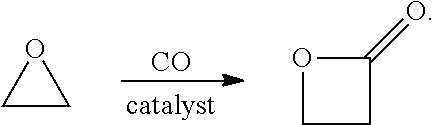Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Propiolactone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Propiolactone may refer to either of two isomeric chemical compounds: Alpha-Propiolactone Beta-Propiolactone
Absorbent article comprising a synthetic polymer derived from a renewable resource and methods of producing said article
An element of an absorbent article is provided. The element has a bio-based content of at least about 50% based on the total weight of the element, and comprises a synthetic polymer derived from a renewable resource via a first intermediate compound selected from the group consisting of crotonic acid, propiolactone, ethylene oxide, i-propanol, butanol, butyric acid, propionic acid, 2-acetoxypropanoic acid, methyl 2-acetoxypropanoate, methyl lactate, ethyl lactate, polyhydroxybutyrate, and a polyhydroxyalkanoate comprising 3-hydroxypropionate monomers. An absorbent article comprising the element and a method of making an element for an absorbent article also are provided.
Owner:THE PROCTER & GAMBLE COMPANY
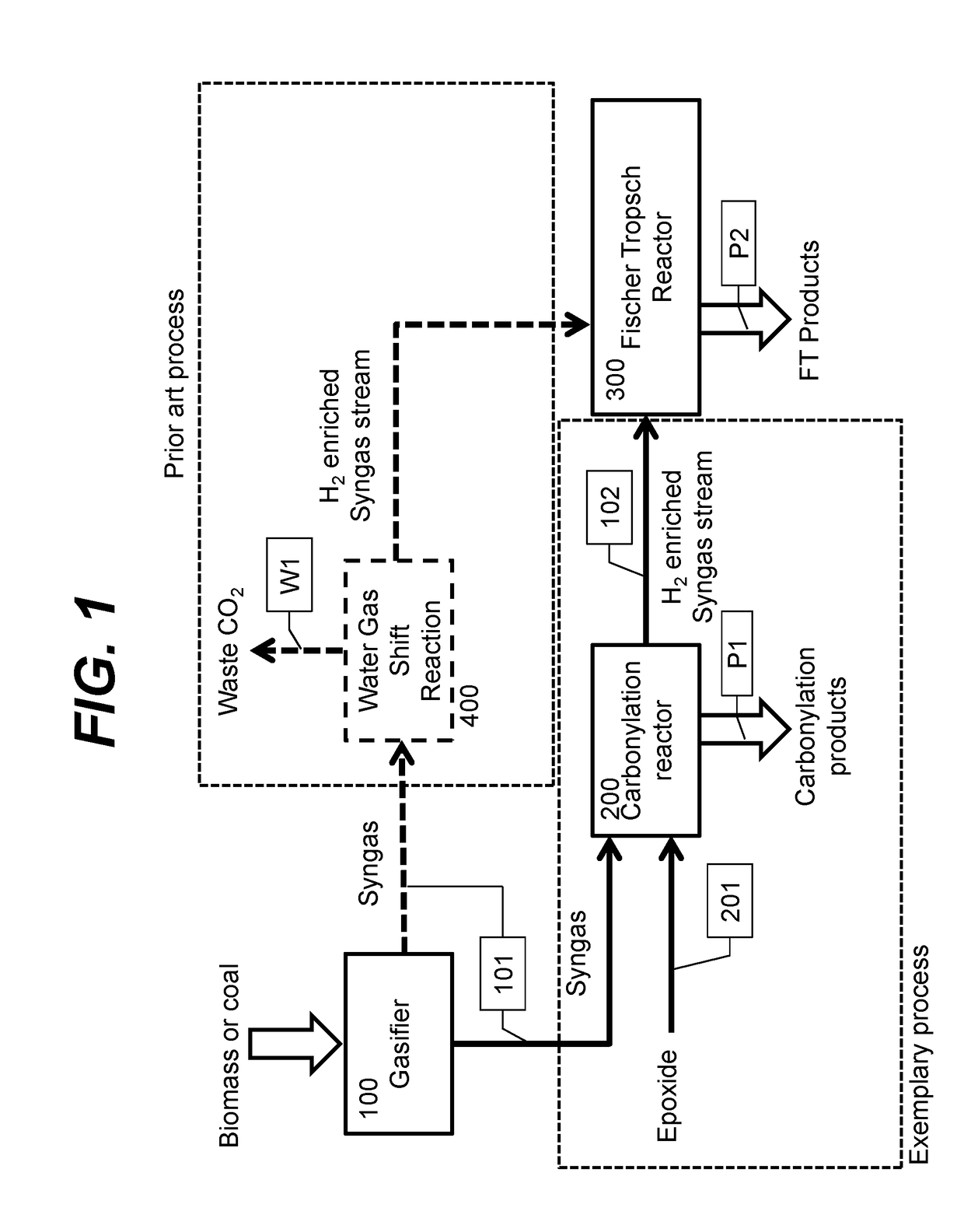
Integrated methods for chemical synthesis
InactiveUS20180030014A1Improve carbon efficiencyImprove efficiencyOrganic chemistryHydrogen separationProtein carbonylChemistry
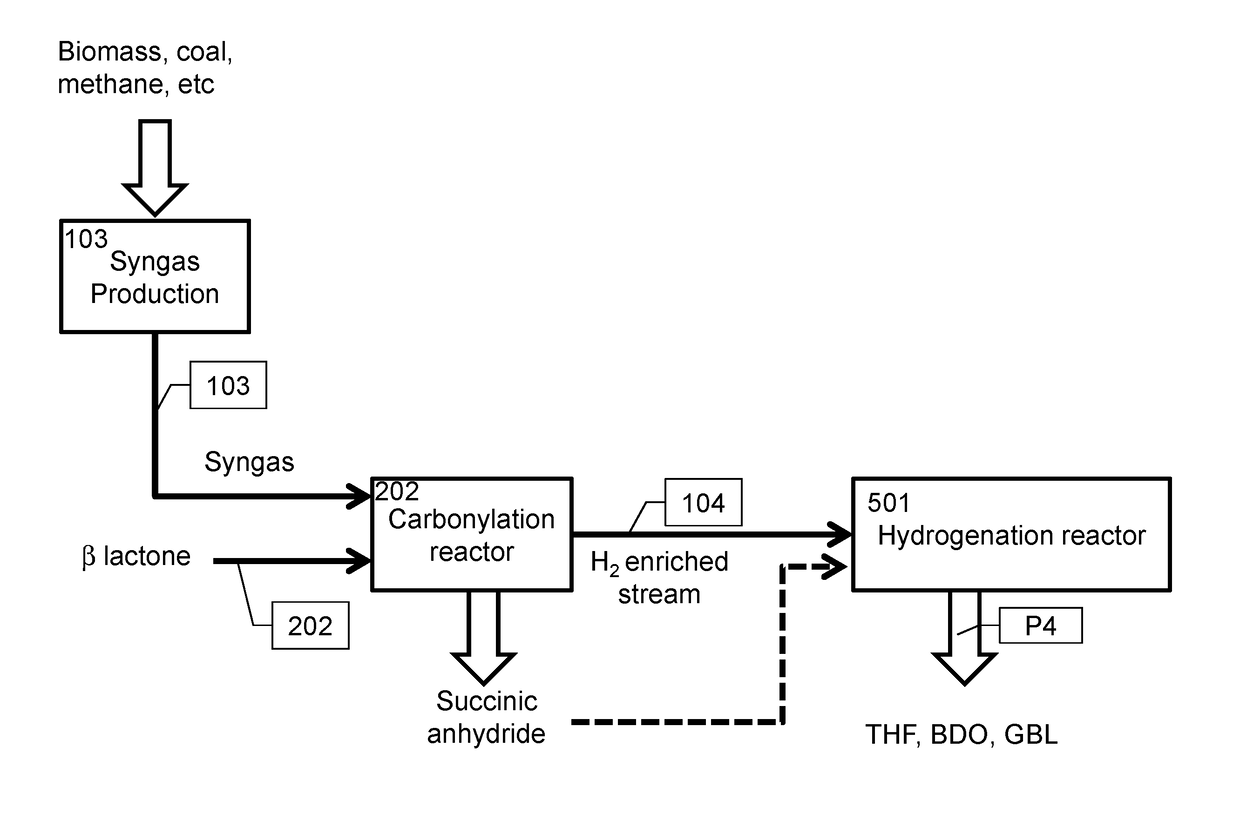
The integrated processes herein provide improved carbon efficiency for processes based on coal or biomass gasification or steam methane reforming. Provided are also ethylene oxide carbonylation products such as beta-propiolactone and succinic anhydride having a bio-based content between 0% and 100%, and methods for producing and analyzing the same.
Owner:NOVOMER INC
Magnetic tumor double-target polymer nano micelle and preparation thereof
InactiveCN101259097AEnhance tumor targeting effectImprove release efficiencyPharmaceutical delivery mechanismPharmaceutical non-active ingredientsAnticarcinogenSide effect
The invention relates to a nanomicelle of magnetic tumor double-targeting polymer and a preparation method thereof. The nanomicelle of magnetic tumor double-targeting polymer is a nuclear shell structure; the surface of an outer shell is connected with targeted ligand, an inner core is enclosed with nano-particles of super paramagnetic ferriferrous oxide and anticarcinogen with hydrophobic property; in virtue of the physical effect of an external magnetic field and induction of the targeting ligand, the tumor double-targeting function of the nanomicelle is realized. The invention also provides the preparation method of the nanomicelle of magnetic tumor double-targeting polymer: polyethylene glycol with end capping by folacin, and poly Epsilon-caprolactone or sandwich copolymer of poly propiolactone are taken as raw materials and enclosed with the nano-particles of super paramagnetic ferriferrous oxide and the anticarcinogen with hydrophobic property, and then dialysed to obtain the nanomicelle of magnetic tumor double-targeting polymer. The drug carrier system of the nanomicelle reinforces the treatment effect of the anticarcinogen with hydrophobic property on tumor, prolongs the circulating time of the anticarcinogen in human body, intensifies the targeting action of drugs, improves the release efficiency and partial concentration of drugs, and reduces the dosage and toxic and side effect of drugs.
Owner:SUN YAT SEN UNIV
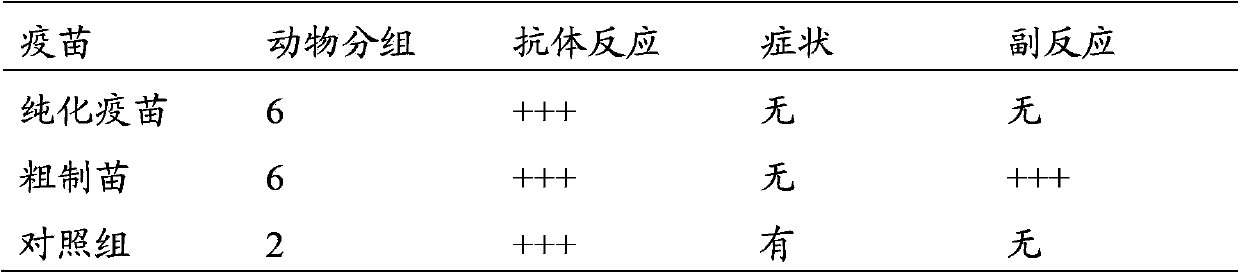
Method for preparing purified foot-and-mouth disease vaccine
InactiveCN103374547ARule out emergency responseReduced risk of contamination with exogenous agentsAntiviralsVertebrate cellsContinuous flow centrifugationSaccharum
The invention discloses a method for preparing a purified foot-and-mouth disease vaccine, a serum or animal-derived ingredient free culture medium and an application of the serum or animal-derived ingredient free culture medium to the preparation of the foot-and-mouth disease vaccine, belonging to the filed of biotechnology. The method for preparing the purified foot-and-mouth disease vaccine comprises the following steps of: culturing a foot-and-mouth disease virus by using the serum or animal-derived ingredient free culture medium, purifying an obtained virus solution to obtain a purified antigen, subjecting a cell strain BHK-21 or BSR to the multiple-generation acclimatization culture and the suspension culture by 300L of a microcarrier through the serum-free culture medium, inoculating the cell strain BHK-21 or BSR against the foot-and-mouth disease vaccine, stirring at the rotating speed of 30-50rpm, microfiltrating, ultrafiltrating, concentrating 50-200 times, carrying out chromatography with a Sephawse6FF molecular sieve or density gradient zonal centrifugation with a continuous flow, and inactivating with beta-propiolactone to obtain the serotype univalent or multivalent vaccine for cattle, sheep and pigs.
Owner:北京必威安泰科技有限公司 +1
Cell-derived viral vaccines with low levels of residual cell DNA
ActiveUS20090304729A1Enhance immune responseLess recognizableSsRNA viruses negative-senseViral antigen ingredientsViral VaccineA-DNA
The present invention relates to vaccine products for the treatment or prevention of viral infections. Further provided are methods of reducing contaminants associated with the preparation of cell culture vaccines. Residual functional cell culture DNA is degraded by treatment with a DNA alkylating agent, such as β-propiolactone (BPL), thereby providing a vaccine comprising immunogenic proteins derived from a virus propagated on cell culture, substantially free of residual functional cell culture DNA.
Owner:SEQIRUS UK LTD
Preparation method and application of swine vaccine specific swine spleen transfer factor (TF)
ActiveCN103566370AReduce the use volumeEasy to mix and prepareAntiviralsAntibody ingredientsHigh concentrationDead volume
The invention discloses a preparation method of a swine vaccine specific swine spleen transfer factor (TF). The preparation method comprises the following steps: slaughtering a swine with a positive swine vaccine antibody to harvest the swine spleen; preserving the swine spleen at low temperature; unfreezing the swine spleen; removing fasciae; mincing the swine spleen; homogenizing the pulp; filling the homogenized pulp into a bottle and storing the pulp; repeatedly freezing and thawing the pulp; centrifuging the pulp; carrying out microfiltration; carrying out ultrafiltration; carrying out inactivation; regulating the pH value; regulating the osmotic pressure; removing bacteria; and detecting the quality. The preparation method has the advantages that the pH value of water for injection is regulated to 4-6 in the pulp homogenizing step, thus being beneficial to increasing of the yields of ribose and polypeptide; a box type membrane coating is adopted for tangential flow filtration, so that the dead volumes of system residues are small and linear amplification production is easy to achieve; a 1-3KD box type membrane coating is adopted to carry out tangential flow nanofiltration on a TF crude product, thus preparing the high-concentration TF and improving the using effects; phenol red is used as an acid-base indicator, thus being convenient for clients to observe the pH value of the TF; the TF uses beta-propiolactone for inactivation instead of traditional formaldehyde and an osmotic pressure regulation process is added, thus being beneficial to combined immunization of the TF and vaccines.
Owner:派生特(福州)生物科技有限公司 +2
Pharmaceutical Compositions Comprising A Pancreatic Enzyme Preparation With Viral Infectivity Reduced Below A Significant Level And Methods Of Preparing And Using The Same
InactiveUS20140017223A1Reduce alkylation activitySure easySsRNA viruses positive-sensePeptide/protein ingredientsAmylasePorcine circovirus
The present invention provides for pharmaceutical compositions comprising pancreatic enzyme preparations (PEPs) with viral infectivity reduced below significant levels and having high enzymatic activity. The PEPs can comprise lipases, proteases, amylases, non-enveloped viruses (e.g., porcine parvovirus (PPV), porcine circovirus type 2 (PCV-2), porcine encephalomyocarditis virus (EMCV)), and enveloped viruses (e.g., vesicular stomatitis virus (VSV), and influenza A (IFA)). The present invention also includes methods of treating pancreatic insufficiency by administering these pharmaceutical compositions and methods of making the same by treating the PEP with beta-propiolactone (BPL) to reduce viral infectivity.
Owner:APTALIS PHARMA CANADA
Systems and processes for polyacrylic acid production
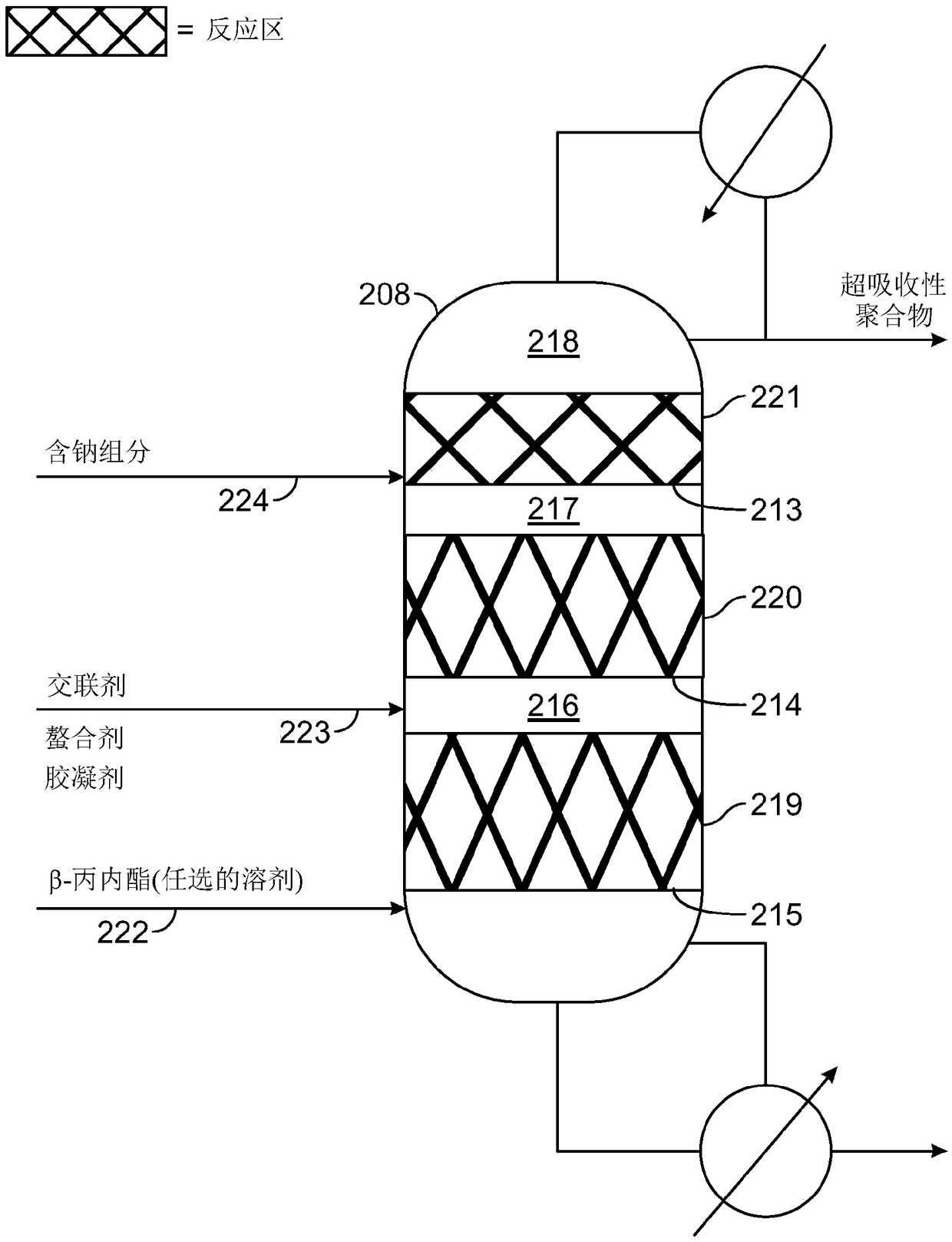
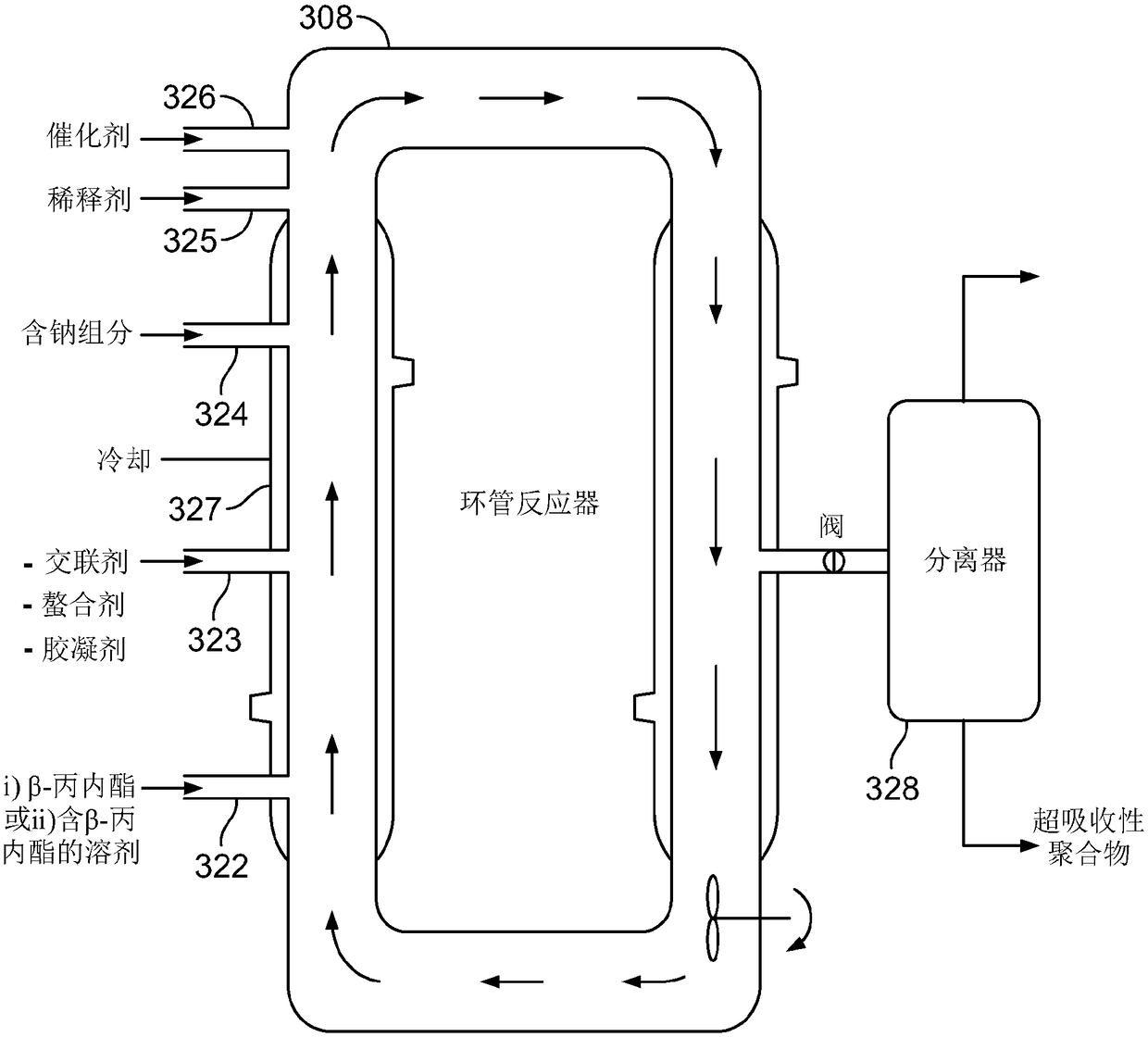
ActiveUS10428165B2Efficient preparationHigh purityOrganic compound preparationPreparation from carboxylic acid esters/lactonesEthylene oxideSuperabsorbent polymer
Disclosed are systems and methods for the production of polyacrylic acid and superabsorbent polymers from ethylene oxidation to form ethylene oxide. Reacting the ethylene oxide with carbon monoxide to form to beta propiolactone (BPL) or polypropiolactone (PPL), or a combination thereof. An outlet configured to provide a carbonylation stream comprising the BPL or PPL, or a combination thereof and using one or more reactors to convert BPL to acrylic acid or to convert at least some of the BPL to PPL, and then to convert PPL to acrylic acid. An outlet configured to provide a PPL stream to a second reactor tm to convert at least some of the PPL to AA or a third reactor to convert at least some of the PPL to AA. The outlet configured to provide an AA stream to a fourth reactor to convert the AA to polyacrylic acid.
Owner:NOVOMER INC
Preparation method of carboxylic acid betaine modified non-woven fabric for hemofiltration
InactiveCN102561033AGood blood compatibilityQuick and easy to manufactureFibre typesFiltration separationPropiolactoneBetaine
The invention provides a preparation method of carboxylic acid betaine modified non-woven fabric for hemofiltration, belonging to the field of biological medical material. The method comprises the following steps of: under the action of photosensitizer and ultraviolet light, grafting N, N dimethyl acrylamide (DMAA) with tert-amido, and then introducing propiolactone to react with the tert-amido to generate a carboxylic acid betaine group. The preparation method is simple in preparation process, easy to realize, explicit and reliable in mechanism; and the carboxylic acid betaine modified non-woven fabric is good in hydrophily and blood compatibility and can be applied to blood filter material and the other biological medical fields.
Owner:SUZHOU YITONG FILTER TECH
Systems and methods for producing superabsorbent polymers
Provided herein are systems, and methods of using such systems, for producing superabsorbent polymers from ethylene oxide and carbon monoxide. The production systems have various unit operations, including, for example, a beta-propiolactone production system configured to produce beta-propiolactone from ethylene oxide and carbon monoxide and a superabsorbent polymer production system configured toproduce superabsorbent polymers from beta-propiolactone and / or acrylic acid.
Owner:NOVOMER INC
Preparation method and application of swine vaccine specific cattle spleen transfer factor (TF)
ActiveCN103565834AReduce the use volumeEasy to mix and prepareUnknown materialsImmunological disordersHigh concentrationDead volume
The invention discloses a preparation method of a swine vaccine specific cattle spleen transfer factor (TF). The preparation method comprises the following steps: slaughtering a cattle with a positive swine vaccine antibody to harvest the cattle spleen; preserving the cattle spleen at low temperature; unfreezing the cattle spleen; removing fasciae; mincing the cattle spleen; homogenizing the pulp; filling the homogenized pulp into a bottle and storing the pulp; repeatedly freezing and thawing the pulp; centrifuging the pulp; carrying out microfiltration; carrying out ultrafiltration; carrying out inactivation; regulating the pH value; regulating the osmotic pressure; removing bacteria; and detecting the quality. The preparation method has the advantages that the pH value of water for injection is regulated to 4-6 in the pulp homogenizing step, thus being beneficial to increasing of the yields of ribose and polypeptide; a box type membrane coating is adopted for tangential flow filtration, so that the dead volumes of system residues are small and linear amplification production is easy to achieve; a 1-3KD box type membrane coating is adopted to carry out tangential flow nanofiltration on a TF crude product, thus preparing the high-concentration TF and improving the using effects; phenol red is used as an acid-base indicator, thus being convenient for clients to observe the pH value of the TF; the TF uses beta-propiolactone for inactivation instead of traditional formaldehyde and an osmotic pressure regulation process is added, thus being beneficial to combined immunization of the TF and vaccines.
Owner:派生特(福州)生物科技有限公司 +1
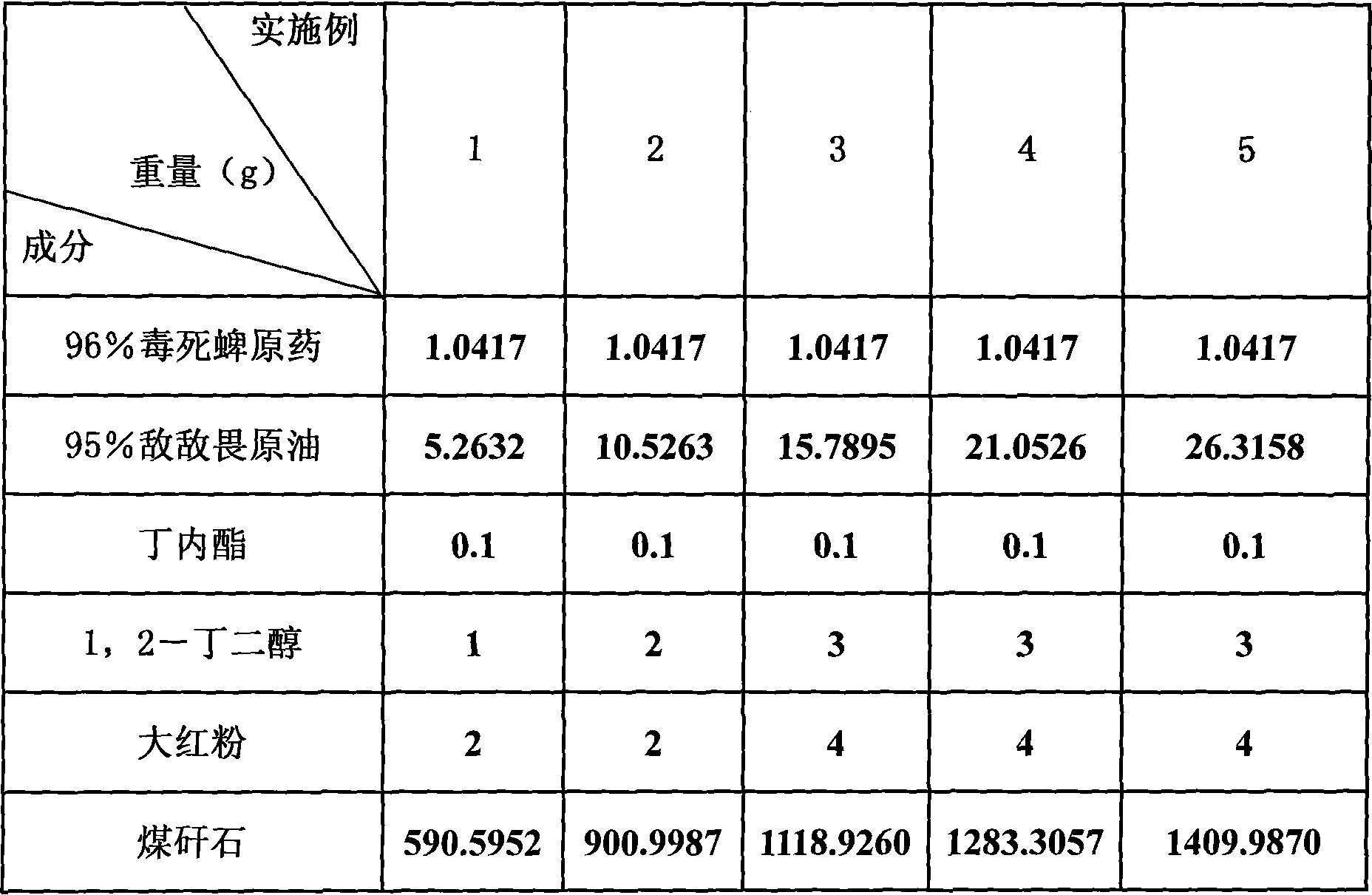
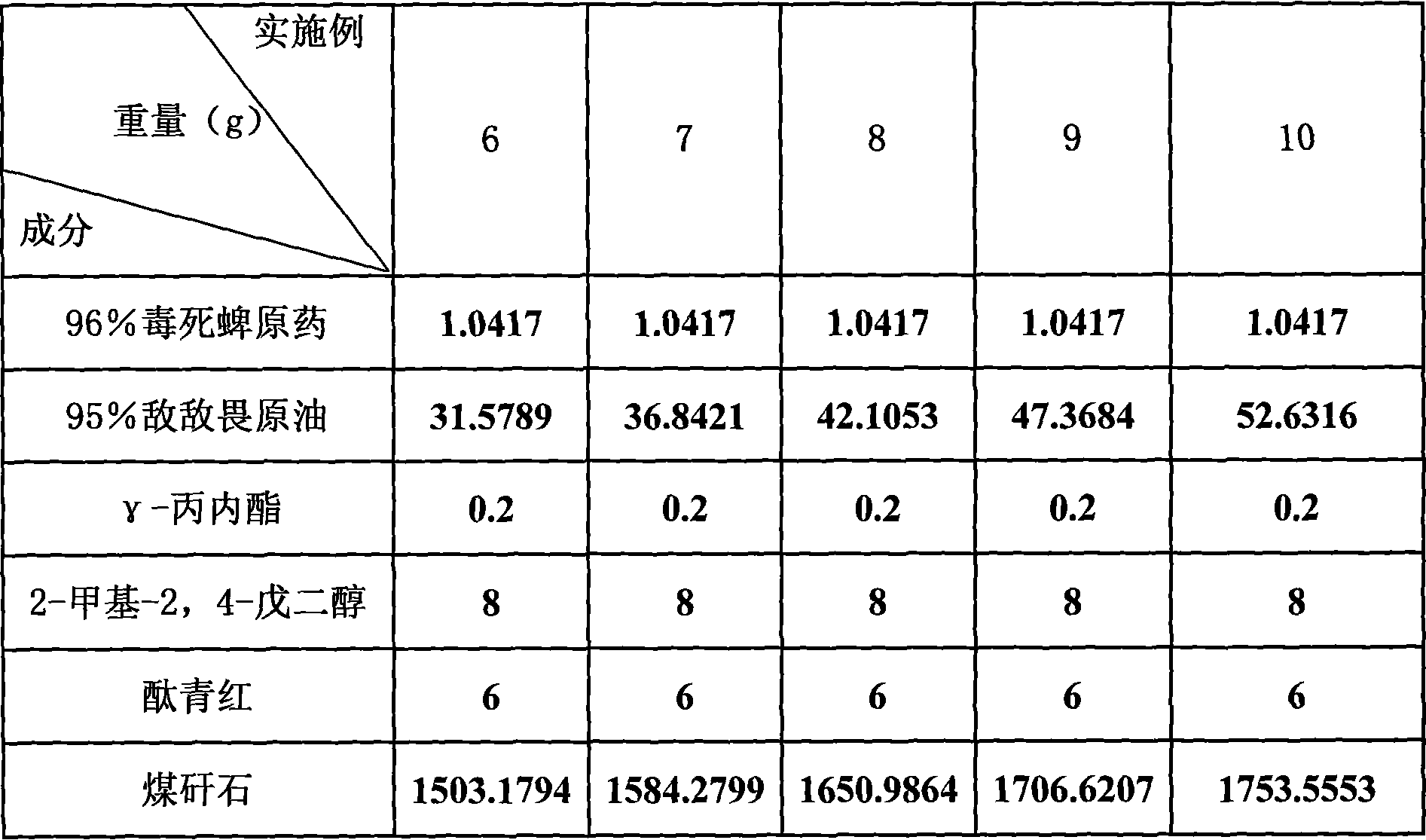
Granules for controlling Laodelphax striatella in wheat fields
InactiveCN101032253AEffective penetrationImprove the effect of prevention and controlBiocideAnimal repellantsPropiolactoneDichlorvos
The granular pesticide preparation for preventing and controlling smaller brown planthopper in wheat field consists of chlorpyrifos, DDVP, stabilizer A, stabilizer B, colorizing agent and carrier in certain weight proportion. The stabilizer A is butyrolactone, gamma-propiolactone or delta-propiolactone; the stabilizer B is 1, 2-butanediol, 2-methyl-2, 4-pentanediol or [1, 5]-pentanediol; the colorizing agent is red powder, phthalocyanine red or acid scarlet; and the carrier is coal slack, perlite or vermiculite. The granular pesticide preparation as re-compounded pesticide has synergistic effect of killing smaller brown planthopper and other effect.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Coffee essence preparation method
InactiveCN107460043AGuaranteed burnt aromaAdd tangEssential-oils/perfumesBenzoic acidN-Hexanoic acid
The invention belongs to the field of coffee essence preparation methods, and relates to a coffee essence preparation method. The method comprises the following steps: S10, raw material weighing; S20, material feeding and production; and S30, packaging, wherein in the step S10, the weighed raw materials and proportions are as follows in weight percentage: 0.07-0.09% of beta-propiolactone, 0.07-0.09% of green cognac oil, 0.09-0.11% of benzoic acid, 0.25-0.29% of n-hexanoic acid, 0.36-0.4% of ethyl acetate, 0.36-0.4% of methyl maltol, 0.29-0.35% of butanedione, 0.6-0.8% of furfuryl mercaptan, 9-11% of red date extract, 8-10% of vanillin, 18-20% of coffee extract, 8-10% of ethyl alcohol, and the balance of distilled water. Through the ingredients and proportions, the rich taste of coffee can be increased while the coke fragrance of coffee is guaranteed; in addition, solids can be dissolved first for preventing the volatilization of ingredients with small proportions.
Owner:潘峰
Thermoplastic elastomer for profile control and water plugging and preparation method thereof
PendingCN112175594AIncrease displacementEnhanced overall recoveryDrilling compositionElastomerPolymer science
The invention discloses a thermoplastic elastomer for profile control and water plugging. The thermoplastic elastomer is prepared from the following raw materials by weight: 8-10 parts of a componentA, 1-3 parts of strong acid, 1-3 parts of a hydrophobic modifier and 100-105 parts of water; wherein the component A is any one of polyethylene glycol, polyvinylmethyl ether, polyvinyl alcohol and a copolymer of acrylamide and acrylonitrile; the strong acid is any one of concentrated hydrochloric acid and concentrated sulfuric acid; the hydrophobic modifier is any one of formaldehyde, acetaldehyde, propionaldehyde, butyraldehyde, beta-propiolactone, gamma-butyrolactone, delta-valerolactone, methyltriacetylsilane, methyltrimethylsilane, butyl acrylate and ethylene glycol dimethacrylate. The thermoplastic elastomer for profile control and water plugging has the advantages of one time preparation molding, high deformation capacity, temperature and salt resistance, high stability and the like,and is suitable for large-scale production and application.
Owner:SOUTHWEST PETROLEUM UNIV
Nucleic acid extraction-free inactivated virus preserving fluid and application thereof
The invention provides a nucleic acid extraction-free inactivated virus preserving fluid and application thereof, and particularly, the virus sample preserving fluid comprises protease K, sodium hydroxide, beta-propiolactone, gelatin and other components, and virus nucleic acid can be effectively released and preserved at room temperature through the synergistic cooperation of the components. Nucleic acid degradation is effectively prevented in the normal-temperature transportation and storage process of the sample, and the method can be used for preparing clinical viral nucleic acid samples.
Owner:JIANGSU COWIN BIOTECH CO LTD
Preparation method of poultry vaccine dilution liquid
InactiveCN108904813AEasy to useGuarantee the safety of useMacromolecular non-active ingredientsAcetic acidFiltration
The invention provides a preparation method of a poultry vaccine dilution liquid, and belongs to the technical field of biomedicine. The preparation method comprises: mixing chicken bursa, chicken intestine and water, homogenizing, carrying out repeated freezing-thawing on the obtained homogenized liquid, mixing the obtained freezing-thawing liquid and acetic acid, sequentially carrying out stirring and centrifugation, mixing the obtained centrifugation supernatant and beta-propiolactone, inactivating, hydrolyzing the obtained inactivated liquid for 1-3 h at a temperature of 35-42 DEG C, carrying out micro-filtration on the obtained hydrolyzate, adjusting the pH value of the obtained micro-filtrate, carrying out ultra-filtration on the obtained adjusted liquid, and sterilizing the obtainedultra-filtrate to obtain the poultry vaccine dilution liquid. According to the present invention, the poultry vaccine dilution liquid prepared through the preparation method can completely kill the non-specific pathogenic bacteria of poultry vaccine, and can ensure the safe use of the vaccine.
Owner:派生特(福州)生物科技有限公司
Absorbent polymers, and methods and systems of producing thereof and uses thereof
Provided herein are absorbent polymers produced from beta-propiolactone, and methods and systems of producing such polymers. The beta-propiolactone may be derived from ethylene oxide and carbon monoxide. The absorbent polymer may be bio-based and / or biodegradable. The absorbent polymers may be used for diapers, adult incontinence products, and feminine hygiene products, as well as for agricultural applications.
Owner:NOVOMER INC
Cell-derived viral vaccines with low levels of residual cell DNA
ActiveUS10655108B2Less recognizableReduce aggregationSsRNA viruses negative-senseViral antigen ingredientsAlkylating antineoplastic agentPropiolactone
Owner:SEQIRUS UK LTD
Main chain type biodegradable liquid crystal polymer and preparation method thereof
ActiveCN110845713AGood biocompatibilityHigh affinityLiquid crystal compositionsPolymer scienceAklanonic acid
The invention belongs to the technical field of biodegradable medical polymer materials, and particularly relates to a main chain type biodegradable liquid crystal polymer and a preparation method thereof. Natural products such as cholesterin, diosgenin, menthol, isosorbide and the like are used as liquid crystal nucleuses; a hydroxyl-terminated alkanoic acid or an amino-terminated acid is used asa flexible spacer to prepare a liquid crystal intermediate; ring opening polymerization reaction on one or a mixture of two or more than two of following monomers or derivatives from trimethylene carbonate, caprolactone, lactide, glycolide, p-dioxanone, propiolactone, butyrolactone, octolactone, caprolactam, morpholine-2, 3-diketone, and a dianhydride are initiated by taking the liquid crystal intermediate as an initiator, so as to obtain the main chain type biodegradable liquid crystal polymer. The main chain type biodegradable liquid crystal polymer provided by the invention has good liquidcrystal performance, is easy to orientate to form an ordered structure, not only generates response to the outside factors of temperature, pH, stress, electric field, magnetic field and the like, butalso has good biocompatibility and degradation performance.
Owner:杨立群
Bovine infectious rhinotracheitis virus ibrv-b strain and its application
ActiveCN107299088BImproving immunogenicityStable potencyViral antigen ingredientsMicroorganism based processesPropiolactoneAdjuvant
The invention discloses an IBRV-B (infectious bovine rhinotracheitis virus-B) strain and application thereof. The IBRV-B strain provided by the invention has the advantages of good immunogenicity and good culture characteristics. The IBRV-B strain is inoculated with MDBK cells; a culture material is harvested; beta-propiolactone is used for inactivation; then, the materials are mixed and emulsified with commercial Montanide<TM> ISA15AVG adjuvants of a French SEPPIC company to be made into an IBRV inactivated vaccine. Experiments prove that when the IBRV inactivated vaccine prepared by using the IBRV-B strain provided by the invention is used for immunizing ablactation health negative cow, the diseases caused by the IBRV can be prevented, so that the vaccine has the advantages of high safety, capability of fast antibody generation, long immunity period and the like.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Rooting liquid for jujube tree scion
InactiveCN107410308APromote growthImprove growth performancePlant growth regulatorsBiocideSaccharumSucrose
The invention provides rooting liquid for a jujube tree scion. The rooting liquid comprises, by weight, 11-16 parts of 2-naphthalene acetic acid, 8-12 parts of geniposide, 5-8 parts of sodium 4-chlorophenoxyacetate, 3-6 parts of beta-propiolactone, 4-8 parts of baicalin, 6-10 parts of zeatin riboside, 15-20 parts of sucrose, 5-9 parts of vitamin B5, 3-7 parts of dimethyl sulfoxide, 160-200 parts of ethanol and 1000-1200 parts of water. The rooting liquid can increase the rooting rate of the jujube tree scion and shorten a rooting cycle.
Owner:安徽金源农林专业合作社
Plasma anticoagulant having virus inactivation function
The invention relates to a plasmid anticoagulant having a virus inactivation function. With 4% sodium citrate which is collected in a blood test as a basic anticoagulant, the virus inactivation function of the plasmid anticoagulant is achieved by adding one or more virus inactivating agents selected from sodium caprylate, formaldehyde and [beta]-propiolactone. With the application of the plasma anticoagulant having a recheck function provided by the invention, the risk of personnel infection due to exposure of positive plasma in a storing, transporting or using process after the collection of the plasma can be effectively prevented, so that the bio-safety of the plasma in an entire life cycle from the collection of the plasma can be guaranteed.
Owner:发贵科技(贵州)有限公司
Cationic polymer gene vector and preparation method thereof
ActiveCN113061254ALow cytotoxicityGood biocompatibilityGenetic material ingredientsGene therapyPolymer sciencePolythylene glycol
The invention discloses a cationic polymer gene vector and a preparation method thereof, the cationic polymer gene vector is modified polyglycidyl amine, and a modification agent is p-methylbenzenesulfonyl terminated polyethylene glycol monomethyl ether, carboxyl terminated polyethylene glycol monomethyl ether, oligosaccharide, an organic hydrophobic substance or zwitter-ions. The oligosaccharide is at least one of maltose, glucose and mannose, the organic hydrophobic substance is at least one of lauric acid, lauric aldehyde, polylactic acid, polycaprolactone, a lactic-co-glycolic acid copolymer and cholesterol, and the zwitterions are at least one of propiolactone, butyrolactone, valerolactone, caprolactone, 1, 3-propane sultone, 1, 4-butane sultone, and 1, 5-pentane sultone; wherein the grafting rate of the modification agent on the polyglycidyl amine is 5%-50%. The modified polyglycidyl amine provided by the invention is used as a non-viral gene transfection vector, and has very high biocompatibility and gene transfection capability and relatively low cytotoxicity.
Owner:ZHEJIANG UNIV OF TECH
Inactivated rabies antigen and preparation method thereof
ActiveCN102205119BProtection attackReduce production processAntiviralsAntibody medical ingredientsAntigenPropiolactone
The invention discloses an inactivated rabies antigen. A method for preparing the antigen comprises the following steps: mixing a rabies virus dG strain virus liquid with a titer of 5.0*10<6> to 1.0*10<7> FFU / mL and a BHK-21 cell suspension with a concentration of 5.0%*10<5> to 1.0*10<6> individuals / mL; cultivating for 72 to 96 h in the environment with a temperature of 33 to 37 DEG C; carrying out freeze thawing and centrifugation, and collecting a supernatant; regulating the pH of the supernatant to 7.4 to 7.6; adding beta-propiolactone with a final concentration of 0.025 to 0.05 V / V% drop by drop; and inactivating and hydrolyzing. According to the present invention, the method has the advantages of needing no condensation, simplifying production technology and saving production cost, and prepared inactivated vaccines can induce the generation of a high level of antibodies in a dog and can effectively protect the dog from virulent attack by the rabies virus. The inactivated rabies antigen prepared through the method allows condensation and purification to be omitted, production technology to be greatly simplified and production cost to be reduced, and is suitable for popularization and application in China.
Owner:GUANGZHOU SOUTH CHINA BIOLOGICAL MEDICINE
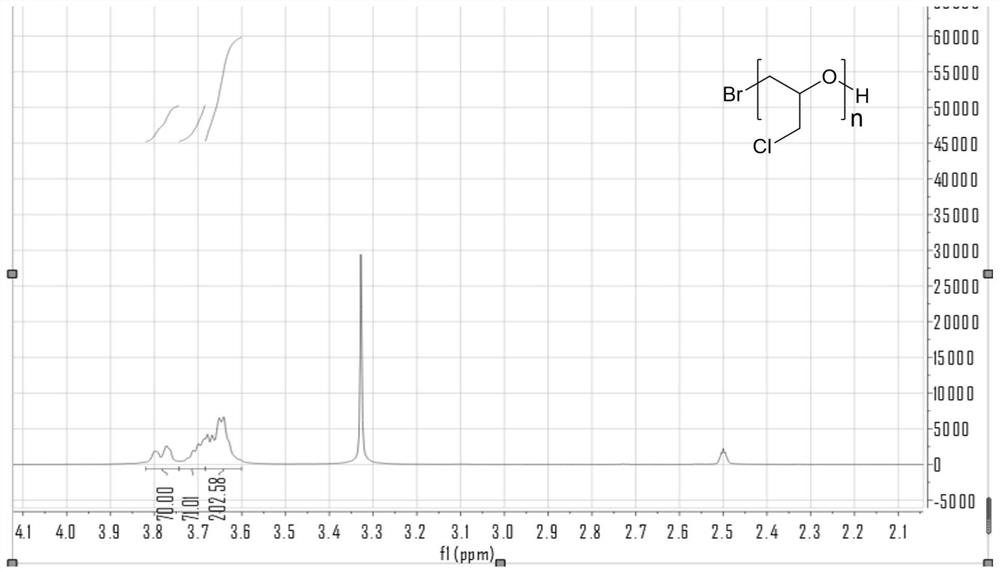
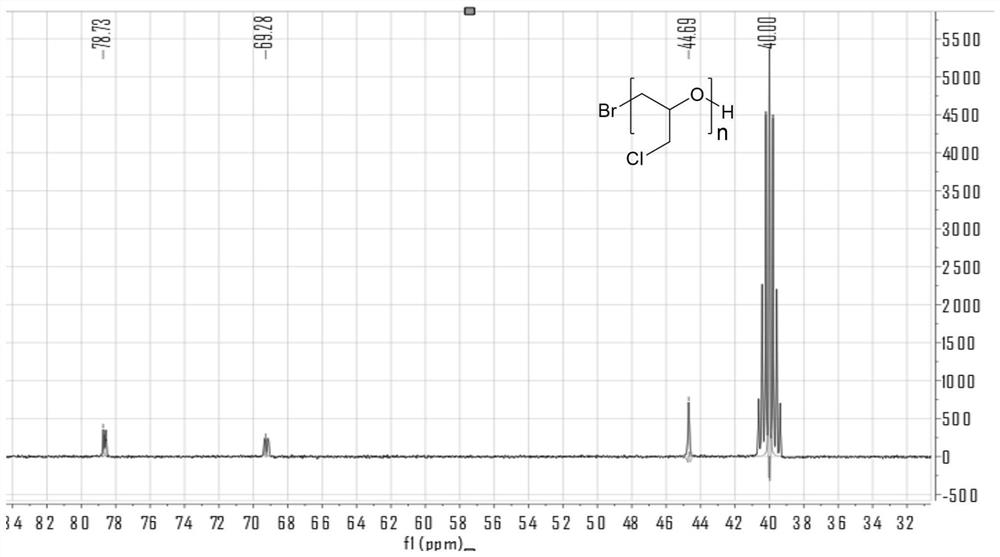
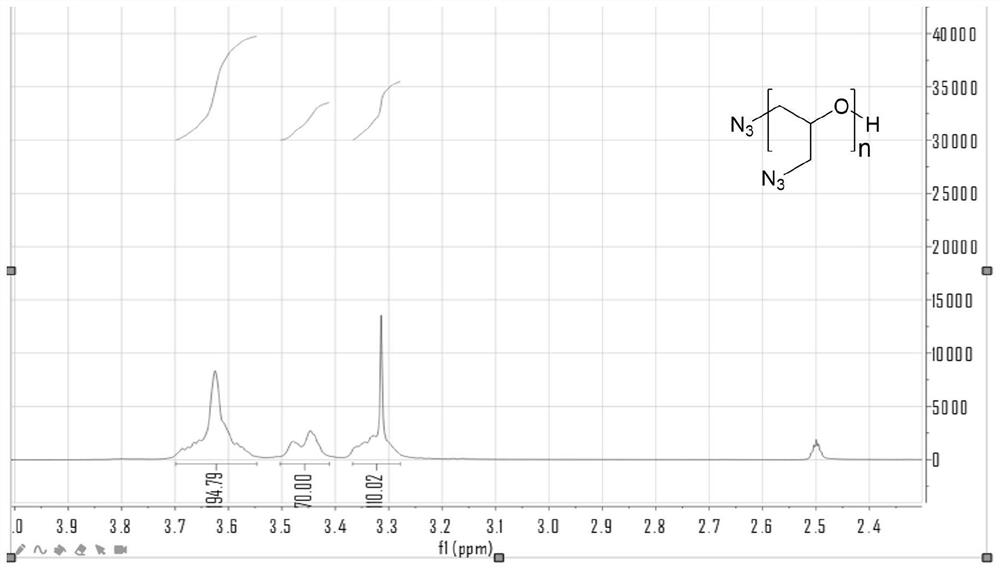
Preparation method of beta-propiolactone
The invention relates to a preparation method of beta-propiolactone, the preparation method comprises the following steps: (1) dissolving 3-halogenated propionic acid in an organic solvent at a room temperature, stirring, cooling to a temperature of 0 DEG C or less, and adding organic amine to obtain a solution A; preparing alkali liquor below 0 DEG C as a solution B; (2) mixing the solution A and the solution B under stirring at 0-2 DEG C, then heating to 4-6 DEG C, carrying out a heat preservation reaction, stopping stirring after the reaction is completed, cooling to 0 DEG C, and standing to generate split phases; and (3) washing the organic phase obtained by phase splitting with salt water, cooling to 0 DEG C or below, washing with water, drying, desolventizing to obtain a crude product beta-propiolactone, and distilling to obtain the product beta-propiolactone. According to the method, the reaction technology combining extraction and phase transfer is adopted, the reaction is carried out at low temperature, beta-propiolactone can be prepared at high yield, the used raw materials are easy to obtain, the cost is low, the preparation process is simple, and the method is suitable for industrial production.
Owner:SINOPHARM CHEM REAGENT
Inactivated respiratory syncytial viral vaccines
An immunogenic composition capable of producing a respiratory syncytial (RS) virus specific immune response in a host immunized therewith comprises purified, inactivated RS virus which is substantially free from cellular and serum components and which is non-infectious, non-immunopotentiating, immunogenic and protective. The virus is grown on a vaccine quality cell line and harvested virus is purified under non-denaturing conditions to be substantially free from cellular and serum components. The purified RS virus is inactivated using β-propiolactone, a non-ionic detergent, particularly n-octyl-α-D-glucopyranoside and n-octyl-β-D-glucopyranoside, or ascorbic acid. The immunogenic composition may be formulated as a vaccine for in vivo administration to a human host. The immunogenic composition also may be used in diagnostic applications.
Owner:CONNAUGHT LAB
Method for removing chick embryo host protein in rabies vaccine product
PendingCN113117067AReduce the impactReduce contentSsRNA viruses negative-senseViral antigen ingredientsRabiesArginine
The invention relates to a method for removing chick embryo host protein in a rabies vaccine product. The method comprises the following steps of: filtering and concentrating harvested virus chick embryo cell culture supernatant, performing washing and filtering by using a phosphate buffer solution containing sodium chloride and arginine, and adding beta-propiolactone for inactivating and hydrolyzing; and adding human serum albumin, performing standing at low temperature, and performing ultrasonic treatment and purification to obtain the rabies vaccine product purified liquid without the chick embryo host protein. According to the method, the human serum albumin is added, so that the influence of ultrasonic waves on virus particles can be effectively reduced, and the content of impure protein in the product is further reduced. The inactivated hydrolysate is subjected to ultrasonic treatment through an ultrasonic cleaning machine, the cleanliness requirement is low, the operation difficulty is low, and the stability is high.
Owner:SHENZHEN WEIGUANG BIOLOGICAL PROD
Thermoplastic elastomer for profile control and water shutoff and method for preparing same
PendingUS20220089935A1The process steps are simpleLow production costDrilling compositionElastomerPolymer science
A thermoplastic elastomer for profile control and water shutoff is prepared from the following raw materials in parts by weight: 8-10 parts of a component A, 1-3 parts of a strong acid, 1-3 parts of a hydrophobic modifier and 100-105 parts of water. The component A is one of polyethylene glycol, polyvinylether, polyvinyl alcohol, copolymer of acrylamide and acrylonitrile. The strong acid is one of concentrated hydrochloric acid and concentrated sulfuric acid. The hydrophobic modifier is any one of formaldehyde, acetaldehyde, propionaldehyde, butyraldehyde, β-propiolactone, γ-butyrolactone, δ-valerolactone, methyltriacetylsilane, methyltrimethylsilane, butyl acrylate and ethylene glycol dimethacrylate. The thermoplastic elastomer for profile control and water shutoff provided by the disclosure has the advantages of one-step preparation and molding, strong deformation capability, temperature resistance, salt tolerance, strong stability and the like, and is suitable for large-scale production and application.
Owner:SOUTHWEST PETROLEUM UNIV
Bovine parainfluenza virus pbiv3-b strain and its application
ActiveCN107338227BImproving immunogenicityStable potencySsRNA viruses negative-senseViral antigen ingredientsBovine parainfluenza virusPropiolactone
The present invention discloses bovine parainfluenza virus PBIV3-B strain and application thereof, the bovine parainfluenza virus PBIV3-B strain has the advantages of good immunogenicity and culture characteristics and the like, the bovine parainfluenza virus PBIV3-B strain is inoculated into MDBK cell to obtain a culture matter, after the culture matter is inactivated with (beta-Propiolactone), the culture matter is mixed with commercial MontanideTMISA15AVG adjuvant of SEPPIC of France for emulsion to obtain a bovine parainfluenza virus inactivated vaccine. Results showed that when the bovine parainfluenza virus inactivated vaccine prepared by the method is used for immunization of a weaned healthy negative cow, diseases caused by the bovine parainfluenza virus type 3 can be prevented, and the bovine parainfluenza virus inactivated vaccine has the advantages of safety, fast antibody production and lasting immunity period and the like.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
A kind of grass carp bacterial septicemia and red skin disease combined vaccine and preparation method thereof
ActiveCN109675024BReduce usageAvoid damageAntibacterial agentsMultivalent vaccineDiseaseEcological environment
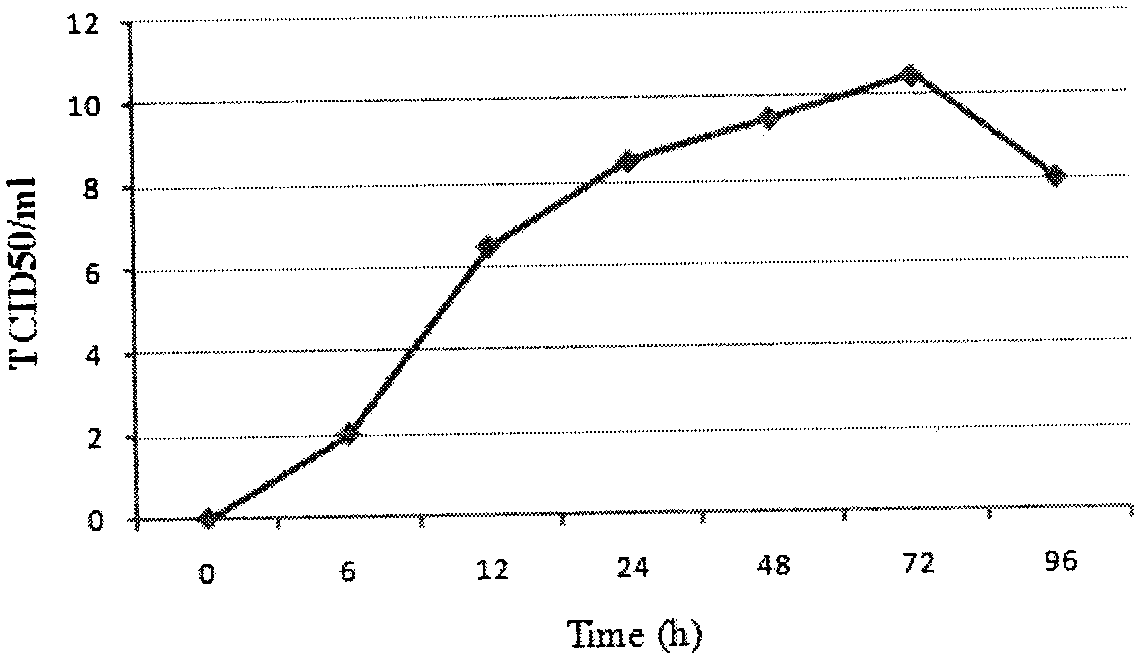
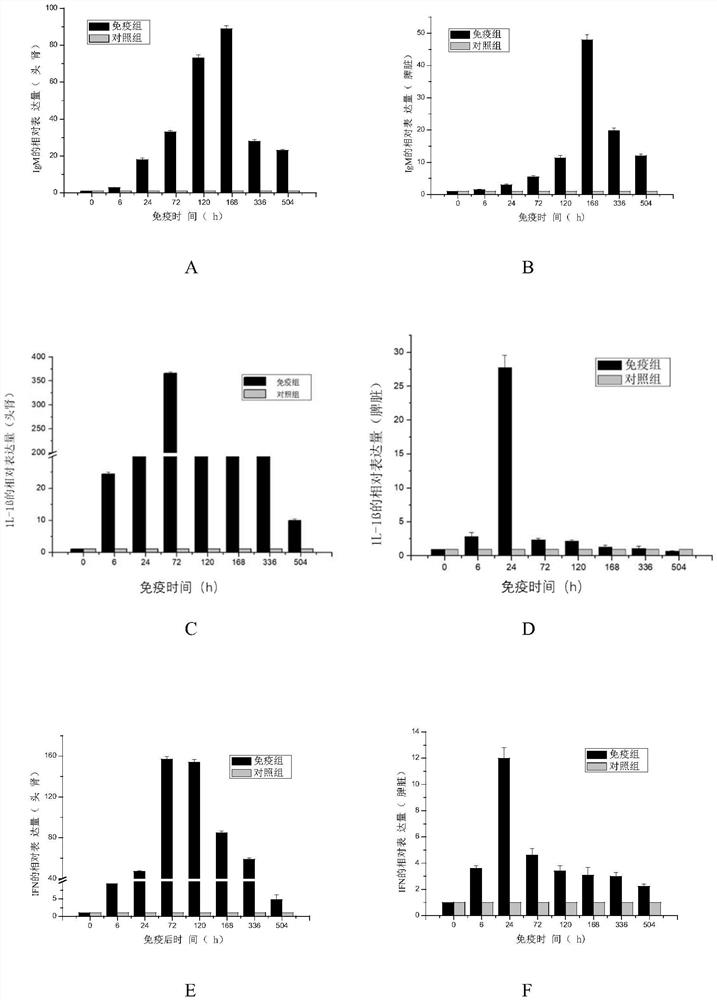
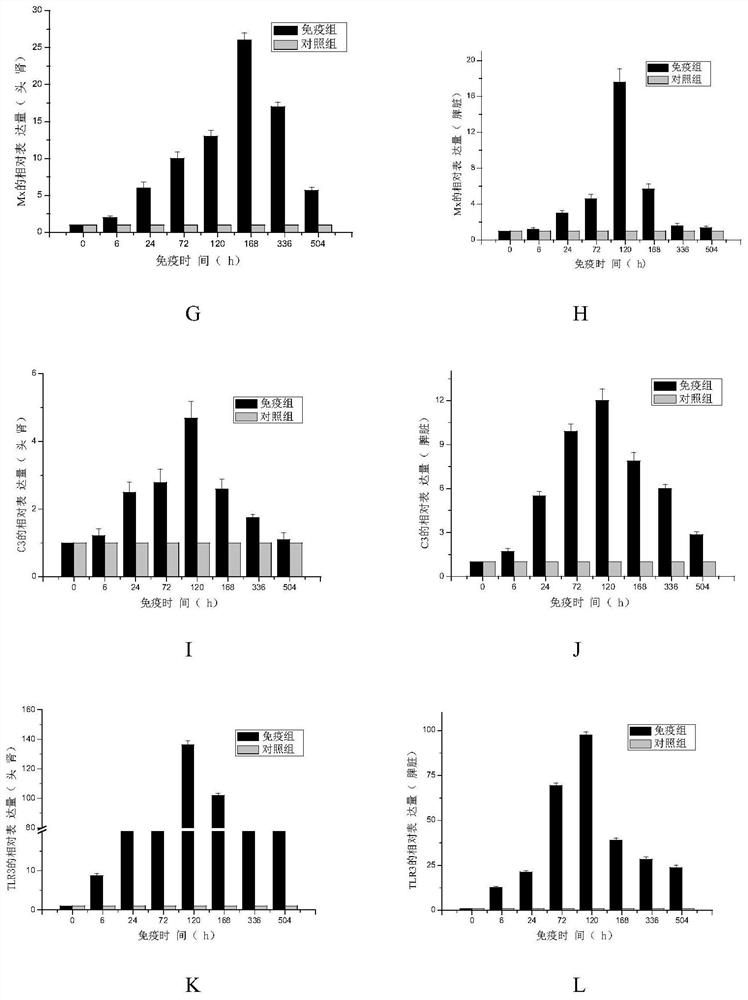
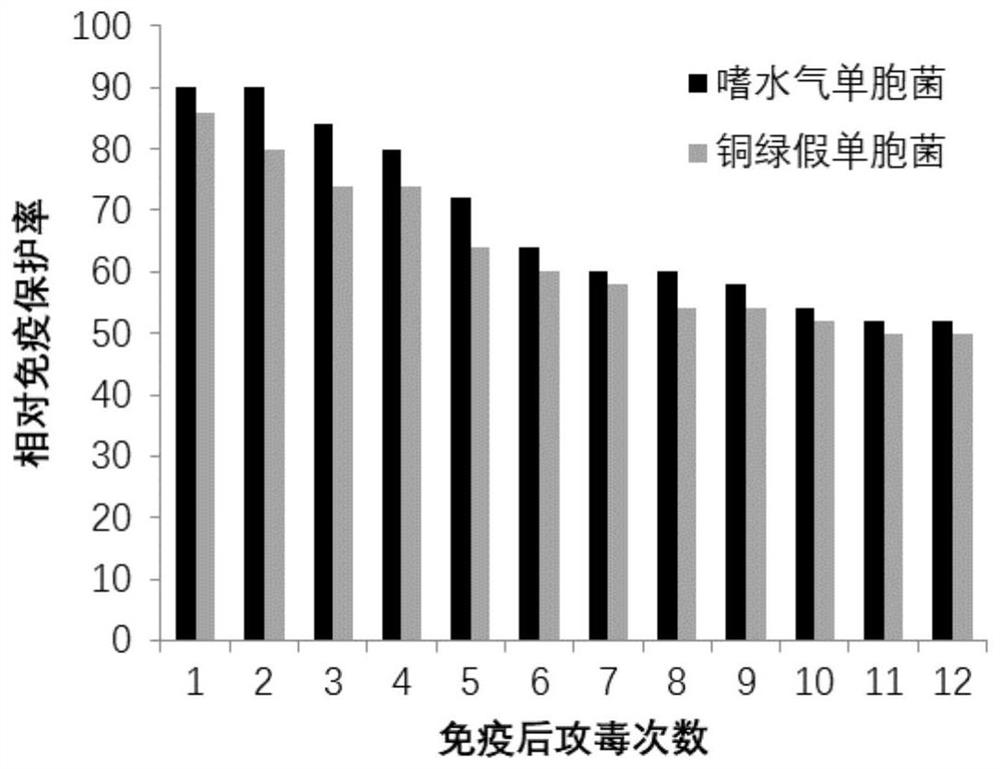
The invention discloses a grass carp bacterial septicemia and red skin disease combined vaccine and a preparation method thereof, using β-propiolactone as an inactivator to develop safe and efficient grass carp Aeromonas hydrophila septicemia and Pseudomonas aeruginosa Bacterial erythroderma dual white oil inactivated vaccine can effectively control the occurrence of bacterial septicemia and erythroderma in grass carp. After immunizing grass carp, it can stimulate the expression of IgM, IL-1β, IFN, Mx, C3 and TLR3 immune-related genes in the spleen and head kidney tissue of the fish, and the vaccine can produce a relative immune protection rate of 80-90% for grass carp , the relative protection rate can still maintain 50% 1 year after immunization; the invention is easy to operate, safe and effective, and two diseases can be prevented by one immunization, which not only reduces the use of antibiotics and chemical drugs, but also reduces the impact on the breeding environment and ecological environment, etc. The pollution and destruction of the external environment ensure the food safety and water ecological safety of grass carp.
Owner:广州普麟生物制品有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com