Method for implementing strong vivo transplantation of human induced pluripotent stem cell-derived hemopoietic stem progenitor cells
A pluripotent stem cell and cell technology, which is applied in the field of potent in vivo transplantation of hematopoietic stem progenitor cells derived from induced pluripotent stem cells, can solve the problems of lack of lymphoid hematopoiesis, inability to reconstruct lymphoid hematopoiesis, low efficiency, etc. The ability of systemic diseases, the effect of overcoming the shortage of original samples, and improving the efficiency of experiments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: MLL-AF4 plasmid construction
[0063] Firstly, the peptide T2A was used to connect MLL-AF4 and EGFP, and the fragment of MLL-AF4-EGFP was cloned by PCR. The PCR amplification template of 3'-MLL-AF4-5'-T2A fragment is pCI-MLL-AF4 plasmid, and the primer of amplification is: MLL-AF4-Klf 1-F (5'-CGTAGGGTCCCAGTCAAGTGCT-3', SEQ ID NO:3) and MLL-AF4-3'-T2A5'-R (5'-GCAGGGATCCTCTGCCCTCAGGTGTTTTGGTTAATTCTTGT-3', SEQ ID NO:4).
[0064] The PCR amplification template of the 3'-T2A-5-EGFP fragment is the pEGFP-N3 plasmid, and the amplified primer is T2A-3'-EGFP-5'-F
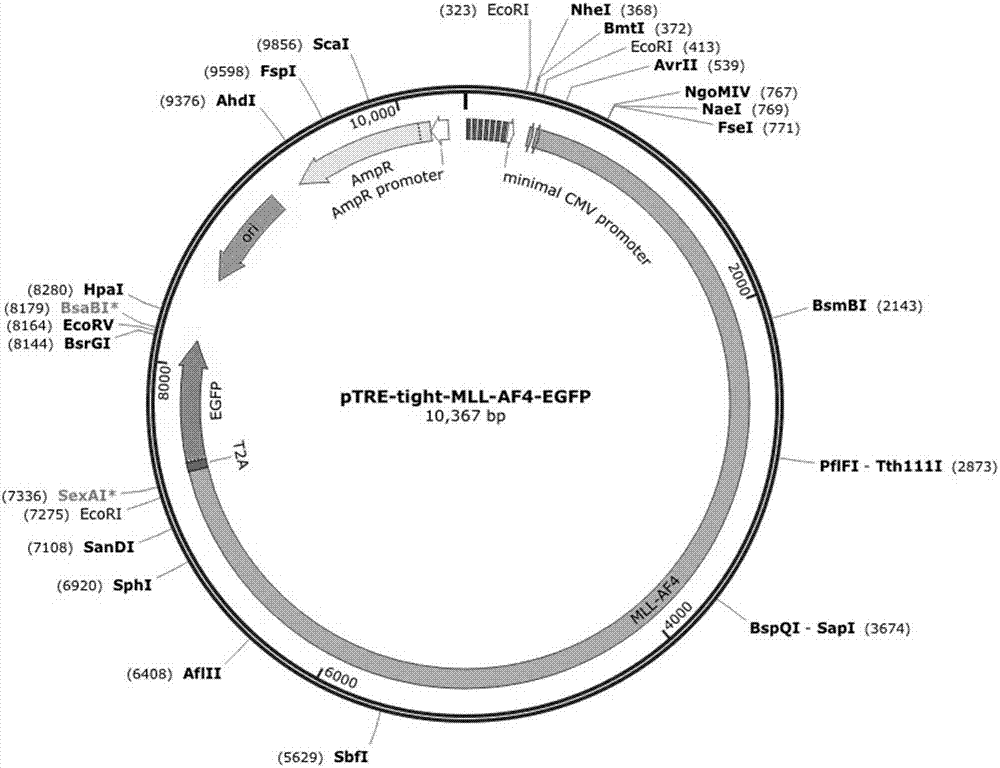
[0065] (5'-CAGAGGATCCCTGCTAACATGTGGTGACGTCGAGGAGAATCCTGGCCCAATGGTGAGCAAGGGCGAGGAGCTGTTC-3', SEQ ID NO:5) and EGFP-3'-R (5'-GAGATATCGGCCGCTTTACTTGTACAGCTCGTCC-3', SEQ ID NO:6). Then, the above-mentioned MLL-AF4-T2A-EGFP fragment was inserted into the downstream of the TRE promoter in the pTRE-Tight plasmid, and then the pTRE-tight-MLL-AF4-EGFP plasmid was constructed ( figure 1 ).
Embodiment 2
[0066] Example 2: Construction of human iPSC cell lines
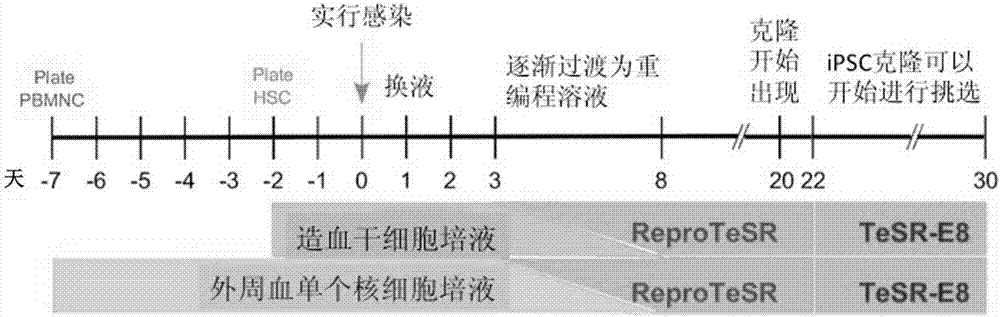
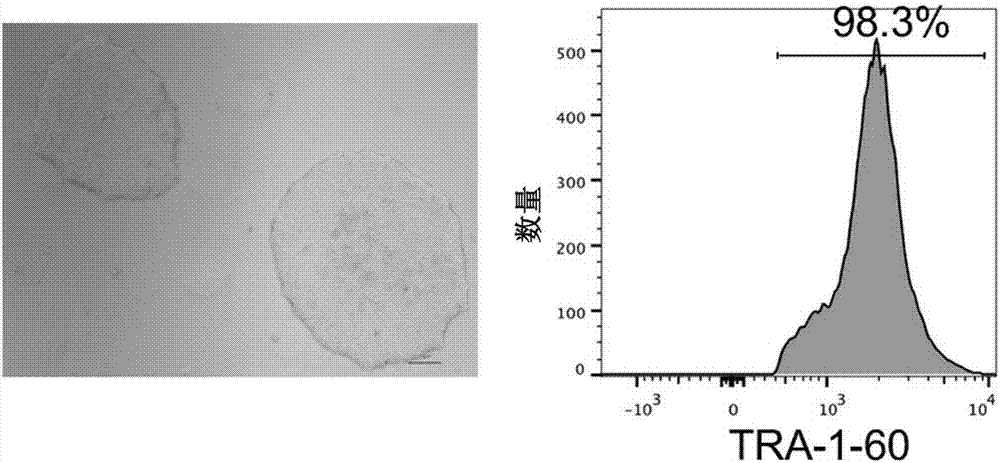
[0067] We selected two different sources of somatic cells for iPSC reprogramming: CD34 mobilized from human peripheral blood + Hematopoietic stem and progenitor cells, and human peripheral blood mononuclear cells (MNCs). The Sendai virus vector (DNAVEC, CytoTune-iPS Reprogramming Kit) containing the four reprogramming factors OCT4, SOX2, KLF4 and cMYC was infected with 2×10 at a titer of 20 Multiplicity of Infection (MOI). 5 of somatic cells. On day 2, the medium was changed to remove Sendai virus. On day 3, all infected cells were transferred to Vitronectin XF TM (StemCellTechnologies) processed six-well plates. From day 4 to day 8, 1 ml ReproTeSR (StemCell Technologies) and 0.25 μM sodium butyrate were added to each well every day. From day 9 to day 22, fresh ReproTeSR was replaced every day, while the formation of iPSC clones was observed under a microscope. From day 23 to day 31, the culture medium was replace...
Embodiment 3
[0069] Example 3: Induction of iPSCs into blood cells in vitro
[0070] We adopted a monolayer culture method (Mono-layer culture) to induce iPSCs to differentiate into blood cells in vitro. Specifically, 24-well culture plates were pre-treated with Vitronectin XF TM (StemCellTechnologies) treatment, followed by iPS cells according to 5 × 10 4 The amount of each hole was spread into the well plate, and cultured with TeSR-E8 medium. On the next day (defined as the first day), the culture medium was replaced with STEMdiffAPEL (StemCell Technologies), and this culture medium was used as the subsequent whole medium, and at the same time, BMP4 (50ng / ml) was added to STEMDiffAPEL. On the third day, replace with fresh culture medium, and add VEGF (50ng / ml) and bFGF (50ng / ml) to the culture medium at the same time. On day 5, replace with fresh culture medium, and add VEGF (50ng / ml), bFGF (50ng / ml) and SB431542 (20μM) into the culture medium at the same time. From the 7th day, half...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com