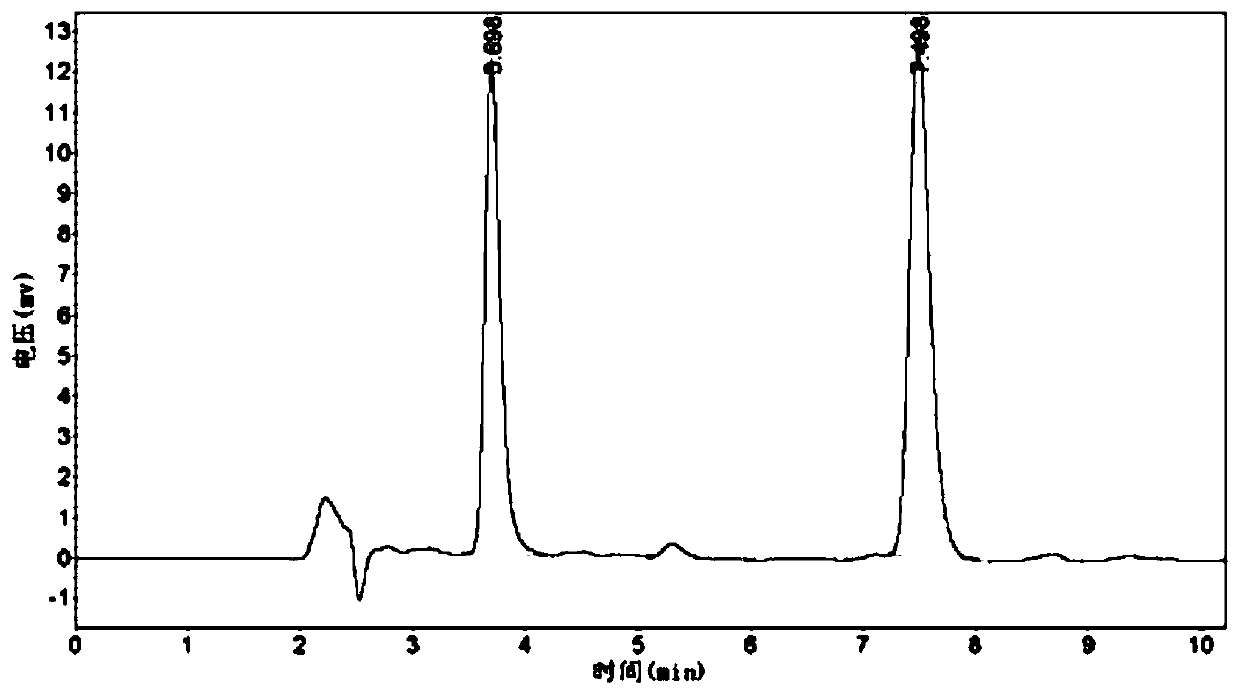
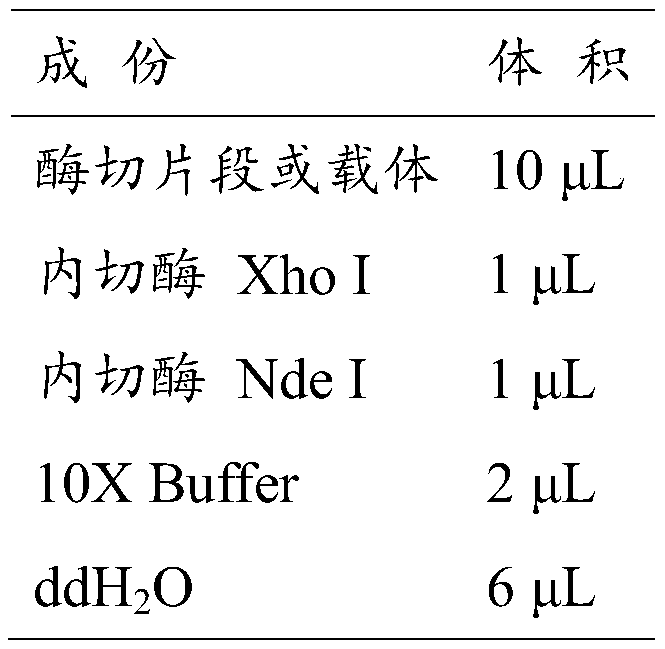
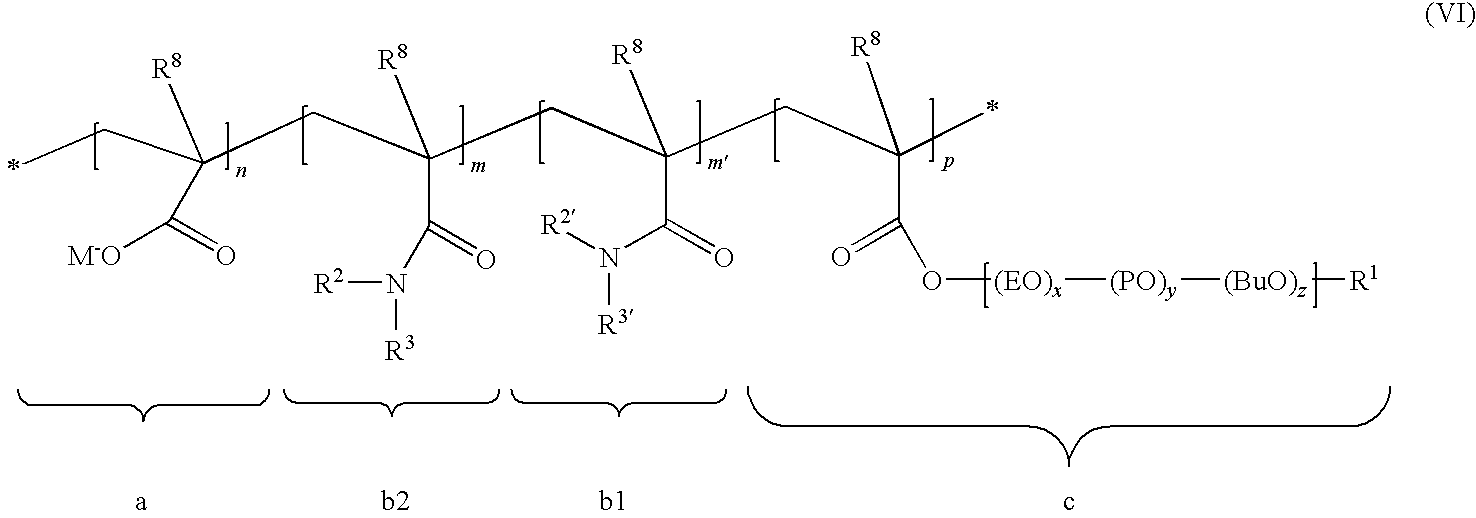
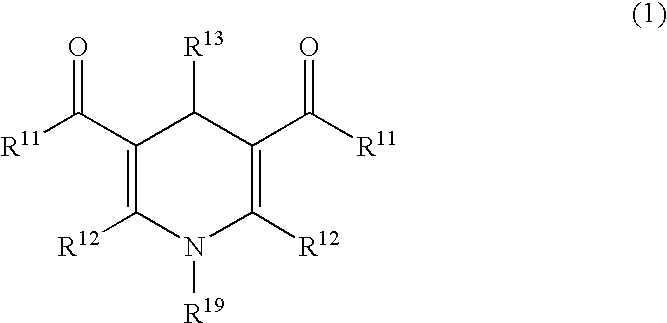
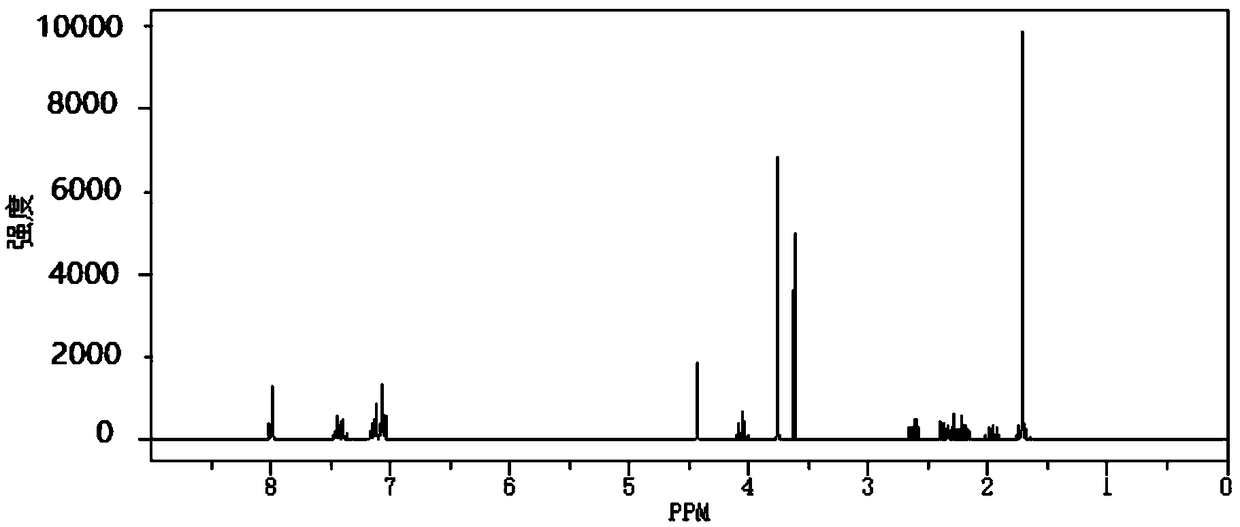
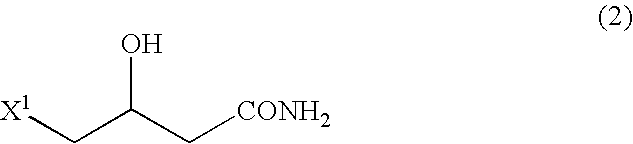Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
232 results about "Crotonic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Crotonic acid ((2E)-but-2-enoic acid) or is a short-chain unsaturated carboxylic acid, described by the formula CH₃CH=CHCO₂H. It is called crotonic acid because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. The cis-isomer of crotonic acid is called isocrotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to butyric acid.
Absorbent article comprising a synthetic polymer derived from a renewable resource and methods of producing said article
An element of an absorbent article is provided. The element has a bio-based content of at least about 50% based on the total weight of the element, and comprises a synthetic polymer derived from a renewable resource via a first intermediate compound selected from the group consisting of crotonic acid, propiolactone, ethylene oxide, i-propanol, butanol, butyric acid, propionic acid, 2-acetoxypropanoic acid, methyl 2-acetoxypropanoate, methyl lactate, ethyl lactate, polyhydroxybutyrate, and a polyhydroxyalkanoate comprising 3-hydroxypropionate monomers. An absorbent article comprising the element and a method of making an element for an absorbent article also are provided.
Owner:THE PROCTER & GAMBLE COMPANY
Pvc/Wood Composite
InactiveUS20080261019A1Synthetic resin layered productsCellulosic plastic layered productsMethacrylateFibrous composites
The present invention relates to a thermoplastic / natural cellulosic fiber composite, and more specifically to a high molecular weight compatibilizer within that composite resulting in both a high flex strength and high modulus and significant reduction in water absorption. The compatibilizer is preferably a terpolymer comprising: a) 0.5-20 percent by weight of monomer units selected from the group consisting of maleic anhydride, substituted maleic anhydride, mono-ester of maleic anhydride, itaconic anhydride, maleic acid, fumaric acid, crotonic acid, acrylic acid and methacrylic acid; b) 0 to 40 percent by weight of monomer units selected from styrene and functionalized styrene; and c) 40 to 98.5 percent by weight of monomer units selected from the group consisting of C1-8 alkyl acrylates and methacrylates, and vinyl acetate.
Owner:ARKEMA INC
A preparation method of gold nanorods whose aspect ratio can be adjusted in a wide range
The present invention is a preparation method of gold nanorods whose length-to-diameter ratio can be adjusted in a large range. The strong reducing agent sodium borohydride reduces tetrachloroauric acid to obtain gold seed solution; the preparation of growth solution: prepare the mixed solution of hexadecyltrimethylammonium bromide, tetrachloroauric acid and silver nitrate, and the mol ratio of the three is 20:1:0.1~20:1:5, add crotonic acid solution to obtain a growth solution; growth of gold nanorods: inject gold seed solution into the growth solution, react for 6-12 hours, and obtain gold nanorods. The invention adopts crotonic acid as growth solution modifier, and solves the biological toxicity and biological modification problems caused by CTAB. The yield of the short-length-diameter ratio gold nanorods prepared can be as high as about 90%, the size distribution is narrow, and the length-diameter ratio is about 1.92. The aspect ratio of the prepared gold nanorods can be adjusted in a wide range from 1.9 to 7.5.
Owner:SOUTHEAST UNIV
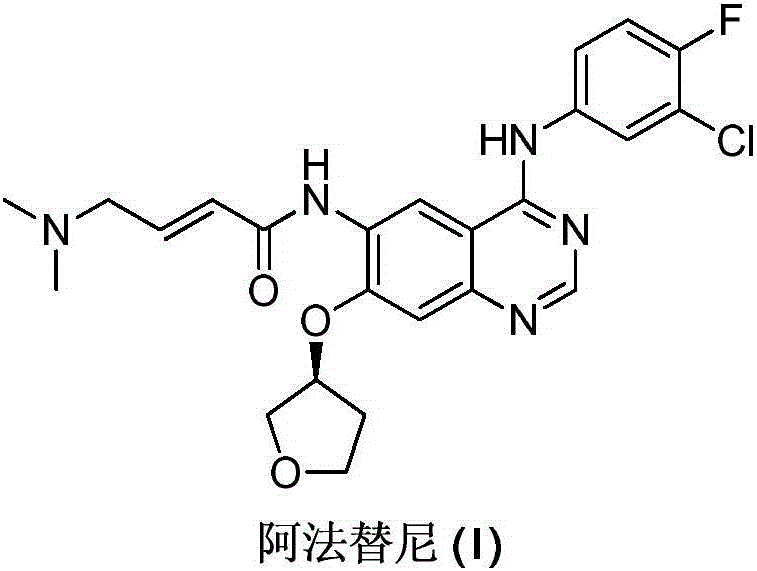
Preparation method for afatinib compound
InactiveCN103755688AReduce pollutionRaw materials are easy to getOrganic chemistrySynthesis methodsQuinazoline
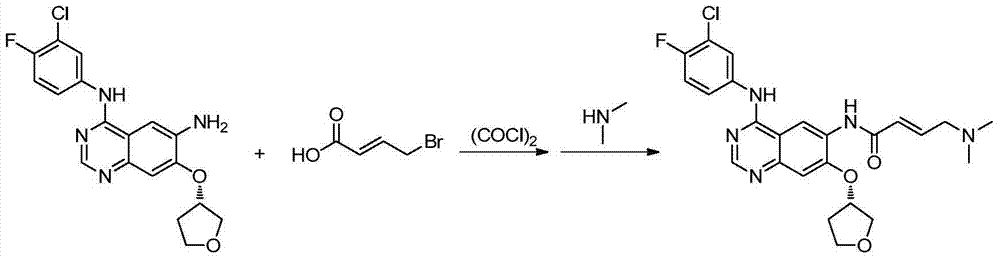
The invention provides a novel synthesis method for afatinib. The afatinib is prepared by activating or chlorinating dimethylamino crotonic acid hydrochloride by using an amide connection reagent and then reacting with N<4>-(3-chloro-4-fluoro-phenyl)-7-((S)-tetrahydrofuran-3-oxy)quinazoline-4,6-diamine. The invention further provides a process of preparing dimethylamino crotonic acid hydrochloride and an intermediate thereof. The method for preparing the afatinib provided by the invention has the advantages of low production cost, small environmental pollution, and simplicity and convenience in operation and is suitable for industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Acrylic pressure sensitive adhesive composition, double coated adhesive sheet, and photovoltaic device
InactiveUS20090250109A1Synthetic resin layered productsPhotovoltaic energy generationEpoxyPolymer science
An acrylic pressure sensitive adhesive composition comprising (A) an acrylic polymer having a carboxyl group and (B) a tetrafunctional epoxy compound, without a substantial amount of a tackifying resin, wherein the acrylic polymer (A) has been prepared by copolymerizing (a) 50% to 80% by weight of butyl acrylate, (b) 5% to 40% by weight of ethyl acrylate, and (c) 7% to 22% by weight of at least one carboxyl group-containing compound selected from the group consisting of acrylic acid, methacrylic acid, itaconic acid, crotonic acid, monobutyl maleate and β-carboxyethyl acrylate; and which has a weight-average molecular weight (Mw) of about 600000 to about 800000 and a glass transition temperature of −35° C. to −10° C., is disclosed. Further, a double-coated adhesive sheet comprising the acrylic pressure sensitive adhesive composition, and a photovoltaic device fabricated, using the double-coated adhesive sheet, is disclosed.
Owner:TOYO INK SC HOLD CO LTD
A high-temperature high-pressure (HTHP) stable synthetic polymer for water based oil-well servicing fluids
Stable water based High-Temperature-High-Pressure (HTHP) and / or Non-HTHP crosslinked copolymers for oil and gas applications consist of (i) a crosslinked, linear polyvinyl amide / polymerizable carboxylic acid) copolymer, having a composition, by weight, of 25-75 wt. % of a vinyl amide monomer selected from vinyl pyrrolidone, vinyl caprolactam, N-vinyl-N-methylacetamide and mixtures thereof, and 25-75 wt. % of a polymerizable carboxylic acid monomer selected from acrylic acid, (meth)acrylic acid, crotonic acid, itaconic acid, maleic acid and mixture thereof; and (ii) a crosslinker in an amount of 0.01-5% based on weight of total monomers. Also discloses relevant compositions comprising said copolymer and method of use thereof.
Owner:ISP INVESTMENTS LLC
Polishing composition and polishing method
A polishing composition includes fumed alumina, alumina other than fumed alumina, colloidal silica, a first organic acid, a second organic acid, an oxidizing agent, and water. When the second organic acid is citric acid, the first organic acid is preferably malic acid, while when the second organic acid is malic acid, the first organic acid is preferably citric acid. When the second organic acid is succinic acid, iminodiacetic acid, itaconic acid, maleic acid, malonic acid, crotonic acid, gluconic acid, glycolic acid, lactic acid, or mandelic acid, the first organic acid is preferably either citric acid or malic acid. The polishing composition can be suitably used for polishing the surface of a substrate for a magnetic disk.
Owner:FUJIMI INCORPORATED
Polycarboxylate-based ceramic water reducer and preparation method as well as application thereof
The invention discloses a polycarboxylate-based ceramic water reducer and a preparation method as well as an application thereof. The water reducer comprises monomers, distilled water, an inorganic chain transfer agent and an initiator; the monomers account for 29-45 percent of the total mass of the monomer and the distilled water; the monomers comprise acrylic acid and crotonic acid; acrylic acid accounts for 66-89 percent of the total mass of the monomers, and crotonic acid accounts for 10-40 percent of the total mass of the monomers. The polycarboxylate-based water reducer based on crotonic acid has the advantages of low mixing amount, high water reducing rate, simplicity in preparation and operation and no need of compounding with inorganic salts, and meet the requirements of ceramic production enterprises; the requirements on equipment during the production of the polycarboxylate-based ceramic water reducer are simple, post treatment is unnecessary, the energy consumption is small, and no environmental pollution is generated; the dose of pottery clay is small during application, the water reducing effect is good, the fluidity of ceramic slurry can be remarkably improved, and the viscosity of ceramic slurry is reduced; no settlement phenomenon of ceramic slurry appears within the adding dose range.
Owner:FOSHAN CENT FOR FUNCTIONAL POLYMER MATERIALS & FINE CHEM
Method for synthesizing crotonic acid by oxidizing croton aldehyde selectively
InactiveCN101003472AEasy to manufactureLow costPhysical/chemical process catalystsOrganic compound preparationPhosphomolybdic acidCrotonaldehyde
This invention relates to a method for synthesizing crotonic acid by selective oxidation of crotonaldehyde. The method adopts crotonaldehyde as the raw material, acetone, acetic acid, benzene or toluene as the solvent, phosphomolybdic acid as the main catalyst, and V2O5 as the cocatalyst, and reacts at 30-100 deg.C and 0.3-0.9 MPa in the presence of O2 to obtain crotonic acid. Phosphomolybdic acid can be supported by such carriers as active carbon, SiO2, gamma-Al2O3 or molecular sieve. Therefore, the catalyst can be separated from the reaction solution by filtration, and dried in air at 120 deg.C for recycling. The supported catalyst has such advantages as simple preparation, high catalytic activity, high selectivity, and high stability, and can be used repeatedly.
Owner:SINOPEC YANGZI PETROCHEM
Method of producing beta-mercaptocarboxylic acids
InactiveCN101801922AMercapto/sulfide group formation/introductionThiol preparationHydrogenSynthetic materials
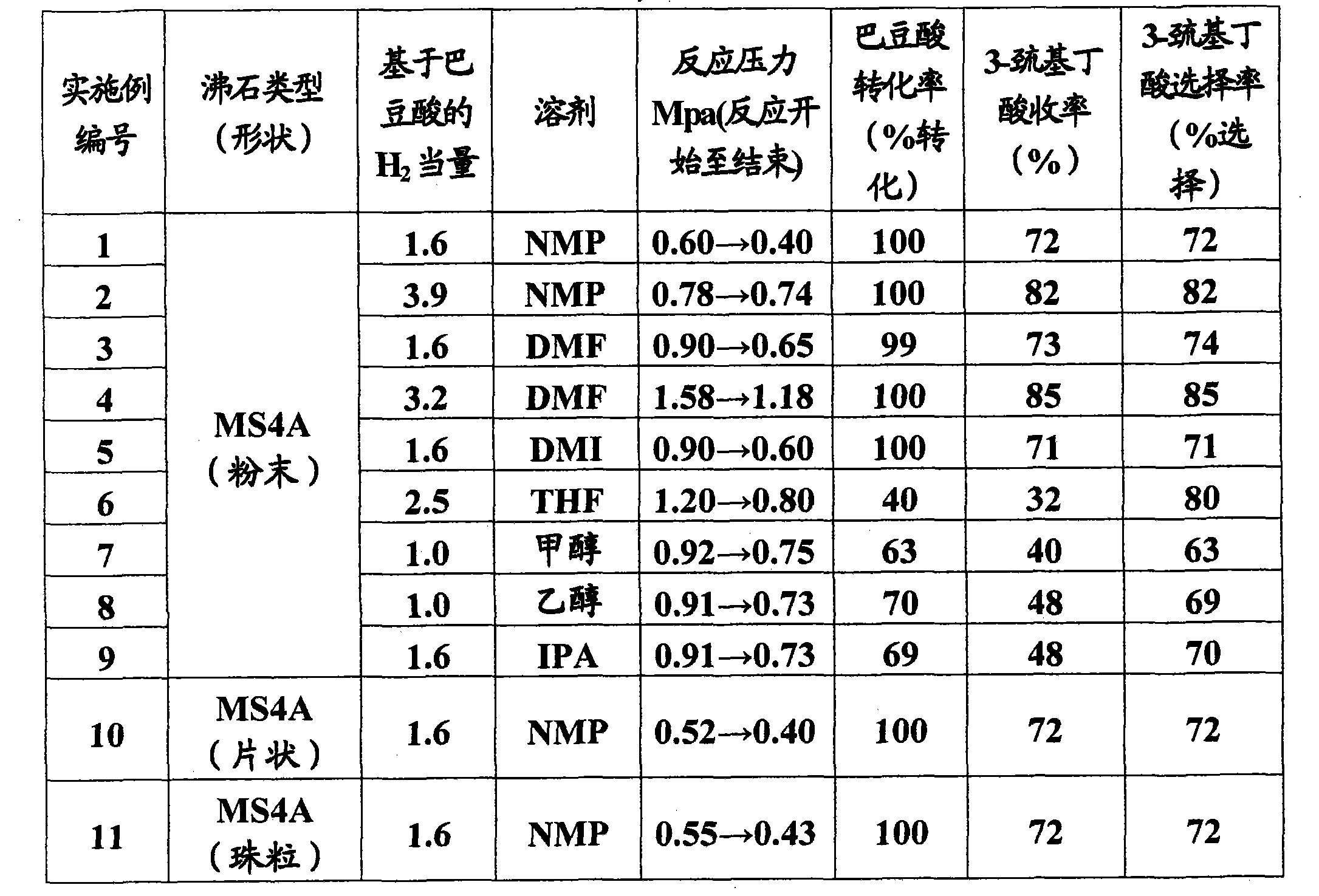
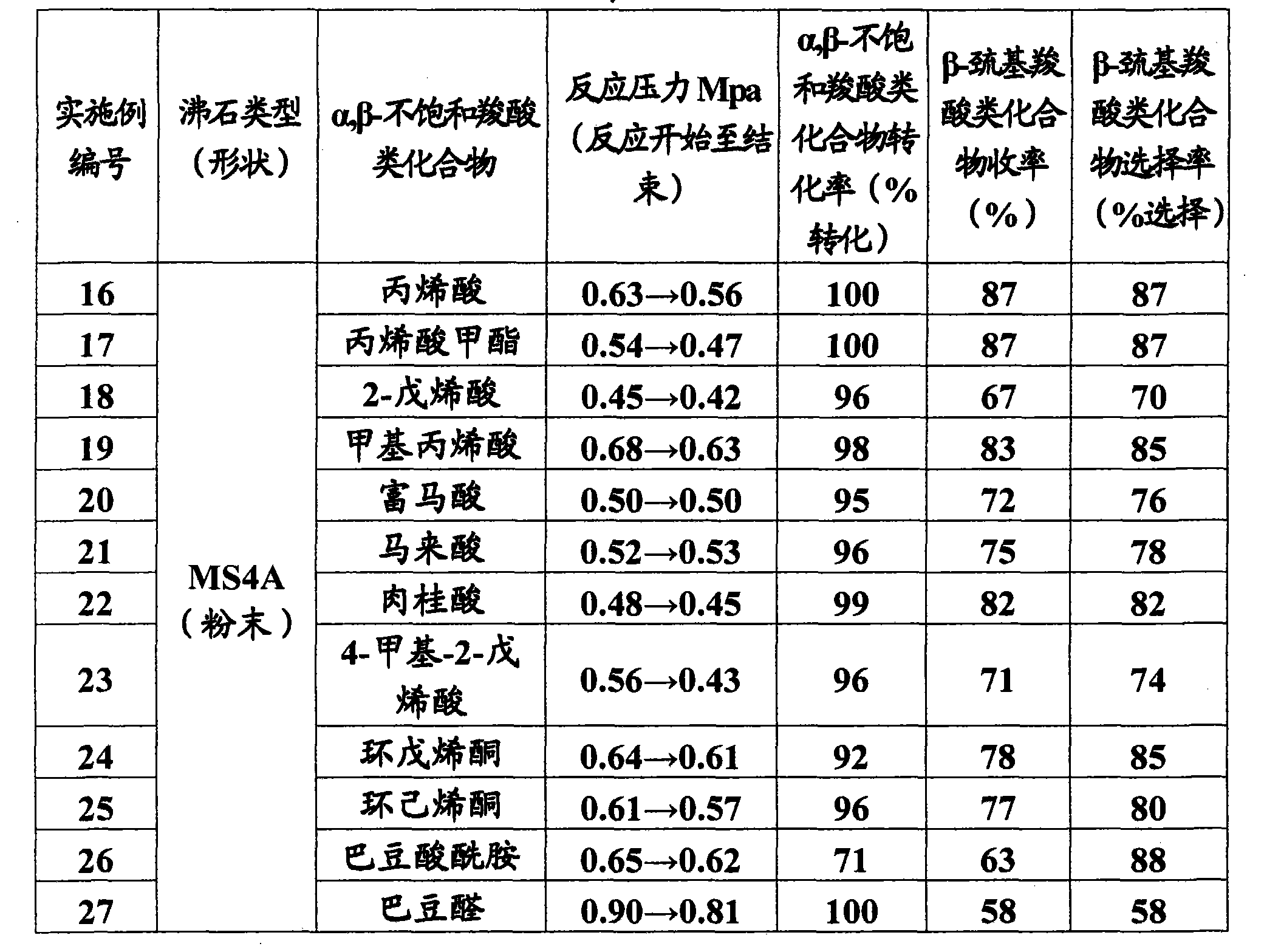
The invention relates to a method for efficiently producing beta-mercaptocarboxylic acids using a solid acid catalyst such as zeolite, which product corresponds to respective starting materials selected from alpha, beta-unsaturated carboxylic acids (alpha, beta-unsaturated carboxylic acid, alpha, beta-unsaturated carboxylic acid ester, alpha, beta-unsaturated amide, alpha, beta-unsaturated aldehide and alpha, beta-unsaturated ketone) and hydrogen sulfides (hydrogen sulfide, sulfide salt and hydrosulfide salt), wherein a solvent compatible with water is used in the reaction. According to the invention, beta-mercaptocarboxylic acids which are useful as additives in synthetic materials for pharmaceutical or agricultural agents and in polymer compounds can be industrially produced efficiently by using easily available alpha, beta-unsaturated carboxylic acid (such as crotonic acid) at high yield.
Owner:RESONAC HOLDINGS CORPORATION
Method for synthesizing crotonic acid by using byproduct of croton aldehyde
InactiveCN101003473AIncrease valuePromote cleaner productionPhysical/chemical process catalystsOrganic compound preparationPhosphomolybdic acidCrotonaldehyde
This invention relates to a method for synthesizing crotonic acid from crotonaldehyde byproduct. The method comprises: distilling 20-40% crotonaldehyde byproduct by controlling the boiling point of the distillate on the top of the column at 25-65 deg.C to remove light components and obtain 45-60% crotonaldehyde in the solution in distillation kettle, adopting the solution in distillation kettle as the raw material, acetone, acetic acid, benzene or toluene as the solvent, phosphomolybdic acid as the main catalyst, and V2O5 as the cocatalyst, and reacting at 30-100 deg.C and 0.3-0.9 MPa in the presence of O2, separating the catalyst, distilling to remove the solvent, and recrystallizing with deionized water for twice to obtain crotonic acid with a purity higher than 99%. Phosphomolybdic acid can be supported by such carriers as active carbon, SiO2, gamma-Al2O3 or molecular sieve. Therefore, the catalyst can be separated from the reaction solution by filtration, and dried in air at 120 deg.C for recycling. The method has such advantages as simple process and high crotonic acid yield.
Owner:SINOPEC YANGZI PETROCHEM
Method for preparing crotonic acid
InactiveCN1415594AHigh yieldShort oxidation timeCarboxylic preparation by oxidationCrotonaldehydeMetal
A process for preparing crotonic acid features that under the catalysis of silver powder as catalyst, the crotonaldehyde is oxidized in oxidizing tower to obtain crotonic acid. Its advantages are high output rate and short reaction time.
Owner:JIANGSU PANOXI CHEM
Preparation method of delayed coagulation type ester polycarboxylate water reducing agent
The invention discloses a preparation method of a delayed coagulation type ester polycarboxylate water reducing agent. The preparation method comprises the following steps: (1) preparing an esterification monomer; (2) performing a copolymerization reaction; and (3) performing a neutralization reaction. The preparation method utilizes a saccharide compound and crotonic acid to prepare the esterification monomer, and has the advantages of low costs and simple and convenient operation; the prepared saccharide esterification product participates in the next step of the copolymerization reaction, so that the main chain of the polycarboxylate water reducing agent has a polyhydroxy structure, and the prepared polycarboxylate water reducing agent has a better delayed coagulation effect, effectively prolongs the initial setting and final setting time of cement, and can reduce negative effects caused by using a retarder; and the polyhydroxy structure of the saccharide compound enables the polycarboxylatewater reducing agent to have better slump loss resistance, so that the pumping construction of concrete in summer is facilitated.
Owner:KZJ NEW MATERIALS GROUP CO LTD
Wall papering adhesive
ActiveUS7122597B2Improve adhesionEasy to disassembleStarch adhesivesPaper coatingPolyvinyl acetateAdhesive
Compositions for securing pre-pasted and non-pre-pasted wall coverings to a surface that is to be decorated are provided. The present compositions are aqueous colloidal dispersions or polymeric solutions comprising from about 3% to about 30% solids. The present compositions comprise water, an alkali, from about 2.5% to about 25% by weight of an alkali-soluble, polyvinyl acetate-crotonic acid copolymer, a thickener, and a glycol.
Owner:RUST OLEUM CORP
Method for preparing cilnidipine used as dihydropyridine calcium antagonist
InactiveCN101602709AShort reaction cycleEasy post-processingOrganic chemistryCardiovascular disorderSolventMethoxyethyl acetate
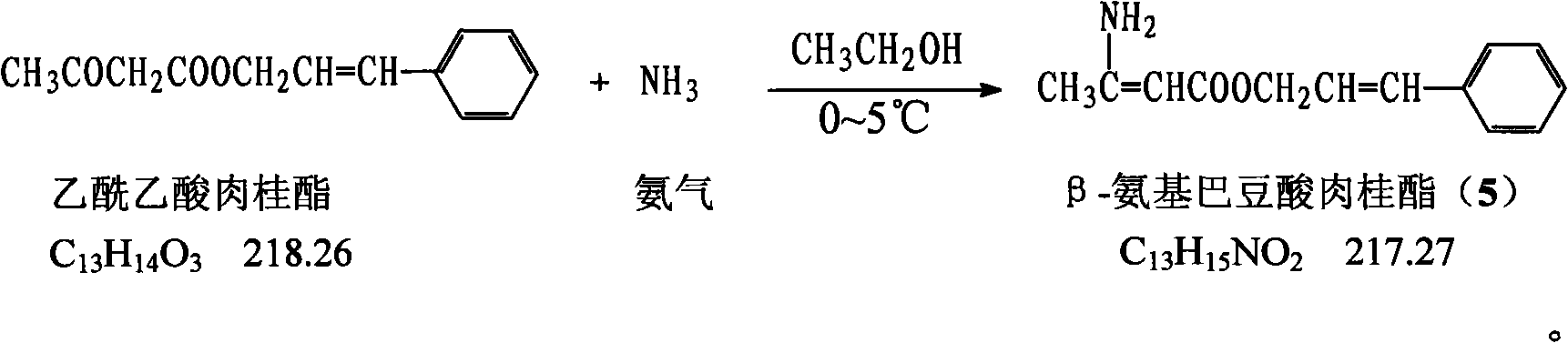
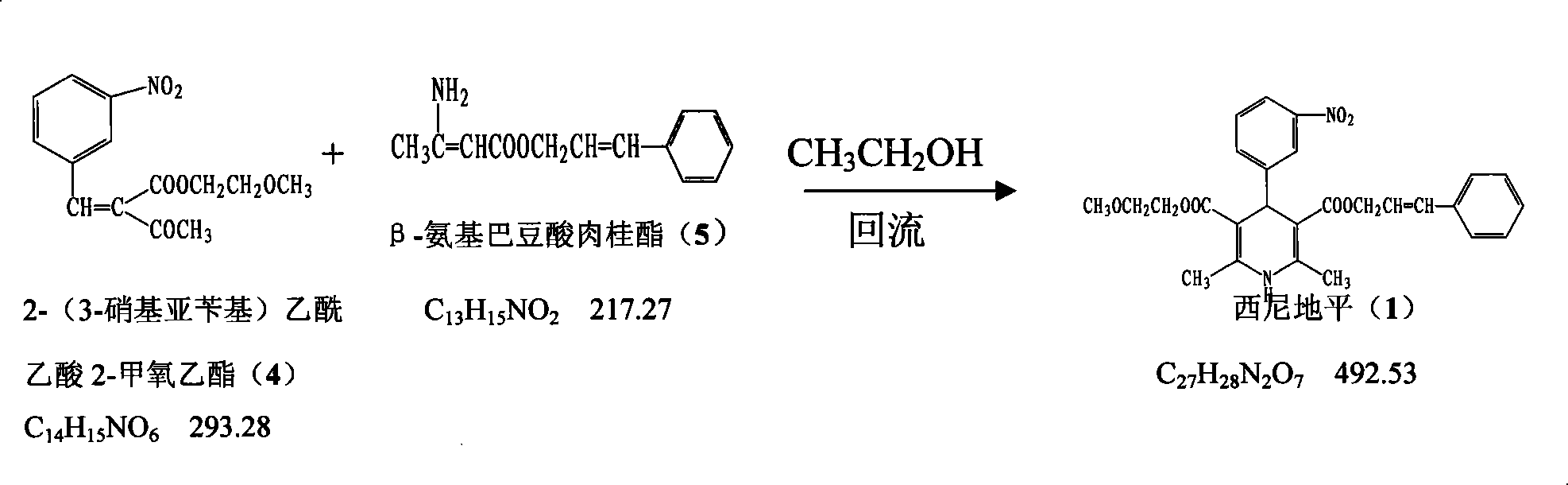
The invention provides a method for preparing cilnidipine used as a dihydropyridine calcium antagonist, which comprises the following steps: 2-(3-nitrobenzyl) acetoacetic acid 2-methoxyethyl acetate 4 and beta-amino crotonic acid cinnamic ester 5 react in an alcohol solvent to obtain a coarse product of cilnidipine 1, and the coarse product of the cilnidipine 1 is recrystallized to obtain the cilnidipine 1. The method has the advantages of short reaction period, simple and convenient after-treatment, simple synthetic route, moderate process condition, and the like and is suitable for industrial production.
Owner:上海医药科技发展有限公司
Synthesis method of all-trans-retinoic acid
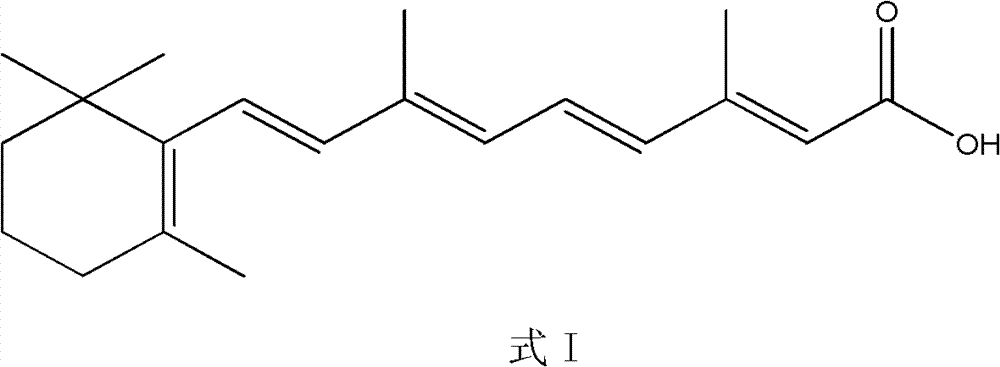
ActiveCN102775338AShort method stepsSimple and fast operationOrganic compound preparationCarboxylic compound preparationIsomerizationSynthesis methods
The invention relates to a synthesis method of all-trans-retinoic acid. The method comprises: taking [3-methyl-5-(2, 6, 6-trimethylcyclohexen-1-yl)-2, 4-pentadiene]-triphenylphosphine salt and beta-formyl crotonic acid as raw materials, which undergo a WITTIG reaction under an alkali effect, then adding a palladium compound or a rhodium compound for a direct isomerization reaction, thus obtaining the needed all-trans configuration product. According to the method, a reaction intermediate product has no need for separation, and the WITTIG reaction and the isomerization reaction undergo continuously in one container. With the advantage of simple operation and production cost saving, the method is suitable for industrialized production.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Recombinant aspartase mutant, coding gene and application of recombinant aspartase mutant
ActiveCN111593039AHigh regional selectivityHigh stereoselectivityCarbon-nitrogen lyasesBacteriaMutantGenetic engineering
The invention provides a recombinant aspartase mutant, a coding gene and an application of the recombinant aspartase mutant in synthesis of R-3-aminobutyric acid, and belongs to the technical field ofgene engineering and biosynthesis. The recombinant aspartase mutant disclosed by the invention can take crotonic acid as a substrate, and has high regioselectivity and stereoselectivity in catalyticsynthesis reaction of R-3-aminobutyric acid; and the catalytic efficiency is high and the cost is lower. The reaction condition for synthesizing the R-3-aminobutyric acid by using the recombinant aspartic acid mutant provided by the invention is mild, and the application is environment-friendly.
Owner:ZHEJIANG HAISEN PHARMACY CO LTD
Process for producing polymers having amide and ester groups in the solid state
The present invention relates to a process for the preparation of a polymer P having amide and ester groups in the solid state of aggregation, in a first step (a) a homo- or copolymer P1 of (meth)acrylic acid, itaconic acid or crotonic acid being reacted with at least one monohydroxy compound E at a temperature up to 200° C. to give a polymer P2, so that anhydride groups form in addition to ester groups, in a second step (b) the polymer P2 prepared in step (a) being cooled to below 60° C. so that the polymer P2 is present in the solid state of aggregation or as supercooled melt, and in a third step (c) the polymer P2 having anhydride groups and present in the solid state of aggregation or as supercooled melt being amidated with at least one amine compound A at temperatures below 60° C., the amine compound A used in the third step (c) being present in the solid state of aggregation or on or in a solid carrier material.
Owner:SIKA TECH AG
Thermal stabilizer compositions for halogen-containing vinyl polymers
Beneficial stabilizer compositions are employed to stabilize halogen-containing vinyl polymers from, for example, degradation and discoloration. The stabilizer compositions comprise a substantially metal-free perchlorate; a first amine-containing stabilizer; and a second amine-containing stabilizer; wherein the first and second amine-containing stabilizers are selected from an aminoalcohol, a dihydropyridine, an aminocrotonate, an aminouracil, a phenyl indole, or a mixture comprising one or more of the foregoing amine-containing stabilizers; and wherein the first and second amine-containing stabilizers are different types of stabilizers.
Owner:AUSTEN STEVEN CHRISTOPHER +2
Liquid for activating plant and method for producing the same
InactiveCN101438708APromote growthPromote photosynthetic reactionBiocidePlant growth regulatorsPropanoic acidCarbonization
The invention relates to a plant activate fluid which comprises organic acids, alcohol, phosphoryl compounds, phenols, a small amount of inorganic matter and water. According to weight percentage, the organic components comprise 48 percent to 55 percent of acetic acid, 0.9 percent to 4.0 percent of formic acid, 0.8 percent to 3.1 percent of propionic acid, 0.04 percent to 0.08 percent of valeric acid, 0.46 percent to 0.8 percent of butylric acid, 0.04 percent to 0.2 percent of crotonic acid, 0.9 percent to 2.1 percent of methanol and 1.8 percent to 2.4 percent of acetaldehyde; and the organic components further comprise 0.3 percent to 1.6 percent of pyridine, 1.6 percent to 3.2 percent of furan, 0.83 percent to 1.87 percent of phenol, 0. 4 percent to 1.6 percent of 2-furfural alcohol, 1.2 percent to 2.9 percent of 2(3H)2 hydrogen furanone, 1.6 percent to 2.8 percent of 2(5H)2 hydrogen furanone, 0.09 percent to 0.32 percent of tetrahydrofuran, 0.1 percent to 1.5 percent of 2-tetrahydrofurfuryl alcohol, 0.89 percent to 2.1 percent of furfurol, 0.3 percent to 1.7 percent of methyl acetate and 0.2 percent to 1.5 percent of hydroxy methyl acetate. The invention takes branch and leaf wastes of ligneous plants as the raw material for carbonization, the smoke is collected during the carbonization process, and the plant activate fluid is obtained through water-cooling, temperature reduction and condensation.
Owner:陈首畅 +1
Preparation method of delayed coagulation type ether polycarboxylate water reducing agent
The invention discloses a preparation method of a delayed coagulation type ether polycarboxylate water reducing agent. The preparation method comprises the following steps: (1) preparing an esterification monomer; (2) performing a copolymerization reaction; and (3) performing a neutralization reaction. The preparation method uses a saccharide compound and crotonic acid to prepare the esterification monomer, and has the advantages of low costs and simple and convenient operation; the prepared saccharide esterification product participates in the next step of the copolymerization reaction, so that the main chain of the polycarboxylate water reducing agent has a polyhydroxy structure, the prepared polycarboxylate water reducing agent has a better delayed coagulation effect, the initial setting and final setting time of cement is effectively prolonged, and negative effects caused by using a retarder can be reduced; and the polyhydroxy structure of the saccharide compound enables the polycarboxylate water reducing agent to have better slump loss resistance, so that the pumping construction of concrete in summer is facilitated.
Owner:KZJ NEW MATERIALS GROUP CO LTD
Preparing method of Afatinib
InactiveCN106045983ASimplify and optimize process routesShort process stepsOrganic chemistryChlorideKetone
The invention relates to a preparing method of Afatinib. The preparing method includes the steps that 6-nitro-7-fluoro-3,4-dihydro quinazoline-4-ketone is used as a raw material and is subjected to etherification reaction with S-3-hydroxy-tetrahydrofuran; obtained 6-nitro-7-[S-(tetrahydrofuran-3-yl)oxyl]-3,4-dihydro quinazoline-4-ketone is subjected to chlorination reaction; obtained 4-chloro-6-nitro-7-[S-(tetrahydrofuran-3-yl)oxyl] quinazoline is subjected to condensation reaction; obtained 4-[(3-chloro-4-fluoro-phenyl)amino]-6-nitro-7-[S-(tetrahydrofuran-3-yl)oxyl] quinazoline is subjected to reduction reaction; E-4-dimethyl amido crotonic acid is subjected to chloroformylation reaction, and E-4-dimethyl amido crotonyl chloride and 4-[(3-chloro-4-fluoro-phenyl)amino]-6-amino-7-[S-(tetrahydrofuran-3-yl)oxyl] quinazoline are subjected to amidation reaction to obtain the finished product.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
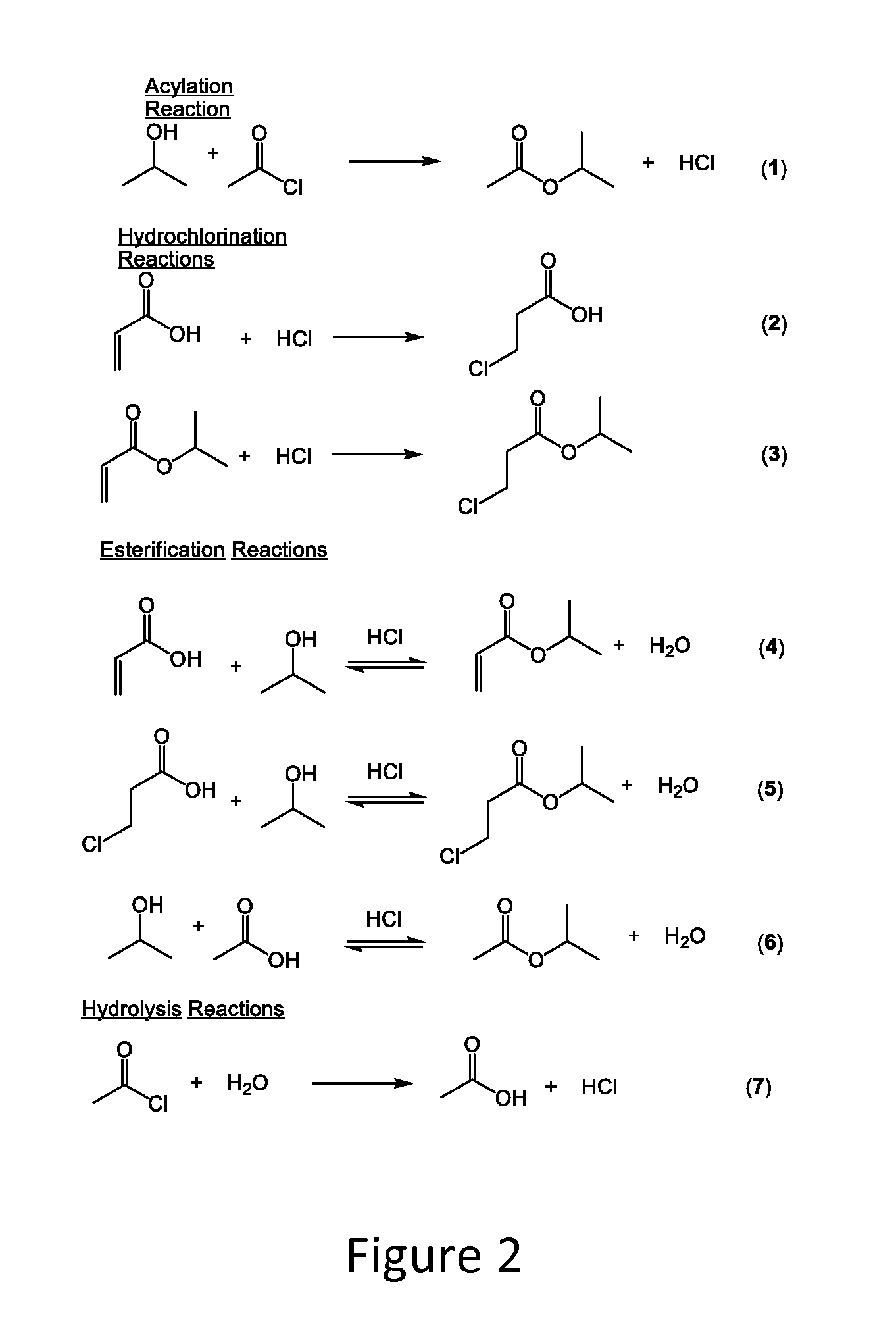
Hydrochlorination of electron-deficient alkenes
InactiveUS20120190879A1Reduce usageMethod securityCarboxylic acid nitrile preparationOrganic compound preparationMethacrylonitrileAcrylic acid
The present invention pertains to a method for the hydrochlorination of electron deficient alkenes, particularly alkenes having the functional groups COOH, CONH2, and CN. Specific alkenes discussed include acrylic acid, crotonic acid, methacrylic acid, acrylonitrile, acrylamide, and methacrylonitrile. The alkene is combined with a primary or secondary alcohol (e.g., isopropanol) and an acid chloride (e.g., acetyl chloride) under conditions suitable to chlorinate the alkene. Products formed by the invention include 3-chorosubstituted carbonyl compounds such as 3-chlorpropionic acid (3-CPA), 3-chloropropionamide (3-CPAD), and 3-chloropropionitrile among other products.
Owner:FUTUREFUEL CHEM
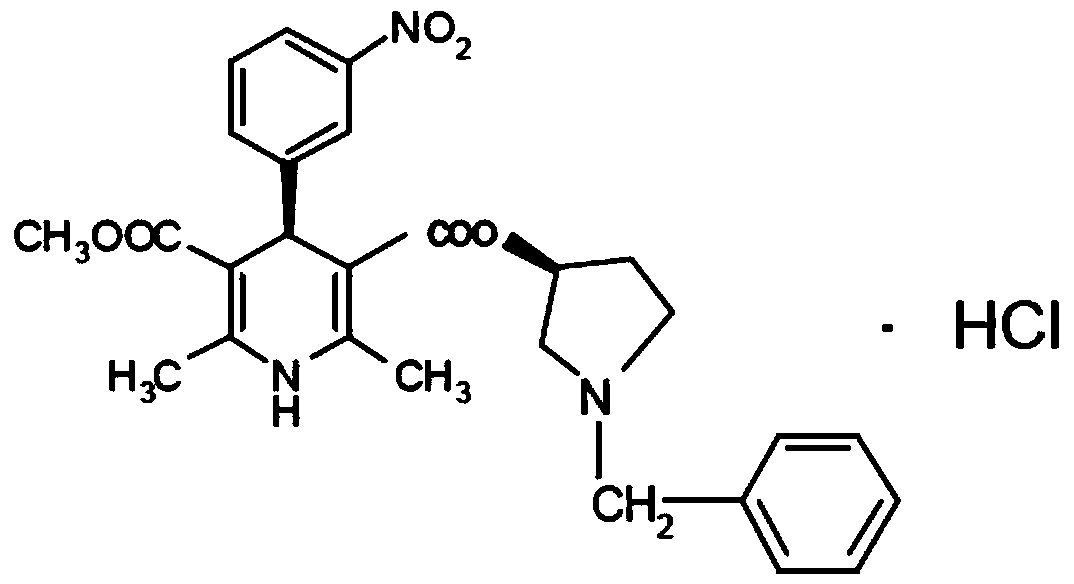
Barnidipine hydrochloride compound and preparation method thereof
The invention discloses a barnidipine hydrochloride compound and a preparation method thereof. The preparation method comprises the following steps: (1) using 3-hydroxypropionitrile to react with diketene, to obtain an intermediate 1; (2) enabling the intermediate 1 to react with m-nitrobenzaldehyde and Beta-amino methyl crotonate, to obtain an intermediate 2; (3) enabling the intermediate 2 to behydrolyzed by strong base, to obtain an intermediate 3; (4) enabling the intermediate 3 to be resolved by chiral organic base, to obtain an intermediate 4; (5) enabling the intermediate 4 to react with thionyl chloride, (S)-1-benzyl-3-pyrrolidinol, and HCI ethanol solution, to obtain a crude product of barnidipine hydrochloride; and (6) performing ethyl alcohol pulping and refining, and ethyl alcohol recrystallization on the crude product of the barnidipine hydrochloride, to obtain the barnidipine hydrochloride.
Owner:森淼(山东)药业有限公司
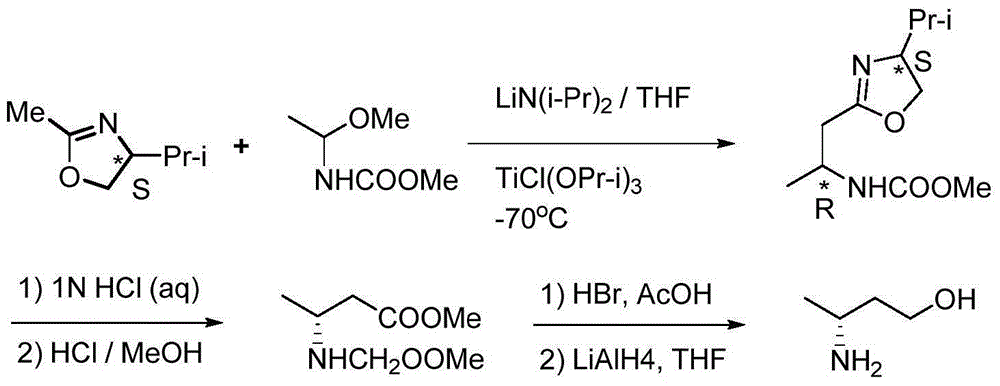
Method for preparing 3(R)/(S)-amidogen-1-butanol
ActiveCN105001098AHigh yieldPromote environmental protectionOrganic compound preparationAmino-hyroxy compound preparationAcetoacetatesDiphosphines
The invention provides a method for preparing 3(R) / (S)-amidogen-1-butanol. The method comprises the steps that firstly, benzamide reacts with acetoacetic ester under the catalyzing action of p-toluenesulfonic acid, so that 3-benzamido-2-butene acid ester is generated; secondly, catalytic hydrogenation is conducted by using a chiral rhodium-diphosphine ligand compound as a catalyst for enantioselective hydrogenation, so that 3(R) / (S)-benzamide butyrate is generated highly selectively; thirdly, benzoyl is removed by means of mixed solvent of concentrated hydrochloric acid and alcohol, so that 3(R) / (S)-amino-crotonic acid ester hydrochloride is generated; finally, optically pure 3(R) / (S)-amidogen-1-butanol is obtained after ester carbonyl is reduced by means of hydronoron. According to the method, the raw materials are low in cost and easy to obtain, the technological operation is easy and convenient, resolution is not needed, the yield is high, cost is low, environmental friendliness is achieved, the optical purity of product is high, and the method is more suitable for industrial production.
Owner:浙江宏康医药化工股份有限公司
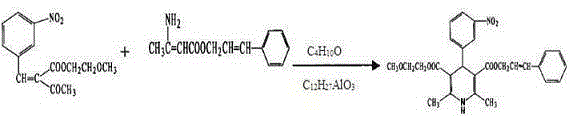
Synthesis method of cilnidipine
The invention discloses a synthesis method of cilnidipine. The synthesis method comprises the following steps: putting methoxyethyl-2-(3-nitrobenzylidene)acetoacetate and beta-amino crotonic acid cinnamyl ester into a reaction kettle, and under the nitrogen protection, adding an isobutyl alcohol solvent and an isobutyl alcohol aluminum catalyst for reflux reaction; cooling and putting over night, and filtering to obtain a cilnidipine crude product; washing with ethanol at 5-10 DEG C, and recrystallizing by using anhydrous ethanol to obtain a cilnidipine fine product. In the synthesis method, the isobutyl alcohol is used as the solvent and the isobutyl alcohol aluminum is used as the catalyst; compared with the prior art, the synthesis method has the advantages as follows: the reaction process is simple and the reaction yield is increased.
Owner:丹阳恒安化学科技研究所有限公司
Low-toxin low-harm nail polish
InactiveCN102415954APlay a nourishing roleImprove stabilityCosmetic preparationsToilet preparationsNitrocelluloseHydrolysate
The invention discloses a low-toxin low-harm nail polish. The low-toxin low-harm nail polish consists of the following raw materials in percentage by weight: 79.5% of vinyl acetate-butenoic acid (crotonic acid)-branched-chain vinyl decanoate copolymer, 0.8% of carboxymethylcellulose, ossein, 6% of keratin hydrolysate, 2% of ethanol, 4% of glycerol and 7.7% of deionized water. In the invention, the vinyl acetate-butenoic acid (crotonic acid)-branched-chain vinyl decanoate copolymer serves as a major raw material; compared with products which mainly consist of nitrocellulose with the addition of chemical solvents such as acetone, acetic ether, ethyl lactate, phthalic acid tincture and the like, the nail polish disclosed by the invention has low toxin and relatively small harm to human body; and the low-toxin low-harm nail polish has a certain nutritional function for nails as nutrients such as collagen and the keratin hydrolysate are added into the nail polish.
Owner:汕头市乾力知识产权代理有限公司
Process for production of betaine
InactiveUS20090325246A1High yieldInhibit side effectsOrganic compound preparationCarboxylic acid amides preparationHalogenBetaine
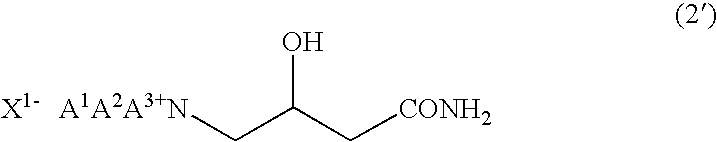
According to the present invention, by using 4-halogeno-3-hydroxybutanamide as a substrate in quaternary amination reaction with trialkylamine which is an important step in betaine (such as carnitine) preparation processes, it becomes possible to reduce the production of crotonic acid derivatives (the major by-product) greatly compared to conventional processes. Consequently, it becomes possible to prepare a betaine, such as carnitine, at a high yield.The present invention also relates to a process for preparing a betaine represented by formula (1) below, comprising a step of quaternary aminating an amide represented by formula (2) below:wherein A1, A2 and A3 individually represent a C1-C20 hydrocarbon group which may have a substituent(s); and X1 is a halogen atom.
Owner:MITSUBISHI RAYON CO LTD
Preparation method of optically pure 4-aryl-2-hydroxy-butyric acid
InactiveCN101941900AEasy to manufactureLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSolventOxygen
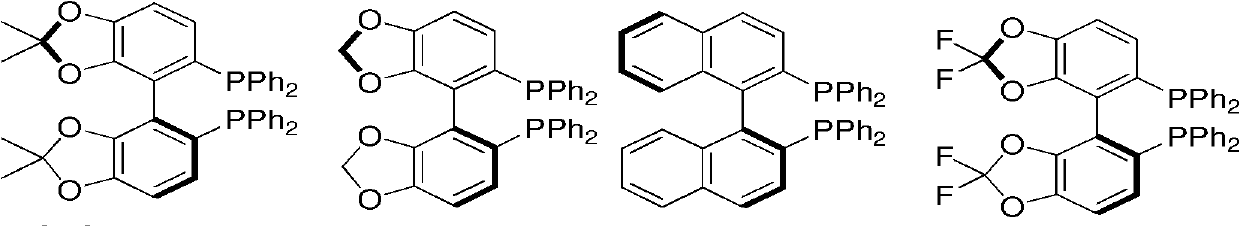
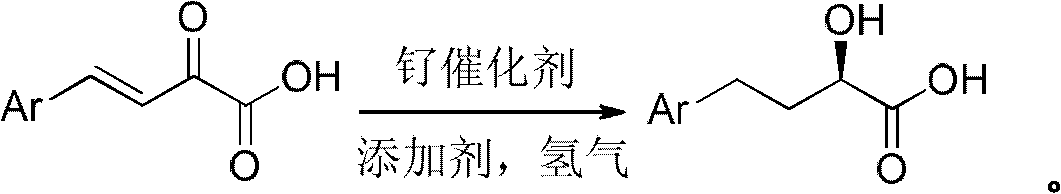
The invention relates to a preparation method of optically pure 4-aryl-2-hydroxy-butyric acid in the technical field of chemical materials. In an organic ether or alcoholic solvent, 4-aryl-2-oxo-3-crotonic acid is catalytically hydrogenated under the coexistence of a ruthenium complex of a united aryl-axis chiral phosphine ligand and an acidic additive to prepare optically pure 4-aryl-2-hydroxy-butyric acid. Starting from the 4-aryl-2-oxo-3-crotonic acid, the invention uses the ruthenium complex of the united aryl-axis chiral phosphine ligand as a catalyst and adds the acidic additive to obtain (R)-4- aryl-2-hydroxy-butyric acid through an asymmetric catalytic hydrogenation. The invention has low cost, stable physical and chemical properties and easy purification and increases an ee value to be larger than 99 percent through a simple and convenient recrystallization method. The reaction formula is carried out as shown in the specification.
Owner:SHANGHAI JIAO TONG UNIV
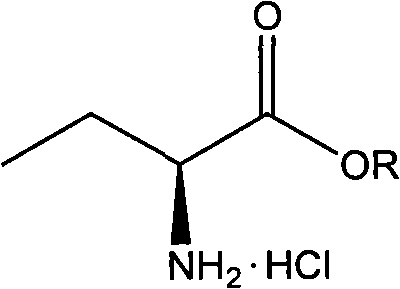
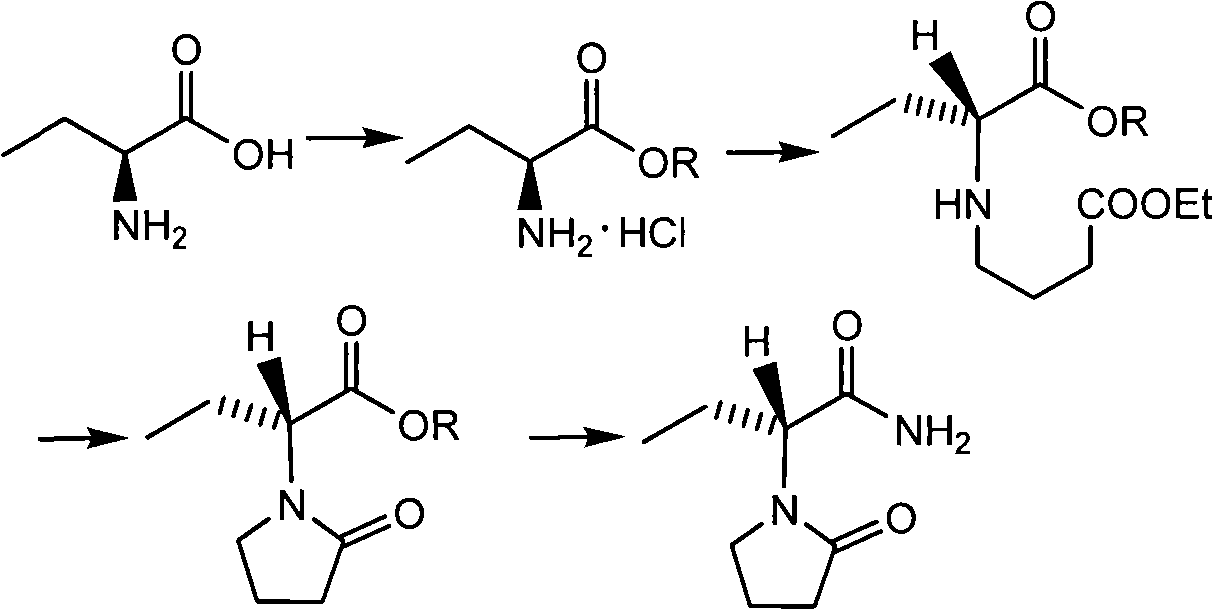
Novel method for preparing levetiracetam midbody S-(+)-2-aminobutyrate hydrochlorate
InactiveCN101492382ASimple stepsTechnical parameters are easy to controlOrganic compound preparationAmino-carboxyl compound preparationEster hydrochloridePhotochemistry
The invention adopts a new method for optically resolving DL-2-amino-crotonic acid ester, thereby producing important intermediate S-(+)-2-amino-crotonic acid ester hydrochloride for preparing levetiracetam.
Owner:SHAOXING STEPCHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com