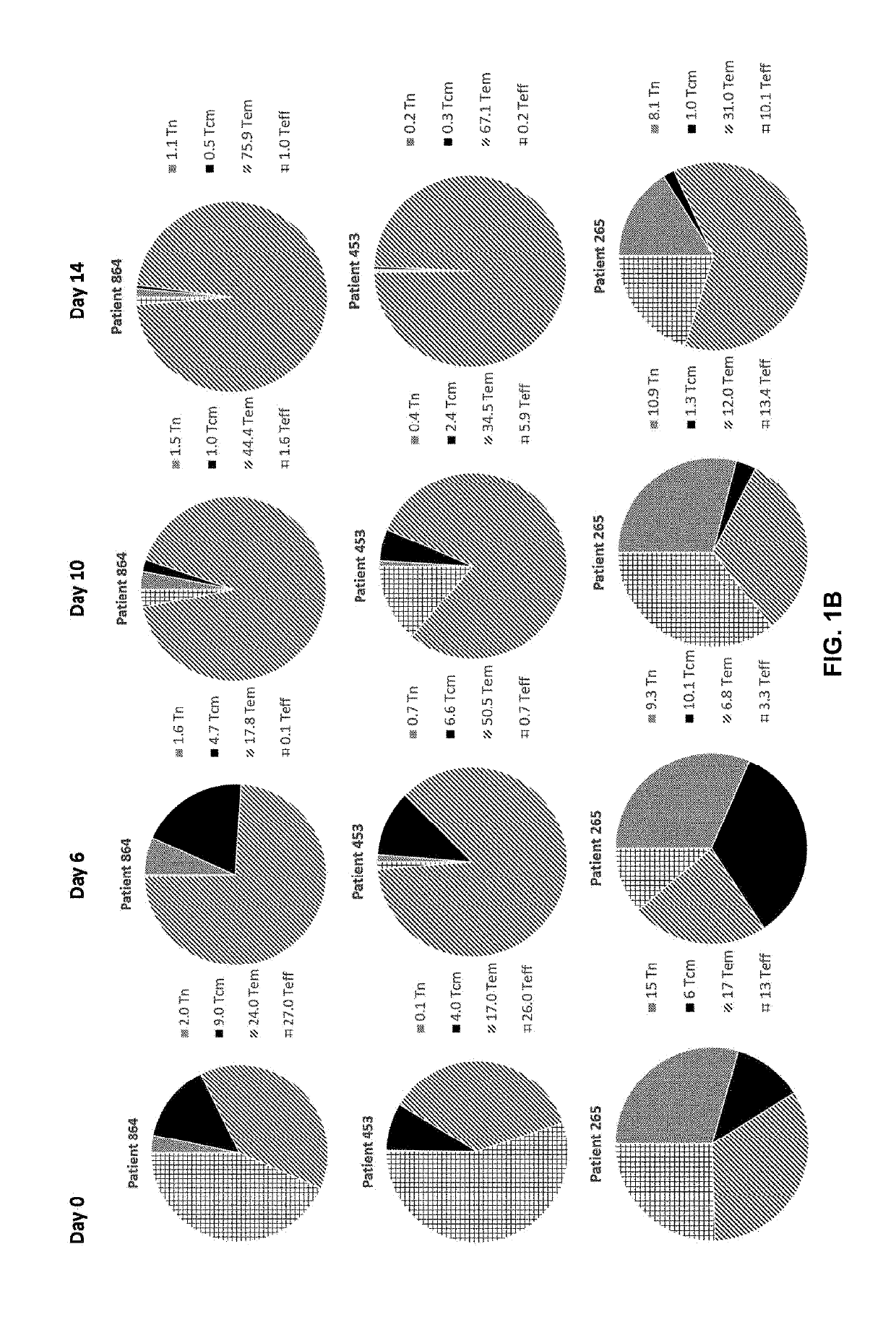
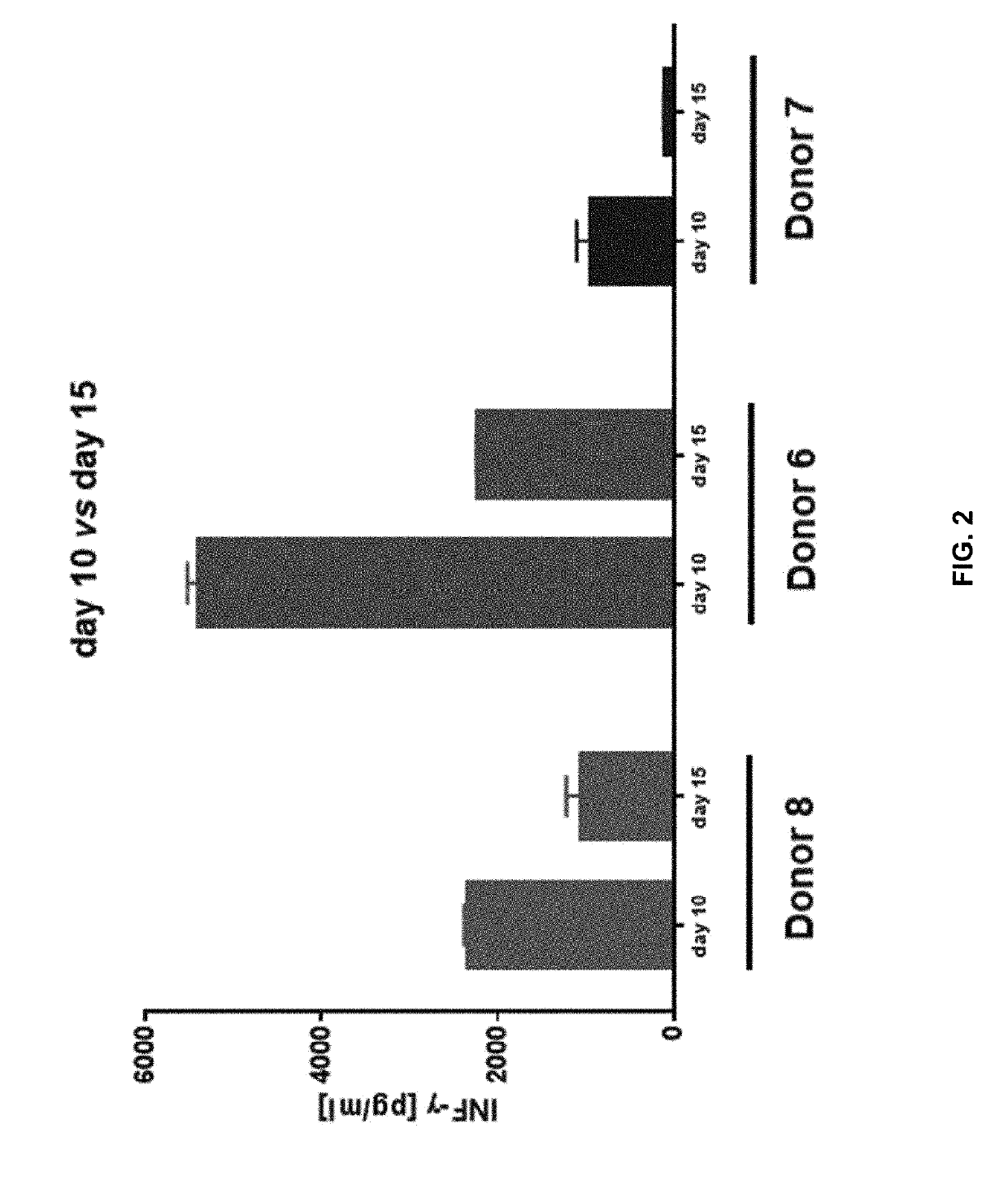
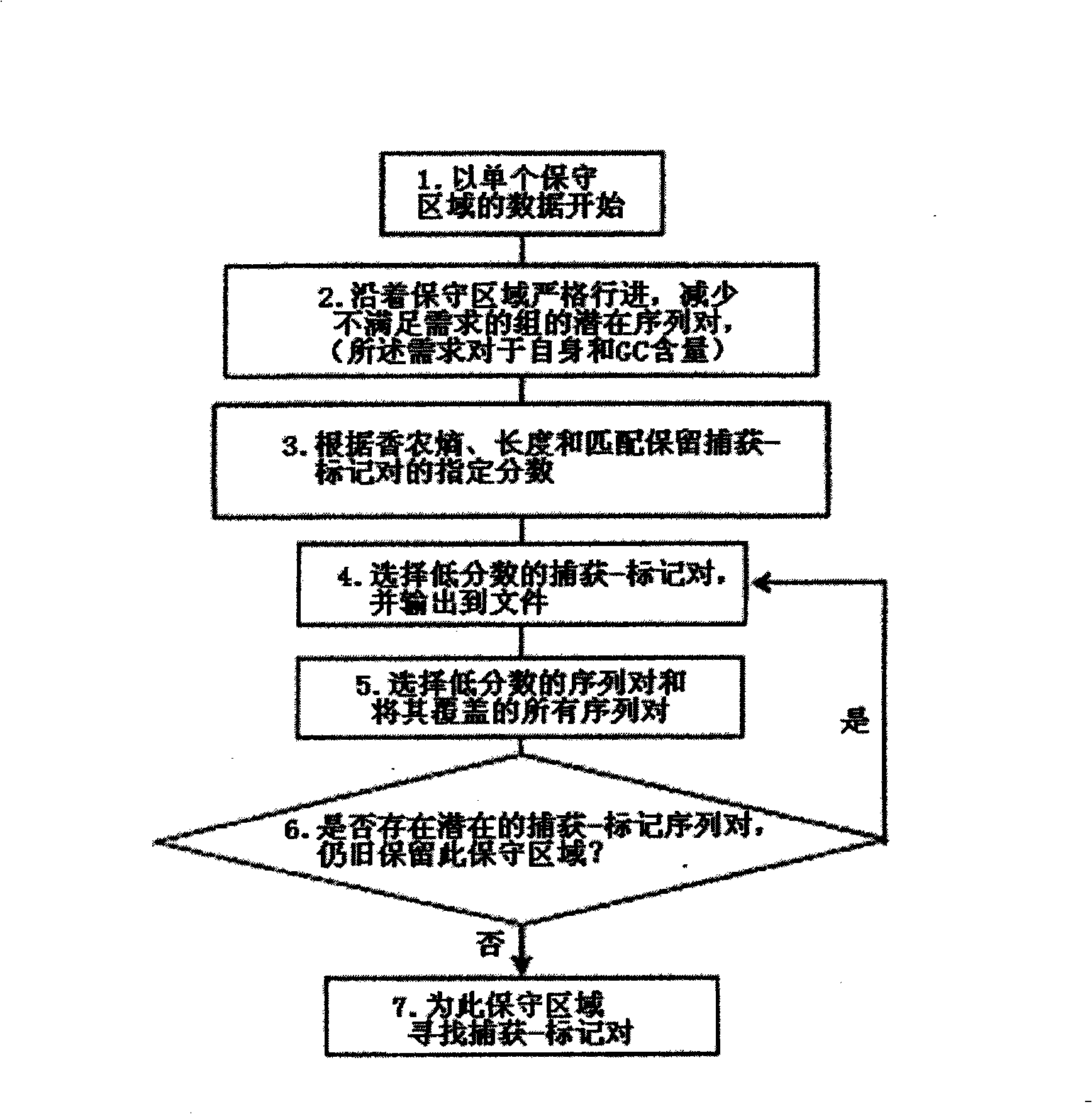
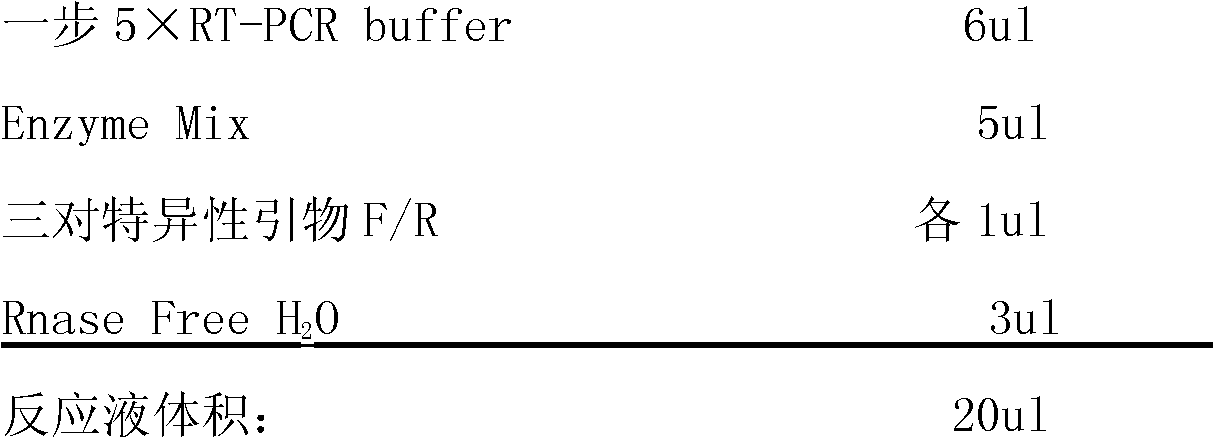Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
183 results about "Virus type" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Three types of viruses cause influenza, more commonly known as the flu. Influenza virus types A and B cause seasonal flu infections, which typically occur from late fall through early spring. Influenza type C infections occur far less frequently and typically cause a mild form of the illness.
Functional influenza virus-like particles (VLPs)
ActiveUS20050009008A1SsRNA viruses negative-senseVirus peptidesMultiple copyVirus Structural Proteins
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Virion Derived Protein Nanoparticles For Delivering Diagnostic Or Therapeutic Agents For The Treatment Of Non-Melanoma Skin Cancer
This invention relates to a transdermal delivery system for treating skin related diseases employing protein nanoparticles to deliver drugs to the keratinocytes and basal membrane cells for the treatment of non-melanoma skin cancer. The current invention presents an effective method for delivering small molecule nucleic acids to the epidermal cells.
Owner:AURA BIOSCI
Method of targeted gene delivery using viral vectors
Methods and compositions are provided for delivering a polynucleotide encoding a gene of interest to a target cell using a virus. The virus envelope comprises a cell-specific binding determinant that recognizes and binds to a component on the target cell surface, leading to endocytosis of the virus. A separate fusogenic molecule is also present on the envelope and facilitates delivery of the polynucleotide across the membrane and into the cytosol of the target cell. The methods and related compositions can be used for treating patients having suffering from a wide range of conditions, including infection, such as HIV; cancers, such as non-Hodgkin's lymphoma and breast cancer; and hematological disorders, such as severe combined immunodeficiency.
Owner:CALIFORNIA INST OF TECH
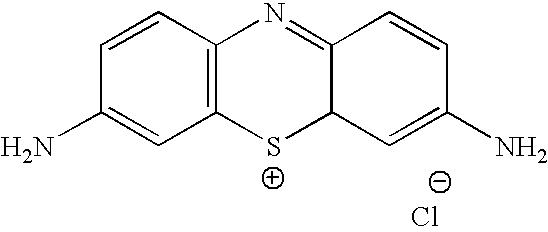
Methylene Blue Therapy of Viral Disease
InactiveUS20060264423A1Avoid infectionHeterocyclic compound active ingredientsAgainst vector-borne diseasesVirus typeHerpes zoster virus
A method for using thiazine dyes, especially methylene blue or methylene blue derivatives, in an immediate or controlled release formulation, alone or in combination with low levels of light or other drugs, to selectively inactivate or inhibit hepatitis infection, has been developed. Clinical trial results demonstrate efficacy in a human clinical trial for treatment of hepatitis C by oral administration of methylene blue immediate release formulation, in a dosage of 65 mg twice daily, over a period of at least 100 days. A method for using thiazine dyes, especially methylene blue or methylene blue derivatives, in an immediate or controlled release formulation, along or in combination with low levels of light or other drugs, to prevent or decrease reactivation of viruses, is also described. The preferred class of patient is infected with, or has been exposed to, viruses such as Herpes simplex virus type 1 & 2, Varicella zoster virus, Epstein-Barr virus, Cytomegalovirus, and Herpes virus type 6 & 7, Adenovirus, and Human polyoma viruses, e.g. JC virus and BK virus. In one embodiment the thiazine dye is administered to a patient experiencing symptoms or disease caused by reactivation of a virus. In a preferred embodiment the thiazine dye is administered to a patient at risk for or experiencing symptoms or disease caused by reactivation of a virus, prior to or during immunosuppression or chemotherapy.
Owner:BIOENVISION
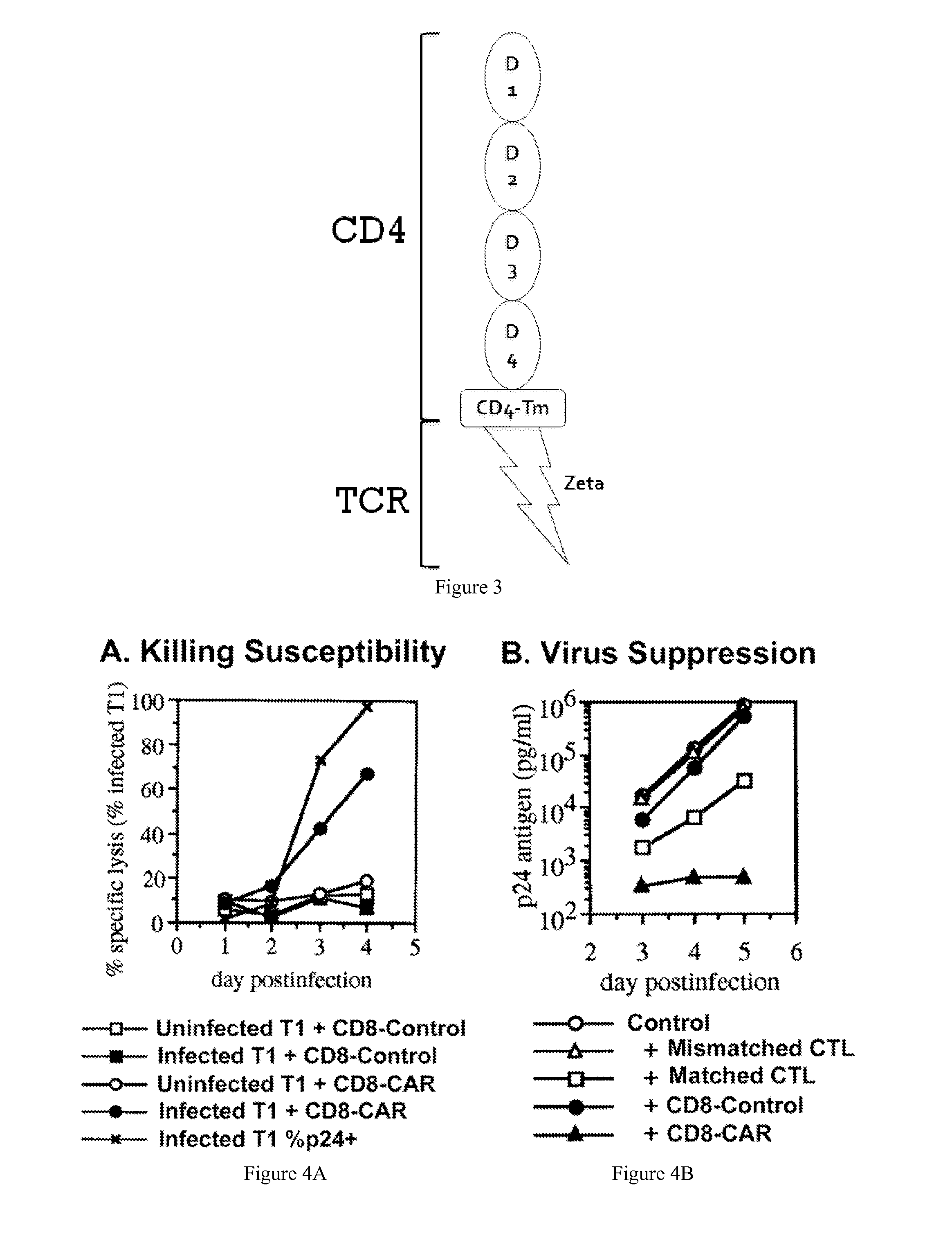
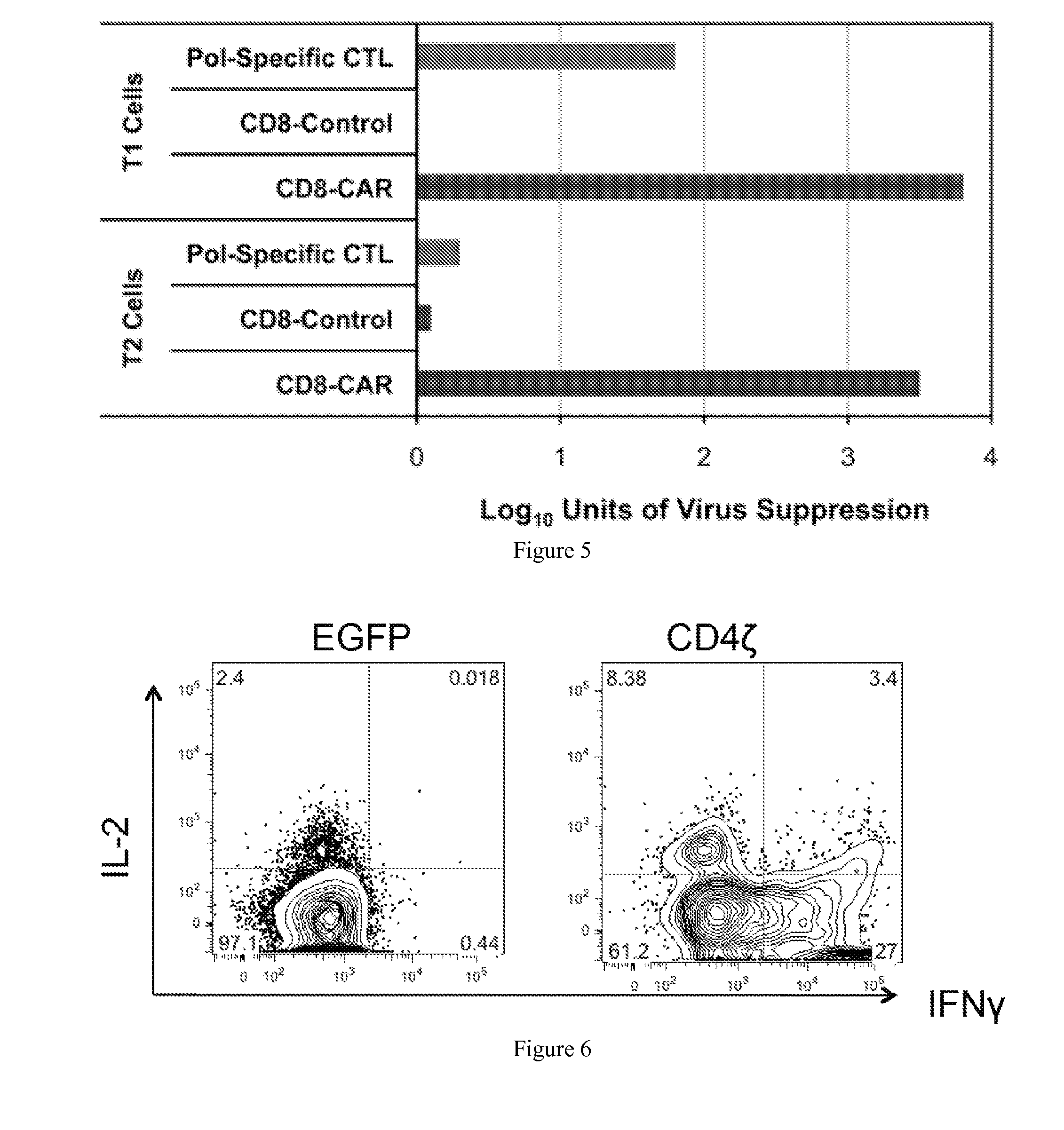
Engineering Antiviral T Cell Immunity through Stem Cells and Chimeric Antigen Receptors
The HIV-specific cytotoxic T lymphocyte (CTL) response is a critical component in controlling HIV replication and is an important part of the ultimate failure to eradicate the virus. Disclosed herein are methods for genetically enhancing the HIV-specific CTL response to allow long-term viral suppression or viral clearance. Human hematopoietic stem cells (HSCs) were genetically modified such that they differentiate into mature CTLs that will kill HIV infected cells. As disclosed herein, the functional effector cells are not human leukocyte antigen (HLA)-restricted. As disclosed herein, stem cells are transduced with non HLA-restricted chimeric antigen receptors (CARs) that allow the recognition of HIV or HIV infected cells when expressed by a CTL.
Owner:RGT UNIV OF CALIFORNIA
Methods for manufacturing t cells
ActiveUS20190247433A1Shorten the resting timeGenetically modified cellsGenetic material ingredientsVirus typeCD28
The disclosure relates to methods of manufacturing T cells for adoptive immunotherapy. The disclosure further provides for methods of genetically transducing T cells, methods of using T cells, and T cell populations thereof. In an aspect, the disclosure provides for methods of thawing frozen peripheral blood mononuclear cells (PBMC), resting the thawed PBMC, activating the T cell in the cultured PBMC with an anti-CD3 antibody and an anti-CD28 antibody immobilized on a solid phase, transducing the activated T cell with a viral vector, expanding the transduced T cell, and obtaining expanded T cells.
Owner:IMMATICS US INC
Cryopreservation method of umbilical cord tissue blocks
ActiveCN104472473AImprove permeabilityAvoid generatingDead animal preservationVirus typeUmbilical cord tissue
The invention discloses a cryopreservation method of umbilical cord tissue blocks, and relates to a cryopreservation method of umbilical cords. The invention aims at solving the problems that the preservation time of umbilical cord tissues is short, and the cell death of the umbilical cord tissues is easily caused at present. The method comprises the following steps: 1, carrying out treatment of the tissue blocks, namely in a sterile operating platform, carrying out disinfection treatment on an umbilical cord, cleaning the umbilical cord normal saline, shearing the umbilical cord into segments, peeling off a wharton jelly, placing the wharton jelly in a clean centrifuging tube and cutting the wharton jelly into pieces; and 2, adding DMEM in the tissue blocks, resuspending the tissue blocks, then adding a pre-cooling cryopreservation liquid, sub-packaging the tissue blocks in cryopreservation tubes, cooling by use of a program-controlled cooler, and transferring the tissue blocks in liquid nitrogen for long-time storage after bacterial detection, microbiological detection and virus detection are all negative, so as to finish the cryopreservation of the umbilical cord tissue blocks. The directly-inoculated adherent survival rate of the cryopreserved and unfrozen tissue blocks in culture bottles is more than 98%, the cell growth is good, and moreover, an osteogenic differentiation experiment carried out on the cultured cells of the cryopreserved tissue blocks indicates that the differentiation function is high. The method is used for storing the umbilical cord tissues.
Owner:天晴干细胞股份有限公司
Foot-and-mouth disease virus detecting test paper tape and its preparation method and using method
ActiveCN1877331ASimple and fast operationIntuitive and easy to judgeBiological testingMedicinePaper tape
Disclosed are a method for preparing and virus type definition test paper for foot and mouth disease and the using method. The test paper comprises O, A, Asia l, C four kinds of serum detecting test paper. The test paper comprises a PVC substrate plate, nitrocellulose membrane, an absorbing pad, a golden mark pad and a sample pad. The PVC substrate plate is installed at the most bottom layer; the nitrocellulose membrane is set on the middle part of the substrate plate; the absorbing pad is stuck on the left end of the nitrocellulose membrane, and the golden mark pad is set on the right end of the nitrocellulose membrane; the sample pad is set on the right upper end of the golden pad. The invention can identify the foot and mouth virus O, A, Asia l and C four serum type.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Preparation method and kit of cytokine-induced killing cell for inducing antibody-dependent cellular cytotoxicity
The invention provides a preparation method of a cytokine-induced killing cell for inducing antibody-dependent cellular cytotoxicity. The preparation method comprises constructing an interferon gamma signal peptide-tumor cell combined peptide, bonding 2th and 3th constant region gene segments of a crystallizable fragment domain heavy chain to the combined peptide, recombining the combined chain to a viral vector, transfecting a human cytokine-induced killing cell and carrying out high expression of a 2th and 3th constant region fusion protein of a novel polypeptide-crystallizable fragment domain heavy chain. The preparation method can cause antibody-dependent cellular cytotoxicity, improve a cytokine-induced killing cell proliferation multiple to 1000 times or more, a CD3+ / CD56+ expression rate to 40% or more and a CD16+ expression rate to 30% or more. In-vivo and in-vitro tests prove that the cytokine-induced killing cell has strong tumor killing toxicity. The invention also provides a kit for autologous cytokine-induced killing cell proliferation culture and has a high-efficiency anti-tumor function.
Owner:ZICHENG RUISHENGHUI BEIJING BIOTECH DEV CO LTD
Lentivirus vector expression system of luciferase and application thereof
InactiveCN102477445AVector-based foreign material introductionForeign genetic material cellsLymphatic SpreadSerial passage
The invention relates to a lentivirus vector expression system of luciferase and an application thereof. Particularly speaking, in the invention a luciferase lentivirus expression system with FG12-Luc2 as core plasmid is successfully constructed, and a PVTT-1 cell line from tumor thrombus tissue of liver cancer patients is established by the system. Through the lentivirus expression system, liver cancer tissue is successfully marked; and by experimental methods such as subcutaneous transplantation and orthotopic liver cancer transplantation, a mouse orthotopic transplantation liver cancer metastasis model is established which is suitable for serial passage, is applicable to in-vivo imaging, and can realize real-time monitoring of the growth and metastasis conditions of tumors in the body.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Immortalized Avian Cell Lines
ActiveUS20110008872A1Efficiently immortalizedSsRNA viruses negative-senseGenetically modified cellsVirus typeViral vector
Owner:TRANSGENE SA
Recombinant influenza virus highly expressing ha protein and preparation method and use thereof
InactiveUS20140227310A1Increase capacityLarge capacitySsRNA viruses negative-senseViral antigen ingredientsVirus typeInfluenza a
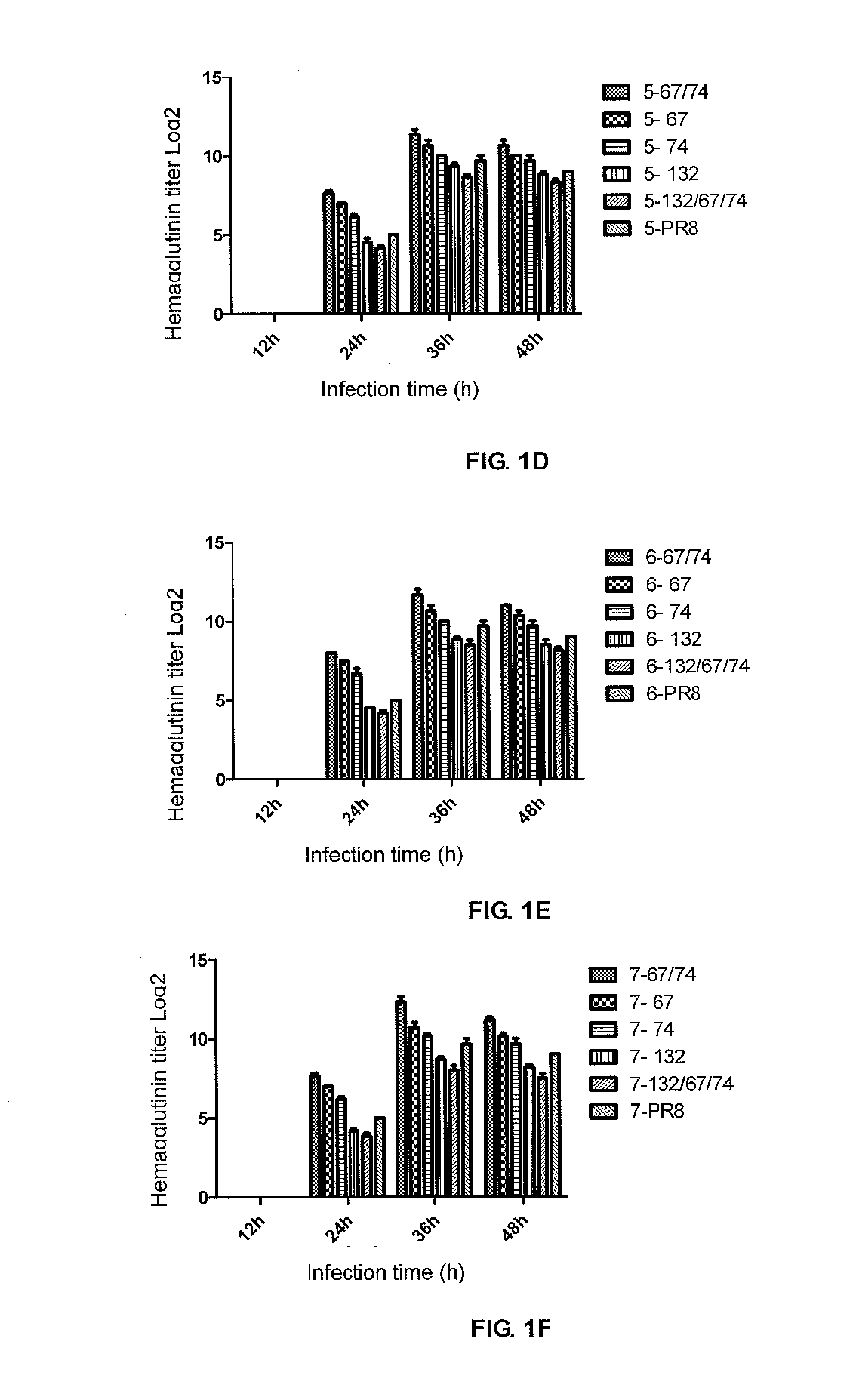
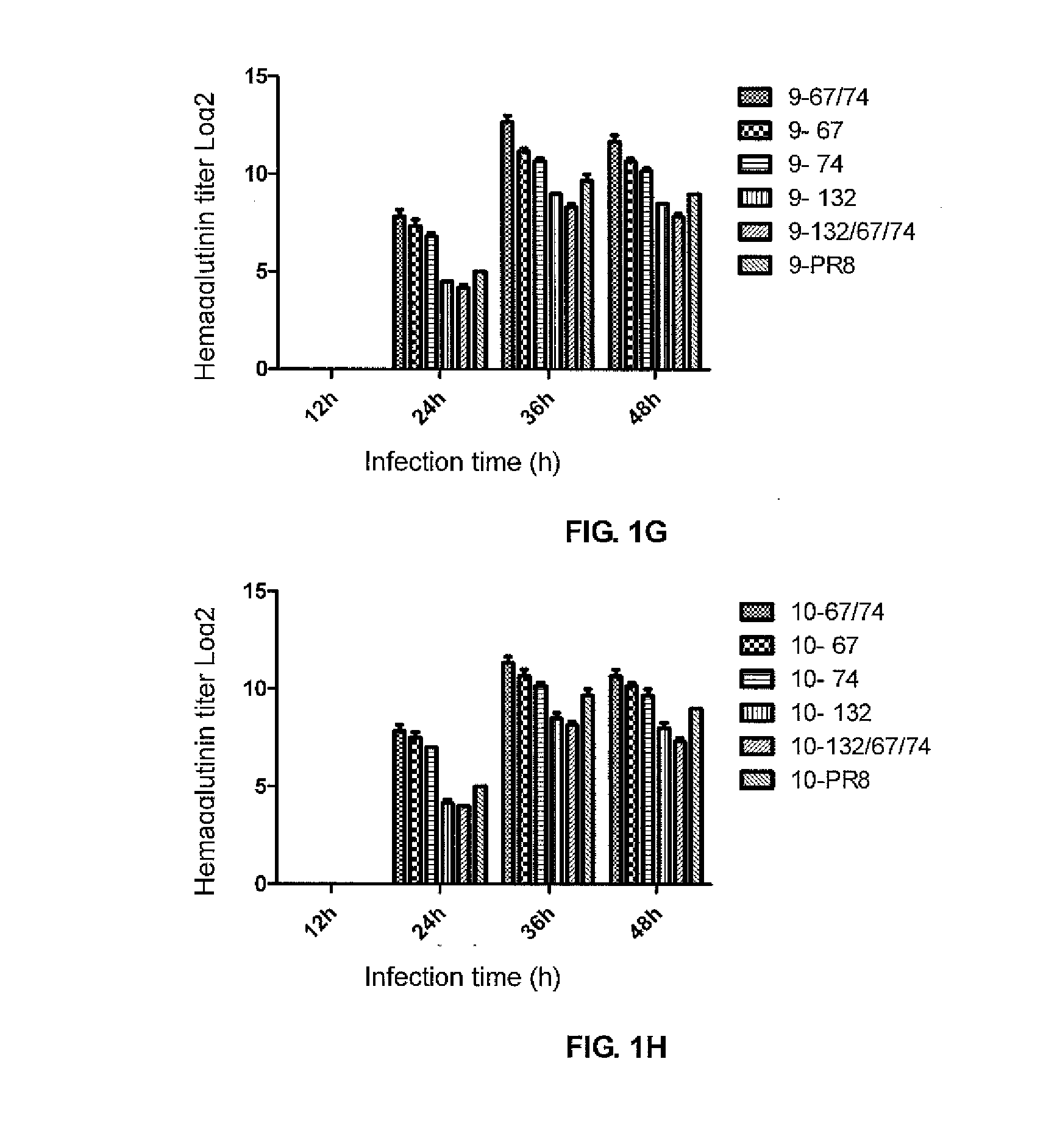
A PR8 recombinant influenza virus contains an HA and / or NA gene of H1, H3, H4, H5, H6, H7, H9 or H10 subtype influenza virus, and 6 internal genes (PB1, PB2, PA, NP, M and NS genes) of PR8 virus, in which the NS and / or NP gene have the following mutation sites: the NS2 protein encoded by the NS gene has an E67S point mutation, E74S point mutation, or E67S / E74S point mutation, and the NP protein encoded by the NP gene has a G132A point mutation.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
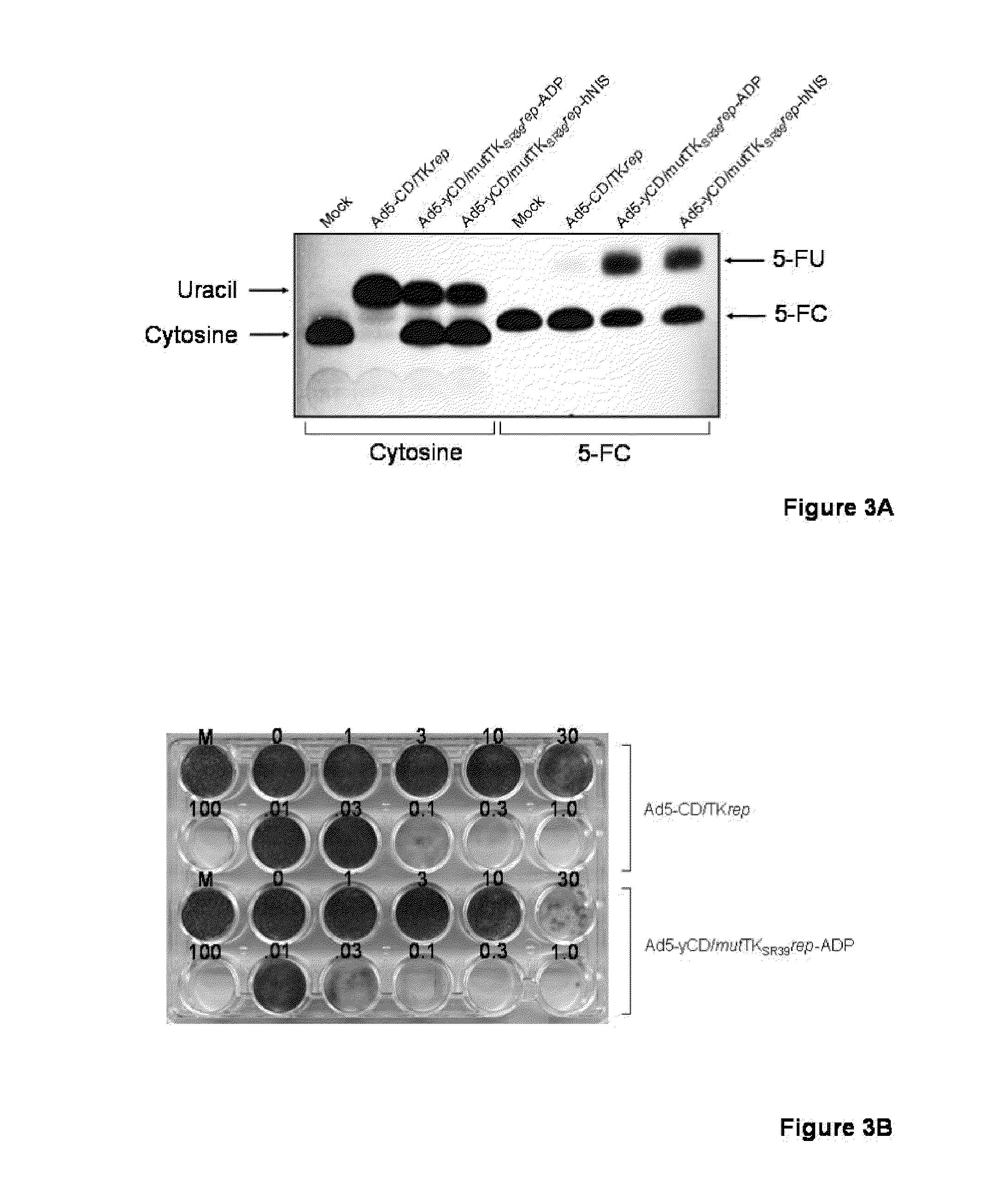
Methods and compositions for cancer therapy using a novel adenovirus
Owner:HENRY FORD HEALTH SYST
Preparation method and application of modified enhanced type targeting immune cell mass
InactiveCN106834354AMammal material medical ingredientsBlood/immune system cellsVirus typePeripheral blood mononuclear cell
The invention discloses a modified enhanced type DC-CIK cell targeting immune cell mass, and a preparation method and application thereof. A method for preparing a DC cell comprises the following steps: performing isolated culture on monocyte, thereby acquiring an immature DC cell; and performing tumor antigen load and induced differentiation culture on the immature DC cell, thereby acquiring a mature DC cell, wherein the monocyte is acquired by separating from a peripheral blood mononuclear cell of a sample. The method for preparing CIK comprises the following steps: performing in vitro differentiation culture on a CIK cell, thereby acquiring an immature CIK cell; preparing a modifying recombined slow virus for performing in vitro modification on the CIK cell; and co-culturing the modified CIK cell and the DC cell, thereby acquiring the mature modified CIK cell (Triumph-DC-CIK), so as to be used for achieving a better tumor immunotherapy effect.
Owner:深圳红石科创生物科技发展有限公司 +2
TRAIL secretion mesenchyma stem cell and purpose thereof for encephaloma treatment
The invention provides a TRAIL secretion mesenchyma stem cell and a purpose thereof for encephaloma treatment, and relates to the field of gene recombination and stem cell application. Particularly, the invention provides a building body for expressing a secretion type TRAIL protein soluble fragment and a slow virus expression vector containing the building body. The invention also provides the mesenchyma stem cell integrating the building body on the genome; the TRAIL protein fragment can be expressed and secreted. The invention also provides application of the building body or the vector or the mesenchyma stem cell to encephaloma treatment.
Owner:SHENZHEN BEIKE BIOTECH
Preparation method of placenta source matrix mesenchymal stem cell
ActiveCN103451150AAvoid damageThe method is simple and fastMammal material medical ingredientsSkeletal/connective tissue cellsSerum free mediaVirus type
The invention provides a preparation method of a placenta source matrix mesenchymal stem cell. The preparation method comprises the following steps: (1) separating the placenta source matrix mesenchymal stem cell; (2) cultivating the placenta source matrix mesenchymal stem cell, wherein a trypLE<TM> enzyme solution is utilized to process a placenta tissue in two steps in the step (1), and a serum-free medium is utilized in the step (2). By adopting the method provided by the invention, the placenta source matrix mesenchymal stem cell is a complete matrix source by short tandem repeat sequence (STR) atlas analysis, and is qualified by chromosome karyotype examination, pathogenic microorganism examination of bacteria, funguses and viruses, cell purity identification and cell biology function identification. Therefore, the placenta source matrix mesenchymal stem cell obtained by the method provided by the invention does not contain foreign protein, can meet the requirements of clinical use, is especially suitable for use by mothers, and also provides a technical support for building an excellent placenta source matrix mesenchymal stem cell bank.
Owner:BEIJING HEALTH & BIOTECH (H&B) CO LTD
Cattle reproductive disease vaccines
InactiveUS20070298053A1Avoid infectionAntibacterial agentsSsRNA viruses negative-senseBovine Viral Diarrhea VirusesVirus type
The present invention relates to combination vaccines and methods for treating or preventing diseases or disorders in an animal caused by infection by Bovine Viral Diarrhea Virus (BVDV) Types 1 and 2, Bovine Herpes Virus Type-1 (BHV-1), Bovine Respiratory Syncytial Virus (BRSV), Parainfluenza Virus (PI3), Campylobacter fetus, Leptospira canicola, Leptospira grippotyphosa, Leptospira hardj-prajitno, Leptospira icterohaemmorrhagiae, Leptospira hardjo-bovis and Leptospira pomona by administering to the animal an effective amount of a combination vaccine. The combination vaccine can be a whole or partial cell inactivated or modified live preparation.
Owner:PFIZER INC
Method for preparing CAR-T cell from gamma delta T cell derived from umbilical cord blood, CAR-T cell and application
PendingCN109609465AEfficient killingEliminate exclusionPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenVirus type
The invention discloses a method for preparing a CAR-T cell from gamma delta T cell derived from umbilical cord blood, the CAR-T cell and an application and also relates to nucleic acid coding a chimeric antigen receptor and a recombinant expression vector expressing the chimeric antigen receptor. The umbilical cord blood is used as a source of the gamma delta T cell, the gamma delta T cell is separated from the umbilical cord blood, after efficient amplification, the T cell is transduced by a lentiviral vector loaded with the chimeric antigen receptor, and the CAR-T cell can be obtained, so that positive tumor cells or other infectious cells which can efficiently or specifically kill related antigens are obtained. The CAR-T cell will play roles in tumor cell therapy and immunotherapy.
Owner:WUHAN BIO RAID BIOTECH CO LTD
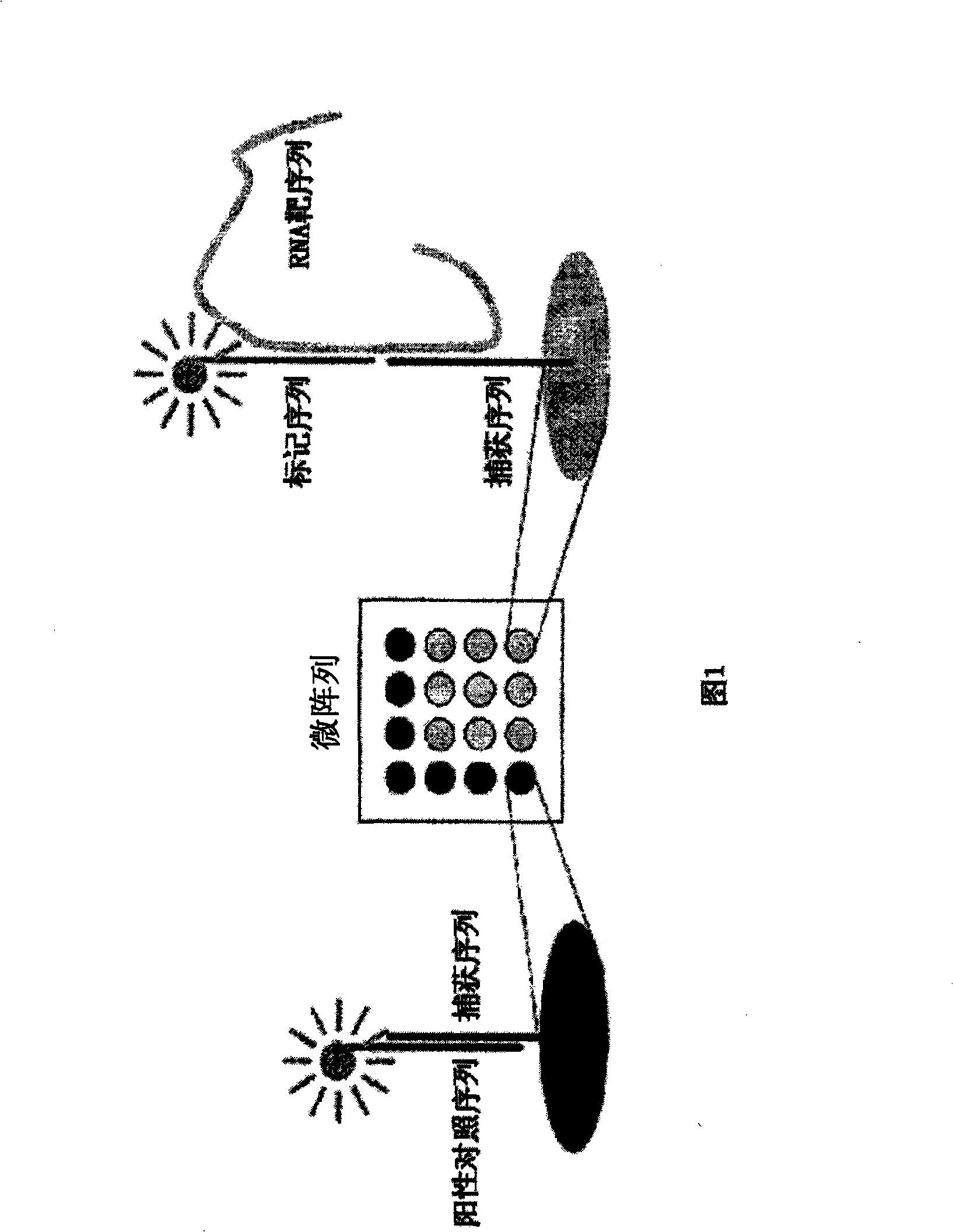
DNA array analysis as a diagnostic for current and emerging strains of influenza
Embodiments herein provide for methods, compositions and apparati for detection and / or diagnosis of virus types, subtypes and / or strains. In particular embodiments, the virus is an influenza virus. The apparatus may include a microarray with attached capture probes, designed to bind to oligonucleotides capable of binding at least a portion of a nucleic acid sequence of one or more target genes in a broad array of influenza types, subtypes or strains. The compositions may include isolated nucleic acids as capture probes, target sequences and / or tagged label probes, of use for diagnosis and / or detection of influenza virus.
Owner:科罗拉多州大学评议会
Method for restraining mesenchymal stem cells from differentiating into fat cells
InactiveCN103103213AEasy to operateVigorous proliferation in vitroGenetic engineeringFermentationIntercellular cell adhesion moleculeVirus type
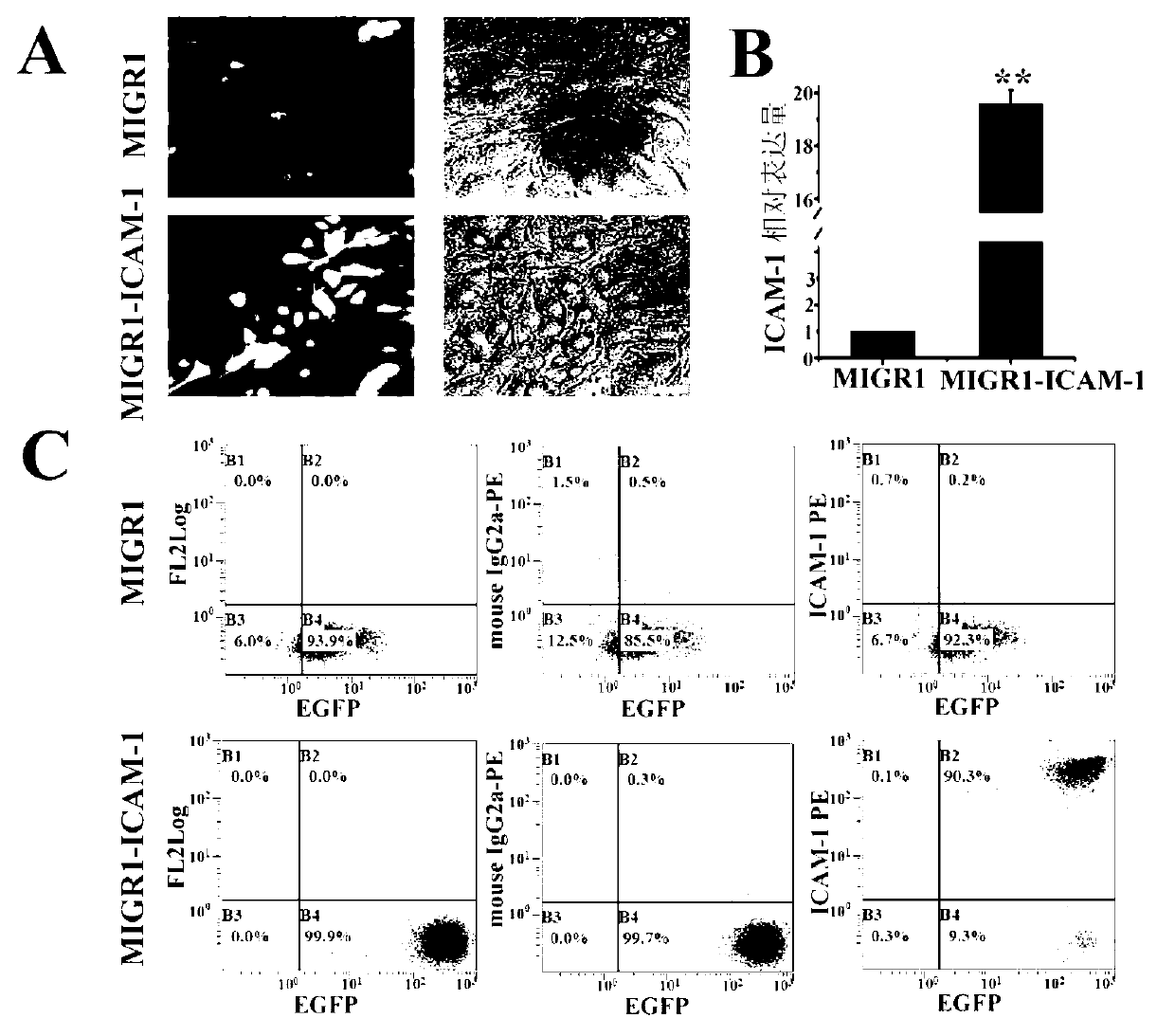
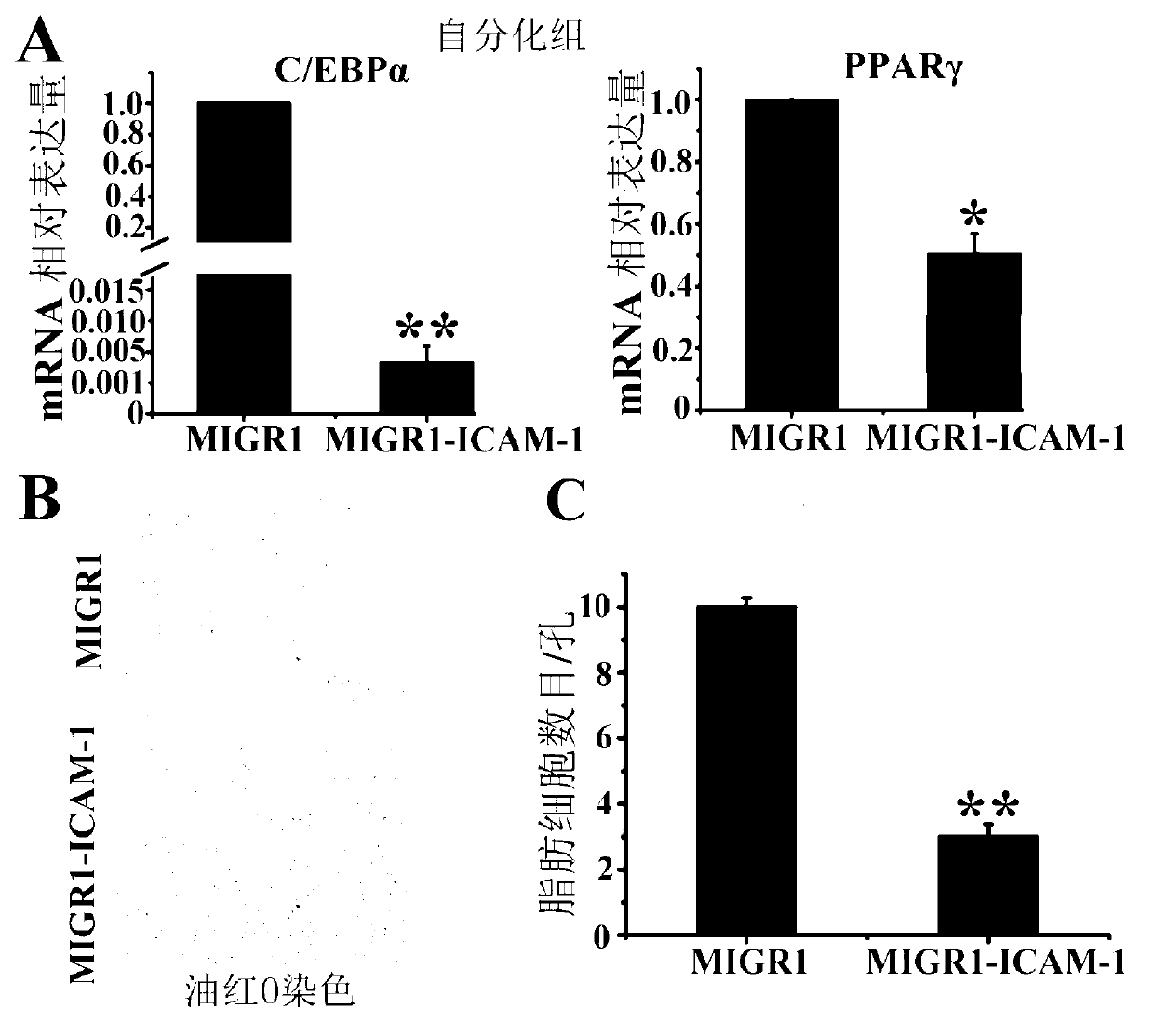
The invention belongs to the technical field of gene engineering and particularly relates to a method for restraining mesenchymal stem cells from differentiating into fat cells. A purpose of restraining the mesenchymal stem cells from differentiating into the fat cells is realized by the following steps of: constructing recombinant retrovirus plasmids containing ICAM-1 (intercellular cell adhesion molecule) genes, carrying out co-transfection on the packaging cells by the recombinant retrovirus plasmids together with packaging plasmids, collecting relevant viral supernatants, and infecting the mesenchymal stem cells. According to the method for restraining the mesenchymal stem cells from differentiating into the fat cells, the separation method is simple in operation, convenient and practical, the efficiency (more than 90% of ICAM-1 can be highly expressed) for transfecting the mesenchymal stem cells is high, and the obtained mesenchymal stem cells are exuberant in in-vitro multiplication and can be passed down for multiple times (more than 50 generations). Thus, as the method and a culture technique system for restraining mesenchymal stem cells from differentiating into fat cells are established, a foundation for research and application of the mesenchymal stem cells is laid.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
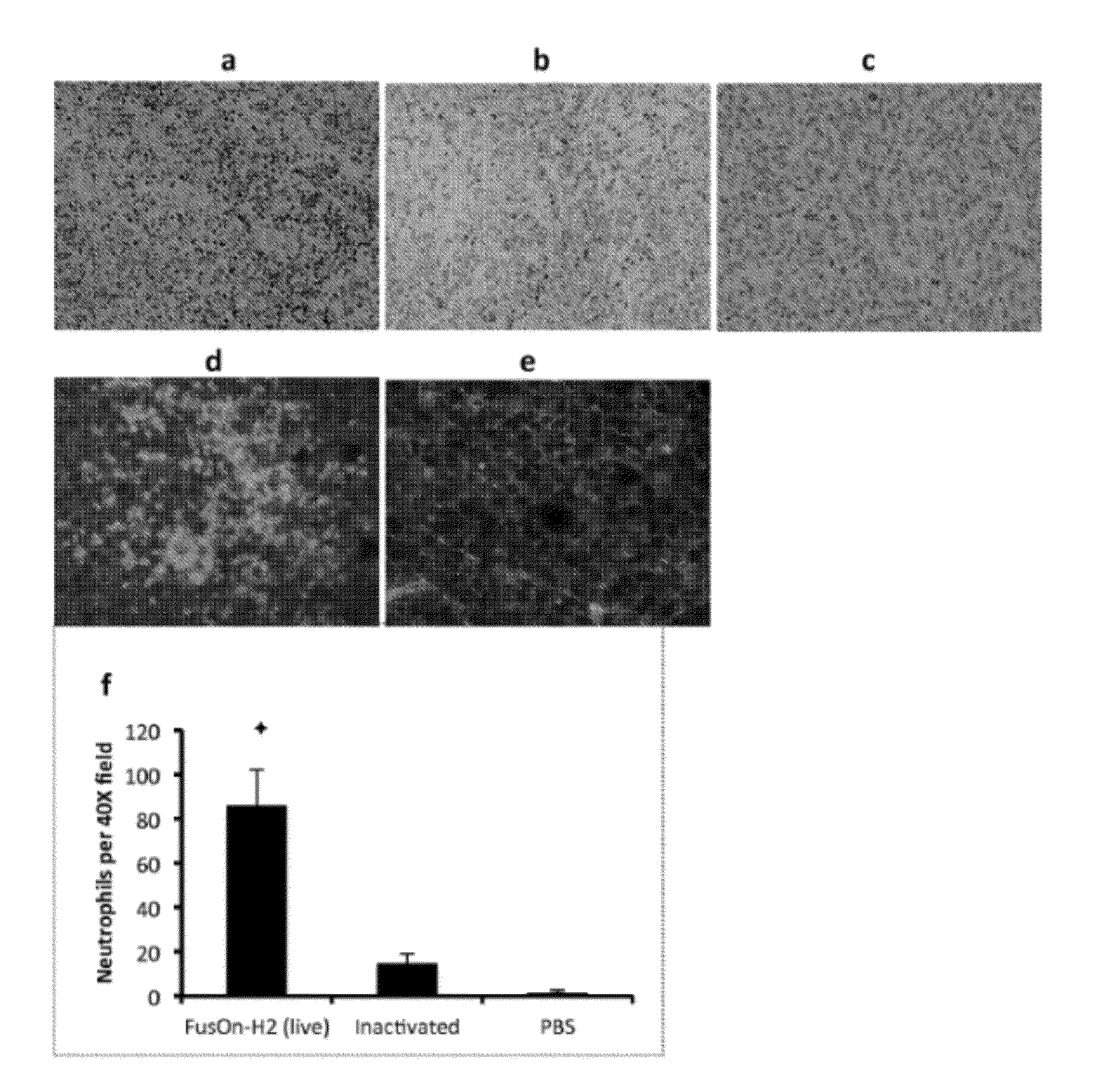
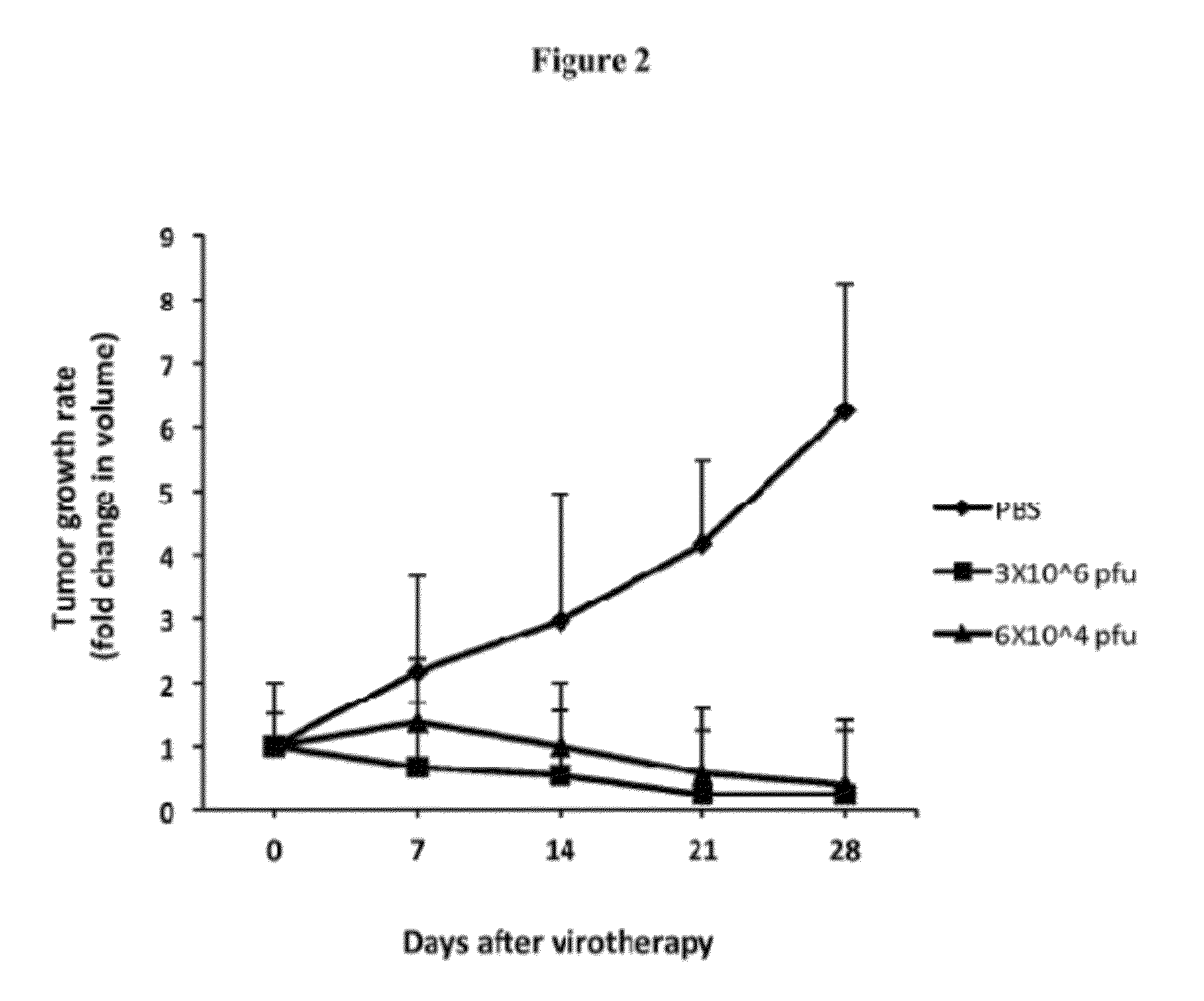
Oncolytic Virus as an Inducer for Innate Antitumor Immunity
InactiveUS20120301506A1Viral/bacteriophage medical ingredientsDisease diagnosisAntitumor immunityVirus type
The present invention is directed to the administration of FusOn-H2, an HSV derived oncolytic virus, to treat tumor cells that are resistant to the lytic effect of the virus. Administration of FusOn-H2 induces the patient's innate immune responses to tumor cells via neutrophils, which are able to destroy tumors efficiently when they migrate to the tumor mass. With the induced innate antitumor immunity, FusOn-H2 is effective at eradicating tumors even when it is used at very low doses.
Owner:UNIV HOUSTON SYST
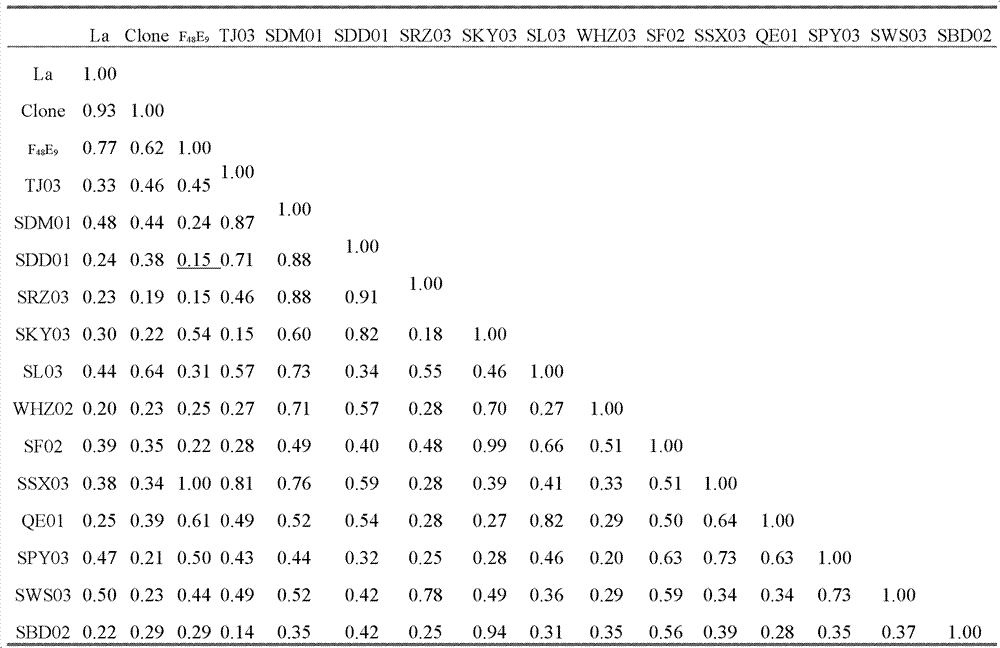
Chicken Newcastle disease virus and its separation method
The invention relates to the field of animal virology and provides a chicken Newcastle disease virus and its separation method. The virus is code-named SDM01 and the invention provides its separation and preparation method. The inventor carries out biological collection for the strain and its collection number is CCTCC NO:V201109. Through chicken embryo passage, biological characteristics tests such as a hemagglutinin and serum neutralization test, an animal regress test and exogenous virus testing, are carried out. The test results confirm that the virus is the Newcastle disease virus. It isproved through chicken embryo mean death time (MDT), one-day-old chick intracebral pathogenicity index (ICPI) measurement and 6-week-old chick intravenous inoculation pathogenicity index (IVPI) measurement that the Newcastle disease virus is a virulent strain. Positive serum is prepared through separated strain and standard strain to carry out an HI cross neutralization test, a chicken embryo neutralization test, a cell neutralization test, an F and HN gene sequence sequencing and comparison and an immunity protective test. Results confirm that there exist large differences between the Newcastle disease virus SDM01 strain and traditional strains (La Sota strain and Clone30 strain) in both genetic typing and antigenicity.
Owner:秦卓明 +1
Method of deriving mature hepatocytes from human embryonic stem cells
ActiveUS20130217752A1Liver inflammation is reducedReduce incidenceHepatocytesPeptide/protein ingredientsDrug metabolismVirus type
A method for producing mature hepatocytes having functional hepatic enzyme activity from human pluripotent cells is disclosed. The method includes the step of transferring an external vector comprising the DNA sequence coding for a microRNA having the seed sequence of the microRNA miR-122, the DNA sequence coding for a microRNA having the seed sequence of the microRNA miR-let-7c, a microRNA having the seed sequence of the microRNA miR-122, a microRNA having the seed sequence of the microRNA miR-let-7c, or a combination thereof into one or more fetal hepatocytes. The resulting cells differentiate into mature hepatocytes that exhibit functional hepatic enzyme activity, and can be used in drug metabolism and toxicity testing, in the study of viruses that target hepatic tissue, and as therapeutics.A related method of maintaining the functional hepatic enzyme activity of primary hepatocytes over time is also disclosed. The method includes the step of transferring an external vector comprising the DNA sequence coding for a microRNA having the seed sequence of the microRNA miR-122 into one or more cultured primary hepatocytes.
Owner:WISCONSIN ALUMNI RES FOUND
Non-Competitive Internal Controls for Use in Nucleic Acid Tests
InactiveUS20110003309A1Sugar derivativesMicrobiological testing/measurementNucleic acid testHuman immunodeficiency
Provided are non-competitive internal controls for use in nucleic acid tests (NATs), which are obtained from the organisms Methanobacterium thermoautrophicum (MET) and Zea mays (Corn). The non-competitive internal controls have utility in DNA and RNA NATs selected from Influenza A, Influenza B, parainfluenza viruses 1 to 4 (PIV-1 to PIV-4), respiratory syncytial virus type A (RSV A), RSV B, human metapneumovirus (hMPV), Chlamydia trachomatis (CT), and Neisseria gonorrhea (GC), Hepatitis B virus (HBV), Hepatitis C virus (HCV), Human Immunodeficiency Virus I (HIV-1), and Severe Acute Respiratory Syndrome (SARS).
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Method for implementing strong vivo transplantation of human induced pluripotent stem cell-derived hemopoietic stem progenitor cells
ActiveCN107964536AHigh in vivo transplantation efficiencyReduce the numberCulture processBlood/immune system cellsProgenitorGenes mutation
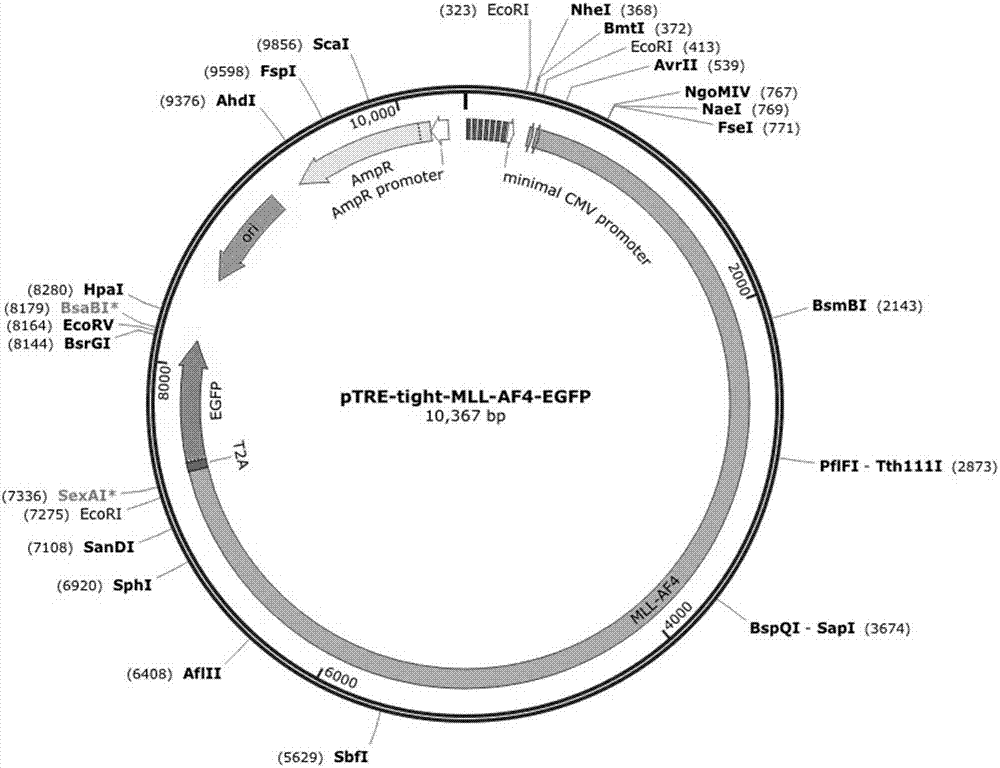
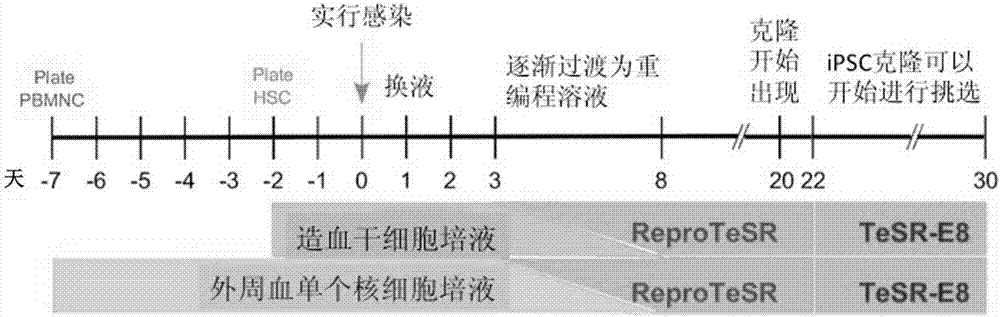
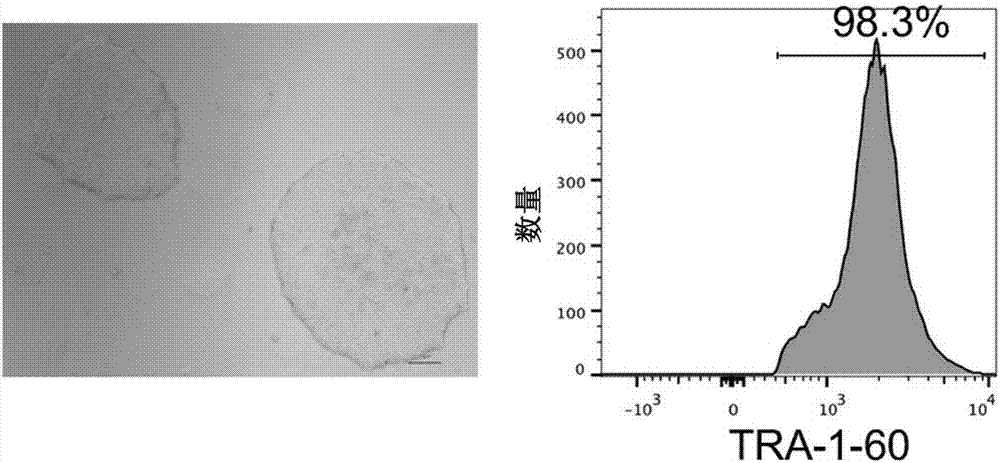
The invention relates to a method for implementing strong vivo transplantation of human induced pluripotent stem cell-derived hemopoietic stem progenitor cells. The method is induced by an MLL-AF4 single factor and has the advantages that the method is simple, convenient and efficient, human iPS (induced pluripotent stem) cells can be induced into HSC (hemopoietic stem cells) with strong vivo transplantation effects and multi-strain hemopoietic reconstruction, and many difficulties in the field are broken through. The method mainly has the advantages that the number of factors needing to be added is greatly decreased by induction with the single factor, experimental operation can be remarkably simplified, experimental efficiency is improved, the risk of gene mutation possibly induced by slow virus mediated genome insertion is avoided by a plasmid-mediated inducing method, vivo transplantation efficiency reaches up to 20% or more, medullary system bias of iPSC-HSPC in vivo is corrected,and a medullary system, a lymphoid system and an erythroid can be comprehensively reconstructed in vivo.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Method for Treating or Preventing Graft Versus Host Disease
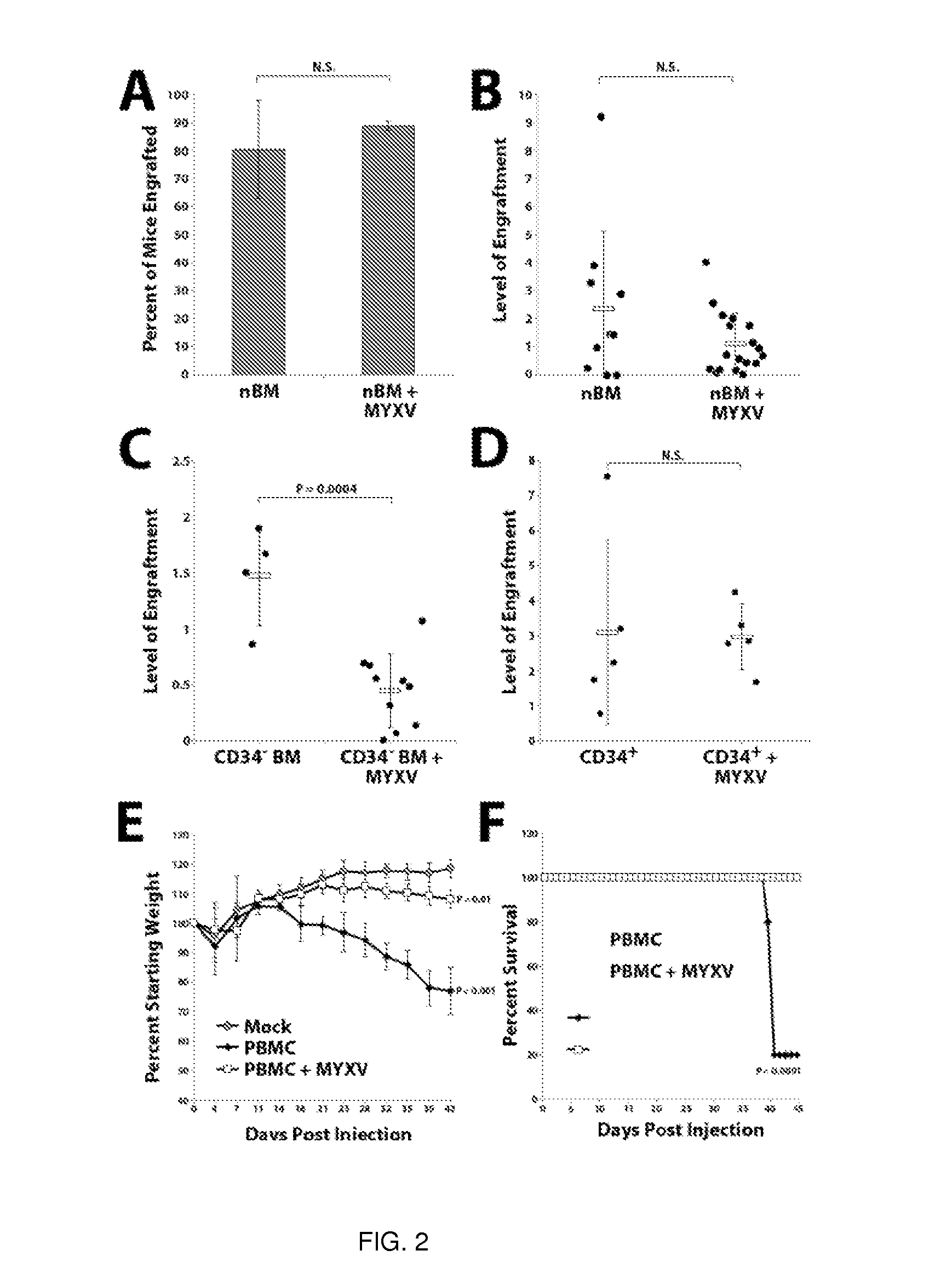
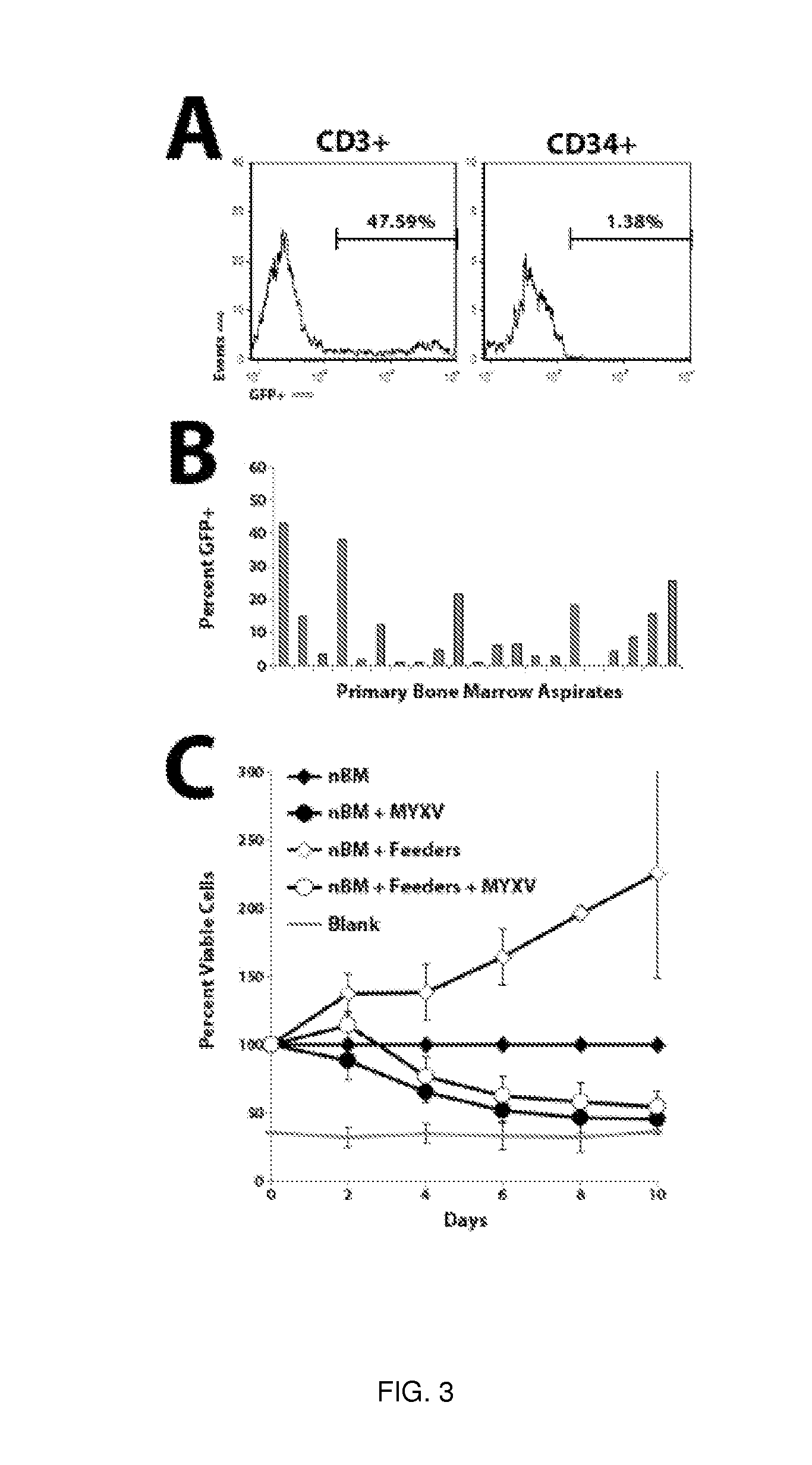
A method of treating or preventing graft versus host disease (GVHD) in a subject receiving a graft comprising hematopoietic cells is provided. The method comprises contacting the graft ex vivo with an amount of a Myxoma Virus effective to inhibit proliferation of T lymphocytes in the graft and to treat or prevent GVHD in the host subject following infusion of the graft into the subject. After the contacting of the graft with the Myxoma Virus, the method comprises transplanting the virus-treated graft into the subject.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Virus searching and killing method, device, equipment and storage medium
ActiveCN110717183AKilling implementationGood killing effectPlatform integrity maintainanceVirus typeIp address
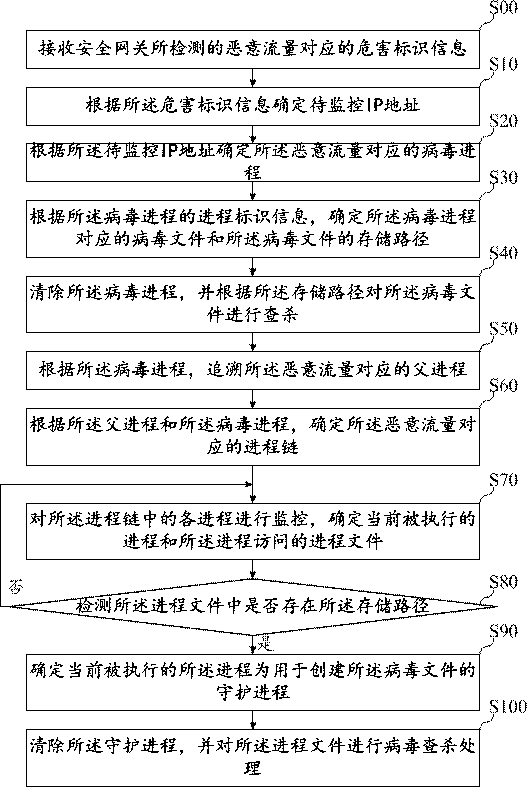
The invention belongs to the technical field of information safety, and discloses a virus searching and killing method, a virus searching and killing device, equipment and a storage medium. The methodcomprises the following steps: receiving hazard recognition information corresponding to malicious traffic detected by a safety gateway; determining a to-be-monitored IP address according to the hazard recognition information; determining a virus process corresponding to the malicious traffic according to the IP address to be monitored; determining a virus file corresponding to the virus processand a storage path of the virus file according to the process recognition information of the virus process; and clearing the virus process, and searching and killing the virus file according to the storage path. By means of the mode, limitation of virus types is avoided, viruses are searched and killed from the source, and therefore the searching and killing effect on the viruses is greatly improved.
Owner:SANGFOR TECH INC
Pig intestinal endothelial cell line for stably expressing CaS9 protein
The invention discloses a pig intestinal endothelial cell line for stably expressing a CaS9 protein. The pig intestinal endothelial cell line is characterized in that non-transformed intestinal endothelial cell line IPEC-J2 of a neonatal pig is taken as a host cell and is transfected with a lentiviral expression vector containing CaS9, and a transformant capable of stably expressing the CaS9 protein is obtained by screening of 20mu g / mL of Blasticidin. A construction method of the pig intestinal endothelial cell line comprises the following steps: firstly, constructing the lentiviral expression vector containing the CaS9; secondly, transforming an HEK293T cell; thirdly, transfecting an IPEC-J2 cell; fourthly, performing monoclonal screening. The pig intestinal endothelial cell line IPEC-J2-CaS9 disclosed by the invention can stably express the CaS9 protein, and has a growth curve identical with that of the non-transformed IPEC-J2 cell, and cell morphology identical with that of the IPEC-J2 cell. The pig intestinal endothelial cell line disclosed by the invention has important application value in aspects of researching the growth and differentiation of pig intestinal endothelial cells as well as dealing with the stimulation from exogenous substances.
Owner:AGRO BIOLOGICAL GENE RES CENT GUANGDONG ACADEMY OF AGRI SCI
One-step RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection kit for influenza virus
InactiveCN102146485AShort measurement time periodImprove detection efficiencyMicrobiological testing/measurementVirus typeQualitative analysis
The invention discloses a one-step RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection kit for an influenza virus, relating to a PCR kit in the technical field of biology. The kit comprises one-step 5xRT-PCR Buffer, positive control, negative control, three pairs of specific primers and Enzyme Mix; a specific primer AF: 5'-ATTCTAACCGAGGTCGAAACGT-3' and a specific primer AR: 5'-GACAAAGCGTCTACGCTGCAGTC-3' are designed according to a gene M of an influenza virus type A; a specific primer is designed according to a gene NS of an influenza virus type B; and a specific primer is designed according to a gene HA of an influenza virus A type H1N1. The detection kit has a short detection time period, high detection efficiency, high virus detection specificity, high accuracy, higher detection sensitivity than an immunological detection method and high experiment result repeatability, can be applied to qualitative analysis of viruses, and is easy to operate and popularize.
Owner:WUHAN UNIV
Preparation method and kit of human stem cell with prolonged service life and enhanced angiogenesis
ActiveCN105018524AExtend your lifeEnhanced angiogenesisMammal material medical ingredientsVector-based foreign material introductionDiseaseVirus type
The invention provides a preparation method of a human stem cell with prolonged service life and enhanced angiogenesis. The preparation method comprises the following steps of establishing gene segments of a RNA (ribonucleic acid) testis signal transduction and activating body and angiogenin-1; and recombining the gene segments to a virus expression vector. The service life of the cell can be prolonged by transfection of the human stem cell, the angiogenesis of the human stem cell is improved, and tumorigenesis is avoided. The invention also provides a kit of the human stem cell with the prolonged service life and the enhanced angiogenesis, and the human stem cell is expected to treat ischemic cardiovascular and cerebrovascular diseases well.
Owner:广州赛琅生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com