Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Hemorrhagic fever virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The term hemorrhagic fever is used to describe several severe and life-threatening viruses, usually spread from either insects or mammals to humans. Some examples of hemorrhagic fever include the Ebola virus, yellow fever, and the Marburg virus. Most viruses resulting in hemorrhagic fever are localized to a specific area.
Antiviral drugs for treatment of arenavrus infection
Compounds, methods and pharmaceutical compositions for treating viral infections, by administering certain novel compounds in therapeutically effective amounts are disclosed. Methods for preparing the compounds and methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by hemorrhagic fever viruses is disclosed, i.e., including but not limited to, Arenaviridae (Junin, Machupo, Guanarito, Sabia, Lassa, Tacaribe, Pichinde, and LCMV), Filoviridae (Ebola and Marburg viruses), Flaviviridae (yellow fever, Omsk hemorrhagic fever and Kyasanur Forest disease viruses), and Bunyaviridae (Rift Valley fever and Crimean-Congo hemorrhagic fever).
Owner:KINETA FOUR LLC
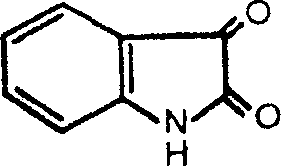
Application of indole-2,3-diketone in preparing medication for antivirus or immunopotenfiator
InactiveCN1759833AImprovement of immune indicatorsShows antiviral effectOrganic active ingredientsAntiviralsNatural productKetone
An application of indole-2.3-bione in preparing the antiviral medicine for HIV, influenza virus, hemorrhagic virus and herpes simplex virus and the immunopotentiator is disclosed.
Owner:QINGDAO UNIV
Fluorescence quantitative polymerase chain reaction (PCR) new method for detecting multiple viruses of yellow fever, dengue fever and epidemicencephalitis B and multiple virus detection PCR system
ActiveCN102191338AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceYellow fever
The invention discloses a fluorescence quantitative polymerase chain reaction (PCR) new method for detecting multiple viruses of yellow fever, dengue fever and epidemicencephalitis B and multiple virus detection PCR system consisting of primers, probes, a Premix Ex Taq reaction solution and a sterilized Tris buffer. With good singularity and high sensitivity, three pairs of primers and probes are very suitable for simultaneously detecting viruses of yellow fever, dengue fever and epidemicencephalitis B. And there is no cross reaction between the primers and probes and several other entomophily hemorrhagic fever viruses, such as Marburg virus and Rift Valley fever virus.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
RPA technology-based marburg virus detection kit and application thereof
InactiveCN106636469AAvoid spreading infectionEfficient amplificationMicrobiological testing/measurementMicroorganism based processesInfection transmissionQuarantine
The invention discloses an RPA technology-based marburg virus detection kit and an application thereof. An experiment proves that a target gene can be effectively amplified by an RPA primer and a probe of a marburg virus, the specificity reaches 100% and sensitivity is 1*10<2>copies / reaction; and the virus can reach the level equivalent to the sensitivity of fluorescent quantitative PCR, and has no cross reaction with a zaire ebola pseudovirus, a dengue virus, a hemorrhagic fever virus with renal syndrome and a Xinjiang hemorrhagic fever virus nucleic acid. The RPA isothermal amplification system is fast in reaction and wide in temperature range, effective amplification of a target gene can be achieved under the condition of 37-42 DEG C, the method can be used for fast field detection of an infectious nucleic acid of the marburg virus, and an available fast detection method is provided for field screening of a pathogen; and meanwhile, the marburg virus detection kit also has the important significance in prevention of infection transmission of the marburg virus in China and inspection and quarantine in affected areas and entry and exit ports.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Azole nucleosides and use as inhibitors of RNA and DNA viral polymerases
InactiveUS20100129317A1Inhibition is effectivePrevent slippingBiocideSugar derivativesCrimean Congo hemorrhagic fever virusPolymerase L
Azole nucleosides represented by the formulae (I) and (II); wherein A=C or N B═C or N X═H; C1-C6 alkyl, cycloalkyl, alkenyl, cycloalkenyl, alkynyl, aryl, heterocyclo, halogen such as F, Cl, Br and I; OH, NH2, NH—(C1-C6 alkyl, cycloalkyl, aryl or heterocyclo); Z═H; C1-C6 alkyl, cycloalkyl, alkynyl, aryl, heterocyclo, halogen such as F, Cl, Br, I; OH NH2, NH—(C1-C6 alkyl, cycloalkyl, aryl or heterocyclo; E=(CH2)HONHR; n is an interger from 0-6 and more typically 0-3; R1= aryl or heterocyclo; each of W, Y, R is individually selected from the group consisting of H; C1-C6 alkyl, cycloalkyl, alkenyl, cycloalkenyl, alkynyl, aryl, heterocyclo, halogen such as F, Cl, Br, and I; O, OH, Oalkyl, Oaryl, NH2, NH(C1-C6 alkyl, cycloalkyl, aryl or heterocyclo); provided that at least one of W, Y, and R is other than H and wherein both W and Y together can be ═O; and each D individually is OH, Oalkyl, Oaryl, FL and H; pharmaceutically acceptable salts thereof, prodrugs thereof and mixtures thereof are provided. Compounds of this disclosure are useful as inhibitors of viral RNA and DNA polymerases such as, but not limited to, Influenza, hantaan Virus, Crimean Congo hemorrhagic fever virus, hepatitis B, hepatitis C, Polio, Coxsackie A and B, Rhino, Echo, orthopoxvirus (small pox), HIV, Ebola, and West Nile virus polymerases; and especially orthopoxvirus, HIV, and hepatitis B.
Owner:SOUTHERN RES INST & IP +1
Marburg and Ebola dual-virus fluorescent quantitative PCR (Polymerase Chain Reaction) detection method and system
ActiveCN102140533AMicrobiological testing/measurementFluorescence/phosphorescenceEbola virusYellow fever
The invention discloses Marburg and Ebola dual-virus fluorescent quantitative PCR (Polymerase Chain Reaction) detection method and system, wherein the detection system comprises primers, probes, a Premix EX Taq reaction solution and sterilizing Tris water. As two pairs of primers and probes have very good specificity, the detection system has high sensitivity and is suitable for simultaneously detecting Marburg and Ebola viruses without having cross reaction with other kinds of hemorrhagic fever arbovirus, such as yellow fever, dengue and rift valley fever.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Preparation method of rabbit hemorrhagic fever virus empty capsid antigen
ActiveCN102304529APromote safe productionReduce consumptionViral antigen ingredientsVirus peptidesAntigenBombyx mori
The invention relates to the field of genetic engineering and particularly relates to a preparation method of a rabbit hemorrhagic fever virus empty capsid antigen. The method provided by the invention comprises the following steps: 1) constructing a rhabdovirus transfer carrier containing a rabbit hemorrhagic fever virus capsid protein VP60 gene or an optimized gene, wherein codon optimization is performed according to the codon frequency of bombyx mori; 2) performing cotransfection on the constructed transfer expression carrier and DNA (deoxyribonucleic acid) of rhabdovirus so as to carry out homologous recombination or transposition to further obtain the recombinant rhabdovirus; 3) infecting the recombinant rhabdovirus with the host cells of an insect; and 4) culturing the infected host of the insect to express the corresponding rabbit hemorrhagic fever virus empty capsid antigen, and harvesting and purifying the expressed antigen. By adopting the method provided by the invention, the production cost of the rabbit hemorrhagic fever virus empty capsid antigen can be greatly reduced, and the method has a plurality of advantages of safety, high efficiency, low energy consumption, low cost and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
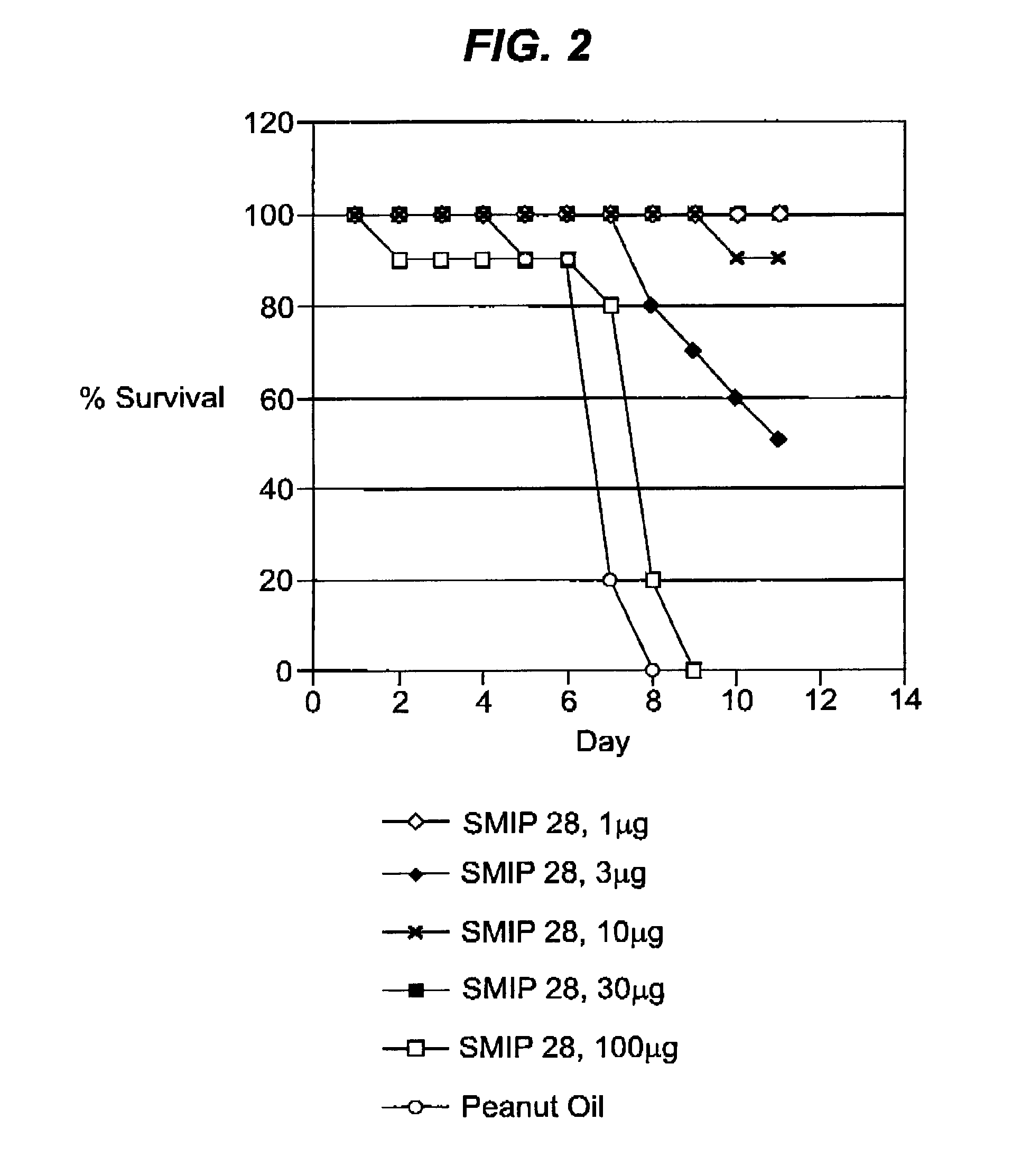
Benzonapthyridine compositions and uses thereof
ActiveUS20130122042A1Organic active ingredientsPeptide/protein ingredientsHemorrhagic fever virusPost exposure
The present invention generally relates to compositions comprising benzonapthyridine small molecule immune potentiators (SMIPs) that are capable of stimulating or modulating an immune response in a subject that has had pre- or post-exposure to a pathogen such as hemorrhagic fever virus. Also provided are methods of preparing and using the SMIP compositions of the invention.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC +1
Detection method of hemorrhagic fever with renal syndrome IgM antibodies and reagent kit
ActiveCN103033616AReduce the impact of stabilityExtended shelf lifeMaterial analysisSerum igeIgm antibody
The invention relates to the technical field of biological detection, in particular to a detection method of hemorrhagic fever with renal syndrome IgM (immunoglobulin M) antibodies and a reagent kit. The detection method comprises the steps that hemorrhagic fever with renal syndrome antigens are fixed on a membrane; to-be-detected serum is added; the hemorrhagic fever with renal syndrome IgM antibodies in the to-be-detected serum are combined with the antigens fixed on the membrane; gold labeled operating fluid containing mouse anti-human IgM monoclonal antibodies is added; the mouse anti-human IgM monoclonal antibodies are combinec with the hemorrhagic fever with renal syndrome IgM antibodies in the to-be-detected serum; a red color is shown; scrubbing liquid is added finally; and the redundant gold labeled operating fluid and other impurities are washed out. The reagent kit has the characteristics of longer quality guarantee period, less cross reaction, better color rendering performance and the like.
Owner:山东康华生物医疗科技股份有限公司
Rapid test paper bar for testing colloidal gold of antibody of epidemic hemorrhagic fever virus
InactiveCN1963516AQuick screeningSave manpower and material resourcesMaterial analysisAntigenGlass fiber
This invention provides one test bar, which comprises the following steps: a, covering antibody IgM linkage single clone antibody and anti-flu hemorrhagic fever virus reset antigen multiple clone antibody two NC fiber films; b, comprising glue gold label flu hemorrhagic fever virus glass fiber film; applying film analysis and glue gold label technique to test sample flu virus antibody.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
Xinjiang hemorrhagic fever virus nucleoprotein antigen gene as well as recombinant protein and application thereof
The invention relates to the technical filed of antigen genes, in particular to a Xinjiang hemorrhagic fever virus nucleoprotein antigen gene (XHFNP235) as well as recombinant protein and application thereof. The gene has a nucleotide sequence as sequence 1. In the invention, the Xinjiang hemorrhagic fever virus nucleoprotein antigen gene (XHFNP235) and the recombinant protein thereof are obtained from Xinjiang hemorrhagic fever virus. Through PCR amplification, cloning, transformation, expression plasmid construction, induction expression and immunoassay detection, the Xinjiang hemorrhagic fever epitope is positioned at the 235th to 305th amino acid region of NP protein, and the region is a highly conservative amino acid region of the NP protein, which further shows that the XHFNP235 recombinant protein can be a candidate diagnosis antigen for Xinjiang hemorrhagic fever, and provides a new way for diagnosing and applying Xinjiang hemorrhagic fever.
Owner:THE CENT FOR DISEASE CONTROL & PREVENTION OF XINJIANG UYGUR AUTONOMOUS REGION
Kidney syndrome hemorrhagic fever Vero cell bivalent purified vaccine and industrialized producing process thereof
InactiveCN1990041ALow costEasy to operateViral antigen ingredientsAntiviralsPediatricsHemorrhagic fever virus
The invention involves nephrotic syndrome hemorrhagic fever Vero cells purified bivalent vaccine and its industrialized preparation methods. The nephrotic syndrome hemorrhagic fever Vero cells bivalent purified vaccine in the inventioncontains effective doses of nephrotic syndrome hemorrhagic fever deactive virus type I, type II and vaccine adjuvants, the type I and type II virus are from PS-6 (C-3) strains, CCTCC-V200503 and L99(C-2) strains, CCTCC-V200504 respectively. The preparation method of the invention includes: 1. Preparating the vaccine stock solution nephrotic syndrome hemorrhagic fever virus PS-6(C-3), CCTCC-V200503 and L99(C-2),CCTCC-V200504, inactivating virus with formaldehyde; 2. combining the deactive vaccine stock solution of PS-6(C-3), CCTCC-V200503 and L99(C-2),CCTCC-V200504 and purifying, the process contains centrifugal separation, hyperfiltration, concentration, zinc acetate deposition and column chromatography; 3. adding adjuvant into the purified vaccine stock solution and getting the purified bivalent vaccine. The nephrotic syndrome hemorrhagic fever Vero cells purified bivalent vaccine in the invention is suitable for the prevention of nephrotic syndrome hemorrhagic fever mainly based on all type I and type II.
Owner:长春生物制品研究所有限责任公司
Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses
Compounds, methods and pharmaceutical compositions for treating viral infections, by administering certain novel semicarbazides, sulfonyl carbazides, ureas and related compounds in therapeutically effective amounts are disclosed. Methods for preparing the compounds and methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by hemorrhagic fever viruses is disclosed, i.e., including but not limited to Arenaviridae (Junin, Machupo, Guanavito, Sabia and Lassa), Filoviridae (ebola and Marburg viruses), Flaviviridae (yellow fever, omsk hemorrhagic fever and Kyasanur Forest disease viruses), and Bunyaviridae (Rift Valley fever).
Owner:KINETA FOUR LLC
Antiviral drugs for treatment of arenavirus infection
Compounds, methods and pharmaceutical compositions for treating viral infections, by administering certain novel compounds in therapeutically effective amounts are disclosed. Methods for preparing the compounds and methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by hemorrhagic fever viruses is disclosed, i.e., including but not limited to, Arenaviridae (Junin, Machupo, Guanarito, Sabia, Lassa, Tacaribe, Pichinde, and LCMV), Filoviridae (Ebola and Marburg viruses), Flaviviridae (yellow fever, Omsk hemorrhagic fever and Kyasanur Forest disease viruses), and Bunyaviridae (Rift Valley fever and Crimean-Congo hemorrhagic fever).
Owner:KINETA FOUR LLC
Antiviral rift valley fever virus peptides and methods of use
ActiveUS9556237B2Easy to produceUseful prophylacticSsRNA viruses negative-sensePeptide/protein ingredientsEbola virusAndes virus
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE ARMY ON BEHALF OF THE U S ARMY MEDICAL RES INST OF INFECTIOUS DISEASES
Compositions and Methods for Generating an Immune Response to a Hemorrhagic Fever Virus
ActiveUS20190117758A1Avoid infectionPrevents and ameliorates BundibugyoSsRNA viruses negative-senseViral antigen ingredientsModified vaccinia AnkaraGenus Ebolavirus
The compositions and methods are described for generating an immune response to a hemorrhagic fever virus such as ebolavirus, Marburgvirus, or arenavirus. The compositions and methods described herein relate to a modified vaccinia Ankara (MVA) vector encoding one or more viral antigens for generating a protective immune response to a member of genus Ebolavirus (such as a member of species Zaire ebolavirus), a member of genus Marburgvirus (such as a member of species Marburg marburgvirus), or a member of genus Arenavirus (such as a member of species Lassa virus) in the subject to which the vector is administered. The compositions and methods of the present invention are useful both prophylactically and therapeutically and may be used to prevent and / or treat an infection caused by ebolavirus, Marburgvirus, or arenavirus.
Owner:GEOVAX INC
Crimean-Congo Hemorrhagic Fever Virus Vaccine
ActiveUS20140050761A1Reduce removalSolve the lack of activitySsRNA viruses negative-senseViral antigen ingredientsBiological bodyISG15
The genetically modified hemorrhagic fever virus of this invention possesses a viral ovarian tumor protease with decreased ability to remove ubiquitin (Ub) and ISG15 tags that the human organism uses to label proteins for removal. Unlike complete knockout strains, the modified virus retains enough activity for replication in a human cell line. This creates an immunogenic and non-pathogenic virus that can be used as an effective live vaccine agent.
Owner:UNITED STATES OF AMERICA +1
Nano-gold biosensor for simultaneously detecting four hemorrhagic fever viruses and detection method thereof
InactiveCN110042173AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationFiberNitrocellulose
The invention discloses a nano-gold biosensor for simultaneously detecting four hemorrhagic fever viruses and a detection method thereof. The biosensor comprises a substrate, a sample pad, an absorption pad, a nitrocellulose film and a bonding pad, wherein the sample pad and the absorption pad are fixed at two ends of the substrate, and the nitrocellulose film is fixed at the middle part of the substrate; a 2-3mm overlap is formed between the absorption pad and the nitrocellulose film, a 2-3mm overlap is formed between the nitrocellulose film and the binding pad, and a 2-3mm overlap is formedbetween the binding pad and the sample pad; and gold nanoparticle DNA probes are arranged on the binding pad. The biosensor has the advantages of high sensitivity, high specificity, short detection time, capability of completing detection within 60 minutes and low detection cost.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Crimean-Congo hemorrhagic fever virus nucleic acid molecule characteristic standard sample and its preparation method
InactiveCN104404168AImprove uniformityImprove stabilityMicrobiological testing/measurementMicroorganism based processesFreeze-dryingCrimean Congo hemorrhagic fever virus
The invention discloses a preparation method of a Crimean-Congo hemorrhagic fever virus nucleic acid molecule characteristic standard sample. The Crimean-Congo hemorrhagic fever virus nucleic acid molecule characteristic standard sample is prepared through sequence synthesis, vector cloning, target fragment and vector connection, plasmid transformation, recombinant plasmid extraction, freeze-drying and preservation, includes virus characteristic sequence information, and is suitable for analyzing the virus and the content value of the virus. The standard sample provided by the invention has the advantages of good uniformity, high stability, long-time storage and good purity. The preparation method has a simple process, can provide the standard sample for detection research and medical research of Crimean-Congo hemorrhagic fever virus to realize the comparison of different laboratory results in order to ensure the laboratory quality control, and also provides the standard sample for the rapid and accurate detection of Crimean-Congo hemorrhagic fever virus by inspection and quarantine institutions, and import and export trade enterprises.
Owner:薛芳
Xinjiang hemorrhagic fever virus immunochromatography fast detection test paper
The invention provides a Xinjiang hemorrhagic fever virus immunochromatography rapid detection test paper comprising a supporting and fixing adhering lining layer on which a sample absorbing layer, a gold-marking binding pad, a fibrin film layer and a water absorbing layer are sequentially adhered to the supporting and fixing adhering lining layer, wherein the fibrin film layer takes a monoclonal antibody which is printed with Xinjiang hemorrhagic fever virus nucleocapsid protein as a detection blot zone. The fibrin film layer of the Xinjiang hemorrhagic fever virus immunochromatography rapid detection test paper is printed with sheep-anti-mouse IgG antibody solution as a contrapositive blot zone. A monoclonal antibody of the Xinjiang hemorrhagic fever virus nucleocapsid protein which is marked by colloidal gold is absorbed on the gold-marking binding pad of the test paper. The Xinjiang hemorrhagic fever virus immunochromatography rapid detection test paper can effectively and rapidly detect the Xinjiang hemorrhagic fever virus.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Reagent kit capable of simultaneously detecting virulent viruses and bacteria and detecting method
PendingCN109852730ARNase resistantImprove stabilityMicrobiological testing/measurementMicroorganism based processesViral nucleic acidHemorrhagic fever virus
The invention provides a reagent kit capable of simultaneously detecting virulent viruses and bacteria and a detecting method. The reagent kit is mainly in accordance with 4 pathogenic microorganismsof ebola viruses, Sinkiang hemorrhagic fever viruses, Brucella and anthrax bacillus. The reagent kit comprises a specific primer probe combination for detecting the ebola viruses, the Sinkiang hemorrhagic fever viruses, the Brucella and the anthrax bacillus, a micro-fluidic solid-phase PCR chip on which a specificity probe is fixed, and an RNase-resistant high-stability positive quality control product consisting of virus-like particles containing viral nucleic acid and thalli containing plasmid carrying the specificity nucleic acid. According to the reagent kit, simultaneous detection of theebola viruses, the Sinkiang hemorrhagic fever viruses, the Brucella and the anthrax bacillus can be realized, and the reagent kit has the advantages of being high in detection flux, high in sensitivity, high in specificity, good in repeatability, short in detection time, low in detection cost, low in operation technique requirements, not liable to pollute and the like, and has great application prospects in the field of quick simultaneous detection of various pathogenic microorganisms.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Test card for rapid detection of epidemic hemorrhagic fever virus antibody, and application of test card
The invention provides a test card for rapid detection of an epidemic hemorrhagic fever virus antibody. The test card comprises a bottom plate, a sample pad, a nitrocellulose membrane, a water absorption pad and a fluorescent microsphere pad, wherein the fluorescent microsphere pad adsorbs fluorescent microspheres marked by epidemic hemorrhagic fever virus antigens and fluorescent microspheres marked by quality control proteins; and an anti-human IgG antibody, an anti-human IgM antibody and an anti-quality control protein antibody are sequentially adsorbed on the nitrocellulose membrane. In addition, the invention provides a preparation method of the test card, an application of the test card to the rapid detection of the epidemic hemorrhagic fever virus human antibody, and the like.
Owner:苏州华益美生物科技有限公司
Virus in deactivated bioproduct and method of removing toxin in bacteria
InactiveCN1786157AEnsure safetyMaintain biological activityInactivation/attenuationBioproductsHemorrhagic fever virus
The invention relates to an inactivation biological product virus and removing bacterial endotoxin method. The biological product or middle product is adjusted its pH value to 12.0+ / -0.2 by NaOH solution, hatched for at least one hour at 25+ / -1 centigrade degree, then adjusted its pH value to need by HCl solution. The method has good inactivation effect for all fat enveloped virus or non. It has done a lot of test for many viruses. And all of them have good inactivation effect. It is qualified tested by TAL and rabbit detection pyrogen.
Owner:李法卿
Replication-deficient modified vaccinia ankara (MVA) expressing marburg virus glycoprotein (GP) and matrix protein (VP40)
PendingUS20220152190A1Avoid infectionPrevents and ameliorates resultingSsRNA viruses negative-senseViral antigen ingredientsModified vaccinia AnkaraViral glycoprotein
The compositions and methods are described for generating an immune response to a hemorrhagic fever virus such as ebolavirus, Marburgvirus, or arenavirus. The compositions and methods described herein relate to a modified vaccinia Ankara (MVA) vector encoding one or more viral antigens for generating a protective immune response to a member of genus Ebolavirus (such as a member of species Zaire ebolavirus), a member of genus Marburgvirus (such as a member of species Marburg marburgvirus), or a member of genus Arenavirus (such as a member of species Lassa virus) in the subject to which the vector is administered. The compositions and methods of the present invention are useful both prophylactically and therapeutically and may be used to prevent and / or treat an infection caused by ebolavirus, Marburgvirus, or arenavirus.
Owner:GEOVAX INC
Fluorescence quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for rapidly detecting XHF (Xinjiang hemorrhagic fever) viruses
InactiveCN102424866AEasy to detectHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesConserved sequenceReverse transcriptase
The invention relates to a fluorescence quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for rapidly detecting XHF (Xinjiang hemorrhagic fever) viruses. In the invention, a simple, rapid and sensitive RT-PCR detection kit is developed on the basis of designing a specific primer according to a specific conserved sequence of an XHF virus and successfully establishing a PCR detection method for XHF viruses. When XHF viruses are detected by using the kit and detection method provided by the invention, the detection process is convenient and rapid, a detection result can be obtained in 2-3 hours, and the detection sensitivity and the detection accuracy are high, therefore, the kit and detection method provided by the invention are especially suitable for the needs of entry-exit inspection and quarantine.
Owner:中华人民共和国江苏出入境检验检疫局
Application of indole-2,3-diketone in preparing medication for antivirus or immunopotenfiator
InactiveCN1316968CImprovement of immune indicatorsShows antiviral effectOrganic active ingredientsAntiviralsNatural productKetone
An application of indole-2.3-bione in preparing the antiviral medicine for HIV, influenza virus, hemorrhagic virus and herpes simplex virus and the immunopotentiator is disclosed.
Owner:QINGDAO UNIV
Crimean-Congo hemorrhagic fever virus vaccine
ActiveUS9474796B2SsRNA viruses negative-senseViral antigen ingredientsISG15Crimean Congo hemorrhagic fever virus
The genetically modified hemorrhagic fever virus of this invention possesses a viral ovarian tumor protease with decreased ability to remove ubiquitin (Ub) and ISG15 tags that the human organism uses to label proteins for removal. Unlike complete knockout strains, the modified virus retains enough activity for replication in a human cell line. This creates an immunogenic and non-pathogenic virus that can be used as an effective live vaccine agent.
Owner:UNITED STATES OF AMERICA +1
Kit for one-step nucleic acid quantitative RT-PCR detection on Xinjiang hemorrhagic fever virus
InactiveCN105779646ARapid Nucleic Acid Quantitative DetectionAccurate nucleic acid quantitative detectionMicrobiological testing/measurementReference genesPositive control
The invention provides a kit for one-step nucleic acid quantitative RT-PCR detection on a Xinjiang hemorrhagic fever virus.The kit comprises RT-PCR reaction liquid, a RT-PCR enzyme mixture, XHFV nucleic acid quantitative primers and probes, an internal reference, negative control, critical positive control, strong positive control and calibrators (1-5).According to the kit, a one-step RT-PCR reaction can be directly performed on extracted XHFV RNA, fluorescent quantitative detection is performed on XHFV RNA in the samples, an internal reference gene sequence is taken as internal control, and contamination is prevented through a UNG enzyme; the kit is simple in one-step amplification method, short in procedure, easy and convenient to operate, capable of preventing contamination, high in specificity of a detection result and sensitivity, clear in result and high in credibility and can be applied to nucleic acid quantitative detection on the Xinjiang hemorrhagic fever virus in serum.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Virus in deactivated bioproduct and method of removing toxin in bacteria
InactiveCN100457896CEnsure safetyMaintain biological activityInactivation/attenuationBioproductsHemorrhagic fever virus
The invention relates to an inactivation biological product virus and removing bacterial endotoxin method. The biological product or middle product is adjusted its pH value to 12.0+ / -0.2 by NaOH solution, hatched for at least one hour at 25+ / -1 centigrade degree, then adjusted its pH value to need by HCl solution. The method has good inactivation effect for all fat enveloped virus or non. It has done a lot of test for many viruses. And all of them have good inactivation effect. It is qualified tested by TAL and rabbit detection pyrogen.
Owner:李法卿
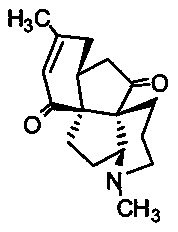
Application of Lycojaponicumin C in preparation of medicine for treating hemorrhagic fever with renal syndrome
ActiveCN103463073AStrong inhibitory activityHighlight substantive featuresOrganic active ingredientsAntiviralsDisease courseMortality rate
The invention discloses a new application of Lycojaponicumin C in preparation of a medicine for treating hemorrhagic fever with renal syndrome. Experimental researches of Lycojaponicumin C in-vitro and in-vivo inhibition on virus of hemorrhagic fever with renal syndrome indicate that Lycojaponicumin C has low toxicity to cells, can directly kill virus of hemorrhagic fever with renal syndrome, can remarkably inhibit multiplication of the virus, and can obviously protect shrewmouse infected by the virus so as to delay the disease time, prolong the course of disease and reduce the death rate. Therefore, the Lycojaponicumin C is an effective and safe medicine for treating hemorrhagic fever with renal syndrome.
Owner:山西明鼎医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com