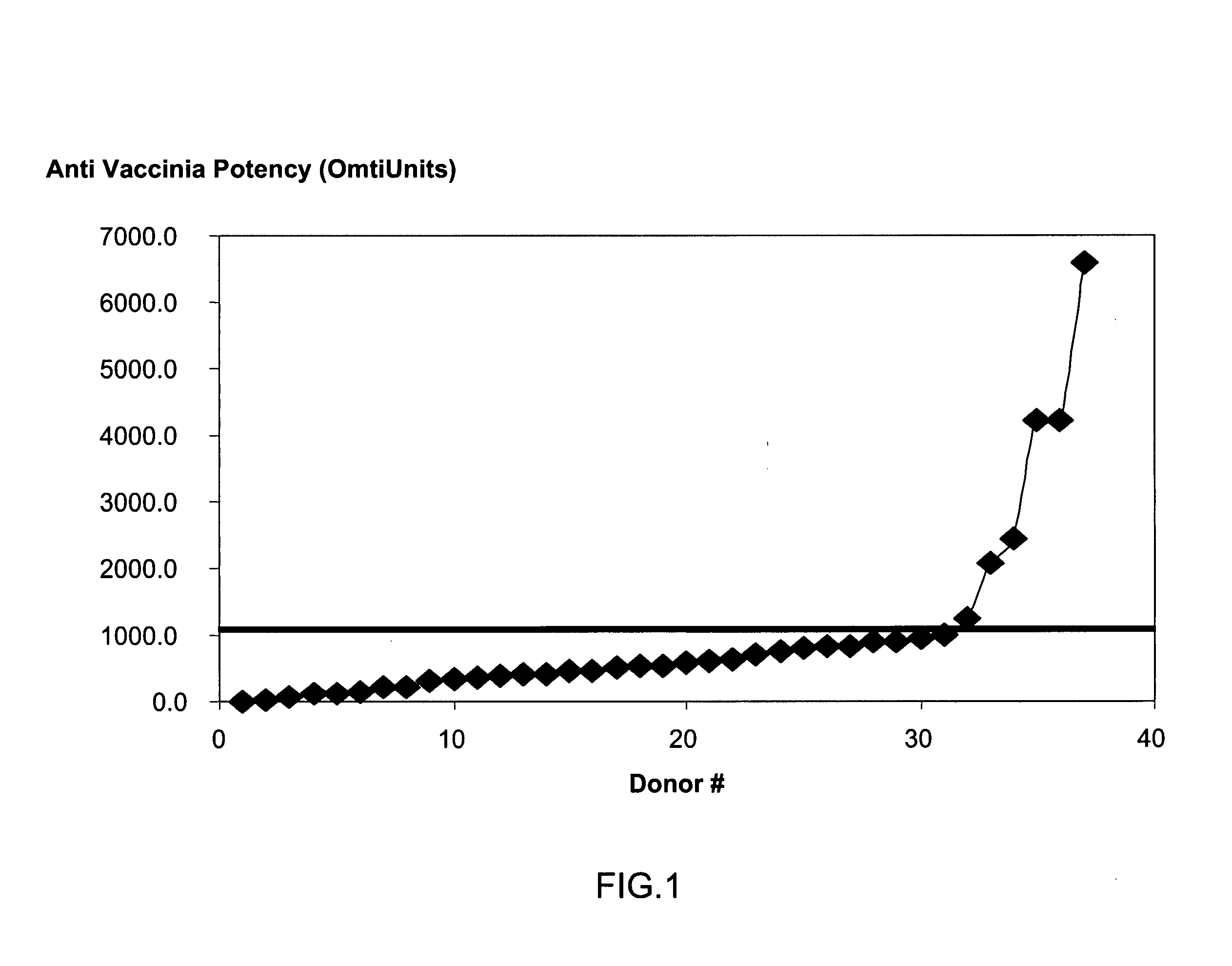
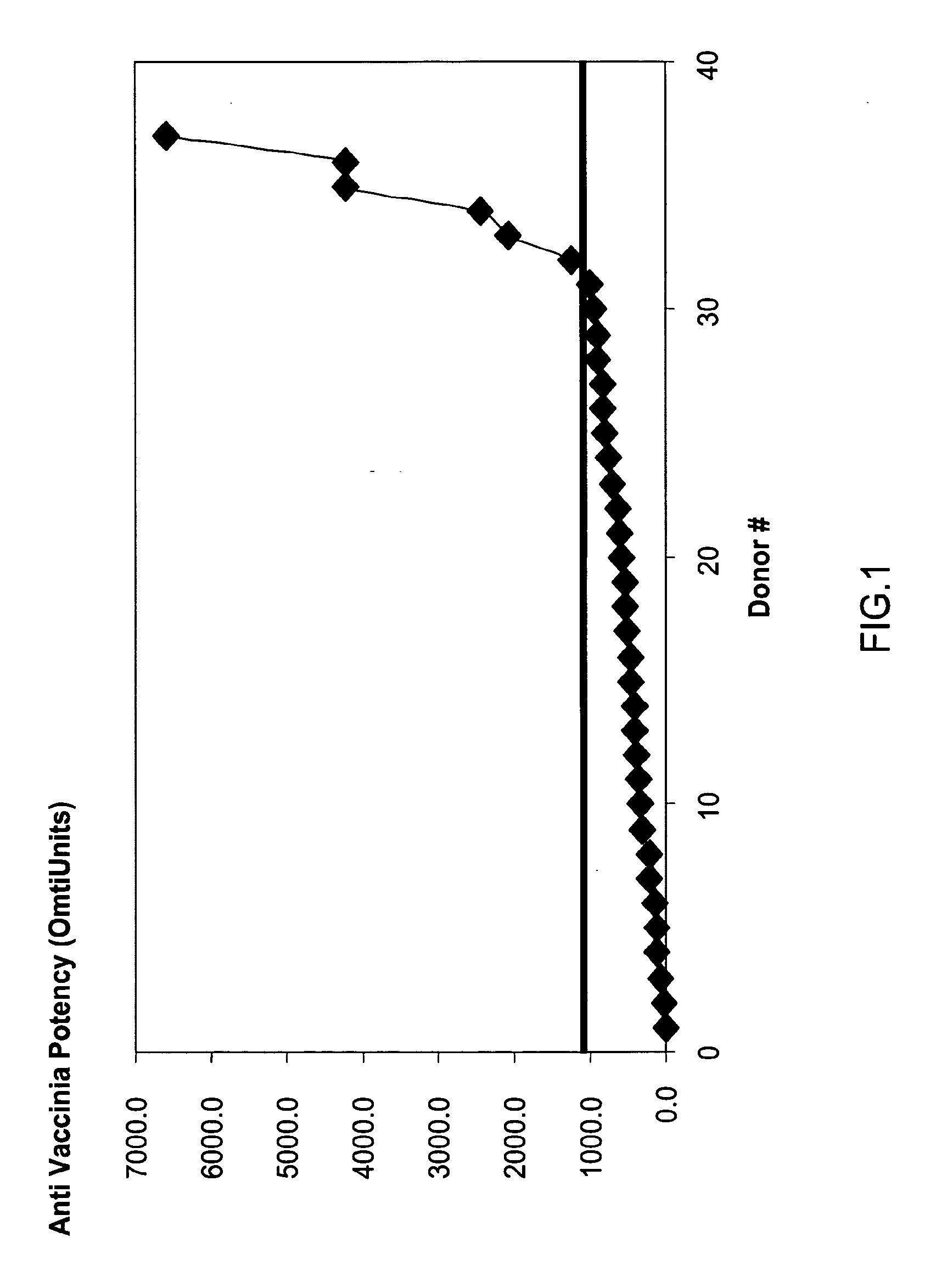
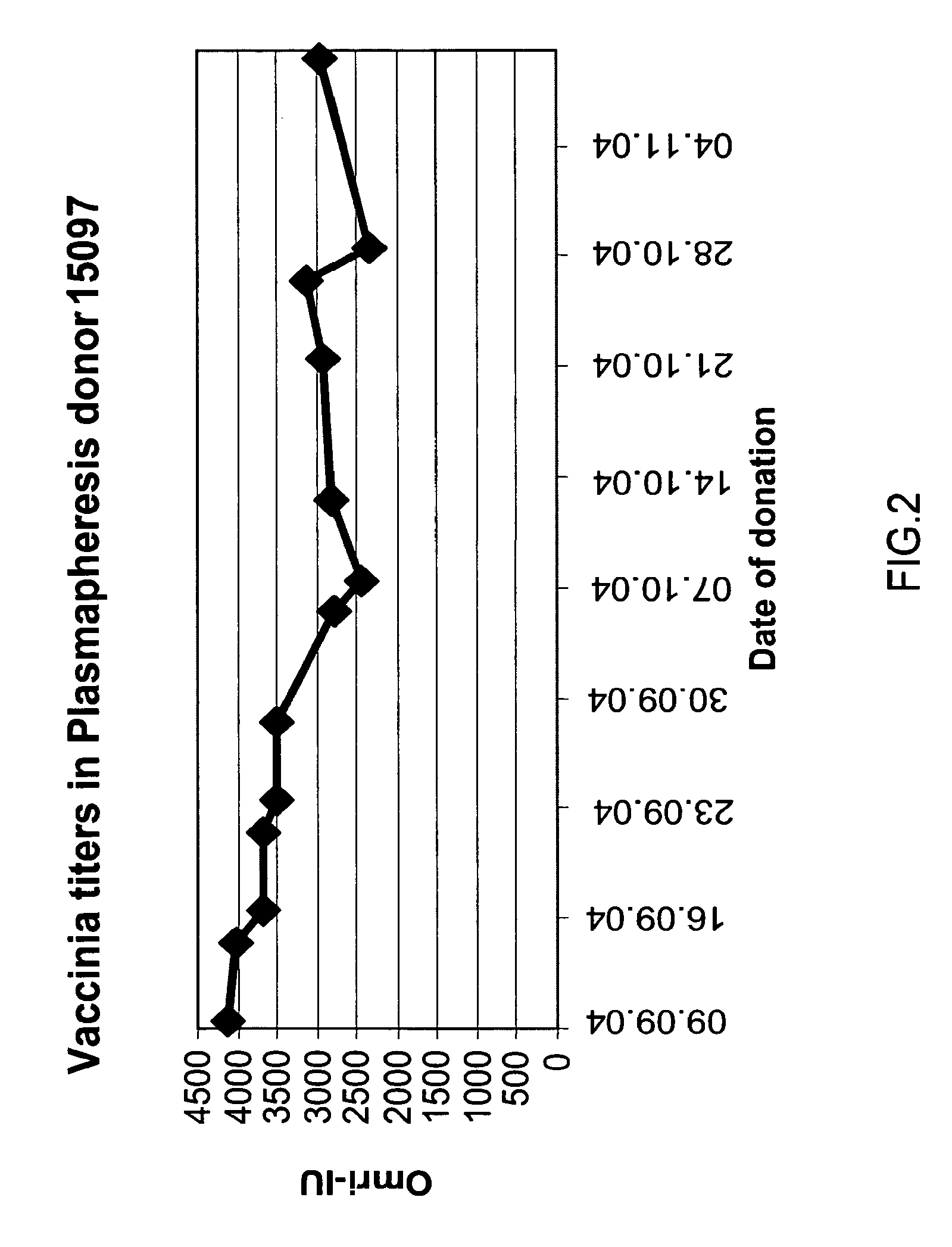
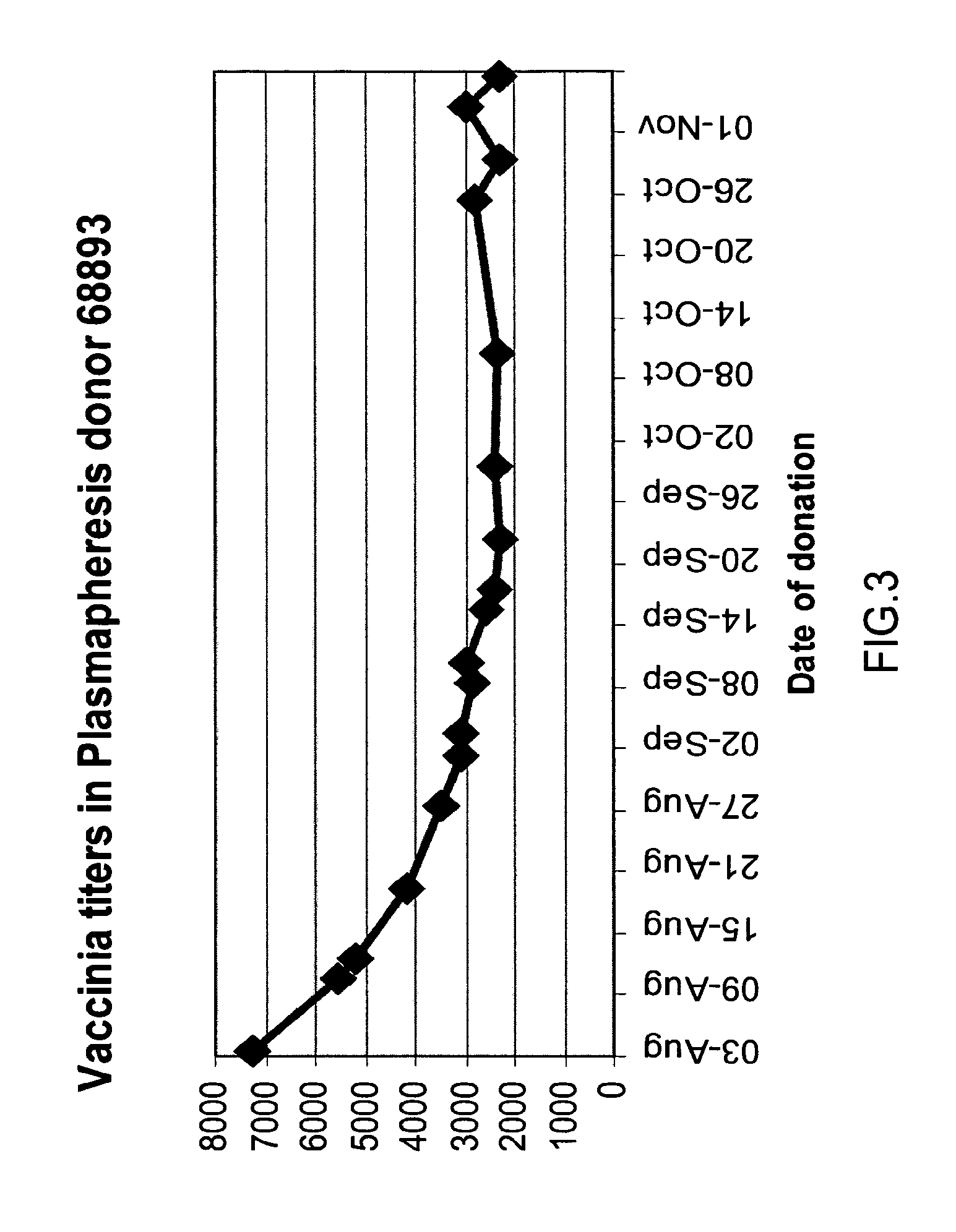
ActiveUS20070037170A1High potencyLow volumePeptide/protein ingredientsAntibody mimetics/scaffoldsPopulationSmallpox virus
A method for preparing a concentrated, immunoglobulin composition for treating subjects vaccinated against or infected with a pathogenic microorganism, comprising: (a) selecting a population of individuals previously vaccinated against one or more antigens associated with the pathogenic microorganism; (b) determining the level of specific antibodies immunoreactive with the pathogenic microorganism in a blood or blood component of the individuals to identify very high titre individuals having a very high titre of the specific antibodies; (c) combining blood or blood components comprising immunoglobulins from the very high titre individuals; and (d) purifying and / or concentrating the product of step (c), thereby obtaining a concentrated immunoglobulin composition. Also disclosed is a concentrated immunoglobulin composition comprising specific antibodies immunoreactive with a pathogenic microorganism, characterized in that the titre of specific antibodies of the composition is at least 5 times higher than the average titre of specific antibodies of a population of individuals previously vaccinated against one or more antigens associated with the pathogenic microorganism. The composition has a relatively high protein concentration and a low percentage of protein aggregates, and is therefore suitable for both iv and im administration. In a preferred embodiment, the pathogenic microorganism is smallpox virus or vaccinia virus.
Owner:OMRIX BIOPHARM
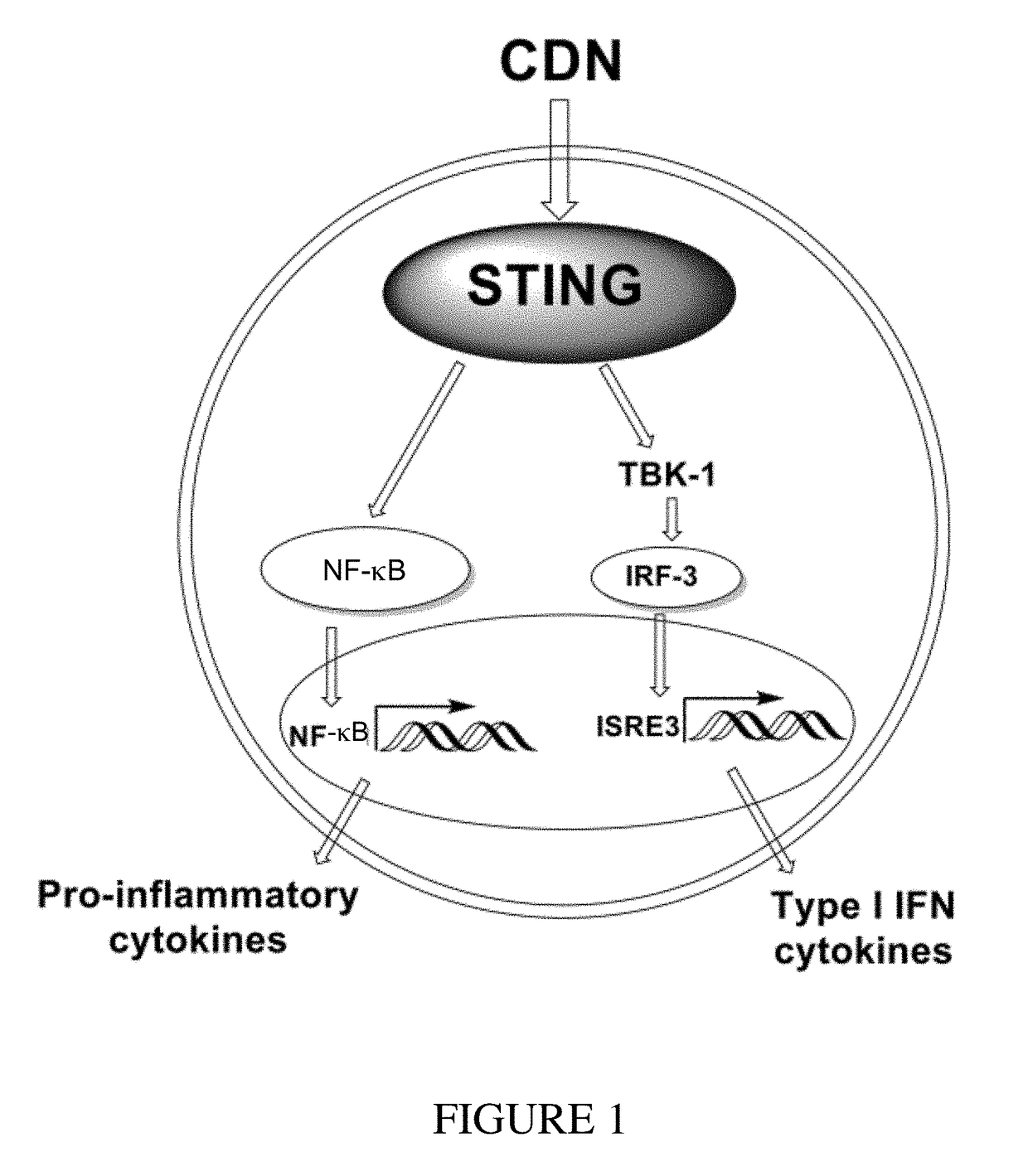
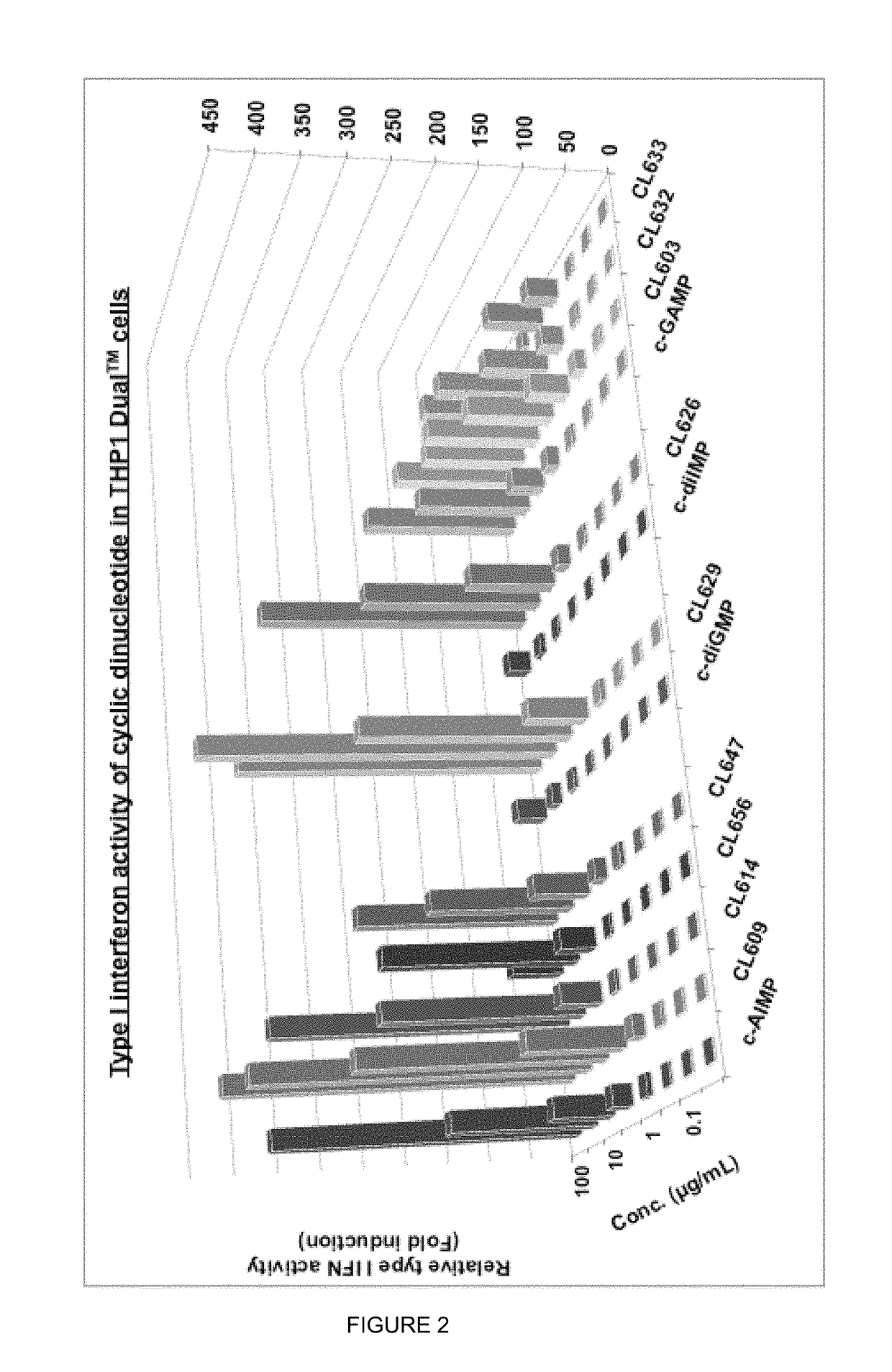
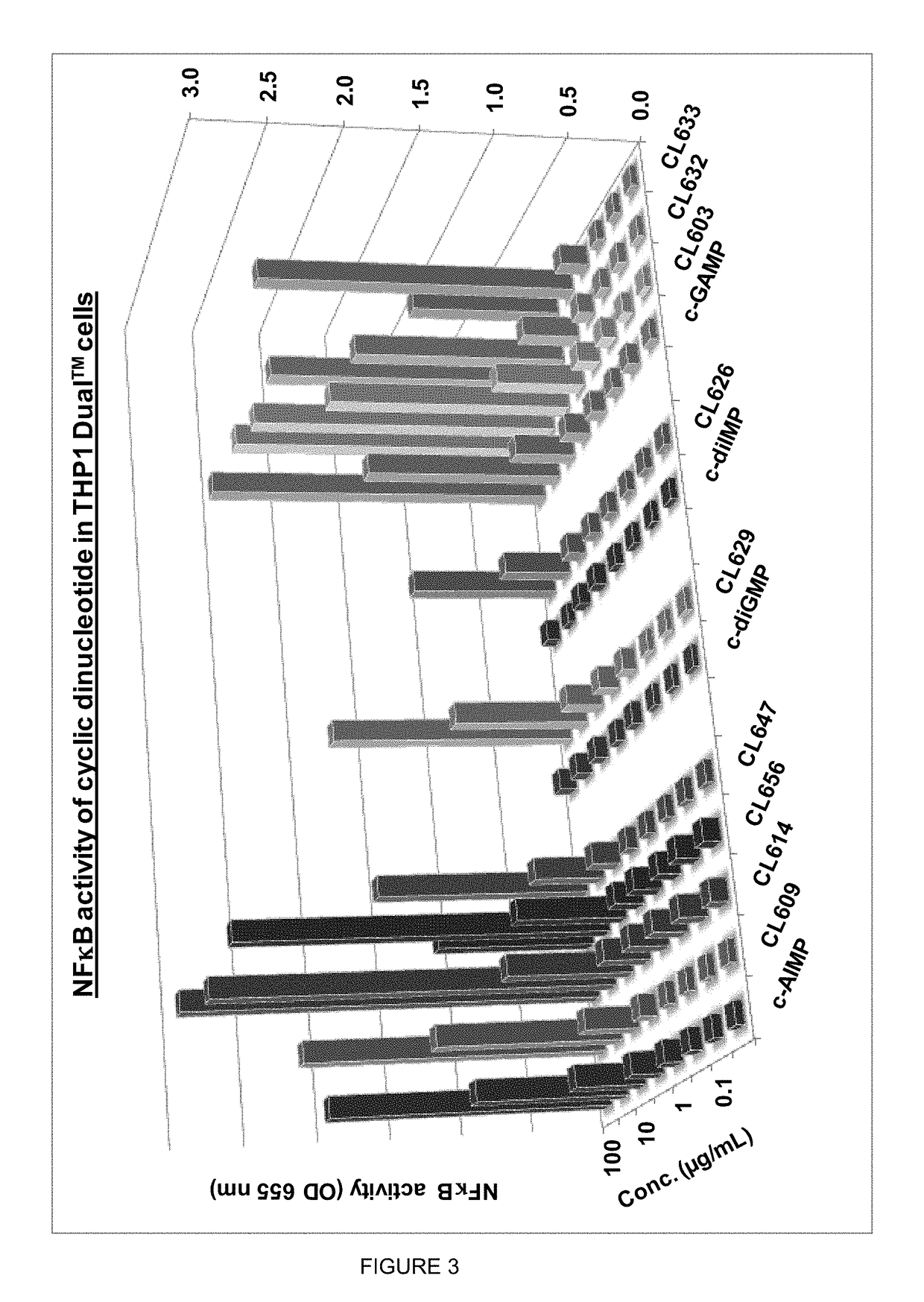
Cyclic dinucleotides for cytokine induction
ActiveUS10011630B2High activityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsPurineIn vivo
A cyclic dinucleotide compound of Formula (I):wherein X1 is H or F; X2 is H or F; at least one among X1 and X2 is a fluorine atom; Z is OH, OR1, SH or SR1, wherein: R1 is Na or NH4, or R1 is an enzyme-labile group which provides OH or SH in vivo such as pivaloyloxymethyl; B1 and B2 are bases chosen from Adenine, Hypoxanthine or Guanine, and B1 is a different base than B2 and a pharmaceutically acceptable salt thereof. Pharmaceutical compositions including the cyclic dinucleotide, as well as their use in the treatment of a bacterial infection, a viral infection or a cancer are also described.
Owner:KAYLA THERAPEUTICS
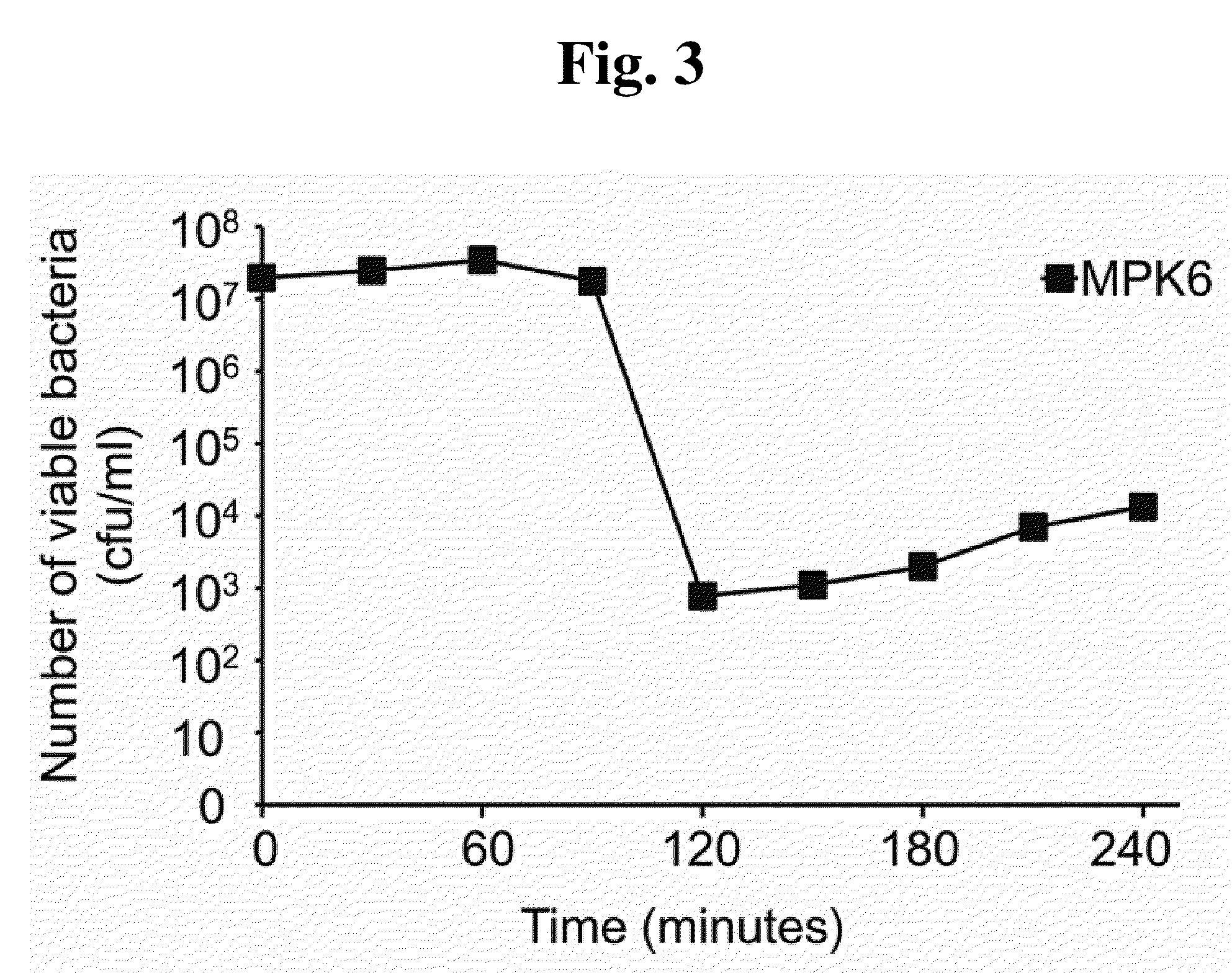
Phage therapy against Pseudomonas aeruginosa
This invention relates to a bacteriophage MPK6 (deposit number: KCCM 11044P) having a lytic activity to Pseudomonas aeruginosa, or a progeny bacteriophage thereof having a RFLP (Restriction fragment length polymorphism) DNA profile substantially equivalent to the bacteriophage MPK6. The present invention provides a bacteriophage MPK6 or a progeny bacteriophage thereof capable of treating a Pseudomonas aeruginosa infection disease, and suggests an anti-bacterial activity of MPK6 and its progeny bacteriophage using a mammalian and non-mammalian infection model. According to the present invention, the present bacteriophage MPK6 or progeny bacteriophage thereof represents very effective efficacy on treatment of P. aeruginosa-induced peritonitis-sepsis.
Owner:IND UNIV COOP FOUND SOGANG UNIV
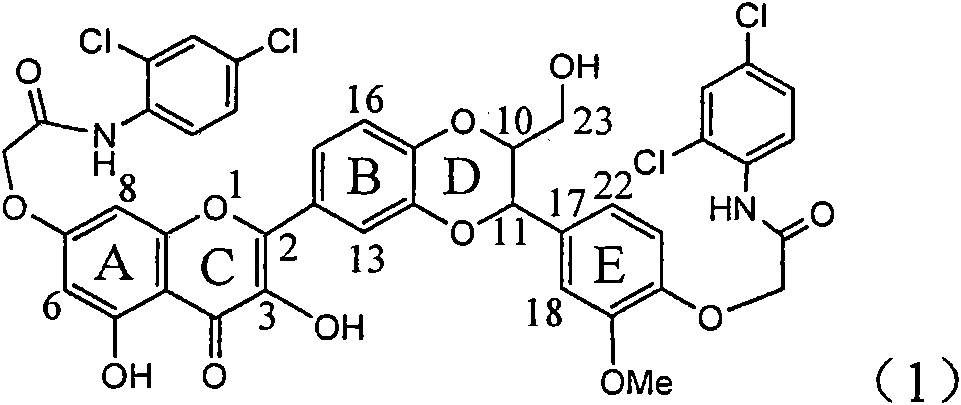
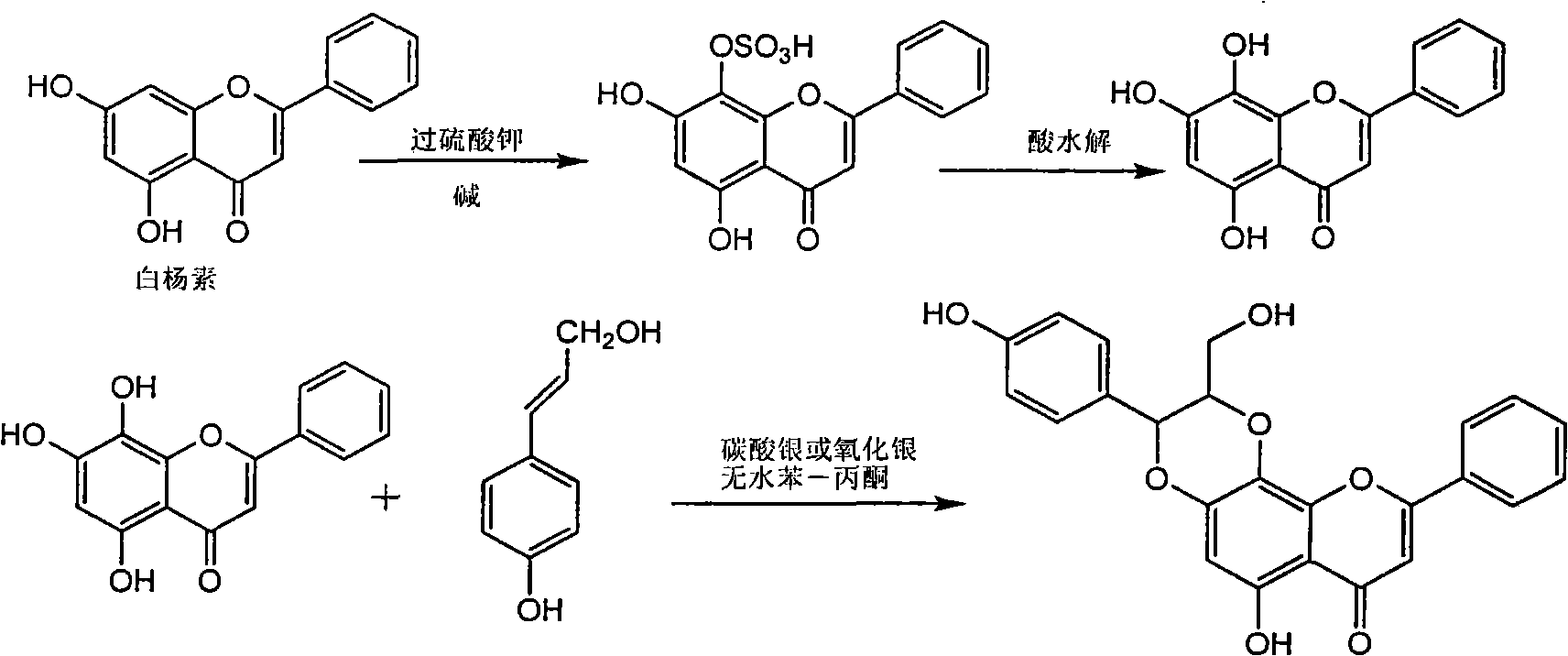
Application of diamine formyl dehydrogenated silybin serving as medicament for curing viral hepatitis B
InactiveCN101829090AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of diamine formyl dehydrogenated silybin serving as a medicament for curing viral hepatitis B, in particular to application of a flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or pharmaceutically acceptable salts thereof in preparation of a medicament for clearing HBsAg and HBeAg and a medicament for inhibiting HBV DNA replication. The flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents has extremely high HBsAg and HBeAg inhibiting activities; when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at a concentration of 20 mu g / ml, the inhibition rates of the HBsAg and the HBeAg are respectively 94.4 percent and 95.7 percent which exceed 5.9 times and 5.7 times those of a positive control alpha-interferon; and simultaneously the inhibition rate of the HBV DNA is 99.7 percent when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at the same concentration, and the inhibition activity of the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is higher than that of lamivudine and the alpha-interferon. In summary, the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or the pharmaceutically acceptable salts thereof can be expected for preparing non-nucleoside medicaments for clearing the HBsAg and the HBeAg, inhibiting the HBV DNA replication, and curing the hepatitis B virus infection diseases.
Owner:DALI UNIV
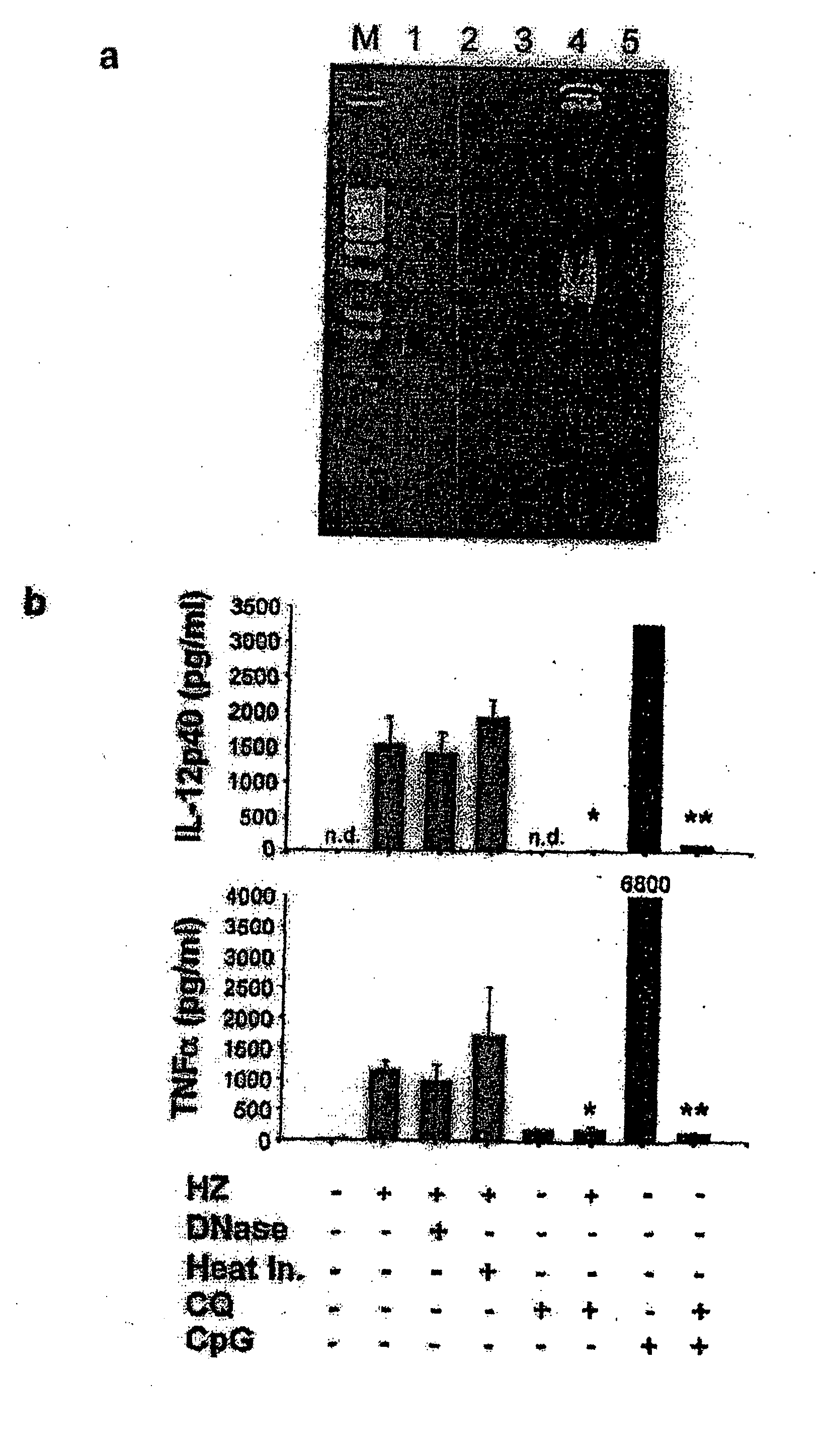
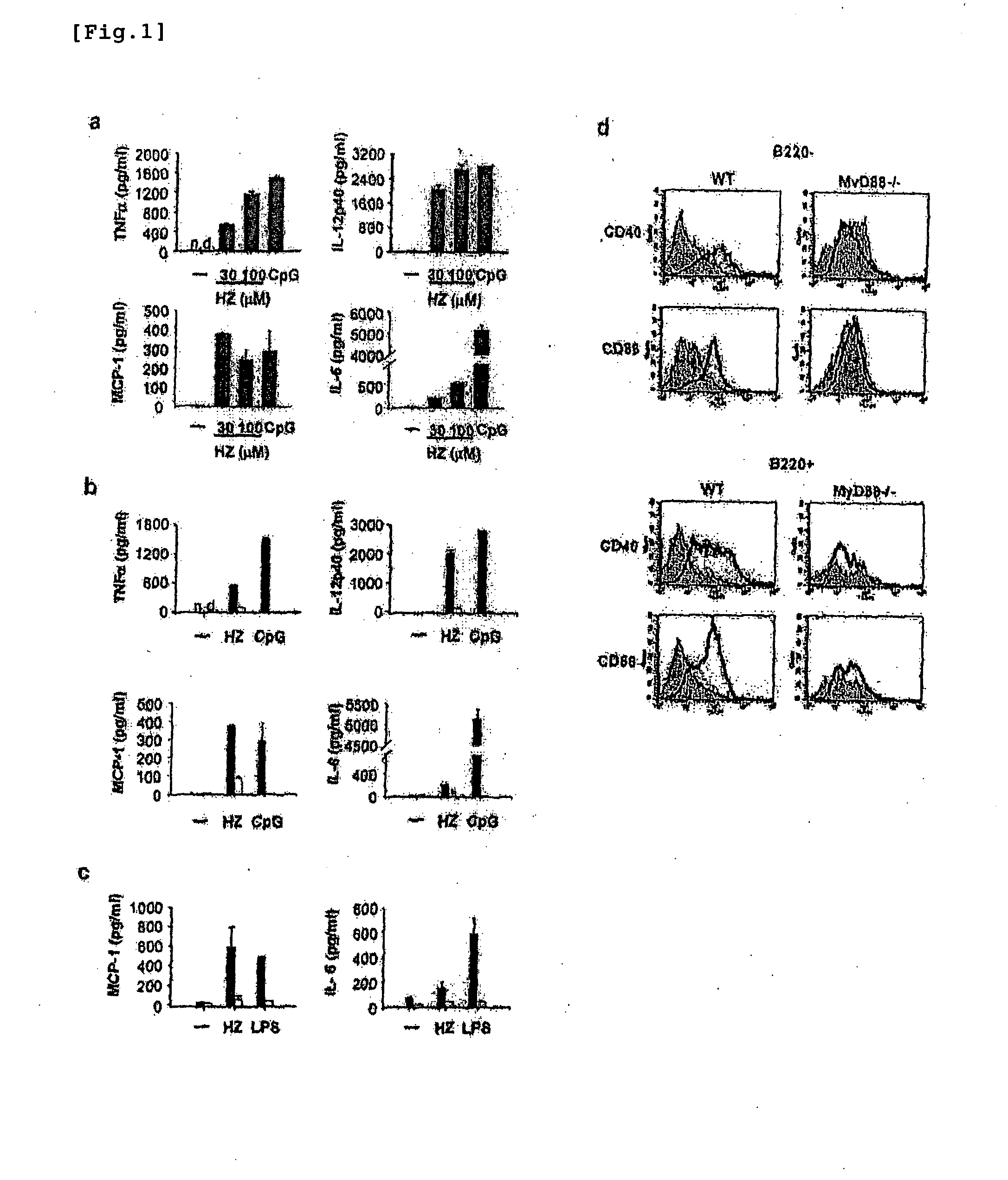
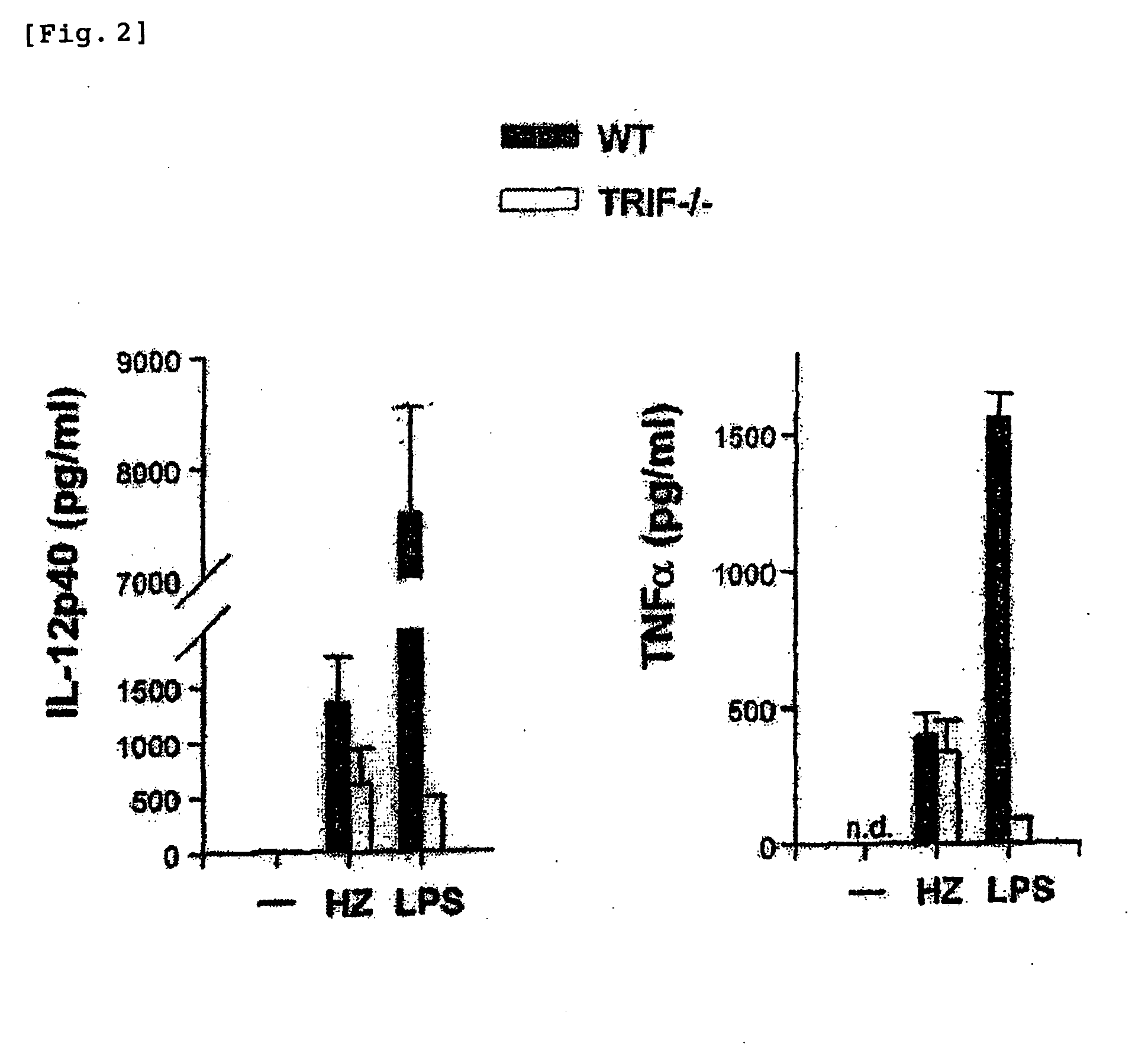
Detection/Measurement Of Malaria Infection Disease Utilizing Natural Immunity By Hemozoin Induction, Screening Of Preventative Or Therapeutic Medicine For Malaria Infection Disease, And Regulation Of Natural Immunity Induction
The instant invention is to provide a method for detecting and measuring malaria infection utilizing the induction by hemozoin (HZ); a method for screening a vaccine for malaria infection and a preventative or therapeutic agent for malaria infection using the method for detecting and measuring; and a means for regulating the induction of innate immunity using the HZ, synthetic HZ, or derivatives thereof as an adjuvant or immunostimulant. Malaria infection is detected and measured of by detecting and measuring HZ-induced, TLR9-mediated, and MyD88-dependent innate immune activity. The detection and measurement of malaria infection can be used to diagnose malaria infection. The method for detecting and measuring is also used for screening a vaccine for malaria infection and a preventative or therapeutic agent for malaria infection. Further, HZ, synthetic HZ, or derivatives thereof are used as an adjuvant or immunostimulant to regulate HZ-induced innate immune induction.
Owner:OSAKA UNIV
Application of aromatic carbamoyl dehydro-silibinin as medicament for treating viral hepatitis B
InactiveCN101829086AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of aromatic carbamoyl dehydro-silibinin as a medicament for treating viral hepatitis B, in particular to application of todehydro-silibinin flavonolignans with a ring A and a ring E which are substituted by double base aromatic carbamoyl methoxyl and pharmaceutically acceptable salt thereof for preparing medicaments for removing HBsAg and HBeAg and medicaments for inhibiting HBV DNA. The todehydro-silibinin flavonolignans has extremely obvious activity on inhibiting the HBsAG and the HBeAg, has the intensity of 46.2 percent and 68.9 percent for respectively removing the HBsAG and the HBeAg in the presence of the concentration of 100 microgram / milliliter, which is 2.9 times and 4.1 times higher than that of positive control medicament alpha-interferon, and has the inhibition ratio of 96 percent on HBV DNA in the presence of the concentration of 100 microgram / milliliter, which is higher than that of lamivudine and the alpha-interferon. Accordingly, the flavonolignans and the pharmaceutically acceptable salt thereof can be expected to be used for preparing non-nucleoside medicaments applied for removing HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
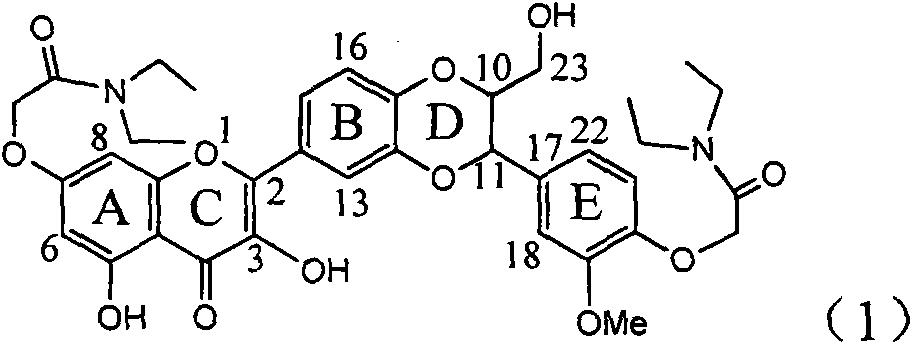
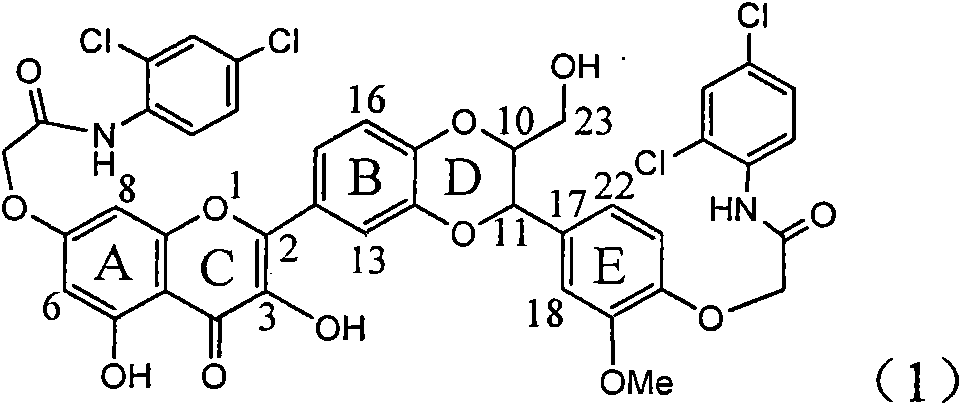
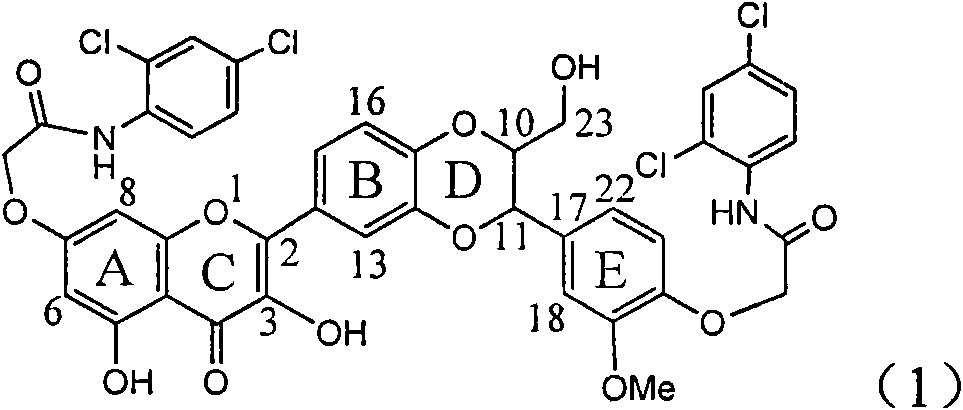
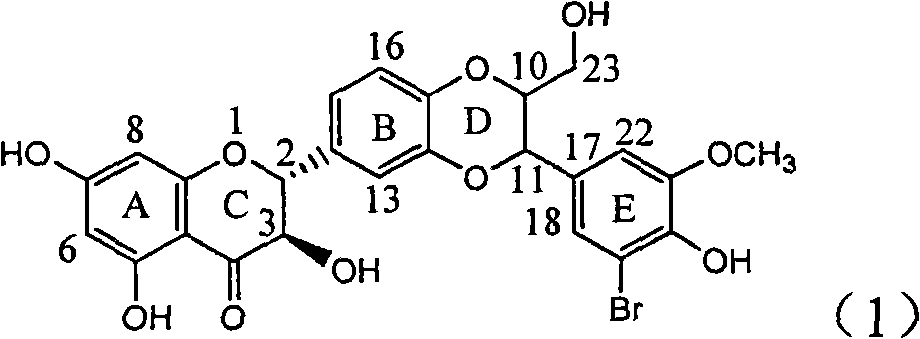
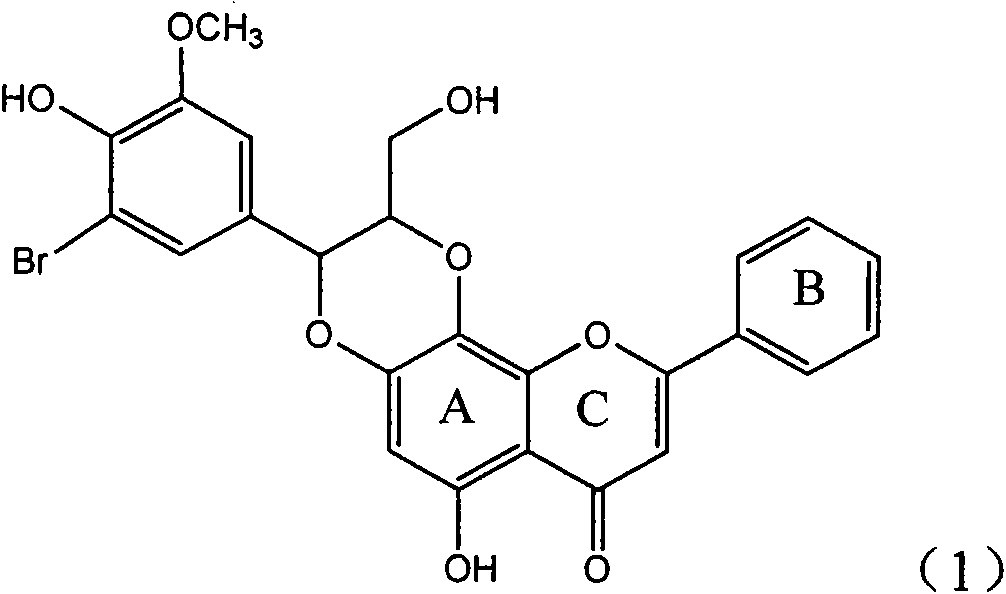
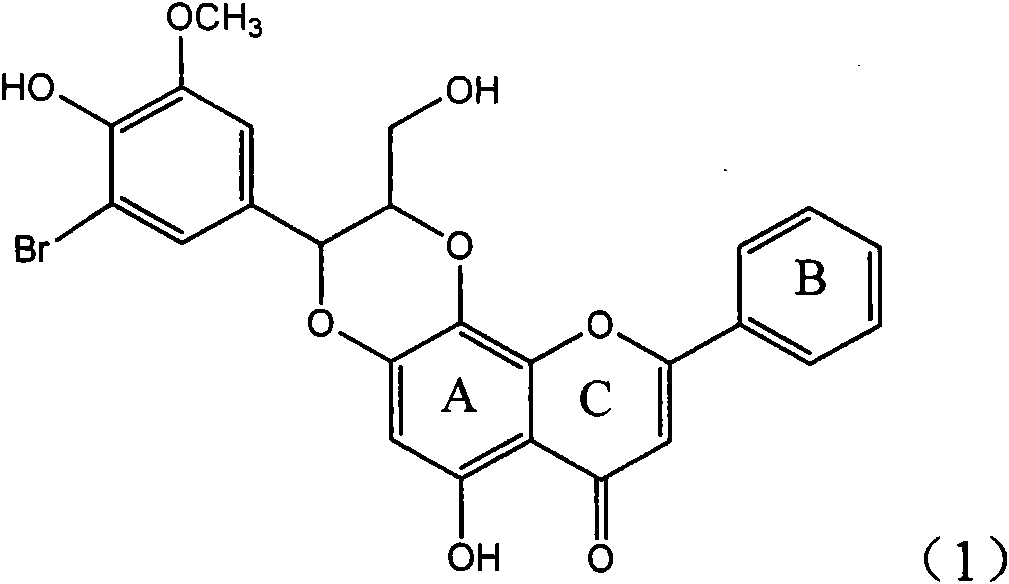
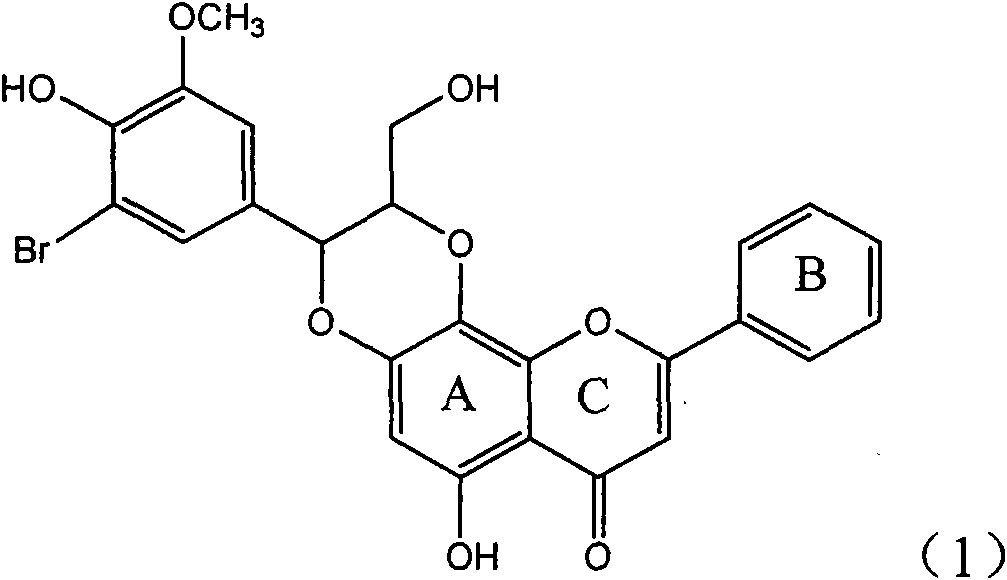
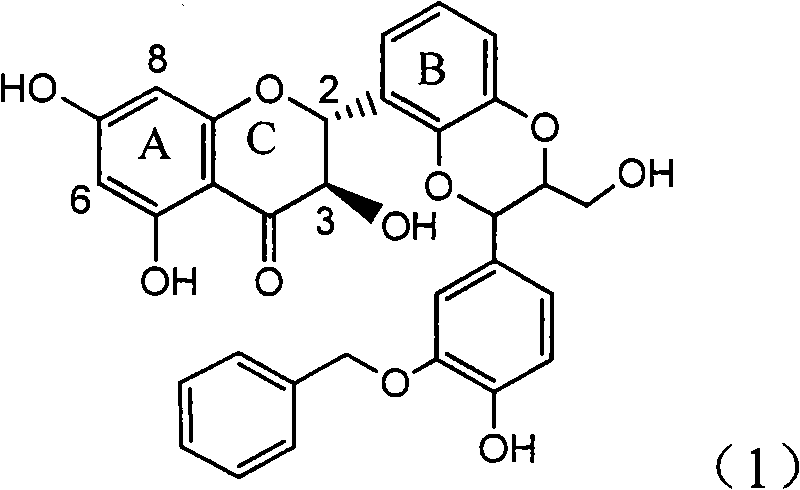
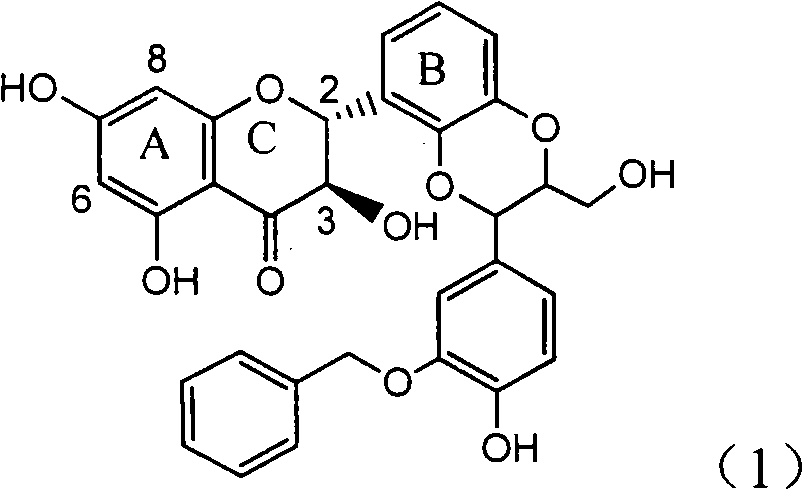
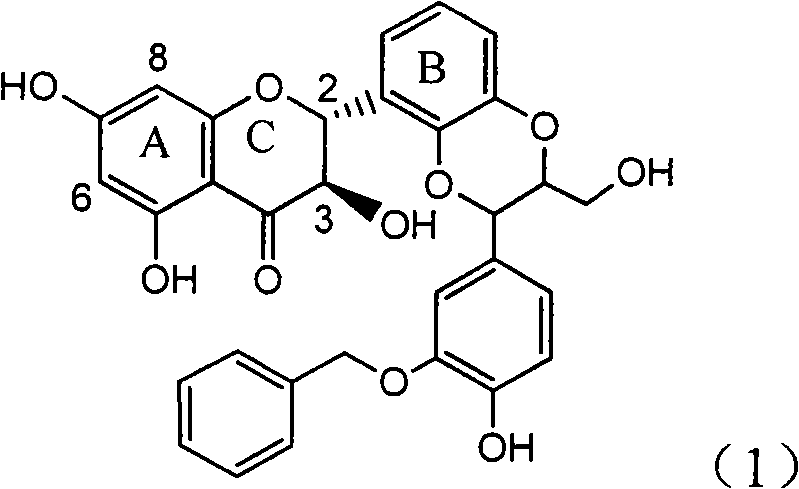
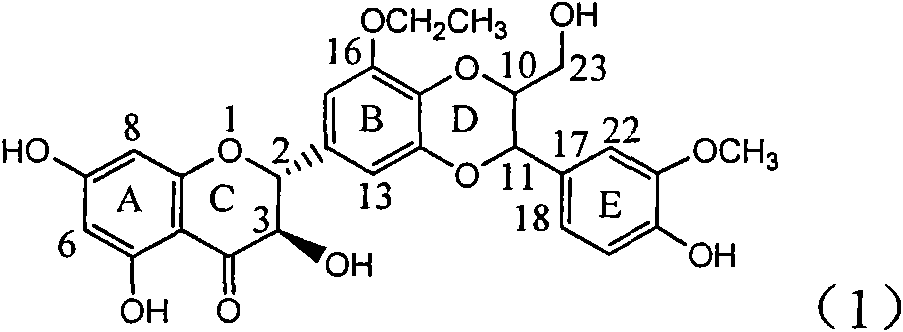
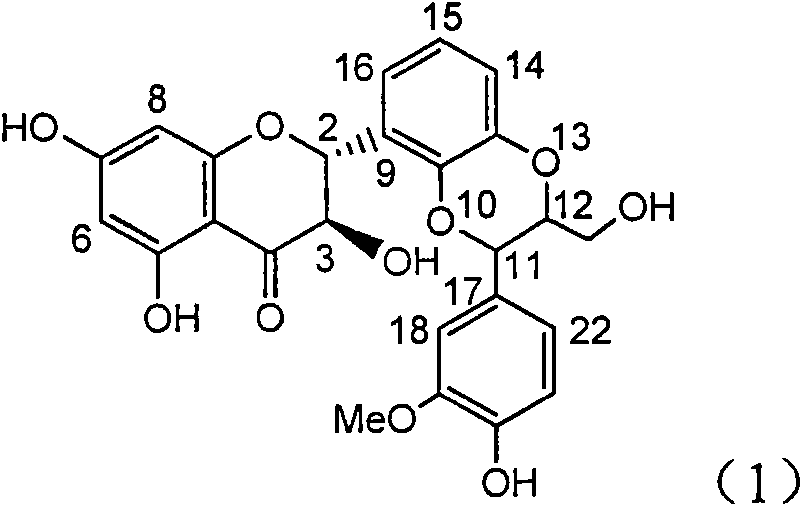
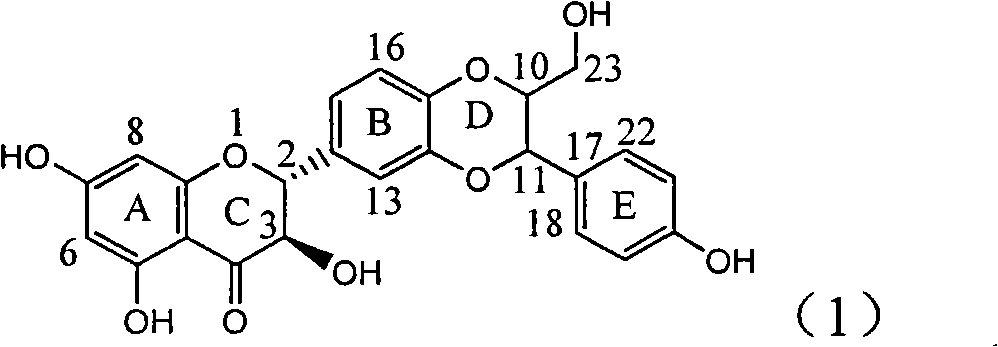
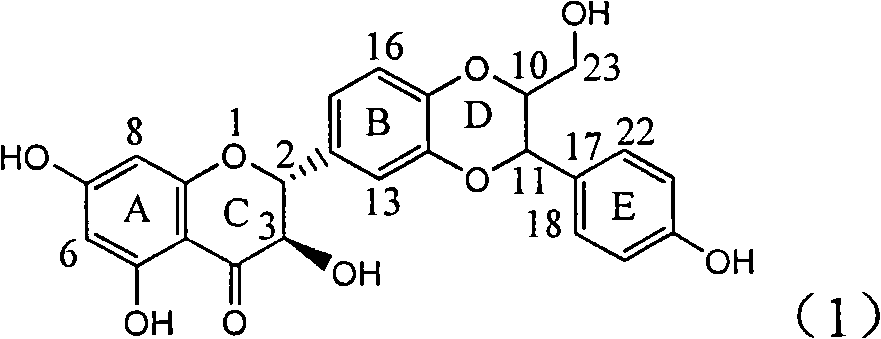
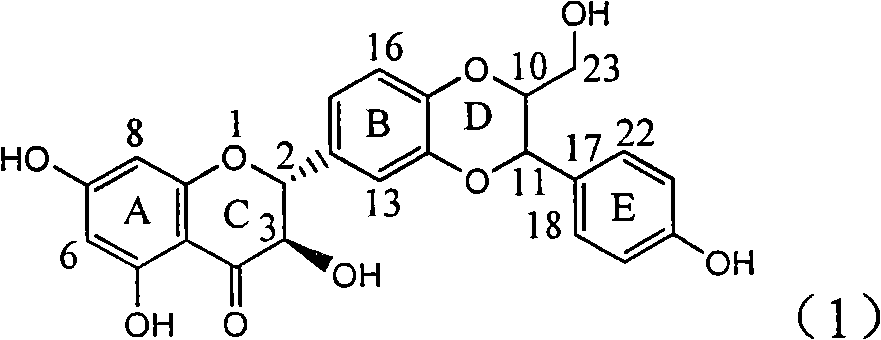
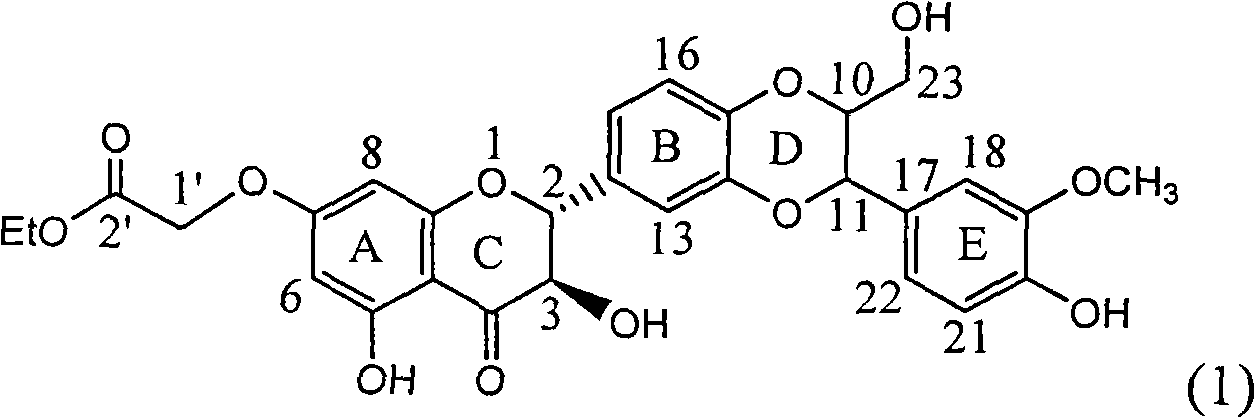
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
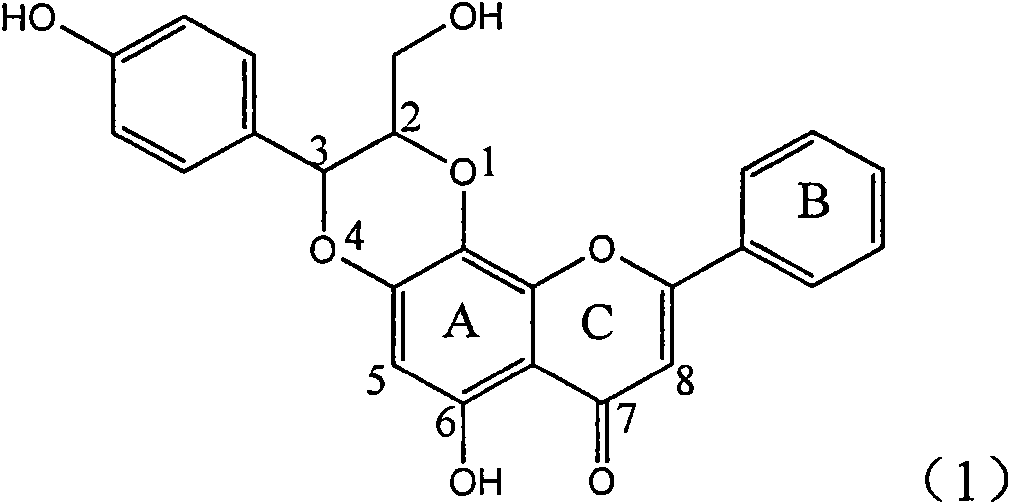
Application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B
InactiveCN101829104AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of the formula (1) or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The intensities of the flavonolignan for clearing away the HBsAg and the HBeAg are respectively 29.4 percent and 29.1 percent in the presence of a concentration of 20 micrograms / milliliter, which is respectively 1.8 times and 1.7 times of the corresponding activity of a positive control medicament (10,000 units / milliliter of alpha-interferon). What is even more exciting is that in the presence of the concentration, the suppression rate of the flavonolignan to the HBV DNA is higher than 83 percent, which is higher than that of Lamivudine which is a positive control and is 2.2 times of that of the alpha-interferon to the HBV DNA. Accordingly, the flavonolignan and the pharmaceutically acceptable salt thereof are indicated to be capable of being expected to be used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
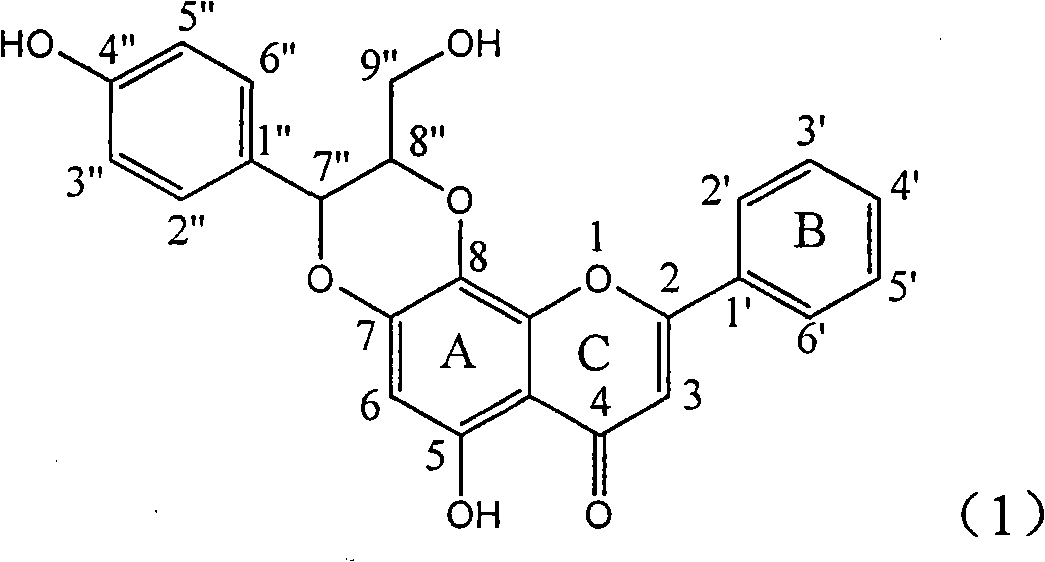
Use of lignanoid containing benzyloxy flavones in preparation of drugs for treating viral hepatitis B
InactiveCN101829095AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to a use of lignanoid containing benzyloxy flavones in the preparation of drugs for treating viral hepatitis B, in particular to the use of a compound as shown in formula (1) or pharmaceutical salts thereof in the preparation of the drugs for eliminating hepatitis B virus surface antigen and hepatitis B e antigen and the drugs for suppressing HBV DNA replication, and the strength of eliminating HBsAg of flavonol lignanoid under the concentration of 20 mu g / ml is 50.8%, which is 3.2 times of the corresponding activity of a positive control drug; the activity of eliminating the HBeAg under the same concentration is equivalent to 10000 units / ml of alpha-interferon; simultaneously, the flavonol lignanoid shows nearly 60% of suppression rate to HBV DNA under the concentration, which is 1.6 times of the corresponding suppression rate of the alpha-interferon. The results show that the lignanoid containing the flavones or the pharmaceutical salts thereof are expected to be used for preparing the non-nucleoside drugs for eliminating the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Intravenous immunoglobulin composition
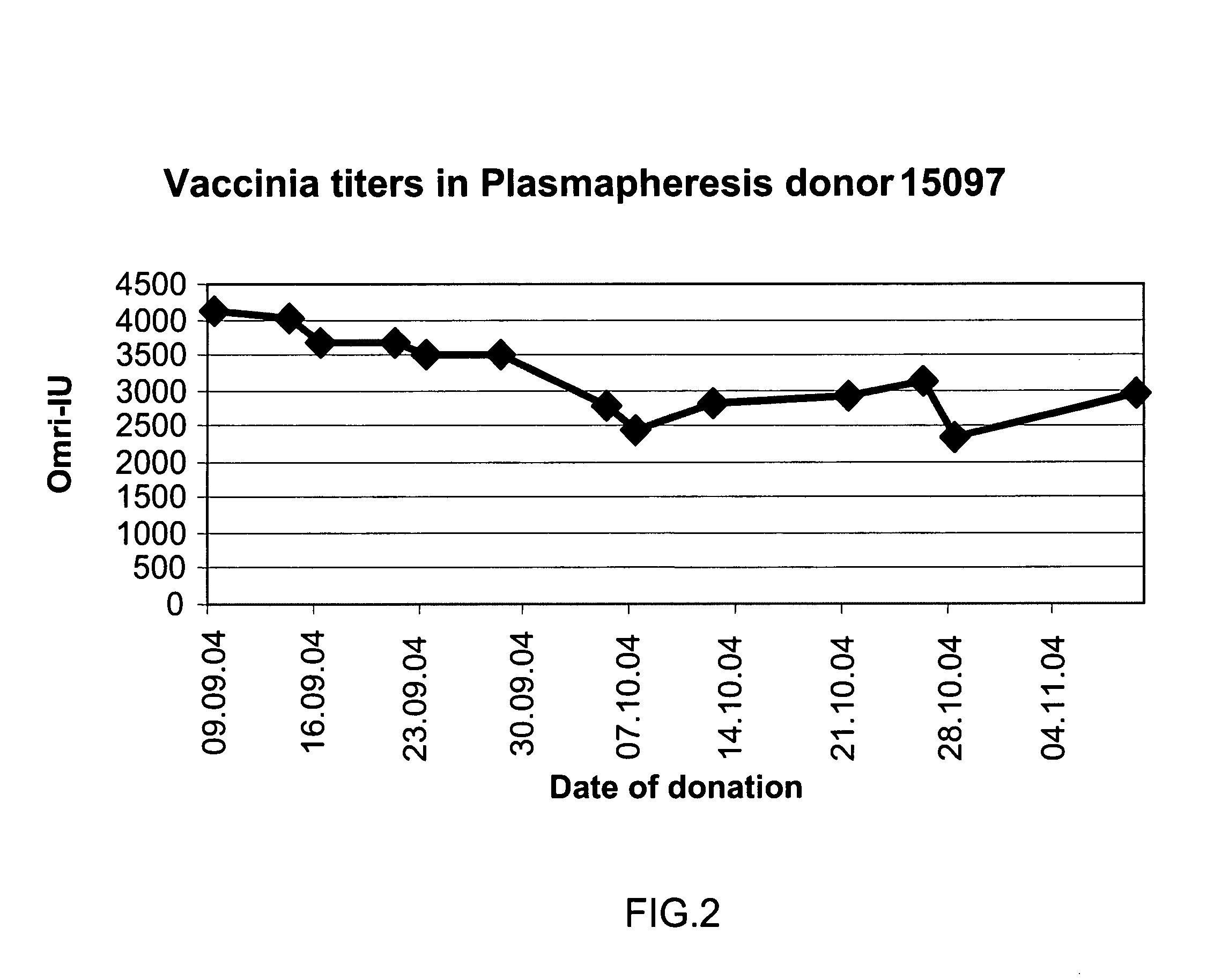
ActiveUS20130011388A1Reduce riskEasy to managePeptide/protein ingredientsAntibody mimetics/scaffoldsSpecific antibodyHigh titre
A concentrated, immunoglobulin composition for treating subjects vaccinated against or infected with a pathogenic microorganism, is made by (a) selecting a population of individuals previously vaccinated against antigens associated with the pathogenic microorganism; (b) identifying very high titre individuals by determining the level of specific antibodies immunoreactive with the pathogenic microorganism in the blood of the individuals; (c) combining blood from the very high titre individuals; and (d) purifying and / or concentrating the product of step (c). A concentrated immunoglobulin composition can include specific antibodies immunoreactive with a pathogenic microorganism, wherein the titre of specific antibodies is at least 5 times higher than the average titre of specific antibodies of a population of individuals previously vaccinated against antigens associated with the pathogenic microorganism. The composition has a relatively high protein concentration and a low percentage of protein aggregates. The pathogenic microorganism is preferably smallpox virus or vaccinia virus.
Owner:OMRIX BIOPHARM
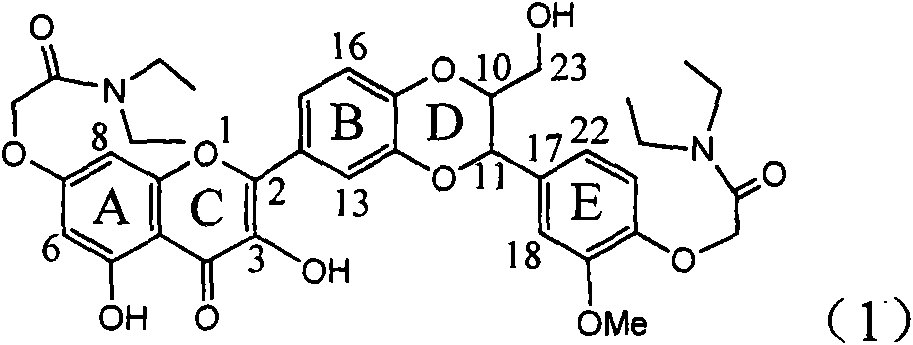
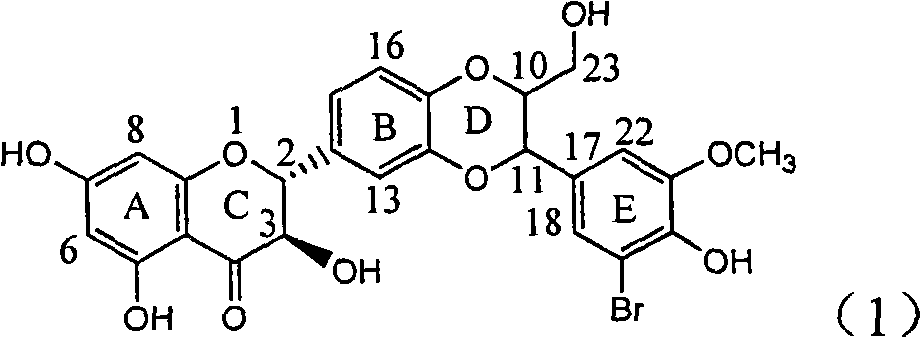
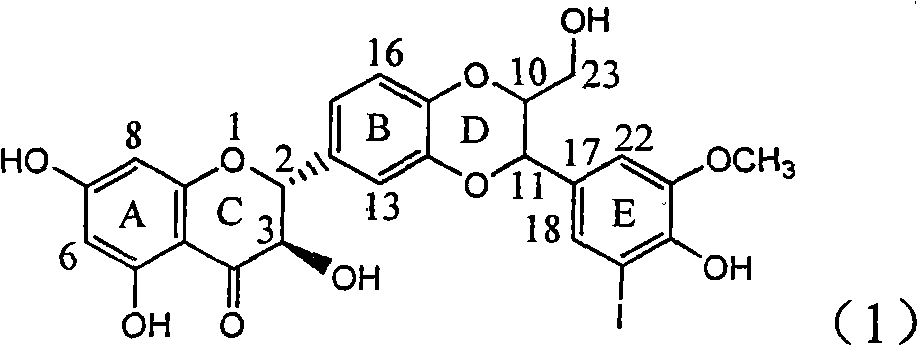
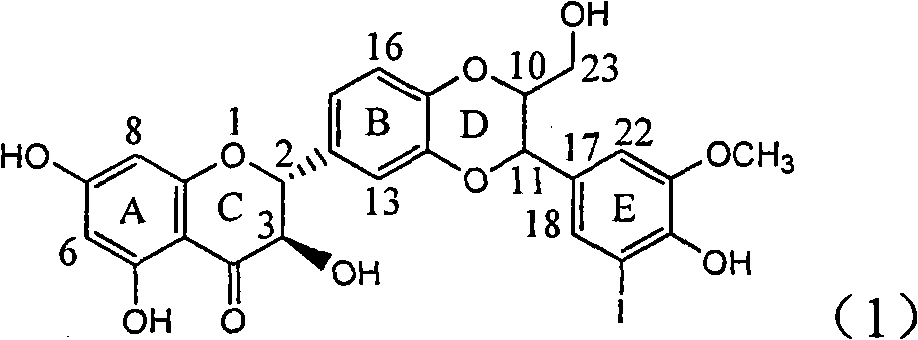
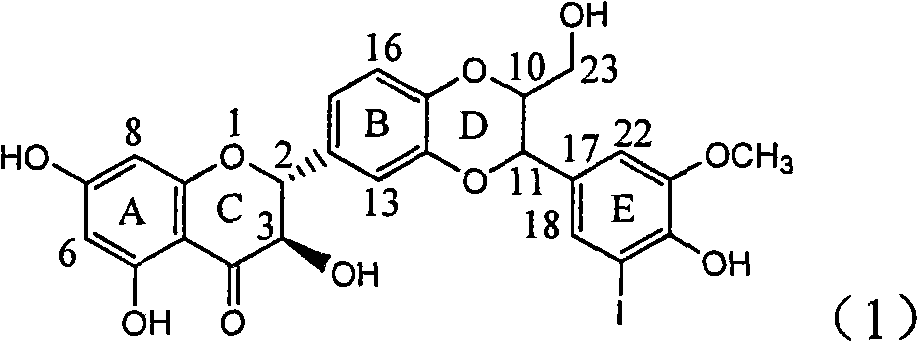
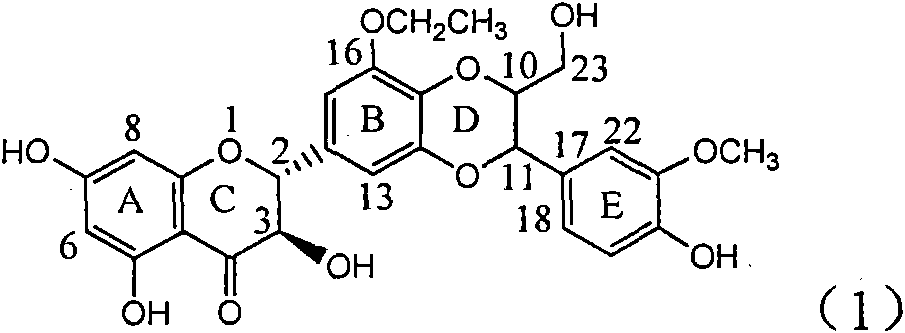
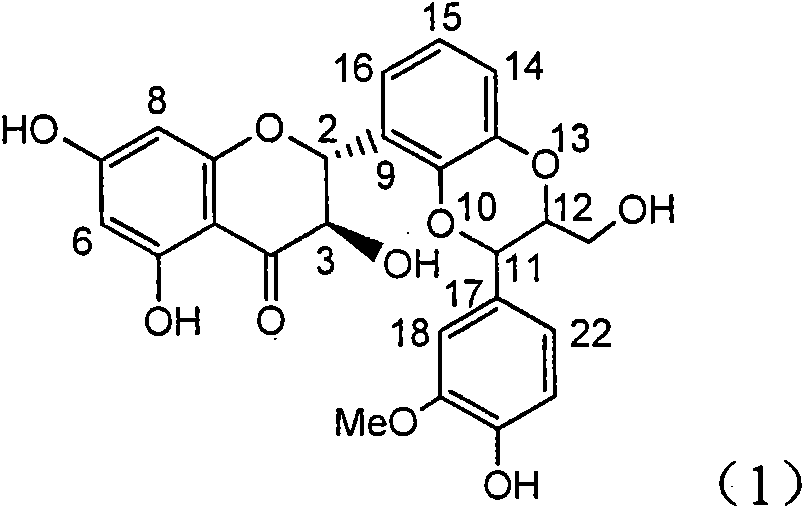
Application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829096AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity of suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 20.0 percent and 29.0 percent which exceed that of a positive control medicament (10,000 units / milliliter of alpha-interferon) by 24 percent and 72 percent. Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 32.6 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
Antiviral, antibacterial and antiinflammatory herbal injection for treating livestock and fowl infectious disease
A Chinese veterinary medicine in the form of injectino for treating infections diseases of livestock and fowls is prepared from 8 Chinese-medicinal materials including honeysuckle flower, forstyhia fruit, coptis root, phellodendron bark, etc through decocting in water and refining for removing impurities (tannin, protein, starch, resin, etc). Its advantages are high cure rate (more than 98%), no toxic by-effect, and no recurrence.
Owner:长春市动物药厂
Application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829089AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B, in particular to application of ring B ethyoxyl substituted silybin ester or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAG (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has strong activity on suppressing the HBsAG and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing the HBsAg and the HBeAg are respectively 64.6 percent and 44.8 percent which are 4.0 times and 2.7 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression rate of the compound on the HBV DNA is 58.1 percent which is 1.5 times of the corresponding activity of the alpha-interferon. Accordingly, the flavonolignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have strong efficacy on suppressing the HBsAg, the HBeAg and the HBV DNA and can be expected to be used for preparing non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
Application of angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B
InactiveCN101953828AInhibition of replicationConvenient sourceOrganic active ingredientsAntiviralsLignanInterferon alpha
The invention relates to application of an angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B, in particular to application of the angle flavonoids lignan or medicinal salts thereof to preparation of medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating HBV infection diseases. The flavonoids lignan can exactly inhibit HBV DNA activity; the inhibition activity of the flavonoids lignan with high dosage (20 microgram / ml) to the HBV DNA replication is 189 percent higher than that of alpha-interferon with the maximum concentration of 10,000 unit / ml; and the flavonoids lignan belongs to a strong-effect non-nucleosides inhibition HBV natural product. The pharmacological results show that the angle flavonoids lignan or the medicinal salts thereof can be expected to be used for preparing the medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
Application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV)
InactiveCN101829085AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV), in particular to application of ring A dioxane coupling type flavone lignan or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B e antigen (HBeAg), suppressing the HBV DNA replication and treating HBV infection diseases. The flavonolignan has certain activity on resisting the HBeAg, and the intensity of the flavonolignan for clearing away the HBeAg is higher than that of Lamivudine which is a positive control and close to that of 10,000 units / milliliter of alpha-interferon. Meanwhile, the suppression ratio of the compound to the HBV DNA replication is higher than 80 percent in the presence of a concentration of 100 micrograms / milliliter. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for clearing away the HBeAg, suppressing the HBV DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
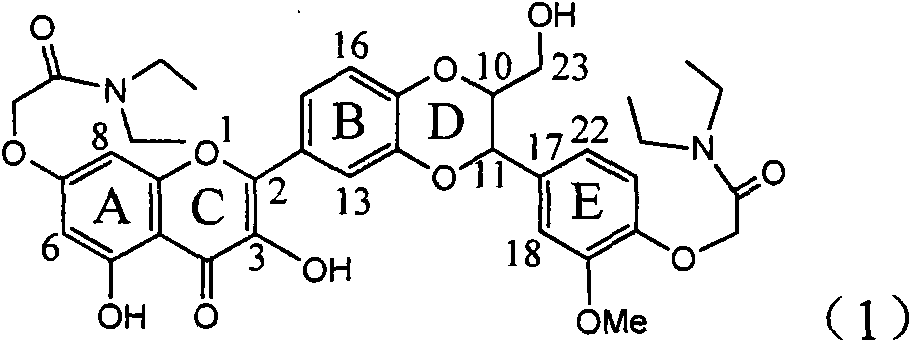
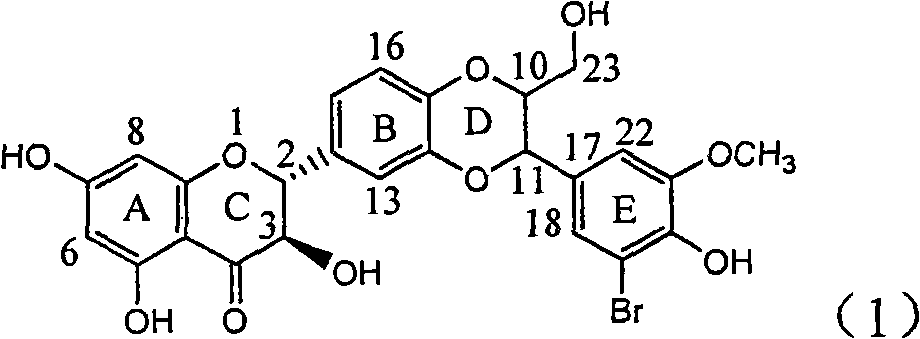
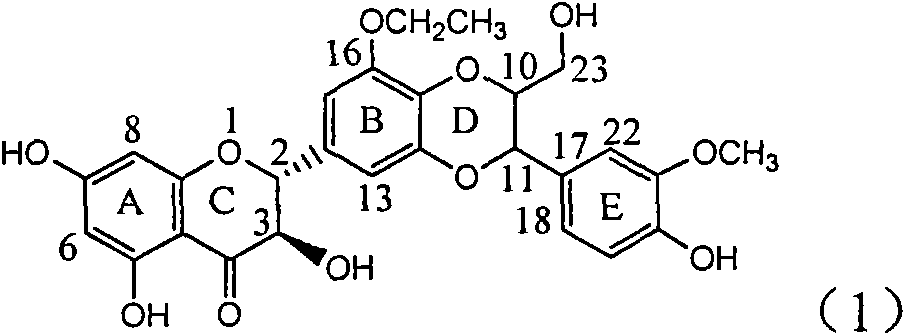
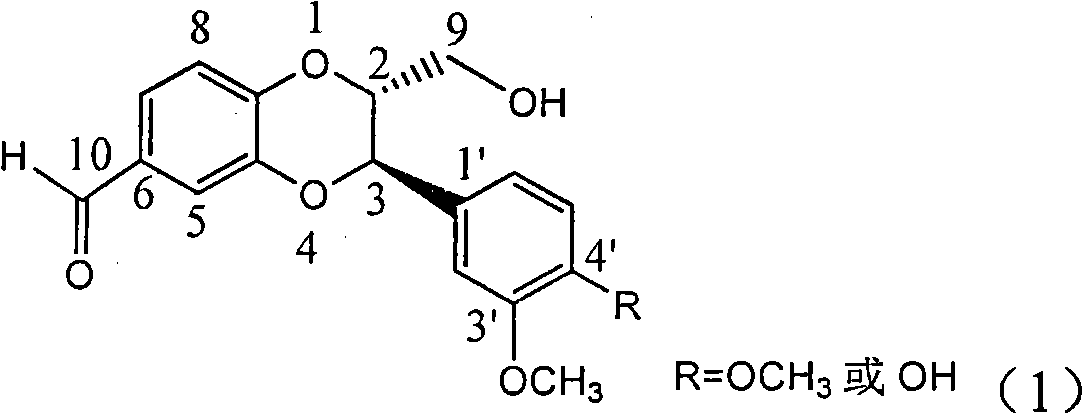
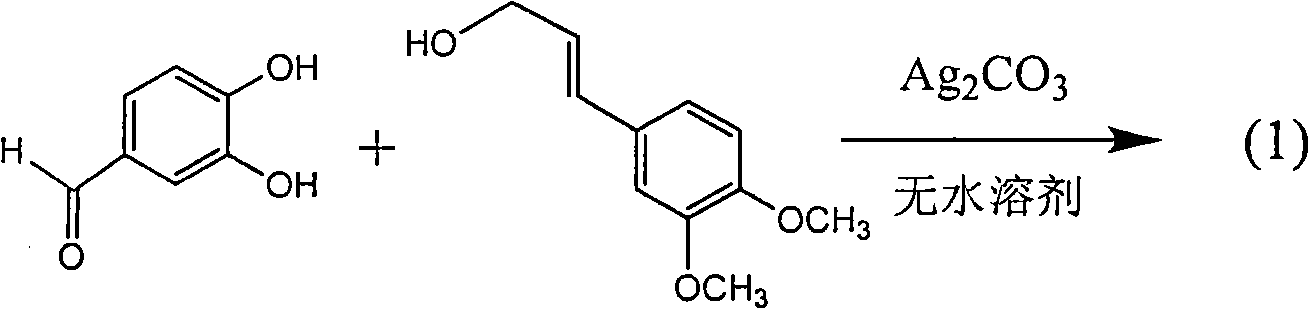
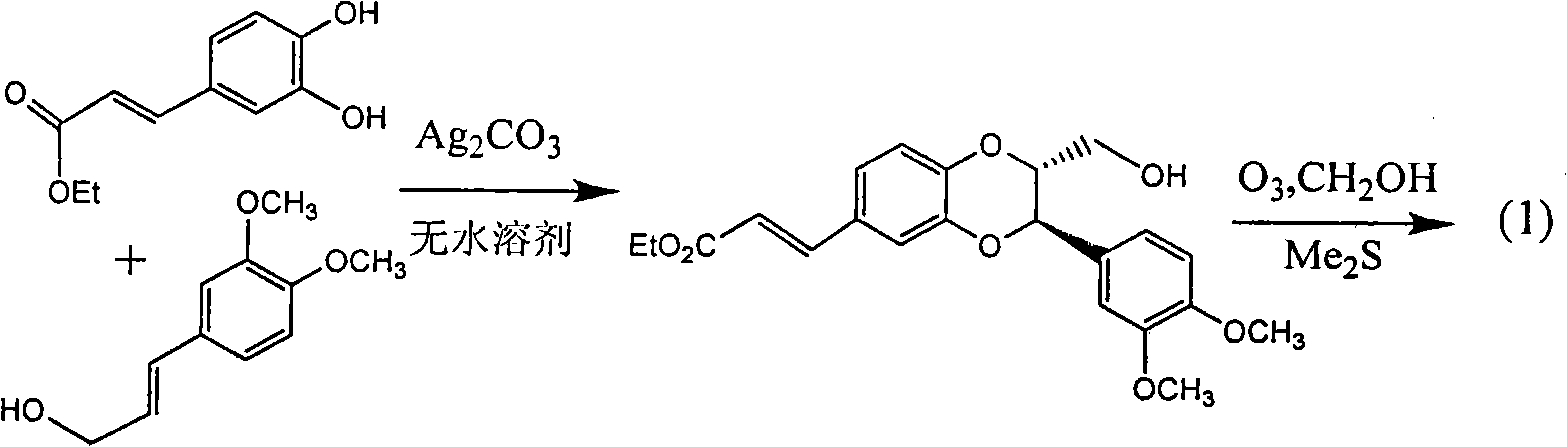
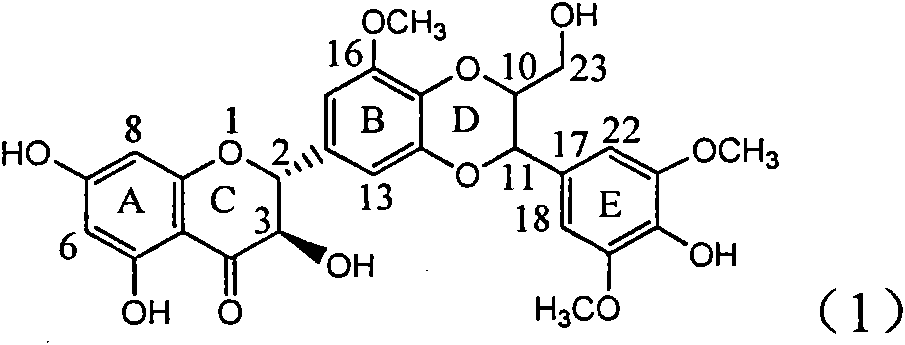
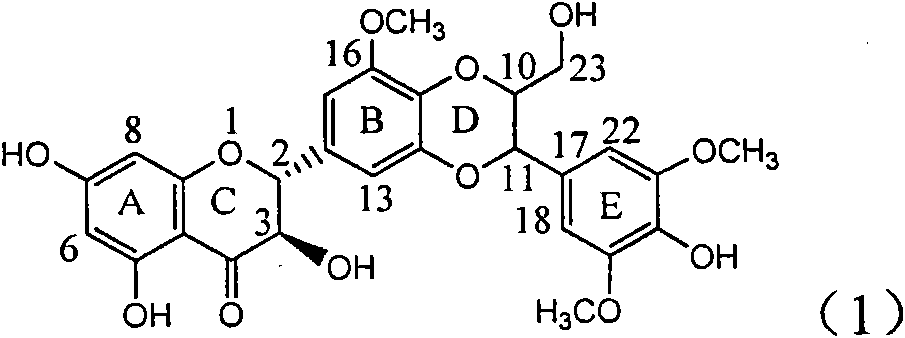
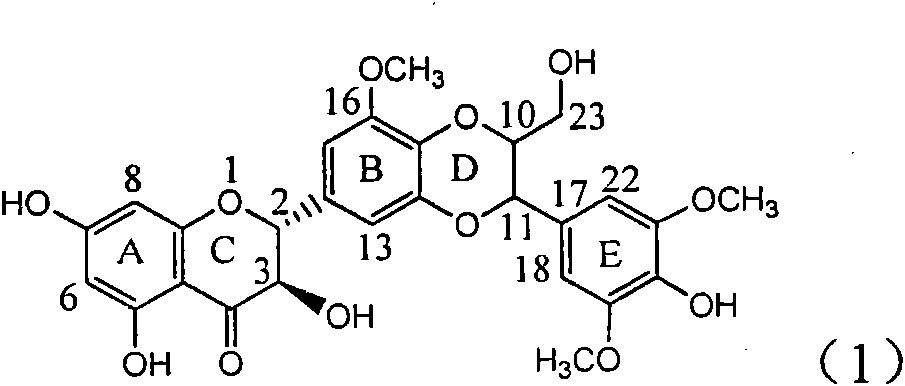
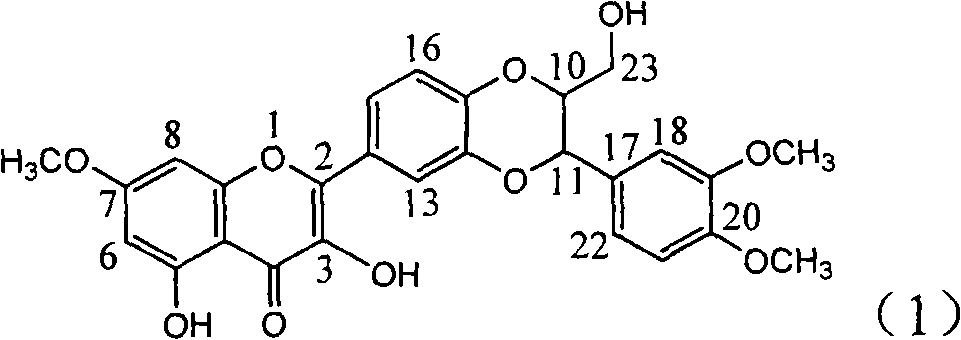
Application of E-ring demethoxy-silibinin for preparing medicament for treating viral hepatitis B
InactiveCN101912383AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of E-ring demethoxy-silibinin for preparing medicaments for treating viral hepatitis B, and particularly to application of compound in formula (1) and pharmaceutically acceptable salt thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV DNA replication. The invention has extremely superactive activity for suppressing the HBsAg and HBeAg; in the presence of the concentration of 20 microgram per millilitre, the intensities for clearing the HBsAg and HBeAg are 95.0% and 34.4% respectively, which are 5.9 and 2.0 times corresponding activity of a positive control medicament alpha-interferon; and it should be noticed that the suppression ratio of the medicament for HBV DNA at the concentration is about 91.5%, which is 13% higher than lamivudine and 2.4 times alpha-interferon suppression activity. In summary, the flavonolignans or pharmaceutically acceptable salt thereof can be prospectively used for preparing non-nucleoside medicaments for clearing the HBsAg and HBeAg, suppressing HBV DNA replication and treating hepatitis B virus infection disease.
Owner:DALI UNIV
Application of benzo-phenylpropanoids in preparing drug for treating viral hepatitis B
InactiveCN101829093AEnhanced inhibitory effectExact originalityOrganic active ingredientsAntiviralsHigh concentrationDisease
The invention relates to an application of benzo-phenylpropanoids in preparing drugs for treating viral hepatitis B, in particular to two benzo-phenylpropanoids or application of pharmaceutically acceptable salt thereof in preparing drugs for inhibiting the replication of hepatitis B virus desoxyribonucleic acid (HBV DNA) and treating hepatitis B virus infection diseases. The two benzo-phenylpropanoids definitely inhibit the activity of the HBV DNA, have the replication inhibition activity on the HBV DNA at high dose (100 microgrammes / milliliter) of 1.3-2.2 times higher than the inhibition activity at the highest concentration (10000 units / milliliter) of an alpha-interferon and belong to an efficient non-nucleoside natural product inhibiting the hepatitis B viruses; pharmacodynamics results show the application of the benzo-phenylpropanoids or the pharmaceutically acceptable salt thereof capable of preparing the drugs for inhibiting the replication of the HBV DNA and treating the hepatitis B virus infection diseases in anticipation.
Owner:DALI UNIV
Application of B/E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829088AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of B / E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester substituted by the methoxy on the ring B and the ring E or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The B / E bi-methoxy silybin has strong activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing away the HBsAg and the HBeAg are respectively 43.9 percent and 43.7 percent which are 2.7 times and 2.6 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression ratio on the HBV DNA is 68.6 percent, and the suppression activity is 1.8 times of that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have the effects of strongly suppressing the HBsAG, the HBeAg and the HBV DNA and can be expected to be used for preparing the non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
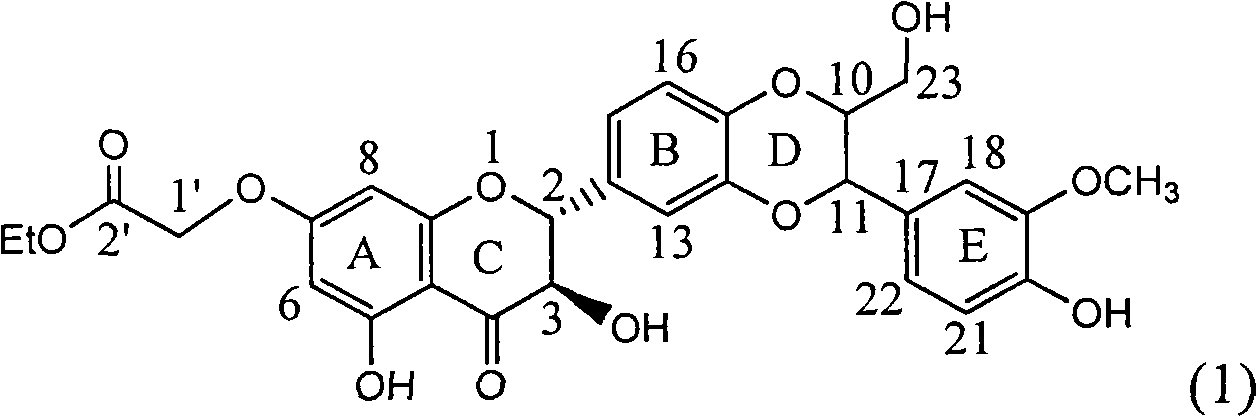
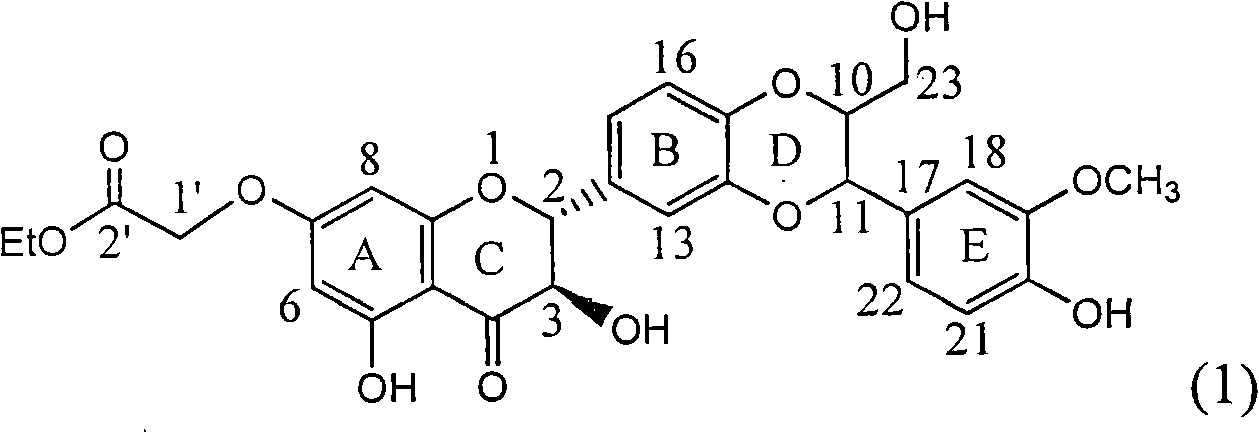
Application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B
InactiveCN101829101AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester flavonolignan substituted by ethoxycarbonyl methyl on the ring A or a pharmaceutically acceptable salt thereof for preparing medicaments for reducing the hepatitis B virus surface antigen (HBsAg), suppressing the HBV (Hepatitis B Virus) DNA replication and treating HBV infection diseases. The flavonolignan has quite obvious activity on suppressing the HBsAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensity of the flavonolignan for clearing away the HBsAG exceeds that of alpha-interferon which is a positive control medicament by 3.3 times. Meanwhile, in the presence of a concentration of 20 micrograms / milliliter, suppression ratio of the compound to the HBV DNA is close to 60 percent. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for treating the HBV infection diseases.
Owner:DALI UNIV
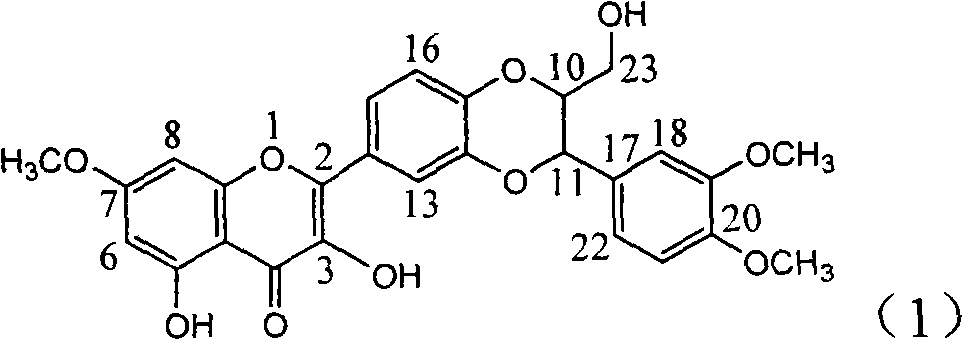
Application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B
InactiveCN101912385AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseNucleoside Reverse Transcriptase Inhibitor
The invention relates to application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B, in particular to the application of 7 and 20-position methyl substituted dehydrated silybin or pharmaceutically acceptable salts thereof in preparing medicaments for removing HBsAG and HBsAg and medicaments for inhibiting HBV DNA replication. The dehydrated silybin has remarkably HBsAg and HBeAg inhibiting activity, wherein the strength for removing the HBsAg and the HBeAg at the concentration of 20 milligram / milliliter is 88.9 percent and 84.1 percent respectively, which are 5.5 times and 5.0 times that of a positive contrast medicament. More importantly, the dehydrated silybin shows the HBV DNA inhibition ratio of about 99.6 percent at the concentration of 20 milligram / milliliter, the activity exceeds lamivudine by 23 percent, which is 2.6 times that of interferon. Therefore, favonolignan or pharmaceutically acceptable salts thereof can be predictably used for preparing the non-nucleoside reverse transcriptase inhibitor medicaments for removing the HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
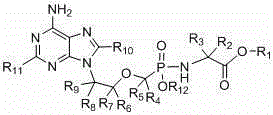
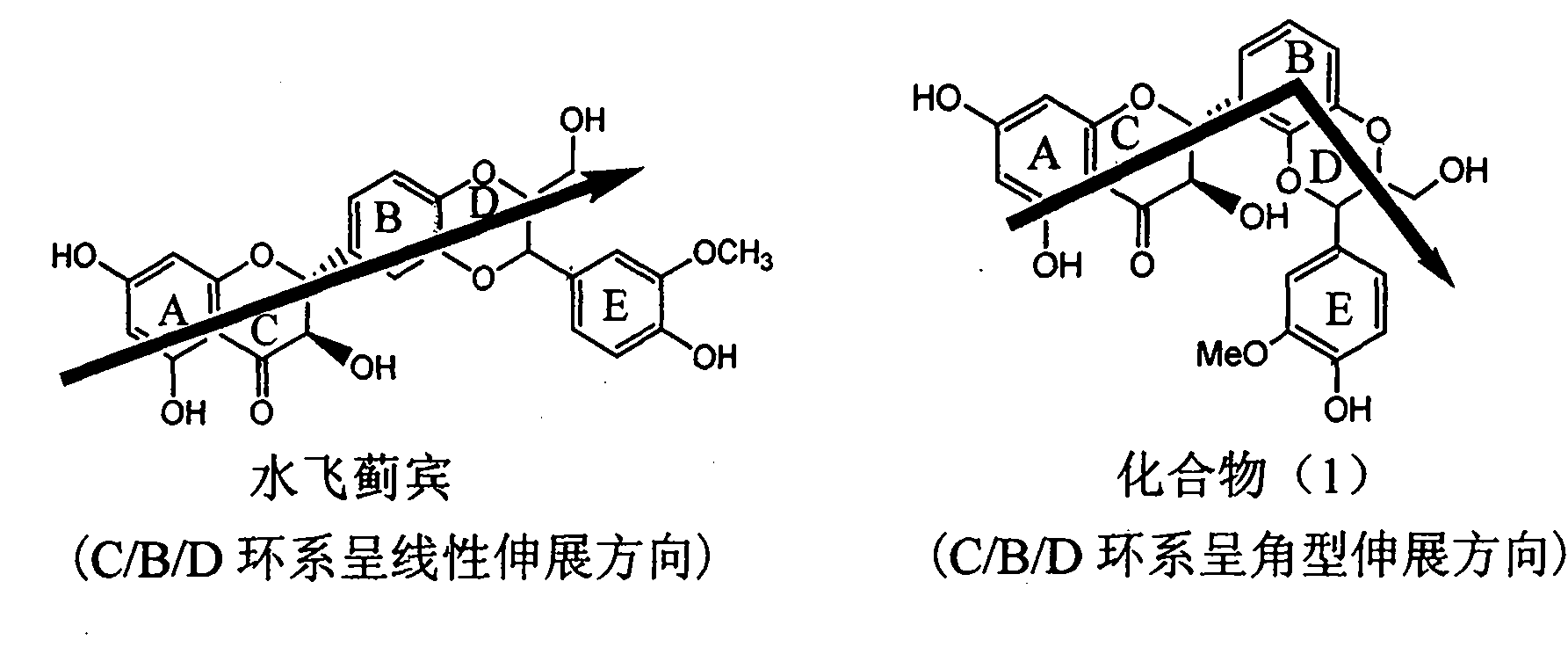
C3/C3 fluoroquinolone dipolymer derivative taking s-triazolo-[3,4-b][1,3,4] thiadiazole as connecting chain and preparation method and application thereof
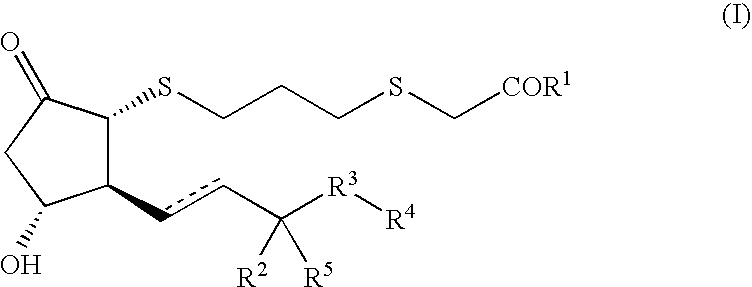
InactiveCN101648962AStrong cytotoxicityHigh activityAntibacterial agentsOrganic active ingredientsChemistryInfection disease
The invention discloses a C3 / C3 fluoroquinolone dipolymer derivative taking s-triazolo-[3,4-b][1,3,4] thiadiazole as a connecting chain and a preparation method and application thereof, wherein the C3 / C3 fluoroquinolone dipolymer derivative taking s-triazolo-[3,4-b][1,3,4] thiadiazole as the connecting chain is a compound having a general structure formula (I). In the general structure formula (I), R1 and R1' are independently selected from alkyl of H,C1-C10, naphthene base of C3-C10, haloalkyl of C1-C10 and substituted aryl radical or substituted heterocycle aryl radical at random; R2 and R2'are independently selected from alkyl of H,C1-C7, naphthene base of C3-C7, substituted aryl radical, substituted heterocycle aryl radical, hydrocarbon acyl or sulfonyl at random; R3 and R3' are independently selected from alkyl of H,C1-C5, naphthene base of C3-C6, substituted aryl radical or substituted heterocycle aryl radical at random; and X and Y are independently selected from CH, N or carbon atoms connected with halogen, alkyl, oxyl, sulfenyl, amino, substituted amino, substituted aryl radical or substituted heterocycle aryl radical. The C3 / C3 fluoroquinolone dipolymer derivative takings-triazolo-[3, 4-b] [1, 3, 4] thiadiazole as the connecting chain can be used to prepare drugs for treating tumors and antimicrobial infection diseases.
Owner:河南省健康伟业生物医药研究股份有限公司
Cytotoxic t cell activator comprising ep4 agonist
InactiveUS20100216689A1Reduce usageUseful in treatmentAntibacterial agentsBiocideDiseaseInfection disease
Disclosed is a substance which has a low molecular weight, can be applied in a simpler manner, and has an immunopotentiating activity against cancer and / or a microorganism-mediated infectious disease.An EP4 agonist exhibits an immunopotentiating activity through the activation of a cytotoxic T cell, and is therefore useful for the prevention and / or treatment of cancer or a microorganism-mediated infection disease.
Owner:HAMAMATSU UNIV SCHOOL OF MEDICINE +1
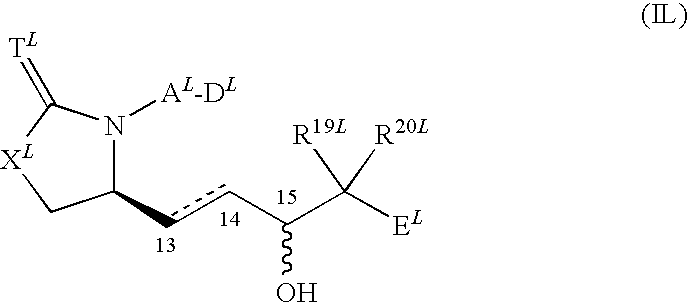
Vista antagonist and methods of use
ActiveUS20160331803A1High affinityImprove stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsCancer preventionInfection disease
The present invention is directed to a peptide, multimer, conjugate, analog, derivative or mimetic thereof that inhibits the activity of VISTA. The invention further contemplates therapeutic use of the VISTA antagonist peptide, multimer, conjugate, derivative or mimetic thereof, including treating or preventing cancer, bacterial infections, viral infections, parasitic infections and fungal infections, as well as research uses of the antagonist.
Owner:KINGS COLLEGE LONDON +1
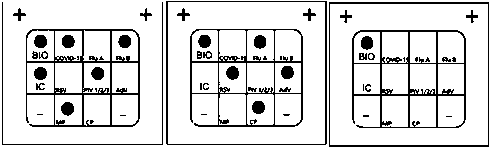
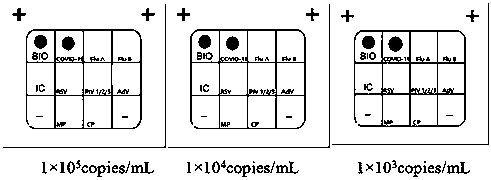
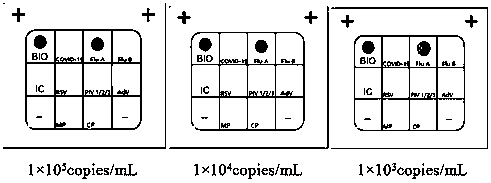
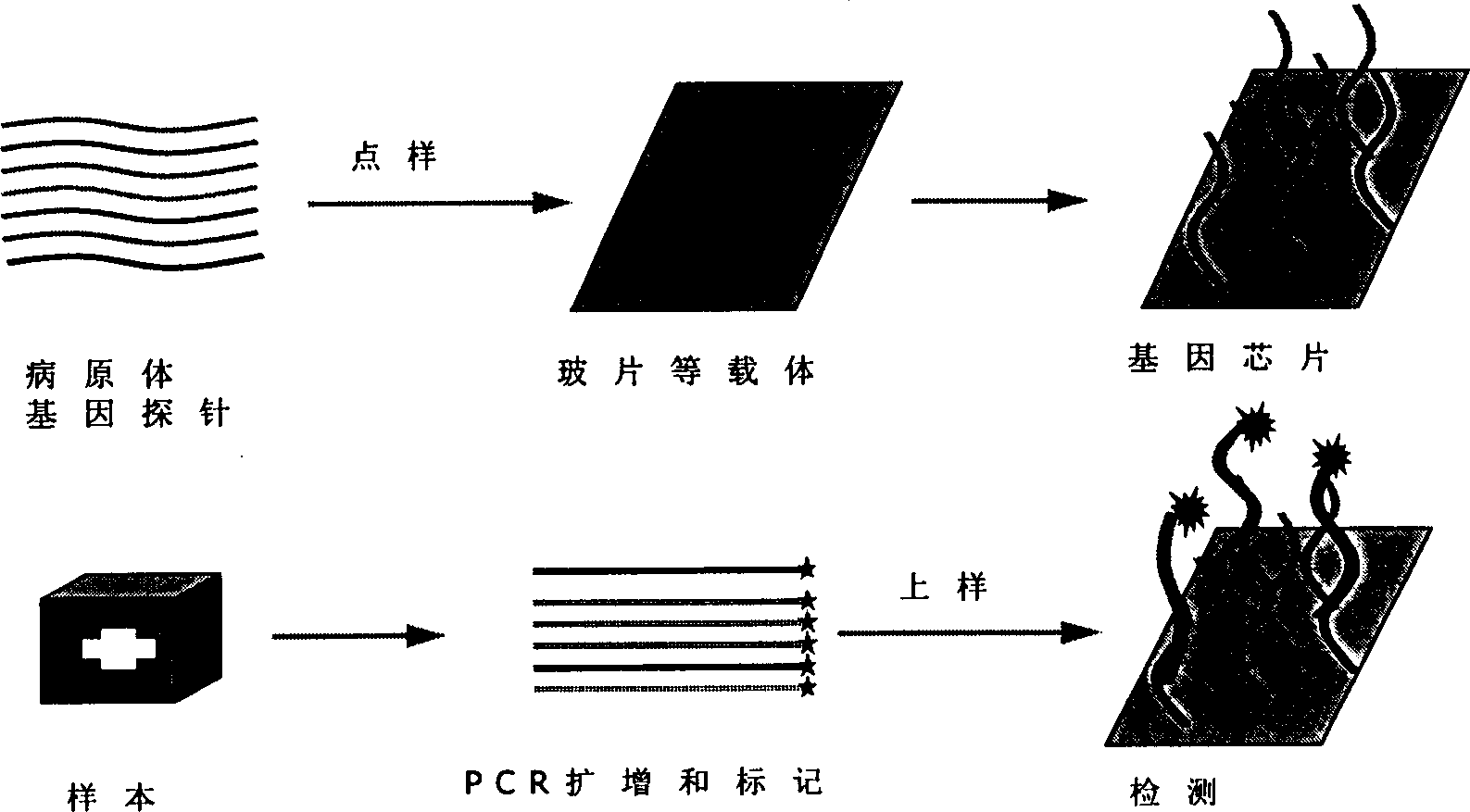
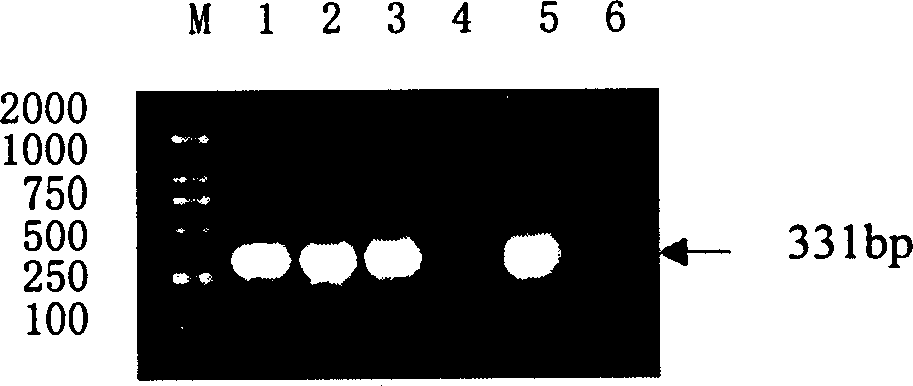
Nucleic acid combined testing kit of respiratory tract infection pathogens
InactiveCN111378789AHigh detection throughputAvoid false negative resultsMicrobiological testing/measurementMicroorganism based processesDiseaseNucleotide
The invention discloses a nucleic acid combined testing kit of respiratory tract infection pathogens. The invention develops a set of primer-probe combinations which can detect multiple types of respiratory tract infection pathogens such as novel coronavirus, influenza virus a, influenza virus b, respiratory syncytial virus, human parainfluenza virus, adenovirus, mycoplasma pneumonia and chlamydiapneumonia through combination of a multiple fluorescence quantitative PCR technology and a flow-through hybridization and gene chip technology, wherein nucleotide sequences thereof are shown by SEQ ID NO:1-36 respectively. The nucleic acid combined testing kit of the respiratory tract infection pathogens is established. The kid can realize synchronous combined testing of the 8 respiratory tract infection pathogens, is high in detection accuracy, specificity and sensitivity, good in repeatability, low in false negativity and false positivity, short in detection time and low in cost, can realize comprehensive detection of a patient, can locate a disease source accurately, can realize treatment in time or make corresponding quarantine measures and is of important significance to effective control of respiratory tract infection and subsequent prevention of outbreak of relevant contagion and infection.
Owner:GUANGZHOU HYBRIBIO MEDICINE TECH LTD +2
Detection type gene chip for detecting various infectious desease and use thereof
InactiveCN1450171AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesEpidemic hemorrhagic fever virusMalaria
The present invention relates to a gene chip for detecting several infections lideases and its application. It can be used for detecting, typing and strain-identifying 7 main infections diseases of epidemic hemorrhagic fever, tsutsugamushi disease, leptospirosis, schistosomiasis, malaria, cholera and hemorrhagic enteritis due to 0157:H7. Said invention can select specific gene probe and PCR primer respectively from S gene of epidemic hemorrhagic fever virus, 56KD protein gene of oriental rickettsia, 23 SRNA gene of leptospirosis, 5D antigen gene of schistosomiasis, SSrRNA gene of malarial parasite, 0157 antigen gene of colibacillus 0157:H7, H7 antigen gene and toxic gene and outer membrane OWP protein gene of cholera vibrio and toxic gene.
Owner:陶开华 +1
Preparation of non-cyclic nucleotide phosphoamides and salts thereof and application of non-cyclic nucleotide phosphoamides and salts thereof in aspect of antivirus
ActiveCN105669751AIntellectual property enhancementReduce exposureOrganic active ingredientsGroup 5/15 element organic compoundsViral infectious diseaseSolvent
The invention belongs to the field of antivirus in medical chemistry, and relates to preparation of non-cyclic nucleotide phosphoamides and salts thereof and application of the non-cyclic nucleotide phosphoamides and salts thereof in treating human immunodeficiency virus (HIV), hepatitis B, hepatitis B virus (HBV) and other virus infection diseases. The non-cyclic nucleotide phosphoamides, and isomers, pharmaceutically acceptable salts, hydrates, solvates or crystals thereof have the general formula I of which the structure is disclosed in the specification. The invention also provides a pharmaceutical composition containing the compounds and isomers, pharmaceutically acceptable salts, hydrates, solvates or crystals thereof, and application of the compounds or composition in treating and / or preventing HIV and HBV infection. The compounds provided by the invention have the advantages of favorable anti-HIV and HBV activities, low oral dosage and low toxicity.
Owner:洛阳聚慧新材料科技有限公司
Preparation of blattodea polypeptide, and uses of the same in anti-gram-positive bacteria and anti-gram-negative bacteria
InactiveCN102743741AHigh yieldBroad biological activityAntibacterial agentsCosmetic preparationsChemical productsGram Positive Bacillus
The present invention relates to preparation of a blattodea polypeptide, and uses of the blattodea polypeptide in anti-gram-positive bacteria and anti-gram-negative bacteria. Specifically the present invention relates to a blattodea extract effective fraction having a function of prevention and treatment of bacterial infection diseases, a preparation method and a medical use thereof. The Periplaneta Americana extract effective fraction of the present invention is a polypeptide substance with a molecular weight less than 5000 dalton, and is prepared by the following steps: carrying out solvent extraction and membrane separation on fresh polypide or dried polypide of Periplaneta Americana to obtain the product of the present invention, wherein the polypeptide active substance of the effective fraction has significant anti-gram-positive bacteria activity and anti-gram-negative bacteria activity, can be prepared into forms of a hydrogel, a cataplasm, lyophilized powder, a water agent, an aerosol, a suppository, a film agent, an external application liniment, and an ointment, and can be used for preparations of drugs for prevention and treatment of diseases related to gram-positive bacteria and gram-negative bacteria and infections, daily chemical products or medical devices.
Owner:DALI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![C3/C3 fluoroquinolone dipolymer derivative taking s-triazolo-[3,4-b][1,3,4] thiadiazole as connecting chain and preparation method and application thereof C3/C3 fluoroquinolone dipolymer derivative taking s-triazolo-[3,4-b][1,3,4] thiadiazole as connecting chain and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/74933943-6de3-4bb2-b8c1-a0bf5fed72d1/A2009100659910002C1.PNG)
![C3/C3 fluoroquinolone dipolymer derivative taking s-triazolo-[3,4-b][1,3,4] thiadiazole as connecting chain and preparation method and application thereof C3/C3 fluoroquinolone dipolymer derivative taking s-triazolo-[3,4-b][1,3,4] thiadiazole as connecting chain and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/74933943-6de3-4bb2-b8c1-a0bf5fed72d1/A2009100659910010C1.PNG)
![C3/C3 fluoroquinolone dipolymer derivative taking s-triazolo-[3,4-b][1,3,4] thiadiazole as connecting chain and preparation method and application thereof C3/C3 fluoroquinolone dipolymer derivative taking s-triazolo-[3,4-b][1,3,4] thiadiazole as connecting chain and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/74933943-6de3-4bb2-b8c1-a0bf5fed72d1/G2009100659910D00021.PNG)