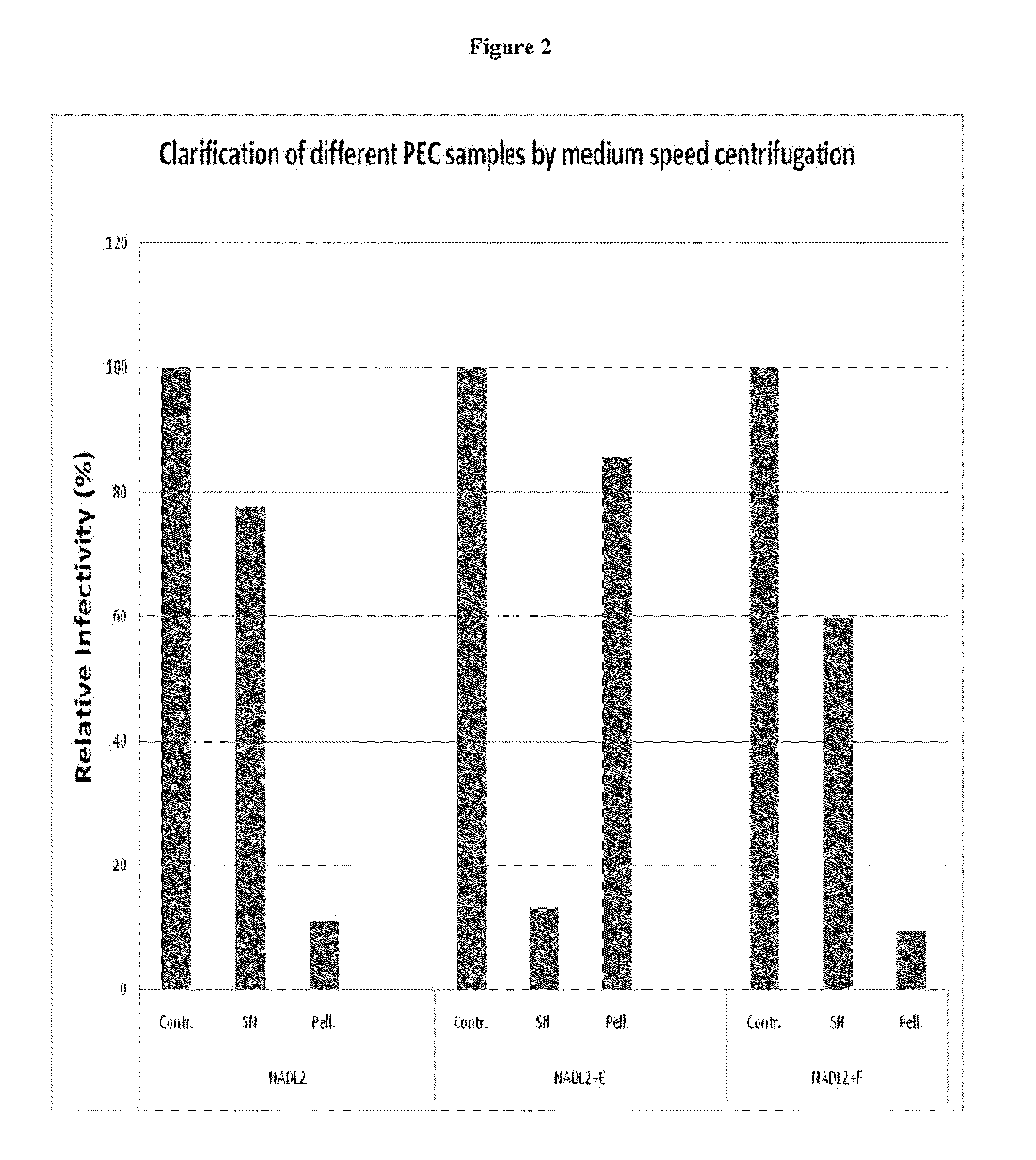
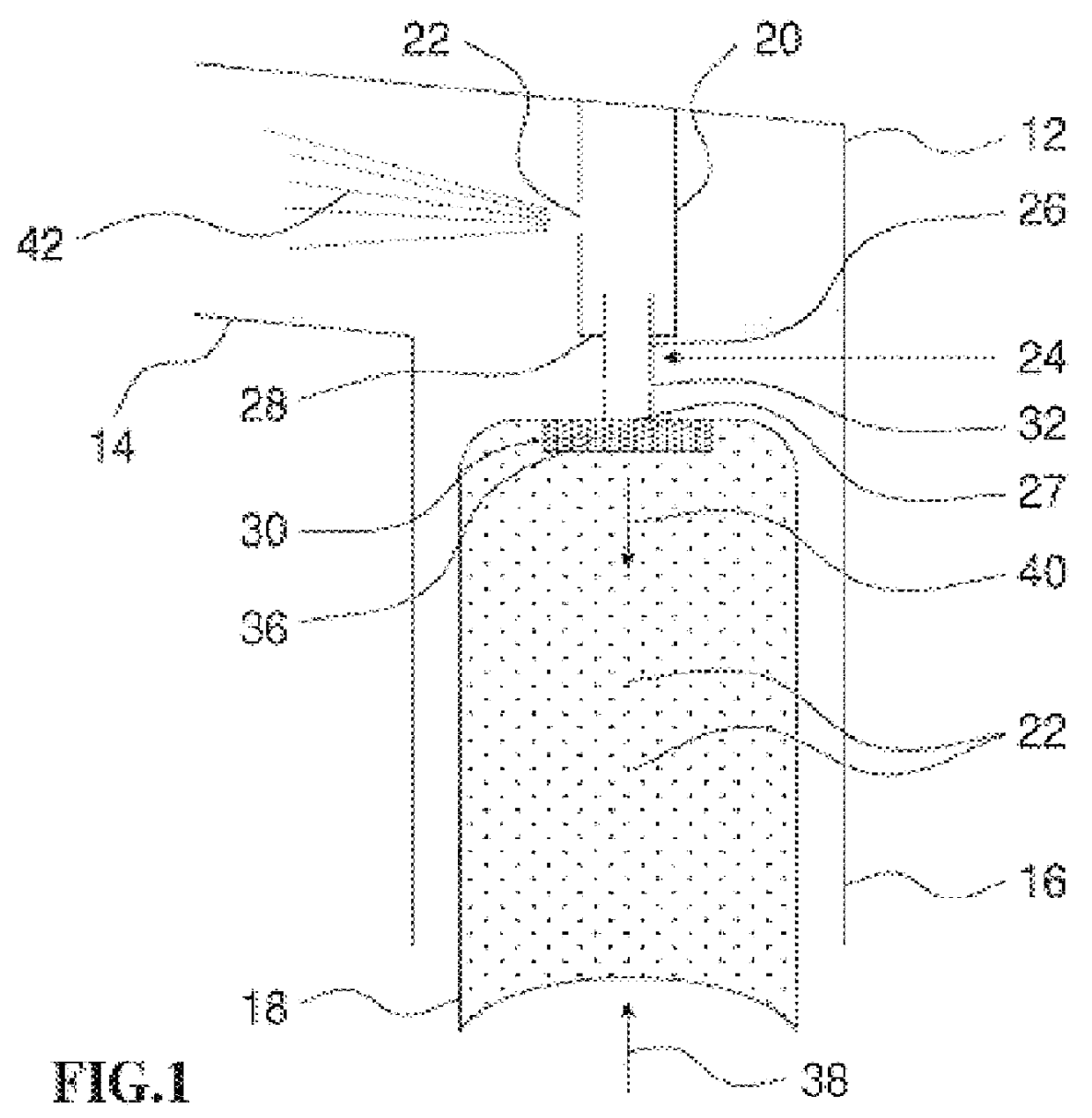
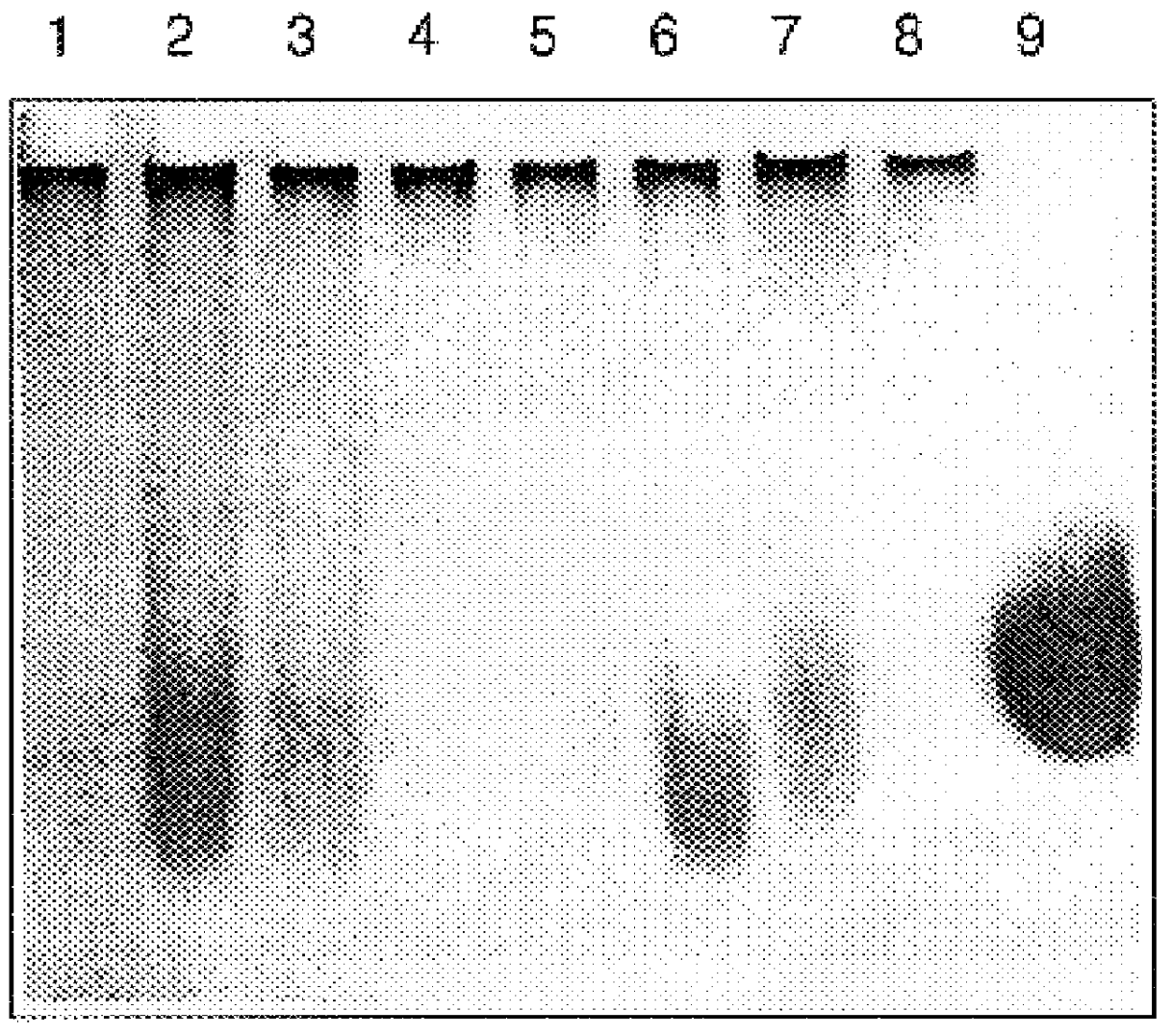
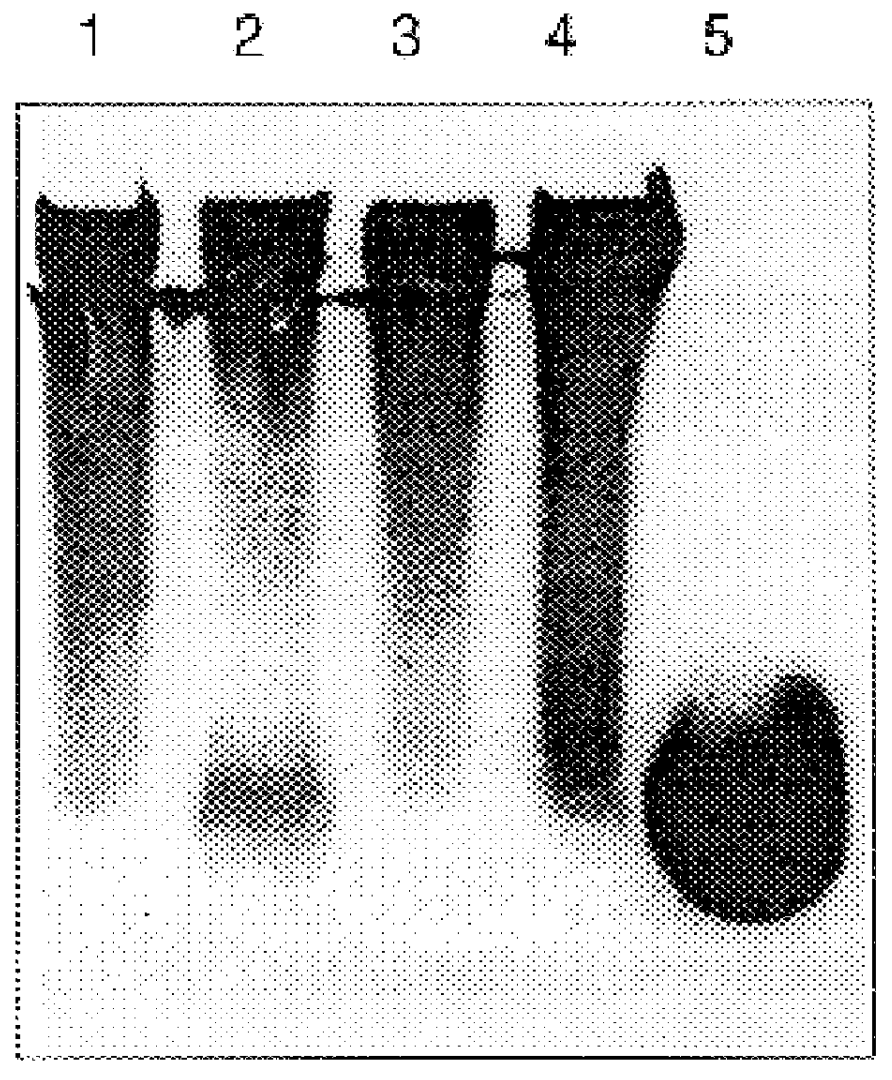Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2162 results about "Infectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In epidemiology, infectivity is the ability of a pathogen to establish an infection. More specifically, infectivity is a pathogen's capacity for horizontal transmission that is, how frequently it spreads among hosts that are not in a parent-child relationship. The measure of infectivity in a population is called incidence.
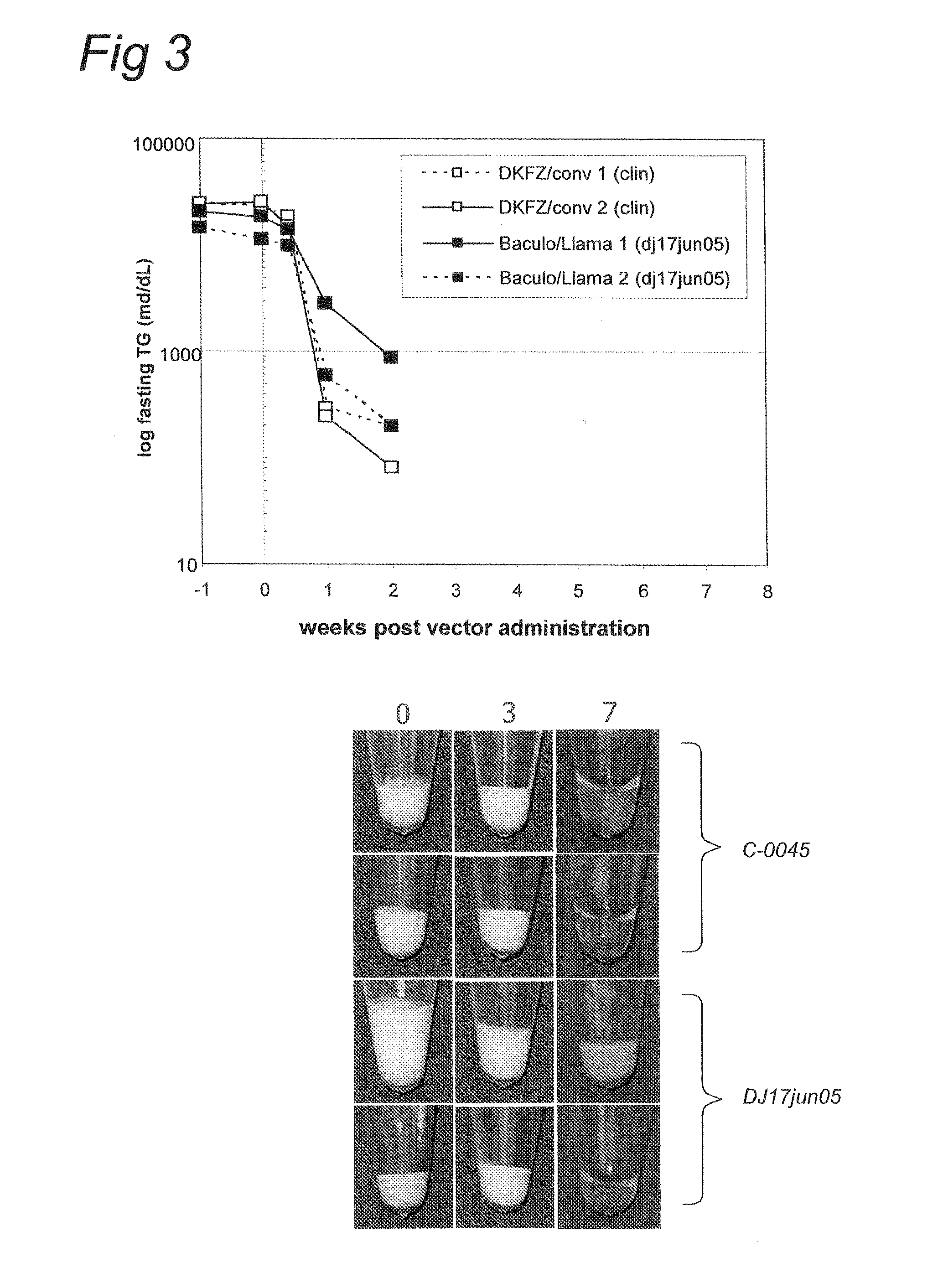
Methods for generating high titer helper-free preparations of released recombinant AAV vectors
InactiveUS6989264B2Genetic therapy composition manufactureGroup 5/15 element organic compoundsGene deliveryHeterologous
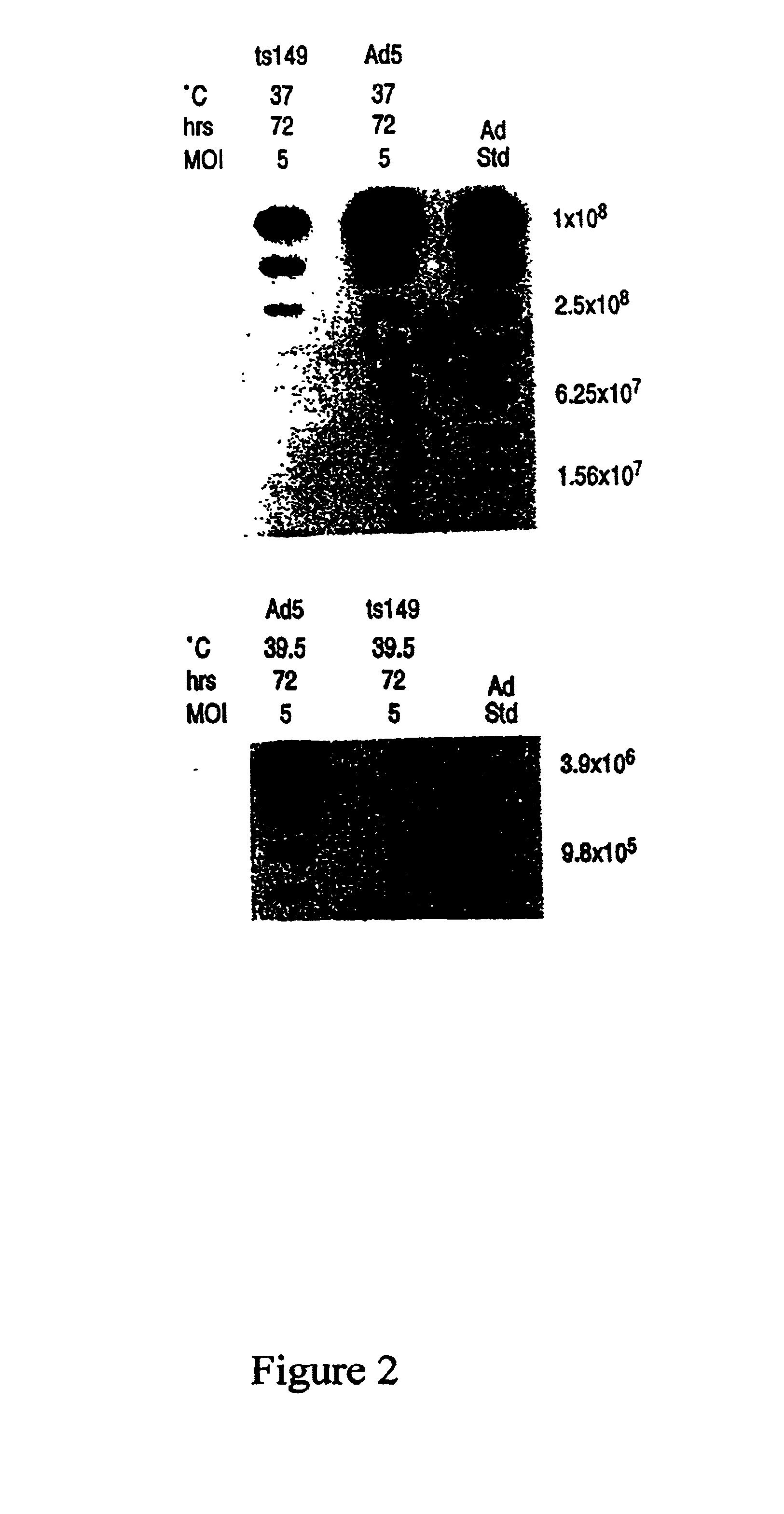
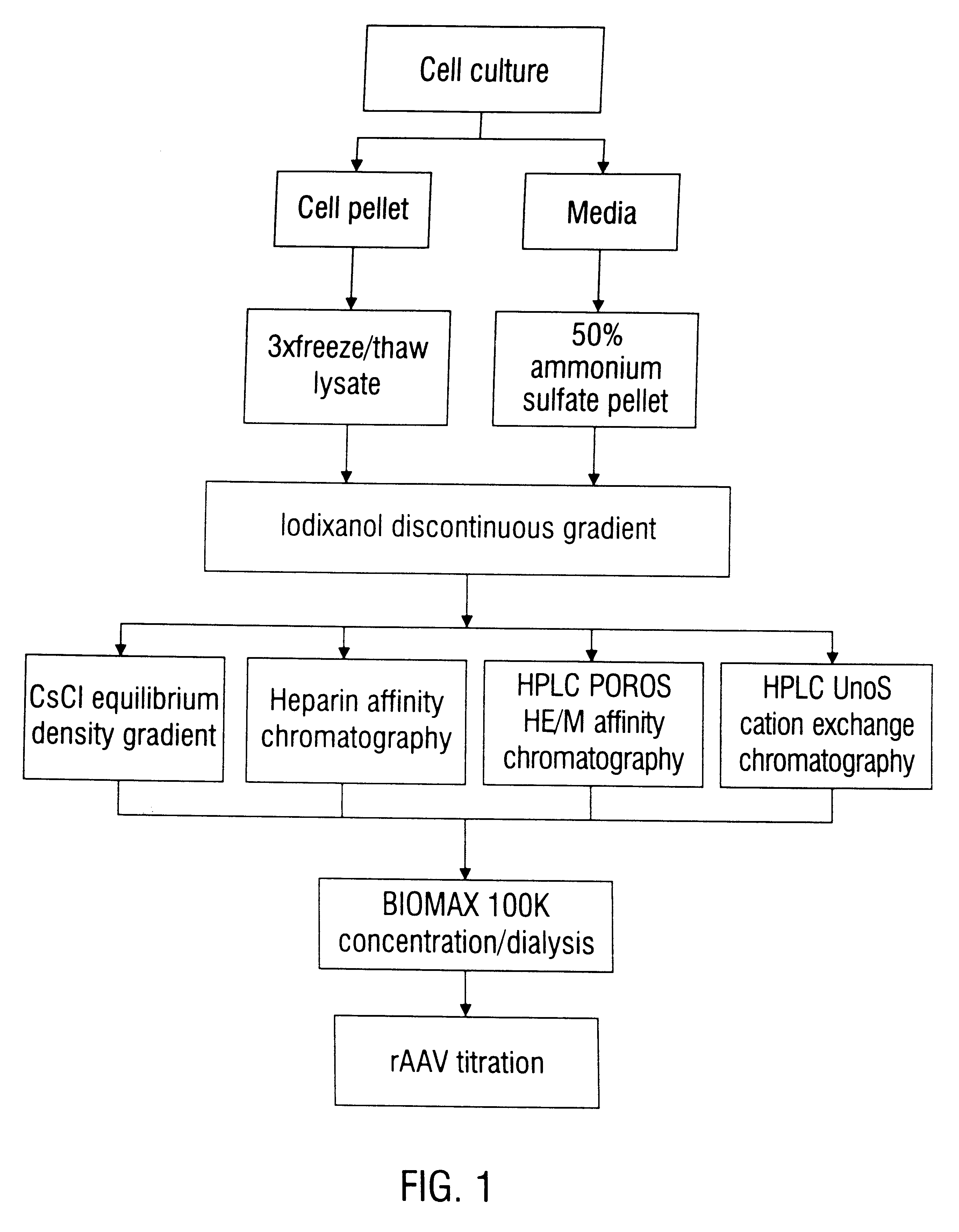
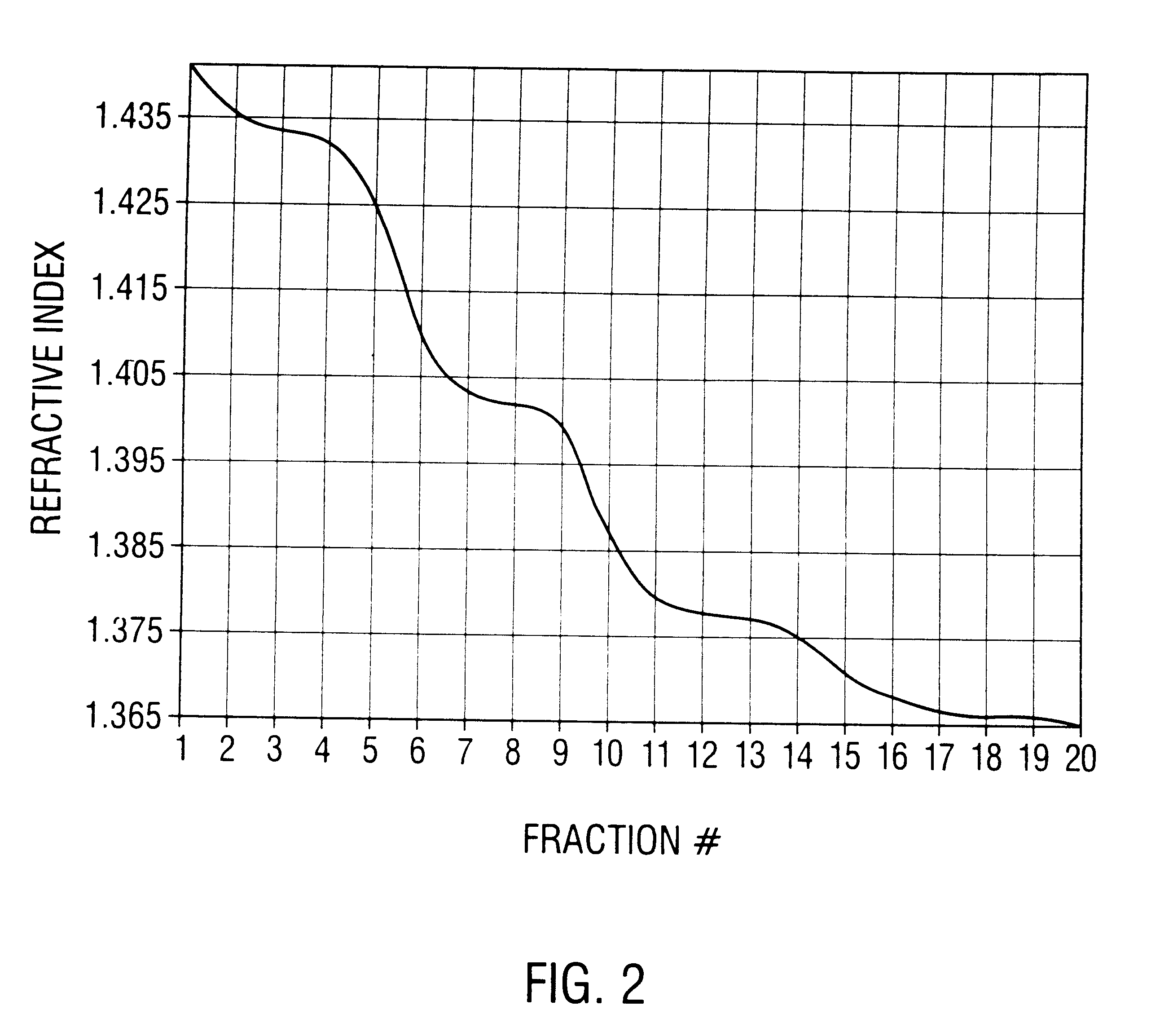
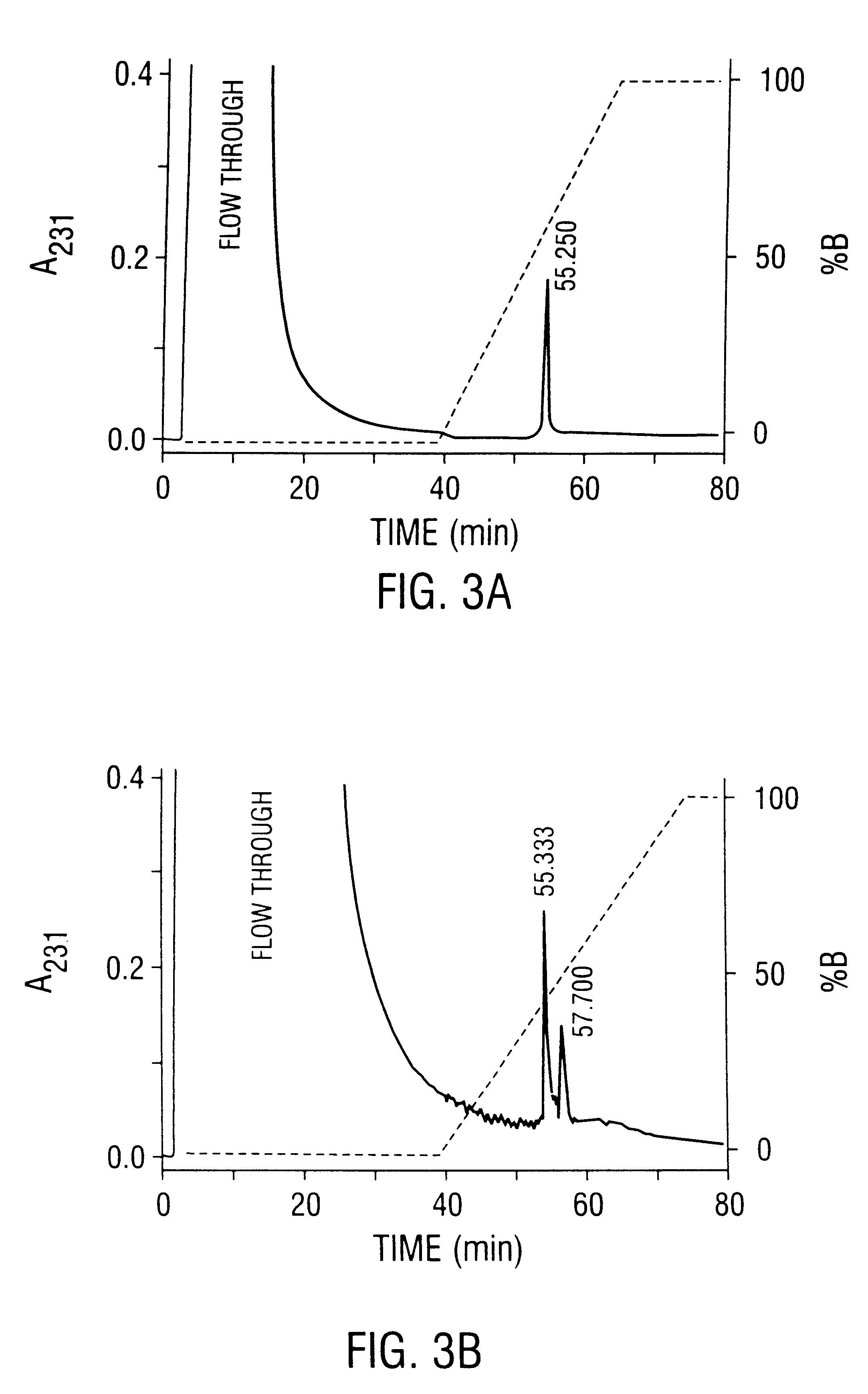
This invention provides methods and compositions for producing high titer, substantially purified preparations of recombinant adeno-associated virus (AAV) that can be used as vectors for gene delivery. At the onset of vector production, AAV producer cells of this invention typically comprise one or more AAV packaging genes, an AAV vector comprising a heterologous (i.e. non-AAV) transgene of interest, and a helper virus such as an adenovirus. The AAV vector preparations produced are generally replication incompetent but are capable of mediating delivery of a transgene of interest (such as a therapeutic gene) to any of a wide variety of tissues and cells. The AAV vector preparations produced according to this invention are also substantially free of helper virus as well as helper viral and cellular proteins and other contaminants. The invention described herein provides methods of producing rAAV particles by culturing producer cells under conditions, such as temperature and pH, that promote release of virus. Also provided is a quantitative, high-throughput assay useful in the assessment of viral infectivity and replication, as well as in the screening of agent that affect viral infectivity and / or replication.
Owner:TARGETED GENETICS CORPORTION
Method of preparing recombinant adeno-associated virus compositions
InactiveUS6660514B1Reduce concentrationImprove yield and recoveryMicrobiological testing/measurementEnzymologyBiochemistryAdeno-associated virus
Disclosed are methods for the isolation and purification of high-titer recombinant adeno-associated virus (rAAV) compositions. Also disclosed are methods for reducing or eliminating the concentration of helper adenovirus in rAAV samples. Methods are disclosed that provide highly-purified rAAV stocks having titers up to about 10<13 >particles / ml at particle-to-infectivity ratios of less than 100 in processes that are accomplished about 24 hours or less.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Mutant adeno-associated virus virions and methods of use thereof
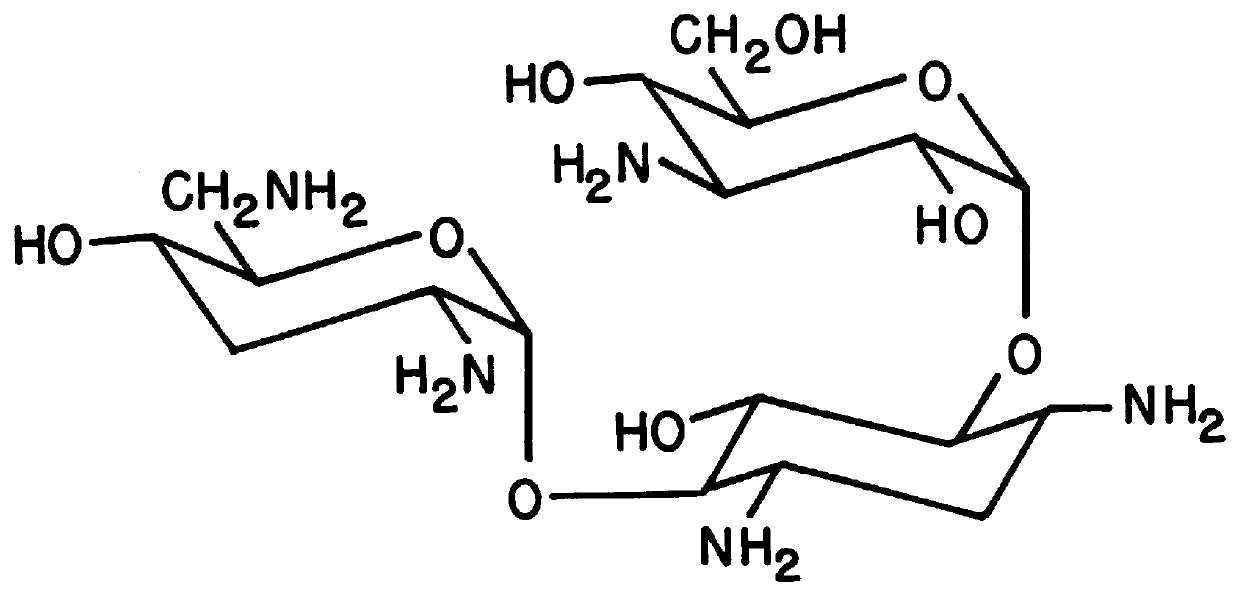
ActiveUS20090202490A1Reduce the binding forceAltered infectivityOrganic active ingredientsBiocideCell type specificNeutralizing antibody
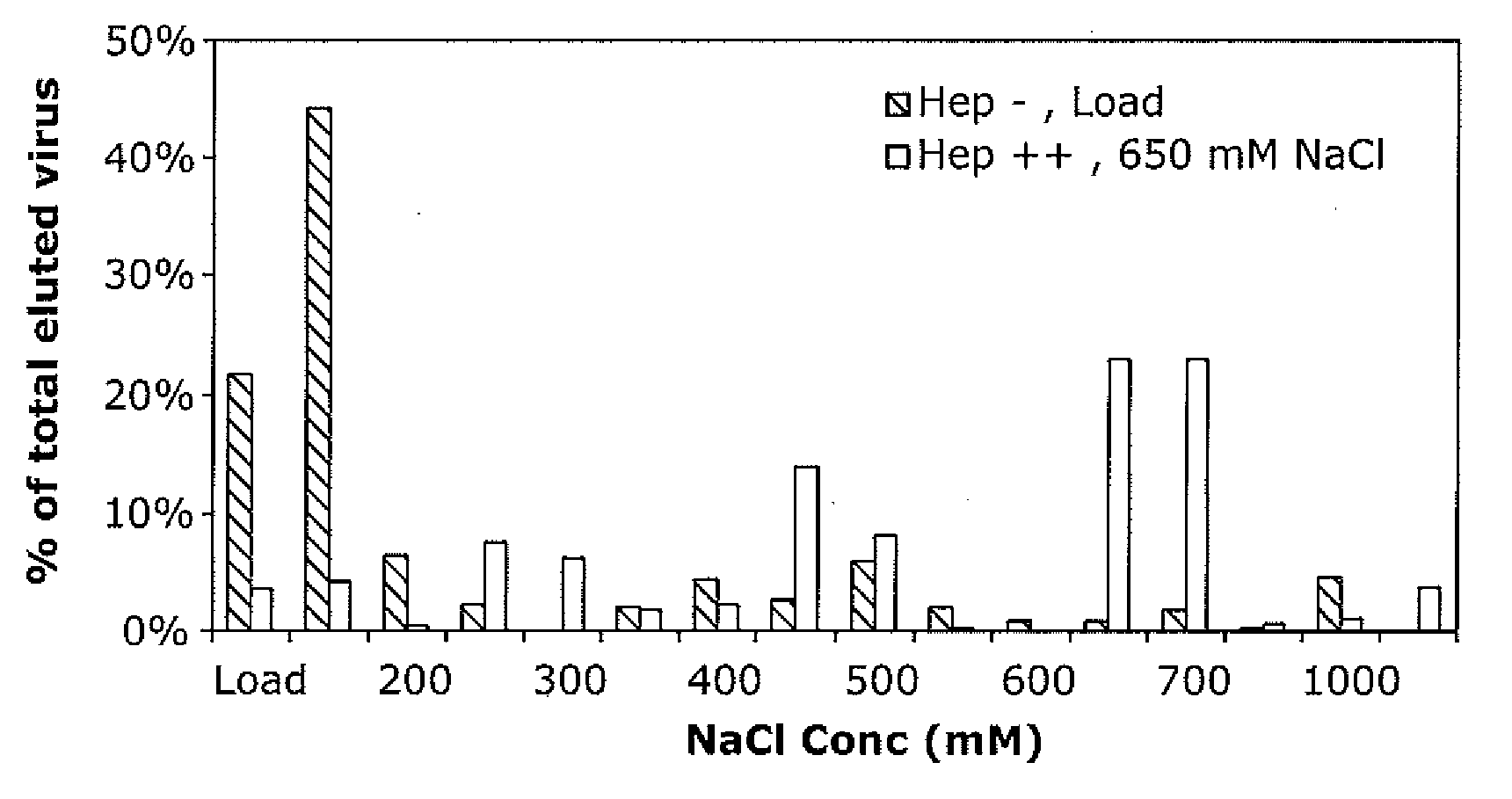
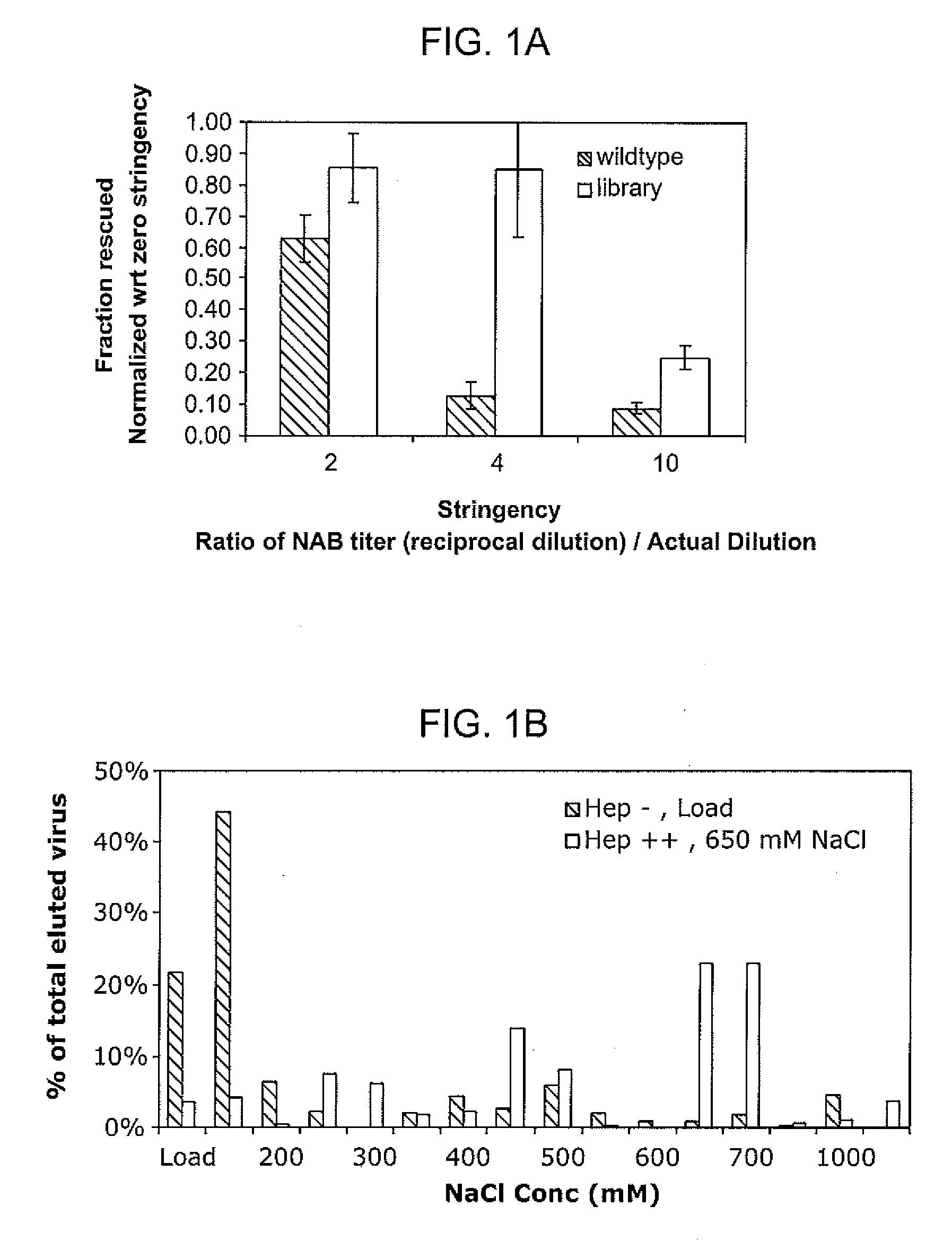
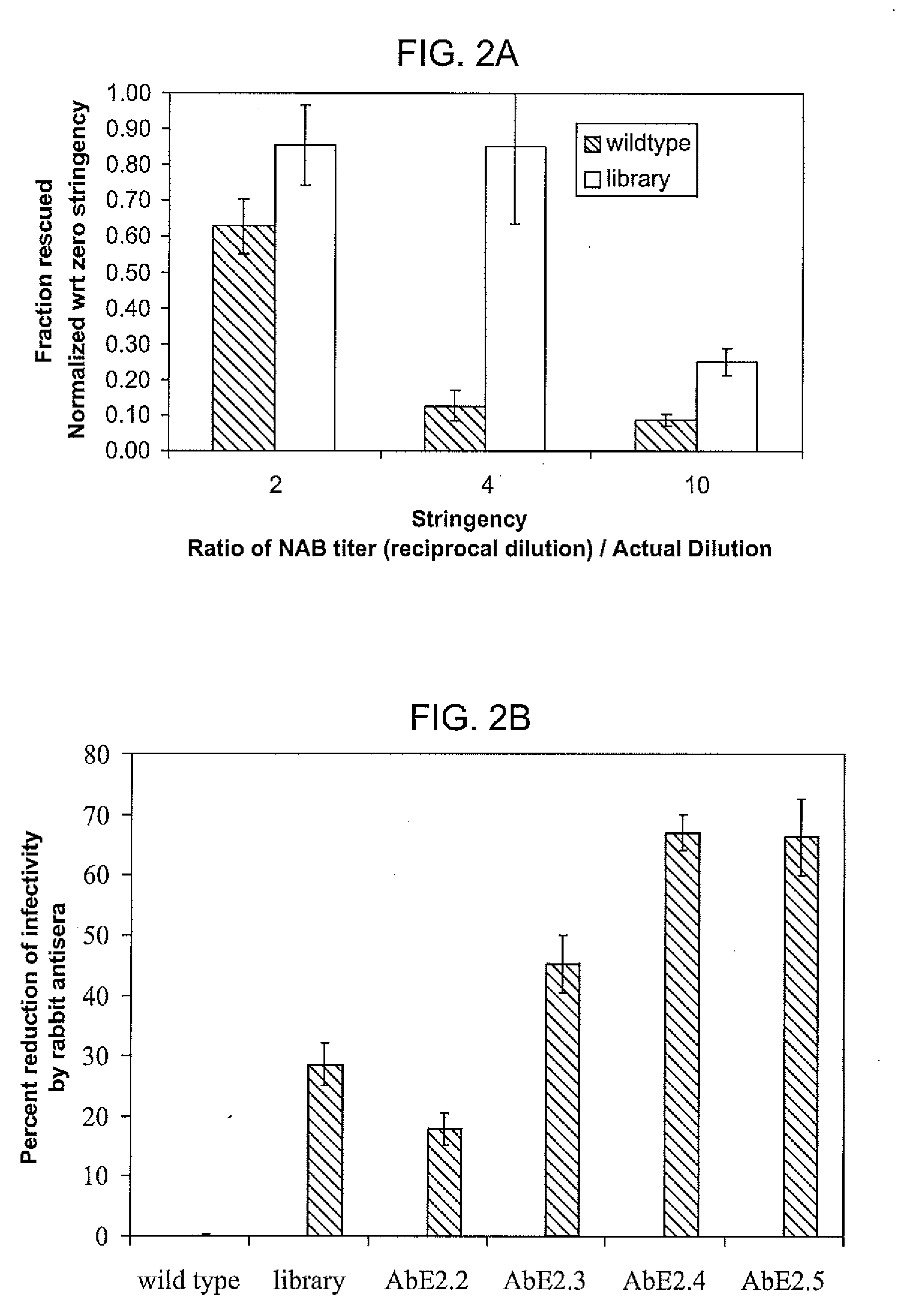
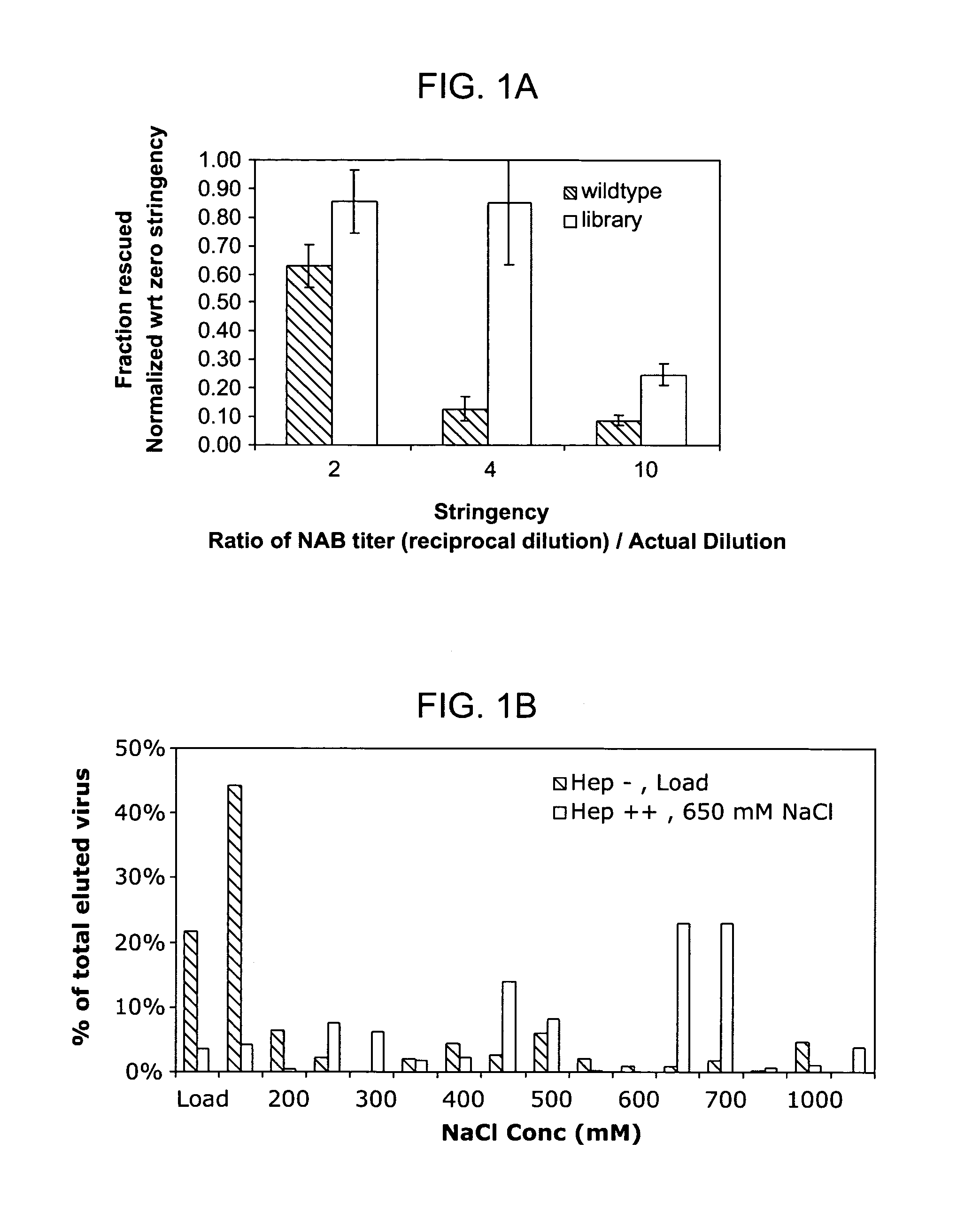
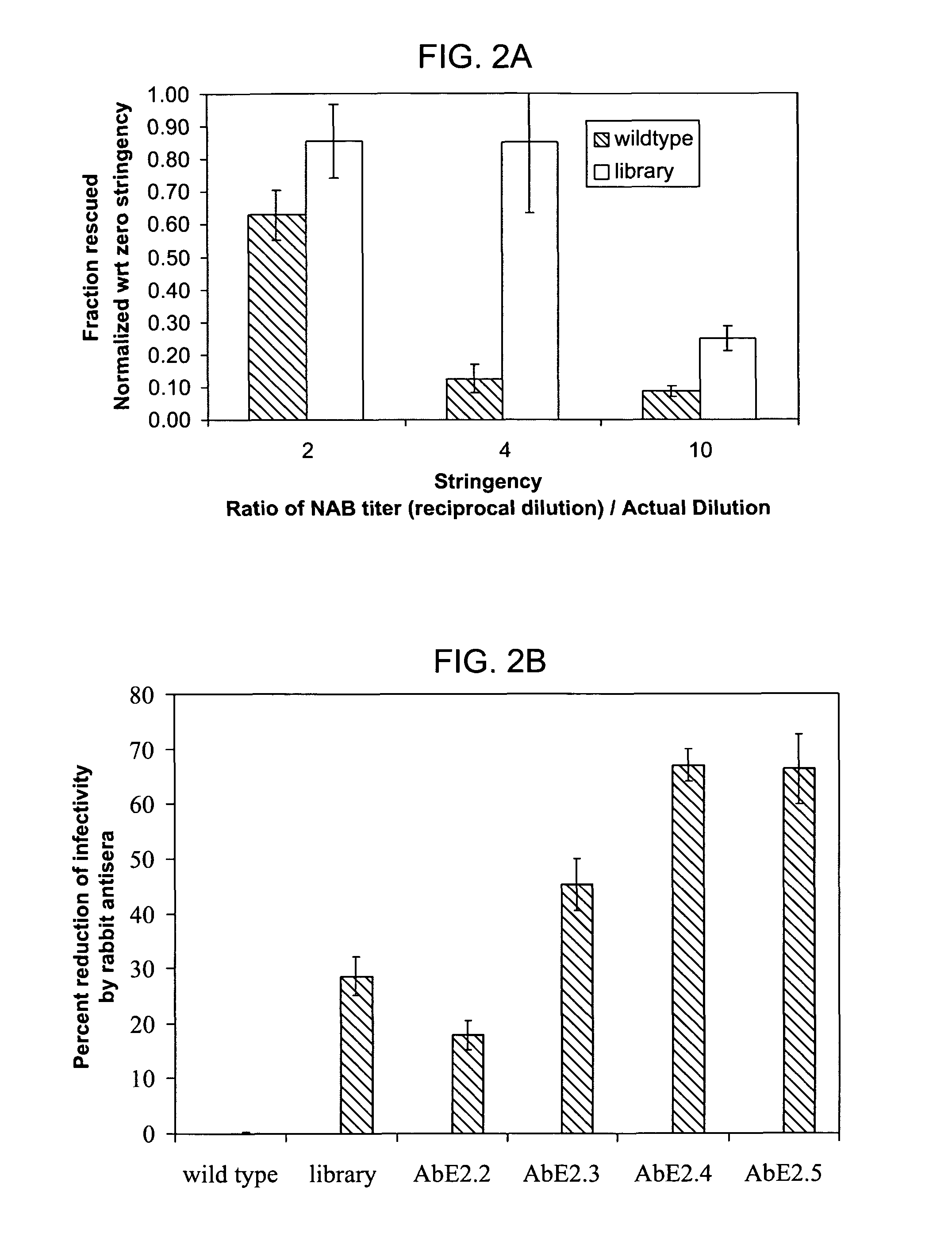
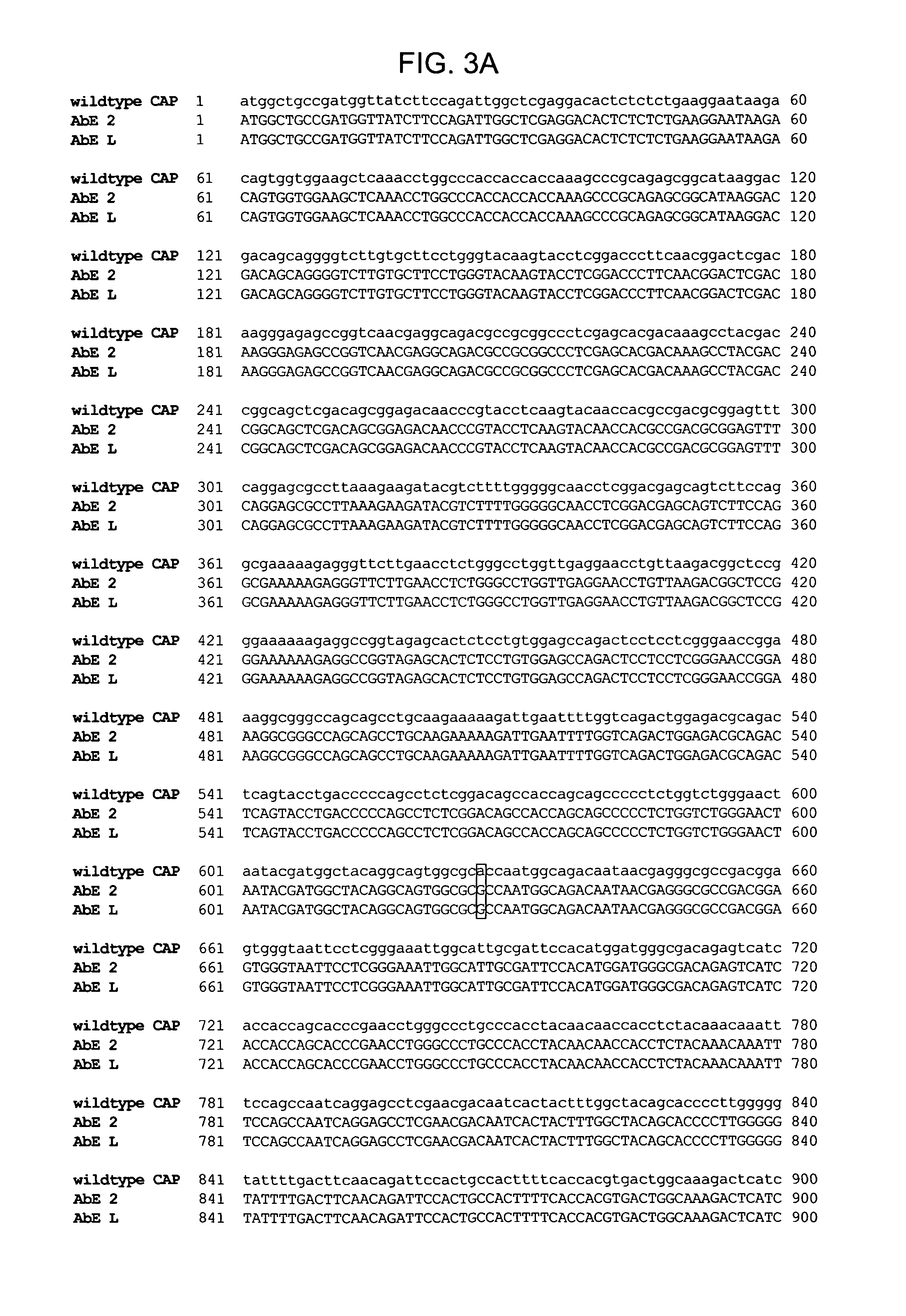
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:RGT UNIV OF CALIFORNIA +1
Method and a tobramycin aerosol formulation for treatment prevention and containment of tuberculosis
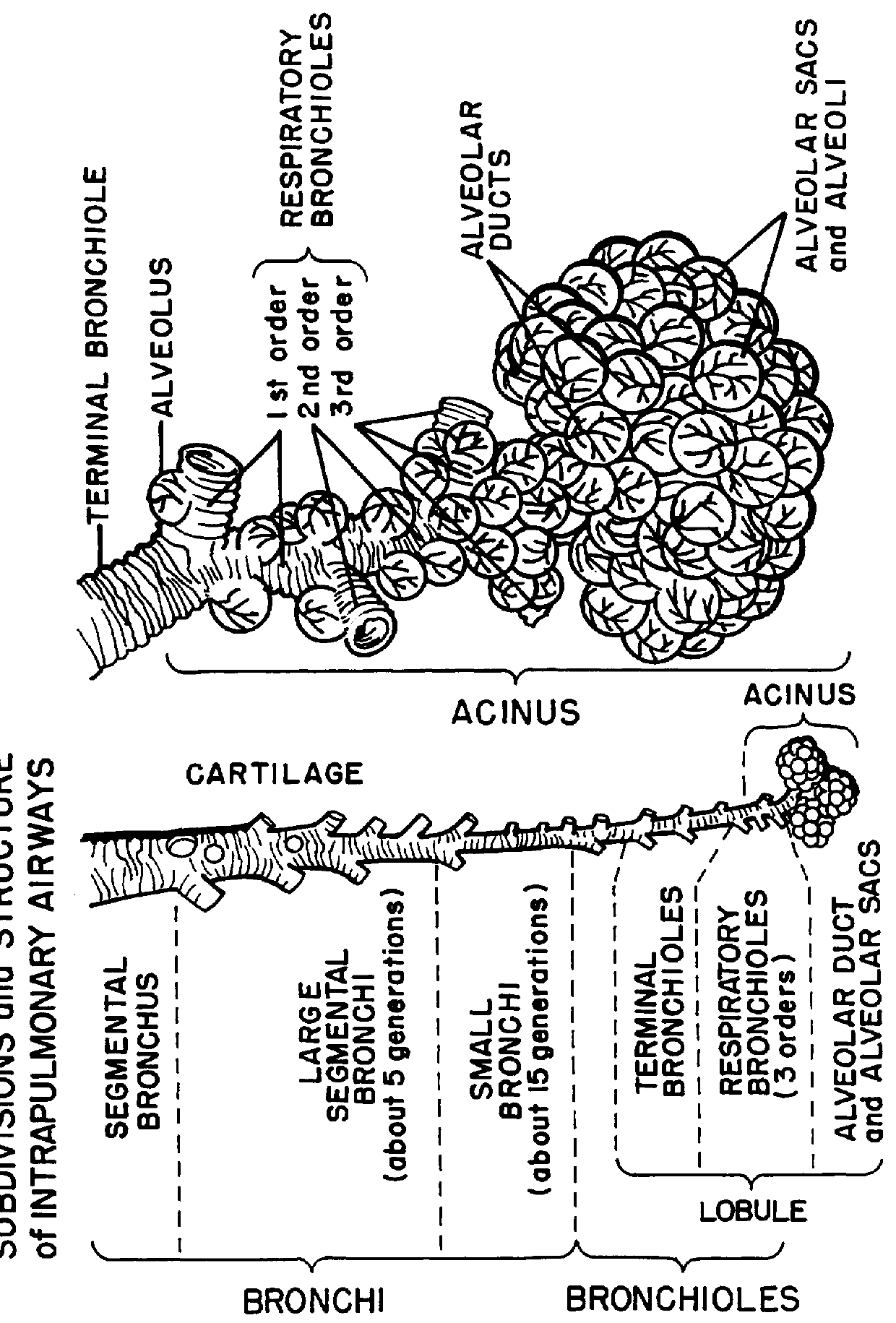
A method for treatment, prevention and containment of acute and chronic tuberculosis using a preservative-free concentrated tobramycin aerosol formulation delivering tobramycin to the lung endobronchial space including alveoli in an aerosol having mass medium average diameter predominantly between 1 to 5 mu . The method comprises administration of tobramycin in concentration one to ten thousand times higher than the minimal inhibitory concentration of Mycobacterium tuberculosis. A method for containment of and decreasing infectivity periods of tuberculosis patients to shorter periods of time.
Owner:CHIRON CORP
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20050053922A1Reduce the binding forceAltered infectivityAntibacterial agentsVirusesReassortant VirusesNeutralizing antibody
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:INTEGRATIVE GENE THERAPEUTICS +1
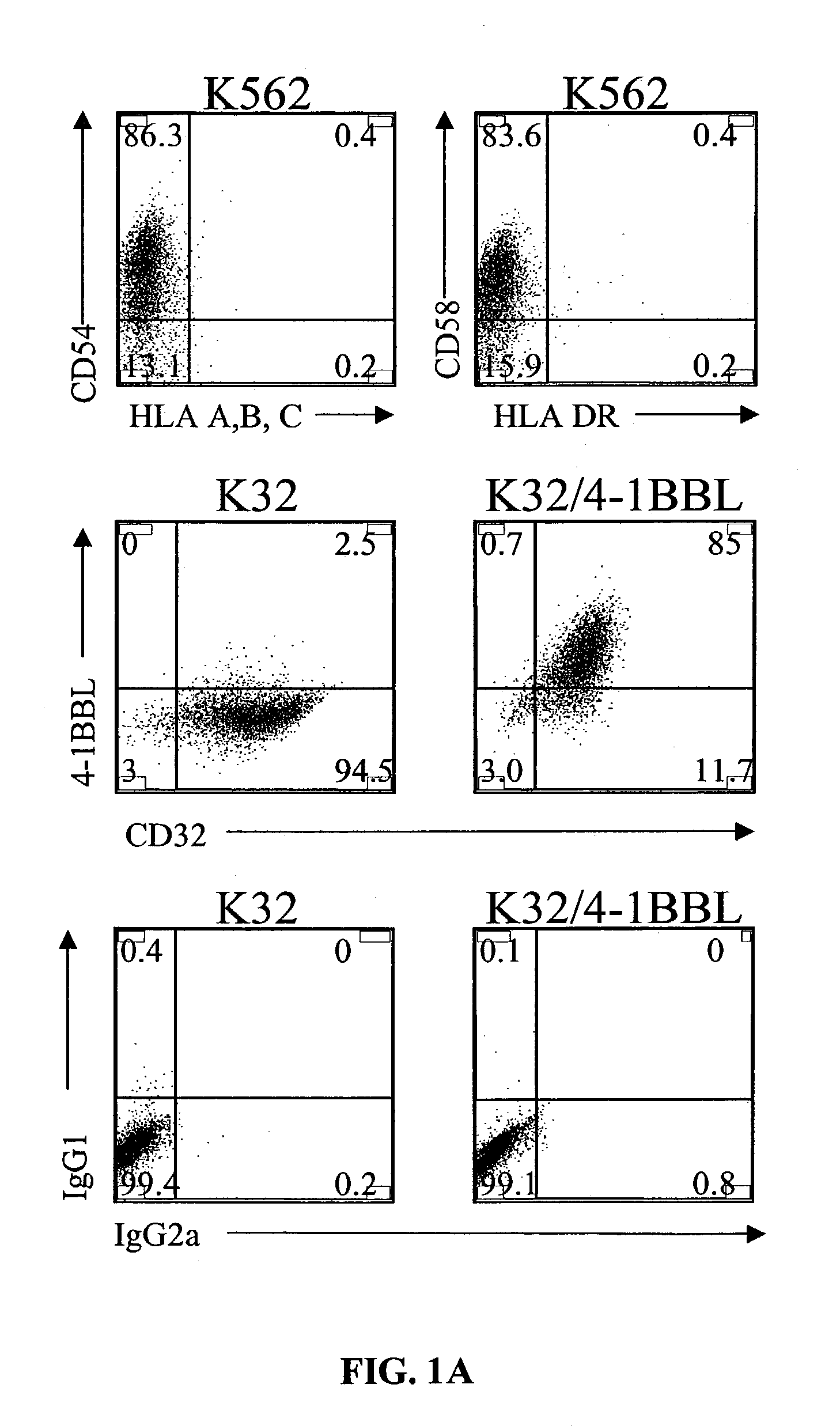
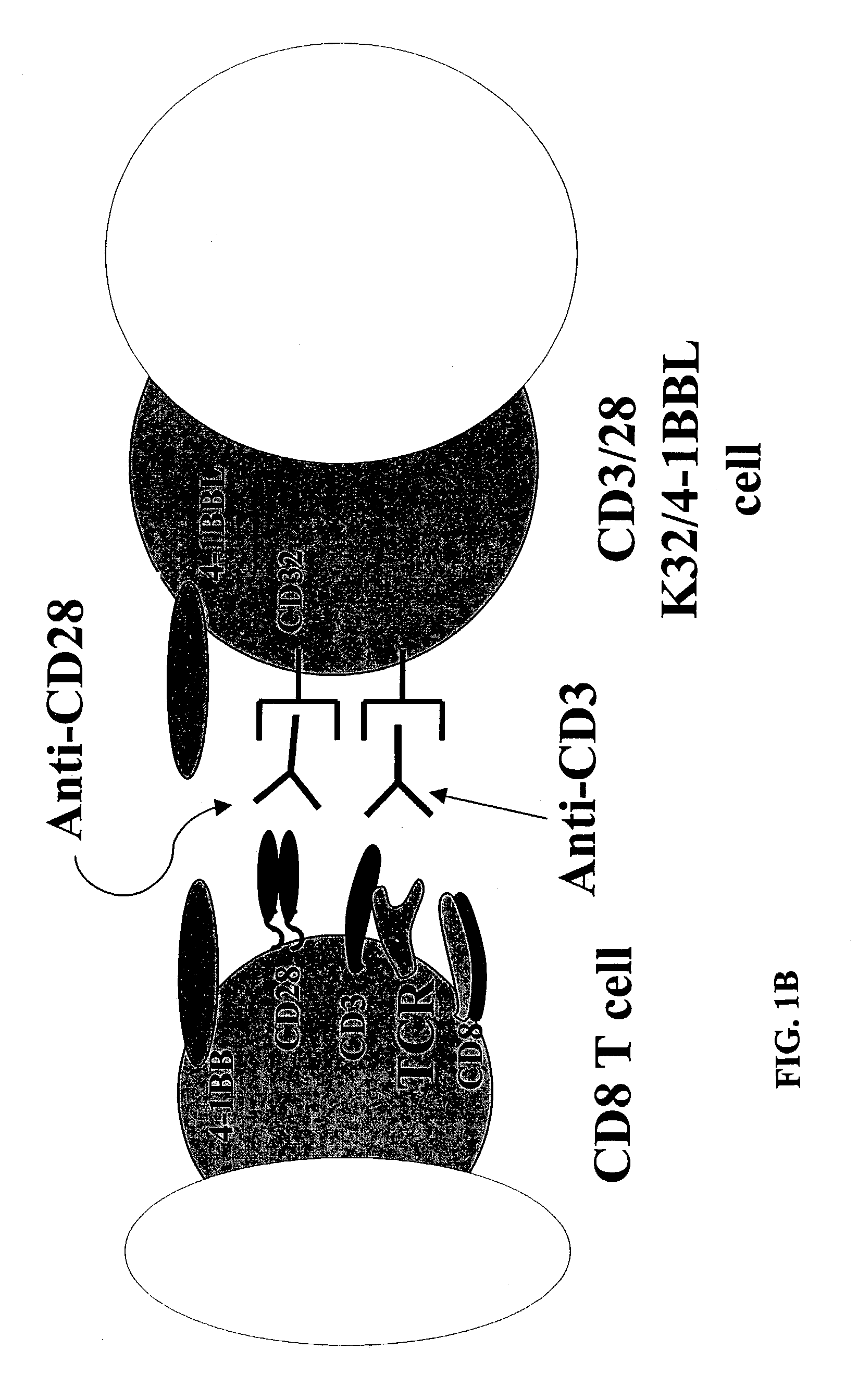
Activation and expansion of T-cells using an engineered multivalent signaling platform as a research tool
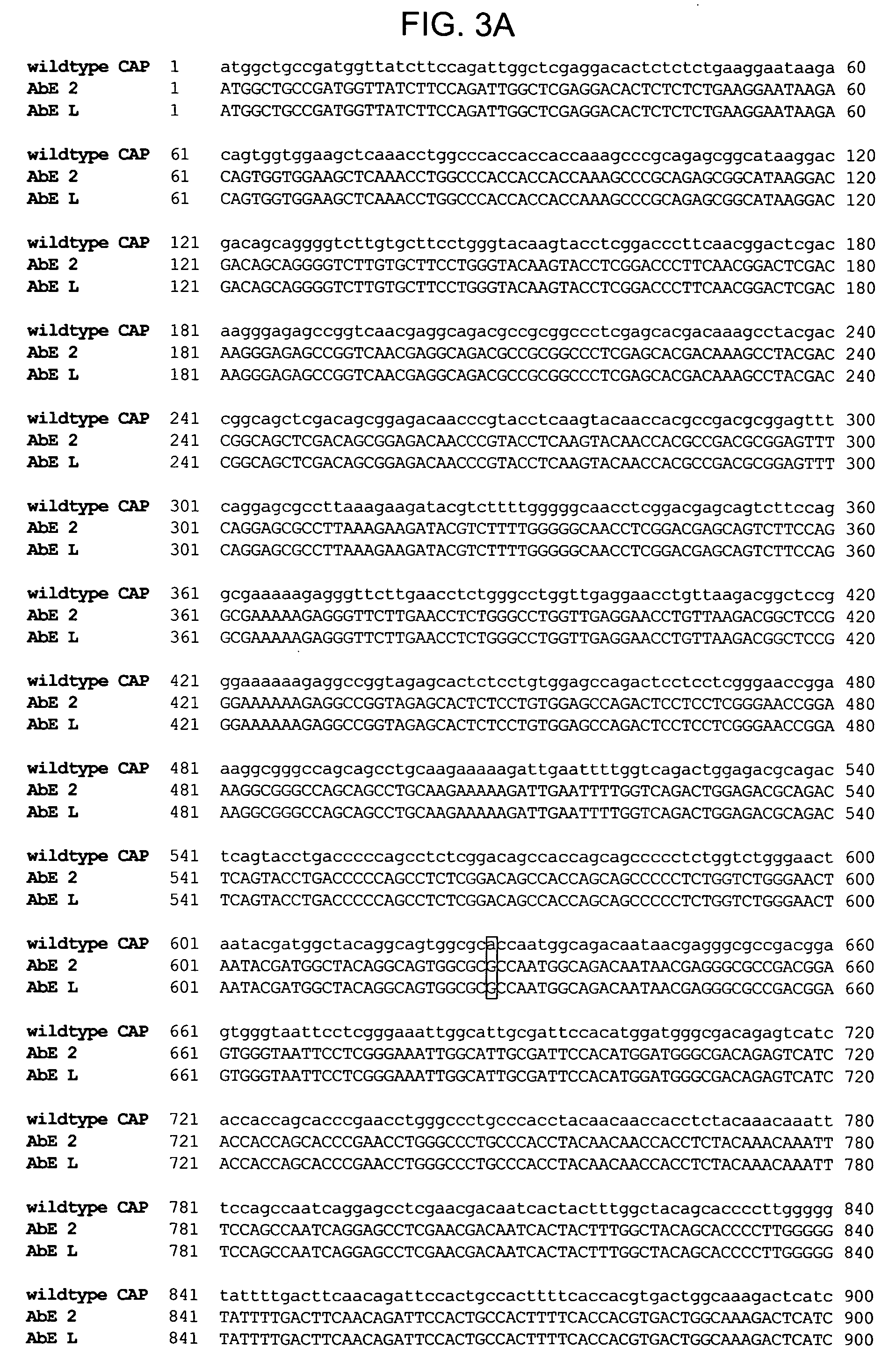
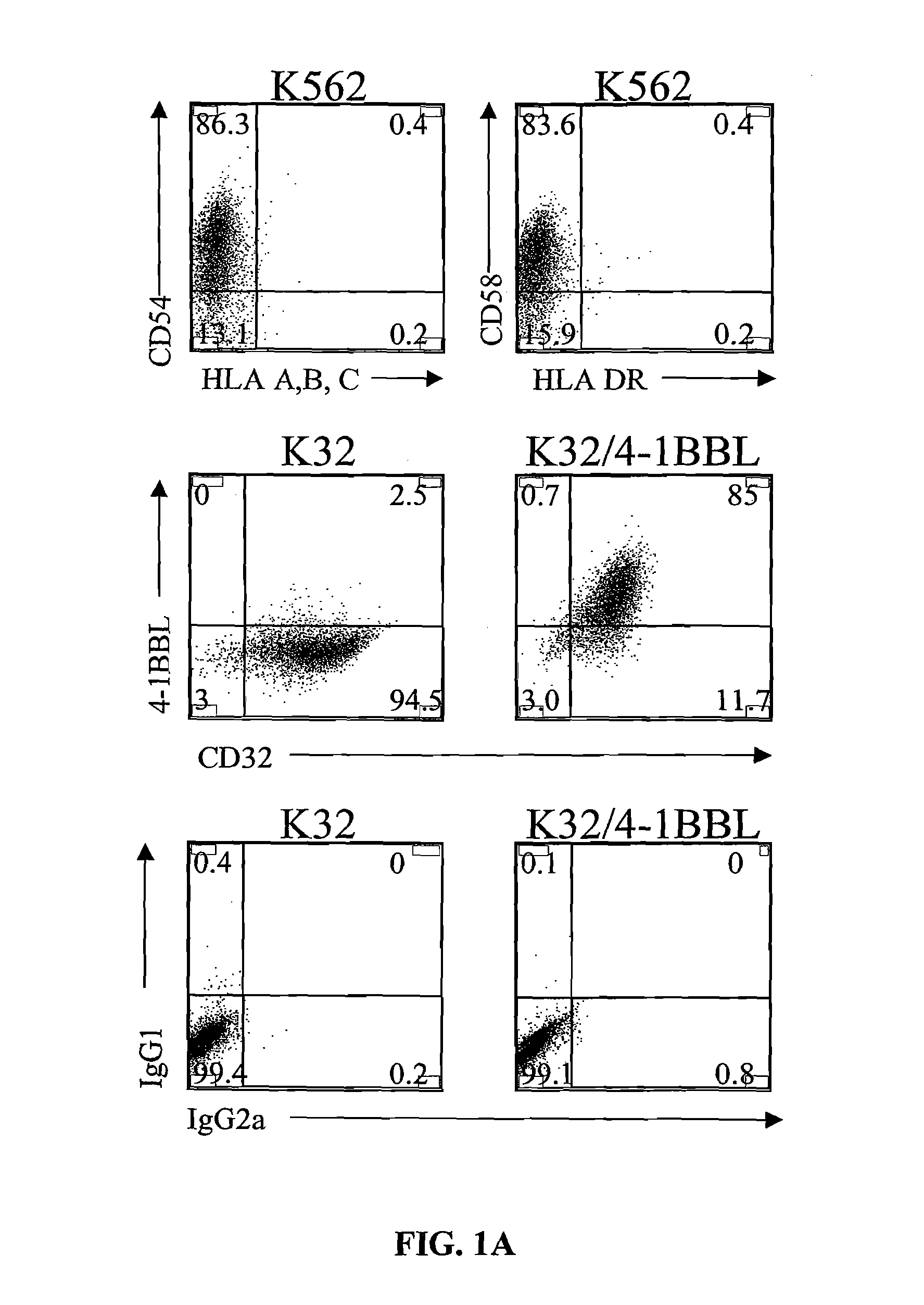
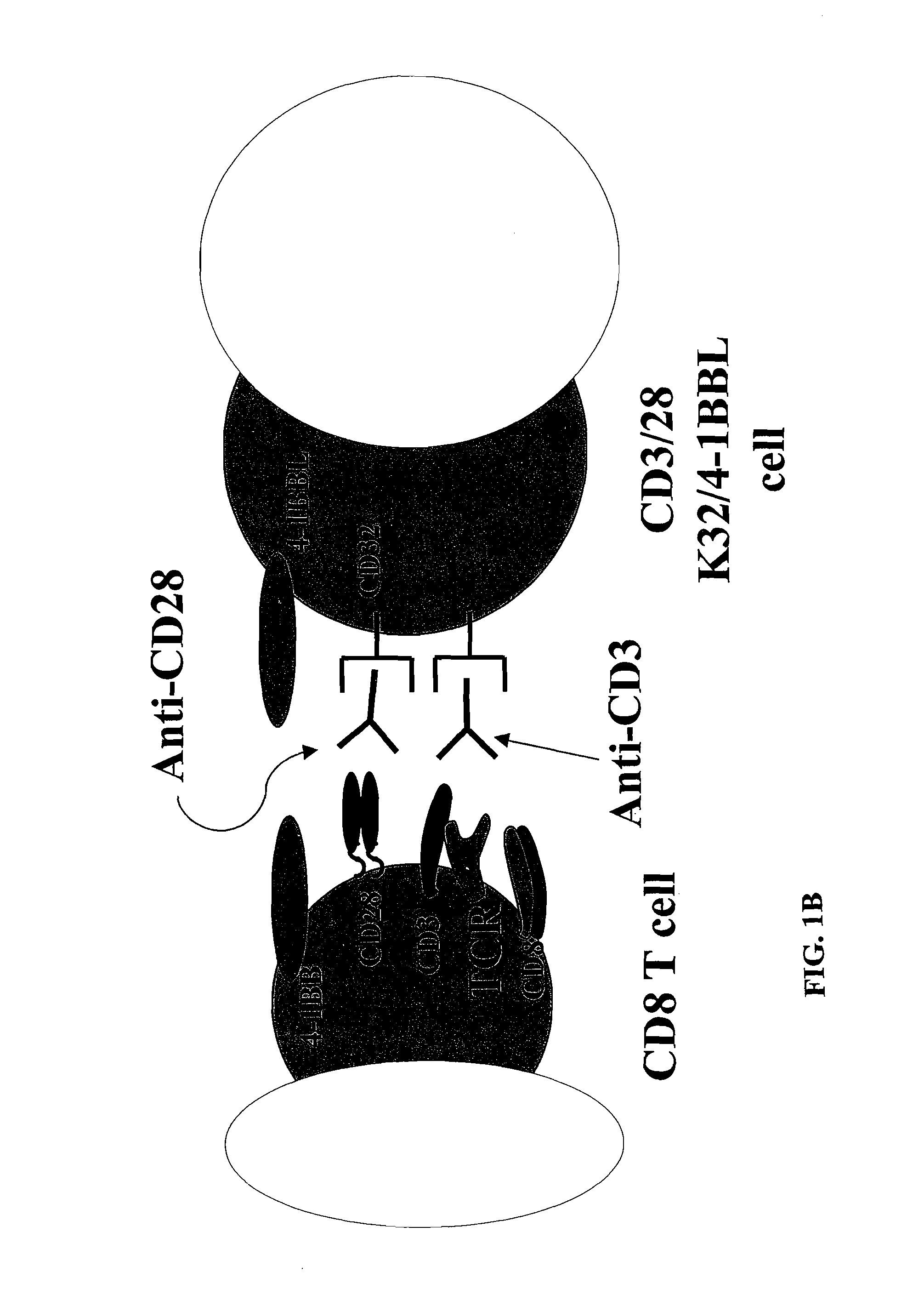
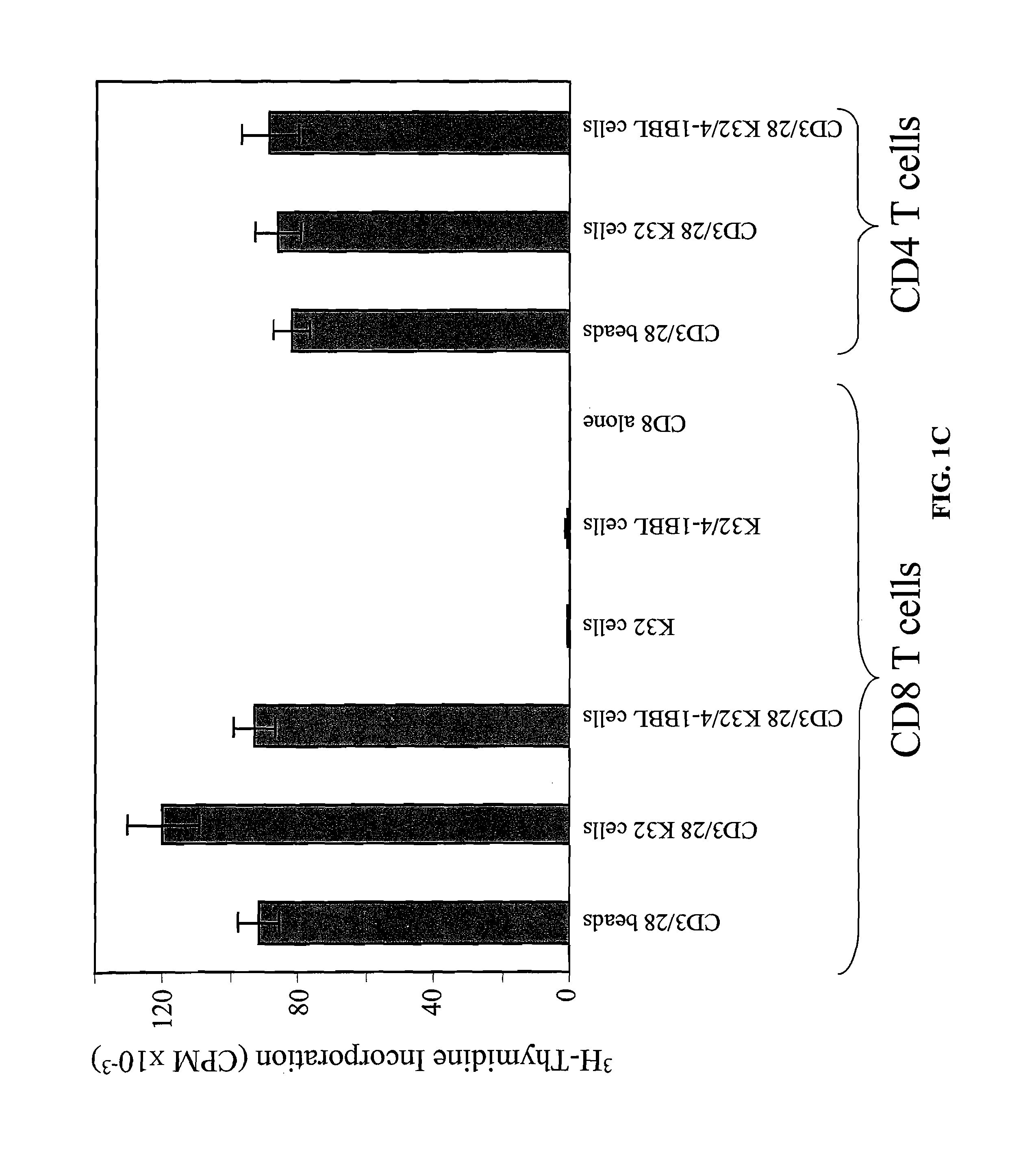
InactiveUS8637307B2Maintain their viabilityLower Level RequirementsGenetically modified cellsMammal material medical ingredientsT cellExogenous growth
Provided are a system and methods for selectively inducing expansion of a population of T cells in the absence of exogenous growth factors, such as lymphokines, and accessory cells for research purposes. The cell based expansion system and methods permit the long-term growth of CTLs, preferably human CTLs. In addition, T cell proliferation can be induced without the need for antigen, thus providing an expanded T cell population that is polyclonal with respect to antigen reactivity. Further provided are methods for using the system and methods to screen and identify antigens related to specific diseases or conditions, tumors, autoimmune disorders, or an infectious disease or pathogen, and to identify target molecule for research purposes, or for developing a vaccine based thereon.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
System and method for inhibiting the decryption of a nucleic acid probe sequence used for the detection of a specific nucleic acid
InactiveUS20060073487A1Reduce or impede a person's ability to designAvoid identificationSugar derivativesMicrobiological testing/measurementGenetic AnomalyNucleic Acid Probes
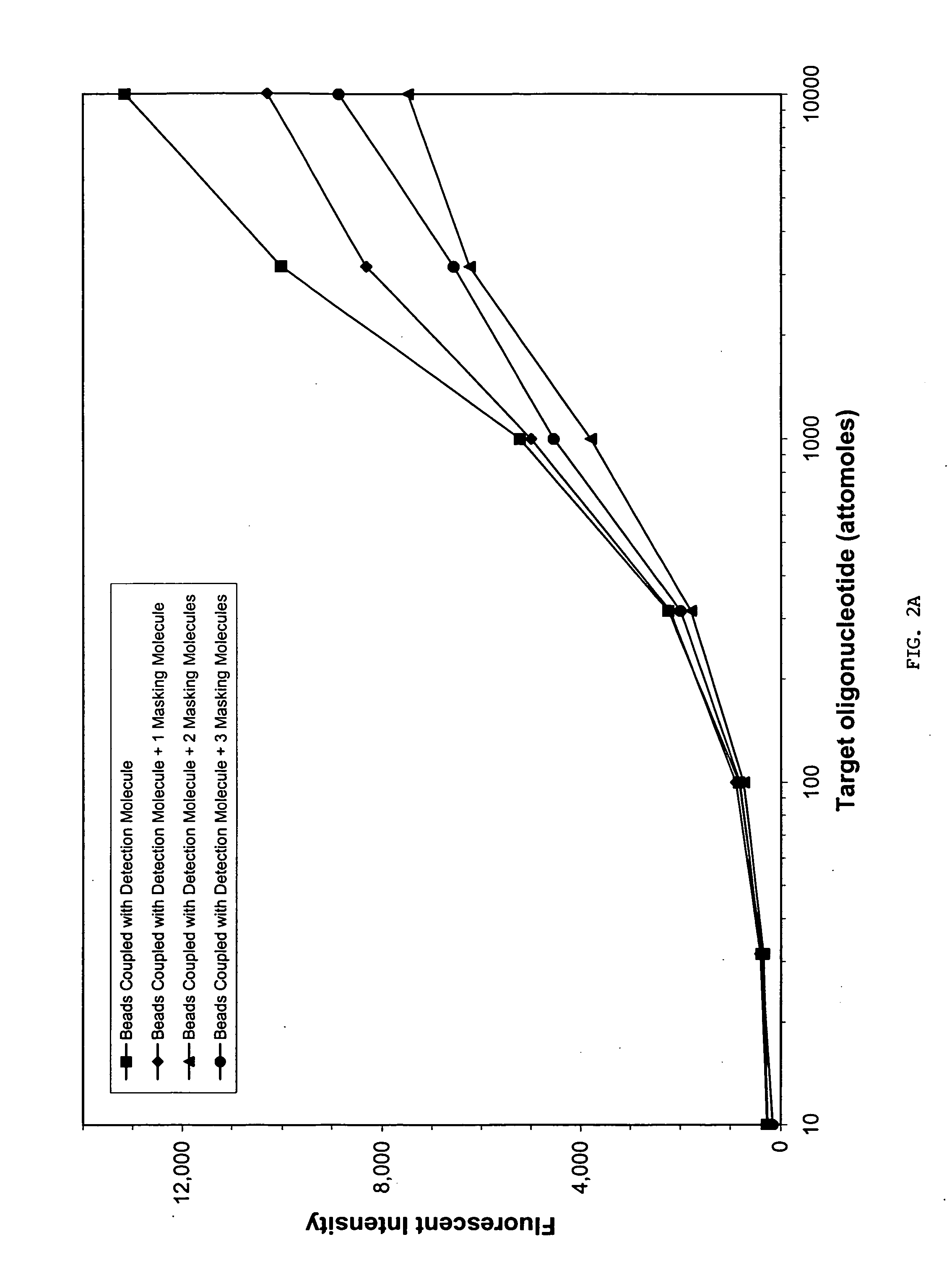
Sequence-specific nucleic acid hybridization assays are used for the detection of specific genetic sequences as indicators of genetic anomalies, mutations, and disease propensity. In addition, they are used for the detection of various biological agents and infectious pathogens. Because a complementary probe or nucleic acid sequence is required to detect a sequence of interest in a hybridization-based assay, nucleic acid sequencing techniques can rapidly determine the specific probe sequence being used for detection. This allows reverse engineered assays to be produced rapidly. In addition, it enables the circumvention of hybridization-based assays for biological agent or infectious pathogen detection by providing the information necessary to create or alter nucleic acid sequences to produce false positives or false negatives. The present invention provides methods and compositions for inhibiting the identification of specific detection sequences. More specifically, the invention provides masking sequences that mask the identity of specific detection sequences.
Owner:RADIX BIOSOLUTIONS
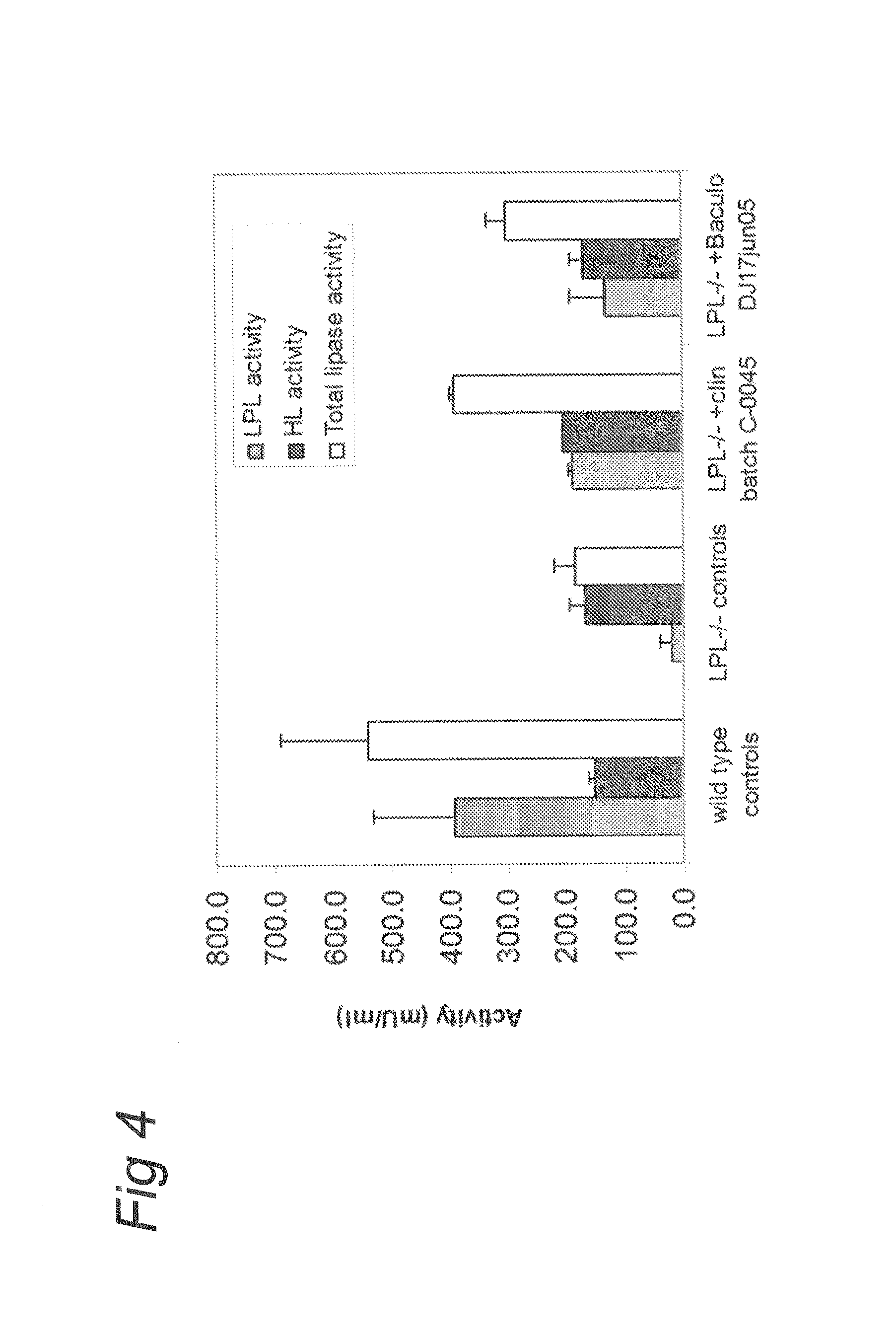
AAV vectors produced in insect cells
ActiveUS8163543B2Improve stabilityReduced expression levelAnimal cellsGenetic material ingredientsNucleotideViral vector
The present invention relates to the production of adeno-associated viral vectors in insect cells. The insect cells therefore comprise a first nucleotide sequence encoding the adeno-associated virus (AAV) capsid proteins, whereby the initiation codon for translation of the AAV VP1 capsid protein is a non-ATG, suboptimal initiation codon. The insect cell further comprises a second nucleotide sequence comprising at least one AAV inverted terminal repeat (ITR) nucleotide sequence; a third nucleotide sequence comprising a Rep52 or a Rep40 coding sequence operably linked to expression control sequences for expression in an insect cell; and, a fourth nucleotide sequence comprising a Rep78 or a Rep68 coding sequence operably linked to expression control sequences for expression in an insect cell. The invention further relates to adeno-associated viral vectors with an altered ratio of the viral capsid proteins that provides improved infectivity of the viral particles.
Owner:UNIQURE IP BV
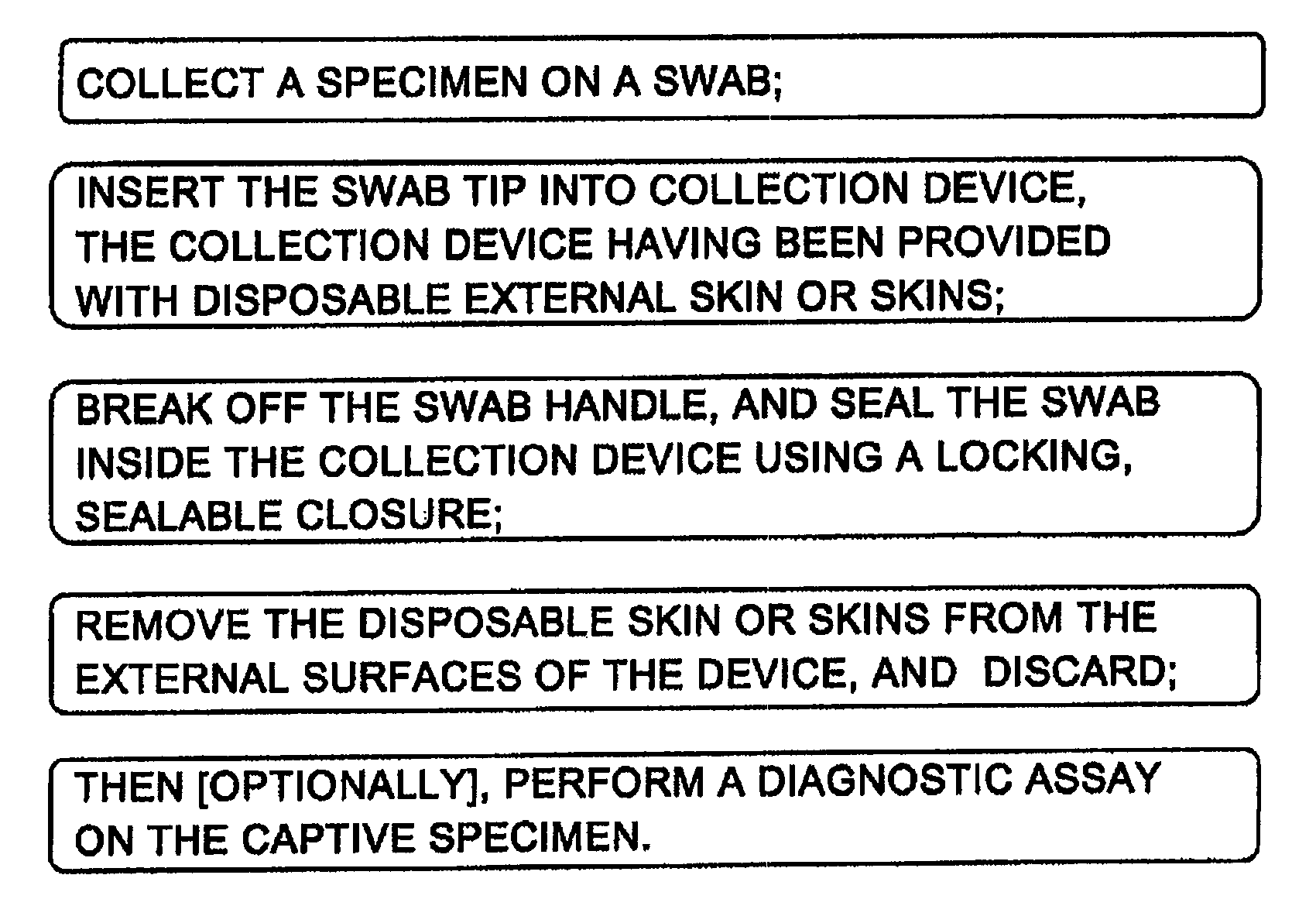
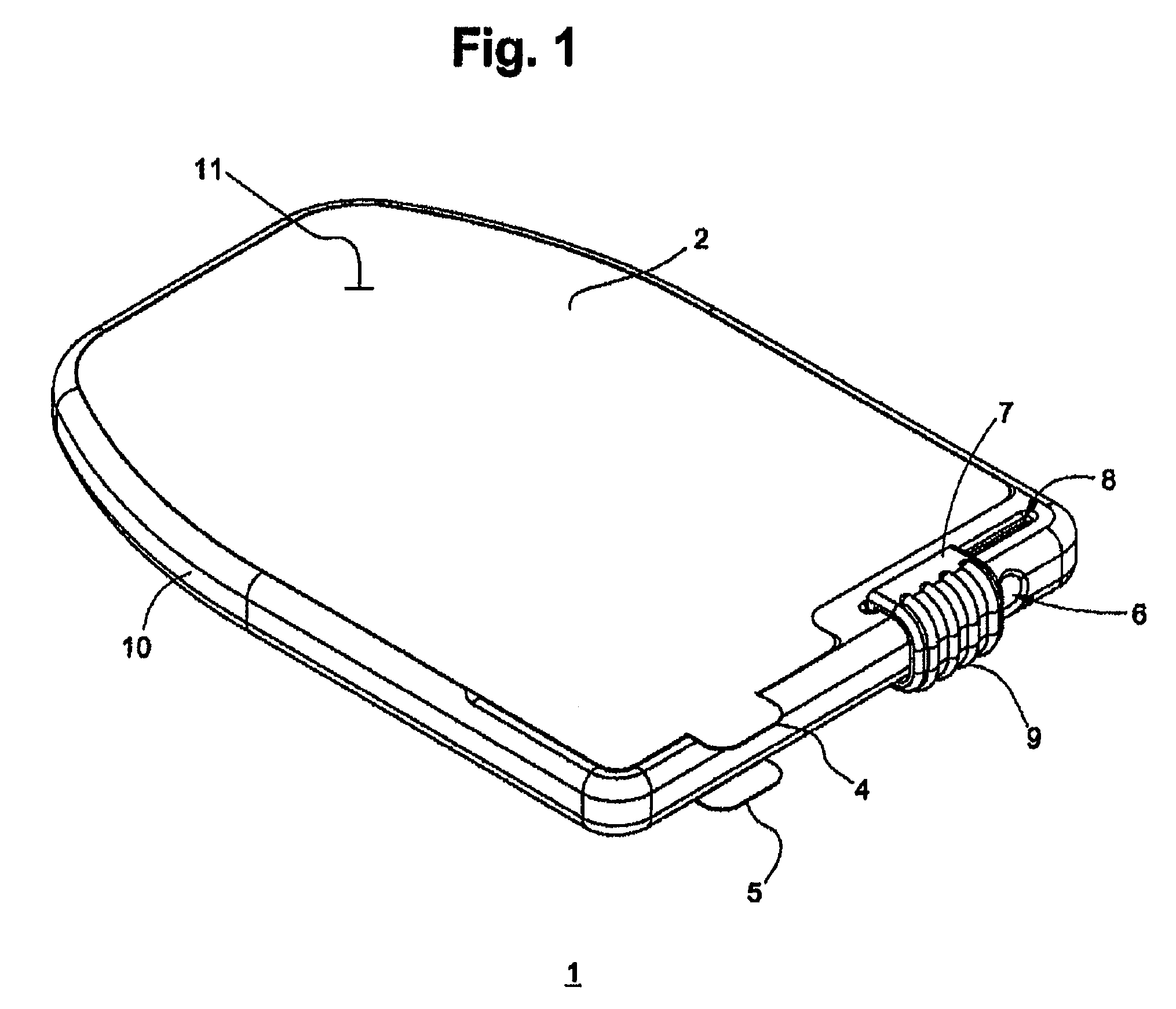
Sanitary swab collection system, microfluidic assay device, and methods for diagnostic assays
ActiveUS8216832B2Contamination riskBioreactor/fermenter combinationsBiological substance pretreatmentsAssayCollection system
Biohazard specimen collection containers are provided with an external disposable skin, that is stripped away and discarded after the biohazardous specimen is collected, thus reducing or eliminating objectionable or dangerous residues on the outside surfaces of the container. Further, we teach that the sample collection container with external disposable skin may also serve as an integrated microfluidic biosample processing and analytical device, thereby providing a single entry, disposable assay unit, kit and system for “world-to-result” clinical diagnostic testing. These integrated assay devices are provided with synergic, multiple safe-handling features for protecting healthcare workers who handle them. The modified collection containers and analytical devices find application, for example, in PCR detection of infectious organisms or pathogenic markers collected on a swab.
Owner:PERKINELMER HEALTH SCIENCES INC
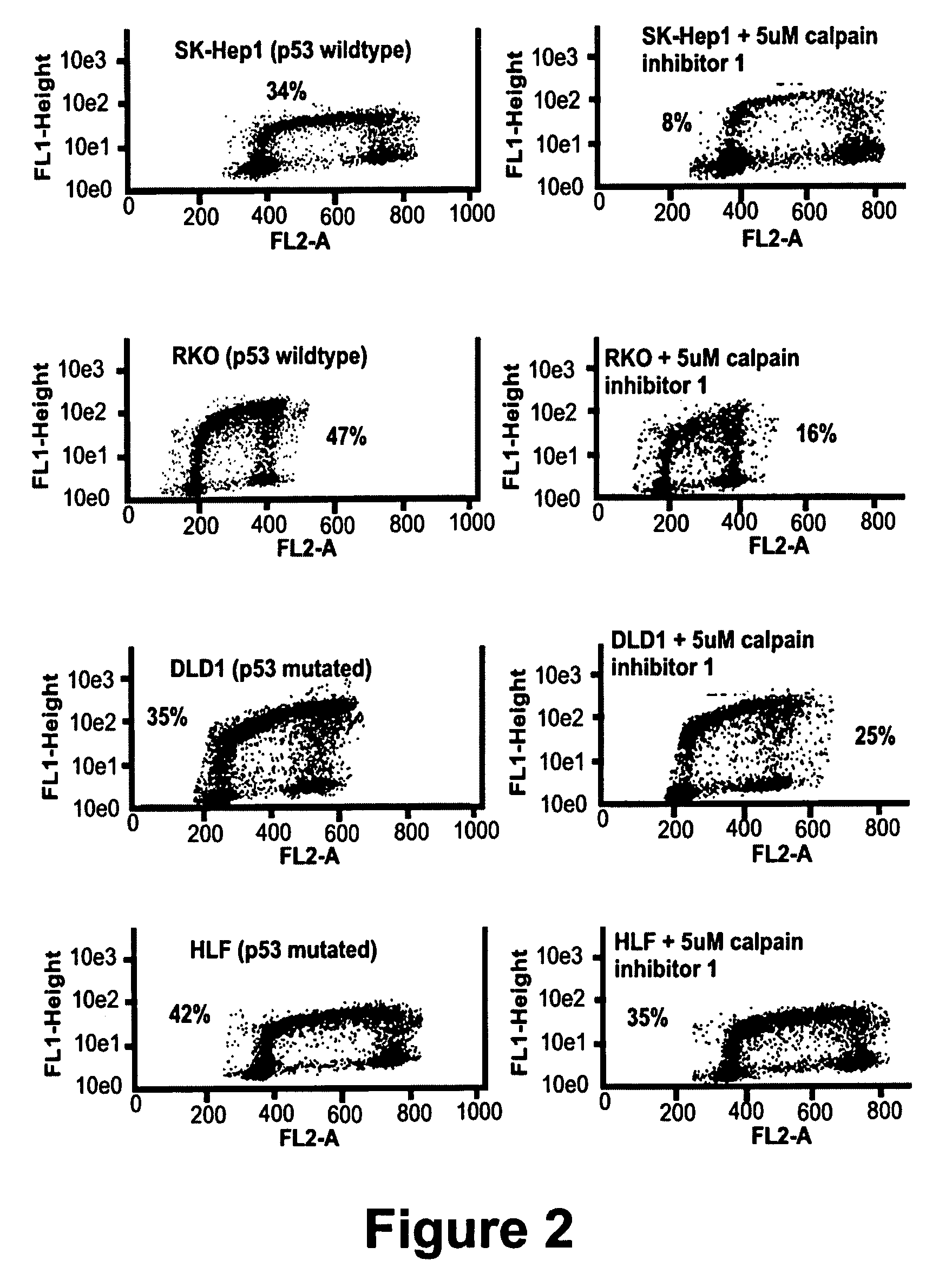
Calpain inhibitors and their applications
InactiveUS7001770B1Enhance p53-mediated apoptosisIncrease infectivityPeptide/protein ingredientsFermentationCo administrationApoptosis
The present invention provides a method to enhance apoptosis in a cell by the administration of p53 in combination with a calpain inhibitor. The present invention provides a method of increasing the infectivity of a cell to a viral vector by treatment of the cell with a calpain inhibitor. the present invention further provides a method of enhancing transciption of a therapeutic transgene from the CMV promoter. The present invention also provides a method of suppress the in vivo CTL response to viral vectors by the use of calpain inhibitors. The present invention further provides a pharmaceutical formulations of p53 and a calpain inhibitor in a pharmaceutically acceptable carrier. The present invention provides a method of ablating neoplastic cells in a mammalian organism in vivo by the co-administration of a calpain inhibitor and p53. The present invention also provides a method of ablating neoplastic cells in a population of normal cells contaminated by said neoplastic cells ex vivo by the administration of a recombinant adenovirus in combination with a calpain inhibitor to said population.
Owner:CANJI
Probe set, probe immobilized carrier and gene examination method
InactiveUS20070134702A1Improve accuracyThe process is fast and accurateSugar derivativesMicrobiological testing/measurementMicroorganismMicrobiology
A probe and a primer capable of collectively detecting microorganisms of the same species while differentiating microorganisms of other species with an object of classification by species of fungus. An oligonucleotide probe for detecting an infectious etiologic microorganism gene includes at least one base sequence selected from the base sequences belonging to one group of the following first to ninth groups. The base sequence groups of first to ninth groups are: first group (SEQ ID NOS:1 to 5); second group (SEQ ID NOS:6 to 10); third group (SEQ ID NOS:11 to 15); fourth group (SEQ ID NOS:16 to 21); fifth group (SEQ ID NOS:22 to 26); sixth group (SEQ ID NOS:27 to 31); seventh group (SEQ ID NOS:32 to 36); eighth group (SEQ ID NOS:37 to 41); and ninth group (SEQ ID NOS:42 to 46).
Owner:CANON KK
Communicable disease barrier digit cover and dispensing package therefor
InactiveUS6179159B2Easy to useEasy to transportDiagnosticsSurgical needlesPulse oximetersEngineering
A disposable digit cover structured to be applied to envelop a portion of a human finger or toe, i.e., digit, for aiding in prevention of the spread of communicable diseases and cross-patient contamination from pulse oximeter probes. The cover is a flexible, tubular structure of material impervious to the passage of infectious agents, and having one open end and an oppositely disposed closed end, and is sufficiently thin and transparent to light to serve as infectious agent barrier between a human digit and a pulse oximeter probe. The covers are stored in a flattened state in a clean condition with a dispensing package containing a plurality of the covers stacked upon each other and secure to the package. The package includes a closeable opening allowing access to the digit covers. The dispensing package is small enough to be carried in a garment pocket.
Owner:GURLEY MARIRUTH D
Pulmonary delivery of protonated/acidified nucleic acids
The present invention provides a method of treating bacterial respiratory infections by pulmonary administration of protonated / acidified nucleic acids. These modified nucleic acids are effective as bactericidal and / or bacteriostatic agents without regard to the class of bacteria, so are especially useful when diagnosis is difficult or when multiple infectious organisms are present. The antibiotic activity of nucleic acids of the invention is not dependent on either the specific sequence of the nucleic acid or the length of the nucleic acid molecule.
Owner:LAKEWOOD AMEDEX
Infectious cDNA clones of porcine reproductive and respiratory syndrome virus and expression vectors thereof
InactiveUS6841364B2SsRNA viruses positive-senseSugar derivativesRespiratory syndrome virusGene mutation
Infectious cDNA clones of North American Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) are provided. Further provided are cDNA clones comprising genetic mutations. Also provided are vaccines comprising cDNAs, including genetically and immunologically marked vaccines for North American PRRSV.
Owner:PROTATEK INT
Method for detecting infectious parvovirus in pharmaceutical preparations
ActiveUS20090226414A1Peptide/protein ingredientsMicrobiological testing/measurementPancrelipaseViral infection
The present invention provides methods for detecting viral infectivity and content in an enzyme preparation. In certain embodiments, the invention relates to methods for producing a pharmaceutical pancreatic enzyme composition. In additional embodiments, the invention relates to detecting infectious porcine parvovirus (PPV) and determining PPV content in pancreatic enzyme preparations (PEPs), including pancrelipase preparations.
Owner:ALLERGAN SALES LLC
Use of glycosaminoglycans degrading enzymes for management of airway associated diseases
InactiveUS6153187AIncrease the gapReduce frequencyPowder deliveryPeptide/protein ingredientsDiseaseMedicine
A method of managing a patient having an accumulation of mucoid, mucopurulent or purulent material containing glycosaminoglycans, the method comprising the step of administering at least one glycosaminoglycans degrading enzyme to the patient in an amount therapeutically effective to reduce at least one of the following: the visco-elasticity of the material, pathogens infectivity and inflammation. An article of manufacture comprising an inhaler including, as an active ingredient, at least one glycosaminoglycans degrading enzyme for generating aerosols including the enzyme for management a patient having an accumulation of mucoid, mucopurulent or purulent material containing glycosaminoglycans.
Owner:INSIGHT BIOPHARMLS
Purification of adenovirus and AAV
InactiveUS7015026B2Rapidly and efficiently purifyHigh molecular weightEnzymologyRecovery/purificationBiomedical engineeringGenetic transfer
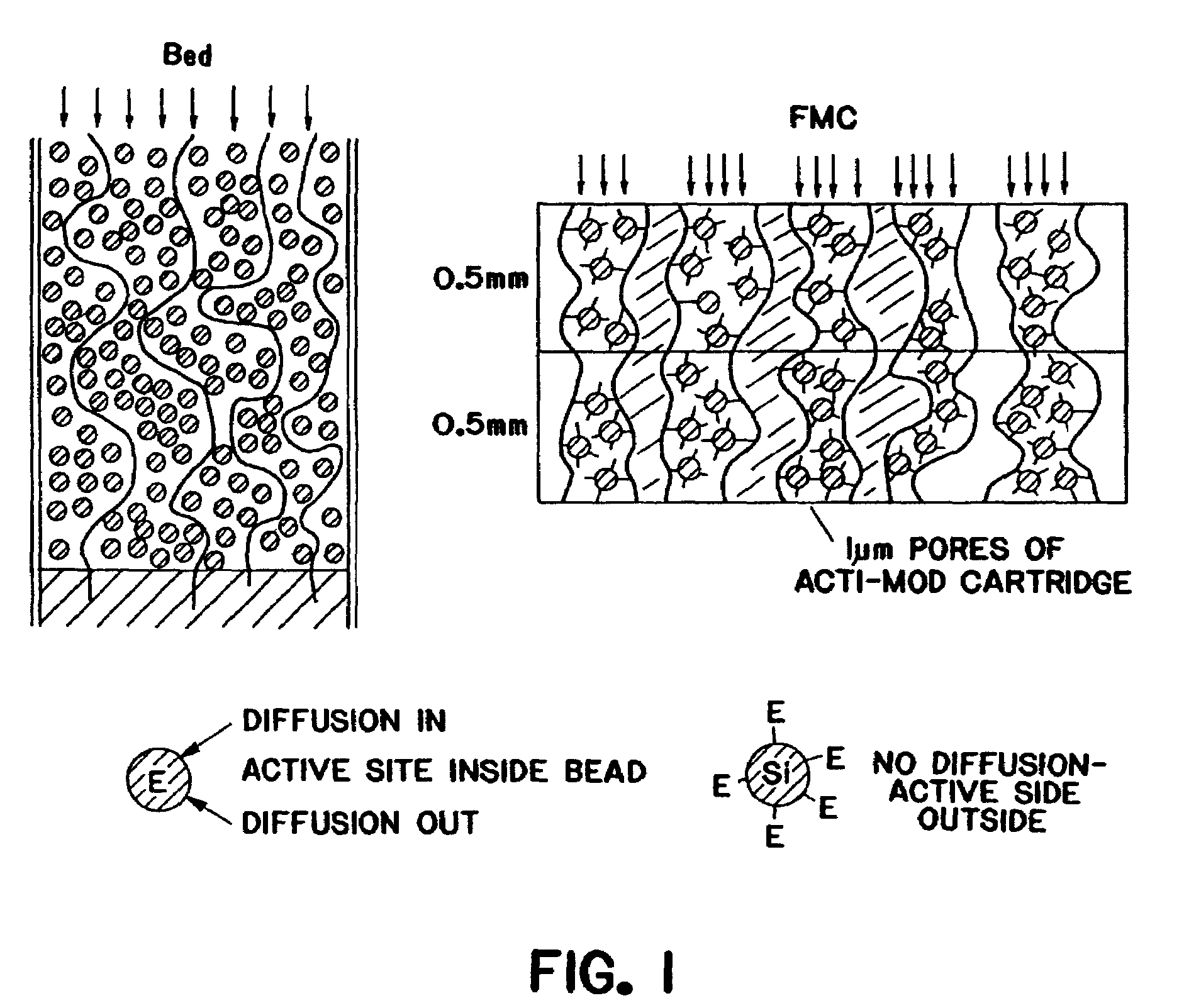
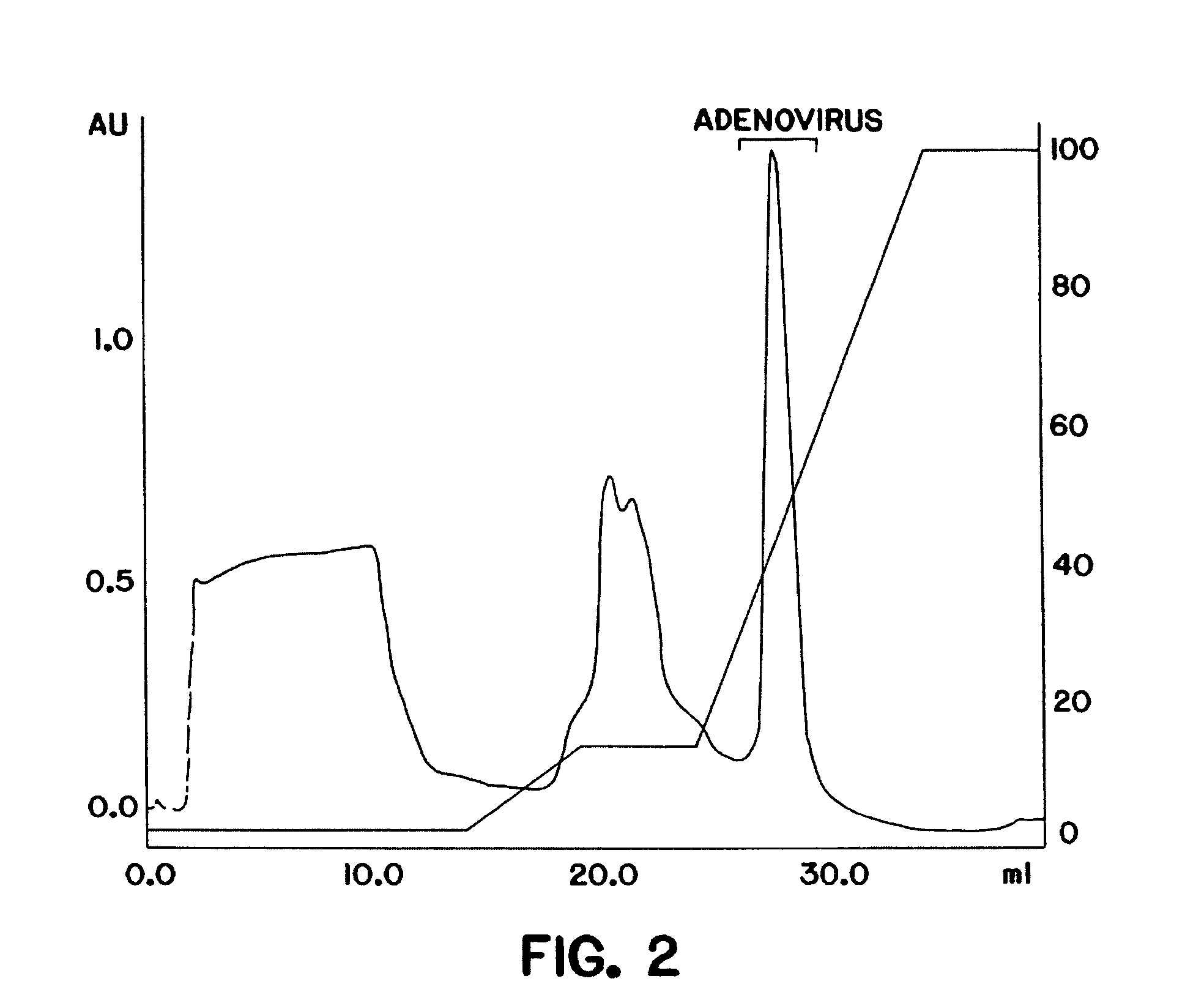
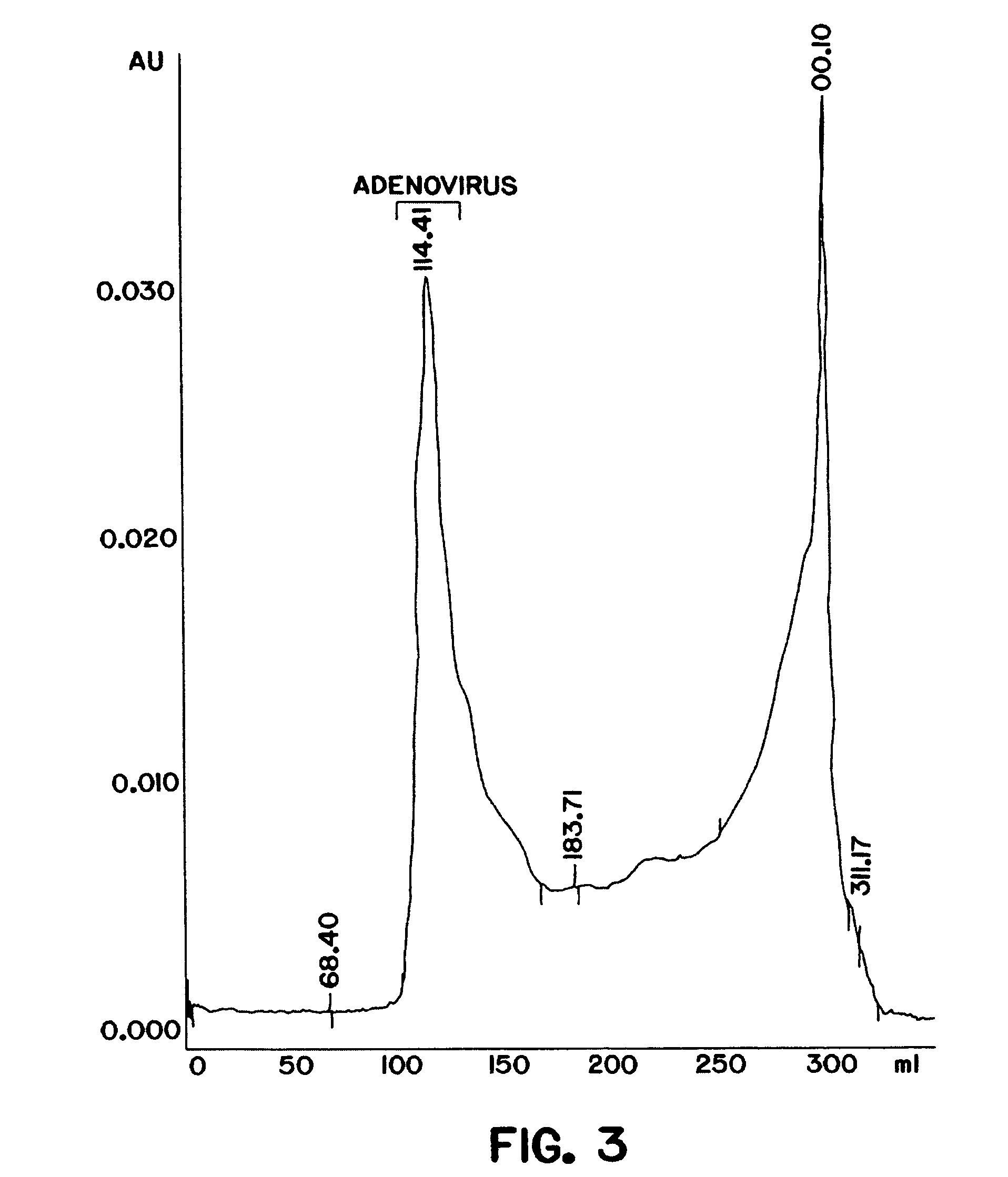
The present invention relates to the purification of large scale quantities of active (infectious) adenovirus and AAV, especially for use in therapeutic applications. In particular, the invention provides improved methods for contacting such viruses with suitable chromatographic materials in a fashion such that any damage to the virus, particularly to surface components thereof, resulting from contact with such chromatographic materials is minimized or eliminated. The result is the ability to rapidly and efficiently purify commercial level quantities of active (infectious) virus suitable for use in therapeutic applications, e.g. gene transfer / therapy procedures.
Owner:GENZYME CORP
Anti-pathogenic air filtration media and air handling devices having protective capabilities against infectious airborne mircoorganisms
The present invention provides an anti-pathogenic air filtration medium comprising a fibrous substrate whose fibers are coated with coating comprising a polymer. The coating provides an environment that is destructive to airborne pathogens. In particular, the filter medium can be used in a building air handling system that both filters the air and eliminates pathogens. The filter medium also can be used to create a new bio-protective gas mask that not only offers protection against chemical warfare agents, but also provides protection against biological pathogens.
Owner:INNOVATIVE CONSTR & BUILDING MATERIALS
Viral purification methods
ActiveUS7223585B2Simple methodViral antigen ingredientsGenetic material ingredientsPurification methodsFiltration
The present invention is directed to an improved method of purifying virus, particularly reovirus. Infectious virus can be extracted from a cell culture with a detergent to produce high titers of virus, and the virus can then be purified by simple steps such as filtration and column chromatography. Viruses and compositions comprising the viruses prepared according to the present invention are also provided.
Owner:ONCOLYTICS BIOTECH
Peptide sequences specific for the hepatic stages of P. falciparum bearing epitopes capable of stimulating the T lymphocytes
The present invention relates to an in vitro diagnostic method for malaria in an individual comprising placing a tissue or a biological fluid taken from an individual in contact with a molecule or polypeptide composition, wherein said molecule or polypeptide composition comprises one or more peptide sequences bearing all or part of one or more T epitopes of the proteins resulting from the infectious activity of P. falciparum, under conditions allowing an in vitro immunological reaction to occur between said composition and the antibodies that may be present in the tissue or biological fluid, and in vitro detection of the antigen-antibody complexes formed. The invention further relates to a polypeptide comprising at least one T epitope from a liver-stage specific protein produced by P. falciparum and a vaccine composition directed against malaria comprising a molecule having one or more peptide sequences bearing all or part of one or more T epitopes resulting from the infectious activity of P. falciparum in the hepatic cells.
Owner:INST PASTEUR
Method of treating tuberculosis with interferons
InactiveUS20100098660A1Increase appetiteReduction in wheezingAntibacterial agentsBiocideInterferon therapyInterferon alpha
A method of treating tuberculosis comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Further, a method of reducing the infectivity of tuberculosis or reducing the number of infectious organisms present in the lungs of a patient suffering from tuberculosis comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Also, pharmaceutical compositions of one or more aerosolized interferon(s) are provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS9441244B2Altered capsid propertiesReduce the binding forceAntibacterial agentsVirusesNucleotideCell type specific
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:INTEGRATIVE GENE THERAPEUTICS +1
Adeno-associated virus virions with variant capsid and methods of use thereof
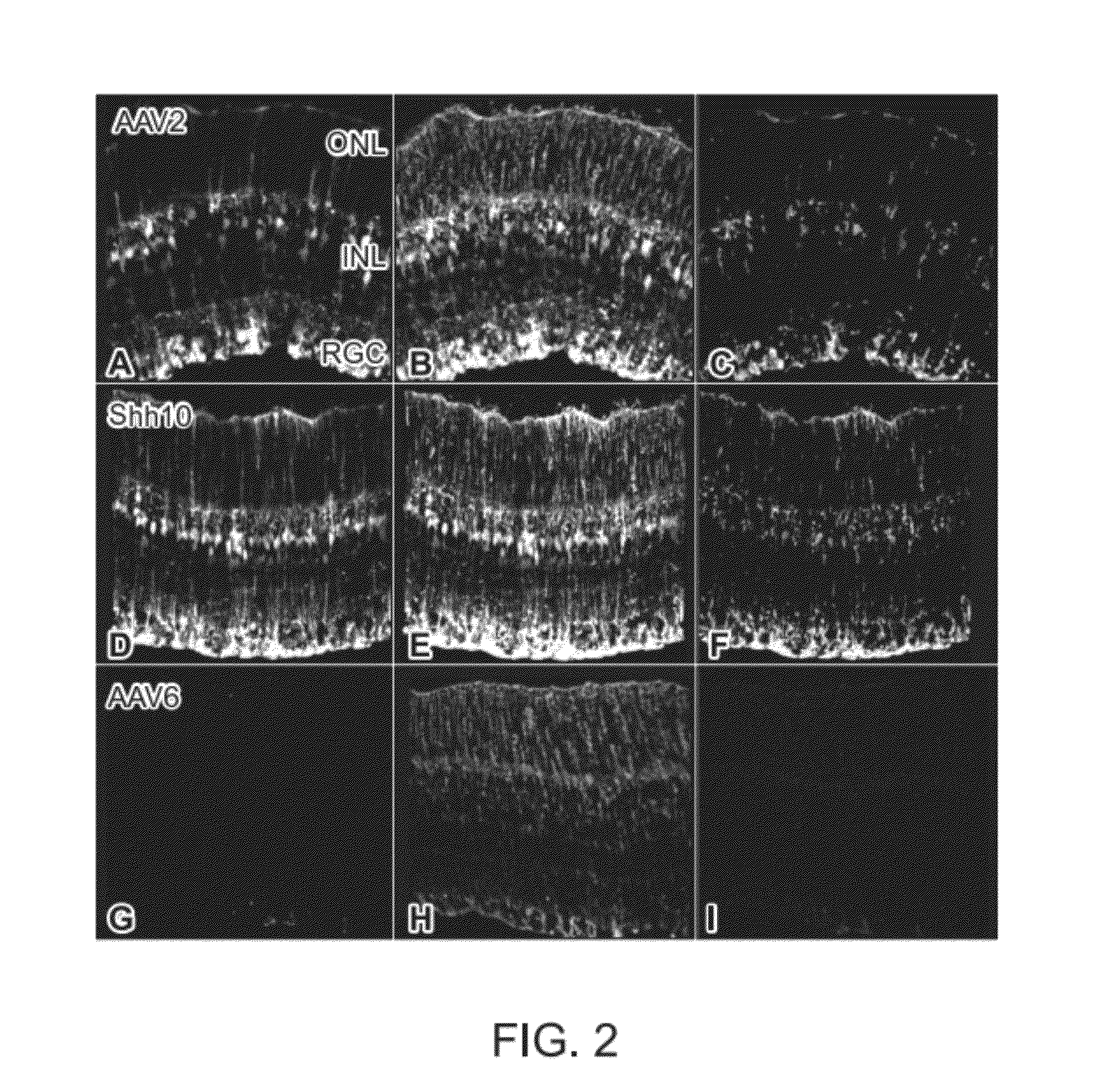
The present disclosure provides adeno-associated virus (AAV) virions with altered capsid protein, where the AAV virions exhibit greater infectivity of retinal cells compared to wild-type AAV. The present disclosure further provides methods of delivering a gene product to a retinal cell in an individual, and methods of treating ocular disease.
Owner:RGT UNIV OF CALIFORNIA
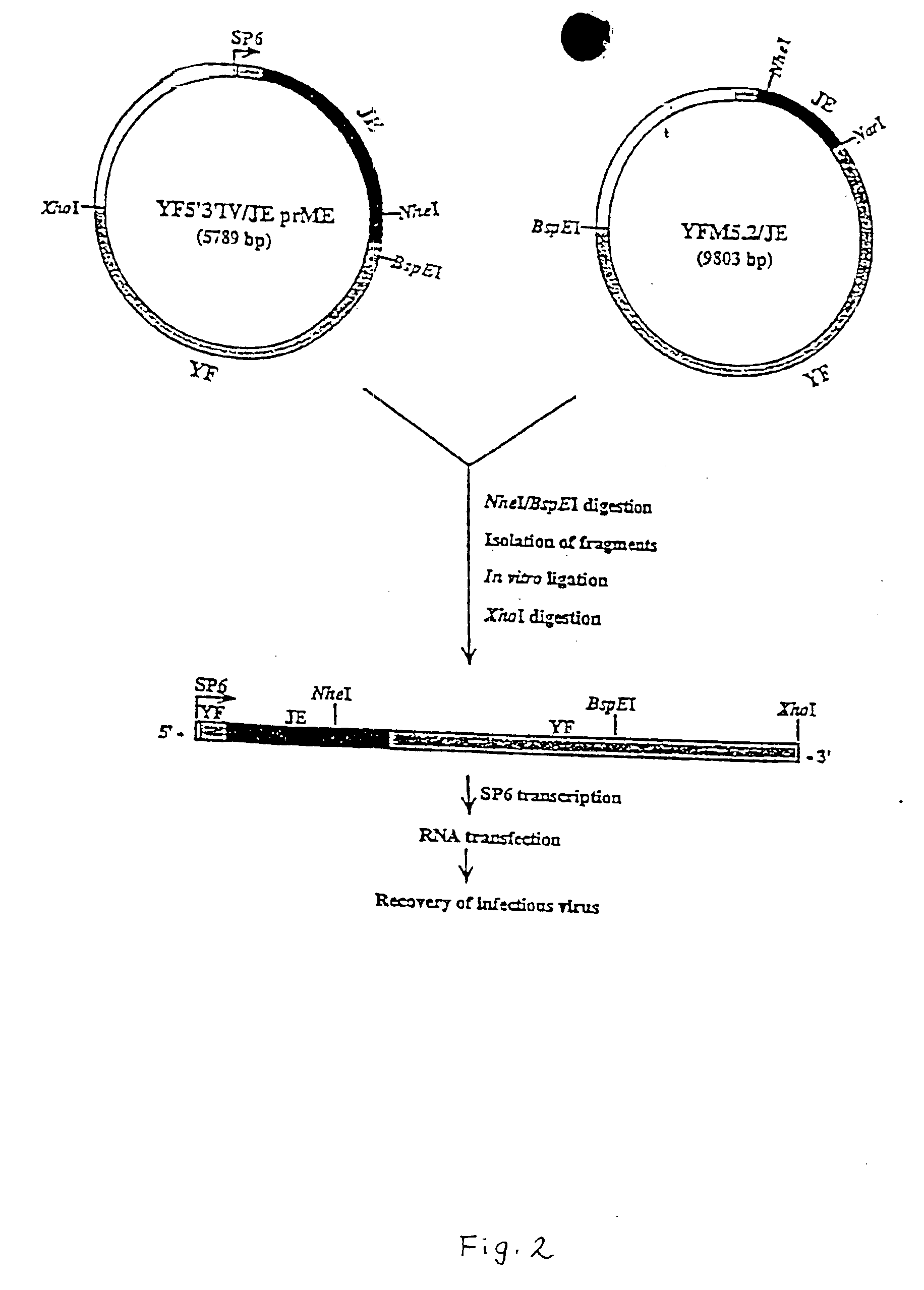
Chimeric flavivirus vaccines
InactiveUS6962708B1Easy to useImproving immunogenicityBiocideSsRNA viruses positive-senseNucleotideNucleotide sequencing
A chimeric live, infectious, attenuated virus containing a yellow fever virus, in which the nucleotide sequence for a prM-E protein is either deleted, truncated, or mutated, so that functional prM-E protein is not expressed, and integrated into the genome of the yellow fever virus, a nucleotide sequence encoding a prM-E protein of a second, different flavivirus, so that the prM-E protein of the second flavivirus is expressed.
Owner:SAINT LOUIS UNIVERSITY +1
Activation and expansion of T-cells using an engineered multivalent signaling platform as a research tool
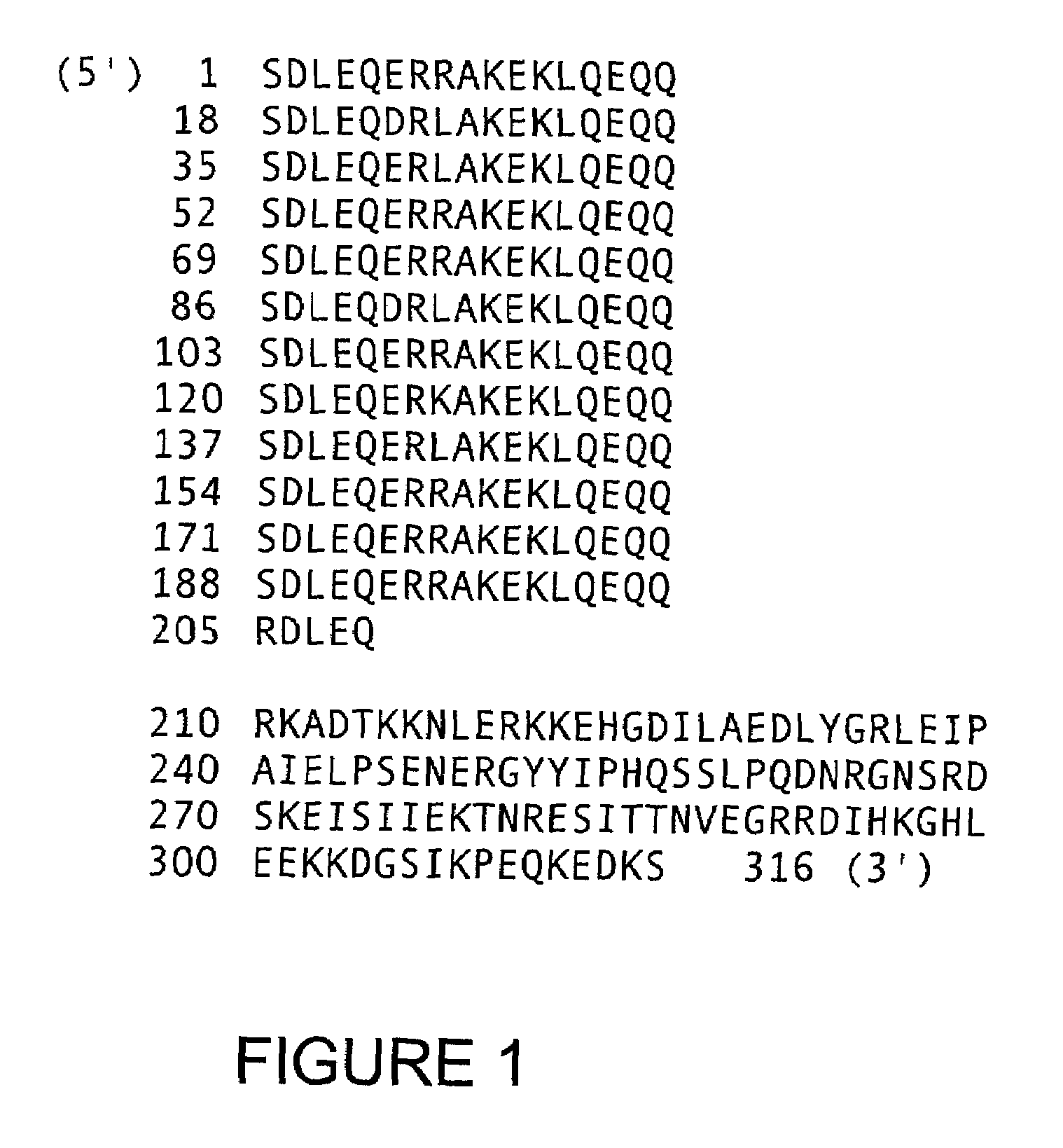
ActiveUS7745140B2Decrease in apoptosis levelImprove survivalBiocideGenetic material ingredientsBiological activationExogenous growth
Provided are a system and methods for selectively inducing expansion of a population of T cells in the absence of exogenous growth factors, such as lymphokines, and accessory cells for research purposes. The cell based expansion system and methods permit the long-term growth of CTLs, preferably human CTLs. In addition, T cell proliferation can be induced without the need for antigen, thus providing an expanded T cell population that is polyclonal with respect to antigen reactivity. Further provided are methods for using the system and methods to screen and identify antigens related to specific diseases or conditions, tumors, autoimmune disorders, or an infectious disease or pathogen, and to identify target molecule for research purposes, or for developing a vaccine based thereon.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
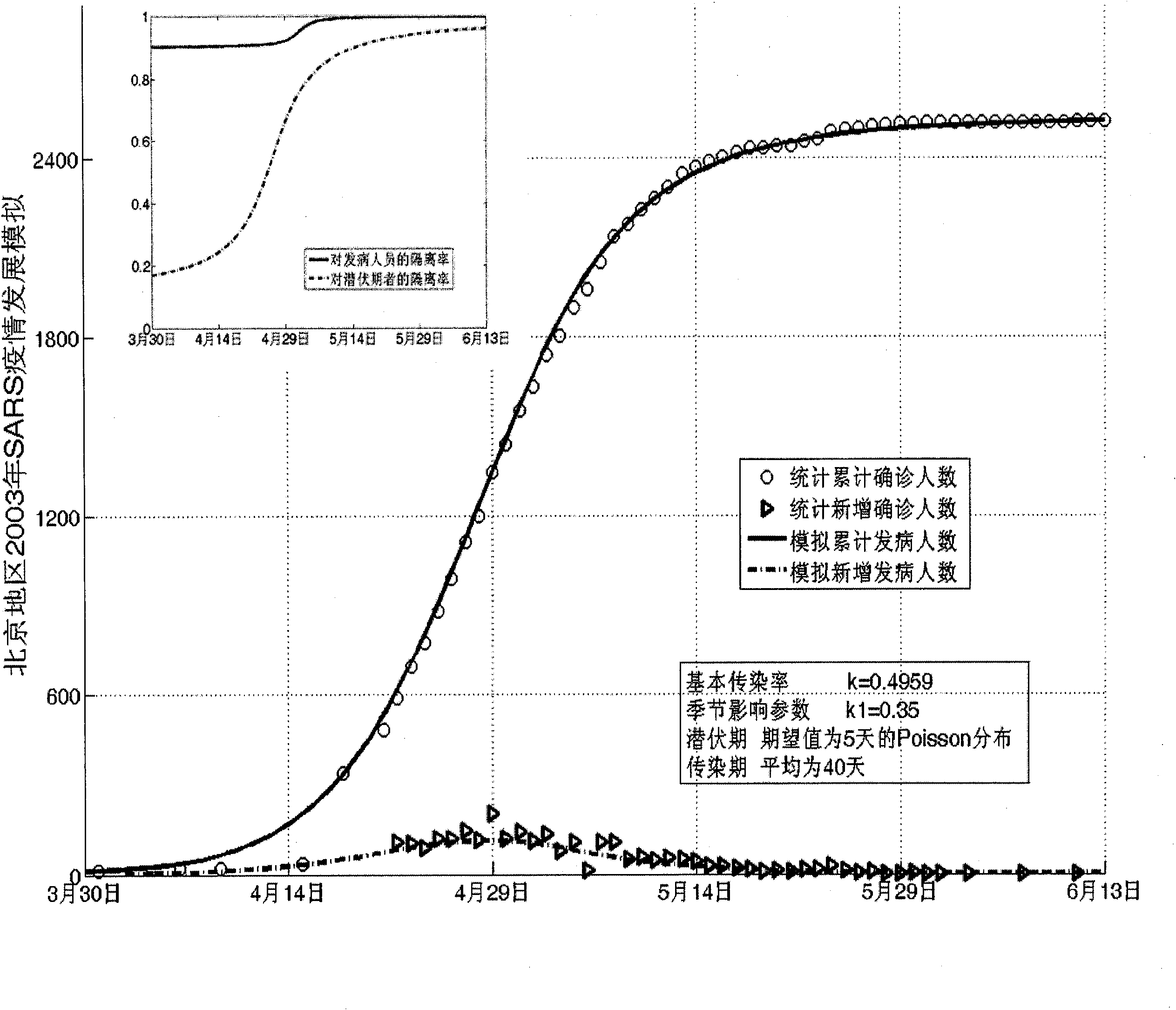
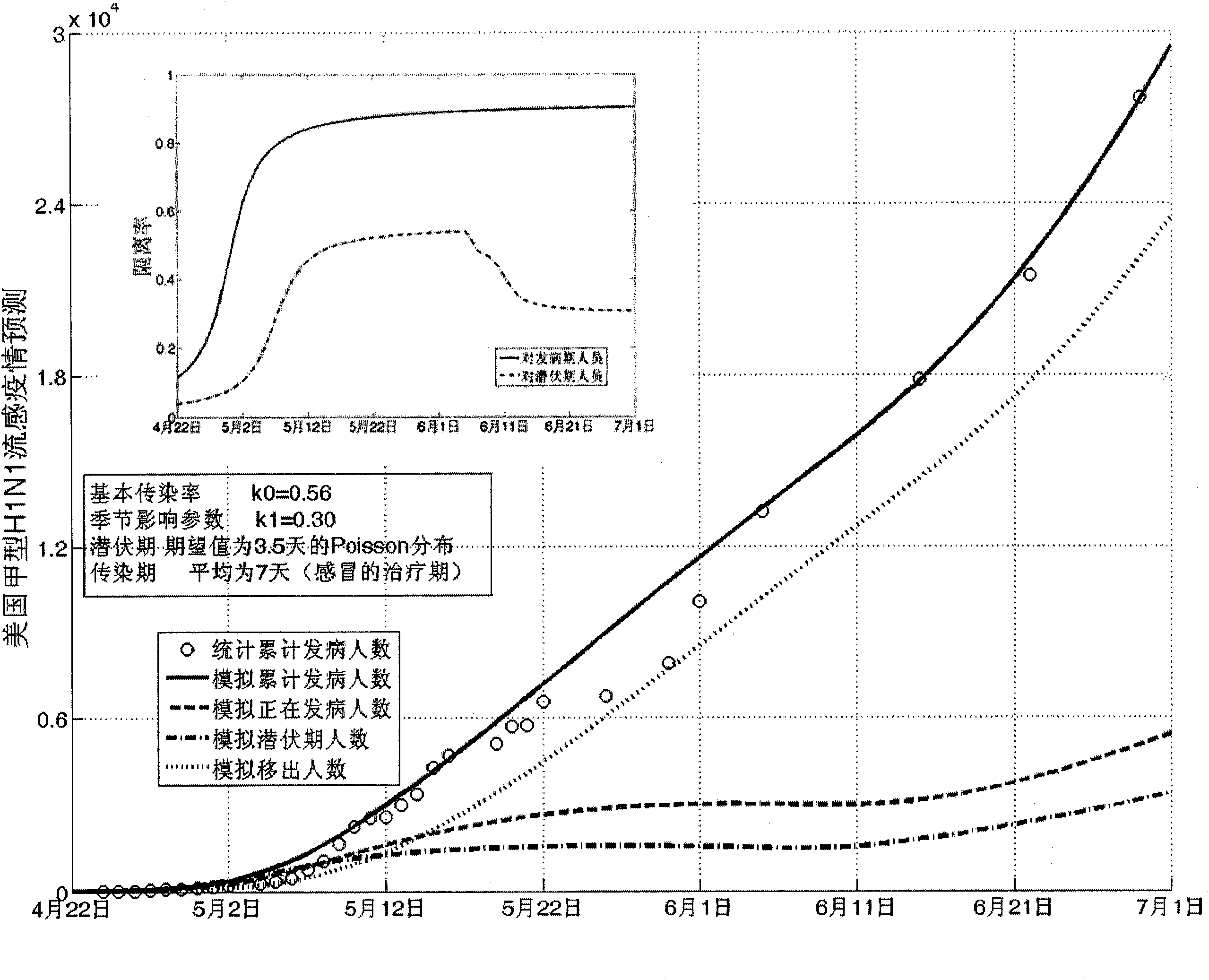
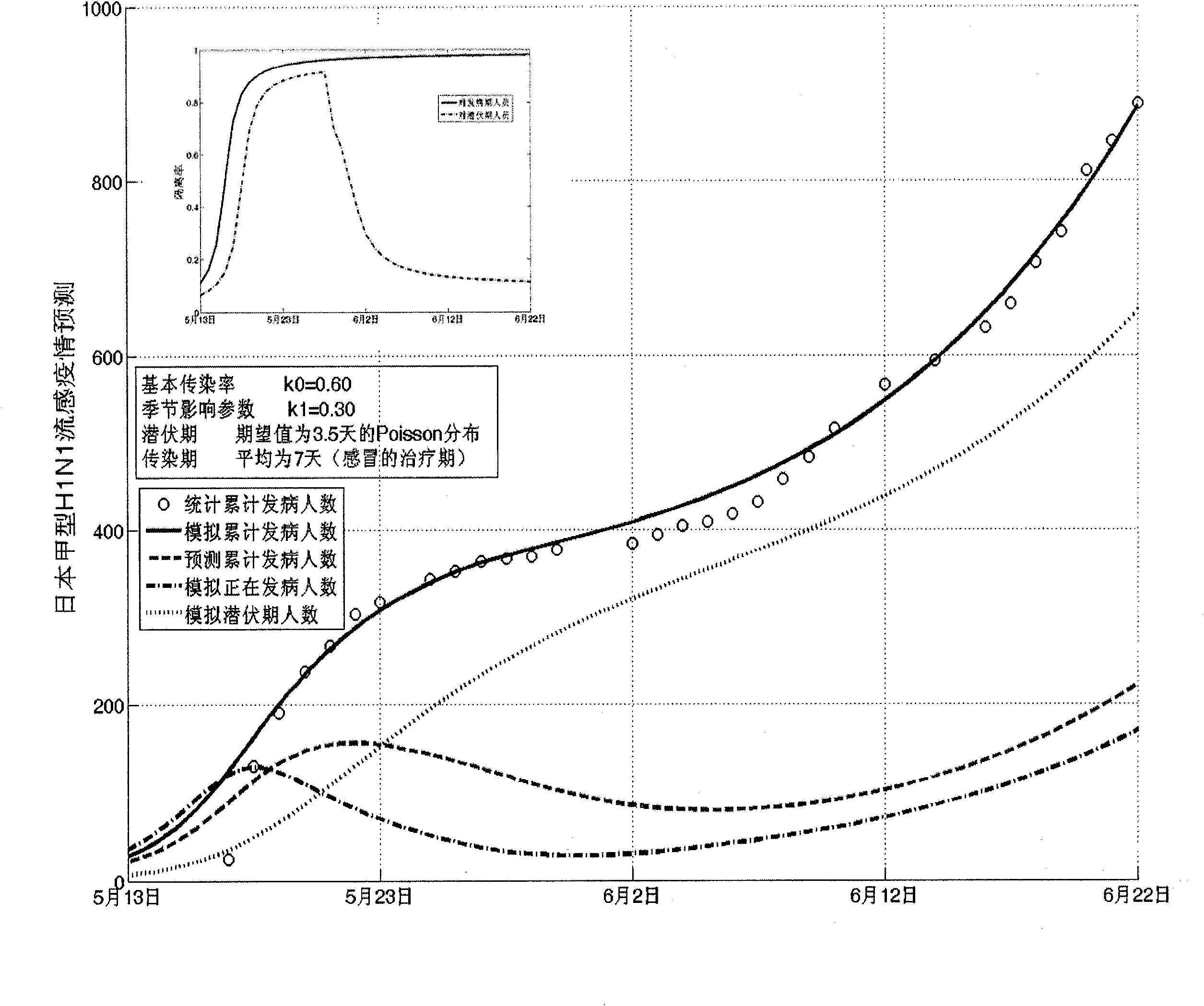
Infectious disease epidemic situation predicative analysis method based on nonlinear and coefficient variation predictive model
InactiveCN101794342ASpecial data processing applicationsSusceptible populationSevere acute respiratory syndrome
The invention establishes a nonlinear and coefficient variation infectious disease predictive model aiming at epidemic diseases with viruses which have infectivity at a latent period and a period of onset, provides an epidemic situation control function directly related to the model, simulating and predicting effects of different control measures and different control degrees on the basis of prediction in consideration of the control measures, considers the epidemic situation control as a continuous change process, integrally simulating and predicting development and control of the epidemic situation and provides crucial quantitative information for decision-making departments to optimally decide and control the epidemic situation with the smallest cost. By adopting the invention, a relative error for simulating SARS (Severe Acute Respiratory Syndromes) in Beijing areas in 2003 is 0.98% and predictive results for influenza A virus subtype H1N1 in US and Japan are well matched with the actual epidemic situation development, a quantified control factor for preventing the influenza A virus subtype H1N1 at an initial development stage and controlling the spread of the epidemic situation is obtained and epidemic situation development conditions of different control intensities and different susceptible people are predicted.
Owner:中国人民解放军防化指挥工程学院
Traditional Chinese medicine mixture for treating livestock and poultry virosis and preparation method thereof
ActiveCN101554414AEasy to takeAbsorb quickly and playDigestive systemAntiviralsHigh grade feverRadix isatidis
The invention relates to a traditional Chinese medicine mixture for treating livestock and poultry virosis and a preparation method thereof. The traditional Chinese medicine mixture contains the contents according to the parts by weight: 10-40 of radix isatidis, 10-40 of folium isatidis, 7-20 of honeysuckle, 3-9 of radix bupleuri, 1-6 of radix lithospermi, 5-20 of forsythia, 5-20 of scutellaria baicalensis, 3-9 of lightyellow sophora root, 2-7 of coptis chinensis, 4-11 of raidx astragali and 1-6 of liquorice. The traditional Chinese medicine mixture has reasonable compositions, convenient mixture taking, obvious effects and no mixture residues, conforms to the safe requirement of animal-derived foods and can be effectively applied to preventing and curing the infective livestock and poultry virosis of chicken flu, newcastle diseases, duck hepatitis viruses, swine fever, virus diarrhea, and the like. The traditional Chinese medicine mixture has the advantages of low cost, simple preparation process and no environmental pollution and is suitable for industrial production.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH
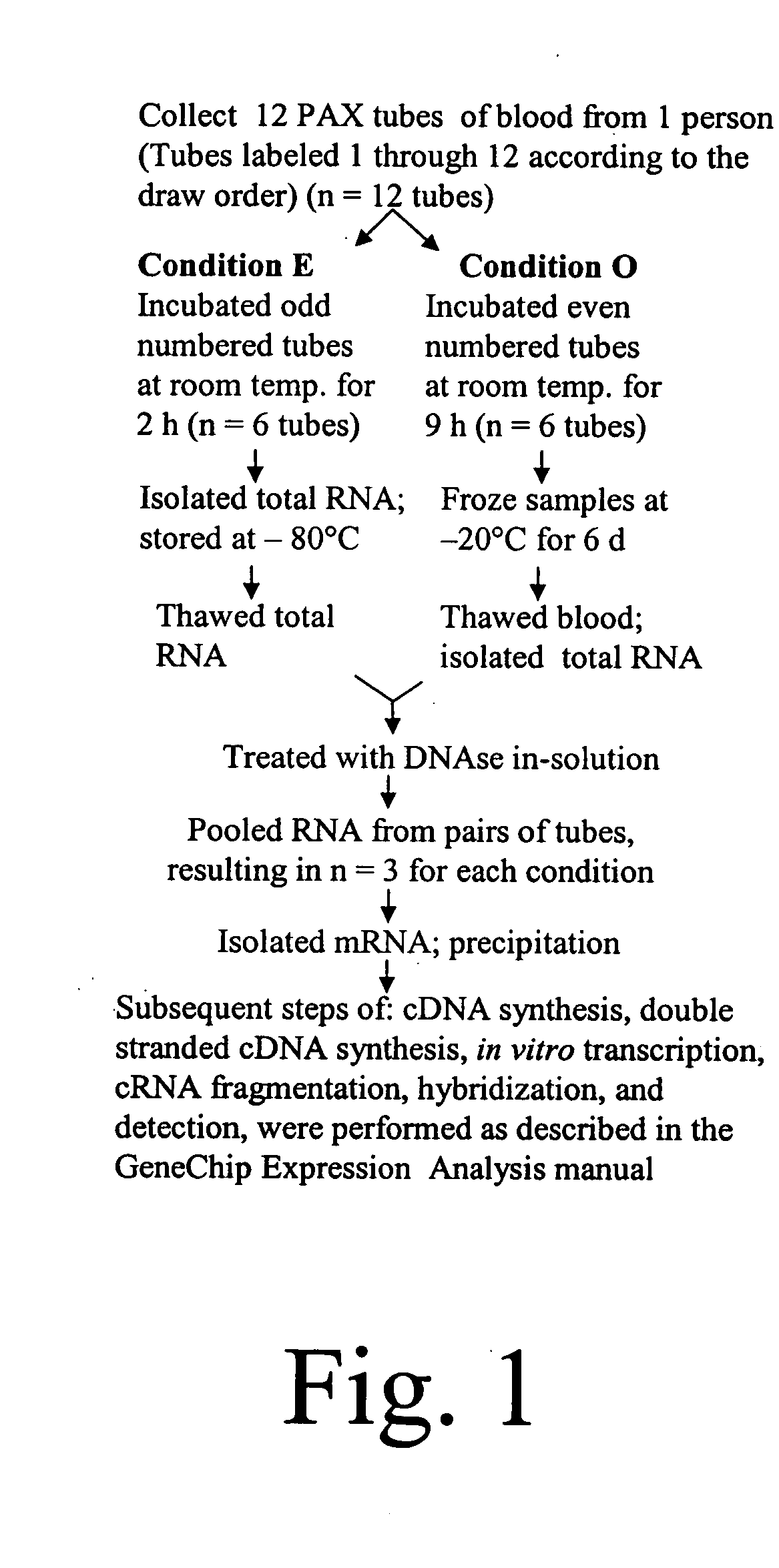
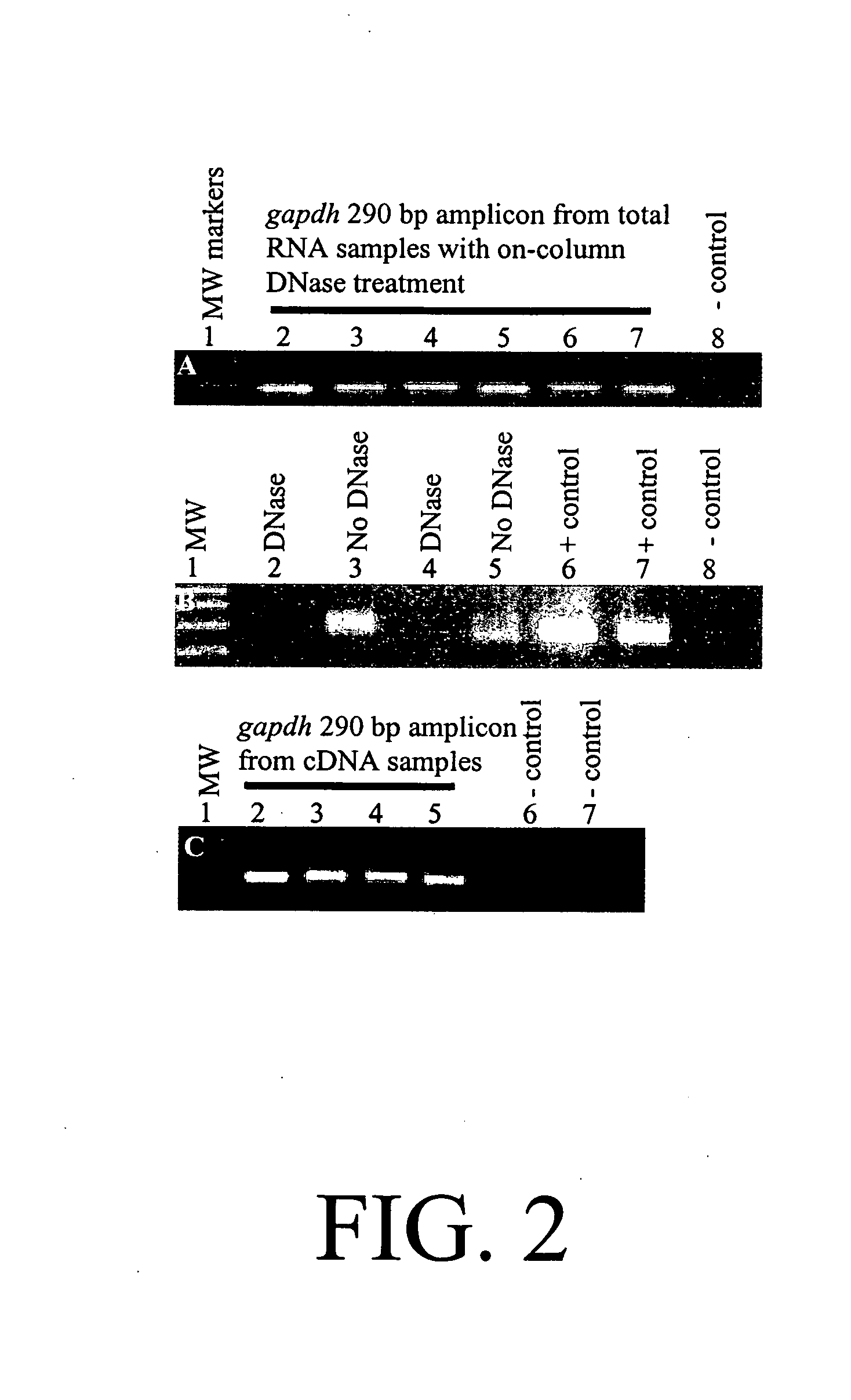
Diagnosis and prognosis of infectious diseases clinical phenotypes and other physiologic states using host gene expression biomarkers in blood
InactiveUS20080020379A1High sensitivityImprove reliabilityMicrobiological testing/measurementWhite blood cellHost gene
The present invention provides a specific set of gene expression markers from peripheral blood leukocytes that are indicative of a host response to exposure, response, and recovery infectious pathogen infections. The present invention further provides methods for identifying the specific set of gene expression markers, methods of monitoring disease progression and treatment of infectious pathogen infections, methods of prognosing the onset of an infectious pathogen infection, and methods of diagnosing an infectious pathogen infection and identifying the pathogen involved.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +1
High Throughput Testing for Presence of Microorganisms in a Biological Sample
InactiveUS20090306230A1Improve throughputFacilitates genotypingBiocideNucleotide librariesMicroorganismMultiplexing
Provided are methods and apparatus for high throughput testing of biological samples that may or may not comprise microorganisms. The methods include the use of a diagnostic multiplexing panel (DMP) specifically designed for the simultaneous identification of a plurality of potential microorganisms that may be present in the biological sample via a primer extension reaction directed a highly conserved nucleic acid sequences in the microorganisms under test. The biological sample is typically immobilised on a solid substrate at a first location before being transferred to a second location for analysis using the DMP. The methods and apparatus of the invention are particularly suited to diagnosis of the presence of infectious pathogens in the biological sample, for example for diagnosis of sexually transmitted infection.
Owner:ROCHESTER INVESTMENT PARTNERS
Plant additive premixed forage for aquatic culture and bait therefrom
InactiveCN1644077AQuality improvementEnhance phagocytosisClimate change adaptationAnimal feeding stuffHerbAnimal Foraging
A vegetative additive for the mixed feed used to culture quatic animals is proportionally prepared from adhesive, allicin, and 8 Chinese-medicinal materials including dandelion herb, phellodendron bark, scutellaria root, liquorice root, etc. Its advantage is high effect to kill bacteria and virus and promote the development of immune organs.
Owner:冯绍宽
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com