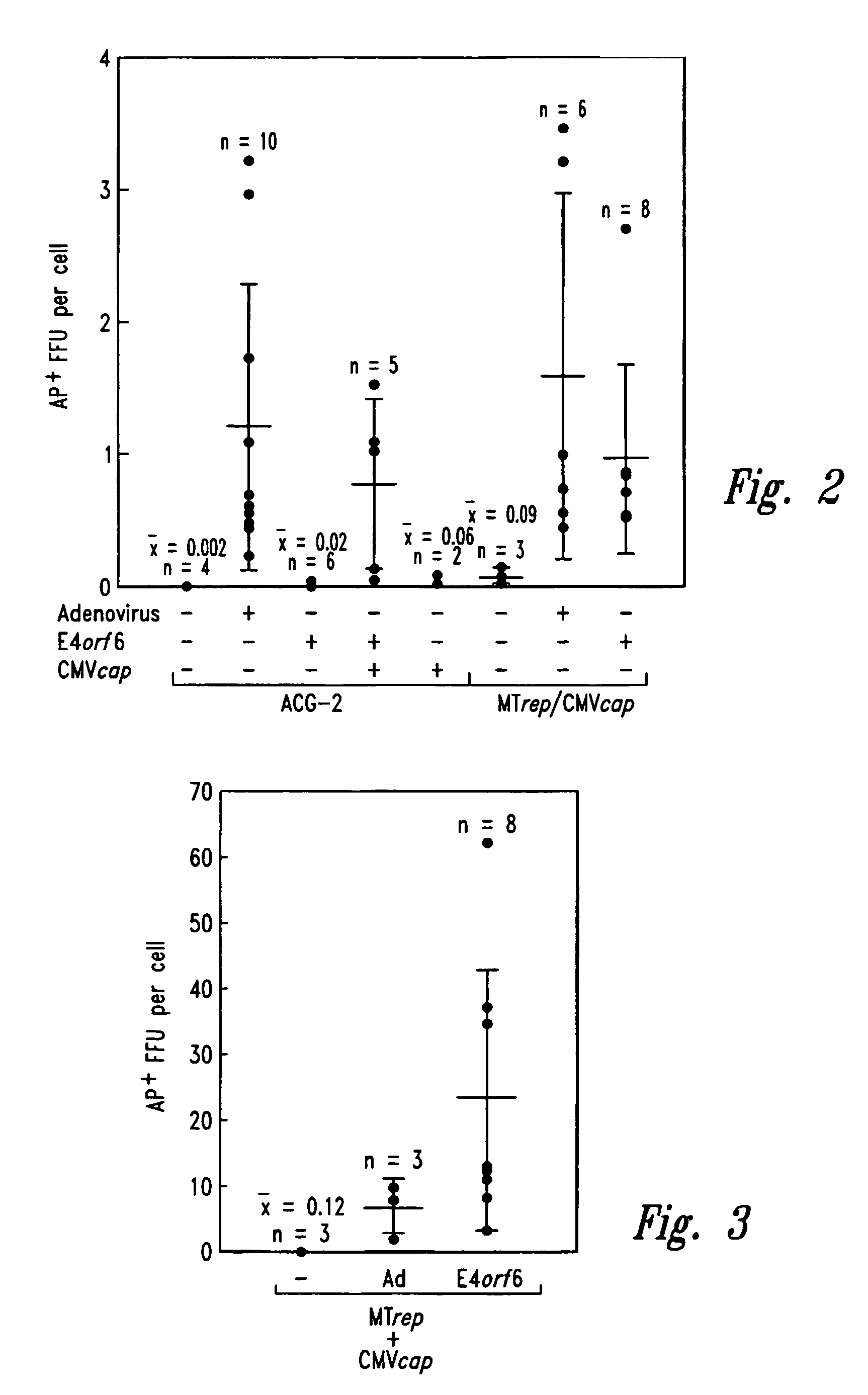
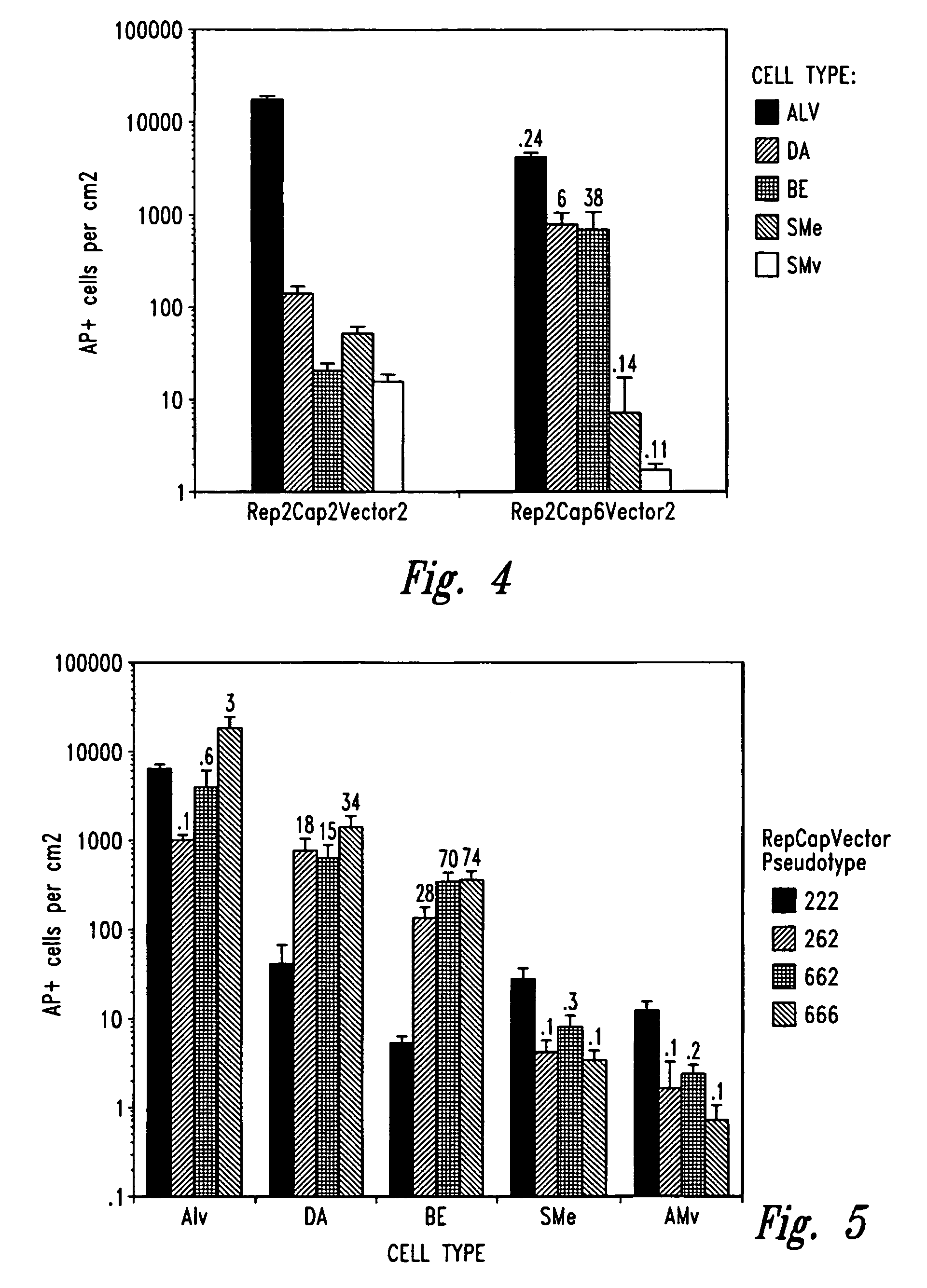
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
91 results about "Helper virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A helper virus is a virus that allows an otherwise-deficient coinfecting virus to replicate. These can be naturally occurring as with Hepatitis D virus, which requires Hepatitis B virus to coninfect cells in order to replicate. Helper viruses are also commonly used to replicate and spread viral vectors for gene expression and gene therapy.
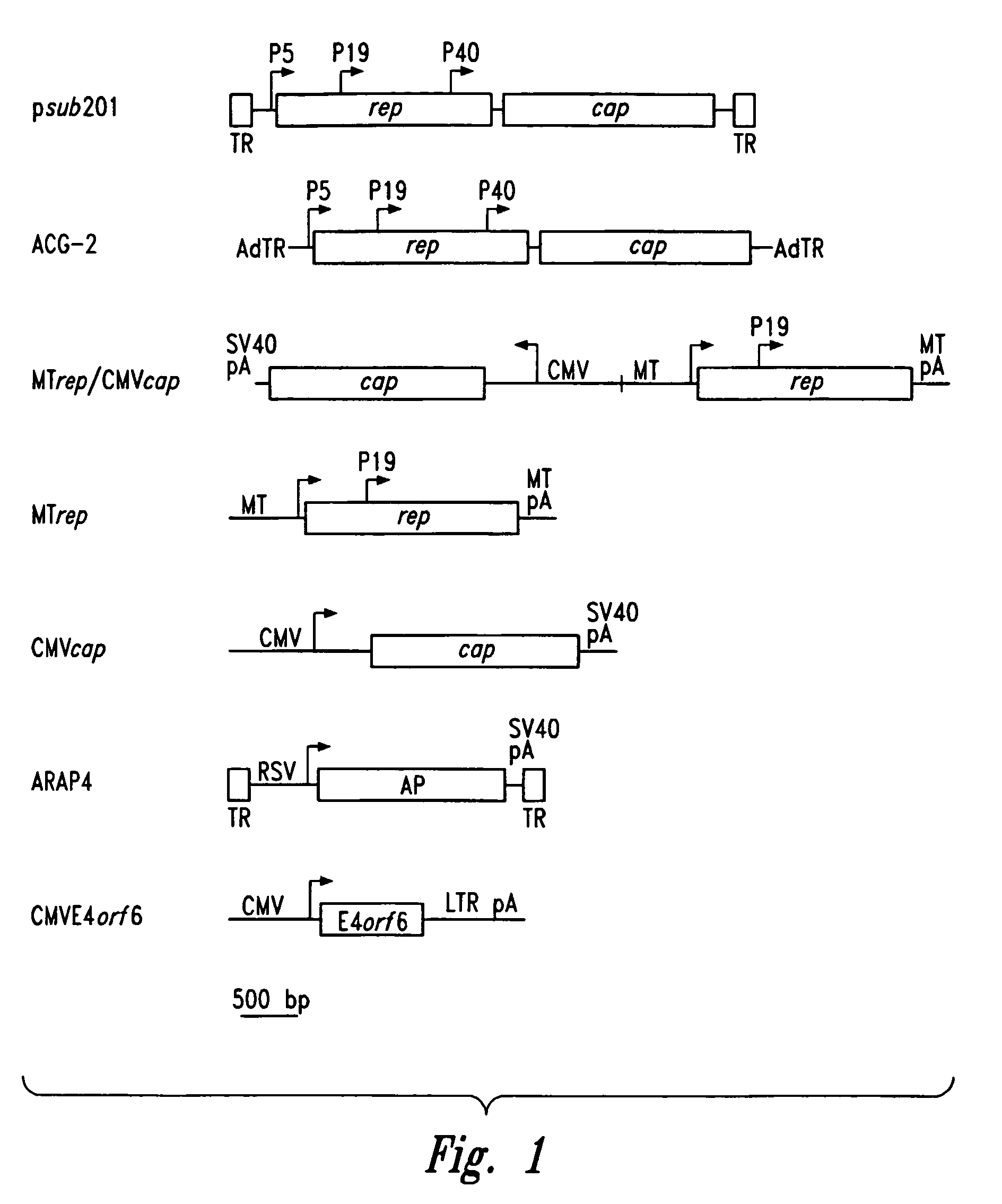
Methods for generating high titer helper-free preparations of released recombinant AAV vectors
InactiveUS6989264B2Genetic therapy composition manufactureGroup 5/15 element organic compoundsGene deliveryHeterologous
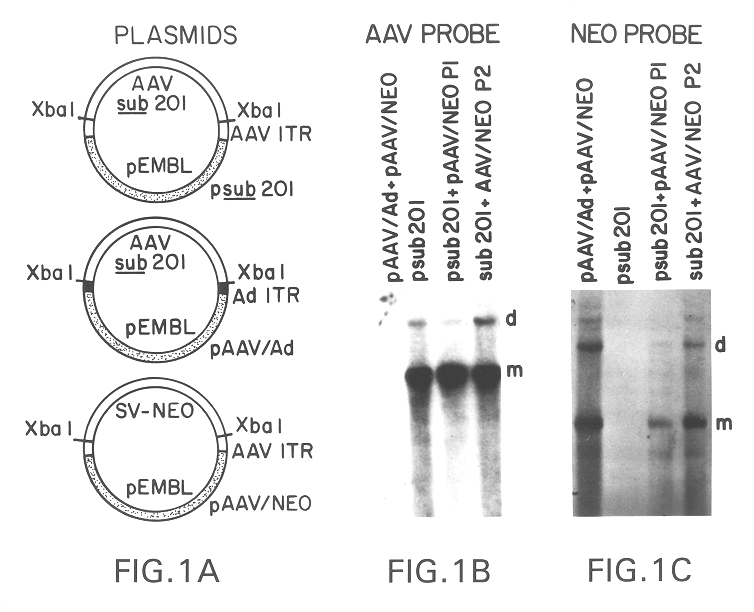
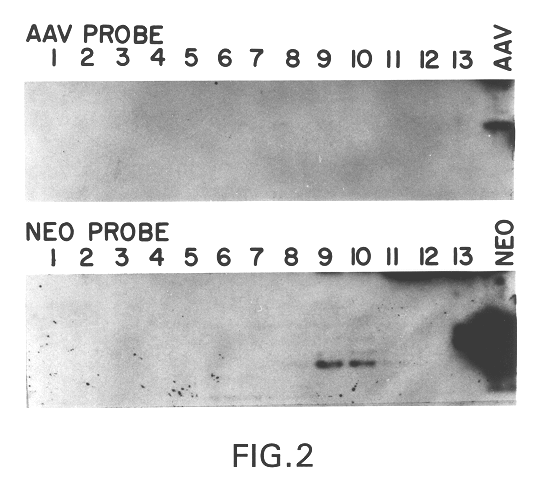
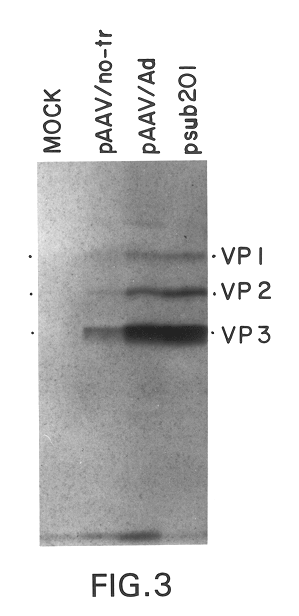
This invention provides methods and compositions for producing high titer, substantially purified preparations of recombinant adeno-associated virus (AAV) that can be used as vectors for gene delivery. At the onset of vector production, AAV producer cells of this invention typically comprise one or more AAV packaging genes, an AAV vector comprising a heterologous (i.e. non-AAV) transgene of interest, and a helper virus such as an adenovirus. The AAV vector preparations produced are generally replication incompetent but are capable of mediating delivery of a transgene of interest (such as a therapeutic gene) to any of a wide variety of tissues and cells. The AAV vector preparations produced according to this invention are also substantially free of helper virus as well as helper viral and cellular proteins and other contaminants. The invention described herein provides methods of producing rAAV particles by culturing producer cells under conditions, such as temperature and pH, that promote release of virus. Also provided is a quantitative, high-throughput assay useful in the assessment of viral infectivity and replication, as well as in the screening of agent that affect viral infectivity and / or replication.
Owner:TARGETED GENETICS CORPORTION
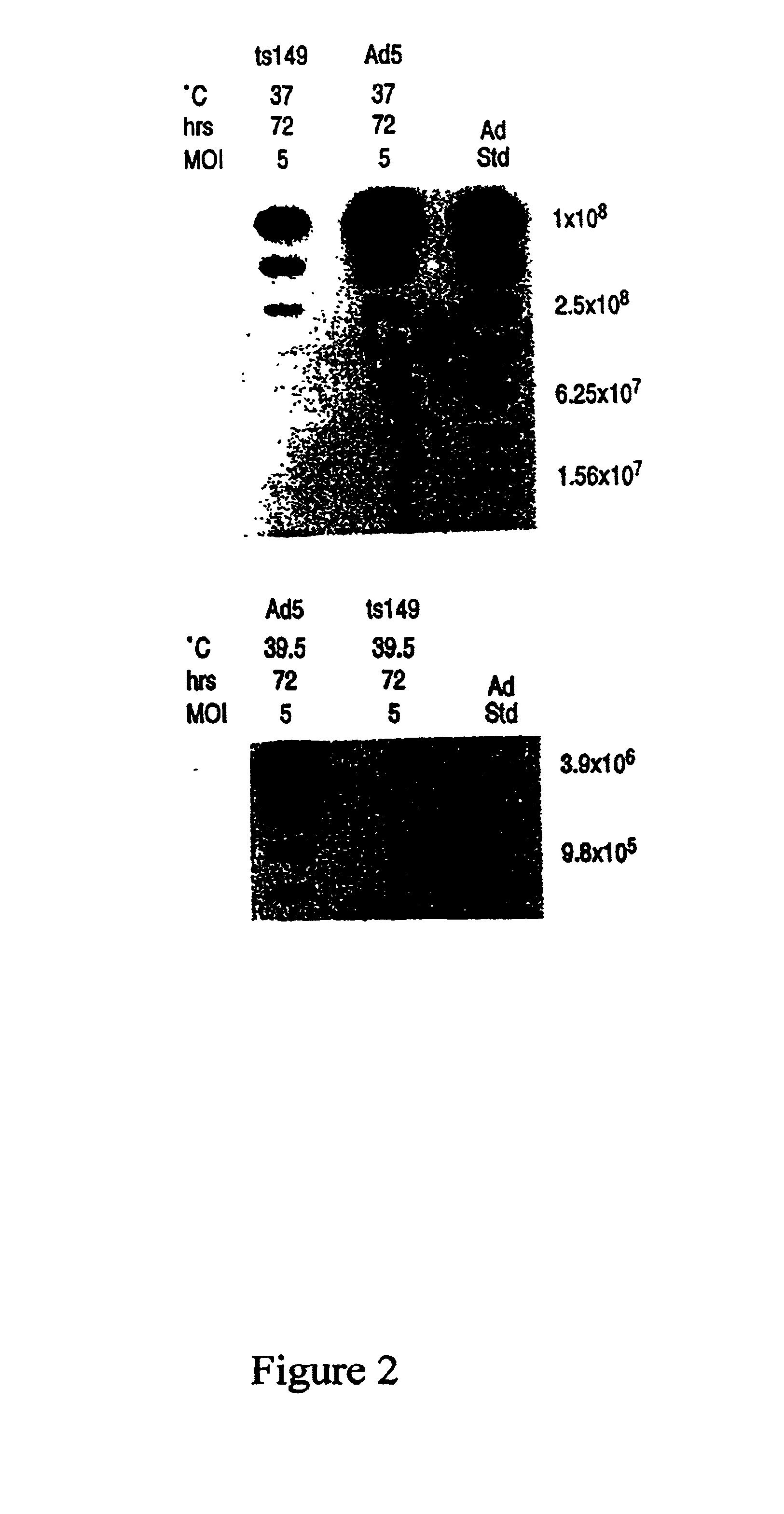
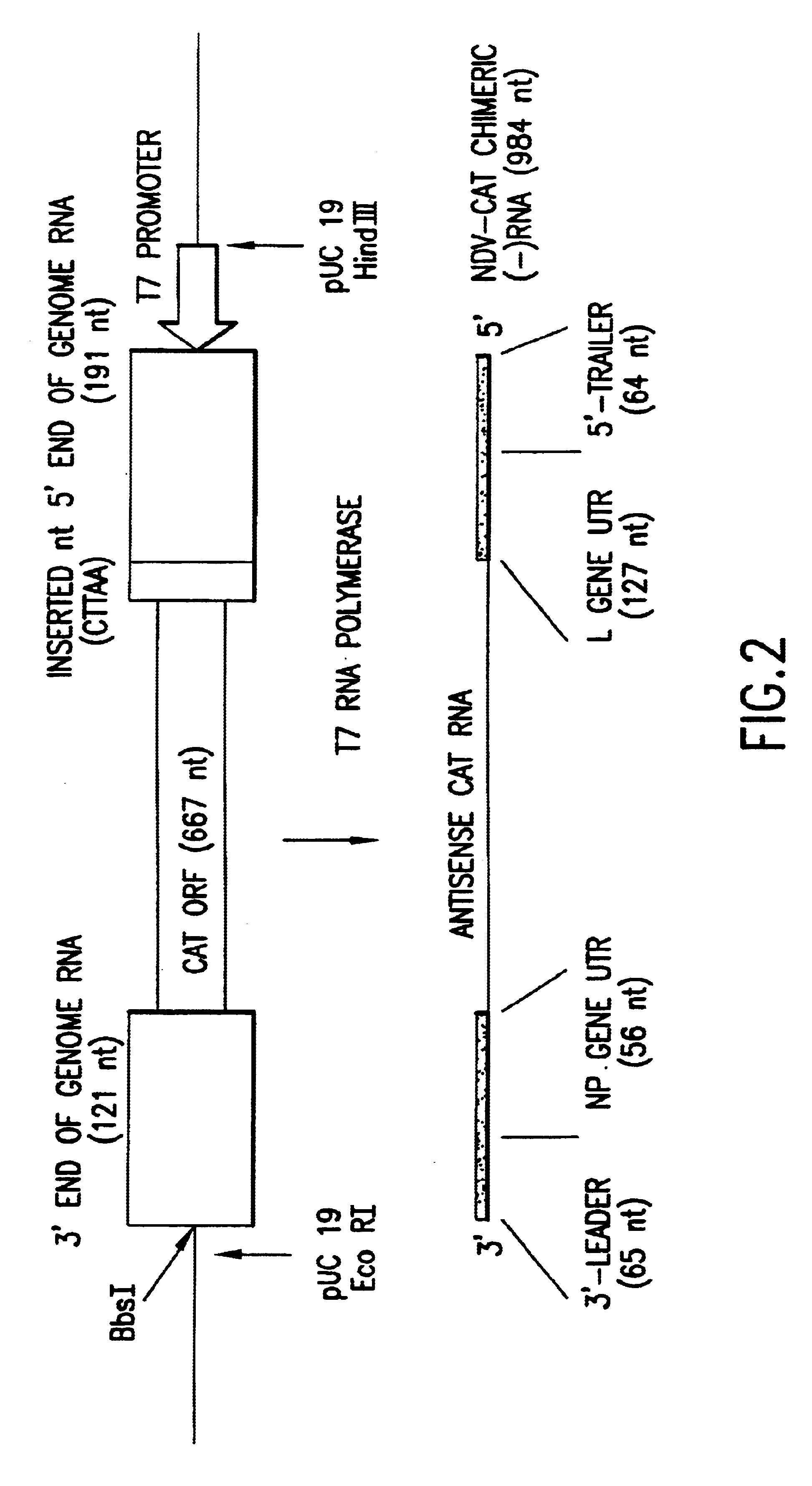
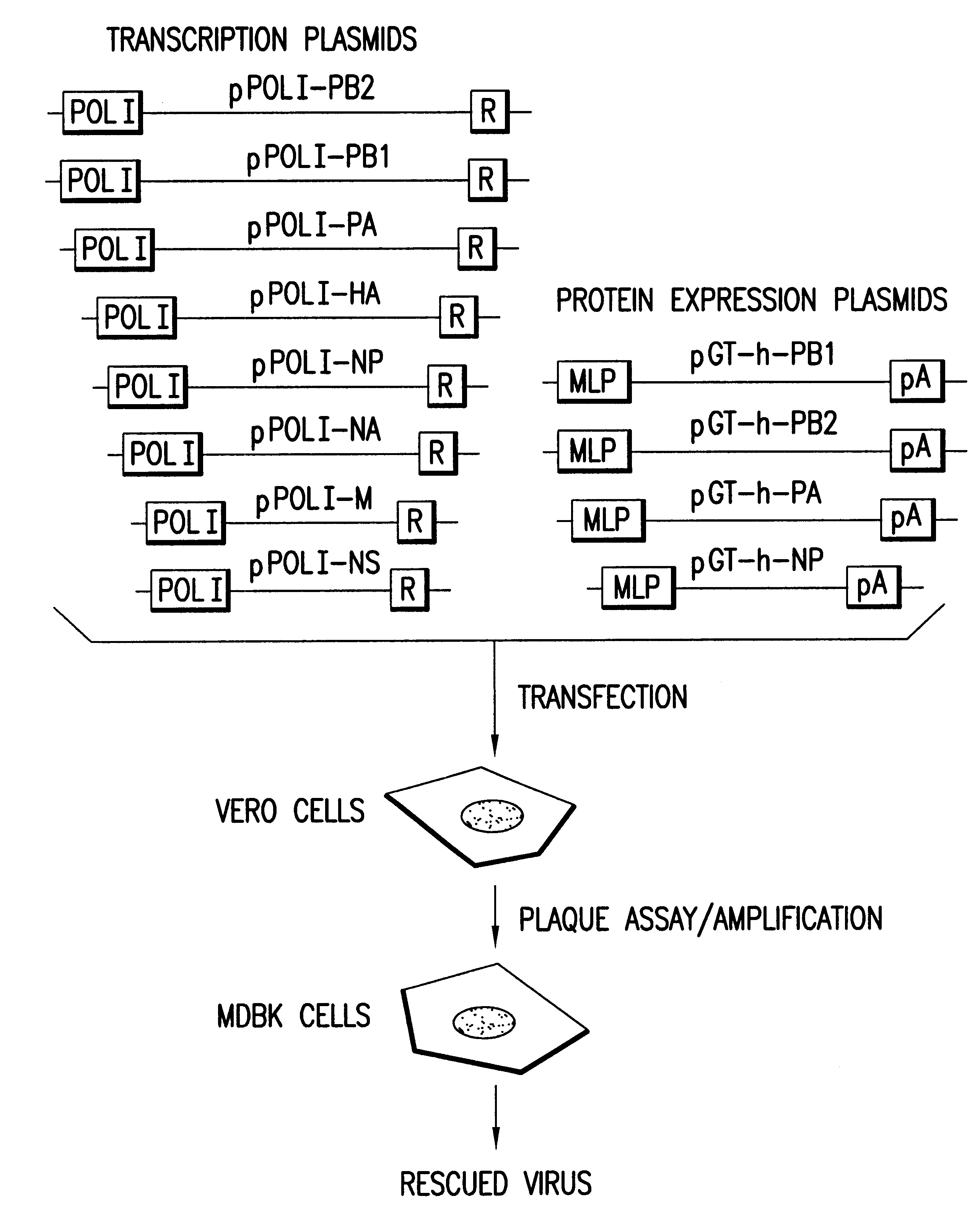
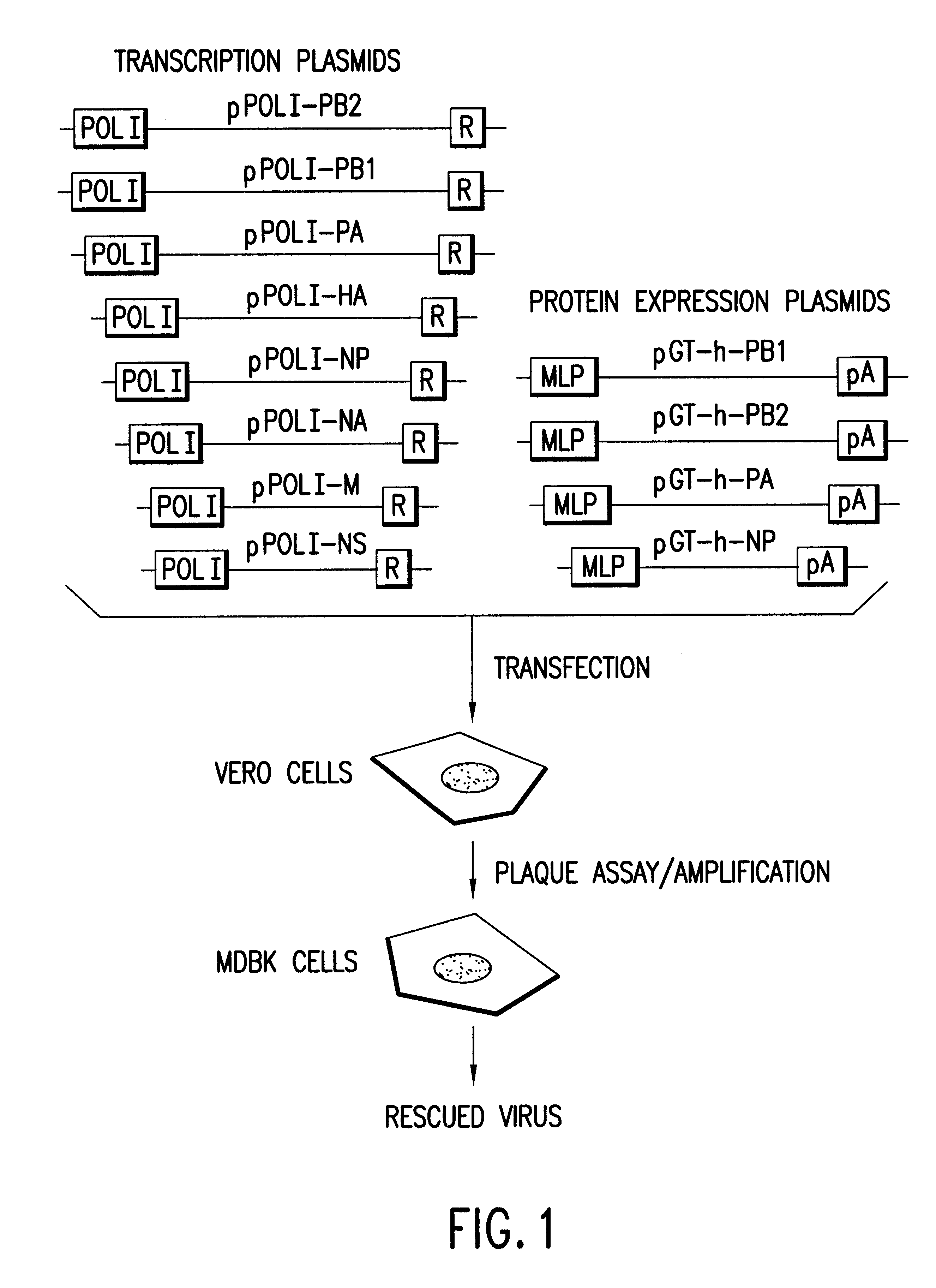
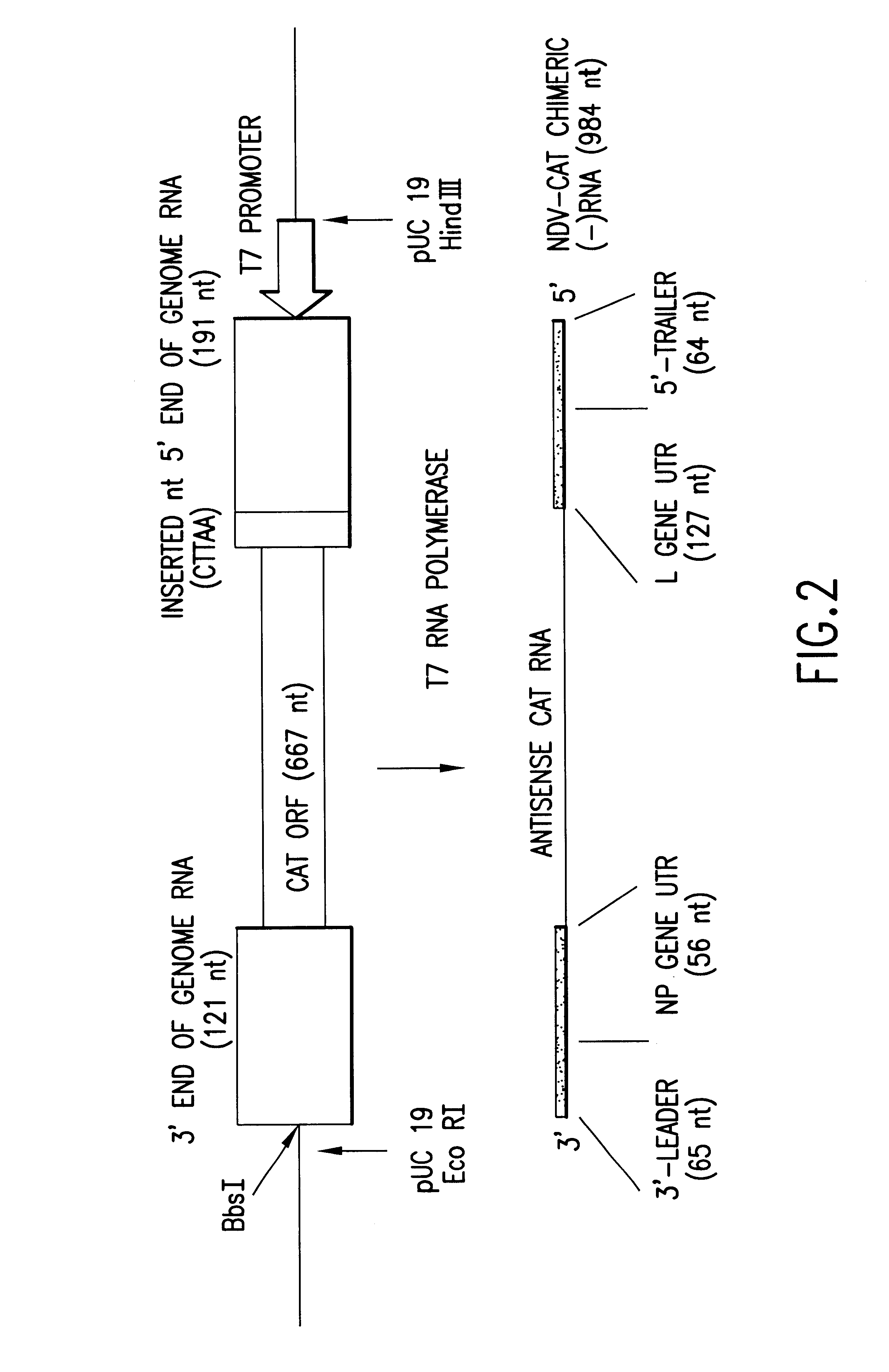
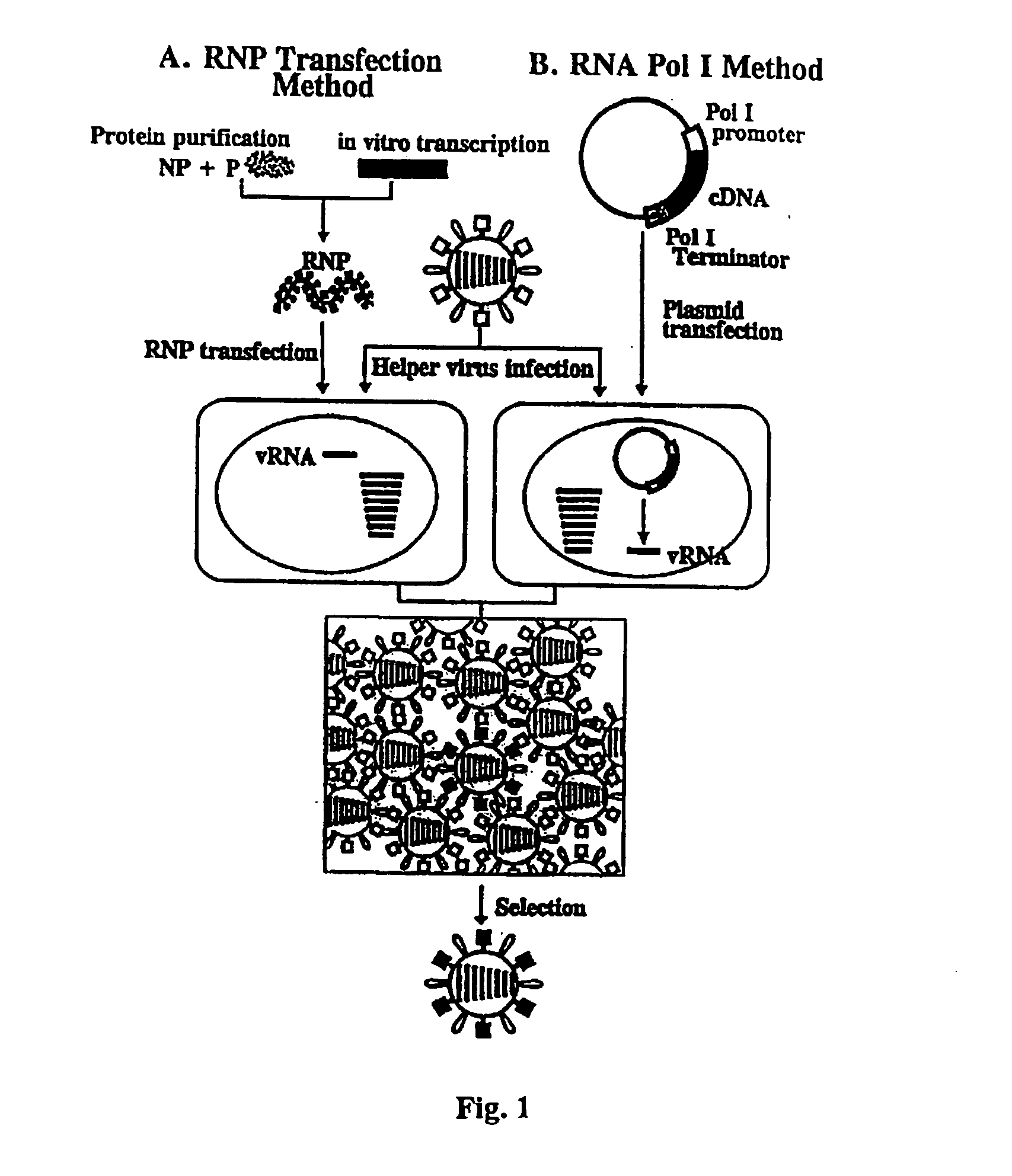
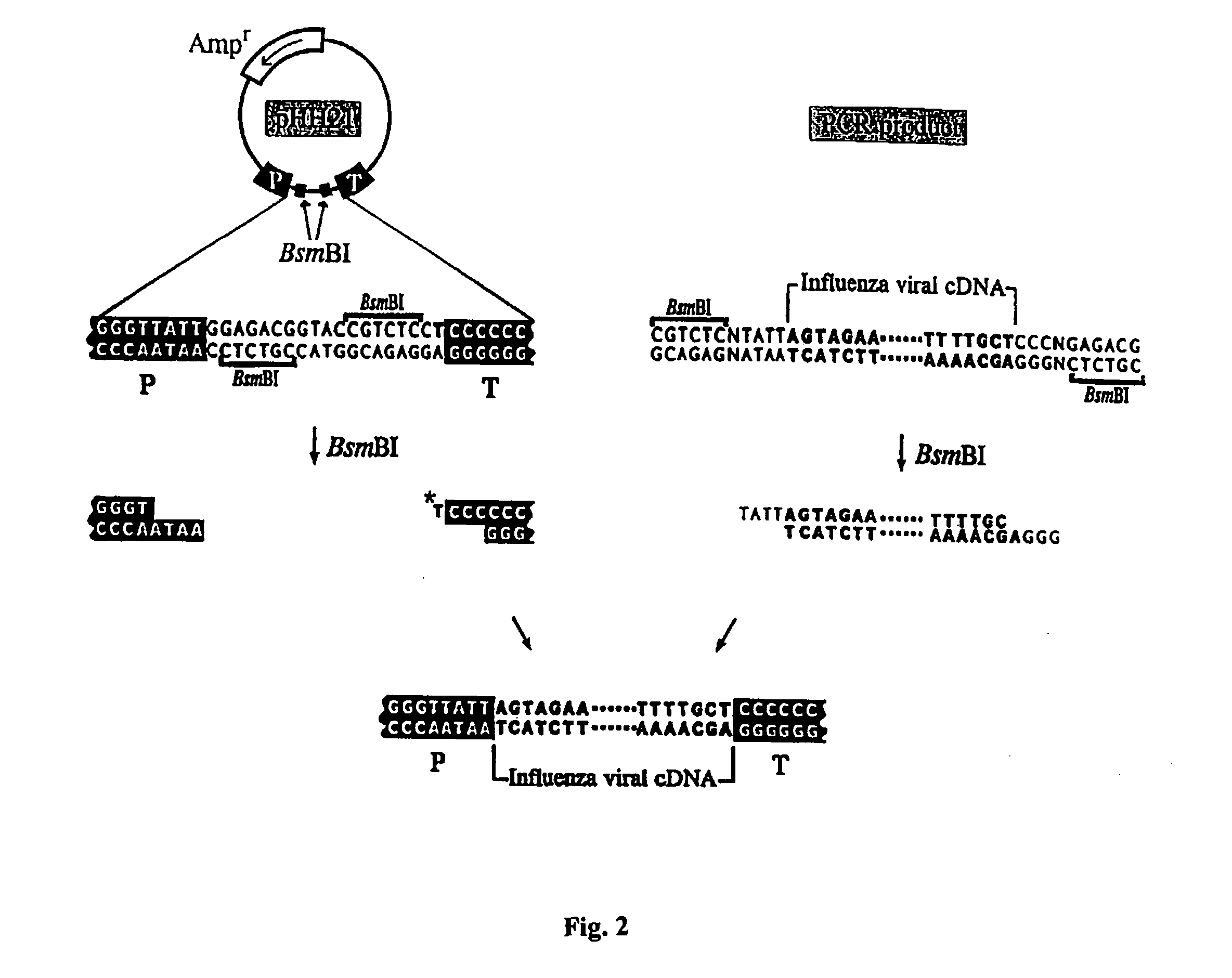
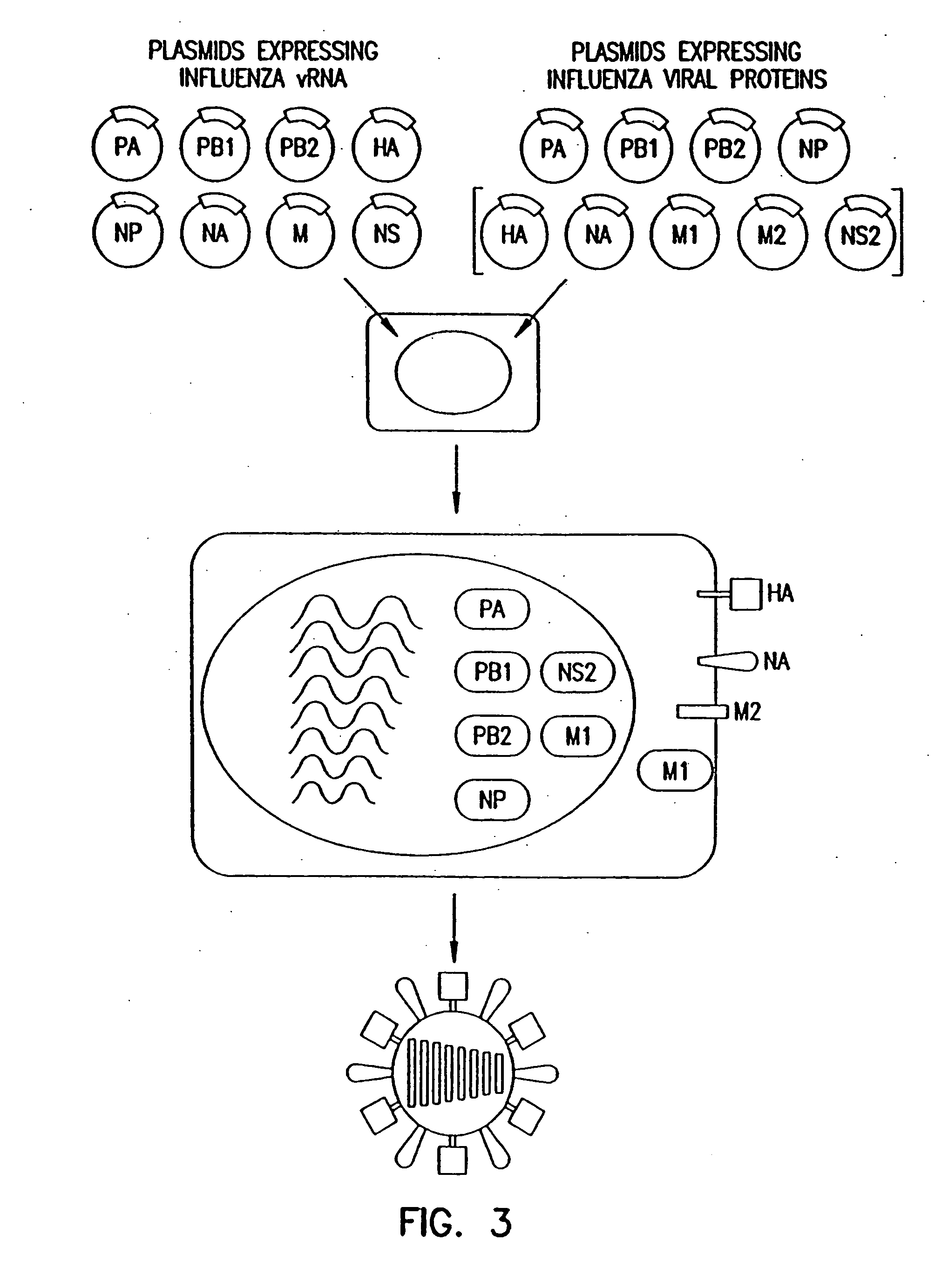
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
Helper-free rescue of recombinant negative strand RNA viruses
InactiveUS6544785B1SsRNA viruses negative-senseGenetic material ingredientsNegative strandNucleic acid sequence
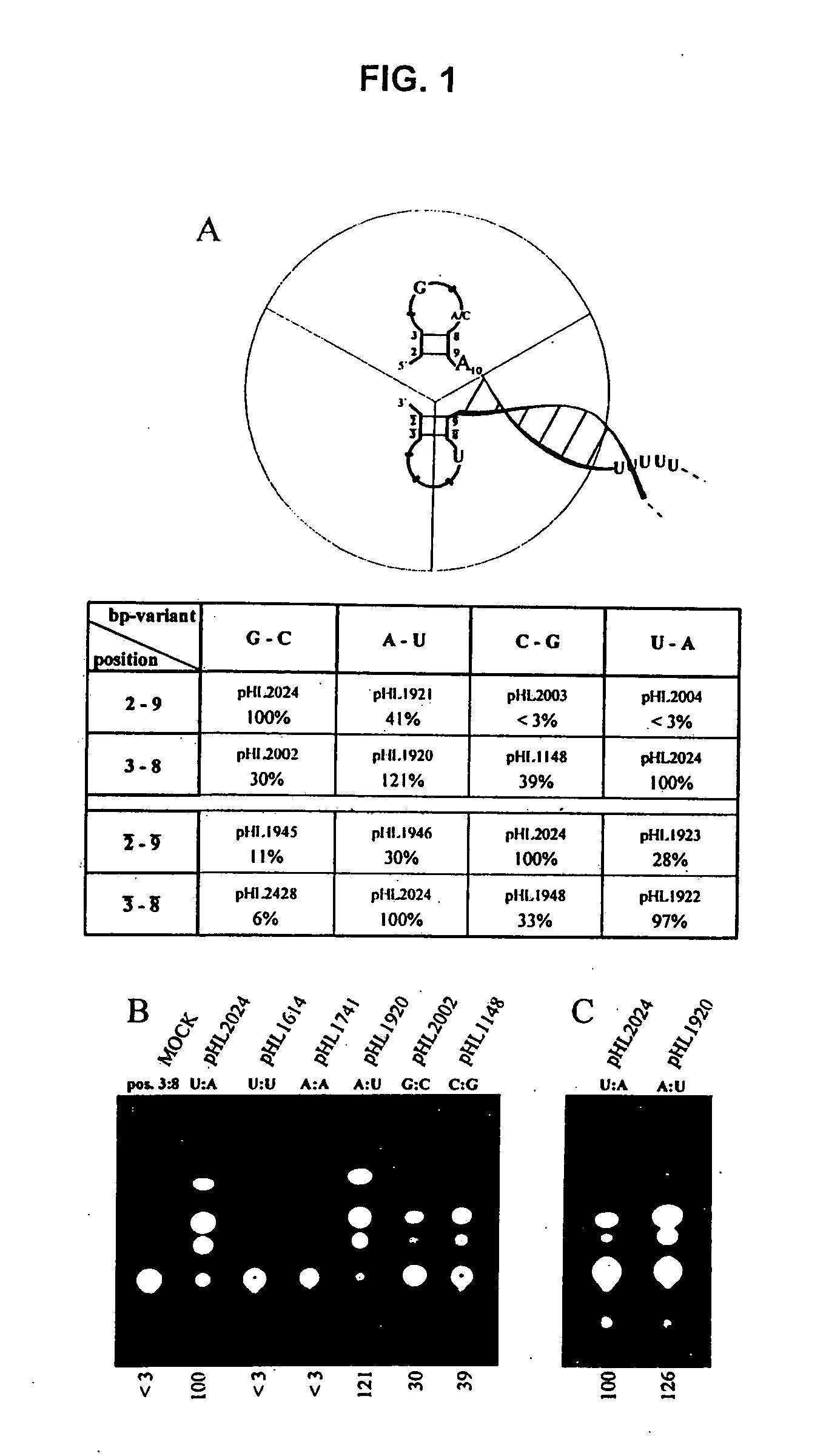
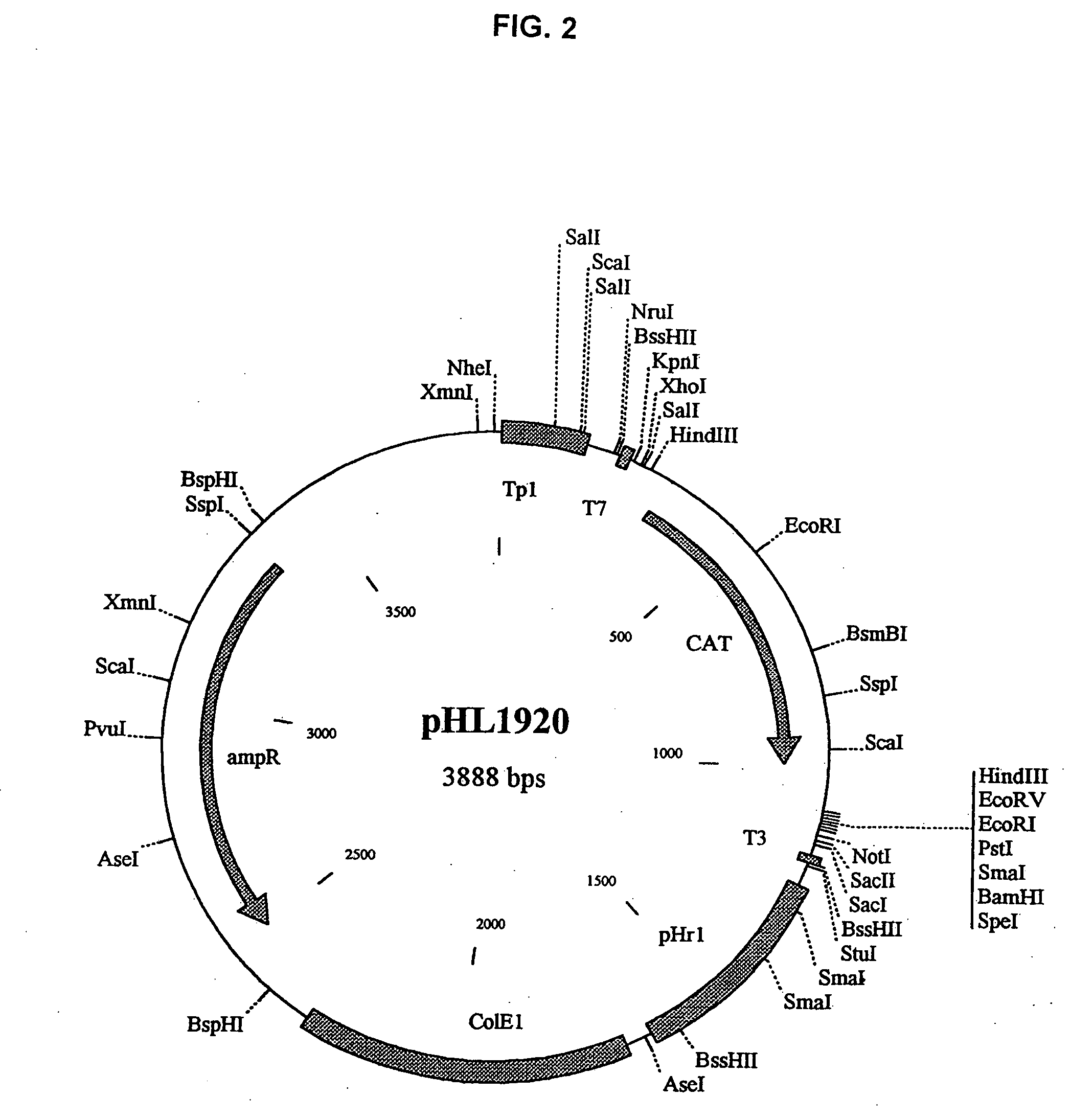
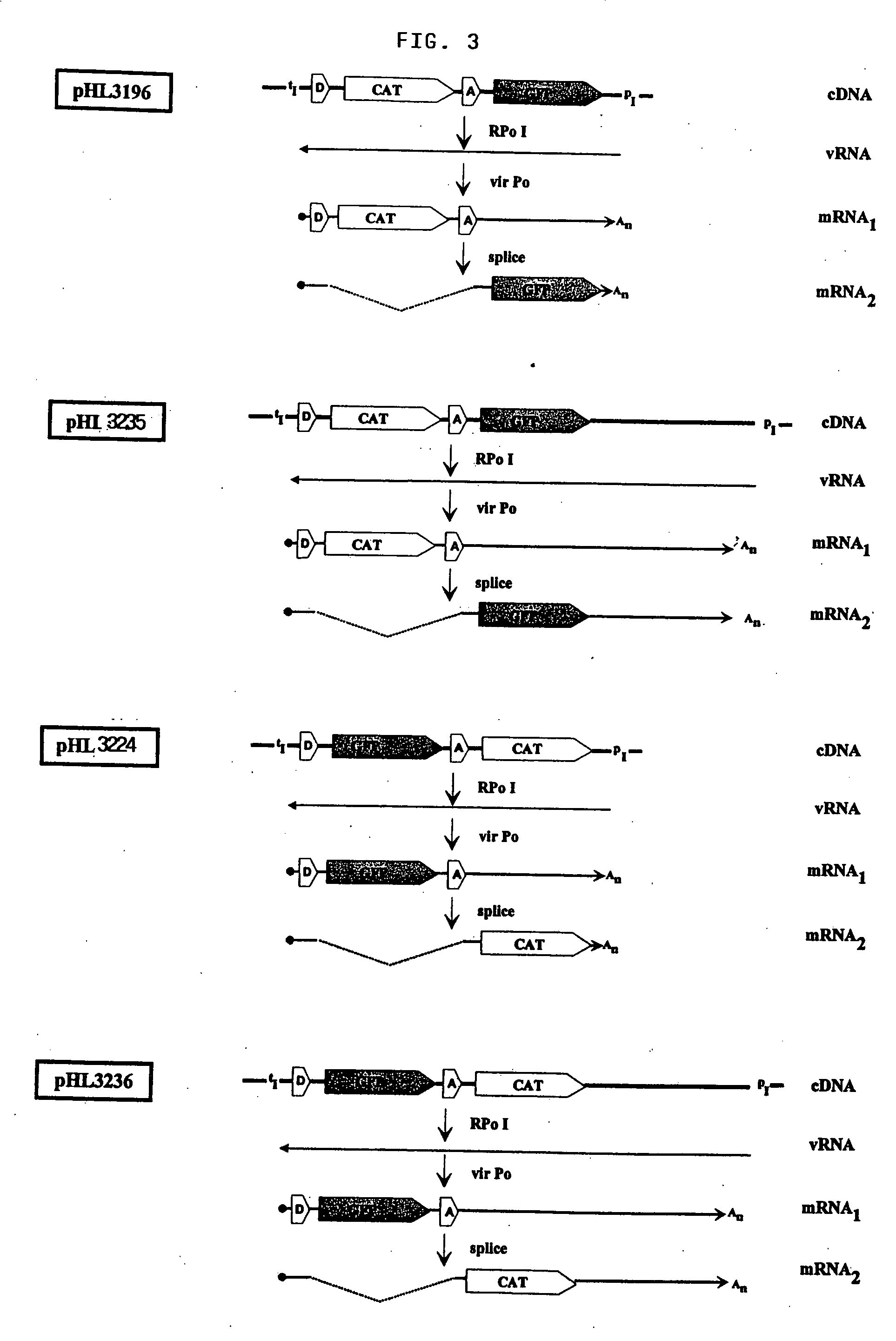
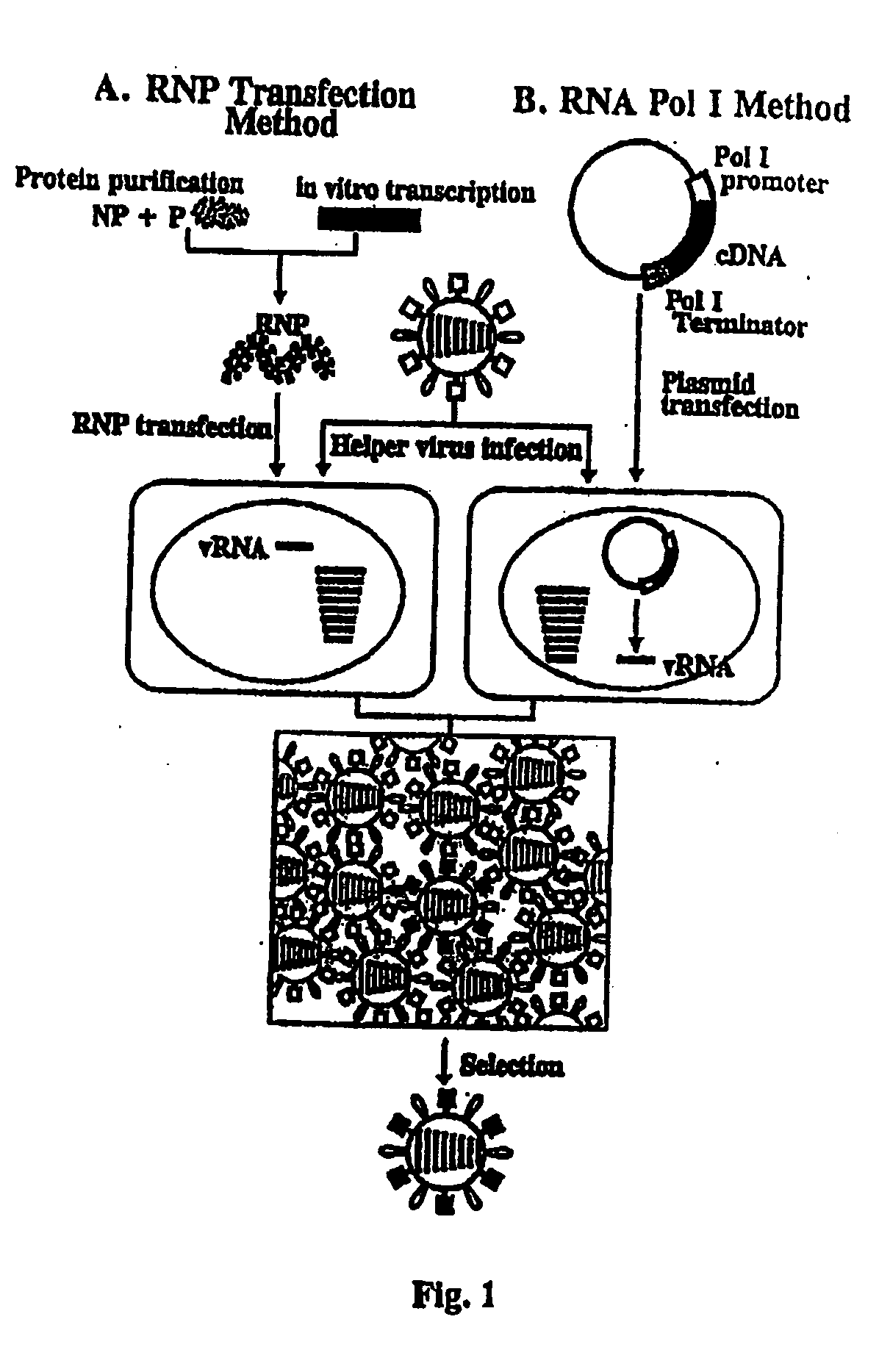
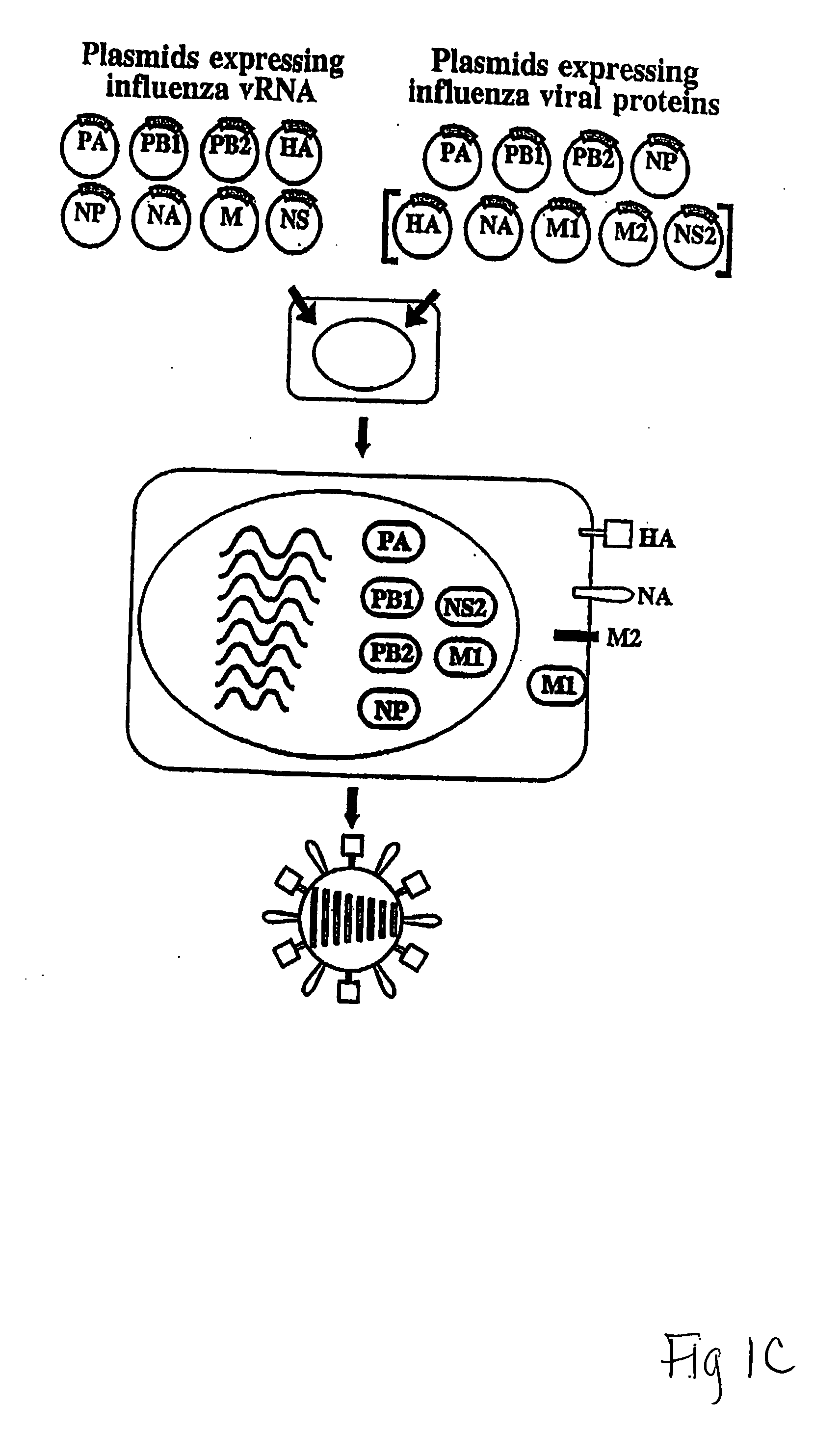
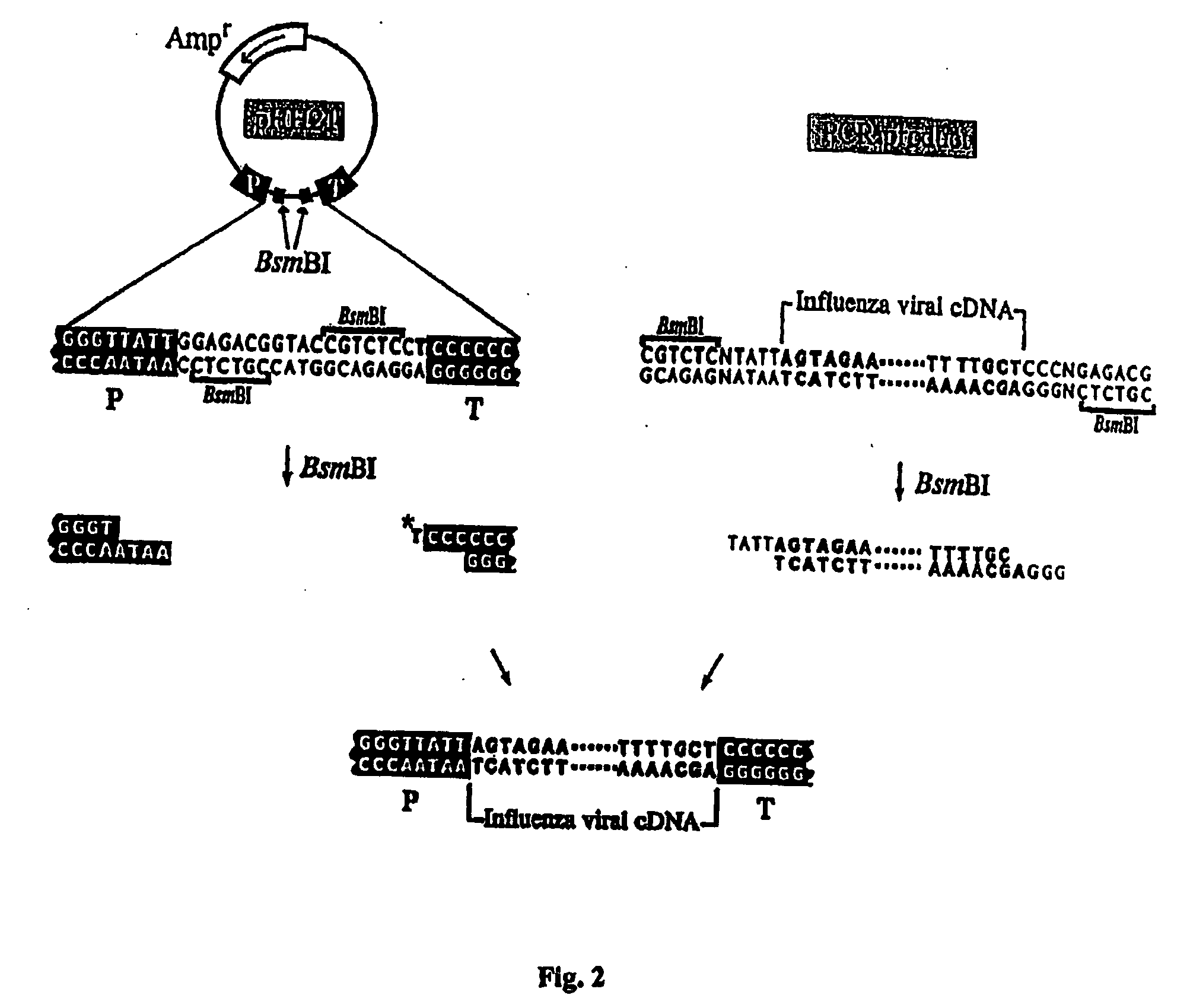
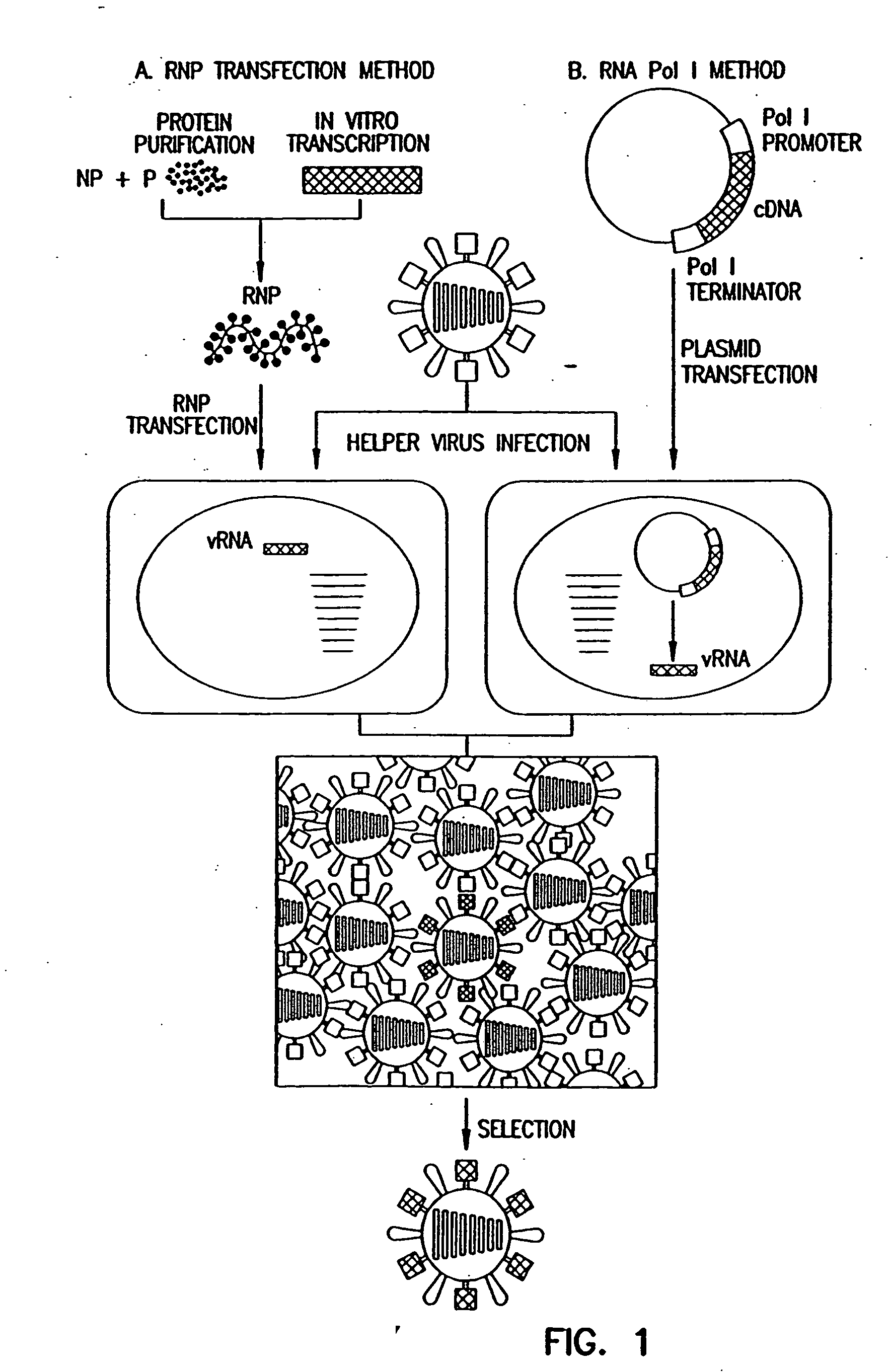
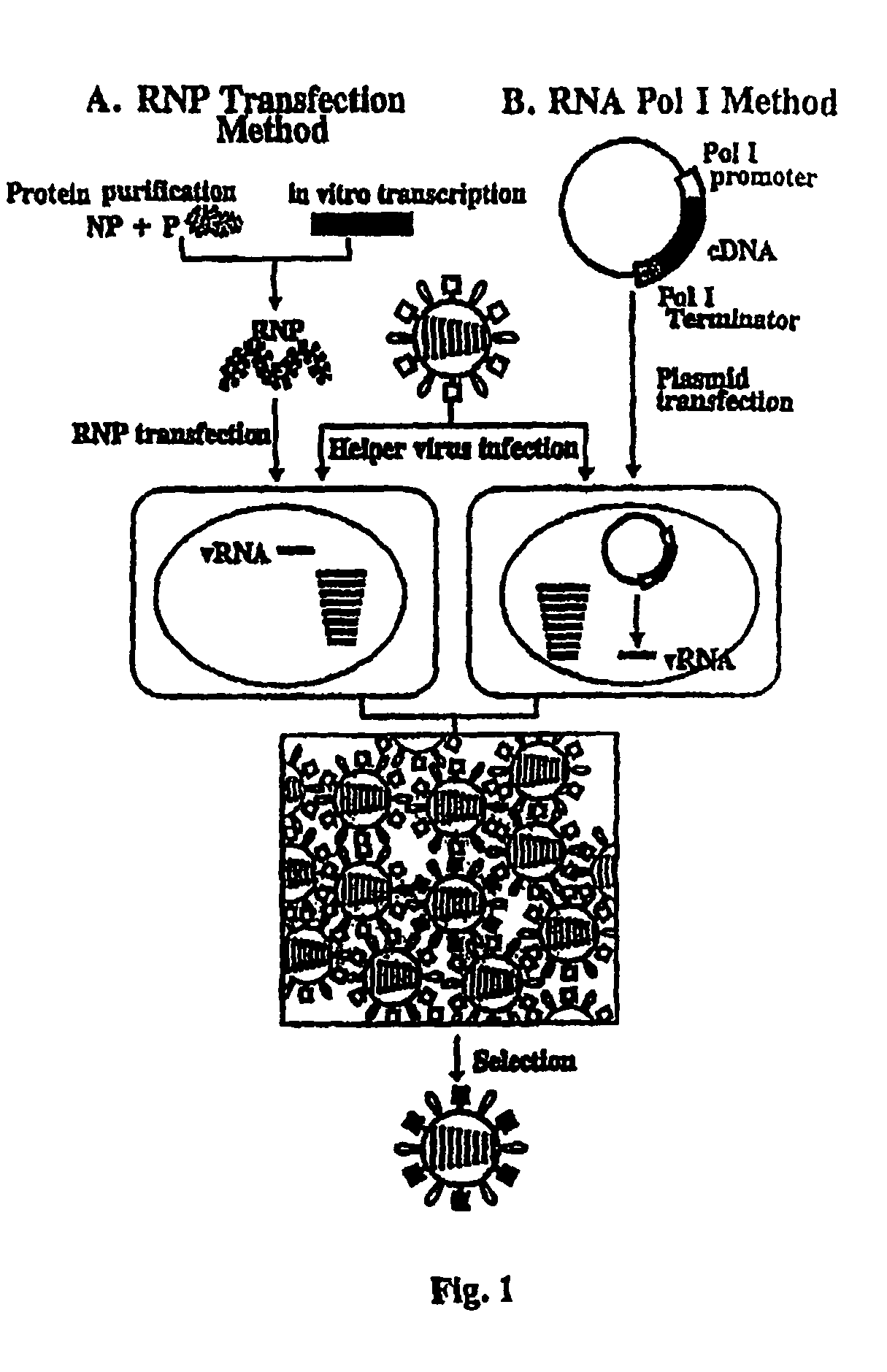
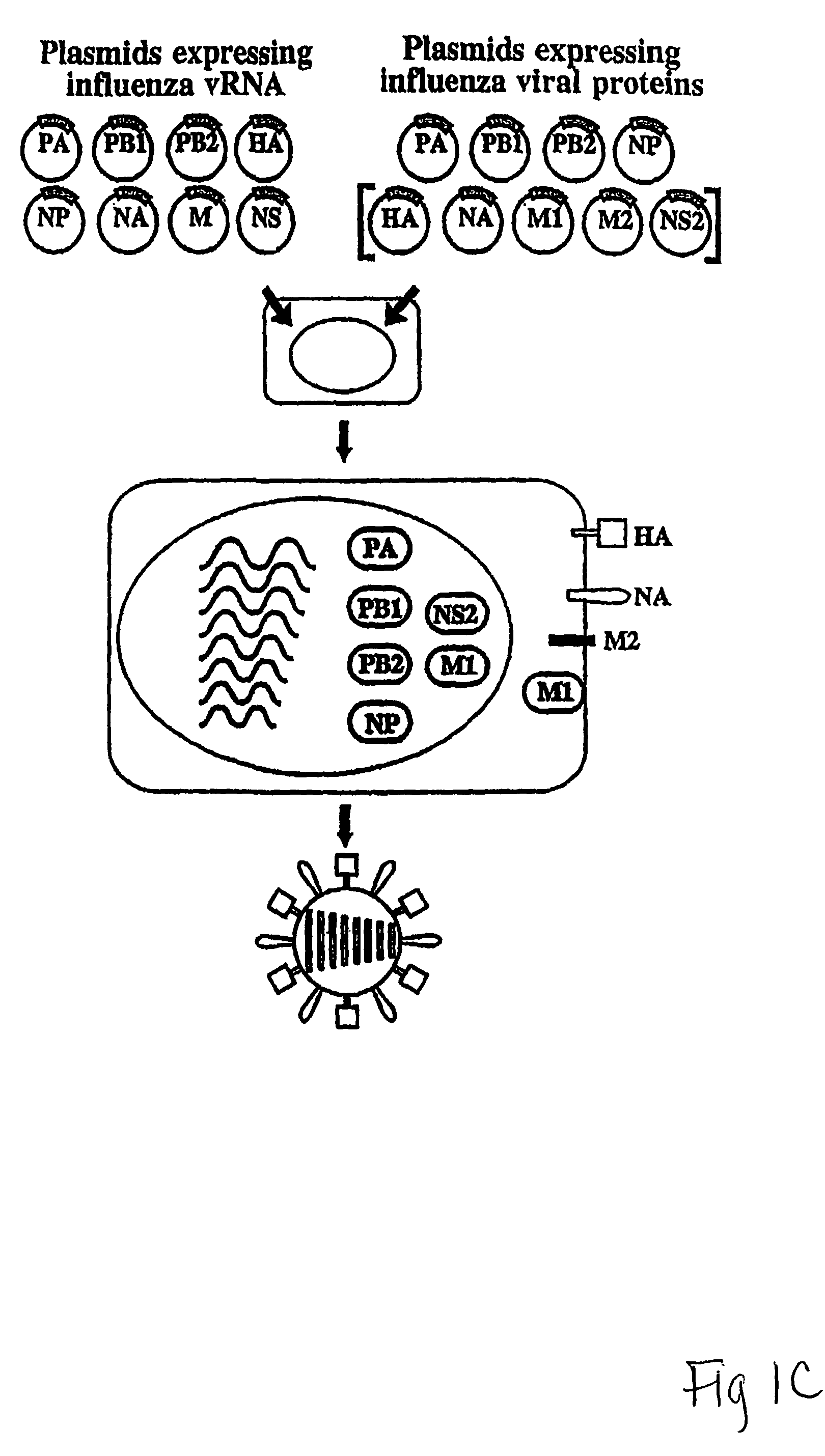
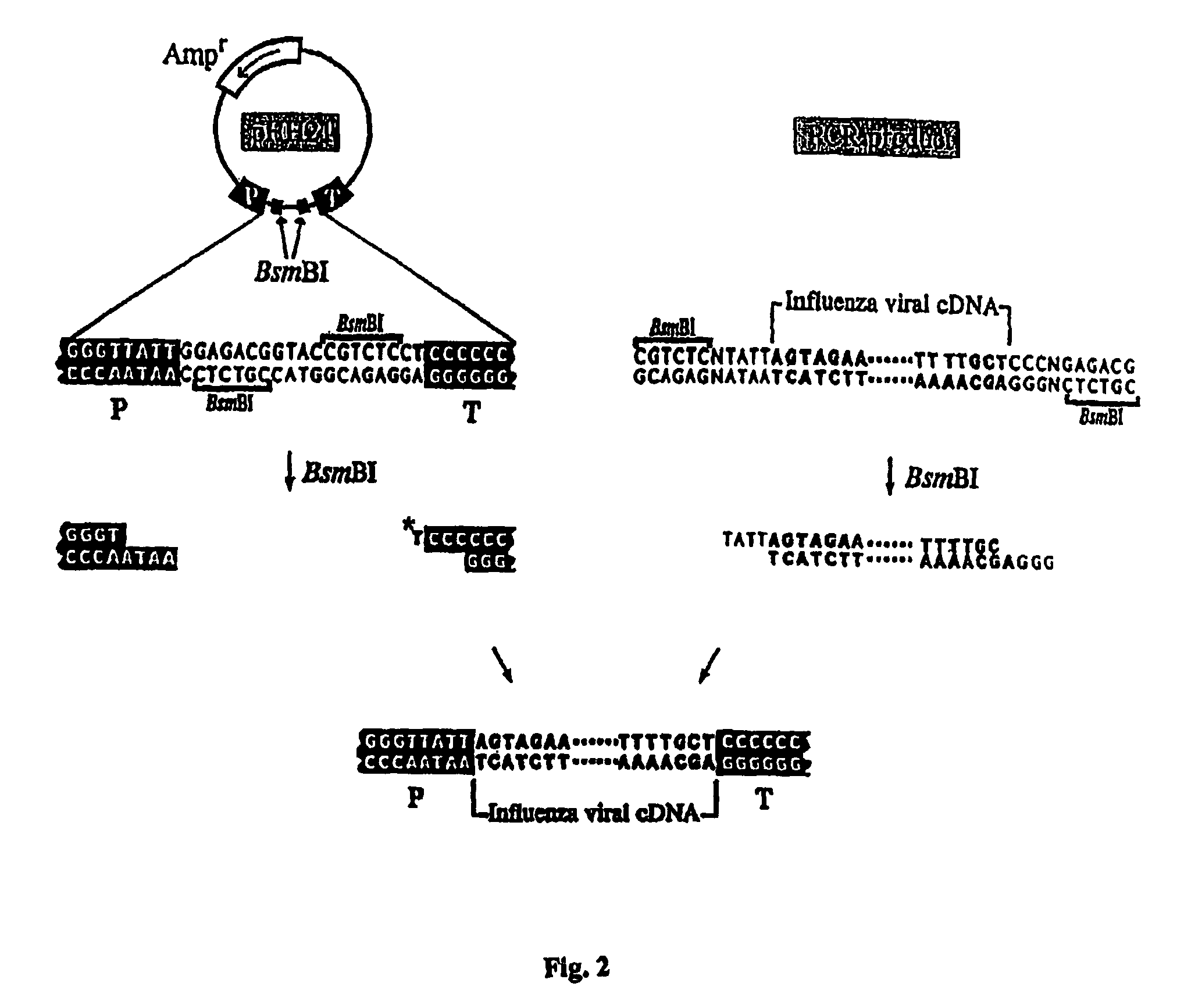
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
Compositions and methods for helper-free production of recombinant adeno-associated viruses
InactiveUS6953690B1Efficient productionIncrease the number ofBiocideGenetic therapy composition manufactureMammalWild type
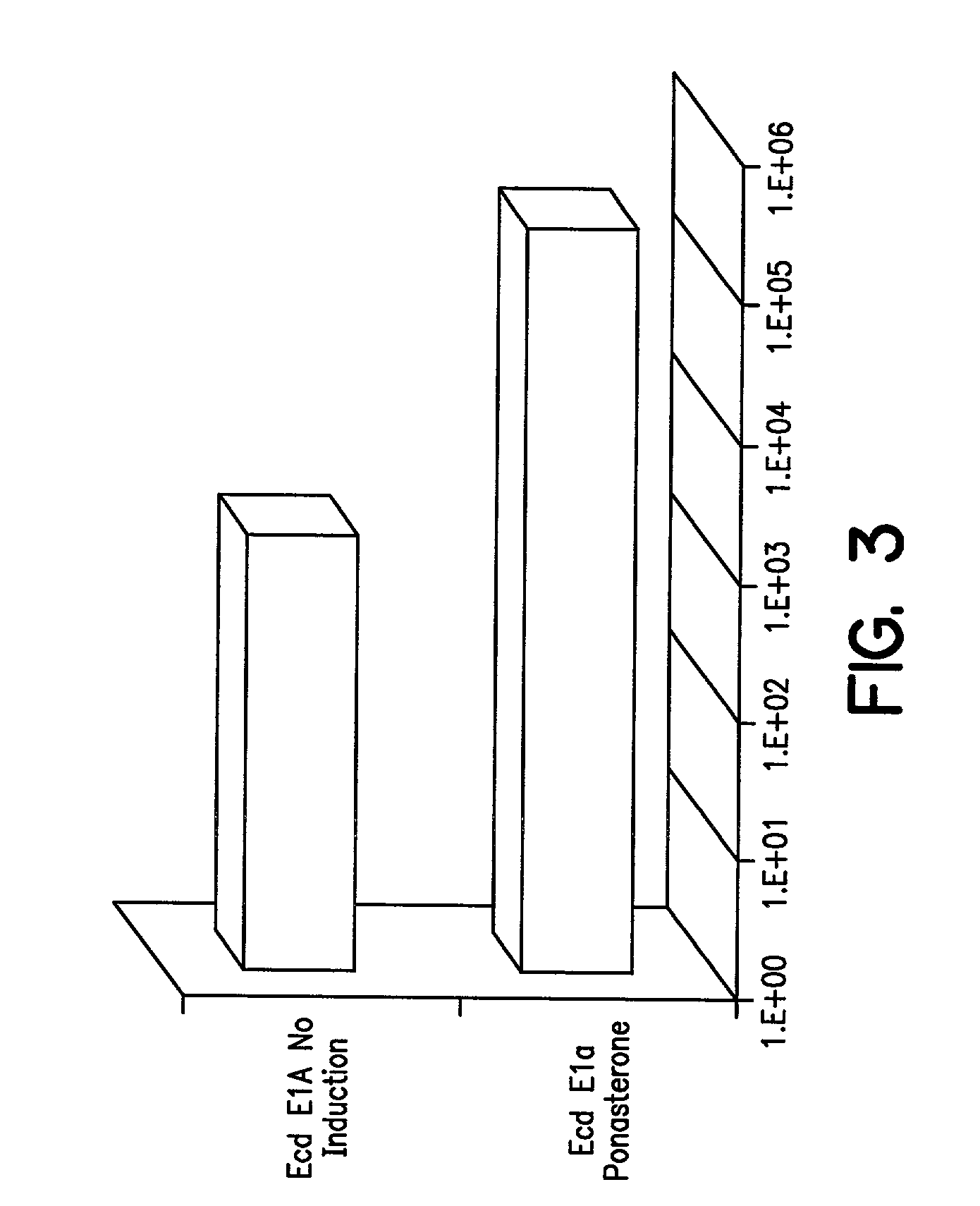
A method for producing recombinant adeno-associated virus in the absence of contaminating helper virus or wild-type virus involves culturing a mammalian host cell containing a transgene flanked by adeno-associated virus (AAV) inverse terminal repeats and under the control of regulatory sequences directing expression thereof, an AAV rep sequence and an AAV cap sequence under the control of regulatory sequences directing expression thereof, and the minimum adenovirus DNA required to express an E1a gene product, an E1b gene product and an E2a gene product, and isolating therefrom a recombinant AAV which expresses the transgene in the absence of contaminating helper virus or wildtype AAV. This method obviates a subsequent purification step to purify rAAV from contaminating virus. Also provided are various embodiments of the host cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods and cell line useful for production of recombinant adeno-associated viruses
InactiveUS7238526B2High yieldInduce expressionGenetically modified cellsGenetic material ingredientsPlasmid VectorAdeno associate virus
Methods for efficient production of recombinant AAV employ a host cell which comprising AAV rep and cap genes stably integrated within the cell's chromosomes, wherein the AAV rep and cap genes are each operatively linked to regulatory sequences capable of directing the expression of the rep and cap gene products upon infection of the cell with a helper virus, a helper gene, and a helper gene product. A method for producing recombinant adeno-associated virus (rAAV) involves infecting such a host cell with a helper virus, gene or gene product and infecting the infected host cell with a recombinant hybrid virus or plasmid vector containing adenovirus cis-elements necessary for replication and virion encapsidation, AAV sequences comprising the 5′ and 3′ ITRs of an AAV, and a selected gene operatively linked to regulatory sequences directing its expression, which is flanked by the above-mentioned AAV sequences.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
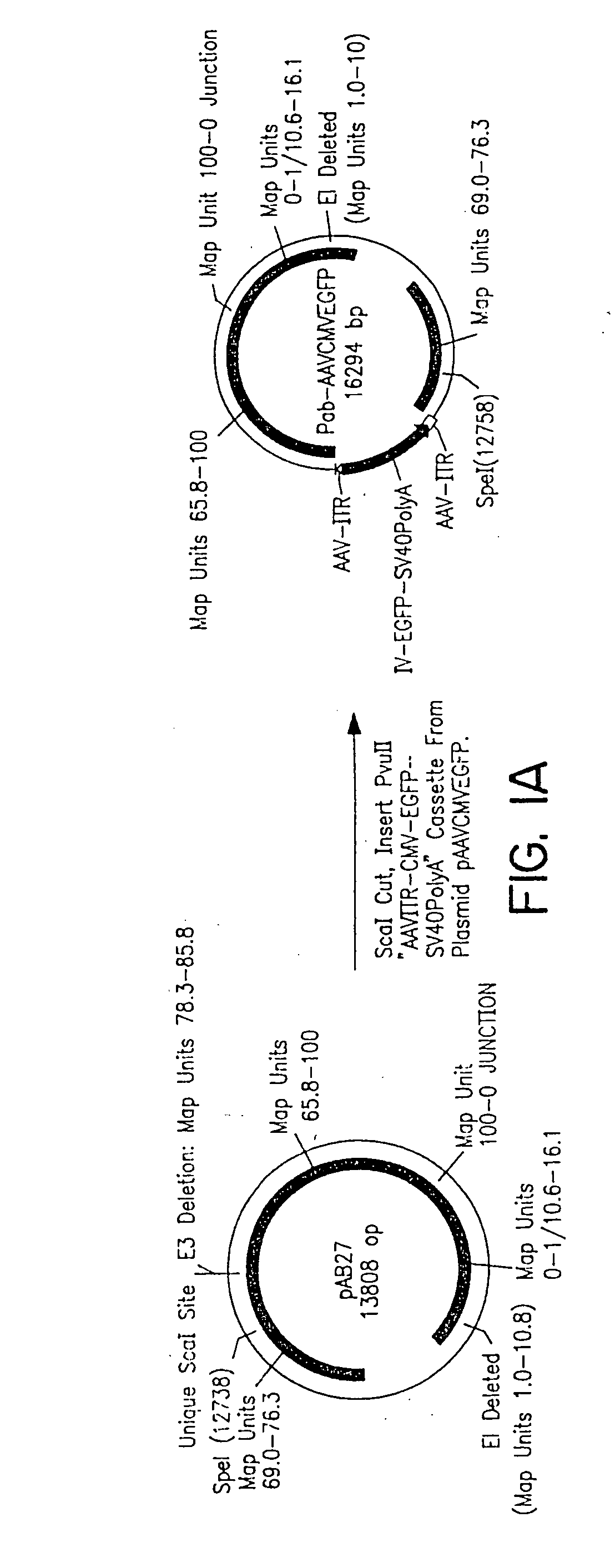
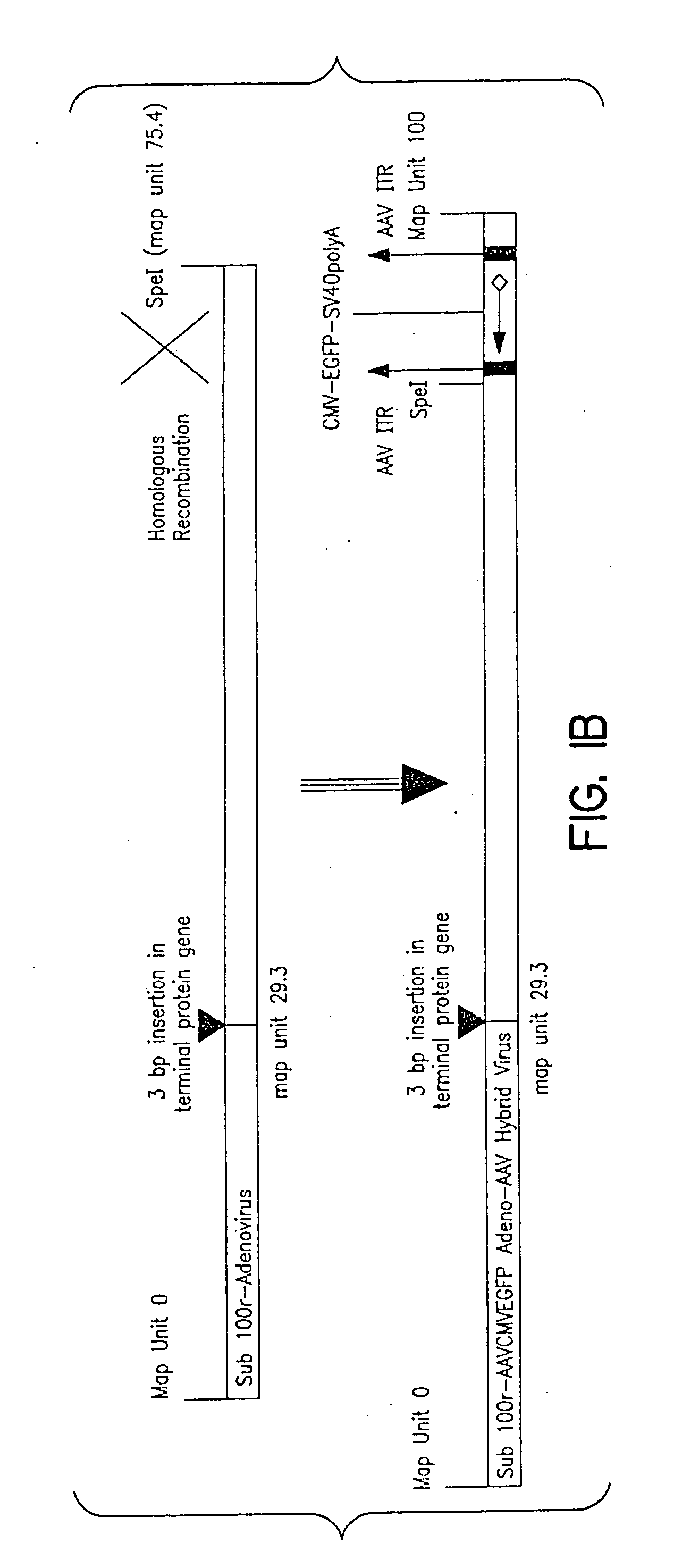
Helper-free, totally defective adenovirus for gene therapy
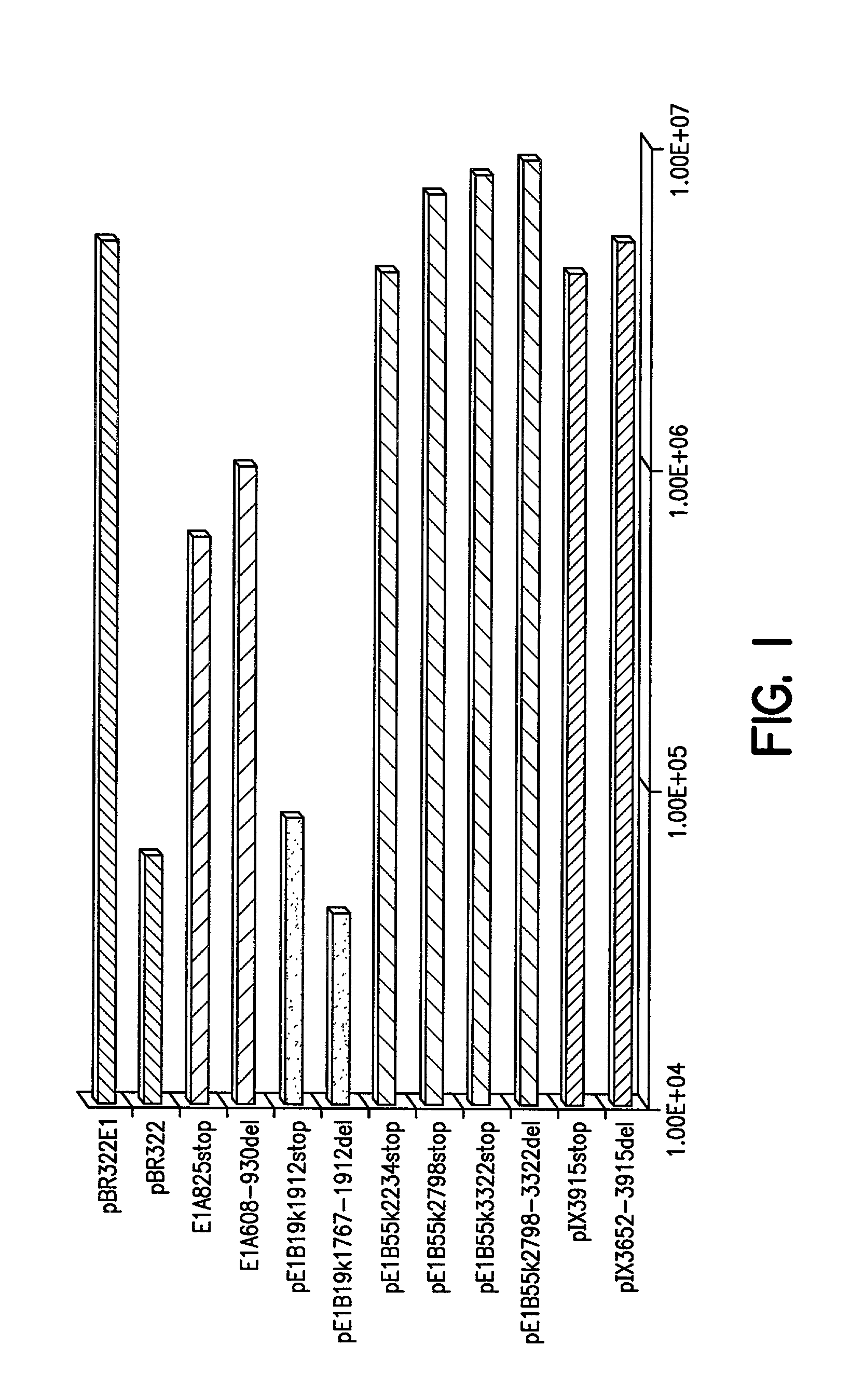
InactiveUS6228646B1Encapsidation efficiencyMaximal recombinationInactivation/attenuationNucleic acid vectorIn vivoHelper virus
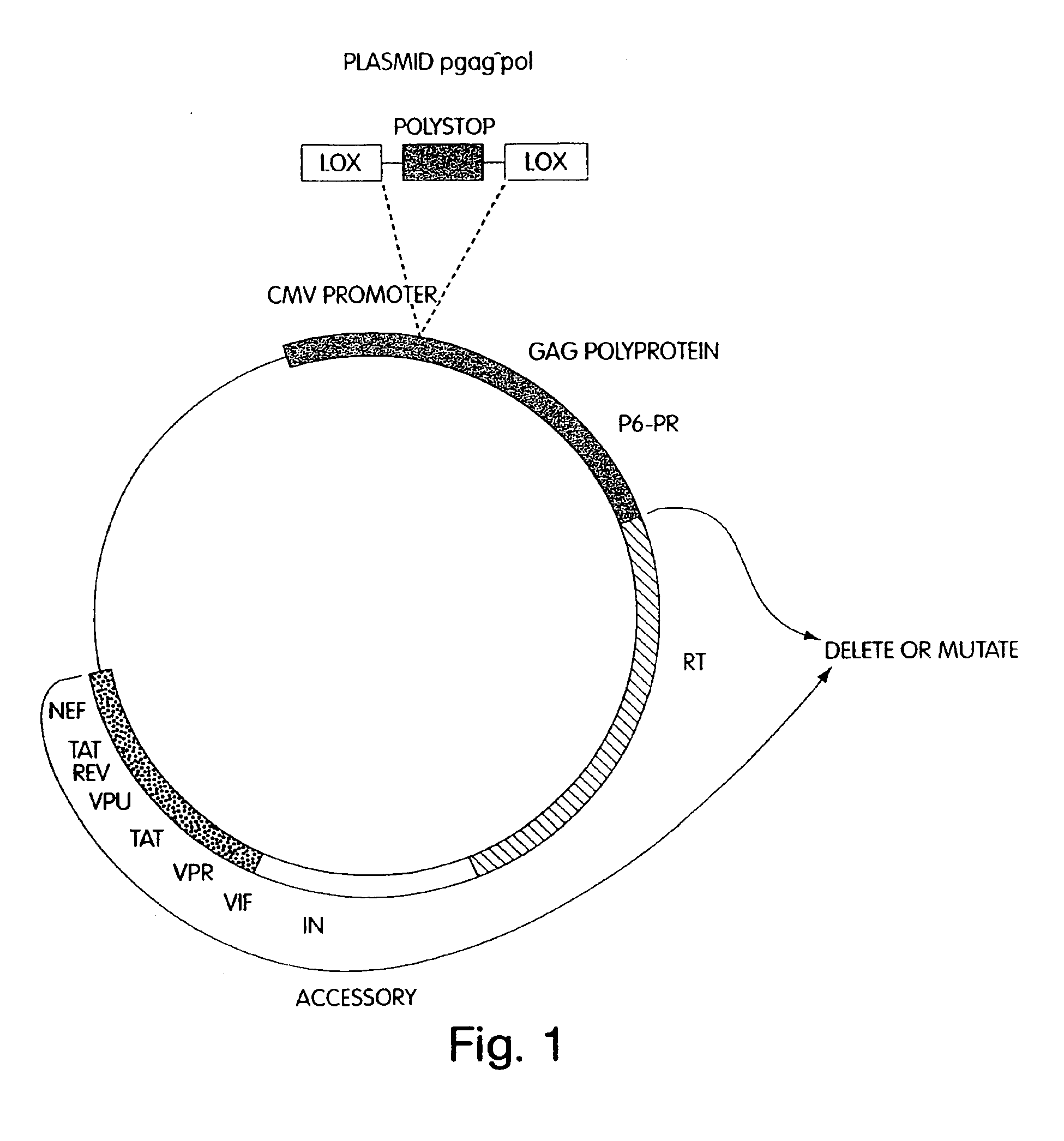
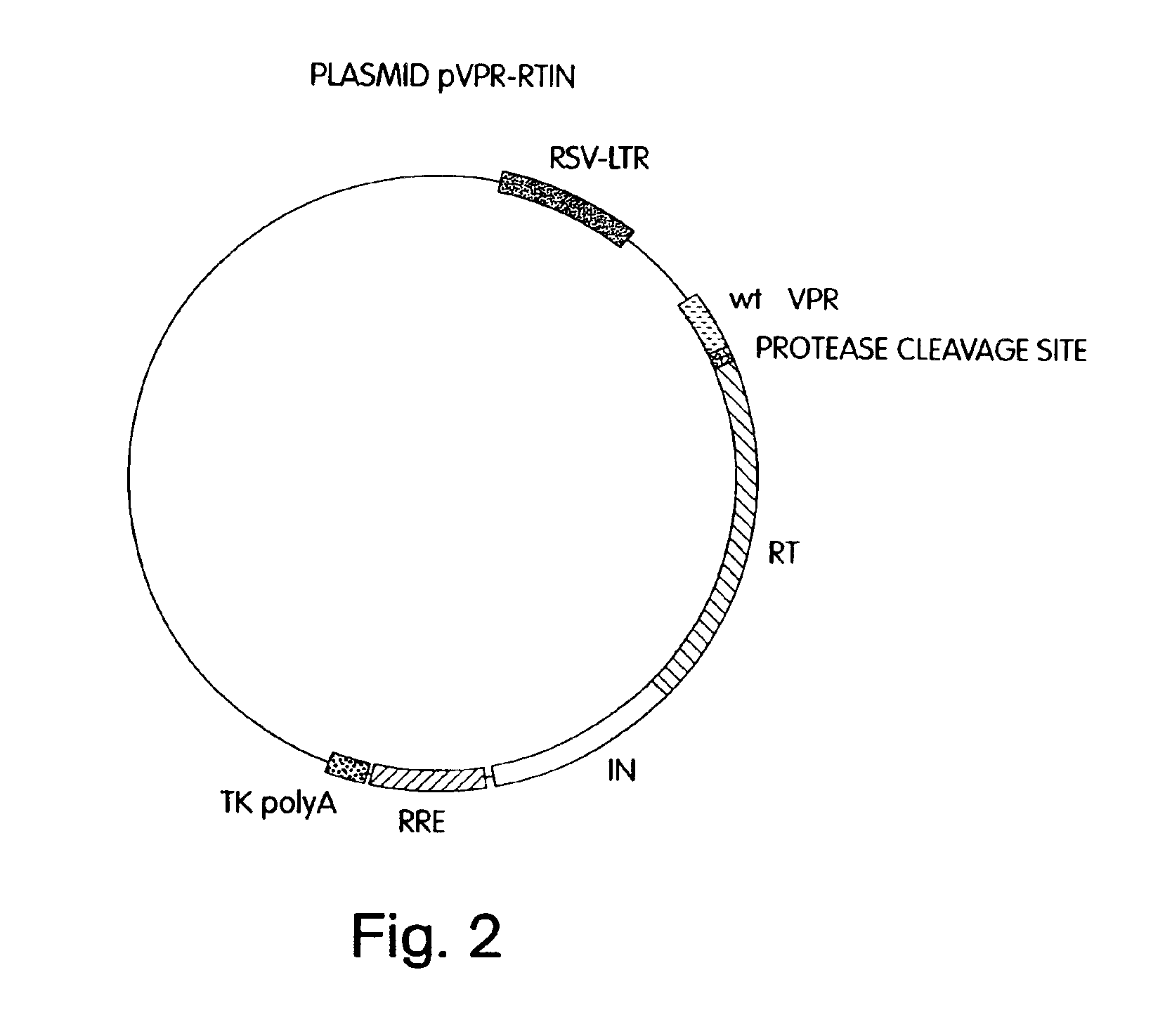
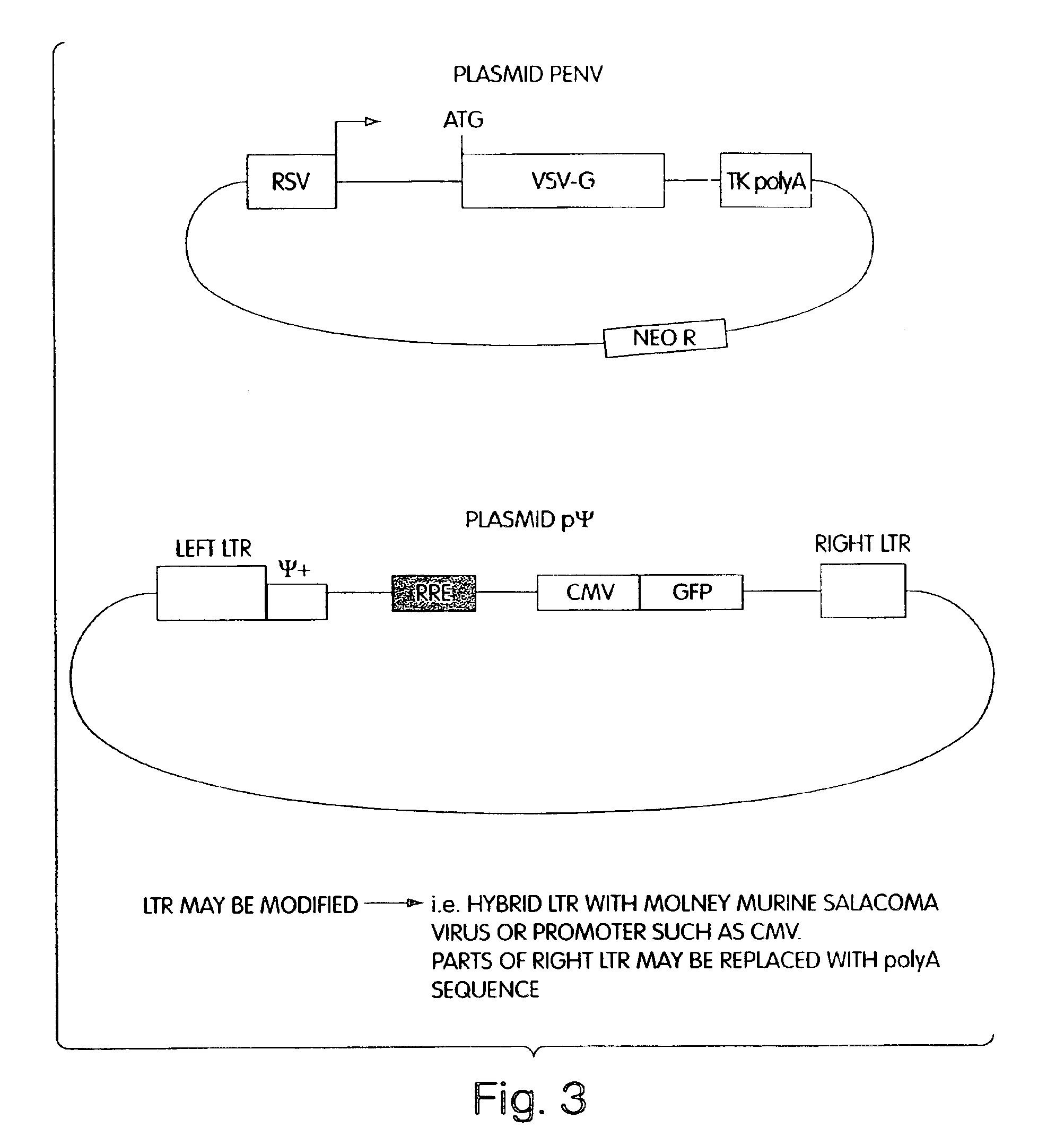
A method for producing in vivo packaged recombinant adenovirus vectors is provided. The recombinant Ad vectors do not contain any Adenovirus genes and are therefore useful for gene therapy. The recombinant Adenovirus vectors are packaged in vivo using a helper virus which is itself very inefficiently packaged, providing a recombinant viral preparation with very little or no contamination with helper virus. In particular, the method makes use of a helper virus in which the packaging site can be easily excised in vivo by recombination mediated by a recombinase. The helper virus is also useful for the in vivo construction of new recombinant adenovirus vectors containing substitutions in the E1 or other adenoviral region.
Owner:RGT UNIV OF CALIFORNIA
Compositions and methods for helper-free production of recombinant adeno-associated viruses
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
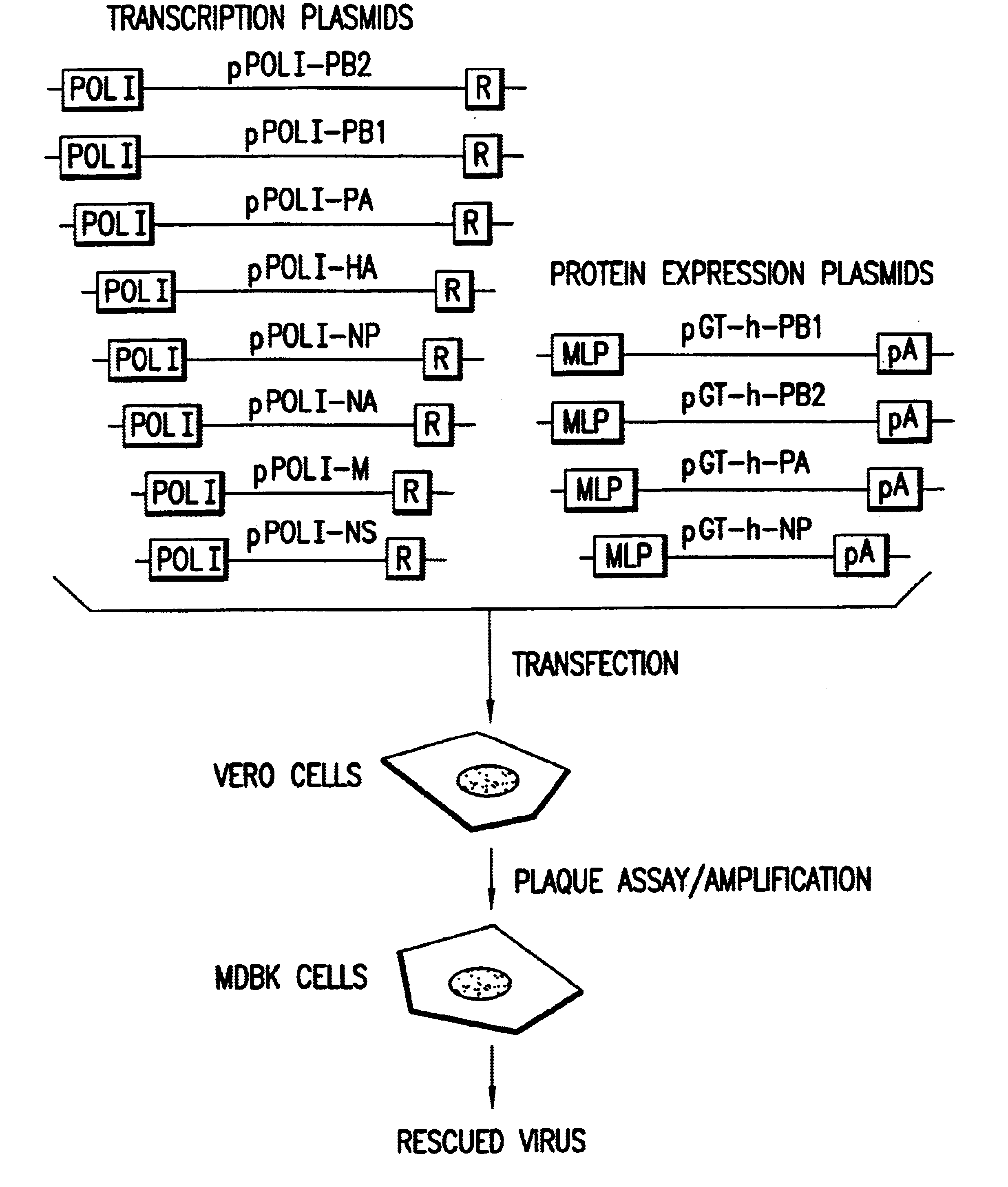
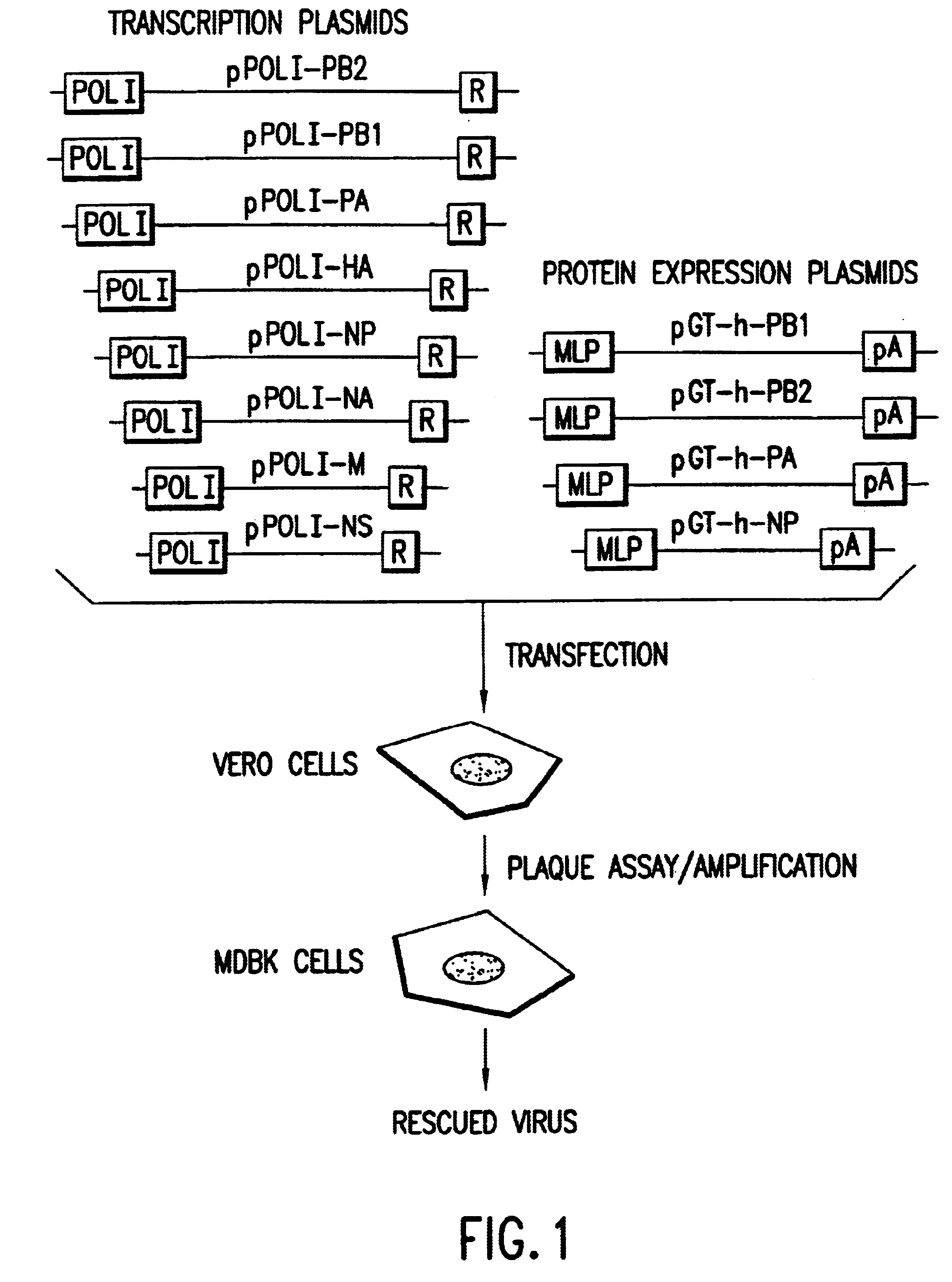
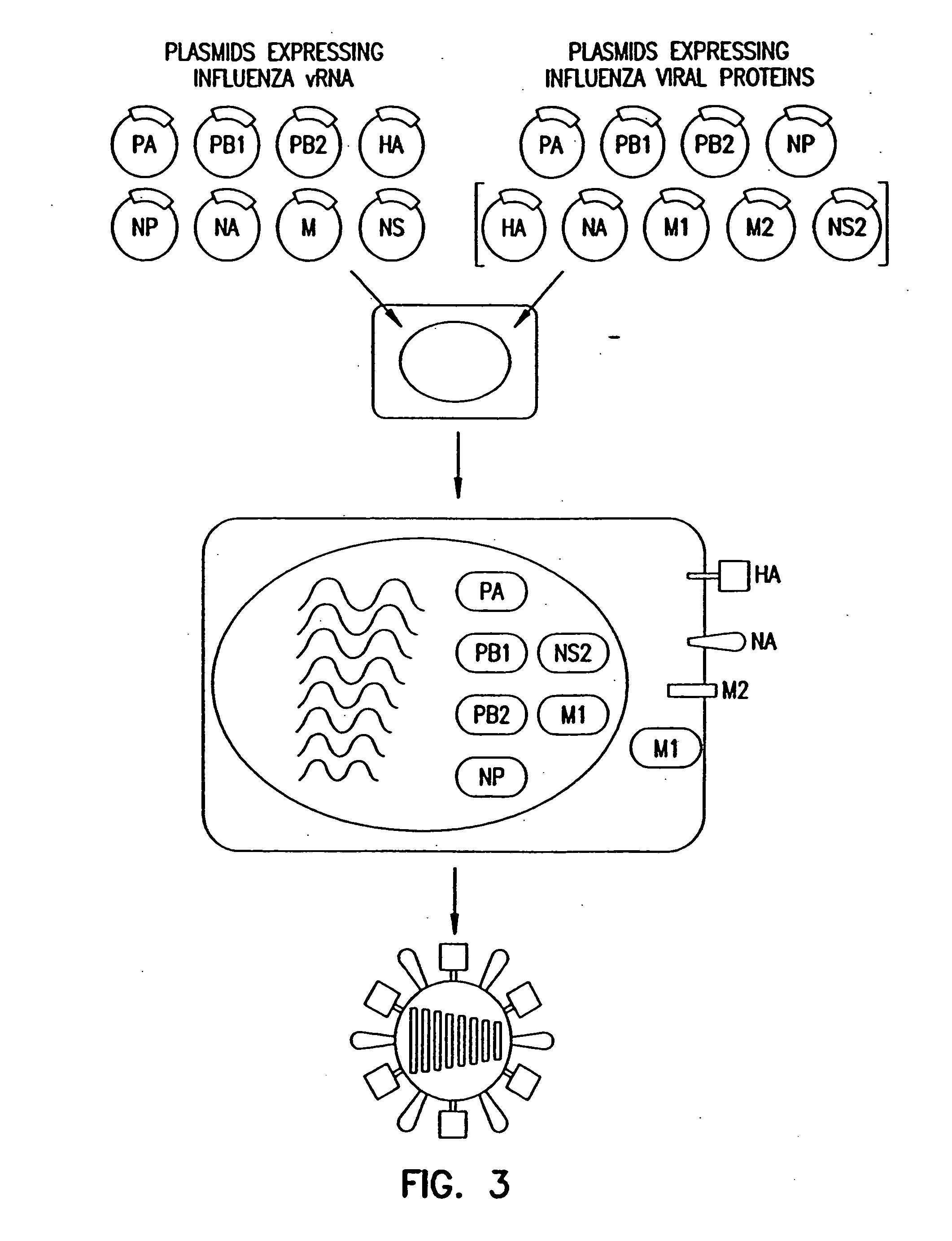
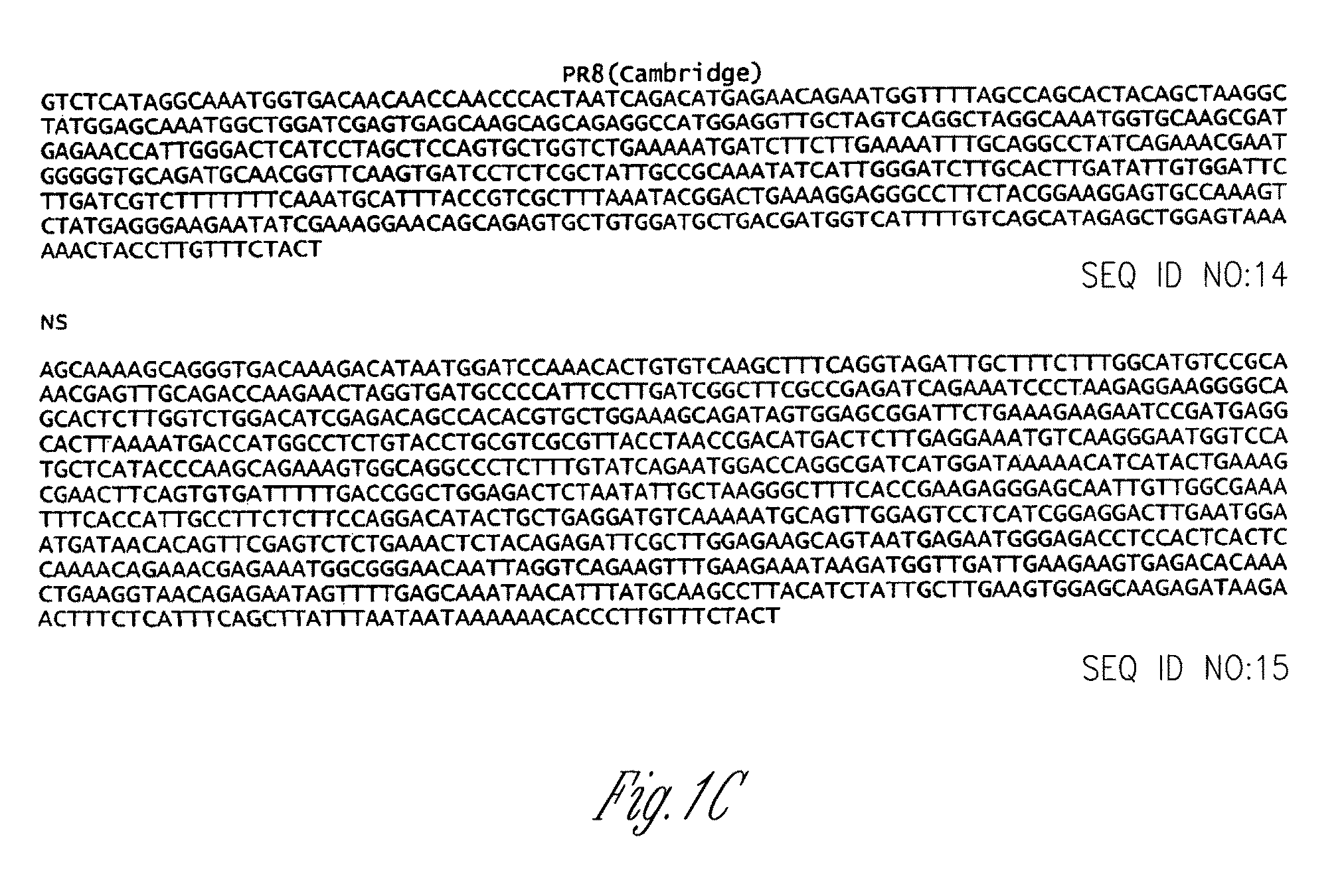
Recombinant influenza vectors with a PolII promoter and ribozymes for vaccines and gene therapy
InactiveUS20050037487A1Easy to operateEnhances these viruses as vaccine vectorsSsRNA viruses negative-senseSugar derivativesHelper virusViral vector
The invention provides a composition useful to prepare influenza viruses, e.g., in the absence of helper virus, using vectors which include Po1II promoters and multiple ribozyme sequences.
Owner:WISCONSIN ALUMNI RES FOUND
Recombinant influenza viruses for vaccines and gene therapy
InactiveUS20060057116A1Improve efficiencyIncrease productionSsRNA viruses negative-senseBiocideHelper virusGene
The invention provides a composition useful to prepare influenza A viruses, e.g., in the absence of helper virus.
Owner:KAWAOKA YOSHIHIRO +1
Recombinant influenza viruses with bicistronic vRNAs coding for two genes in tandem arrangement
The invention relates to recombinant influenza viruses for high-yield expression of incorporated foreign gene(s), which are genetically stable in the absence of any helper virus and which comprise at least one viral RNA segment being a tandem bicistronic RNA molecule coding for two genes in tandem, in said tandem bicistronic RNA molecule one of the standard viral genes being in covalent junction with a foreign, recombinant gene and having an upstream splice donor and a downstream splice acceptor signal surrounding the proximal coding region. The invention further provides a method for obtaining attenuated viruses which resist reassortment dependent progeny production in case of chance superinfections by wild-type influenza viruses; a method for the production of said recombinant influenza viruses; pharmaceutical compositions comprising said recombinant influenza viruses; and the use of said recombinant influenza viruses for preparing medicaments for vaccination purposes.
Owner:HOBOM GERD +2
Recombinant influenza vectors with tandem transcription units
InactiveUS20060166321A1Efficient and robust generationReduce in quantitySsRNA viruses negative-senseBiocideViral vectorHelper virus
The invention provides a composition useful to prepare influenza viruses, e.g., in the absence of helper virus, using vectors which include tandem transcription cassettes containing PolI and / or PolII promoters.
Owner:WISCONSIN ALUMNI RES FOUND
Recombinant influenza viruses for vaccines and gene therapy
InactiveUS20060134138A1Improve efficiencyEnhances these viruses as vaccine vectorsSsRNA viruses negative-senseAnimal cellsHelper virusGene
The invention provides a composition useful to prepare influenza A viruses, e.g., in the absence of helper virus.
Owner:WISCONSIN ALUMNI RES FOUND
Modified baculovirus expression system for production of pseudotyped rAAV vector
InactiveUS20060166363A1Reduce lossesEfficient and high productionAnimal cellsSugar derivativesBaculovirus expressionHelper virus
The invention provides modifications to a baculovirus-based recombinant adeno associated virus (AAV) system including enhancement of the helper virus stability and construction of novel baculovirus vectors for rAAV pseudotyping. The modified system extends the flexibility of rAAV vector production and promotes the utility of AAV as, a clinically applicable gene therapy vector.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Methods for purification of recombinant aav vectors
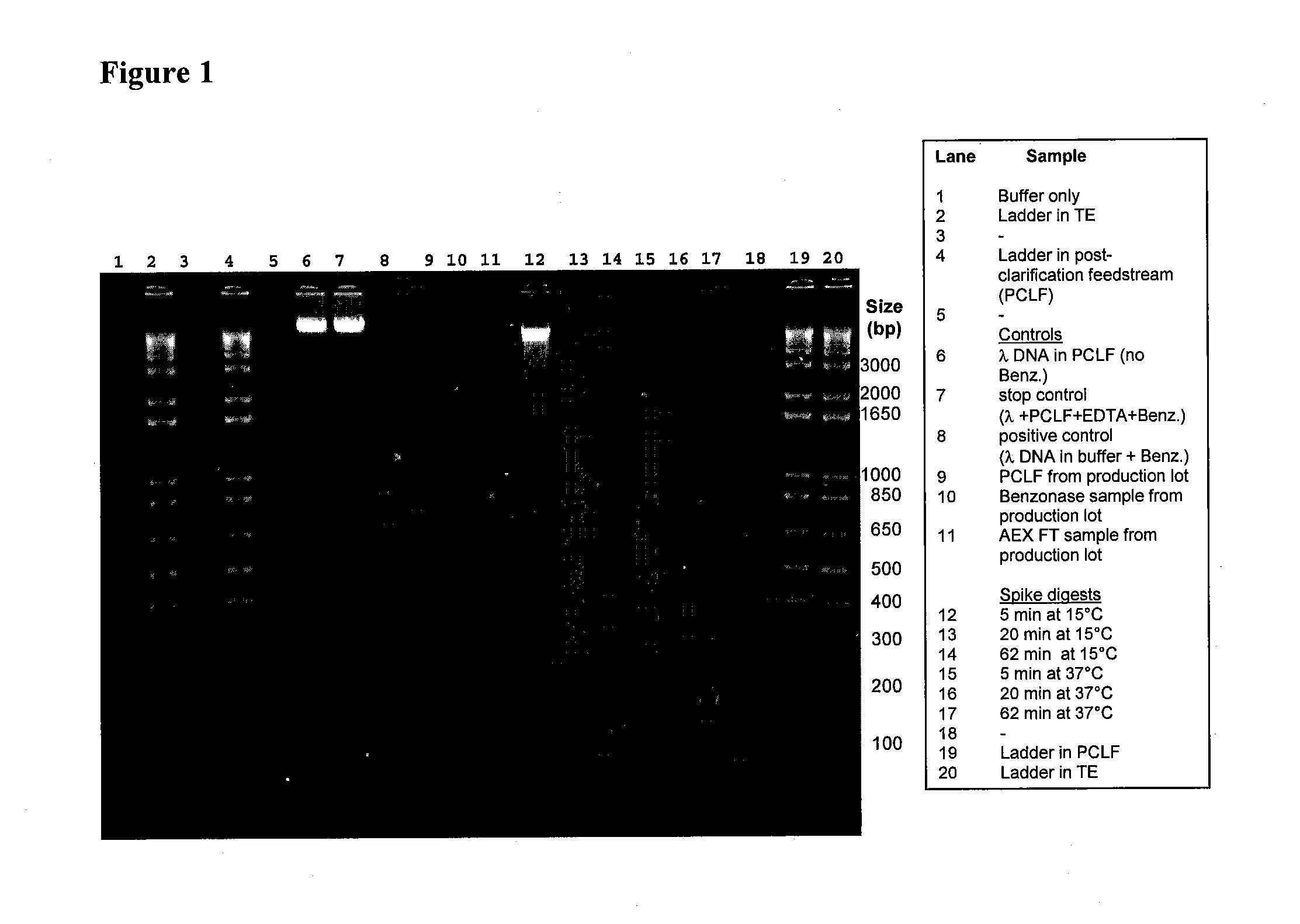
Provided herein are methods for the purification of recombinant adeno-associated virus (rAAV) vectors that can be used for gene transfer and specifically for gene therapy or vaccination. Recombinant AAV vectors of the invention are substantially free of in-process impurities, including production components such as cellular nucleic acids, cellular proteins, helper virus, and media components.
Owner:GENZYME CORP
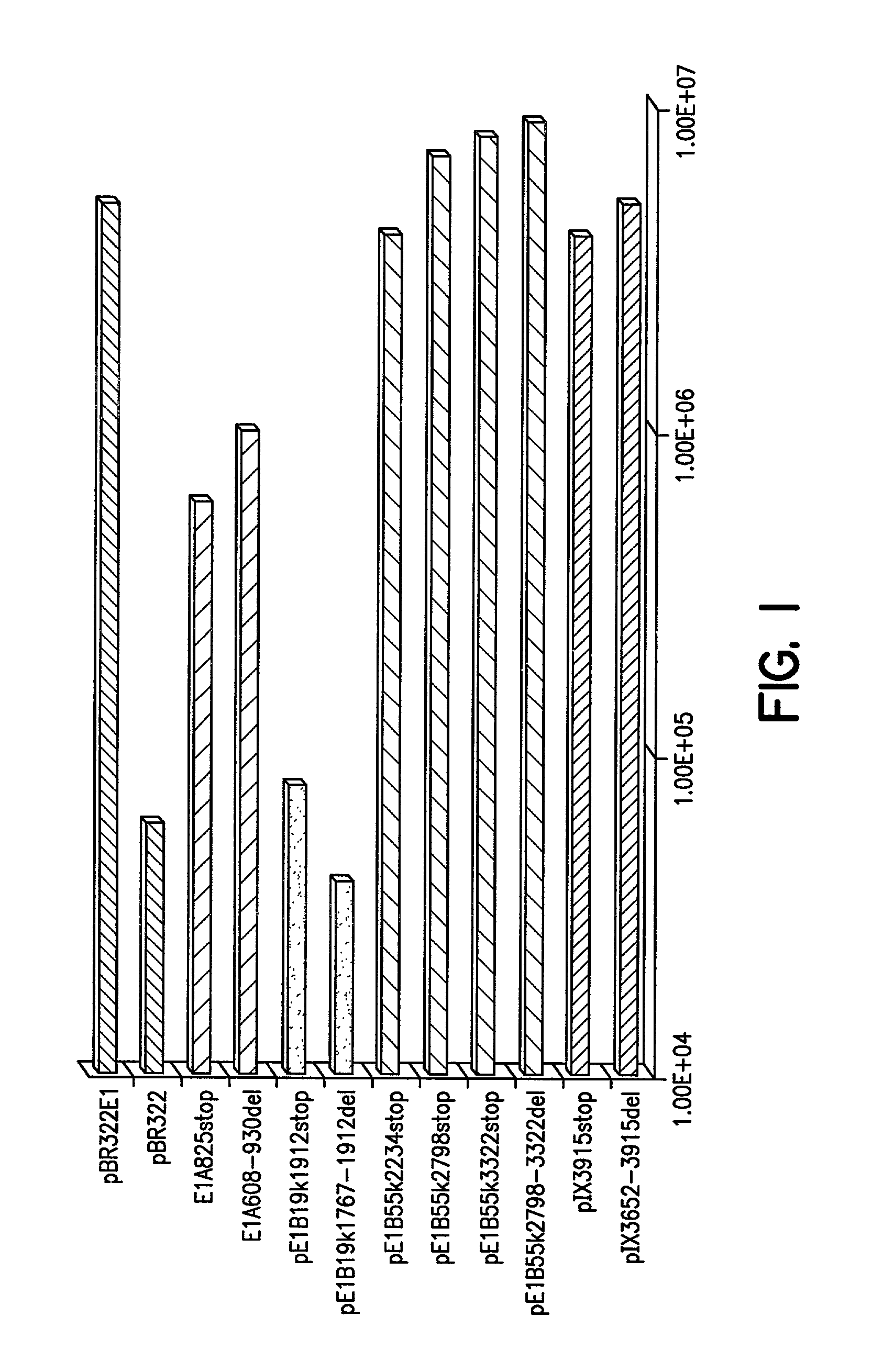
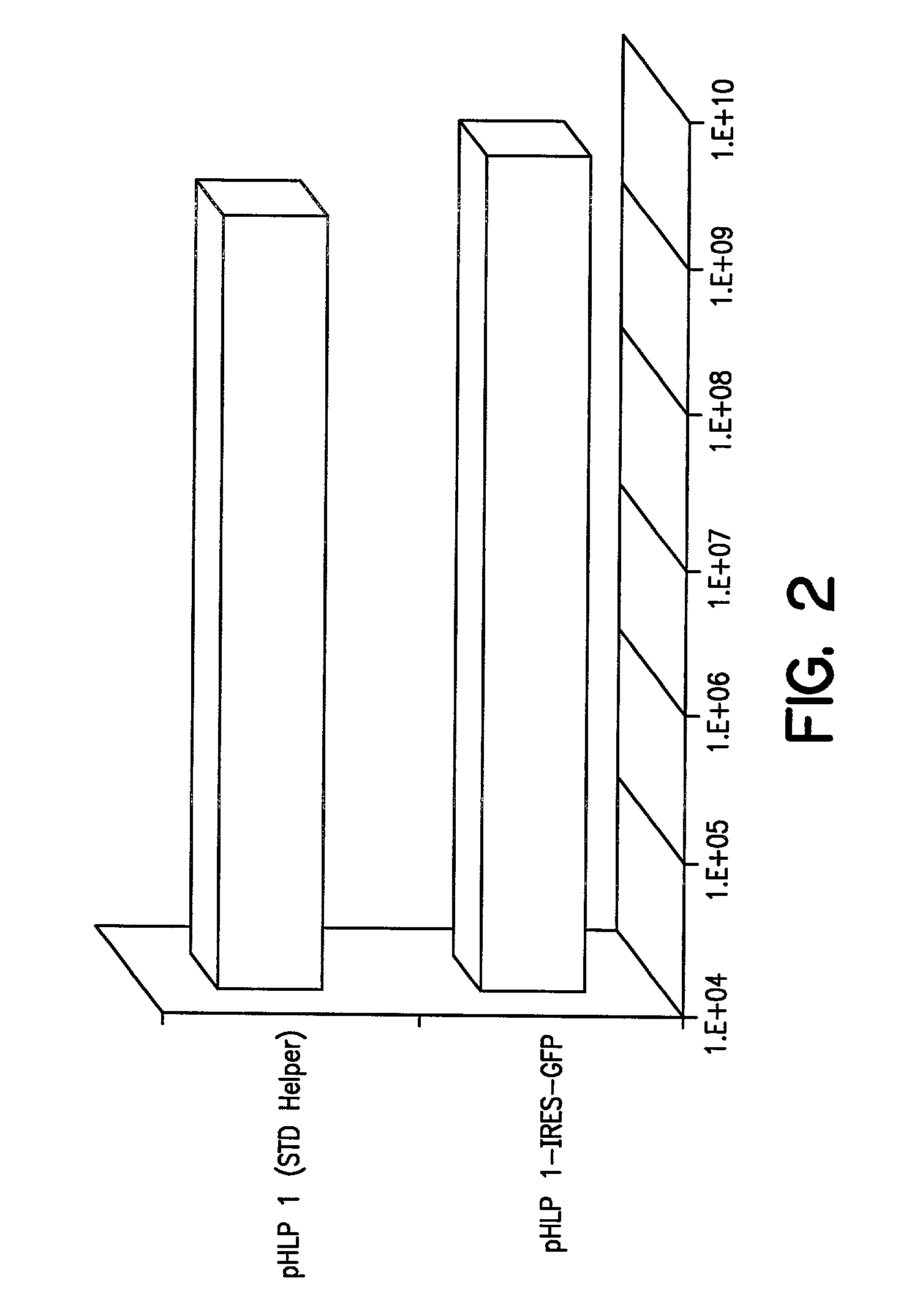
Adenovirus vectors generated from helper viruses and helper-dependent vectors
InactiveUS6080569ALarge capacityReduce in quantityMicrobiological testing/measurementGenetic material ingredientsVector systemHelper virus
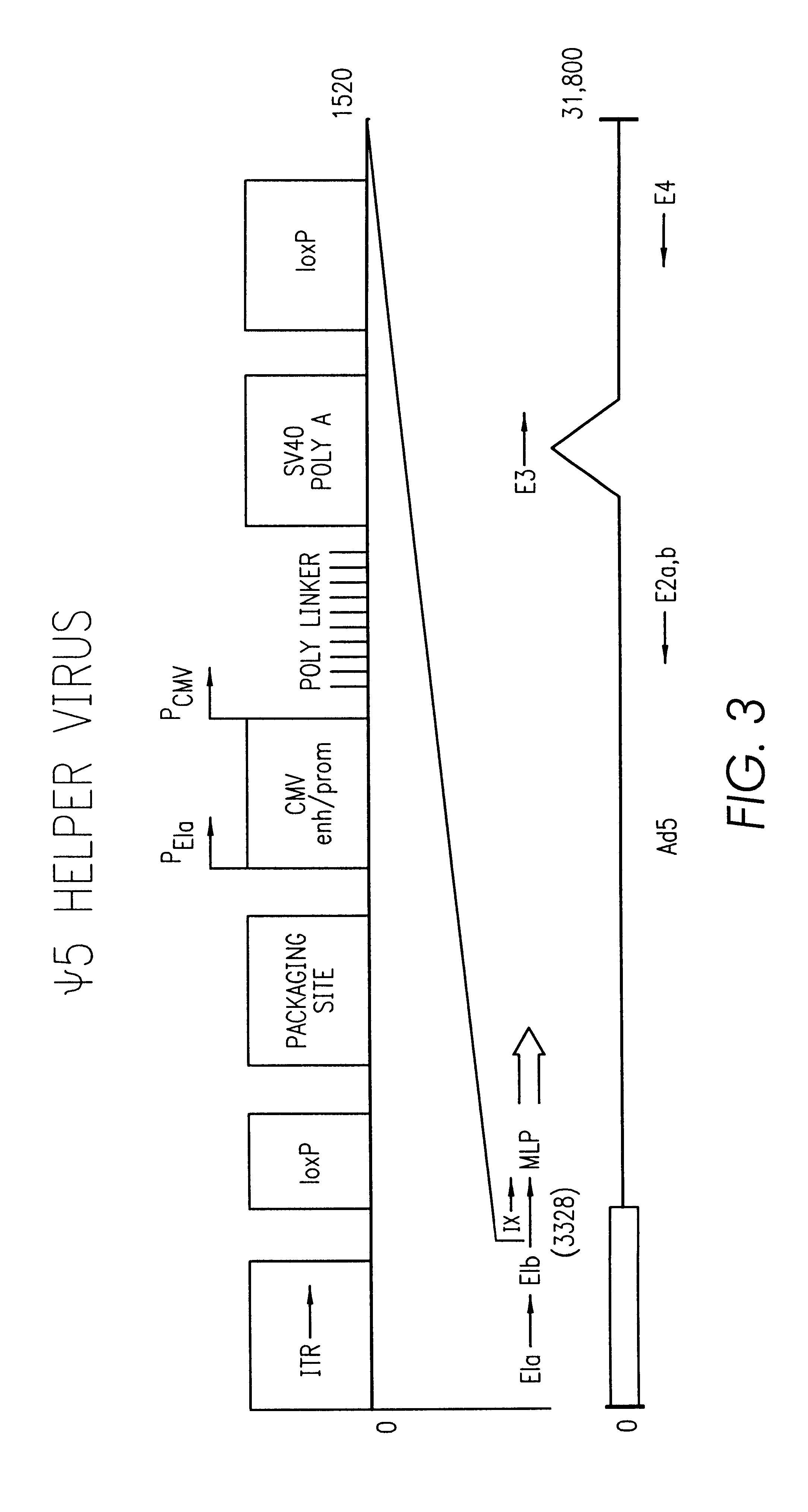
The present invention provides an improved helper-dependent vector system for production of high capacity adenoviral cloning vectors. The invention makes use of the DNA size packaging constraints imposed on a pIX-defective Ad virion that prevent such virions from packaging DNA larger than approximately 35 kb. This constraint can be used to develop helper viruses that do not package their DNA. In one embodiment, the invention combines this methodology with the Cre-loxP helper-dependent system to decrease the quantity of contaminating helper virus in vector preparations. In another embodiment the invention is used for vector growth.
Owner:ADVEC
Recombinant influenza vectors with tandem transcription units
InactiveUS7968101B2Efficient and robust generationReduce in quantitySsRNA viruses negative-senseBiocideHelper virusViral vector
The invention provides a composition useful to prepare influenza viruses, e.g., in the absence of helper virus, using vectors which include tandem transcription cassettes containing PolI and / or PolII promoters.
Owner:WISCONSIN ALUMNI RES FOUND
Lentiviral packaging cells and uses therefor
InactiveUS6955919B2Improve securityRule out the possibilitySugar derivativesGenetic material ingredientsPol genesVpr Protein
Owner:GENETIX PHARMA
Methods for generating high titer helper-free preparations of released recombinant AAV vectors
InactiveUS20050266567A1Genetic therapy composition manufactureGroup 5/15 element organic compoundsGene deliveryHeterologous
This invention provides methods and compositions for producing high titer, substantially purified preparations of recombinant adeno-associated virus (AAV) that can be used as vectors for gene delivery. At the onset of vector production, AAV producer cells of this invention typically comprise one or more AAV packaging genes, an AAV vector comprising a heterologous (i.e. non-AAV) transgene of interest, and a helper virus such as an adenovirus. The AAV vector preparations produced are generally replication incompetent but are capable of mediating delivery of a transgene of interest (such as a therapeutic gene) to any of a wide variety of tissues and cells. The AAV vector preparations produced according to this invention are also substantially free of helper virus as well as helper viral and cellular proteins and other contaminants. The invention described herein provides methods of producing rAAV particles by culturing producer cells under conditions, such as temperature and pH, that promote release of virus. Also provided is a quantitative, high-throughput assay useful in the assessment of viral infectivity and replication, as well as in the screening of agent that affect viral infectivity and / or replication.
Owner:ATKINSON EDWARD M +5
Helper-free rescue of recombinant negative strand RNA virus
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
High titer recombinant influenza viruses with enhanced replication in vero cells
ActiveUS20110110978A1Enhanced influenza virus replicationHigh pHSsRNA viruses negative-senseVirus peptidesDiseaseInfluenza virus vaccine
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, and genes from vaccine seed virus isolates which include a HA gene segment with a HA2 sequence encoding a HA2 that confers enhanced growth in cells in culture, such as Vero cells.
Owner:WISCONSIN ALUMNI RES FOUND
Helper-free stocks of recombinant adeno-associated virus vectors
InactiveUS6489162B1Efficient yieldImprove efficiencyMicrobiological testing/measurementBiological testingWild typeViral vector
The present invention relates to a method for producing helper-free stocks of recombinant adeno-associated virus (rAAV) which can be used to efficiently and stably transduce foreign genes into host cells or organisms. The method comprises the cotransfection of eukaryotic cells with rAAV and with helper AAV DNA in the presence of helper virus (e.g. adenovirus or herpesvirus) such that the helper AAV DNA is not associated with virion formation. The crux of the invention lies in the inubility of the helper AAV DNA to recombine with rAAV vector, thereby preventing the generation of wild-type virus. In a specific embodiment of the invention, the vector comprises a recombinant AAV genome containing only the terminal regions of the AAV chromosome bracketing a non-viral gene, and the helper AAV DNA comprises a recombinant AAV genome containing that part of the AAV genome which is not present in the vector, and in which the AAV terminal regions are replaced by adenovirus sequences. In a further embodiment of the invention, cell lines are created which incorporate helper AAV DNA which can directly produce substantially pure recombinant AAV virus. The pure stocks of recombinant AAV produced according to the invention provide an AAV viral expression vector system with increased yield of recombinant virus, improved efficiency, higher definition, and greater safety than presently used systems.
Owner:THE TRUSTEES FOR PRINCETON UNIV
High titer recombinant influenza viruses with enhanced replication in mdck or vero cells or eggs
ActiveUS20170354730A1Improve scalabilitySignificantly higher viral titersSsRNA viruses negative-senseAntiviralsDiseaseEgg cell
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, that confer enhanced growth in cells in culture, such as MDCK cells, or in eggs.
Owner:WISCONSIN ALUMNI RES FOUND
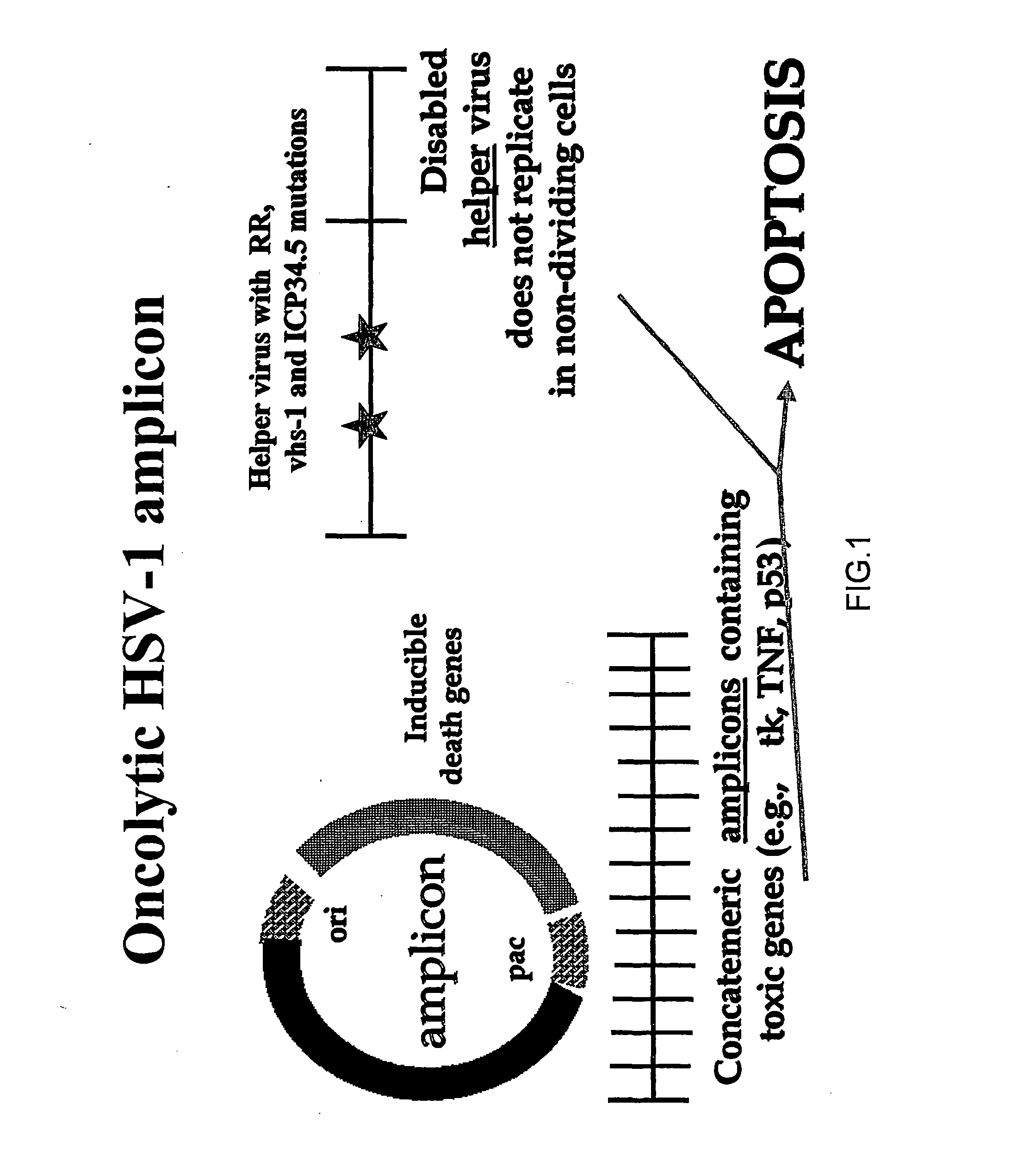
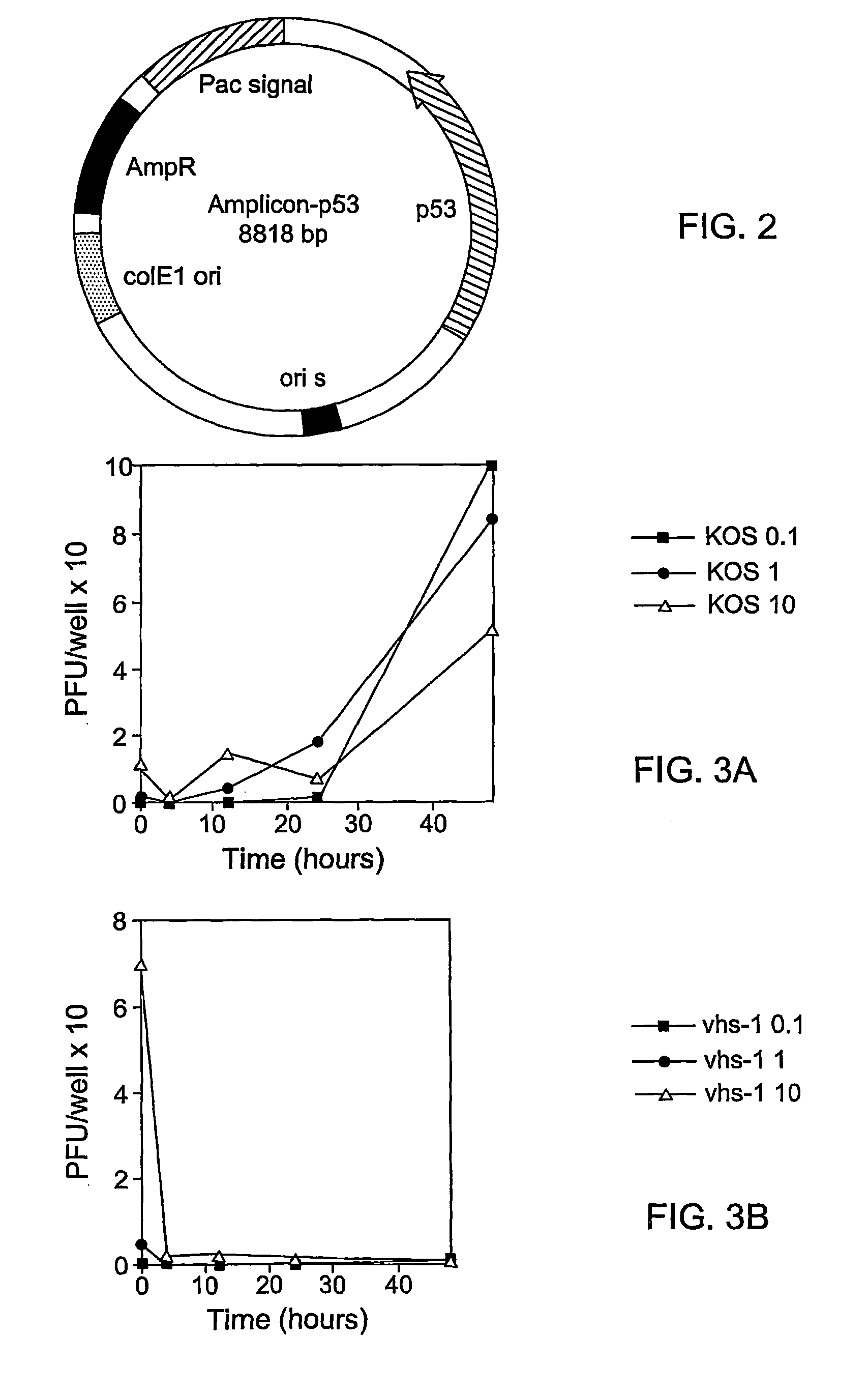
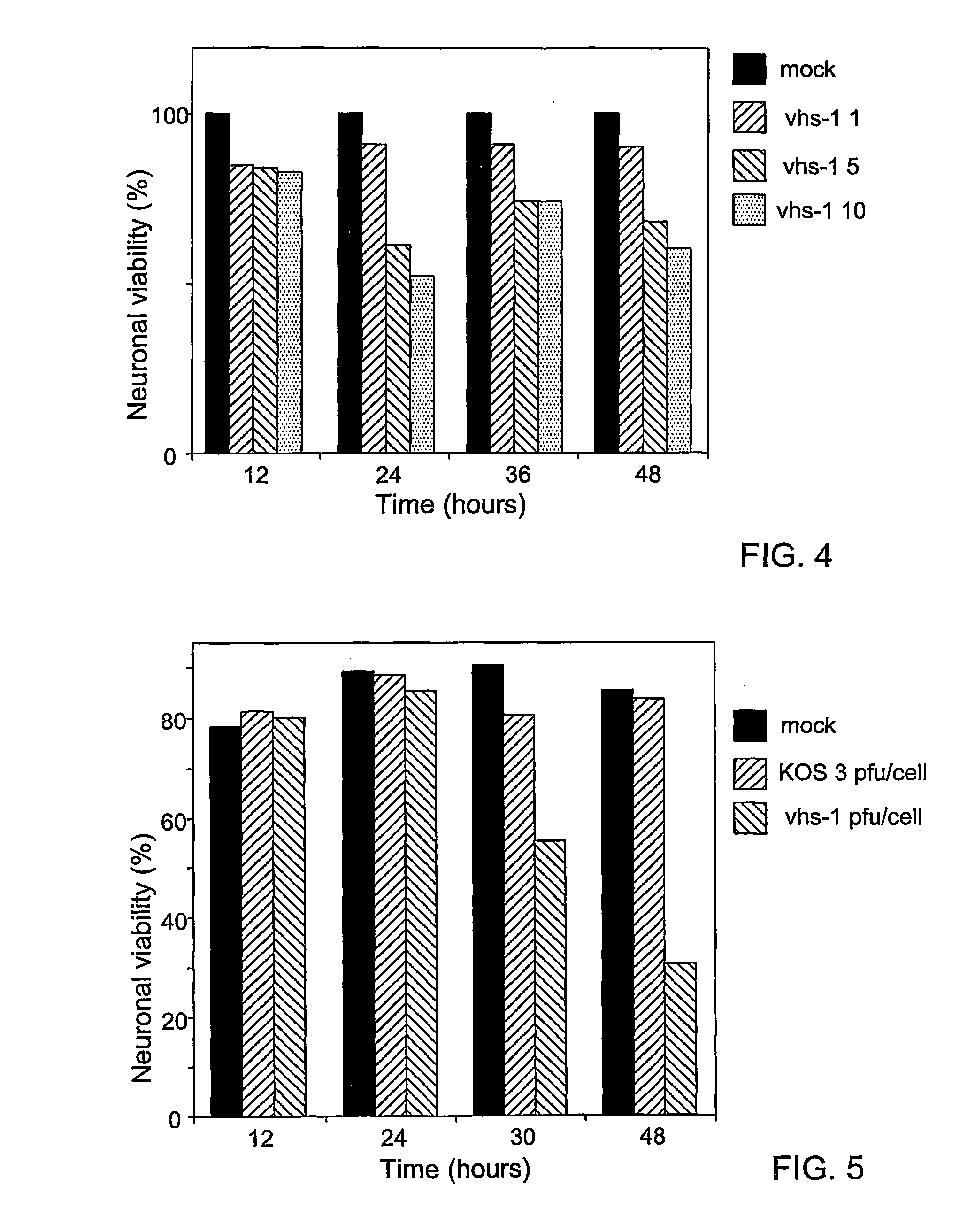
Composite oncolytic herpes virus vectors
InactiveUS20040120928A1Enhancing immune systemGood curative effectBiocidePeptide/protein ingredientsBrain malignant tumorsMalignancy
A highly efficient and safe HSV derived composite oncolytic vector is provided for the treatment of solid tumors in an individual. The vector comprises two main components being a defective viral genome with multiple reiterations of amplicon type repeat units each carrying inducible toxic genes with cell destructive capabilities and, as a second component, an HSV mutant helper virus which is incapable of replication in non-dividing cells. The vector may be used for the treatment of various kinds of solid tumors including brain malignancies as well as lung, pancreatic, kidney, colon, stomach and other types of cancers. The provided vector is also suitable for administration to an individual in combination with an additional treatment.
Owner:FRENKEL NIZA PROF
Compositions and methods for efficient AAV vector production
InactiveUS7208315B2Improve efficiencyHigh potencySugar derivativesGenetic therapy composition manufactureAdeno associate virusAdeno-associated virus
The present invention provides packaging cell lines for the efficient production of an Adeno-associated virus (AAV) vector which does not require “helper” virus function for the replication and encapsidation of the AAV vector particles. Packaging cells, methods for their production and methods for producing recombinant AAV vector particles useful for human gene therapy are provided.
Owner:FRED HUTCHINSON CANCER RES CENT
Method and application for fast retrograde transsynaptic labeling of nerve cells
The invention belongs to the construction field of auxiliary viruses, and discloses a method and an application of fast retrograde transsynaptic labeling of nerve cells. The invention uses double-stranded adeno-associated virus (SCAAV) as a helper virus vector to load TVA receptor and rabies virus outer membrane glycoprotein (RVG) respectively, and packaged into virus for specific recognition andretrograde transsynaptic marker of ENVA outer membrane wrapped defective recombinant rabies virus. As shown by that live test result, the recombinant defective rabies virus RV-[delta]G-X-ENVA and helper virus SCAAV combined system, can realize fast retrograde transsynaptic labeling, which saves 1-2 week experiment time, saves material resources and manpower, provides a better research tool for theapplication of defective rabies virus in neural network reverse transsynaptic marker, and lays a good technical support for the structure and function analysis of neural network.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Polynucleotides for use in recombinant adeno-associated virus virion production
ActiveUS20020052485A1Efficient productionEliminate the effects ofSugar derivativesVirus peptidesNucleotidePolynucleotide
Accessory functions capable of supporting efficient recombinant AAV (rAAV) virion production in a suitable host cell are provided. The accessory functions are in the form of one or more vectors that are capable of being transferred between cells. Methods of producing rAAV virions are also provided. The methods can be practiced to produce commercially significant levels of rAAV particles without also generating significant levels of infectious helper virus or other contaminating by-products.
Owner:GENZYME CORP
High titer recombinant influenza viruses with enhanced replication in vero cells
ActiveUS9109013B2Improve scalabilityEasy to copySsRNA viruses negative-senseVirus peptidesDiseaseInfluenza virus vaccine
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, and genes from vaccine seed virus isolates which include a HA gene segment with a HA2 sequence encoding a HA2 that confers enhanced growth in cells in culture, such as Vero cells.
Owner:WISCONSIN ALUMNI RES FOUND
Attenuated, brightened and replication-controllable HSV recombinant virus, preparation method and applications thereof
InactiveCN107630009AEasy genetic manipulationEasy to insertViruses/bacteriophagesFermentationCell specificNervous system
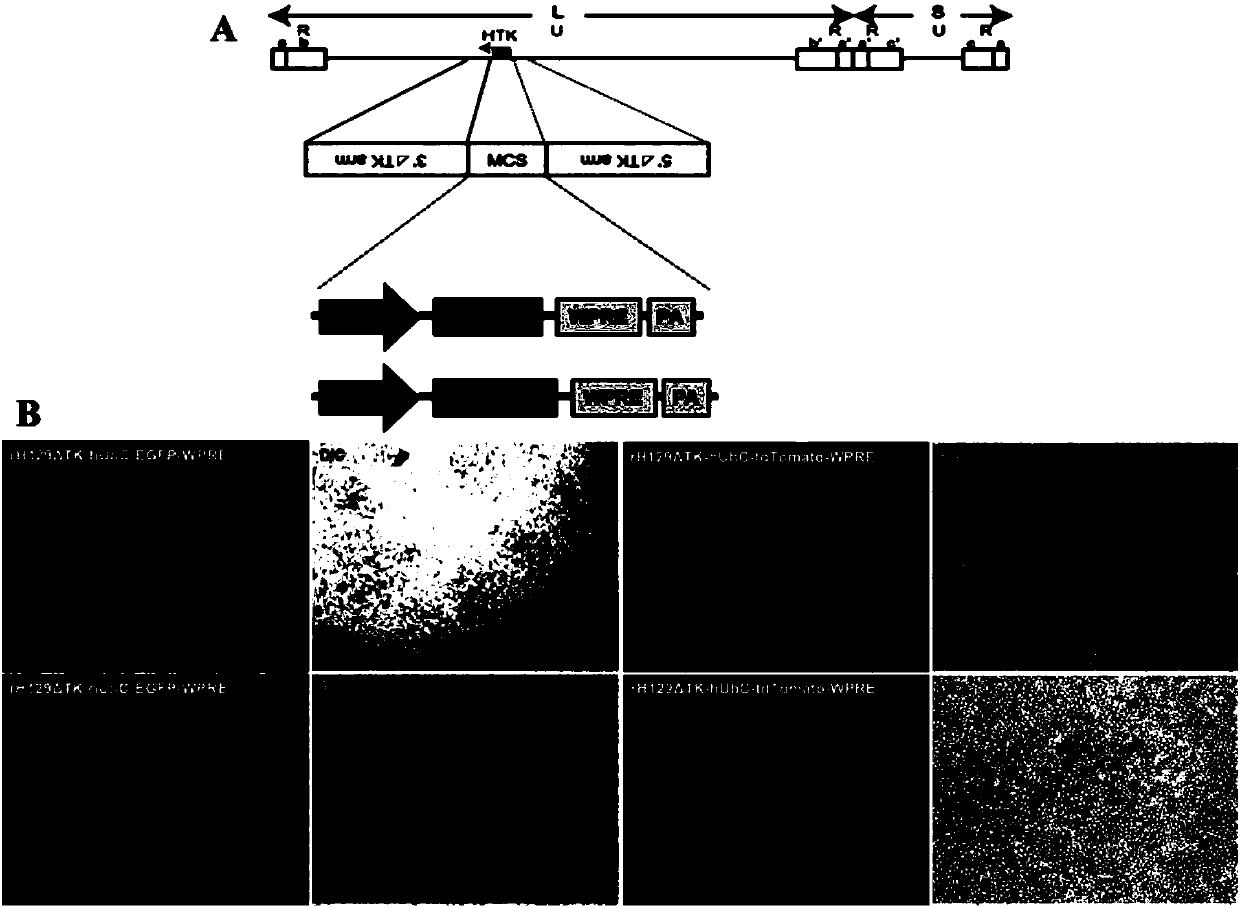
The invention discloses attenuated, brightened and replication-controllable HSV recombinant virus, a preparation method and applications thereof. According to the present invention, thymidine kinase (TK) gene essential for replicating viruses in neurons and being a main virulence factor is knocked out by using a homologous recombination method, and subsequently a red or green fluorescent gene enhancement expression cassette is recombined into the genome of the virus to construct a series of novel recombinants viruses, wherein the toxicity is markedly low, the states of infected mice are good,the fluorescence signal is strong, and the expression of the recombinant viruses is limited at the injection site after the recombinant viruses are used in in-vivo animal center labeling; by combiningwith Cre-dependent AAV helper viruses capable of expressing TK in a compensated manner, the cell-specific transmonosynaptic loop tracing is achieved; and the recombinant HSV has wide application value in nervous system targeted gene transduction, neural network transsynaptic tracing, tumor disintegration, viral replication and pathogenesis mechanism, antiviral drug screening and other fields.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Influenza virus replication for vaccine development
ActiveUS20170067029A1Increase productionImprove scalabilitySsRNA viruses negative-senseVirus peptidesInfluenza virus vaccineViral Vaccine
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, that confer enhanced growth in cells in culture, such as MDCK cells, or in eggs.
Owner:WISCONSIN ALUMNI RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com