Human umbilical cord mesenchymal stem cell membrane granules, preparation and applications thereof
A technology of stem cells and human umbilical cord, applied in the field of synthetic biology, can solve problems such as the limitation of clinical application of stem cells, and achieve the effect of avoiding immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation method of human umbilical cord mesenchymal stem cell membrane particles mainly comprises the following steps:
[0037] (1) Healthy full-term umbilical cord and amniotic membrane delivered by caesarean section were obtained from the Department of Obstetrics and Gynecology of the Second Affiliated Hospital of Shantou University School of Medicine with the consent of the family members and the approval of the school ethics committee;
[0038] (2) After washing with PBS to remove red blood cells, cut Wharton's jelly into small pieces of 3-4cm, remove blood vessels, cut into smaller pieces, and transfer to a 10cm culture plate, and incubate for 30 minutes until the tissue can be attached on the culture plate;
[0039] (3) Carefully add 5ml of high-sugar DMEM (10% fetal bovine serum, 100mg / ml penicillin, 100mg / ml streptomycin) culture solution, cultivate in a 5% CO2 humidified incubator at 37 degrees, replace the culture solution every two days, 5 - Fibrous c...
Embodiment 2
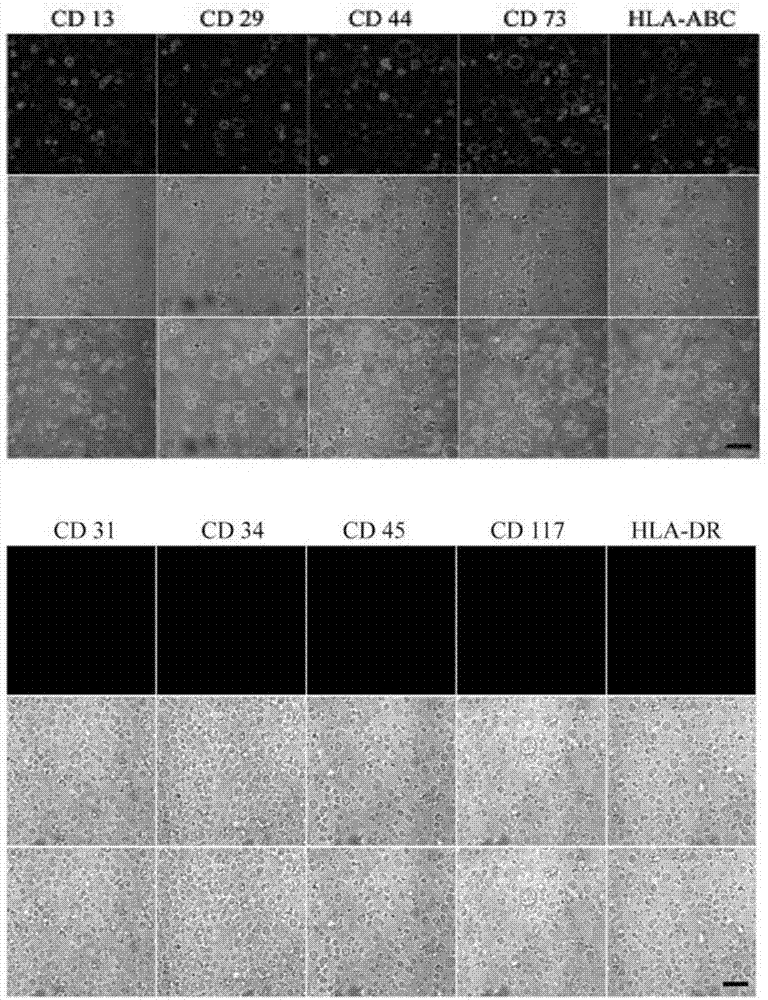
[0045] Collect 2x10 according to the method of embodiment 1 6 Human umbilical cord mesenchymal stem cells were resuspended in 200 μl of culture medium, and then added 1:50 diluted PE fluorescently labeled antibodies, including CD13, CD29, CD44, CD73, HLA-ABC, CD45, CD117, CD31, CD34 and HLA-DR antibody was incubated at 4°C for 1-2h, fixed with 1% paraformaldehyde, and then analyzed by flow cytometry. Analysis results such as figure 2 As shown, the human umbilical cord mesenchymal stem cells cultured in Example 1 express typical undifferentiated cell surface marker proteins, such as CD13, CD29, CD44, CD73 and HLA-ABC, but do not express cell differentiation coding marker proteins, such as CD45 , CD117, CD31, CD34 and HLA-DR.
Embodiment 3
[0047] Collect 2x10 6 Human umbilical cord mesenchymal stem cells were resuspended in 200 μl of culture medium, and then added 1:50 diluted PE fluorescently labeled antibodies, including CD13, CD29, CD44, CD73, HLA-ABC, CD45, CD117, CD31, CD34 and HLA-DR antibody, incubated at 4°C for 2h, washed 3 times, and then resuspended in 3mL culture medium. Place the polycarbonate membrane with a pore size of 3 μm in a 25mm replaceable membrane syringe filter, and tighten the filter, draw the cell suspension with a 10ml syringe, and forcefully push the cells from one side of the filter to the other. Collect in a 15ml centrifuge tube.
[0048] Collect the obtained human umbilical cord mesenchymal stem cell membrane particles, centrifuge at 1000g for 10min, gently absorb the supernatant, leave about 200μl of culture medium, and gently shake the centrifuge tube to obtain human umbilical cord mesenchymal stem cells with a size of about 1-5μm. Stem cell membrane granules. The obtained hum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com