Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
107 results about "Viral glycoprotein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
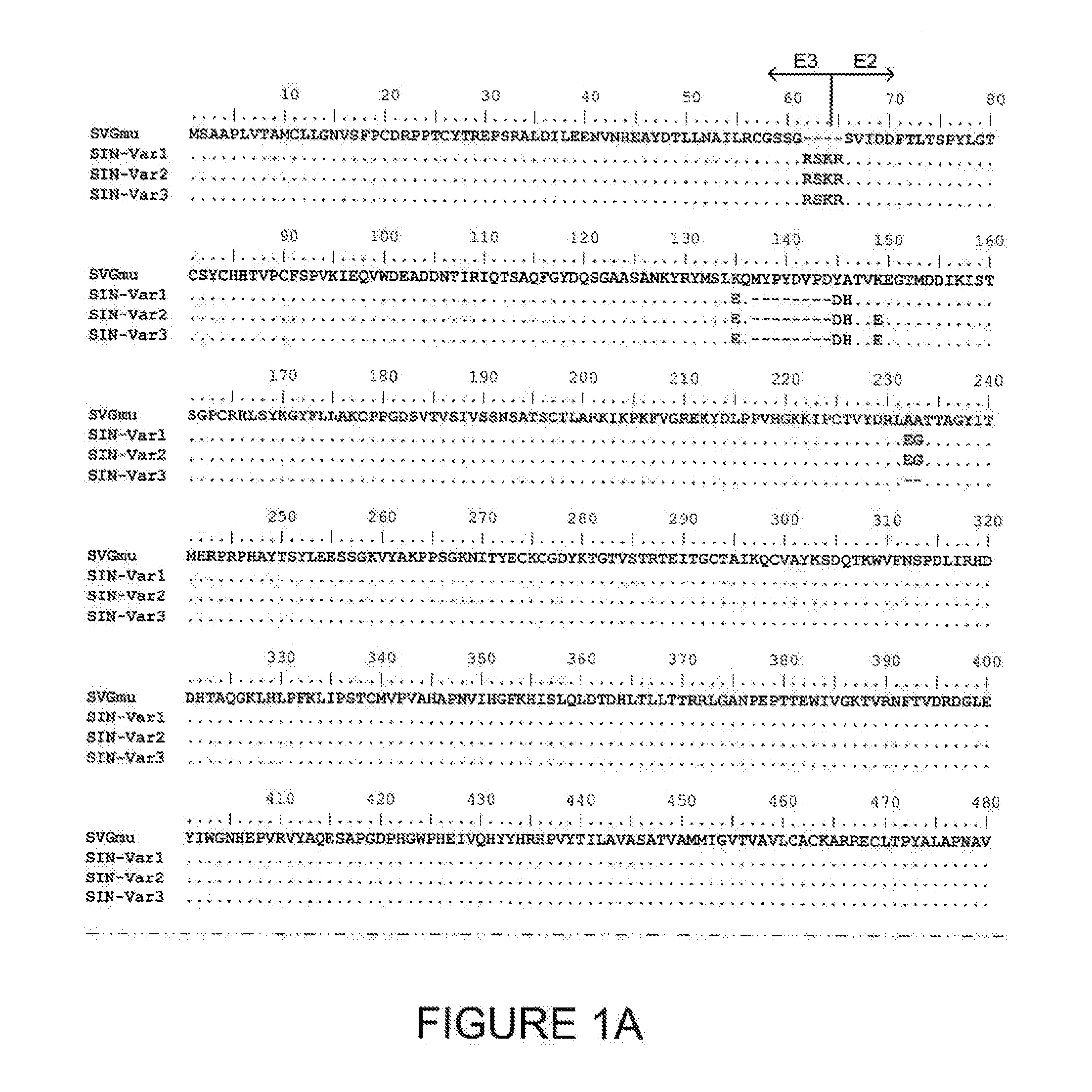
Lentiviral vectors pseudotyped with a sindbis virus envelope glycoprotein
InactiveUS20110064763A1Promote infectionSsRNA viruses positive-senseAntiviralsDendritic cellSindbis virus
Lentiviral vector particles comprising a Sindbis virus E2 glycoprotein variant and a lentiviral vector genome comprising a sequence of interest are provided. A lentiviral vector particle comprising: (a) an envelope comprising a Sindbis virus E2 glycoprotein variant; and (b) a lentiviral vector genome comprising a sequence of interest; wherein the E2 glycoprotein variant facilitates infection of dendritic cells by the lentiviral vector particle, and wherein the E2 glycoprotein variant has reduced binding to heparan sulfate compared to a reference sequence (HR strain).
Owner:IMMUNE DESIGN CORP
Human Antibodies to Ebola Virus Glycoprotein
ActiveUS20160215040A1Inhibiting and neutralizing activityAvoid enteringImmunoglobulins against virusesAntiviralsViral glycoproteinAntigen Binding Fragment
The present invention provides monoclonal antibodies, or antigen-binding fragments thereof, that bind to Ebola virus glycoproteins, pharmaceutical compositions comprising the antibodies and methods of use. The antibodies of the invention are useful for inhibiting or neutralizing Ebola virus activity, thus providing a means of treating or preventing Ebola virus infection in humans. In some embodiments, the invention provides for use of one or more antibodies that bind to the Ebola virus for preventing viral attachment and / or entry into host cells. The antibodies of the invention may be used prophylactically or therapeutically and may be used alone or in combination with one or more other anti-viral agents or vaccines.
Owner:REGENERON PHARM INC
Avipox virus containing DNA sequences encoding herpesvirus glycoproteins
InactiveUS6183750B1SsRNA viruses negative-senseSsRNA viruses positive-senseCanarypox virusViral glycoprotein
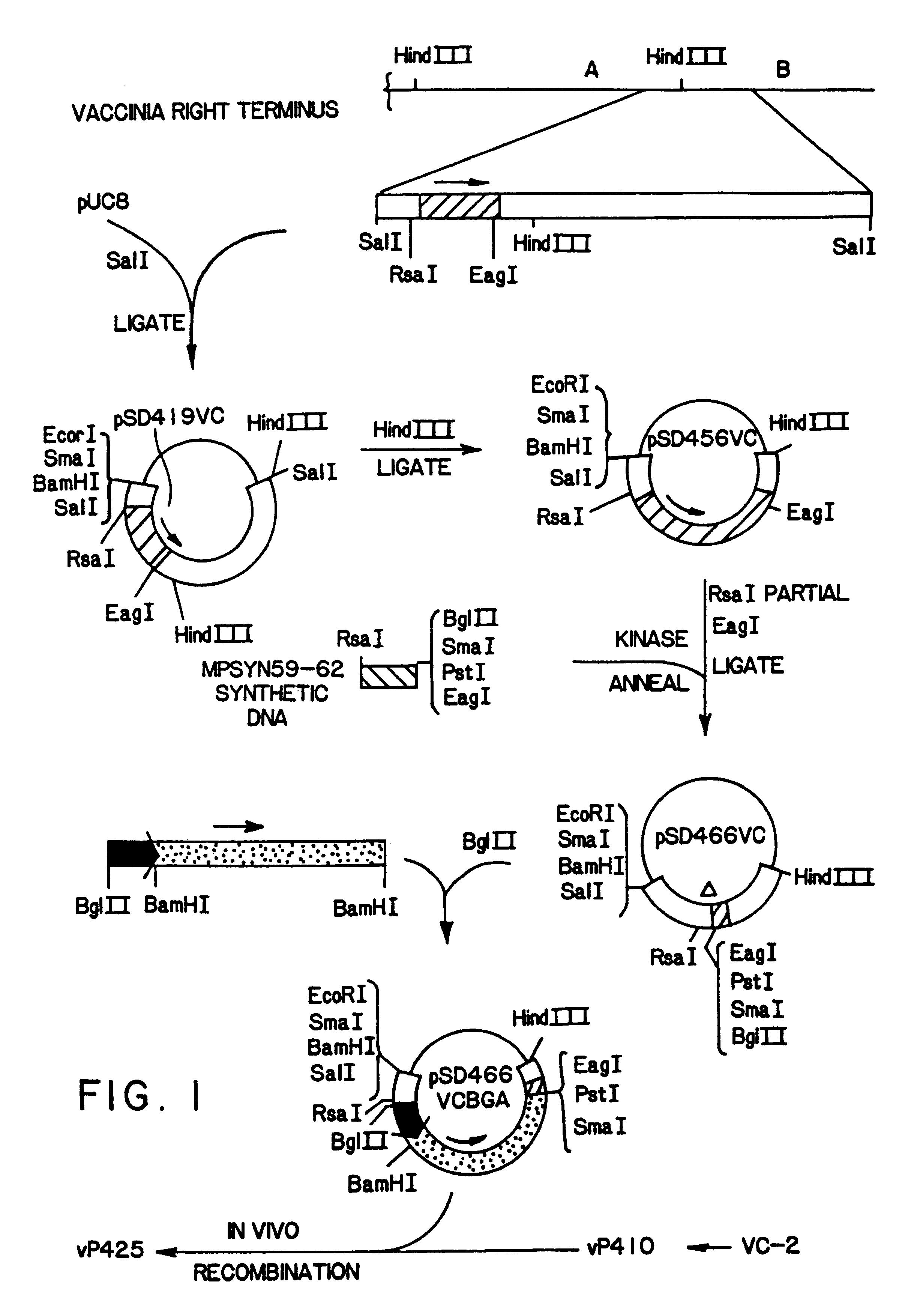
What is described is a recombinant poxvirus, such as vaccinia virus, fowlpox virus and canarypox virus, containing foreign DNA from herpesvirus. In one embodiment, the foreign DNA is expressed in a host by the production of a herpesvirus glycoprotein. In another embodiment, the foreign DNA is expressed in a host by the production of at least two, particularly two or three, herpesvirus glycoproteins. What is also described is a vaccine containing the recombinant poxvirus for inducing an immunological response in a host animal inoculated with the vaccine. By the present invention, the barrier of maternal immunity in a newborn offspring can be overcome or avoided.
Owner:HEALTH RES INC
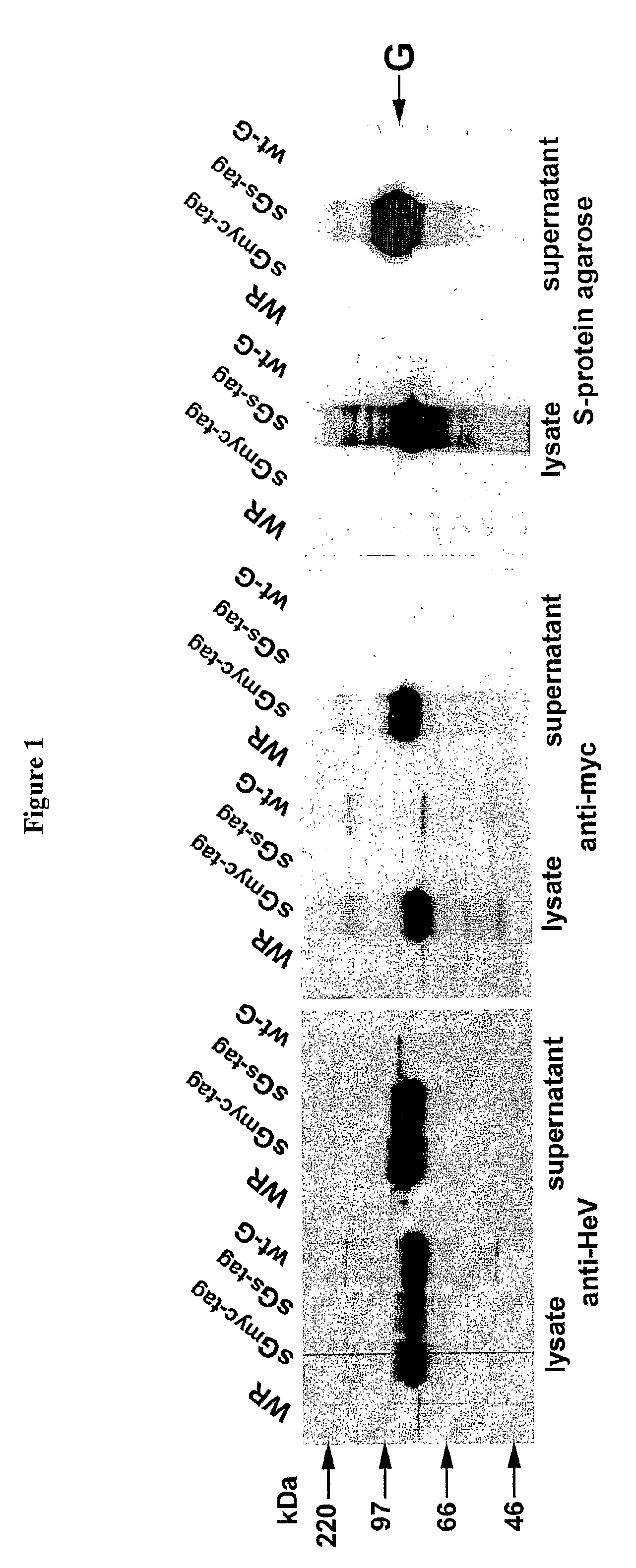
Soluble Forms of Hendra and Nipah Virus G Glycoprotein
ActiveUS20090041772A1Improve stabilityImproving immunogenicitySsRNA viruses negative-sensePeptide/protein ingredientsTherapeutic antibodyNeutralizing antibody
This invention relates to soluble forms of G glycoprotein from Hendra and Nipah virus. In particular, this invention relates to compositions comprising soluble forms of G glycoprotein from Hendra and Nipah virus and also to diagnostic and therapeutic methods using the soluble forms of G glycoprotein from Hendra and Nipah virus. Further, the invention relates to therapeutic antibodies including neutralizing antibodies, and vaccines for the prevention and treatment of infection by Hendra and Nipah viruses.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Nipah virus vaccines
ActiveUS20070031455A1Easy to storeSsRNA viruses negative-senseSugar derivativesViral glycoproteinViral Vaccine
The present invention relates to recombinant anti-Nipah virus vaccines and the administration of such vaccines to animals, advantageously pigs. Advantageously, the anti-Nipah virus vaccine may comprise a recombinant avipox virus containing a Nipah virus glycoprotein gene. The invention encompasses methods of vaccinating animals, advantageously pigs, by administration of anti-Nipah virus vaccines that may comprise a recombinant avipox virus that may contain a Nipah virus glycoprotein gene.
Owner:MERIAL INC
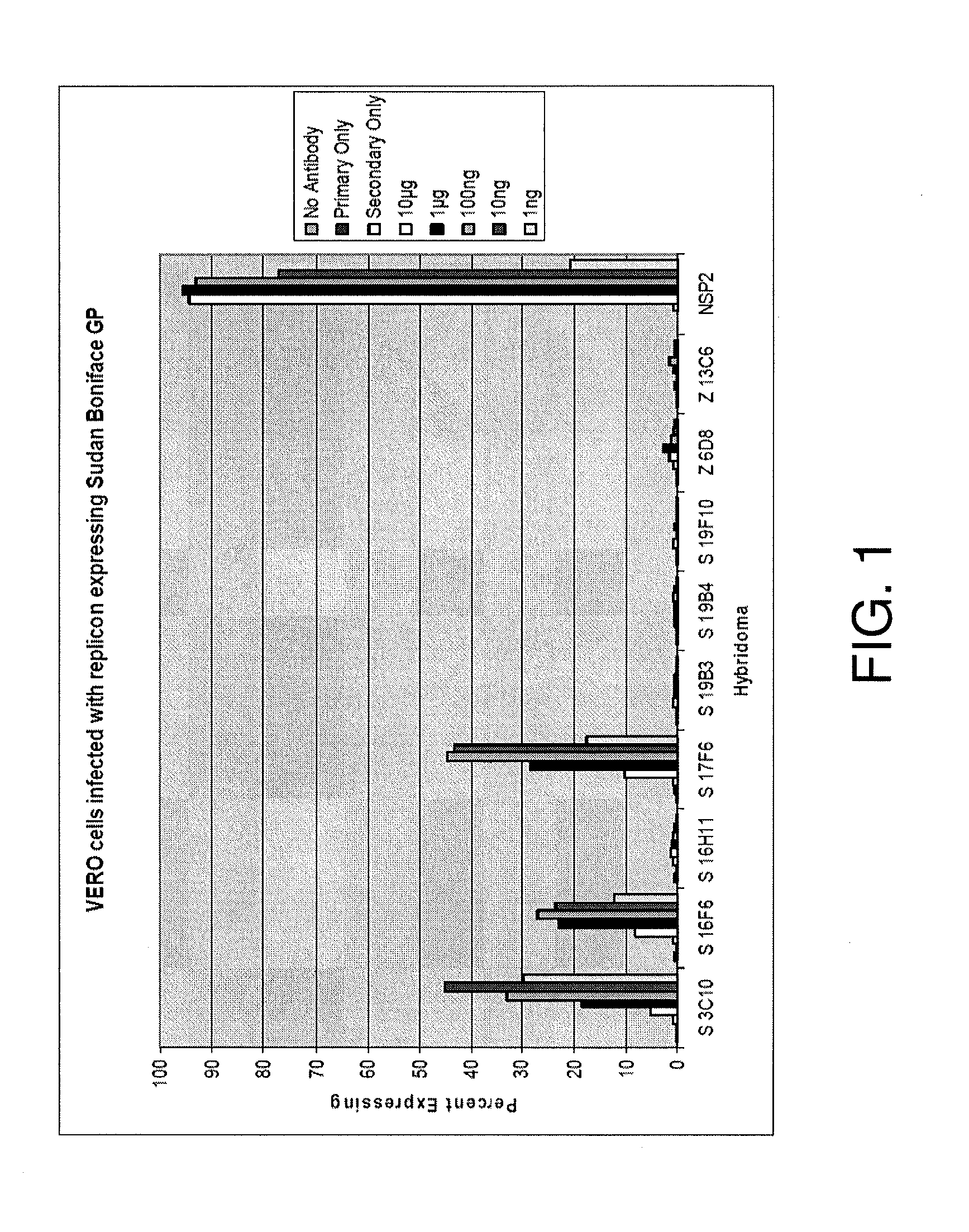
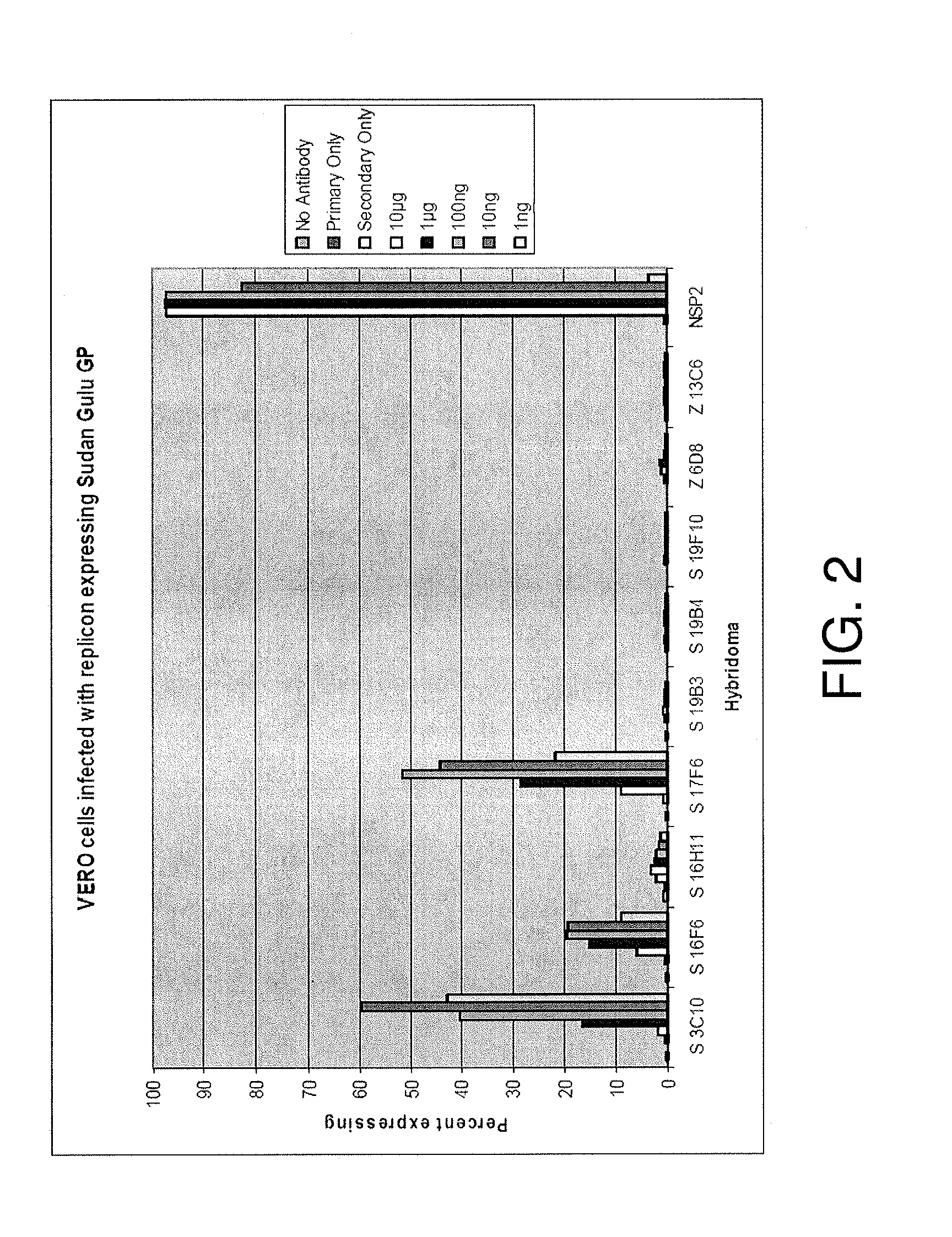
Monoclonal antibodies against glycoprotein of ebola sudan boniface virus
ActiveUS20120164153A1Facilitate identificationSsRNA viruses negative-senseGenetic material ingredientsGlycoproteinTherapeutic treatment
We disclose Ebola Sudan Boniface virus GP Monoclonal antibodies, epitopes recognized by these monoclonal antibodies, and the sequences of the variable regions of some of these antibodies. Also provided are mixtures of antibodies of the present invention, as well as methods of using individual antibodies or mixtures thereof for the detection, prevention, and / or therapeutic treatment of Ebola Sudan Boniface virus infections in vitro and in vivo.
Owner:THE US SEC THE ARMY ON BEHALF OF USAMRMC
Method for immobilizing viral glycoproteins for use in solid-phase immunoassays
InactiveUS6165710ABioreactor/fermenter combinationsBiological substance pretreatmentsAntigenViral antibody
A process for selectively immobilizing viral glycoproteins on lectin-coated surfaces for use in solid phase immunoassays is disclosed. This method does not require that the virus or antigen be purified prior to immobilization. This method provides an inexpensive and effective immunoassay method to screen fluids for the presence of viral antibodies.
Owner:ROBINSON JAMES E
Kit for testing neutralizing antibody racing ELISA in human and animal rabies
InactiveCN101251537AAccurate quantitative determinationEasy to operateBiological testingAntigenRabies
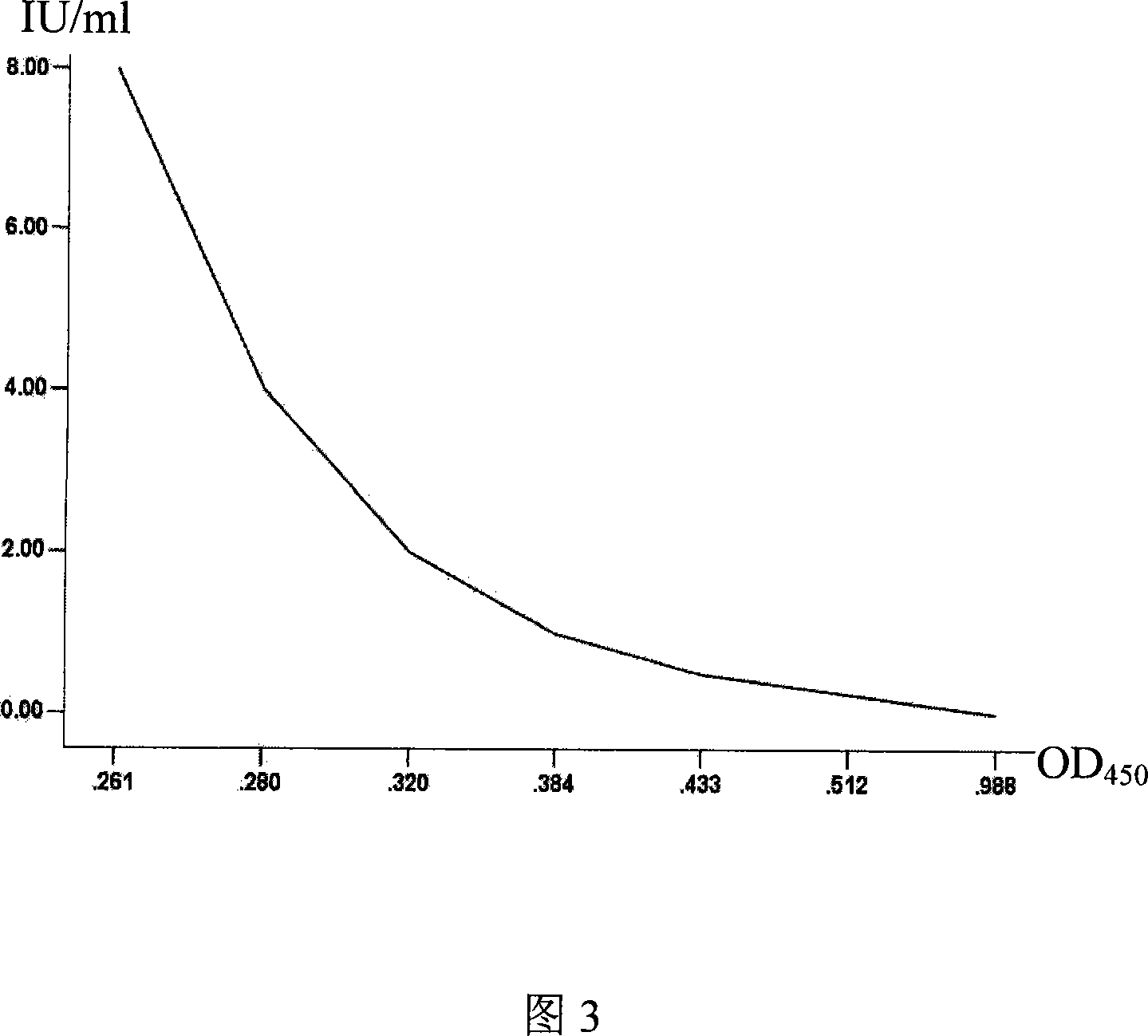
The invention discloses a reagent box for detecting hydrophobia neutralizing antibody competition ELISA of human beings and animals, wherein the reagent box can easily, quickly, accurately and quantitatively detect the hydrophobia neutralizing antibody in blood serums of human beings and animals by marking the hydrophobia neutralizing antibody, the standard serum and the envelope antigen. By using hydrophobia virosome or virus glycoprotein to coat enzyme synapticulae, the enzyme labeling hydrophobia neutralizing antibody is mixed with the blood serum to be tested and the standard serum respectively according to a certain ratio and reacts with the hydrophobia virus glycoprotein antigen coated on the enzyme synapticulae, a standard curve is drawn according to the OD value of the standard blood serum reaction and the known neutralizing titer after the color development, and the titer of the corresponding neutralizing antibody is obtained from the standard curve according to the OD value of the reaction of the blood serum to be tested. The reagent box has the advantages of accurately and quantitatively detecting the neutralizing antibody of the hydrophobia virus, along with simple operation and short time; moreover, the test result of the invention keeps a good consistence with test results of neutralizing test methods recommended by WHO and OIE.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Attenuated Live Triple G Protein Recombinant Rabies Virus Vaccine for Pre- and Post-Exposure Prophylaxis of Rabies
The invention provides a recombinant rabies viruses comprising three copies of a mutated G gene wherein each G gene encodes a rabies virus glycoprotein having the amino acid 194 mutated to a serine and the amino acid 333 is mutated to a glutamic acid. The recombinant rabies virus is nonpathogenic in immunodeficient mammals and can be used in a vaccine to induce an immune response protect mammals from infection by rabies virus as well as clear a pre-existing rabies virus infection from neural tissues.
Owner:THOMAS JEFFERSON UNIV
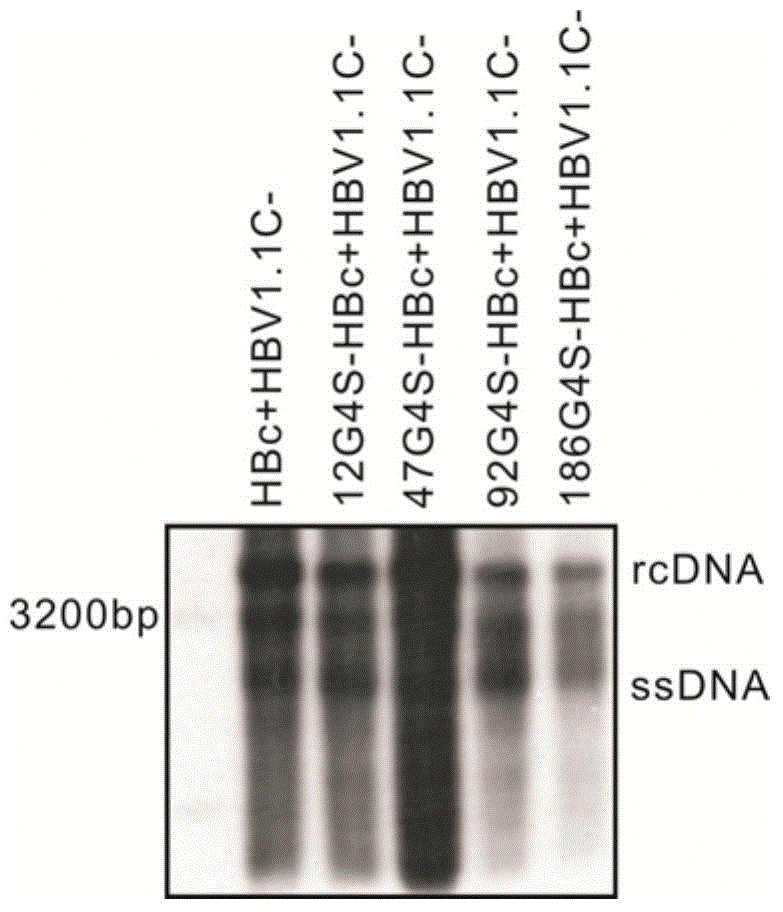
Recombinant human hepatitis B virus core protein fused protein
The invention discloses a recombinant human hepatitis B virus core protein fused protein. The fused protein comprises a protein (X), a linker peptide (L) and a hepatitis B virus core protein (HBc) from the end N to the end C in sequence; the linker peptide (L) has the amino acid sequence of Gly-Ser-(Gly-Gly-Gly-Gly-Ser)n, and n is an integer between 2 and 20 and is 9 or 18, particularly; the end C of the linker peptide (L) is connected with the end N of the hepatitis B virus core protein (HBc); the end C of the protein (X) is connected with the end N of the linker peptide (L); and the protein (X) is a red fluorescent protein or vesicular stomatitis virus G glycoprotein. The hepatitis B virus core protein (HBc) is connected with the functional protein, and the functions of the proteins on two ends of the linker peptide (L) can be both guranteed. The problem in the prior art that the functions of the hepatitis B virus core protein (HBc) and the functions of the functional protein can not be both guaranteed after the hepatitis B virus core protein (HBc) is fused with the functional protein is solved. The fused protein is of great importance to the research on the hepatitis B virus (HBV).
Owner:CHONGQING MEDICAL UNIVERSITY
Dog anti rabies virus antibody colloidal gold immunochromatography assay detection reagent plate and preparation method thereof
This invention relates to one anti-rabies virus antigen gold immune analysis test agent board and its process method, which comprises the following steps: paving glass fiber paper and aqua fortis fiber film on the underlay film of polyethylene board and polychloroethylene underlay film; covering the film onto the test line and comparison line; the test line is added with gold detector ester polymer film; the comparison line side is added with water absorptive pad; the test line is to cover the virus protein. This invention process method is to cover virus protein analysis film by glue gold label rabbit anti-virus IgG multi-clone antigen to process gold detection combination pad.
Owner:WUHAN J H BIO TECH
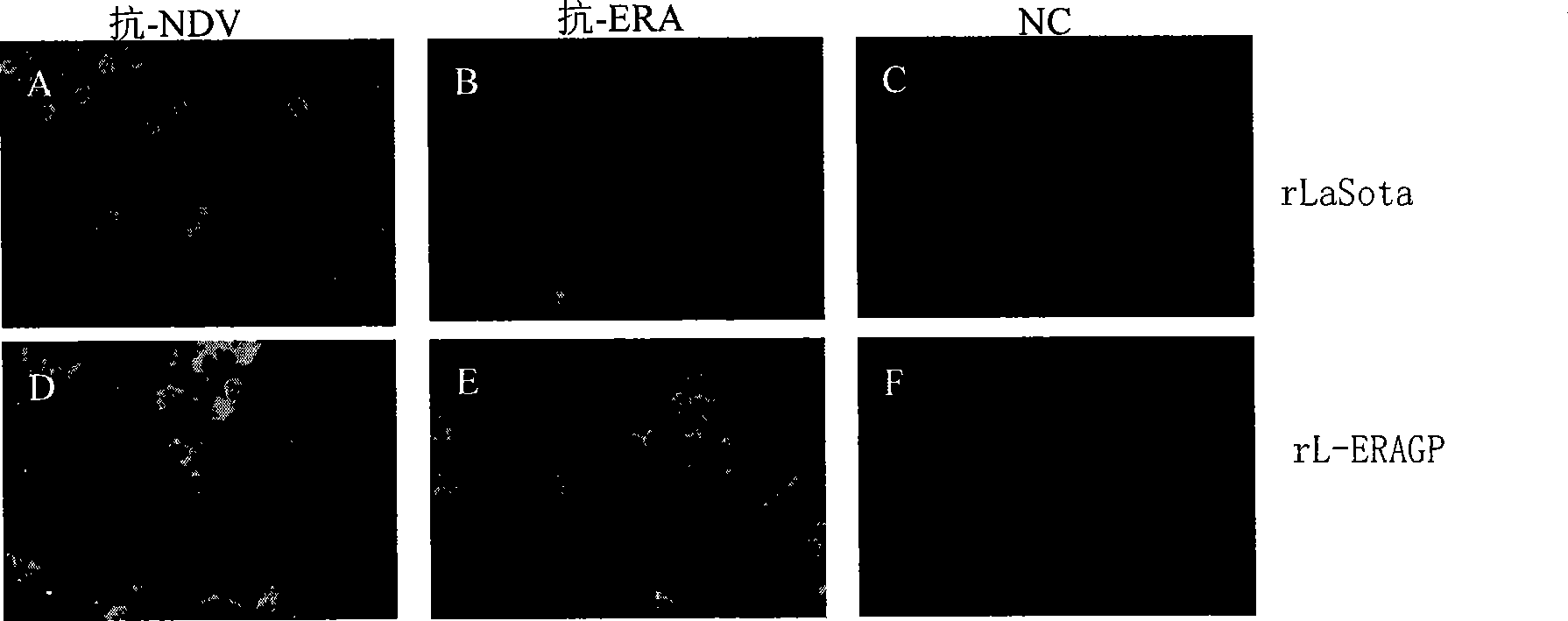
Recombinant new castle disease LaSota attenuated vaccine strain expressing rabies virus glucoprotein (GP protein)
The invention relates to a Newcastle disease LaSota lentogen vaccine which expresses rabiesvirus glycosidoprotein (GP protein); more specifically, the Newcastle disease LaSota lentogen vaccine is rL-ERAGP. The invention also discloses a method for preparing the Newcastle disease LaSota lentogen vaccine and an application of the Newcastle disease LaSota lentogen vaccine in preparing bivalent vaccine used to prevent rabies and Newcastle diseases.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Rabies virus glycoprotein and nucleoprotein antigen epitope polypeptides, and screening and identification method and application thereof
The invention belongs to the technical field of biology, and particularly relates to screening and identification of antigen epitope polypeptides. The invention discloses screening, identification and application of a series of rabies virus glycoprotein and nucleoprotein antigen epitope polypeptides. The rabies virus glycoprotein and nucleoprotein are predicted by biological information means to obtain the candidate epitope polypeptides; and a lymphopoiesis experiment, ELISPOT experiment and a stream-type cell method are utilized to carry out in-vitro experimental verification on the subsequent epitope polypeptides to obtain the four rabies virus protein antigen epitope polypeptides. The invention is characterized in that the antigen epitope polypeptides respectively comprise a Th epitope and a CTL epitope, can stimulate the lymphopoiesis of the vaccine-immunized mouse in vitro and induce the cells to secrete related cell factors, and have the functions of killing virus-infected cells and stimulating the generation of the antibody. The invention can be used for developing rabies virus epitope vaccines and detecting the vaccine effect, and has important value for developing and producing immunologic function detection kits for rabies virus vaccines.
Owner:FUDAN UNIV
Inhibitors of filovirus entry into host cells
Organic compounds showing the ability to inhibit viral glycoprotein (GP)-mediated entry of a filovirus into a host cell are disclosed. The disclosed filovirus entry inhibitor compounds are useful for treating, preventing, or reducing the spread of infections by filovirus including the type species Marburg virus (MARV) and Ebola virus (EBOV). Preferred inhibitors of the invention provide therapeutic agents for combating the Ivory Coast, Sudan, Zaire, Bundibugyo, and Reston Ebola virus strains.
Owner:MICROBIOTIX
Monoclonal antibodies against glycoprotein of Ebola sudan boniface virus
ActiveUS9097713B2SsRNA viruses negative-senseImmunoglobulins against virusesEpitopeViral glycoprotein
We disclose Ebola Sudan Boniface virus GP Monoclonal antibodies, epitopes recognized by these monoclonal antibodies, and the sequences of the variable regions of some of these antibodies. Also provided are mixtures of antibodies of the present invention, as well as methods of using individual antibodies or mixtures thereof for the detection, prevention, and / or therapeutic treatment of Ebola Sudan Boniface virus infections in vitro and in vivo.
Owner:US DEPT OF THE ARMY REPRESENTED BY THE SEC OF THE ARMY
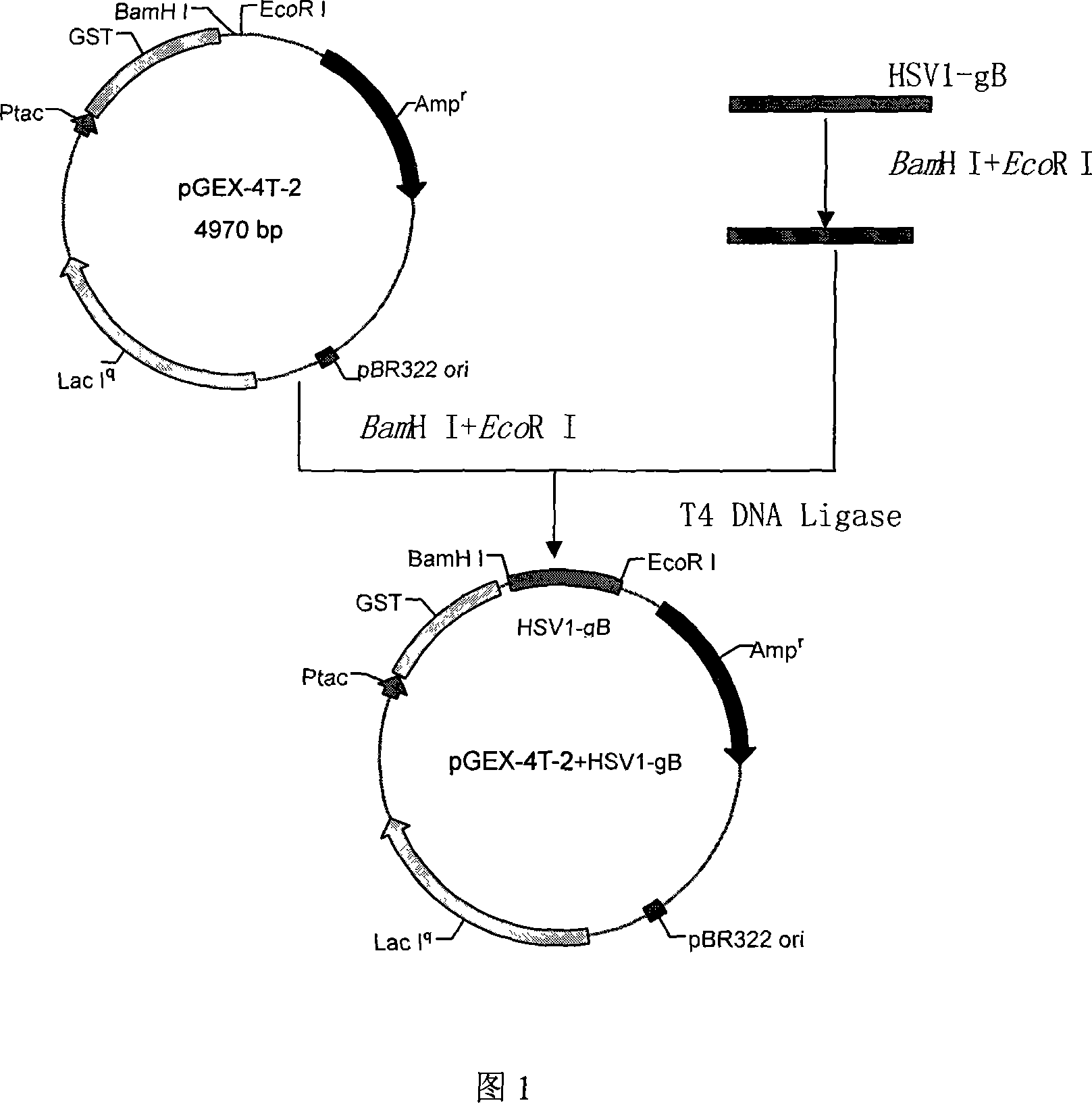
Chemically synthesized HSV1 virus gB glucoprotein extracellular region gene fragment, representation and application of the same
InactiveCN101173290APromote safe productionLow costGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsGenetic engineeringGlycoprotein
The chemically synthesized HSV1 viral gB glycoprotein extracellular region gene fragment and its expression and application relate to the fields of genetic engineering technology, vaccines and diagnostic reagents. The present invention screens out the strong epitope in the gB glycoprotein of HSV1 virus through computer analysis, from the first amino acid to the 696th amino acid, a total of 696 amino acids, selects codons favored by both eukaryotic and prokaryotic organisms, and chemically synthesizes The brand-new gene sequence of the antigenic epitope uses genetic engineering technology to express the gene fragment and prepare a strong antigenic epitope fragment of the gB glycoprotein of the HSV1 virus. The expressed strong antigenic epitope fragment of gB glycoprotein of HSV1 virus can be used for the detection of vaccine, HSV1 virus antibody or antigen, and for immunization preparation of anti-HSV1 virus monoclonal antibody and polyclonal antibody and the like.
Owner:李越希
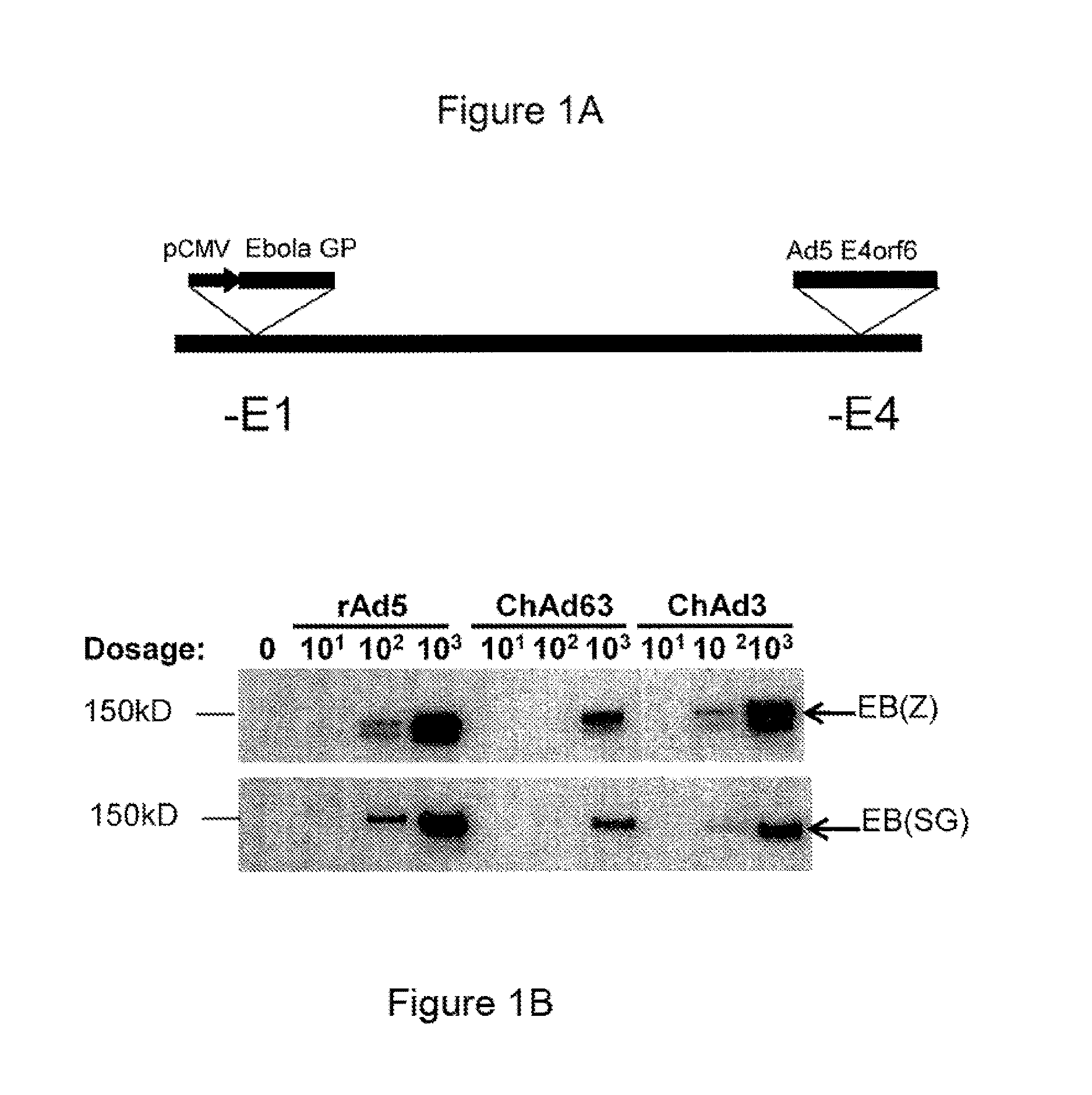
Methods for the induction of ebola virus-specific immune responses comprising administering a replication-defective chimpanzee adenovirus vector expressing the ebola virus glycoprotein
ActiveUS9526777B2SsRNA viruses negative-senseViral antigen ingredientsViral glycoproteinMarburg virus
This invention provides vaccines for inducing an immune response and protection against filovirus infection for use as a preventative vaccine in humans. In particular, the invention provides chimpanzee adenoviral vectors expressing filovirus proteins from different strains of Ebola virus (EBOV) or Marburg virus (MARV).
Owner:UNITED STATES OF AMERICA +1
Nipah Virus Vaccines
ActiveUS20100278862A1SsRNA viruses negative-senseViral antigen ingredientsViral glycoproteinPox viruses
The present invention relates to recombinant anti-Nipah virus vaccines and the administration of such vaccines to animals, advantageously pigs. Advantageously, the anti-Nipah virus vaccine may comprise a recombinant avipox virus containing a Nipah virus glycoprotein gene. The invention encompasses methods of vaccinating animals, advantageously pigs, by administration of anti-Nipah virus vaccines that may comprise a recombinant avipox virus that may contain a Nipah virus glycoprotein gene.
Owner:MERIAL INC
Antibodies against f glycoprotein of hendra and nipah viruses
The present invention relates to antibodies or antibody fragments that bind, neutralize, and / or inhibit Hendra or Nipah virus. The invention provides antibodies or antibody fragments that selectively bind to the F glycoprotein of Hendra or Nipah virus, and pharmaceutical compositions including such antibodies and / or fragments. The invention further provides polynucleotides encoding the antibodies and fragments of the invention and host cells transformed therewith. Additionally, the invention discloses prophylactic, therapeutic, and diagnostic methods employing the antibodies, fragments, polynucleotides, and / or compositions of the invention.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Method for constructing virus live vector recombinant vaccine by utilizing transposon
InactiveCN101850116AGenetic material ingredientsViruses/bacteriophagesSwine Fever VirusRecombinant vaccines
The invention discloses a method for constructing virus live vector recombinant vaccine by utilizing transposon. Green fluorescent protein is taken as a report gene, expression boxes respectively expressing rabies virus glycoprotein and swine fever E2 protein genes are constructed and are cloned to the shuttle vector of the transposon, under the action of mediation of transposase, recombination with purified canine adenovirus type II virus and herpes virus type I entire genome are respectively carried out, then transfection agent (liposome and the like) is utilized to respectively transfect the recombination product with MDCK and Vero cells, thus obtaining four strains of recombinant viruses taking green fluorescent protein as report gene, namely recombinant canine adenovirus type II virus expressing glycoprotein, recombinant canine adenovirus type II virus expressing E2 protein, recombinant herpes virus type I expressing glycoprotein and recombinant herpes virus type I expressing E2 protein. Immunity test shows that the canine adenovirus type II virus expressing E2 gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to swine fever virus infection in swine and canine adenovirus type II virus expressing glycoprotein gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to rabies virus infection in dog.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Pseudotyped retroviruses and stable cell lines for their production
InactiveUS7033595B1Effective blockingSsRNA viruses positive-senseVectorsViral glycoproteinNucleotide
Cells that produce inventive pseudotyped retroviruses having a broad host range have been produced. In one aspect of the invention, the cells produce retroviruses pseudotyped with at least two different viral glycoproteins, such as togaviral glycoproteins. In alternative embodiments, the cells produce retroviruses pseudotyped with filoviral glycoproteins. Methods of producing the above-described cells, as well as the pseudotyped retroviruses thus produced, are also provided. In other embodiments, methods of screening agents effective in blocking viral entry into a cell, including filoviral entry or entry of viruses having at least two different viral glycoproteins disposed in their lipid bilayer, such as togaviruses, are provided. Moreover, methods of using the inventive pseudotyped retroviruses for introducing nucleotide sequences into target cells, and kits for forming the inventive pseudotyped retroviruses, are also provided.
Owner:PURDUE RES FOUND INC
Herpes Simplex Virus Complex
ActiveUS20110244576A1Improve efficiencyHigh affinityGenetic material ingredientsVirus peptidesDiseaseCancer cell
There is provided an HSV complex which comprises an avirulent HSV and a targeting agent which allows the HSV particle to infect and lyse a specific targeted cell. The inventors have found a way in which avirulent HSV can be targeted to disease cells, e.g. cancer cells, by incorporating an antibody binding domain into one or more viral glycoproteins.
Owner:VIRTTU BIOLOGICS
Human umbilical cord mesenchymal stem cell membrane granules, preparation and applications thereof
InactiveCN107446884AHas a repairing effectYouthfulCell dissociation methodsSkeletal/connective tissue cellsSurface markerCell membrane
The invention relates to human umbilical cord mesenchymal stem cell membrane granules, preparation and applications thereof. The surfaces of the cell membrane granules express surface markers of human umbilical cord mesenchymal stem cells and wrap biomacromolecules and organelles; the biomacromolecules comprise mRNA products of dry genes of the human umbilical cord mesenchymal stem cells; the organelles comprise mitochondria, proteasome, lysosome and autophagosome; and the cell membrane granules does not contain cell nucleuses, cell nucleuse DNA and nucleoproteins as well as virus glycoproteins VSV-G. 1-5mum cell membrane granules can be formed by a filtering membrane with the pore diameter of 3mum in mechanical extrusion, are larger, can be used for delivering the biomacromolecules and the organelles in vitro and delivering the biomacromolecules and the organelles to the liver and the spleen, and has a function of repairing aged cells and injured cells. The human umbilical cord mesenchymal stem cell membrane granules, the preparation and the applications have the advantages that due to no containing of the VSV-G, the generation of immunoreactions for viral proteins is avoided; and due to no containing of nuclear DNA, the probability of inducing canceration of target cells is almost zero.
Owner:SHANTOU UNIV
Chimeric lyssavirus nucleic acids and polypeptides
InactiveUS7235245B2Improve the level ofImprove protectionSsRNA viruses negative-senseSsRNA viruses positive-senseDNA vaccinationEpitope
The present invention provides chimeric nucleic acids, preferably contained on an expression vector, that encode chimeric immunogenic polypeptides. The nucleic acids encode at least site III of a lyssavirus glycoprotein, which has been found to improve the immunogenicity of lyssavirus epitopes for protection from rabies. The chimeric nucleic acids and proteins can also contain antigenic determinants for epitopes other than those of lyssavirus. Thus, the invention provides chimeric nucleic acids and polypeptides that elicit a strong immune response to multiple antigens. Use of the methods of the present invention permits DNA vaccination without the need to supply multiple antigens on separate DNA molecules.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Test paper bar for testing colloidal gold of protective antibody of rabies virus
InactiveCN1963509AThe result is clear and easy to distinguishEasy to operateMaterial analysisViral glycoproteinColloid
This invention provides one glue gold test bar to test rabies virus protective antibody, which covers rabies virus protein and rabies multiple clone antibodies on the NC film and combines glue gold rabies virus antigen and applies film analysis double antigen clamper method and tests specimen rabies virus protective antibody.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
Hendra and nipah virus g glycoprotein immunogenic compositions
InactiveCN104244974ASsRNA viruses negative-senseViral antigen ingredientsViral glycoproteinNipah Virus Infection
Immunogenic compositions directed against Hendra and / or Nipah viruses, and methods of its use, are provided. In addition, methods of distinguishing subjects vaccinated with the immunogenic compositions of the invention from those infected with Hendra and / or Nipah virus are provided.
Owner:ZOETIS SERVICE LLC +1
Raccoon Poxvirus Expressing Rabies Glycoproteins
ActiveUS20090010963A1Effective immunizationEffective immunizingSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininViral glycoprotein
The present invention relates to recombinant raccoon poxvirus vectors that express the rabies virus glycoprotein gene at the hemagglutinin (ha) locus of the poxvirus genome or express the glycoprotein gene of the same or different rabies strains at the thymidine kinase (tk) and the hemagglutinin (ha) loci of the poxvirus genome, and their use as adjuvant-free vaccines. The raccoon poxvirus vector comprises the nucleic acid molecules encoding the glycoprotein of a Challenge Virus Standard rabies strain inserted and expressed at the tk locus of the poxvirus genome and of a Pasteur-Paris rabies strain inserted and expressed at the ha locus of the poxvirus genome. The vaccine may optionally contain a mixture of additional feline and canine antigens for immunization of animals. Also disclosed are methods for inducing an immune response to rabies in a mammal by administering to the mammal an effective immunizing amount of the vaccine of the invention.
Owner:ELANCO US INC
Murine original monoclonal antibody 3D8 identified hantaan virus glycoprotein neutralizing epitope peptide and application thereof
ActiveCN102731623ADirect determination of linear epitopesViral antigen ingredientsAntiviralsViral glycoproteinProtein target
The invention discloses a neutralizing monoclonal antibody 3D8 identified HTNV-GP specific B cell epitope and key amino acid residue sequence of the epitope for treating hemorrhagic fever with renal syndrome (HFRS) caused by hantaan virus (HTNV) infection. The B cell epitope has an amino acid sequence of 882GFLCPEFPGSFRKKC896. The epitope peptide can be applied to study of mechanism of 3D8 neutralizing monoclonal antibody in treatment of HFRS, or preparation of drug for treating HFRS and preparation of novel vaccine strain aiming at hantaan virus 76-118, or development of novel diagnostic kitfor hantaan virus 76-118 as a target protein. The invention has good prospects of development and application in the field of HFRS specific immunotherapy.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Lphavirus replicon vector-based rabies virus infectious clone, and preparation method and application thereof
ActiveCN110643632AControllable positioningEasy way to getSsRNA viruses negative-senseViral antigen ingredientsViral glycoproteinTGE VACCINE
The invention belongs to the technical field of biology, and particularly discloses an alphavirus replicon vector-based rabies virus infectious clone, and a preparation method and application thereof.The infectious clone of the strain is constructed by taking Venezuelan Equine Encephalitis Virus (VEEV) vaccine strain TC-83 replicon which lacks structural protein genes as a vector and inserting rabies virus Glycoprotein (G) genes into a deletion position of the vector, and the rescued strain serving as an attenuated live vaccine not only provides effective immune protection, but also is saferto use.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI +1
Recombinant human bivalent diabody against rabies virus and uses thereof
InactiveCN103596975ASsRNA viruses negative-senseImmunoglobulins against virusesVaccine PotencyViral glycoprotein
The present invention provides recombinant human bivalent diabody against rabies virus capable of recognizing rabies virus glycoprotein and neutralizing rabies viruses and a method for production thereof. The present invention further provides polynucleotide encoding the recombinant bivalent diabody. The bivalent diabody disclosed in the present invention is also useful for quantitation of the rabies virus glycoprotein for evaluating the vaccine quality and predicting the vaccine potency.
Owner:INDIAN IMMUNOLOGICALS LIMITED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com