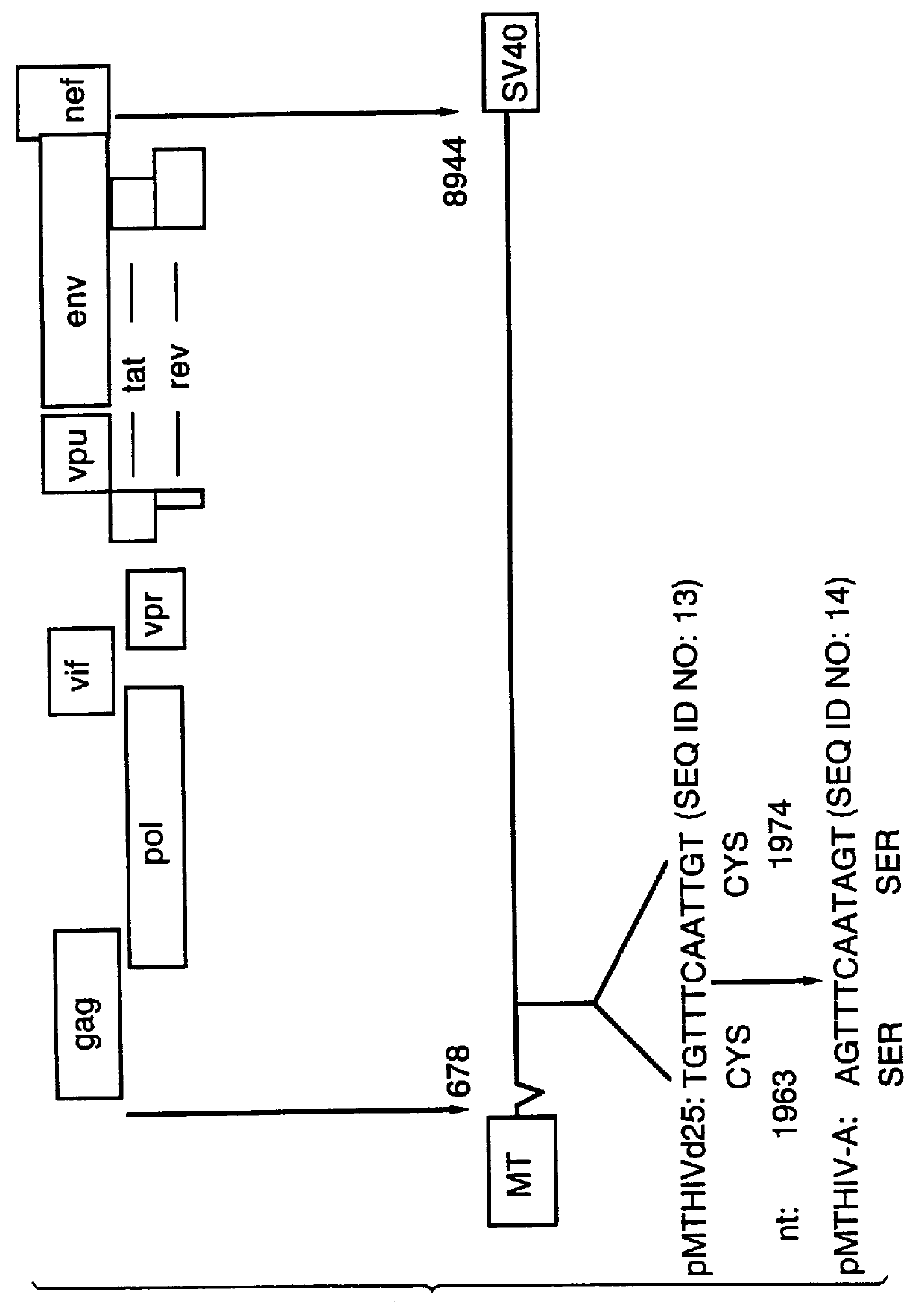
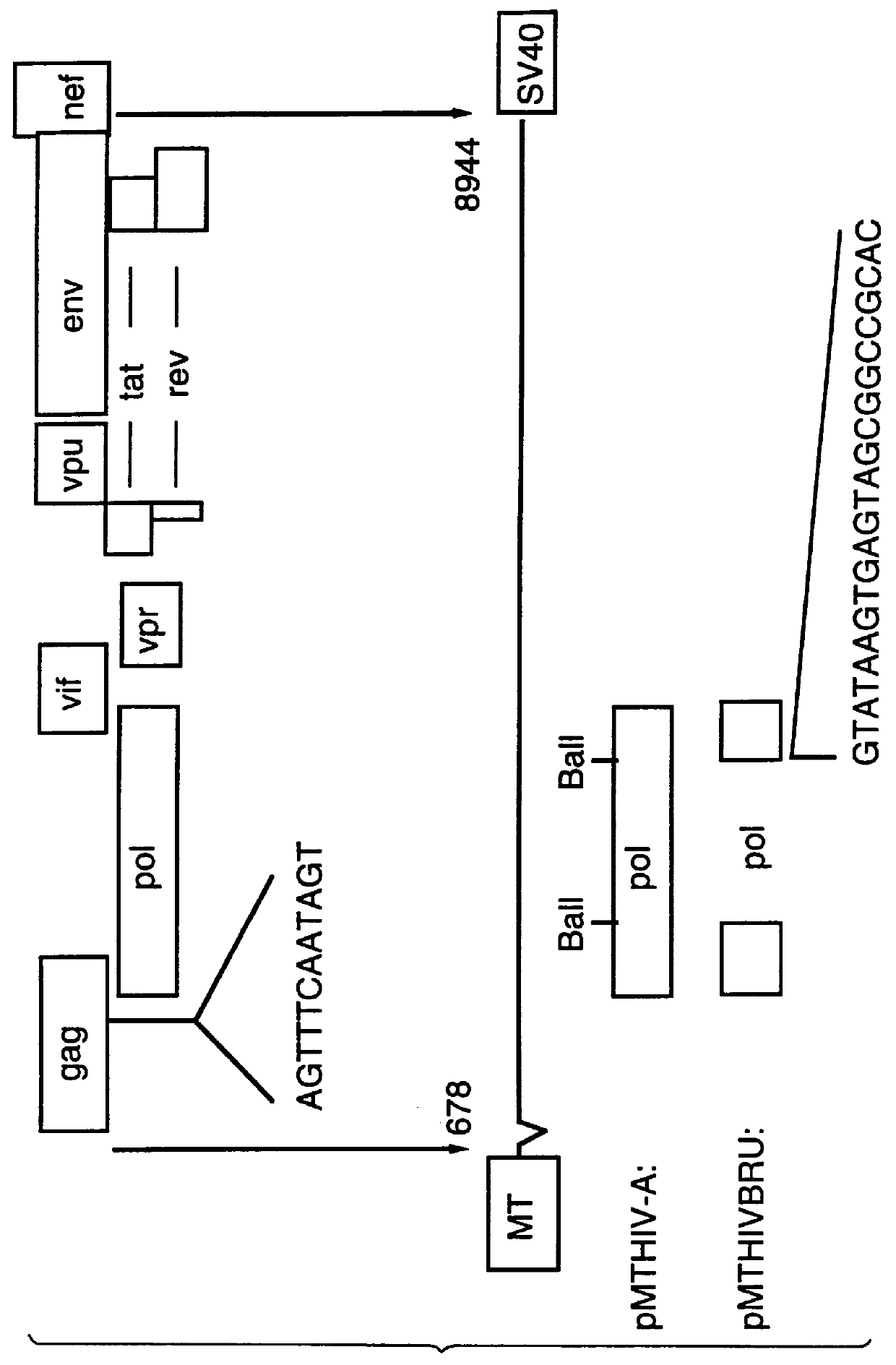
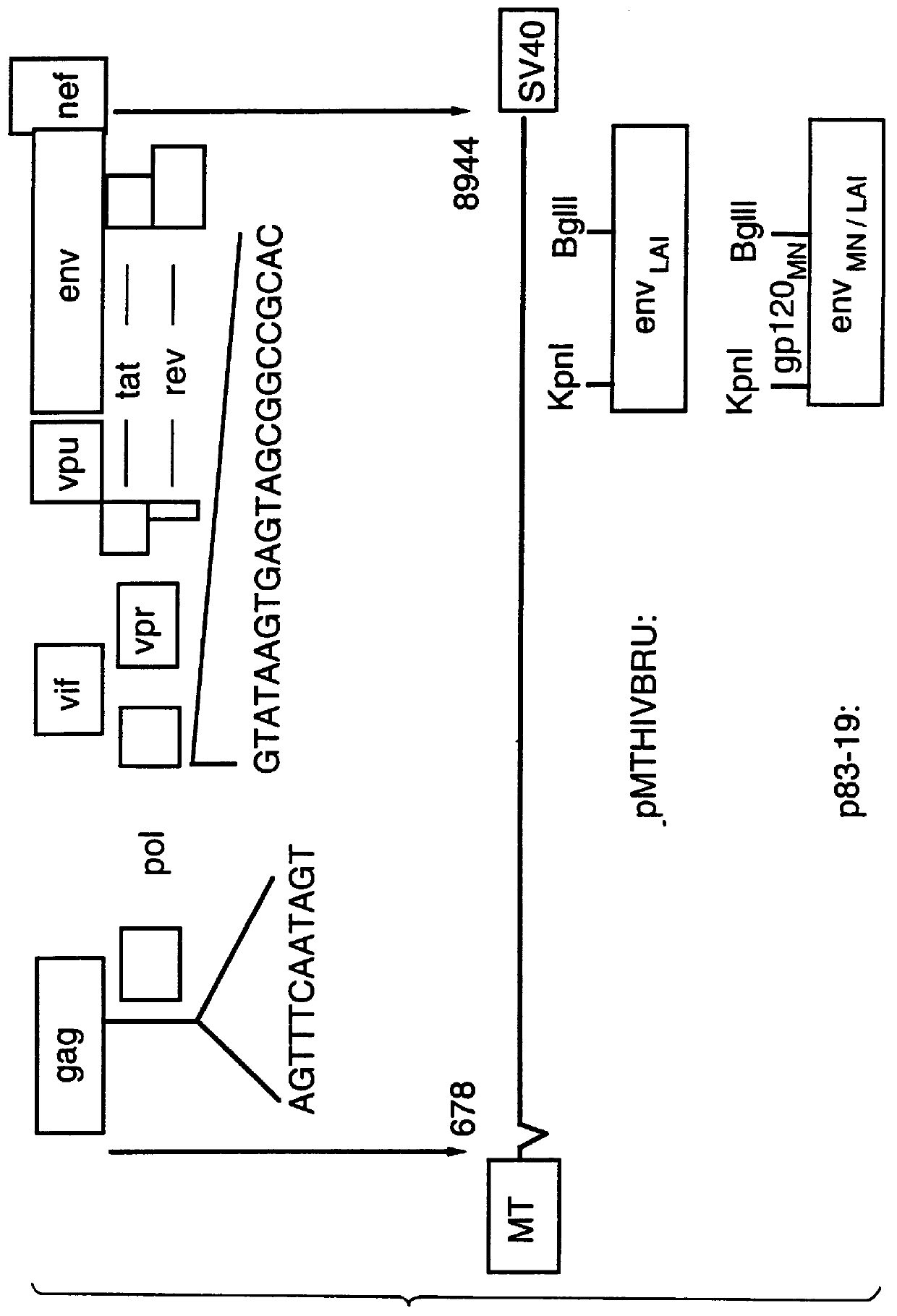
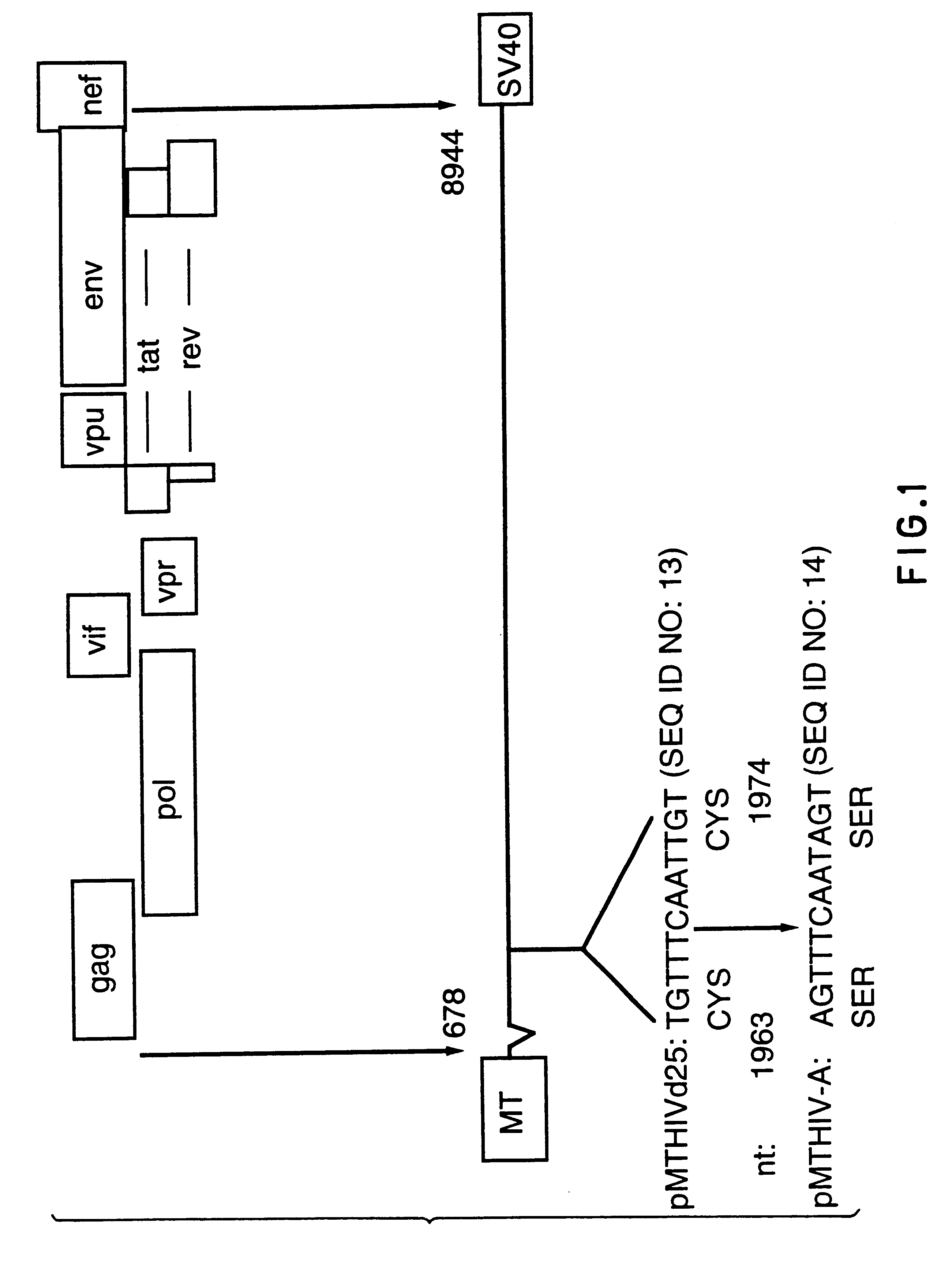
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Pol genes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
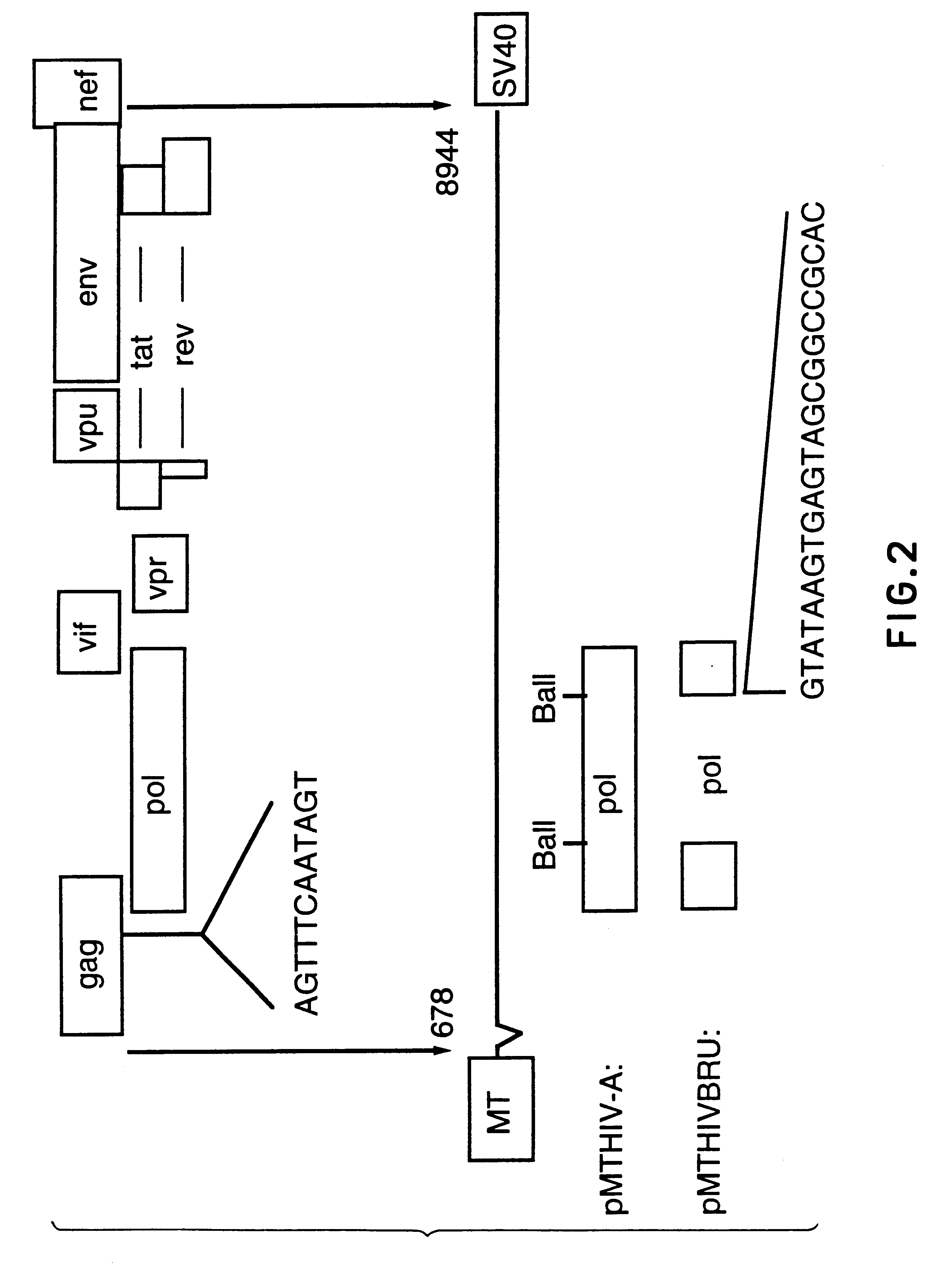
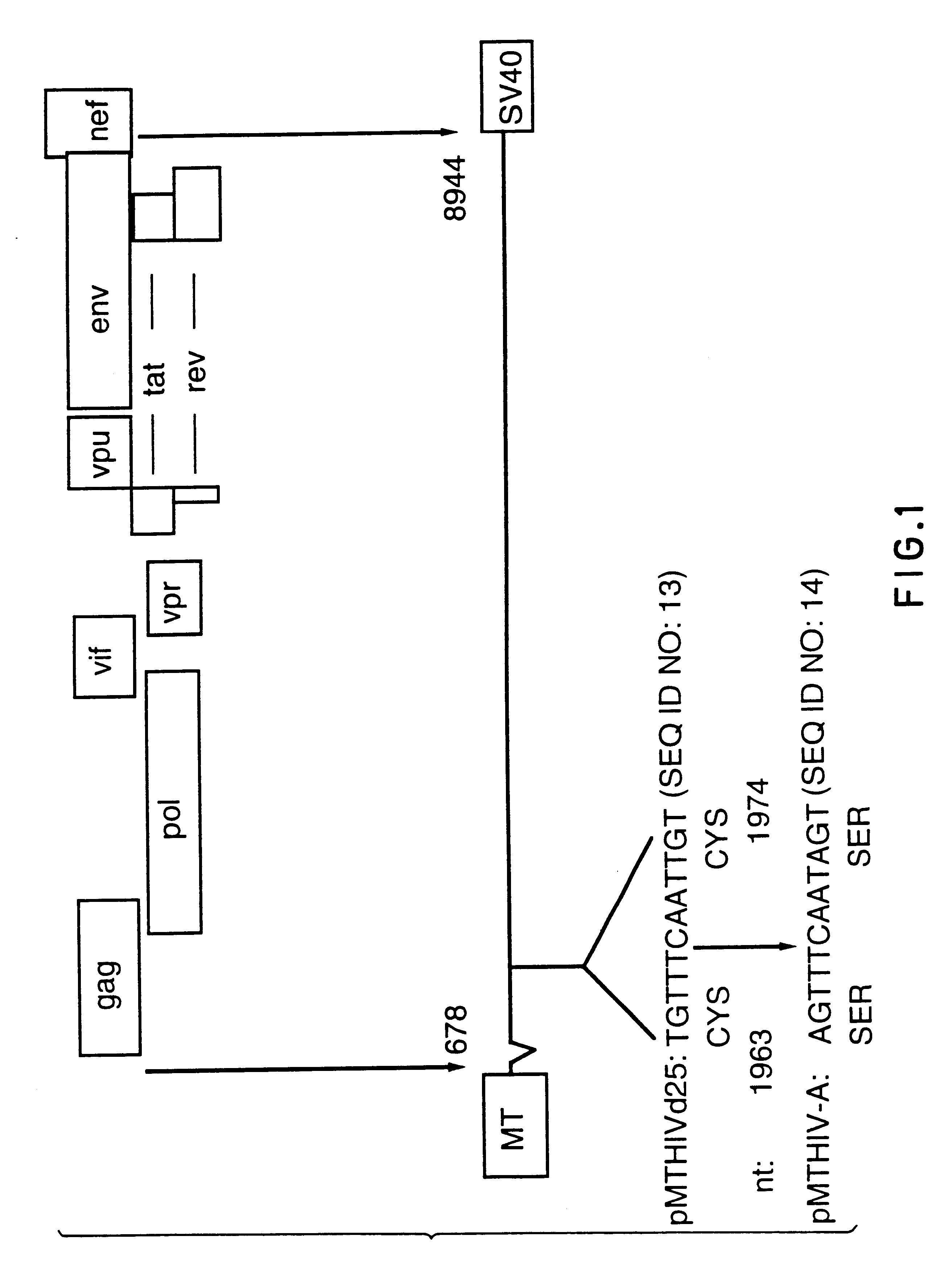
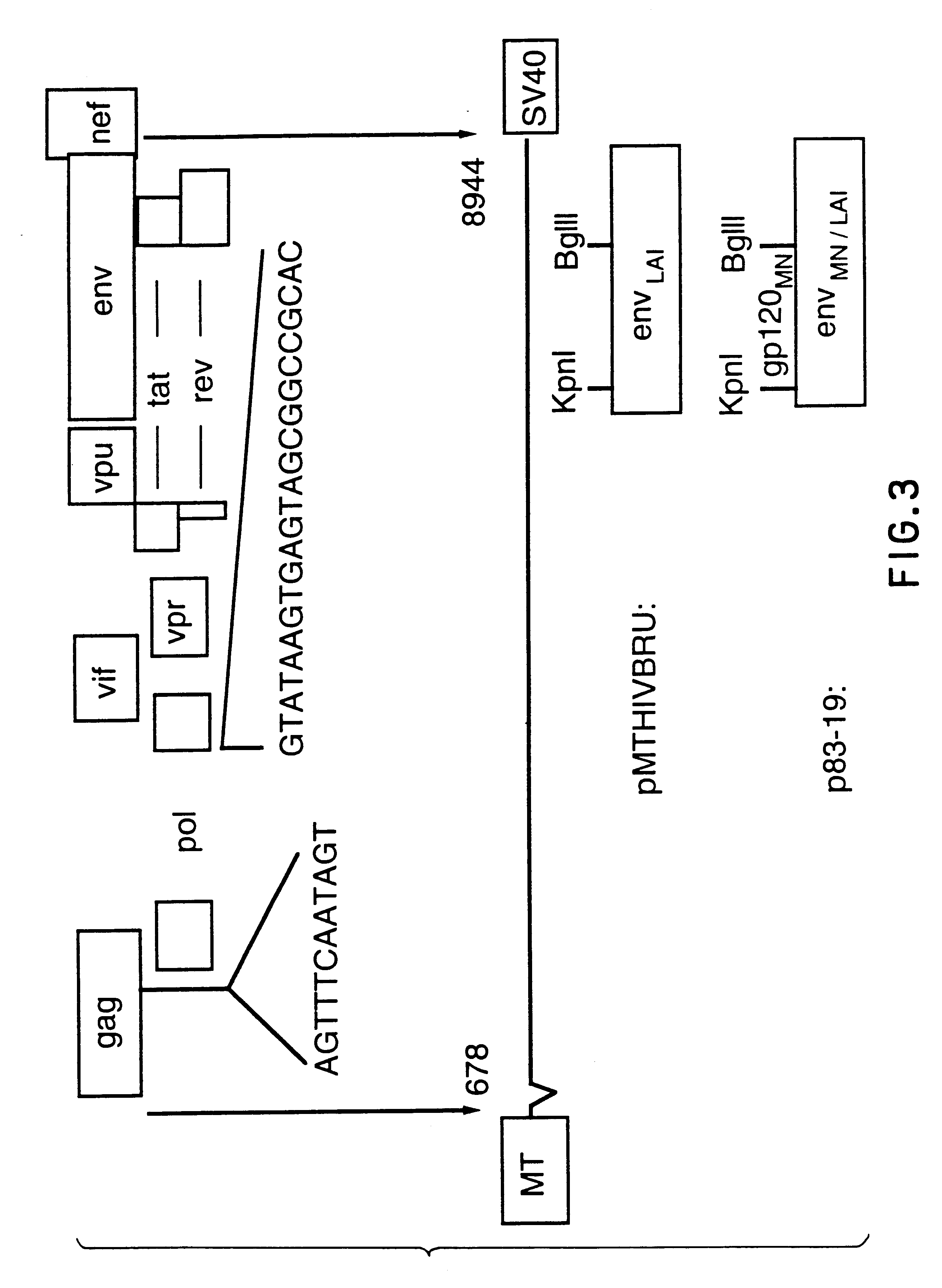
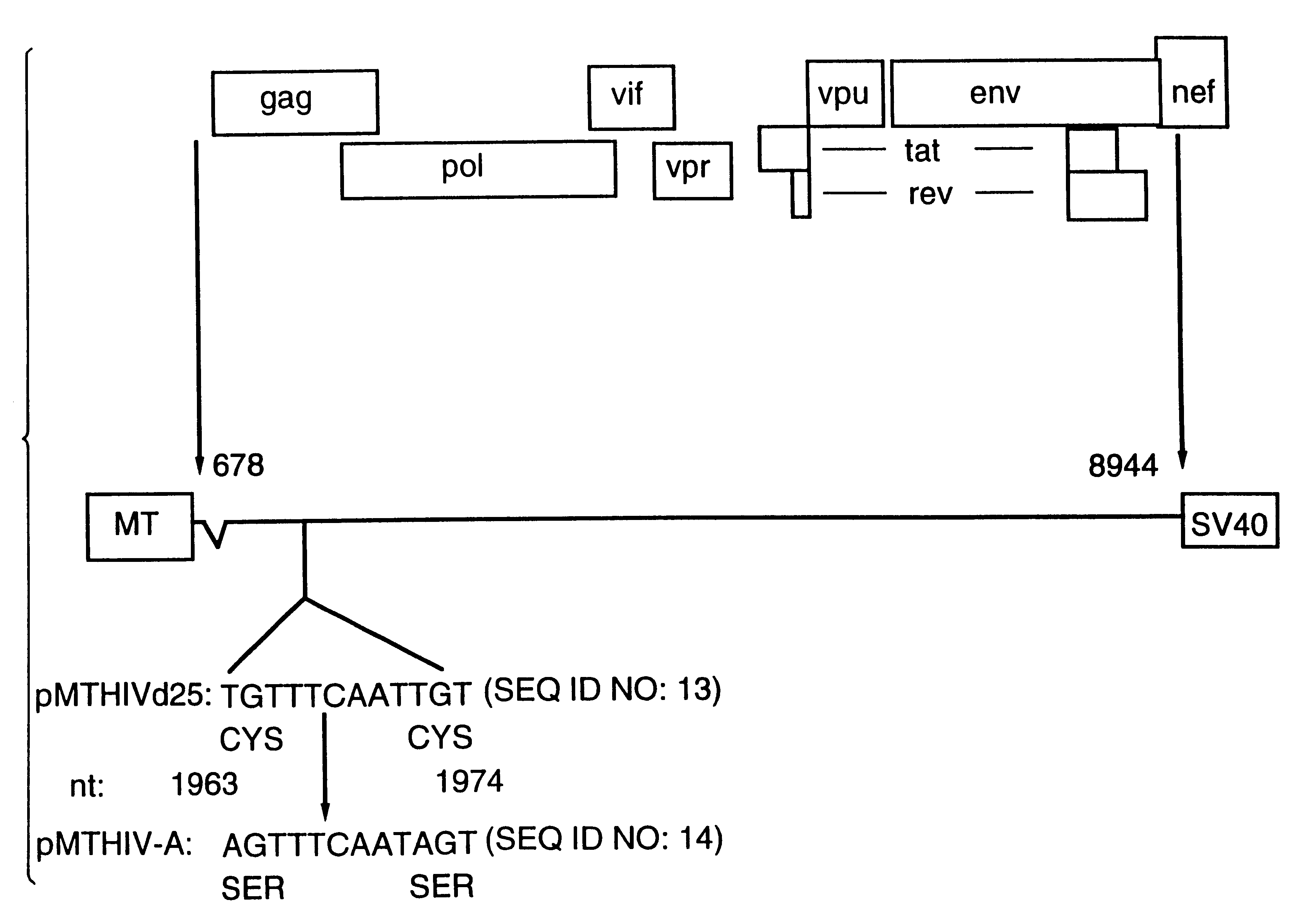
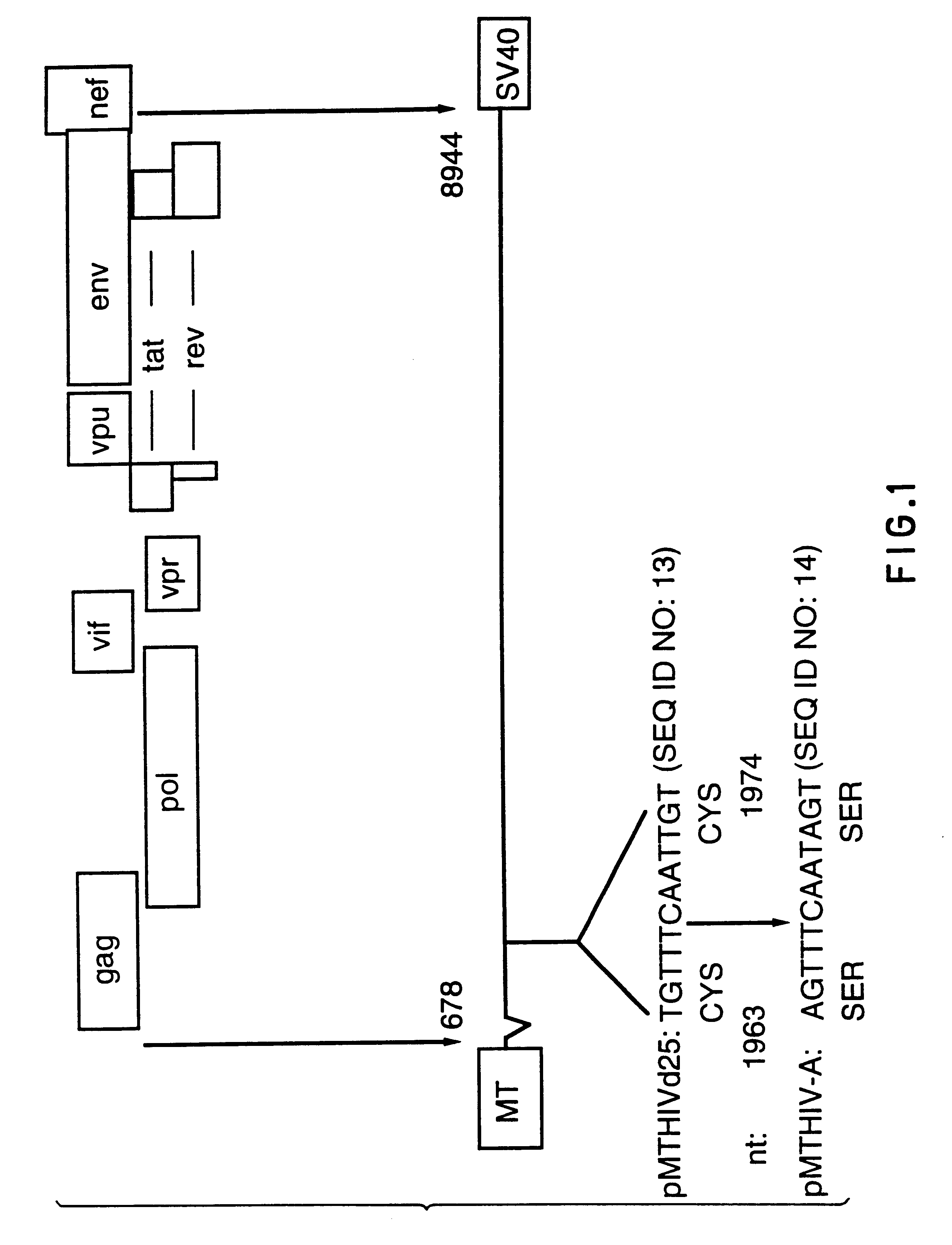
Pol (HIV) Pol (DNA polymerase) refers to a gene in retroviruses, or the protein produced by that gene.
Human immunodeficiency virus type 1 nucleic acids devoid of long terminal repeats capable of encoding for non-infectious, immunogenic, retrovirus-like particles
InactiveUS6080408AImprove efficiencyLow backgroundSugar derivativesViral antigen ingredientsPol genesReverse transcriptase activity
Non-infectious, retrovirus-like particles contain mutations to reduce gag-dependent RNA-packaging of the gag gene product, eliminate reverse transcriptase activity of the pol gene product, eliminate integrase activity of the pol gene product and eliminate RNase H activity of the pol gene product through genetic manipulation of the gag and pol genes. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in diagnosis.
Owner:CONNAUGHT LAB
Antigenically-marked non-infectious retrovirus-like particles
InactiveUS6291157B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesIn vivo
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:CONNAUGHT LAB
Diagnostic kits comprising genetically engineered human immunodeficiency virus-like particles containing heterologous antigenic markers
InactiveUS6342228B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesHeterologous
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:AVENTIS PASTEUR LTD
Lentiviral packaging cells and uses therefor
InactiveUS6955919B2Improve securityRule out the possibilitySugar derivativesGenetic material ingredientsPol genesVpr Protein
Owner:GENETIX PHARMA
Antigentically-marked non-infectious retrovirus-like particles
InactiveUS6518030B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesIn vivo
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:AVENTIS PASTEUR LTD
Retrovirus like particles made non infectious by a plurality of mutations
InactiveUS6451322B1Improve efficiencyLow backgroundSugar derivativesViral antigen ingredientsPol genesReverse transcriptase activity
Non-infectious, retrovirus-like particles contain mutations to reduce gag-dependent RNA-packaging of the gag gene product, eliminate reverse transcriptase activity of the pol gene product, eliminate integrase activity of the pol gene, product and eliminate RNase H activity of the pol gene product through genetic manipulation of the gag and pol genes. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis.
Owner:AVENTIS PASTEUR LTD
HIV pharmaccines
InactiveUS20050175627A1Increase the number ofEnhance immune responsePeptide/protein ingredientsGenetic material ingredientsPol genesVaccination
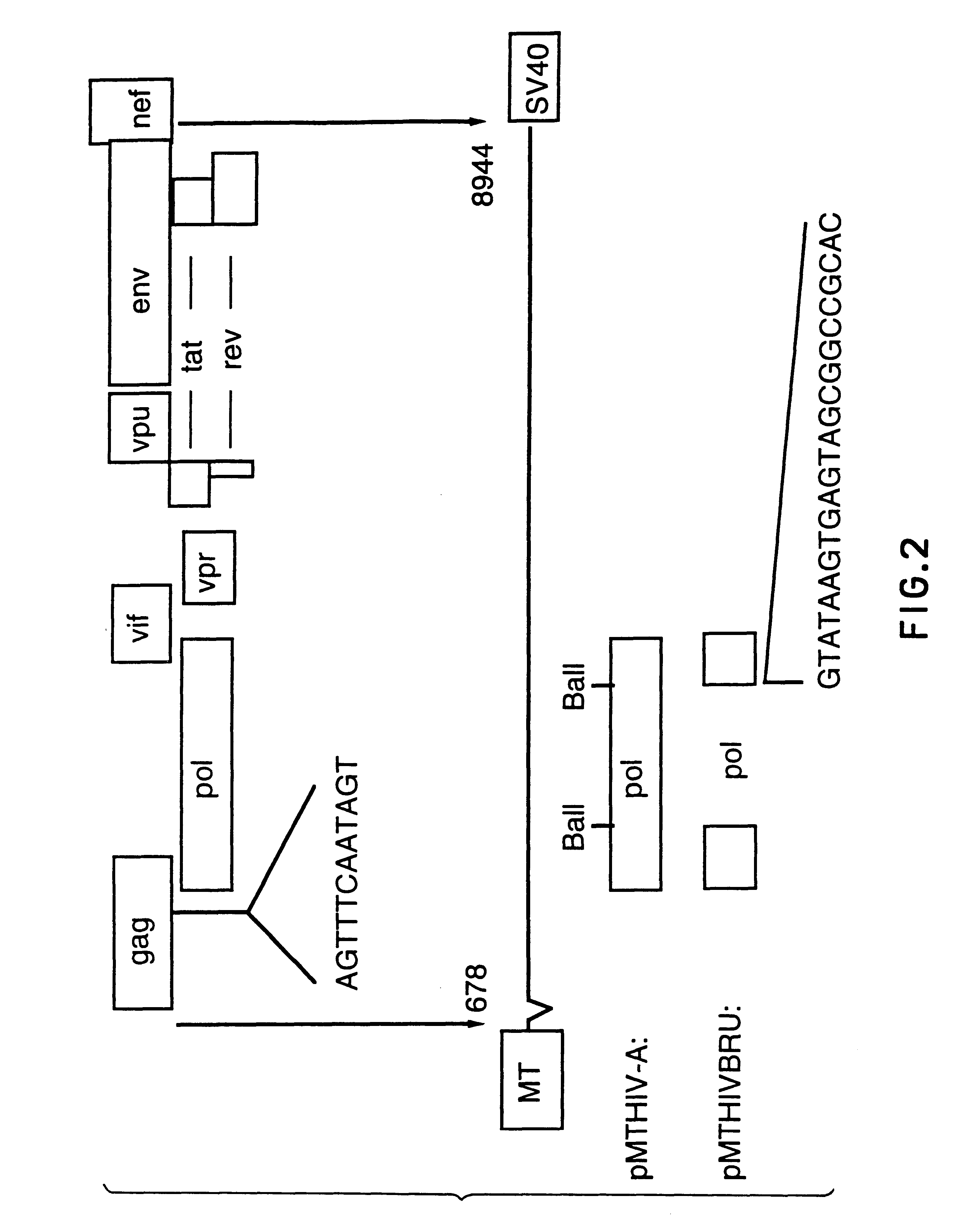
The invention relates to a recombinant polypeptide comprising amino acid sequence derived from at least one of an HIV gag gene product; an HIV pol gene product; or an HIV nef gene product, said sequence being mutated with respect to the natural sequence of said gene product, and said sequence maintaining each of the naturally occurring CD8+ T cell epitopes of said gene product as defined in p17 and p24 (gag), amino acids 1-440 of RT (pol) and nef shown in Example 8. Furthermore the invention relates to nucleic acids encoding same, and viral vectors encoding same, and to their use in medicine and in immunisation and vaccination.
Owner:OXXON THERAPEUTICS LTD
Method for preparing retrovirus vector for gene therapy
InactiveUS6743620B1High recovery rateStably produced at a high titerVectorsSugar derivativesPol genesA-DNA
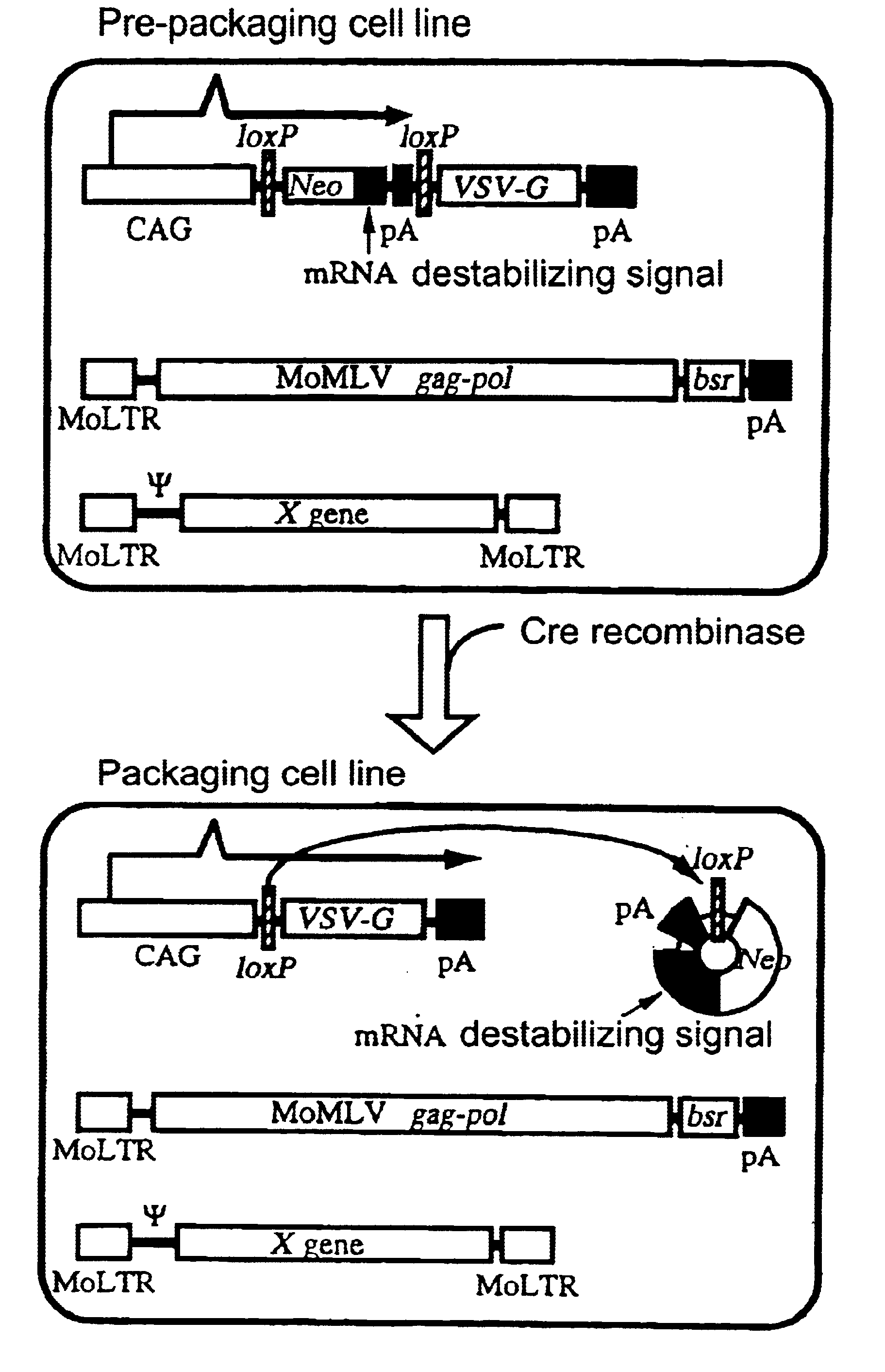
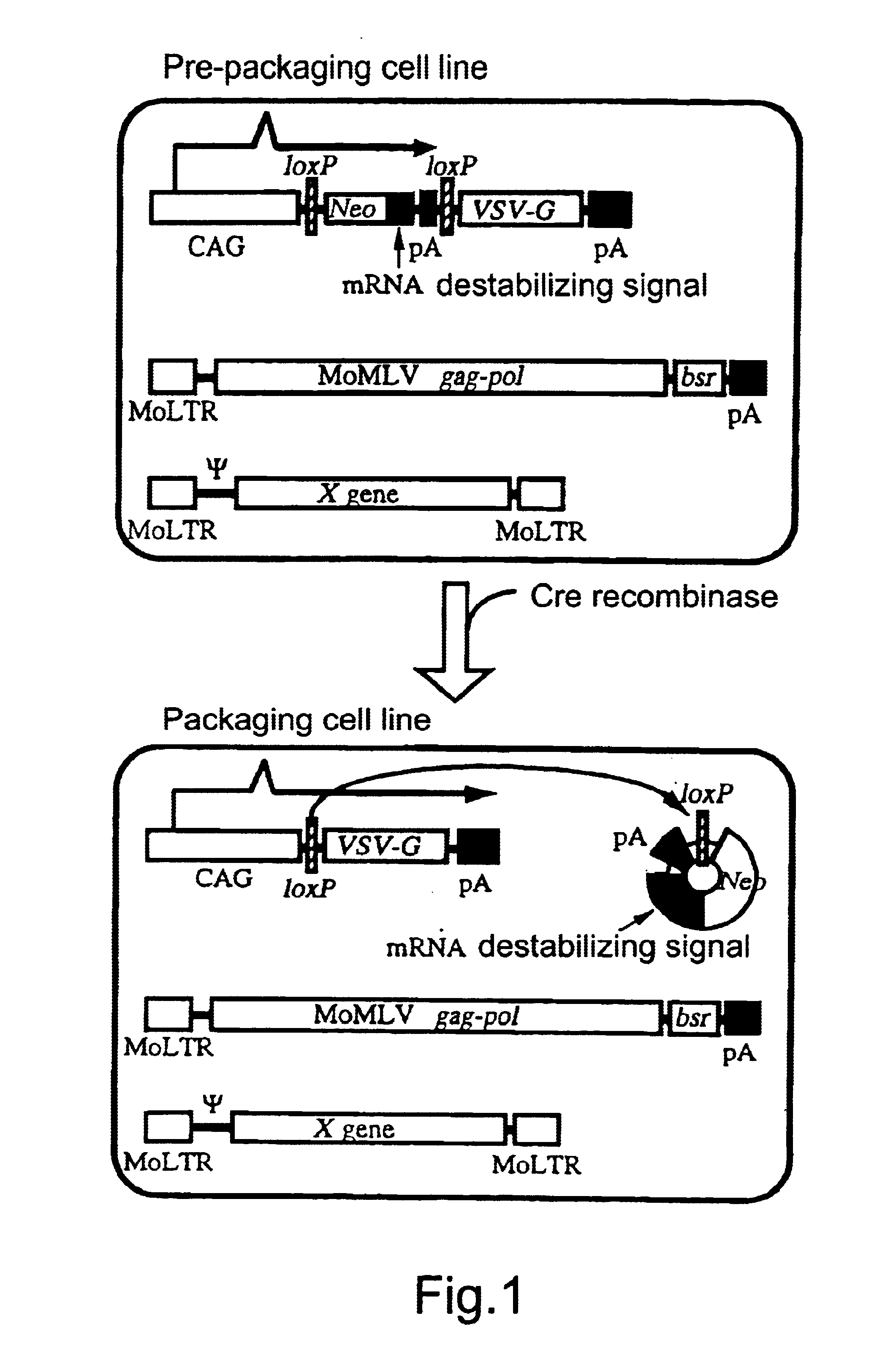
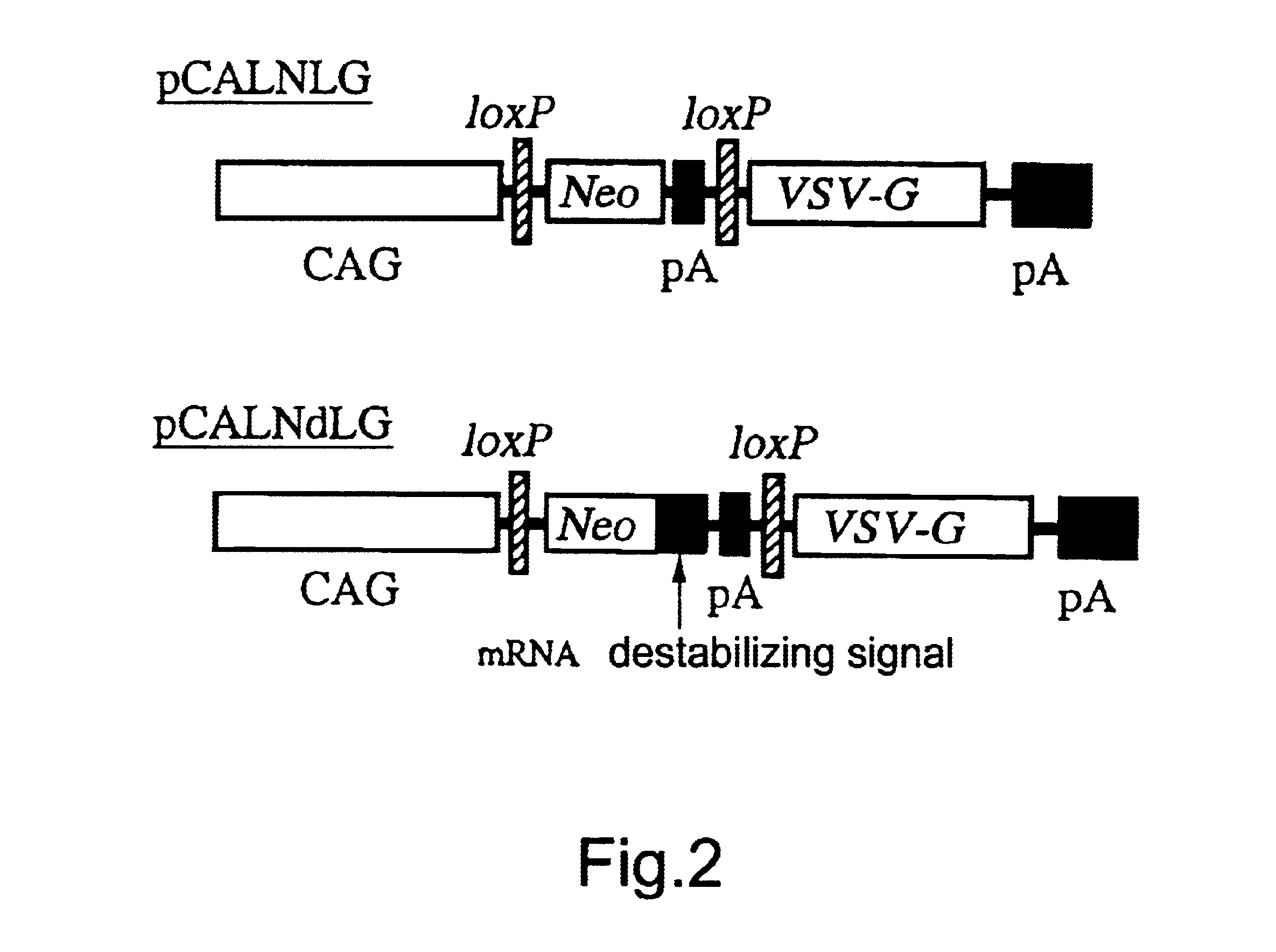
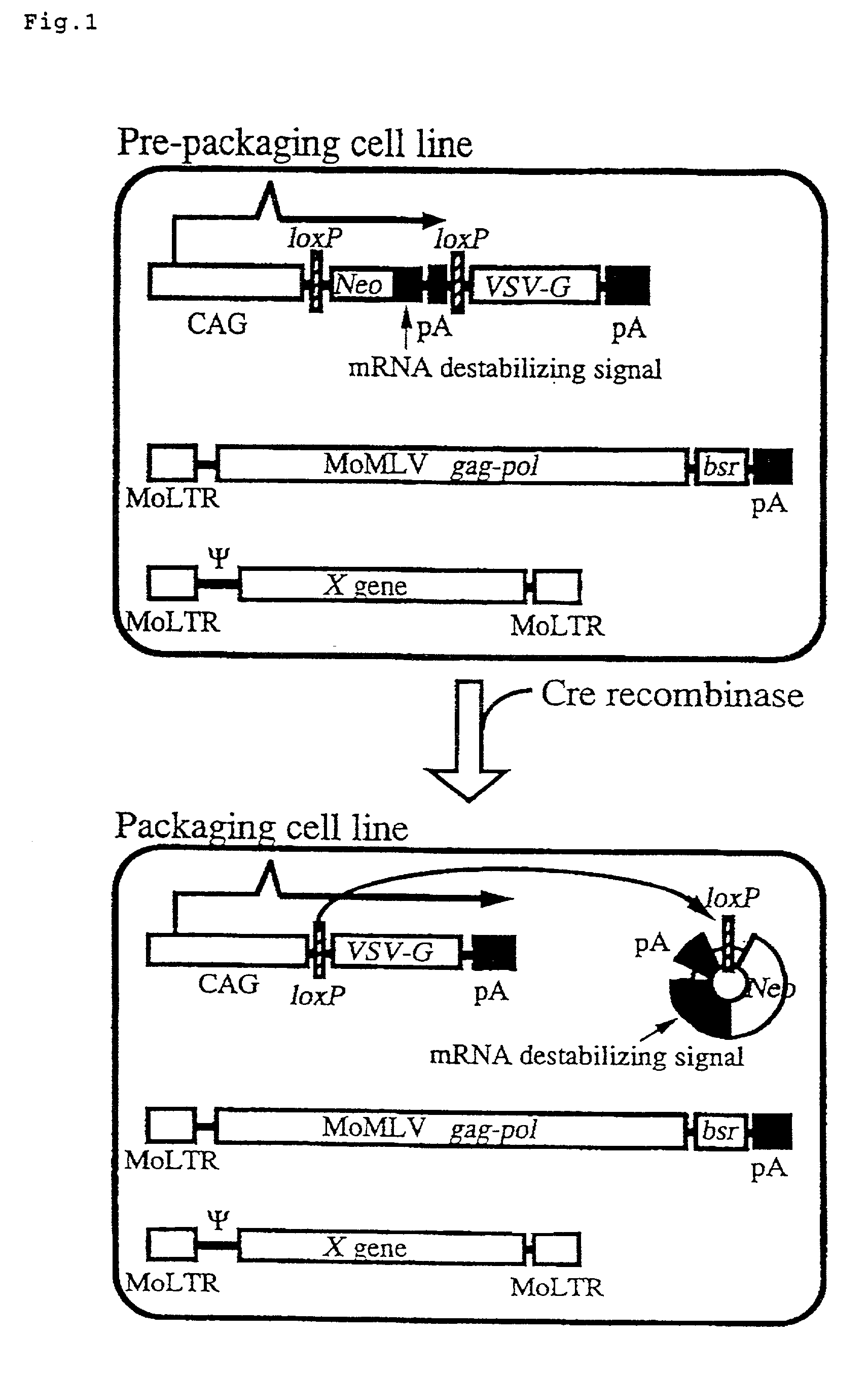
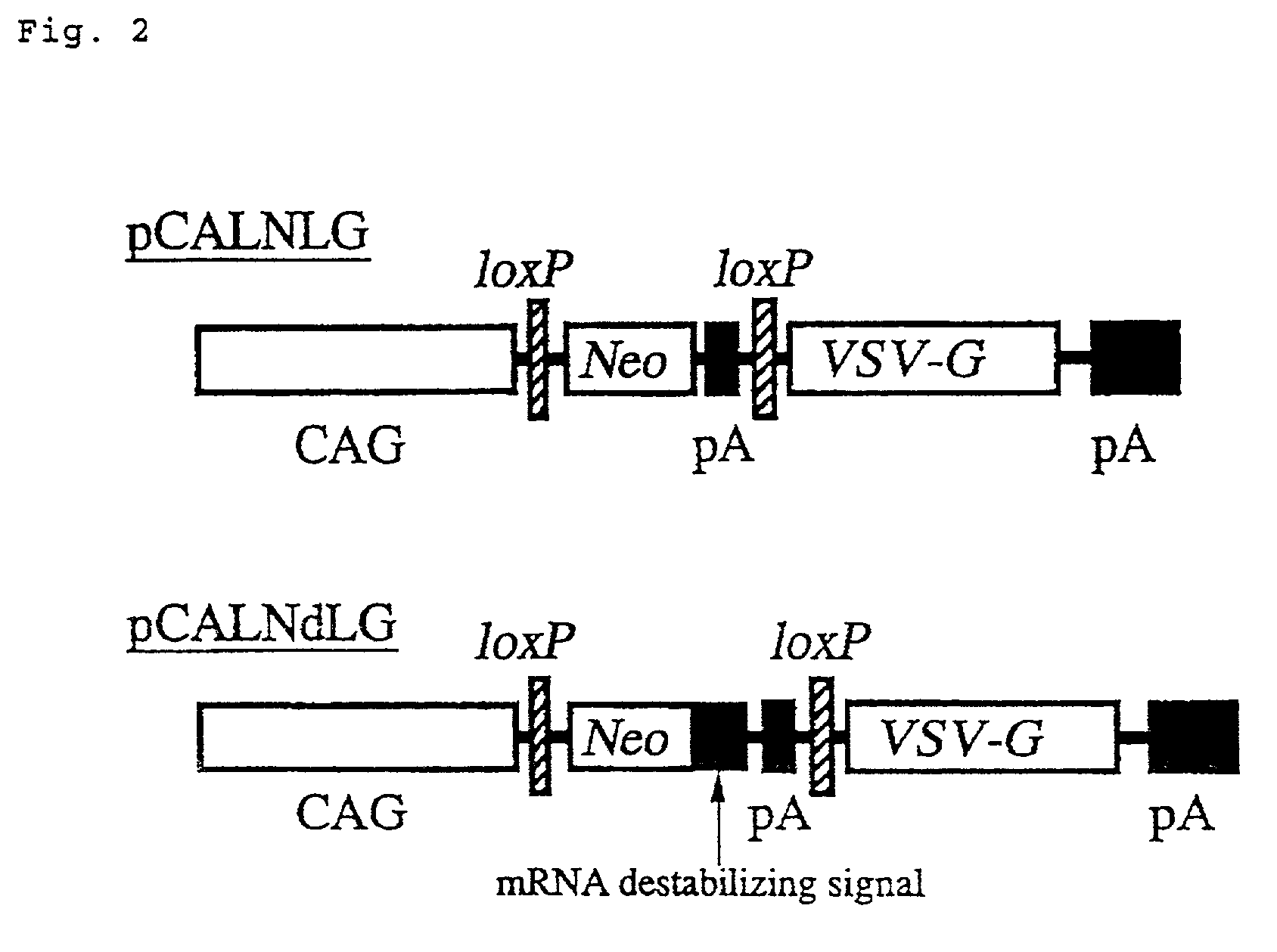
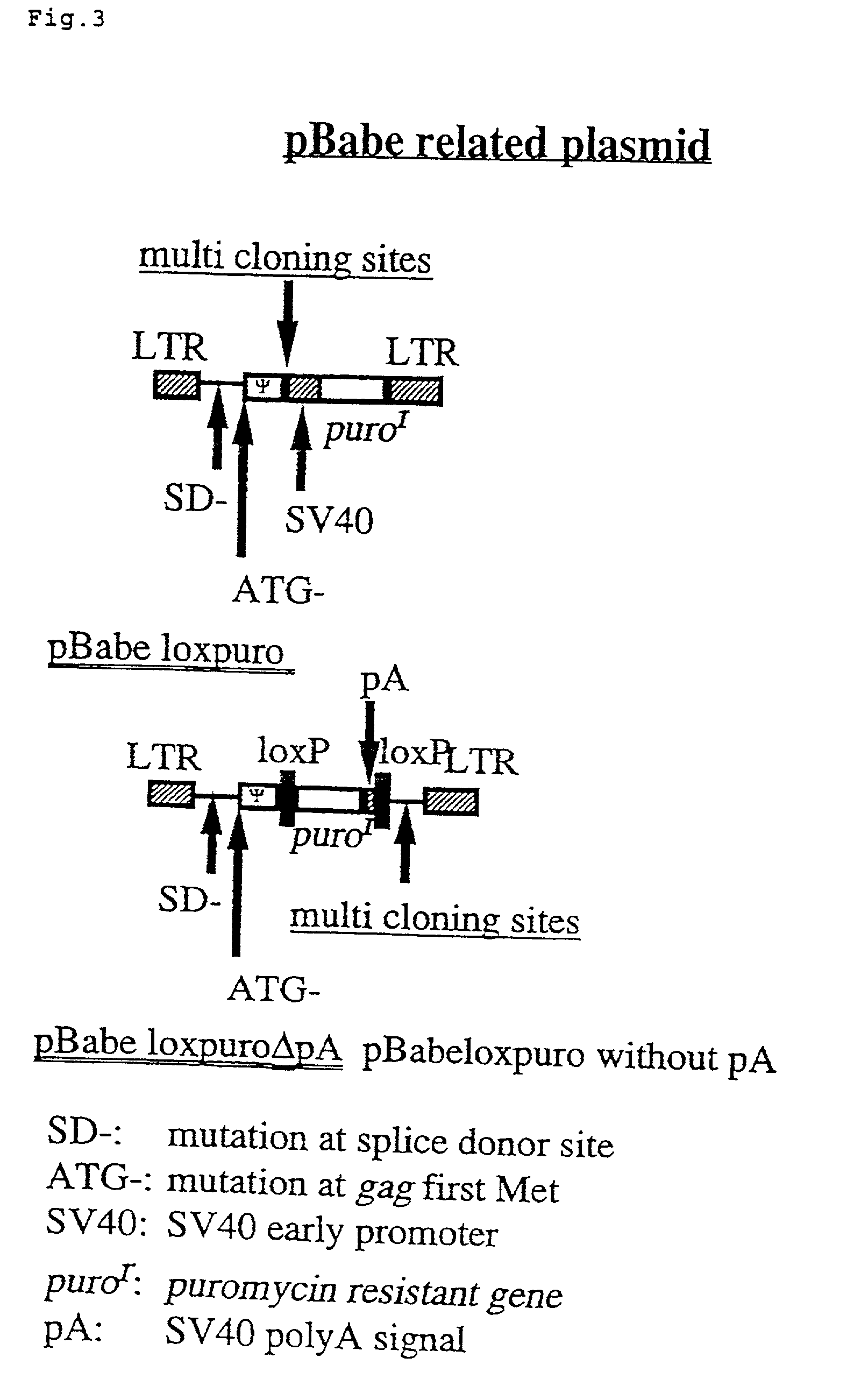
The present invention provides a process for preparing a retrovirus to be expressed at a high titer by specifically transferring a desired foreign gene into target cells. A pseudotyped retrovirus vector having a high titer can be prepared by transferring a DNA construction wherein a promoter, an loxP sequence, a VSV-G gene and a polyA addition signal are arranged in this order is transferred into cells carrying the retrovirus gag and pol gene expression systems, and then transferring a retrovirus vector containing the desired foreign gene thereinto, followed by treatment with a recombinase.
Owner:EISIA R&D MANAGEMENT CO LTD
Triple minRNA for resisting virus infection of aids and construction method thereof
InactiveCN103184224APrevent escapeHigh infection efficiencyAntiviralsFermentationPol genesRna expression
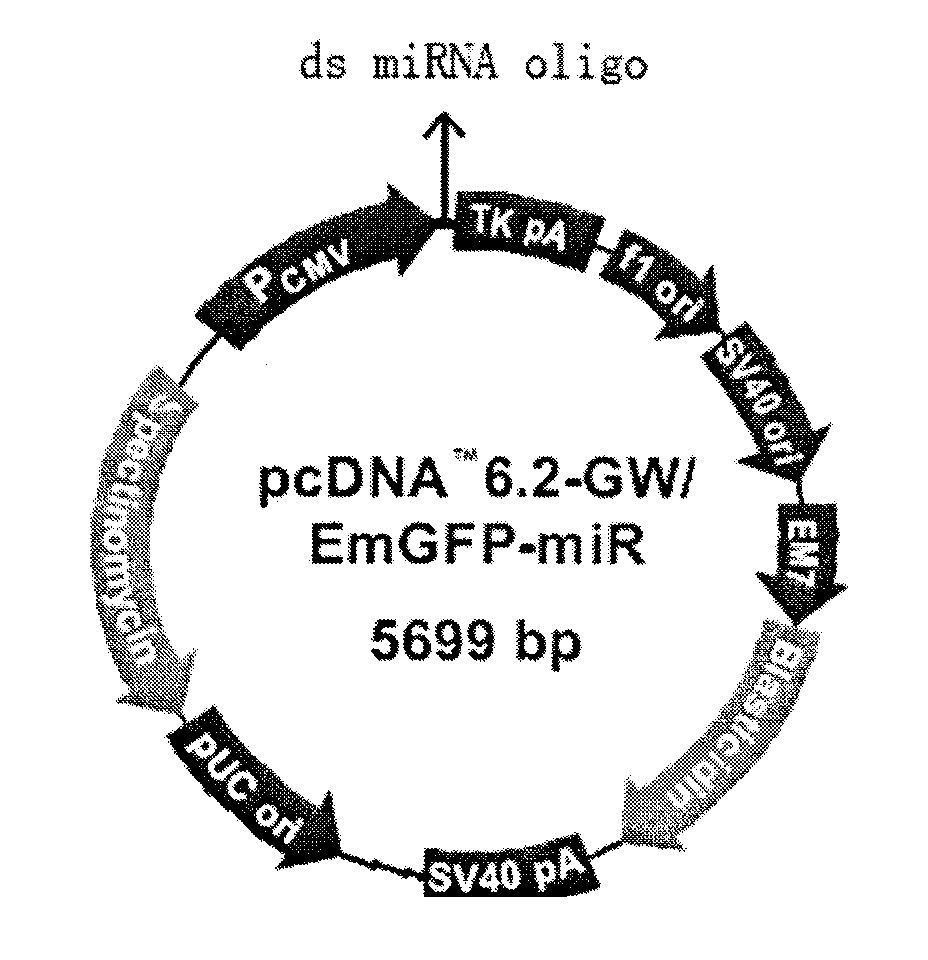
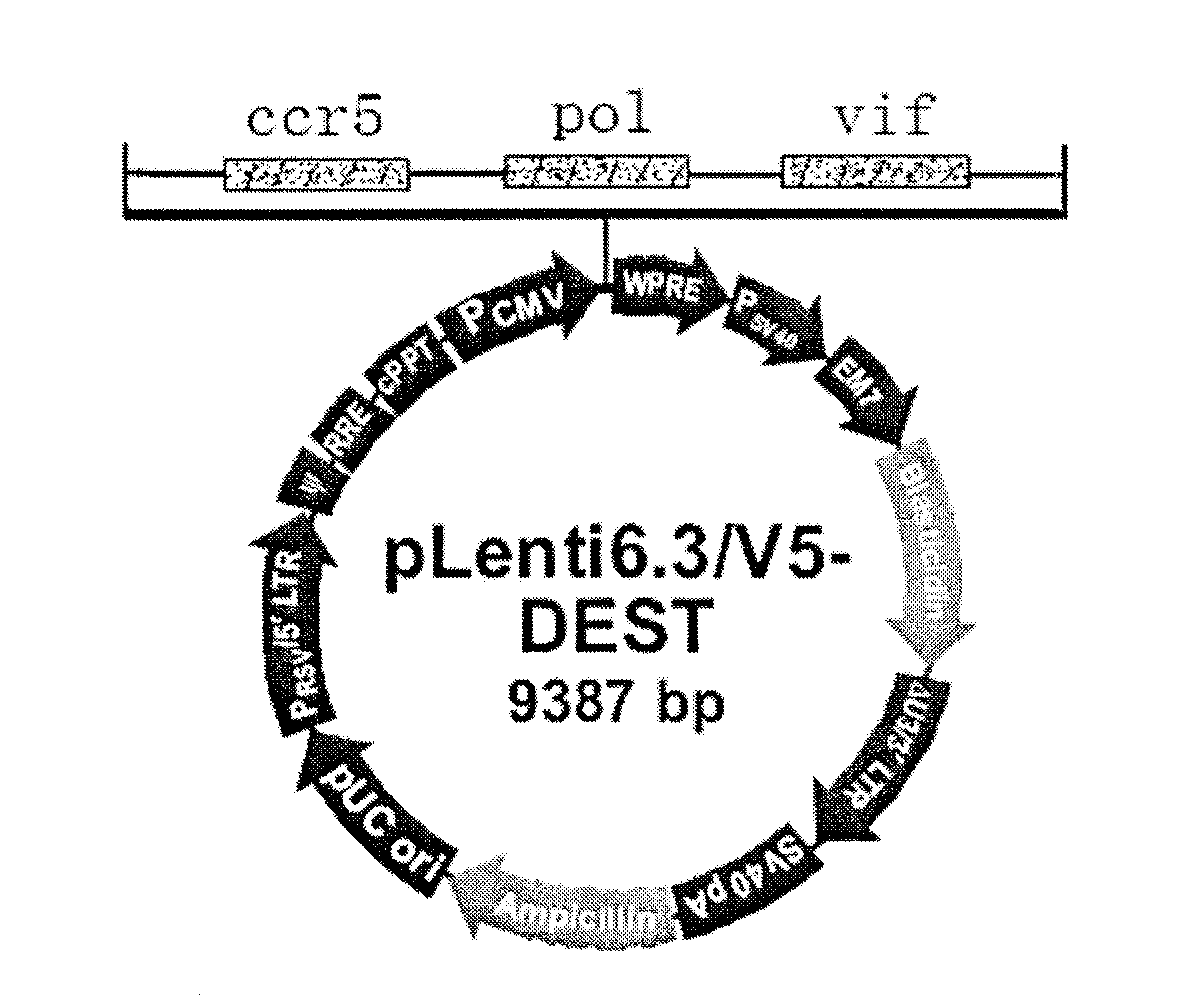
The invention discloses a triple minRNA for resisting virus infection of aids and the construction method thereof. The sequences of the triple minRNA are respectively designed according to gene sequences of ccr5, pol and vif and combined to be miRNA oligomeric and single strand DNA, then double-strand DNA is formed through annealing, then double-strand DNA is respectively inserted into the expression vector of pcDNA<TM> 6.2-GW / EmGFPmiR of miRNA to built the mi RNA expression plasmid, and the jamming effect of mi RNA expression plasmid to target gene can be detected through a Qpcr method. A miRNA string with the best gene interference effect to ccr5, vif and pol is inserted into a carrier of pcDNA 6.2 <TM>-GW / EmGFP, so as to obtain a series-wound interference vector of pcDNA6.2<TM>-GW / EmGFP-ccr5-pol-vif, and then the miR-ccr5-pol-vif in the series-wound interference vector is transferred to a lentiviral vector pLenti6.3 / V5-DEST so as to construct pLenti6.3 / V5-DEST miR-ccr5-pol-vif. The recombinant lentiviral vector can product three miRNA at the same time to cause interference effects to gene ccr5, gene pol and gene vif, so as to prevent the gene ccr5, the gene pol and the gene vif from expressing corresponding protein. Therefore, the effect of controlling Hiv infection and replication can be achieved.
Owner:HENGYANG NORMAL UNIV
Non-infectious, non-replicating, immunogenic human immunodeficiency virus-like particles
InactiveUS7008784B1Improving immunogenicitySafe preparationNanotechVirus peptidesHeterologousPol genes
The present invention is directed toward methods for the production of non-infectious, replication-deficient, immunogenic human immunodeficiency virus (HIV)-like particles. These particles are prepared from a recombinant expression vector comprising a heterologous promoter operatively connected to a DNA molecule comprising a modified HIV genome devoid of the long terminal repeat (LTR) regulatory regions but containing at least the gag and pol genes in their natural genomic arrangement. This vector is introduced into mammalian cells to produce the particles of interest. These particles should prove useful in a number of diagnostic, virologic, and immunologic applications.
Owner:AVENTIS PASTEUR LTD
Primer for detecting main drug-resistant mutation sites of aids therapeutic nucleoside reverse transcriptase inhibitor and application thereof
InactiveCN105624333AEfficient detectionOvercome the cycleMicrobiological testing/measurementMicroorganism based processesNucleoside Reverse Transcriptase InhibitorPol genes
Owner:GUANGXI MEDICAL UNIVERSITY
Process for preparing retrovirus vector for gene therapy
InactiveUS20010018203A1High recovery rateStably produced at a high titerVectorsGenetic material ingredientsPol genesA-DNA
The present invention provides a process for preparing a retrovirus to be expressed at a high titer by specifically transferring a desired foreign gene into target cells. A pseudotyped retrovirus vector having a high titer can be prepared by transferring a DNA construction wherein a promoter, an loxP sequence, a VSV-G gene and a polyA addition signal are arranged in this order is transferred into cells carrying the retrovirus gag and pol gene expression systems, and then transferring a retrovirus vector containing the desired foreign gene thereinto, followed by the treatment with a recombinase.
Owner:EISIA R&D MANAGEMENT CO LTD
LAMP (Loop-Mediated Isothermal Amplification) kit for detecting main subtype avian leukemia virus
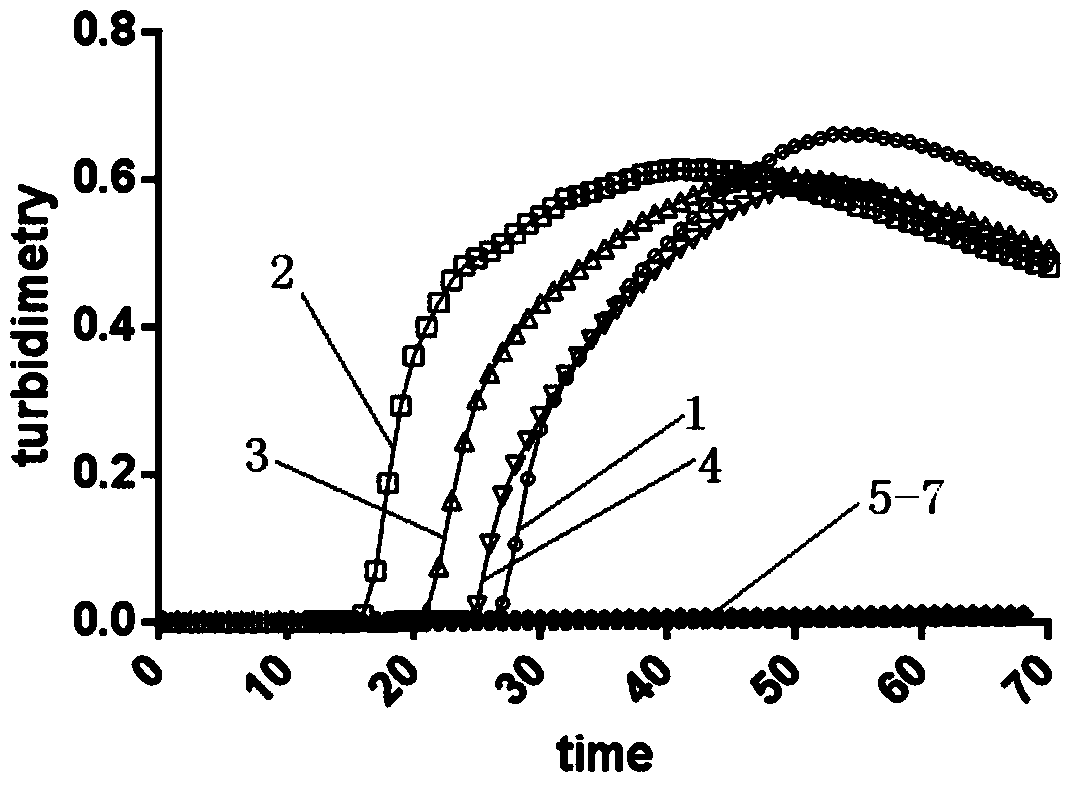
The invention discloses an LAMP (Loop-Mediated Isothermal Amplification) kit for detecting a main subtype avian leukemia virus. A loop-mediated isothermal amplification (LAMP) technology is adopted, and two pairs of specific primers (inner primers FIP and BIP, outer primers F3 and B3) are designed according to a POL (Point Of Load) gene sequence of the avian leukemia virus. By applying the LAMP kit and a detecting method established by the LAMP kit, an LAMP real-time turbidity meter is utilized to carry out real-time, quantitative and whole-course sealed monitoring analysis on LAMP reaction primers of the ALV (Avian Leukemia Virus), a reaction system and a reaction process, so that LAMP primers of the ALV are efficiently and specifically amplified. The LAMP kit disclosed by the invention has the advantages that the specificity is strong, the sensitivity is high, a result is quickly obtained, pollution is not caused and a product can be detected in real time; the avian leukemia virus can be detected out in real time by sampling according to the established system, and the detected result can be quickly and accurately obtained, so that convenience is brought for simply and quickly detecting the avian leukemia virus.
Owner:GUANGXI UNIV
HIV medicament screening cell model and special pseudotype lentivirus therefor
InactiveCN101353666AIncreased sensitivityImprove securityMicrobiological testing/measurementViruses/bacteriophagesPol genesHerpetic stomatitis
The invention discloses an HIV drug screening cell model and special pseudotype slow virus thereof; the invention constructs recombinant plasmid for expressing Gag gene and Pol gene of HIV, recombinant slow-virus plasmid for expressing reporter gene and recombinant plasmid for expressing Rev gene of HIV; the three plasmids and recombinant plasmid for expressing glycoprotein gene of capsid G of herpetic stomatitis are transfected in the cells of mammals so as to obtain pseudotype slow virus; the pseudotype slow virus is used for infecting the cells of mammals, thus obtaining the HIV drug screening cell model based on the reporter gene. The HIV drug screening cell model provided by the invention uses the virus with one-time infection with good safety and the reporter gene leads the cell model to have super high sensitivity.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
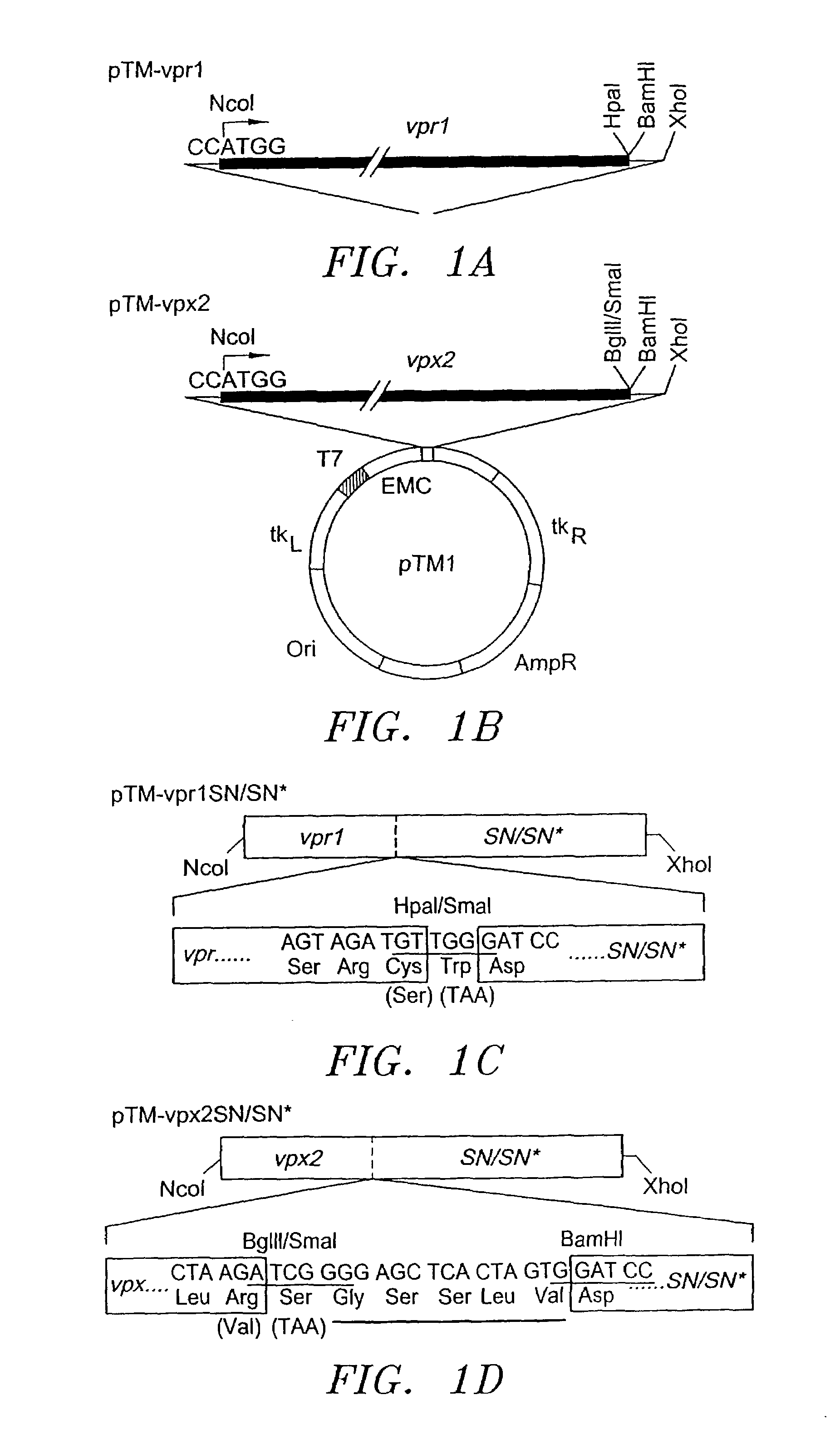
Fusion protein delivery system and uses thereof
The present invention provides a composition of matter, comprising: DNA encoding a viral Vpx protein fused to DNA encoding a protein. In another embodiment of the present invention, there is provided a composition of matter, comprising: DNA encoding a viral Vpr protein fused to DNA encoding a protein. The present invention further provides DNA, vectors and methods for expressing a lentiviral pol gene in trans, independent of the lentiviral gag-pol. A gene transduction element is optionally delivered to a lentiviral vector according to the present invention. Also provided are various methods of delivering a virus inhibitory molecule to a target in an animal. Further provided is a pharmaceutical composition.
Owner:UAB RES FOUND
Cell for Producing Retrovirus Vector
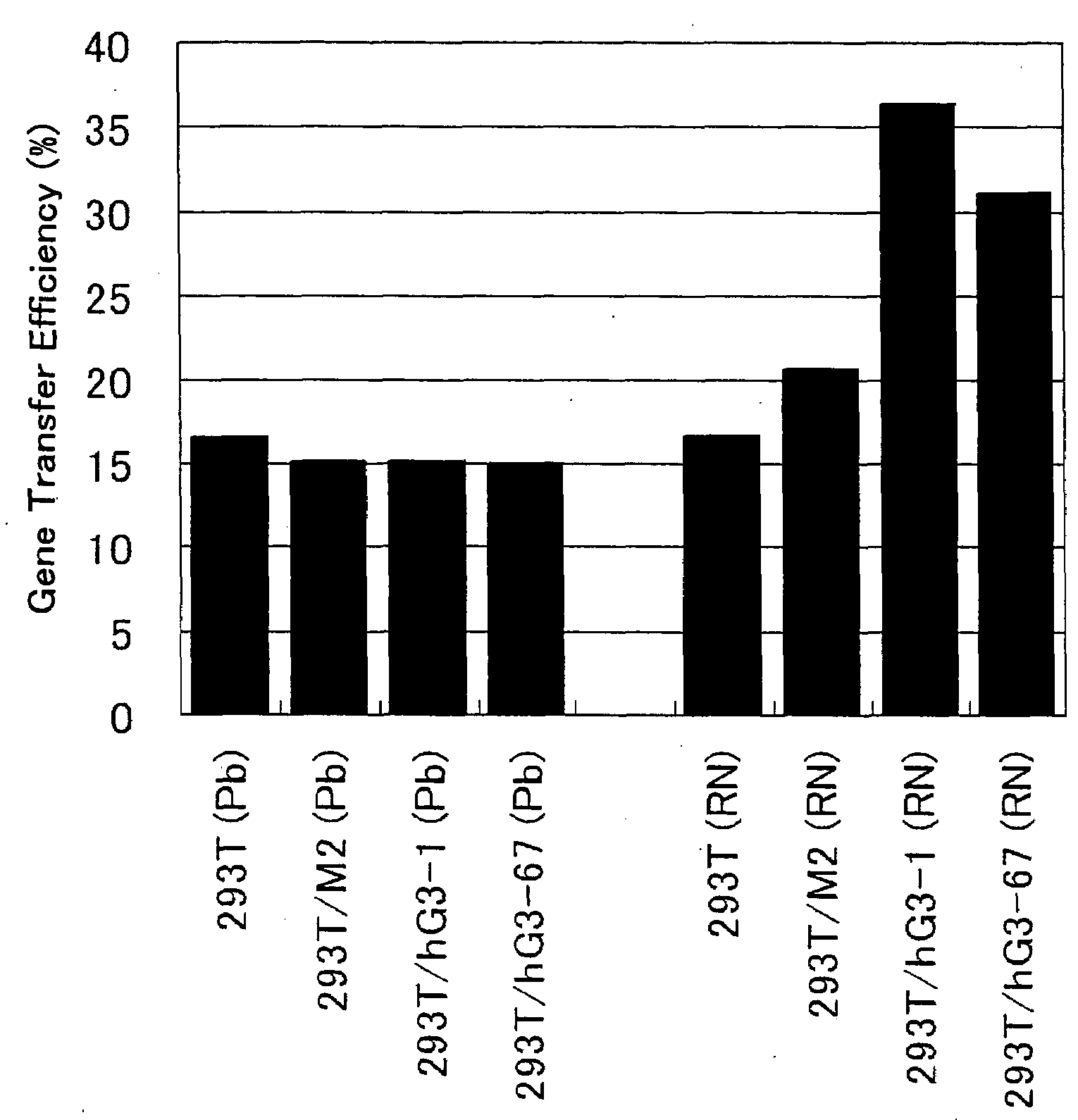
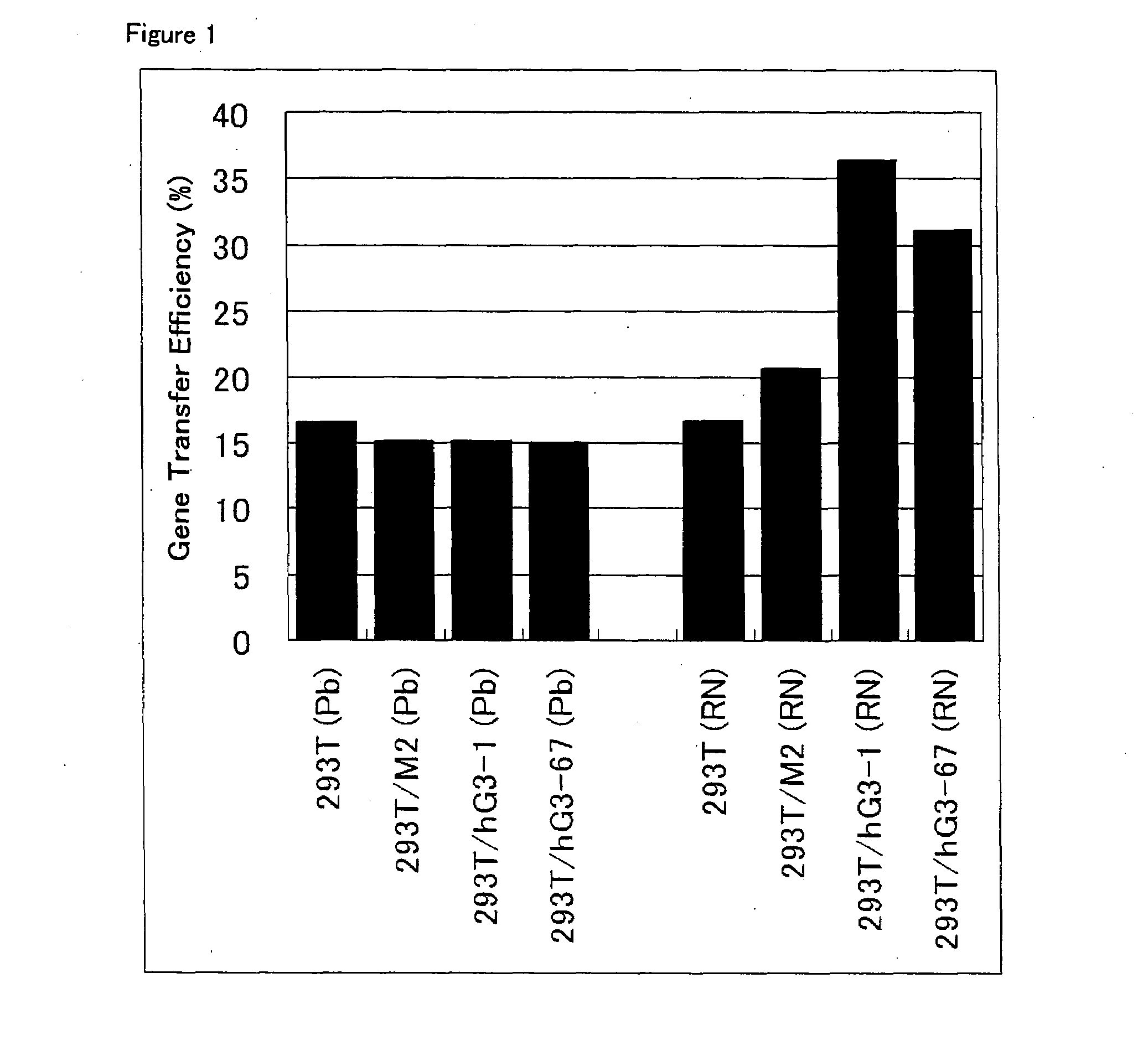
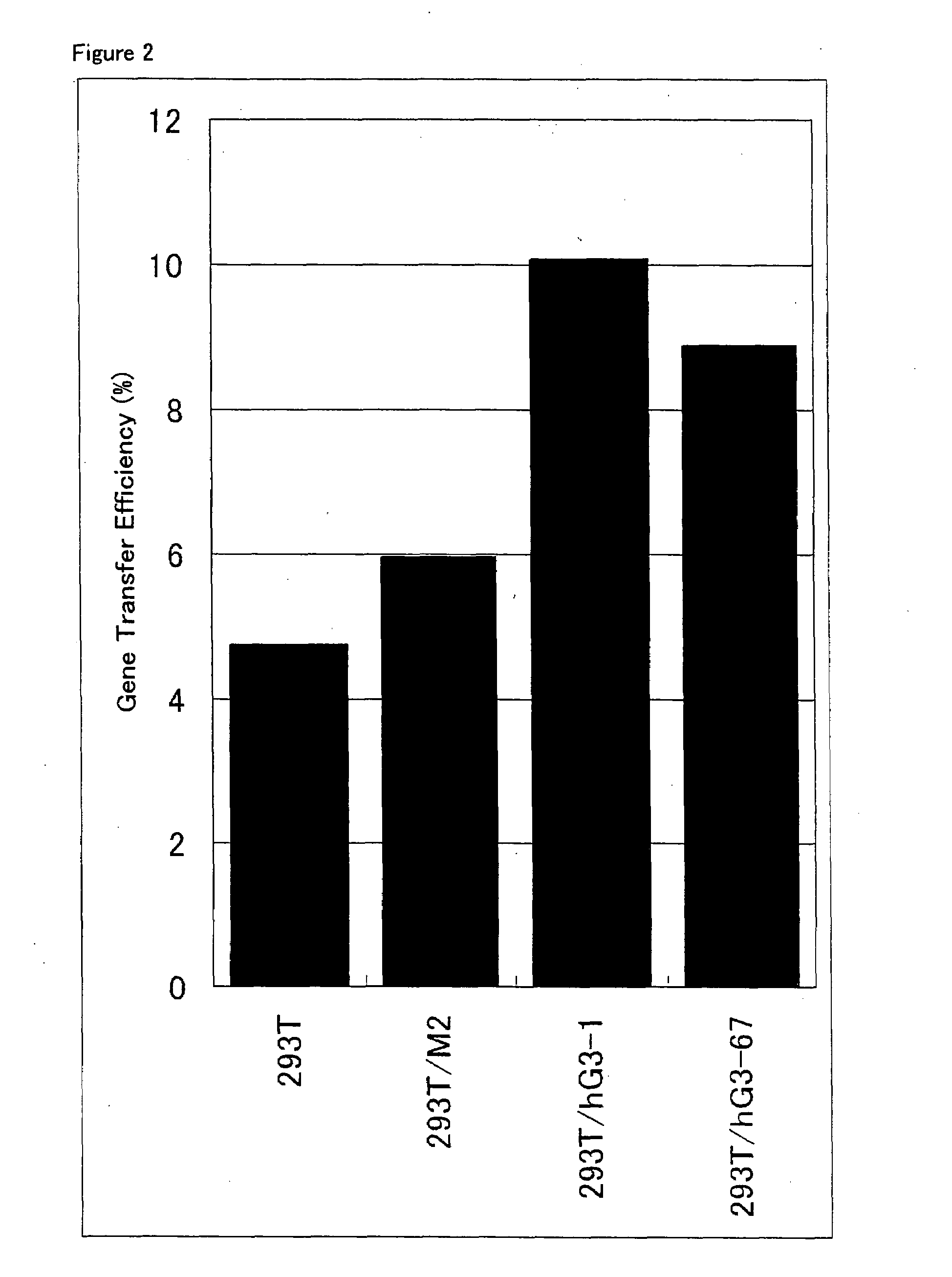
ActiveUS20080293141A1Improve gene transfer efficiencySmooth transferVectorsHybrid cell preparationPol genesEnvelope Gene
The N-acetylglucosaminyltransferase III activity is enhanced in a cell carrying retrovirus-origin gag-pol gene and env gene. By constructing a retrovirus vector with the use of the above cell, a retrovirus vector having a modified sugar chain structure can be obtained. The retrovirus vector constructed by this method shows a high infection efficiency particularly in the presence of a functional substance.
Owner:TAKARA HOLDINGS
Kit and method for detecting genetic mutation of PR regions and RT regions of HIV-1 genes
ActiveCN109439800AEfficient amplificationStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesPol genesMutation detection
The invention provides a kit and method for detecting genetic mutation of PR regions and RT regions of HIV-1 genes, and particularly discloses method for detecting genetic mutation of PR regions and RT regions of HIV-1 virus pol genes in plasmas of HIV patient, a primer and a kit comprising mixed liquor of the primer. Based on a generation of sequencing platform, the method has the advantages of being multiple in mutation detection sites, high in accuracy and easy in sample acquisition.
Owner:DAAN GENE CO LTD
Two-chain small-molecular interference ribonucleic acid and its compination for treating preventing and treating AIDS
The invention relates to double bond small molecule disturbance ribonucleic acid to prevent and cure AIDS. The double bond small molecule disturbance ribonucleic acid is RNA double bond molecule, which is called siRNA double bond molecule that has 19 base pairings. Two protrude basic groups dT are on the ends of 5' of positive bond and negative bond. GC content is 40-55%. Target sequence is gag gene, pol gene, vif gene, vpr gene, env gene, and nef gene selected from HIV gene. The positive bond sequence of siRNA double bond molecule is selected from SEQ ID NO.1-116, and the negative bond is corresponding to positive sequence. The invention conquers the problem of validity decreasing or losing effectiveness of siRNA because of the mutation of HIV-I virus.
Owner:李宝健
Stable pseudotyped lentiviral particles and uses thereof
PendingUS20190300902A1High physical titersHigh infectious titersGenetic material ingredientsVirus peptidesPol genesHeterologous
The present invention relates to a method for obtaining stable pseudotyped lentiviral particles including a heterologous gene of interest, comprising the following steps: a) transfecting at least one plasmid in appropriate cell lines, wherein said at least one plasmid comprises the gene of interest, the rev. gag and pol genes, and a sequence coding for an ERV syncytin, wherein the rev, gag and pol genes are retroviral genes; b) incubating the transfected cells obtained in a), so that they produce the stable pseudotyped lentiviral particles in the supernatant; and c) harvesting and concentrating the stable lentiviral particles obtained in b). The present invention also relates to a method to transduce immune cells using lentiviral vectors pseudotyped with an ERV syncytin glycoprotein. The method can be performed on non-stimulated blood cells or on cells stimulated briefly with IL7, and the cells can be expanded. The stable pseudotyped lentiviral particles obtained are particularly useful in gene therapy.
Owner:GENETHON +2
High-titer retroviral packaging cells
The present invention relates to non-replicative recombinant retrovirus packaging cells able to grow in suspension in a serum-free medium. In particular, the present invention relates to a human embryonic 293SF-based cell line stably expressing gag and pol gene products from the murine Moloney leukemia virus (MLV) and the feline RD114 env gene. This particular combination allows the production of high titer of non-replicative retrovirus pseudotyped and prevents the recombination of plasmids. The recombinant retroviruses produced from these cells are safer and easier to produce for clinical use in gene therapy.
Owner:UNIV LAVAL
High-titer retroviral packaging cells
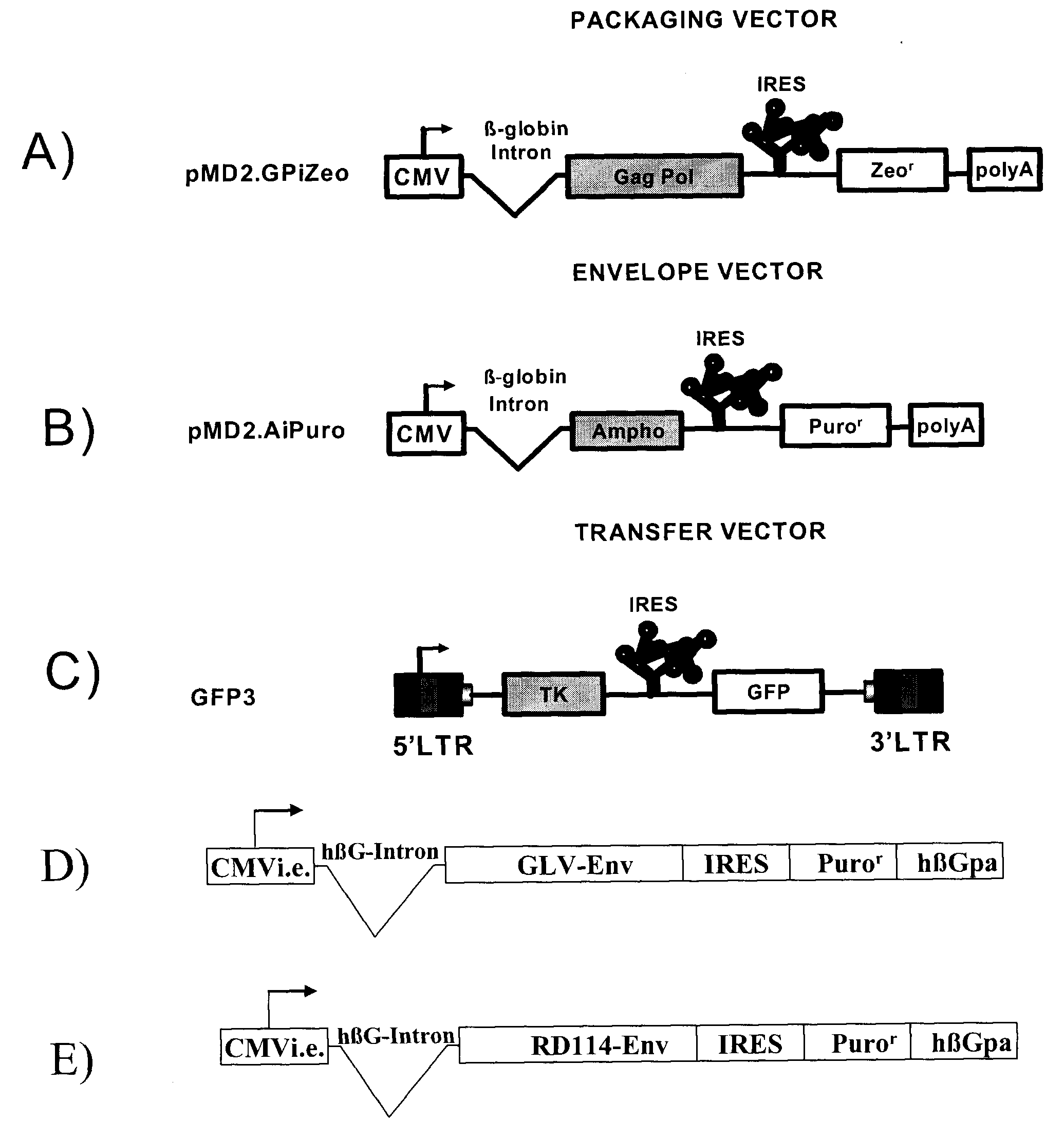
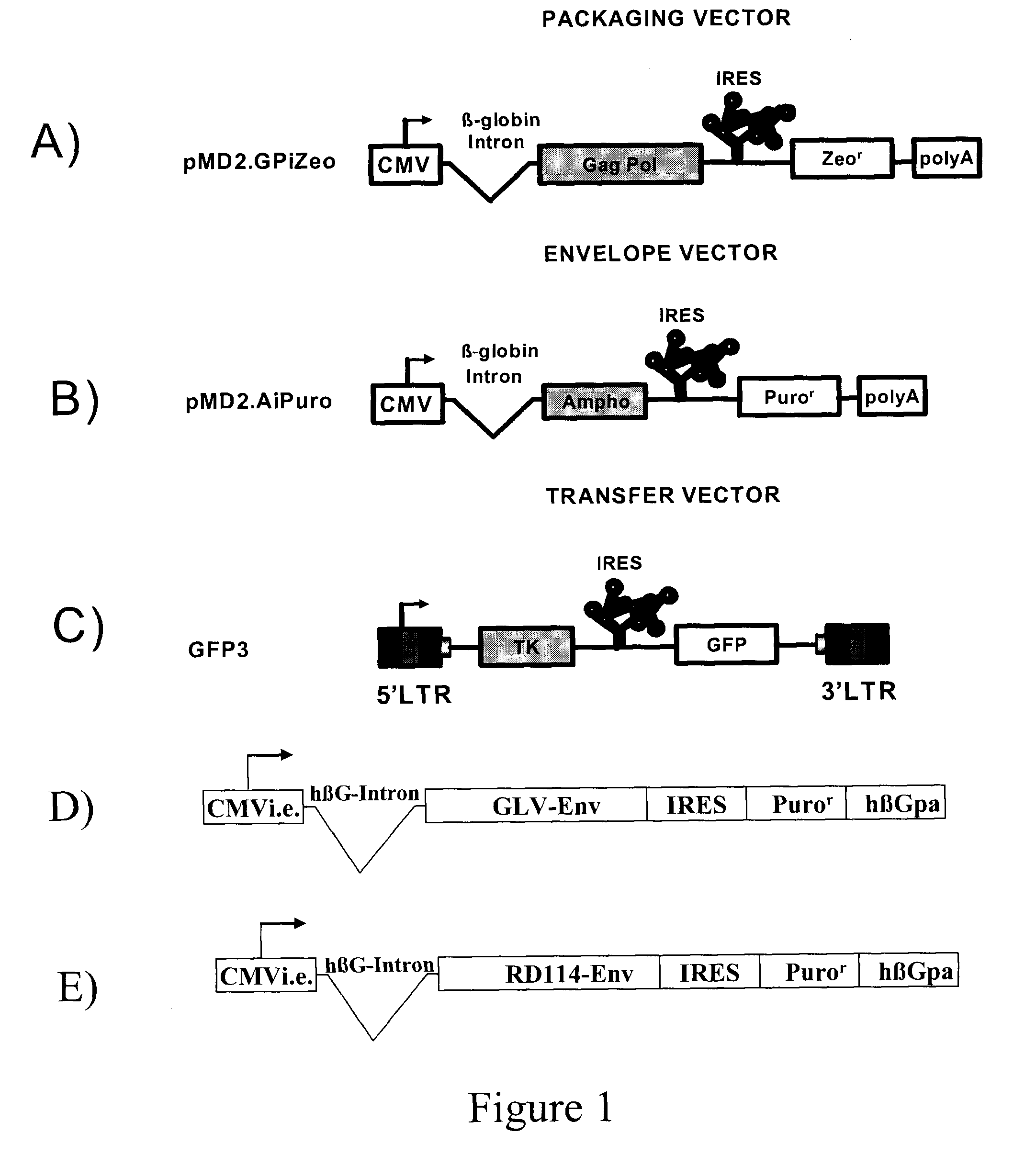
The present invention relates to non-replicative recombinant retrovirus packaging cells able to grow in suspension in a serum-free medium. In particular, the present invention relates to a human embryonic 293SF-based cell line stably expressing gag and pol gene products from the murine Moloney leukemia virus (MLV) and either the feline RD114 env gene, the gibbon ape leukemia virus (GLV) env gene, or the amphotropic 4070Aenv gene. This particular combination allows the production of high titer of non-replicative retrovirus pseudotyped and prevents the recombination of plasmids. The recombinant retroviruses produced from these cells are safer and easier to produce for clinical use in gene therapy.
Owner:UNIV LAVAL
Real-time fluorescent quantitative PCR detection method and reagent kit for simian foamy virus
InactiveCN101831510AImprove accuracyGood precisionMicrobiological testing/measurementFluorescence/phosphorescencePol genesNucleotide
The invention discloses a real-time fluorescent quantitative PCR detection method and a reagent kit for simian foamy virus. Primers and a TaqMan probe are designed according to the conserved regions of SFV Pol genes and are used for quantitatively detecting the number of the copied nucleic acids in the SFV of the simian lymphocytes or related biological products. Specifically, the upstream primer has the nucleic acid sequence of SEQ ID NO: 1 in the sequence table, the downstream primer has the nucleic acid sequence of SEQ ID NO: 2 in the sequence table, and the TaqMan probe has the nucleic acid sequence of SEQ ID NO: 3 in the sequence table. The real-time fluorescent quantitative PCR detection method and the reagent kit can be used for accurately detecting the infection with the SFV of the simian group and the residue of the SFV of related biological products, are very important to the control of the quality of the experimental simian group, the transmission of the SFV, the safety of the medicament use of people and the inspection and the quarantine of import and export of animals and can ensure the quality of elated biological products. The real-time fluorescent quantitative PCR detection method and the reagent kit can be used for the simian foamy virus and has broad application prospect.
Owner:NAT INST FOR THE CONTROL OF PHARMA & BIOLOGICAL PROD
RD114-based retroviral packaging cell line and related compositions and methods
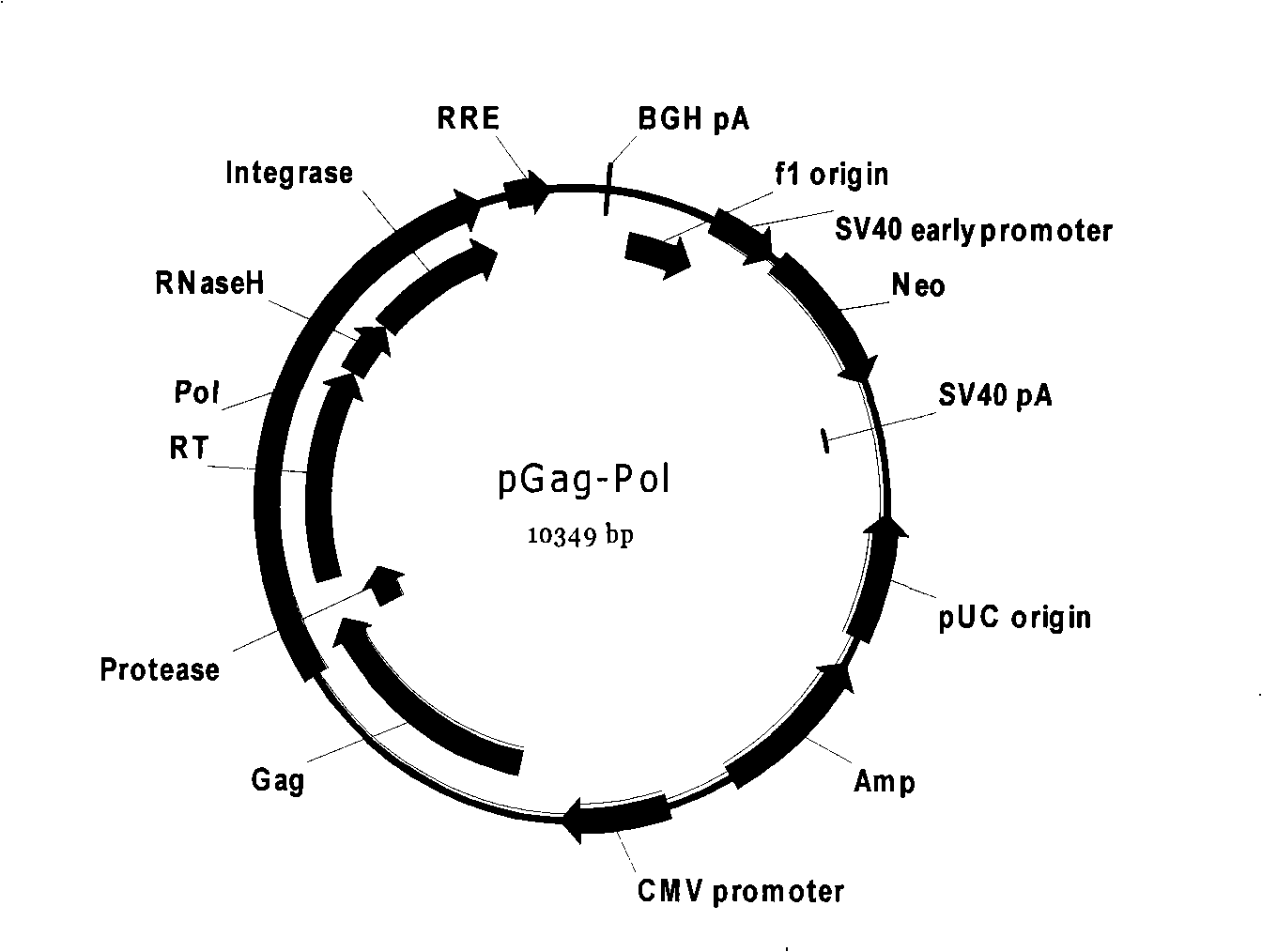
This invention provides a retroviral packaging cell comprising a suitable mammalian cell having therein (i) a first recombinant nucleic acid comprising MMLV gag and pol genes and a selectable marker, and (ii) a second recombinant nucleic acid comprising RD114 envelope gene and a selectable marker, wherein the MMLV gag and pol genes and the RD114 envelope gene are stably expressed in the cell. This invention further provides related production methods, virions and kits.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
High-titer retroviral packaging cells
The present invention relates to non-replicative recombinant retrovirus packaging cells able to grow in suspension in a serum-free medium. In particular, the present invention relates to a human embryonic 293SF-based cell line stably expressing gag and pol gene products from the murine Moloney leukemia virus (MLV) and either the feline RD114 env gene, the gibbon ape leukemia virus (GLV) env gene, or the amphotropic 4070Aenv gene. This particular combination allows the production of high titer of non-replicative retrovirus pseudotyped and prevents the recombination of plasmids. The recombinant retroviruses produced from these cells are safer and easier to produce for clinical use in gene therapy.
Owner:UNIV LAVAL
Primer pair and probe for detecting ABC (Abacavir) drug-resistant mutation sites of AIDS (Acquired immune deficiency syndrome) curing drugs and application of primer pair and probe
PendingCN110079635AStrong specificityEnrichmentMicrobiological testing/measurementDNA/RNA fragmentationPol genesSocial benefits
The invention discloses a primer pair and probe for detecting ABC (Abacavir) drug-resistant mutation sites of AIDS (Acquired immune deficiency syndrome) curing drugs. The primer pair and probe are characterized by comprising an ARMS (Amplification refractory mutation system) primer and a Taqman probe for detecting the mutation sites of K65R, K70E, L74V, L74I, Y115F and Q151M at the 65th, 70th, 74th, 115th and 151th sites of a pol gene of HIV-1 (Human immunodeficiency virus) viral RNA. The invention further provides application of the primer pair and probe in the detection of the ABC major drug-resistant mutation sites of K65R, K70E, L74V, L74I, Y115F and Q151M. According to the primer pair and probe disclosed by the invention, a kit is high in detection sensitivity, good in specificity andlow in detection cost; medication guidance is provided for the treatment of clinical AIDS patients; individualized treatment of the AIDS patients is realized; the effectiveness of the drugs can be improved; the survival time of the AIDS patients is prolonged; wide application prospect and social benefit are realized.
Owner:JIANGSU FAST BIOTECH
Method for mutation detection in HIV-1 using pol sequencing
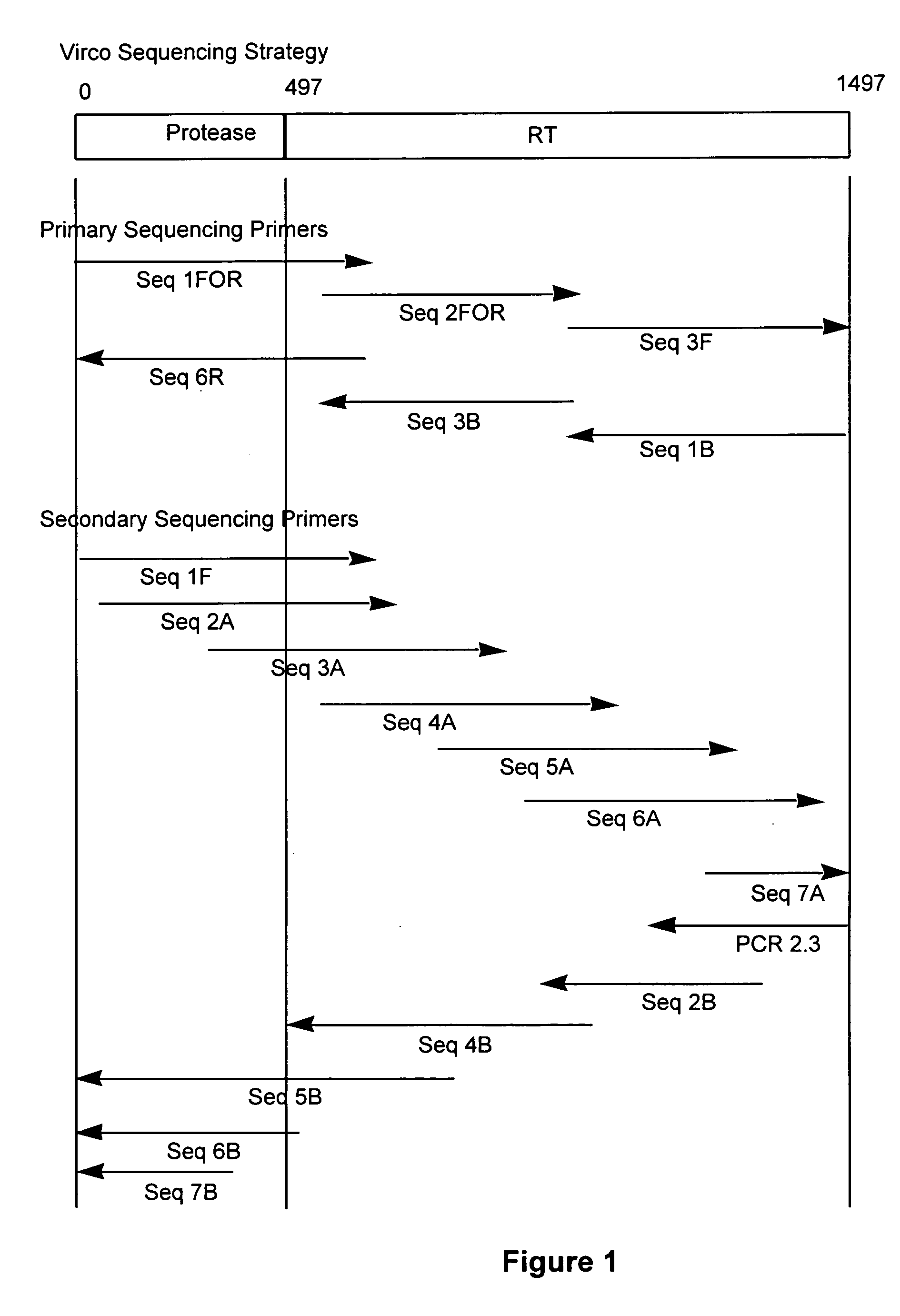
The present invention relates to a method for mutation analysis of the HIV pol gene of HIV virions comprising amplifying virion RNA or DNA via nested PCR using outer primers as represented in SEQ ID No. 1 and 2, amplifying said PCR product via nested PCR using a 5′ and 3′ primer chosen from the inner primers SEQ ID No. 3, 4, 5, and 6, and sequencing this secondary obtained PCR product using at least one sequencing primer chosen from any of SEQ ID No. 7 to 12 or variants thereof. In the alternative, at least one secondary sequencing primer may be used chosen from any of SEQ ID No. 13 to 24. The benefit of the sequences present in the invention resides in the fact that, with the aid of the oligonucleotides, the sequences of all presently known HIV subtypes and all mutations of the pol gene presently known to yield resistance towards antiretroviral therapy can be determined. The present invention also relates to kits for performing such a method as well as primers for performing the same.
Owner:VIRCO NV
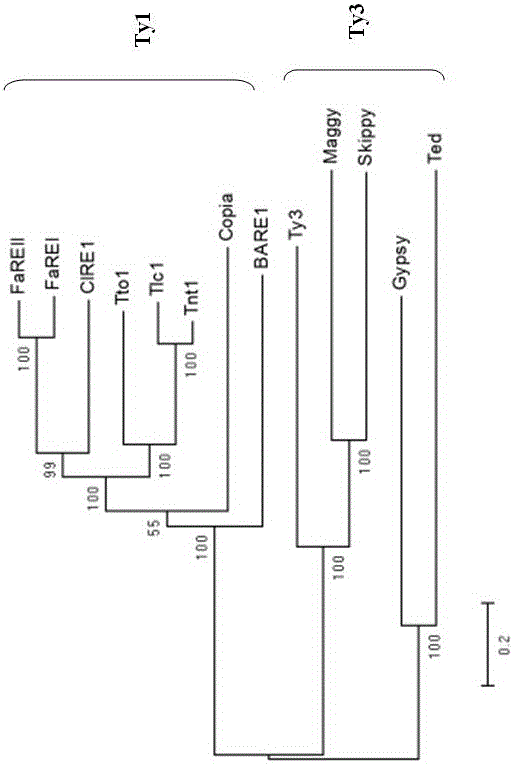
Strawberry reverse transcription transposon gene and transcription characteristic
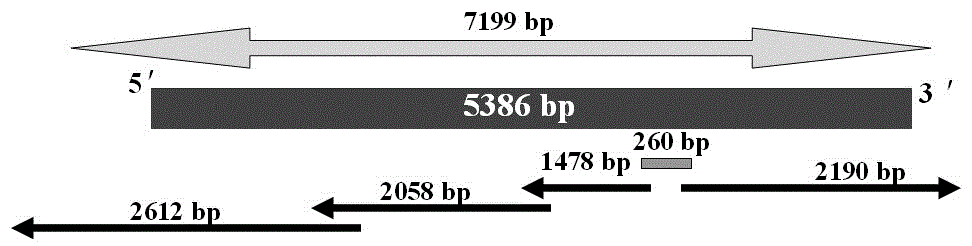
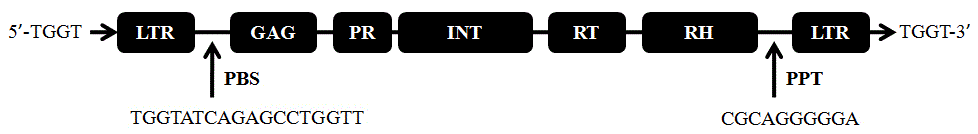
The invention discloses a strawberry reverse transcription transposon gene sequence and a transcription characteristic analysis result. A full length of a gene DNA sequence is 5386 bp, and is named as FaREII, the gene sequence has a Ty1-copia reverse transcription transposon gene characteristic sequence, and is arranged according to gag and pol genes, wherein pol gene contains the characteristic motifs of GAG, PR, IN, RT and RH genes, a long terminal repeat sequence of 393 bp is positioned at two ends of the gene, and the 393 bp is a typical Ty1-copia reverse transcription transposon. According to the invention, the transcription of FaREII can be happened under hormone stress through Nothern Blot.
Owner:SHANDONG INST OF POMOLOGY
Method for detecting integrated copy number of porcine endogenous retrovirus (PERV) through fluorescence quantitative polymerase chain reaction (PCR) and application thereof
InactiveCN101845518AAccurate detectionHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescencePol genesFluorescence
The invention discloses a method for detecting the integrated copy number of a porcine endogenous retrovirus (PERV) through a fluorescence quantitative polymerase chain reaction (PCR) and application thereof. In the method, the copy number of the porcine endogenous retrovirus which is integrated in a genome is detected by using SYBR Green I dye-based fluorescence quantitative PCR technology, and a PERV pol gene conservative region is taken as a detection object. The detection method has the advantages of high sensitivity, high specificity, good repeatability, rapidness, simpleness and convenience, low cost, capability of rapidly and correctly detecting the copy number of the PERV and estimating the copy number of the PERV which is integrated in a single cell genome, suitability for rapidly screening the copy number of the PERV integrated in a heteroplastic small pig donor and a porcine biological material genome and performing long-term monitoring and forward evaluation on the spreading of heteroplastic PERV, deep practical meaning and wide application prospect.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY +1
Retroviral vectors
ActiveUS20200277629A1Reduce riskImprove efficiencySsRNA viruses negative-senseVectorsPol genesViral Genes
The present invention relates to nucleic acid molecules comprising viral genes or derivatives thereof for use in the production of retroviral packaging vectors, and retroviral packaging and producer cell lines. In one embodiment, the nucleic acid molecules comprise env and gag-pol genes wherein the coding sequences of the env and gag-pol genes are in opposing orientations.
Owner:OXFORD GENETICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com