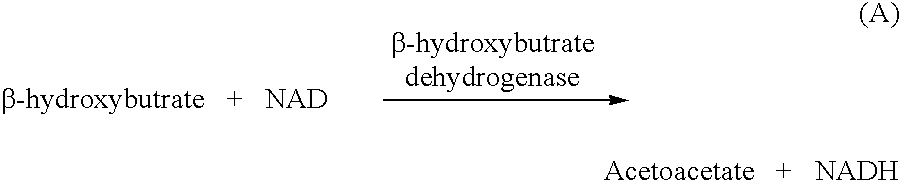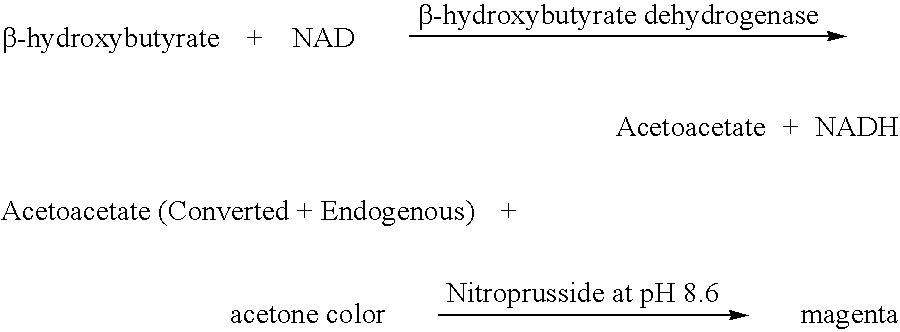Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1719 results about "Butyrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Butyrate or butanoate is the traditional name for the conjugate base of butyric acid (also known as butanoic acid). The formula of the butyrate ion is C4H7O−2. The name is used as part of the name of esters and salts of butyric acid...
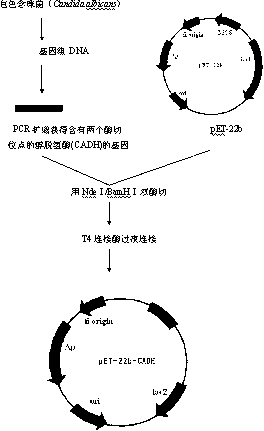
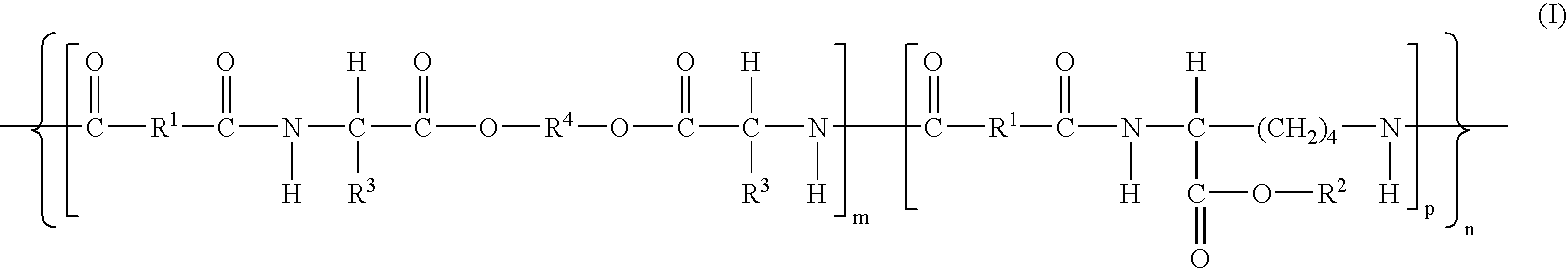
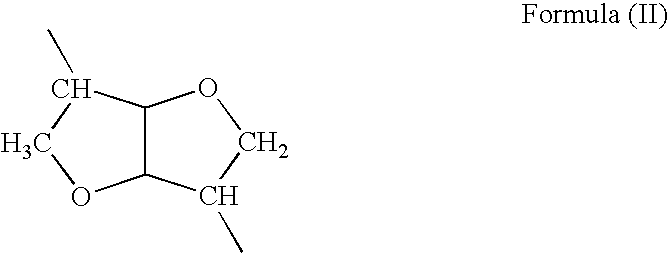
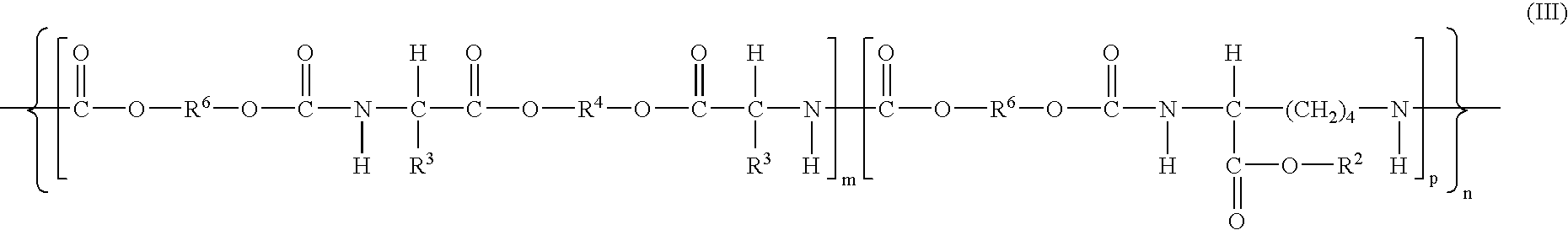
Wound healing polymer compositions and methods for use thereof
The present invention provides bioactive polymer compositions that can be formulated to release a wound healing agent at a controlled rate by adjusting the various components of the composition. The composition can be used in an external wound dressing, as a polymer implant for delivery of the wound healing agent to an internal body site, or as a coating on the surface of an implantable surgical device to deliver wound healing agents that are covalently attached to a biocompatible, biodegradable polymer and / or embedded within a hydrogel. Methods of using the invention bioactive polymer compositions to promote natural healing of wounds, especially chronic wounds, are also provided. Examples of biodegradable copolymer polyesters useful in forming the blood-compatible, hydrophilic layer or coating include copolyester amides, copolyester urethanes, glycolide-lactide copolymers, glycolide-caprolactone copolymers, poly-3-hydroxy butyrate-valerate copolymers, and copolymers of the cyclic diester monomer, 3-(S)[(alkyloxycarbonyl)methyl]-1,4-dioxane-2,5-dione, with L-lactide. The glycolide-lactide copolymers include poly(glycolide-L-lactide) copolymers formed utilizing a monomer mole ratio of glycolic acid to L-lactic acid ranging from 5:95 to 95:5 and preferably a monomer mole ratio of glycolic acid to L-lactic acid ranging from 45:65 to 95:5. The glycolide-caprolactone copolymers include glycolide and ε-caprolactone block copolymer, e.g., Monocryl or Poliglecaprone.
Owner:MEDIVAS LLC
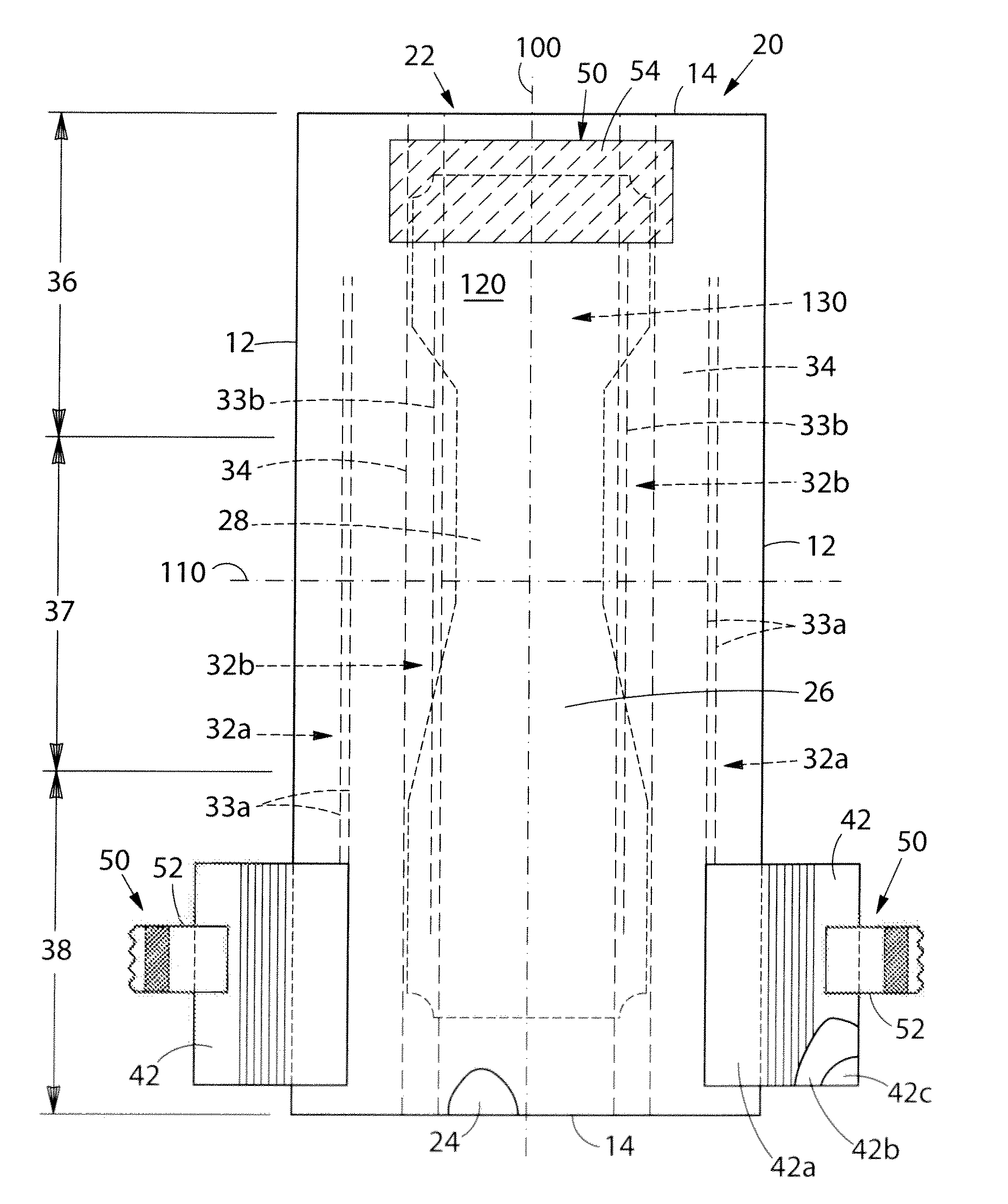
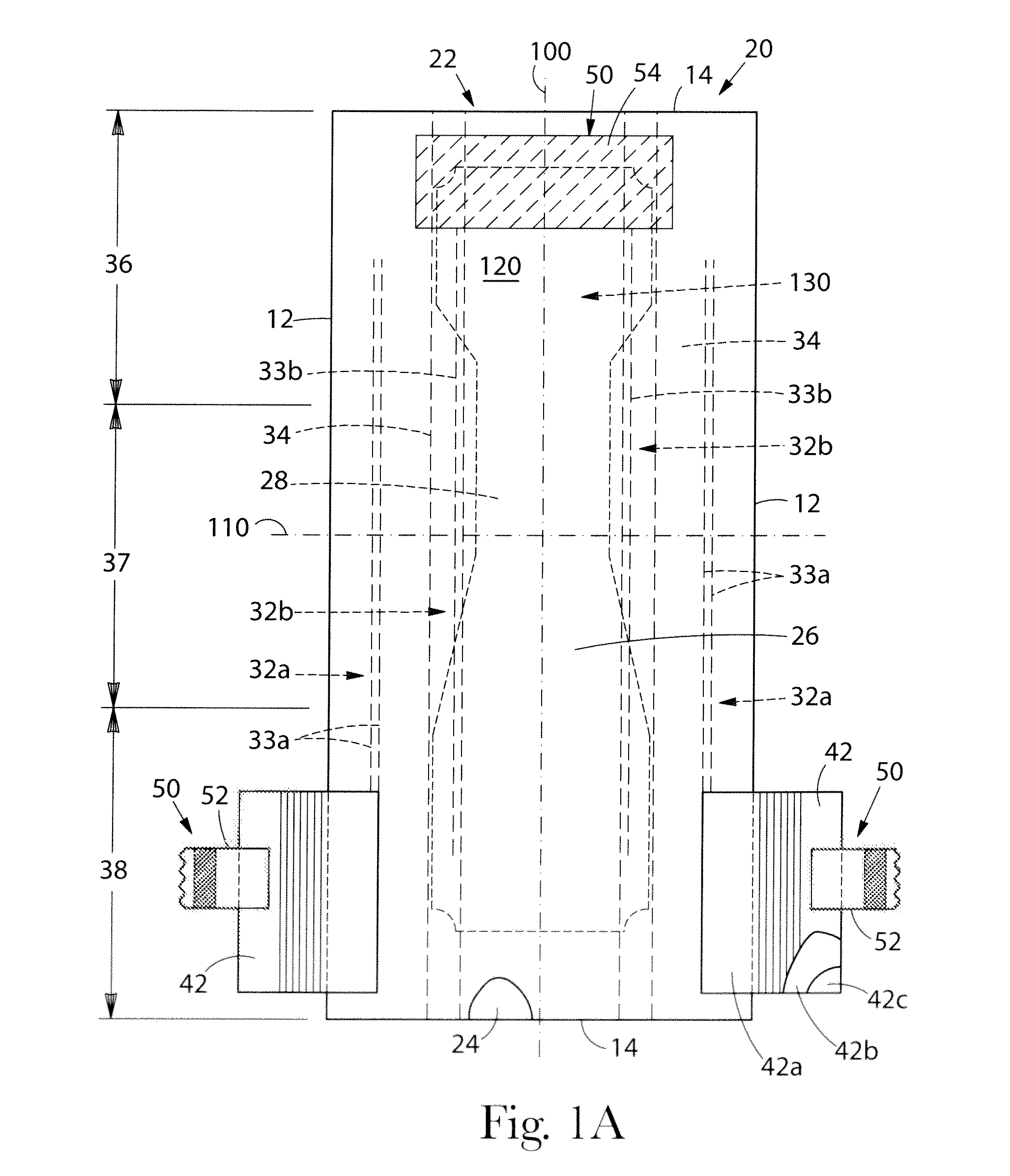
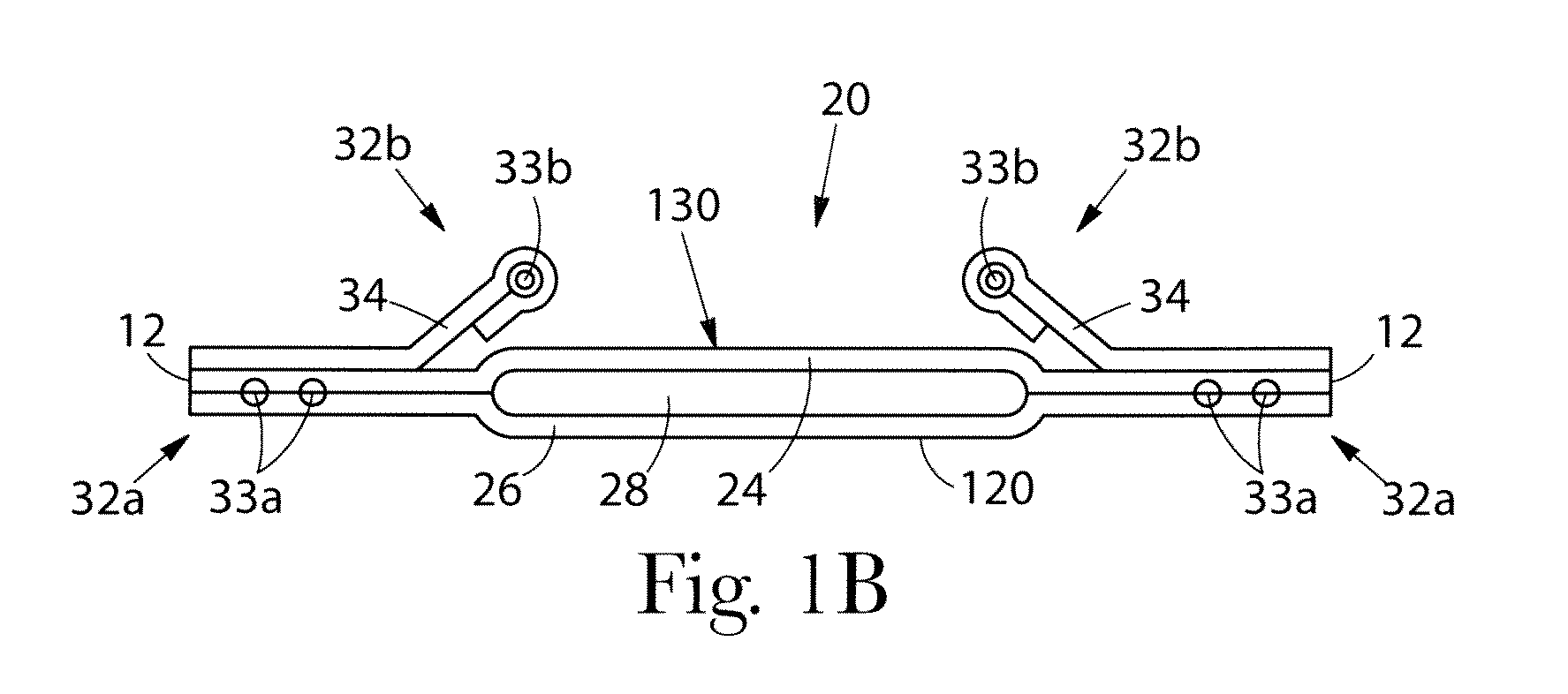
Absorbent article comprising a synthetic polymer derived from a renewable resource and methods of producing said article
An element of an absorbent article is provided. The element has a bio-based content of at least about 50% based on the total weight of the element, and comprises a synthetic polymer derived from a renewable resource via a first intermediate compound selected from the group consisting of crotonic acid, propiolactone, ethylene oxide, i-propanol, butanol, butyric acid, propionic acid, 2-acetoxypropanoic acid, methyl 2-acetoxypropanoate, methyl lactate, ethyl lactate, polyhydroxybutyrate, and a polyhydroxyalkanoate comprising 3-hydroxypropionate monomers. An absorbent article comprising the element and a method of making an element for an absorbent article also are provided.
Owner:THE PROCTER & GAMBLE COMPANY
Hydroxybutyrate ester and medical use thereof
A compound which is 3-hydroxybutyl 3-hydroxybutyrate enantiomerically enriched with respect to (3R)-hydroxybutyl (3R)-hydroxybutyrate of formula (I) is an effective and palatable precursor to the ketone body (3R)-hydroxybutyrate and may therefore be used to treat a condition which is caused by, exacerbated by or associated with elevated plasma levels of free fatty acids in a human or animal subject, for instance a condition where weight loss or weight gain is implicated, or to promote alertness or improve cognitive function, or to treat, prevent or reduce the effects of neurodegeneration, free radical toxicity, hypoxic conditions or hyperglycaemia.
Owner:UNITED STATES OF AMERICA +1
Microorganisms and methods for conversion of syngas and other carbon sources to useful products
A non-naturally occurring microbial organism having an isopropanol, 4-hydroxybutryate, or 1,4-butanediol pathway includes at least one exogenous nucleic acid encoding an isopropanol, 4-hydroxybutryate, or 1,4-butanediol pathway enzyme expressed in a sufficient amount to produce isopropanol, 4-hydroxybutryate, or 1,4-butanediol. The aforementioned organisms are cultured to produce isopropanol, 4-hydroxybutryate, or 1,4-butanediol.
Owner:GENOMATICA INC
Melamine-formaldehyde microcapsule slurries for fabric article freshening
Described is a method for freshening fabric articles by means of spraying the articles with an aqueous slurry of microcapsules having rupturable melamine-formaldehyde polymeric walls, containing substantive and efficacious functional substances, e.g., malodour counteractants and / or fragrances. The slurry may optionally contain non-confined functional substances, e.g. malodour counteractants and / or fragrances. The method is effective for the deposition of effectively-rupturable malodour suppressant and / or fragrance emitting microcapsules onto fabrics wherein the resulting emitted fragrance activity and / or malodour counteractant activity is long-lasting and where the resulting substantive aroma is aesthetically pleasing over the long period of time for which it is effective. Also described are efficacious and substantive malodour counteractant compositions useful for the aforementioned process containing a malodour composition composed of zinc ricinoleate and at least one of: 1-cyclohexylethan-1-yl butyrate; 1-cyclohexylethan-1-yl acetate; 1-cyclohexylethan-1-ol; 1-(4′-methylethyl)cyclohexylethan-1-yl propionate; and / or 2′-hydroxy-1′-ethyl(2-phenoxy)acetate. In addition, described are efficacious microcapsule slurries useful for the aforementioned process containing microcapsules having melamine-formaldehyde polymeric capsule walls with the microcapsules being in contact with one or more polymeric silicone phospholipids.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Special-purpose energy-saving environment-friendly type nano coating for aluminum alloy sections (door and window) and preparing method thereof
InactiveCN101012350AStrong UV resistanceImprove insulation efficiencyLiquid surface applicatorsEpoxy resin coatingsEpoxyPolyvinyl butyral
The invention discloses a specific nanometer paint of energy-saving environment-protective typed aluminium alloy section bar (door and window), which comprises the following parts: 90-100% filming agent, 0.1-10% hardener, 0.5-8% levelling agent, 0.5-10% nanometer material, 20-50% fill and 10% hollow microball, wherein the filming agent is one or more of epoxy resin, polyester resin, acrylic acid resin, amino resin, phenol resin or alkide resin; the hardener is one or more of dicyandiamide, imidazole, dihydrazide, polybasic carboxylic acid, beta-hydroxyalkyl amide or triglycidol isocyanuric ester hardener; the levelling agent is one or more of polyacrylic resin, siliceous acryl resin, polyvinyl butyral, benzoin, hydrocastor oil, cellulose acetate butyrate or epoxy soy oil; the nanometer material is nanometer zinc oxide; the hollow micro-ball is hollow ceramic microball.
Owner:苏州裕丰装饰门窗有限公司
Eubacterium, Clostridium preparation and use thereof
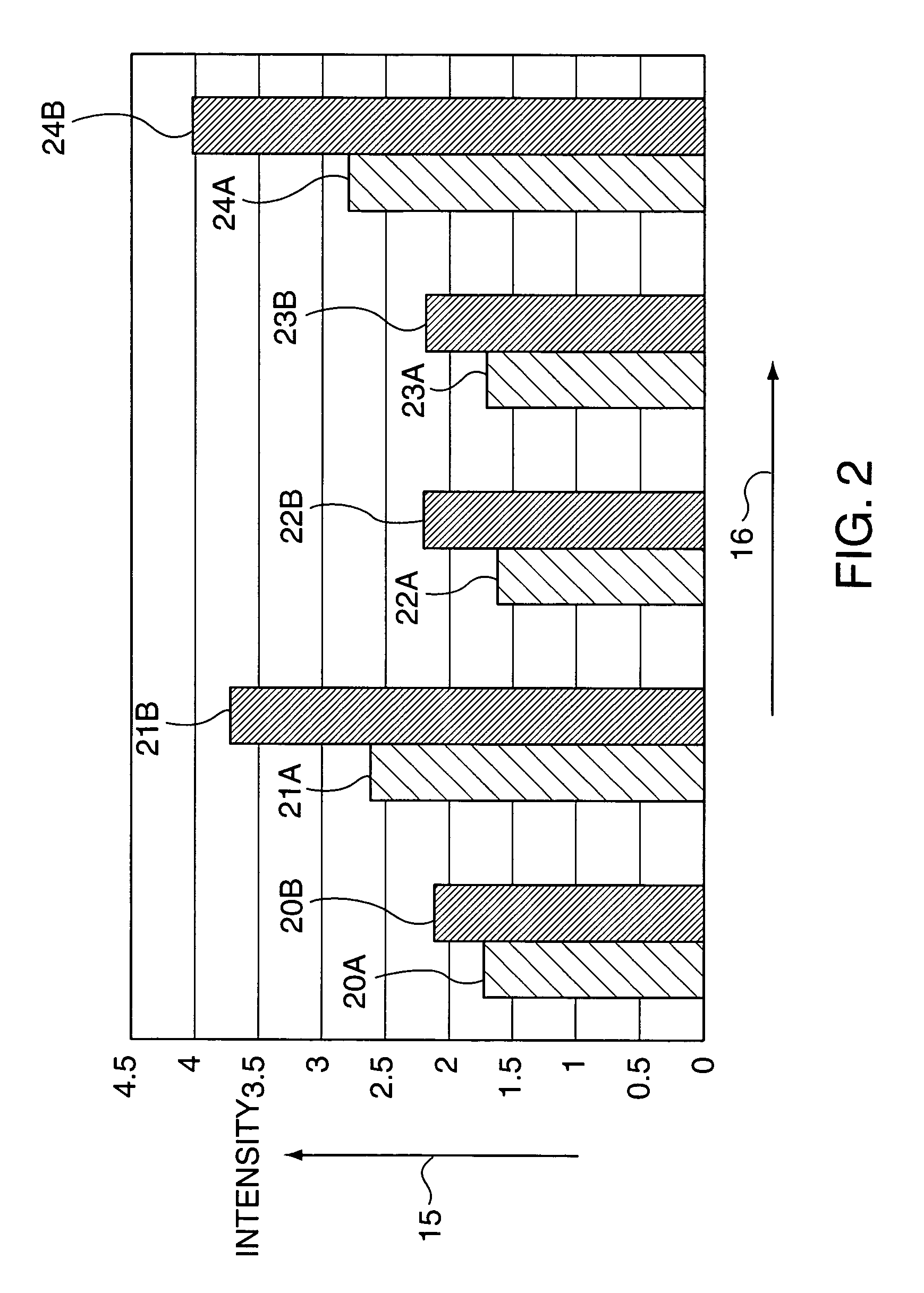
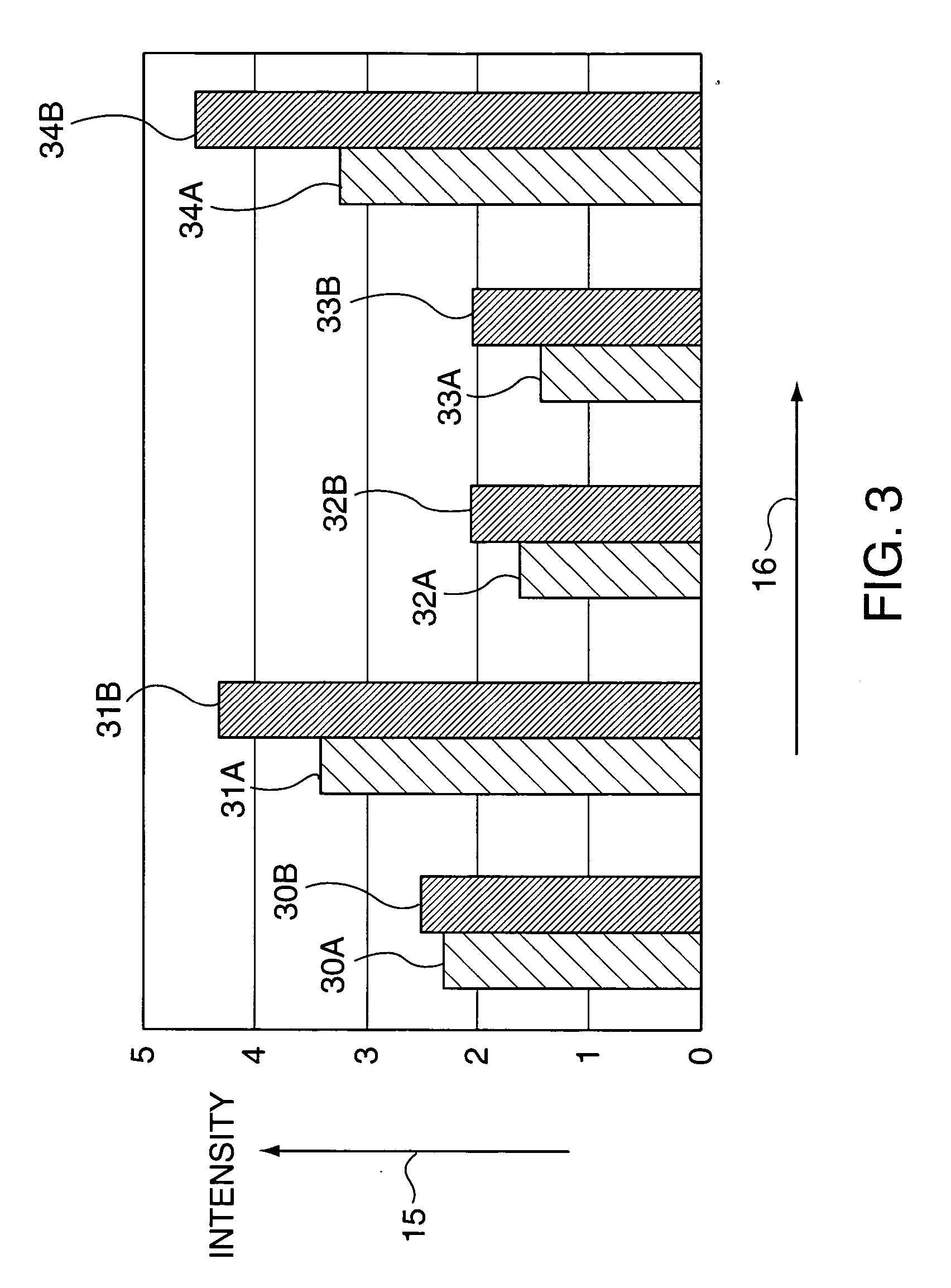
The invention relates to a eubacterium and clostridium preparation and application of the same, in particular to a microecological preparation which is prepared for replenishing butyric acid bacteria and butyric acid produced by intestinal tract by taking single eubacterium, single clostridium or a eubacterium and clostridium composition as a main active composition, and application of the same in treating related diseases through butyric acid production, and belongs to the field of biological medicine.
Owner:QINGDAO EASTSEA PHARMA +1
Weight loss medication and method
The invention involves a medication for weight loss by means of appetite suppression and a method for administering this medication to humans and other mammals. The medication comprises potassium butyrate or closely related chemical compounds, together with chemicals which facilitate the dispersion of the medication in the stomach.
Owner:AXCESS GLOBAL SCI LLC +1
Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations
A compound of an angiotensin receptor antagonist (ARB), a neutral endopeptidase inhibitor (NEPi) and one or more monovalent cations are useful for the treatment of hypertension and / or heart failure. ARB includes S—N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine in the anion form, NEPi includes (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester in the anion form and cation includes monovalent cations such as Na+. The compound includes trisodium [3-((1S,3R)-1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-(S)-3′-methyl-2′-(pentanoyl{2″-(tetrazol-5-ylate)biphenyl-4′-ylmethyl}amino)butyrate] hemipentahydrate.
Owner:NOVARTIS PHARM CORP
Reduction of 2,3-dihydroxy-2-methyl butyrate (DHMB) in butanol production
The invention relates generally to the field of industrial microbiology and butanol production. More specifically, the invention relates methods of reducing 2,3-dihydroxy-2-methyl butyrate (DHMB) in butanol production. DHMB can be reduced by inhibiting the reduction of acetolactate to DHMB, for example, by knocking out enzymes that catalyze the reduction or by removing DHMB during or after fermentation. Yeast strains, compositions, and methods for reducing DHMB and increasing butanol yield are provided.
Owner:BUTAMAXTM ADVANCED BIOFUELS
Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament
InactiveCN103893163AStrong inhibitory activitySelectiveOrganic active ingredientsNervous disorderMedicineMonoamine Oxidase A Gene
The invention belongs to the field of medicine, and in particular relates to a medical application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in a selective histone lysine-specific demethylase 1 (LSD1) inhibitor, especially the application in anti-tumor medicaments. Pharmacodynamic tests indicate that the 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate has a remarkable LSD1 inhibiting effect, and has selectivity to homologous proteins MAO-A (monoamine oxidase-A) and MAO-B.
Owner:CHINA PHARM UNIV
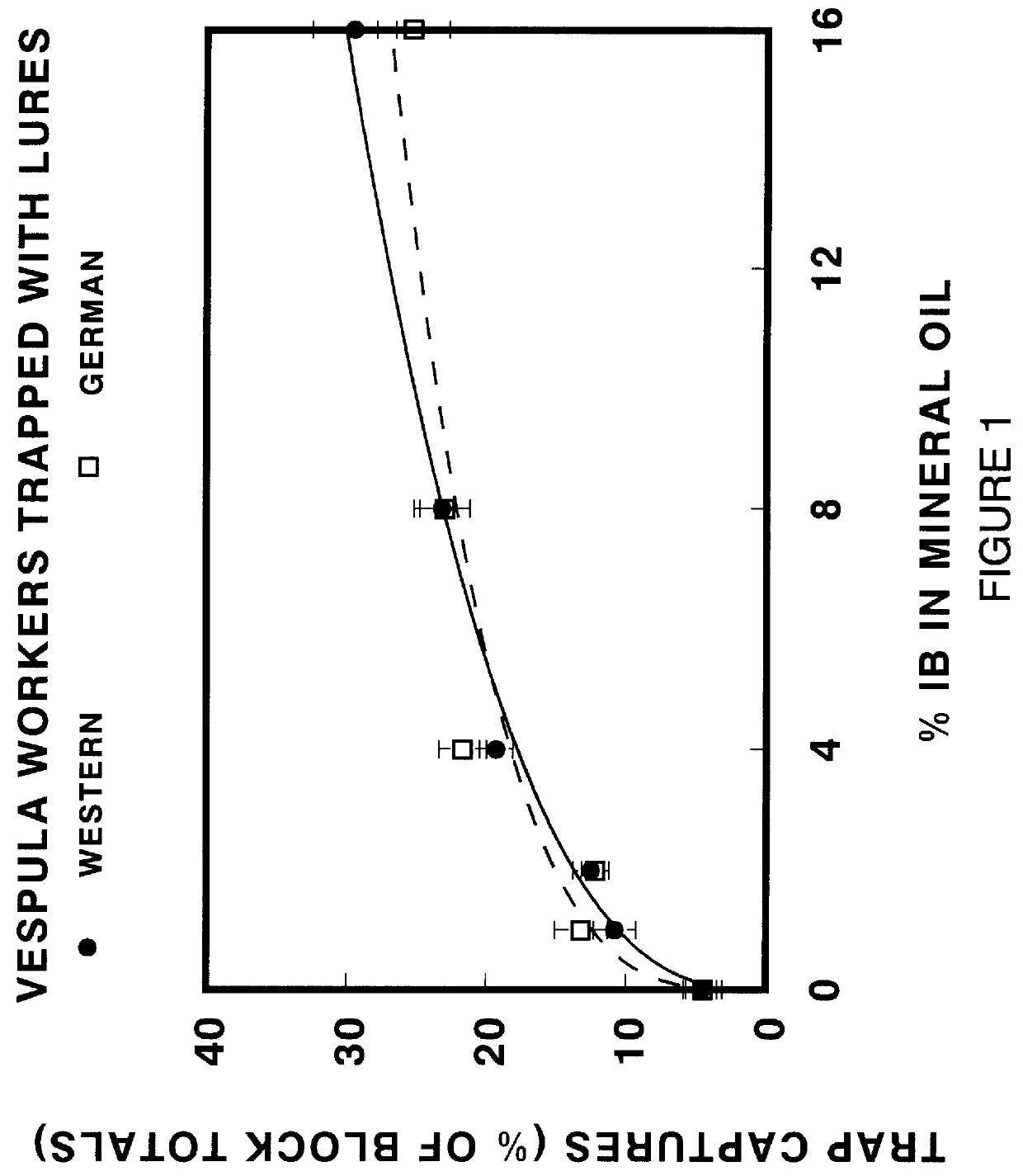
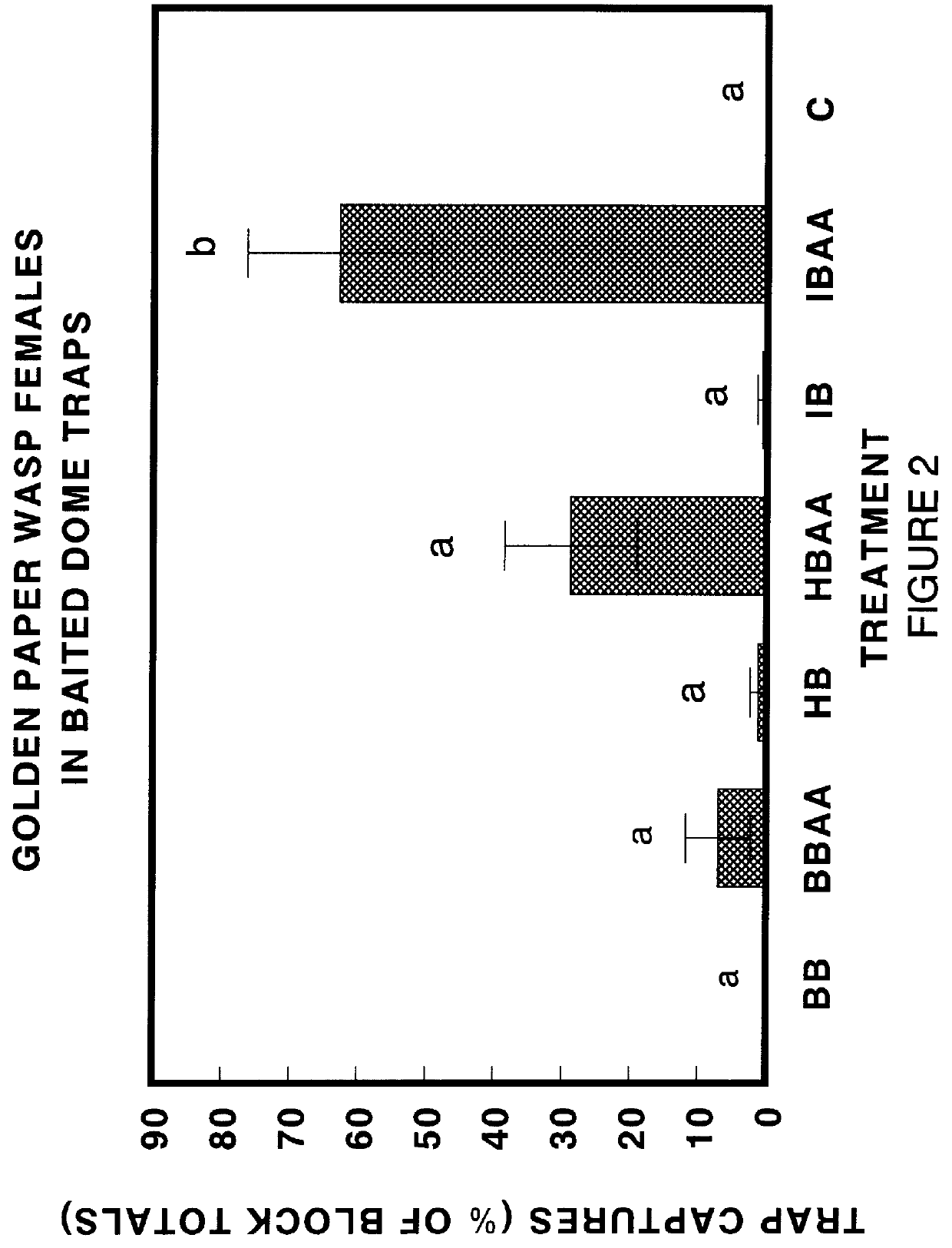
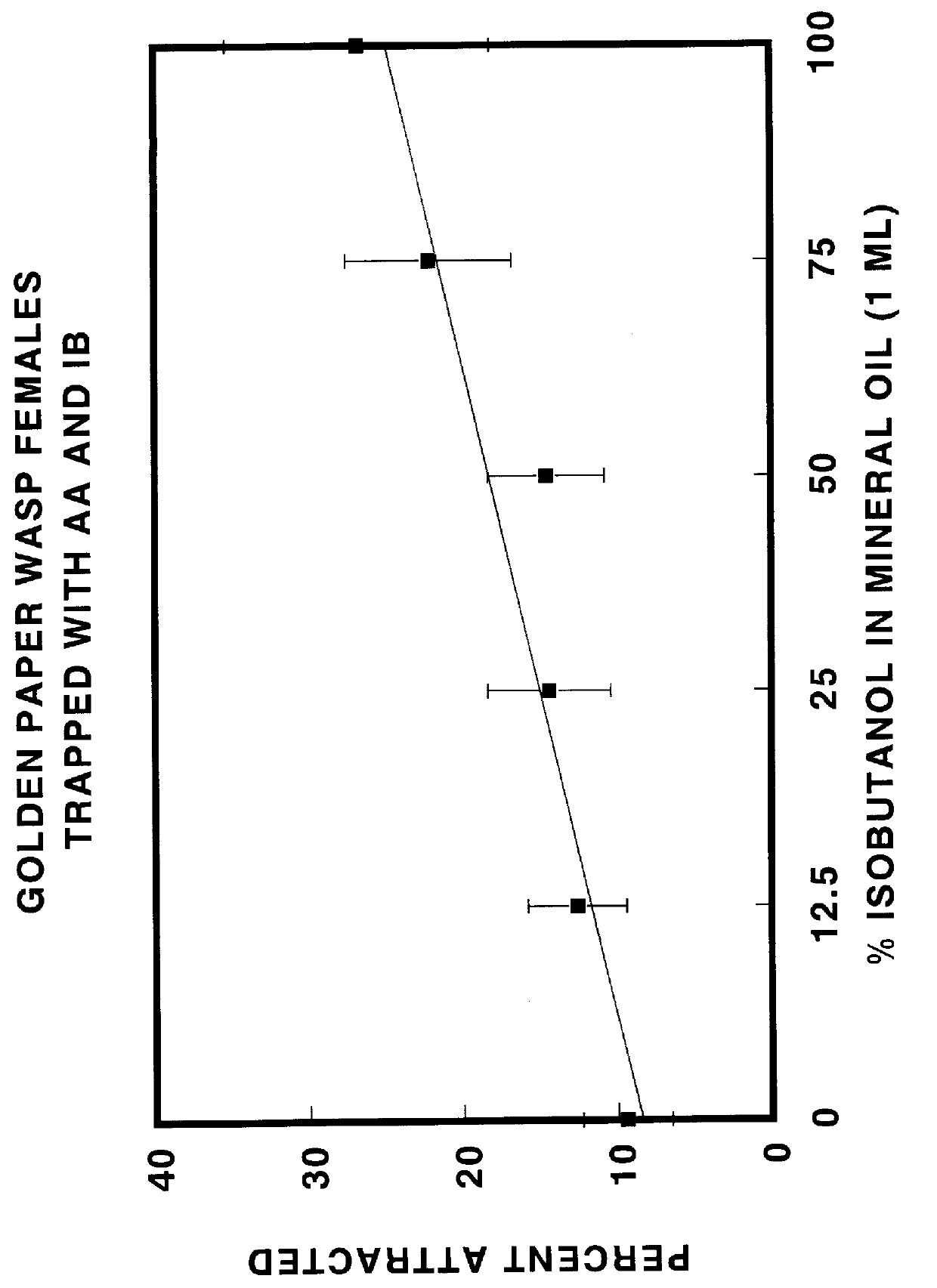
Chemical attractants for yellowjackets and paper wasps
InactiveUS6083498AEnhanced informationEfficient methodBiocidePeptide/protein ingredientsIsobutanolPropanol
Compositions and lures are described which provide vapor blends of acetic acid and one or more compounds selected from the group consisting of isobutanol, racemic 2-methyl-1-butanol, S-(-)-2-methyl-1-butanol, 2-methyl-2-propanol, heptyl butyrate, and butyl butyrate which function as highly effective attractants for yellowjacket wasps and paper wasps. By attracting wasps to traps or baits, the chemical attractants provide a means for detecting, surveying, monitoring, and controlling the wasps.
Owner:US SEC AGRI
Hydroxybutyrate ester and medical use thereof
ActiveUS20110237666A1Increase alertnessImprove cognitive functionBiocideNervous disorderFatty acidAnimal subject
A compound which is 3-hydroxybutyl 3-hydroxybutyrate enantiomerically enriched with respect to (3R)-hydroxybutyl (3R)-hydroxybutyrate of formula (I) is an effective and palatable precursor to the ketone body (3R)-hydroxybutyrate and may therefore be used to treat a condition which is caused by, exacerbated by or associated with elevated plasma levels of free fatty acids in a human or animal subject, for instance a condition where weight loss or weight gain is implicated, or to promote alertness or improve cognitive function, or to treat, prevent or reduce the effects of neurodegeneration, free radical toxicity, hypoxic conditions or hyperglycaemia.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Ketone bodies and ketone body esters as blood lipid lowering agents
ActiveUS9211275B2Reduce serum cholesterol and/or triglyceride levelLowering of total serum cholesterol levelHydroxy compound active ingredientsMetabolism disorderChemistryNutritional composition
The subject disclosure provides compositions for reducing serum cholesterol and / or triglyceride levels in subjects. These compositions can comprise racemic β-hydroxybutyrate or D-β-hydroxybutyrate, optionally in the acid form, physiologically compatible salts of racemic β-hydroxybutyrate or D-β-hydroxybutyrate, esters of D-β-hydroxybutyrate, oligomers of D-β-hydroxybutyrate containing from 2 to 20 or more monomeric units in either linear or cyclic form, racemic 1,3 butandiol or R-1,3 butandiol alone and can be, optionally, administered in conjunction with a low fat diet to a subject. Alternatively, compositions comprising racemic β-hydroxybutyrate or D-β-hydroxybutyrate, optionally in the acid form, physiologically compatible salts of racemic β-hydroxybutyrate or D-β-hydroxybutyrate, esters of D-β-hydroxybutyrate, oligomers of D-β-hydroxybutyrate containing from 2 to 20 or more monomeric units in either linear or cyclic form, racemic 1,3 butandiol, R-1,3 butandiol or combinations thereof can be formulated as nutritional supplements (also referred to as nutritional compositions) or incorporated into therapeutic compositions containing a) anti-hypertensive agents; b) anti-inflammatory agents; c) glucose lowering agents; or d) anti-lipemic agents) which are administered to a subject, optionally in combination with a low fat diet, in order to cause a reduction or lowering of: serum cholesterol levels; triglyceride levels; serum glucose levels, serum homocysteine levels, inflammatory proteins (e.g., C reactive protein) and / or hypertension in treated subjects. Alternatively, compositions disclosed herein can be administered alone, or in combination with other therapeutic agents to prevent or reverse vascular disease.
Owner:OXFORD UNIV INNOVATION LTD +1
Electroplating effect imitated plastic paint and preparation method, diluent and process of using same
The invention discloses a plastic paint with simulated electroplating effect. The composition and the weight ratio of the invention are as follows: 30 to 45 of acrylic resin with 50 percent of solid content and 70 to 90 DEG C of Tg, 20 to 30 of cellulose acetate butyrate with 20 percent of solid content, 8 to 12 of triad copolymer vinyl chloride-acetate resin with 30 percent of solid content, 6 to 10 of non-floating aluminum and silver pulp with an average diameter less than or equal to 20 Mum, 0.3 to 0.5 of dispersant, 0.2 to 0.4 of flatting agent, 0.4 to 0.6 anti-settling agent, 6.0 to 10.0 of toluene, 3.5 to 6.0 of ethyl acetate, 3.5 to 6.0 methy isobutyl ketone, 3.0 to 5.0 of isobutanol and 4.0 to 7.0 of glycol butyl ether. The special diluent of the invention, according to the weight ratio, is made from the following components evenly mixed: 20 of white gas, 10 of toluene, 22 of ethyl acetate, 15 of acetone, 25 of isobutanol and 8 of glycol butyl ether. The main paint which comprises the composition and the diluent are evenly mixed according to the ratio of 1: 2-3, then the mixture is used for spray coating; the metallic appearance of the paint film obtained is very close to the effect of the electroplating, and the paint film has good alcohol resistance with high hardness and strong wear resistance, and also has simple process. The aluminum and silver pulp used is a common type, the cost of which is only 5 percent to 10 percent of the simulated electroplating aluminum and silver pulp and about 2 percent of electrosilvering.
Owner:SHENZHEN GRANDLAND ENVIRONMENTAL COATING CO LTD
Valbenazine salts and polymorphs thereof
Provided herein are salts of (S)-2-amino-3-methyl-butyric acid (2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl ester in amorphous and crystalline forms, and processes of preparation, and pharmaceutical compositions thereof. Also provided are methods of their use for treating, preventing, or ameliorating one or more symptoms of neurological disorders and diseases including hyperkinetic movement disorders or diseases.
Owner:NEUROCRINE BIOSCI INC
Pest control using natural pest control agent blends
ActiveUS20110135764A1Increase volumeBiocideHydroxy compound active ingredientsMethyl salicylateBULK ACTIVE INGREDIENT
Embodiments of the invention relate to a composition for controlling a target pest, wherein the composition includes at least two active ingredients selected from the group consisting of thymyl acetate, linalyl acetate, amyl butyrate, anise star oil, black seed oil, p-cymene, geraniol, isopropyl myristate, d-limonene, linalool, lilac flower oil, methyl salicylate, alpha-pinene, piperonal, piperonyl alcohol, tetrahydrolinalool, thyme oil white, thyme oil red, thymol, vanillin, and winter-green oil, wherein the composition causes synergistic control of the target pest.
Owner:TYRATECH +1
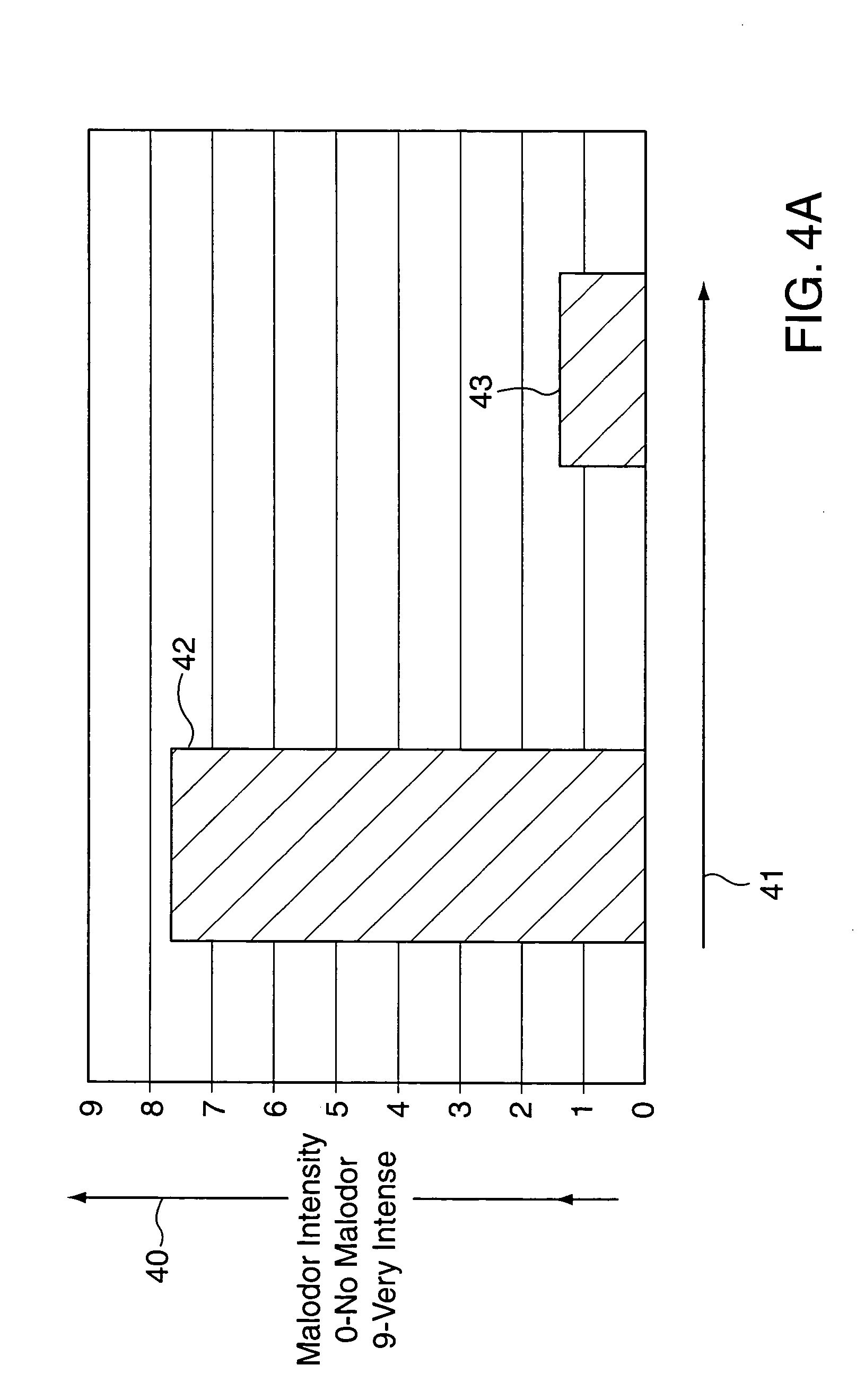
Synergistically-effective composition of zinc ricinoleate and one or more substituted monocyclic organic compounds and use thereof for preventing and/or suppressing malodors
InactiveUS20050106192A1Eliminate perceptionReduction of perceptionCosmetic preparationsToilet preparationsPolymer scienceEthyl group
Described are synergistically-effective compositions consisting essentially of zinc ricinoleate or solutions thereof and one or more of the substituted monocyclic organic compounds: 1-cyclohexylethan-1-yl butyrate; 1-cyclohexylethan-1-yl acetate; 1-cyclohexylethan-1-ol; 1-(4′-methylethyl)cyclohexylethan-1-yl propionate; and / or 2′-hydroxy-1′-ethyl(2-phenoxy)acetate and uses of same and compositions containing same for preventing and / or suppressing malodors. Also described for the purpose of application to an inanimate laminar substantially solid surface are malodor-suppressing composition-containing stick articles containing an ester-terminated polyamide or an amide-terminated polyamide structural support polymer in combination with the aforementioned synergistically-effective zinc ricinoleate-substituted monocyclic organic compound composition. Also described is a package for conveniently handling the aforementioned stick article.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Peel-off coating compositions
ActiveUS20100167075A1High strengthAvoid lack of toughnessNon-fibrous pulp additionAlkaline-earth metal silicatesEmulsionWater resistant
Methods and compositions are provided for protecting exterior surfaces of automobiles and other products, or components of products, against abrasion, abrasive dust, water, acid rain, etc. The methods involve applying to a surface a protective coating composition comprising a polyvinyl butyrate emulsion and a relatively inert extender. The emulsion is dried to form a water-resistant protective coating that can be removed from the underlying surface by peeling when no longer desired.
Owner:CAL WEST SPECIALTY COATINGS
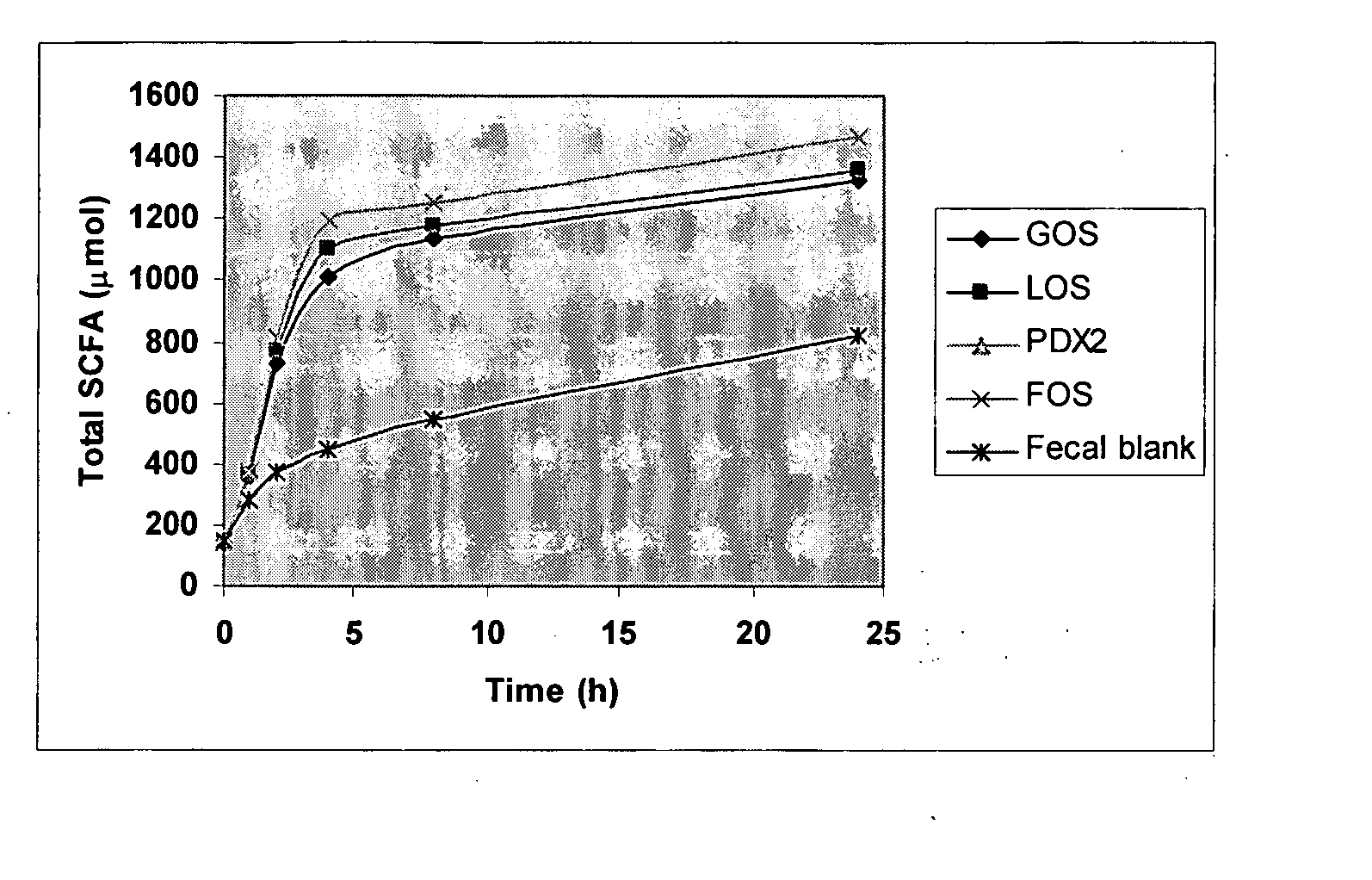
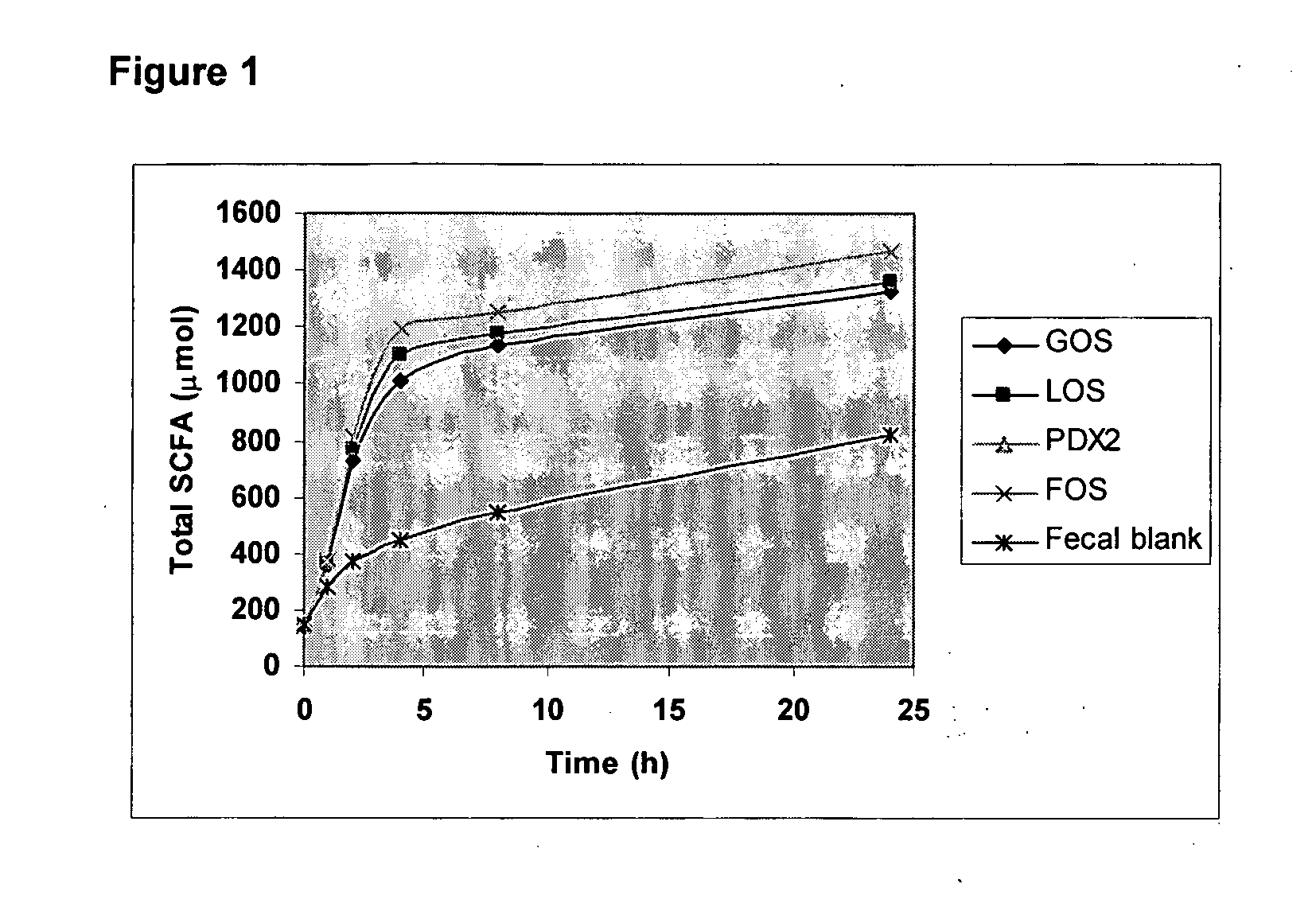
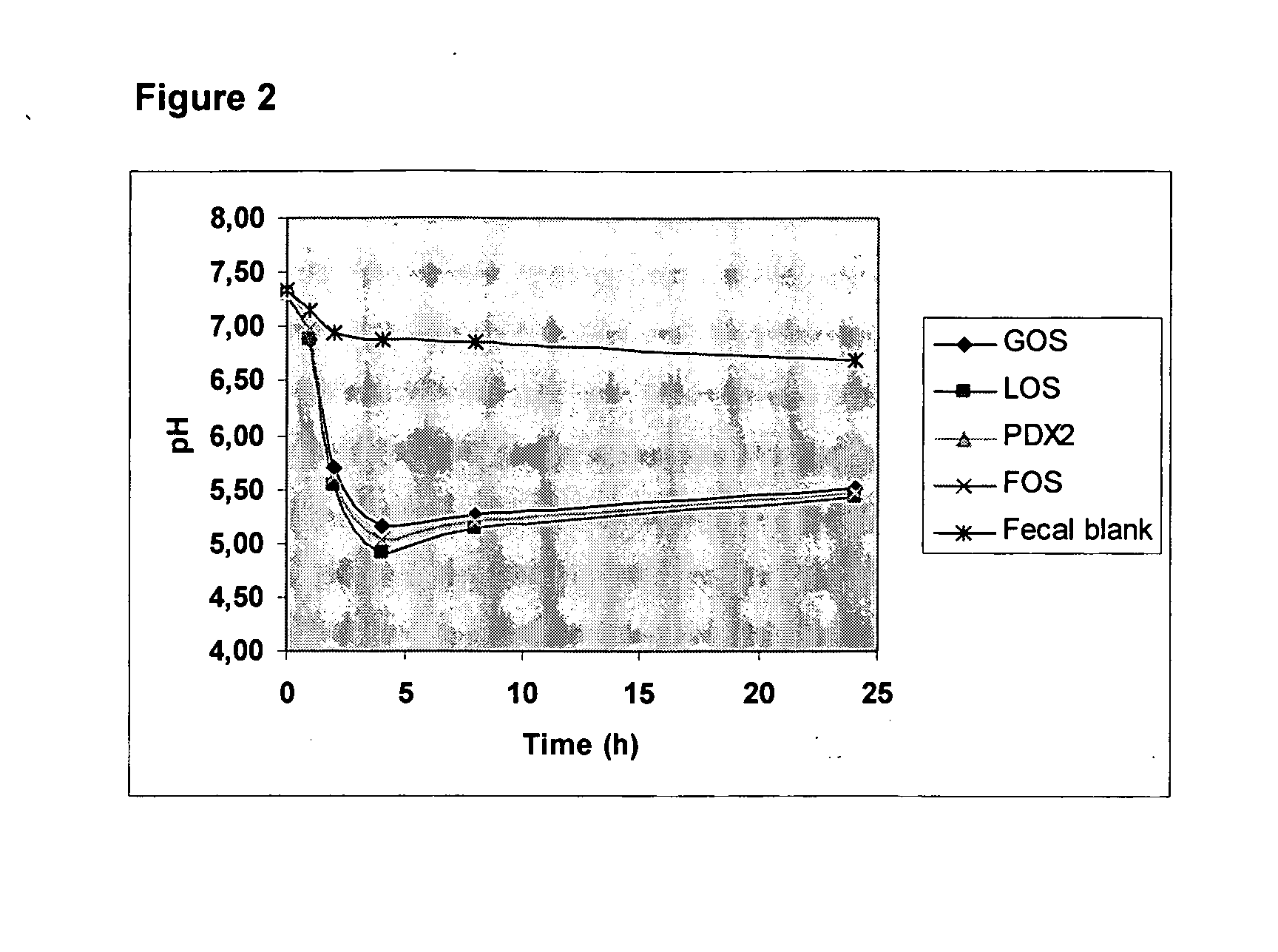
Method for simulating the functional attributes of human milk oligosaccharides in formula-fed infants
ActiveUS20060286258A1Improve the level ofDecrease in butyrateOrganic active ingredientsDigestive systemButyrateFormula fed
The present invention is directed to a novel method for increasing the production of acetate, decreasing the production of butyrate, increasing the population and species of beneficial bacteria and slowing the rate of fermentation of prebiotics within the gut of a formula-fed infant. The method comprises administration of a therapeutically effective amount of PDX to the infant.
Owner:MEAD JOHNSON NUTRITION
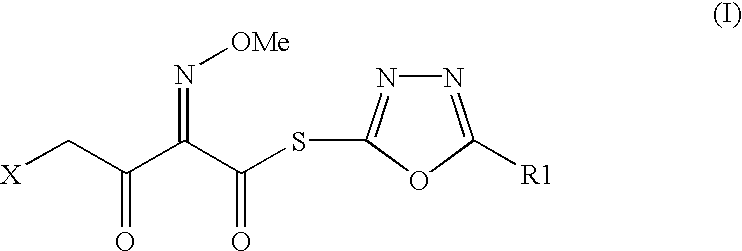
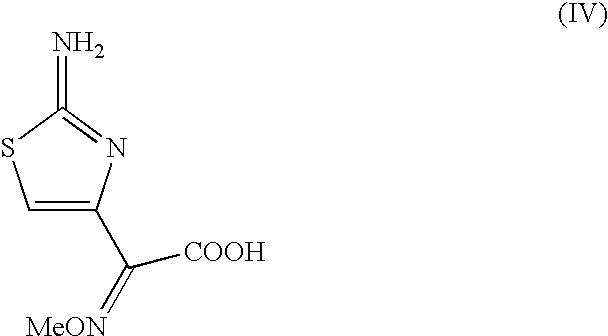
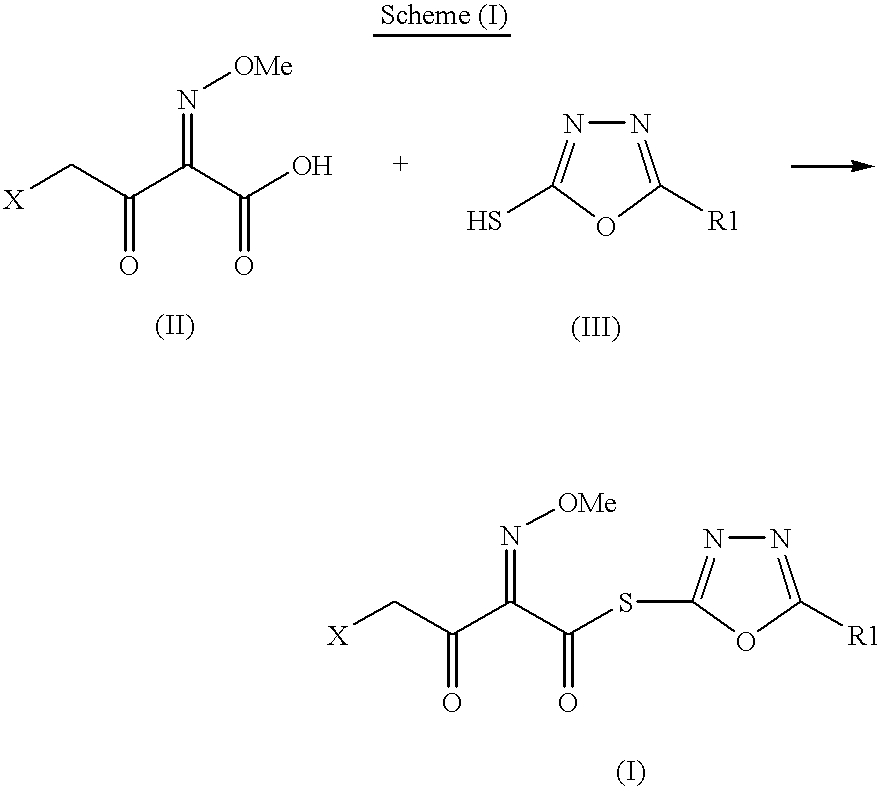
Preparation of new intermediates and their use in manufacturing of cephalosporin compounds
The present invention provides new thioester derivatives of 4-halogeno-2-methoxyimino-3-oxo-butyric acid of the general formula (I), also, the invention provides a method by which the said thioester derivatives can be prepared by reacting 4-halogeno-2-methoxyimino-3-oxo-butyric acid of the general formula (II) with 2-mercapto-5-substituted-1,3,4-oxadiazoles of the general formula (III) in a solvent, in the presence of DMF / POCl3 and in presence of an organic base and if desired the so obtained thioester derivatives so obtained are reacted with 7-amino-cephem carboxylic acids of the general formula (V) to produce condensed products which are insitu reacted with thiourea to get cephalosporin antibiotic compounds having the general formula (VI).
Owner:ORCHID CHEM &PHARMA LIMITED INDIA
Valbenazine salts and polymorphs thereof
Provided herein are salts of (S)-2-amino-3-methyl-butyric acid (2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl ester in amorphous and crystalline forms, and processes of preparation, and pharmaceutical compositions thereof. Also provided are methods of their use for treating, preventing, or ameliorating one or more symptoms of neurological disorders and diseases including hyperkinetic movement disorders or diseases.
Owner:NEUROCRINE BIOSCIENCES INC
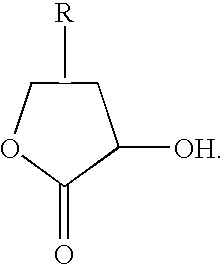
Method for producing optically active 2-(n-substituted aminomethyl)-3-hydroxybutyric acid ester
The present invention relates to a method for producing optically active 2-(N-substituted aminomethyl)-3-hydroxybutyric acid esters wherein a 2-(N-substituted aminomethyl)-3-oxobutyric acid ester is treated with an enzyme source capable of stereoselectively reducing said ester to the corresponding optically active 2-(N-substituted aminomethyl)-3-hydroxybutyric acid ester having the (2S,3R) configuration. The present invention provides an efficient method for industrially producing optically active 2-(N-substituted aminomethyl)-3-hydroxybutyric acid esters, in particular such compounds having the (2S,3R) configuration, which are useful as intermediates for the production of medicinal compounds, among others.
Owner:KANEKA CORP
Full-biological degradation nonwoven cloth material
InactiveCN101220534AConjugated synthetic polymer artificial filamentsNon-woven fabricsEpoxyCyclohexene oxide
The invention relates to a total biodegradable non-woven fabric material which is produced by adding 10 to 40 percent of low molecular weight carbon dioxide-cyclohexene oxide copolymer or 10 to 40 percent of high molecular weight poly L-polylactic acid or 10 to 40 percent of poly-hydroxylic butyrate to biodegradable carbon dioxide-propylene oxide or carbon dioxide-epoxy ethane copolymer materials, thus improving the dimensional stability of the carbon dioxide copolymer under high temperature. The number average molecular weight of the carbon dioxide-cyclohexene oxide copolymer is between 4000 and 8000 and the number average molecular weight of the poly L-polylactic acid is above 100,000. By using the carbon dioxide copolymer material of the invention, manufacture can be carried out by adopting a spun-bond method which is commonly used in non-woven fabric manufacturing technique. The obtained non-woven fabrics can maintain dimensional stability at the temperature between 70 DEG C and 75 DEG C, have biodegradation characteristics under the situation of composts or landfill with carbon dioxide and water as degradation end products, and cannot pollute environment when being burnt.
Owner:吉林金源北方科技发展有限公司
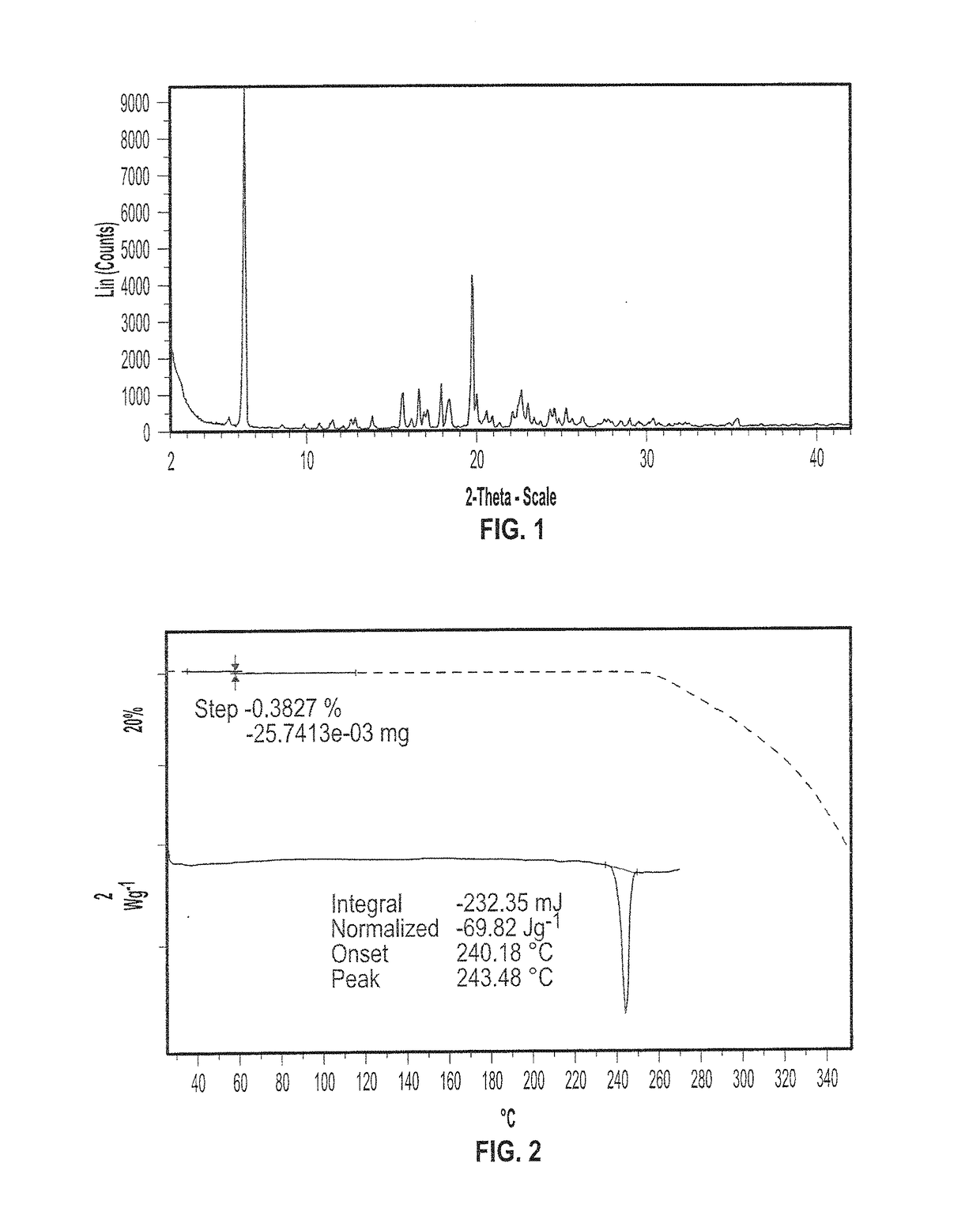
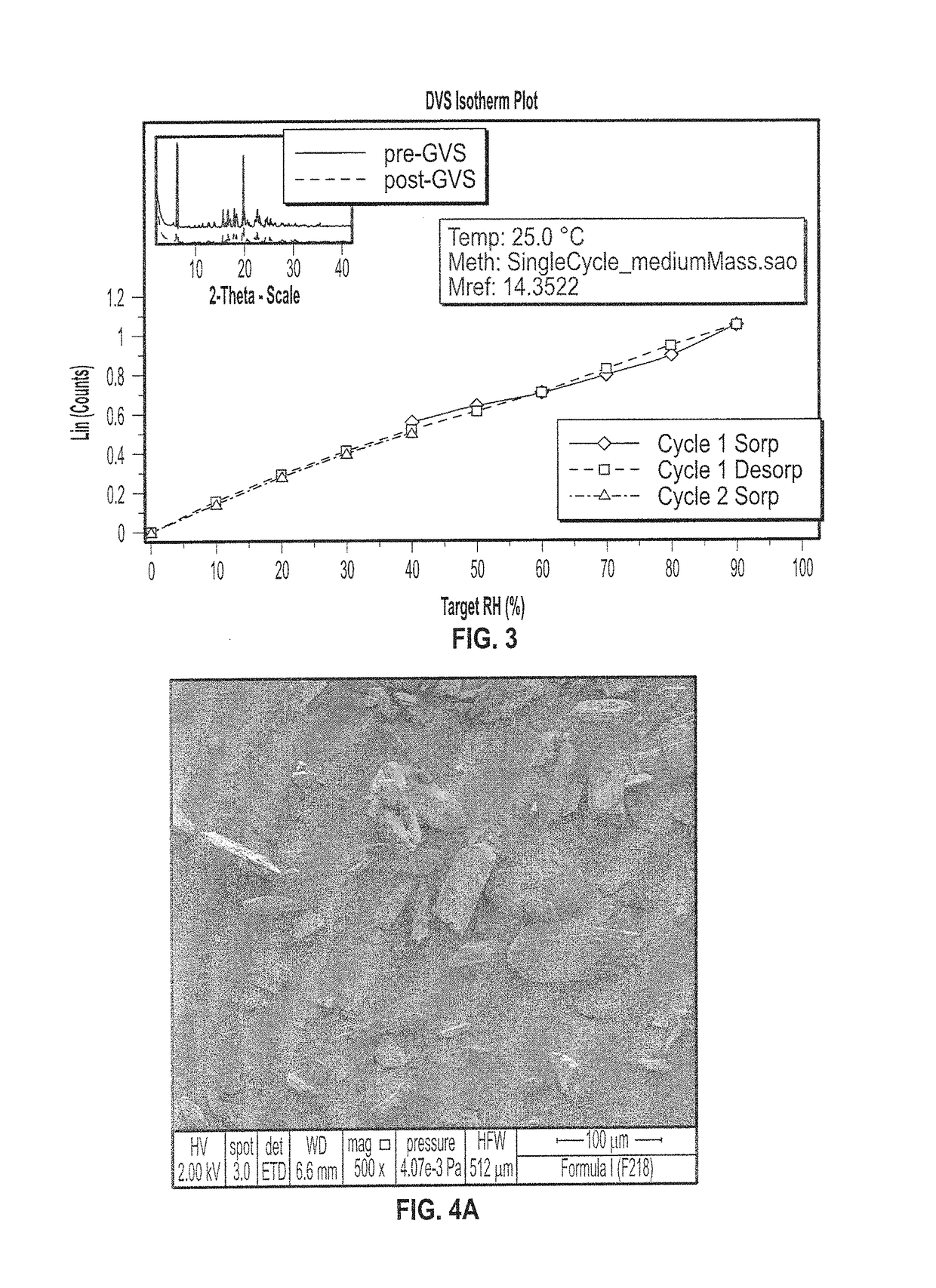
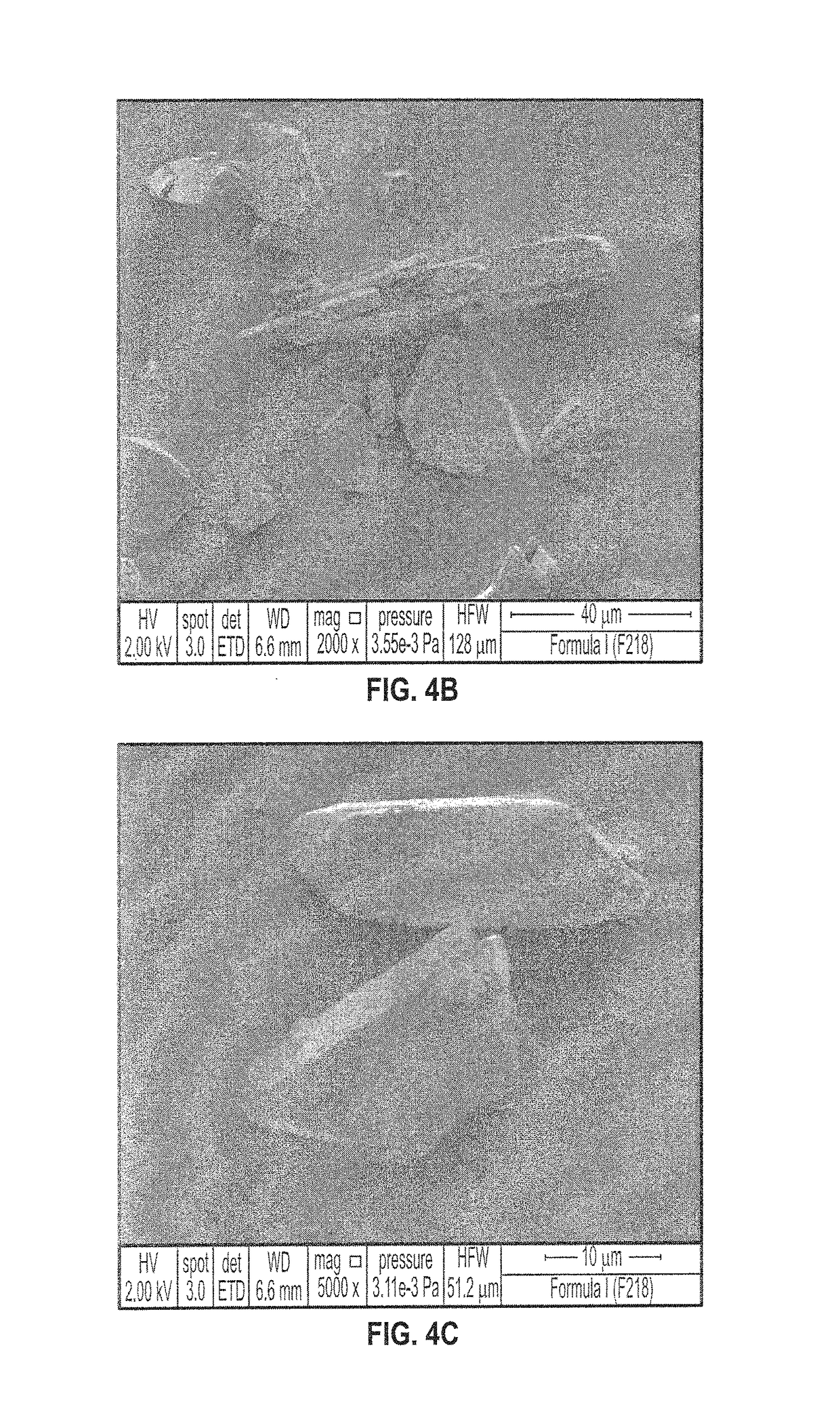
SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)
ActiveUS20170183346A1Safe and efficient and cost-effective and readily scalableSafe and efficient and and readily methodOrganic chemistryPyridine3-Methylbutanoic acid
Provided herein are processes for the preparation of (S)-(2R,3R,11bR-3-isobuty-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl 2-amino-3-methylbutanoate di(4-methylbenzenesulfonate), or a solvate, hydrate, or polymorph thereof.
Owner:NEUROCRINE BIOSCI INC
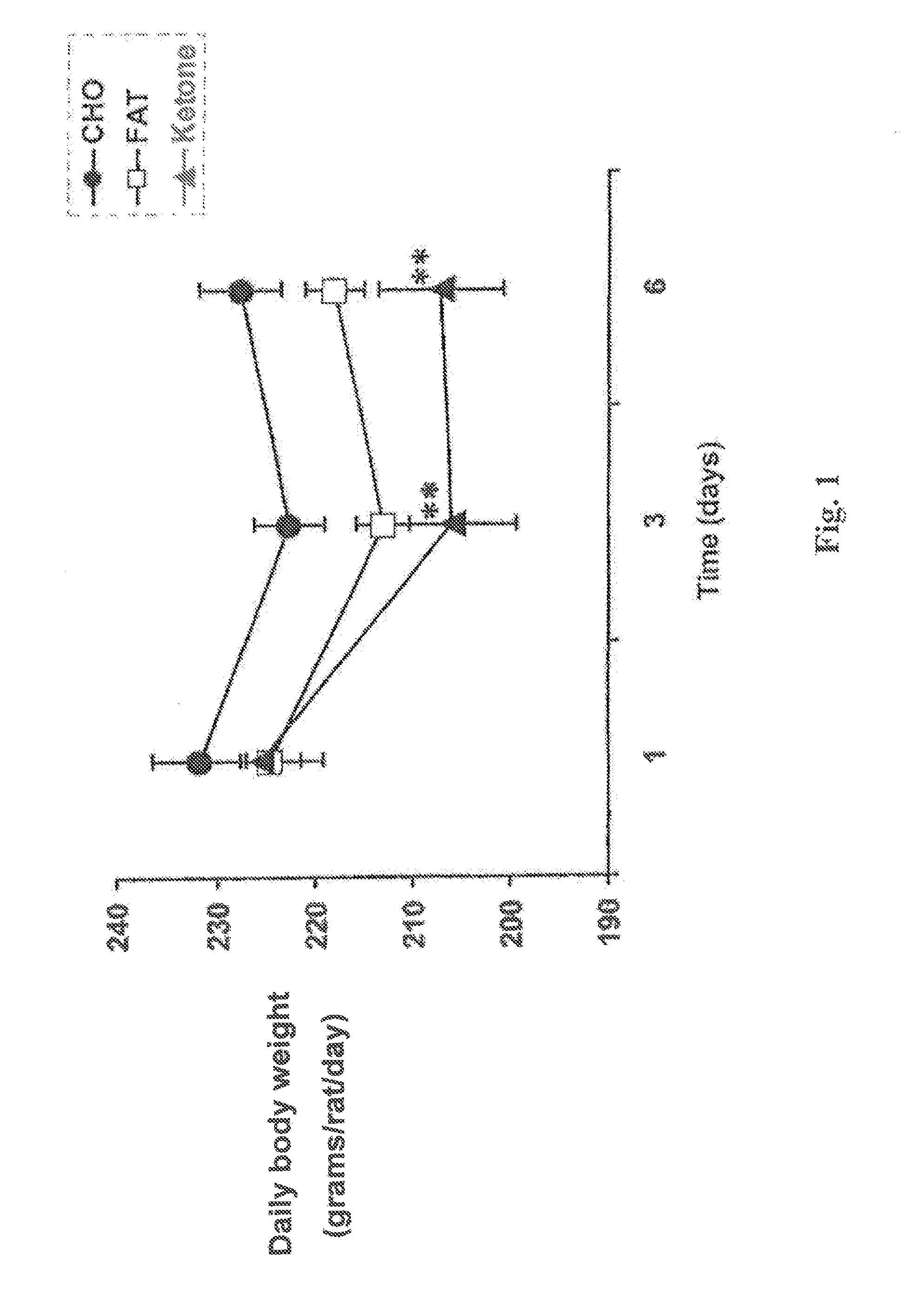
Method and test strips for the measurement of fat loss during weight loss programs
InactiveUS20040043376A1Great advantageEasy to manufactureMicroorganismsMicrobiological testing/measurementPhysiologyAcyl CoA dehydrogenase
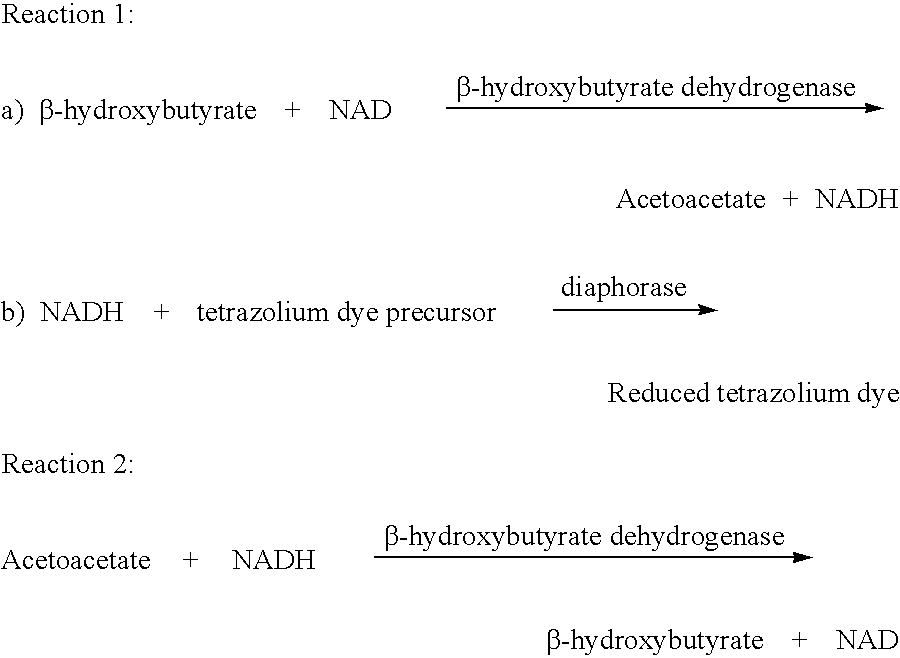
Disposable test strips and a wet chemistry method for measuring each of beta-hydroxybutyrate alone, combined beta-hydroxybutyrate and acetoacetate or total ketone bodies (i.e., beta-hydroxybutyrate, acetoacetate and acetone) in human bodily fluid samples, including but not limited to urine, saliva or sweat are described. The test strips need only be dipped in the sample and can be used by anyone in almost any milieu. Measurement can be made electrochemically, spectrophotometrically, fluorometrically or by comparision to a color standard. Combined acetoacetate and beta-hydroxybutyrate which account for 97-98% of total ketone bodies and may be measured in a cyclic reaction that occurs at pH about 7.0 to about 8.3 with beta-hydroxybutyrate dehydrogenase, (beta-HBD), nicotinamide adenine dinucleotide, a tetrazolium dye precursor and an electron mediator. Using this reaction, false positive results obtained from urine samples taken from patients on sulfhydryl drugs are avoided. beta-HBD from some sources was found to cause false negative results in samples (e.g. urine) containing high chloride content due to chloride inhibition of beta-HBD. Using a simple test for chloride inhibition, it was found that beta-HBD from Alcaligenes is not so inhibited. Using either beta-HBD that is not inhibited by chloride or using 10-20 times the normal concentration of this enzyme eliminates false negatives in samples having substantial chloride content, such as urine, both in the reaction described above and in other reactions disclosed for measuring each of beta-hydroxybutyrate alone, combined beta-hydroxybutyrate and acetoacetate and total ketone bodies, all of which reactions occur in the pH range of about 8.6 to about 9.5.
Owner:GUPTA SURENDRA
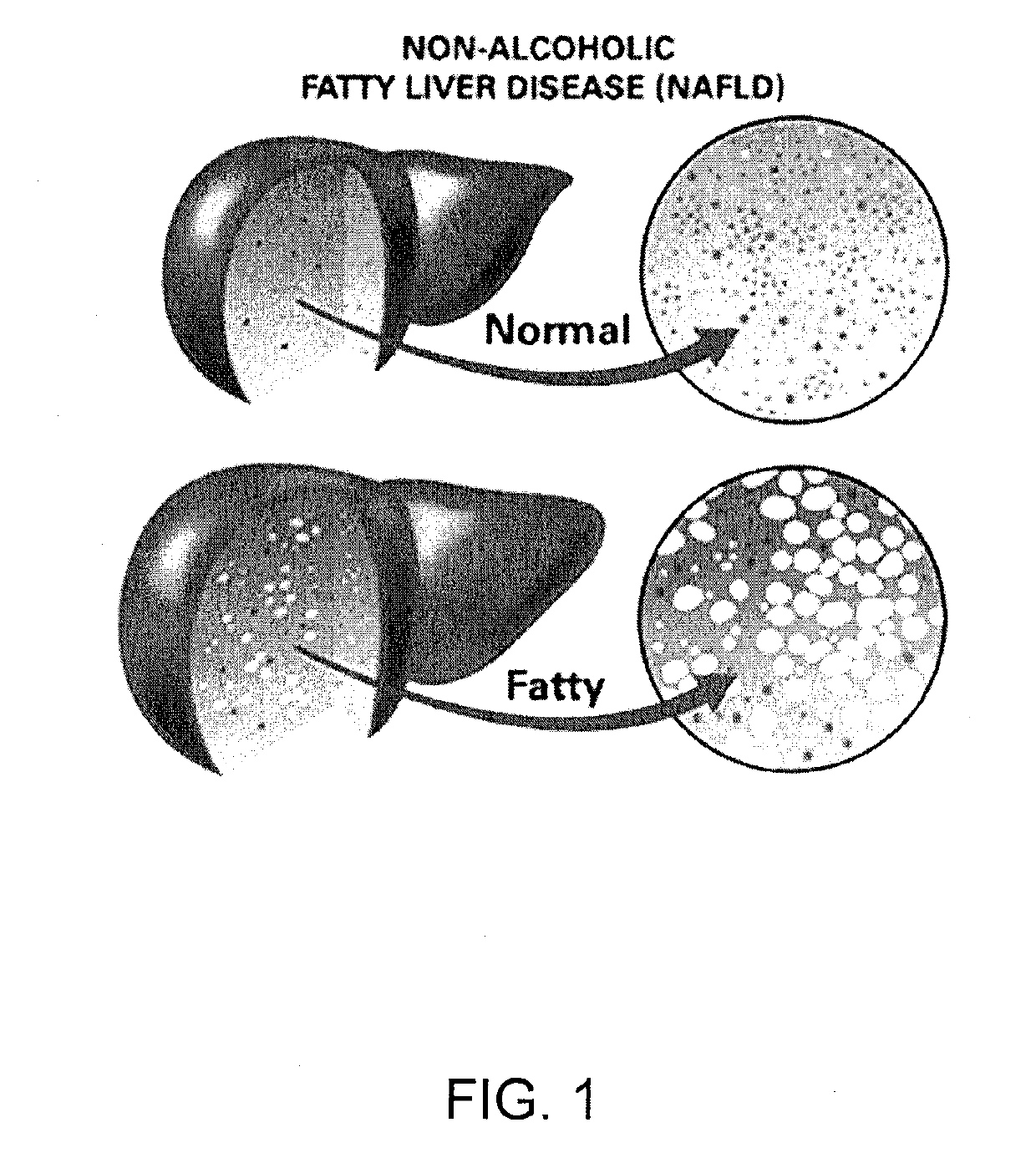
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS20190117709A1Inhibit progressEarly detectionOrganic active ingredientsDigestive systemFiberMonoacylglycerol acyltransferase
A method for reducing the likelihood of developing non-alcoholic steatohepatitis (NASH) in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and at least 6 grams per day of fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and are also encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced.
Owner:SEED HEALTH INC
Application of alcohol dehydrogenase in catalytic generation of ethyl (R)-4-chloro-3-hydroxy butyrate
InactiveCN103160547AHigh yieldHigh optical activityMicroorganism based processesFermentationHydroxybutyric acidPtru catalyst
The invention discloses application of alcohol dehydrogenase with amino acid sequence disclosed as SEQ ID NO:2 in preparing ethyl (R)-4-chloro-3-hydroxy butyrate from ethyl 4-chloroacetoacetate by asymmetric reduction. By using alcohol dehydrogenase with amino acid sequence disclosed as SEQ ID NO:2 as a catalyst, ethyl 4-chloroacetoacetate as a substrate and NADH (nicotinamide adenine dinucleotide) as a cofactor, asymmetric reduction is carried out to prepare the ethyl (R)-4-chloro-3-hydroxy butyrate. The invention applies the alcohol dehydrogenase with amino acid sequence disclosed as SEQ ID NO:2 in preparing ethyl (R)-4-chloro-3-hydroxy butyrate from ethyl 4-chloroacetoacetate by asymmetric reduction for the first time, and has favorable effect. The enzyme activity is up to 5.6 U / mg, the yield of the substrate is up to 94%, and the enantiomeric excess value of the product is 100%. The yield is high, and the production cost is greatly lowered.
Owner:NANJING UNIV OF TECH
Prepn process of beta-hydroxyl-beta-methyl butyric calcium (HMB-Ca)
InactiveCN1417190ASimple preparation processHigh purityCarboxylic acid salt preparationIsobutanolHaloform reaction
The present invention belongs to an organic compound preparing technology. Beta-hydroxyl-beta-methyl butyric calcium (HMB-Ca) is prepared using 4-methyl-4-hydroxy-2-propione as raw material and wateras solvent and through haloform reaction in water solution of NaOBr, acidification, isobutanol extraction, reaction of HMB acid in isobutanol extract with Ca(OH)2 to produce salt HMB-Ca. The said process has high product purity and less environmental pollution. The product HMB-Ca may be used in promoting the growth of muscles, strengthening immunity, reducing cholesterin and low-density lipoprotein level, preventing coronary heart disease and cardiac vascular disease, strengthening body's nitrogen-fixing capacity and maintaining body's protein level.
Owner:MANTE SHANGHAI BIAOLOGICAL SCI TECH
Preparation method of two-dimension nitrogen- and phosphorus-doped graphene
The invention discloses a preparation method of two-dimension nitrogen- and phosphorus-doped graphene. The preparation method particularly includes following steps: adding hydrotalcite to a proper amount of a sodium butyrate solution, stirring the mixture, aging the mixture, adding 4-dimethyl aminopyridine and n-butyl phosphate, stirring the mixture in a constant-temperature water bath at 60-70 DEG C for 6-8 h, separating a precipitation from liquid, washing the precipitation with deionized water for 2-3 times, drying and grinding the precipitation, sieving the precipitation through a sieve being 20-40 in meshes to obtain modified hydrotalcite powder, heating the modified hydrotalcite powder in a vacuum tubular furnace to 400-600 DEG C under a vacuum condition for calcining the powder for 2-4 h, adding the calcined powder to a hydrochloric acid solution, separating the precipitation and finally heating the precipitation to 2000-2500 DEG C under the vacuum condition to perform heat treatment for 3-6 h, and cooling a product to obtain the two-dimension nitrogen- and phosphorus-doped graphene. The preparation method is simple in raw materials and is mild in conditions.
Owner:JINING XINRUIDA INFORMATION TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00000.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00001.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-C00001.png)


![Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7e706329-04e4-4b5b-b8c9-1a182cd2dbef/HSA0000102396500000011.PNG)
![Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7e706329-04e4-4b5b-b8c9-1a182cd2dbef/HSA0000102396500000012.PNG)
![Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7e706329-04e4-4b5b-b8c9-1a182cd2dbef/HSA0000102396500000013.PNG)

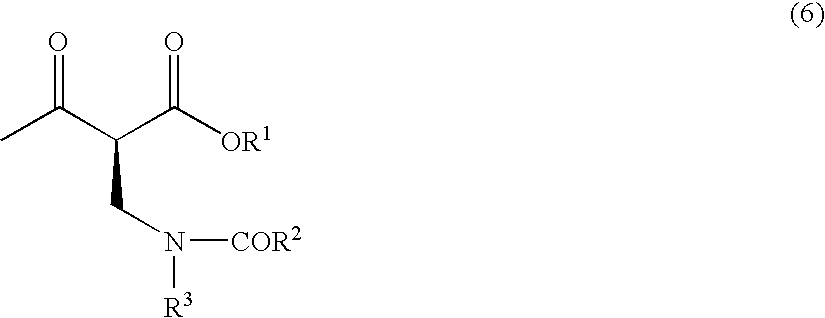

![SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE) SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/af5a93d0-aa6a-4674-8cd4-51408de0087f/US20170183346A1-20170629-C00001.png)
![SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE) SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/af5a93d0-aa6a-4674-8cd4-51408de0087f/US20170183346A1-20170629-C00002.png)
![SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE) SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/af5a93d0-aa6a-4674-8cd4-51408de0087f/US20170183346A1-20170629-C00003.png)